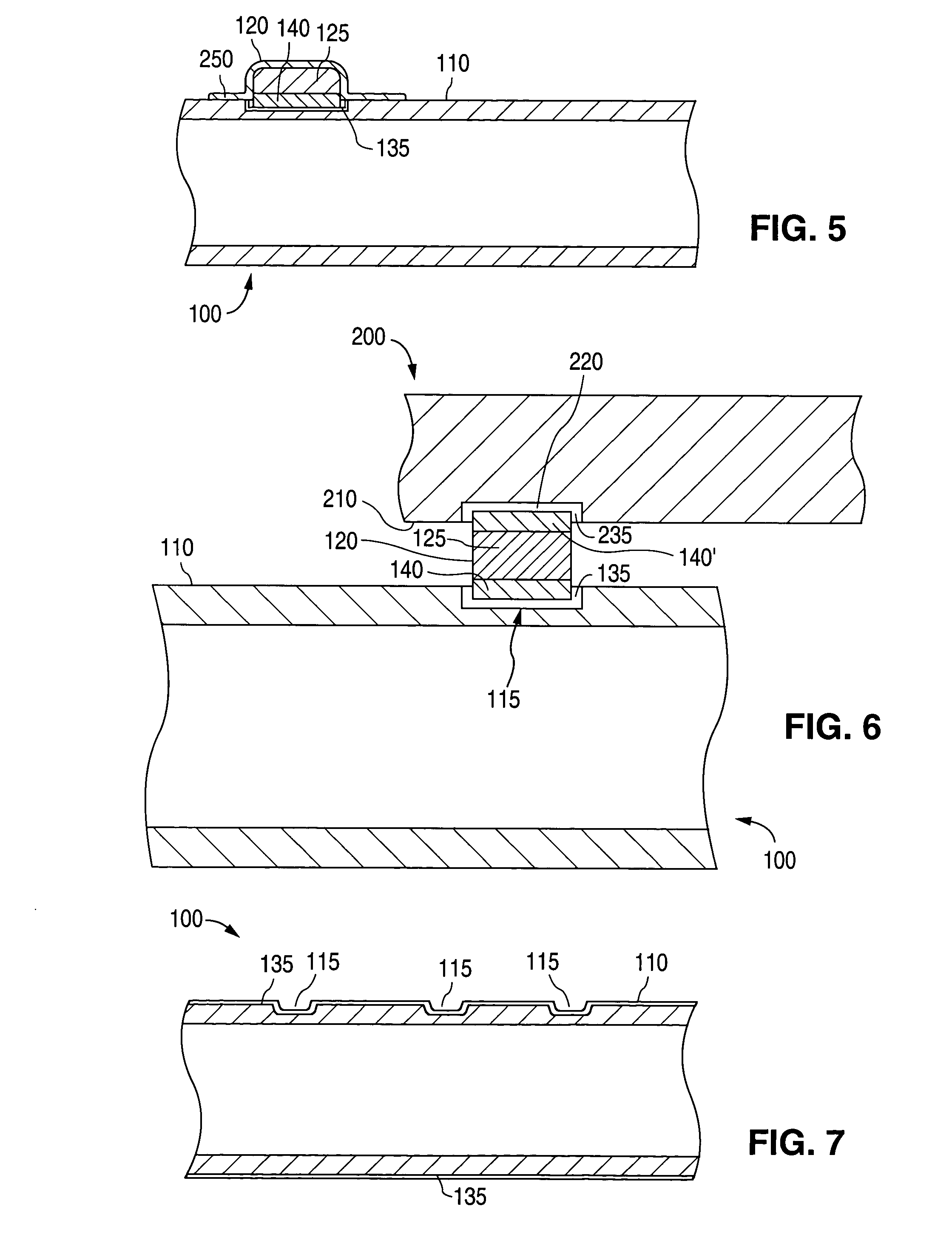
Patents
Literature
341 results about "Drug reservoir" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Ommaya Reservoir Drug Infusion. Also known as a subcutaneous cerebrospinal fluid (CSF) reservoir, the Ommaya reservoir allows delivery of long-term drug therapy to the CSF by way of the brain’s ventricles. The reservoir spares the patient repeated lumbar punctures to administer chemotherapeutic drugs, analgesics, antibiotics, and antifungals.
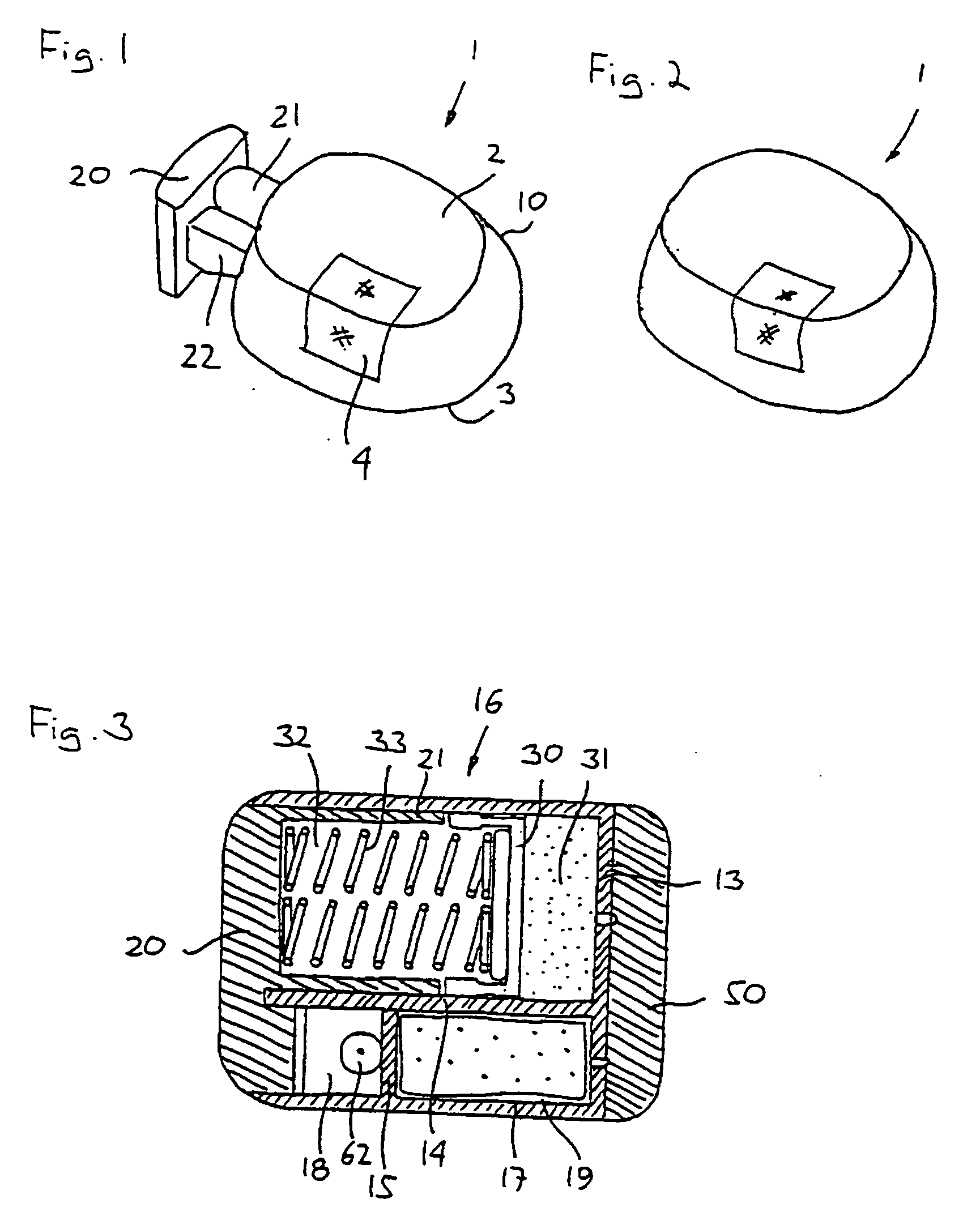
Solid solution perforator for drug delivery and other applications
InactiveUS6945952B2Fast biodegradationBarrier property can be diminished and controlledSurgeryMicroneedlesDrug reservoirDrugs solution
A solid drug solution perforator (SSP) system and an associated drug reservoir are provided for delivering therapeutic, prophylactic and / or cosmetic compounds, for nutrient delivery and for drug targeting. For drug delivery, the SSP system includes an active drug ingredient and a matrix of perforator material that biodegrades or dissolves quickly upon contact with a patient's body. The SSP system provides a skin barrier perforator and a controller for prompt initiation and cut-off drug delivery. In a preferred method of transdermal drug delivery, an SSP system containing a selected drug penetrates into an epidermis or dermis, and the drug is promptly released from the (dissolving) SSP system perforator. An additional drug is optionally delivered from a patch reservoir through skin pores created by insertion of the perforator. Formulation and fabrication procedures for the SSP and associated reservoir are also provided. An SSP system can be fabricated with variety of shapes and dimensions.
Owner:THERAJECT INC.
Delivery device
InactiveUS20060069382A1Safe and easy identificationImprove securityInfusion syringesMedical devicesDrug reservoirEngineering
This invention relates to a delivery device of the bleeding hole type, where a primary drive fluid, e.g. silicon oil, is used to expel a secondary fluid, e.g. a drug, contained in a reservoir. To provide a desired drug flow rate, the primary fluid is forced from a first reservoir through a flow restrictor into a second reservoir displacing a portion of the drug reservoir, thereby expelling the drug from its reservoir. The idea is to provide a drive fluid outlet, i.e. a flow restrictor inlet, which protrudes into the first reservoir. By this arrangement the amount of particles and air-bubbles entering the narrow flow restrictor will be reduced. The reduction is achieved because particles and air-bubbles will normally concentrate in the top or bottom of the reservoir, whereas the protrusion will primarily connect to the centre of the first reservoir.
Owner:NOVO NORDISK AS
Implantable Drug Delivery Device and Methods for Treatment of the Bladder and Other Body Vesicles or Lumens
ActiveUS20090149833A1High plasma concentrationMinimize irritationBiocideMedical devicesDrug reservoirControlled drugs
An implantable medical device is provided for controlled drug delivery within the bladder, or other body vesicle. The device may include at least one drug reservoir component comprising a drug; and a vesicle retention frame which comprises an elastic wire having a first end, an opposing second end, and an intermediate region therebetween, wherein the drug reservoir component is attached to the intermediate region of the vesicle retention frame. The retention frame prevents accidental voiding of the device from the bladder, and it preferably has a spring constant selected for the device to effectively stay in the bladder during urination while minimizing the irritation of the bladder.
Owner:MASSACHUSETTS INST OF TECH
Device and Method for Treating Weight Disorders
An apparatus and a method for treating a weight disorder in a subject are provided. The apparatus comprising an implantable device such as an inflatable balloon and electrodes capable of sensing a physiological change associated with food ingestion or hunger and a mechanism adapted for directly stimulating a region such as the duodenum which is responsive to a gastrointestinal satiety agent, such a mechanism can be a drug reservoir containing a drug such as CCK or analogs thereof which is contained within an inflatable balloon being implantable in a stomach of the subject. The apparatus and method provided here combine synergistic approaches to limiting meal size, i.e., chemo and mechano receptor activation of vagal satiety stimuli, electric stimulation of specific vagal pathways and limitations of gastric space.
Owner:DUOCURE
Systems and methods for delivering flow restrictive element to airway in lungs
A method and system for catheter-based delivery of implants in the body. Implants can include stents, plugs, coils, baskets, filters, valves, grafts, prosthesis', drugs, drug reservoirs, biologics, or pumps. The catheter system comprises a uniquely configured grasper mechanism that allows holding the implant during the unsheathing delivery step prior to full release. With this delivery system, the implant can be unsheathed, positioned, and the position can be evaluated prior to releasing the implant from the catheter. Upon evaluation of the position of the implant, if it is found to be inaccurately placed, then removal of the implant can be done easily and without a device exchange. If the implant is found to be positioned correctly, the grasper mechanism can be actuated to release the implant from the catheter.
Owner:PULMONX
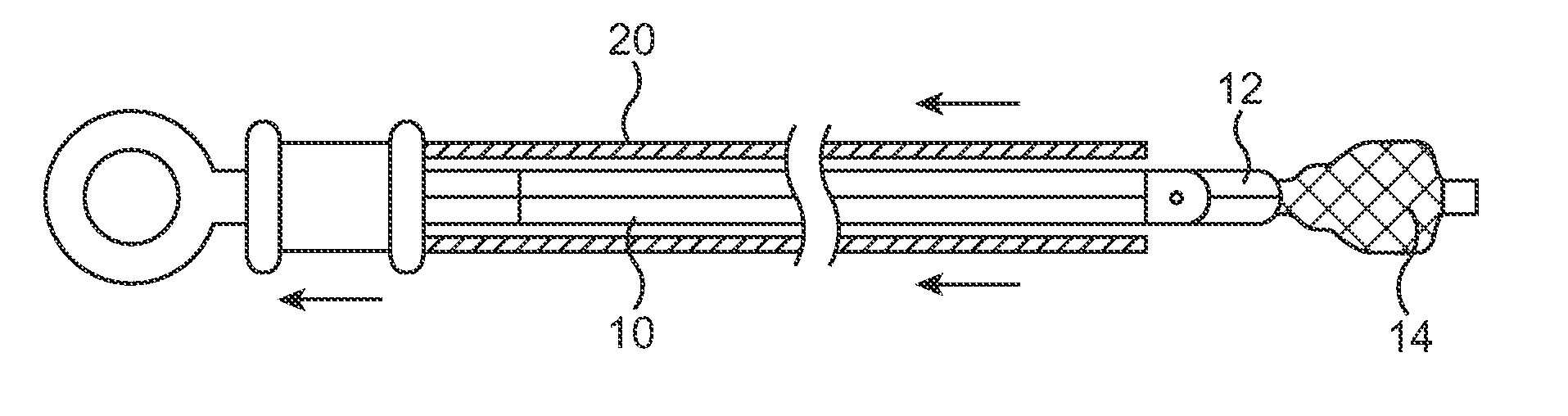
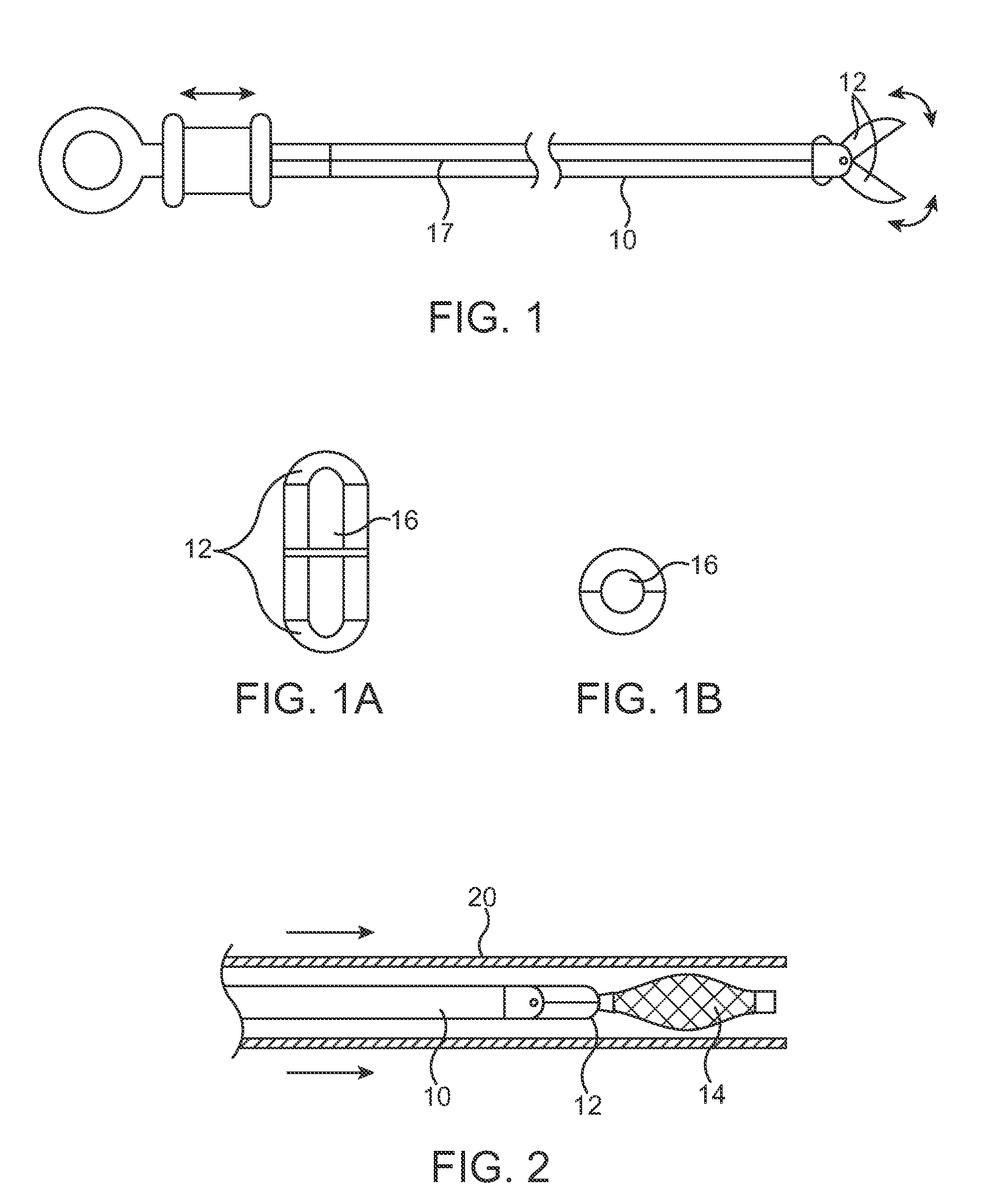
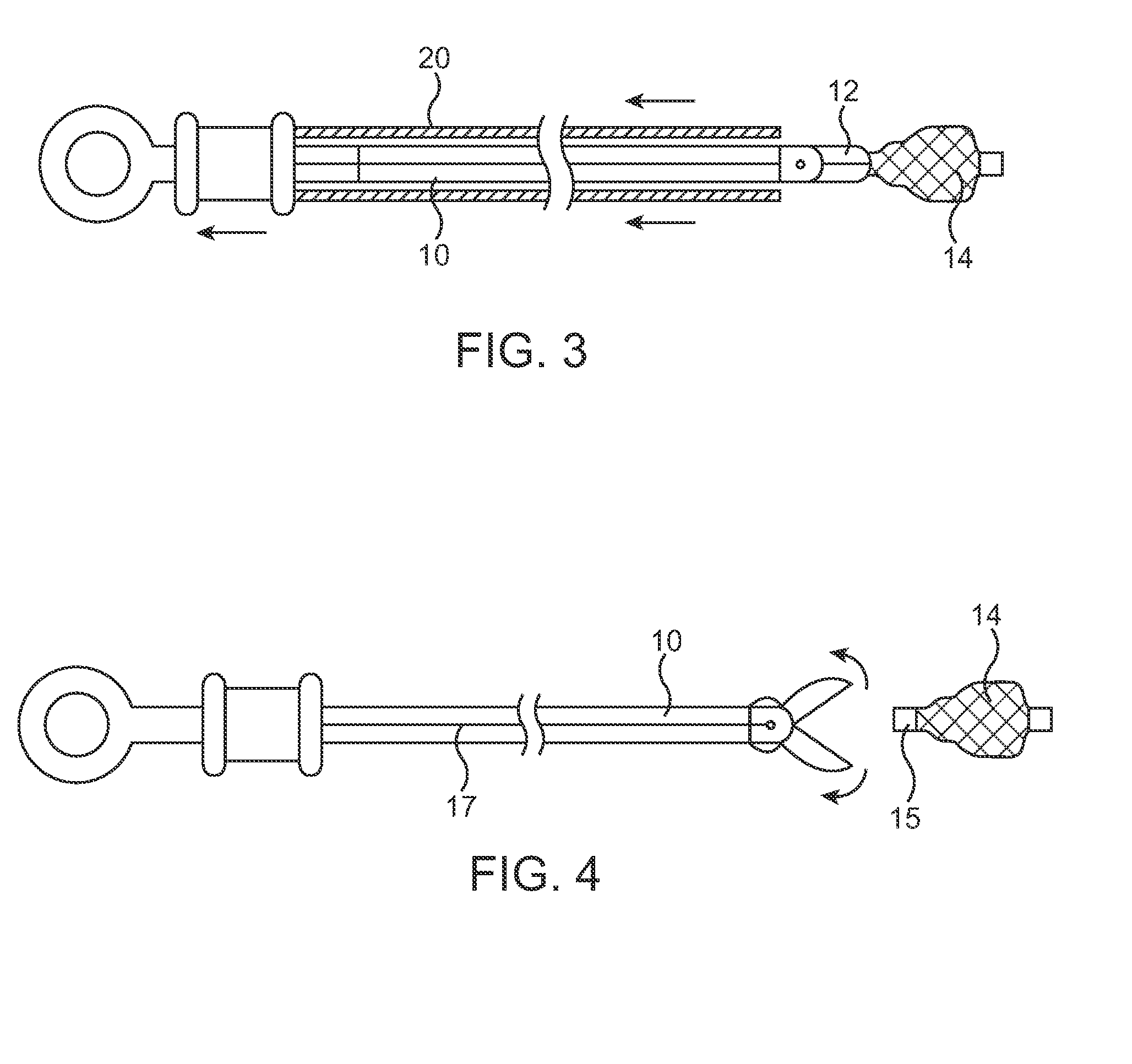
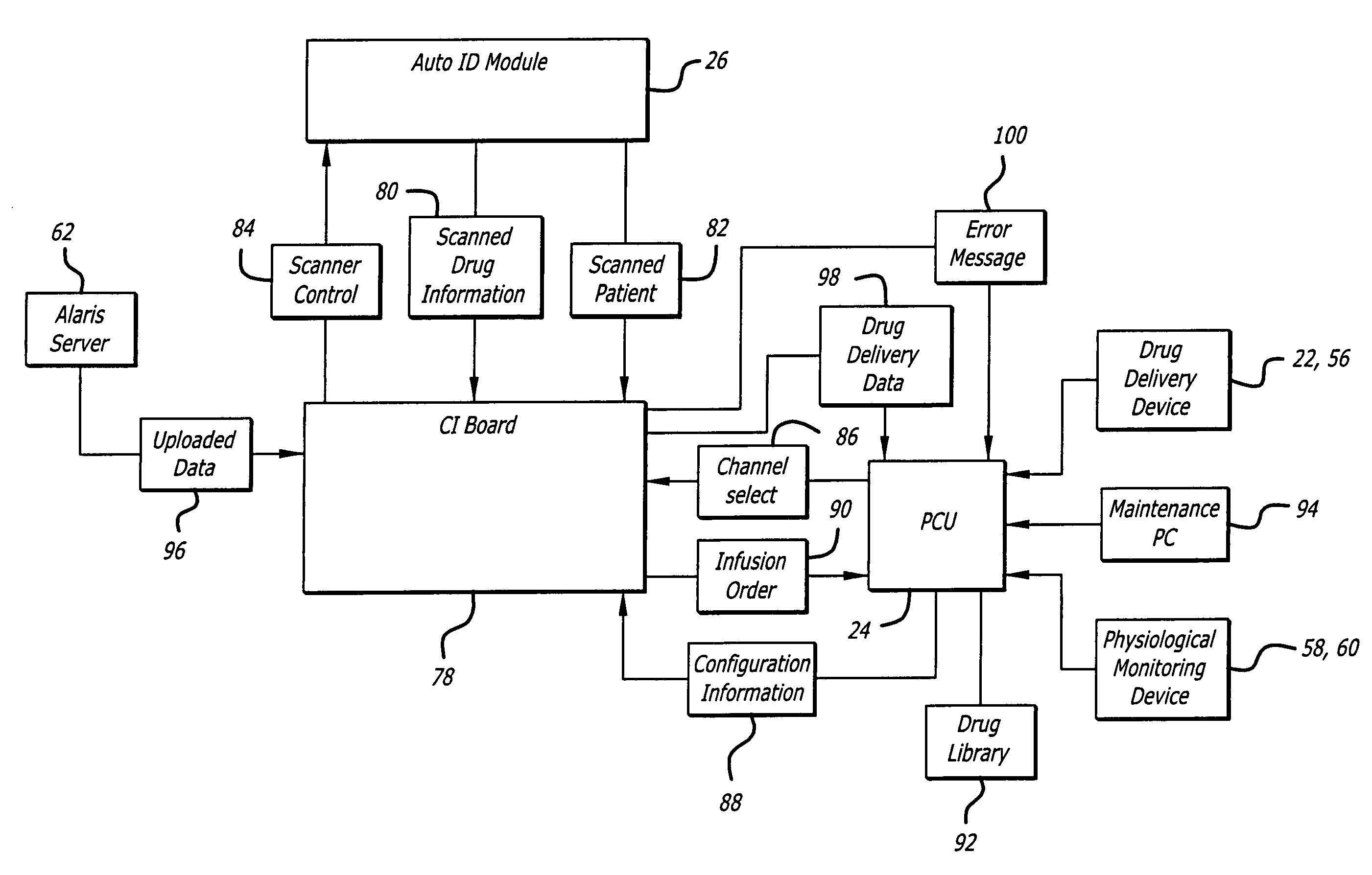
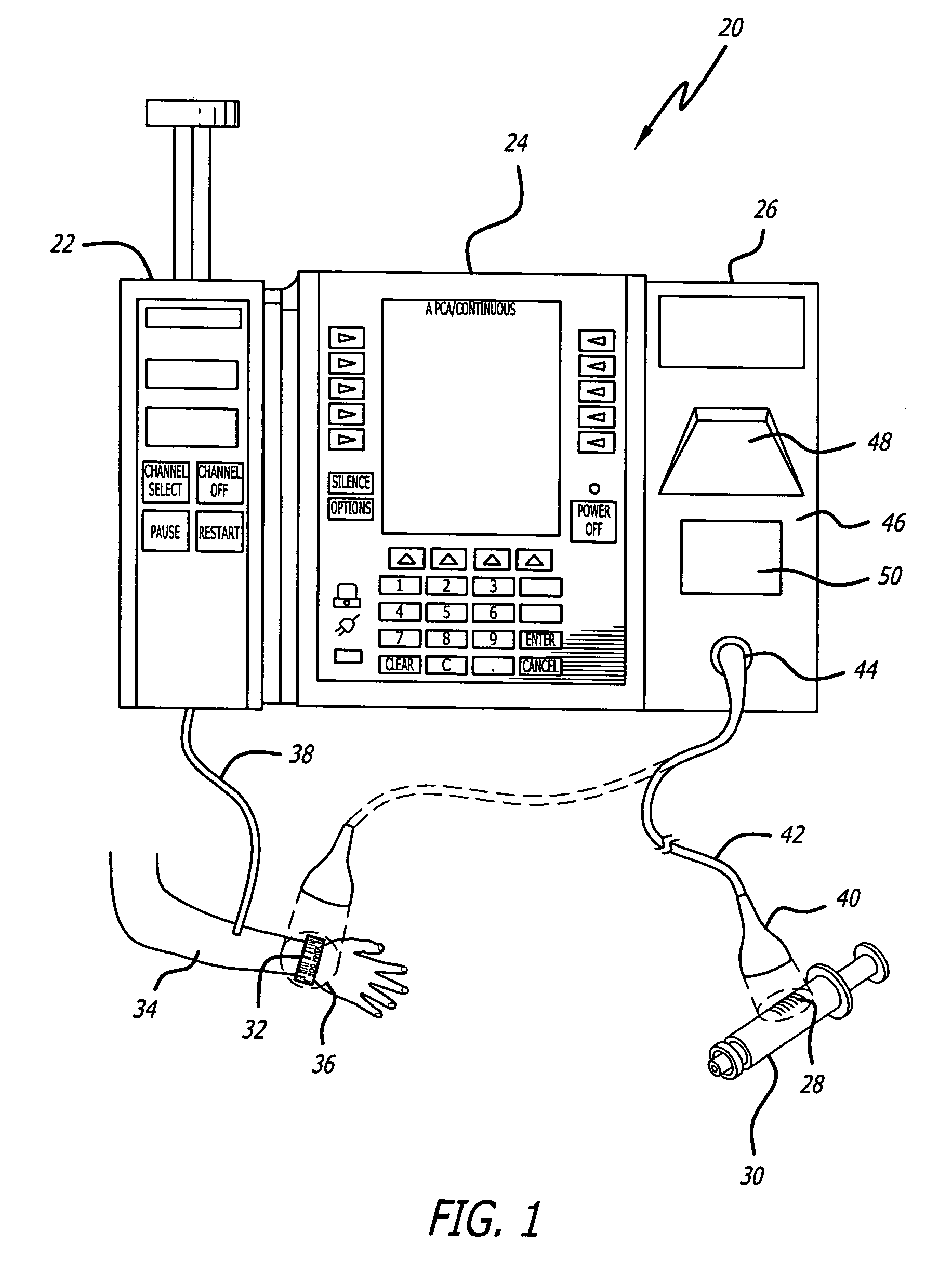
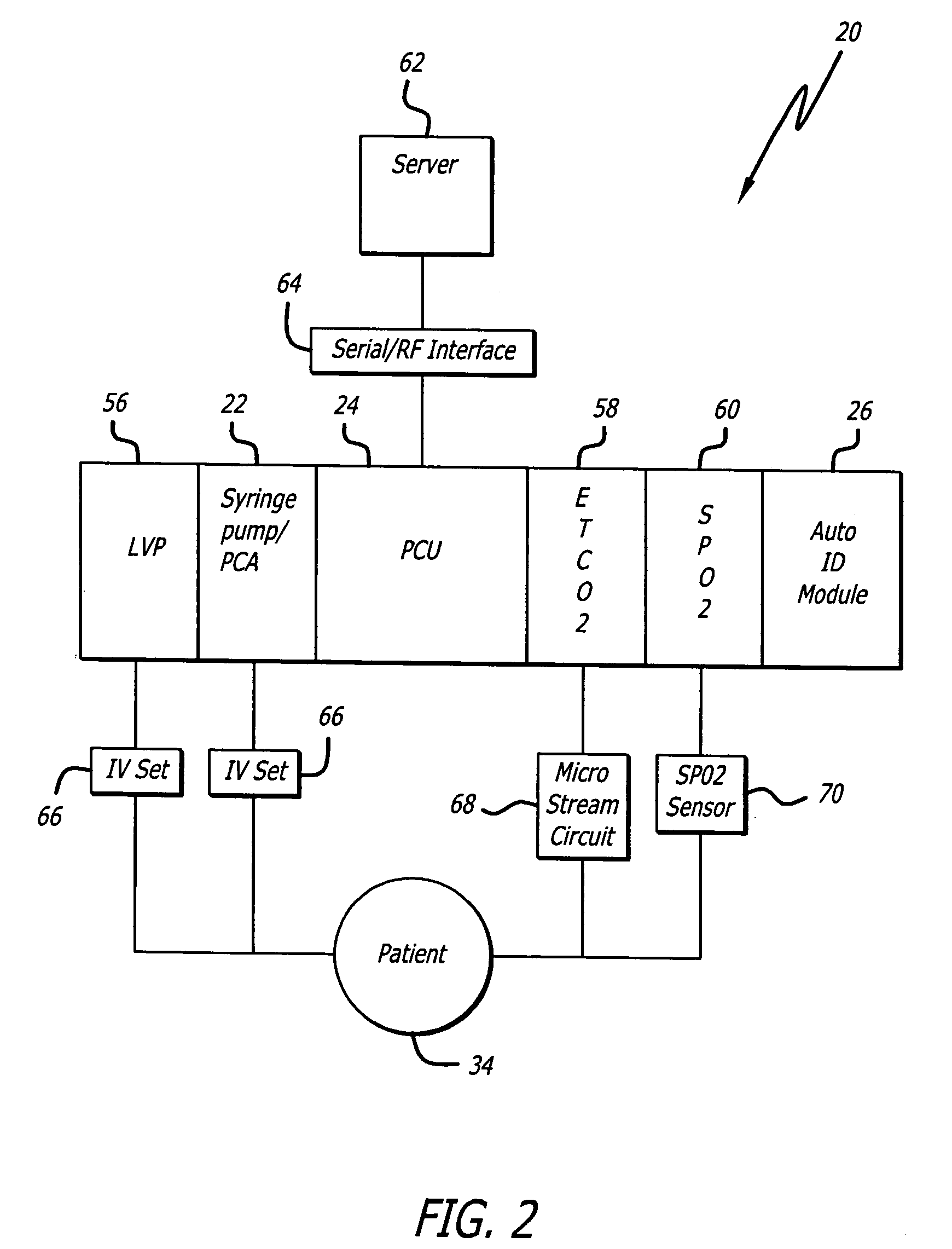
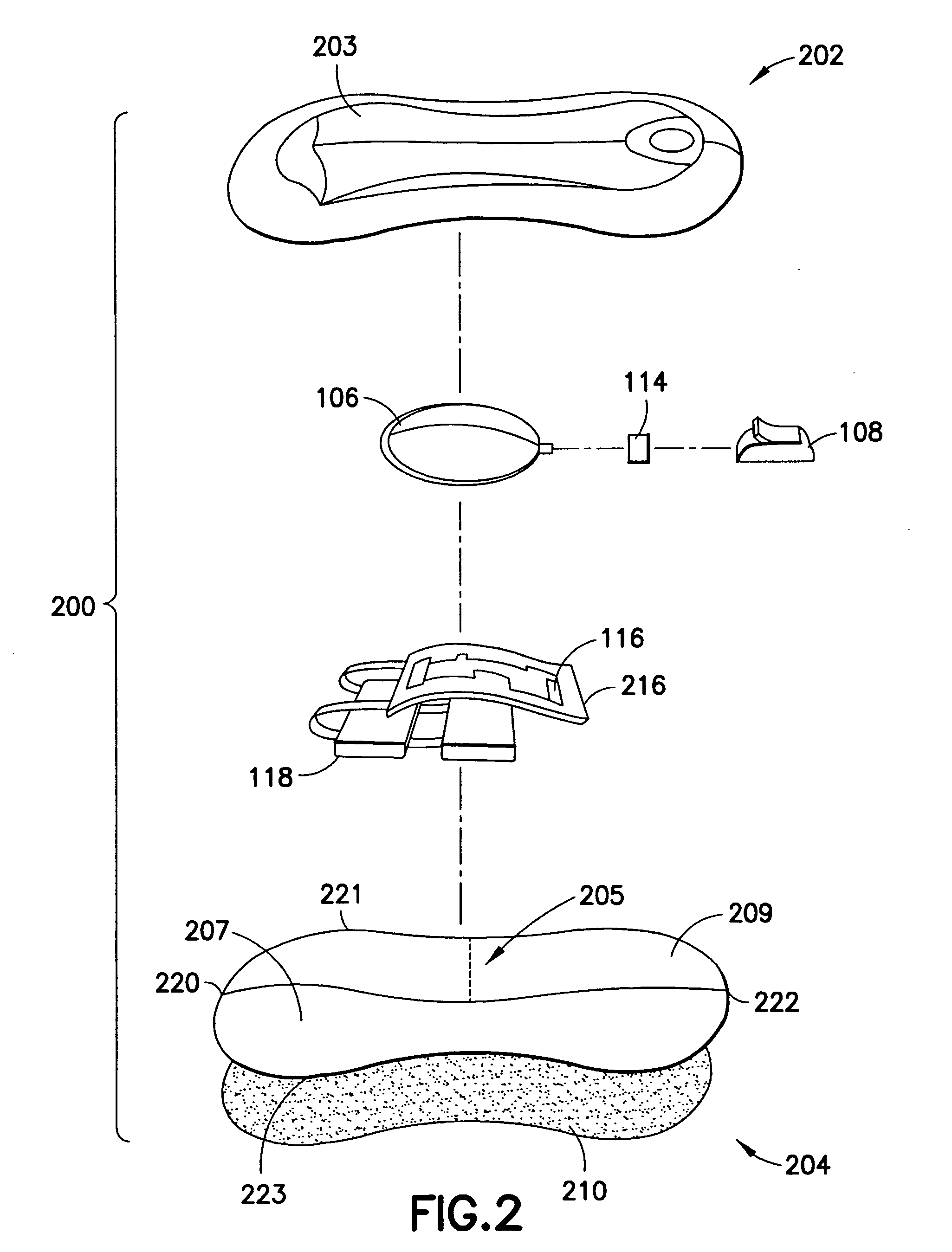
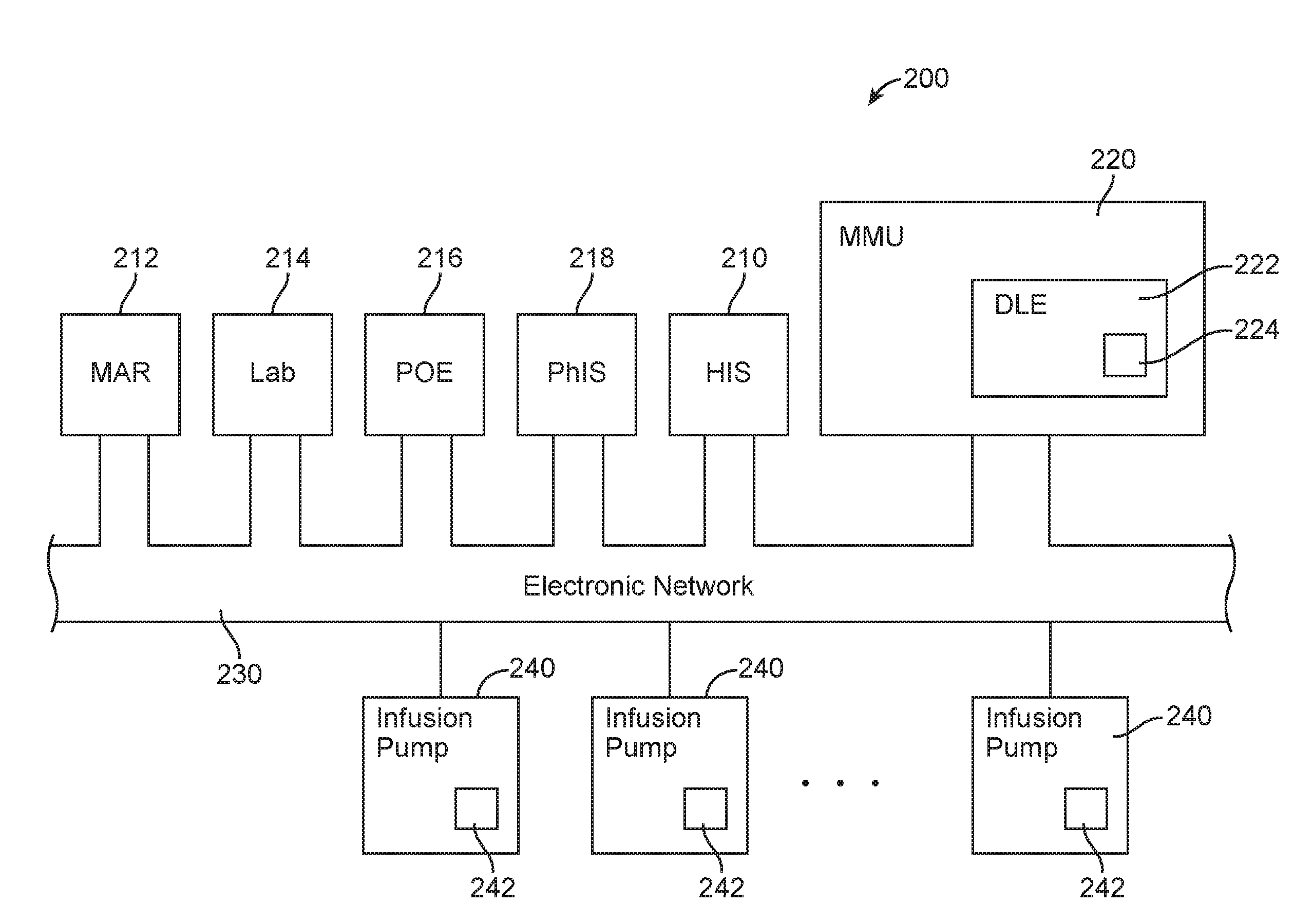
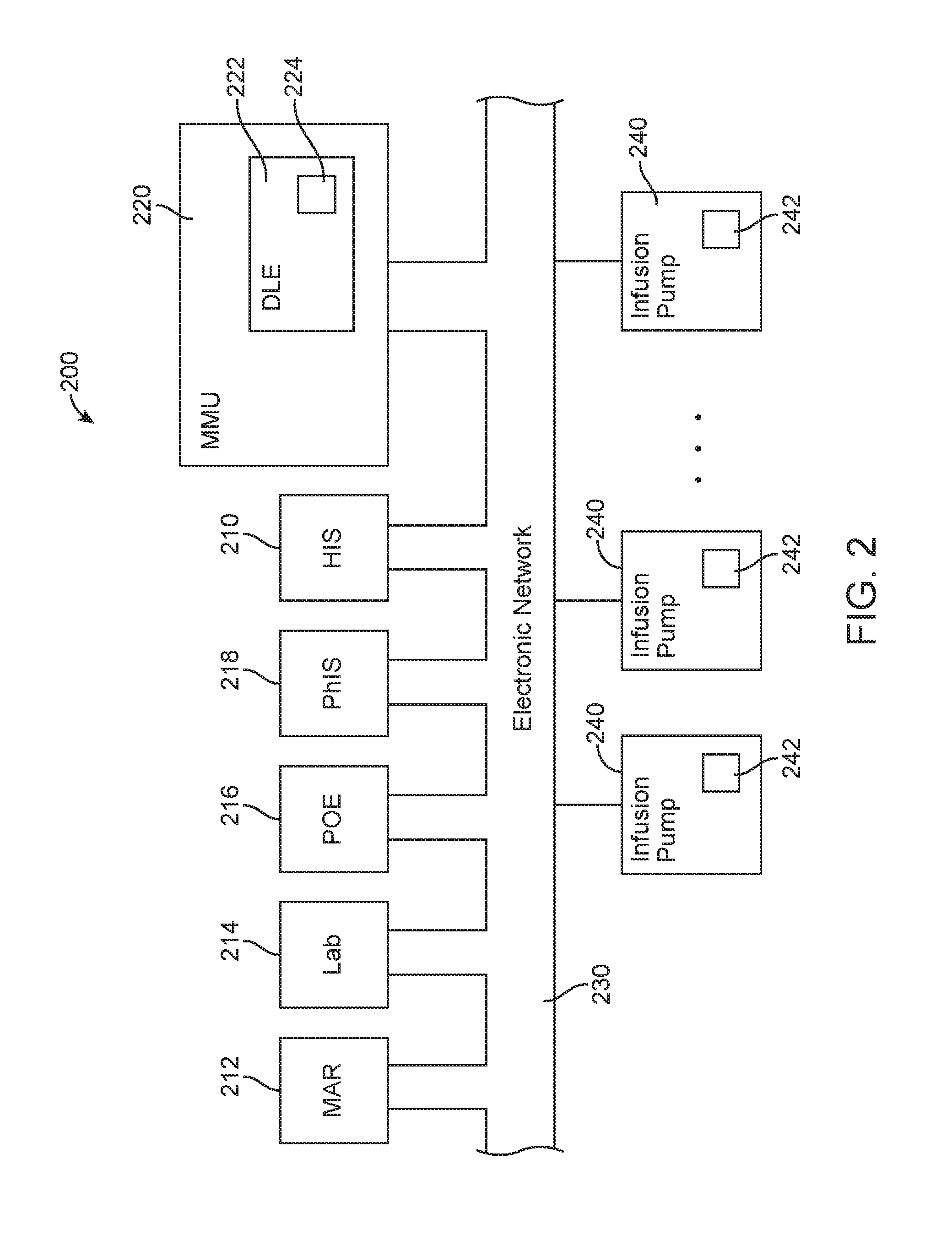
Identification system and method for medication management
An identification system and method for medication management comprises a controller, a drug library accessible by the controller, the drug library containing drug library profiles including a data set of drug information, and an identification module configured to read patient identification information from machine-readable identification devices worn by patients and to read drug information from machine-readable identification devices affixed to drugs or containers of drugs. The controller compares the read information to each other and to the drug library profile and provides alerts or error signals in the event of an inconsistency. In one case, identification devices are read by an optical reader fixedly mounted to the identification module. In another case, identification devices are read by an optical reader that is hand-held and mobile and can be moved to the location of the identification device. The hand-held reader communicates with the identification module by wired or wireless means. In another case, a third reader that is non-optical and wireless is fixedly mounted to the identification module. The controller is also configured to provide alerts or error messages in the event that there is an inconsistency between the drug information on the drug or drug container when compared to the drug library profile associated with the controller.
Owner:CAREFUSION 303 INC
Porous glass fused onto stent for drug retention
Owner:ABBOTT CARDIOVASCULAR
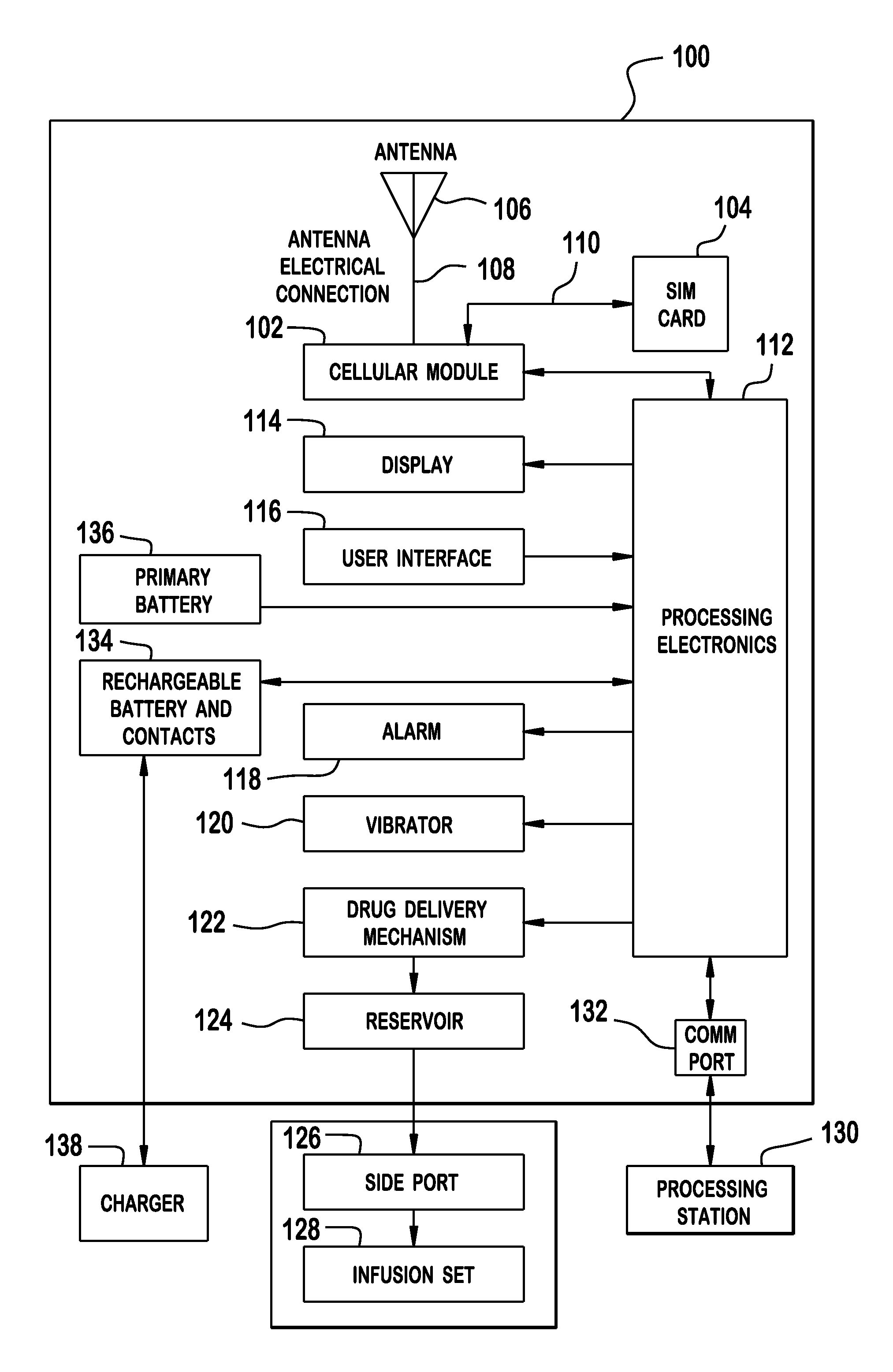
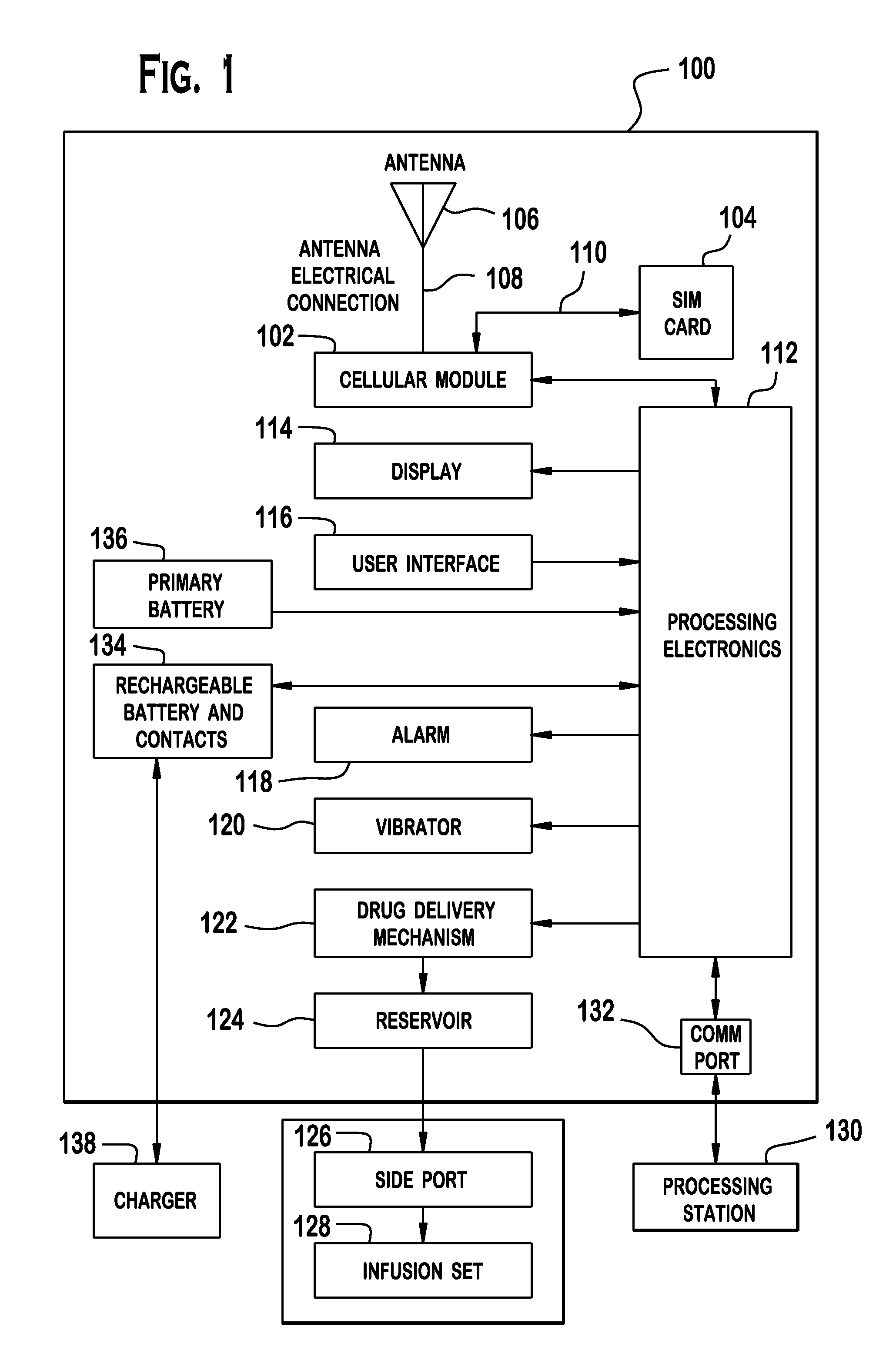
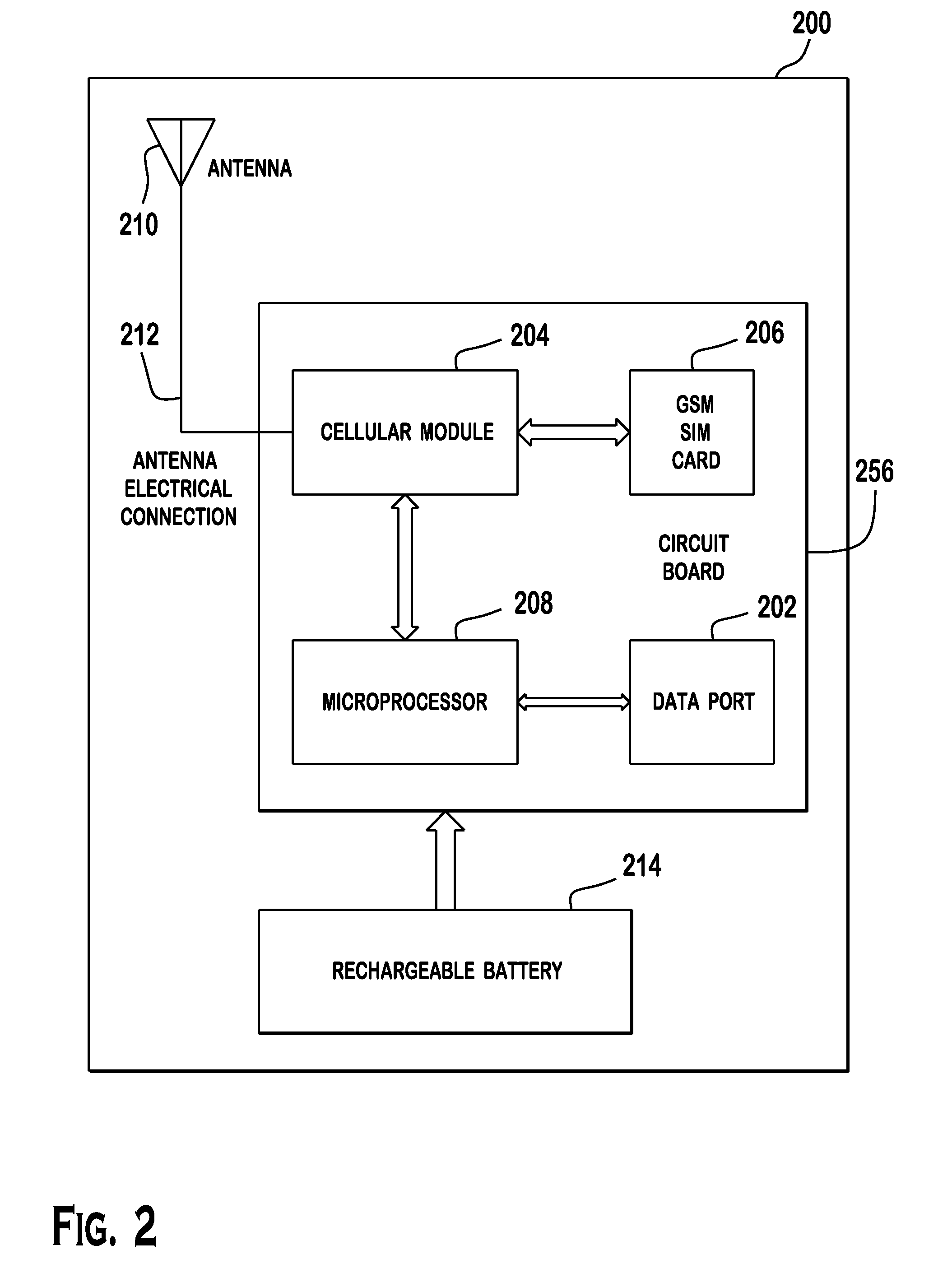
Cellular-Enabled Medical Monitoring and Infusion System
Described is a medical infusion device that is configured to send and receive data on a real-time basis through the use of circuitry that enables the connection of the device to a cellular network. In one embodiment, a medical infusion device is provided that includes a housing having a drug reservoir, a first processing system and a drive mechanism adapted to receive and execute instructions from the first processing system. The drive mechanism is in mechanical cooperation with the drug reservoir for controlling expulsion of the drug from the reservoir. Medical infusion device also includes an input device for receiving external instructions and configured to transmit the external instructions to the first processing system, a display for receiving and displaying information from the first processing system, and a second processing system in communication with the first processing system for controlling communication between the first processing system and a remote processing system via a cellular network.
Owner:ANIMAS CORP
Drug infusion system with reusable and disposable components
InactiveUS20100049128A1Low profileAutomatic syringesPressure infusionMedication infusionDrug reservoir
The application discloses a drug infusion system comprising a base and a drug dispenser. The base is configured to receive a cannula that delivers a drug to beneath a wearer's skin. The base is attachable to the skin of the wearer. The reusable drug dispenser is removably attachable to the base and has a pump unit configured to establish fluid communication between a removably attachable drug reservoir and the cannula. The pump unit pumps the drug to the wearer upon activation by the wearer. The pump unit may have an inlet to contact the drug within the reservoir, which may be a needle. The pump unit may also have a receiving unit to receive the reservoir. Such a receiving unit may be a tubular for a cylindrical reservoir, or may have a cavity or an encasing unit to hold the reservoir.
Owner:CALIBRA MEDICAL
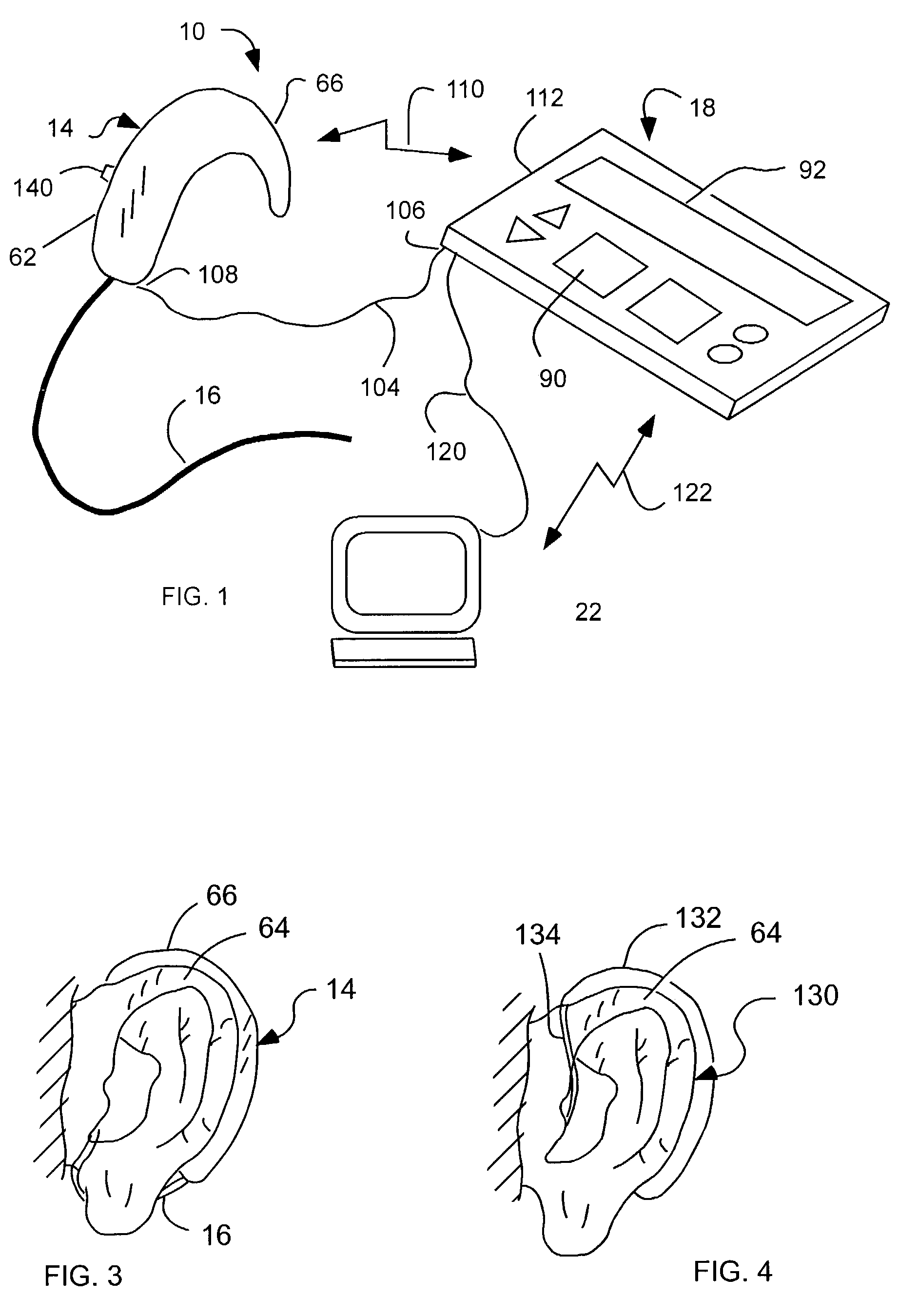
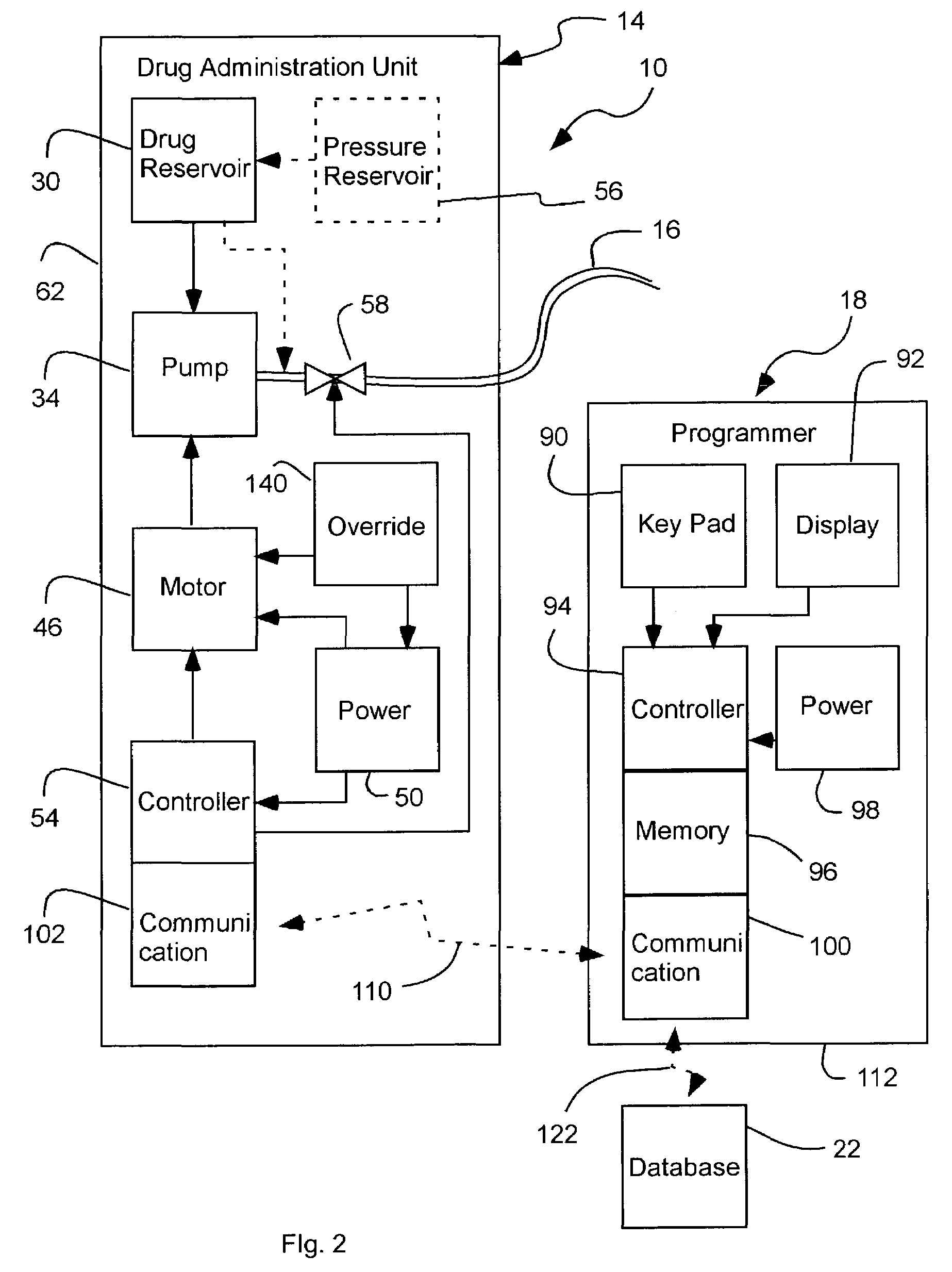
Cochlear drug delivery system and method
InactiveUS7206639B2Convenient, unobtrusive, and inexpensiveElectrotherapyEar treatmentDrug reservoirMiddle ear
A drug administration system configured to administer a drug to a user's ear includes a housing, sized and shaped to substantially fit behind the user's ear, and configured to pump the drug in controlled amounts to the user's middle ear. A drug reservoir is disposed in the housing and includes a drug configured to treat an inner ear condition. A catheter is operatively coupled to the drug reservoir and is sized and shaped to extend from the drug administration unit into the user's middle ear.
Owner:STERLING INVESTMENTS LC
Drug reservoir loading and unloading mechanism for a drug delivery device using a unidirectional rotated shaft
A drug reservoir loading and unloading mechanism for drug delivery device using unidirectional rotated lead screw and method thereof are disclosed. The drug reservoir loading / unloading mechanism allows exchanging the drug reservoir quickly with very few steps and with more safety. The invention neither requires rewinding of the drive system either automatically or manually while replacing the drug reservoir nor requires an additional adapter to secure the drug reservoir.
Owner:ROCHE DIABETES CARE INC +1
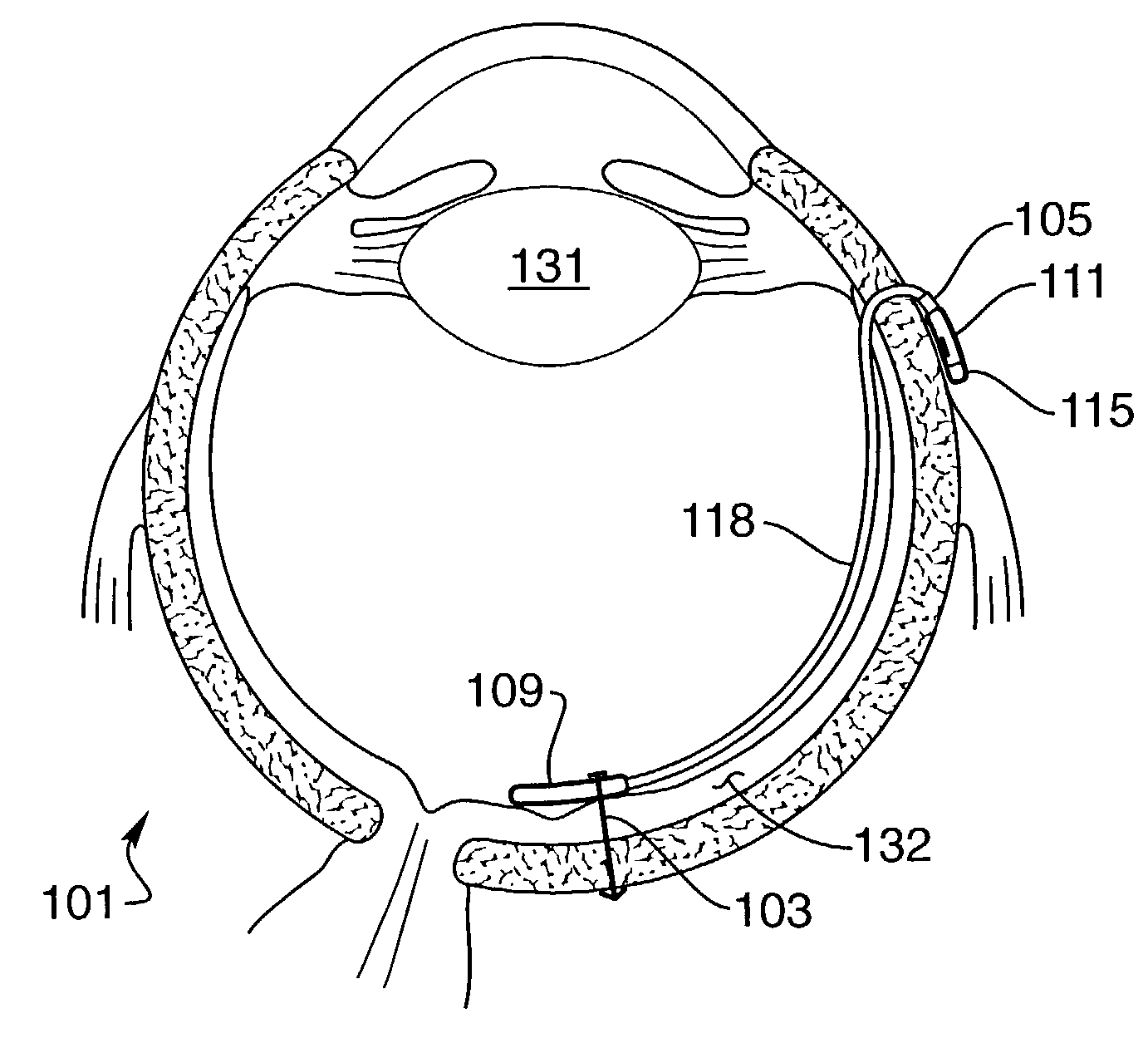
Implantable drug delivery device
InactiveUS7181287B2Avoiding and minimizing harmful stressPrecise positioningHead electrodesMedical devicesDrug reservoirMedicine
The invention is directed to an implantable device to enable delivery of drugs to the retina. The device minimizes stress to the retina by virtue of its softness and smooth shape that conform to the retina. Drugs are delivered by osmosis or by the device dissolving. It may be connected to an externally mounted pump and drug reservoir that control the amount of drug. It contains one or more holes that are positioned to deliver drugs to the desired location. Drugs may stimulate the retina to enable vision in blind patients. Drugs may be injected directly inside the eye by a trans-scleral pump and valve drug delivery device.
Owner:SECOND SIGHT MEDICAL PRODS
Drug reservoir loading and unloading mechanism for a drug delivery device using a unidirectional rotated shaft
A drug reservoir loading and unloading mechanism for drug delivery device using unidirectional rotated lead screw and method thereof are disclosed. The drug reservoir loading / unloading mechanism allows exchanging the drug reservoir quickly with very few steps and with more safety. The invention neither requires rewinding of the drive system either automatically or manually while replacing the drug reservoir nor requires an additional adapter to secure the drug reservoir.
Owner:ROCHE DIABETES CARE INC +1
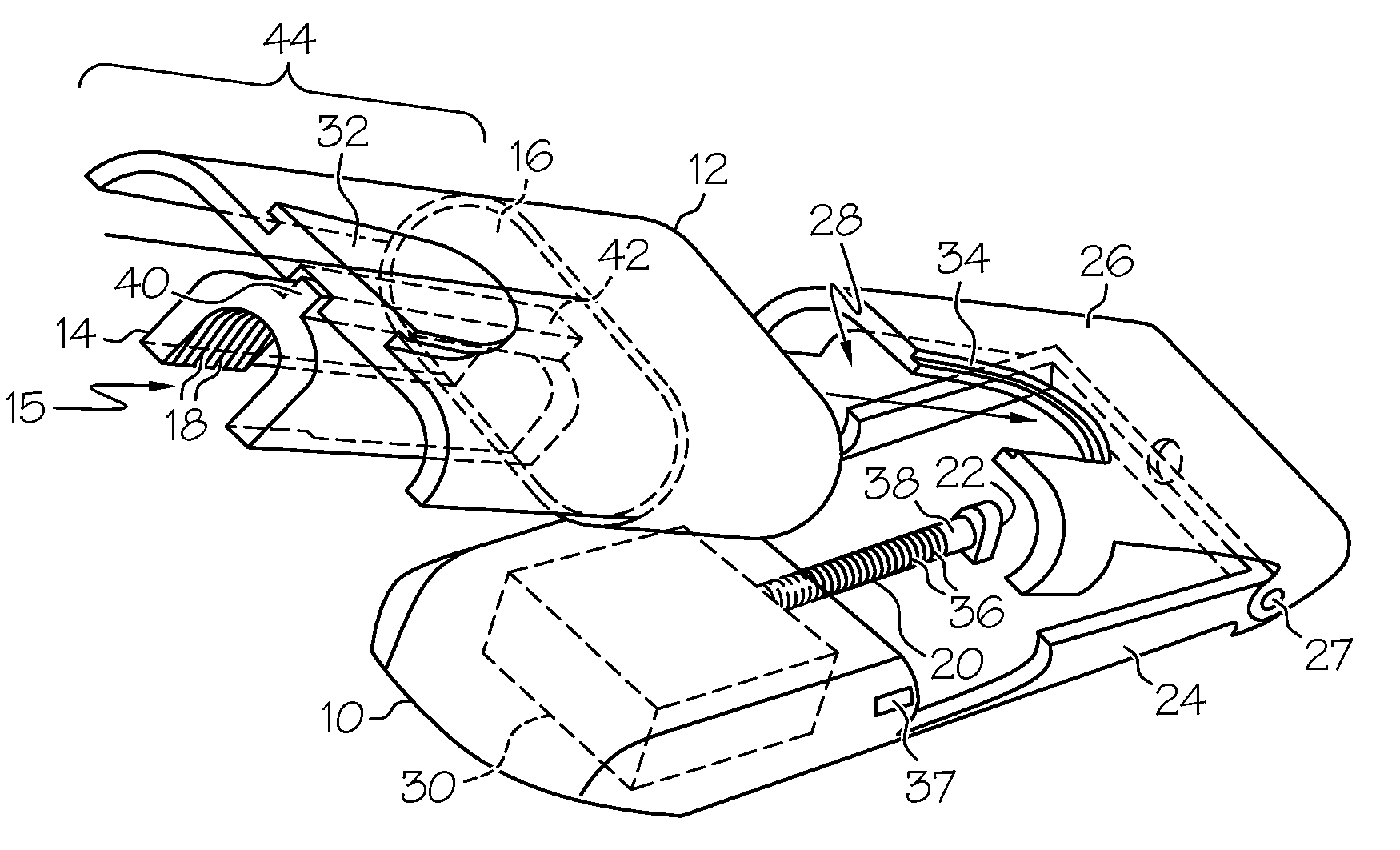
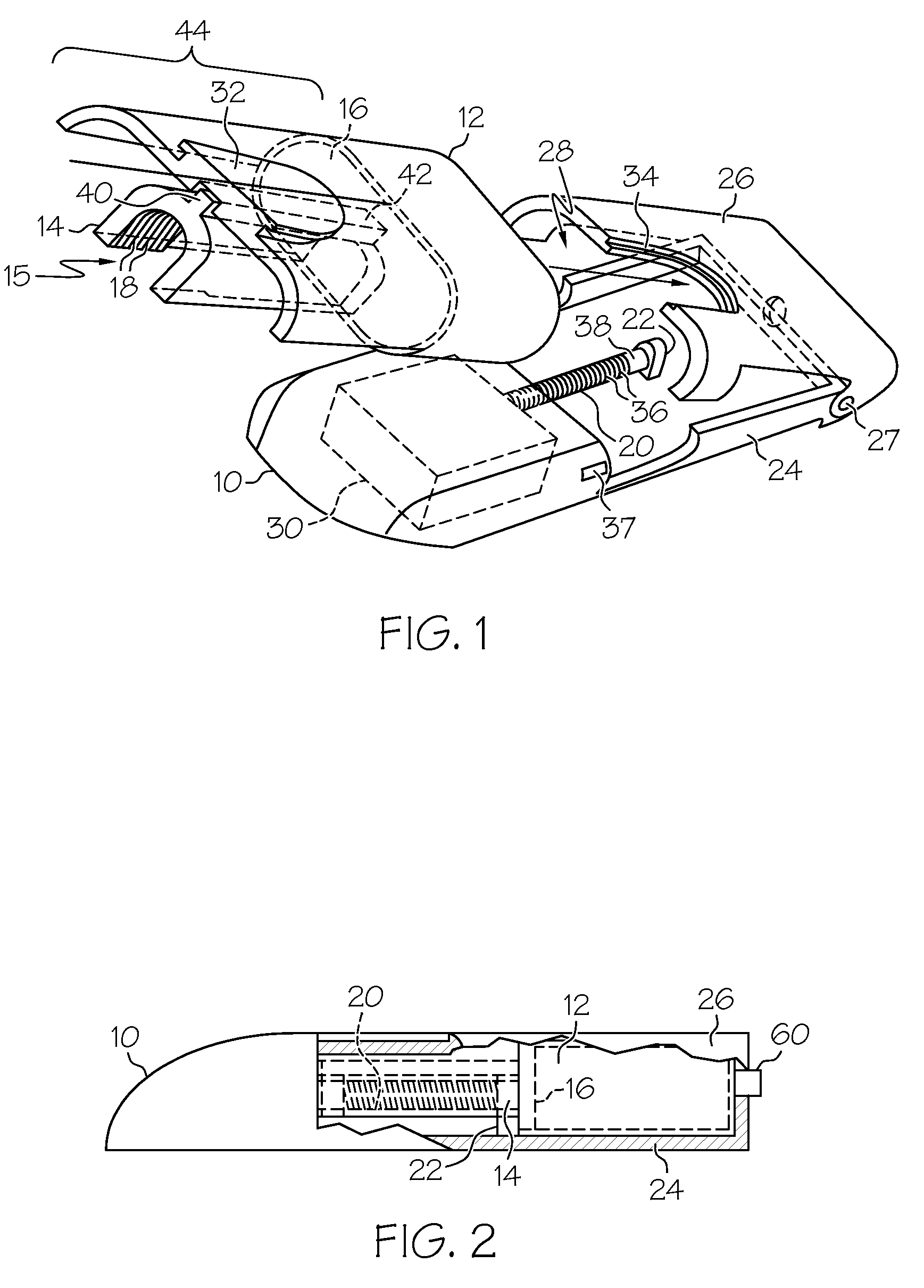
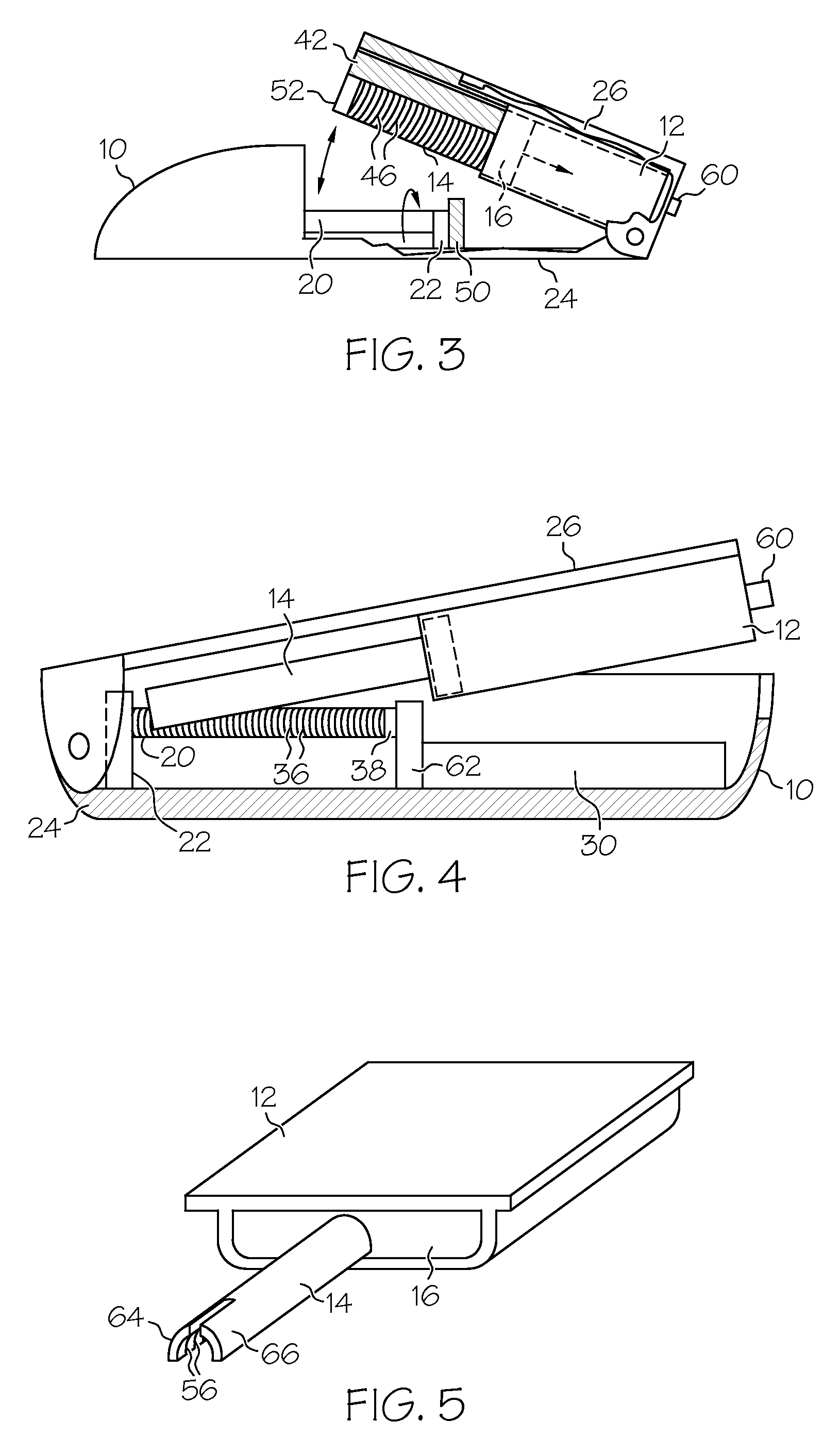
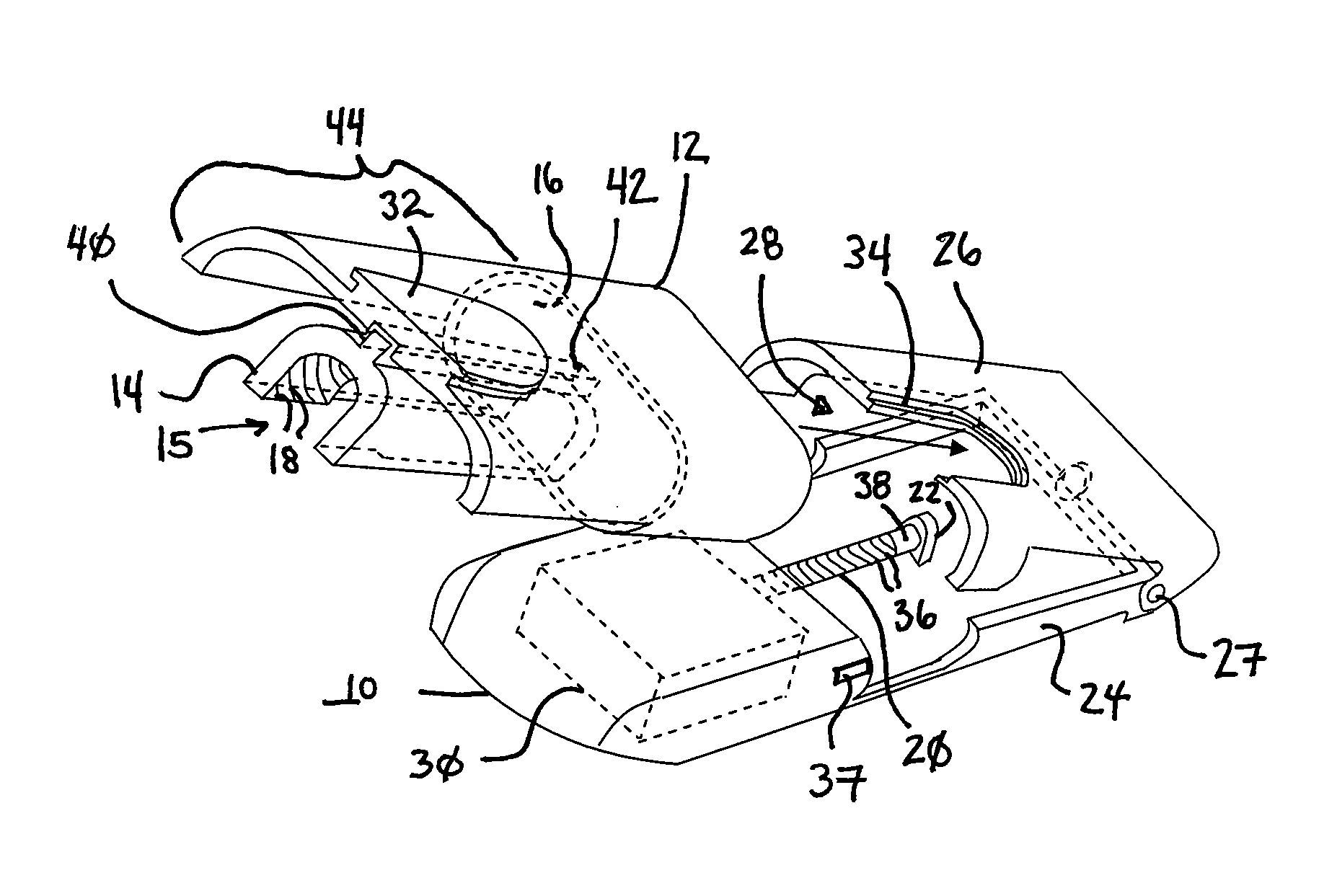
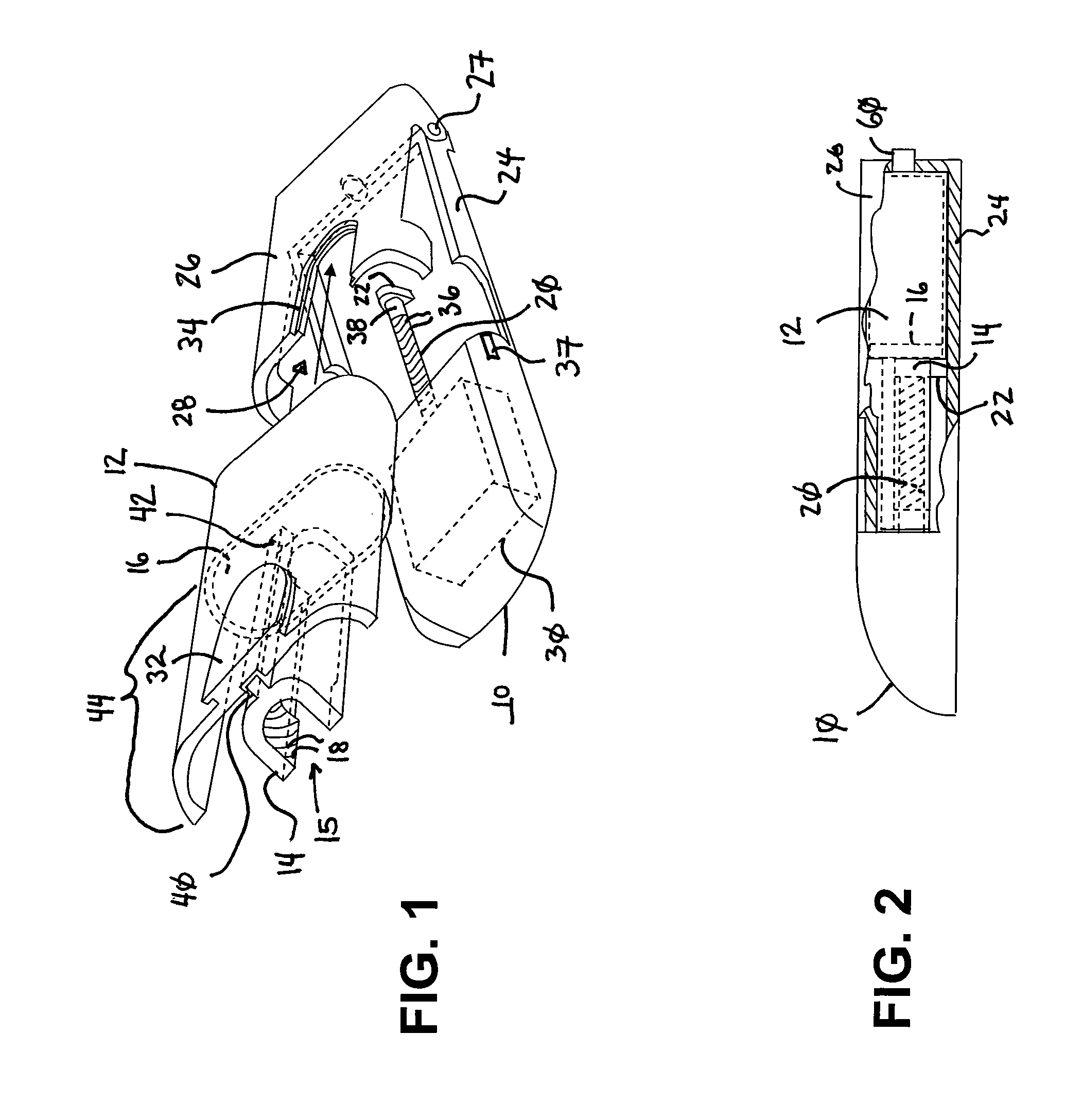
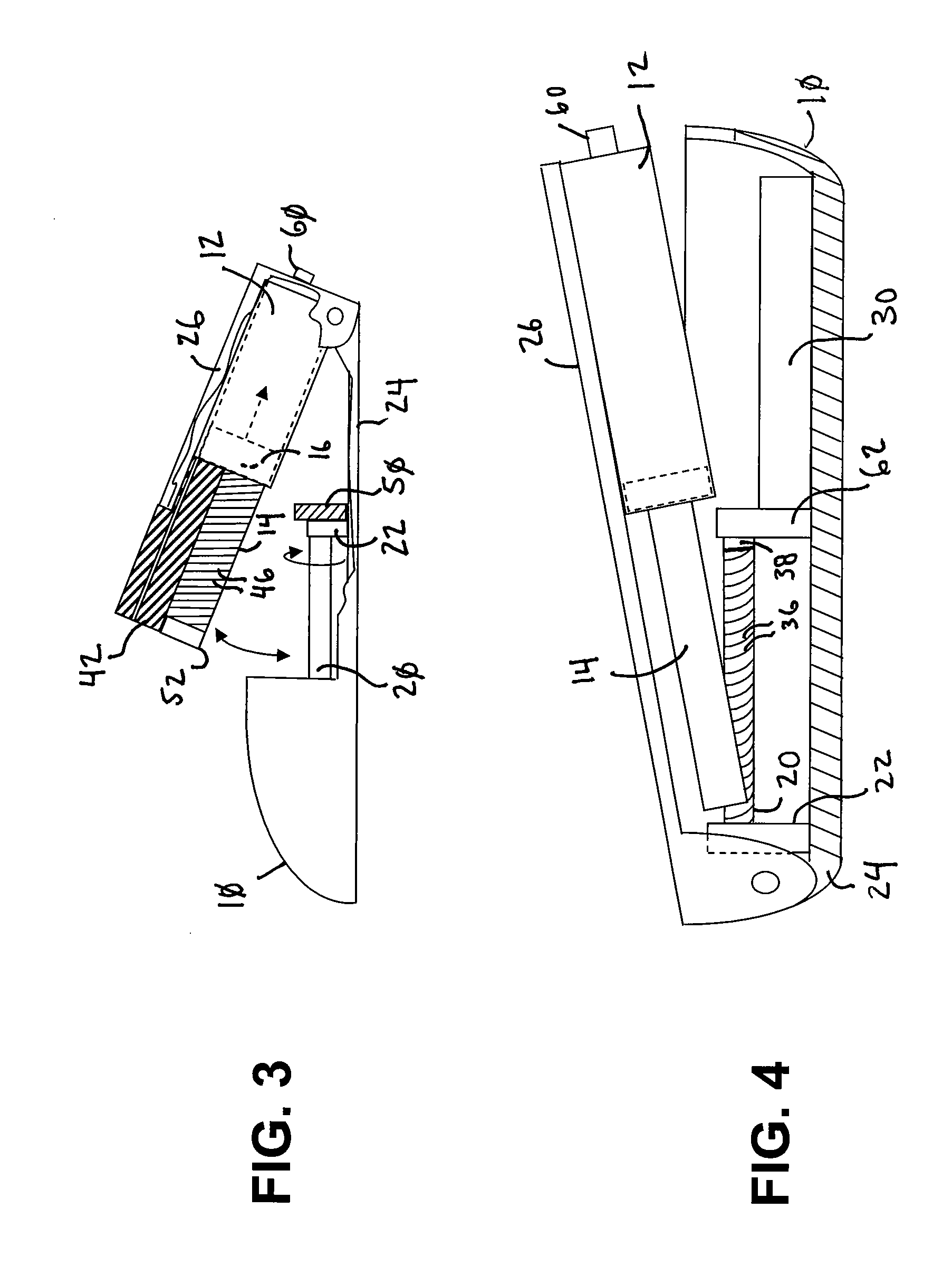
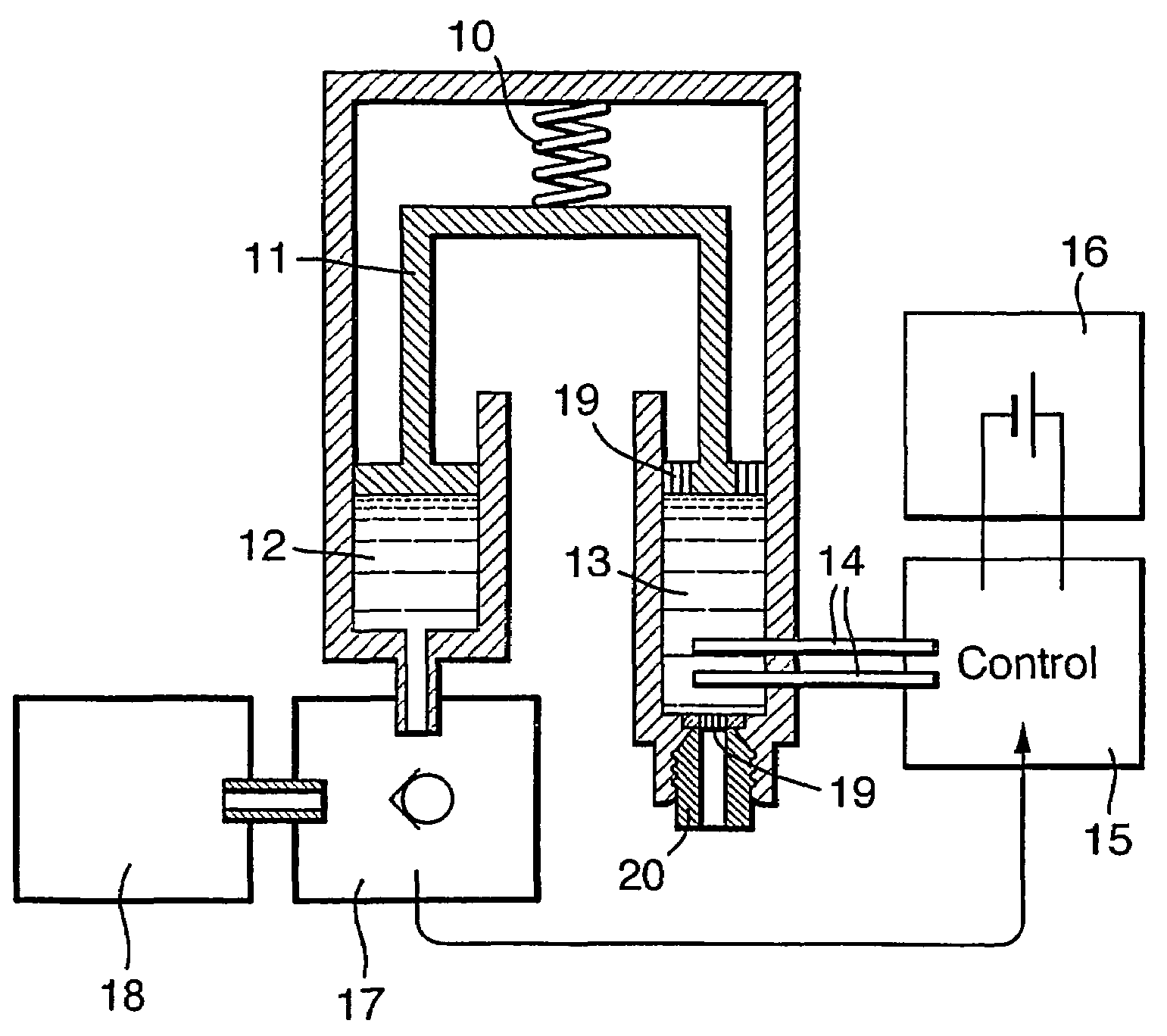
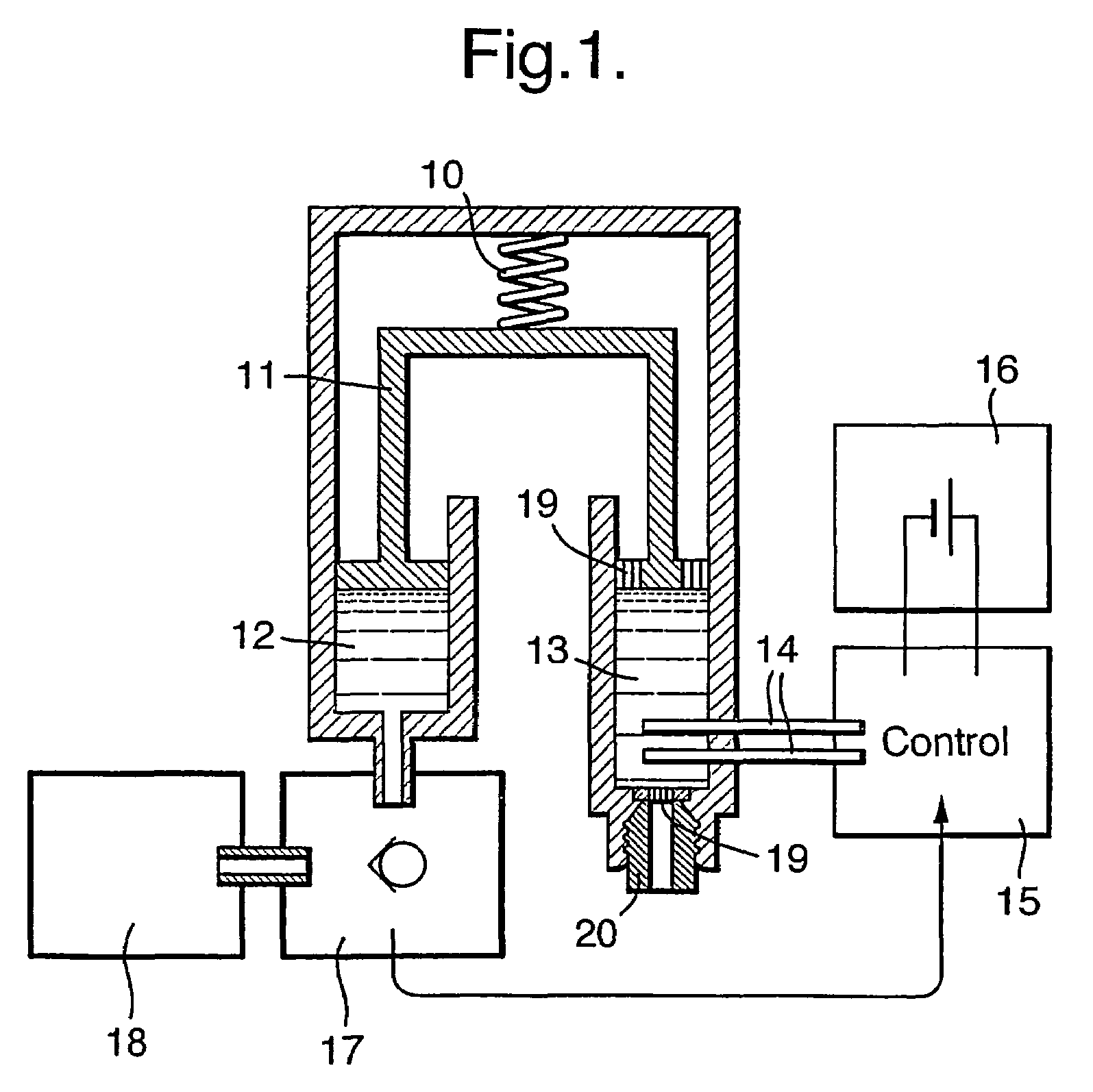
Drug infusion device
ActiveUS7524304B2Stable and constant infusion deviceFriction effect is negligibleAutomatic syringesPharmaceutical delivery mechanismDrug reservoirMedication infusion
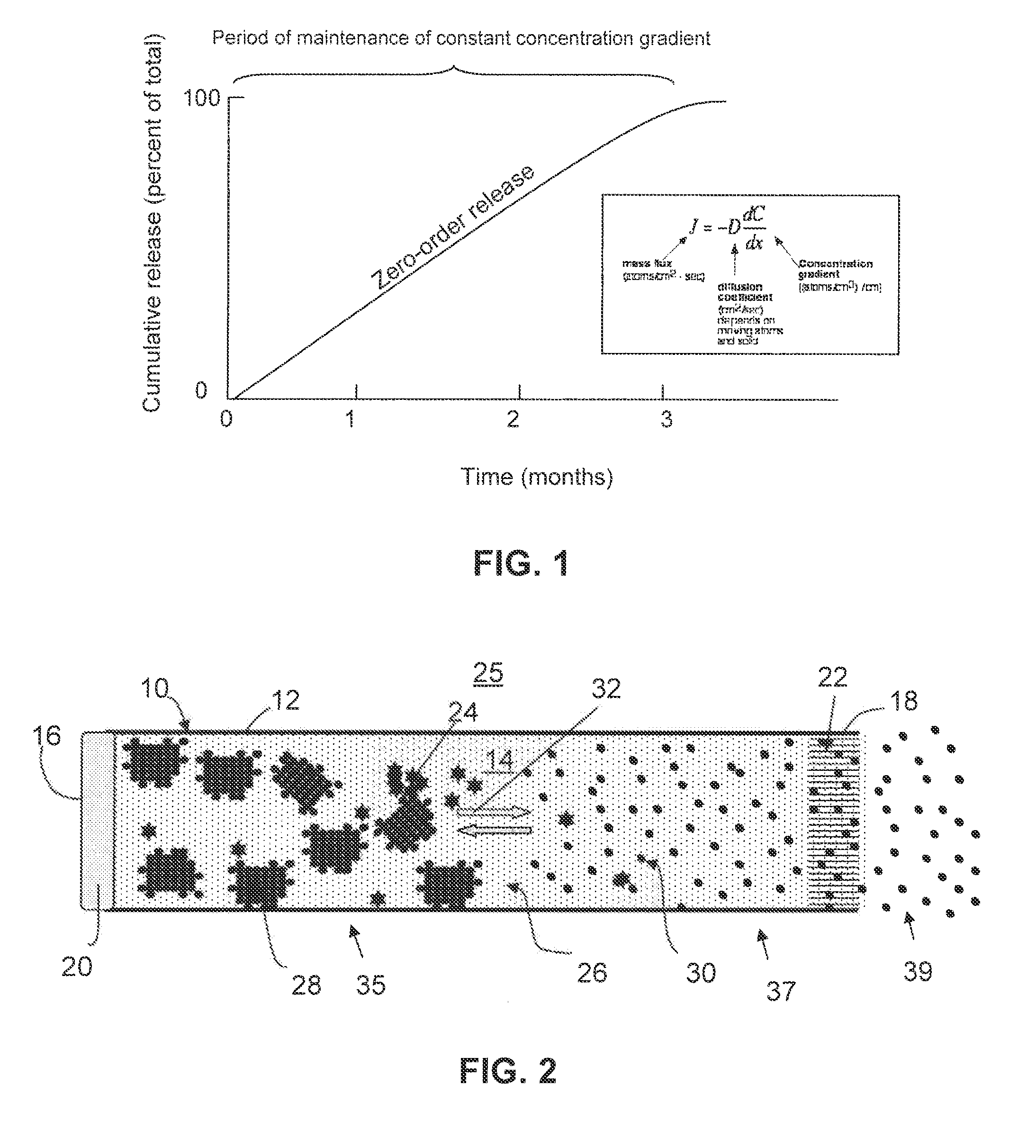
The invention provides a drug delivery infusion device comprising an injection means (18) in fluid connection with a drug reservoir chamber (12) and pressure-generation means (10) coupled to both the drug reservoir (12) and to a liquid-filled control chamber (13), wherein the coupling is such that the liquid-filled control chamber (13) serves to constrain the motion of the pressure-generation means (10), thereby controlling the drug infusion rate, wherein the liquid-filled control chamber (13) is associated with means for controlled depletion of the liquid therein whereby depletion of the volume of liquid in the control chamber enables the pressure-generation means to drive the drug in the reservoir chamber therefrom for infusion thereof.
Owner:UNITED THERAPEUTICS CORP
Expandable medical device with beneficial agent matrix formed by a multi solvent system
A multi solvent drug delivery matrix formation method is used to place layers into a reservoir in a stent in a stepwise manner to achieve extended delivery of water soluble, sensitive, or difficult to deliver drugs. The multi solvent matrix formation method allows the formation of a drug reservoir with a layered morphology in which the mixing between layers is limited to allow the different layers to perform different functions in controlling drug delivery. A stent having a drug delivery matrix includes a first beneficial agent layer affixed to the stent by depositing a first solution of a first polymer and a first solvent, and a second beneficial agent layer affixed to the first beneficial agent layer by depositing a second solution of a second polymer and a second solvent. The second solvent is selected so that the first polymer is substantially insoluble in the second solvent to prevent degradation of the first polymer during deposition of the second polymer. A therapeutic agent is provided in the first beneficial agent layer or the second beneficial agent layer to form a drug delivery matrix.
Owner:INNOVATIONAL HLDG LLC
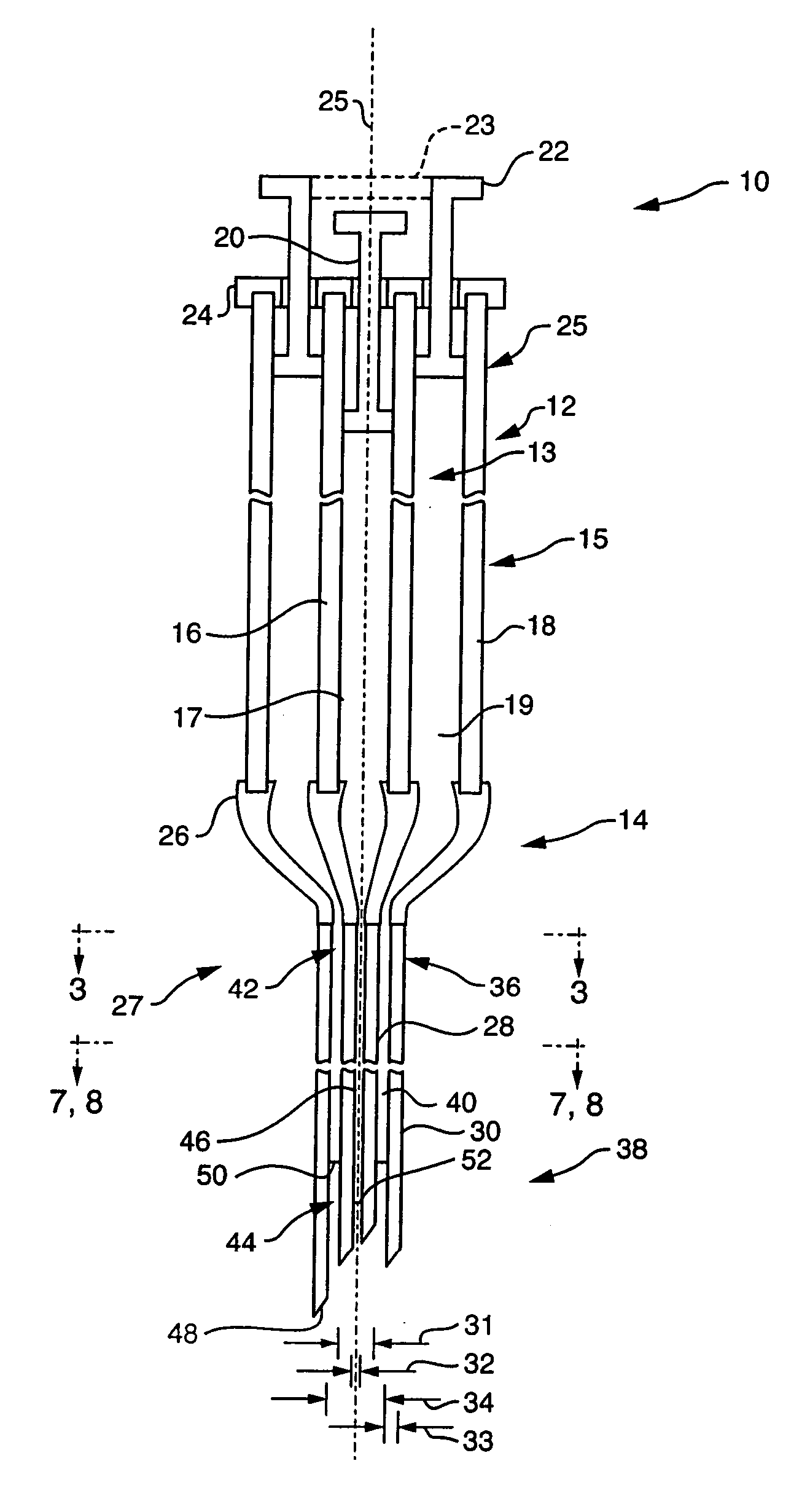
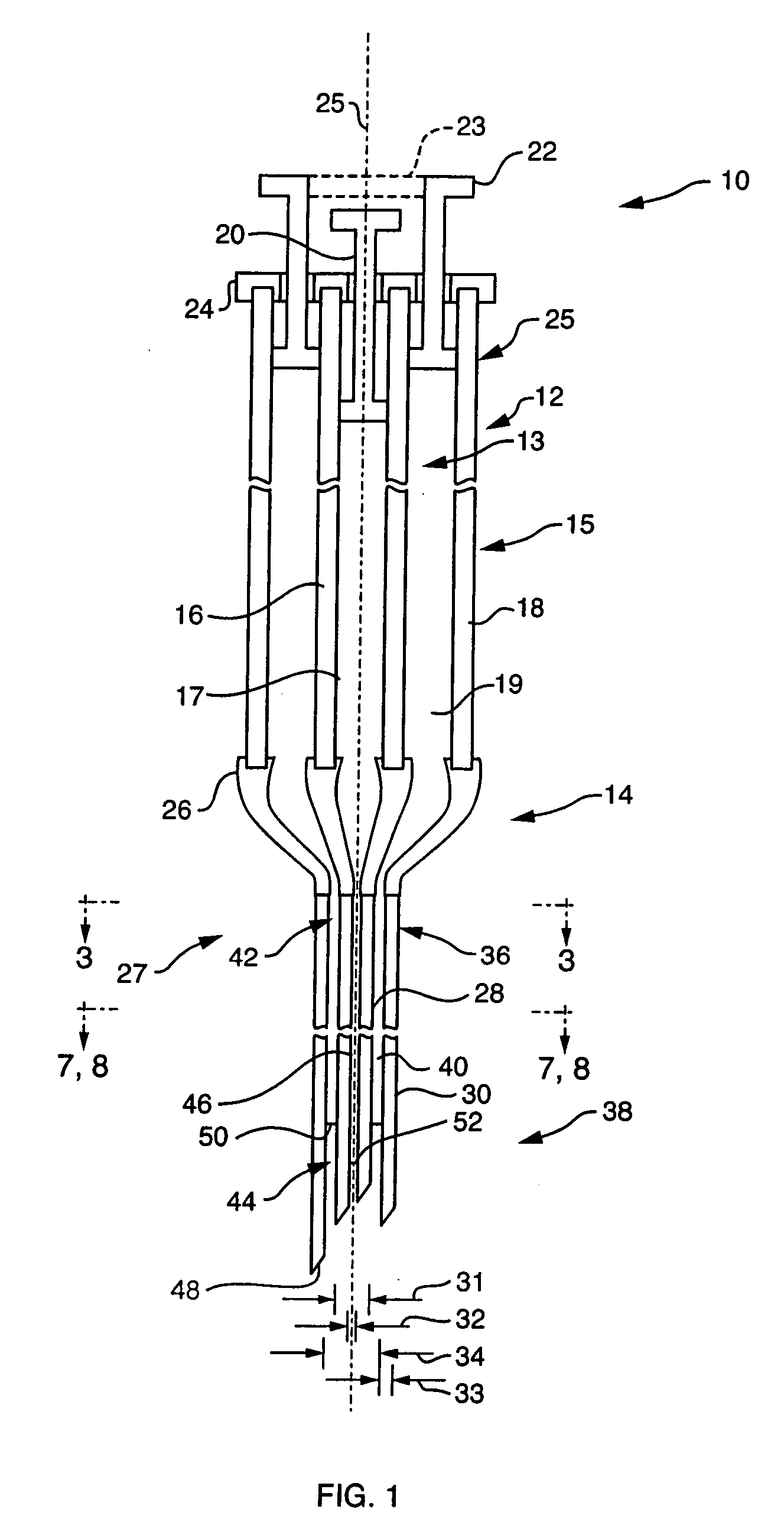
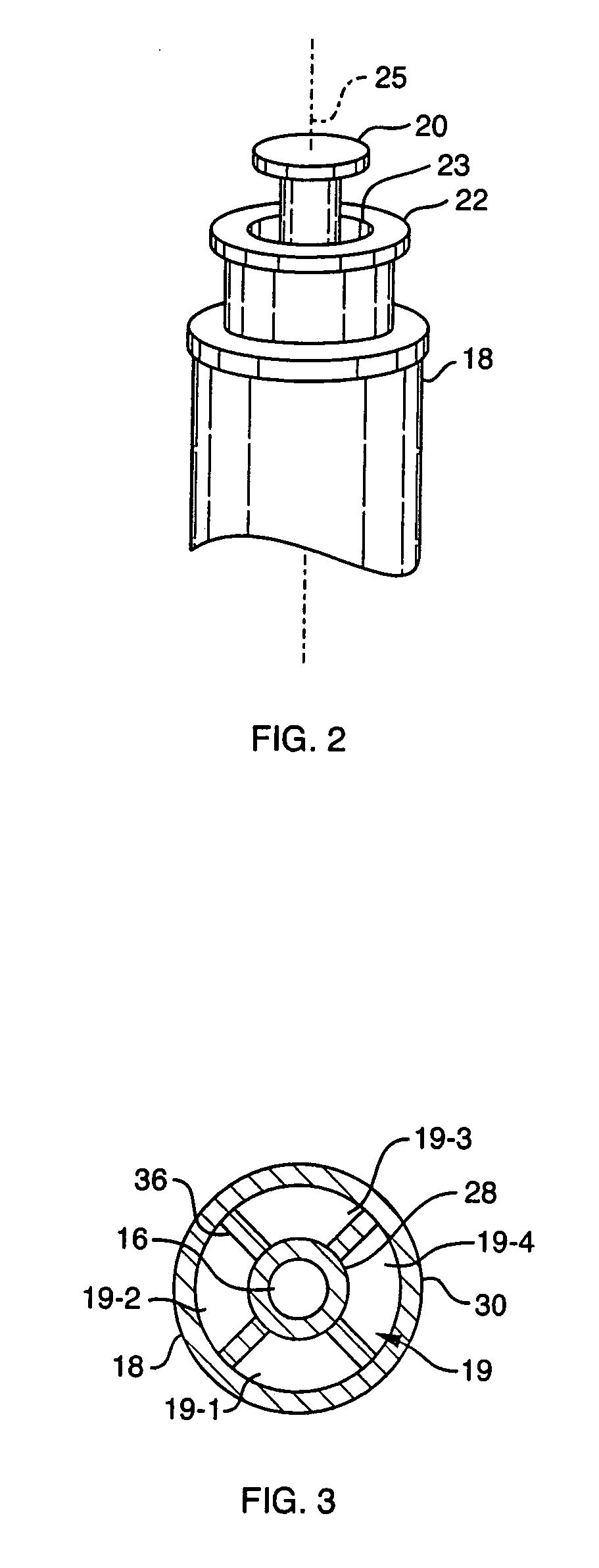
Low-loss multi-lumen injection apparatus
InactiveUS20070073267A1Reduce residual lossInfusion syringesMedical devicesDrug reservoirInjection site
Injection devices, systems and methods are disclosed for injecting two or more medicaments to a patient at a single injection site while preferably minimizing any mixing of the medicaments prior to delivery to the patient. The invention can also be used to sequentially deliver the medicaments to the patient in a repetitive manner. For example, the injection apparatus can sequentially provide a first medicament and then a second medicament to the patient during a first injection procedure. During a second injection procedure, the injection apparatus can again sequentially provide the first medicament and the second medicament to the patient either at the injection site of the first injection procedure or at a different injection site. Multi-lumen manifolds are disclosed for coupling to conventional drug ampoules, to permit the user to sequentially delivery different medicaments via a single skin penetration and to reduce losses associated with usage. Systems including multiple drug reservoirs and filling adaptors are also disclosed.
Owner:MILE CREEK CAPITAL
Method and articles for remote magnetically induced treatment of cancer and other diseases, and method for operating such article
InactiveUS20070196281A1Avoid damagePowder deliveryHeart defibrillatorsAbnormal tissue growthCancer cell
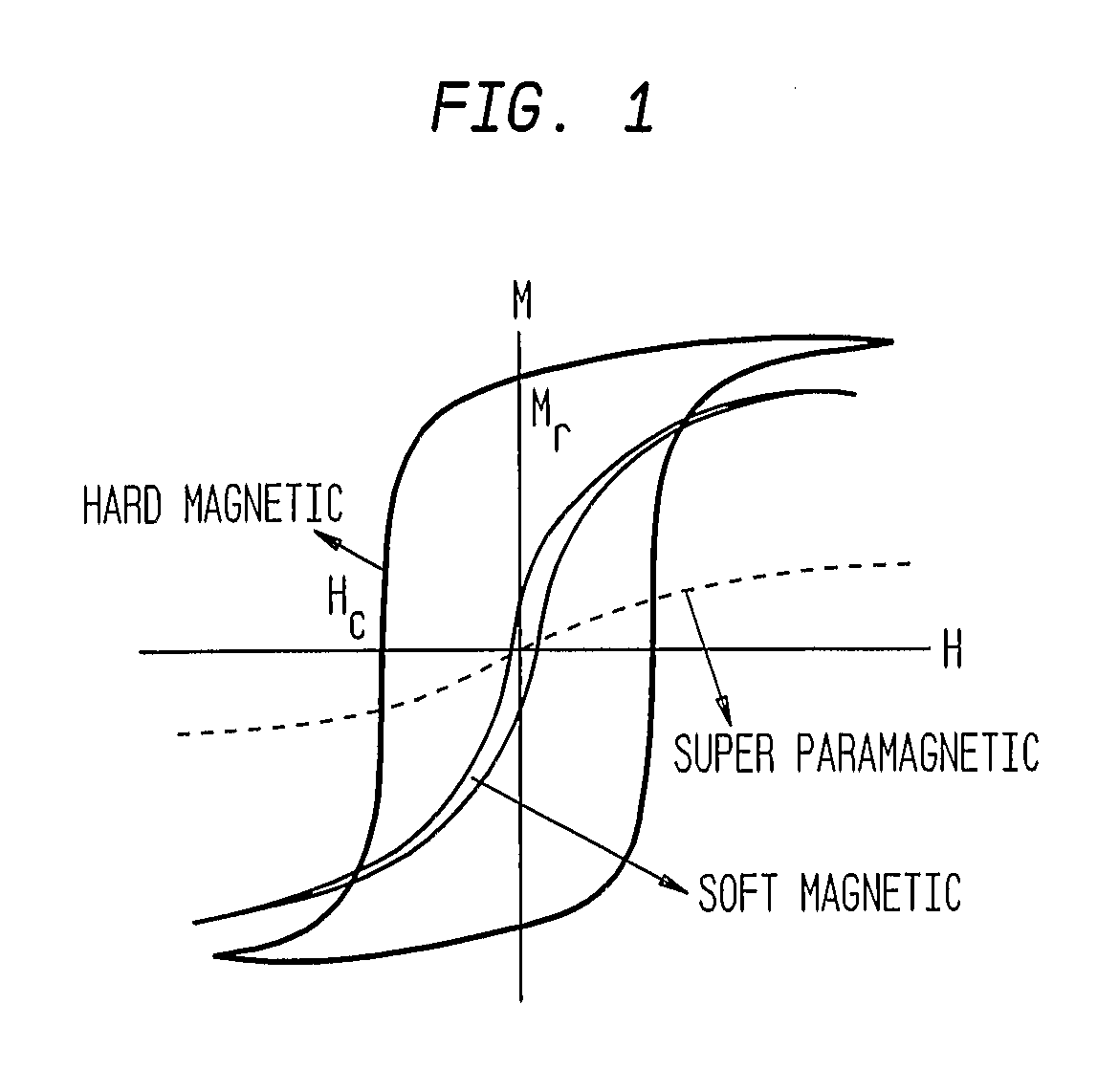
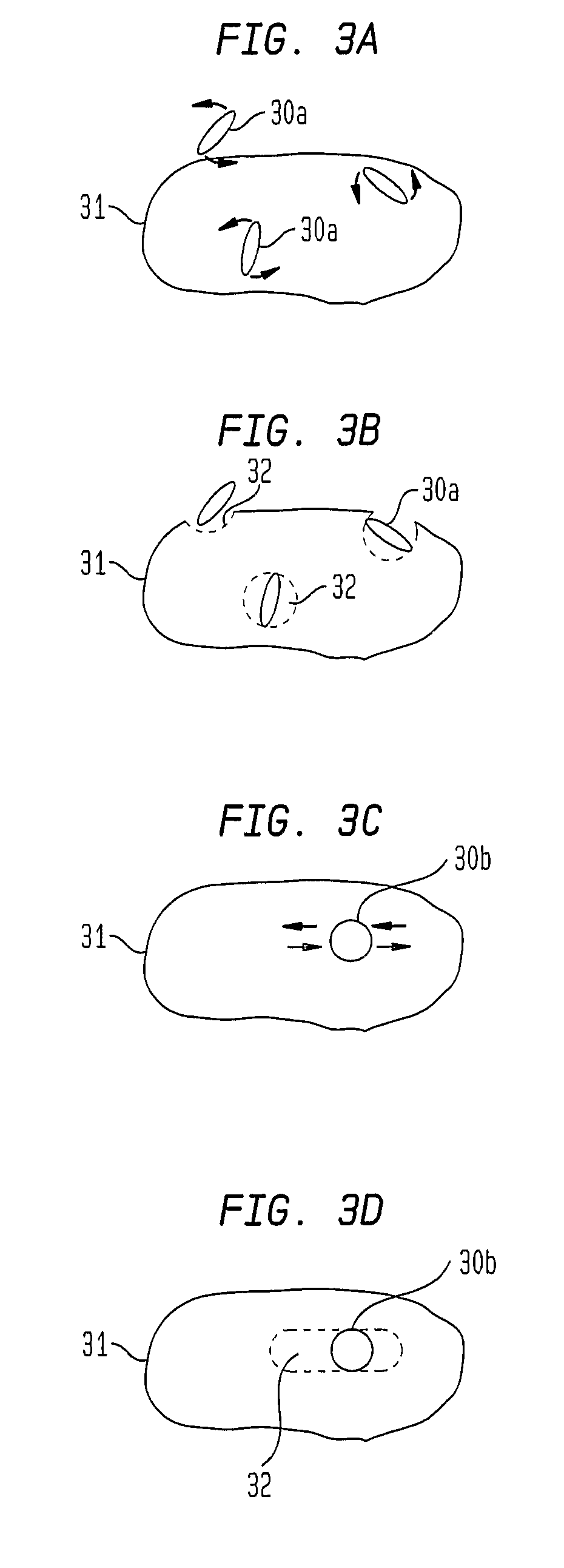
This invention describes unique treatment methods and innovative articles that can be placed in a human or animal body to enable controlled destruction of diseased tissue. The methods include destruction of diseased cells and tissues by magnetically controlled motion and an externally controllable drug delivery process with a capability to start and stop the drug delivery at any time, for any duration. This invention provides two approaches to diseased cell destruction, (1) magneto-mechanical disturbance of cell structure (e.g. cancer cells) for cell lysis and (2) magnetically activated drug release at local regions (e.g. tumors) from a magnetic-particle-containing drug reservoir. The invention also provides combinations of both the above treatments for dual therapy. It further combines one or both of the treatments with magnetic hyperthermia for multifunctional cell destruction therapy. The approaches can be combined with magnetic MRI for monitoring the accuracy of placement as well as for following up the cancer destruction progress and appropriate reprogramming of the magneto-mechanical therapy and remote-controlled drug release.
Owner:RGT UNIV OF CALIFORNIA
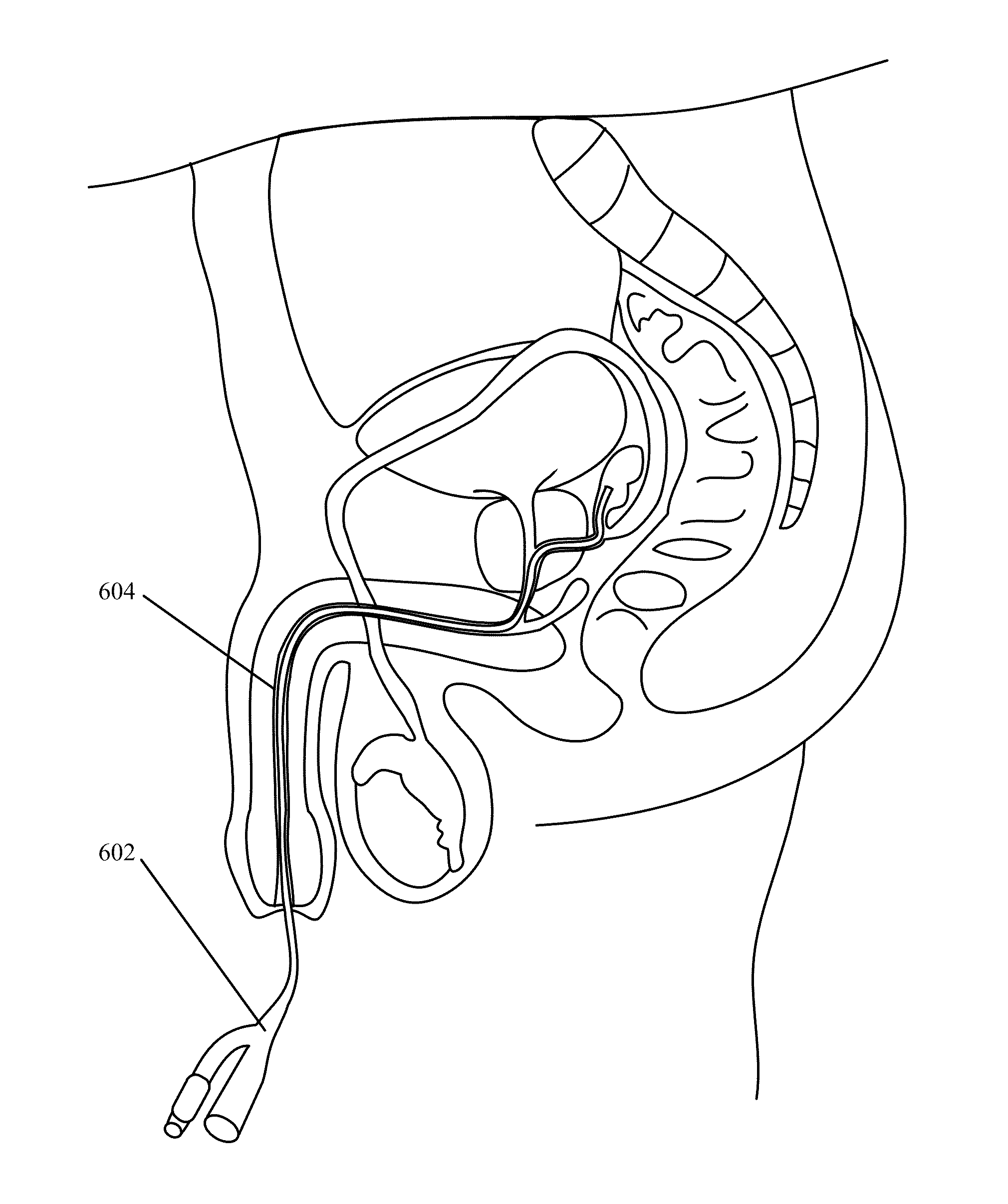
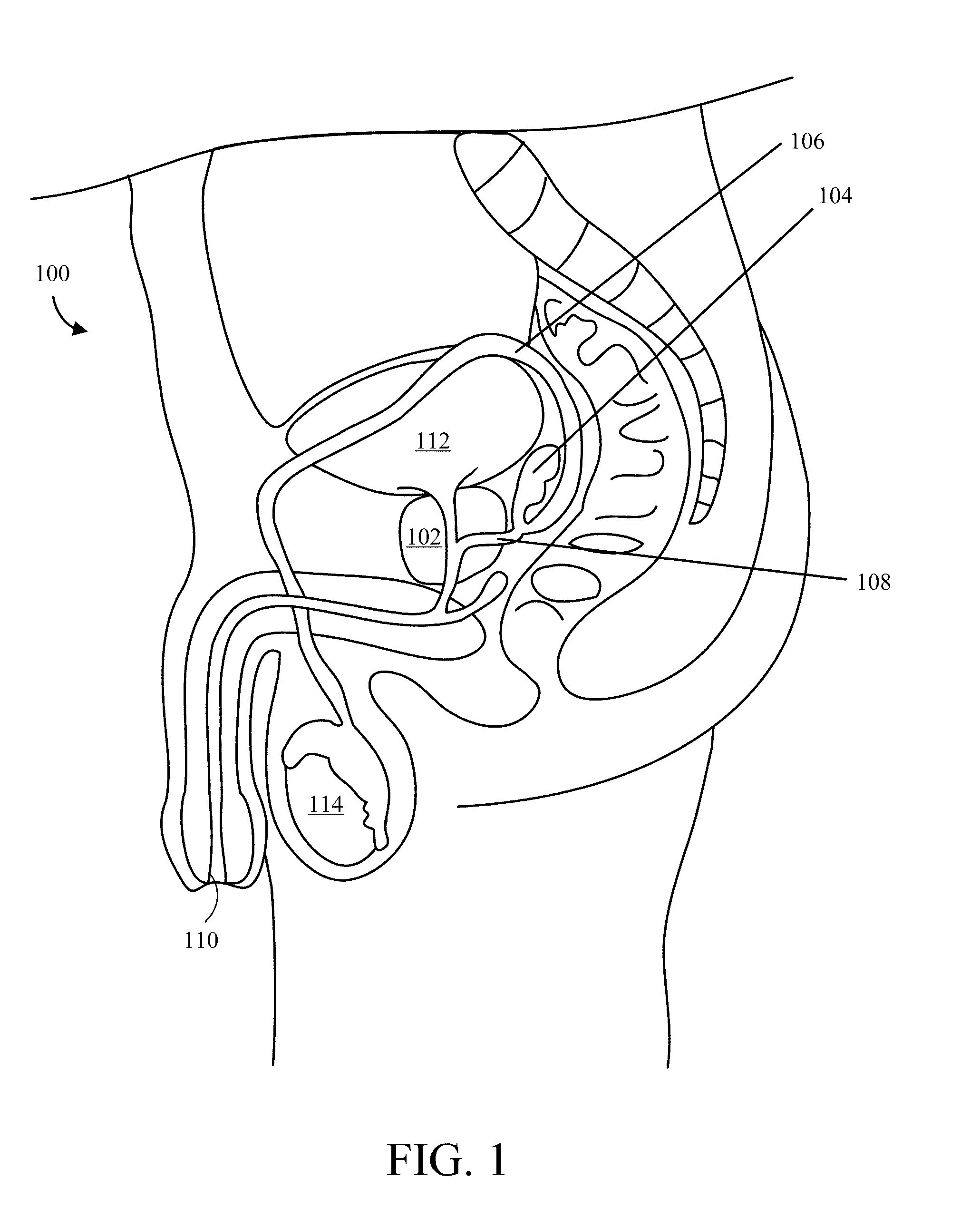
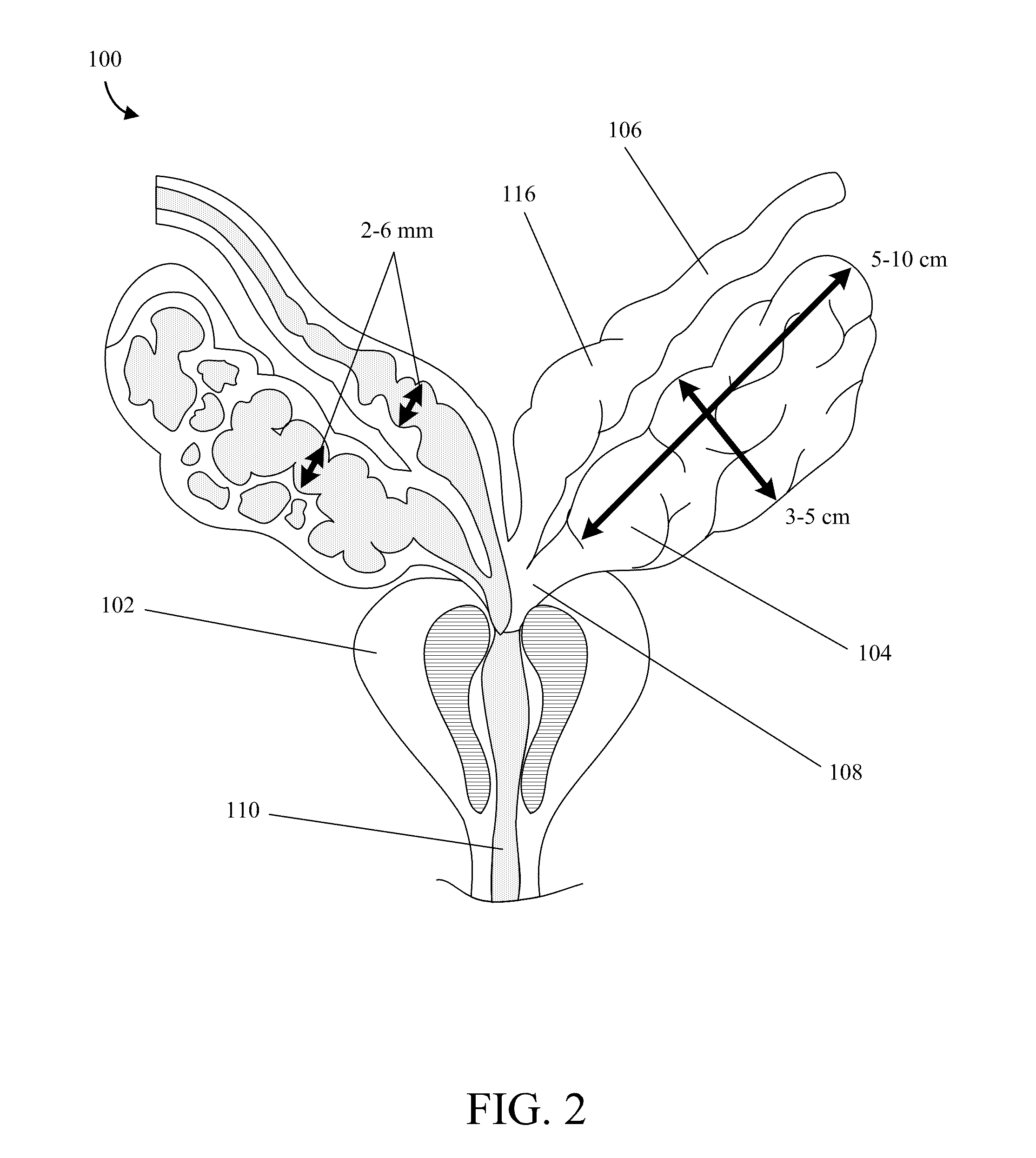
Implantable Drug Delivery Device and Methods of Treating Male Genitourinary and Surrounding Tissues
A method is provided for local controlled delivery of a drug to the seminal vesicle, the prostate, the ejaculatory duct, or the vas deferens of a patient in need of treatment. In one embodiment, the method includes implanting a resorbable drug delivery device within the seminal vesicle, the prostate, the ejaculatory duct, or the vas deferens of the patient. The drug delivery device may include an elastic device body housing at least one drug reservoir which contains at least one drug. In a preferred embodiment, the method further includes releasing the drug from the device in a controlled manner to, typically directly to, the seminal vesicle, the prostate, the ejaculatory duct, or the vas deferens.
Owner:MASSACHUSETTS INST OF TECH +1
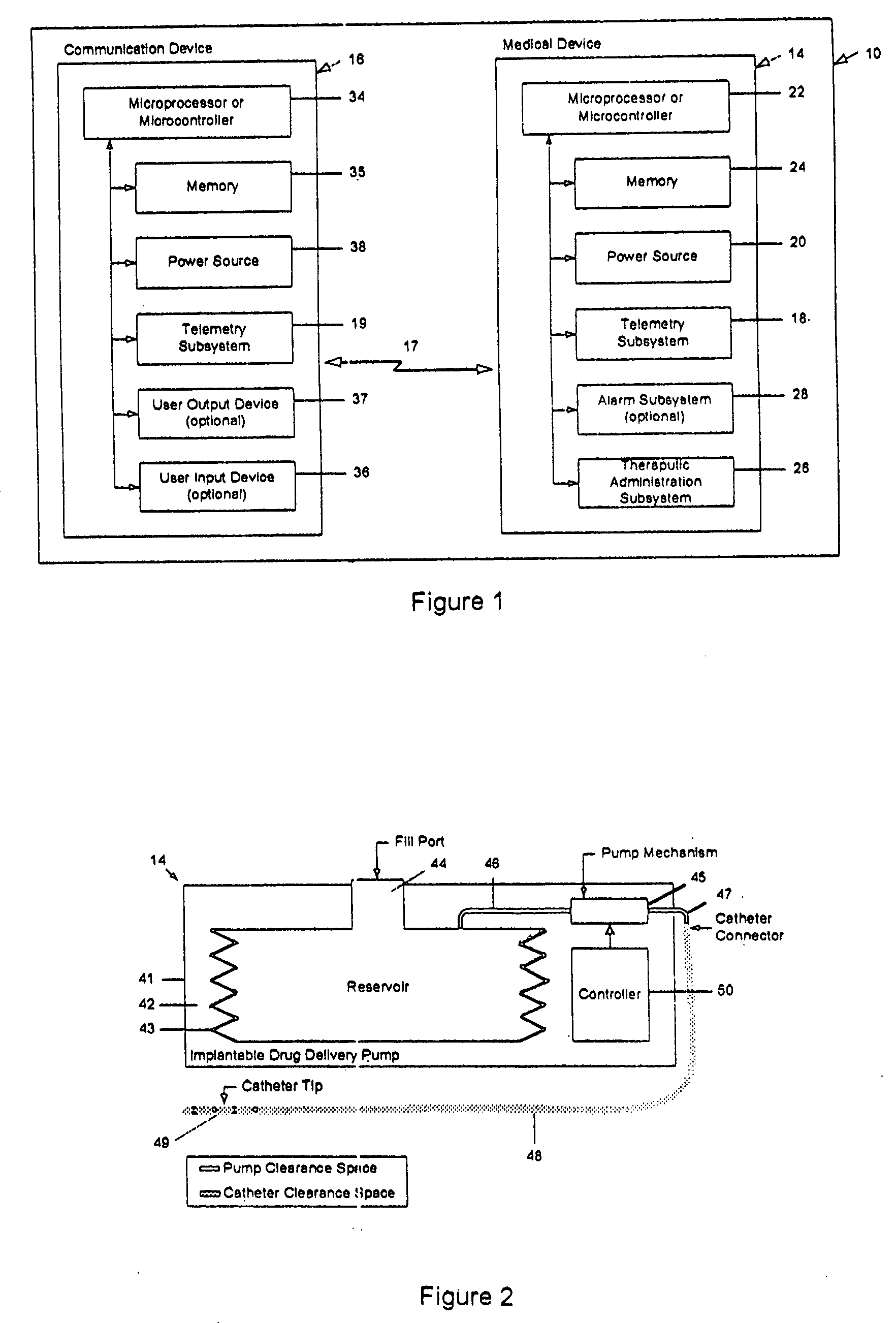
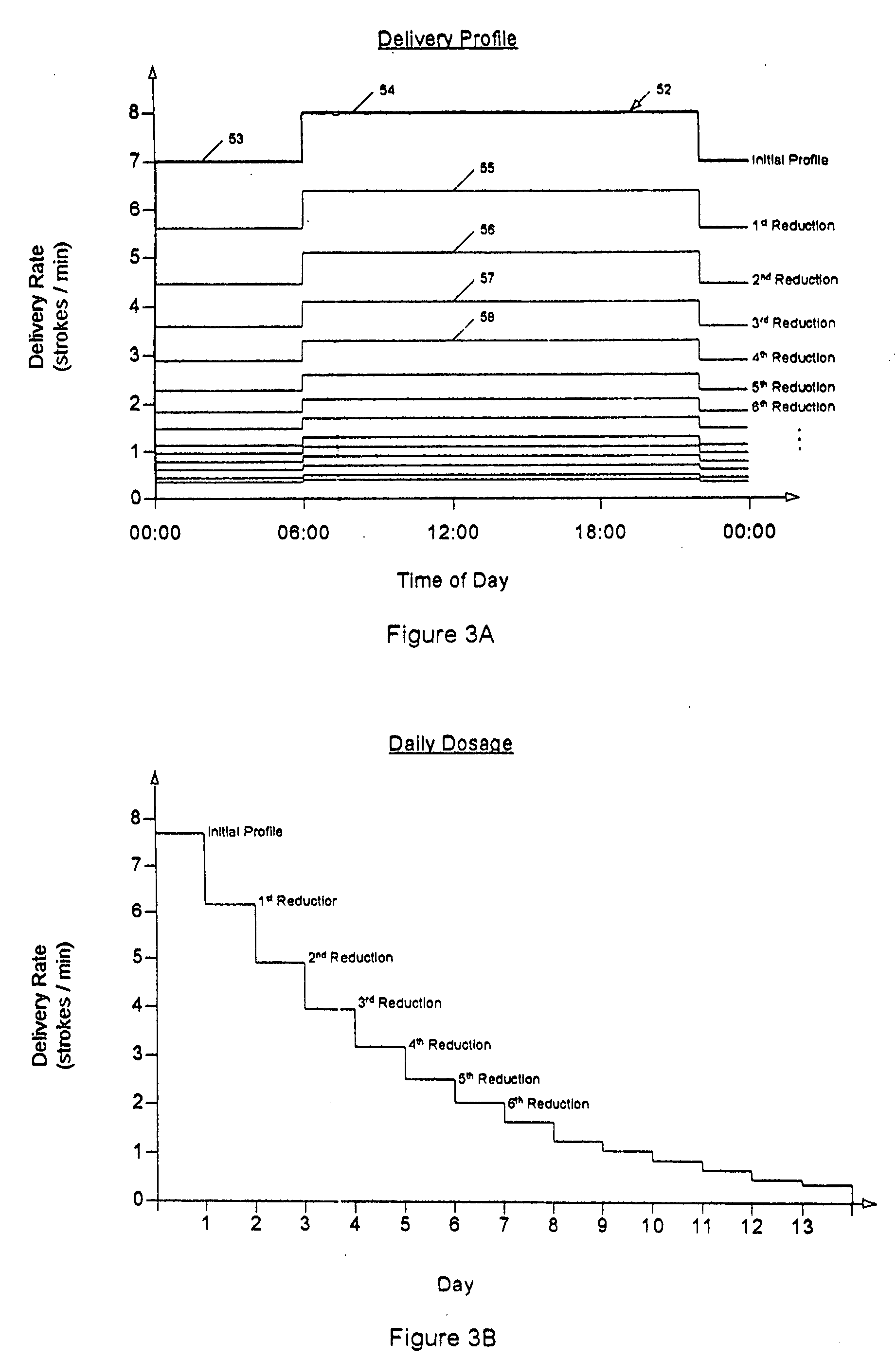
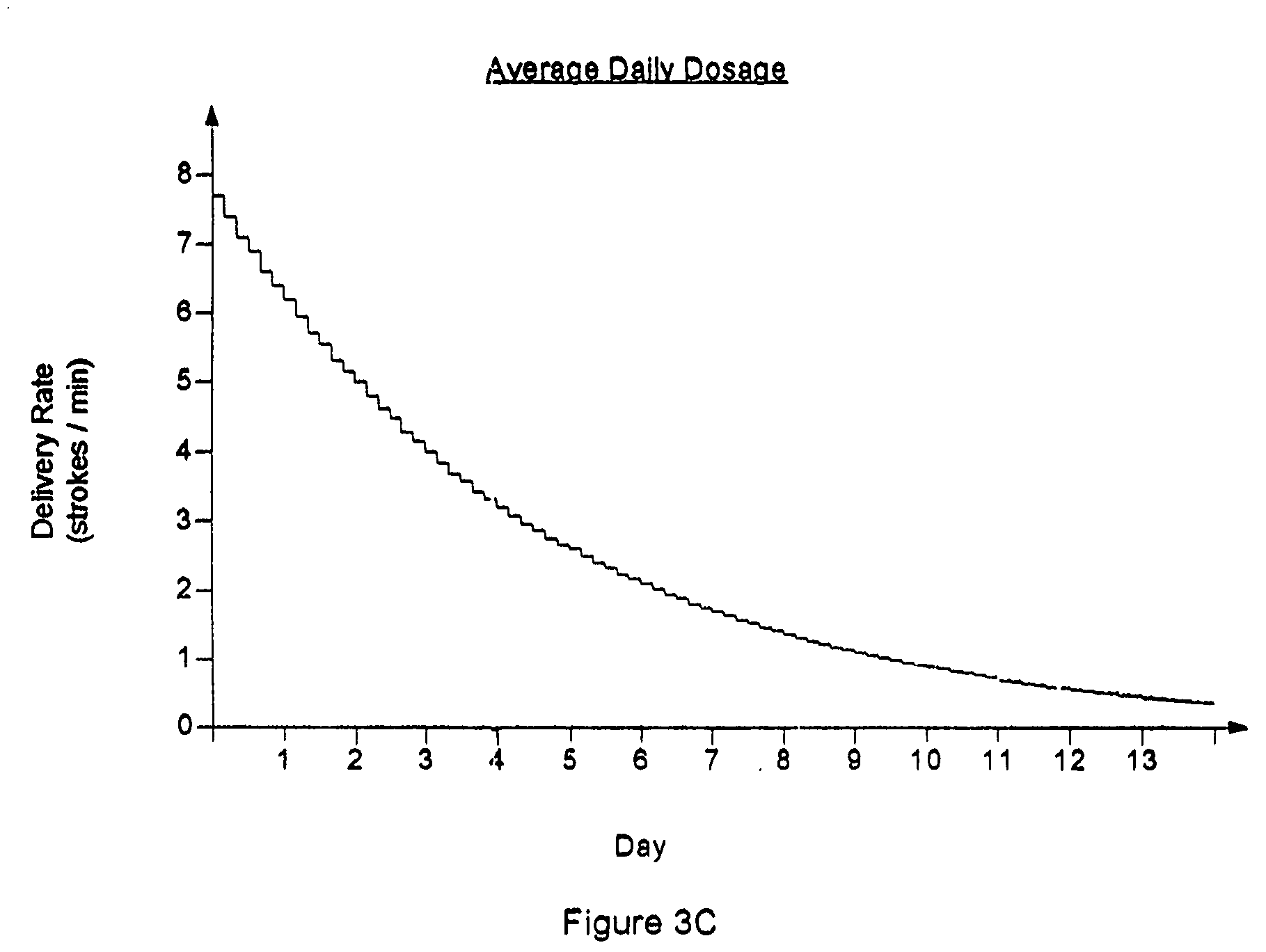
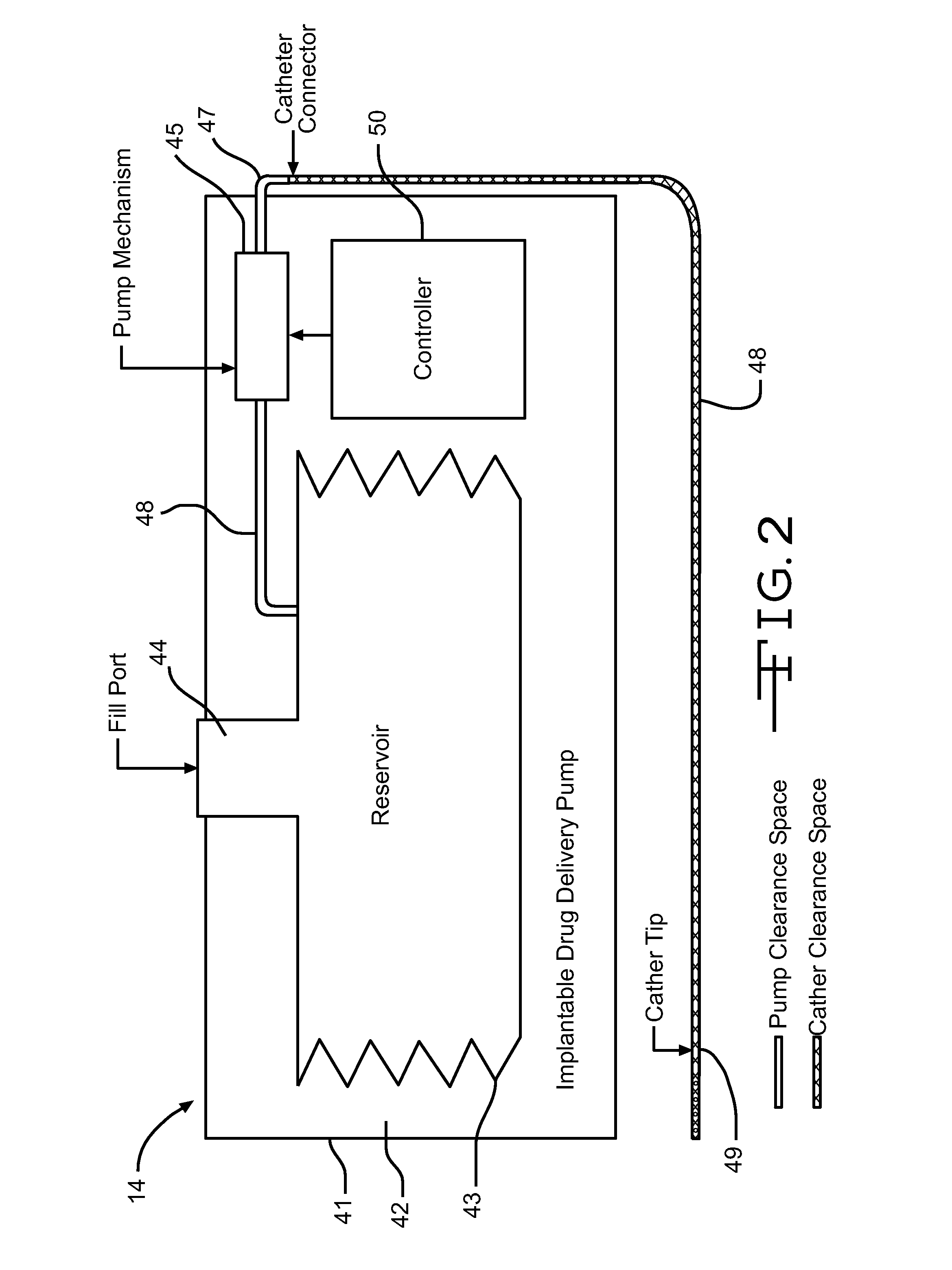
Drug delivery apparatus and method for automatically reducing drug dosage
InactiveUS20060047270A1Reduce probabilityMedical devicesPressure infusionMicrocontrollerDrug reservoir
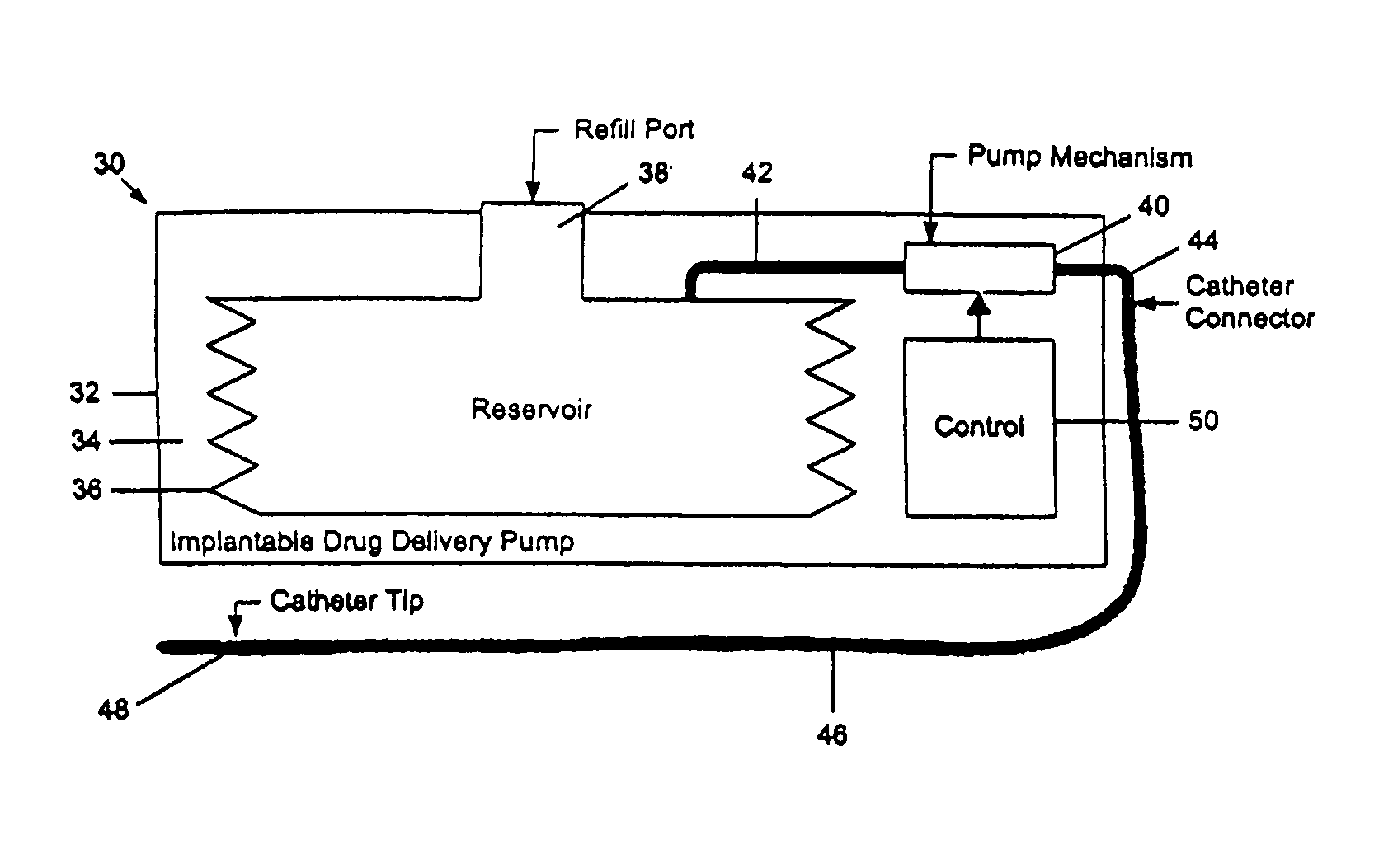
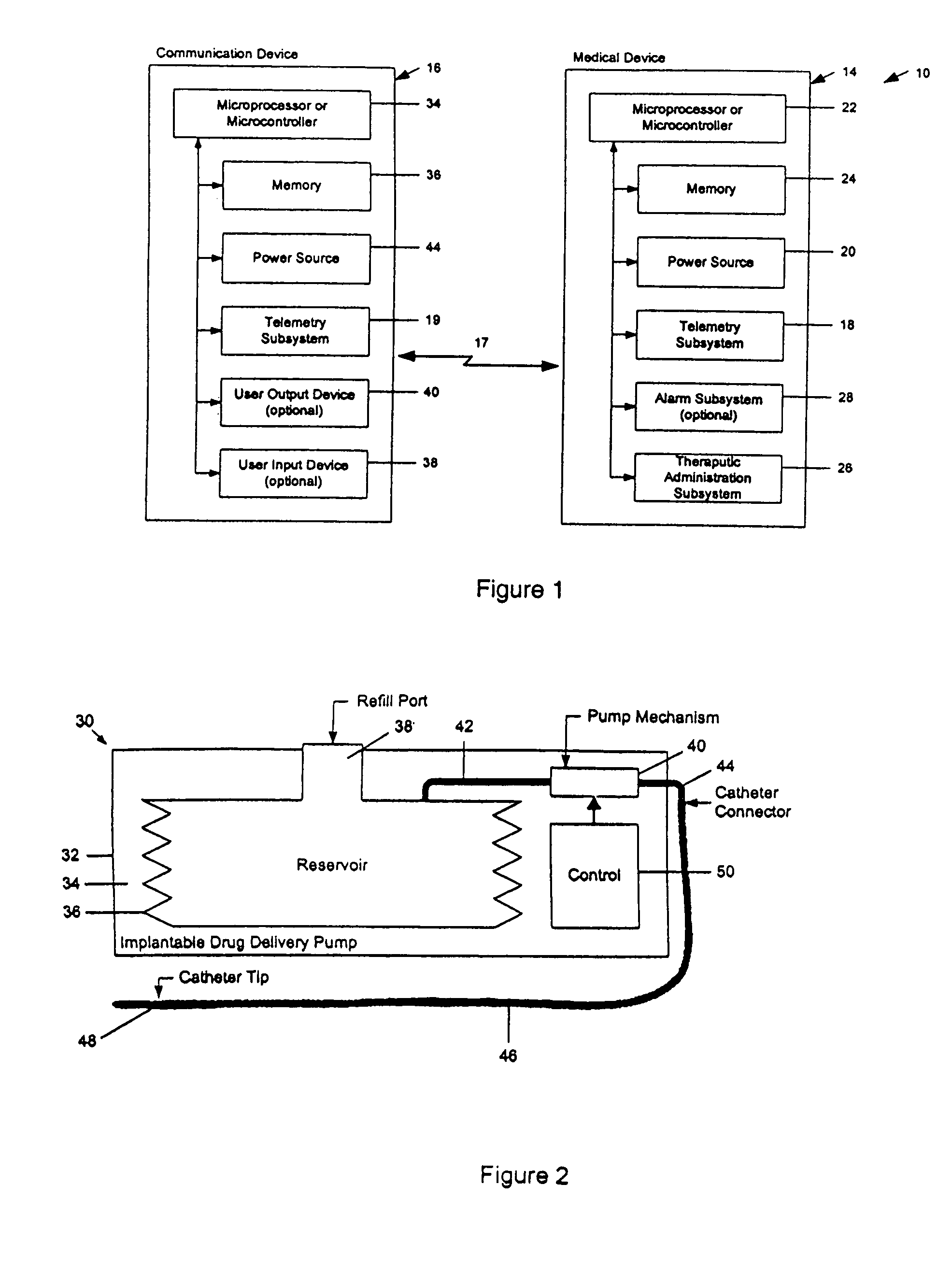
A drug delivery device which includes a fluid drug reservoir, a catheter, a controllable fluid transfer device, e.g., a pump mechanism or valve, and a drug delivery control means. The drug delivery control means comprises a controller, e.g., a microprocessor or microcontroller which is operable to automatically reduce the rate of drug delivery over a certain reduction interval (e.g., multiple days) from an initial dosage value to a final dosage value.
Owner:INFUSION SYST
Methods, devices, and kits for microjet drug delivery
ActiveUS20080091139A1Reduce leakageEnhance nozzle contactJet injection syringesIntravenous devicesDrug reservoirEngineering
Described here are methods, devices, and kits for microjet drug delivery. The devices described here may be modular or non-modular. The modular devices typically include a first module having a drug reservoir and a nozzle in fluid communication with the drug reservoir and a second module having an actuator and a power supply. The power supply provides power to the actuator and when the first and second modules are coupled, the actuator is capable of acting on a dispensing member causing it to dispense a drug in liquid form from the drug reservoir via the nozzle at a velocity sufficient to penetrate skin. Other devices described include a nozzle, a reservoir in fluid communication with the nozzle, a dispensing member, and an actuator. In these devices, the nozzle has at least one feature that enhances nozzle contact with the skin in order to reduce lateral drug leakage about the nozzle.
Owner:CORIUM PHARMA SOLUTIONS INC
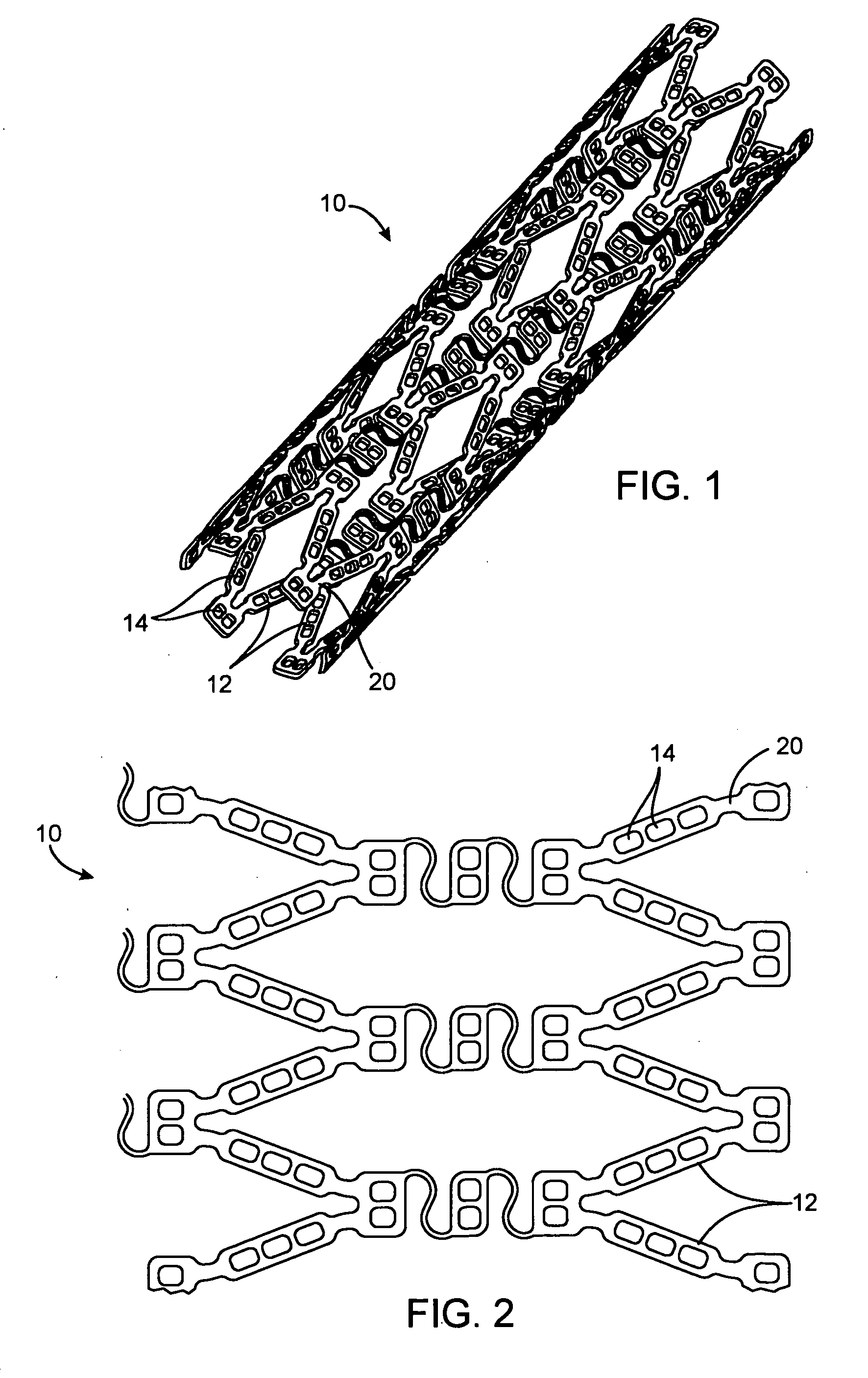
Stent with drug-delivery system
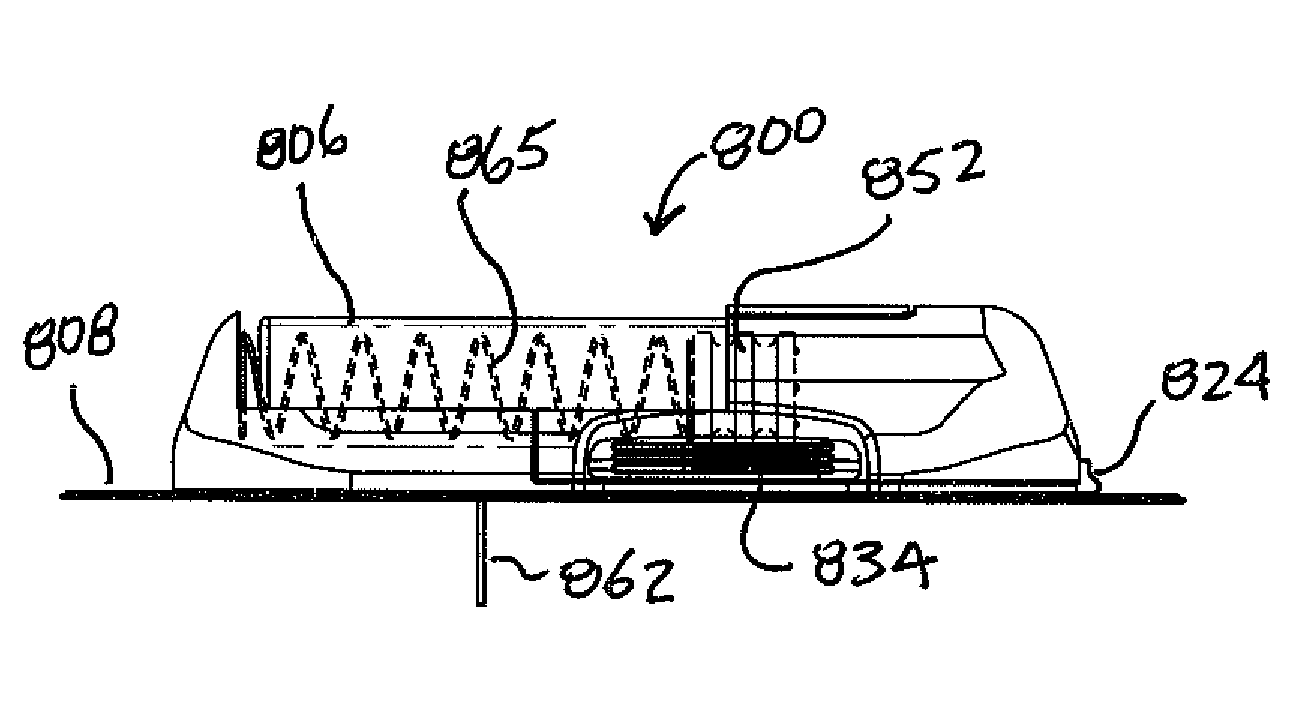
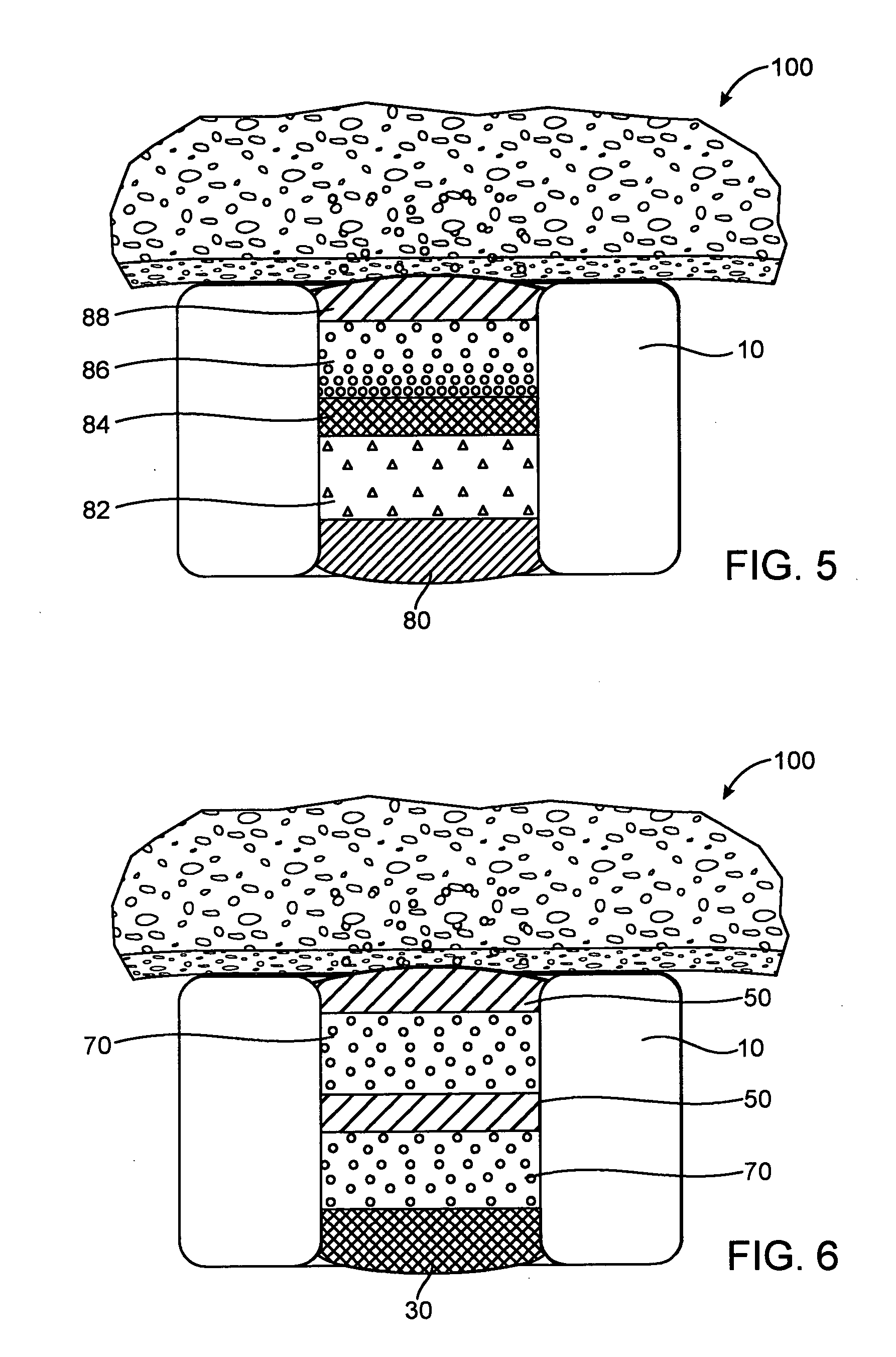
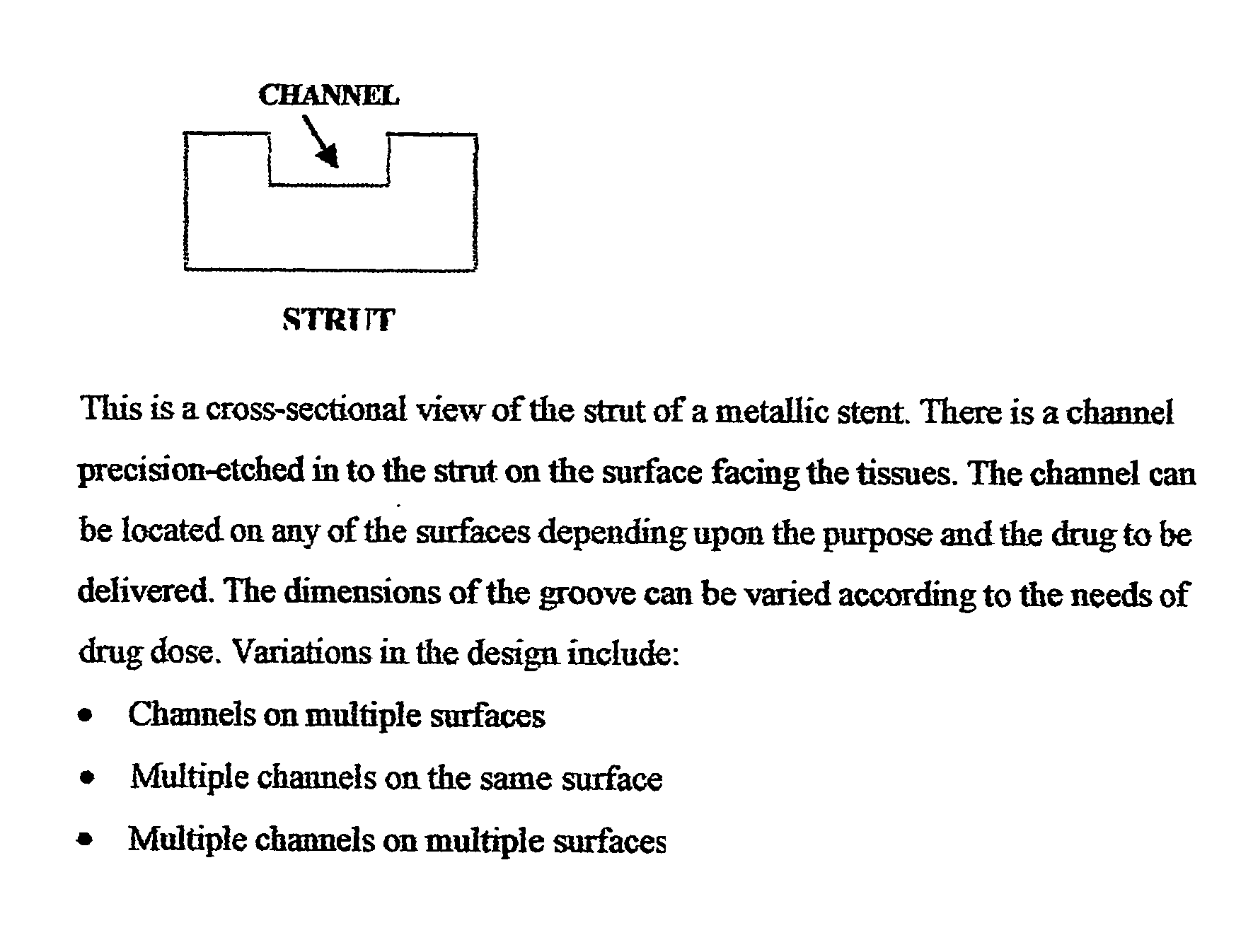
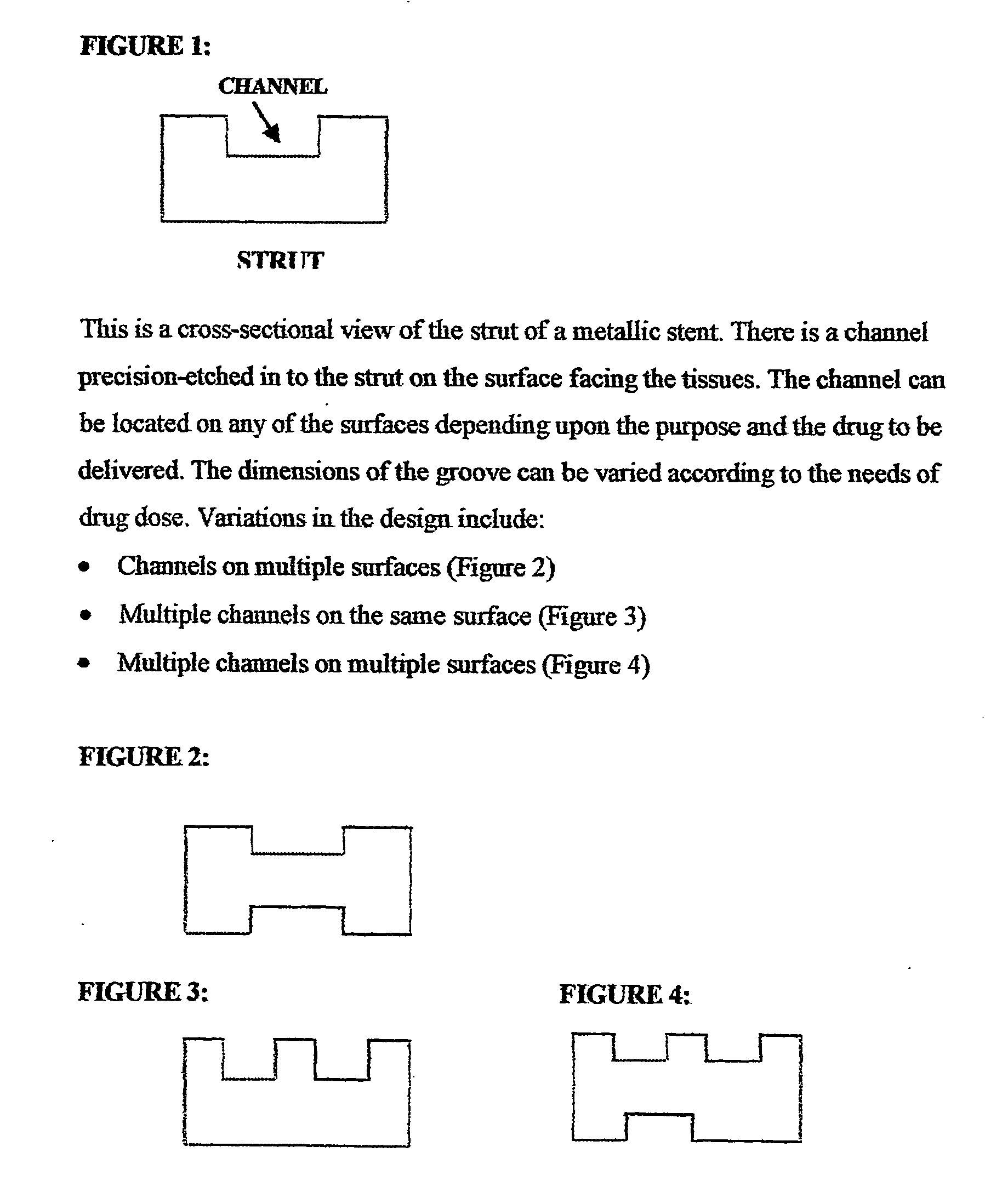
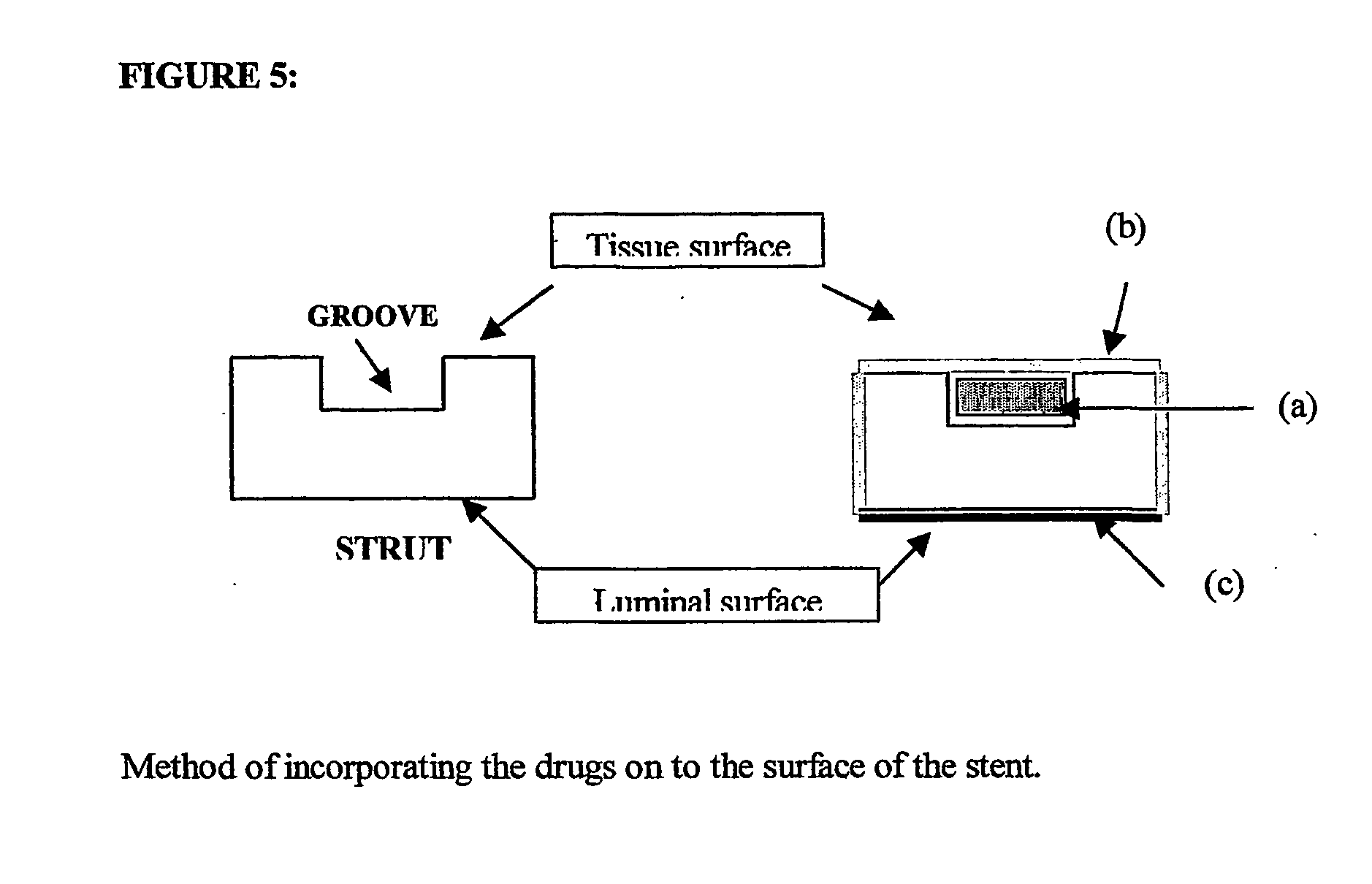
A unique design of the stent that incorporates a drug-reservoir running along the stent struts. One or more drugs can be incorporated within the reservoir. Additionally other drugs can be coated on the surface of the strut enclosing the reservoir drug to facilitate sequential release of drugs. Thus, the design incorporating sequential release of multiple drugs facilitates to tackle the sequential complex biologic processes involved in renarrowing following stent implantation. The design also ensures that the drug present in the reservoir is released exclusively into the adjacent tissue without getting washed away into the blood flowing within the lumen.
Owner:BADARI NAYANAN NAGARADA GADDE +1
Implantable device with reservoirs for increased drug loading
A method of manufacturing a drug loaded stent includes applying a photo resistant coating to at least a portion of a stent framework and removing at least a portion of the photo resistant coating from the stent framework. The method further includes applying an etchant to at least a portion of the stent framework and forming a plurality undercut drug reservoirs in the stent framework based on applying the etchant. A stent for treating a vascular condition includes a stent framework, a plurality of undercut reservoirs formed within the stent framework and a therapeutic agent disposed within at least a portion of the plurality of undercut reservoirs.
Owner:MEDTRONIC VASCULAR INC
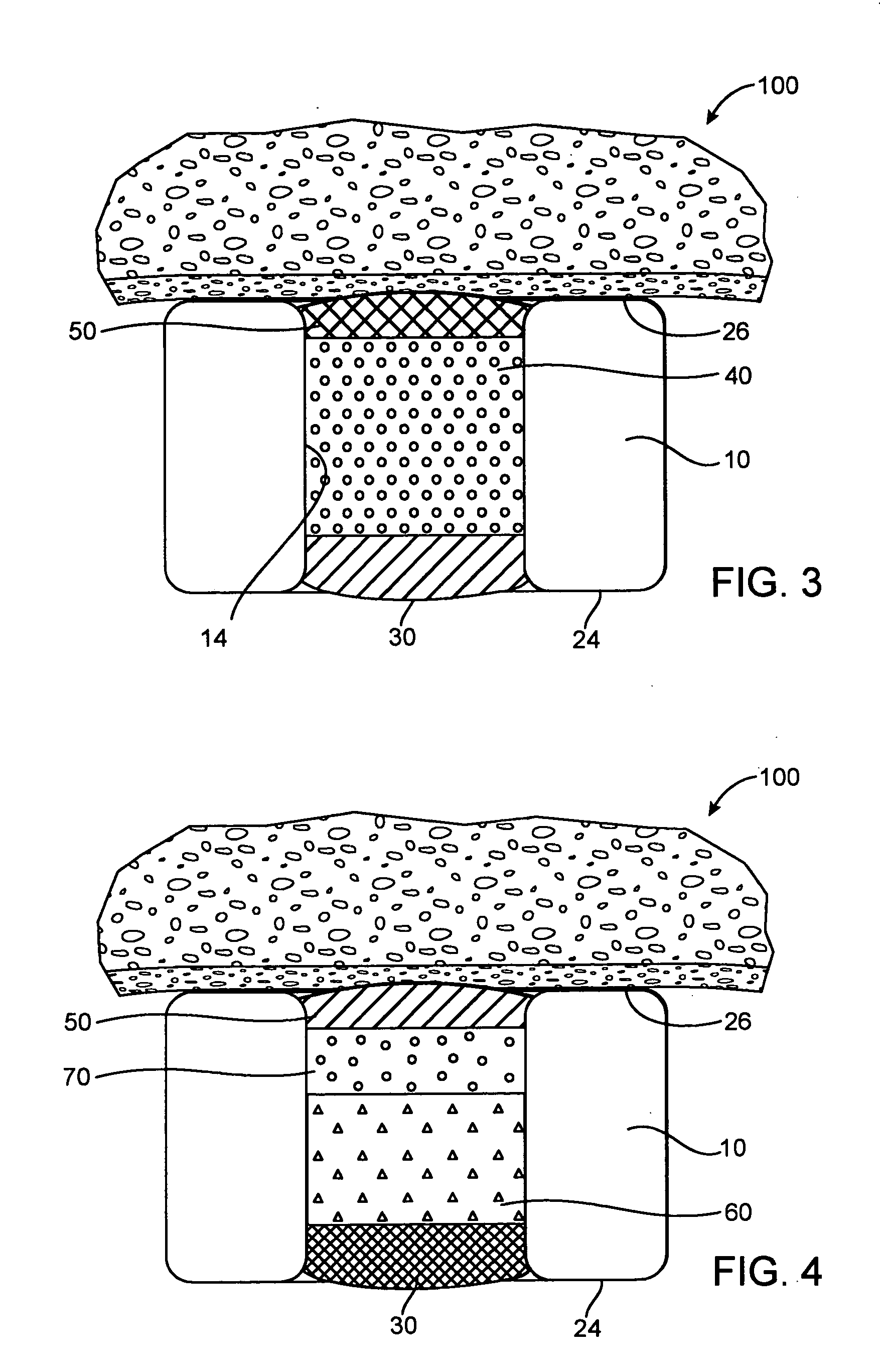
Medical Device Having Coating With Zeolite Drug Reservoirs
A medical device having a drug-eluting coating that includes a pharmaceutical compound or, more generally, a therapeutic material housed within pores of a zeolite carrier. The zeolite carrier has an open porous structure with reservoirs for holding the therapeutic material. The therapeutic material loaded zeolites may be suspended or dispersed within a bioerodible polymer matrix to provide controlled delivery of the therapeutic material. Zeolite drug carriers may have enhanced or optimally engineered pore sizes for a particular therapeutic material and release profile. Along with a therapeutic material, reservoirs of a zeolite drug delivery system may include a release agent. The release agent may be used to entrap the therapeutic material until such time as a triggering condition is met that prompts the release agent to activate and thereby release the therapeutic material from the zeolite reservoir.
Owner:MEDTRONIC VASCULAR INC
Drug delivery system
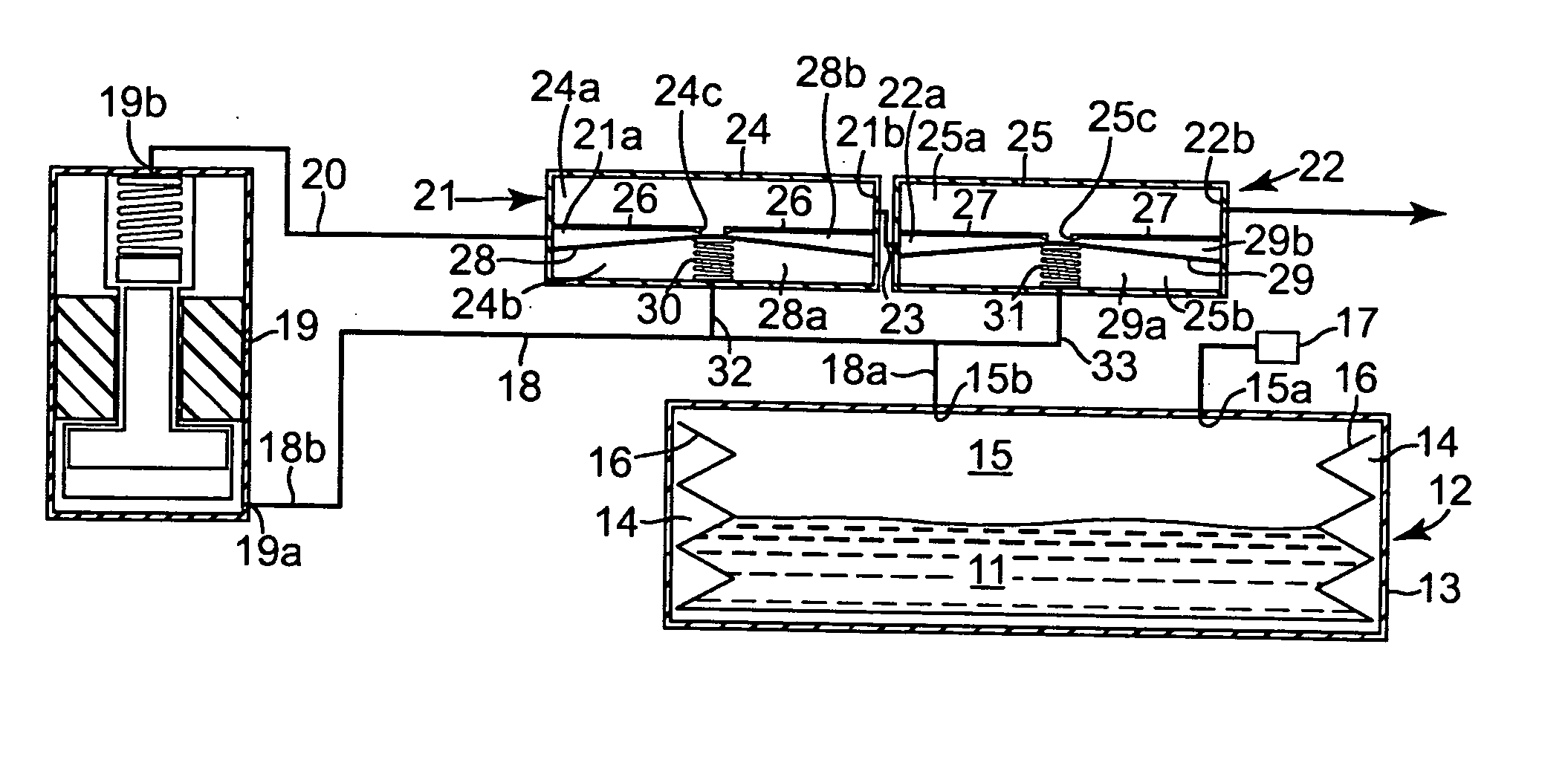
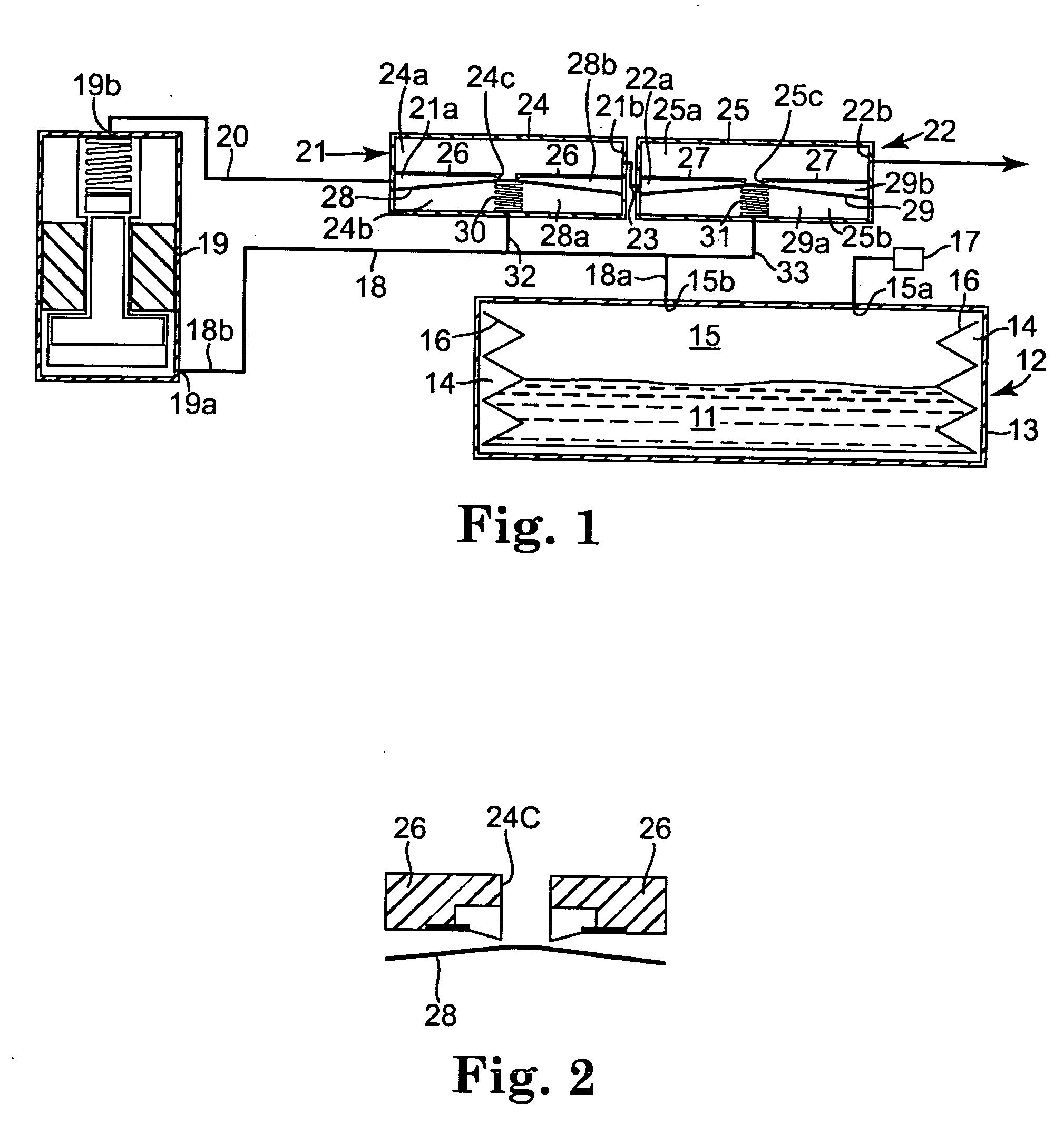
ActiveUS20050273082A1Avoid flowPharmaceutical delivery mechanismMedical devicesDrug reservoirPositive pressure
A drug delivery system (10) has a positive pressure drug reservoir (15). A pump (19) is in fluid communication with the reservoir (15) via a first fluid passageway (18). The outlet (19b) of the pump (19) is in fluid communication with a first regulator (21). The regulator (21) includes a housing (24) that has a first subchamber (24a) and a second subchamber (24b). The second subchamber (24b) is divided into a lower chamber (28a) and a middle chamber (28b) by a diaphragm (28). A third fluid passageway (32) is positioned between the lower chamber (28a) and the drug reservoir (15), whereby accidental flow from the reservoir (15) to an outlet (21b) is prevented.
Owner:MEDTRONIC INC
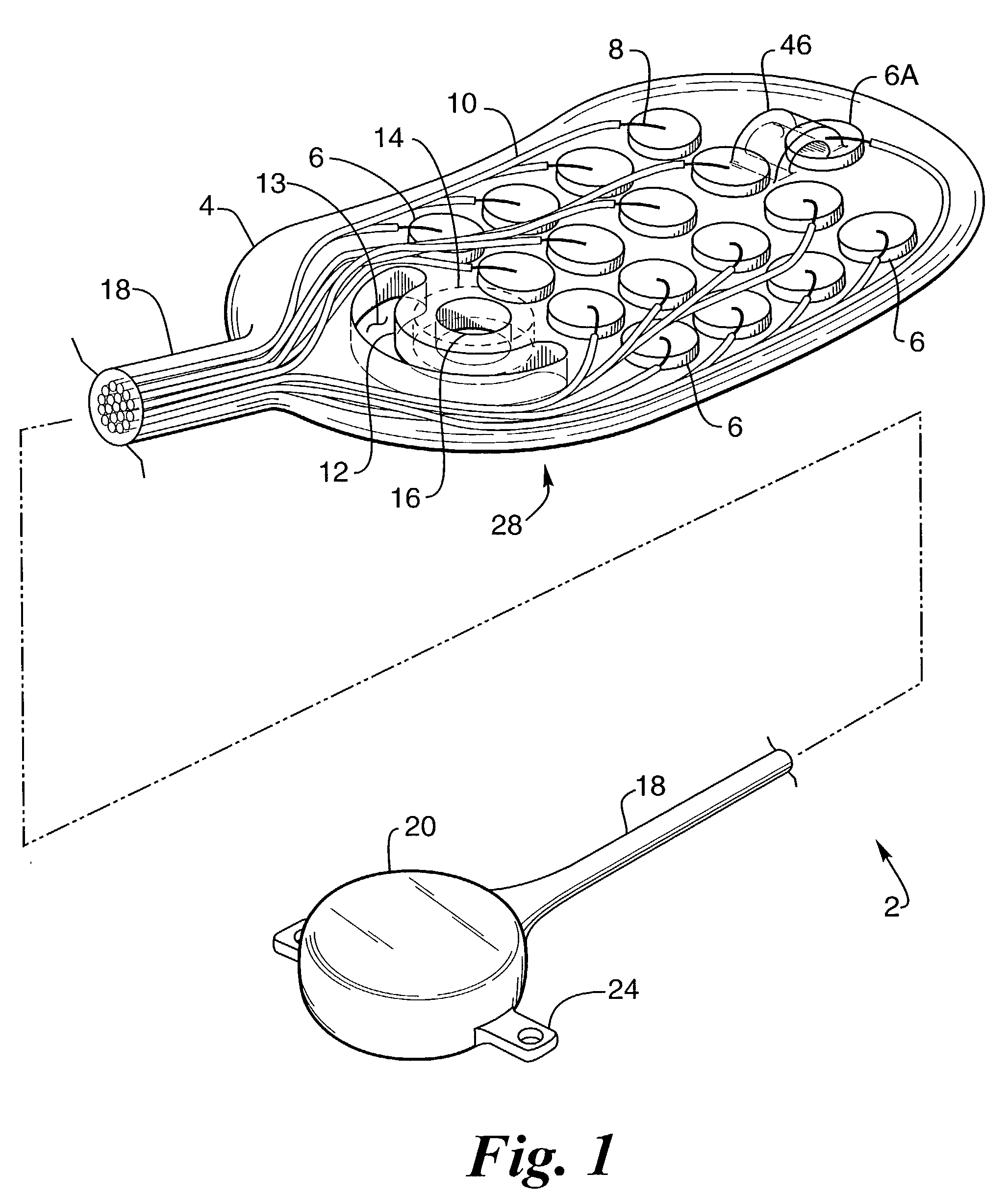
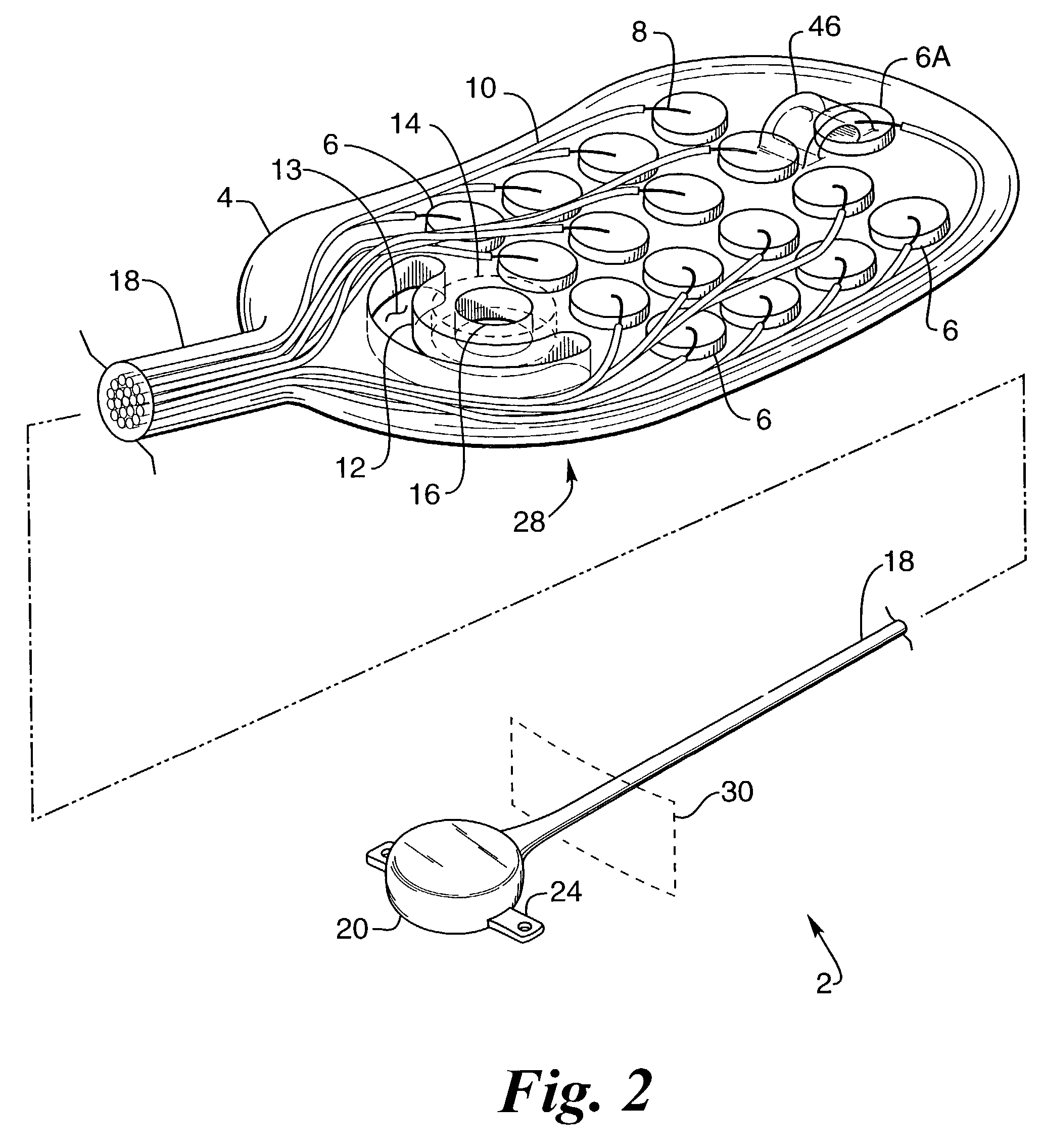
Flexible and Conformal Patch Pump
Provided is a flexible and conformal wearable, self-contained medical device. The medical device comprises an integral housing formed by a flexible upper portion and a flexible lower portion joined along their perimeters. The medical device is also provided in a plurality of shapes and configurations for increasing the flexibility and conformability of the housing. The components contained within the housing, such as a drug reservoir, printed circuit board, and power supply are preferably constructed from flexible materials and are formed, connected and positioned according to the configuration of the housing in a manner for enhancing flexibility of the housing. A thermal bubble micropump is provided for controlling flow of a drug from the flexible reservoir, that utilizes a thermal resistor provided locally to a thermal expansion fluid that causes a surrounding membrane to expand and displace a volume of drug to be provided to the user.
Owner:BECTON DICKINSON & CO
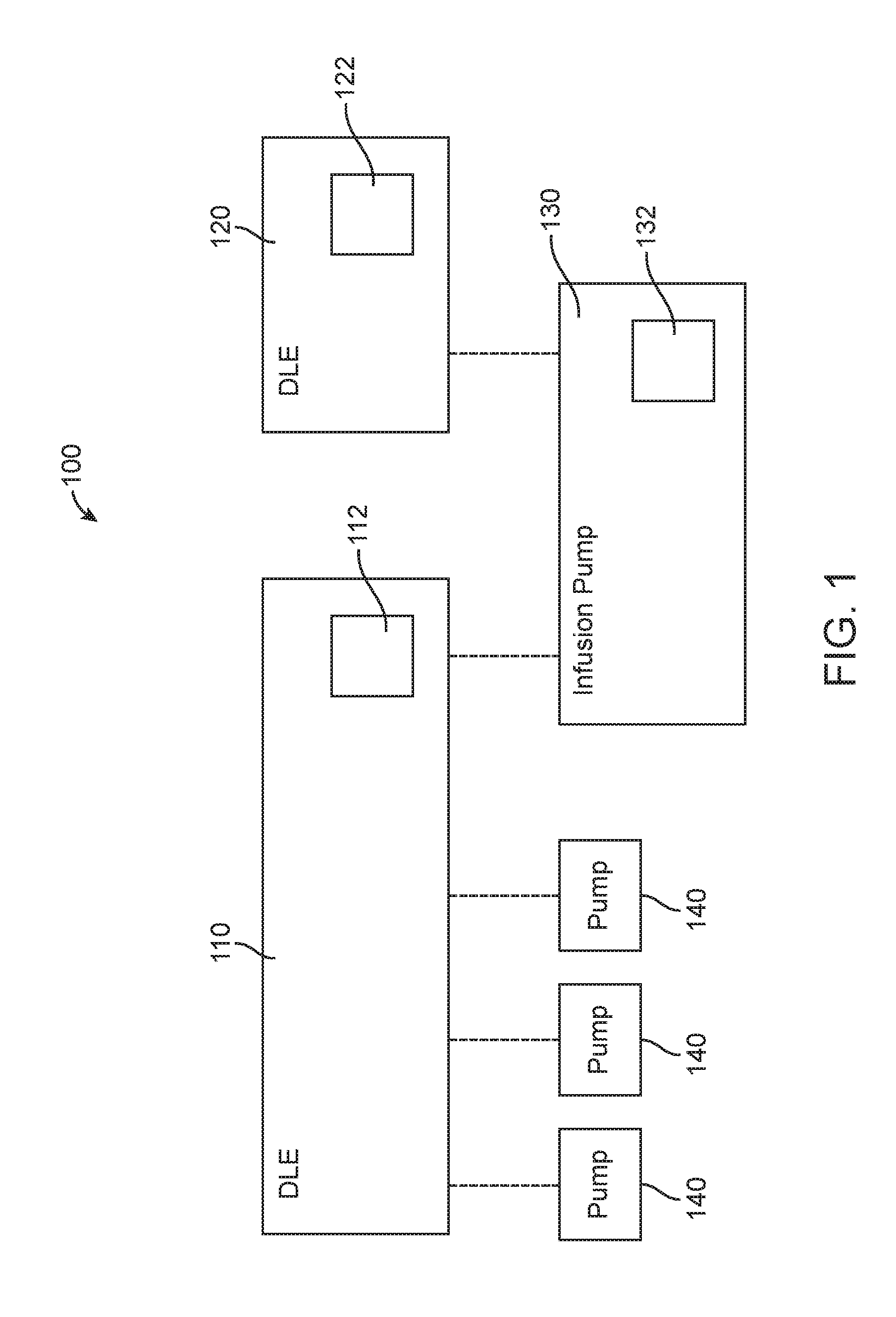
Infusion pump system and method with multiple drug library editor source capability
An infusion pump system and method with multiple drug library editor source capability including: an infusion pump system having a first drug library editor operable to generate a first drug library; a second drug library editor operable to generate a second drug library; and an infusion pump operable to connect to either one of the first drug library editor and the second drug library editor, the infusion pump having an operational drug library being one of the first drug library received from the first drug library editor and the second drug library received from the second drug library editor. The first drug library editor is one of a dedicated drug library editor and an enterprise drug library editor, and the second drug library editor is the other of the one of the dedicated drug library editor and the enterprise drug library editor.
Owner:ICU MEDICAL INC
Method and apparatus for automatically modifying delivery profile of drug delivery system
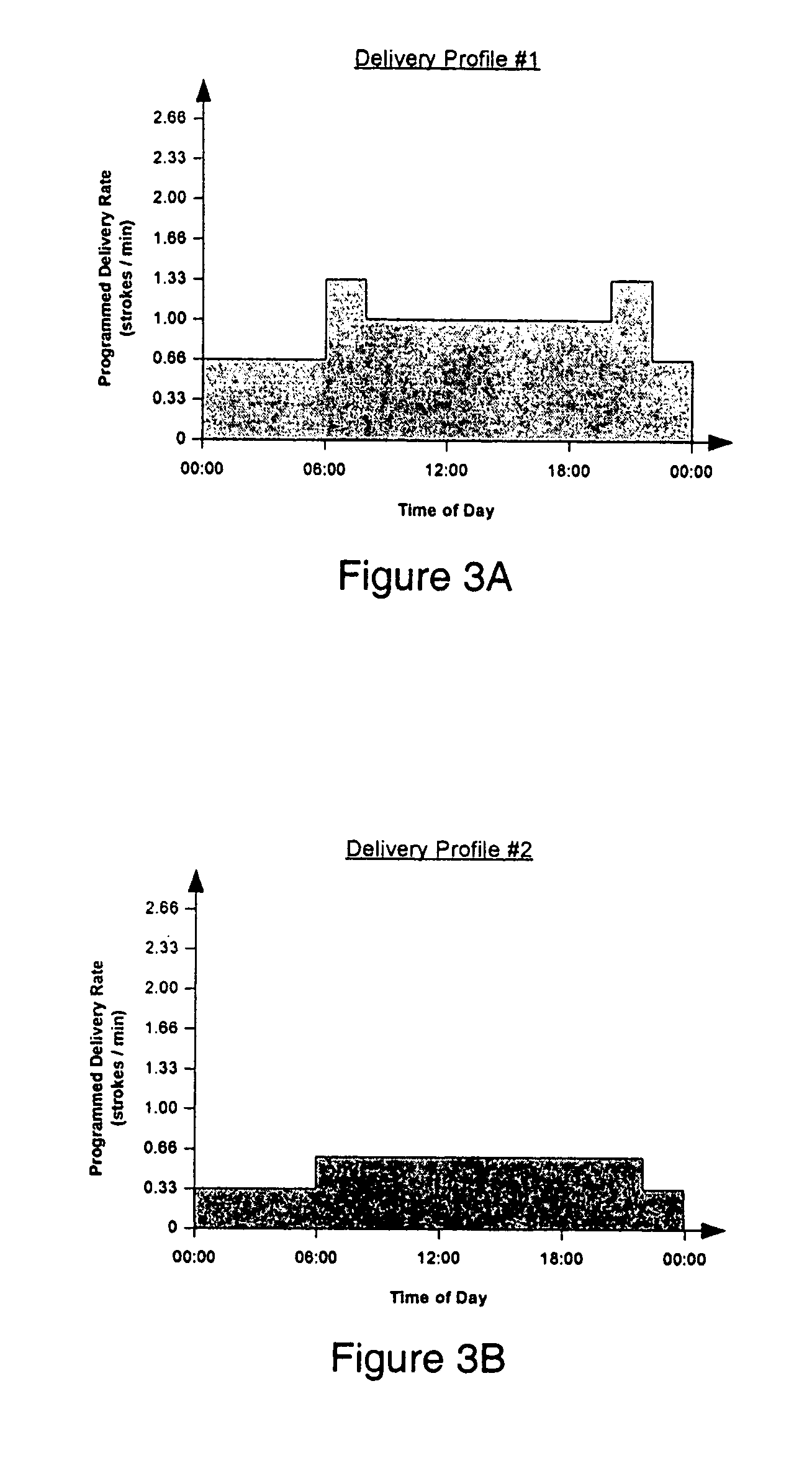
The present invention relates to an implantable drug delivery device which includes a fluid drug reservoir, a catheter, a controllable fluid transfer device, e.g., a pump or valve, and a drug delivery control means. The control means in accordance with the invention is configured to initially clear a first, or old, drug from the device based on the content of the Current Profile data containing a first, or old, delivery profile. After the old drug is cleared, then the control means automatically modifies the Current Profile data to match a second, or new, delivery profile for controlling delivery of a second, or new, drug.
Owner:MEDTRONIC MIMIMED INC
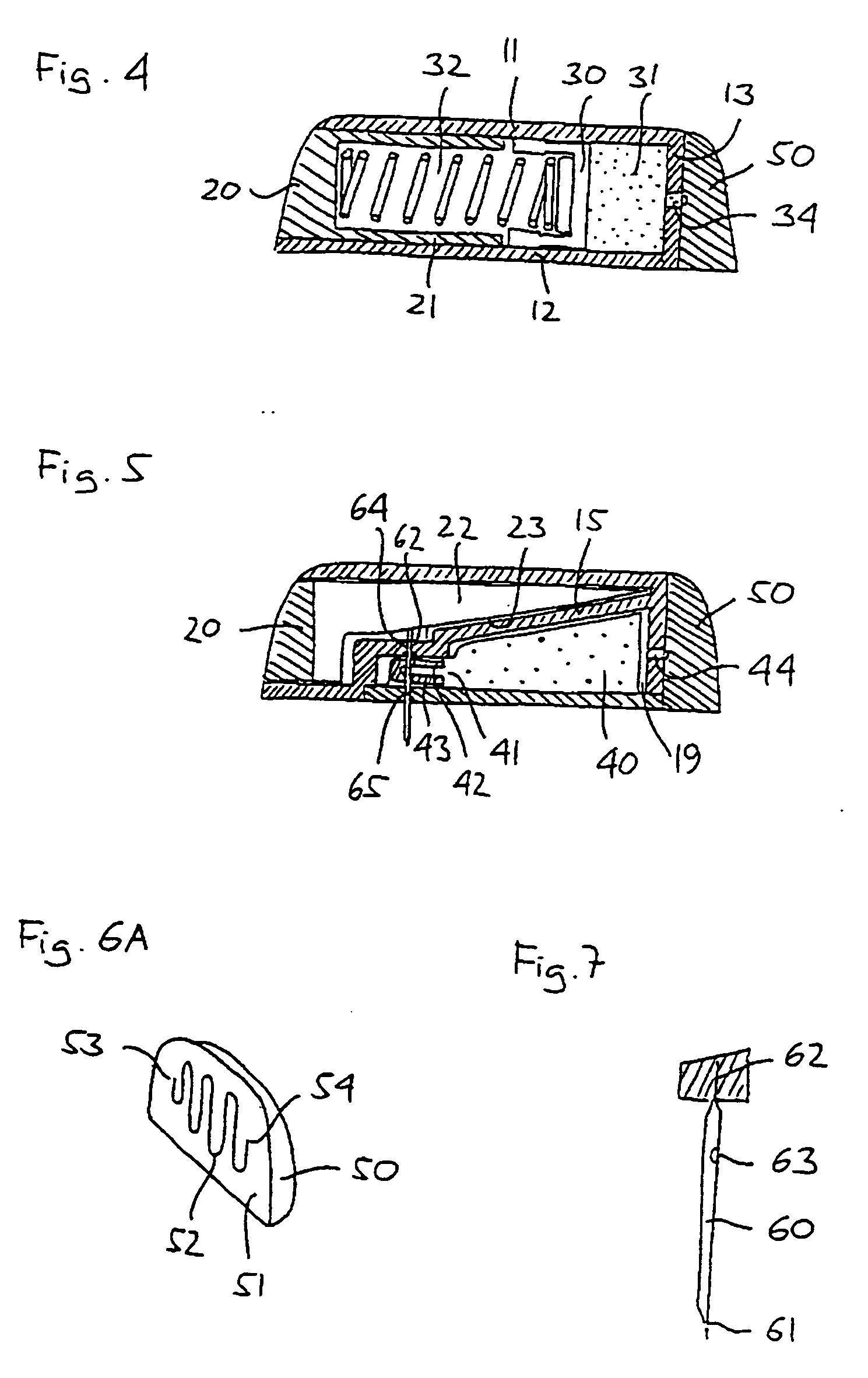
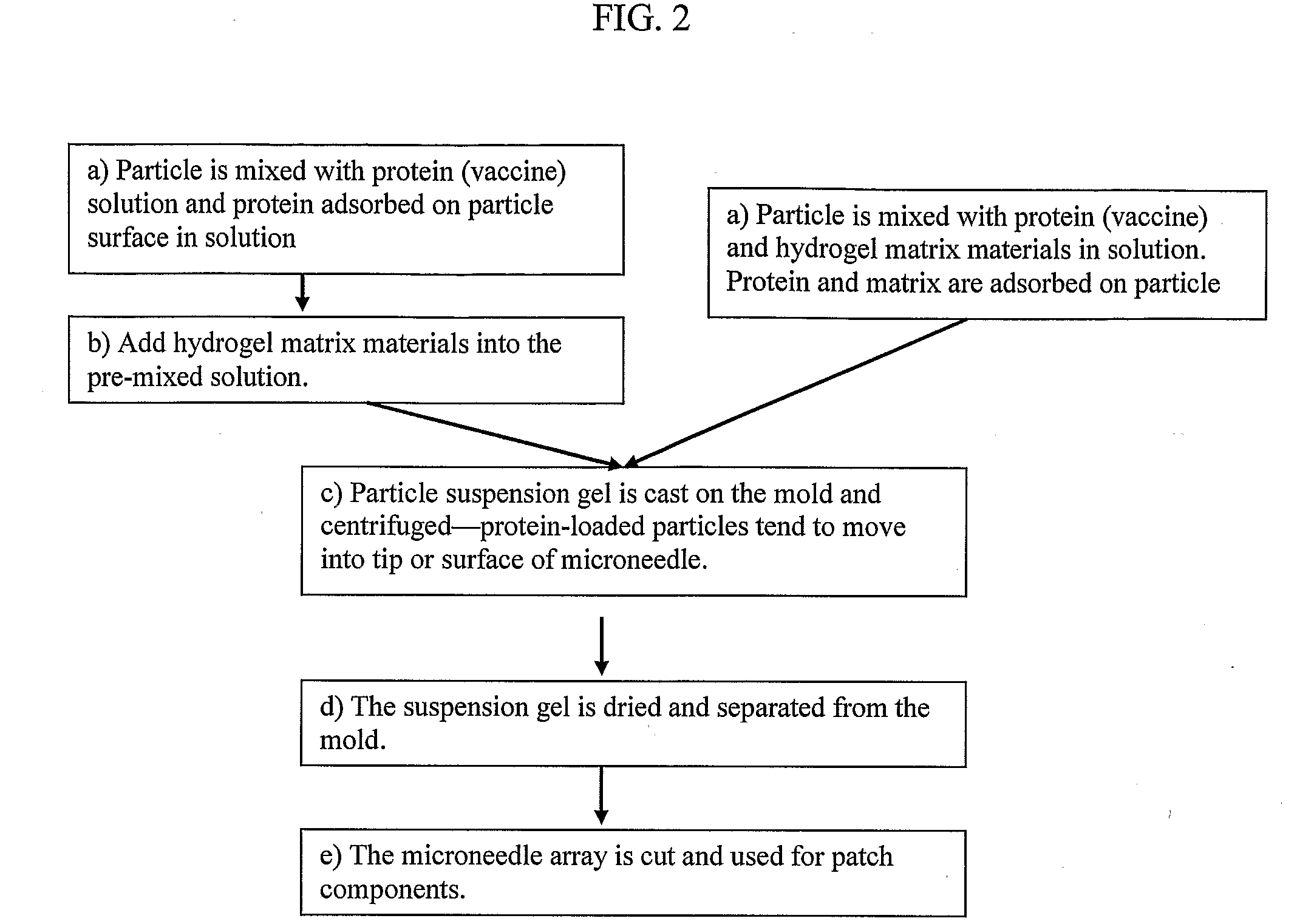
Solid Solution Perforator Containing Drug Particle and/or Drug-Adsorbed Particles
InactiveUS20090035446A1Barrier property can be diminished and controlledFast biodegradationPeptide/protein ingredientsMicroneedlesDrugs solutionDrug reservoir
A solid drug solution perforator containing drug particles and / or drug-adsorbed or loaded particles with an associated drug reservoir (SSPP system) are provided for delivering therapeutic, prophylactic and / or cosmetic compounds, diagnostics, and for nutrient delivery and drug targeting. For drug delivery, the SSPP system includes an active drug ingredient in particulate form or drug adsorbed on the particle surface in a matrix material that dissolves upon contact with a patient's body. In a preferred method of transdermal drug delivery, an SSPP system containing a drug-adsorbed microparticle penetrates into the epidermis or dermis, and the drug is released from the (dissolving) SSPP system perforator and desorbed from the particles. An additional drug is optionally delivered from a patch reservoir through skin pores created by insertion of the perforator Formulation and fabrication procedures for the SSPP and associated reservoir are also provided. An SSPP system can be fabricated with variety of shapes and dimensions.
Owner:THERAJECT INC.
Drug Delivery Apparatus and Method for Automatically Reducing Drug Dosage
InactiveUS20080172044A1Reduce probabilityMedical devicesPressure infusionMicrocontrollerDrug reservoir
Owner:INFUSION SYST
Implantable device for long-term delivery of drugs
A device for sustained delivery of a poorly water soluble drug is described. A drug reservoir within the device, when in operation, contains an aqueous suspension of the drug mixed with a suspension of an excipient that, in one embodiment, generates acidic groups for a sustained period of time to maintain a desired pH in the aqueous suspension that in turn provides a constant concentration of a soluble form of the drug.
Owner:DELPOR
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com