Methods, compositions, formulations, and uses of cellulose and acrylic-based polymers
a technology of acrylic-based polymers and cellulose, which is applied in the field of cellulose and acrylic-based polymers, can solve the problems of vaginal infections, affecting the quality of cellulose-based products, and infecting millions of people worldwide, and achieves excellent stability against phase separation, enhanced thickening or delivery aspects of polymers, and improved viscoelastic properties. the effect of the polymer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Acrylic-Based Polymers, Copolymers or Oligomers
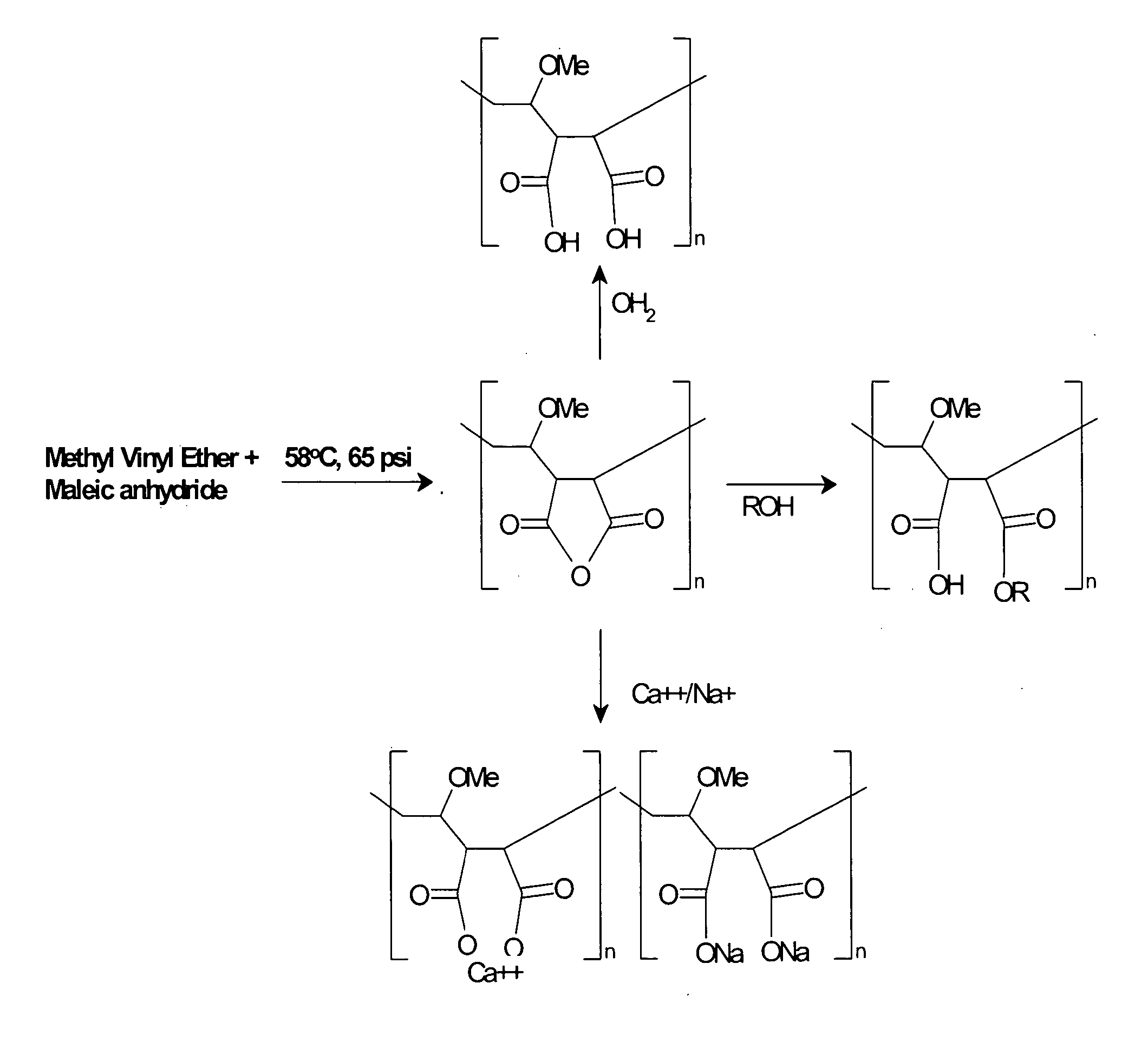
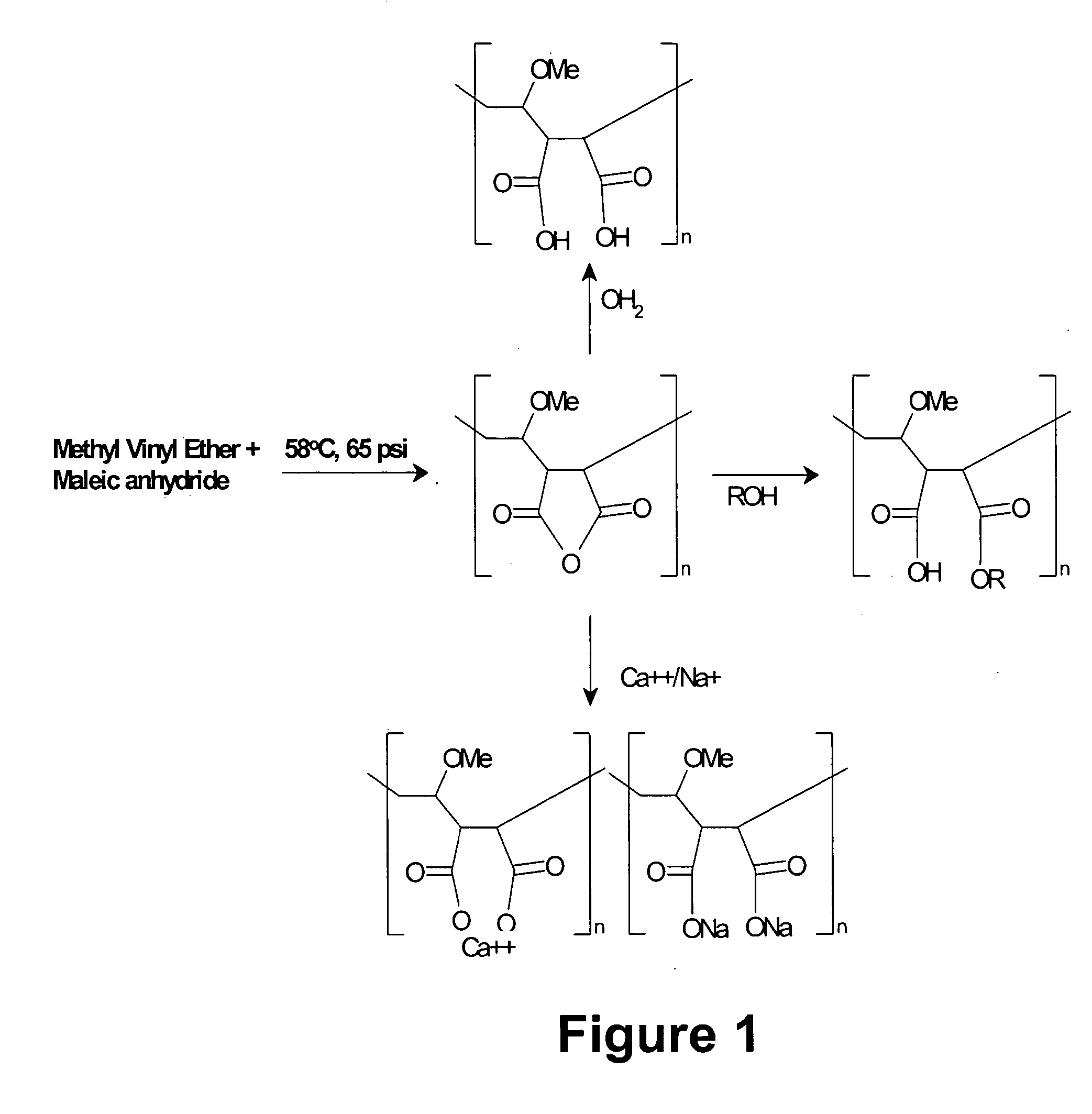
[0172] Acrylic based polymers and copolymers can be obtained using a variety of techniques that would be apparent to one skilled in the art. For example, a synthetic scheme that one could employ to synthesize MVE / MA involves the addition of 404.4 parts cyclohexane, and 269.6 parts ethyl acetate into a I liter pressure reactor. Next 0.3 parts of t-butylperoxypivilate are added at 58° C. in three installments of 0.1 part each at times 0, 60 and 120 minutes from the first addition. Seventy-five parts of molten maleic anhydride and 49.0 parts of methyl vinyl ether are mixed together and gradually added to the reaction vessel at 58° C. and 65 psi over a 2 hour period of time. The reaction mixture is then held at 58° C. for two hours after the last addition of initiator. The presence of maleic anhydride is followed by testing with triphenyl phosphene. The product precipitates out of solution. After the reaction is complete the p...
example 2
Derivitization of Acrylic-Based Polymers, Copolymers or Oligomers to Achieve Enhanced Solubility at Low pH
[0173] One skilled in the art could imagine several different mechanisms for creating diversity within the acrylic polymer or copolymer motif that will allow for variation in charge density or hydrophobicity. One mechanism would be to interchange maleic anhydride in Example 1 above with any anhydride derivative of moieties containing one or more carboxylic acid group as shown in, but not limited to, Table 1. Alternatively a mixture of two or more anhydride containing moieties, derived from examples shown in Table 1, could be used to generate a polymer with alternating charged moieties. These moieties could be aliphatic or aromatic.
[0174] A second mechanism that could be employed to modify the hydrophobicity or electrostatic charge of an acrylic based polymer would be to replace methyl vinyl ether described in Example 1 above with styrene, methyl methacrylate phthalic acid, tri...
example 3
Synthesis of Cellulose-Based Polymers and Copolymers or Oligomers
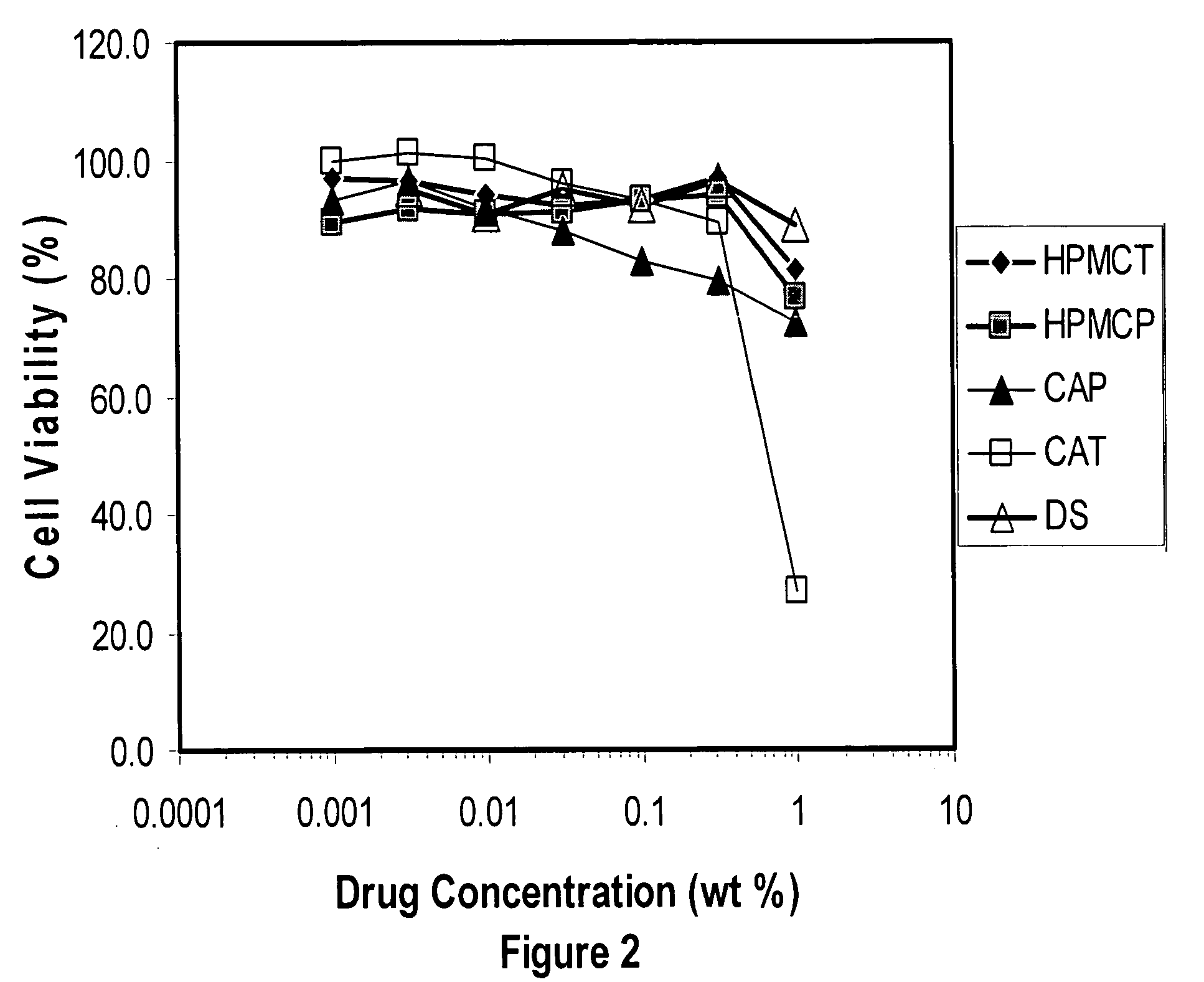
[0176] For the synthesis of hydroxypropyl methylcellulose trimellitate (HPMCT), 700 grams of HMPC 2910 or 2208 is dissolved in 2100 grams of acetic acid (reagent grade) in a 5 liter kneader at 70° C. Then an appropriate amount of trimellitic anhydride (Wako Pure Chemical Industries) and 275 grams of sodium acetate (reagent grade) as a catalyst are added and the reaction is allowed to proceed at 85 to 90° C. for 5 hours. After the reactions, 1200 grams of purified water is poured into the reaction mixture, and the resultant mixture is poured into an excess amount of purified water to precipitate the polymer. The crude polymer is washed well with water and then dried to yield HPMCT. Hydroxypropyl methylcellulose acetate maleate (HPMCAM) is synthesized similarly using a mixture of acetic and maleic anhydride in place of trimellitic anhydride. Other methods can be employed to generate carboxylic acid substituted polymers ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com