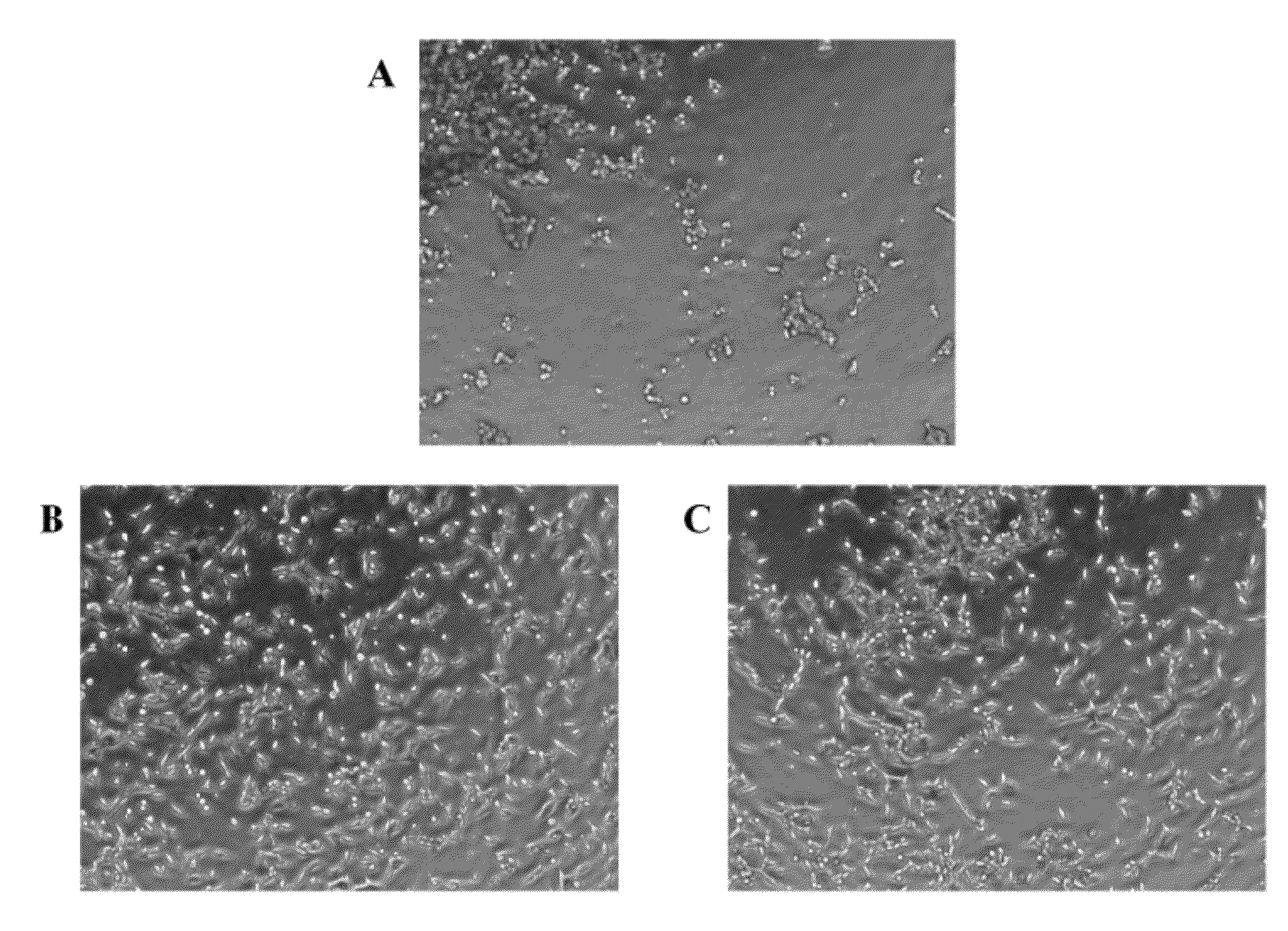Patents
Literature
576 results about "Fibronectin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
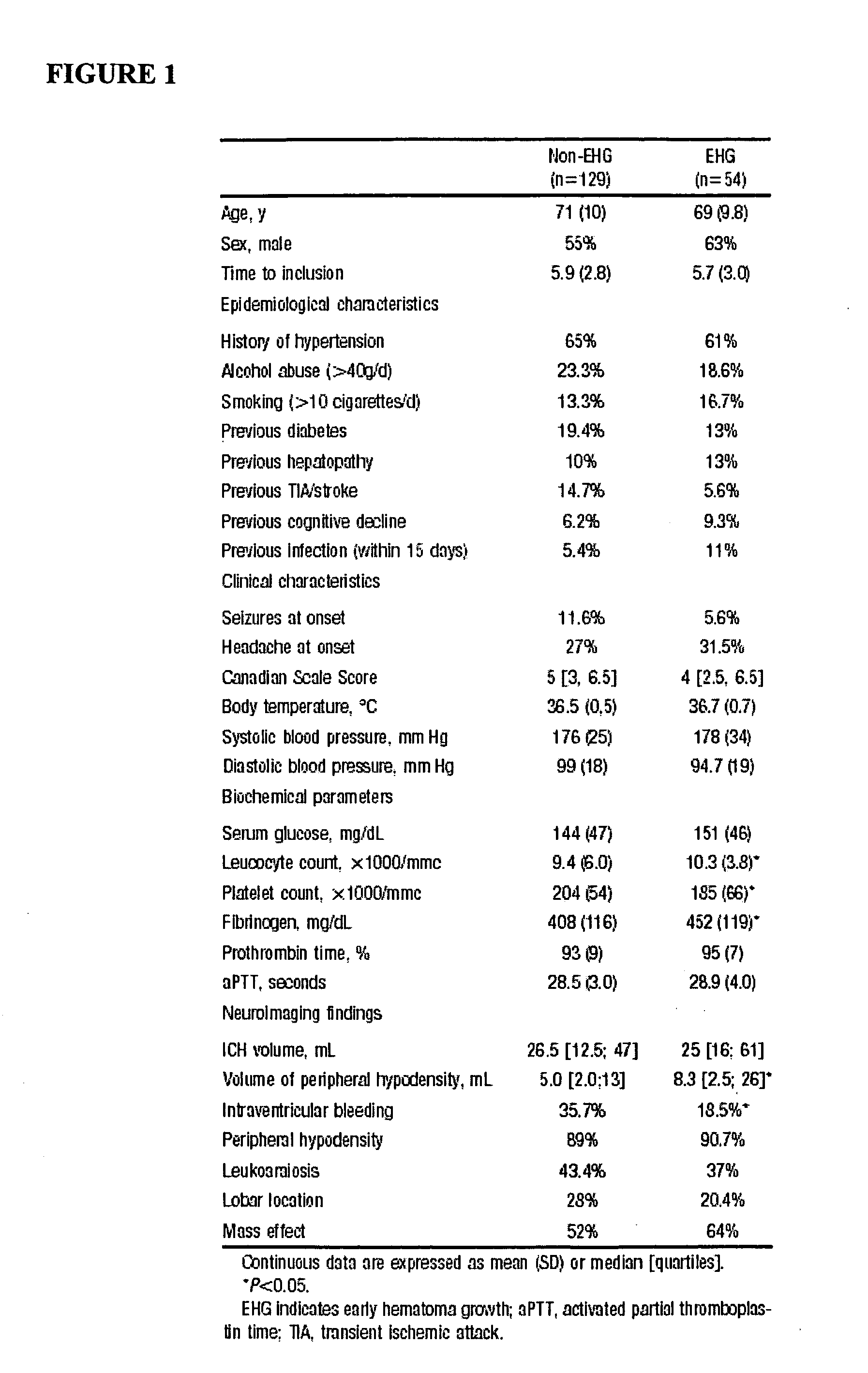
Fibronectin is a high-molecular weight (~440kDa) glycoprotein of the extracellular matrix that binds to membrane-spanning receptor proteins called integrins. Fibronectin also binds to other extracellular matrix proteins such as collagen, fibrin, and heparan sulfate proteoglycans (e.g. syndecans).
Bioactive coating composition and methods
InactiveUS6921811B2Promotes desired biologicalPromotes therapeutic effectSuture equipmentsOrganic active ingredientsArylBody fluid
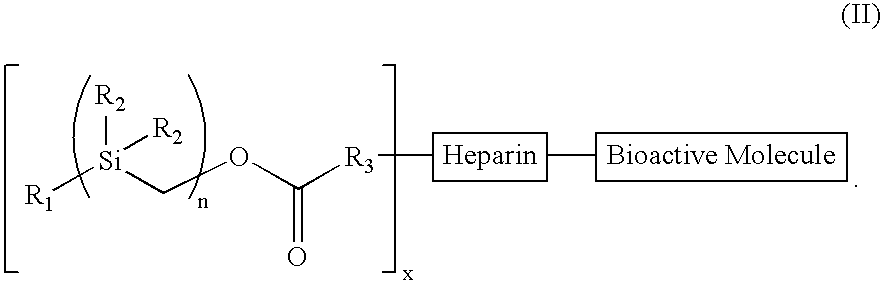
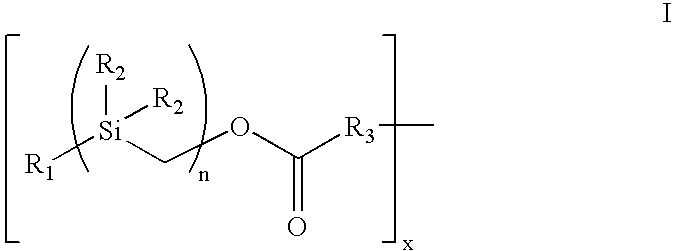
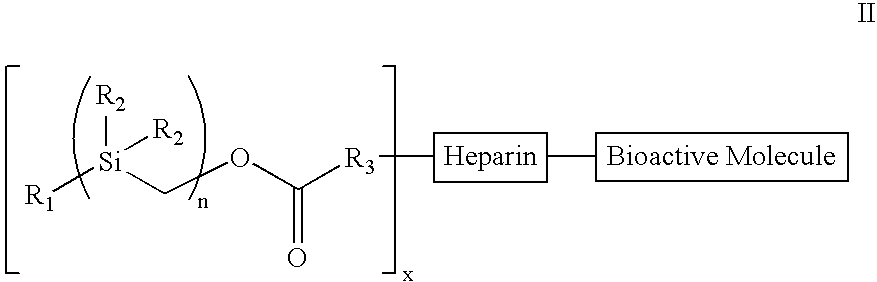
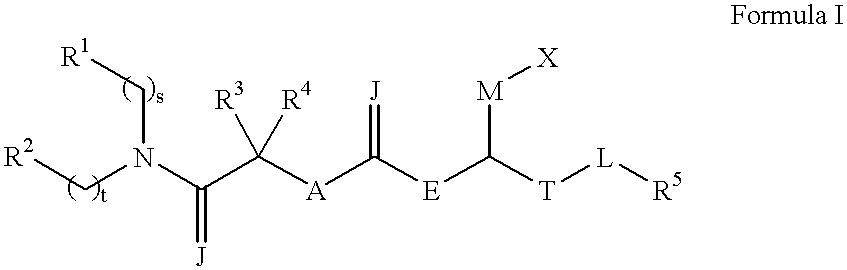
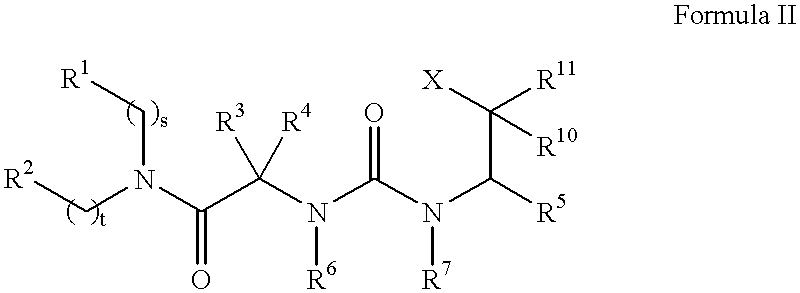
The present invention provides a bioactive coating composition, method and devices for bodily fluid-contacting surfaces. The coating comprises a complex of Formula II: wherein R1 is an C1-18alkyl or C6-32aryl group, each R2 is independently selected from the group consisting of C1-18alkyl and C6-32aryl, R3 is N or O, n is a number from 1 to 10, and x in a number from 1 to about 30, directly bound to a heparin-activity molecule via covalent bonding, with one or more bioactive molecules bound to the heparin-activity molecule. The bioactive molecule may be an adhesive molecule such as fibronectin, a growth factor such as basic fibroblast growth factor, or any other bioactive molecule that binds, by any mechanism, to a heparin-activity molecule
Owner:BIOSURFACE ENG TECH
Compositions and methods for intraocular delivery of fibronectin scaffold domain proteins
InactiveUS20080220049A1Easy to optimizeImprove bioavailabilitySenses disorderPeptide/protein ingredientsMedicineFibronectin
The present disclosure relates to novel sustained-release intraocular drug delivery systems and improvements in the treatment of retinopathies. In particular, fibronectin scaffold domain proteins that selectively inhibit VEGFR-2 are contemplated.
Owner:BRISTOL MYERS SQUIBB CO
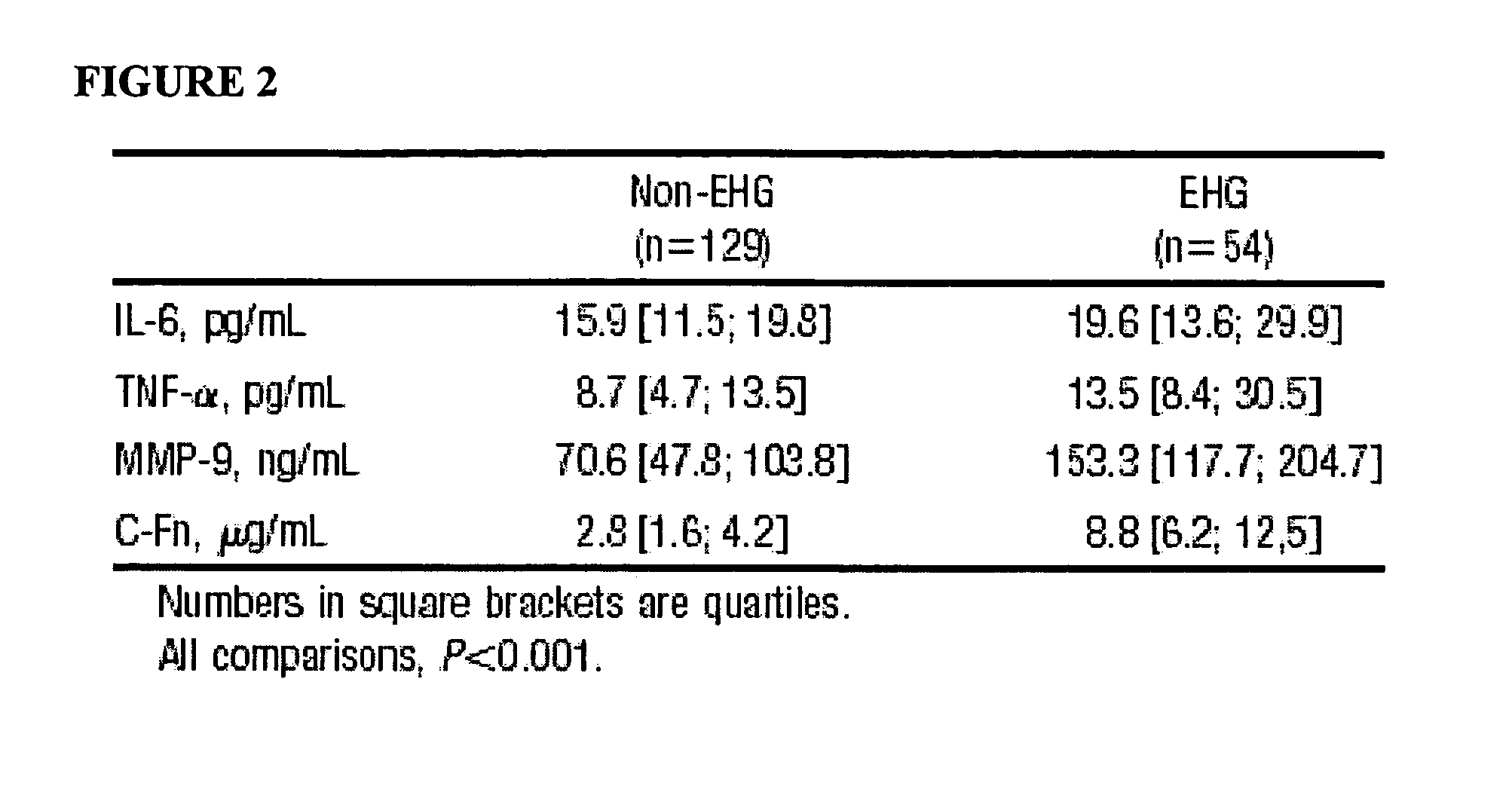
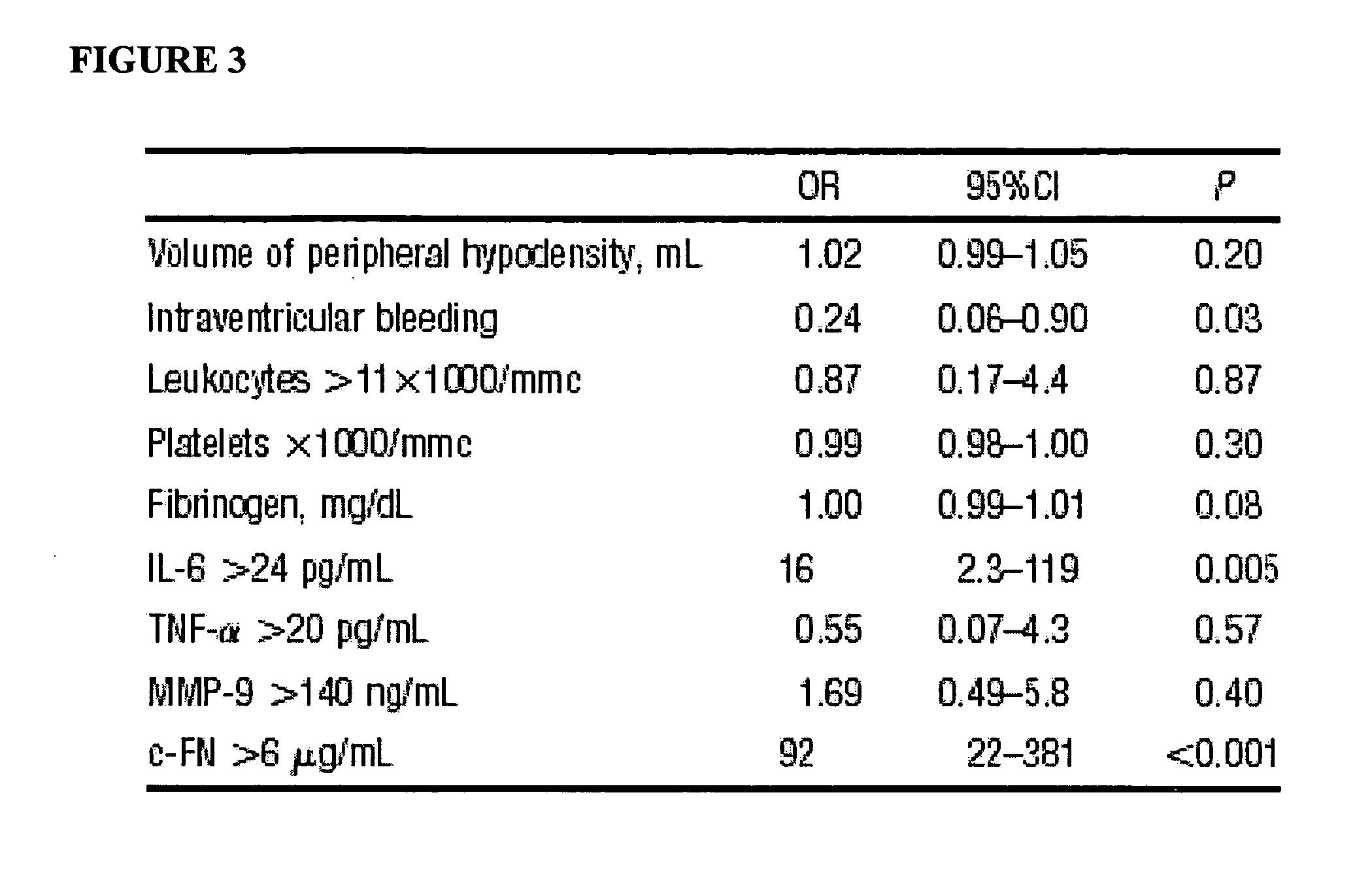
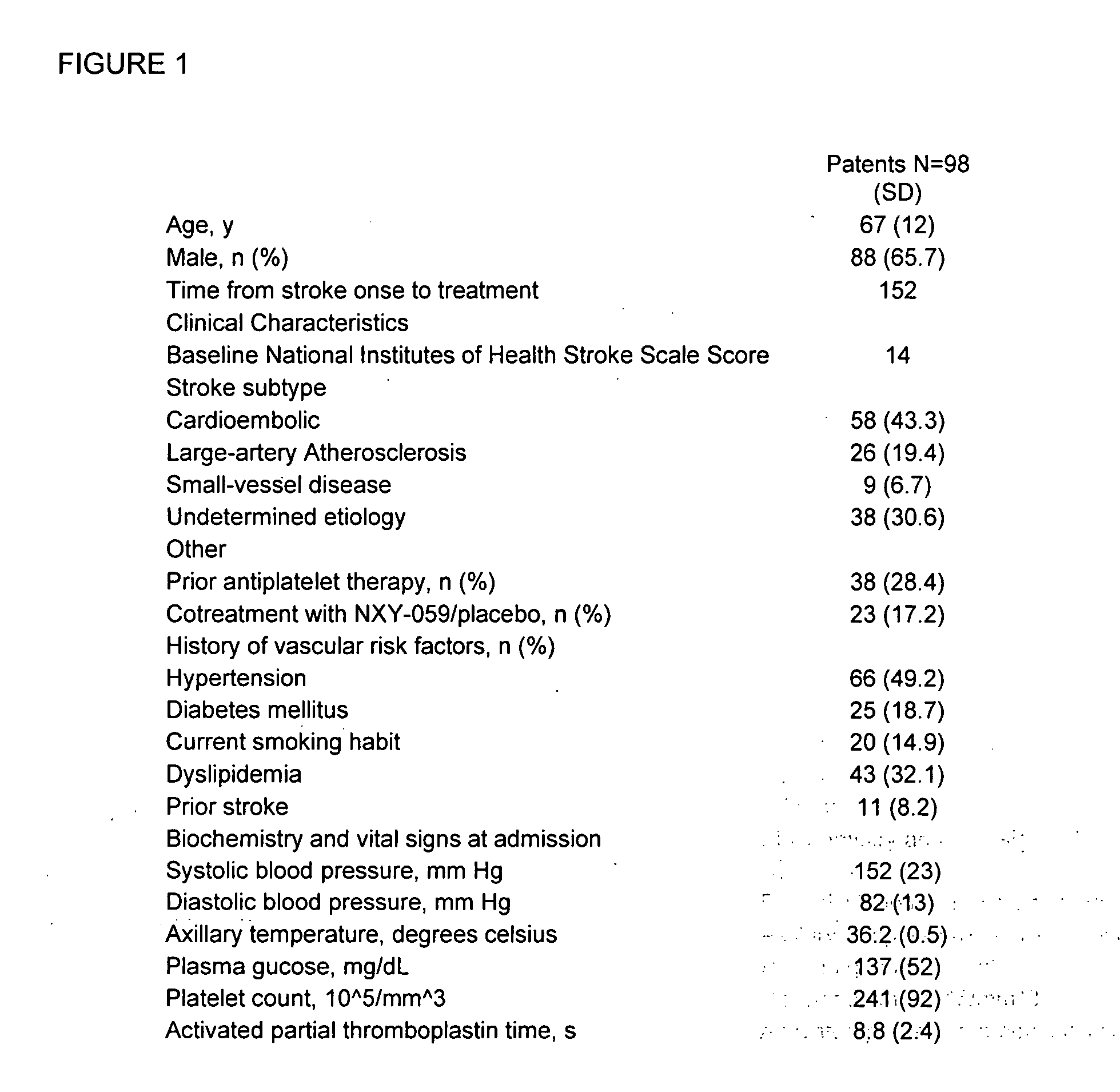
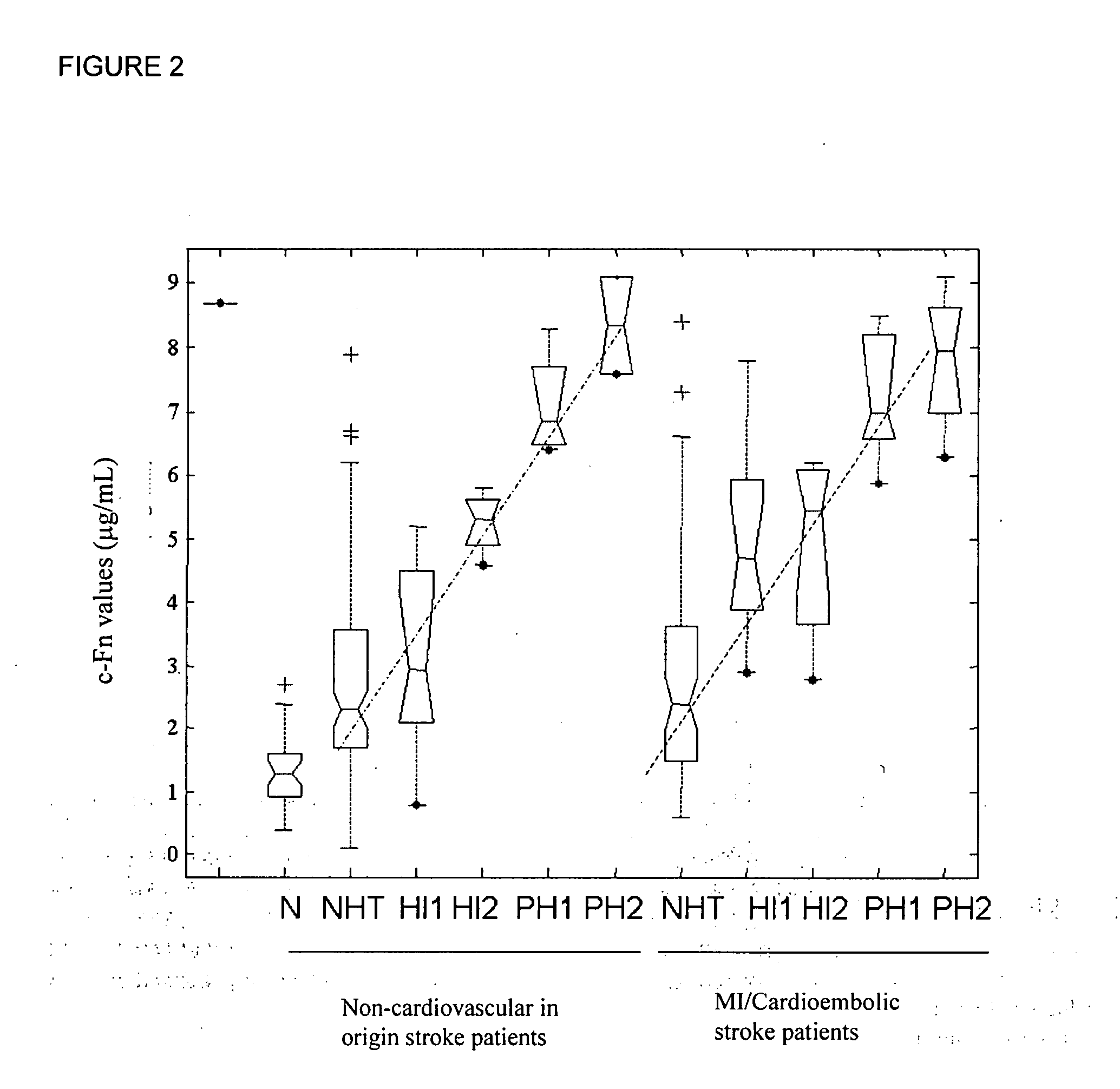
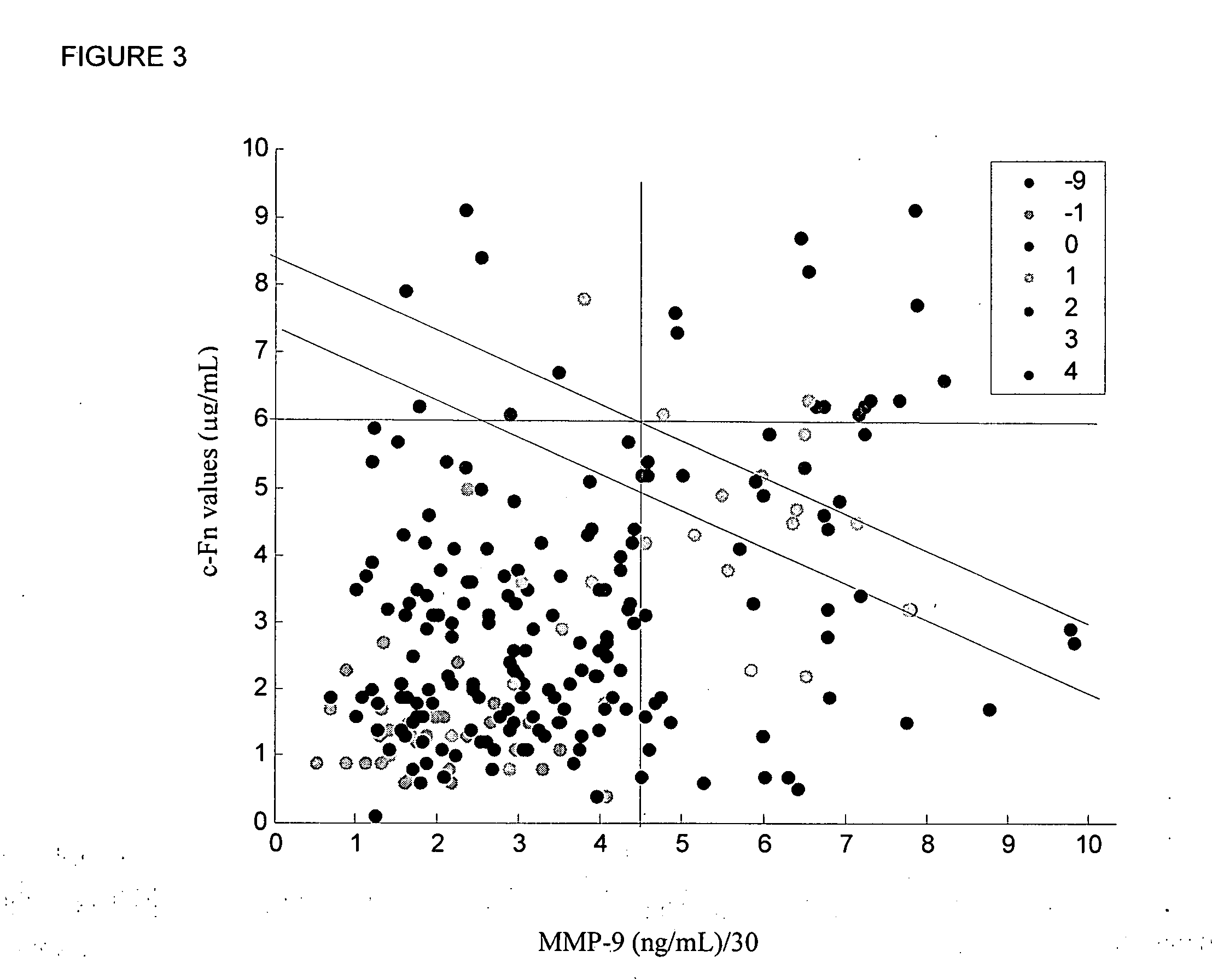
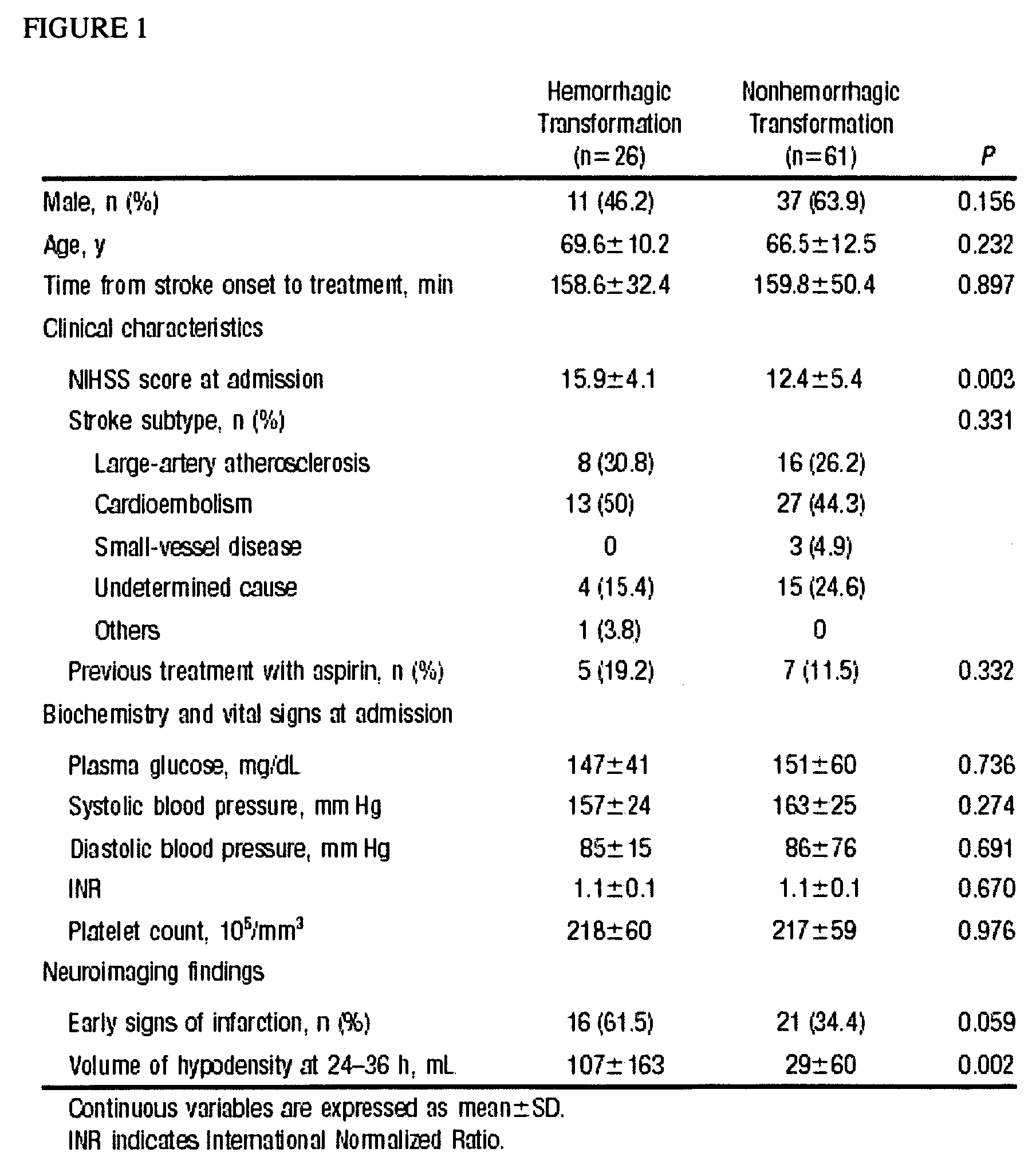
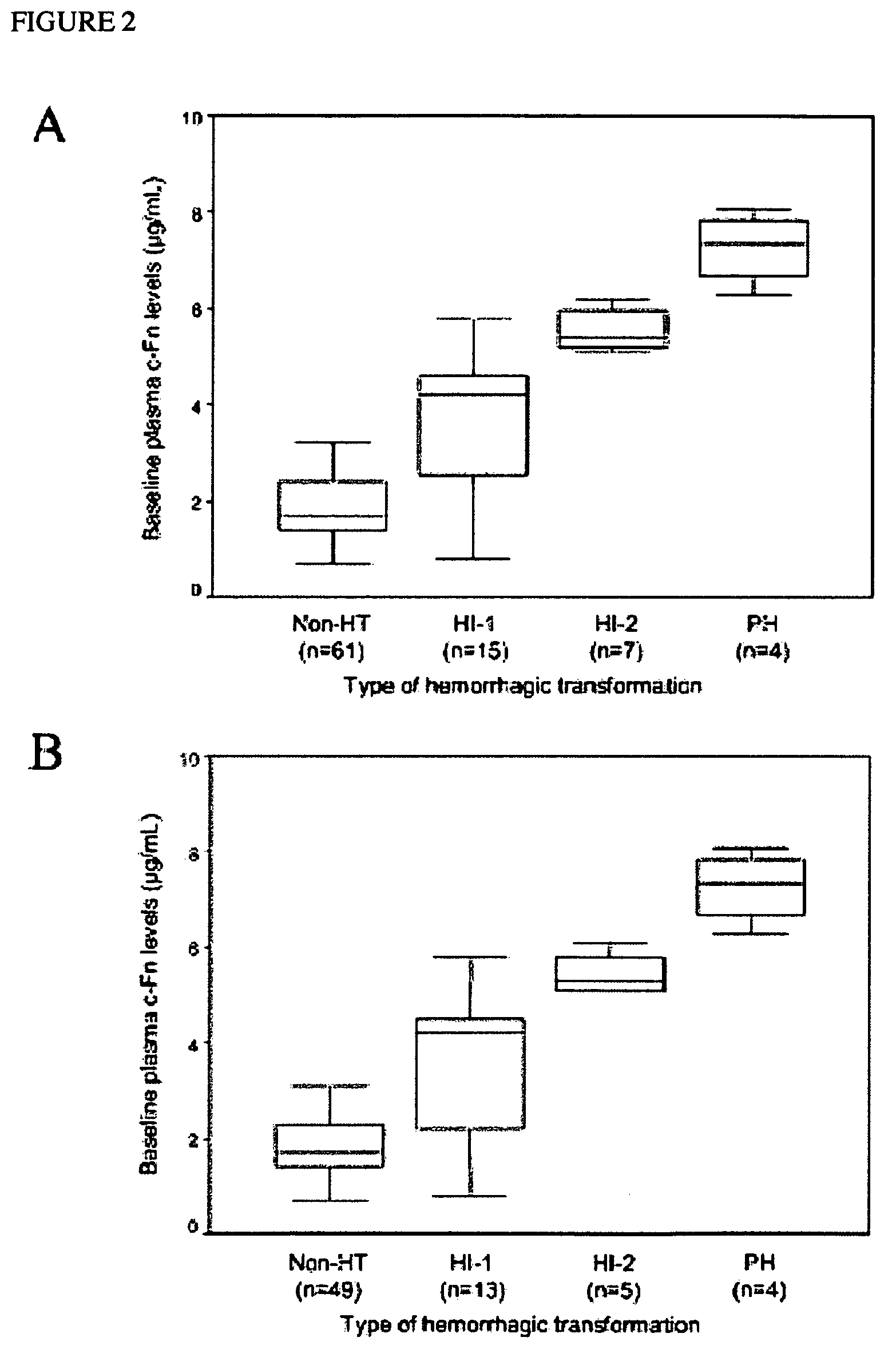
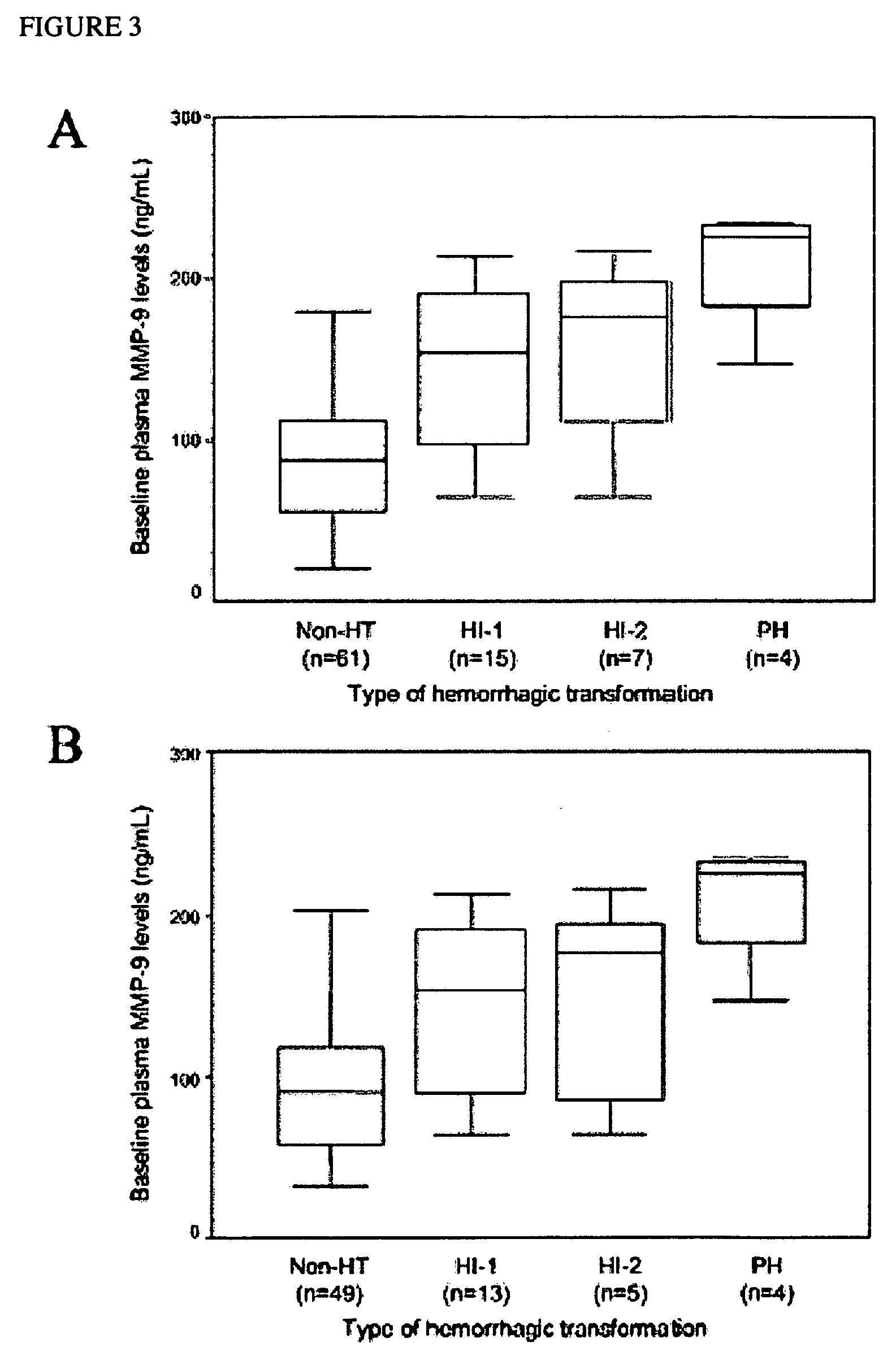
Cellular fibronectin as a diagnostic marker in stroke and methods of use thereof
InactiveUS20050130230A1Rapidly and accurately determinedMaximal sensitivityBiostatisticsDisease diagnosisStroke treatmentAssay
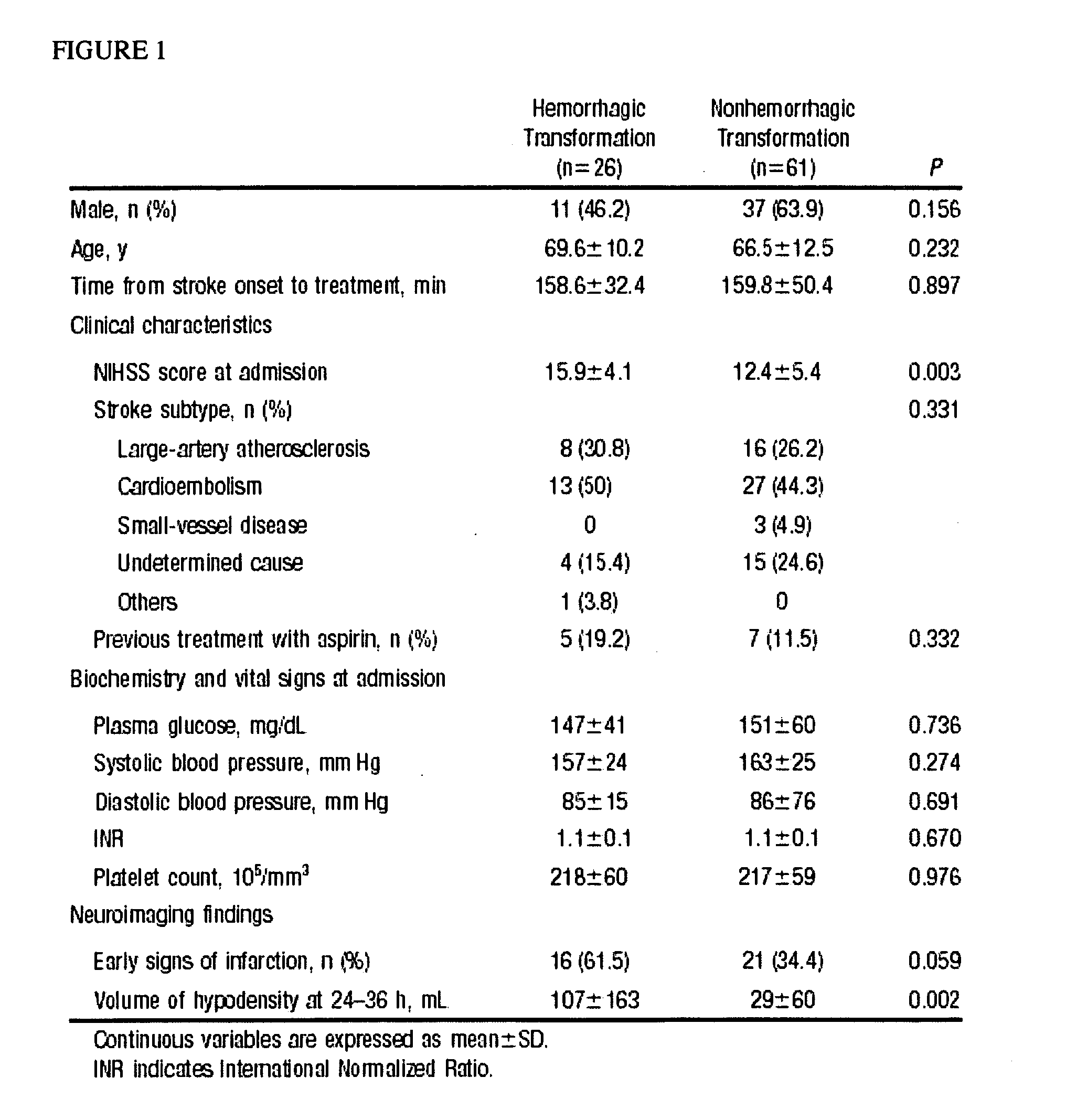
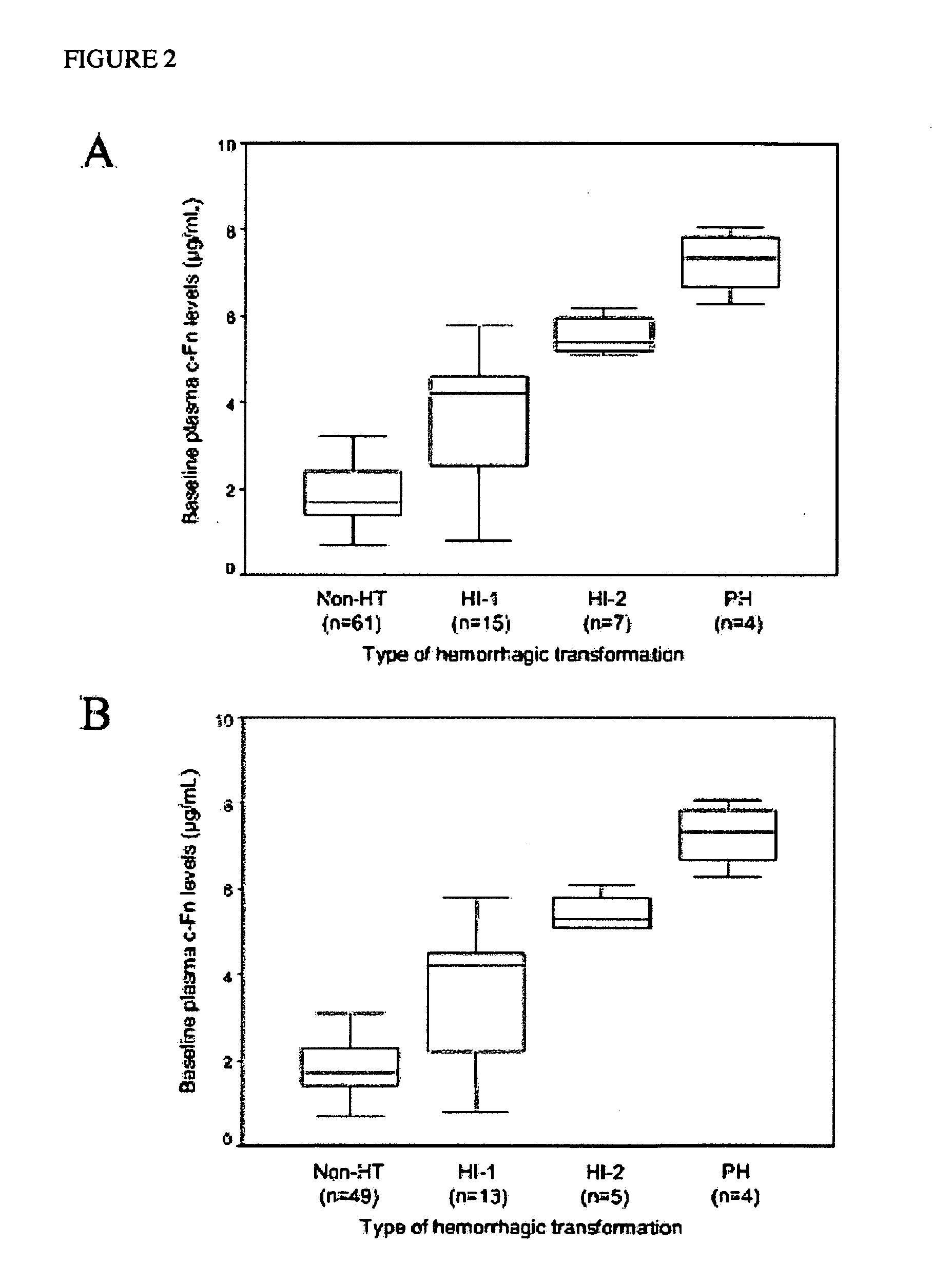
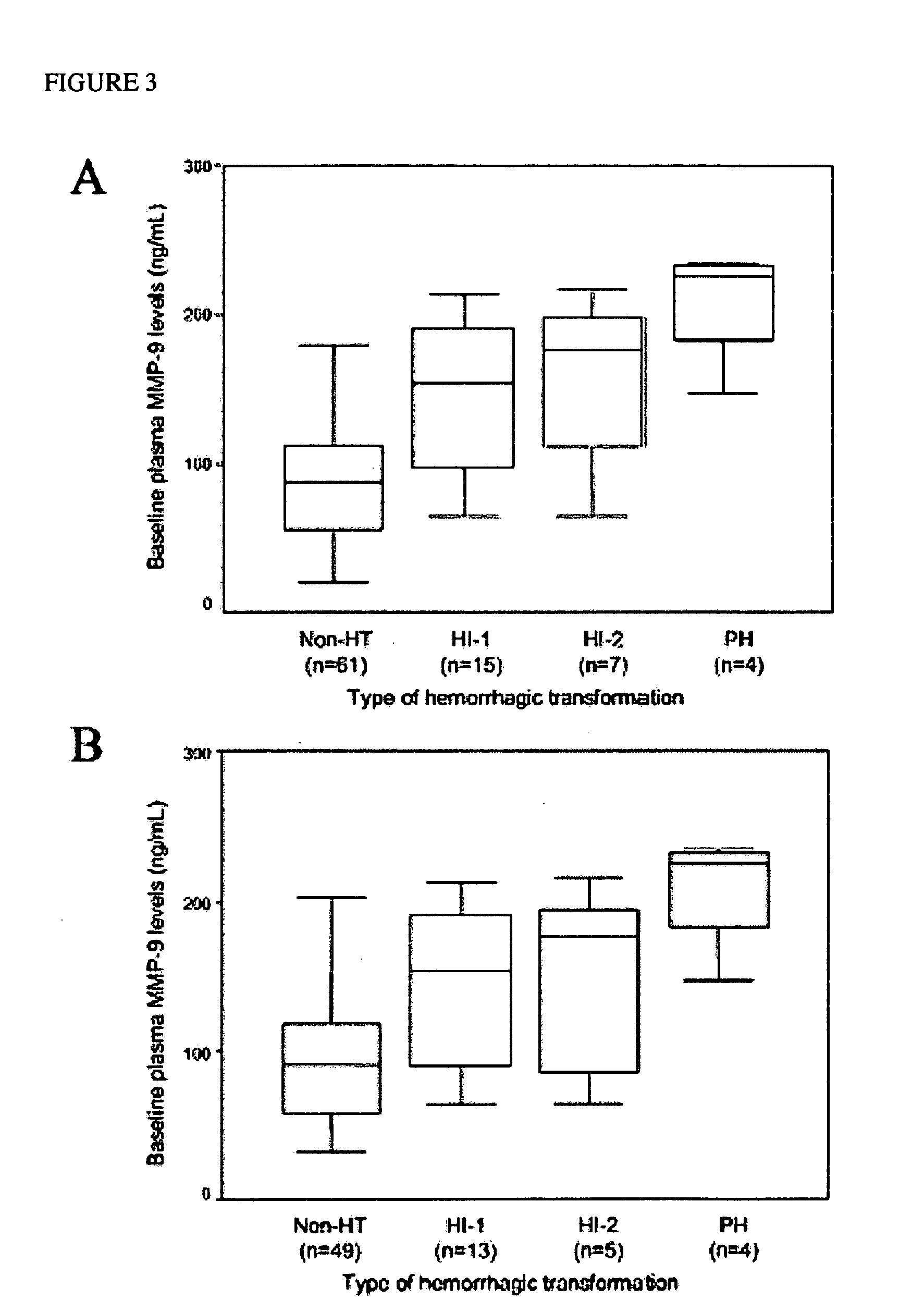
The present invention relates to methods for the diagnosis and evaluation of stroke and stroke sub-type. A variety of bio-markers are disclosed for assembling a panel for such diagnosis and evaluation. Methods are disclosed for selecting markers and correlating their combined levels with a clinical outcome of interest. In various aspects: the invention provides methods for early detection and differentiation of stroke subtypes, for determining the prognosis of a patient presenting with stroke symptoms, and identifying a patient at risk for hemorrhagic transformation after thrombolyic therapy. Methods are disclosed that provide rapid, sensitive and specific assays to greatly increase the number of patients that can receive beneficial stroke treatment and therapy, and reduce the costs associated with incorrect stroke diagnosis.
Owner:PREDICTION SCI
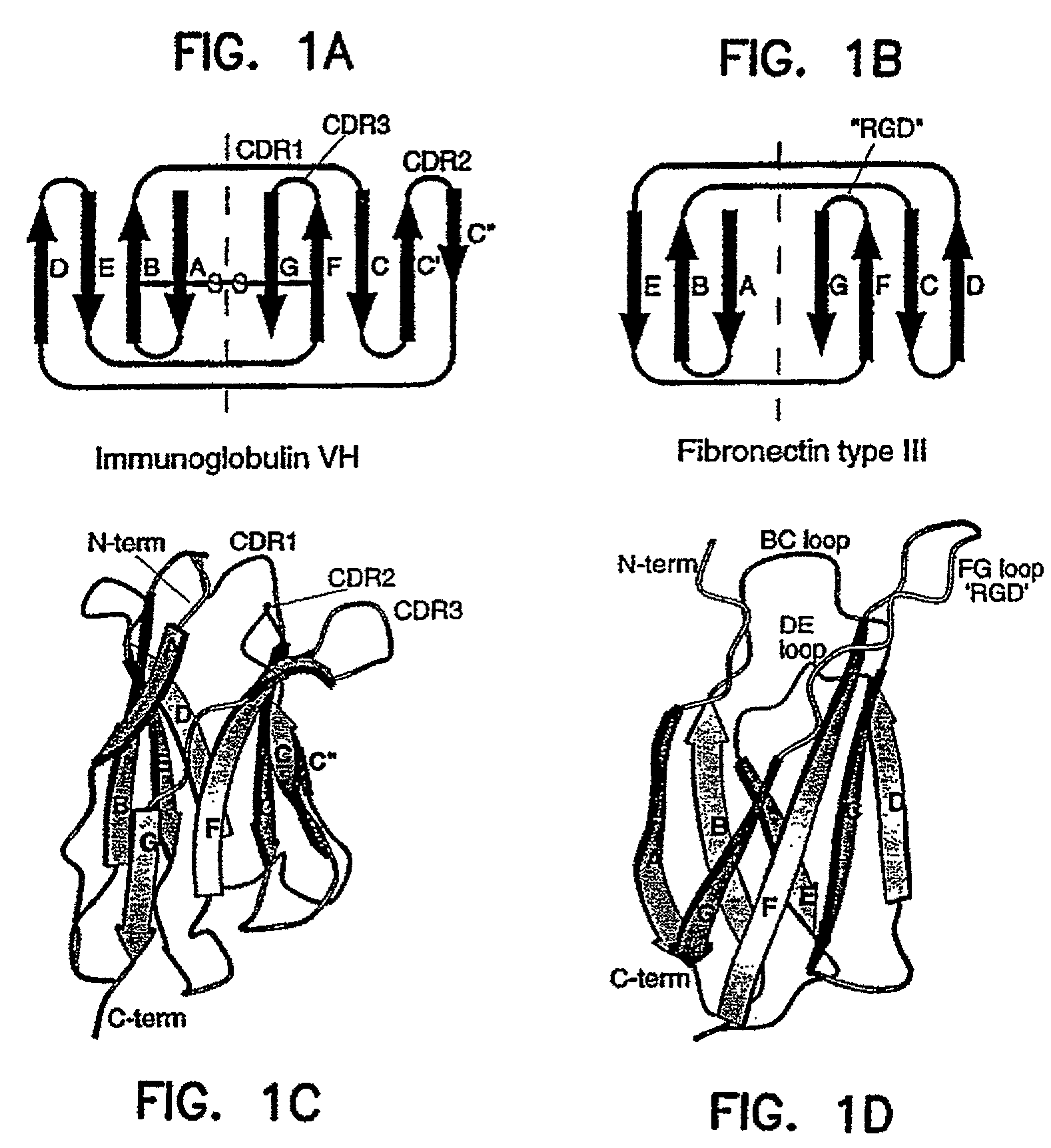
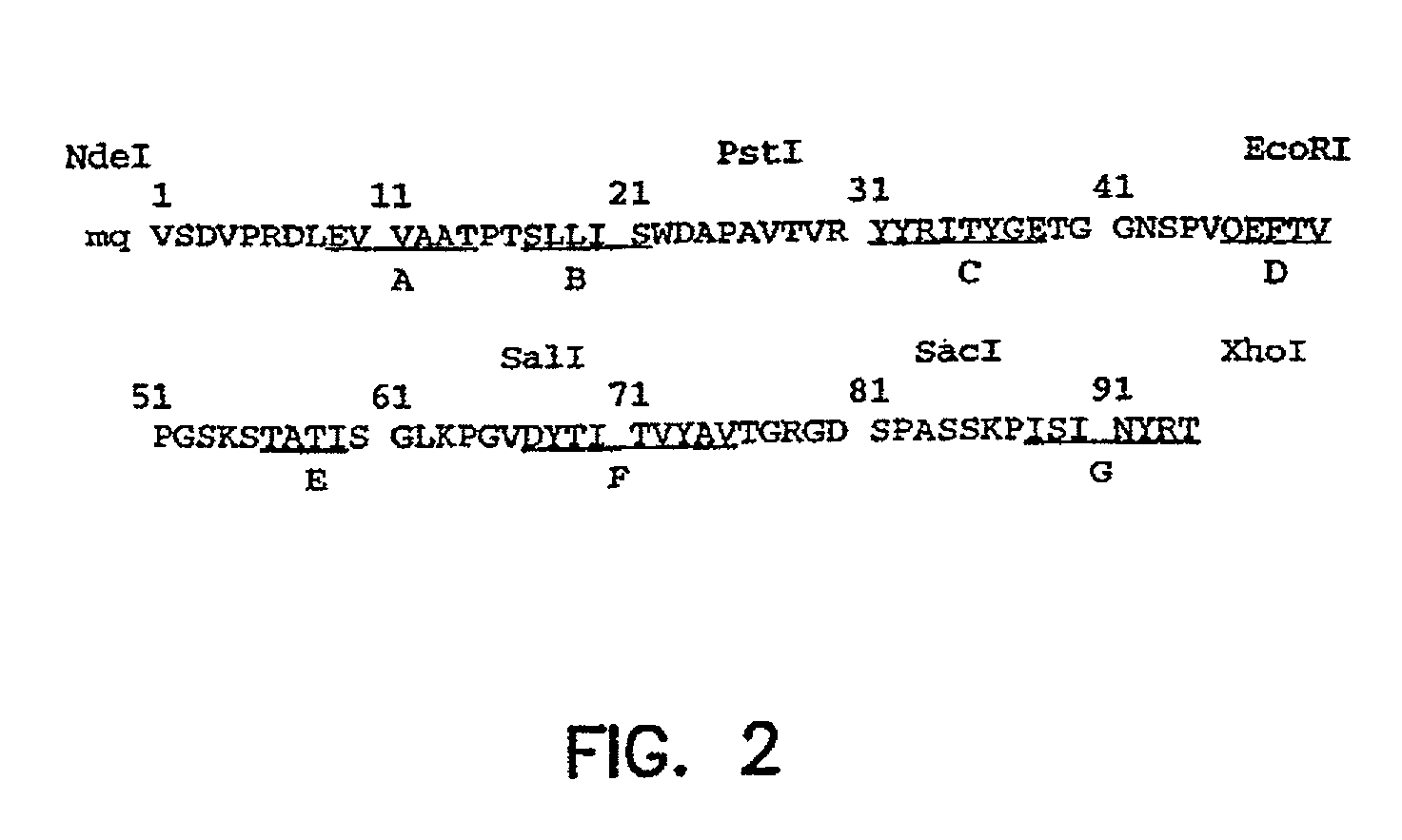
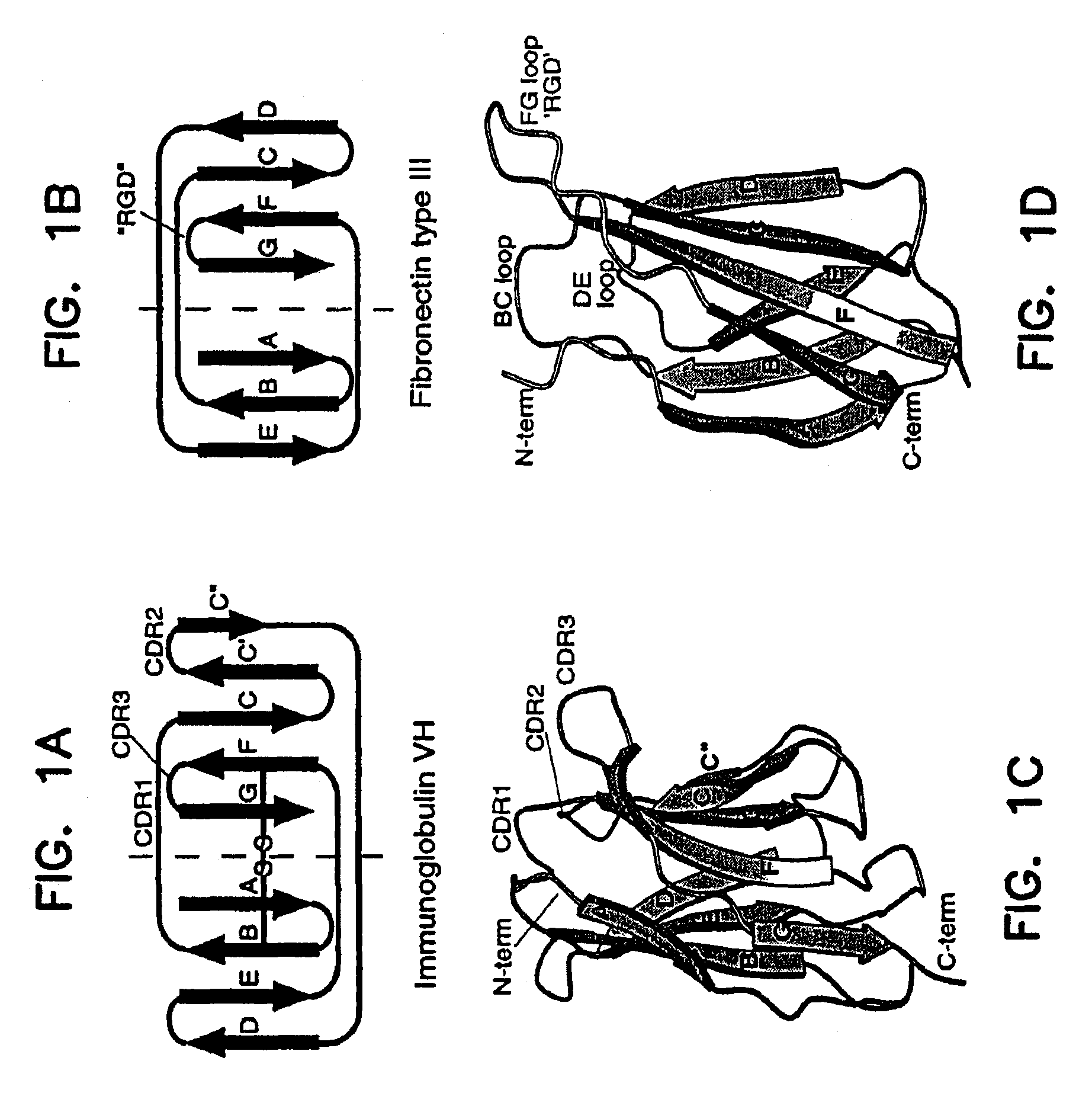
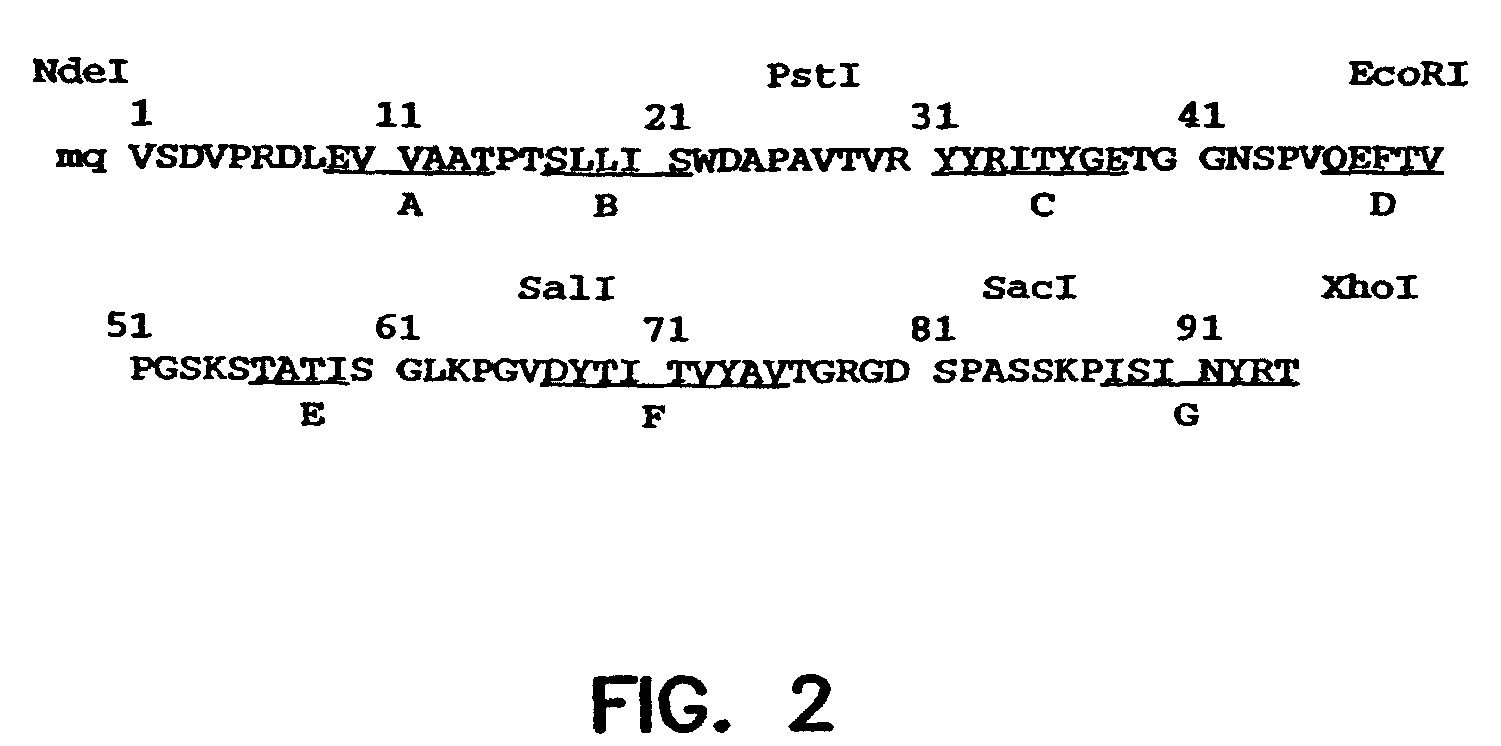
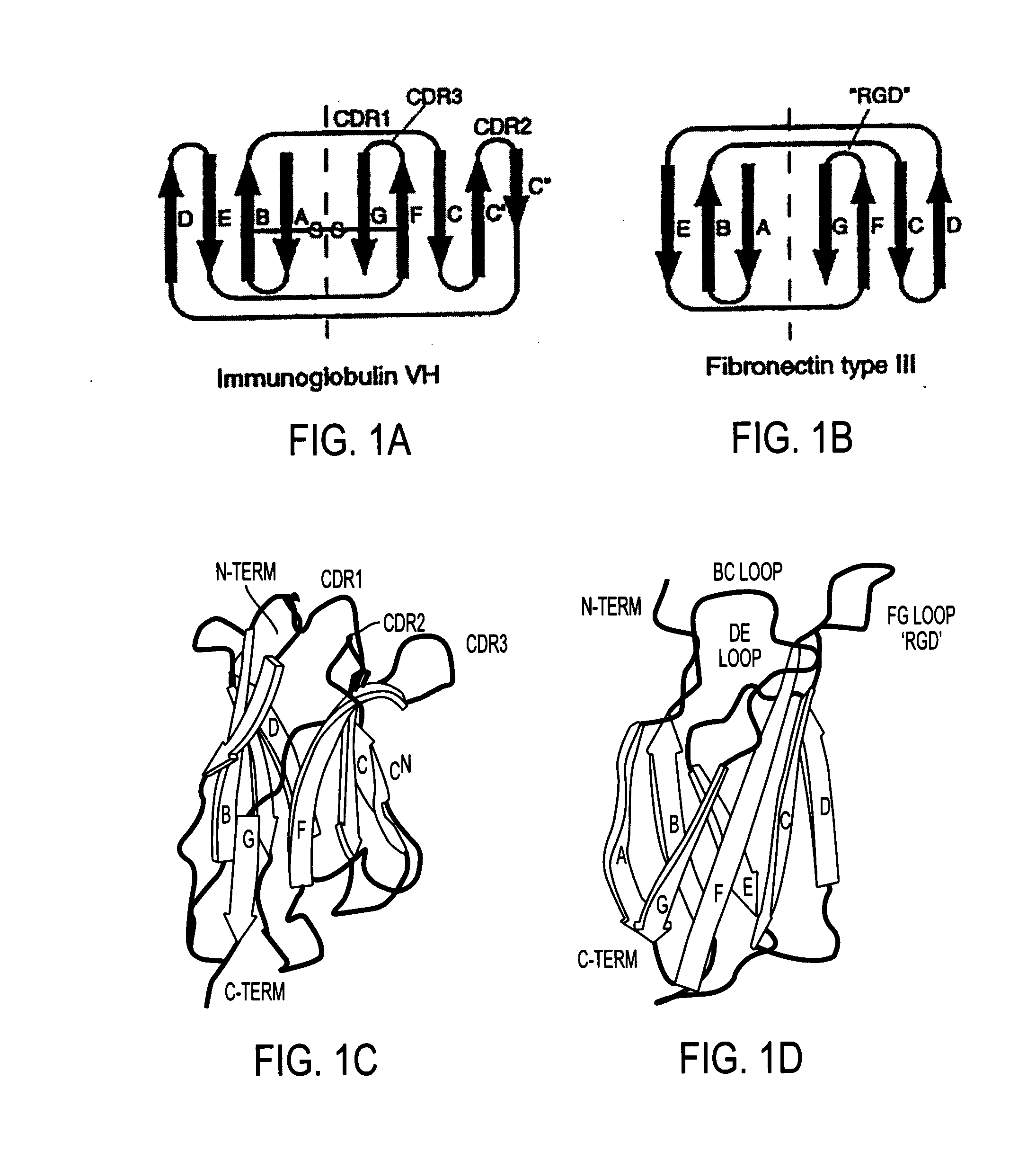
Universal fibronectin type III binding-domain libraries
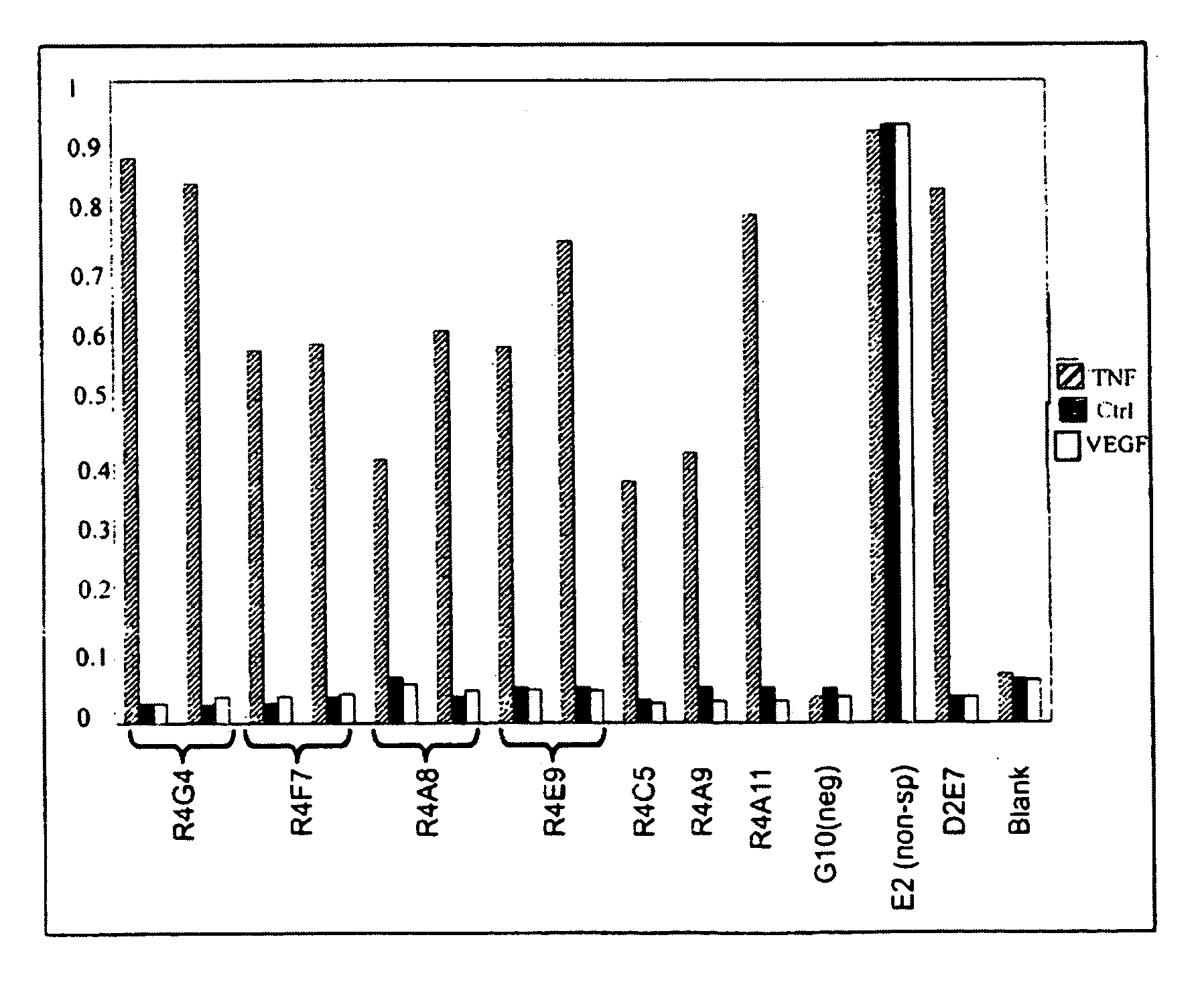
Walk-through mutagenesis and natural-variant combinatorial fibronectin Type III (FN3) polypeptide libraries are described, along with their method of construction and use. Also disclosed are a number of high binding affinity polypeptides selected by screening the libraries against a variety of selected antigens.
Owner:PROTELIX
Binding polypeptides
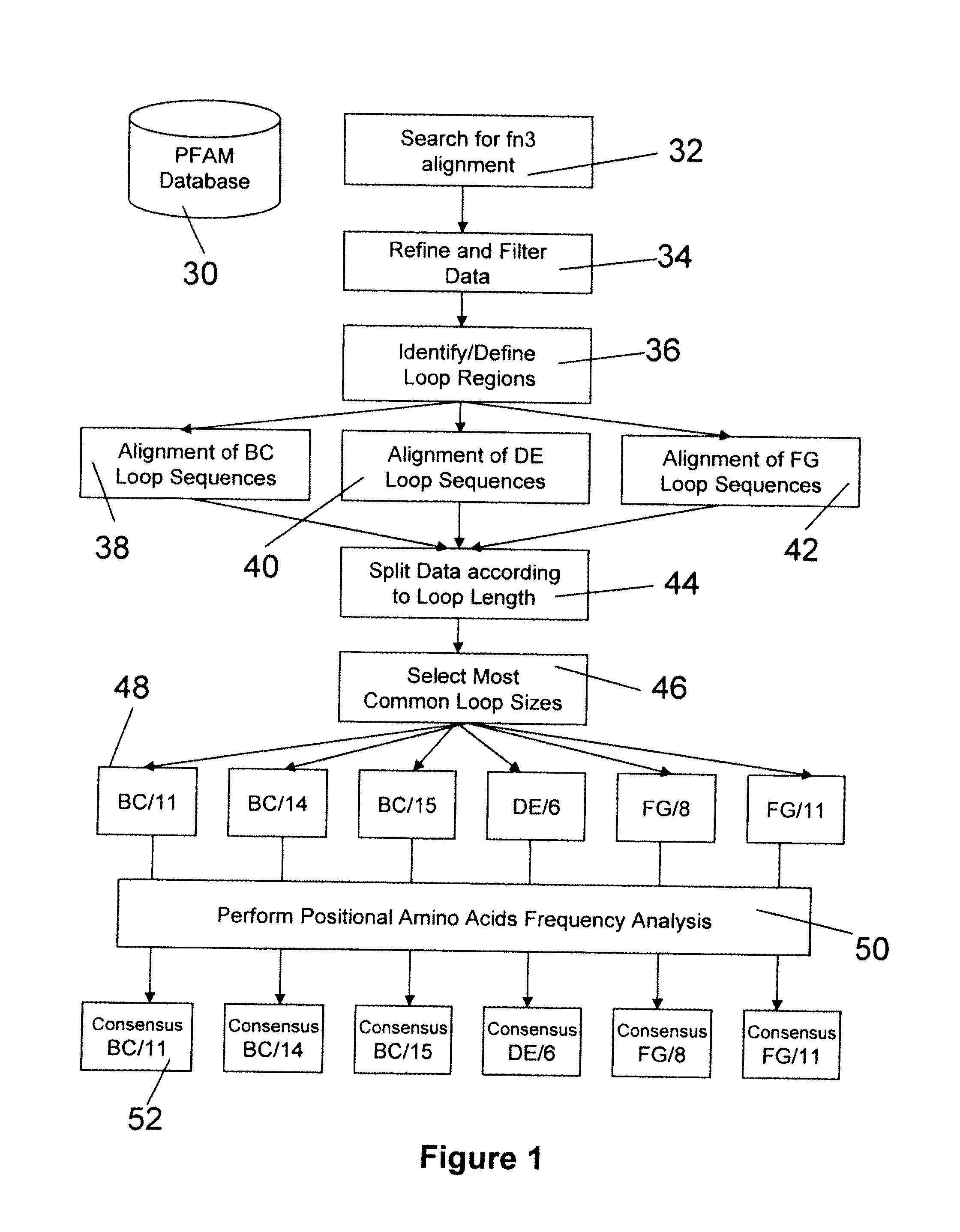
The present invention provides modified fibronectin type III (Fn3) molecules, nucleic acid molecules encoding the modified Fn3 molecules, and related vectors and host cells.
Owner:NOVARTIS AG
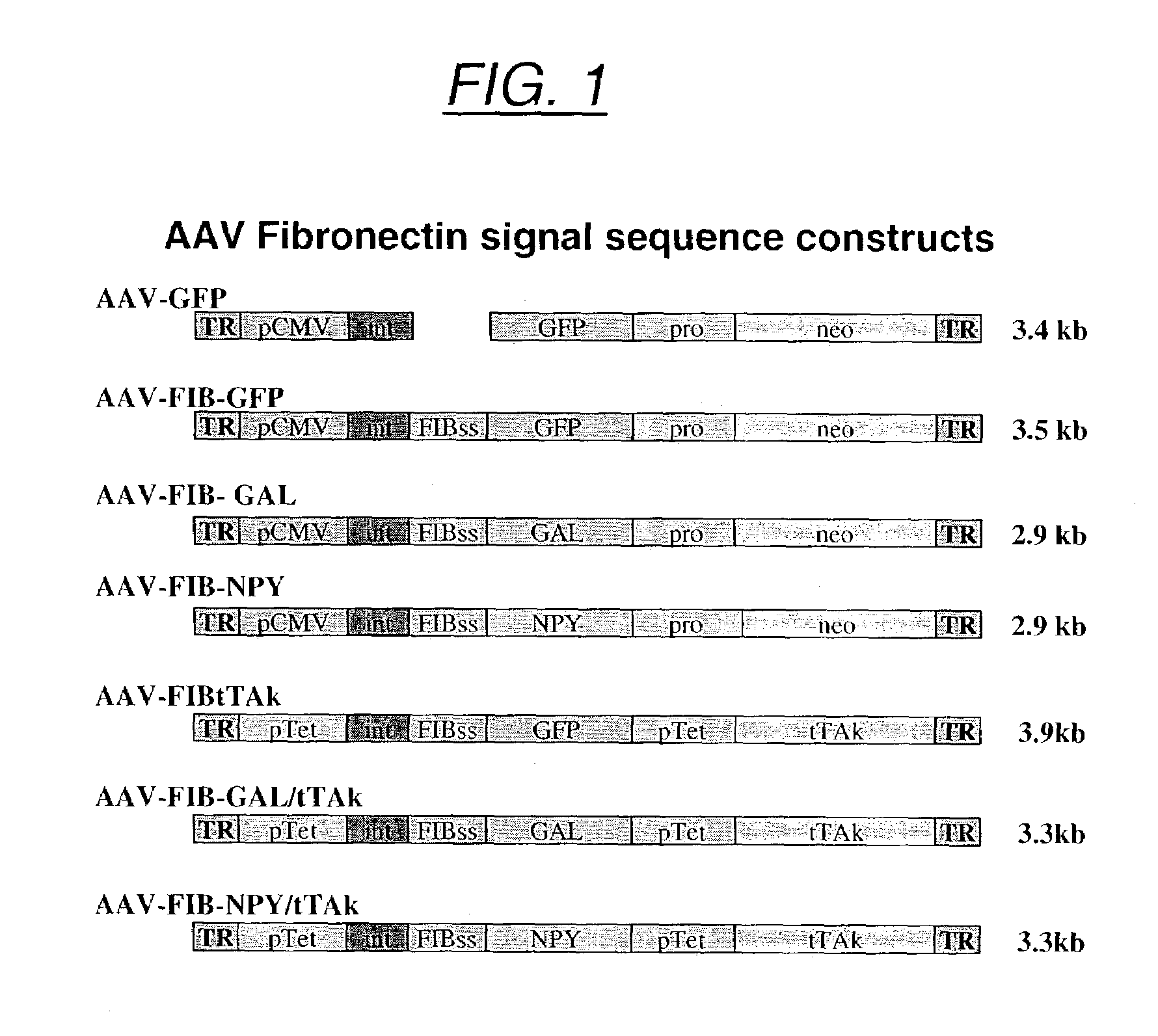
Secretion signal vectors
InactiveUS7071172B2Minimize potential deleterious side effectAttenuate seizure activityBiocideAnimal repellantsHeterologousAdeno associate virus
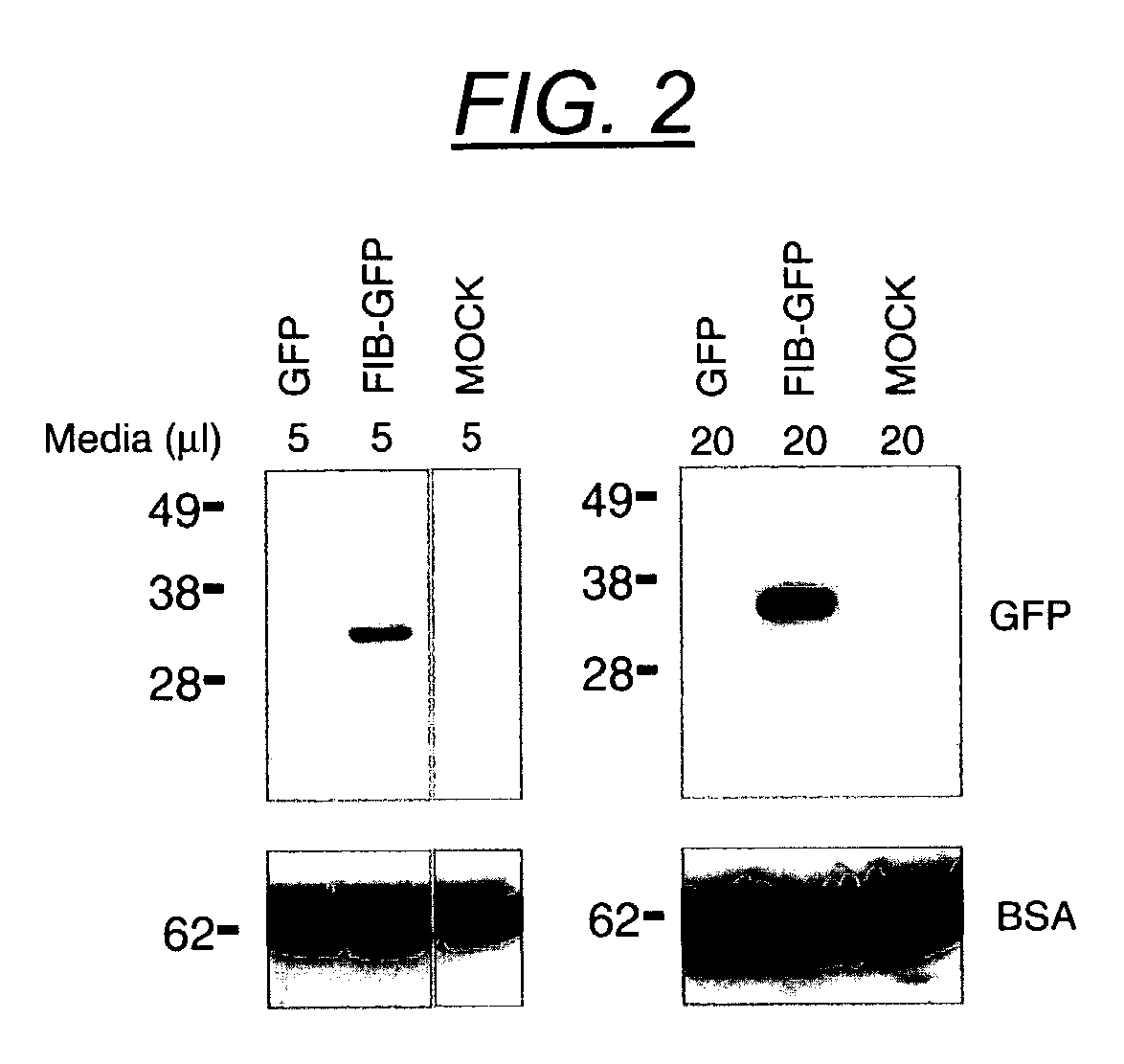
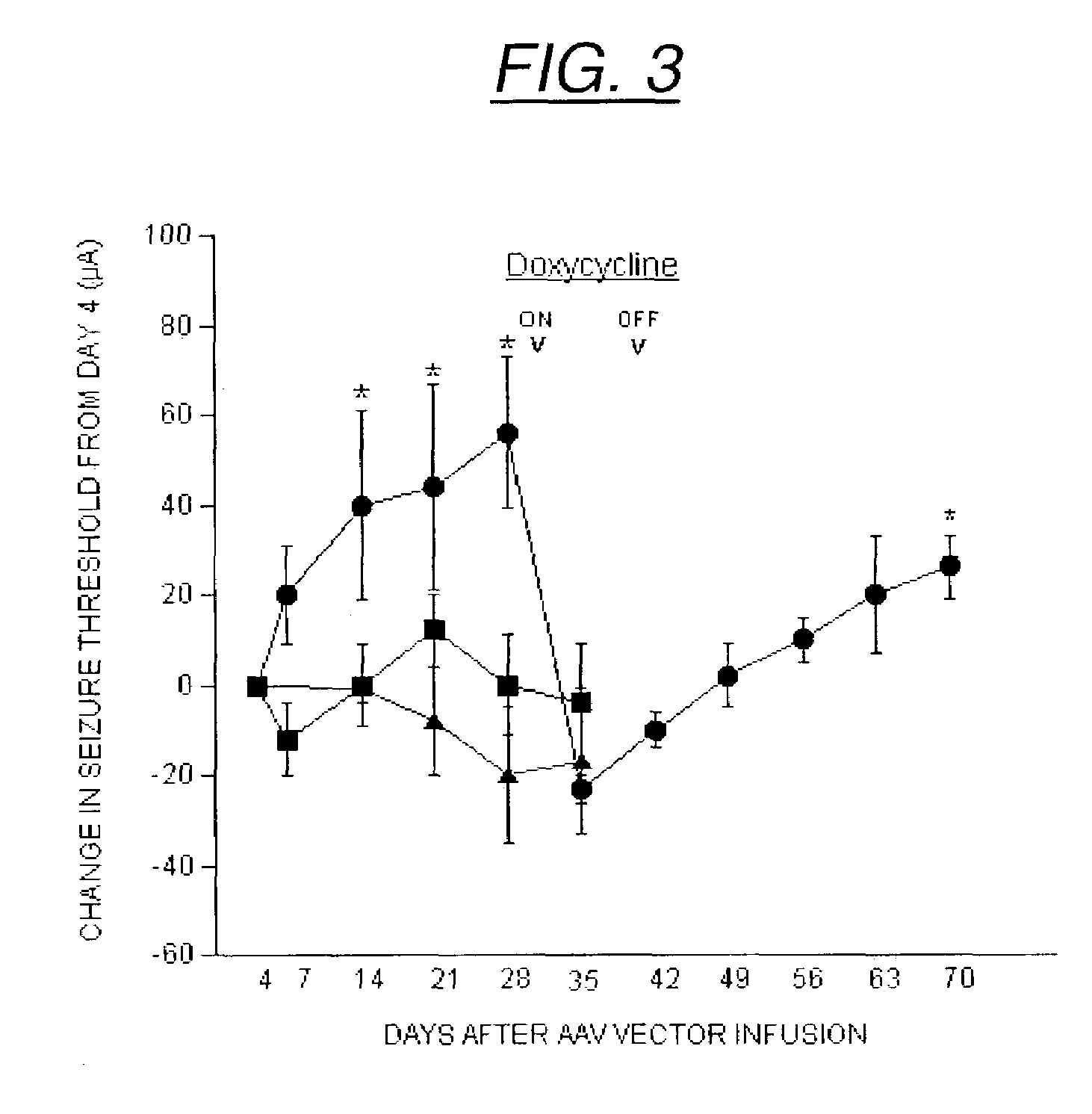
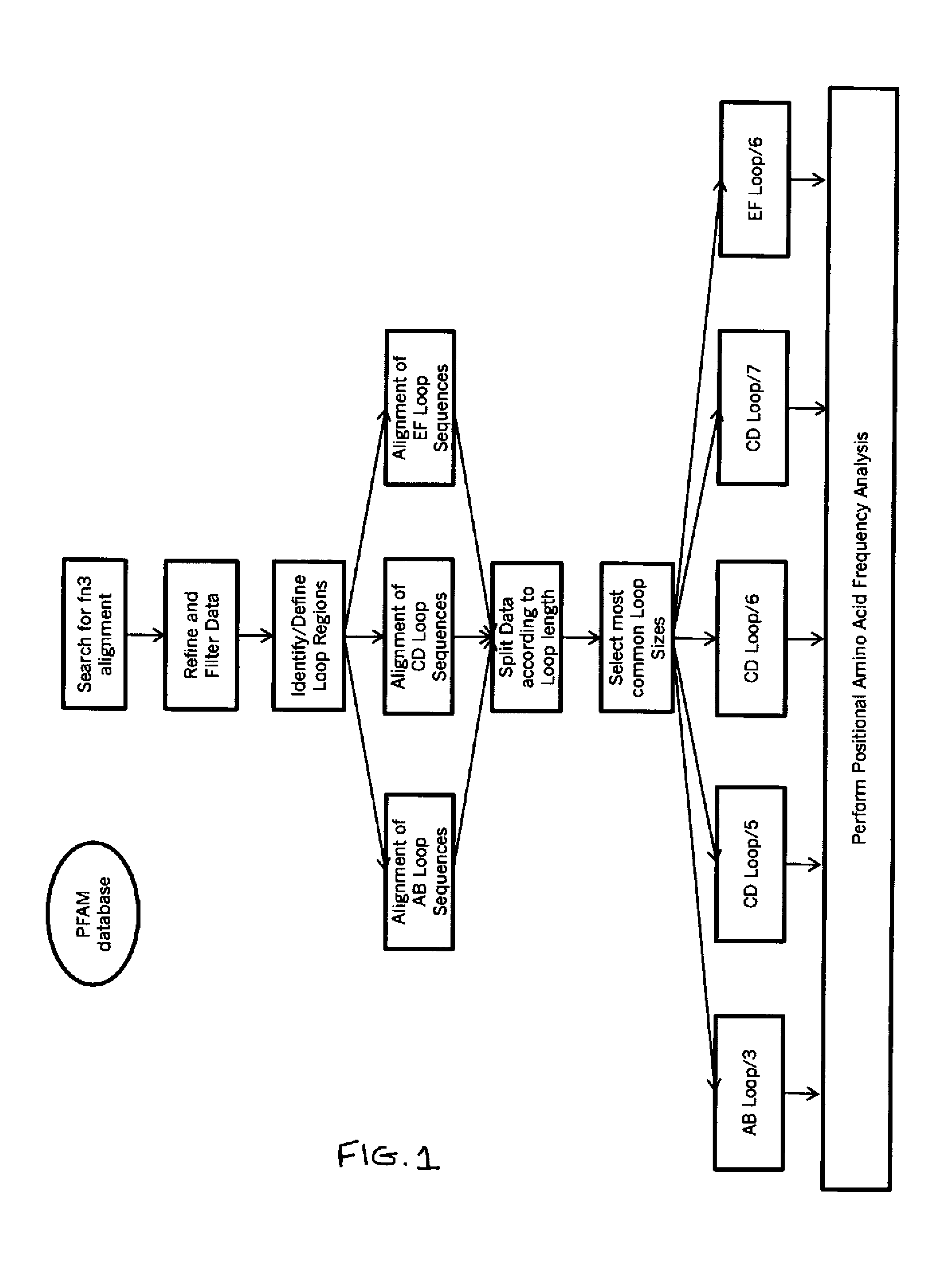
The present invention provides delivery vectors for transferring a nucleic acid sequence to a cell in vitro, ex vivo or in vivo. The delivery vector comprises a segment encoding a secretory signal peptide. In embodiments of the invention, the delivery vector is an adeno-associated virus (AAV) vector. In other embodiments, the secretory signal peptide is a fibronectin secretory signal peptide (including variations and modifications, thereof). The delivery vectors of the invention may further comprise a heterologous nucleic acid sequence encoding a polypeptide of interest for transfer to a target cell, where the polypeptide of interest is operably associated with the secretory signal. Also disclosed are methods of transferring a nucleic acid of interest to a cell using the delivery vectors of the invention.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Universal fibronectin type iii binding-domain libraries
Walk-through mutagenesis and natural-variant combinatorial fibronectin Type III (FN3) polypeptide libraries are described, along with their method of construction and use. Also disclosed are a number of high binding affinity polypeptides selected by screening the libraries against a variety of selected antigens.
Owner:PROTELIX
Reconstituted polypeptides
ActiveUS8258265B2Connective tissue peptidesImmunoglobulins against animals/humansFibronectinCell biology
The present invention provides modified fibronectin type III (Fn3) molecules, and nucleic acid molecules encoding the modified Fn3 molecules. Also provided are methods of preparing these molecules, and kits to perform the methods.
Owner:RES CORP TECH INC
Universal fibronectin type iii bottom-side binding domain libraries
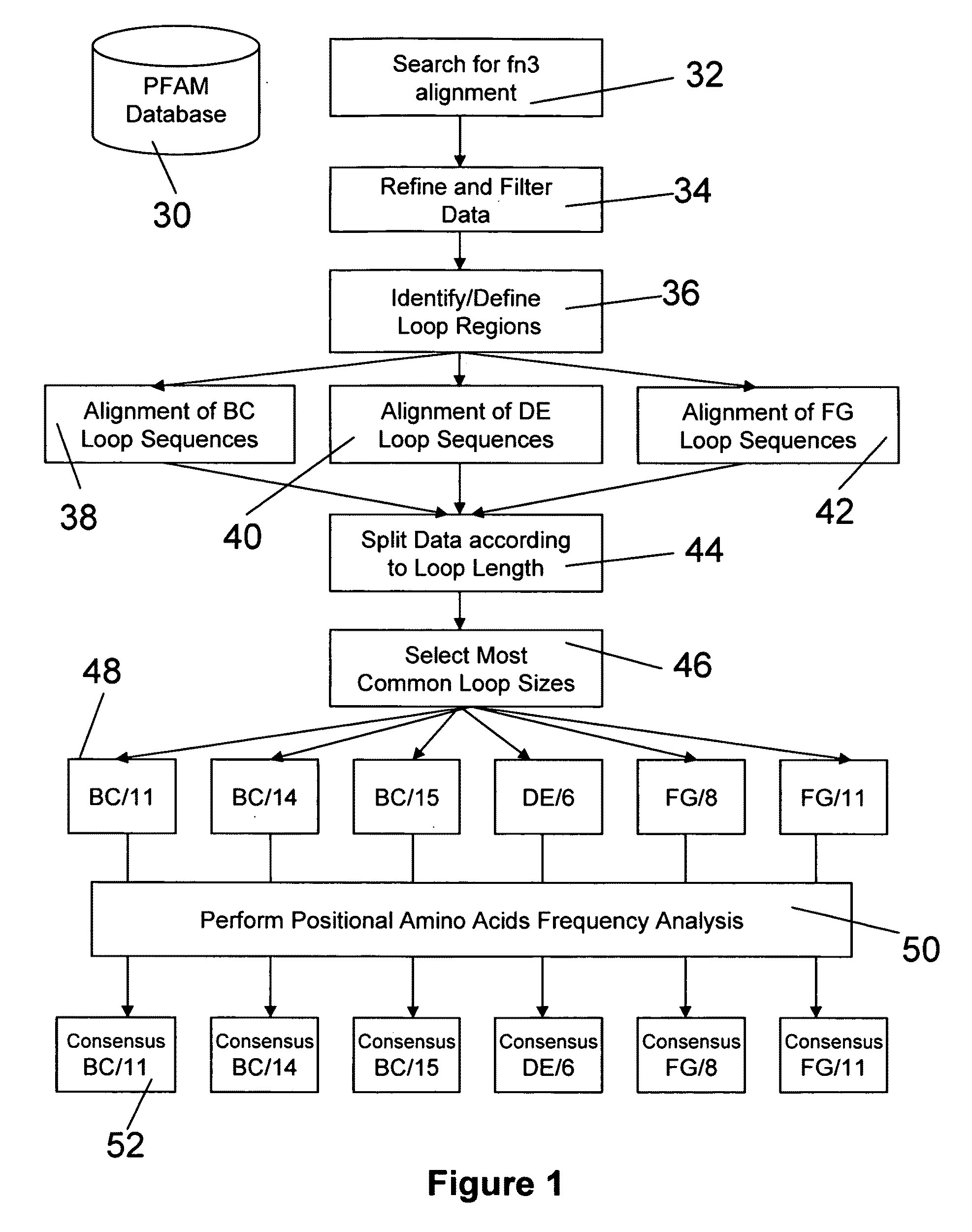
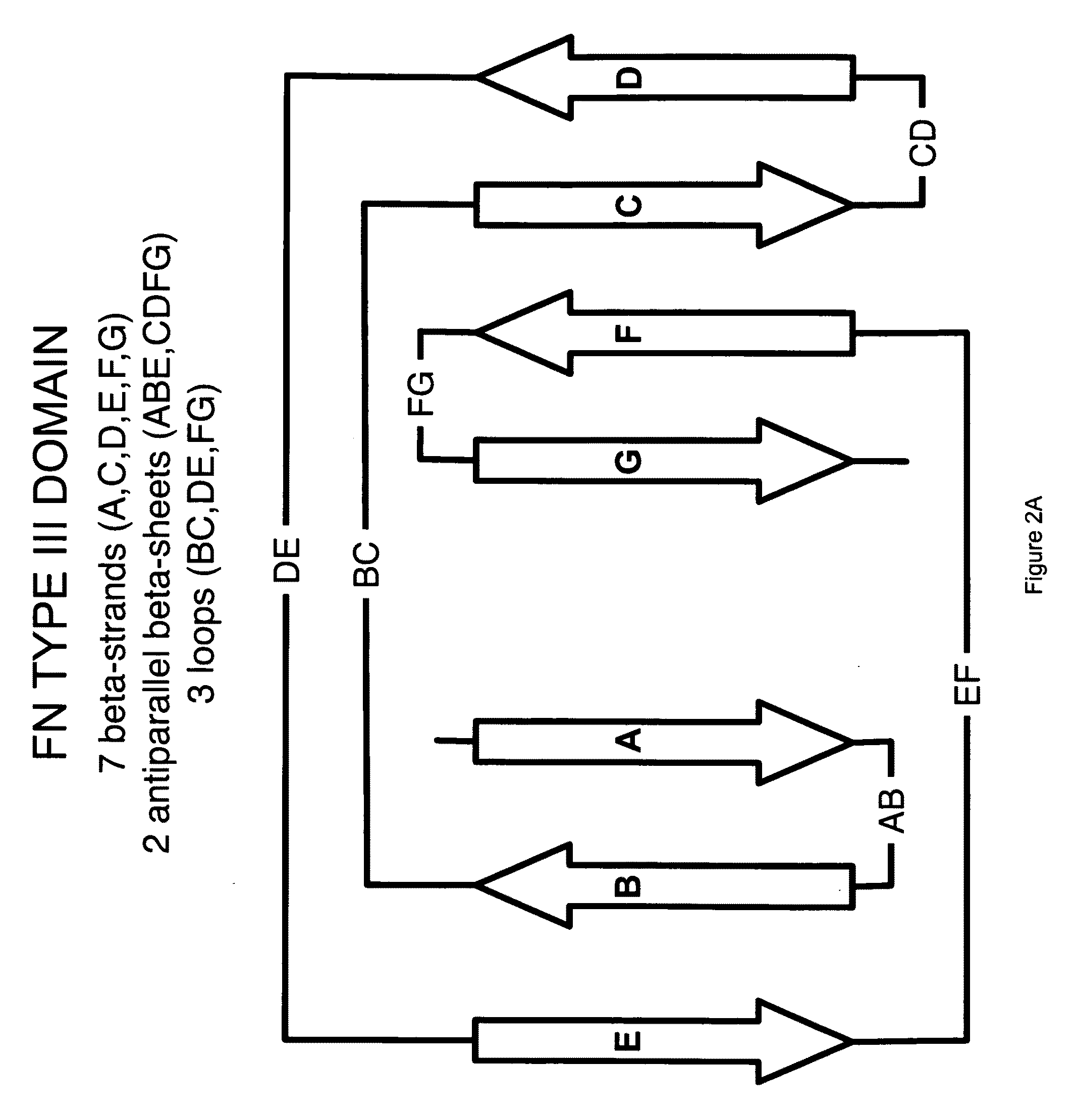
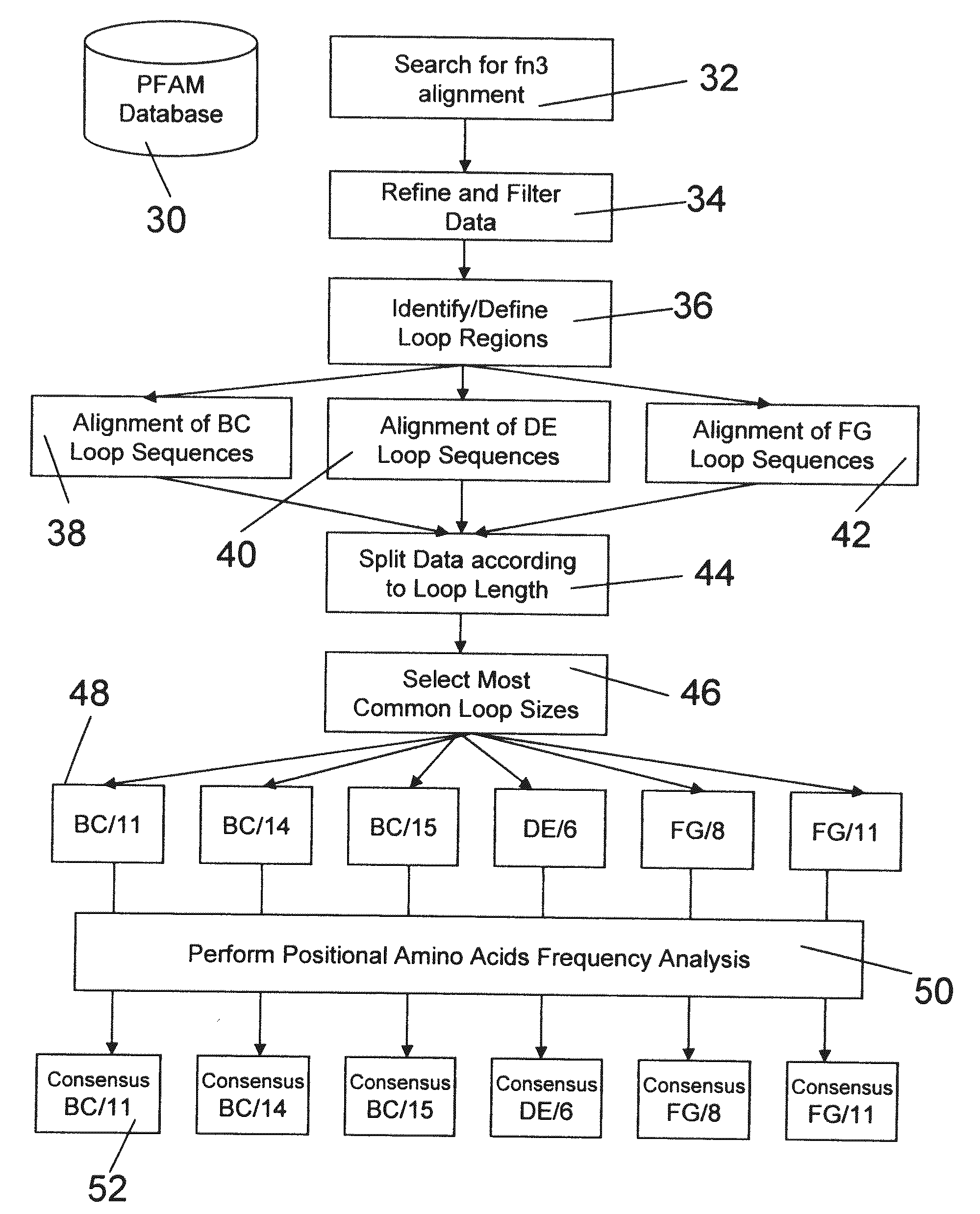
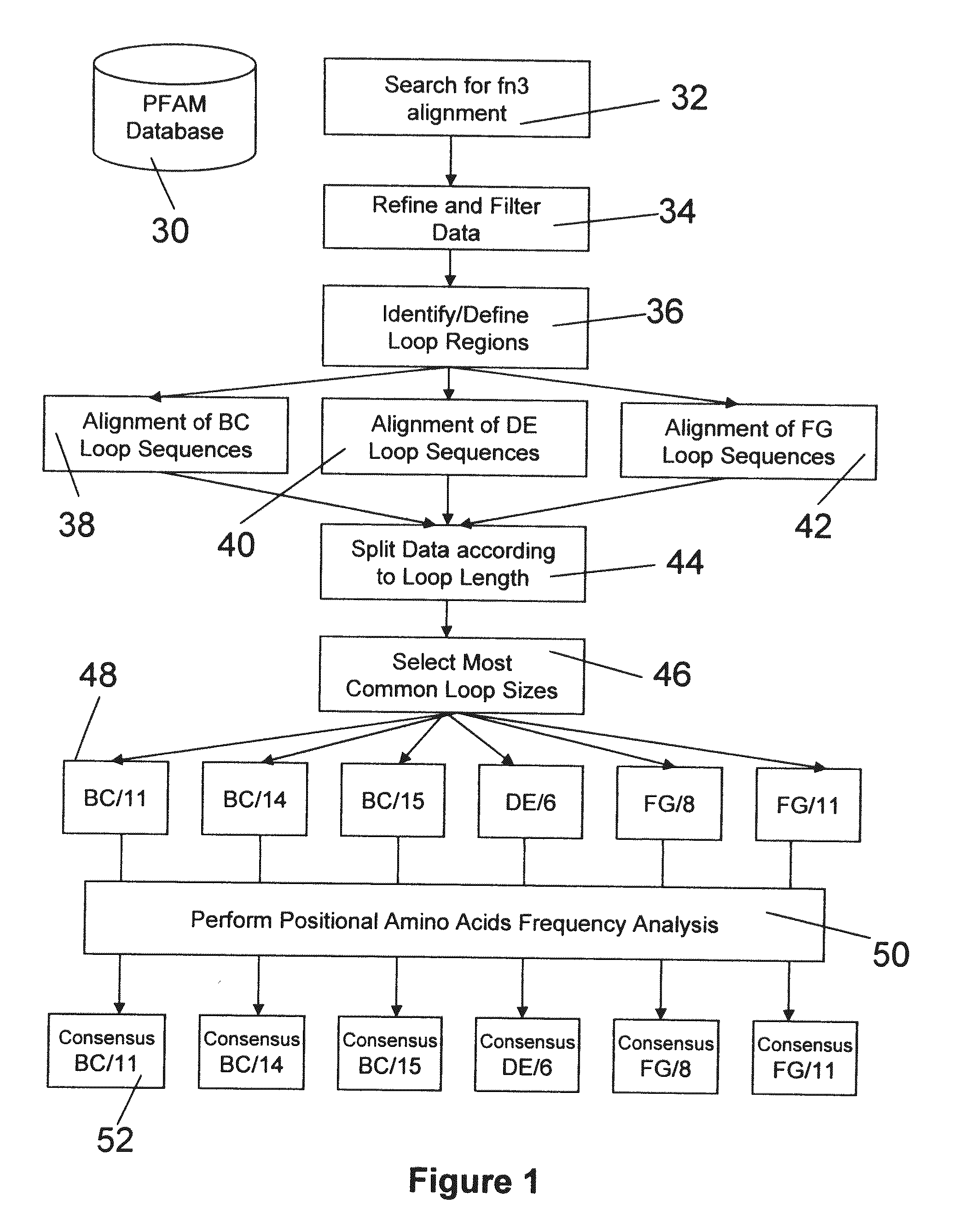
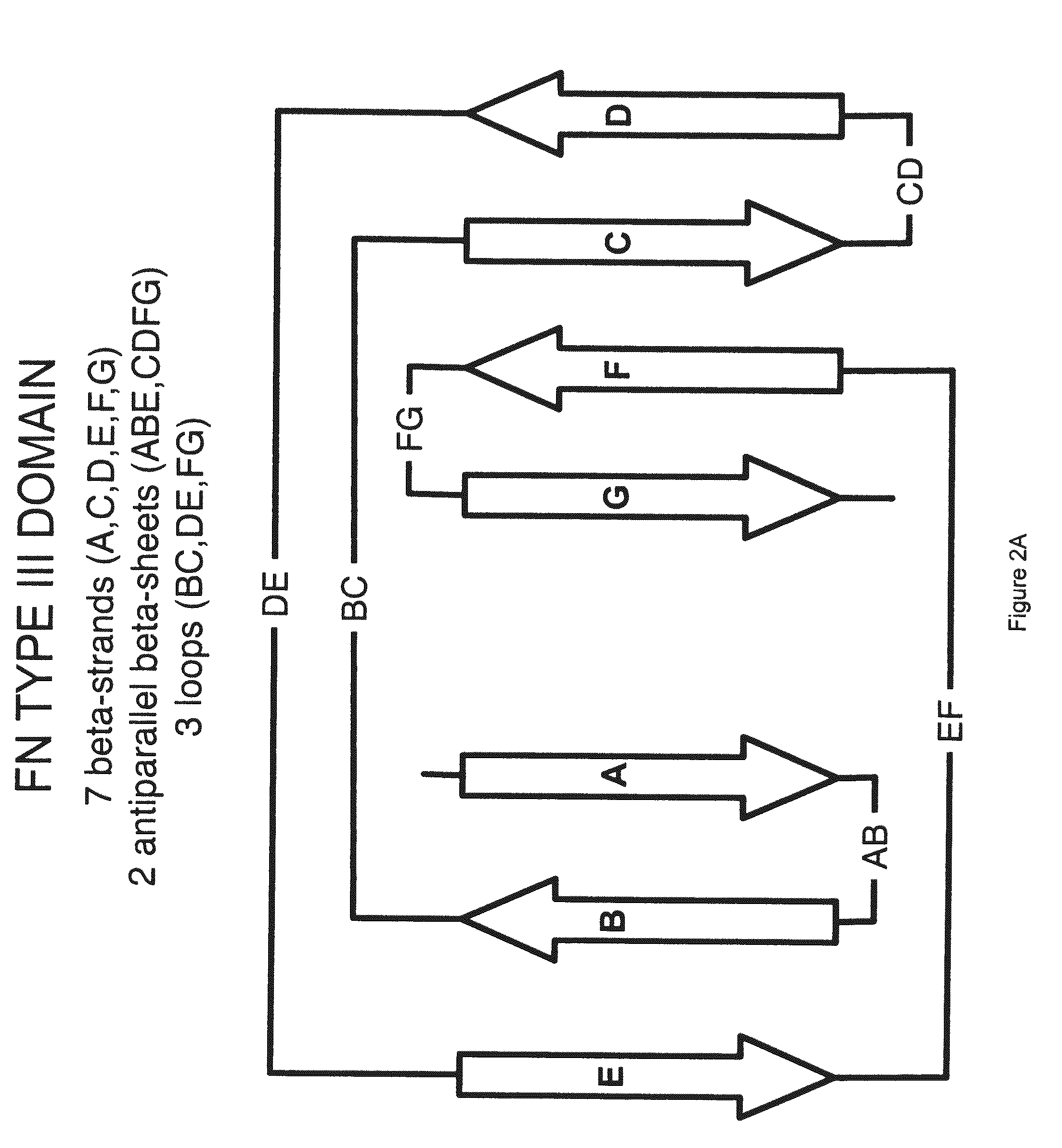
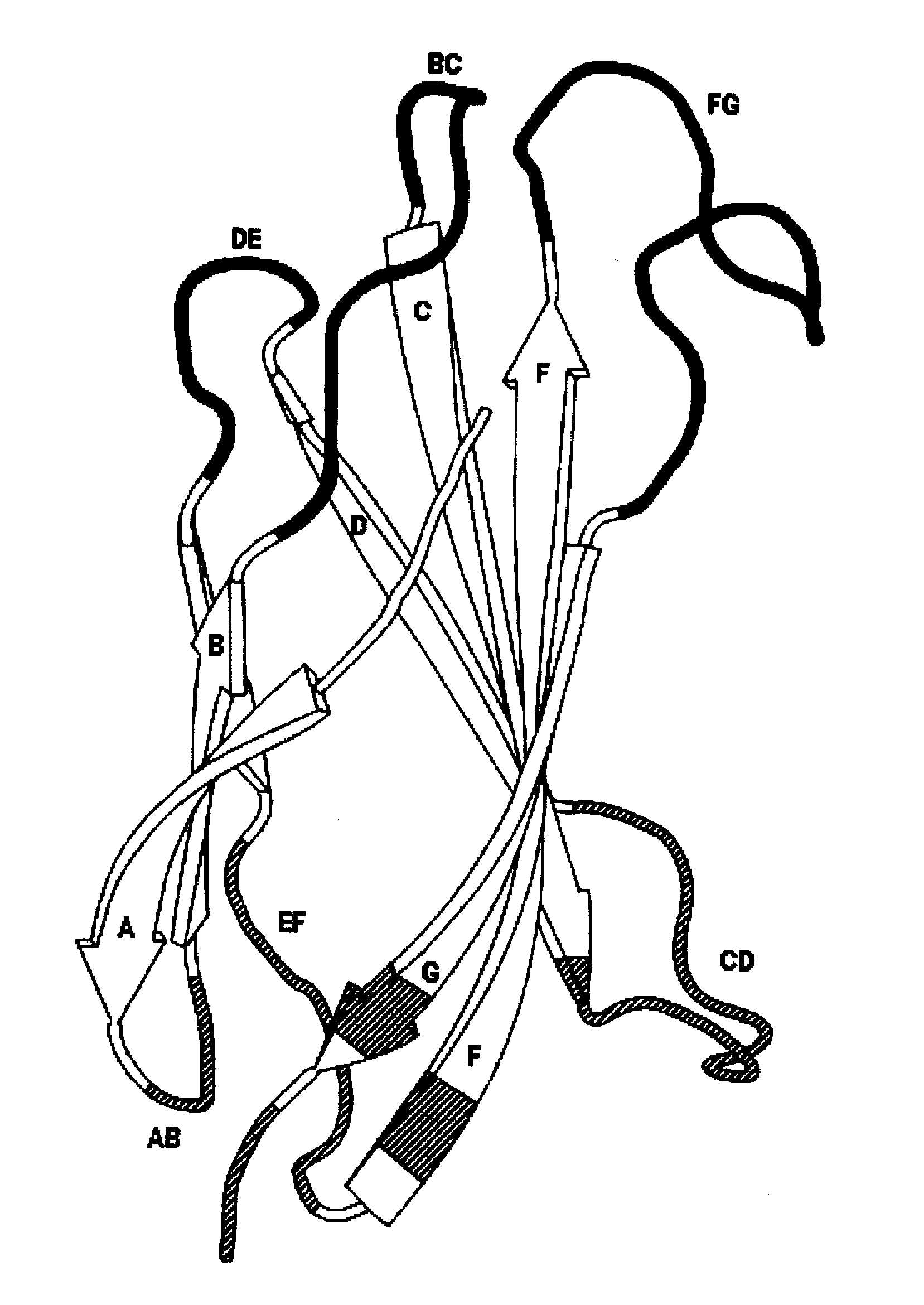
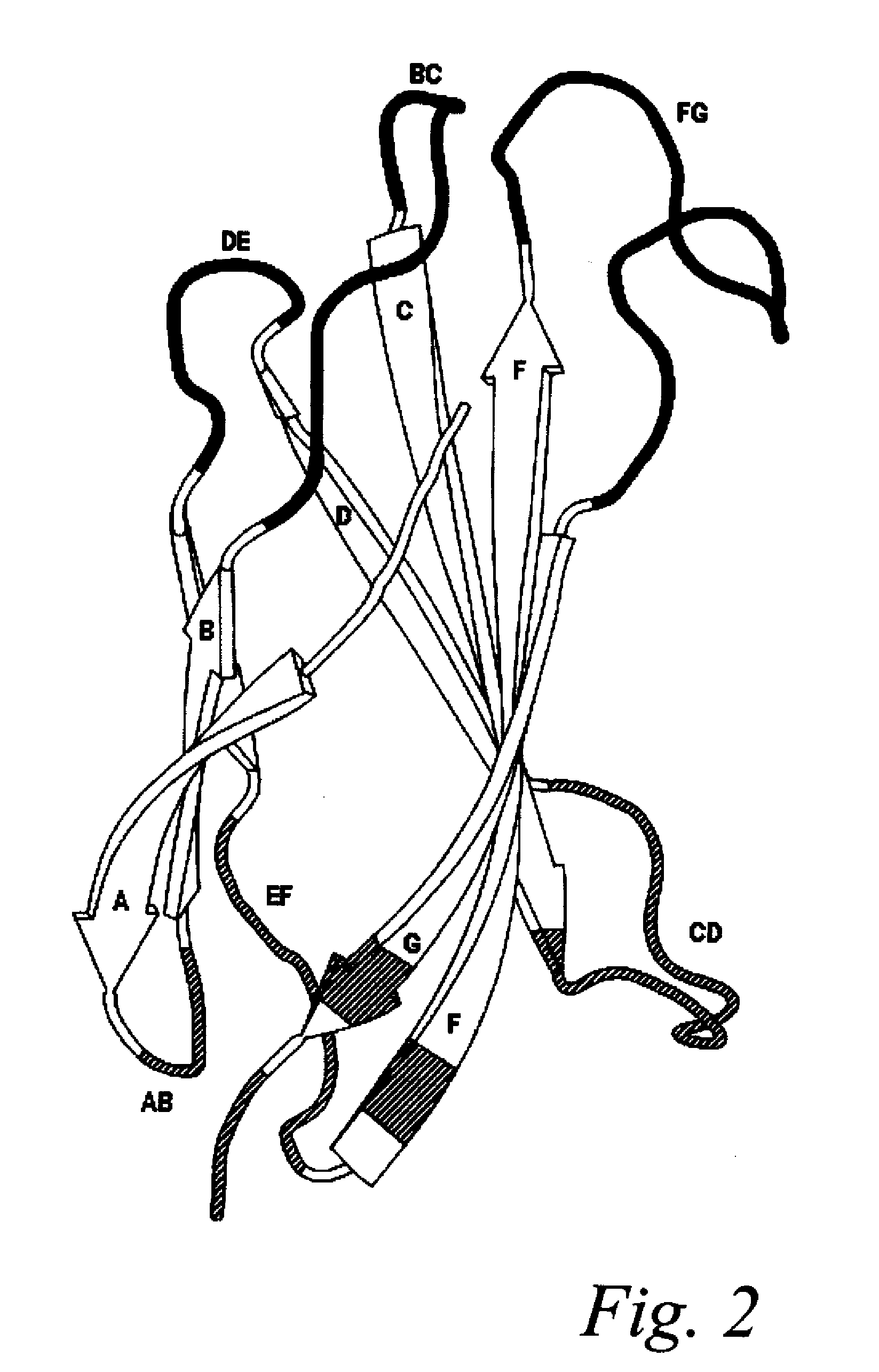
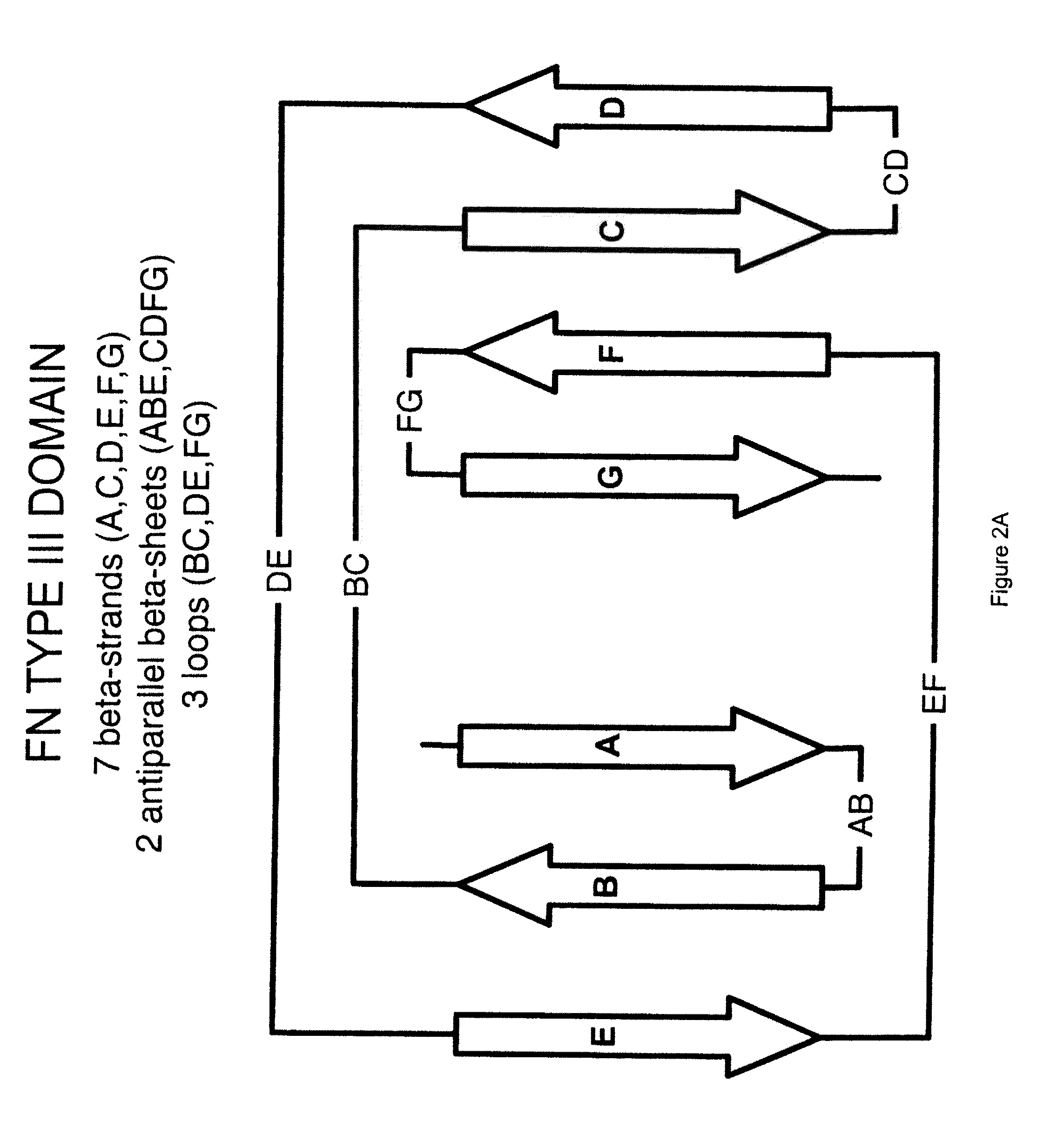
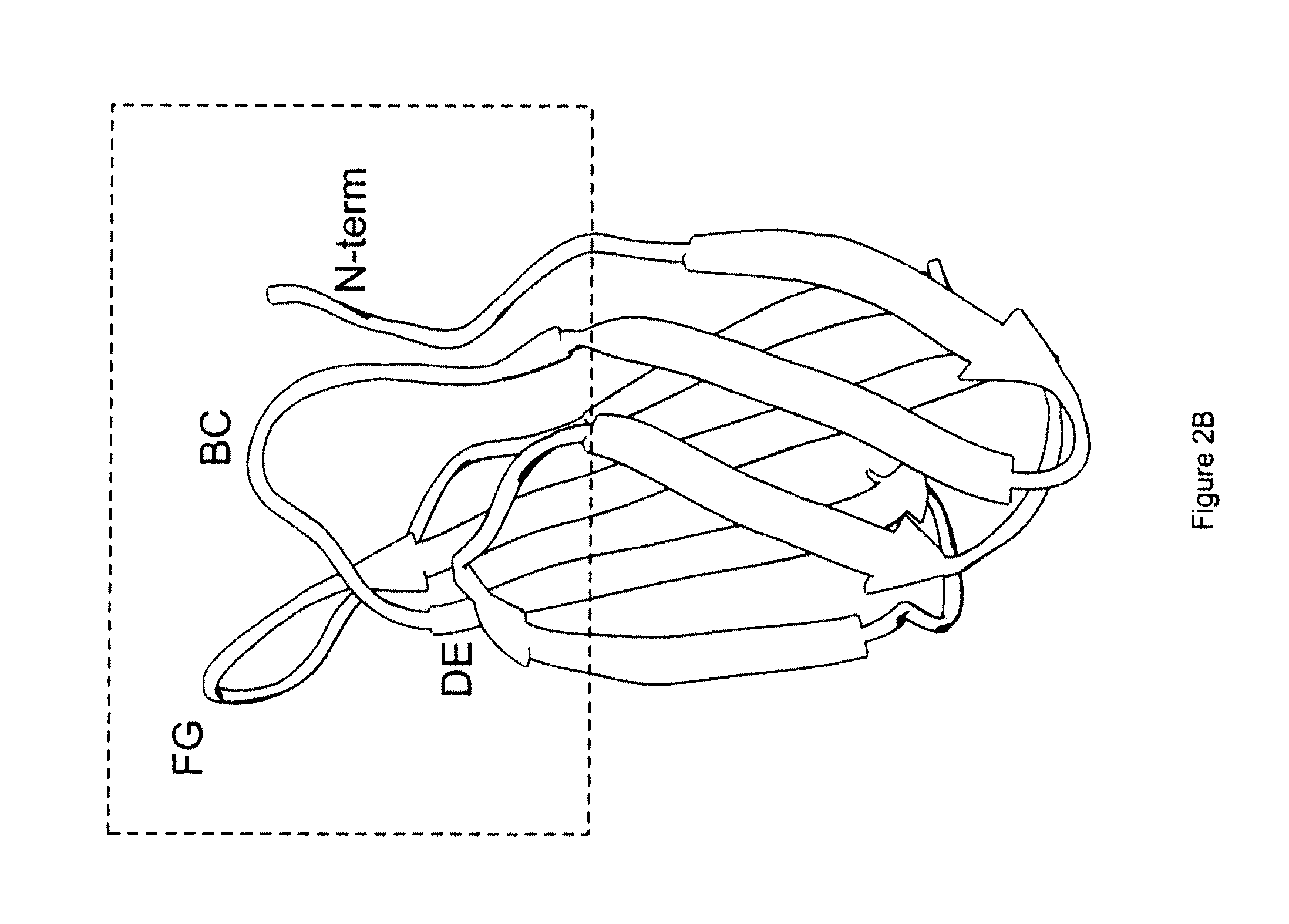
The invention pertains to a natural-variant combinatorial library of fibronectin Type 3 domain (Fn3) polypeptides useful in screening for the presence of one or more polypeptides having a selected binding or enzymatic activity. The library polypeptides include (a) regions A, AB, B, C, CD, D, E, EF, F, and G having wildtype amino acid sequences of a selected native fibronectin Type 3 polypeptide or polypeptides, and (b) loop regions AB, CD, and EF having selected lengths (Bottom Loops). The Fn3 may also have loop regions BC, DE, and FG having wildtype amino acid sequences, having selected lengths, or mutagenized amino acid sequences (Top Loops).
Owner:NOVARTIS AG
Universal fibronectin type iii binding-domain libraries
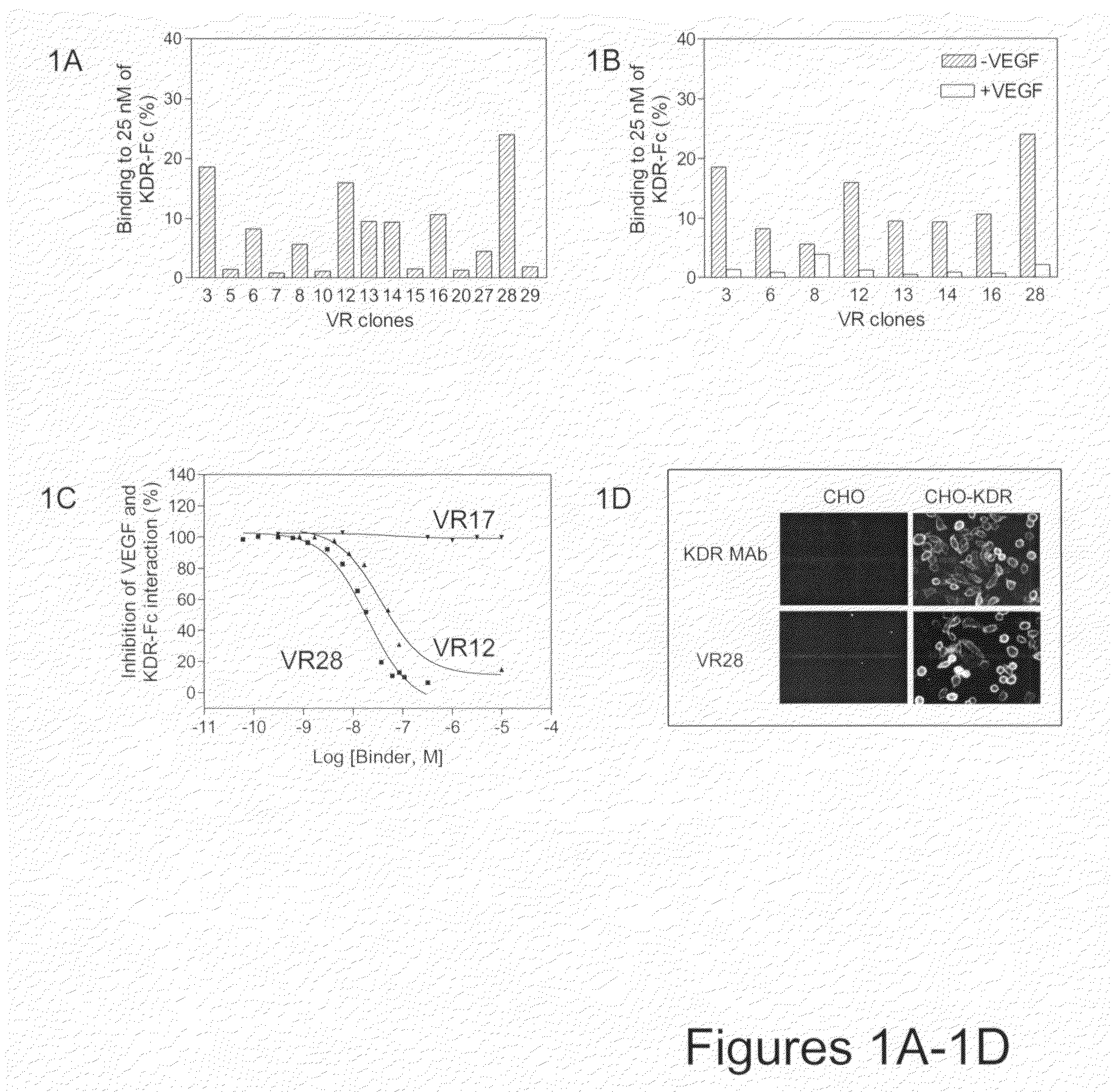
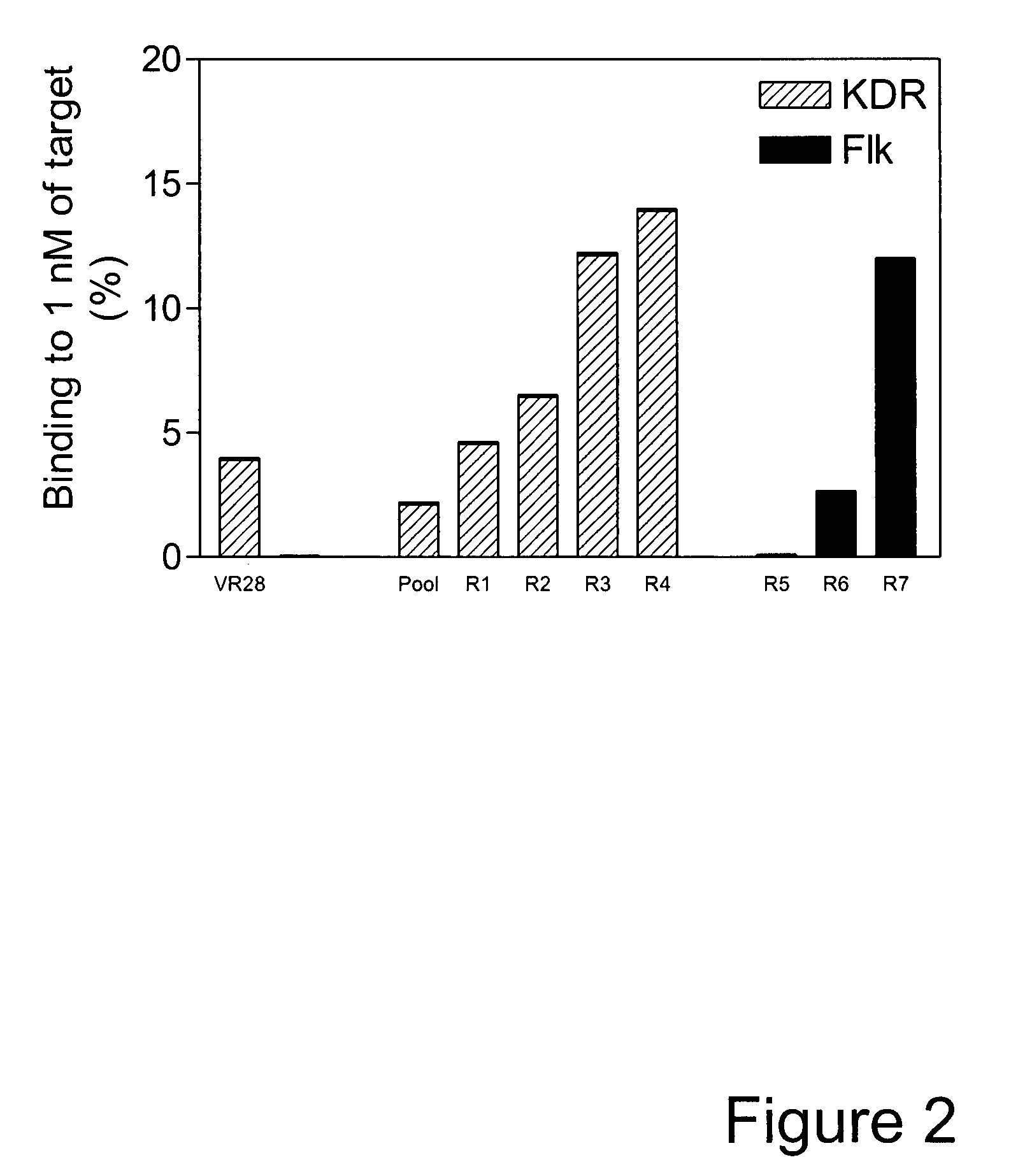
Fibronectin Type III (FN3) polypeptide libraries are described, along with their use in identifying fibronectin-type binding peptides having hign binding affinities, e.g., greater than 300 nM, for VEGFR2 or Axl proteins.
Owner:PROTELICA
Clinical-grade human mesenchymal stem cell serum-free complete medium
ActiveCN103243071APromote growthLow toxicitySkeletal/connective tissue cellsInsulin-like growth factorCuticle
The invention relates to a human mesenchymal stem cell culture medium. According to the culture medium, the basal culture medium comprises the following components based on the final concentration: 1-2g / L of human serum albumin, 5-10mg / L of transferring, 2-8mg / L of fibronectin, 1-4mg / L of laminin, 50g / L of Fe(NO3)3.9H2O, 417g / L of FeSO4.7H2O, 1-3mu g / L of estradiol, 2-5mu g / L of testosterone, 1-3mu g / L of progesterone, 39.25-117.74 mu g / L of dexamethasone, 5-10mg / L of insulin, 376.36mg / L of riboflavin, 80.96-242.87mg / L of coenzyme A, 4.41-6.17mg / L of butanediamine, 1-2mg / L of taurine, 0.61-1.85mg / L of aminoethanol, 8.81-26.42mg / L of pyruvic acid, 3.78-7.56mu g / L of sodium selenate, 292.3-584.6mg / L of L-glutamine, 2-8mu g / L of vascular endothelial growth factor, 4-10mu g / L of epidermal growth factor, 4-10mu g / L of basic fibroblast growth factor, 1-5mu g / L of leukaemia inhibitory factor, 1-5mu g / L of insulin-like growth factor-I and 2-8mu g / L of stem cell factor. The culture medium does not contain the animal serum, the potential animal endogenous endotoxin or virus of the animal serum is eliminated, and the culture medium is conveniently applied to clinics.
Owner:QINGDAO RESTORE BIOTECHNOLOGY CO LTD
Cosmetic compositions
ActiveUS20120288478A1Reducing activity of hyaluronidaseSkin stimulationBiocideCosmetic preparationsTrifluoroacetic acidHyaluronidase
Disclosed are compositions and methods for their use that can be used in cosmetic applications. The composition can include an effective amount of a Centella asiatica stem cells to reduce the activity of hyaluronidase in skin, an effective amount of tetradecyl aminobutyroylvalylamino butyric urea trifluoroacetate or Alpinia galanga leaf extract to promote the production of hyaluronic acid in skin, an effective amount of tripeptide-1 to promote the production of fibronectin and laminin in skin, and a dermatologically acceptable vehicle.
Owner:MARY KAY INC
Fibronectin-based binding molecules and their use
InactiveUS20100322930A1Improve stabilityExtended half-lifeSenses disorderPeptide/protein ingredientsHalf-lifeMammalian cell
The invention provides fibronectin-based binding molecules and methods for introducing donor CDRs into a fibronectin-based binding scaffold, in particular, Fn3. The fibronectin-based binding molecules of the invention may be further conjugated to another moiety, for example, Fc, anti-FcRn, HSA, anti-HSA, and PEG, for improved half life and stability, particularly in mammalian cells. The invention also provides methods for screening such molecules for binding to a target antigen as well as the manufacture and purification of a candidate binder.
Owner:NOVARTIS AG
N, N-disubstituted amides that inhibit the binding of integrins to their receptors
A method for the inhibition of the binding of .alpha..sub.4.beta..sub.1 integrin to its receptors, for example VCAM-1 (vascular cell adhesion molecule-1) and fibronectin; compounds that inhibit this binding; pharmaceutically active compositions comprising such compounds; and the use of such compounds either as above, or in formulations for the control or prevention of diseases states in which .alpha..sub.4.beta..sub.1 is involved.
Owner:ENCYSIVE PHARMA INC
Cellular fibronectin as a diagnostic marker in stroke and methods of use thereof
InactiveUS7392140B2Convenient treatmentAdditional diagnostic and/or prognostic indicatorsMedical data miningDisease diagnosisStroke subtypeGastroenterology
Methods for the diagnosis and evaluation of stroke and stroke sub-type employ a variety of bio-markers including cellular fibronectin (c-Fn) assembled as a panel for stoke diagnosis and evaluation. Methods are disclosed for selecting markers and correlating their combined levels with a clinical outcome of interest. In various aspects the methods permit early detection and differentiation of stroke subtypes, determination of the prognosis of a patient presenting stroke symptoms, and identification of a patient at risk for early hematoma growth and / or malignant massive cerebral artery infarction. The disclosed methods provide rapid, sensitive and specific assays to greatly increase the number of patients that can receive beneficial stroke treatment and therapy, and to reduce the human and economic costs associated with incorrect stroke diagnosis.
Owner:PREDICTION SCI
Methods and compositions for topical delivery
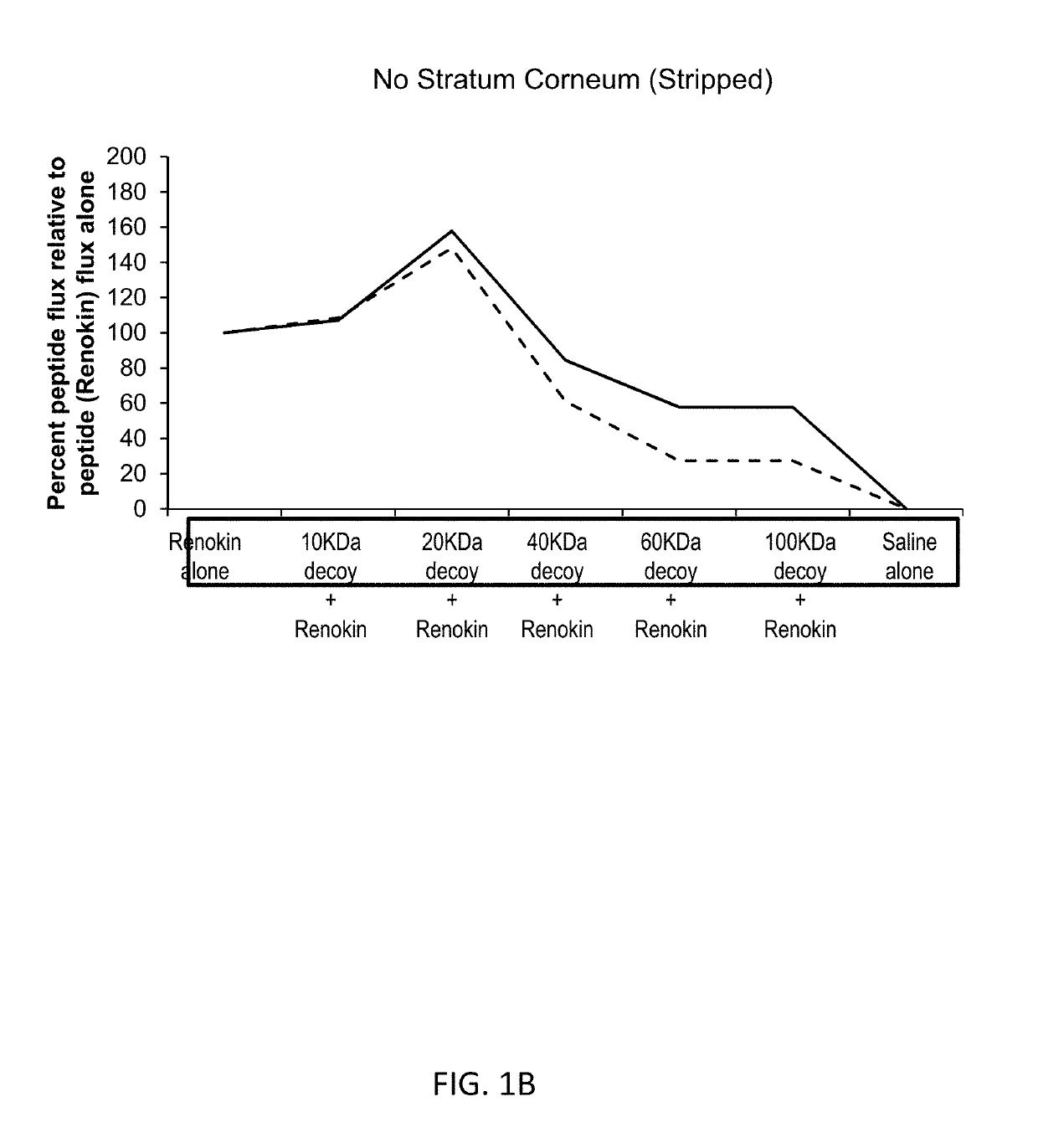
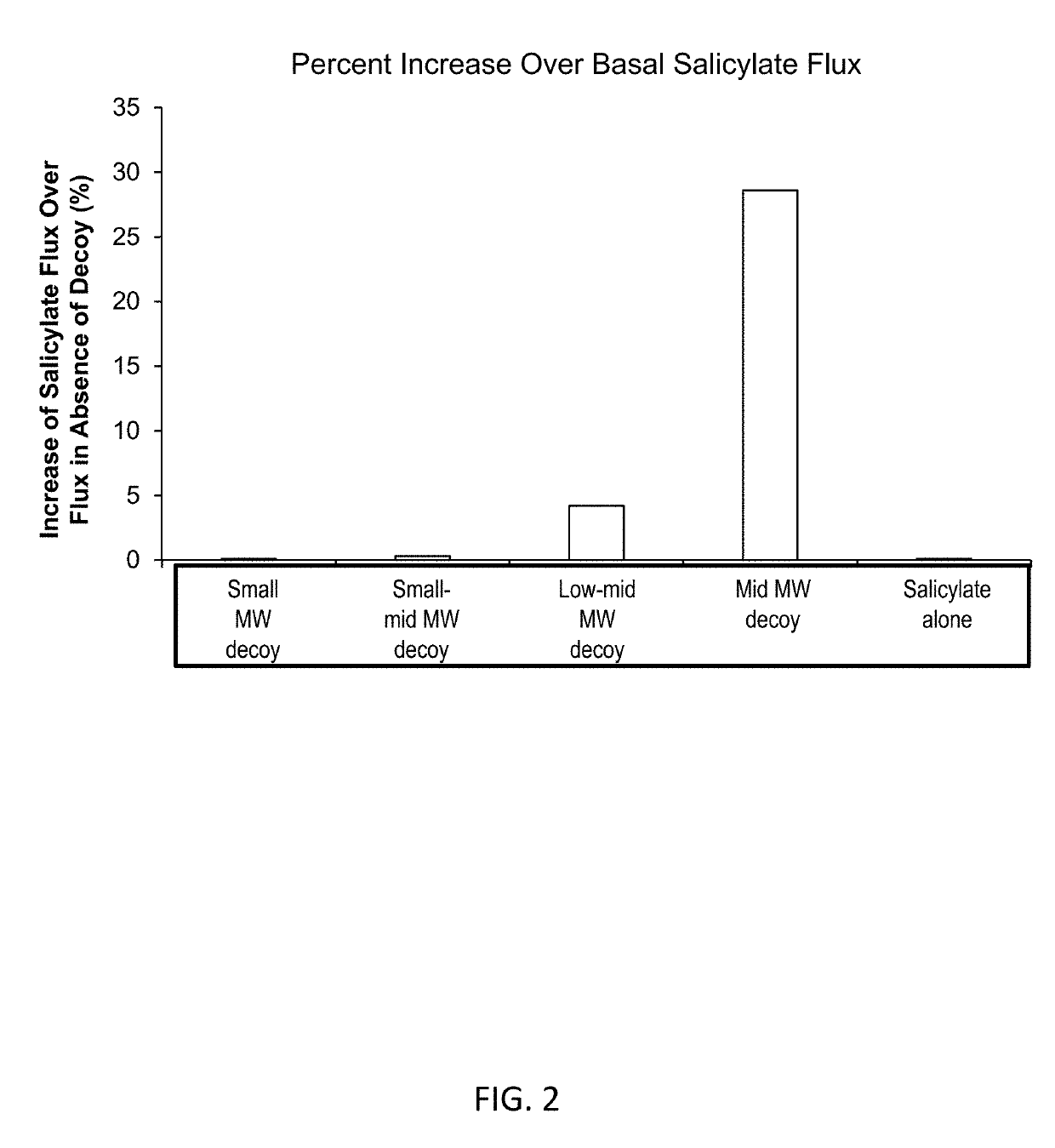
PendingUS20190105261A1Thin skinAvoid inconvenienceSalicyclic acid active ingredientsCosmetic preparationsActive agentMedicine
Compositions for topical delivery of an active agent and methods for using such compositions are described herein. Compositions include one or more active agents and about 0.001 wt. % to about 10 wt. % of a extracellular matrix component having average molecular weight of about 2,000 daltons to about 20,000 daltons. The extracellular components include hyaluronic acid, collagen, fibronectin, elastin, lectin, and fragments thereof and combinations thereof.
Owner:ILLUSTRIS PHARMA INC
Cellular fibronectin as a diagnostic marker in cardiovascular disease and methods of use thereof
InactiveUS20080010024A1Reduce eliminateProlonged plasma half-lifeDisease diagnosisBiological testingCardiovascular InjuryCardiovascular Disorder
Thrombolytic therapy in the treatment of a cardiovascular event such as myocardial infarction (MI) carries with it a chance of suffering a hemorrhagic incident leading to severe disability and often death. Methods for the evaluation of proper therapy for a specific patient who has suffered a cardiovascular event employ a variety of bio-markers including cellular fibronectin (c-Fn) assembled as a panel for evaluation. Methods are disclosed for selecting markers and correlating their combined levels with a clinical outcome of interest. In various aspects the methods permit early detection of potential bleeding events, determination of the prognosis of a patient presenting cardiovascular damage, and identification of a patient at risk for hemorrhage when given thrombolytic therapy. The disclosed methods provide rapid, sensitive and specific assays to greatly reduce the risk of bleeding or the number of patients that can receive the most beneficial treatment for their cardiovascular event, and to reduce the human and economic costs associated with bleeding following such treatments.
Owner:PREDICTION SCI
Cellular fibronectin as a diagnostic marker in stroke and methods of use thereof
InactiveUS7634360B2Rapidly and accurately determinedBiostatisticsDisease diagnosisStroke treatmentThrombus
The present invention relates to methods for the diagnosis and evaluation of stroke and stroke sub-type. A variety of bio-markers are disclosed for assembling a panel for such diagnosis and evaluation. Methods are disclosed for selecting markers and correlating their combined levels with a clinical outcome of interest. In various aspects: the invention provides methods for early detection and differentiation of stroke subtypes, for determining the prognosis of a patient presenting with stroke symptoms, and identifying a patient at risk for hemorrhagic transformation after thrombolyic therapy. Methods are disclosed that provide rapid, sensitive and specific assays to greatly increase the number of patients that can receive beneficial stroke treatment and therapy, and reduce the costs associated with incorrect stroke diagnosis.
Owner:PREDICTION SCI
Methods and compositions for diagnosis and prognosis of renal injury and renal failure
ActiveUS20110201038A1Easy to adaptMicrobiological testing/measurementDisease diagnosisInterleukin 10Soluble P-Selectin
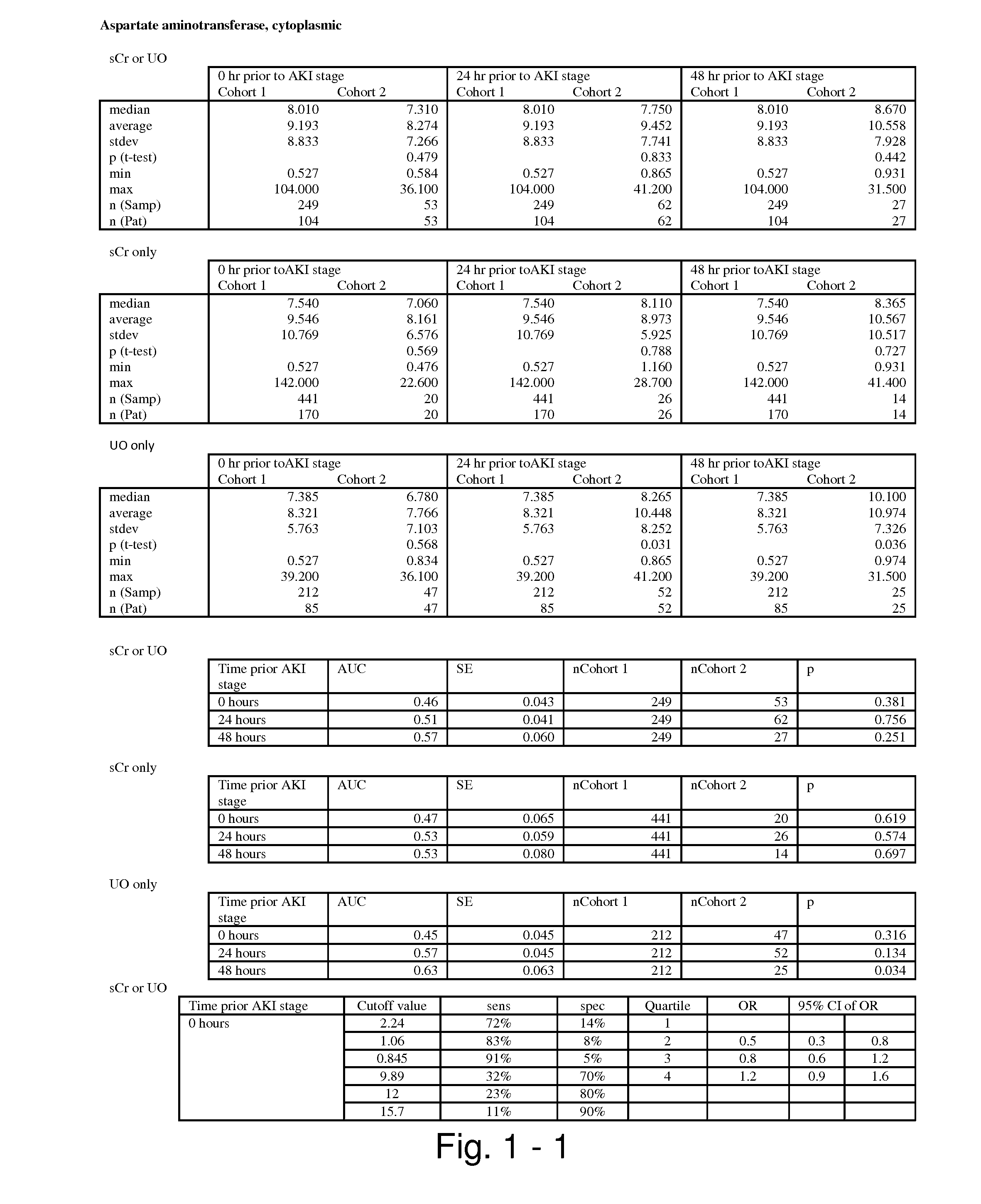
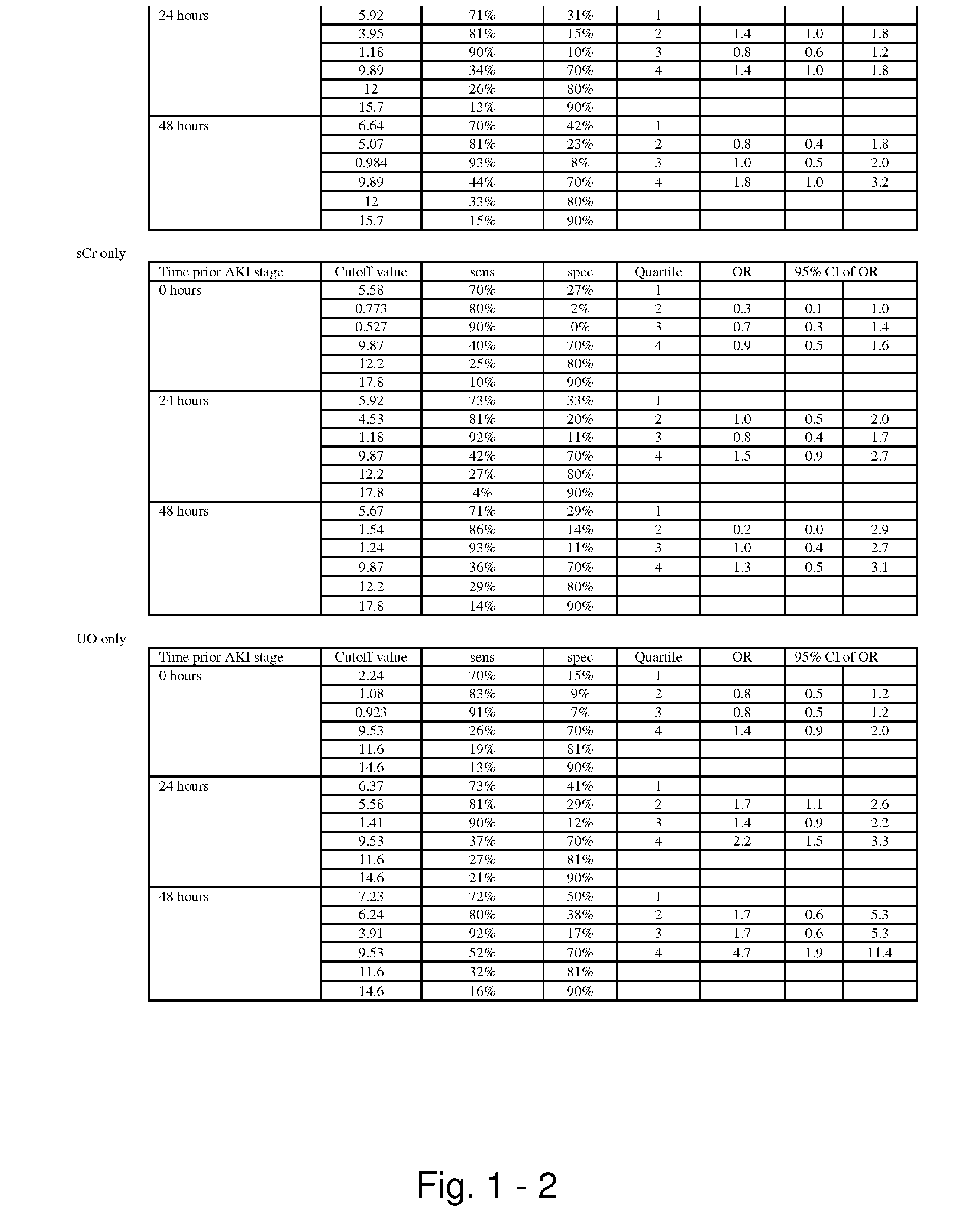
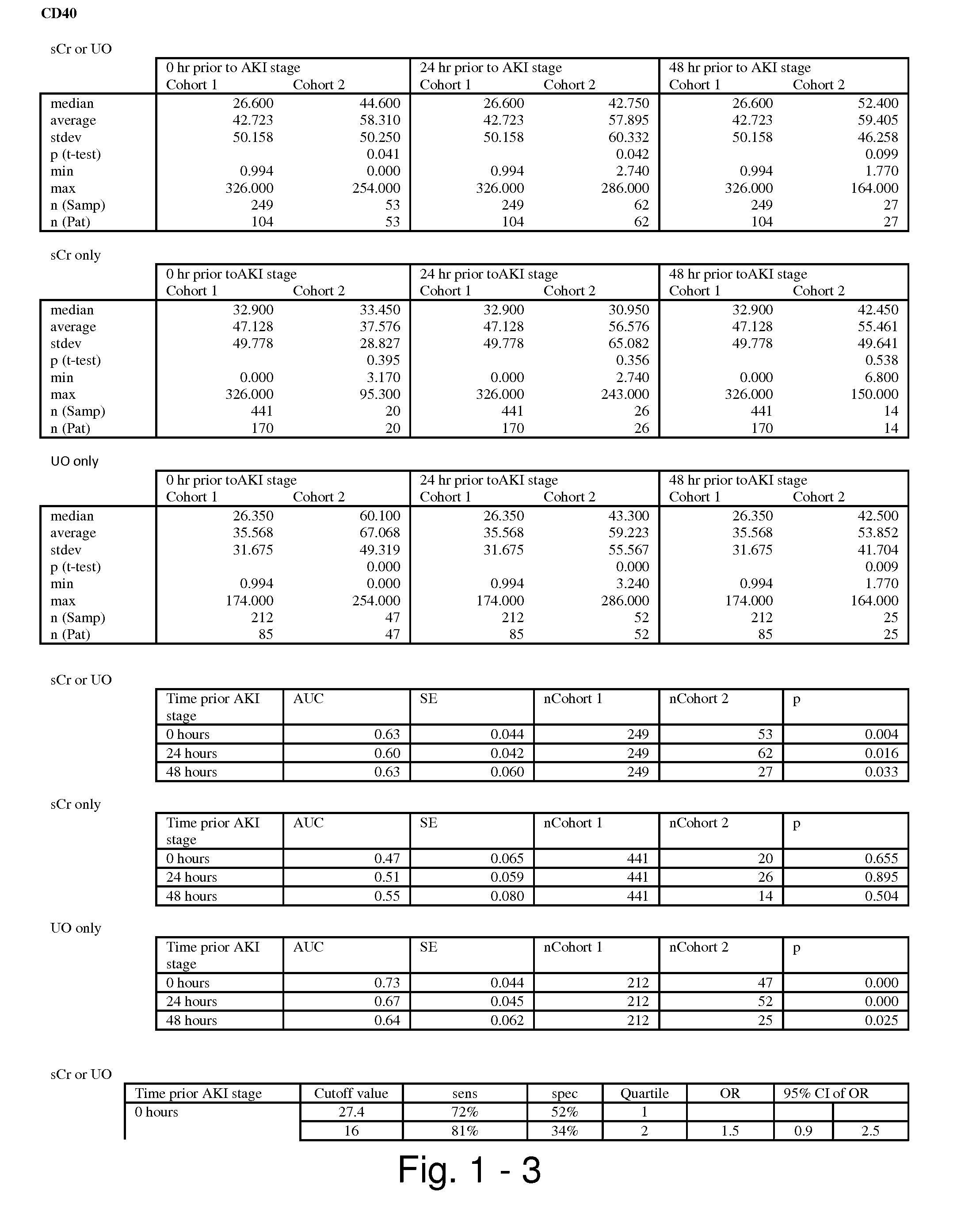
The present invention relates to methods and compositions for monitoring, diagnosis, prognosis, and determination of treatment regimens in subjects suffering from or suspected of having a renal injury. In particular, the invention relates to using assays that detect one or more markers selected from the group consisting of Cytoplasmic aspartate aminotransferase, soluble Tumor necrosis factor receptor superfamily member 5, soluble CD40 Ligand, soluble C-X-C Motif chemokine 16, S100-A12, Eotaxin, soluble E-selectin, Fibronectin, Granulocyte colony-stimulating factor, Granulocyte-macrophage colony-stimulating factor, Heparin-binding growth factor 2, soluble Hepatocyte growth factor receptor, Interleukin-1 receptor antagonist, Interleukin-1 beta, Interleukin-10, Interleukin-15, Interleukin-3, Myeloperoxidase, Nidogen-1, soluble Oxidized low-density lipoprotein receptor 1, Pappalysin-1, soluble P-selectin glycoprotein ligand 1, Antileukoproteinase, soluble Kit ligand, Tissue inhibitor of metalloproteinase 1, Tissue inhibitor of metalloproteinase 2, soluble Tumor necrosis factor, soluble Vascular cell adhesion molecule 1, and Vascular endothelial growth factor A as diagnostic and prognostic biomarkers in renal injuries.
Owner:ASTUTE MEDICAL
Artificial antibody polypeptides
The present invention provides a fibronectin type III (Fn3) molecule, wherein the Fn3 contains a stabilizing mutation. The present invention also provides Fn3 polypeptide monobodies, nucleic acid molecules encoding monobodies, and variegated nucleic acid libraries encoding such monobodies. Also provided are methods of preparing a Fn3 polypeptide monobody, and kits to perform the methods.
Owner:RES CORP TECH INC
Collagen-coated carrier and method for manufacturing collagen-coated carrier
InactiveUS20060270037A1Promote cell growthPeptide/protein ingredientsPretreated surfacesCalcium biphosphateProtein composition
A collagen-coated carrier that has excellent cell adhesion properties and that allows excellent cell growth thereon is provided. Further, a method for manufacturing such a collagen-coated carrier efficiently and reliably is also provided. The collagen-coated carrier includes a base material (carrier) composed of a calcium phosphate-based compound and a coating layer provided so as to cover the surface of the base material. The coating layer is composed of collagen and a protein having a high affinity for the collagen. Such a coating layer covering the surface of the base material is formed by allowing the base material to firmly adsorb the collagen via the protein. The protein preferably has a collagen receptor. More preferably, the protein contains at least one of fibronectin and integrin as a main ingredient.
Owner:ASAHI KOGAKU KOGYO KK
Fibronectin based scaffold proteins having improved stability
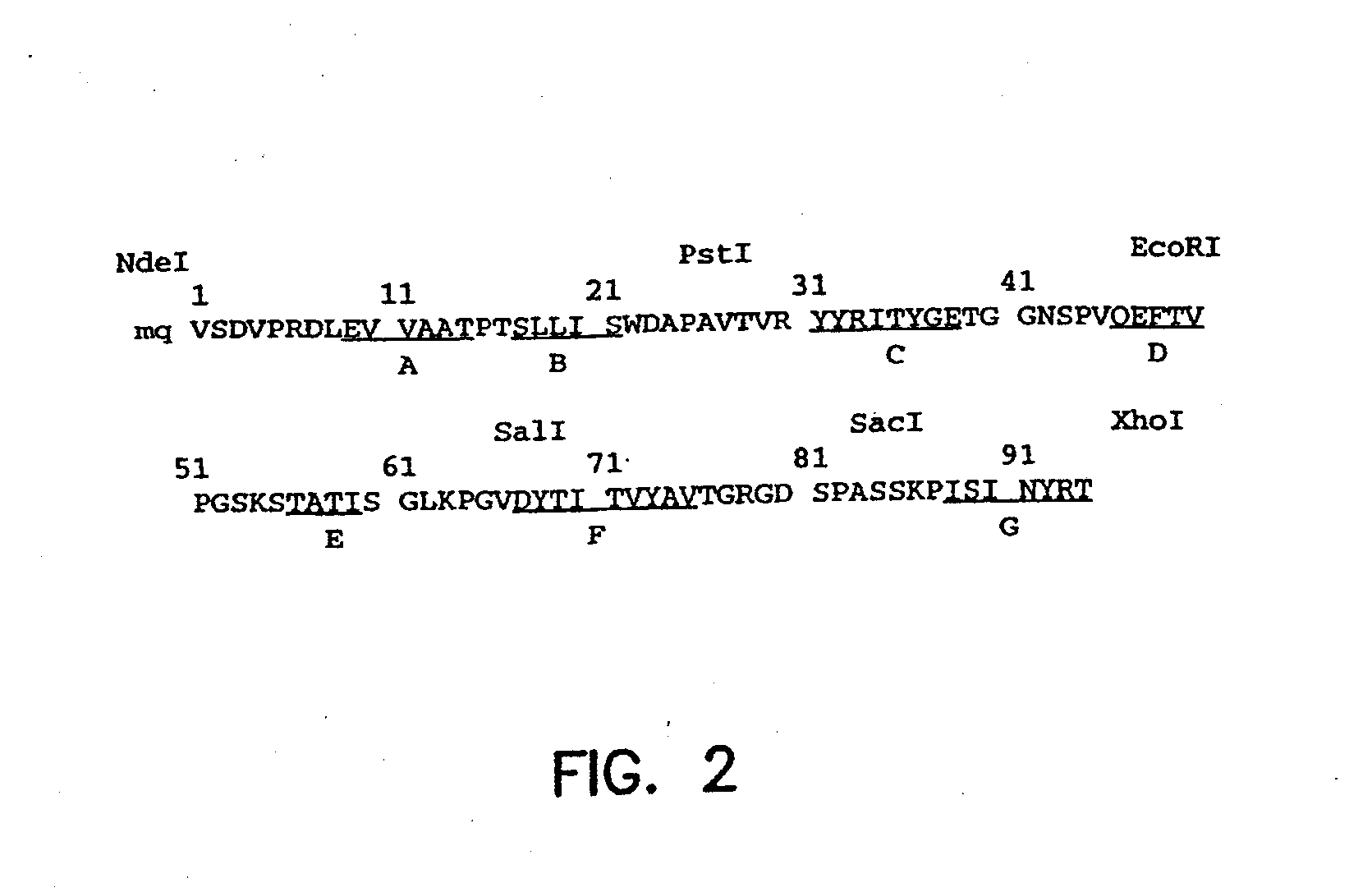
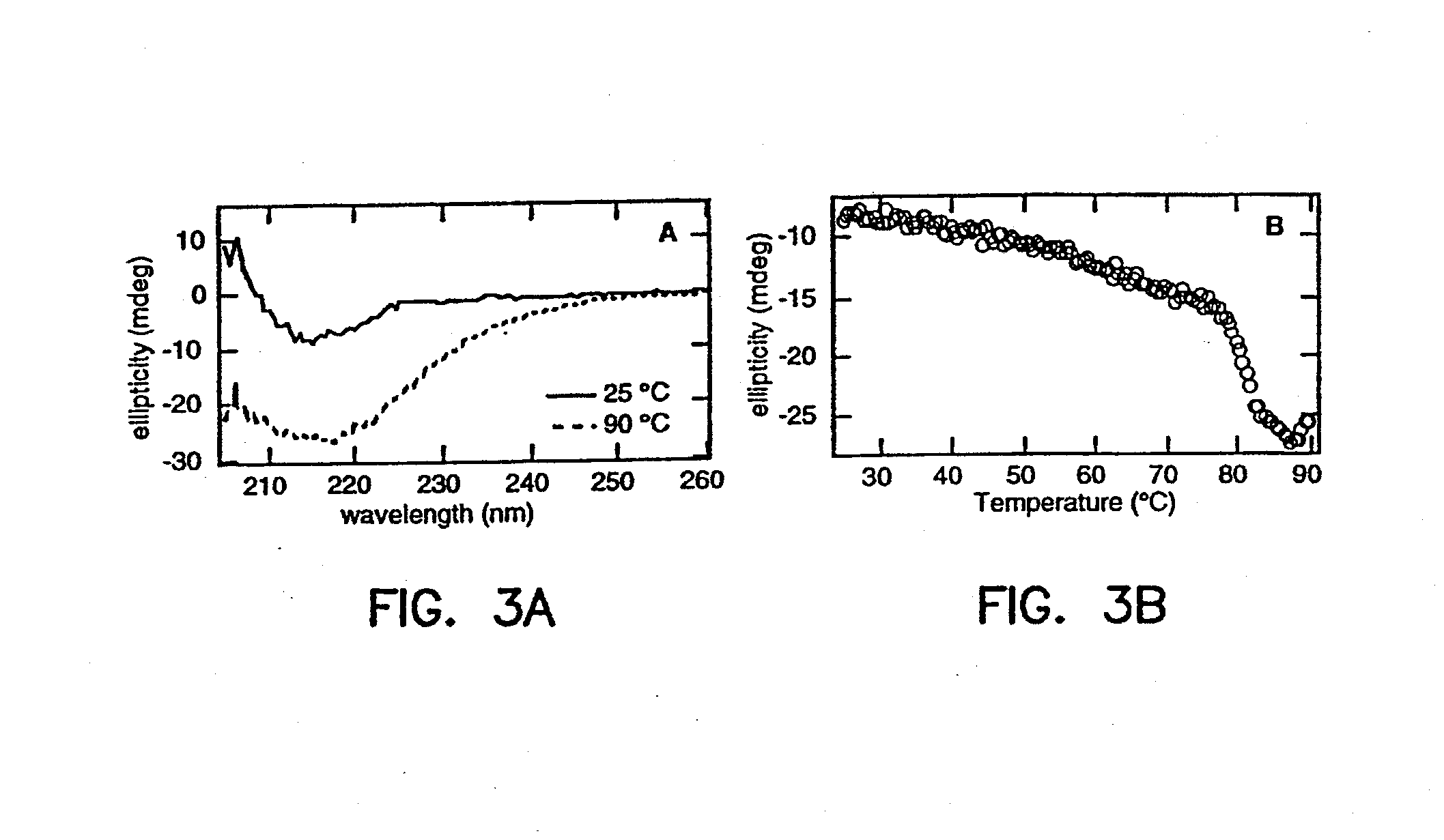
ActiveUS20130184212A1Improve stabilityReduce fragmentationBacteriaPeptide/protein ingredientsScaffold proteinProteinoid
The present application provides fibronectin based scaffold proteins associated with improved stability. The application also relates to stable formulations of fibronectin based scaffold proteins and the use thereof in diagnostic, research and therapeutic applications. The application further relates to cells comprising such proteins, polynucleotides encoding such proteins or fragments thereof, and to vectors comprising such polynucleotides.
Owner:BRISTOL MYERS SQUIBB CO
Serum-free culture medium for mesenchymal stem cells
ActiveCN102433302AGood growthFast growthSkeletal/connective tissue cellsINSULIN HUMANPancreatic hormone
The invention discloses a serum-free culture medium for mesenchymal stem cells. The serum-free culture medium for the mesenchymal stem cells comprises the following ingredients: fibronectin at the final concentration of 25mu g / ml, basic fibroblast growth factors at the final concentration of 10ng / ml, human epidermal growth factors at the final concentration of 15ng / ml, recombinant human insulin at the final concentration of 1mg / ml, human transferrin at the final concentration of 0.55mg / ml, human blood albumin in a volume ratio of 5 percent, sodium selenite at the final concentration of 0.67mug / ml, L-carnitine at the final concentration of 5mM and resveratrol at the final concentration of 30mu M. When the mesenchymal stem cells are cultured by the serum-free culture medium for the mesenchymal stem cells, animal-derived serum is not contained, so infection risks can be controlled; the L-carnitine and the resveratrol which are added into the serum-free culture medium for the mesenchymalstem cells can effectively improve the growth state of the mesenchymal stem cells; and the growth speed of the mesenchymal stem cells is remarkably improved, and the biological characteristics of themesenchymal stem cells are kept unchanged.
Owner:CHENGDU QINGKE BIOTECH
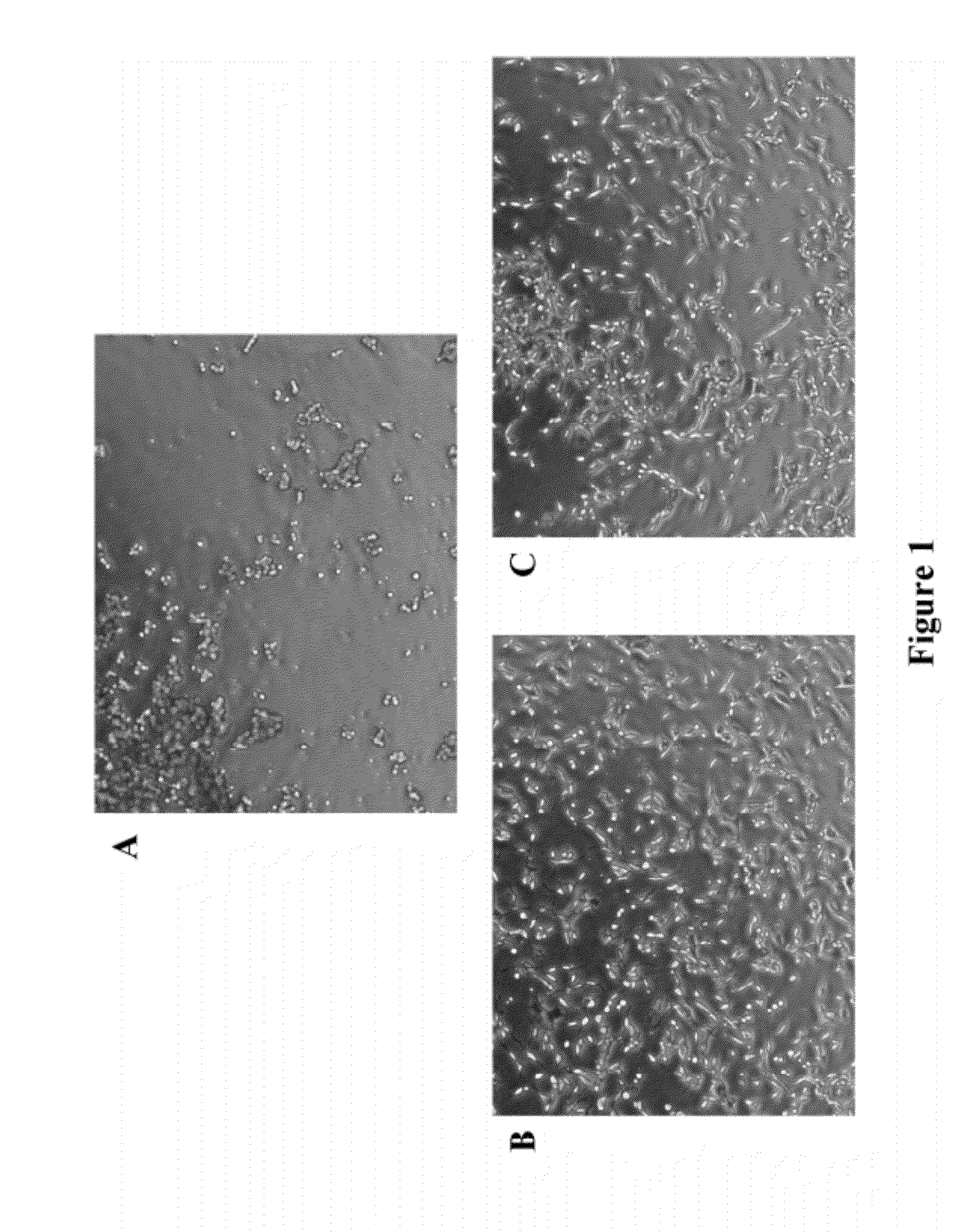
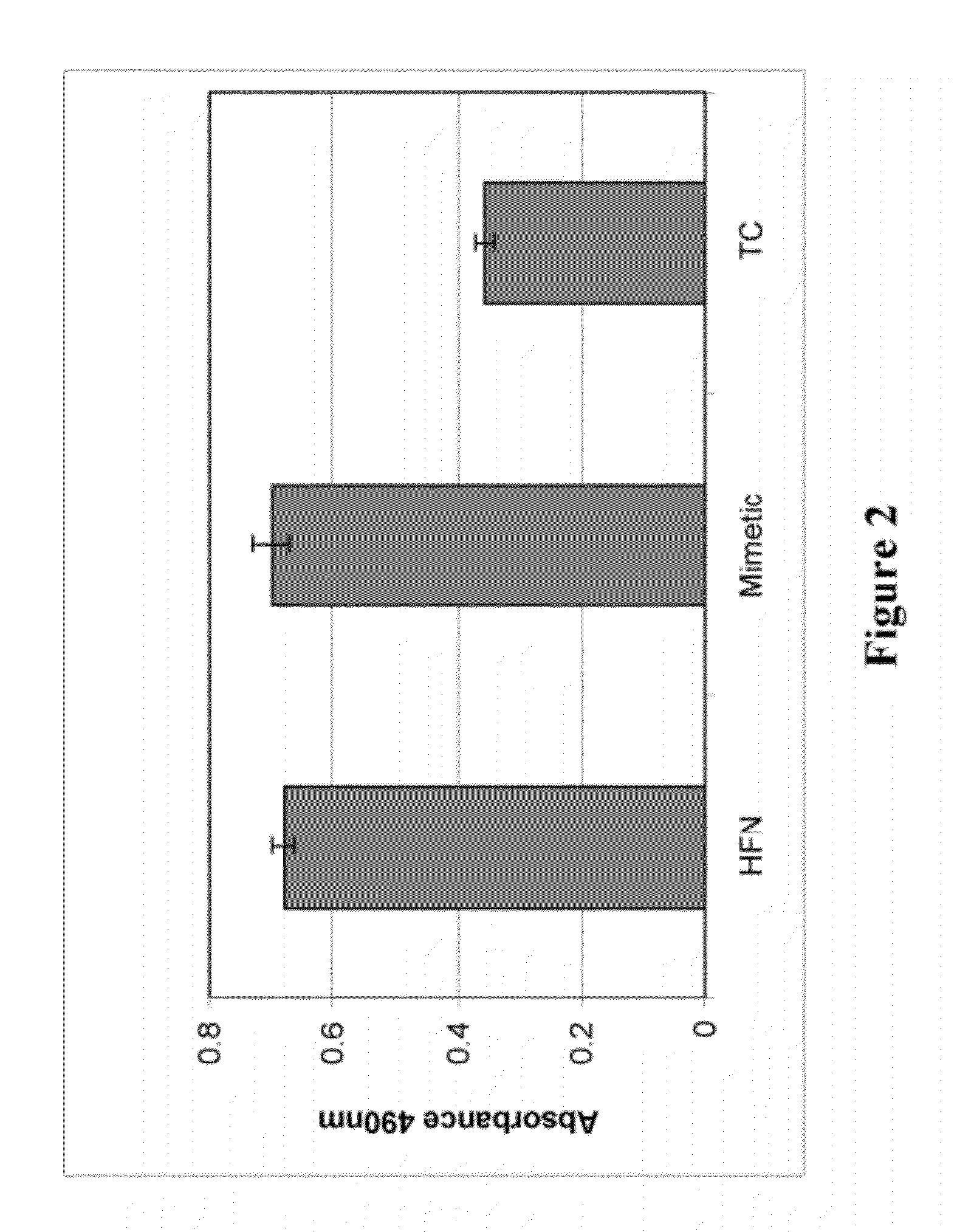
Synthetic, Defined Fibronectin Mimetic Peptides And Surfaces Modified With The Same
ActiveUS20120149871A1Avoid issuingConnective tissue peptidesPeptide/protein ingredientsFibronectinCell biology
The present invention discloses compositions for applications that mimic fibronectin coated surfaces. Advantageously, such compositions provide an animal free (xeno-free, and human-component-free), synthetic, chemically defined surface that mimics at least one of the functionalities of fibronectin.
Owner:CORNING INC
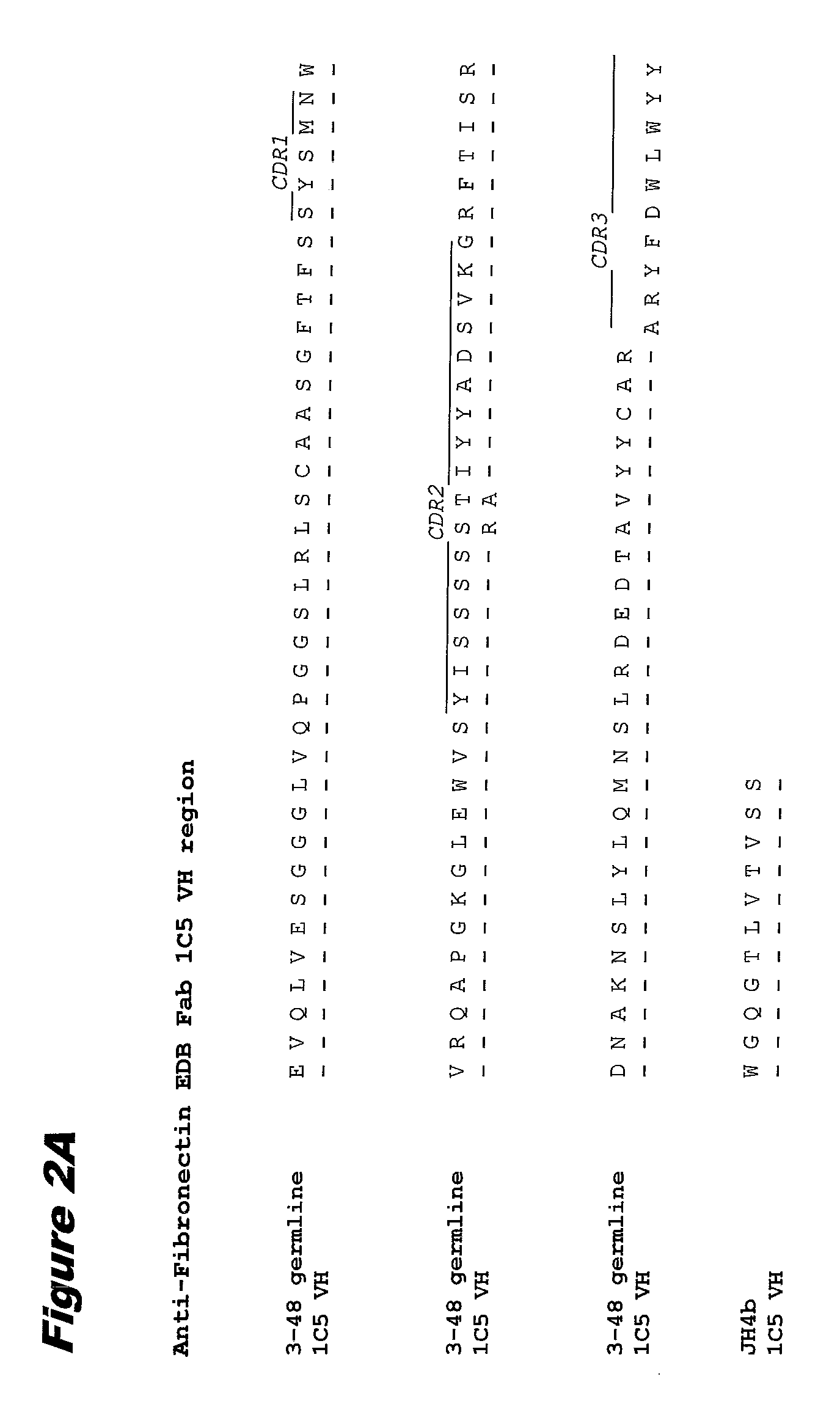
Fibronectin ed-b antibodies, conjugates thereof, and methods of use
InactiveUS20090162372A1Immunoglobulins against animals/humansAntibody ingredientsAntibody conjugateAntibody fragments
The present invention provides anti-ED-B antibodies, antibody fragments, and antibody mimetics and such antibodies conjugated to a partner molecule, wherein the antibody or the antibody-partner molecule conjugate provides a therapeutic effect regardless of whether the ED-B-antibody or ED-B-conjugate complex is internalized within a targeted cell.
Owner:MEDAREX INC
Gene transfer method with the use of serum-free medium
InactiveUS6287864B1Effective maintenanceImprove efficiencyBiocideMicroorganismsVirus-RetrovirusGene transfer
A method for transferring a gene into target cells by a retrovirus with the use of serum-free medium. This method comprises infecting target cells with a retrovirus in serum-free medium optionally containing low-density lipoprotein and / or cytokines in the presence of a functional substance such as fibronectin in an amount effective in elevating the gene transfer efficiency of the retrovirus into the target cells by co-localizing the retrovirus and the target cells.
Owner:TAKARA HOLDINGS
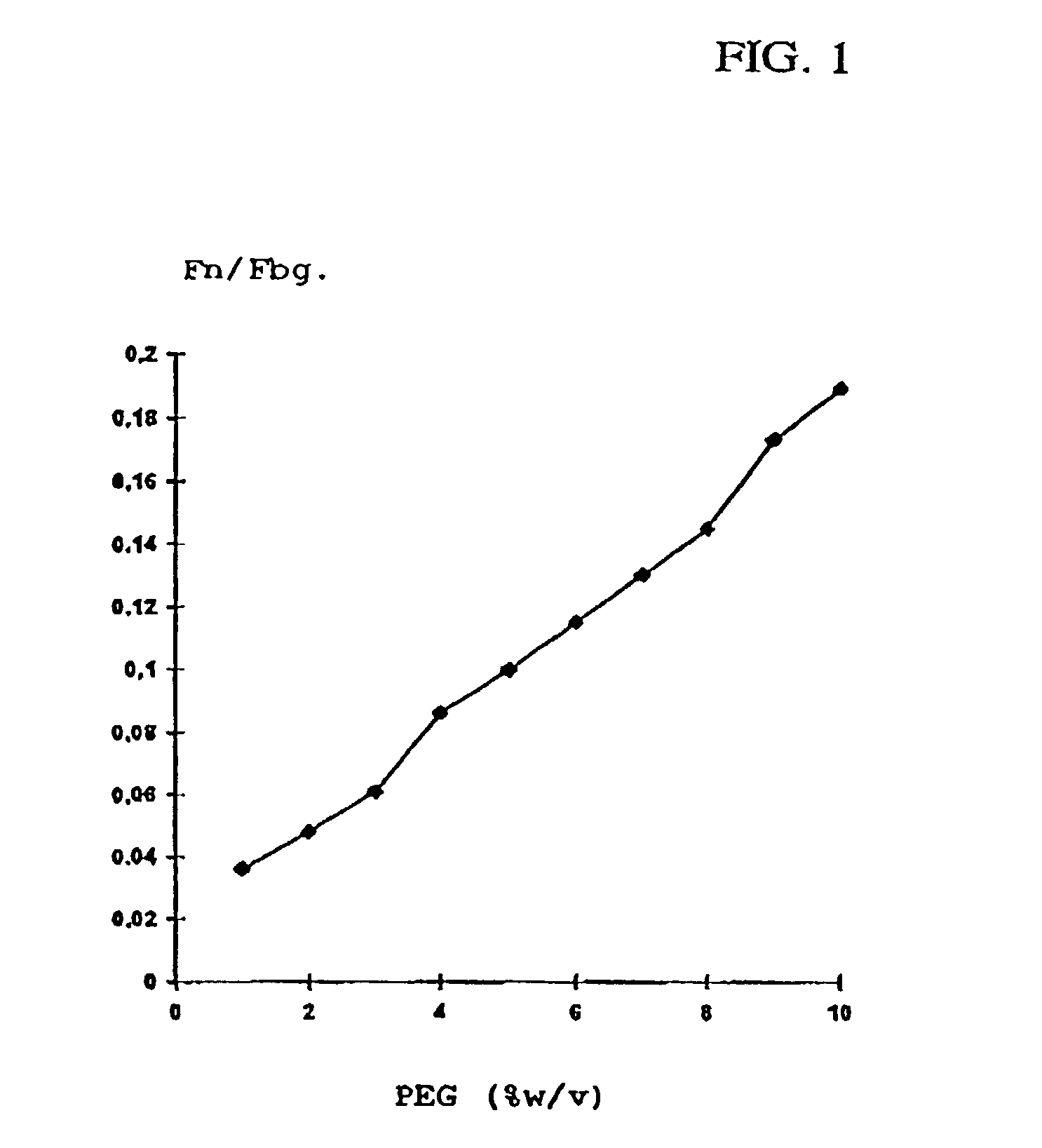
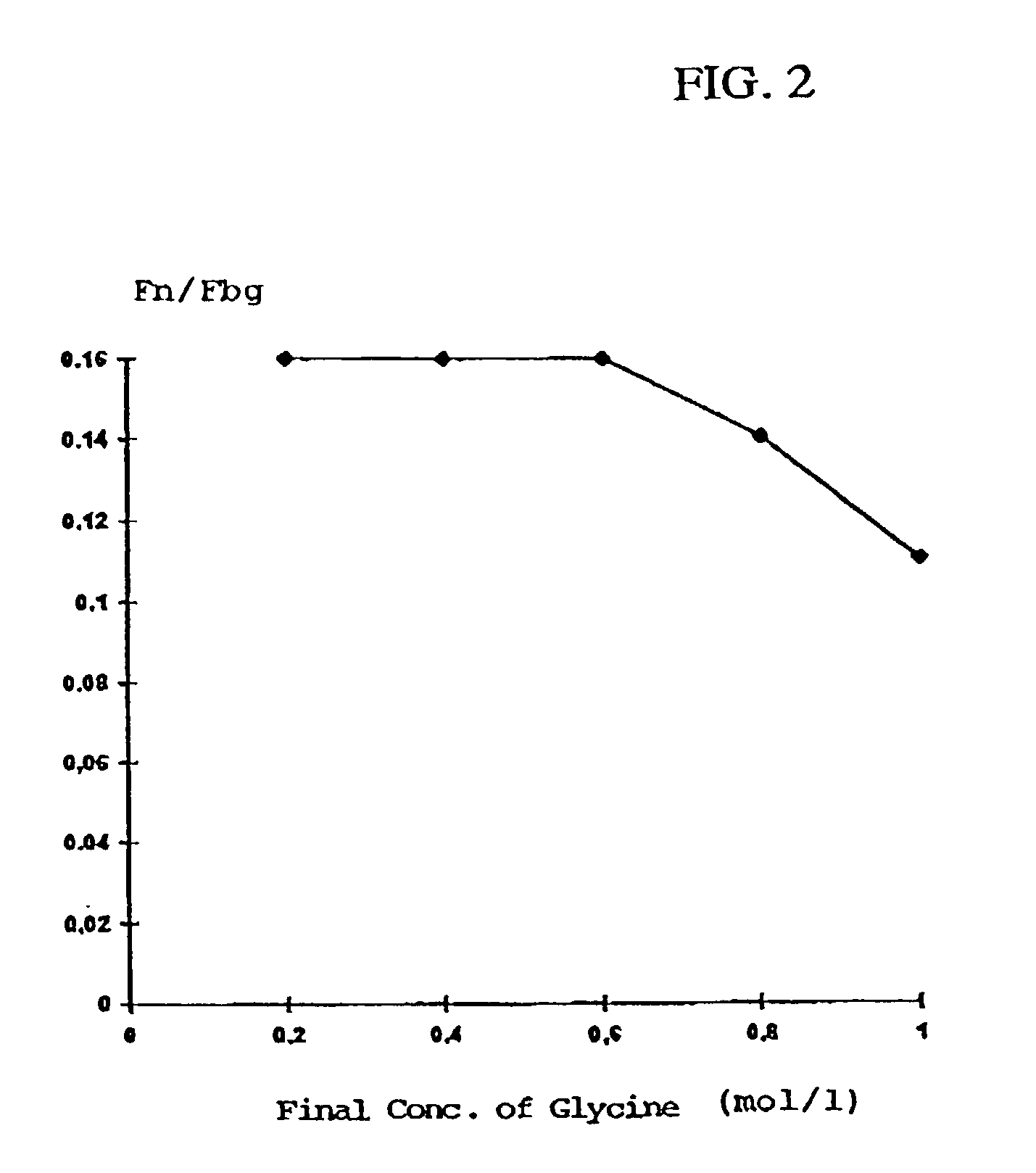
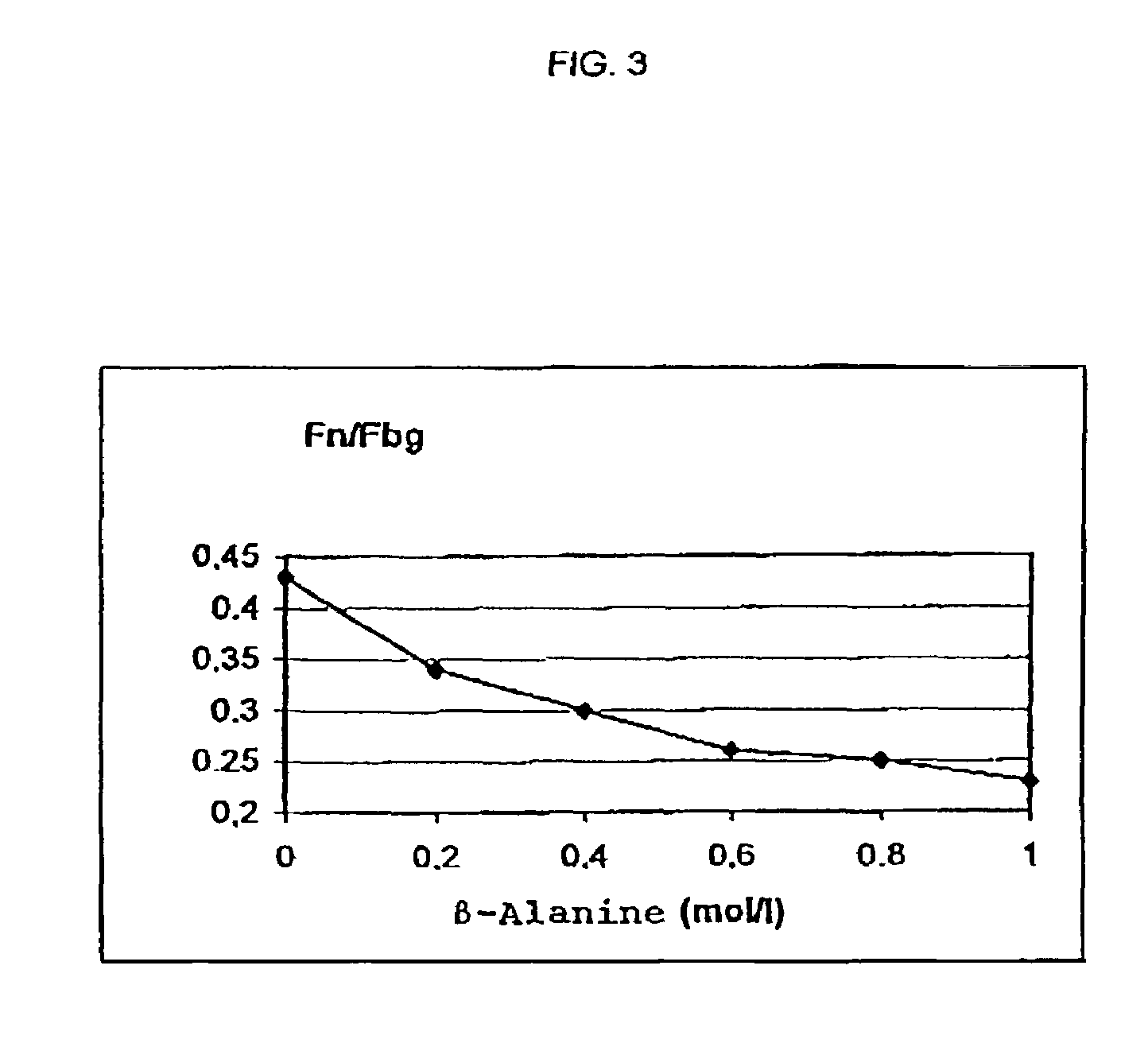
Method for producing a preparation based on fibrinogen and fibronectin as well as protein compositions obtainable according to this method
InactiveUS7241603B2Promote wound healingEliminate pollutionFactor VIIConnective tissue peptidesSAA proteinSolubility
A method for producing protein compositions comprising fibrinogen and fibronectin is disclosed, wherein a fibrinogen and fibronectin-containing starting solution is treated with a precipitating composition which comprises two different components that modify the solubility of fibrinogen and / or fibronectin, so that in a single-step precipitation a precipitate is formed which comprises fibrinogen and fibronectin, and the precipitate formed optionally is further treated by methods known per se.
Owner:BAXTER INT INC +1
Enzyme-mediated modification of fibrin for tissue engineering: fibrin formulations with peptides
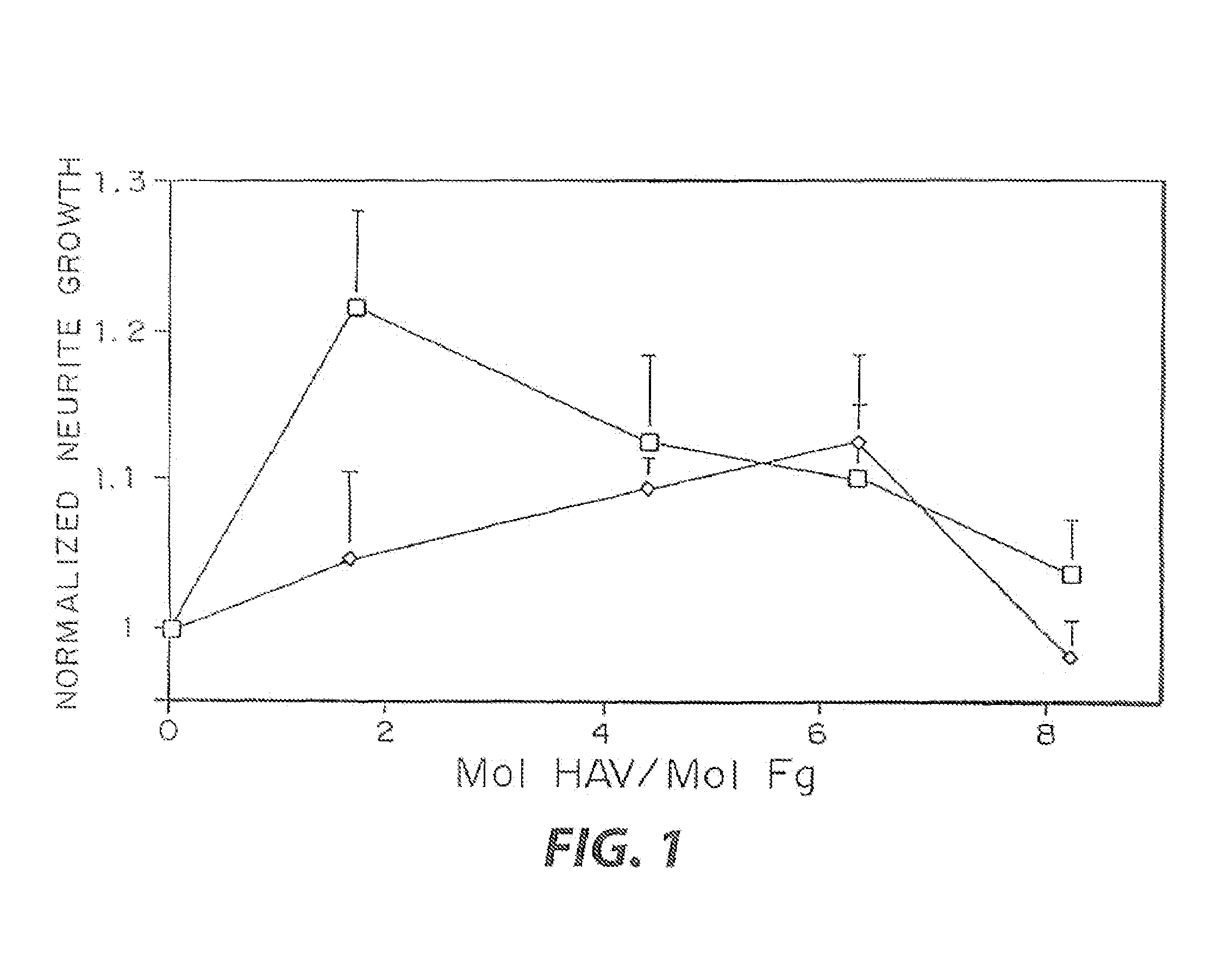
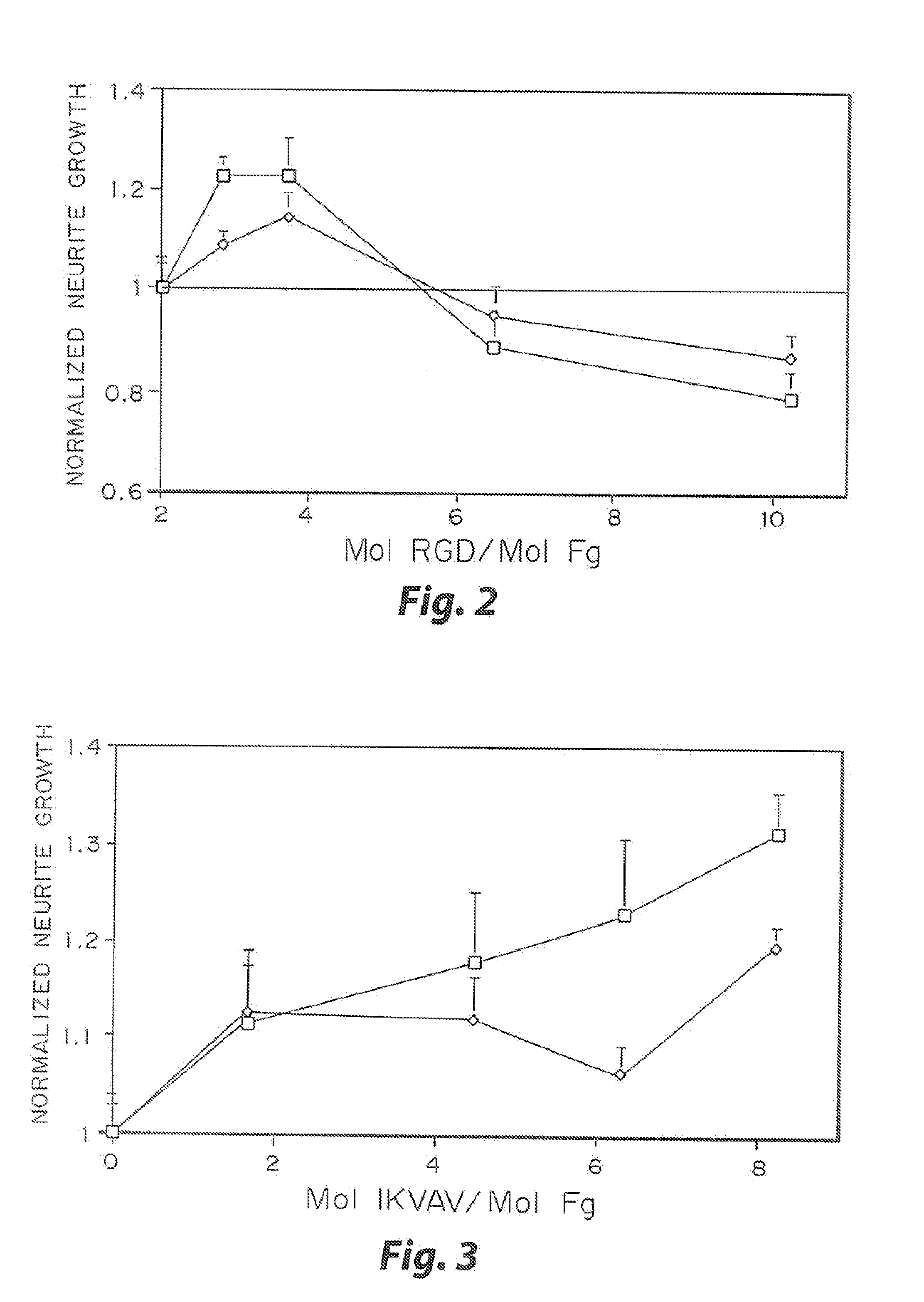
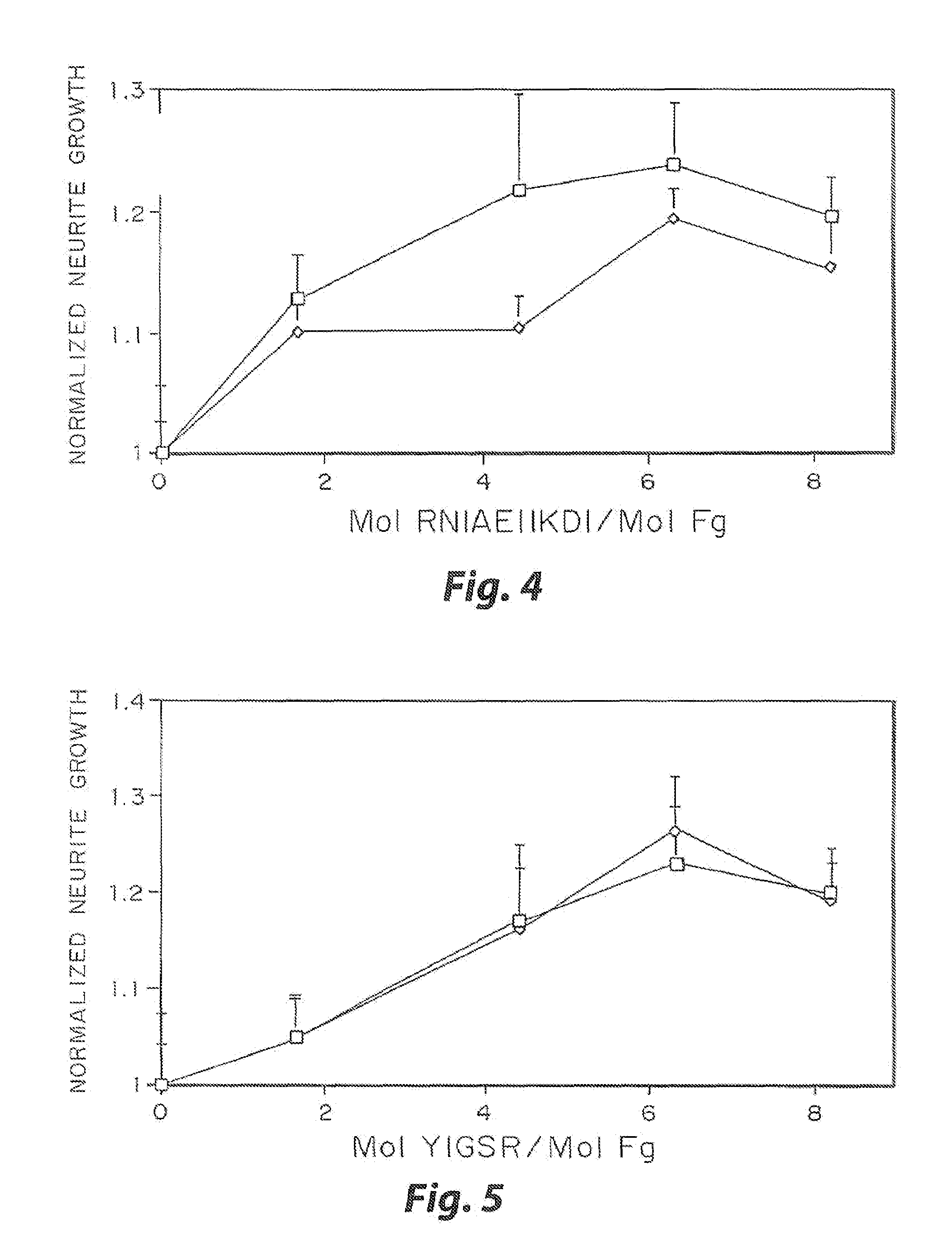
InactiveUS7241730B2Efficacious platformEnhanced andPeptide/protein ingredientsTransferasesCell Surface ProteinsADAMTS Proteins
Heparin-binding regions of several proteins, such as neural cell adhesion molecule, fibronectin, laminin, midkine, and anti-thrombin III have been shown to promote neurite extension on two-dimensional surfaces. The effect of heparin-binding peptides on neurite extension through three-dimensional matrices was investigated by culturing embryonic chick dorsal root ganglia (DRG) within fibrin gels containing chemically attached heparin-binding peptide (HBP). The length of neurites within fibrin gels containing cross-linked HBP was increased by more than 70% over extension through fibrin gels containing no peptide. The HBP sequence of antithrombin III was incorporated into the fibrin gel as the C-terminal domain of a bidomian, chimeric peptide; the N-terminal second domain of this peptide contained the ∀2-plasmin inhibitor substrate for Factor XIIIa. Factor XIIIa, a transglutaminase, was used to chemically attach the HBP-containing chimeric peptide to the fibrin gels during polymerization. The amount of HBP cross-linked into the fibrin gels was determined, after degradation by plasmin using gel permeation chromatography, to be approximately 8 moles of peptide per mole fibrinogen. A peptide (HBP), where the cross-linking glutamine was replaced with glycine, showed no increase in extension in comparison with fibrin gels. The additional of heparin to the gel percursors resulted in no increase in neurite extension in comparison with fibrin gels. HBPs promote neurite extension by binding to cell surface proteoglycans on the DRG.
Owner:UNIV ZURICH +1
Methods and compositions for the treatment of fibrotic conditions & impaired lung function & to enhance lymphocyte production
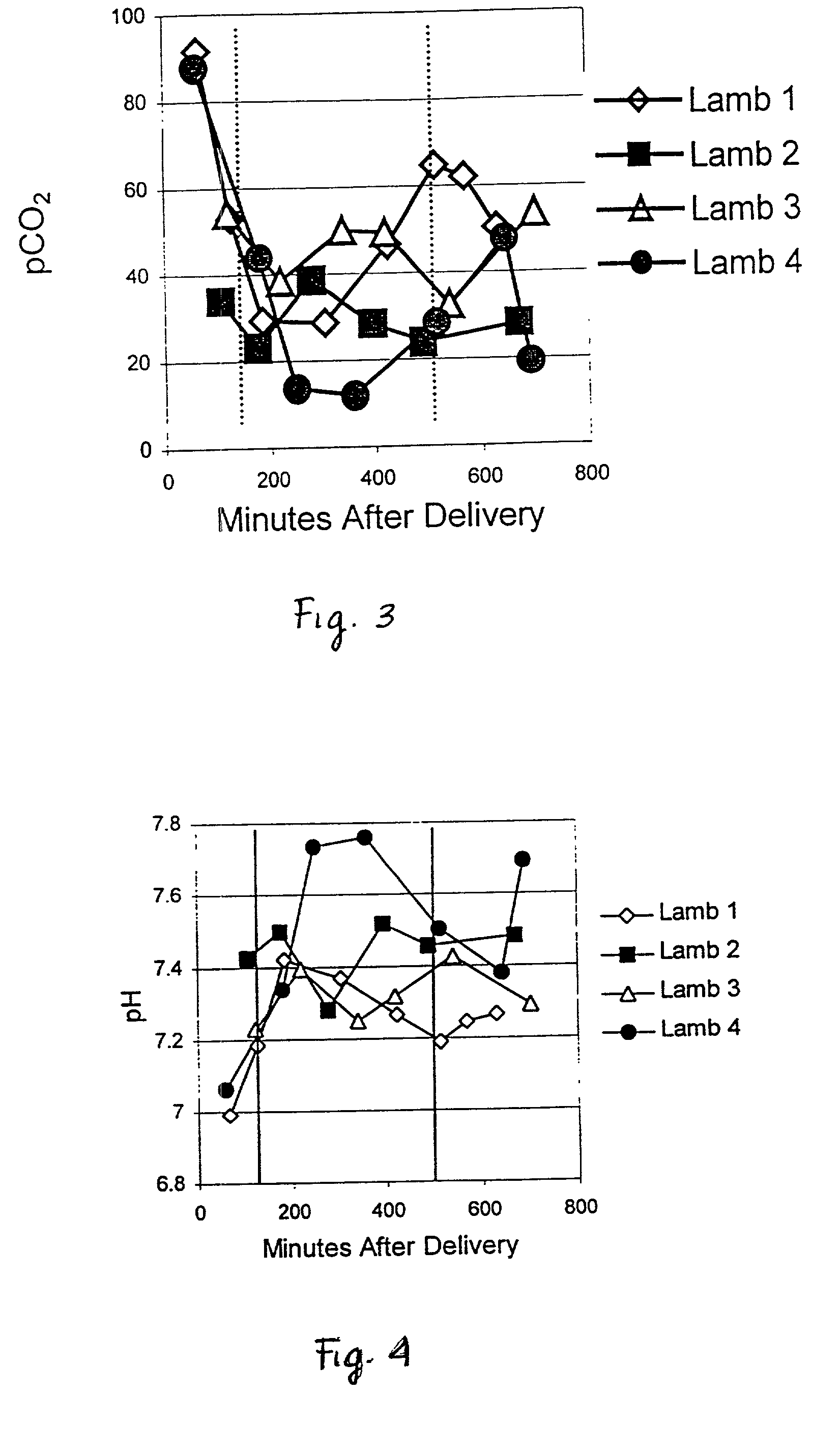
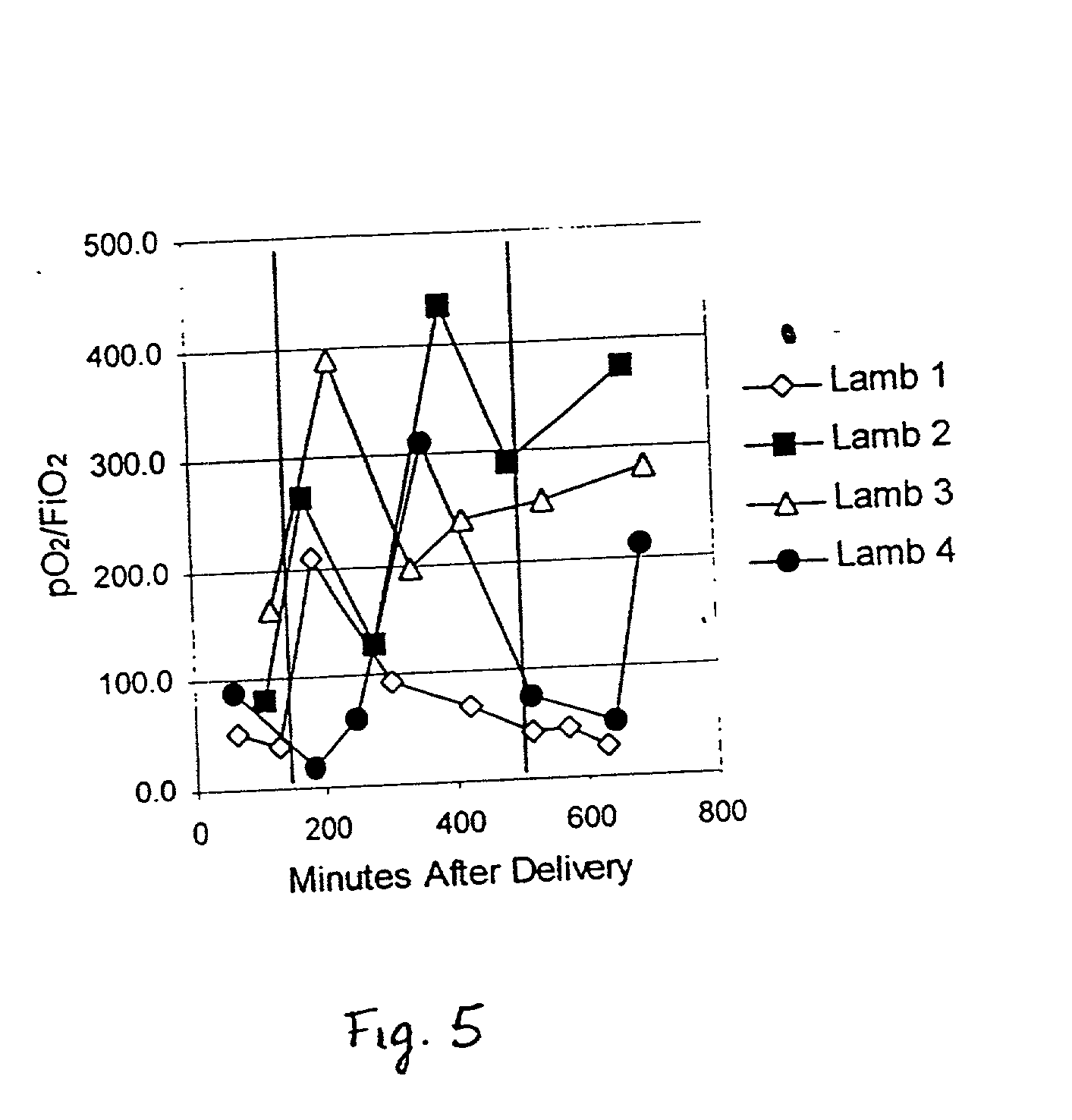
InactiveUS20030008816A1Improve complianceFunction increaseAntibacterial agentsCosmetic preparationsCell adhesionPulmonary compliance
The present invention provides methods and compositions to treat fibrotic conditions, to increase lymphocyte production in vivo, and to improve and / or normalize lung function, pulmonary compliance, blood oxygenation, and blood pH to inhibit inflammatory processes to stimulate or inhibit pro-inflammatory and immune cells, and to inhibit migration of vascular endothelial cells. The invention contemplates the administration of human uteroglobin, native or recombinant, as a means of achieving these ends. Specifically, it has been found that uteroglobin inhibits cell adhesion to fibronectin, increases lymphocyte production in vivo, and improves and / or normalizes lung function, pulmonary compliance, blood oxygenation, and blood pH, and inhibits inflammatory process. In addition it has been found that uteroglobin can stimulate or inhibit pro-inflammatory and immune cells and inhibitor migration of vascular endothelial cells.
Owner:CC10 SWEDEN +4
Efficient multiplication CTL preparation method killing tumors in targeted mode
InactiveCN103923880AEfficient proliferative abilityInhibition of differentiationBlood/immune system cellsSerum free mediaPeripheral blood mononuclear cell
The invention discloses an efficient multiplication CTL preparation method killing tumors in a targeted mode. The CTL preparation method comprises the following steps: (a) removing CD4+CD25+Treg cells through immunomagnetic bead negative sorting; (b) arranging mixed cells in a serum-free medium for cultivation, and obtaining suspension cells and adherent cells; (c) adding GM-SCF and IL-4 in the adherent cells, culturing the cells for five days; in the sixth day, adding a tumour cell holoantigen, and in the seventh day, adding TNF-alpha and IL-27; (d) transferring the suspension cells to a culture flask wrapped by a CD3 monoclonal antibody and recombinant human fibronectin, adding IFN-gamma, in the second day, adding IL-2, IL-12 and the IL-27, and culturing the mixture till the eighth day to obtain CIK cells; (e) mixing the CIK cells and mature DC cells, and adding the IL-12, IL-7 and an anti-CD 28 monoclonal antibody for cultivation; in the third day, adding an anti-CTLA-4 monoclonal antibody, and then culturing the mixture for four days. According to the efficient multiplication CTL preparation method killing tumors in the targeted mode, efficiency of in-vitro CTL cell proliferation is improved, activity of killing the tumor cells in the targeted mode is improved, transformation of peripheral blood mononuclear cells to the CD4+CD25+Treg cells is inhibited.
Owner:四川全组生命科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com