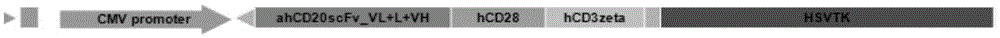Chimeric antigen receptor immune cell provided with safety switch as well as preparation method and application of chimeric antigen receptor immune cell
A chimeric antigen receptor and safety switch technology, applied in the field of biomedicine, can solve problems such as cell mutation and canceration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1: Synthesis of CAR expression cassette and construction of expression vector
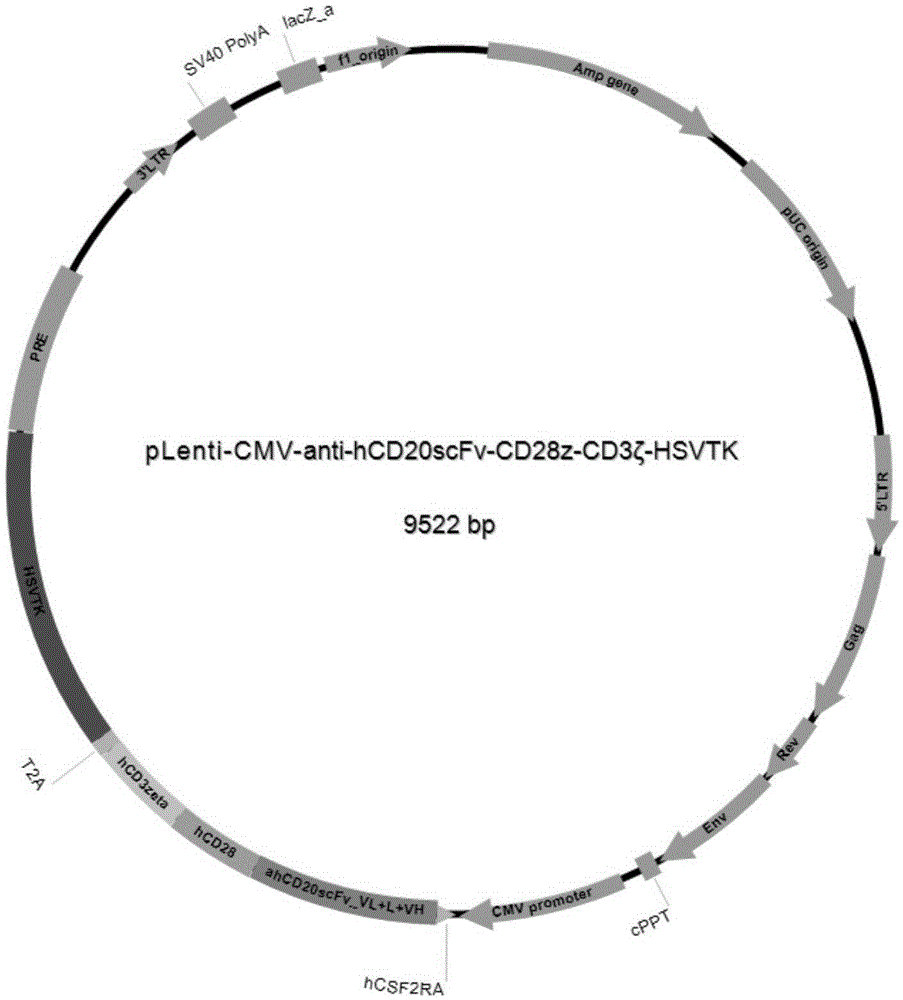
[0052]According to the amino acid sequence and coding sequence of each component of CAR, splicing into the entire fused amino acid sequence and coding DNA expression cassette, the full-length sequence contains anti-hCD20scFv (SEQ ID NO: 2 or SEQ ID NO: 6) and human CD28 Transmembrane and intracellular segment (SEQ ID NO:3 or SEQ ID NO:7), CD3ζ chain (SEQ ID NO:4 or SEQ ID NO:8) and HSVTK suicide gene (SEQ ID NO:5 or SEQ ID NO:9 ), collectively referred to as anti-hCD20scFv-CD28z-CD3ζ-HSVTK sequence (such as figure 1 shown), the two ends of the sequence introduced the coding sequence of XbaI and SalI restriction endonuclease cutting sites. In the present invention, the full-length sequence of anti-hCD20scFv-CD28z-CD3ζ-HSVTK is artificially synthesized, and BGI Gene Co., Ltd. is commissioned to synthesize it for a fee. The third-generation lentiviral vector pLenti-CMV is selected, an...
Embodiment 2
[0054] Example 2: Viral packaging comprising a CAR structure
[0055] Use 293FT as packaging cells, and when they reach 90% confluence, replace with serum-free DMEM medium, and use Lipofectamine3000 as the transfection reagent. For a six-well plate, the amount of plasmids used for transfection is respectively, vsv-G0.37μg +tat 0.19μg+rev 0.19μg+gag 0.19μg+vector(pLenti-CMV-anti-hCD20scFv-CD28z-CD3ζ-HSVTK) 2μg, operate according to the instructions of Lipofectamine3000, after preparing the transfection solution, add it to the serum-free medium for culture In the 293FT, after 6 hours, replace it with 10% fetal bovine serum DMEM complete medium, continue to culture for 48-72 hours, collect the virus liquid, filter and centrifuge at 90,000gx for 2 hours, add 500μl PBS / tube, and freeze in - Standby at 80°C.
Embodiment 3
[0056] Example 3: Preparation of anti-hCD20-CAR-T (CAR20-T)
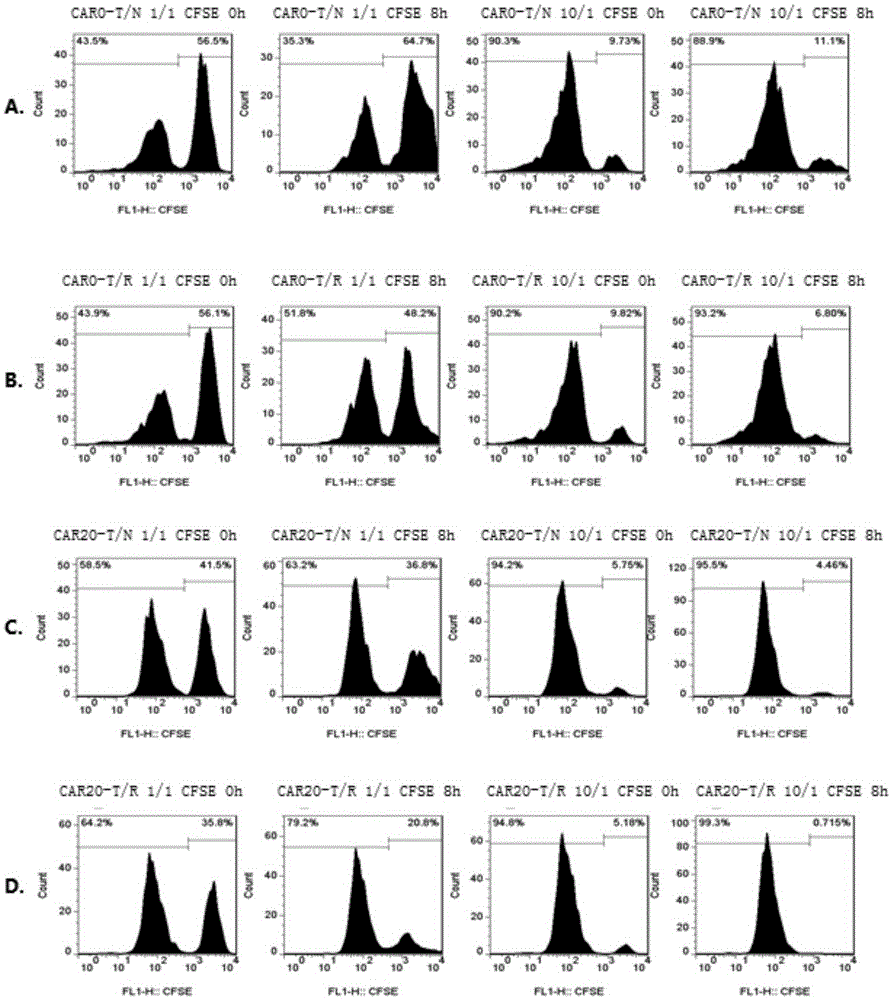
[0057] Using a 50ml syringe, collect 50ml of peripheral blood from a lymphoma patient at one time, dilute it with PBS at a rate of 1:2, and slowly add it to the Ficoll separation solution at a ratio of 2:1. Centrifuge at 1500rpm for 10 minutes at room temperature, it can be seen that it is divided into three layers, and the white layer in the middle is lymphocytes; gently suck the lymphocyte layer into 6ml~8mlRPMI1640 to elute; centrifuge at 1500rpm for 10 minutes; remove the supernatant, add 2mlRPMI1640, and count Press 0.5x 10 6 / ml added to OpTmizer TM In T-cell Expansion SFM medium, cultivate overnight. Mix the virus liquid obtained in the previous step with MOI 5, polybrene 10 μg / ml / , 1000 IU / ml recombinant human interleukin 2 (rhIL-2) and add it to T cells after overnight culture, the cell concentration is 0.5×10 6 / ml, after culturing overnight, replace with OpTmizer containing 1000IU / ml rhIL-2 TM T-cell ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com