Tablets of mosapride citrate and preparation method thereof
A technology of mosapride citrate and dispersible tablets is applied in the field of medicine and can solve the problem of low solubility of insoluble drugs, low mosapride citrate, and poor dissolution effect of mosapride citrate dispersible tablets. ideals etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053]Mosapride Citrate Dispersible Tablets
[0054] Mosapride Citrate 6g
[0055] Povidone K15 12g
[0056] Lactose 150g
[0057] Starch 150g
[0058] Low-substituted hydroxypropyl cellulose 6g
[0059] 5% povidone K30 aqueous solution appropriate amount
[0060] Micronized silica gel 1.5g
[0061] Magnesium Stearate 1.5g
[0062] Made into 3000 pieces
[0063] Preparation method: each raw and auxiliary material is weighed according to the prescription amount. Add mosapride citrate to 600ml of absolute ethanol, heat in a water bath at 60°C until completely dissolved, then add povidone K15 and stir to dissolve; transfer to a rotary evaporator, evaporate ethanol under reduced pressure at 60°C, Make a solid dispersion; dry the solid dispersion at 60°C for 3 hours under reduced pressure, take it out, crush it, and pass it through a 100-mesh sieve for later use; pass lactose, starch and low-substituted hydroxypropyl cellulose through a 80-mesh sieve, and then add the solid...
Embodiment 2
[0065] Mosapride Citrate Dispersible Tablets
[0066] Mosapride Citrate 7.5g
[0067] Povidone K30 30g
[0068] Lactose 210g
[0069] Starch 60g
[0070] Low-substituted hydroxypropyl cellulose 15g
[0071] 5% povidone K30 aqueous solution appropriate amount
[0072] Micronized silica gel 1.5g
[0073] Magnesium Stearate 1.5g
[0074] Made into 3000 pieces
[0075] Preparation method: each raw and auxiliary material is weighed according to the prescription amount. Add mosapride citrate to 1500ml of absolute ethanol, heat in a water bath at 60°C until completely dissolved, then add povidone K30 and stir to dissolve; transfer to a rotary evaporator, evaporate ethanol under reduced pressure at 60°C, and prepare into a solid dispersion; dry the solid dispersion at 60°C for 4 hours, take it out, crush it, and pass it through a 100-mesh sieve for later use; pass lactose, starch and low-substituted hydroxypropyl cellulose through a 80-mesh sieve, and then add the solid disper...
Embodiment 3
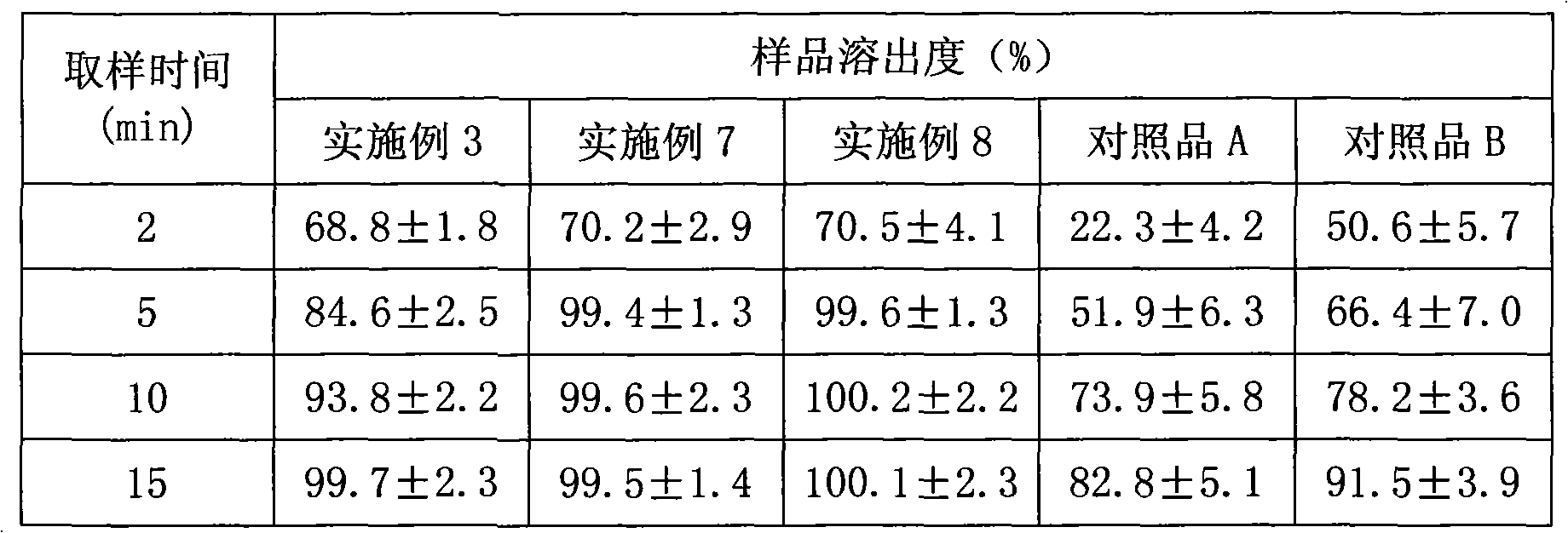
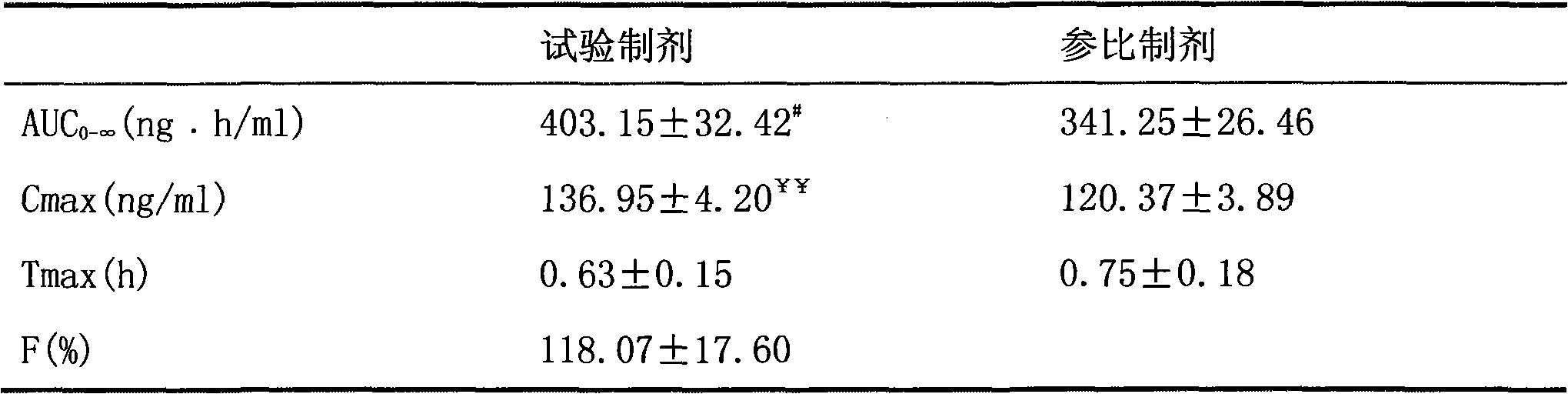
[0076] Embodiment 3 mosapride citrate dispersible tablet
[0077] Mosapride Citrate 15g
[0078] Macrogol 4000 75g
[0079] Lactose 90g
[0080] Starch 150g
[0081] Low-substituted hydroxypropyl cellulose 8g
[0082] 5% povidone K30 aqueous solution appropriate amount
[0083] Micronized silica gel 3g
[0085] Made into 3000 pieces
[0086] Preparation method: each raw and auxiliary material is weighed according to the prescription amount. Add mosapride citrate to 2500ml of absolute ethanol, heat in a water bath at 60°C until completely dissolved, then add polyethylene glycol 4000 and stir to dissolve; transfer to a rotary evaporator, evaporate ethanol under reduced pressure at 60°C, Make a solid dispersion; dry the solid dispersion at 60°C for 4 hours, take it out, crush it, and pass it through a 100-mesh sieve for later use; pass lactose, starch and low-substituted hydroxypropyl cellulose through a 80-mesh sieve, and then add the solid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com