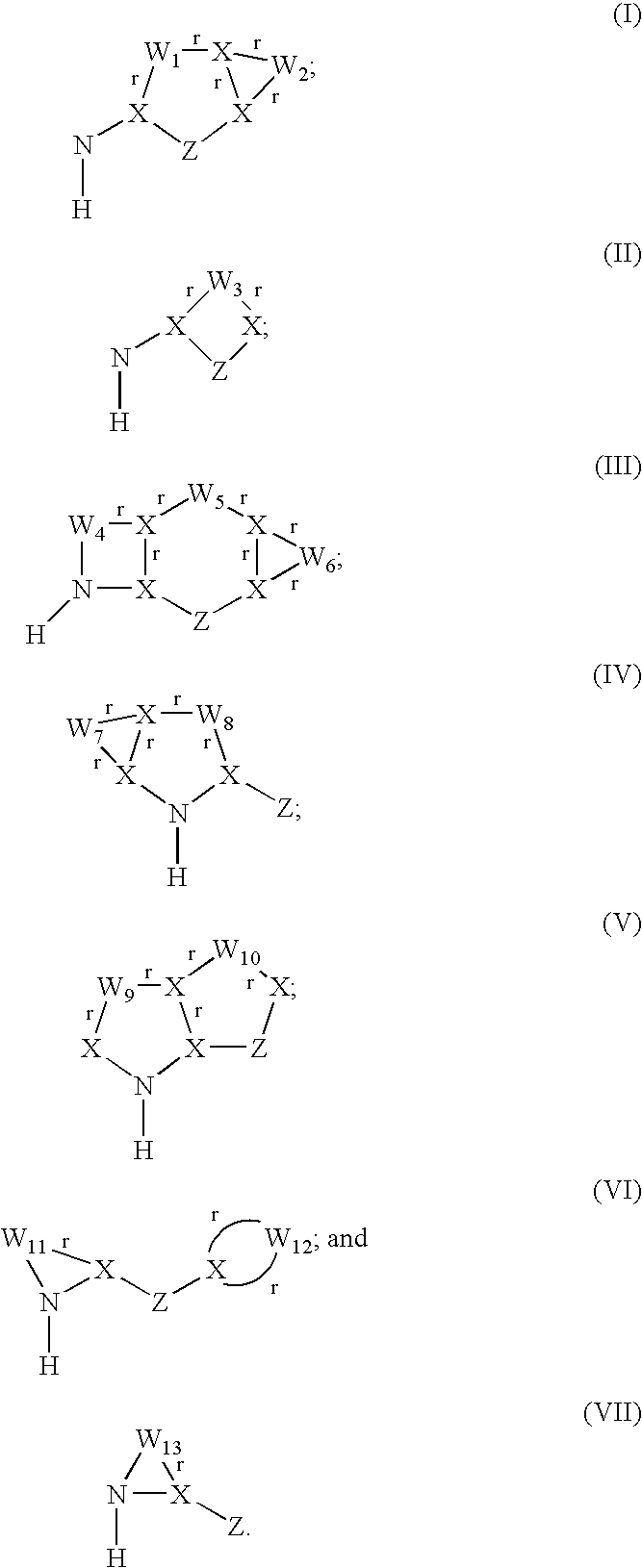
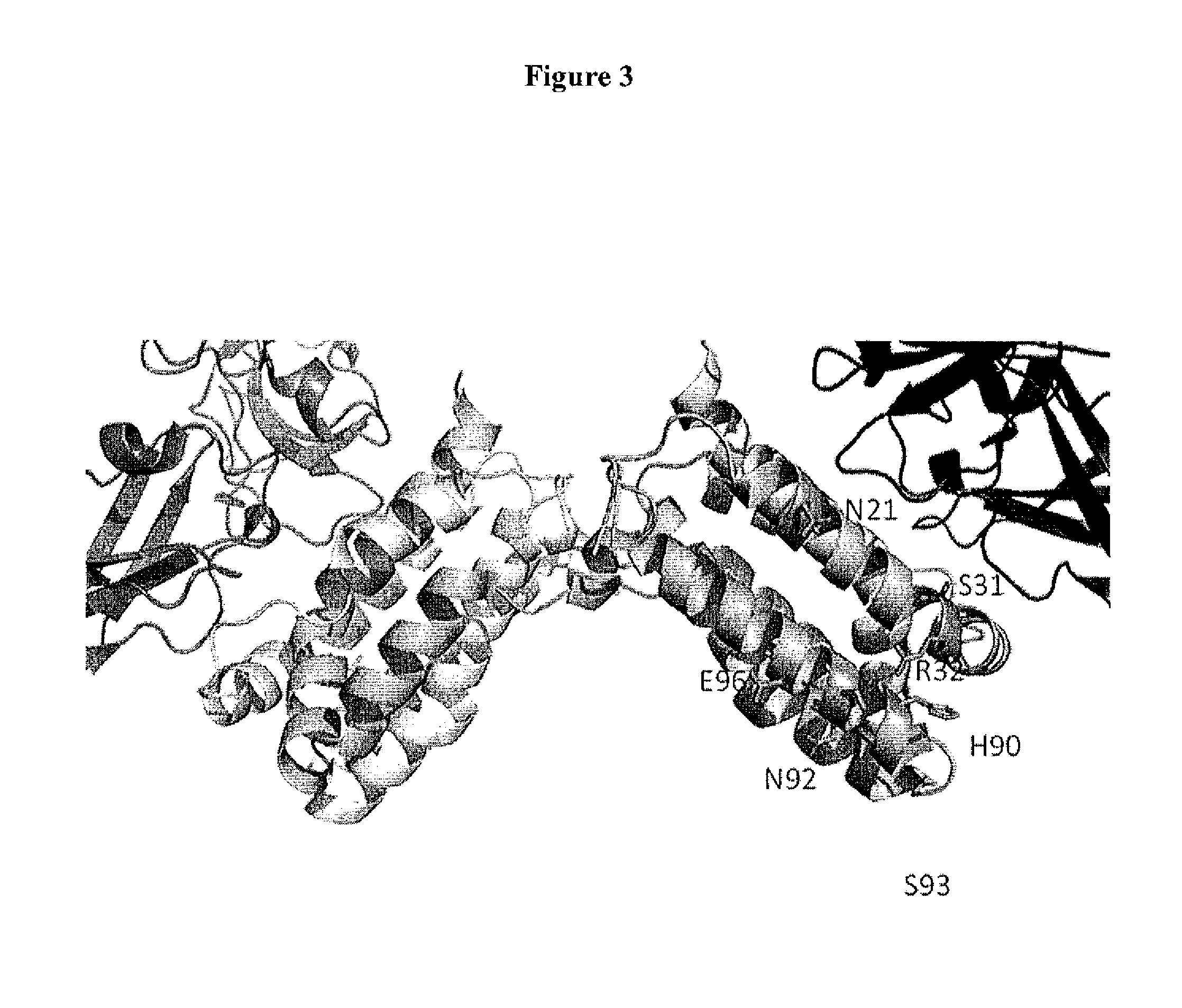
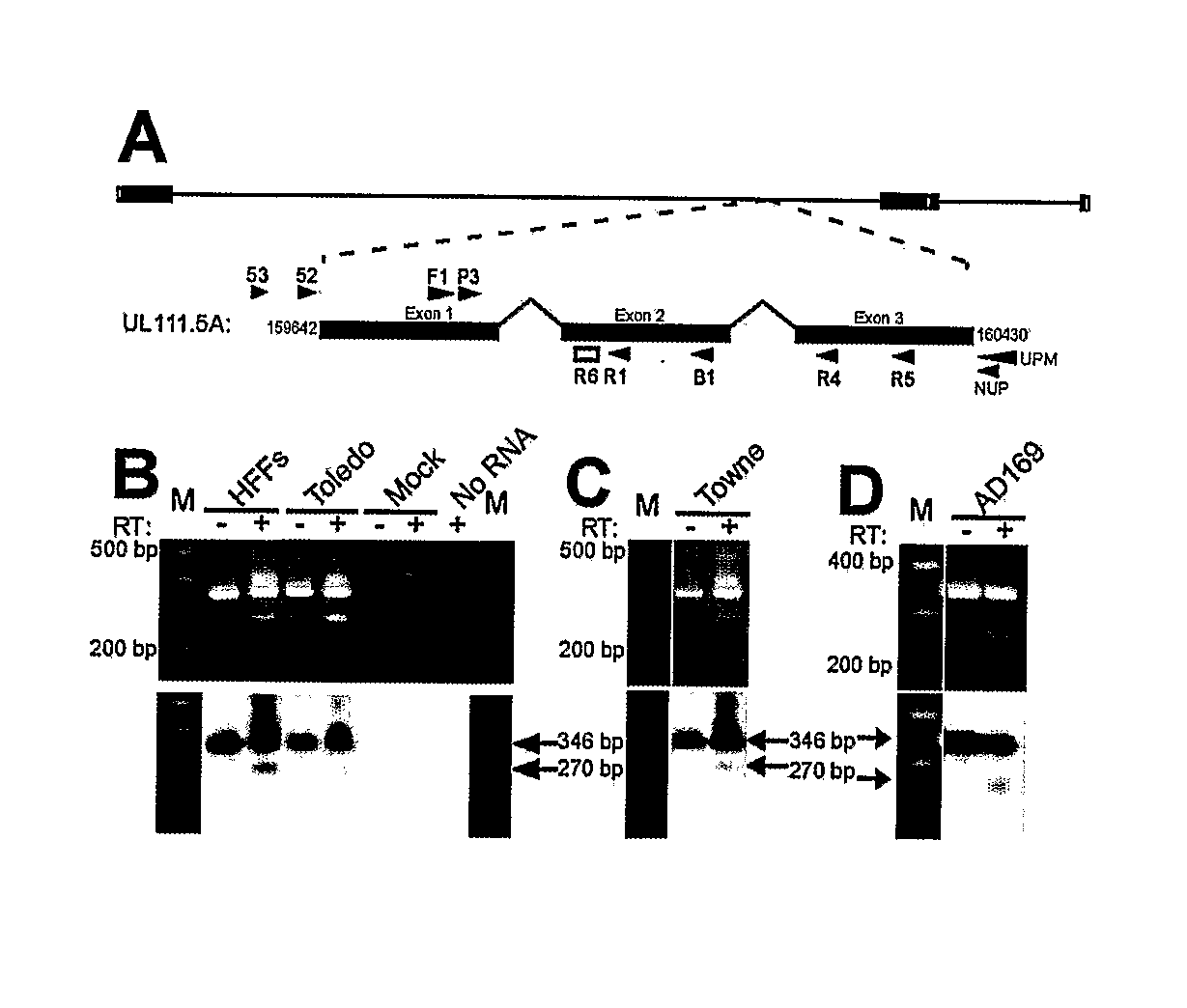
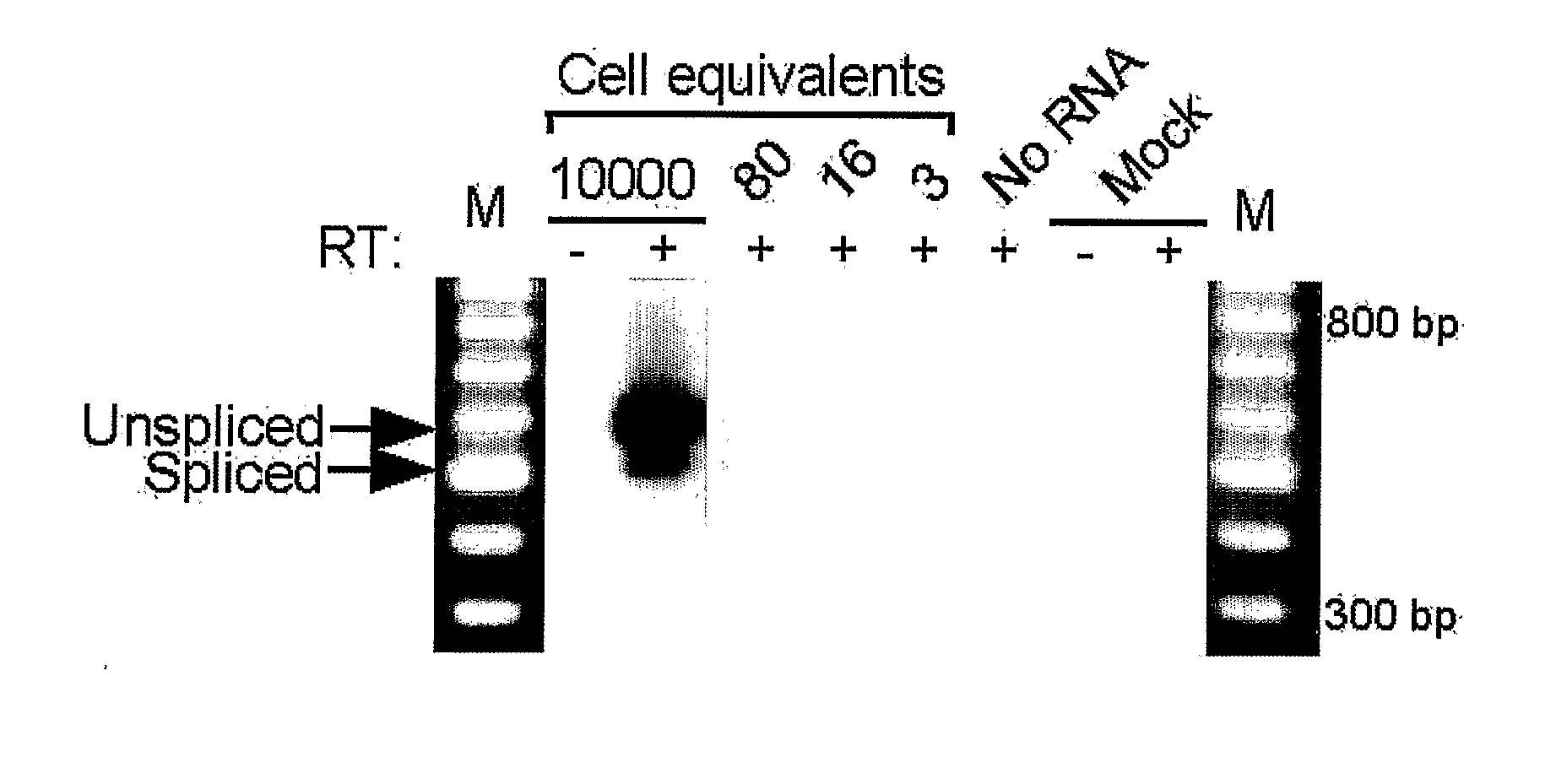
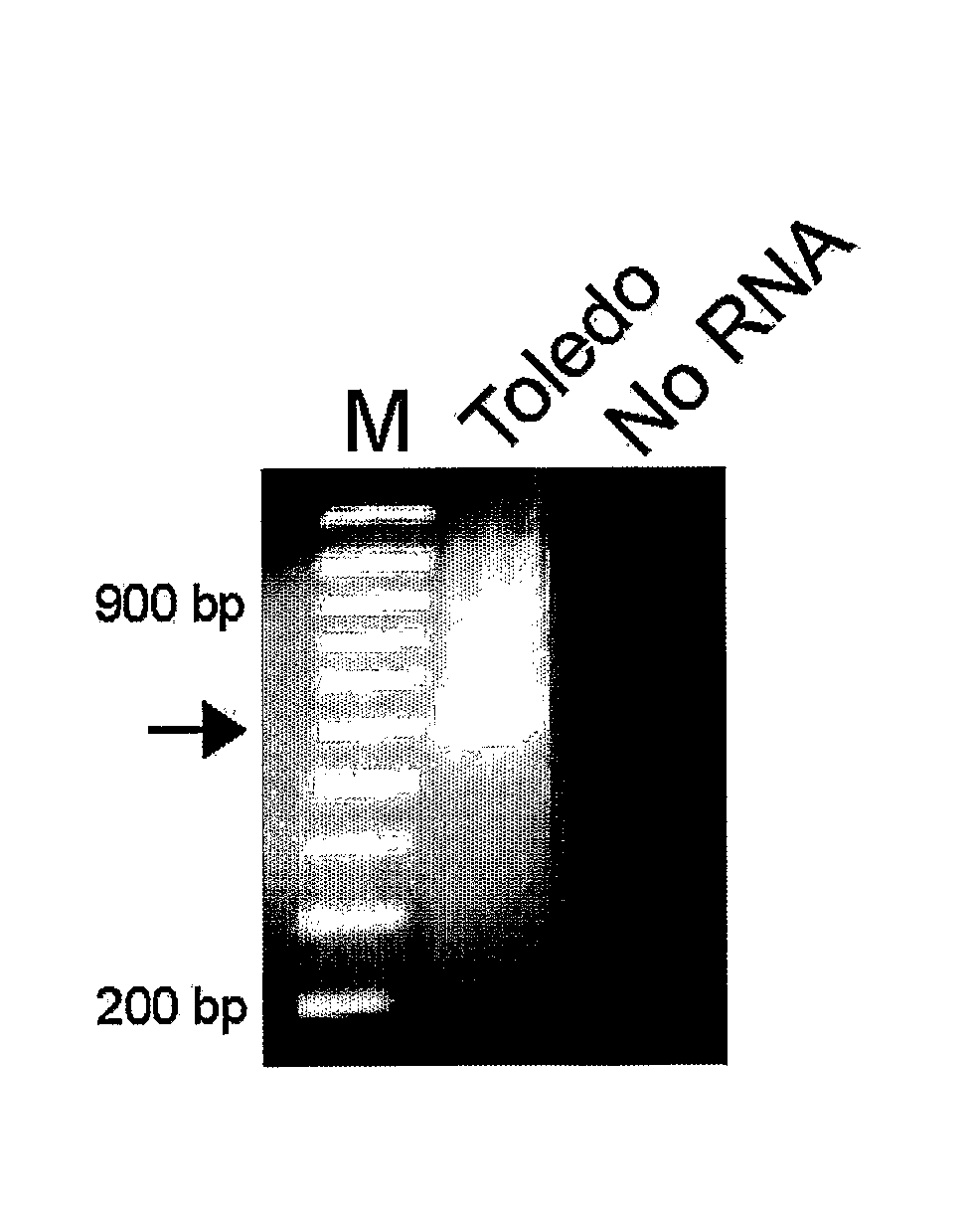
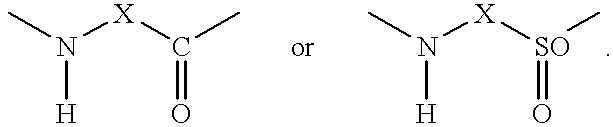Patents
Literature
149 results about "Interleukin 10" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Interleukin 10 (IL-10), also known as human cytokine synthesis inhibitory factor (CSIF), is an anti-inflammatory cytokine. In humans, interleukin 10 is encoded by the IL10 gene. IL-10 signals through a receptor complex consisting of two IL-10 receptor-1 and two IL-10 receptor-2 proteins. Consequently, the functional receptor consists of four IL-10 receptor molecules. IL-10 binding induces STAT3 signalling via the phosphorylation of the cytoplasmic tails of IL-10 receptor 1 + IL-10 receptor 2 by JAK1 and Tyk2 respectively.
Pegylated interleukin-10
InactiveUS7052686B2Minimize disruptionPeptide/protein ingredientsAntipyreticInterleukin 10White blood cell
Interleukin-10 (IL-10) conjugated via a linker to one or more polyethylene glycol (PEG) molecules at a single amino acid residue of the IL-10, and a method for preparing the same, are provided. The method produces a stable mono-pegylated IL-10, which retains IL-10 activity, where pegylation is selective for the N-terminus on one subunit of IL-10 with little or no formation of monomeric IL-10. The method also provides a substantially homogenous population of mono-PEG-IL-10.
Owner:MERCK SHARP & DOHME CORP
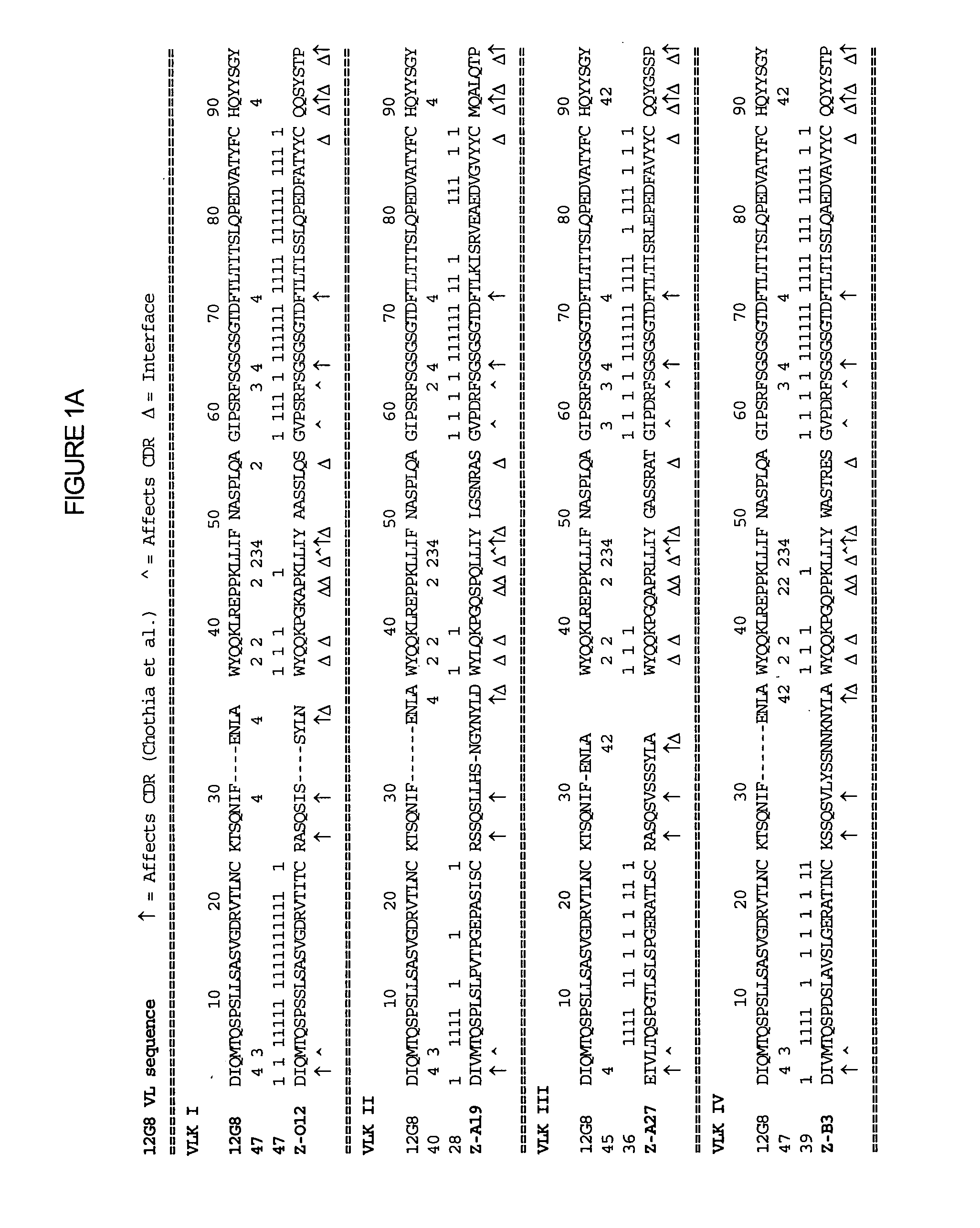
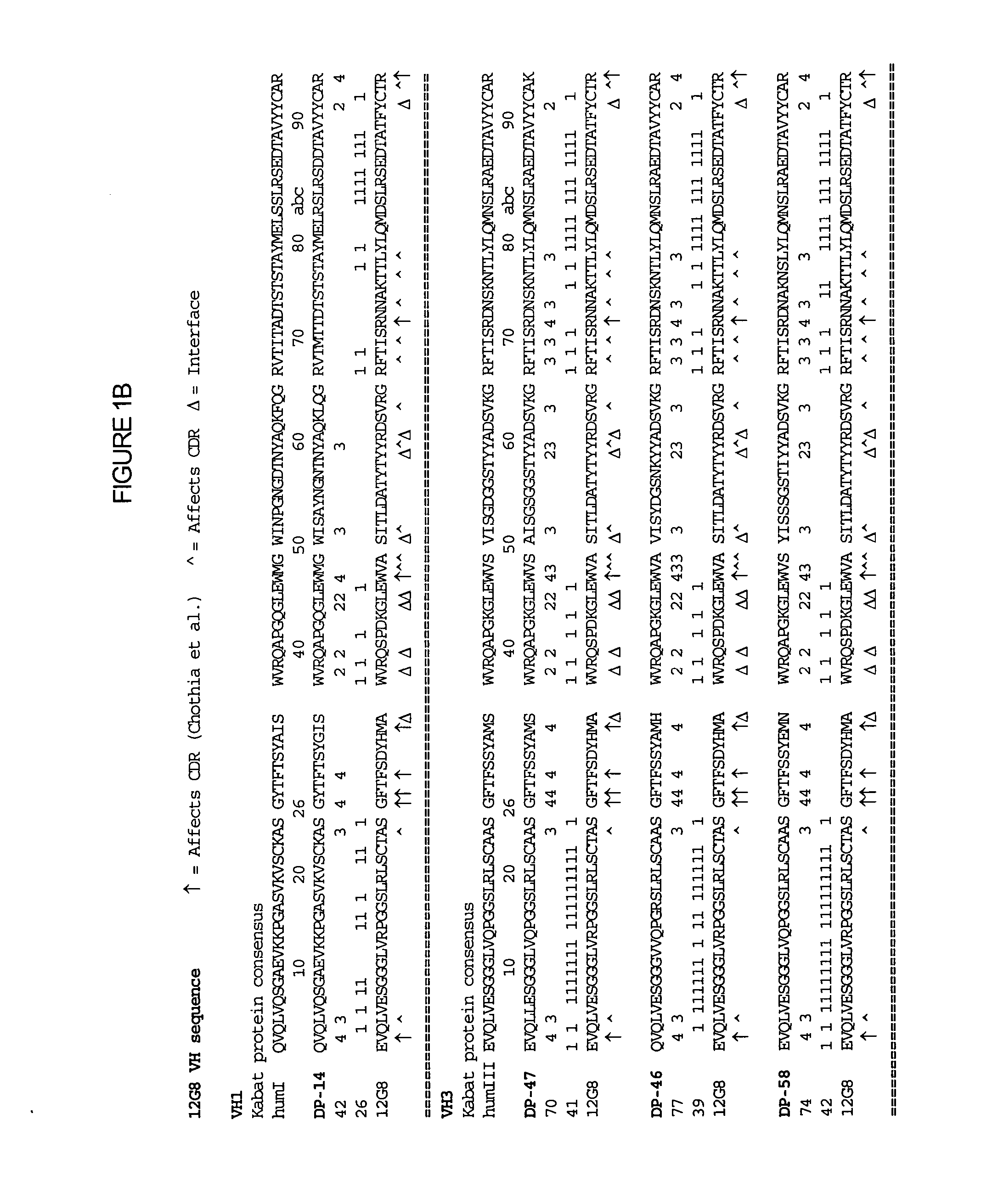
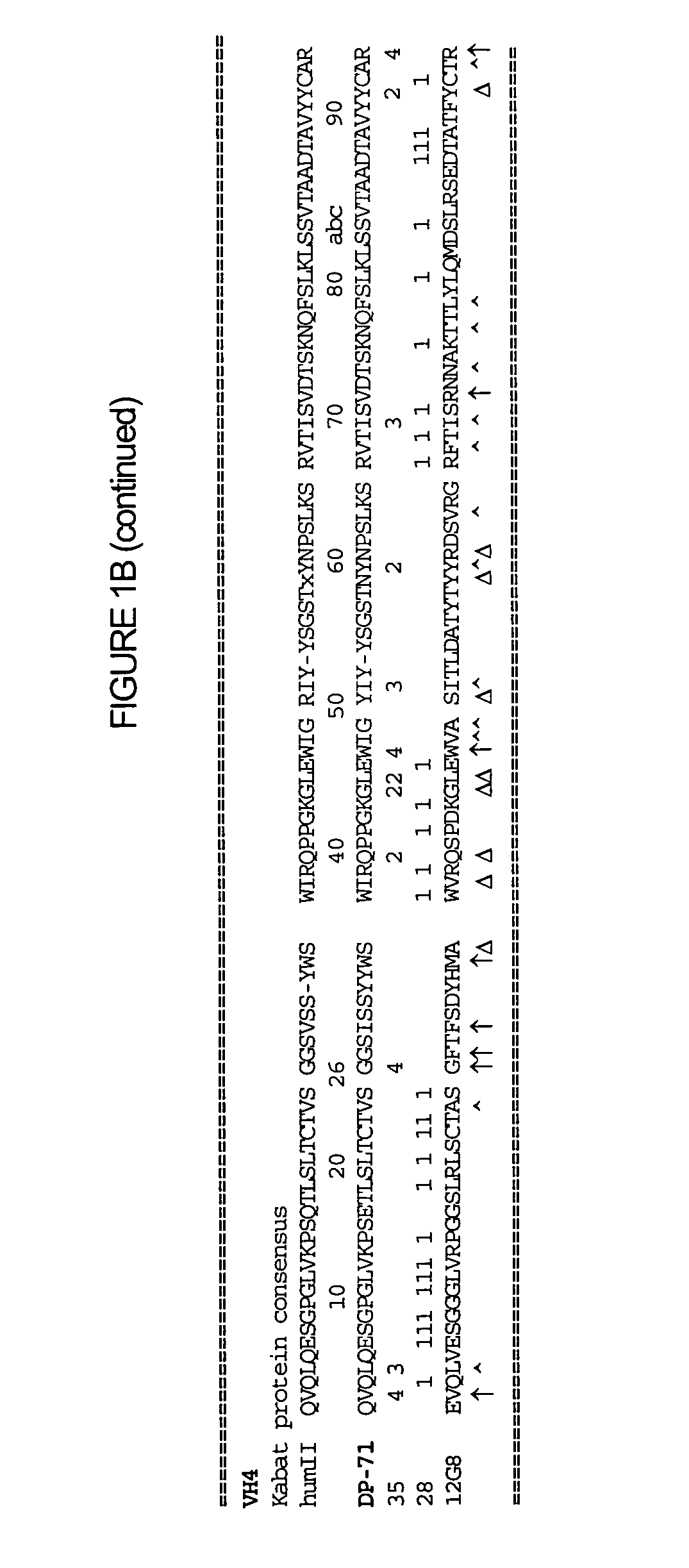
Interleukin-10 antibodies
The methods and compositions provided herein relate generally to IL-10 specific antibodies and uses thereof. More specifically, compositions of humanized IL-10 specific antibodies and methods to use such antibodies in modulating the biological activity of IL-10, particularly in autoimmune disorders and pathogen-mediated immunopathology.
Owner:SCHERING CORP
Pegylated interleukin-10
InactiveUS20060210534A1Minimize disruptionPeptide/protein ingredientsAntipyreticInterleukin 10White blood cell
Interleukin-10 (IL-10) conjugated via a linker to one or more polyethylene glycol (PEG) molecules at a single amino acid residue of the IL-10, and a method for preparing the same, are provided. The method produces a stable mono-pegylated IL-10, which retains IL-10 activity, where pegylation is selective for the N-terminus on one subunit of IL-10 with little or no formation of monomeric IL-10. The method also provides a substantially homogenous population of mono-PEG-IL-10.
Owner:SCHERING CORP
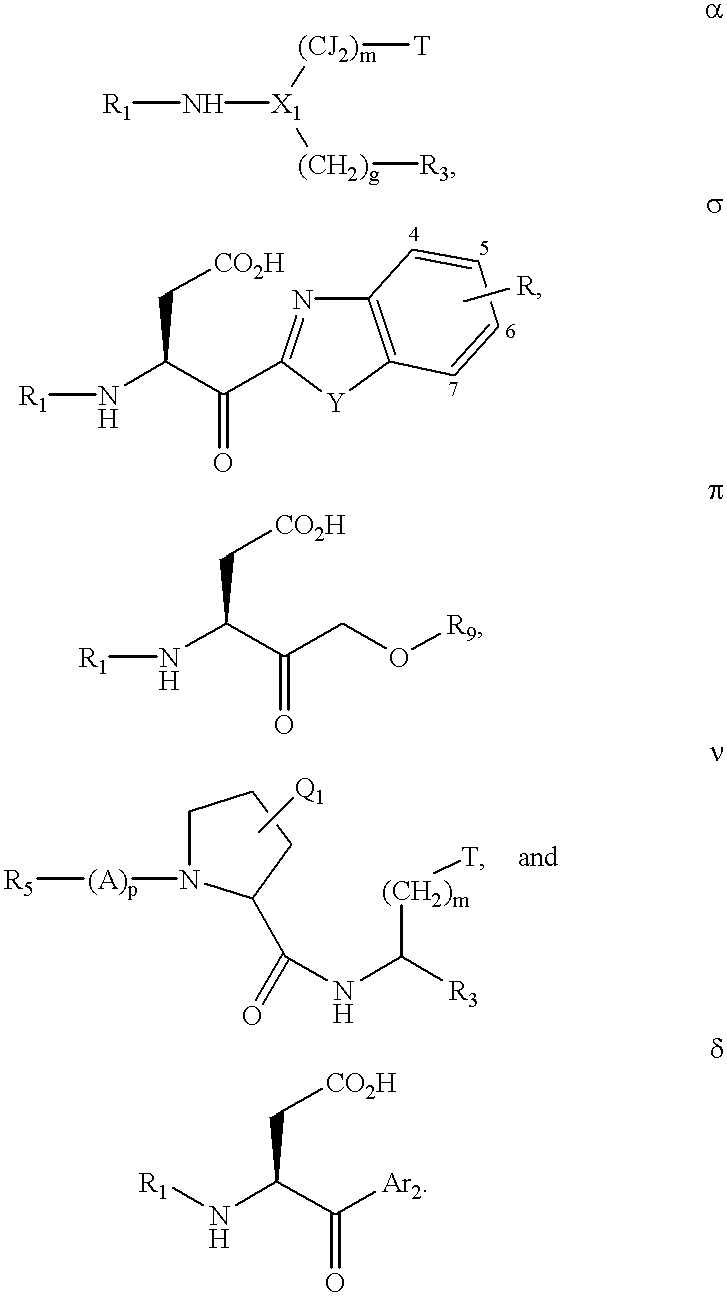
Inhibitors of interleukin-1β converting enzyme
InactiveUS7288624B2Inhibit enzyme activityBiocideTripeptide ingredientsInterleukin 1β converting enzymeInterleukin 10
The present invention relates to novel classes of compounds which are inhibitors of interleukin-1β converting enzyme. The ICE inhibitors of this invention are characterized by specific structural and physicochemical features. This invention also relates to pharmaceutical compositions comprising these compounds. The compounds and pharmaceutical compositions of this invention are particularly well suited for inhibiting ICE activity and consequently, may be advantageously used as agents against interleukin-1 mediated diseases, including inflammatory diseases, autoimmune diseases and neurodegenerative diseases. This invention also relates to methods for inhibiting ICE activity and methods for treating interleukin-1 mediated diseases using the compounds and compositions of this invention.
Owner:VERTEX PHARMA INC
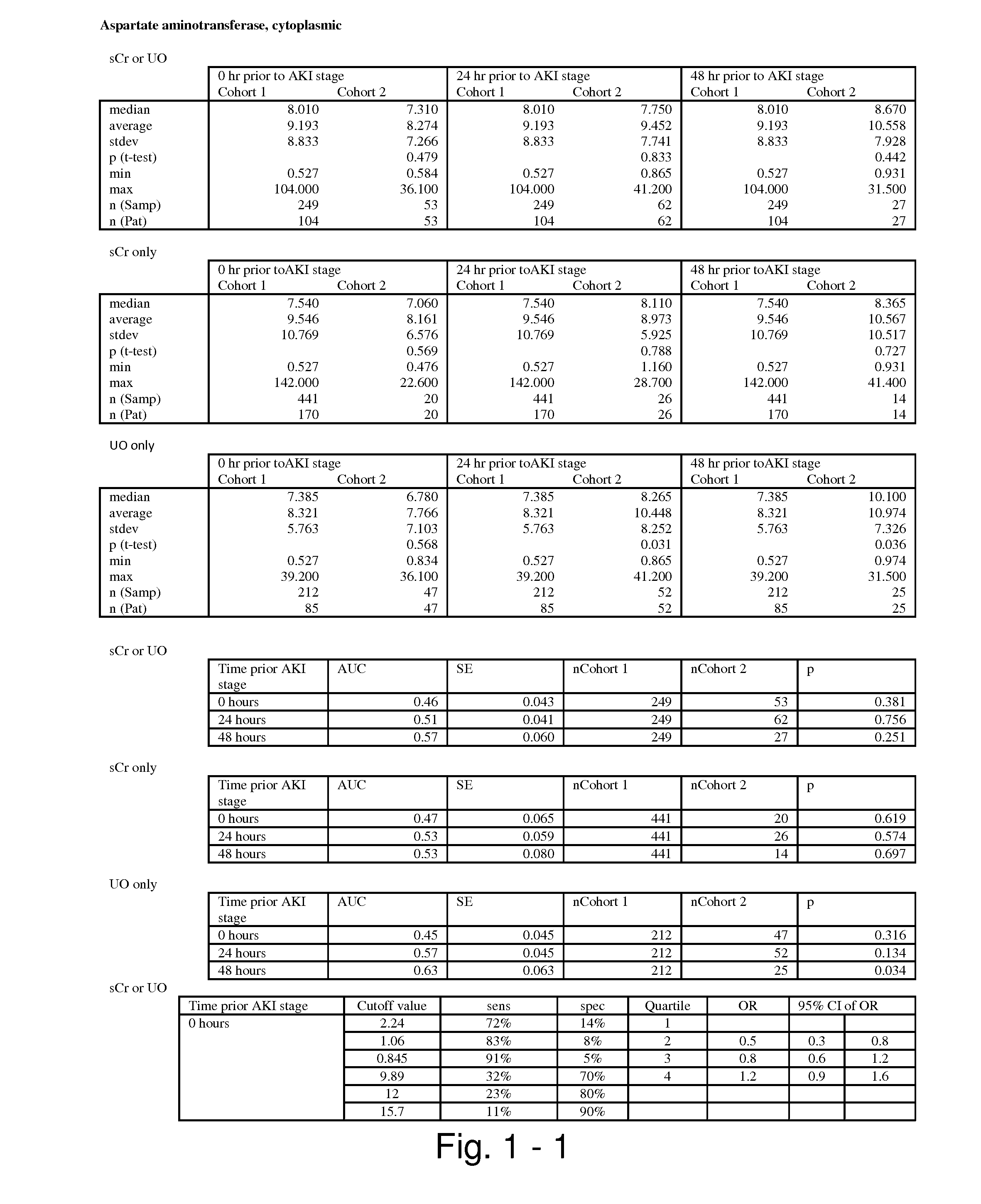
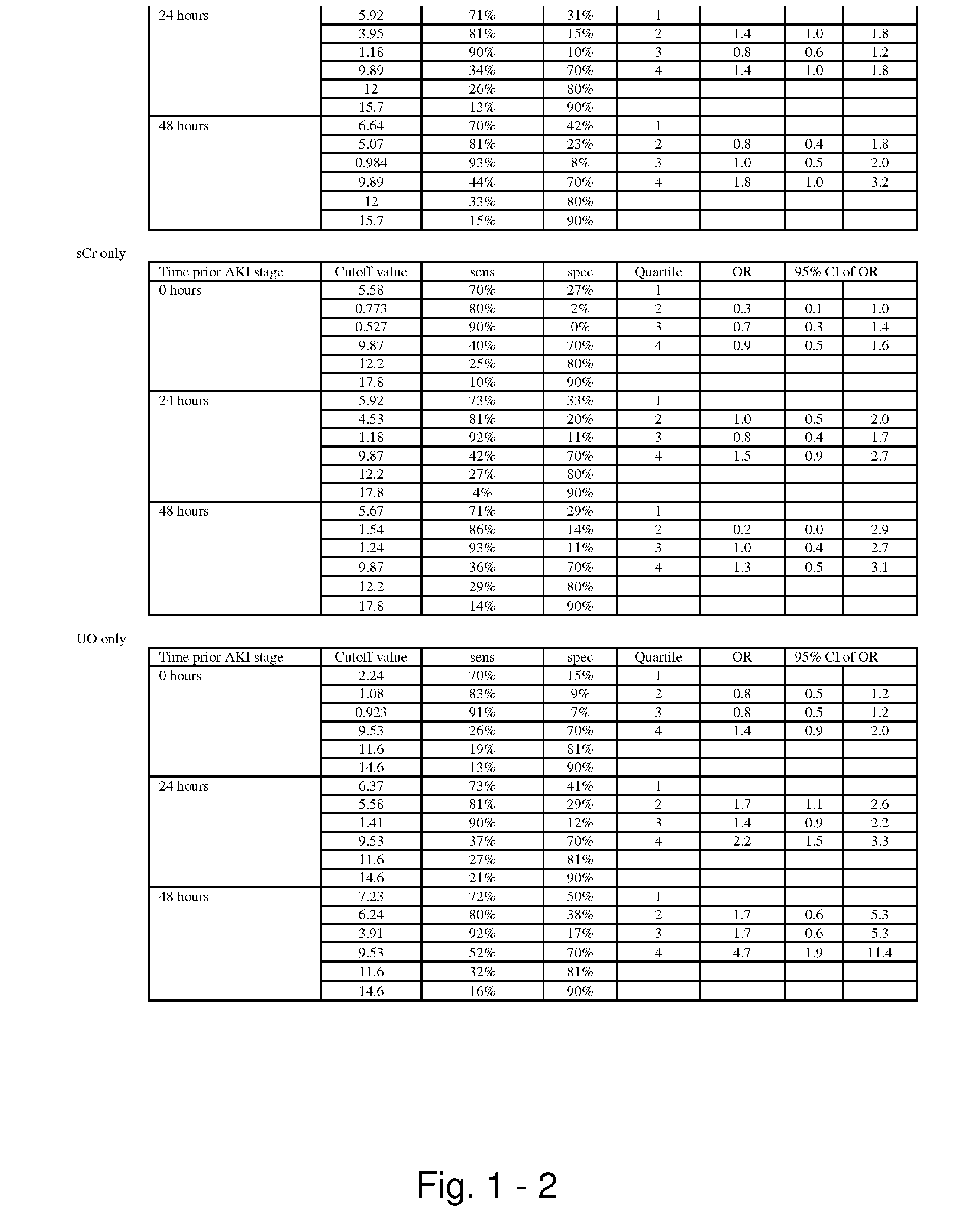
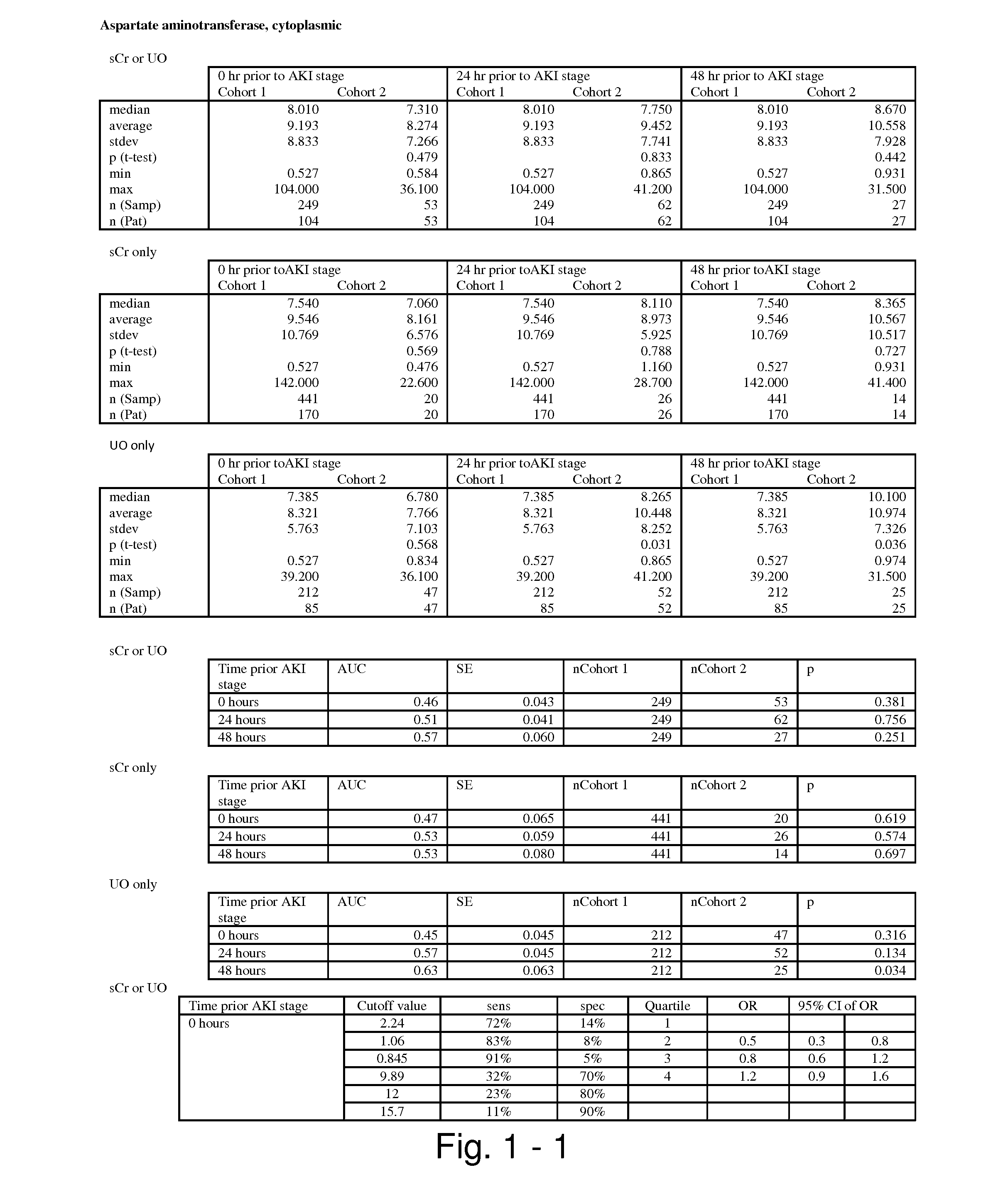
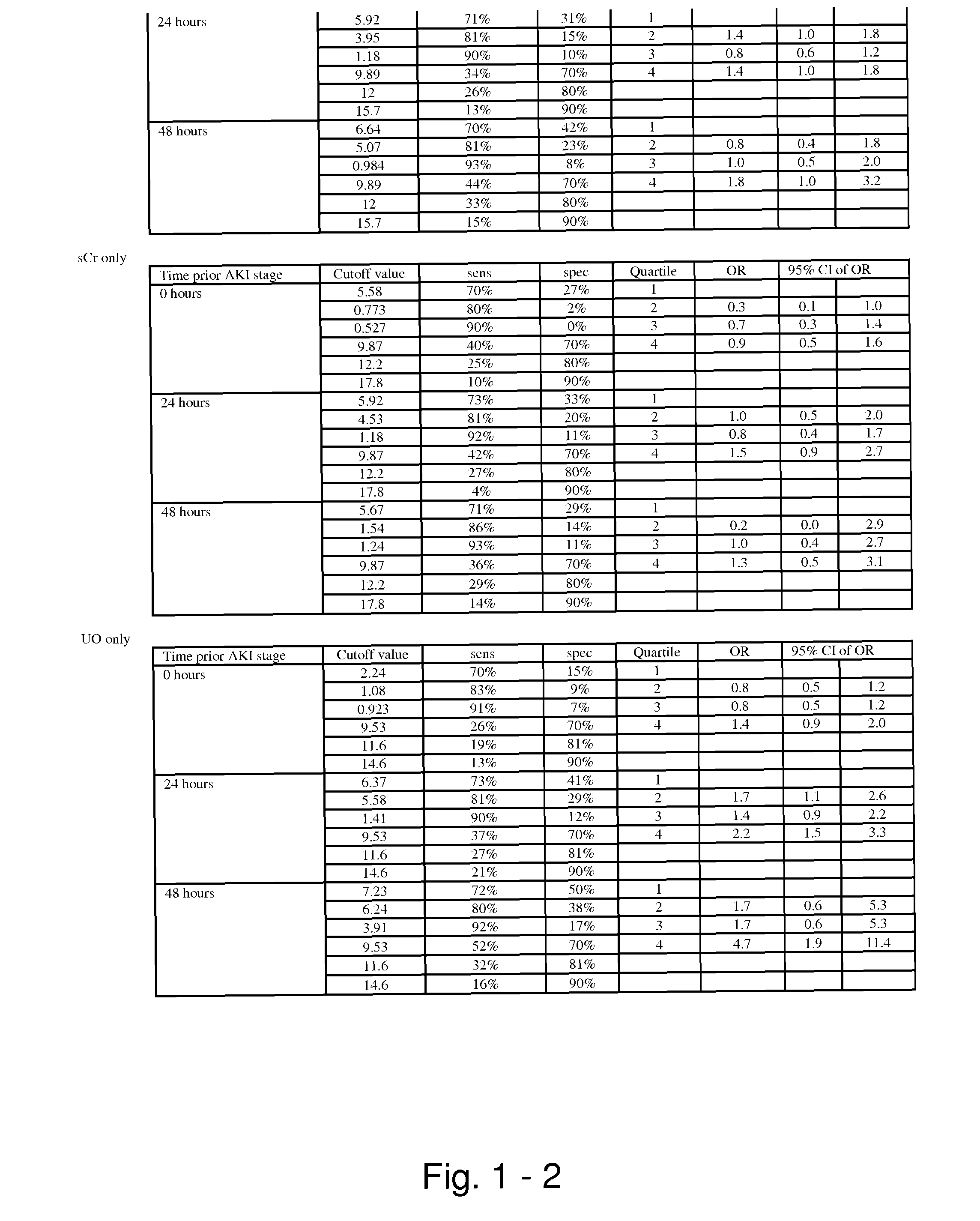
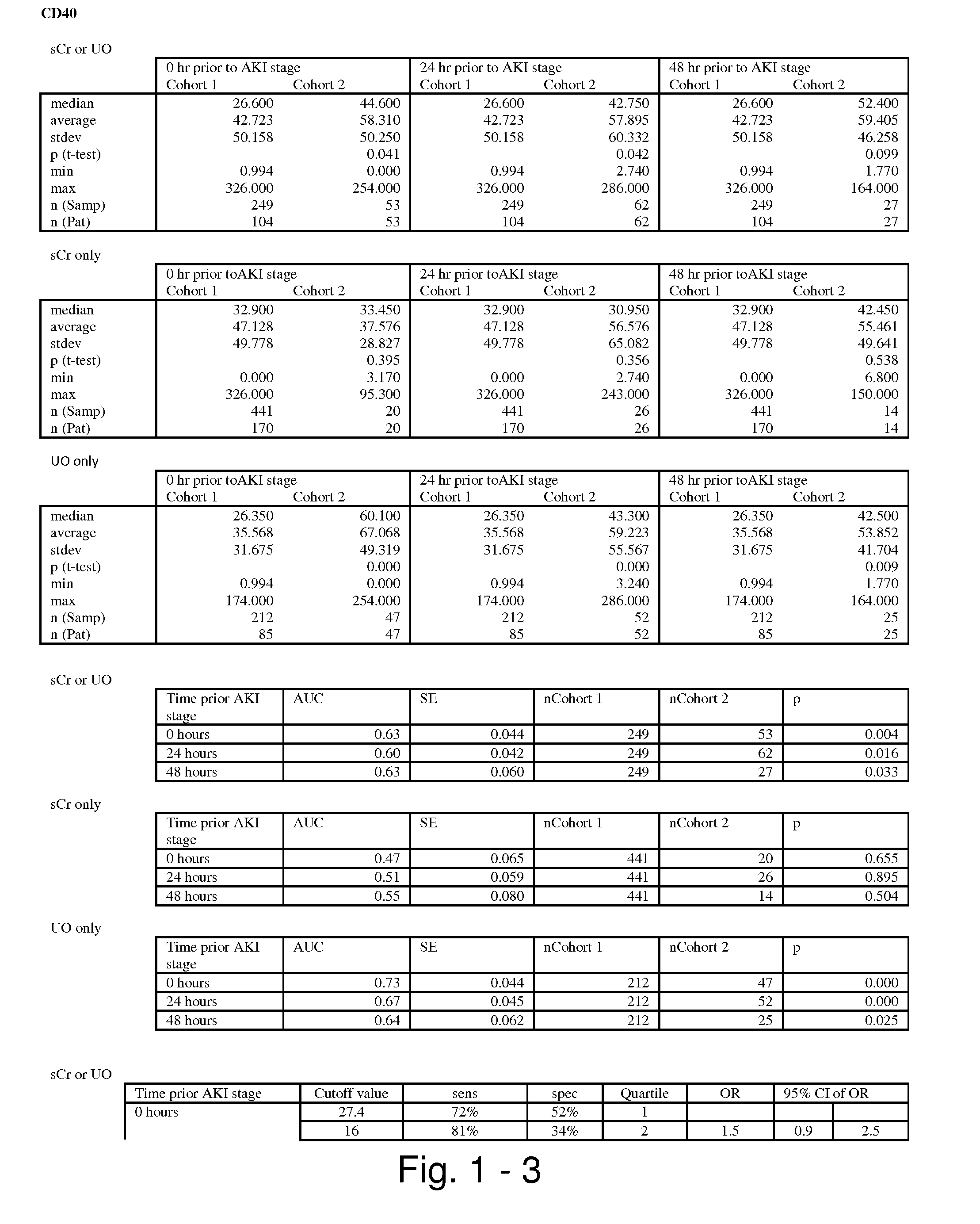
Methods and compositions for diagnosis and prognosis of renal injury and renal failure
ActiveUS20110201038A1Easy to adaptMicrobiological testing/measurementDisease diagnosisInterleukin 10Soluble P-Selectin
The present invention relates to methods and compositions for monitoring, diagnosis, prognosis, and determination of treatment regimens in subjects suffering from or suspected of having a renal injury. In particular, the invention relates to using assays that detect one or more markers selected from the group consisting of Cytoplasmic aspartate aminotransferase, soluble Tumor necrosis factor receptor superfamily member 5, soluble CD40 Ligand, soluble C-X-C Motif chemokine 16, S100-A12, Eotaxin, soluble E-selectin, Fibronectin, Granulocyte colony-stimulating factor, Granulocyte-macrophage colony-stimulating factor, Heparin-binding growth factor 2, soluble Hepatocyte growth factor receptor, Interleukin-1 receptor antagonist, Interleukin-1 beta, Interleukin-10, Interleukin-15, Interleukin-3, Myeloperoxidase, Nidogen-1, soluble Oxidized low-density lipoprotein receptor 1, Pappalysin-1, soluble P-selectin glycoprotein ligand 1, Antileukoproteinase, soluble Kit ligand, Tissue inhibitor of metalloproteinase 1, Tissue inhibitor of metalloproteinase 2, soluble Tumor necrosis factor, soluble Vascular cell adhesion molecule 1, and Vascular endothelial growth factor A as diagnostic and prognostic biomarkers in renal injuries.
Owner:ASTUTE MEDICAL
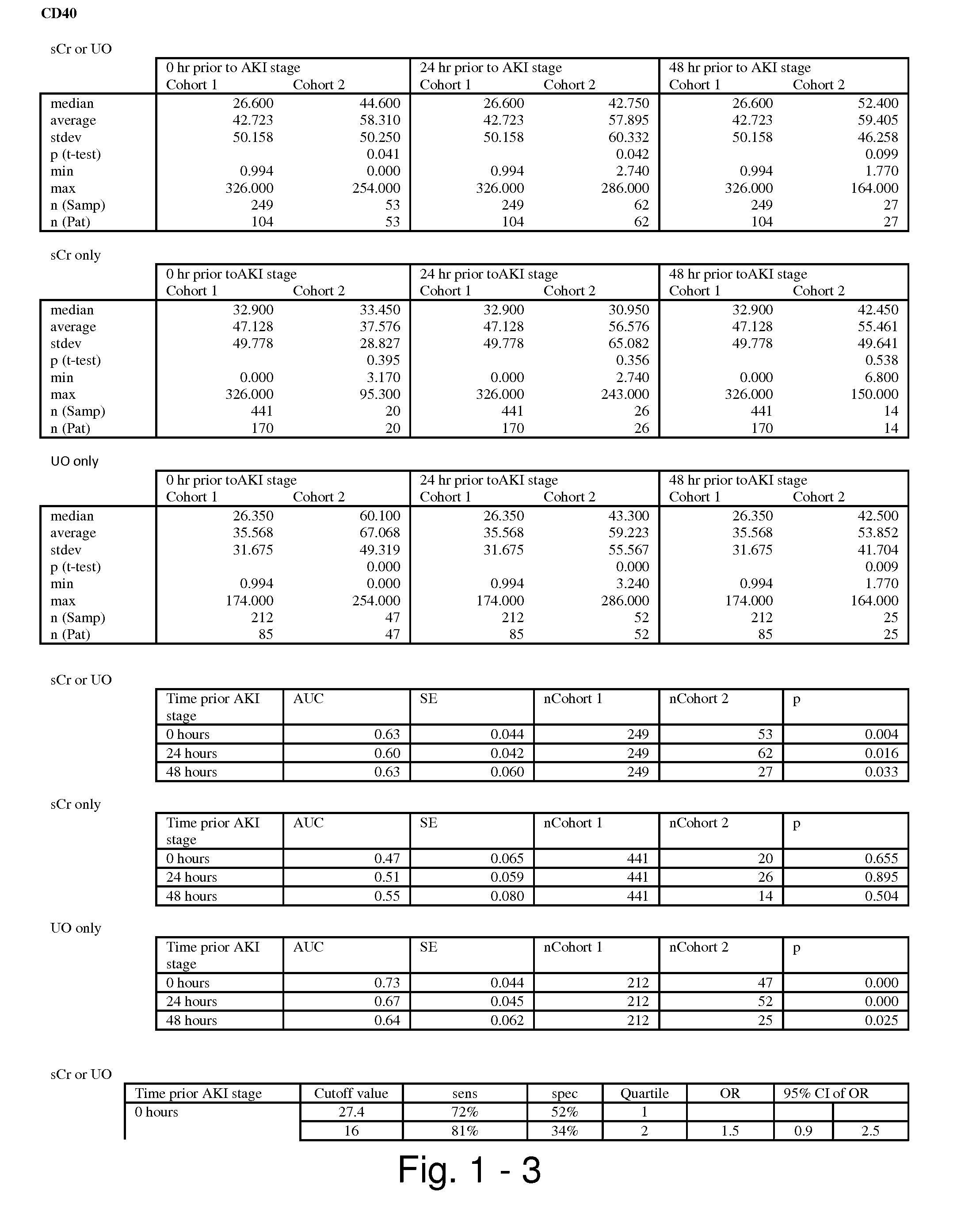
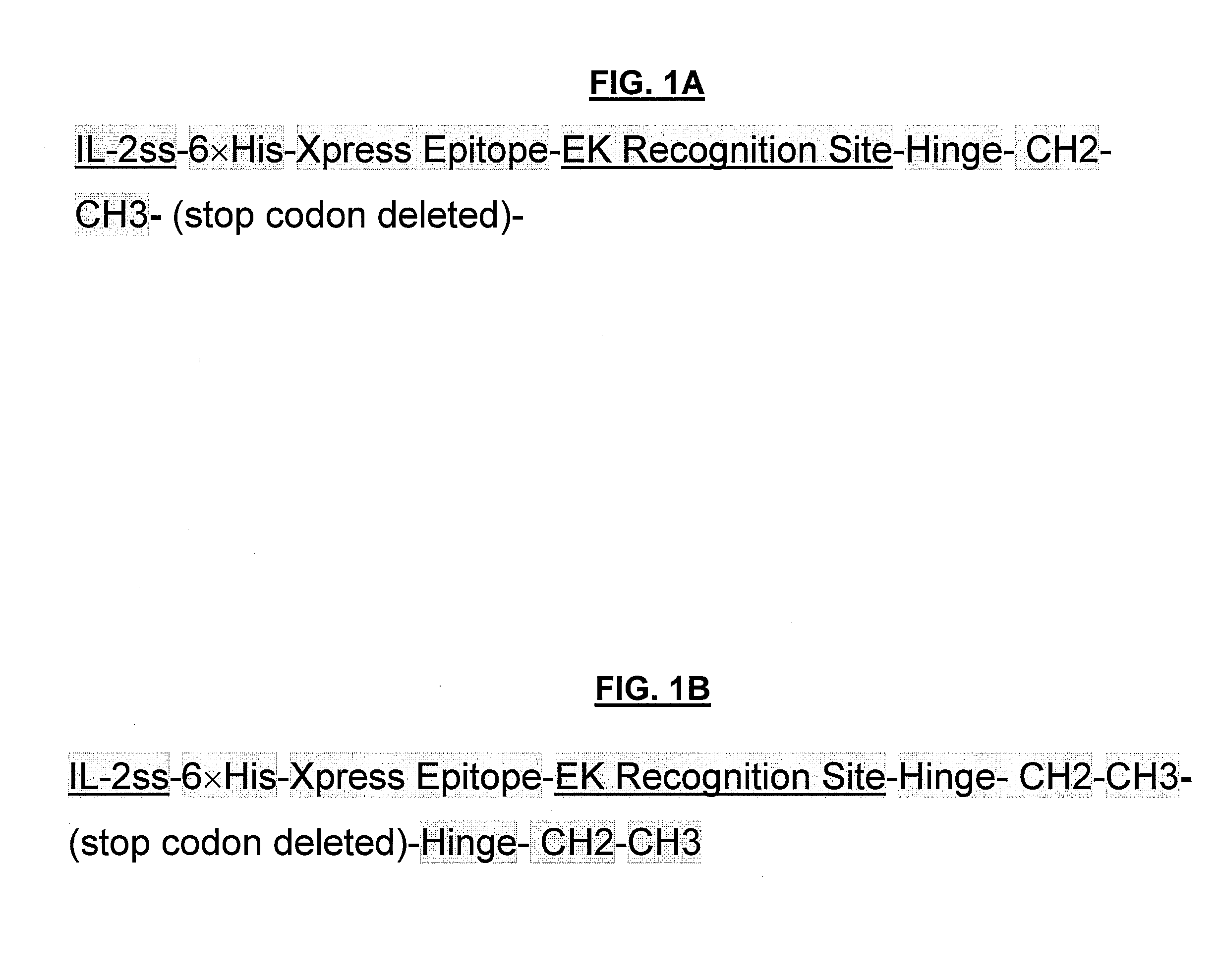
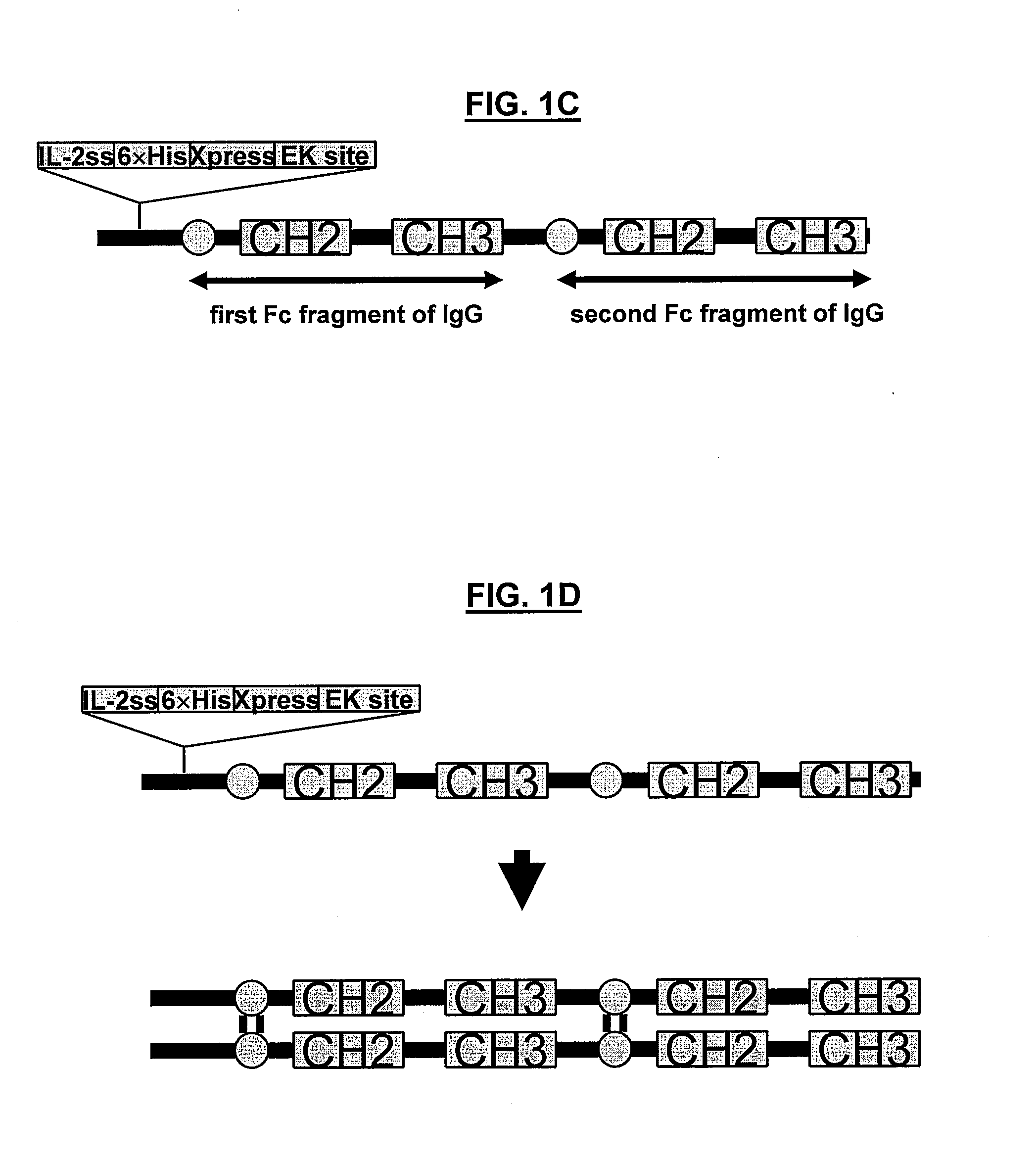
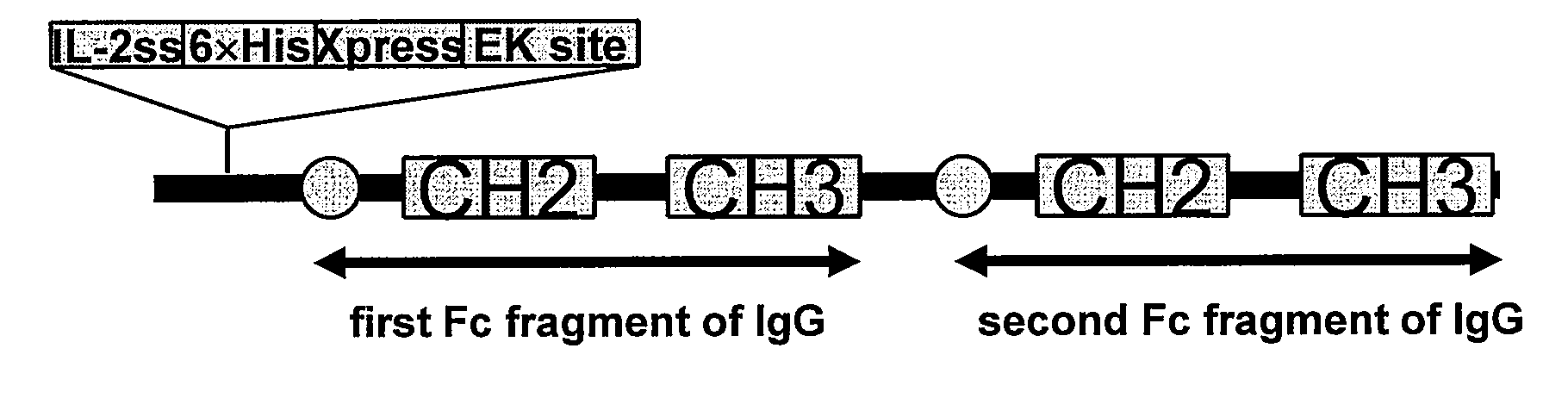
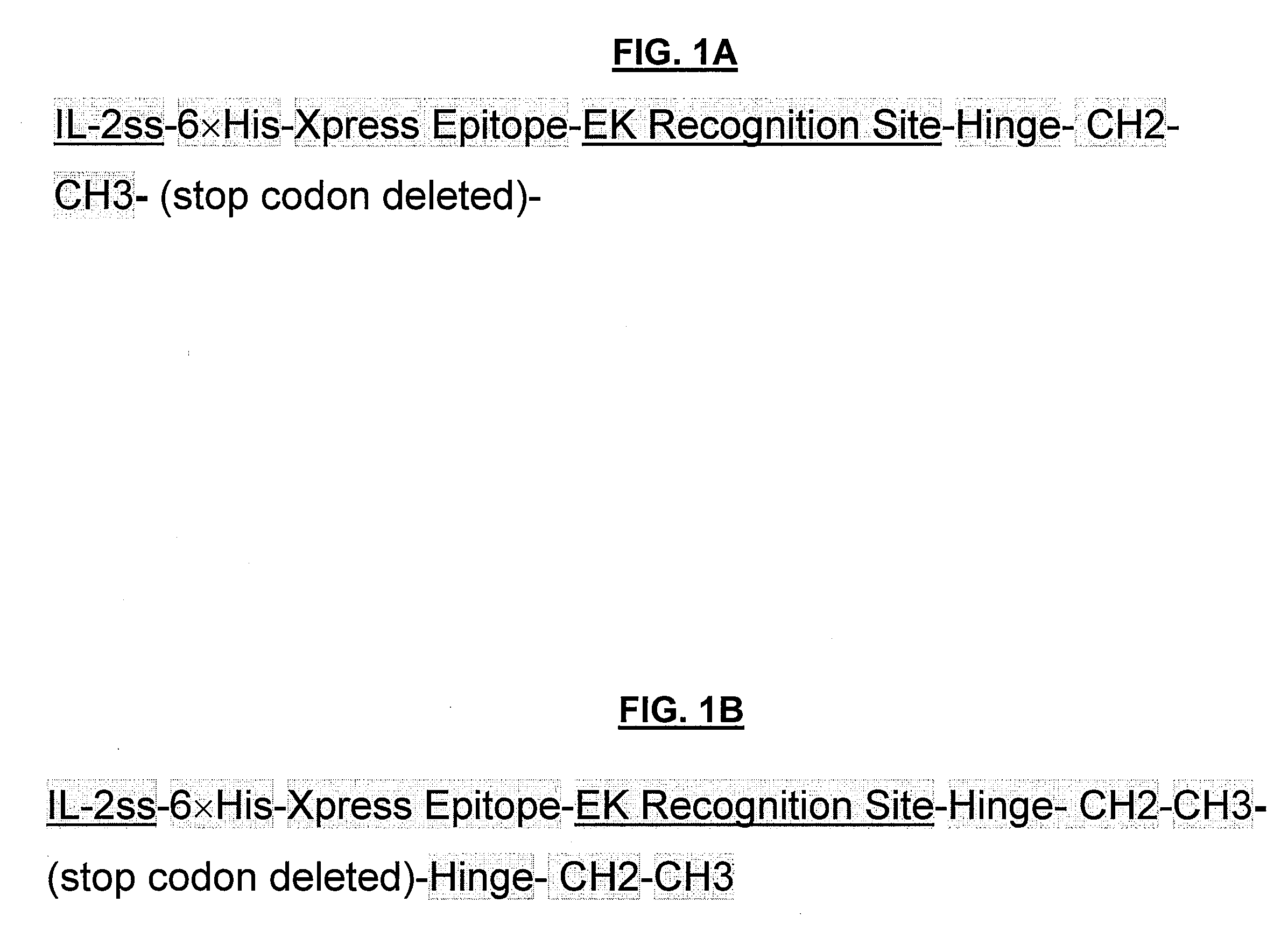
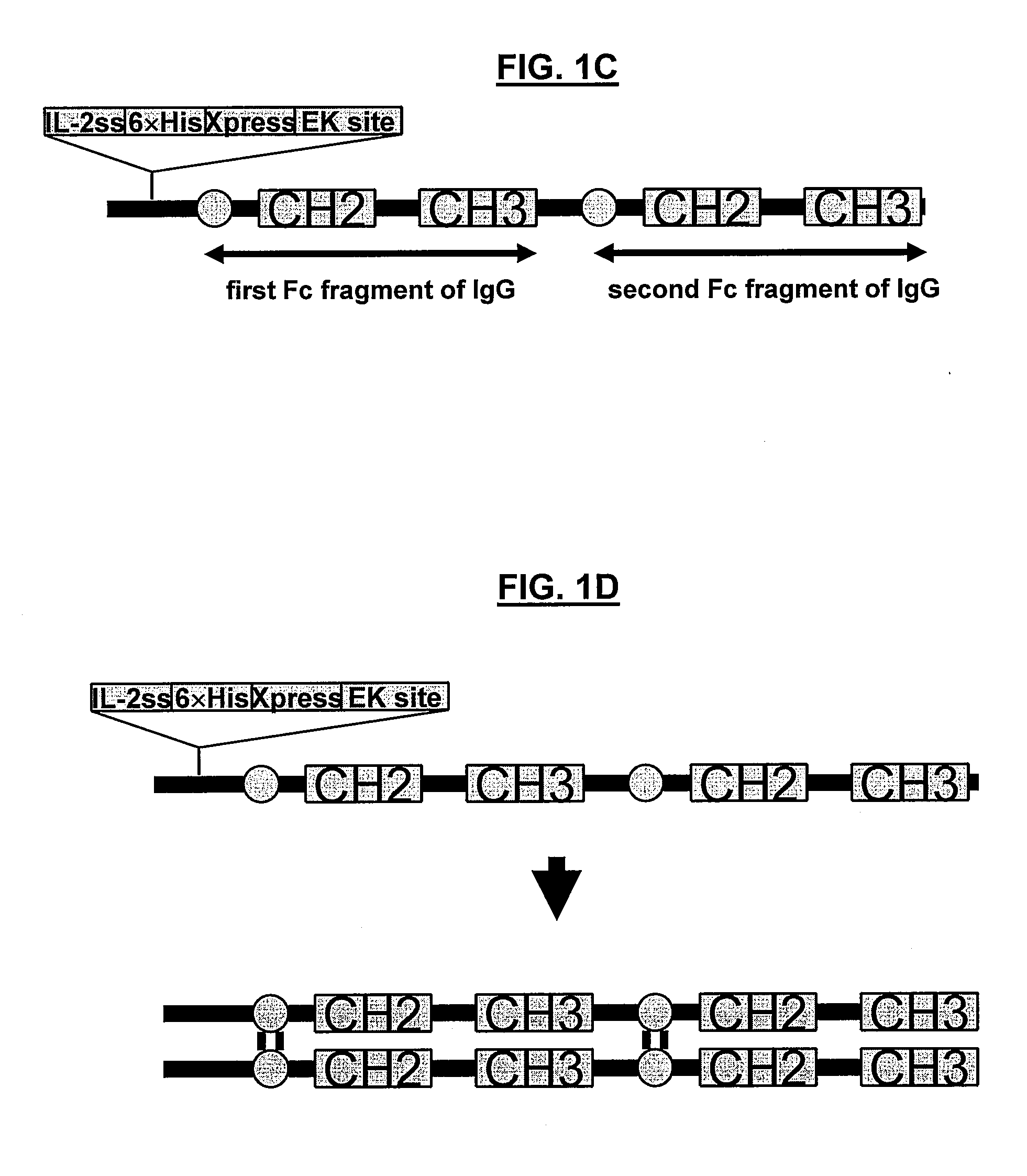
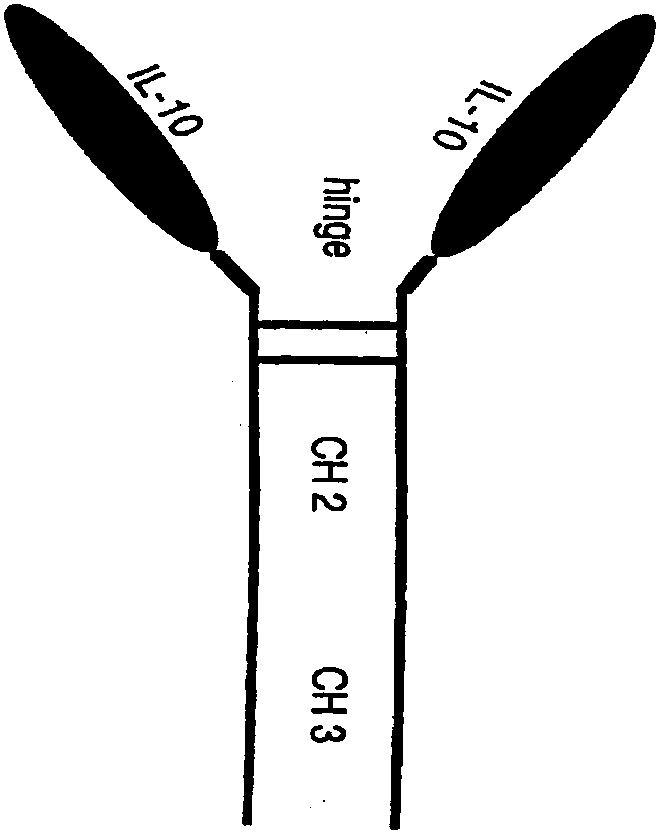
POLYPEPTIDES COMPRISING Fc FRAGMENTS OF IMMUNOGLOBULIN G (lgG) AND METHODS OF USING THE SAME
Polypeptides comprising at least a first and second Fc fragment of IgG that can be used to induce a stimulated cell to produce the anti-inflammatory cytokine Interleukin-10 and methods of using the same are disclosed herein.
Owner:LEUKOSIGHT +1
Algorithm for estimating the outcome of inflammation following injury or infection
InactiveUS20030087285A1Medical simulationMicrobiological testing/measurementInterleukin 6Interleukin 10
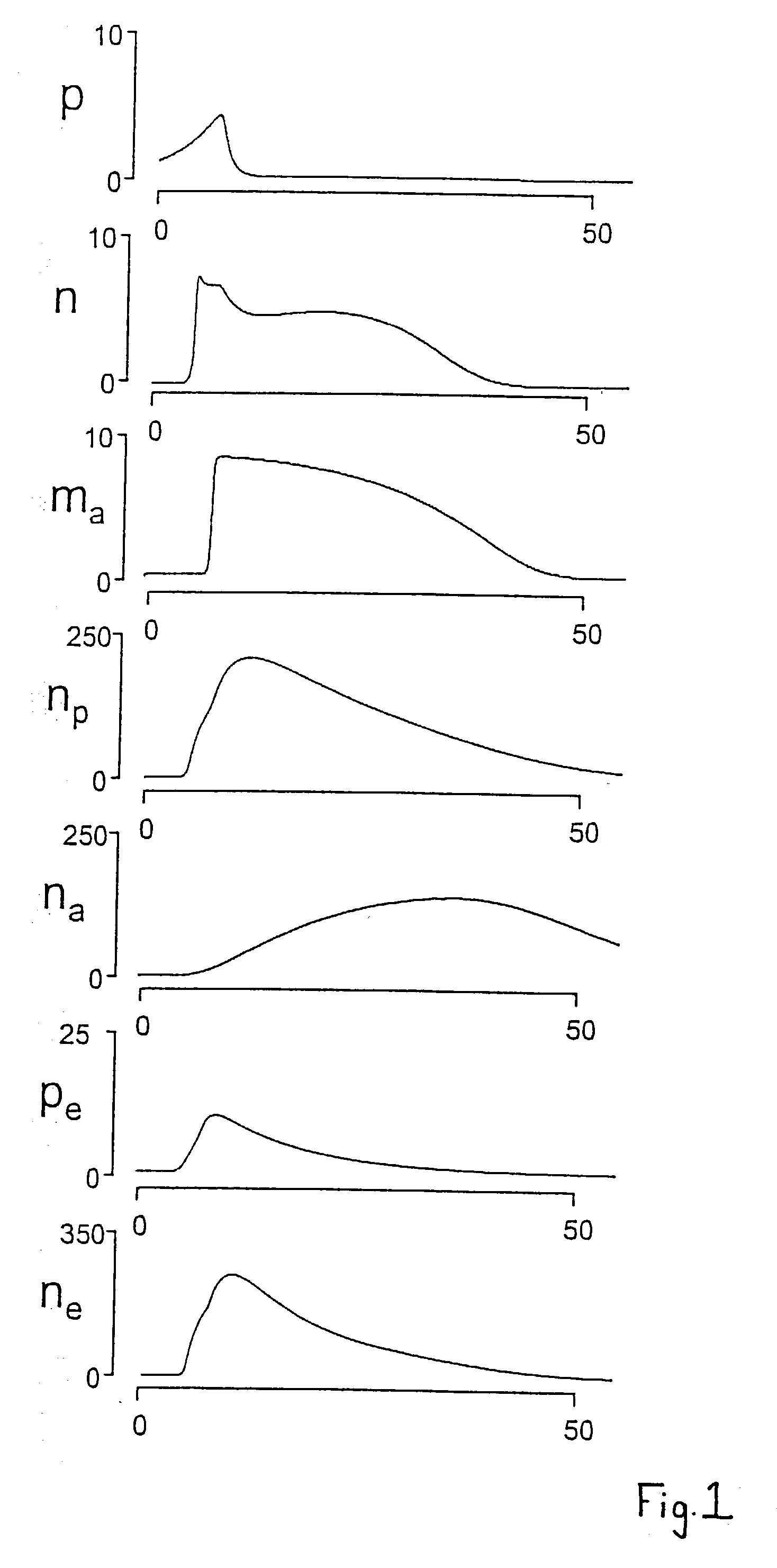
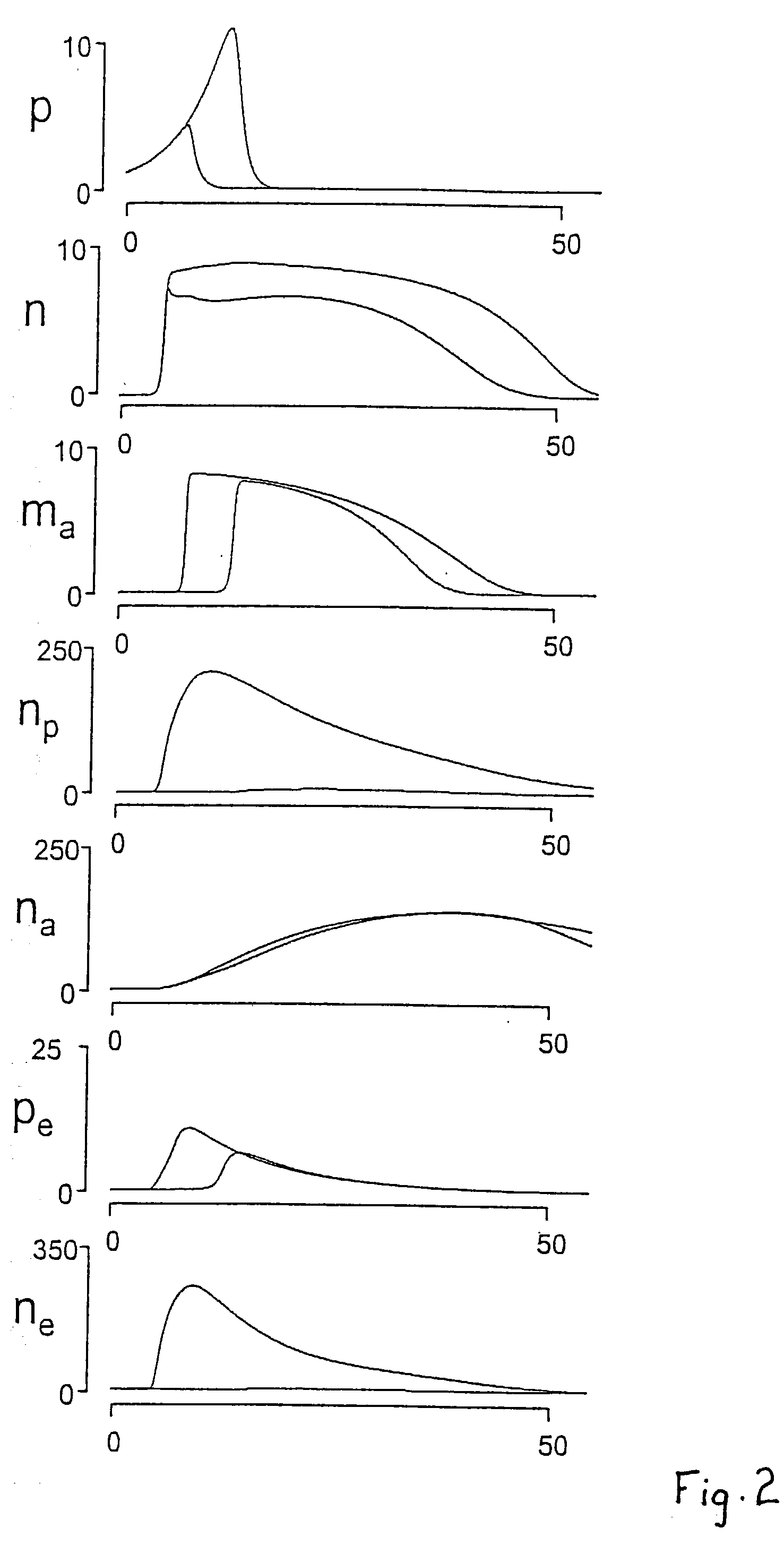
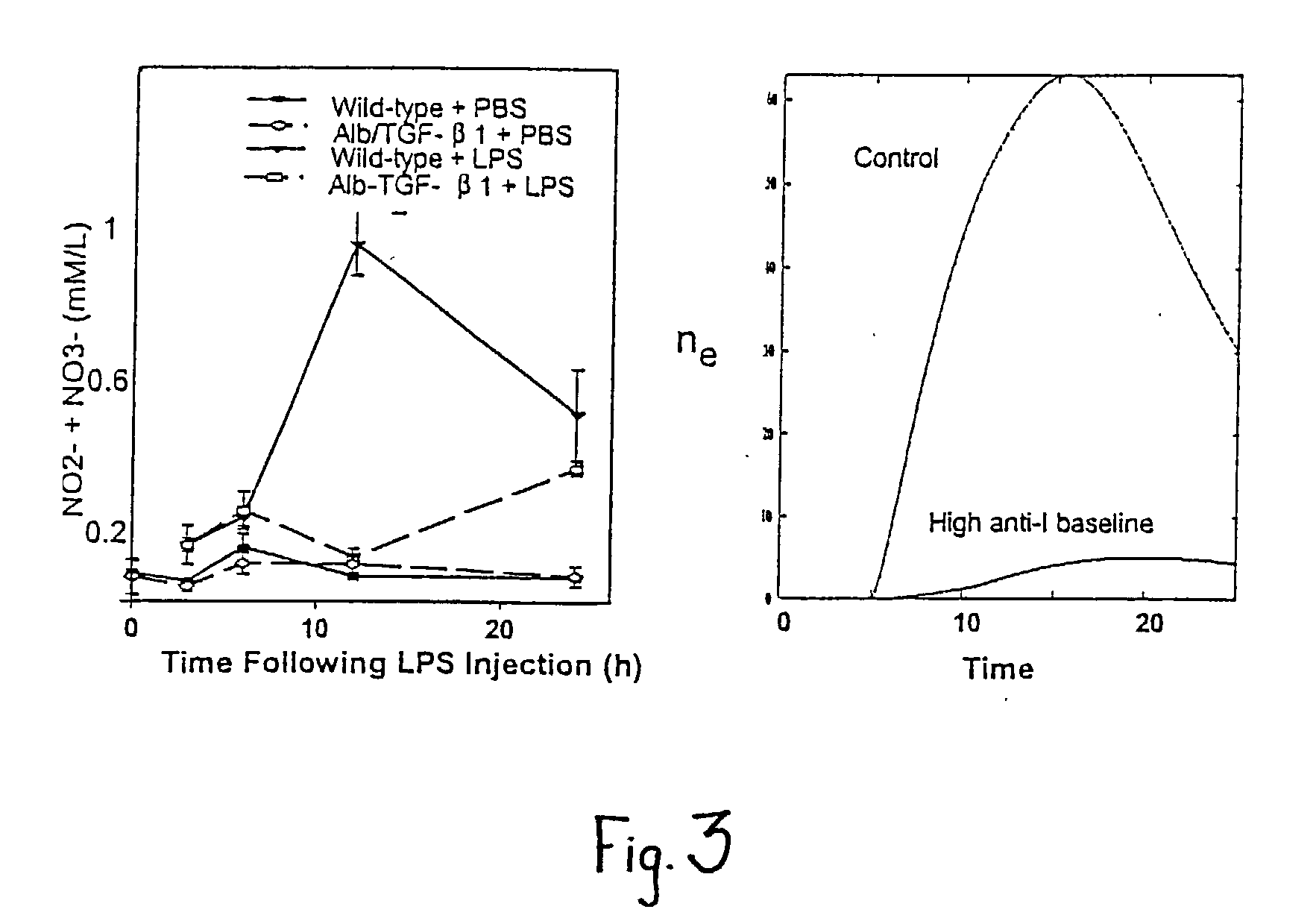
A mathematical prognostic in which changes in a number of physiologically significant factors are measured and interpolated to determine a "damage function" incident to bacterial infection or other serious inflammation. By measuring a large number of physiologically significant factors including, but not limited to, Interleukin 6 (IL6), Interleukin 10 (IL10), Nitric Oxide (NO), and others, it is possible to predict life versus death by the damage function, dD / dt, which measures and interpolates differential data for a plurality of factors. In mammals, an IL6 / NO ratio <8 at 12 hours post infection is highly predictive (60%) of mortality; also in mammals, an IL6 / NO ratio <4 at 24 hours post infection is highly predictive (52%) of mortality; and an IL6 / IL10 ratio in mammals of <7.5 at 24 hours post infection is highly predictive (68%) of mortality.
Owner:PITTSBURGH UNIV OF
Interleukin-10 antibodies
The methods and compositions provided herein relate generally to IL-10 specific antibodies and uses thereof. More specifically, the methods and compositions provided herein relate to humanized IL-10 specific antibodies and methods to use such antibodies in modulating the biological activity of IL-10, particularly in autoimmune disorders and pathogen-mediated immunopathology.
Owner:MERCK SHARP & DOHME LLC
Use of parasitic biological agents for prevention and control of allergic and other IgE-mediated disorders
InactiveUS20070087020A1Reduce and eliminate and ameliorate inappropriate immune responseStimulate immune responseProtozoa antigen ingredientsLeech/worm material medical ingredientsDiseaseInterleukin 10
The present invention describes using, on a repetitive basis, a non-human colonizing helminth compound, in an amount sufficient to establish a transitory parasitic helminth infection and or to simulate in a parasitic helminth infection, thereby having immunosuppressive effect against benign antigens and or stimulating a regulatory immune response characterized by the production of T helper cells 2 (Th2), T regulatory helper cells (TREG) and certain cytokines, including, but not limited to interleukin 10 (IL-10), as a therapy or prophylaxis of allergy and other IgE-mediated disorders, which are marked by an inappropriate IgE immune response including, but not limited to an aberrant and or enhanced IgE antibody production to benign antigens. The invention presents using helminth compound by administering it in a frequency and amount sufficient to eliminate or ameliorate the inappropriate immune response in an asthmatic and or allergic individual.
Owner:MILESTONE RES
Generation of human regulatory T cells by bacterial toxins for the treatment of inflammatory disorders
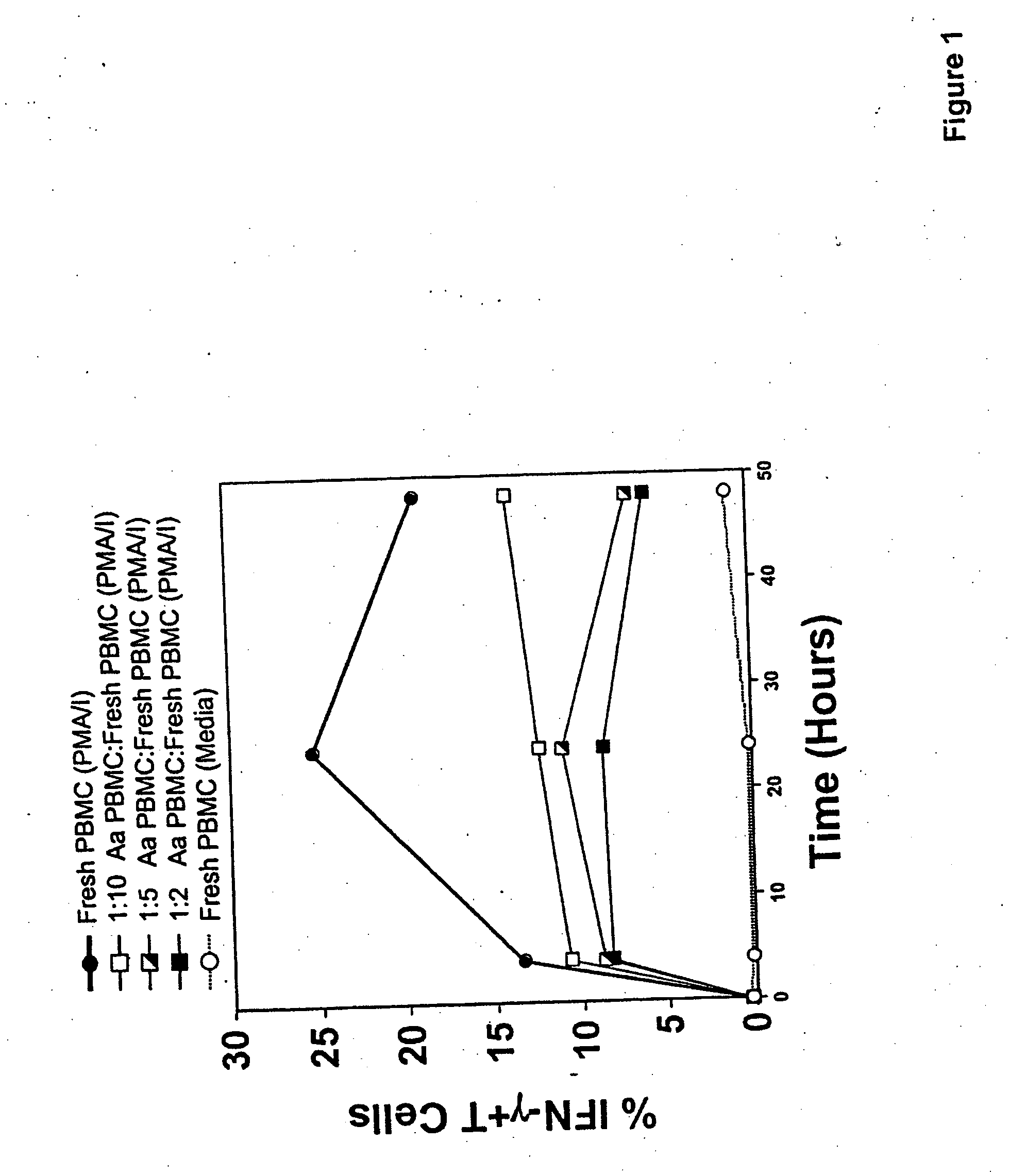
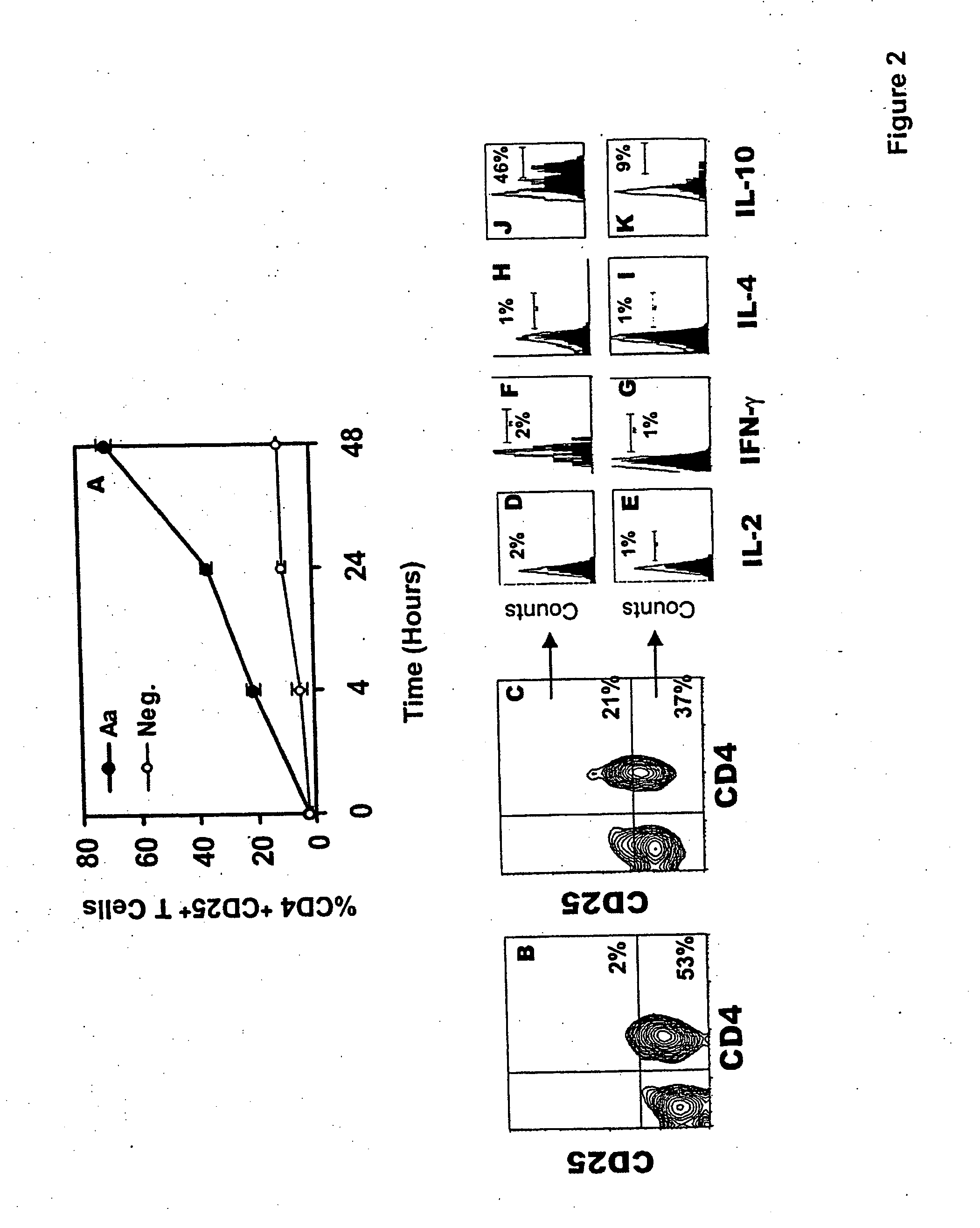
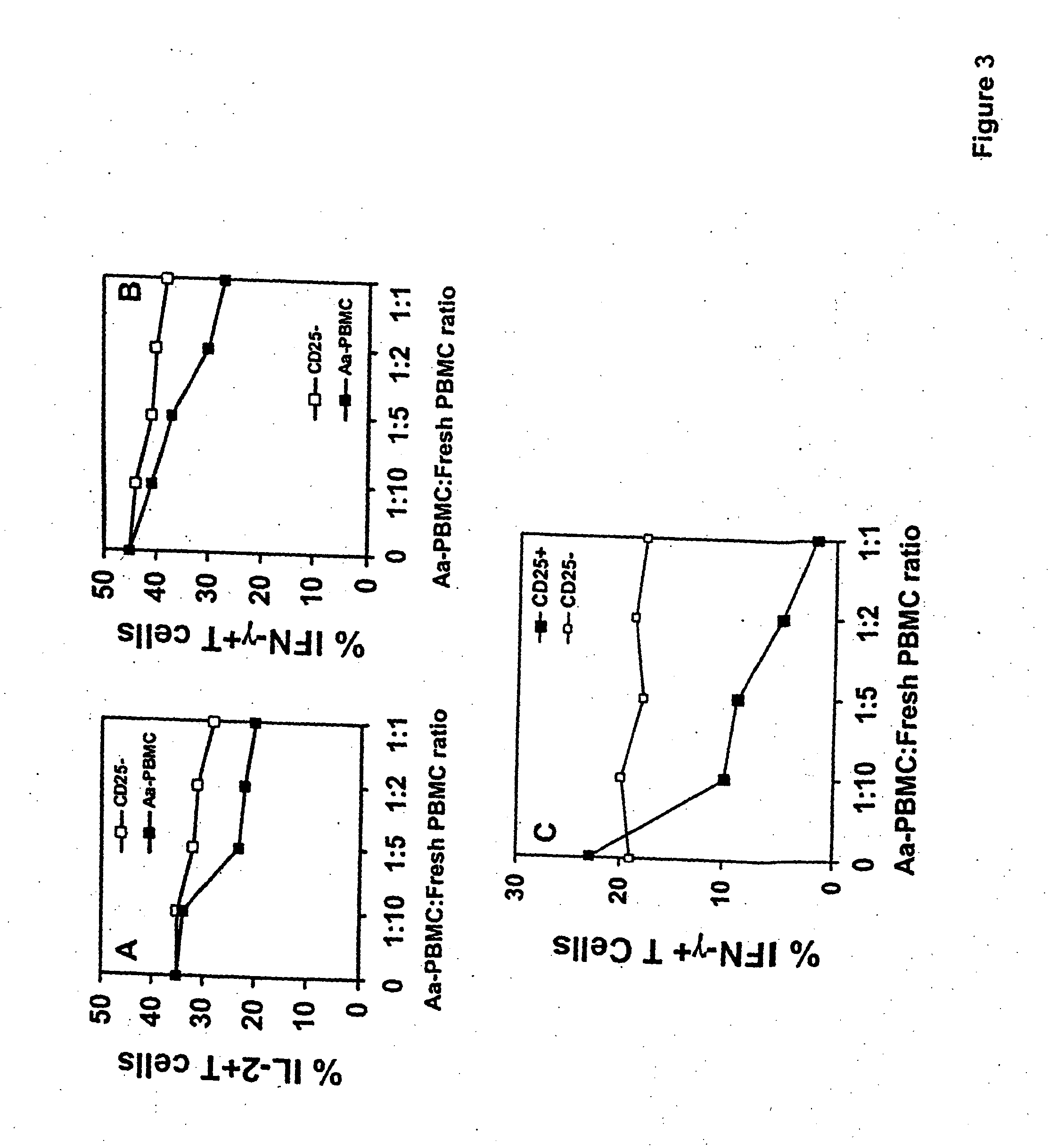
An adoptive immunotherapy using ex vivo-generated regulatory T cells may be used for the suppression of undesireable immune response. T cells are to be obtained from the patient's blood, and upon exposure to a set of toxins from the pathogen A. actinomycetemcomitans, the population of regulatory T cells will be enriched ex vivo and adoptively transferred back to the patient. The novel aspect of the present invention is that it generates large numbers of type 1 regulatory T cells, which secrete Interleukin-10.
Owner:UNIV OF SOUTHERN CALIFORNIA
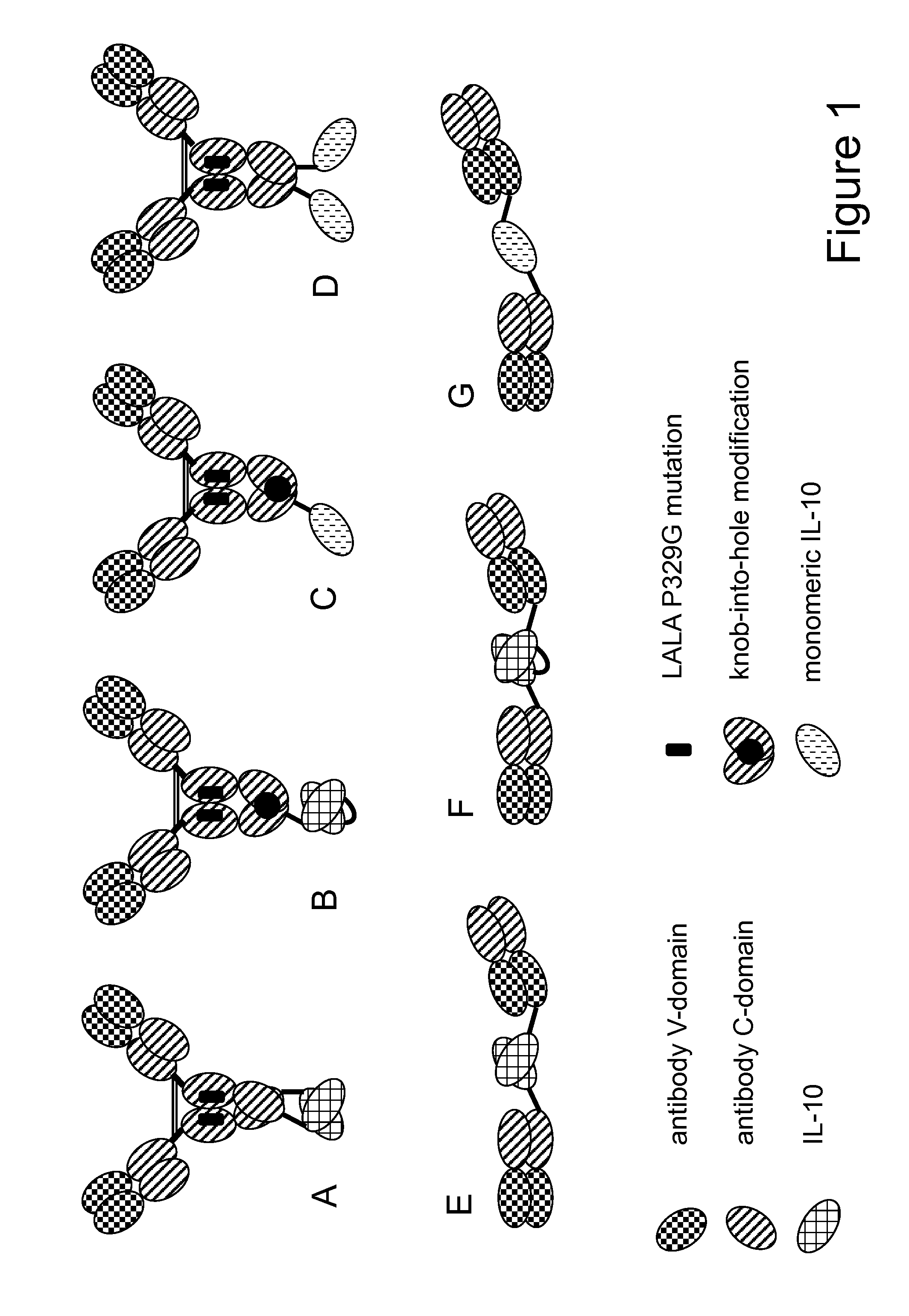
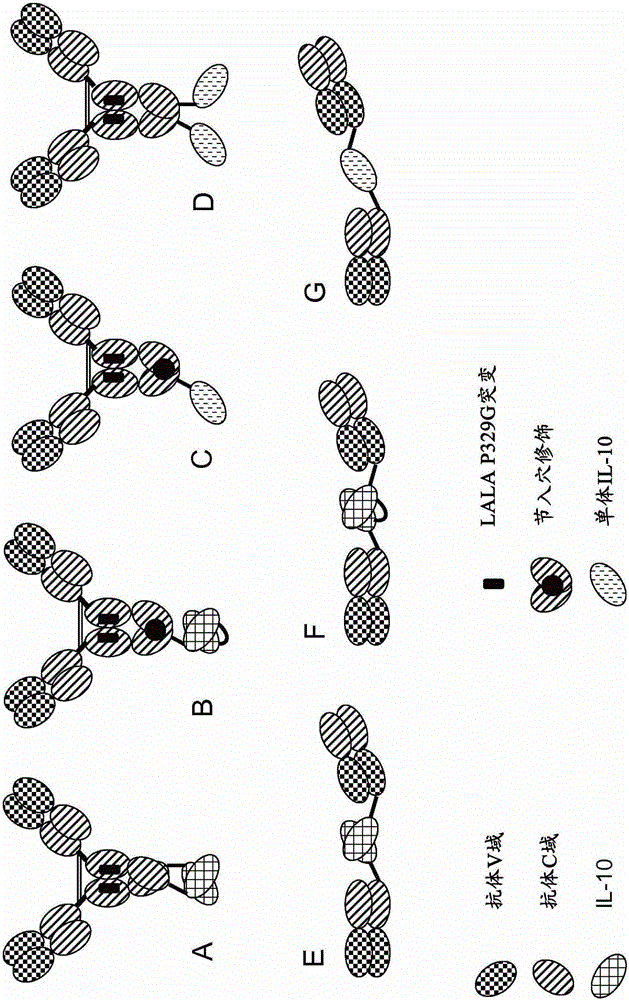
Interleukin-10 fusion proteins
The present invention generally relates to fusion proteins of antibodies and interleukin-10 (IL-10). In addition, the present invention relates to polynucleotides encoding such fusion proteins, and vectors and host cells comprising such polynucleotides. The invention further relates to methods for producing the fusion proteins of the invention, and to methods of using them in the treatment of disease.
Owner:ROCHE GLYCART AG
Interleukin-10 Polypeptide Conjugates and Their Uses
InactiveUS20150038678A1Long half-lifeEnhance tumor killing activityDepsipeptidesPeptide preparation methodsInterleukin 10Cytokine synthesis
This invention relates to interleukin-10 (IL-10) polypeptide conjugates comprising at least one non-naturally-encoded amino acid.
Owner:AMBRX
Latent phase viral interleukin-10-(VII-10) and uses thereof
InactiveUS20090214463A1Reduced activityOrganic active ingredientsPeptide/protein ingredientsInterleukin 10Nucleic acid sequencing
The present invention relates to a purified nucleic acid sequence encoding a homologue of human interleukin 10 (IL-10), wherein said IL-10 homologue is expressed during the latent phase of infection by a virus of the herpesvirideae group. The present invention also relates to uses of this polypeptide, in particular for diagnosing disease states and screening for modulator and inhibitor compounds of such polypeptides and in turn the virus itself, screening for infection in vertebrates and biological tissue, cleansing of infected biological tissues, and in the treatment and / or prophylaxis and / or diagnosis of disease caused by a virus of the herpesvirideae group.
Owner:THE UNIV OF SYDNEY
Methods and compositions for diagnosis and prognosis of renal injury and renal failure
ActiveUS8778615B2Easy to adaptMicrobiological testing/measurementAntibody ingredientsInterleukin 10Soluble P-Selectin
The present invention relates to methods and compositions for monitoring, diagnosis, prognosis, and determination of treatment regimens in subjects suffering from or suspected of having a renal injury. In particular, the invention relates to using assays that detect one or more markers selected from the group consisting of Cytoplasmic aspartate aminotransferase, soluble Tumor necrosis factor receptor superfamily member 5, soluble CD40 Ligand, soluble C-X-C Motif chemokine 16, S100-A12, Eotaxin, soluble E-selectin, Fibronectin, Granulocyte colony-stimulating factor, Granulocyte-macrophage colony-stimulating factor, Heparin-binding growth factor 2, soluble Hepatocyte growth factor receptor, Interleukin-1 receptor antagonist, Interleukin-1 beta, Interleukin-10, Interleukin-15, Interleukin-3, Myeloperoxidase, Nidogen-1, soluble Oxidized low-density lipoprotein receptor 1, Pappalysin-1, soluble P-selectin glycoprotein ligand 1, Antileukoproteinase, soluble Kit ligand, Tissue inhibitor of metalloproteinase 1, Tissue inhibitor of metalloproteinase 2, soluble Tumor necrosis factor, soluble Vascular cell adhesion molecule 1, and Vascular endothelial growth factor A as diagnostic and prognostic biomarkers in renal injuries.
Owner:ASTUTE MEDICAL
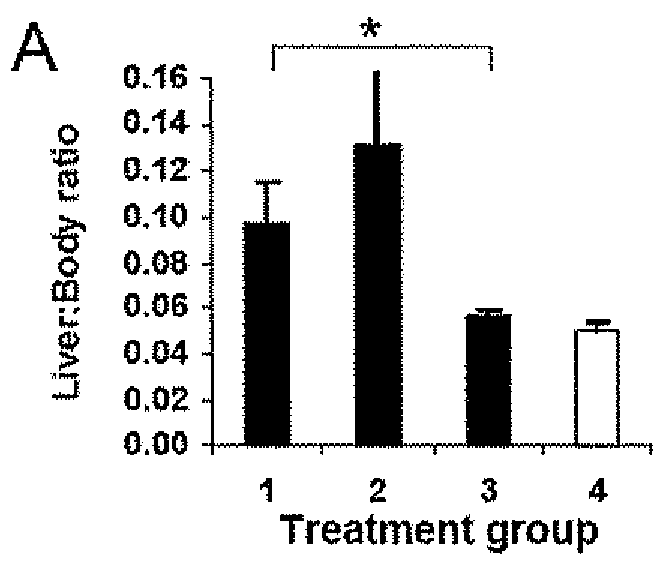
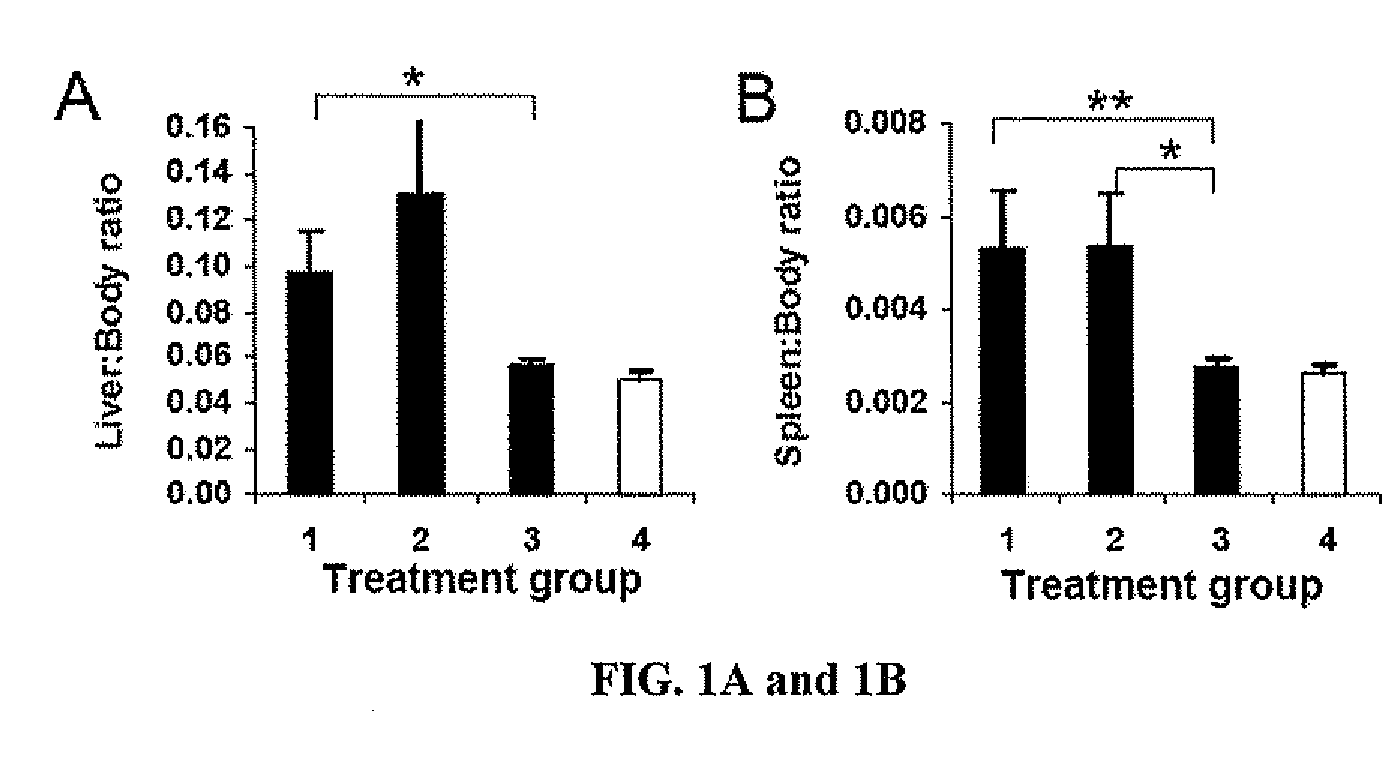
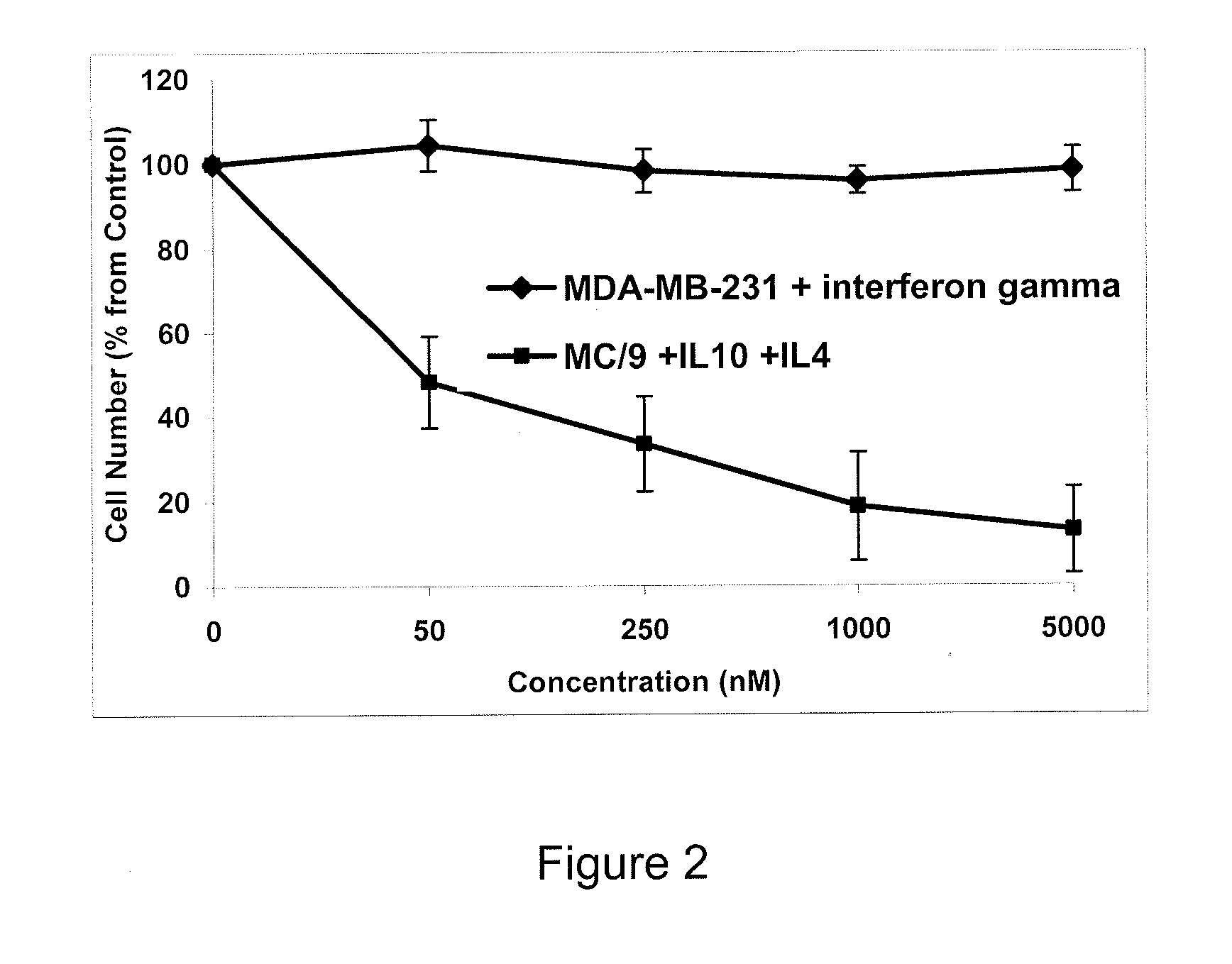
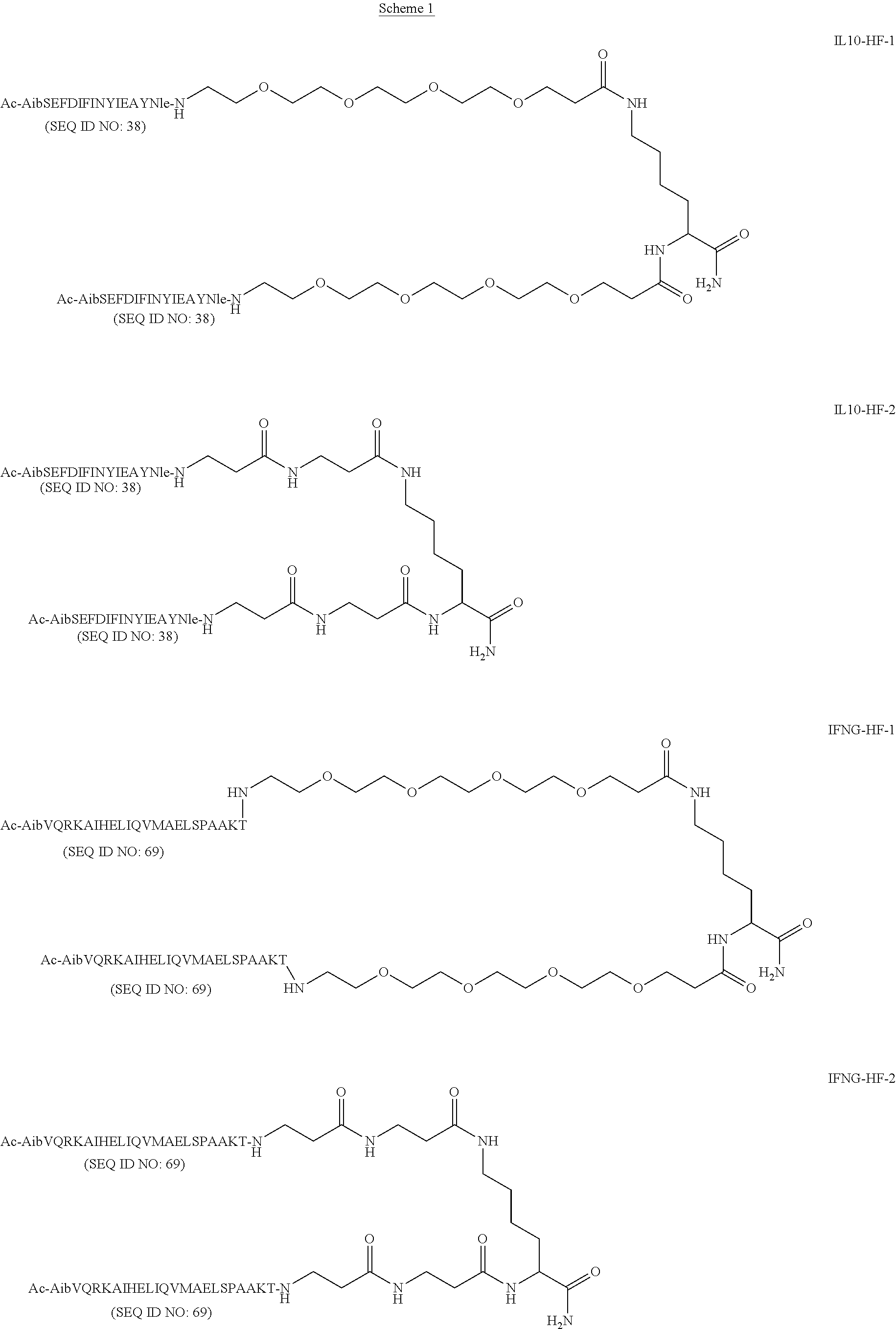
Methods of using interleukin-10 for treating diseases and disorders
ActiveUS10143726B2Improve propertiesLess immunogenicNervous disorderPeptide/protein ingredientsDiseaseInterleukin 10
Methods of treating subjects having a disease or disorder responsive to IL-10, including methods of administration and dosing regimens associated therewith, are provided.
Owner:ARMO BIOSCI
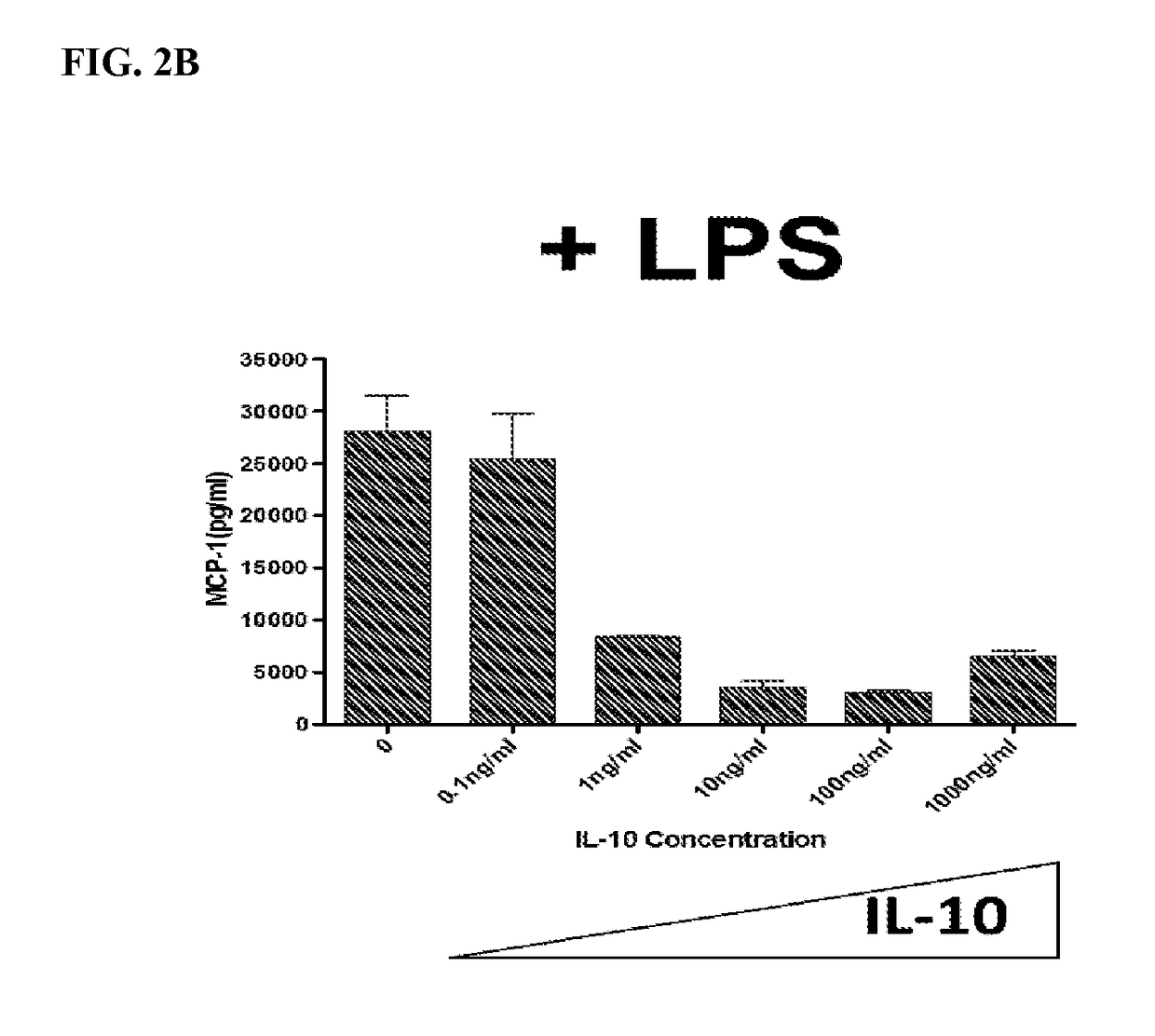
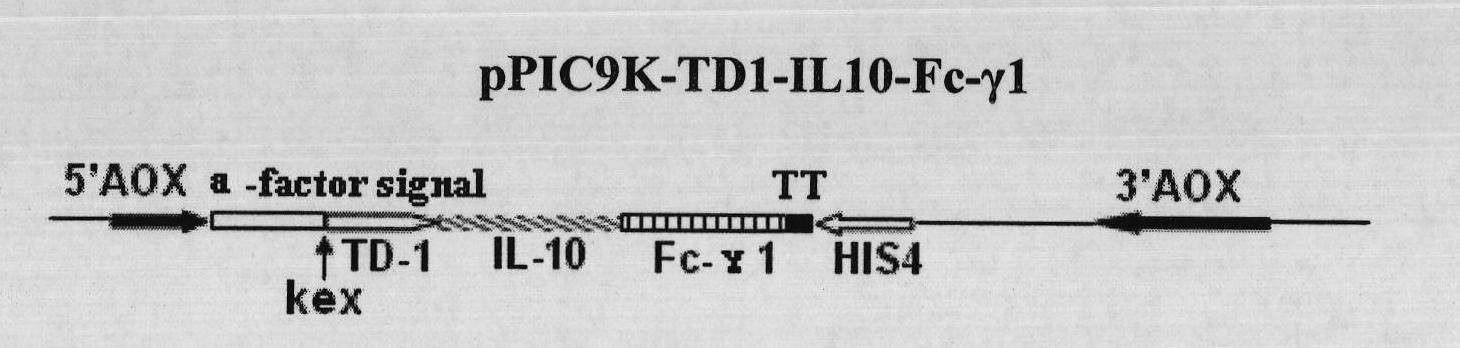
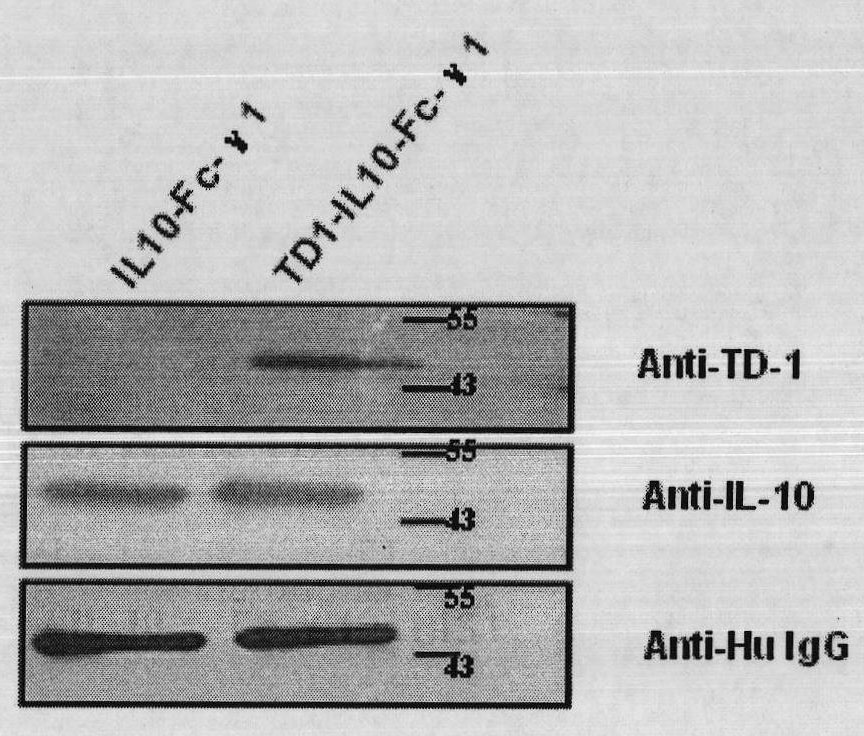
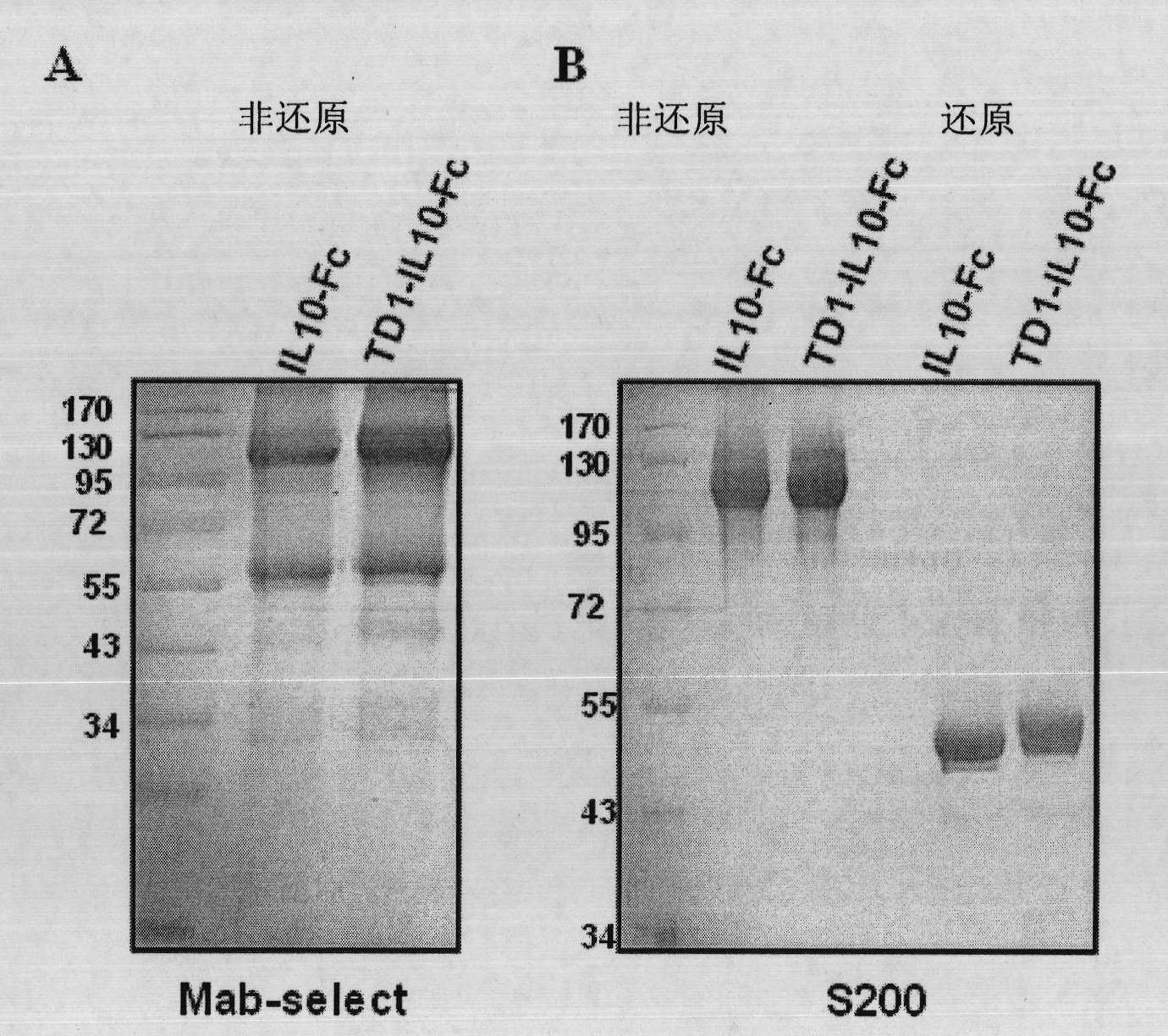
Fusion protein with transdermal capability and interleukin-10 activity as well as coding gene and application thereof
InactiveCN101962413AEasy to purifyShorten the production cycleFungiBacteriaInterleukin 10White blood cell
The invention discloses a fusion protein with transdermal capability and interleukin 10 activity as well as a coding gene and application thereof. The fusion protein provided by the invention is formed by connecting any one protein of (1) human IgG Fc-gamma1 or the mutain thereof and (2) human IgG Fc-gamma2 or the mutain thereof on the carboxyl terminal of a human interleukin 10 protein, and connecting a transit peptide on an amino terminal; wherein the amino acid sequence of the human interleukin 10 protein is the amino acid sequence from the 12th site to the 171st site on the amino terminal of a sequence 2 in a sequence table; and the amino acid sequence of the transit peptide is the amino acid sequence from the 1st site to the 11th site on the amino terminal of the sequence 2 in the sequence table. Tests prove that the fusion protein provided in the invention has the transdermal capability and the interleukin 10 activity simultaneously, and has greater practical significance and broad application prospect in the field of the preparation of medicaments for treating autoimmunity reaction and especially psoriasis.
Owner:UNIV OF SCI & TECH OF CHINA
Use Of Low Doses Of Oligonucleotides Antisense To TGF-Beta, VEGF, Interleukin-10, C-Jun, C-Fos Or Prostaglandin E2 Genes In The Treatment Of Tumors
InactiveUS20090306176A1Improve securityLess side effectsOrganic active ingredientsNervous disorderDiseaseInterleukin 10
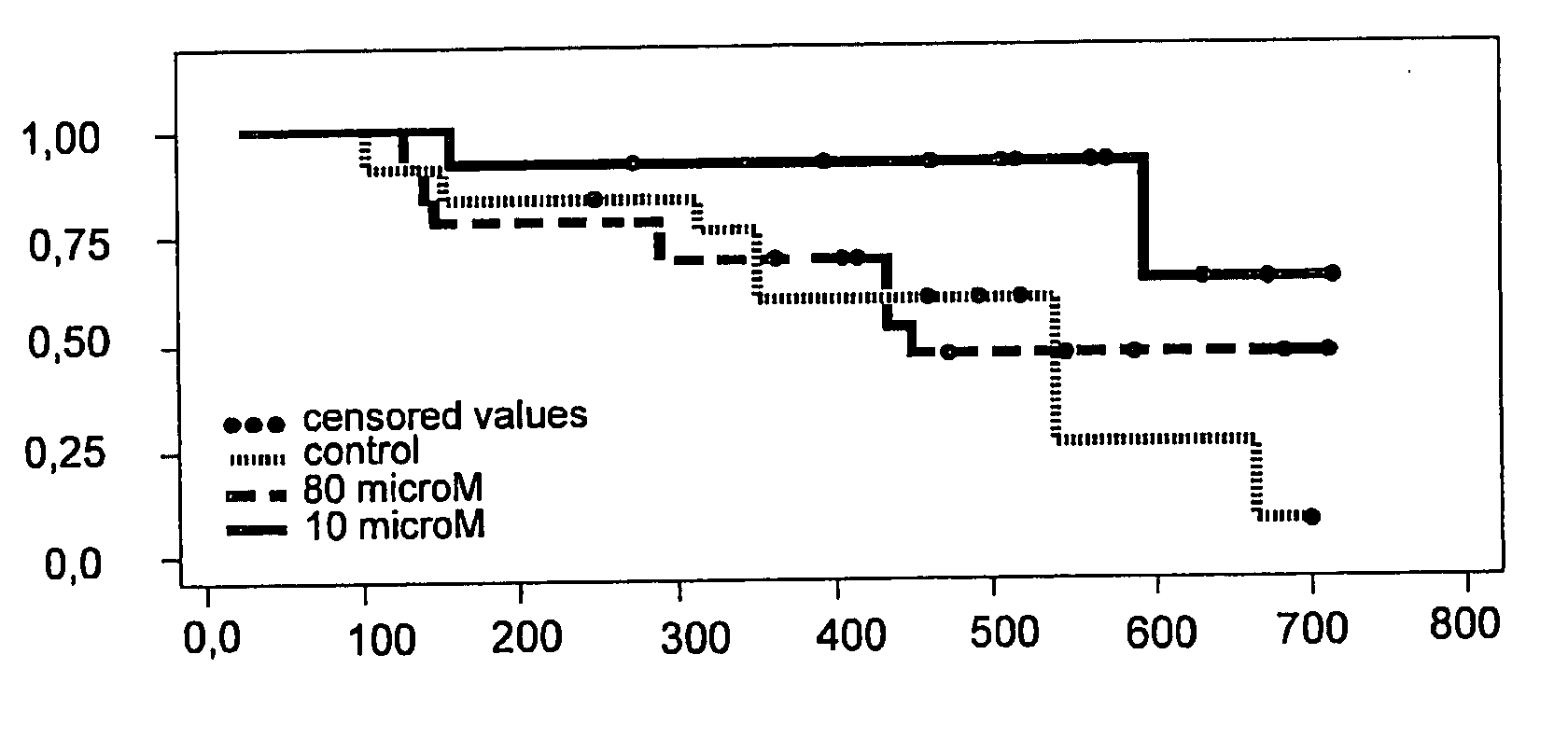
This invention is related to the use of at least one oligonucleotide with a length of from about 8 to about 30 nucleotide building blocks for manufacturing a pharmaceutical preparation for the prophylaxis and / or the treatment of diseases, that are modulated by TGF-beta2, TGF-beta1, TGF-beta3, VEGF, interleukin-10, c-jun, c-fos, and / or prostaglandin E2 in a mammal, wherein said oligonucleotide hybridizes with a messenger RNA of a TGF-beta2, TGF-beta1, TGF-beta3, VEGF, interleukin-10, c-jun, c-fos and / or prostaglandin E2 gene and wherein said preparation comprises said oligonucleotide in a concentration of about 1 microM to about 25 microM.
Owner:ANTISENSE PHARMA GMBH
POLYPEPTIDES COMPRISING Fc FRAGMENTS OF IMMUNOGLOBULIN G (IgG) AND METHODS OF USING THE SAME
Polypeptides comprising at least a first and second Fc fragment of IgG that can be used to induce a stimulated cell to produce the anti-inflammatory cytokine Interleukin-10 and methods of using the same are disclosed herein.
Owner:UNIV OF MARYLAND +1
Fusion protein and coding gene and application thereof
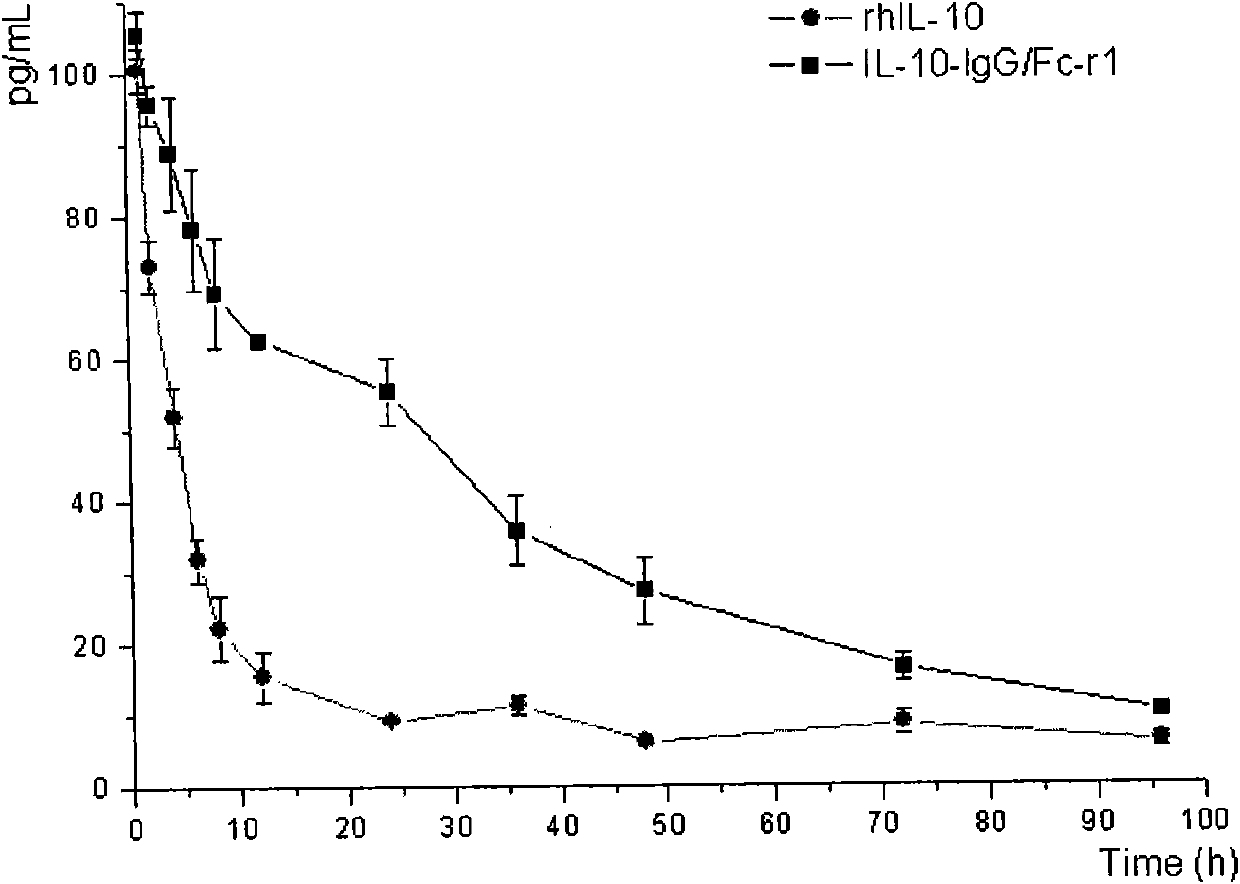
The invention discloses a fusion protein and a coding gene and application thereof. The fusion protein provided by the invention is formed by connecting any one of the following proteins at the carboxyl terminal of human interleukin-10 protein: 1) human IgGFc-gamma 1 or mutant protein thereof, and 2) human IgGFc-gamma 2 or mutant protein thereof, wherein the amino acid sequence of the human interleukin-10 protein is an amino acid sequence from 1st to 160th site of the amino terminal of the sequence 1 in a sequence table. Proved by experiments, the IL-10-IgG / Fc fusion protein of interleukin-10activity is produced by using a pichia pastoris fermentation expression system, and a foundation for clinically researching the treatment effect of the IL-10-IgG / Fc fusion protein in the next step.
Owner:UNIV OF SCI & TECH OF CHINA
Method and composition for treatment of renal disease with antibodies and their equivalents
InactiveUS20070218063A1Improve the quality of lifeMore pathogenetic treatmentPeptide/protein ingredientsImmunoglobulins against animals/humansInterleukin 6Interleukin 10
A method for treating renal disease includes administering to a patient suffering from renal disease an effective amount of antibody or of a functional equivalent thereof to at least two of urea, creatinine, tumor necrosis factor alpha, interferon gamma, interleukin 6 and interleukin 1 beta. Soluble cytokine receptors also can be employed. Antibody, functional equivalent thereof or soluble cytokine receptor to interleukin 10 or interleukin 13 also can be administered. The method can be used as a supplement to or as partial or complete replacement for dialysis. A pharmaceutical composition includes antibody or functional equivalent thereof to urea, creatinine, or both; antibody, functional equivalent or soluble cytokine receptor to tumor necrosis factor alpha, interferon gamma, interleukin 6, interleukin 1 beta or any combination thereof; and, optionally, antibody, functional equivalent or soluble cytokine receptor to interleukin 10, interleukin 13 or both. The composition can be included in a kit.
Owner:SKURKOVICH BORIS +2
Interleukin-10 peptides and antibodies thereof for inhibiting adverse effects of protozoan infection
ActiveUS20140017248A1Easy maintenanceStay highBiocideEgg immunoglobulinsInterleukin 10White blood cell
The present disclosure is directed to interleukin-10 (IL-10) peptides and isolated antibodies that specifically bind to the IL-10 peptides. The IL-10 peptides and the isolated antibodies may be administered alone or as an animal feed additive to treat gastrointestinal protozoan infection in animals.
Owner:WISCONSIN ALUMNI RES FOUND
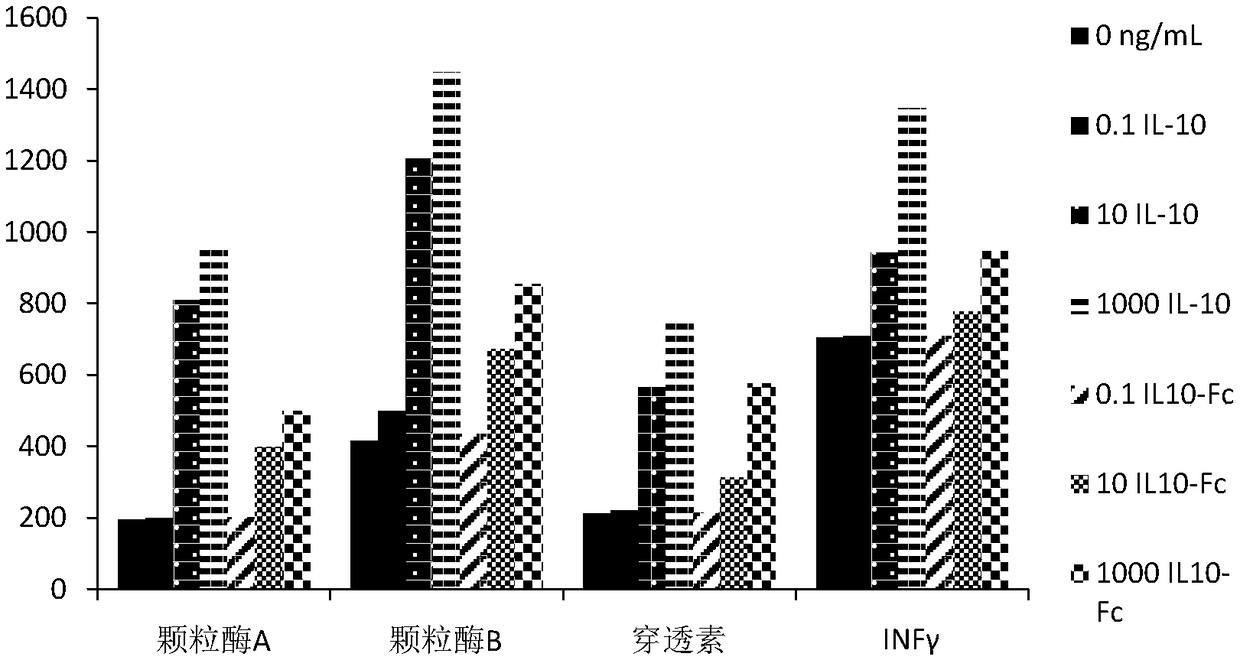
Human interleukin 10-Fc fusion protein, and coding gene and application thereof
The invention relates to the field of genetic engineering medicines, in particular to a human interleukin 10-Fc (IL10-Fc) fusion protein, and a coding gene and application thereof. By replacing aminoacids at multiple positions in an IgG4Fc part, the modified IL10-Fc fusion protein has superior performance over existing Fc fusion proteins, such as increasement of in-vivo stability, elimination ofunnecessary effector functions and reduction of immunogenicity of the fusion proteins in living organisms. The invention also discloses a method for treating diseases by using an IL10-Fc fusion protein medicament, and the method comprises administering a therapeutically effective amount of the medicament to a subject suffering from diseases, wherein the diseases comprise an inflammatory symptom, immune related diseases, fibrosis diseases, cancers and the like.
Owner:HANGZHOU BOHU BIOTECH CO LTD
Compositions and methods for modulating interleukin-10
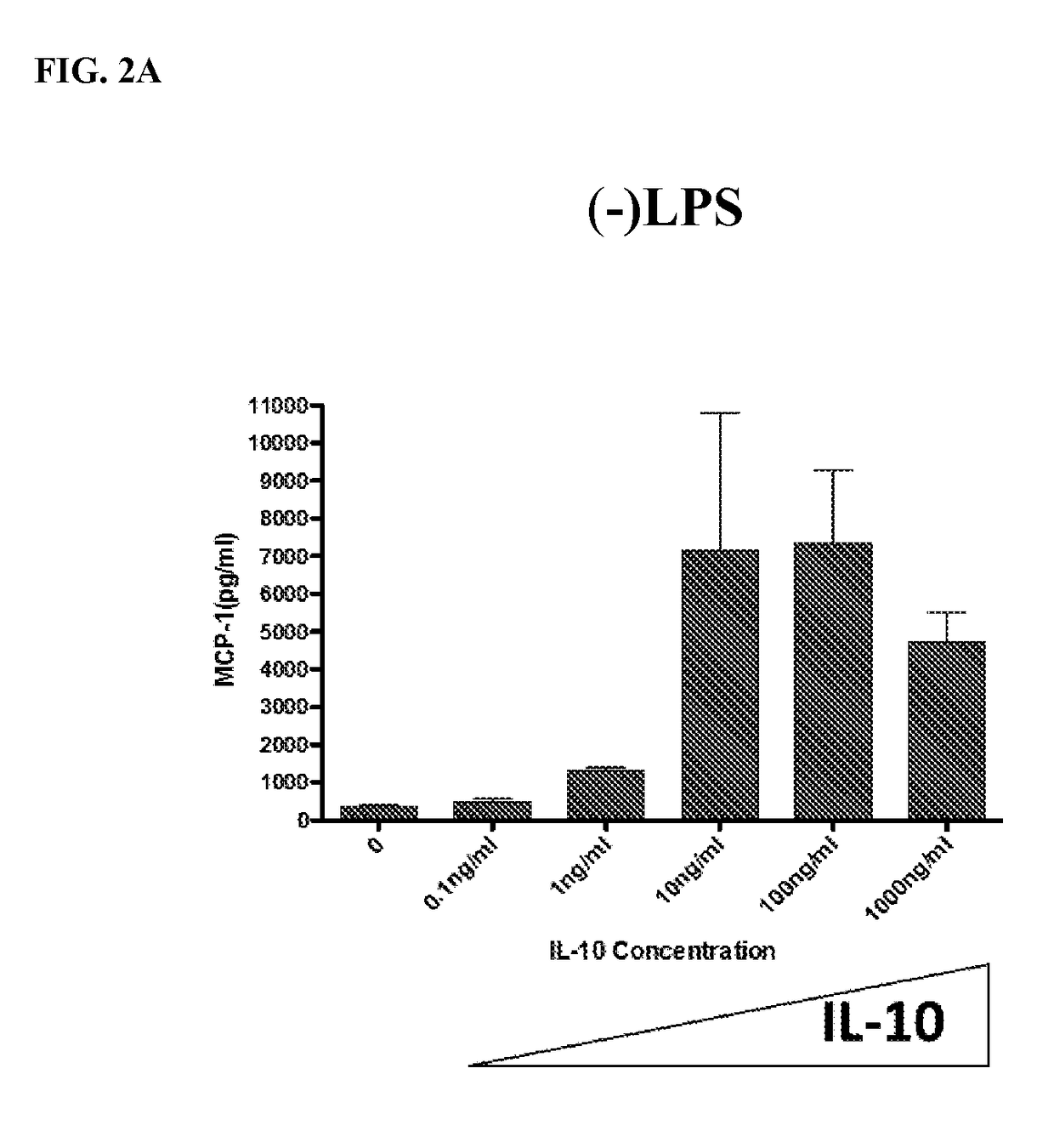
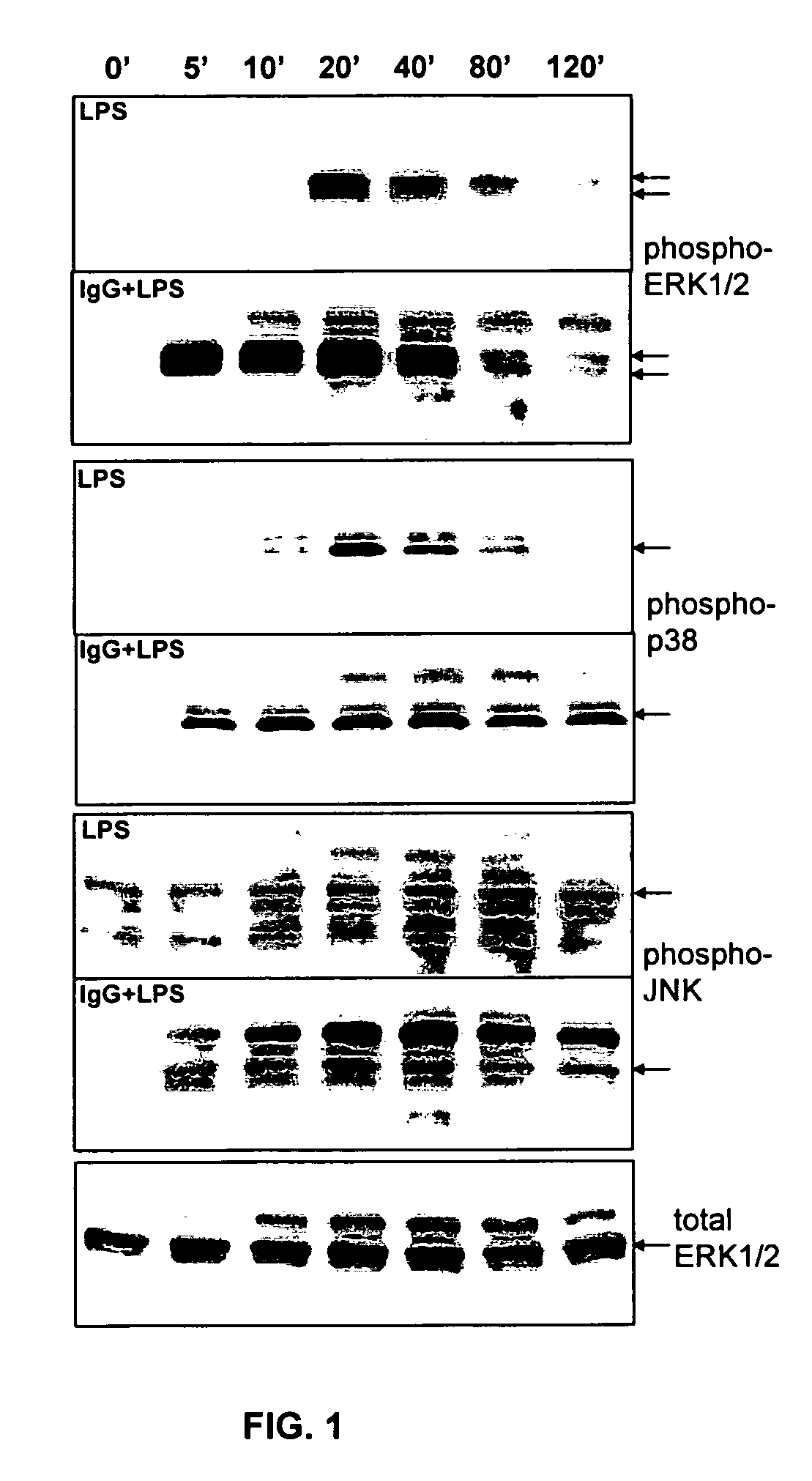
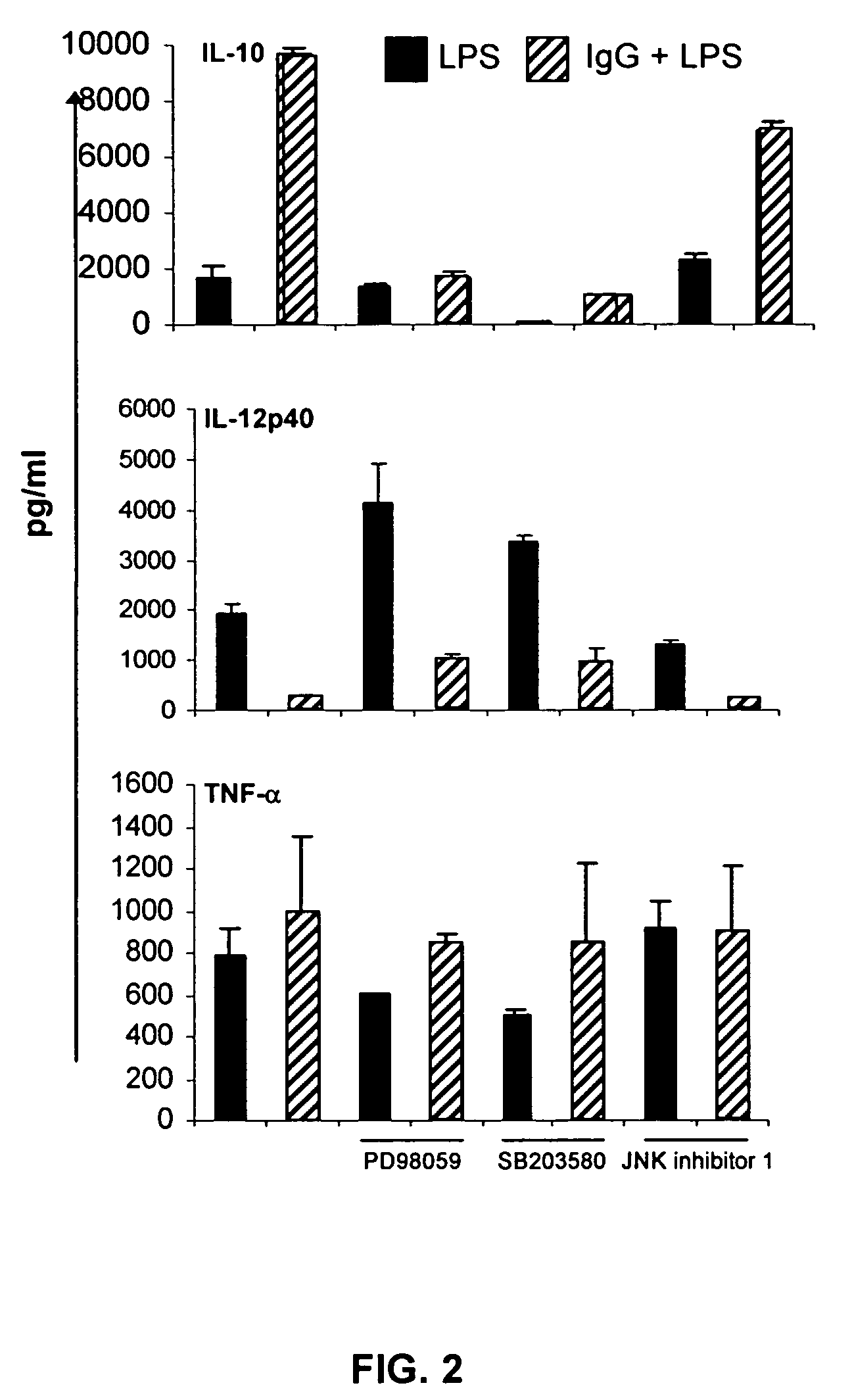
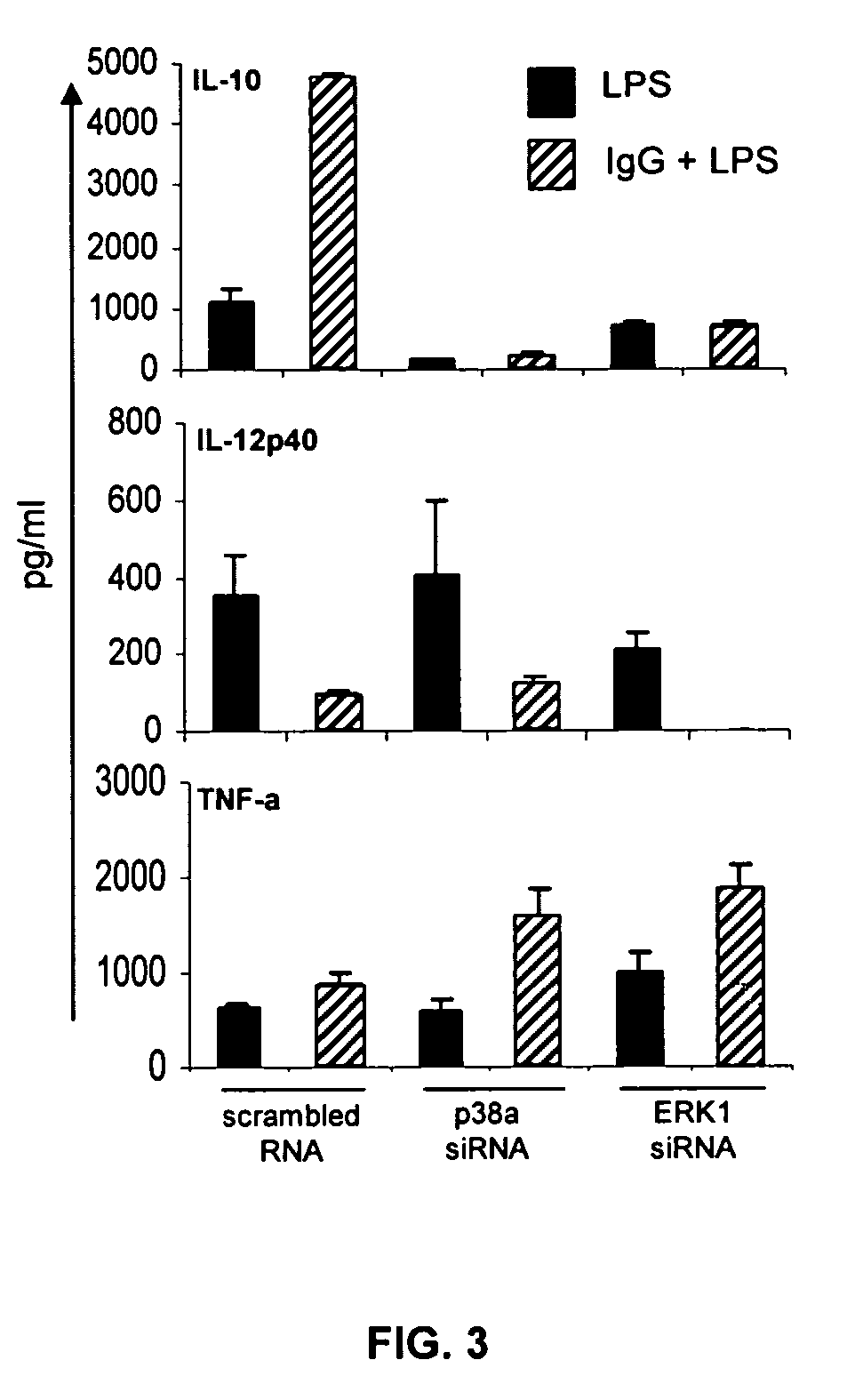
The present invention provides compositions and methods for upregulating IL-10 production in a stimulated cell. In one aspect, the invention provides methods of identifying ERK activating agents capable of activating and amplifying the ERK MAPK pathway in a cell. Such ERK activating agents are capable of upregulating the production of IL-10 in stimulated cells. In another aspect, the invention provides ERK activating agents identified by the screening methods of the invention. Methods are also provided for preventing and treating inflammation in a susceptible patient by administering to the patient, a therapeutically effective amount of an ERK activating agent identified in accordance with the invention.
Owner:MARYLAND UNIV OF
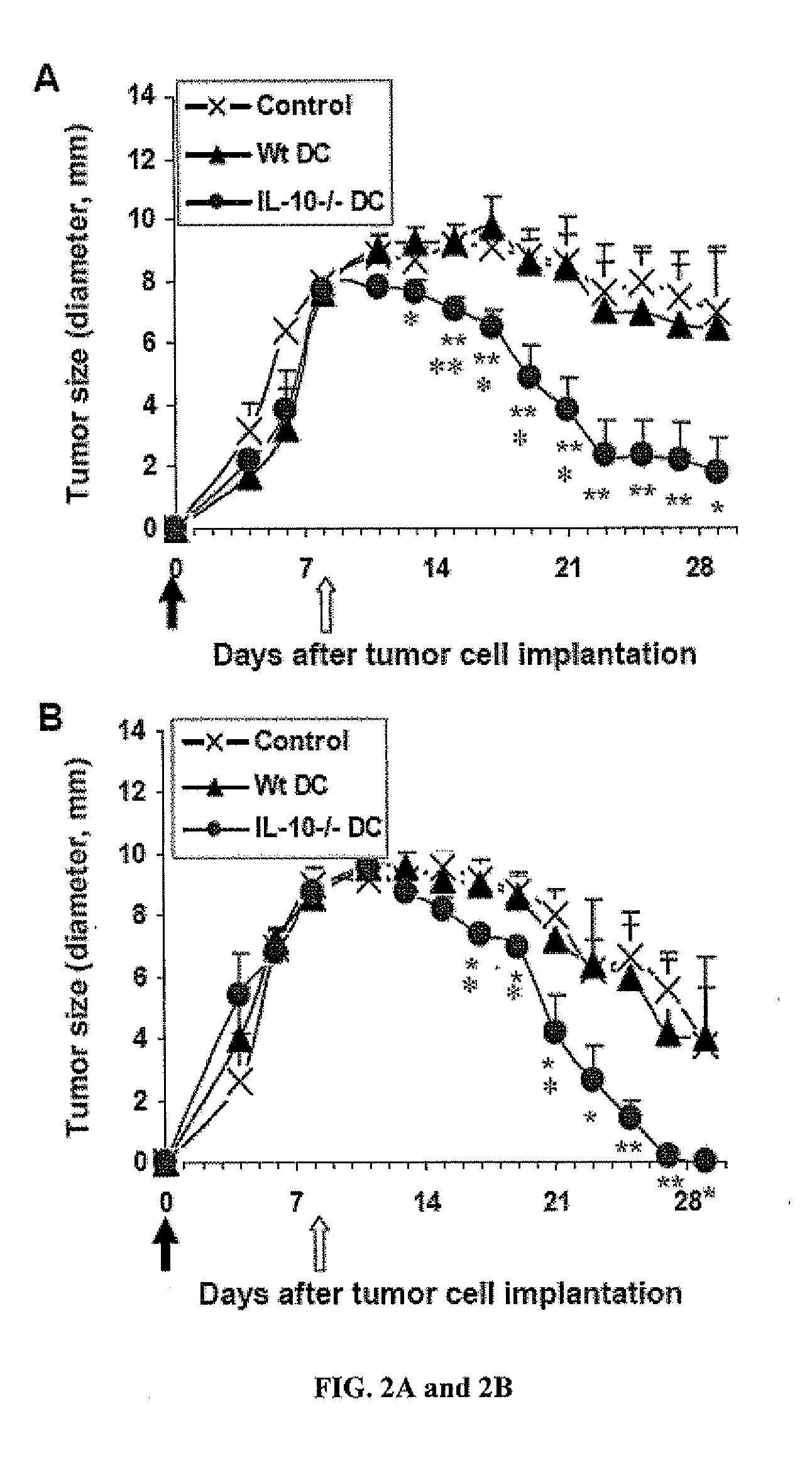
Anti-tumor vaccines delivered by dendritic cells devoid of interleukin-10
InactiveUS20090010948A1Highly effectiveBiocideGenetic material ingredientsDendritic cellInterleukin 10
It has been discovered that reducing, inhibiting or preventing the expression of immunosuppressive cytokines or tolergenic agents in antigen presenting cells improves the ability of the antigen presenting cell to promote an immune response. One embodiment provides a genetically engineered antigen presenting cell that has reduced or no expression of IL-10. Preferred antigen presenting cells are dendritic cells. Expression of IL-10 can be inhibited or blocked by genetically engineering the antigen presenting cell to express inhibitory nucleic acids that inhibit or prevent the expression mRNA encoding immunosuppressive cytokines. Inhibitory nucleic acids include siRNA, antisense RNA, antisense DNA, microRNA, and enzymatic nucleic acids that target mRNA encoding immunosuppressive cytokines. Immunosuppressive cytokines include, but are not limited to IL-10, TGF-β, IL-27, IL-35, or combinations thereof. Tolerogenic agents include but are not limited to indoleamine 2,3-dioxygenase.
Owner:THE UNIVERSITY OF HONG KONG
Interleukine 10 immunoconjugates
The present invention generally relates to fusion proteins of antibodies and interleukin-10 (IL-0). More particularly, the invention concerns fusion proteins of antibodies and mutant IL-10 that exhibit improved properties for use as therapeutic agents, e.g. in the treatment of inflammatory diseases. In addition, the present invention relates to polynucleotides encoding such fusion proteins, and vectors and host cells comprising such polynucleotides. The invention further relates to methods for producing the fusion proteins of the invention, and to methods of using them in the treatment of disease.
Owner:F HOFFMANN LA ROCHE & CO AG
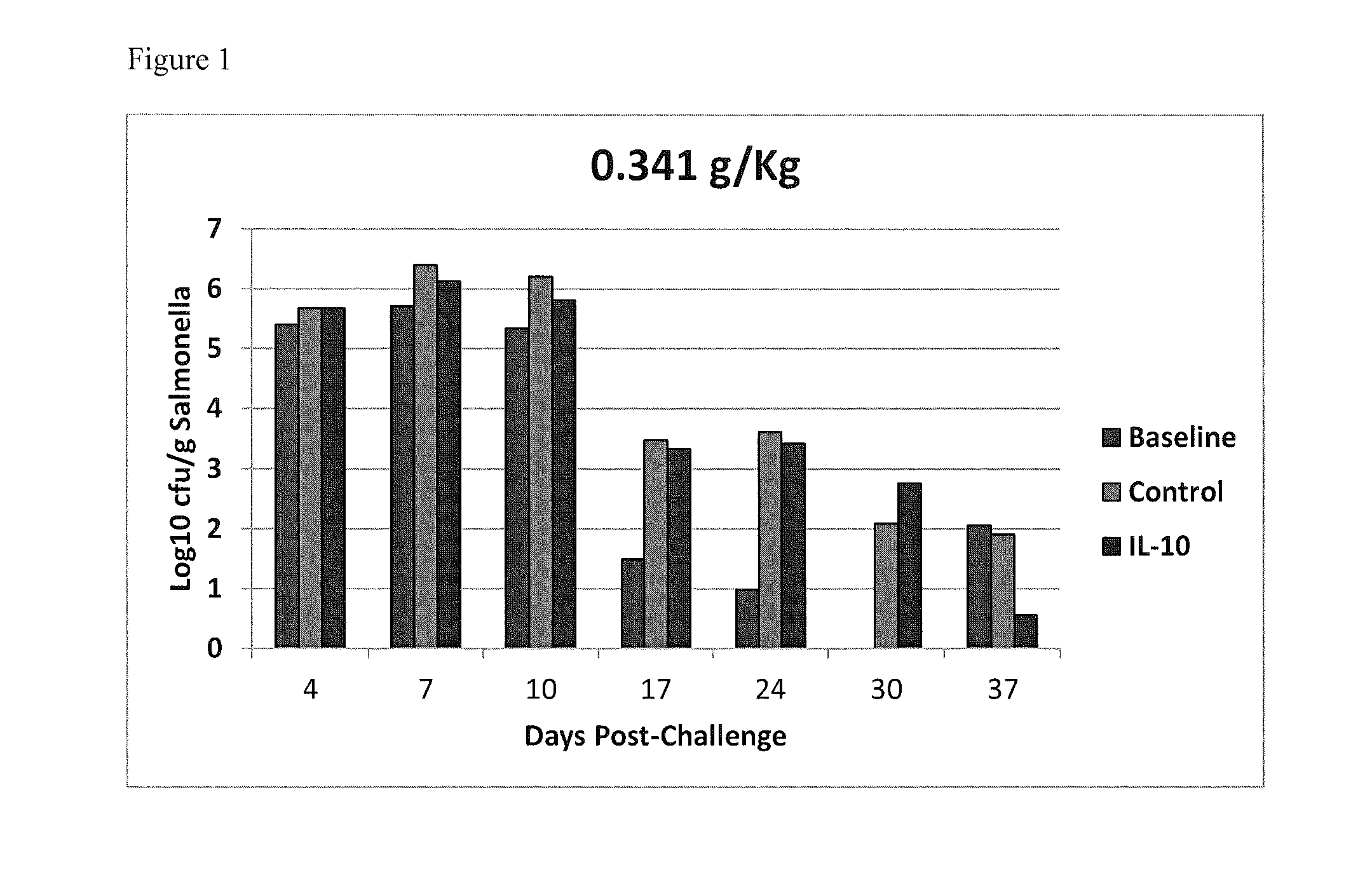
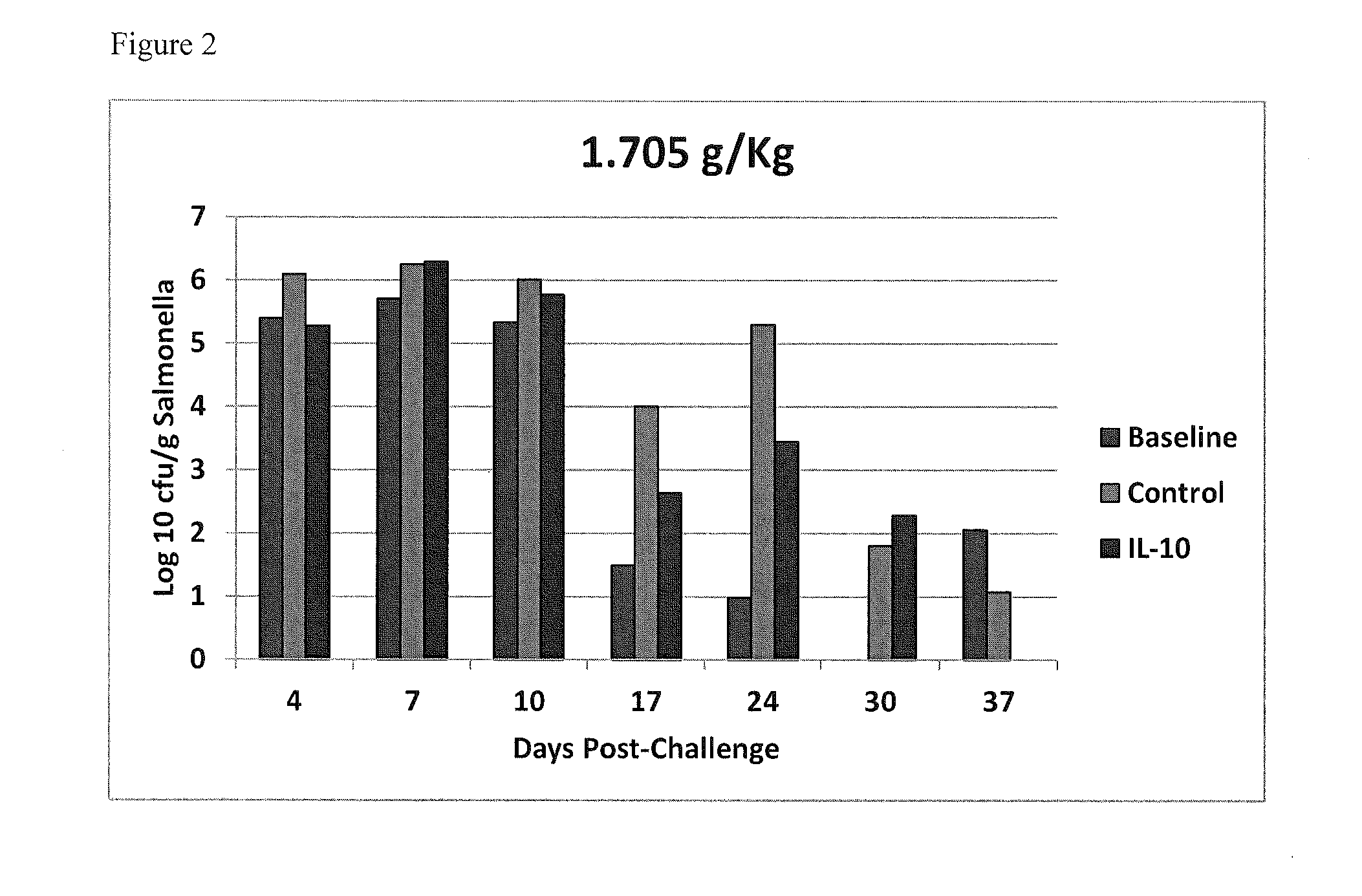
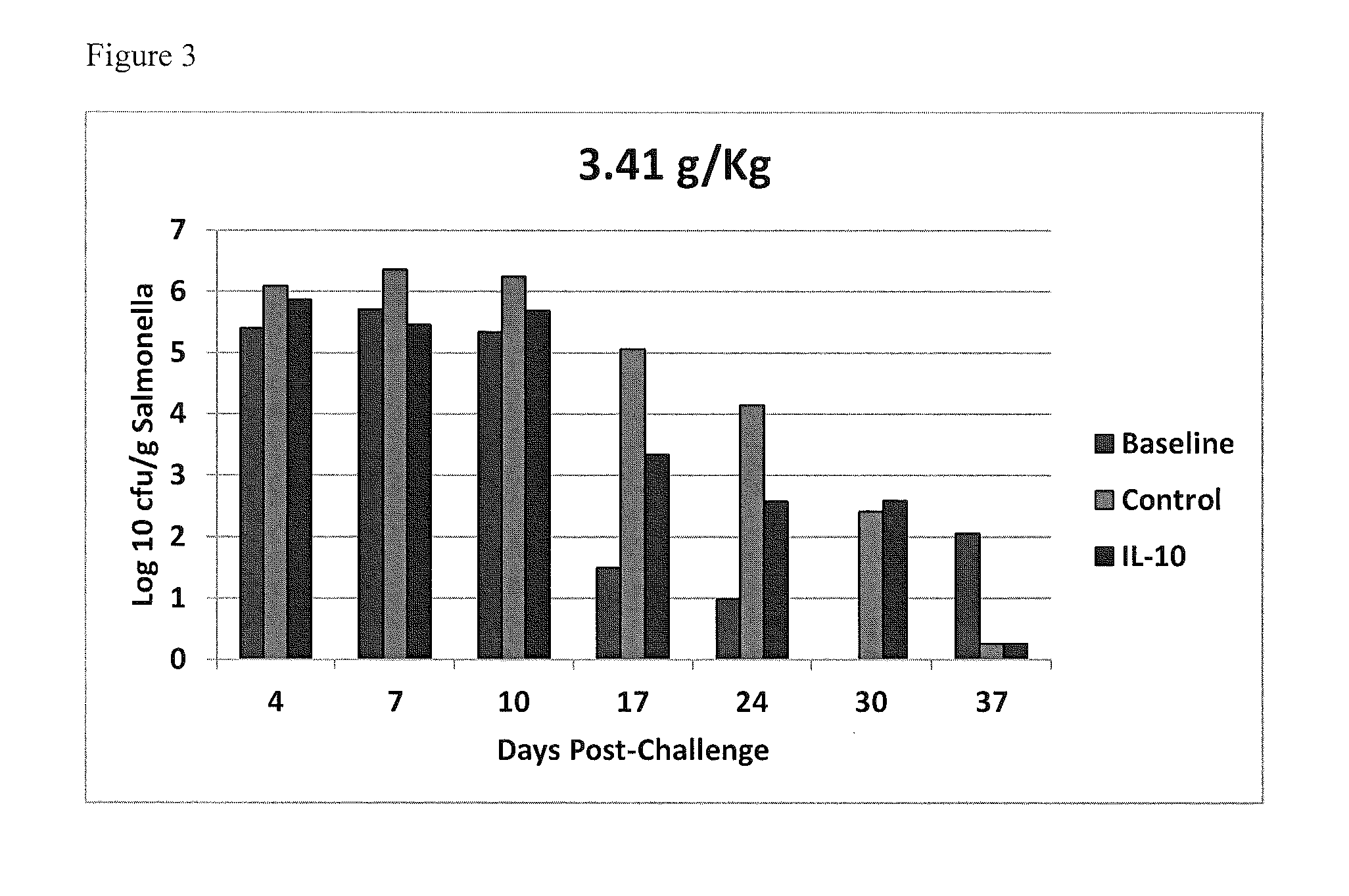
Methods of reducing salmonella in poultry
InactiveUS20150037277A1Egg immunoglobulinsPeptide/protein ingredientsIntestinal structureInterleukin 10
Described herein are methods of reducing Salmonella in the intestines of poultry in need thereof by administering to a poultry bird an effective amount of an interleukin-10 peptide or an isolated antibody that specifically binds an interleukin-10 peptide. Administering may be performed within 1 to 4 weeks of harvest of the poultry in order to reduce Salmonella transmission to human consumers. Also included herein are finishing feeds that include an interleukin-10 peptide or an isolated antibody that specifically binds an interleukin-10 peptide.
Owner:US SEC AGRI +1
Liquid band-aid and preparing method thereof
The invention discloses a liquid band-aid, comprising, active materials, a film-forming material and a solvent. The active materials include cytokines 0.042 to 0.0095%, which are chosen from at least two of EGF (epidermal growth factor), PDGF (platelet derived growth factor), FGF (fibroblast growth factor) and IL-10 (interleukin-10). The film-forming material comprises chitosan hydrochloride 2% to 8%. The solvent is deionized water or distilled water. The invention further discloses a method for preparing the liquid band-aid. By the use of the preparation method and / or the liquid band-aid comprising various biological active materials and prepared by the preparation method, activity of the biological active materials can be retained, and wounds can be efficiently repaired without a scar.
Owner:ACADEMY OF MILITARY MEDICAL SCI +1
Interleukin-10 peptides and antibodies thereof for inhibiting adverse effects of protozoan infection
The present disclosure is directed to interleukin-10 (IL-10) peptides and isolated antibodies that specifically bind to the IL-10 peptides. The IL-10 peptides and the isolated antibodies may be administered alone or as an animal feed additive to treat gastrointestinal protozoan infection in animals.
Owner:WISCONSIN ALUMNI RES FOUND
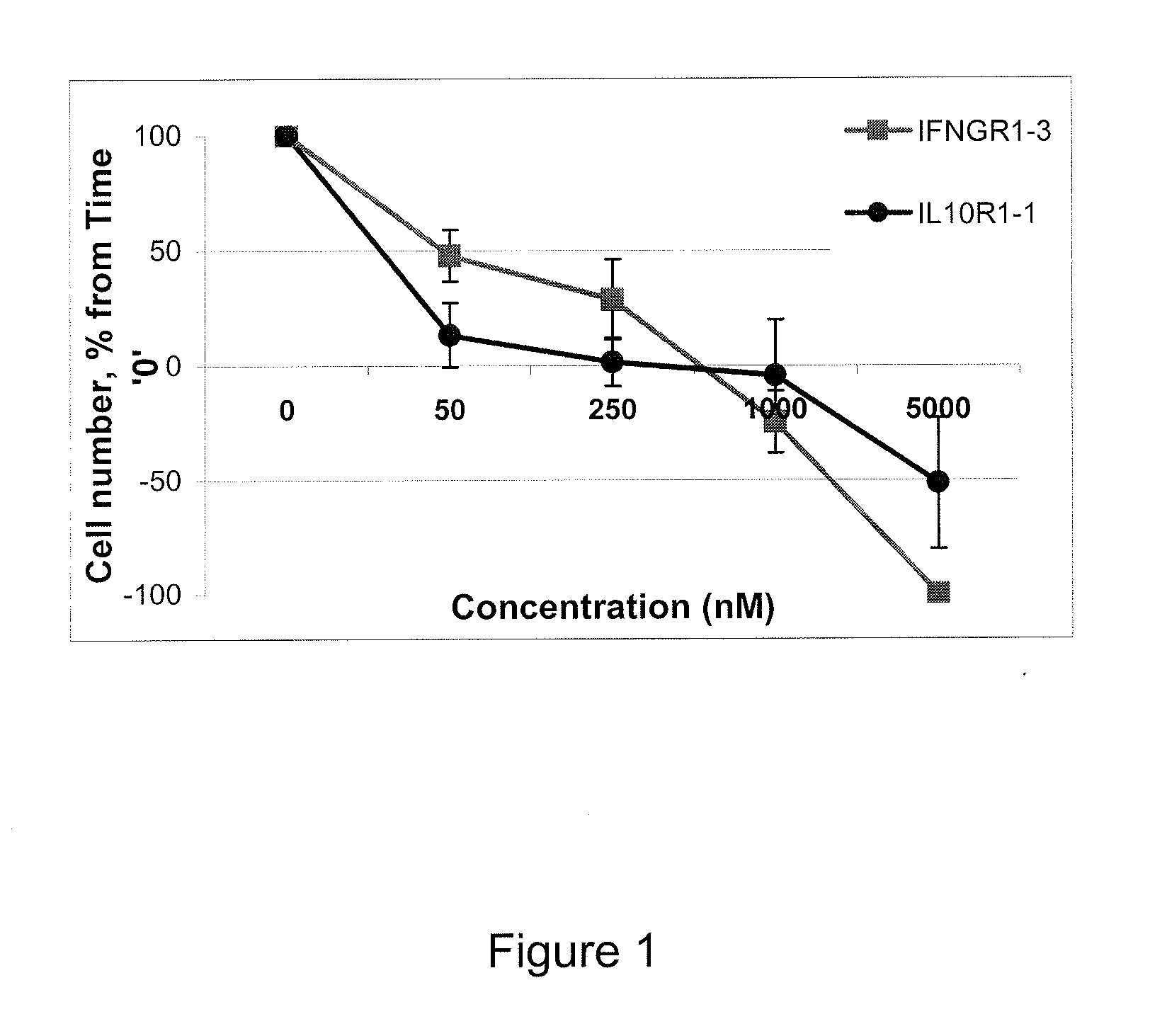
Peptide-based inhibitor of interleukin-10 or interferon-gamma signaling
ActiveUS20130109619A1Suppression problemInhibition of activationBiocideAntipyreticInterleukin 10White blood cell
A peptide or peptidomimetic comprising an amino acid sequence based on conserved regions of IL10 or IFN-gamma receptor sequences, and related compounds and compositions, as well as methods for the use thereof to inhibit cytokine signaling.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Application of nitidine chloride to preparation of medicament for resisting autoimmunity disease and graft versus host disease
InactiveCN102008474ARich sourcesLow cost of treatmentOrganic active ingredientsSenses disorderInterleukin 10Dendritic cell
The invention provides application of nitidine chloride to the preparation of a medicament for resisting autoimmunity disease and graft versus host disease. An experiment shows that the nitidine chloride can inhibit the proliferation of human dendritic cells in vitro, inhibit the human dendritic cells from promoting the proliferation reaction of allogeneic T cells and strengthen the capacity of secreting interleukin 10 of the human dendritic cells. Meanwhile, in an experimental autoimmunity disease cerebrospinal meningitis model, when used in vivo, the nitidine chloride can reduce the average clinical score of a mouse and inhibit the spinal marrow infiltration degree of monocytes and can obviously improve the secretion level of the interleukin 10 in the blood serum of the mouse. In a heart transplantation experiment, when used in vivo, the nitidine chloride can prolong the survival time of the transplanted heart. In a delayed type hypersensitivity reaction model, when used in vivo, the nitidine chloride can reduce the ear swelling degree of the mouse. Both in-vivo and in-vitro results show that the nitidine chloride has a certain effect of resisting the autoimmunity disease and the graft versus host disease. The medicament is a preparation which consists of the nitidine chloride serving as an active ingredient and a pharmaceutical carrier. The medicament can be prepared into an oral preparation, an injection, a suppository or an external preparation and the like.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com