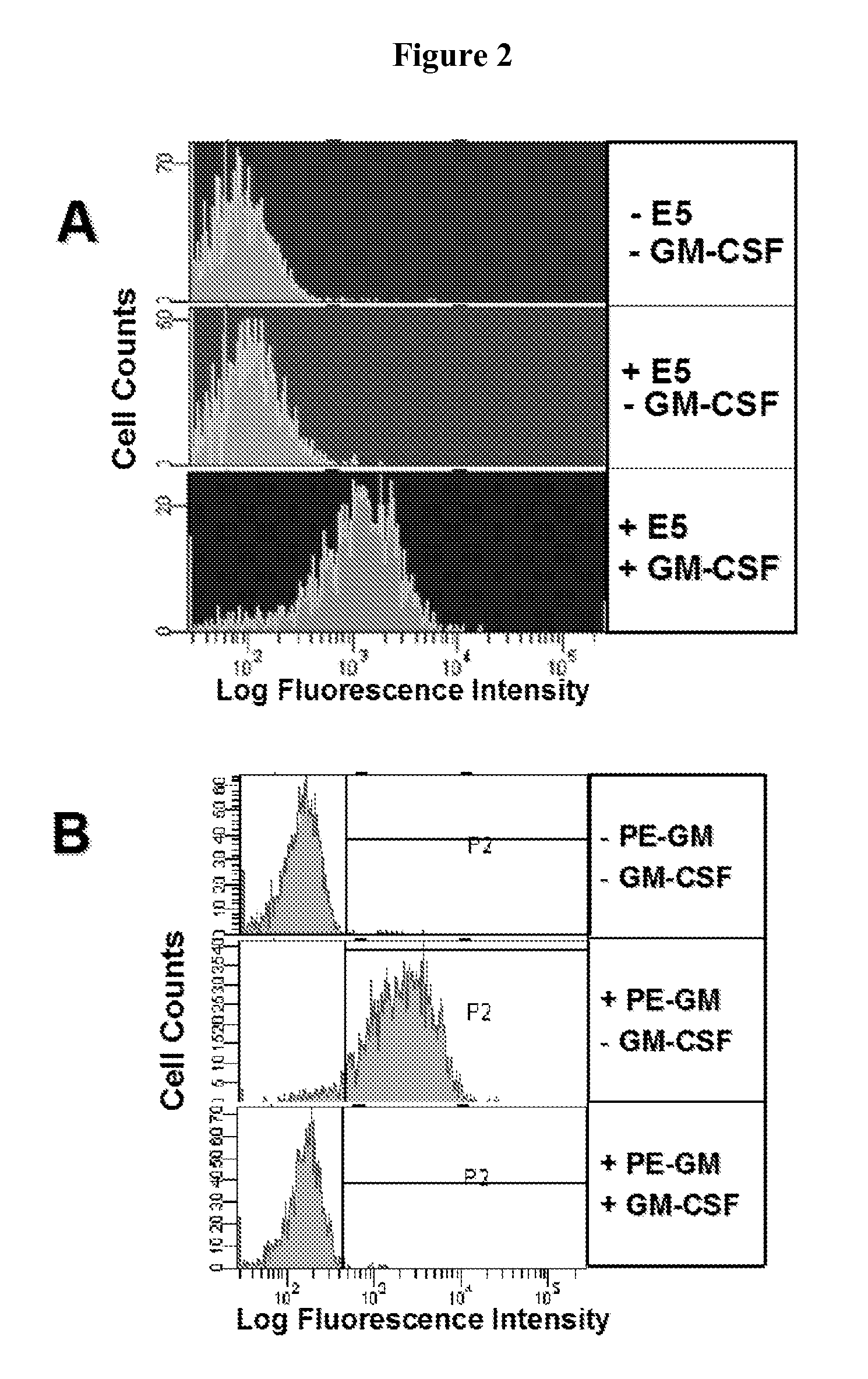
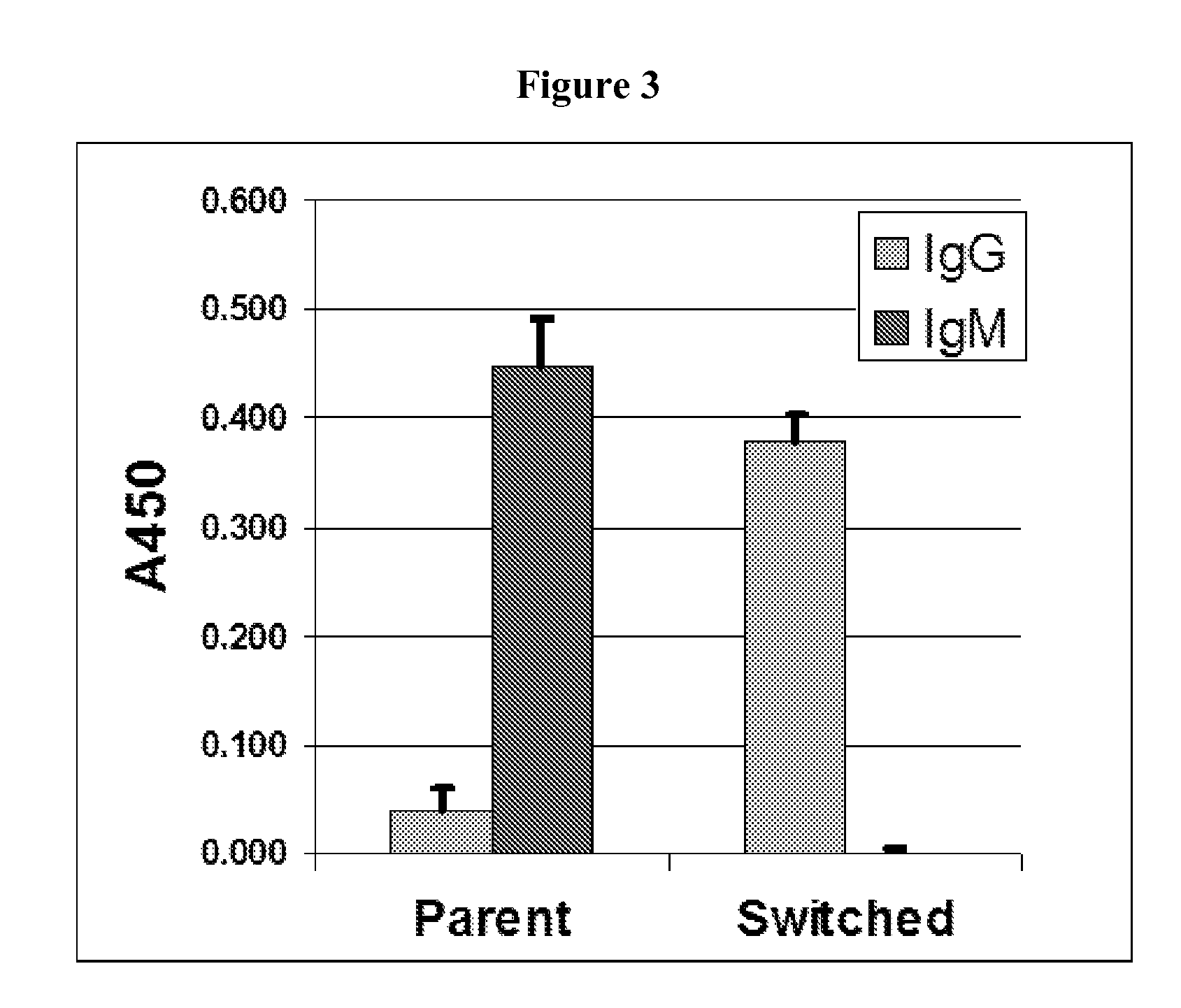
Patents
Literature
474 results about "Granulocyte" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Granulocytes are a category of white blood cells characterized by the presence of granules in their cytoplasm. They are also called polymorphonuclear leukocytes or polymorphonuclear neutrophils (PMN, PML, or PMNL) because of the varying shapes of the nucleus, which is usually lobed into three segments. This distinguishes them from the mononuclear agranulocytes. In common parlance, the term polymorphonuclear leukocyte often refers specifically to "neutrophil granulocytes", the most abundant of the granulocytes; the other types (eosinophils, basophils, and mast cells) have lower numbers. Granulocytes are produced via granulopoiesis in the bone marrow.
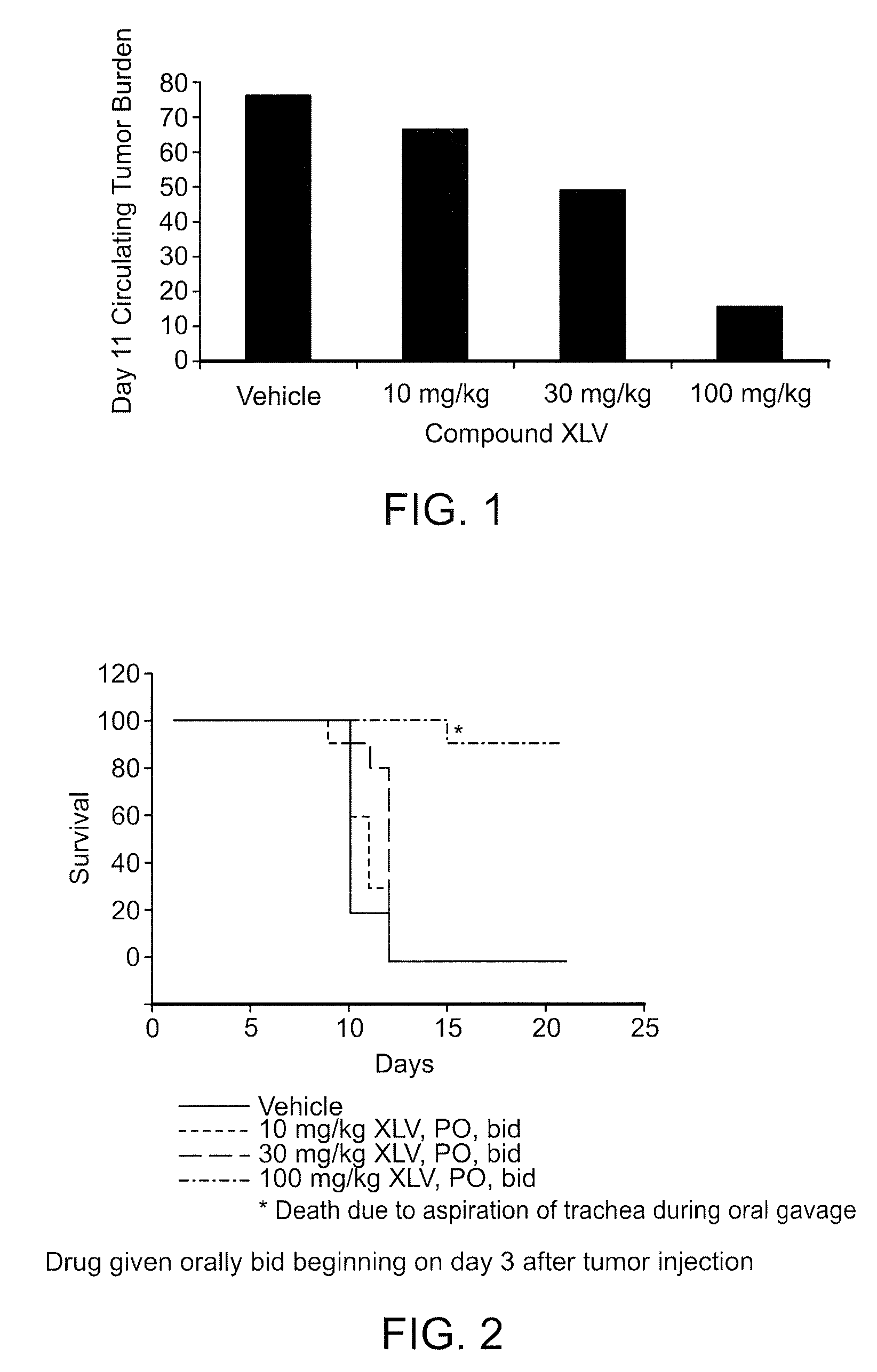
Methods and compositions for the treatment of myeloproliferative diseases and other proliferative diseases
Compounds of the present invention, alone and in combination with other active agents, find utility in the treatment of hyperproliferative diseases, mammalian cancers and especially human cancers including but not limited to for example malignant melanomas, myeloproliferative diseases, chronic myelogenous leukemia, acute lymphocytic leukemia, a disease caused by c-ABL kinase, oncogenic forms thereof, aberrant fusion proteins thereof and polymorphs thereof.
Owner:DECIPHERA PHARMA LLC
Process to study changes in gene expression in granulocytic cells
InactiveUS6365352B1Resistant to digestionDigestion of every cDNA is assuredMicrobiological testing/measurementBacteroidesNeutrophil granulocyte
The present invention comprises a method to identify granulocytic cell genes that are differentially expressed upon exposure to a pathogen or in a sterile inflammatory disease by preparing a gene expression profile of a granulocytic cell population exposed to a pathogen or isolated from a subject having a sterile inflammatory disease and comparing that profile to a profile prepared from quiescent granulocytic cells. The present invention is particularly useful for identifying cytokine genes, genes encoding cell surface receptors and genes encoding intermediary signaling molecules. The invention also includes methods to identify a therapeutic agent that modulates the expression of at least one gene in a granulocytic population. Genes which are differentially expressed during neutrophil contact with a pathogen, such as a virulent bacteria, or that are differentially expressed in a subject having a sterile inflammatory disease are of particular importance.
Owner:YALE UNIV +2
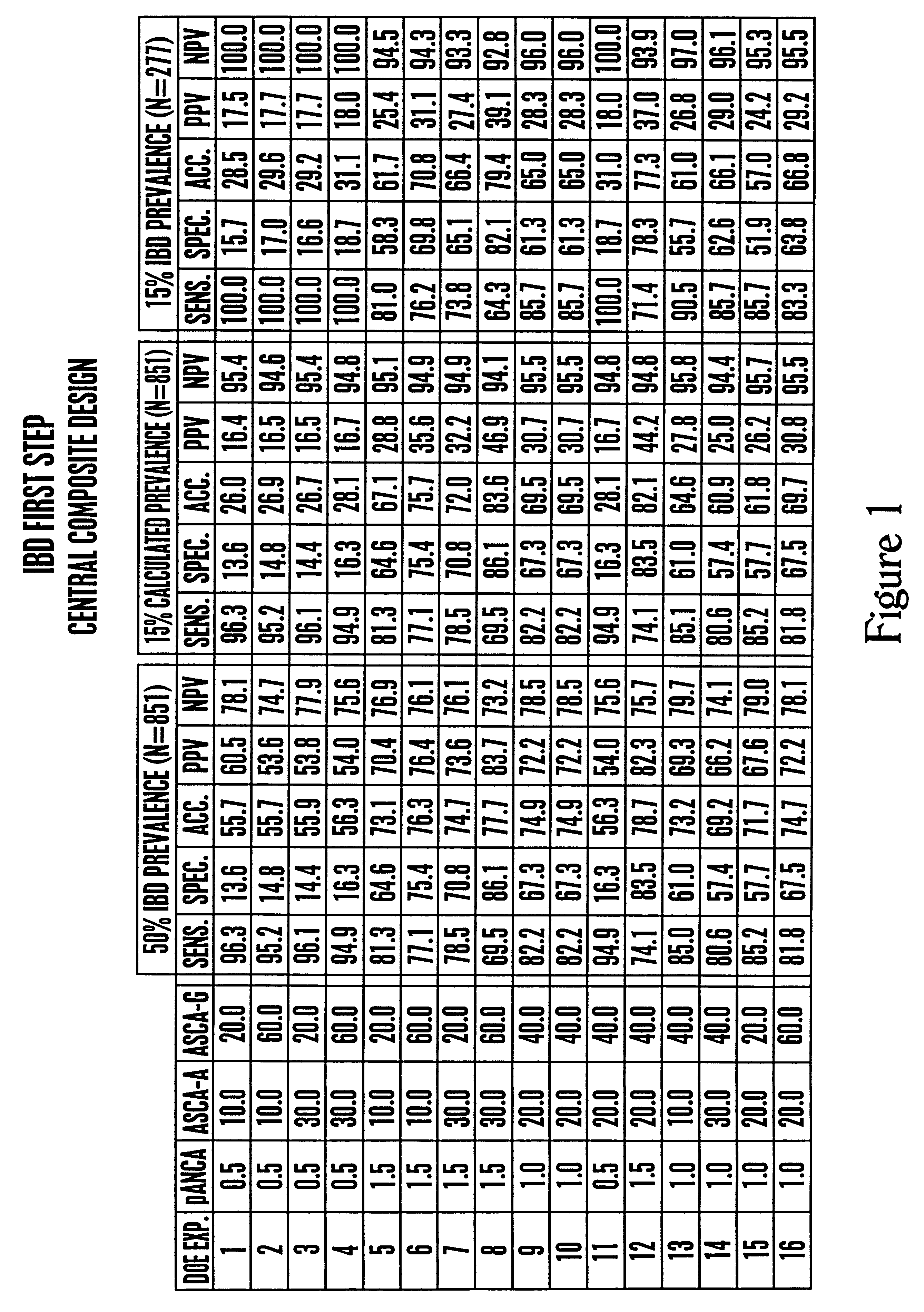
Inflammatory bowel disease first step assay system
The present invention provides a highly sensitive method of diagnosing inflammatory bowel disease (IBD) in an individual. The method includes the steps of isolating a sample from the individual; determining by non-histological means whether the sample is positive for anti-neutrophil cytoplasmic antibodies (ANCA); determining whether the sample is positive for anti-Saccharomyces cerevisiae immunoglobulin A (ASCA-IgA); determining whether the sample is positive for anti-Saccharomyces cerevisiae immunoglobulin G (ASCA-IgG); and diagnosing the individual as having IBD when the sample is positive for ANCA, ASCA-IgA or ASCA-IgG, and diagnosing the individual as not having IBD when the sample is negative for ANCA, ASCA-IgA and ASCA-IgG, provided that the method does not include histological analysis of neutrophils.
Owner:PROMETHEUS LAB +1
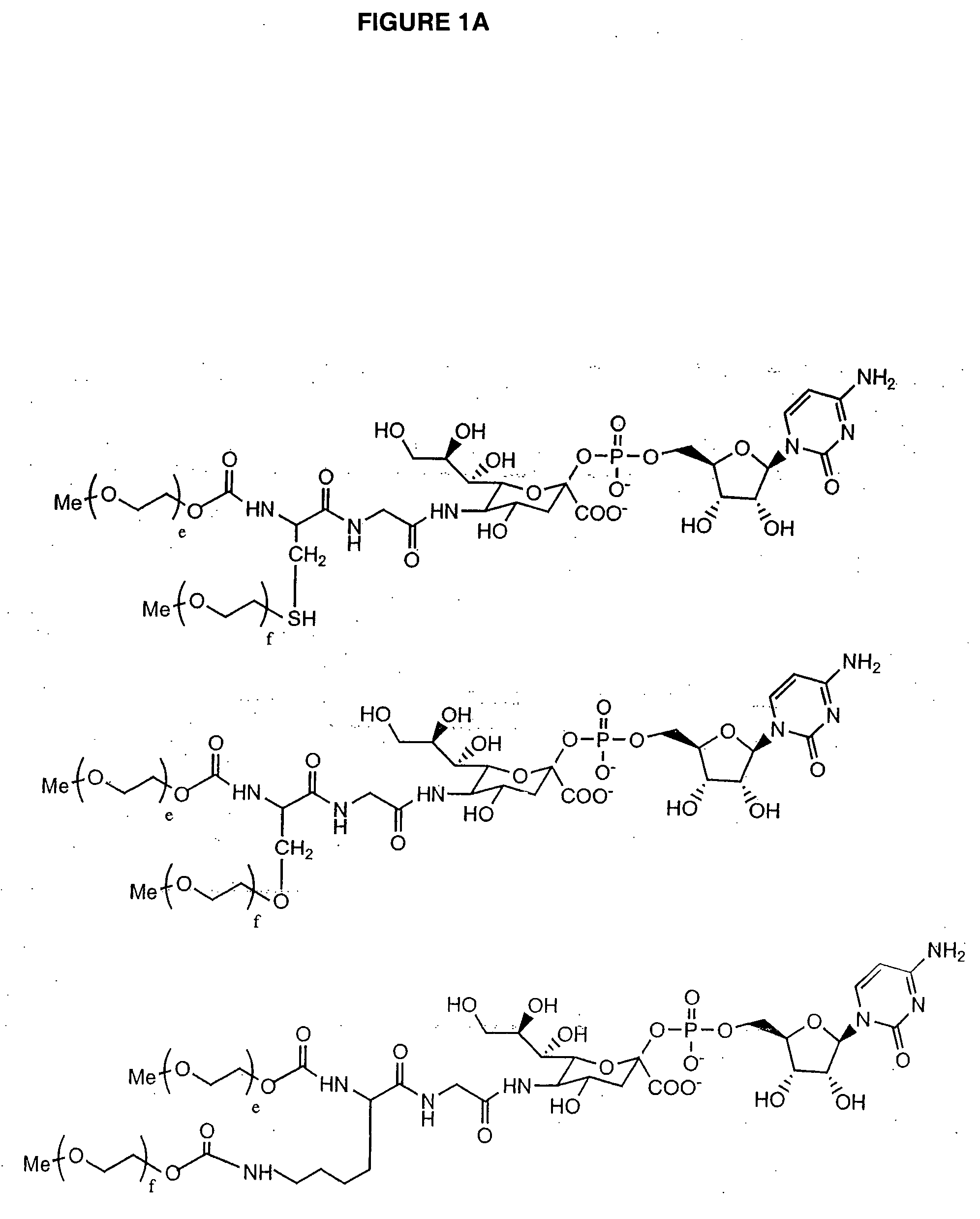
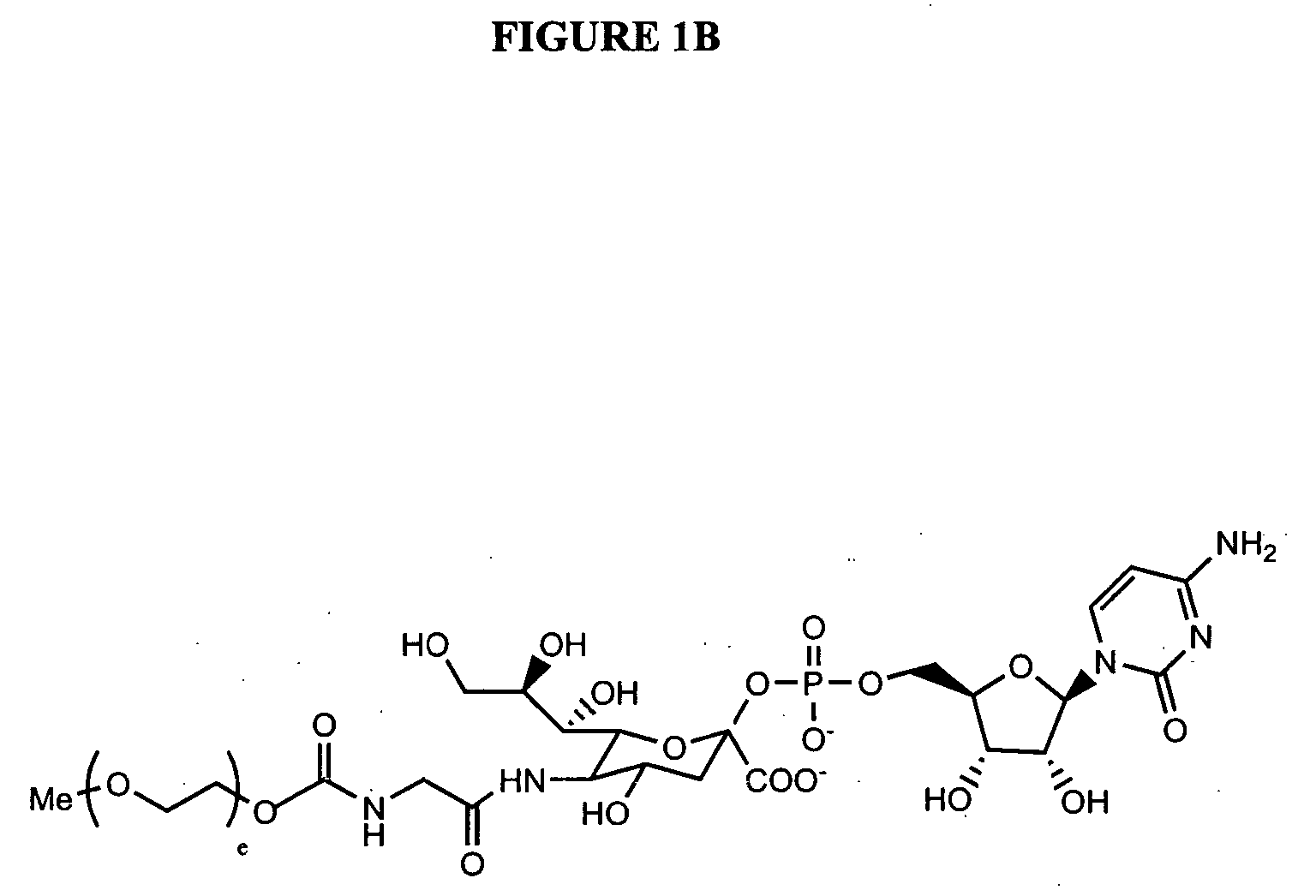
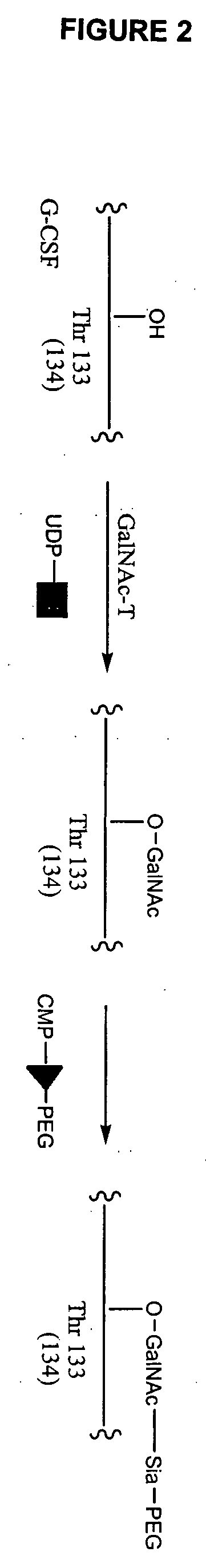
Glycopegylated granulocyte colony stimulating factor
ActiveUS20070014759A1Improve pharmacokinetic propertyImproved pharmacokinetic propertiesPeptide/protein ingredientsFermentationPeptideSugar moiety
The present invention provides conjugates between Granulocyte Colony Stimulating Factor and PEG moieties. The conjugates are linked via an intact glycosyl linking group that is interposed between and covalently attached to the peptide and the modifying group. The conjugates are formed from both glycosylated and unglycosylated peptides by the action of a glycosyltransferase. The glycosyltransferase ligates a modified sugar moiety onto either an amino acid or glycosyl residue on the peptide. Also provided are pharmaceutical formulations including the conjugates. Methods for preparing the conjugates are also within the scope of the invention.
Owner:NOVO NORDISK AS
Nicotinamide acids, amides, and their mimetics active as inhibitors of PDE4 isozymes
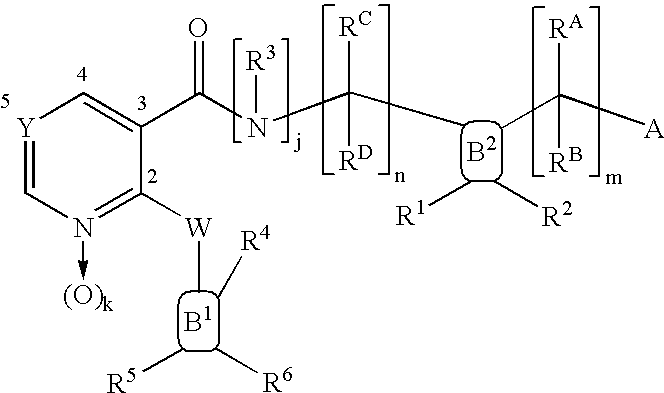
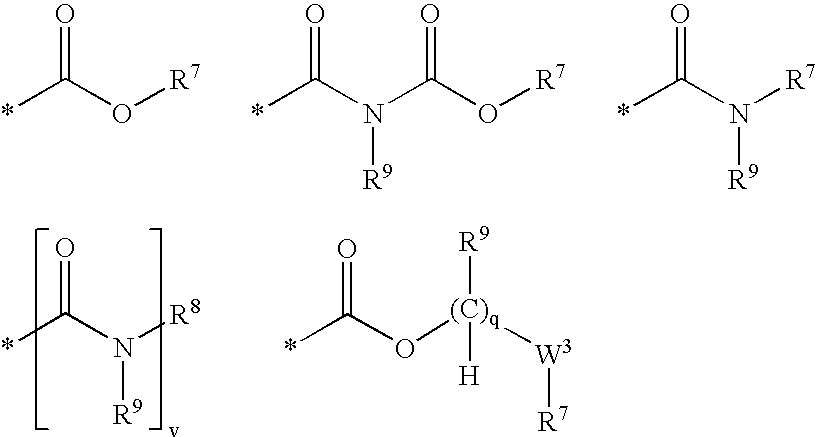
Compounds useful as inhibitors of PDE4 in the treatment of diseases regulated by the activation and degranulation of eosinophils, especially asthma, chronic bronchitis, and chronic obstructuive pulmonary disease, of the formula: wherein j is 0 or 1, k is 0 or 1, m is 0, 1, or 2; n is 1 or 2; A is selected from the partial Formulas: where q is 1, 2, or 3, W3 is -O-; -N(R9)-; or -OC(=O)-; R7 is selected from -H; -(C1-C6) alkyl, -(C2-C6) alkenyl, or -(C2-C6) alkynyl substituted by 0 to 3 substituents R10; -(CH2)u-(C3-C7) cycloalkyl where u is 0, 1 or 2, substituted by 0 to 3 R10; and phenyl or benzyl substituted by 0 to 3 R14; R8 is tetrazol-5-yl; 1,2,4-triazol-3-yl; 1,2,4-triazol-3-on-5-yl; 1,2,3-triazol-5-yl; imidazol-2-yl; imidazol-4-yl; imidazolidin-2-on-4-yl; 1,3,4-oxadiazolyl; 1,3,4-oxadiazol-2-on-5-yl; 1,2,4-oxadiazol-3-yl; 1,2,4-oxadiazol-5-on-3-yl; 1,2,4-oxadiazol-5-yl; 1,2,4-oxadiazol-3-on-5-yl; 1,2,5-thiadiazolyl; 1,3,4-thiadiazolyl; morpholinyl; parathiazinyl; oxazolyl; isoxazolyl; thiazolyl; isothiazolyl; pyrrolyl; pyrazolyl; succinimidyl; glutarimidyl; pyrrolidonyl; 2-piperidonyl; 2-pyridonyl; 4-pyridonyl; pyridazin-3-onyl; pyridyl; pyrimidinyl; pyrazinyl; pyridazinyl; indolyl; indolinyl; isoindolinyl; benzo[b]furanyl; 2,3-dihydrobenzofuranyl; 1,3-dihydroisobenzofuranyl; 2H-1-benzopyranyl; 2-H-chromenyl; chromanyl; benzothienyl; 1H-indazolyl; benzimidazolyl; benzoxazolyl; benzisoxazolyl; benzothiazolyl; benzotriazolyl; benzotriazinyl; phthalazinyl; 1,8-naphthyridinyl; quinolinyl; isoquinolinyl; quinazolinyl; quinoxalinyl; pyrazolo[3,4-d]pyrimidinyl; pyrimido[4,5-d]pyrimidinyl; imidazo[1,2-a]pyridinyl; pyridopyridinyl; pteridinyl; or 1H-purinyl; or A is selected from phosphorous and sulfur acid groups; W is -O-; -S(=O)t-, where t is 0, 1, or 2; or -N(R3)-; Y is =C(R1a)-, or -[N<custom-character file="US20020111495A1-20020815-P00900.TIF" wi="20" he="20" id="custom-character-00001" / >(O)k] where k is 0 or 1; R4, R5 and R6 are (1) -H; provided that R5 and R6 are not both -H at the same time, -F; -Cl; -(C2-C4) alkynyl; -R16; -OR16; -S(=O)pR16; -C(=O)R16, -C(=O)OR16, -C(=O)OR<highlight><sup
Owner:PFIZER INC
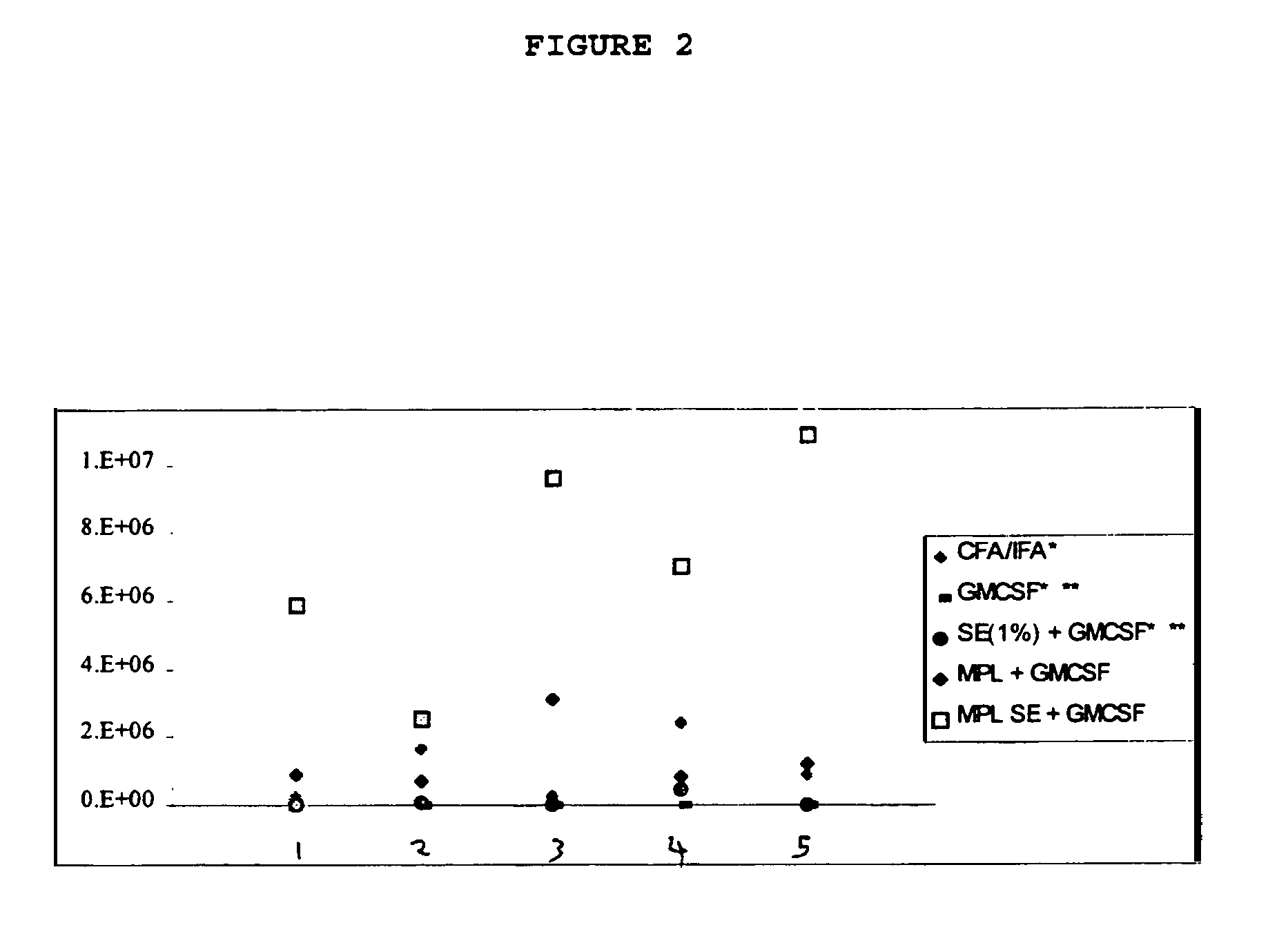
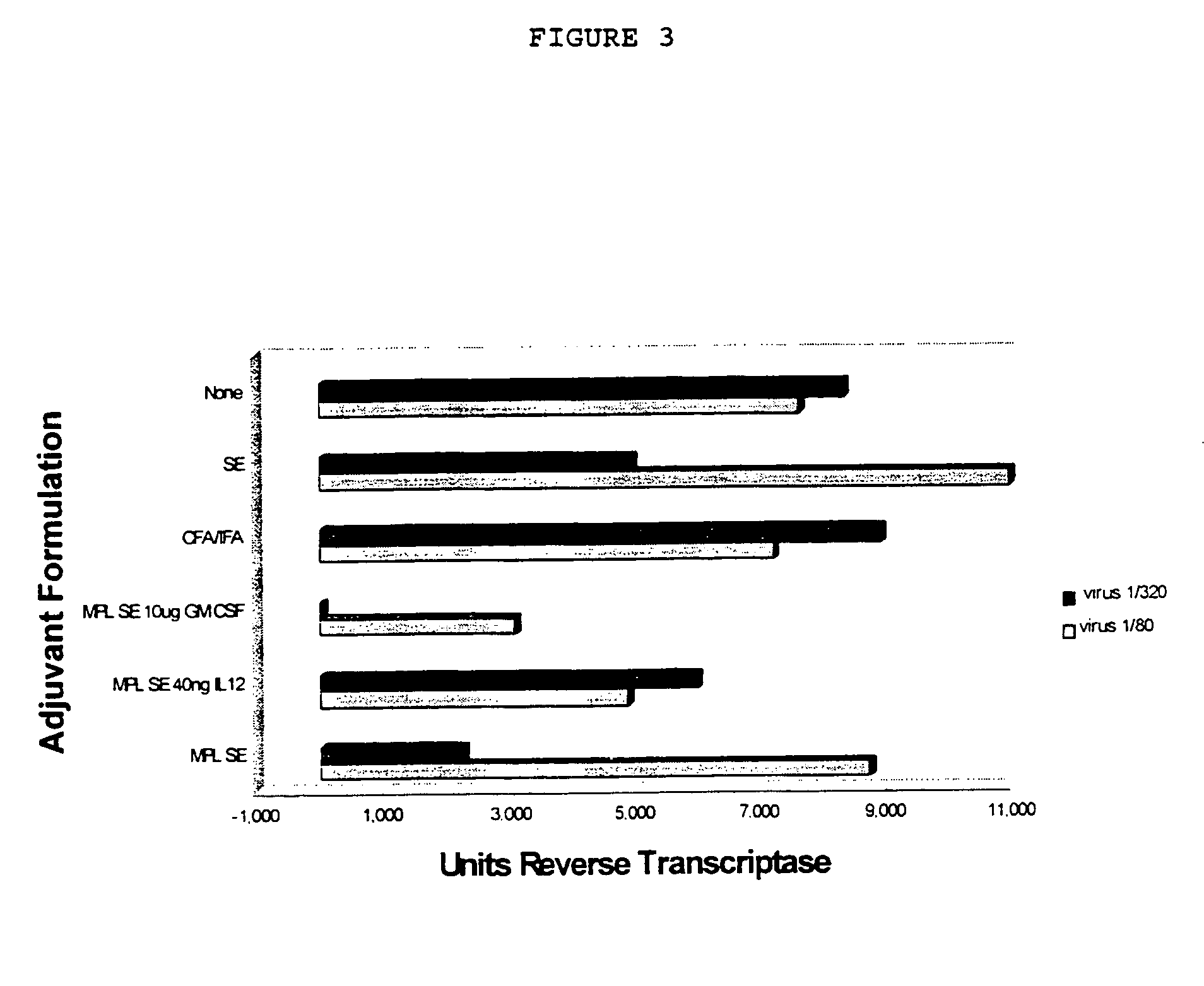
Compositions containing immunogenic molecules and granulocyte-macrophage colony stimulating factor, as an adjuvant
Granulocyte-macrophage colony stimulating factor ("GM-CSF") has been found to act as an adjuvant when administered to subjects. Compositions of GM-CSF and immunogenic compositions are presented, as is the use of GM-CSF alone and in these compositions.
Owner:LUDWIG INST FOR CANCER RES
Chemical address tags
The present invention provides methods and compositions related to the fields of chemoinformatics, chemogenomics, drug discovery and development, and drug targeting. In particular, the present invention provides subcellular localization signals (e.g., chemical address tags) that influence (e.g., direct) subcellular and organelle level localization of associated compounds (e.g., drugs and small molecule therapeutics, radioactive species, dyes and imagining agents, proapoptotic agents, antibiotics, etc) in target cells and tissues. The compositions of the present invention modulate the pharmacological profiles of associated compounds by influencing the compound's accumulation, or exclusion, from subcellular loci such as mitochondria, endoplasmic reticulum, cytoplasm, vesicles, granules, nuclei and nucleoli and other subcellular organelles and compartments. The present invention also provides methods for identifying chemical address tags, predicting their targeting characteristics, and for rational designing chemical libraries comprising chemical address tags.
Owner:RGT UNIV OF MICHIGAN
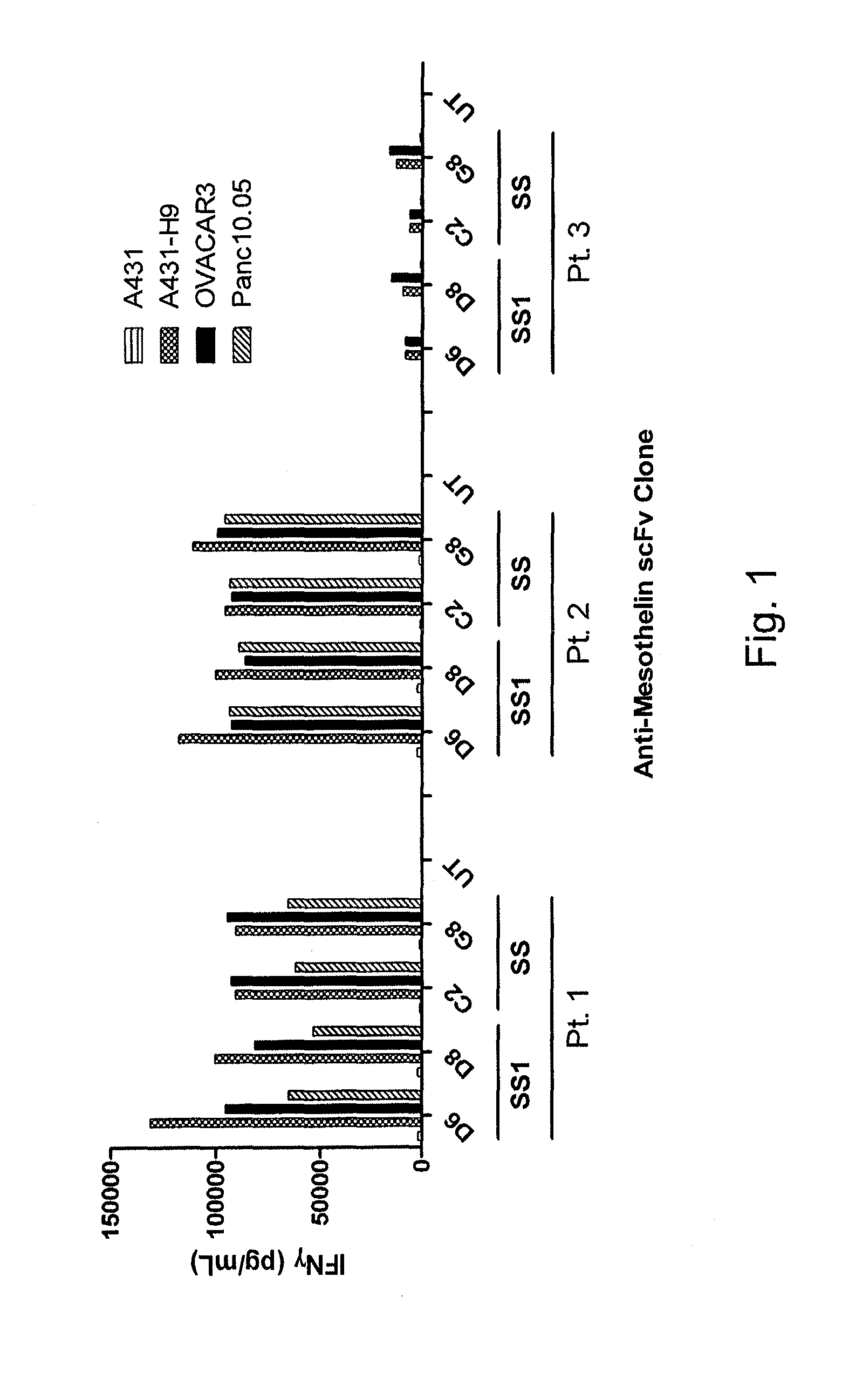
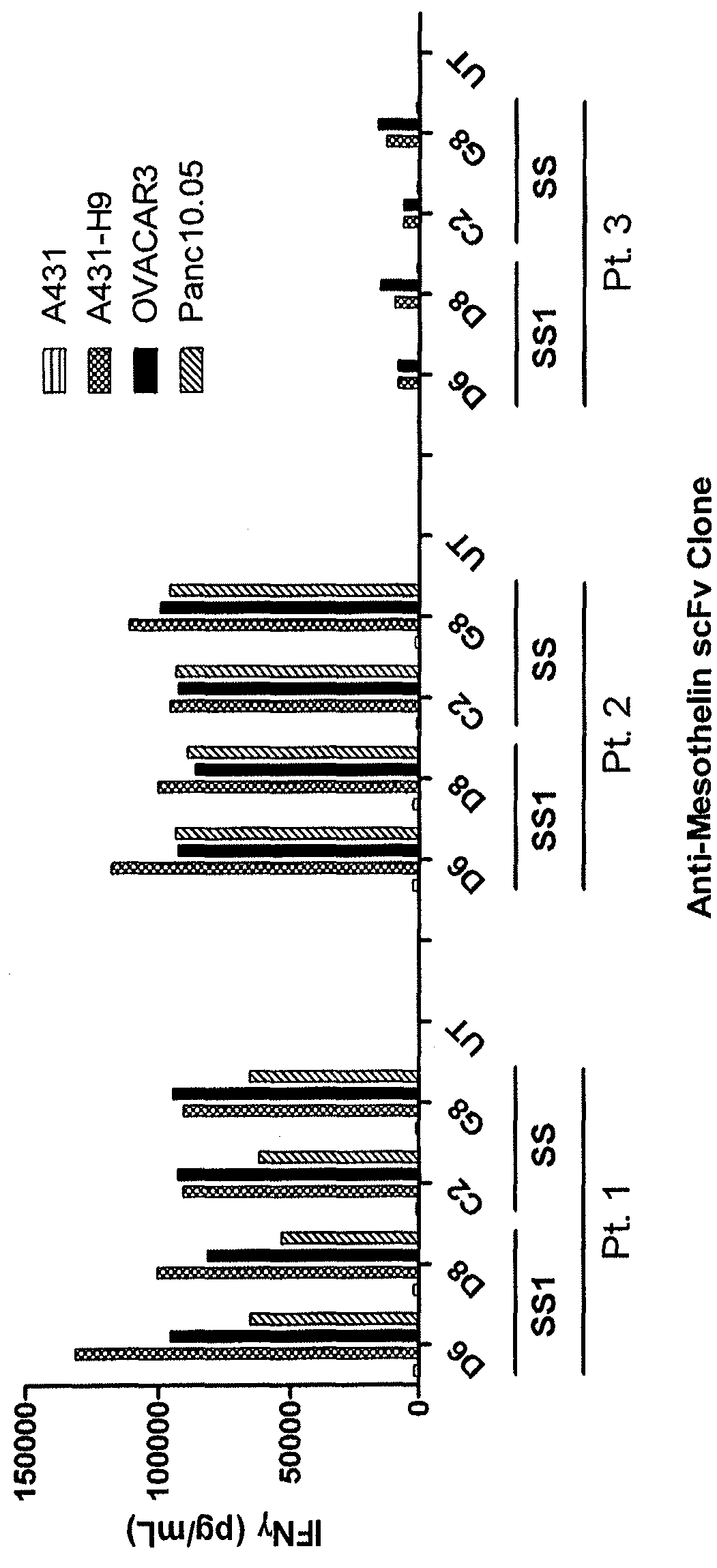
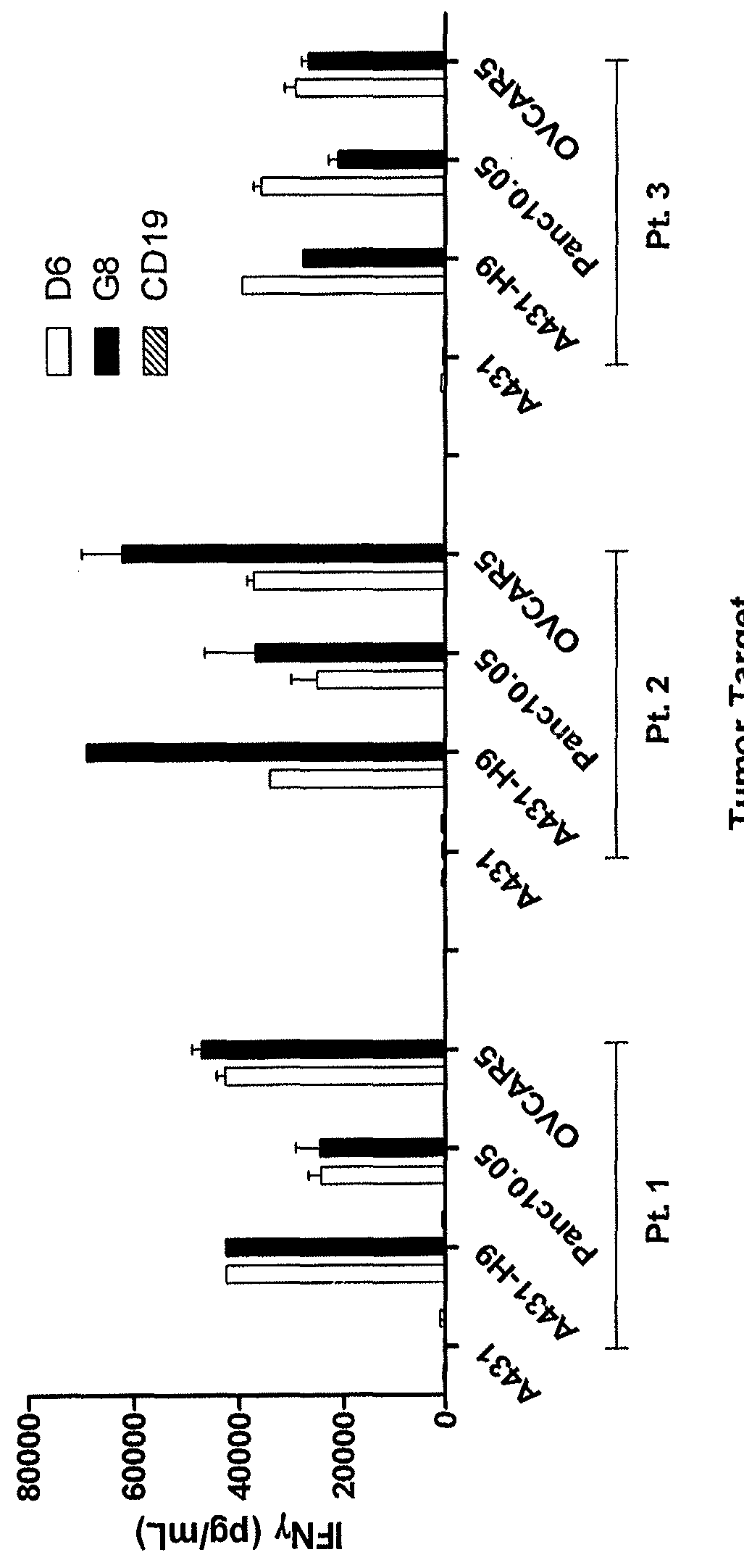
Anti-mesothelin chimeric antigen receptors
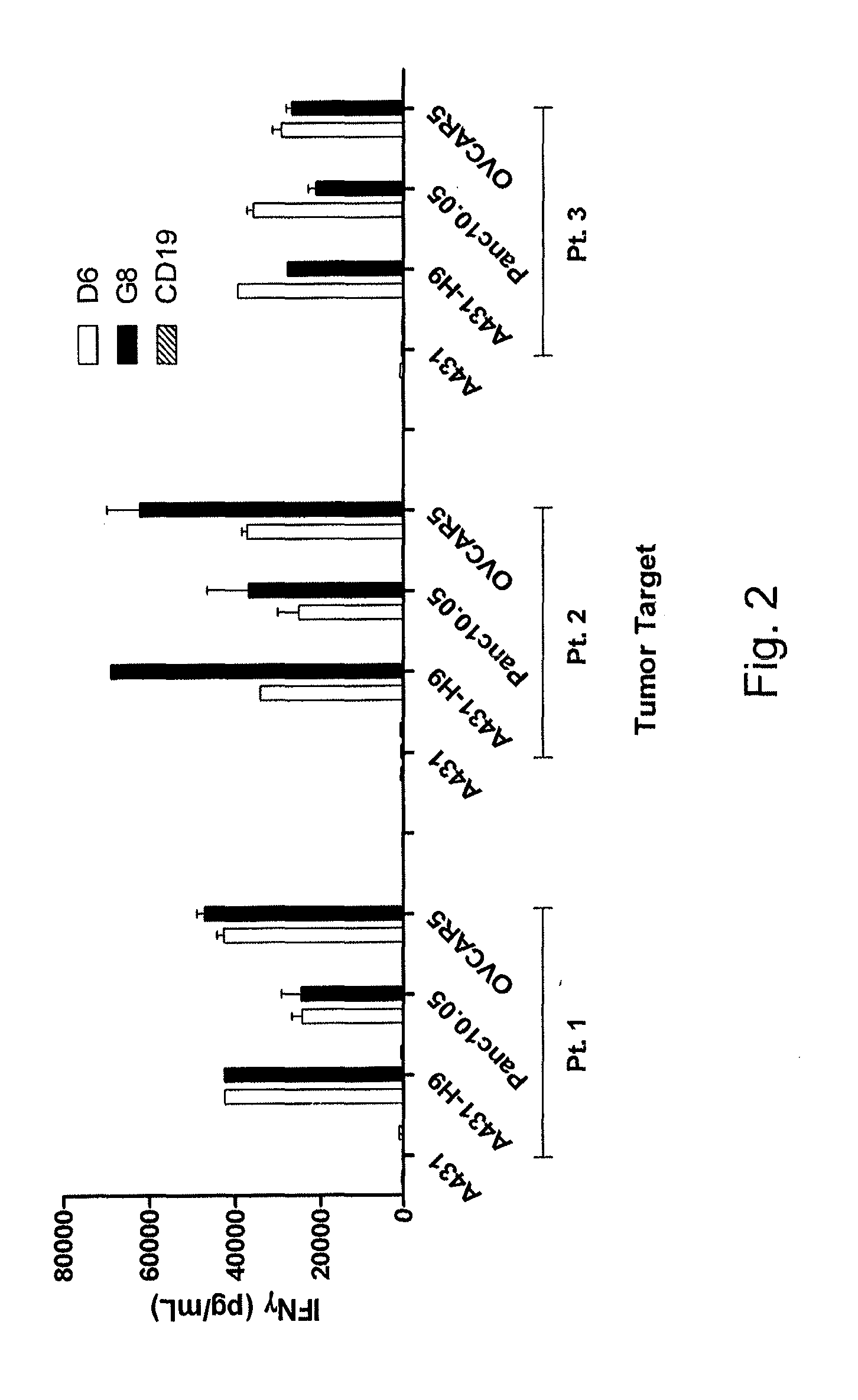
The invention provides a chimeric antigen receptor (CAR) (a) an antigen binding domain of HN1 or SS, a transmembrane domain, and an intracellular T cell signaling domain, or (b) an antigen binding domain of SS1, a transmembrane domain, an intracellular T cell signaling domain, and a granulocyte-macrophage colony-stimulating factor (GM-CSF) receptor 2 leader. Nucleic acids, recombinant expression vectors, host cells, populations of cells, antibodies, or antigen binding portions thereof, and pharmaceutical compositions relating to the CARs are disclosed. Methods of detecting the presence of cancer in a mammal and methods of treating or preventing cancer in a mammal are also disclosed.
Owner:UNITED STATES OF AMERICA
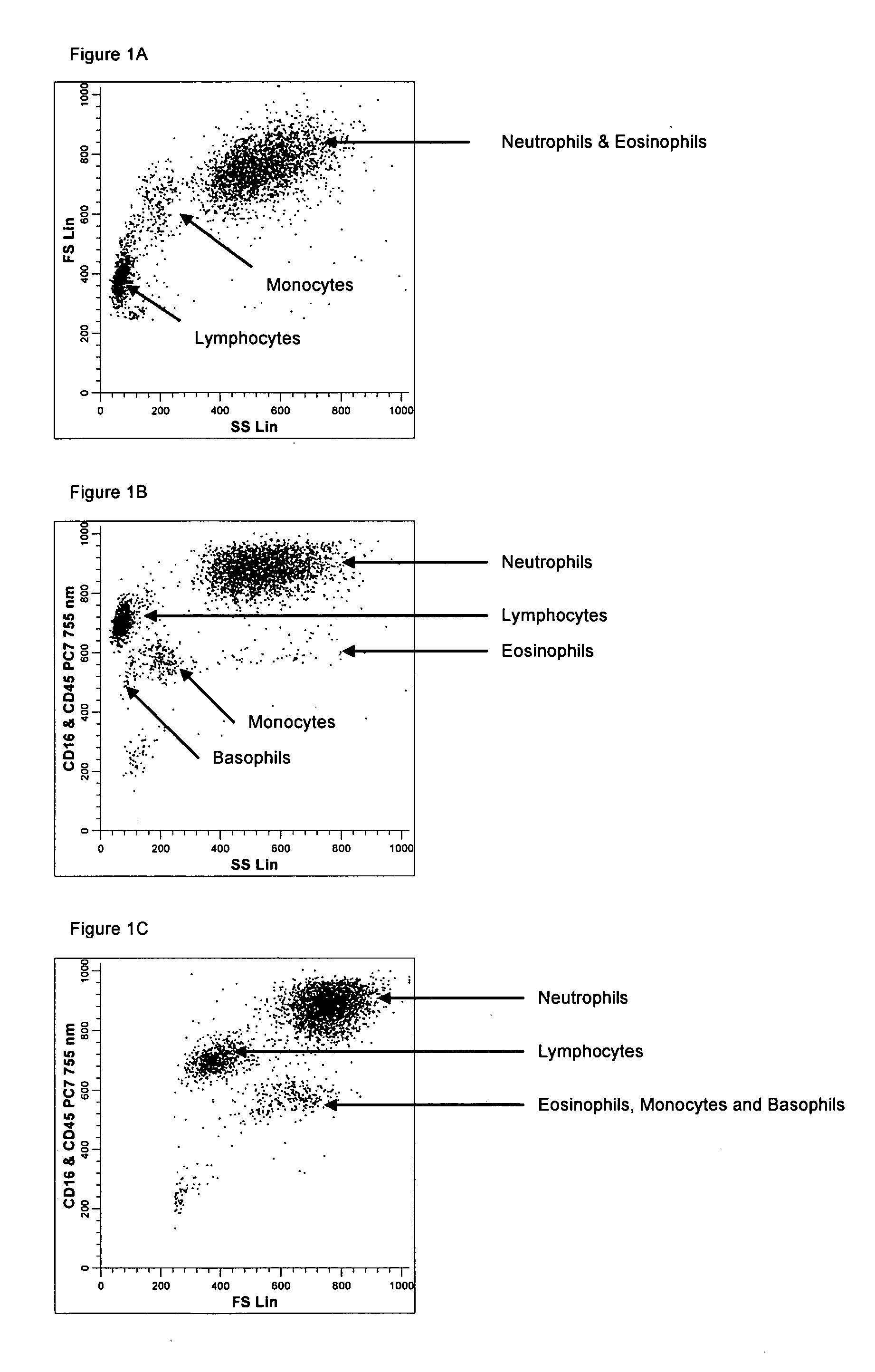
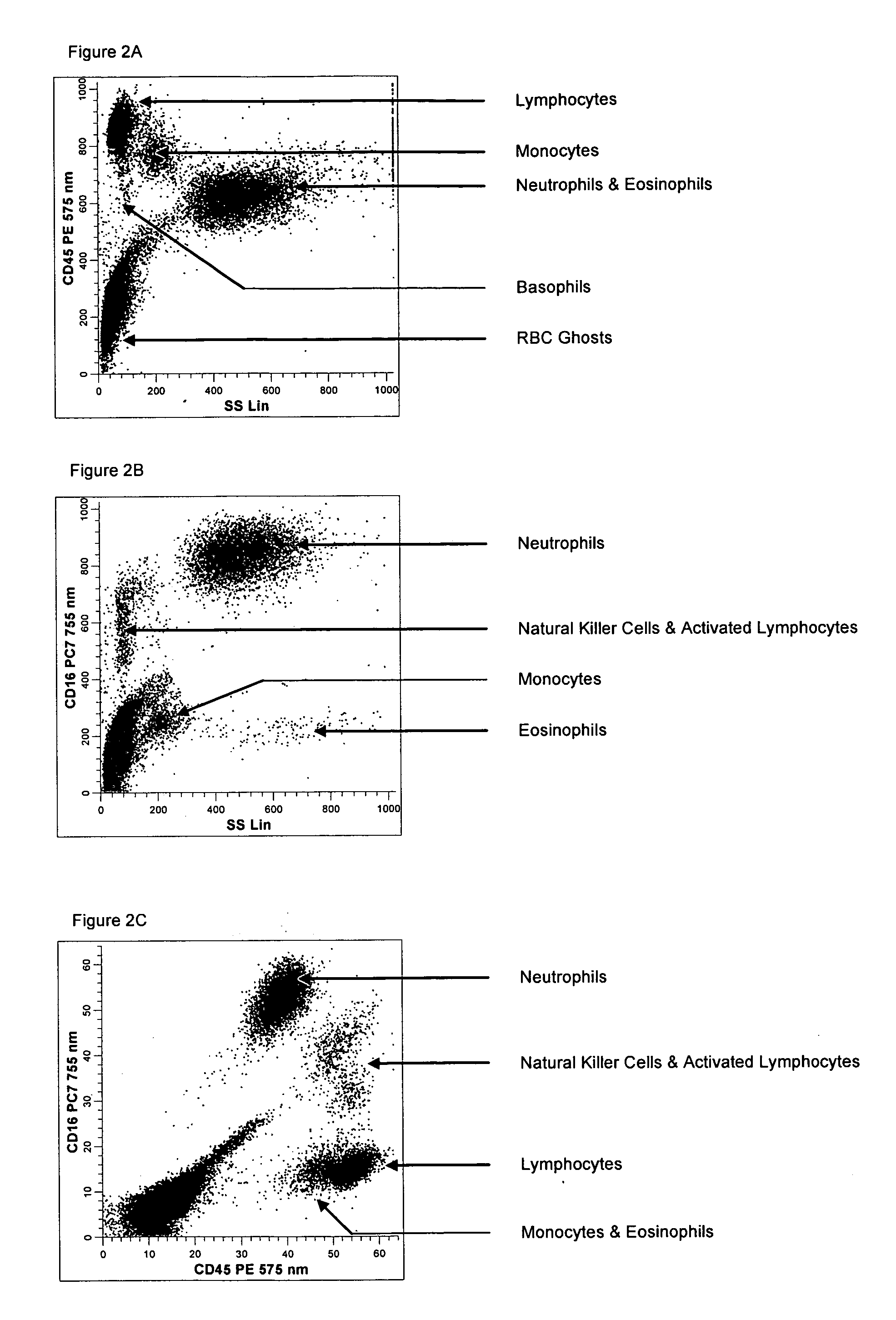
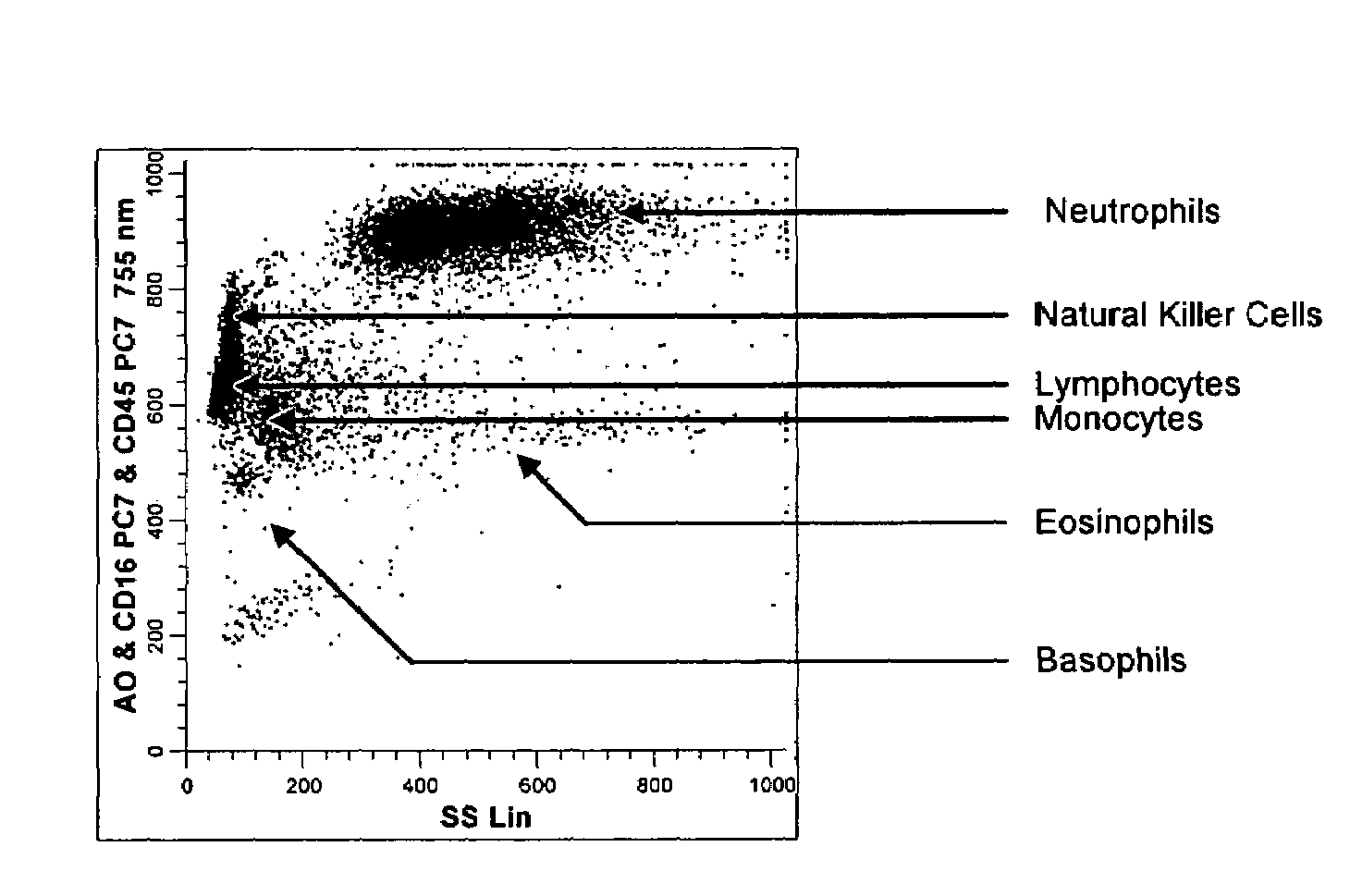
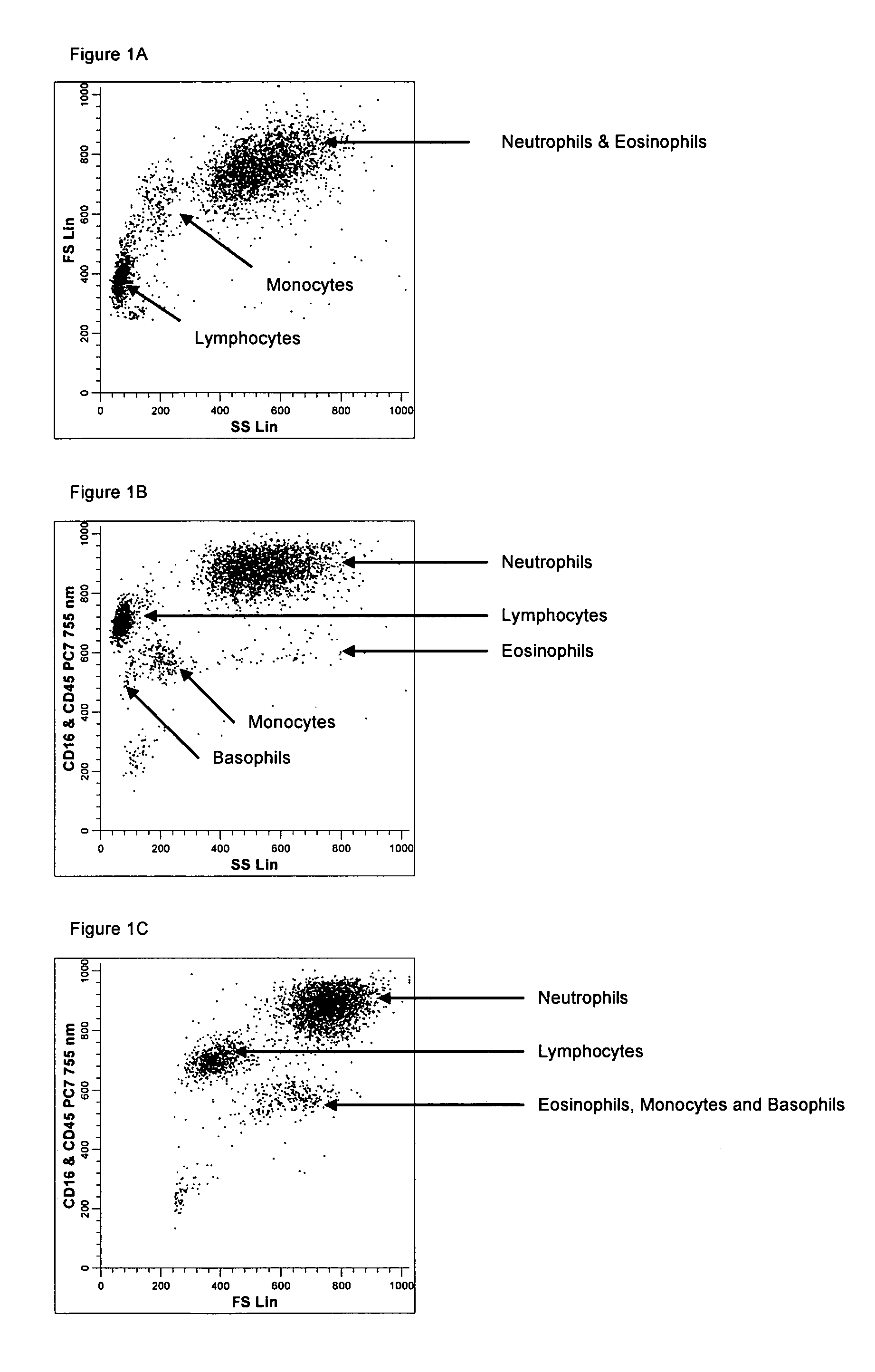
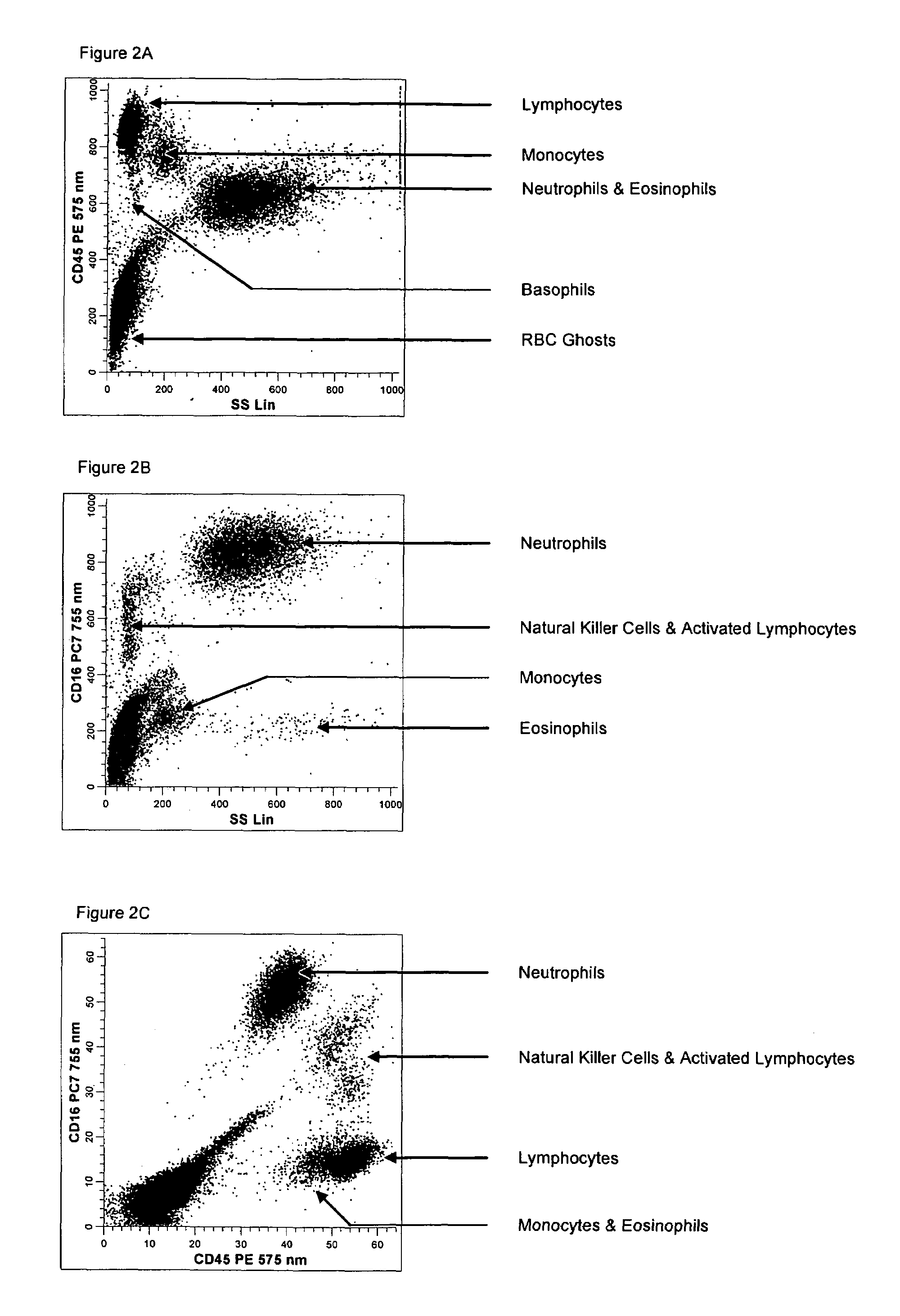
Method for a fully automated monoclonal antibody-based extended differential
ActiveUS20050260766A1Great advanceHigh degree of sensitivityBioreactor/fermenter combinationsBiological substance pretreatmentsAntigenWhite blood cell
A method useful for the enumeration of cell populations in a biological sample includes the steps of reacting in a single reaction mixture a sample, a first antibody labeled with a fluorochrome having a first emission spectrum and an additional antibody. The first antibody binds to an antigenic determinant differentially expressed on leukocytes and non-leukocytes. The additional antibody binds to an antigenic determinant differentially expressed on mature and immature granulocytes or myeloid cells, and is labeled either with the first fluorochrome or an additional fluorochrome having an emission spectrum distinguishable from the first emission spectrum. The reaction mixture can be mixed with a nucleic acid dye having an emission spectrum that overlaps with one of the first or additional emission spectra. The reaction mixture may be treated with a lytic system that differentially lyses non-nucleated red blood cells and conserves leukocytes. Populations of hematological cells are detected and enumerated using at least two parameters (fluorescence, optical, and electrical) for each population.
Owner:BECKMAN COULTER INC
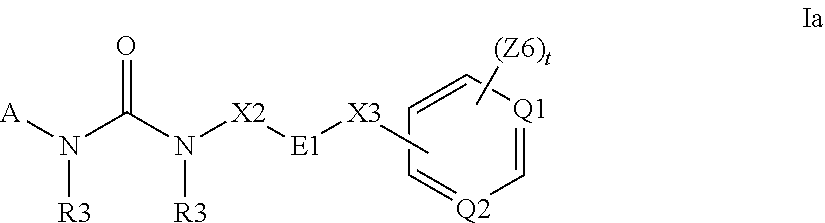
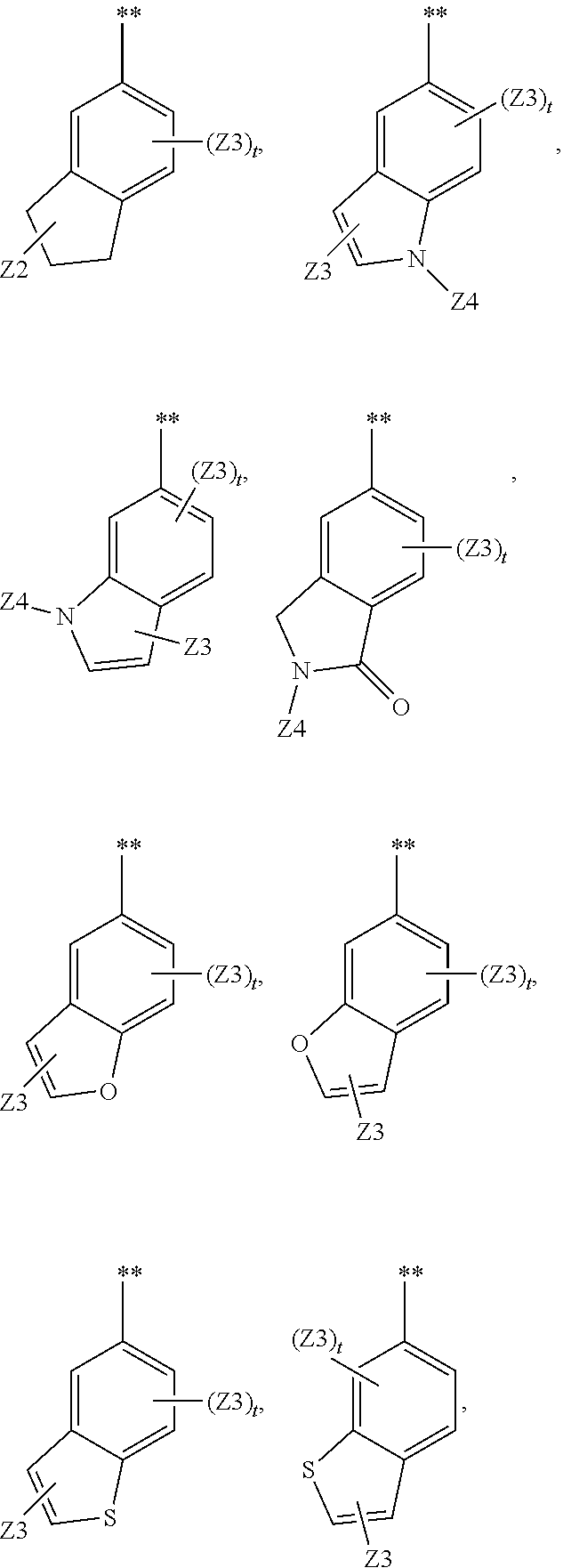
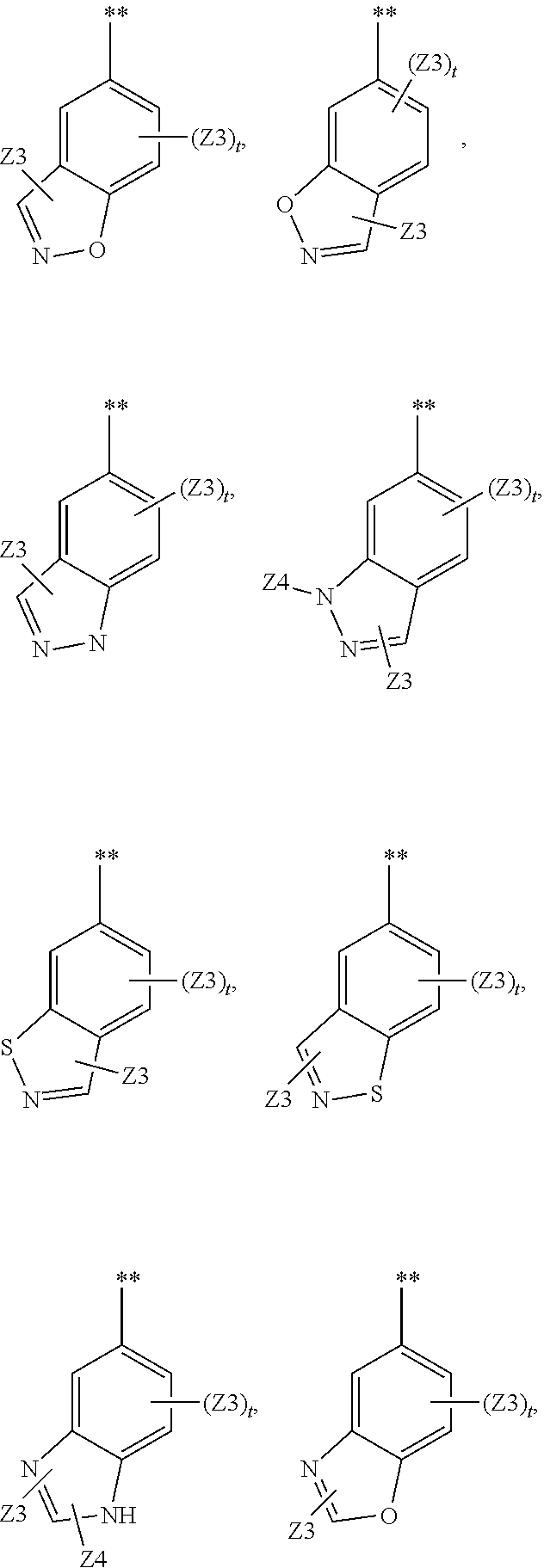
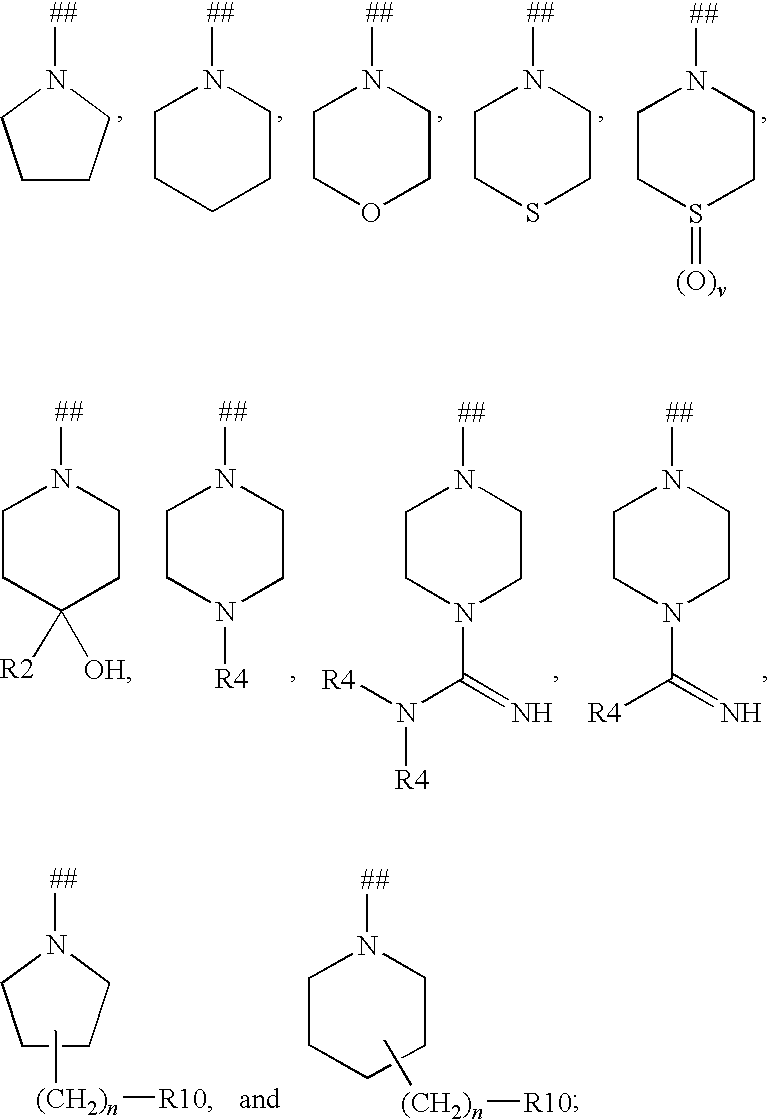
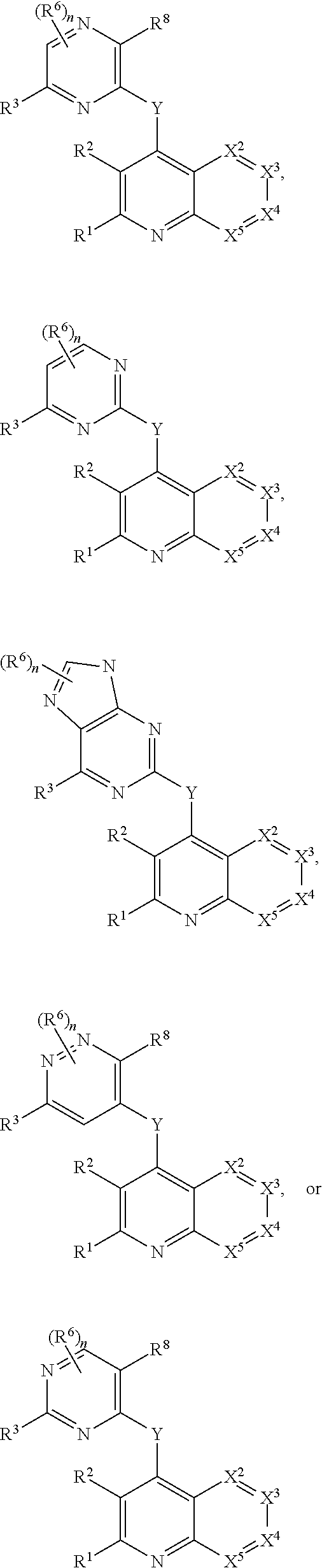
Heterocyclic compounds and their uses
Substituted bicyclic heteroaryls and compositions containing them, for the treatment of general inflammation, arthritis, rheumatic diseases, osteoarthritis, inflammatory bowel disorders, inflammatory eye disorders, inflammatory or unstable bladder disorders, psoriasis, skin complaints with inflammatory components, chronic inflammatory conditions, including but not restricted to autoimmune diseases such as systemic lupus erythematosis (SLE), myestenia gravis, rheumatoid arthritis, acute disseminated encephalomyelitis, idiopathic thrombocytopenic purpura, multiples sclerosis, Sjoegren's syndrome and autoimmune hemolytic anemia, allergic conditions including all forms of hypersensitivity. The present invention also enables methods for treating cancers that are mediated, dependent on or associated with pi 105 activity, including but not restricted to leukemias, such as Acute Myeloid leukaemia (AML) Myelo-dysplastic syndrome (MDS) myelo-proliferative diseases (MPD) Chronic Myeloid Leukemia (CML) T-cell Acute Lymphoblastic leukaemia (T-ALL) B-cell Acute Lymphoblastic leukaemia (B-ALL) Non Hodgkins Lymphoma (NHL) B-cell lymphoma and solid tumors, such as breast cancer.
Owner:AMGEN INC
Bi-Aryl Meta-Pyrimidine Inhibitors of Kinases
The invention provides methods of treating a disease selected from systemic sclerosis, rheumatoid arthritis, mastocytosis, and chronic eosinophilic leukemia comprising administering biaryl meta-pyrimidine compounds having the general structure (A) to a subject in need thereof. The pyrimidine compounds of the invention are capable of inhibiting kinases, such as members of the JAK kinase family, and various other specific receptor and non-receptor kinases.
Owner:IMPACT BIOMEDICINES INC
Hybrid protein for inhibiting the degranulation of mastocytes and the use thereof
InactiveUS6822076B2Avoid allergic reactionsAvoid symptomsHydrolasesPeptide/protein ingredientsTetanusBasophilia
A hybrid protein contains a protein that binds to a receptor of mastocytes and basophils and is endocyted by them. The protein can be IgE; IgE fragment; IgE Fc fragment; antibody against IgE receptor of mastocytes and basophils; fragment of the antibody against the IgE receptor of mastocytes and basophils; antibody against mastocyte specific potassium channel; and mast cell degranulating peptide. The hybrid protein also contains a protease cleaving proteins of the secretion process of the mastocytes and basophils so as to inhibit the secretion process without killing the mastocytes and basophils. The protease can be light chain Clostridium botulinum toxin; proteolytically active fragment of the light chain of a Clostridium botulinum toxin containing an amino acid sequence His-Xaa-Xaa-Xaa-His-Xaa-Xaa-His wherein Xaa is an amino acid; light chain of the tetanus toxin; proteolytically active fragment of the light chain of the tetanus toxin containing His-Asp-Leu-lIe-His-Val-Leu-His; IgA protease of Neisseria gonorrhoeae; and proteolytic domain of the IgA protease of Neisseria gonorrhoeae.
Owner:MERZ PHARMA GMBH & CO KGAA
N-acyl ureas exhibiting anti-cancer and anti-proliferative activities
Compounds of the present invention find utility in the treatment of mammalian cancers and especially human cancers including, but not limited to, malignant melanomas, solid tumors, glioblastomas, ovarian cancer, pancreatic cancer, prostate cancer, lung cancers, breast cancers, kidney cancers, hepatic cancers, cervical carcinomas, metastasis of primary tumor sites, myeloproliferative diseases, chronic myelogenous leukemia, leukemias, papillary thyroid carcinoma, non-small cell lung cancer, mesothelioma, hypereosinophilic syndrome, gastrointestinal stromal tumors, colonic cancers, ocular diseases characterized by hyperproliferation leading to blindness including various retinopathies, diabetic retinopathy, rheumatoid arthritis, asthma, chronic obstructive pulmonary disease, mastocytosis, mast cell leukemia, and diseases caused by PDGFR-α kinase, PDGFR-β kinase, c-KIT kinase, cFMS kinase, c-MET kinase, and oncogenic forms, aberrant fusion proteins and polymorphs of any of the foregoing kinases.
Owner:DECIPHERA PHARMA LLC
Anti-mesothelin chimeric antigen receptors
ActiveUS9359447B2Polypeptide with localisation/targeting motifImmunoglobulin superfamilyCancer preventionAntiendomysial antibodies
Owner:UNITED STATES OF AMERICA
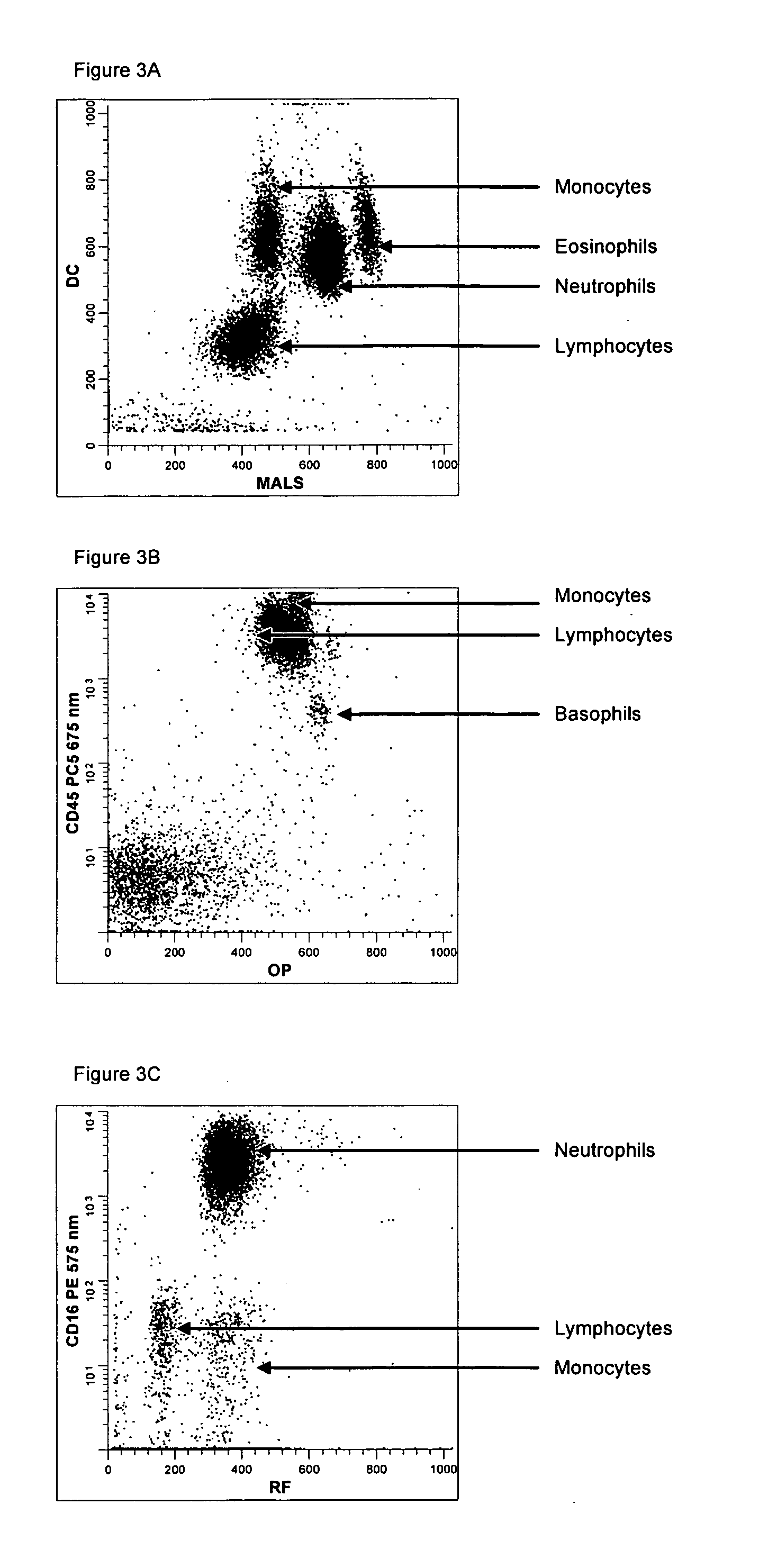
Method for a fully automated monoclonal antibody-based extended differential
ActiveUS7625712B2Bioreactor/fermenter combinationsBiological substance pretreatmentsFluorescenceWhite blood cell
A method useful for the enumeration of cell populations in a biological sample includes the steps of reacting in a single reaction mixture a sample, a first antibody labeled with a fluorochrome having a first emission spectrum and an additional antibody. The first antibody binds to an antigenic determinant differentially expressed on leukocytes and non-leukocytes. The additional antibody binds to an antigenic determinant differentially expressed on mature and immature granulocytes or myeloid cells, and is labeled either with the first fluorochrome or an additional fluorochrome having an emission spectrum distinguishable from the first emission spectrum. The reaction mixture can be mixed with a nucleic acid dye having an emission spectrum that overlaps with one of the first or additional emission spectra. The reaction mixture may be treated with a lytic system that differentially lyses non-nucleated red blood cells and conserves leukocytes. Populations of hematological cells are detected and enumerated using at least two parameters (fluorescence, optical, and electrical) for each population.
Owner:BECKMAN COULTER INC
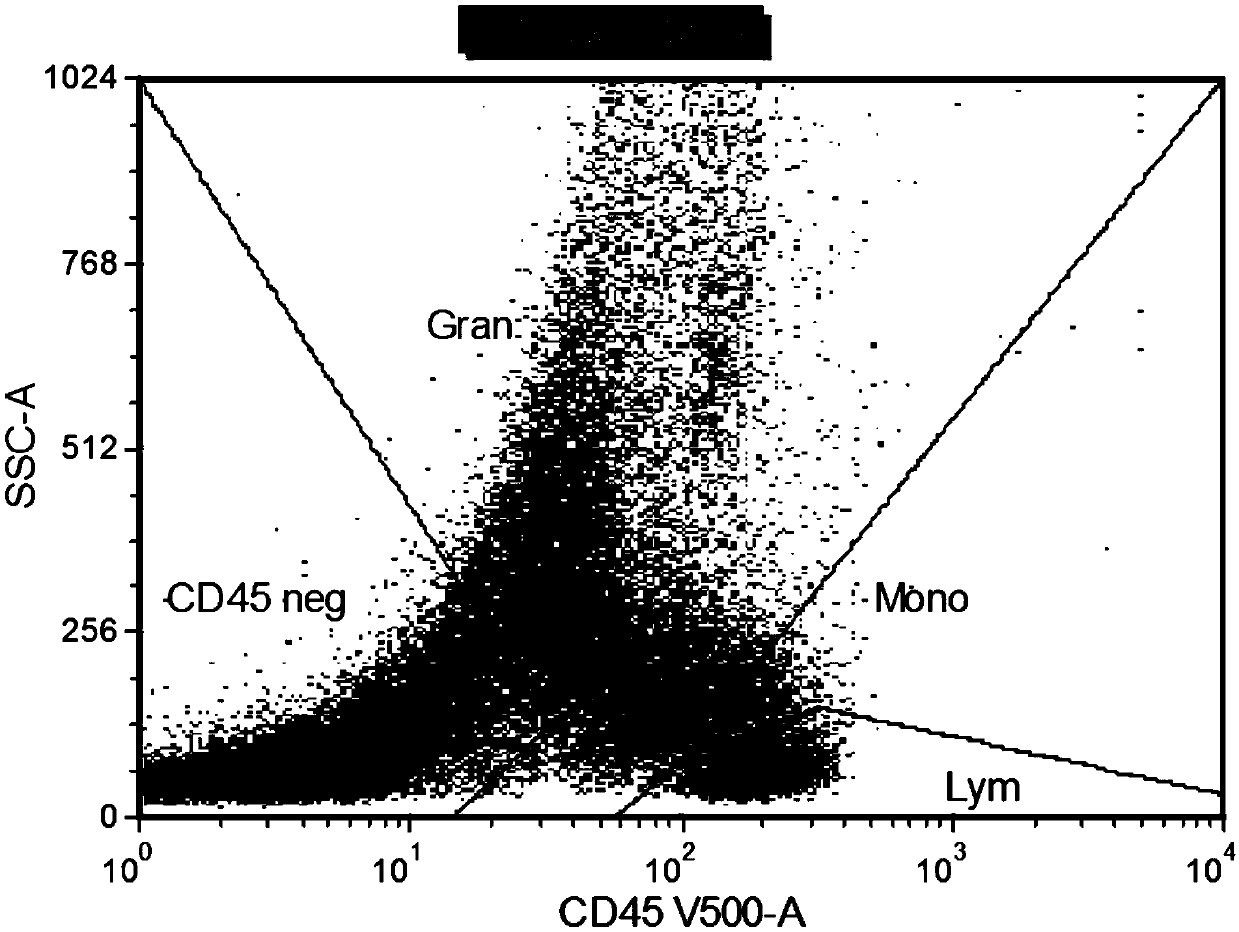
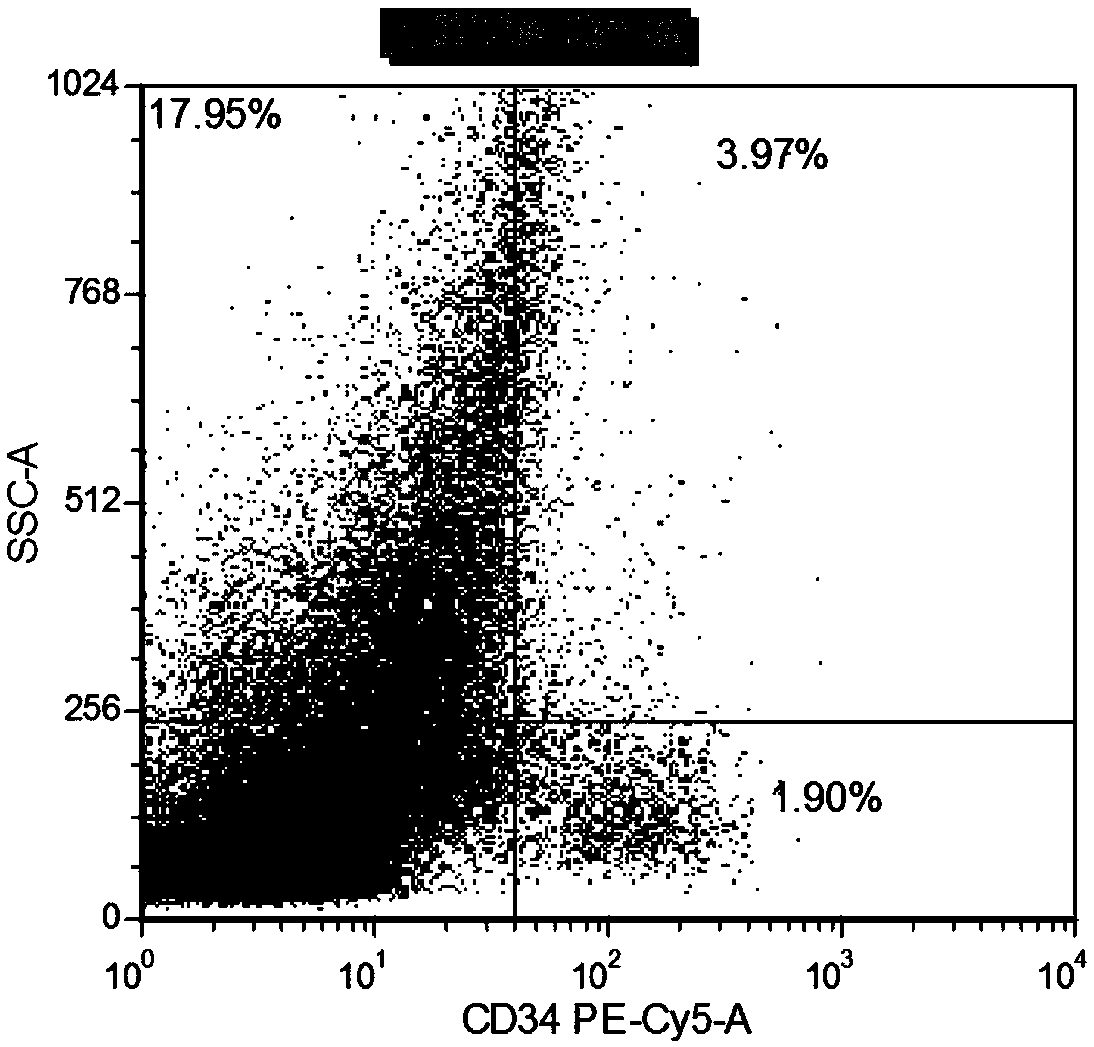
Combined reagent for detecting acute myelocytic leukemia cells and system thereof
ActiveCN109655616AWide coverageThere is no problem of reciprocal inhibition of expressionMaterial analysisCD33CD15
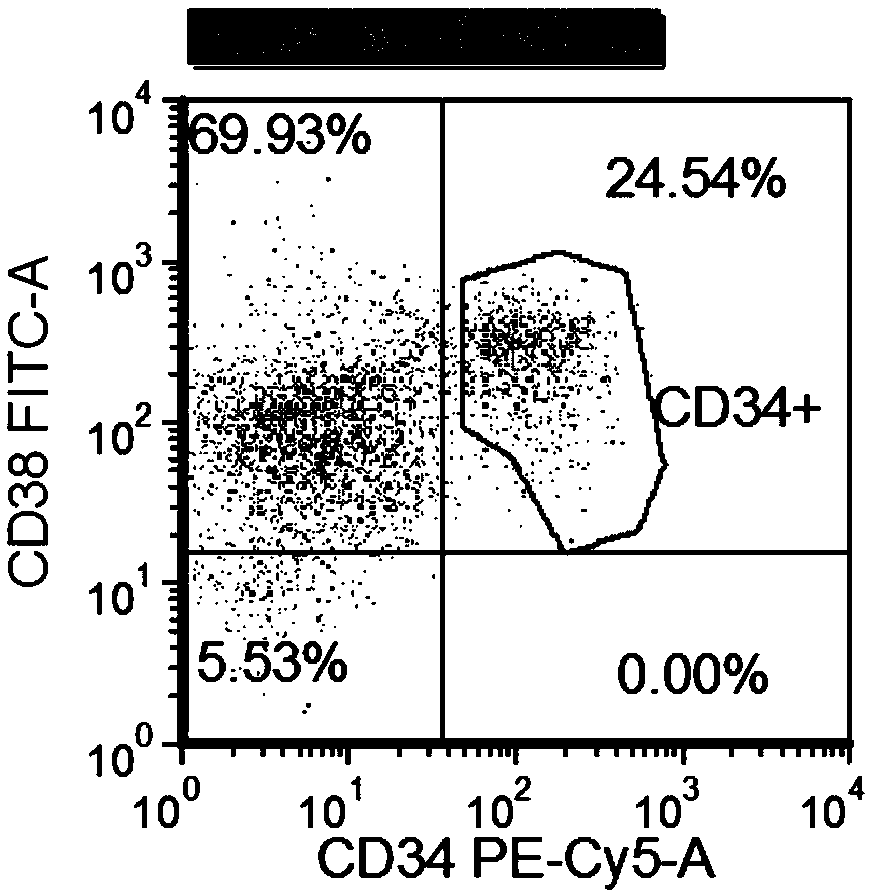
The invention relates to a combined reagent for detecting acute myelocytic leukemia cells and a system thereof, wherein the combined reagent and the system thereof belong to the field of medical technology. The combined reagent comprises at least one selected from the following antibody combinations: a first antibody combination which comprises CD38, CD13, CD34, CD117, CD33, CD19, HLA-DR and CD45antibodies; a second antibody combination which comprises CD38, CD64, CD34, CD123, CD56, CD14, HLA-DR and CD45 antibodies; and a third antibody combination which comprises CD38, CD7, CD34, CD5, CD11b,CD15 and CD45 antibodies. The antibody combinations of the invention cover the expression marks of three systems of granulocyte, single cell and lymphocyte. A normal antibody expression mode is established. Tumor cells can be identified maximally. Furthermore, through a large number of experiment data, the antibodies in each combination have no problem of mutual expression inhibition. FurthermoreAML-MRD can be comprehensively and quickly detected with high sensitivity through multi-parameter flow type cell analysis.
Owner:GUANGZHOU KINGMED DIAGNOSTICS CENT
Recombinant human granulocyte colony stimulating factor and one-step purifying process for chemical modifications thereof
InactiveCN1663962AHigh purityCarrier-bound/immobilised peptidesCytokines/lymphokines/interferonsG-csf therapyHigh activity
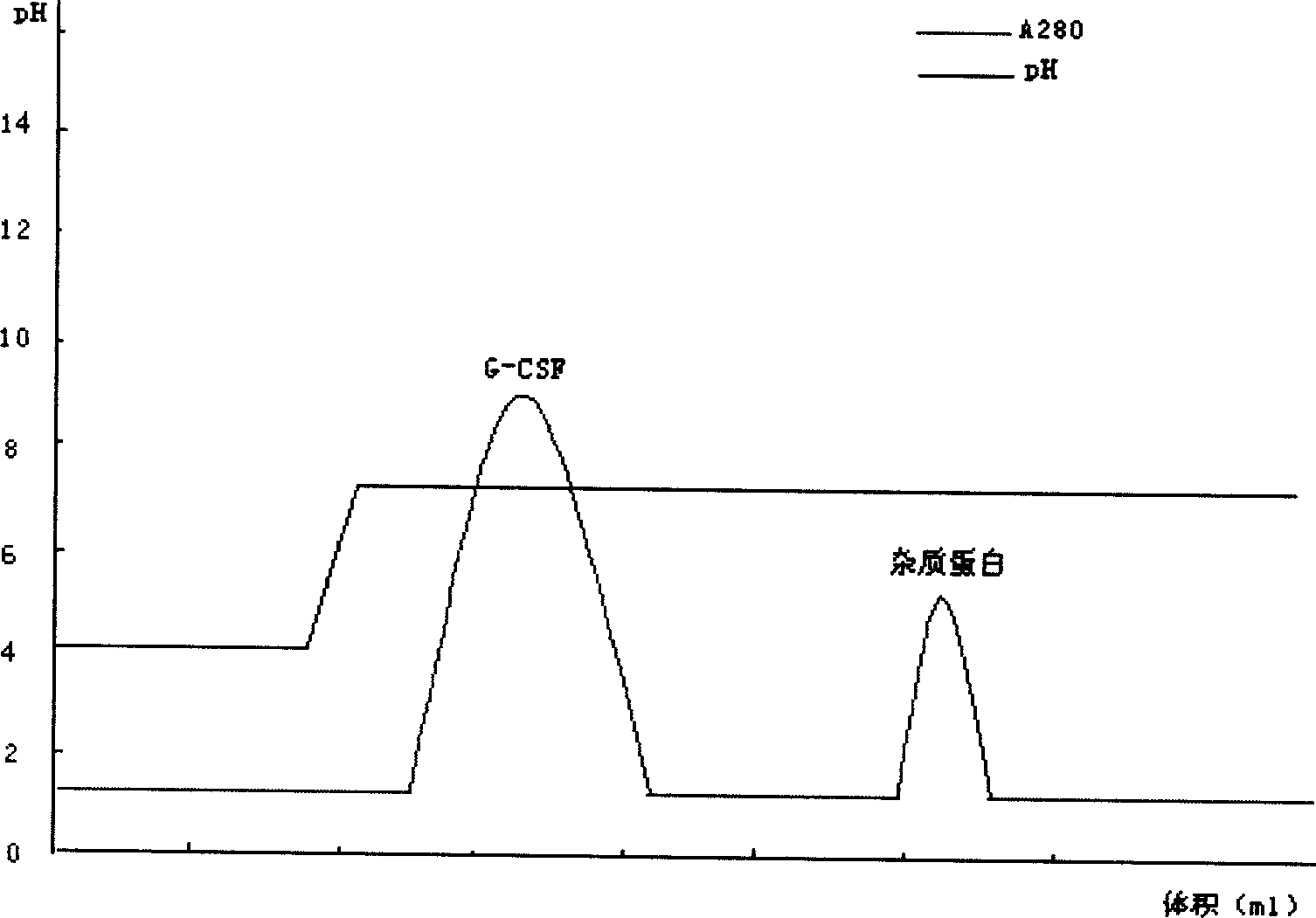
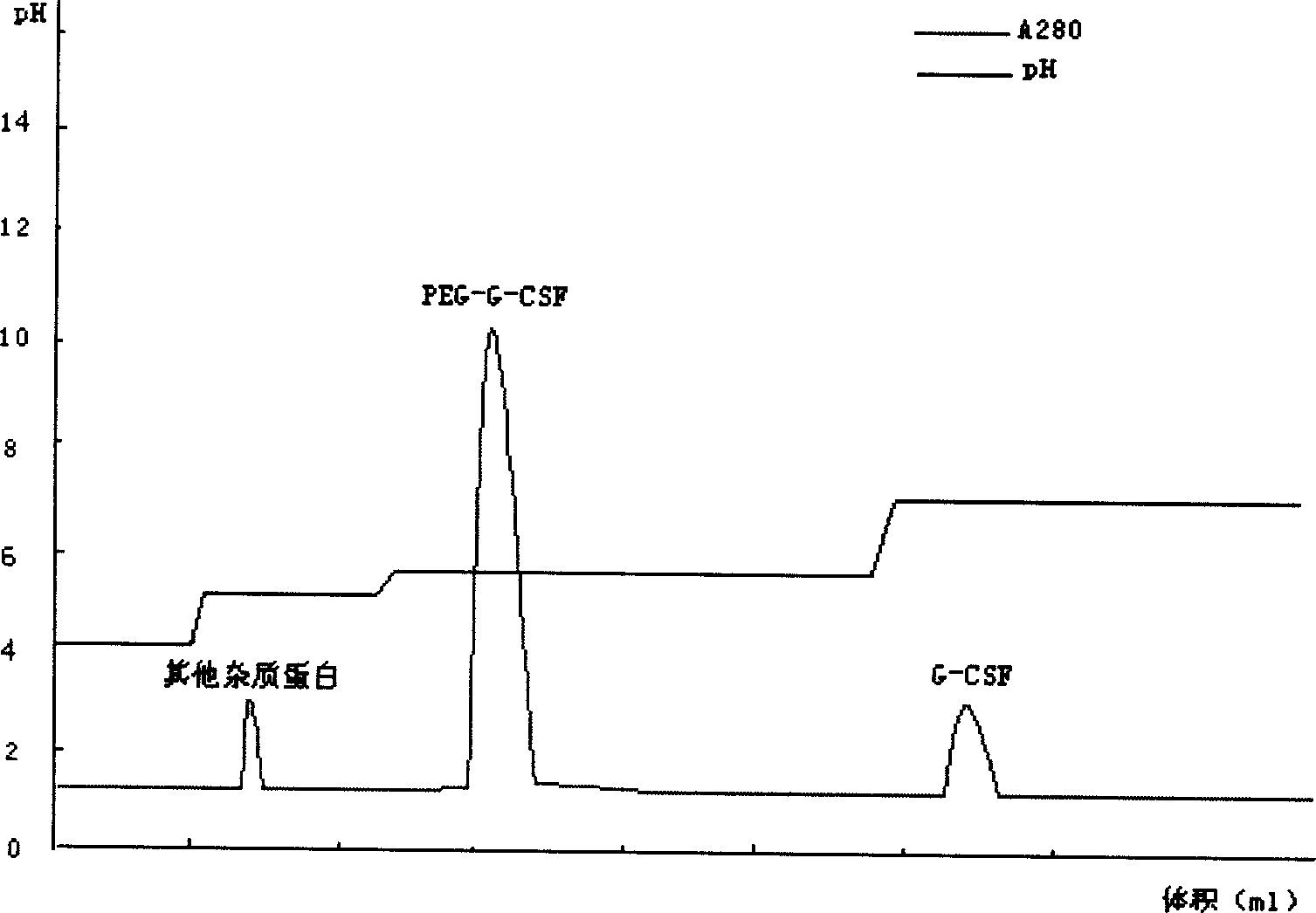
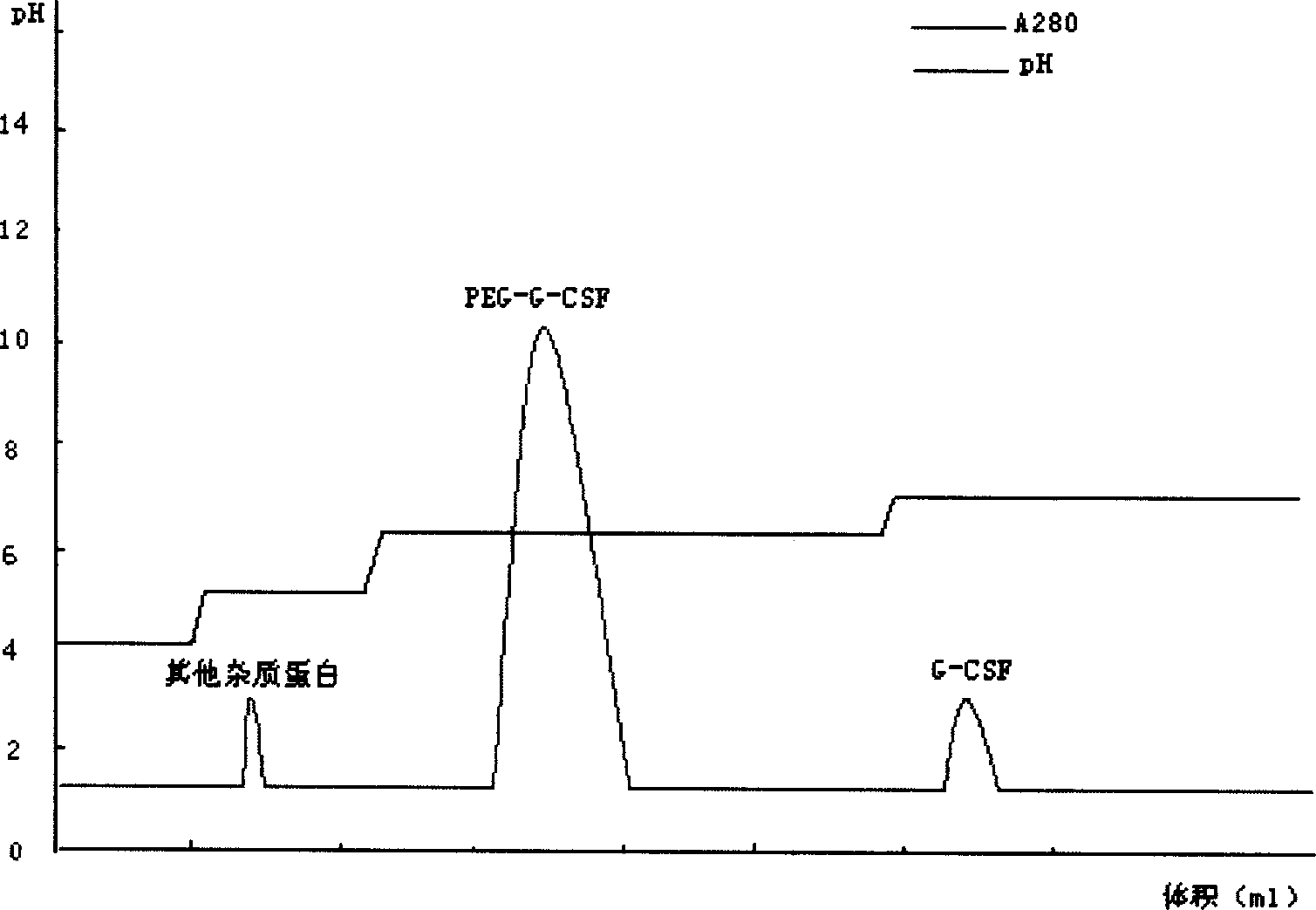
The invention relates to a recombinant human granulocyte colony stimulating factor and one-step purifying process for chemical modifications, which employs the method of cation exchange chromatography, wherein one-step chromatography is utilized for obtaining high activity, high purity and high recovery ratio rhG-CSF and polyethylene glycol chemically modified rhG-CSF. The process is especially suitable for industrial production.
Owner:CHONGQING FAGEN BIOMEDICAL
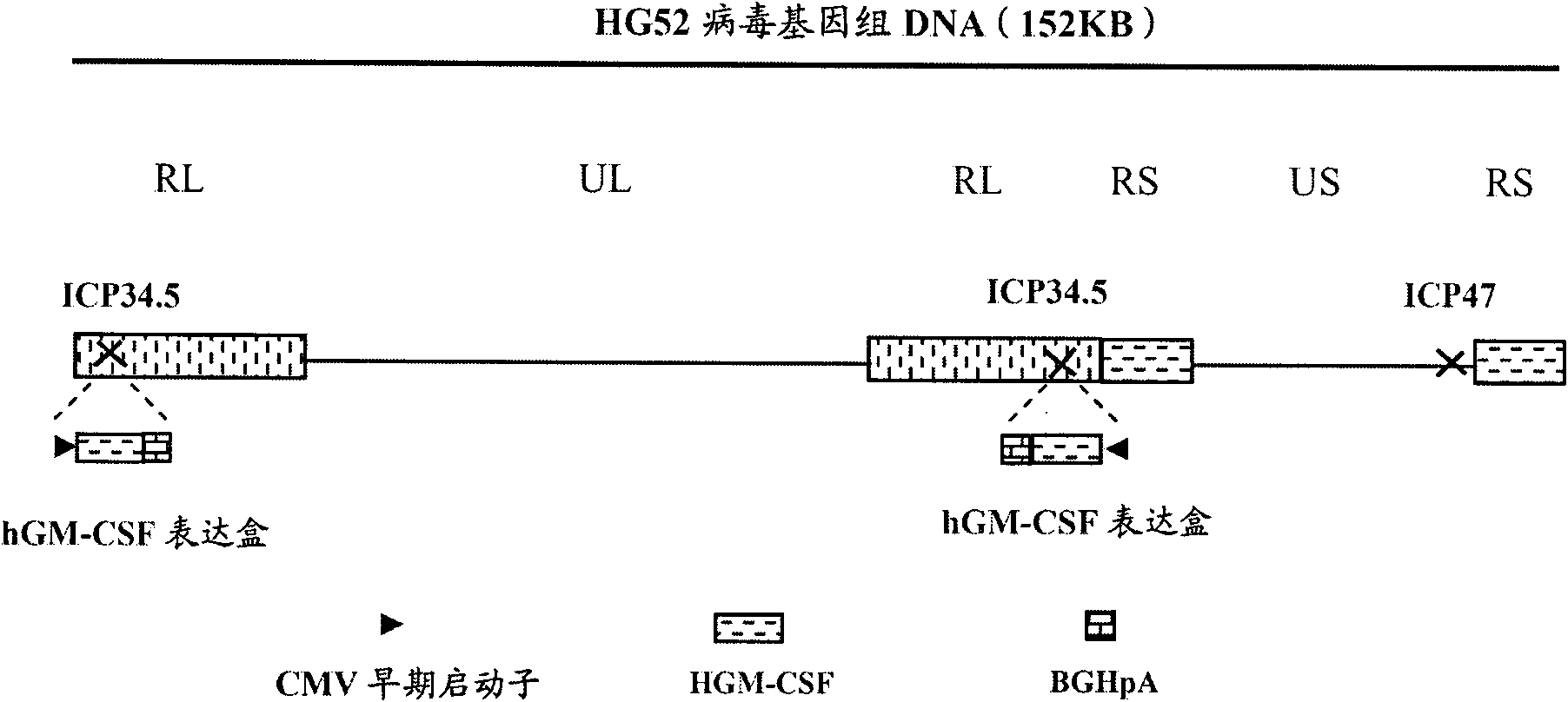
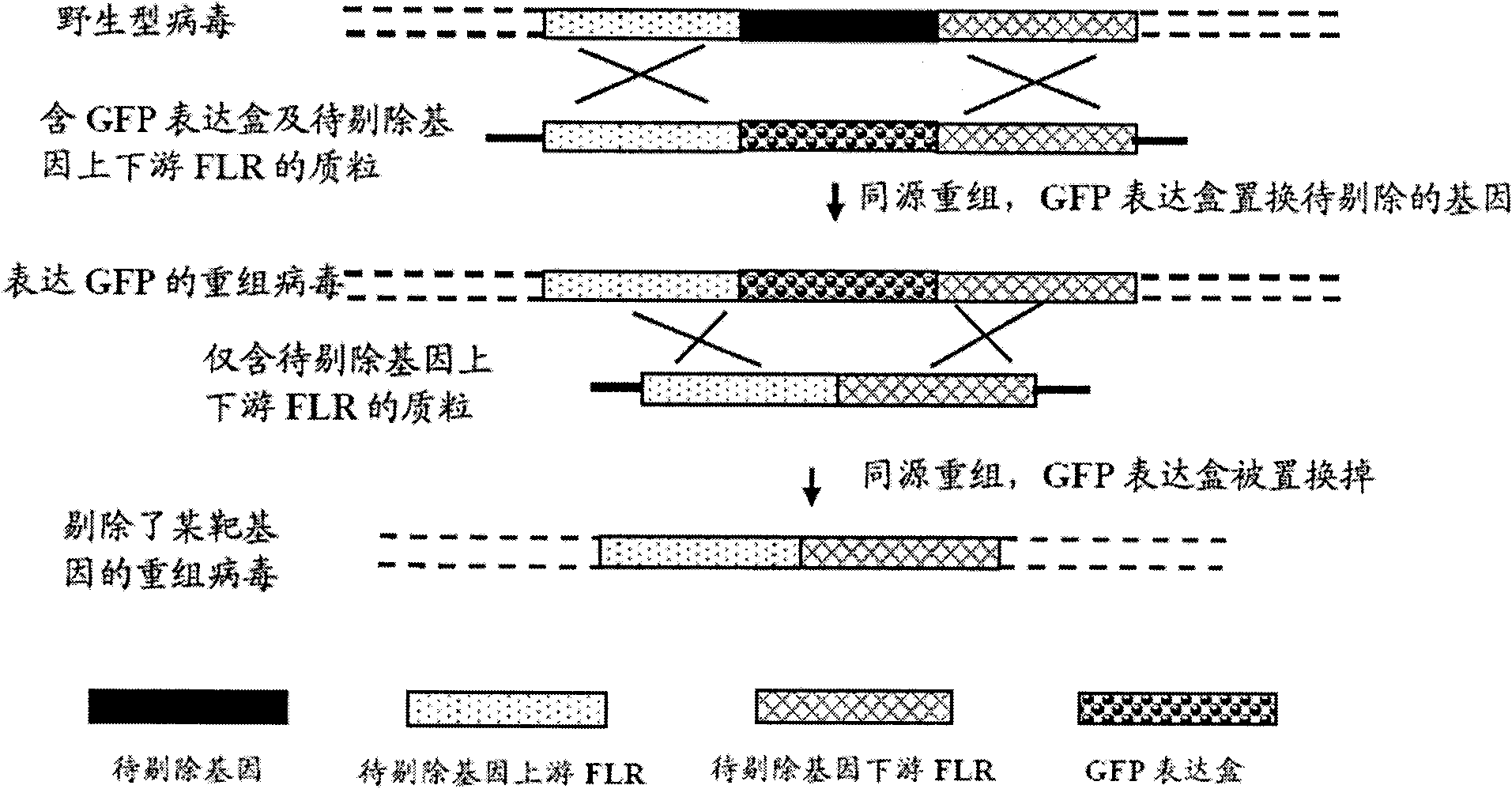
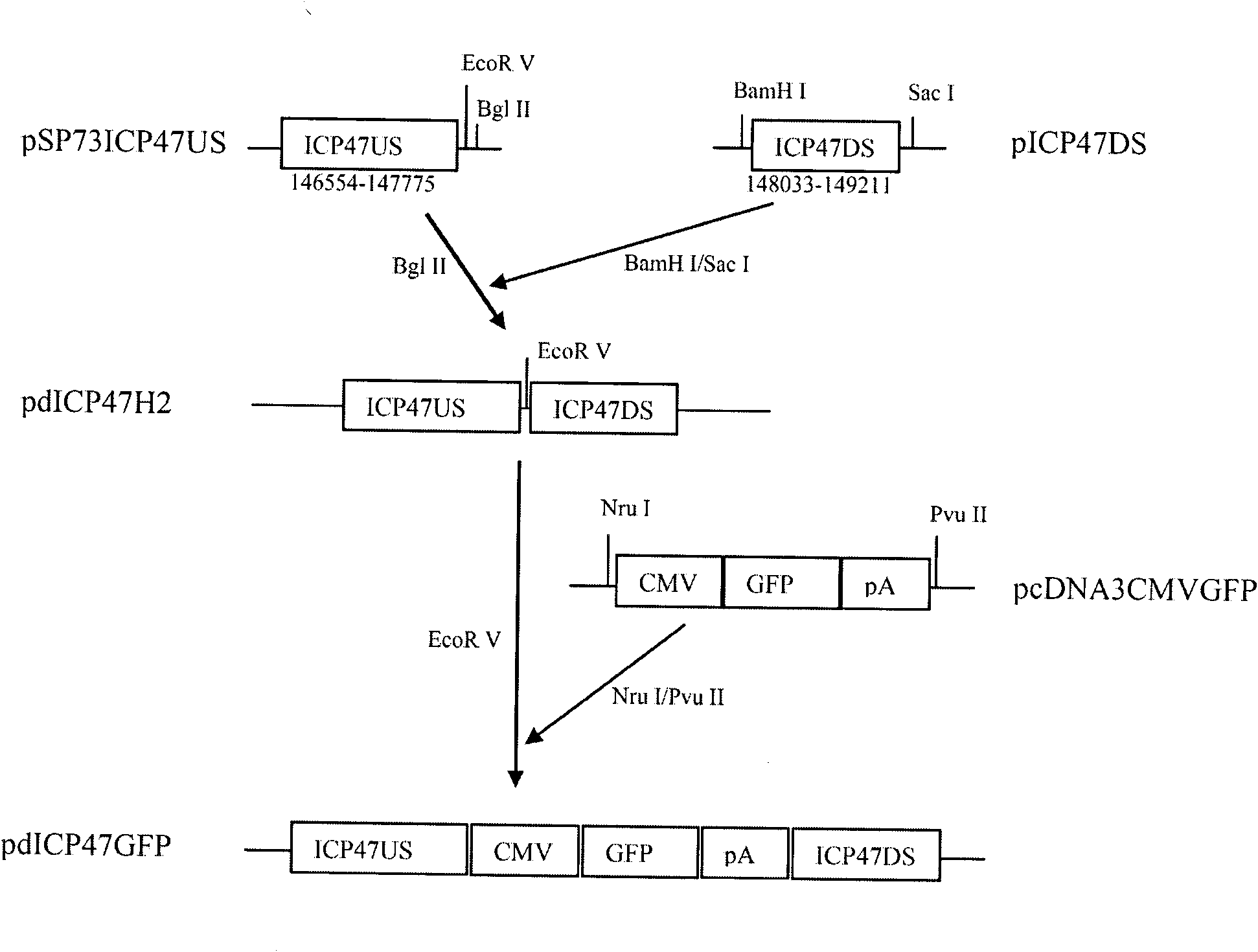
Recombinant II type herpes simplex virus vector, preparation method of recombinant II type herpes simplex virus vector, recombinant virus, medicinal composition and application
ActiveCN102146418AGenetic material ingredientsViral/bacteriophage medical ingredientsCurative effectRecombinant virus vaccine
The invention provides a recombinant II type herpes simplex virus vector. An ICP34.5 gene and an ICP47 gene of a wild II type herpes simplex virus HG52 strain are removed in the virus vector, and preferably a human granulocyte macrophage-colony stimulating factor (hGM-CSF) expression box is inserted into the position where the ICP34.5 gene is removed. The invention also provides a preparation method of the recombinant II type herpes simplex virus vector, a recombinant virus using the recombinant II type herpes simplex virus as a vector, a medicinal composition consisting of the recombinant II type herpes simplex virus vector and a pharmaceutically acceptable vector or excipient, and application of the recombinant II type herpes simplex virus vector in preparation of a gene medicament for treating tumors. As the ICP34.5 gene is removed in the recombinant II type herpes simplex virus vector provided by the invention, the oncolysis virus is safe and can selectively grow and propagate in tumor cells; the ICP47 gene is removed to promote immune response and enhance oncolysis activity; and the curative effect of the recombinant II type herpes simplex virus vector is superior to that of the conventional recombinant I type herpes simplex virus vector, and the recombinant II type herpes simplex virus vector has high safety.
Owner:WUHAN BINHUI BIOTECH CO LTD
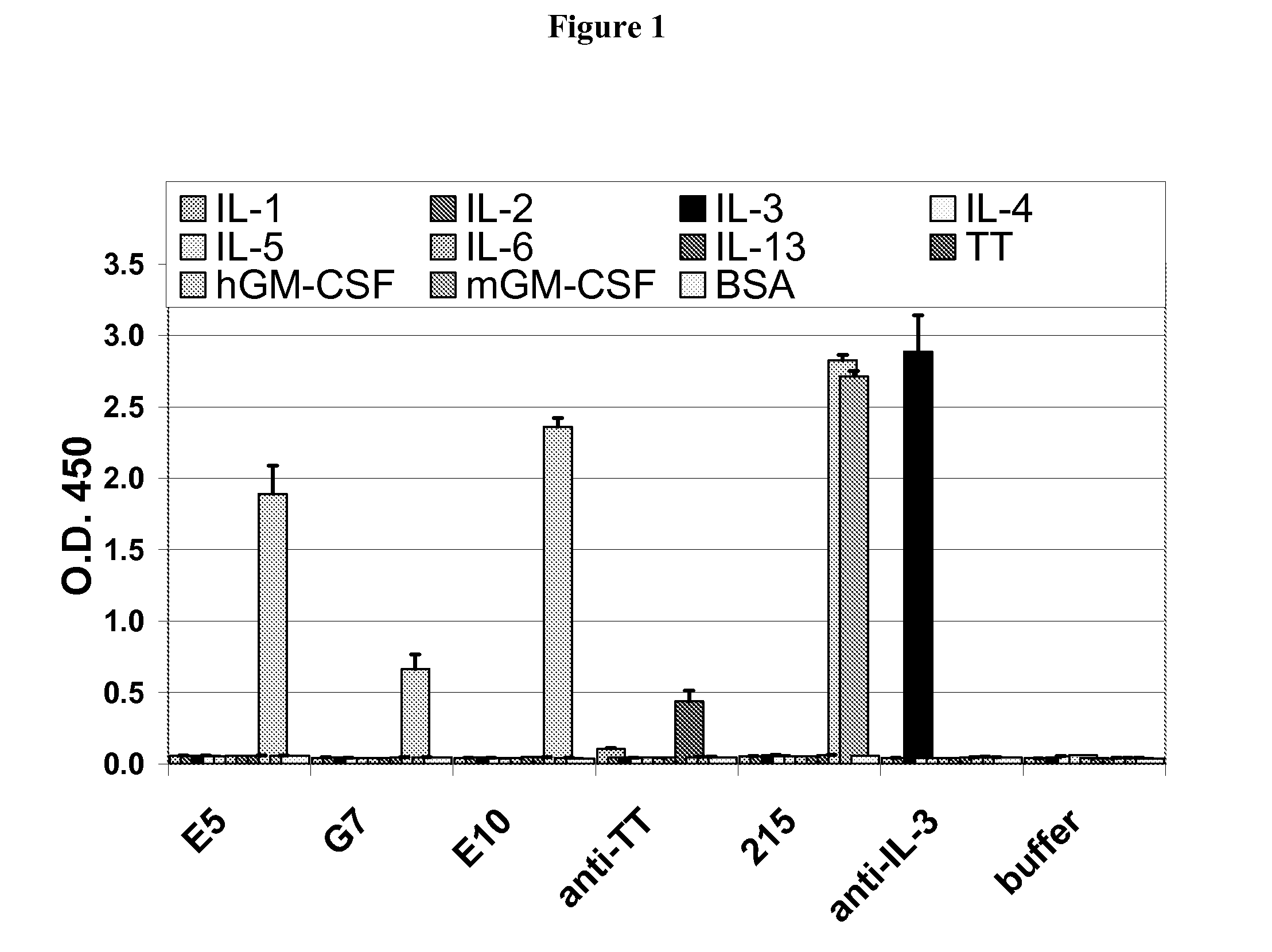
Antibodies to GM-CSF
Owner:EISAI INC
Novel heterocycles
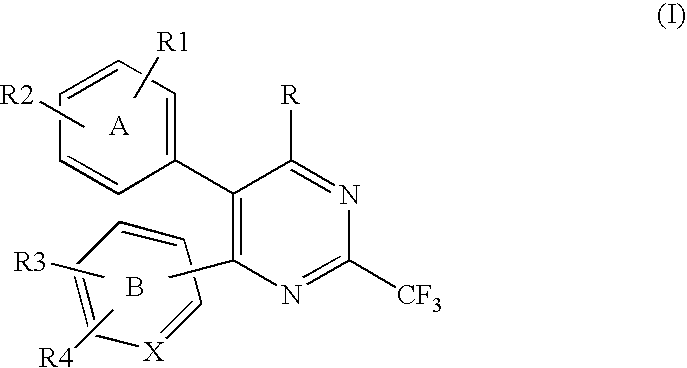
InactiveUS20070167413A1Useful in treatmentOrganic active ingredientsBiocideRESPIRATORY DISTRESS SYNDROME ADULTContact dermatitis
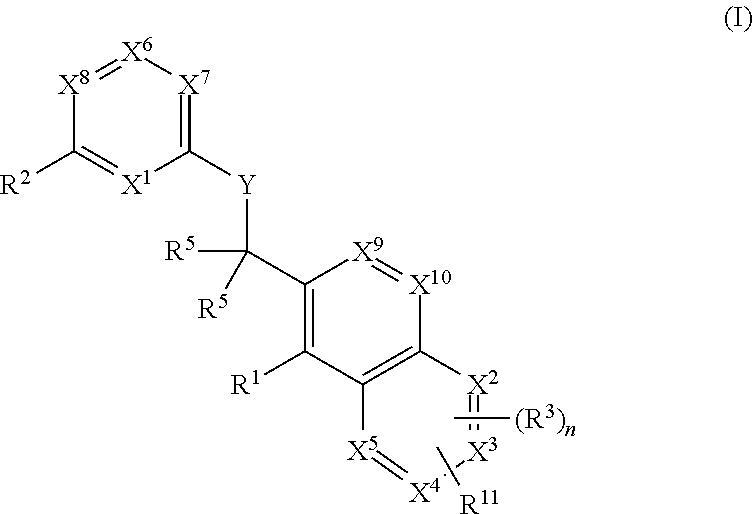
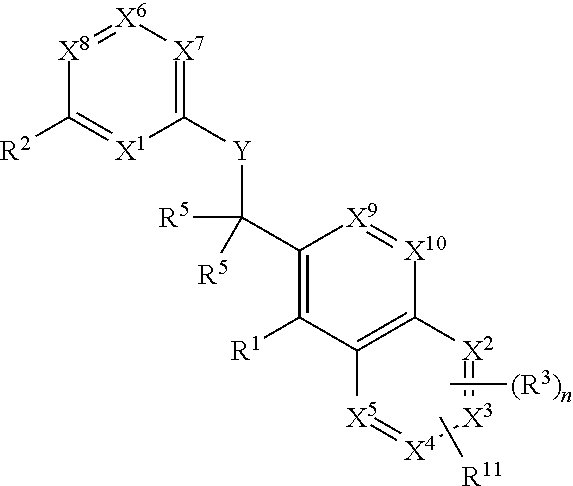
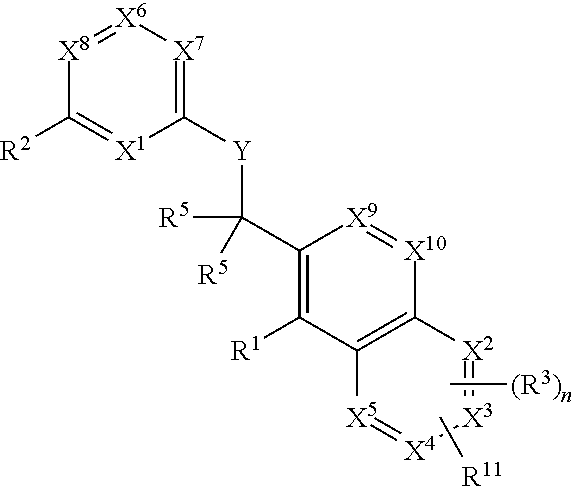
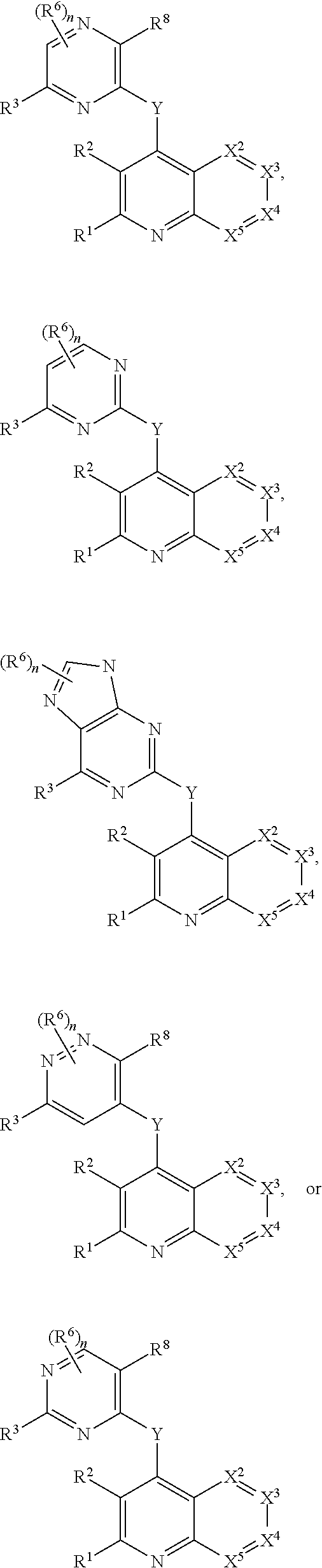
The present invention relates to novel heterocyclic compounds of the general formula (I), their derivatives, analogs, tautomeric forms, stereoisomers, polymorphs, hydrates, solvates, pharmaceutically acceptable salts and compositions, metabolites and prodrugs thereof. The present invention more particularly provides novel hetereocycles of the general formula (I). Also included is a method of treatment of immunological diseases, inflammation, pain disorder, rheumatoid arthritis; osteoporosis; multiple myeloma; uveititis; acute and chronic myelogenous leukemia; ischemic heart disease; atherosclerosis; cancer; ischemic-induced cell damage; pancreatic beta cell destruction; osteoarthritis; rheumatoid spondylitis; gouty arthritis; inflammatory bowel disease; adult respiratory distress syndrome (ARDS); psoriasis; Crohn's disease; allergic rhinitis; ulcerative colitis; anaphylaxis; contact dermatitis; muscle degeneration; cachexia; asthma; bone resorption diseases; ischemia reperfusion injury; brain trauma; multiple sclerosis; sepsis; septic shock; toxic shock syndrome; fever, and myalgias due to infection in a mammal comprising administering an effective amount of a compound of formula (I) as described above.
Owner:ORCHID RES LAB +1
Polyethylene glycol modified protein separating and purifying method
The invention relates to a polyethylene glycol modified protein separating and purifying method. The invention discloses a novel cation chromatography media MacroCap Sp which can induct protein surface charge distribution relation sensitively. The more positive charge on a protein surface, the stronger combination capability of the protein with the MacroCap Sp, and the higher ion gradient is needed for elution. By utilizing the above property, polyethylene glycol modified protein molecule can be effectively separated. The method comprises the following steps: (1) after modification of object protein by polyethylene glycol with active reaction group, adjusting pH value of a reaction solution, wherein the pH value should be lower than isoelectric point of the polyethylene glycol modified protein; (2) loading the reaction solution to balanced MacroCap Sp cation exchange columns; (3) adding a sodium salt solution to carry out ion gradient elution based on a balanced buffer solution, and collecting object peak. The method of the present invention is suitable for effective separation of recombinant human granulocyte stimulating factor modified by the polyethylene glycol and exenatide.
Owner:HANGZHOU JIUYUAN GENE ENG
Adjuvant combination formulations
InactiveUS7611721B1Improve abilitiesSsRNA viruses negative-senseAntibacterial agentsWhite blood cellVertebrate Animals
The use of 3-O-deacylated monophosphoryl lipid A or monophosphoryl lipid A and derivatives and analogs thereof, in combination with a cytokine or lymphokine such as granulocyte macrophage colony stimulating factor or interleukin-12 is useful as an adjuvant combination in an antigenic composition to enhance the immune response in a vertebrate host to a selected antigen.
Owner:ZOETIS SERVICE LLC
Cyanine compound, composition containing same and application in cell detection thereof
ActiveCN102115456AAccurate countEliminate distractionsOrganic chemistryMethine/polymethine dyesOrganic chemistryNonionic surfactant
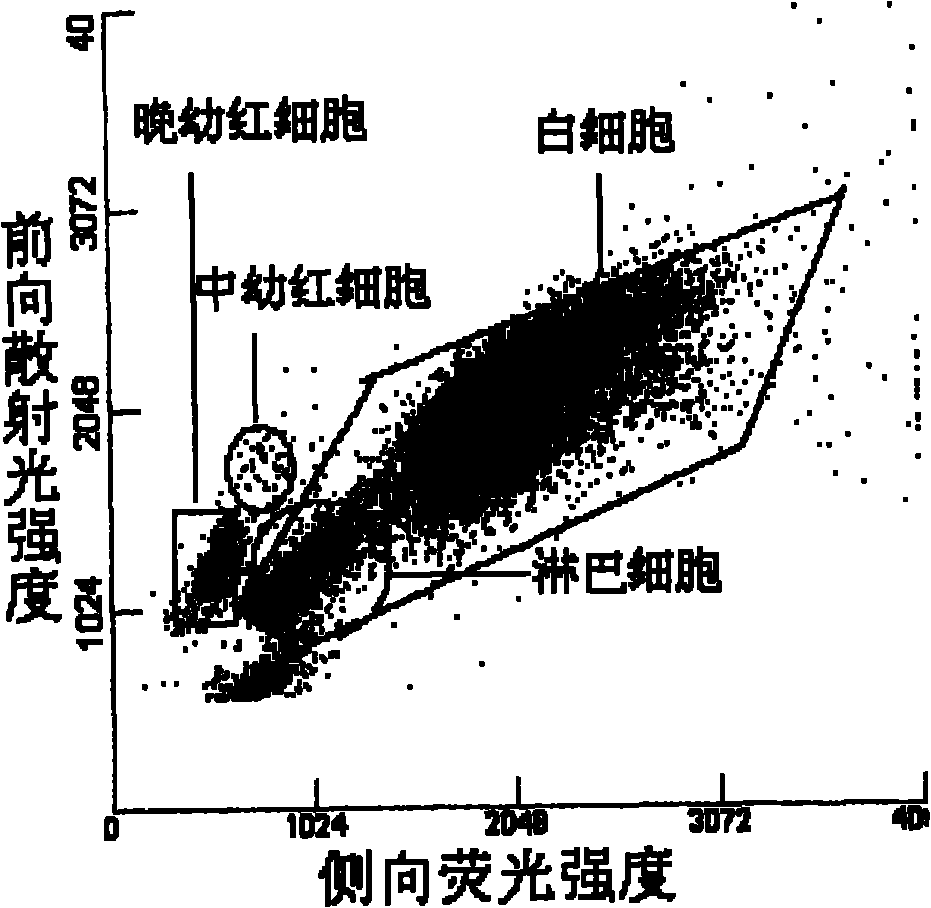
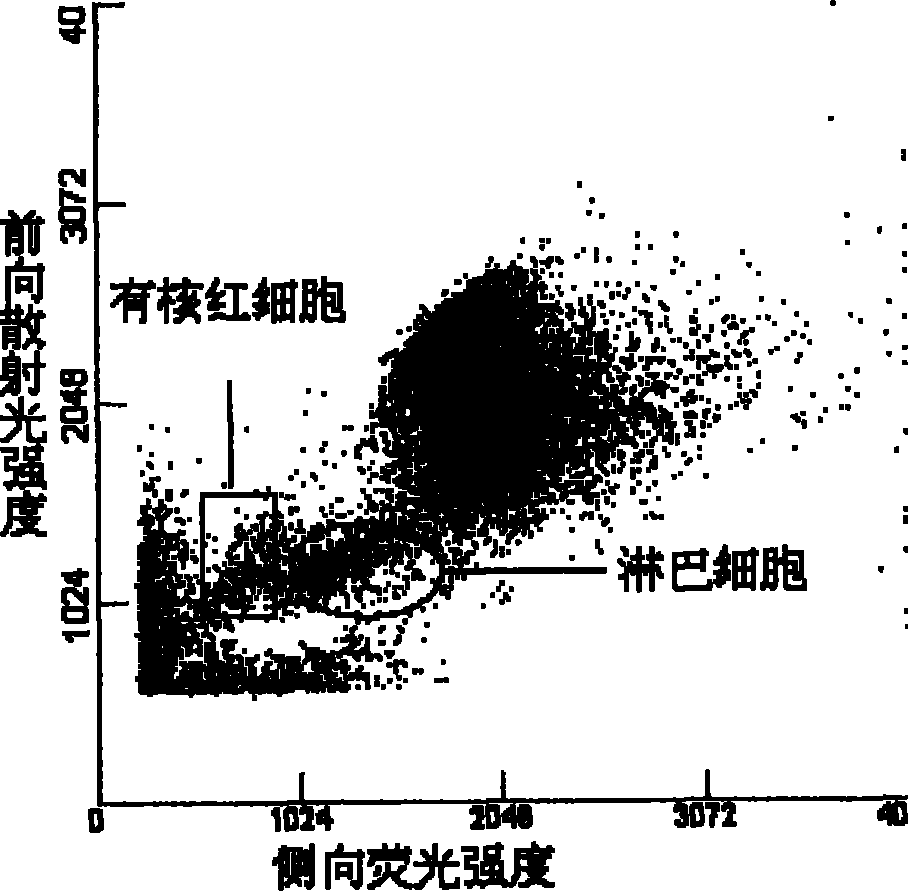
On one hand, the invention provides a compound with a structure of general formula I or a conjugate thereof, wherein each group is as defined in the description. On the other hand, the invention provides a composition comprising (i) the compound of formula I or the conjugate thereof, and (ii) at least one surfactant selected from cationic surfactants and nonionic surfactants. The invention also provides a preparation method of the composition and a kit containing the composition. The invention still provides a method for the simultaneous identification of nucleated erythrocyte, basophilic granulocyte and lymphocyte and the classification by using the composition disclosed in the invention.
Owner:SHENZHEN MINDRAY BIO MEDICAL ELECTRONICS CO LTD
Methods and compositions for diagnosis and prognosis of renal injury and renal failure
The present invention relates to methods and compositions for monitoring, diagnosis, prognosis, and determination of treatment regimens in sepsis patients. In particular, the invention relates to using assays that detect one or more biomarkers selected from the group consisting of Insulin-like growth factor-binding protein 7, Beta-2-glycoprotein 1, Metalloproteinase inhibitor 2, Alpha-1 Antitrypsin, Leukocyte elastase, Serum Amyloid P Component, C-X-C motif chemokine 6, Immunoglobulin A, Immunoglobulin G subclass I, C-C motif chemokine 24, Neutrophil collagenase, Cathepsin D, C-X-C motif chemokine 13, Involucrin, Interleukin-6 receptor subunit beta, Hepatocyte Growth Factor, CXCL-1, -2, -3, Immunoglobulin G subclass II, Metalloproteinase inhibitor 4, C-C motif chemokine 18, Matrilysin, C-X-C motif chemokine 11, and Antileukoproteinase as diagnostic and prognostic biomarker assays of renal injury in the sepsis patient.
Owner:ASTUTE MEDICAL
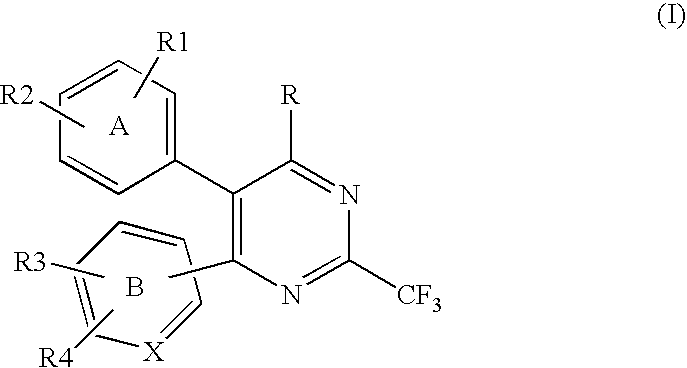
Heterocyclic compounds and their uses
InactiveUS20130079342A1Organic active ingredientsSenses disorderB-cell acute lymphoblastic leukaemiaAutoimmune disease
Substituted bicyclic heteroaryls of the following formulae and compositions containing them, for the treatment of general inflammation, arthritis, rheumatic diseases, osteoarthritis, inflammatory bowel disorders, inflammatory eye disorders, inflammatory or unstable bladder disorders, psoriasis, skin complaints with inflammatory components, chronic inflammatory conditions, including but not restricted to autoimmune diseases such as systemic lupus erythematosis (SLE), myestenia gravis, rheumatoid arthritis, acute disseminated encephalomyelitis, idiopathic thrombocytopenic purpura, multiples sclerosis, Sjoegren's syndrome and autoimmune hemolytic anemia, allergic conditions including all forms of hypersensitivity, The present invention also enables methods for treating cancers that are mediated, dependent on or associated with p110 activity, including but not restricted to leukemias, such as Acute Myeloid leukaemia (AML) Myelo-dysplastic syndrome (MDS) myelo-proliferative diseases (MPD) Chronic Myeloid Leukemia (CML) T-cell Acute Lymphoblastic leukaemia (T-ALL) B-cell Acute Lymphoblastic leukaemia ALL) Non Hodgkins Lymphoma (NHL) B-cell lymphoma and solid tumors, such as breast cancer.
Owner:AMGEN INC
Anti-Siglec-8 antibodies and methods of use thereof
ActiveUS9546215B2High activityVertebrate antigen ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsMast cellEosinophil
The invention provides humanized anti-Siglec-8 antibodies and their use in treating and preventing eosinophil-mediated disorders and / or mast cell-mediated disorders, as well as compositions and kits comprising the humanized anti-Siglec-8 antibodies.
Owner:ALLAKOS
Production method for recombinant human granulocyte colony-stimulating factor
ActiveCN103233053ALarge amount of processingReduce difficultyMicroorganism based processesPeptide preparation methodsEscherichia coliYeast
The invention discloses production method for a recombinant human granulocyte colony-stimulating factor. According to the invention, an engineered strain of an escherichia coli expressed recombinant human granulocyte colony-stimulating factor is cultured to obtain an inclusion body, the engineered strain of the escherichia coli expressed recombinant human granulocyte colony-stimulating factor is pKG931 / HB101, a culture method comprises multiplication culture and culture in a fermentation cylinder, an antibiotic-free medium containing yeast and peptone is employed in both culture, a high pressure homogenizer is used for breaking of bacteria, and denaturation, renaturation and chromatography are carried out so as to obtain a high purity G-CSF stock solution. The production method provided by the invention has the advantages of a short production period, high production efficiency, a great production scale, especial suitability for industrial production and reduction in production cost.
Owner:BEIJING FOUR RINGS BIOPHARM
Nucleic acid molecules encoding cytotoxic conjugates that contain a chemokine receptor targeting agent
InactiveUS7192736B2Enhance and aid in survivalPrevent proliferationBacteriaPeptide/protein ingredientsCell biologyWhite blood cell
Nucleic acid moleucles that encode conjugates containing as a ligand a chemokine receptor targeting agent, such as a chemokine, and a targeted agent, such as a toxin are provided. These conjugates are used to treat inflammatory responses associated with activation, proliferation and migration of immune effector cells, including leukocyte cell types, neutrophils, macrophages, and eosinophils.
Owner:SAMSUNG ENGINEERING CO LTD +1
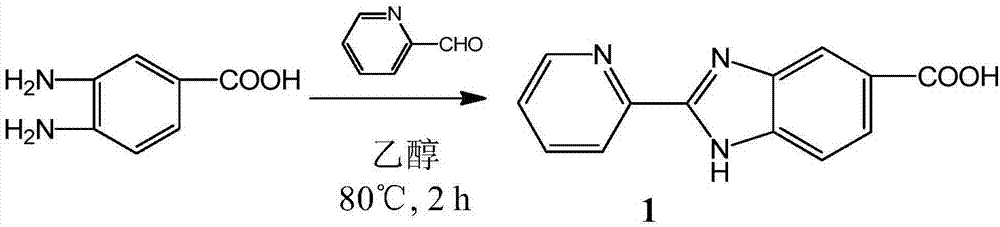
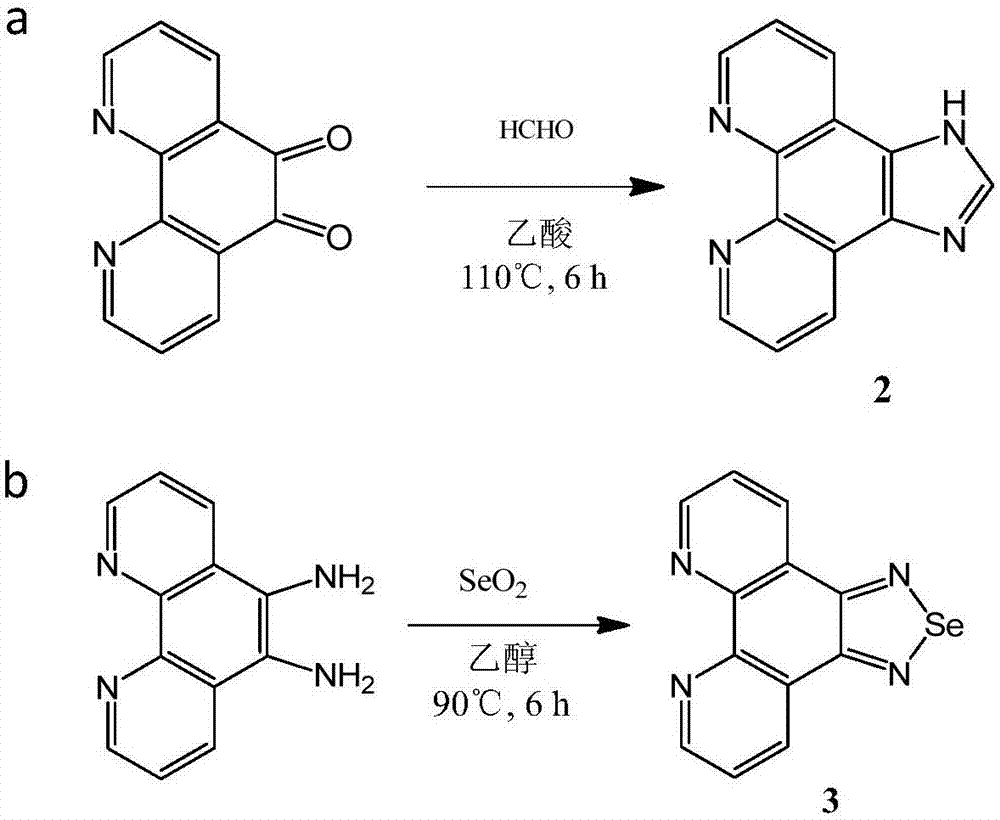
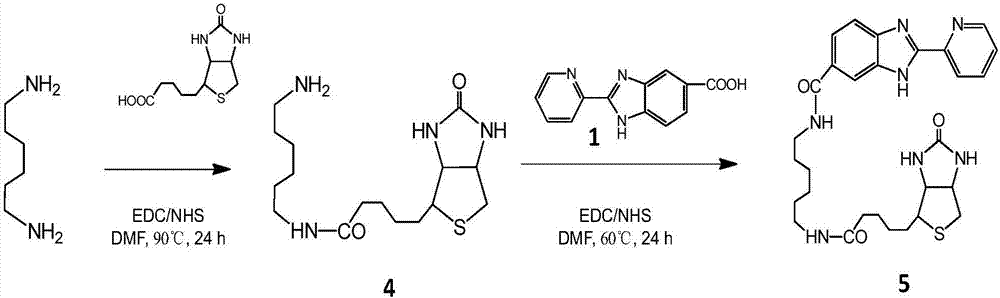
Tumor targeting metal complex, synthetic method and application
ActiveCN106866743AImprove utilizationSmall toxicityRuthenium organic compoundsOrganic active ingredientsAbnormal tissue growthApoptosis
The invention discloses a tumor targeting metal complex, a synthetic method and application. The synthetic method comprises the steps of connecting a ligand with a substituent group and a targeting molecule, complexing the ligand and metal ions, and synthesizing to obtain the tumor targeting metal complex, wherein the ligand is one of a phenanthroline derivative, a dipyridyl derivative, a benzimidazole derivative, a phenyl-pyridine derivative and a thiophene-pyridine derivative; the targeting molecule is one of biotin, folic acid, integrin, transferrin, cell-penetrating peptide, octa-poly-L-arginine, MUC-1 attached membrane protein, galactosamine, neovascular targeting peptide and a granulocyte-macrophage colony-stimulating factor; the metal ions are ruthenium, osmium, rhodium or iridium ions. The complex obtained by the method provided by the invention can be selectively absorbed by tumor cells, and induces apoptosis of the tumor cells. In a nude mouse tumor-bearing model, the tumor targeting metal complex can image and treat tumors, and the toxicity of organs and tissues is reduced.
Owner:JINAN UNIVERSITY
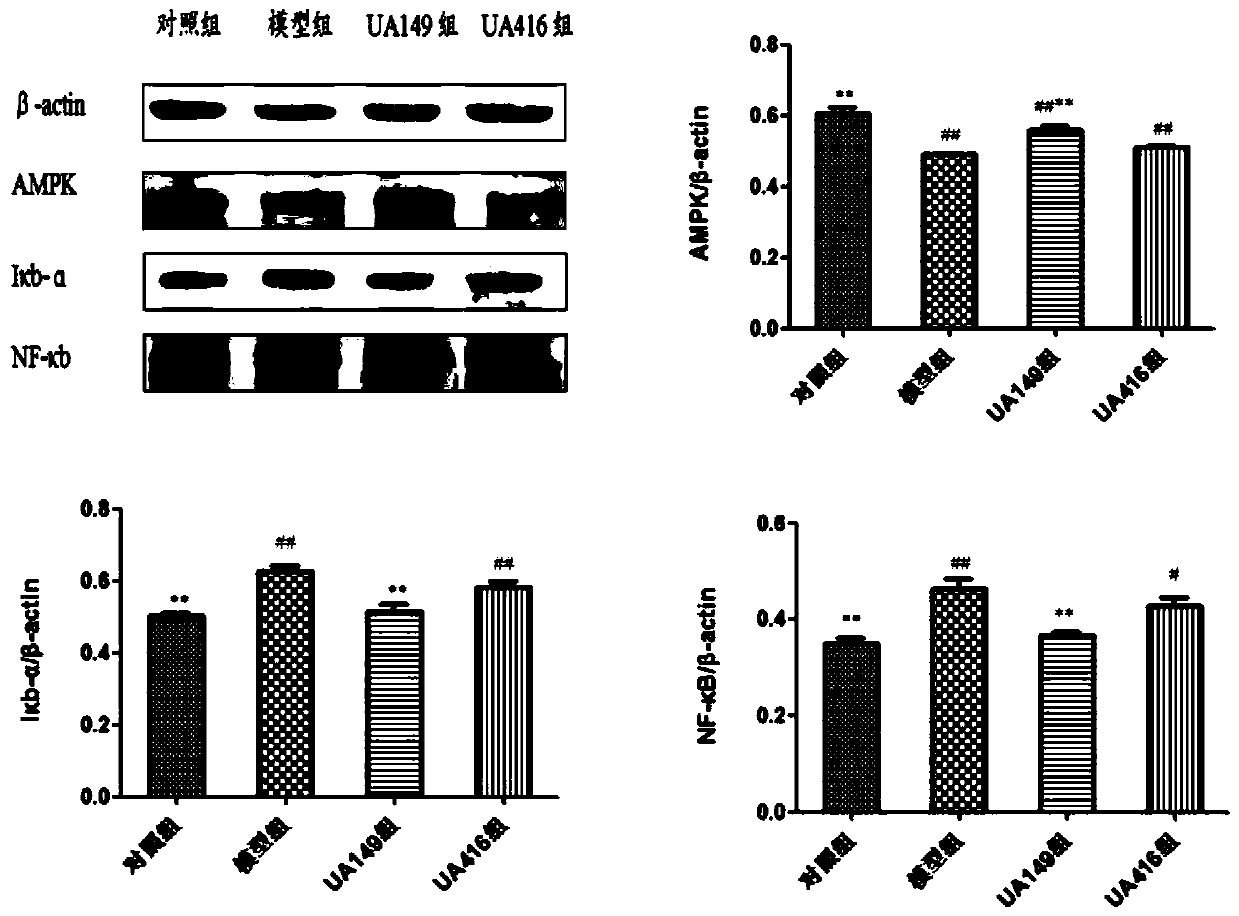
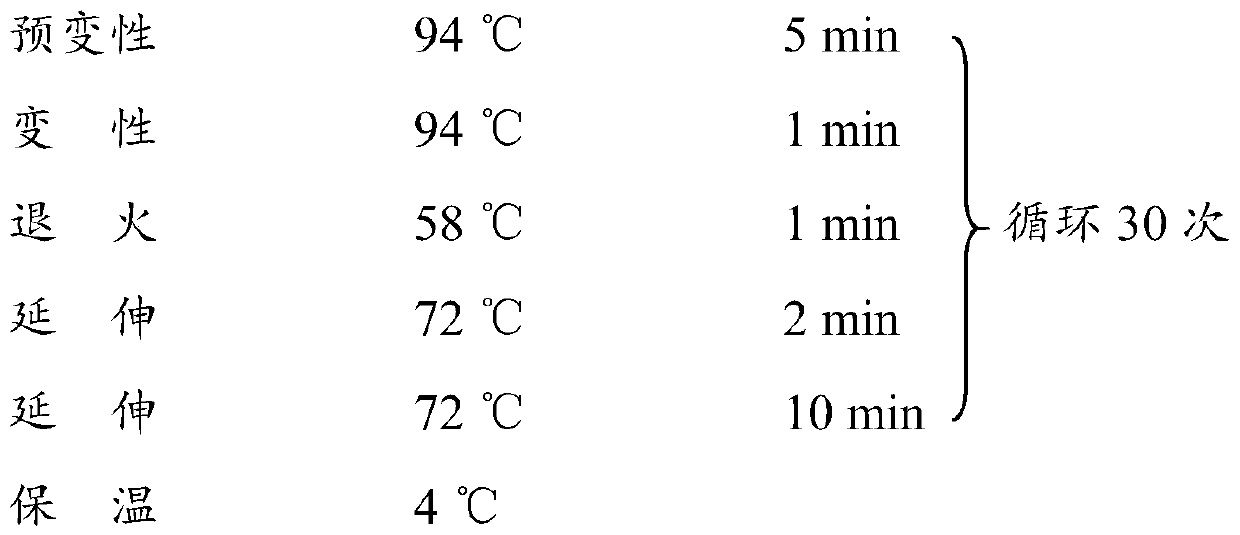
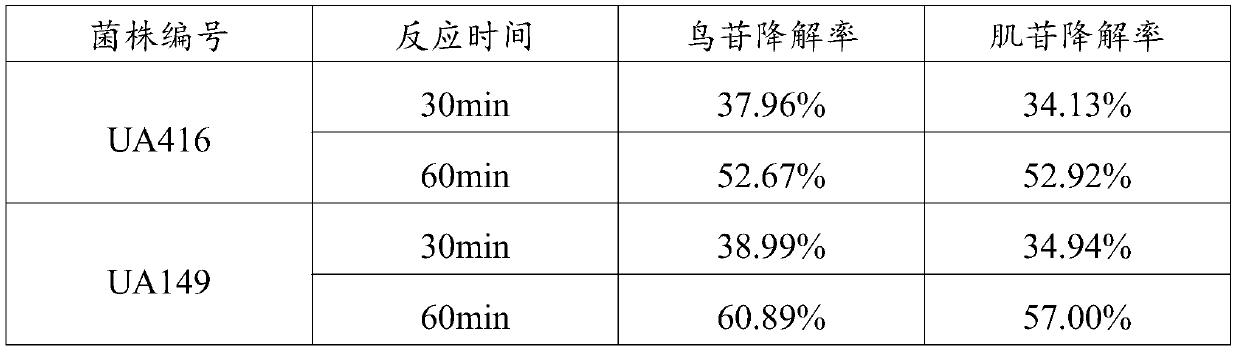
L.plantarum UA149 strain and application thereof
ActiveCN110055199AReduce synthesisLower uric acid levelsBacteriaMicroorganism based processesInflammatory factorsResearch Object
The invention discloses an L.plantarum UA149 strain and an application thereof, and belongs to the field of functional food microorganisms. The L.plantarum UA149 strain is deposited in China Typical Culture Collection Center on November 29, 2018 with the preservation number of CCTCC No: M2018842. The strain can be apply to preparing products with a uric acid reducing function or a gout resisting function. Lactic acid bacteria separated and identified from the surface of fleshy plant leaves are taken as research objects, and a new strain of lactic acid bacteria is screened through a large number of experiments. Hyperuricemia model rats are established by potassium oxazinate combined with fructose water, continuous intragastric administration of the lactobacillus plantarum UA149 strain for 14 days can significantly reduce the level of uric acid; and during gout attack, the release of inflammatory factors thromboxane and leukotriene mediated by neutrophils is reduced, the influx of neutrophils into joints is avoided, and the symptoms of redness, swelling, pain, heat and the like are reduced.
Owner:JILIN MINGZHIYUAN BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com