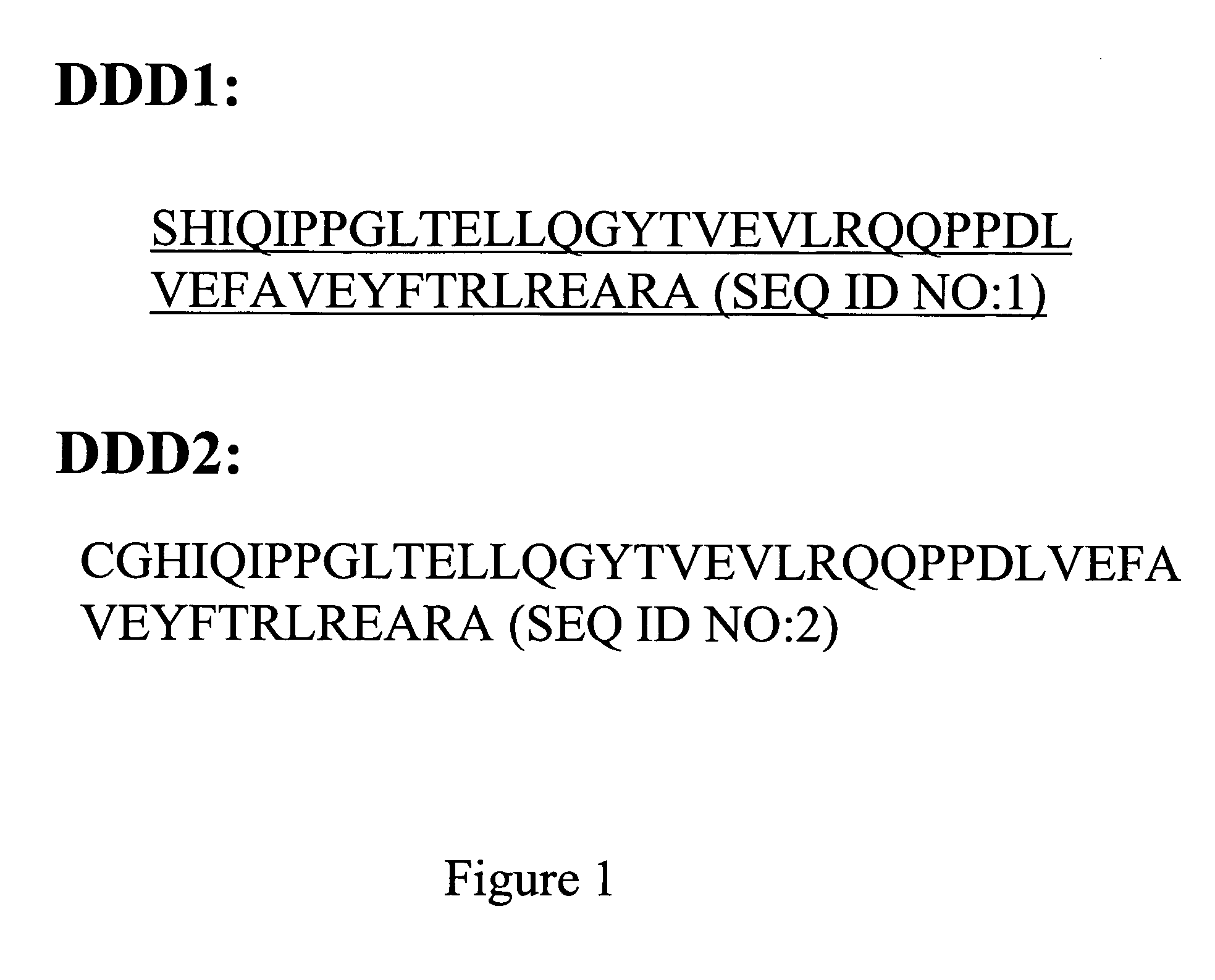
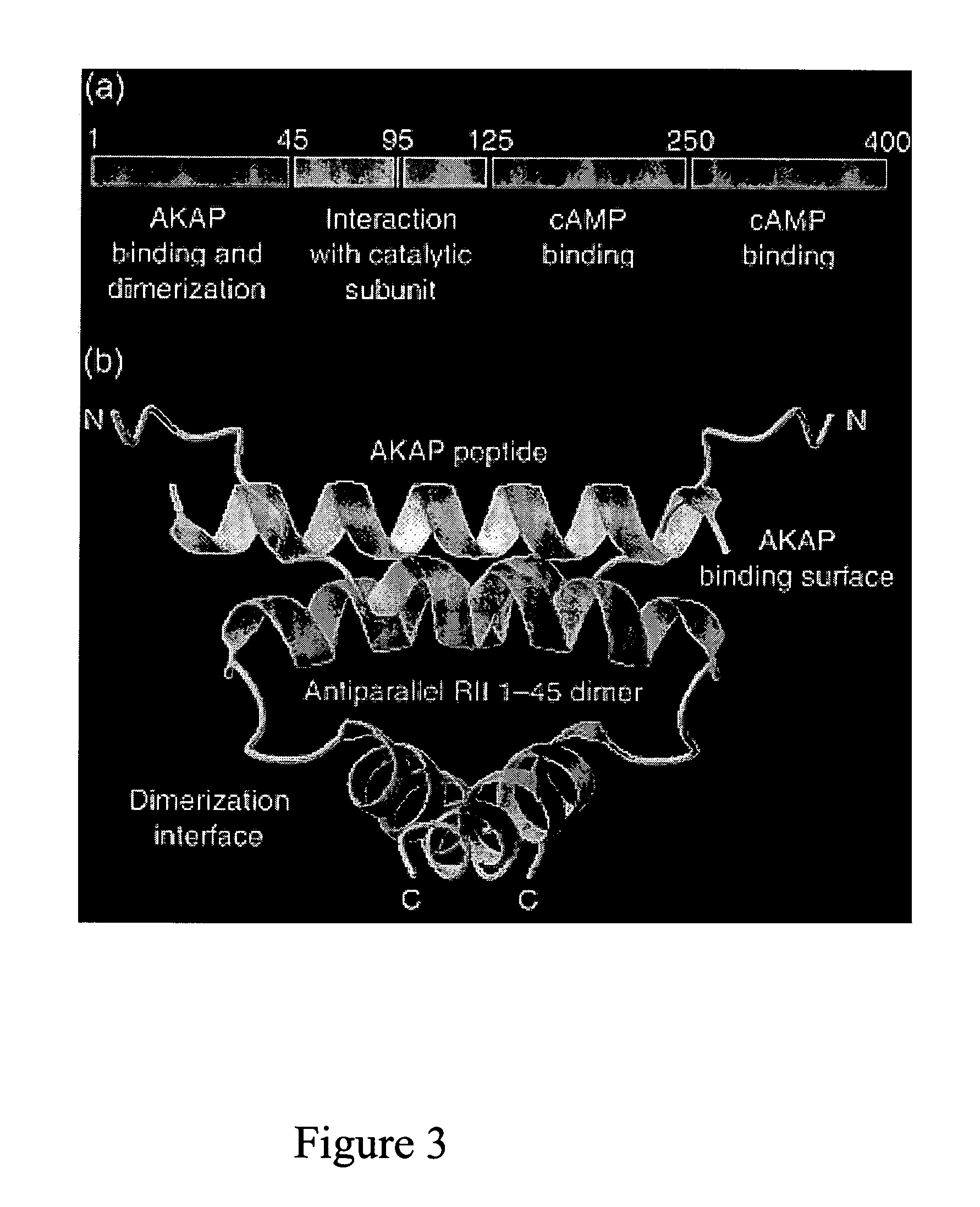
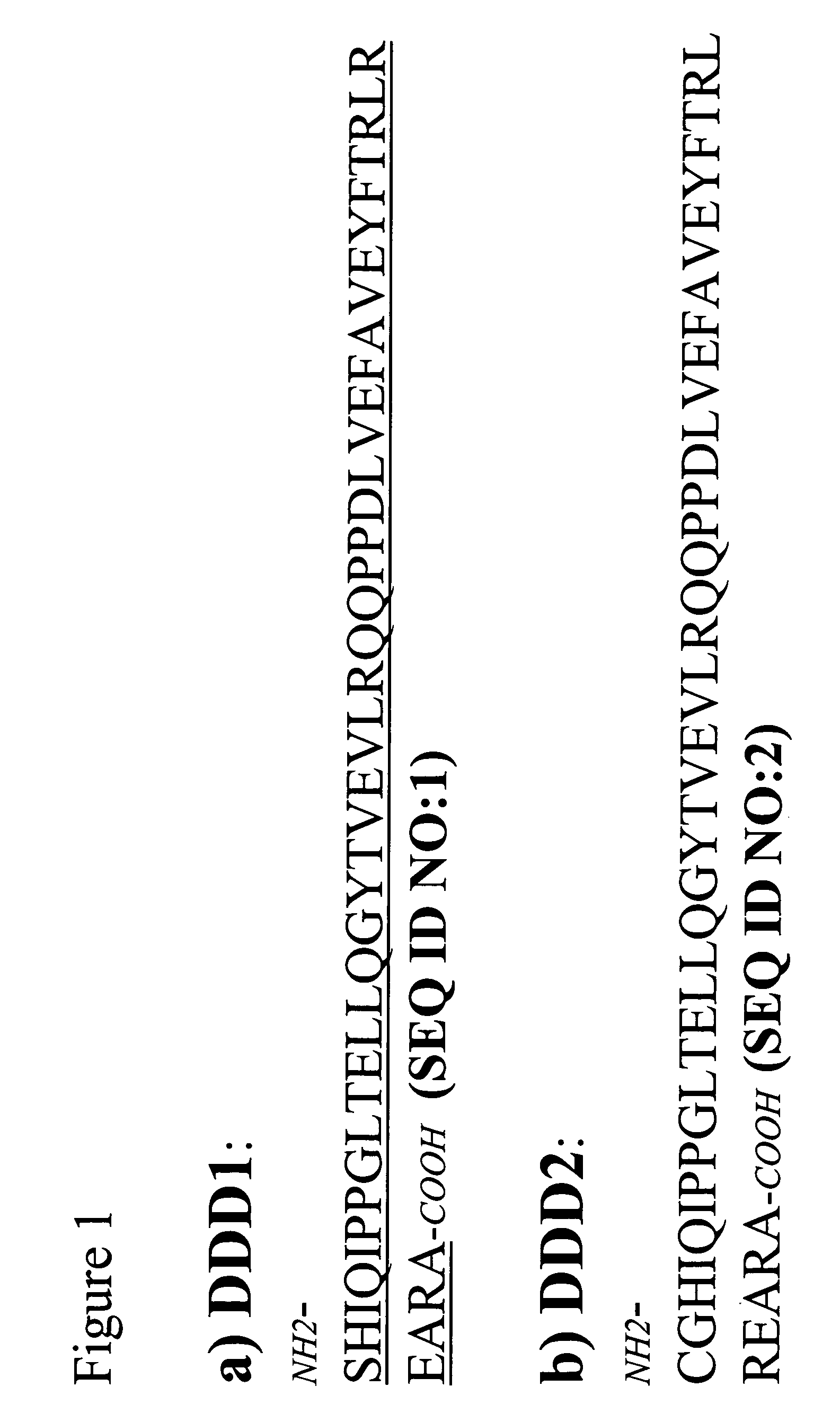
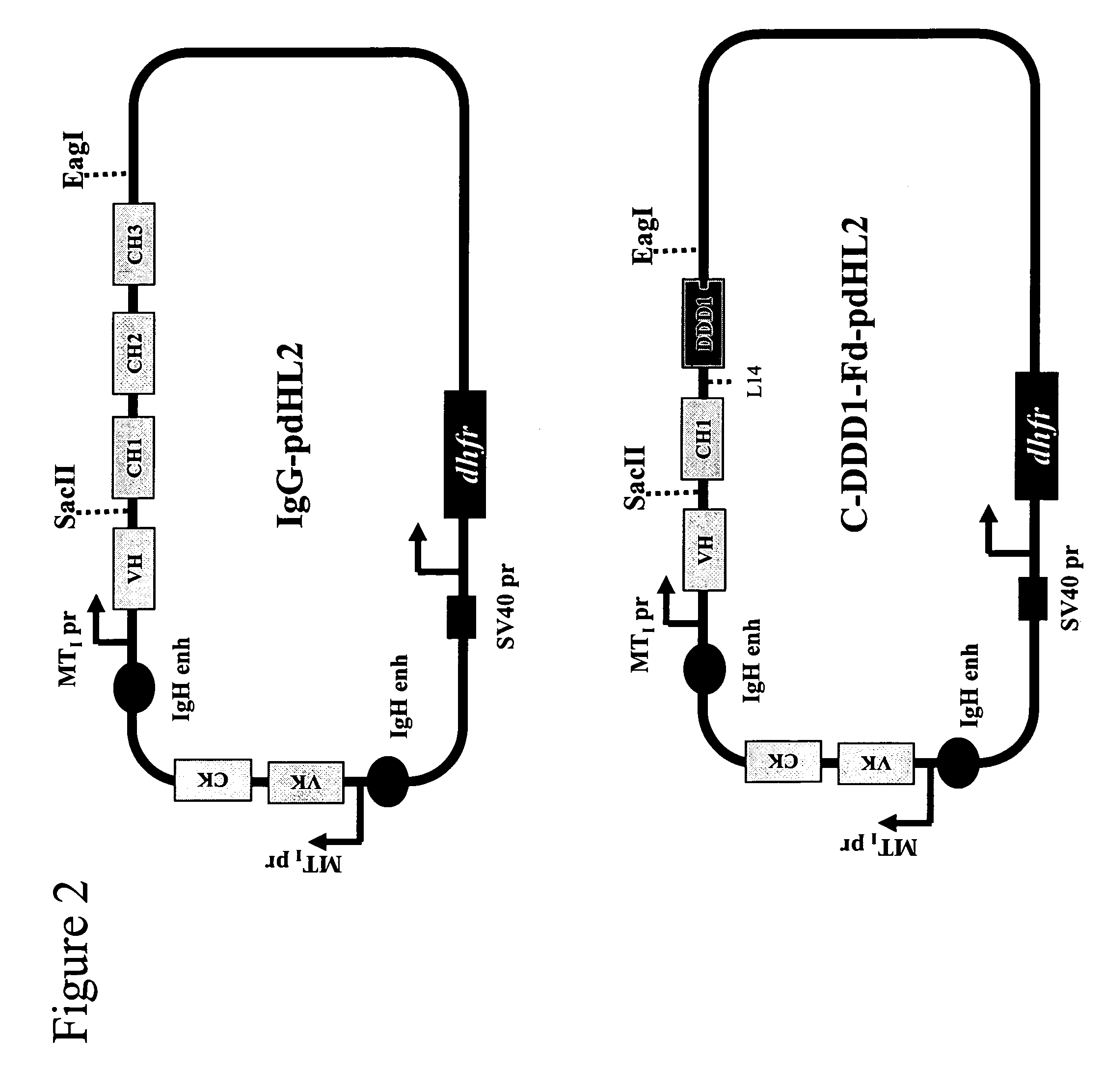
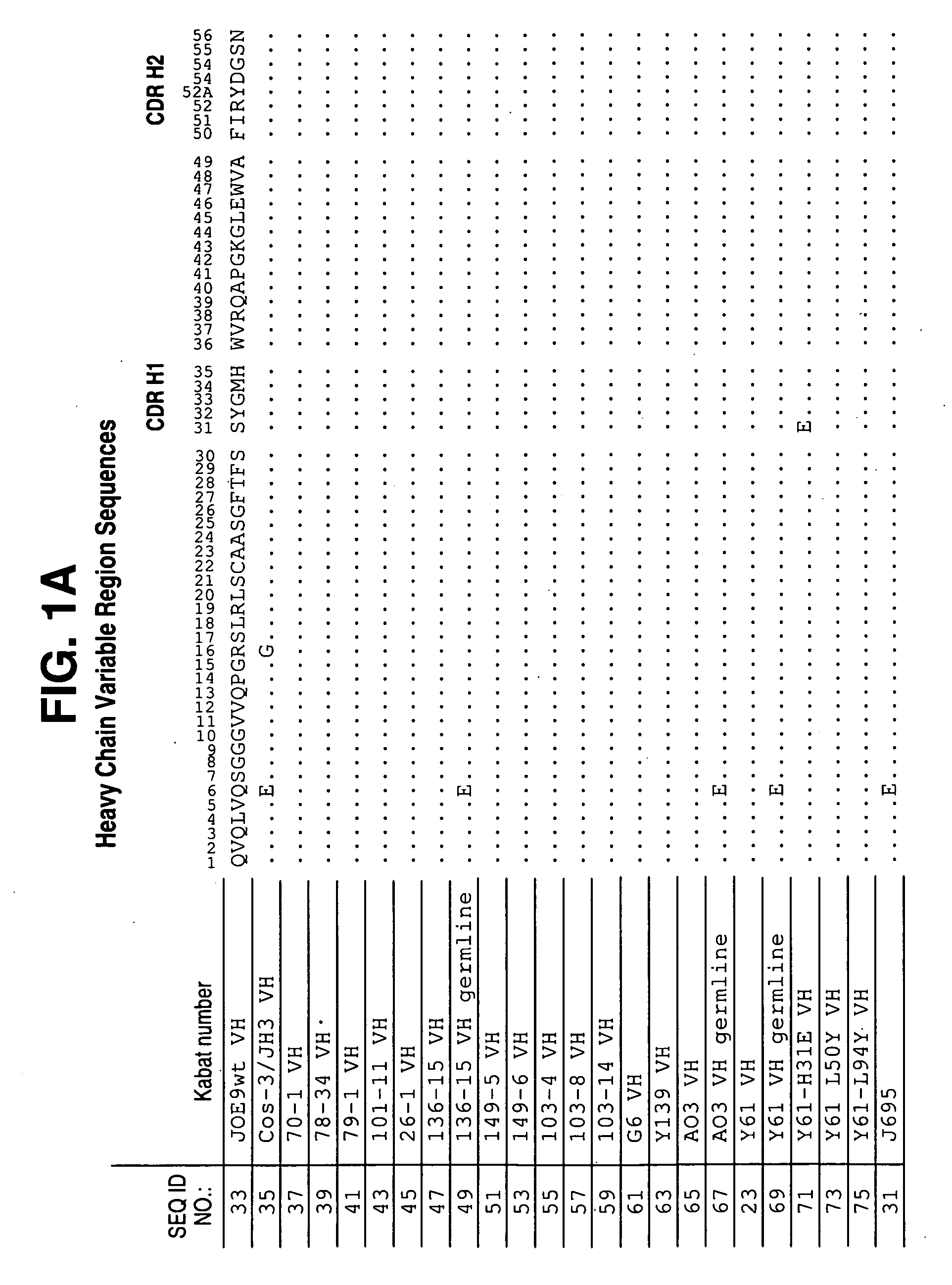
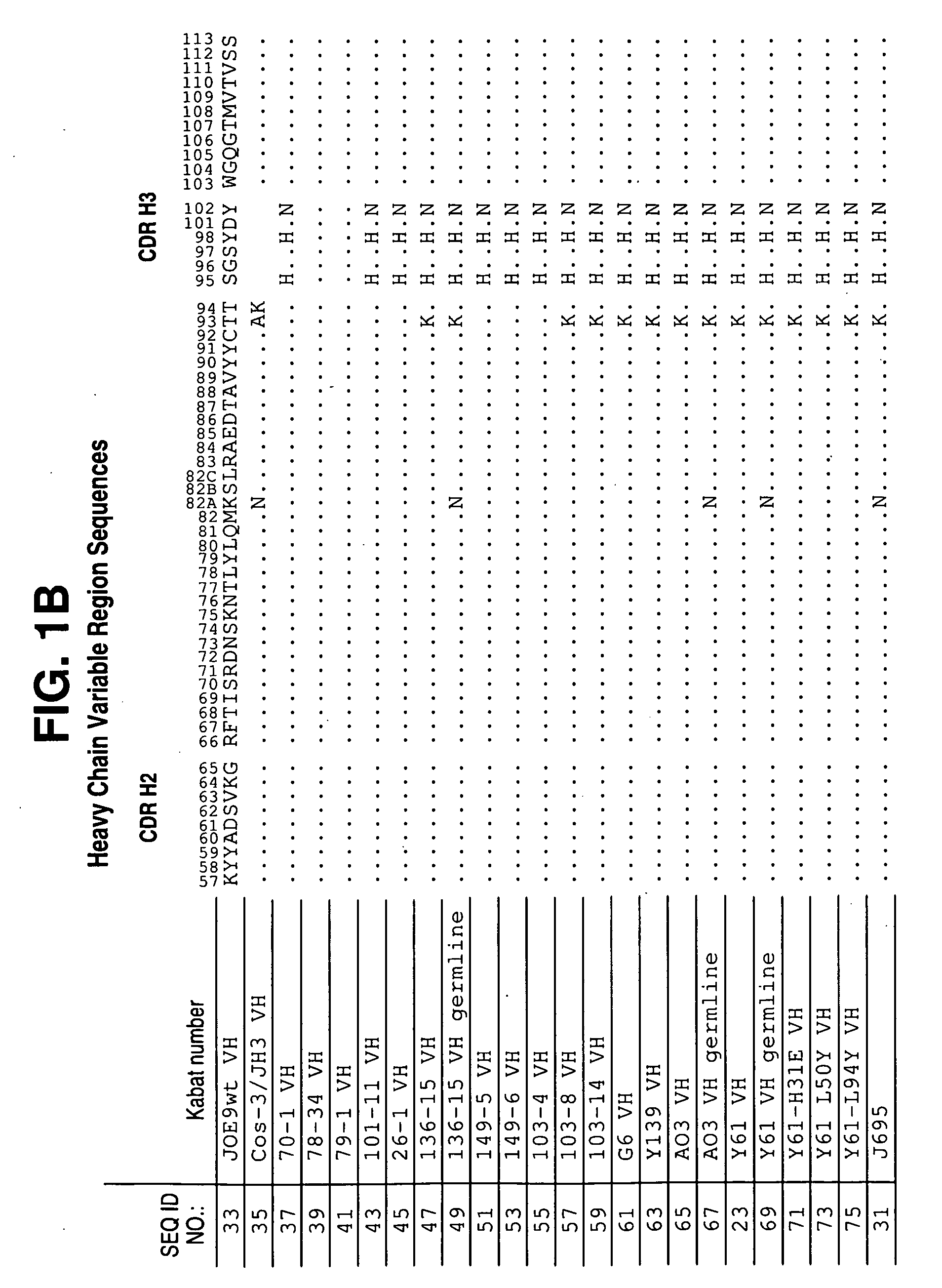
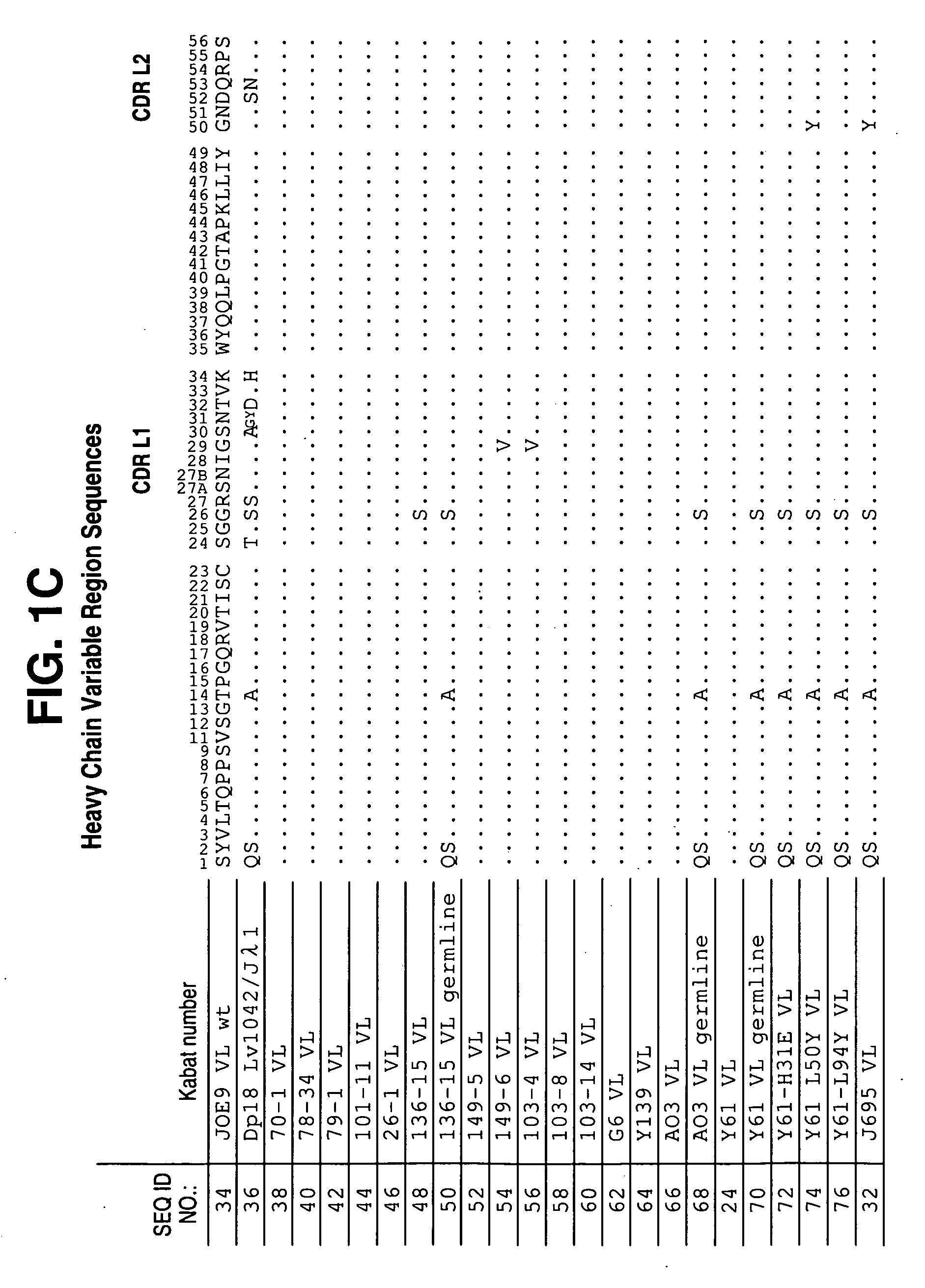
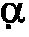Patents
Literature
1147 results about "Interleukin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
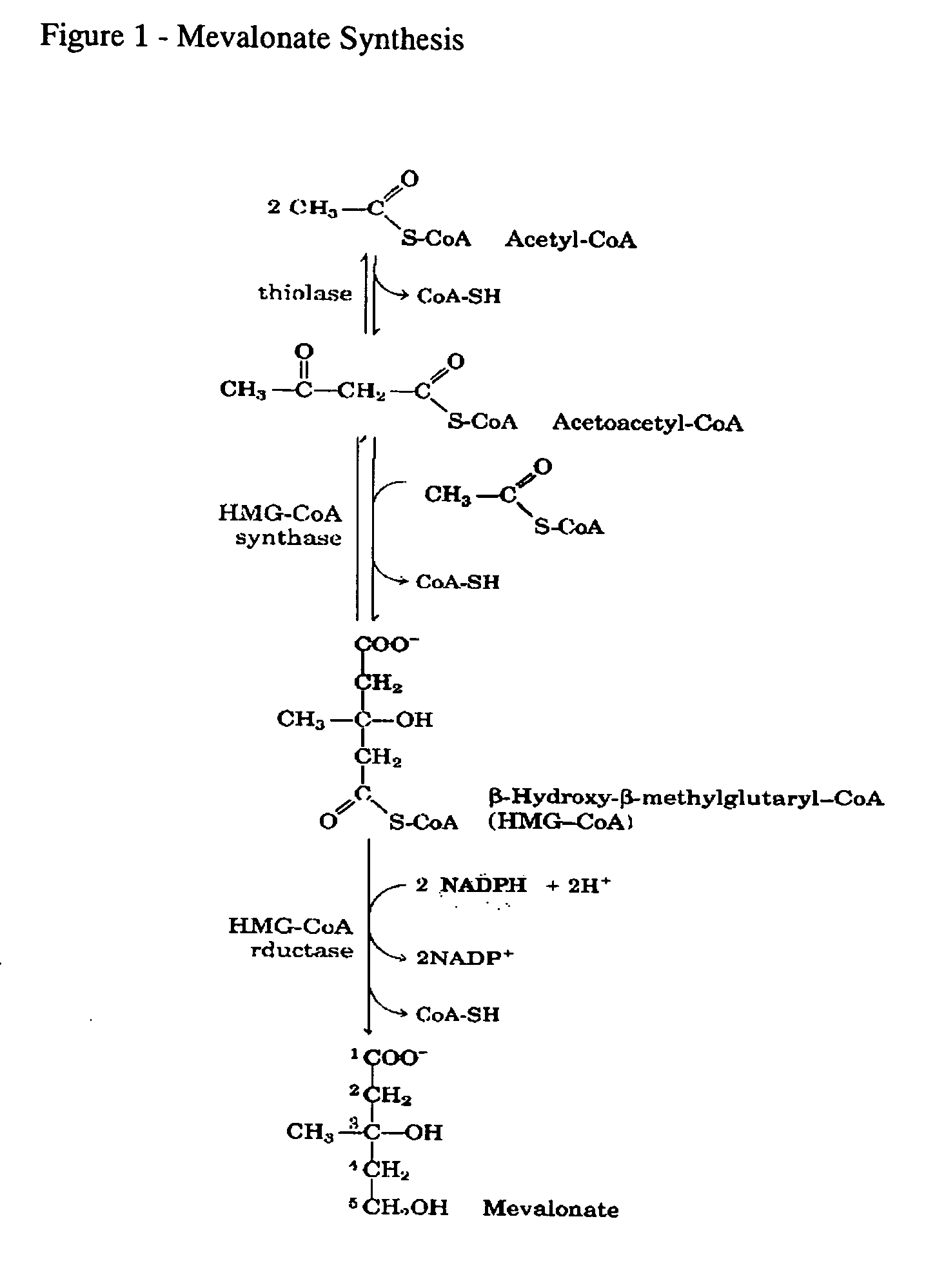
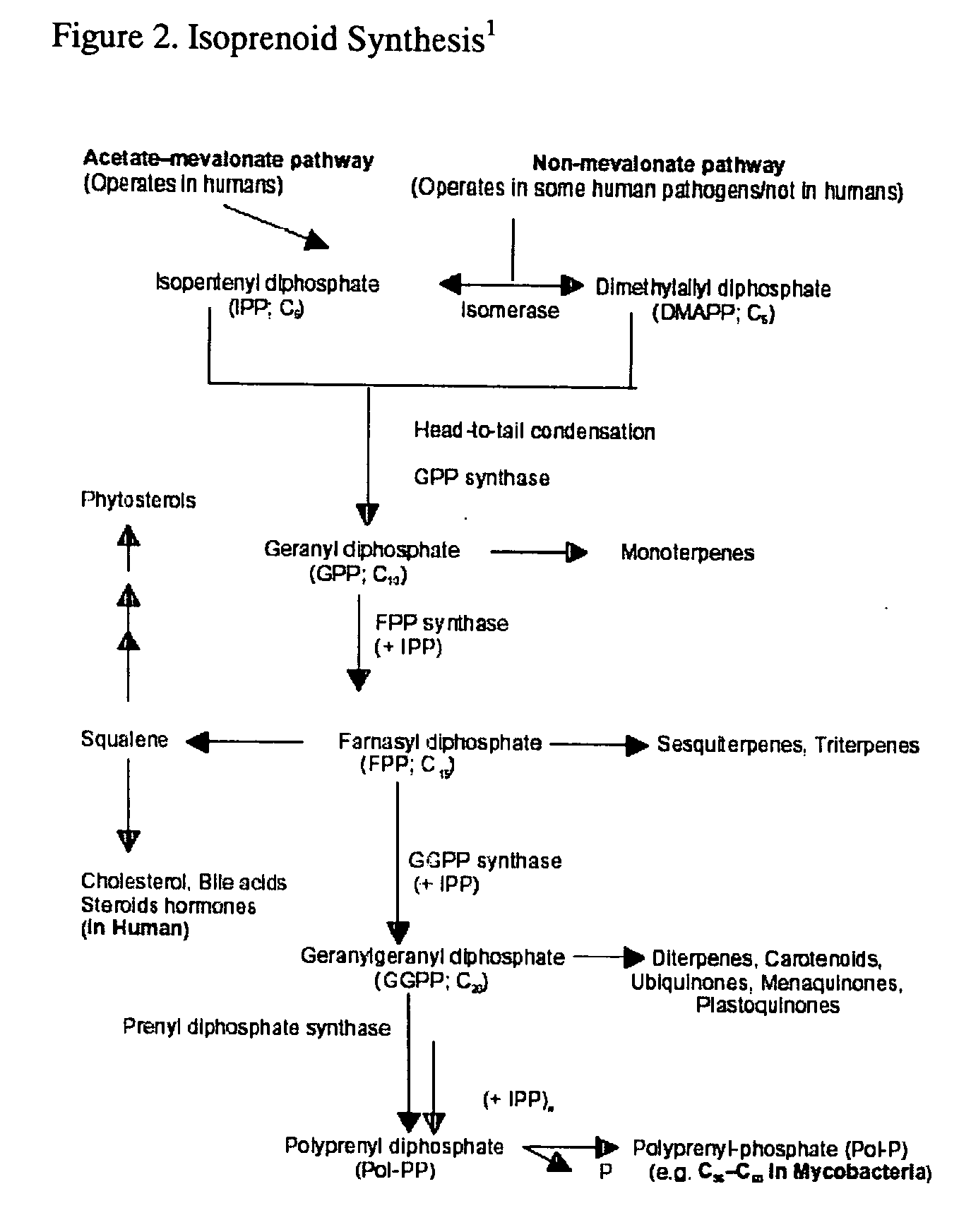
Interleukins (ILs) are a group of cytokines (secreted proteins and signal molecules) that were first seen to be expressed by white blood cells (leukocytes). ILs can be divided into four major groups based on distinguishing structural features. However, their amino acid sequence similarity is rather weak (typically 15–25% identity). The human genome encodes more than 50 interleukins and related proteins.
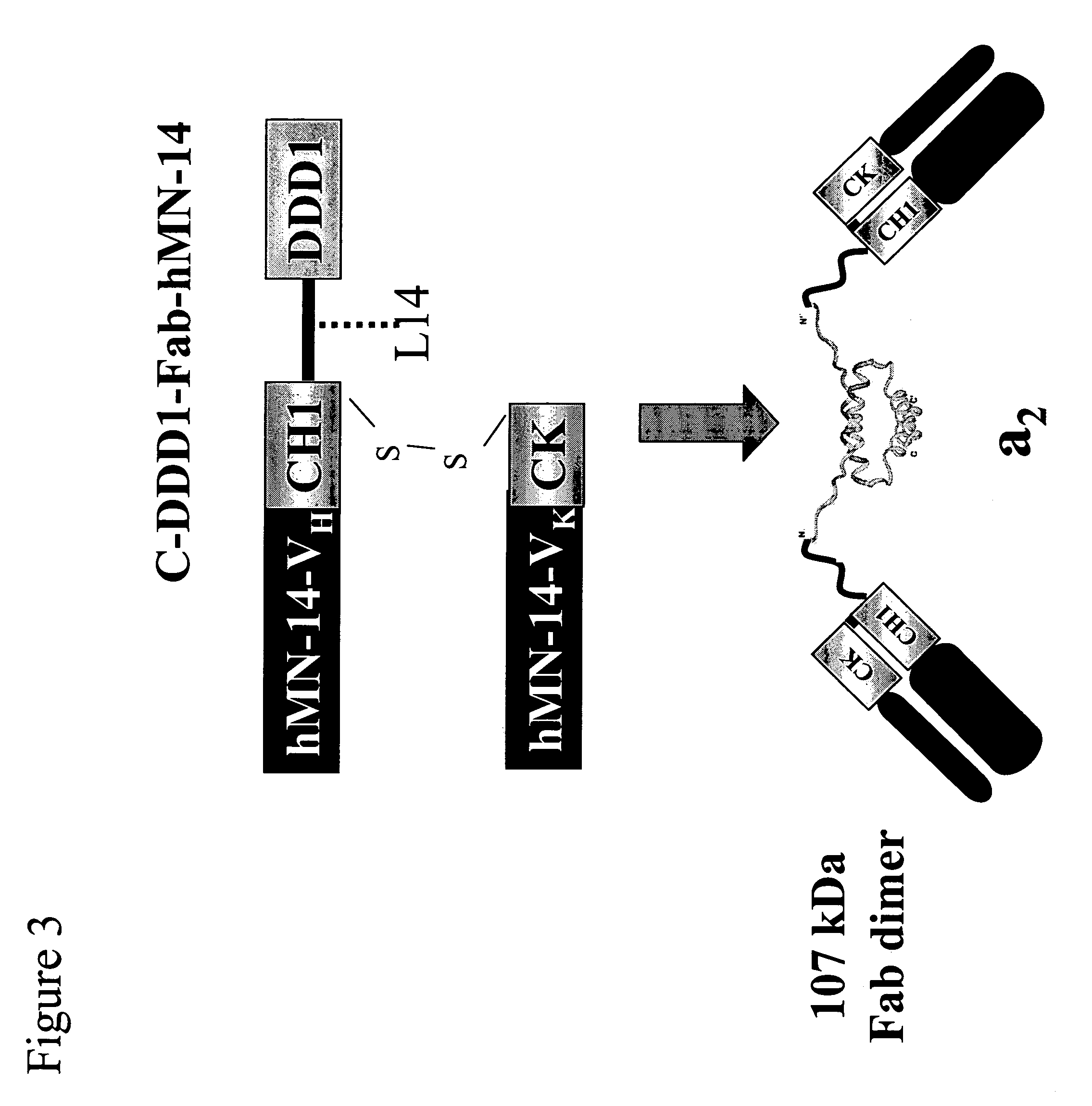
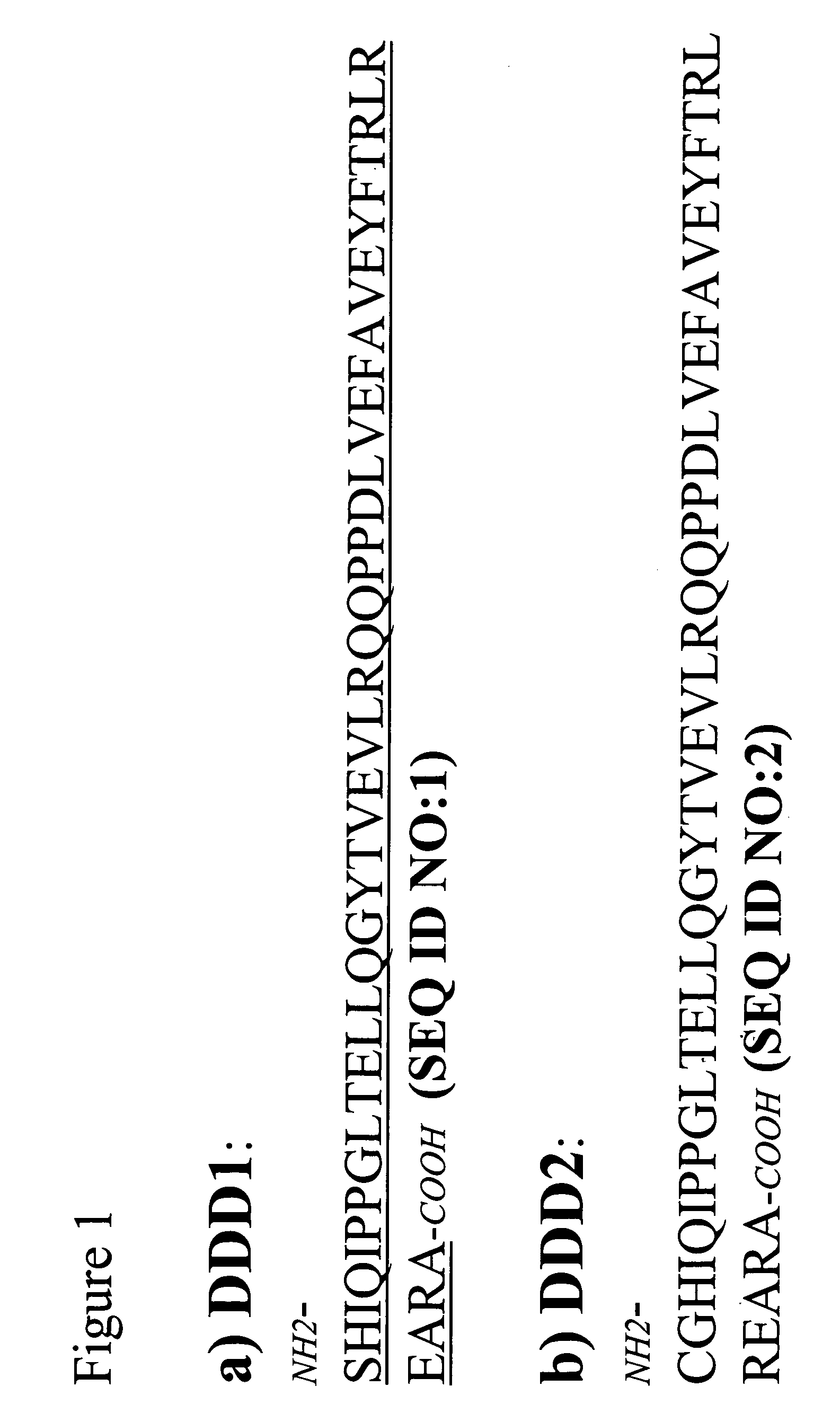
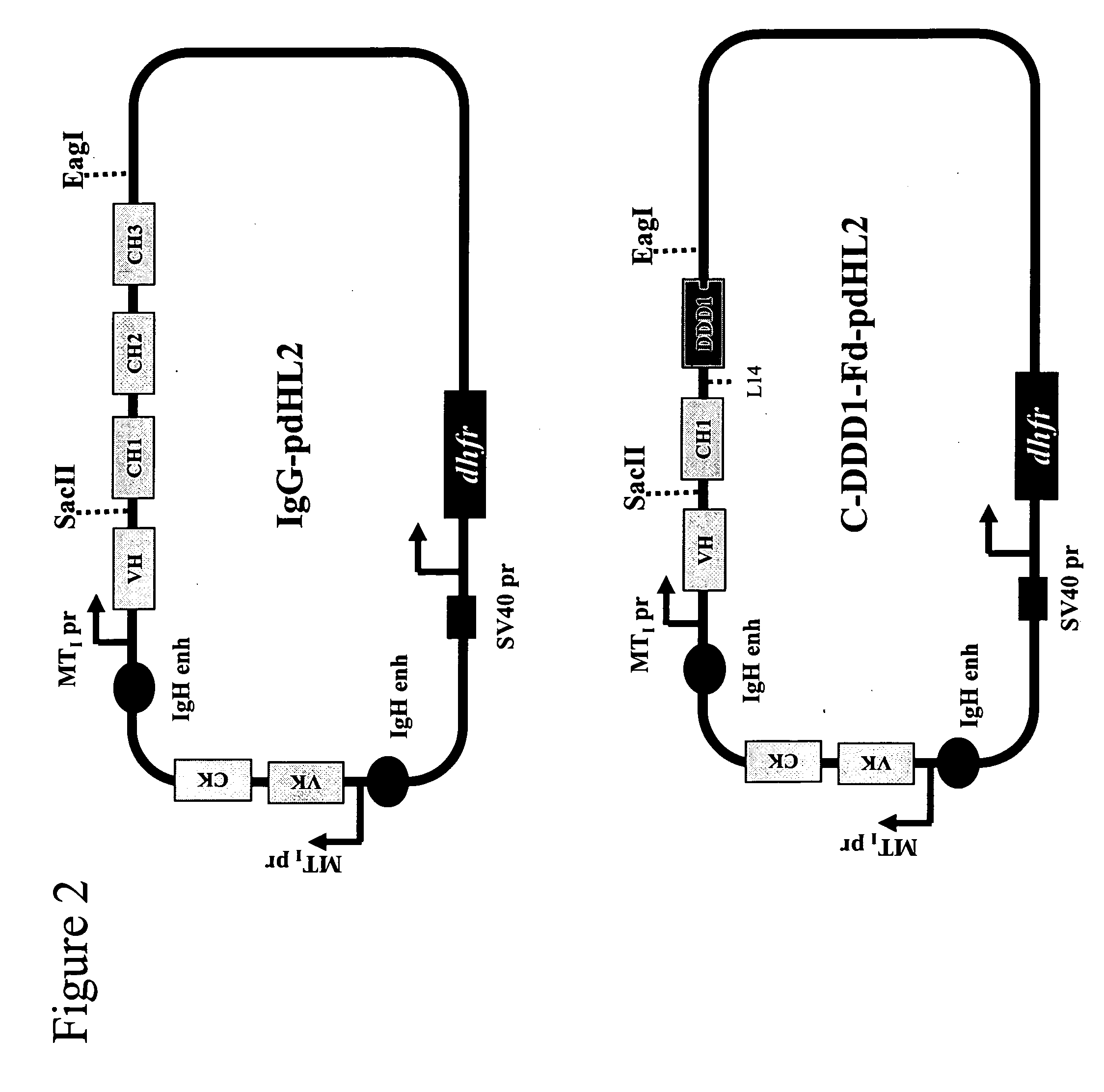
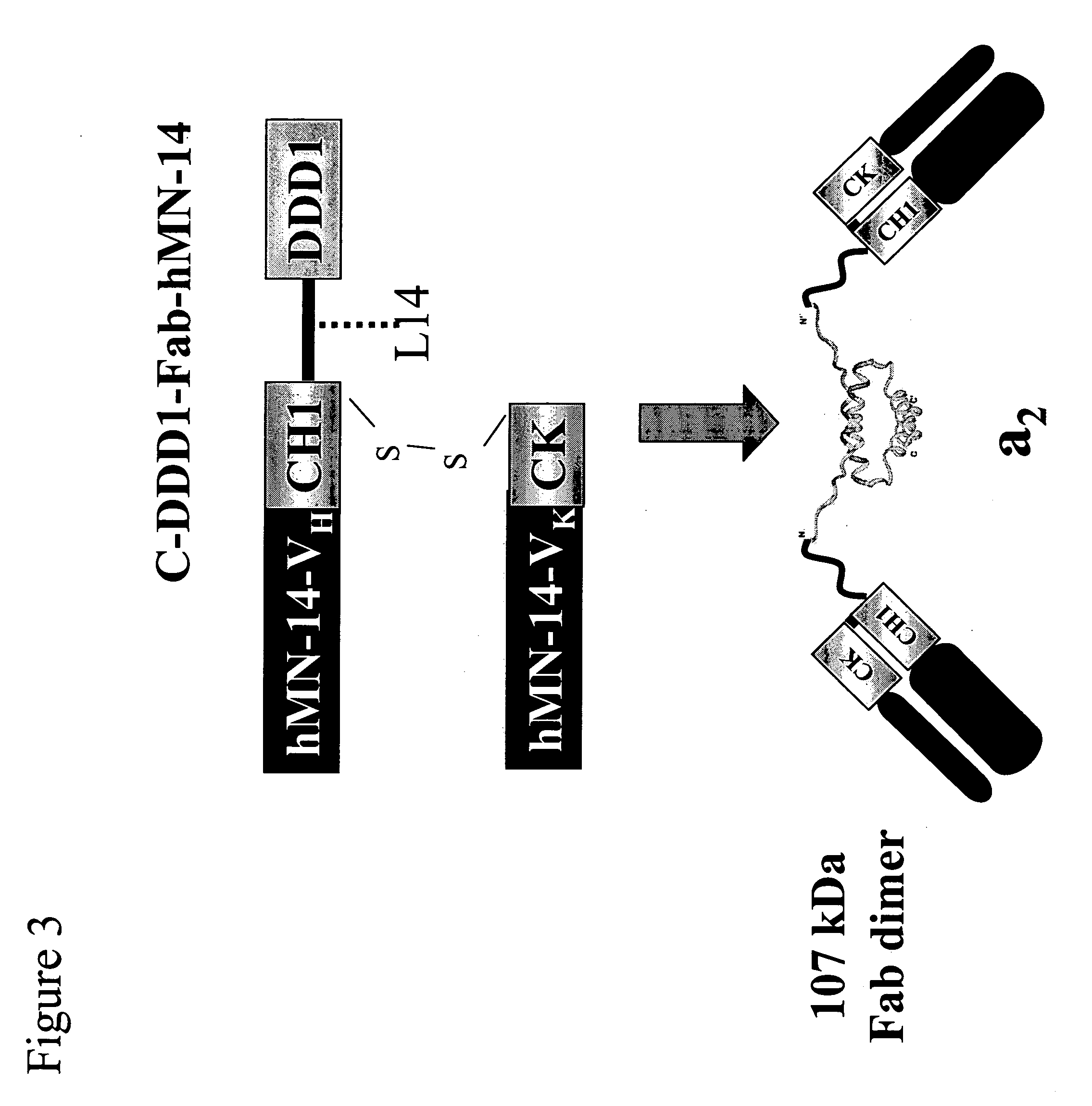
Stably tethered structures of defined compositions with multiple functions or binding specificities
Owner:IBC PHARMACEUTICALS INC
Methods for generating stably linked complexes composed of homodimers, homotetramers or dimers of dimers and uses
ActiveUS7550143B2Improve functionalityStrong specificityPeptide/protein ingredientsAntibody mimetics/scaffoldsHomotetramerAptamer
The present invention concerns methods and compositions for stably tethered structures of defined compositions, which may have multiple functionalities and / or binding specificities. Particular embodiments concern homodimers comprising monomers that contain a dimerization and docking domain attached to a precursor. The precursors may be virtually any molecule or structure, such as antibodies, antibody fragments, antibody analogs or mimetics, aptamers, binding peptides, fragments of binding proteins, known ligands for proteins or other molecules, enzymes, detectable labels or tags, therapeutic agents, toxins, pharmaceuticals, cytokines, interleukins, interferons, radioisotopes, proteins, peptides, peptide mimetics, polynucleotides, RNAi, oligosaccharides, natural or synthetic polymeric substances, nanoparticles, quantum dots, organic or inorganic compounds, etc. Other embodiments concern tetramers comprising a first and second homodimer, which may be identical or different. The disclosed methods and compositions provide a facile and general way to obtain homodimers, homotetramers and heterotetramers of virtually any functionality and / or binding specificity.
Owner:IBC PHARMACEUTICALS INC
Pharmaceutical composition for treatment of diseases caused by IL-6 production
Pharmaceutical compositions for prevention or treatment of diseases caused by interleukin-6 production, comprising an antibody to interleukin-6 receptor (IL-6R antibody). As the IL-6R antibody, an antibody of animals other than the human such as mice, rats, etc., a chimeric antibody between these and a human antibody, a reshaped human antibody, etc. may be used. The pharmaceutical compositions are useful for prevention or treatment of diseases caused by interleukin-6 production such as plasmacytosis, anti-IgGl-emia, anemia, nephritis, etc.
Owner:KISHIMOTO TADAMITSU +2
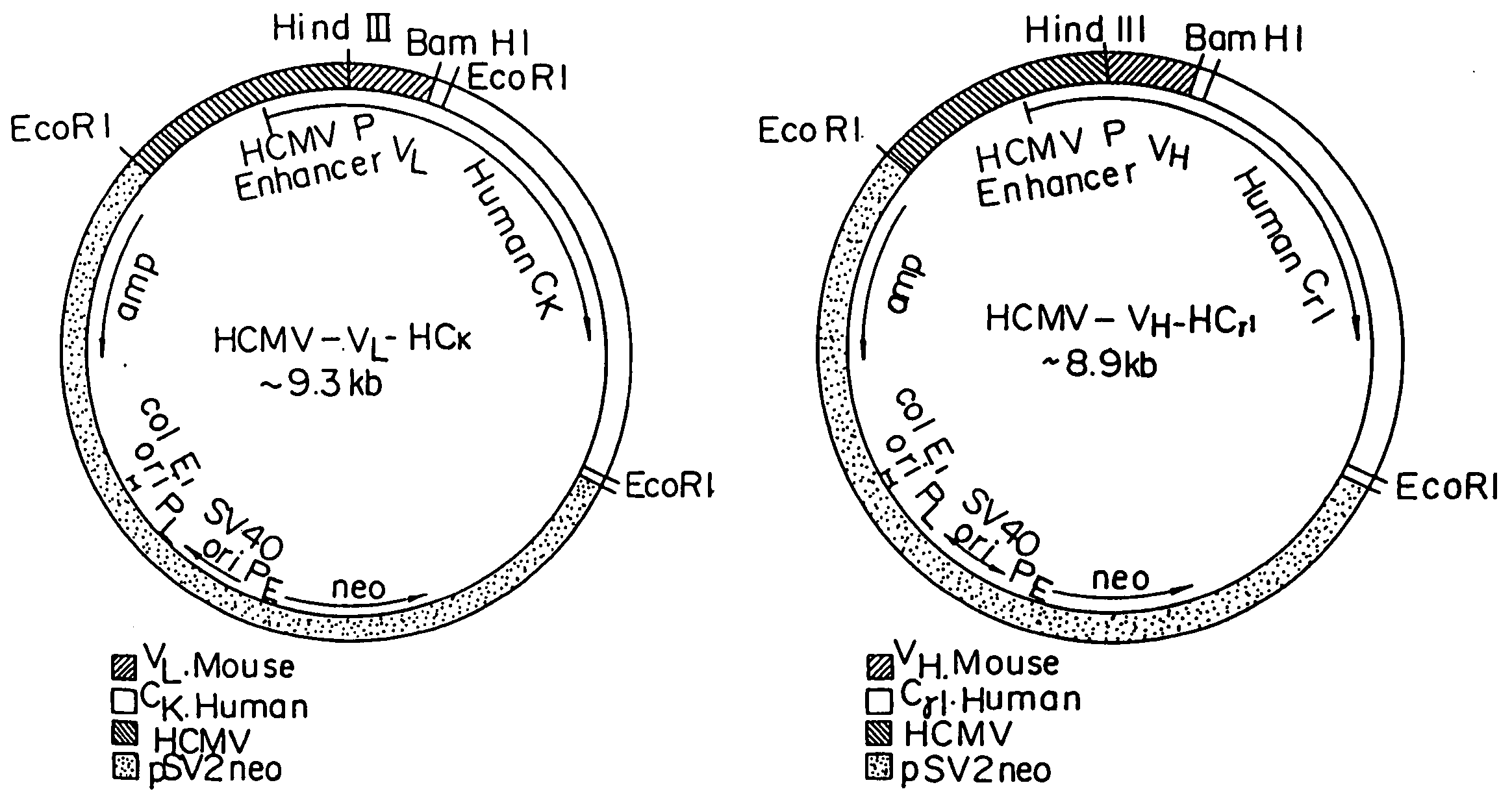
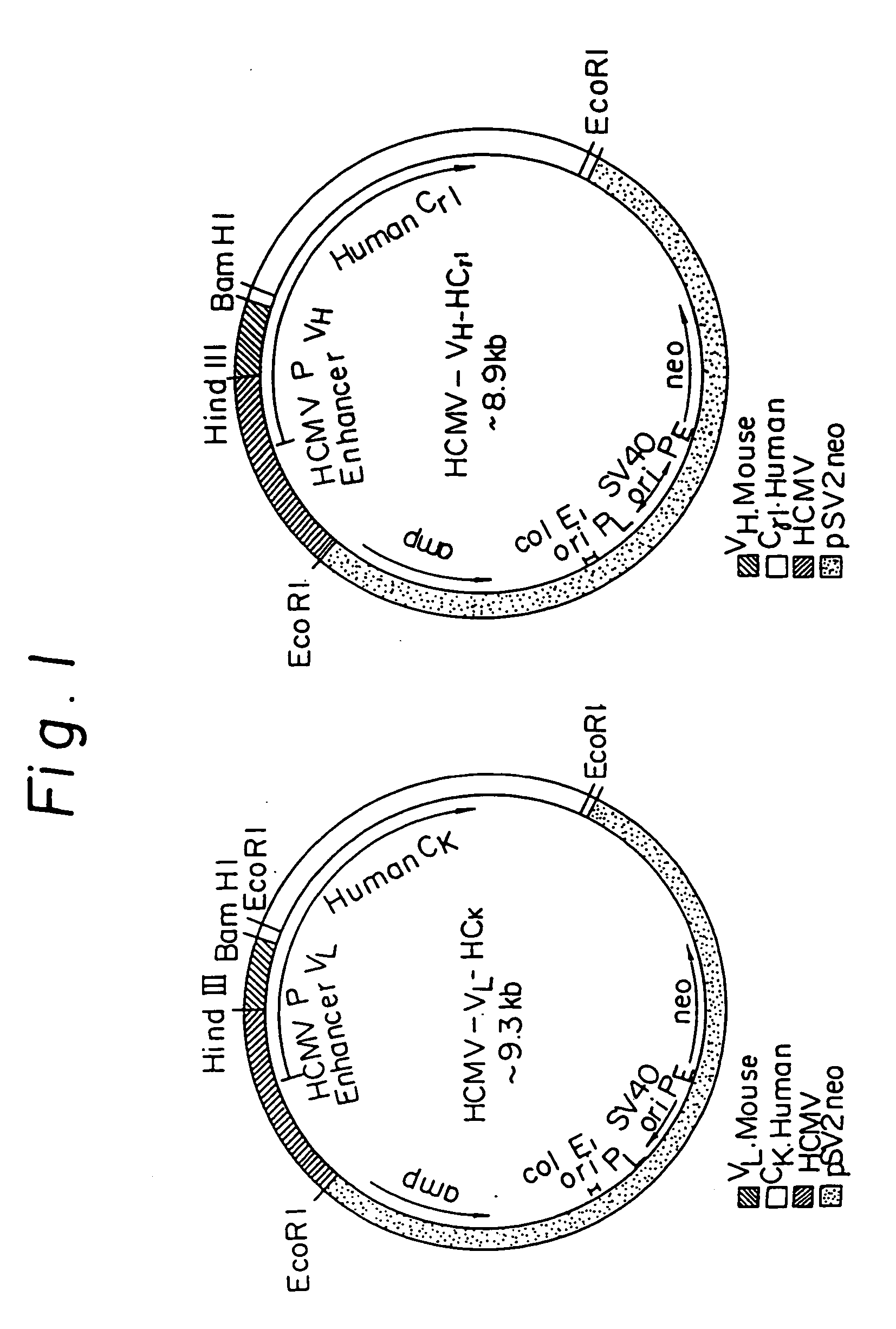
Reshaped human antibody to human interleukin-6 receptor
InactiveUS20050142635A1Less immunogenicPeptide/protein ingredientsHydrolasesComplementarity determining regionV region
A reshaped human antibody to the human IL-6R, comprising: (A) an L chain comprising, (1) a human L chain C region, and (2) an L chain V region comprising human L chain framework regions (FRs), and mouse L chain complementary determination regions (CDRs) of a momoclonal antibody to the IL-6 receptor (IL-6R); and (B) an H chain comprising, (1) a human H chain C region, and (2) an H chain V region comprising human H chain FRS, and mouse H chain CDRs of a monoclonal antibody to the IL-6R. Since major portion of the reshaped human antibody is derived from a human antibody and the mouse CDRs which are less immunogenic, the present reshaped human antibody is less immunogenic to human, and therefor is promised for therapeutic uses.
Owner:CHUGAI PHARMA CO LTD
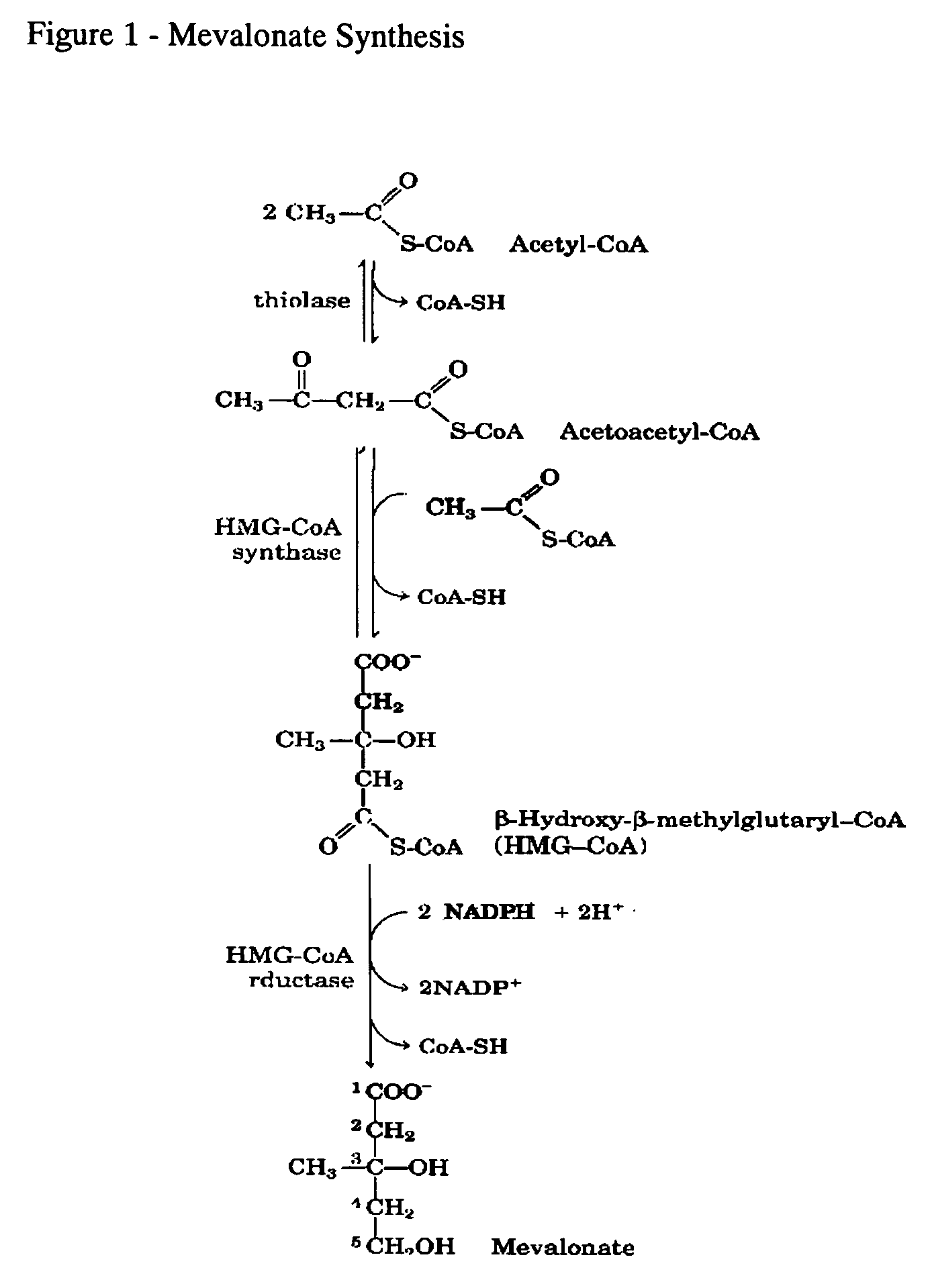
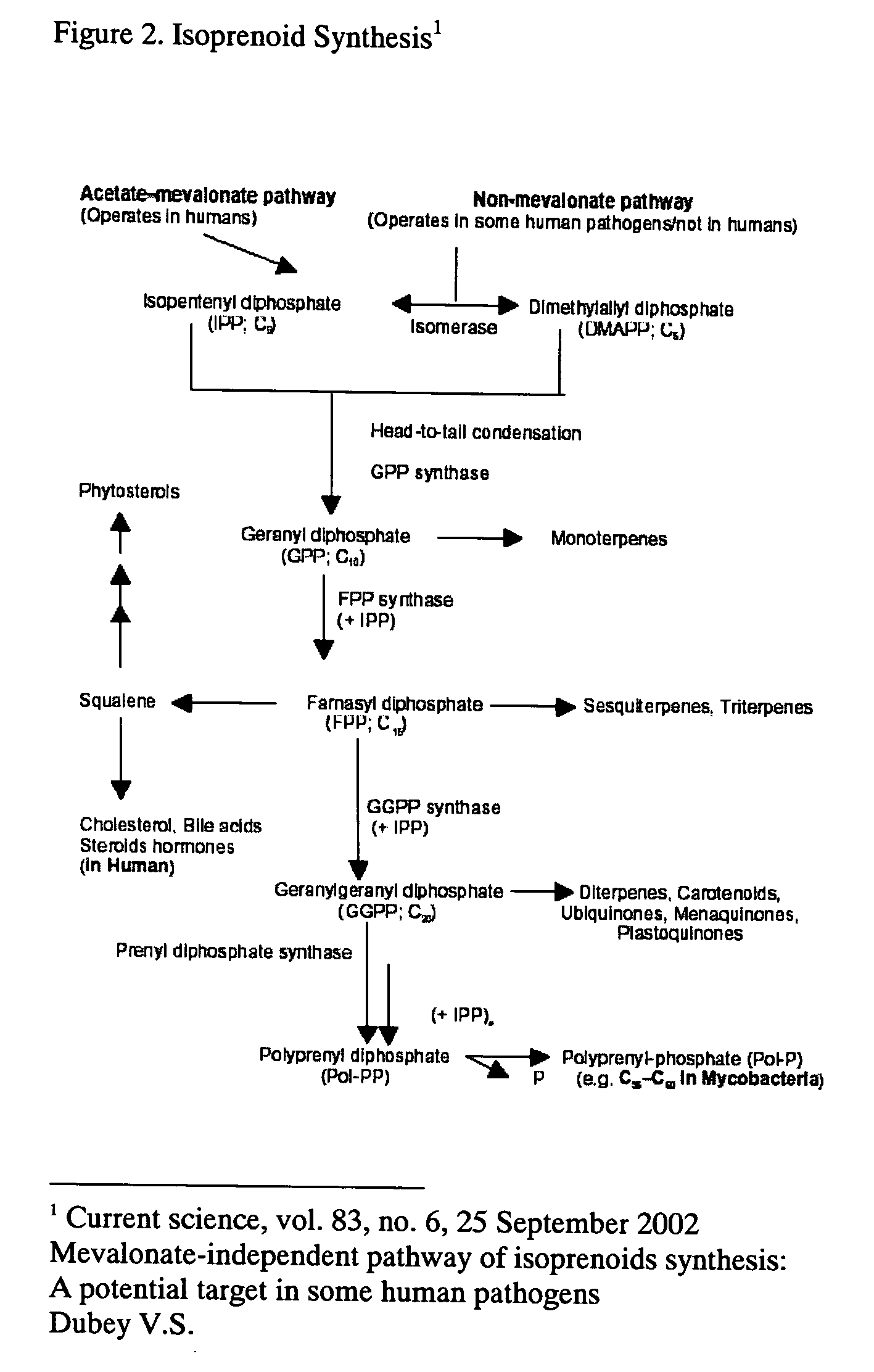
Method of prevention and treatment of aging, age-related disorders and/or age-related manifestations including atherosclerosis, peripheral vascular disease, coronary artery disease, osteoporosis, arthritis, type 2 diabetes, dementia, alzheimers disease and cancer
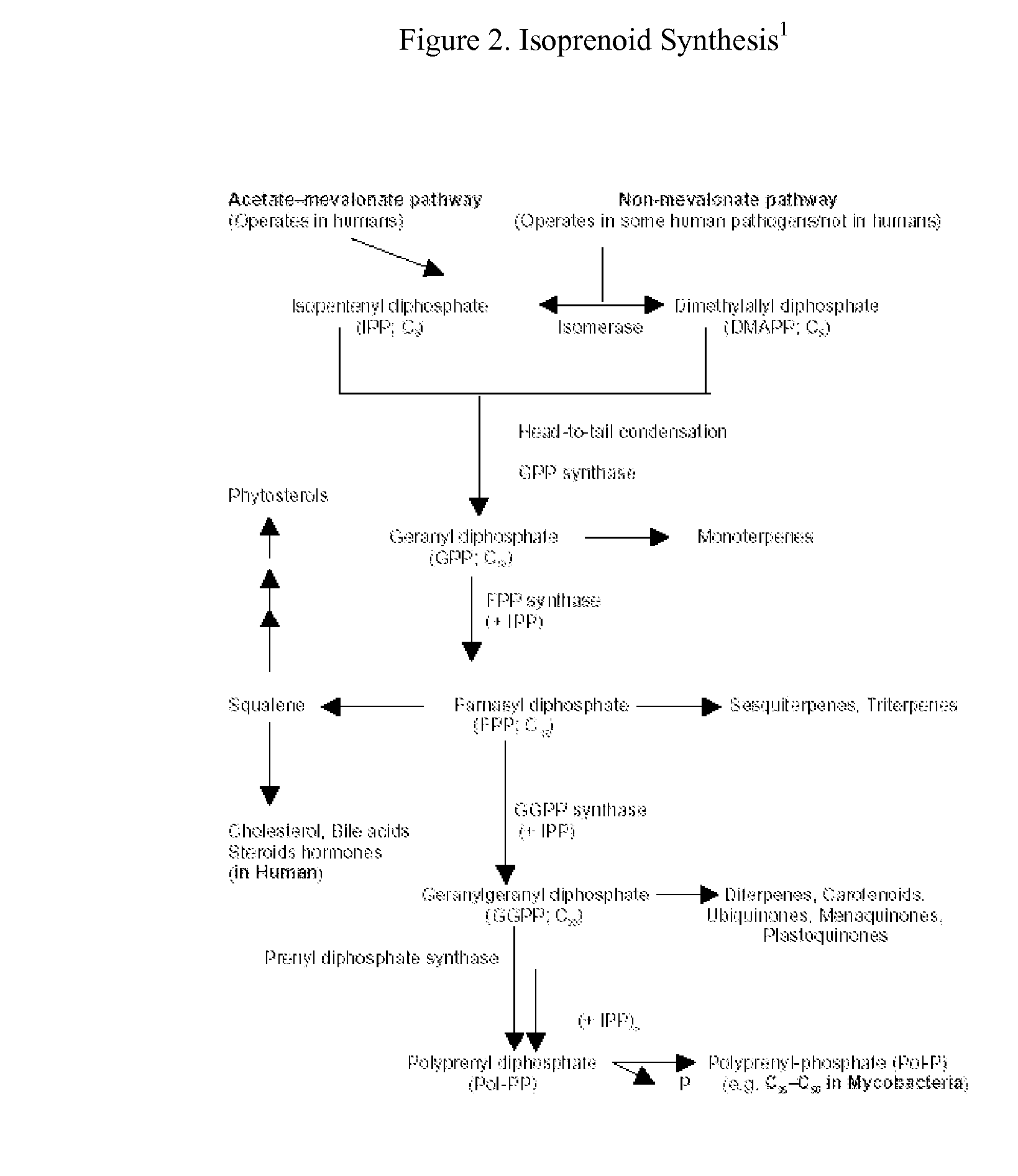
InactiveUS20060275294A1Halogenated hydrocarbon active ingredientsBiocideAbnormal tissue growthSTAT Transcription Factors
This invention relates to a method for prevention and treatment of aging, age-related disorders and / or age-related manifestations including atherosclerosis, peripheral vascular disease, coronary artery disease, osteoporosis, type 2 diabetes, dementia and some forms of arthritis and cancer in a subject comprising administering to said subject, separately, sequentially or simultaneously a therapeutically effective dosage of each component or combination of statins, bisphosphonates, cholesterol lowering agents or techniques, interleukin-6 inhibitor / antibody, interleukin-6 receptor inhibitor / antibody, interleukin-6 antisense oligonucleotide (ASON), gp130 protein inhibitor / antibody, tyrosine kinases inhibitors / antibodies, serine / threonine kinases inhibitors / antibodies, mitogen-activated protein (MAP) kinase inhibitors / antibodies, phosphatidylinositol 3-kinase (PI3K) inhibitors / antibodies, Nuclear factor κB (NF-κB) inhibitors / antibodies, IκB kinase (IKK) inhibitors / antibodies, activator protein-1 (AP-1) inhibitors / antibodies, STAT transcription factors inhibitors / antibodies, altered IL-6, partial peptides of IL-6 or IL-6 receptor, or SOCS (suppressors of cytokine signaling) protein, or a functional fragment thereof, administered separately, in sequence or simultaneously. Inhibition of the signal transduction pathway for Interleukin 6 mediated inflammation is key to the prevention and treatment of atherosclerosis, peripheral vascular disease, coronary artery disease, aging, age-related disorders and / or age-related manifestations including osteoporosis, type 2 diabetes, dementia and some forms of arthritis and tumors. Inhibition of Interleukin 6 mediated inflammation may be achieved indirectly through regulation of endogenous cholesterol synthesis and isoprenoid depletion or by direct inhibition of the signal transduction pathway utilizing interleukin-6 inhibitor / antibody, interleukin-6 receptor inhibitor / antibody, interleukin-6 antisense oligonucleotide (ASON), gp130 protein inhibitor / antibody, tyrosine kinases inhibitors / antibodies, serine / threonine kinases inhibitors / antibodies, mitogen-activated protein (MAP) kinase inhibitors / antibodies, phosphatidylinositol 3-kinase (PI3K) inhibitors / antibodies, Nuclear factor κB (NF-κB) inhibitors / antibodies, IκB kinase (IKK) inhibitors / antibodies, activator protein-1 (AP-1) inhibitors / antibodies, STAT transcription factors inhibitors / antibodies, altered IL-6, partial peptides of IL-6 or IL-6 receptor, or SOCS (suppressors of cytokine signaling) protein, or a functional fragment thereof. Said method for prevention and treatment of said disorders is based on inhibition of Interleukin-6 inflammation through regulation of cholesterol metabolism, isoprenoid depletion and / or inhibition of the signal transduction pathway
Owner:OMOIGUI OSEMWOTA SOTA
Methods for generating stably linked complexes composed of homodimers, homotetramers or dimers of dimers and uses
ActiveUS20060228357A1Improve functionalityStrong specificityPeptide/protein ingredientsAntibody mimetics/scaffoldsChemistryToxin
The present invention concerns methods and compositions for stably tethered structures of defined compositions, which may have multiple functionalities and / or binding specificities. Particular embodiments concern homodimers comprising monomers that contain a dimerization and docking domain attached to a precursor. The precursors may be virtually any molecule or structure, such as antibodies, antibody fragments, antibody analogs or mimetics, aptamers, binding peptides, fragments of binding proteins, known ligands for proteins or other molecules, enzymes, detectable labels or tags, therapeutic agents, toxins, pharmaceuticals, cytokines, interleukins, interferons, radioisotopes, proteins, peptides, peptide mimetics, polynucleotides, RNAi, oligosaccharides, natural or synthetic polymeric substances, nanoparticles, quantum dots, organic or inorganic compounds, etc. Other embodiments concern tetramers comprising a first and second homodimer, which may be identical or different. The disclosed methods and compositions provide a facile and general way to obtain homodimers, homotetramers and heterotetramers of virtually any functionality and / or binding specificity.
Owner:IBC PHARMACEUTICALS INC
Cytokine antagonists for the treatment of sensorineural hearing loss
InactiveUS6423321B2Improve hearingReduce developmentPeptide/protein ingredientsImmunoglobulins against cytokines/lymphokines/interferonsIntravenous routeSensorineural hearing loss
Specific Cytokine Antagonists, including TNF antagonists and / or Interleukin-1 antagonists, are used as novel therapeutic agents for the treatment of hearing loss, including presbycusis and other forms of sensorineural hearing loss. The present invention provides a method for inhibiting the action of TNF and / or IL-1 antagonists for treating hearing loss in a human by administering a TNF antagonist and / or an IL-1 antagonist for reducing the inflammation affecting the auditory apparatus of said human, or for modulating the immune response affecting the auditory apparatus of said human, by administering a therapeutically effective dosage level to said human of a TNF antagonist and / or an IL-1 antagonist. Administration may be systemic, through the subcutaneous, intramuscular, oral, or intravenous routes; or by delivering an anatomically localized application in the region of the head. The TNF antagonist is selected from the group consisting of etanercept, infliximab, D2E7, CDP 571, or thalidomide; and the IL-1 antagonist is either IL-1 RA or IL-1R type II receptor. Antiviral agents may be added for treating certain patients.
Owner:TACT IP
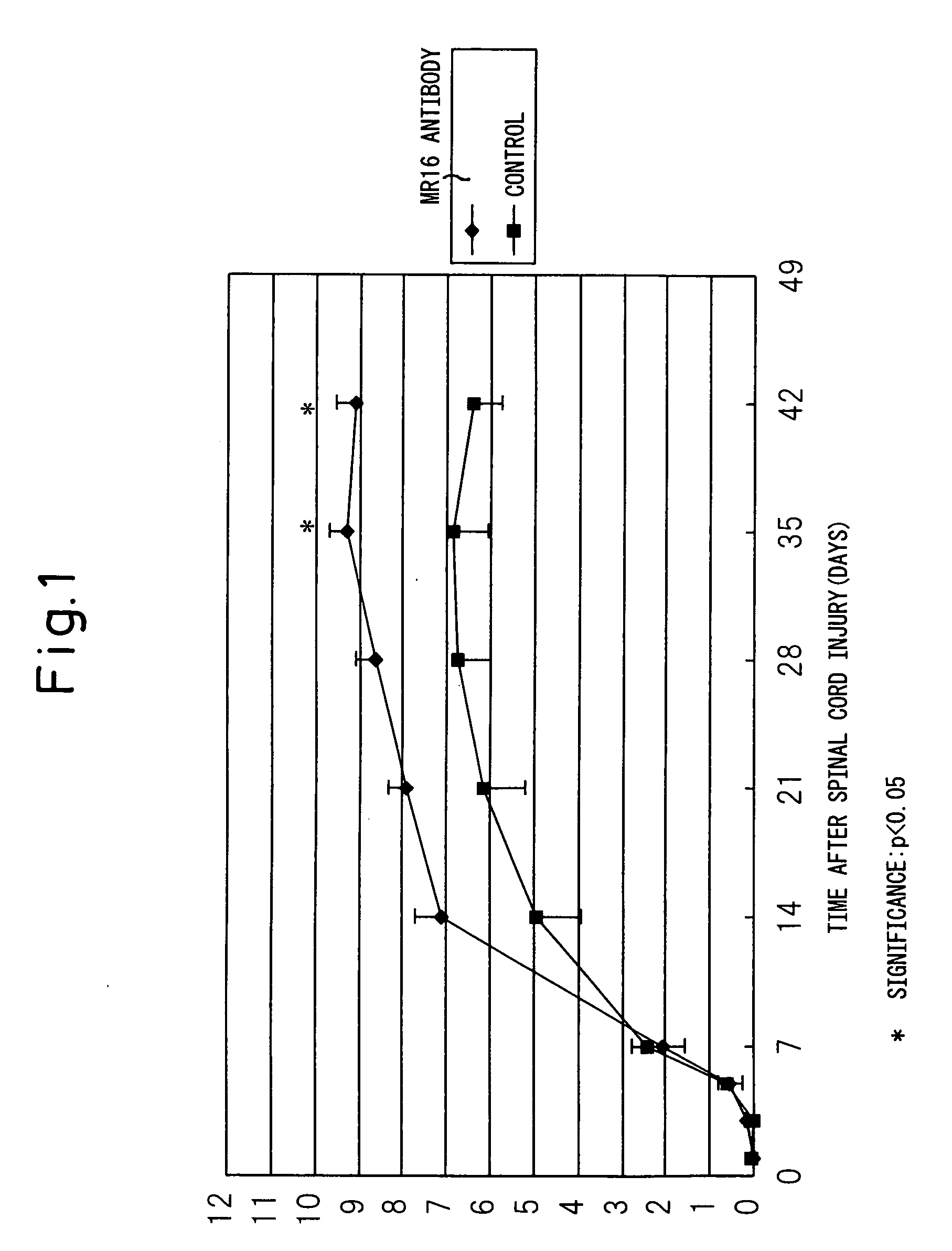
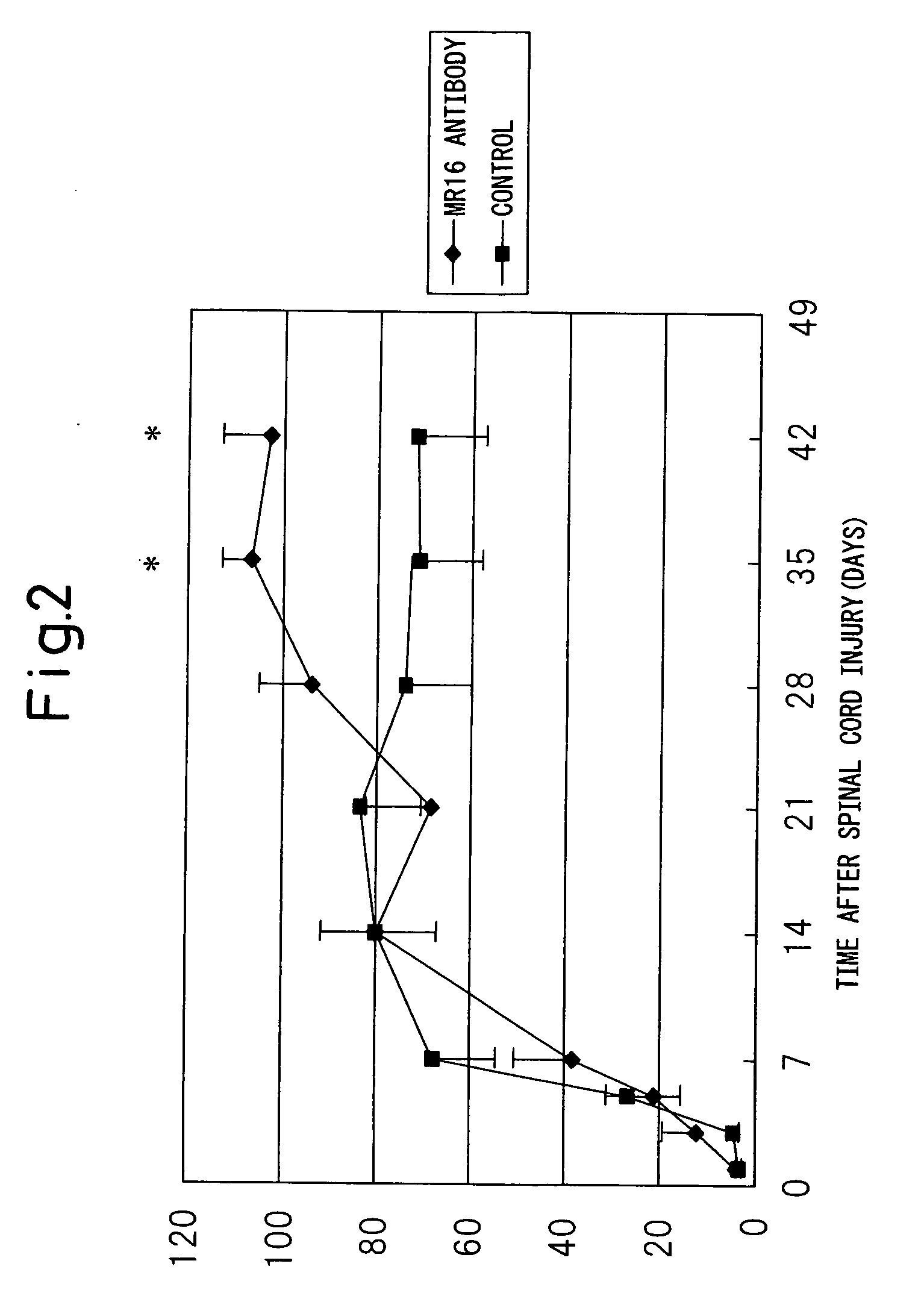
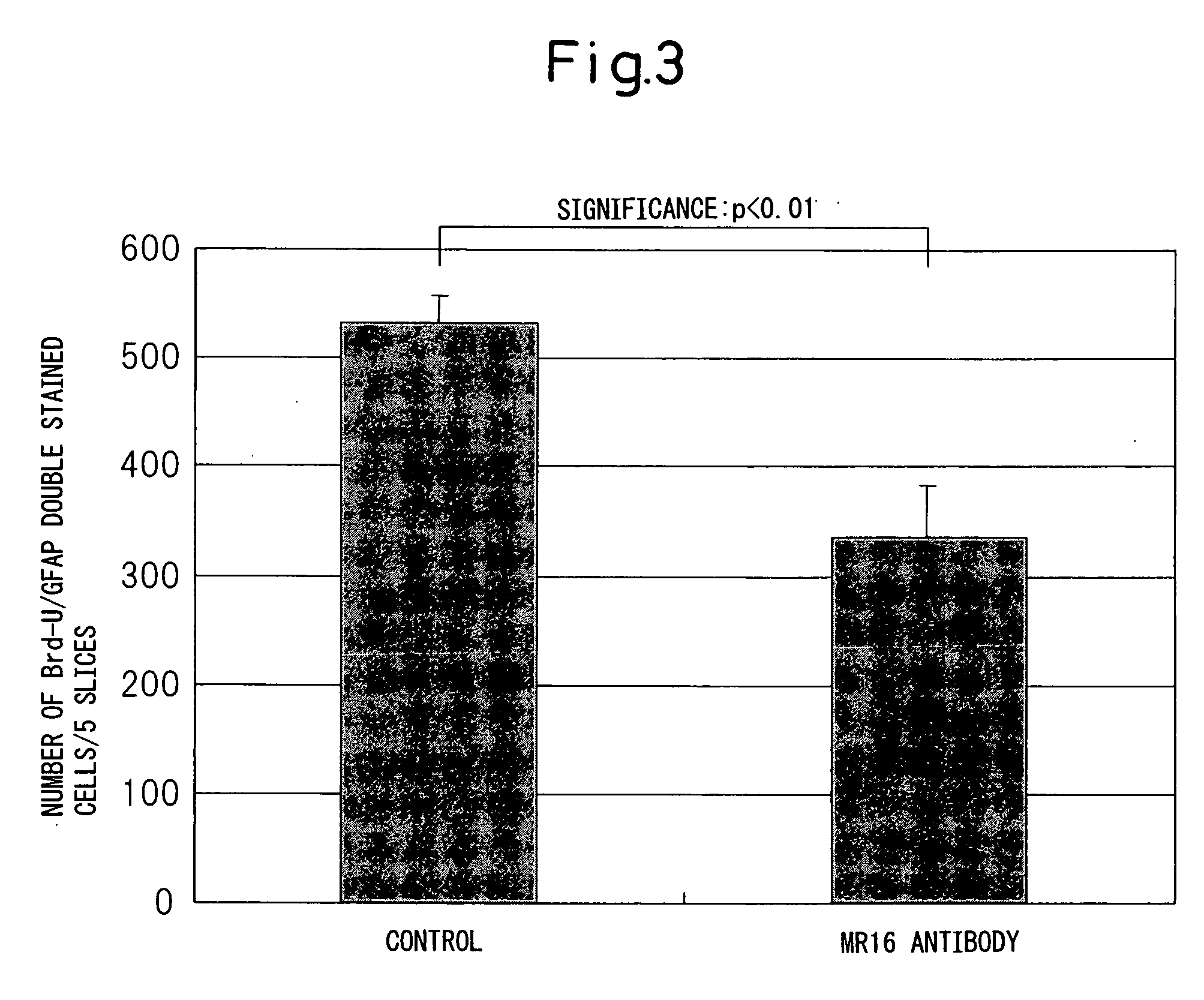
Remedy for spinal injury containing interleukin-6 antagonist
InactiveUS20060165696A1RecoveryPromote recoveryNervous disorderPeptide/protein ingredientsInterleukin 6Medicine
A therapeutic agent for spinal cord injury, a modulator of differentiation of neural stem cells and an inhibitor of differentiation into glia cells comprising an interleukin-6 antagonist as an active ingredient.
Owner:CHUGAI PHARMA CO LTD +1
Method of prevention and treatment of aging and age-related disorders including atherosclerosis, peripheral vascular disease, coronary artery disease, osteoporosis, arthritis, type 2 diabetes, dementia, alzheimer's disease and cancer
This invention relates to a method for prevention and treatment of aging and age-related disorders including atherosclerosis, peripheral vascular disease, coronary artery disease, osteoporosis, type 2 diabetes, dementia and some forms of arthritis and cancer in a subject comprising administering to said subject, separately, sequentially or simultaneously a therapeutically effective dosage of each component or combination of statins, bisphosphonates, cholesterol lowering agents or techniques, interleukin-6 inhibitor / antibody, interleukin-6 receptor inhibitor / antibody, interleukin-6 antisense oligonucleotide (ASON), gp130 protein inhibitor / antibody, tyrosine kinases inhibitors / antibodies, serine / threonine kinases inhibitors / antibodies, mitogen-activated protein (MAP) kinase inhibitors / antibodies, phosphatidylinositol 3-kinase (PI3K) inhibitors / antibodies, Nuclear factor κB (NF-κB) inhibitors / antibodies, IκB kinase (IKK) inhibitors / antibodies, activator protein-1 (AP-1) inhibitors / antibodies, STAT transcription factors inhibitors / antibodies, altered IL-6, partial peptides of IL-6 or IL-6 receptor, or SOCS (suppressors of cytokine signaling) protein, or a functional fragment thereof, administered separately, in sequence or simultaneously. Inhibition of the signal transduction pathway for Interleukin 6 mediated inflammation is key to the prevention and treatment of atherosclerosis, peripheral vascular disease, coronary artery disease, aging and age-related disorders including osteoporosis, type 2 diabetes, dementia and some forms of arthritis and tumors. Inhibition of Interleukin 6 mediated inflammation may be achieved indirectly through regulation of endogenous cholesterol synthesis and isoprenoid depletion or by direct inhibition of the signal transduction pathway utilizing interleukin-6 inhibitor / antibody, interleukin-6 receptor inhibitor / antibody, interleukin-6 antisense oligonucleotide (ASON), gp130 protein inhibitor / antibody, tyrosine kinases inhibitors / antibodies, serine / threonine kinases inhibitors / antibodies, mitogen-activated protein (MAP) kinase inhibitors / antibodies, phosphatidylinositol 3-kinase (PI3K) inhibitors / antibodies, Nuclear factor κB (NF-κB) inhibitors / antibodies, IκB kinase (IKK) inhibitors / antibodies, activator protein-1 (AP-1) inhibitors / antibodies, STAT transcription factors inhibitors / antibodies, altered IL-6, partial peptides of IL-6 or IL-6 receptor, or SOCS (suppressors of cytokine signaling) protein, or a functional fragment thereof. Said method for prevention and treatment of said disorders is based on inhibition of Interleukin-6 inflammation through regulation of cholesterol metabolism, isoprenoid depletion and / or inhibition of the signal transduction pathway.
Owner:OMOIGUI OSEMWOTA SOTA
Reshaped human antibody to human interleukin-6
InactiveUS6121423AUseful purposeAntibody mimetics/scaffoldsImmunoglobulins against cytokines/lymphokines/interferonsDiseaseV region
A reshaped antibody comprising: (A) L chains comprising: (1) a human C region, and (2) an L chain V region comprising human L chain FRs and L chain CDRs of a mouse monoclonal antibody; and (B) H chains comprising: (1) a human H chain C region, and (2) an H chain V region comprising human H chain FRs, and H chain CDRs of a mouse monoclonal antibody to human IL-6. Since the major portions of the reshaped human antibody are derived from human, and the mouse CDRs are less immunogenic, then the present reshaped human antibody is less immunogenic, and therefore inhibits information transfer by IL-6, and is promising as a therapeutic agent for diseases caused by IL-6.
Owner:CHUGAI PHARMA CO LTD
Trans-capsular administration of high specificity cytokine inhibitors into orthopedic joints
ActiveUS20050025765A1Extended half-lifeEliminate side effectsOrganic active ingredientsPeptide/protein ingredientsWhite blood cellOrthopedic department
The present invention relates to trans-capsularly administering into a diseased joint a high specificity antagonist selected from the group consisting of: i) an inhibitor of a pro-inflammatory interleukin; ii) an inhibitor of TNF-α synthesis; iii) an inhibitor of membrane-bound TNF-α; iv) an inhibitor of a natural receptor of TNF-α; v) an inhibitor of NO synthase, vi) an inhibitor of PLA2 enzyme; vii) an anti-proliferative agent; viii) an anti-oxidant; ix) an apoptosis inhibitor selected from the group consisting of EPO mimetic peptides, EPO mimetibodies, IGF-I, IGF-II, and caspase inhibitors, and x) an inhibitor of MMPs; and xi) an inhibitor of p38 kinase.
Owner:DEPUY SYNTHES PROD INC
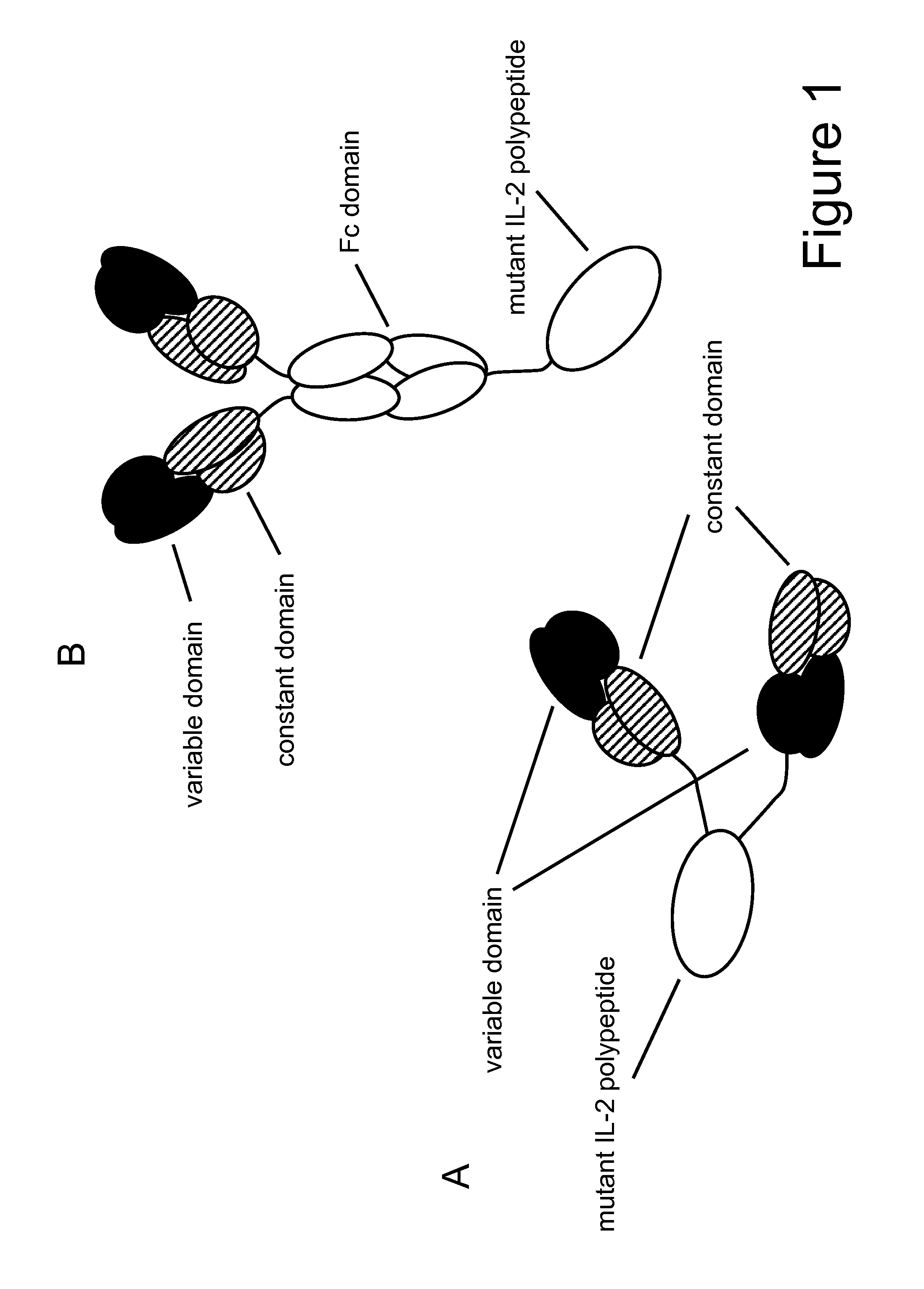
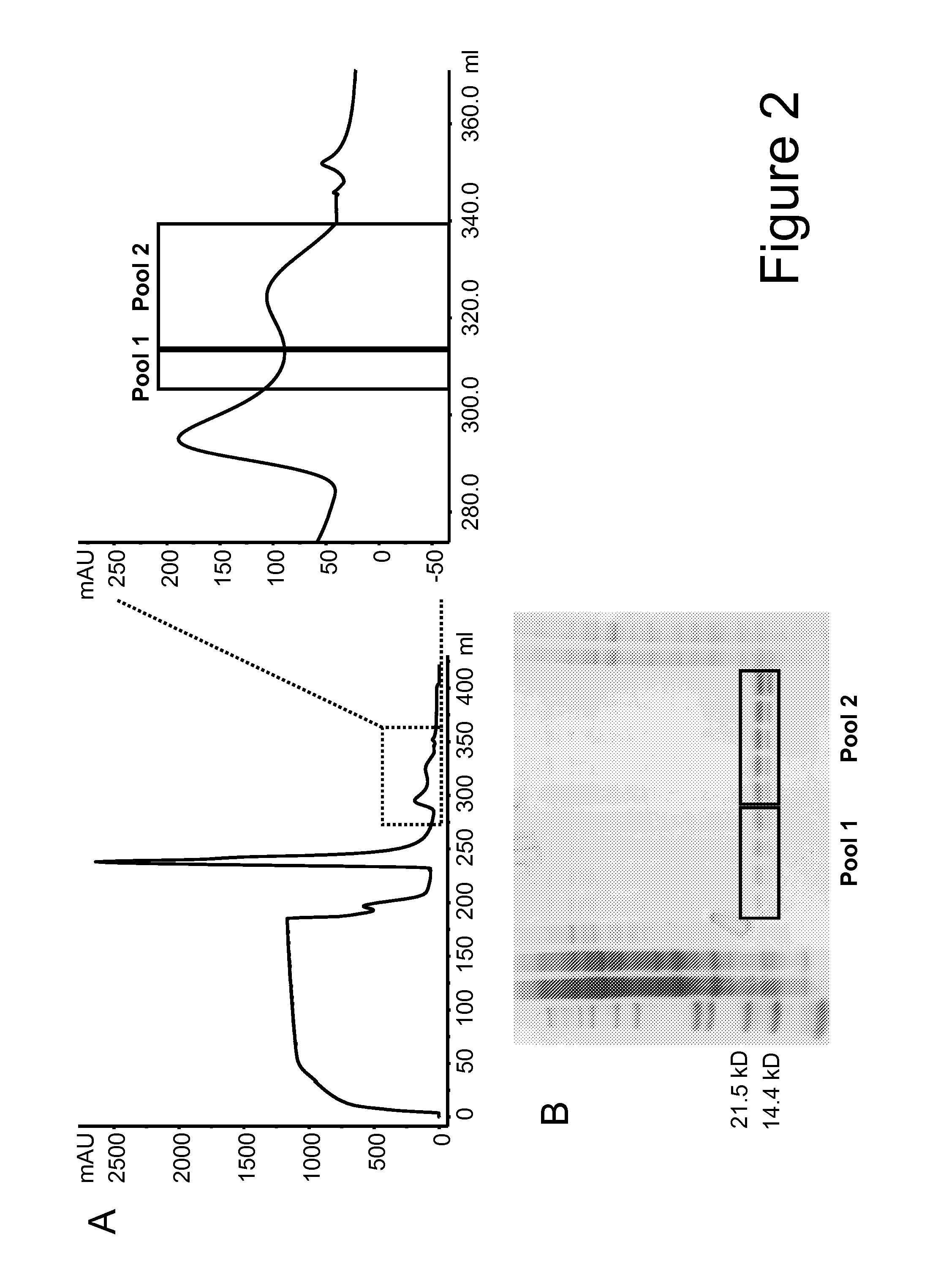
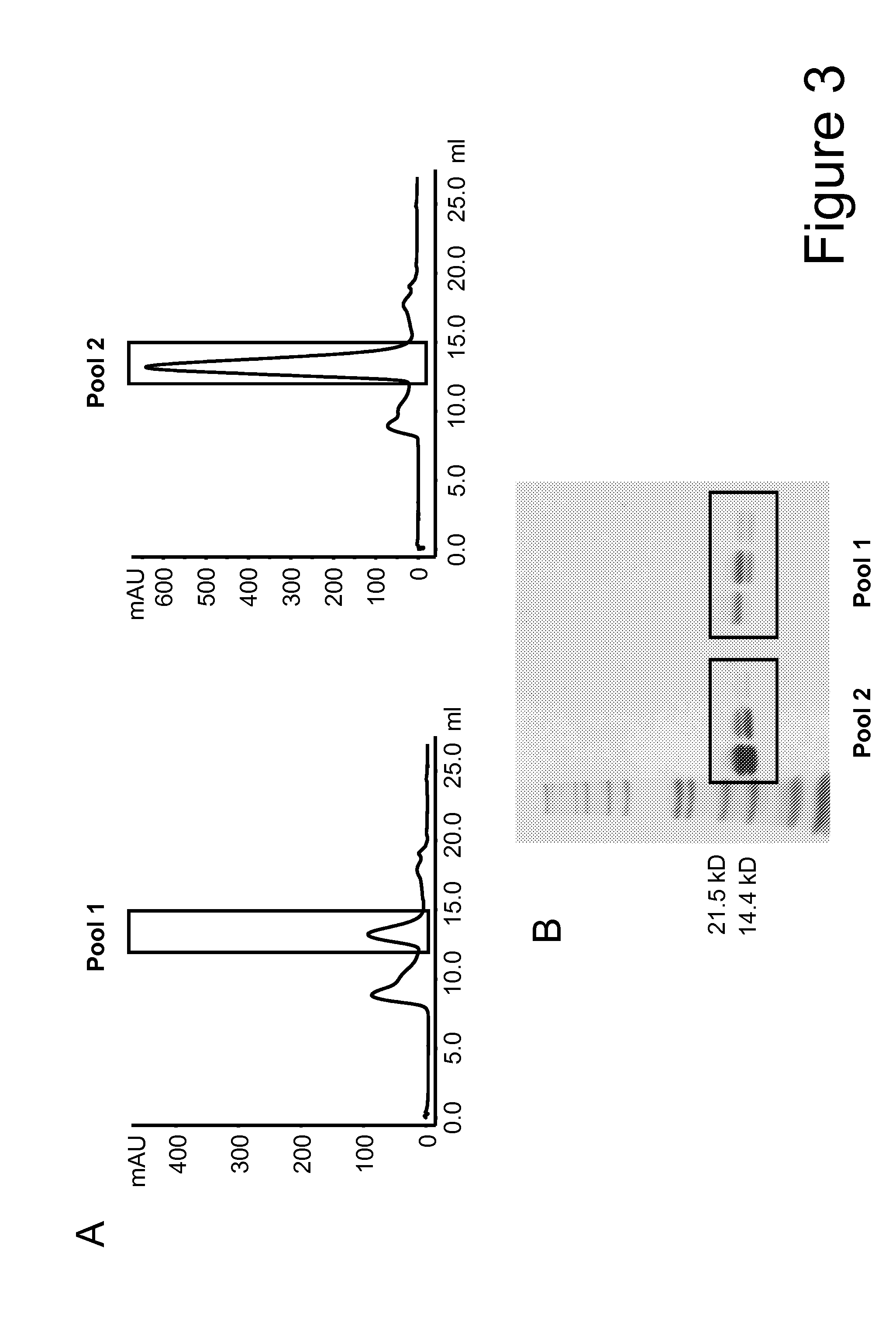
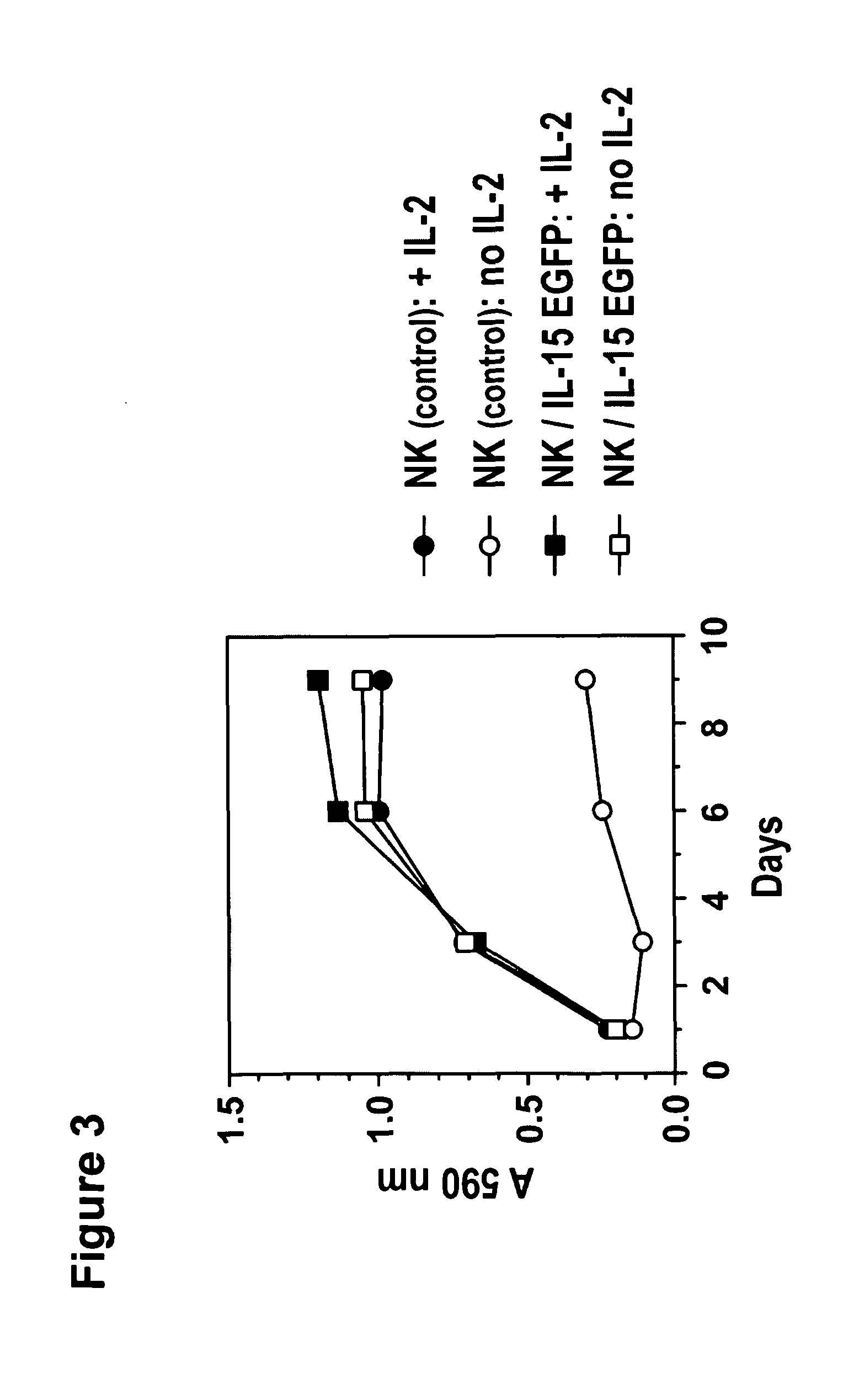
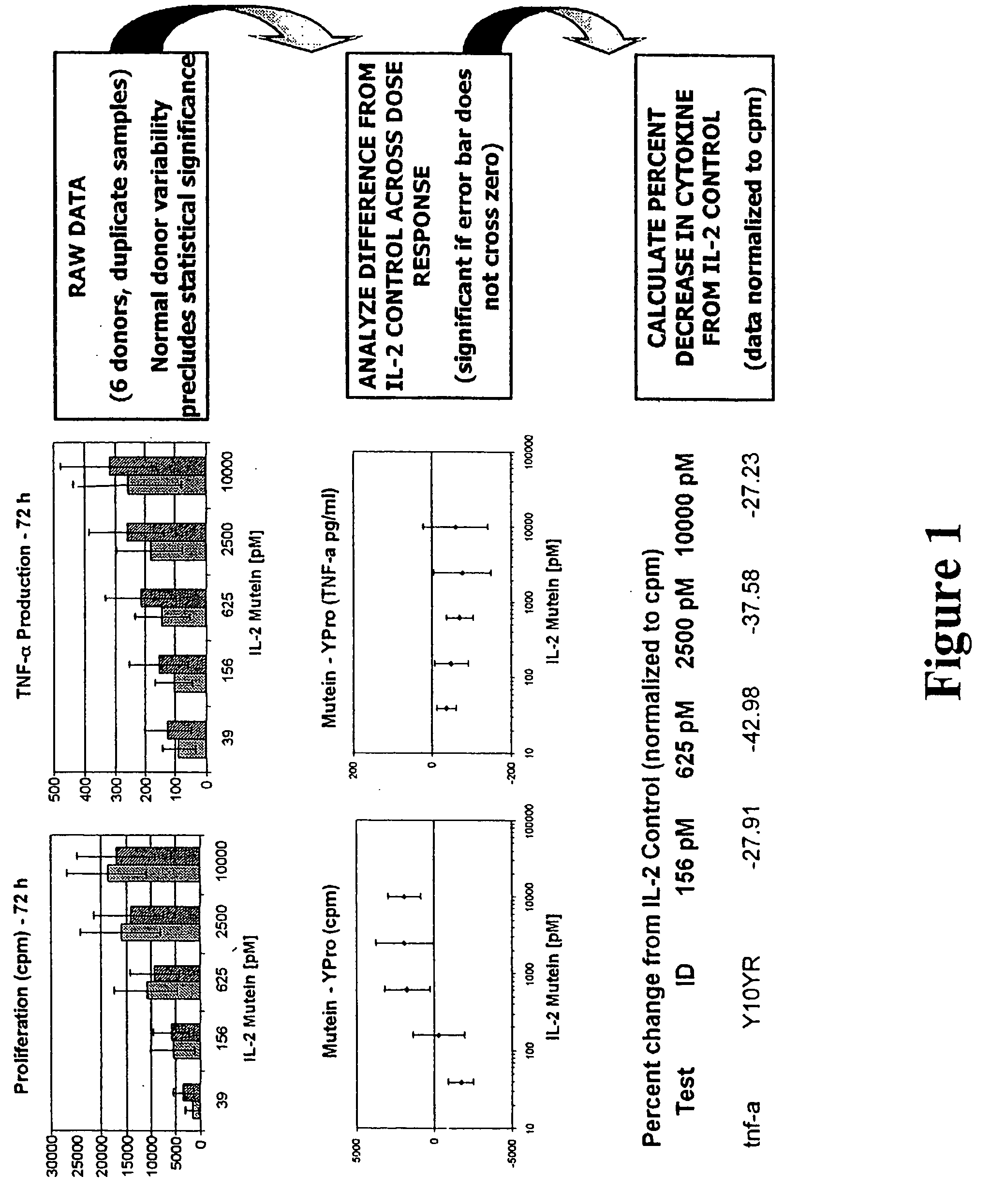
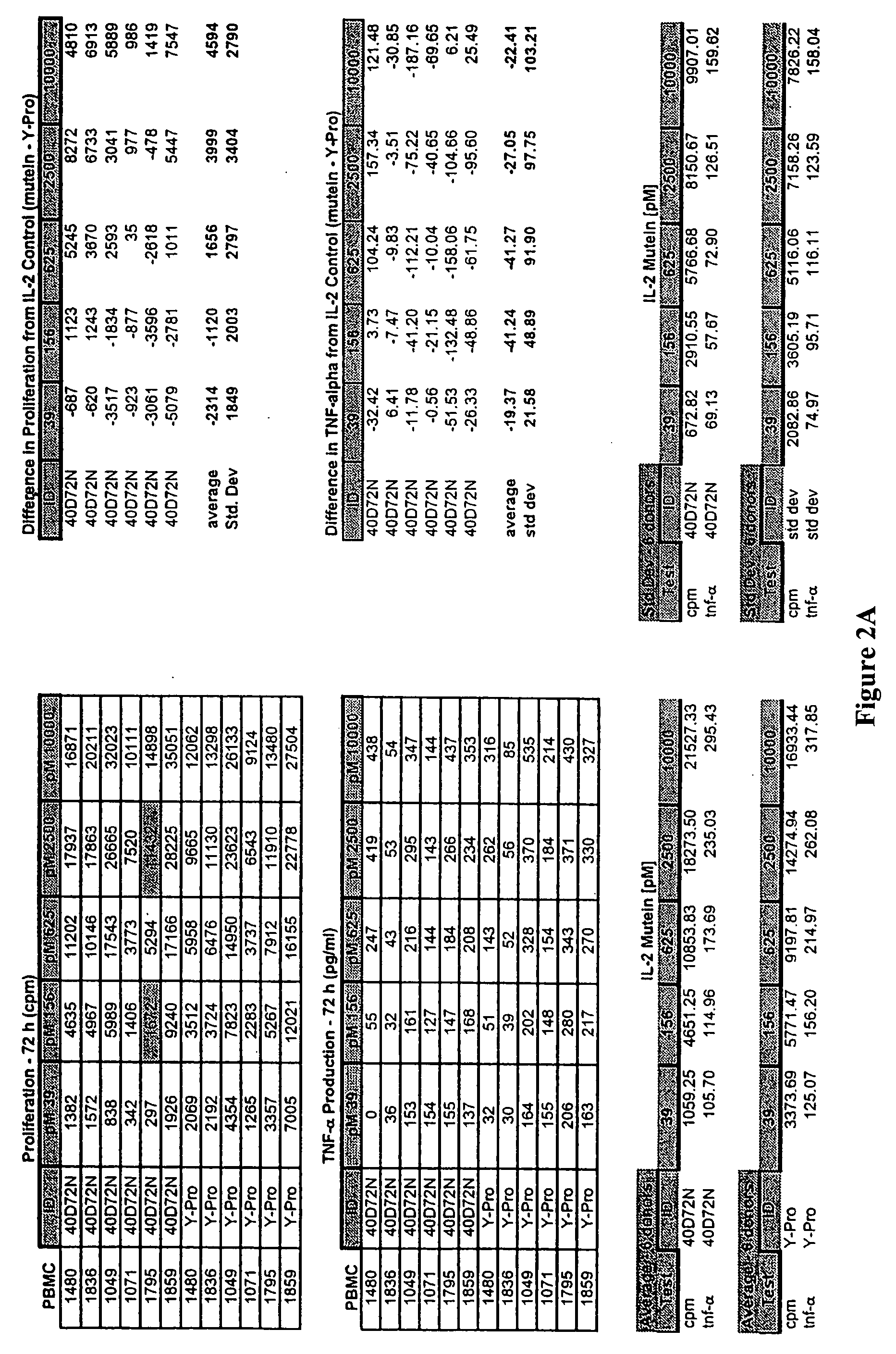
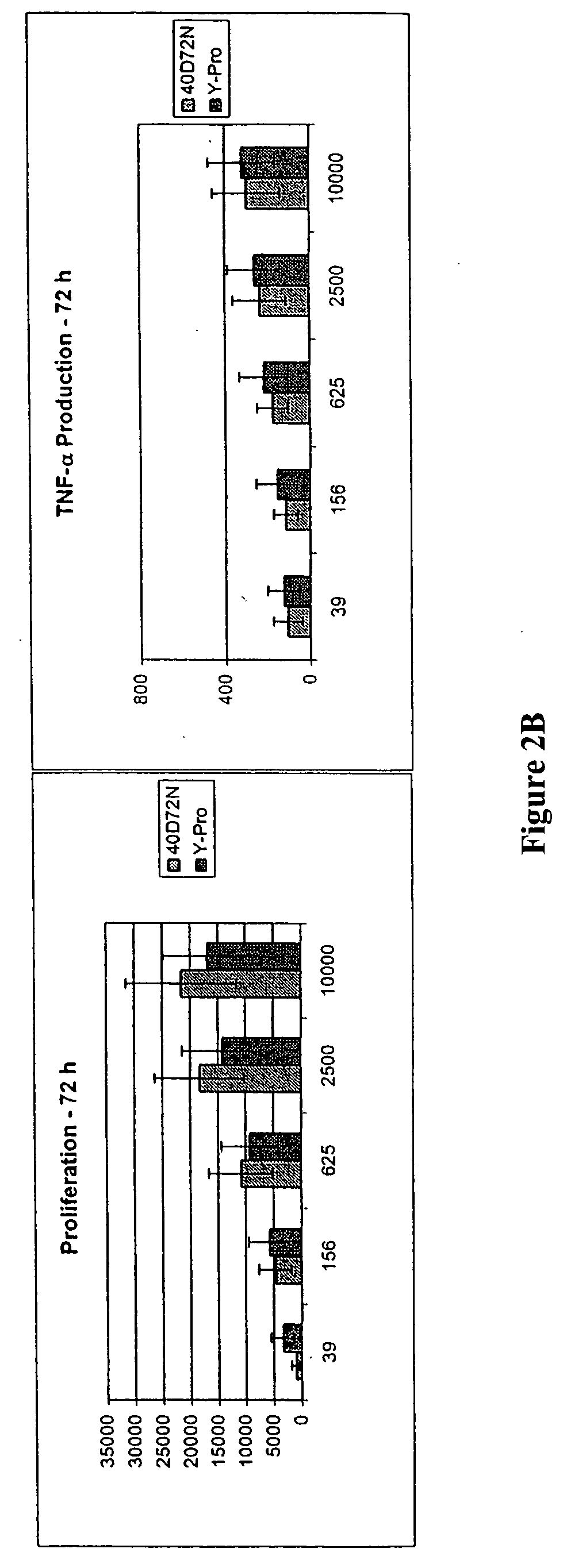
Mutant interleukin-2 polypeptides
ActiveUS20120244112A1Eliminates and decrease and delayEliminates and decrease and and and effectPeptide/protein ingredientsAntibody mimetics/scaffoldsImmunotherapeutic agentReceptor
The present invention generally relates to mutant interleukin-2 polypeptides that exhibit reduced affinity to the α-subunit of the IL-2 receptor, for use as immunotherapeutic agents. In addition, the invention relates to immunoconjugates comprising said mutant IL-2 polypeptides, polynucleotide molecules encoding the mutant IL-2 polypeptides or immunoconjugates, and vectors and host cells comprising such polynucleotide molecules. The invention further relates to methods for producing the mutant IL-2 polypeptides or immunoconjugates, pharmaceutical compositions comprising the same, and uses thereof.
Owner:ROCHE GLYCART AG
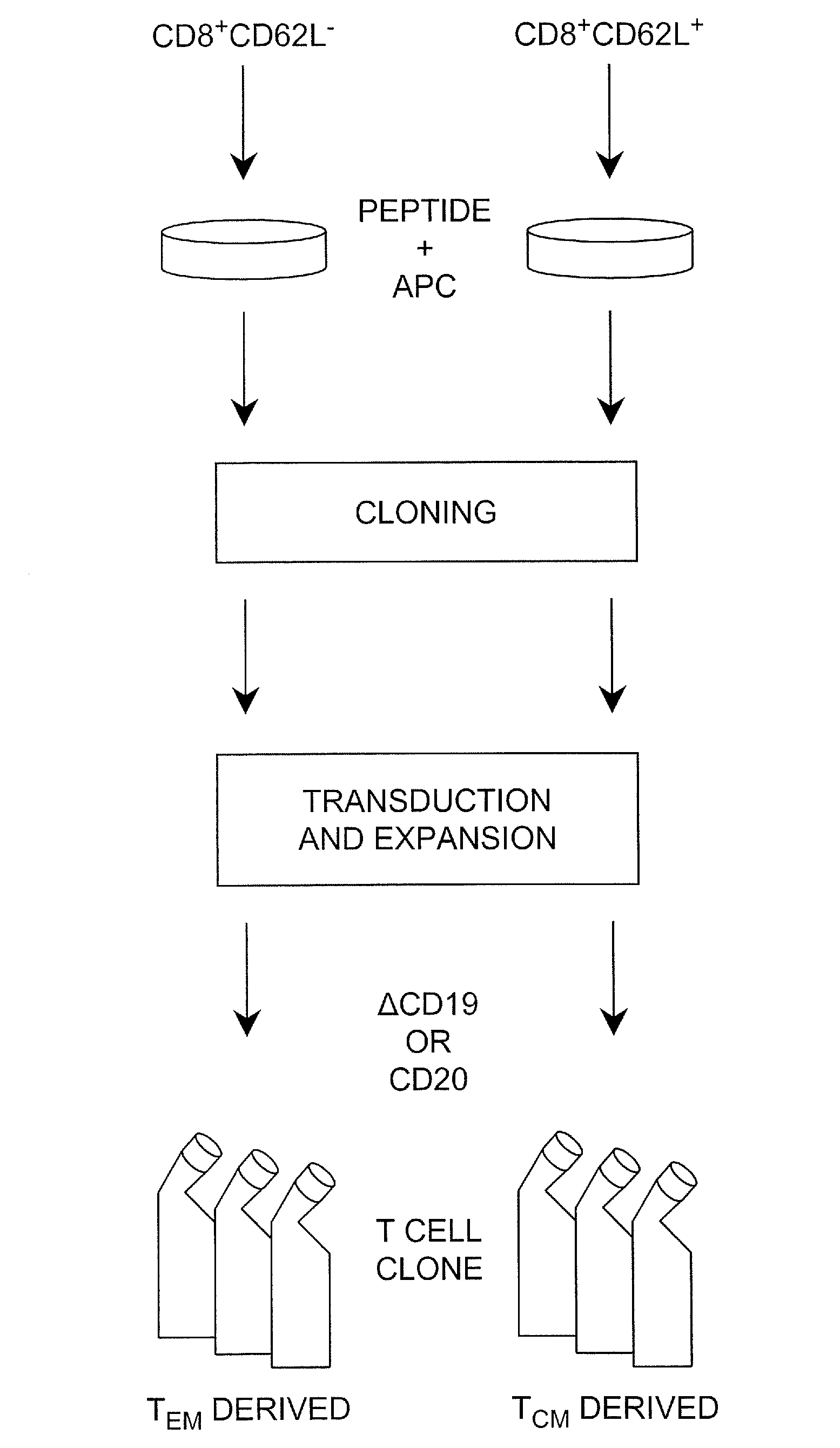
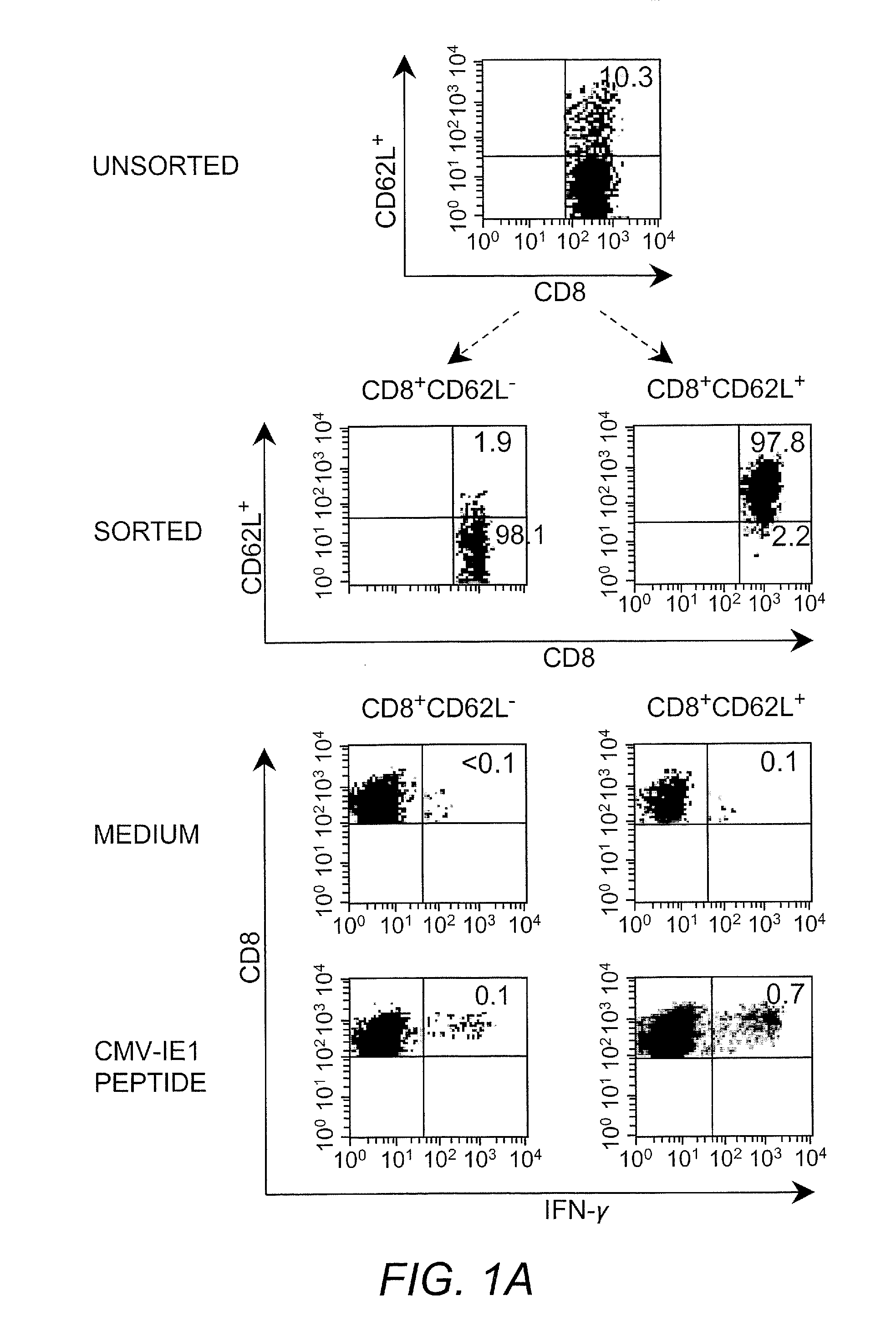
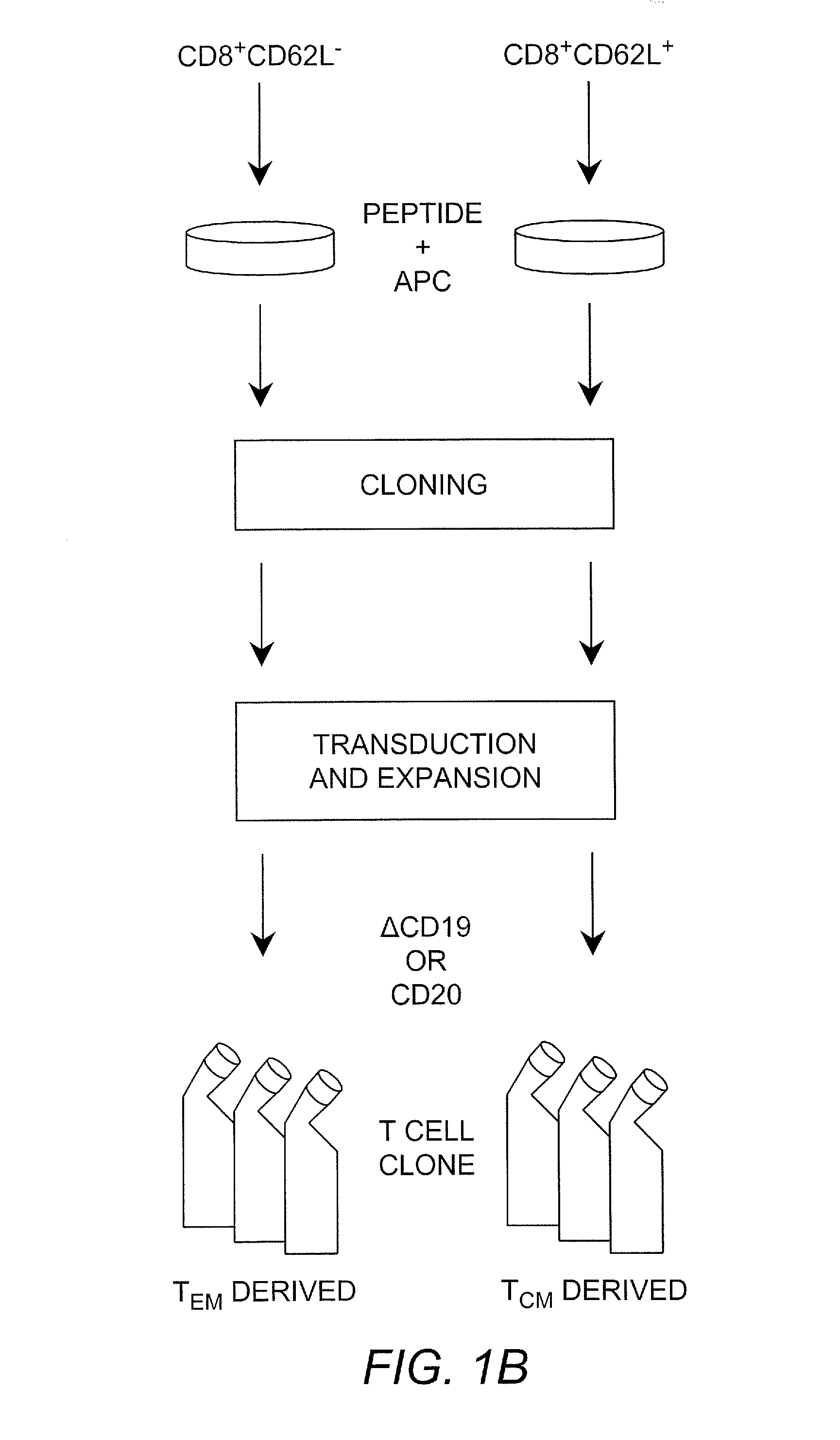
Adoptive transfer of cd8 + t cell clones derived from central memory cells
InactiveUS20080131415A1Increased proliferationBiocideArtificial cell constructsWhite blood cellPharmaceutical formulation
The present invention provides a method of carrying out adoptive immunotherapy in a primate subject in need thereof by administering the subject a cytotoxic T lymphocytes (CTL) preparation in a treatment-effective amount. The method comprises administering as the CTL preparation a preparation consisting essentially of an in vitro expanded primate CTL population, the CTL population enriched prior to expansion for central memory T lymphocytes, and depleted prior to expansion of effector memory T lymphocytes. In some embodiments, the method may further comprise concurrently administering Interleukin-15 to the subject in an amount effective to increase the proliferation of the central memory T cells in the subject. Pharmaceutical formulations produced by the method, and methods of using the same, are also described.
Owner:CITY OF HOPE +1
Method of prevention and treatment of Atherosclerosis, Peripheral vascular disease, Coronary artery disease, aging and age-related disorders including osteoporosis, arthritis, type 2 diabetes, dementia and Alzheimer's disease
InactiveUS20060078532A1BiocidePhosphorous compound active ingredientsInterleukin 6Age related disease
This invention relates to a method for prevention and treatment of Atherosclerosis, Peripheral Vascular Disease, Coronary Artery Disease, and age-related disorders including Osteoporosis, Arthritis, Type II Diabetes, Dementia and Alzheimer's disease in a subject comprising administering to said subject a therapeutically effective dosage of each component or combination of statins, bisphosphonates, cholesterol lowering agents or techniques, interleukin-6 inhibitor / antibody, interleukin-6 receptor inhibitor / antibody, gp130 protein inhibitor / antibody, tyrosine kinases inhibitors / antibodies, STAT transcription factors inhibitors / antibodies, altered IL-6, partial peptides of IL-6 or IL-6 receptor, or SOCS (suppressors of cytokine signaling) protein, or a functional fragment thereof, administered separately, in sequence or simultaneously. Inhibition of the signal transduction pathway for Interleukin 6 mediated inflammation is key to the prevention and treatment of atherosclerosis, peripheral vascular disease, coronary artery disease, aging and age-related disorders including osteoporosis, type 2 diabetes, dementia and some forms of arthritis and tumors. Inhibition of Interleukin 6 mediated inflammation may be achieved indirectly through regulation of endogenous cholesterol synthesis and isoprenoid depletion or by direct inhibition of the signal transduction pathway including interleukin-6 inhibitor / antibody, interleukin-6 receptor inhibitor / antibody, gp130 protein inhibitor / antibody, tyrosine kinases inhibitors / antibodies, STAT transcription factors inhibitors / antibodies, altered IL-6, partial peptides of IL-6 or IL-6 receptor, or SOCS (suppressors of cytokine signaling) protein, or a functional fragment thereof. Said method for prevention and treatment of said disorders is based on inhibition of Interleukin-6 inflammation through regulation of cholesterol metabolism, isoprenoid depletion and inhibition of the signal transduction pathway.
Owner:OMOIGUI OSEMWOTA SOTA
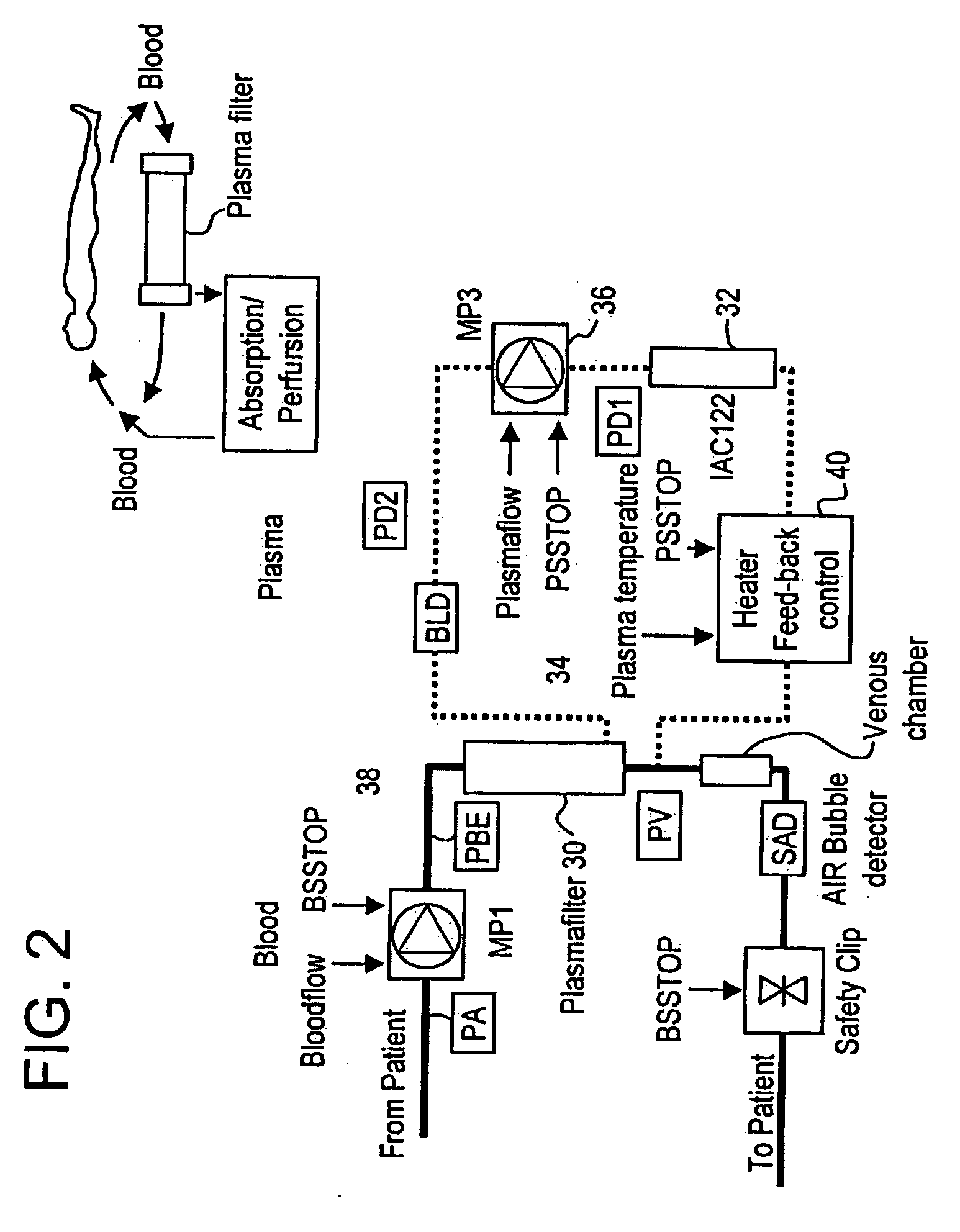
Septicemia prevention and treatment system
InactiveUS6193681B1Electrolysis componentsOther blood circulation devicesStaphylococcus cohniiFiltration
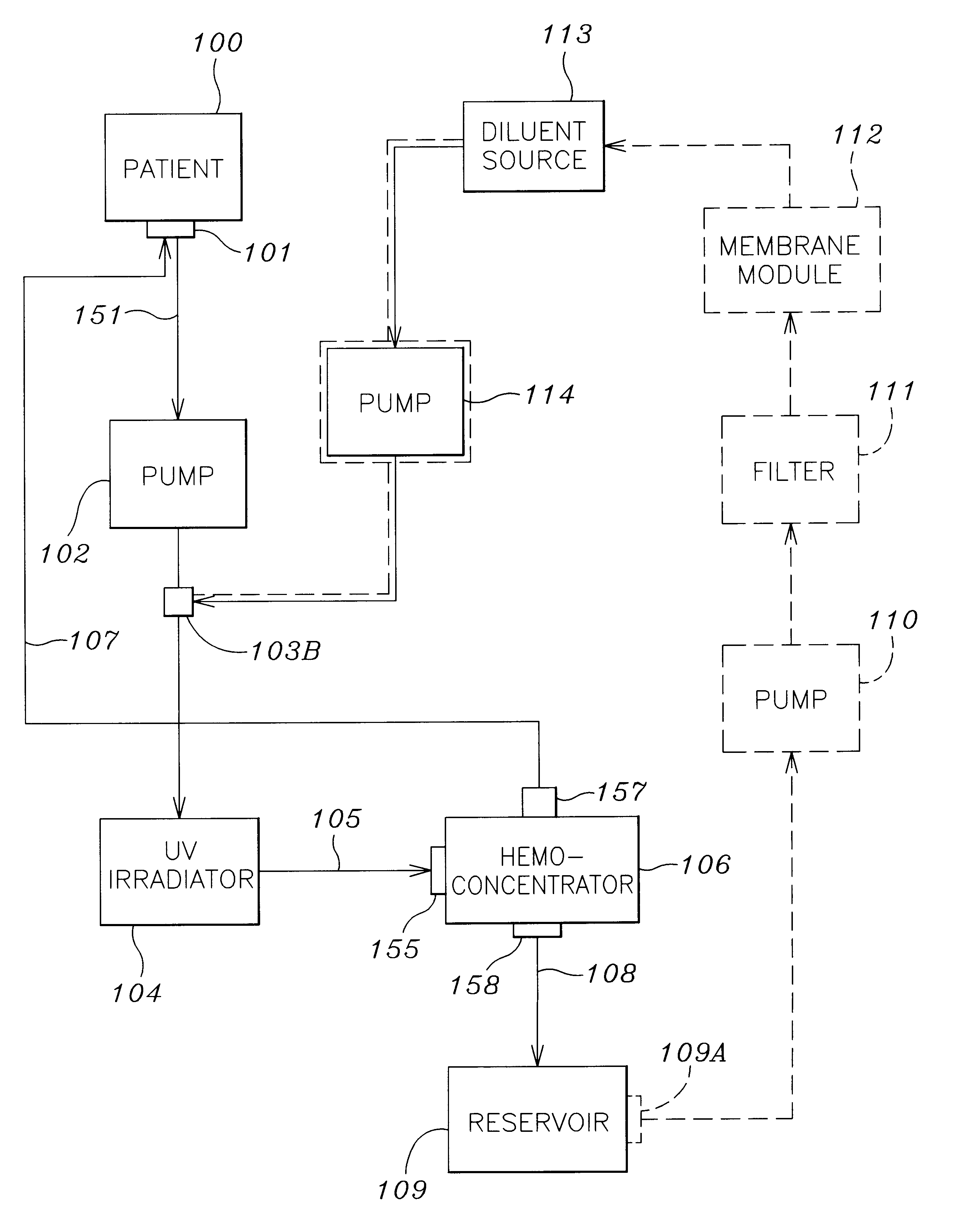
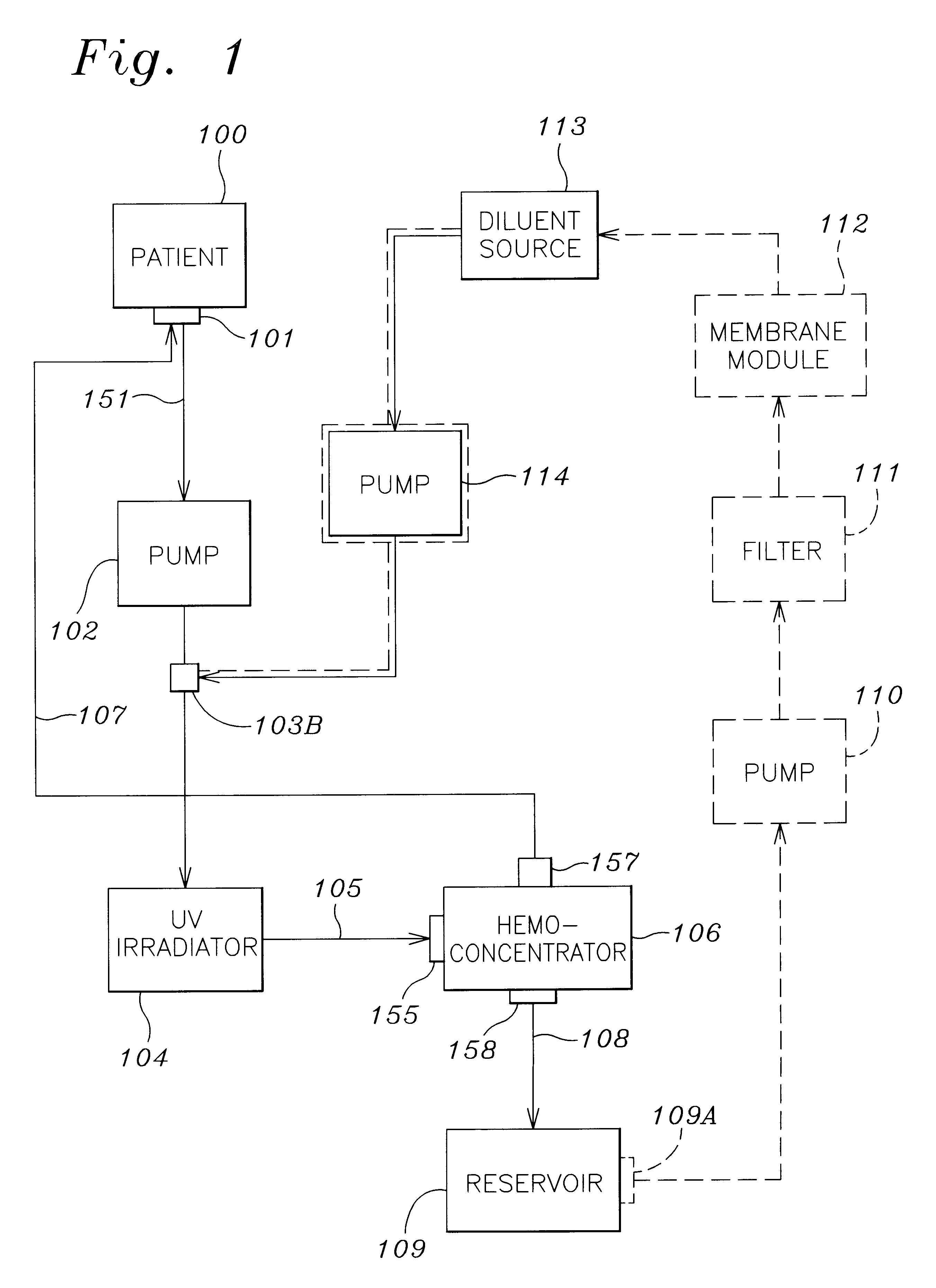
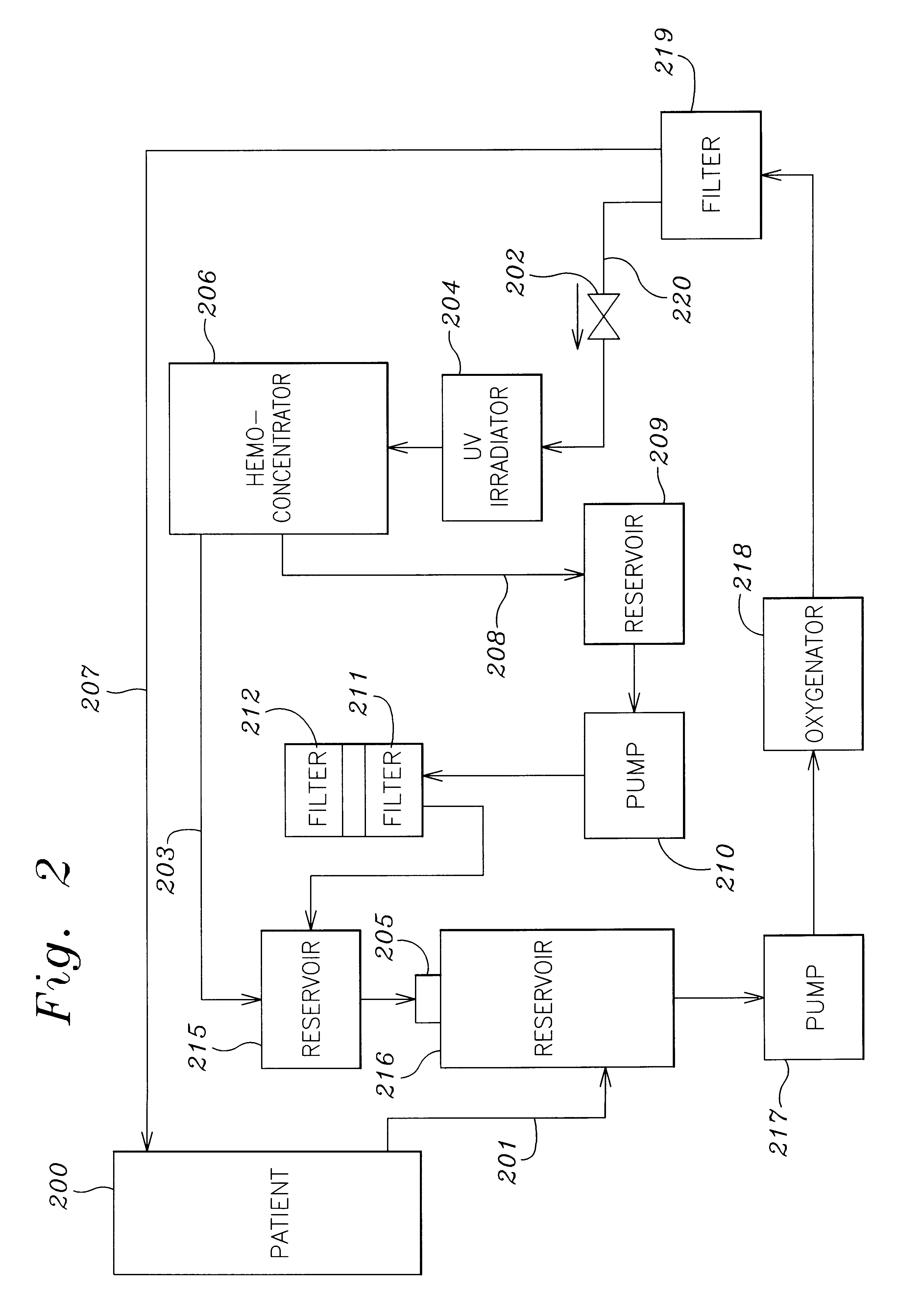
A method and apparatus for preventing and treating septicemia in patient blood. The extracorporeal system includes an anti-microbial device to kill at least 99% of bloodborne microorganisms, a hemoconcentrator / filtration unit to remove approximately 90% of target molecules from the patient blood and a filter unit to remove target molecules from patient blood from the sieved plasma filtrate. Target molecules are produced by microorganisms as well as the patient's cells and include endotoxins from gram negative bacteria, exotoxins from gram negative and gram positive bacteria, as well as RAP protein mediator from Staphylococcus aureus, and cell mediators such as tumor necrosis factor-alpha, and interleukin 1-beta, complement proteins C3a and C5a, and brandykinin.
Owner:HEMAVATION
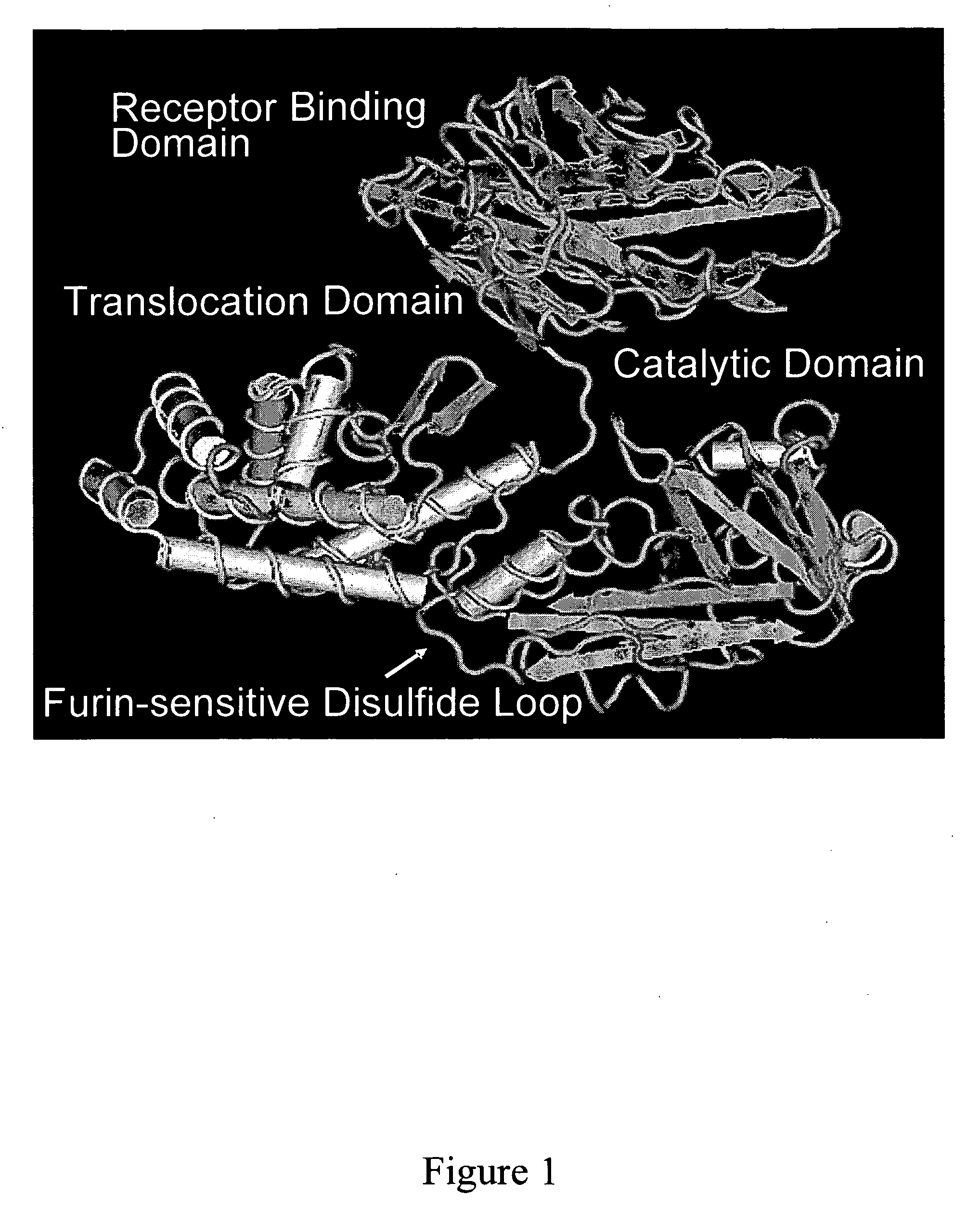
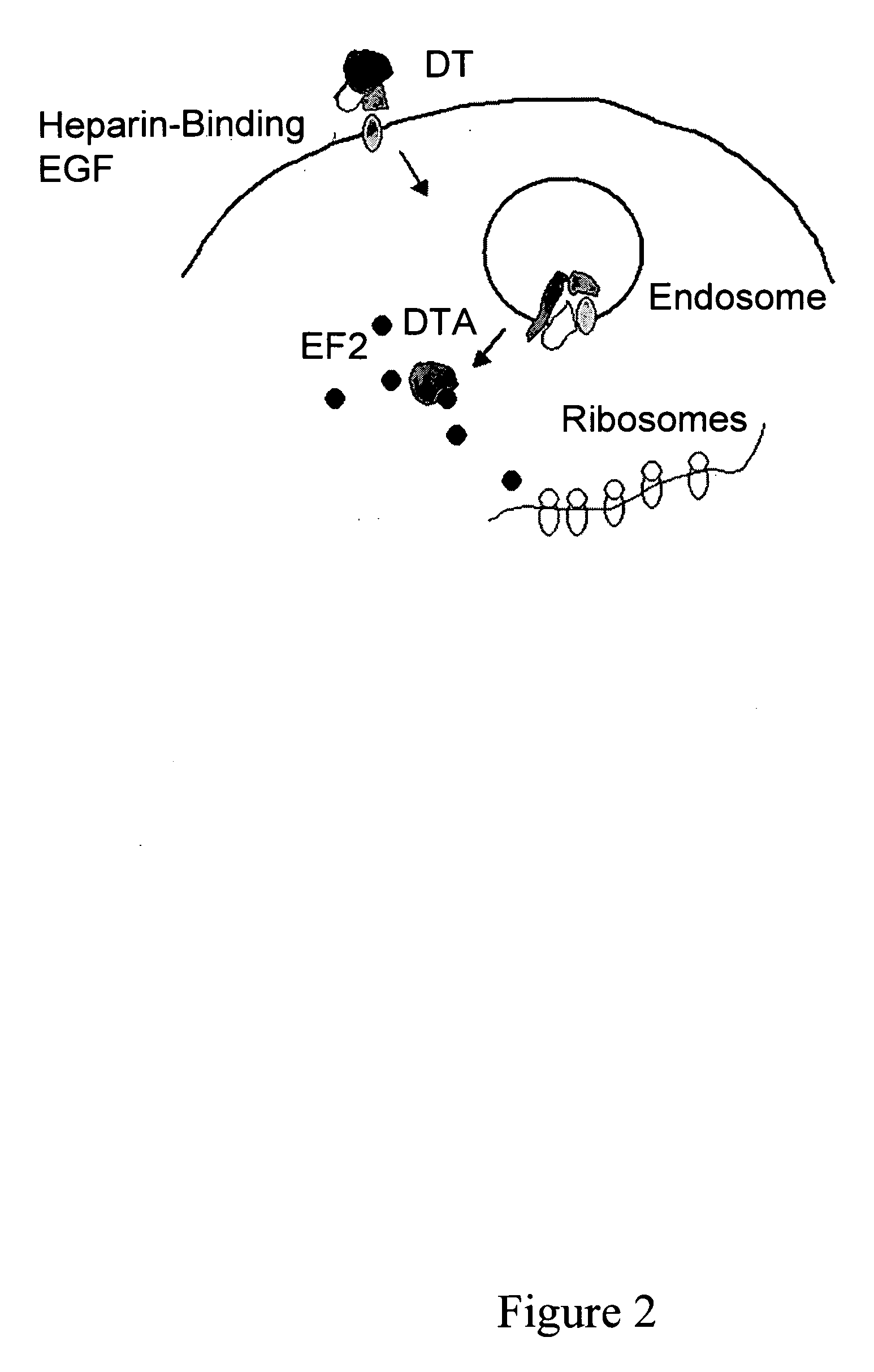
Methods and compositions based on diphtheria toxin-interleukin-3 conjugates
ActiveUS20080138313A1Enhance or improve the prophylactic effect(s) of another therapyShorten the durationBiocidePeptide/protein ingredientsCancer cellWhite blood cell
The present invention provides methods for inhibiting interleukin-3 receptor-expressing cells, and, in particular, inhibiting the growth of such cells by using a diphtheria toxin-human interleukin-3 conjugate (DT-IL3) that is toxic to cells expressing the interleukin-3 receptor. In preferred embodiments, the DT-IL3 conjugate is a fusion protein comprising amino acids 1-388 of diphtheria toxin fused via a peptide linker to full-length, human interleukin-3. In certain embodiments, the methods of the present invention relate to the administration of a DT-IL3 conjugate to inhibit the growth of cancer cells and / or cancer stem cells in humans, which cells express one or more subunits of the interleukin-3 receptor. Exemplary cells include myeloid leukemia cancer stem cells. In other embodiments, the methods of the present invention relate to ex vivo purging of bone marrow or peripheral blood to remove cells that express one or more subunits of the interleukin-3 receptor such that the purged bone marrow or peripheral blood is suitable, e.g., for autologous stem cell transplantation to restore hematopoietic function.
Owner:SCOTT & WHITE MEMORIAL HOSPITAL
Pegylated interleukin-10
InactiveUS20060210534A1Minimize disruptionPeptide/protein ingredientsAntipyreticInterleukin 10White blood cell
Interleukin-10 (IL-10) conjugated via a linker to one or more polyethylene glycol (PEG) molecules at a single amino acid residue of the IL-10, and a method for preparing the same, are provided. The method produces a stable mono-pegylated IL-10, which retains IL-10 activity, where pegylation is selective for the N-terminus on one subunit of IL-10 with little or no formation of monomeric IL-10. The method also provides a substantially homogenous population of mono-PEG-IL-10.
Owner:SCHERING CORP
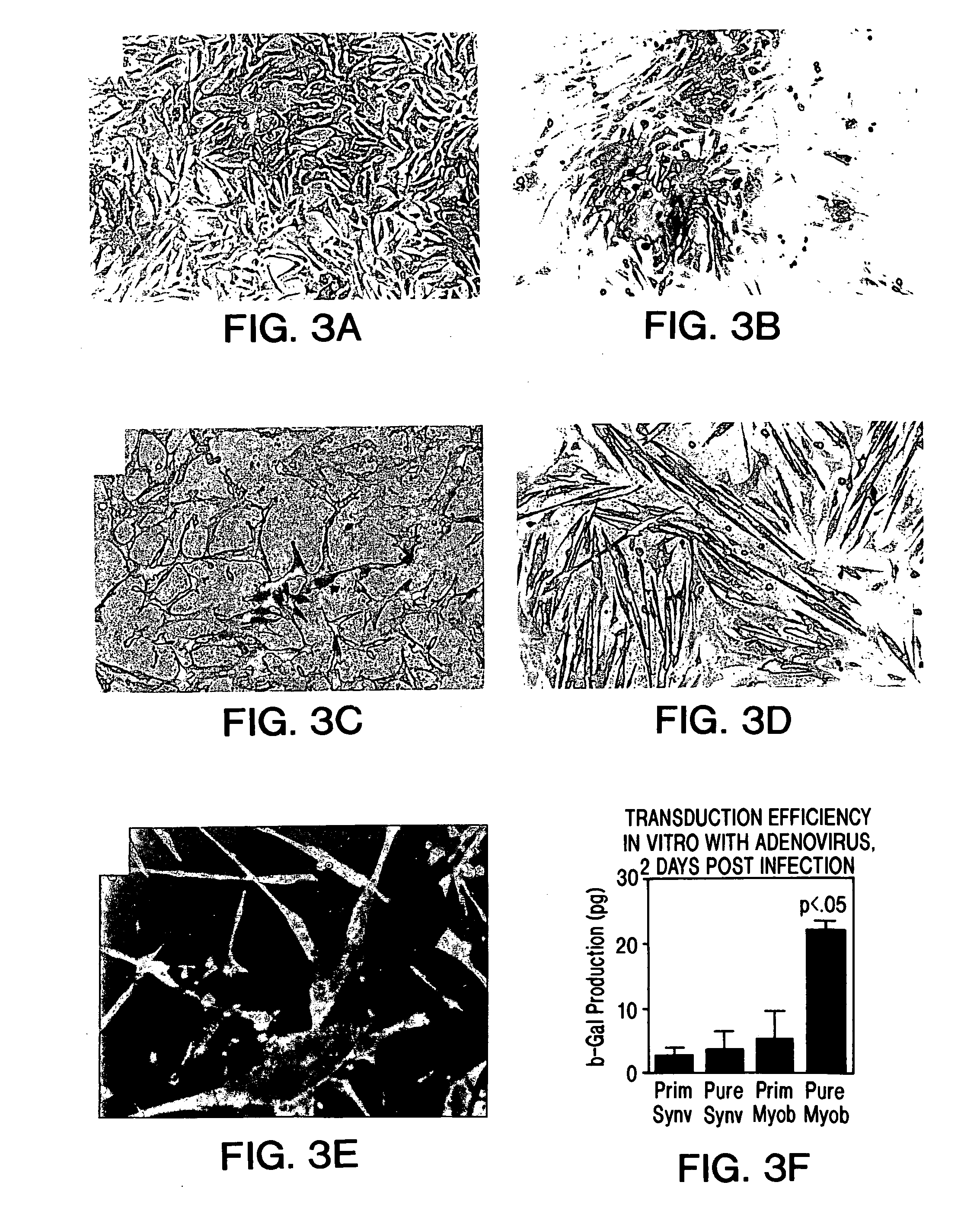
Muscle-derived cells (MDCs) for treating muscle- or bone-related injury or dysfunction
The present invention provides muscle-derived cells, preferably myoblasts and muscle-derived stem cells, genetically engineered to contain and express one or more heterologous genes or functional segments of such genes, for delivery of the encoded gene products at or near sites of musculoskeletal, bone, ligament, meniscus, cartilage or genitourinary disease, injury, defect, or dysfunction. Ex vivo myoblast mediated gene delivery of human inducible nitric oxide synthase, and the resulting production of nitric oxide at and around the site of injury, are particularly provided by the invention as a treatment for lower genitourinary tract dysfunctions. Ex vivo gene transfer for the musculoskeletal system includes genes encoding acidic fibroblast growth factor, basic fibroblast growth factor, epidermal growth factor, insulin-like growth factor, platelet derived growth factor, transforming growth factor-β, transforming growth factor-α, nerve growth factor and interleukin-1 receptor antagonist protein (IRAP), bone morphogenetic protein (BMPs), cartilage derived morphogenetic protein (CDMPs), vascular endothelial growth factor (VEGF), and sonic hedgehog proteins.
Owner:UNIVERSITY OF PITTSBURGH
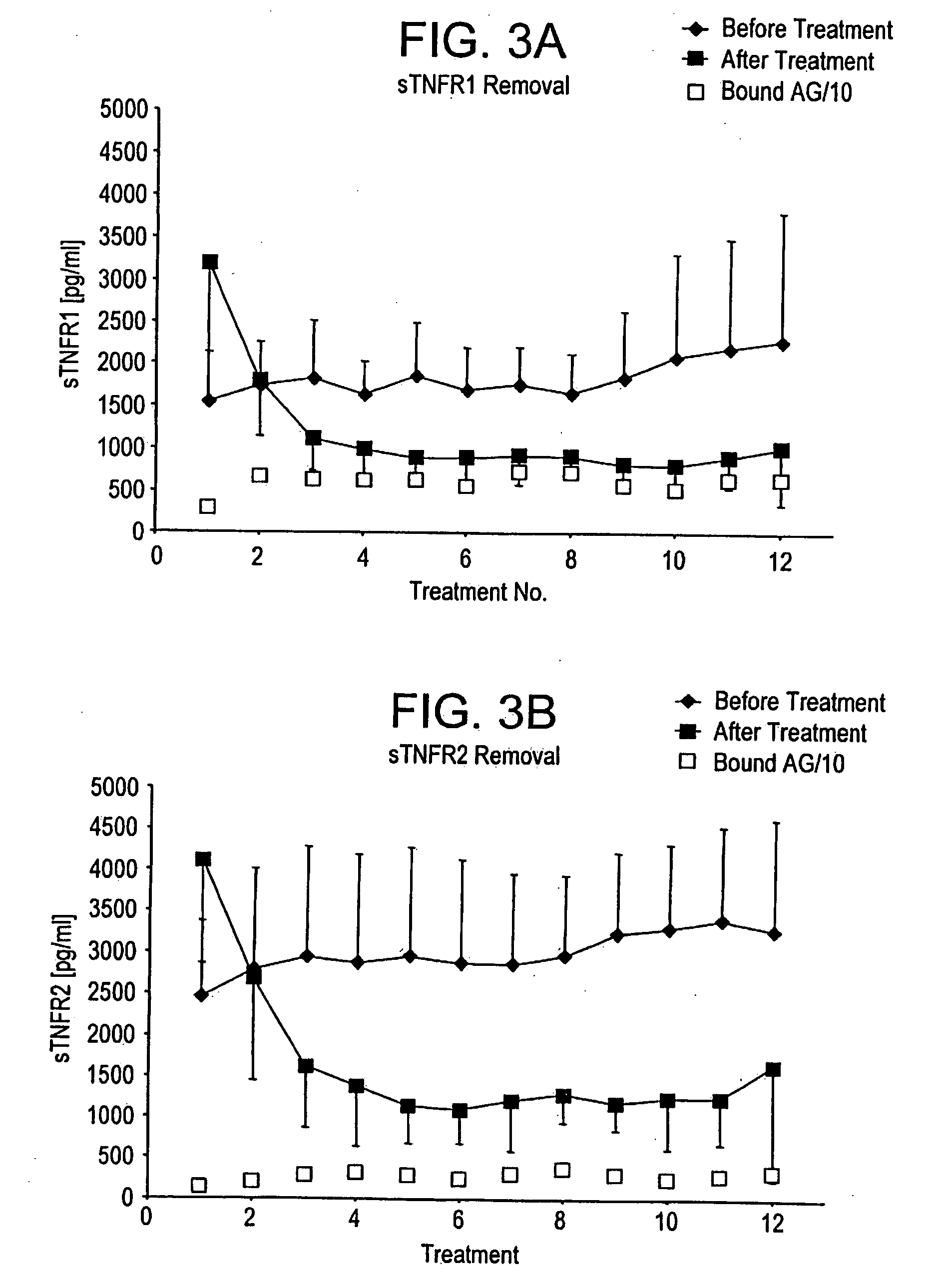
Method and system to remove soluble TNFR1, TNFR2, and IL2 in patients
InactiveUS20050265996A1Induce remissionPeptide/protein ingredientsHaemofiltrationDiseaseAntiendomysial antibodies
A method, and system, to induce remission in diseases characterized by excess production of sTNR and interleukin 2 has been developed. In the most preferred embodiment, the system consists of antibodies to sTNFR1, sTNFR2 and sIL2R immobilized in a column containing a material such as SEPHAROSE™. The patient is connected to a pheresis machine which separates the blood into the plasma and red cells, and the plasma is circulated through the column until the desired reduction in levels of sTNFR1, sTNFR2, and IL2 is achieved, preferably to less than normal levels. In the preferred method, patients are treated three times a week for four weeks. This process can be repeated after a period of time. Clinical studies showed reduction in tumor burden in patients having failed conventional chemotherapy and radiation treatments.
Owner:INNATUS CORP
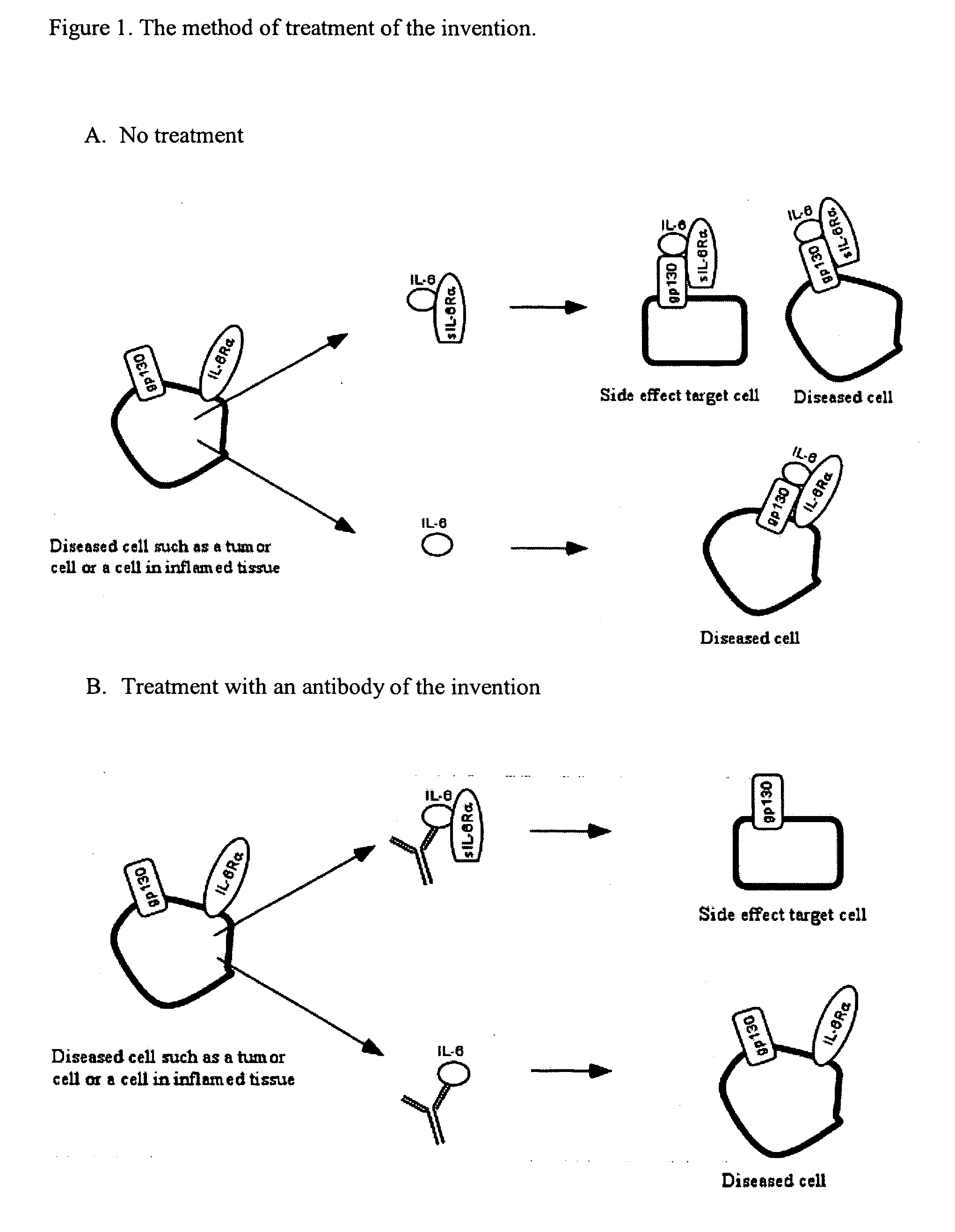
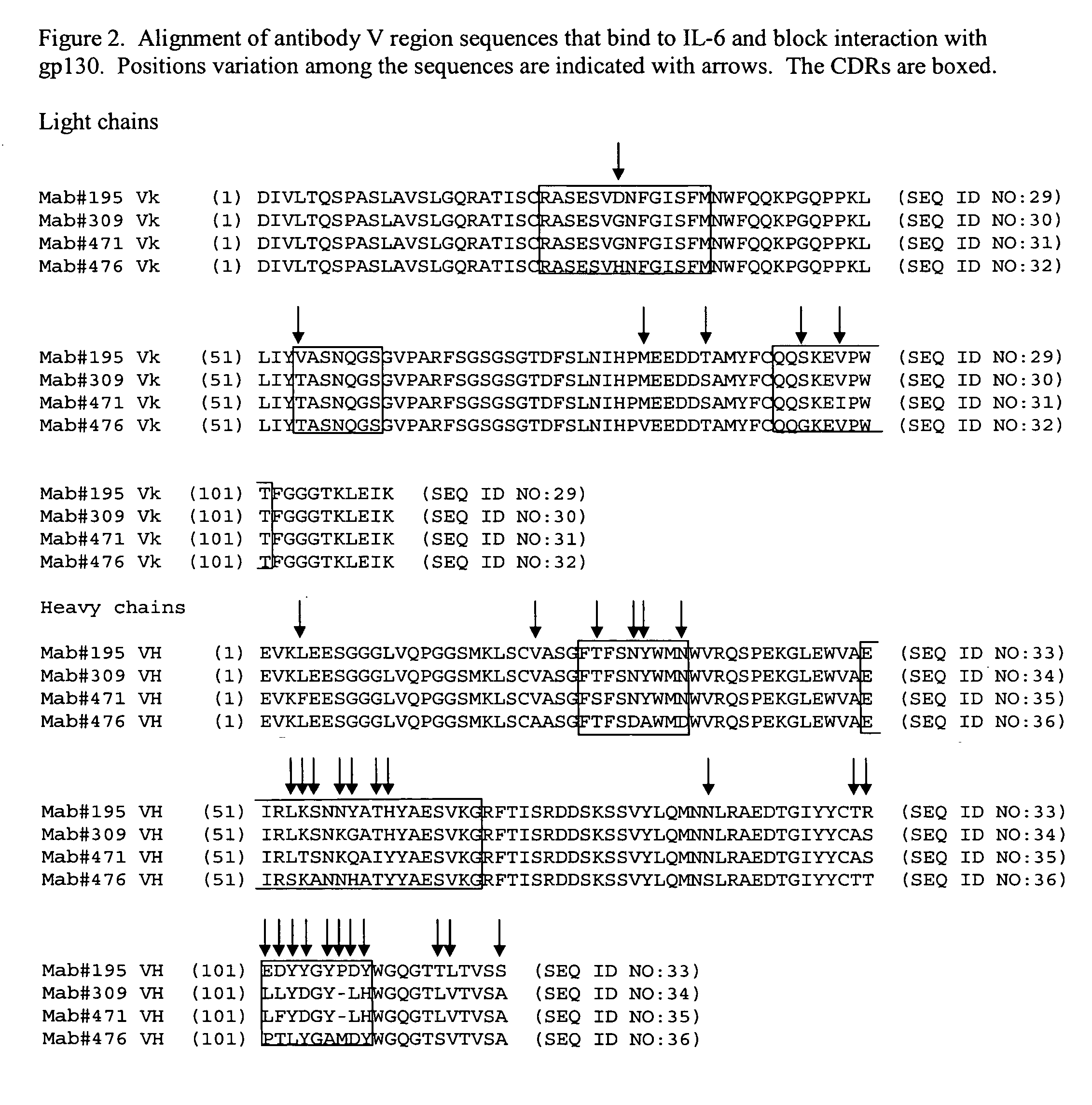
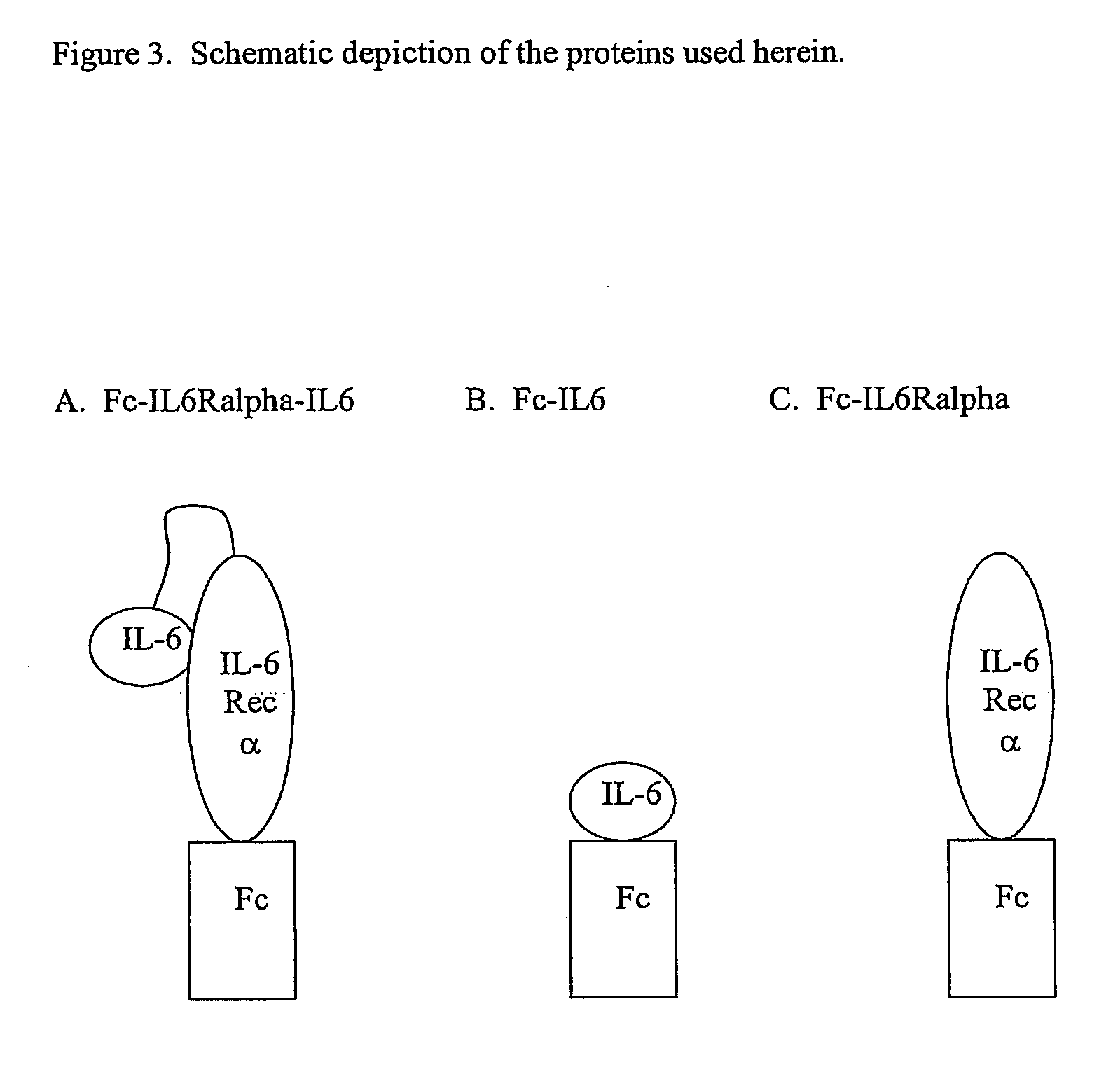
Interleukin-6 antagonists
InactiveUS20070178098A1Avoid interactionPromote formationAntibody mimetics/scaffoldsImmunoglobulins against cytokines/lymphokines/interferonsInterleukin 6Antagonist
The present invention provides an isolated IL-6 antagonist including an antibody variable region that prevents IL-6 from binding to gp130. The present invention also provides compositions and methods for treating IL-6 related diseases based on the IL-6 antagonists of the invention.
Owner:MERCK PATENT GMBH
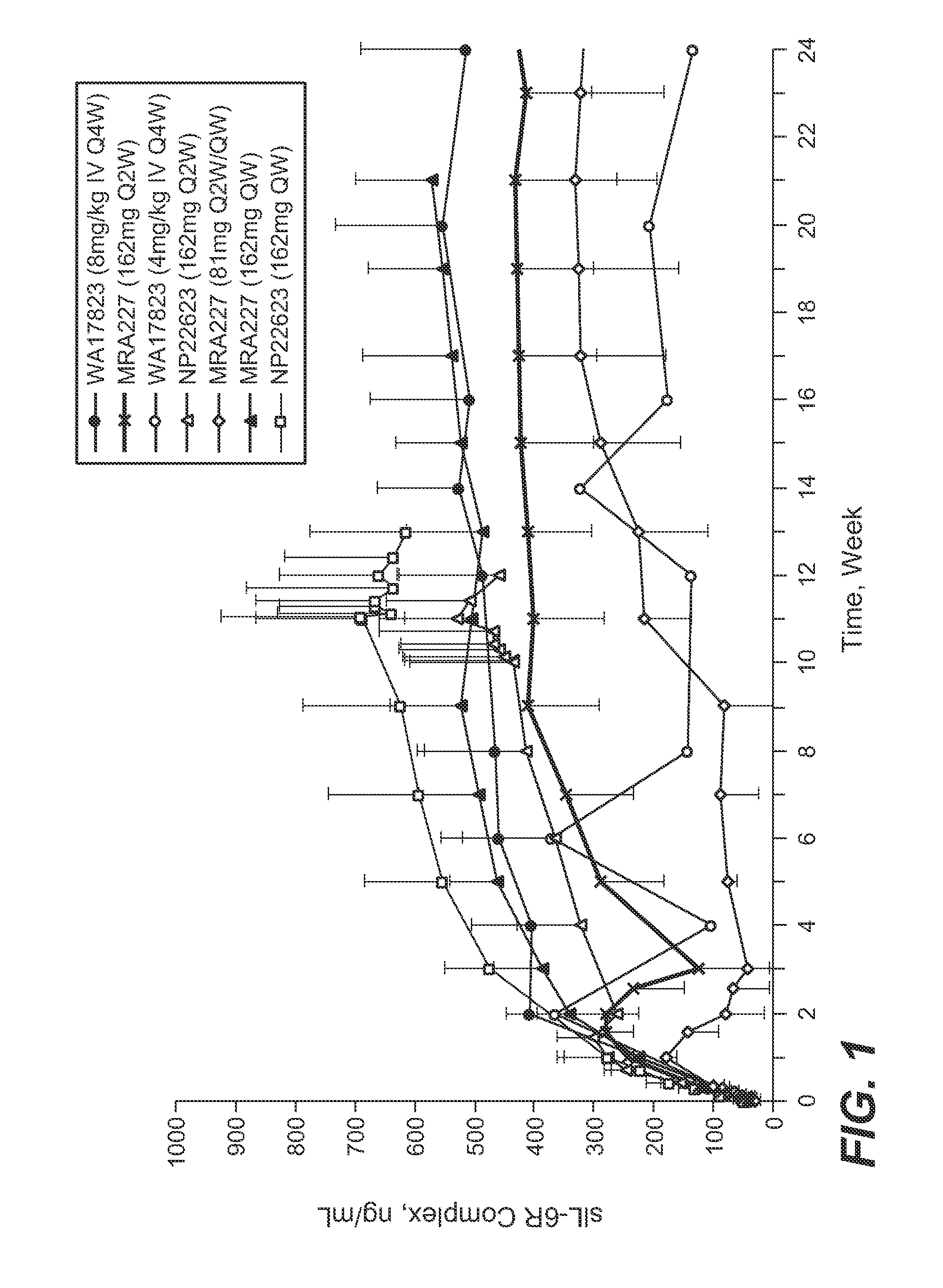
Subcutaneously administered Anti-il-6 receptor antibody
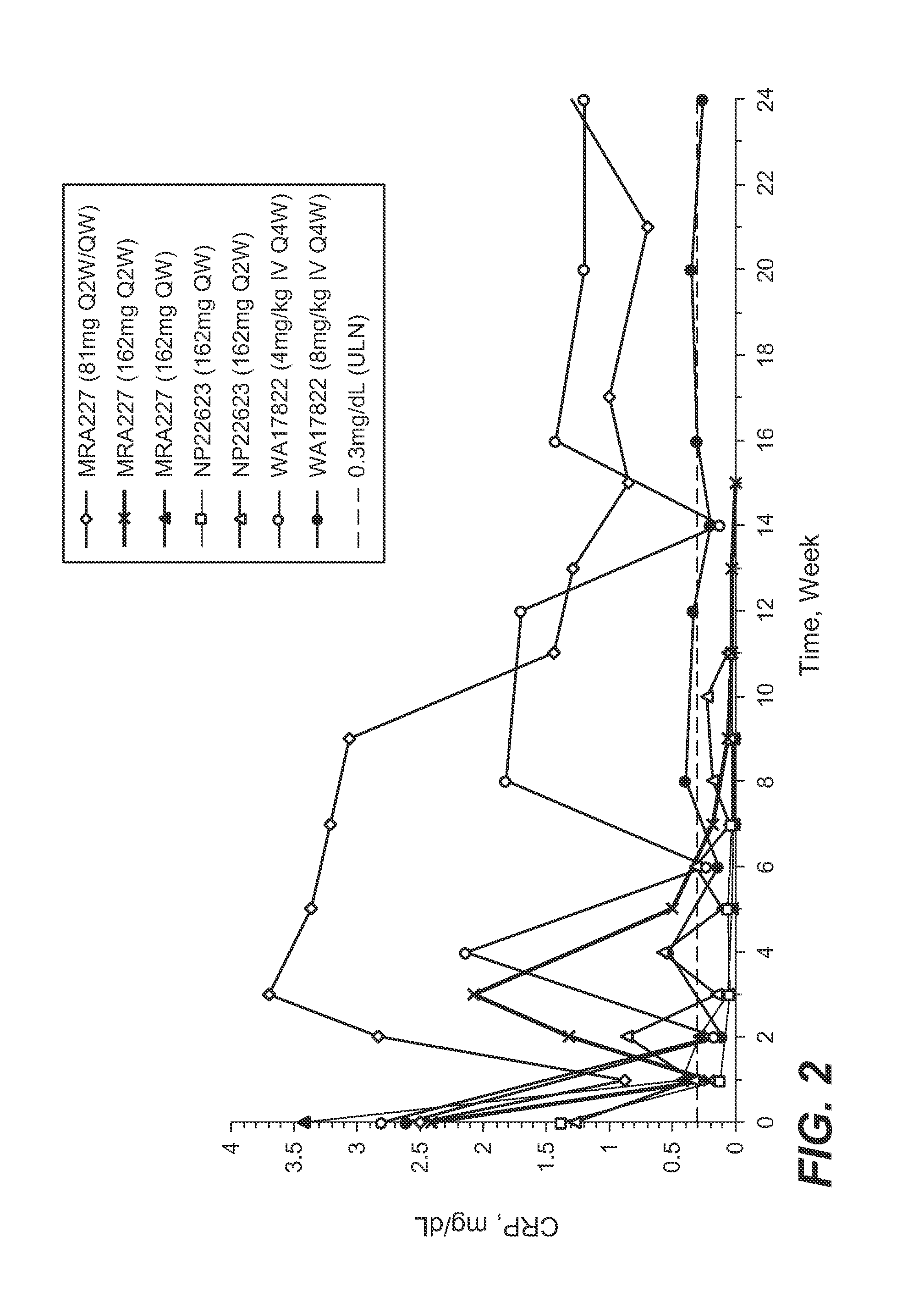
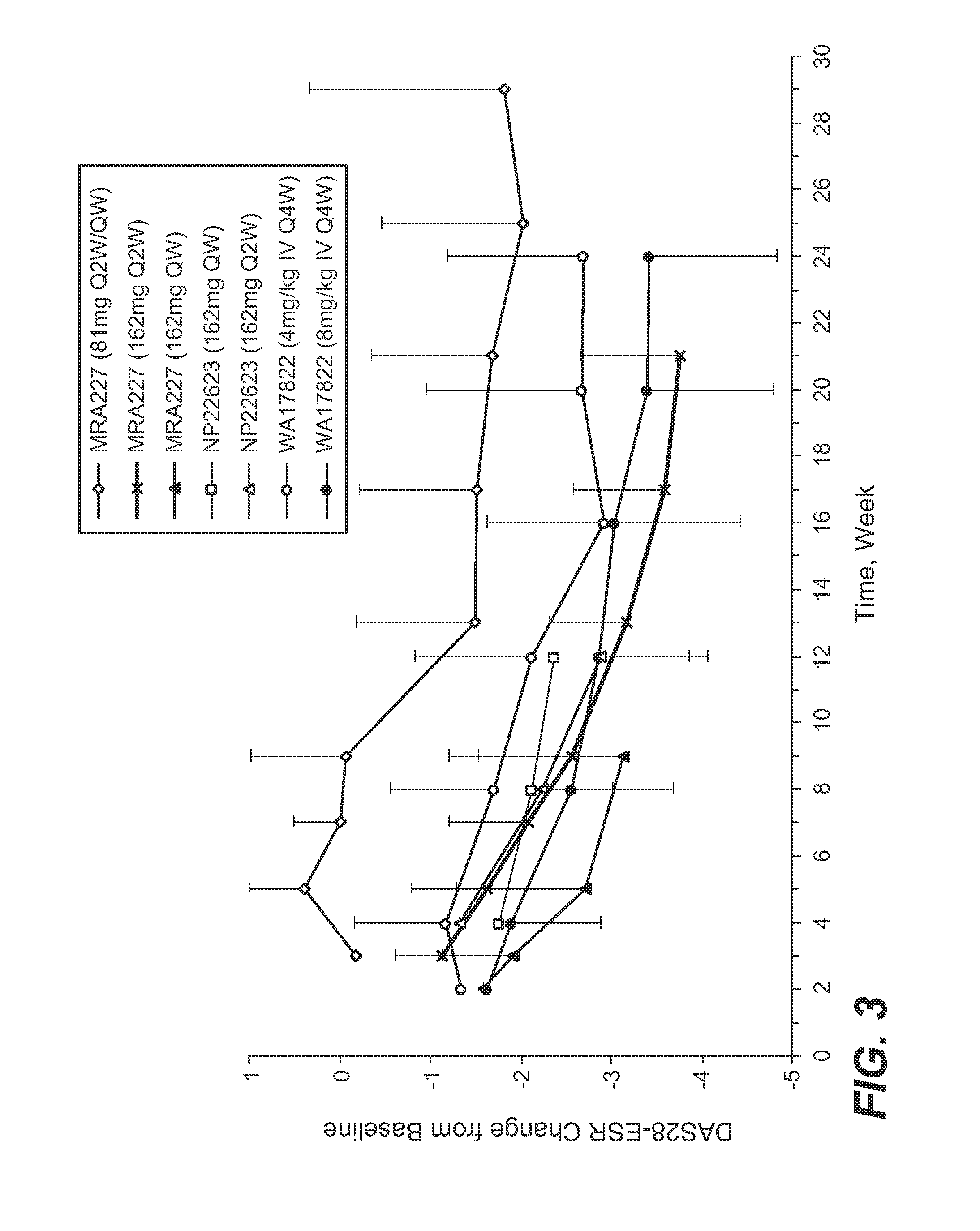
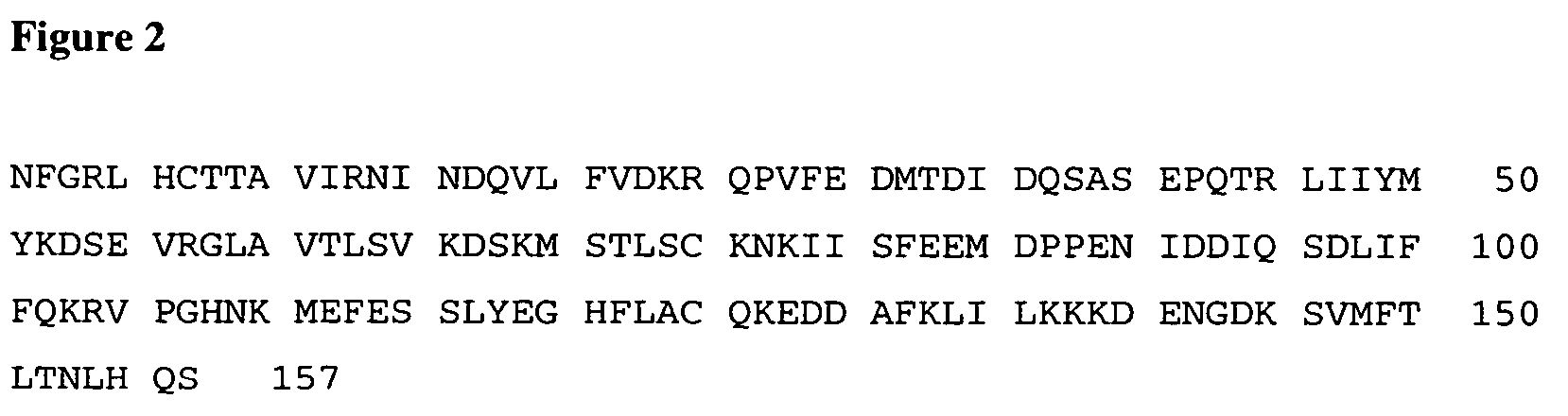
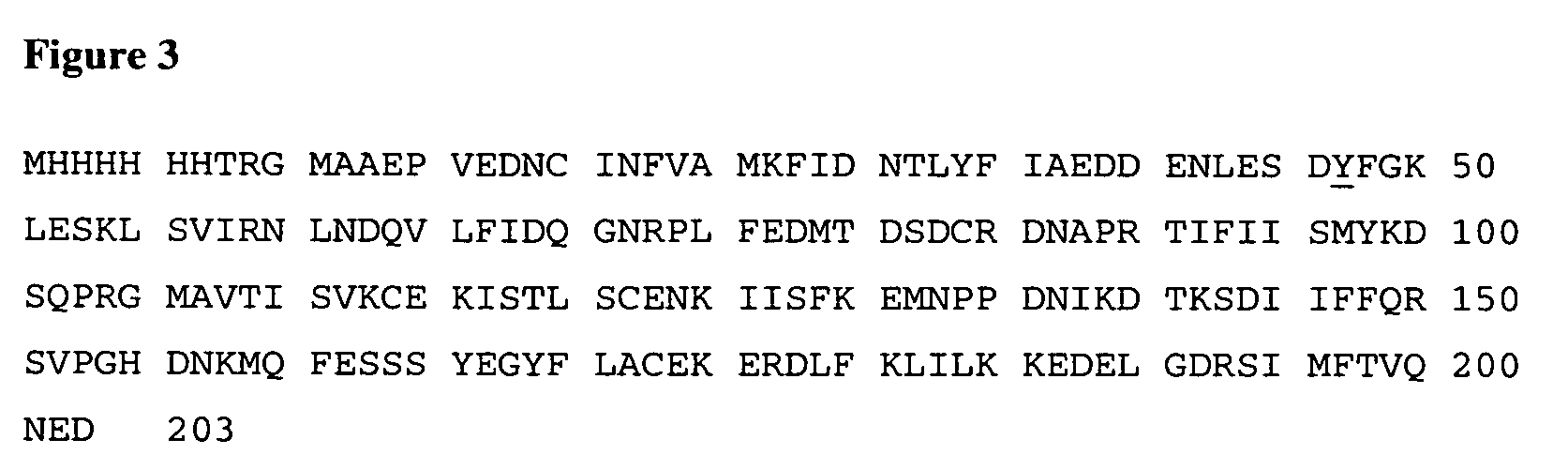
The present application discloses methods for treating an IL-6-mediated disorder such as rheumatoid arthritis (RA), juvenile idiopathic arthritis (JIA), systemic JIA (sJIA), polyarticular course JIA (pcJIA), systemic sclerosis, or giant cell arteritis (GCA), with subcutaneously administered antibody that binds interleukin-6 receptor (anti-IL-6R antibody). In particular, it relates to identification of a fixed dose of anti-IL-6R antibody, e.g. tocilizumab, which is safe and effective for subcutaneous administration in patients with IL-6-mediated disorders. In addition, formulations and devices useful for subcutaneous administration of an anti-IL-6R antibody are disclosed.
Owner:CHUGAI PHARMA CO LTD
Conjugates comprising human IL-18 and substitution mutants thereof
Human interleukin-18 (IL-18) polypeptides and substitution mutants thereof were conjugated to water-soluble polymers at specific sites on the human IL-18 protein. These conjugated human IL-18 and substitution mutants thereof retain biological activity. These conjugated cytokines demonstrate enhanced and unexpected biological properties when compared to the corresponding unconjugated cytokines.
Owner:GLAXO SMITHKLINE LLC
Method and composition for treatment of renal failure with antibodies and their equivalents as partial or complete replacement for dialysis
InactiveUS7504106B2Lower Level RequirementsSlow onsetBiocidePeptide/protein ingredientsInterleukin 6Creatinine rise
A method for treating patients with renal failure includes administering to them an effective amount of antibody or of a functional equivalent thereof to at least two of urea, creatinine, tumor necrosis factor alpha, interferon gamma, interleukin 6 and interleukin 1 beta. Soluble cytokine receptors also can be employed. The method can be used as a supplement to or as partial or complete replacement for dialysis. A pharmaceutical composition includes antibody or functional equivalent thereof to urea, creatinine, or both; antibody, functional equivalent or soluble cytokine receptor to tumor necrosis factor alpha, interferon gamma, interleukin 6, interleukin 1 beta or any combination thereof The composition can be included in a kit.
Owner:SKURKOVICH BORIS +2
Human antibodies that bind human il-12 and methods for producing
InactiveUS20080305114A1Avoid interferencePreservationNervous disorderPeptide/protein ingredientsDiseaseAntigen binding
Human antibodies, preferably recombinant human antibodies, that specifically bind to human interleukin-12 (hIL-12) are disclosed. Preferred antibodies have high affinity for hIL-12 and neutralize hIL-12 activity in vitro and in vivo. An antibody of the invention can be a full-length antibody or an antigen-binding portion thereof. The antibodies, or antibody portions, of the invention are useful for detecting hIL-12 and for inhibiting hIL-12 activity, e.g., in a human subject suffering from a disorder in which hIL-12 activity is detrimental. Nucleic acids, vectors and host cells for expressing the recombinant human antibodies of the invention, and methods of synthesizing the recombinant human antibodies, are also encompassed by the invention.
Owner:ABBVIE DEUTSHLAND GMBH & CO KG
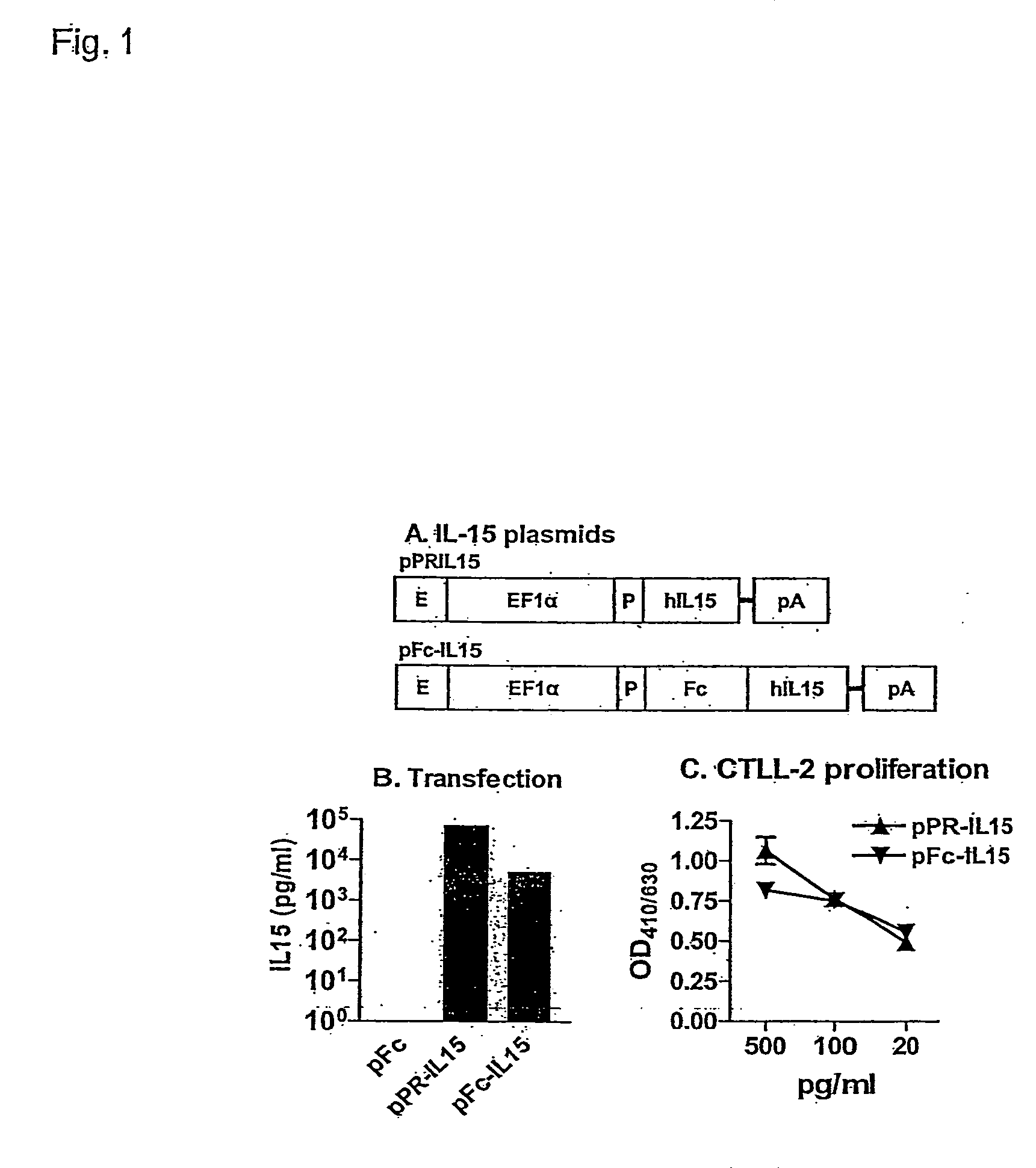
Novel form of interleukin-15, Fc-IL-15, and methods of use
InactiveUS20060257361A1Eliminates any neoantigenicityShort half-lifeHybrid immunoglobulinsPeptide/protein ingredientsAbnormal tissue growthT cell
The present invention relates to Fc-IL-15 hybrids, which may or may not include peptide linkers between the IL-15 and the Fc portion, for methods of treatment of tumors and viral infections. The IL-15 hybrids can be Fc-IL-15 or IL-15-Fc hybrids. The Fc-IL-15 hybrids include variants, including the IL-15 and Fc variants. The hybrids preferably (but not necessarily) include peptide linkers between the IL-15 and the Fc portion. These linkers are preferably composed of a T cell inert sequence, or any non-immunogenic sequence.
Owner:DEPT OF HEALTH & HUMAN SERVICES GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC
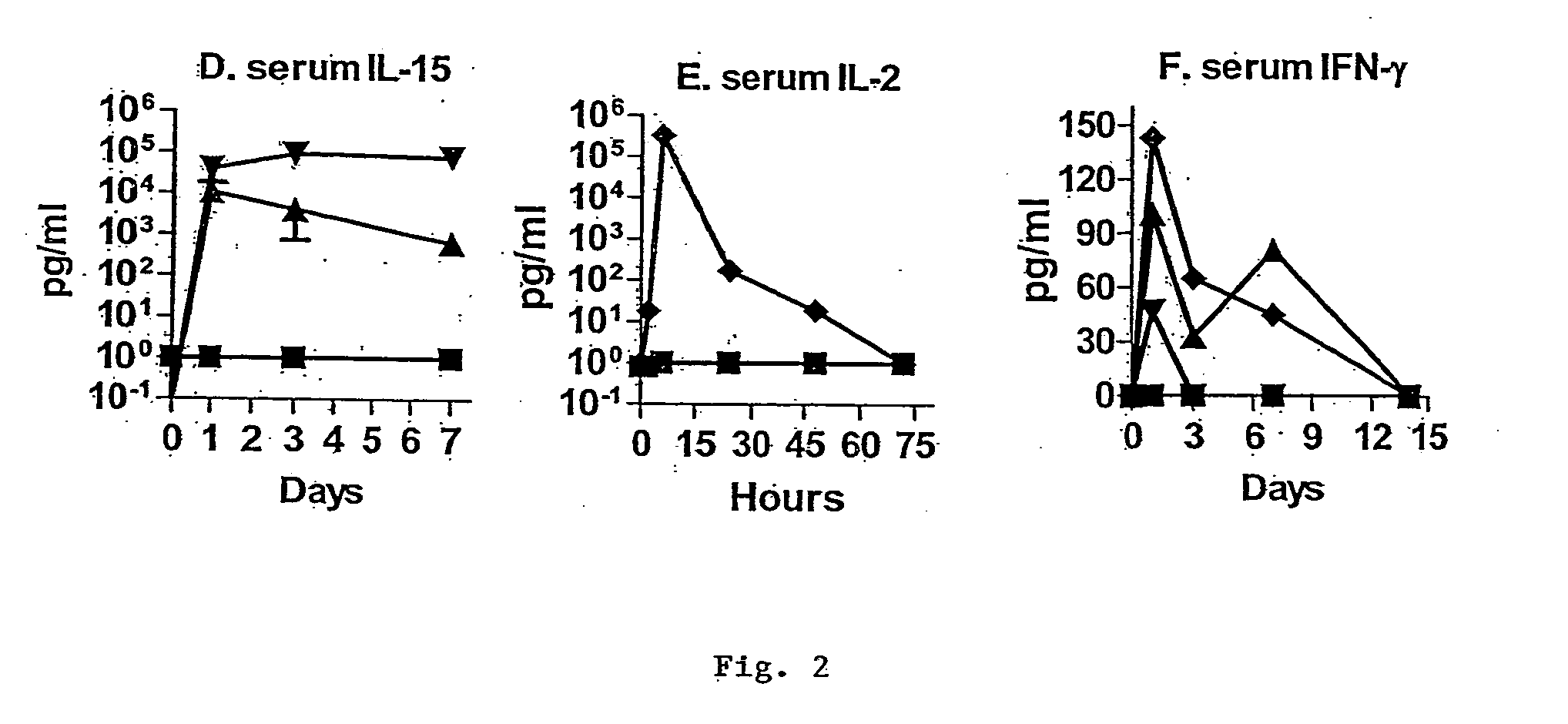
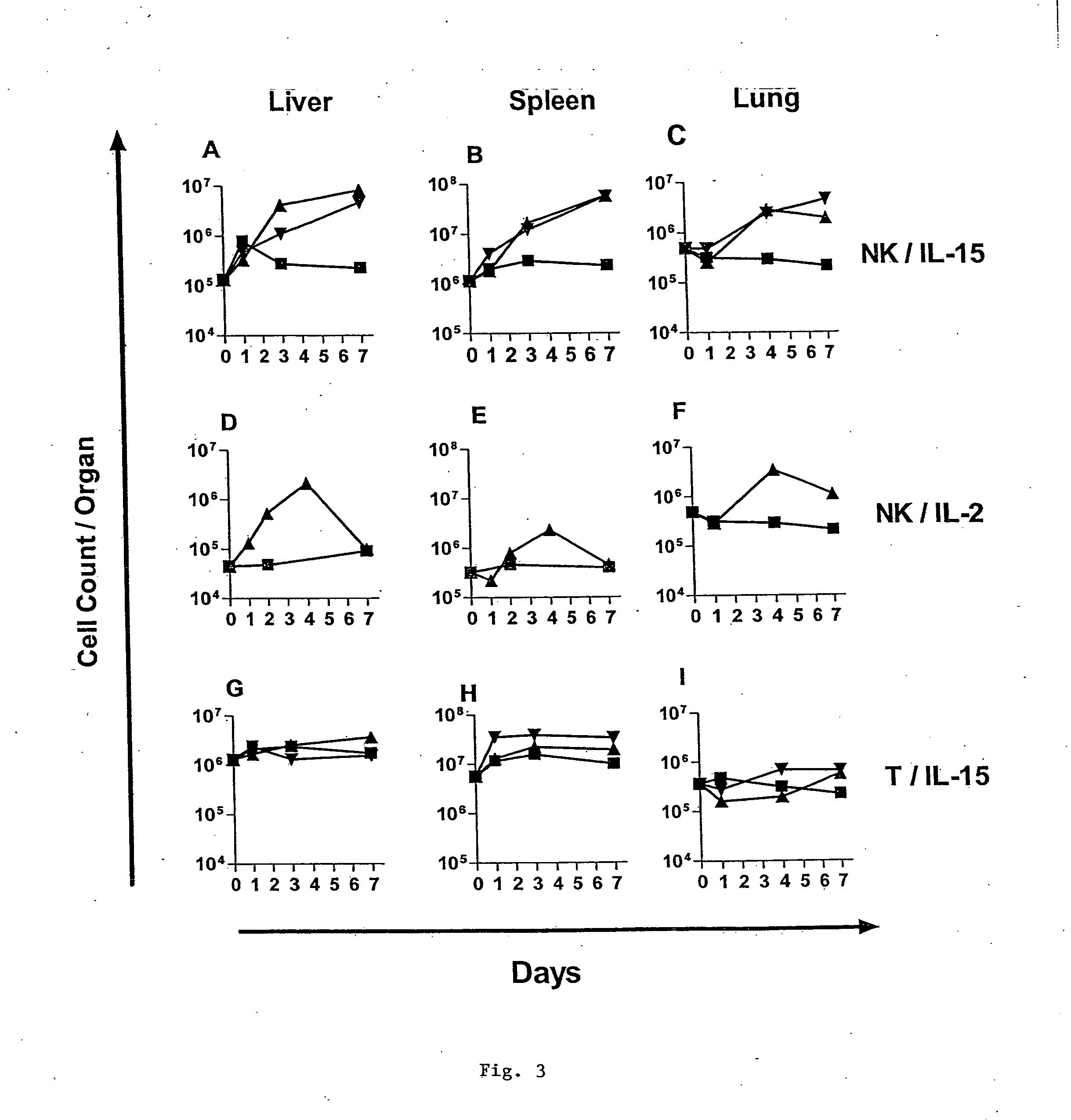
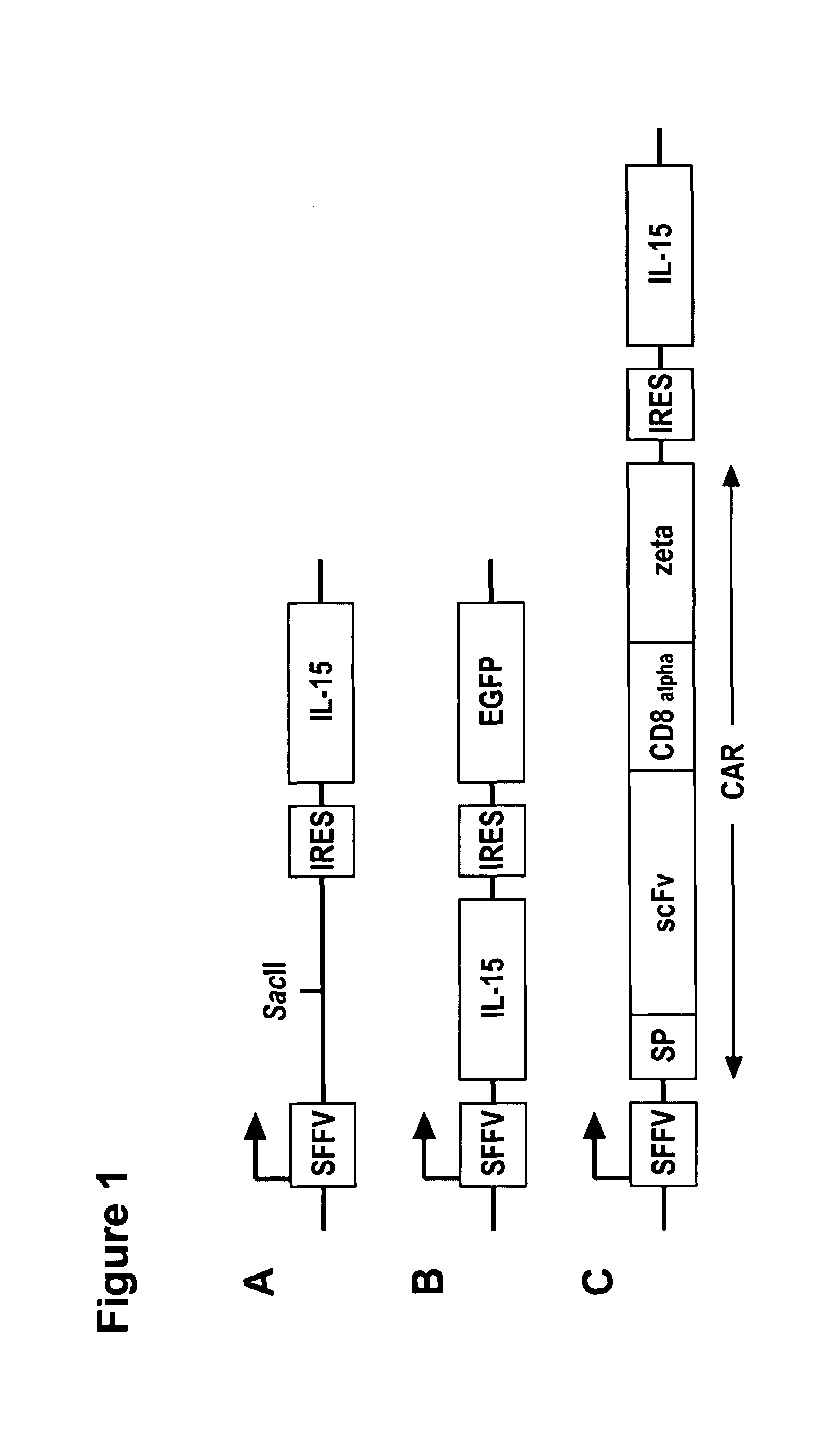
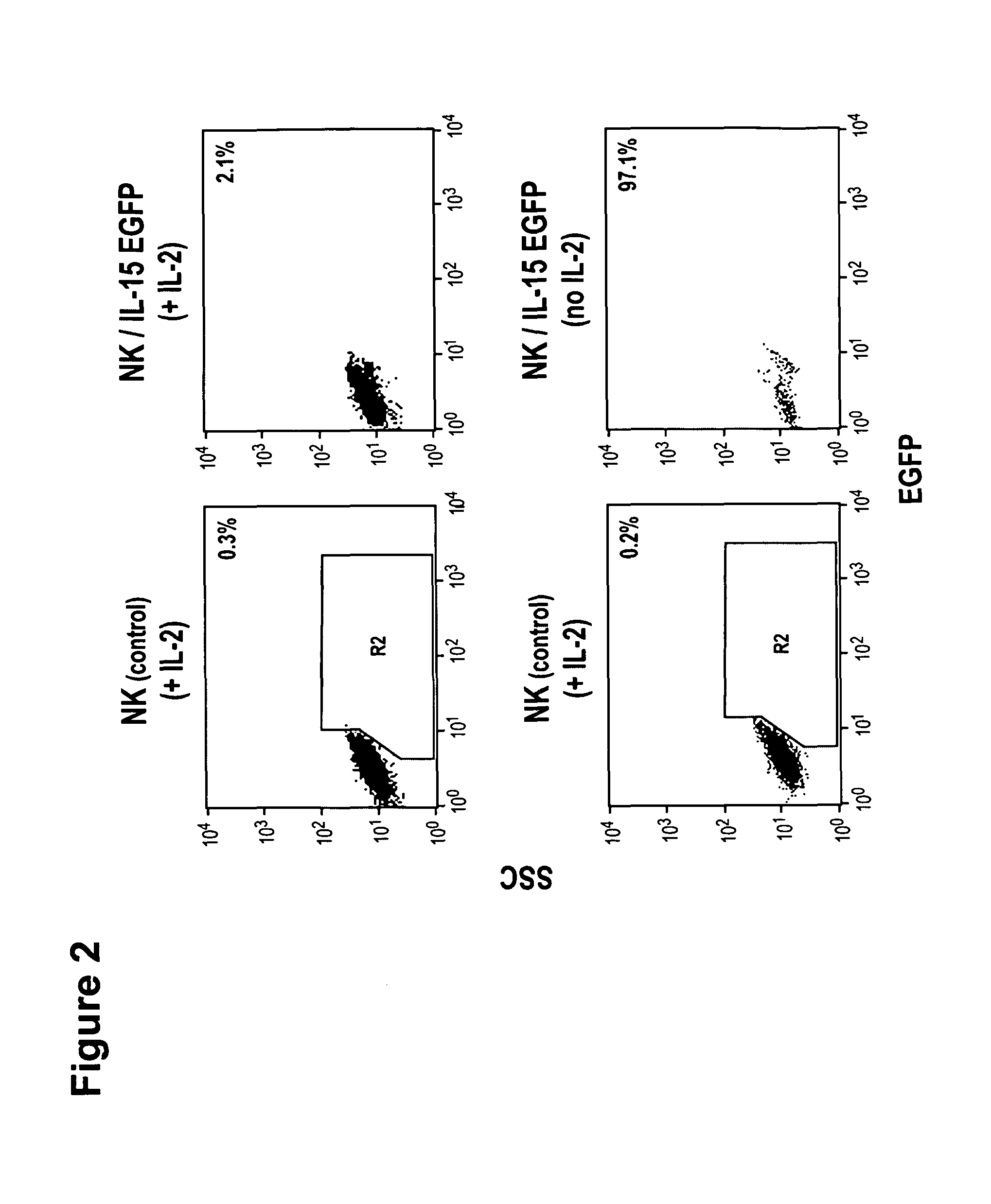
Interleukin 15 as Selectable Marker for Gene Transfer in Lymphocytes
ActiveUS20130280221A1Opening possibilityBiocideAntibody mimetics/scaffoldsADAMTS ProteinsAntigen receptors
The present invention relates to the use of interleukin-15 (IL-15) as selectable marker for gene transfer, preferably of at least one gene of therapeutic interest, into a mammalian cell or cell line, in particular a human cell or cell line. The present invention furthermore relates to transgenic mammalian cells or cell lines expressing IL-15 as selectable marker and co-expressing at least one protein of interest encoded by at least one gene of interest, which is preferably a protein of therapeutic interest. The present invention is in particular suitable for chimeric antigen receptors (CARs) as the gene or protein of interest and their expression in lymphocytes. The transgenic mammalian cells and cell lines are furthermore suitable for use as a medicament, in particular in the treatment of cancer and in immunotherapy, such as adoptive, target-cell specific immunotherapy.
Owner:CHEMOTHERAPEUTISCHES FORSCHUNGINSTITUT GEORG SPEYER HAUS
Combinatorial interleukin-2 muteins
InactiveUS20060160187A1Maintaining and enhancing proliferationLower Level RequirementsPeptide/protein ingredientsTissue cultureNatural Killer Cell Inhibitory ReceptorsInterleukin II
Novel human interleukin-2 (IL-2) muteins or variants thereof, and nucleic acid molecules and variants thereof are provided. Methods for producing these muteins as well as methods for stimulating the immune system of an animal are also disclosed. In addition, the invention provides recombinant expression vectors comprising the nucleic acid molecules of this invention and host cells into which expression vectors have been introduced. Pharmaceutical compositions are included comprising a therapeutically effective amount of a human IL-2 mutein of the invention and a pharmaceutically acceptable carrier. The IL-2 muteins have lower toxicity than native IL-2 or Proleukin® IL-2, while maintaining or enhancing NK cell-mediated effects, and can be used in pharmaceutical compositions for use in treatment of cancer, and in stimulating the immune response.
Owner:CHIRON CORP
IL-12/p40 binding proteins
The present invention encompasses IL-12p40 binding proteins, particularly antibodies that bind human interleukin-12 (hIL-12) and / or human IL-23 (hIL-23). Specifically, the invention relates to antibodies that are chimeric, CDR grafted and humanized antibodies. Preferred antibodies have high affinity for hIL-12 and / or hIL-23 and neutralize h IL-12 and / or hIL-23 activity in vitro and in vivo. An antibody of the invention can be a full-length antibody or an antigen-binding portion thereof. Method of making and method of using the antibodies of the invention are also provided. The antibodies, or antibody portions, of the invention are useful for detecting hIL-12 and / or hIL-23 and for inhibiting hIL-12 and / or hIL-23 activity, e.g., in a human subject suffering from a disorder in which hIL-12 and / or hIL-23 activity is detrimental.
Owner:ABBVIE INC
Diagnostic Methods Of Multiple Organ Amyloidosis
InactiveUS20080038192A1In-vivo radioactive preparationsMicrobiological testing/measurementElevated C-reactive proteinPredictive biomarker
Described are methods of assessing whether a subject has or is at risk of having multiple organ amyloidosis (MOA) The method includes detecting a diagnostically predictive collection of biomarkers of multiple organ amyloidosis, wherein the detection of a diagnostically predictive collection of biomarkers indicates the subject has or is at risk of having multiple organ amyloidosis Also described are methods of monitoring treatment of subjects with multiple organ amyloidosis and evaluating therapeutic compounds Representative biomarkers for use in the methods may be selected from variant serum amyloid A (SAA) allele, elevated SAA level, elevated C-reactive protein (CRP) level, depressed glycosammoglycan (GAG) level, elevated interleukin-18 (IL-18) level, elevated macrophage-colony stimulating factor (M-CSF) level, elevated hepatocyte growth factor (HGF) level, presence of an antibody against citrullmated vimentm (Sa), presence of a monoclonal immunoglobulin light chain, increased serum albumin, and increased creatinine clearance
Owner:NEUROCHEM INT
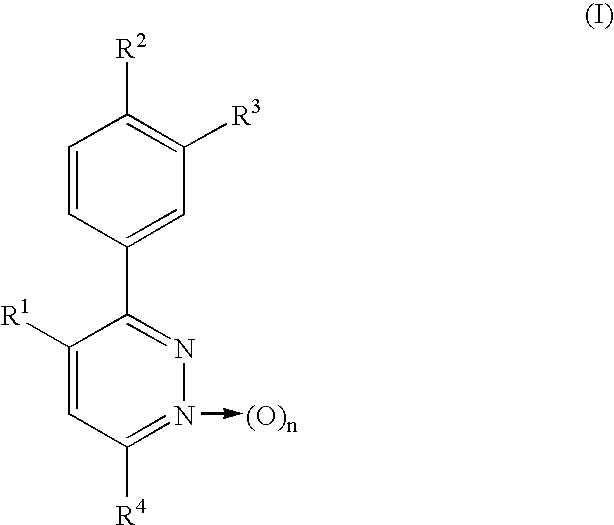
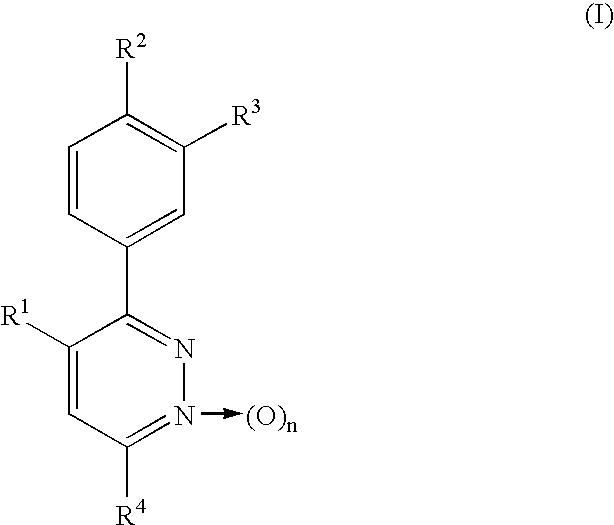
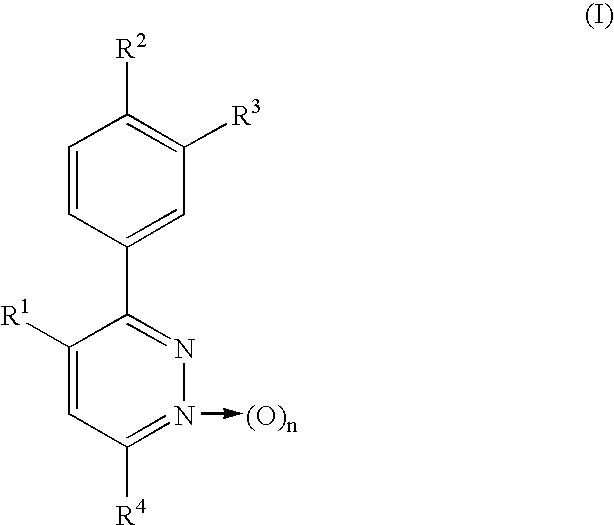
Phenylpyridazine compounds and medicines containing the same
InactiveUS6664256B1Excellent inhibitory activityStrong inhibitory activityBiocideOrganic chemistryIschemic diseaseWhite blood cell
Phenylpyridazine compounds represented by the following formula (I): ##STR1## are provided, wherein R.sup.1, R.sup.2, R.sup.3, R.sup.4, and n are as defined herein having excellent inhibitory activity against interleukin-1.beta. production, and useful in the treatment of prevention of diseases caused by stimulation of interleukin-1.beta. production, such as immune system diseases, inflammatory diseases, and ischemic diseases.
Owner:KOWA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com