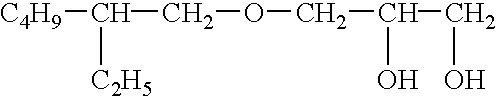Patents
Literature
9148 results about "Irritation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Irritation, in biology and physiology, is a state of inflammation or painful reaction to allergy or cell-lining damage. A stimulus or agent which induces the state of irritation is an irritant. Irritants are typically thought of as chemical agents (for example phenol and capsaicin) but mechanical, thermal (heat), and radiative stimuli (for example ultraviolet light or ionising radiations) can also be irritants. Irritation also has non-clinical usages referring to bothersome physical or psychological pain or discomfort.
Avenanthramide-containing compositions
Methods and compositions for treating or preventing a skin condition, an inflammation, an irritation or an allergy associated with an ectoparasitic infection or infestation on an animal. The methods involve applying to the skin of the animal a pharmaceutical composition that contains a therapeutically effective amount of one or more than one avenanthramide, an optional ecto and / or endo-parasiticidal agent, and a pharmaceutically acceptable diluent or carrier
Owner:CEAPRO
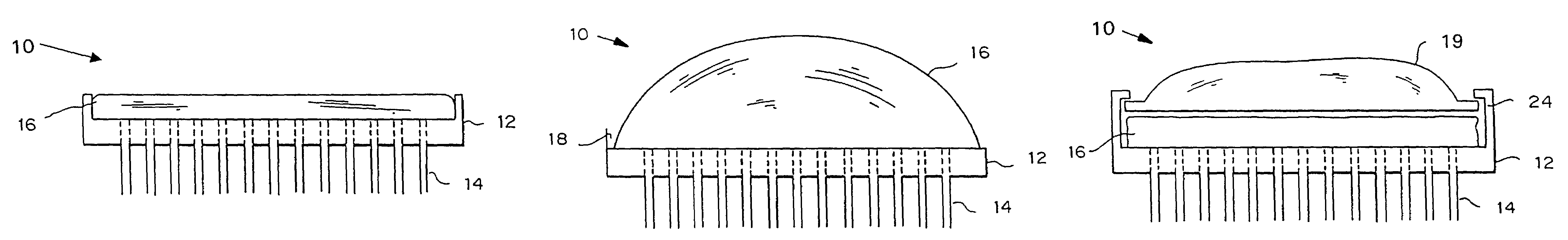
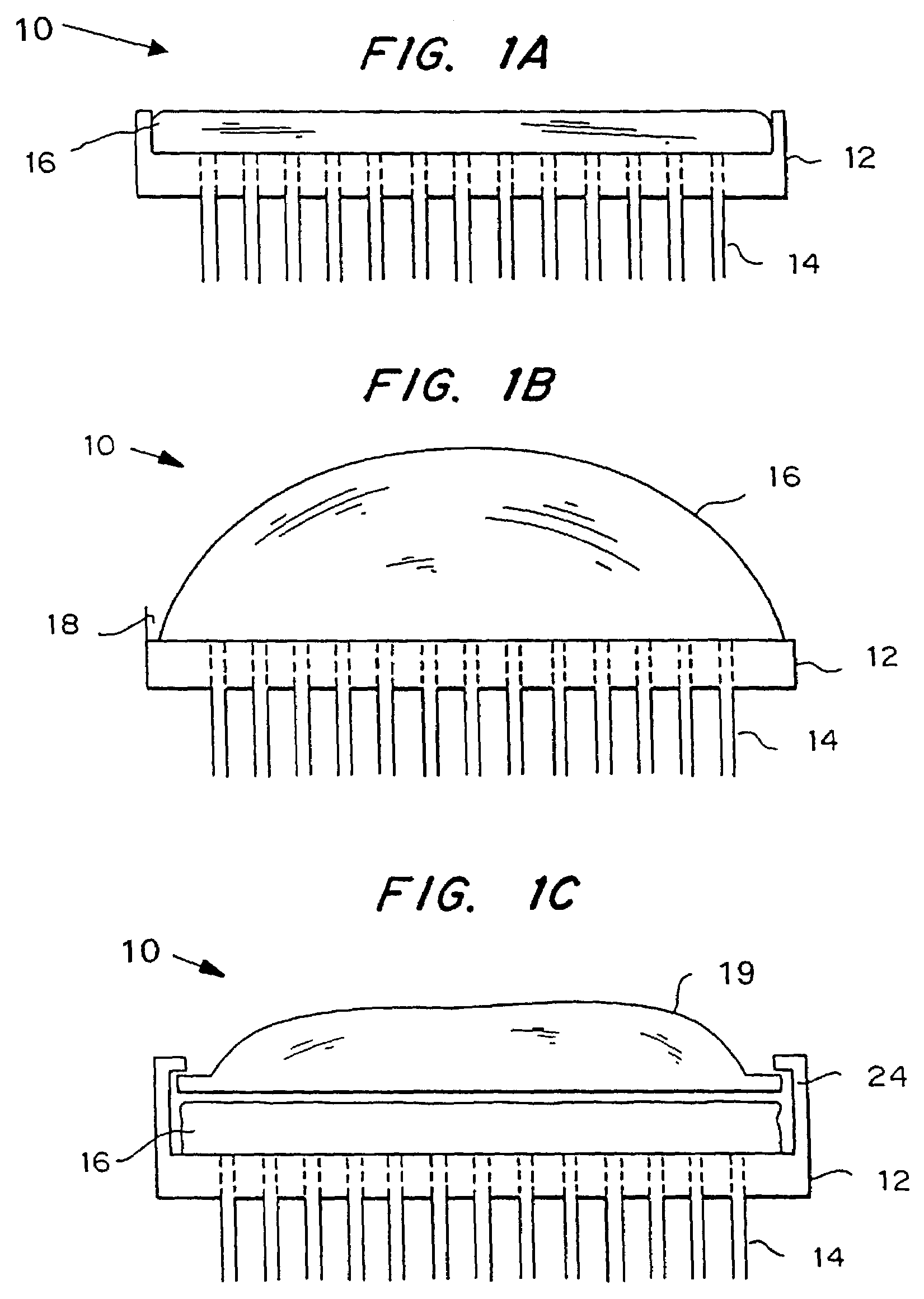
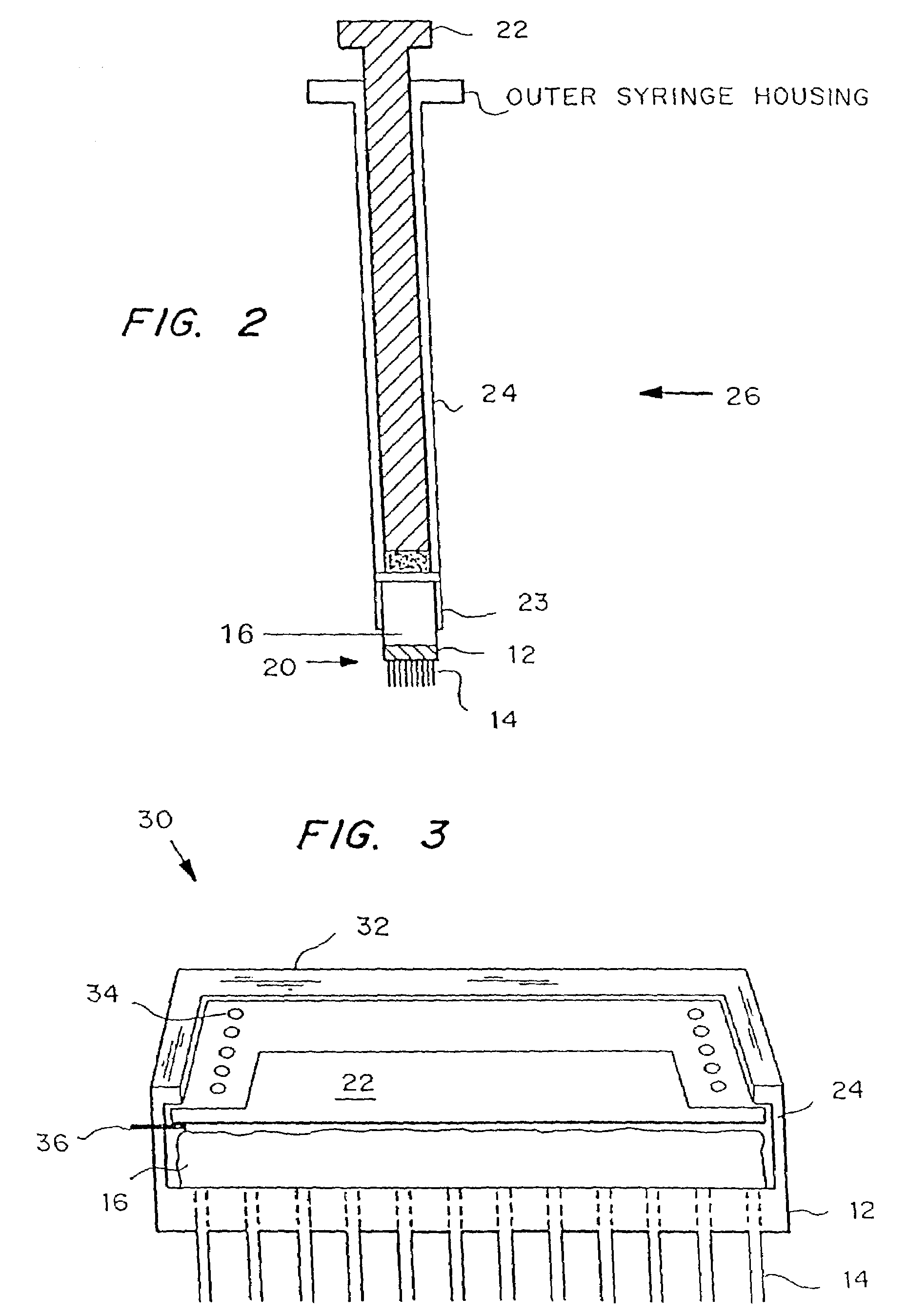
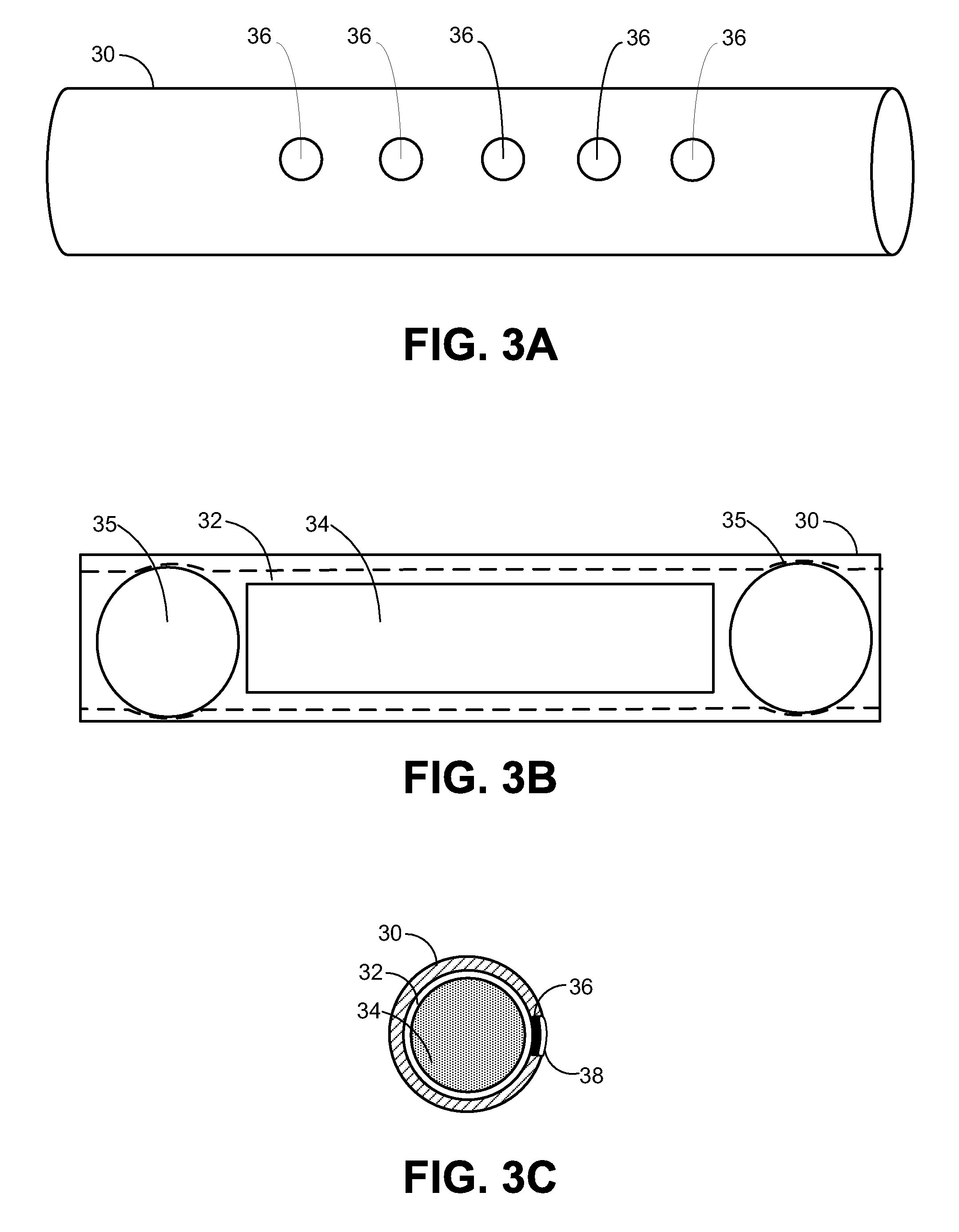
Microneedle device for extraction and sensing of bodily fluids
InactiveUS7344499B1Simple wayMinimal and no damageAdditive manufacturing apparatusMicroneedlesMetaboliteIrritation
Microneedle devices are provided for controlled sampling of biological fluids in a minimally-invasive, painless, and convenient manner. The microneedle devices permit in vivo sensing or withdrawal of biological fluids from the body, particularly from or through the skin or other tissue barriers, with minimal or no damage, pain, or irritation to the tissue. The microneedle device includes one or more microneedles, preferably in a three-dimensional array, a substrate to which the microneedles are connected, and at least one collection chamber and / or sensor in communication with the microneedles. Preferred embodiments further include a means for inducing biological fluid to be drawn through the microneedles and into the collection chamber for analysis. In a preferred embodiment, this induction is accomplished by use of a pressure gradient, which can be created for example by selectively increasing the interior volume of the collection chamber, which includes an elastic or movable portion engaged to a rigid base. Preferred biological fluids for withdrawal and / or sensing include blood, lymph, interstitial fluid, and intracellular fluid. Examples of analytes in the biological fluid to be measured include glucose, cholesterol, bilirubin, creatine, metabolic enzymes, hemoglobin, heparin, clotting factors, uric acid, carcinoembryonic antigen or other tumor antigens, reproductive hormones, oxygen, pH, alcohol, tobacco metabolites, and illegal drugs.
Owner:GEORGIA TECH RES CORP +1
Microneedle drug delivery device
InactiveUS7226439B2Minimal and no damage and pain and irritationAdditive manufacturing apparatusSurgical needlesIrritationMicro-needle
Simple microneedle devices for delivery of drugs across or into biological tissue are provided, which permit drug delivery at clinically relevant rates across or into skin or other tissue barriers, with minimal or no damage, pain, or irritation to the tissue. The devices include a substrate to which a plurality of hollow microneedles are attached or integrated, and at least one reservoir, containing the drug, selectably in communication with the microneedles, wherein the volume or amount of drug to be delivered can be selectively altered. The reservoir can be formed of a deformable, preferably elastic, material. The device typically includes a means, such as a plunger, for compressing the reservoir to drive the drug from the reservoir through the microneedles. In one embodiment, the reservoir is a syringe or pump connected to the substrate.
Owner:VALERITAS LLC (US)
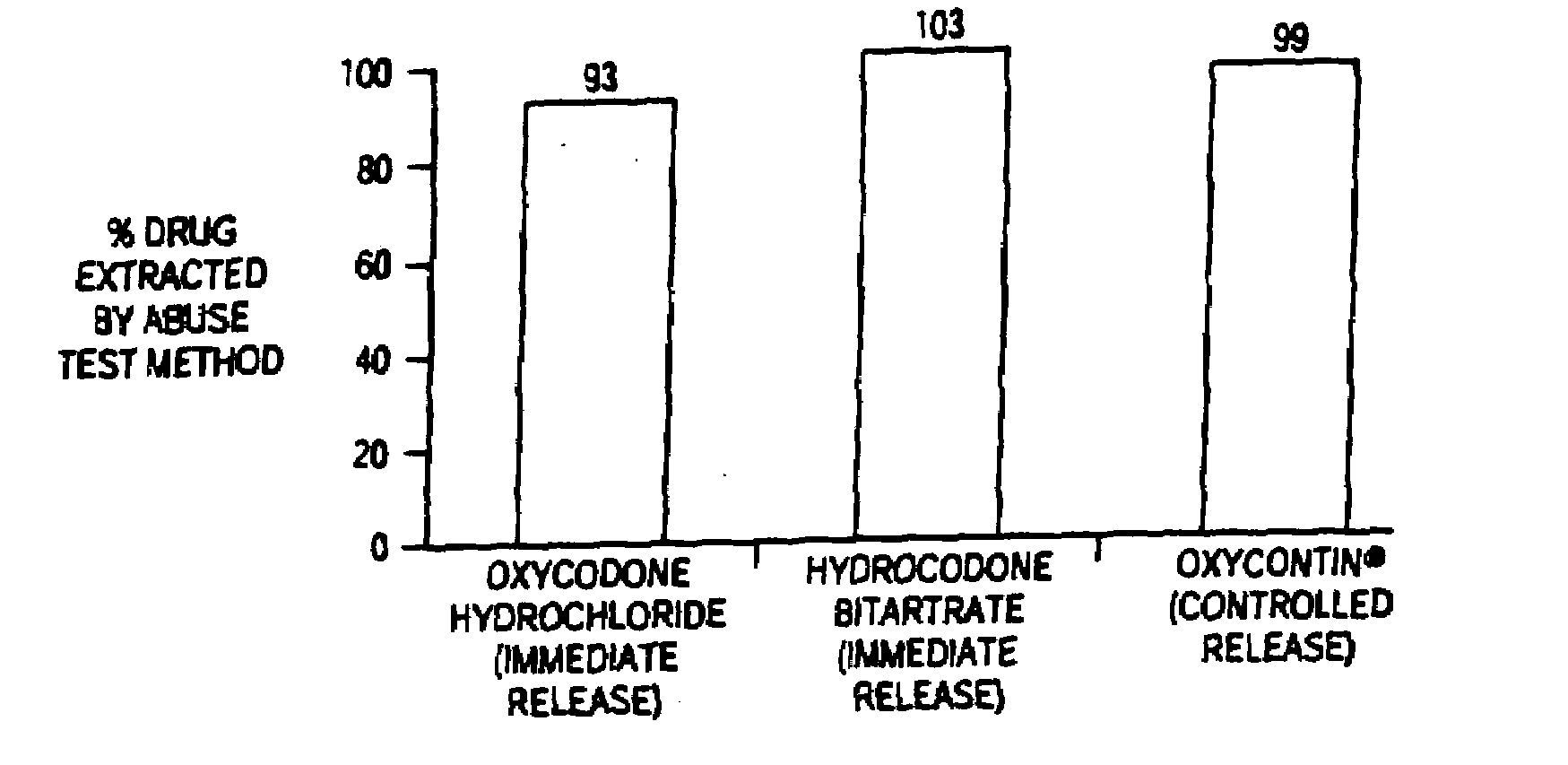
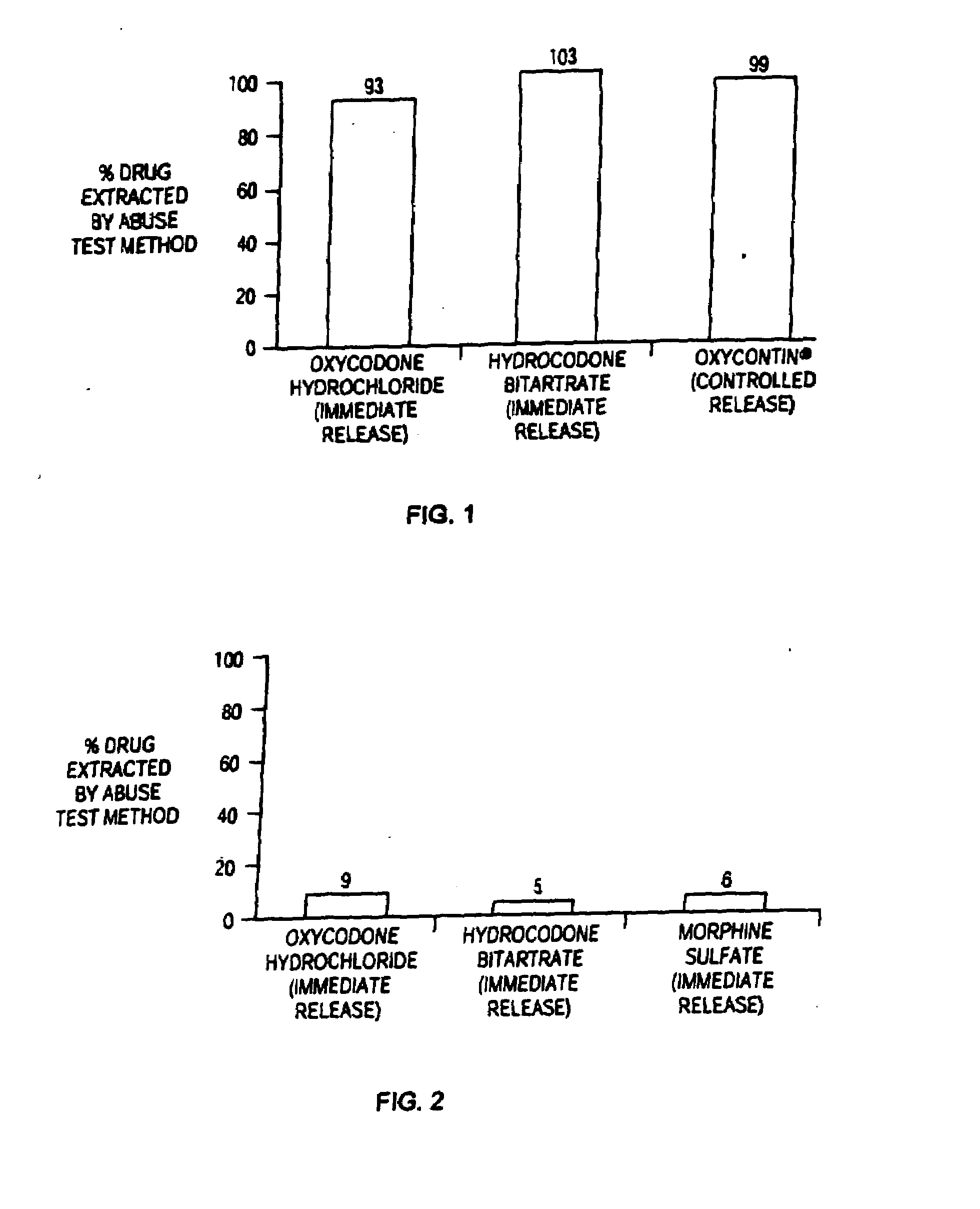
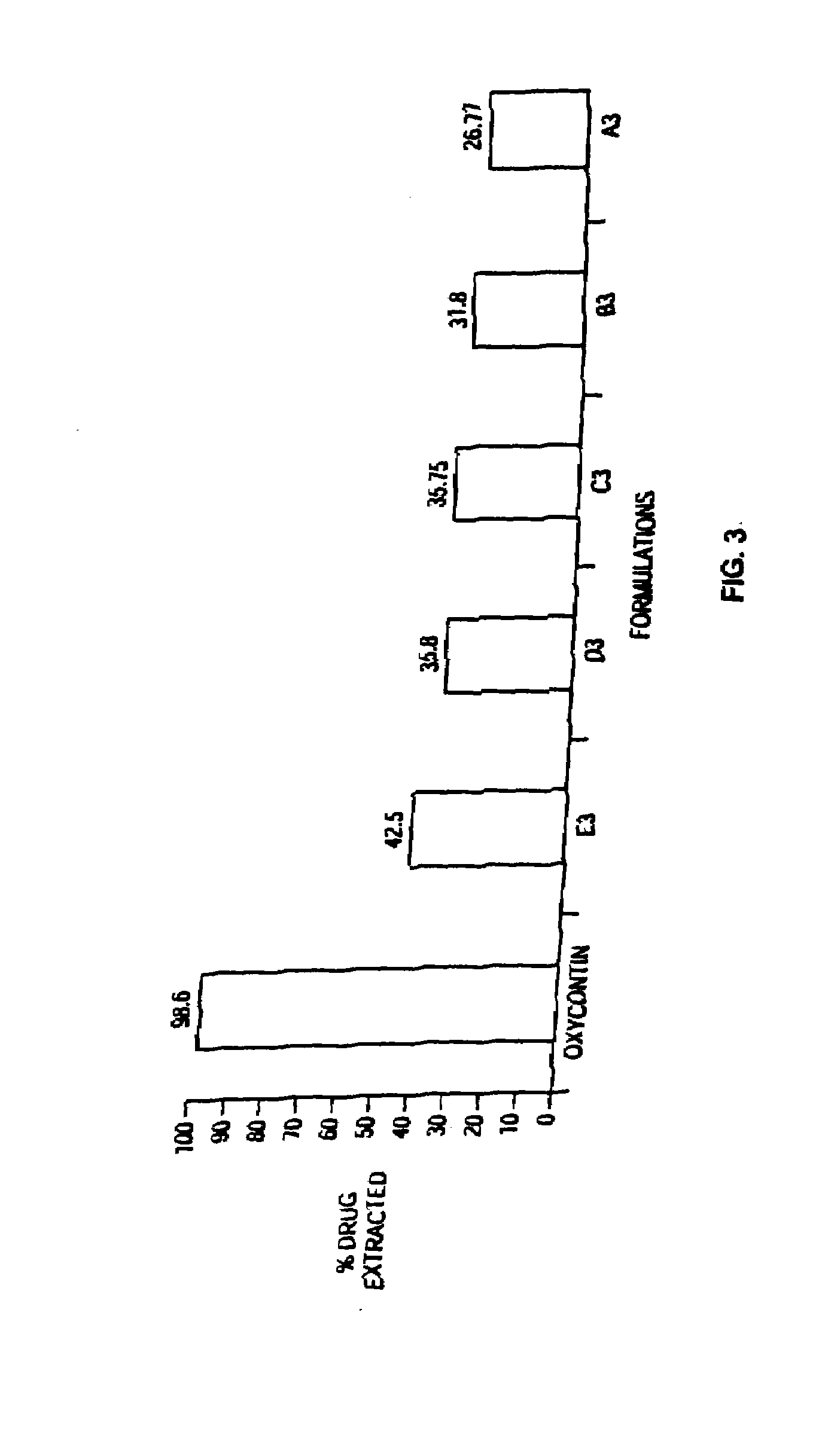
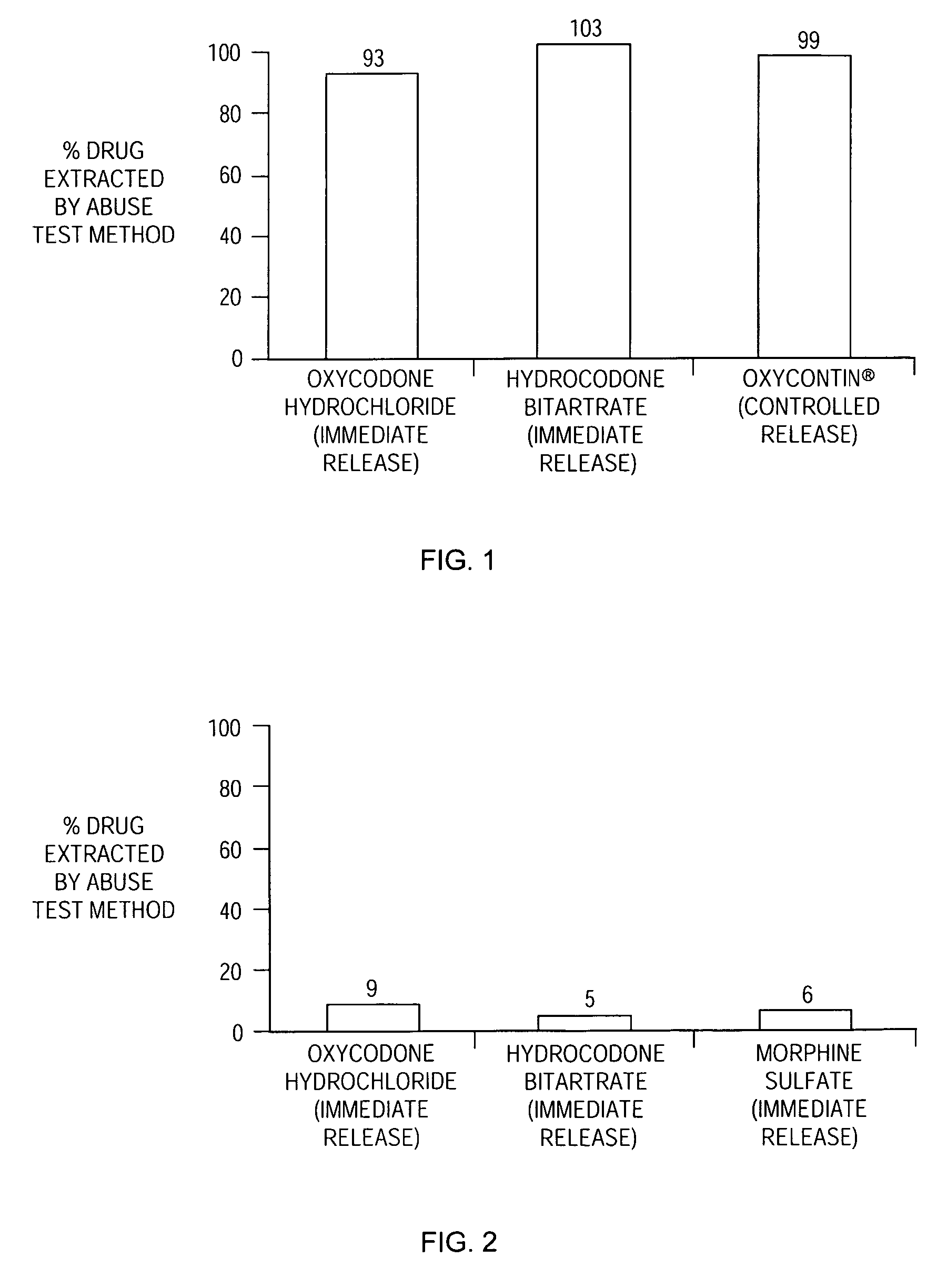
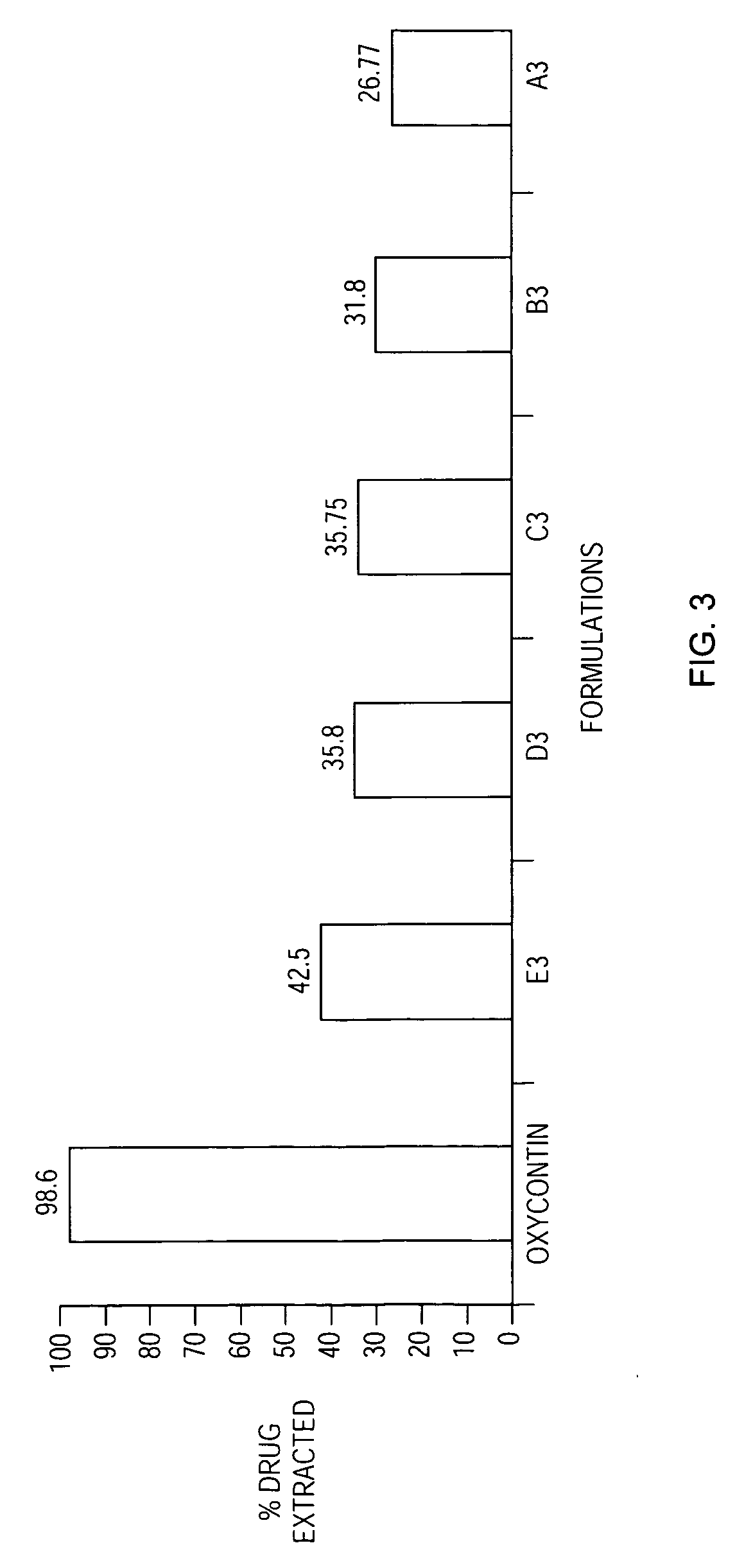
Methods and compositions for deterring abuse of orally administered pharmaceutical products
This invention relates to an abuse deterrent formulation of an oral dosage form of a therapeutically effective amount of any active drug substance that can be subject to abuse combined with a gel forming polymer, a nasal mucosal irritating surfactant and a flushing agent. Such a dosage form is intended to deter abuse of the active drug substance via injection, nasal inhalation or consumption of quantities of the dosage unit exceeding the usual therapeutically effective dose.
Owner:ACURA PHARMA
Implantable Drug Delivery Device and Methods for Treatment of the Bladder and Other Body Vesicles or Lumens
ActiveUS20090149833A1High plasma concentrationMinimize irritationBiocideMedical devicesDrug reservoirControlled drugs
An implantable medical device is provided for controlled drug delivery within the bladder, or other body vesicle. The device may include at least one drug reservoir component comprising a drug; and a vesicle retention frame which comprises an elastic wire having a first end, an opposing second end, and an intermediate region therebetween, wherein the drug reservoir component is attached to the intermediate region of the vesicle retention frame. The retention frame prevents accidental voiding of the device from the bladder, and it preferably has a spring constant selected for the device to effectively stay in the bladder during urination while minimizing the irritation of the bladder.
Owner:MASSACHUSETTS INST OF TECH
Topically Bioavailable Acne and Rosacea Treatment Compositions
InactiveUS20040156873A1Reduce stimulationSynergistic superior anti-acneBiocideCosmetic preparationsAdditive ingredientIrritation
The present invention relates to acne and rosacea compositions by a six-prong synergistic combination treatment strategy that includes (1) control of excess sebum production, (2) control of undesirable bacteria or mites, (3) control of inflammation, (4) enhanced desquamation of follicular infundibulum cells, (5) reduction of irritation from anti-acne or rosacea compositions themselves, and (6) enhancement of the topical bioavailability of anti-acne and rosacea compositions. This is achieved by a synergistic combination of commonly utilized topical anti-acne and rosacea ingredients with a topical bioavailability enhancement composition, which results in enhanced anti-acne and rosacea action from such ingredients. Moreover, additional inclusion of an anti-inflammatory composition, and also a vascular micro-circulation enhancement composition, further results in synergistic superior anti-acne and rosacea benefits from such compositions. The present invention discloses additional surprising synergistic combinations for the control of acne and rosacea that are suitable for a variety of delivery systems and packaging forms.
Owner:GUPTA SHYAM K
Gentle-acting skin disinfectants
InactiveUS6846846B2Minimize skin irritationUnexpected antimicrobial effectivenessCosmetic preparationsBiocideOctoxyglycerinMedicine
Antimicrobial compositions having synergistic combinations of octoxyglycerin and at least one other antimicrobial agent in formulations which are more effective than prior art compositions without causing increased irritation to the skin of the average user. In certain embodiments, skin irritation may be minimized by low concentrations of antimicrobials and / or the presence of soothing compounds such as zinc. Preferred embodiments include combinations of octoxyglycerin, a quaternary compound, and at least one other antimicrobial agent. Without being bound to any particular theory, it is hypothesized that the unexpected antimicrobial effectiveness of combinations of octoxyglycerin may result from an enhancement of the permeability of microbes to antimicrobials caused by octoxyglycerin.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
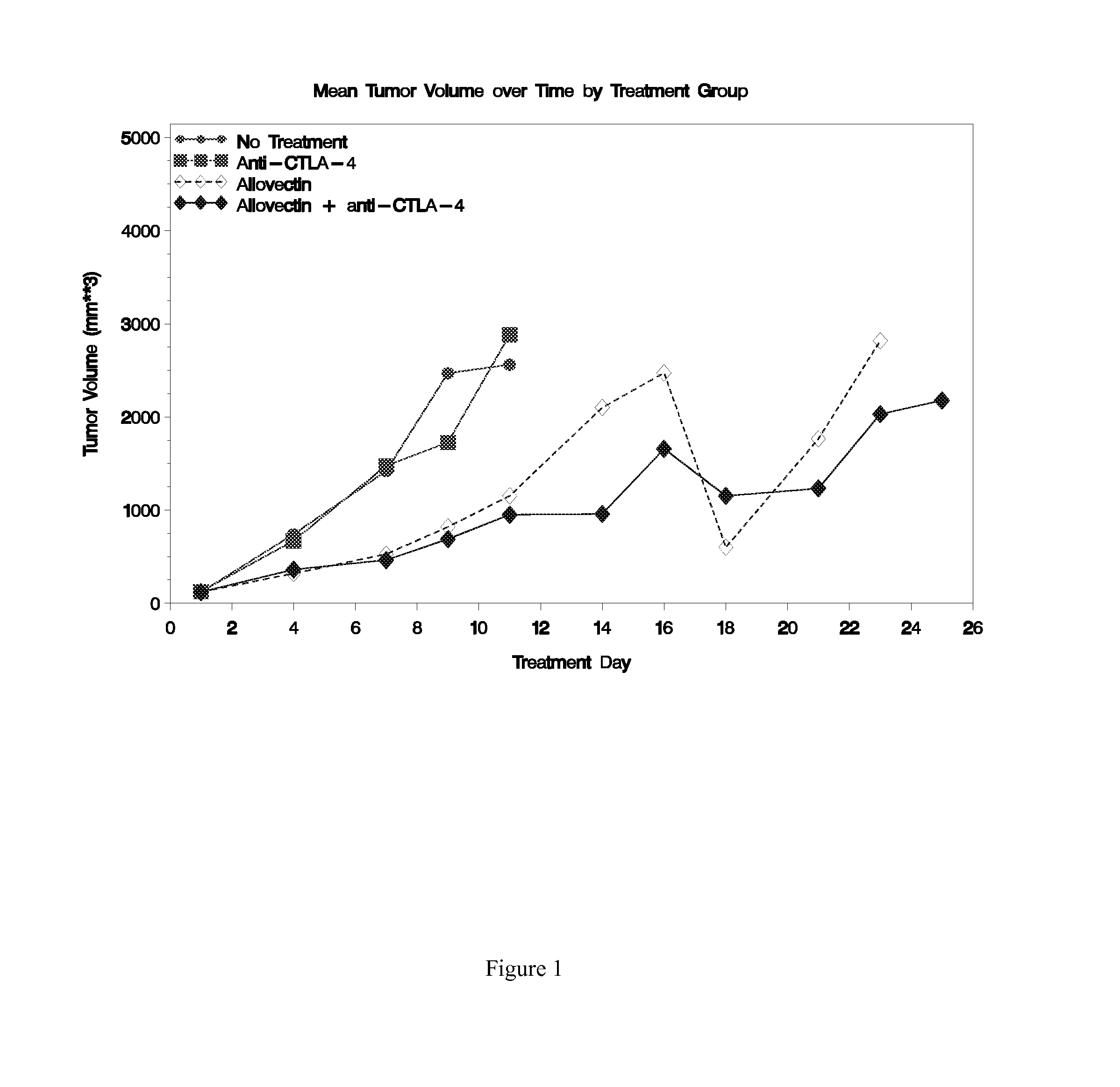
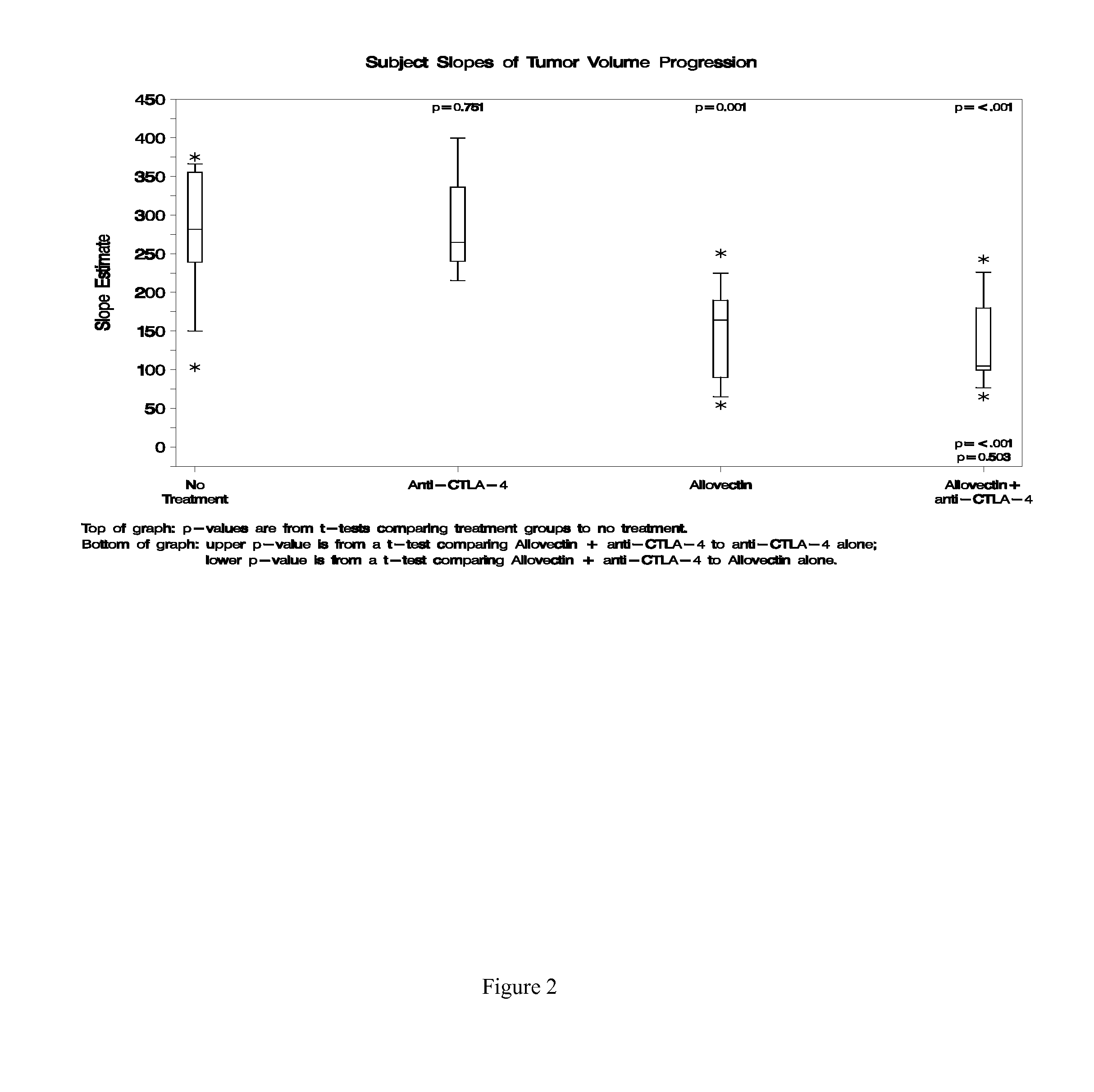
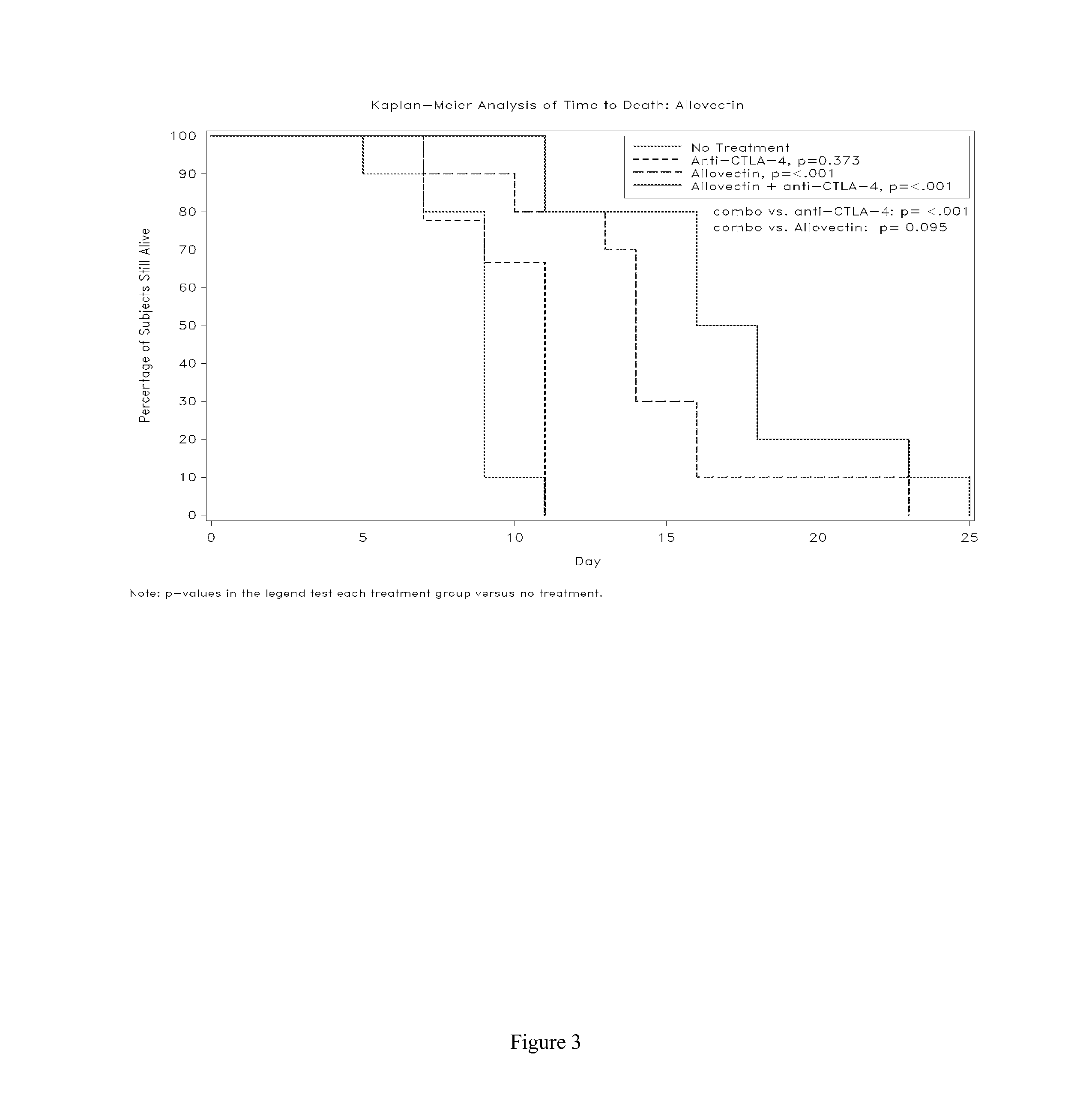
Synergistic Anti-tumor efficacy using alloantigen combination immunotherapy
InactiveUS20130071403A1Increased activationOrganic active ingredientsAntibody ingredientsImmunotherapeutic agentIrritation
The present disclosure provides combinations of immunotherapeutics and methods for treating medical conditions that are characterized by the lack of an effective immune response, for example as would result following a down-regulation of MHC class I, such as in cancer. The immunotherapeutic compositions of the invention, which can be used to treat the medical conditions, include one or more immunostimulatory antibodies or molecules having specificity for CTLA-4, PD-1, PD-L1, PD-L2, CD40, OX40, CD137, GITR, ILT2, or ILT3, or ligands for these molecules (e.g., an isolated fully-human monoclonal antibody) in association with one or more alloantigens, such as, vector(s) capable of expressing protein(s) or peptide(s) that stimulate T-cell immunity against tissues or cells, formulated in a pharmaceutically acceptable carrier. The proteins or peptides may comprise class I major histocompatibility complex (MHC) antigens, β2-microglobulins, or cytokines. The MHC antigen may be foreign to the subject. The MHC antigen may be HLA-B7.
Owner:VICAL INC
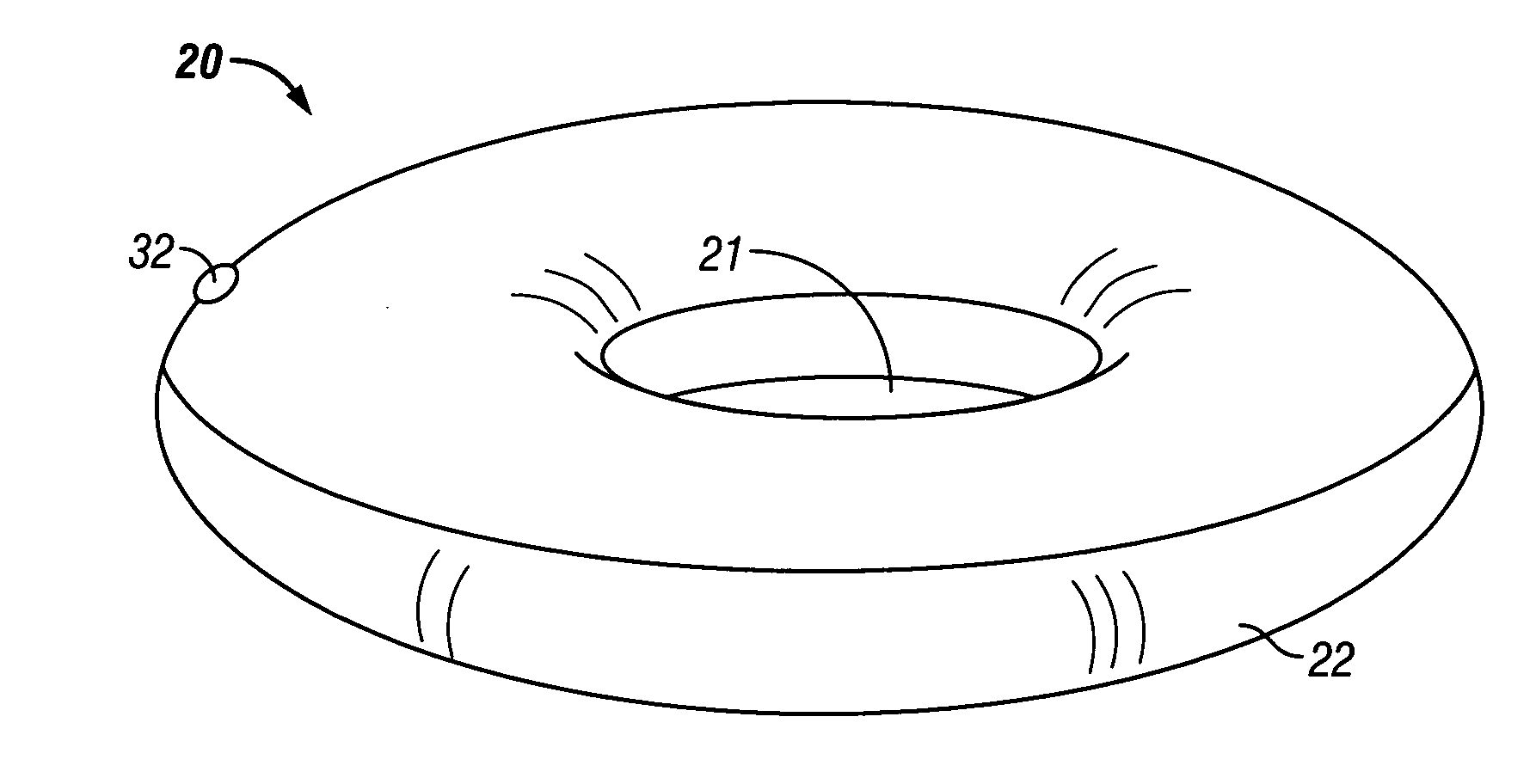
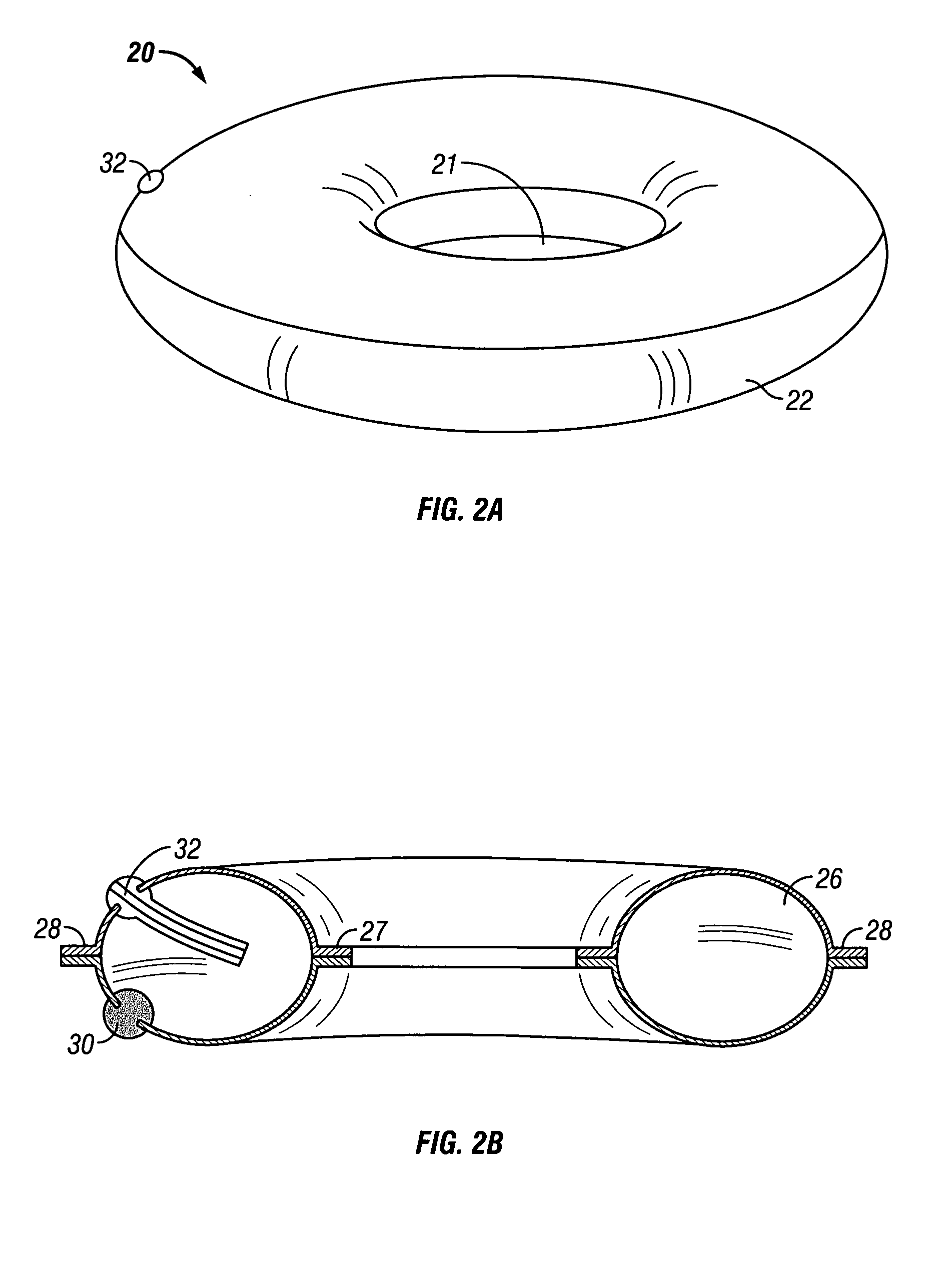
Overweight control apparatuses for insertion into the stomach
A gastric balloon apparatus. An apparatus is disclosed that is insertable into a patent's stomach for treatment of overweight. The balloon occupies a volume of the gastric lumen to provide a sensation of fullness after the consumption of only modest amounts of food. The balloon apparatus has a basic toroidal shape to prevent blockage of the entrance or exit lumens of the stomach and promote proper passage of food through the stomach, while protecting the stomach lining from ulceration and irritation. A series of toroidal balloons of graduated diameter may be joined by inner and outer sleeves to define a funnel-shaped apparatus which expands when food is ingested, thus satiating the patient with substantially reduced quantity of food. A balloon storage and insertion apparatus also is disclosed, whereby a gastric balloon according to the disclosure may be pre-inflated and stored ion a tube for later use, whereupon the pre-inflated balloon is deployed into the stomach. Various mechanisms are disclosed for providing a pre-determined deflation of an inserted balloon, permitting the deflated balloon to be excreted from the body.
Owner:HULL WENDELL C SR +1
Tape formulation for percutaneous administration containing fentanyi
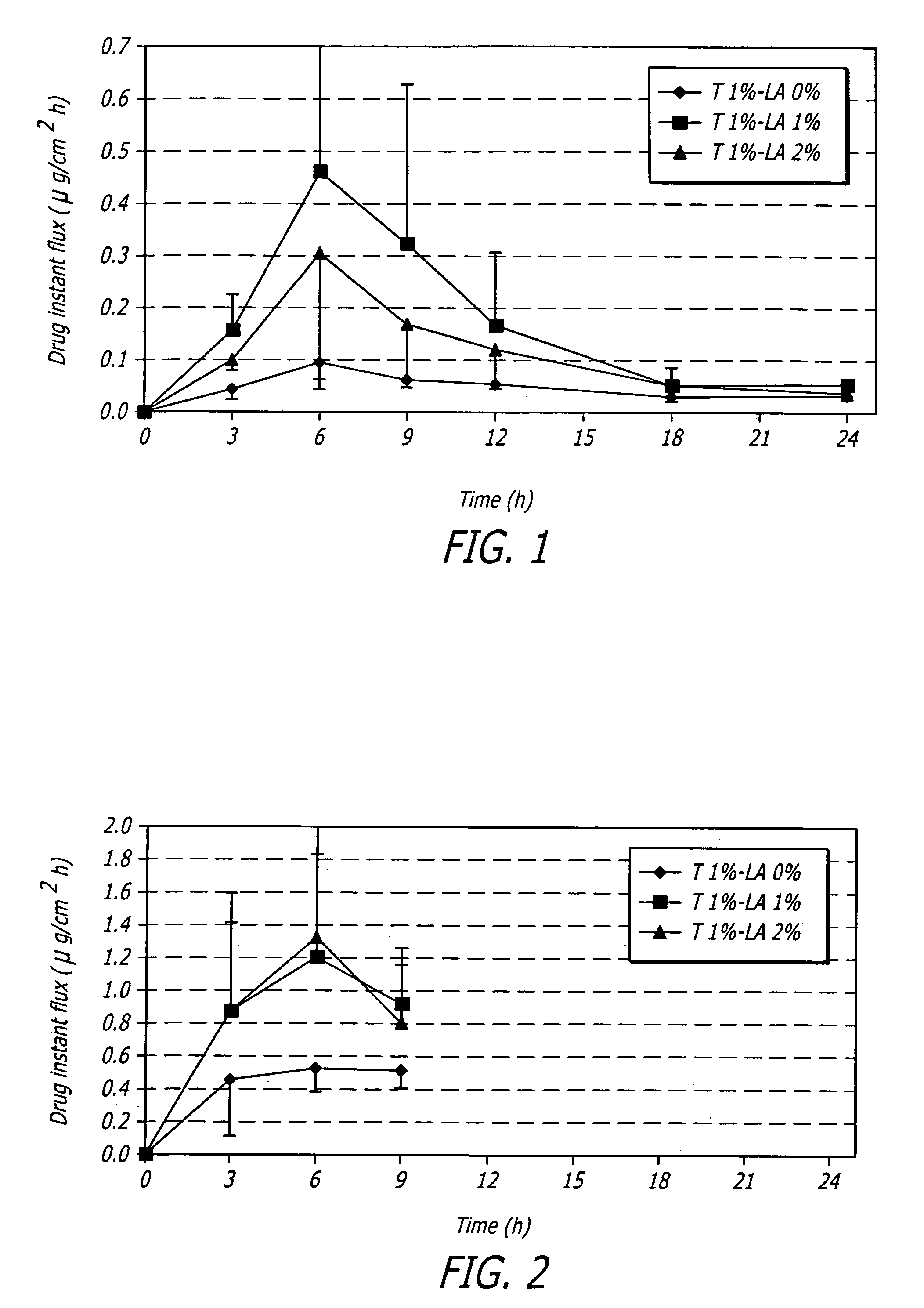
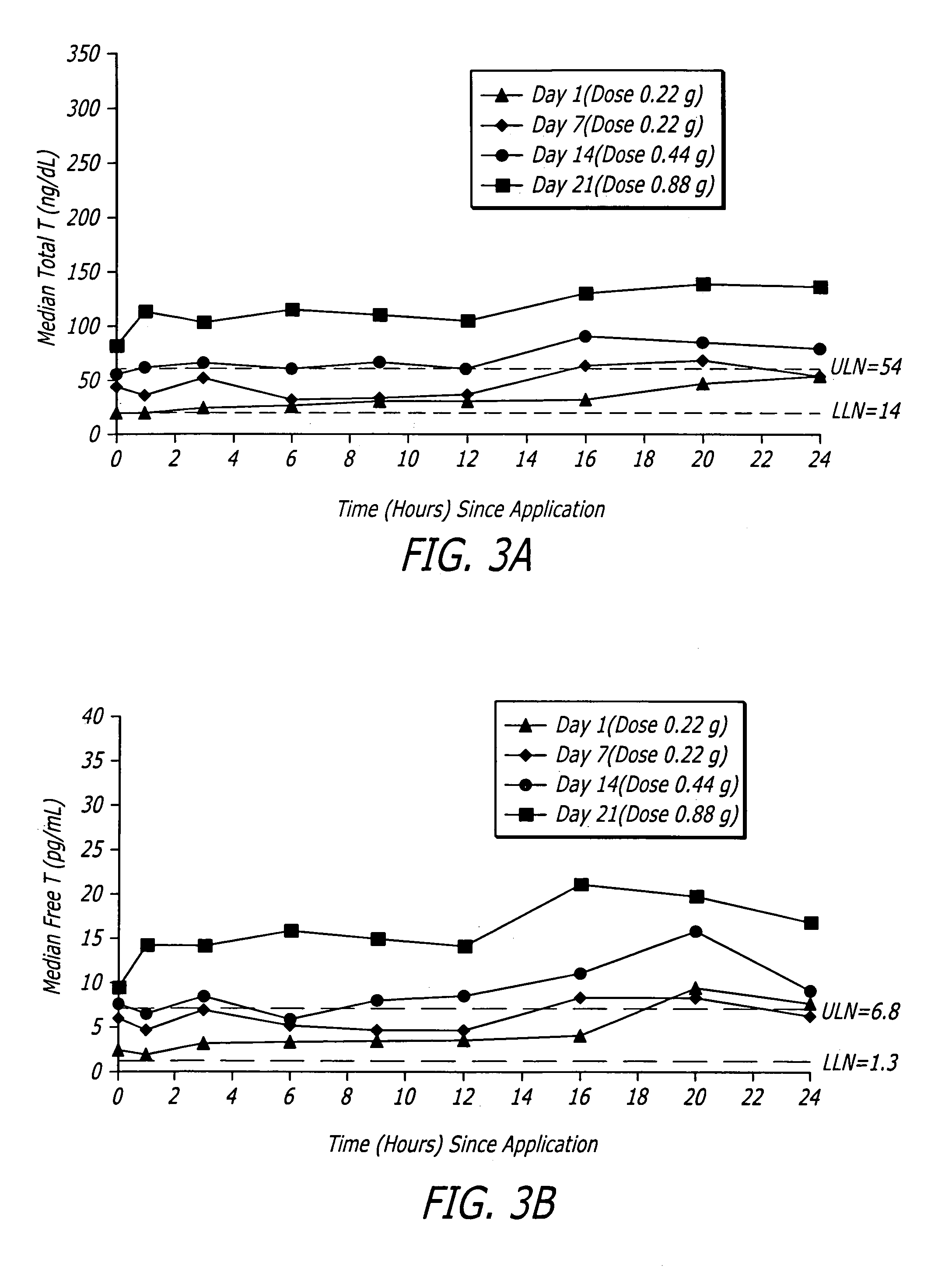
PCT No. PCT / JP97 / 01595 Sec. 371 Date Jan. 13, 1998 Sec. 102(e) Date Jan. 13, 1998 PCT Filed May 13, 1997 PCT Pub. No. WO97 / 42952 PCT Pub. Date Nov. 20, 1997A tape formulation for percutaneous administration containing fentanyl which comprises fentanyl or a salt thereof, a pressure sensitive adhesive and sodium acetate, is disclosed. The salt of fentanyl is preferably fentanyl citrate. The tape formulation of the present invention is little irritation to the skin and excellent in the percutaneous permeation of fentanyl and has a high stability even after the passage of time.
Owner:HISAMITSU PHARM CO INC
Morinda citrifolia-based oral care compositions and methods
InactiveUS20050084551A1Reduce penetrationReduce enzyme activityCosmetic preparationsBiocideDiseaseIrritation
Owner:TAHITIAN NONI INT INC
Implantable Stimulation Electrode with a Coating for Increasing Tissue Compatibility
InactiveUS20080234790A1Avoid tissue irritationGood biocompatibilitySurgeryInternal electrodesImplantable Stimulation ElectrodesIrritation
An implantable stimulation electrode for use with an implantable tissue stimulator, especially a pacemaker, a defibrillator, a bone stimulator or a neurostimulator includes a metal base body, optionally one or more intermediate layers disposed on the base body and a coating covering the base body and, optionally, intermediate layers in order to increase tissue compatibility. The coating should prevent tissue irritations after implantation and more particularly increase the stimulus threshold associated therewith, have very high biocompatibility and also has an anti-inflammatory effect. An increase in tissue compatibility is achieved by virtue of the fact that the coating has a polysaccharide layer made of hyaluronic acid and / or hyaluronic acid derivatives.
Owner:BIOTRONIK MESS UND THERAPIEGERAETE GMBH & CO
Therapeutic/cosmetic compositions comprising CGRP antagonists for treating sensitive human skin
Topically applicable pharmaceutical / dermatological / cosmetic compositions well suited for the therapeutic treatment or care of sensitive human skin, hair, mucous membranes, nails and / or the scalp, in particular for reducing or avoiding the skin-irritant side effects of a variety of bioactive agents, for example the alpha -hydroxy acids, comprise a therapeutically / cosmetically effective amount of at least one calcitonin gene related peptide ("CGRP") antagonist, e.g., CGRP 8-37 or an anti-CGRP antibody.
Owner:LOREAL SA
Topical dermal anaesthetic
A liquid composition applied transdermally for relief of pain comprising alcohol in an amount by weight of about 57 to about 91 percent; glycerin in an amount by weight of about 1 to about 12 percent; an analgesic agent in an amount by weight of about 2 to about 28 percent, the analgesic agent comprising a derivative of salicylic acid; methylsulfonylmethane in an amount by weight of about 0.02 to 5 percent; and emu oil in an amount by weight of about 0.01 to 3 percent, the liquid composition permeating skin to relieve pain. The composition further comprising, as an additional feature, aloe vera in an amount by weight of at least about 0.05 percent and having an amount by weight of about 0.05 to 4 percent. The composition features transdermal pain relief such that a patient can apply the analgesic agent directly to an area of pain without such side effects as stomach irritation which is normally associated with aspirin. The composition may be sprayed or rolled directly onto the painful area. Because of the unique formula, the composition is safe to vital internal organs, requires no mixing before use, and is shelf stable for marketing purposes.
Owner:VELTRAN LP
Formulations for transdermal or transmucosal application
InactiveUS7198801B2Improve permeabilityOrganic active ingredientsBiocideLong chain fatty acidIrritation
The present invention relates generally to formulations for transdermal or transmucosal administration of an active agent. The invention is a substantially malodorous-free and irritation free transdermal formulation which is substantially free of long chain fatty alcohols, long-chain fatty acids, and long-chain fatty esters.
Owner:ANTARES PHARMA IPL
Zinc salt compositions for the prevention of dermal and mucosal irritation
InactiveUS20040102429A1Minimize and prevent irritationReduce transmissionAntibacterial agentsOrganic active ingredientsHigh concentrationFungicide
The addition of low concentrations of combinations of water-soluble organic salts of zinc to gels, creams, lotions or ointments can increase the ability of these products to reduce or prevent exogenous irritants from causing irritation of the underlying substrate. The addition of low concentrations of combinations of water-soluble organic zinc salts to these gels, creams, lotions or ointments also can reduce the irritation of skin or mucous membranes caused by the addition of potentially-irritating substances such as spermicides, microbicides, fungicides or other therapeutic agents to the gel, cream, lotion or ointment. The advantages of this anti-irritant approach over others, which generally employ high concentrations of single zinc salts, are the reduced potential for zinc toxicity, the reduced potential for toxicity related to zinc itself, and the preservation of the desirable biological properties of potentially-irritating therapeutic substances added to the gel, cream, lotion or ointment.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Methods and compositions for deterring abuse of orally administered pharmaceutical products
This invention relates to an abuse deterrent formulation of an oral dosage form of a therapeutically effective amount of any active drug substance that can be subject to abuse combined with a gel forming polymer, a nasal mucosal irritating surfactant and a flushing agent. Such a dosage form is intended to deter abuse of the active drug substance via injection, nasal inhalation or consumption of quantities of the dosage unit exceeding the usual therapeutically effective dose.
Owner:ACURA PHARMA
Nasal cannula retainer
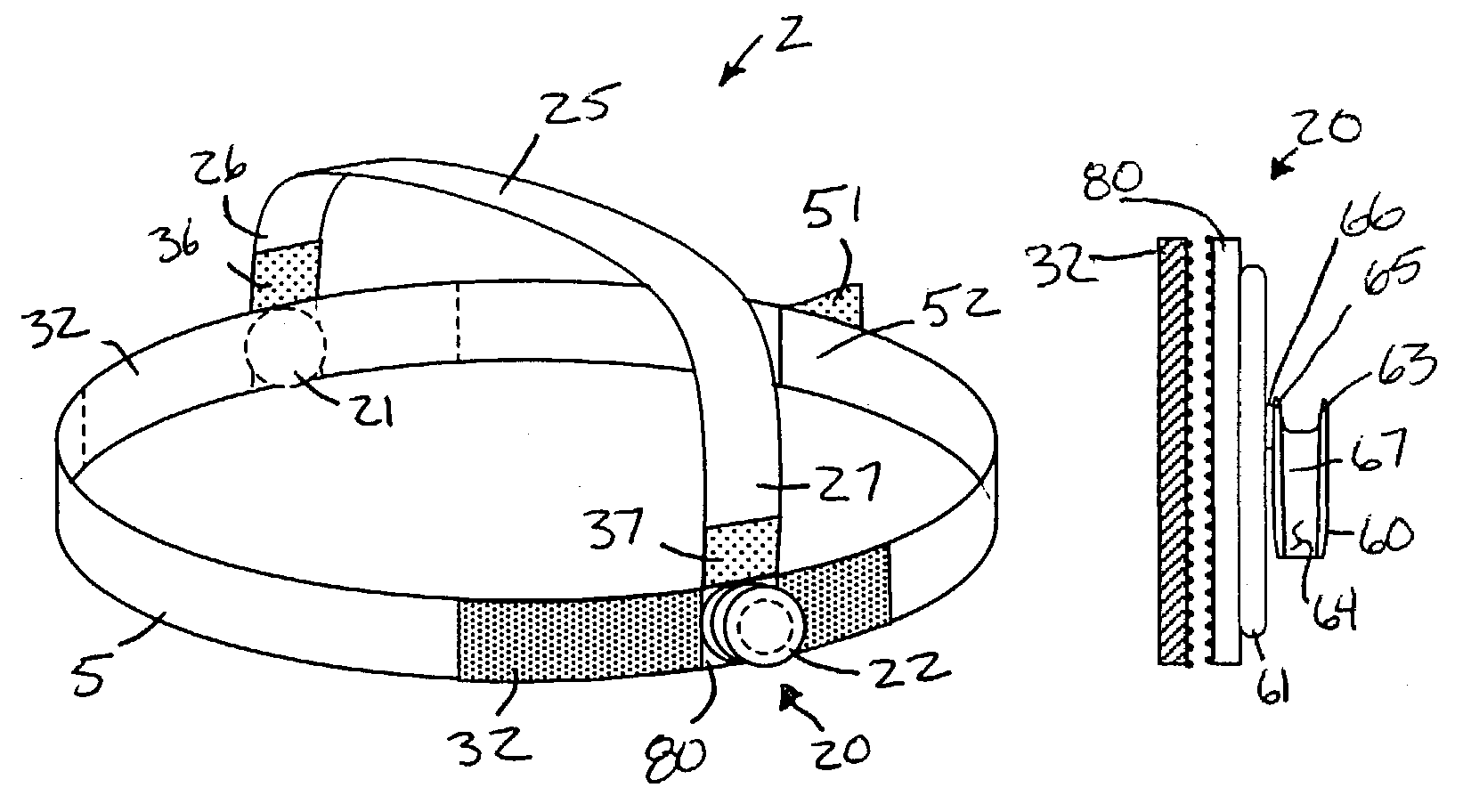
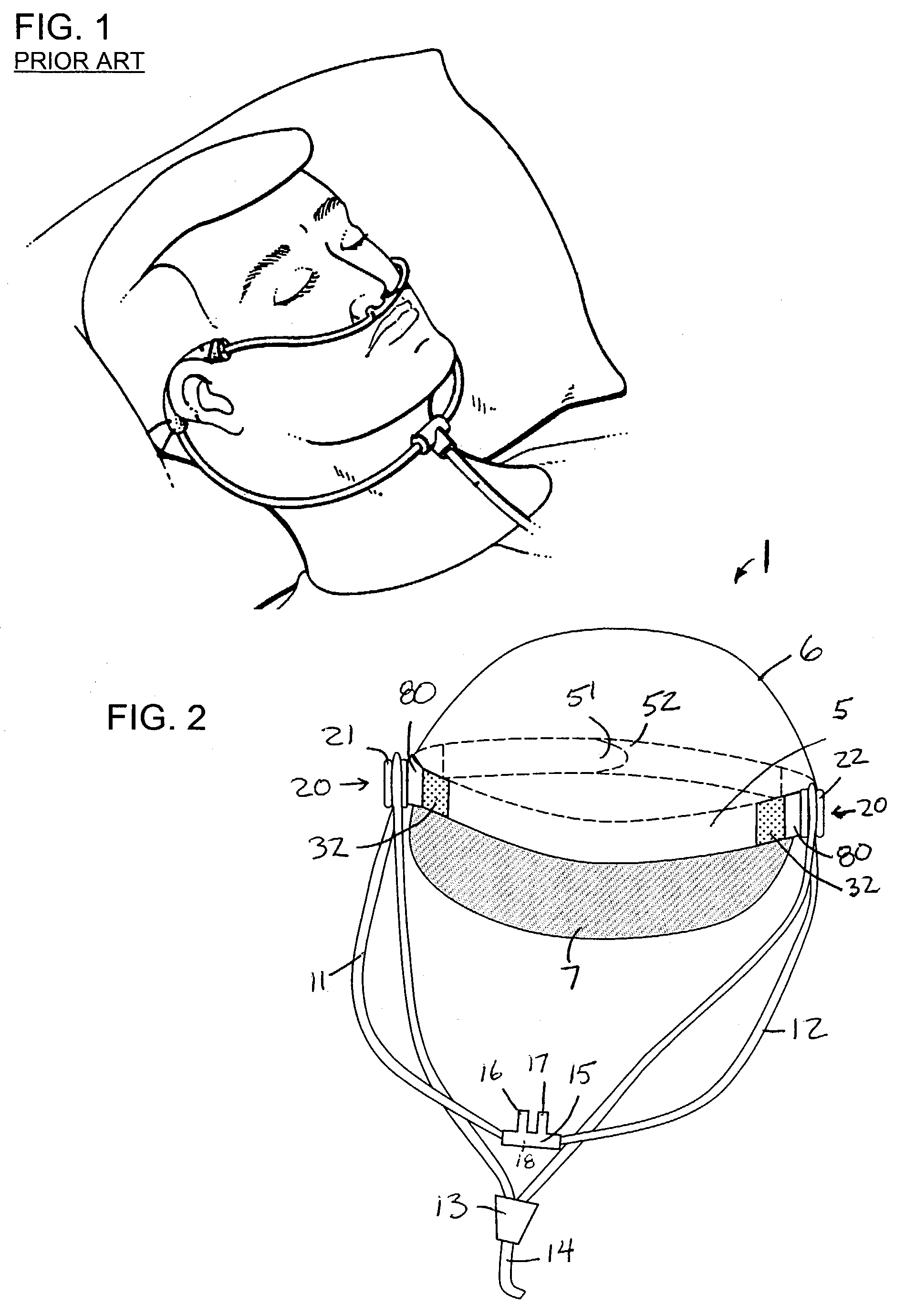
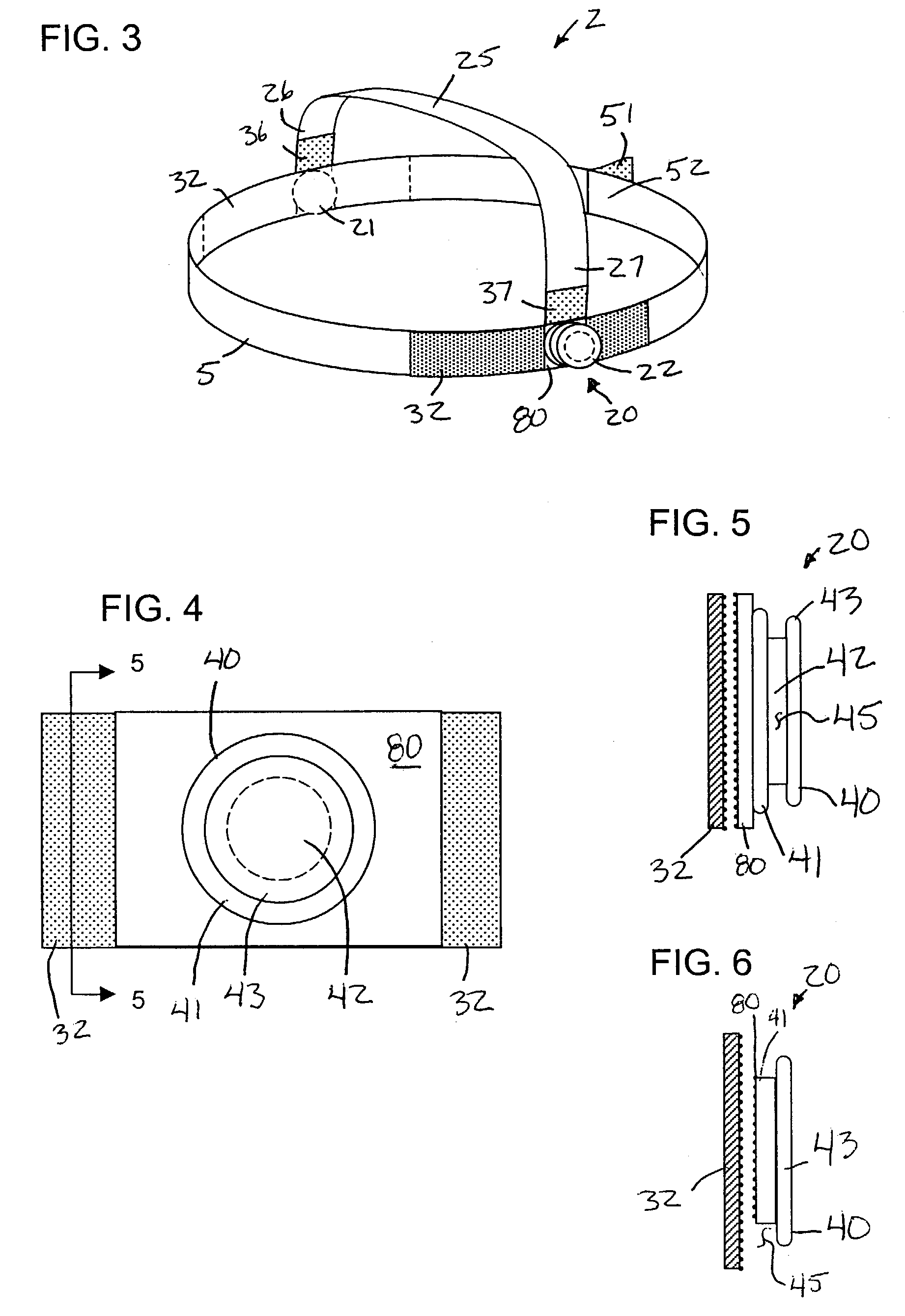
InactiveUS7146976B2Avoid interferenceEliminate irritationRespiratory masksBreathing masksIrritationNose
A nasal cannula apparatus is provided for use by patients desiring a comfortable arrangement. The nasal cannula apparatus is particularly suited for long-term oxygen users, for extended wear in both standing, resting and supine positions. The nasal cannula apparatus has headgear and retainers for holding the gas supply tubes, which are adjustable to allow fast and easy adjustment to size. The headgear and retainers allow the cannula tubes to be held in a position which prevents skin discomfort and irritation, and which promotes healing of such irritation, injuries and sores.
Owner:MCKOWN JOSEPH R
Foam and gel oat protein complex and method of use
InactiveUS6514487B1Smoothness and eleganceNormal skinCosmetic preparationsToilet preparationsAdditive ingredientIrritation
A composition containing enhanced colloidal oatmeal which utilizes other avena sativa ingredients to neutralize the discomfort, irritation and inflammation of the skin, as well as maintaining normal skin, and can be used to treat many types of discomforts, including itching; due to poison ivy, oak and sumac, insect bites, sunburn, chicken pox, hives, prickly heat, chafing, and the like while maintaining the normal pH of the skin.
Owner:BARR TERESA LEIGH
Bandage, methods of producing and using same
ActiveUS6967261B1Improve health environmentPromote rapid healingAbsorbent padsCoatingsAcute woundIrritation
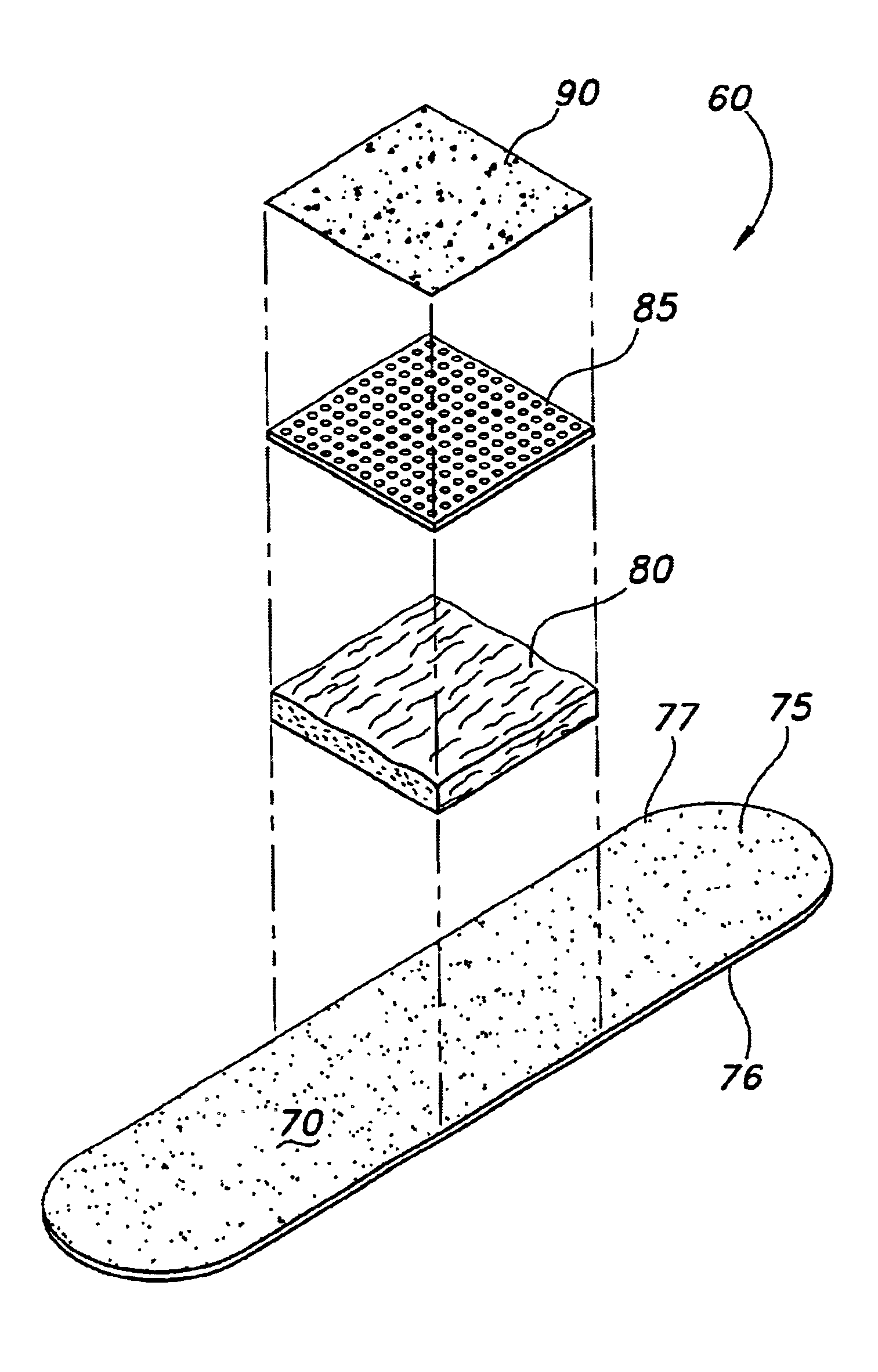
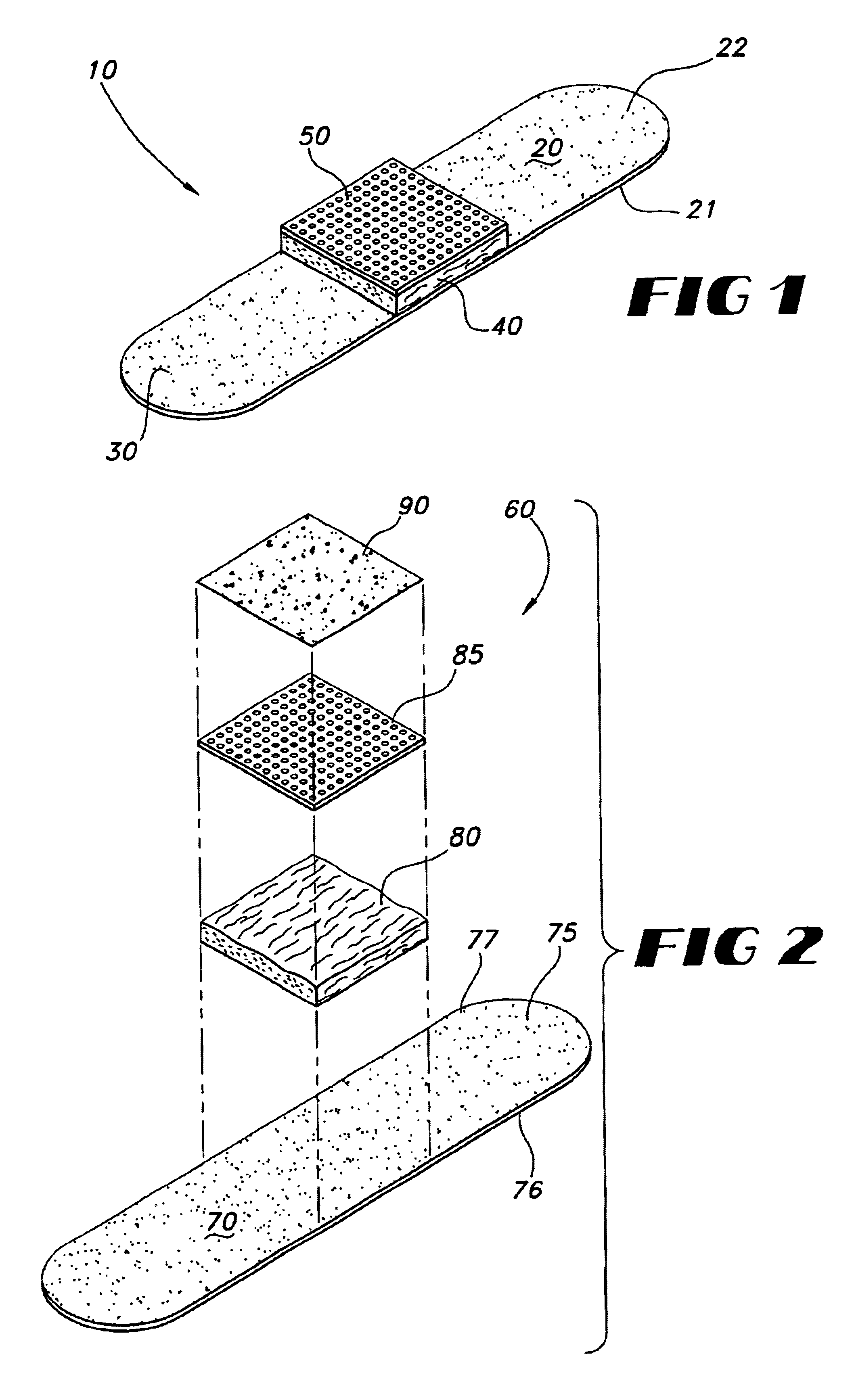
A bandage of the type used on acute wounds, minor wounds, burn wounds and irritations, includes a first layer for covering the wound site and an area around the wound site, with the first layer including a top surface and bottom surface; a second layer over the first layer bottom surface, for absorbing exudates from the wound site; the second layer including a poly(ethyleneoxide)-based compound and a chitosan-based compound. A third layer is situated over the second layer, the third layer being of a perforated film, and wherein, at least one antimicrobial agent is associated with the bandage in a position where the antimicrobial agent will come in contact with the wound site, and which is transferable from the bandage to the wound site, upon contact with the wound site.
Owner:O&M HALYARD INC
Preparation method of multifunctional beauty concentrated liquor
ActiveCN105997815AEnhance the inherent beauty functionEnhance beauty functionCosmetic preparationsToilet preparationsFruit juiceIrritation
The invention relates to the technical field of cosmetics, and particularly discloses a preparation method of multifunctional beauty concentrated liquor. Transparent, clear and golden yellow beauty concentrated liquor is obtained through the technological steps of fermentation of fructus hippophae juice and traditional Chinese medicine concentrated liquid raw materials, sedimentation of fermentation liquor, after-sedimentation extraction of liquor and the like. The beauty concentrated liquor can be applied in skincare products, has functions of being free of irritation, resisting allergy, whitening skin and fading freckles, resisting ageing and the like, and is stable in product property and outstanding in effect.
Owner:卓雍皓
Penetration Enhancer Combinations for Transdermal Delivery
InactiveUS20070269379A1Easy to transportLess irritatingOrganic active ingredientsBiocideHigh-Throughput Screening MethodsIrritation
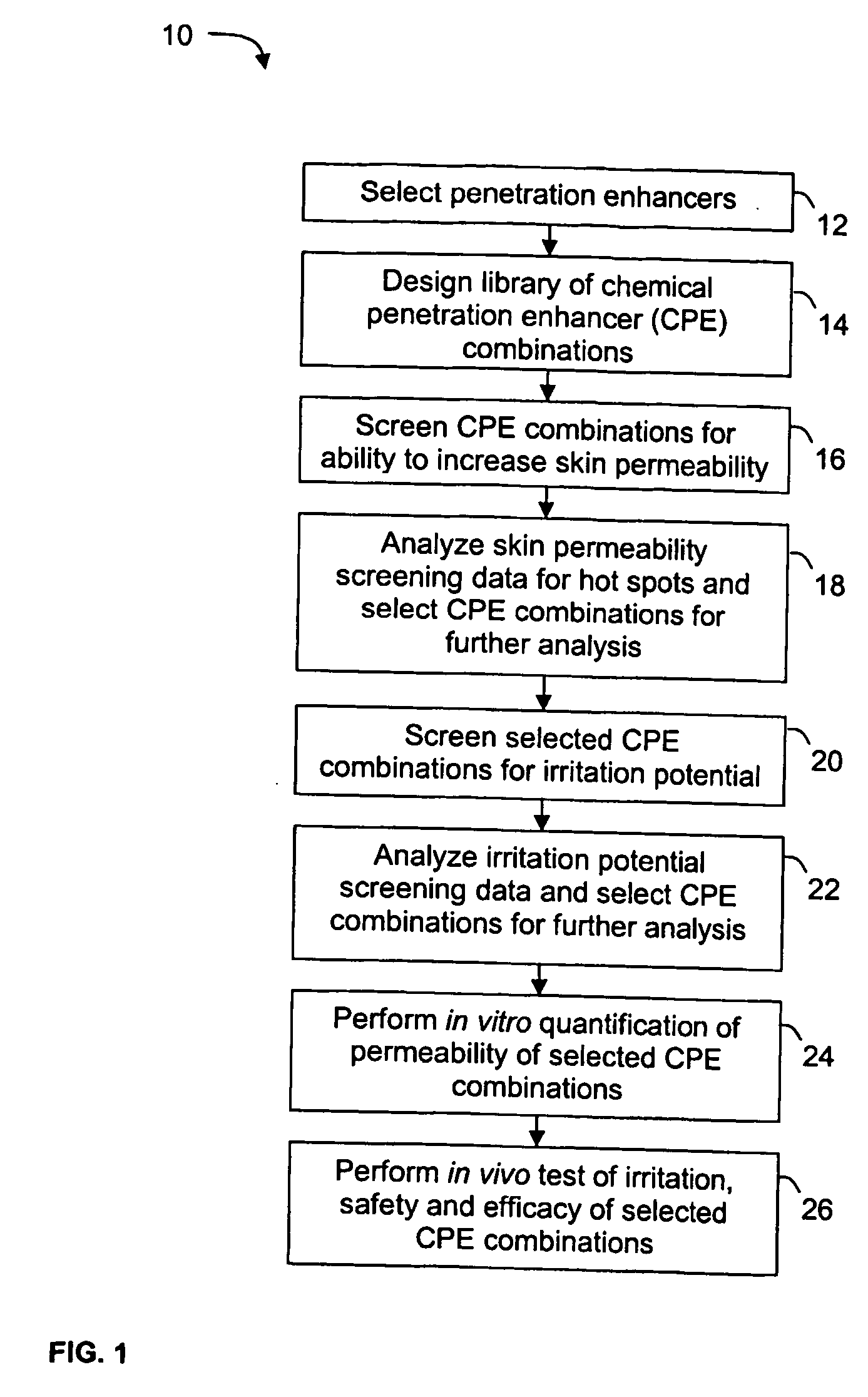
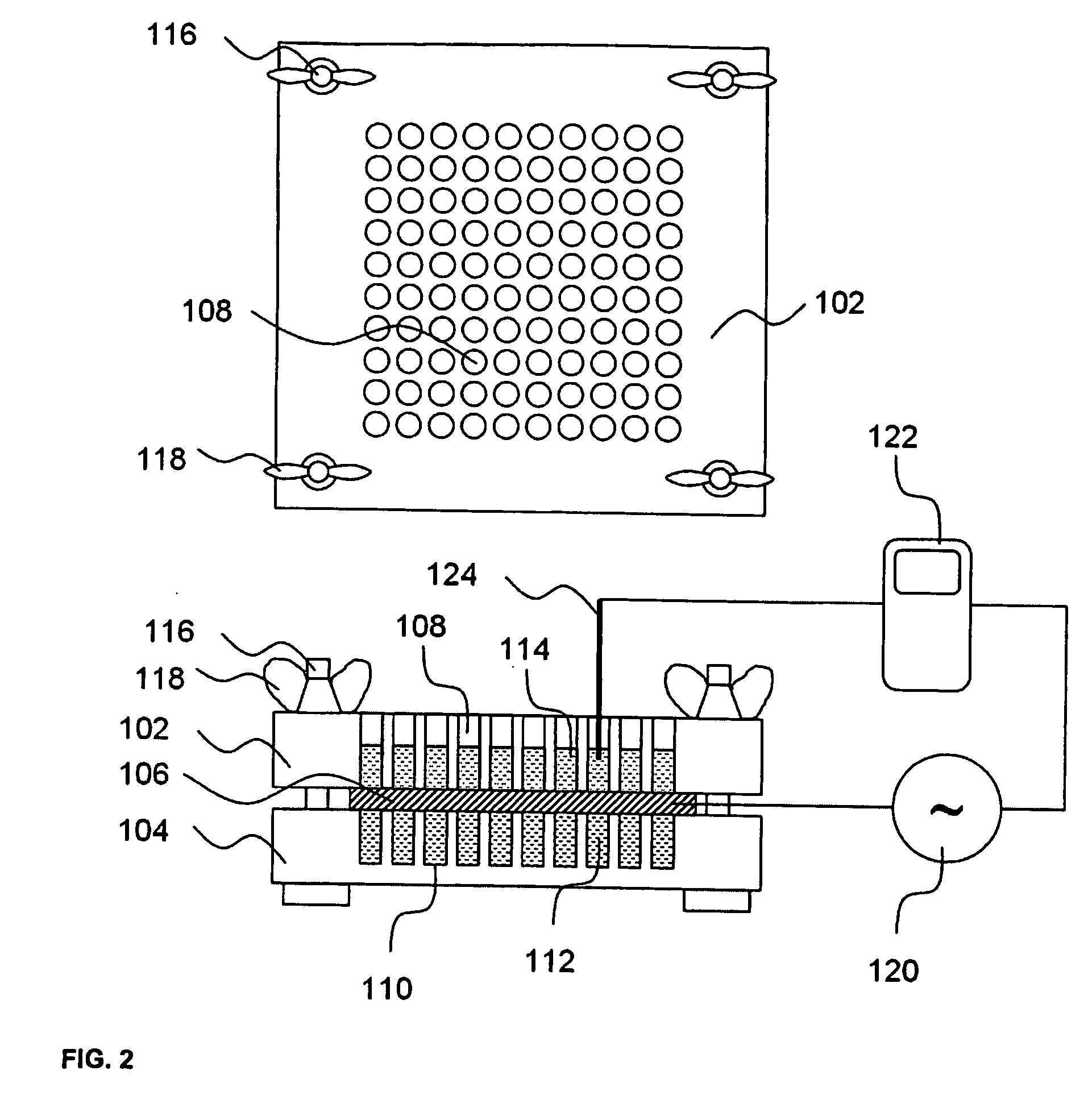
A high throughput screening and isolation system identifies rare enhancer mixtures from a candidate pool of penetration enhancer combinations. The combinations are screened for high penetration but low irritation potential using a unique data mining method to find new potent and safe chemical penetration enhancer combinations. The members of a library of chemical penetration enhancer combinations are screened with a high throughput device to identify “hot spots”, particular combinations that show higher chemical penetration enhancement compared to neighboring compositions. The irritation potentials of the hot spot combinations are measured to identify combinations that also show low irritation potential. A active component, such as a drug, is then combined with the combination in a formulation which is tested for the ability of the drug to penetrate into or through skin. It is then assessed whether the formulation can deliver the quantity of drug required, and animal tests are conducted to confirm in vivo the ability of the chemical penetration enhancer combinations to facilitate transport of sufficient active molecules across the skin to achieve therapeutic levels of the active molecule in the animal's blood. The invention provides specific unique and rare mixtures of chemical penetration enhancers that enhance skin permeability to hydrophilic macromolecules by more than 50-fold without inducing skin irritation, such as combinations of sodium laurel ether sulfate and 1-phenyl piperazine, and combinations of N-lauryl sarcosine and Span 20 / sorbitan monolaurate.
Owner:RGT UNIV OF CALIFORNIA
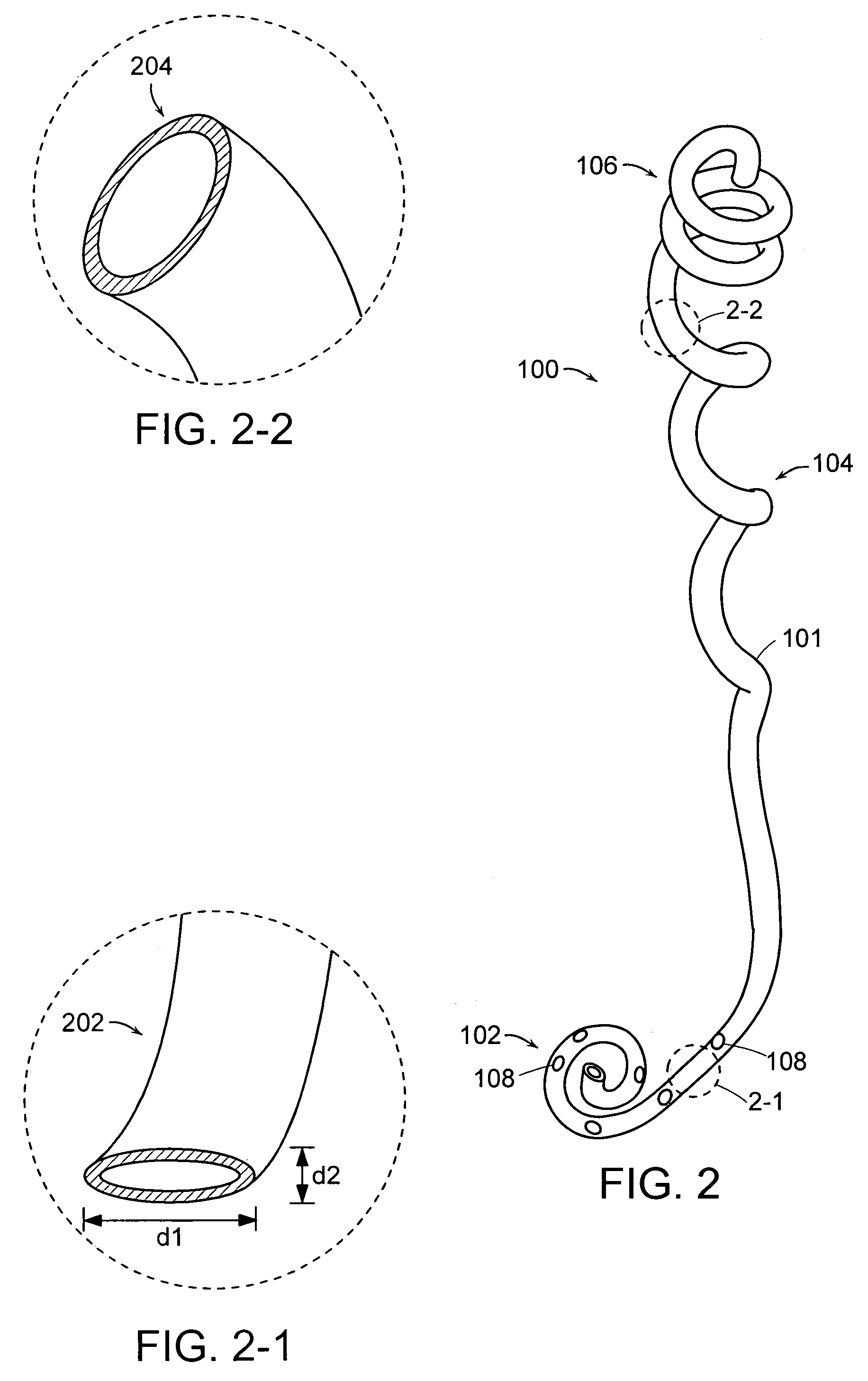
Ureteral stent configured for improved patient comfort and aftercare
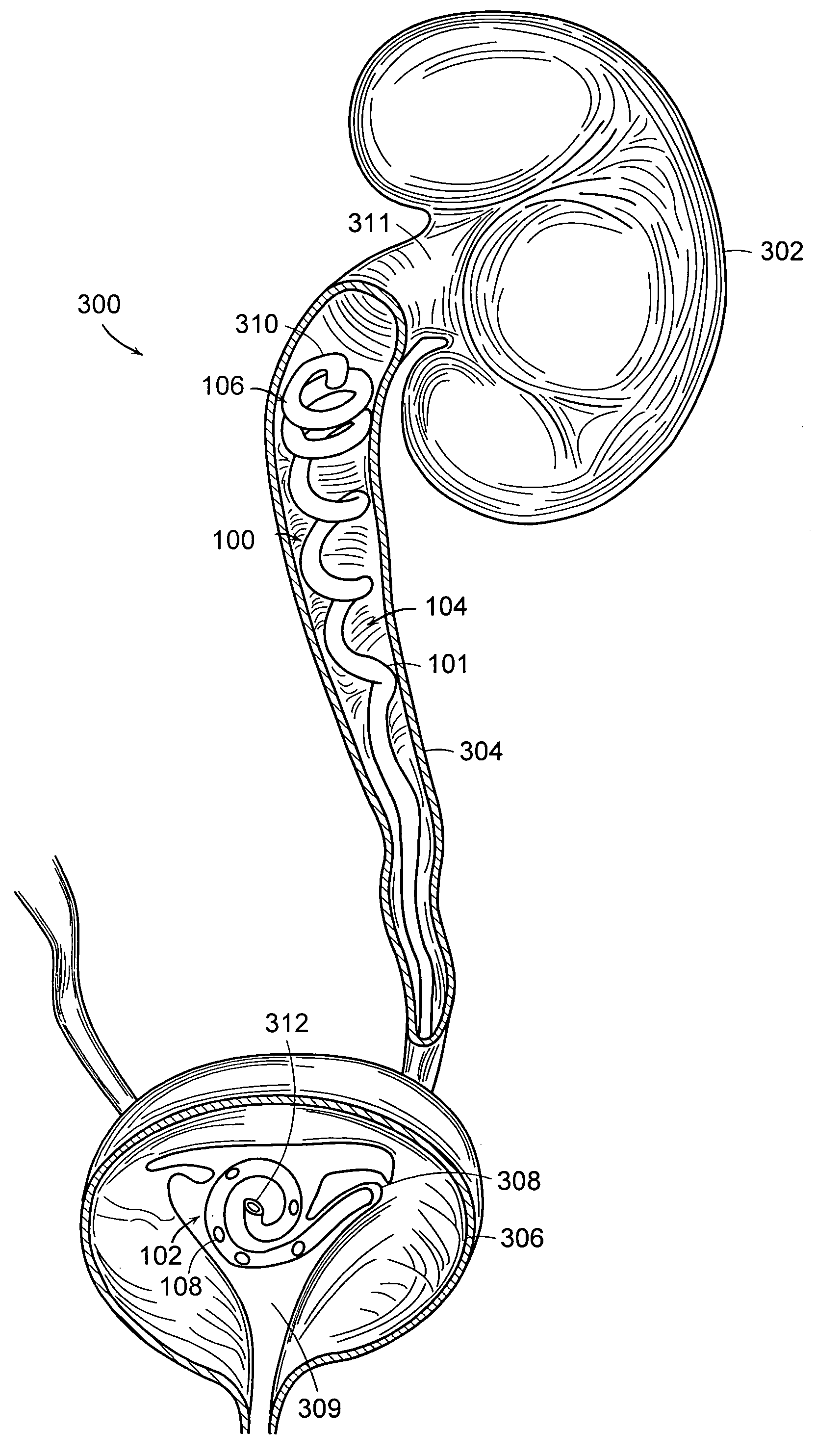
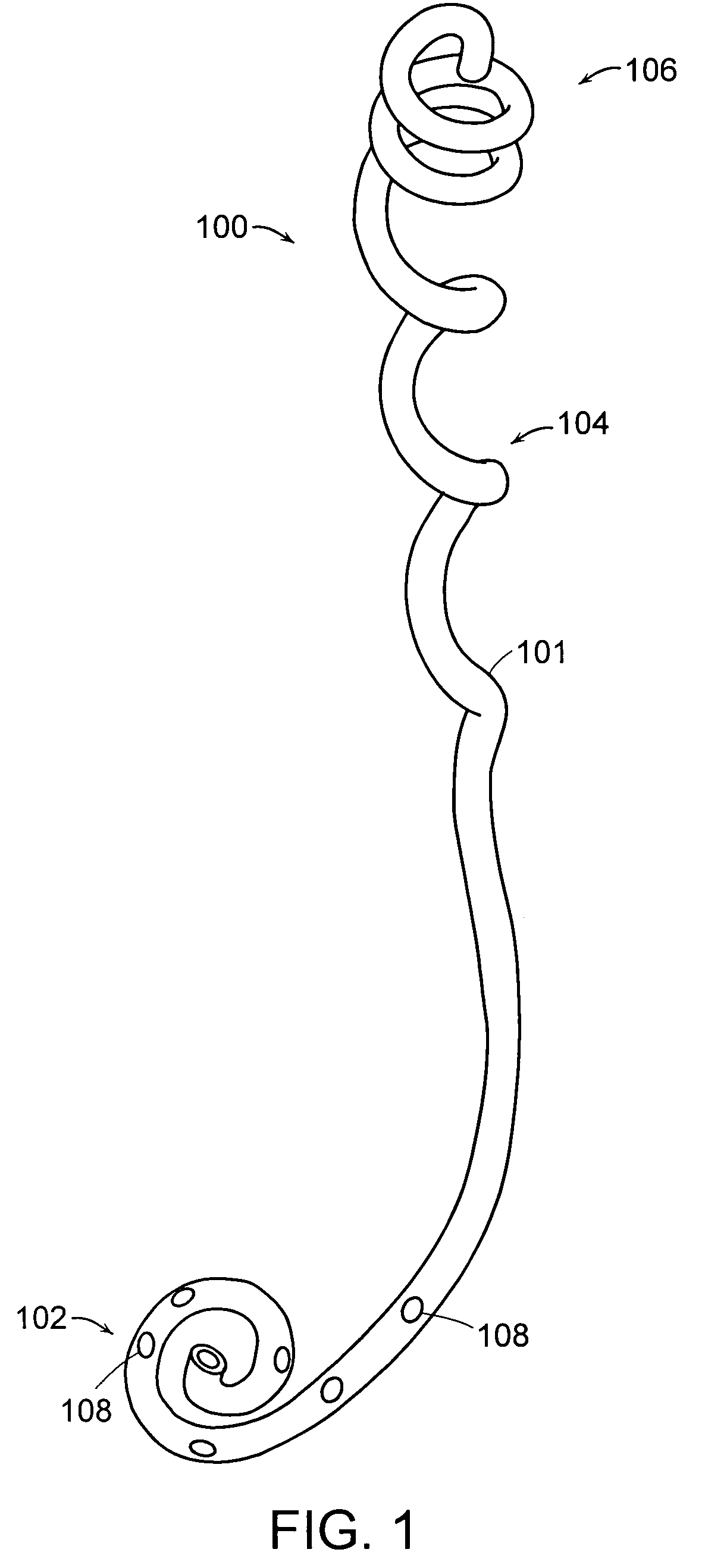
A ureteral stent is configured for improved patient comfort and aftercare. The stent can have one or more of the following features: a distal portion with a somewhat flattened, non-circular cross-section that provides reduced irritation and elimination of urine reflux; a proximal portion with a helical coil shape that allows self-anchoring of the stent below the kidney; and a portion along the body of the stent having a coil shape that allows self-adjustment of the stent with ureteral movement.
Owner:BOSTON SCI SCIMED INC
Zinc salt compositions for the prevention of dermal and mucosal irritation
InactiveUS20050238602A1Minimize and prevent irritationReduce transmissionCosmetic preparationsBiocideMedicineIrritation
The present invention provides for compositions and methods that may offer protection from irritants, as well as antimicrobial protection. Preferred embodiments of the invention include topical antimicrobial compositions comprising two or more water-soluble zinc salts in low concentrations.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
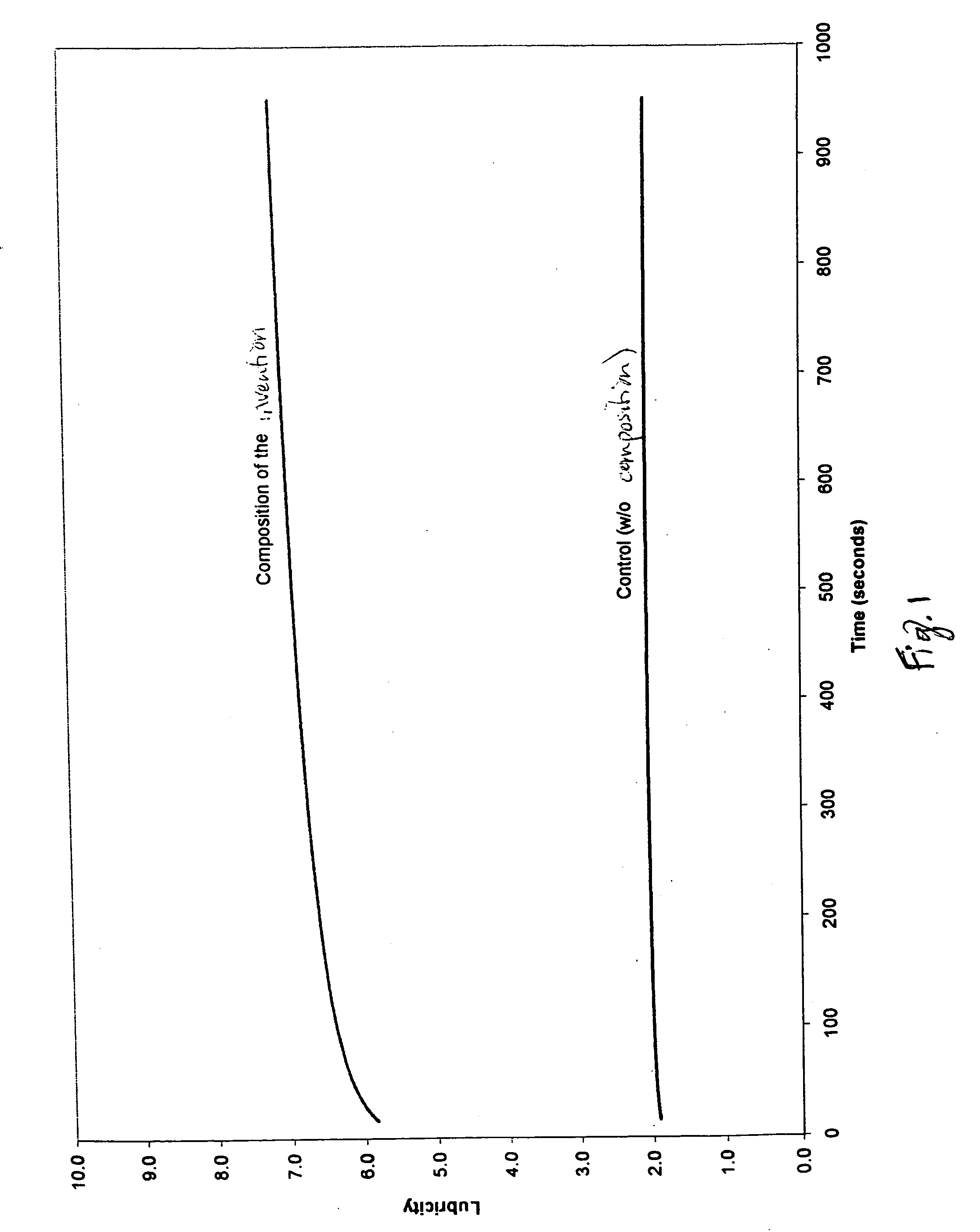
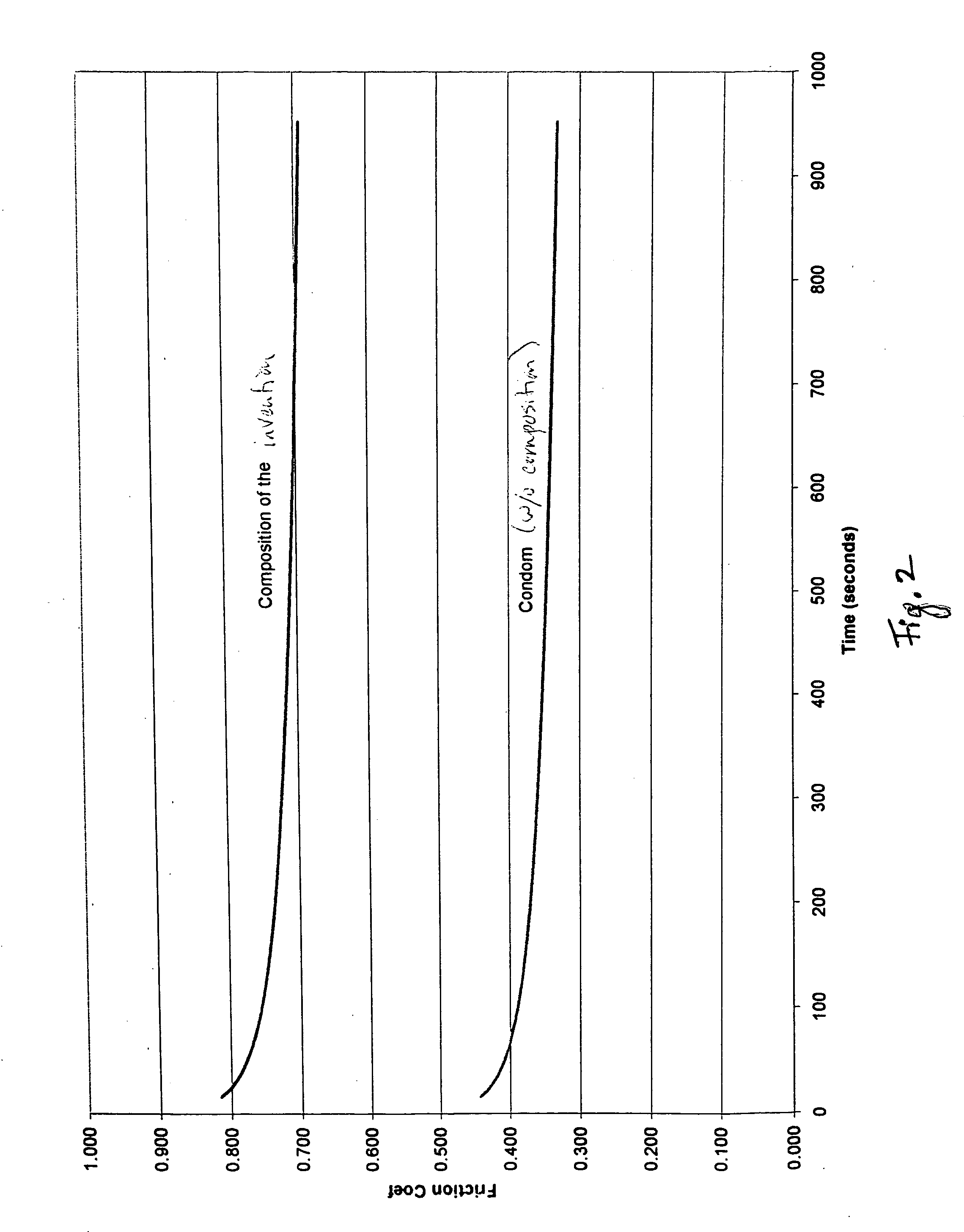
Method of providing lubricious surfaces
This invention relates to a method of providing lubricious surfaces, more particularly to a method of providing lubricious characteristics to skin surfaces that come into contact with other surfaces so as to prevent or treat chafing and / or irritation.
Owner:MILLER JONATHAN +2
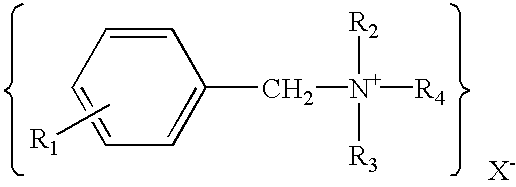
Transdermal drug delivery systems containing quaternary ammonium salts and methods of using the same
InactiveUS20030091620A1Improve permeabilityReduce skin irritationAntibacterial agentsNervous disorderIrritationReducer
A transdermal drug delivery system is disclosed, which includes a polymer, a drug and an amount of a quaternary ammonium salt that is sufficient to act as a penetration enhancer. The quaternary ammonium salt may also be present in an amount sufficient to act as an irritation reducer. Further, the transdermal drug delivery system may also contain a co-enhancer, which provides a synergistic skin permeation enhancing effect when combined with the quaternary ammonium salt. A method for enhancing the transdermal delivery of a drug is also disclosed.
Owner:FIKSTAD DAVID +3
Cosmetic or dermopharmaceutical composition comprising an enzyme which is insoluble in an aqueous medium, as well as its uses
InactiveUS20040120917A1Lowering melanic indexEffective formulationCosmetic preparationsHair cosmeticsIrritationAqueous medium
The invention relates to a cosmetic or dermopharmaceutical composition, as well as these uses. The invention relates mainly to a cosmetic or dermopharmaceutical composition, notably an anti-wrinkle cosmetic or dermopharmaceutical composition, comprising an enzyme which is insoluble in an aqueous medium, in admixture with at least one cosmetically or dermopharmaceutically acceptable excipient. This composition is mainly used for limiting reactions of irritation and / or of allergy during topical use of this composition.
Owner:BASF BEAUTY CARE SOLUTIONS FRANCE SAS
Transdermal and topical administration of drugs using basic permeation enhancers
InactiveUS20050074487A1Improve throughputEffective amountCosmetic preparationsBiocideActive agentIrritation
Methods are provided for enhancing the permeability of skin or mucosal tissue to topical or transdermal application of pharmacologically or cosmeceutically active agents. The methods entail the use of a base in order to increase the flux of the active agent through a body surface while minimizing the likelihood of skin damage, irritation or sensitization. The permeation enhancer can be an inorganic or organic base. Compositions and transdermal systems are also described.
Owner:DERMATRENDS INC
Aripiprazole complex formulation and method
An aripiprazole formulation is provided which includes the antipsychotic agent aripiprazole in the form of an inclusion complex in a β-cyclodextrin, preferably, sulfobutyl ether β-cyclodextrin (SBECD), which in the form of an injectable produces reversible generally minimal to mild irritation at the intramuscular injection site. A method for minimizing or reducing irritation caused by aripiprazole at an intramuscular injection site and a method for treating schizophrenia employing the above formulation are also provided.
Owner:OTSUKA PHARM CO LTD
Warming and nonirritating lubricant compositions and method of comparing irritation
InactiveUS7005408B2Capacitive effectIncrease temperatureAntibacterial agentsOrganic active ingredientsPolyolAlcohol
This invention relates to substantially anhydrous warming, non-toxic and nonirritating lubricating compositions containing polyhydric alcohols and an insulating agent. The invention also relates to methods of using such compositions for lubrication, administration of active ingredients and for preventing or treating dysmenorrhea.
Owner:RECKITT BENCKISER HEALTH LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com