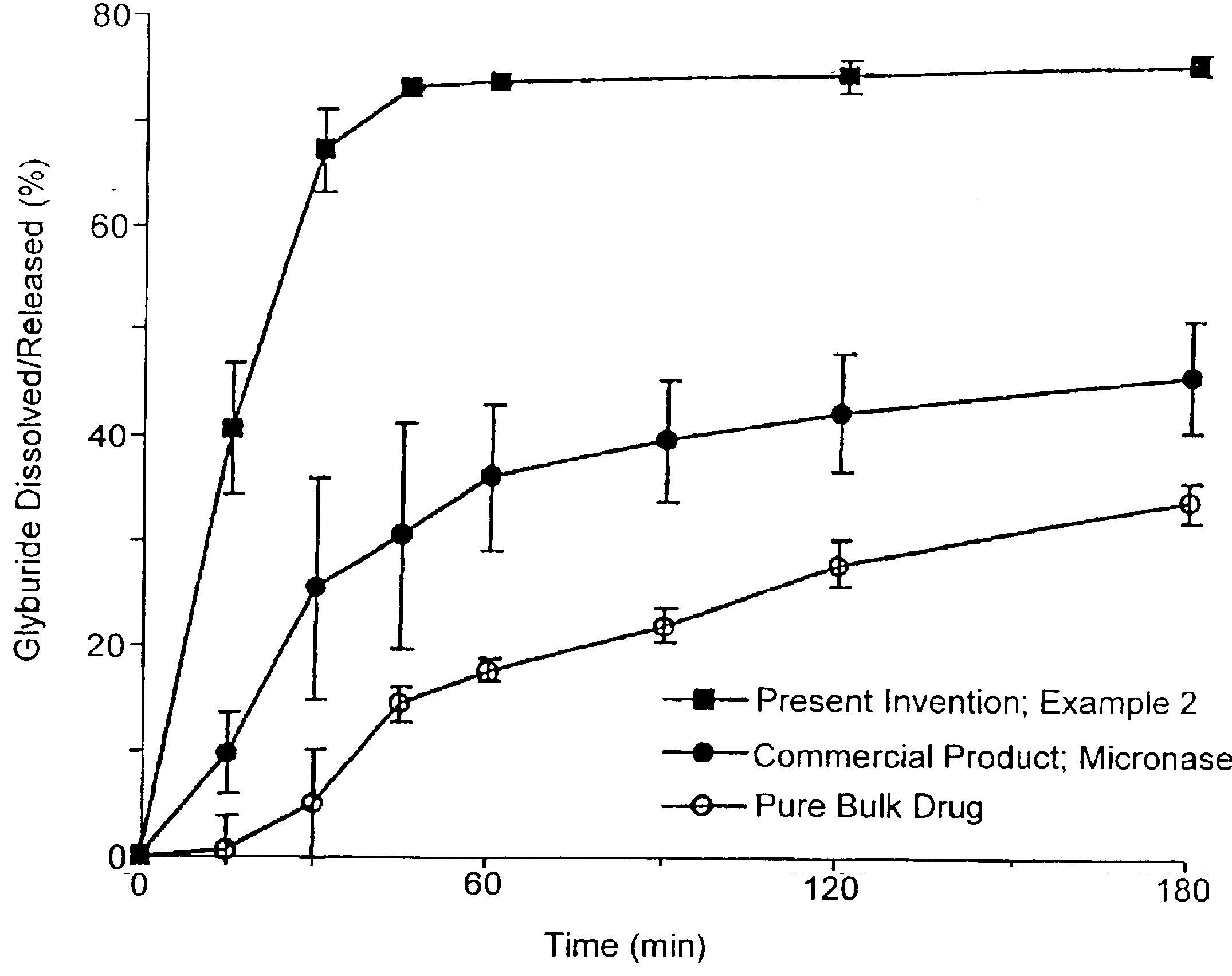
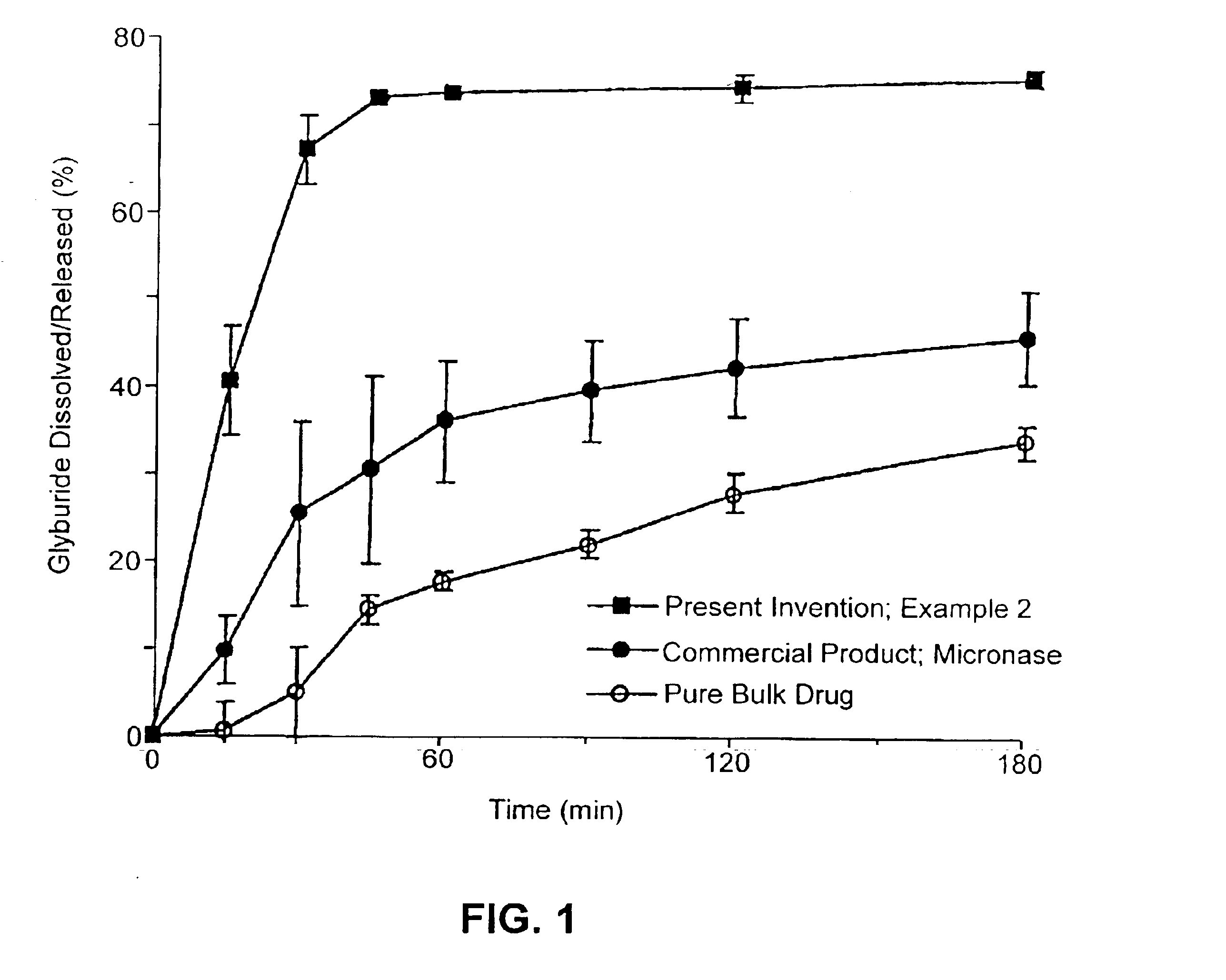
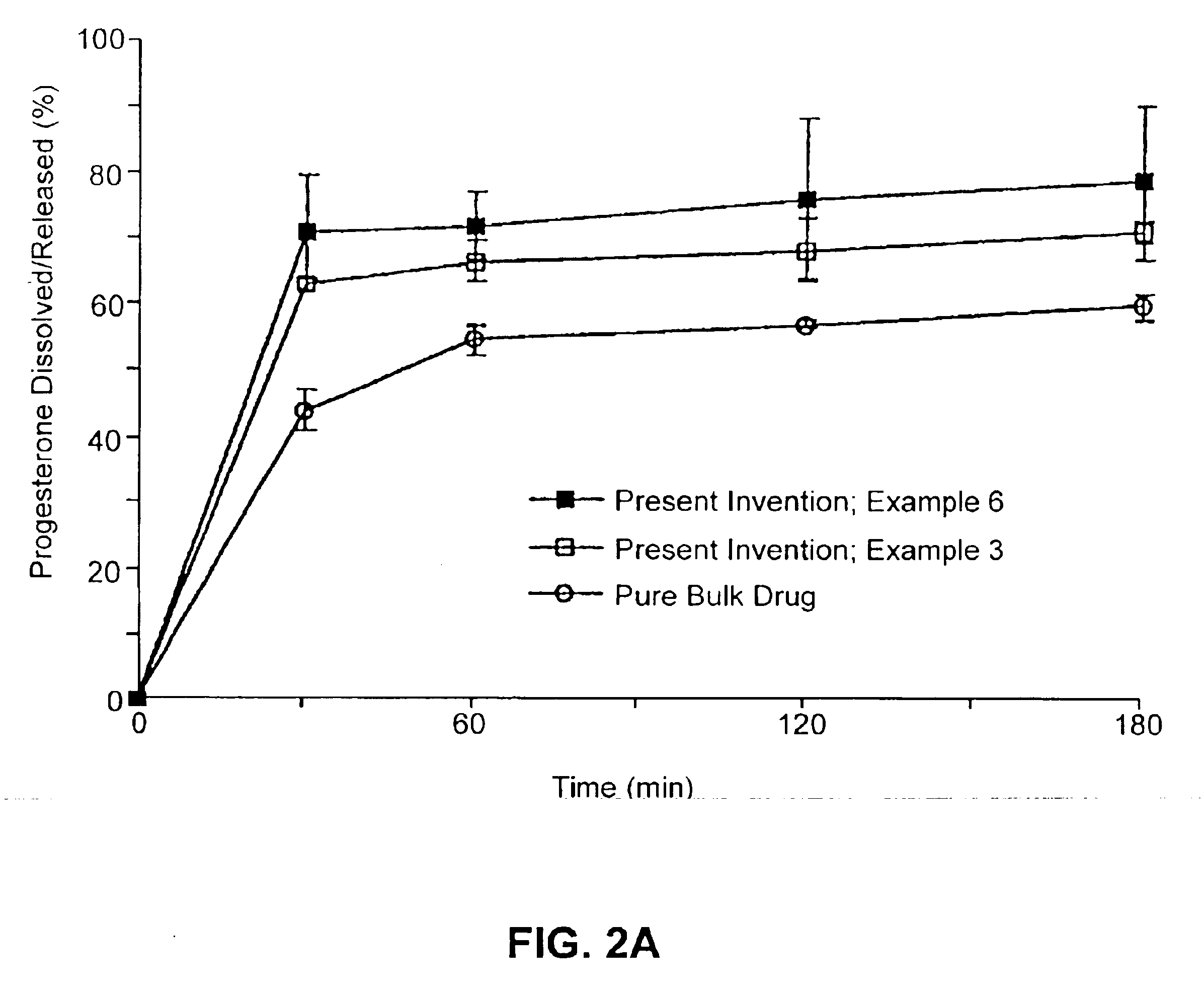
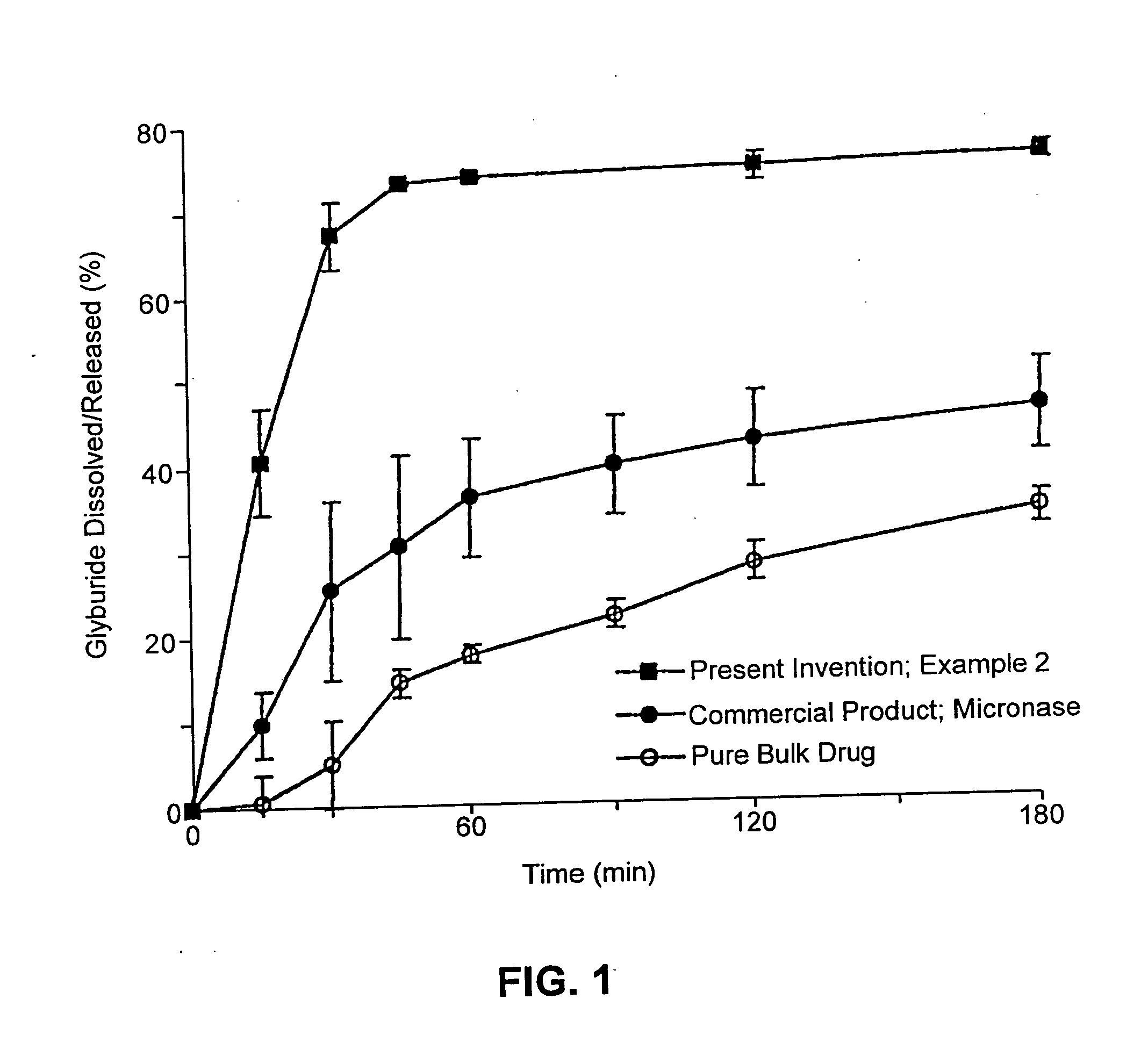
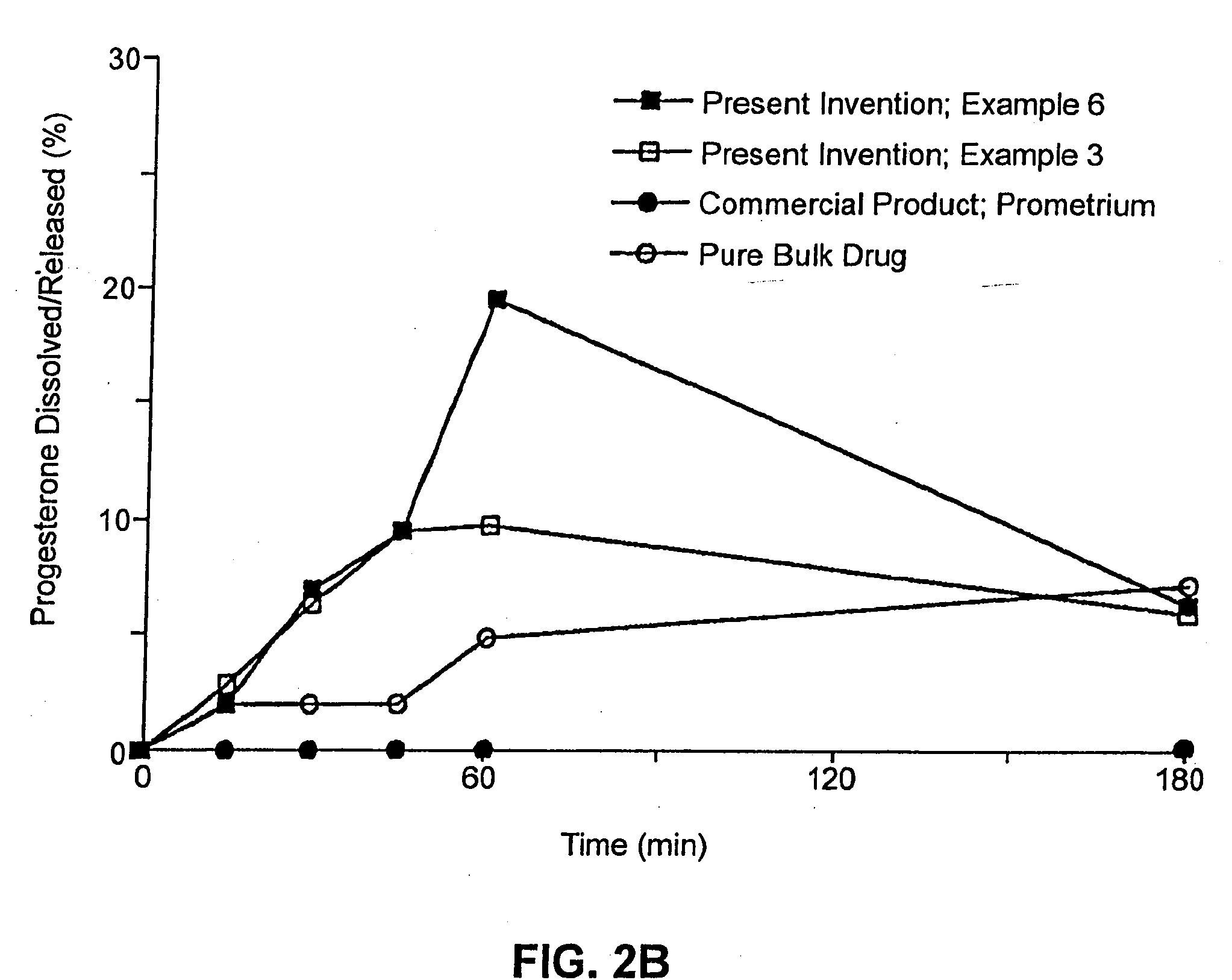
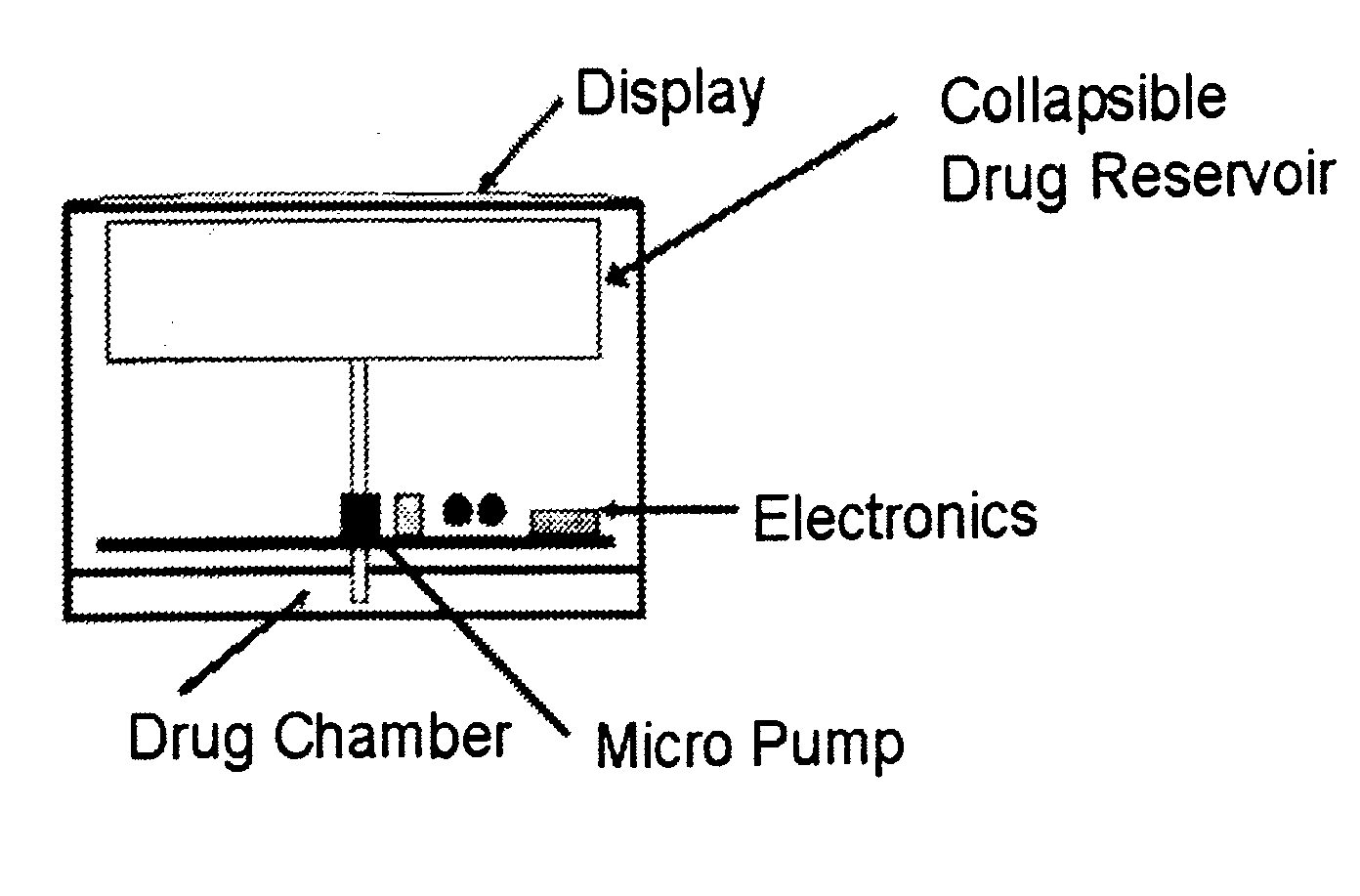
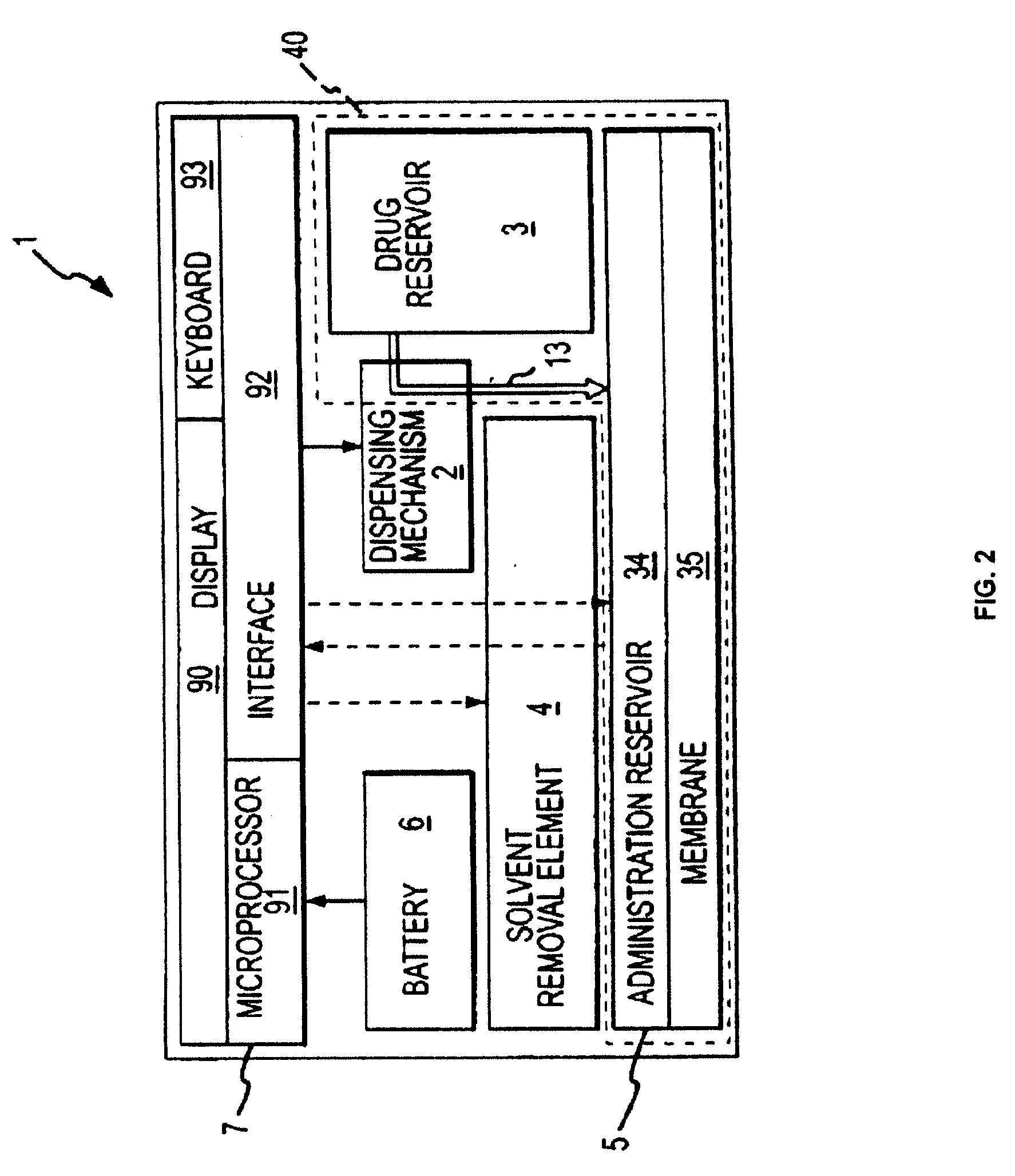
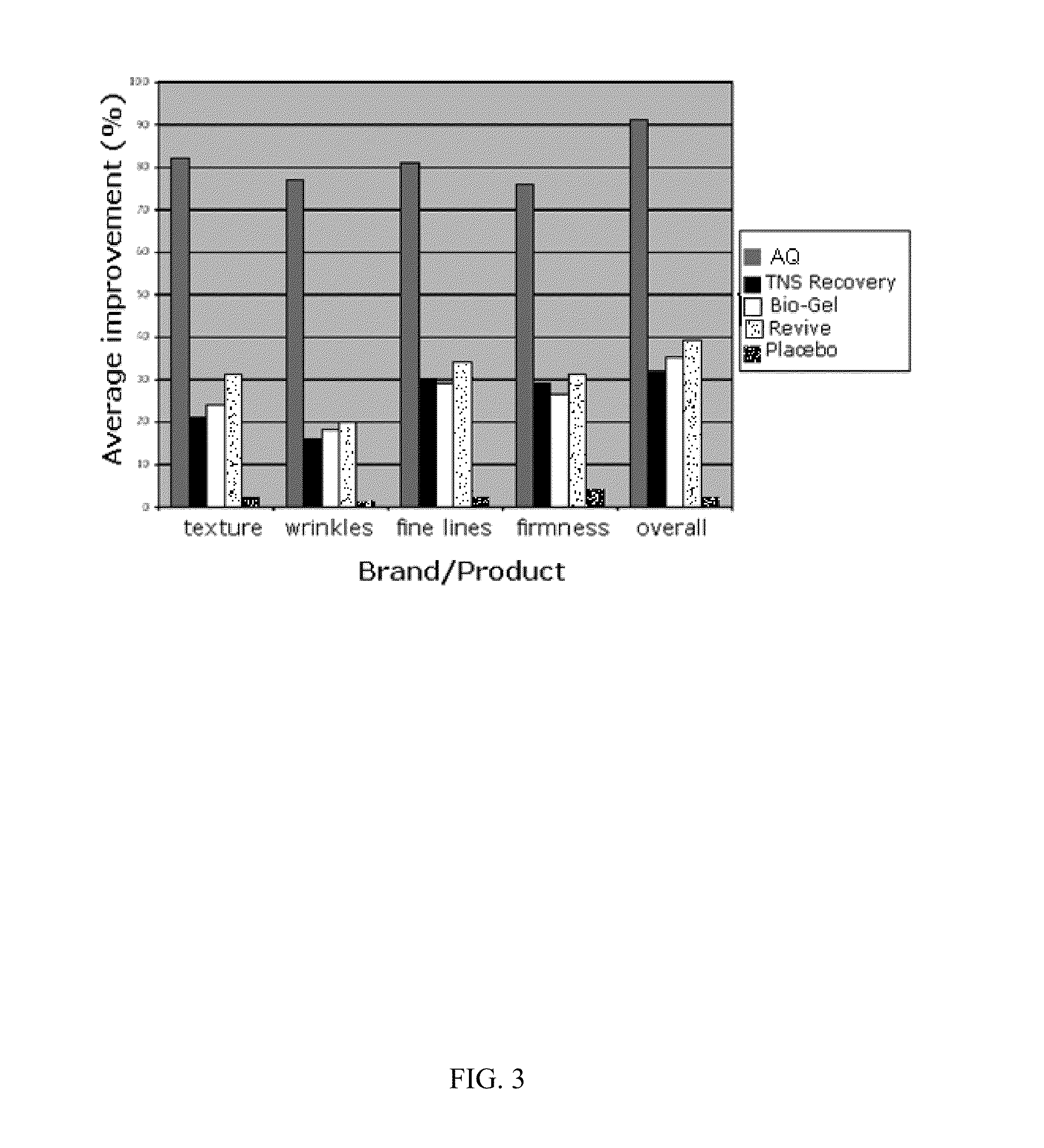
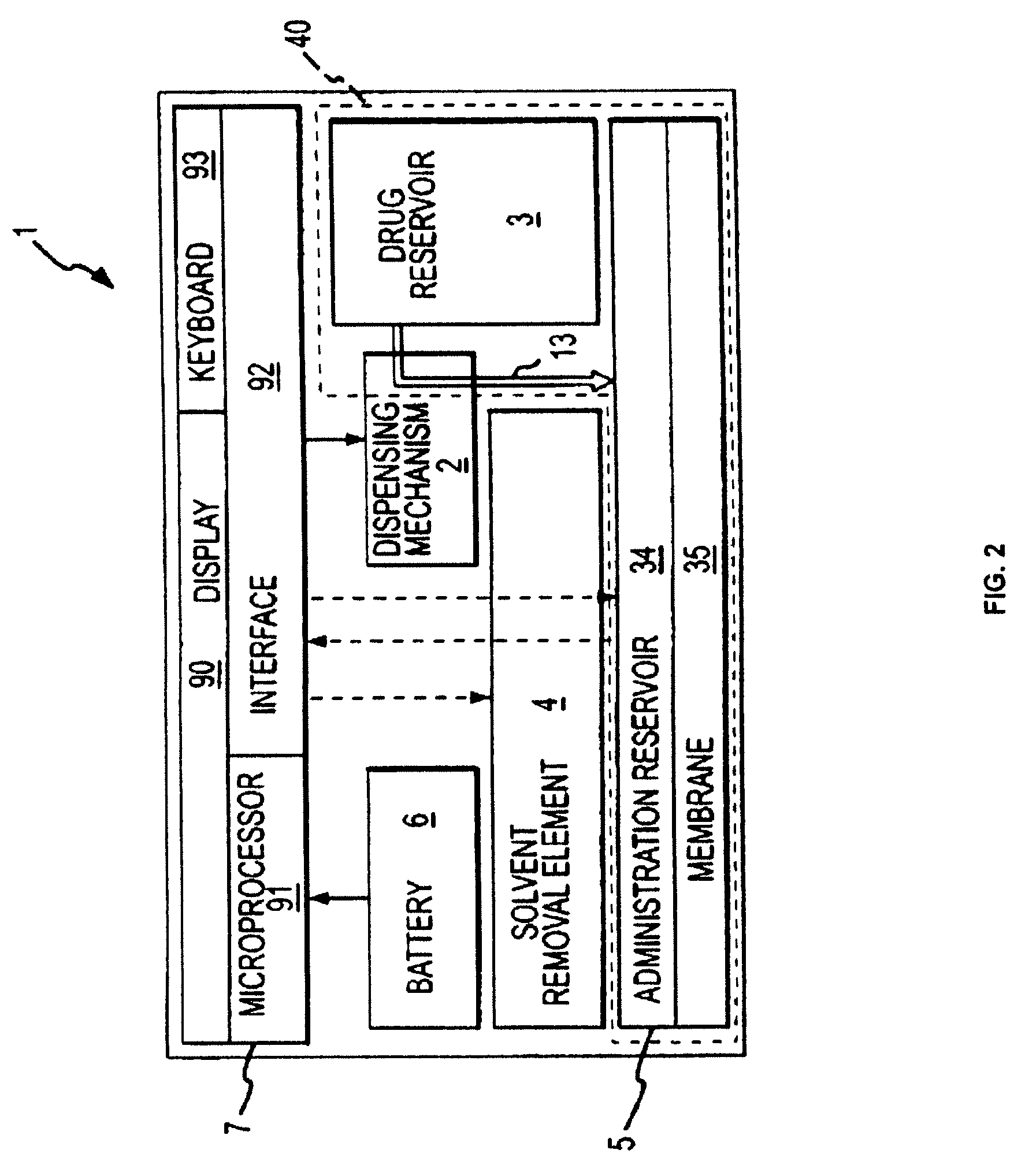
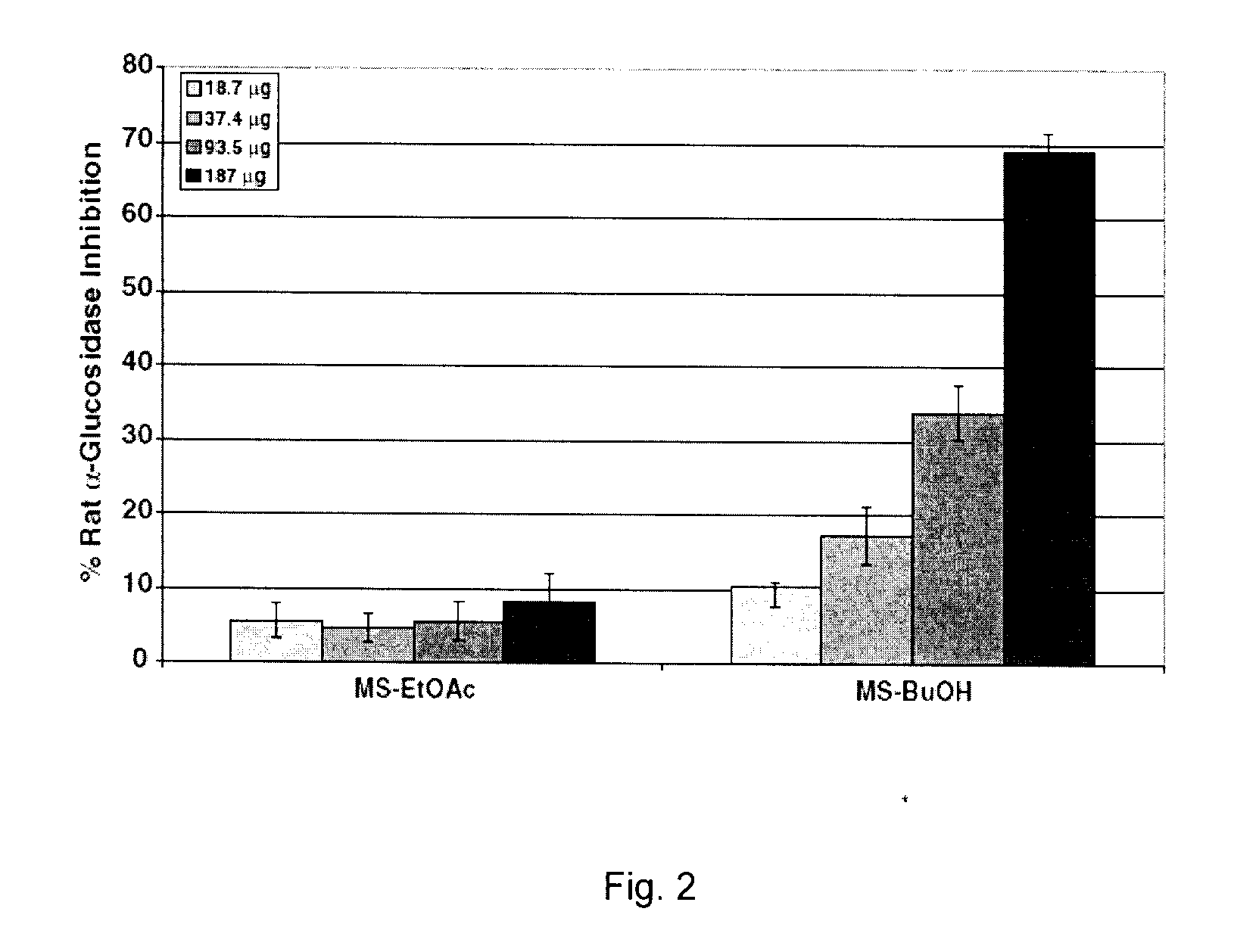
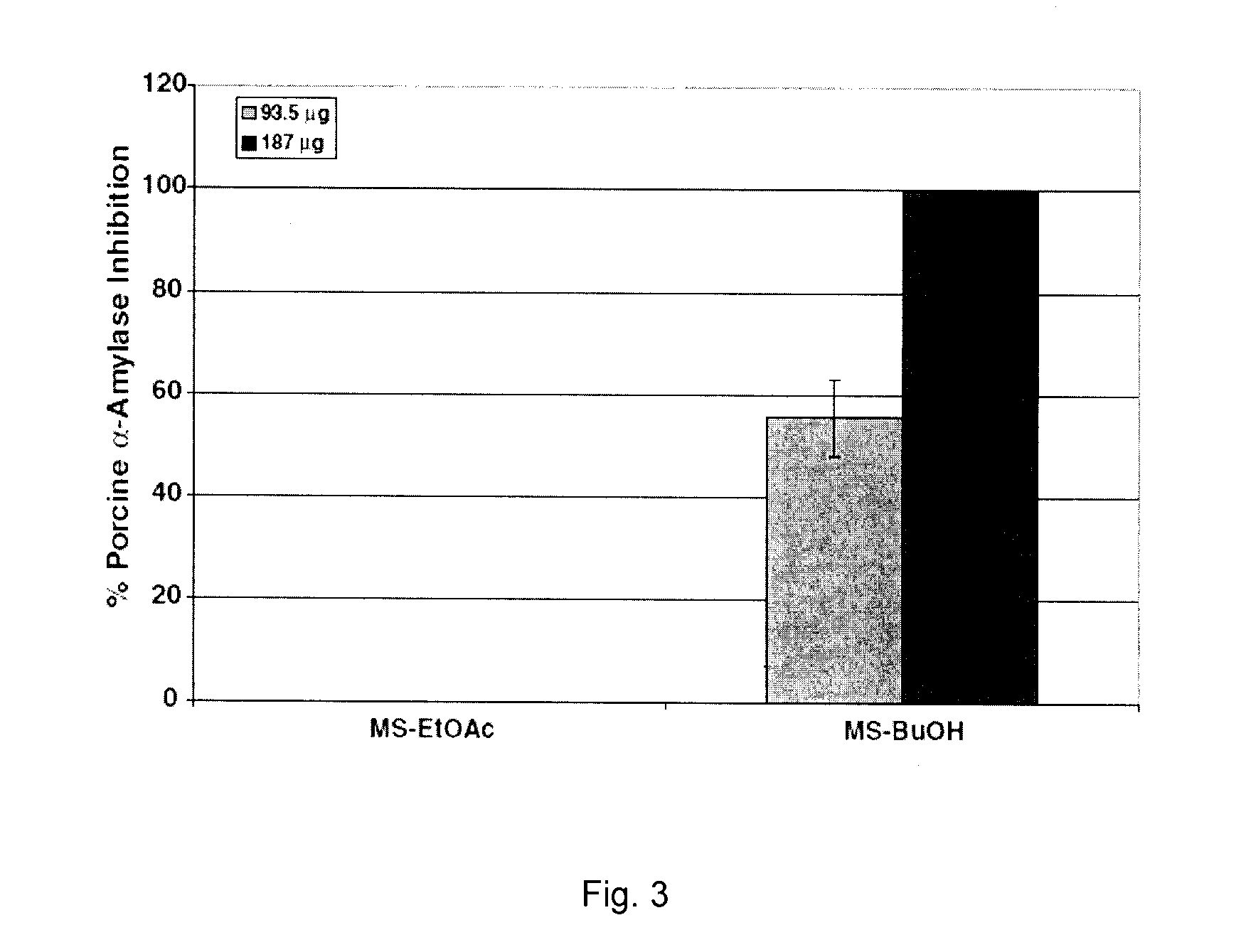
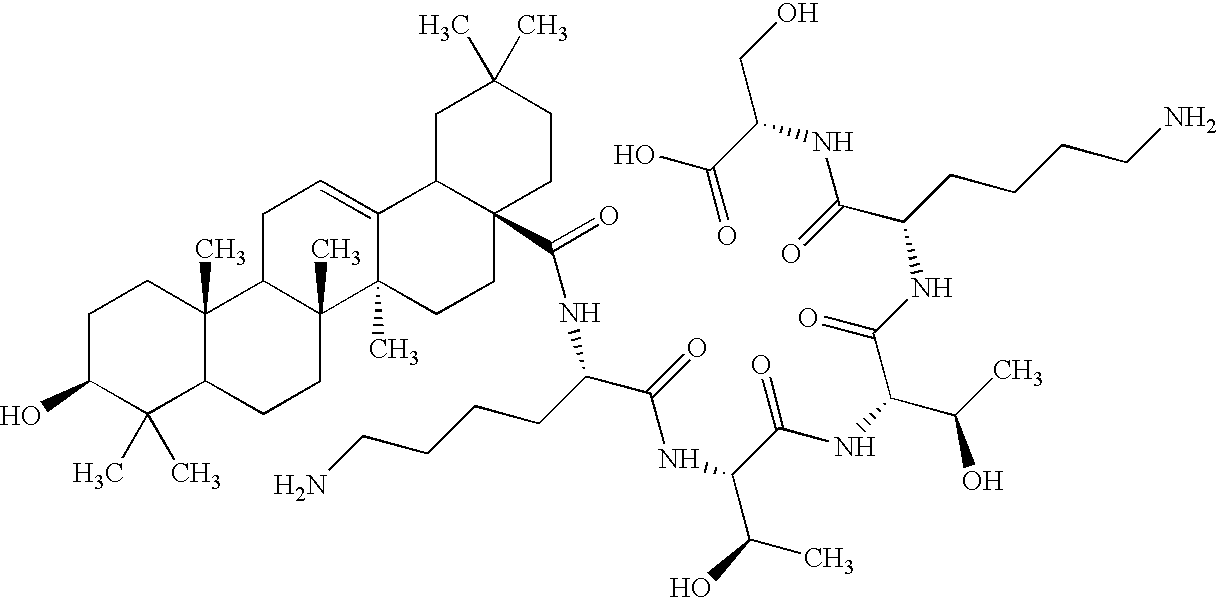
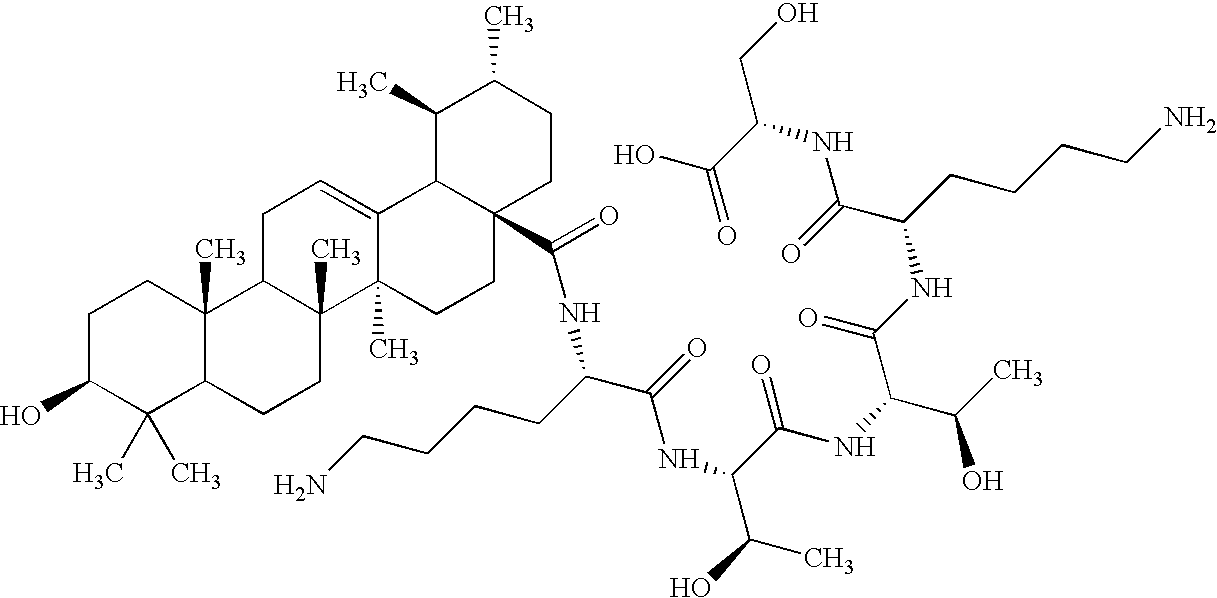
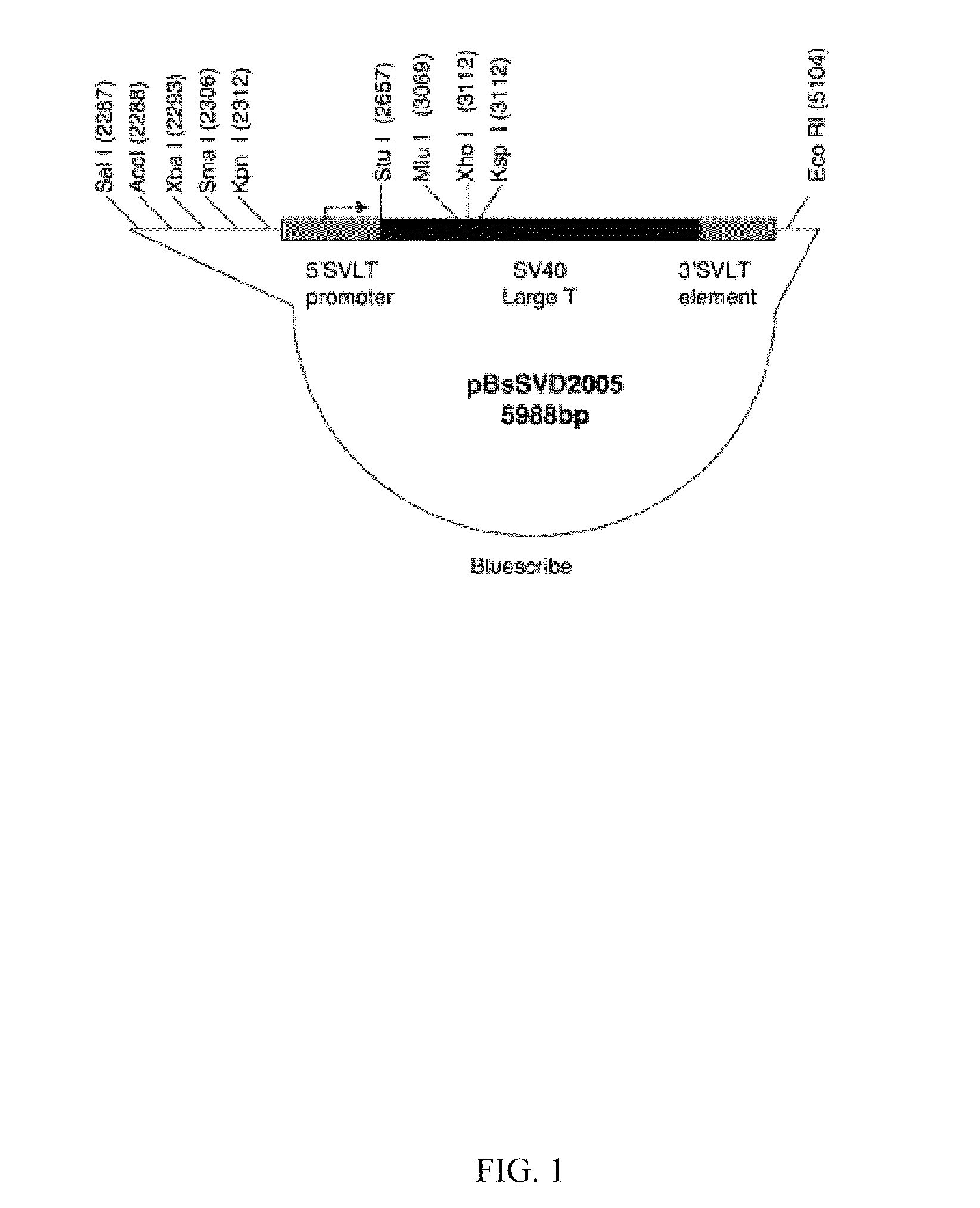
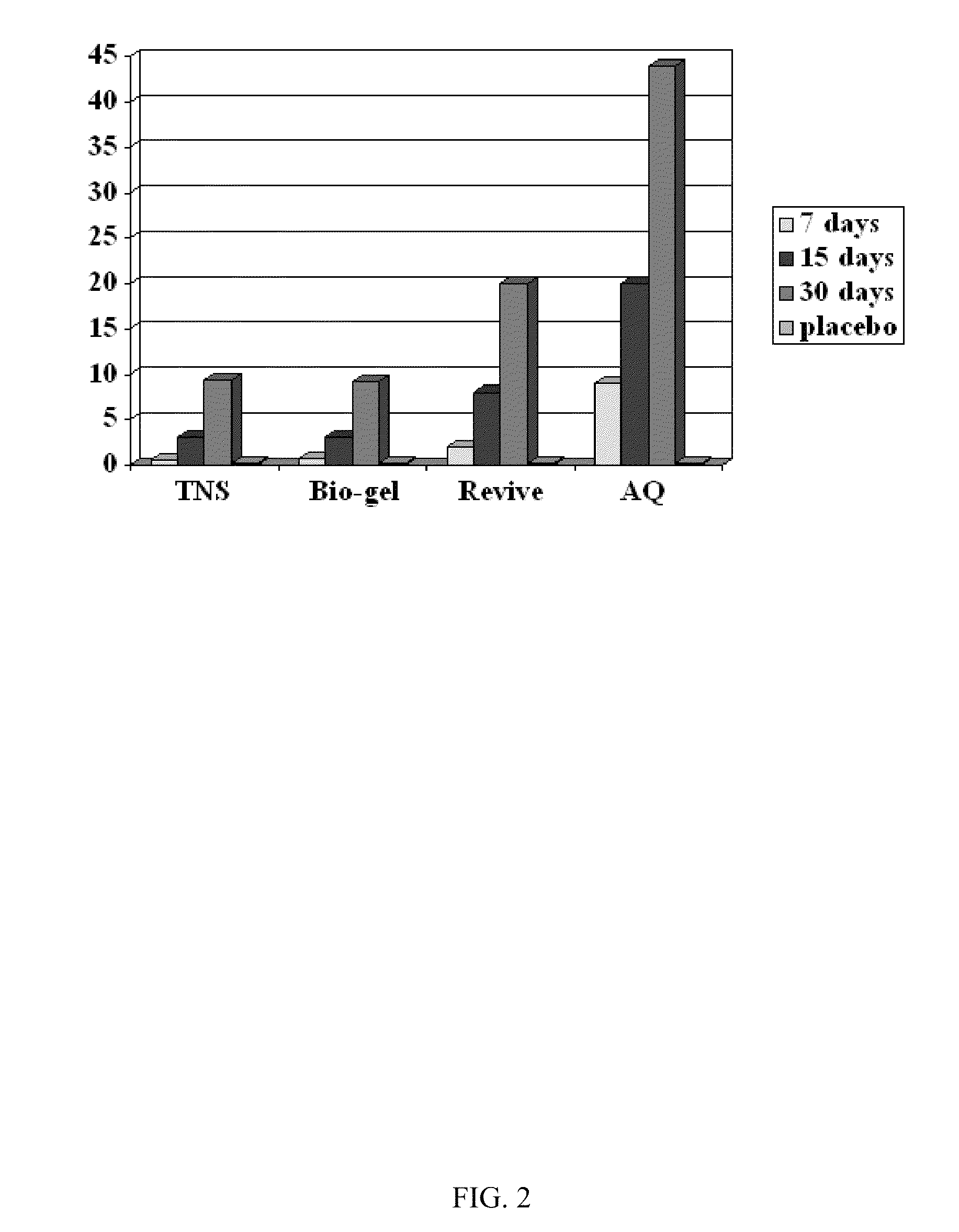
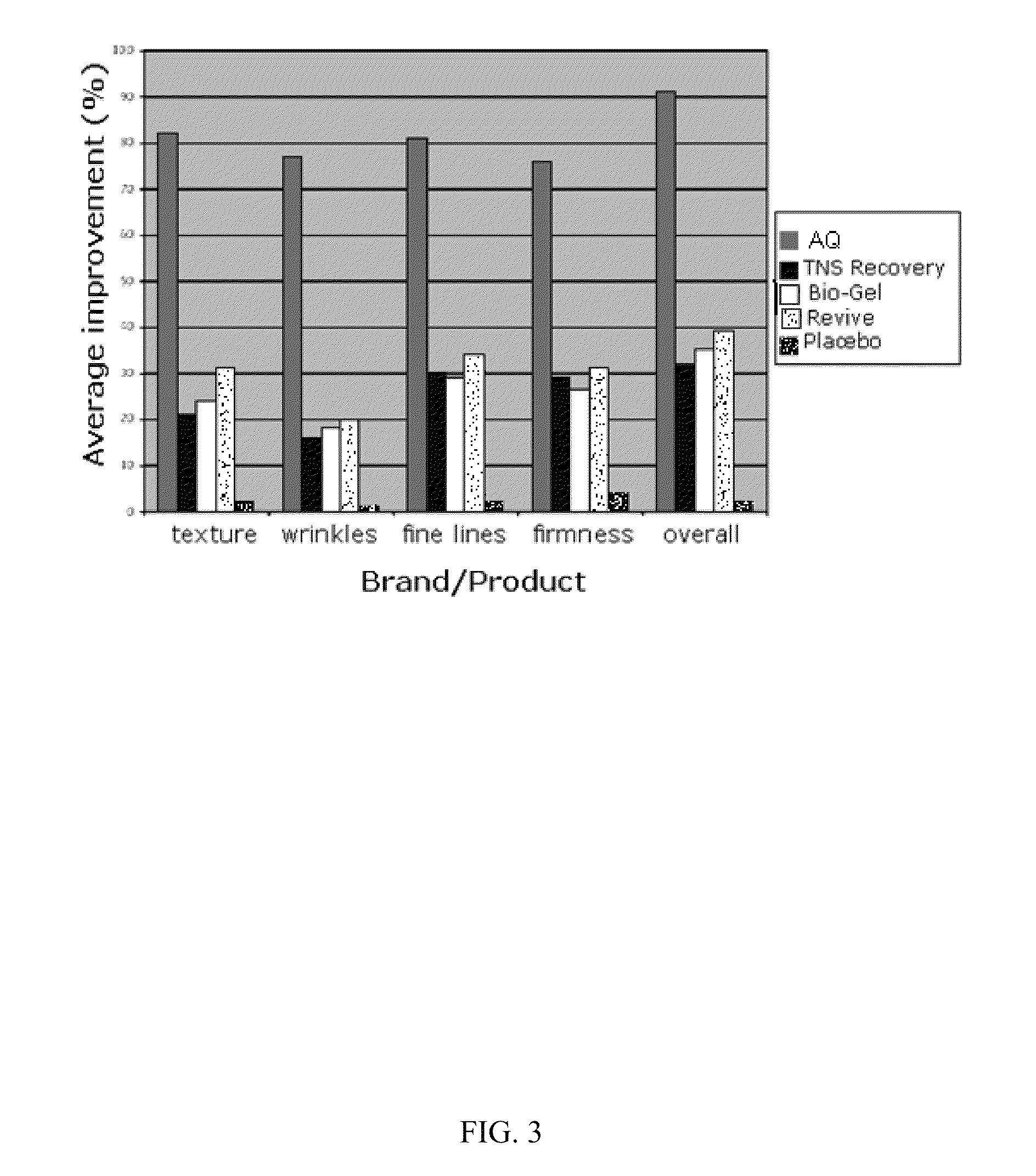
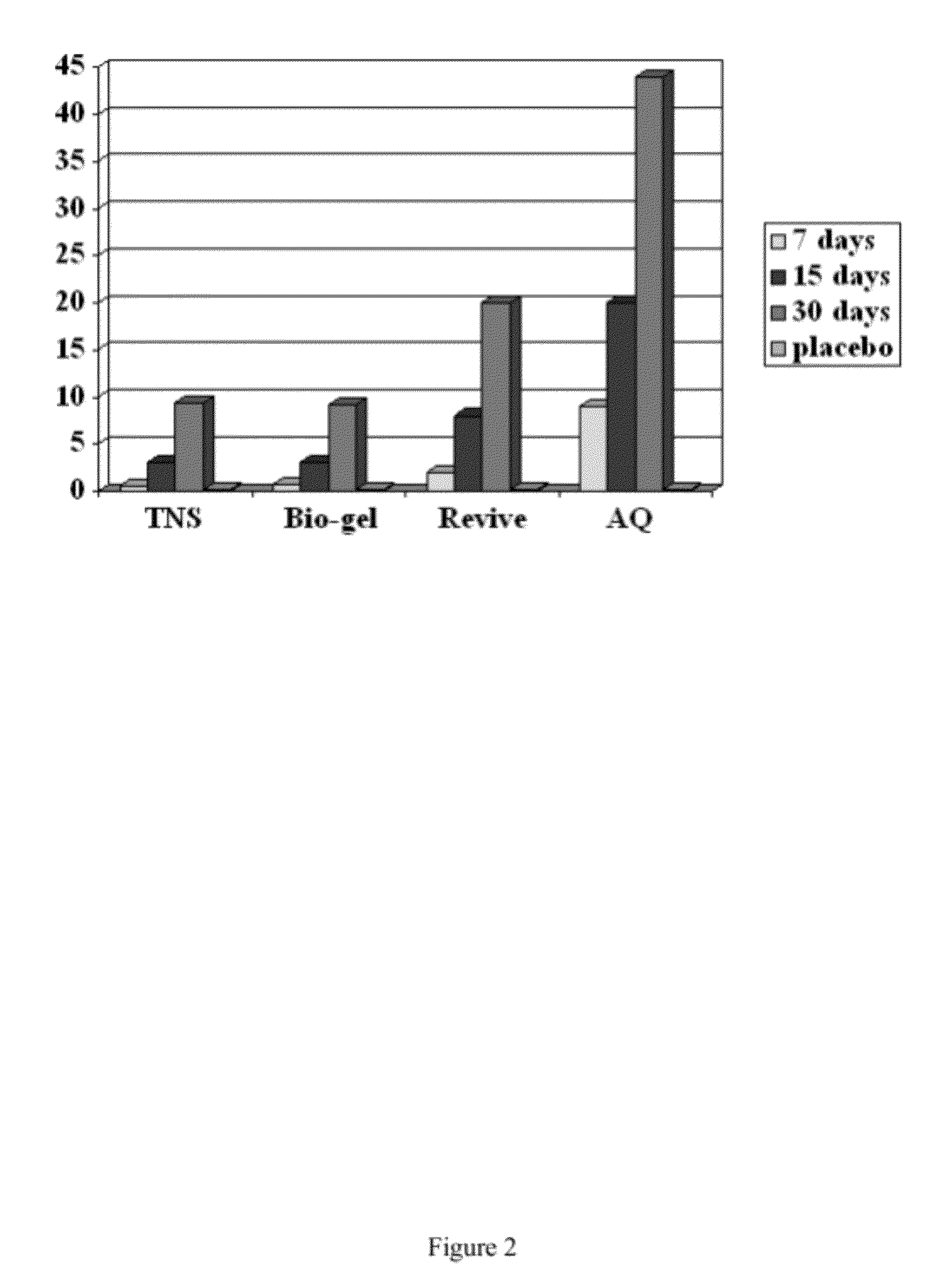
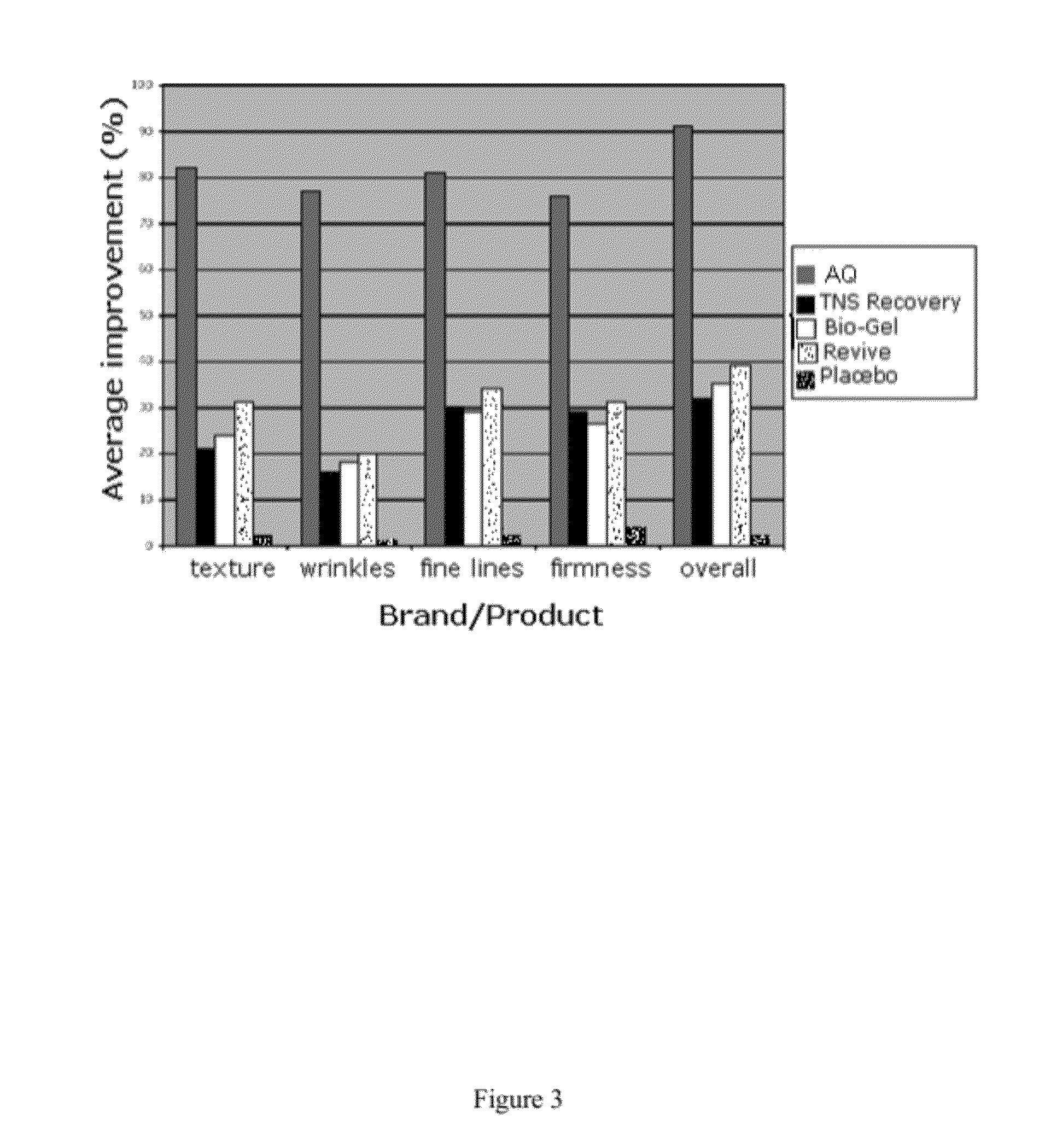
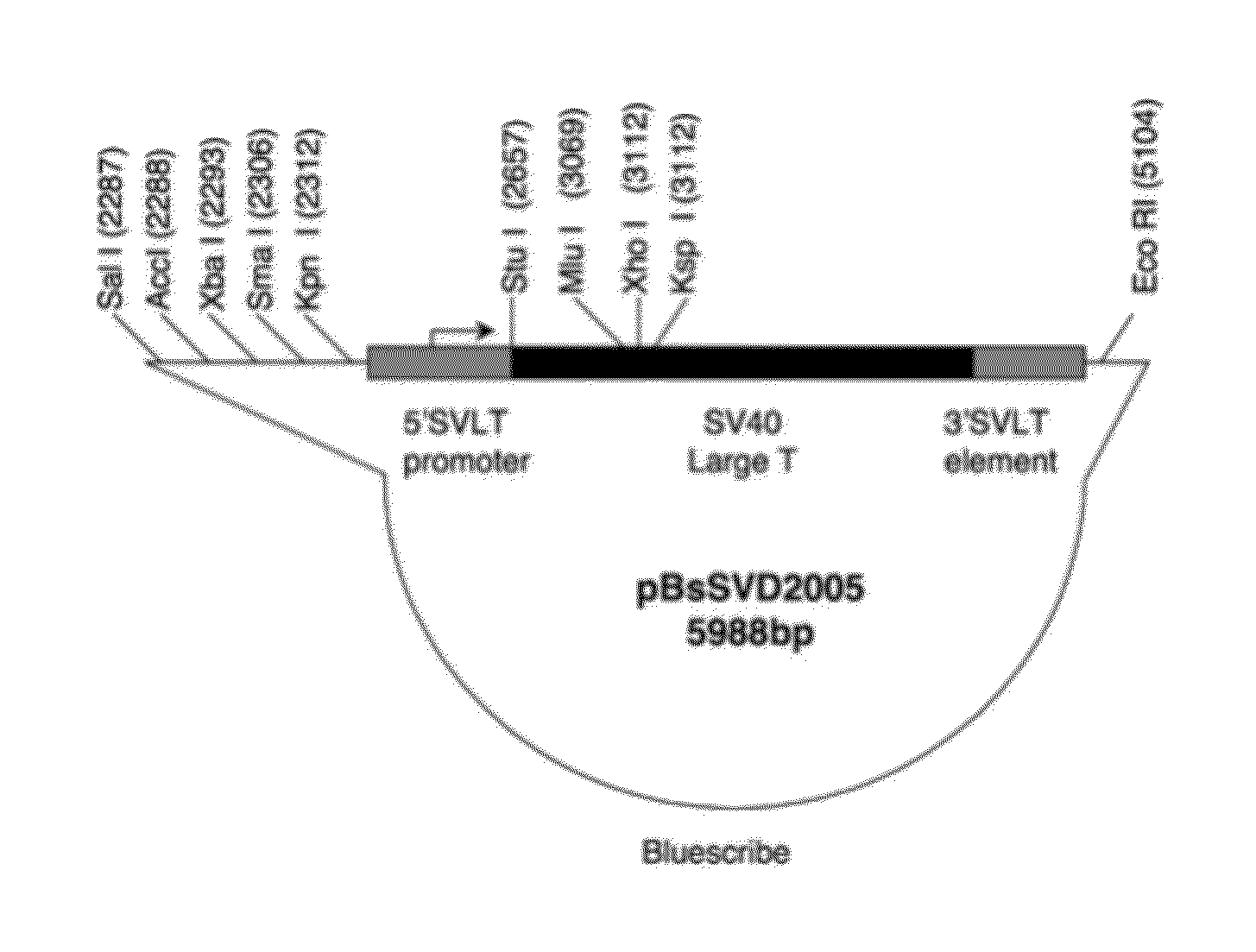
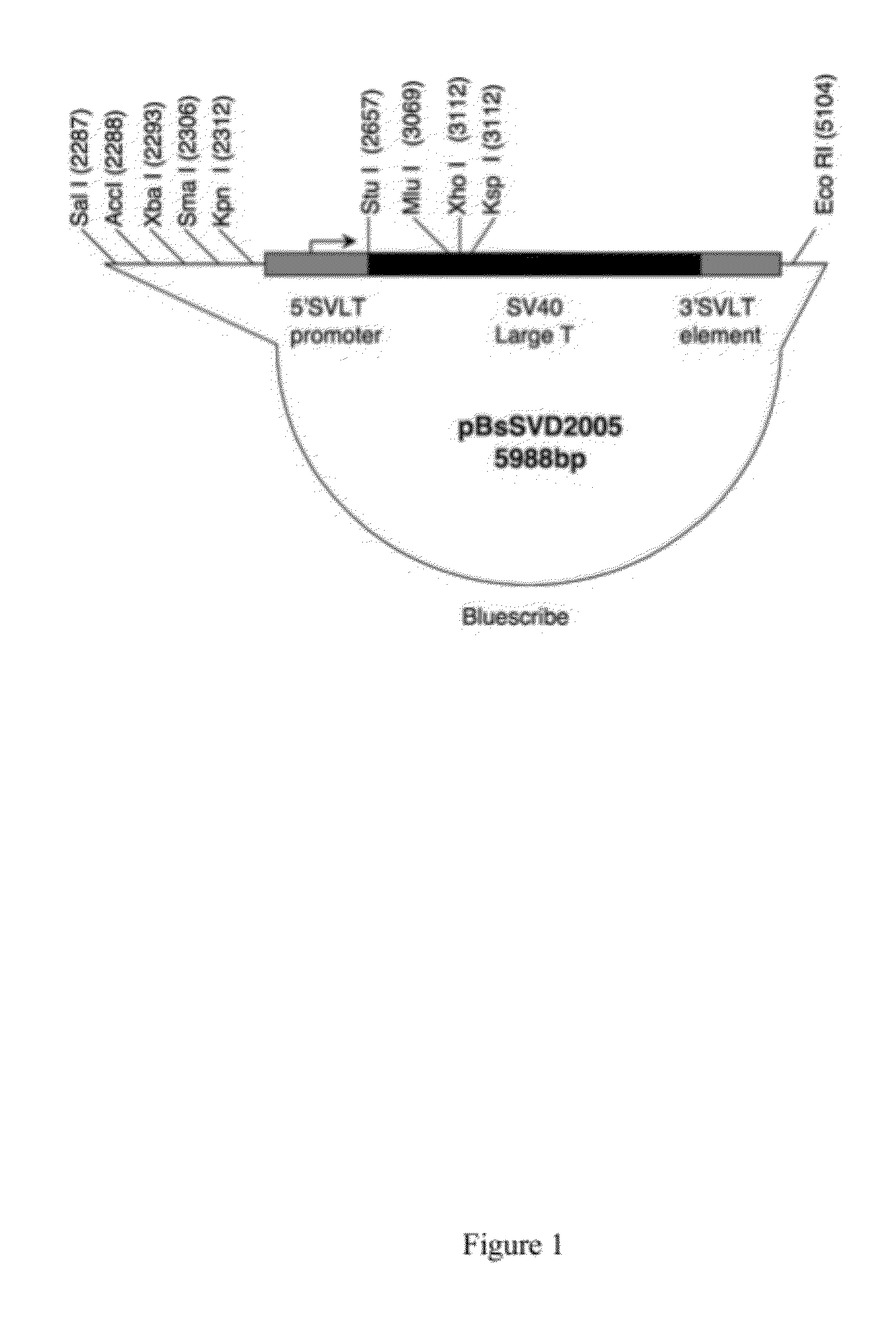
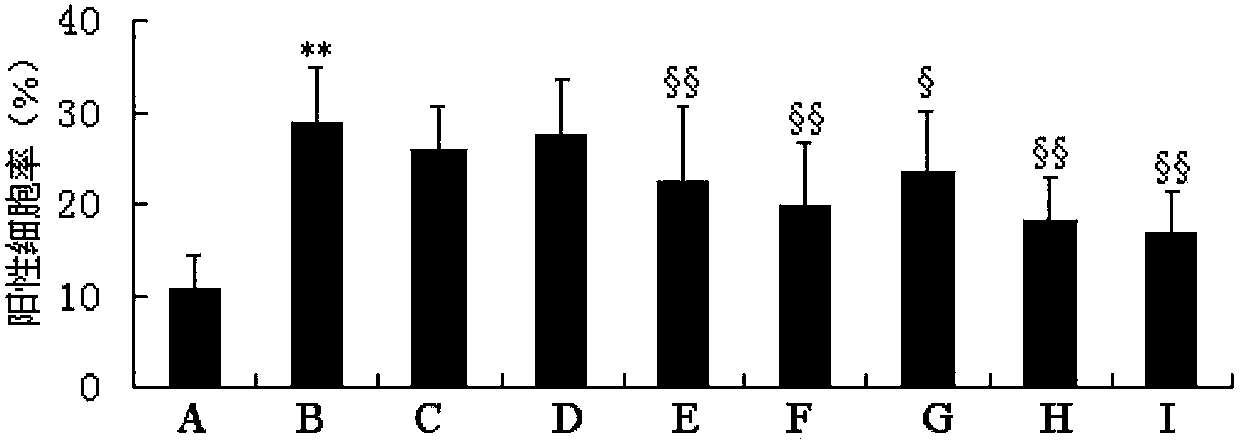Patents
Literature
59 results about "Cosmeceutical" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cosmeceuticals are cosmetic products with bioactive ingredients purported to have medical benefits. There are no legal requirements to prove that these products live up to their claims. The name is a combination of "cosmetics" and "pharmaceuticals". "Nutricosmetics" are related dietary supplement or food or beverage products with additives that are marketed as having medical benefits that affect appearance.
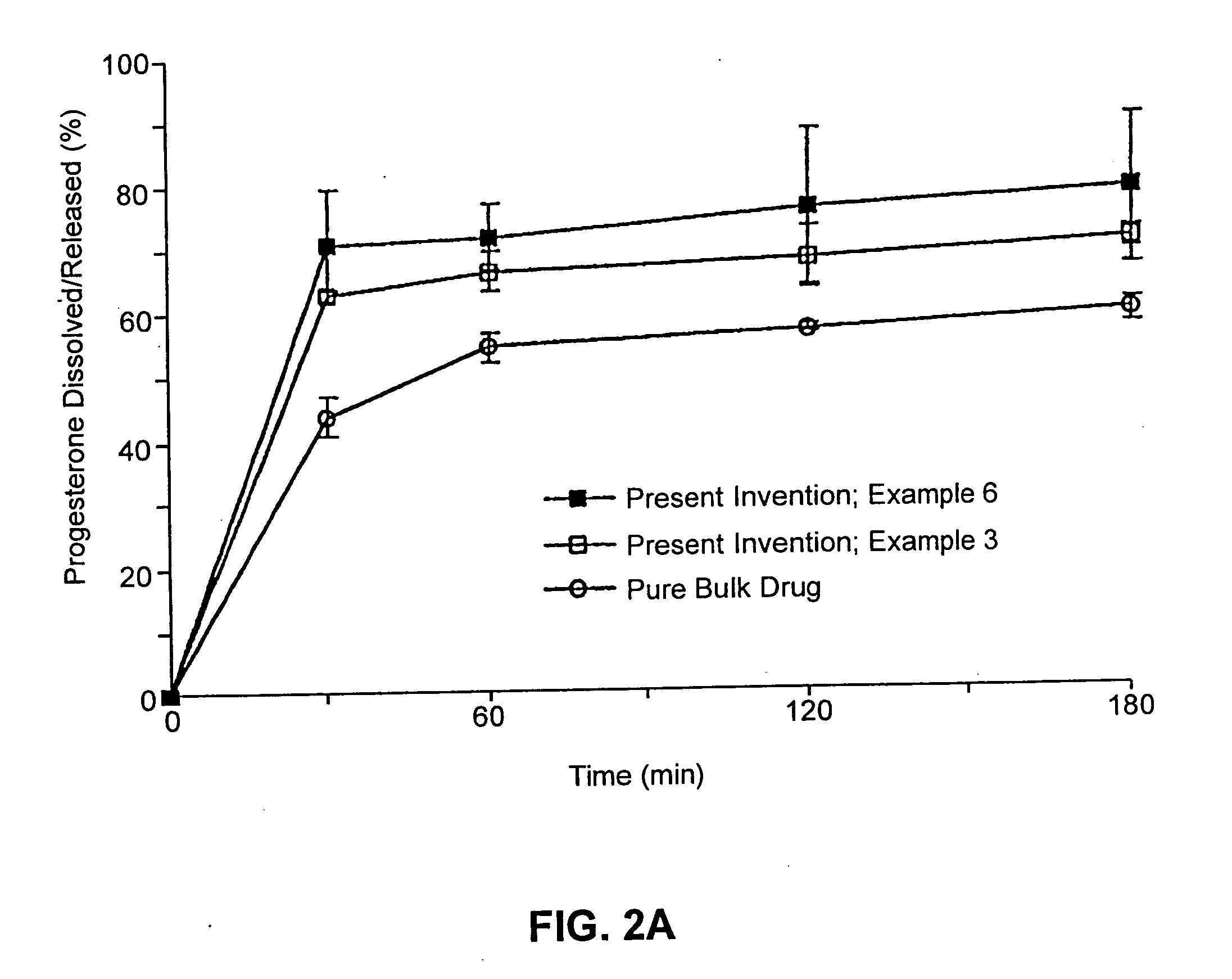
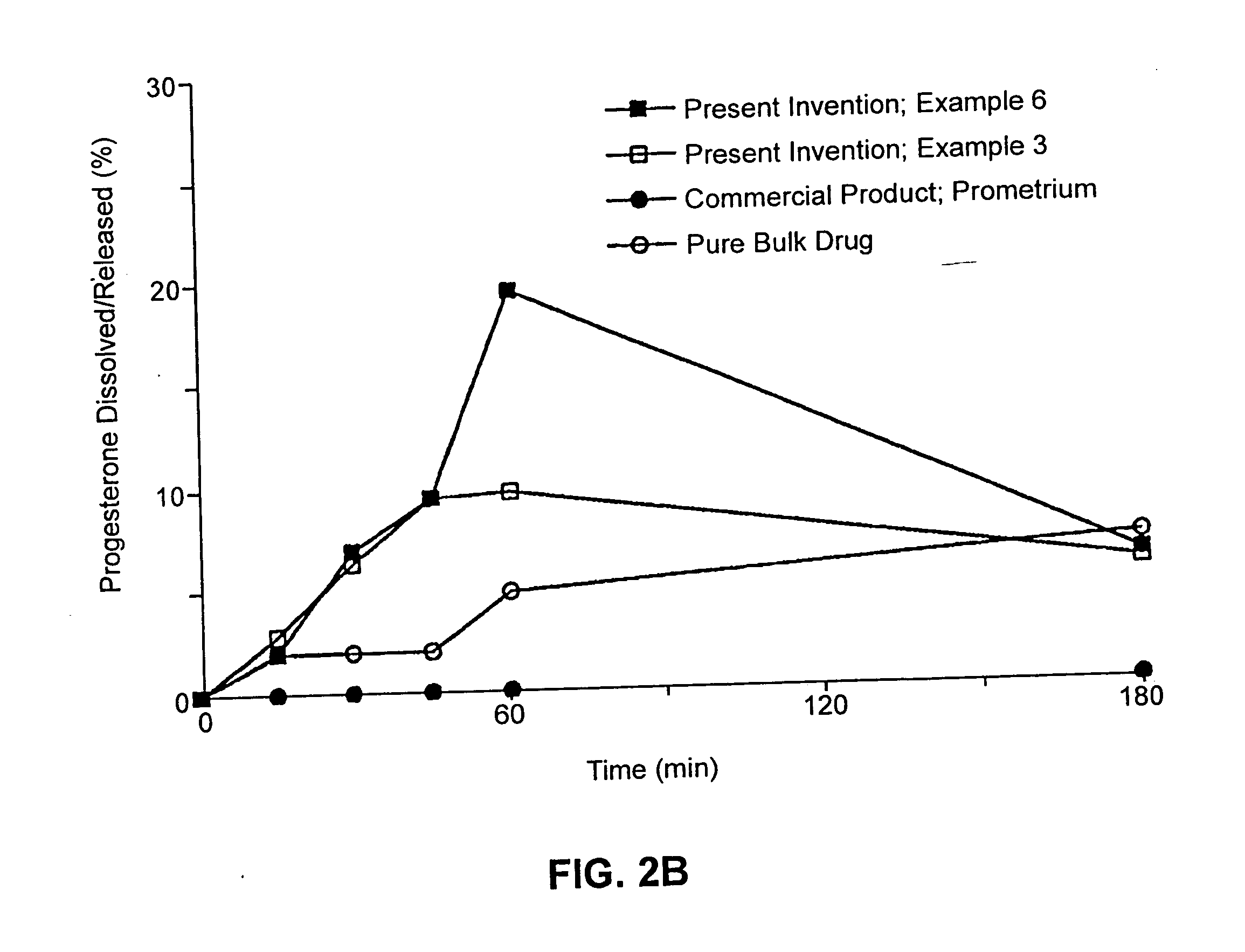
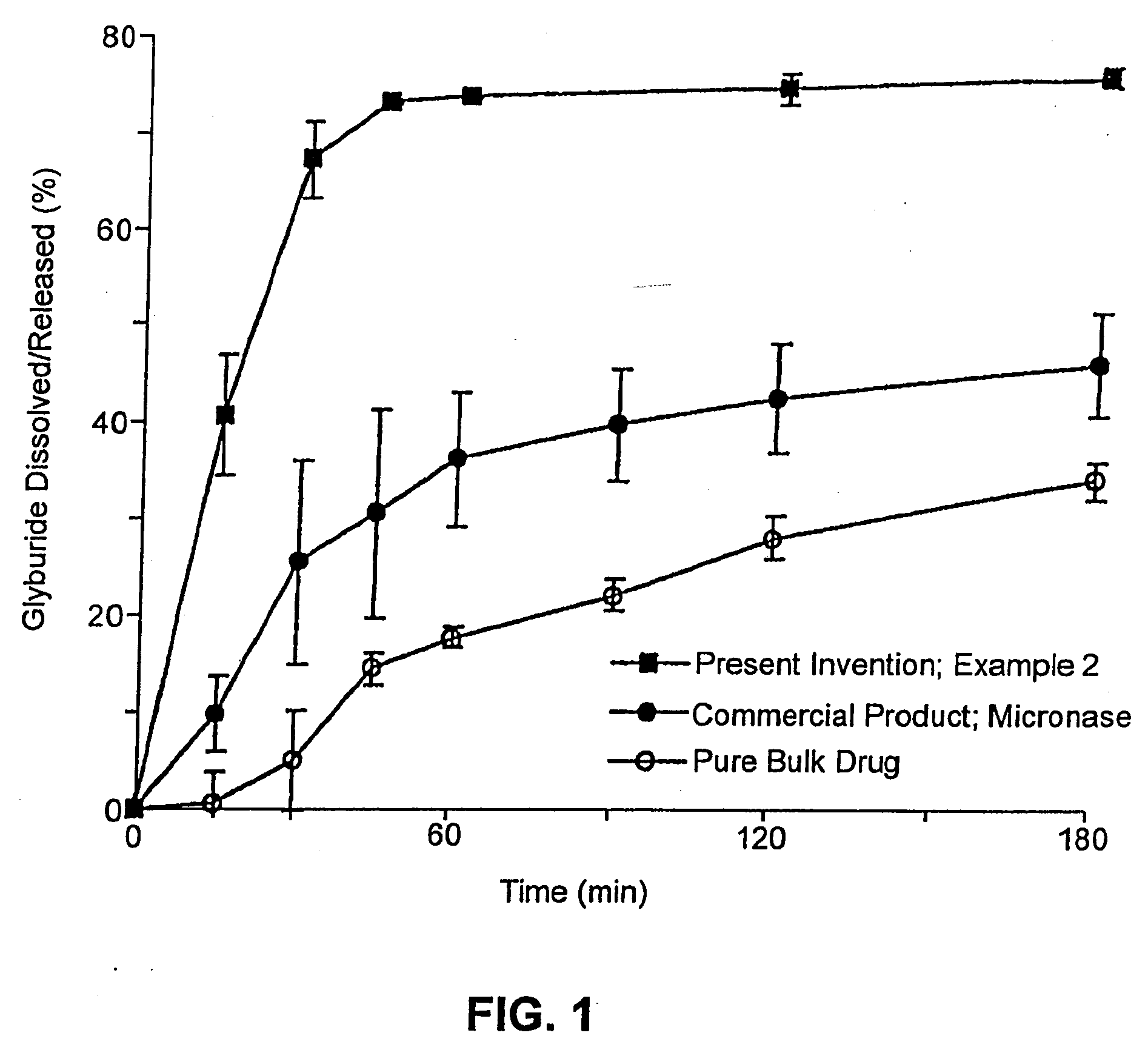
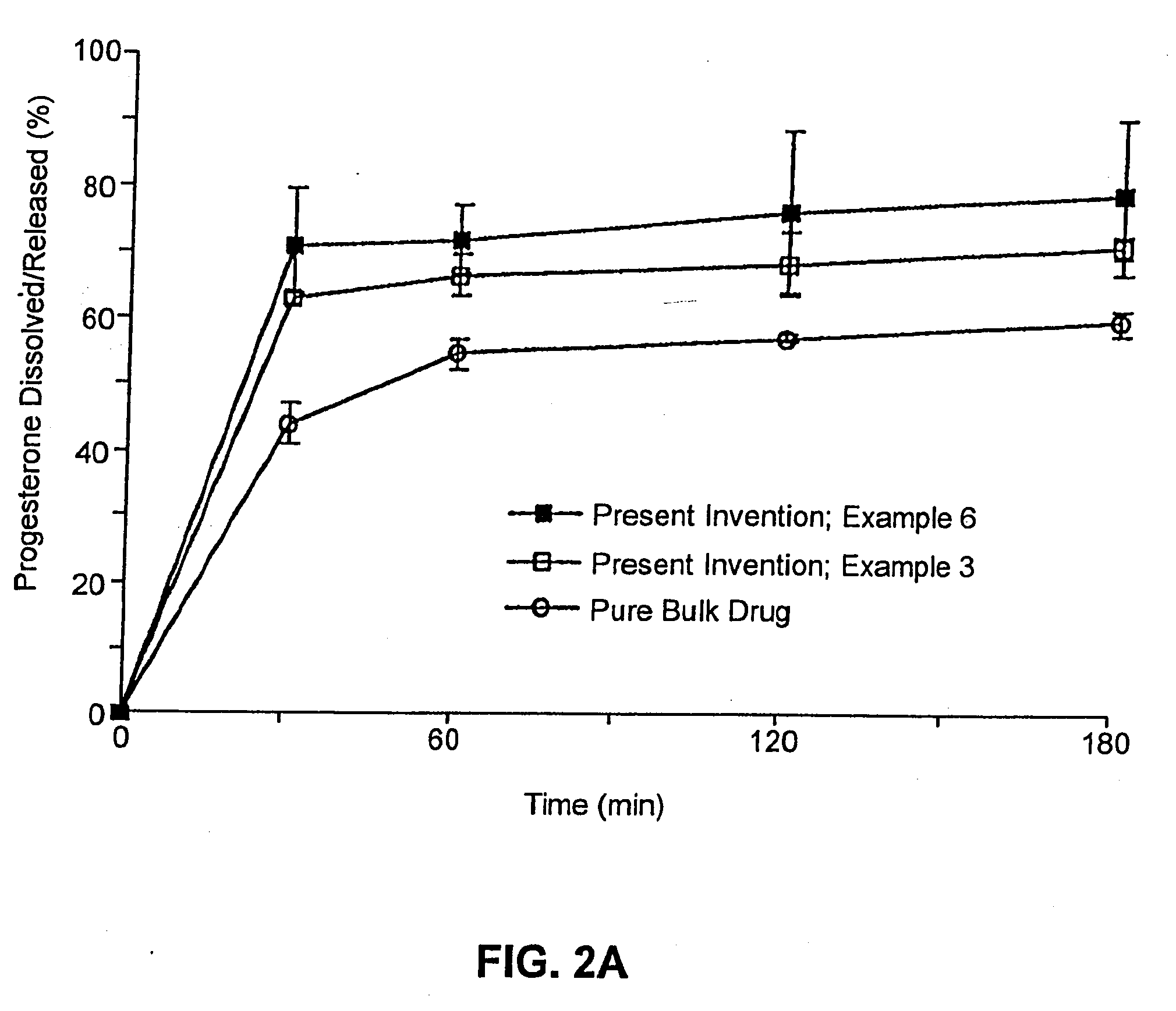
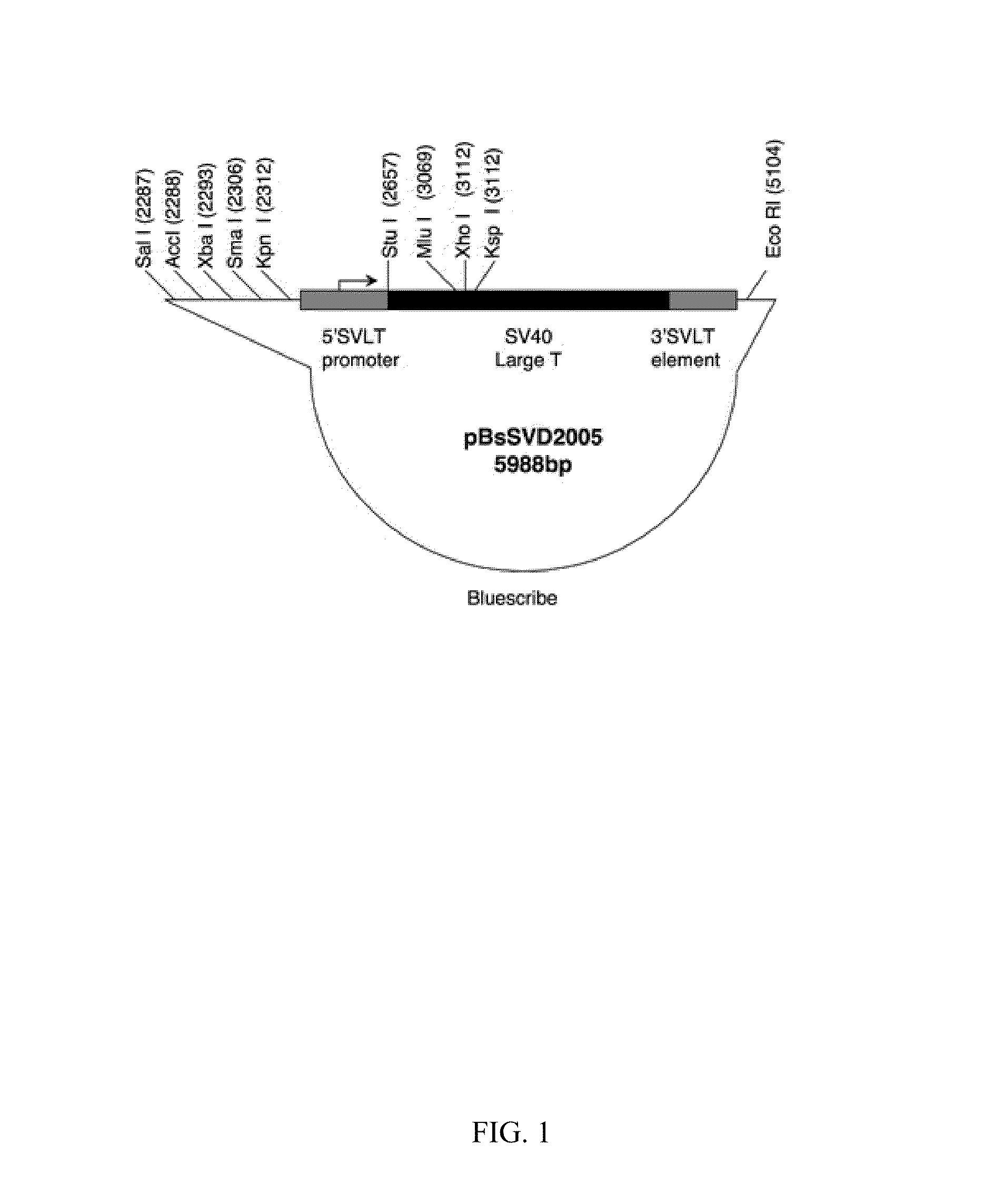
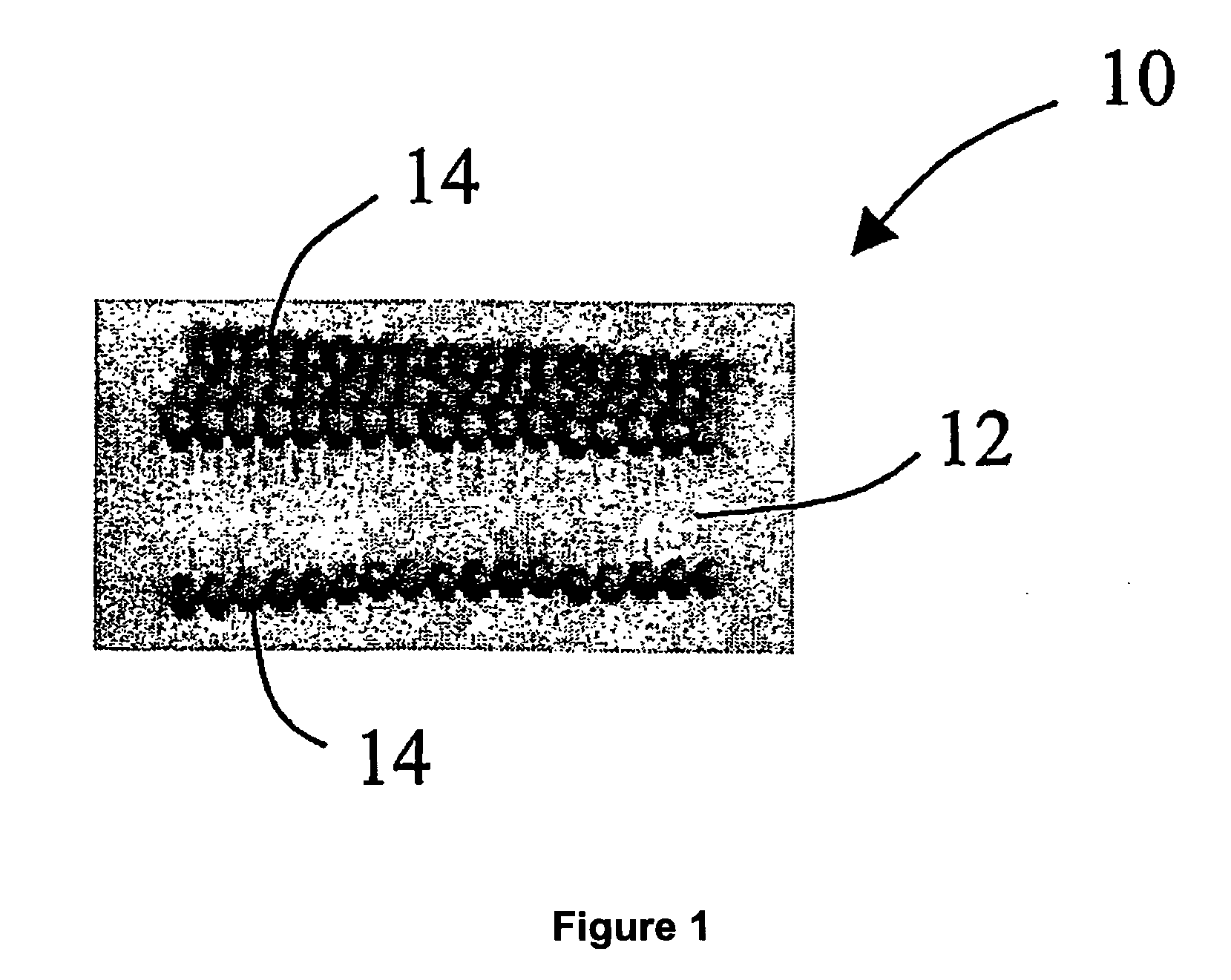
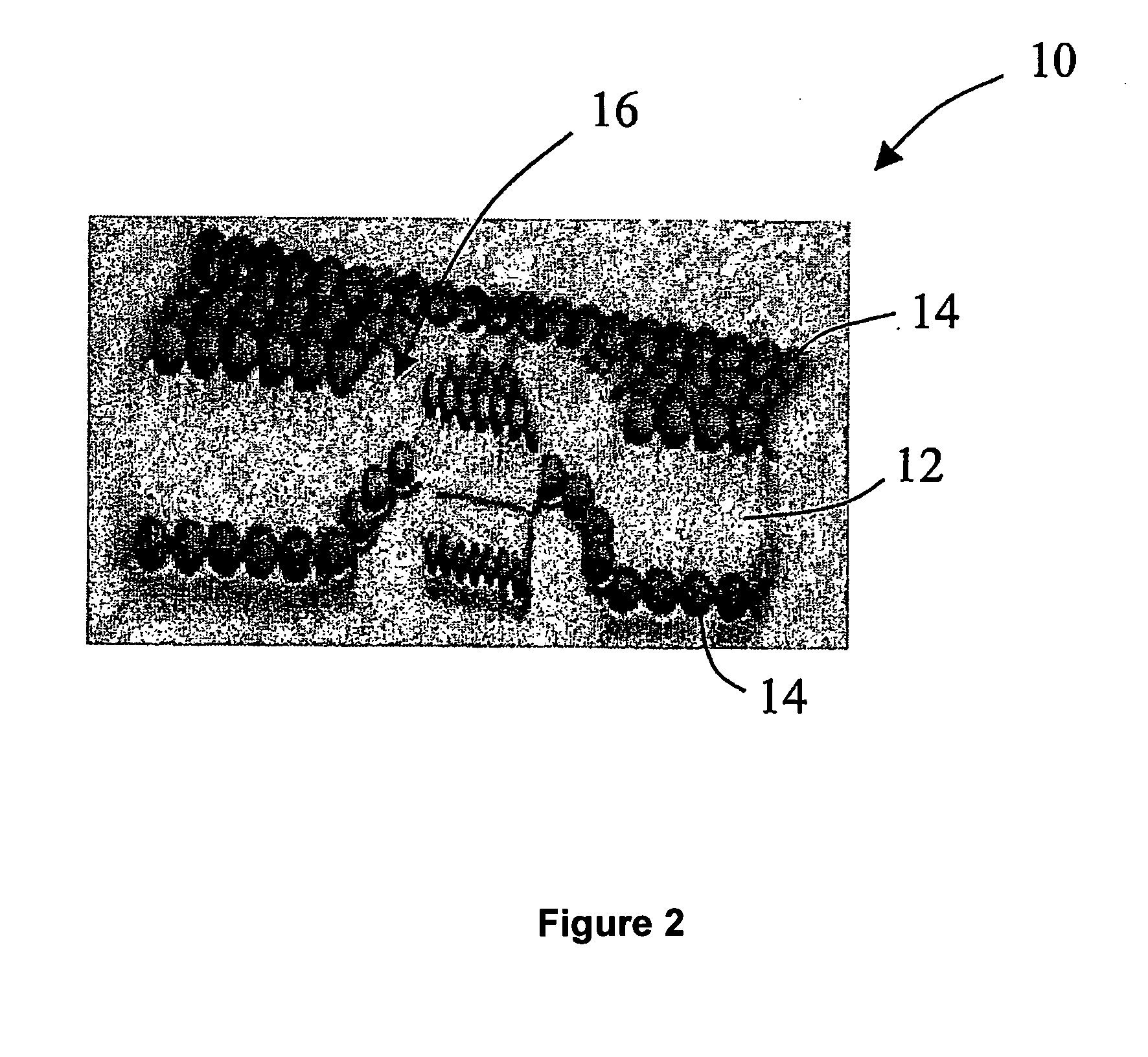
Solid carriers for improved delivery of active ingredients in pharmaceutical compositions
InactiveUS6923988B2Rapid dissolvableMore solubilizedAntibacterial agentsOrganic active ingredientsDiagnostic agentTG - Triglyceride
The present invention provides solid pharmaceutical compositions for improved delivery of a wide variety of pharmaceutical active ingredients contained therein or separately administered. In one embodiment, the solid pharmaceutical composition includes a solid carrier, the solid carrier including a substrate and an encapsulation coat on the substrate. The encapsulation coat can include different combinations of pharmaceutical active ingredients, hydrophilic surfactant, lipophilic surfactants and triglycerides. In another embodiment, the solid pharmaceutical composition includes a solid carrier, the solid carrier being formed of different combinations of pharmaceutical active ingredients, hydrophilic surfactants, lipophilic surfactants and triglycerides. The compositions of the present invention can be used for improved delivery of hydrophilic or hydrophobic pharmaceutical active ingredients, such as drugs, nutritional agents, cosmeceuticals and diagnostic agents.
Owner:LIPOCINE
Solid carriers for improved delivery of active ingredients in pharmaceutical compositions
InactiveUS20060034937A1Good chemical stabilityPromote absorptionGranular deliveryMicrocapsulesDiagnostic agentMedicine
The present invention provides solid pharmaceutical compositions for improved delivery of a wide variety of pharmaceutical active ingredients contained therein or separately administered. In one embodiment, the solid pharmaceutical composition includes a solid carrier, the solid carrier including a substrate and an encapsulation coat on the substrate. The encapsulation coat can include different combinations of pharmaceutical active ingredients, hydrophilic surfactant, lipophilic surfactants and triglycerides. In another embodiment, the solid pharmaceutical composition includes a solid carrier, the solid carrier being formed of different combinations of pharmaceutical active ingredients, hydrophilic surfactants, lipophilic surfactants and triglycerides. The compositions of the present invention can be used for improved delivery of hydrophilic or hydrophobic pharmaceutical active ingredients, such as drugs, nutritional agents, cosmeceuticals and diagnostic agents.
Owner:LIPOCINE
Solid carriers for improved delivery of active ingredients in pharmaceutical compositions
InactiveUS20090074859A1Dissolve fastReduce deliveryAntibacterial agentsPowder deliveryDiagnostic agentTG - Triglyceride
The present invention provides solid pharmaceutical compositions for improved delivery of a wide variety of pharmaceutical active ingredients contained therein or separately administered. In one embodiment, the solid pharmaceutical composition includes a solid carrier, the solid carrier including a substrate and an encapsulation coat on the substrate. The encapsulation coat can include different combinations of pharmaceutical active ingredients, hydrophilic surfactant, lipophilic surfactants and triglycerides. In another embodiment, the solid pharmaceutical composition includes a solid carrier, the solid carrier being formed of different combinations of pharmaceutical active ingredients, hydrophilic surfactants, lipophilic surfactants and triglycerides. The compositions of the present invention can be used for improved delivery of hydrophilic or hydrophobic pharmaceutical active ingredients, such as drugs, nutritional agents, cosmeceuticals and diagnostic agents.
Owner:LIPOCINE
Biosynchronous transdermal drug delivery for longevity, anti-aging, fatigue management, obesity, weight loss, weight management, delivery of nutraceuticals, and the treatment of hyperglycemia, alzheimer's disease, sleep disorders, parkinson's disease, aids, epilepsy, attention deficit disorder, nicotine addiction, cancer, headache and pain control, asthma, angina, hypertension, depression, cold, flu and the like
ActiveUS20080220092A1Improve performanceReduce the amount requiredHeavy metal active ingredientsBiocidePhytochemicalAntioxidant
Systems and methods for longevity, anti-aging, fatigue management, obesity, weight loss, weight management, delivery of nutraceuticals, and treating hyperglycemia, Alzheimer's disease, sleep disorders, Parkinson's disease, Attention Deficit Disorder and nicotine addiction involve synchronizing and tailoring the administration of nutraceuticals, medications and other substances (for example, stimulants) in accordance with the body's natural circadian rhythms, meal times and other factors. Improved control of blood glucose levels, extended alertness, and weight control, and counteracting of disease symptoms when they are at their worst are possible. An automated, pre-programmable transdermal administration system is used to provide pulsed doses of medications, pharmaceuticals, hormones, neuropeptides, anorexigens, pro-drugs, stimulants, plant extracts, botanicals, nutraceuticals, cosmeceuticals, phytochemicals, phytonutrients, enzymes, antioxidants, essential oils, fatty acids, minerals, vitamins, amino acids, coenzymes, or other physiological active ingredient or precursor. The system can utilize a pump, pressurized reservoir, a system for removing depleted carrier solution, or other modulated dispensing actuator, in conjunction with porous membranes or micro-fabricated structures.
Owner:MORNINGSIDE VENTURE INVESTMENTS
Delivery devices for hair-promoting cosmetic agent
InactiveUS20070160562A1Limiting and eliminating concernPromote growthBiocideCosmetic preparationsMedicineEyelash
The field of the present invention relates to improved cosmeceuticals capable of stimulating the growth of hair, e.g., natural eyelashes, in a human subject. More specifically, the present invention relates to delivery devices that provide for effective, targeted application of a therapeutically effective amount of the improved cosmeceuticals. Importantly, the targeted application helps to limit and / or eliminate concerns about the cosmeceutical active ingredient effecting unintended areas of the body.
Owner:BRINKENHOFF MICHAEL C
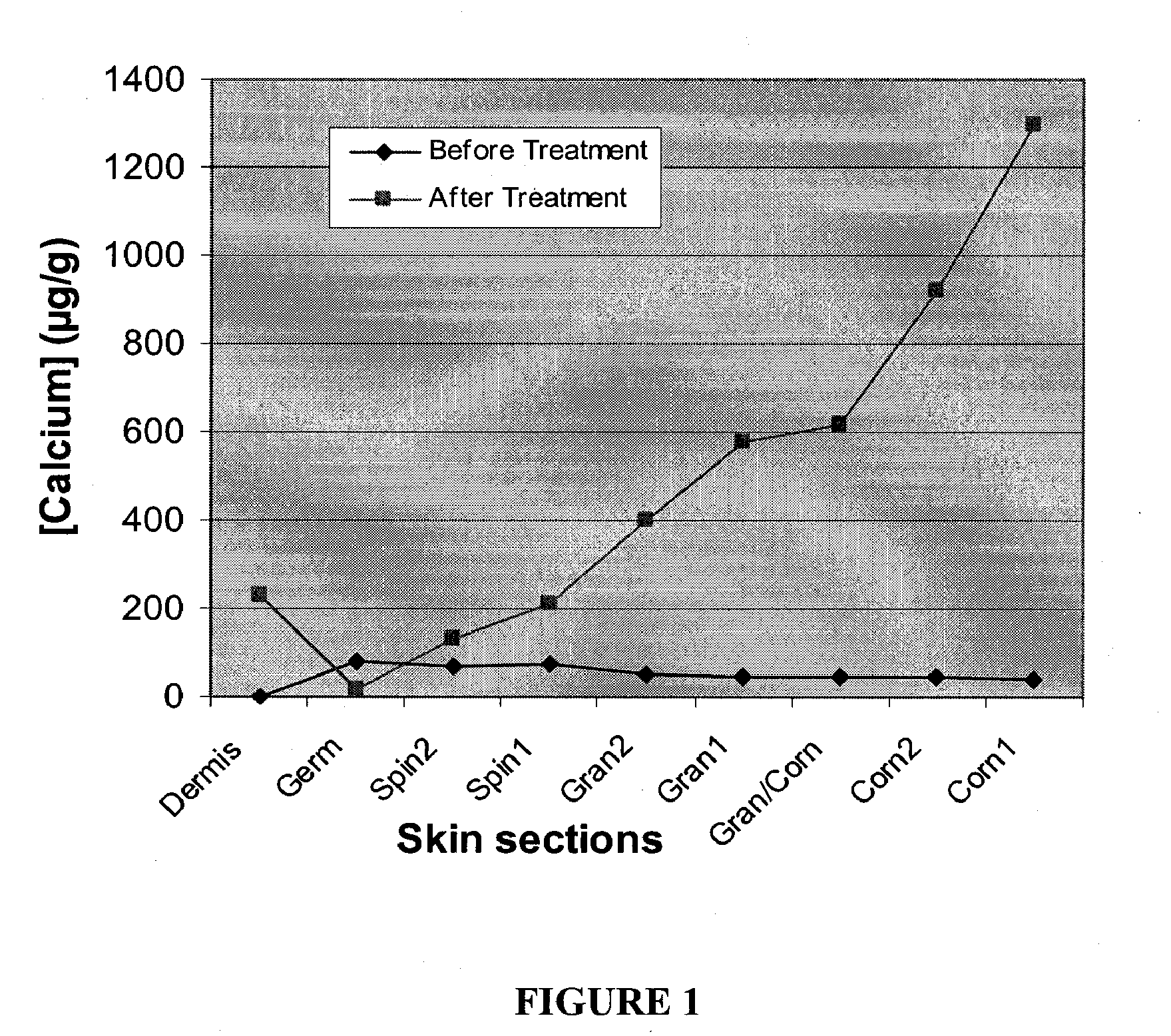
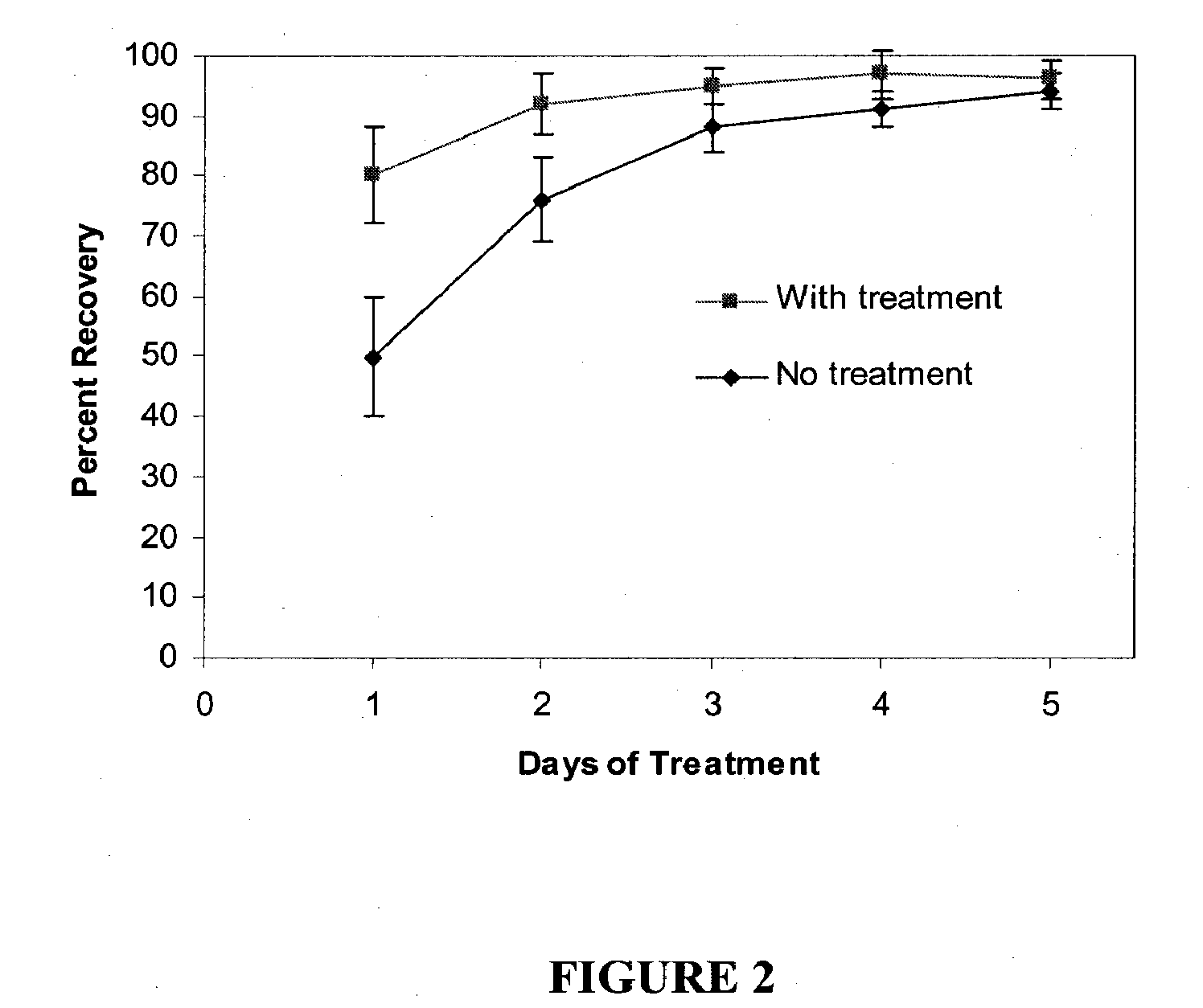
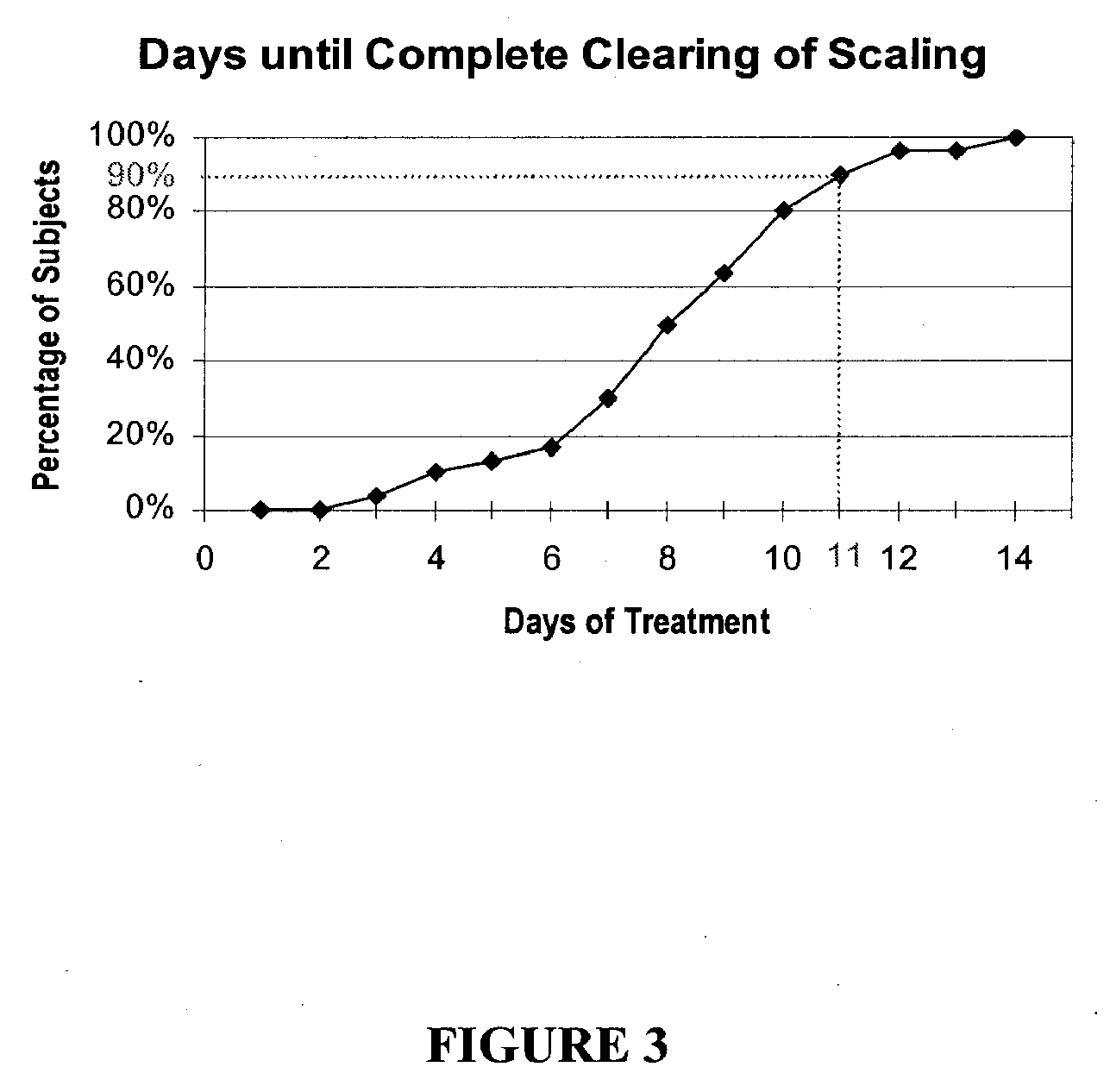
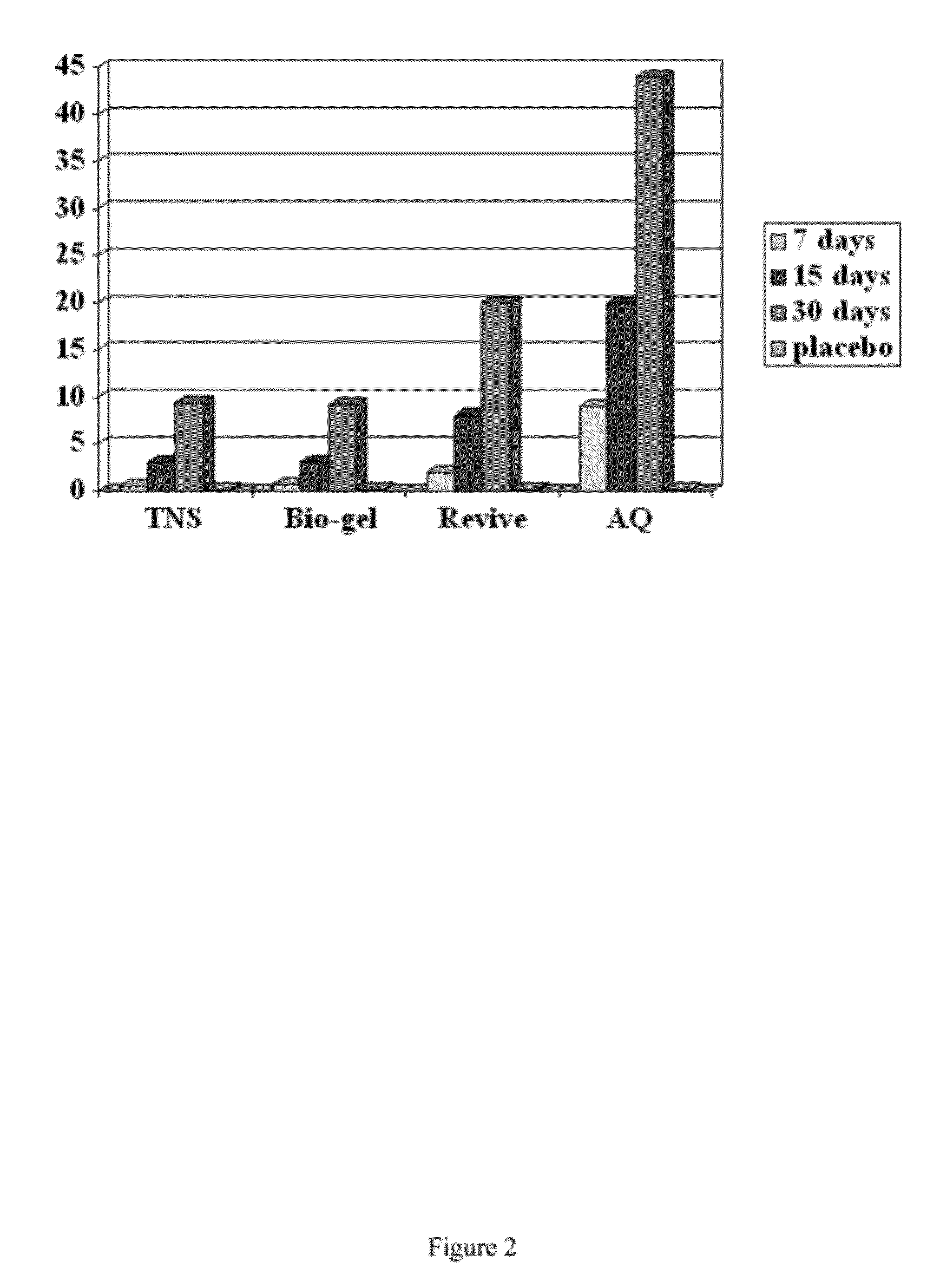
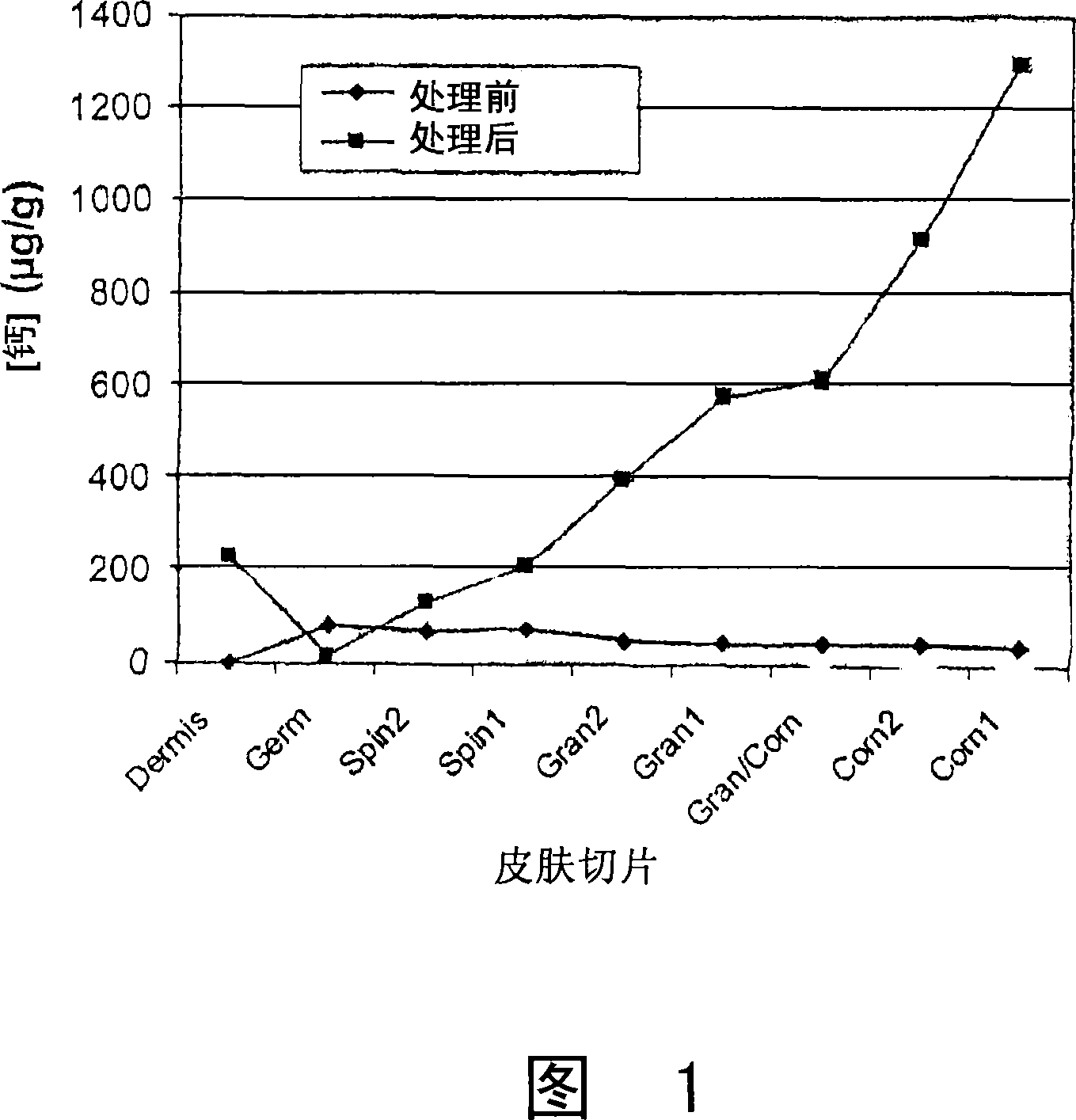
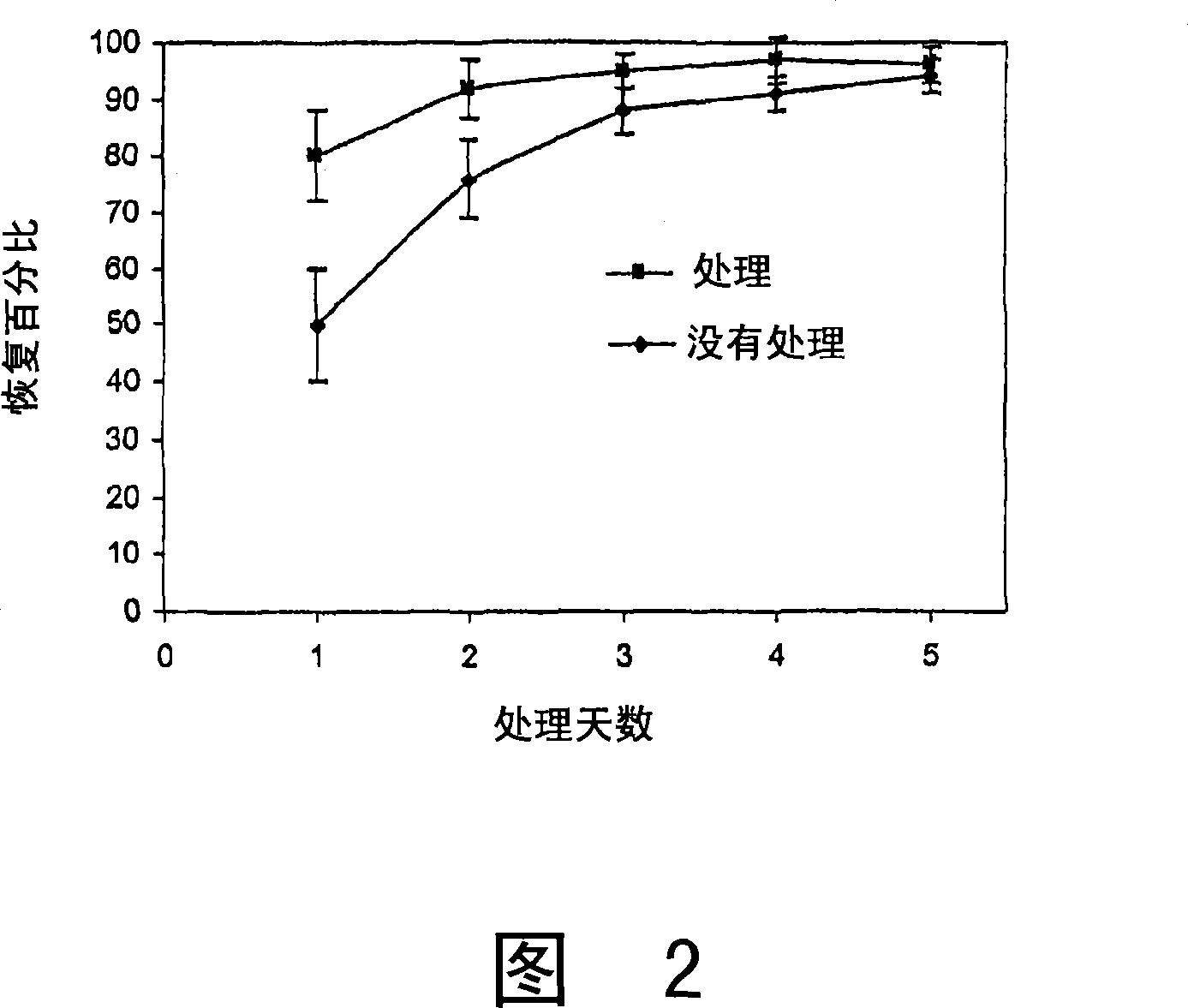
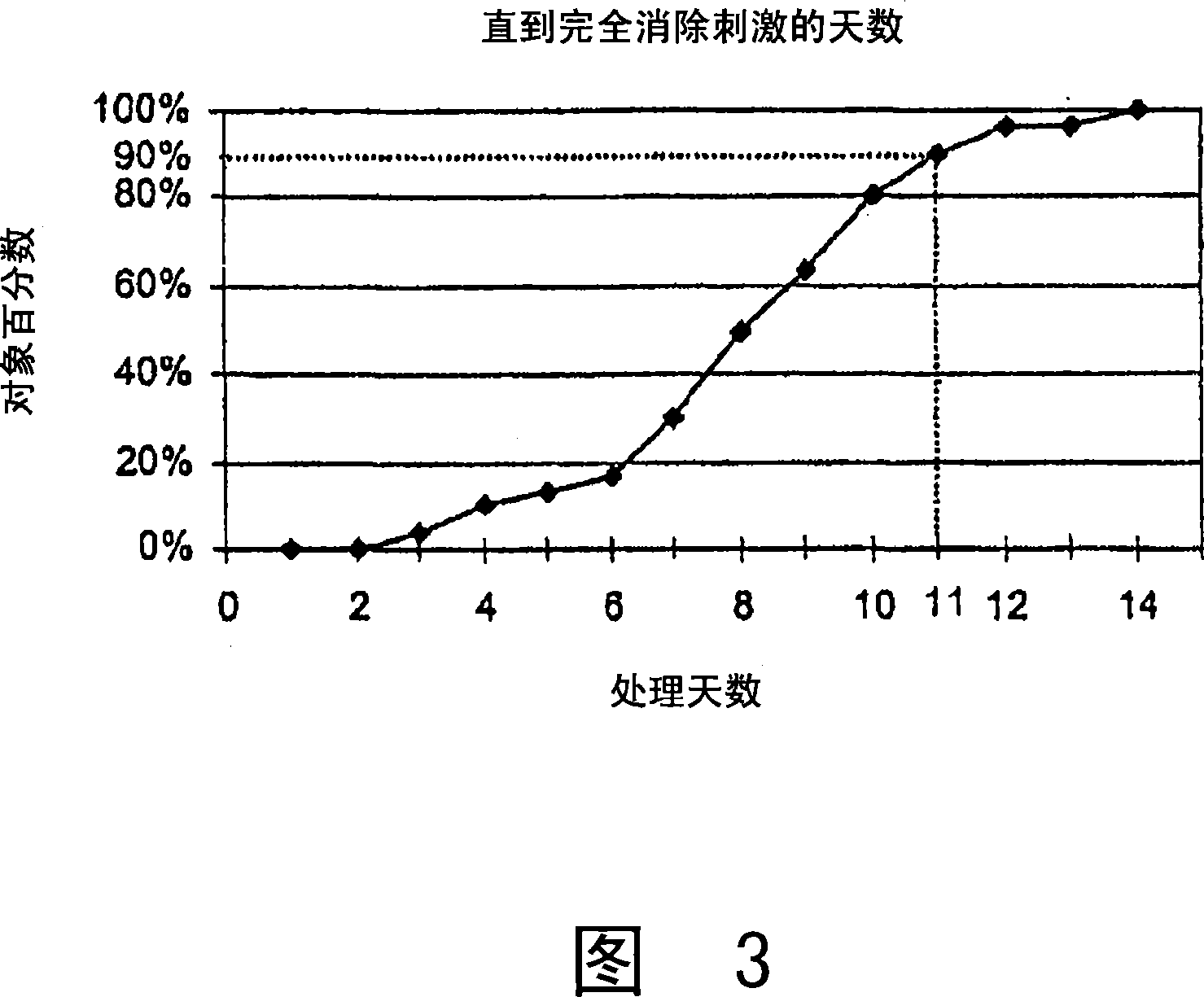
Cosmetic and cosmeceutical compositions for restoration of skin barrier function
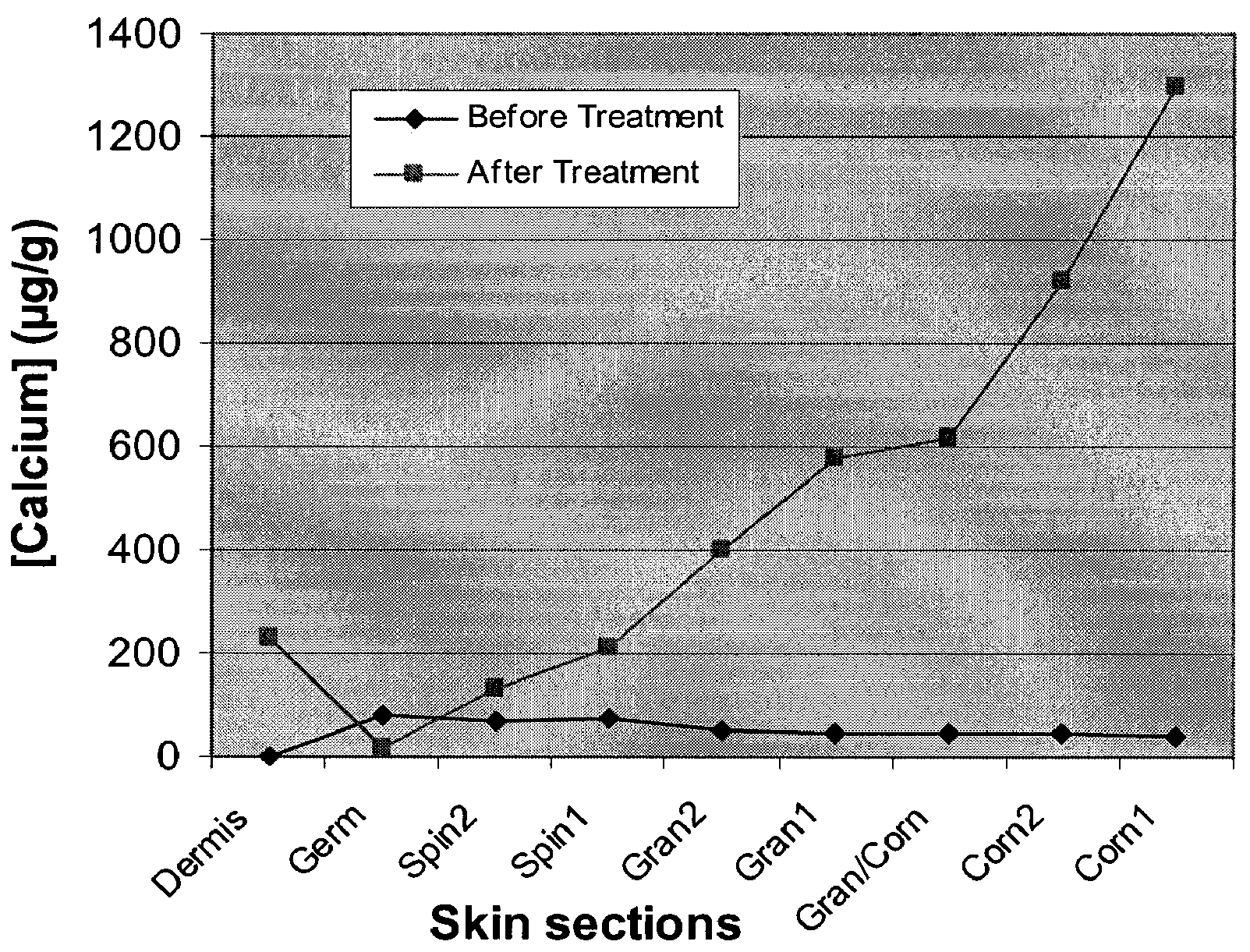
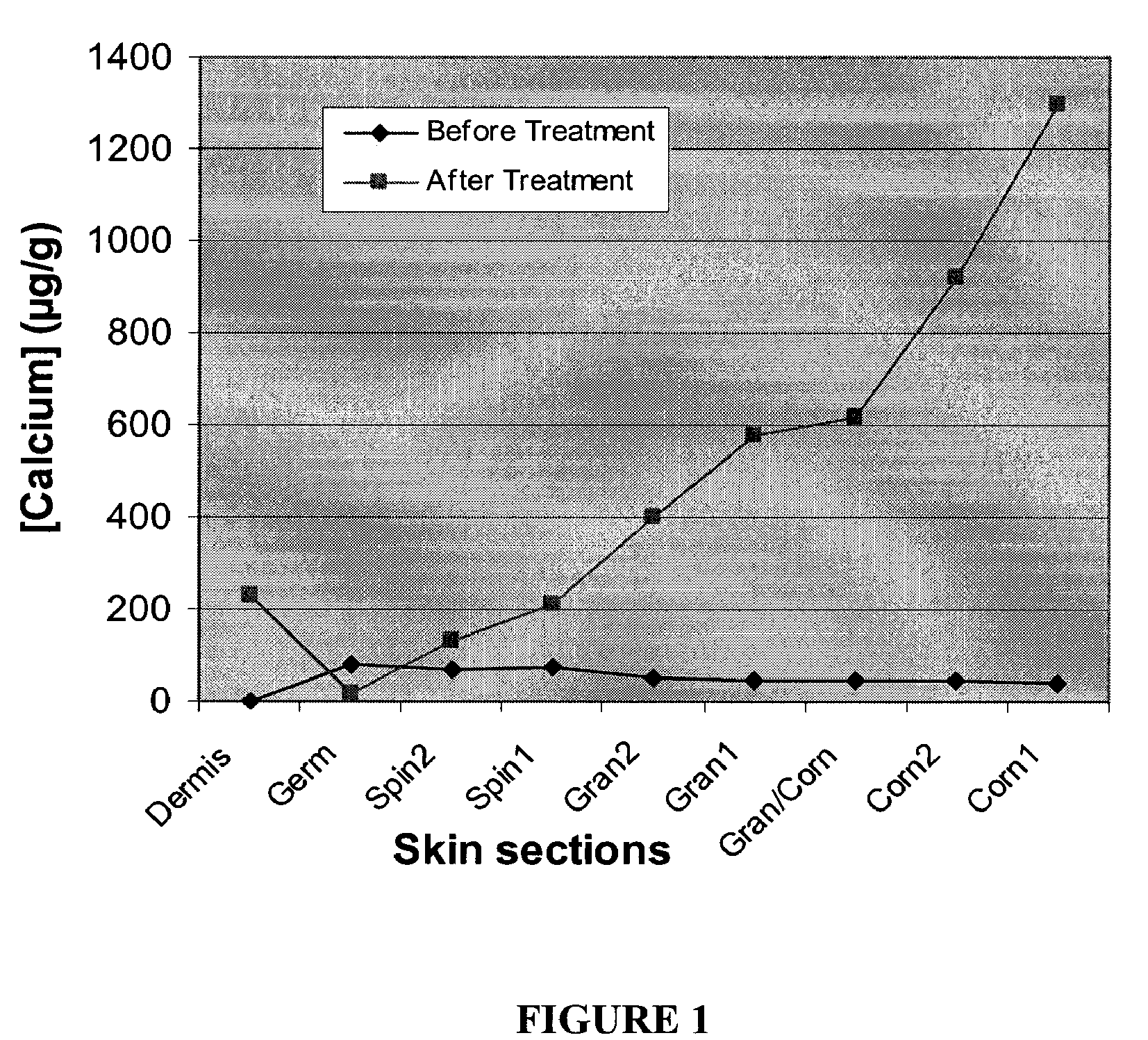
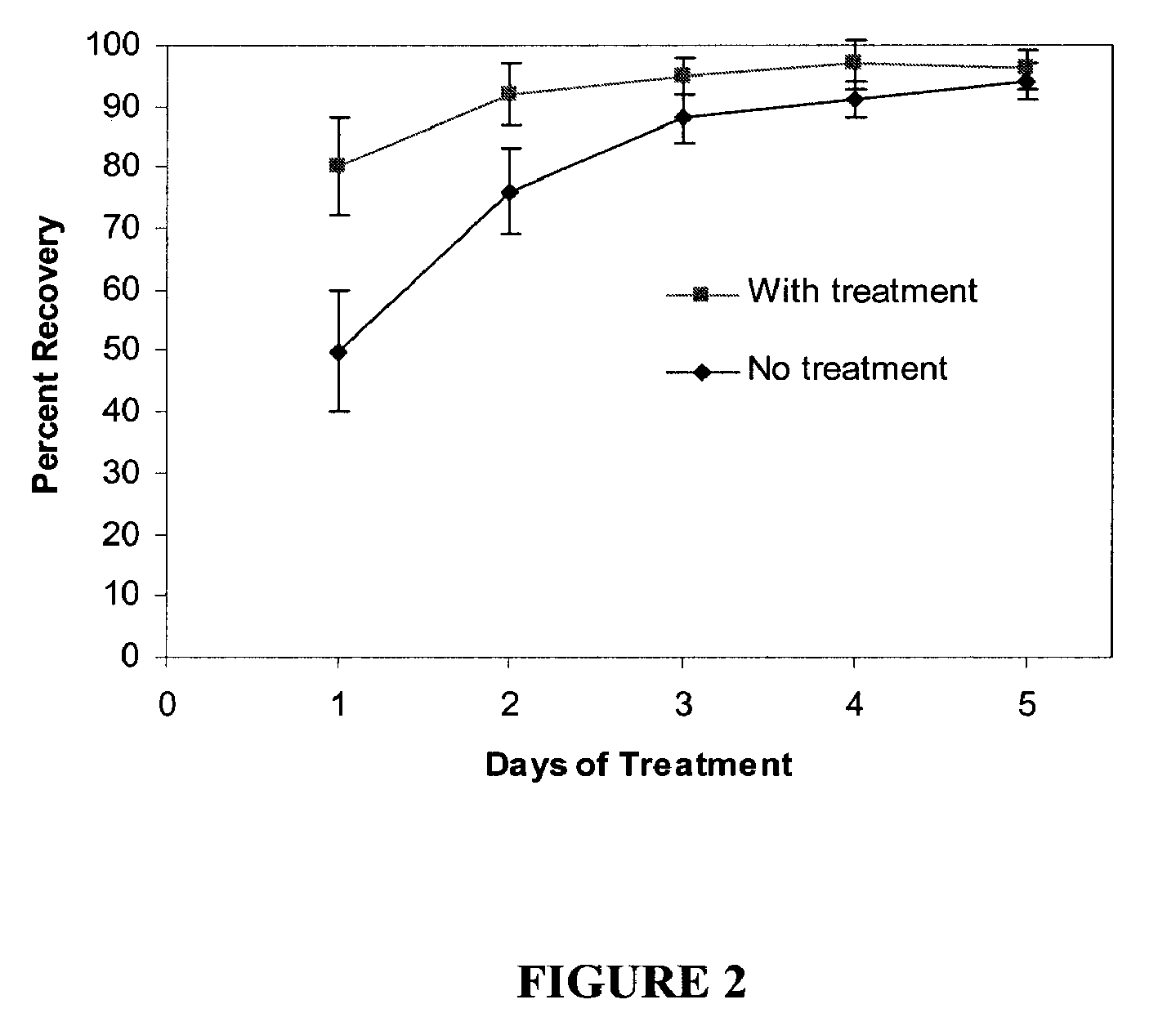
Compositions, kits and methods are provided for restoring skin barrier function to skin exposed to environmental elements and / or in a pathological condition. In general, divalent cations such as calcium ions and / or magnesium ions are included in a physiologically acceptable medium. In some embodiments, divalent cations are balanced with monovalent cations such as sodium and potassium ions at appropriate ratios in order to maintain the homeostasis of skin barrier. The compositions, kits and methods can be used as cosmetics, cosmeceuticals or pharmaceuticals for improving skin condition, and preventing or treating dermatological diseases and skin disorders.
Owner:GENEPHARM
Skin cream
ActiveUS20150023908A1Increase blood flowIncrease skin temperatureCosmetic preparationsHair cosmeticsMedicineCell culture media
The present invention relates to skin care compositions, including cosmeceuticals, for topical application, and more particularly, a skin cream, comprising exosomes and cell culture medium conditioned by cells grown in two-dimensional culture. Also included are methods of making and using such compositions and kits comprising the skin cream therein.
Owner:AL QAHTANI AHMED H
Biosynchronous transdermal drug delivery for longevity, anti-aging, fatigue management, obesity, weight loss, weight management, delivery of nutraceuticals, and the treatment of hyperglycemia, alzheimer's disease, sleep disorders, parkinson's disease, aids, epilepsy, attention deficit disorder, nicotine addiction, cancer, headache and pain control, asthma, angina, hypertension, depression, cold, flu and the like
ActiveUS8252321B2Improve performanceReduce the amount requiredHeavy metal active ingredientsBiocideDiseasePhytochemical
Owner:MORNINGSIDE VENTURE INVESTMENTS
Cosmetic and cosmeceutical compositions for restoration of skin barrier function
InactiveUS20060182770A1Restoring skin barrier functionMaintain steady stateBiocideOrganic active ingredientsSkin barrier functionPotassium ions
Compositions, kits and methods are provided for restoring skin barrier function to skin exposed to environmental elements and / or in a pathological condition. In general, divalent cations such as calcium ions and / or magnesium ions are included in a physiologically acceptable medium. In some embodiments, divalent cations are balanced with monovalent cations such as sodium and potassium ions at appropriate ratios in order to maintain the homeostasis of skin barrier. The compositions, kits and methods can be used as cosmetics, cosmeceuticals or pharmaceuticals for improving skin condition, and preventing or treating dermatological diseases and skin disorders.
Owner:GENEPHARM
Skin cream
InactiveUS20110294731A1Prevent skinDefect in skinCosmetic preparationsPeptide/protein ingredientsMedicineCell culture media
Preferred embodiments of the invention relate to compositions, including anti-aging cosmeceuticals for topical application, and more particularly, a skin cream, comprising cell culture medium conditioned by cells grown in two-dimensional culture.
Owner:INVITRX
Maple tree-derived products and uses thereof
The present document describes a neutraceutical, cosmeceuticals, functional food, pharmaceutical, food ingredient, and non-food ingredient compositions comprising sugar maple extract, essential oil compositions comprising oil extracted from an Acer tree, sweetening compositions containing sugar extracted from maple tree leaves, food ingredients comprising maple tree extract, cosmetic composition comprising maple tree extracts, infusion compositions prepared from maple tree leaves, maple roots, maple wood, maple stems of leaves and samara, and stems / twigs as well as compounds isolated from sugar maple biomass and the methods of extracting the same.
Owner:UNIVERSITY OF RHODE ISLAND +1
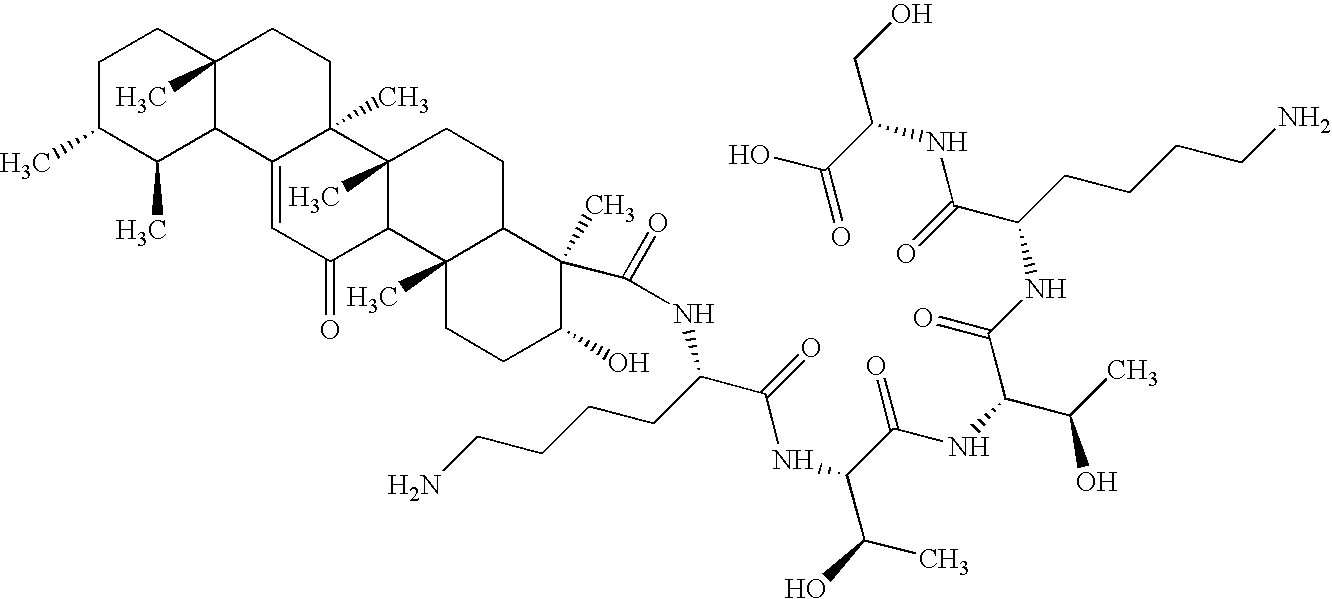
Peptides Modified with Triterpenoids and Small Organic Molecules: Synthesis and use in Cosmeceuticals
ActiveUS20100034758A1Reduce wrinklesReduce appearanceCosmetic preparationsPeptide/protein ingredientsWrinkle skinAdditive ingredient
The present invention relates to the Synthesis of Triterpenoid peptides and mechanism of action for Anti ageing and skin care. The present invention is directed towards anti-aging skin care compositions comprising peptides which are made by linking herbal actives to a pentapeptide for enhanced anti ageing activity by regenerating the dermal matrix. In detail, the present invention relates to the synthesis of Triterpenoid peptides, providing an enhanced and synergistic activity for reducing the consequences of ageing such as appearance of fine expression lines and wrinkles on the skin by cosmetic modes of application. The Triterpenoid peptides of the present invention with its novel dual action mode can be used for skin ageing & collagen insufficiency. Its Triterpenoid group acts by preventing oxidation and excess activity of serine proteases like elastase and collagenase that result in wrinkling of skin. With added peptides which boost the collagen and other matrix protein, Triterpenoid peptides provide a complete protection against pre mature ageing and functions as a best anti ageing ingredient.
Owner:SAMI LABS LTD
Gastropod biological fluid, method of making and refining and use
InactiveUS20100233111A1High activitySkin stimulationBiocideCosmetic preparationsMedicineCosmeceuticals
The present technology generally relates to skin care compositions, cosmeceuticals or formulations and methods of making or using the same. More specifically, the presently described technology generally relates to methods of making and using compositions, cosmeceuticals or formulations including a unique composition collected and refine from a gastropod, namely Helix Aspersa Müller, among others.
Owner:PHARMACOM INT
Skin cream
ActiveUS9119974B2Improve integrityIncrease contentCosmetic preparationsHair cosmeticsCell culture mediaSkin Cream
The present invention relates to skin care compositions, including cosmeceuticals, for topical application, and more particularly, a skin cream, comprising exosomes and cell culture medium conditioned by cells grown in two-dimensional culture. Also included are methods of making and using such compositions and kits comprising the skin cream therein.
Owner:AL QAHTANI AHMED H
Skin cream
ActiveUS8518879B2Prevent skinPromote secretionCosmetic preparationsPeptide/protein ingredientsSkin CreamCell culture media
The present invention relates to skin care compositions, including cosmeceuticals, for topical application, and more particularly, a skin cream, comprising cell culture medium conditioned by cells grown in two-dimensional culture. Also included are methods of using such compositions and kits comprising the skin cream therein.
Owner:AL QAHTANI AHMED H
Skin Cream
ActiveUS20120225029A1Promote secretionPrevent skinCosmetic preparationsHair cosmeticsMedicineCell culture media
The present invention relates to skin care compositions, including cosmeceuticals, for topical application, and more particularly, a skin cream, comprising cell culture medium conditioned by cells grown in two-dimensional culture. Also included are methods of using such compositions and kits comprising the skin cream therein.
Owner:AL QAHTANI AHMED H
Cosmetic and cosmeceutical compositions for restoration of skin barrier function
InactiveCN101146508ACosmetic preparationsToilet preparationsSkin barrier functionChemical composition
Compositions, kits and methods are provided for restoring skin barrier function to skin exposed to environmental elements and / or in a pathological condition. In general, divalent cations such as calcium ions and / or magnesium ions are included in a physiologically acceptable medium. In some embodiments, divalent cations are balanced with monovalent cations such as sodium and potassium ions at appropriate ratios in order to maintain the homeostasis of skin barrier. The compositions, kits and methods can be used as cosmetics, cosmeceuticals or pharmaceuticals for improving skin condition, and preventing or treating dermatological diseases and skin disorders.
Owner:GENEPHARM
Dry emollient compositions
The present invention are dry-feel emollient compositions comprising jojoba oil based esters that have use in personal care, cosmetic, cosmaceutical and pharmaceutical products. These compositions are essentially solid at room temperature, can be provided in various shapes and sizes (especially as particulates such as spheres), and can be produced from combinations of fatty alcohols, isopropyl esters and wax esters obtained from the oil contained in the seed of the jojoba plant. These new compositions also increase the range of applications for cosmetic compositions through an emollient that is more polar and hydrophilic than is found in jojoba oils. The compositions of the present invention may be obtained by a novel process of a base catalyzed alcoholysis reaction between jojoba oil and an alcohol, such as isopropyl alcohol. These components, whether exclusively jojoba esters or when combined with other carrier and vehicle components (including other emollients or binders) can form excellent carrier composition for use in the cosmetic, personal care and / or pharmaceutical field. Typical materials with which the compositions of the present invention may be blended include, but are not limited to, cosmetic oils and waxes, both natural and synthetic, (including hydrogenated or partially hydrogenated oils), silicone oils, mineral oils, long chain esters, vitamins, long chain fatty acids, cosmeceuticals, pigments, botanical extracts, esters and ethers, dimers, trimers, oligomers, and polymers, and the like. The proportions of the jojoba esters should be chosen to provide the dry-feel to the composition, which is highly desired. This will usually require a weight percent that ranges from 10% to 100% by weight of carrier material in the composition.
Owner:INT FLORA TECH
Delivery systems
InactiveUS20120045486A1Outstanding wettingOutstanding dispersing propertyBiocidePowder deliveryParticulatesAdditive ingredient
The present invention provides a delivery system for active and functional ingredients. In particular, the delivery systems of the present invention find particular application in the delivery of active and functional ingredients, such as medicaments, pharmaceuticals, nutritional supplements, botanicals, cosmeceuticals etc. Further, the invention relates to a delivery system for oral or topical administration of such active and functional ingredients. The invention is a delivery system based on a particulate gel precursor that acts both as the bodying agent,as well as the dispersing and suspending agent in the formulation. By modifying the level of precursor and process conditions, a broad range of product consistencies can be achieved ranging from thin liquid suspension to firm or semi solid gel. The precursor gel is a particulate linear chain fuctan gel. Inulin is a preferred fructan.
Owner:OXFORD PHARMASCI
Growth factor mediated cosmeceuticals and use thereof to enhance skin quality
InactiveUS20080234194A1Promote migrationReduce wrinklesCosmetic preparationsVirusesDiseaseWrinkle skin
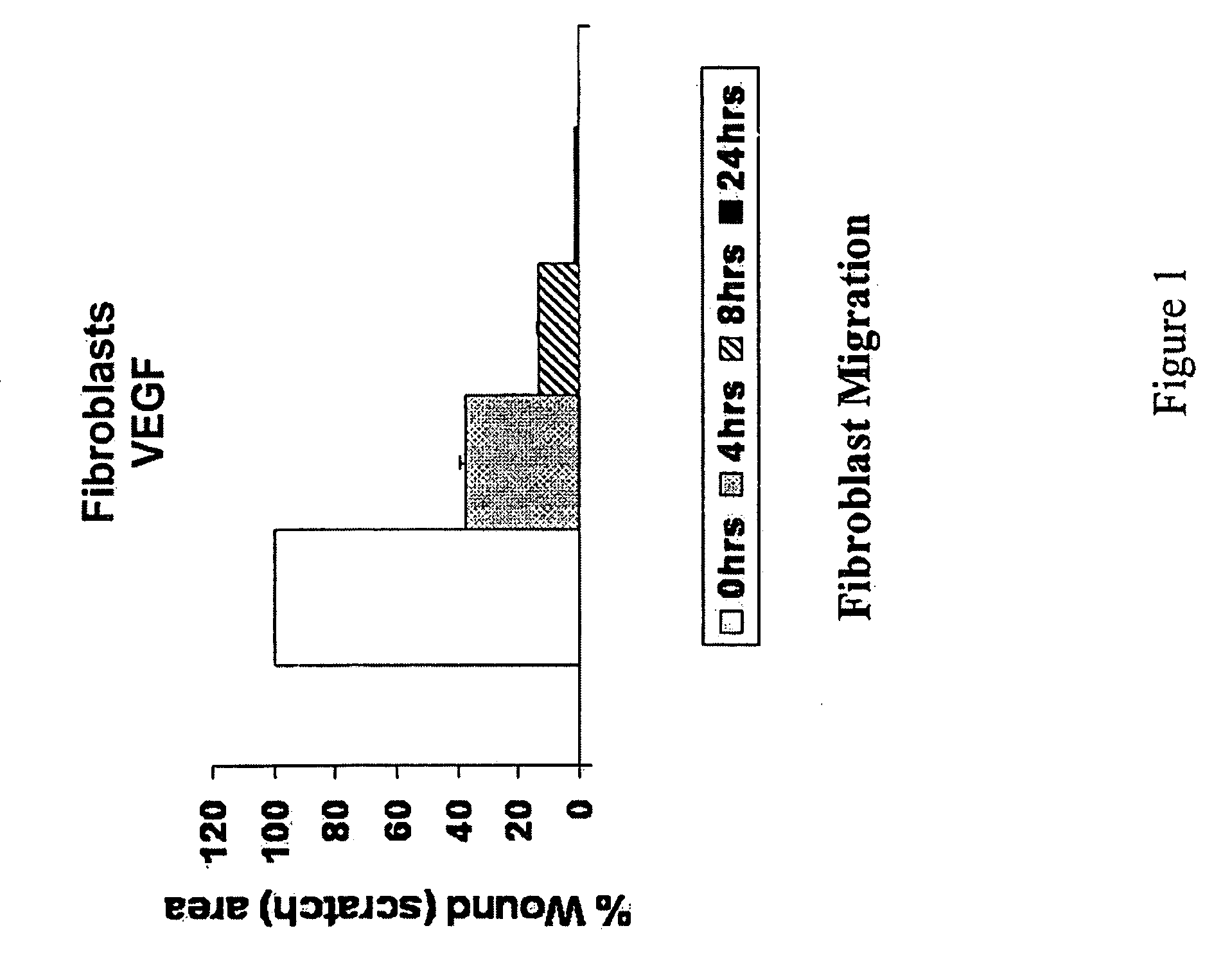
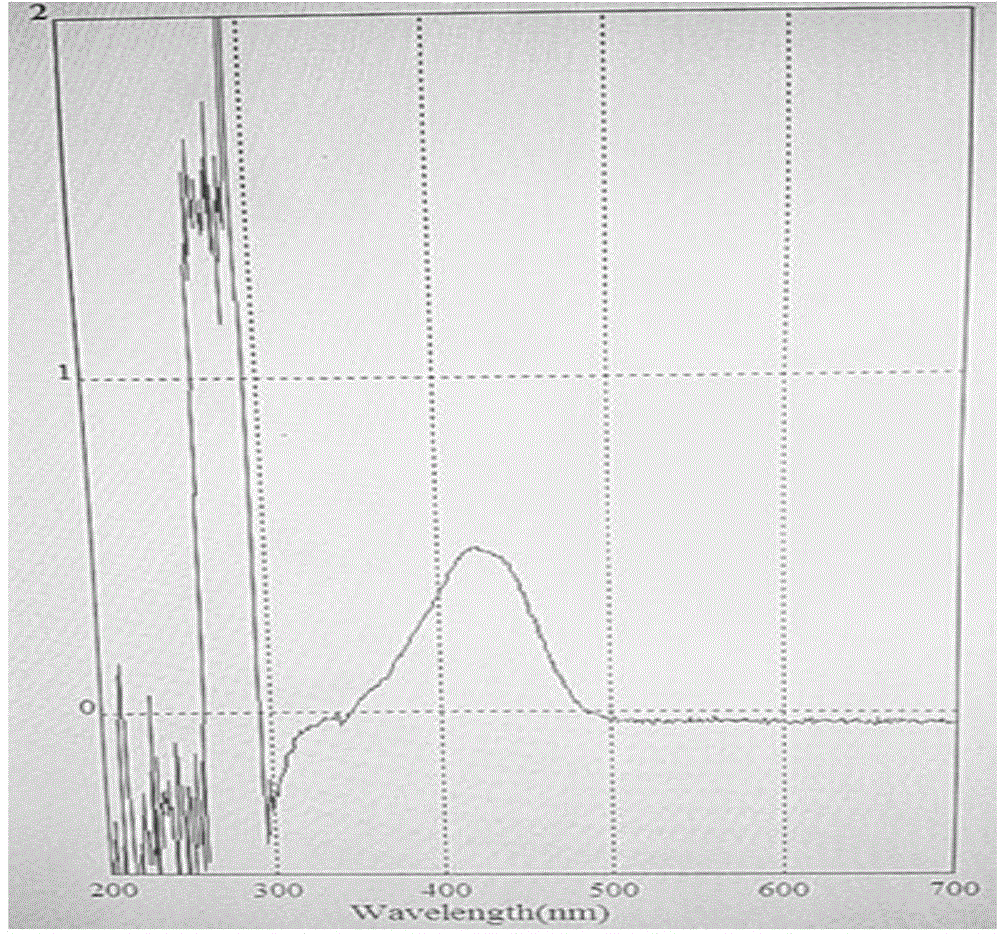
It has been discovered that vascular endothelial growth factor (“VEGF”) promotes migration of activated (but not differentiating) keratinocytes to skin. This growth factor specifically increases migration of keratinocytes of the “wounded skin” phenotype but does not have significant effects upon differentiated keratinocytes. It also increases collagen deposition and reduces wrinkles, enhances skin quality, and increases skin thickness to normal levels in individuals where skin has thinned due to age or disorder such as diabetes. It is particularly well suited for use as cosmeceuticals when applied in purified form and in known amounts. The data presented in the examples demonstrate efficacy and specificity of VEGF in enhancing migration of normal human keratinocytes as well as formation of new granulation tissue including collagen formation. VEGF induces keratinocyte and fibroblast migration, formation of new tissue, and not only induces deposition of collagen but improves alignment of the collagen fibers. Accordingly, this growth factor is highly suitable for use as a cosmeceutical, especially for skin resurfacing and reduction in wrinkles.
Owner:NEW YORK UNIV
Preparation method of curcumin flexible liposome cream
InactiveCN104434551AGood effectImprove antioxidant functionCosmetic preparationsToilet preparationsCurcumin LiposomeGynecology
The invention relates to a preparation method of a curcumin flexible liposome cream. The preparation method comprises the following three steps: preparing a curcumin flexible liposome, preparing freeze-dried powder from the curcumin flexible liposome, and preparing a curcumin flexible liposome cream. According to the preparation method disclosed by the invention, a curcumin liposome and a flexible liposome are prepared by virtue of a thin film dispersion process, and emulsible pastes of the curcumin liposome and the flexible liposome are prepared by virtue of an emulsification process so as to ensure that an optimal prescription is screened out and is subjected to stability evaluation and sensory evaluation, the utilization degree of curcumin can be improved, the emulsible pastes can be finer and more lubricant, and more choices can be provided in the application of cosmeceuticals in the future.
Owner:YANTAI UNIV
Apparatus and method of treatment utilizing a varying electromagnetic energization profile
The present invention relates to an apparatus and method for improving the delivery of desirable substances across a membrane utilizing a varying electromagnetic energisation profile. More particularly, the invention provides methods for modifying the cellular environment of such membranes and the behaviour of substance molecules during transport across a membrane. In use, the profile will have application in, enhancing transmembrane delivery of substances (such as pharmaceuticals, nutraceuticals, biopharmaceuticals and cosmeceuticals), and / or enhancing or expanding a cellular cohort of membranes and surrounding tissues.
Owner:INT SCI
Compositions and methods for delivery of skin cosmeceuticals
InactiveUS20030165552A1Improve skin appearanceAvoid irritationAntibacterial agentsSalicyclic acid active ingredientsOrganic acidSloughing
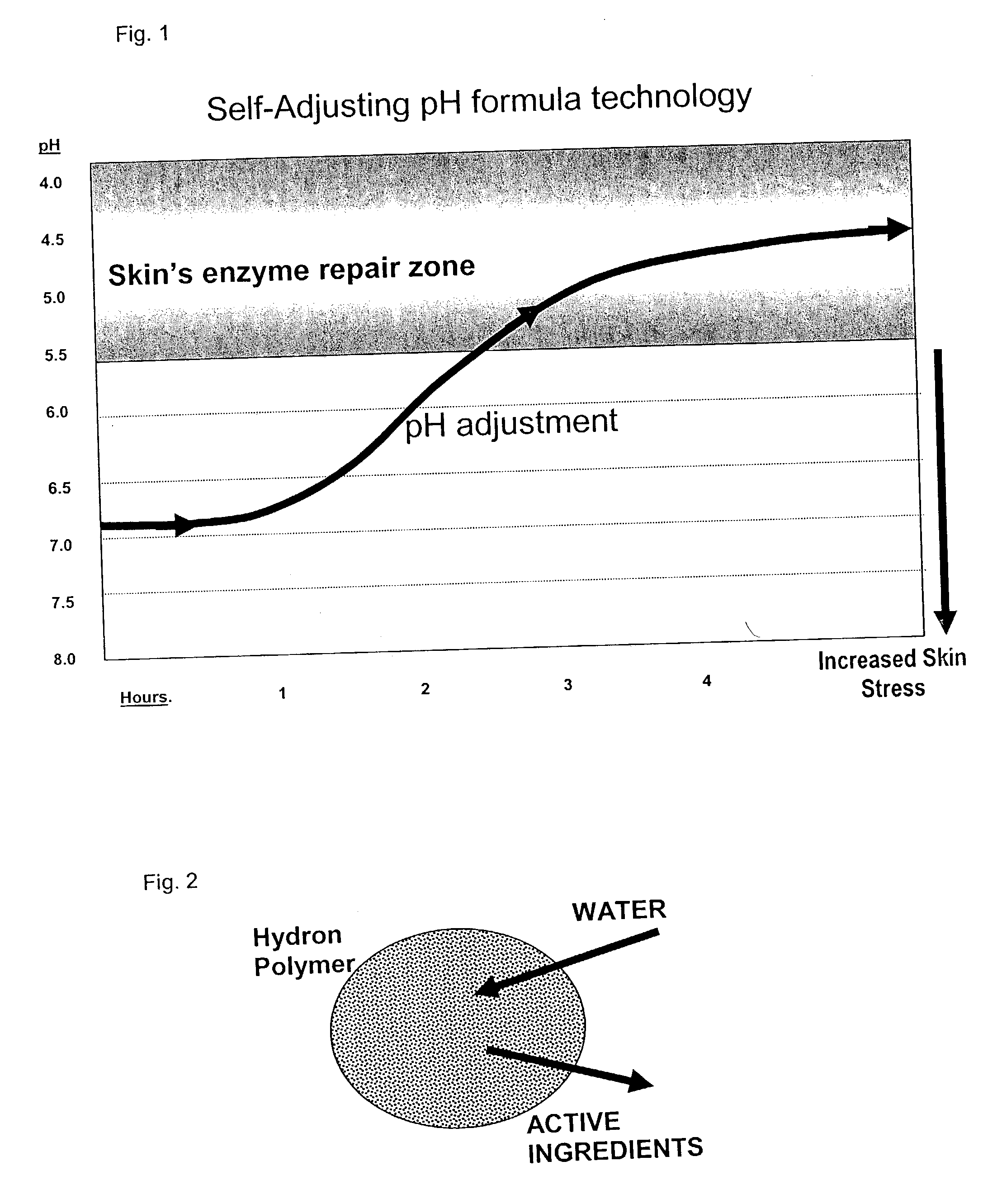
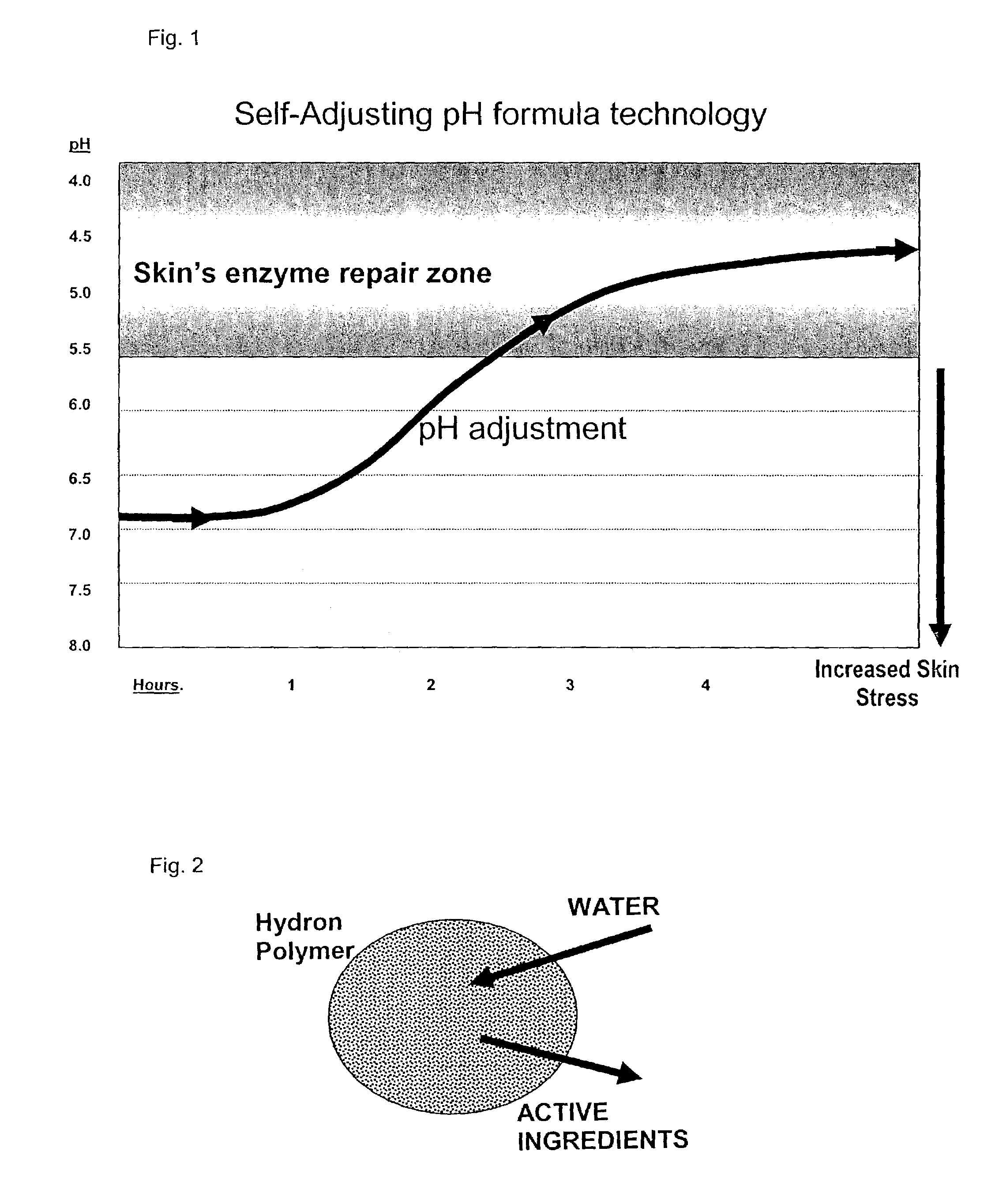
New cosmetic compositions are disclosed that are effective both as moisturizers and skin sloughing agents. The compositions contain neutralized weak organic acids that when applied over time to the skin in appropriate formulations will gradually increase in acidity to pH 5.5 or less, for example about pH 4.5, without causing skin irritation while exhibiting increasing activity in skin renewal effects.
Owner:HYDRON TECH
Collagen-containing beauty food composition
InactiveCN103222625ASuppress generationSolve the defects of whiteningFood preparationCuticleIngested food
The invention discloses a collagen-containing beauty food composition which comprises collagens and enzymes RAJ-326 as principal components and can promote generation of collagens in a human body to repair damaged skin. The invention also discloses another collagen-containing beauty food composition which is characterized by comprising collagens, enzymes RAJ-326 and more than one or two cysteine derivatives and / or salts of cysteine derivatives as principal components and can promote generation of collagens in the human body, restrain tyrosinase activities and prevent melanin formation to achieve the effect of skin whitening. The beauty food composition can serve as a healthcare food or a pharmaceutical product in the form of powder, beverages, capsules or tablets, and can also serve as a skin care product, a cosmetic product or an agent for external use in the form of gels, oil-in-water emulsions or water-in-oil emulsions.
Owner:百岳特生物科技(上海)有限公司 +3
Traditional Chinese medicine itching-relieving gel and preparation method thereof
ActiveCN103041054AHeat-clearing and detoxifyingLess medicinalAntipyreticAerosol deliveryCarrageenanGlycerol
The invention belongs to the technical field of medicines and relates to traditional Chinese medicine itching-relieving gel. The traditional Chinese medicine itching-relieving gel is prepared from raw materials comprising Chinese violet, cortex dictamni and sophora flavescens at a mass ratio of 2:1:1. The invention further relates to a preparation method of the traditional Chinese medicine itching-relieving gel. The preparation method comprises the following steps: (1) performing preparation of concentrated solution decocted with water of a traditional Chinese medicine; (2) performing preparation of gel substrate; and (3) finally adding the concentrated solution decocted with the water of the itching-relieving traditional Chinese medicine to the gel substrate, uniformly stirring, and cooling down at low temperature. According to the preparation method provided by the invention, the auxiliary of the gel comprises the following components in parts by weight: 12-14 parts of hydroxyethyl cellulose, 5-8 parts of avicel CL-611, 100 parts of glycerol, 50 parts of absolute ethyl alcohol, 15 parts of azone, 300-400 parts of distilled water, 0-3 parts of carrageen and 0-1 part of sodium hydroxide. The traditional Chinese medicine itching-relieving gel disclosed by the invention is applied to medicines for treating skin itch caused by various reasons, such as eczema, urticaria and pruritus; and in addition, cosmeceuticals can be prepared by adding 5-10 parts of lavender essential oil or menthol essential oil to the traditional Chinese medicine itching-relieving gel.
Owner:上海国创医药股份有限公司
Preservative composition with anti-acne function
InactiveCN104800130AImprove the safety of useLess irritatingAntibacterial agentsCosmetic preparationsPersonal careBacillus acnes
The invention provides a preservative composition containing various plant anti-acne components. The usage amount of the preservative can be reduced by sufficiently bringing the synergistic antibacterial effect of each component in the composition into play; the preservative composition has broad-spectrum bacteriostasis, is easy to use, safe and effective, harmless to a human body, and can be used for microbial sterilization and corrosion prevention in products of personal care, functional cosmetics, cosmeceuticals and the like; a natural plant preservative raw material is natural, safe, non-toxic, environment-friendly, and harmless to the human body, the used synergetic effect additive and solvent are alcohols, and the three achieve synergistic interaction, so that the preservative composition especially has a good inhibitory effect on propionibacterium acnes and staphylococcus epidermidis.
Owner:BEIJING SUNPU BIOCHEM TECH
Recombinant human fibronectin III1-C, and preparation method and application thereof
ActiveCN111217903AIncrease productionShorten the production cycleCosmetic preparationsConnective tissue peptidesBALB/cCell adhesion
The invention discloses a recombinant human fibronectin III1-C (rhFNIII1-C), and a preparation method and application thereof. The preparation method comprises the following steps: optimizing a targetgene segment of the target protein according to codon expression preference, and inserting the obtained gene segment into pET-32a to construct a recombinant plasmid; transforming and introducing therecombinant plasmid into an expression vector BL21(DE3), and carrying out screening to obtain positive clone bacteria capable of realizing efficient soluble expression; carrying out enlarging fermentation culture on the positive clone bacteria, carrying out induced expression, performing crushing and centrifuging to obtain a supernatant, and carrying out purification through elution with a His-tagaffinity chromatography column, dialysis, filtration and other steps to obtain a high-quality rhFNIII 1-C solution with a protein concentration of 1.0 mg / mL or above and a purity of 90% or above. According to detection and observation results of cell adhesion promoting tests, the rhFNIII1-C has the performance of obviously promoting adherence and adhesion of MDBK cells and Balb / c / 3T3 cells and rapid division and growth of the cells, which indicates that the rhFNIII1-C has huge potential of being used as a raw material and a finished product for cosmeceuticals and medical skincare.
Owner:ANHUI MEDICAL UNIV
Anti-light aging preparation for skin
InactiveCN103505378AHigh in collagenHigh activityCosmetic preparationsToilet preparationsP38 MAPK Signaling PathwayCosmeceuticals
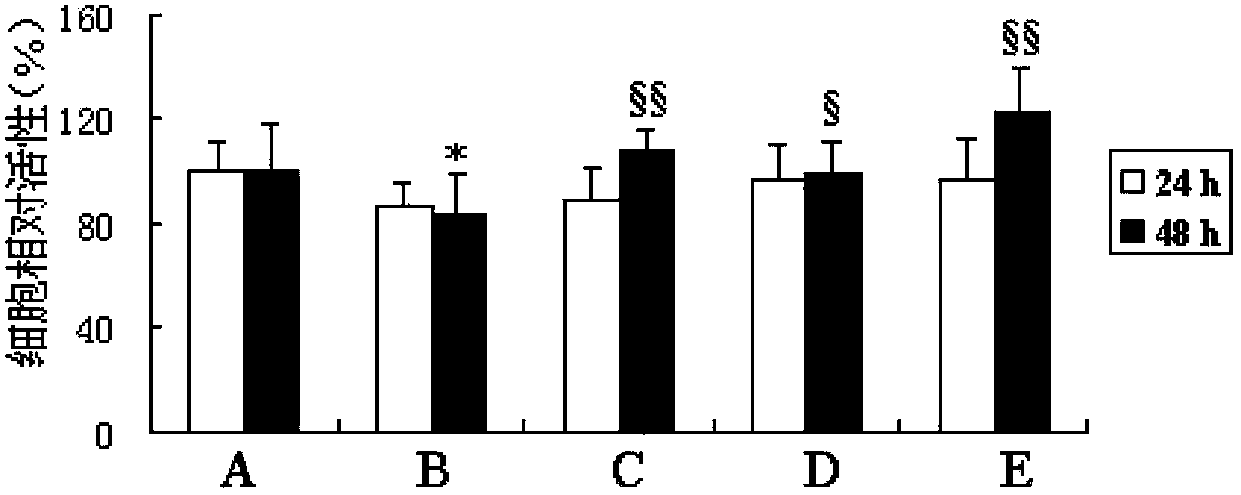
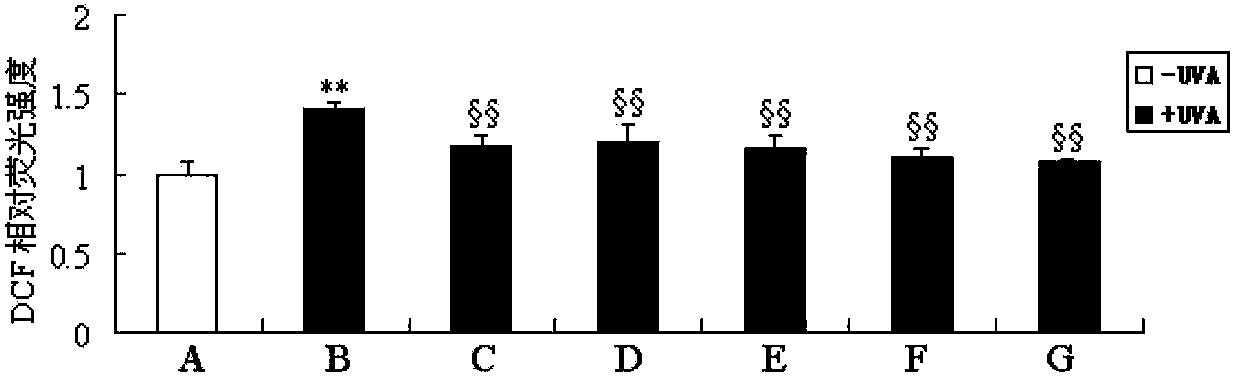
The invention discloses an anti-light aging preparation for the skin, wherein the preparation comprises royal jelly acid (10-HAD) and AA-2G. Meanwhile, the invention further discloses application of royal jelly acid and AA-2G in preparation of skin care products, cosmetics and cosmeceuticals. The anti-light aging preparation can substantially improve activity of cells irradiated by UVA, inhibits UVA-induced ROS generation and UVA-induced cell aging and has a substantial anti-light aging effect. According to research on action mechanism of the anti-light aging preparation, 10-HDA included in the anti-light aging preparation can inhibit activation of p-JNK and p38 MAPK signaling pathways and reduce expression of UVA-induced MMP-1 and MMP-3, so the content of collagen in cells can be increased; thus, the preparation is an effective anti-light aging preparation for the skin.
Owner:郑锦芬
Compositions and methods for delivery of skin cosmeceuticals
InactiveUS6984391B2Reduced pHReduce deliveryAntibacterial agentsSalicyclic acid active ingredientsOrganic acidSloughing
New cosmetic compositions are disclosed that are effective both as moisturizers and skin sloughing agents. The compositions contain neutralized weak organic acids that when applied over time to the skin in appropriate formulations will gradually increase in acidity to pH 5.5 or less, for example about pH 4.5, without causing skin irritation while exhibiting increasing activity in skin renewal effects.
Owner:HYDRON TECH
Anti-skin photoaging preparation and application thereof
InactiveCN103505374AHigh activityInhibitionCosmetic preparationsToilet preparationsP38 MAPK Signaling PathwayUltraviolet
The invention discloses an anti-skin photoaging preparation containing royal jelly acid (10-HDA), and also discloses application of 10-HDA (10-hydroxy-2-decenoic acid) in preparation of skin care products, cosmetics and cosmeceuticals. The anti-photoaging preparation provided by the invention can significantly improve the activity of UVA (ultra violet A) irradiated cells, inhibit UVA induction produced ROS (reactive oxygen species), inhibit UVA induced cell senescence, and has a significant anti-photoaging effect. Study on the action mechanism of the anti-skin photoaging preparation shows that the 10-HDA contained in the anti-photoaging preparation can inhibit activation of p-JNK and p38 MAPK signaling pathway and reduce UVA induced MMP-1 and MMP-3 expression, thereby increasing the collagen content in cells. Thus, the preparation is an effective anti-skin photoaging preparation.
Owner:郑锦芬 +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com