Patents
Literature
2575 results about "Semi solid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
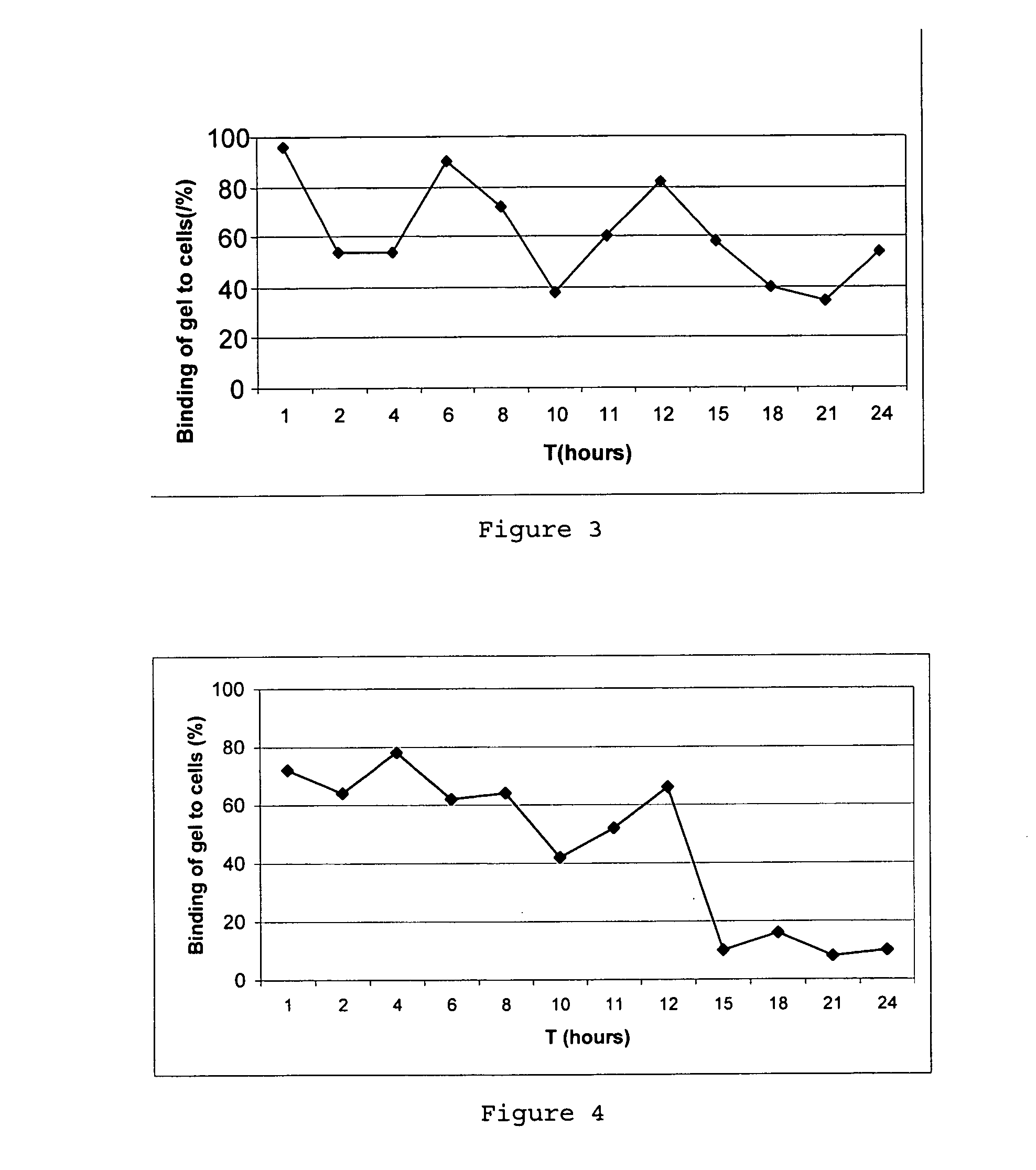
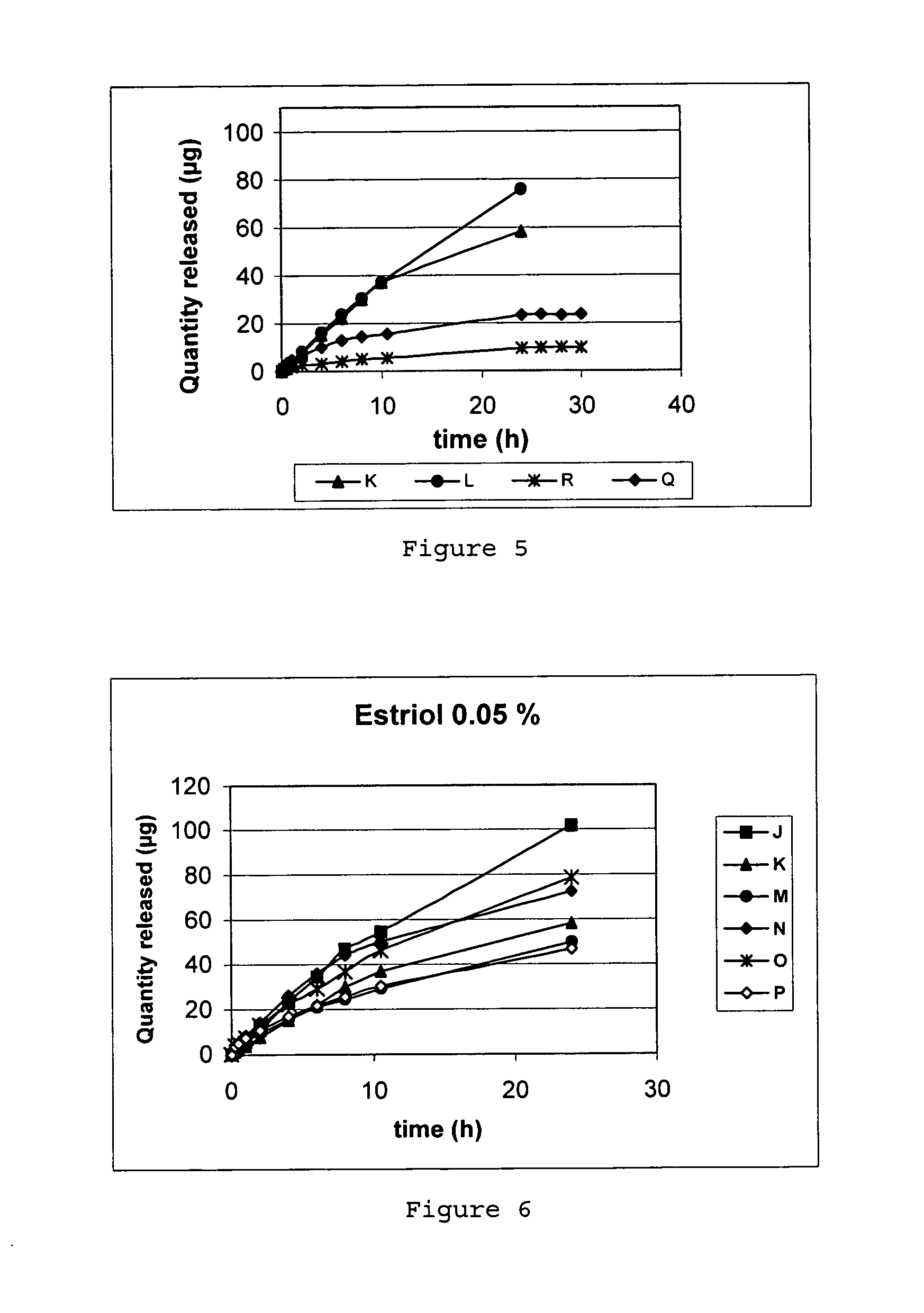
Jump to navigation Jump to search. A semisolid is a substance that is in between a solid and a liquid. Another name for a semi-solid is a quasi-solid.
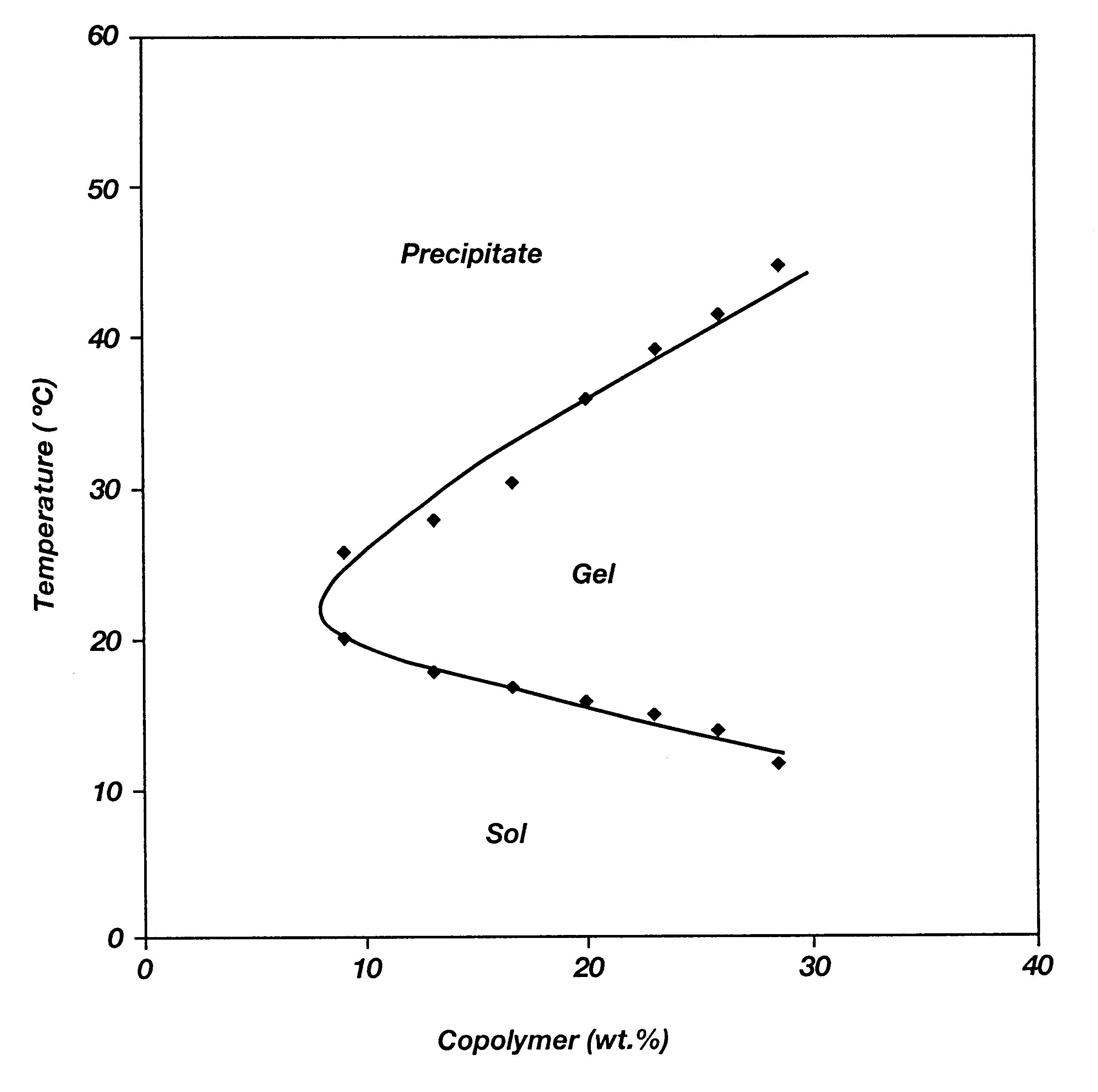
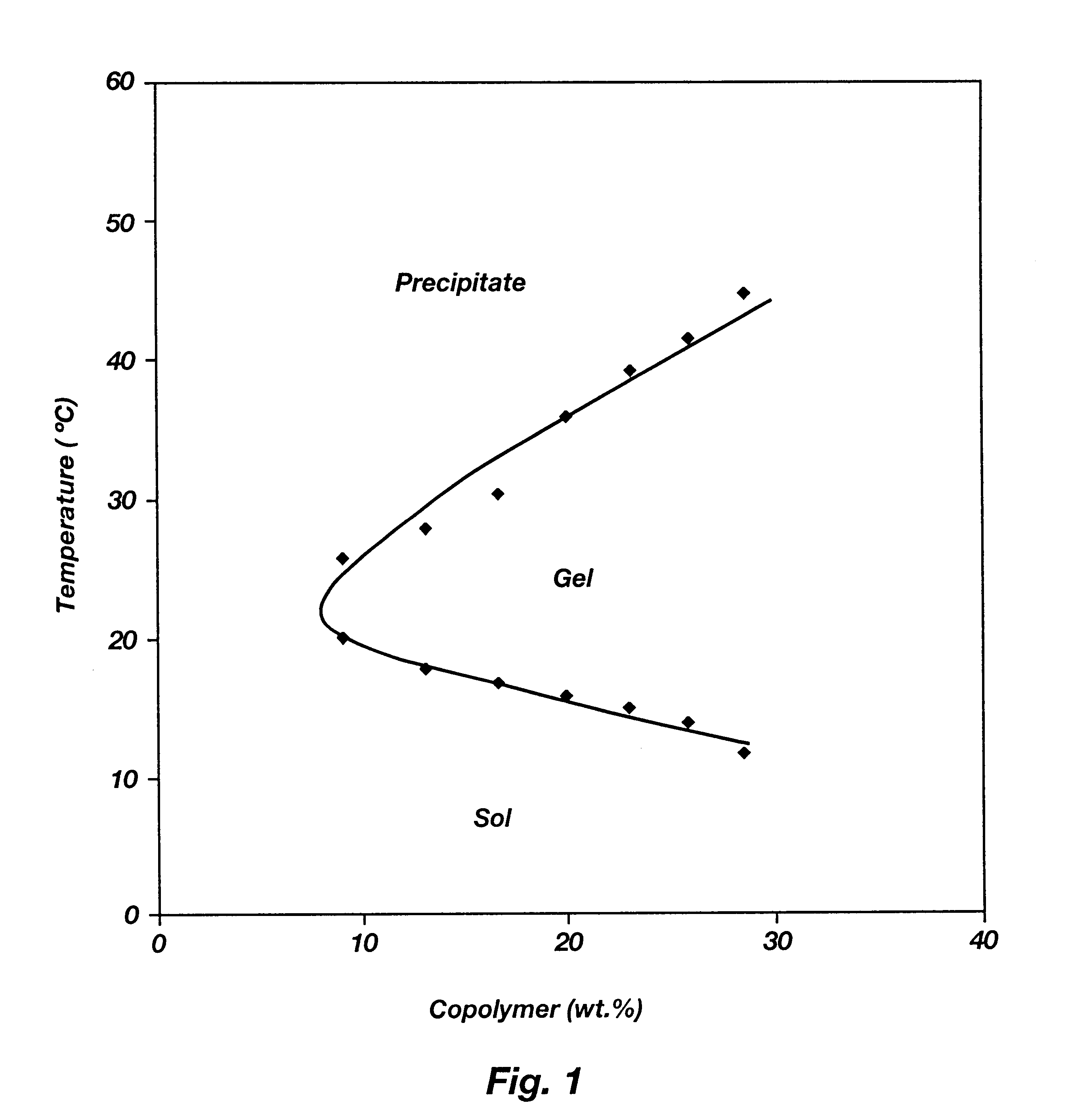
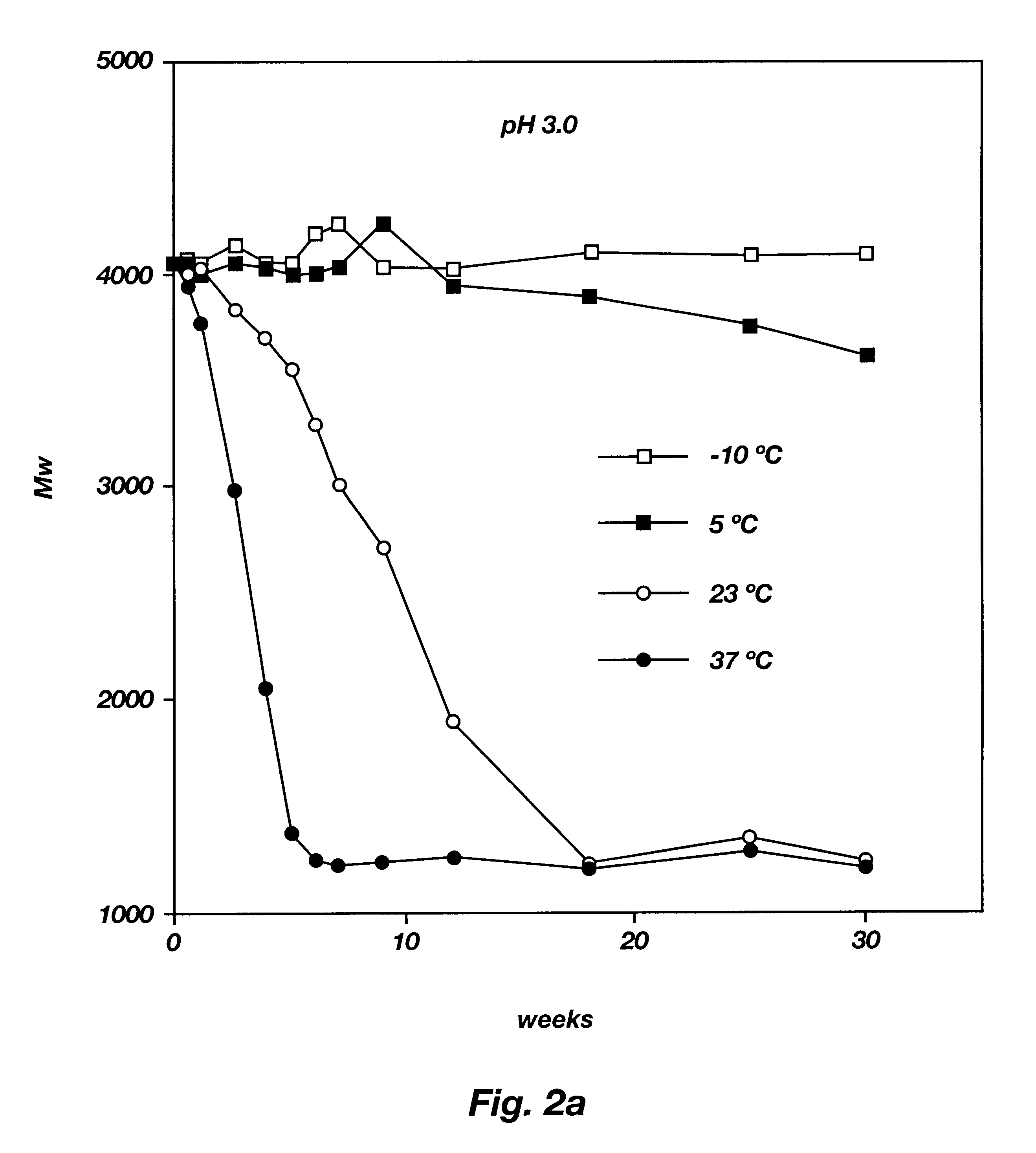
Biodegradable low molecular weight triblock poly(lactide-co- glycolide) polyethylene glycol copolymers having reverse thermal gelation properties
InactiveUS6201072B1Difficult to formulateDifficult to administerOrganic active ingredientsPowder deliverySolubilityPolymer science
A water soluble, biodegradable ABA- or BAB-type tri-block polymer is disclosed that is made up of a major amount of a hydrophobic A polymer block made of a biodegradable polyester and a minor amount of a hydrophilic polyethylene glycol(PEG) B polymer block, having an overall average molecular weight of between about 2000 and 4990, and that possesses reverse thermal gelation properties. Effective concentrations of the tri-block polymer and a drug may be uniformly contained in an aqueous phase to form a drug delivery composition. At temperatures below the gelation temperature of the tri-block polymer the composition is a liquid and at temperatures at or above the gelation temperature the composition is a gel or semi-solid. The composition may be administered to a warm-blooded animal as a liquid by parenteral, ocular, topical, inhalation, transdermal, vaginal, transurethral, rectal, nasal, oral, pulmonary or aural delivery means and is a gel at body temperature. The composition may also be administered as a gel. The drug is released at a controlled rate from the gel which biodegrades into non-toxic products. The release rate of the drug may be adjusted by changing various parameters such as hydrophobic / hydrophilic component content, polymer concentration, molecular weight and polydispersity of the tri-block polymer. Because the tri-block polymer is amphiphilic, it functions to increase the solubility and / or stability of drugs in the composition.
Owner:KIM PH D SUNG WAN +2
Pharmaceutical and cosmetic carrier or composition for topical application
A pharmaceutical or cosmetic carrier or composition for topical application characterized by rheological properties which render the carrier or composition semi-solid at rest and a liquid upon application of shear forces thereto. The composition or carrier are prepared by mixing 1-25 percent of a solidifying agent and 75-99 percent of a hydrophobic solvent, by weight, wherein at least one of them has therapeutic or cosmetic benefits, in the presence or absence of a biologically active substance.
Owner:VYNE PHARMA LTD
Compositions and methods for use in three dimensional model printing
Compositions for use in the manufacture of three-dimensional objects including compositions for use as a support and / or release material in the manufacture of the three-dimensional objects are provided. There is thus provided, in accordance with an embodiment of the present invention, a composition suitable for building a three-dimensional object. The compositions may include, inter alia, a curable component, having a functional group, wherein if the functional group is a polymerizable reactive functional group, then the functional group is a (meth)acrylic functional group, a photo-initiator, a surface-active agent and a stabilizer; wherein said composition has a first viscosity of about 50–500 cps at a first temperature, wherein said first temperature is ambient temperature, and a second viscosity lower than 20 cps at a second temperature wherein said second temperature is higher than said first temperature, wherein, after curing, the composition results in a solid form. There is thus provided, in accordance with another embodiment of the present invention, a composition suitable for support in building a three-dimensional object. The compositions may include, inter alia: a non-curable component, a curable component, wherein the non-curable component is not reactive with said curable component, a surface-active agent and a stabilizer; wherein said composition has a first viscosity of about 20–500 cps at a first temperature, wherein said first temperature is ambient temperature, and a second viscosity lower than 20 cps at a second temperature wherein said second temperature is higher than said first temperature, wherein, after irradiation, the composition results in a solid, a semi-solid or liquid material. A method for the preparation of a three-dimensional object by three-dimensional printing is provided in accordance with embodiments of the present invention. Embodiments of the present invention further provide a three-dimensional object prepared according to the methods of the invention.
Owner:STRATASYS LTD
High energy density redox flow device
ActiveUS20110200848A1Avoid accumulationHigh enough specific energyOrganic chemistryFlow propertiesElectrochemical responseHigh energy
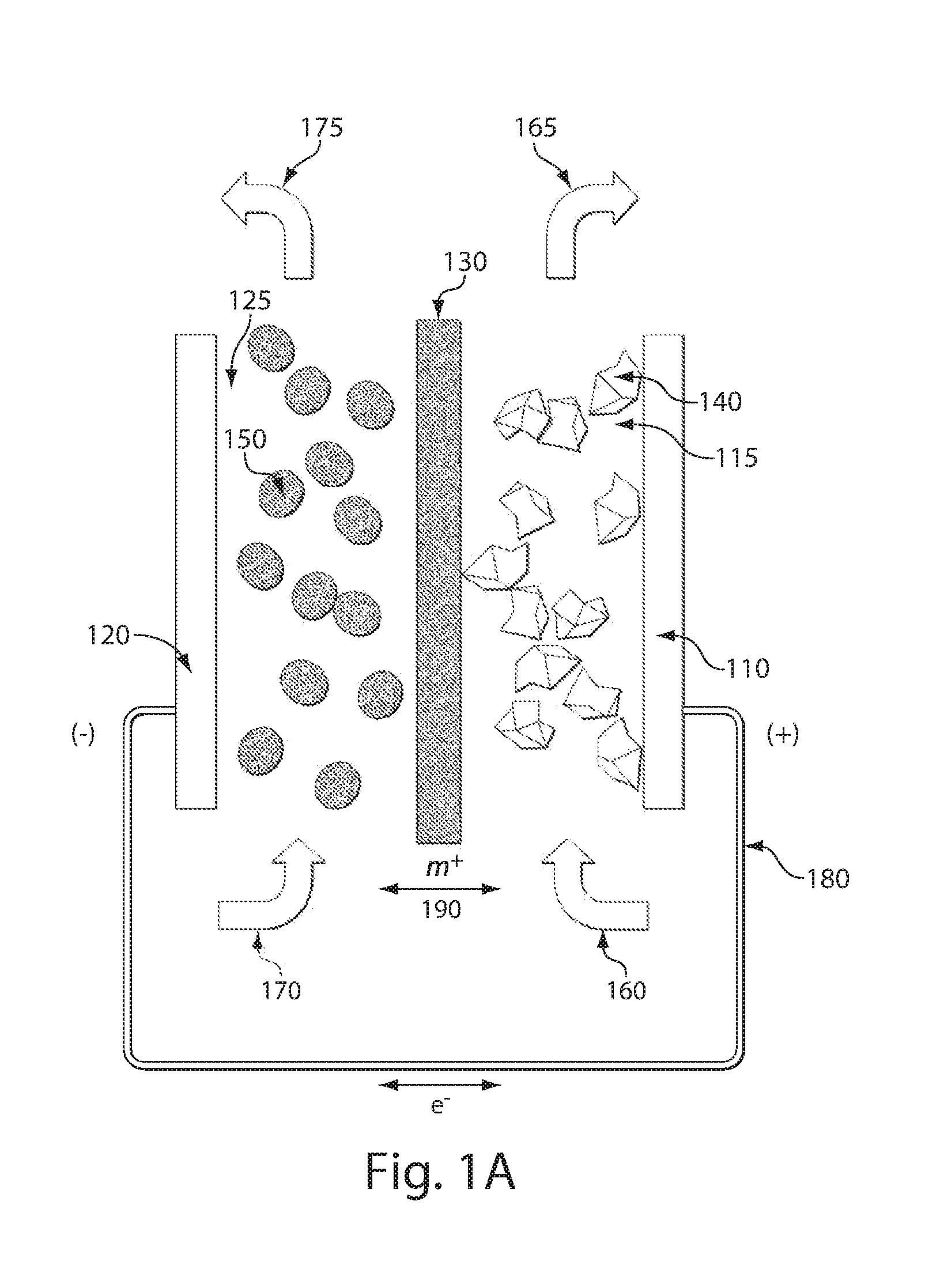
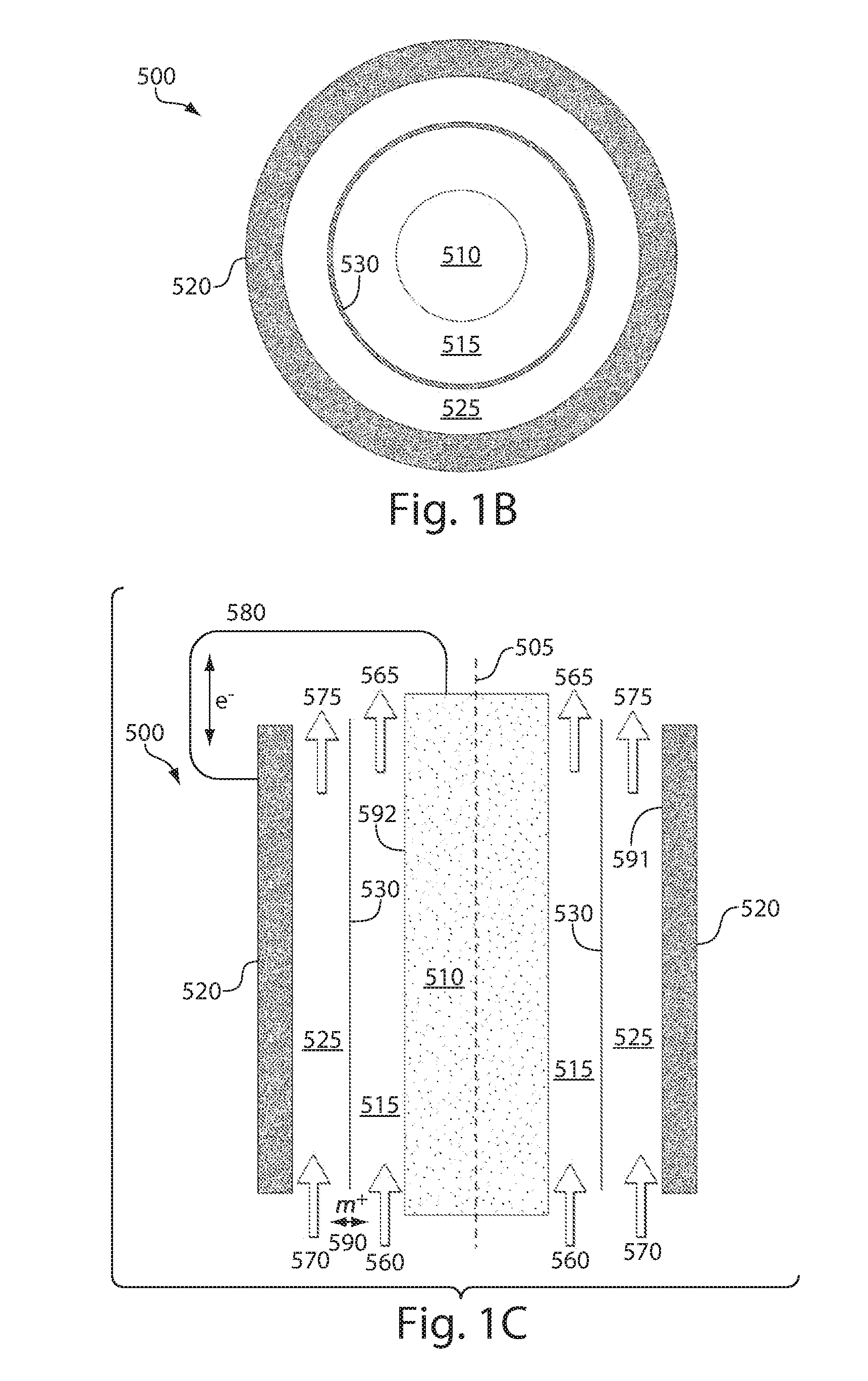
Redox flow devices are described in which at least one of the positive electrode or negative electrode-active materials is a semi-solid or is a condensed ion-storing electroactive material, and in which at least one of the electrode-active materials is transported to and from an assembly at which the electrochemical reaction occurs, producing electrical energy. The electronic conductivity of the semi-solid is increased by the addition of conductive particles to suspensions and / or via the surface modification of the solid in semi-solids (e.g., by coating the solid with a more electron conductive coating material to increase the power of the device). High energy density and high power redox flow devices are disclosed. The redox flow devices described herein can also include one or more inventive design features. In addition, inventive chemistries for use in redox flow devices are also described.
Owner:MASSACHUSETTS INST OF TECH +2
Delivery of tetrahydrocannabinol
InactiveUS20070104741A1Avoiding hepatic first-pass metabolismGood choiceBiocideNervous disorderChylomicronTG - Triglyceride
A self-emulsifying drug delivery system to improve dissolution, stability, and bioavailability of drug compounds of dronabinol or other cannabinoids. The drug compound(s) are dissolved in an oily medium (e.g. triglycerides and / or mixed glycerides and / or free fatty acids containing medium and / or long chain saturated, mono-unsaturated, and / or poly-unsaturated free fatty acids) together with at least one surfactant. The surfactant promotes self-emulsification, thereby promoting targeted chylomicron delivery and optimal bioavailability to a mammalian intestinal lumen. A dosage form can optionally include co-solvents, anti-oxidants, viscosity modifying agents, cytochrome P450 metabolic inhibitors, P-GP efflux inhibitors, and amphiphilic / non-amphiphilic solutes to induce semi-solid formation for targeted release rates.
Owner:MURTY PHARMA
Remelted ingestible products
ActiveUS20130274296A1Reduce exposureHigh heating temperatureBiocideTobacco preparationSemi solidTemperature sensitive
A method of preparing an orally ingestible hard boiled product, comprising: i) heating a sugar material to a first temperature sufficient to liquefy the sugar material; ii) cooling the liquefied sugar material to provide a cooled sugar material having a solid or semi-solid form; iii) heating the cooled sugar material to a second temperature, which is lower than the first temperature; iv) combining the sugar material with one or more temperature sensitive ingredients before, during, or after said heating step iii), but after said cooling step ii), such that an intimate mixture of the second liquefied sugar material and the one or more temperature sensitive ingredients is provided; and v) cooling the intimate mixture to form an orally ingestible product. Orally ingestible hard boiled products prepared according to this method are also provided.
Owner:R J REYNOLDS TOBACCO COMPANY
Polymeric precursors of non-absorbable, in situ-forming hydrogels and applications thereof
InactiveUS20070202074A1Pharmaceutical delivery mechanismPharmaceutical non-active ingredientsInvasive treatmentsSemi solid
The present invention is directed toward an injectable, single- or multiple-component polymeric liquid precursor of an in situ-forming, non-absorbable, flexible, and resilient hydrogel or semi-solid that can be used in non-surgical, minimally invasive treatment of herniated disc.
Owner:POLY MED
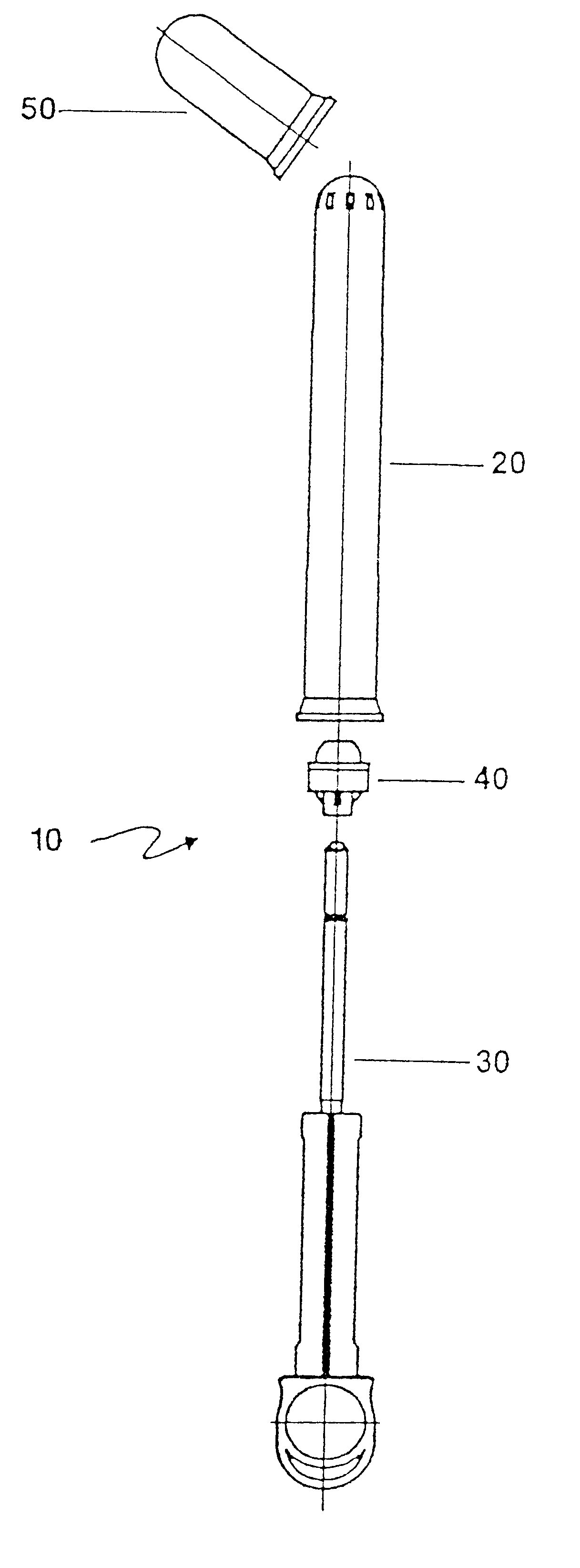
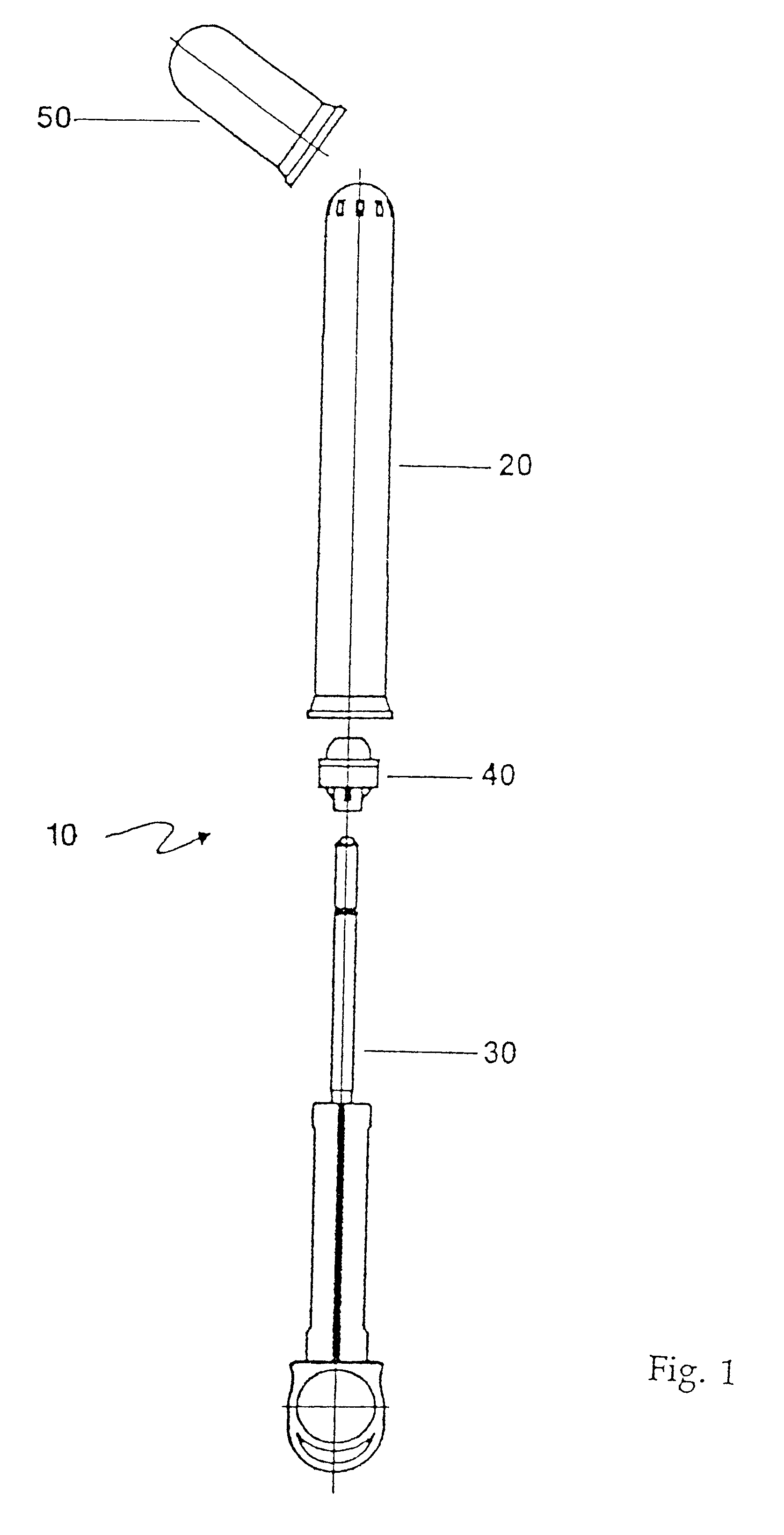
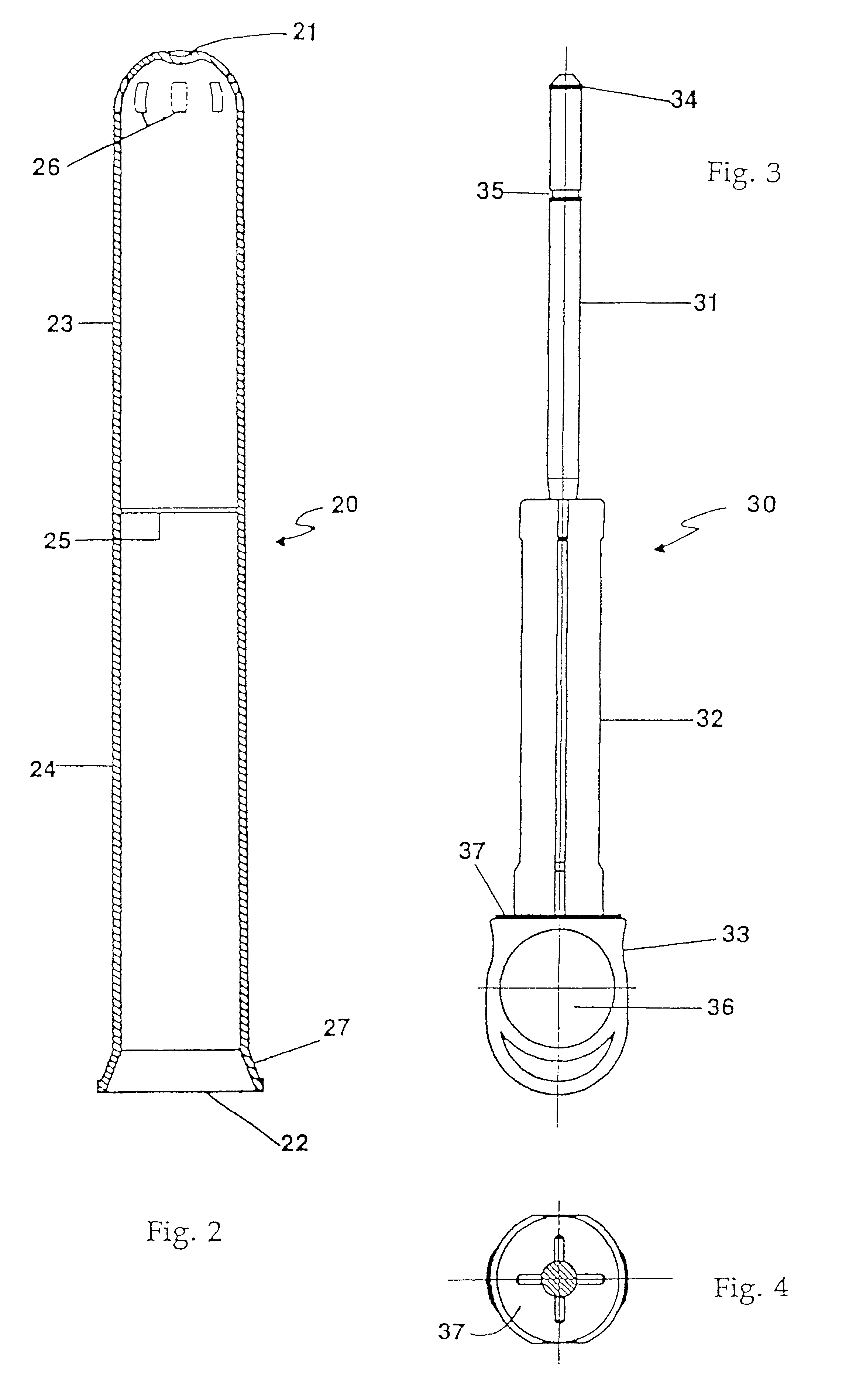
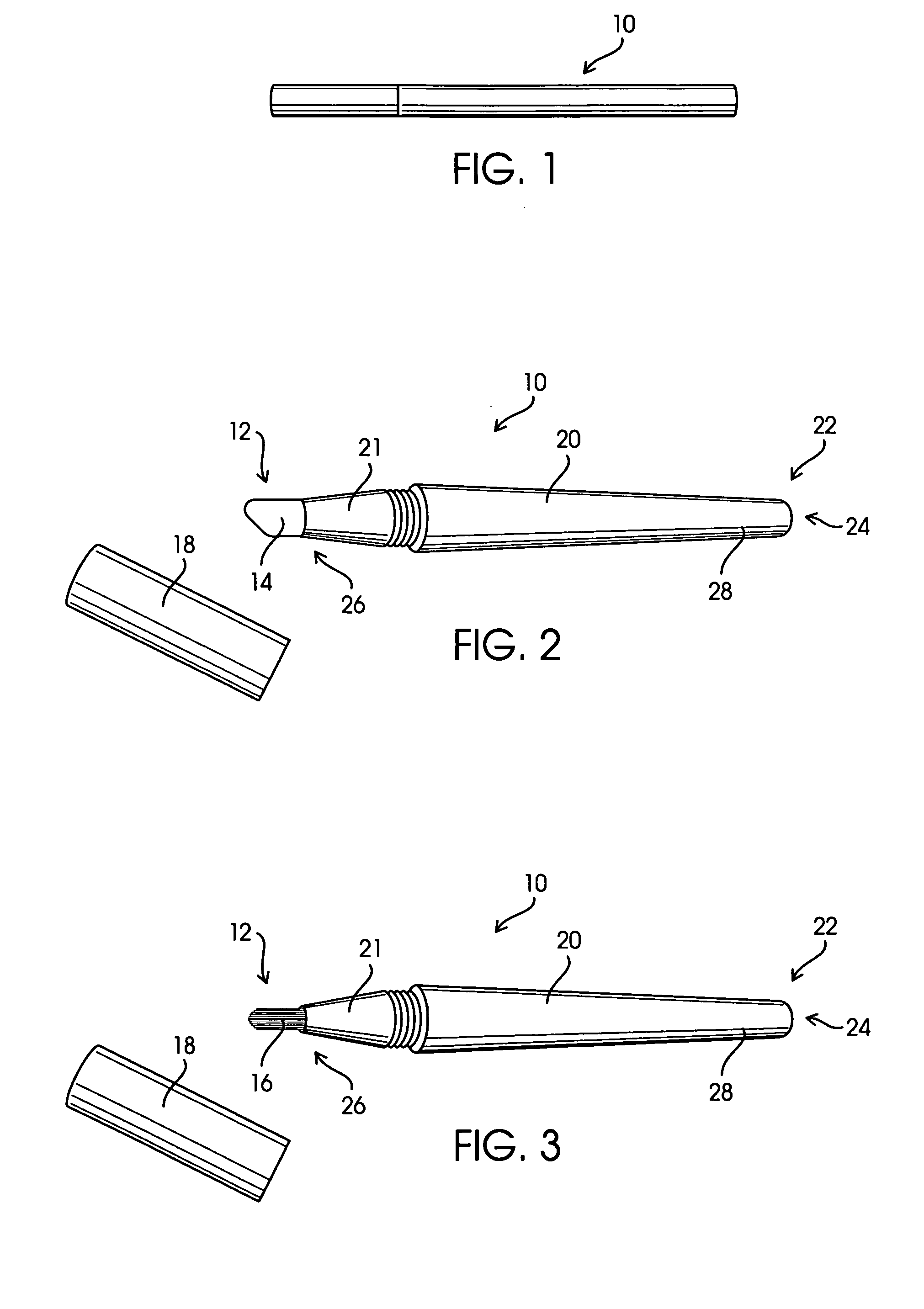
Applicator for semi-solid medications
An applicator for semi-solid medications comprises a tubular body (20) having a rounded dispensing end (21) provided with at least one opening (26) and a grasping end (22), the inner surface of a proximal portion of said tubular body defining a reservoir for the medication, a plunger (30), initially housed inside the tubular body, which plunger is provided with a rod (31) and grasping means (33), a piston (40), positioned in sealing contact with the inner surface of said tubular body providing a closure for the medication reservoir, wherein said piston has a longitudinal hole (45) through which rod (31) of the plunger is disposed, and wherein said piston initially abuts on a stop means (25) when the applicator is received by the user, a coupling means (35, 46) between rod (31) and piston (40), a closure means for sealingly closing off said opening (26) on said dispensing end, wherein the plunger (30) is extractable from the tubular body until the rod (31) becomes engaged with the piston (40) by the coupling means, whereupon the plunger together with the piston is displaceable along the tubular body towards the dispensing end (21) for expelling the medication through opening (26), and wherein the stops means (25) is provided in the form of a projection on the inner surface of the tubular body.
Owner:J URIACH Y CIA SA
Method of introducing leak detection dye into an air conditioning or refrigeration system including solid or semi-solid fluorescent dyes
InactiveUSRE36951E1Reduce riskDetection of fluid at leakage pointOther chemical processesFluorescenceSemi solid
PCT No. PCT / US95 / 04262 Sec. 371 Date Sep. 21, 1995 Sec. 102(e) Date Sep. 21, 1995 PCT Filed Apr. 6, 1995 PCT Pub. No. WO96 / 07088 PCT Pub. Date Mar. 7, 1996A method of introducing a leak detection dye into a closed refrigeration system through circulation of the refrigerant. A predetermined amount of the leak detection dye, which is soluble in the refrigerant and the system lubricant, is installed in a component of the refrigeration system, such as in a desiccant bag placed in a dehydrator. The leak detection dye may come in various forms including as a leak detection additive having the leak detection dye implanted on and absorbed into a host swatch of a substrate material, as a powder, as a solid pellet of powdered dye concentrate and inert ingredients, or as a slurry. The refrigeration system is assembled, charged and operated, by which the refrigerant and system lubricant flowing through the component, such as a desiccant bag in the dehydrator, and mixes the dye with the refrigerant and system lubricant.
Owner:SPECTRONICS
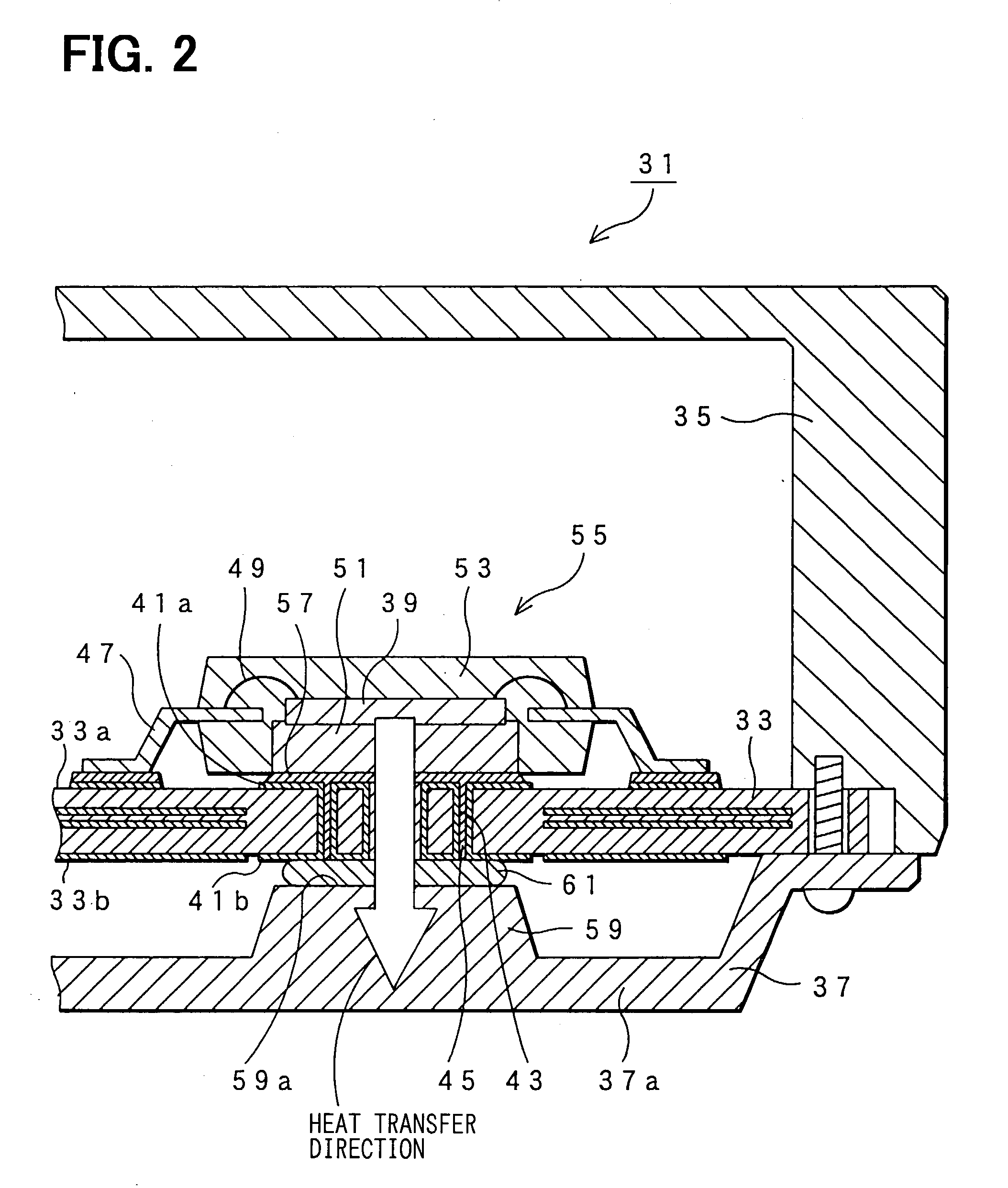
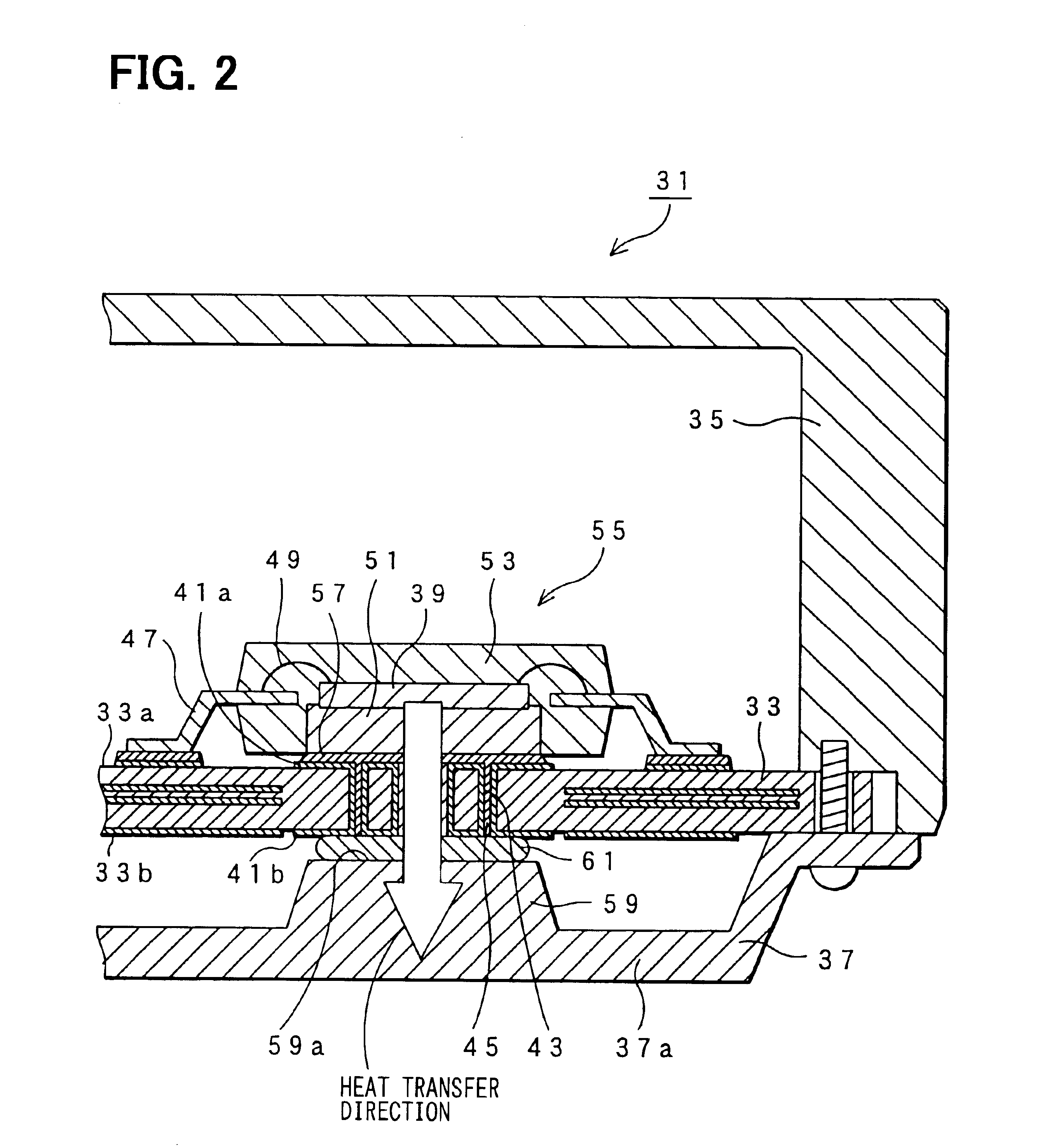
Electronic control unit
InactiveUS20030184969A1Semiconductor/solid-state device detailsPrinted circuit aspectsSemi solidConductive materials
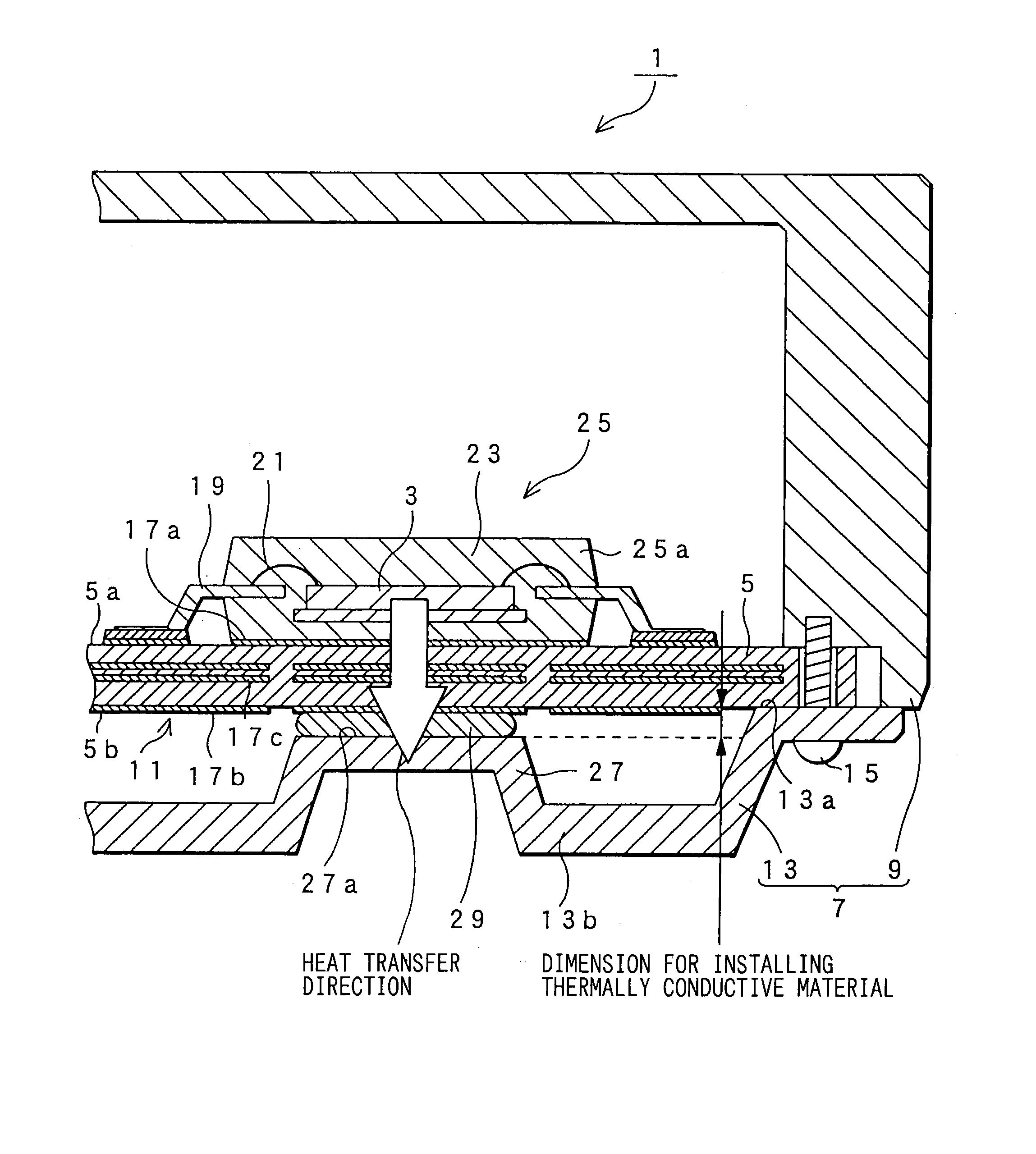
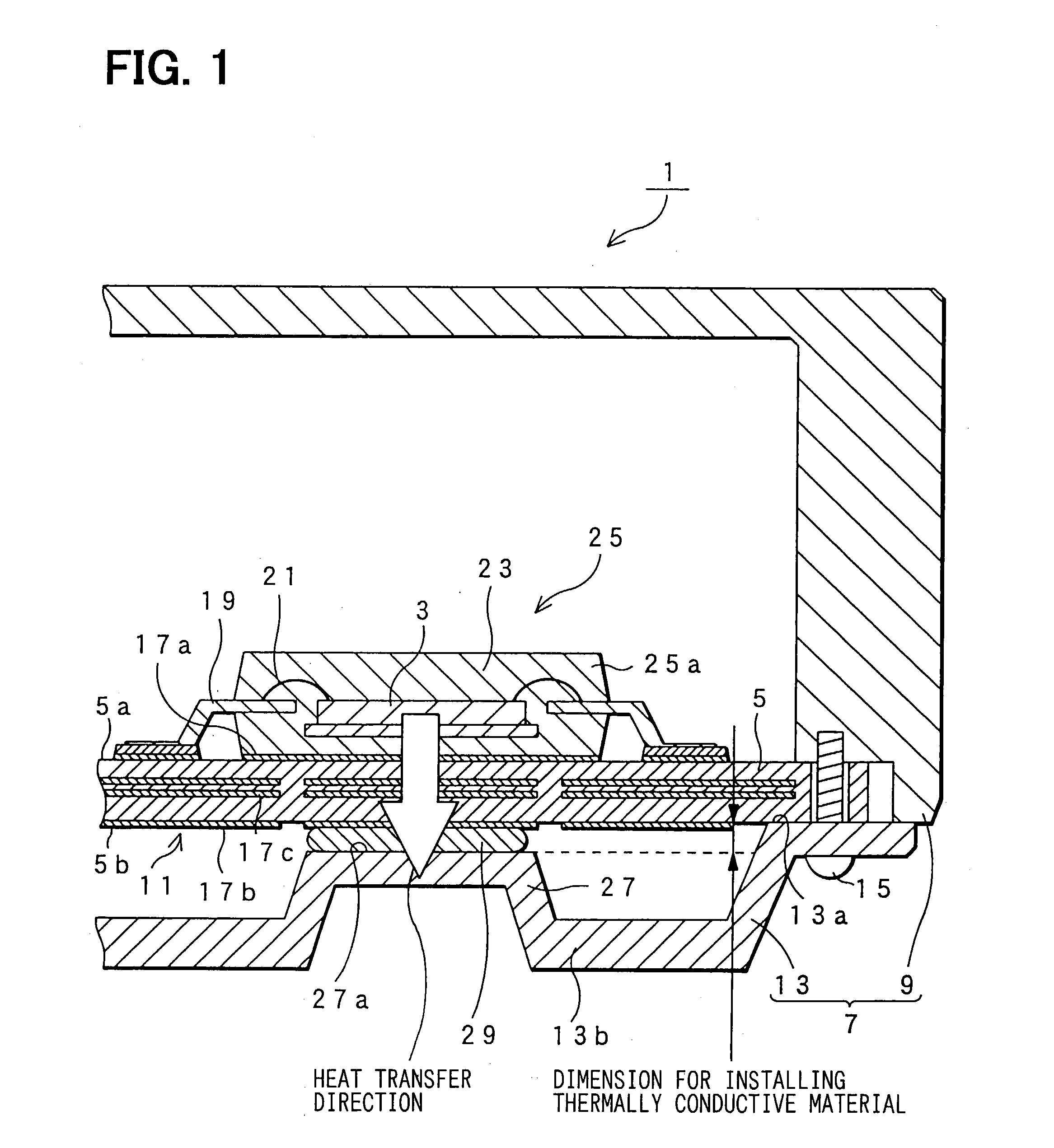
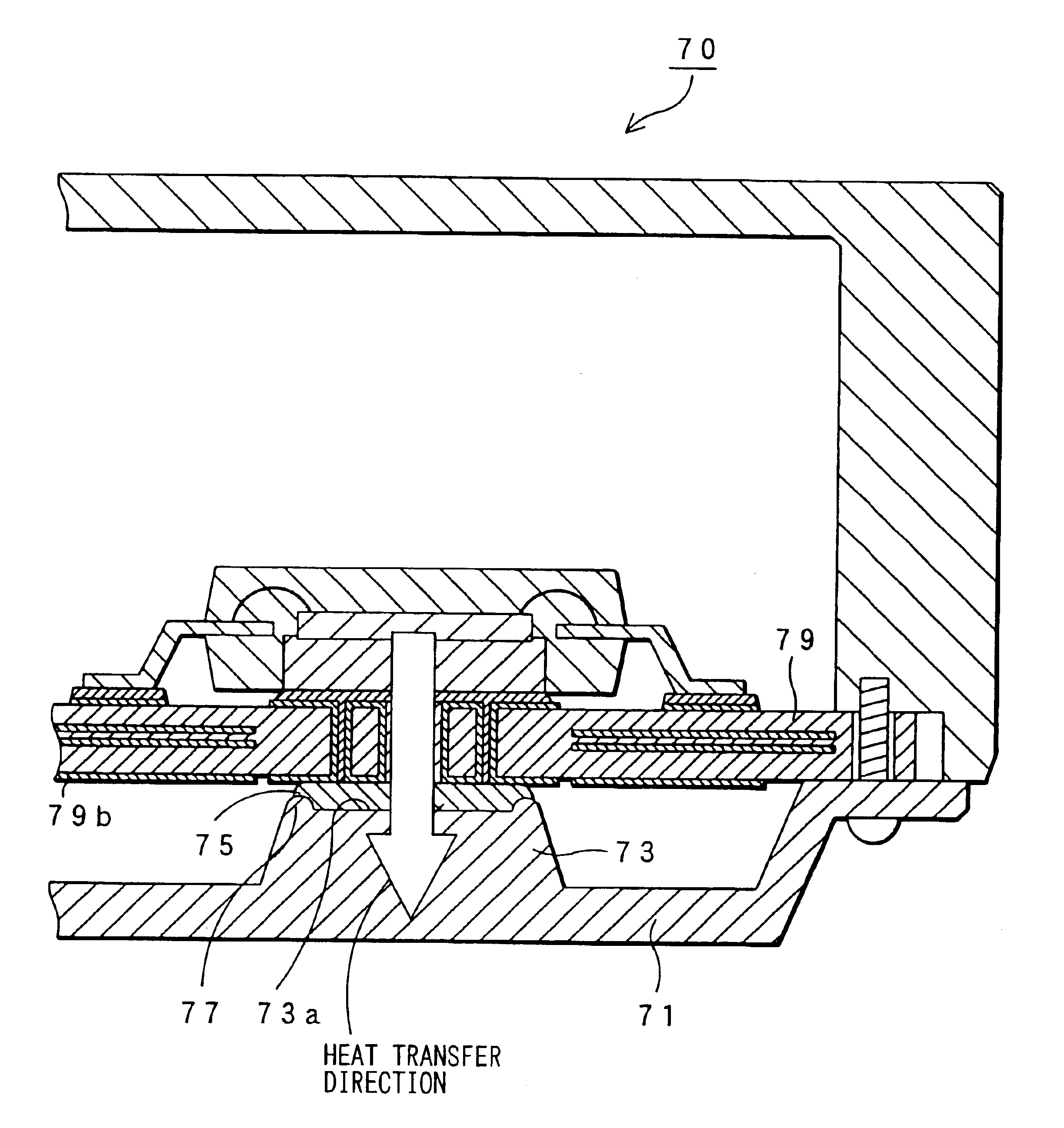
An electronic control device having a high heat dissipating ability includes a printed board secured to an enclosure and interposed between a case and a cover with screws passing through the printed board. Thermally conductive thin film layers made of copper foil are formed in parallel on a mount face and an opposite mount face of the printed board and inside the printed board so as to be thermally separated from each other. A protrusion is provided on the cover and protrudes beyond a bottom part of the cover toward the position where an electronic component is mounted. A flexible, semi-solid thermally conductive material is placed between an end face of the protrusion and the opposite mount face of the printed board corresponding to the position where the electronic component is mounted to be in contact with the end face and the opposite mount face.
Owner:DENSO CORP
Thermally reversible implant and filler
The invention relates to the use of a thermal reversible gel, such as a copolymer composition, as a biological filler or implant. The gel has a semi-solid form at body temperature, but upon cooling to a temperature below a threshold level, the gel is liquefied and can be re-shaped, re-sized, manipulated or removed from the body. The gel may be used as a subcutaneous implant, a biological filler, joint or tissue spacer, for wrinkle filling or other cosmetic implants, as a soft-tissue replacement for reconstructive surgery, or as a barrier within the lumen of a biological structure, such as a blood vessel. The implant may be used to provide reversible birth control by providing, for example, a reversible barrier to the cervix or a reversible blockage of the lumen of the vas deferens.
Owner:CHENG YU LING +3
Therapeutic dental composition
InactiveUS20050008584A1Eliminate needFacilitate easy dispensingCosmetic preparationsToilet preparationsWater insolubleSemi solid
Owner:DISCUS DENTAL LLC
Dermatological application with solidified fat compositions
InactiveUS20030157138A1Antibacterial agentsEdible oils/fats ingredientsFirming agentCosmetic vehicle
A pharmaceutical or cosmetic carrier or composition for topical application characterized by rheological properties which render the carrier or composition semi-solid at rest and a liquid upon application of shear forces thereto. The composition or carrier are prepared by mixing 1-25 percent of a solidifying agent and 75-99 percent of a hydrophobic solvent, by weight, wherein at least one of them has therapeutic or cosmetic benefits, in the presence or absence of a biologically active substance.
Owner:VYNE PHARMA LTD
Method for treatment of uterine fibroid tumors
InactiveUS20060251581A1Improve delivery efficiencyMinimize adverse effectsUltrasonic/sonic/infrasonic diagnosticsPowder deliverySemi solidViscosity
Various injectable or insertable uterine fibroid treatment formulations are provided, which comprise a uterine fibroid treatment agent in an amount effective to cause shrinkage or elimination of uterine fibroids. The injectable or insertable formulations are typically solids, semi-solids or high-viscosity fluids. Other aspects of the invention are directed to systems and methods for treatment of uterine fibroids.
Owner:SCI MED LIFE SYST
Pharmaceutical compositions using semi-solid delivery vehicle
A semi-solid delivery vehicle contains a polyorthoester and an excipient, and a semi-solid pharmaceutical composition contains an active agent and the delivery vehicle. The pharmaceutical composition may be a topical, syringable, or injectable formulation; and is suitable for local delivery of the active agent. Methods of treatment are also disclosed.
Owner:HERON THERAPEUTICS
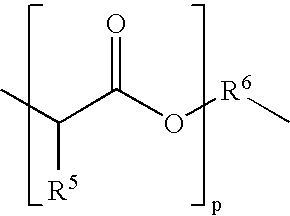
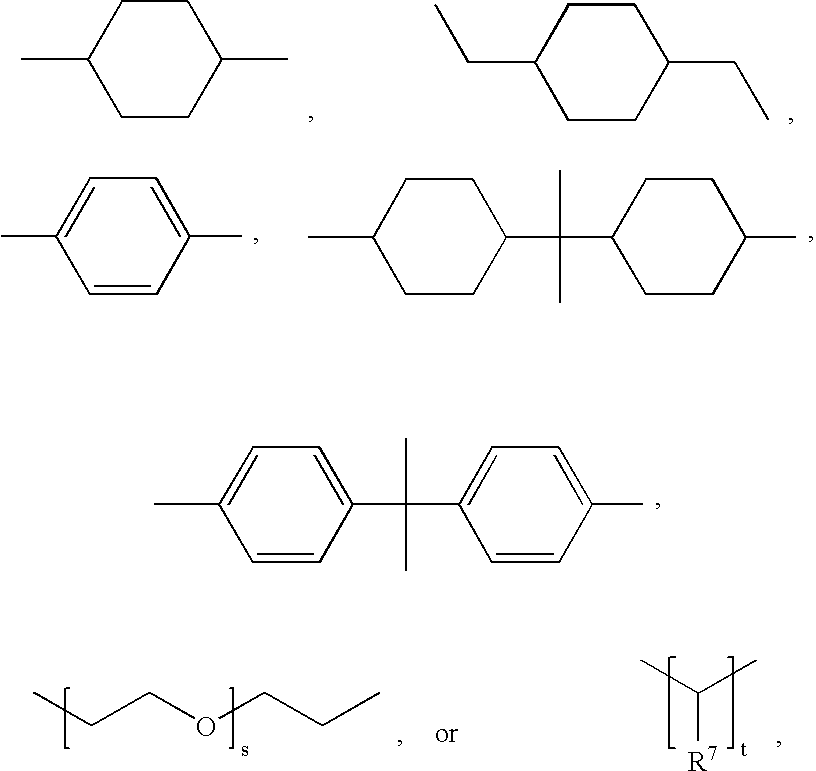
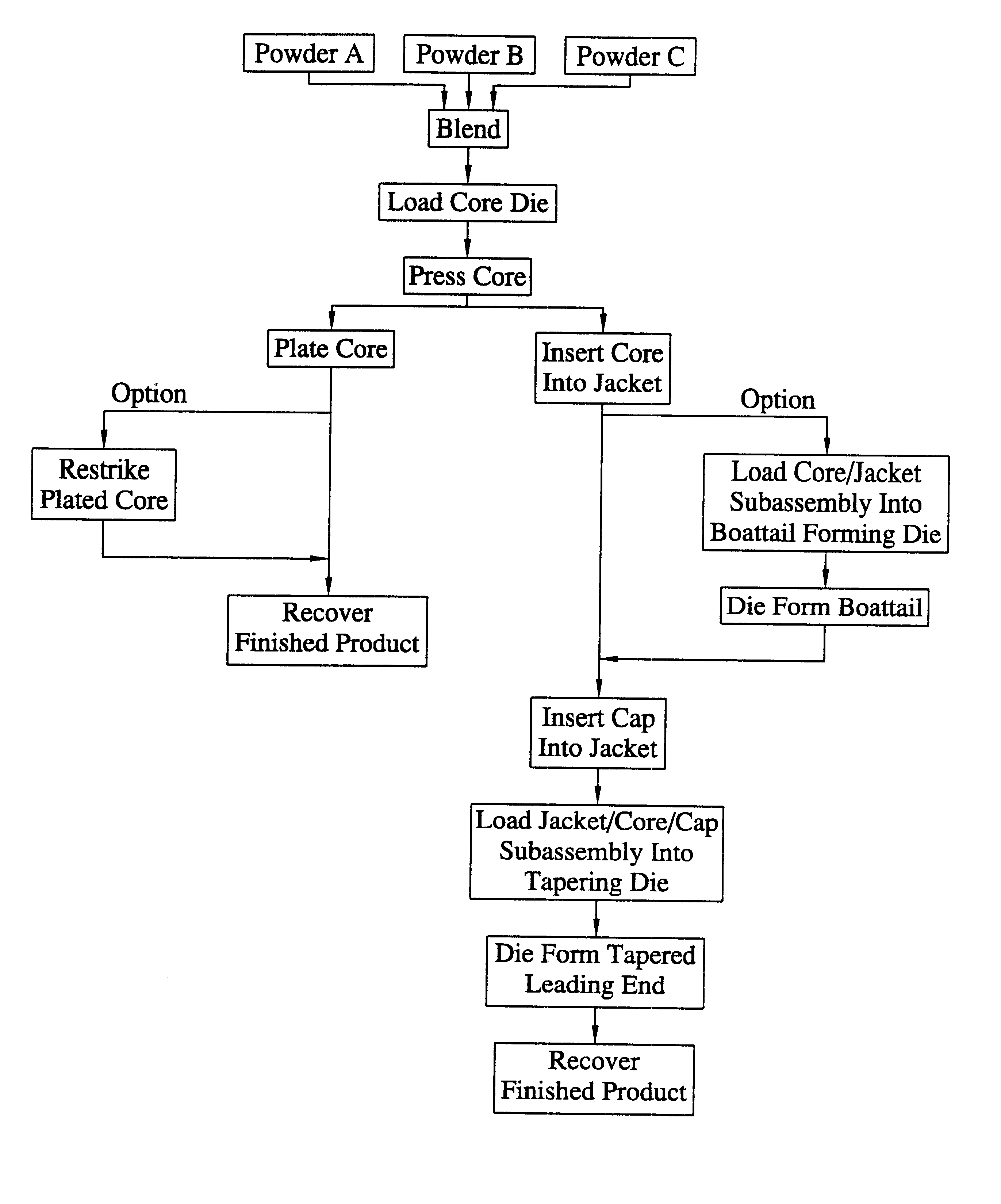
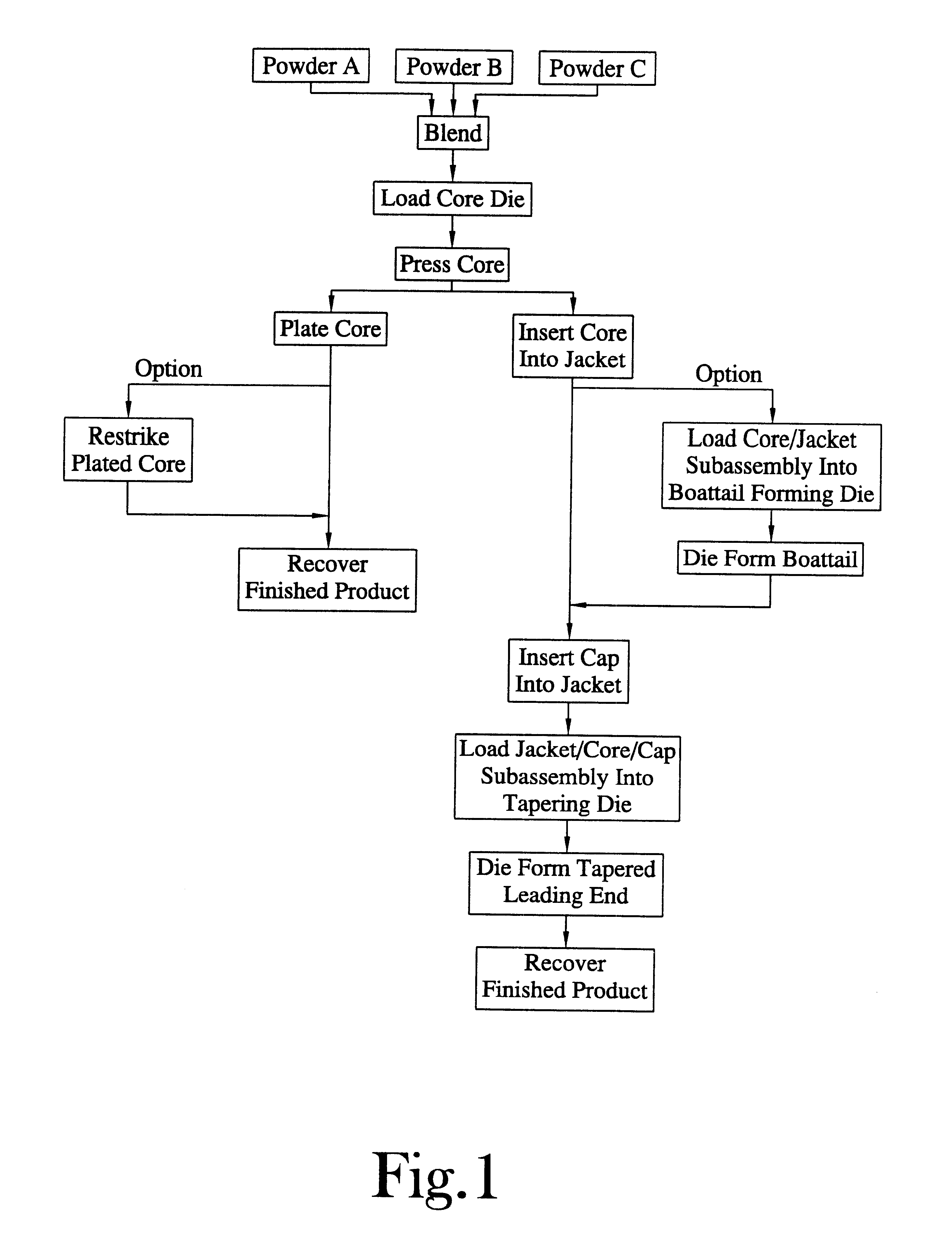
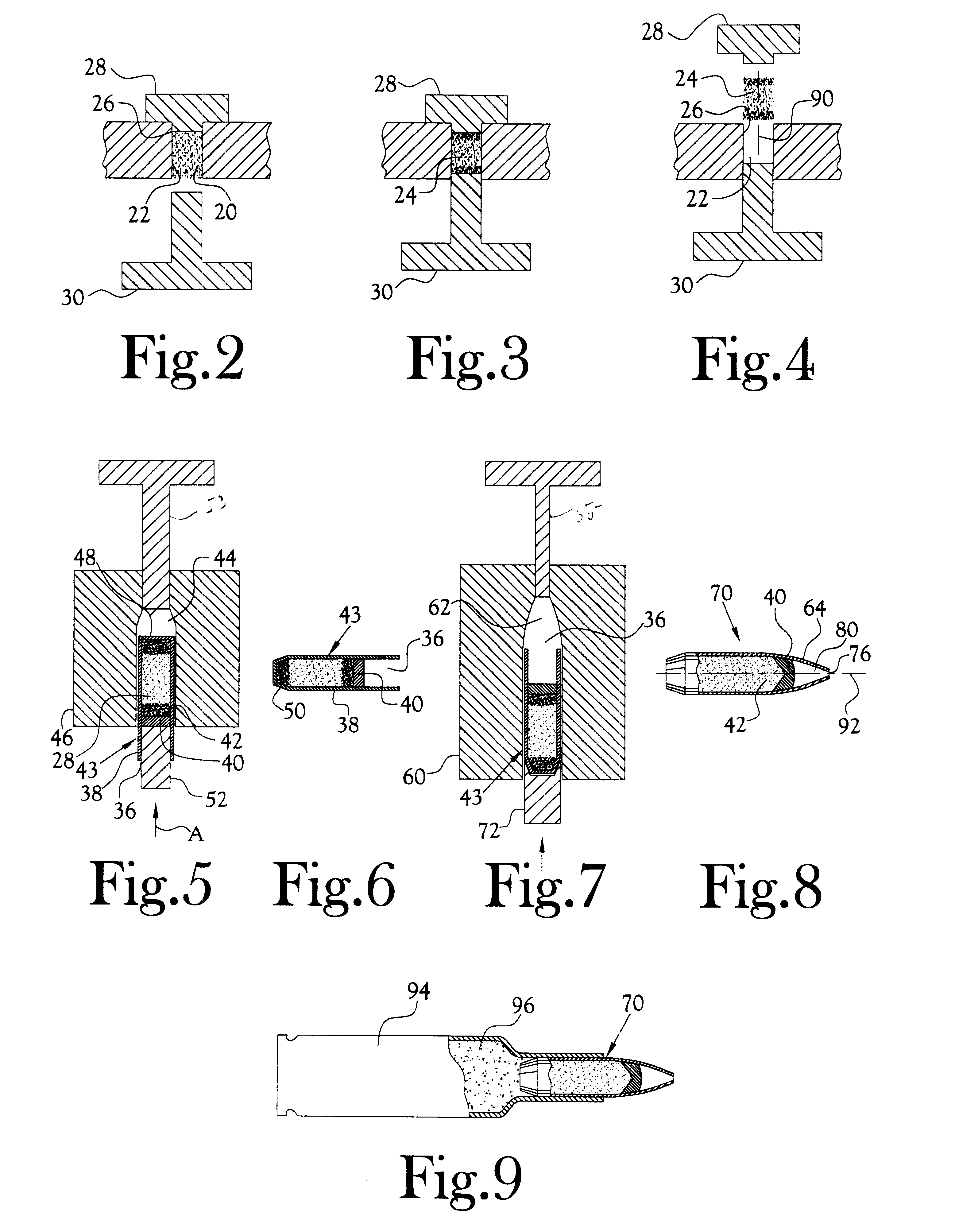
Method for the manufacture of a frangible nonsintered powder-based projectile for use in gun ammunition and product obtained thereby
InactiveUS6457417B1Easy and inexpensive to manufactureAmmunition projectilesTraining ammunitionSemi solidRicochet
A method for the manufacture of heavy metal powder-based frangible projectiles which are relatively easy and inexpensive to manufacture and which exhibit a selectable variety of desirable physical and / or performance properties. The projectiles of the present invention are powder-based, preferably including predominately tungsten powder as a heavy metal, particularly a tungsten powder which includes a predominate portion of finely sized particles. Lighter metal powders, also preferably having a predominate portion of finely sized particles, may be employed in combination with the tungsten to achieve certain desired results. Importantly, the present inventor has found that inclusion of a non-metal matrix powder, also of finely sized particles, in a mixture of a heavy metal powder, such as tungsten powder, and a light metal powder, may be employed in a variety of combinations to produce a projectile which is fully frangible upon striking a target (no ricochet), or which is frangible after either partial or full penetration of a selected target, either a semi-solid (e.g., a gel block) or a solid (e.g., a ¼ inch thick cold rolled steel plate at an angle of about 90 degrees).
Owner:COVE CORP +1
Swellable Dosage Form Comprising Gellan Gum
ActiveUS20080299199A1Easily can be orally ingestedIncrease intakeHeavy metal active ingredientsOrganic active ingredientsParticulatesGellan gum
A novel dosage form. The dosage form is presented in particulate form and before oral ingestion the particulate material is subjected to an aqueous medium, whereby it is converted to a semi-solid form by swelling or gelling of one or more of the components, especially of a gellan gum, of the particulate matter. The invention also relates to a vehicle for oral administration of one or more active substances, the vehicle comprising a gellan gum arranged in a configuration allowing optimal water diffusion so that upon addition of a predetermined amount of an aqueous medium, without the necessity of applying shear forces or other mixing forces, within a time period of 5 minutes or less swells and / or gels and the texture of the swelled vehicle being similar to that of a soft pudding and having a viscosity of at least about 10,000 cps as measured by a Brookfield Viscometer with a #4 LV spindle at 6 rpm and at 20-25° C. In one embodiment of the invention, the particulate matter can be moulded into a desired shape or pressed onto a dispensing unit such as a spoon.
Owner:ADARE PHARM INC
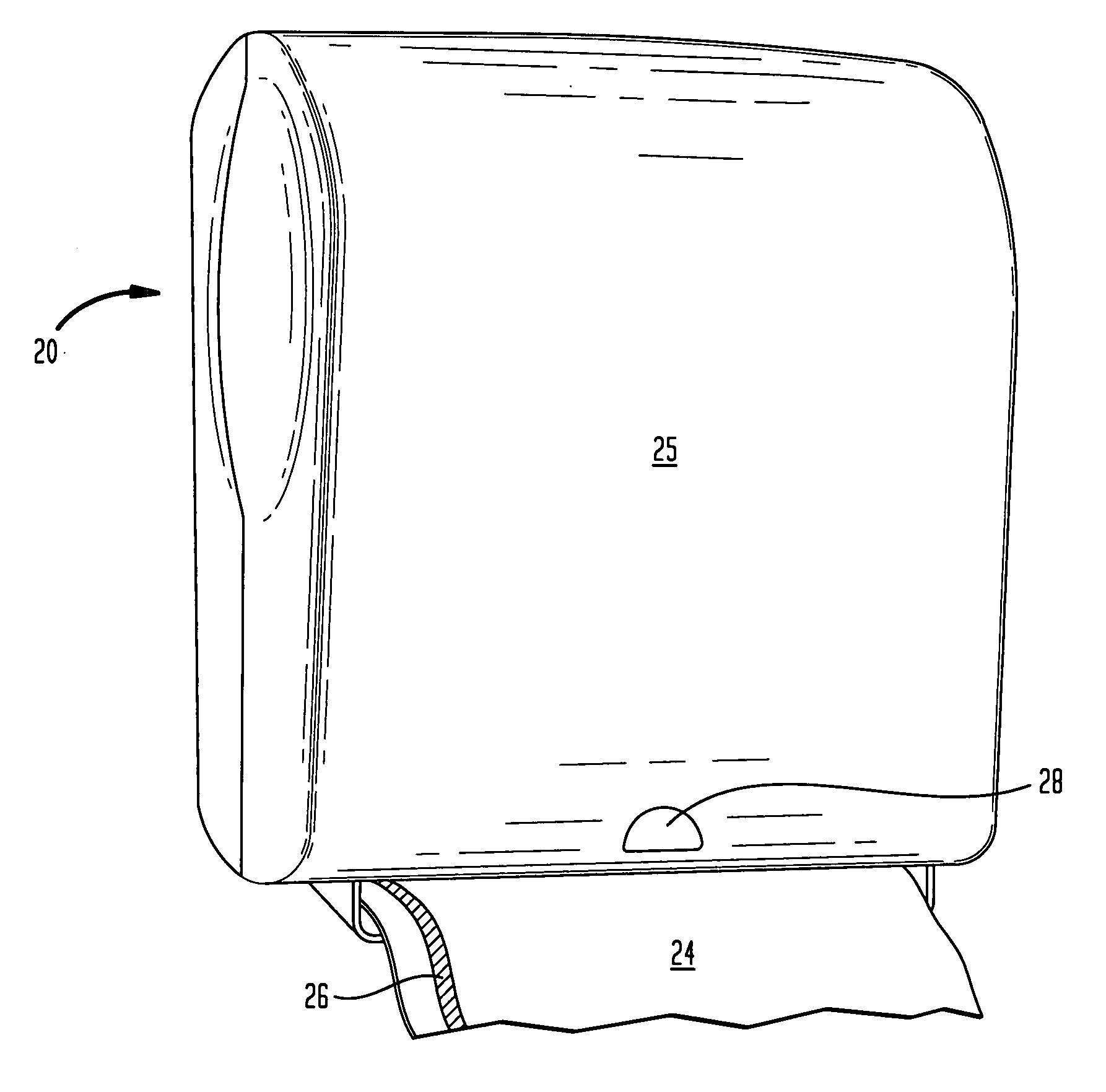
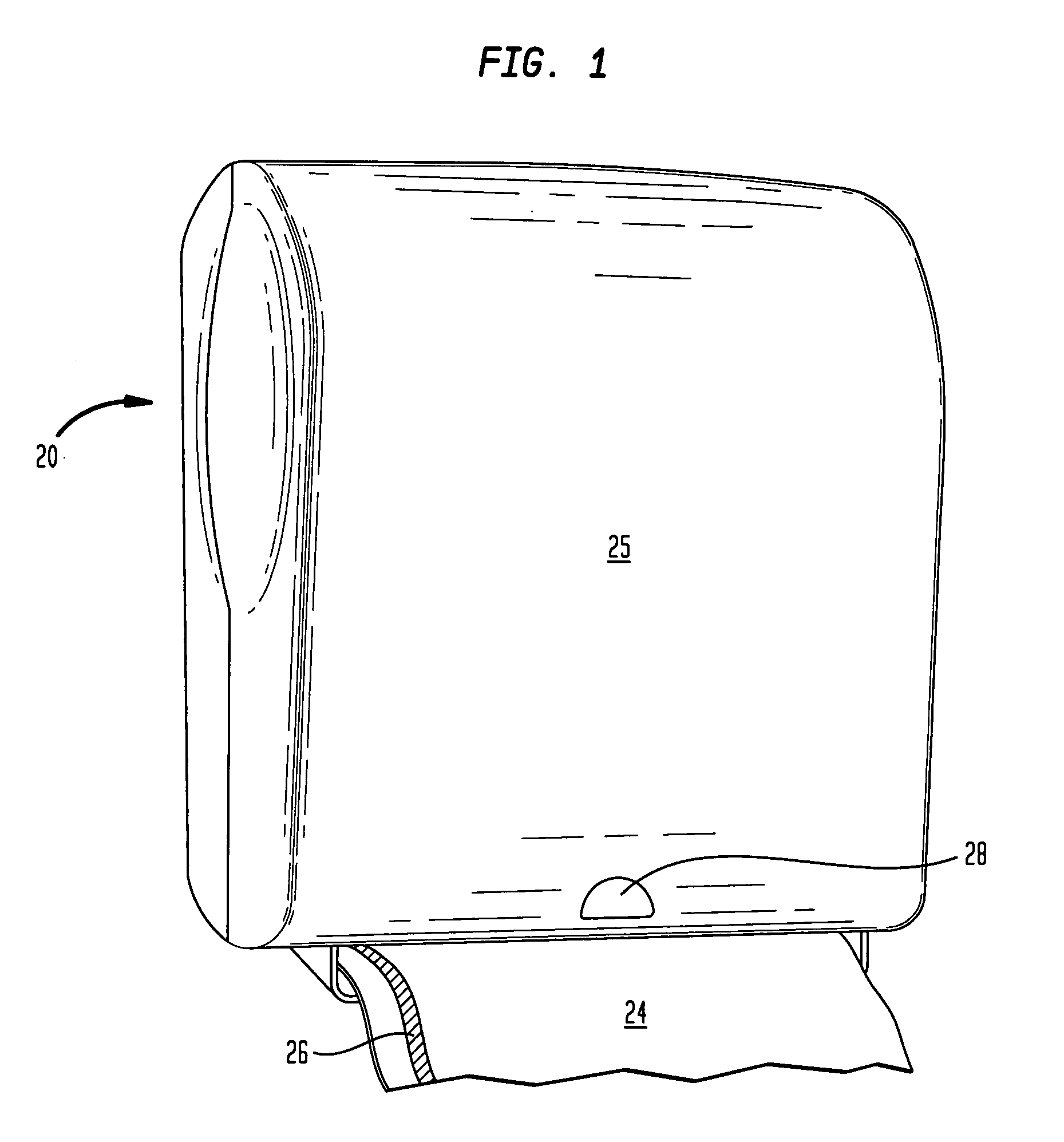
Antimicrobial hand towel for touchless automatic dispensers
InactiveUS20080008865A1Increased WAR timeEasy transferNatural cellulose pulp/paperPaper after-treatmentCelluloseBiochemical engineering
A disposable anti-microbial paper towel and dispensing method includes disposing paper towel in an automatic touchless dispenser which is adapted to generate a touchless proximity signal upon nearness of a consumer, and dispensing the paper towel in response to the proximity signal. A typical invention towel has: (i) a cellulosic web characterized in that the web is substantially without crepe bars and has an unlotioned MD bending length of at least 3.5 cm; and (ii) a transferable lotion composition comprising an emollient and anti-microbial agent, the lotion composition being immobilized on the cellulosic web in a semi-solid or solid form. The transferable lotion composition is selected from lotion compositions which are transferable upon contact with water or lotion compositions which are transferable upon application of body heat.
Owner:GEORGIA PACIFIC CONSUMER PRODS LP
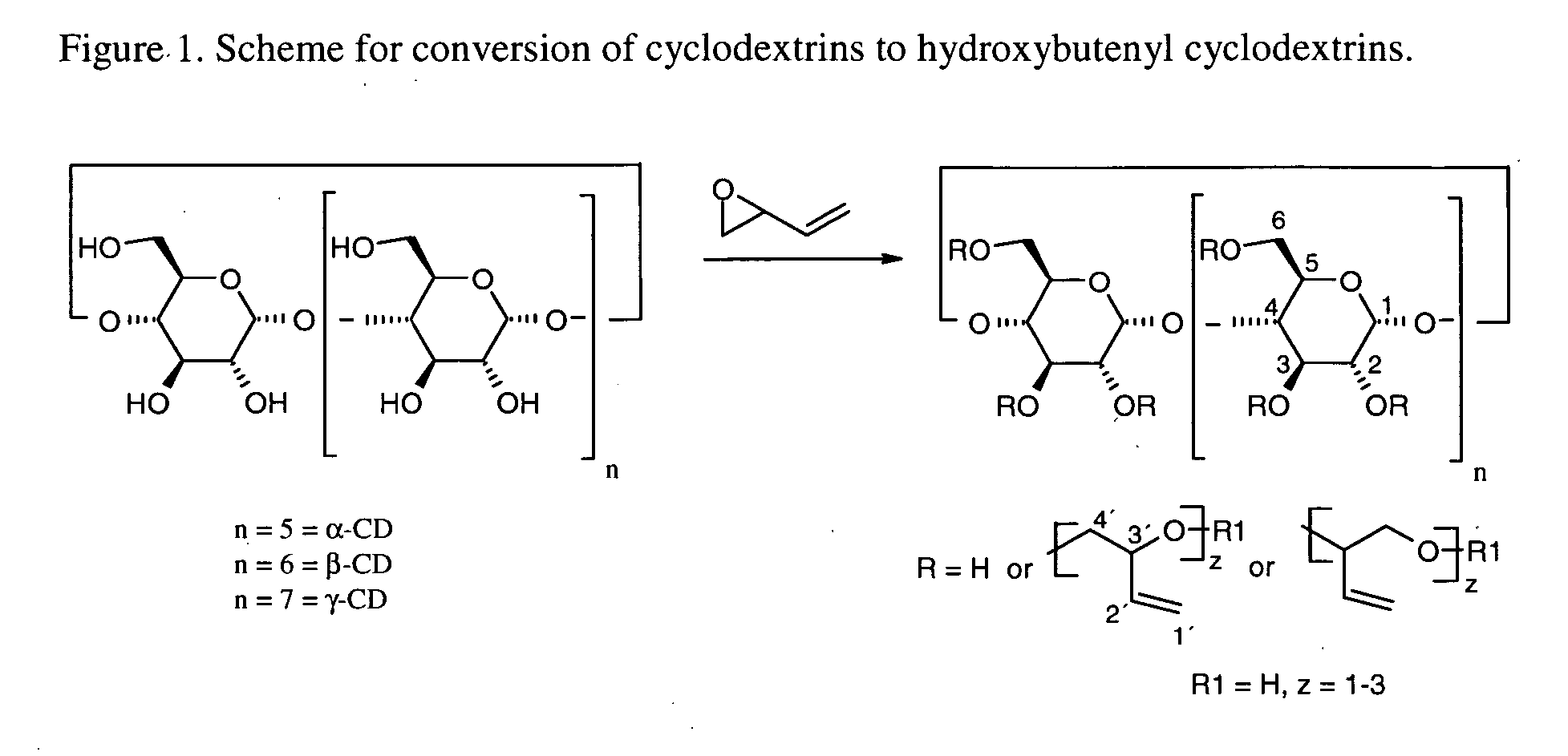
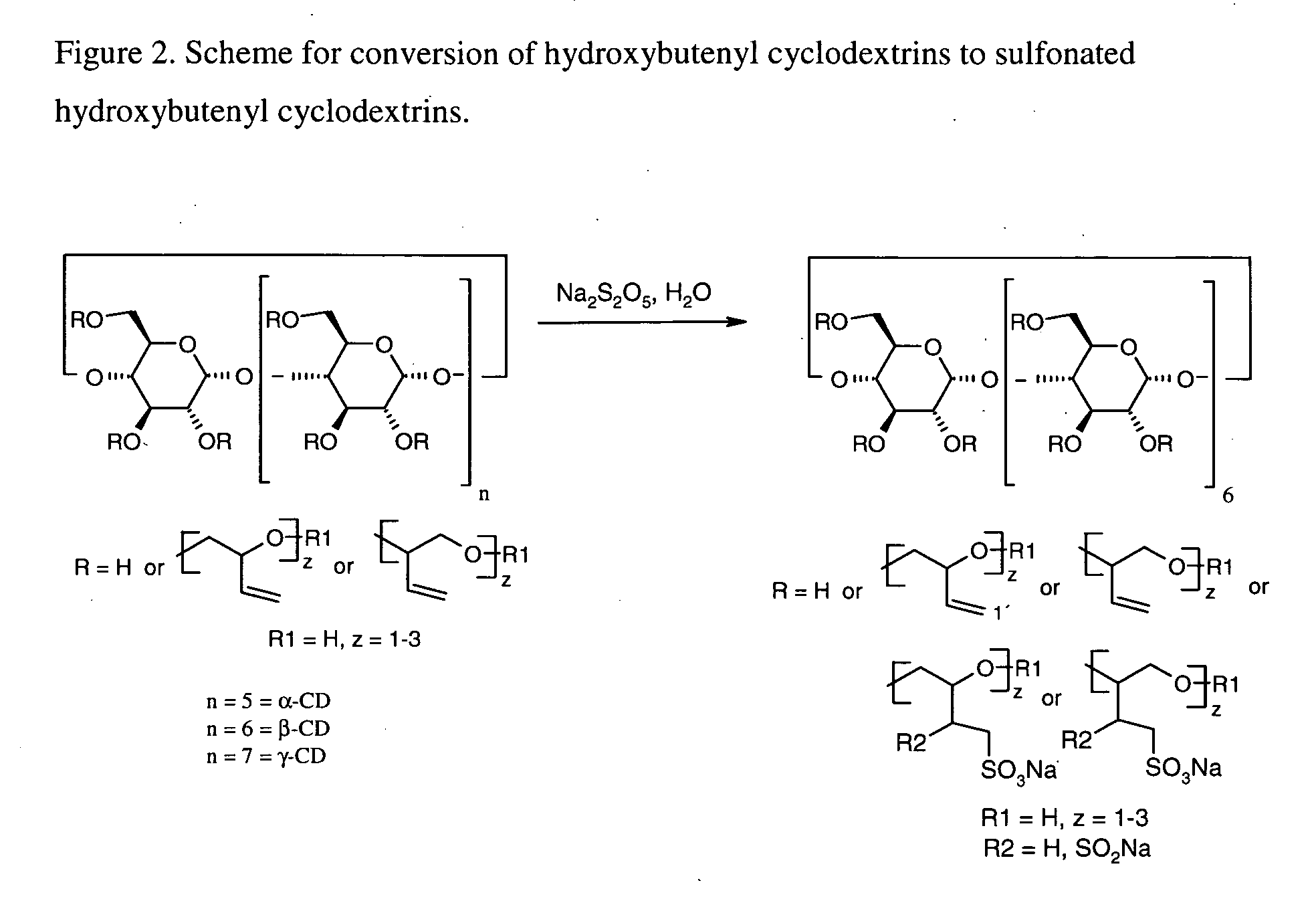
Cyclodextrin solubilizers for liquid and semi-solid formulations
InactiveUS20060105045A1Reduce capacityImprove solubilityPowder deliveryBiocideSolubilityOrganic solvent
This invention is directed to compositions comprising a water-miscible organic solvent or solvent mixture and one or more cyclodextrin derivatives soluble in the organic water-miscible organic solvent or solvent mixture. In some embodiments, the composition comprises one or more compounds. The invention is further directed to capsules containing the compositions of the present invention and the administration of pharmaceutically active compounds or molecules to subjects. The invention also includes methods of enhancing the solubility of a compound comprising forming a complex or mixture of a compound with a hydroxybutenyl cyclodextrin and a water-miscible solvent.
Owner:EASTMAN CHEM CO
Drug delivery materials made by sol/gel technology
InactiveUS20060171990A1Easy to produceLow costPowder deliveryOrganic active ingredientsActive agentMedicine
The present invention relates to a method of producing a drug delivery material by encapsulating a biologically or therapeutically active agent in a shell, combining the encapsulated agent with a sol, and converting the combination into a solid or semi-solid drug delivery material. The present invention further relates to drug delivery materials produced by this exemplary method, and to implants formed at least in part from these materials.
Owner:CINVENTION AG
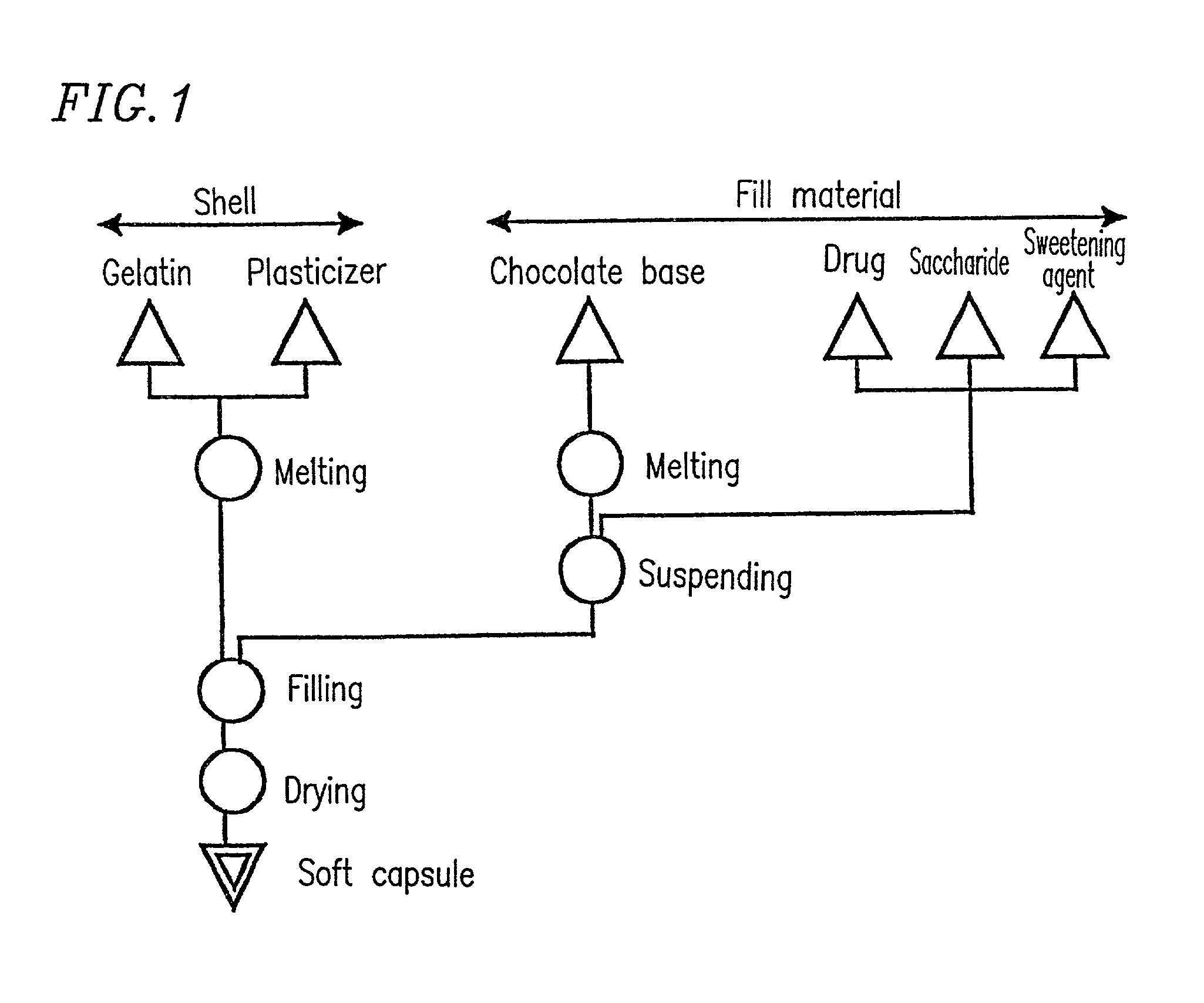
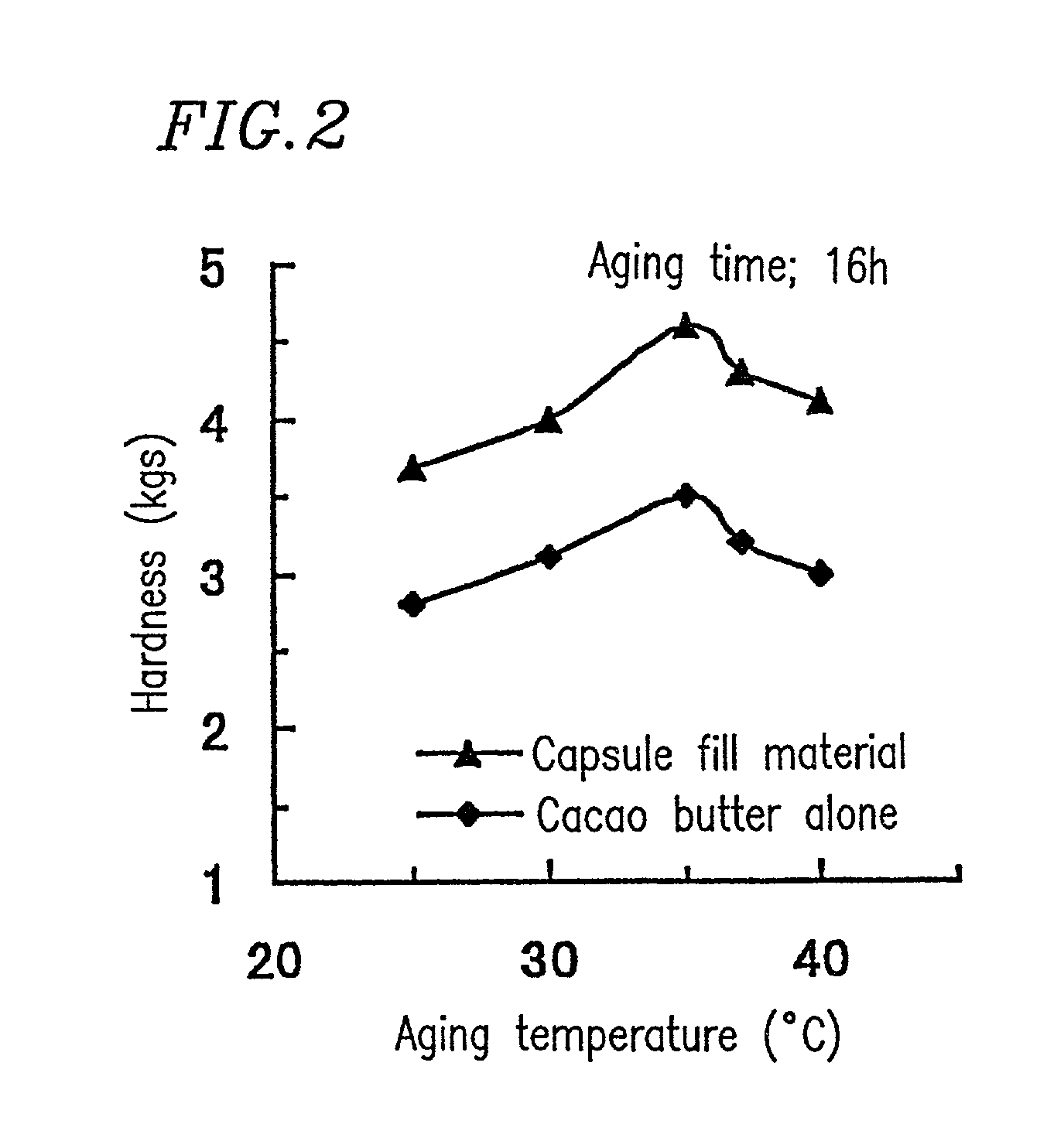
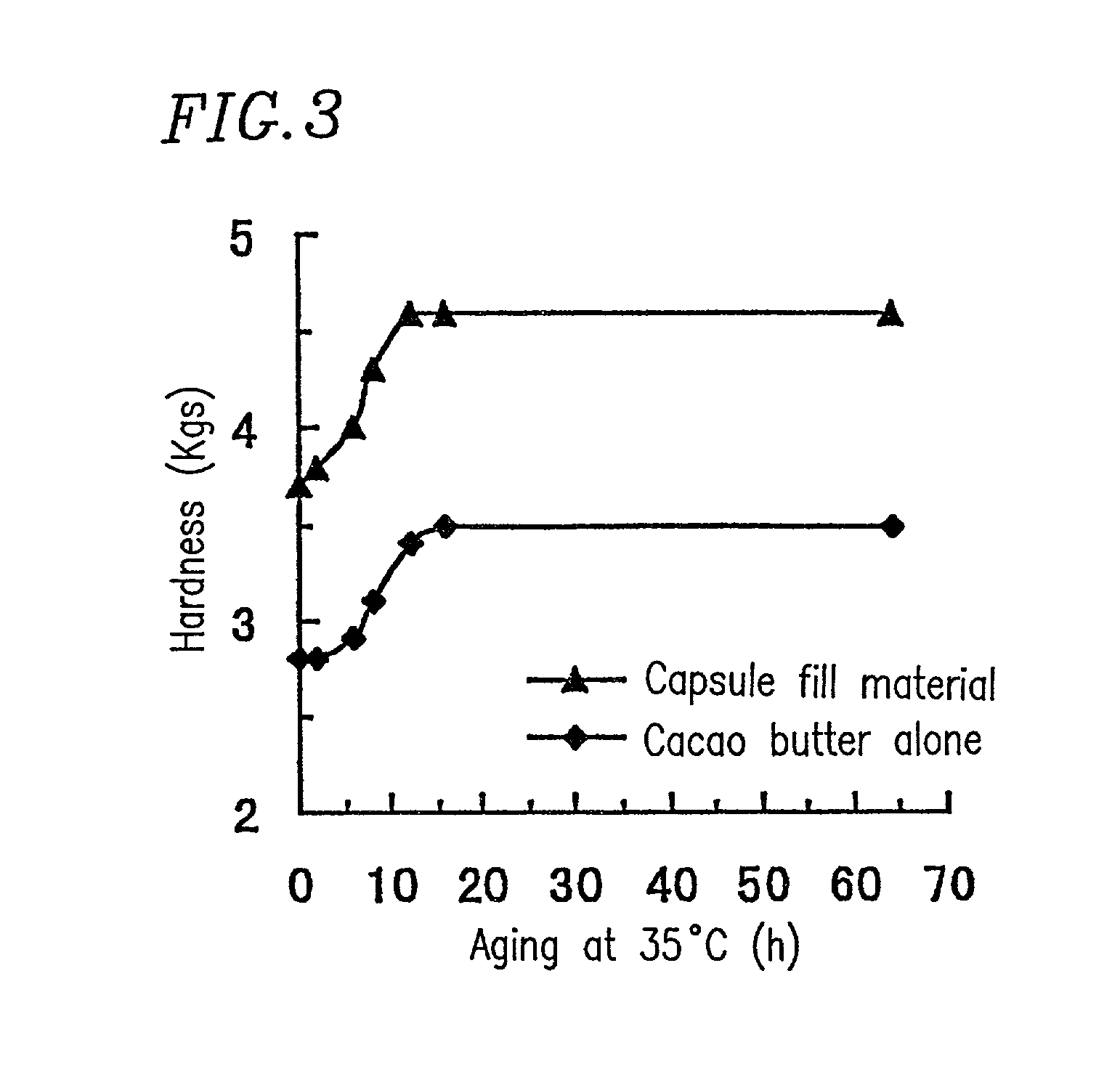
Chewable soft capsules having improved administration properties and process for producing the same
InactiveUS7763276B1Low melting pointEasily chewed in mouthAntipyreticAnalgesicsFilling materialsCoconut oil
A soft capsule in which a shell is filled with fill material, and the fill material is in a solid or semi-solid form at room temperature. The soft capsule may be a chewable capsule, and the fill material may comprise a low melting point additive. The content of the low melting point additive may be 10% or more with respect to the total weight of the fill material, and may have a melting point of about 20 to 50° C. The low melting point additive may be selected from the group consisting of chocolate base, lard, coconut oil and macrogol (polyethylene glycol) as well as a combination thereof.
Owner:SHIONOGI & CO LTD
Encapsulated cure systems
ActiveUS20060073334A1Extensive curingLiquid surface applicatorsGlass/slag layered productsSemi solidBiomedical engineering
Encapsulated cure systems are provided wherein a curative is incorporated into a solid or semi-solid carrier material whereby mere fracturing or failure of the capsule wall encapsulating such cure systems will not provide for or allow sufficient release of the curative. Also provided are adhesive systems incorporating said encapsulated cure systems.
Owner:ENCAPSYS LLC
Blender/food processor blade arrangement for small throated blender jars
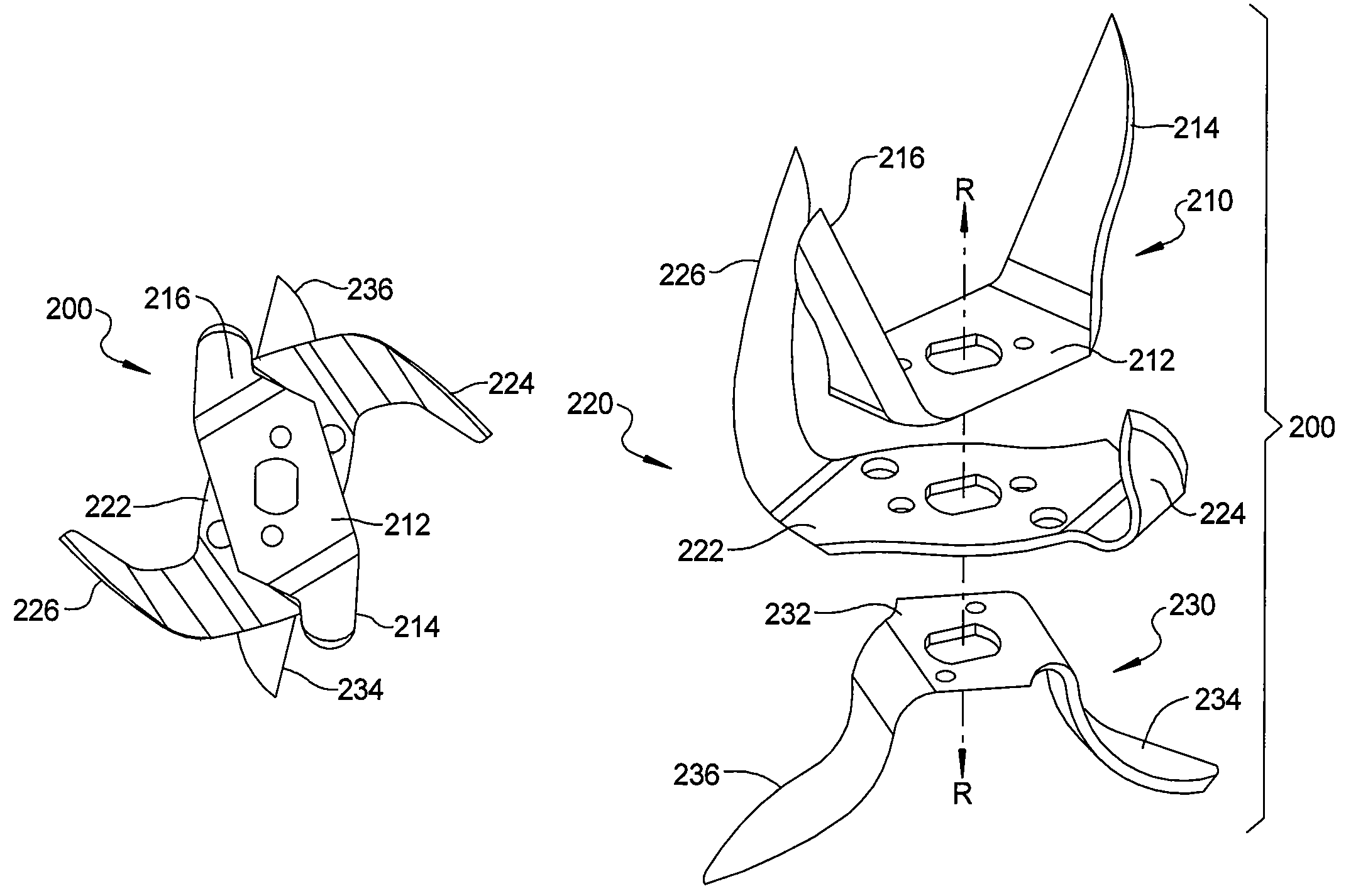
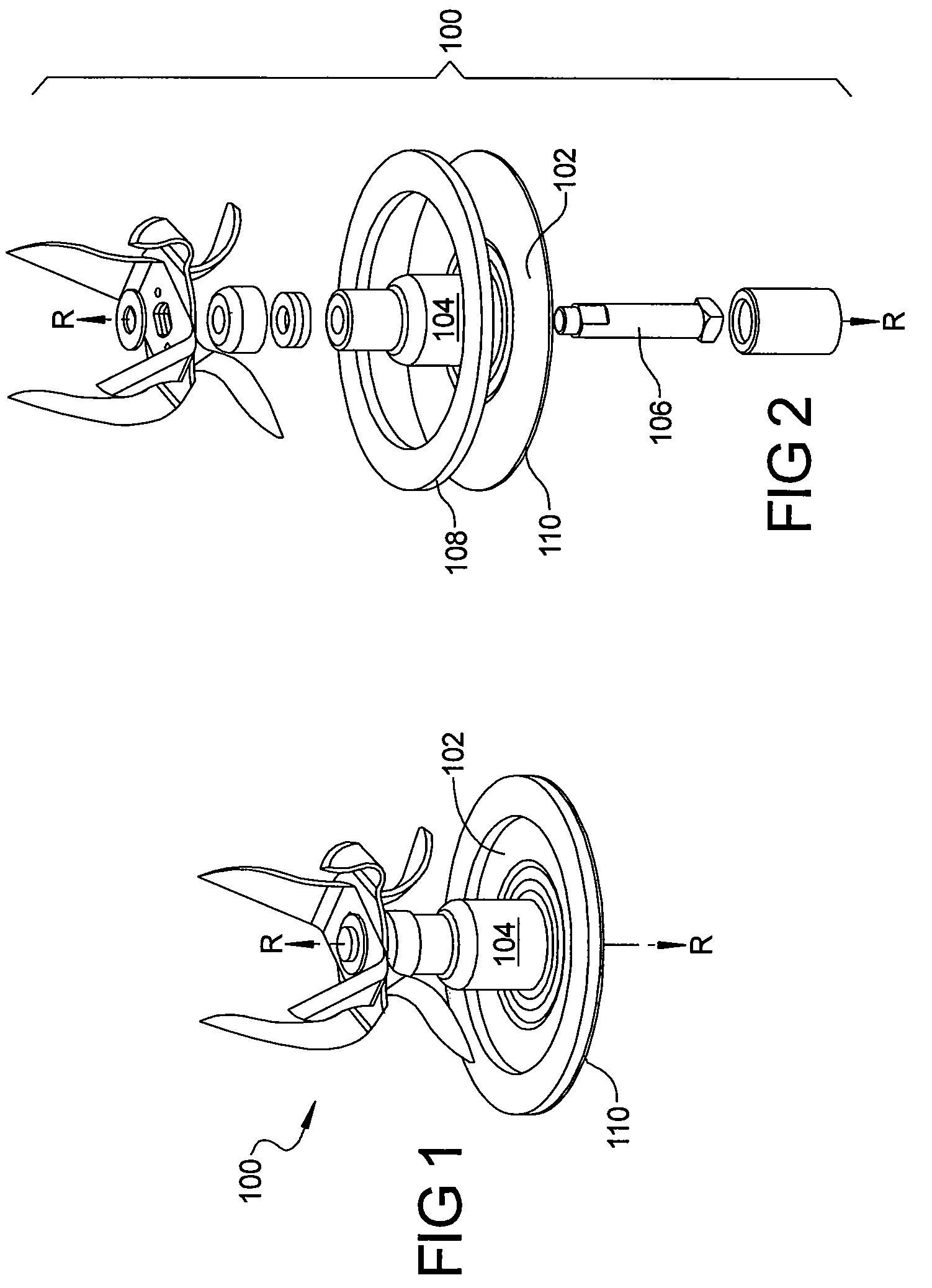
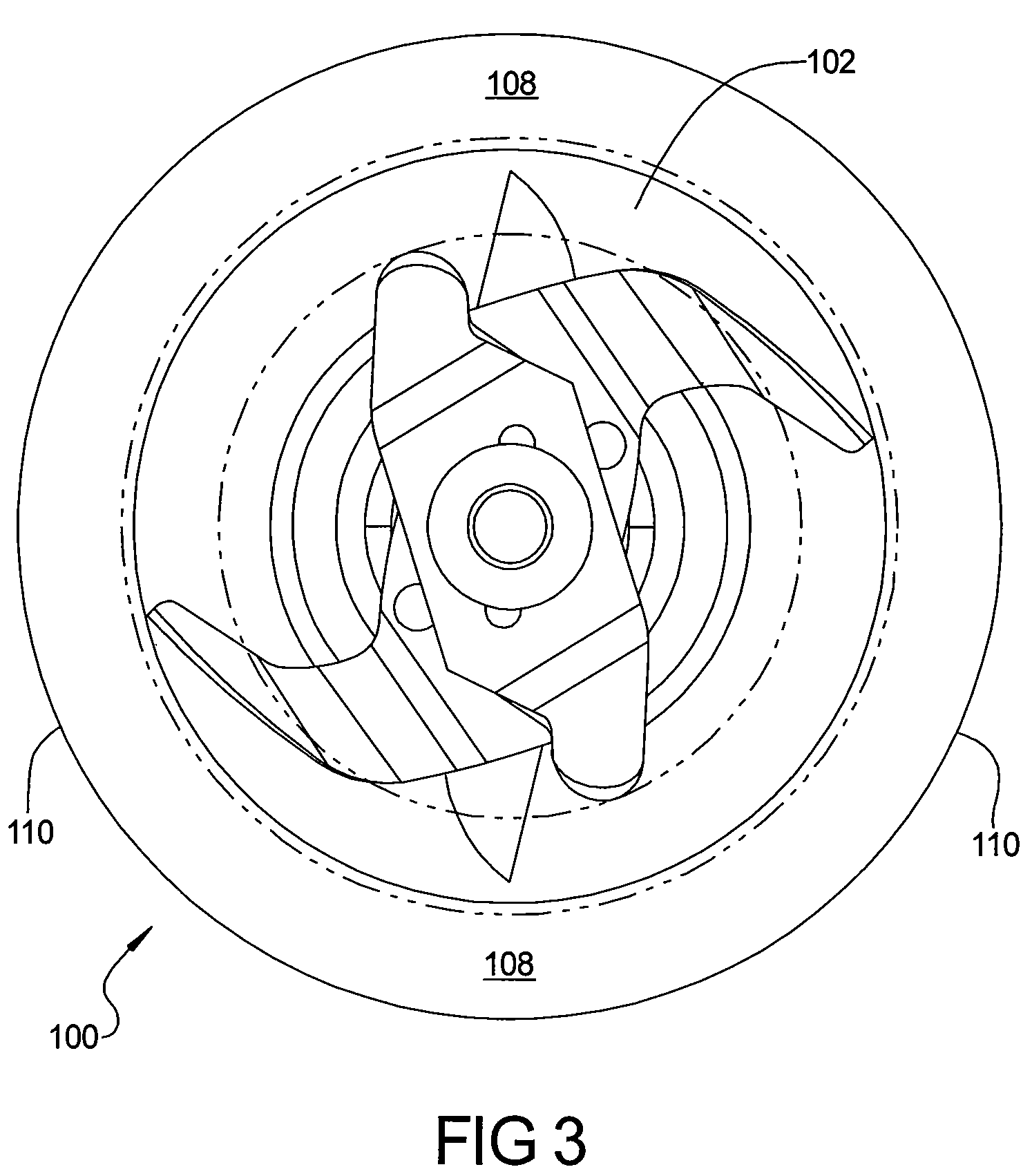
ActiveUS20080198691A1Improve abilitiesEasy to operateRotary stirring mixersKitchen equipmentSide effectSemi solid
A blade assembly suited for both blending and food processing in small throat blender jars is provided. The blade assembly comprises a plurality of blade forms each designed to perform a specific blending or processing task while simultaneously working together and with the geometric restrictions of the jar to optimize the assembly's capability to crush ice, blend or mix liquids and / or semi-solid materials, and to chop, cut, or slice solid food items without the need for user interaction to clear compacted items from the blades and / or the walls or bottom of the jar during its operation. The improved performance also serves the beneficial side effect of improved bearing and motor life in the blender / food processor.
Owner:SUNBEAN PROD INC
Blender/food processor blade arrangement for small throated blender jars
ActiveUS7641380B2Improve abilitiesEasy to operateRotary stirring mixersKitchen equipmentSide effectSemi solid
A blade assembly suited for both blending and food processing in small throat blender jars is provided. The blade assembly comprises a plurality of blade forms each designed to perform a specific blending or processing task while simultaneously working together and with the geometric restrictions of the jar to optimize the assembly's capability to crush ice, blend or mix liquids and / or semi-solid materials, and to chop, cut, or slice solid food items without the need for user interaction to clear compacted items from the blades and / or the walls or bottom of the jar during its operation. The improved performance also serves the beneficial side effect of improved bearing and motor life in the blender / food processor.
Owner:SUNBEAN PROD INC
Semi-solid mucoadhesive formulations
ActiveUS20060240111A1Good bioadhesionEfficient use ofPowder deliveryOintment deliveryDiseaseGynecology
Semisolid mucoadhesive formulations for vaginal application, with improved technical and organoleptic characteristics, which contain at least two bioadhesive gelling polymers and an active ingredient, useful in the prevention and / or treatment of various pathologies and disorders in human beings or animals.
Owner:ITF RISECH FARMA S L U
Electronic control unit
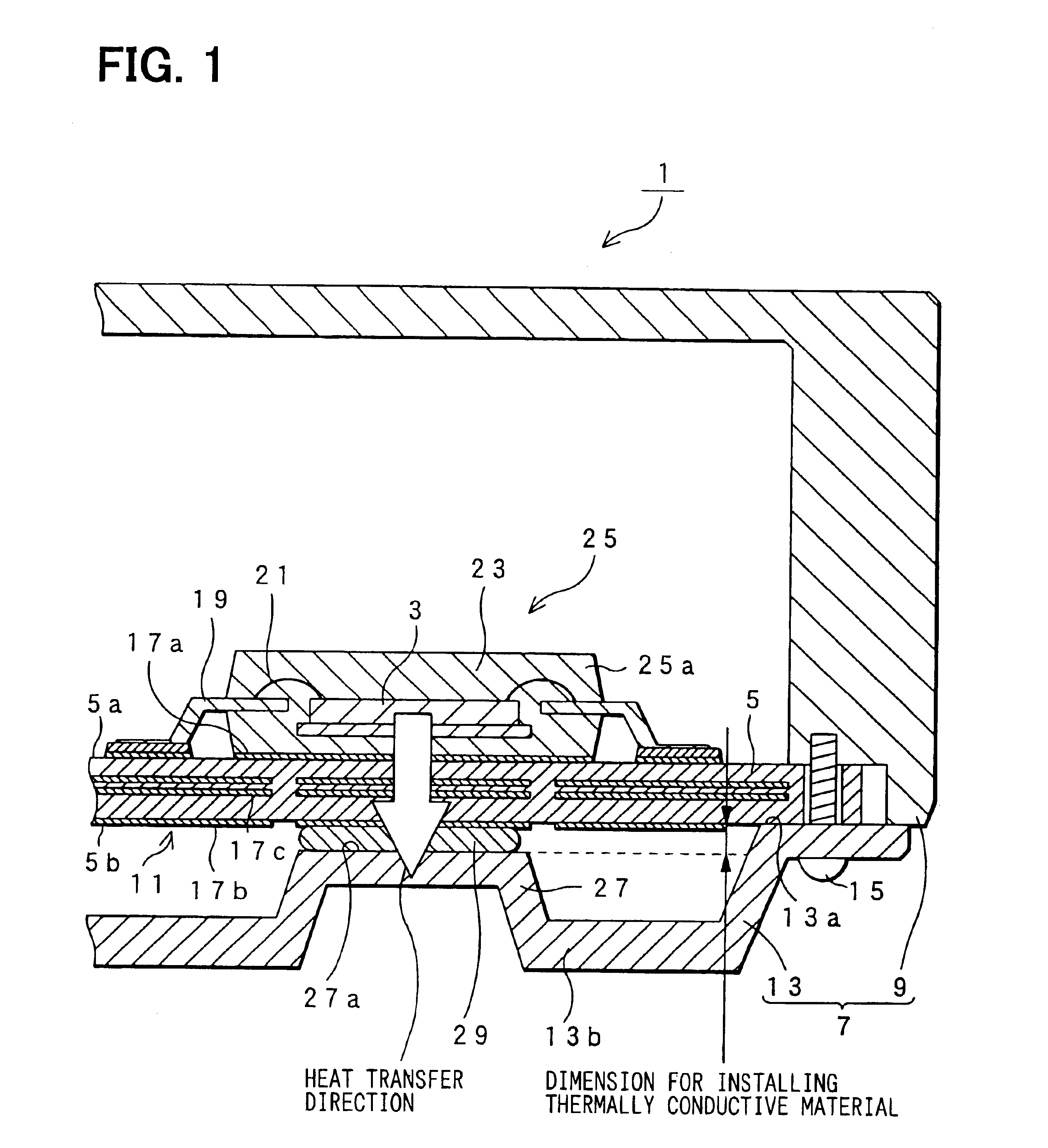
InactiveUS6816377B2Semiconductor/solid-state device detailsPrinted circuit aspectsSemi solidEngineering
An electronic control device having a high heat dissipating ability includes a printed board secured to an enclosure and interposed between a case and a cover with screws passing through the printed board. Thermally conductive thin film layers made of copper foil are formed in parallel on a mount face and an opposite mount face of the printed board and inside the printed board so as to be thermally separated from each other. A protrusion is provided on the cover and protrudes beyond a bottom part of the cover toward the position where an electronic component is mounted. A flexible, semi-solid thermally conductive material is placed between an end face of the protrusion and the opposite mount face of the printed board corresponding to the position where the electronic component is mounted to be in contact with the end face and the opposite mount face.
Owner:DENSO CORP
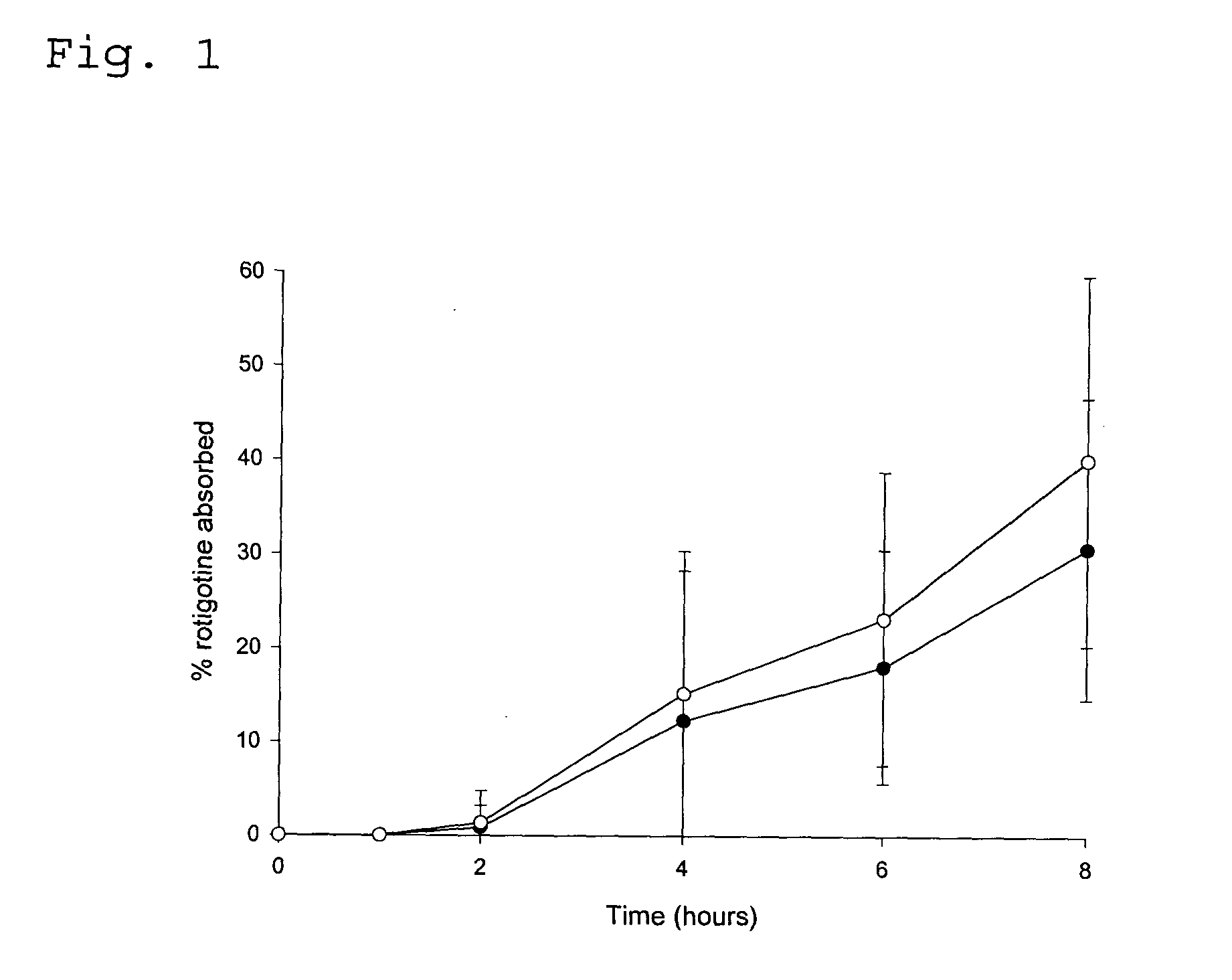
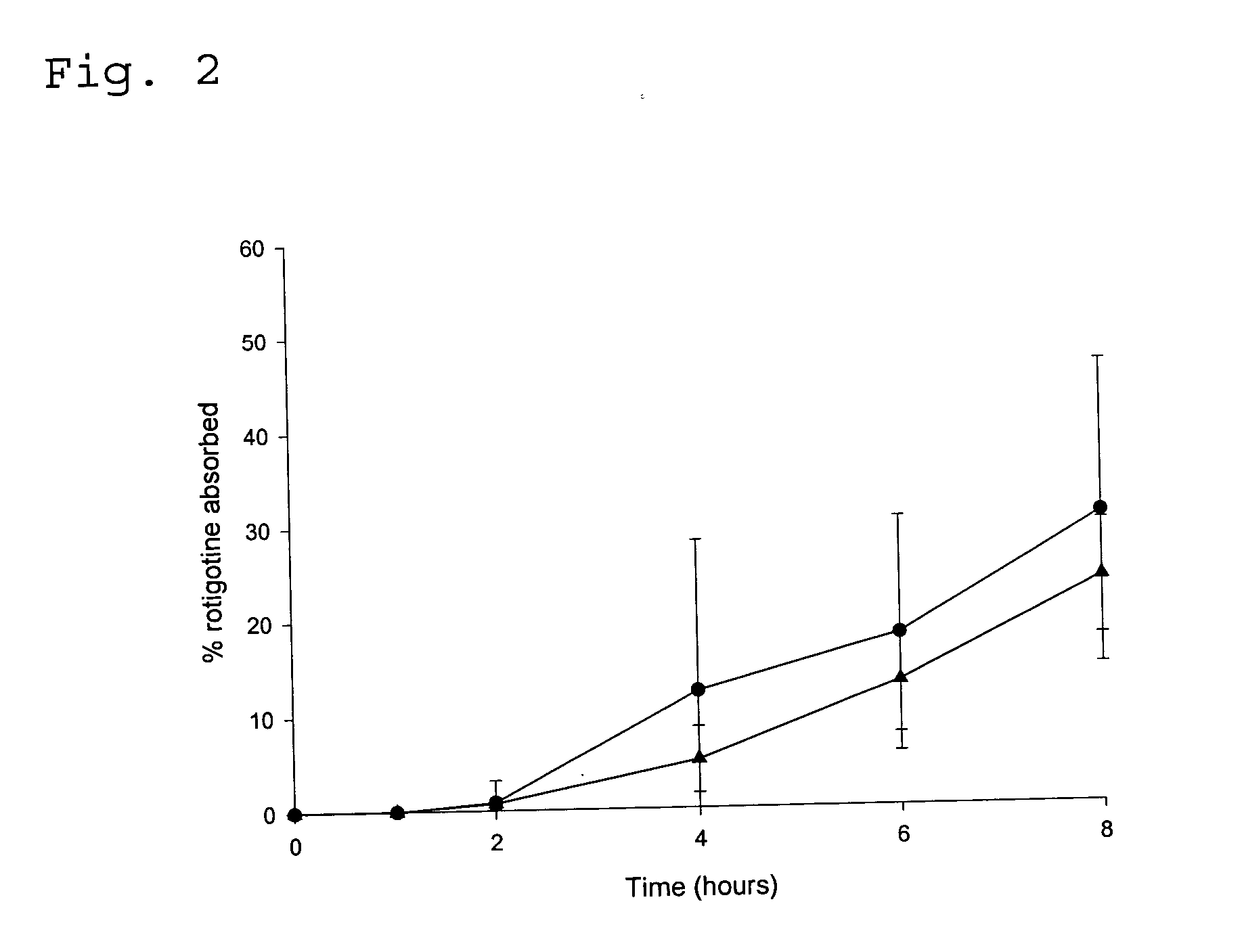
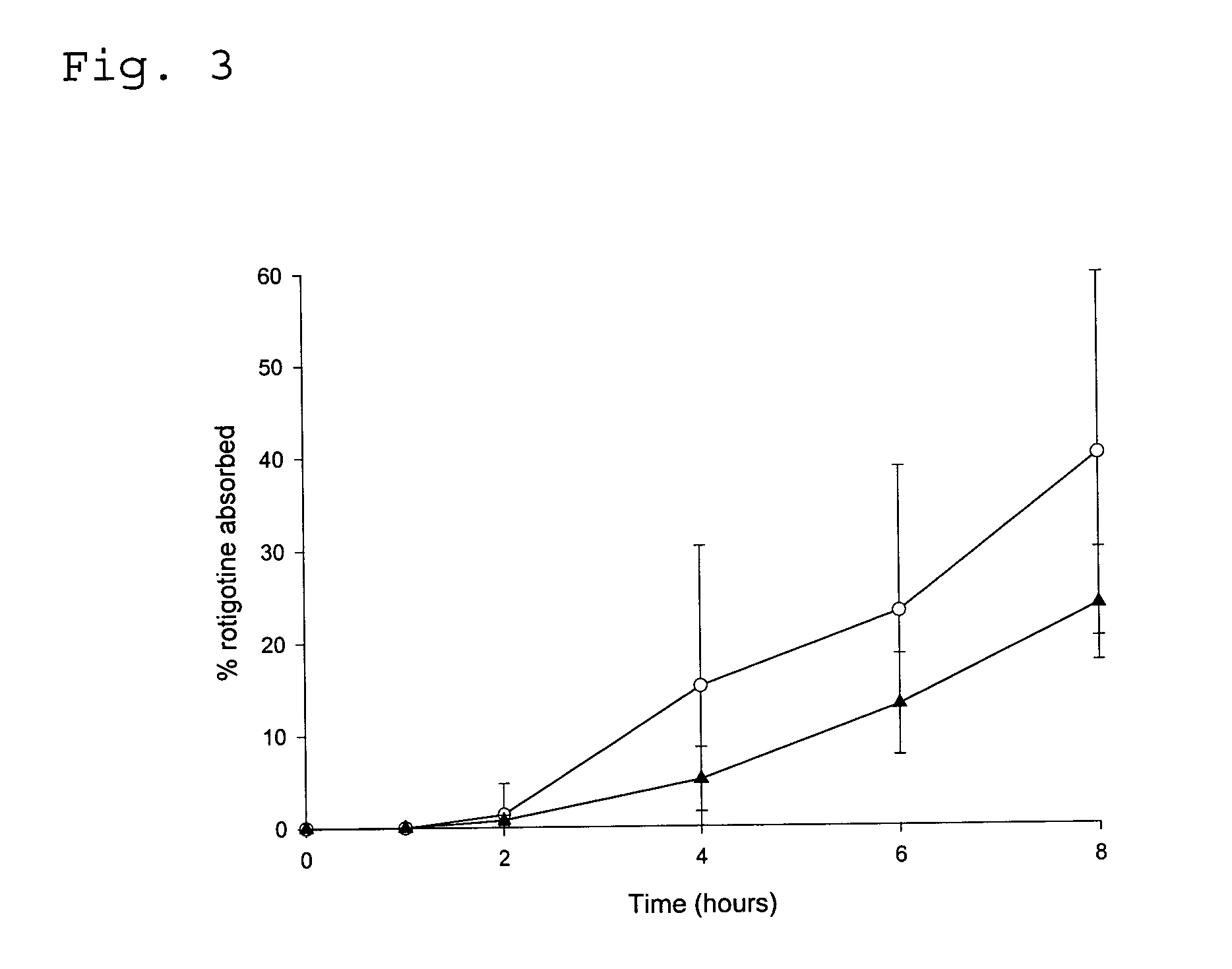
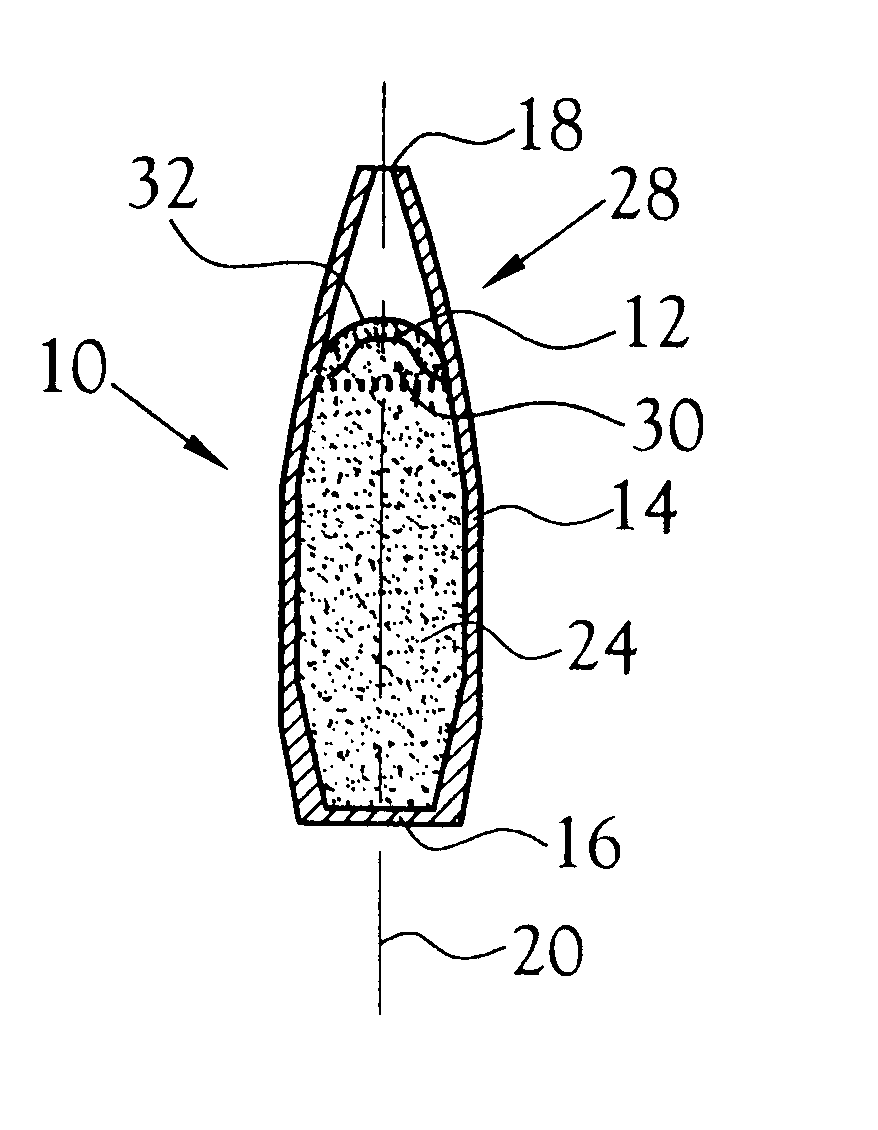
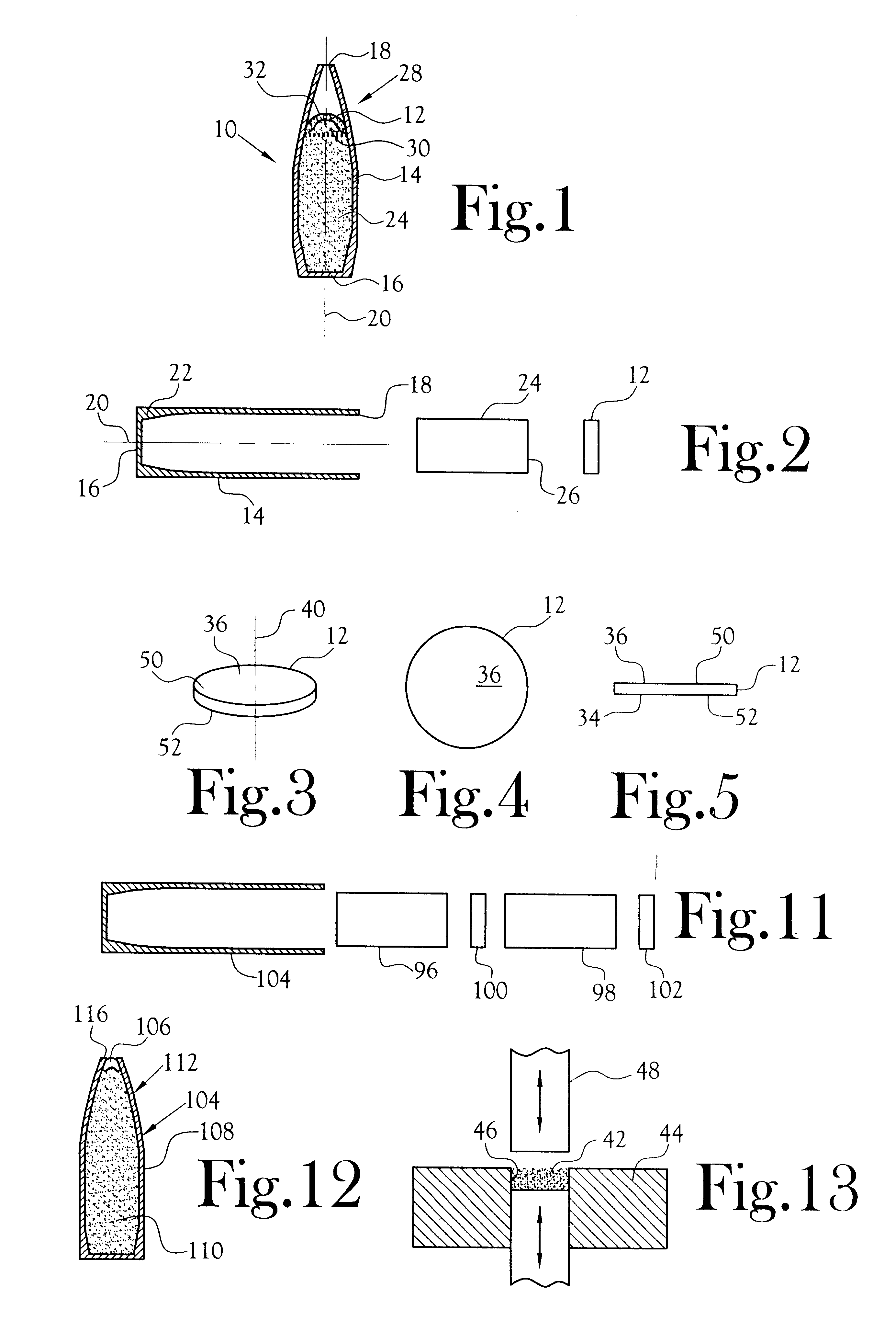
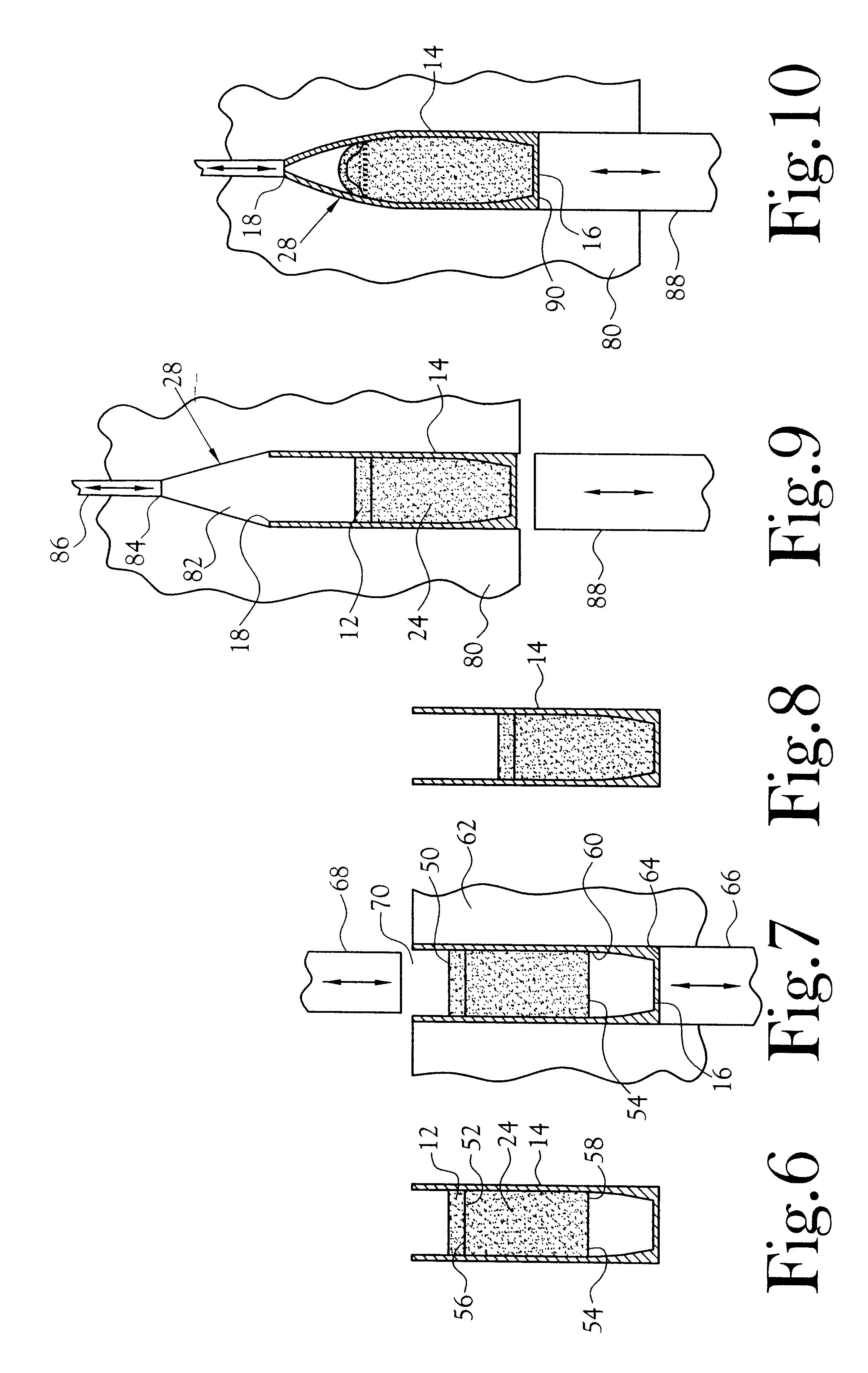
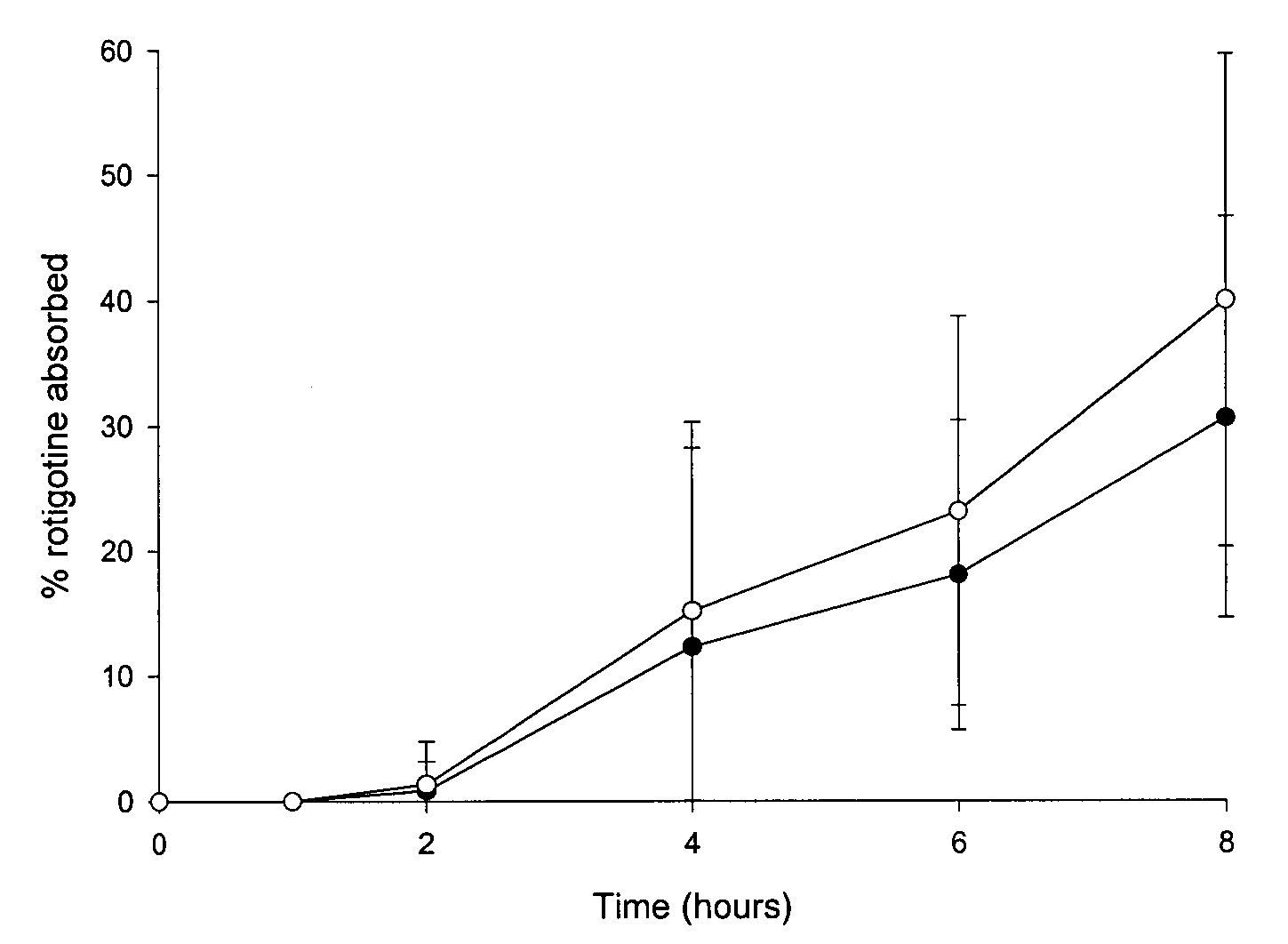
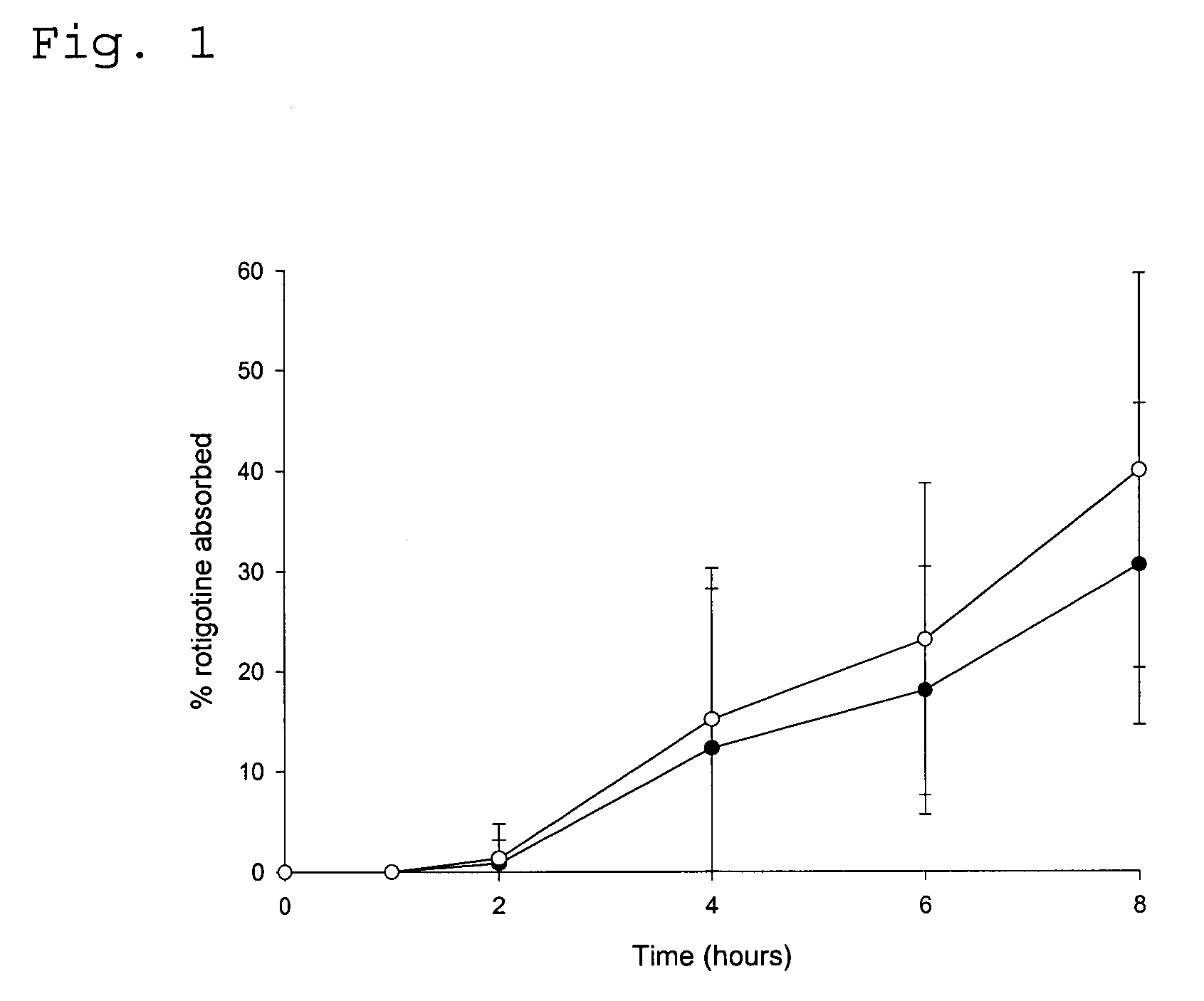
Transdermal delivery system for the administration of rotigotine
An improved Transdermal Delivery System (TDS) comprising a backing layer inert to the components of the matrix, a self-adhesive matrix containing rotigotine and a protective foil or sheet to be removed prior to use, characterized in that the self-adhesive matrix consists of a solid or semi-solid semi-permeable polymer (1) wherein rotigotine in its free base form has been incorporated, (2) which is saturated with rotigotine and contains said rotigotine as a multitude of microreservoirs within the matrix, (3) which is highly permeable for the free base of rotigotine, (4) which is impermeable for the protonated form of rotigotine, (5) wherein the maximum diameter of the microreservoirs is less than the thickness of the matrix. is provided. Said TDS provides for enhanced flux of rotigotine across the TDS / skin interface.
Owner:UCB SA
Powder-based disc for gun ammunition having a projectile which includes a frangible powder-based core disposed within a metallic jacket
InactiveUS6371029B1Minimizing non-uniformityAmmunition projectilesProjectilesCircular discSemi solid
A disc for use in the manufacture of gun ammunition and a round of gun ammunition which includes the disc. A preferred disc comprises a mixture of metal powders compressed into self-supporting deformable disc that is incorporated along with a core into a jacket to define the projectile of a round of gun ammunition. The disc is frangible upon the projectile striking a solid or semi-solid target. Preferably the core is likewise frangible. In one embodiment the disc is incorporated into the ogive of a projectile. In another embodiment, a disc may be incorporated into a projectile comprising multiple cores, the disc being disposed between adjacent surfaces of the cores. This latter disc commonly is in addition to the disc which is incorporated into the ogive of the projectile. A round of gun ammunition incorporating a powder-based disc in the projectile thereof is disclosed.
Owner:DORIS NEBEL BEAL INTER VIVOS PATENT TRUST PAWLEYS ISLAND
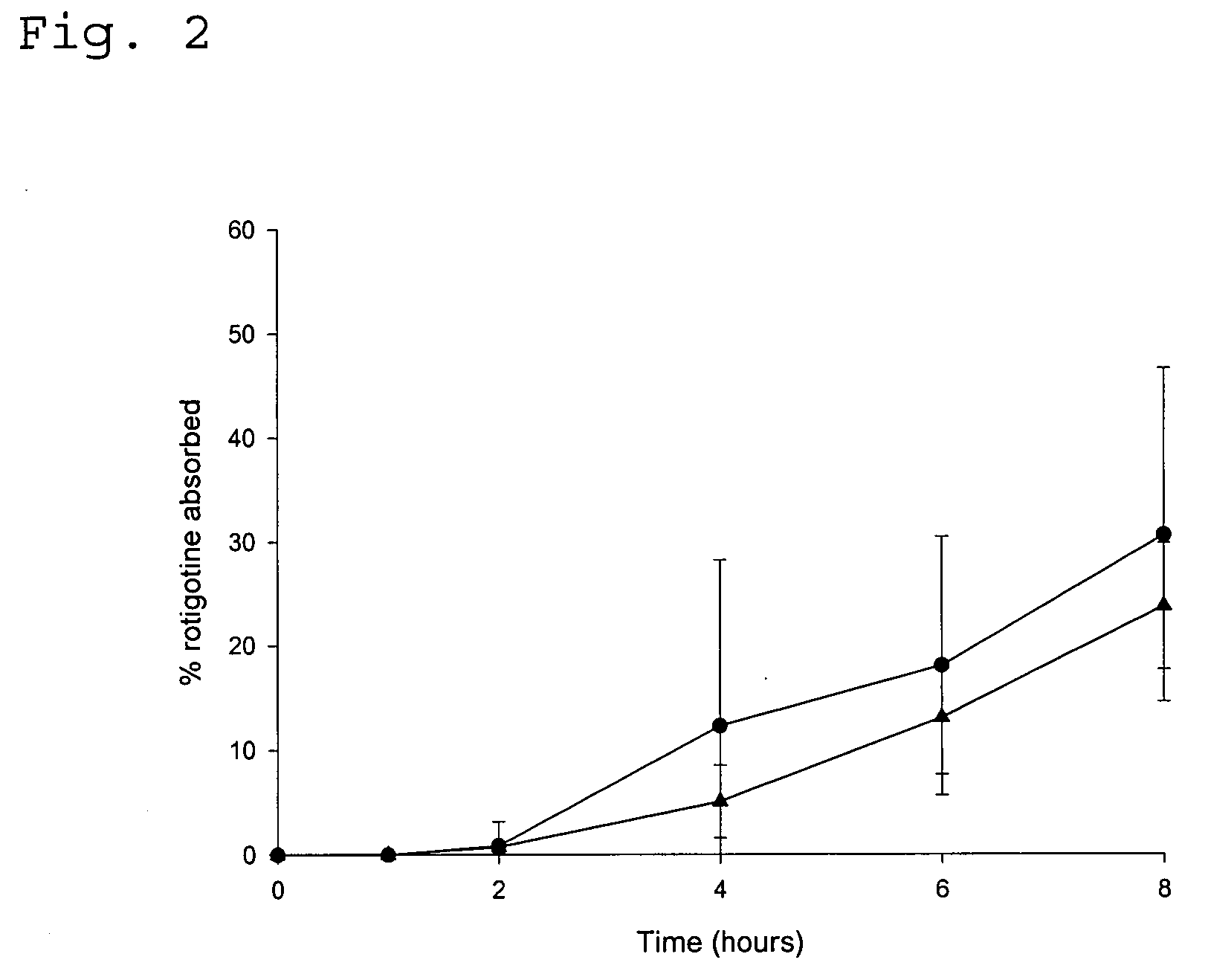
Transdermal delivery system
ActiveUS20040081683A1Facilitated releaseGood release effectPowder deliveryBiocideProtonationMedicine
An improved Transdermal Delivery System (TDS) comprising a backing layer inert to the components of the matrix, a selfadhesive matrix containing an amine-functional drug and a protective foil or sheet to be removed prior to use, characterized in that the self-adhesive matrix consists of a solid or semi-solid semi-permeable polymer (1) wherein an amine functional drug in its free base form has been incorporated, (2) which is saturated with the amine functional drug and contains said drug as a multitude of microreservoirs within the matrix, (3) which is highly permeable for the free base of the amine functional drug, (4) which is impermeable for the protonated form of the amine functional drug, (5) wherein the maximum diameter of the microreservoirs is less than the thickness of the matrix. is provided. Said TDS provides for enhanced flux of the amine functional drug across the TDS / skin interface.
Owner:UCB SA
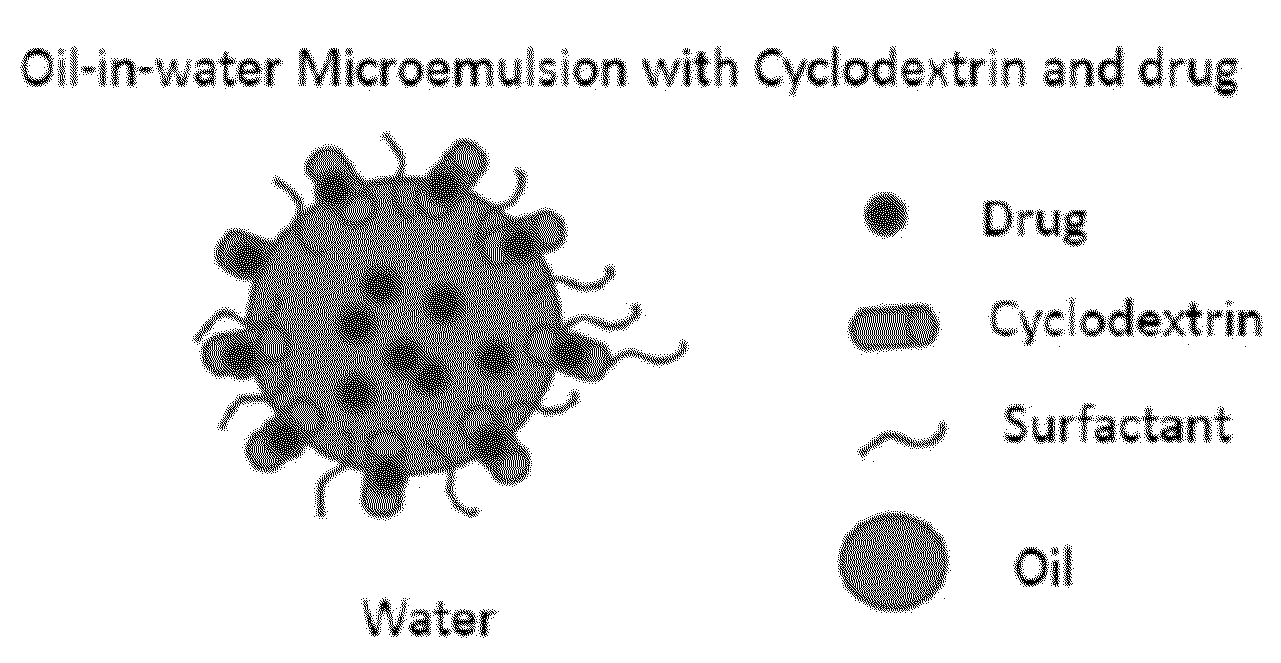
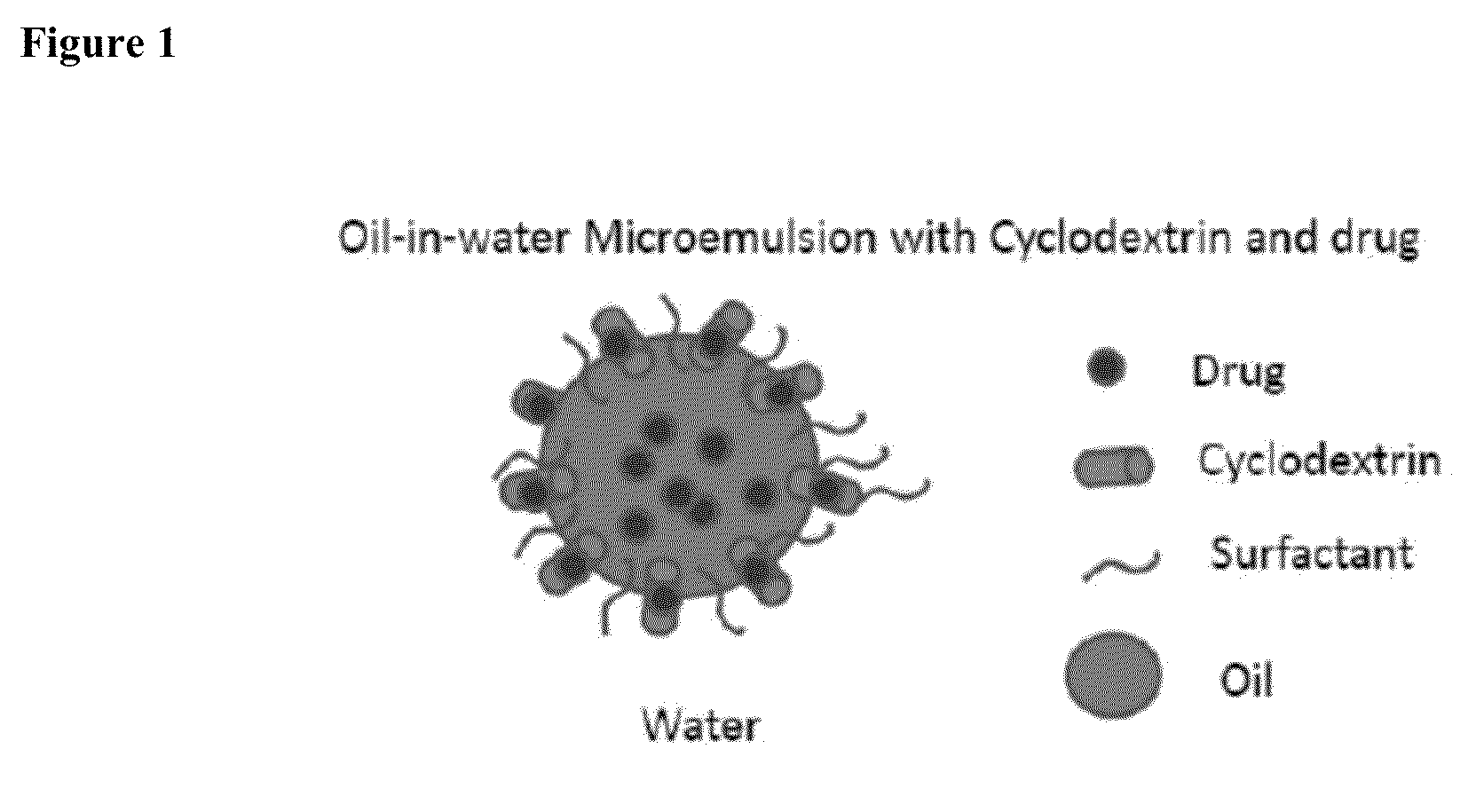
Cyclodextrin-Based Microemulsions, and Dermatological Uses Thereof
InactiveUS20130251644A1Large average pore sizeUneven skinBiocideCosmetic preparationsSolubilityActive agent
Described herein are cyclodextrin-stabilized microemulsion systems useful for increasing the solubility, stability, bioavailability, or safety of an active agent for delivery to the skin. The microemulsions may reduce the occurrence of skin irritation or odor upon application. In certain embodiments, the active agent is substantially insoluble in water. The microemulsions may be formulated as semi-solids, for example creams, or as aerosol or non-aerosol foams. Also described are methods of treating skin disorders, comprising the step of applying to an affected area of a subject in need thereof a therapeutically-effective amount of an inventive microemulsion.
Owner:PRECISION DERMATOLOGY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com