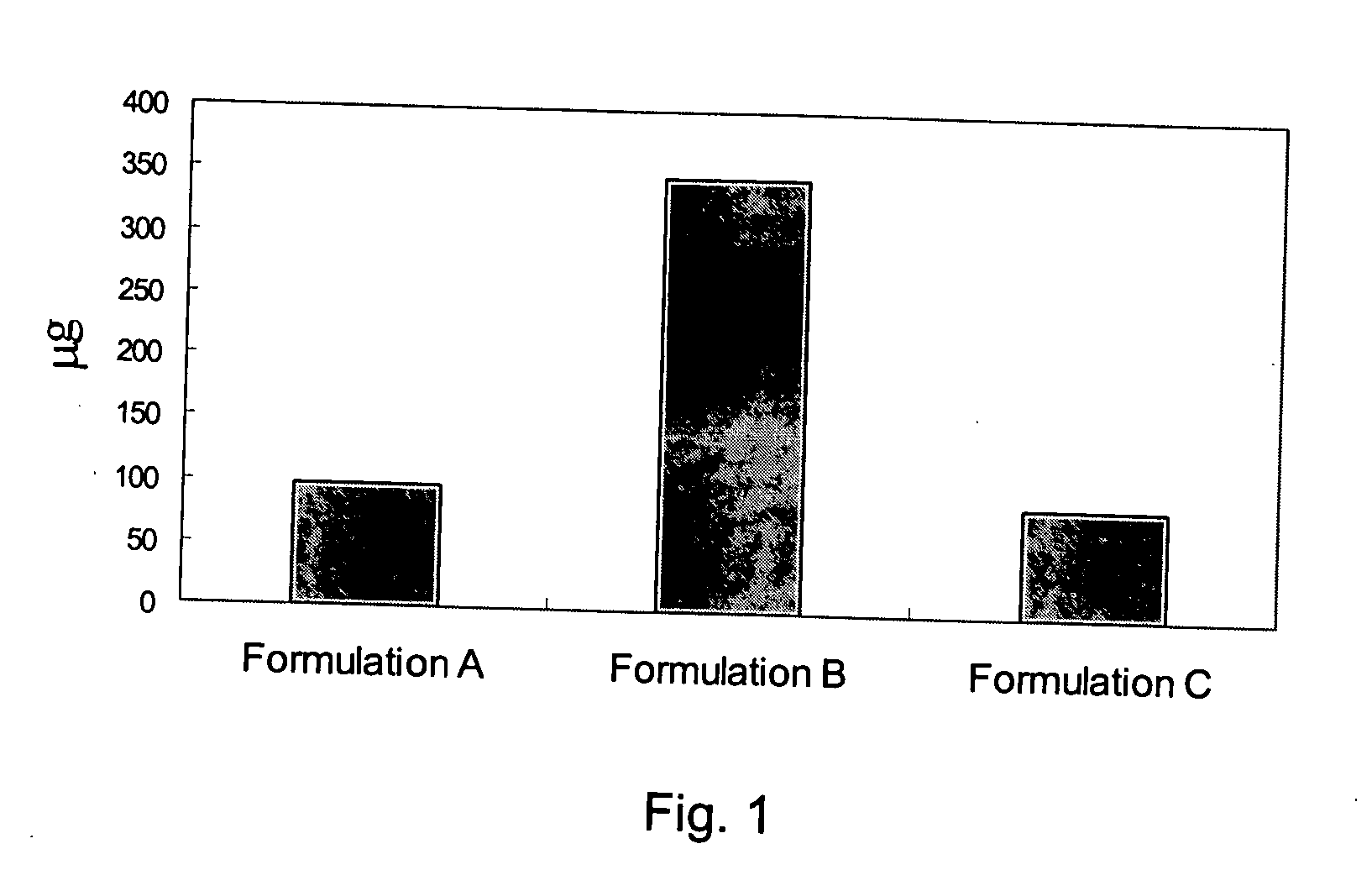
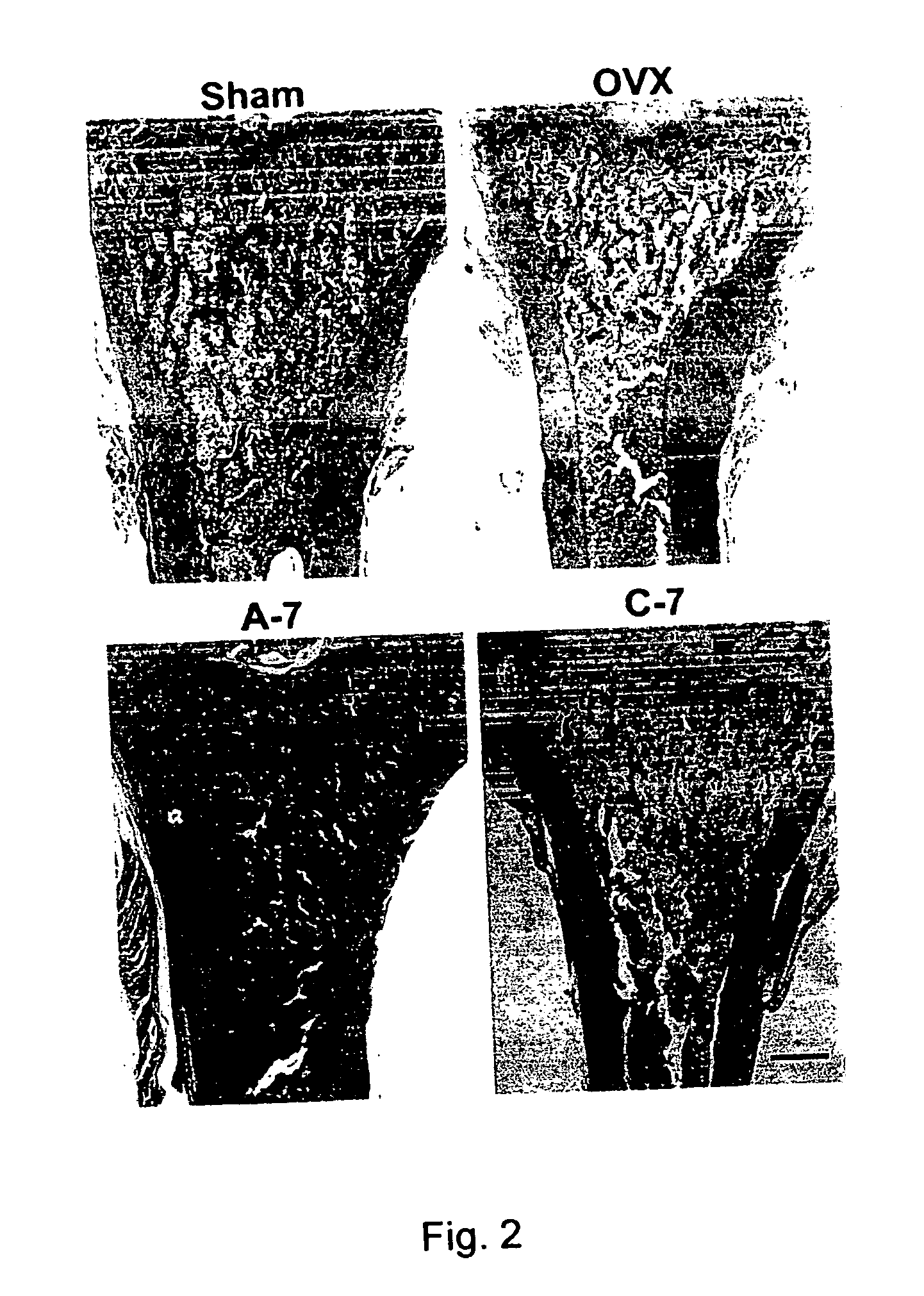
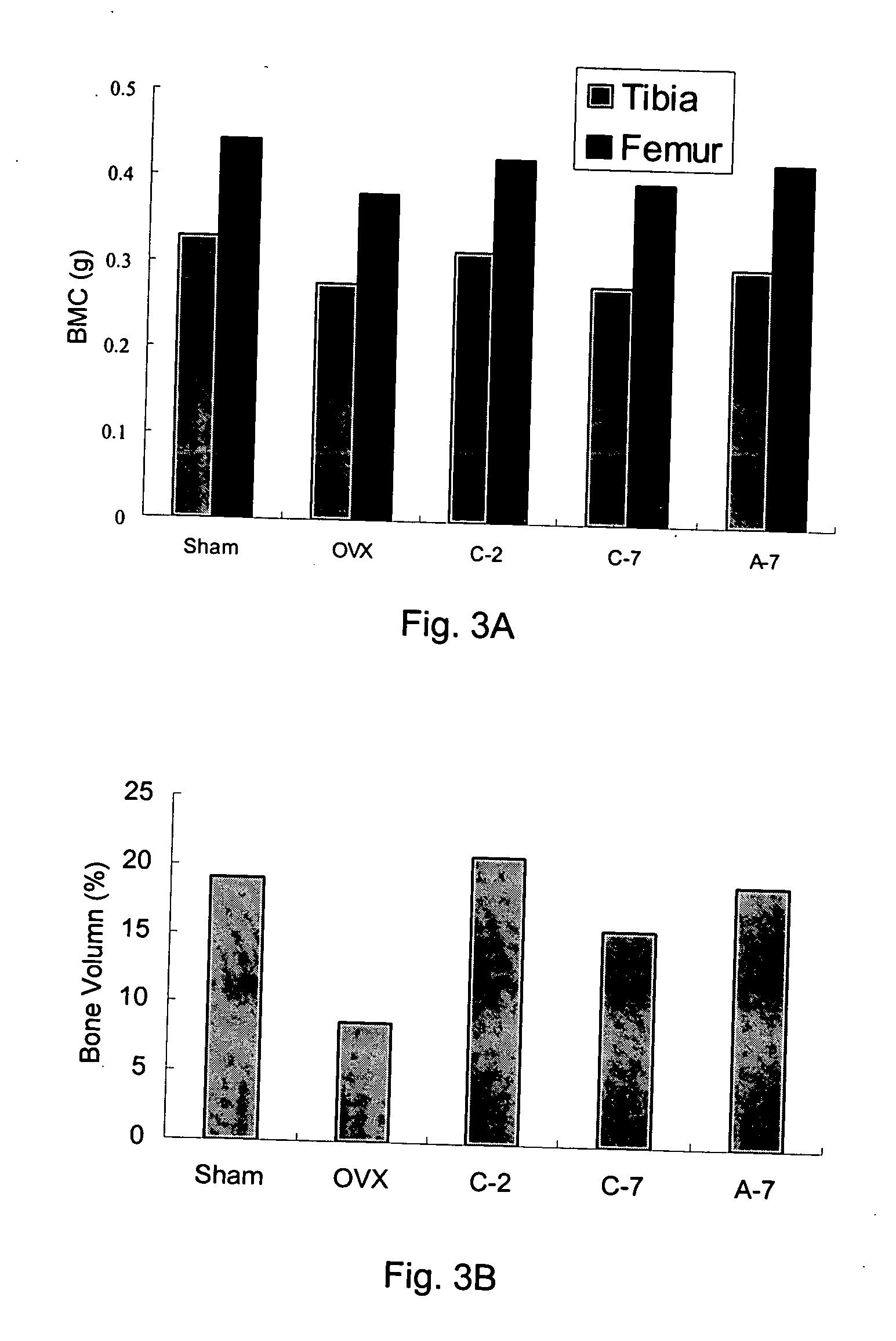
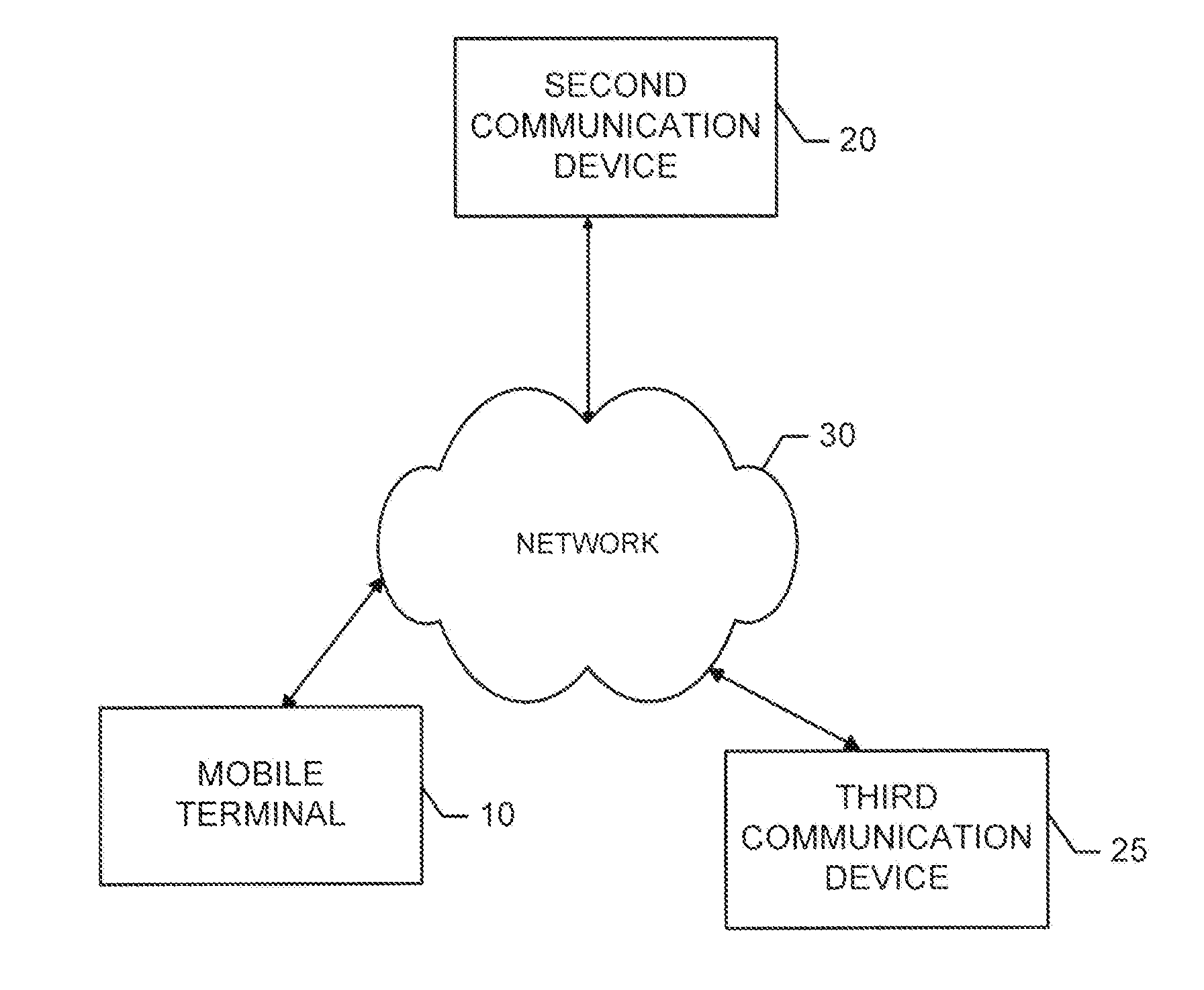
Patents
Literature
94results about How to "Minimize irritation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
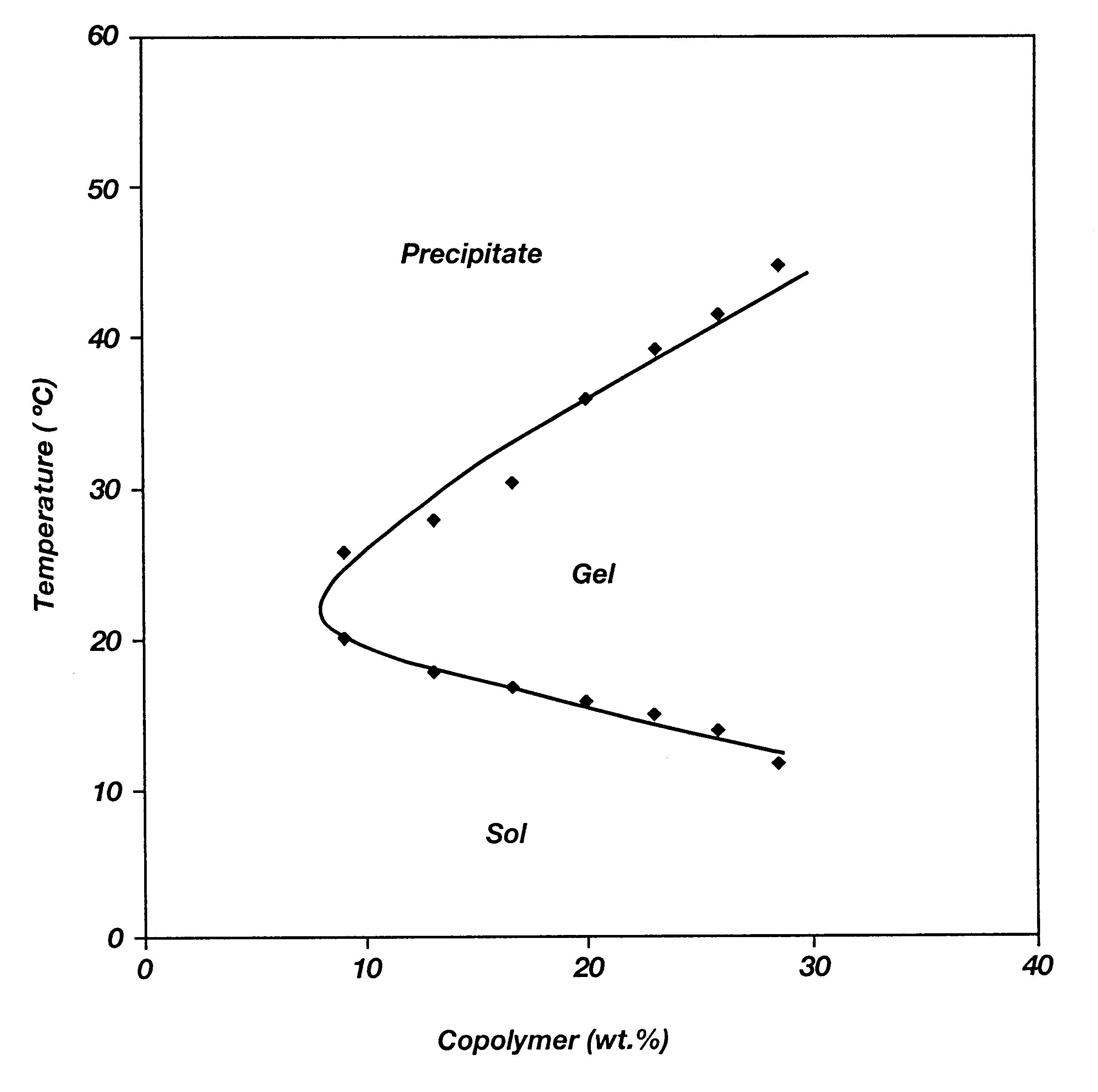
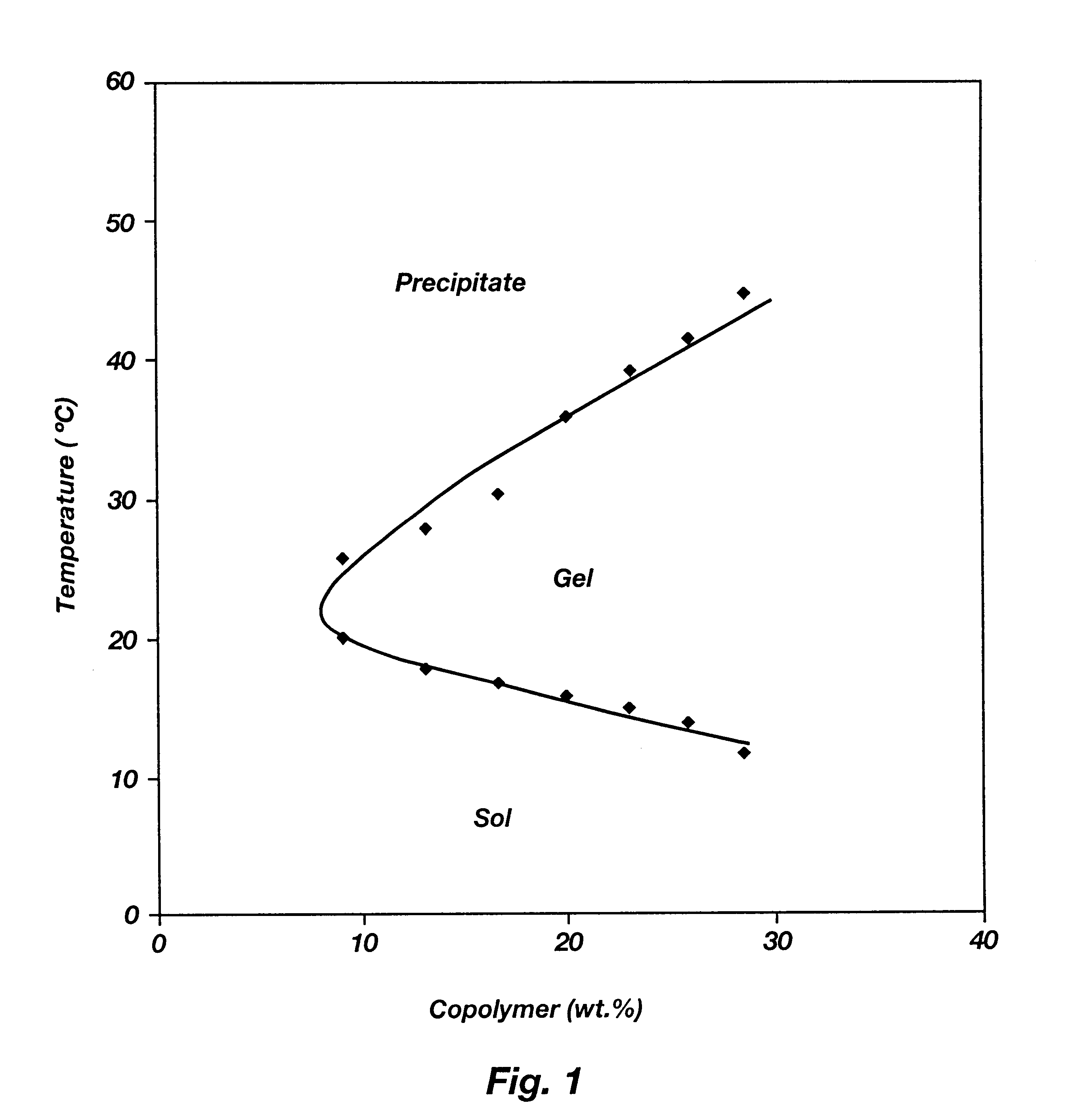
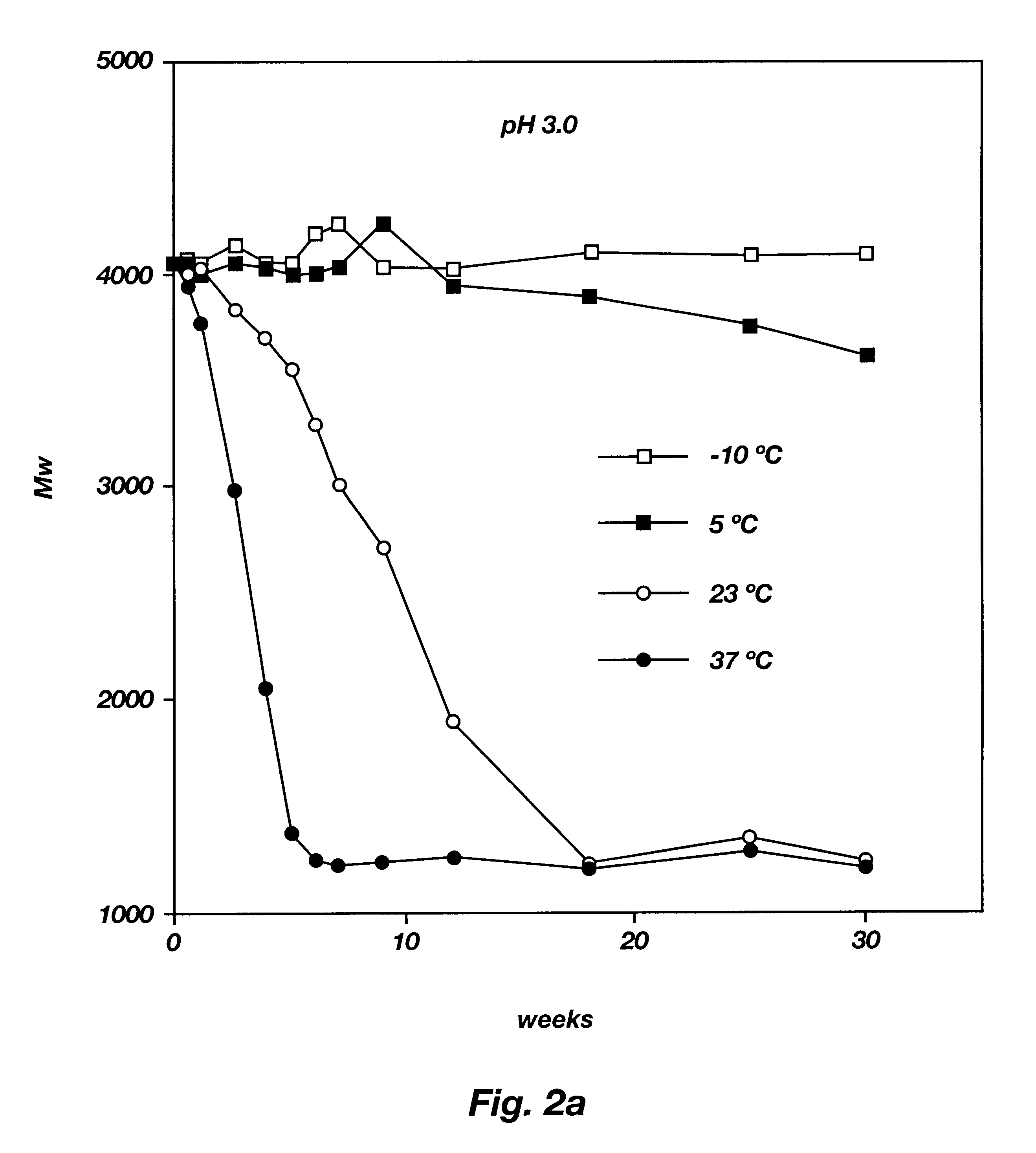
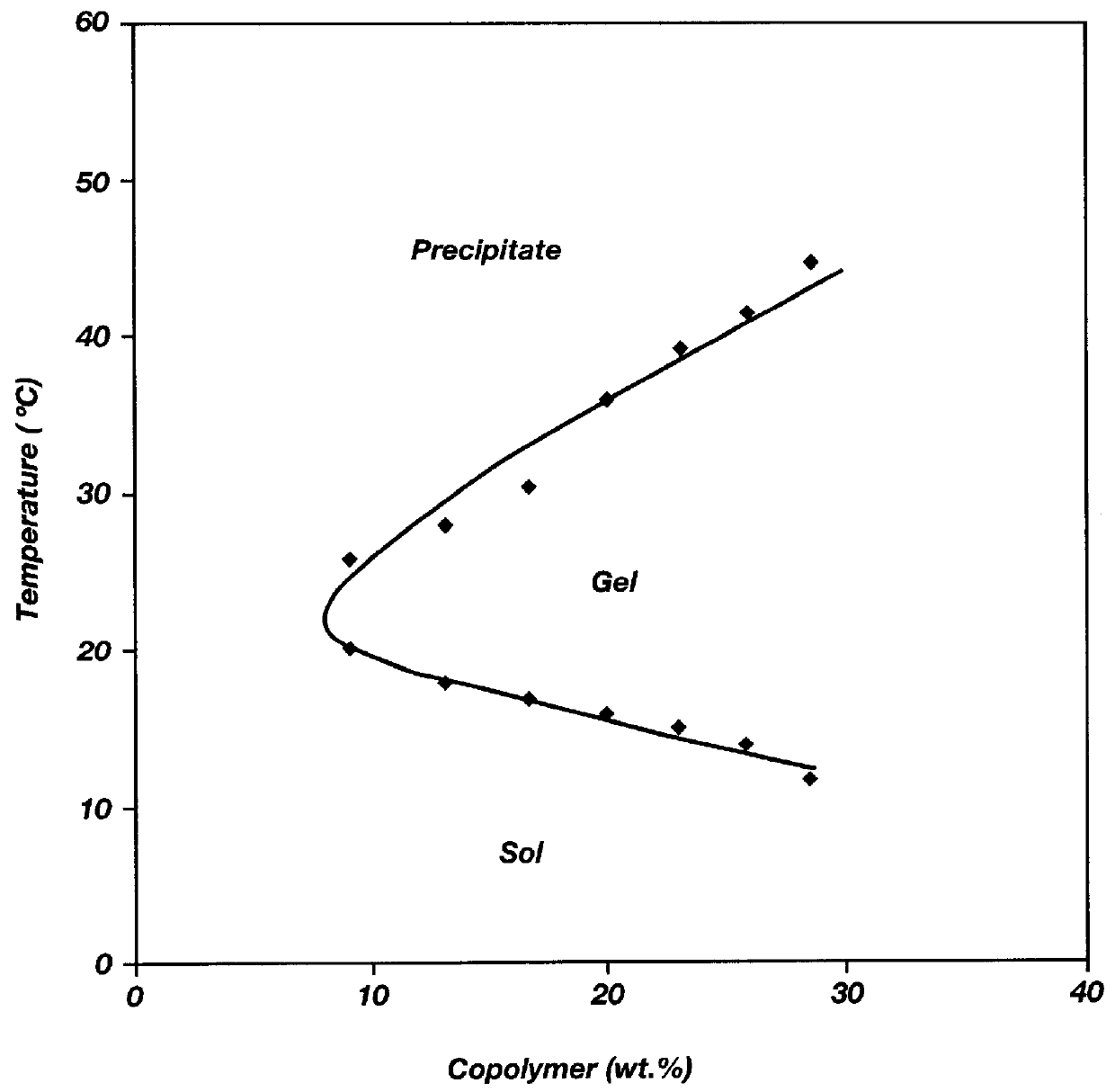
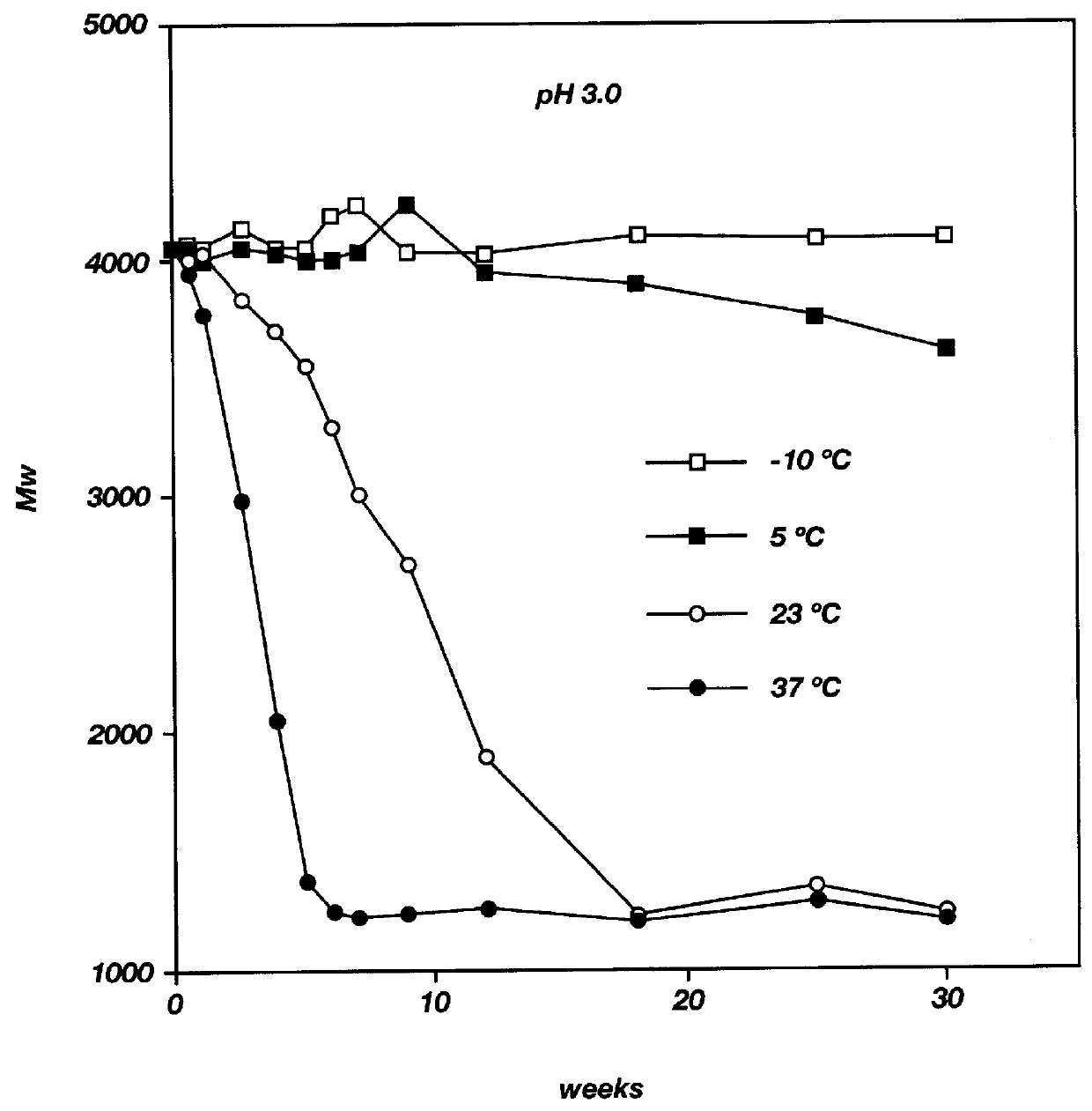
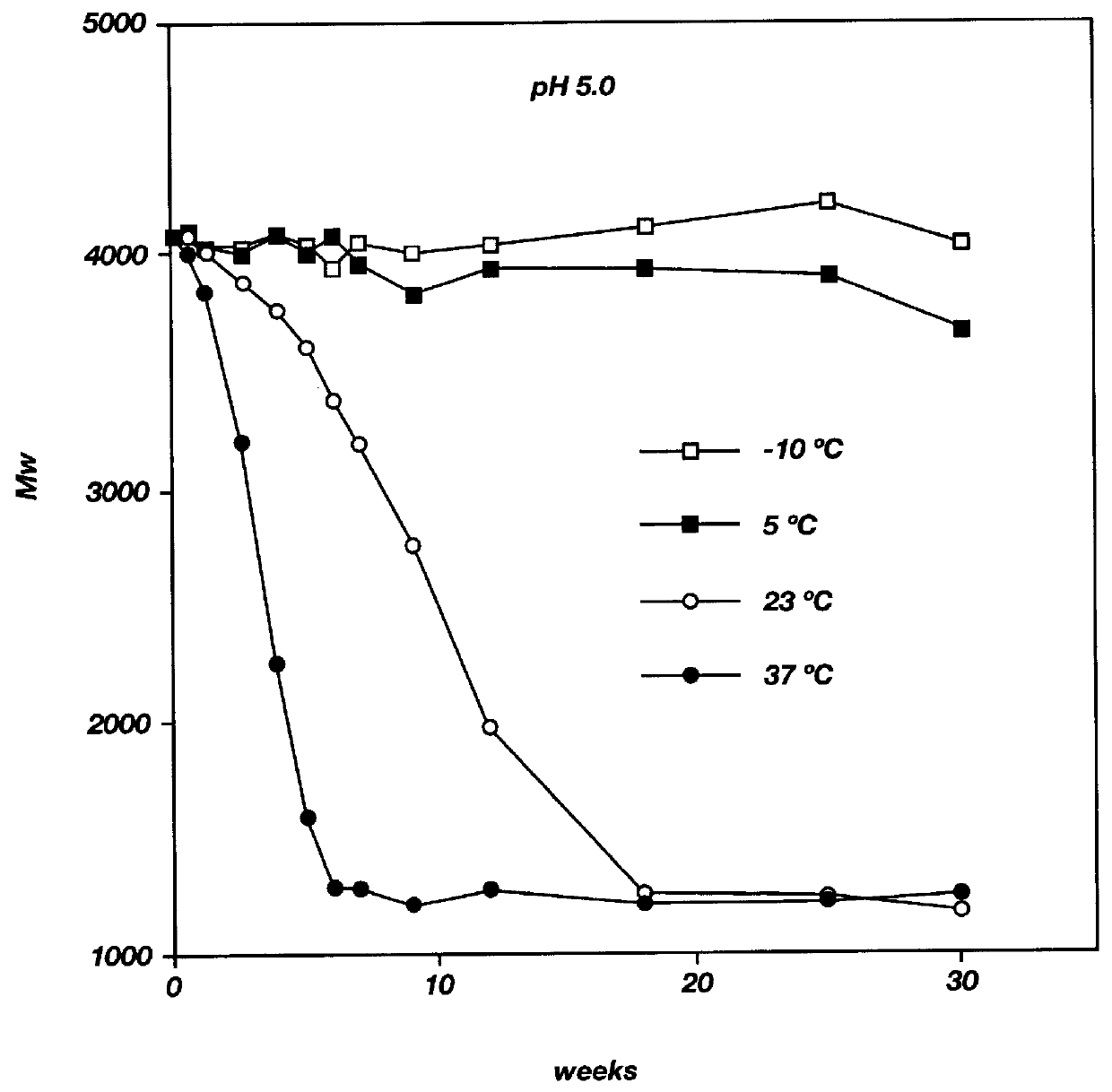
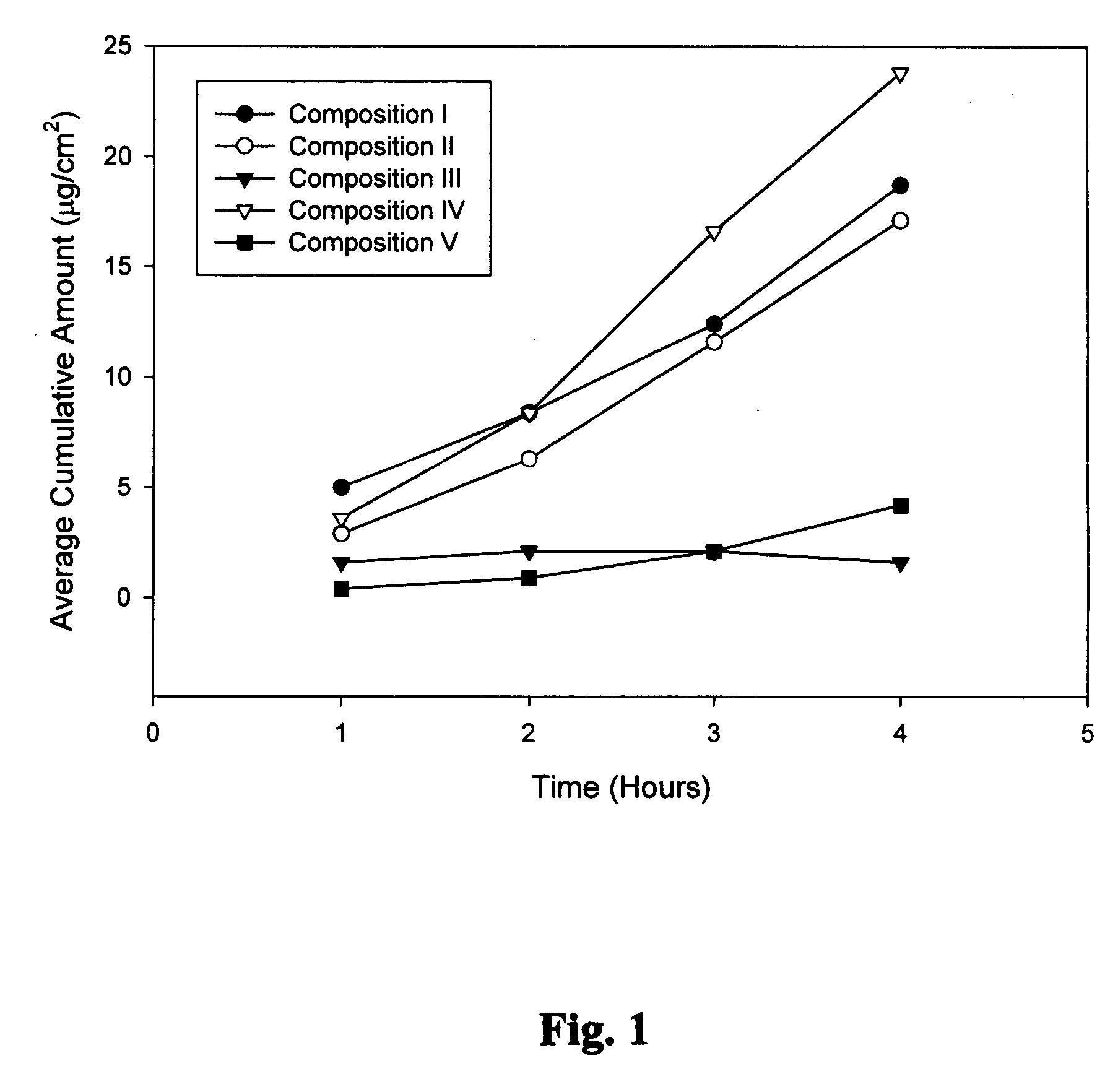
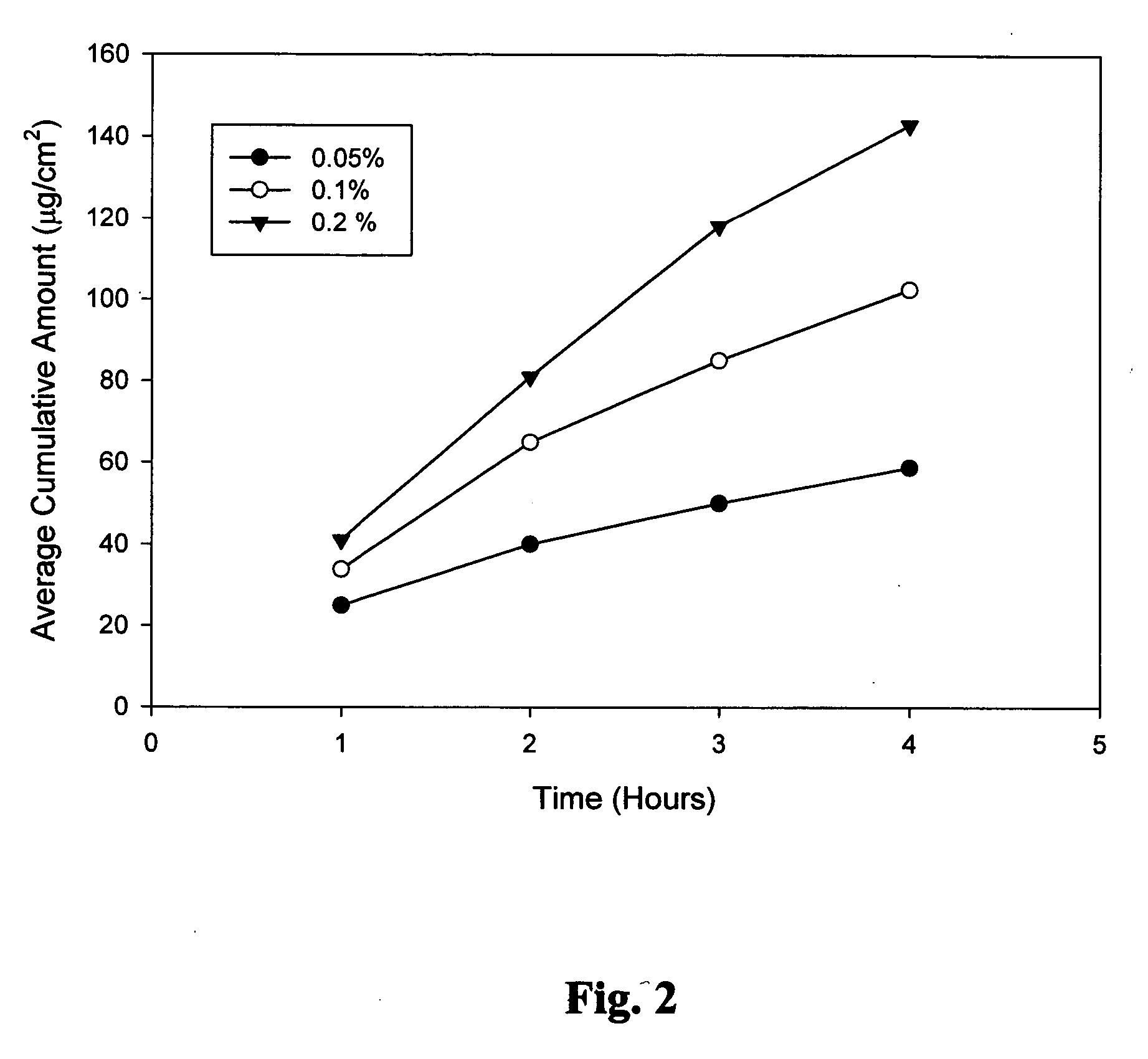
Biodegradable low molecular weight triblock poly(lactide-co- glycolide) polyethylene glycol copolymers having reverse thermal gelation properties
InactiveUS6201072B1Difficult to formulateDifficult to administerOrganic active ingredientsPowder deliverySolubilityPolymer science
A water soluble, biodegradable ABA- or BAB-type tri-block polymer is disclosed that is made up of a major amount of a hydrophobic A polymer block made of a biodegradable polyester and a minor amount of a hydrophilic polyethylene glycol(PEG) B polymer block, having an overall average molecular weight of between about 2000 and 4990, and that possesses reverse thermal gelation properties. Effective concentrations of the tri-block polymer and a drug may be uniformly contained in an aqueous phase to form a drug delivery composition. At temperatures below the gelation temperature of the tri-block polymer the composition is a liquid and at temperatures at or above the gelation temperature the composition is a gel or semi-solid. The composition may be administered to a warm-blooded animal as a liquid by parenteral, ocular, topical, inhalation, transdermal, vaginal, transurethral, rectal, nasal, oral, pulmonary or aural delivery means and is a gel at body temperature. The composition may also be administered as a gel. The drug is released at a controlled rate from the gel which biodegrades into non-toxic products. The release rate of the drug may be adjusted by changing various parameters such as hydrophobic / hydrophilic component content, polymer concentration, molecular weight and polydispersity of the tri-block polymer. Because the tri-block polymer is amphiphilic, it functions to increase the solubility and / or stability of drugs in the composition.
Owner:KIM PH D SUNG WAN +2
Biodegradable low molecular weight triblock poly (lactide-co-glycolide) polyethylene glycol copolymers having reverse thermal gelation properties
InactiveUS6117949AReduce solubilityReduced stabilityPowder deliveryPeptide/protein ingredientsSolubilityPolymer science
A water soluble biodegradable ABA- or BAB-type triblock polymer is disclosed that is made up of a major amount of a hydrophobic polymer made of a poly(lactide-co-glycolide) copolymer or poly(lactide) polymer as the A-blocks and a minor amount of a hydrophilic polyethylene glycol polymer B-block, having an overall weight average molecular weight of between about 2000 and 4990, and that possesses reverse thermal gelation properties. Effective concentrations of the triblock polymer and a drug may be uniformly contained in an aqueous phase to form a drug delivery composition. At temperatures below the gelation temperature of the triblock polymer the composition is a liquid and at temperatures at or above the gelation temperature the composition is a gel or semi-solid. The composition may be administered to a warm-blooded animal as a liquid by parenteral, ocular, topical, inhalation, transdermal, vaginal, transurethral, rectal, nasal, oral, pulmonary or aural delivery means and is a gel at body temperature. The composition may also be administered as a gel. The drug is released at a controlled rate from the gel which biodegrades into non-toxic products. The release rate of the drug may be adjusted by changing various parameters such as hydrophobic / hydrophilic componenet content, polymer concentration, molecular weight and polydispersity of the triblock polymer. Because the triblock polymer is amphiphilic, it functions to increase the solubility and / or stability of drugs in the composition.
Owner:BTG INT LTD +2
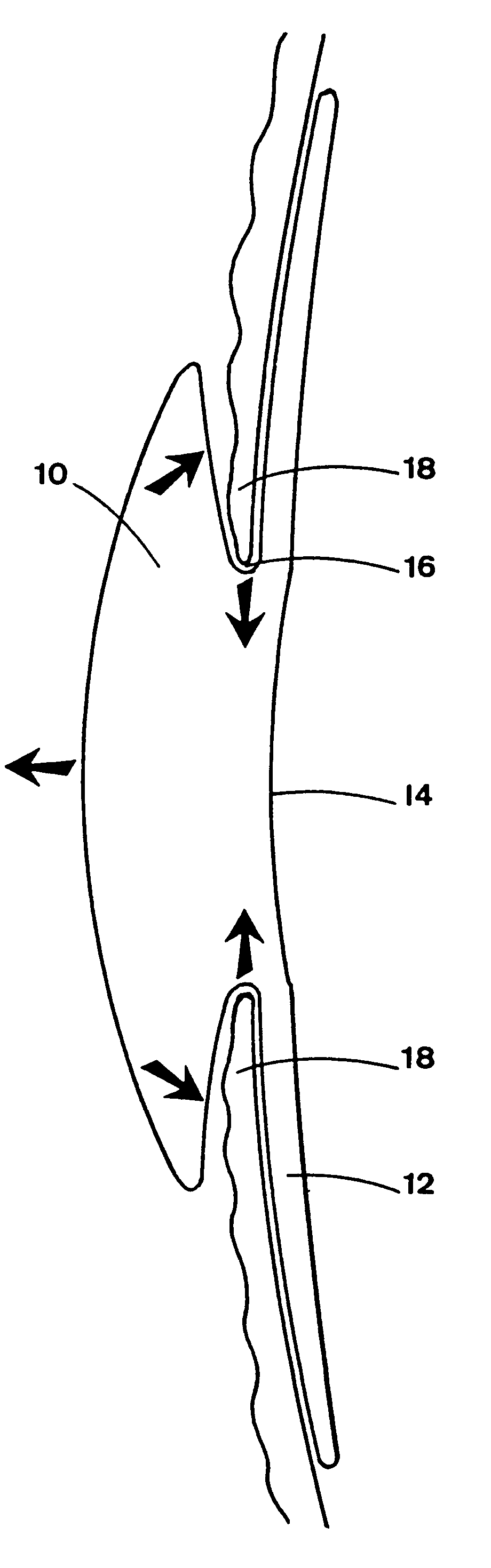
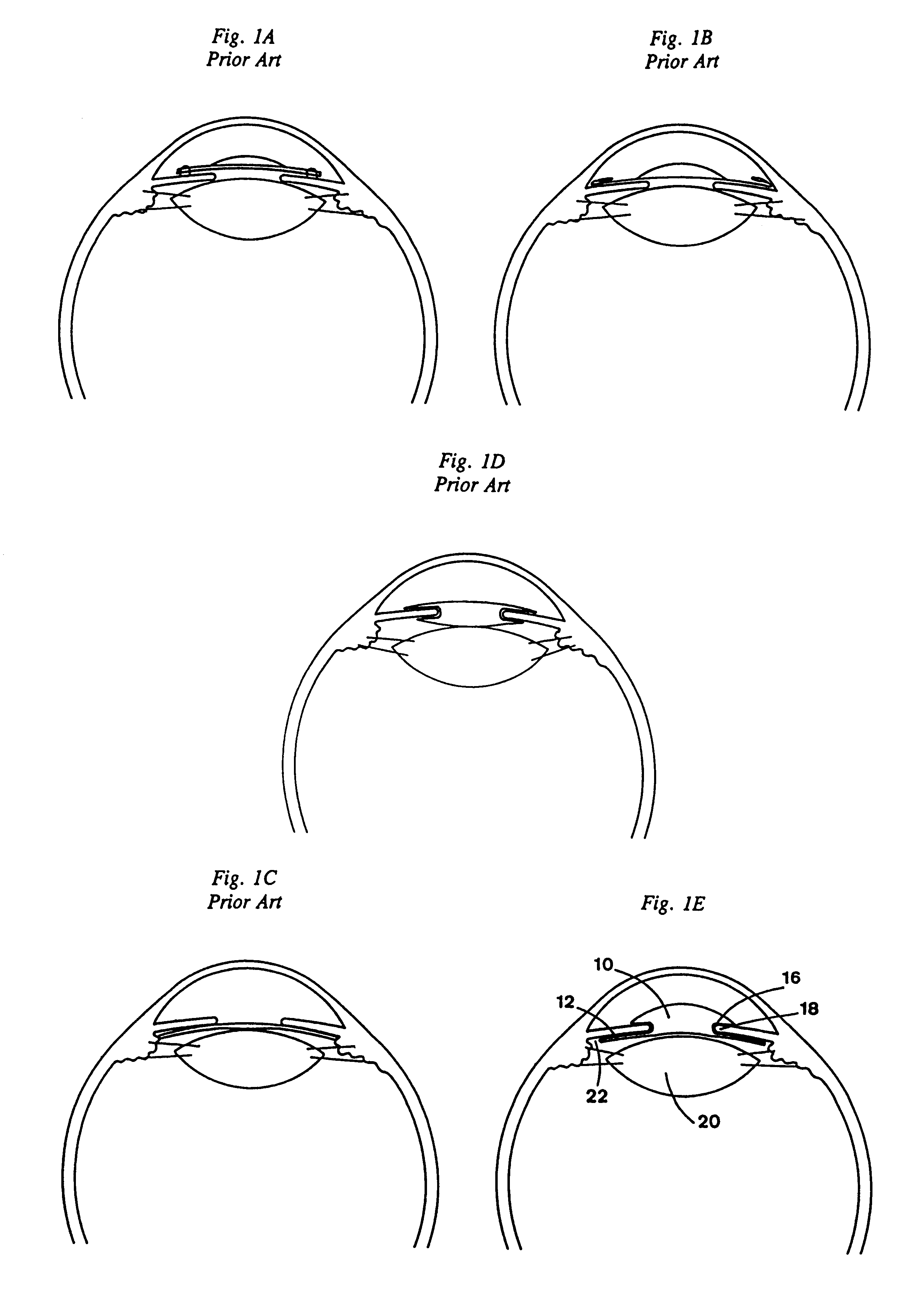
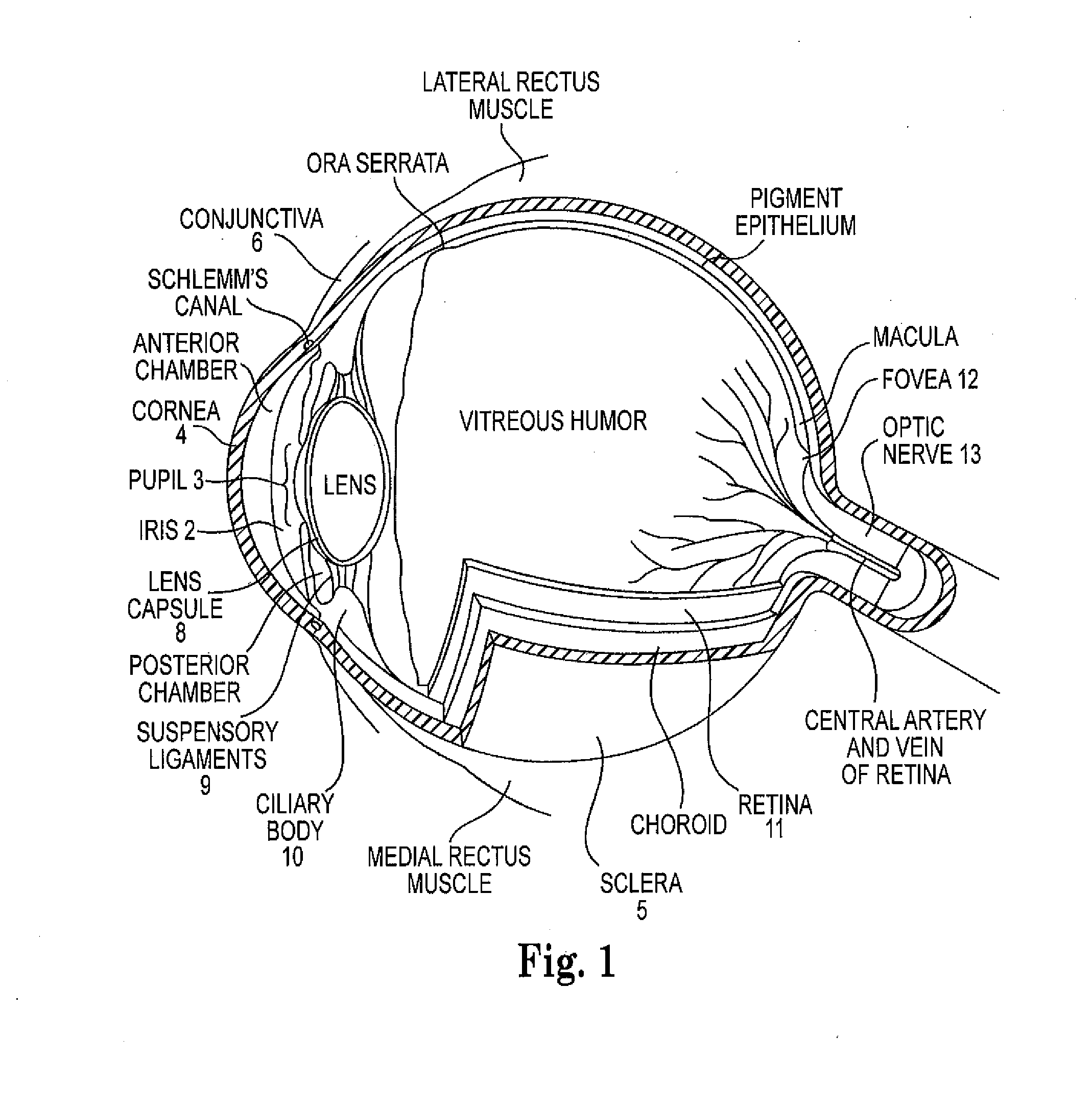
Intraocular lens with accommodative properties
InactiveUS6200342B1Focus assistPrevent excessive lateral movement and luxationIntraocular lensPupil diameterIntraocular lens
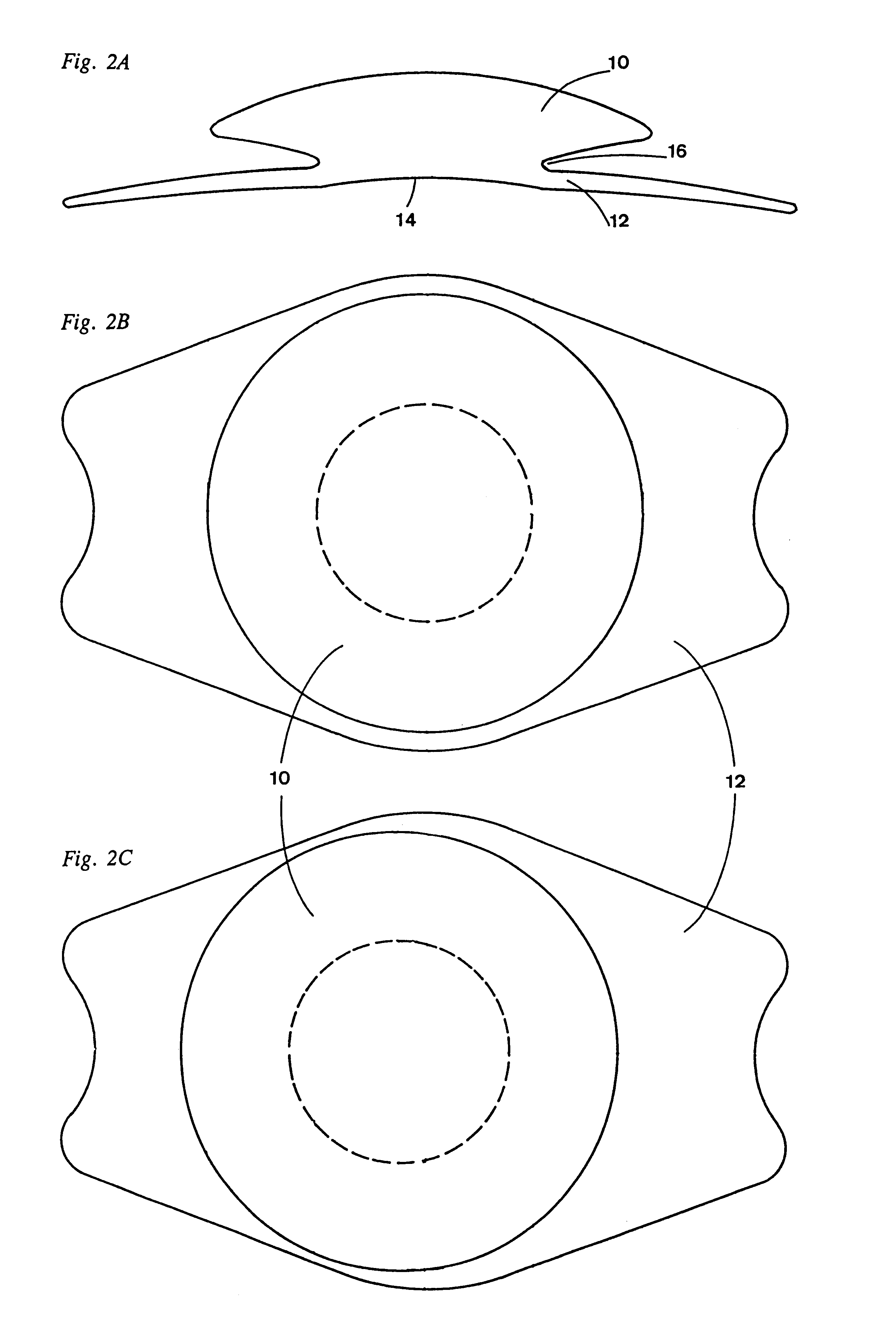
A new lens design and method of implantation uses the change in pupil diameter of the eye concurrent with the changes induced by a contraction of the ciliary muscle during the accommodative reflex, in order to assist in focusing of nearby objects. This new intraocular lens consists of two parts. The posterior part or haptic part is inserted behind the iris and in front of the natural lens or artificial implant. Its main purpose is to participate in the accommodative mechanism and to prevent excessive lateral movement and luxation of the lens. An anterior or optical part is made of flexible material and is placed before the iris. Its diameter is variable but should be large enough to cover the pupillary margins to some degree under various conditions of natural dilation. The anterior and posterior part of the lens are separated by a compressible circular groove in which the iris will settle. The diameter of this groove is slightly larger than the pupillary diameter measured under normal photopic daylight conditions and for distance vision. Since the pupil becomes smaller in near vision, the iris will exert a slight pressure at the level of the groove of the lens which will cause a progressive and evenly distributed flexing of the anterior part of the intraocular lens, as the diameter of the compressible circular groove slightly decreases. This flexing will induce an increase in refractive power which corresponds to a variable part of the amount necessary for focusing nearby objects.
Owner:TASSIGNON MARIE JOSE B
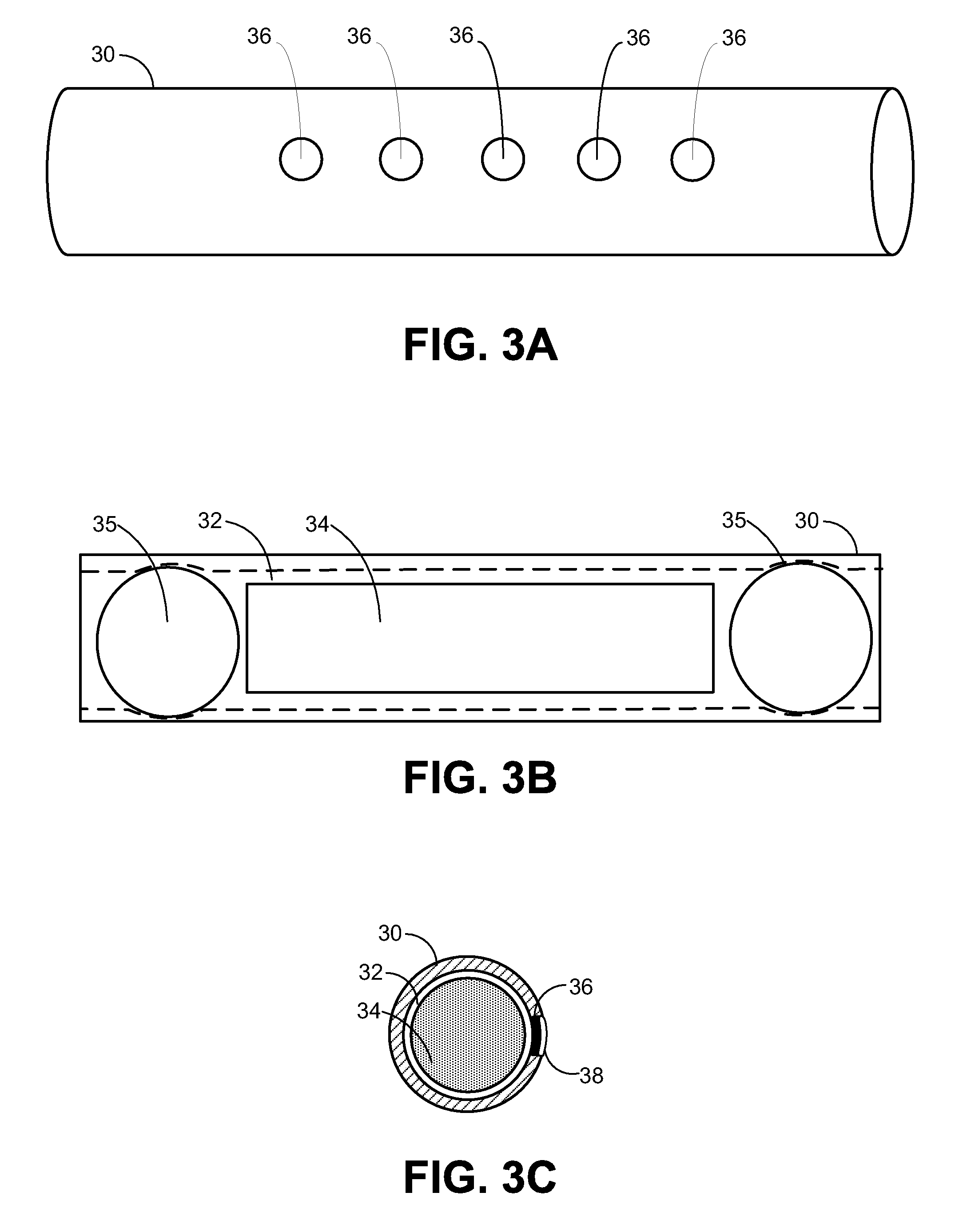
Implantable Drug Delivery Device and Methods for Treatment of the Bladder and Other Body Vesicles or Lumens
ActiveUS20090149833A1High plasma concentrationMinimize irritationBiocideMedical devicesDrug reservoirControlled drugs
An implantable medical device is provided for controlled drug delivery within the bladder, or other body vesicle. The device may include at least one drug reservoir component comprising a drug; and a vesicle retention frame which comprises an elastic wire having a first end, an opposing second end, and an intermediate region therebetween, wherein the drug reservoir component is attached to the intermediate region of the vesicle retention frame. The retention frame prevents accidental voiding of the device from the bladder, and it preferably has a spring constant selected for the device to effectively stay in the bladder during urination while minimizing the irritation of the bladder.
Owner:MASSACHUSETTS INST OF TECH
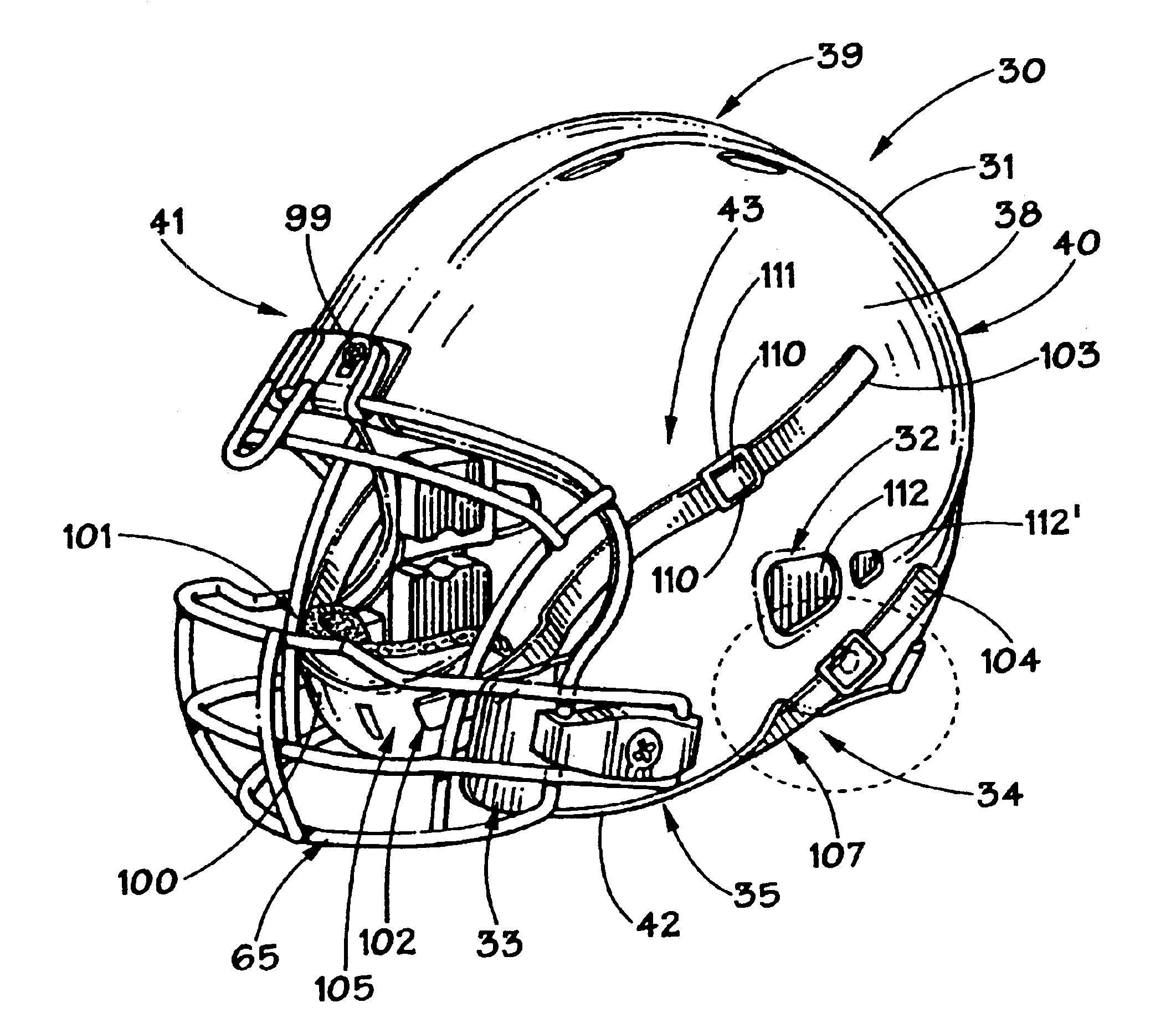
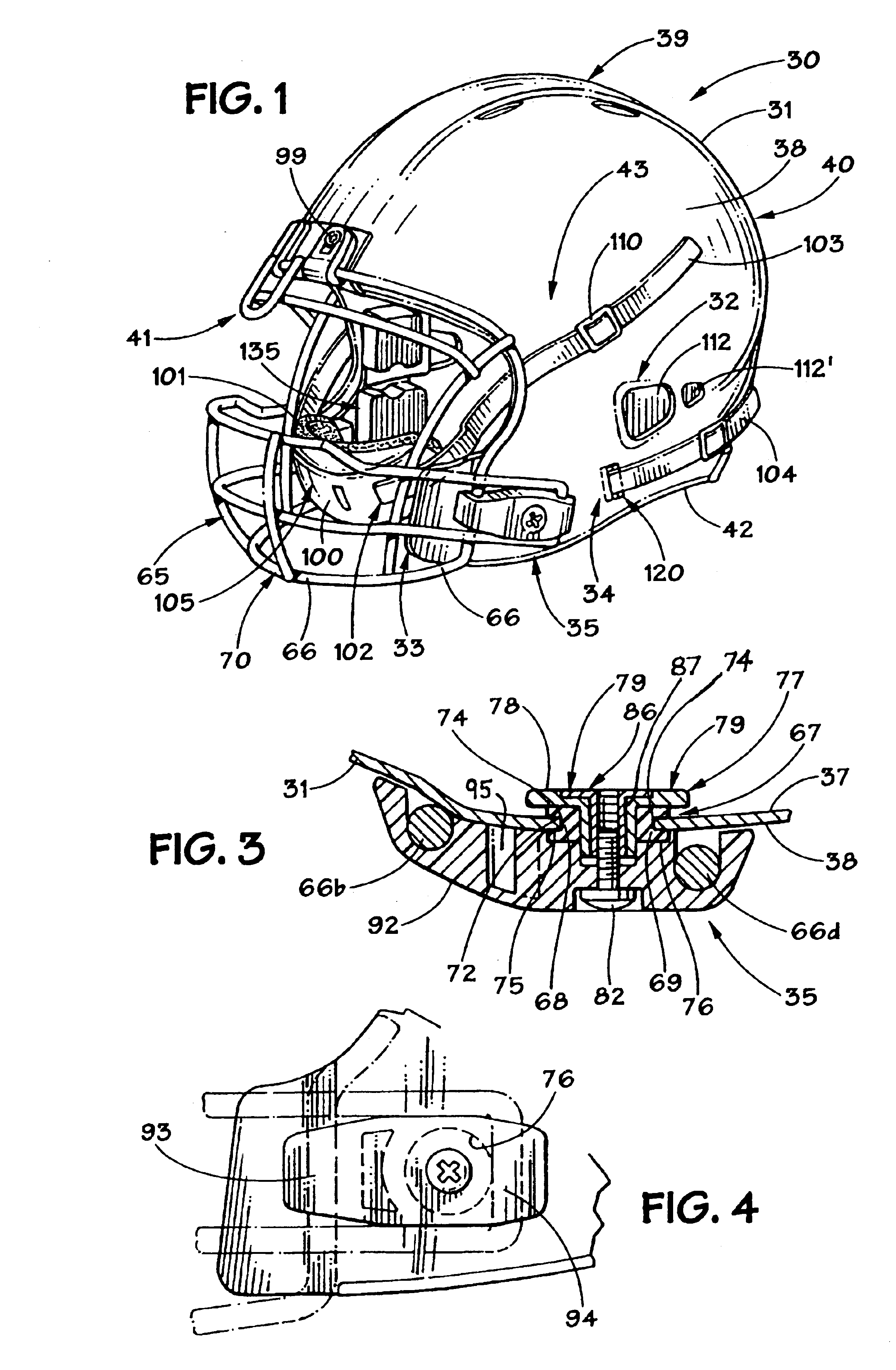
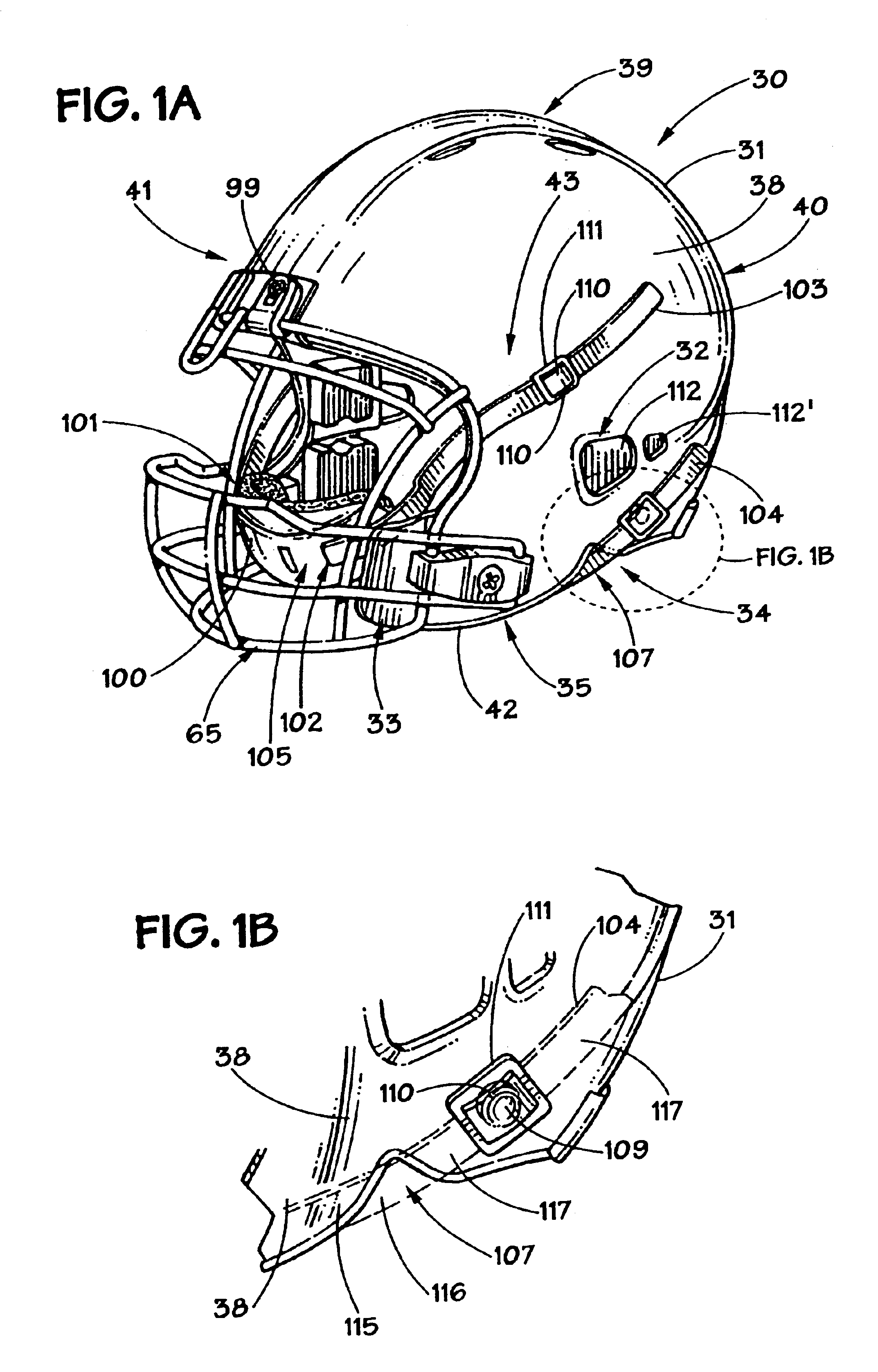
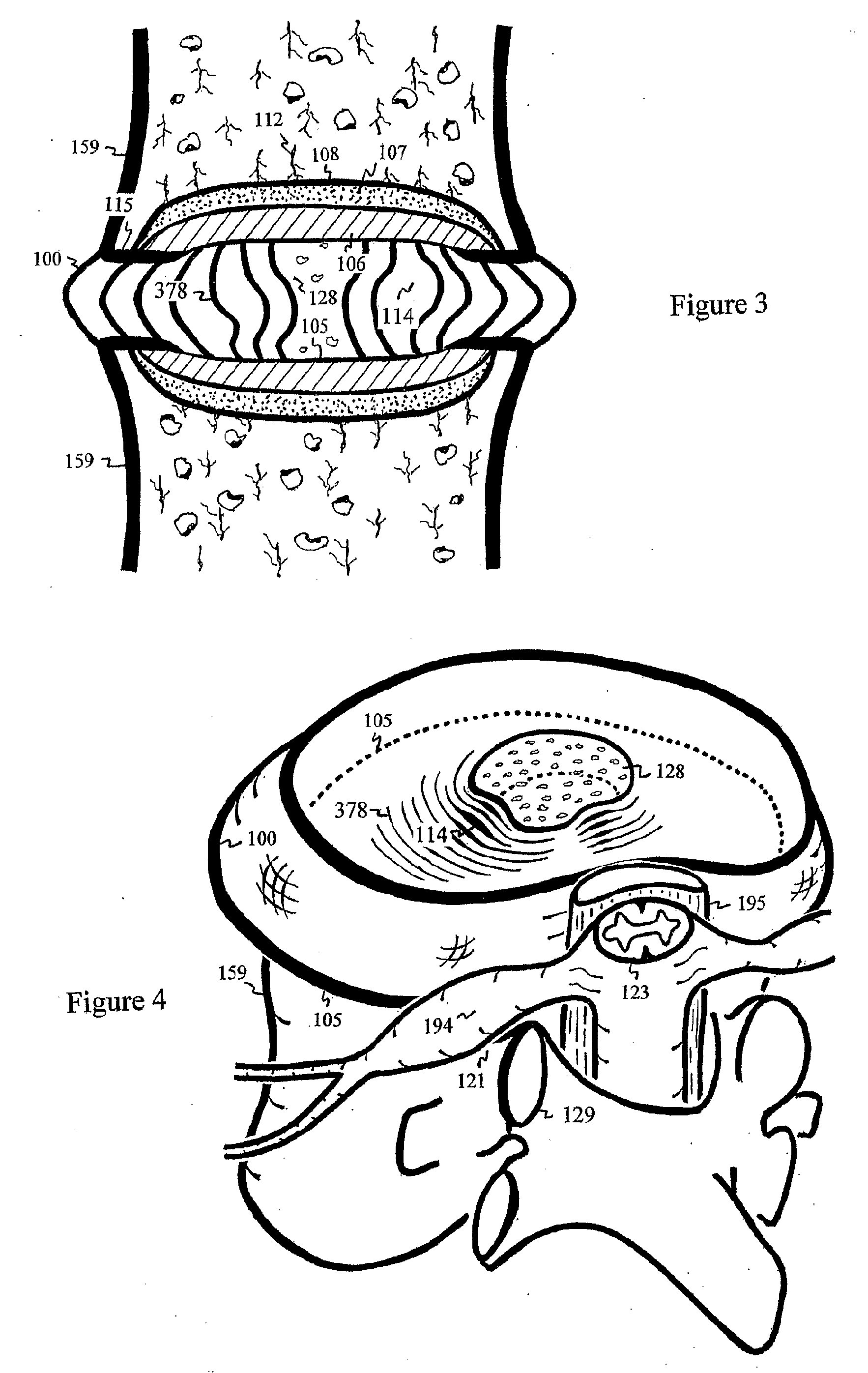
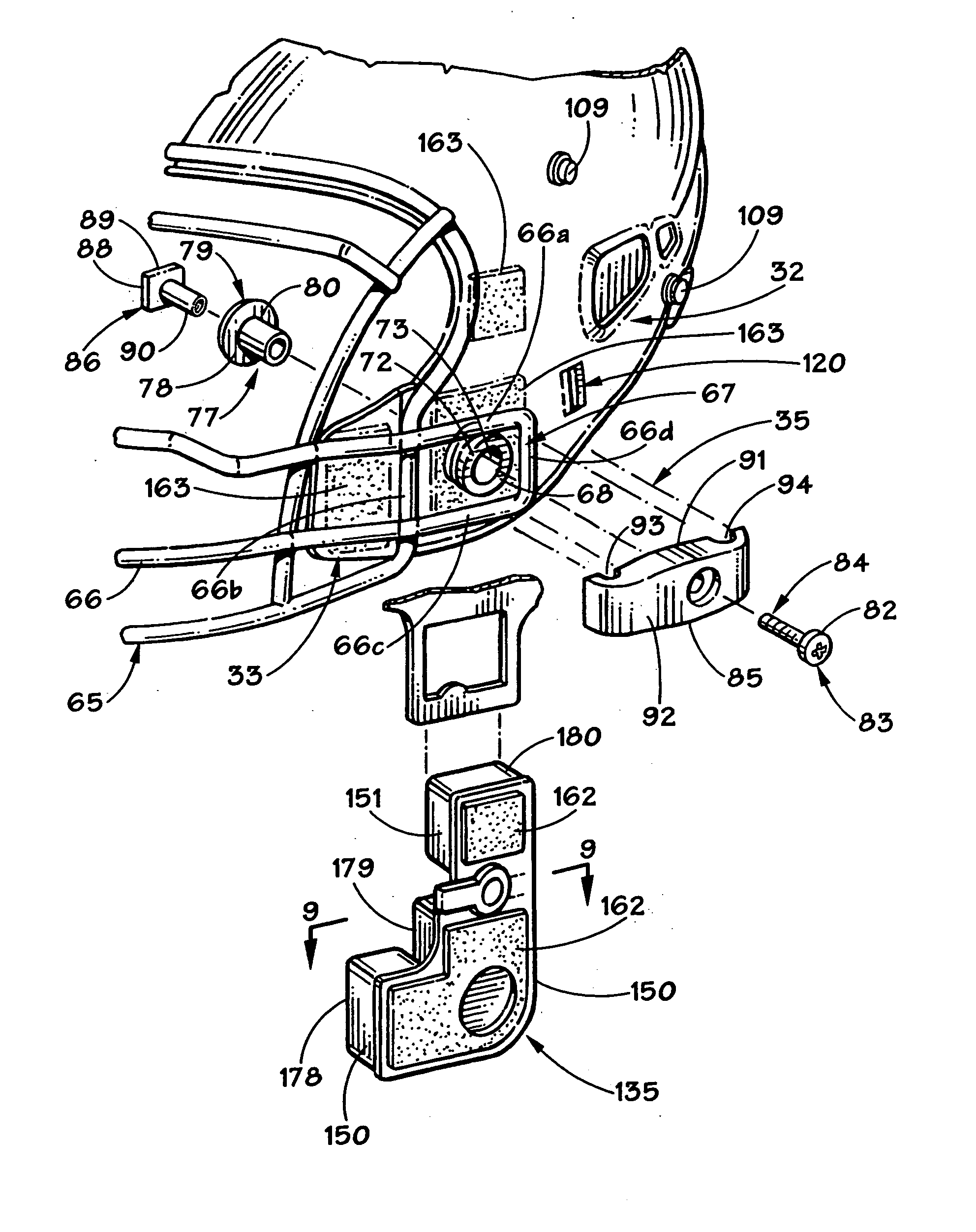
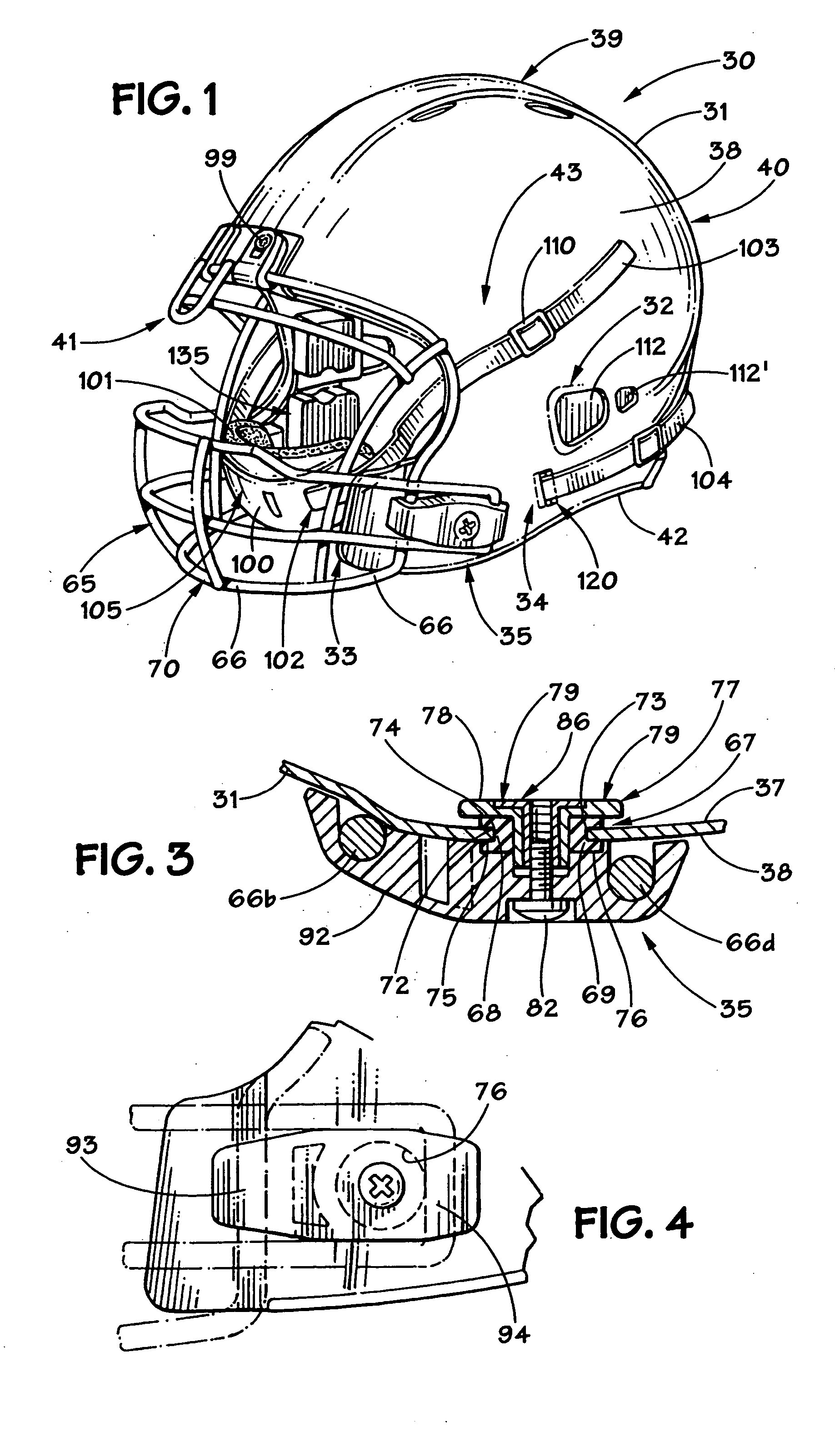
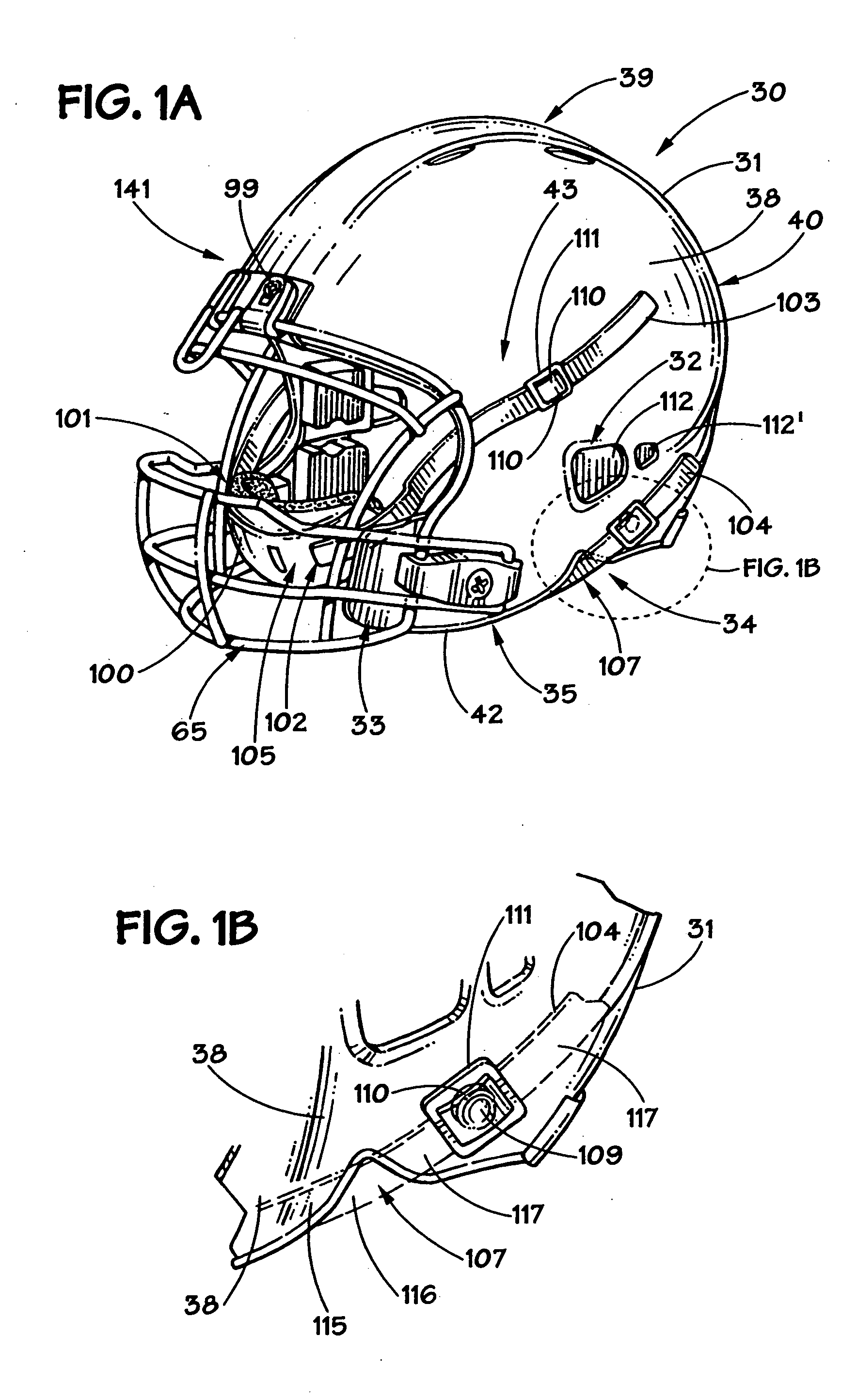
Football helmet
Owner:RIDDELL
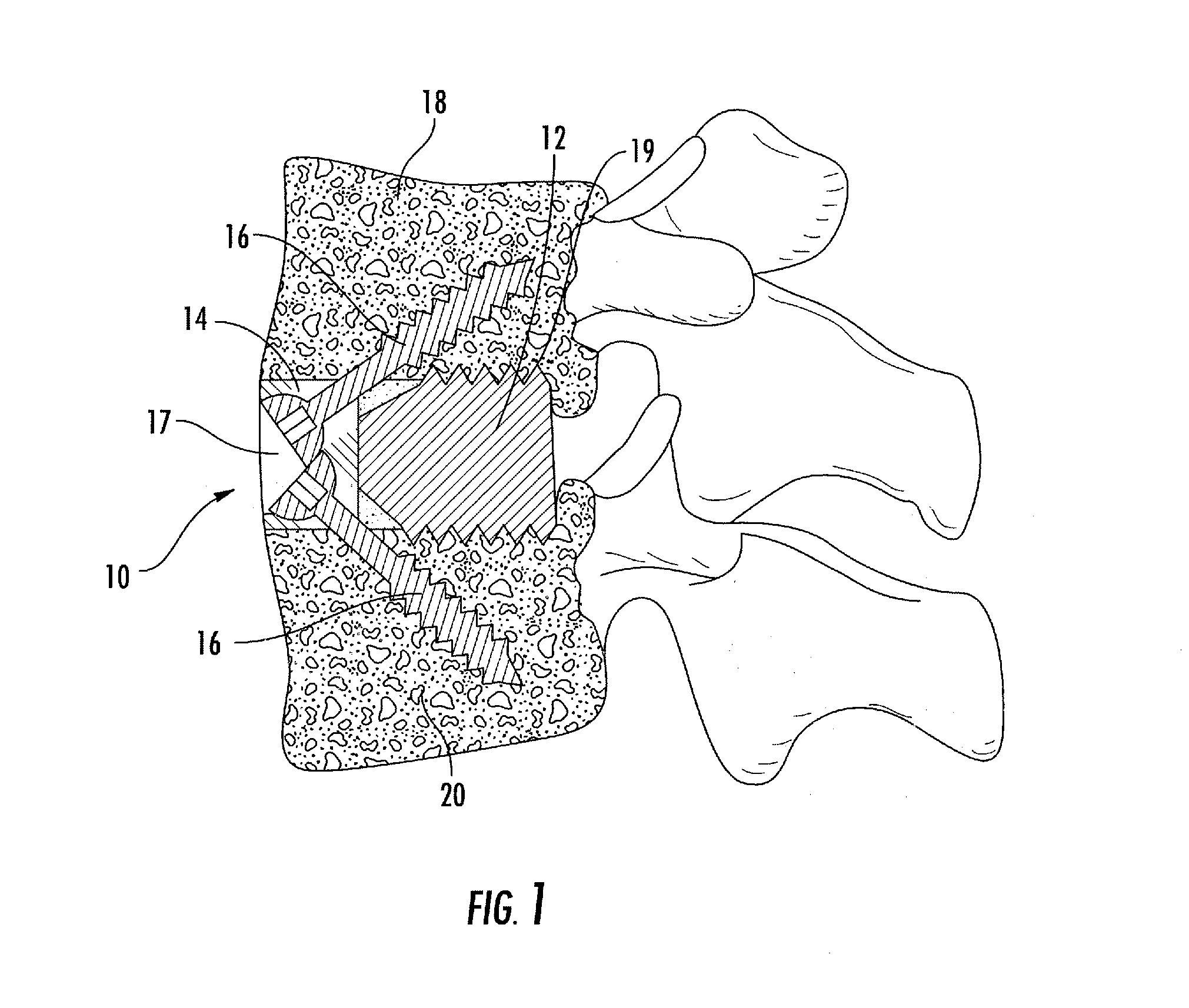
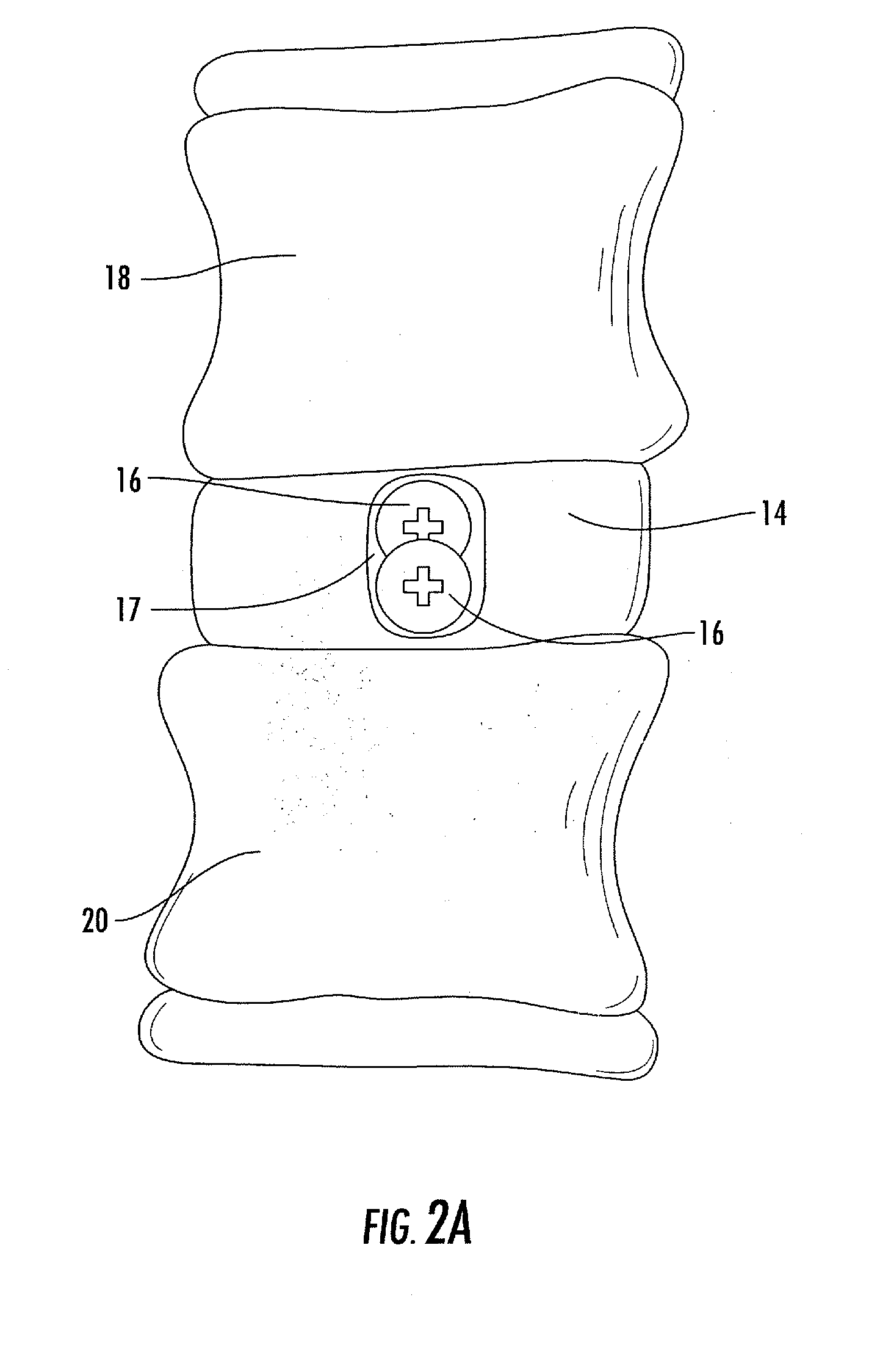
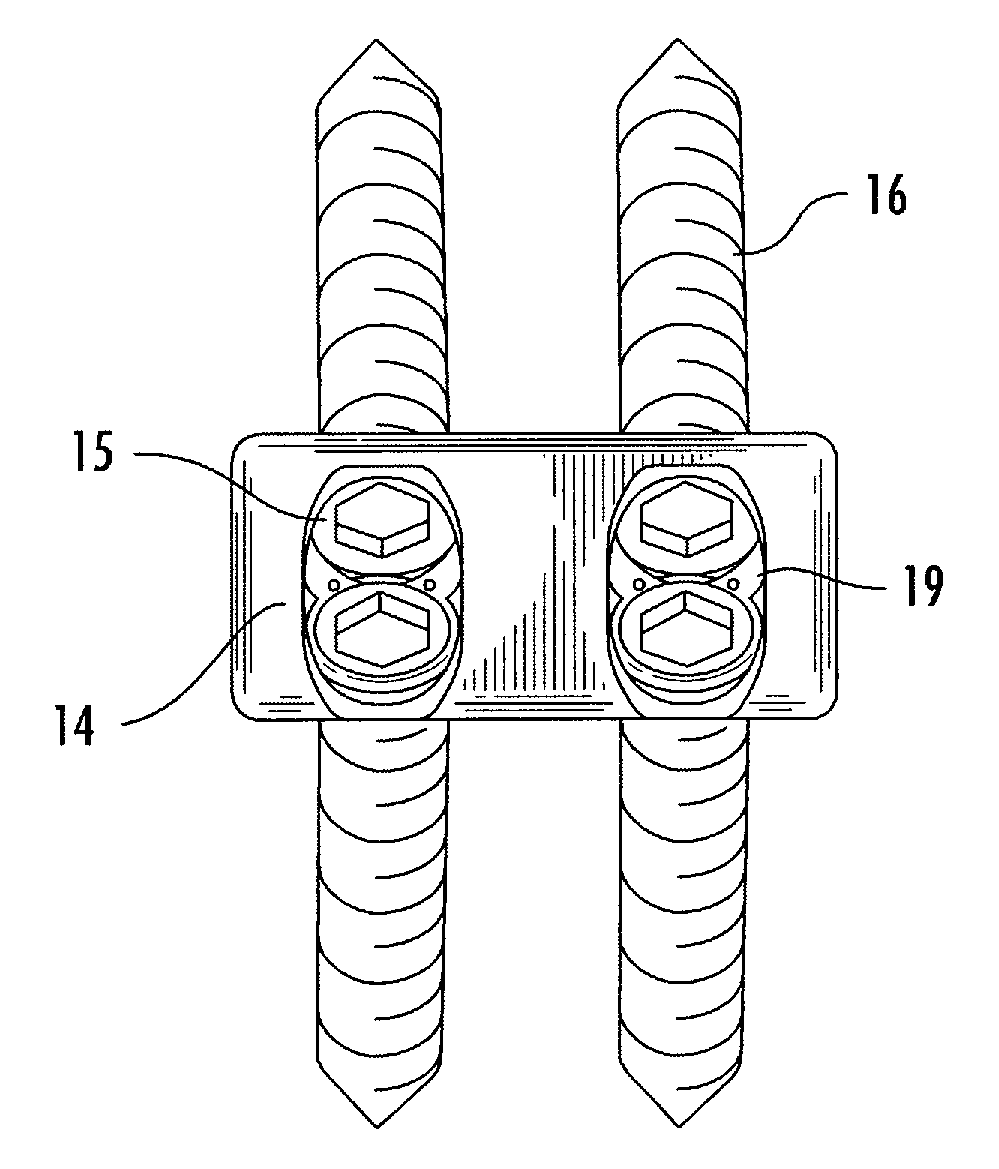
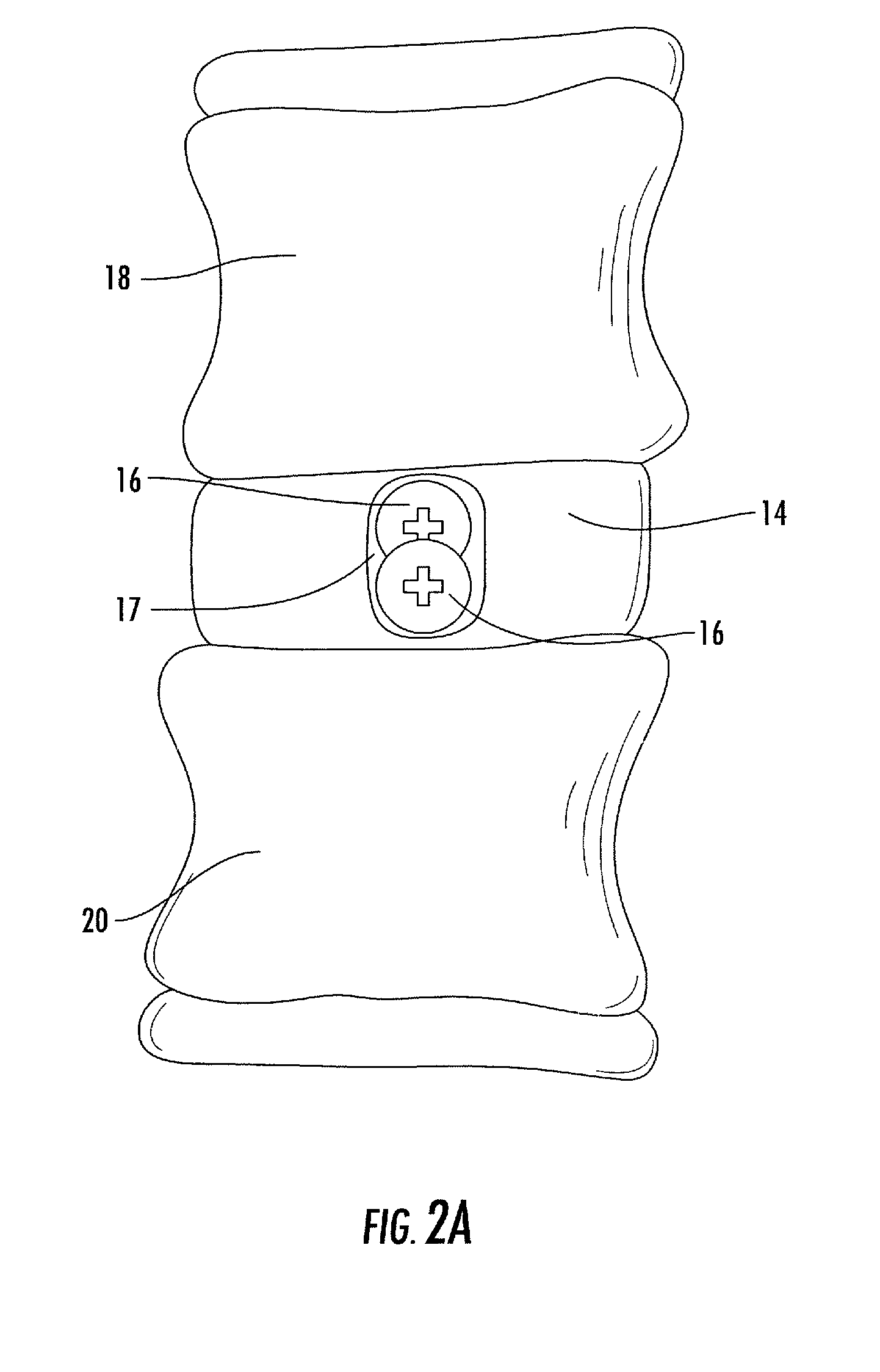
Interbody fusion system with intervertebral implant retention assembly
ActiveUS20100217393A1Eliminating exacerbation of instabilityImprove stabilitySpinal implantsFastenersPosterior instrumentationCorpectomy
The present disclosure is directed towards a biomechanical implant and anterior, lateral or posterior instrumentation construct. The construct may be of unitary or modular construction, whereby a single molded construction can form the entire assembly, in which case the through holes may be adapted to receive a metallic insert for screw fixation; or alternatively be of a modular construction wherein the anterior / lateral instrumentation and intervertebral spacer are designed for removable locking engagement, one with the other, for insertion by the surgeon as a unitary construct. A unique feature of the construct resides within the instrumentation construction, whereby a single opening formed therein permits two bone screws, or the like fastener device, to be positioned within both the superior and inferior vertebral bodies surrounding the spacer implant, or, for example in the case of a corpectomy or diskectomy with cage insertion, wherein two screws can be fixed within a single vertebral body through a single through hole, and wherein the bone screws are constructed and arranged to cooperate with the retention plate so as to provide locking engagement, one to the other, with the retention plate, upon final fixation thereof. Screw retention elements of alternative shape, based upon the choice of vertical or horizontal orientation, based upon an opened figure eight design, are provided for insertion in a groove formed in the borehole of the instrumentation plate which allows insertion of each fixation element but will prevent a loosened fixation element from falling out of the plate.
Owner:SPARTAN CAGE HLDG
Pharmaceutical and cosmeceutical wash-off mousse shampoo compositions, processes for preparing the same and uses thereof
InactiveUS20060057075A1Minimize irritationAntibacterial agentsCosmetic preparationsHead scalpAdditive ingredient
A pharmaceutical or cosmeceutical mousse shampoo composition formulated for application to hirsute areas, such as the human scalp, and containing an active pharmaceutical ingredient as well as a cleansing agent is disclosed. Methods of treatment using the same and processes of preparing the same are also disclosed.
Owner:AGIS INDUSTRIES (1983) LTD
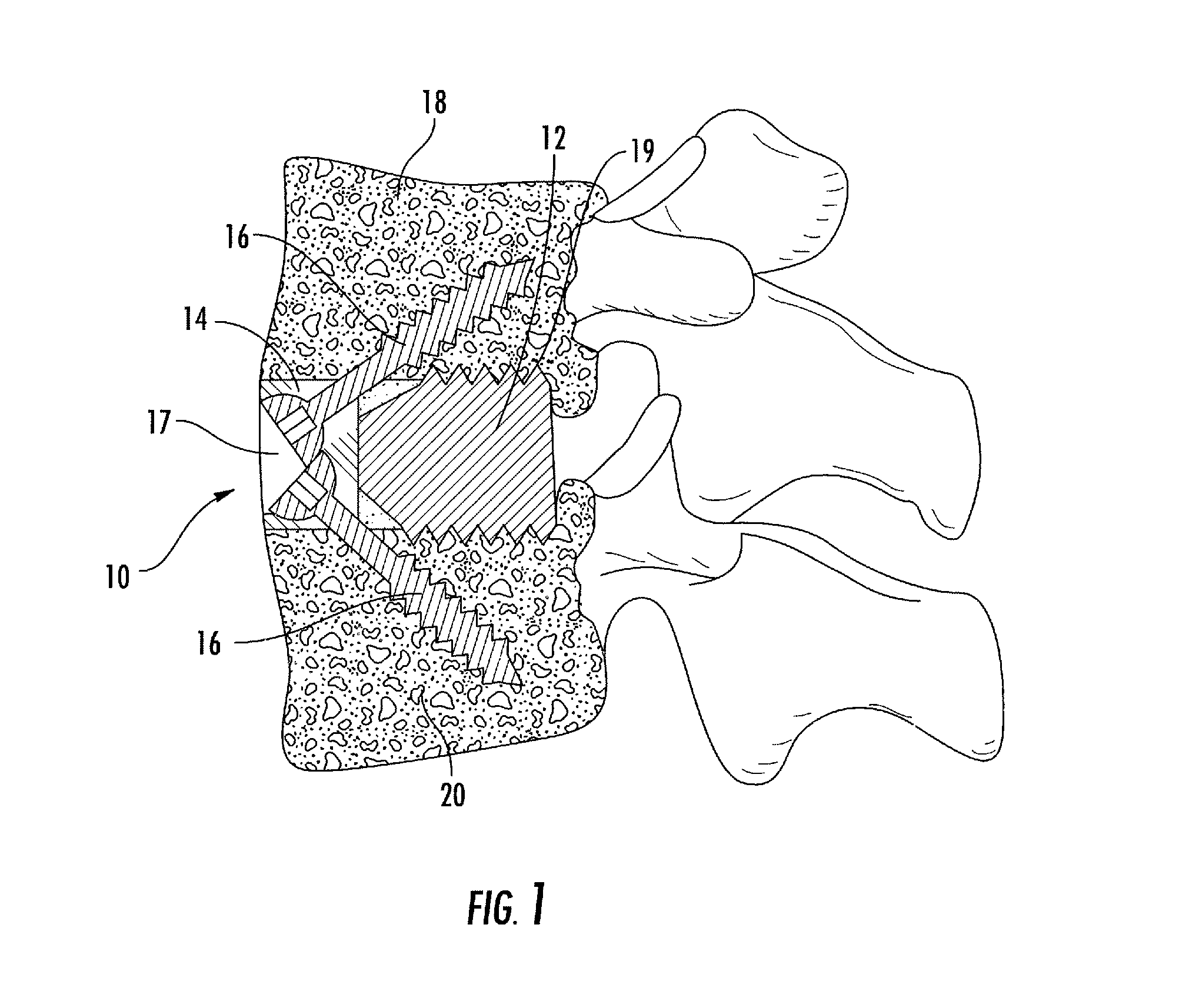
Interbody fusion system with intervertebral implant retention assembly
ActiveUS8187329B2Effective protectionLower profile of implantSpinal implantsFastenersPosterior instrumentationEngineering
The present disclosure is directed towards a biomechanical implant and anterior, lateral or posterior instrumentation construct. The construct may be of unitary or modular construction, whereby a single molded construction can form the entire assembly, in which case the through holes may be adapted to receive a metallic insert for screw fixation; or alternatively be of a modular construction wherein the anterior / lateral instrumentation and intervertebral spacer are designed for removable locking engagement, one with the other, for insertion by the surgeon as a unitary construct. A unique feature of the construct resides within the instrumentation construction, whereby a single opening formed therein permits two bone screws, or the like fastener device, to be positioned within both the superior and inferior vertebral bodies surrounding the spacer implant, or, for example in the case of a corpectomy or diskectomy with cage insertion, wherein two screws can be fixed within a single vertebral body through a single through hole, and wherein the bone screws are constructed and arranged to cooperate with the retention plate so as to provide locking engagement, one to the other, with the retention plate, upon final fixation thereof. Screw retention elements of alternative shape, based upon the choice of vertical or horizontal orientation, based upon an opened figure eight design, are provided for insertion in a groove formed in the borehole of the instrumentation plate which allows insertion of each fixation element but will prevent a loosened fixation element from falling out of the plate.
Owner:SPARTAN CAGE HLDG
Devices for integrated, repeated, prolonged, and/or reliable sweat stimulation and biosensing
ActiveUS20150112164A1Effective simulationMitigate interference of stimulatingElectrotherapySurgerySkin surfaceIrritation
A sweat sensing device includes a plurality of sweat collection pads communicating with a sensor. Each of the pads is activated by a timing circuit which allows one or more of the pads to be activated at a selected time and subsequent deactivated after a defined period of time. This allows for selective collection of sweat from a plurality of pads over a prolonged period of time. An impedance measuring circuit can be employed to determine if one or more of the pads becomes disconnected, in order to avoid irritation. Further, the devices can use a common microfluidic device which both transports sweat activating substance, such as pilocarpine, to the surface of the skin and directs sweat away from the skin to a sensing device.
Owner:UNIVERSITY OF CINCINNATI
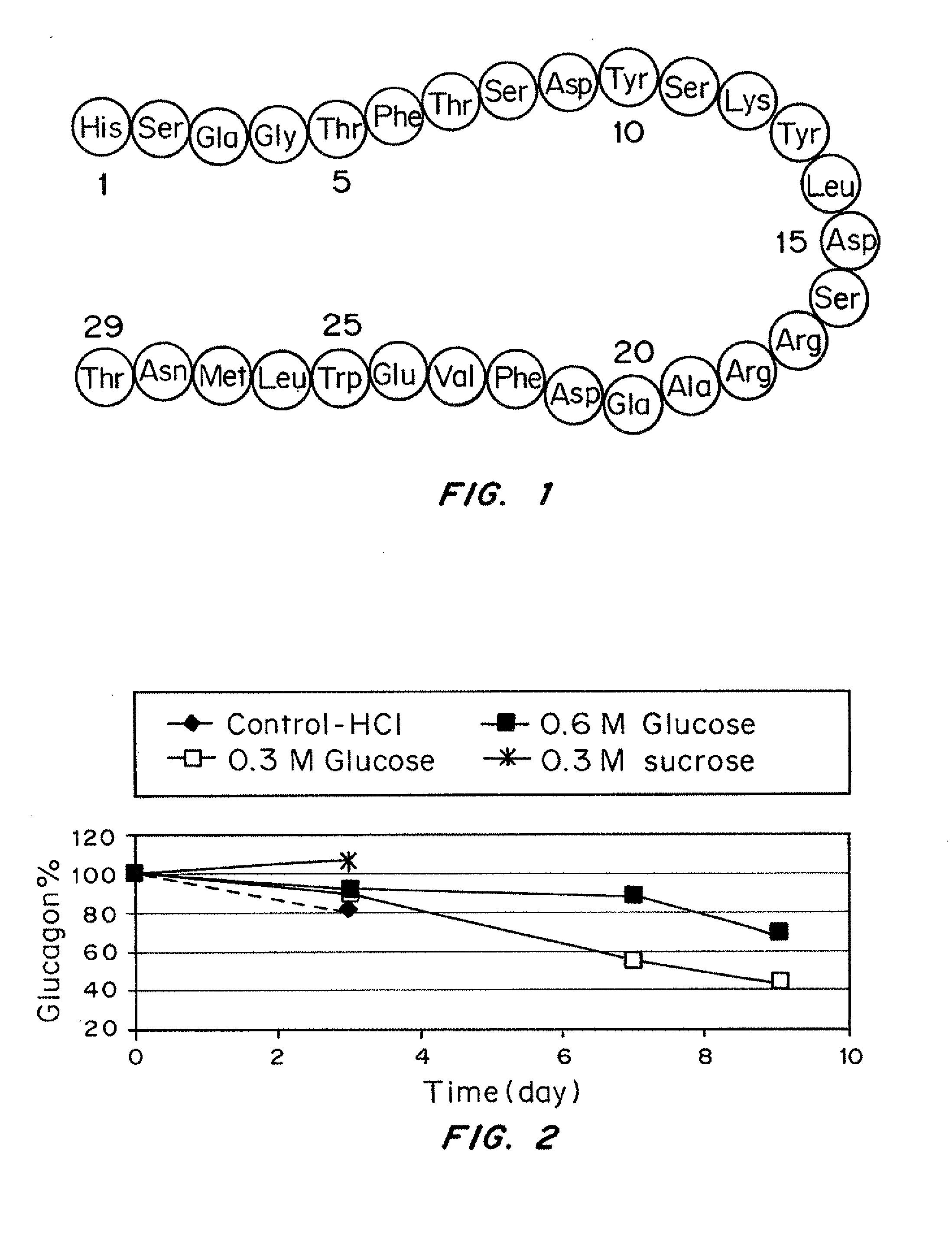
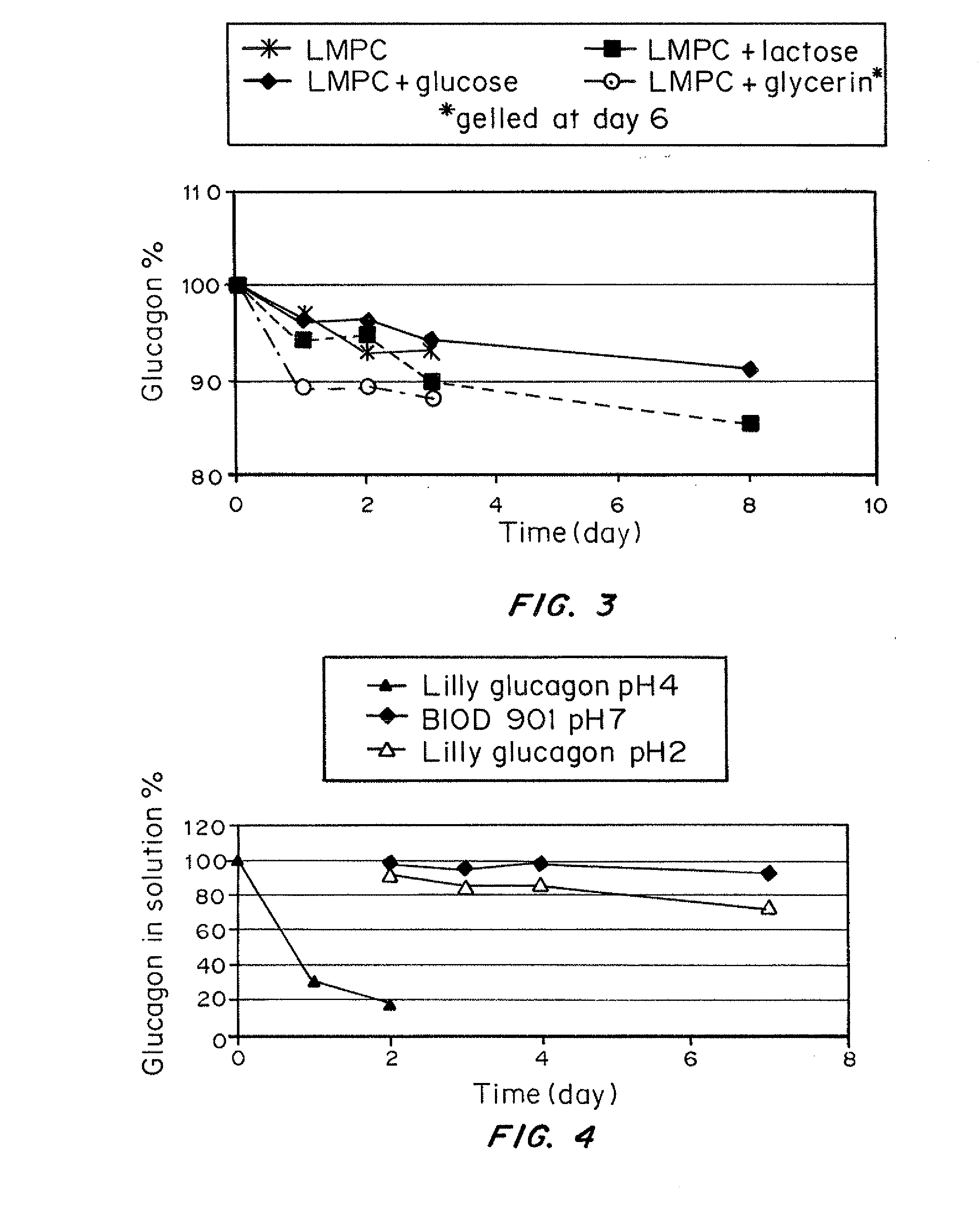
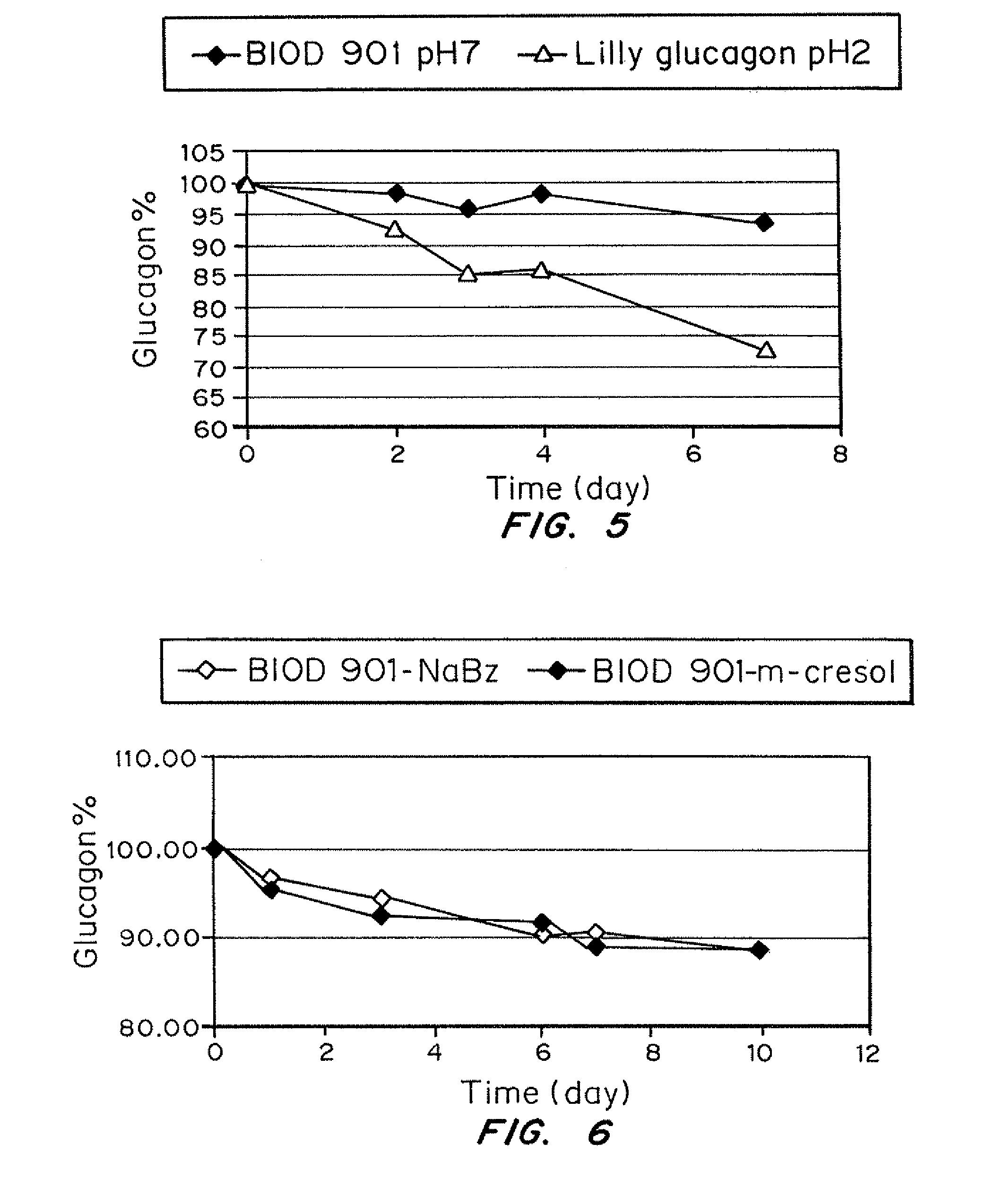
Stabilized glucagon solutions
InactiveUS20110097386A1Assist in elevationStabilize hydrophilic regionPeptide/protein ingredientsMetabolism disorderIrritationGlucose polymers
A formulation composed of a sugar such as glucose and a surfactant such as myristoyl lysophosphocholine (LMPC) has been designed to stabilize both hydrophilic and hydrophobic portions of the glucagon molecule, under prolonged physiological conditions, in a formulation that is sufficiently similar to the pH and osmolarity of plasma so as not to induce or to minimize site irritation. The combination of a simple sugar and an surfactant stabilizes the glucagon molecule in an aqueous solution for seven days at 37° C.
Owner:ALBIREO PHARMA INC
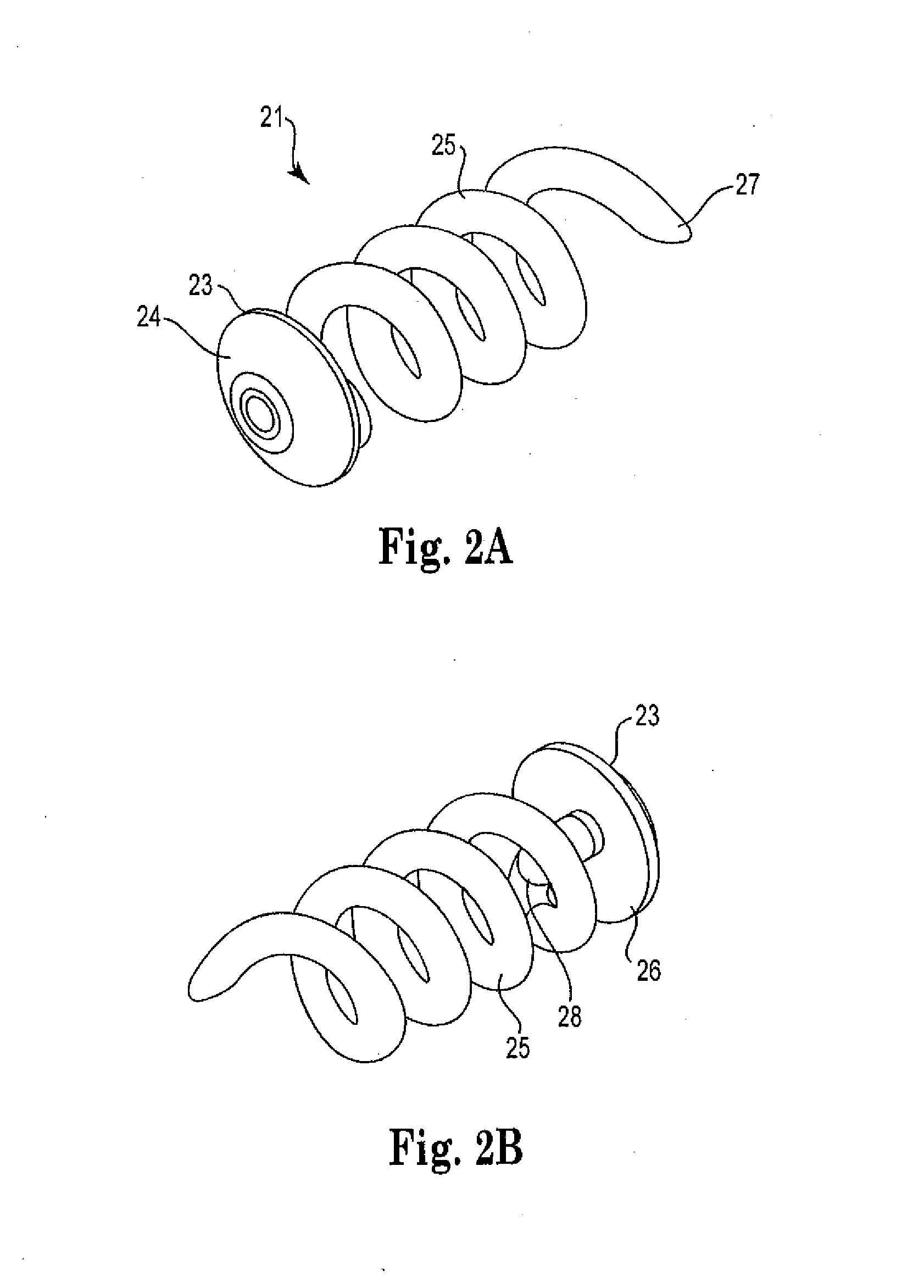
Implantable ocular drug delivery device and methods
InactiveUS20100189765A1Improve stabilityMinimize movementOrganic active ingredientsBiocideDistal portionMedical device
The present invention provides implantable ocular drug delivery devices. Generally, the devices have a distal portion with a coil shaped body member and a proximal portion which contacts the sclera. In one aspect, the coil-shaped body member includes a unique configuration including two coiled portions with different pitches, which improves insertion of the device into the eye. In another aspect, the device has a proximal portion that includes a unique cap configuration having a concave distal face that improves stabilization of the device in the eye. In another aspect, the device includes a transitional portion between the cap and the coil-shaped body member that also improves stabilization of the device in the eye. The invention also provides methods for inserting the medical device into the eye, and methods for the treatment of an ocular condition.
Owner:SURMODICS INC
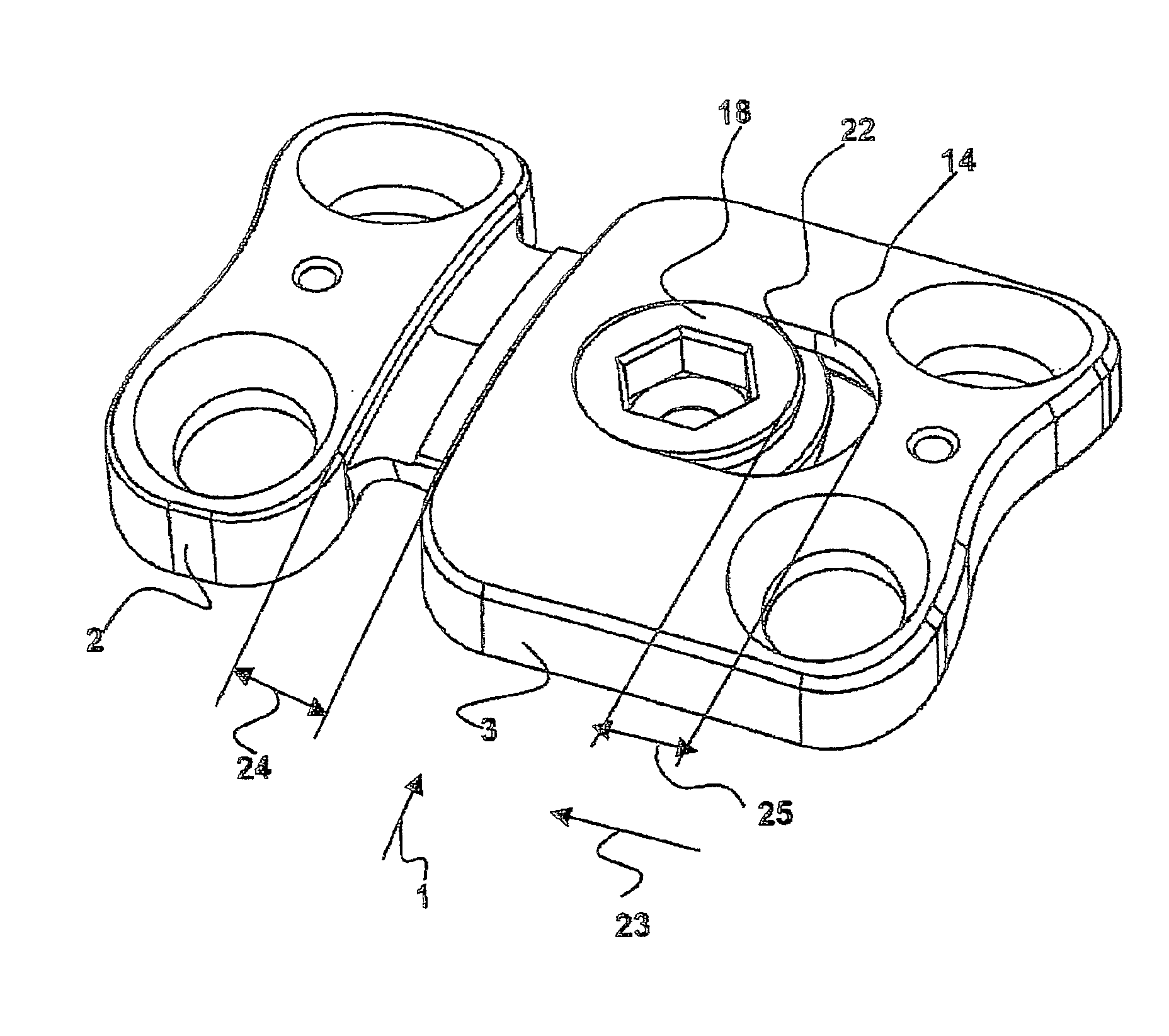
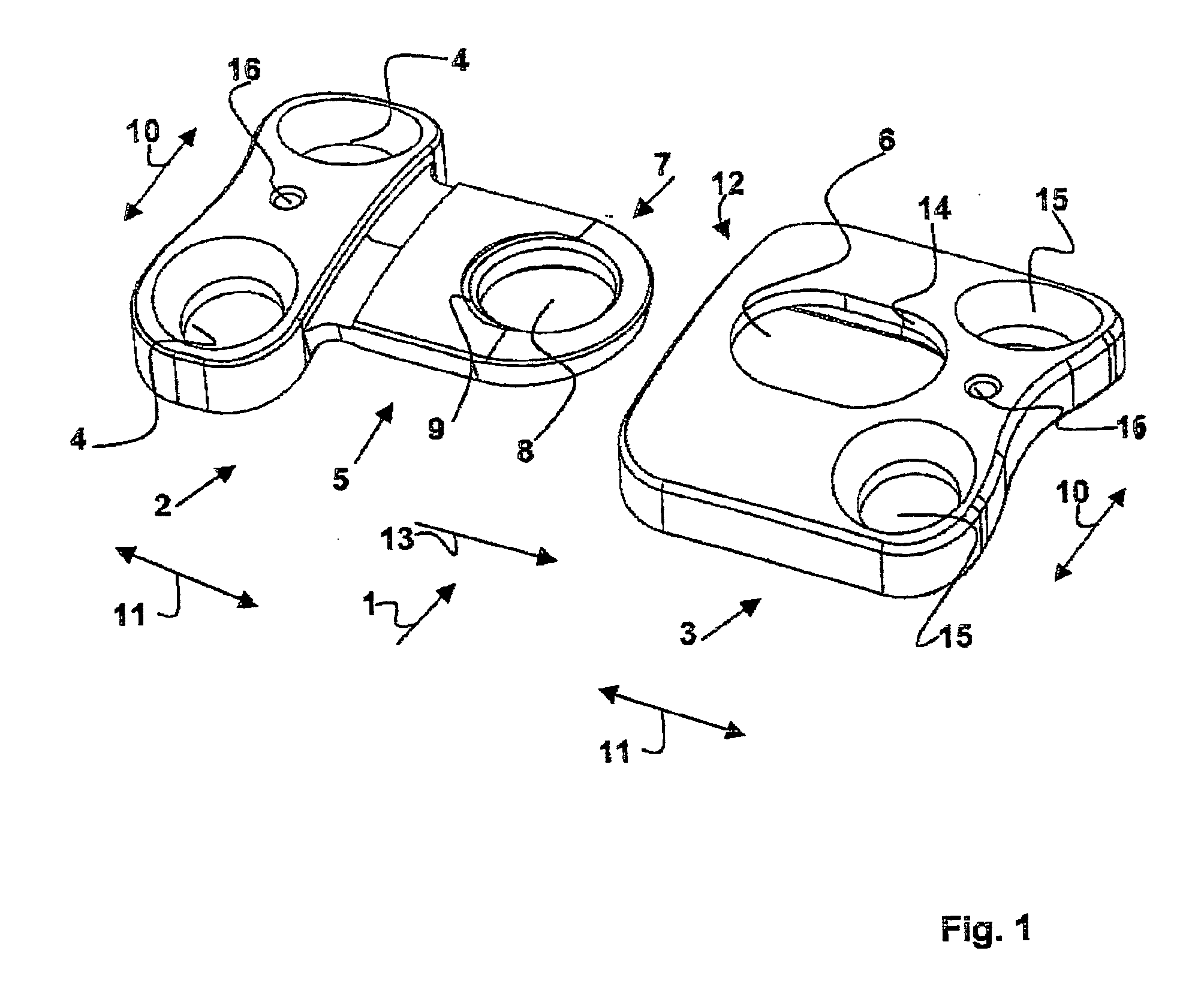
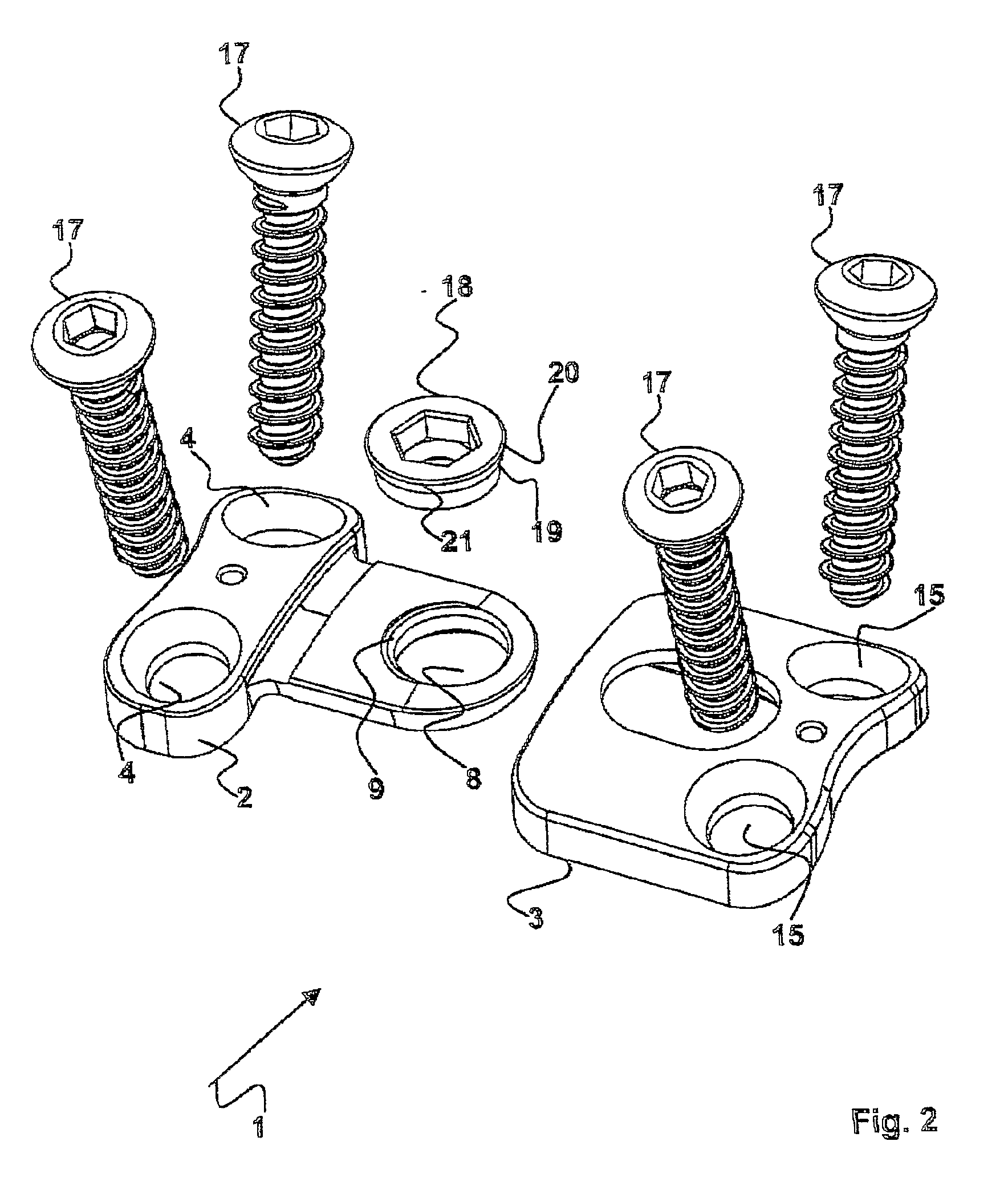
Plate implant, in particular for use on a spinal column
The invention relates to a plate-implant for use in osteosynthesis, comprising at least one first plate component with throughbores for receiving fastening screws and a connection means, at least one second plate component with throughbores for receiving fastening screws and a receiving means for receiving a connection means. According to the invention, each plate component is slidable in one direction relative to the other plate component, the plate components being provided with a device limiting their slide path relative to each other. The device comprises a clamping screw (18) having a thread and a screw head and, in the mounted state of the two plate components (2, 3), the thread cooperates with the connection means (5) and the screw head with a longitudinal borehole (14) provided in the second plate component (3) in such a way that a clamping effect is obtained between the screw head and the longitudinal borehole (14) and between the connection means (5) and the receiving means (6).
Owner:ULRIKH GMBKH & KO KG
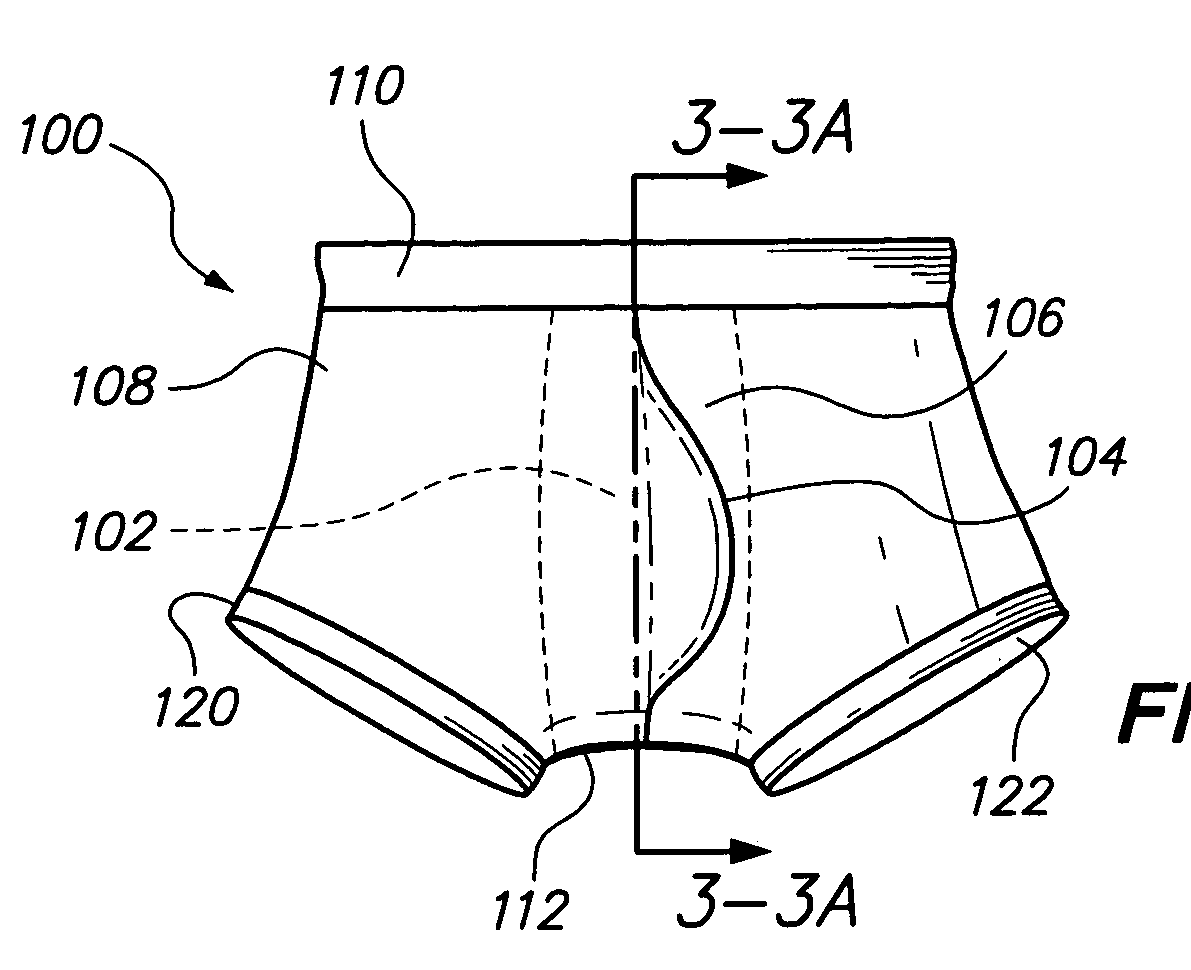
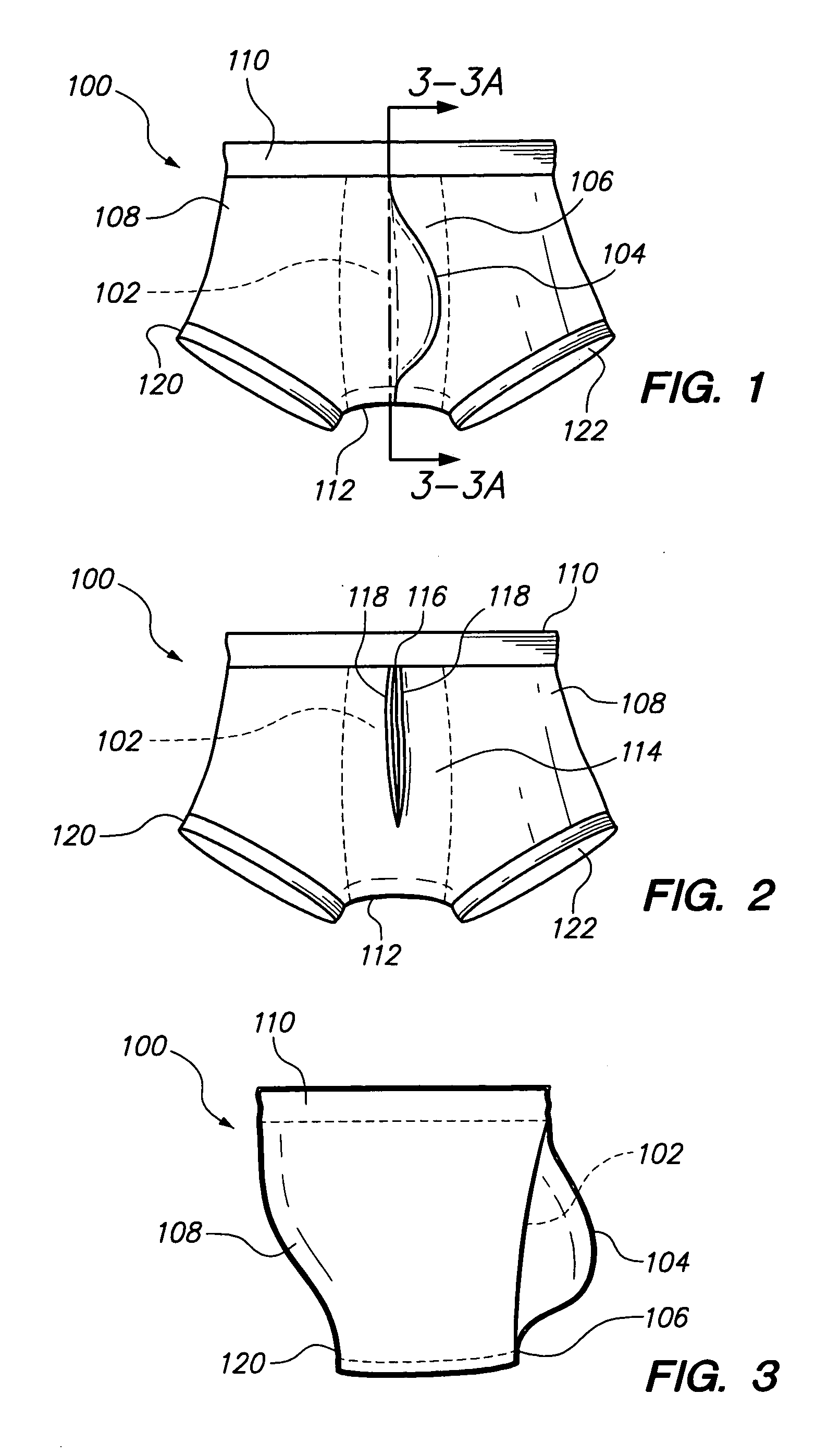
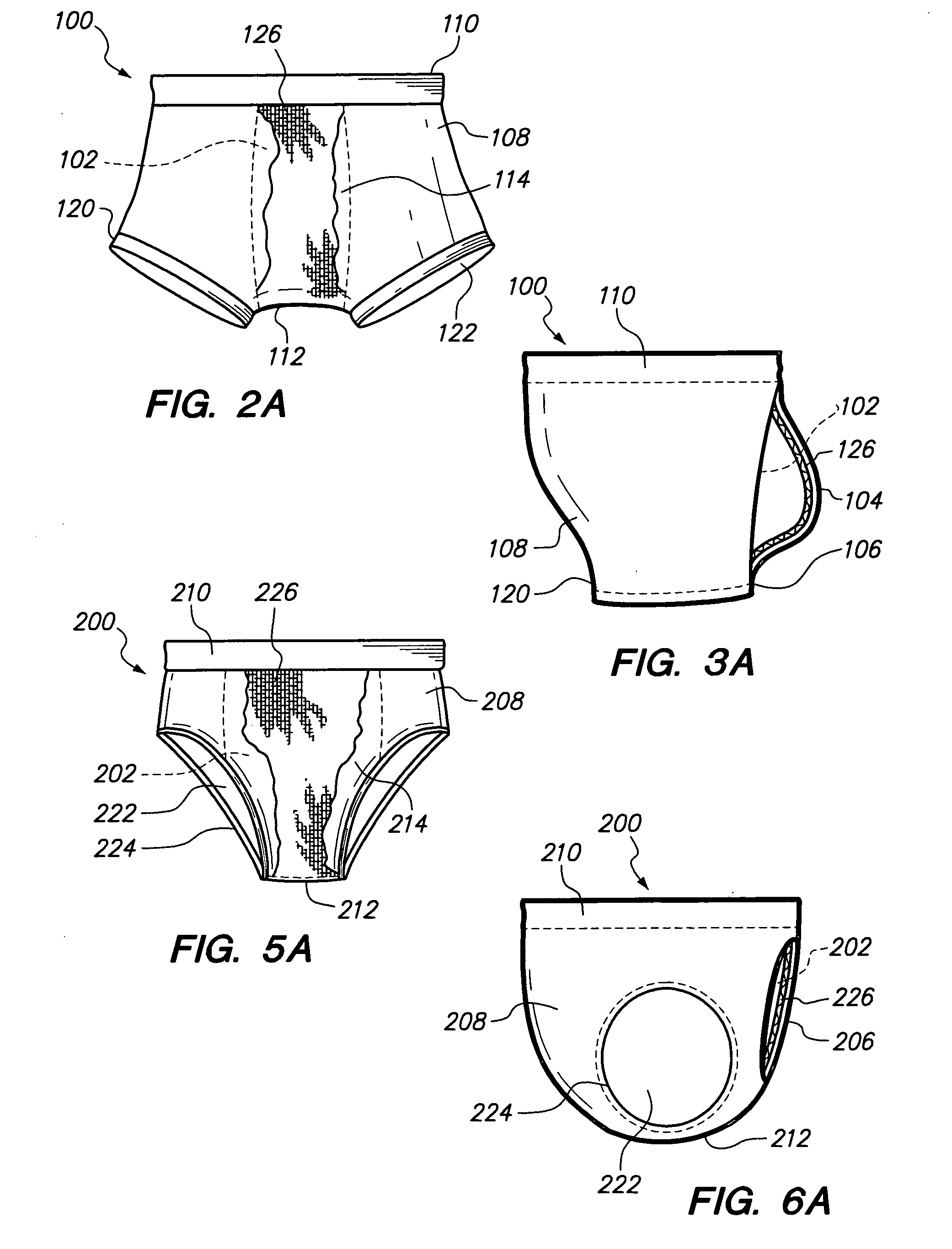
Underwear having internal pocket and pouch
A pair of underwear for use by a male includes a fabric fashioned to form a boxer-brief type undergarment, the fabric having a bilateral symmetry and including a front panel. A rear panel is formed within the fabric and positioned immediately behind the front panel to form an internal pocket. A slit-shaped portal is formed within the rear panel for providing access to the internal pocket for depositing a scrotum and genitalia of the male. Finally, an external pouch extending outward from the front panel is provided as a depository for the genitalia of the male. The boxer-brief type undergarment minimizes perspiration and irritation, absorbs accidental leakage of fluid body waste, and eliminates exposure to the fluid body waste and stains on the body of the male. A thin layer of absorbent material can be included to minimize the effects of accidental leakage.
Owner:SHLUSH JACOB
Method and apparatus for treating subcutaneous histological features
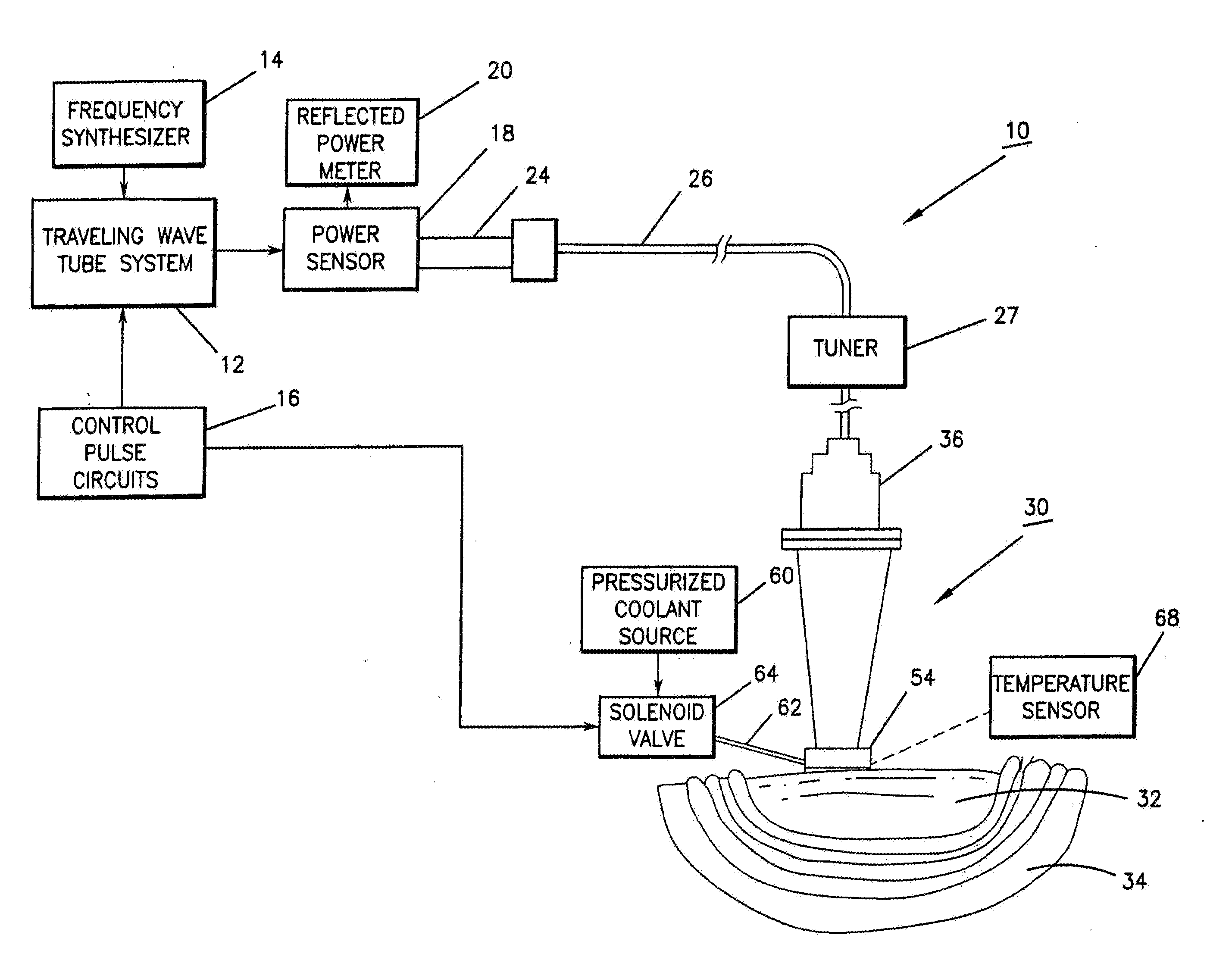
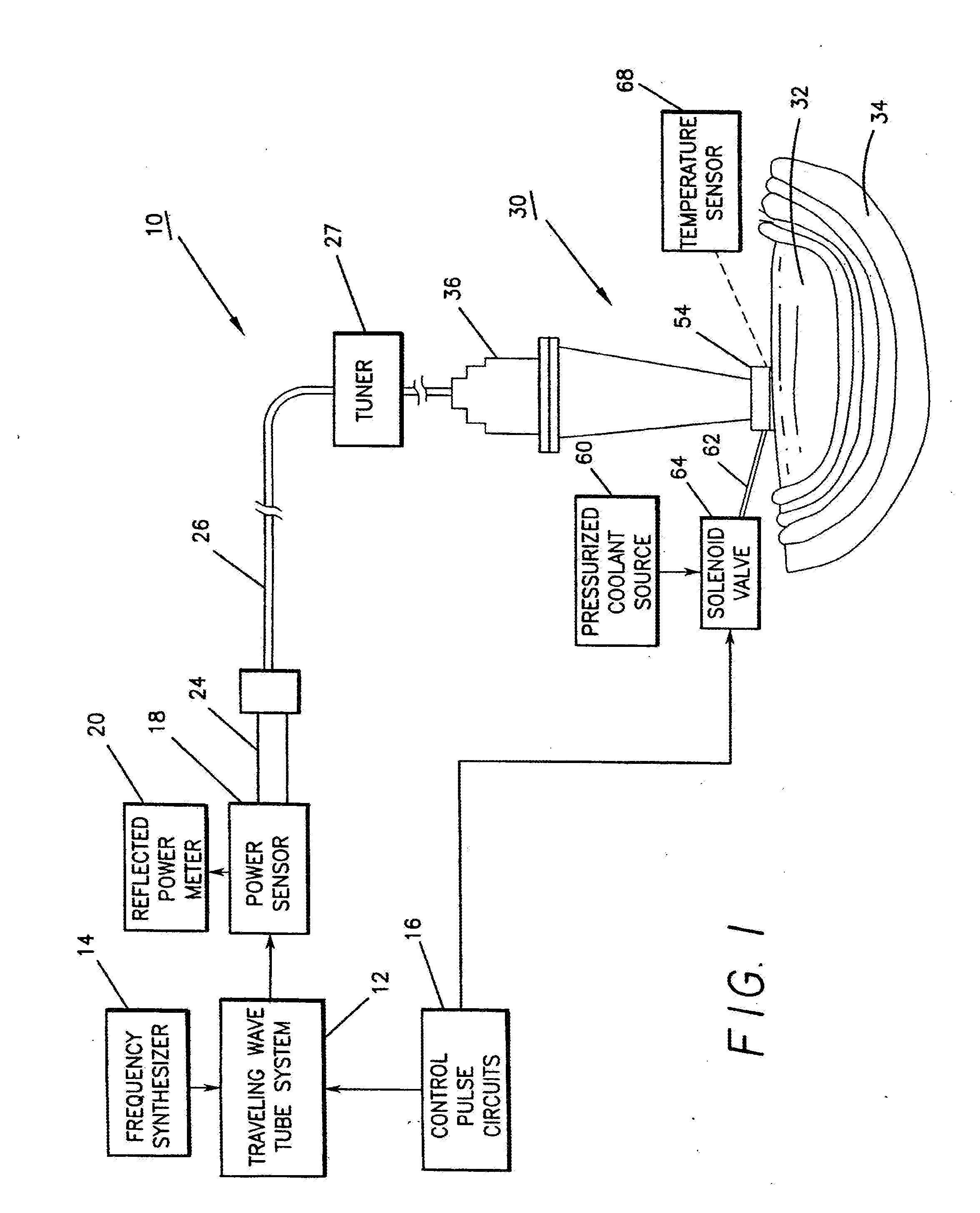
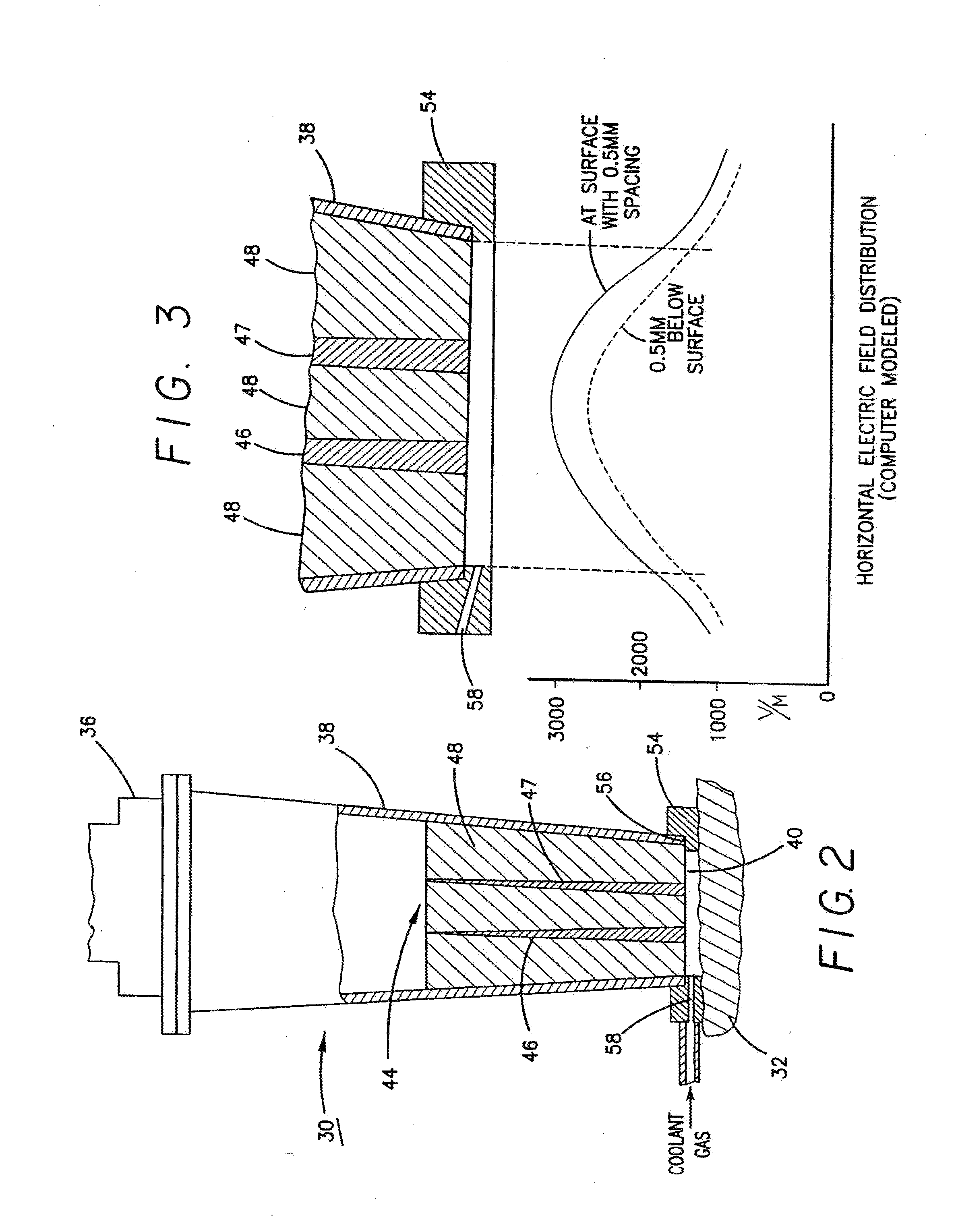
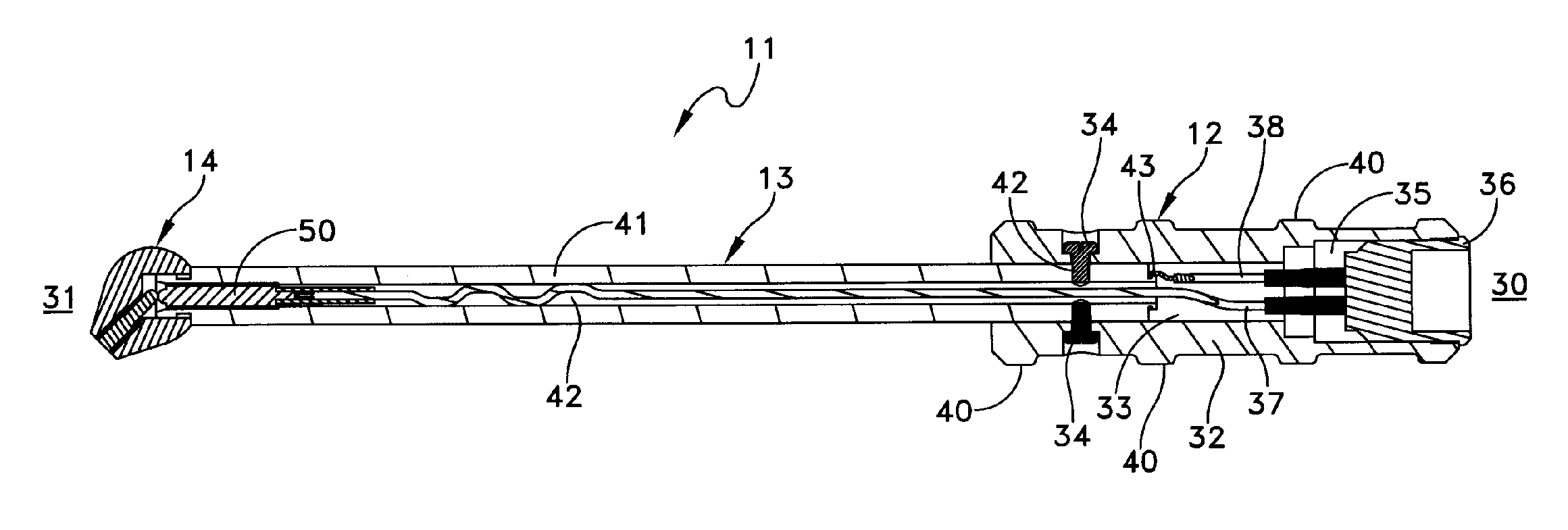
InactiveUS8073550B1Overcome deficienciesMaximize microwave energyDiagnosticsMicrowave therapySide effectMicrowave
A system and method for treating subcutaneous histological features without affecting adjacent tissues adversely employs microwave energy of selected power, frequency and duration to penetrate subcutaneous tissue and heat target areas with optimum doses to permanently affect the undesirable features. The frequency chosen preferentially interacts with the target as opposed to adjacent tissue, and the microwave energy is delivered as a short pulse causing minimal discomfort and side effects. By distributing microwave energy at the skin over an area and adjusting power and frequency, different conditions, such as hirsuitism and telangiectasia, can be effectively treated.
Owner:MIRADRY INC
Immune modulation device for use in animals
InactiveUS6958158B2Minimize irritationEnhance immune responseElectrotherapySnake antigen ingredientsFiberMammal
The present invention is directed to an implantable immune modulation device that is useful for modulating an immune response in mammals, comprising a plurality of fibers, within a porous shell. The fiber filling is loaded with single or multiple antigens, and optionally one or more biologically active compounds such as cytokines (e.g. lymphokines, chemokines etc.), attachment factors, genes, peptides, proteins, nucleotides, carbohydrates or cells depending on the application.
Owner:ORTHO MCNEIL PHARM INC
Oral compositions containing peroxide and methods for use
InactiveUS20050163729A1Effective tooth-whiteningShorten the timeCosmetic preparationsToilet preparationsIrritationTooth whitening
A peroxide-containing dual component tooth-whitening system providing enhanced whitening efficacy, minimal gingival irritation, and tooth sensitivity is described. The system is comprised of a first component containing a peroxide compound and a peroxide-compatible abrasive and a second component containing an alkaline compound. When the components are combined and contact the surface of a tooth an enhanced whitening effect is obtained with minimal tooth sensitivity and gum irritation. A desensitizer compound, a color indicator, and a stabilizing carrier can be included in the system. In certain embodiments, the composition is substantially free of chelating agents.
Owner:COLGATE PALMOLIVE CO
Method and device to prevent cardiac dysrhythmias
InactiveUS6520927B1Avoid mixingMinimize irritationTransvascular endocardial electrodesDiagnosticsCardiac dysrhythmiaCardiac wall
A method and to treat dysrhythmias of the heart, for example atrial fibrillation, by creating artificial conduction pathways, fields or patterns. These artificial pathways or fields are created by injecting materials having desired electrical properties into the walls of the heart.
Owner:UNSWORTH JOHN D
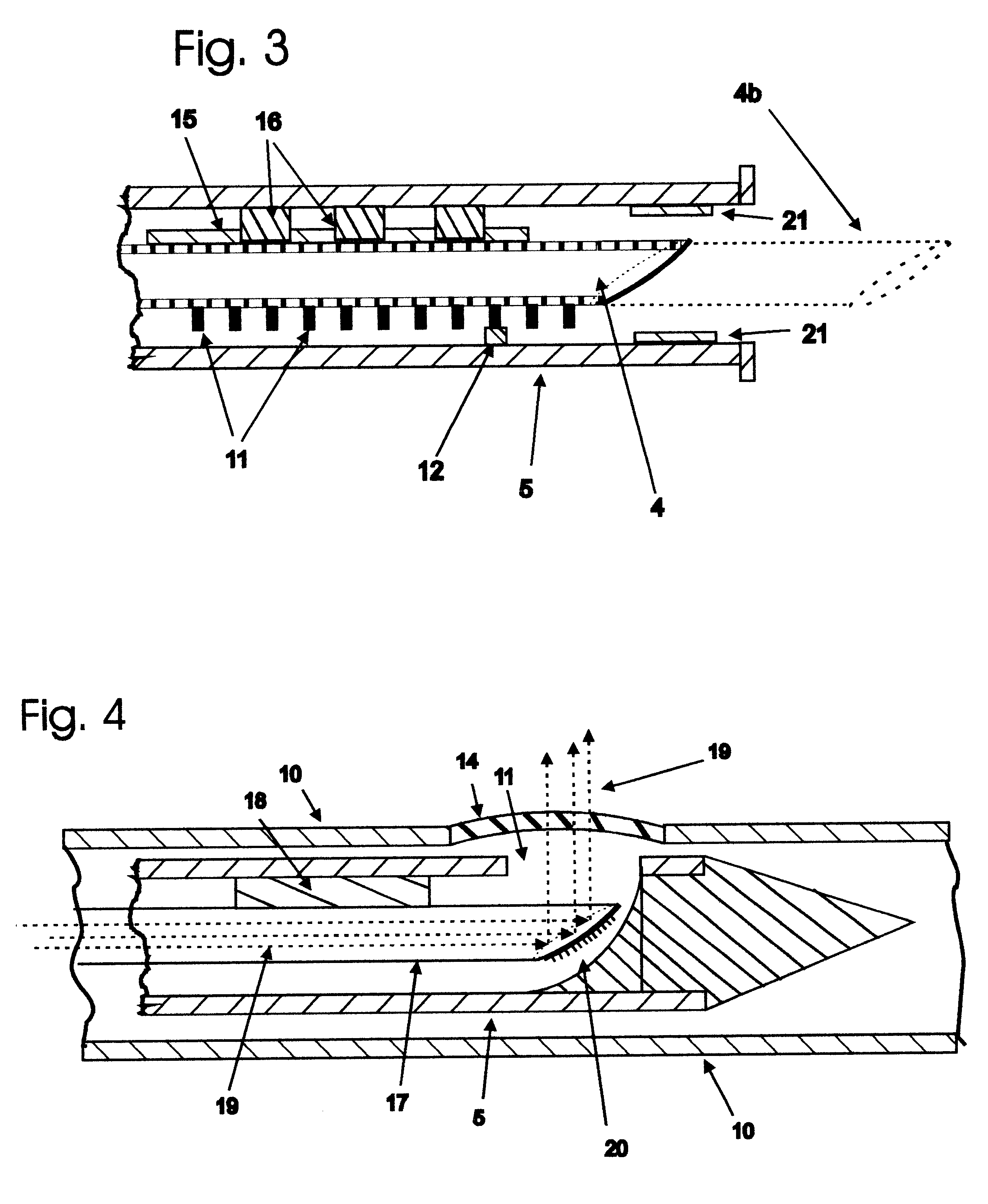
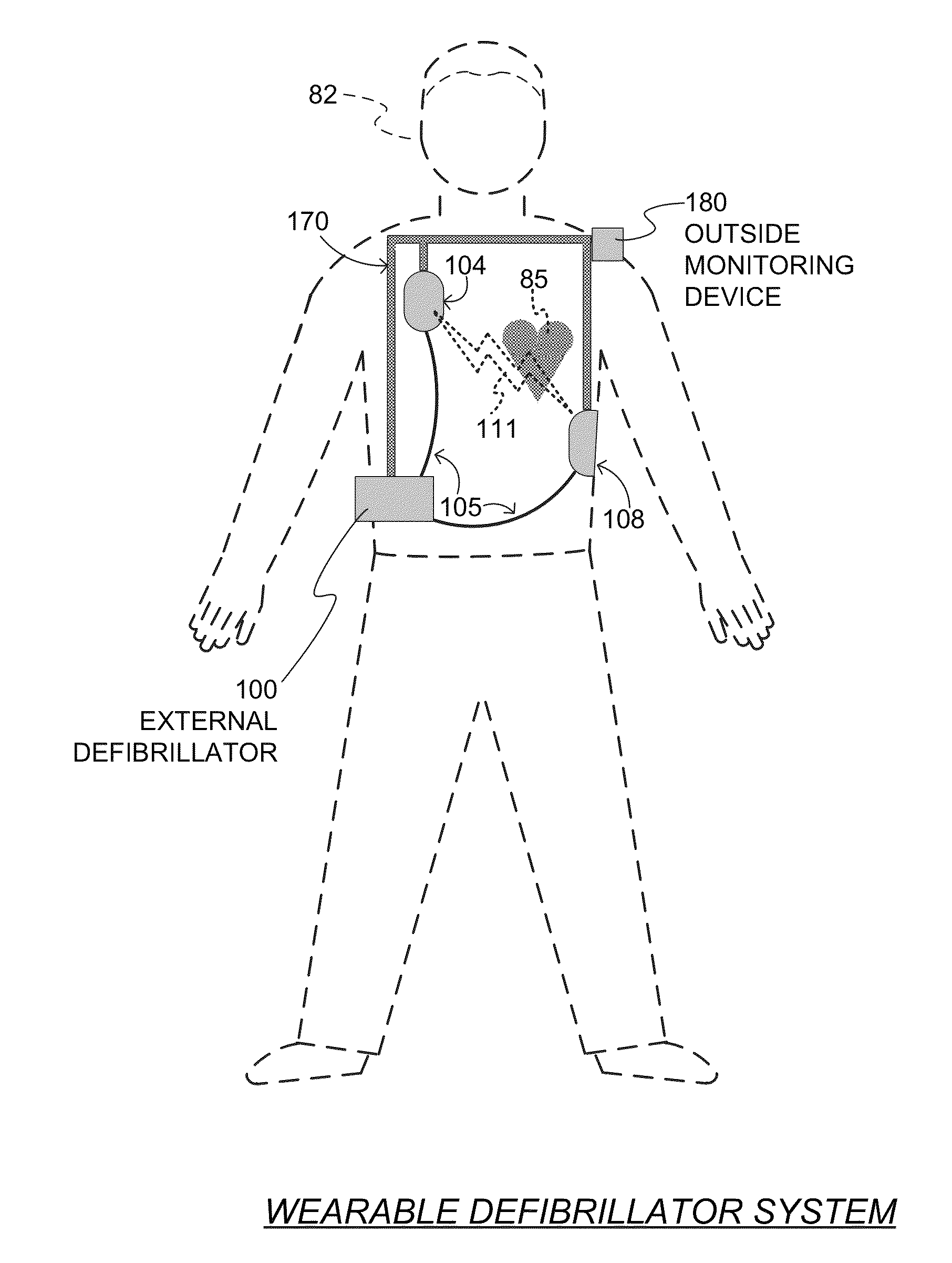
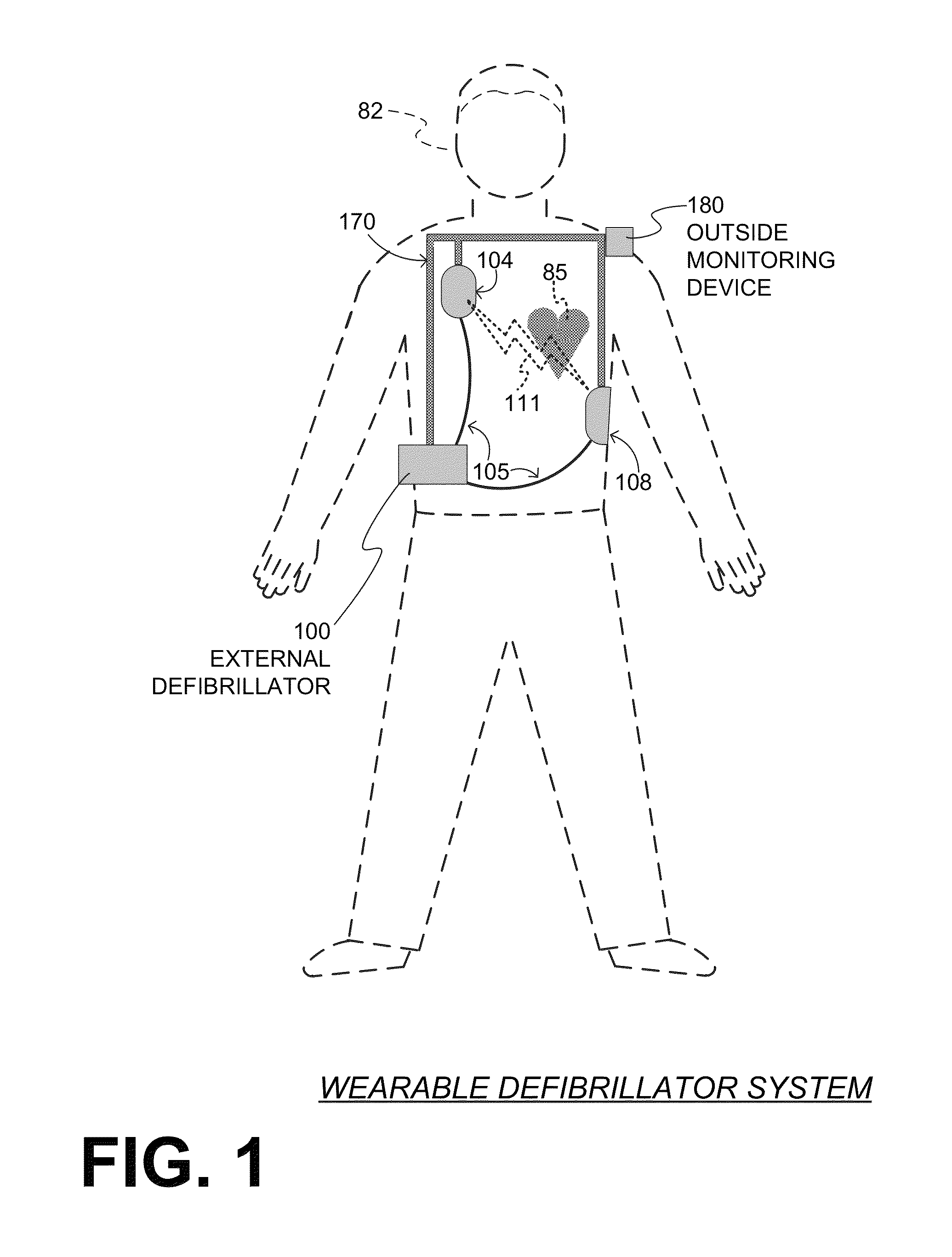
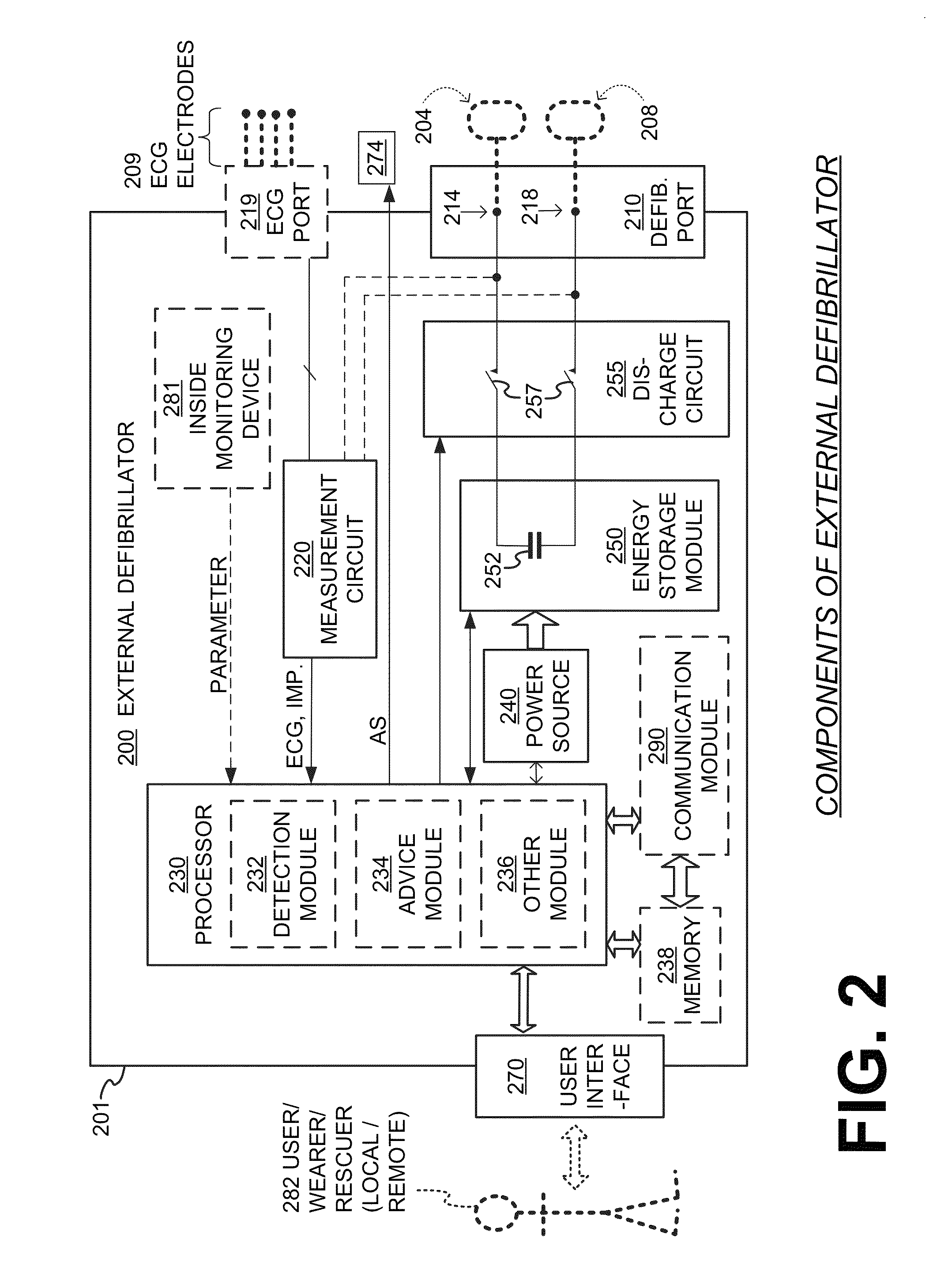
Wearable cardiac defibrillator system controlling conductive fluid deployment
ActiveUS9345898B2Minimize irritationControl releaseHeart defibrillatorsExternal electrodesEngineeringElectrical impedance
In embodiments, a wearable cardiac defibrillator system includes an energy storage module configured to store a charge. Two electrodes can be configured to be applied to respective locations of a patient. One or more reservoirs can store one or more conductive fluids. Respective fluid deploying mechanisms can be configured to cause the fluids to be released from one or more of the reservoirs, which decreases the impedance at the patient location, and decreases discomfort for the patient. In some embodiments an impedance is sensed between the two electrodes, and the stored charge is delivered when the sensed impedance meets a discharge condition. In some embodiments, different fluids are released for different patient treatments. In some embodiments, fluid release is controlled to be in at least two doses, with an intervening pause.
Owner:WEST AFFUM HLDG DAC
Electrode patch and method for neurostimulation
ActiveUS8175718B2Minimize skin irritationReduce placementSensorsExternal electrodesAdhesiveElectrode array
A system for stimulating a nerve or nerves in a patient includes an electrode patch having an array of equally spaced concentric electrodes each having a central cathode and a concentrically surrounding anode. The electrode array patch is used to determine the location of the nerve or nerves to be stimulated by electrical pulses. Once the location of the nerve is determined, a concentric electrode patch having a central cathode and a concentrically surrounding anode is positioned at the optimal location on the patient's skin to effect neurostimulation. The concentric electrode patch may be removably affixed to the patient's skin by adhesive or magnets.
Owner:ETHICON INC
Long-acting oxytocin analogues for the treatment and prevention of breast cancer and psychiatric disorders
InactiveUS6894026B1Prevent and alleviate symptomInhibit initiation and growthOrganic active ingredientsNervous disorderDiseaseVein
Methods and compositions are provided for prophylaxis and treatment of breast cancer involving administration of a therapeutically effective amount of carbetocin and / or other long-acting oxytocin analogues. 1-Butanoic acid-2-(O-methyl-L-tyrosine)-1-carbaoxytocin (carbetocin) and / or other long-acting oxytocin analogues are formulated with a pharmaceutically acceptable carrier and administered in an amount sufficient to inhibit initiation or growth of breast cancer in the patient. The carbetocin and / or other long-acting oxytocin analogues may also be formulated with a pharmaceutically acceptable carrier and administered in an amount sufficient to treat, prevent or alleviate the symptoms of a psychiatric disorder in the patient. Carbetocin may be administered prophylactically or to treat existing conditions in patients by a variety of administration modes, including intramuscular, intravenous, intranasal, intrapulmonary, subcutaneous, parenteral, oral, or transdermal delivery methods and formulations. Preferably, the carbetocin is administered to a mucosal surface of the patient via intranasal delivery. For this purpose, pharmaceutical compositions are provided for intranasal delivery that incorporate carbetocin in a powder or aqueous formulation for intranasal delivery.
Owner:KYALIN BIOSCI
Compositions and methods for amelioration of human female sexual dysfunction
InactiveUS20050004226A1Modulate sexual responseIncrease secretionBiocideElcosanoid active ingredientsDiseaseFemale Sexual Arousal Disorder
The invention provides compositions and methods suitable for ameliorating female sexual dysfunction, and in particular, female sexual arousal disorder. In preferred embodiments, the invention provides a semisolid composition suitable for topical application comprising: an effective amount of a vasoactive prostaglandin, a penetration enhancer, a polymer thickener, a lipophilic component, and an acidic buffer system. In other embodiments, the invention provides a method of treating female sexual arousal disorder by applying an effective dose of a topical semisolid prostaglandin composition to the anterior wall of the vagina.
Owner:NEXMED HLDG INC
Indwelling urinary catheter with self-retaining mechanism
A urinary catheter with an improved retaining and activating feature is provided which is a safe device with reduced irritation and discomfort to a patient. The retaining mechanism positioned at the proximal end of the catheter assumes a “close” state for introduction and removal of the catheter into and from the urethral tract, and is transitioned into the “open” state when the catheter is in the bladder by mechanically manipulating the retaining mechanism through the activation mechanism. An actuating linkage wire connected between the retaining mechanism and activation mechanism controllably reciprocates in the channel of the catheter to transition the catheter between the “open” and “closed” states.
Owner:LOTUS MEDICAL TECH
Strap-Based Exercise System
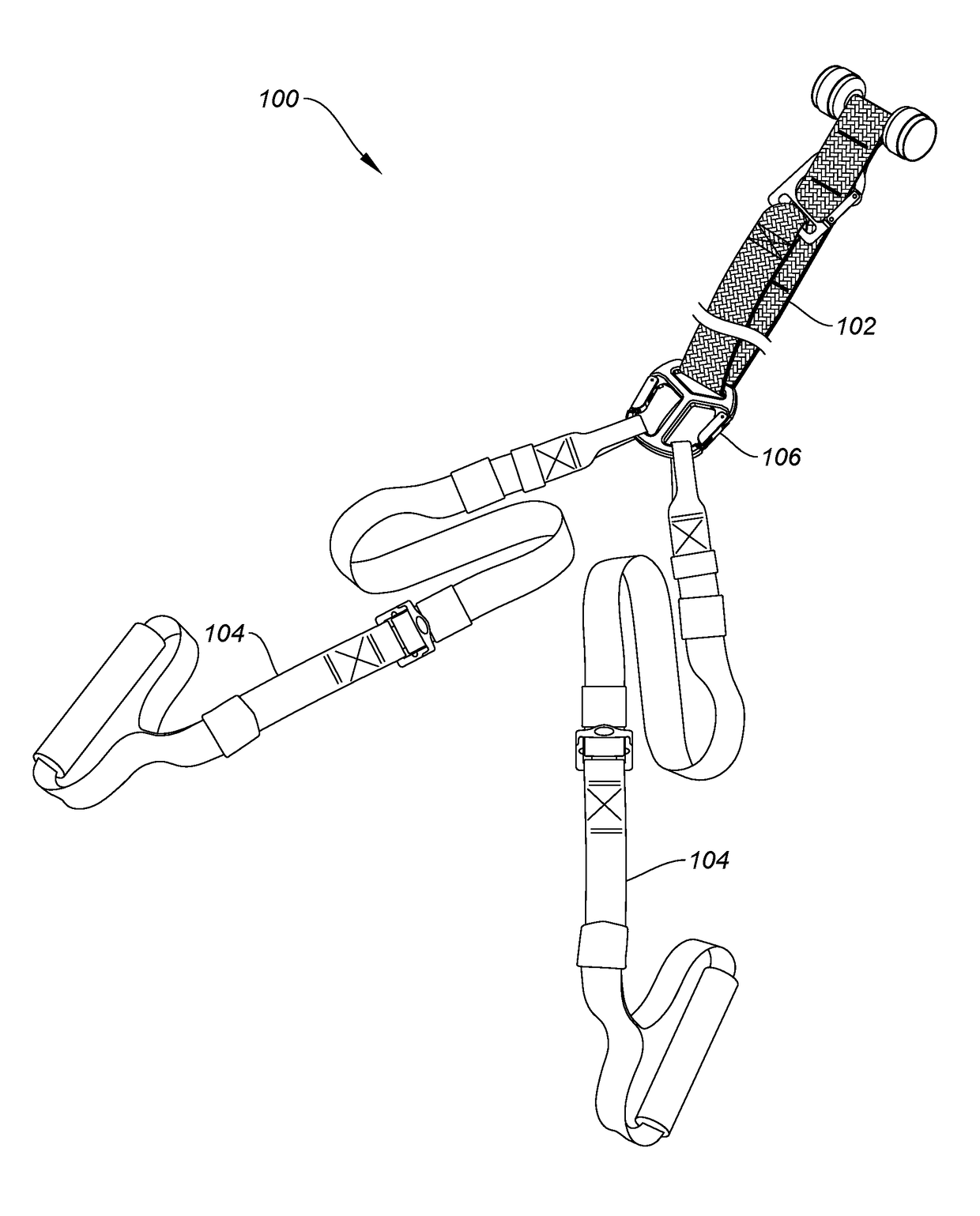
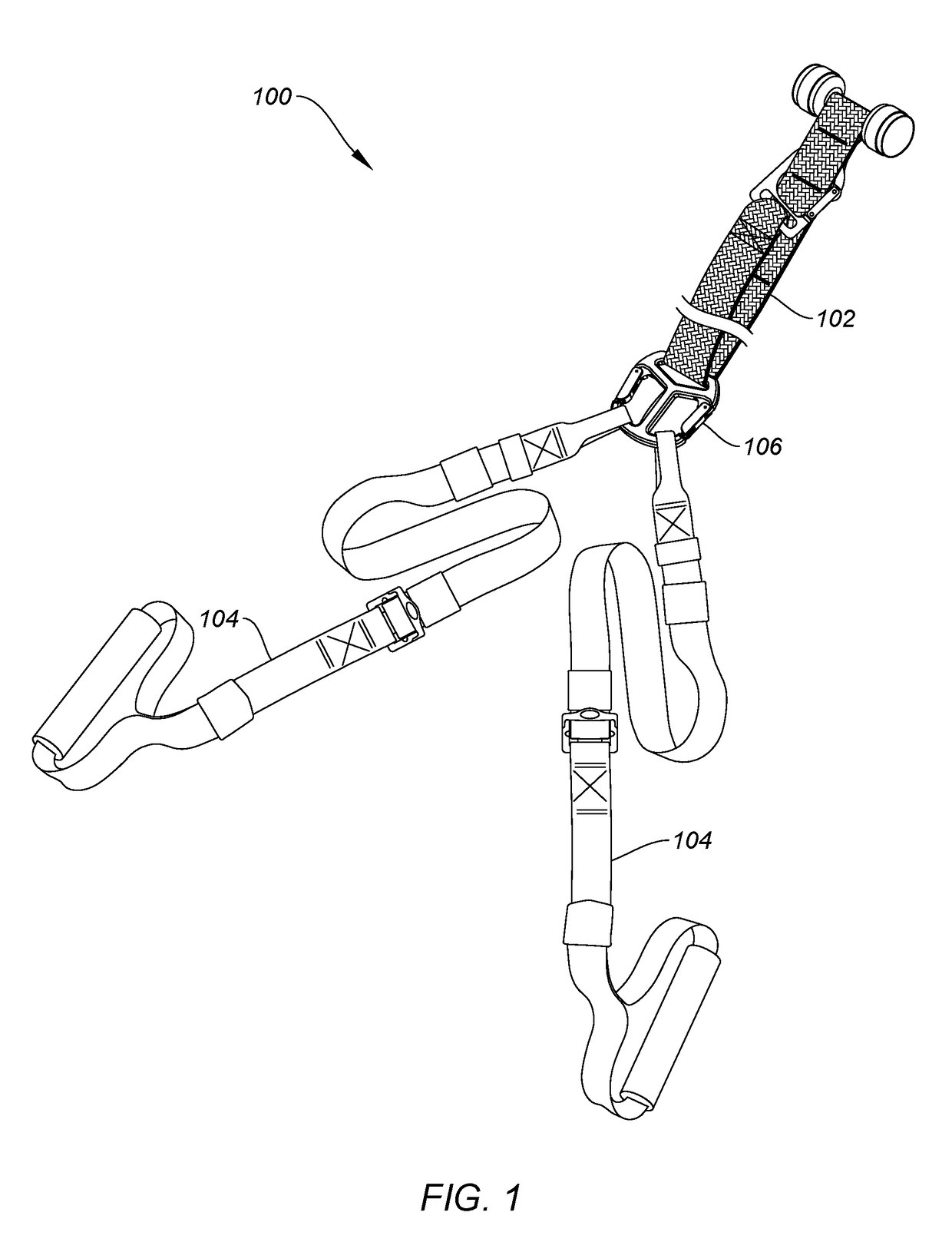
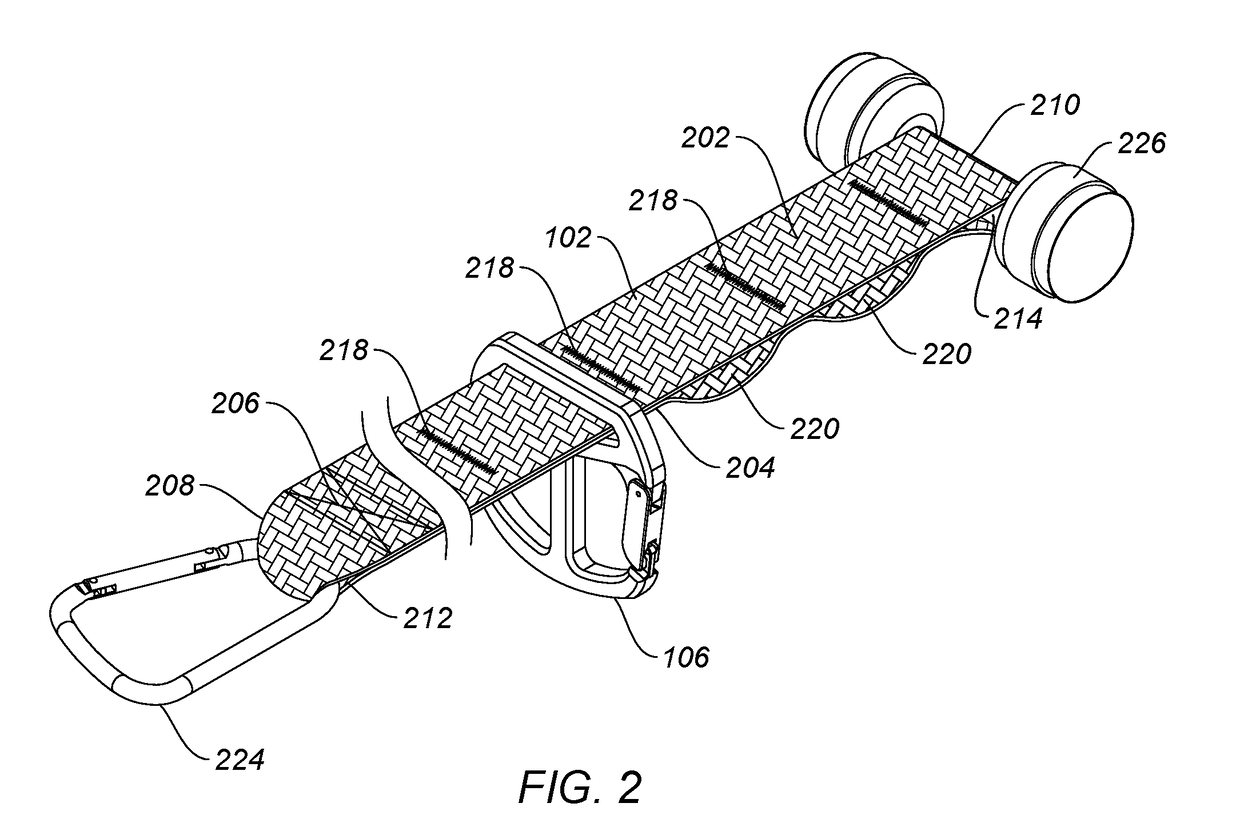
ActiveUS20180318631A1Minimize irritationMountaineeringMuscle exercising devicesEngineeringStationary object
The invention is a strap-based suspension exercise system that includes a main strap that is attached to a fixed or stationary object to anchor the exercise system and exercise straps that are used to perform a variety of exercises. Different main straps are provided that enable anchoring the exercise system virtually anywhere. The exercise straps, which may be inelastic or elastic are attached to the main strap using a uniquely designed buckle and include a safety strap. Various optional equipment, such as a foot hammock, a wrist or foot strap, additional handles, exercise straps with gym rings, and a vest worn by the user are provided.
Owner:AUSTER ENTERPRISES LTD
Method and Apparatus for Treating Subcutaneous Histological Features
InactiveUS20120041432A1Maximize microwave energyEfficient deliveryMicrowave therapySurgical instruments for heatingSide effectMicrowave
A system and method for treating subcutaneous histological features without affecting adjacent tissues adversely employs microwave energy of selected power, frequency and duration to penetrate subcutaneous tissue and heat target areas with optimum doses to permanently affect the undesirable features. The frequency chosen preferentially interacts with the target as opposed to adjacent tissue, and the microwave energy is delivered as a short pulse causing minimal discomfort and side effects. By distributing microwave energy at the skin over an area and adjusting power and frequency, different conditions, such as hirsuitism and telangiectasia, can be effectively treated.
Owner:MICROWAVE MEDICAL
Dermal phase meter with improved replaceable probe tips
InactiveUS20070222467A1Minimizes tissue irritationMinimize irritationElectronic circuit testingFault location by increasing destruction at faultElastomerElectricity
A probe for a dermal phase meter includes a handle with an extension that terminates with a displaceable center conductor. A replaceable tip attaches to the distal end of the extension and includes a center conductor that engages the center conductor in the extension and an outer conductor that establishes electrical connection through the extension. Substituting different replacement tips provides a probe with an articulation capability. Probe tips with a layer of a patient compatible, compressible elastomer overmolded onto the outer conductor provides insulation and minimizes the potential for irritating tissues during a measurement in an anatomical cavity.
Owner:NOVA TECH CORP
Injection device for the intervertebral disc
ActiveUS20090082719A1Reduce bendingReduce droopInternal osteosythesisSurgical needlesNutrientSacroiliac joint
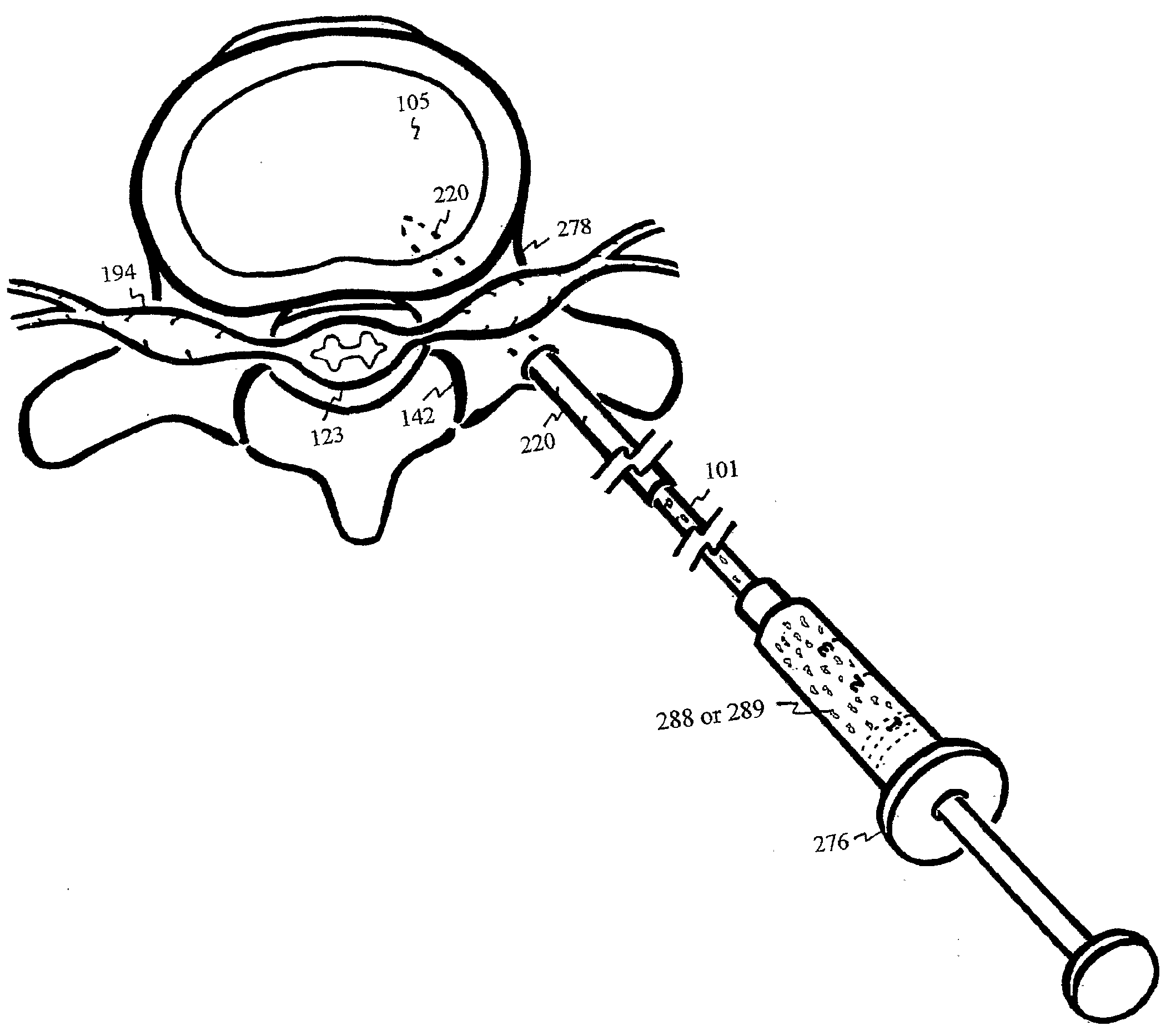
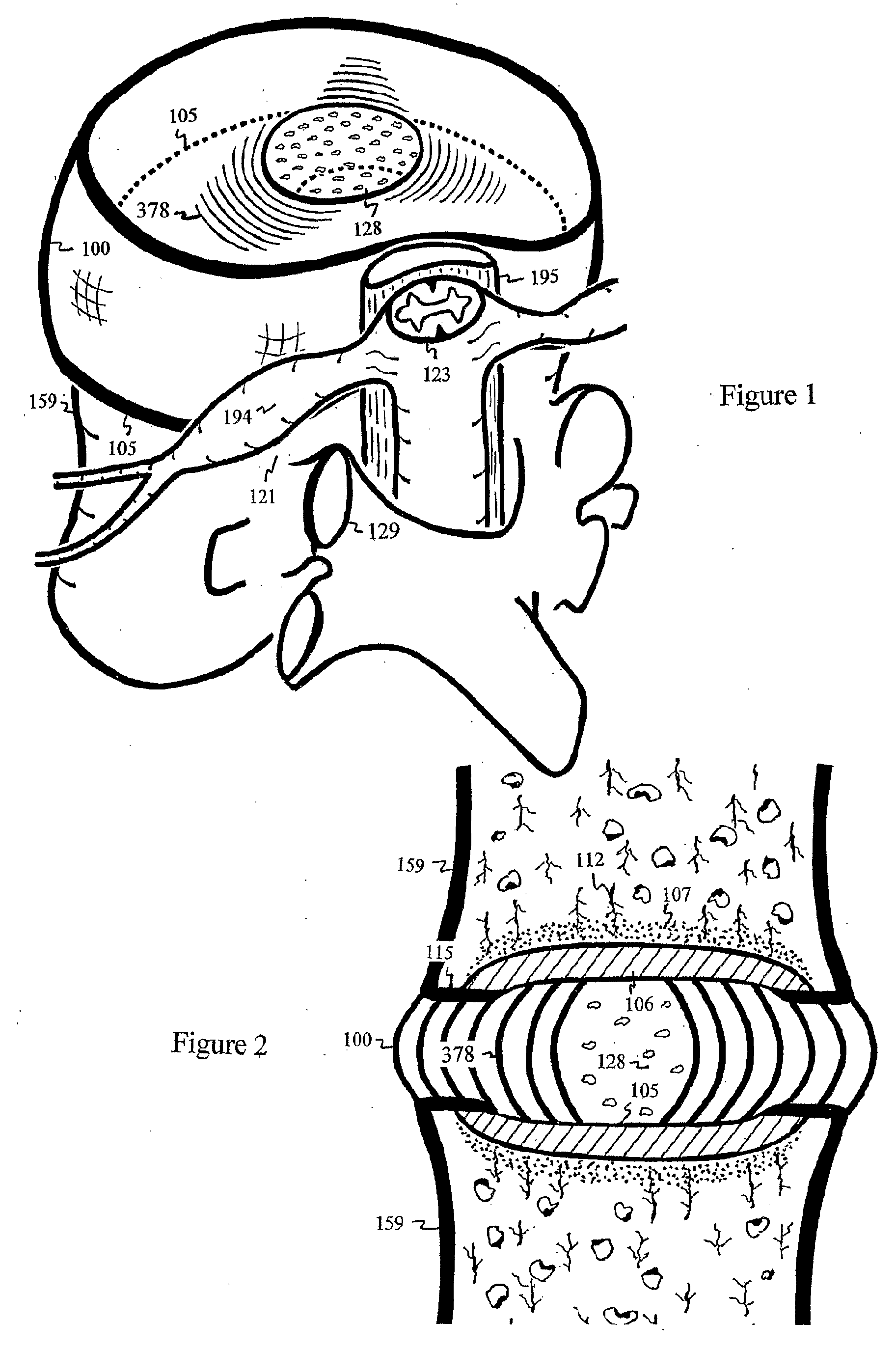
The intervertebral disc is avascular. With aging, calcified layers occlude the cartilaginous endplates, blocking the diffusion of nutrients and oxygen into the avascular disc. Under anaerobic condition, excessive production of lactic acid irritates nerves and further hinders transport of sodium sulfate essential for biosynthesis of the water retaining and load sustaining sulfated glycosaminoglycans. As the result of acid irritation and load shifting to facet joints, pain ensues. Through the pedicle, calcified endplate is punctured by a well-supported and elastically curved needle, injecting antacid to neutralize the lactic acid and enhance transport of sodium sulfate into the shielded discs between ilia. Disc filler or nutrients can also be injected through the curved needle into the degenerated disc.
Owner:YEUNG JEFFREY E
Face guard for a sports helmet
The present invention provides a face guard for a sports helmet having at least two ear holes. The face guard includes a main body having an arrangement of elongated members. The face guard also includes a pair of quadrilateral projections for securing the face guard to the helmet, wherein the projections are integral with and extend from the main body. The projections are positioned below the ear holes in the helmet when the face guard is secured to the helmet. Also, the projections are positioned adjacent a lower edge of the helmet when the face guard is secured to the helmet. The projections are positioned below a rear lower edge of the helmet when the face guard is secured to the helmet. Each projection has first and second vertical members that are configured to engage a connector that secures the face guard to the helmet. Furthermore, each projection includes a first and second substantially horizontal members that, when combined with the first and second vertical members, define a rectangle or a trapezoid.
Owner:RIDDELL
Pharmaceutical formulation for oral delivery of bisphosphates
InactiveUS20050182028A1Minimizing potential esophageal irritationMinimize irritationBiocidePhosphorous compound active ingredientsDosing regimenPharmacy
The present invention discloses a method for treating or preventing a bone disorder in a mammal in need thereof comprising orally administering to said mammal a pharmaceutically effective amount of a pharmaceutical composition of at least one bisphosphonate, or a pharmaceutically acceptable salt or esters thereof, and at least one aminoalky methacrylate copolymer, according to a dosing schedule having a dosing interval selected from once-weekly dosing, twice-monthly dosing, once-monthly, once-quarterly and once-annually dosing. The present invention further discloses a method for treating or preventing a bone disorder in a mammal in need thereof comprising continuously orally administering a unit dosage per-day to said mammal in a short time for a long time therapy.
Owner:CHEN CHIH MING JAMES
Method for managing device behavior during increased load or congestion using policies
InactiveUS20150103651A1Minimize congestionNetwork load increaseError preventionNetwork traffic/resource managementNetwork conditionsCommunication device
An apparatus for enabling provision of one or more policies to manage behavior of one or more communications devices may include a processor and memory storing executable computer code causing the apparatus to at least perform operations including analyzing data of at least one policy. The policy includes information instructing a communication device(s) regarding a manner to behave according to a designated network condition(s) designated by a network operator. The computer program code may further cause the apparatus to apply the policy responsive to detecting a load of a network is increased or that the network is congested. The computer program code may further cause the apparatus to behave in the manner designated by the network operator according to the applied policy responsive to detecting the increased load or that the network is congested to minimize congestion in the network. Corresponding methods and computer program products are also provided.
Owner:NOKIA TECHNOLOGLES OY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com