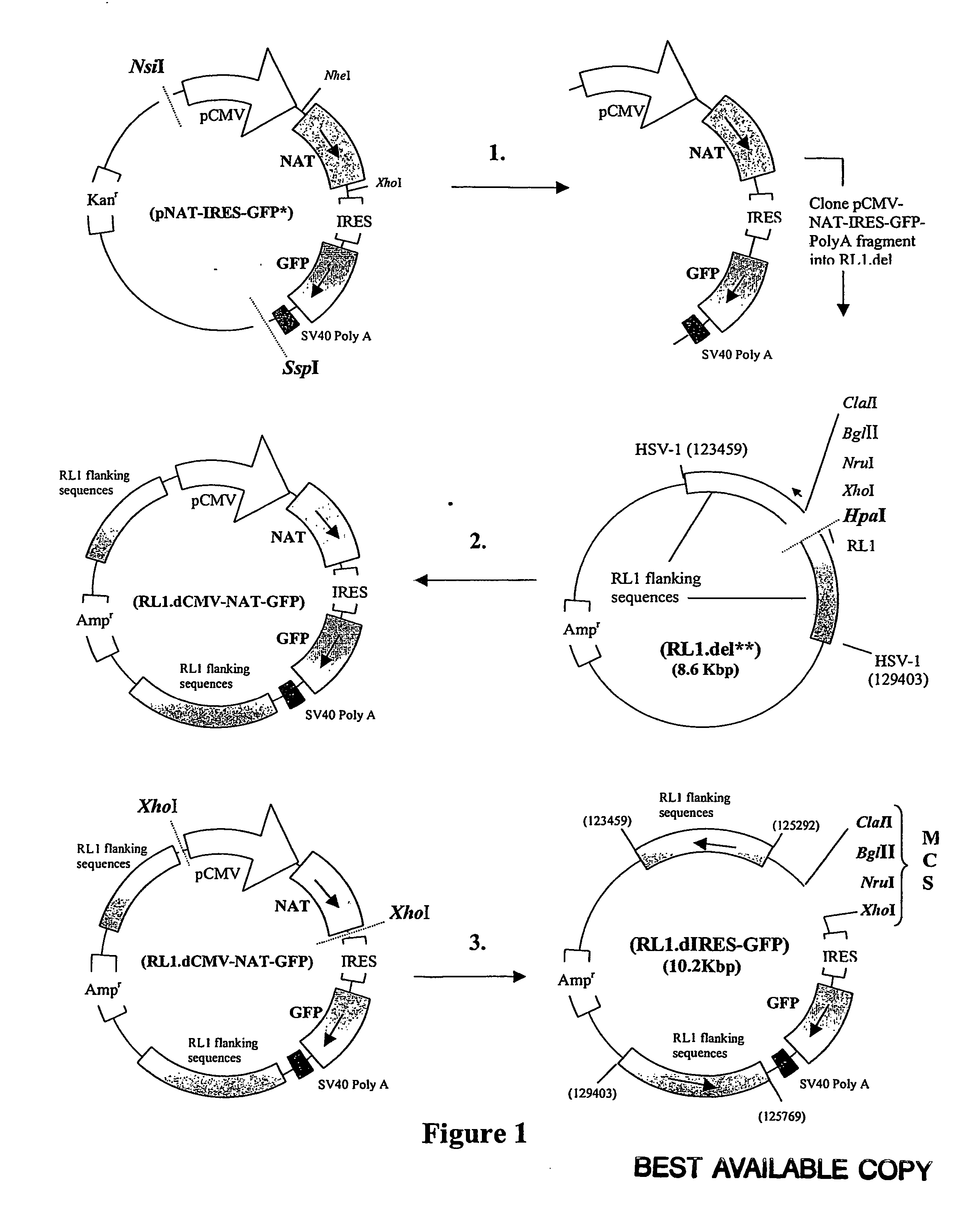
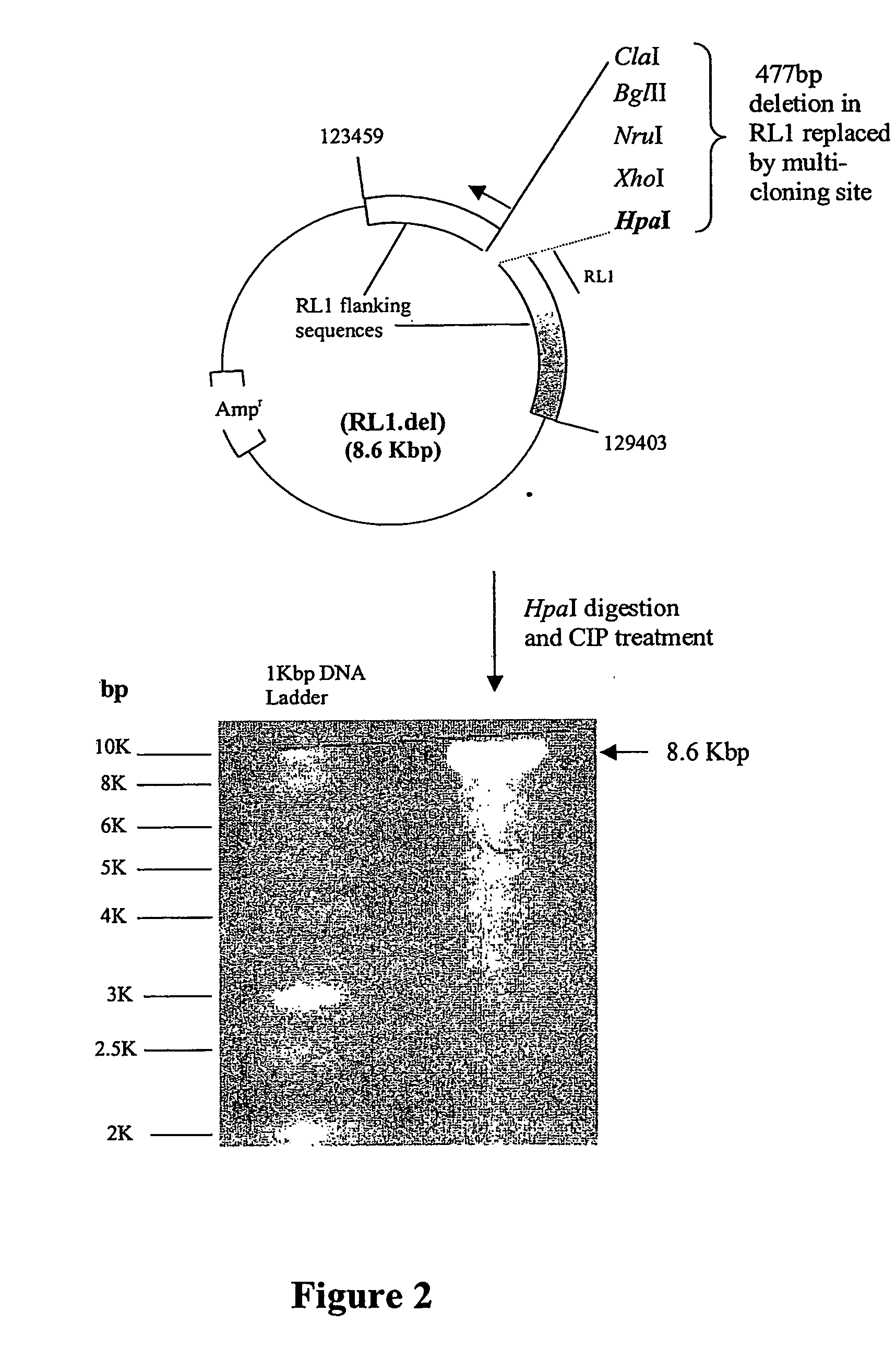
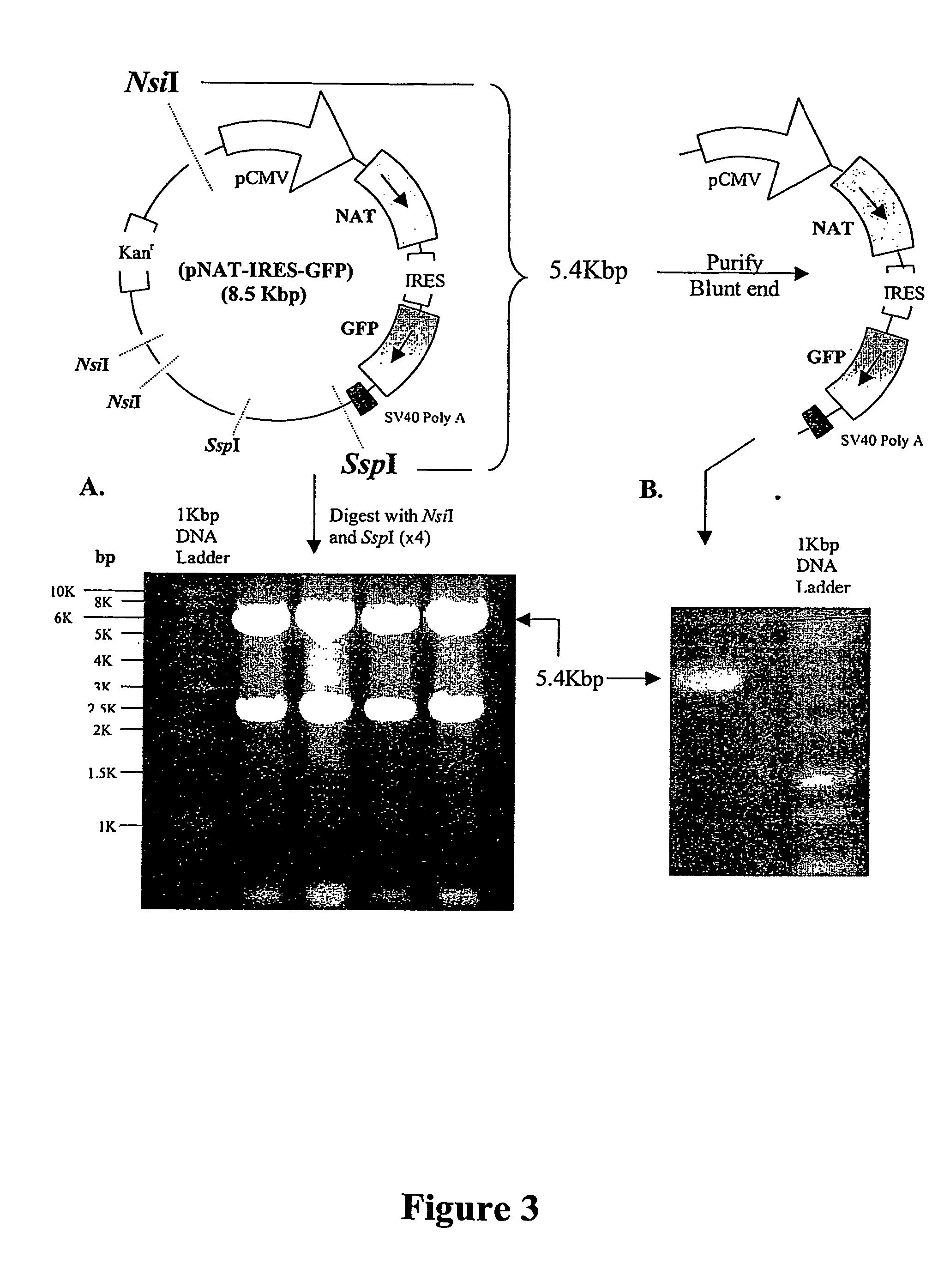
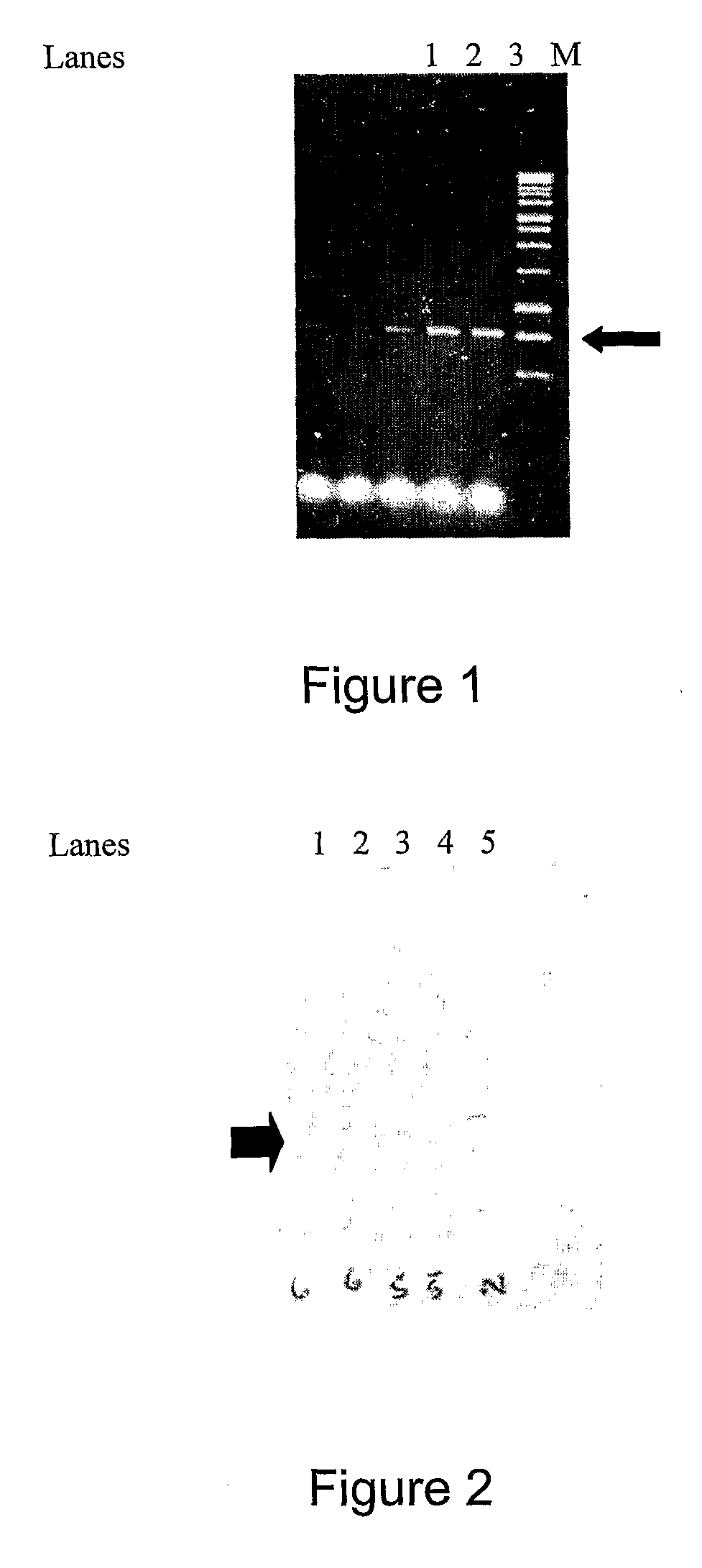
Patents
Literature
456 results about "Herpes simplex virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An infection caused by the virus HSV.
Use of laggera plant abstract in inhibiting herpes simplex virus and hepatitis B virus
InactiveCN1989989AReduced expression functionDigestive systemPharmaceutical delivery mechanismDiseaseCaffeoylquinic acid
The invention involves novel drug use of six-rowed chrysanthemum plant extracts which is used to treating herpes simplex virus (type 1 and / or type 2) and various disease caused by hepatitis B virus infection. The six-rowed chrysanthemum plant extracts is prepared by six-rowed chrysanthemum plant fresh or dry goods through the refining of alcohol-water extraction, column chromatography, alcohol solvent elution, the amount of caffeoyl guinic acid chemical compound is below 30%. The six-rowed chrysanthemum plant extracts prepared in the invention has significant function of inhibiting herpes simplex virus with type 1 (HSV-1), herpes simplex virus type 2 (HSV-2) and hepatitis B virus (HBV) replication, and can reduce effectiveness of HBV e antigen (HBeAg) in the HepG 2.2.15 cell lines, it can be used for treatment various disease caused by said correlate virus infection.
Owner:ZHEJIANG HISUN PHARMA CO LTD
Complementing cell lines
InactiveUS6974695B2Low efficiencyEfficient disseminationBiocideGenetic material ingredientsHeterologousVaccination
A packaging cell line capable of complementing recombinant adenoviruses based on serotypes from subgroup B, preferably adenovirus type 35. The cell line is preferably derived from primary, diploid human cells (e.g., primary human retinoblasts, primary human embryonic kidney cells and primary human amniocytes) which are transformed by adenovirus E1 sequences either operatively linked on one DNA molecule or located on two separate DNA molecules, the sequences being operatively linked to regulatory sequences enabling transcription and translation of encoded proteins. Also disclosed is a cell line derived from PER.C6 (ECACC deposit number 96022940), which cell expresses functional Ad35 E1B sequences. The Ad35-E1B sequences are driven by the E1B promoter or a heterologous promoter and terminated by a heterologous poly-adenylation signal. The new cell lines are useful for producing recombinant adenoviruses designed for gene therapy and vaccination. The cell line can also be used for producing human recombinant therapeutic proteins such as human growth factors and human antibodies. In addition, the cell lines are useful for producing human viruses other than adenovirus such as influenza virus, herpes simplex virus, rotavirus, measles virus.
Owner:JANSSEN VACCINES & PREVENTION BV
Avirulent oncolytic herpes simplex virus strains engineered to counter the innate host response
ActiveUS20060039894A1Preserving abilityContinual and sustained deliveryBiocideGenetic material ingredientsAutoimmune conditionAutoimmune disease
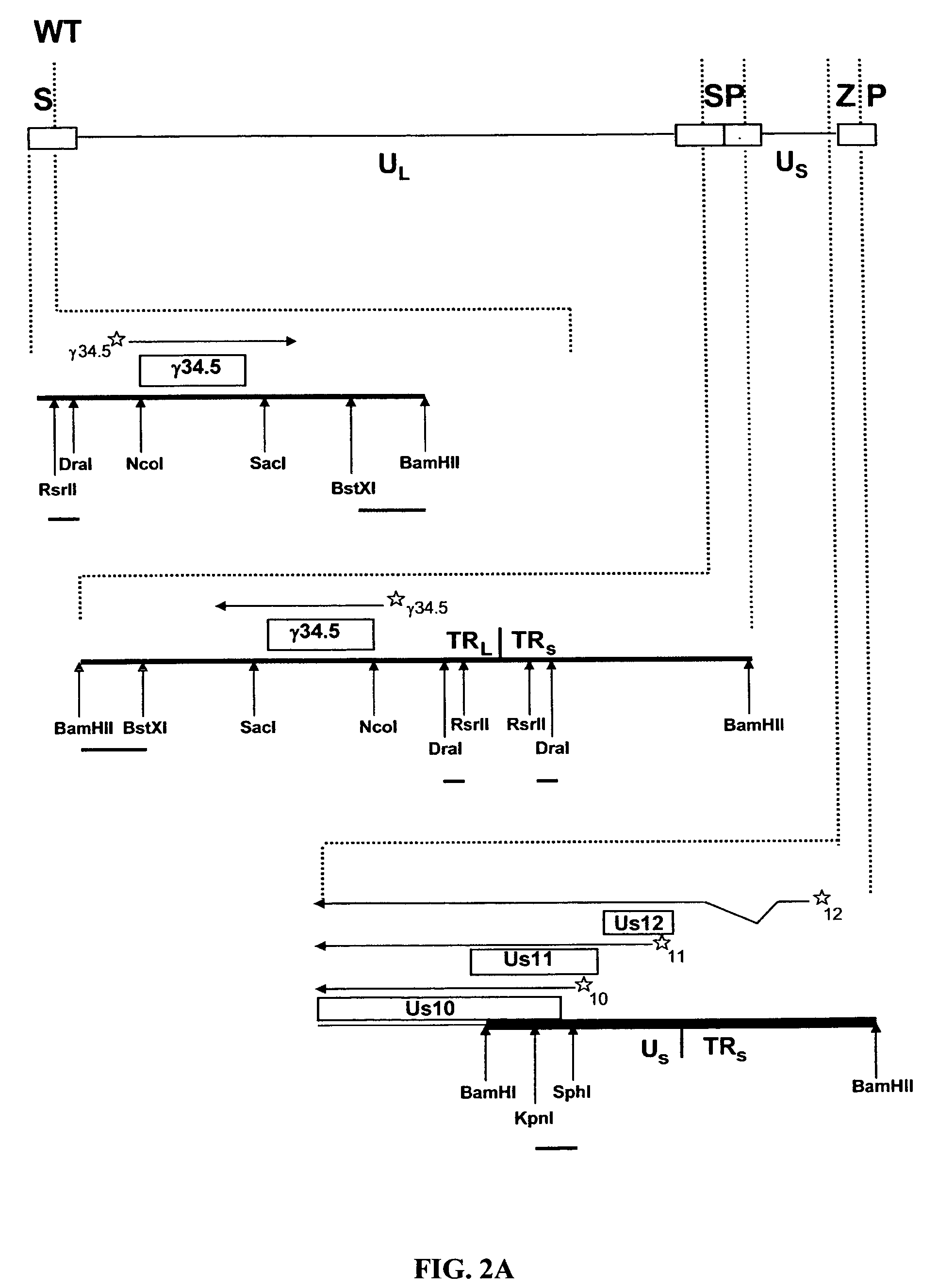
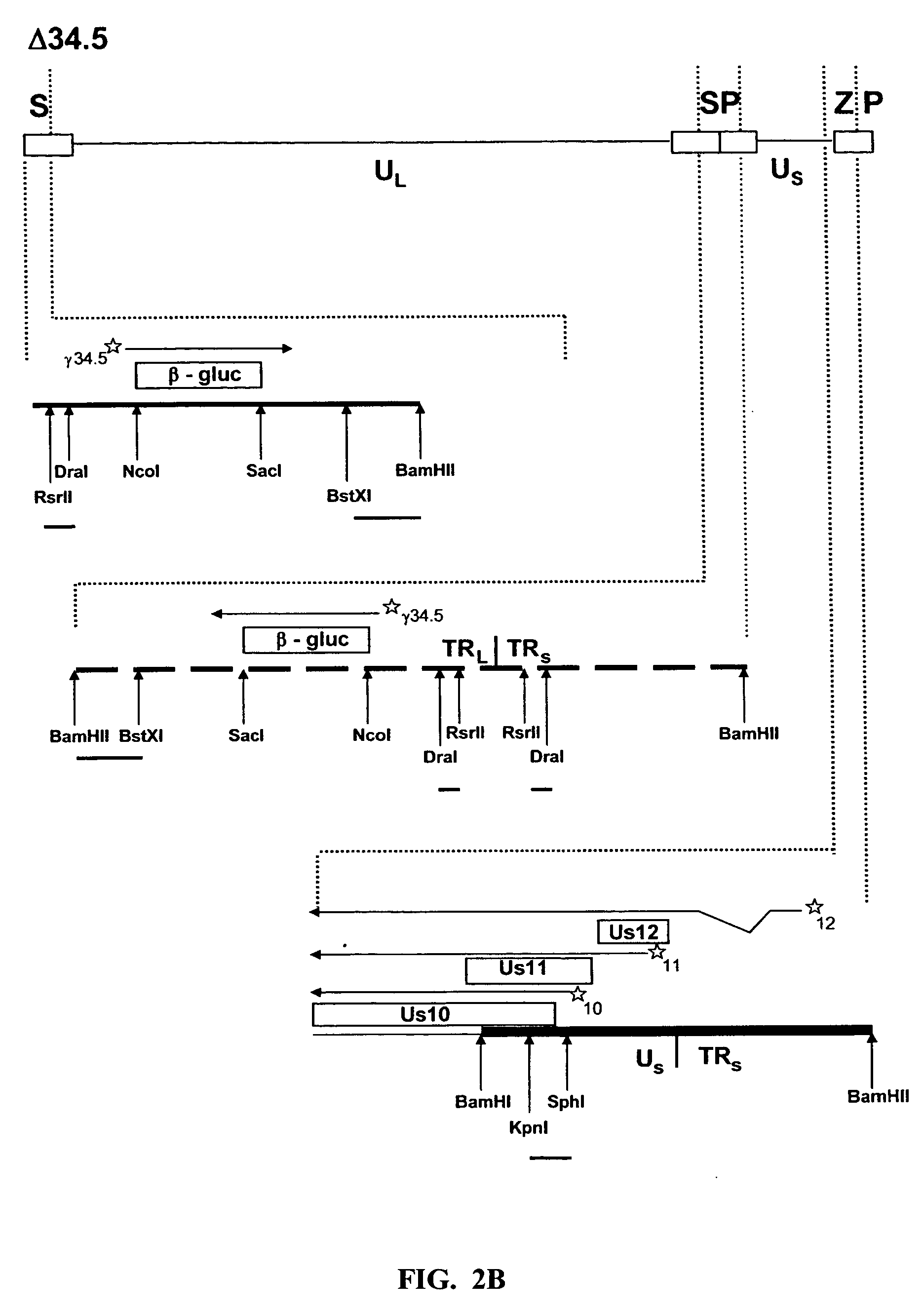
The present invention relates to an avirulent, oncolytic herpes simplex virus modified from a wild-type herpes simplex virus so that both γ134.5 genes of the virus have been deleted and each replaced with an interferon-resistance gene that is expressed as an immediate-early gene. The present invention also relates to a pharmaceutical composition that includes the modified herpes simplex virus of the present invention and a pharmaceutically acceptable vehicle for in situ administration to tumor cells. Also provided in the present invention are methods for killing tumor cells in a subject and for immunizing a subject against an infectious disease, cancer, or an autoimmune disease that involve administering to a subject the modified avirulent, oncolytic herpes simplex virus of the present invention.
Owner:NEW YORK UNIV
Treatment using herpes simplex virus
ActiveUS7897146B2Successful tumour treatmentConvenient treatmentBiocideOrganic active ingredientsTreatment useWilms' tumor
The use of Herpes Simplex Virus (HSV) in the treatment of tumour by extratumoural administration of HSV, and the use of HSV in treatment of tumour by combination therapy with a pharmaceutical wherein the HSV and / or pharmaceutical is administered at an extratumoural location, is disclosed.
Owner:VIRTTU BIOLOGICS
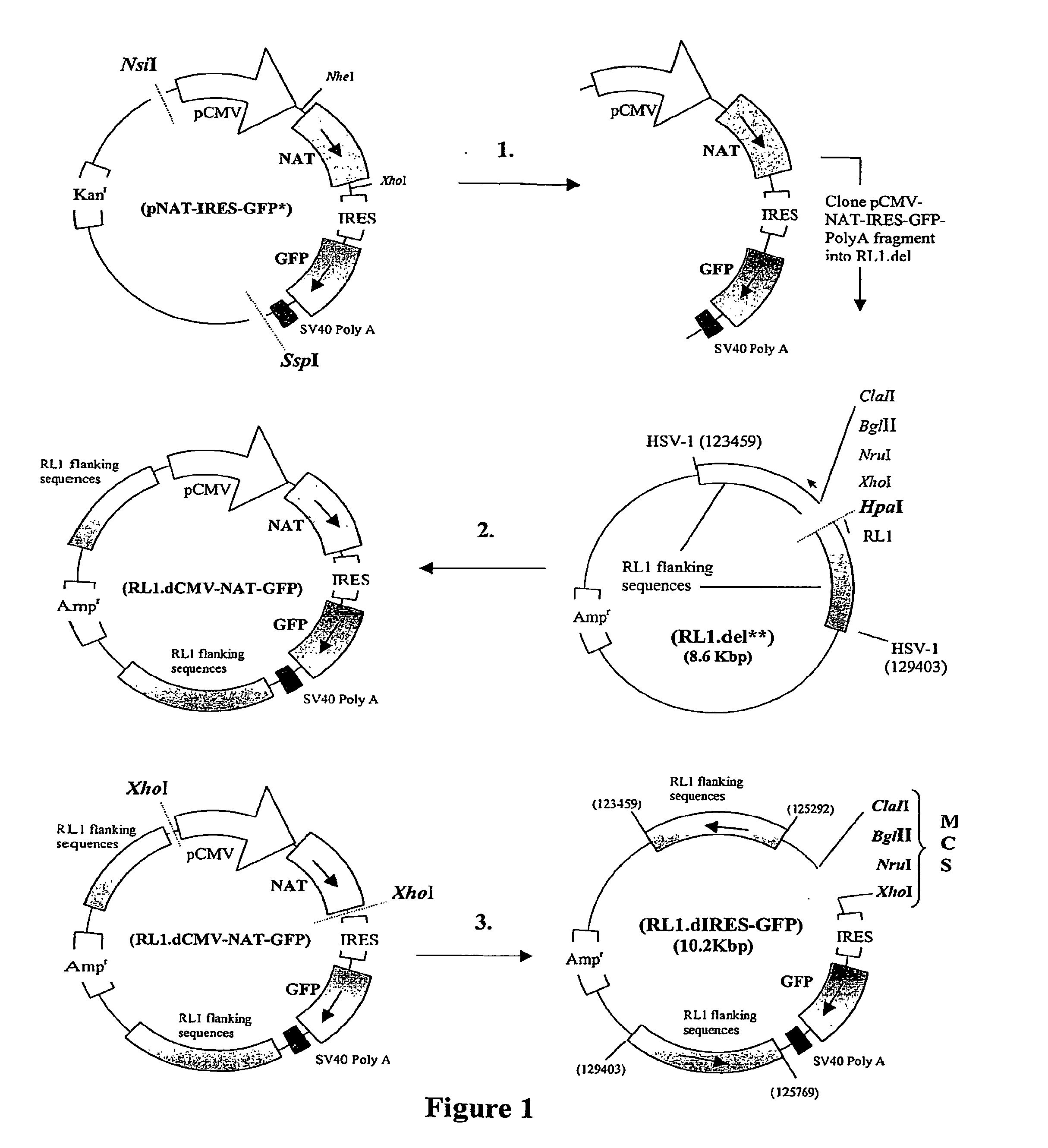
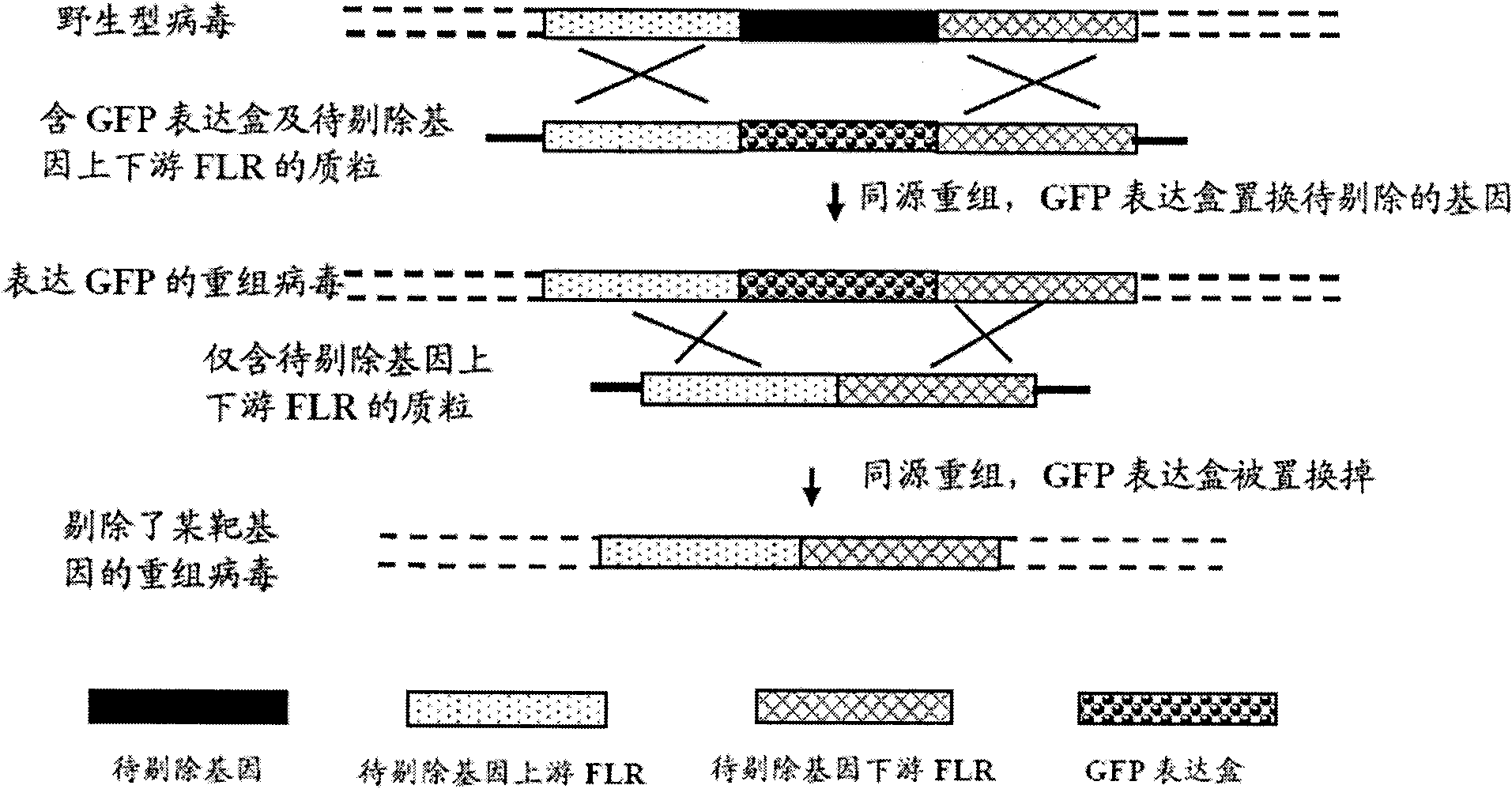
Vectors, mutant viruses and methods for generating mutant viruses
InactiveUS20070110720A1Easy to identifyEnhanced cytotoxic potentialBiocideGenetic material ingredientsHeterologousBinding site
A nucleic acid vector comprising first and second nucleotide sequences corresponding to nucleotide sequences flanking an insertion site in the genome of a selected herpes simplex virus strain; and a cassette located between said first and second nucleotide sequences comprising nucleic acid encoding: (a) one or a plurality of insertion sites and / or a nucleotide sequence of interest; and (b) a ribosome binding site or a regulatory nucleotide sequence; and (c) a marker is disclosed. Herpes simplex viruses generated using said vector, methods for their generation and herpes simplex viruses having a genome comprising heterologous nucleic acid are also disclosed.
Owner:CRUSADE LAB
Topical use of probiotic Bacillus spores to prevent or control microbial infections
Compositions including an isolated Bacillus species, spores or an extracellular product of B. coagulans, suitable for topical application, for inhibiting growth of yeast, fungus, bacteria or Herpes simplex virus are disclosed. Methods of inhibiting growth of yeast, fungus, bacteria or Herpes simplex virus by topical application of compositions that include an isolated Bacillus species, spores or an extracellular product of a B. coagulans strain are disclosed.
Owner:GANEDEN BIOTECH
Topical Use of Probiotic Bacillus Spores to Prevent or Control Microbial Infections
Compositions including an isolated Bacillus species, spores or an extracellular product of B. coagulans, suitable for topical application, for inhibiting growth of yeast, fungus, bacteria or Herpes simplex virus are disclosed. Methods of inhibiting growth of yeast, fungus, bacteria or Herpes simplex virus by topical application of compositions that include an isolated Bacillus species, spores or an extracellular product of a B. coagulans strain are disclosed.
Owner:GANEDEN BIOTECH
Herpes simplex viruses and methods of viral replication
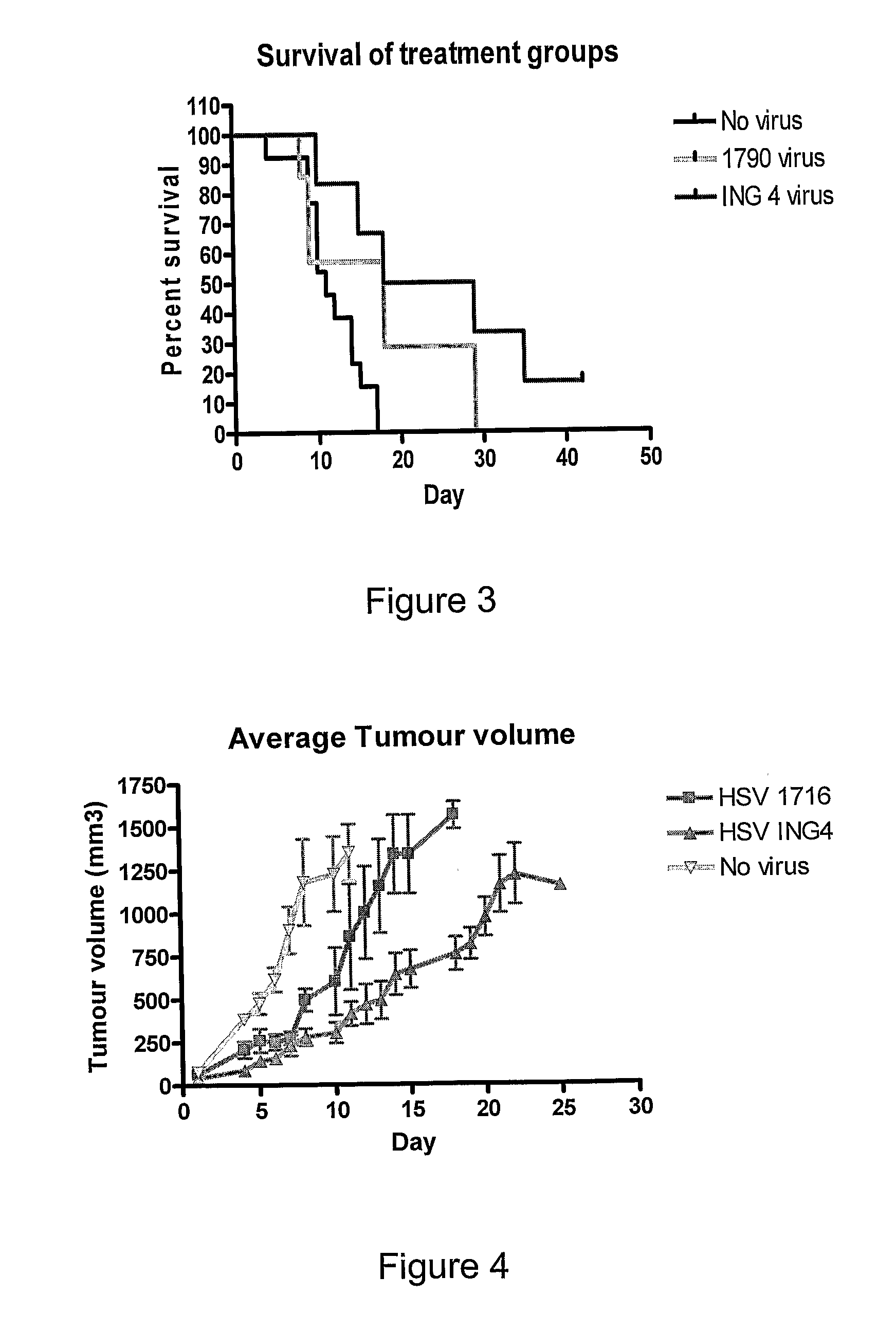
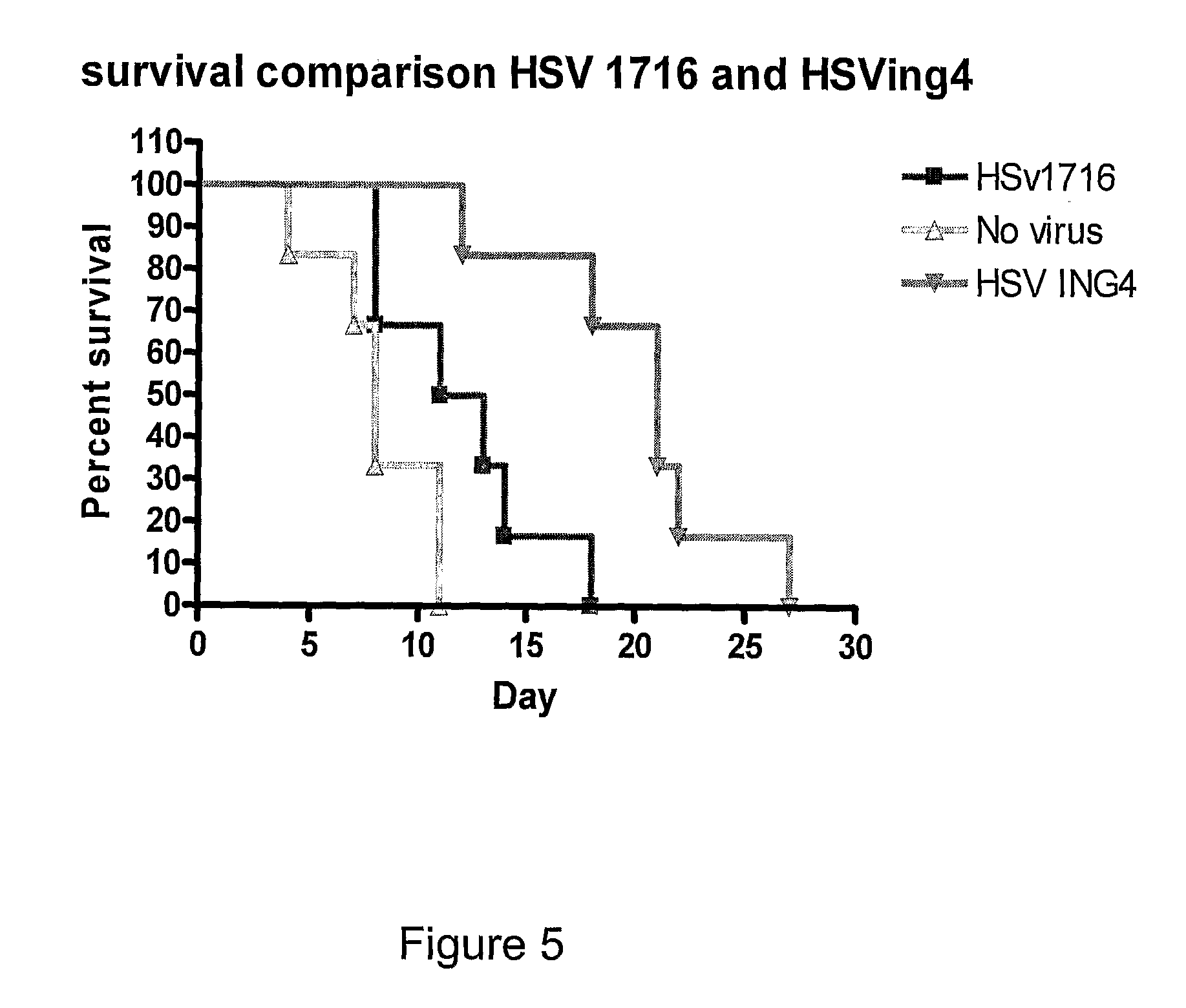
ActiveUS20100092515A1Enhanced ability to retard tumour growthSmall average tumour volumeBiocideBacteriaNucleic acid sequencingViral replication
A herpes simplex virus is disclosed in which the herpes simplex virus genome comprises a nucleic acid sequence encoding an ING4 polypeptide.
Owner:VIRTTU BIOLOGICS
Mutant Viruses
An herpes simplex virus wherein the herpes simplex virus genome comprises nucleic acid encoding an antisense to the squamous cell carcinoma related oncogene (asSCCRO); and an herpes simplex virus wherein the herpes simplex virus genome comprises nucleic acid encoding a short interfering ribonucleic acid (siRNA) molecule that is capable of repressing or silencing expression of squamous cell carcinoma related oncogene (SCCRO) nucleic acid or polypeptide are disclosed together with methods for generation and applications of such viruses.
Owner:SLOAN KETTERING INST FOR CANCER RES +1
Topical Use Of Probiotic Bacillus Spores To Prevent Or Control Microbial Infections
Compositions including an isolated Bacillus species, spores or an extracellular product of B. coagulans, suitable for topical application, for inhibiting growth of yeast, fungus, bacteria or Herpes simplex virus are disclosed. Methods of inhibiting growth of yeast, fungus, bacteria or Herpes simplex virus by topical application of compositions that include an isolated Bacillus species, spores or an extracellular product of a B. coagulans strain are disclosed.
Owner:GANEDEN BIOTECH
Treatment Using Herpes Simplex Virus
ActiveUS20090010889A1Successful tumour treatmentConvenient treatmentBiocideOrganic active ingredientsCombination therapyVirology
The use of Herpes Simplex Virus (HSV) in the treatment of tumour by extratumoural administration of said HSV, and the use of HSV in treatment of tumour by combination therapy with a pharmaceutical wherein the HSV and / or pharmaceutical is administered at an extratumoural location, is disclosed.
Owner:VIRTTU BIOLOGICS
Compositions and methods for vaccinating against HSV-2
InactiveUS7628993B2Stimulate immune responseEnhance cellular immune responseSugar derivativesViral antigen ingredientsWhole bodyImmune activation
This invention relates to a method for systemic immune activation which is effective for eliciting both a systemic, non-antigen specific immune response and a strong antigen-specific immune response in a mammal. The method is particularly effective for protecting a mammal from herpes simplex virus. Also disclosed are therapeutic compositions useful in such a method.
Owner:UNIV OF WASHINGTON +1
Method and topical treatment composition for herpesvirus hominis
Improved topical treatment of active phase lesions resulting from recurrent viral infection by herpes simplex virus which includes the use of two primary agents, namely, an aqueous solution of benzalkonium halide, preferably benzalkonium chloride, and a dry form of the herb Echinacea purpurea, preferably in powder form. Active phase herpes lesions are wetted with the benzalkonium chloride solution and dusted with the powder form of Echinacea purpurea to create a coating on the wetted lesion surface. The coating is maintained on the lesion throughout treatment, and unexpected rapid resolution of the lesions results.
Owner:MERITUS +1
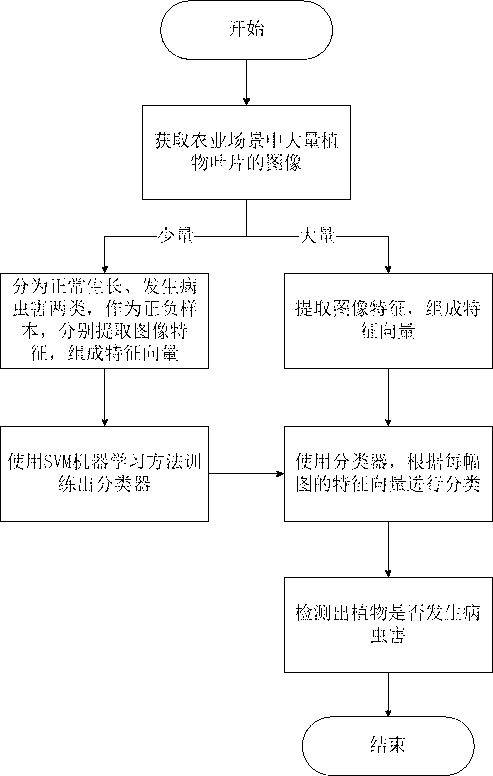
Plant disease and pest detection method based on SVM (support vector machine) learning
InactiveCN102915446AImprove efficiencyRealize continuous computingCharacter and pattern recognitionDiseaseFeature vector
The invention belongs to the technical field of digital image processing and pattern recognition and particularly relates to a plant disease and pest detection method based on SVM (support vector machine) learning. The plant disease and pest detection method comprises the following steps: acquiring a large number of regularly grown plant leaves and the plant leaves with diseases and pests from a large number of monitoring videos of agricultural scenes; extracting a part of pictures of the regularly grown plant leaves and the plant leaves with diseases and pests as samples; extracting characteristics [including color characteristic, HSV (herpes simplex virus) characteristic, edge characteristic and HOG (histogram of oriented gradient) characteristic] of each leaf picture; combining the characteristics into characteristic vectors; training the characteristic vectors of each leaf picture through an SVM learning method; forming a classifier after training; and detecting the large number of plant leaf pictures by the classifier to detect whether diseases or pests occur to the plant leaves. Compared with a biologic plant disease and pest detection method, the plant disease and pest detection method based on the SVM learning is higher in real time and easier to implement; and the shortcoming of detecting the plant diseases and pests by working in the fields is overcome.
Owner:FUDAN UNIV
Tetracycline repressor regulated oncolytic viruses
The present invention is directed oncolytic Herpes simplex-l viruses whose replication is controlled using a tetracycline operator / repressor system. The invention also includes DNA sequences used in making the viruses and methods in which these viruses are used in the treatment of cancer patients with solid tumors.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
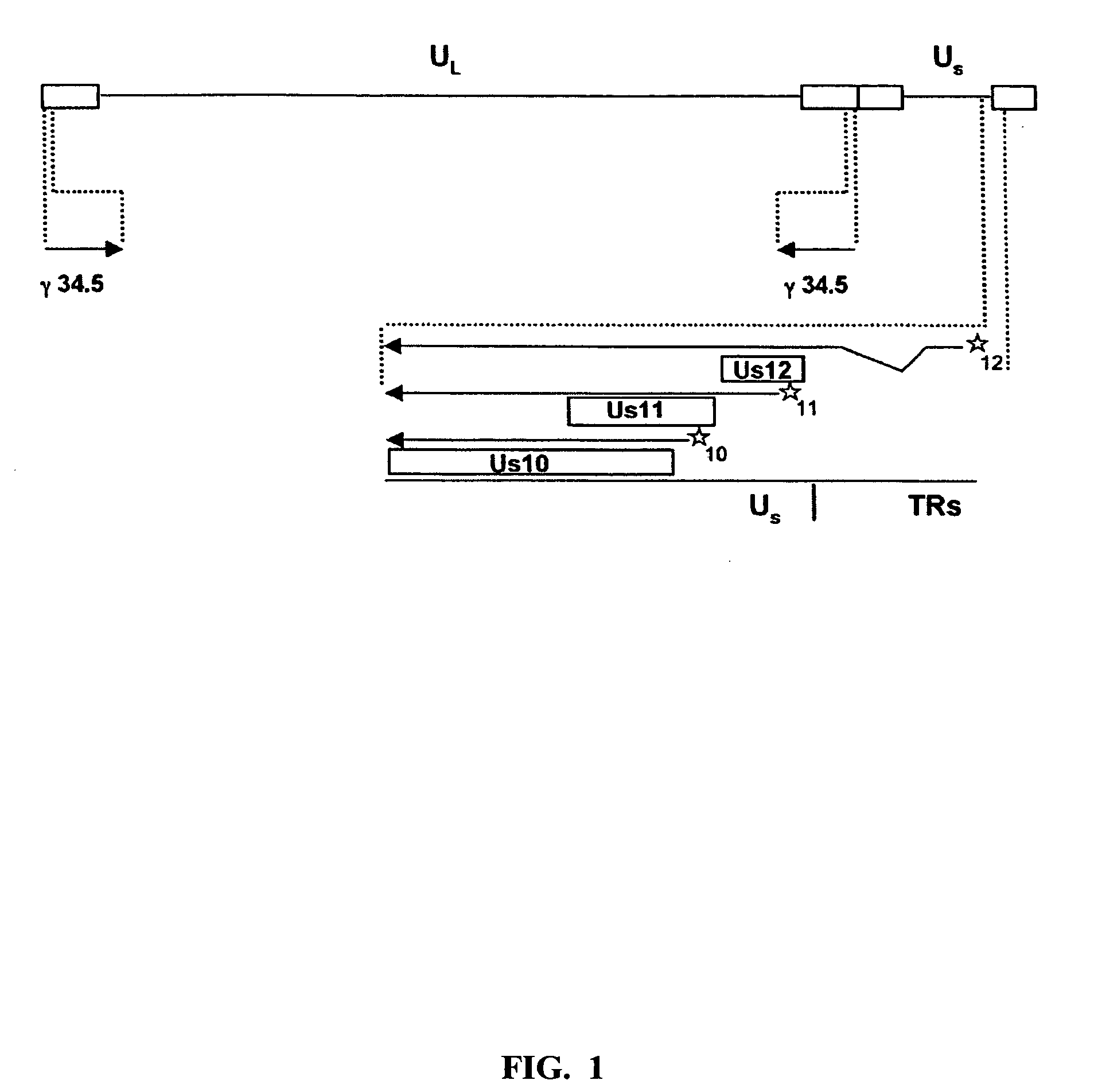
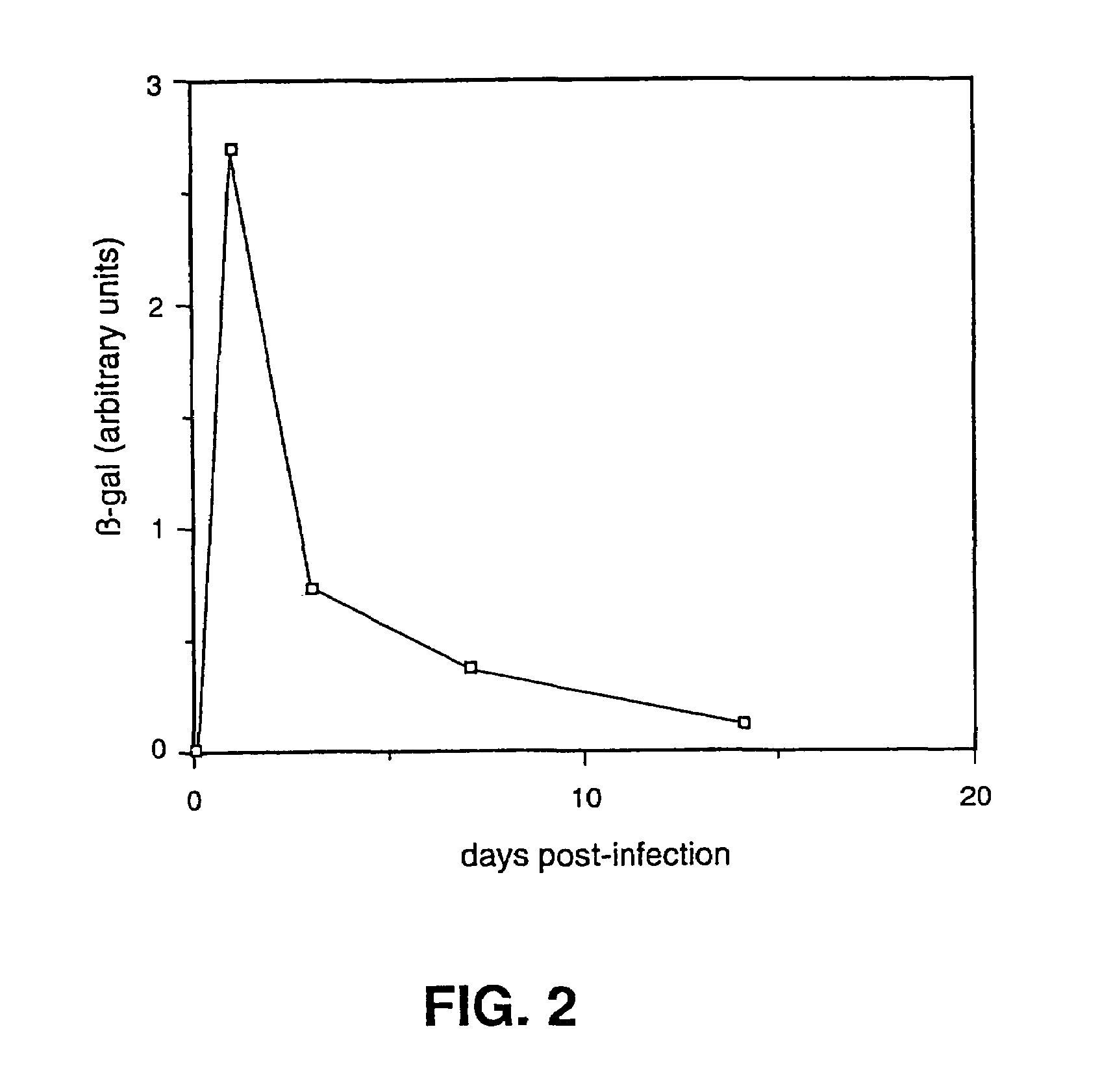
HSV strain lacking functional ICP27 and ICP34.5 genes
The present invention provides a herpes simplex virus strain which lacks a functional ICP34.5 gene and a functional ICP27 gene. It also provides the use of a herpes simplex virus strain which lacks a functional ICP34.5 gene and a functional ICP27 gene in the treatment of disorders of, or injuries to, the nervous system of a mammal.
Owner:UNIV COLLEGE OF LONDON +1
Compositions and methods for vaccinating against hsv-2
InactiveUS20080102087A1Stimulate immune responseStrong cellular responseOrganic active ingredientsGenetic material ingredientsWhole bodyMammal
This invention relates to a method for systemic immune activation which is effective for eliciting both a systemic, non-antigen specific immune response and a strong antigen-specific immune response in a mammal. The method is particularly effective for protecting a mammal from herpes simplex virus. Also disclosed are therapeutic compositions useful in such a method.
Owner:UNIV OF WASHINGTON +1
Methods, compositions, formulations, and uses of cellulose and acrylic-based polymers
InactiveUS20050244365A1Easy to chargeLow pKaAntibacterial agentsCosmetic preparationsDisinfectantReverse transcriptase
Compositions, formulations, and methods for the treatment or prevention, or decreasing the frequency of transmission of a virus (such as human immunodeficiency virus type 1 (HIV-1), Herpes Simplex virus type 1 (HSV1), or Herpes Simplex Virus Type 2 (HSV2), or other virus), or a bacterial infection (such as Trichomonas vaginalis, Neisseris gonorrhoeae Haemopholus ducreyl, or Chlamydia trachomatis, or other bacterial species), or a fungal infection, using an anionic cellulose- or acrylic-based oligomer, polymer, or copolymer. The present invention also includes administering a therapeutically effective amount of said oligomer, polymer, or copolymer, or a pharmaceutically acceptable salt thereof, or with a pharmaceutically acceptable carrier or diluent, thereof. The invention relies on the unique biochemical substitution of the cellulose or acrylic backbone such that the resultant molecule can remain molecularly dispersed in solution (or gel or other formulation) and mostly dissociated over a wide range of physiological microenvironments, such as the low pH found within the vaginal lumen, preferably from a pH of 14 to below 3.5. These specific substitutions also impart on the resultant molecule potent antiviral, anti-bacterial, and anti-fungal properties. In addition, these compositions can be used as general disinfectants for human use such as in contact lens solutions, mouthwashes, toothpastes, suppositories, or as more generalized disinfectants found in soaps, household cleaning products, paints, water treatments modalities, or can be incorporated into cosmetic, and can be used as vehicles for drug delivery, an adjuvant in a therapeutic formulation, or as a preservative. These compounds can be delivered in a liquid or solid dosage form and can be incorporated into barrier devices such as condoms, diaphragms, or cervical caps, to help prevent the transmission of STDs. The compounds of this invention can also be used in combination therapies with other classes of antiviral, antibacterial, or antifungal agent having similar or differing mechanisms of action including, but not limited to, anionic or cationic polymers, copolymers, or oligomers, surfactants, protease inhibitors, DNA or RNA polymerase inhibitors (including reverse transcriptase inhibitors), fusion inhibitors, cell wall biosynthesis inhibitors, integrase inhibitors, or virus or bacterial attachment inhibitors.
Owner:NOVAFLUX INC +1
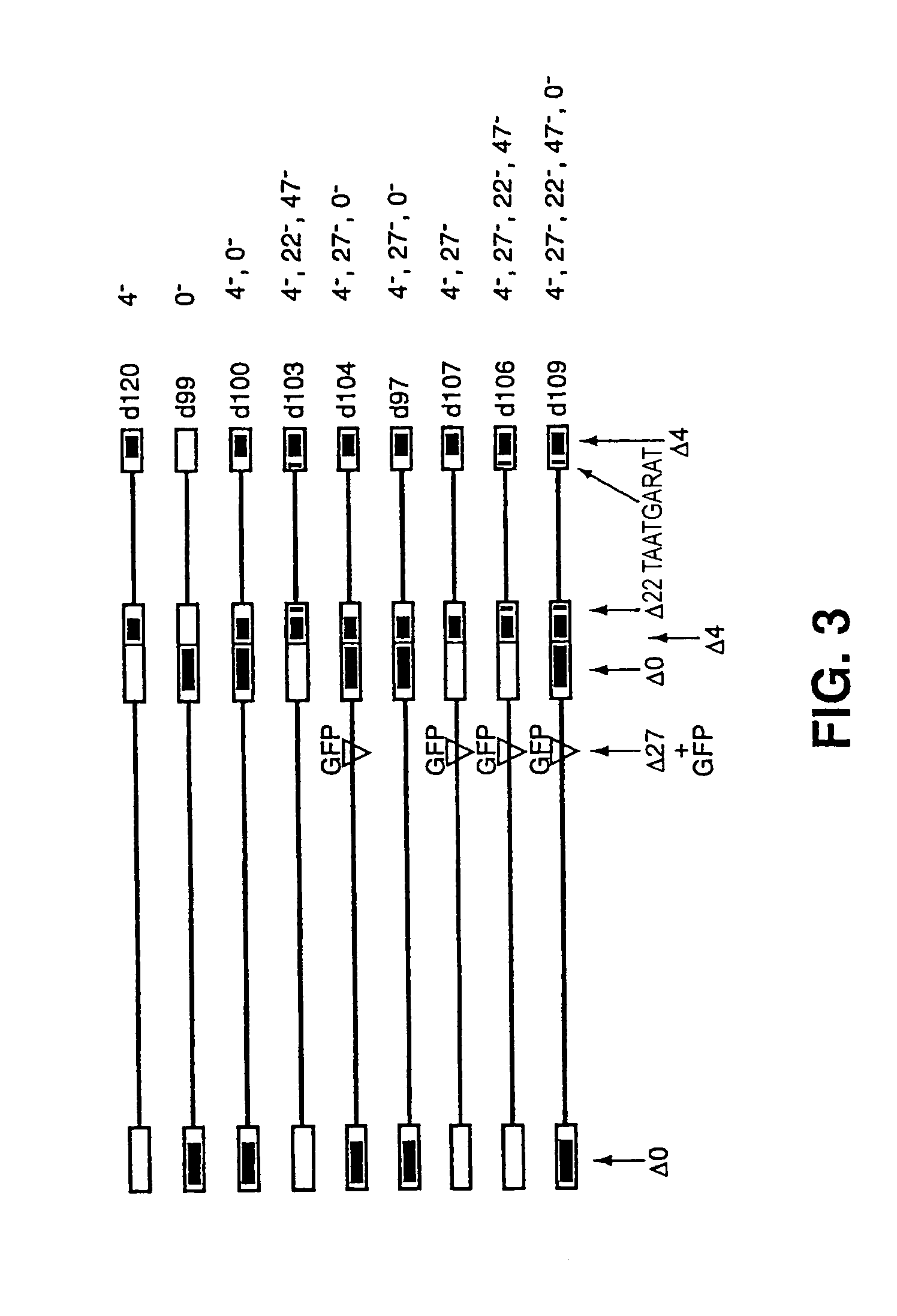
Non-toxic hsv vectors for efficient gene delivery applications and complementing cells for their production
The invention provides a herpes simplex virus (HSV) vector that does not express toxic HSV genes in non-complementing cells and which comprises a genome comprising one or more transgenes, wherein the vector is capable of expression of a transgene for at least 28 days in non-complementing cells. The disclosed vectors include vectors having deletions in the genes ICP0, ICP4, TCP22, TCP27 and TCP47, or alternative inactivating mutations, or vectors which express one or more of these genes with modified kinetics. The invention also relates to viral stocks of the inventive vectors, compositions thereof suitable for use therapeutically or for in vitro applications, and methods relating thereto. In another aspect, the invention provides a complementing cell, in particular a U20S cell, engineered to express ICP4 and ICP27 when the cell is infected with HSV for the production of the inventive vector. Said cells are disclosed as naturally complementing ICP0.
Owner:UNIVERSITY OF PITTSBURGH
Methods of treating diseases which are mediated by cutaneous lymphocyte antigen positive cells
ActiveUS8388964B2Improves and prevents and inhibits and reduces skinImproves and prevents and inhibits and reduces pruritisCompounds screening/testingLuminescence/biological staining preparationDiseaseContact dermatitis
The present invention relates to methods of treating patients suffering from itching and puritis mediated by cutaneous lymphocyte antigen positive T cell. In particular, diseases or disorders including contact dermatitis, drug induced delayed type cutaneous allergic reactions, toxic epidermal necrolysis, cutaneous T cell lymphoma, bullous pemphigoid, alopecia aereata, vitiligo, acne rosacea, prurigo nodularis, and herpes simplex virus, or combination thereof will benefit from the administration of an IL-31 antagonist. The invention also includes methods of predicting a therapeutically responsive patient population.
Owner:ZYMOGENETICS INC +1
Oncolytic hsv vector
ActiveUS20170035819A1Prevent vector pathogenesisSafe and effectiveVirus peptidesX-ray constrast preparationsLipid formationCancer cell
The present invention provides a recombinant oncolytic Herpes Simplex Virus (oHSV) comprising a non-HSV ligand specific for a molecule (protein, lipid, or carbohydrate determinant) present on the surface of a cell (such as a cancer cell) and one or more copies of one or more microRNA target sequences inserted into one or more HSV gene loci, preferably one or more HSV gene(s) required for replication of HSV in normal (i.e., non-cancerous) cells. The invention further provides stocks and pharmaceutical compositions comprising the inventive oHSV and methods for killing tumor cells employing the inventive oHSV.
Owner:UNIVERSITY OF PITTSBURGH
Herpes simplex virus strains
InactiveUS7078029B2Reduction in wild-type reversionInterference minimizationBiocideGenetic material ingredientsGene productEarly response gene
The present invention provides an HSV having a genome with a mutation of a TAATGARAT sequence such that, in the presence of a ICP4 gene product, a native immediate early gene is expressed from the genome with delayed kinetics, the genome having a further inactivating mutation of each of the genes encoding ICP4.
Owner:UNIVERSITY OF PITTSBURGH
Fatty acids for use as a medicament
ActiveUS20100113387A1Clear and significant laxative effectAntibacterial agentsBiocideDiseasePharmaceutical formulation
The invention relates to fatty acid stimulation of rectal mucosa initiating the process of defecation, acting as a laxative. Furthermore, the invention relates to the usage of free fatty acids, fatty acid mixtures and fatty acid extracts from marine lipids in pharmaceutical formulations such as suppositories, ointments, tablets and gelatin capsules for treatment and prevention of multiple disorders like constipation, hemorrhoids, bacterial infections (e.g. helicobacter pylori), viral infections (e.g. herpes simplex virus infections) and inflammations, as well as against fissura ani and pruritus ani.
Owner:LIPID PHARMA EHF
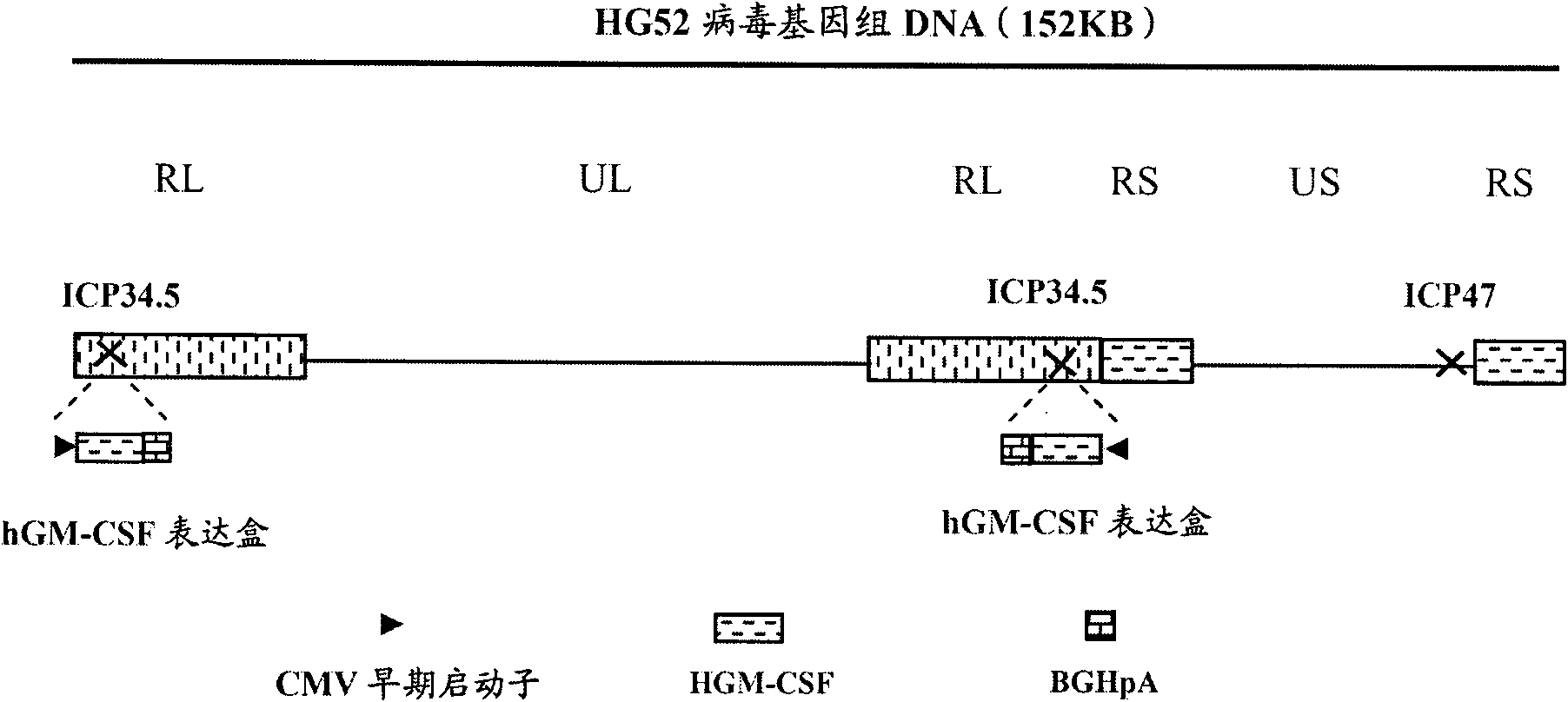
Recombinant II type herpes simplex virus vector, preparation method of recombinant II type herpes simplex virus vector, recombinant virus, medicinal composition and application
ActiveCN102146418AGenetic material ingredientsViral/bacteriophage medical ingredientsCurative effectRecombinant virus vaccine
The invention provides a recombinant II type herpes simplex virus vector. An ICP34.5 gene and an ICP47 gene of a wild II type herpes simplex virus HG52 strain are removed in the virus vector, and preferably a human granulocyte macrophage-colony stimulating factor (hGM-CSF) expression box is inserted into the position where the ICP34.5 gene is removed. The invention also provides a preparation method of the recombinant II type herpes simplex virus vector, a recombinant virus using the recombinant II type herpes simplex virus as a vector, a medicinal composition consisting of the recombinant II type herpes simplex virus vector and a pharmaceutically acceptable vector or excipient, and application of the recombinant II type herpes simplex virus vector in preparation of a gene medicament for treating tumors. As the ICP34.5 gene is removed in the recombinant II type herpes simplex virus vector provided by the invention, the oncolysis virus is safe and can selectively grow and propagate in tumor cells; the ICP47 gene is removed to promote immune response and enhance oncolysis activity; and the curative effect of the recombinant II type herpes simplex virus vector is superior to that of the conventional recombinant I type herpes simplex virus vector, and the recombinant II type herpes simplex virus vector has high safety.
Owner:WUHAN BINHUI BIOTECH CO LTD
Herpes simplex virus expressing foreign genes and method for treating cancers therewith
An anti-cancer pharmaceutical composition includes a herpes simplex virus (HSV) vector into which a nucleic acid sequence encoding for an anti-cancer agent selected from interleukin-12, GM-CSF, and CD has been inserted. A method of treatment of a patient suffering from cancer includes administering to the patient the anti-tumor pharmaceutical composition including a HSV vector having a nucleic acid sequence encoding for an anti-cancer agent selected from interleukin-12, GM-CSF, and CD inserted therein.
Owner:UAB RES FOUND
Oncolytic hsv vector
ActiveUS20160250267A1Avoid problemsSafer and effectiveVirus peptidesX-ray constrast preparationsCancer cellMicroRNA
The present invention provides a recombinant oncolytic Herpes Simplex Virus (oHSV) comprising a non-HSV ligand specific for a molecule (protein, lipid, or carbohydrate determinant) present on the surface of a cell (such as a cancer cell) and one or more copies of one or more microRNA target sequences inserted into one or more HSV gene loci, preferably one or more HSV gene(s) required for replication of HSV in normal (i.e., non-cancerous) cells. The invention further provides stocks and pharmaceutical compositions comprising the inventive oHSV and methods for killing tumor cells employing the inventive oHSV.
Owner:UNIVERSITY OF PITTSBURGH
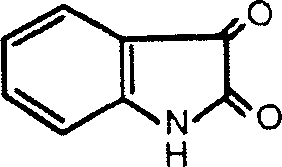
Application of indole-2,3-diketone in preparing medication for antivirus or immunopotenfiator
InactiveCN1759833AImprovement of immune indicatorsShows antiviral effectOrganic active ingredientsAntiviralsNatural productKetone
An application of indole-2.3-bione in preparing the antiviral medicine for HIV, influenza virus, hemorrhagic virus and herpes simplex virus and the immunopotentiator is disclosed.
Owner:QINGDAO UNIV
Treatment of cancer
InactiveUS20170319638A1Strong specificityImprove securityViral/bacteriophage medical ingredientsMammal material medical ingredientsLymphocyteChimeric antigen receptor
A method of treating cancer in a subject is disclosed, the method comprising administration of an oncolytic herpes simplex virus and administration of lymphocyte cells modified to express a chimeric antigen receptor (CAR) or modified to express a T cell receptor (TCR).
Owner:VIRTTU BIOLOGICS
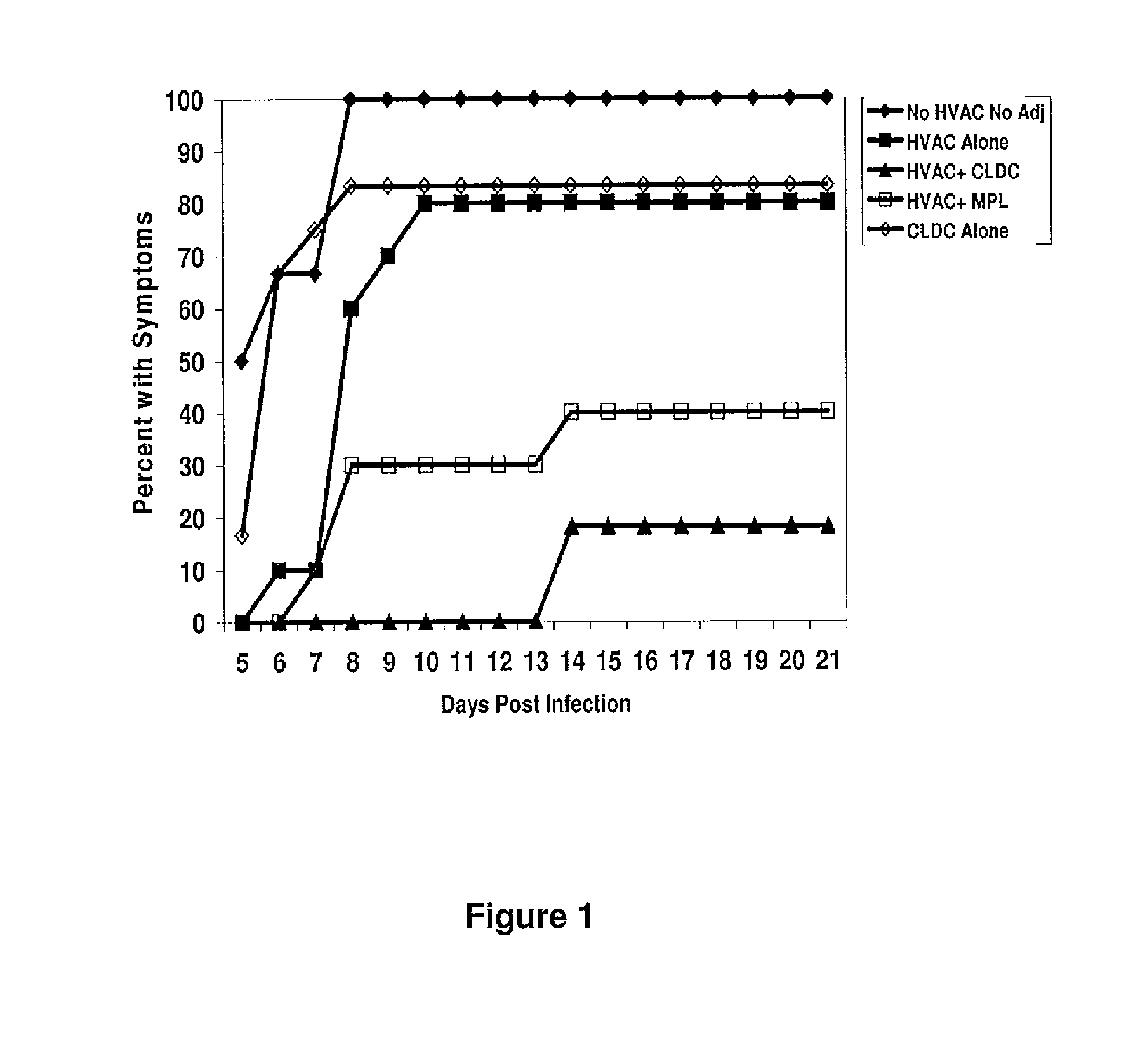
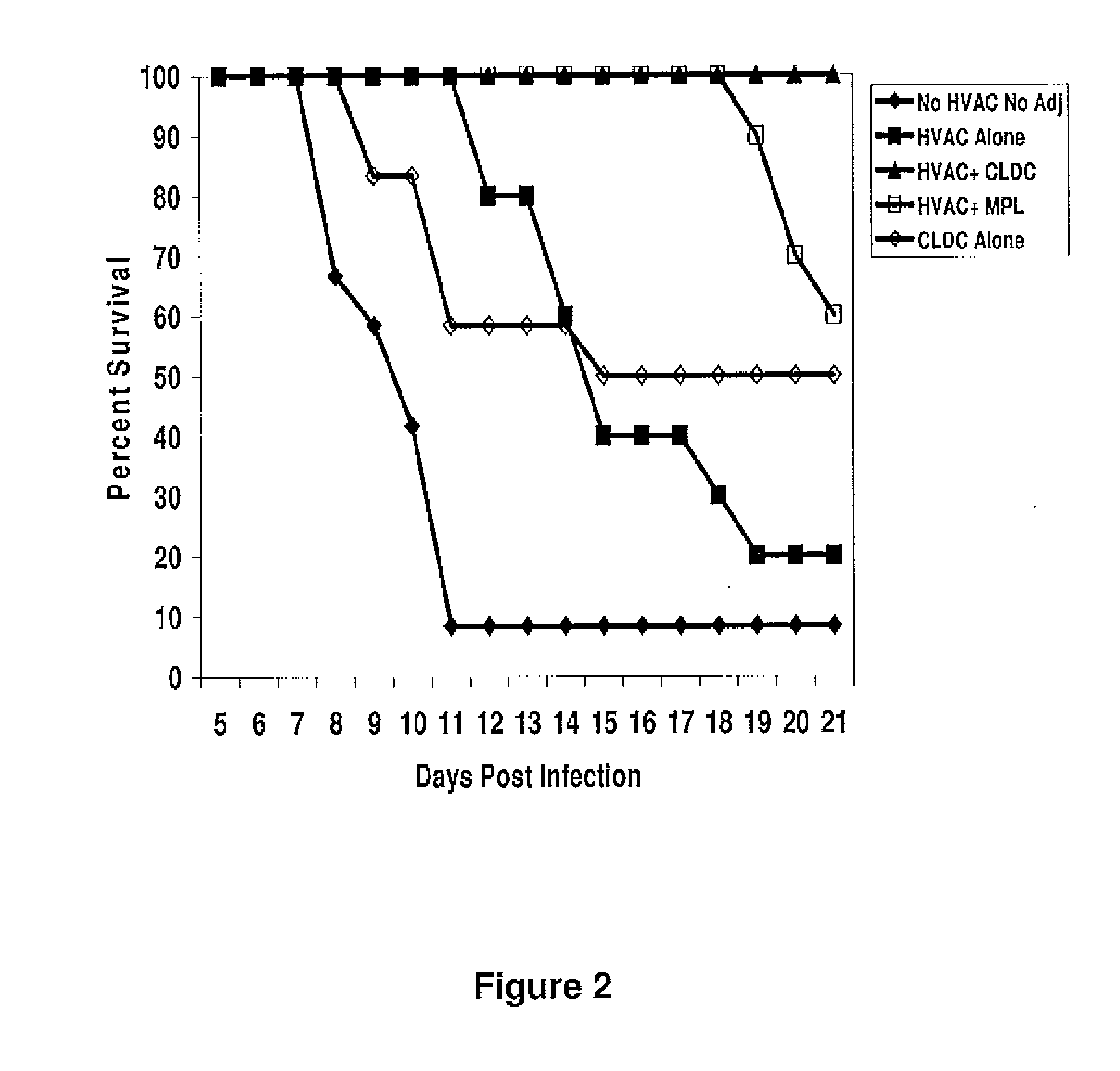
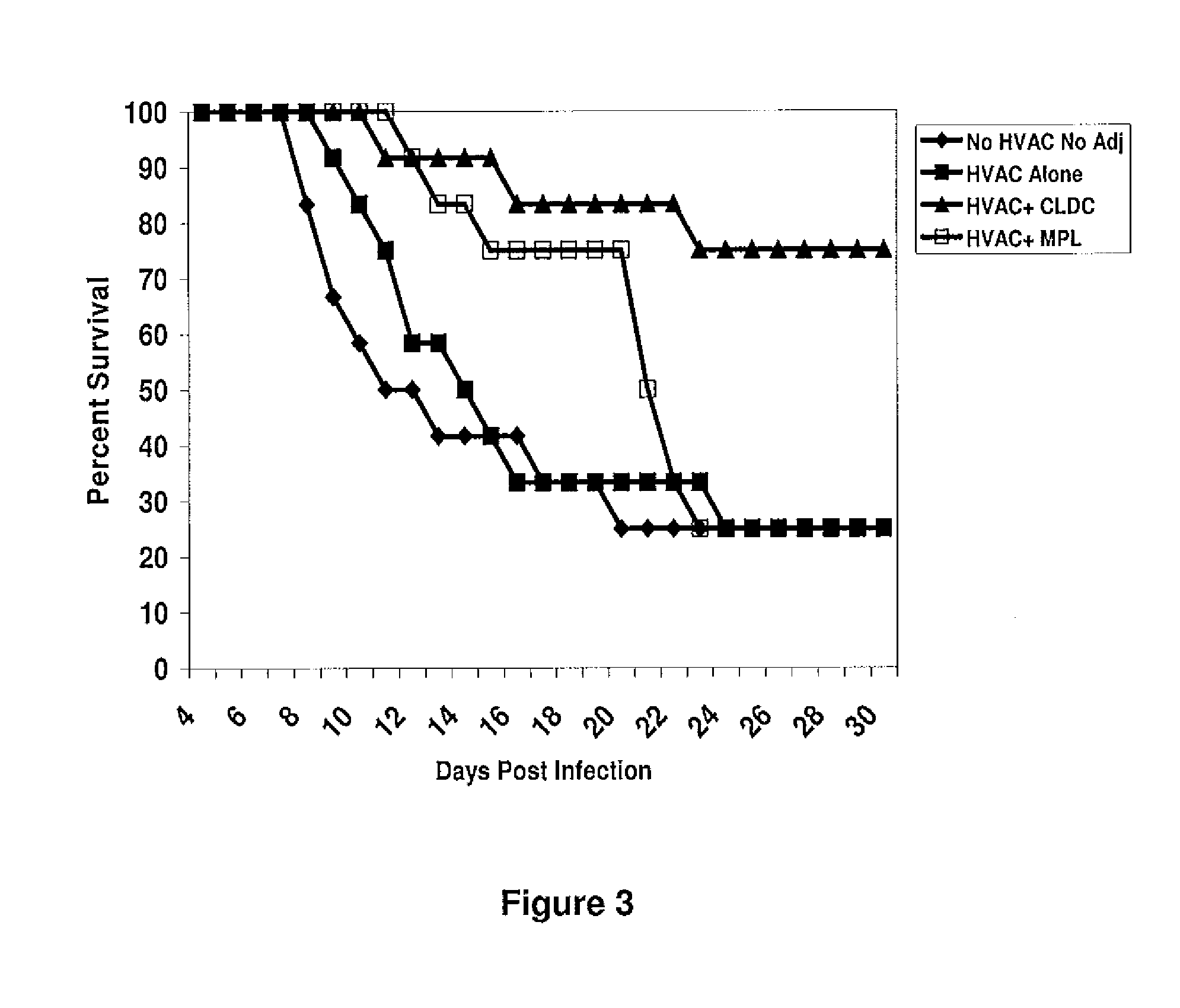
Subunit Vaccines for Herpes Viruses and Methods of Use
The present disclosure generally relates to vaccine compositions for Herpes Simplex Viruses (HSV) types 1 and / or 2. The vaccines comprise isolated antigens or glycoprotein subunits of the viruses, optionally with an adjuvant, such as a cationic liposome DNA complex (CLDC). Also the present disclosure contains methods of vaccinating a subject utilizing these compositions.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +1
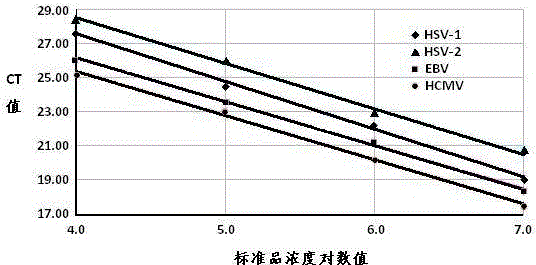
Fluorescent quantitation PCR (polymerase chain reaction) detection kit for four conventional herpesvirus hominises
InactiveCN103820573AMicrobiological testing/measurementMicroorganism based processesFluoProbesHerpesvirus hominis
The invention provides a real-time fluorescent quantitation PCR (polymerase chain reaction) kit for simultaneously detecting conventional herpesvirus hominises HSV (herpes simplex virus)-1, HSV-2, EBV and HCMV (human cytomegalovinis). The kit consists of quantitation PCR reaction liquid, quantitation reaction liquid, an HSV-1 standard substance, an HSV-2 standard substance, an EBV standard substance, an HCMV standard substance, a negative reference substance, an HSV-1 positive reference substance, an HSV-2 positive reference substance, an EBV positive reference substance, an HCMV positive reference substance, a specification and a kit body. The kit disclosed by the invention adopts a real-time fluorescent quantitation PCR technology and two specific fluorescent probes in the same reaction system; the detection range covers the four most conventional herpesvirus hominises, so that the four herpesvirus hominises in a sample can be simultaneously subjected to parting detection; real-time and accurate quantitation can be realized; the need for early, quick and accurate diagnosis can be met; a powerful basis is supplied to epidemiological investigation and immediate formulation of a targeted treating scheme.
Owner:ZHEJIANG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com