Patents
Literature
83 results about "Midkine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Midkine (MK or MDK) also known as neurite growth-promoting factor 2 (NEGF2) is a protein that in humans is encoded by the MDK gene. Midkine is a basic heparin-binding growth factor of low molecular weight, and forms a family with pleiotrophin (NEGF1, 46% homologous with MK). It is a nonglycosylated protein, composed of two domains held by disulfide bridges. It is a developmentally important retinoic acid-responsive gene product strongly induced during mid-gestation, hence the name midkine. Restricted mainly to certain tissues in the normal adult, it is strongly induced during oncogenesis, inflammation and tissue repair.
Methods for detecting early cancer
InactiveUS7090983B1Easy to implementSensitive highPeptide/protein ingredientsMicrobiological testing/measurementBacteriuriaUrine production
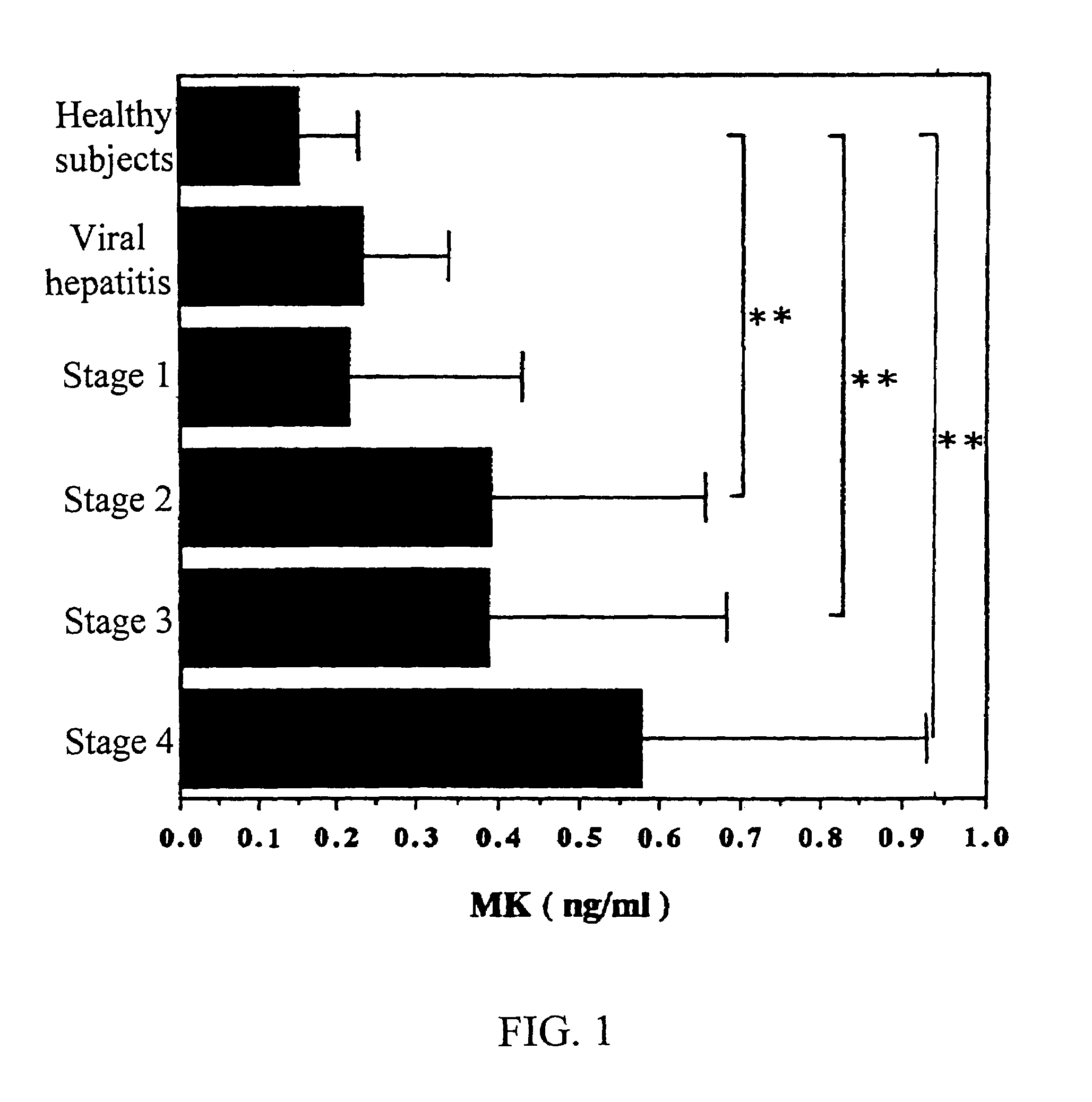
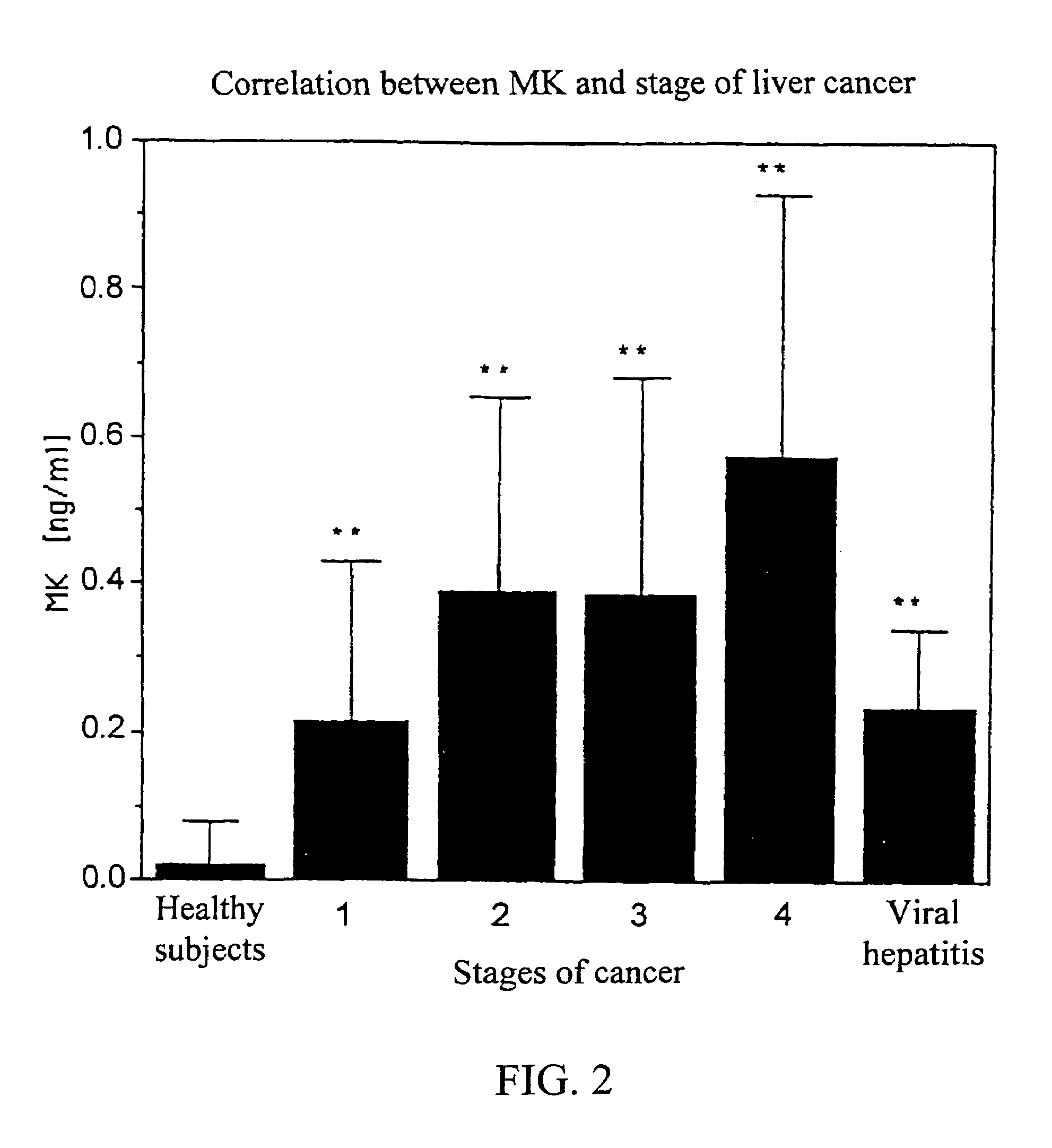
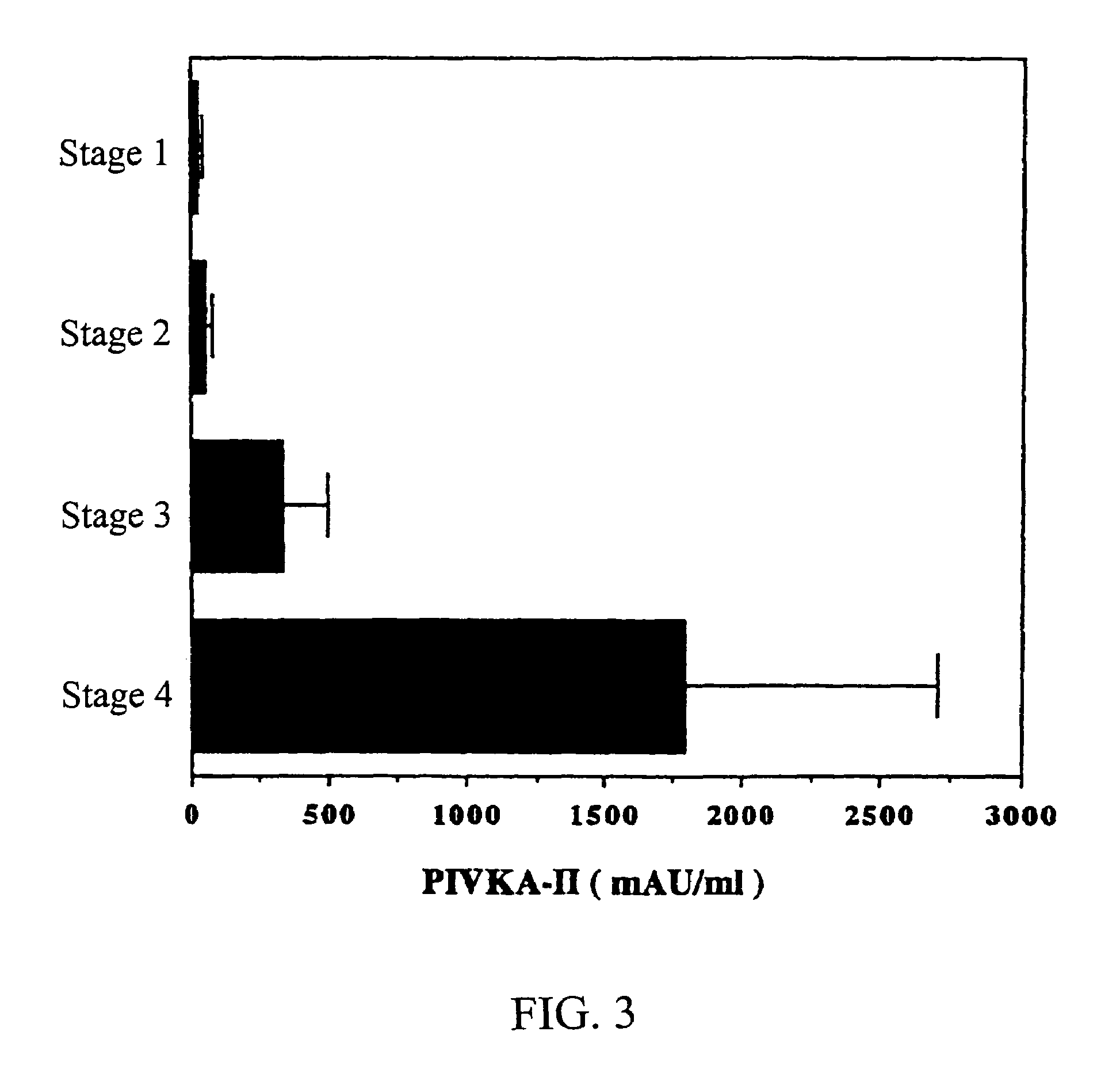
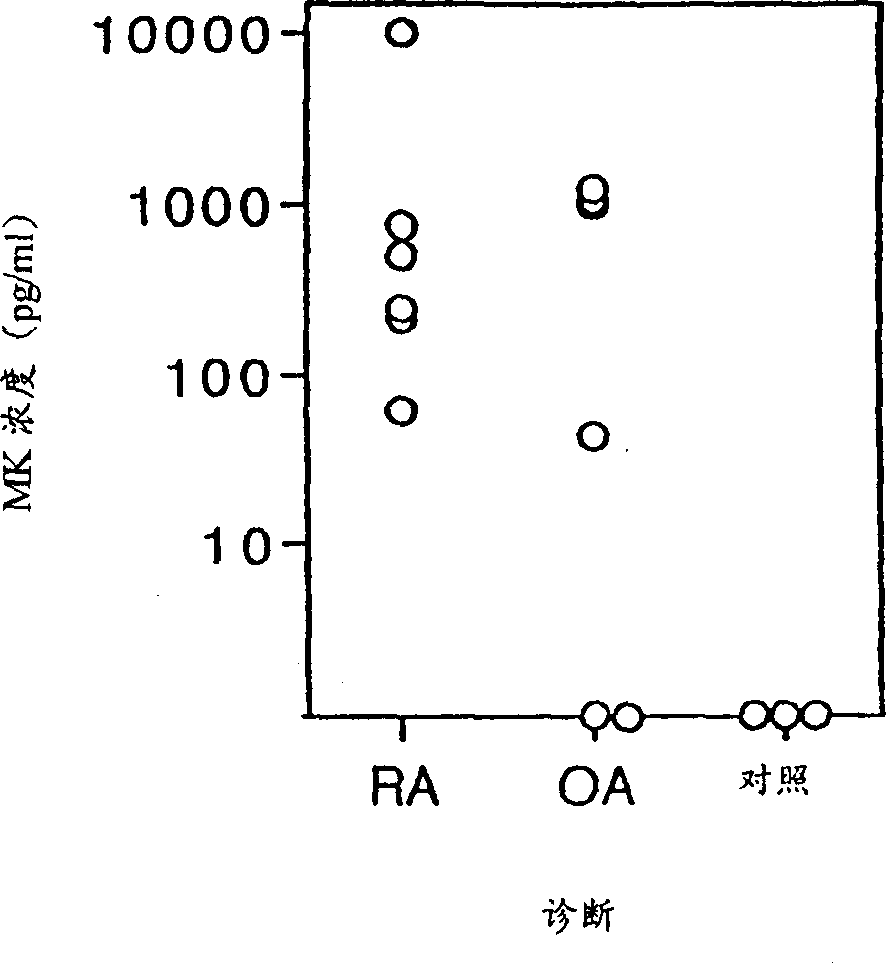
MK (midkine) was found to rise in the blood or urine of patients with various types of cancers at early stage. Based on this finding, a method for detecting early cancer, comprising the step of measuring MK in blood or urine was completed.
Owner:MEDICAL THERAPIES
Enzyme-mediated modification of fibrin for tissue engineering: fibrin formulations with peptides
InactiveUS7241730B2Efficacious platformEnhanced andPeptide/protein ingredientsTransferasesCell Surface ProteinsADAMTS Proteins
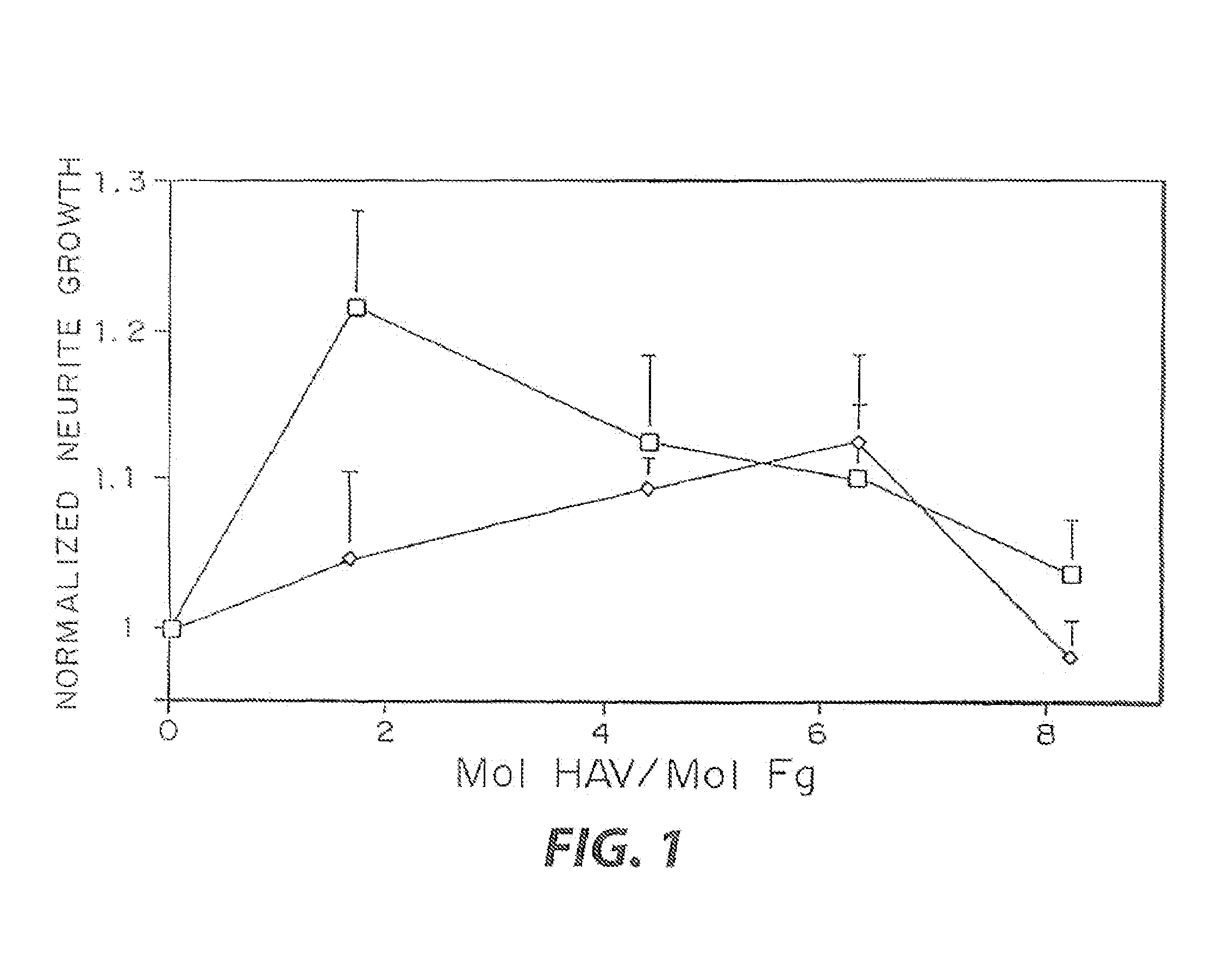
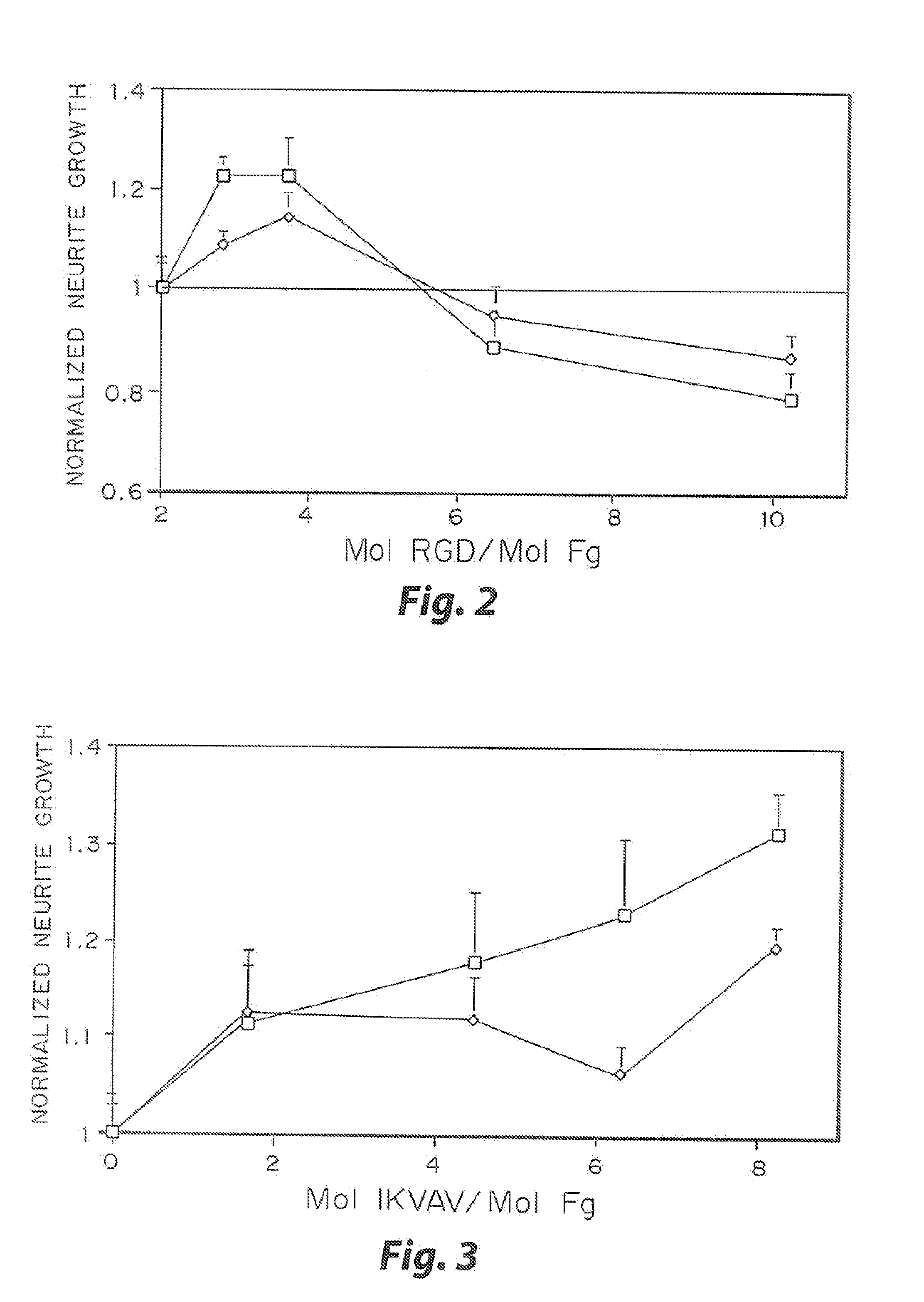
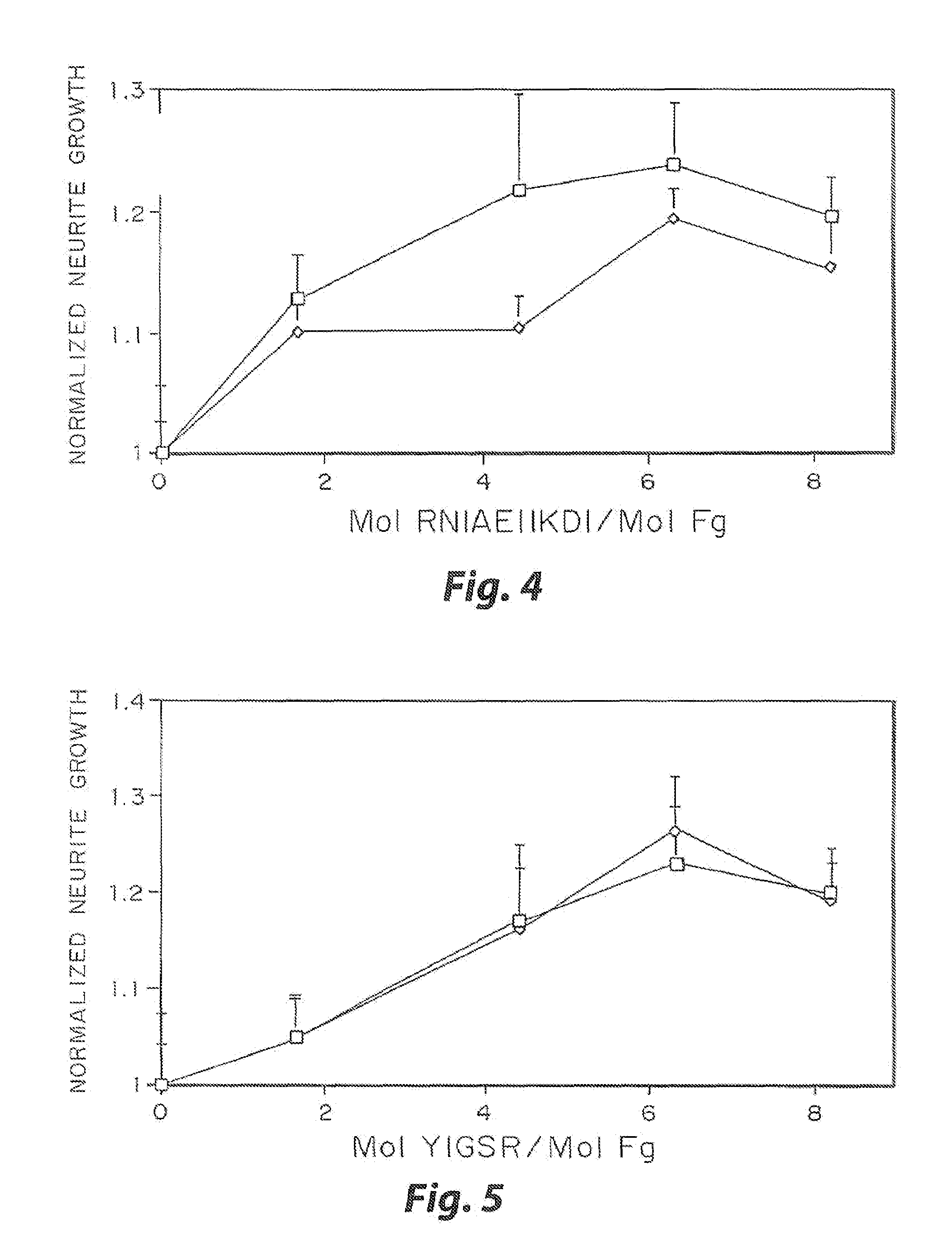
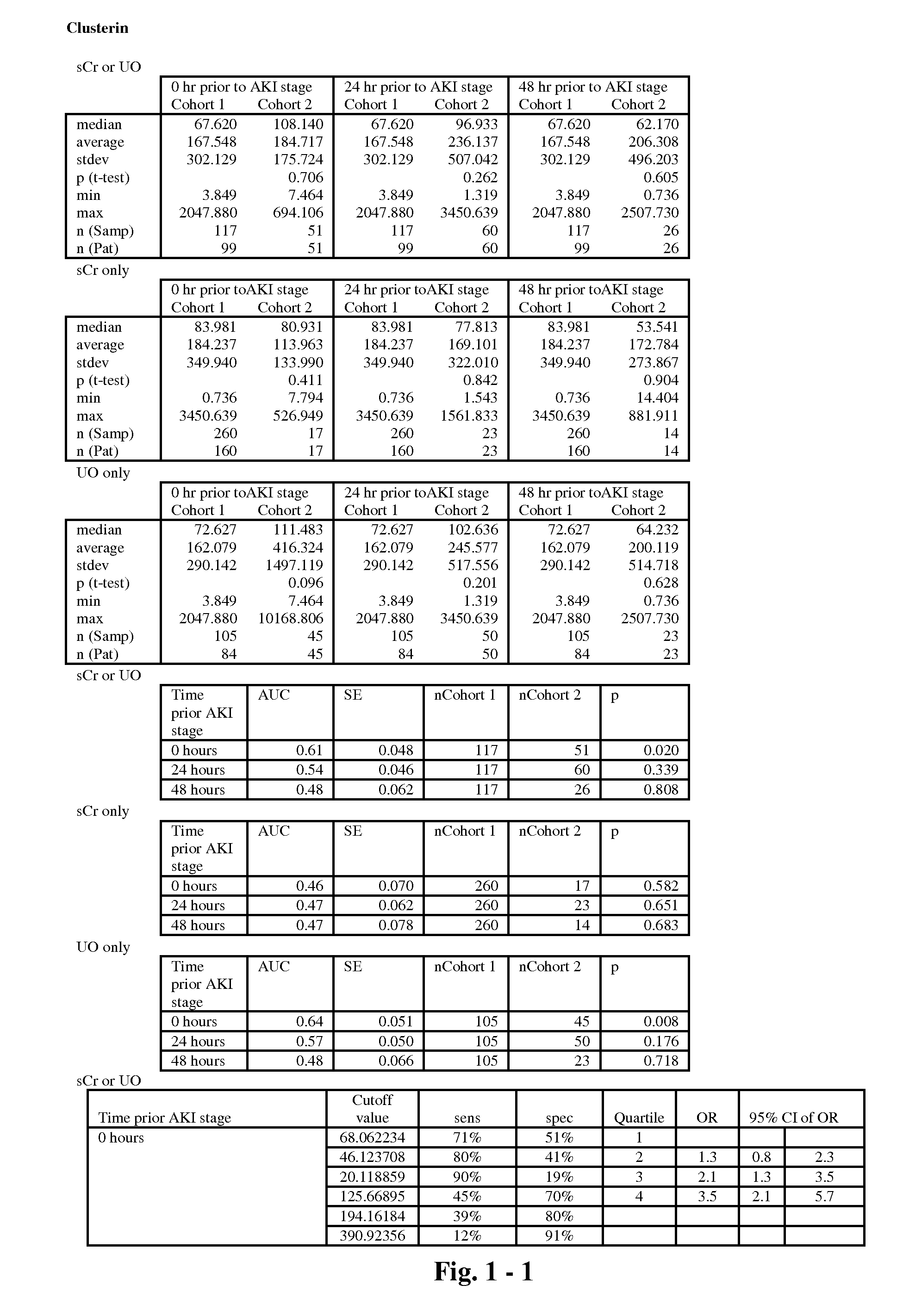
Heparin-binding regions of several proteins, such as neural cell adhesion molecule, fibronectin, laminin, midkine, and anti-thrombin III have been shown to promote neurite extension on two-dimensional surfaces. The effect of heparin-binding peptides on neurite extension through three-dimensional matrices was investigated by culturing embryonic chick dorsal root ganglia (DRG) within fibrin gels containing chemically attached heparin-binding peptide (HBP). The length of neurites within fibrin gels containing cross-linked HBP was increased by more than 70% over extension through fibrin gels containing no peptide. The HBP sequence of antithrombin III was incorporated into the fibrin gel as the C-terminal domain of a bidomian, chimeric peptide; the N-terminal second domain of this peptide contained the ∀2-plasmin inhibitor substrate for Factor XIIIa. Factor XIIIa, a transglutaminase, was used to chemically attach the HBP-containing chimeric peptide to the fibrin gels during polymerization. The amount of HBP cross-linked into the fibrin gels was determined, after degradation by plasmin using gel permeation chromatography, to be approximately 8 moles of peptide per mole fibrinogen. A peptide (HBP), where the cross-linking glutamine was replaced with glycine, showed no increase in extension in comparison with fibrin gels. The additional of heparin to the gel percursors resulted in no increase in neurite extension in comparison with fibrin gels. HBPs promote neurite extension by binding to cell surface proteoglycans on the DRG.
Owner:UNIV ZURICH +1
Methods and compositions for diagnosis and prognosis of renal injury and renal failure
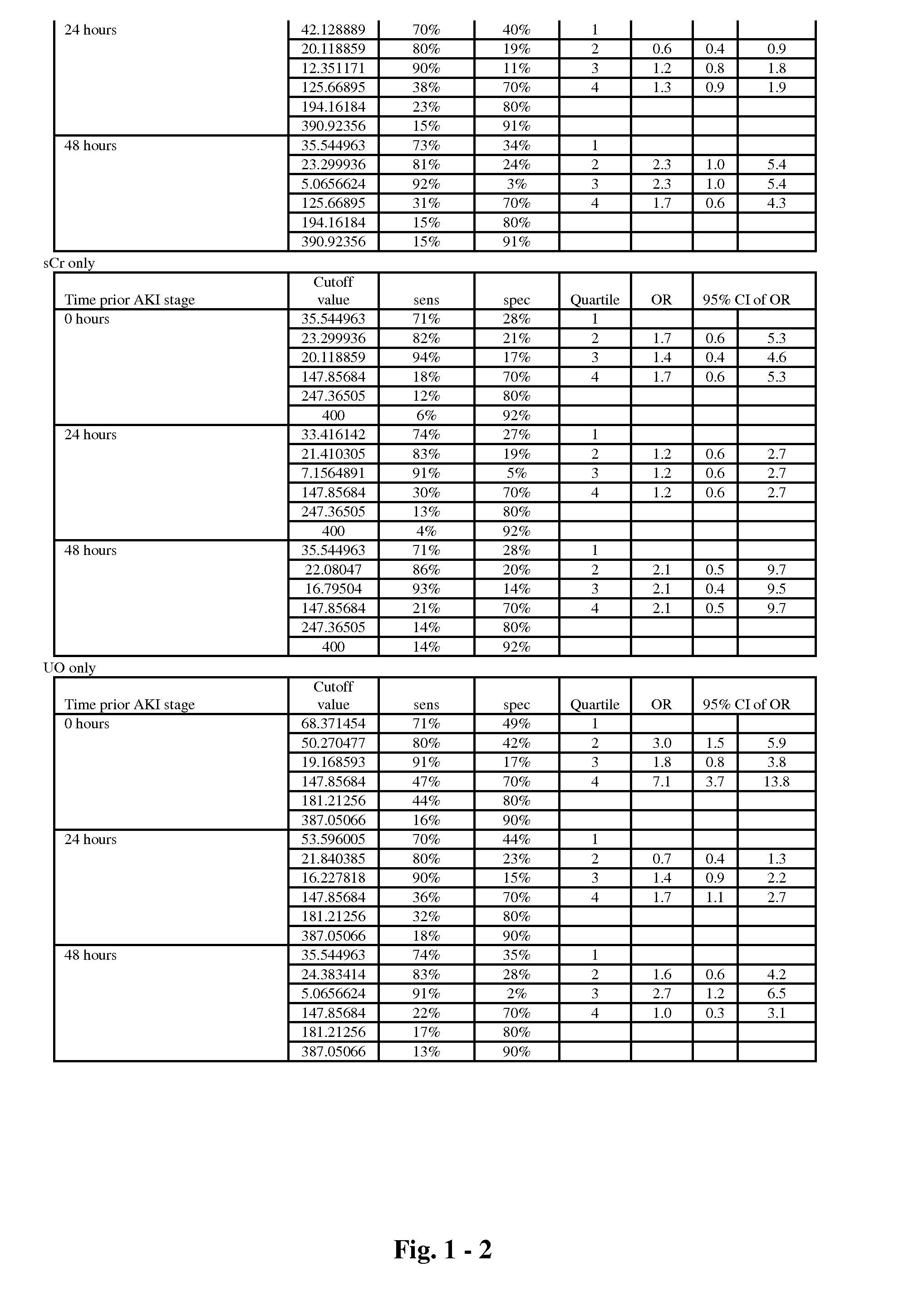
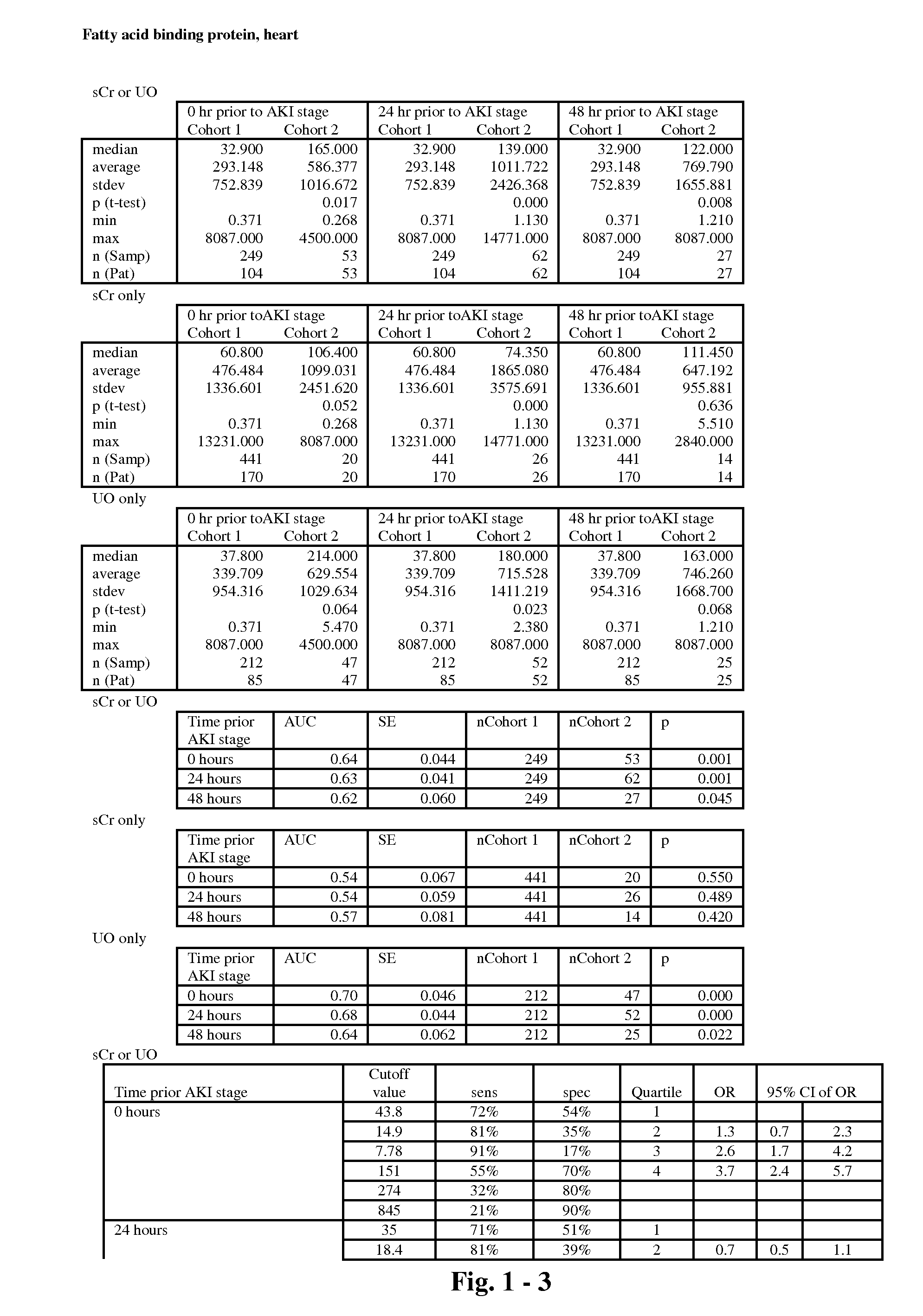
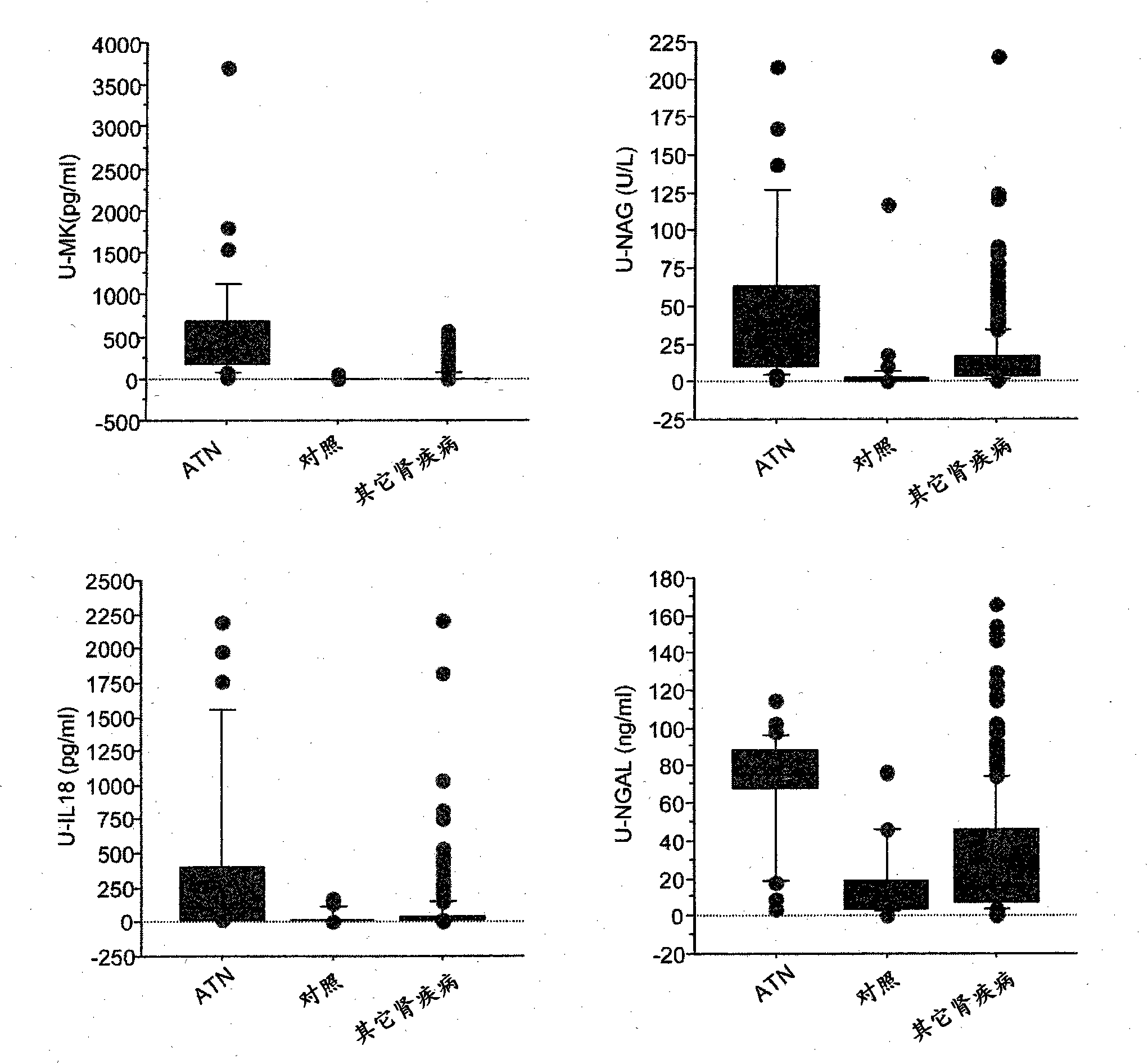
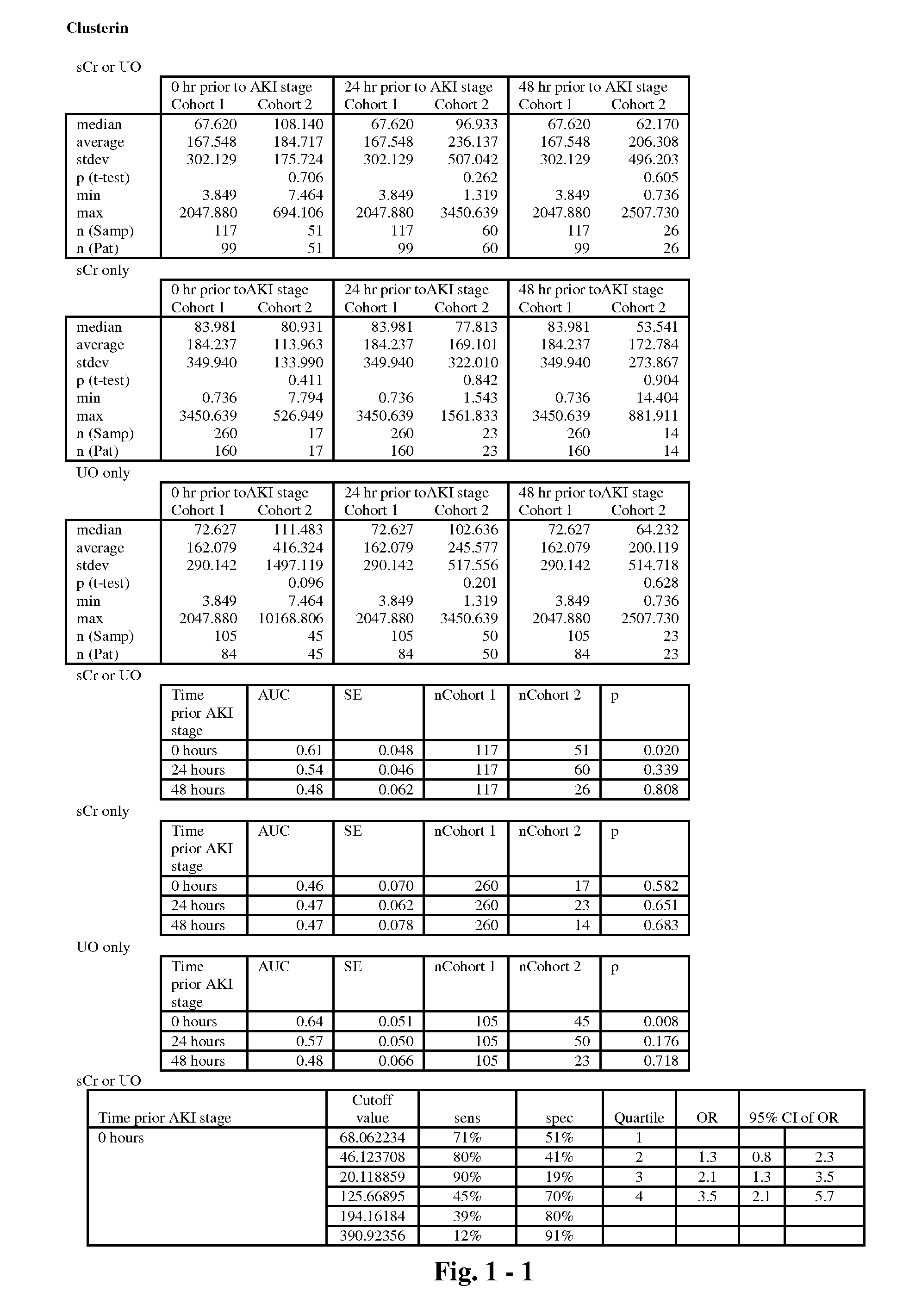
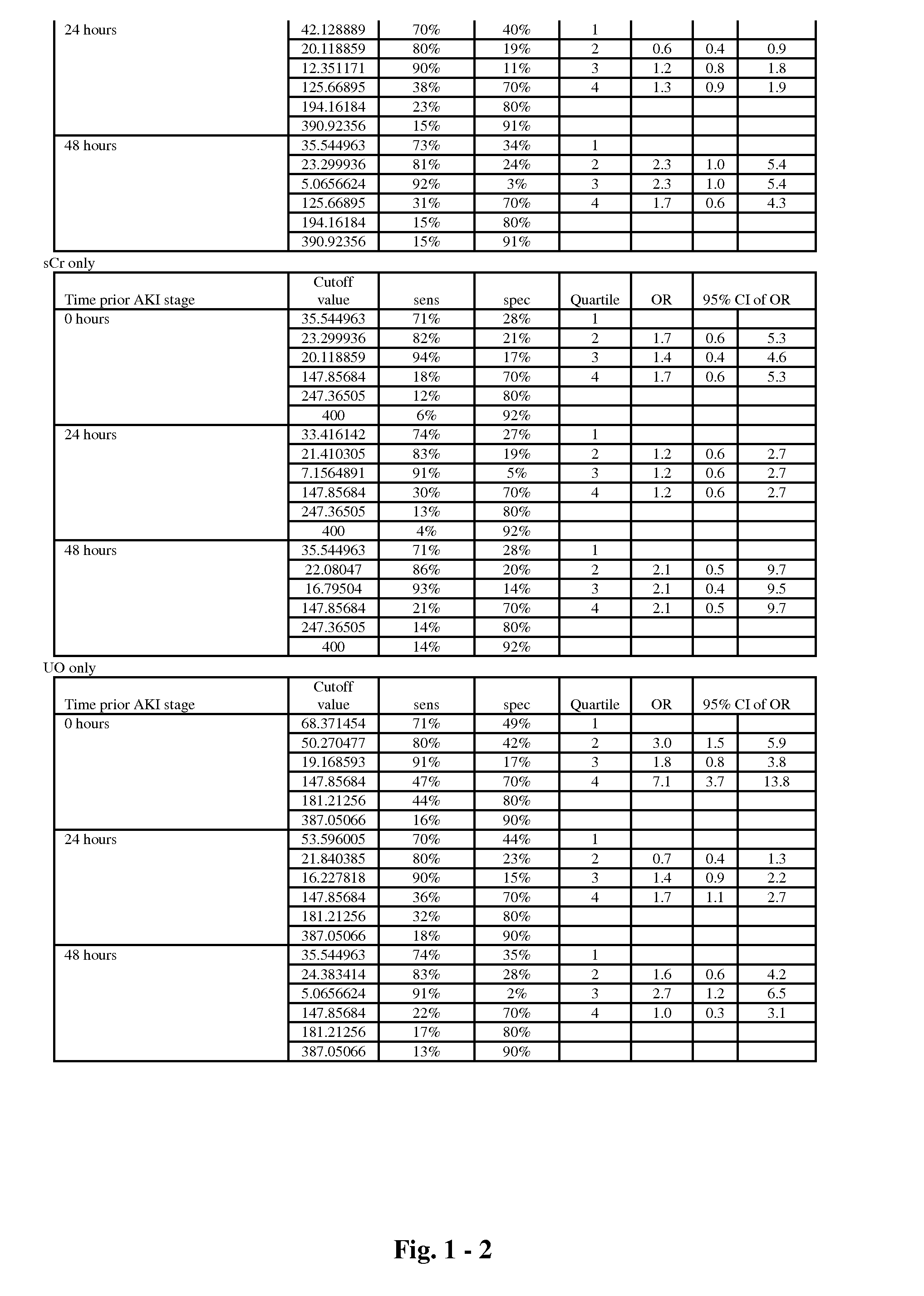
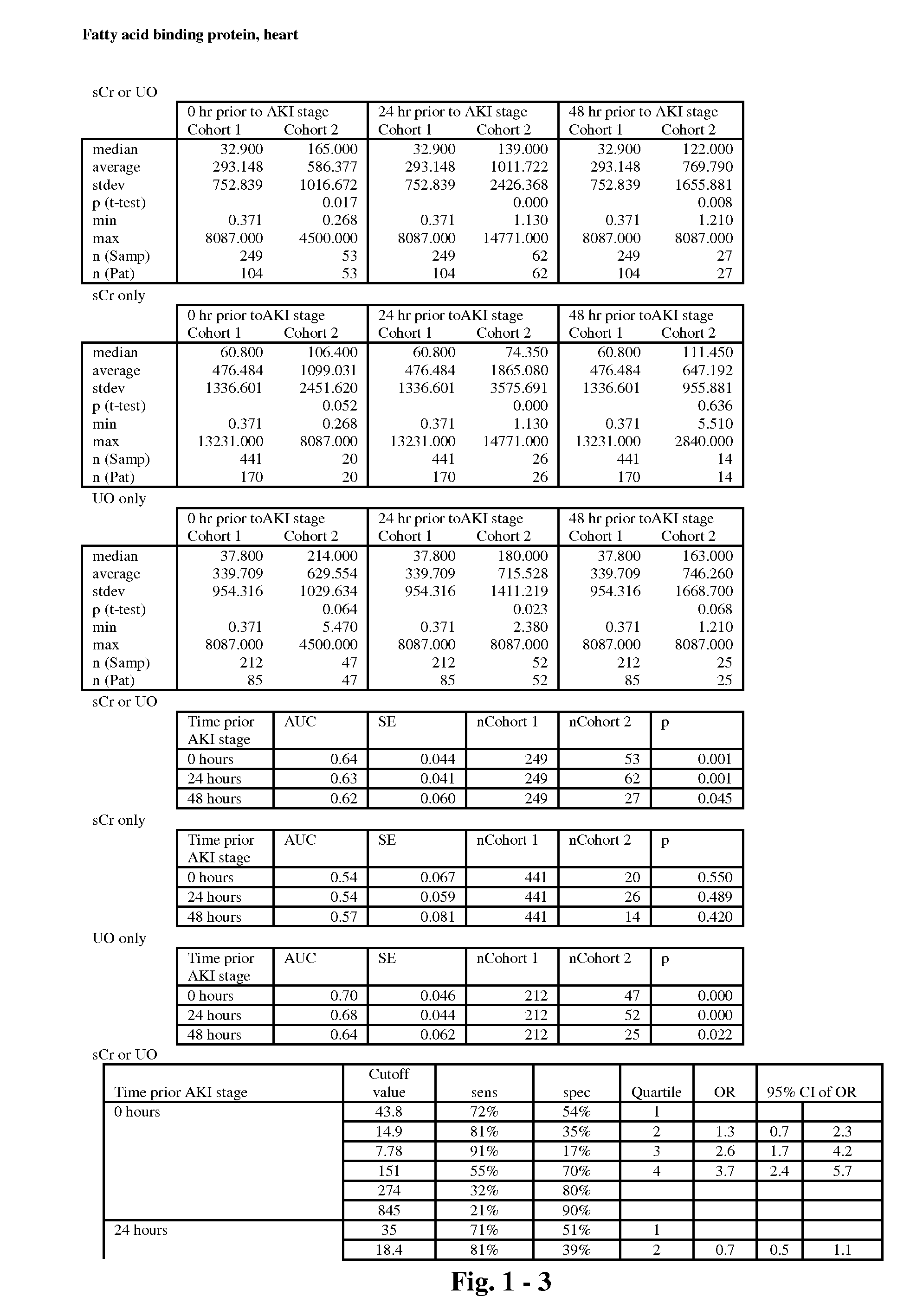
The present invention relates to methods and compositions for monitoring, diagnosis, prognosis, and determination of treatment regimens in subjects suffering from or suspected of having a renal injury. In particular, the invention relates to using assays that detect one or more markers selected from the group consisting of Clusterin, Heart-type fatty acid binding protein, Hepatocyte growth factor, Interferon gamma, Interleukin-12 subunit beta, Interleukin-16, Interleukin-2, 72 kDa type IV collagenase, Matrix metalloproteinase-9, Midkine, and Serum amyloid P-component as diagnostic and prognostic biomarkers in renal injuries.
Owner:ASTUTE MEDICAL
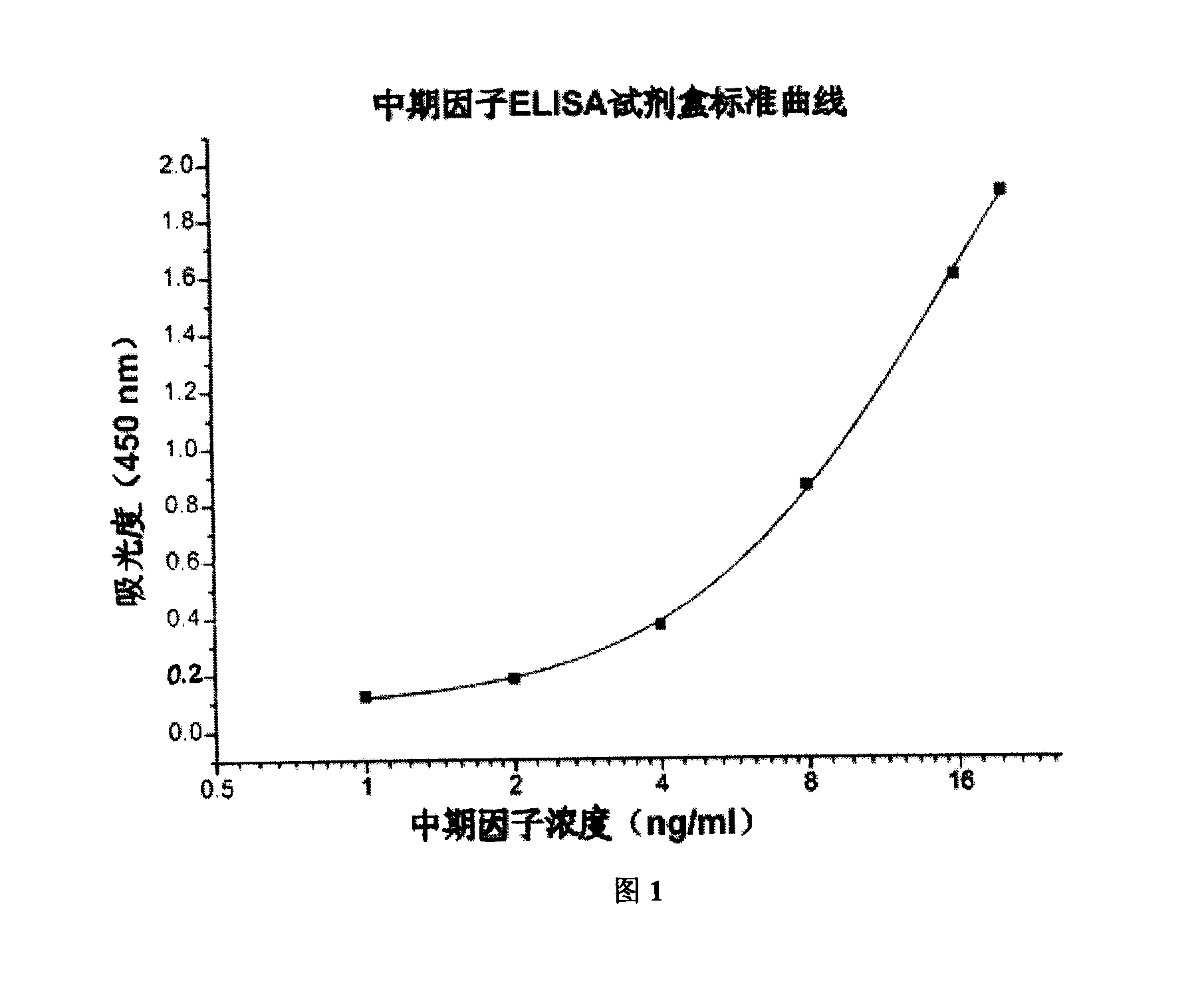
ELISA reagent box for detecting metaphase factor concentration and method of use thereof
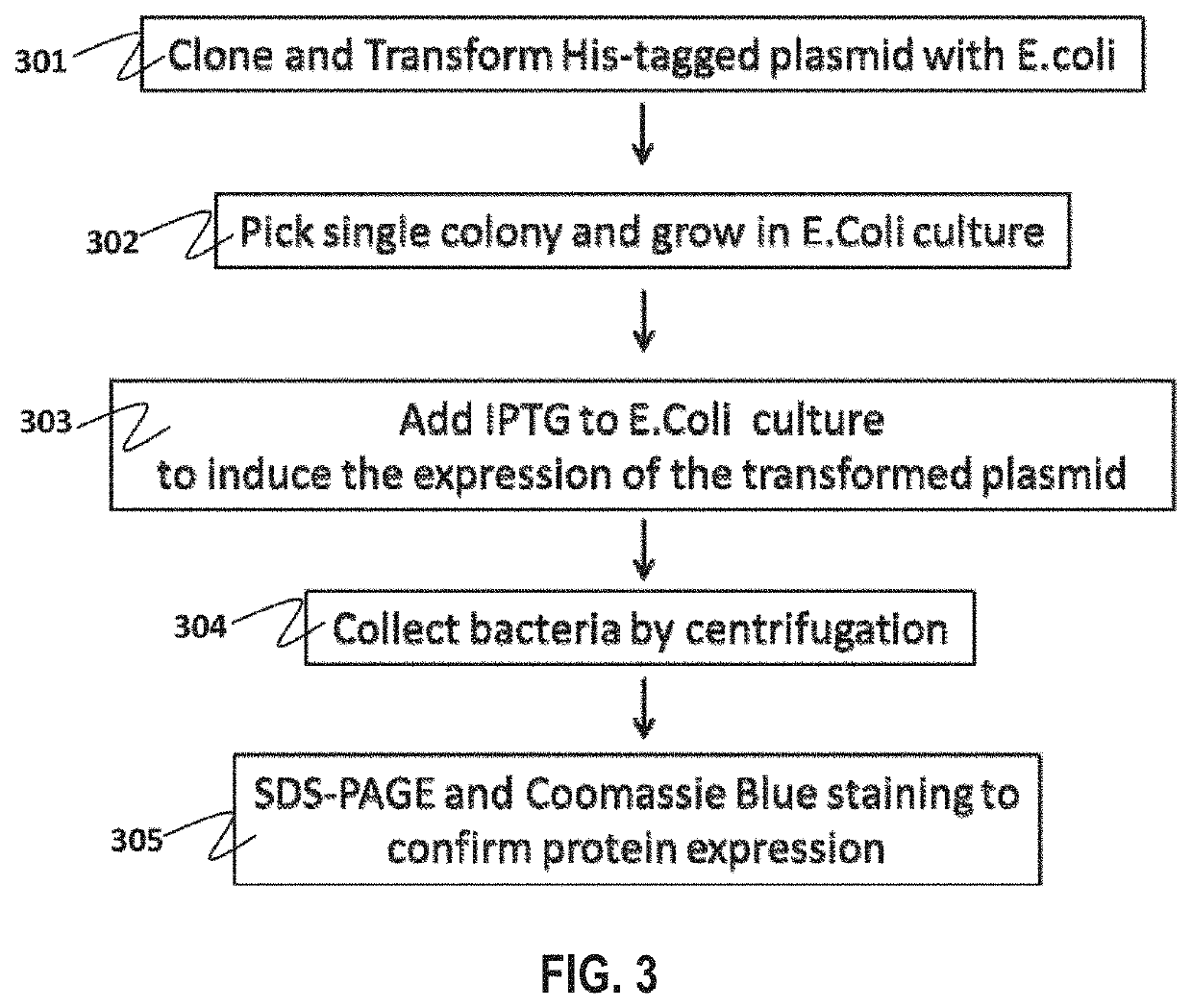
The invention provides an ELISA kit to detect the concentration of Midkine and a method of application thereof. The kit comprises monoclonal antibodies of mouse anti-human MK coated on an enzyme marker; polyclonal antibodies of rabbit anti-human; polyclonal antibodies of goatanti rabbit IgG marked with horse radish peroxidase (HRP); His-MK expressed with procaryon in colibacillus E.coli and affinity purified with N ends having six His labels. The application method comprises coating a microlon ELISA plate with commercial monoclonal antibodies of Midkine of mouse anti-human; adding specimens to be detected, and multiple resistance of Midkine of rabbit anti-human and rabbit anti-human IgG marked with HRP for joint incubation after the plate is sealed; adding substrate solution of TMB for reaction and visualization; finally, terminating the reaction with vitriol, and detecting the absorbance at the position of 450nm. A standard curve is drawn with the concentration of standard samples and corresponding absorbance. In this way, the concentration of Midkine in the specimens to be detected can be got through the absorbance and the corresponding standard curve.
Owner:ZHEJIANG UNIV
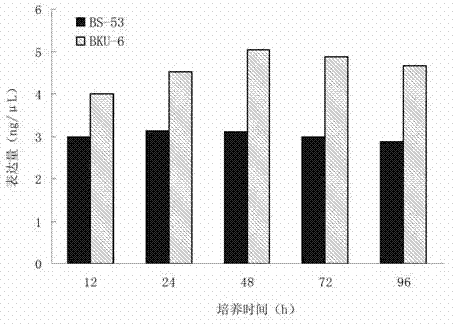
Vitamin K2 high-yielding strain
The invention aims to disclose a vitamin K2 high-yielding strain. Compared with the prior art, bacillus natto BS-5 is taken as an original strain, after the ultraviolet and nitroso arc composite mutation, the mutant strain BS-53 is obtained after screening, the mutant strain BS-53 and bacillus natto CICC (China center of industrial culture collection)10262 which has low vitamin K2 yield but high growth rate, are subjected to protoplast fusion, fusant is obtained through the screening of a flat screen, and the fusant is finally obtained after the selecting of the fermenting property experiment; and the vitamin K2 high-yielding strain has excellent characteristics of parents, i.e. has the dual effects of high MK (midkine) yield and high growth rate, an effective method is provided for realizing high MK yield, important scientific meaning and application value are obtained, and the purpose of the invention is realized.
Owner:上海红马饲料有限公司
Antibody recognizing c-domain of midkine
ActiveUS20100311187A1Inhibit functioningInhibit cell migrationAntipyreticAnalgesicsEpitopeDiagnostic agent
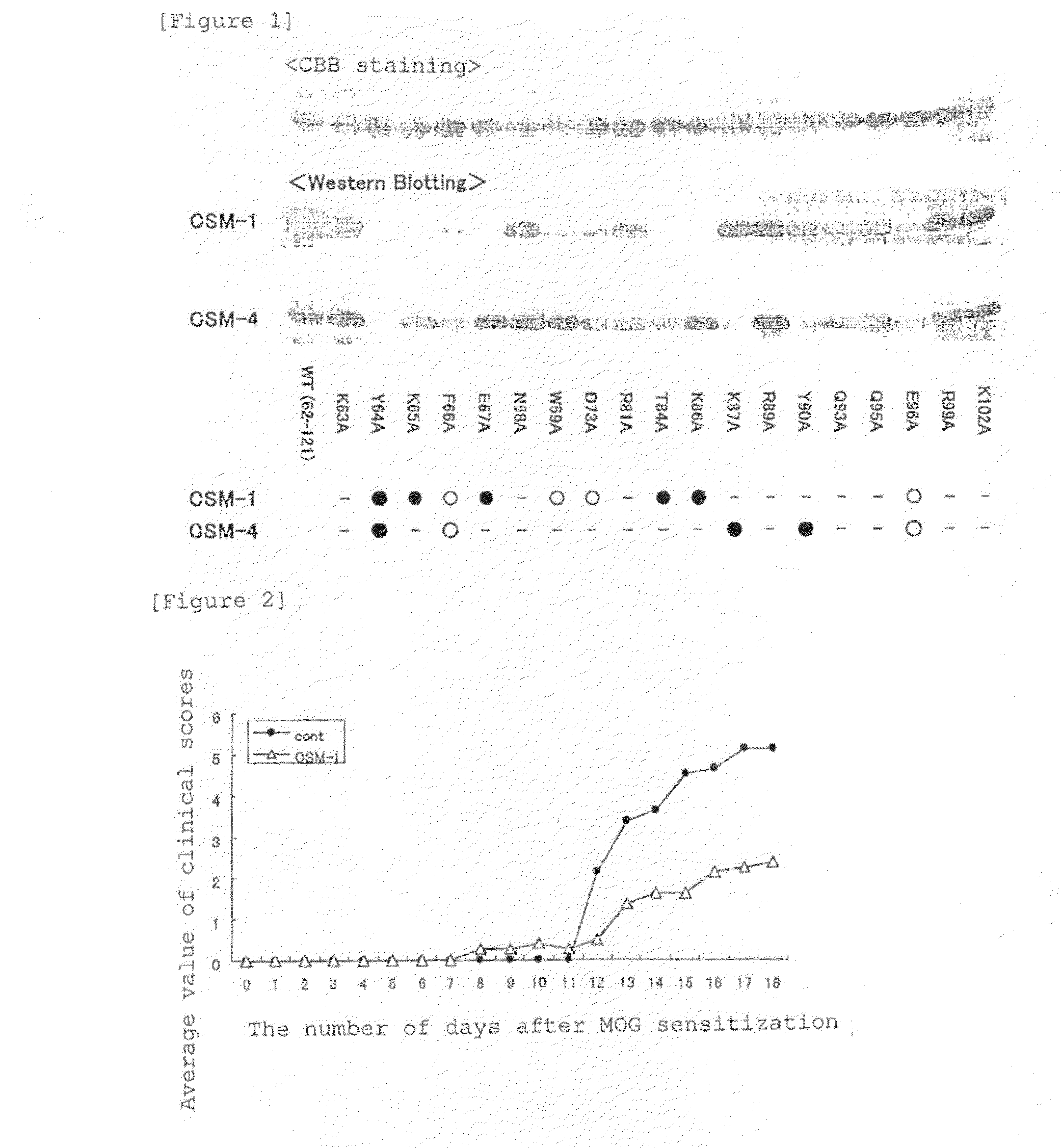
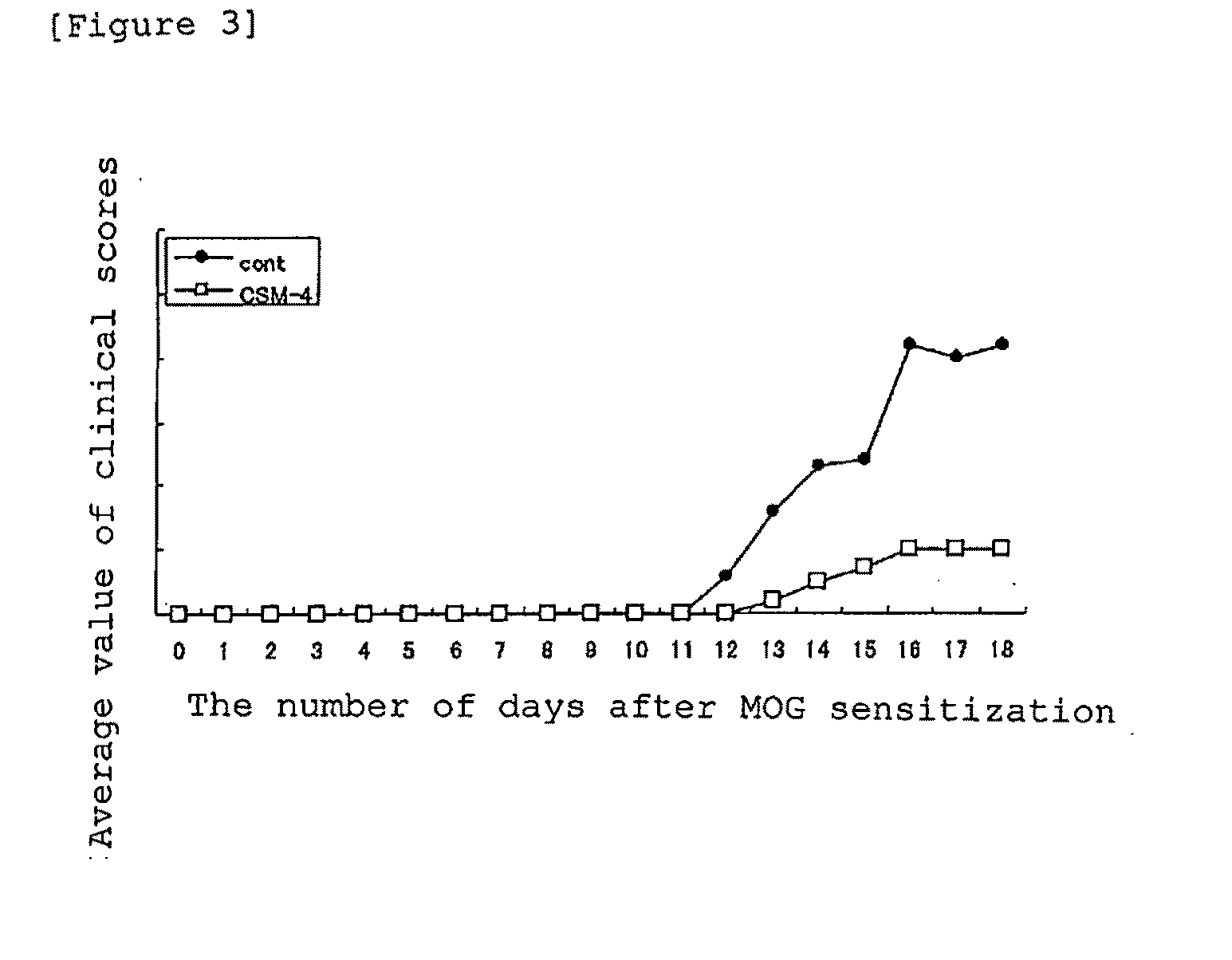
It is intended to provide an antibody which inhibits the function of midkine. An antibody which recognizes an epitope consisting of amino acid residues 62 to 104 of midkine or a fragment thereof; DNA encoding the antibody or a fragment thereof; a recombinant vector which contains the DNA; a transformant which has the vector or a hybridoma which produces the antibody; a method for producing the antibody by allowing the transformant or hybridoma to produce the antibody or a fragment thereof and collecting the resulting antibody or fragment thereof; a pharmaceutical composition containing the antibody or a fragment thereof as an active ingredient; and a diagnostic agent containing the antibody or a fragment thereof as an active ingredient.
Owner:MEDICAL THERAPIES
LM3 cell line with Midkine stable low expression and construction method of LM3 cell line
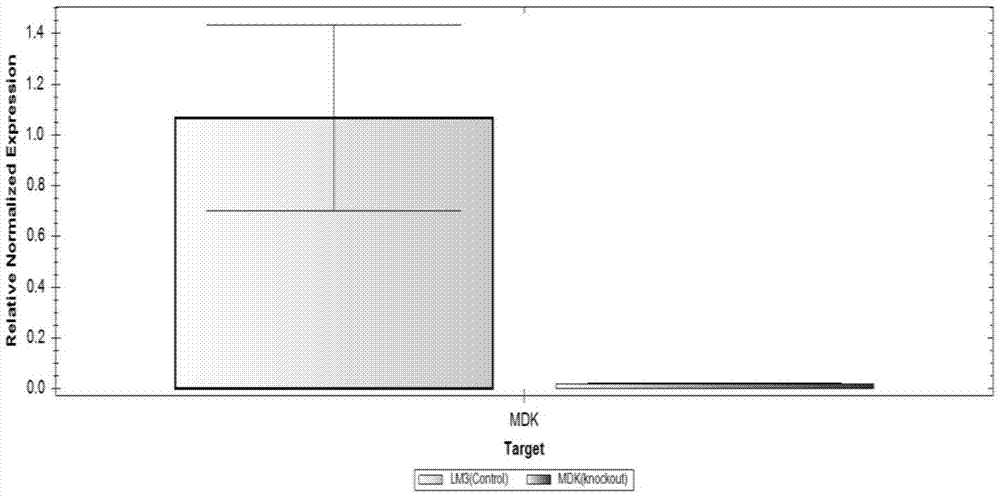
InactiveCN106867967AStable expressionHigh transfection efficiencyTumor/cancer cellsGenetic engineeringLiquid mediumStable cell line
The invention relates to the field of biomedical research, and in particular to a construction method of an LM3 stable cell line with Midkine low expression. The LM3 cell line with Midkine stable low expression is constructed by virtue of an RNA interference technology. The construction method comprises the following steps: conducting primer design and target fragment amplification, and constructing Midkine shRNA recombinant plasmid; then, collecting a liquid medium containing viruses, filtering the liquid medium, adding the filtered liquid medium to a culture dish inoculated with LM3 cells in advance and conducting co-culture; and finally, conducting Puromycin resistance screening and sub-culturing on the LM3 cells accepting transfection, so that the LM3 stable cell line with Midkine low expression is finally obtained. With the establishment of the cell line, a new experimental material is provided for researching a molecular action mechanism of Midkine in tumors and a regulatory effect of the Midkine in tumor energy metabolism.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Tumor-specific promoter
InactiveUS20060099188A1High tumor-specificity and promoter activityHigh promoter activityBiocideGenetic material ingredientsPromoter activityC erbb 2
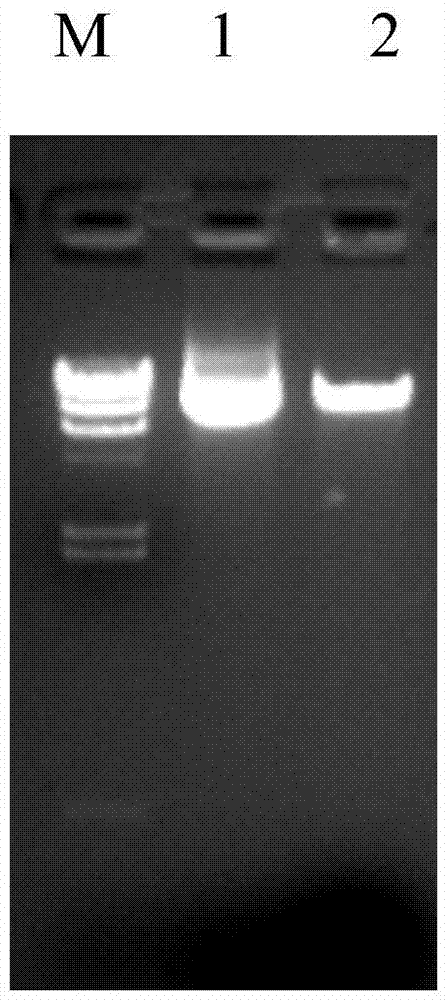
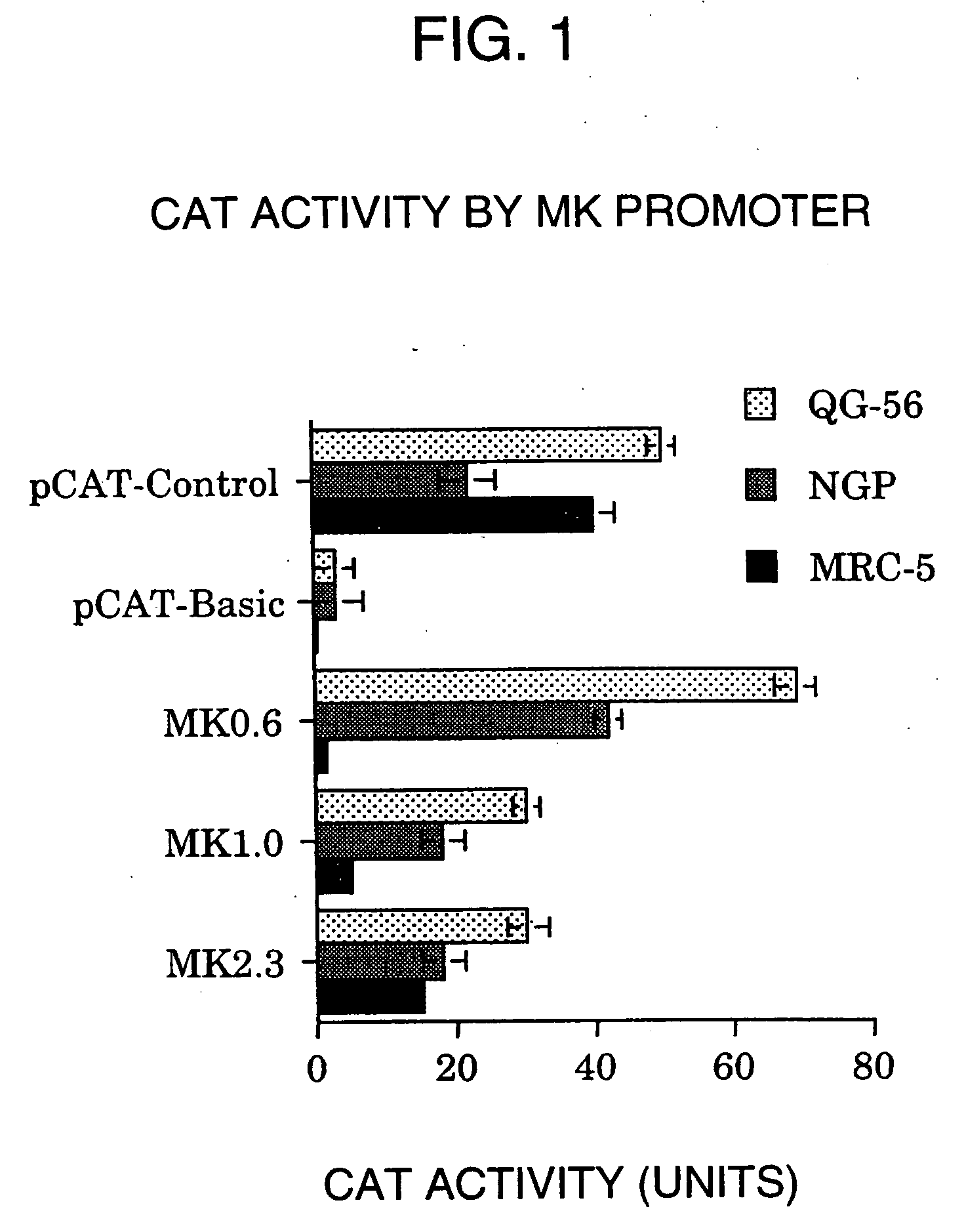
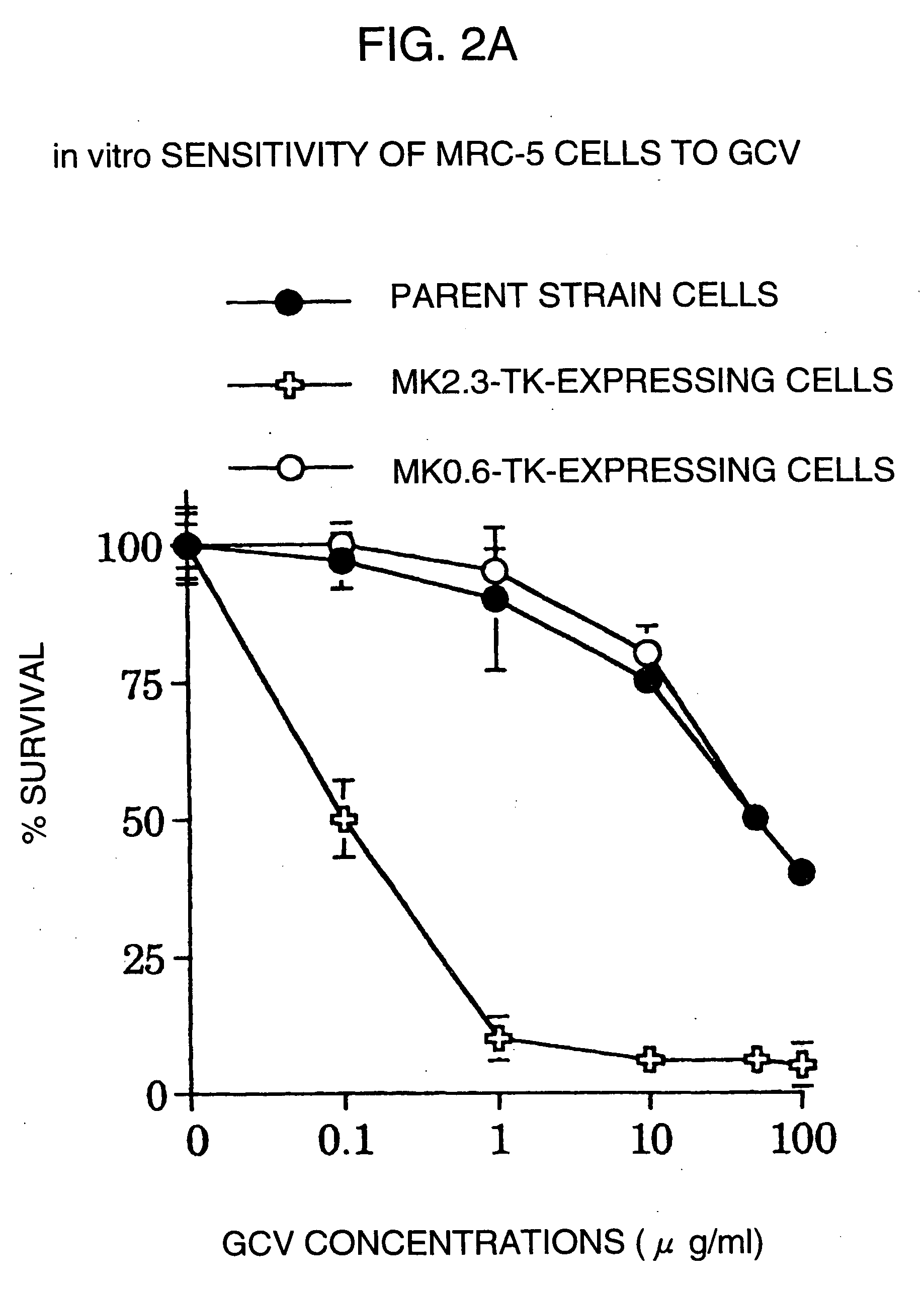
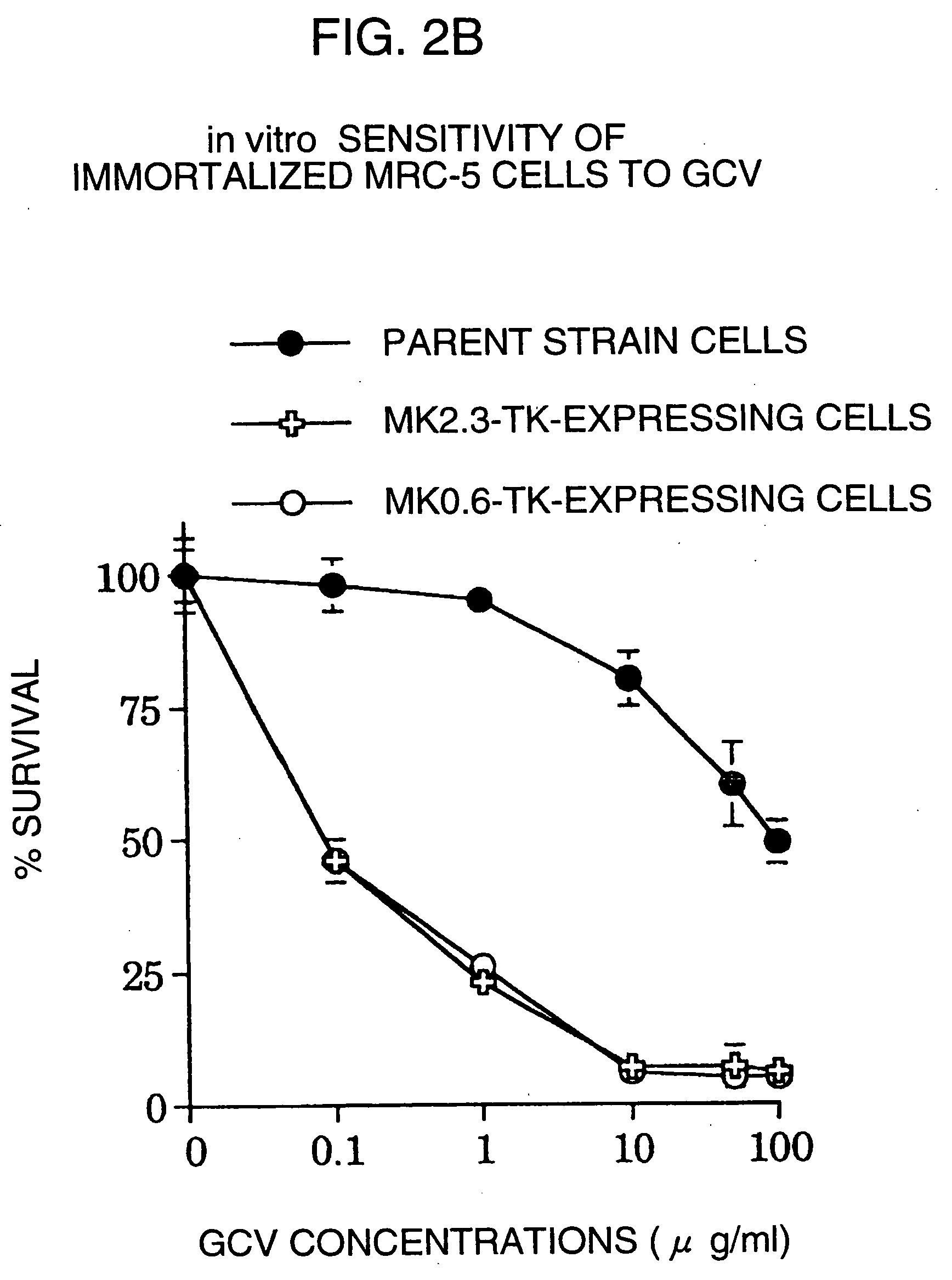
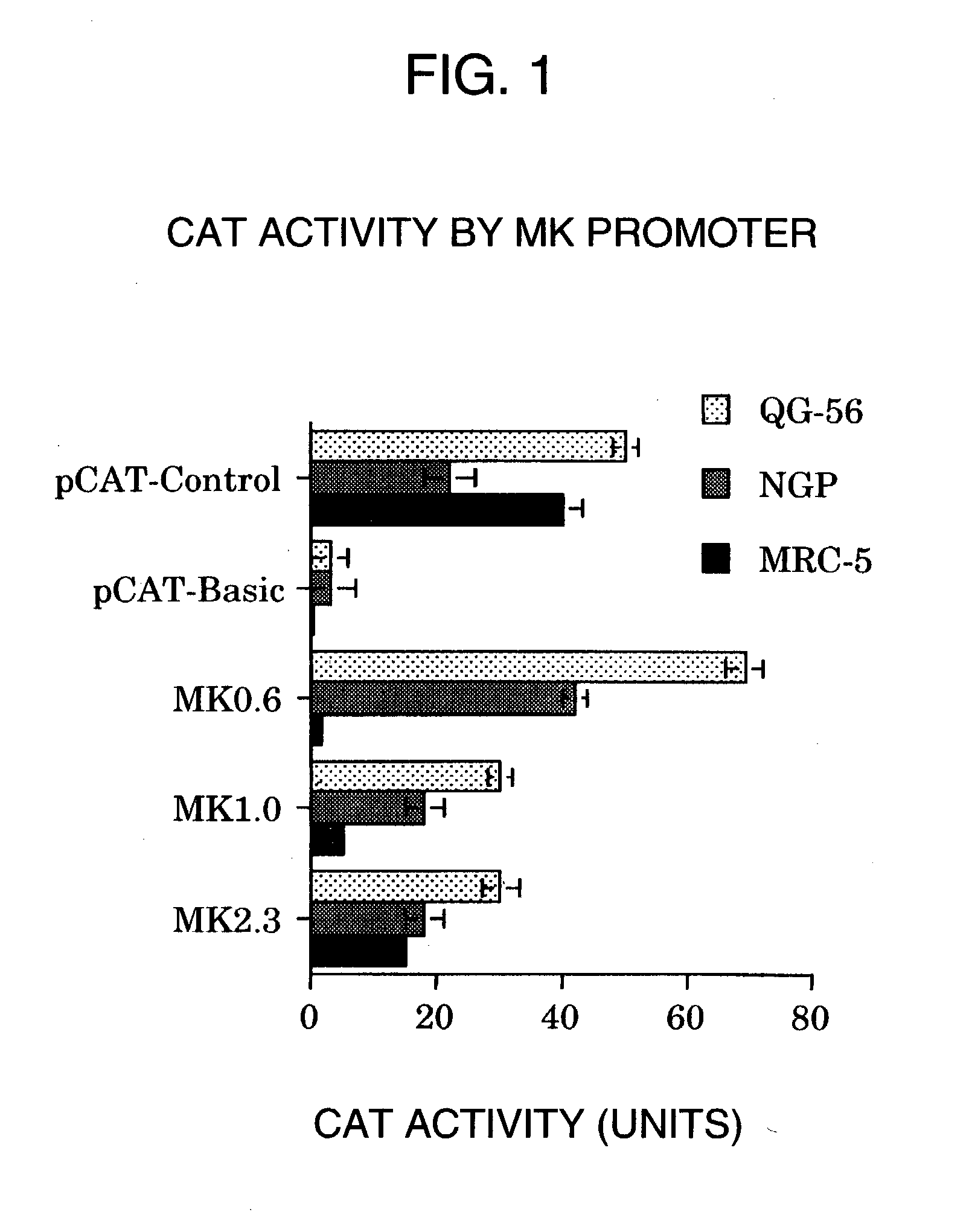
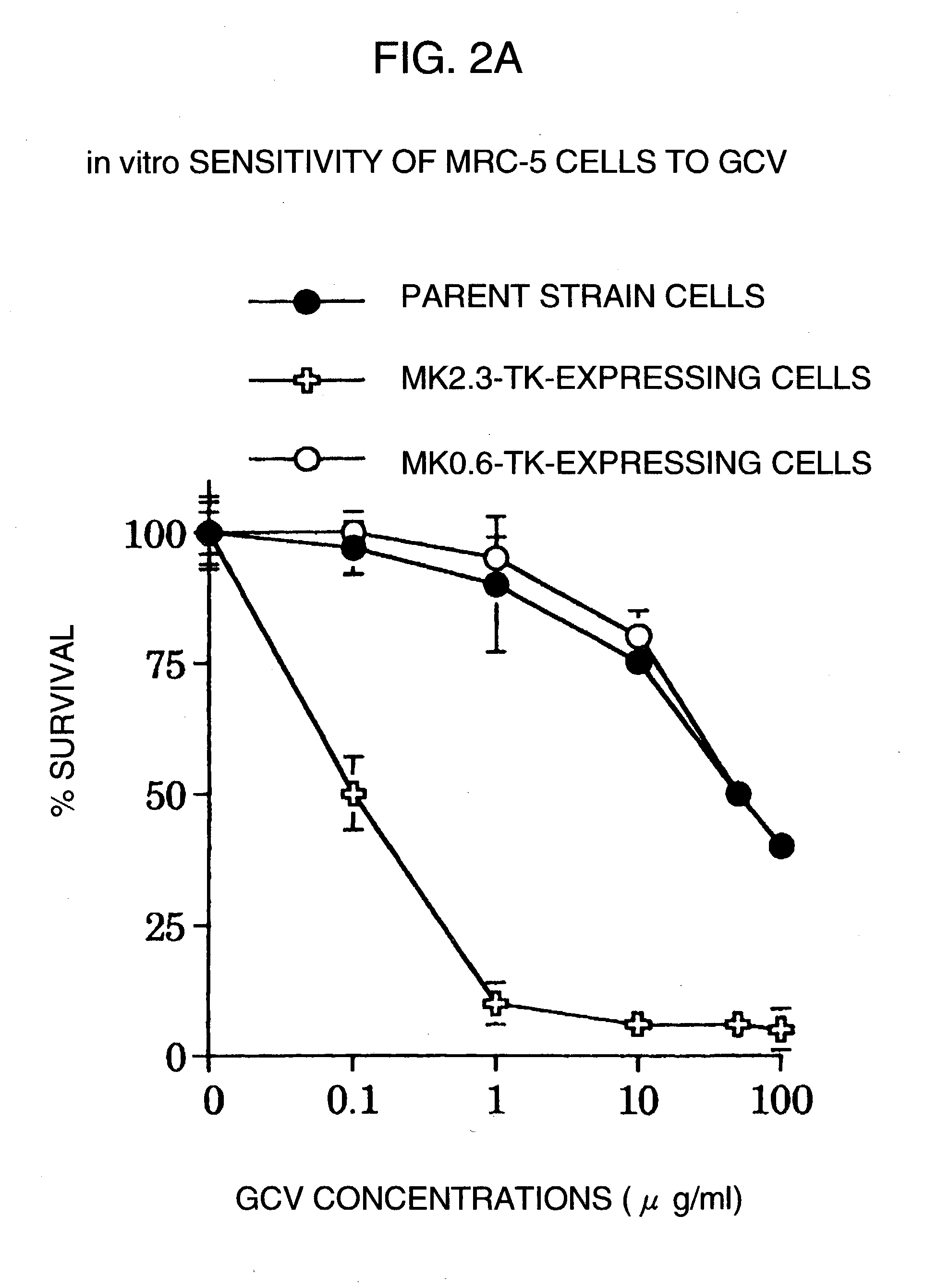
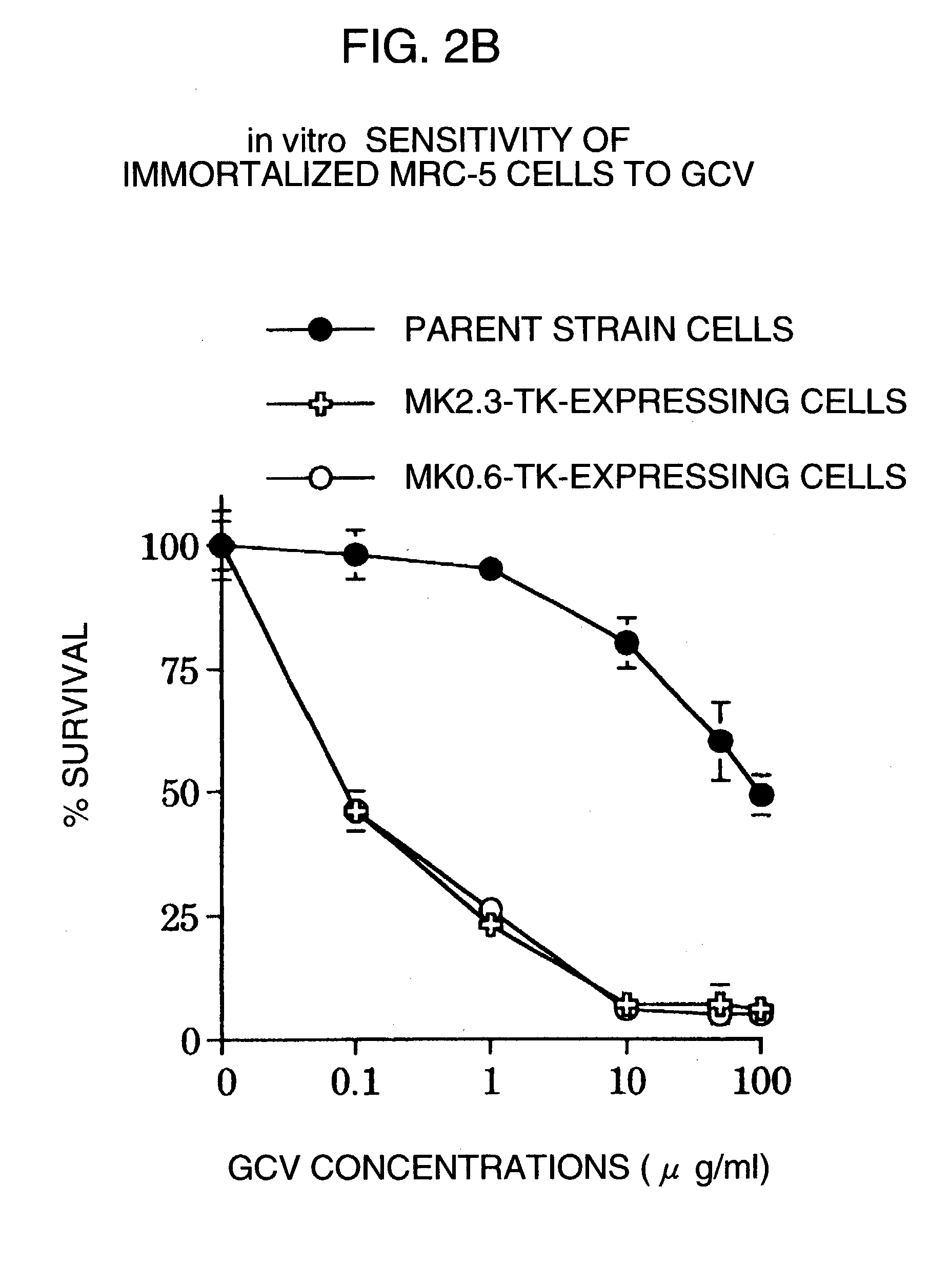
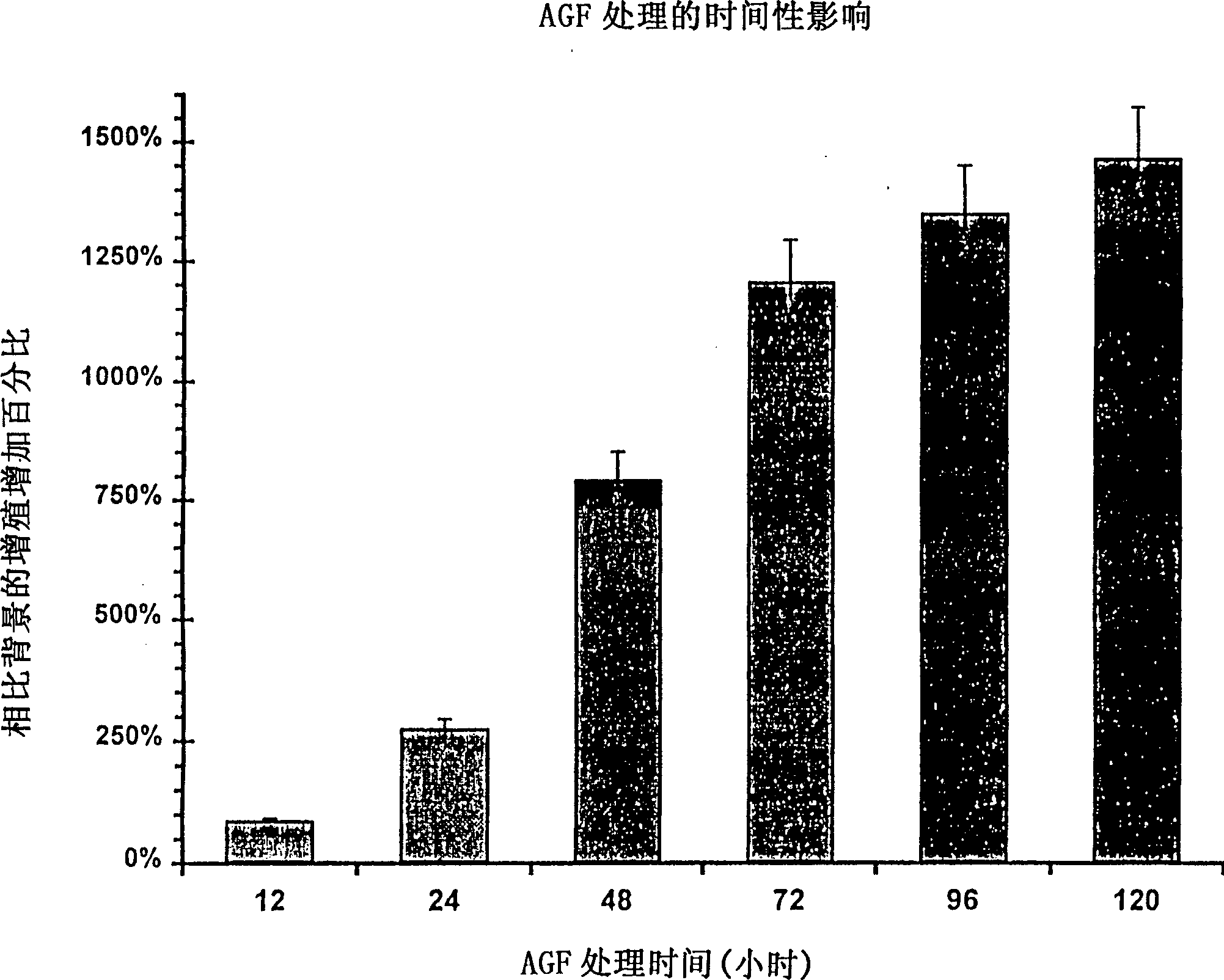
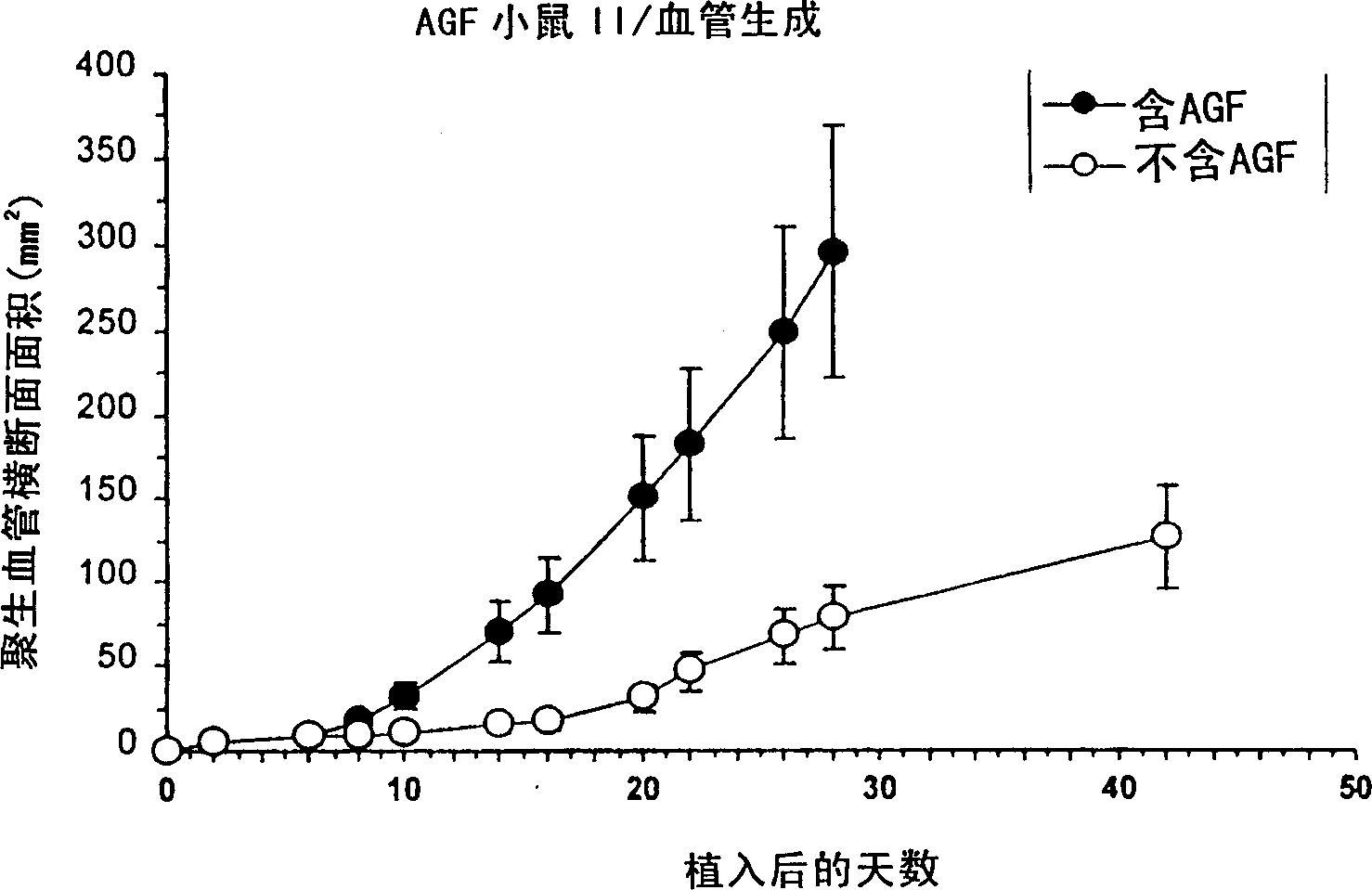
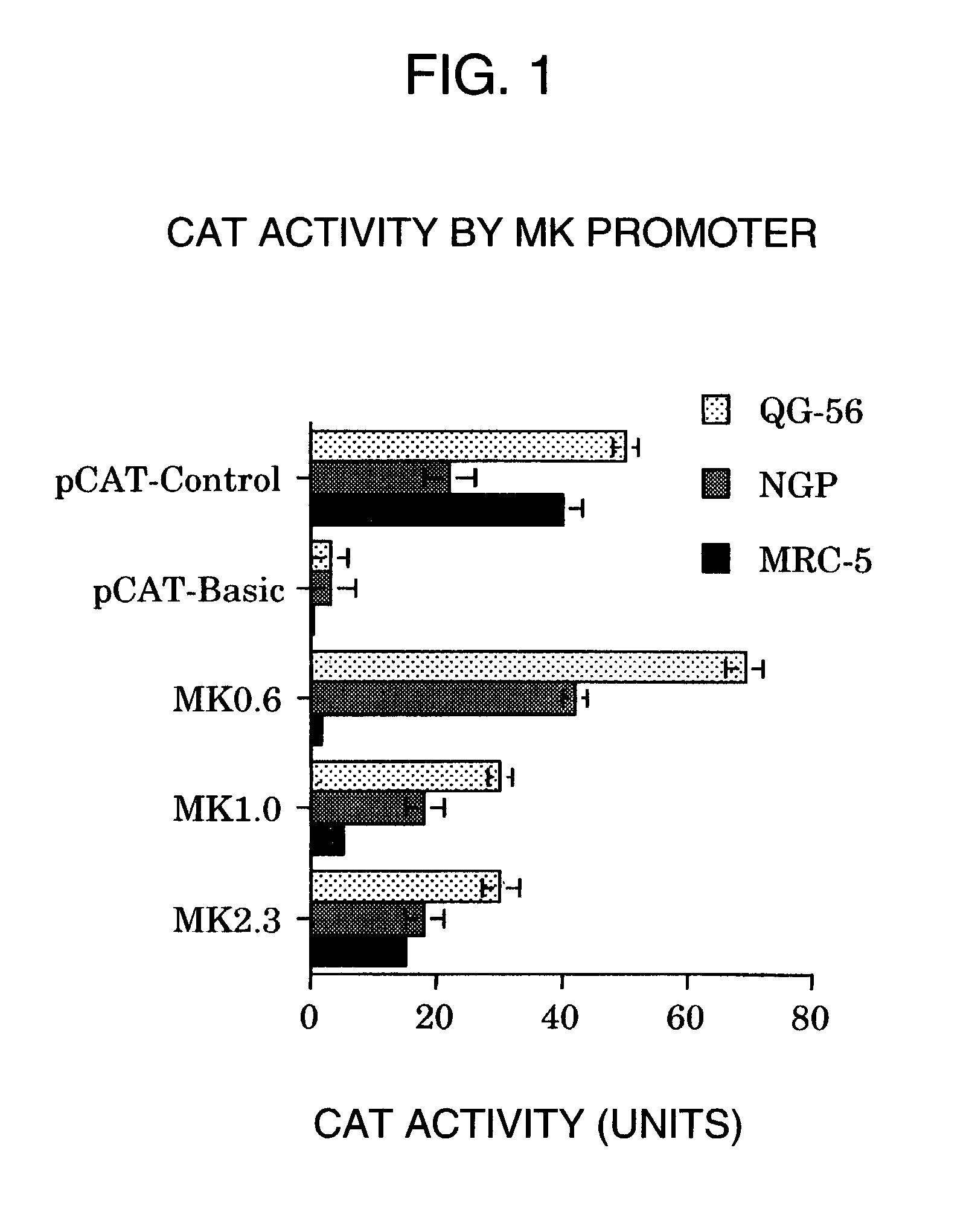
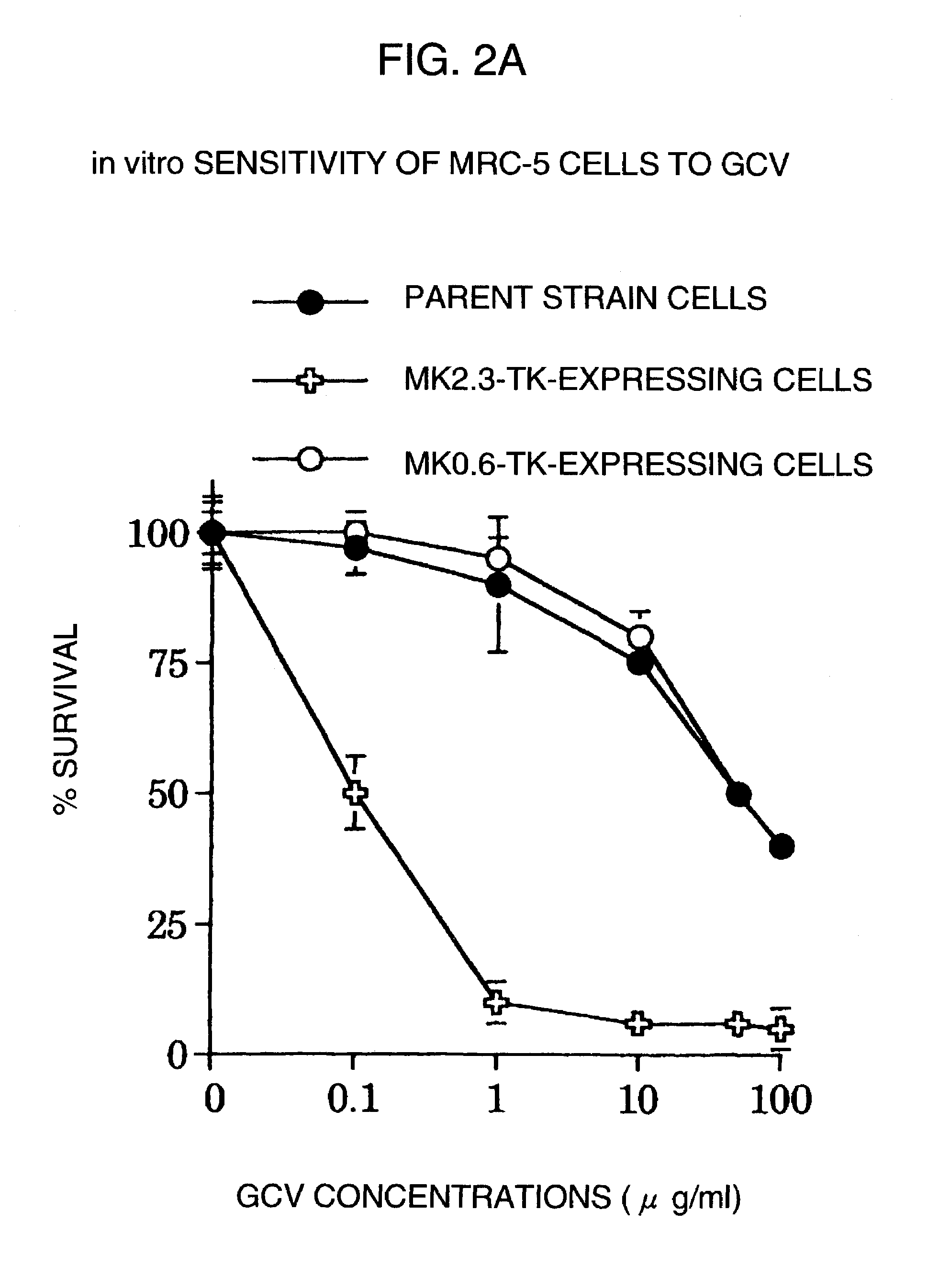
A DNA comprising a 609 bp base sequence from −559 to +50 when the first base sequence of exon 1 of the midkine gene, a human retinoic acid-responsive growth / differentiation factor was set as +1, or a DNA comprising a 251 bp base sequence from −213 to +38 when the transcription initiation point of the c-erbB-2 gene belonging to the EGF receptor family and having a tyrosine kinase activity was set as +1 has a tumor-specific transcription activity, and the promoter activity thereof is high, and therefore is very important as a tumor-specific promoter for use in the suicide gene therapy that combines the use of a gene for a drug metabolizing enzyme and a prodrug for cancer therapy, the gene therapy of cancer using an expression vector that contains a gene encoding a cytokine, and the gene therapy of cancer using an oncolytic virus.
Owner:PRIMMUNE CORP +1
Biomarker for the estimation of acute renal disorder and prognosis of the disorder, and use of the biomarker
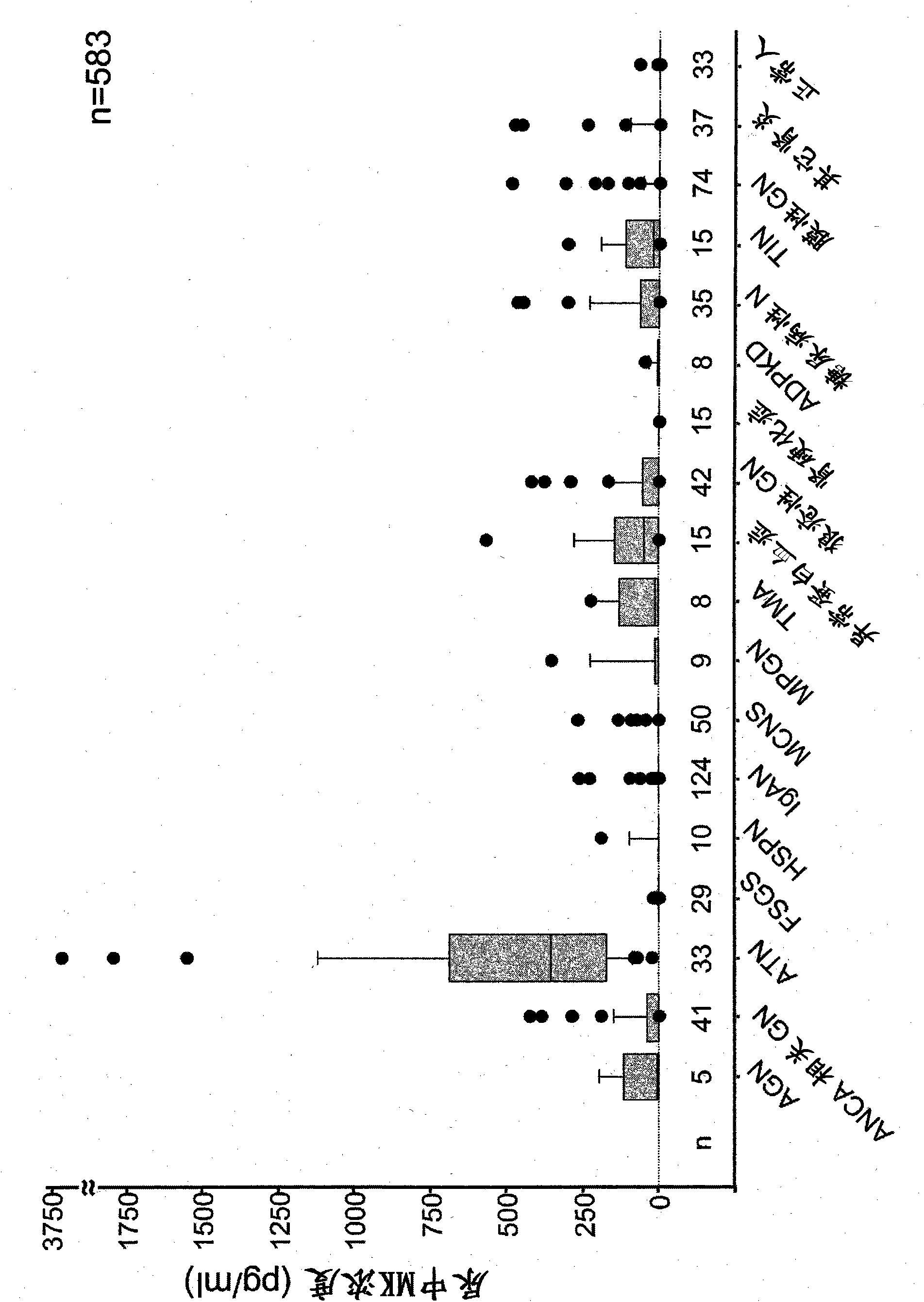
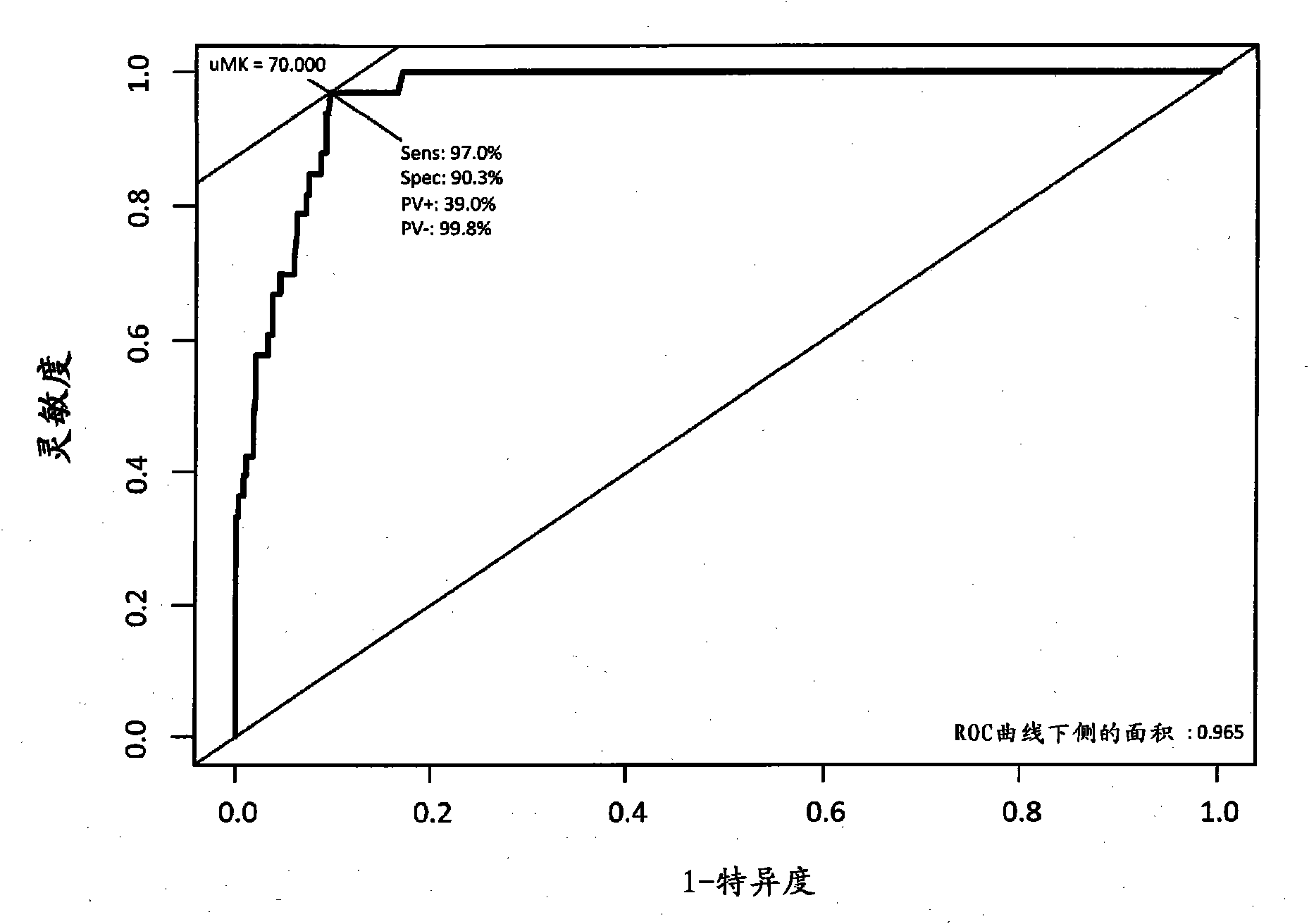
Disclosed is a novel biomarker which is useful for the prediction of early onset of acute renal disorder, the estimation of prognosis associated with a renal function, and the diagnosis of acute renal disorder. Also disclosed is use of the novel biomarker. A midkine is used as the biomarker. The determination of the possibility of the onset of acute renal disorder, the estimation of prognosis associated with a renal function or the diagnosis of acute renal disorder can be achieved based on the results of the detection of a midkine in urine.
Owner:NAGOYA UNIVERSITY
Method for diagnosis and treatment of pulmonary disorders
InactiveUS20050130928A1Preferentially modulatingIncrease and decrease midkine expression levelVectorsMicrobiological testing/measurementDiseasePulmonary vasculature
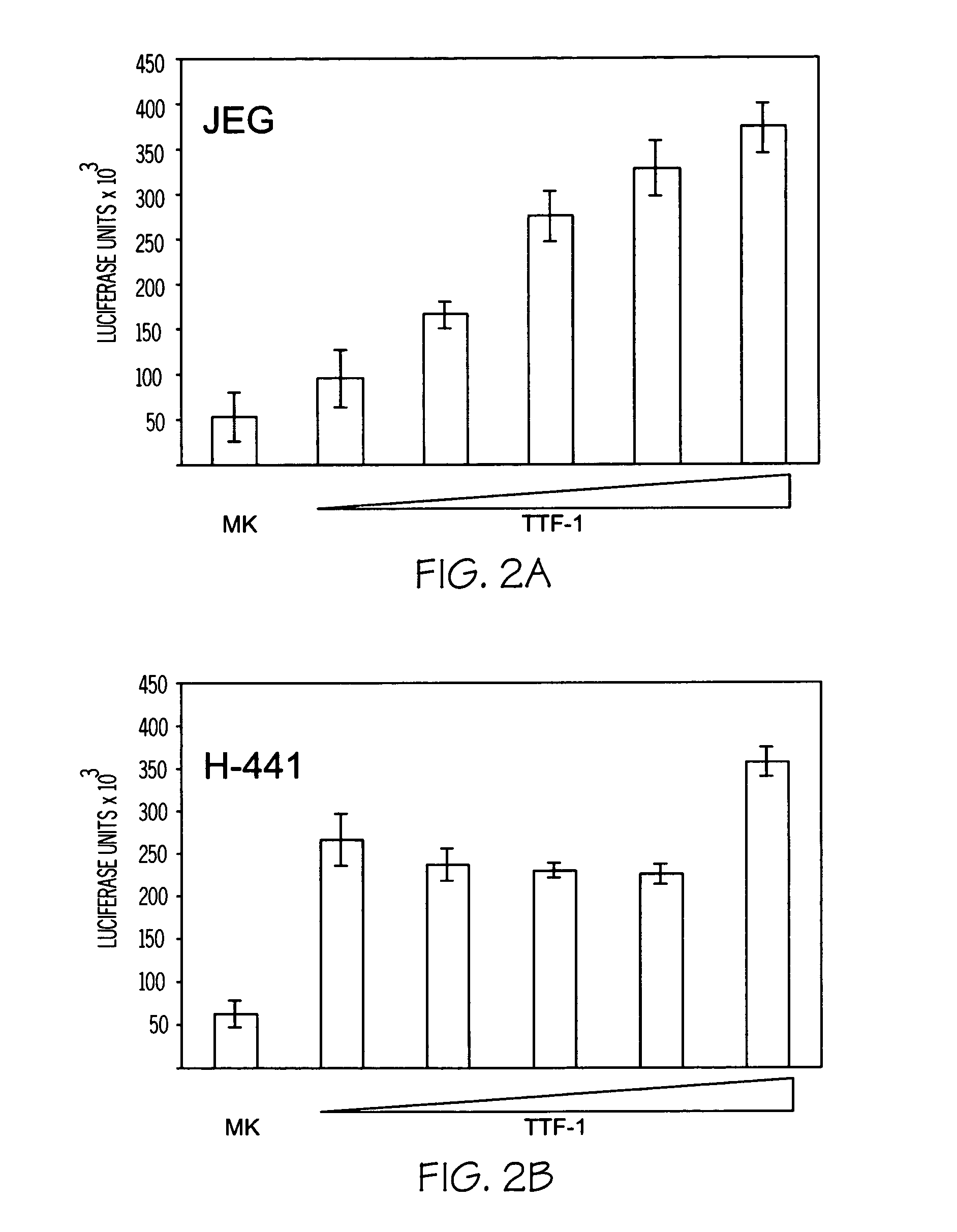
This invention relates to the discovery that midkine modulates pulmonary vasculature development and smooth muscle cell development. Modulation of midkine activity thus alters pulmonary vasculature development and smooth muscle cell development. The invention provides methods of modulating pulmonary disorders, smooth muscle cell related disorders, and pulmonary smooth muscle cell disorders. Disorders of particular interest include, but are not limited to, asthma and pulmonary hyperplasia. Further the invention relates to the discovery that TTF1 and HIF1 -α exhibit midkine modulating activity. The invention pertains to the discovery that midkine modulates myocardin activity.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
Activation of endothelial nitric oxide synthase by midkine and uses therefor in effecting vasodilation
ActiveUS8288343B2Less side effectsTherapy or prophylaxis of diseasesPeptide/protein ingredientsMetabolism disorderCoronary arteriesEndothelial NOS
Owner:CELLMID LTD
Assay to measure midkine or pleiotrophin level for diagnosing a growth
ActiveUS20150301057A1High sensitivityHigh affinitySurgical needlesDisease diagnosisMalignant GrowthBiology
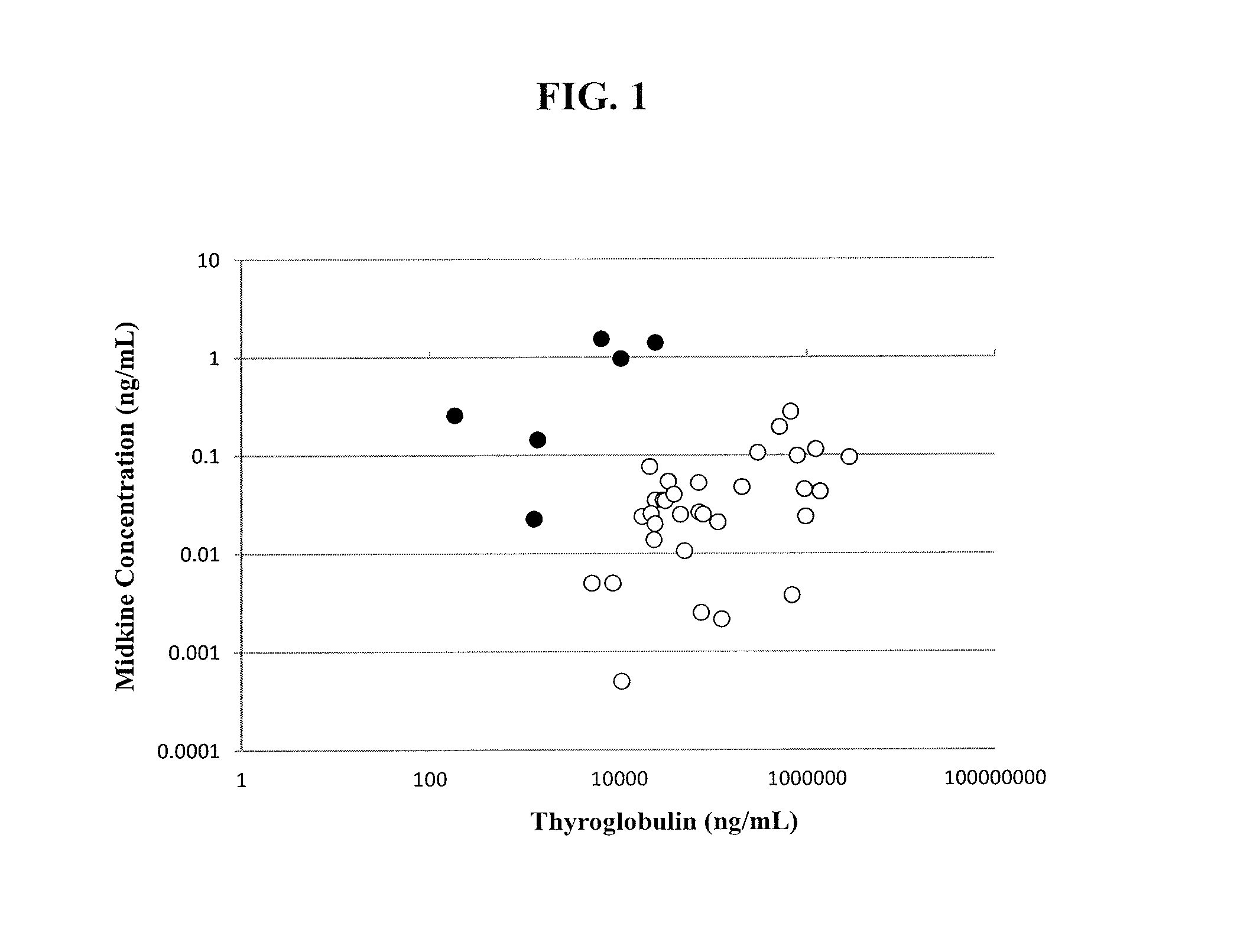
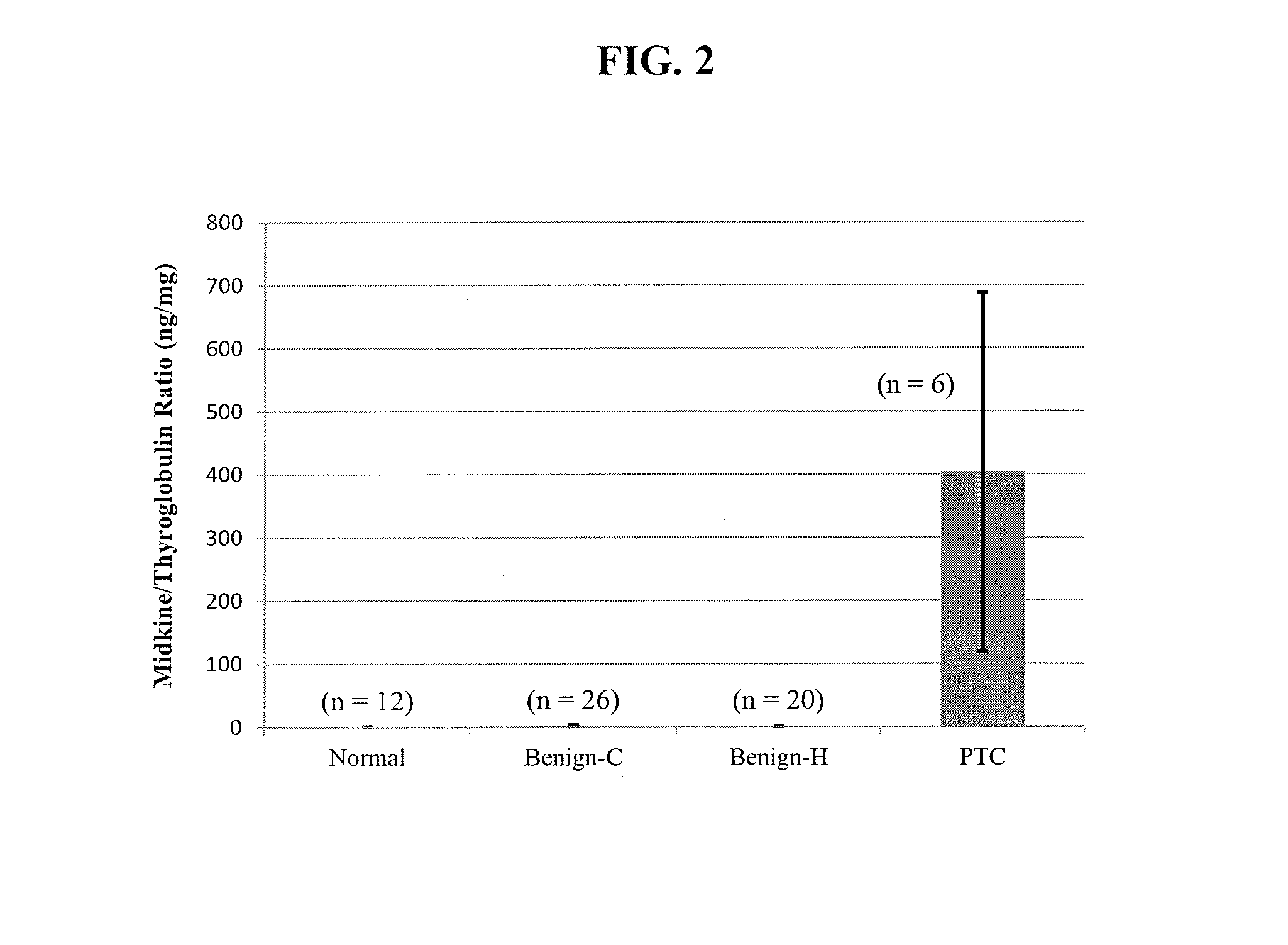
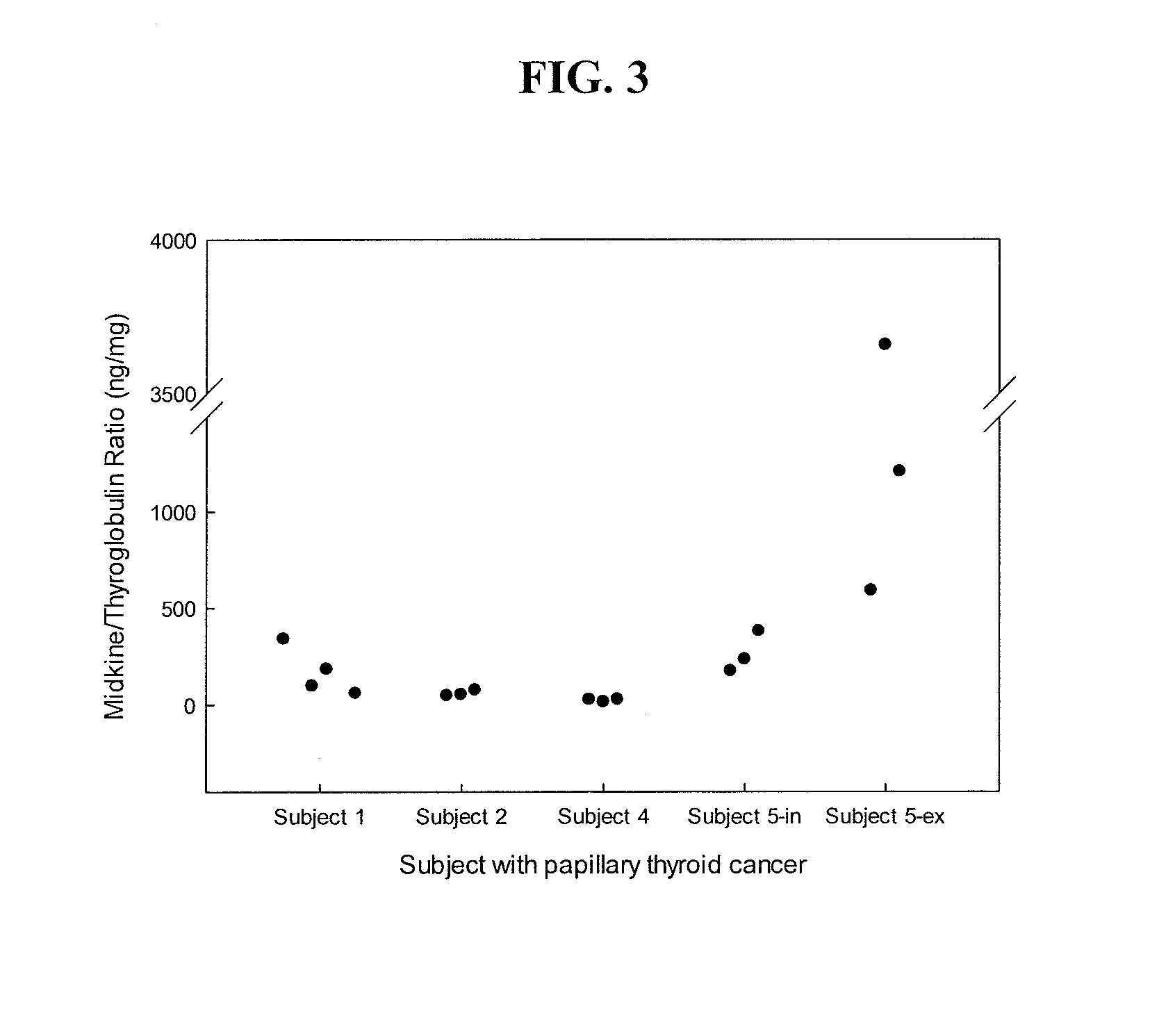
The invention provides methods and kits for diagnosing a growth in a subject by providing a sample of a growth taken from a subject, determining the level of midkine or pleiotrophin in the sample by an immunoassay, and comparing the level of midkine or pleiotrophin determined from the sample with a control. An increased level of midkine or pleiotrophin in the sample as compared to the control is diagnostic of a malignant growth, whereas an equivalent or decreased level of midkine or pleiotrophin in the sample as compared to the control is diagnostic of a benign growth. Growth refers to, for example, papillary thyroid cancer (PTC).
Owner:UNITED STATES OF AMERICA
Methods and compositions for diagnosis and prognosis of renal injury and renal failure
InactiveUS20110306063A1Eliminate needEasy to adaptMaterial analysis by observing effect on chemical indicatorBiostatisticsMatrix metalloproteinase 9Serum amyloid P component
The present invention relates to methods and compositions for monitoring, diagnosis, prognosis, and determination of treatment regimens in subjects suffering from or suspected of having a renal injury. In particular, the invention relates to using assays that detect one or more markers selected from the group consisting of Clusterin, Heart-type fatty acid binding protein, Hepatocyte growth factor, Interferon gamma, Interleukin-12 subunit beta, Interleukin-16, Interleukin-2, 72 kDa type IV collagenase, Matrix metalloproteinase-9, Midkine, and Serum amyloid P-component as diagnostic and prognostic biomarkers in renal injuries.
Owner:ASTUTE MEDICAL
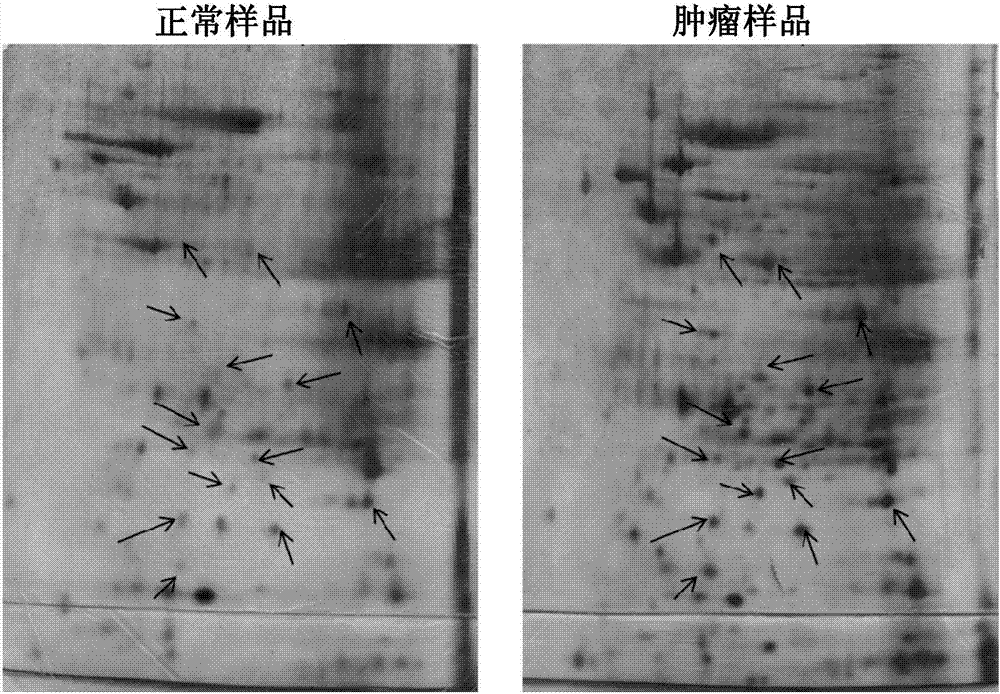
Specific biomarker set for non-invasive diagnosis of liver cancer
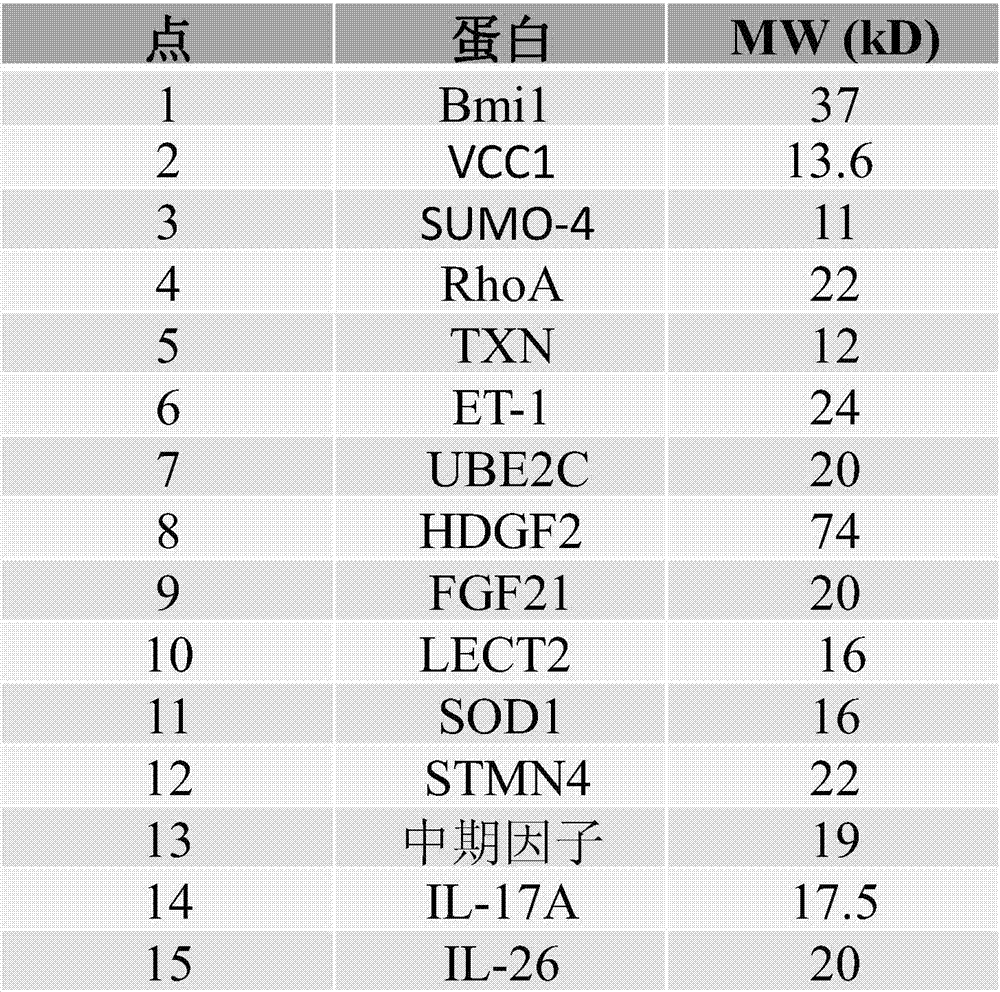
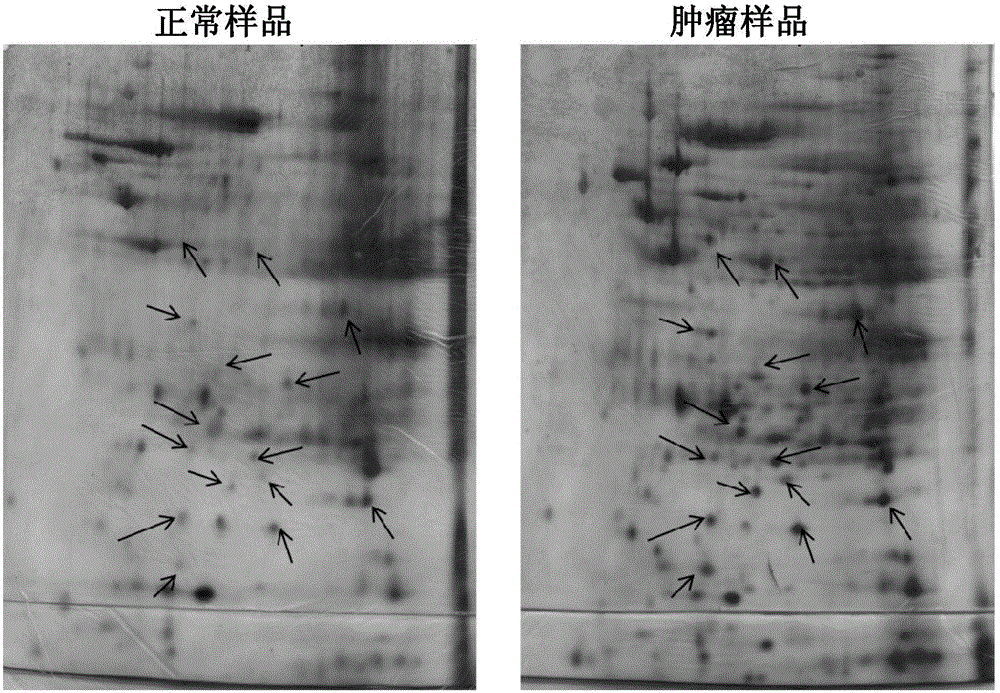
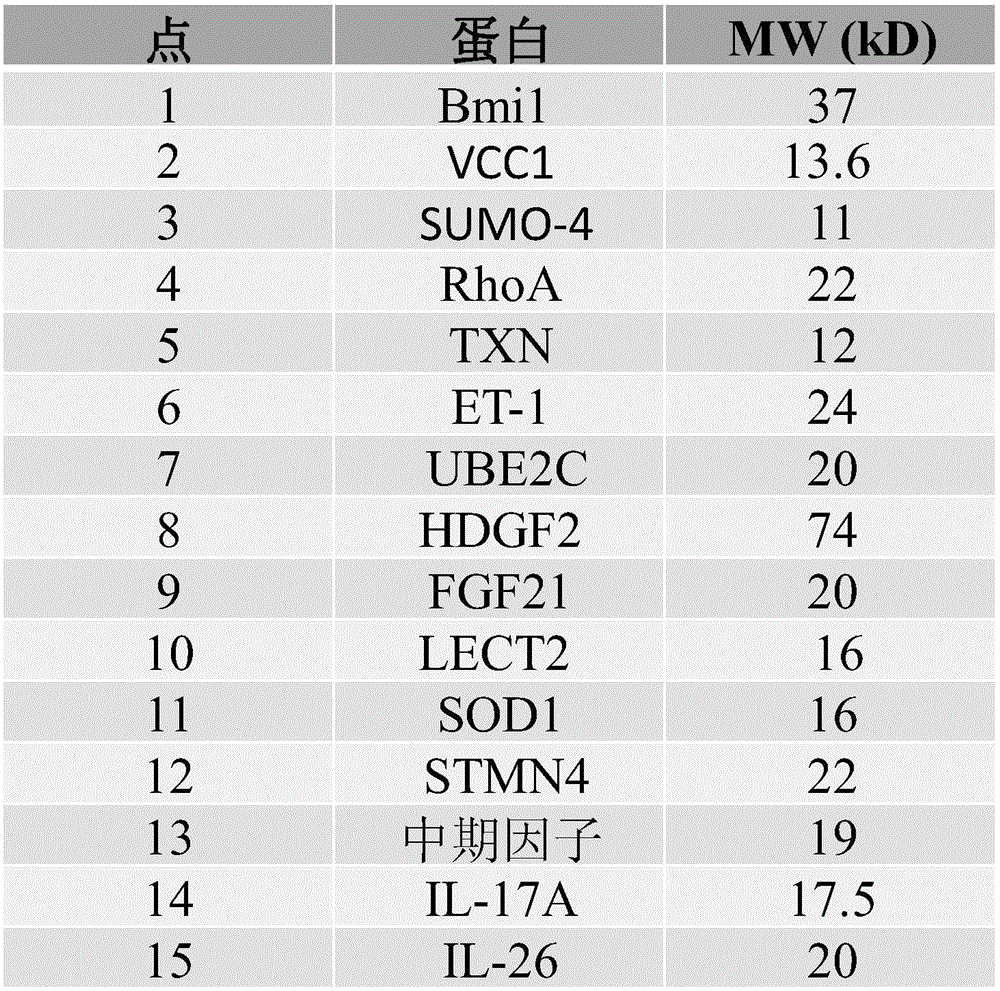
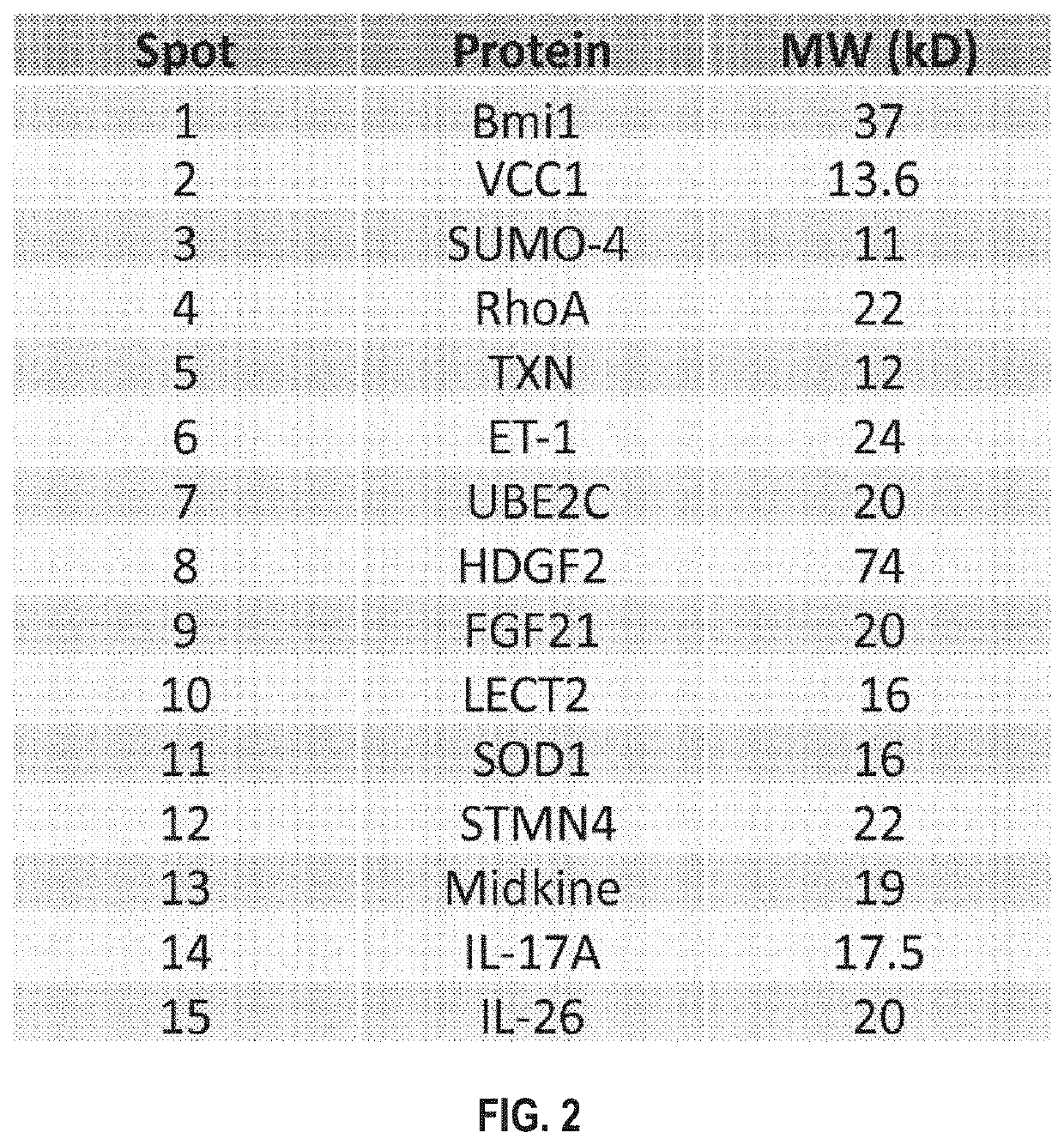
Cells within liver tumour mass comprise a unique set of proteins / tumour antigens when compared to the normal liver tissues epithelial cells juxtaposed to the tumour. The presence of tumour antigens couples the production of auto-antibodies against these tumour antigens. The present invention relates to the identification and elucidation of a protein set that can act as a novel marker set for liver cancer diagnosis and prognosis. Specifically, it relates to a kit that enables diagnostic and prognostic measurement of auto-antibodies in serum of liver cancer patients. The present invention provides a non-invasive, specific, sensitive, and cost effective detection and quantification method by evaluating a set of validated liver cancer proteins / tumour antigens, which includes Bmi-1, VCC1, SUMO-4, RhoA, TXN, ET-1, UBE2C, HDGF2, FGF21, LECT2, SOD1, STMN4, Midkine, IL-17A or IL26, to complement the conventional diagnostic methods.
Owner:DRAGON VICTORY DEV
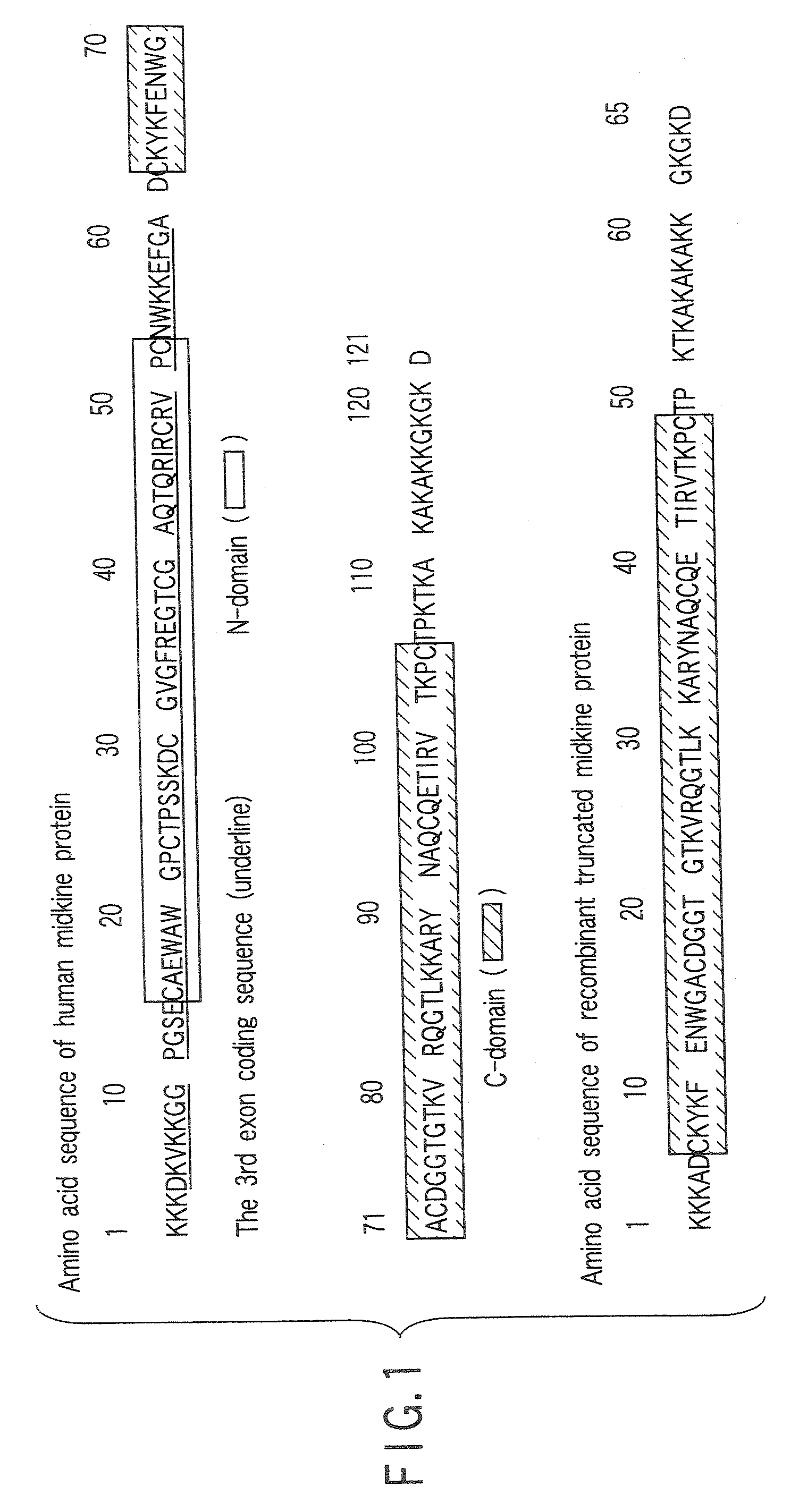
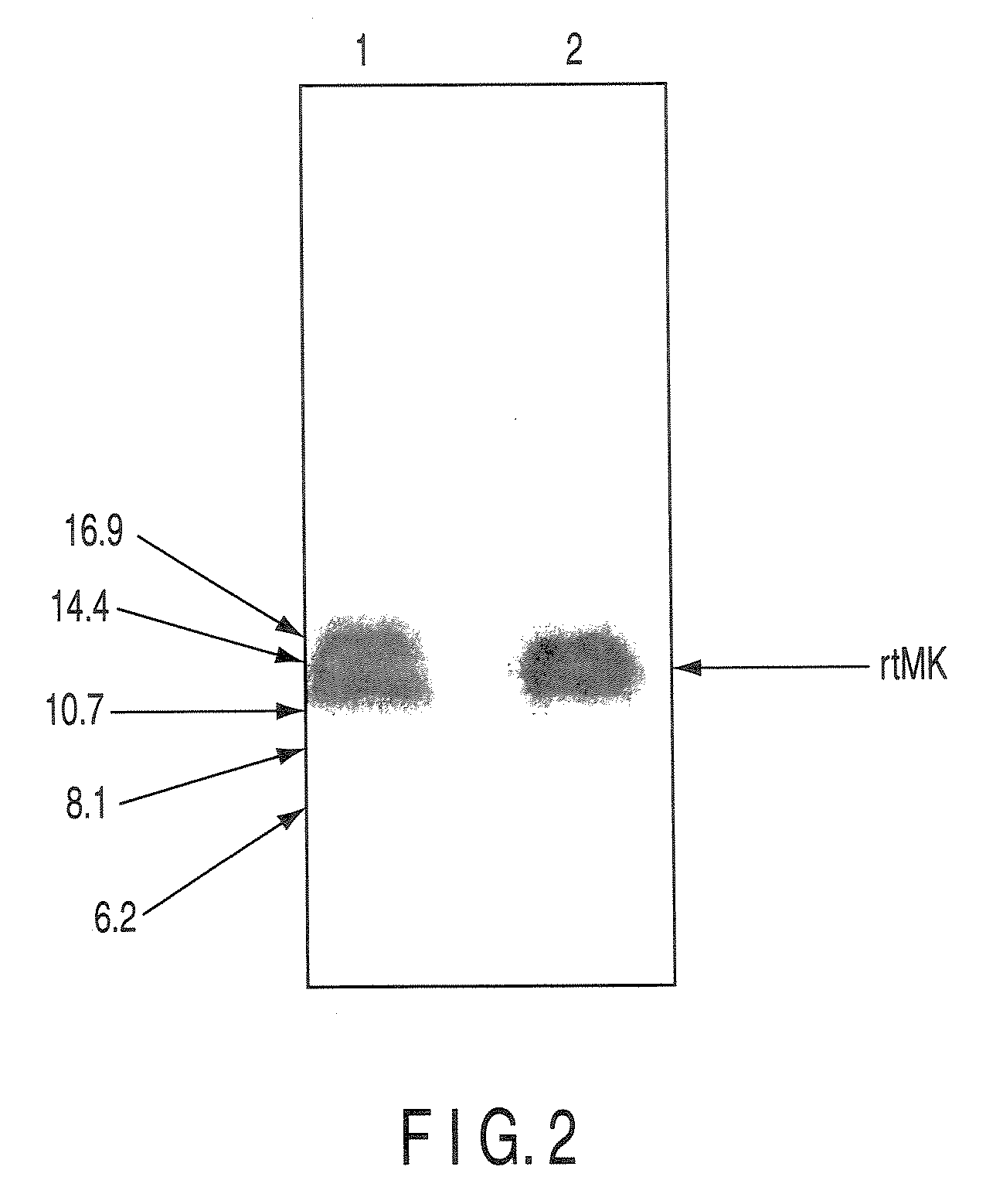
Monoclonal antibody specific to truncated midkine (TMK) protein and uses thereof
InactiveUS20070154949A1Animal cellsImmunoglobulins against cytokines/lymphokines/interferonsMonoclonal antibodyTumor cells
The present invention relates to a monoclonal antibody or a fragment thereof specific to truncated Midkine (tMK) protein, hybridoma producing the monoclonal antibody, a method of detecting truncated Midkine protein (tMK) by use of the monoclonal antibody, a method of detecting a tumor cell, and a kit containing the monoclonal antibody for detecting truncated Midkine (tMK) protein.
Owner:MITSUMOTO TOMOHIRO +1
Tumor-specific promoters
InactiveUS20030157065A1High tumor-specificityHigh promoter activityBiocideGenetic material ingredientsPromoter activityC erbb 2
A DNA comprising a 609 bp base sequence from -559 to +50 when the first base sequence of exon 1 of the midkine gene, a human retinoic acid-responsive growth / differentiation factor was set as +1, or a DNA comprising a 251 bp base sequence from -213 to +38 when the transcription initiation point of the c-erbB-2 gene belonging to the EGF receptor family and having a tyrosine kinase activity was set as +1 has a tumor-specific transcription activity, and the promoter activity thereof is high, and therefore is very important as a tumor-specific promoter for use in the suicide gene therapy that combines the use of a gene for a drug metabolizing enzyme and a prodrug for cancer therapy, the gene therapy of cancer using an expression vector that contains a gene encoding a cytokine, and the gene therapy of cancer using an oncolytic virus that exhibits cytotoxic effects only on tumor cells, etc.
Owner:CHIBA PREFECTURE +1
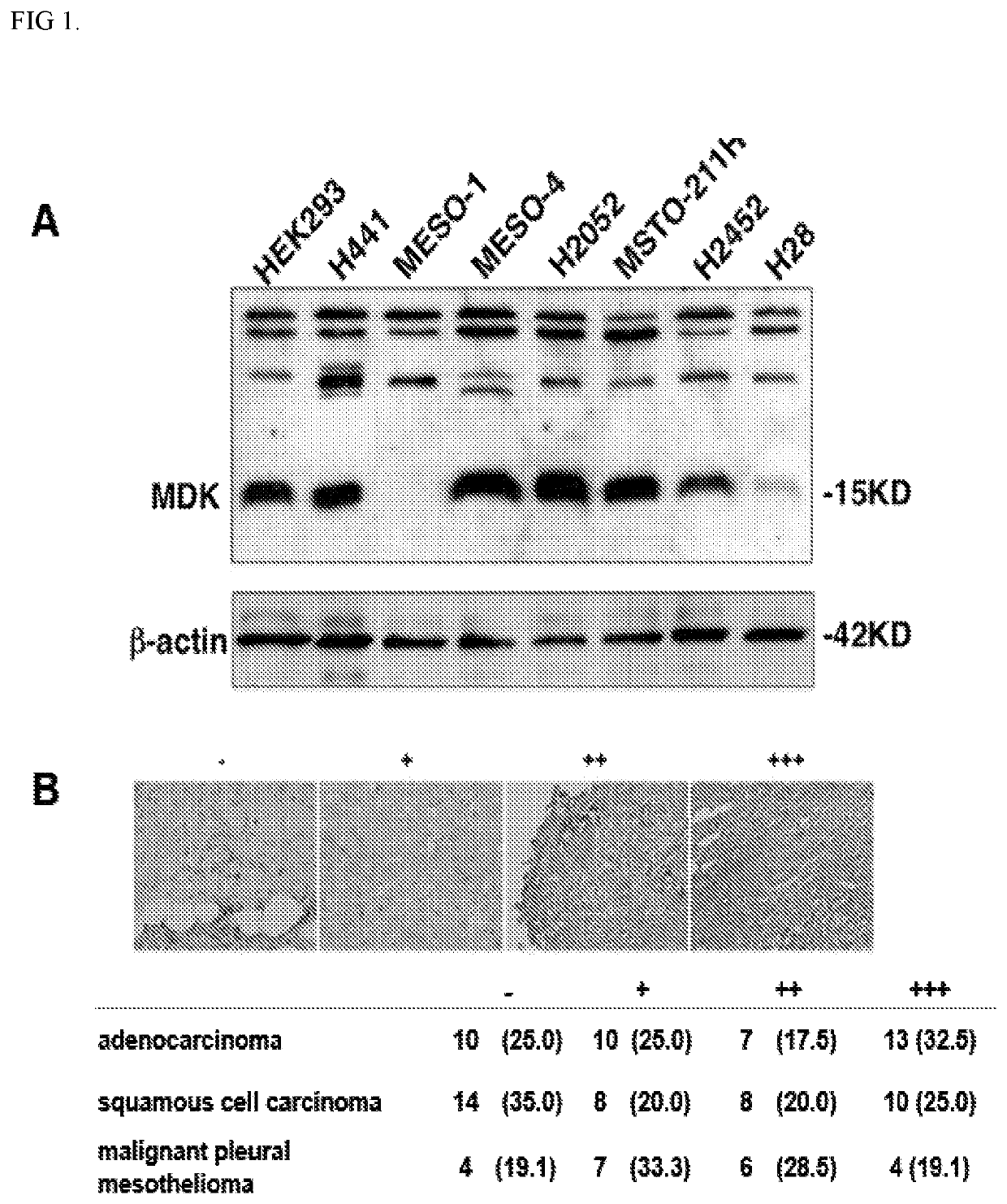
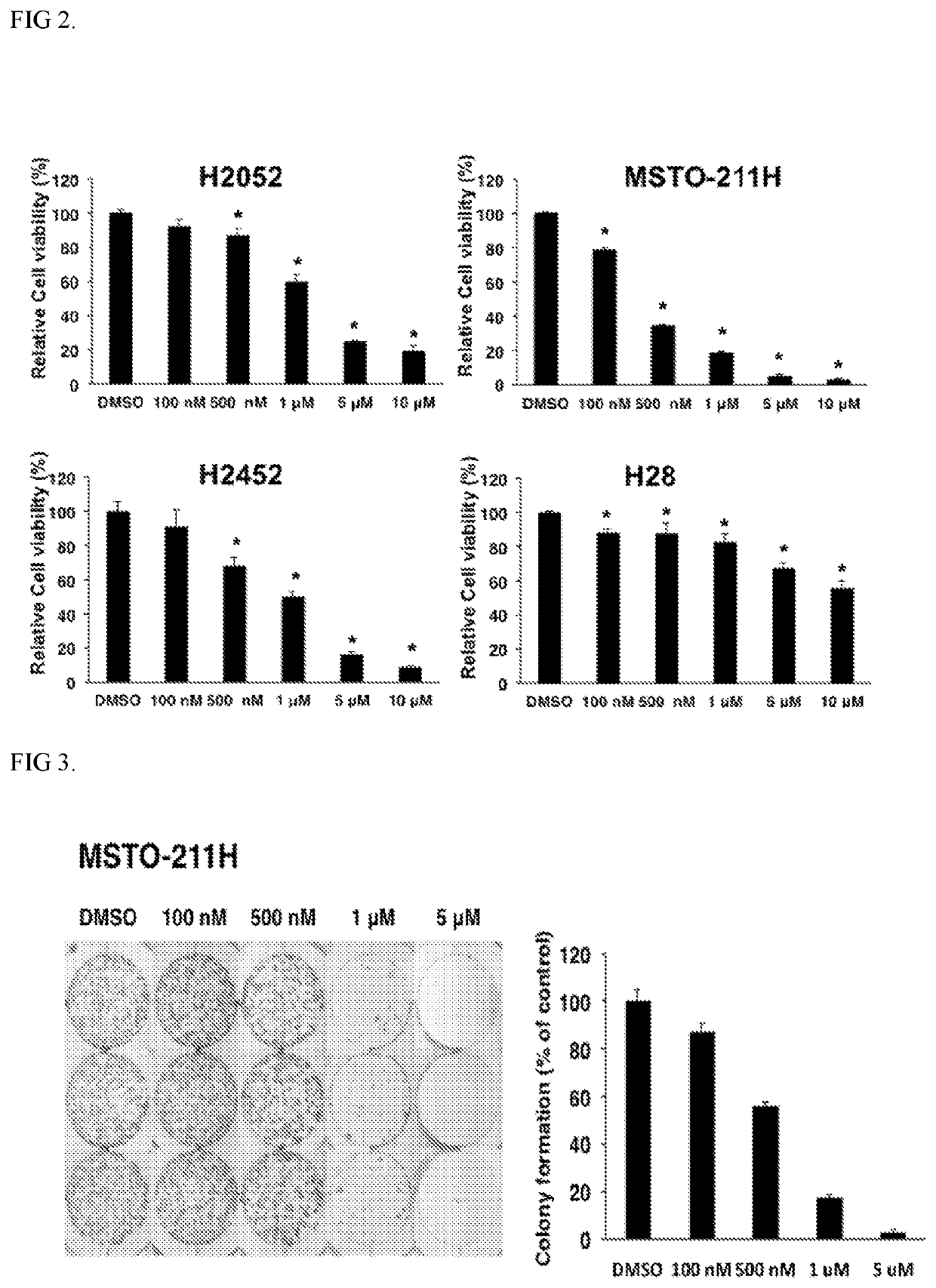
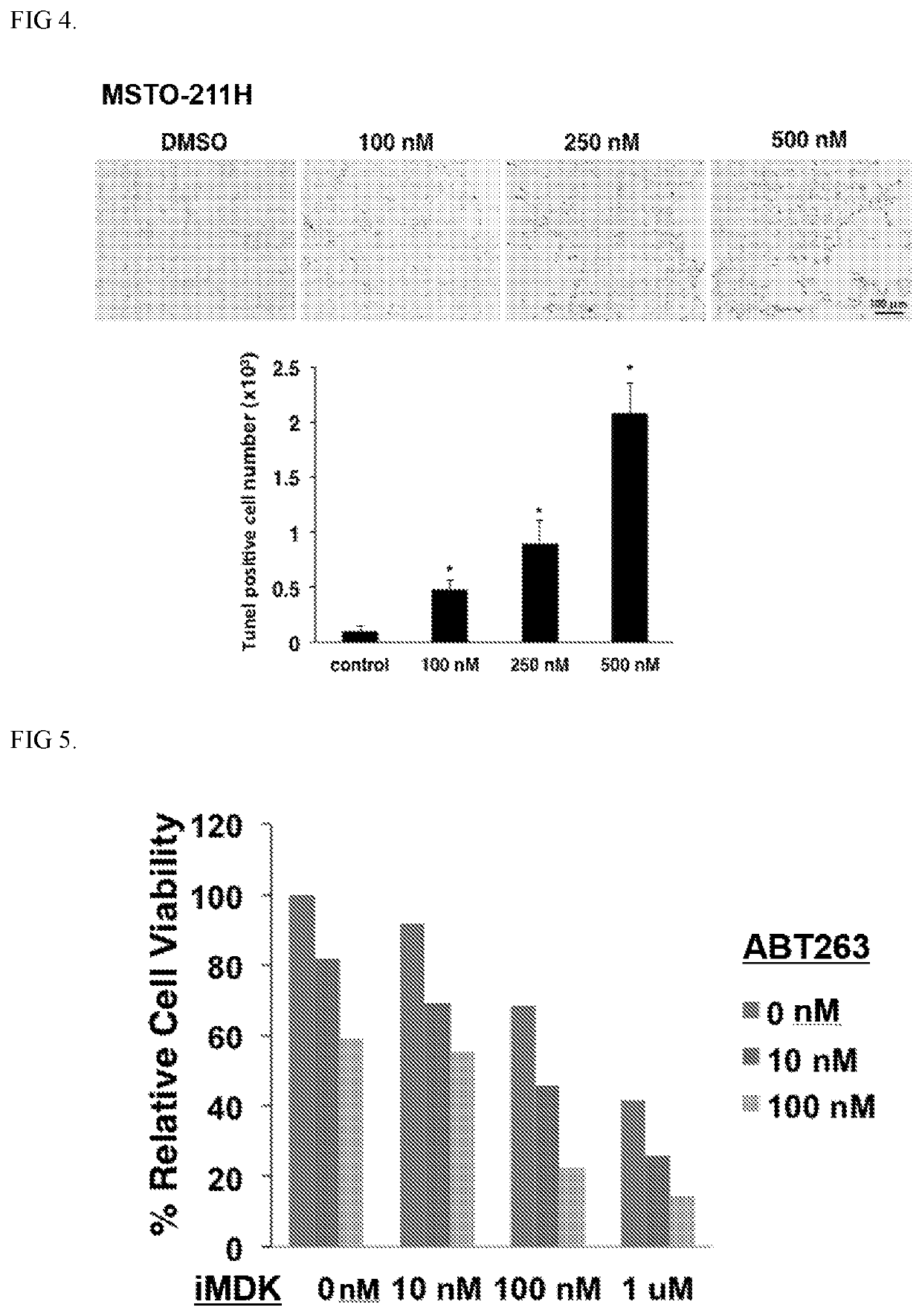
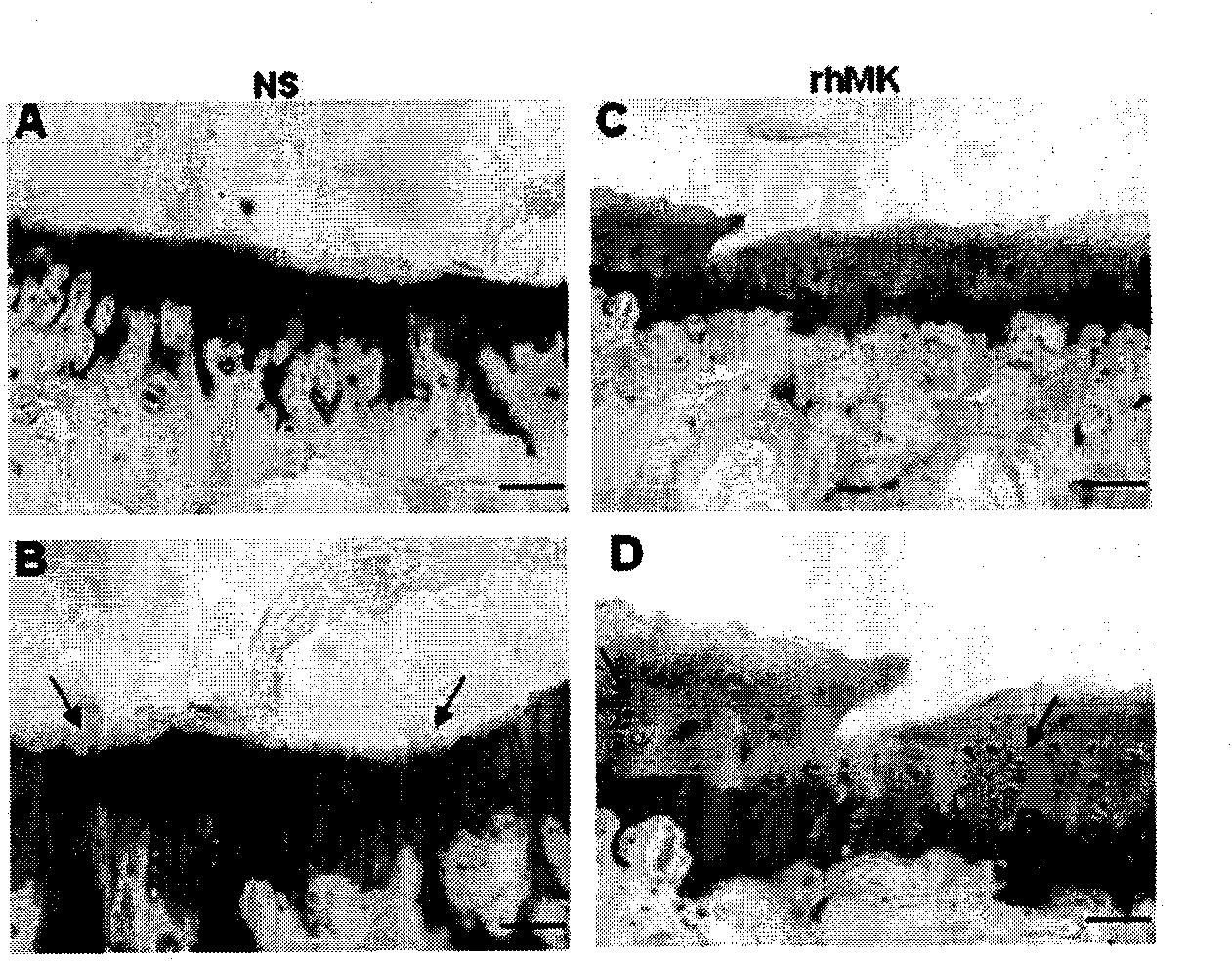
Methods and compositions for treating mesothelioma and small lung cancer that express midkine
Embodiments provided herein relate to methods and compositions for treating mesothelioma and / or a small cell lung cancer that express midkine.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
Application of midkine protein and medical device containing protein
ActiveCN101601858AProliferation effect is goodStentsPeptide/protein ingredientsCartilage cellsDisease
The invention relates to application of midkine protein and a medical device containing the protein in the technical field of biology. The invention discloses an application of protein shown as (a) or (b) for preparing a medicament for promoting the growth of cartilage tissues or a medicament for treating cartilage tissue diseases, an application of the protein for promoting cartilage tissue cell growth factors, and a medical device containing the protein; wherein the (a) is the protein of which amino acid sequence is expressed as SEQ ID No.1; the (b) is the protein which has at least 60 percent of homology of the amino acid sequence in the (a). The invention is fully different from the prior art on the cognition for MK proteins; experiments show that the MK has good proliferation effect on three kinds of cartilage cells in vivo so that the MK can be used for preparing the medicament for treating the cartilage diseases and provides a new selection for clinically treating the cartilage diseases in future, and simultaneously the MK also can be used for in vitro amplification of the cartilage cells in the cartilage tissue engineering.
Owner:USYNOVA PHARM
Specific biomarker set for non-invasive diagnosis of liver cancer
Cells within liver tumour mass comprise a unique set of proteins / tumour antigens when compared to the normal liver tissues epithelial cells juxtaposed to the tumour. The presence of tumour antigens couples the production of auto-antibodies against these tumour antigens. The present invention relates to the identification and elucidation of a protein set that can act as a novel marker set for liver cancer diagnosis and prognosis. Specifically, it relates to a kit that enables diagnostic and prognostic measurement of auto-antibodies in serum of liver cancer patients. The present invention provides a non-invasive, specific, sensitive, and cost effective detection and quantification method by evaluating a set of validated liver cancer proteins / tumour antigens, which includes Bmi-1, VCC1, SUMO-4, RhoA, TXN, ET-1, UBE2C, HDGF2, FGF21, LECT2, SOD1, STMN4, Midkine, IL-17A or IL26, to complement the conventional diagnostic methods.
Owner:DRAGON VICTORY DEV
Method for massively preparing midkine antisense oligodeoxynucleotide nano-liposomes for injection
ActiveCN102989007AGood curative effectAchieve targeted deliveryGenetic material ingredientsDigestive systemAntisense oligodeoxynucleotidesOrganosolv
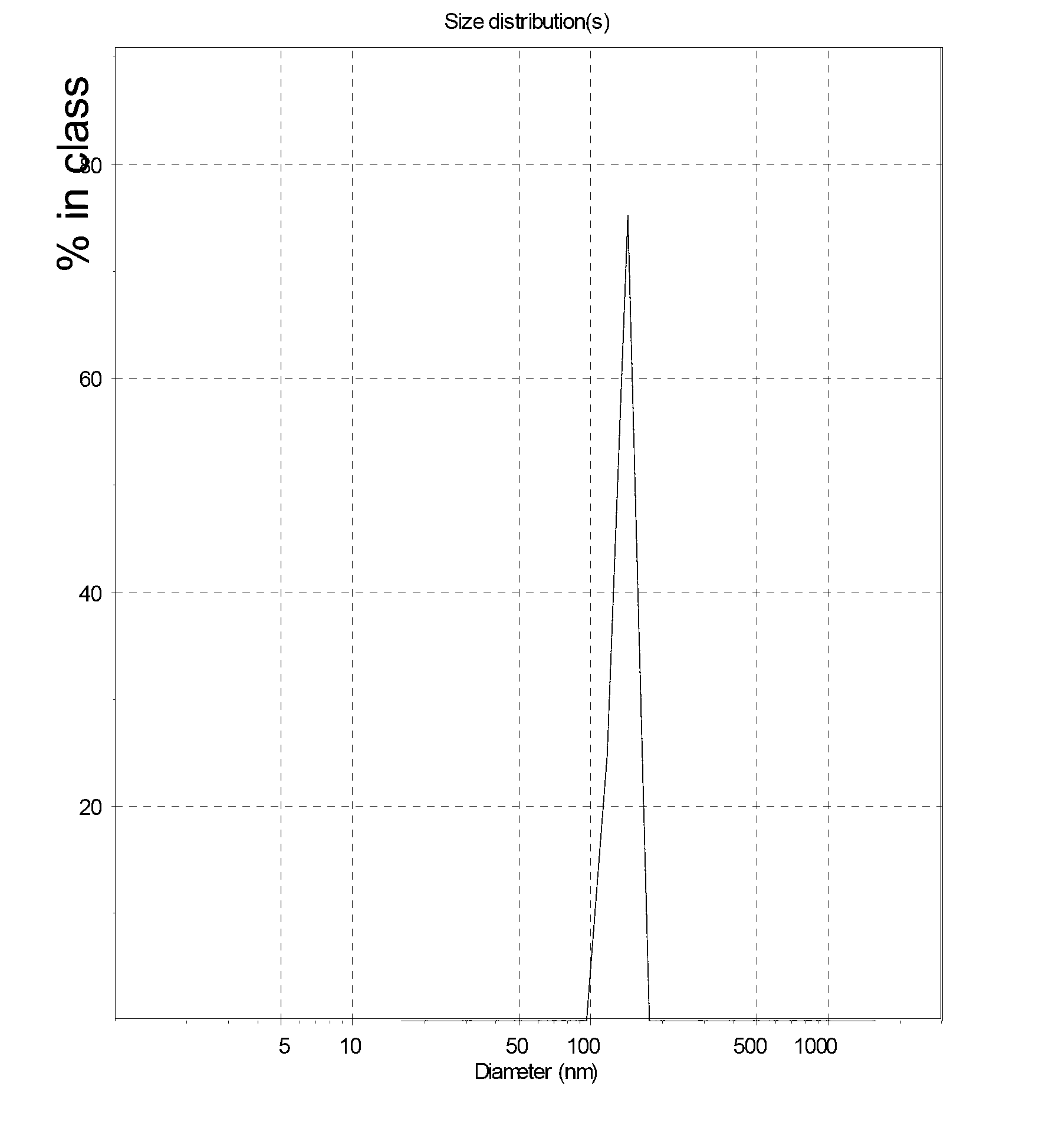
The invention relates to a method for massively preparing midkine antisense oligodeoxynucleotide nano-liposomes for injection. The method comprises the following steps: dissolving a mixture of cationic liposomes and lecithoid liposomes in an organic solvent to obtain a liposome liquor; then, initially dispersing the liposomes by an ultrasonic instrument, and ultrafiltering to remove the organic solvent, and finally squeezing by a squeezer at a high pressure to uniform grain size to obtain blank nano-liposomes; and then, mixing with an MK-ASODN liquor to obtain MK-ASODN nano-liposomes. The grain size of the midkine antisense oligodeoxynucleotide nano-liposomes for injection produced by the method is within 100-500nm, and the midkine antisense oligodeoxynucleotide nano-liposomes for injection is stable in property and the anti-hepatoma effect of the midkine antisense oligodeoxynucleotide nano-liposomes for injection is remarkably enhanced. The method is simple in process, easy to realize, very easy to amplify, and suitable for industrial production.
Owner:HUZHOU CENT HOSPITAL
Specific biomarker set for non-invasive diagnosis of liver cancer
PendingUS20200386761A1Facilitate early detectionEarly detectionFluorescence/phosphorescenceAssay labelsAutoantibodyRHOA
Cells within liver tumor mass comprise a unique set of proteins / tumor antigens when compared to the normal liver tissues epithelial cells juxtaposed to the tumor. The presence of tumor antigens couples the production of auto-antibodies against these tumor antigens. The present invention relates to the identification and elucidation of a protein set that can act as a novel marker set for liver cancer diagnosis and prognosis. Specifically, it relates to a kit that enables diagnostic and prognostic measurement of auto-antibodies in serum of liver cancer patients. The present invention provides a non-invasive, specific, sensitive, and cost effective detection and quantification method by evaluating a set of validated liver cancer proteins / tumor antigens, which includes Bmi-1, VCC1, SUMO-4, RhoA, TXN, ET-1, UBE2C, HDGF2, FGF21, LECT2, SOD1, STMN4, Midkine, IL-17A, IL26, or DCP to complement the conventional diagnostic methods.
Owner:DRAGON VICTORY DEV
Drugs contg. as active ingredient midkine or inhibitors thereof
Drugs for treating neutrophilic functional disorders or inflammatory diseases, containing as the active ingredient midkine (MK), which is a retinoic acid-inducible heparin-binding growth factor found to enhance the neutrophile migration and to exist at a high concentration in an inflammatory state, or inhibitors thereof.
Owner:MEDICAL THERAPIES
Use of human midkine protein blocking peptide in preparation of antitumor medicament
InactiveCN101255190AGrowth inhibitionSuppress generationPeptide/protein ingredientsPeptidesMedicineAngiogenesis growth factor
The invention relates to an application of human midkine protein blocking peptide for preparing antineoplastic medicine, wherein the human midkine protein blocking peptide comprises the following amino acid sequence: CTSTAMDNC. The invention provides an application of human midkine protein blocking peptide for preparing antineoplastic medicine, the polypeptide can inhibit the growth of malignant tumor cells, particularly for inhibiting the generation of malignant tumor neovascularization, provides foundation for screening new medicines, and has great clinical significance.
Owner:HUZHOU CENT HOSPITAL
Therapeutic angiogenic factors and methods for their use
The present invention provides a method of stimulating angiogenesis in a human or animal in need thereof, and also provides a composition comprising an angiogenic factor and a pharmaceutically acceptable carrier. In one embodiment, the method comprises administering to a human or other animal a therapeutically effective amount of an angiogenic factor, such as pleiotropin or a midkine protein, contained in a pharmaceutically acceptable carrier. In one embodiment, the carrier includes a controlled release matrix, such as a polymer, that allows the controlled release of angiogenic factors. The polymer is biodegradable and / or bioerodible, preferably biocompatible. Polymers useful for controlled release include, for example, poly(esters), poly(anhydrides) and poly(amino acids). Polymers include, for example, silk elastin poly(amino acid) block copolymers and poly-lactide-co-glycolide. In another embodiment, angiogenic factors may be provided in a carrier comprising liposomes, such as heterocystic liposomes. Carriers such as liposomes may contain targeting ligands capable of targeting the liposomes to predetermined sites in vivo. Angiogenic factors may be administered to the vasculature, such as the cardiovascular system or the peripheral vasculature. In preferred embodiments, the angiogenic factor is a pleiotrophin or a midkine protein. In another embodiment, there is provided a method of stimulating angiogenesis in a human or animal, the method comprising administering to the human or animal a therapeutically effective amount of a gene transfer vector contained in a pharmaceutically acceptable carrier, the gene transfer vector Can encode pleiotropin or midkine protein. Gene transfer vectors can be, for example, naked DNA or viral vectors, and can be used, for example, in combination with liposomes.
Owner:ANGIOGENIX
Tumor specific promoters of the midkine gene that allow for selective expression in P53-inactivated cells
InactiveUS7030099B2High tumor-specificity and promoter activityHigh promoter activityBiocideSugar derivativesPromoter activityC erbb 2
A DNA comprising a 609 bp base sequence from −559 to +50 when the first base sequence of exon 1 of the midkine gene, a human retinoic acid-responsive growth / differentiation factor was set as +1, or a DNA comprising a 251 bp base sequence from −213 to +38 when the transcription initiation point of the c-erbB-2 gene belonging to the EGF receptor family and having a tyrosine kinase activity was set as +1 has a tumor-specific transcription activity, and the promoter activity thereof is high, and therefore is very important as a tumor-specific promoter for use in the suicide gene therapy that combines the use of a gene for a drug metabolizing enzyme and a prodrug for cancer therapy, the gene therapy of cancer using an expression vector that contains a gene encoding a cytokine, and the gene therapy of cancer using an oncolytic virus.
Owner:CHIBA PREFECTURE +1
Gene associated with cancer
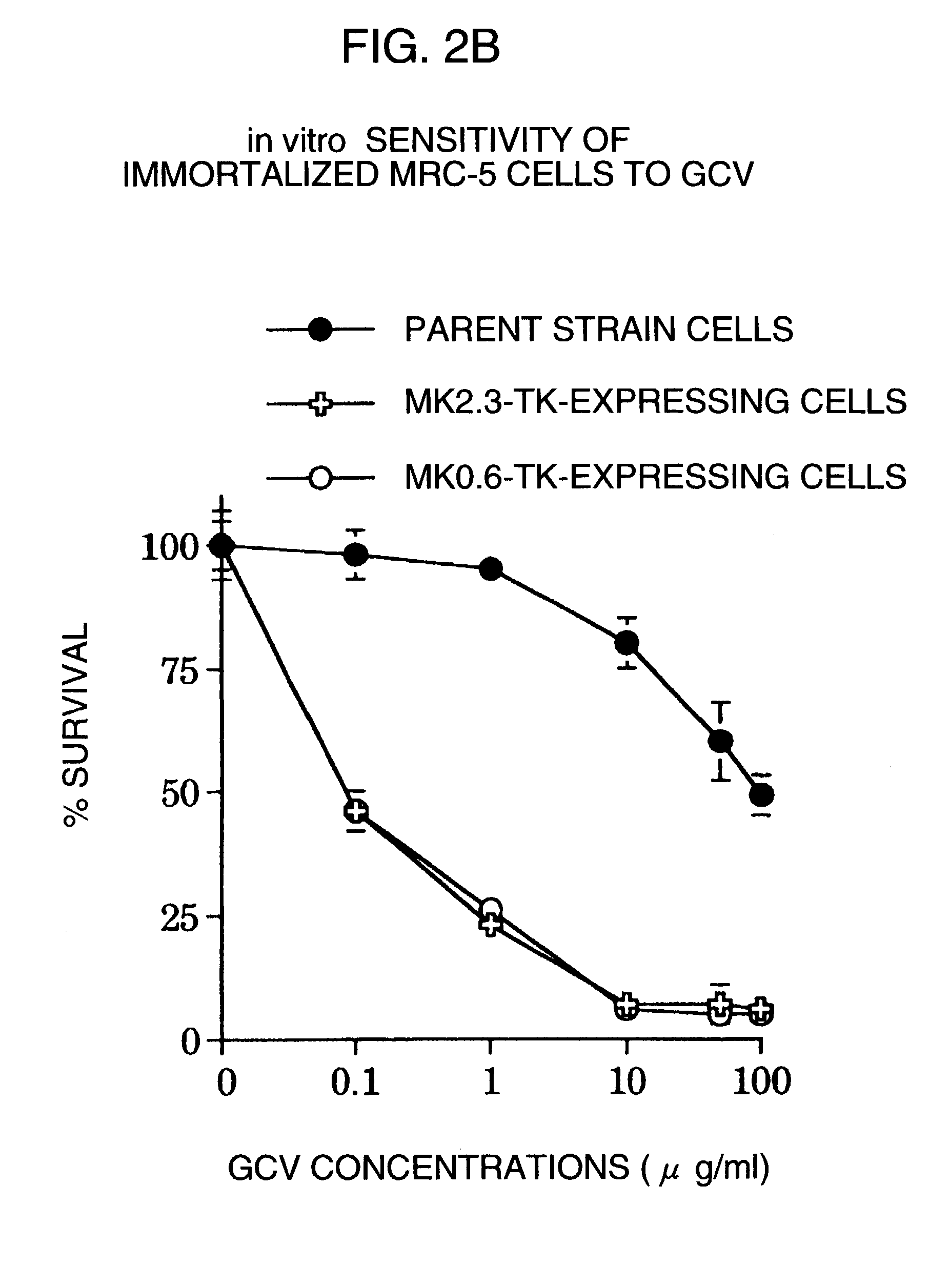
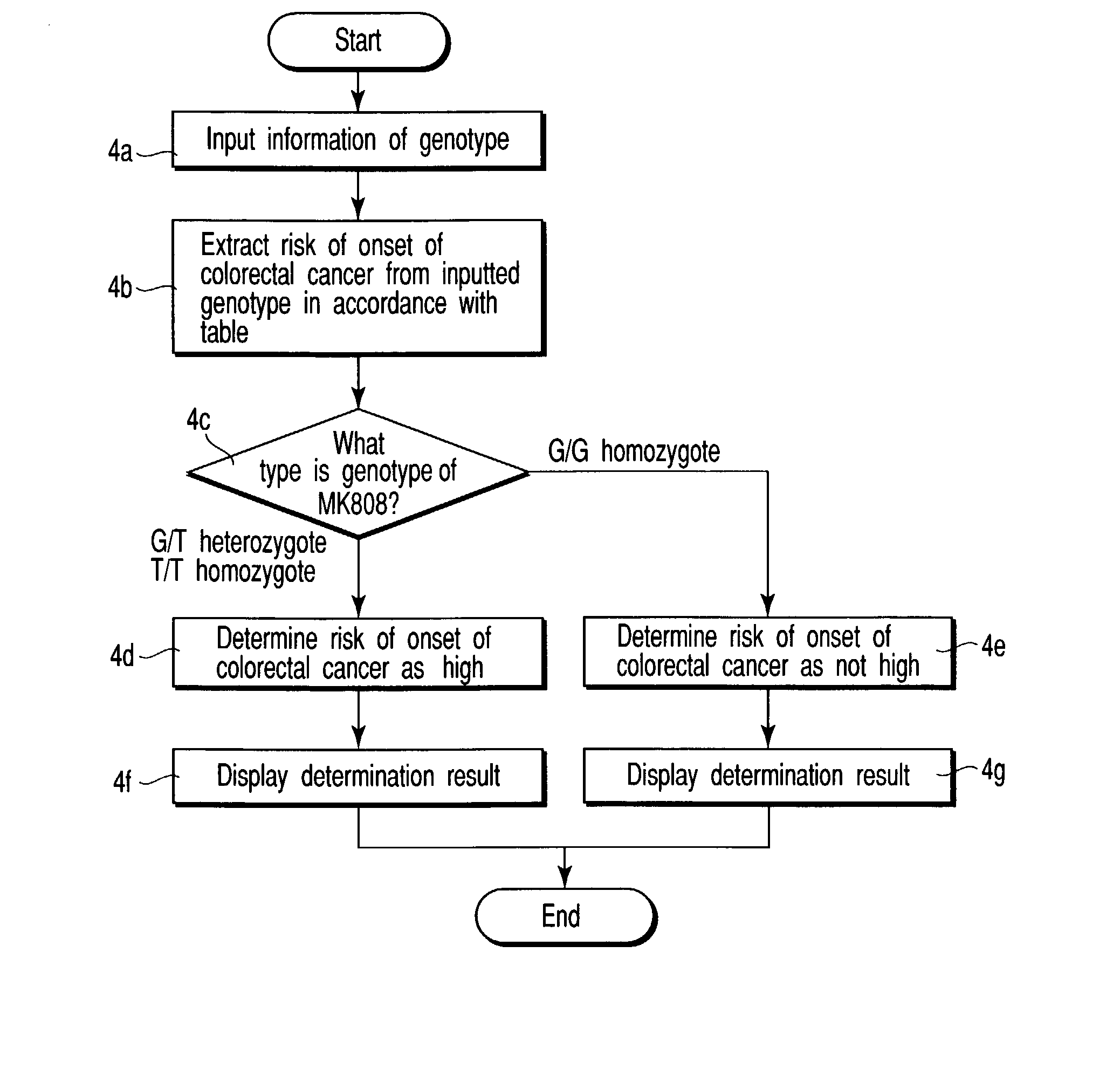
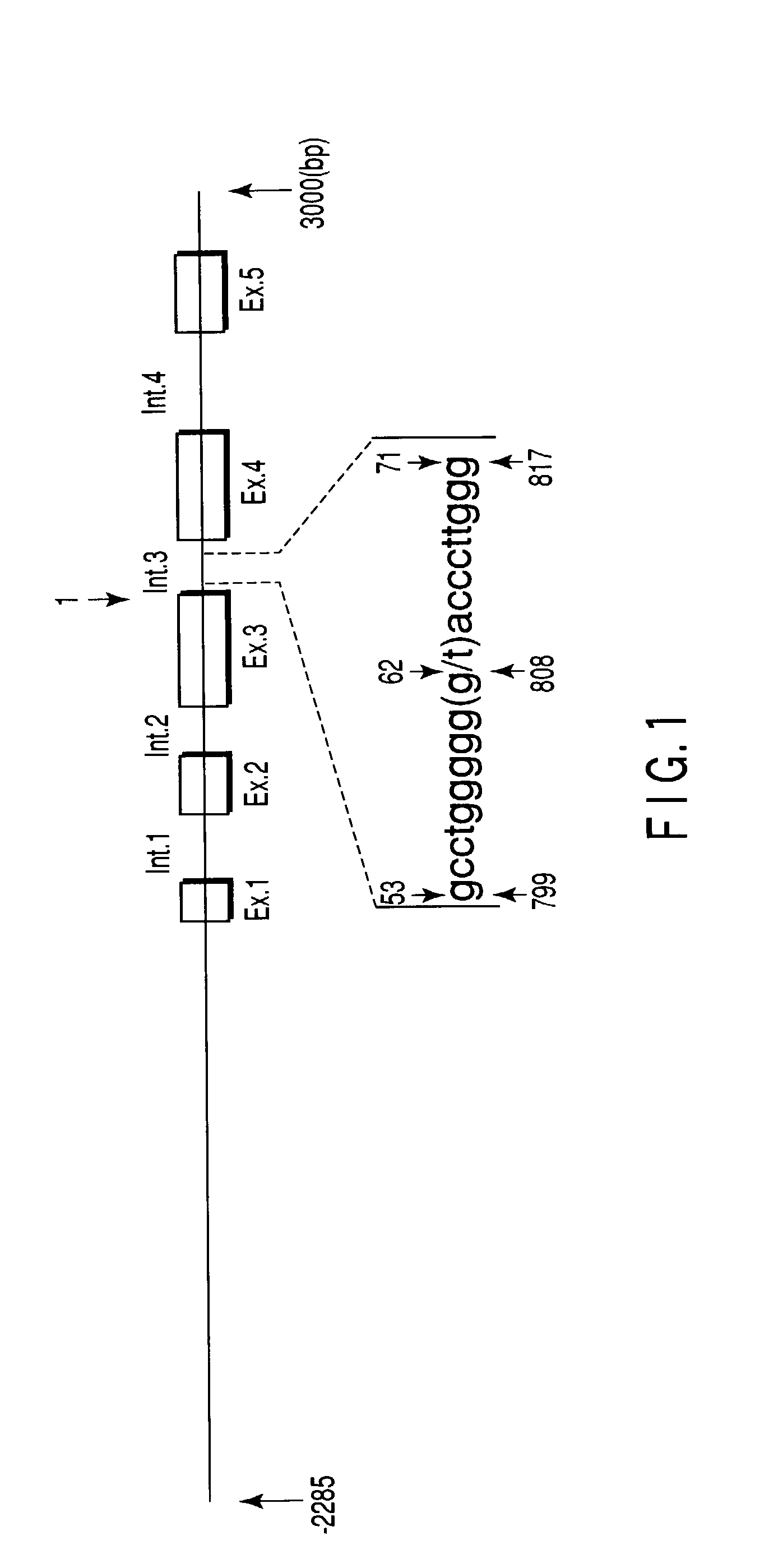
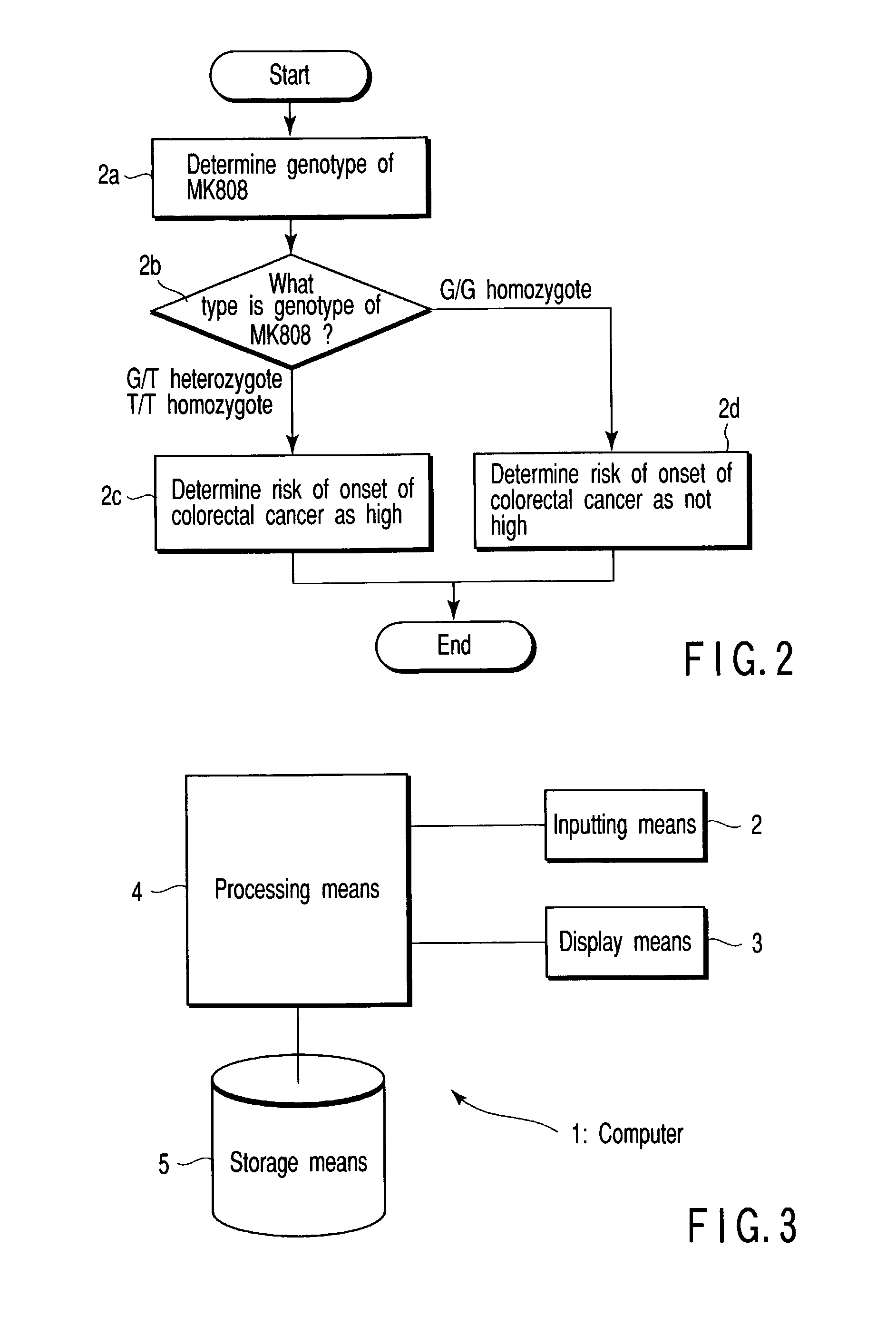
A method of anticipating risk of the onset of cancer in an individual, including (1) obtaining a sample derived from the individual, (2) analyzing a polymorphism of a Midkine gene concerning the sample of (1), and (3) anticipating risk of the onset of cancer based on the polymorphism determined in (2) above.
Owner:KUDOH NORIO
Method for in vitro collecting Midkine proteins and treating cells with collected Midkine proteins
ActiveCN111254175AAvoid enteringGuaranteed biological activityCell culture active agentsTumor/cancer cellsExtracellularIntracellular
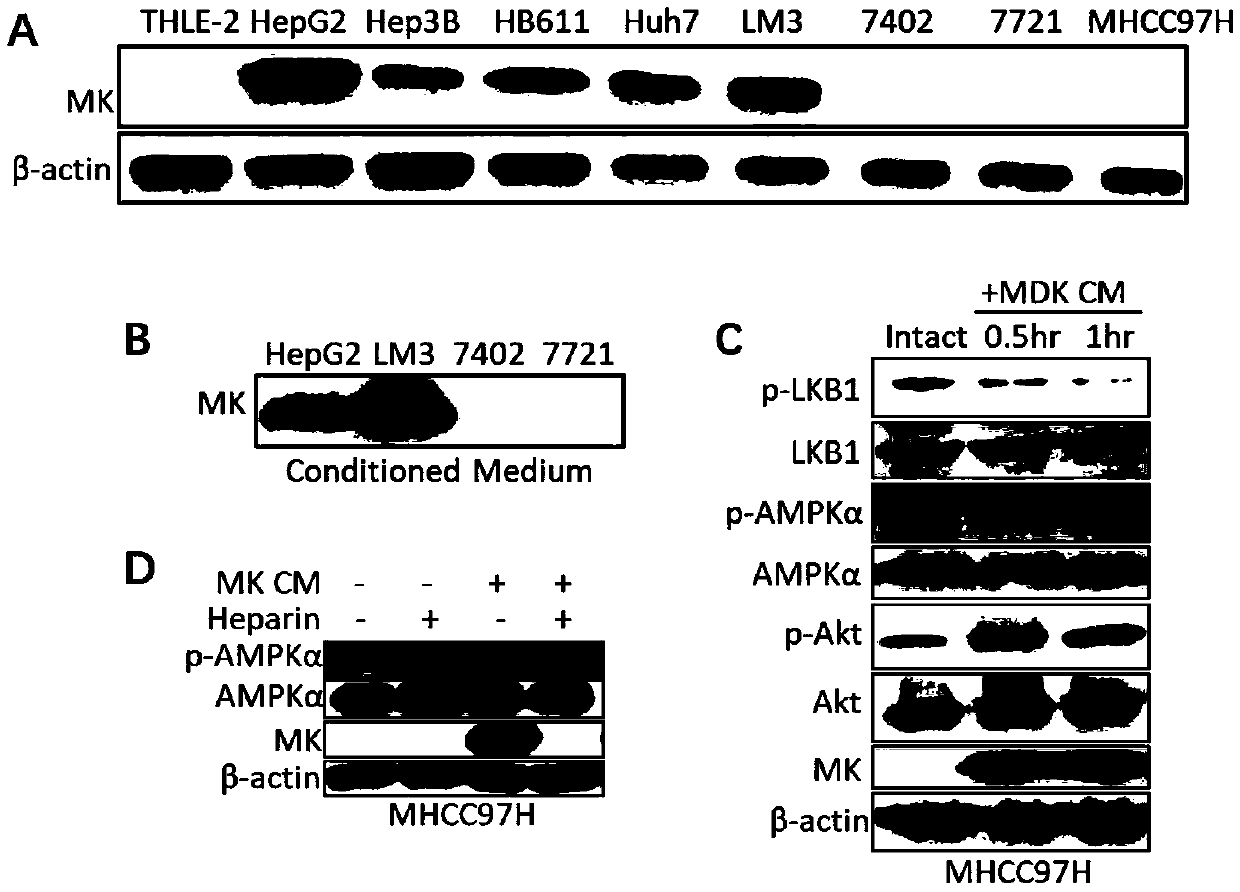
The invention discloses a method for in vitro collecting Midkine proteins and treating cells with the collected Midkine proteins. Cells for highly expressing Midkine proteins are cultured, through thesecretion characteristics of the Midkine proteins, the Midkine proteins are collected in culture mediums, and the secretion quantity of the Midkine proteins is detected through Western blot experiment; through detection with the Western blot experiment, the inventor finds that MK proteins can be transferred into the cells, and the phosphorylation level of AMPK is restrained; when the Midkine proteins are applied outside the cells, heparin (heparin) is added, through detection with the Western blot experiment, the inventor finds that Heparin restrains the Midkine proteins from entering the cells, and the activity of the AMPK proteins is improved. The method provides a novel method for researching the action mechanism of the Midkine.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Midkine-like protein
InactiveUS20060216709A1Inhibit and extinguish its activityInhibit biological activitySugar derivativesMicrobiological testing/measurementCell biologyMidkine
Owner:LEVITA CARMIT +3
Immunogenic peptides derived from the midkine protein, as an anticancer vaccine
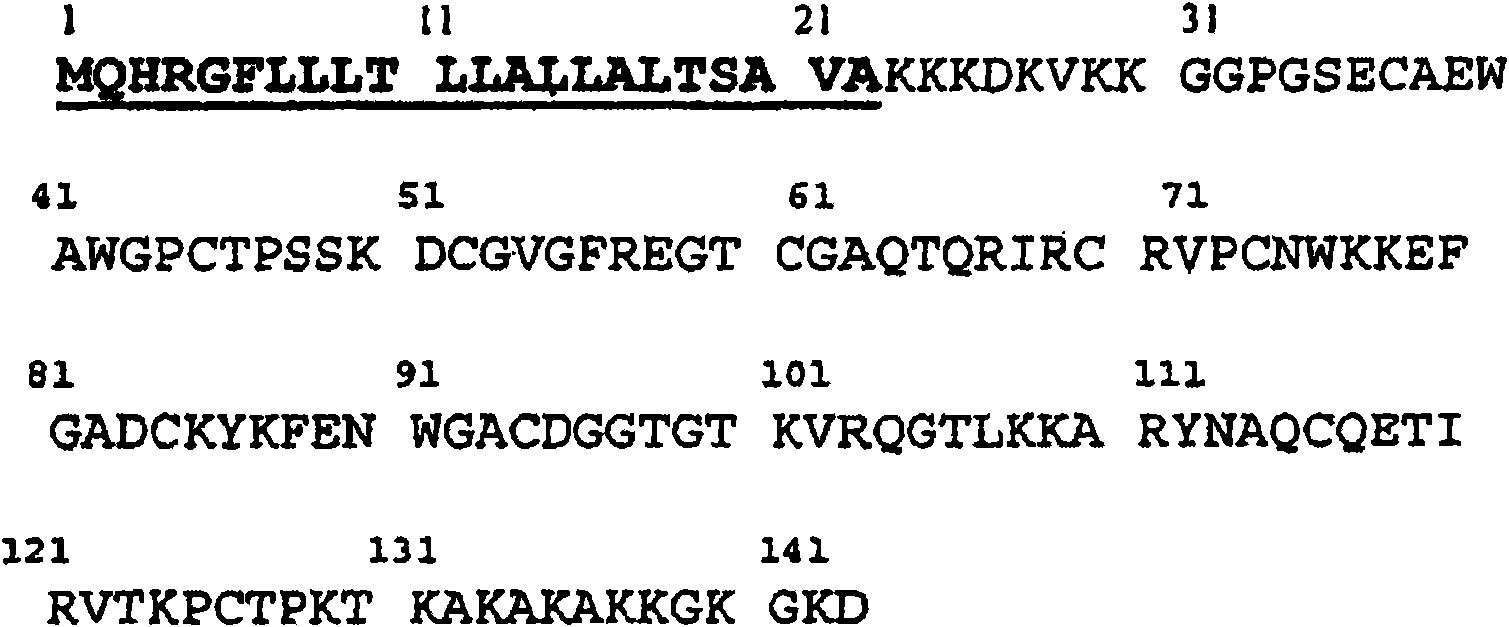
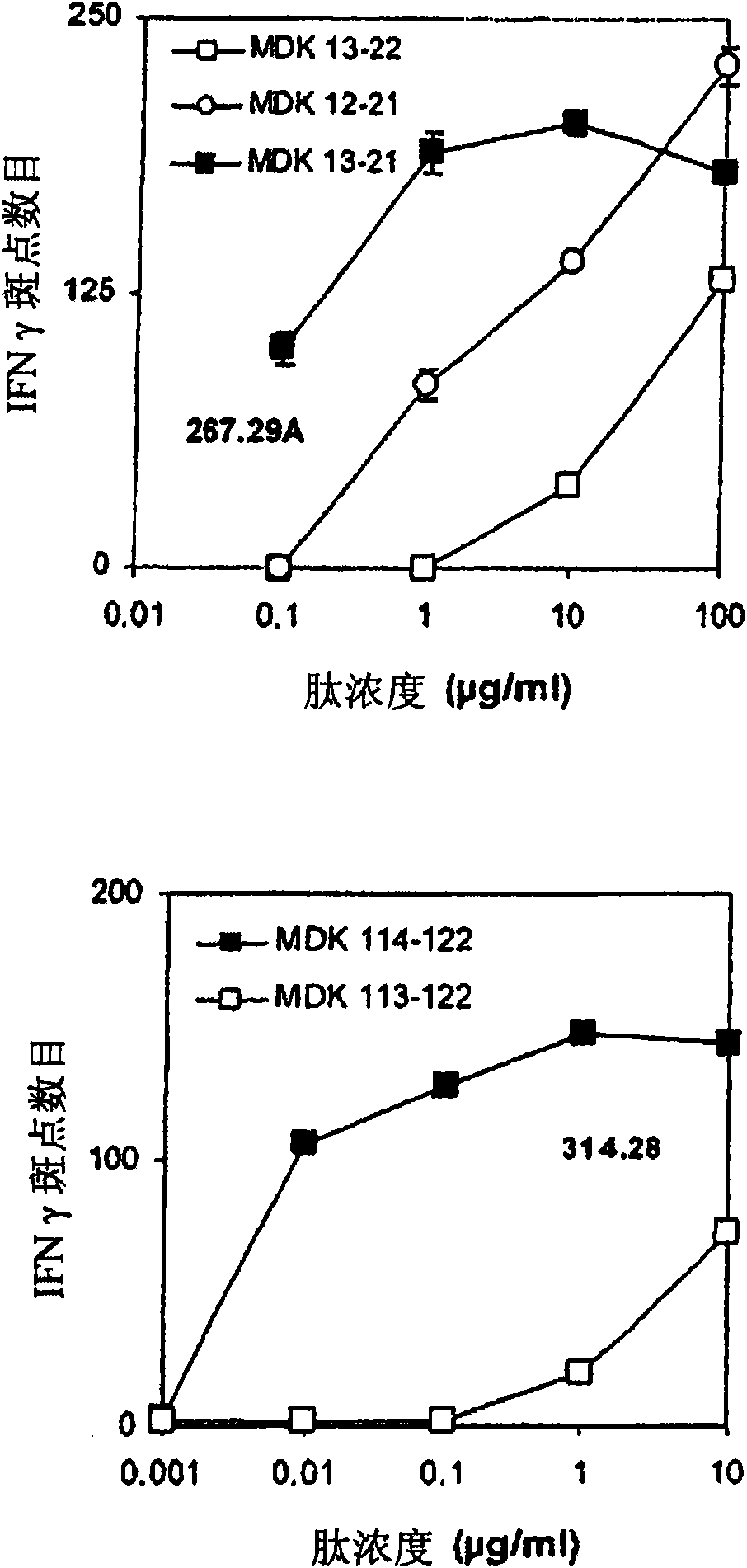
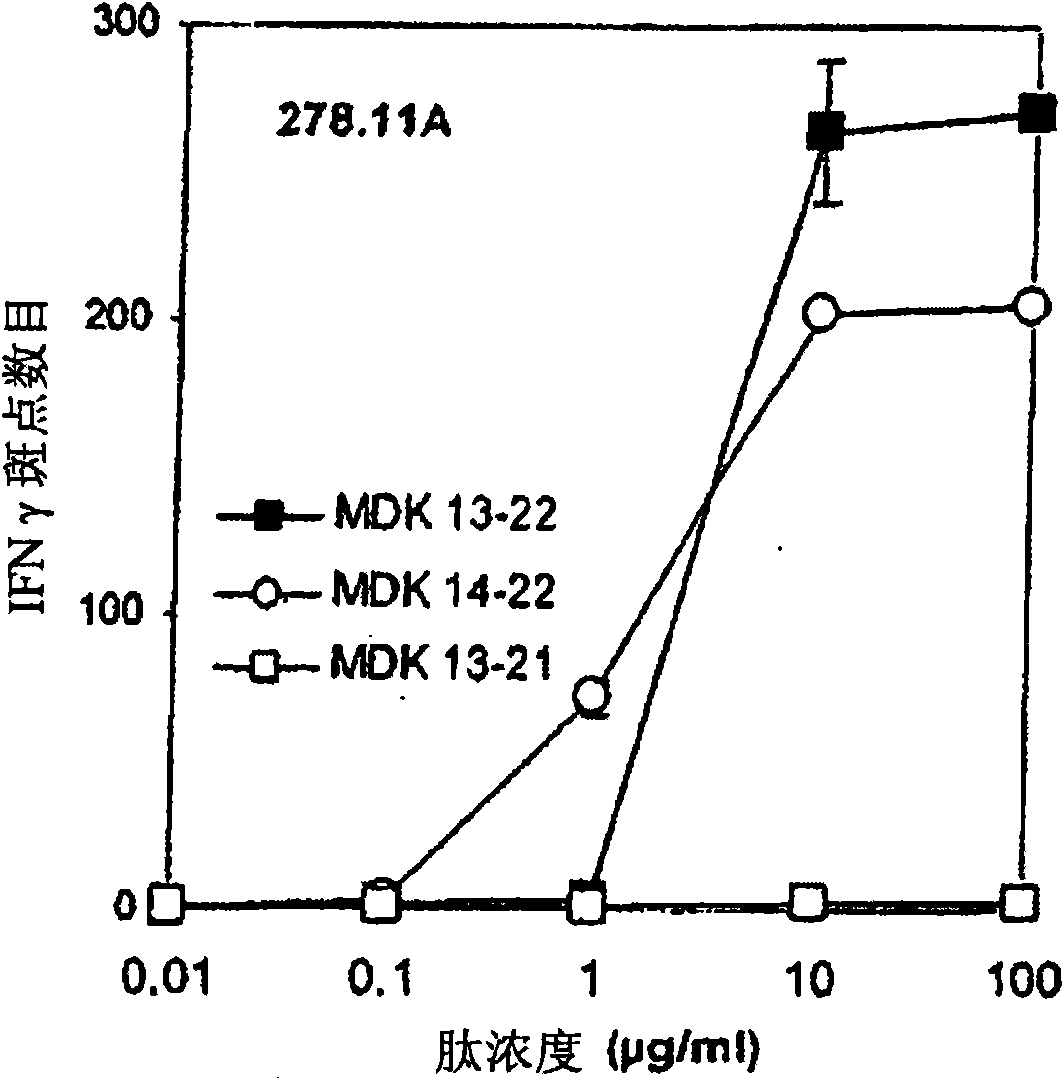
A peptide is derived from the Midkine protein, comprising at least one CD4<+>T or CD8<+>T epitope restricted by the HLA molecules predominant in the Caucasian population, or a polynucleotide encoding said peptide, as an anticancer vaccine or as a reagent for immunomonitoring of the cellular response against Midkine over the course of a cancer or of an anticancer treatment.
Owner:法国原子能与替代能源委员会
Therapeutic Method Targeting Midkine
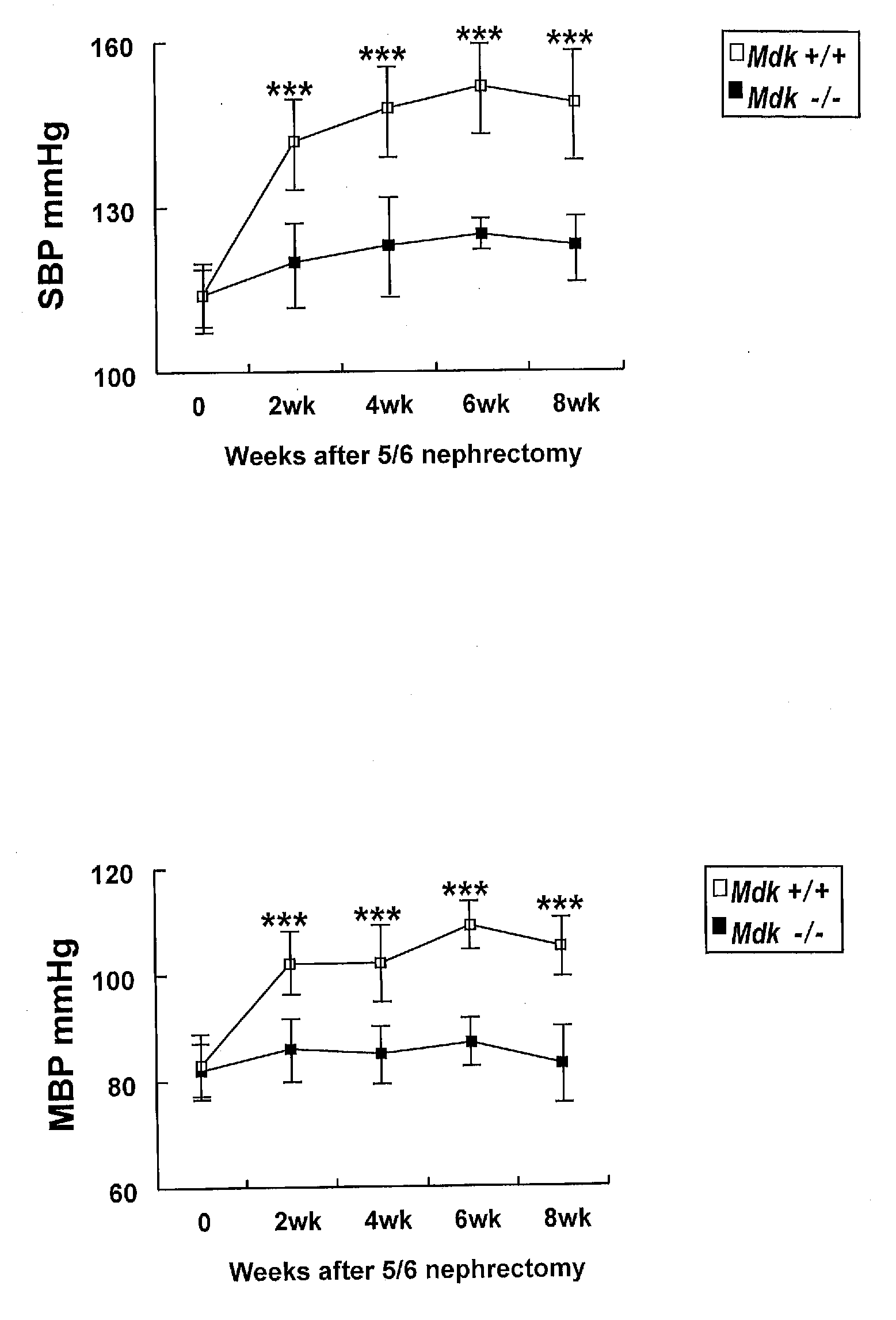
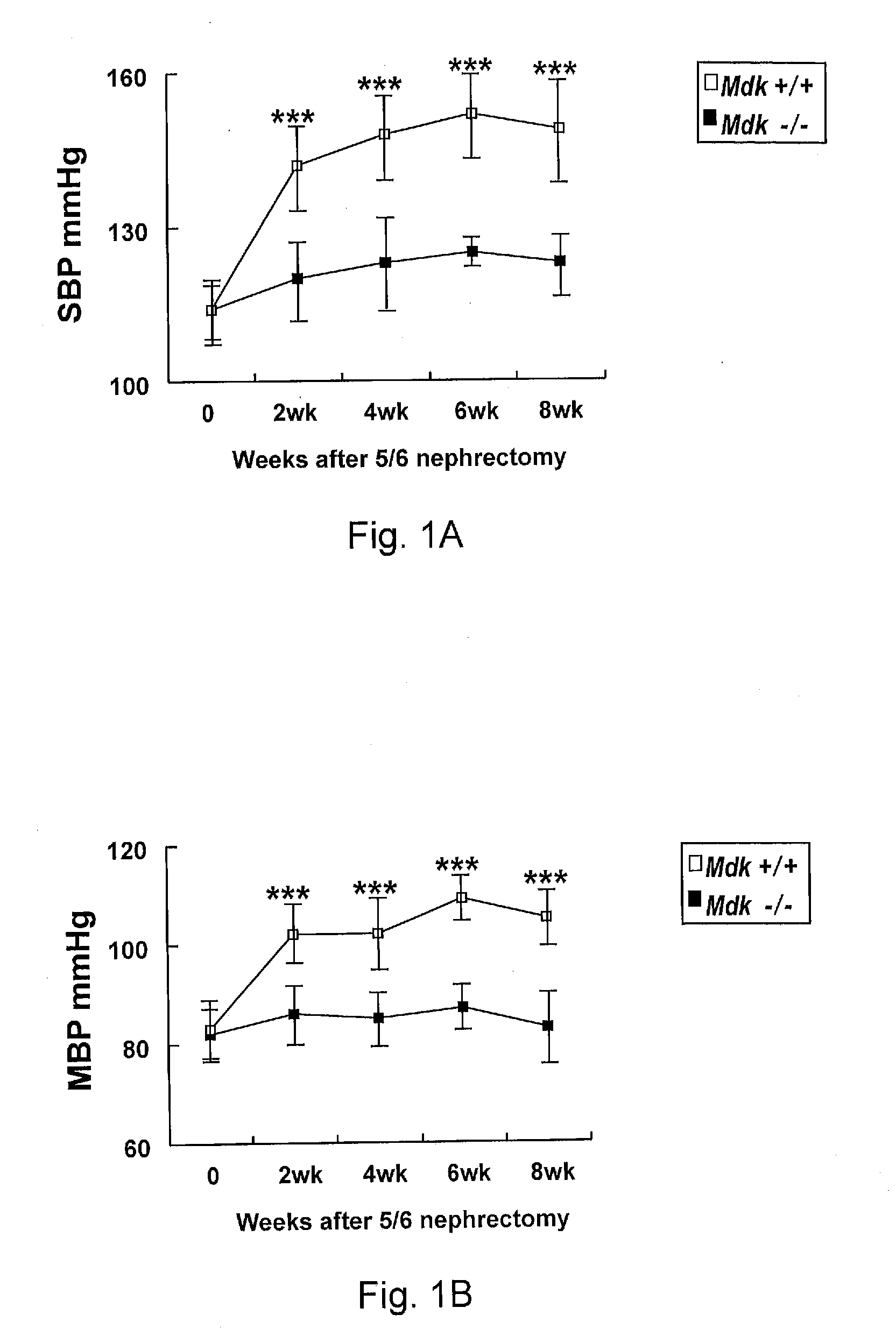
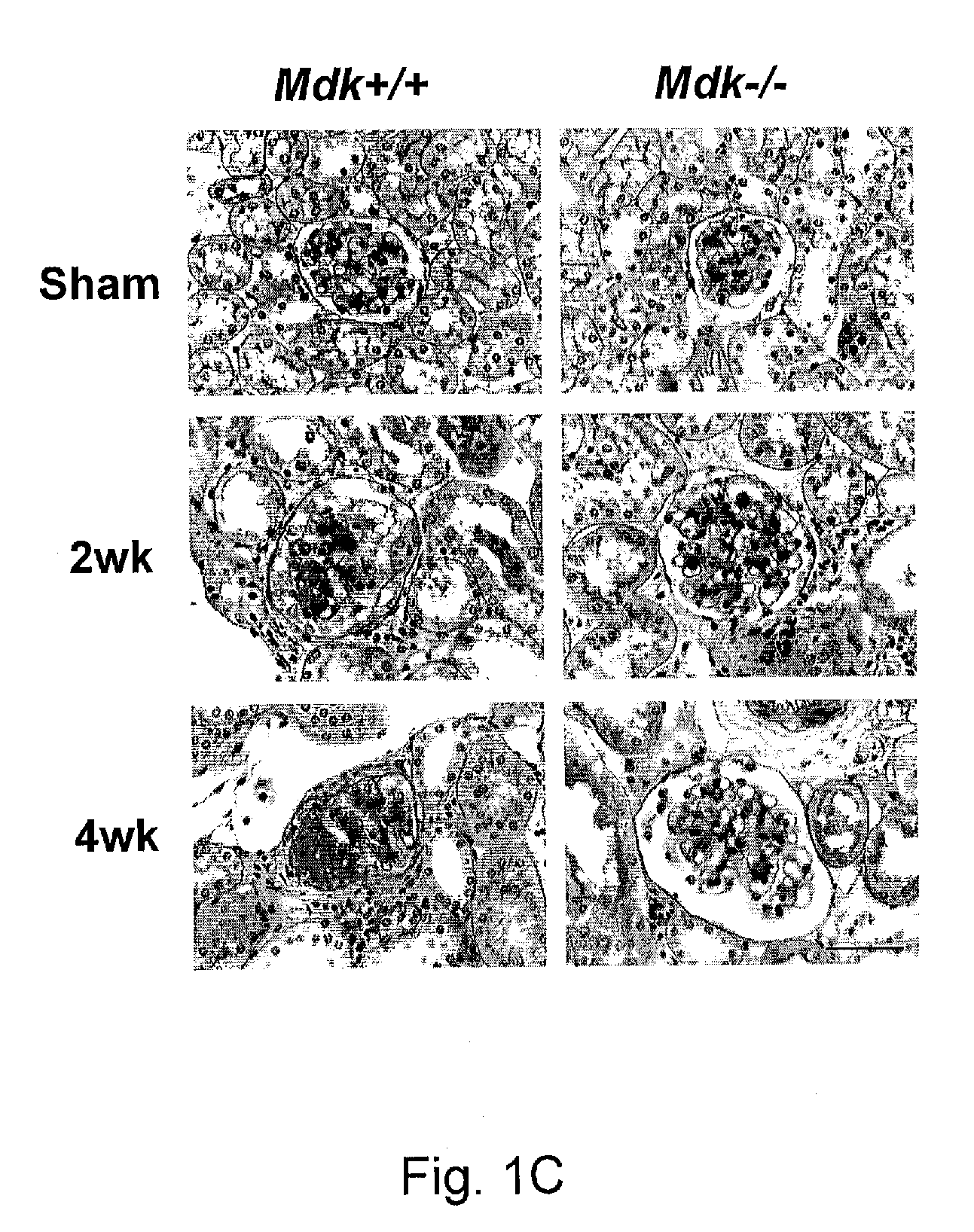
InactiveUS20110059102A1Suppressing midkine expressionInhibit expressionOrganic active ingredientsGenetic material ingredientsNephropathyMidkine
Provided is a therapeutic method targeting midkine for treating mammalian kidney disorders, primary kidney disorders, hypertension that follows secondary kidney disorders such as diabetic nephropathy, and hypertension secondary to chronic kidney disease.
Owner:NAGOYA UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com