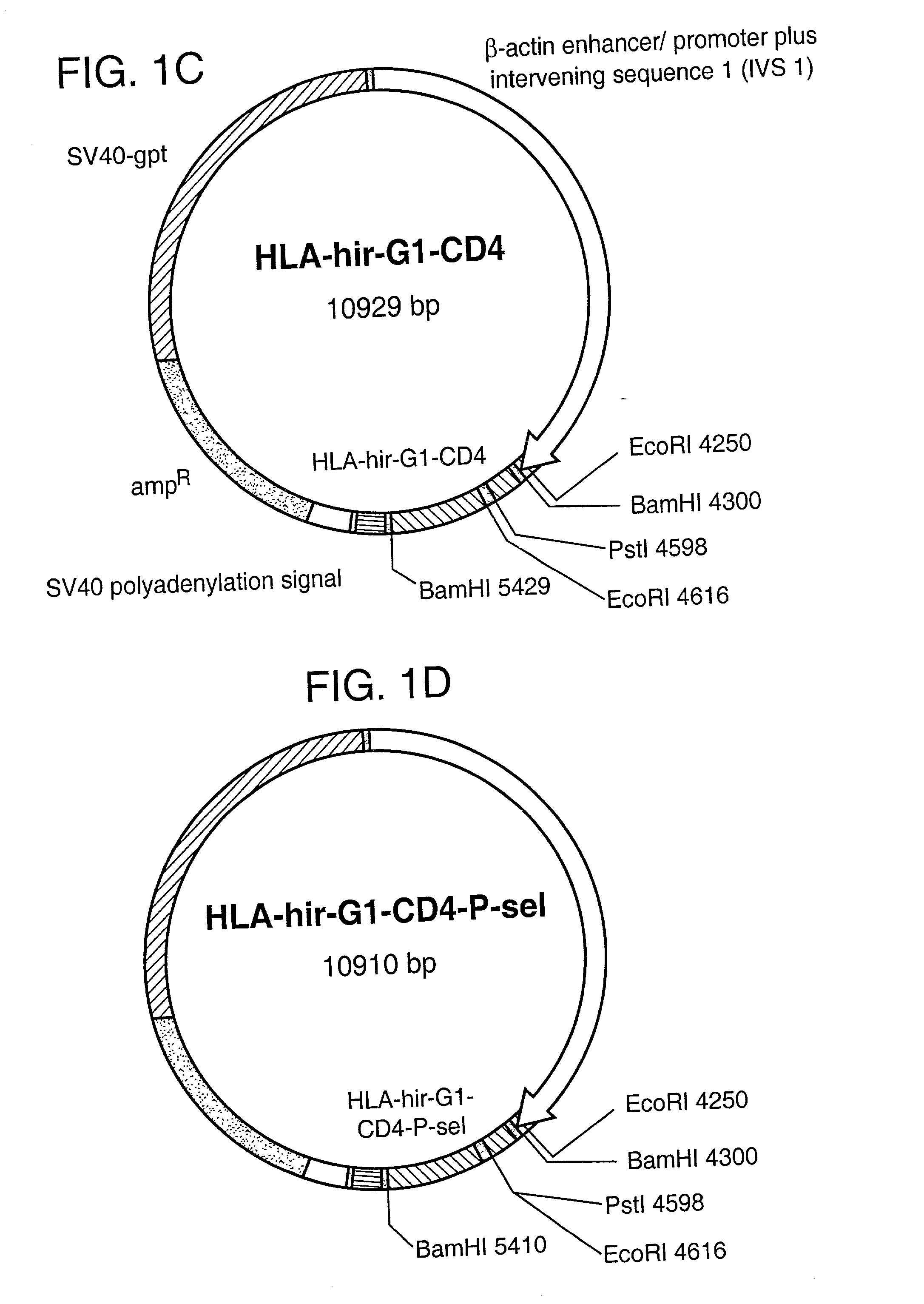
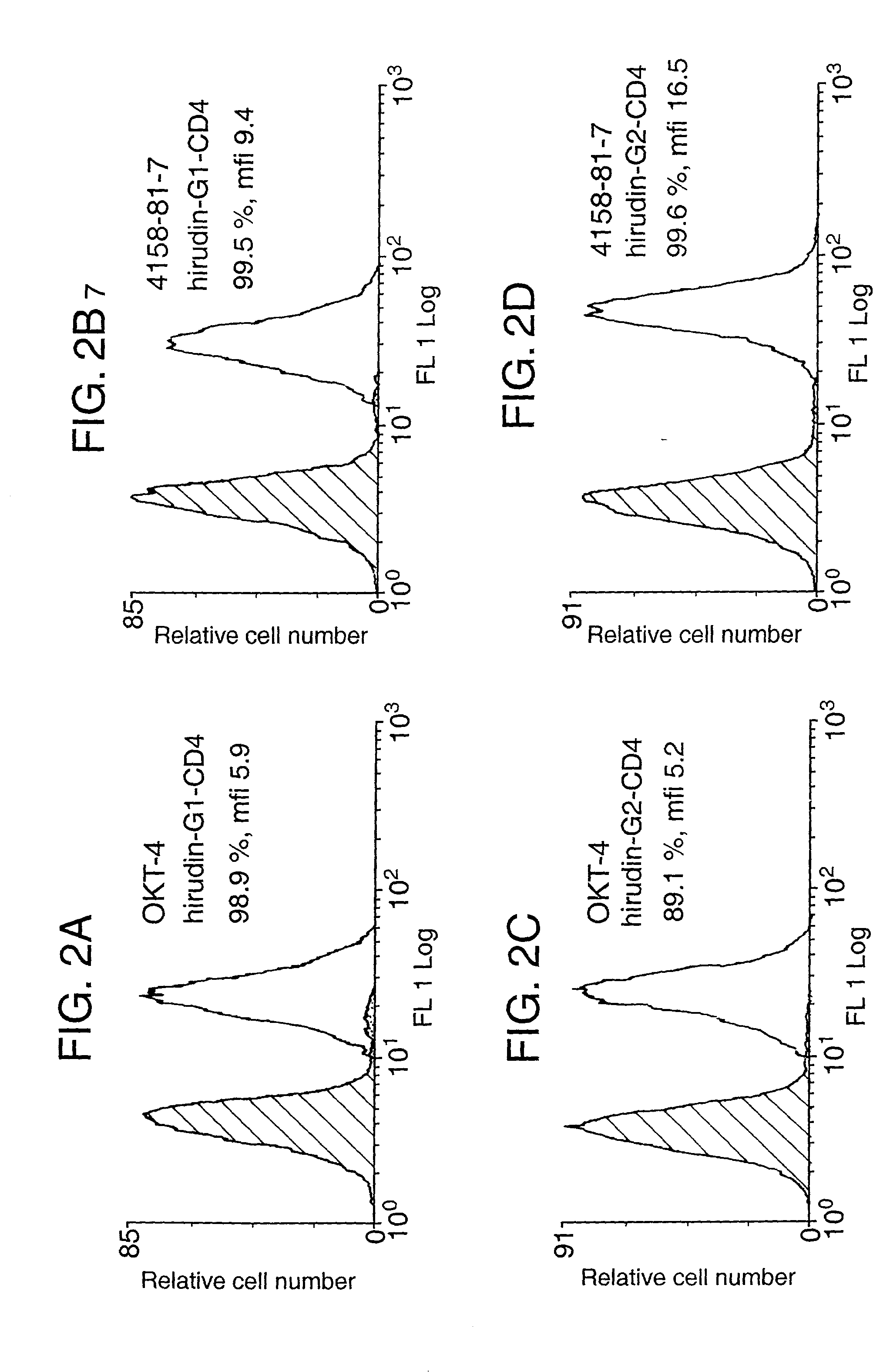
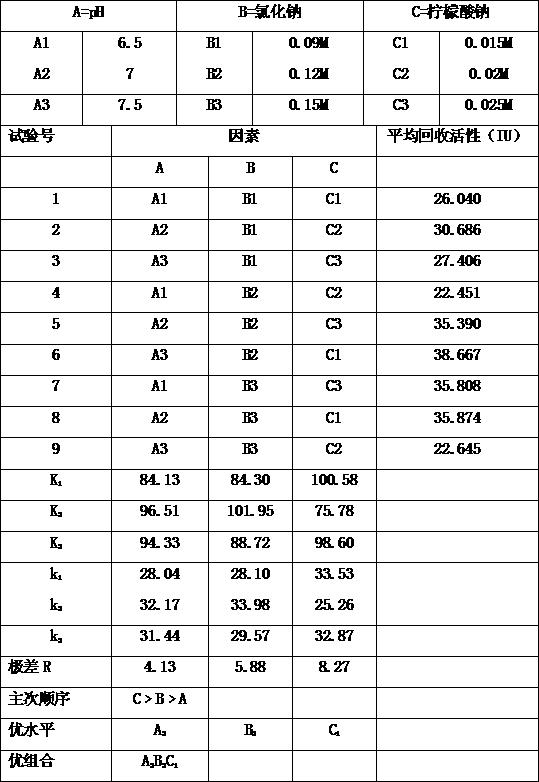
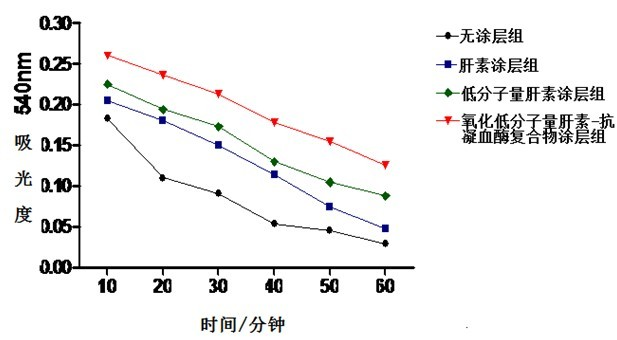
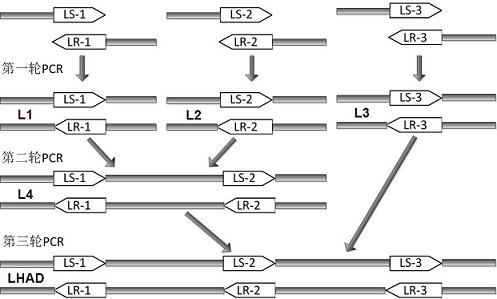
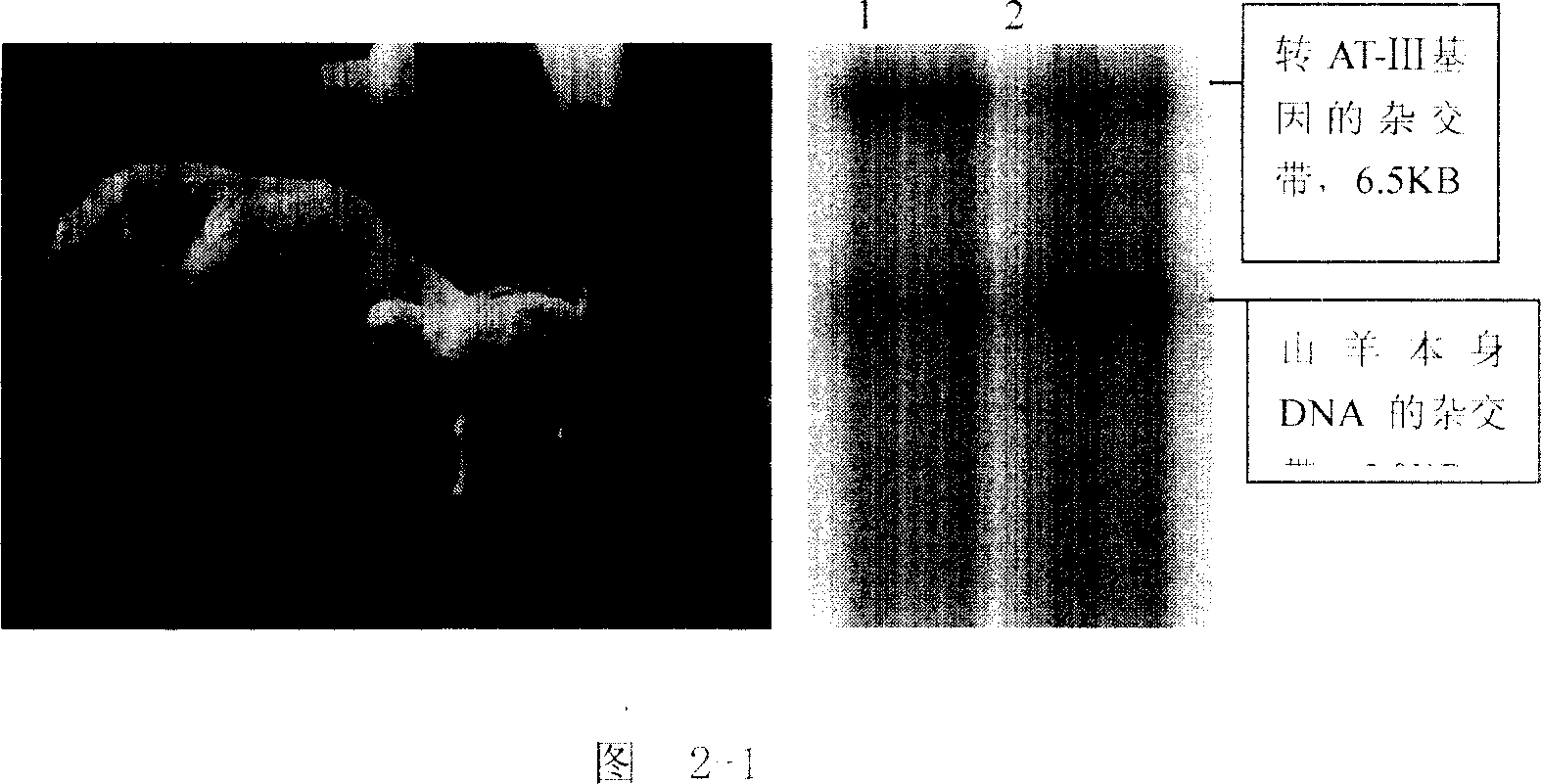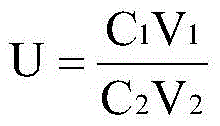Patents
Literature
79 results about "Antithrombin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
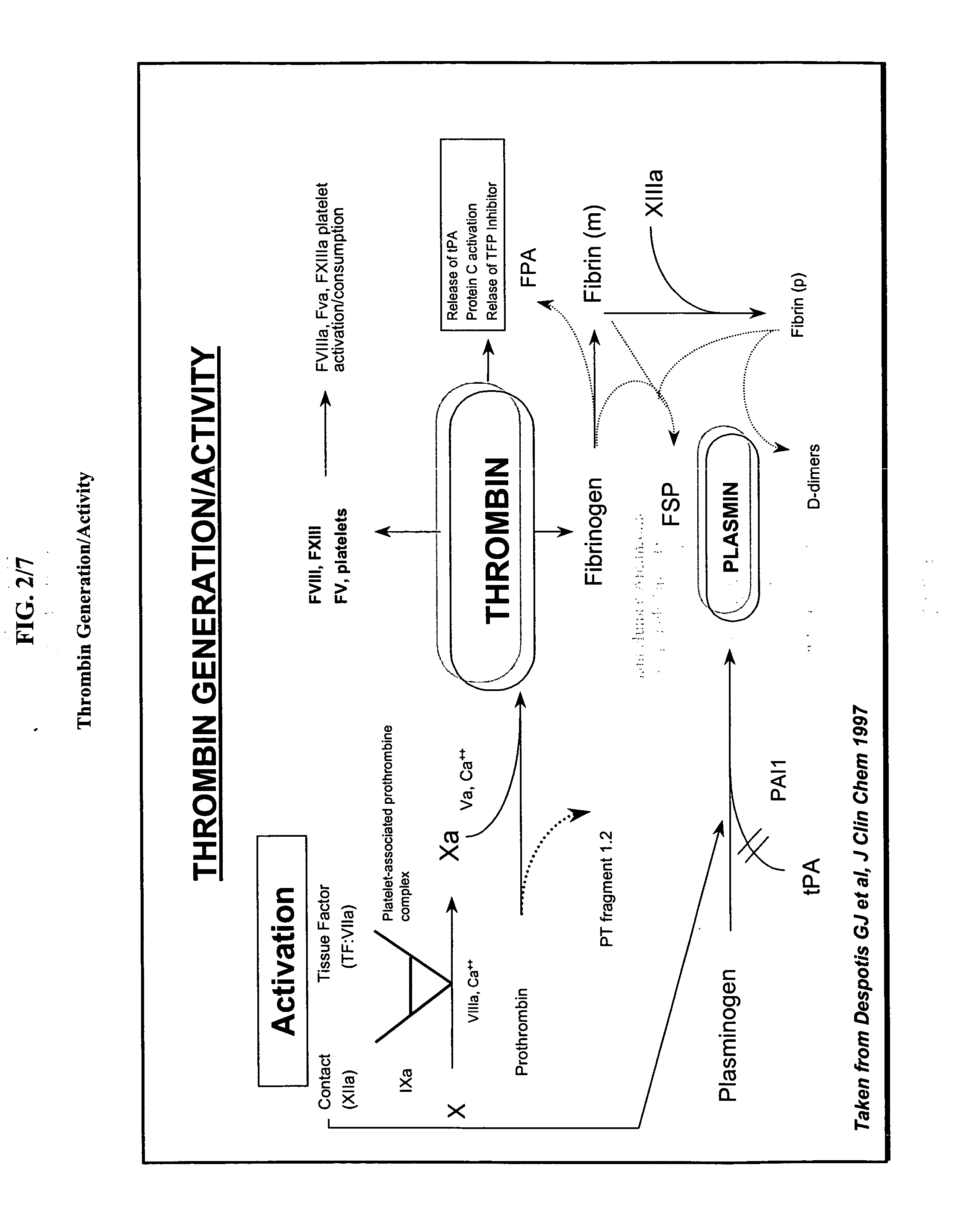
Antithrombin (AT) is a small protein molecule that inactivates several enzymes of the coagulation system. Antithrombin is a glycoprotein produced by the liver and consists of 432 amino acids. It contains three disulfide bonds and a total of four possible glycosylation sites. α-Antithrombin is the dominant form of antithrombin found in blood plasma and has an oligosaccharide occupying each of its four glycosylation sites. A single glycosylation site remains consistently un-occupied in the minor form of antithrombin, β-antithrombin. Its activity is increased manyfold by the anticoagulant drug heparin, which enhances the binding of antithrombin to factor IIa (Thrombin) and factor Xa.
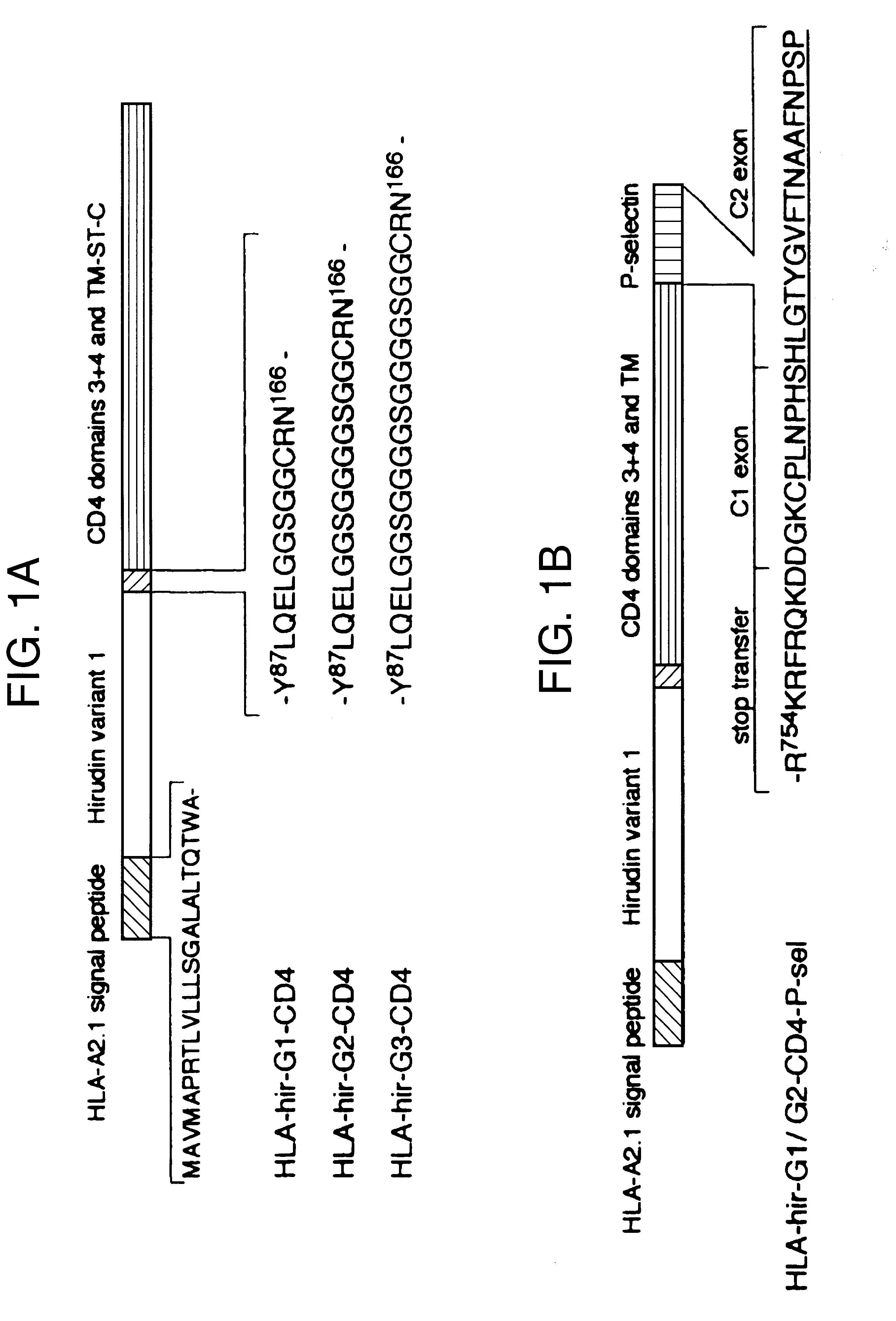
Anticoagulant fusion protein anchored to cell membrane
InactiveUS6423316B1Prolong clotting timeGood curative effectFungiVirusesCell membraneBlood coagulations
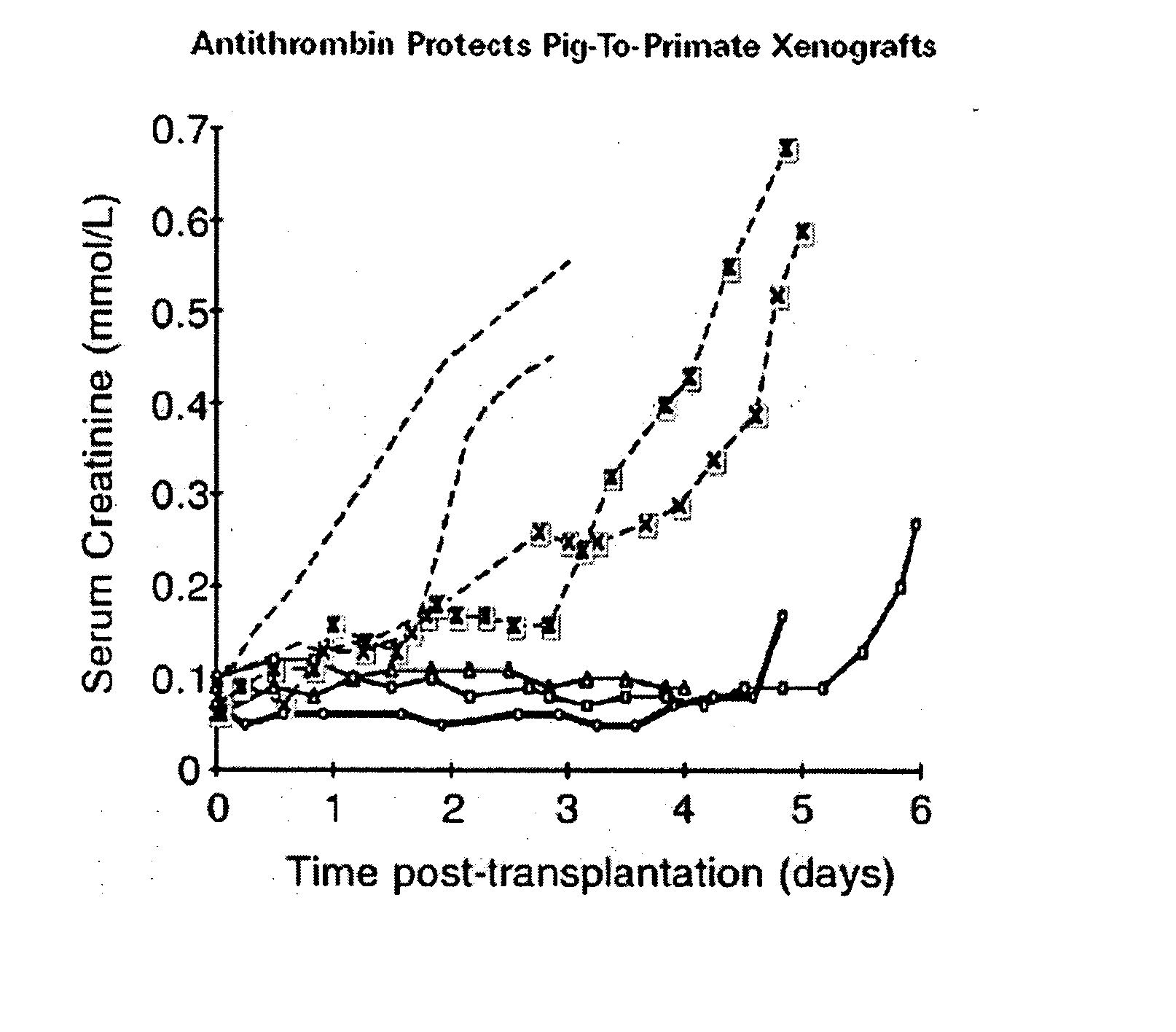
The invention relates to the inhibition of blood coagulation, especially during organ rejection, and in particular the inhibition of delayed vascular rejection. The invention provides anticoagulant proteins which are anchored to cell membranes. The anticoagulant function preferably provided by heparin, antithrombin, hirudin, TFPI, tick anticoagulant peptide, or a snake venom factor. These anticoagulant proteins are preferably prevented from being constitutively expressed at the cell surface. In particular, expression at the cell surface is regulated according to cell activation, for instance by targeting the protein to a suitable secretory granule. Expression of these proteins renders cells, tissues and organs less vulnerable to rejection after transplantation (e.g. after xenotransplantation).
Owner:IMPERIAL INNOVATIONS LTD
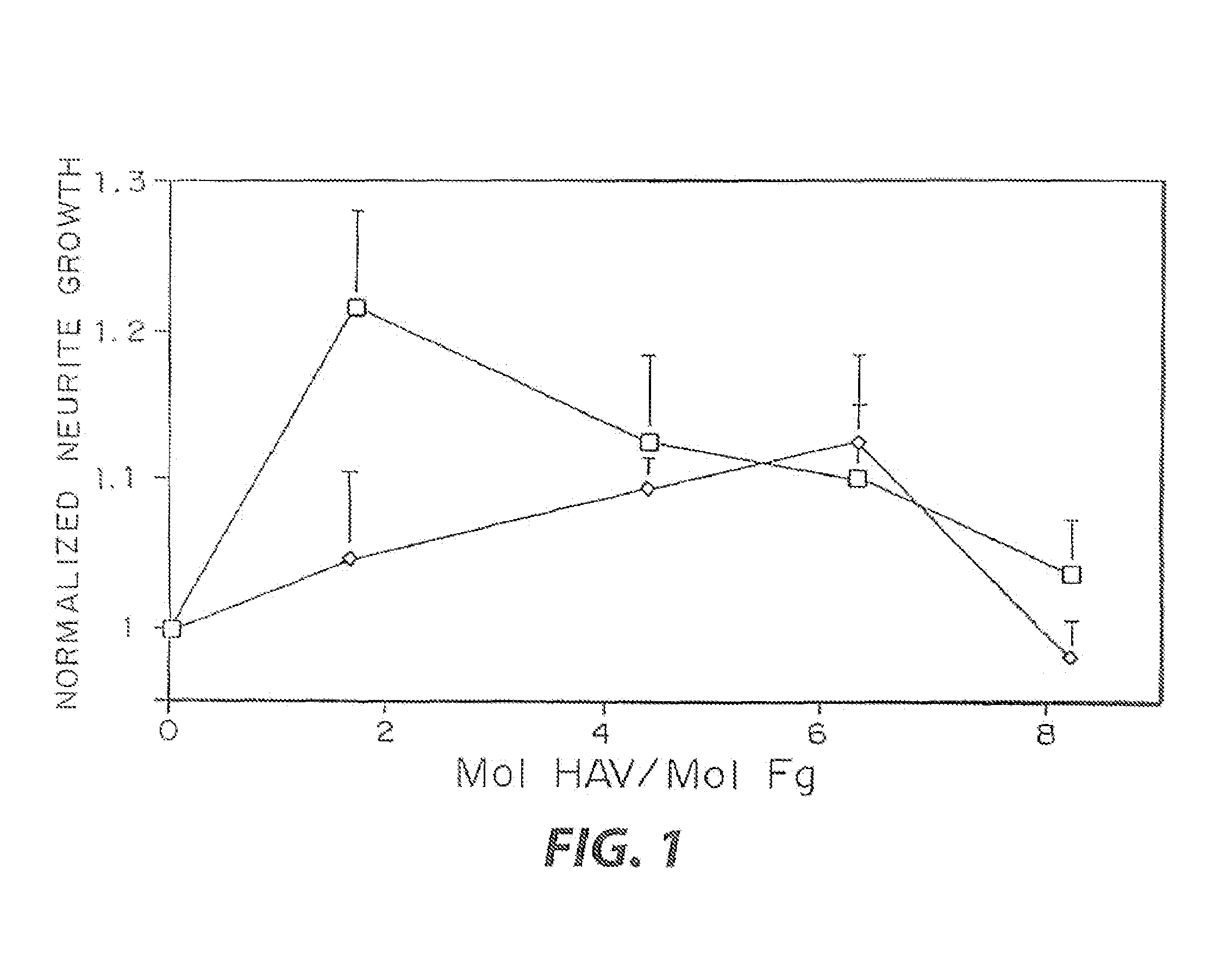
Enzyme-mediated modification of fibrin for tissue engineering: fibrin formulations with peptides
InactiveUS7241730B2Efficacious platformEnhanced andPeptide/protein ingredientsTransferasesCell Surface ProteinsADAMTS Proteins
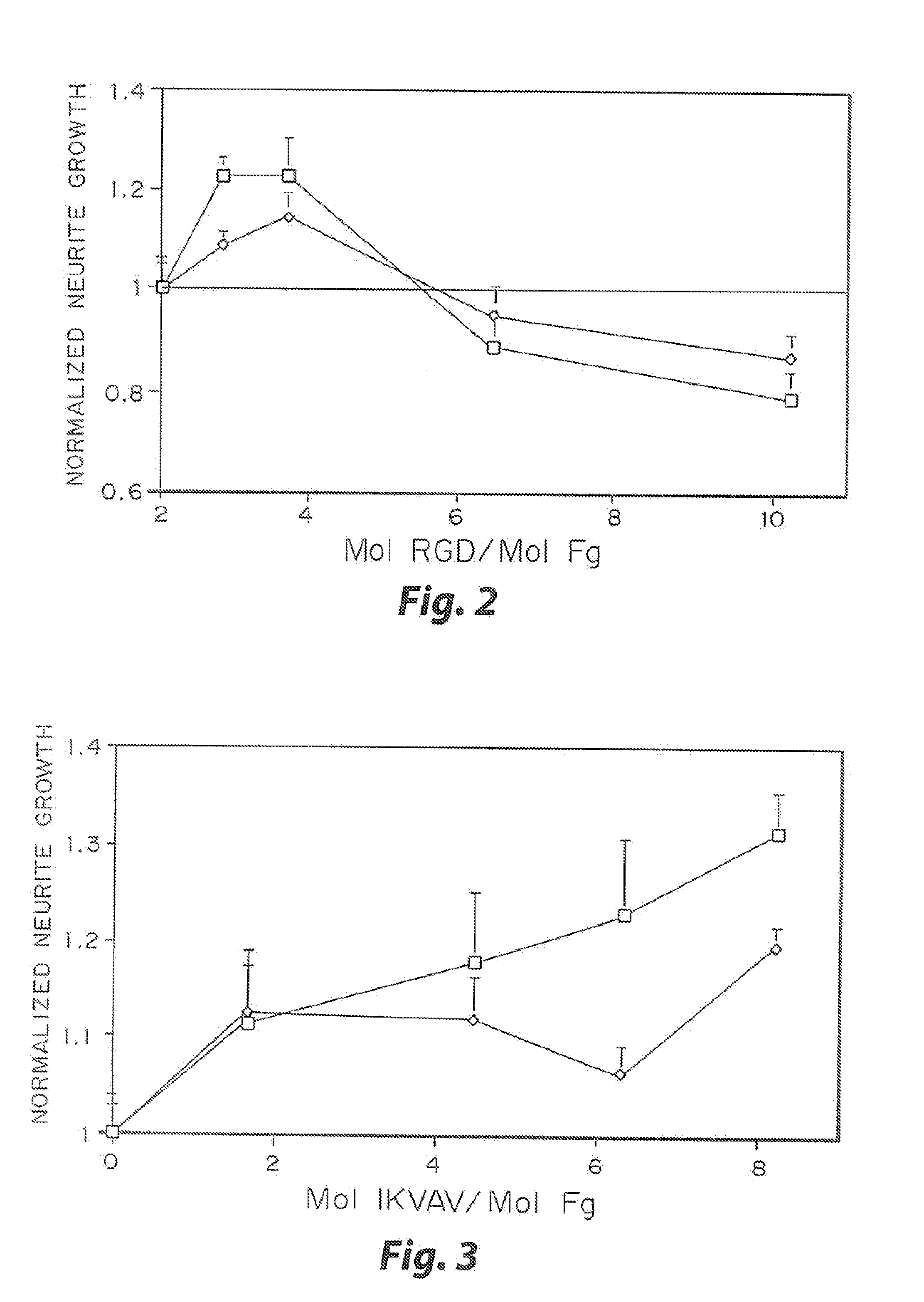
Heparin-binding regions of several proteins, such as neural cell adhesion molecule, fibronectin, laminin, midkine, and anti-thrombin III have been shown to promote neurite extension on two-dimensional surfaces. The effect of heparin-binding peptides on neurite extension through three-dimensional matrices was investigated by culturing embryonic chick dorsal root ganglia (DRG) within fibrin gels containing chemically attached heparin-binding peptide (HBP). The length of neurites within fibrin gels containing cross-linked HBP was increased by more than 70% over extension through fibrin gels containing no peptide. The HBP sequence of antithrombin III was incorporated into the fibrin gel as the C-terminal domain of a bidomian, chimeric peptide; the N-terminal second domain of this peptide contained the ∀2-plasmin inhibitor substrate for Factor XIIIa. Factor XIIIa, a transglutaminase, was used to chemically attach the HBP-containing chimeric peptide to the fibrin gels during polymerization. The amount of HBP cross-linked into the fibrin gels was determined, after degradation by plasmin using gel permeation chromatography, to be approximately 8 moles of peptide per mole fibrinogen. A peptide (HBP), where the cross-linking glutamine was replaced with glycine, showed no increase in extension in comparison with fibrin gels. The additional of heparin to the gel percursors resulted in no increase in neurite extension in comparison with fibrin gels. HBPs promote neurite extension by binding to cell surface proteoglycans on the DRG.
Owner:UNIV ZURICH +1
Compositions, methods and kits relating to thrombin degradation resistant fibroblast growth factor-1
InactiveUS6982170B1Improve efficiencyEnhancing FGF- responseSugar derivativesTissue cultureDiseaseThrombin activity
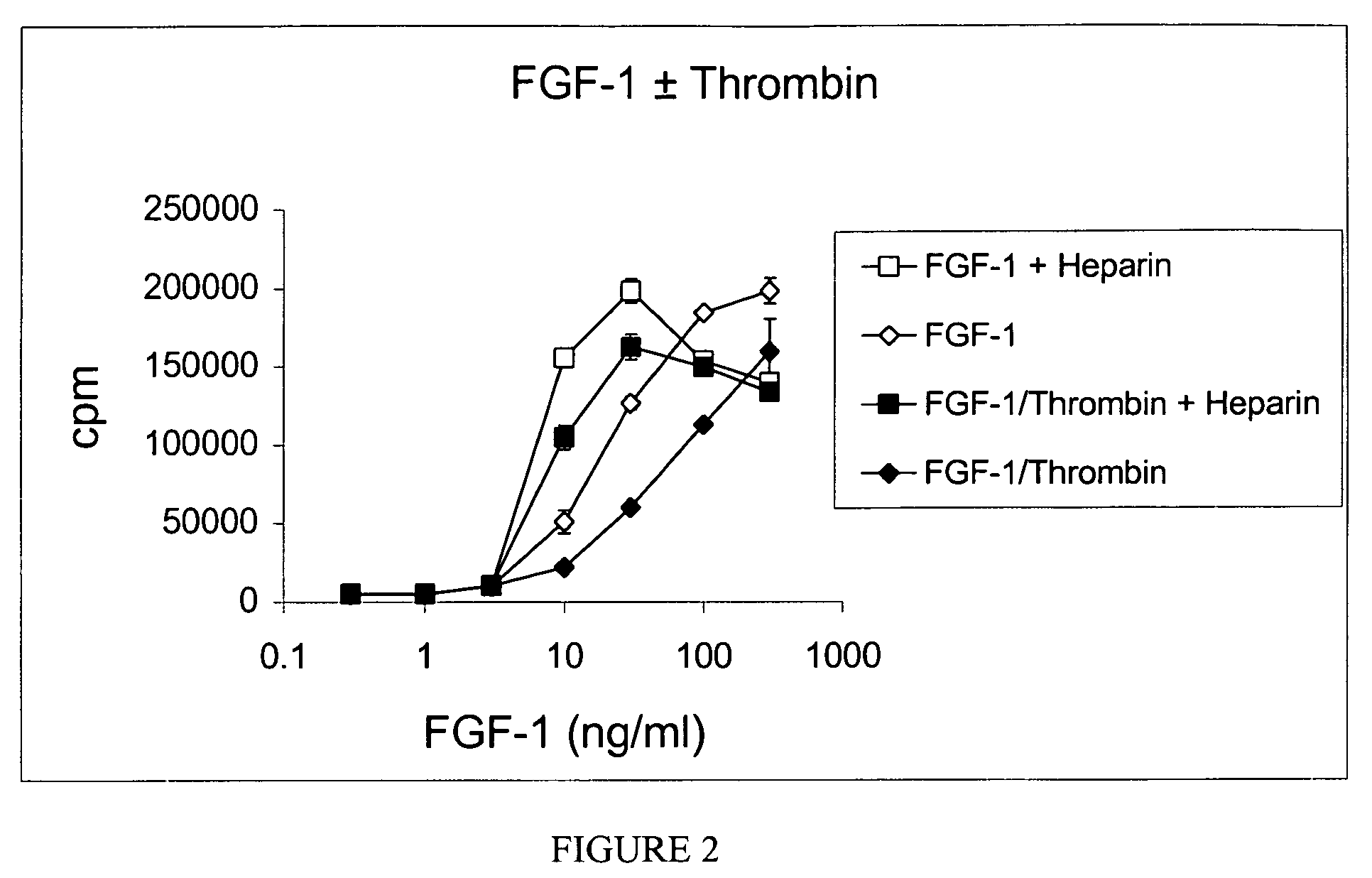
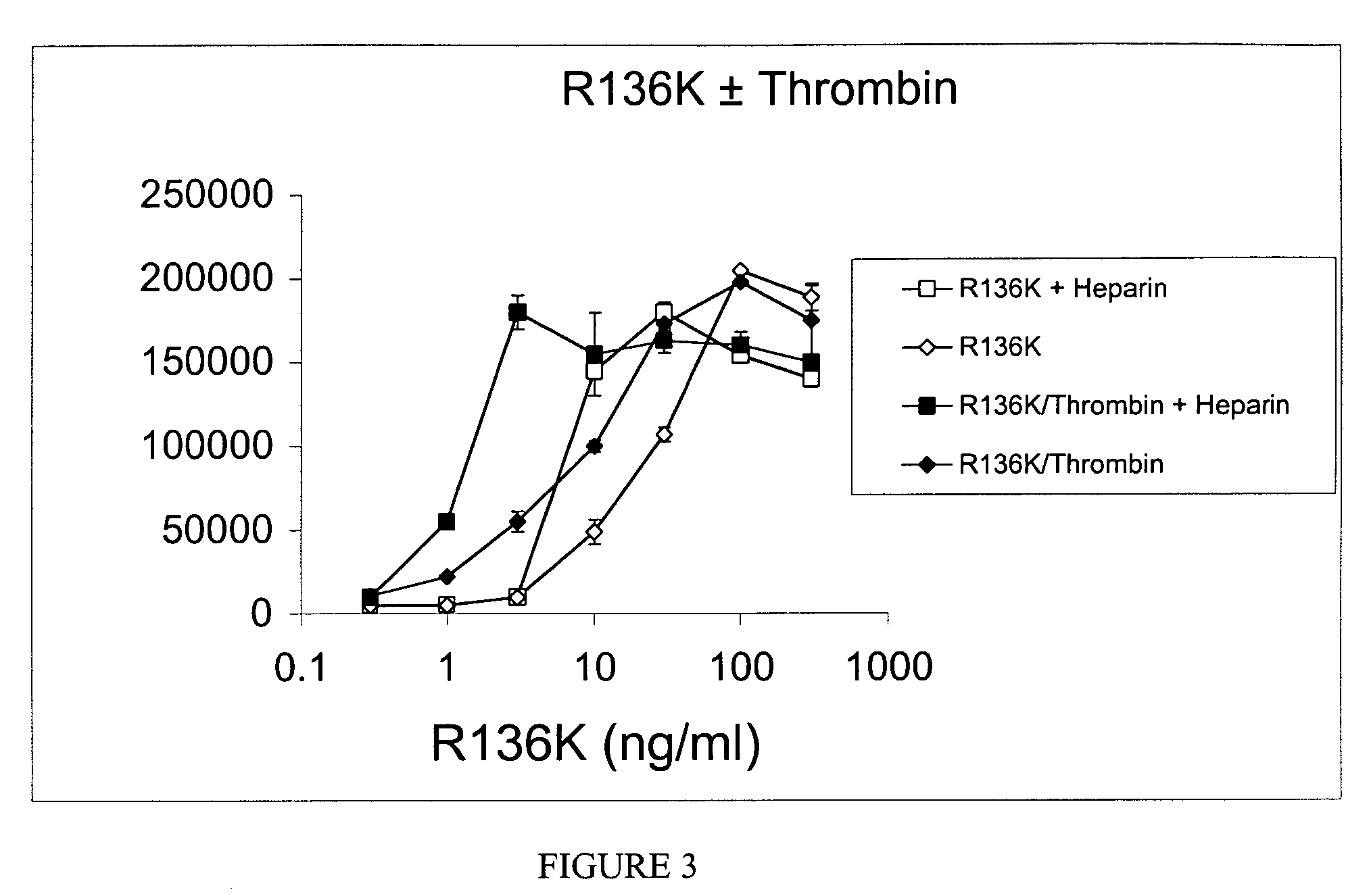
The invention relates to novel degradation resistant FGF-1, and methods for producing and using the same. More specifically, the invention relates to identification of a thrombin degradation resistant FGF-1, an a nucleic acid encoding the same. The thrombin degradation resistant FGF-1 can elicit responses that are otherwise typically impeded by degradation of FGF-1 by thrombin. Thrombin degradation resistant FGF-1 is an important molecule for effecting an FGF-1 response that would be otherwise inhibited by thrombin. Thus, the present invention provides a powerful therapeutic for diseases or disorders wherein an FGF-1 response can mediate a reduction in the frequency or intensity of a symptom of the disease or disorder but for degradation of FGF-1 before it can effect the response.
Owner:MAINE MEDICAL CENT +1
Combination and method using EDTA, cystine, zinc and selenium for anti-thrombin effect and for anti-platelet aggregation and measurement of efficacy
InactiveUS20020182585A1Increased platelet depositionIncrease blood flowOrganic active ingredientsBiocideEtiologyVitamin C
The invention is for the combination of EDTA, cystine, selenium, Vitamin C, Vitamin E, and zinc for anti-thrombotic effect and for the effect of restoring platelet aggregation, an integral component of thrombus formation, to normal and for the monitoring of the response to therapy with the combination. Methods for use of the components and method for performing the monitoring are included. The combination and method are particularly efficacious for vascular deficiency ailments including atherosclerotic vascular disease, reduction of ischemic cerebal event, complications from surgical procedures including restenosis, neurogenerative disease, and erectile disfunction, and vascular deficiency resulting from etiology of sepsis and chronic infection.
Owner:KINDNESS GEORGE +2
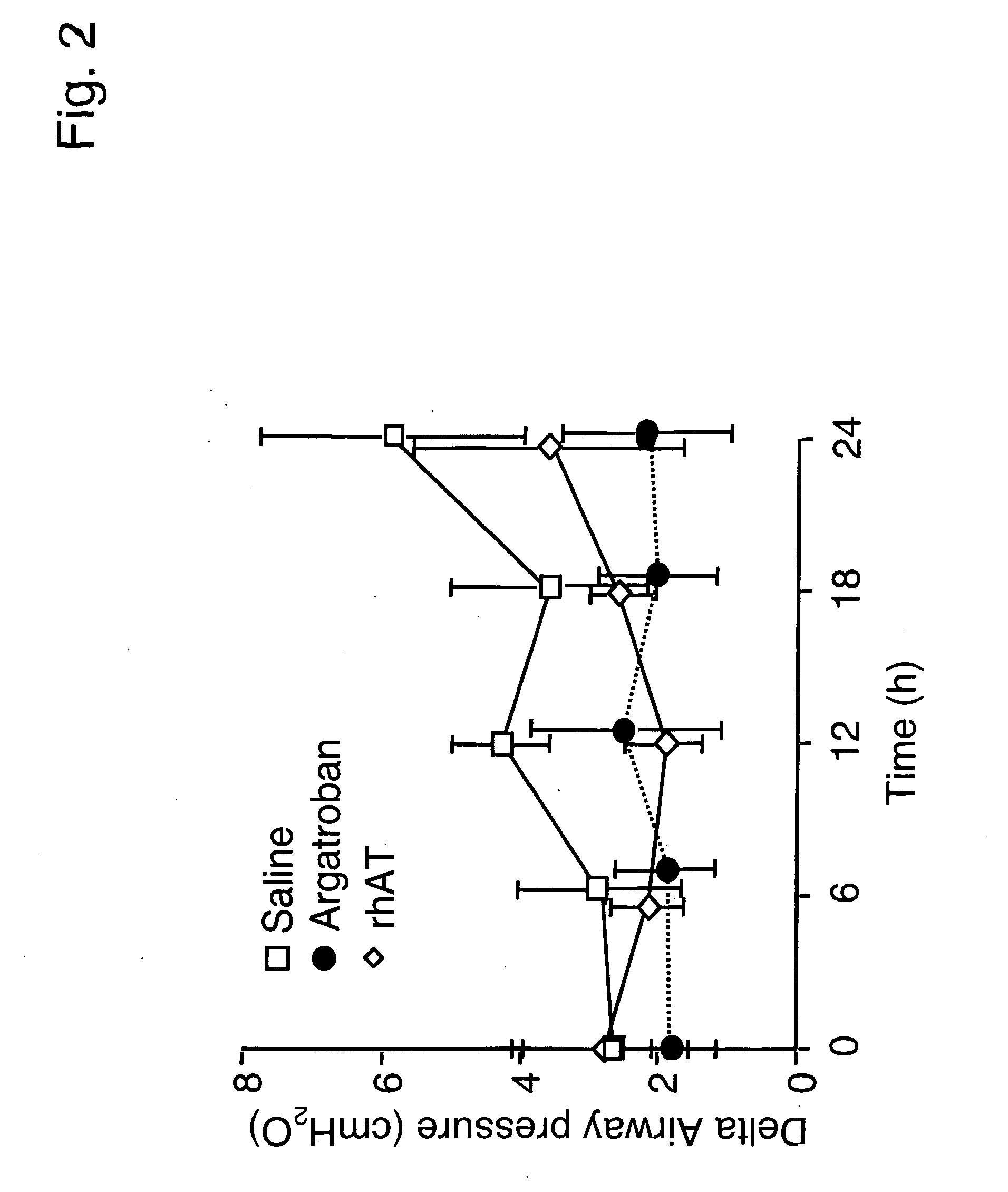
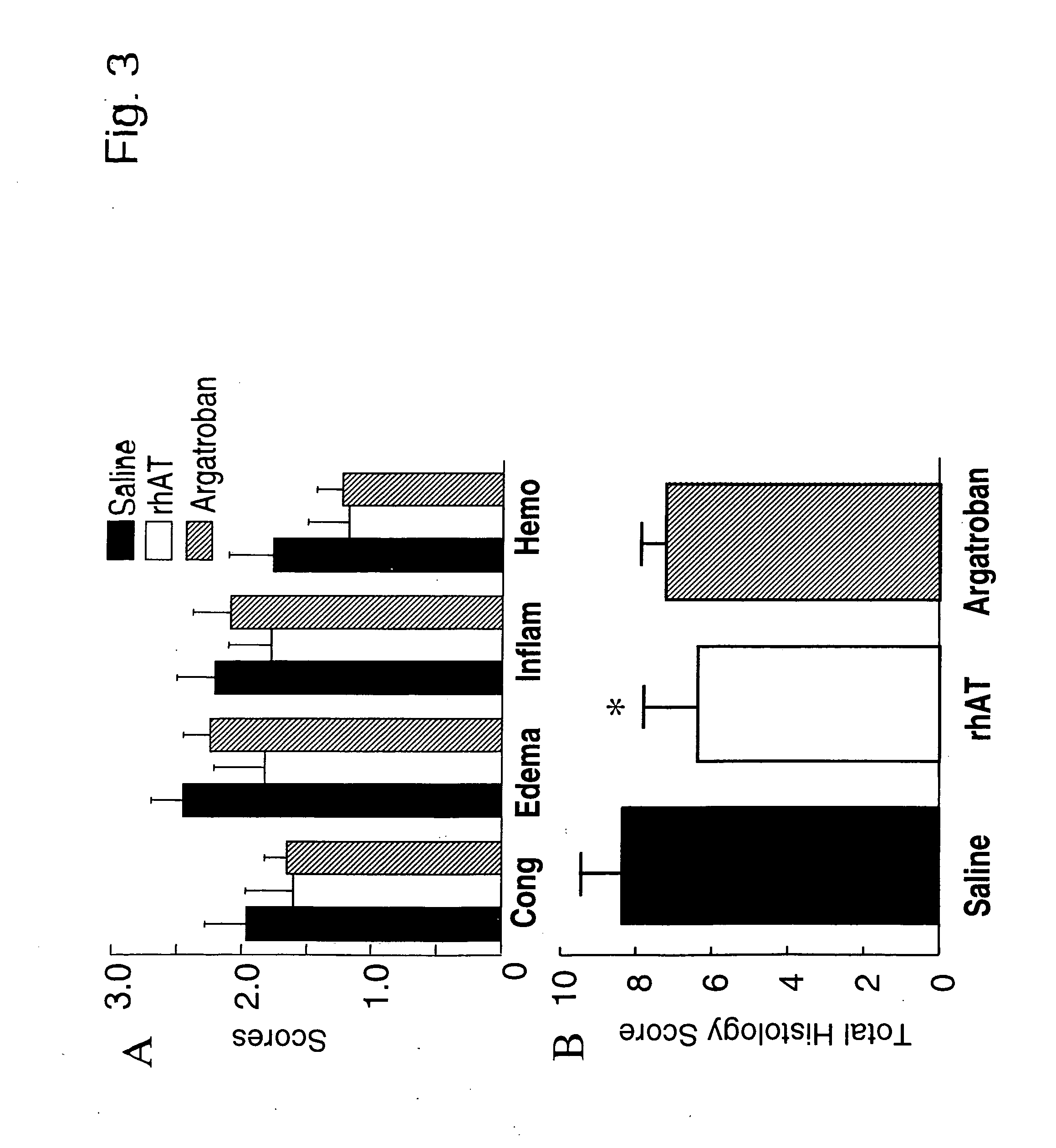
Use of aerosolized antithrombin to treat acute lung injury
InactiveUS20050169908A1Effective treatmentReduced gas exchangePeptide/protein ingredientsAerosol deliveryDiseaseALI - Acute lung injury
The invention features methods of treating a subject having a lung disorder such as lung inflammation and injury, by administering antithrombin III through pulmonary delivery.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST +1
Glycosaminoglycans derived from k5 polysaccharide having high anticoagulant and antithrombotic activities and process for their preparation
InactiveUS20110281820A1High activityReduce bleeding riskOrganic active ingredientsBiocideSulfationAntithrombotic Agent
Glycosaminoglycans derived from K5 polysaccharide having high anticoagulant and antithrombotic activity and useful for the control of coagulation and as antithrombotic agents are obtained starting from an optionally purified K5 polysaccharide by a process comprising the steps of N-deacetylation / N-sulfation, C5 epimerization, O-oversulfation, selective O-desulfation, 6-O-sulfation, N-sulfation, and optional depolymerization, in which said epimerization is performed with the use of the enzyme glucoronosyl C5 epimerase in solution or in immobilized form in the presence of divalent cations. New, particularly interesting antithrombin compounds are obtained by controlling the reaction time in the selective O-desulfation step and submitting the product obtained at the end of the final N-sulfation step to depolymerization.
Owner:GLYCORES 2000 SRL
Combination comprising thrombin agent and examining agent
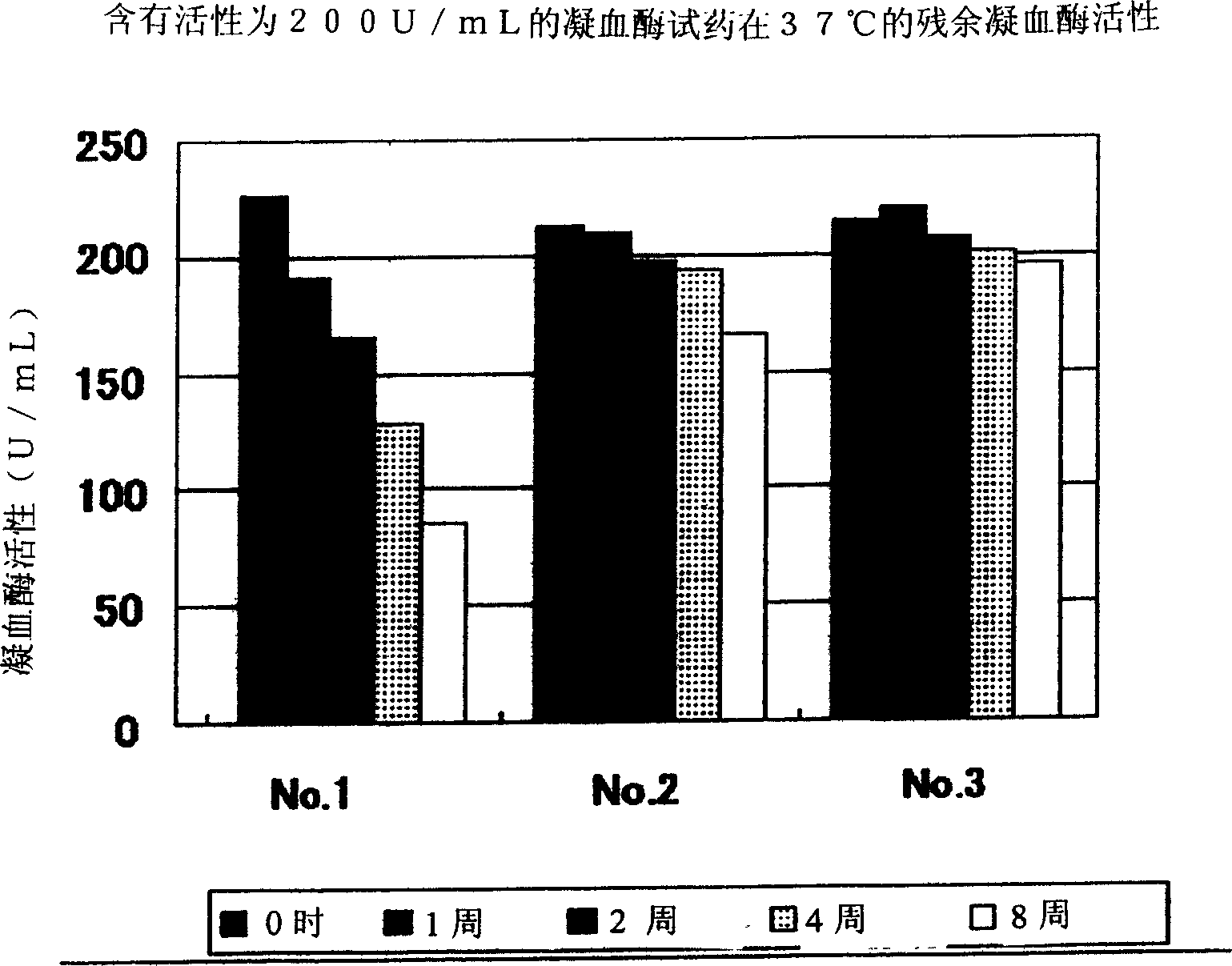
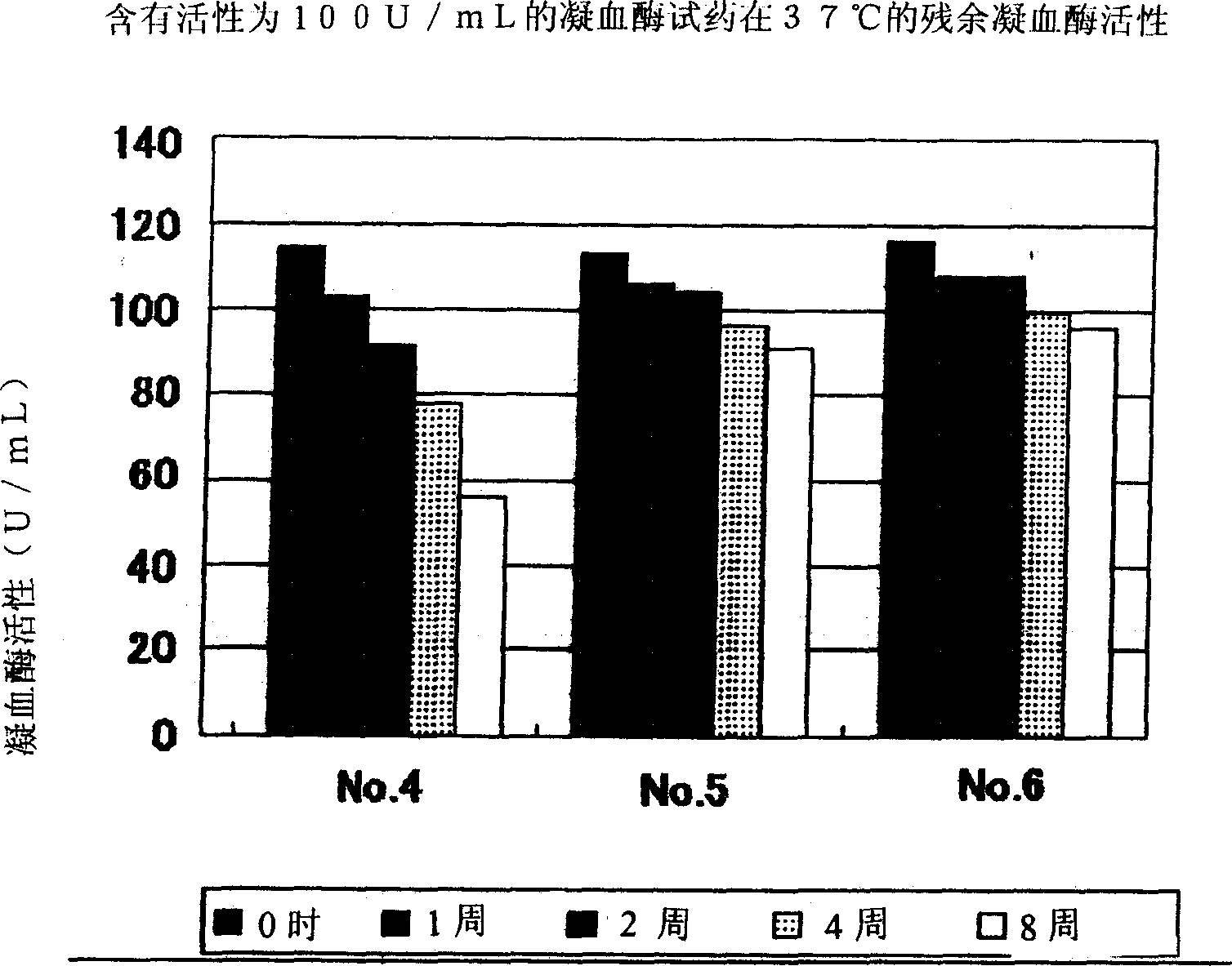
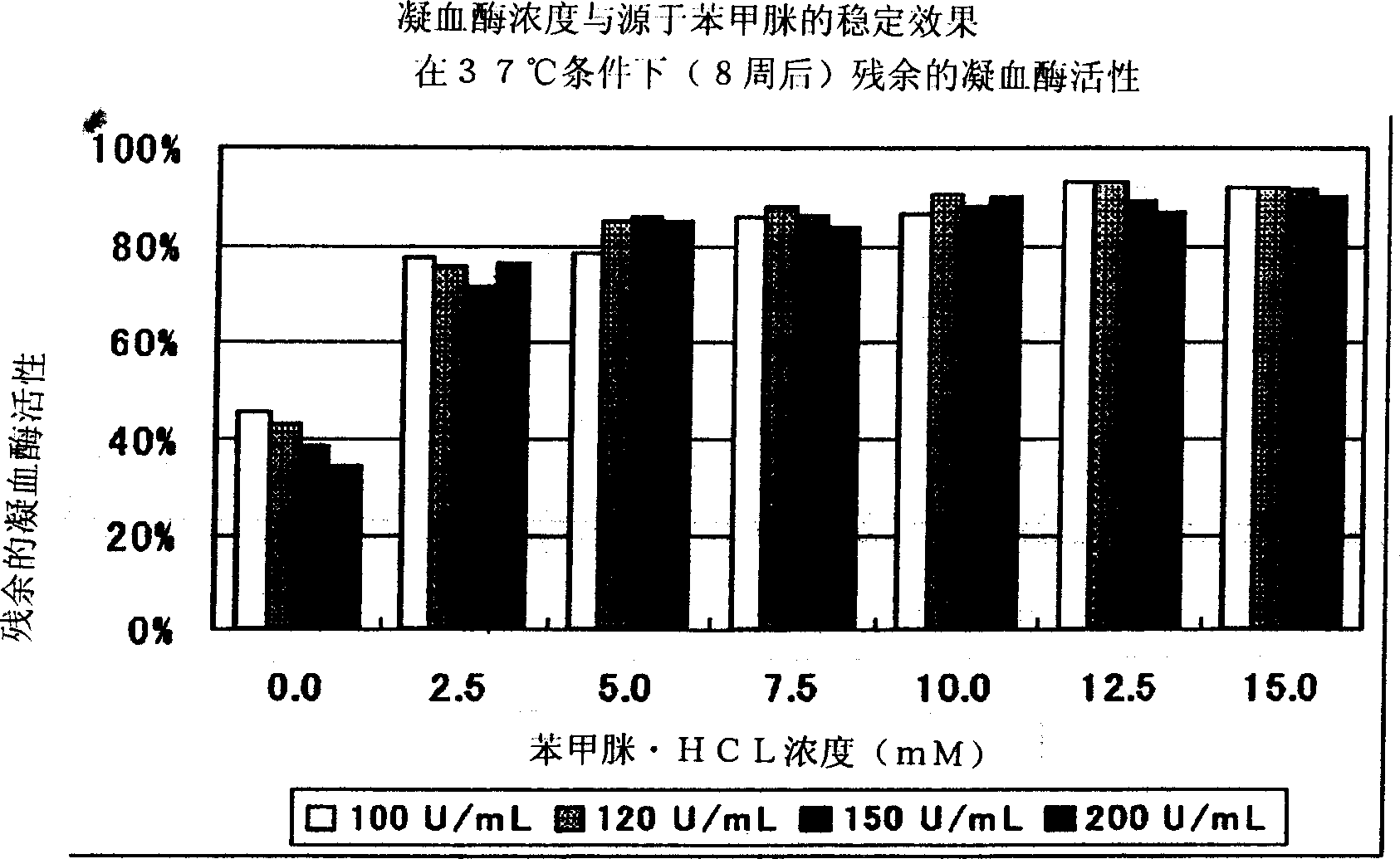
Provided is a stabilized thrombin containing a test reagent and the test reagent kit. The test reagent contains thrombin and an inhibitor having thrombin inhibiting effect, the inhibitor is a protease antagonist having thrombin inhibiting effect, and is preferably selected from the group consisting of benzamidine, p-aminobenzamidine, m-aminobenzamidine, phenylguanidine, argatroban, gabexate mesylate and nafamostat mesylate. The test reagent of the present invention can contain one or more compounds with thrombin stabilizing function, selected from the group consisting of a calcium ion, an organic acid, a surfactant and a protein. The test reagent is useful as a solidifying ability test reagent, especially, fibrinogen metering reagent.
Owner:SYSMEX CORP
Recombined dimerization antithrombin III-Fc fusion protein and mammalian cell efficient expression system thereof
ActiveCN102690354AHigh expressionSimplified purification stepsPeptide/protein ingredientsPharmaceutical non-active ingredientsSide effectHalf-life
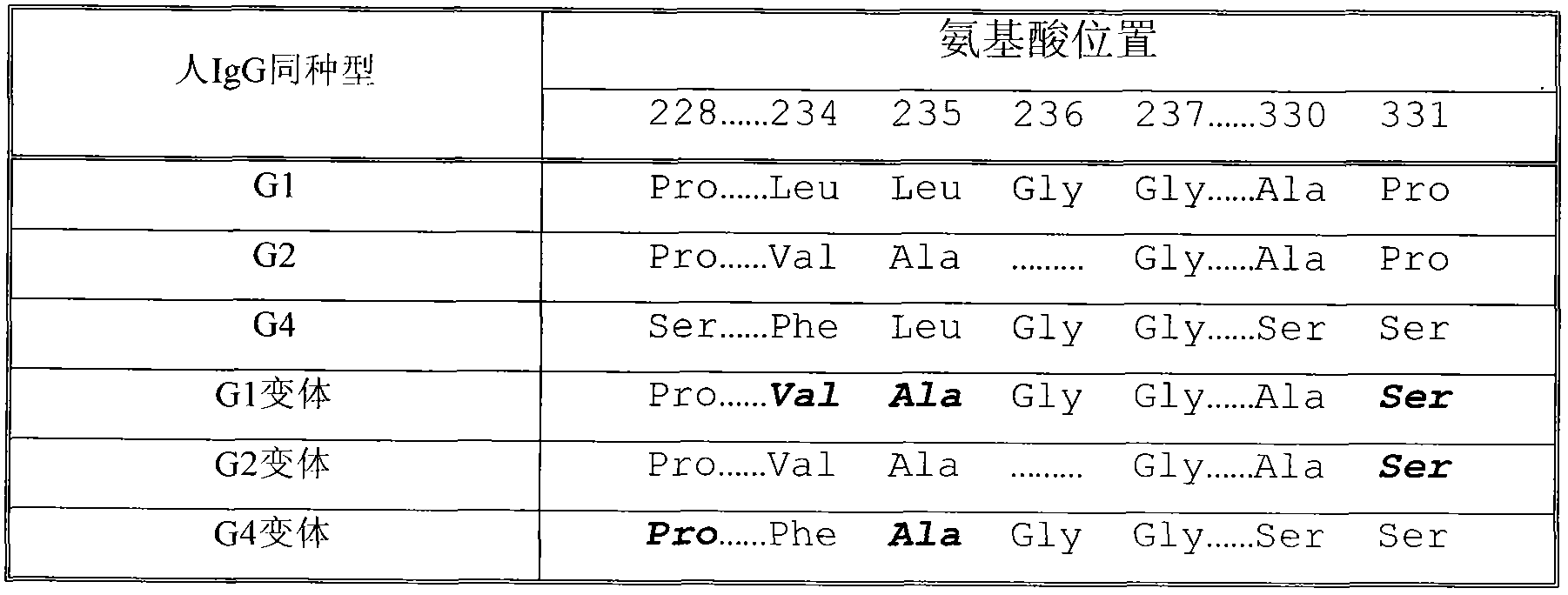
The invention discloses a recombined dimerization anti-thrombin III-Fc fusion protein, of which the in vitro biological activity is similar to or higher than that of the serum derived anti-thrombin III, and the in vivo half-life period is prolonged. The fusion protein provided by the invention contains human anti-thrombin III (hAT), a flexible peptide joint (L) containing about 20 or less amino acids, and a human IgG Fc mutant (vFC) which is represented by hAT-L-vFC (Fc). Such Fc mutant excludes cracking property and shows extremely low bad-Fc-induced side effect. Such hAT-L-vFC fusion protein is prolonged in serum half-life period and enhanced in the biological activity, so that the pharmacokinetics effect and the pesticide effect are improved. The invention further discloses a method for efficiently expressing or producing such recombined fusion proteins by adopting the mammalian cells.
Owner:AMPSOURCE BIOPHARMA (SHANGHAI) INC
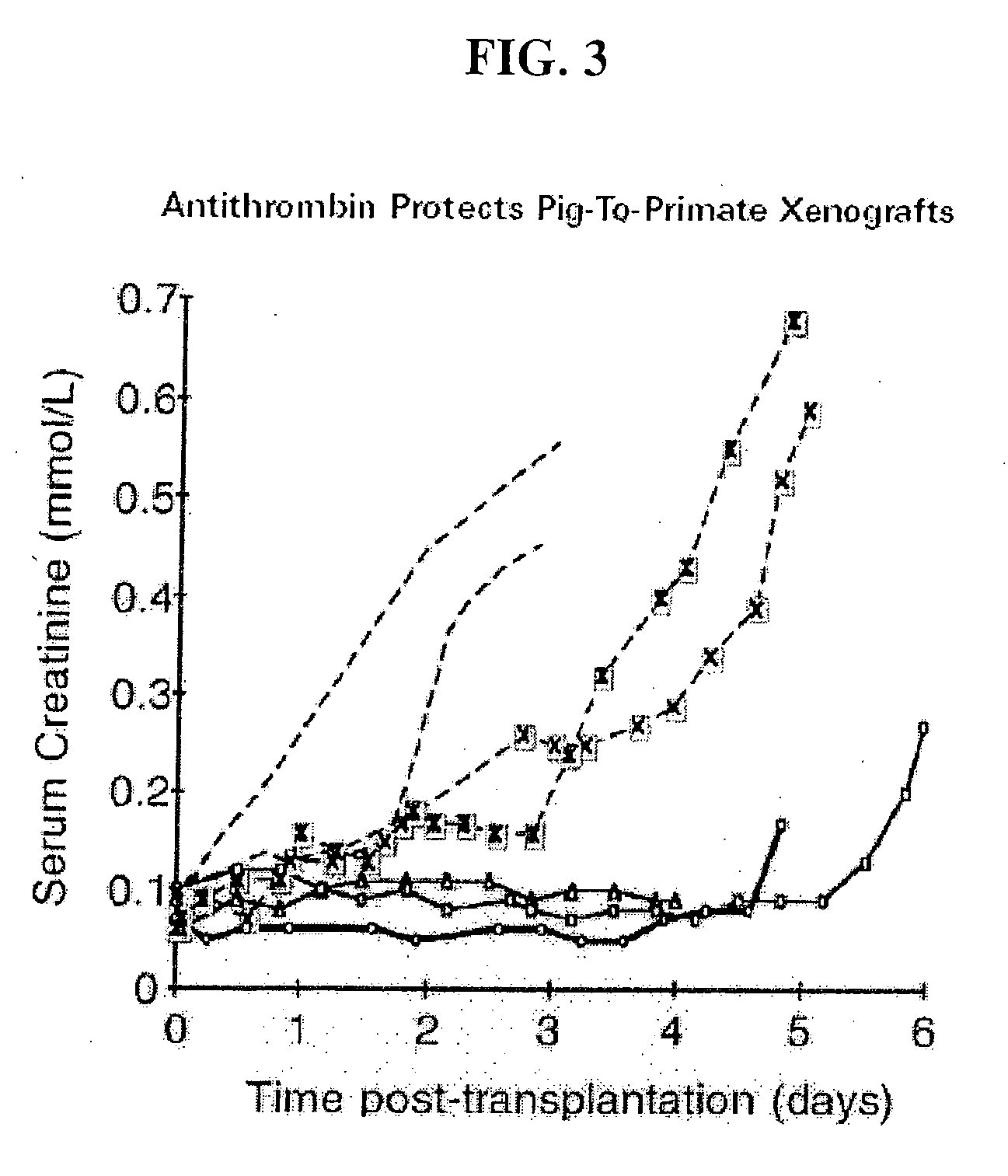
Methods of reducing the incidence of rejection in tissue transplantation through the use of recombinant human antithrombin
InactiveUS20060121004A1Reduce complicationsReducing immune system reactionBiocidePeptide/protein ingredientsTreatment effectOrgan transplantation
Owner:ECHELARD YANN +2
Determination method for heparin activity
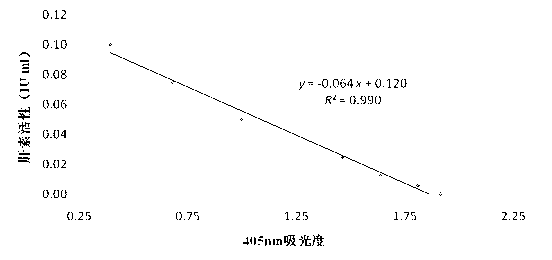
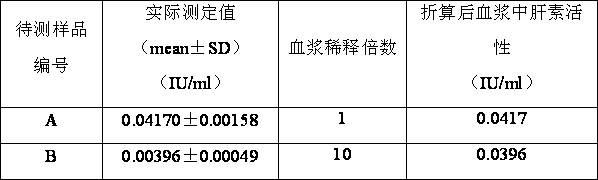
ActiveCN103063592AImprove detection efficiencyHigh precisionColor/spectral properties measurementsAcetic acidBovine blood
The invention discloses a determination method for heparin activity. The method comprises three steps of treatment of standard substances and samples, establishment of a standard curve and sample measurement, and calculation of heparin activity contents in the samples, wherein reaction systems and time are as follows: (1) 10-40 microliters standard curve establishment solution and samples with 0-0.1 IU / ml heparin activity concentration are mixed with 10-40 microliters antithrombase with 1 IU / ml concentration, and are incubated for 3 minutes at the temperature of 37 DEG C; (2) 10-40 microliters activated bovine blood coagulation factor X with 10 nkat / ml concentration is added for mixing, and is incubated for 1.5 minutes at the temperature of 37 DEG C; (3) 10-40 ml color development substrate S-2765 with 2.5 mmol / L concentration is added for mixing, and is incubated for 3 minutes at the temperature of 37 DEG C; and (4) 10-40 microliters acetic acid with 50% of volume concentration is added for evenly mixing, and then the reaction is stopped. The method has the advantages of lower reagent dosage, high detection efficiency and high precision of measurement results, and is more suitable for detecting the samples with lower heparin activity.
Owner:BLOOD TRASFUSION INST CHINESE ACAD OF MEDICAL SCI +1
Method of preventing fibrin clots in pulmonary tissue through the use of aerosolized anticoagulants
InactiveUS20050192226A1Avoid problemsFunction increaseBiocideOrganic active ingredientsThrombusBlood vessel
ATIII is a serine proteinase inhibitor (serpin) with anti-coagulant, anti-inflammatory, anti-proliferative and anti-angiogenic properties. The invention features methods of treating a subject having lung injury due to burns and smoke inhalation by administering a synergistic combination of antithrombin III and heparin through pulmonary delivery means.
Owner:BOARD OF REGENTS +1
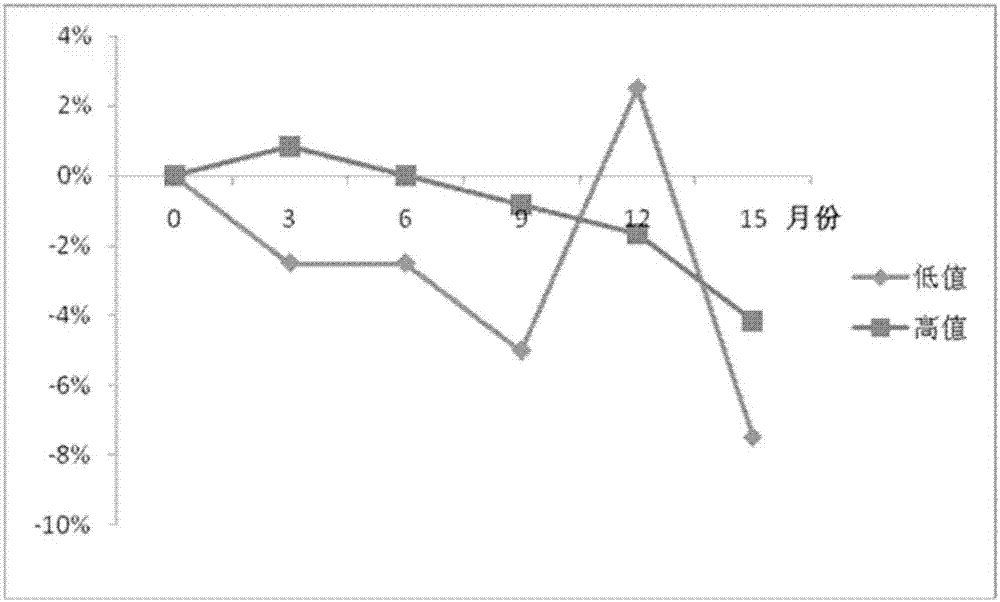
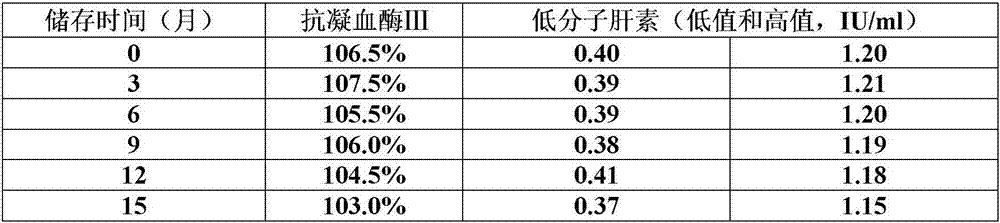
Preparation method of long-term preservable plasma matrix universally used in quality control products
The invention discloses a preparation method of long-term preservable plasma matrix universally used in quality control products. The universally used plasma matrix includes, but is not limited to, plasma which can be used for preparing blood coagulation quality control products, anticoagulant quality control products, and antithrombase quality control products. The invention also relates to a lyoprotectant and a lyophilization process of the universally used plasma. The lyoprotectant is prepared by mixing polyols, amino acids and inorganic salts according to certain proportions.
Owner:SHANGHAI VASCUTECH DIAGNOSIS CO LTD
Method for measuring content of heparin in human antithrombase-III concentrate
ActiveCN103063593AImprove accuracyReduce dosageColor/spectral properties measurementsAntithrombinChemistry
The invention discloses a method for measuring the content of heparin in a human antithrombase-III concentrate. The method is characterized by comprising the steps of treating a sample to be measured, making a standard curve and measuring the sample, wherein the step of treating the sample to be measured includes diluting and heating, and a micro color developing substrate method is adopted in the steps of making the standard curve and measuring the sample. The method has the benefits as follows: the micro color developing substrate method is utilized to perform the measurement operation and has the advantages of high sensitivity, simplicity and time saving for operation, low usage amount of required detection reagent and the like; the sample to be measured-the antithrombase-III concentrate is heated for 20-50 min at the temperature of 60-80 DEG C, so that antithrombase-III components with great influence on results in the sample to be measured can be inactivated, but the activity of the heparin is not affected, and the accuracy of the measurement results is improved; and the stability and the accuracy are high as the results are calculated by drawing the standard curve.
Owner:BLOOD TRASFUSION INST CHINESE ACAD OF MEDICAL SCI +1
Early prediction marker for gestational diabetes mellitus and detection method thereof
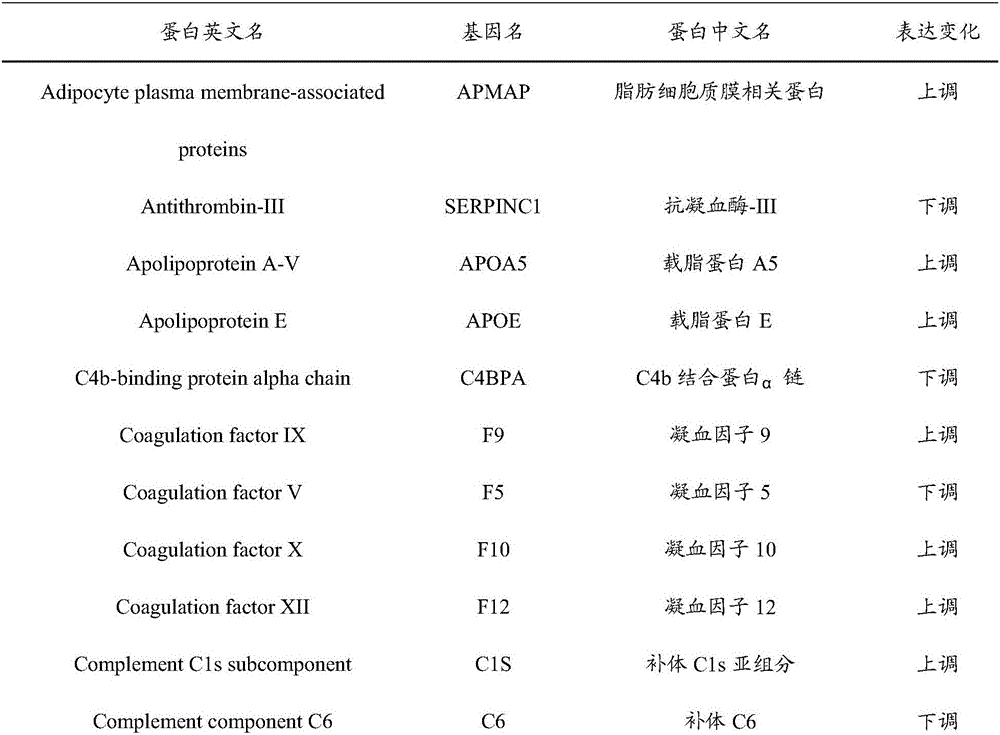
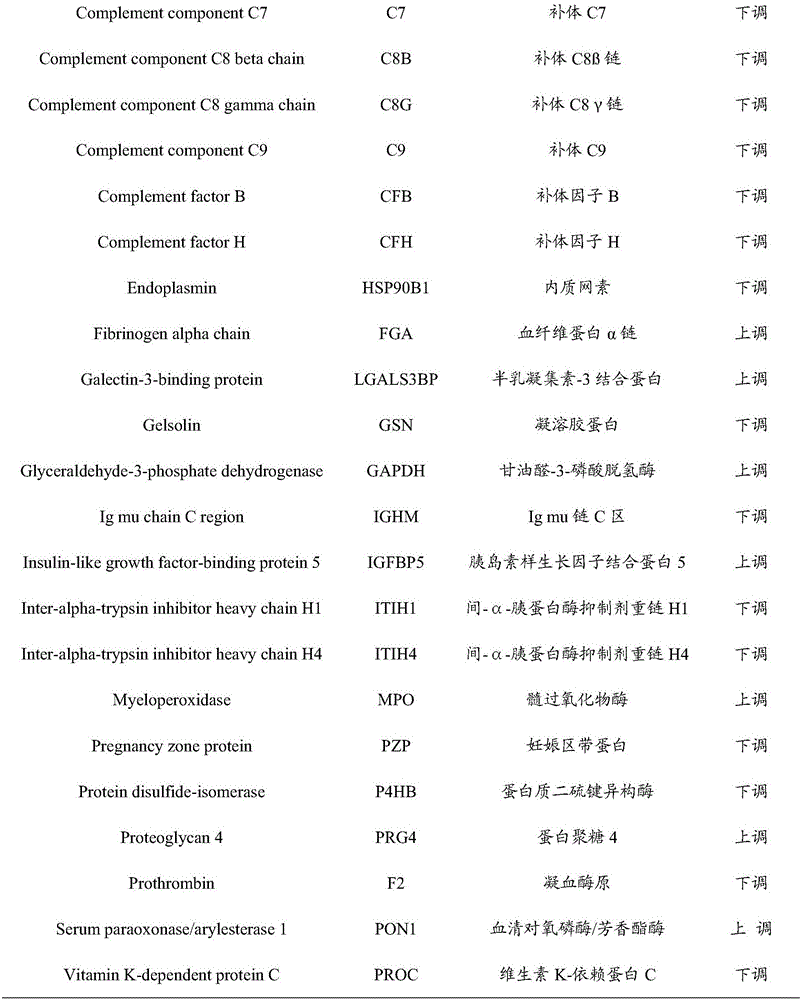
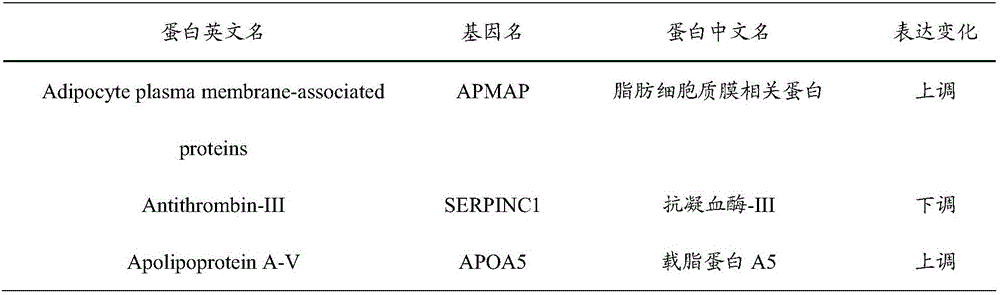
InactiveCN106706928AImprove forecast accuracyEasy to operateBiological testingNeonatal diabetesProteomic Profile
The invention provides an early prediction marker for gestational diabetes mellitus and a detection method thereof. The marker is serum differential protein which comprises 33 proteins including fat plasma membrane related protein, antithrombin-III, apolipoprotein A5, apolipoprotein E and the like; the concentration change of the serum differential protein is detected by one or more of a high-throughput proteomics method, an enzyme linked immunosorbent assay technology and a protein chip technology so as to predict whether a to-be-detected object in the 12th-16th week of pregnancy is to suffer from gestational diabetes mellitus in the 24th-28th weeks of pregnancy; the prediction accuracy of the marker is high; and the detection method is simple to operate and can assist in early diagnosis of potential patients suffering from gestational diabetes mellitus so as to avoid adverse impacts of the disease on the health of patients and fetuses thereof.
Owner:SHENZHEN UNIV
Method of using recombinant human antithrombin for neurocognitive disorders
InactiveUS20050245444A1Reduce in quantityReduce morbidityFactor VIISenses disorderMedicineEffective treatment
The present invention provides for the production of recombinant human antithrombin (rhAT) for the treatment or prophylaxis of neurocognitive disorders typically associated with major surgical procedures. The recombinant processes of the current invention as well as more efficient methods of treatment, formulation and production have been developed to treat the incidence of neurocognitive problems associated with visuoconstruction, parieto-occipital watershed area injury, hypoperfusion, microemboli or other larger embolic factors and / or CABG procedures secondary to surgery.
Owner:GTC BIOTHERAPEUTICS INC
Anticoagulant fusion protein anchored to cell membrane
The invention relates to the inhibition of blood coagulation, especially during organ rejection, and in particular the inhibition of delayed vascular rejection. The invention provides anticoagulant proteins which are anchored to cell membranes. The anticoagulant function preferably provided by heparin, antithrombin, hirudin, TFPI, tick anticoagulant peptide, or a snake venom factor. These anticoagulant proteins are preferably prevented from being constitutively expressed at the cell surface. In particular, expression at the cell surface is regulated according to cell activation, for instance by targeting the protein to a suitable secretory granule. Expression of these proteins renders cells, tissues and organs less vulnerable to rejection after transplantation (e.g. after xenotransplantation).
Owner:IMPERIAL INNOVATIONS LTD
Method for preparing human antithrombin-III product
ActiveCN103059129ALittle loss of activityShort manufacturing timePeptide preparation methodsProtease inhibitorsVirus inactivationMedicine
The invention relates to a method for preparing a human antithrombin-III product, which comprises the following steps of: separating a component IV in a plasma protein process by using a Cohn low-temperature ethanol method, precipitating the component IV serving as a raw material; and finally, preparing the high-purity antithrombin-III lyophilized product through extracting, filter pressing, carrying out heparin affinity chromatography, ultra-filtering, degerming, filtering, lyophilizing and the like and a necessary S / D and dry heat virus inactivating process. According to the invention, the process is adjusted, so that AT-III is dissolved as much as possible; the Pasteur virus inactivating process is replaced by the S / D virus inactivating process by adding proper protective agent; activity loss is reduced; the preparation time is shortened; furthermore, high-purity AT-III solution can be obtained only in the need of heparin affinity chromatography for one step; after carrying out the steps of ultra-filtering, freezing, drying, inactivating dry heat virus and the like, the purity of a final product is above 95%; and the specific activity is above 6.5 IU / mg and is 3.0 IU / mg more than specified limits in the European pharmacopoeia and united state pharmacopoeia.
Owner:GUIZHOU TAIBANG BIOLOGICAL PROD +1
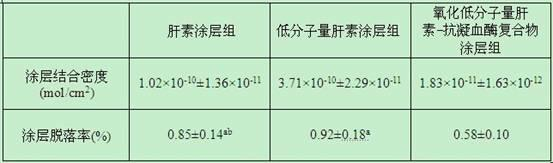
Preparation method and application of oxidized low-molecular-weight heparin-antithrombin compounds
InactiveCN101879335AReduce consumptionHigh synthesis ratePharmaceutical containersMedical packagingExtracorporeal circulationHeparin coating
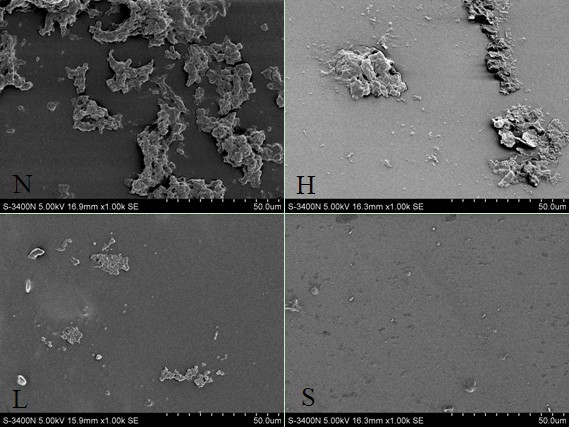
The invention discloses a method for preparing oxidized low-molecular-weight heparin-antithrombin compounds and applying the oxidized low-molecular-weight heparin-antithrombin compounds to coating layer used for improving extracorporeal circulation pipeline blood and biocompatibility. The method comprises the following steps: firstly, oxidizing low-molecular-weight heparin through sodium periodate; then, carrying out nonenzymatic glycosylation reaction with antithrombin; obtaining the purified oxidized low-molecular-weight heparin-antithrombin compounds through centrifugal ultrafiltration and diethylaminoethyl-agarose high-flowing-speed anion exchange chromatography; and then, using the polymine-glutaric dialdehyde combining technology for immobilizing the low-molecular-weight heparin-antithrombin compounds on the surface of the extracorporeal circulation pipeline. The extracorporeal circulation pipeline of the oxidized low-molecular-weight heparin-antithrombin compound coating layer prepared by the invention has better coating layer stability, anticoagulation performance and biocompatibility than the extracorporeal circulation pipeline of the heparin coating layer and low-molecular-weight heparin coating layer, the inflammatory mediator generation and release and the blood coagulation system activation caused by the contact of blood with the surface of the extracorporeal circulation pipeline can be lightened.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Method for preparing recombinant human antithrombase III protein using mammary gland biological reactor
InactiveCN1944645AImprove efficiencyPeptide/protein ingredientsTissue cultureBiotechnologyHuman body
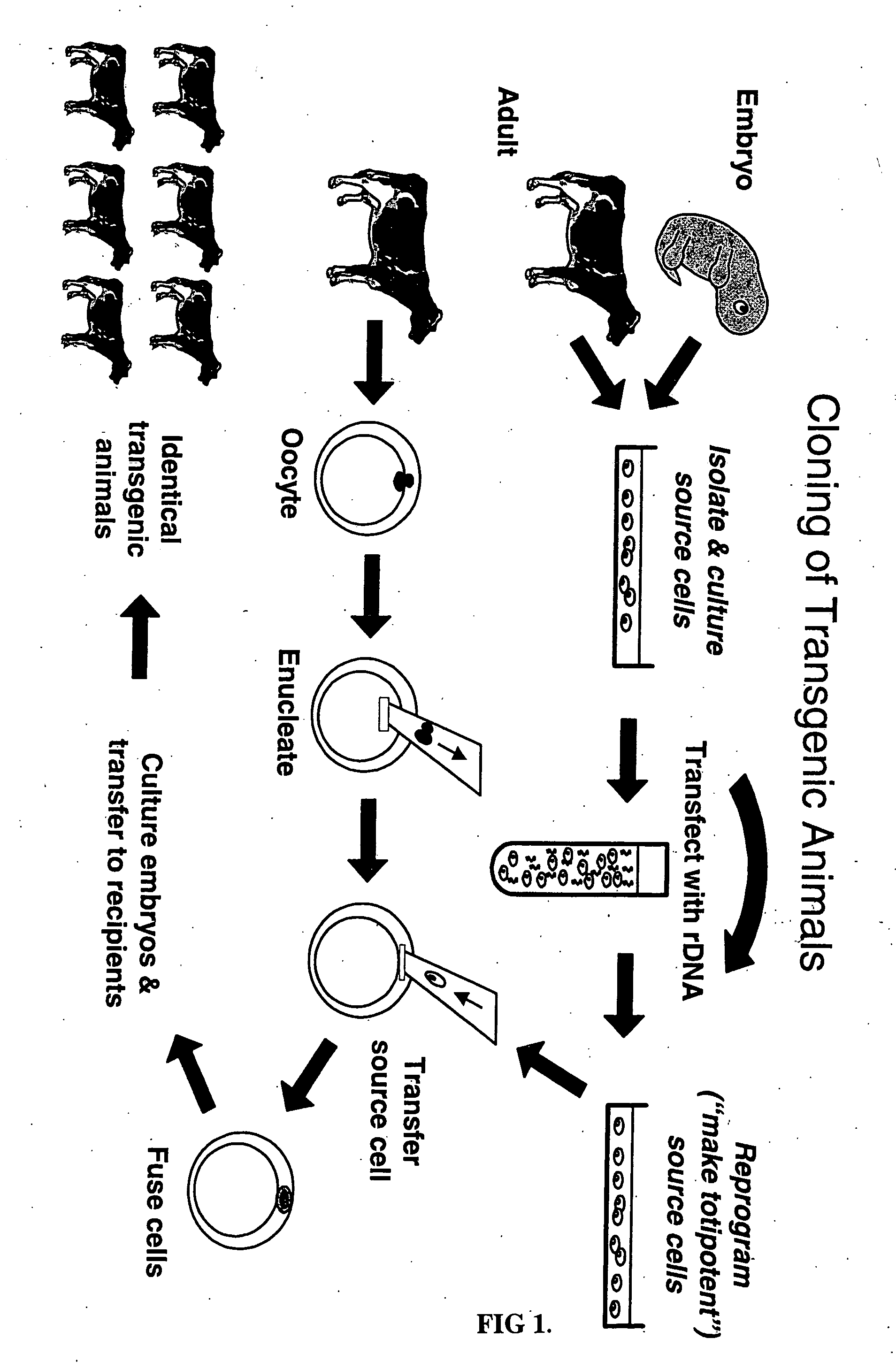
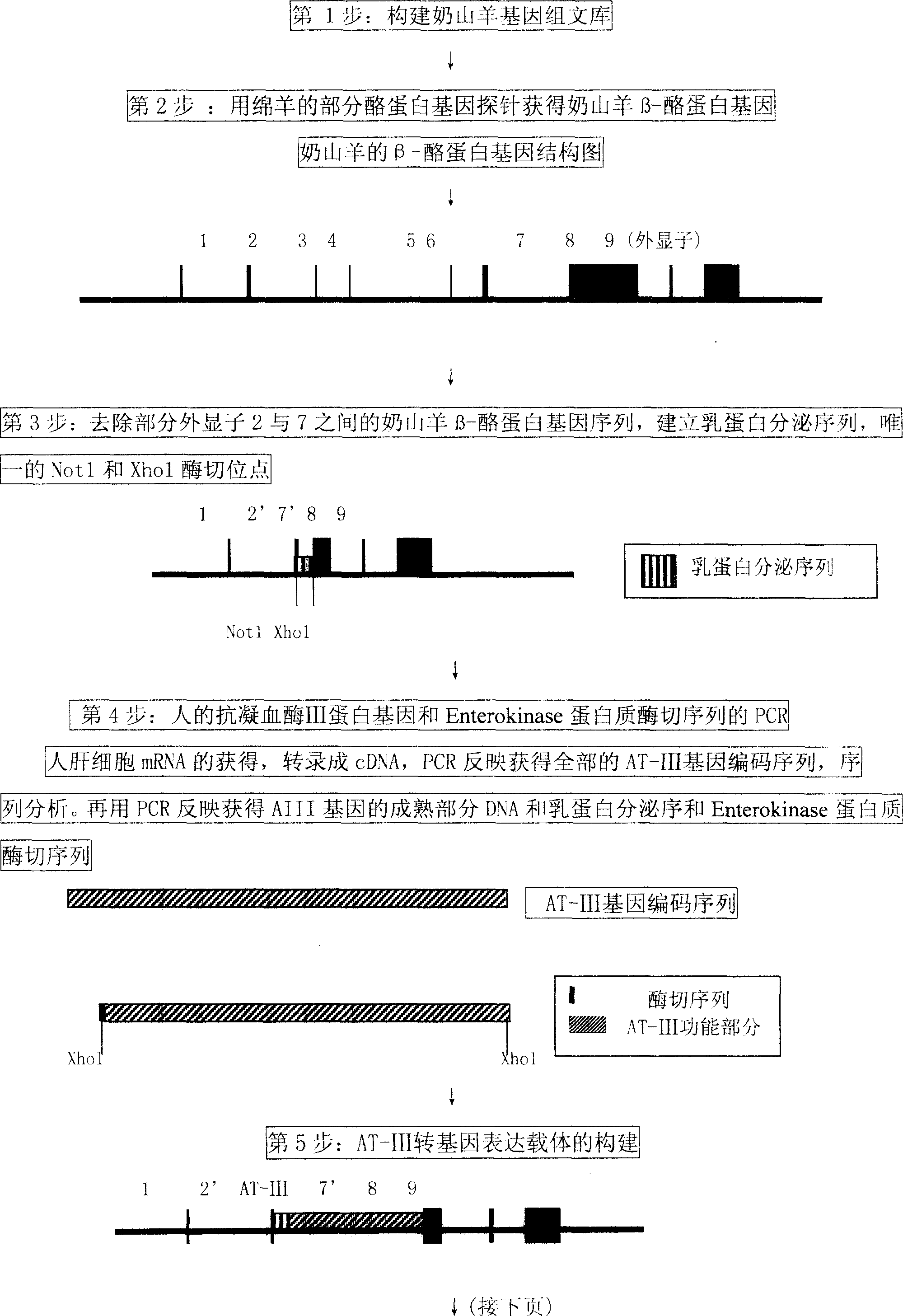
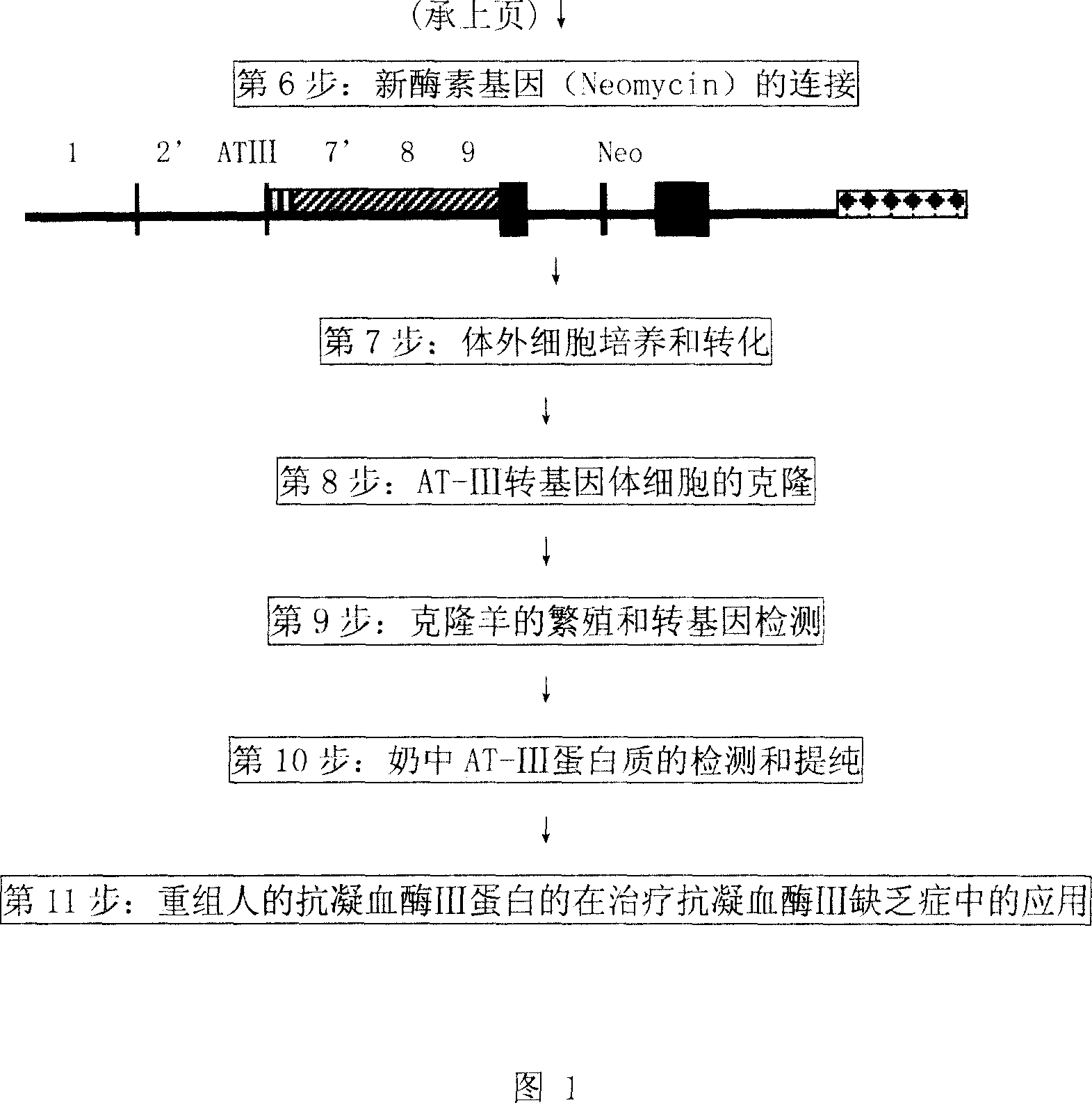
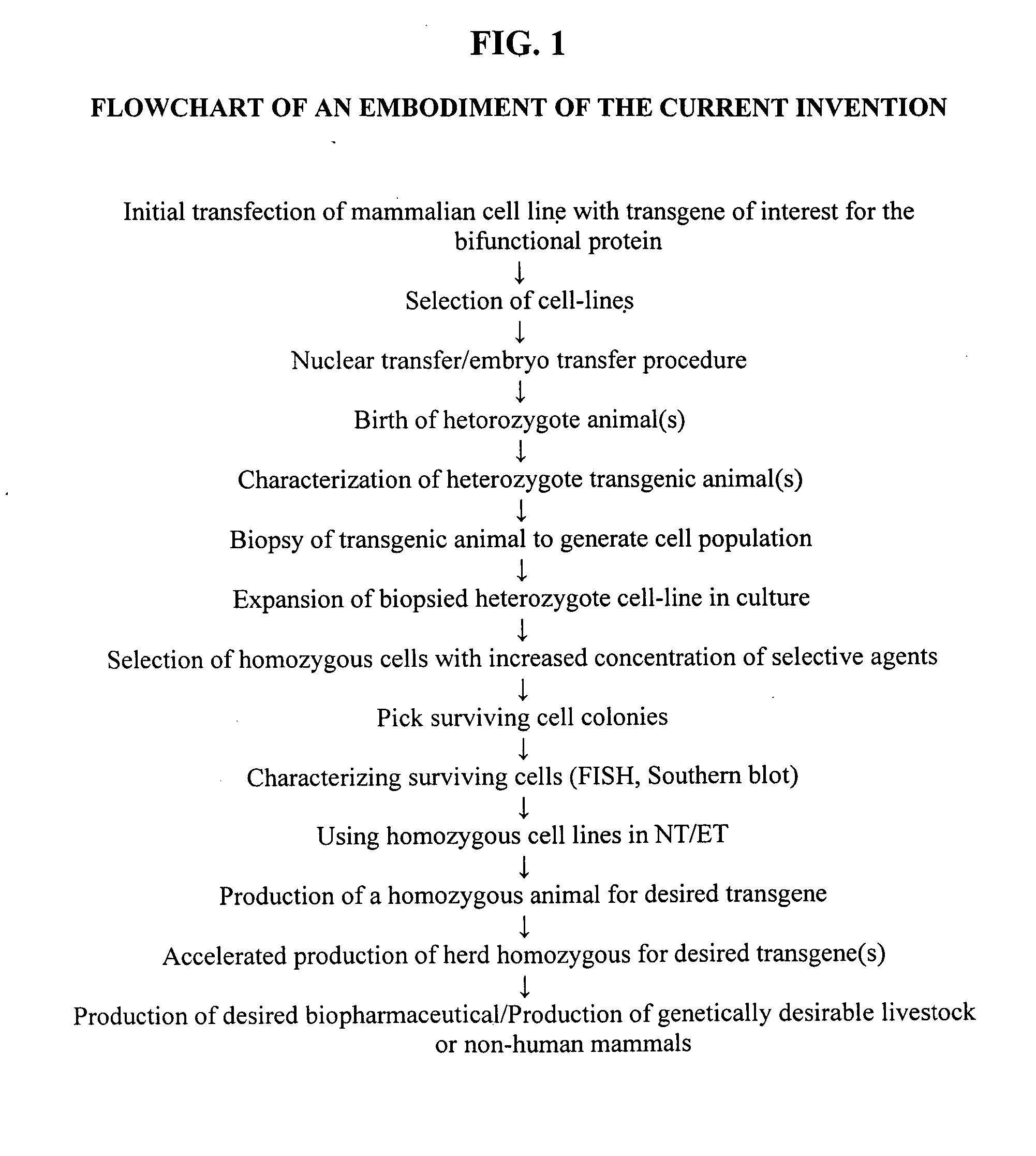
The process of preparing recombinant human antithrombase III protein with mammary gland bioreactor includes the following steps: selecting mammalian lactoprotein gene as the control region and expression frame of the transgene, establishing unique cleavage site on the frame, connecting human AT-III protein gene function part, lactoprotein secretion sequence and enterokinase protein cleaving sequence gene to the expression frame, and connecting one screening gene to the end of the vector; transferring the expression vector to in vitro cultured animal somatic cell to obtain cell strain containing human AT-III protein gene; transferring the transgenic cell strain to mature denucleated oocyte of goat to fuse the nucleus donor cell and the denucleated oocyte; transferring the cloned embryo to yow oviduct to obtain transgenic goat; detecting transgenic milk and purifying; animal experiment and human body experiment; and applying recombinant AT-III protein in human body.
Owner:青岛森淼生物技术研究所
Process for synthesizing antithrombin inhibitor of non-asymmetric non-peptide kind
InactiveCN100509799CHigh yieldEasy to operate and separateOrganic chemistryChemical synthesisReagent
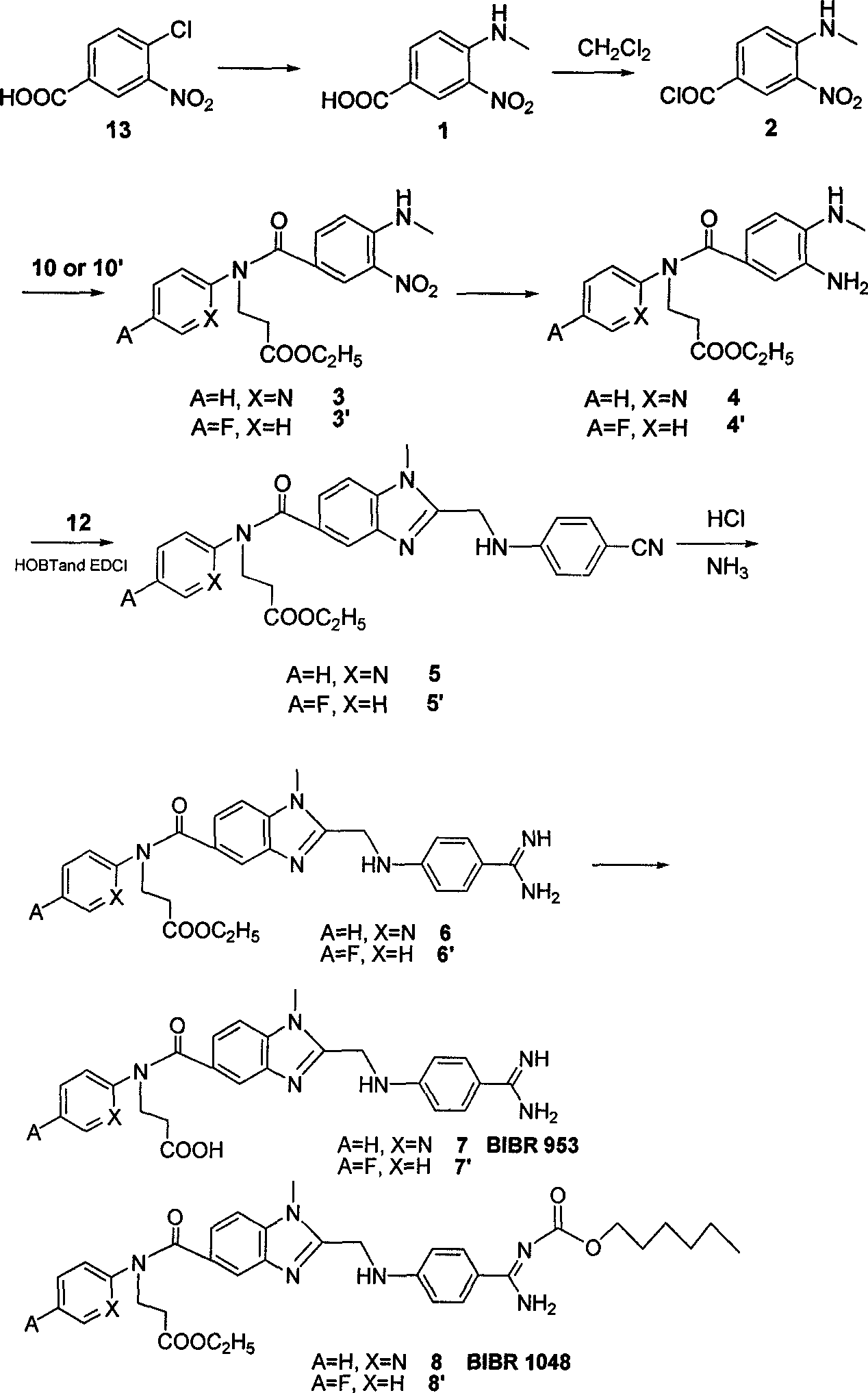
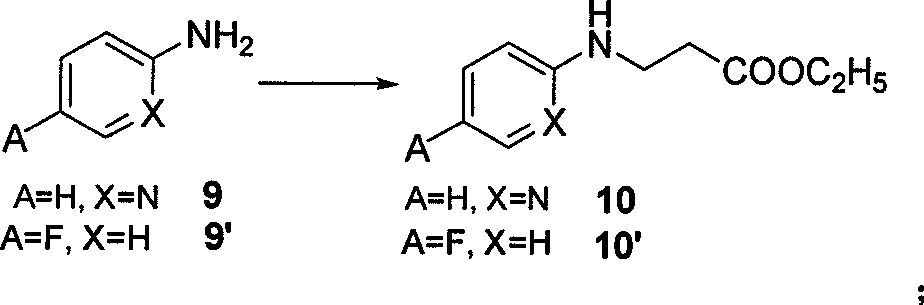
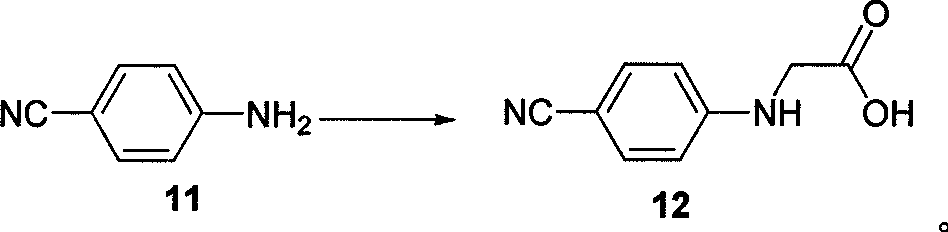
The invention belongs to the field of chemical synthesis, and relates to a synthesis method of an inhibitor containing a heterocyclic compound, especially an achiral, a Synthesis of peptide antithrombin inhibitors. The present invention uses cheap and easy-to-obtain 3-nitro-4-chlorobenzoic acid as a starting material, a highly efficient and high-yield synthetic raw material compound and a condensing agent with better effect to finally synthesize the target compound antithrombin inhibitor BIBR- 953 and BIBR-1048 and its analogs. The method of the invention has simple step operation and separation, high yield of each step, cheap and easy-to-obtain reagents, short route and high total yield of the target compound, which is nearly 50%.
Owner:FUDAN UNIV
Coagulation factor vii polypeptides
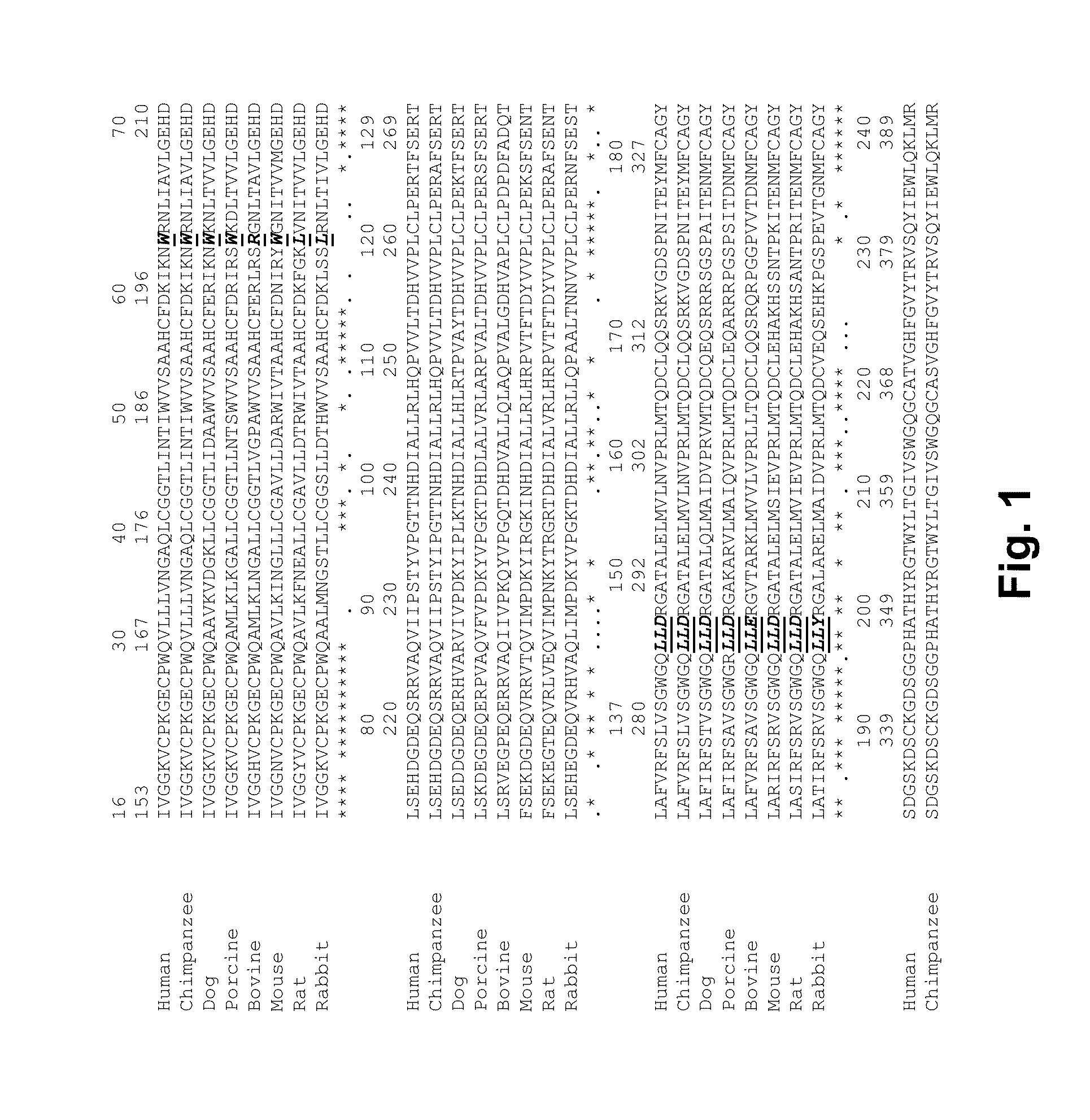
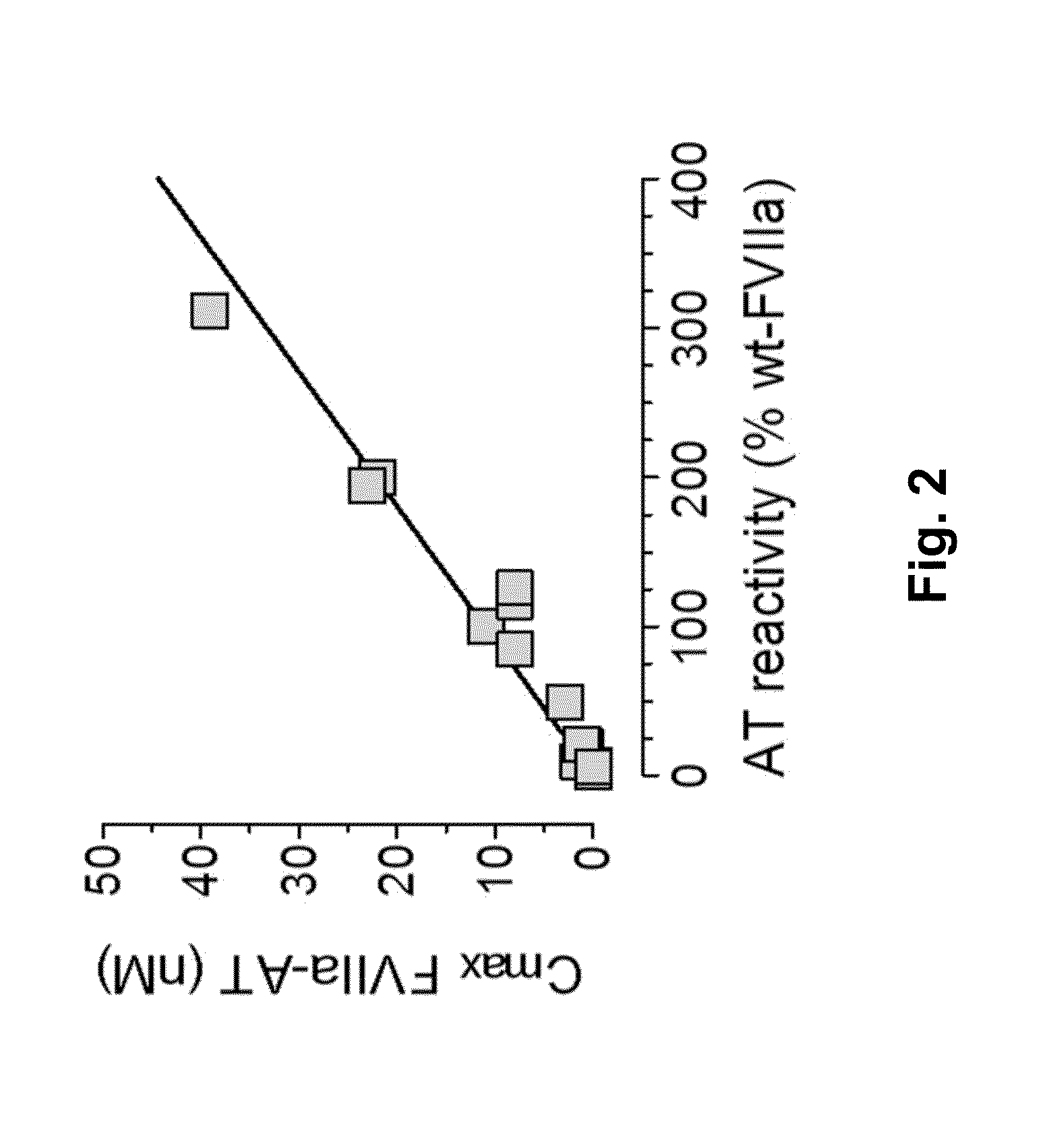
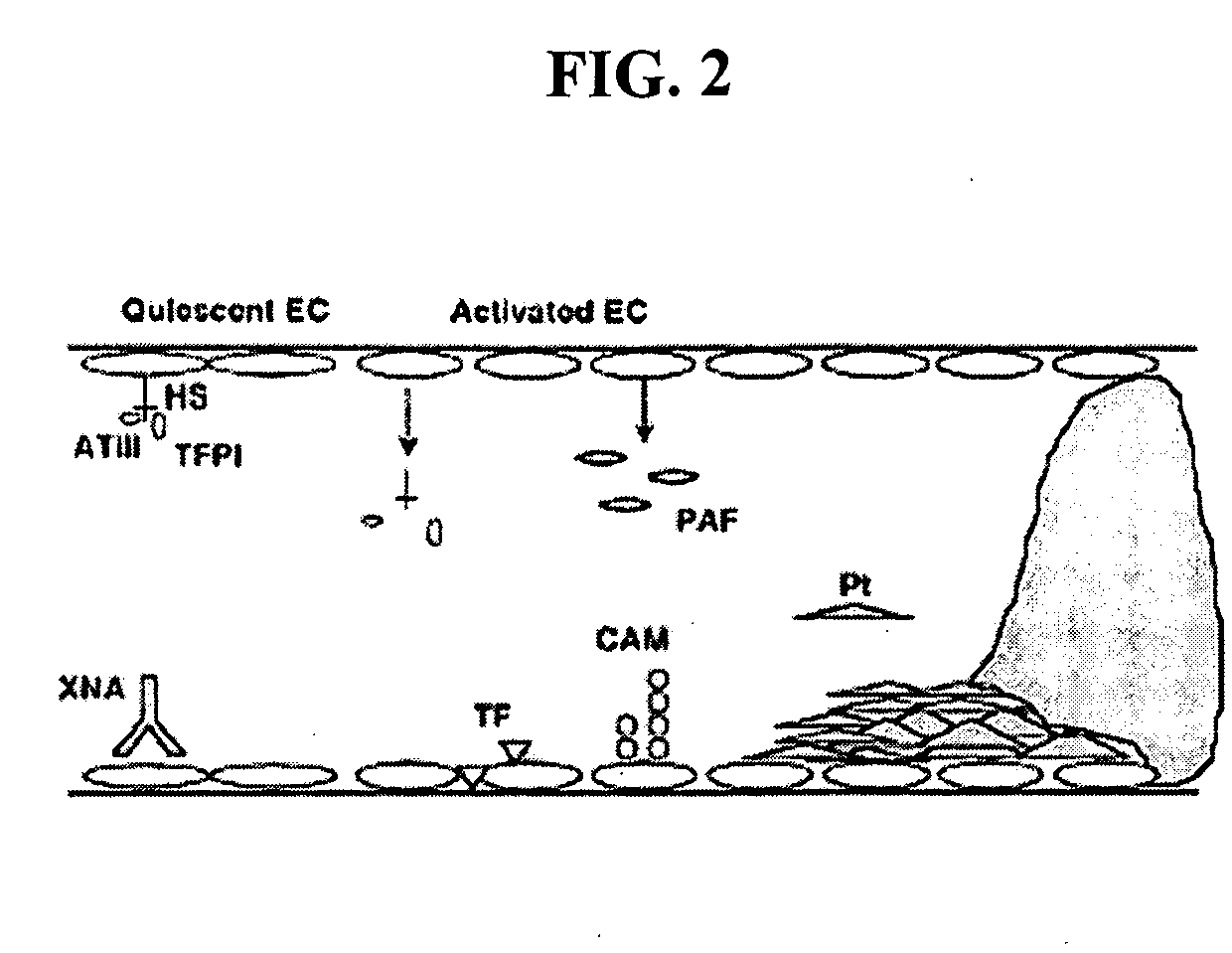
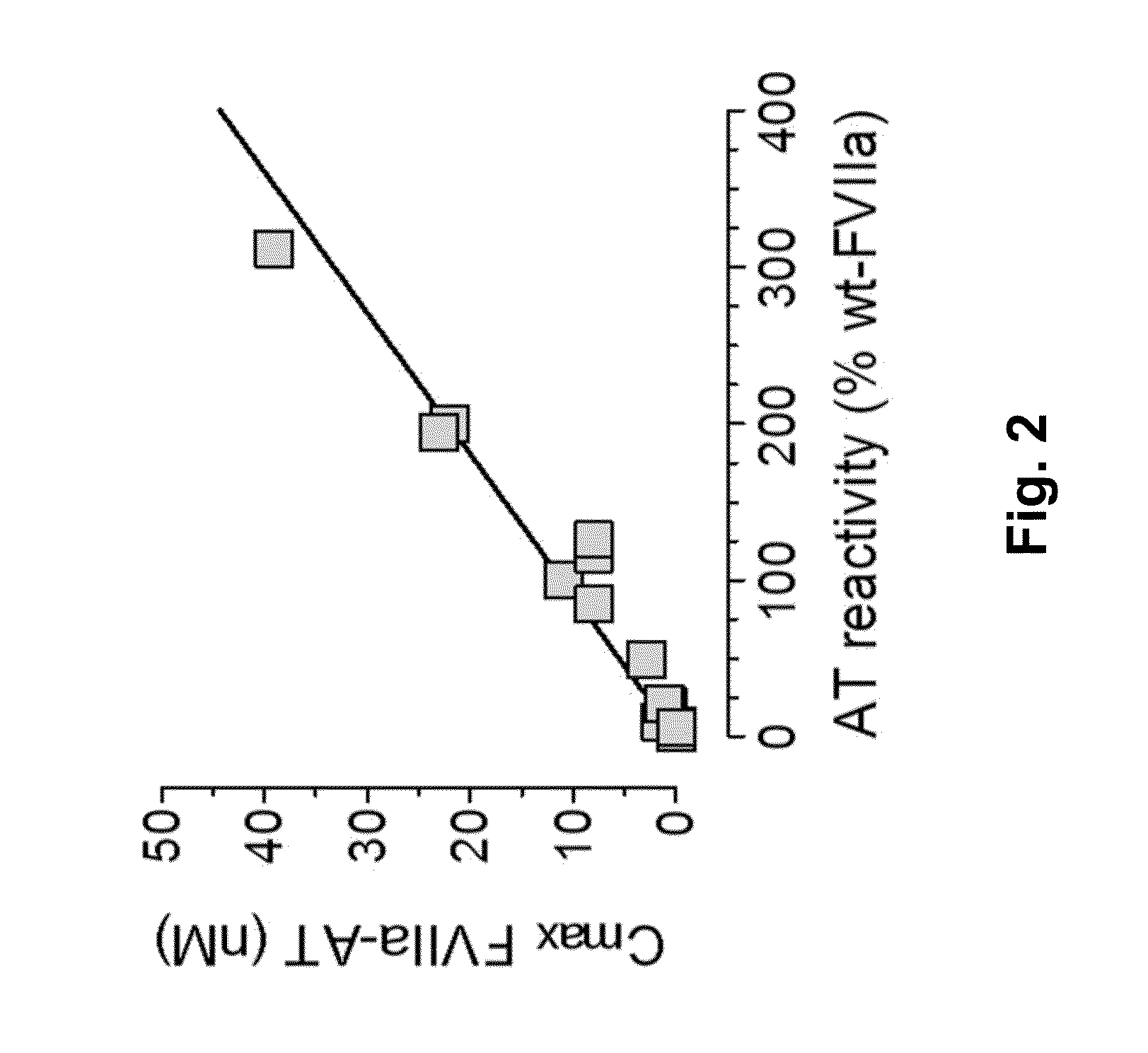
InactiveUS20150105321A1Function increaseEnhanced and little and no loss of proteolytic activityPeptide/protein ingredientsMammal material medical ingredientsNucleotideFactor ii
The present invention relates to modified coagulation Factor VII polypeptides exhibiting increased resistance to antithrombin inactivation and enhanced proteolytic activity. The present invention also relates to polynucleotide constructs encoding such polypeptides, vectors and host cells comprising and expressing such polynucleotides, pharmaceutical compositions, uses and methods of treatment.
Owner:NOVO NORDISK AS
Formulations that stabilize proteins
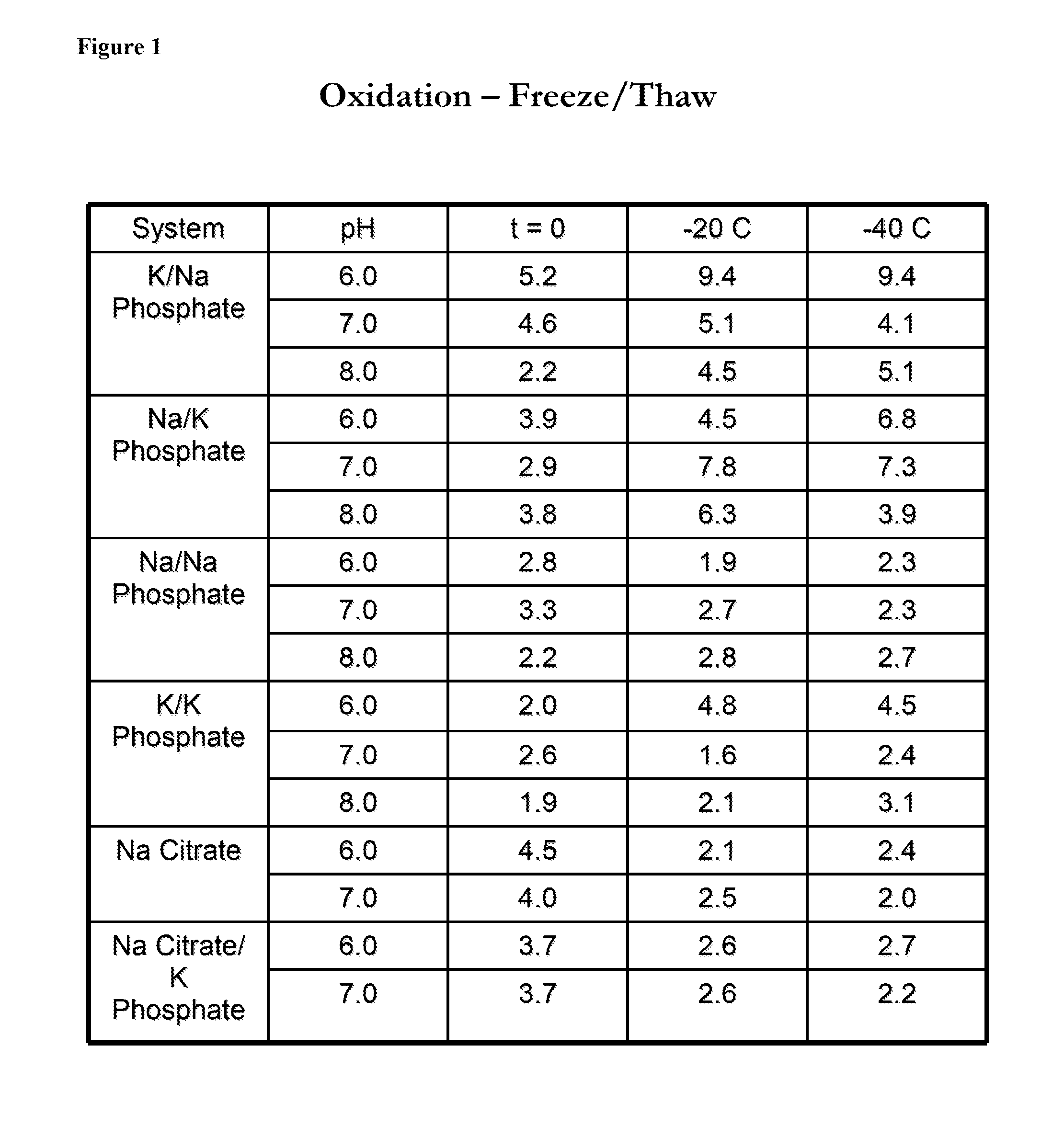
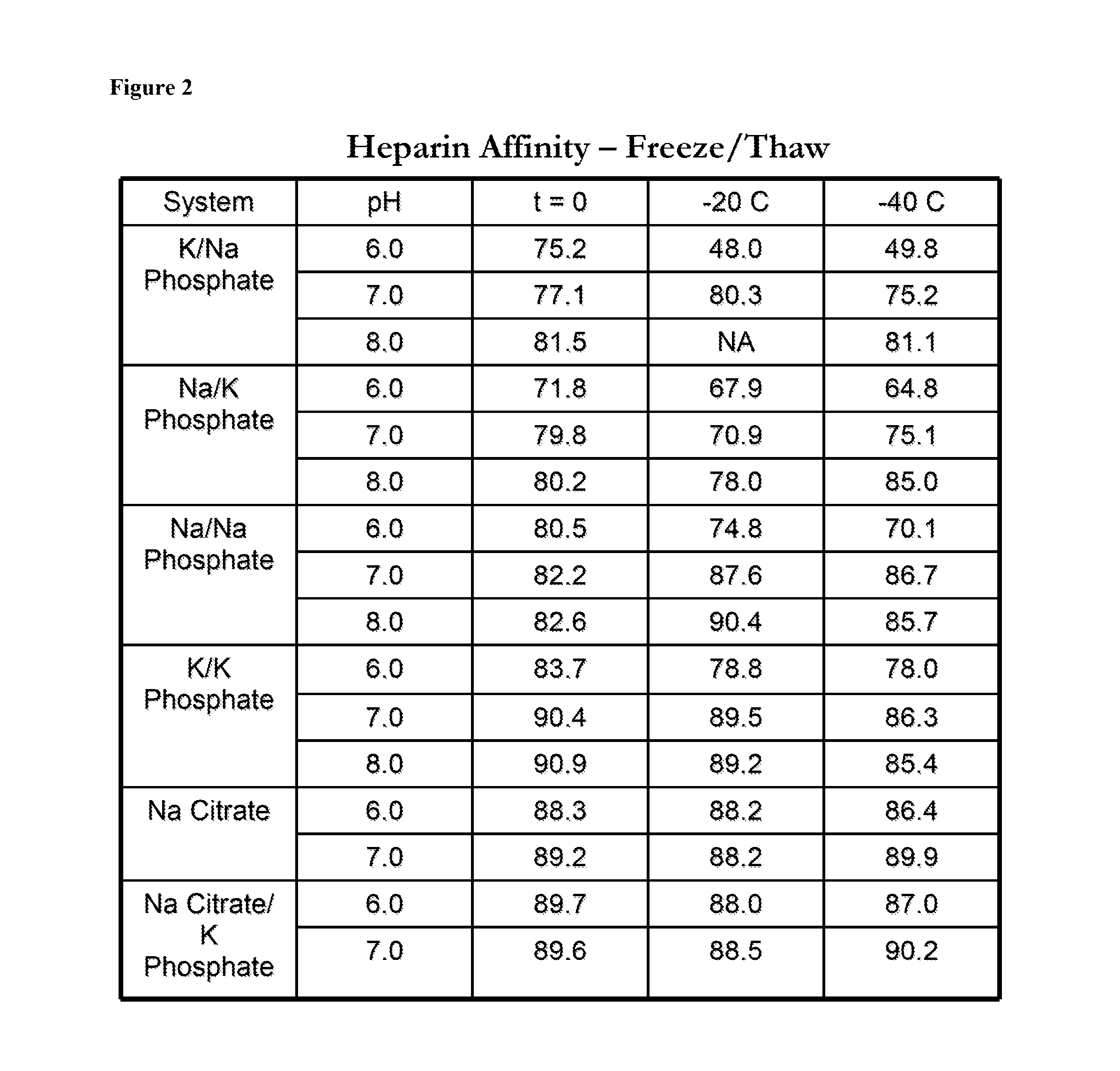
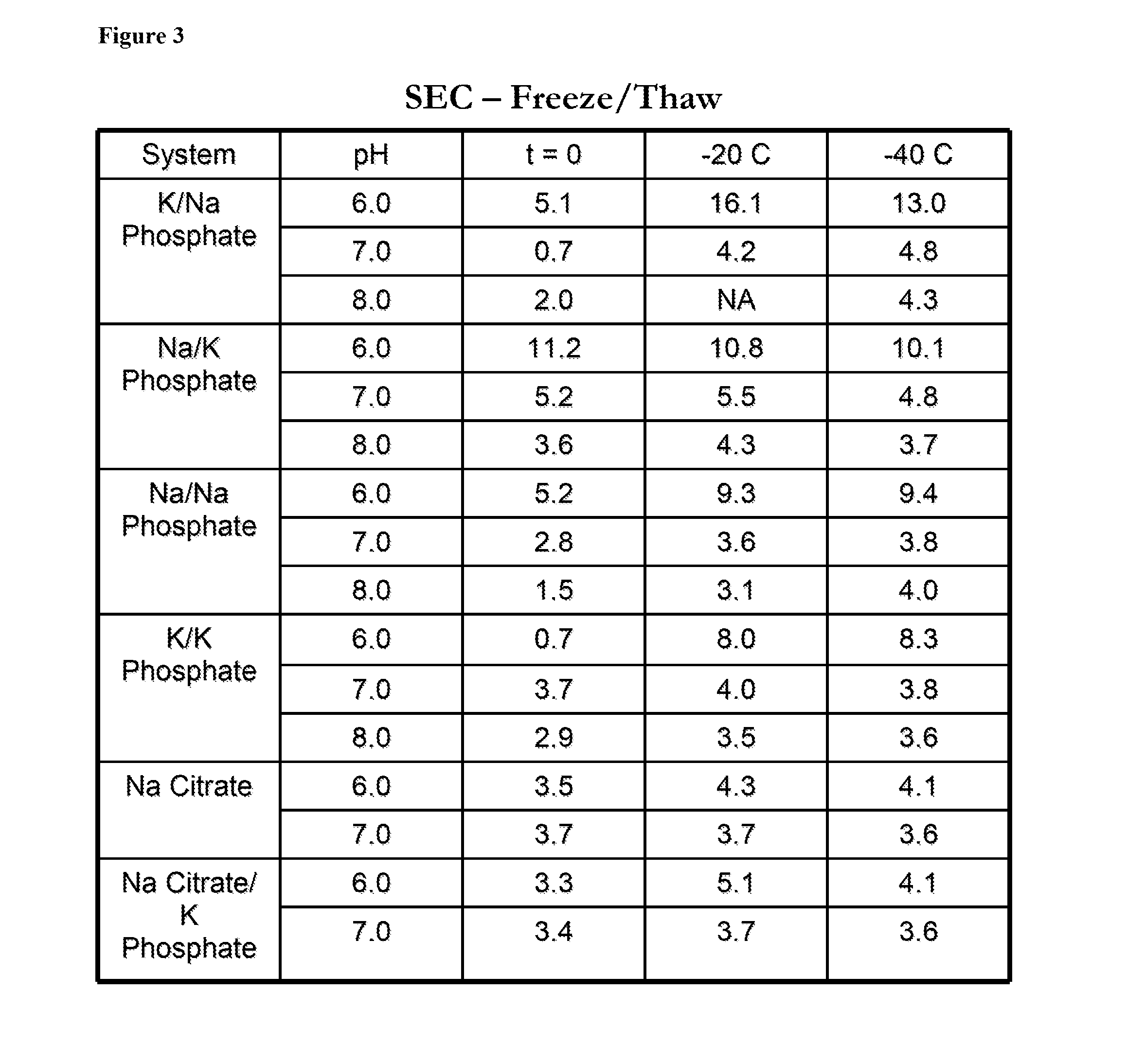
InactiveUS20140242182A1Peptide/protein ingredientsPharmaceutical delivery mechanismHydrogen phosphateTherapeutic protein
In one aspect, the disclosure provides formulations that stabilize proteins, wherein the formulations comprise a buffer. In some embodiments, the buffer comprises potassium mono-hydrogen-phosphate and potassium di-hydrogen-phosphate, or the buffer comprises sodium mono-hydrogen-phosphate and sodium di-hydrogen-phosphate. In some embodiments, the protein is a therapeutic protein. In some embodiments, the therapeutic protein is antithrombin.
Owner:LFB USA
Protein marker for myocardial infarction in urine, and applications thereof in diagnosis and prognosis
The invention relates to a protein marker for myocardial infarction in urine and applications thereof in diagnosis and prognosis, and particularly, relates to applications of identification reagents of following proteins, including antithrombin-III, complement-C3, [alpha]-1-acid glycoprotein 1, serum transferrin, cathepsin Z, and a combination thereof, in preparation of reagents for diagnosis and / or prognosis of the myocardial infarction of patients. Herein, the five proteins have excellent clinical application prospects in the fields of diagnosis of myocardial infarction and detection on diseases status and therapy effects.
Owner:THE INST OF BASIC MEDICAL SCI OF CHINESE ACAD OF MEDICAL SCI
Target multifunctional anti-embolism fusion protein as well as preparation method and application thereof
InactiveCN102180973AInhibition formationInhibition of developmentFungiPeptide/protein ingredientsArginineCoagulation Factor Xa
The invention belongs to the technical field of genetic engineering, relating to a target multifunctional anti-embolism fusion protein an amino acid sequence of which is as shown in SEQ ID NO.1. The invention also relates to a gene for encoding the fusion protein, a recombinant expression vector containing the gene, a transformant containing the recombinant expression vector and a method for preparing the fusion protein. The fusion protein can reasonably splice human antibacterial peptide LL-37, leech peptide Hirudin-12, Agkistrodon acutus peptide (AAP), arginine-glycin-aspartate (RGD) and human blood coagulation factor Xa identification sites, so that the functions among a target component, an antibacterial component, a thrombin resisting component and a platelet aggregation resisting component are complementary and in synergistic effect; and the fusion protein can well exert target anti-inflammatory and anticoagulation activities at a thrombus position, and simultaneously can repair a vessel endothelial cell, can commonly inhibit the formation and development of thrombus by virtue of multiple ways, and can be used for preparing a medicament for preventing and treating thrombotic diseases.
Owner:CHONGQING UNIV
Methods and compositions for diagnosis and prognosis of renal injury and renal failure
The present invention relates to methods and compositions for monitoring, diagnosis, prognosis, and determination of treatment regimens in subjects suffering from or suspected of having a renal injury. In particular, the invention relates to using a one or more assays configured to detect a kidney injury marker selected from the group consisting of Stanniocalcin-1, Antithrombin-III, Toll-like receptor 2, Triiodothyronine (T3), Thyroxine (T4), Extracellular matrix protein 1, Coagulation factor XIII A chain, Coagulation factor XIII B chain, Interleukin-17F, Interleukin-22, Vitronectin, Progesterone, Estradiol, Growth / differentiation factor 15, and Proprotein convertase subtilisin / kexin type 9 as diagnostic and prognostic biomarkers in renal injuries.
Owner:ASTUTE MEDICAL
Expression method of human anti-zymoplasm III, special-purpose expression vector and engineering bacterium thereof
InactiveCN101402968AHigh purityShorten the growth cycleFungiMicroorganism based processesBiotechnologyPichia pastoris
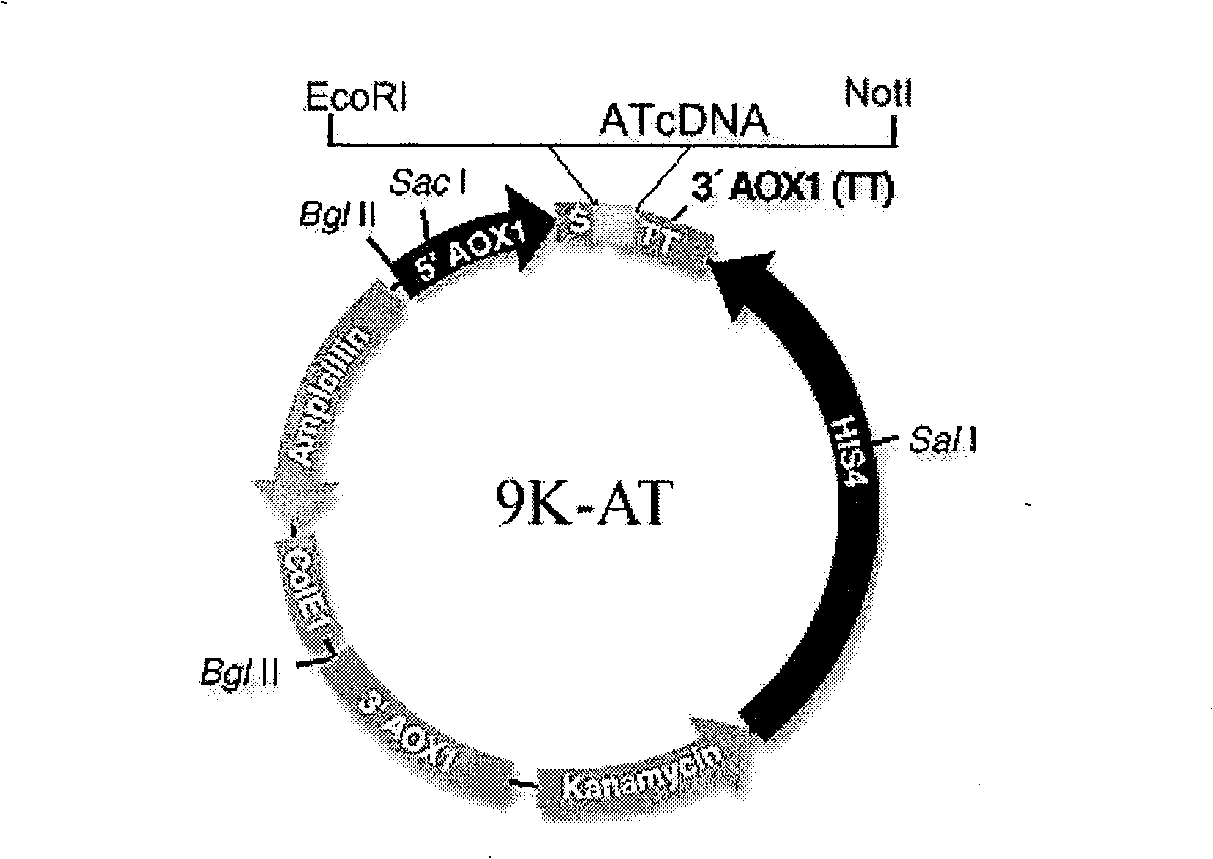
The invention discloses a fermentation expression method of a human anti-thrombin III, a special expression vector and an engineered strain thereof. The Pichia pastoris expression vector for expressing the human anti-thrombin III is the recombinant Pichia pastoris expression vector containing human anti-thrombin III code gene. The Pichia pastoris engineered strain for expressing the human anti-thrombin III is obtained by introducing the recombinant Pichia pastoris expression vector containing the human anti-thrombin III into Pichia pastoris. The use of the Pichia pastoris expression vector and the engineered strain for carrying out the expression of the human anti-thrombin III can make up for the deficiencies of the traditional preparation method of the human anti-thrombin III and the expression mode of a prokaryotic expression system; in addition, if the invention is applied to the industrial production of the human anti-thrombin III, the advantages of simple operation, short growth cycle of raw material organisms, large production scale (high-density fermentation), low extraction cost and high enzyme activity are significant, thereby having important industrial application prospect and practical significance.
Owner:FIELD OPERATION BLOOD TRANSFUSION INST OF PLA SCI ACAD OF MILITARY
Raising bait for sucking-blood leeches as well as preparation method and application of raising bait
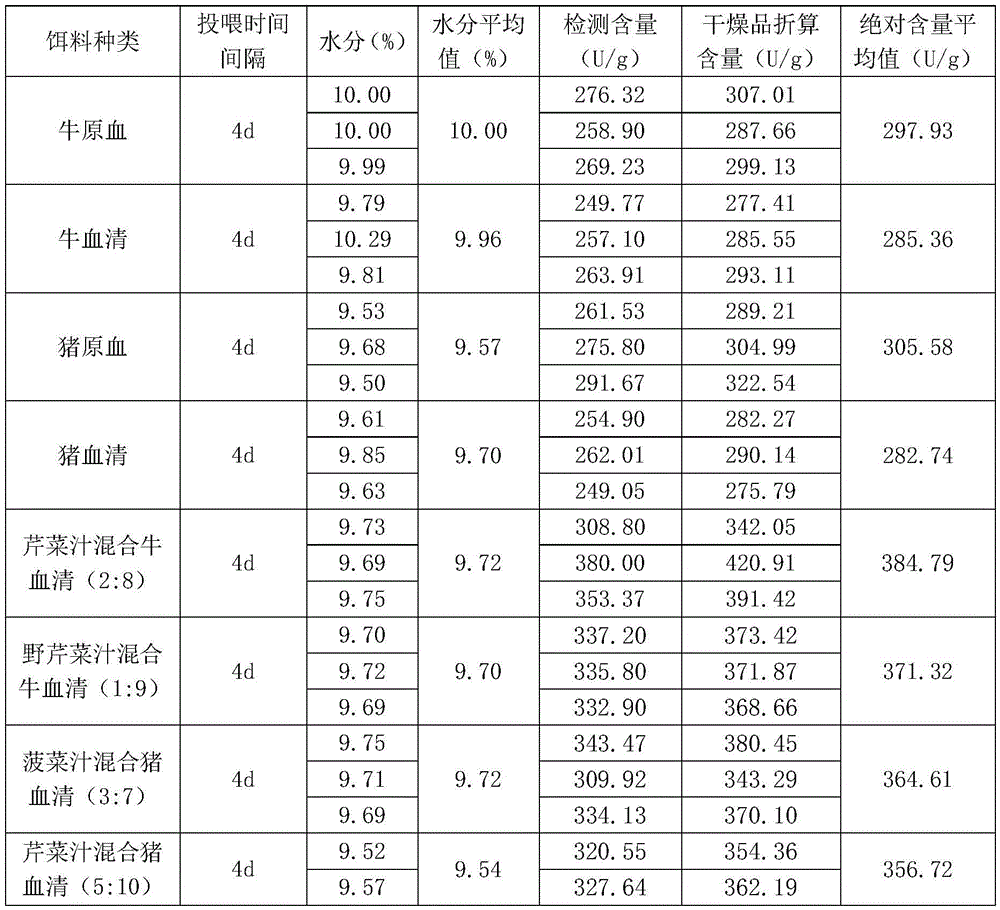
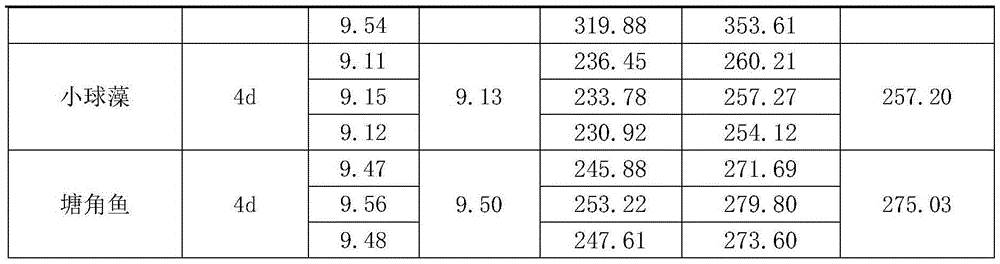
InactiveCN105558313AHigh medicinal valueImprove the economic benefits of artificial breedingClimate change adaptationAnimal feeding stuffFiberFiltration
The invention discloses raising bait for sucking-blood leeches as well as a preparation method and application of the raising bait. The raising bait is prepared by mixing vegetable juice with animal serum in the volume ratio of the vegetable juice to the animal serum being (1-5) to (6-10). The preparation method comprises the following steps of (1) taking animal blood clods, stirring and breaking the taken animal blood clods, and performing filtration with a 40-mesh sieve so as to obtain filtrate namely the animal serum; (2) thoroughly cleaning vegetables, draining the cleaned vegetables, chopping the drained vegetables to obtain vegetable segments of 3-5 cm, mashing the vegetable segments, and performing filtration with a 40-mesh sieve so as to remove fibers and obtain filtrate namely the vegetable juice; and (3) mixing the animal serum with the vegetable juice in proportion. The raising feed for sucking-blood leeches provided by the invention is prepared by mixing the vegetable juice with the animal serum, so that the content of antithrombins and the individual weight of the leeches in unit mass can be greatly increased, and besides, the nutrient structure of the bait for the leeches is enriched; the raising efficiency is improved, and the medical value of the leeches is notably increased, so that the artificial raising economic benefits of the leeches are improved. The preparation method is simple, the raw materials are easy to obtain, and the vegetable juice and the animal serum are mixed and used, so that the supply pressure of the animal blood in the market can be relieved.
Owner:GUIZHOU XINBANG PHARMACEUTICAL CO LTD
Methods of reducing the incidence of rejection in tissue transplantation through the use of recombinant human antithrombin
InactiveUS20080004212A1Reduce activationReduce complicationsPeptide/protein ingredientsMammal material medical ingredientsMedicineOrgan transplantation
Owner:GTC BIOTHERAPEUTICS INC
Method for producing antithrombin composition
ActiveUS20100113754A1High yieldPeptide/protein ingredientsMammal material medical ingredientsSulfateAqueous solution
The provision of an antithrombin composition having a desired α-form content rate or β-form content rate is required. The invention provides a process for producing an antithrombin composition having a desired α-form content rate or β-form content rate which is prepared by contacting an antithrombin-containing aqueous solution with a Cellufine Sulfate chromatography carrier.
Owner:KYOWA HAKKO KIRIN CO LTD
Coagulation factor VII polypeptides
InactiveUS9370583B2Function increaseEnhanced and little and no loss of proteolytic activityPeptide/protein ingredientsPharmaceutical non-active ingredientsNucleotideFactor ii
The present invention relates to modified coagulation Factor VII polypeptides exhibiting increased resistance to antithrombin inactivation and enhanced proteolytic activity. The present invention also relates to polynucleotide constructs encoding such polypeptides, vectors and host cells comprising and expressing such polynucleotides, pharmaceutical compositions, uses and methods of treatment.
Owner:NOVO NORDISK HEALTH CARE AG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com