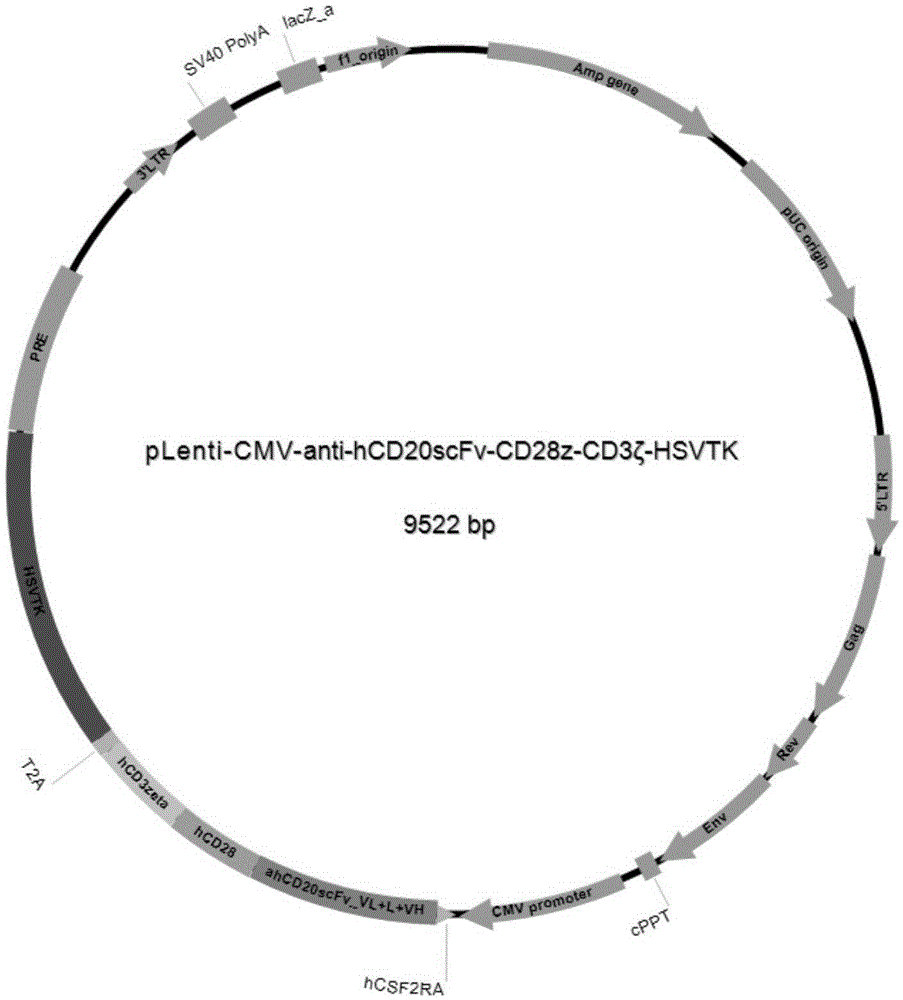
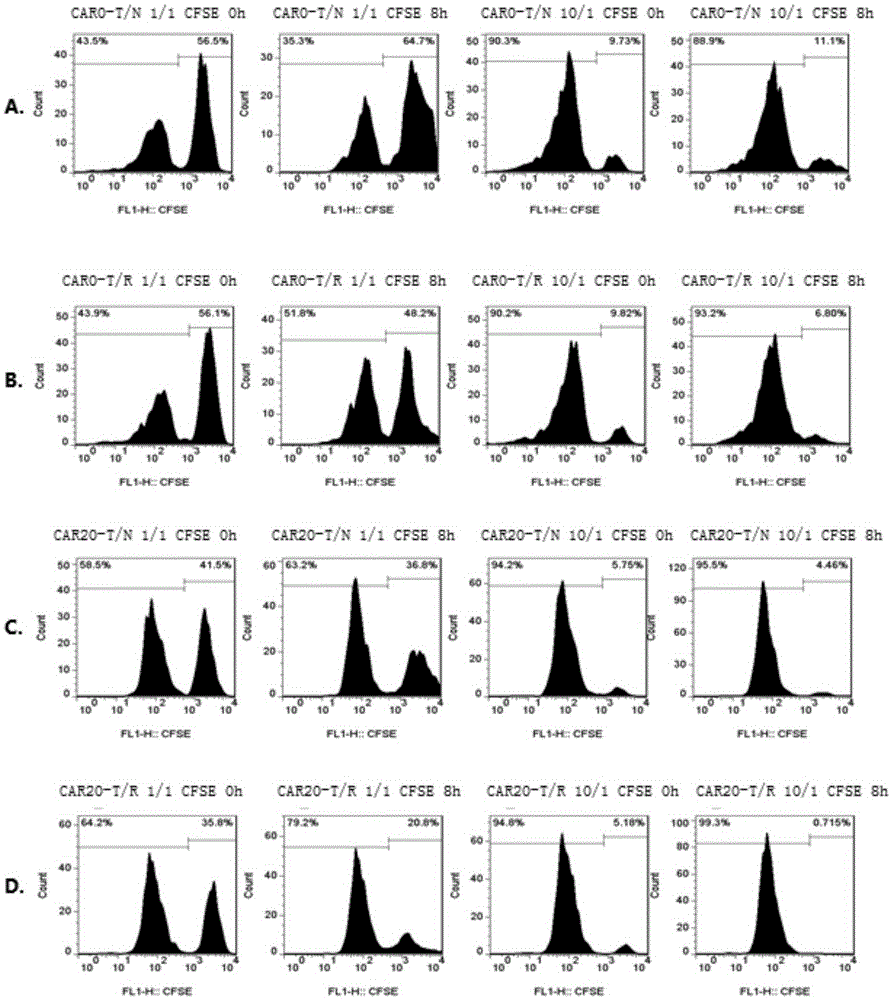
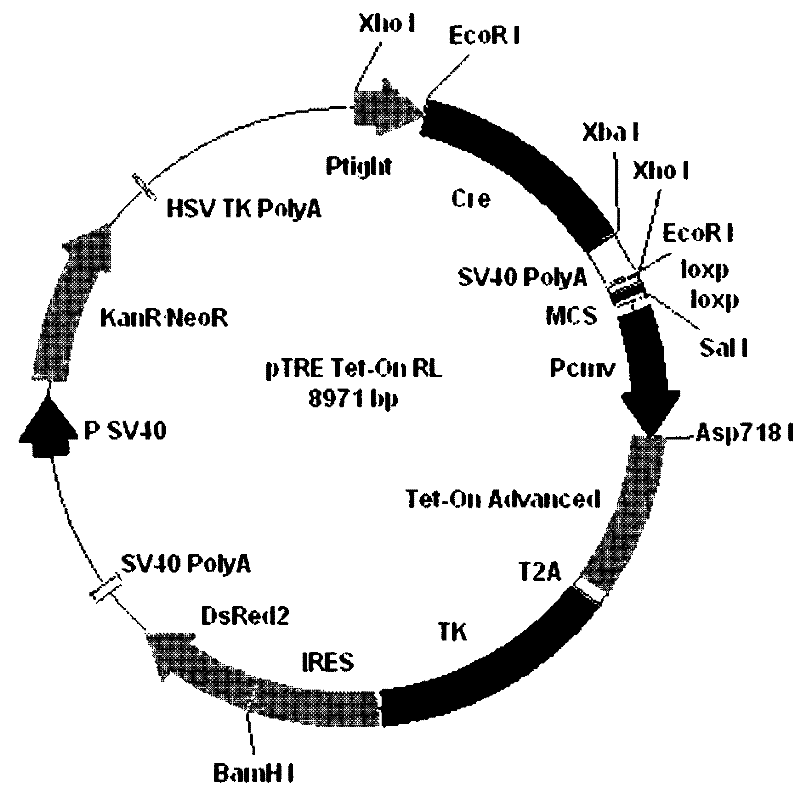
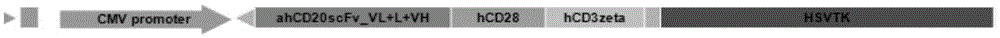Patents
Literature
127 results about "Suicide gene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A suicide gene, in genetics, will cause a cell to kill itself through apoptosis. Activation of these genes can be due to many processes, but the main cellular "switch" to induce apoptosis is the p53 protein. Stimulation or introduction (through gene therapy) of suicide genes is a potential way of treating cancer or other proliferative diseases. Suicide genes form the basis of a strategy for making cancer cells more vulnerable, more sensitive to chemotherapy. The approach has been to attach parts of genes expressed in cancer cells to other genes for enzymes not found in mammals that can convert a harmless substance into one that is toxic to the tumor. Most suicide genes mediate this sensitivity by coding for viral or bacterial enzymes that convert an inactive drug into toxic antimetabolites that inhibit the synthesis of nucleic acid. Suicide genes must be introduced into the cells in ways that ensure their uptake and expression by as many cancer cells as possible, while limiting their expression by normal cells. Suicide gene therapy for cancer requires the vector to have the capacity to discriminate between target and non target cells, between the cancer cells and normal cells.
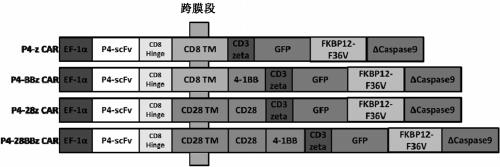
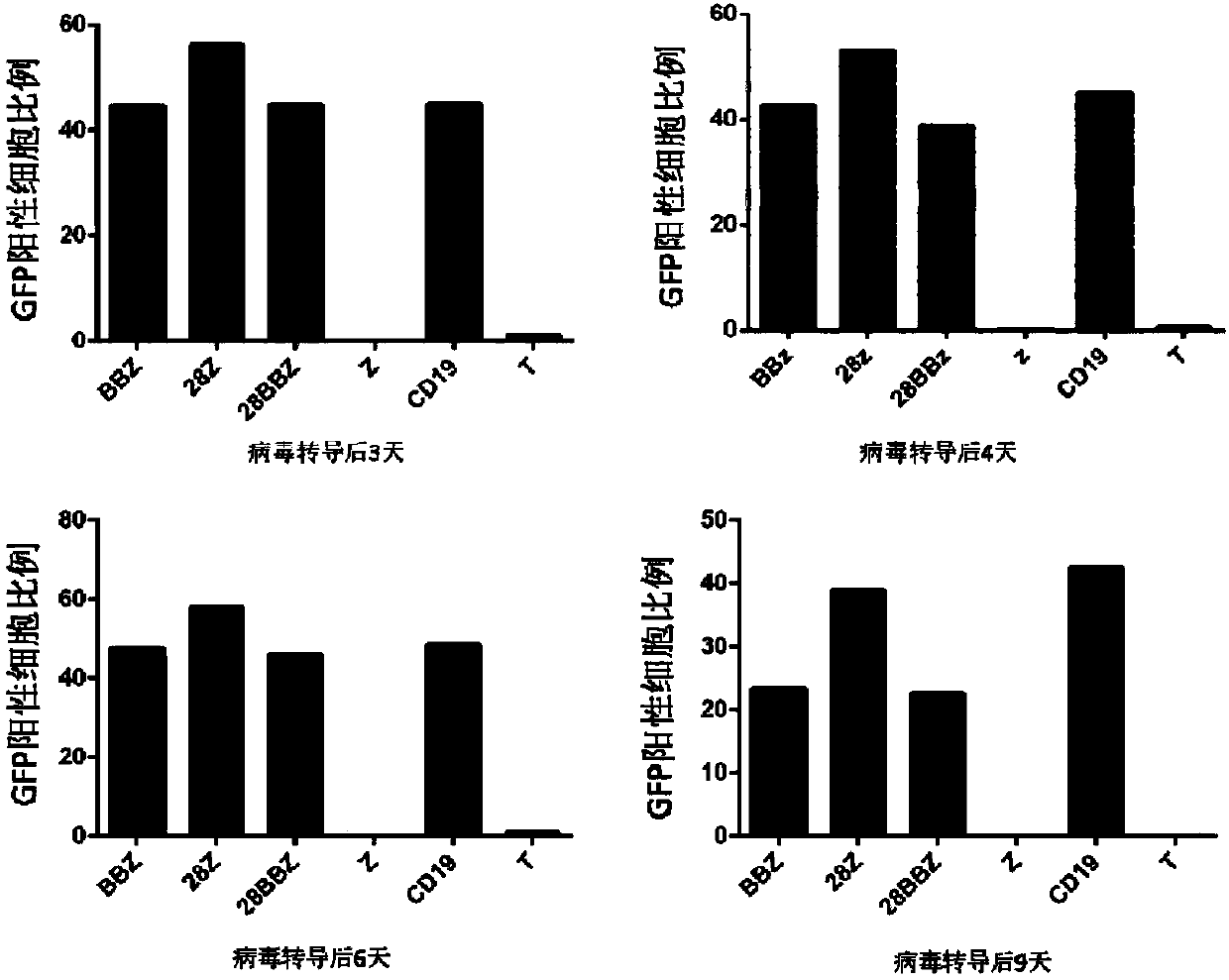
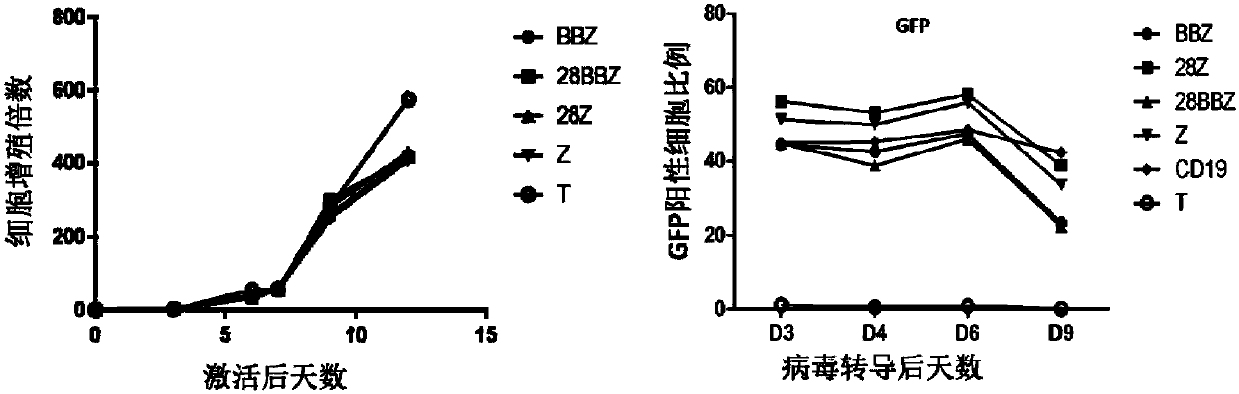
Generation and application of universal T cells for B-ALL
InactiveUS20070036773A1Hinder recognitionEnhanced siRNA effectBiocideGenetic material ingredientsAntigenNatural Killer Cell Inhibitory Receptors
The present invention is directed to universal T cells and their use in treating diseases and other physiological conditions. More specifically, the present invention is directed to universal T cells and their use in treating treating B-lineage acute lymphoblastic leukemia (B-ALL) in particular and malignancy in general. The universal T cells contain (i) nucleic acid encoding a chimeric antigen receptor (CAR) to redirect their antigen specificity and effector function and (ii) nucleic acids encoding shRNA and / or siRNA molecules to down-regulate cell-surface expression of T cell classical HLA class I and / or II genes to avoid recognition by recipient T cells. The universal T cells may also contain a nucleic acid encoding a non-classical HLA gene, such as an HLA E gene to enforce expression of HLA E genes and / or an HLA G gene to enforce expression of HLA G genes, to avoid recognition by recipient NK cells. The universal T cells may further contain a nucleic acid encoding a selection-suicide gene.
Owner:CITY OF HOPE
Vectors for the diagnosis and treatment of solid tumors including melanoma
The present invention is directed to the isolation and use of super-infective, tumor-specific vectors that are strains of parasites including, but not limited to bacteria, fungi and protists. In certain embodiments the parasites include, but are not limited to, the bacterium Salmonella spp., such as Salmonella typhimurium, the bacterium Mycobacterium avium and the protozoan Leishmania amazonensis. In other embodiments, the present invention is concerned with the isolation of super-infective, tumor-specific, suicide gene-containing strains of parasites for use in treatment of solid tumors.
Owner:YALE UNIV
Vectors for the diagnosis and treatment of solid tumors including melanoma
The present invention is directed to the isolation and use of super-infective, tumor-specific vectors that are strains of parasites including, but not limited to bacteria, fungi and protists. In certain embodiments the parasites include, but are not limited to, the bacterium Salmonella spp., such as Salmonella typhimurium, the bacterium Mycobacterium avium and the protozoan Leishmania amazonensis. In other embodiments, the present invention is concerned with the isolation of super-infective, tumor-specific, suicide gene-containing strains of parasites for use in treatment of solid tumors.
Owner:YALE UNIV
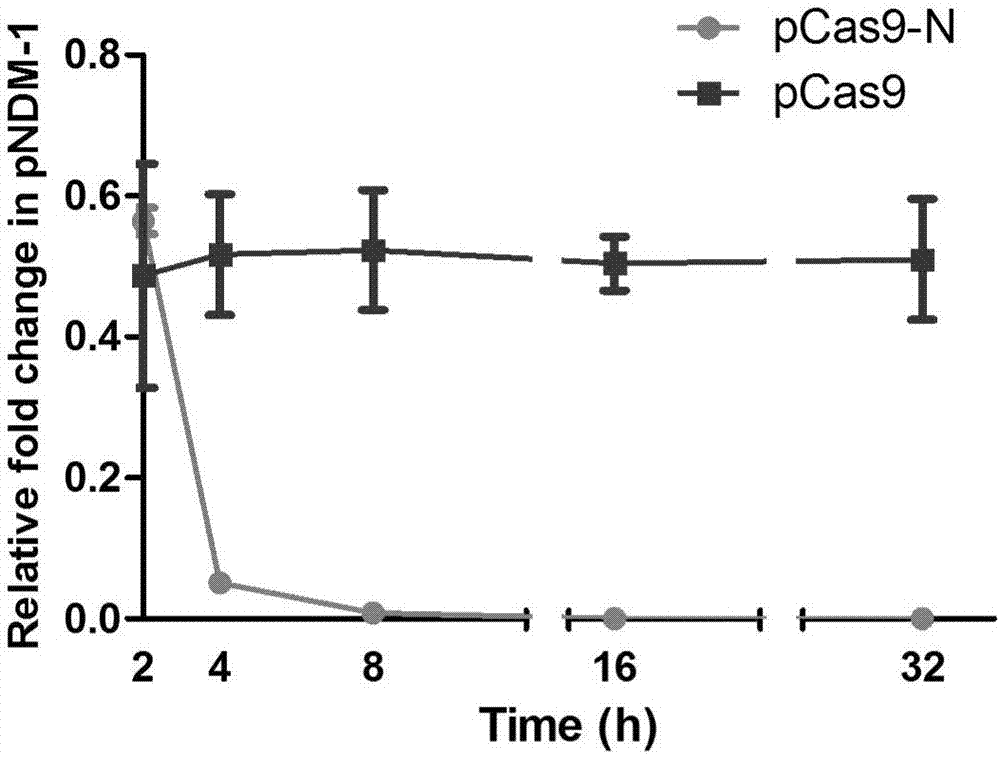
Method of packaging CRISPR-Cas9 (clustered regularly interspaced short palindromic repeat-associated 9) system by using temperate phage vector
ActiveCN107365804ADelay drug resistanceBlock horizontal transferHydrolasesStable introduction of DNAEscherichia coliPhage lysis
The invention discloses a method of packaging a CRISPR-Cas9 (clustered regularly interspaced short palindromic repeat-associated 9) system by using a temperate phage vector. The method comprises the steps of: (1) constructing suicide genes, target spot sequences bound with specific gRNA (guide ribonucleic acid), and downstream PAM (protospacer adjacent motif) sequences into pSTK (protein serine threonine kinase) plasmids, (2) transforming the pSTK plasmids into escherichia coli host bacteria, (3) transforming CRISPR-Cas9 sequence recombination template double-chain DNA (deoxyribonucleic acid) linear fragments carrying phage sequence homologous arms at the two ends into the host bacteria, (4) inducing expression of homologous recombination related enzymes and the suicide genes SacB, (5) screening the host bacteria subjected to homologous recombination, and (6) inducing temperate phages to crack the host bacteria, and harvesting the recombined temperate phages packaging the CRISPR-Cas9 system, wherein chromosomes of the escherichia coli host bacteria are integrated with the temperate phages; and plasmids capable of expressing the homologous recombination related enzymes are transformed into the escherichia coli host bacteria. According to the packaging method, a secondary recombination step of deleting a resistance marker is removed, and the technical support is provided for the phage vector presenting CRISPR-Cas9 system to resist drug-resistance bacteria efficiently and quickly.
Owner:INST OF PLA FOR DISEASE CONTROL & PREVENTION
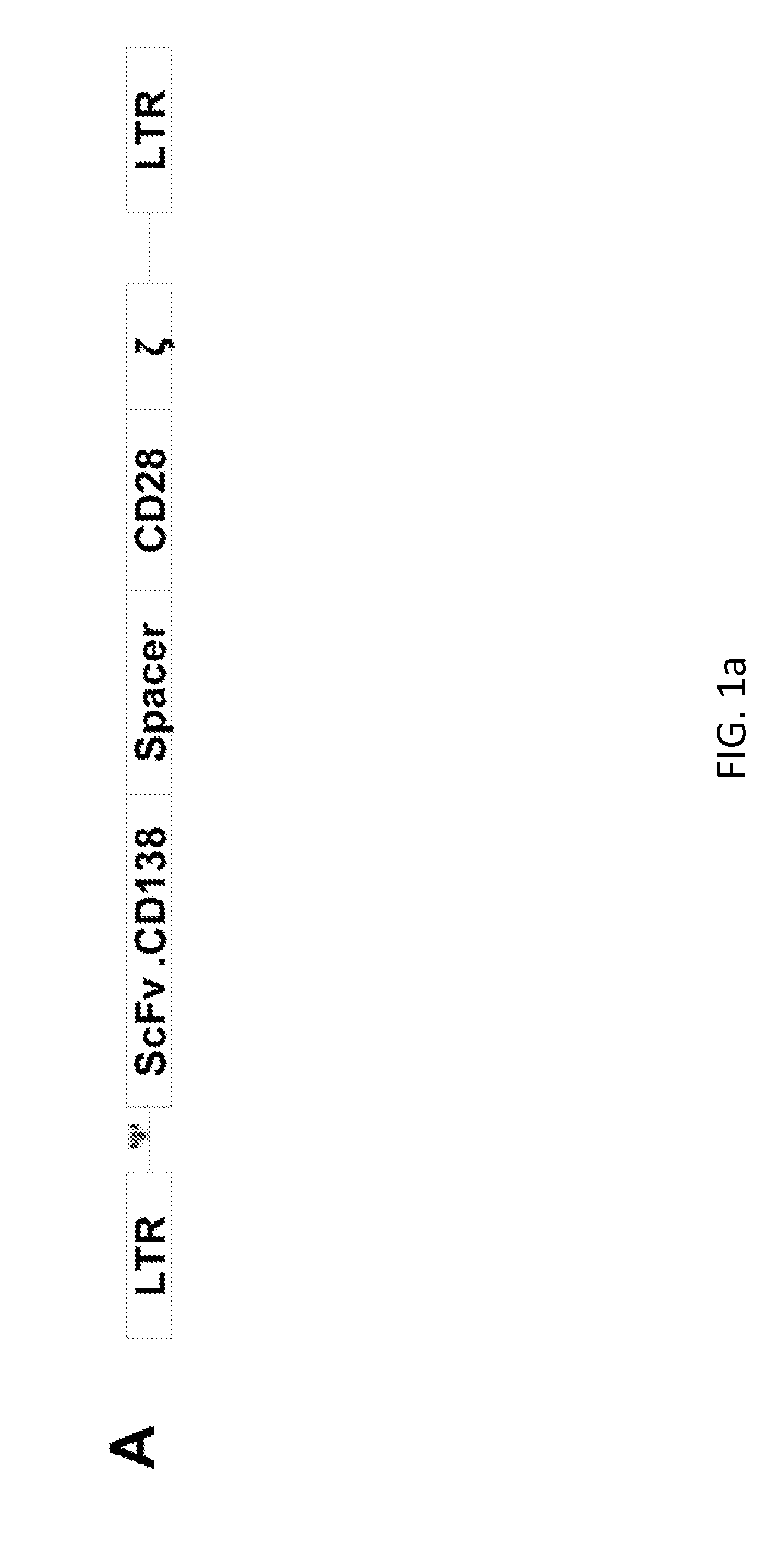
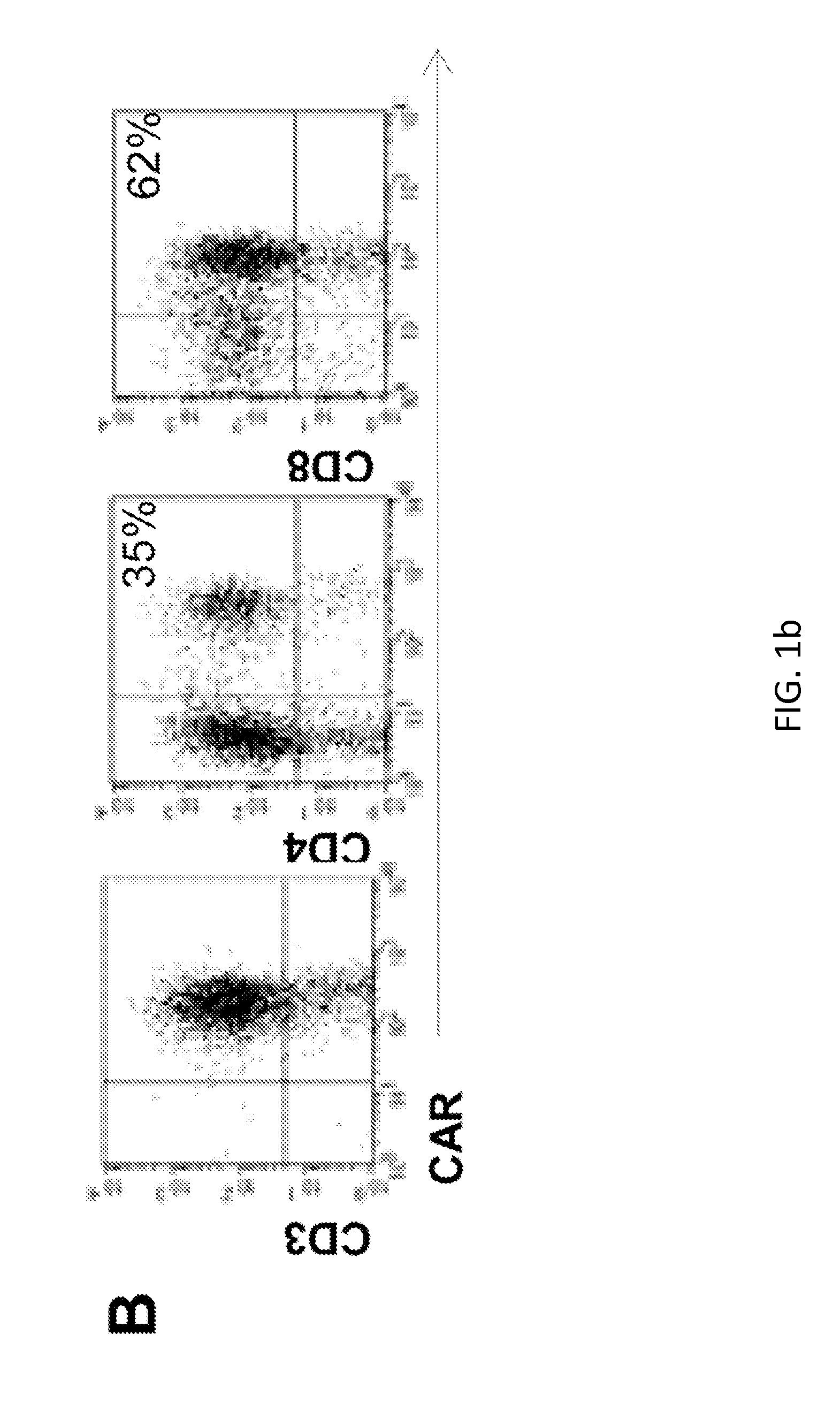
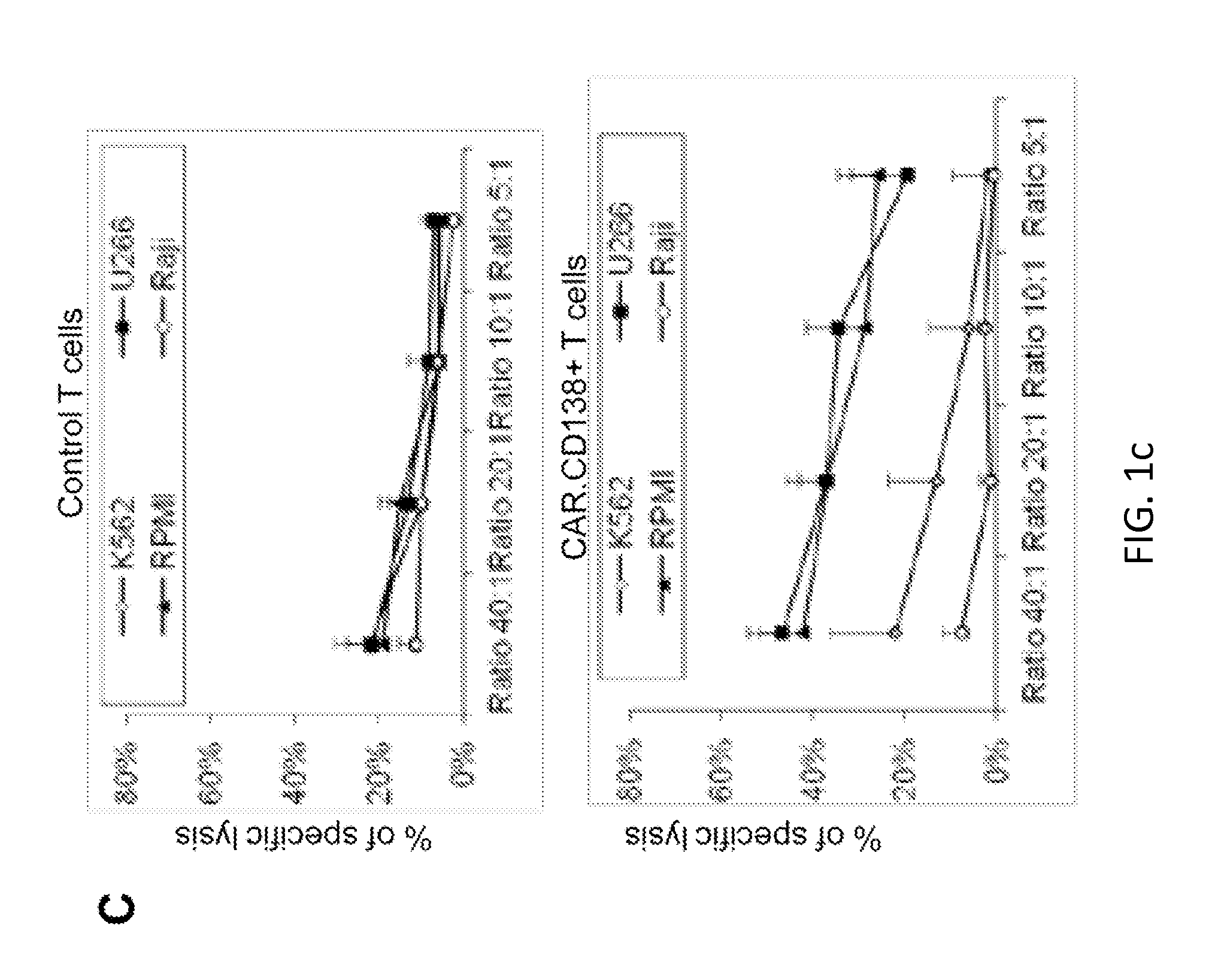
Targeting cd138 in cancer
Embodiments of the present disclosure concern therapeutic vectors and cells that target certain cancer cells but do not other cells having the same antigen. In specific embodiments, the methods and compositions of the disclosure concern cells having a CD138-specific chimeric antigen receptor whose expression is under the control of environment-specific regulation. In specific embodiments the environment is hypoxia. In some cases, the compositions comprise a suicide gene.
Owner:BAYLOR COLLEGE OF MEDICINE
Chimeric antigen receptor immune cell provided with safety switch as well as preparation method and application of chimeric antigen receptor immune cell
InactiveCN106755023AImprove effectivenessImprove securityGenetic material ingredientsMammal material medical ingredientsAbnormal tissue growthAntigen receptors
The invention relates to a chimeric antigen receptor (CAR) immune cell provided with a safety switch as well as a preparation method and an application of the CAR immune cell. The CAR immune cell carrying the safety mechanism (the safety switch) comprises a CAR coding nucleotide sequence, wherein the structure of the nucleotide sequence comprises a receptor structural domain for recognizing tumor-specific antigen or tumor-associated antigen, a transmembrane-stimulation structural domain, a CD3[zeta] stimulating signal transduction region and a suicide gene region. The CAR immune cell can be obtained as different immunological effect cells are amplified and a CAR sequence carrying a suicide mechanism is transduced; corresponding antigens of tumor cells are recognized by virtue of CAR; and the CAR immune cell can generate a specific killing effect on the tumor cells. The CAR immune cell, when used, is infused in a gradient mode and dynamic change in related cell factor levels is monitored; in case of need, a suicide gene can be started by virtue of drugs to scavenge the immunological effect cells, so that optimal balance between safety and a curative effect is achieved; therefore, the safety of the technology applied to the treatment of tumors in the clinical field is guaranteed to the greatest extent.
Owner:AFFILIATED HOSPITAL CHINA ACADEMY OF MILITARY MEDICAL SCI +1
Expression vector, and its construction and application
InactiveCN102226201AImprove securityVector-based foreign material introductionAnimal husbandryAgricultural scienceGene
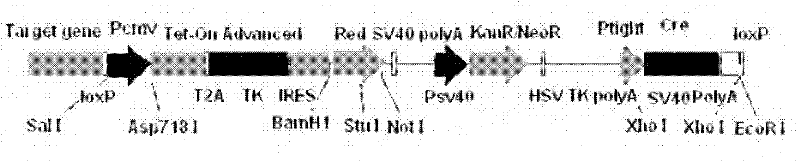
The invention discloses an expression vector, and its construction and application. The construction of the expression vector comprises adding LoxP sequences at two ends of an untargeted gene of a linear vector and introducing inducible expression Cre enzyme and TK suicide gene components simultaneously. The expression vector improves securities of transgenic animals.
Owner:XINJIANG ACADEMY OF AGRI & RECLAMATION SCI
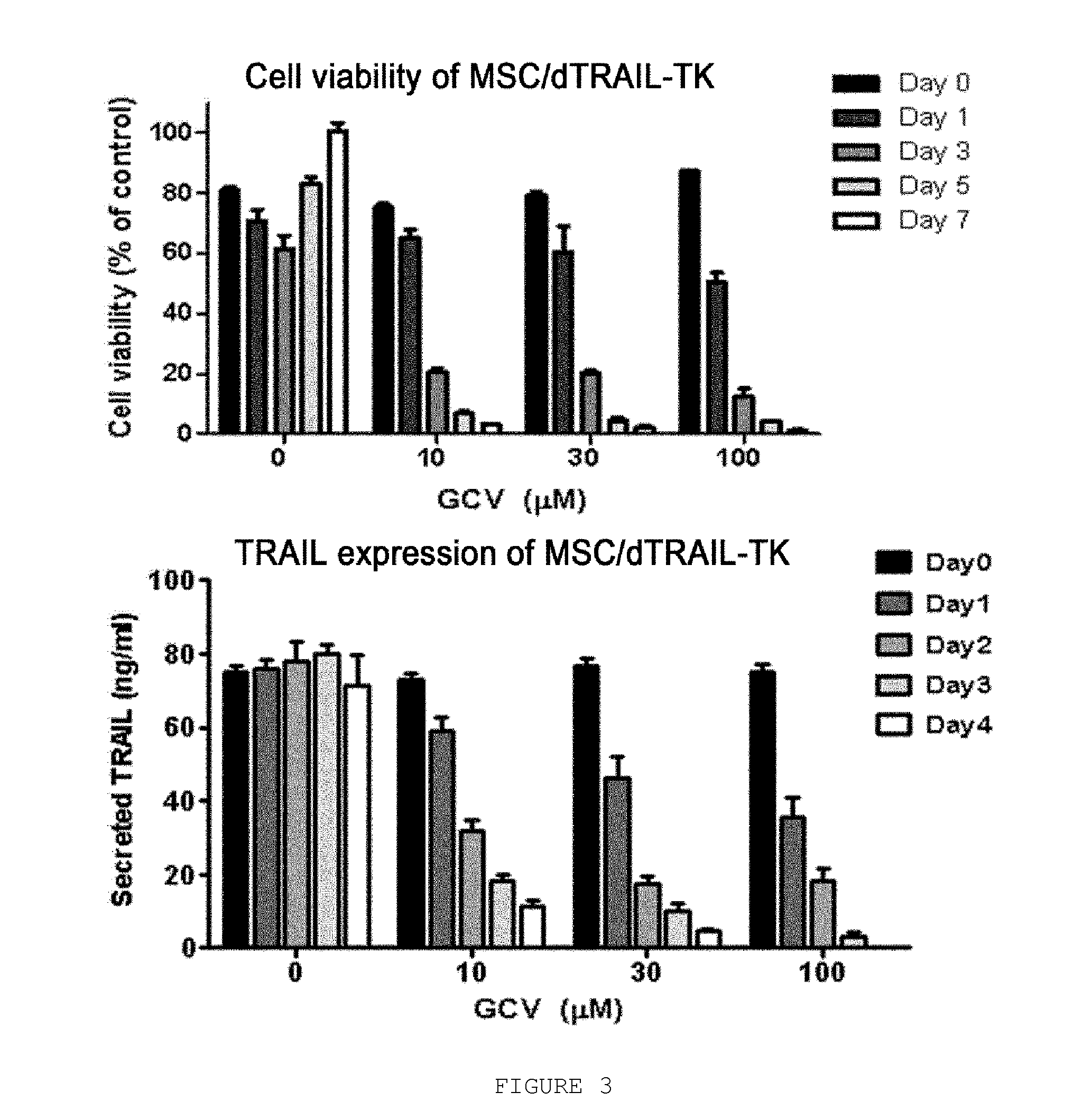
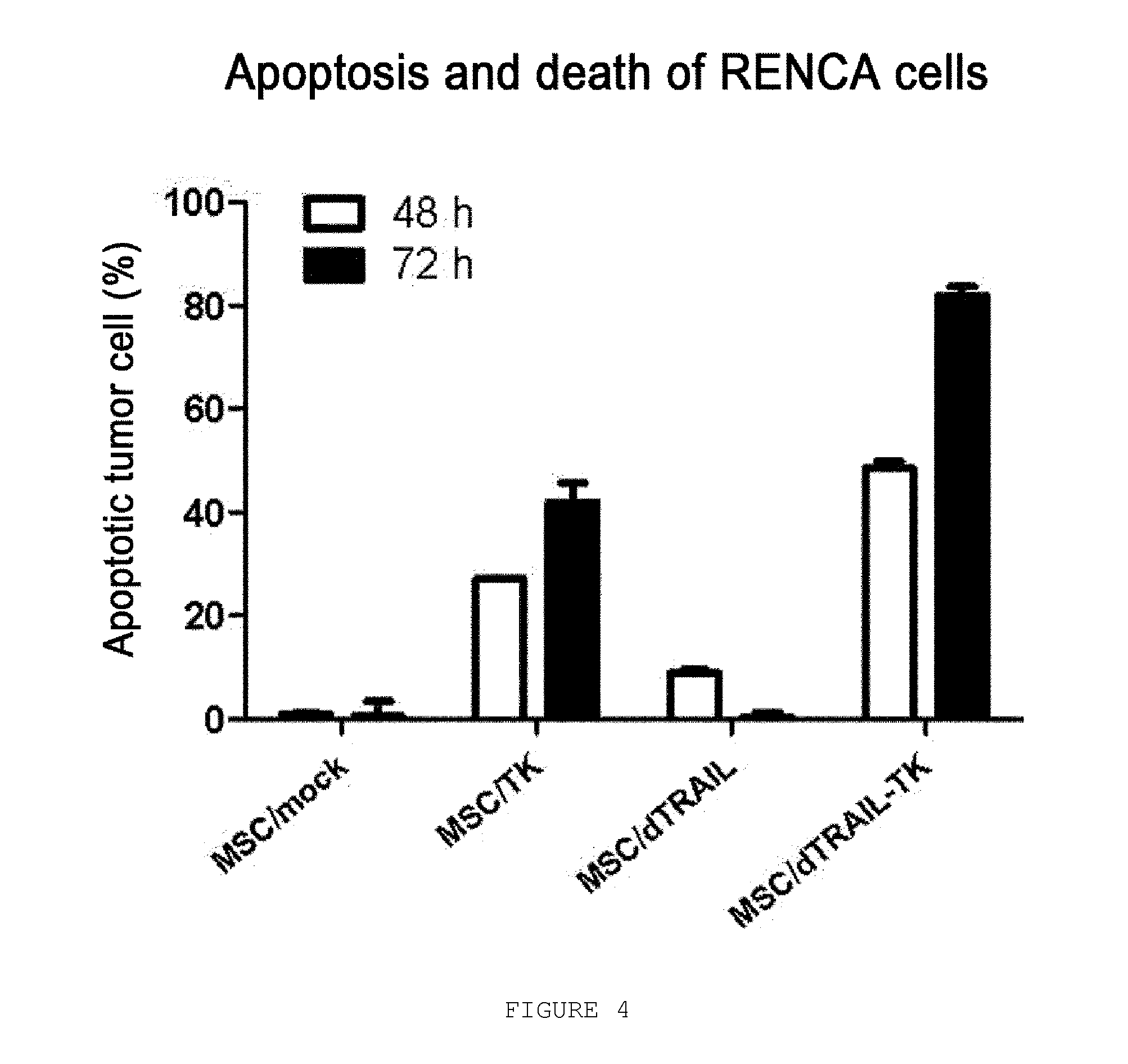
Use Of Mesenchymal Stem Cells Genetically Modified To Express A Suicide Gene For Treating A Cancer
InactiveUS20080241115A1Reduced viabilityIncrease the number ofBiocideGenetic material ingredientsAnticarcinogenMesenchymal stem cell
Mesenchymal stem cells expressing a suicide gene show excellent and highly selective anticancer effects against cancer tissues through the selective conversion of a prodrug of an anticancer agent to the anticancer agent at around the cancer. Also disclosed herein are a pharmaceutical composition for treating a cancer comprising the mesenchymal stem cell; a kit for treating a cancer comprising an expression vector comprising the suicide gene, the mesenchymal stem cell and the prodrug; and a method for treating a cancer patient, which comprises successively administering the mesenchymal stem cell and the prodrug to the patient.
Owner:AJOU UNIV IND ACADEMIC COOP FOUND
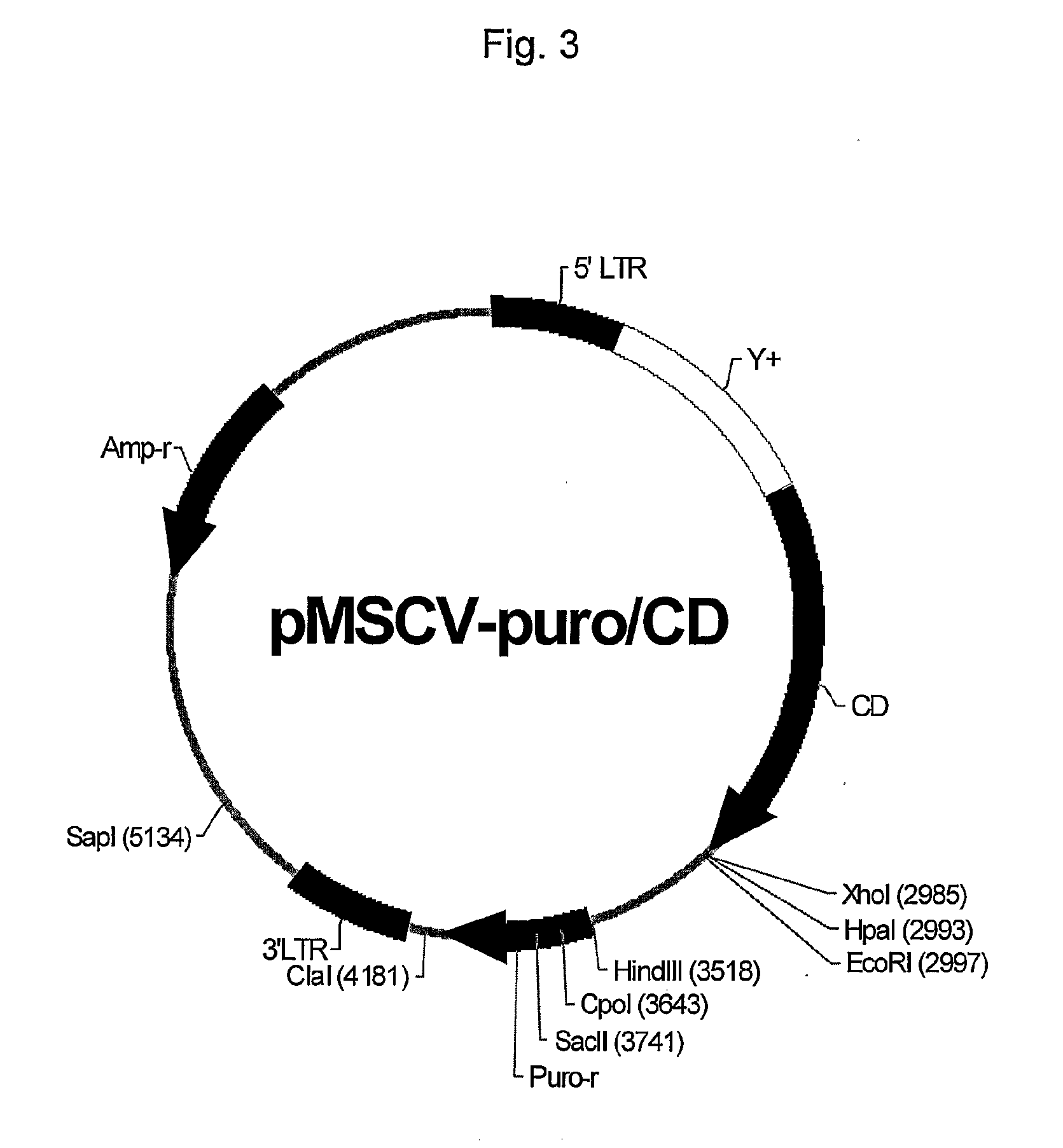
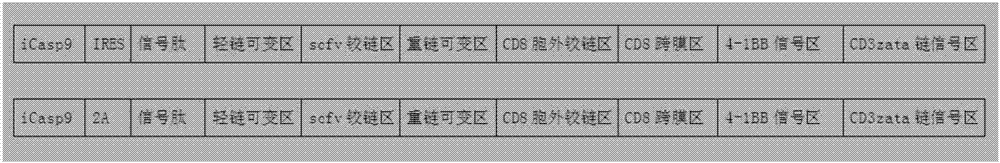
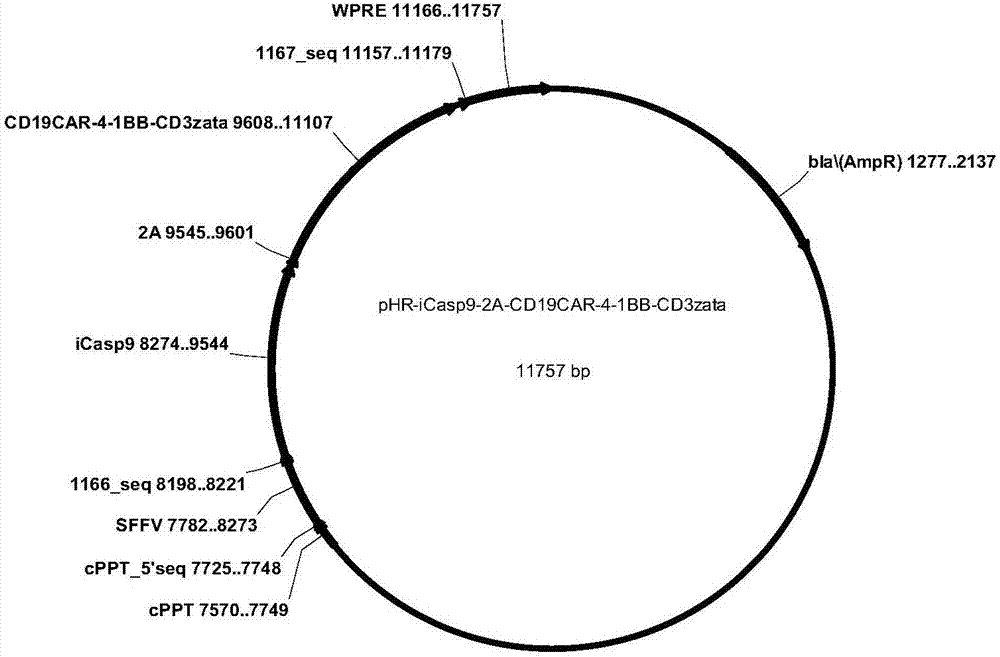
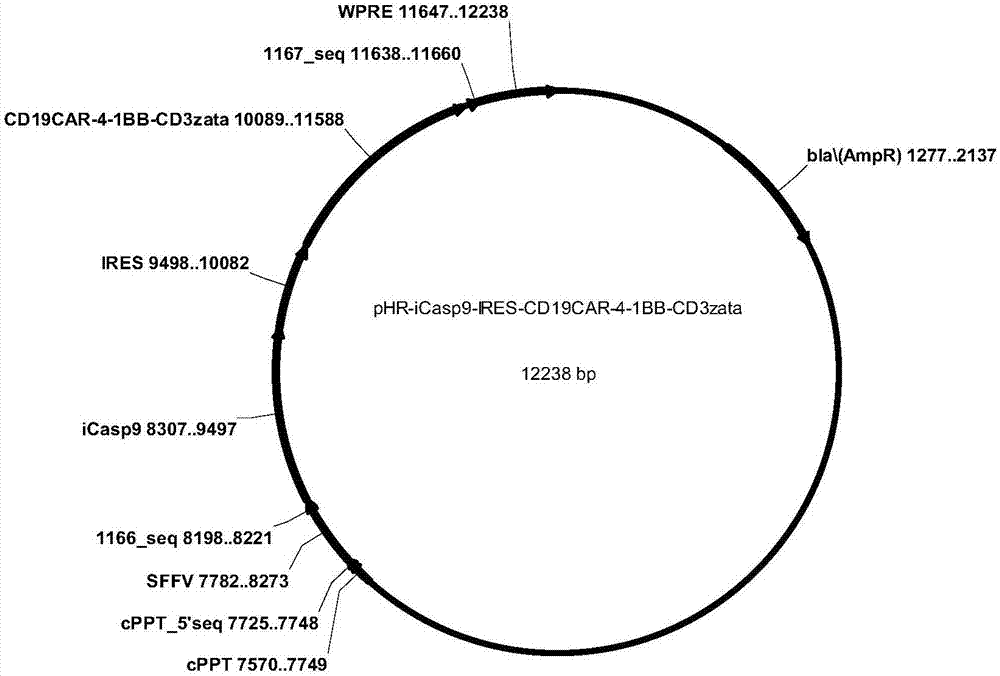
CD19-CAR-T cell carrying iCasp9 suicide gene and use thereof
ActiveCN107365798AImprove securityImmunoglobulin superfamilyGenetically modified cellsT-cell apoptosisSide effect
The invention discloses a lentivirus expression vector. The lentivirus expression vector comprises a gene encoding a chimeric antigen receptor and an iCasp9 apoptotic gene. The invention also discloses a CD19-CAR-T cell carrying an iCasp9 suicide gene. The CD19-CAR-T cell is a T lymphocyte comprising a lentivirus expression vector or a T lymphocyte of which the chromosome is integrated with a gene encoding a chimeric antigen receptor and an iCasp9 apoptotic gene. The invention also discloses a use of the CD19-CAR-T cell carrying an iCasp9 suicide gene in preparation of drugs for preventing and / or treating and / or assistantly treating cancers. Compared with the existing CD19-CAR-T cell, the CD19-CAR-T cell carrying an iCasp9 suicide gene utilizes a suicide mechanism and can express CAR and co-express a suicide gene iCasp9. When serious side effects occur, through inducing T cell apoptosis, CRS side effects are controlled and safety is improved.
Owner:山东省齐鲁细胞治疗工程技术有限公司
Engineering immune cell with suicide gene switch of targeting human mesothelin
ActiveCN109593721APolypeptide with localisation/targeting motifImmunoglobulin superfamilyDiseaseAbnormal tissue growth
The invention provides an engineering immune cell with a suicide gene switch of targeting human mesothelin (MSLN). Particularly, the invention provides a chimeric antigen receptor T cell of the targeting human mesothelin, a CAR (Chimeric Antigen Receptor) structure of the cell comprises a cell suicide element, and the expression of a PD1 gene in the cell is silent. Particularly, the CAR structurecomprises a CAR basic structure and the cell suicide element at the same time, the CAR basic structure and the cell suicide element are independent of each other, and the corresponding functions of the CAR basic structure and the cell suicide element are non-interfering. In addition, the silent expression of the PD1 gene in the cell has a synergistic effect with the CAR structure, the tumor killing effect is strengthened, and the disease does not relapse easily.
Owner:GRACELL BIOTECH SHANGHAI CO LTD
Recoverable immortalized hepatic cell line carrying double suicide genes and establishing method of recoverable immortalized hepatic cell line
ActiveCN104450620AAchieve knockoutRealize regulationGenetic engineeringFermentationHuman bodyProgenitor cell
The invention provides an establishing method of a recoverable immortalized hepatic cell line. The establishing method comprises the following steps: separating a hepatic progenitor cell from liver in a mouse which is 12.5-14.5 days old in an embryonic period; guiding a retrovirus containing genes SV40T and HSV-TK into the hepatic progenitor cell; screening a monoclonal cell strain having a hepatic progenitor cell marker and having a function of differing to a mature hepatic cell; then guiding a retrovirus containing CD gene into the cell strain so as to obtain recoverable immortalized hepatic cell carrying double suicide genes. The cell strain can multiply in vitro to obtain the phenotype and functions of a normal hepatic cell, the safety of the immortalized cell becomes adjustable through controllable modes such as locus recombination and drug screening; when the cell is used in a human body, the biological safety of the immortalized cell is ensured to the greatest extent and the dangerousness of the immortalized cell is reduced; therefore, a reliable, safe and ideal hepatic cell material is provided for bioartificial liver technology.
Owner:重庆多沃生物科技有限公司
Tumor targeted therapeutic drug carrier as well as preparation method and application thereof
ActiveCN102133404AImprove targetingGood tumor therapy targetingPowder deliveryOrganic active ingredientsTumor targetLiposome
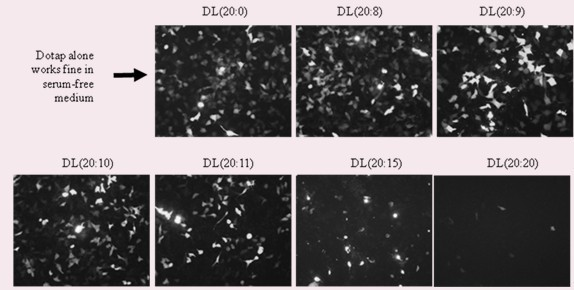
The invention discloses a drug carrier for general administration through intravenous injection, a preparation method and technology thereof applied to tumor gene therapy, belonging to the field of tumor targeted therapy. The carrier provided by the invention is a novel liposome formed by DOTAP or other analogues and lecithin or derivatives thereof according to a mole ratio of 20: (7-13), can form a stable composite with bioactive substances, and can directionally and efficiently carry the bioactive substances out of the human body to culture and carry into target cells in the human body. Thecomposite provided by the invention has larger carrying capacity; the particle size is greatly decreased, namely, the particle size is optimized to 200nm and below; high transfection efficiency is kept under the condition of high serum concentration; when the carrier package provided by the invention carries cancer suppressor gene or cell suicide gene DNA and the like, the formed composite can betargeted and carried out of the human body to culture, carried away from the human body and carried into the tumor cells in the human body so as to achieve the purpose of carrying out gene therapy onthe tumor.
Owner:CHENGDU NUOEN BIOLOGICAL TECH
Feeder cells for target cell induction
InactiveUS20120149598A1Simple and easy cultureMicrobiological testing/measurementGenetically modified cellsMicrobiologyCell sheet
The present invention provides a feeder cell for target cell induction, wherein the feeder cell is transduced with an immortalizing gene and a suicide gene; and a production process for a target cell sheet using the feeder cell for target cell induction.
Owner:OSAKA UNIV
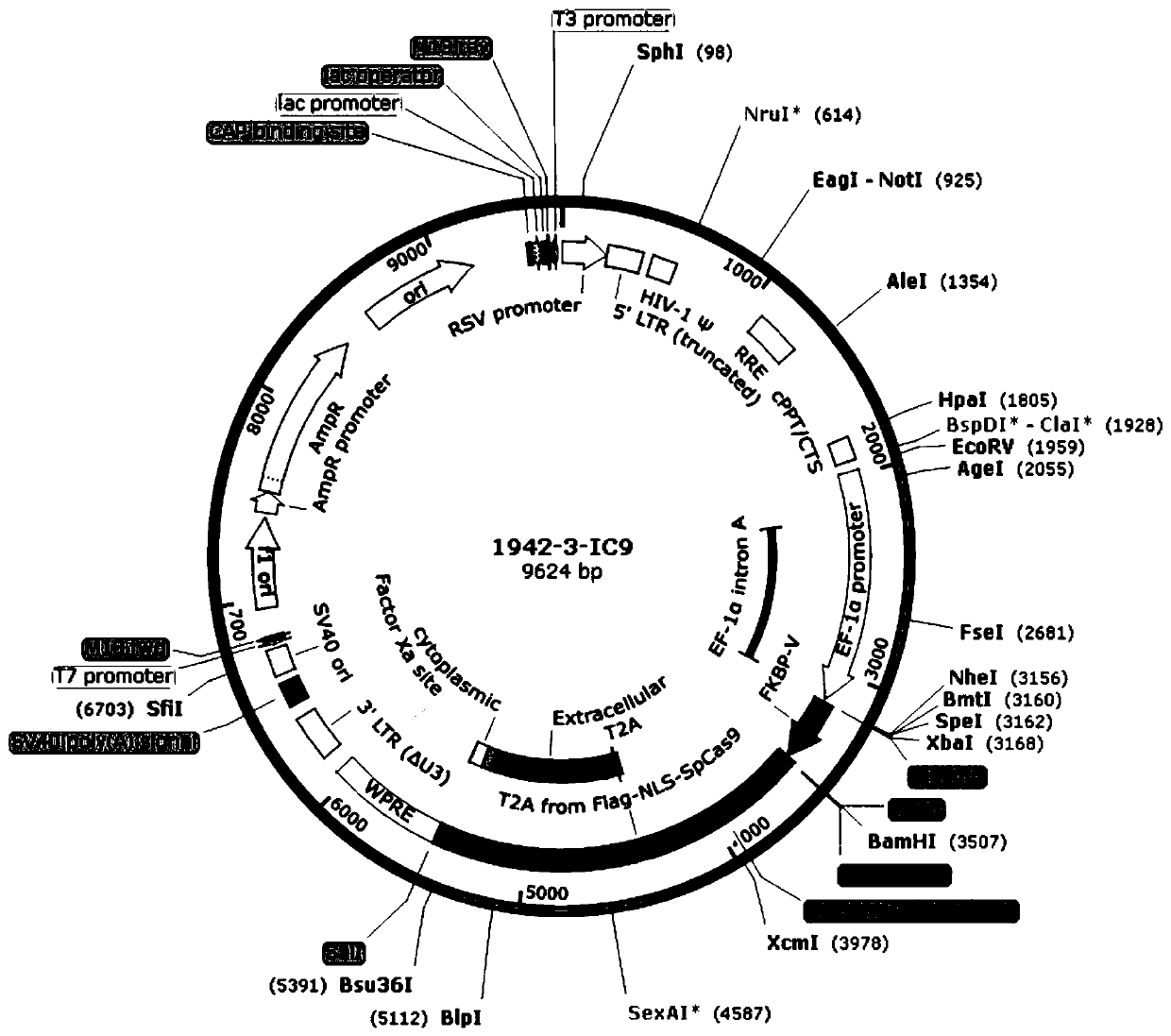
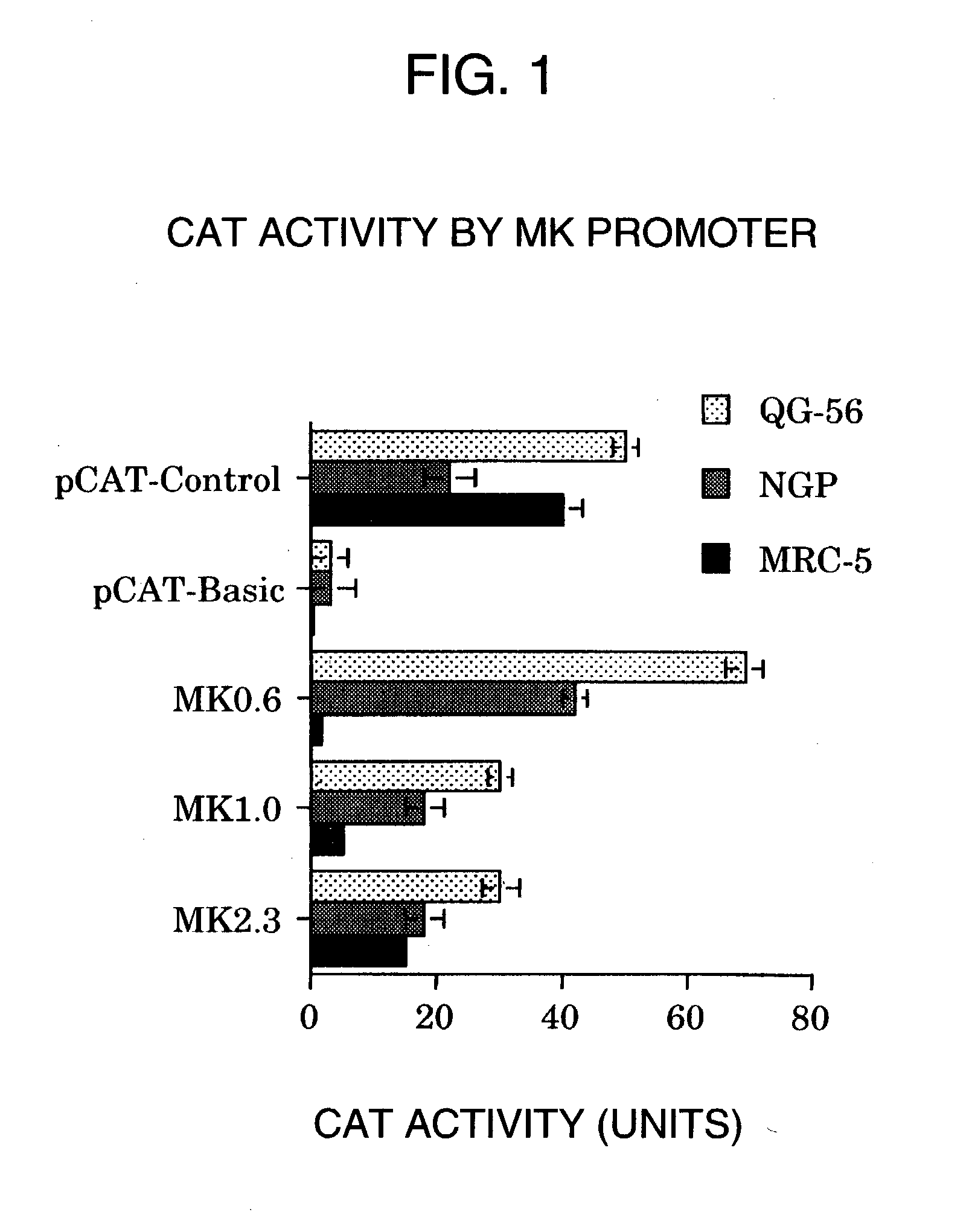
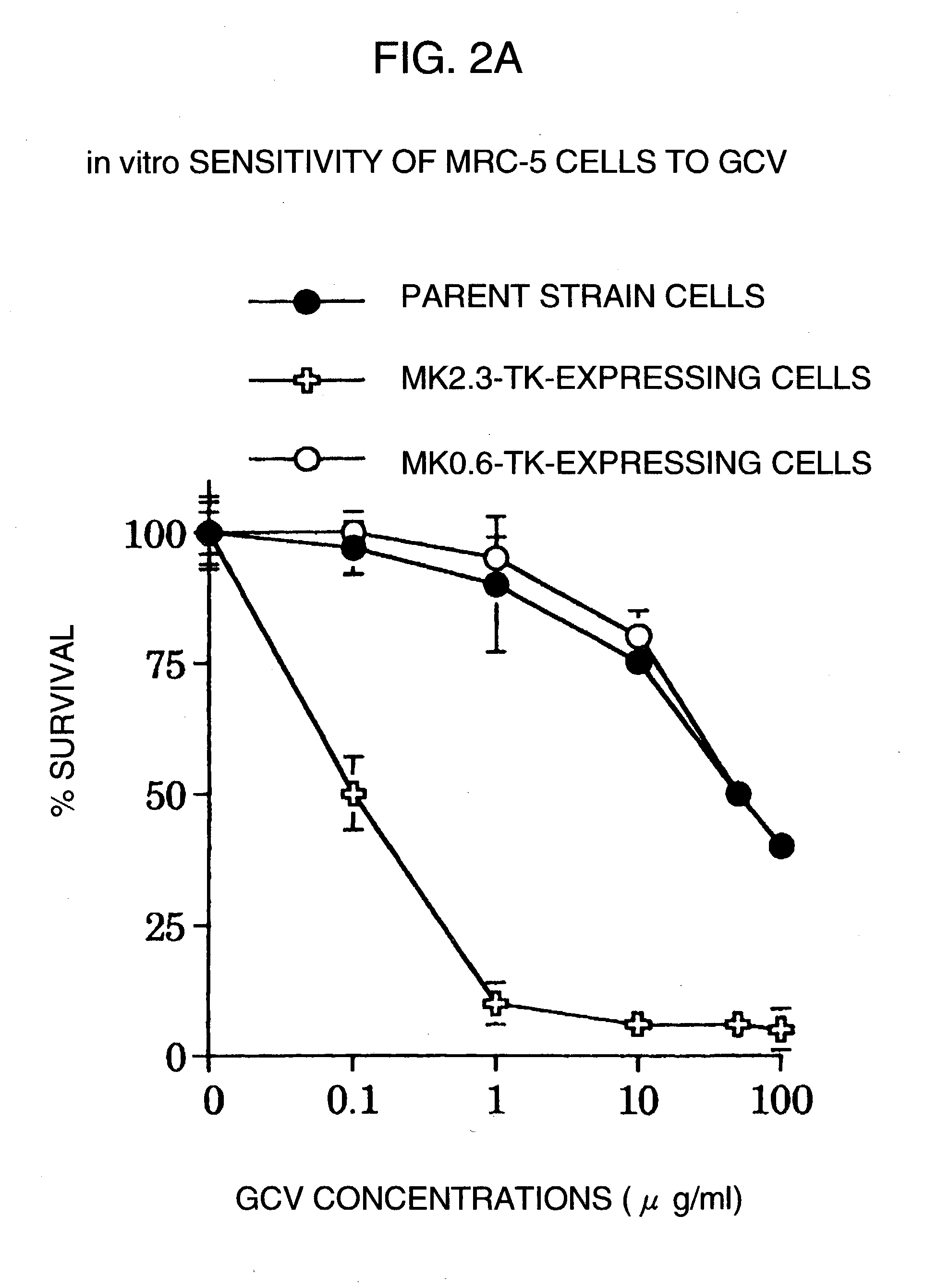
Tumor-specific promoter
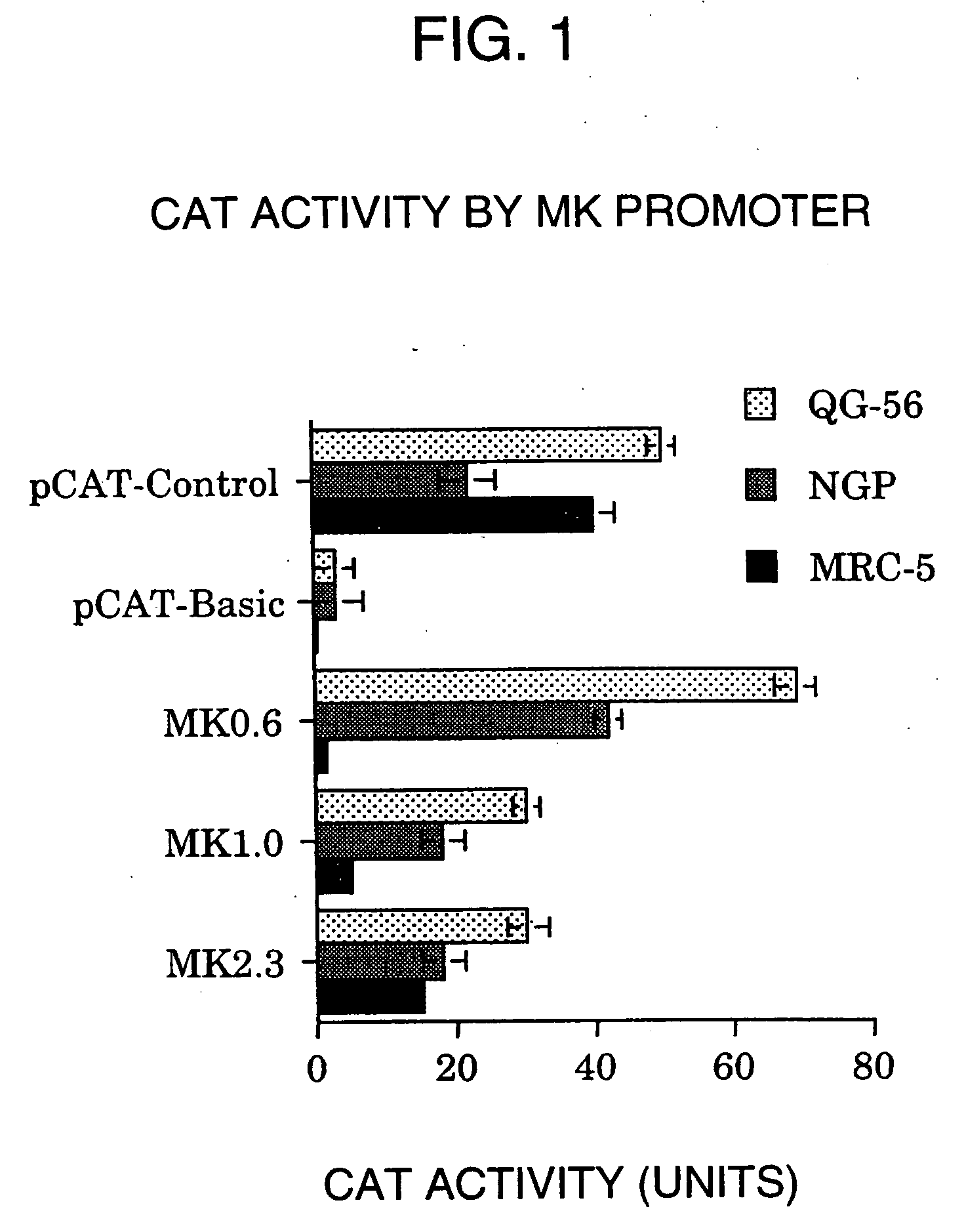
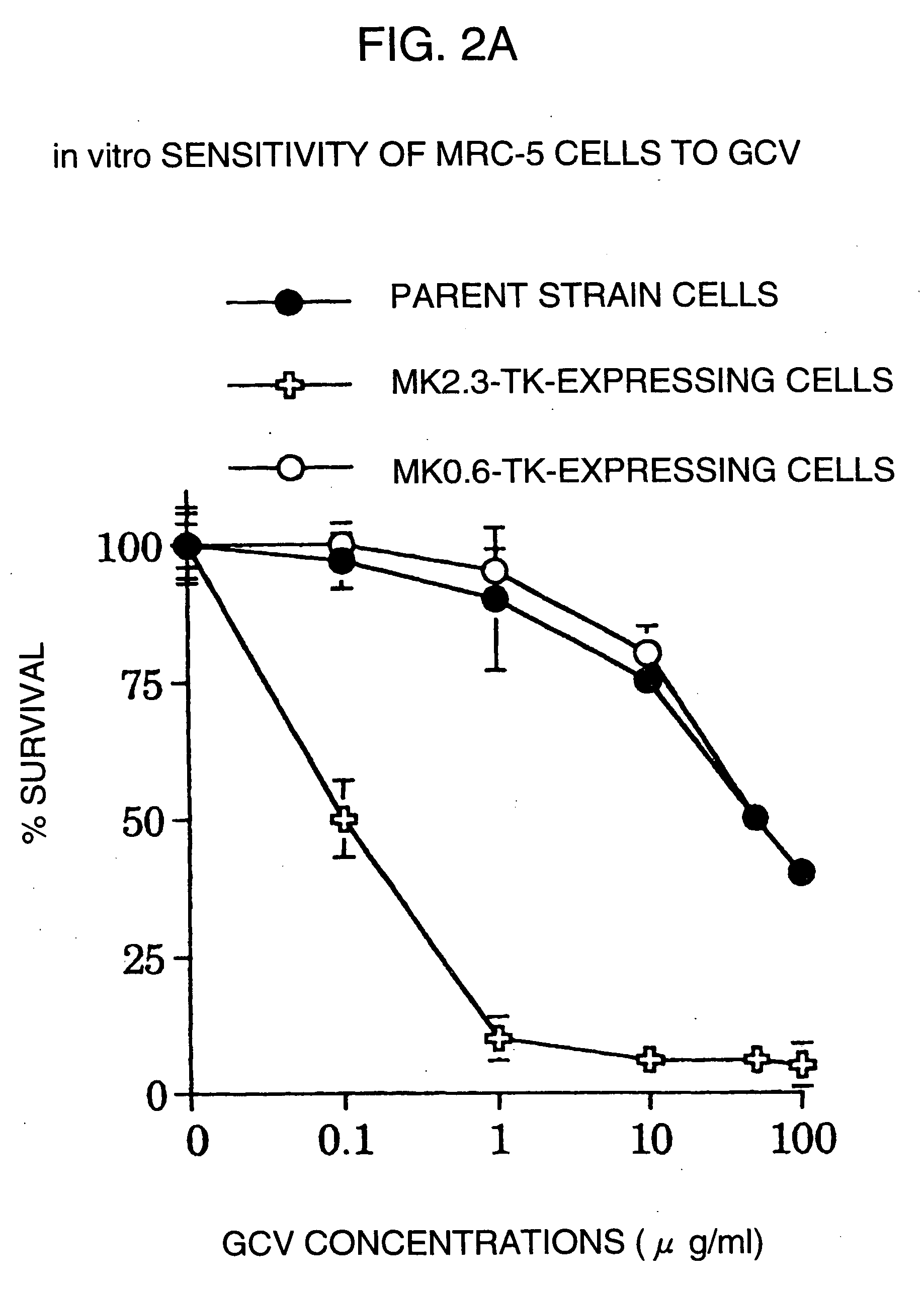
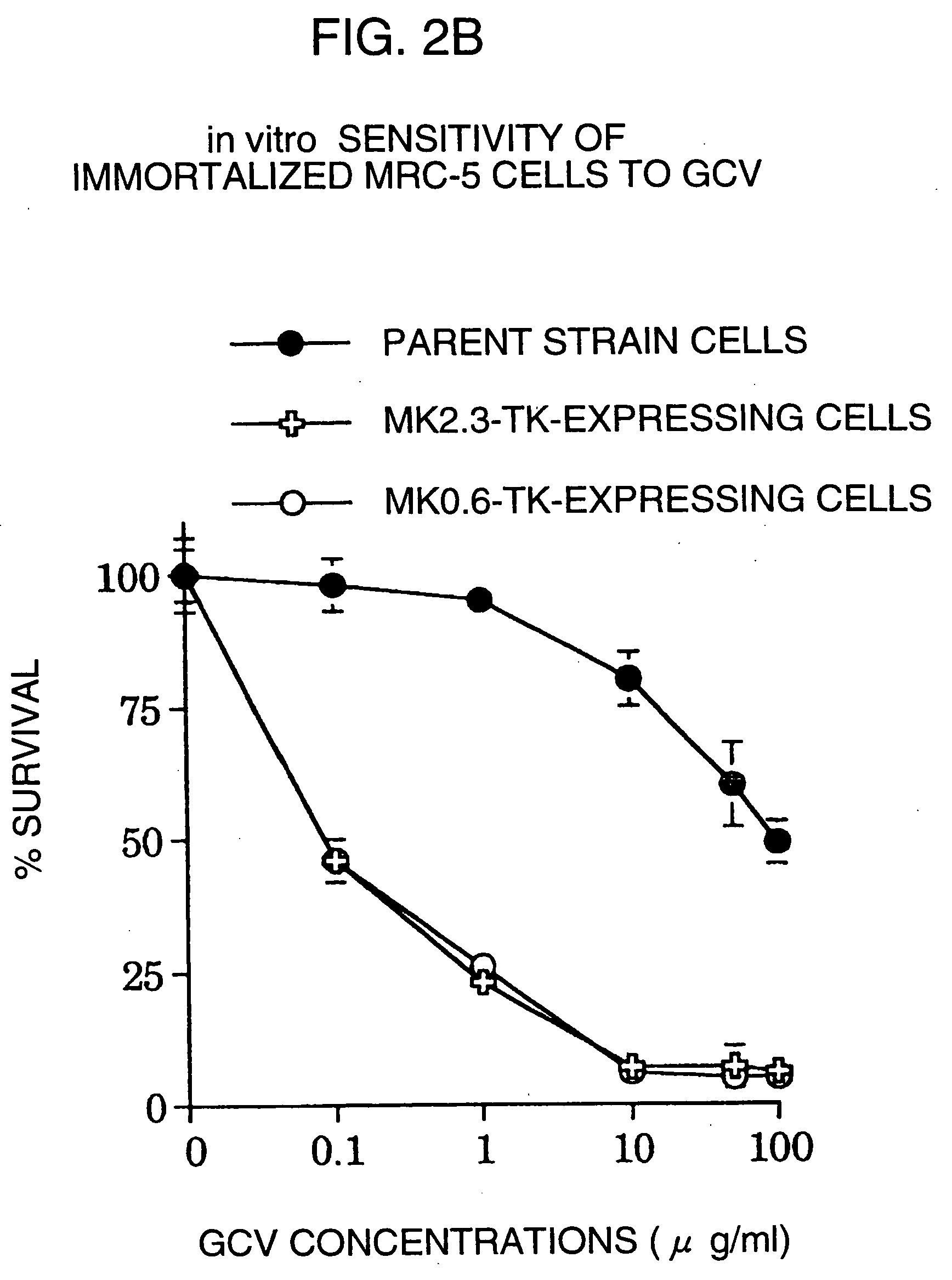
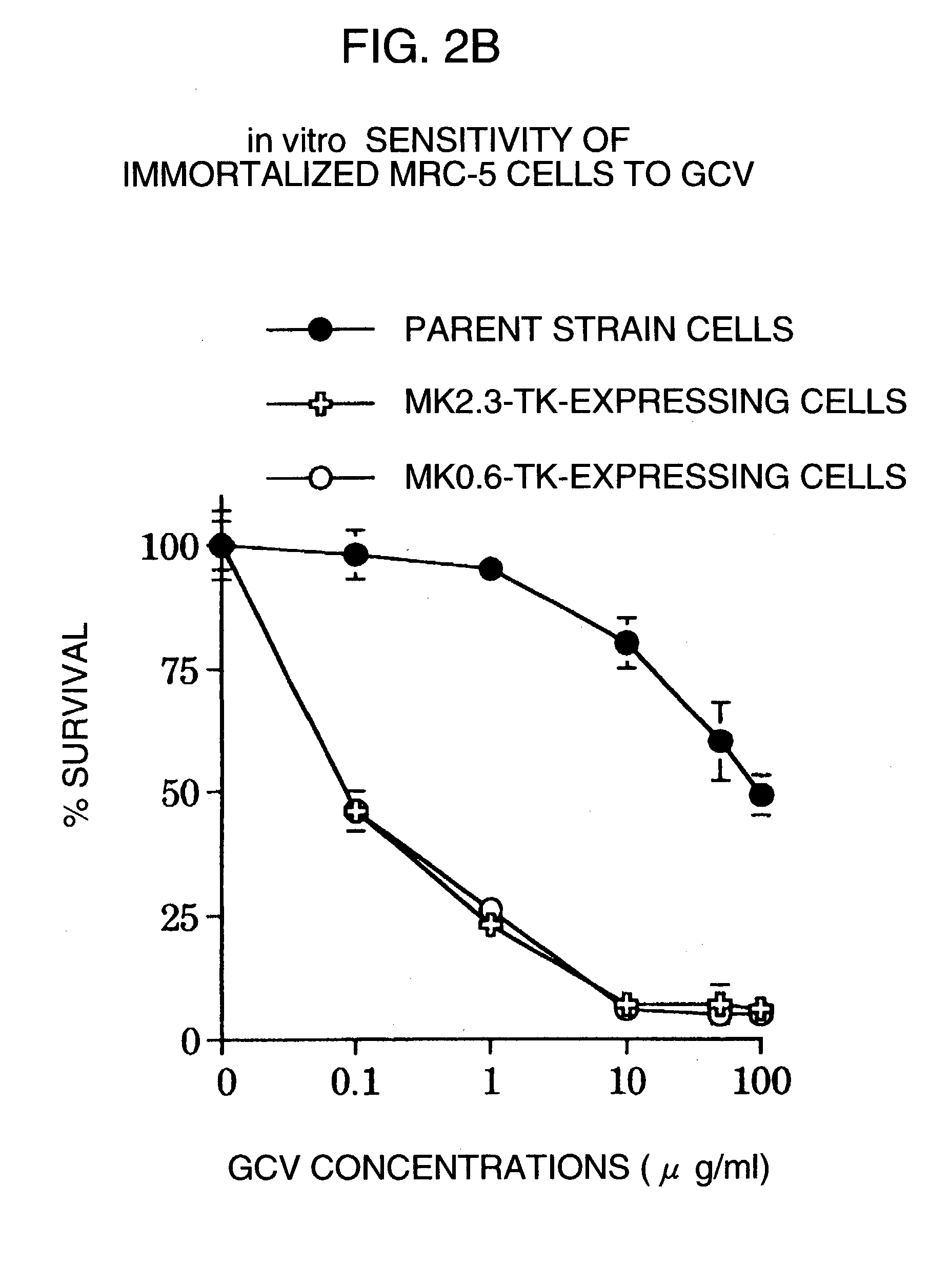
InactiveUS20060099188A1High tumor-specificity and promoter activityHigh promoter activityBiocideGenetic material ingredientsPromoter activityC erbb 2
A DNA comprising a 609 bp base sequence from −559 to +50 when the first base sequence of exon 1 of the midkine gene, a human retinoic acid-responsive growth / differentiation factor was set as +1, or a DNA comprising a 251 bp base sequence from −213 to +38 when the transcription initiation point of the c-erbB-2 gene belonging to the EGF receptor family and having a tyrosine kinase activity was set as +1 has a tumor-specific transcription activity, and the promoter activity thereof is high, and therefore is very important as a tumor-specific promoter for use in the suicide gene therapy that combines the use of a gene for a drug metabolizing enzyme and a prodrug for cancer therapy, the gene therapy of cancer using an expression vector that contains a gene encoding a cytokine, and the gene therapy of cancer using an oncolytic virus.
Owner:PRIMMUNE CORP +1
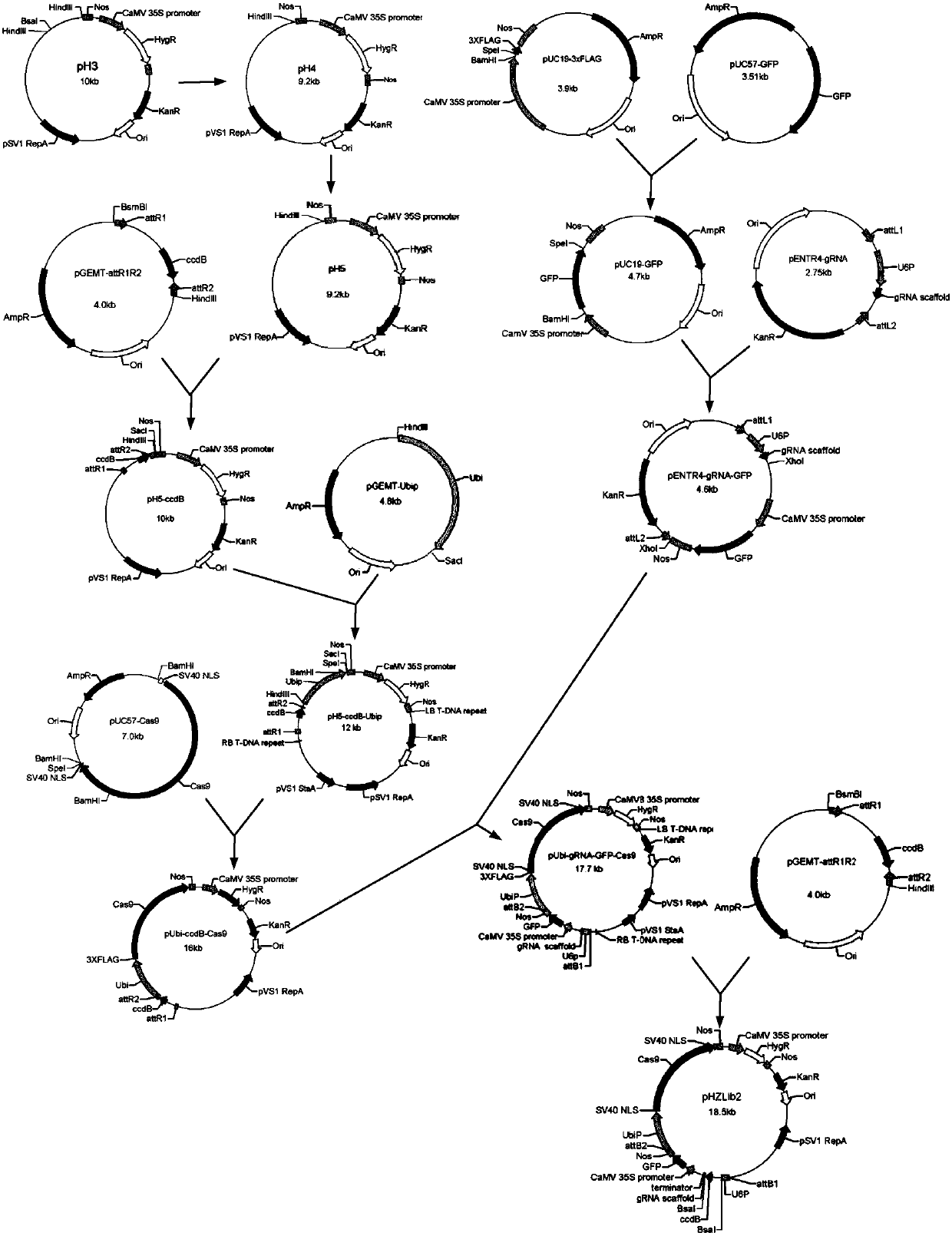
Plasmid vector and method for building plant population by using plasmid vector
ActiveCN108034671AIncrease positive rateReduce the probability of false positive plantsHydrolasesVector-based foreign material introductionOrigin of replicationPlant population
The invention relates to a plasmid vector and a method for building a plant population by using the plasmid vector. The plasmid vector contains, at a replication origin, a gene expression box for expressing Cas9 protein, a first promoter, nucleotide sequences containing suicide gene sequences, a gRNA scaffold element and a termination signal, and at least one first enzyme cutting site located on the nucleotide sequence 5' end containing the suicide gene sequence and at least one second enzyme cutting site located at the nucleotide sequence 3' end containing the suicide gene sequence; furthermore, no first enzyme cutting site and no second enzyme cutting site exist in other positions of the plasmid vector. The first promoter is located at the upstream of the termination signal; the nucleotide sequences containing suicide gene sequences and the gRNA scaffold element are all located between the first promoter and the termination signal.
Owner:INST OF PLANT PROTECTION CHINESE ACAD OF AGRI SCI
Infertility control of genetically modified fish
A method of inducing male and / or female infertility in a genetically modified (GM) fish is disclosed. Also disclosed is a method of generating an infertile GM fish with a phenotype and / or genotype of interest. The method involves generation of a GM fish whose genome comprises a foreign transgene operably linked to a fish gonad-specific promoter selected from the group consisting of an ovary-specific promoter and a testis-specific promoter. The transgene comprises a suicide gene selected from the group consisting of a reductase and a photosensitizer, in which the reductase is operably linked to a reporter gene. Infertility of the GM fish is induced if the GM fish expressing the reductase is treated with an effective amount of a reductase-activated cytotoxic prodrug or if the GM fish expressing the photosensitizer is treated with light irradiation.
Owner:ACAD SINIC +1
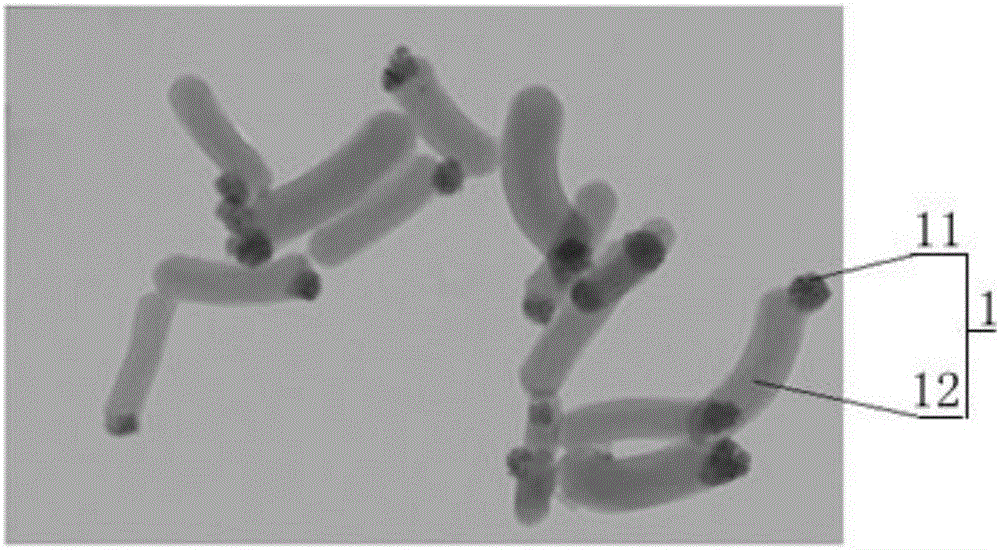
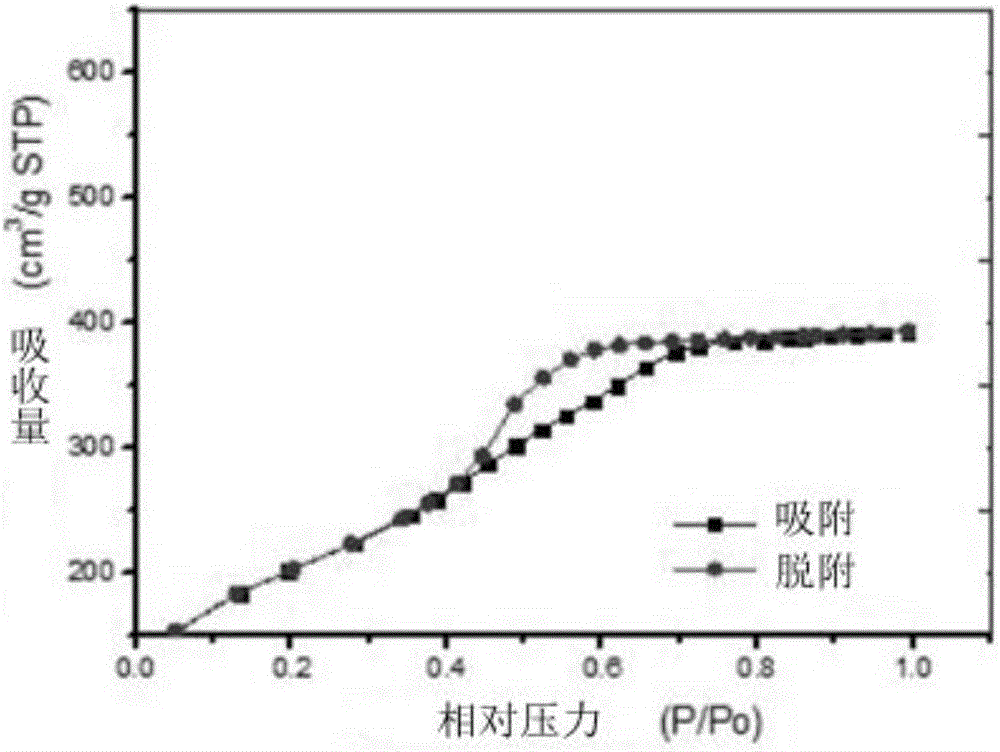
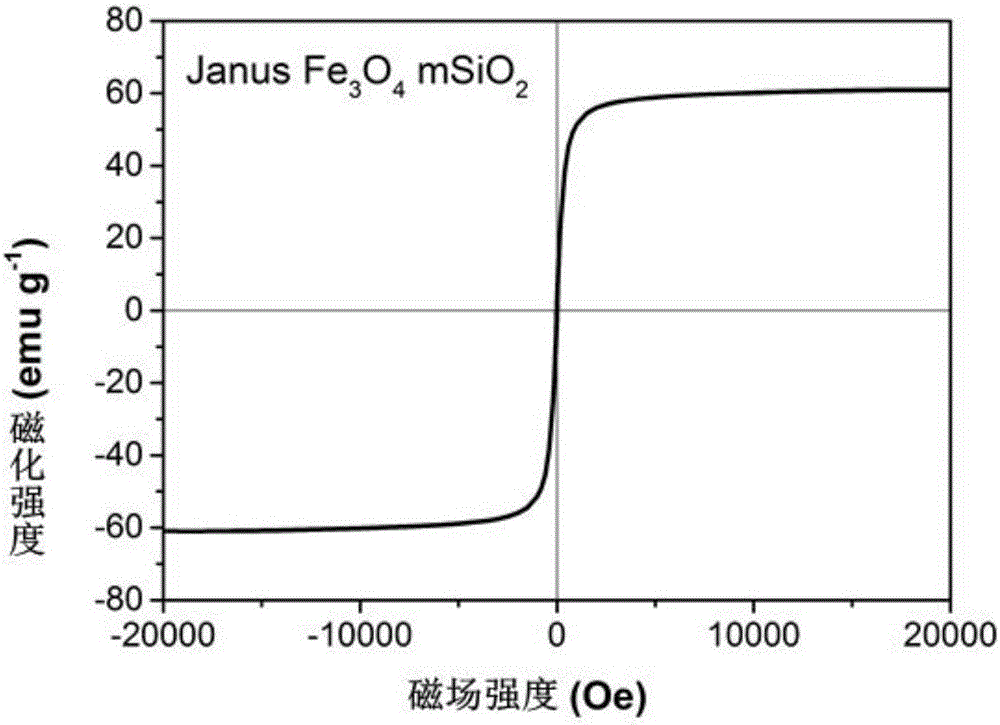
Nano drug-carrying system, and preparation and application thereof
ActiveCN106822921AGood biocompatibilityLow cytotoxicityEnergy modified materialsGenetic material ingredientsPolyethylene glycolMesoporous silica
The invention relates to the technical field of medicine, and provides a nano drug-carrying system. The nano drug-carrying system comprises a magnetic-mesoporous silicon dioxide nanorod carrier, a pro-drug GCV (ganciclovir) carried on the carrier, and a graft copolymer PLL-g-PEG (poly-L-lysine graft polyethylene glycol) formed by PLL and PEG, wherein the PLL-g-PEG also carries a suicide gene TK. The magnetic-mesoporous silicon dioxide nanorods are used as the magnetic targeted carrier to carry the suicide gene TK / pro-drug GCV to enter cells, thereby implementing the time and space consistency of the suicide gene and pro-drug transfer, and further implementing accurate drug release. The magnetic-mesoporous silicon dioxide silicon dioxide nanorods and suicide gene / pro-drug treatment method are effectively combined, thereby enhancing the combined treatment effect on the liver cancer. The preparation method of the nano drug-carrying system is simple in technique and suitable for large-scale industrial production.
Owner:SUZHOU INST OF BIOMEDICAL ENG & TECH CHINESE ACADEMY OF SCI
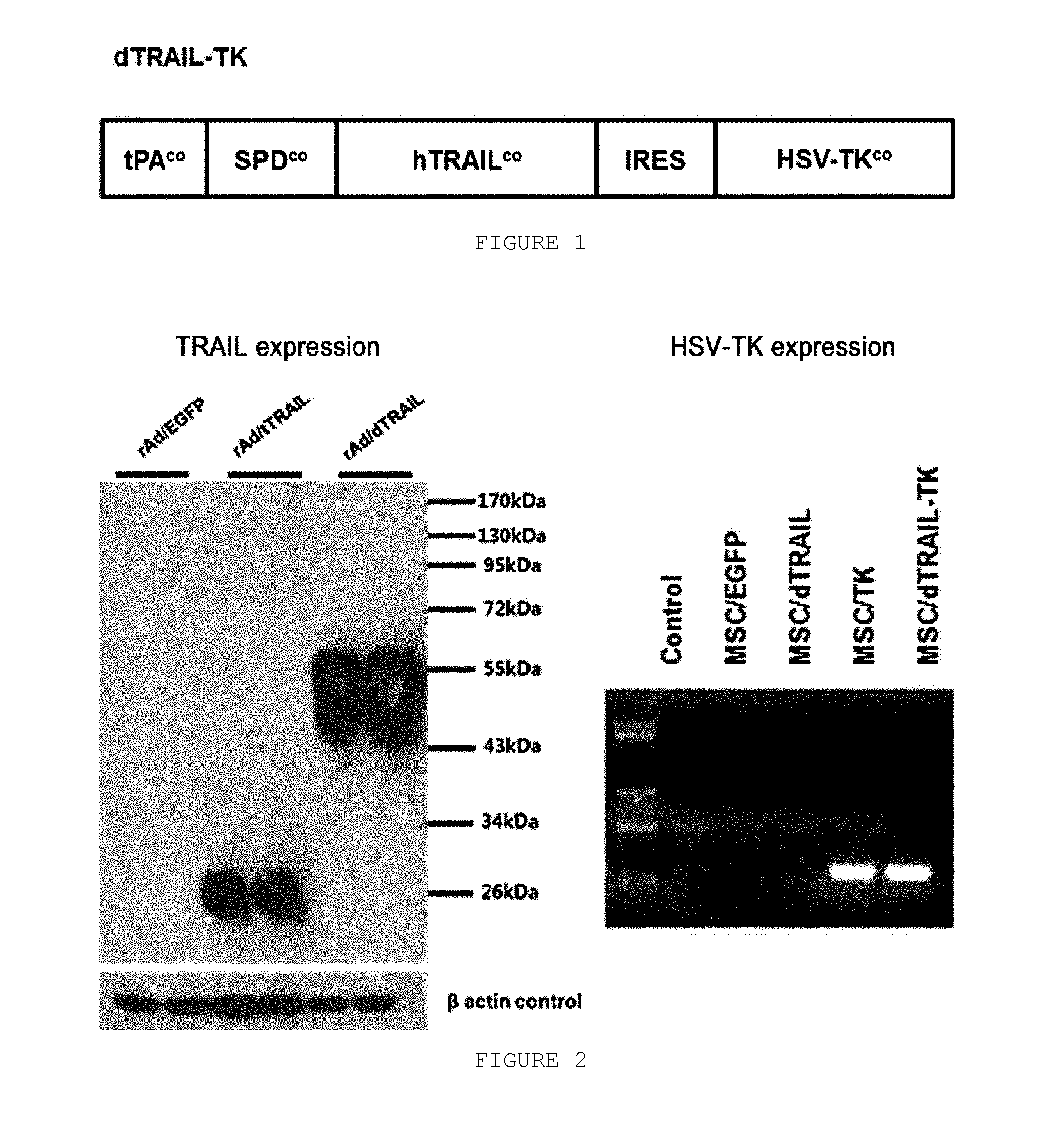
Vector simultaneously expressing dodecameric trail and hsv-tk suicide genes, and anticancer stem cell therapeutic agent using same
The present invention relates to a DNA cassette comprising a nucleotide sequence encoding dodecameric TRAIL and a suicide gene nucleotide sequence, a recombinant expression vector comprising the DNA cassette, a recombinant adenovirus prepared by using the recombinant expression vector, a host cell transduced with the recombinant adenovirus, a composition for treating cancer comprising the host cell, and a method for treating cancer comprising the step of administering the composition for treating cancer to a subject. The stem cell therapy coexpressing dodecameric TRAIL and HSV-TK by introduction of the DNA cassette of the present invention has more excellent anticancer effects than the known therapy, and thus can be effectively used in the treatment of many different types of solid tumors and metastatic tumors.
Owner:POSTECH ACAD IND FOUND
Method for rapidly screening bacterial quorum sensing inhibitor (QSI) by utilizing suicide gene
The invention provides a method for rapidly screening a bacterial quorum sensing inhibitor (QSI) by utilizing a suicide gene. The method is characterized in that under natural light, bacteria not infected with a phage do not turn blue and bacteria infected with the phage turn blue; induced by an added lethal condition inducer, a specific polypeptide with the function of inhibiting QS does not start expression of the suicide gene because of inhibiting the activities of QS molecules, so the bacteria survive; a non-specific polypeptide can not inhibit the QS molecules, so the suicide gene starts lethal; then survival candidate clones are picked out, functional verification is further carried out and finally a DNA (deoxyribonucleic acid) sequence corresponding to a specific polypeptide sequence is obtained by a method of DNA sequencing, thus obtaining an efficient and specific QSI polypeptide. The method is simple in steps, and multiple QSI candidate polypeptides can be obtained only within three days to about one weak, so that the method has very obvious advantages.
Owner:FUJIAN AGRI & FORESTRY UNIV
Vector coexpressing dodecameric trail and suicide gene hsv-tk, and anticancer stem cell medicine using thereof
InactiveCN104160027AGood anticancer effectEffective treatmentPeptide/protein ingredientsTransferasesNucleotideA-DNA
Owner:POSTECH ACAD IND FOUND +1
Method for efficiently establishing pure line gene knock-out mouse model
InactiveCN1580251AImprove the efficiency of culling miceOther foreign material introduction processesFermentationMammalBlastosphere
The invention provides a kind of transgenic inhuman mammal by producing klone gene without mouse model. Roll outside self murder gene into the mammal. The expression box contains promoter, self murder gene relation with promoter and termination codon. The natural masculine gene will express in testes organize after transgenic animal builds system. The transgenic animal can provide blastosphere for eliminating gene technic to improve the efficiency of the technic and to solve the problem that mouse genetic background is impure.
Owner:SHANGHAI BIOMODEL ORGANISM SCI & TECH DEV +4
Recombinant slow virus vector, recombinant slow virus and stem cell containing recombinant slow virus
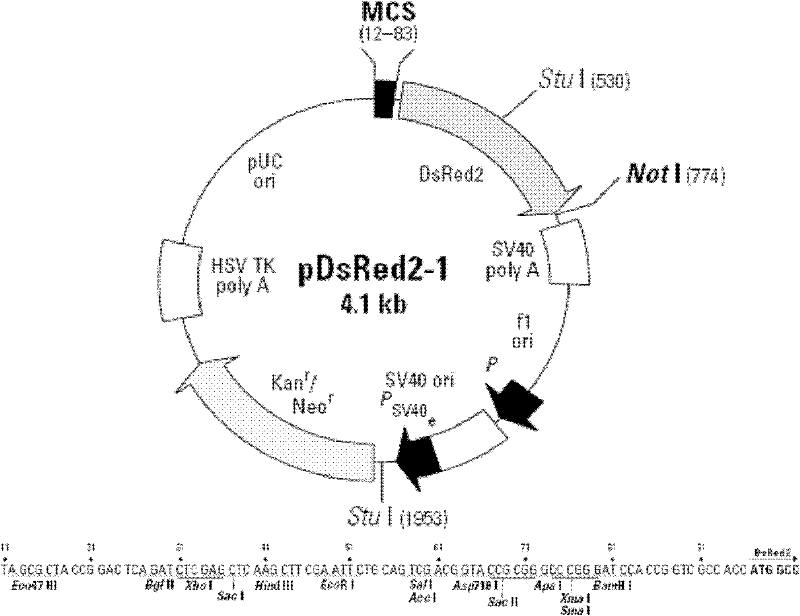
The invention discloses a recombinant slow virus vector, a recombinant slow virus and a stem cell containing recombinant slow virus, belonging to the field of medical molecular biology. The recombinant slow virus vector is characterized in that: a Survivin prompter, an Apoptin gene and a SV40Poly A sequence are sequentially inserted into a plurality of cloning sites of the slow virus vector; and the slow virus vector is FG-12. Due to the application of the recombinant slow virus, such as transfection applied to a transplanted stem cell, the stem cell transplanted into the body of a patient is under the monitoring of the promoter of the recombinant slow virus vector. Once the transplanted stem cell is transformed severely, the expression of a suicide gene Apoptin in the recombinant slow virus is started immediately, so that the severely-transformed cell is dead by apoptosis. During clinical application, the recombinant slow virus plays a role in specifically pre-warning, reacting, tracing and clearing the transformed stem cell.
Owner:ARMY MEDICAL UNIV
A human immortalised neural precursor cell line
InactiveCN101189327ANervous system cellsForeign genetic material cellsWilms' tumorGap Junction Proteins
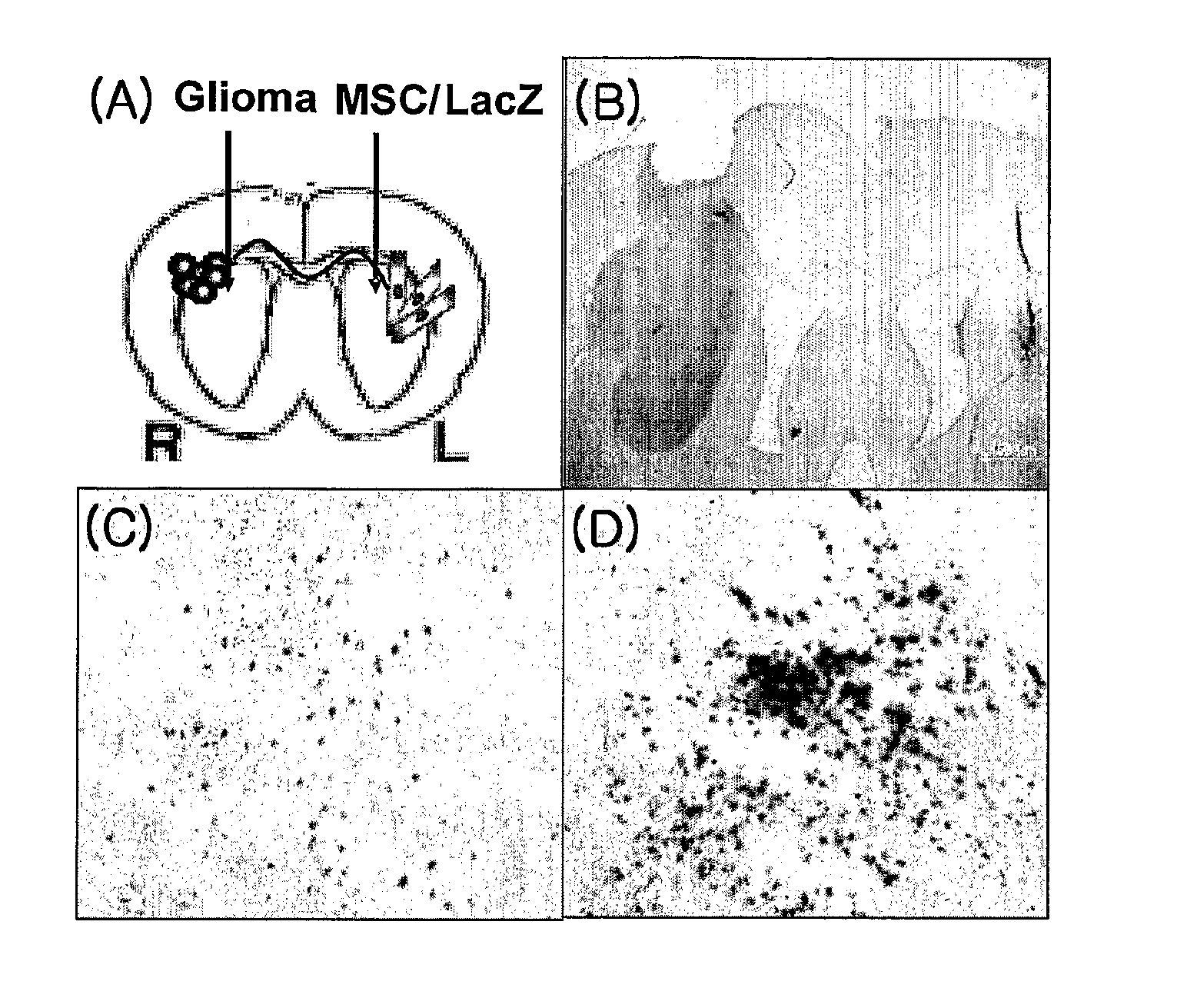
The present invention relates to an immortalised human neural precursor cell line, NGC-407. The cell line has been established from human foetal tissue. The cell line has been immortalised using a retroviral vector containing the v-myc oncogene. The cell line is a neural progenitor cell line capable of differentiating into to astrocytes and neurons including dopaminergic neurons. NGC-407 cells are capable of migrating to glioblastoma tumours implanted into rat brains and form gap junctions with the tumour cells. NGC-407 cells expressing a suicide gene can be be used for delivering activated prodrugs in the form of activated nucleoside analogs to tumours.
Owner:NSGENE AS
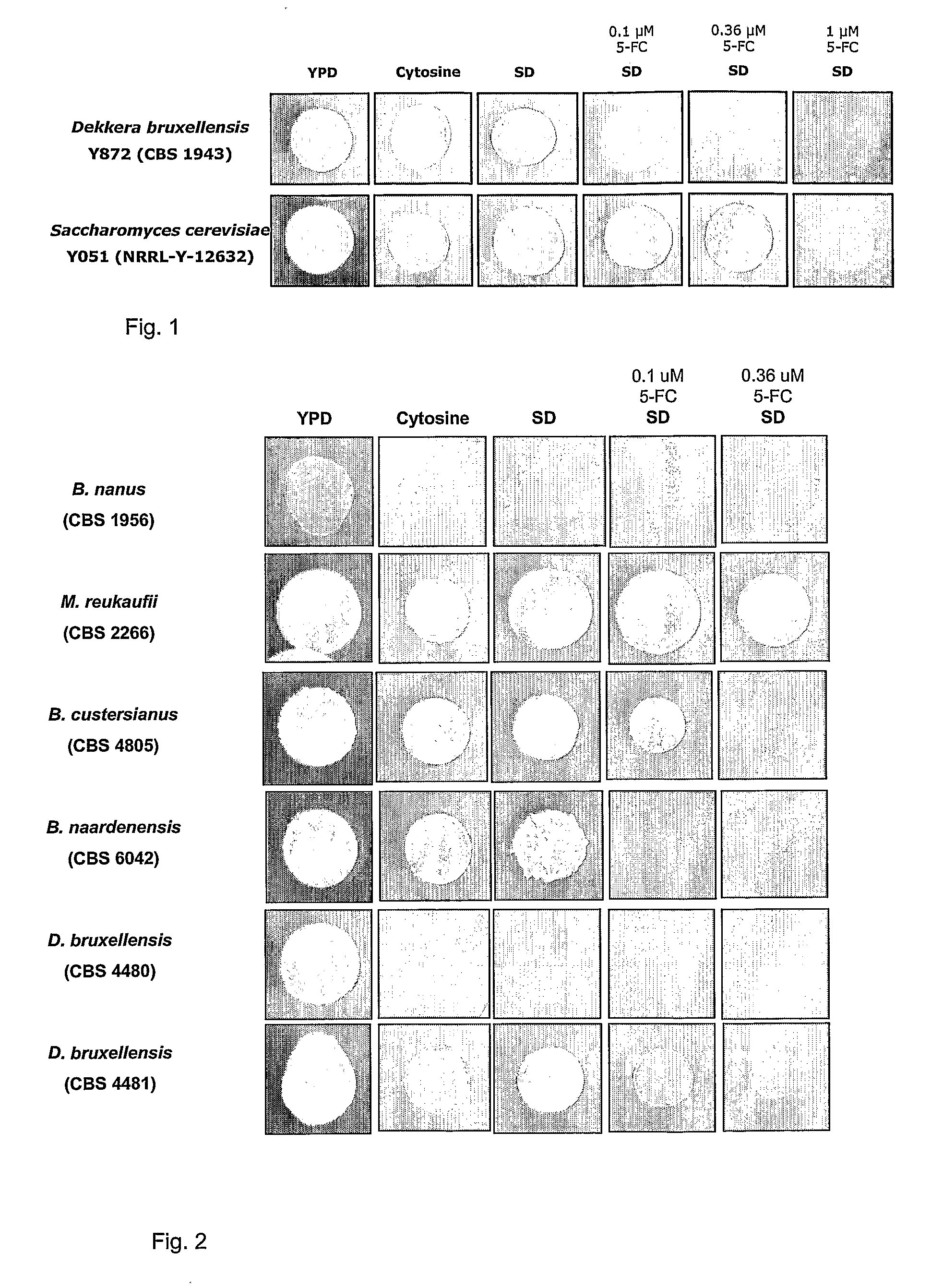
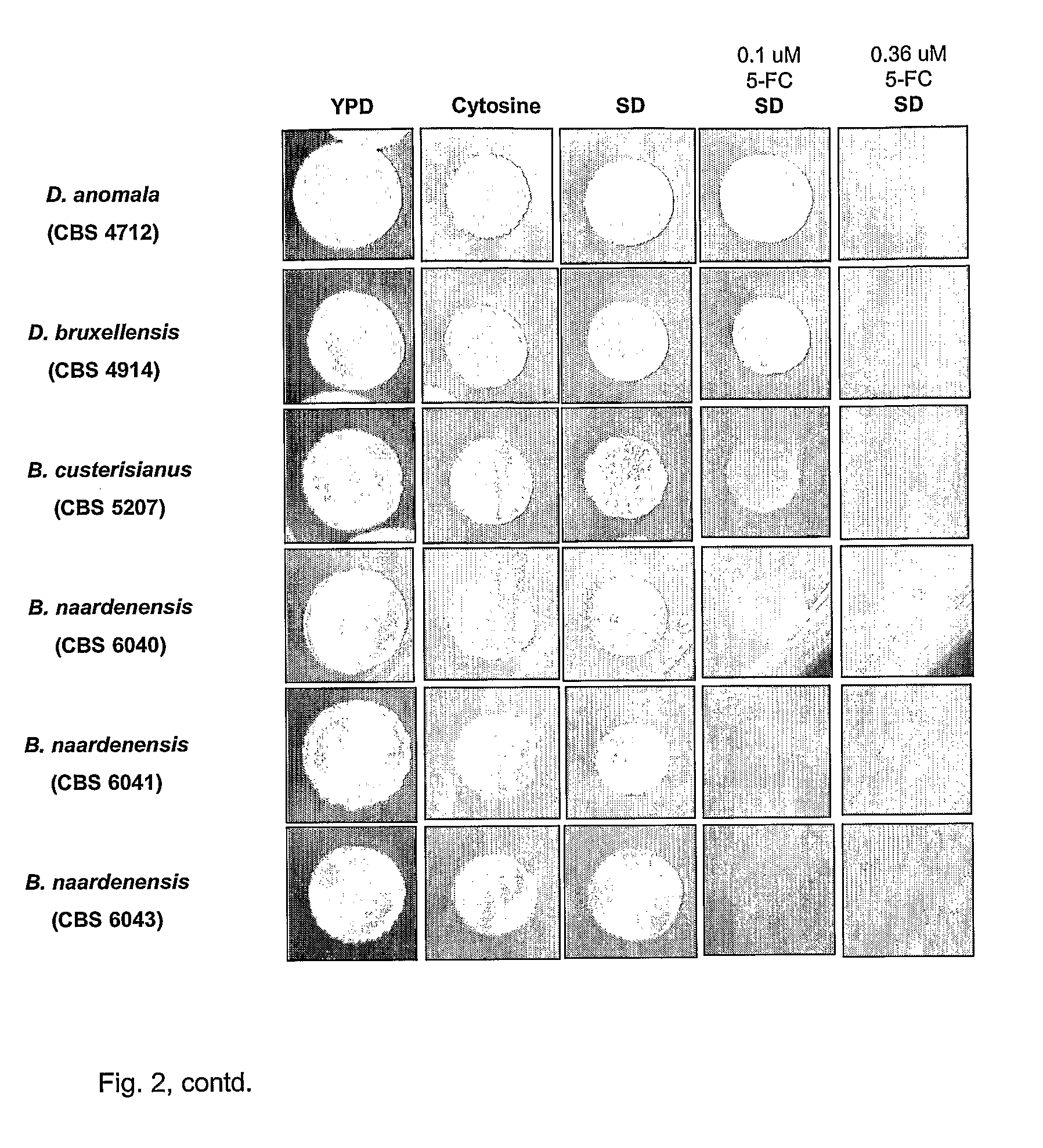
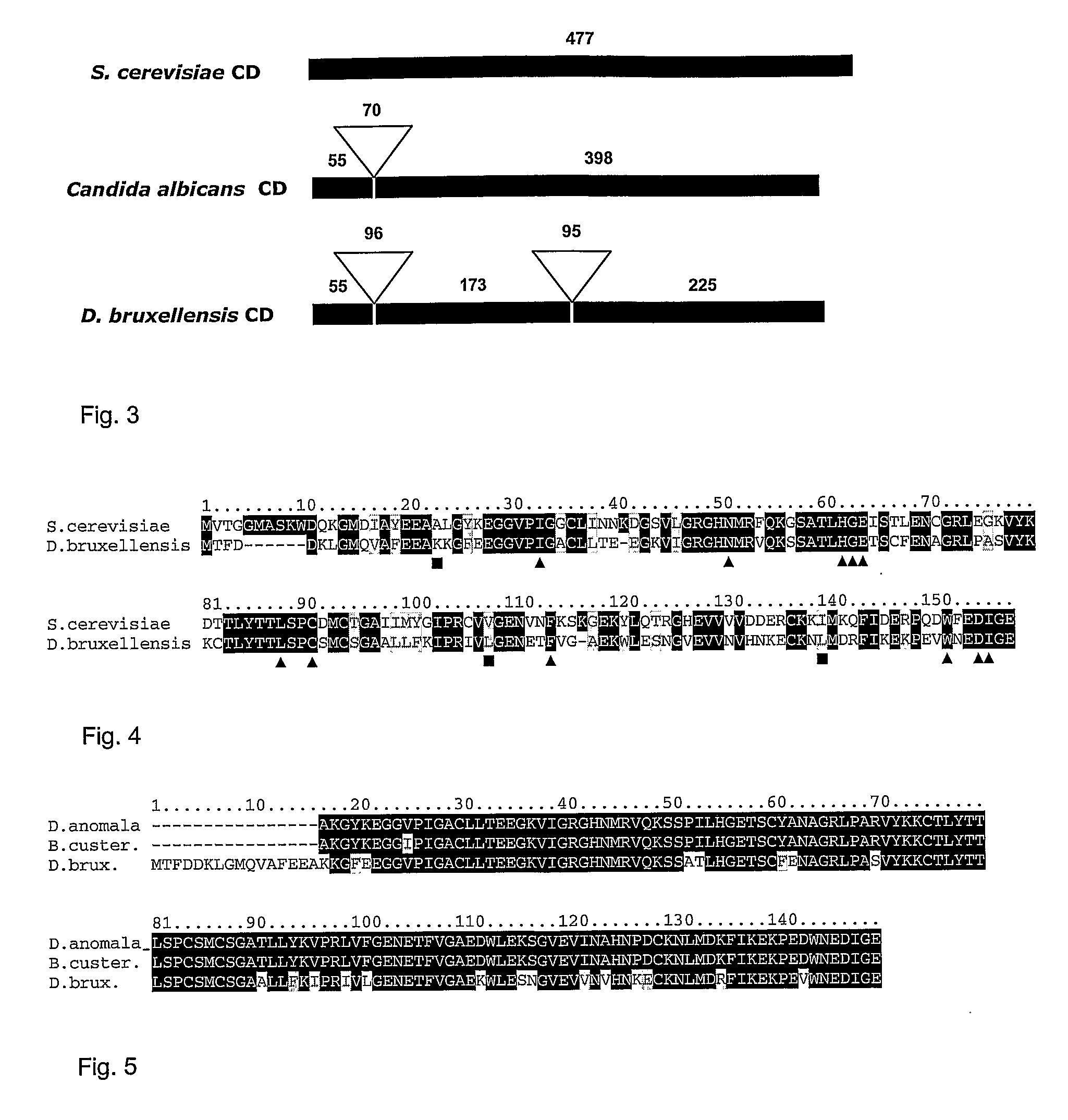
Dekkera/Brettanomyces Cytosine Deaminases And Their Use
The present invention relates to cytosine deaminase protein and cDNA from various species of the yeast genus Dekkera / Brettanomyces. Compared to yeast cytosine deaminase the novel cytosine deaminases are more efficient and have a higher stability. The invention also relates to the field of suicide gene therapy based on activation of a non-toxic prodrug, 5-fluorocytosine to a toxic drug 5-fluorouracil based on the enzymatic activity of novel cytosine deaminses. Finally the invention provides use of 5-fluorocytosine for controlling the growth of Dekkera / Brettanomyces yeast.
Owner:ZGENE
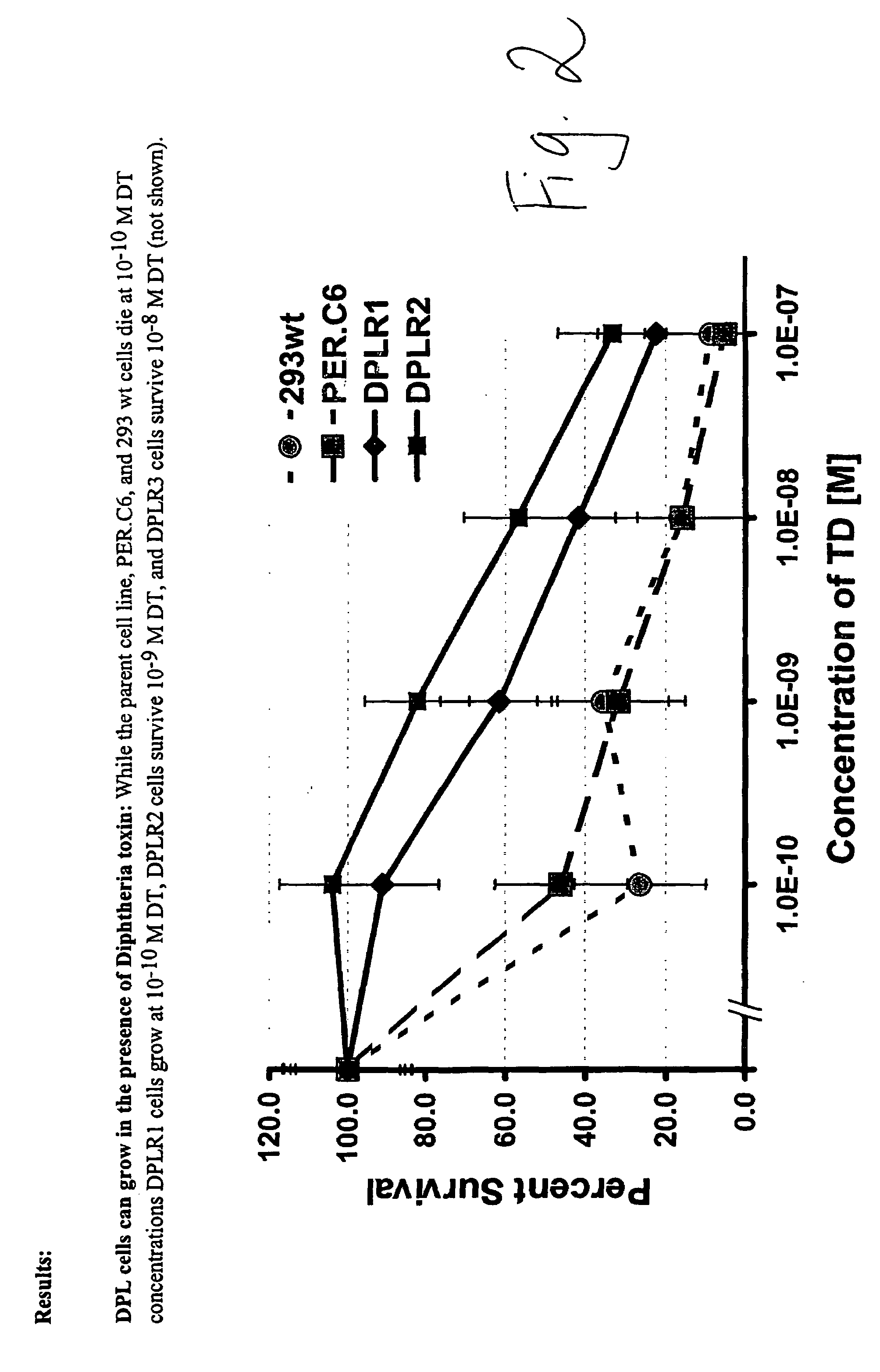
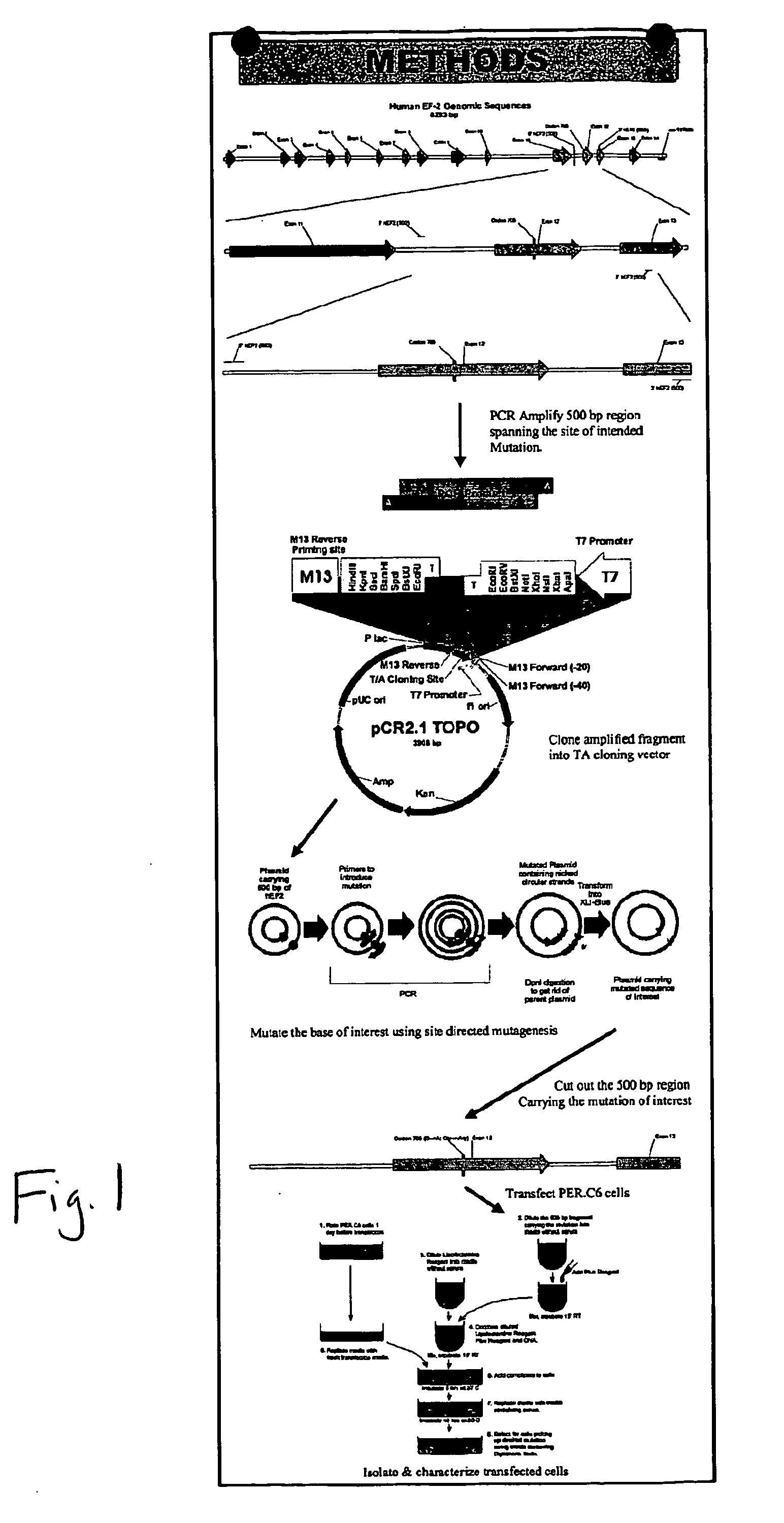
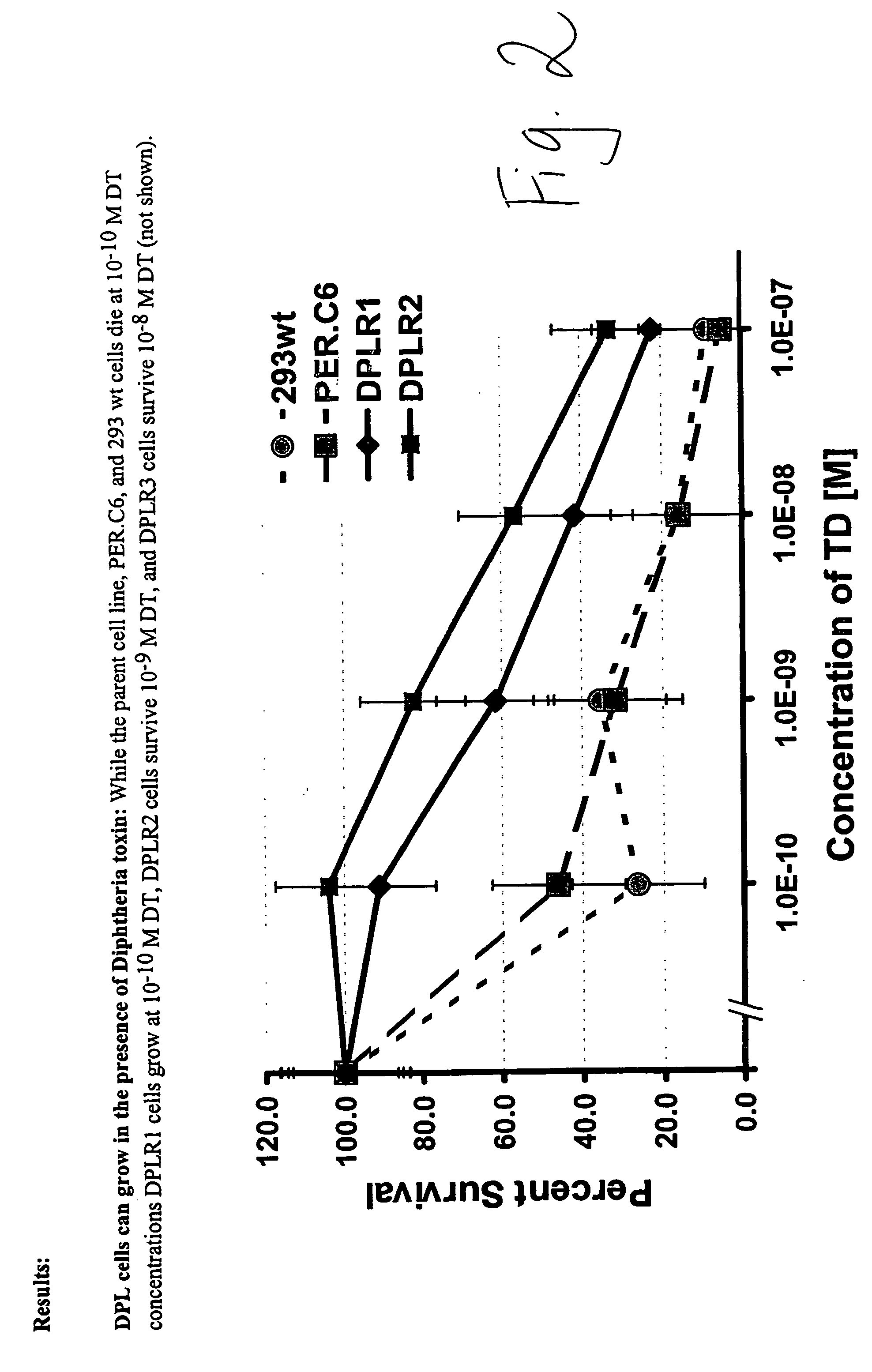
Packaging cell line for diphtheria toxin expressing non-replicating adenovirus
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Proliferation and tumor cell specific gene operating system for gene therapy of malignant tumor
InactiveCN101671666AMeet the characteristicsFulfil requirementsGenetic material ingredientsFermentationEucaryotic cellAbnormal tissue growth
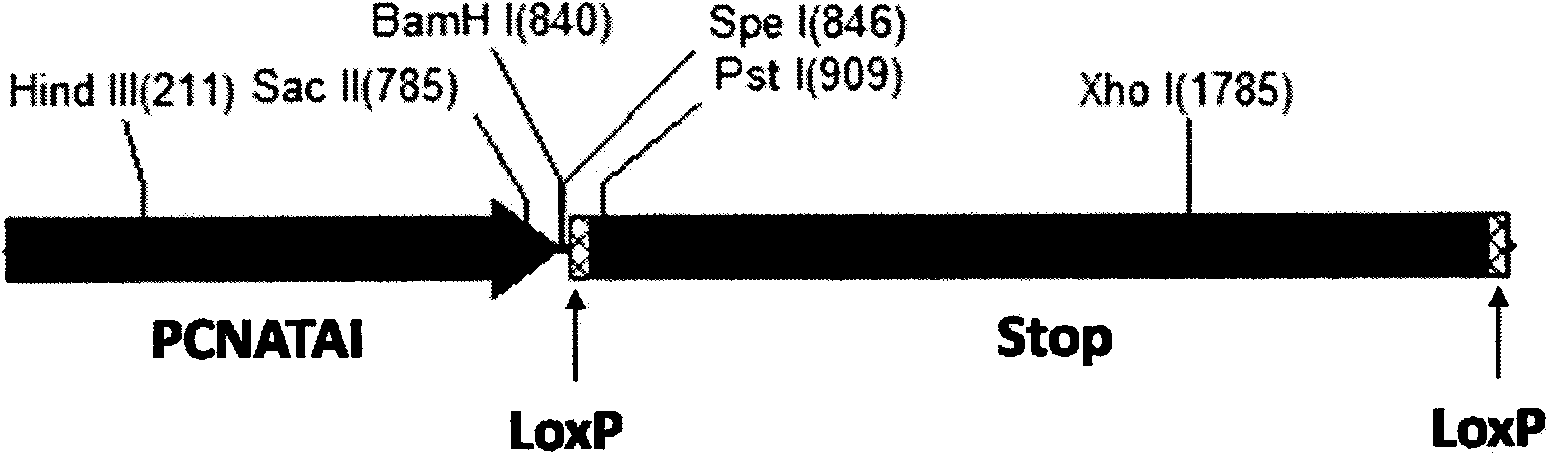
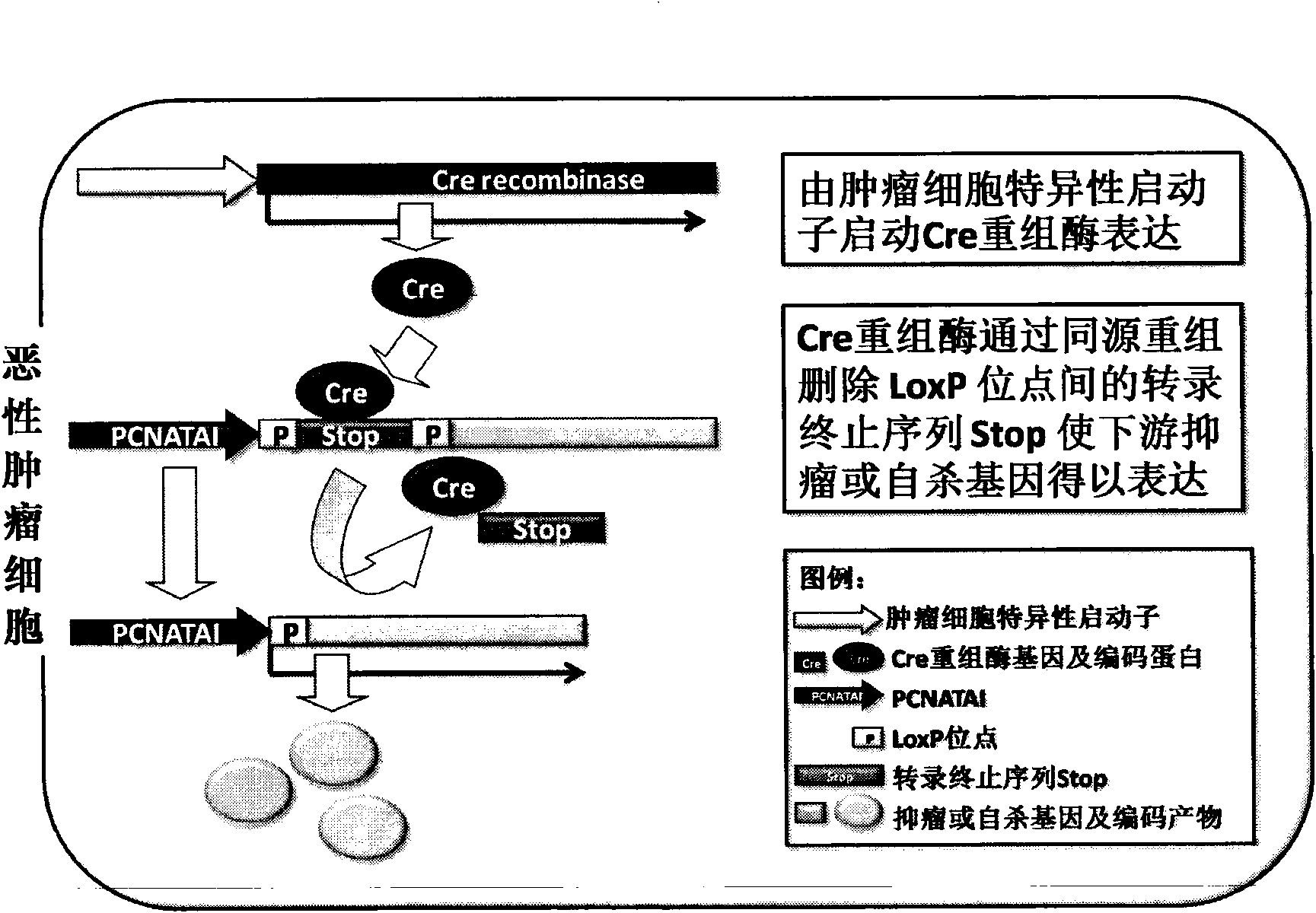
The invention relates to the field of biotechnology and discloses a high-proliferation specific recombination eucaryotic cell promoter PCNATAI; an eucaryotic cell operating system PCNATAI-LoxP-stop-LoxP taking a PCNATAI as a core transcription initiator and a LoxP-stop-LoxP as a transcriptional repression element; and a set of general scheme for eliminating the repression effect of the LoxP-stop-LoxP to the transcription process of the PCNATAI promoter through a Cre recombinase provided by a tumor cell specific dependent model trans form and enabling the downstream exogenous tumor suppressor gene or the suicide gene to be effectively and specifically expressed in the malignant tumor cell in a tumor cell specific pattern and a proliferation specific pattern. The scheme not only can remarkably improve the tumor inhibition rate during the gene therapy of the malignant tumor or the expression efficiency of the suicide gene in the malignant tumor cell, but also can ensure the security of the gene therapy.
Owner:GENERAL HOSPITAL OF TIANJIN MEDICAL UNIV
Packaging cell line for diptheria toxin expressing non-replicating adenovirus
The invention provides a packaging cell line for production of diphtheria toxin (DT) expressing, non-replicating adenovirus for use in suicide gene therapy of cancer cells, as well as production of immunotoxins. Also provided are methods for producing diphtheria toxin (DT) expressing, non-replicating adenovirus, methods for producing immunotoxins, and adenovirus and immunotoxins produced by those methods. Further provided are methods for making a cell resistant to diphtheria toxin.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
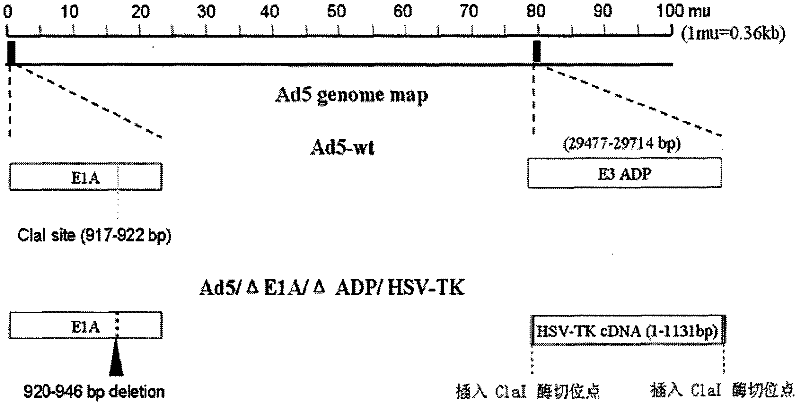
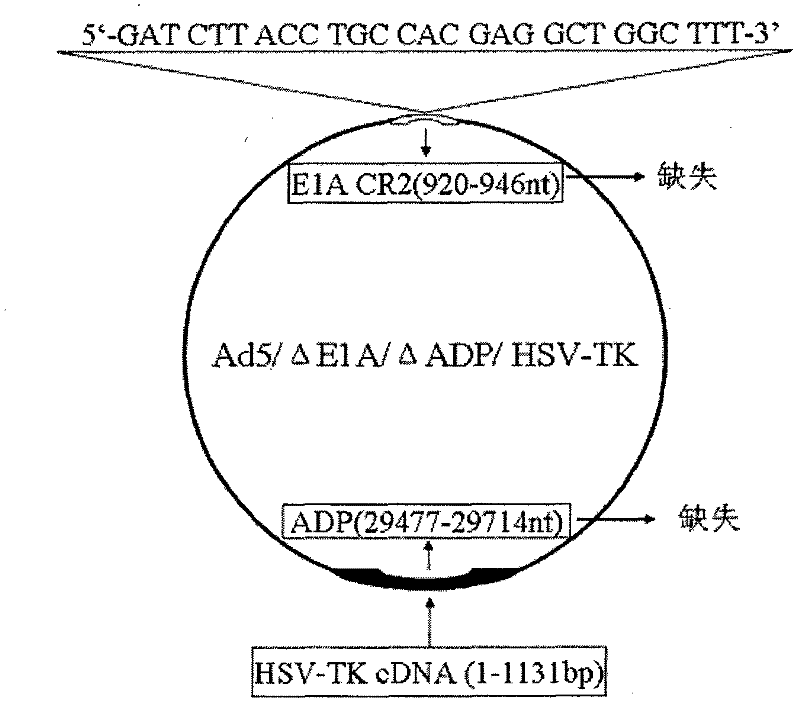
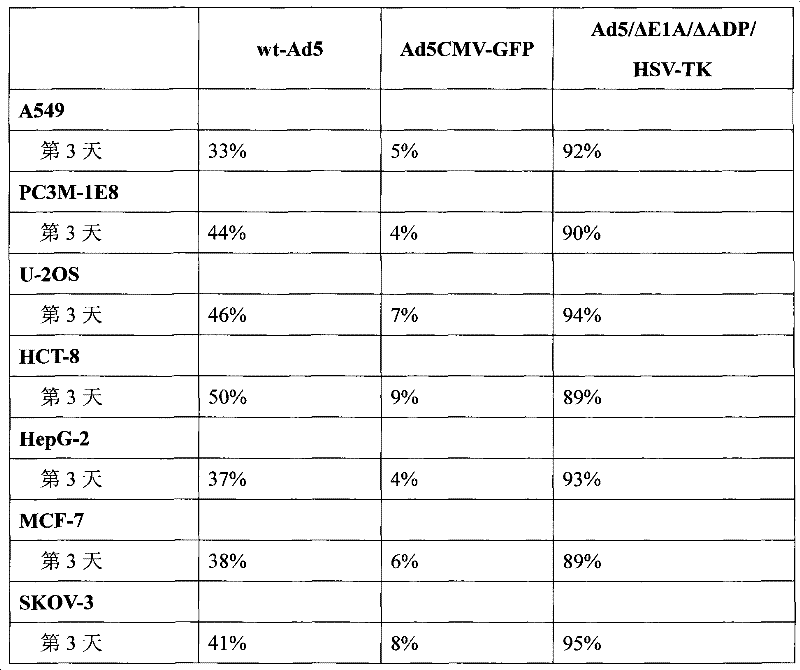
Acquisition and application of a novel oncolytic adenovirus-thymidine kinase gene construct
InactiveCN102286433AHas therapeutic effectIncrease productionGenetic material ingredientsViruses/bacteriophagesEnzyme digestionConserved sequence
The invention discloses a construction scheme of artificially transforming human type 5 adenovirus (Ad5), and a specific application of a novel oncolytic adenovirus construct obtained by the scheme in tumor treatment, and belongs to the technical field of medical genetic engineering. A recombinant adenovirus construct obtained by PCR amplification site-directed deletion, enzyme digestion, ligation, cloning, homologous recombination, transfection, adenovirus monoclonal purification, etc., its technical characteristics are: Ad5 genome E1A conserved sequence 2 27 bases were deleted in the (CR2) region; 29477-29714nt of the ADP gene in the E3 region was deleted; and the full-length coding sequence (1131bp) of the HSV-TK gene was inserted in the deleted region. This construct is a new type of oncolytic adenoviral vector with higher tumor selective replication ability, which utilizes the suicide gene function of HSV-TK and the double killing effect of oncolytic virus to dissolve tumor cells, and has great potential in the biological treatment of tumors. Unique practical value.
Owner:马丁
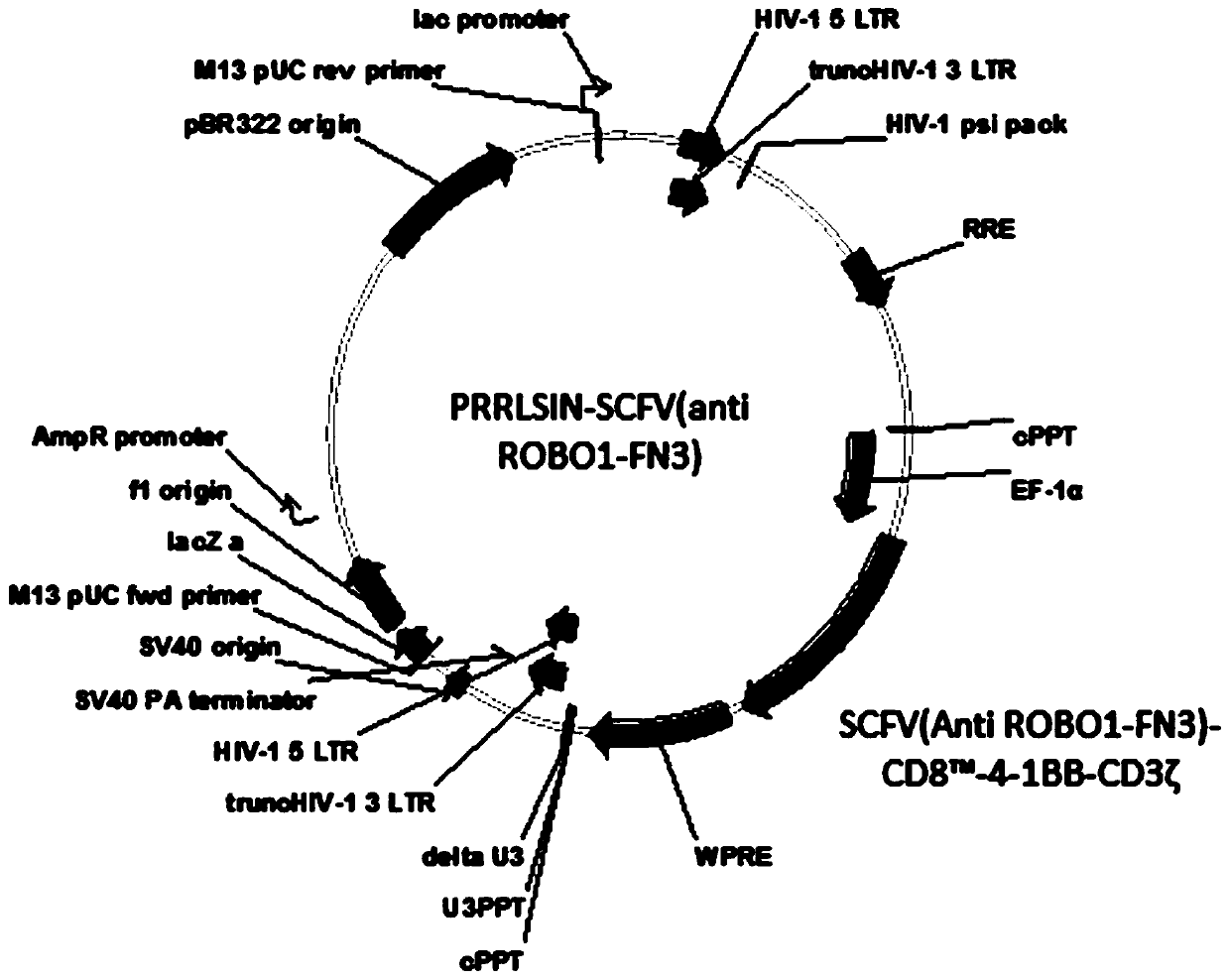
ROBO1 CAR-NK cell carrying suicide gene as well as preparation method and application of ROBO1 CAR-NK cell
PendingCN111269925ASmall side effectsGood killing effectVirusesHydrolasesNatural Killer Cell Inhibitory ReceptorsROBO1
The invention discloses an ROBO1 CAR-NK cell carrying a suicide gene as well as a preparation method and application of the ROBO1 CAR-NK cell. In order to improve the safety and controllability of theCAR-NK therapy, a suicide gene switch element is integrated into a genome through a lentiviral transfection technology on the basis of the current ROBO1 CAR-NK cell to form the CAR-NK with the suicide gene. By adding the suicide gene, the CAR-NK cells can be better controlled, and the clinical safety is further improved.
Owner:ASCLEPIUS SUZHOU TECH CO GRP CO LTD
Tumor-specific promoters
InactiveUS20030157065A1High tumor-specificityHigh promoter activityBiocideGenetic material ingredientsPromoter activityC erbb 2
A DNA comprising a 609 bp base sequence from -559 to +50 when the first base sequence of exon 1 of the midkine gene, a human retinoic acid-responsive growth / differentiation factor was set as +1, or a DNA comprising a 251 bp base sequence from -213 to +38 when the transcription initiation point of the c-erbB-2 gene belonging to the EGF receptor family and having a tyrosine kinase activity was set as +1 has a tumor-specific transcription activity, and the promoter activity thereof is high, and therefore is very important as a tumor-specific promoter for use in the suicide gene therapy that combines the use of a gene for a drug metabolizing enzyme and a prodrug for cancer therapy, the gene therapy of cancer using an expression vector that contains a gene encoding a cytokine, and the gene therapy of cancer using an oncolytic virus that exhibits cytotoxic effects only on tumor cells, etc.
Owner:CHIBA PREFECTURE +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com