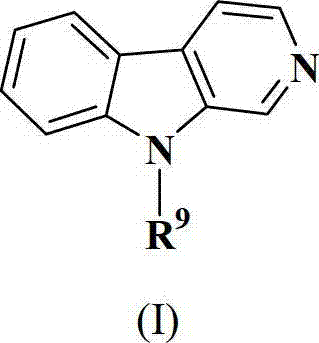
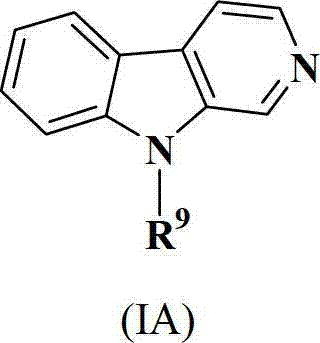
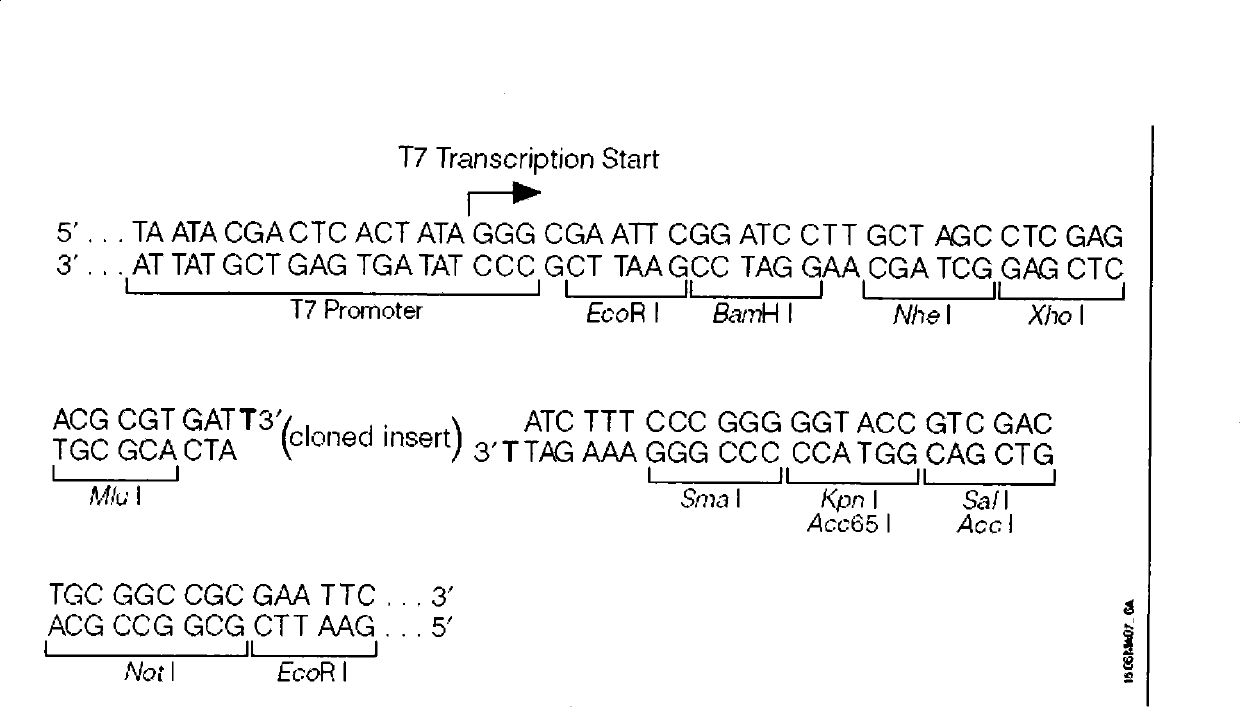
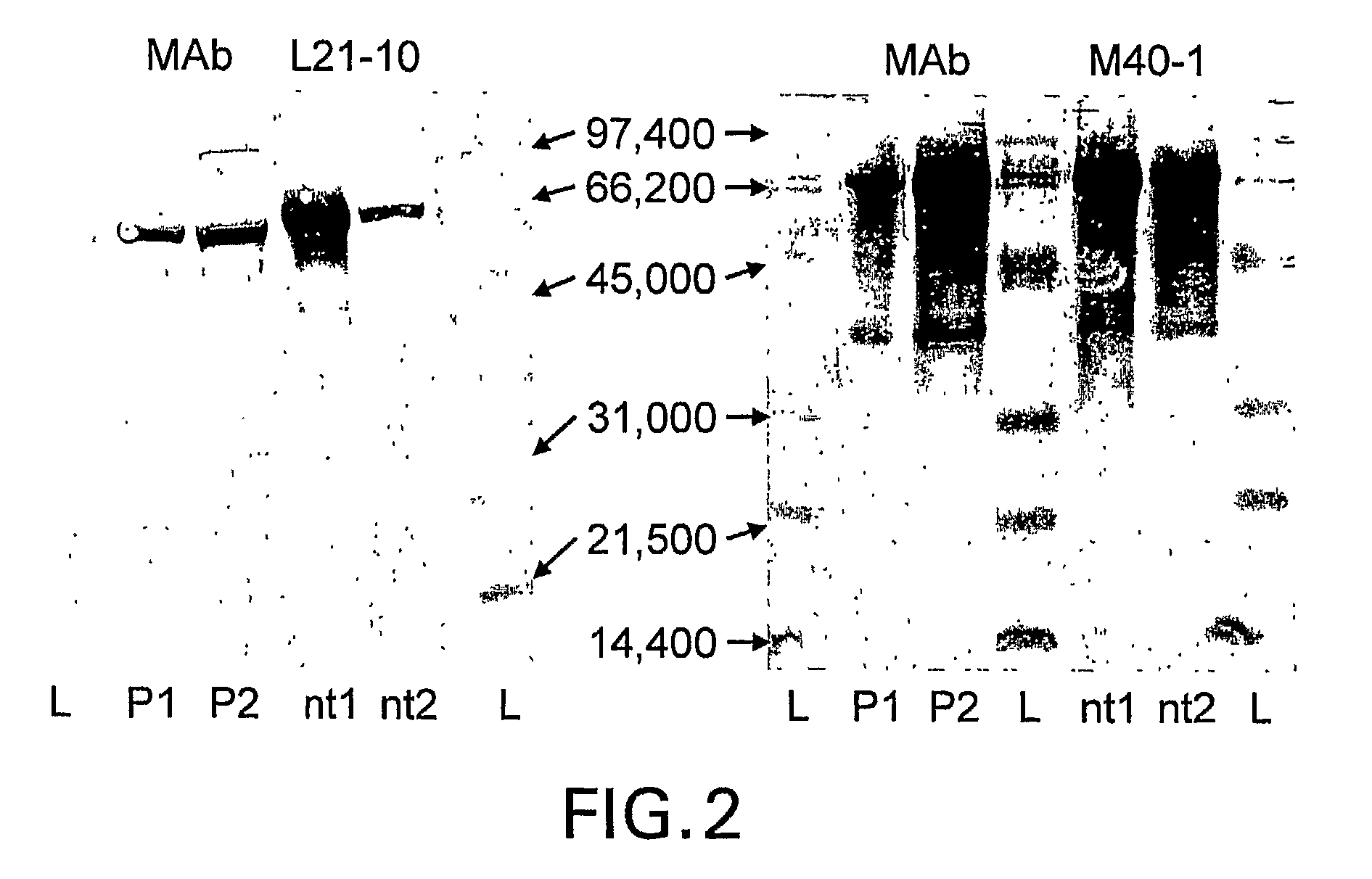
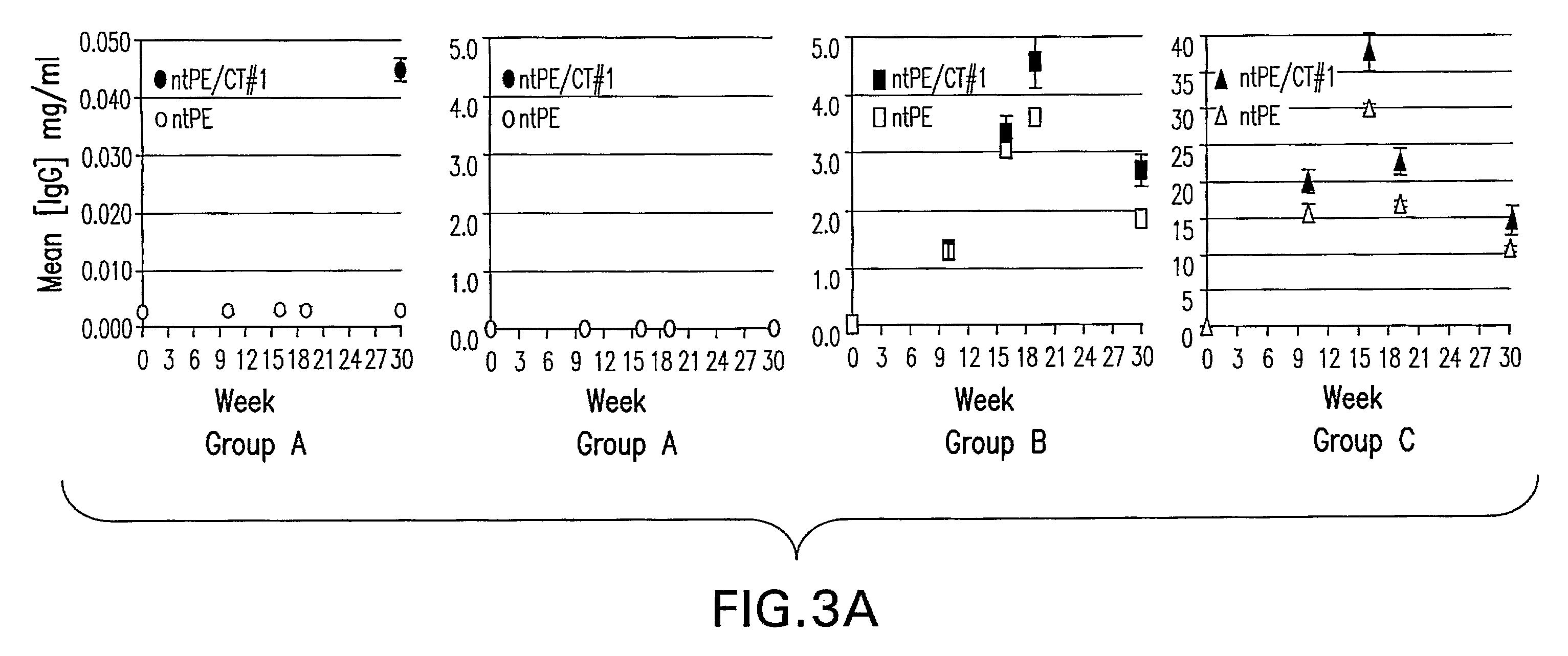
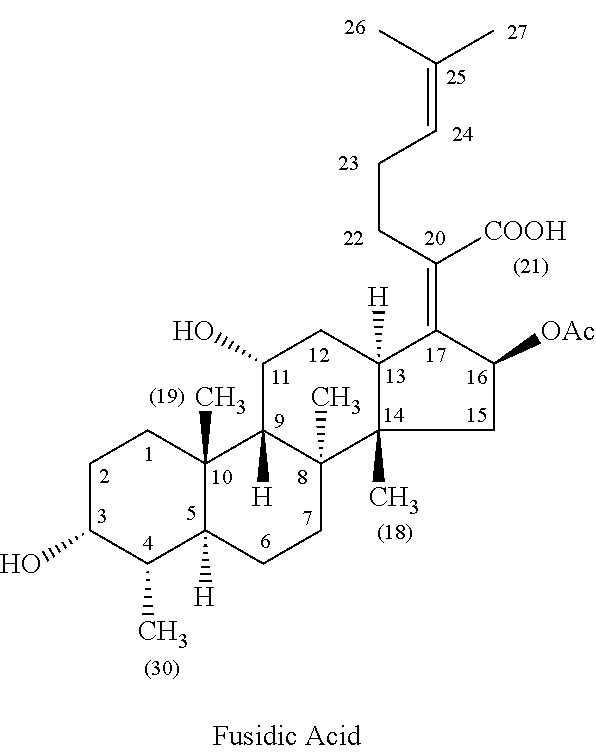
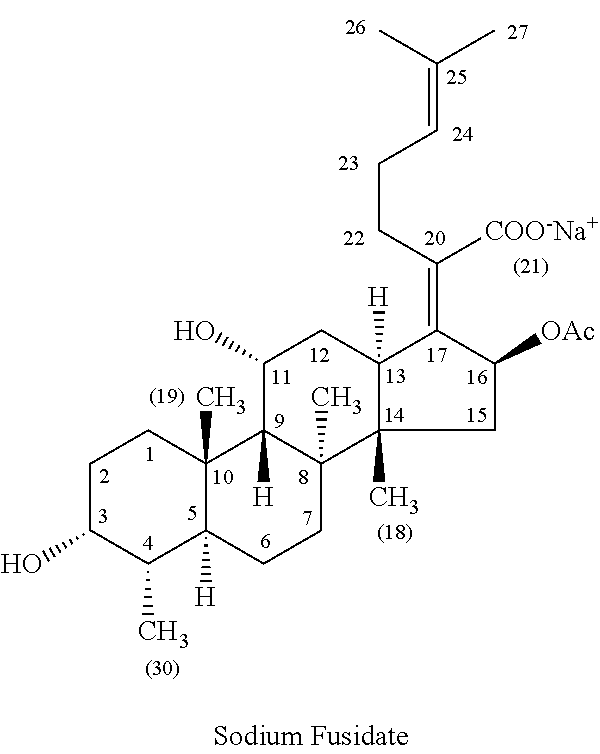
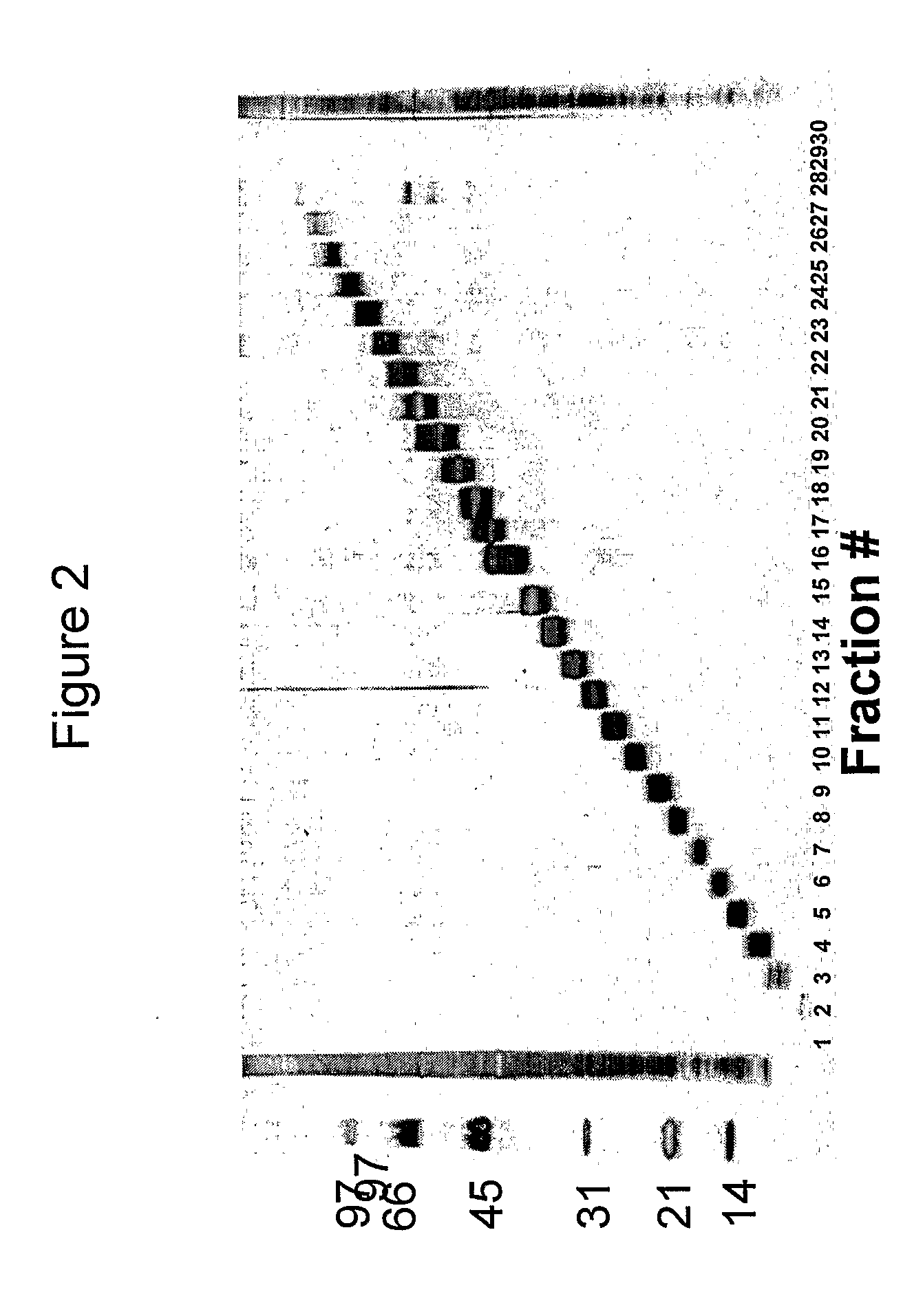
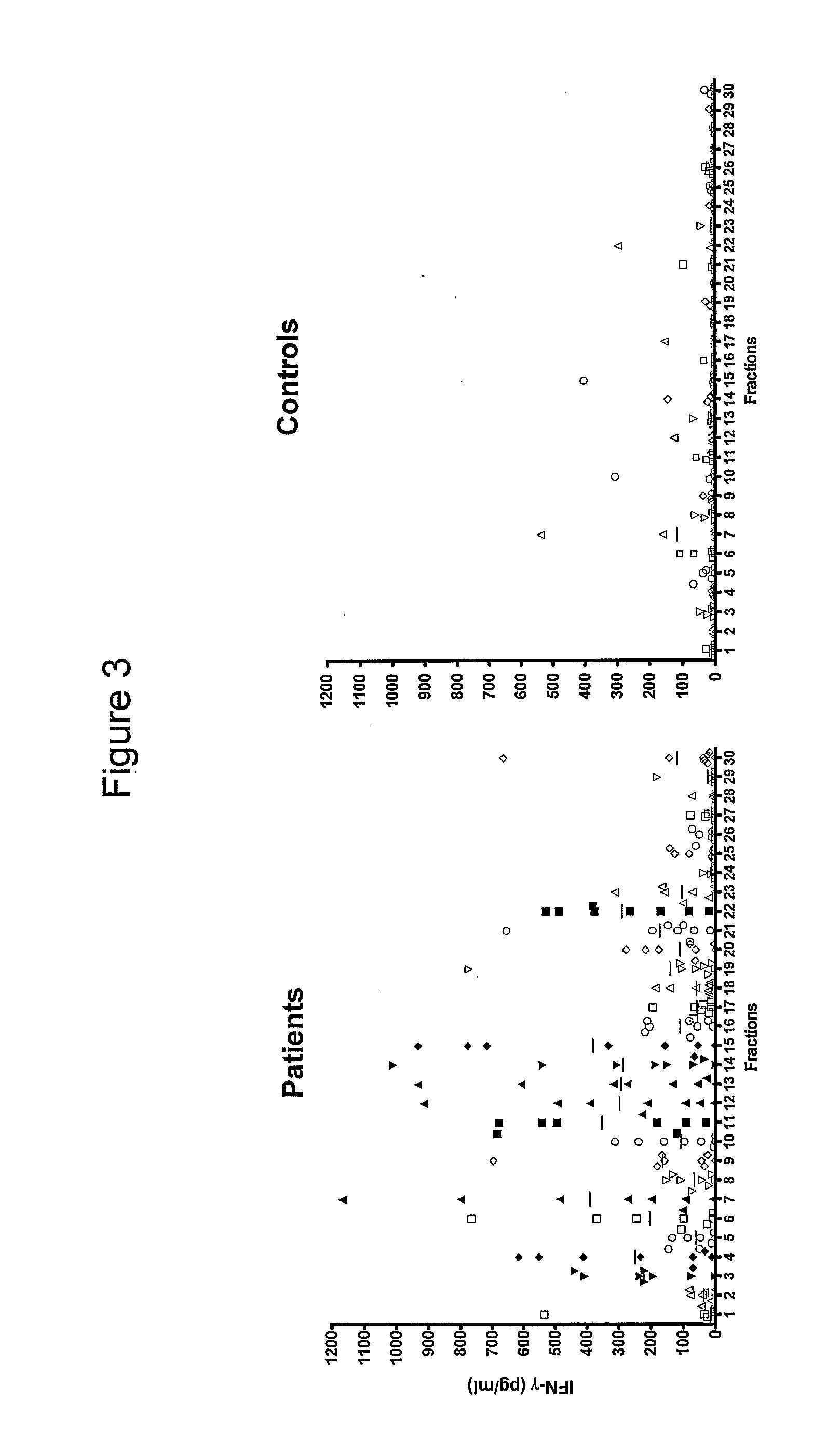
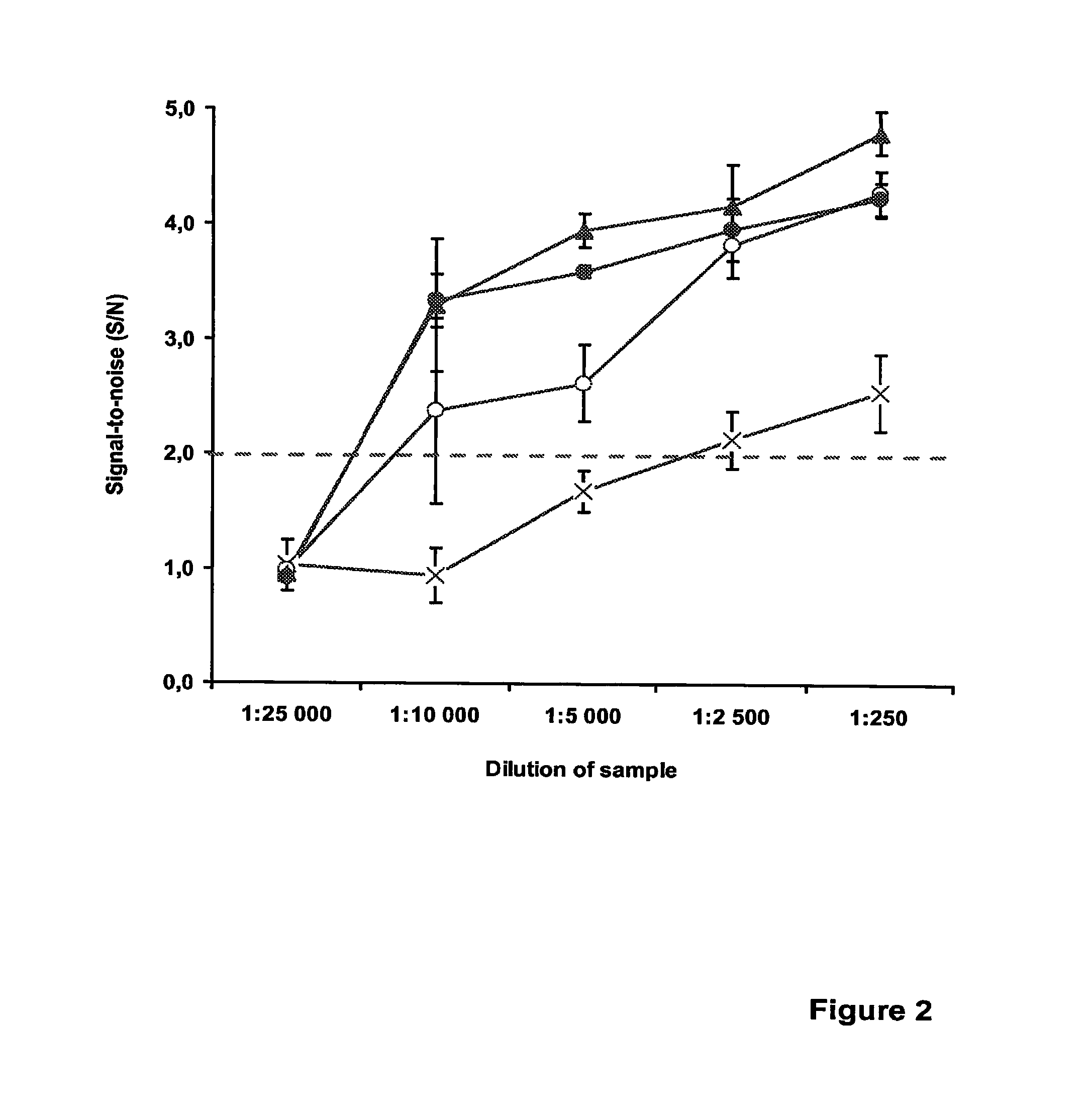
Patents
Literature
119 results about "Chlamydia trachomatis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Chlamydia trachomatis (/kləˈmɪdiə trəˈkoʊmətɪs/), commonly known as chlamydia, is a bacterium that causes chlamydia, which can manifest in various ways, including: trachoma, lymphogranuloma venereum, nongonococcal urethritis, cervicitis, salpingitis, pelvic inflammatory disease. C. trachomatis is the most common infectious cause of blindness and the most common sexually transmitted bacterium.
Nanosilver-containing antibacterial and antifungal granules and methods for preparing and using the same
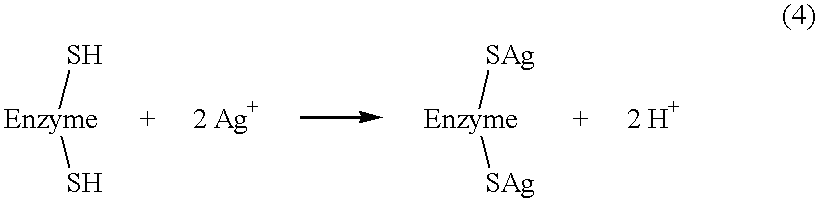
InactiveUS6379712B1Improve solubilityPreventing mold build-upPowder deliveryOrganic active ingredientsEscherichia coliDisease
The present invention relates to nanosilver-containing antibacterial and antifungal granules ("NAGs"). The NAGs have longlasting inhibitory effect on a broad-spectrum of bacteria and fungi, which include, but are not limited to, Escherichia coli, Methicillin resistant Staphylococcus aureus, Chlamydia trachomatis, Providencia stuartii, Vibrio vulnificus, Pneumobacillus, Nitrate-negative bacillus, Staphylococcus aureus, Candida albicans, Bacillus cloacae, Bacillus allantoides, Morgan's bacillus (Salmonella morgani), Pseudomonas maltophila, Pseudomonas aeruginosa, Neisseria gonorrhoeae, Bacillus subtilis, Bacillus foecalis alkaligenes, Streptococcus hemolyticus B, Citrobacter, and Salmonella paratyphi C. The NAGs contain ground stalk marrow of the plant Juncus effusus L. which has been dispersed with nanosilver particles. The nanosilver particles are about 1-100 nm in diameter. Each of the nanosilver particles contain a metallic silver core which is surrounded by silver oxide. The present invention also provides a process for making the NAGs. The NAGs can be used in a variety of healthcare and industrial products. Examples of the healthcare products include, but are not limited to, ointments or lotions to treat skin trauma, soaking solutions or cleansing solutions for dental or women hygiene, medications for treating gastrointestinal bacteria infections, sexual related diseases, and eye diseases. Examples of industrial products include, but are not limited to, food preservatives, water disinfectants, paper disinfectants, construction filling materials (to prevent mold formation).
Owner:LEGEND WIN FINANCE
Immunogenic compositions for Chlamydia trachomatis
PendingUS20060034871A1Enhance immune responseAntibacterial agentsBacterial antigen ingredientsDiseaseAdjuvant
The invention relates to immunogenic compositions comprising combinations of Chlamydia trachomatis antigens and their use in vaccines. The composition may comprise at least two components, one component of which comprises Chlamydia trachomatis antigens for eliciting a Chlamydia trachomatis specific TH1 immune response and another component of which comprises antigens for eliciting a Chlamydia trachomatis specific TH2 immune response. The invention further relates to an immunogenic composition comprising a Chlamydia trachomatis Type III secretion system (TTSS) regulatory protein and a Chlamydia trachomatis Type III secretion system (TTSS) secreted protein or a fragment thereof. The invention further relates to the use of combinations of adjuvants for use with antigens associated with a sexually transmissible disease, such as Chlamydia trachomatis antigens. Preferred adjuvant combinations include mineral salts, such as aluminium salts and oligonucleotides comprising a CpG motif. The invention further provides a combination of Chlamydia trachomatis antigens comprising a Chlamydia trachomatis antigen that is conserved over at least two serovars.
Owner:NOVARTIS AG
Methods, compositions, formulations, and uses of cellulose and acrylic-based polymers
InactiveUS20050244365A1Easy to chargeLow pKaAntibacterial agentsCosmetic preparationsDisinfectantReverse transcriptase
Compositions, formulations, and methods for the treatment or prevention, or decreasing the frequency of transmission of a virus (such as human immunodeficiency virus type 1 (HIV-1), Herpes Simplex virus type 1 (HSV1), or Herpes Simplex Virus Type 2 (HSV2), or other virus), or a bacterial infection (such as Trichomonas vaginalis, Neisseris gonorrhoeae Haemopholus ducreyl, or Chlamydia trachomatis, or other bacterial species), or a fungal infection, using an anionic cellulose- or acrylic-based oligomer, polymer, or copolymer. The present invention also includes administering a therapeutically effective amount of said oligomer, polymer, or copolymer, or a pharmaceutically acceptable salt thereof, or with a pharmaceutically acceptable carrier or diluent, thereof. The invention relies on the unique biochemical substitution of the cellulose or acrylic backbone such that the resultant molecule can remain molecularly dispersed in solution (or gel or other formulation) and mostly dissociated over a wide range of physiological microenvironments, such as the low pH found within the vaginal lumen, preferably from a pH of 14 to below 3.5. These specific substitutions also impart on the resultant molecule potent antiviral, anti-bacterial, and anti-fungal properties. In addition, these compositions can be used as general disinfectants for human use such as in contact lens solutions, mouthwashes, toothpastes, suppositories, or as more generalized disinfectants found in soaps, household cleaning products, paints, water treatments modalities, or can be incorporated into cosmetic, and can be used as vehicles for drug delivery, an adjuvant in a therapeutic formulation, or as a preservative. These compounds can be delivered in a liquid or solid dosage form and can be incorporated into barrier devices such as condoms, diaphragms, or cervical caps, to help prevent the transmission of STDs. The compounds of this invention can also be used in combination therapies with other classes of antiviral, antibacterial, or antifungal agent having similar or differing mechanisms of action including, but not limited to, anionic or cationic polymers, copolymers, or oligomers, surfactants, protease inhibitors, DNA or RNA polymerase inhibitors (including reverse transcriptase inhibitors), fusion inhibitors, cell wall biosynthesis inhibitors, integrase inhibitors, or virus or bacterial attachment inhibitors.
Owner:NOVAFLUX INC +1
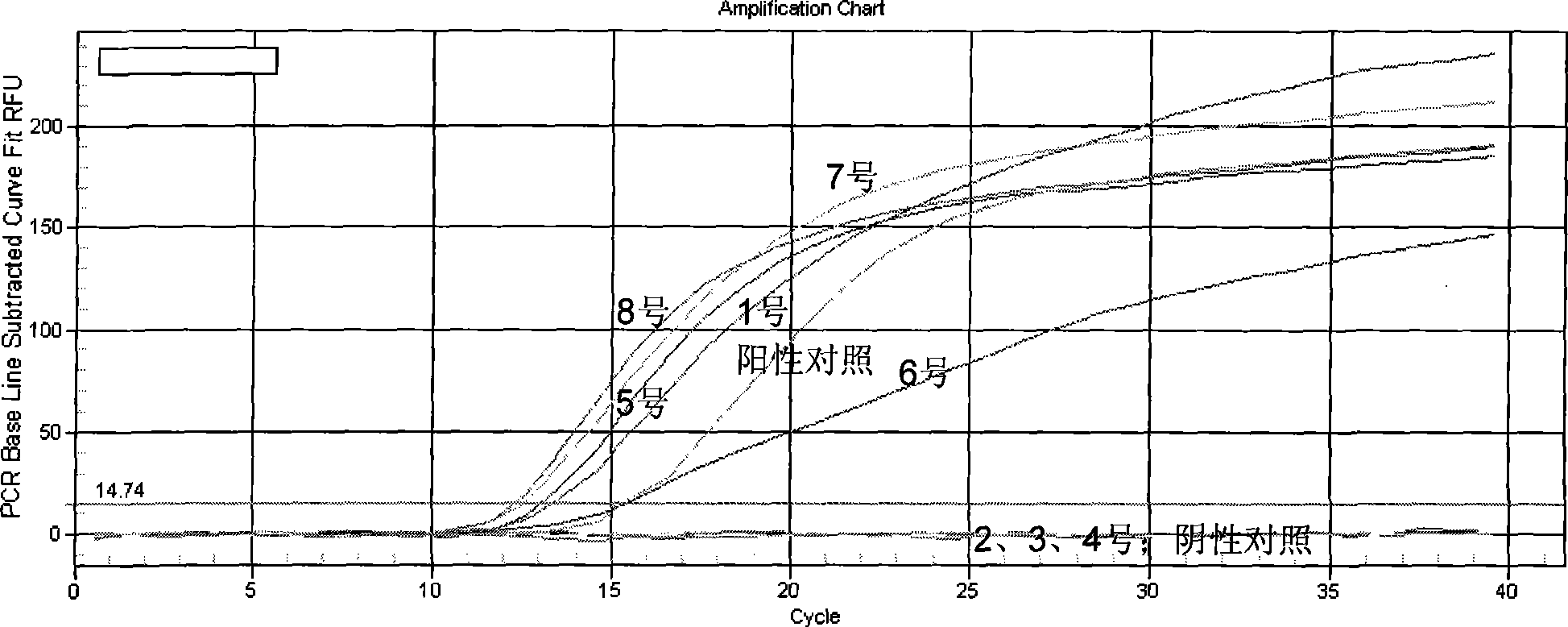
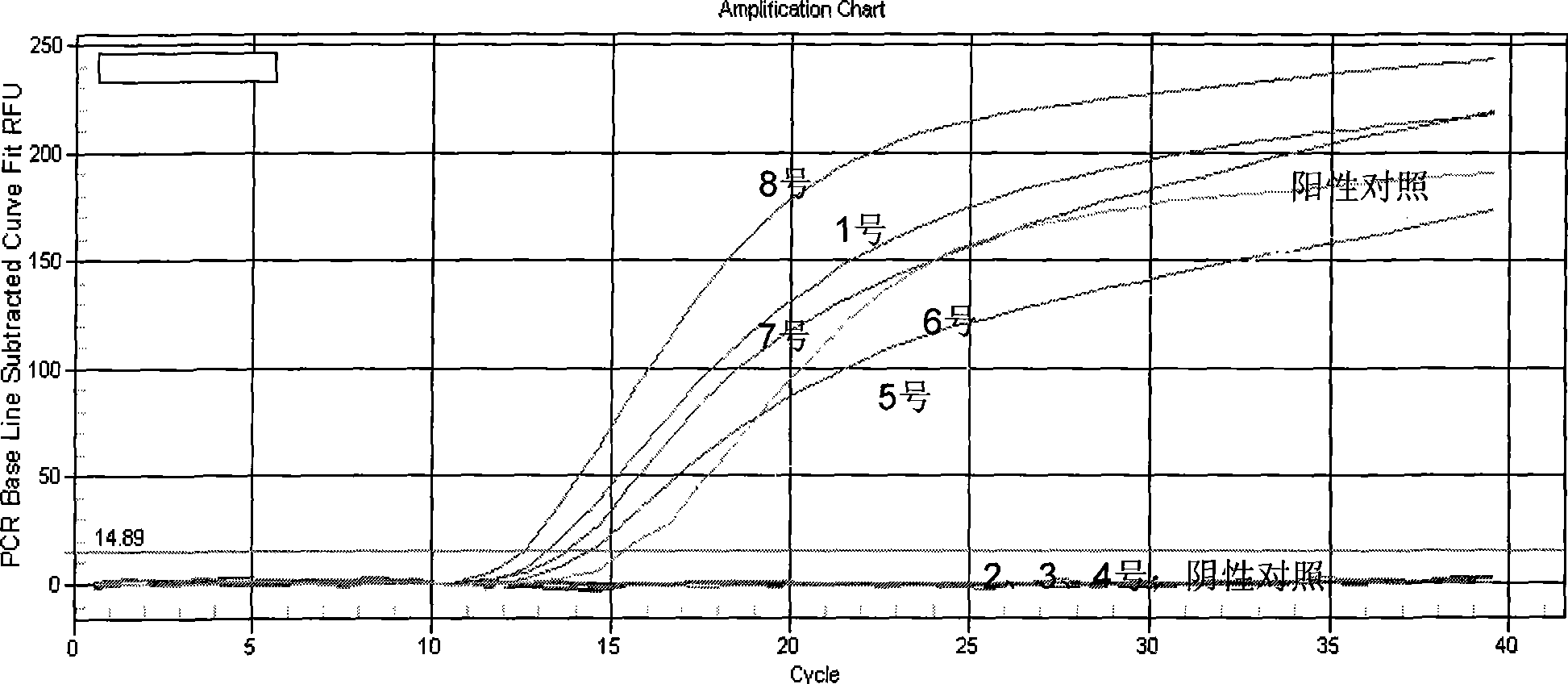
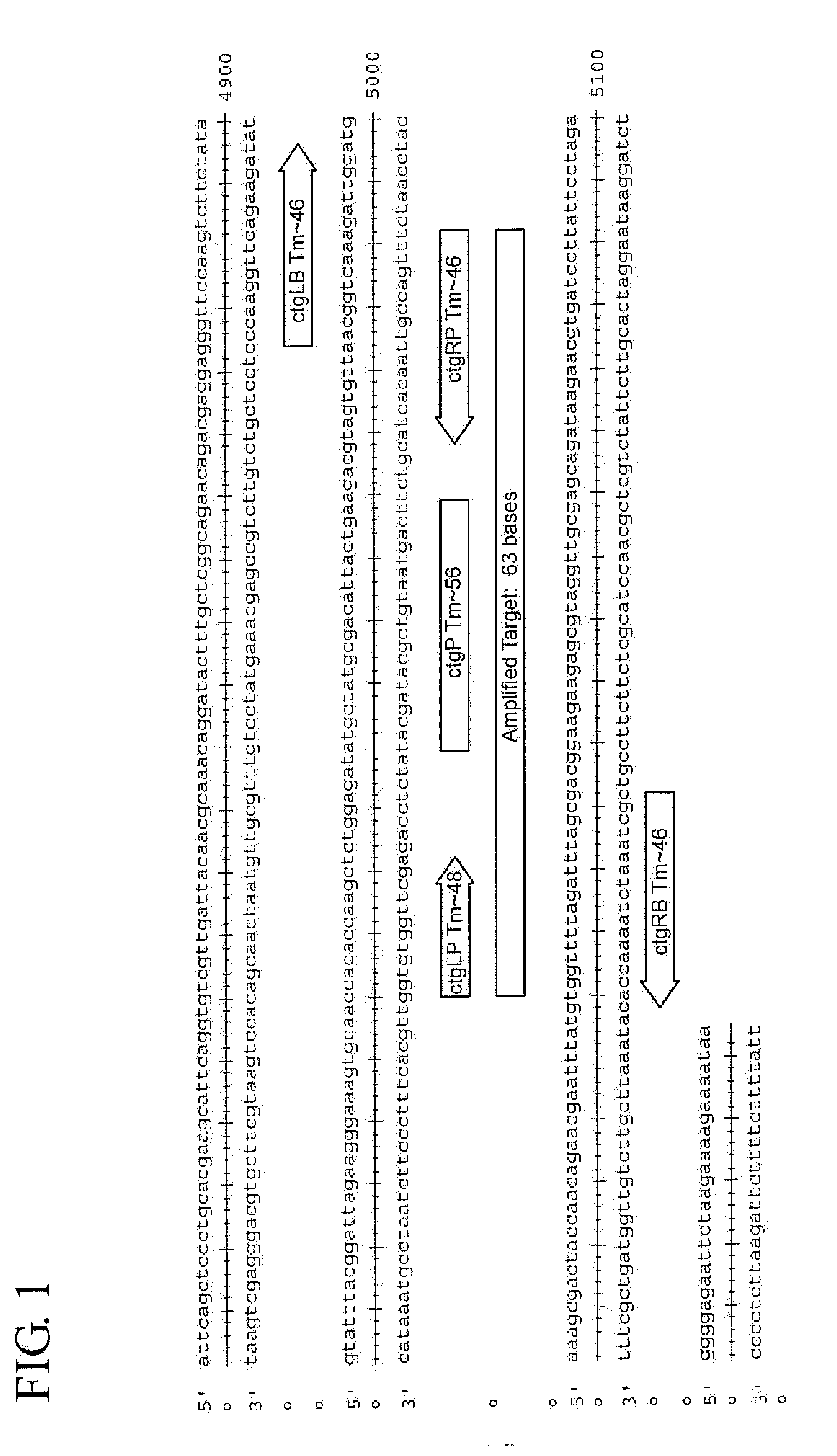
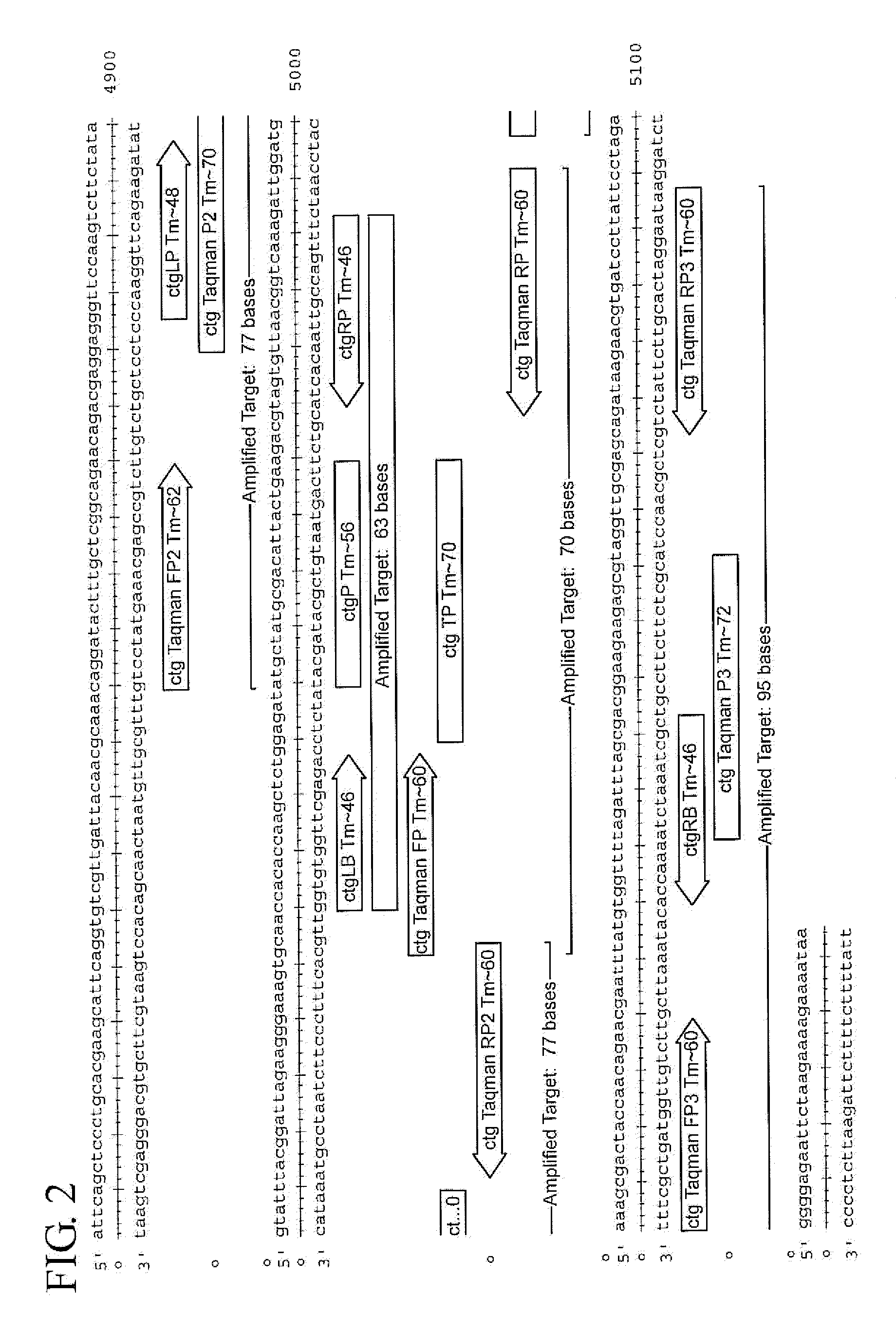
Chlamydia trachomatis nucleic acid detection kit for constant-temperature amplification by using RNA
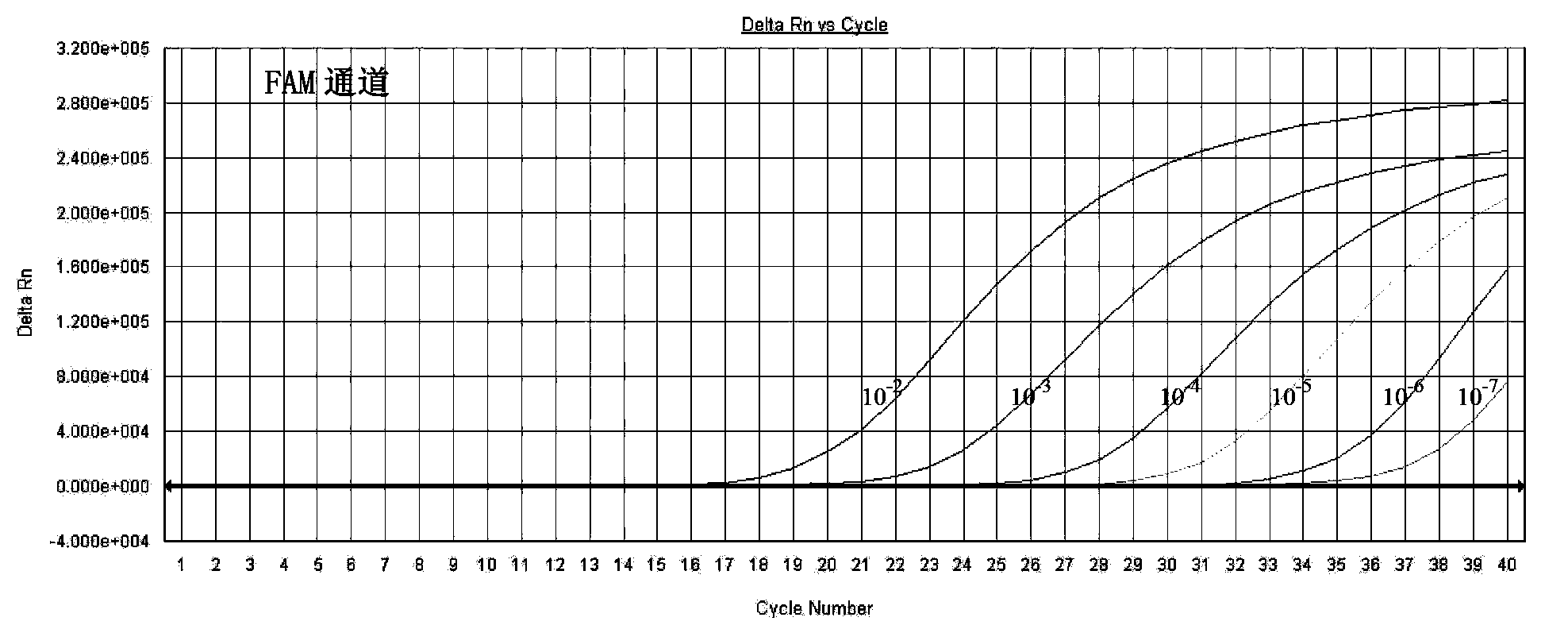
InactiveCN101509041AMicrobiological testing/measurementMicroorganism based processesChlamydia trachomatis nucleic acid detectionPositive control
The invention relates to a kit for utilizing a magnetic bead-RNA concentrating technology to extract purified target RNA as well as testing chlamydia trachomatis (CT) by using constant temperature nucleic acid simultaneous amplification detection technology (SAT). The kit comprises urine sample preservation solution, nucleic acid extracting solution, cleaning solution, CT reaction solution, CT detection solution, STA enzyme liquid, CT positive control and CT negative control. The kit has high specificity and sensitivity; furthermore, the amplified product RNA is easy for degradation in natural environment with little pollution.
Owner:SHANGHAI RENDU BIOTECH
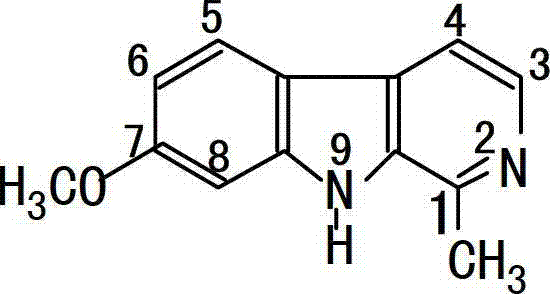
Application of Harmine derivative to preparation of antibacterial medicine
The invention discloses application of a Harmine derivative to preparation of antibacterial medicines. The bacteria is selected from Acinetobacter, Bacillus, Campylobacter, Chlamydia, Chlamydia trachomatis, Clostridium, Citrobacter, Escherichia, enterohemorrhagic escherichia coli, enteric bacteria, Enterococcus, Francisella, Haemophilus, helicobacter, Klebsiella Bacillus, Lester monocytogenes, Moraxella, Mycobacterium, Neisseria, proteus, Pseudomonas, Salmonella, shewanella oneidensis, Shigella, Stenotrophomonas, Staphylococcus, Streptococcus and Yersinia.
Owner:XINJIANG HUASHIDAN PHARMA RES
Water dispersible film
InactiveUS20050070501A1Avoid difficultyAntibacterial agentsOrganic active ingredientsDiseaseComposite film
Owner:NEW YORK BLOOD CENT
Rapidly dispersible vaginal tablet that provides a bioadhesive gel
InactiveUS20110159091A1Safe and relatively inexpensive methodAvoid spreadingAntibacterial agentsBiocideBacterial vaginosisSpiroplasma
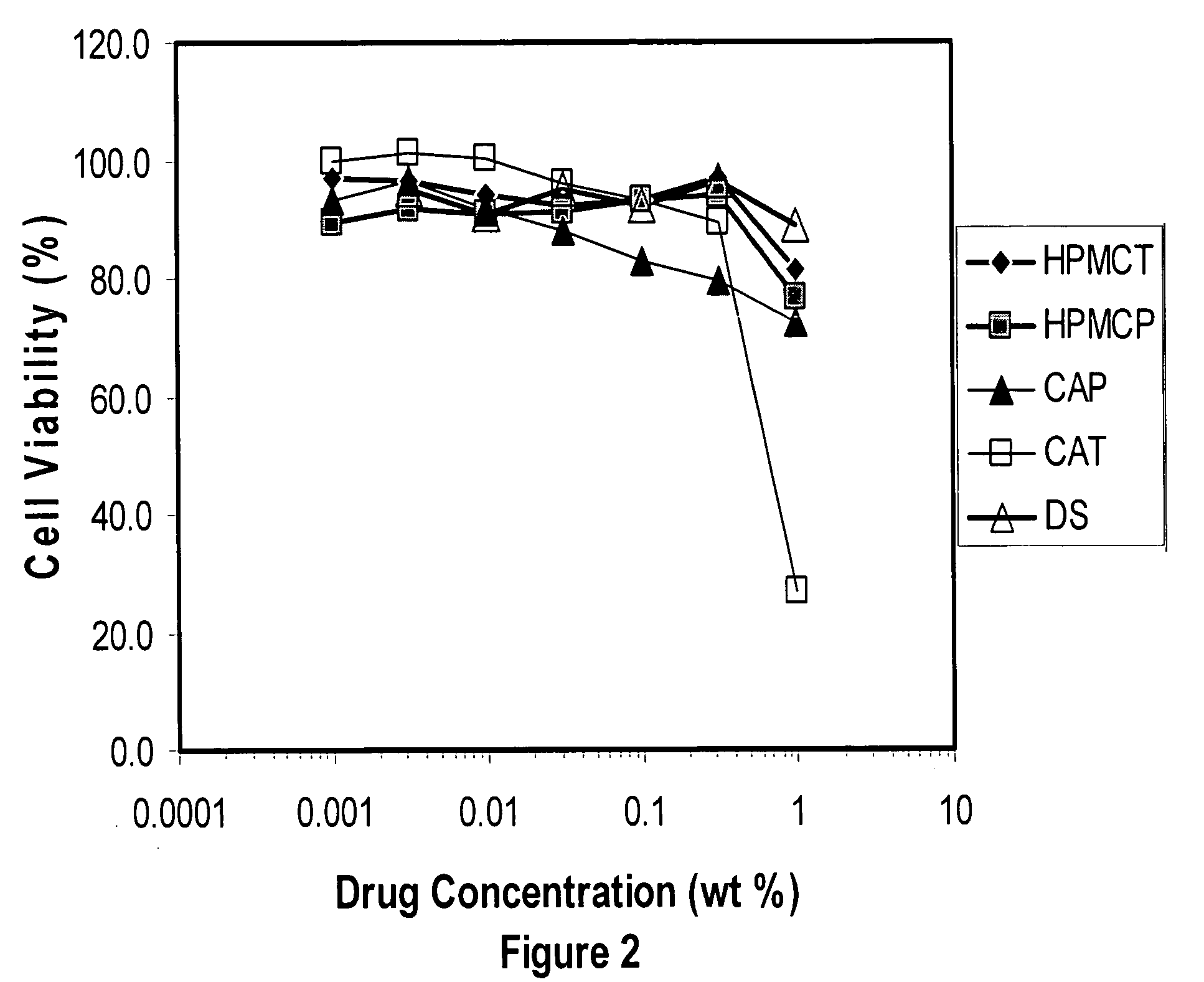
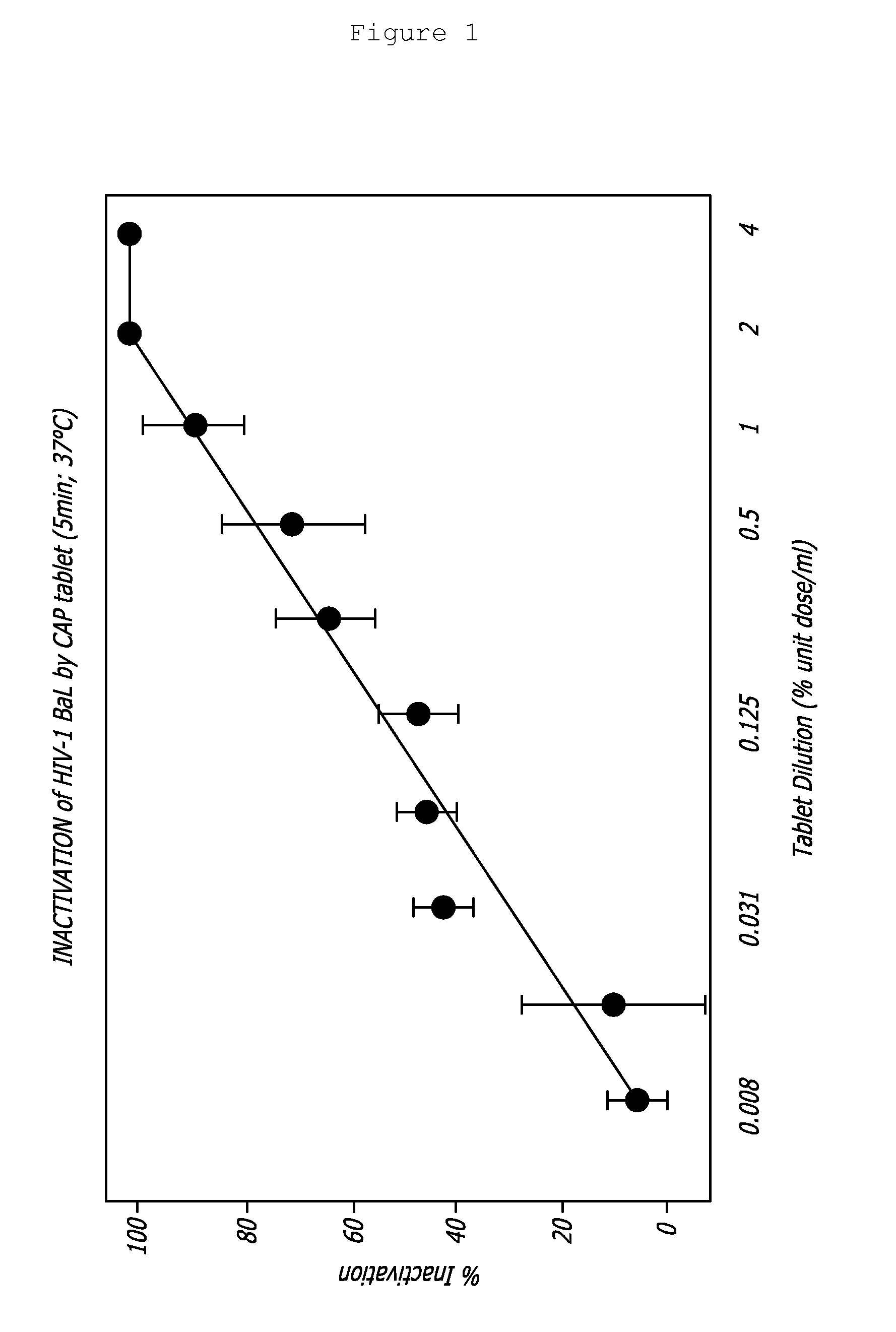
A tablet for insertion into a vagina including 0.01 to 500 mg of a vaginal medication, such as a microbicide, such as cellulose acetate 1,2-benzenedicarboxylate (CAP); 100 to 500 mg of mannitol powder; 50 to 300 mg of inert microcrystalline cellulose; 10 to 80 mg of hydroxypropyl methylcellulose; 50 to 250 mg of glycerol and optionally 2 to 4 mg of at least one preservative which protects against microbicidal contamination and discourages the growth of yeast in the vagina. The tablet which includes CAP as the vaginal medication is vaginally administered before coitus in methods for preventing the sexual transmission of HIV-1, HIV-2, herpesvirus, or an infection caused by Neisseria gonorrhoeae, Chlamydia trachomatis, Trichomonas vaginalis, Haemophilus ducreyi or Treponema pallidum. The tablet which includes CAP as the vaginal medication is vaginally administered to prevent or treat bacterial vaginosis.
Owner:NEW YORK BLOOD CENT
Methods and compositions for the detection of Chlamydia trachomatis
InactiveUS20070065837A1High stringency conditionSugar derivativesMicrobiological testing/measurementOmpa geneChlamydia trachomatis
The present invention provides novel methods for determining the presence or absence of Chlamydia in a patient, as well as diagnostic kits useful in practicing the methods of the invention. The methods of the invention are based on nucleic acid amplification reactions to detect both Chlamydia genomic and cryptic plasmid sequences. In one embodiment, the methods involve using nucleic acid primers to specifically amplify the Chlamydia trachomatis ompA gene and cryptic plasmid. These methods provide both enhanced reliability and sensitivity of detection, thereby providing an accurate determination of the presence or absence of Chlamydia trachomatis in a patient.
Owner:QIAGEN DIAGNOSTICS
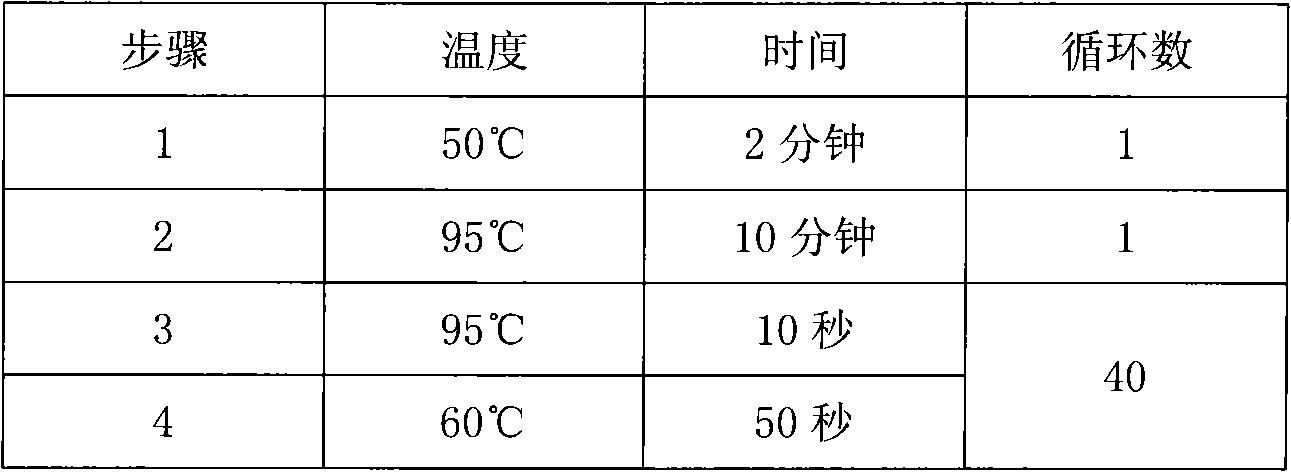
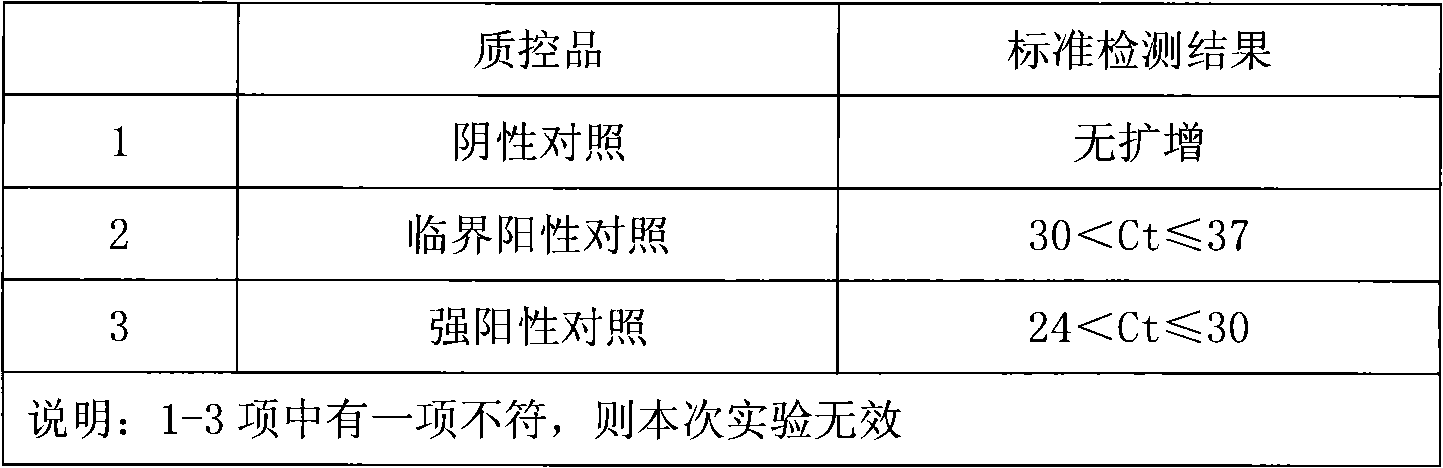
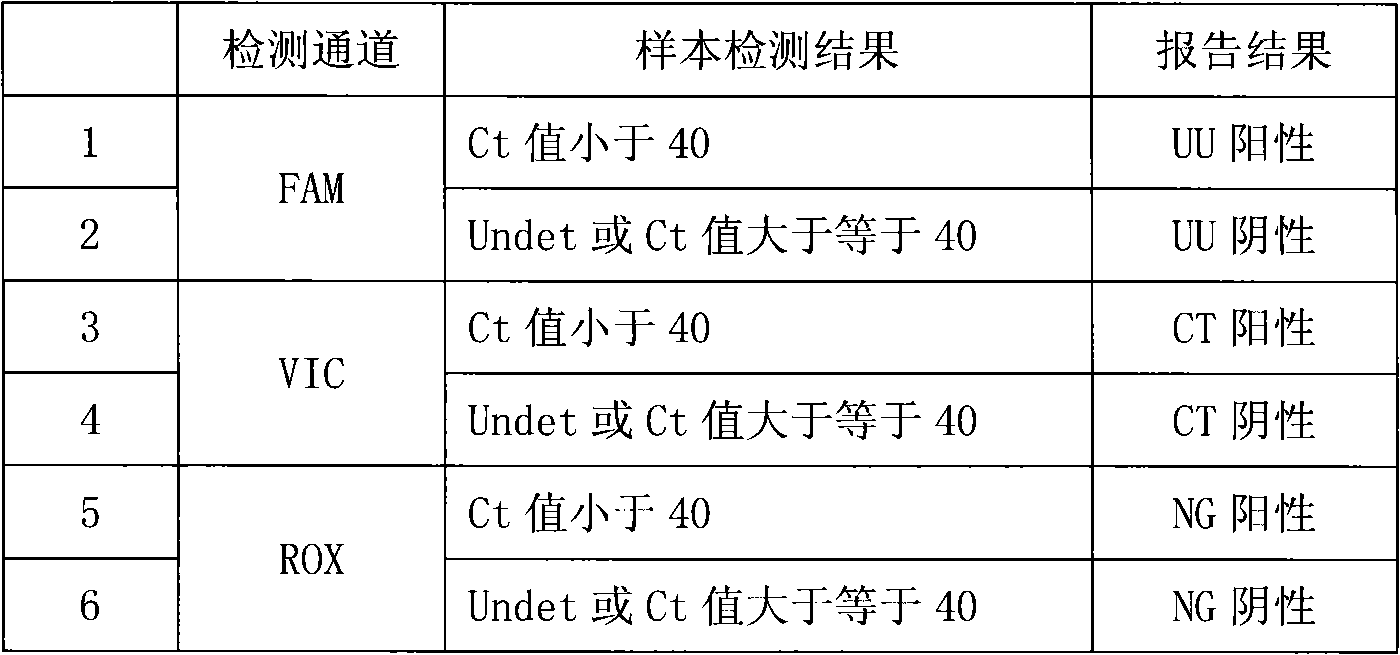
Fluorescence PCR method for diagnosing infection of Chlamydia trachomatis, neisseria gonorrhoeae and ureaplasma urealyticum
ActiveCN101613763AMicrobiological testing/measurementMicroorganism based processesForward primerFluorescence
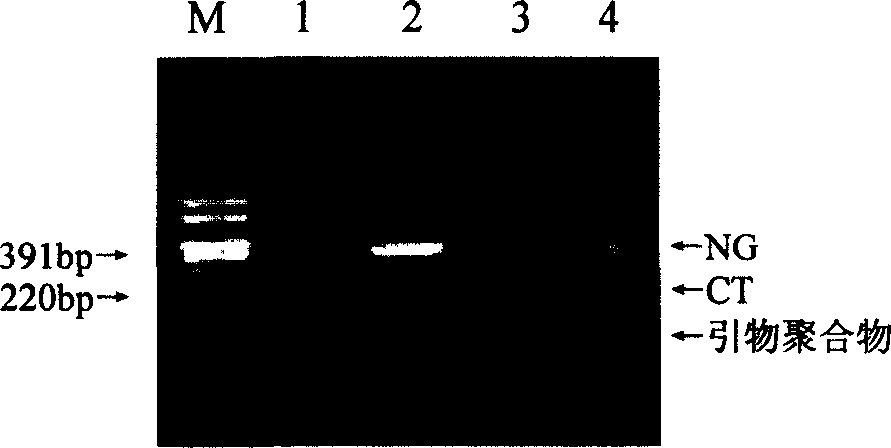
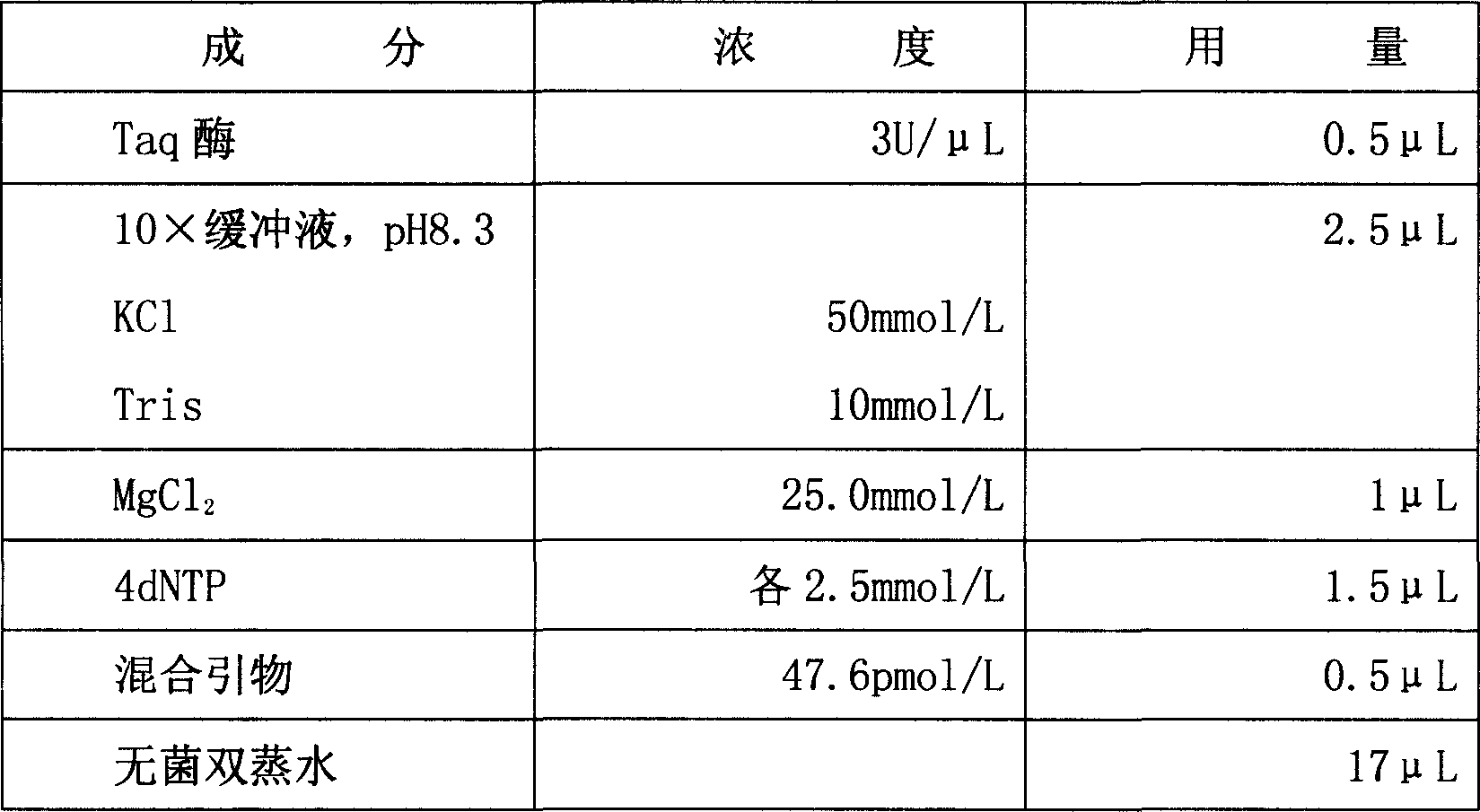
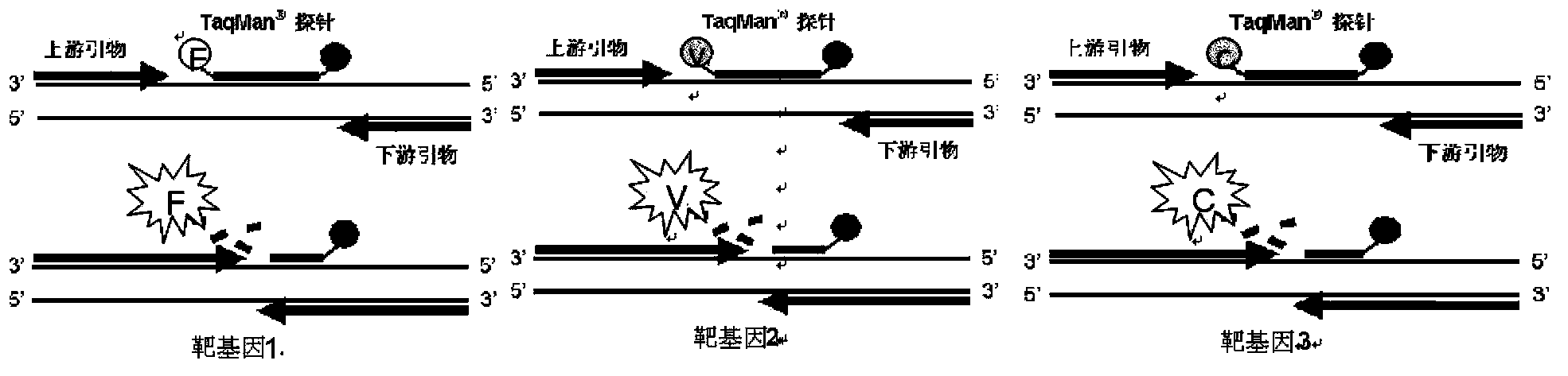
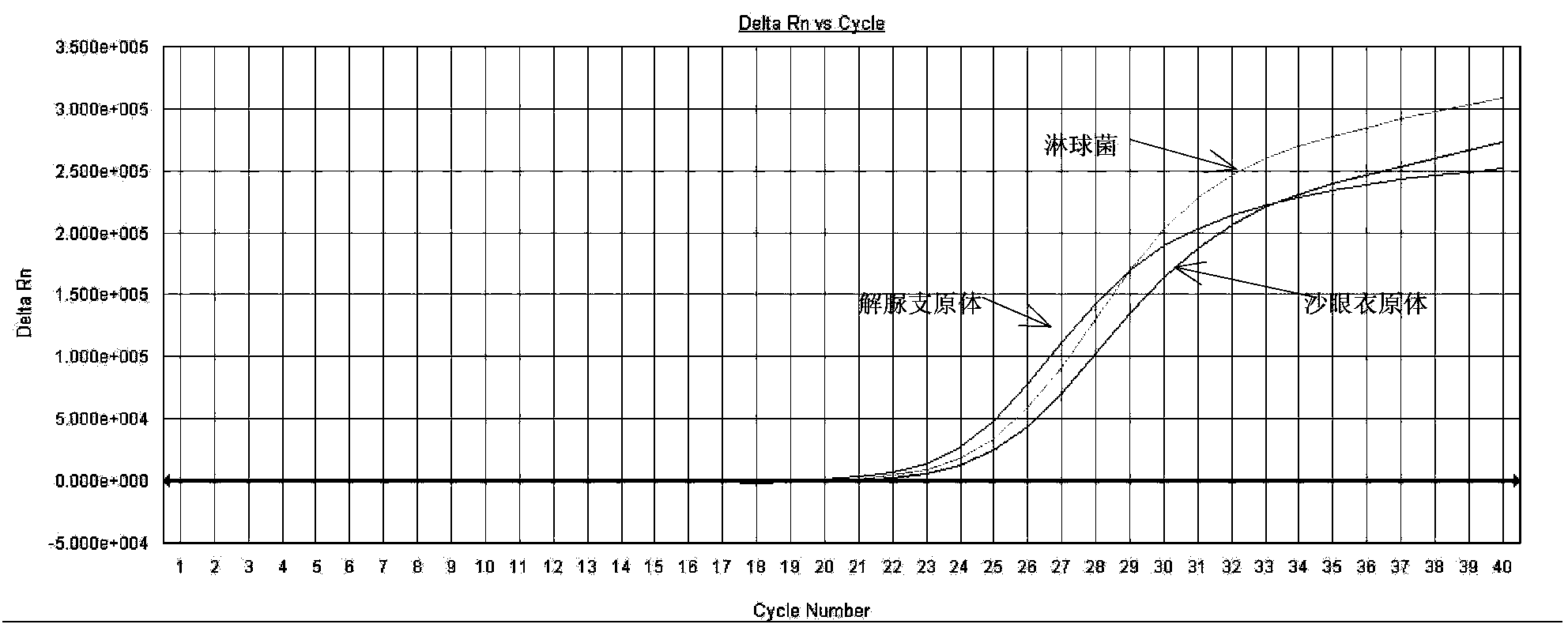
The invention relates to a fluorescence PCR (polymerase chain reaction) detection method for diagnosing infection of Chlamydia trachomatis (CT), neisseria gonorrhoeae (NG) and ureaplasma urealyticum (UU), which belongs to the field of nucleic acid in vitro diagnosis. The method comprises a polymerase chain reaction (PCR) system based on fluorescence PCR technology, contains a forward primer and areverse primer aiming at the CT / the NG / the UU and a fluorescent probe, and can detects DNA of three pathogens such as the CT, the NG, the UU and the like simultaneously in a reaction tub under suitable PCR condition. The method can diagnosing the infection of the CT / the NG / the UU in a clinical sample simply, conveniently and rapidly, has high sensitivity and specificity, and has important clinical value to early control and prevention of relevant genitourinary tract infections, blocking of an infection sources, and infection reduction of related pathogen.
Owner:CITY UNIVERSITY OF HONG KONG
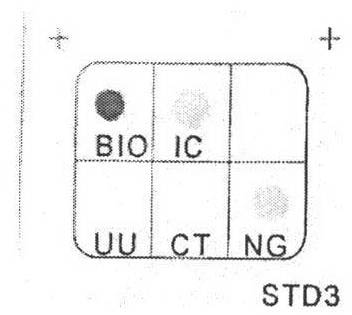
Quality control substance for detecting chlamydi trachomatis
InactiveCN101363041AReduce generationStable storageMicrobiological testing/measurementVector-based foreign material introductionSubstance useBiotechnology
The invention discloses a quality control substance used for testing chlamydia trachomatis, belonging to the field of clinical ecsomatics and biotechnology. The quality control substance used for testing chlamydia trachomatis contains nucleotide sequence or complementary sequence thereof which is shown by sequence tables of SEQ ID No.1 to SEQ ID No.4. The quality control substance has the advantages that (1) by adopting overlapping PCR and the method of double enzyme digestion, a simple and effective method for connecting and constructing segments with a plurality of intervals into the same carrier is set up, and the probability of lower efficiency in long distance PCR is avoided by directed cloning, so that the two methods can be combined for use to make up for the deficiencies of each other. (2) Clinical specimen which can be simulated and has no biological infection fatalness is successfully obtained, and the quality control substance for testing polymerase chain reaction of the chlamydia trachomatis can be produced in large quantity, is stored stably and has better applicability.
Owner:BEIJING HOSPITAL
Methods and compositions for immunizing against Chlamydia infection
InactiveUS7964200B2Reduce decreaseReduce adhesionAntibacterial agentsPeptide/protein ingredientsNucleotideChlamydia trachomatis Antigen
The present invention relates, in part, to methods and compositions for immunizing against infection by Chlamydia trachomatis. The methods and compositions rely, in part, on administering an immunogenic composition comprising one or more peptides derived from C. trachomatis major outer membrane protein (MOMP) to a subject to be immunized. In some embodiments, the compositions comprise a chimeric immunogen comprising a receptor binding domain, a translocation domain, and a Chlamydia trachomatis antigen. Polynucleotides encoding the chimeric immunogens, expression vectors comprising the polynucleotides, and kits comprising the compositions are also provided.
Owner:CHILDREN S HOSPITAL &RES CENT AT OAKLAN
Compounds and methods of early diagnosis of cervical cancer and genital condyloma with HPV, CHSP60 tumor suppressor h-ras, k-ras and PTEN derived peptides modified
InactiveUS20060154238A1Easy to synthesizeStrong specificityAntibody mimetics/scaffoldsViral antigen ingredientsAbnormal tissue growthHuman papillomavirus
An isolated sequence or peptide isolated from an E2, E4, E6, E7 early or late coding region of human papillomavirus (HPV) that is soluble in aqueous medium, and characterized by a linkage to another protein sequence or peptide isolated from the E2, E4, E6, E7 early or late coding region of HPV by a spacer sequence, wherein the isolated protein sequence or peptide consists of more than 50% hydrophilic amino acids, and is recognized by a specific antibody of HPV. Also disclosed are isolated protein sequences or peptides from Harvey Ras (H-Ras), Kirsten Ras (K-Ras), and phosphatase and tensin homologue (PTEN) tumor suppressor proteins and Chlamydia trachomatis heat shock protein 60 (CHSP60 groEL1) and methods for detecting or diagnosing cancer or cellular abnormalities.
Owner:HU YAO XIONG
Compounds and methods of early diagnosis of cervical cancer and genital condyloma with HPV, CHSP60 tumor suppressor H-Ras, K-Ras and PTEN derived peptides modified
InactiveUS7314630B2Easy to synthesizeStrong specificityAntibody mimetics/scaffoldsViral antigen ingredientsHuman papillomavirusADAMTS Proteins
Owner:HU YAO XIONG
Immunisation against Chlamydia trachomatis
InactiveUS20060216308A1Stable maintenanceAntibacterial agentsBacterial antigen ingredientsChlamydia trachomatisAntigenic protein
The present invention provides antigenic proteins of Chlamydia trachomatis. The proteins of the present invention are useful for eliciting an immune response to Chlamydia in a patient. For example, an effective amount of protein of the present invention or fraction thereof may be administered to a patient for eliciting a Chlamydia specific immune response. In another example, a method of raising an antibody specific for Chlamydia trchomatis elementary bodies (EB) is provided in which a protein of the present invention is administered to a patient.
Owner:CHIRON CORP
Fluorescence quantitative kit PCR for quick testing chlamydia trachomatis
InactiveCN1873023AQuantitatively accurateThe detection process is fastMicrobiological testing/measurementFluorescenceBiology
This invention discloses a fluorescent PCR test kit for quantitatively detecting Chlamydia trachomatis, which is composed of DNA lysis solution, fluorescent quantitative PCR reaction solution, positive standard template and negative standard sample. The fluorescent quantitative PCR reaction solution comprises forward and reverse primers and fluorescent probes. The test kit has such advantages as high specificity, high sensitivity, high accuracy, simple operation and good repeatability.
Owner:WUHAN BIOTECH GENE ENG
Chlamydia trachomatis antigens for vaccine and diagnostic use
InactiveUS20090304722A1High expressionImprove abilitiesAntibacterial agentsOrganic active ingredientsGamma interferonWhole cell lysate
The present invention is related to antigens from Chlamydia trachomatis which are recognized by specific antibodies from individuals infected with Chlamydia or which can induce T cells from the same individuals to secrete gamma-interferon. The T cell reactive antigens are present in a whole-cell lysate and have apparent molecular weights of 5-12, 16-20, 25-35 and 58-74 kDa as determined by SDS-PAGE. The antigens of the invention are useful in vaccines but also as diagnostic compositions.
Owner:STATENS SERUM INST
Primer and probe sequences for detecting chlamydia trachomatis
ActiveUS20080299567A1Easy to detectSugar derivativesMicrobiological testing/measurementChlamydia trachomatisDisease cause
Owner:ABBOTT MOLECULAR INC
Method for detecting nucleic acids
InactiveUS20120040349A1Microbiological testing/measurementChlamydia trachomatisNucleotide sequencing
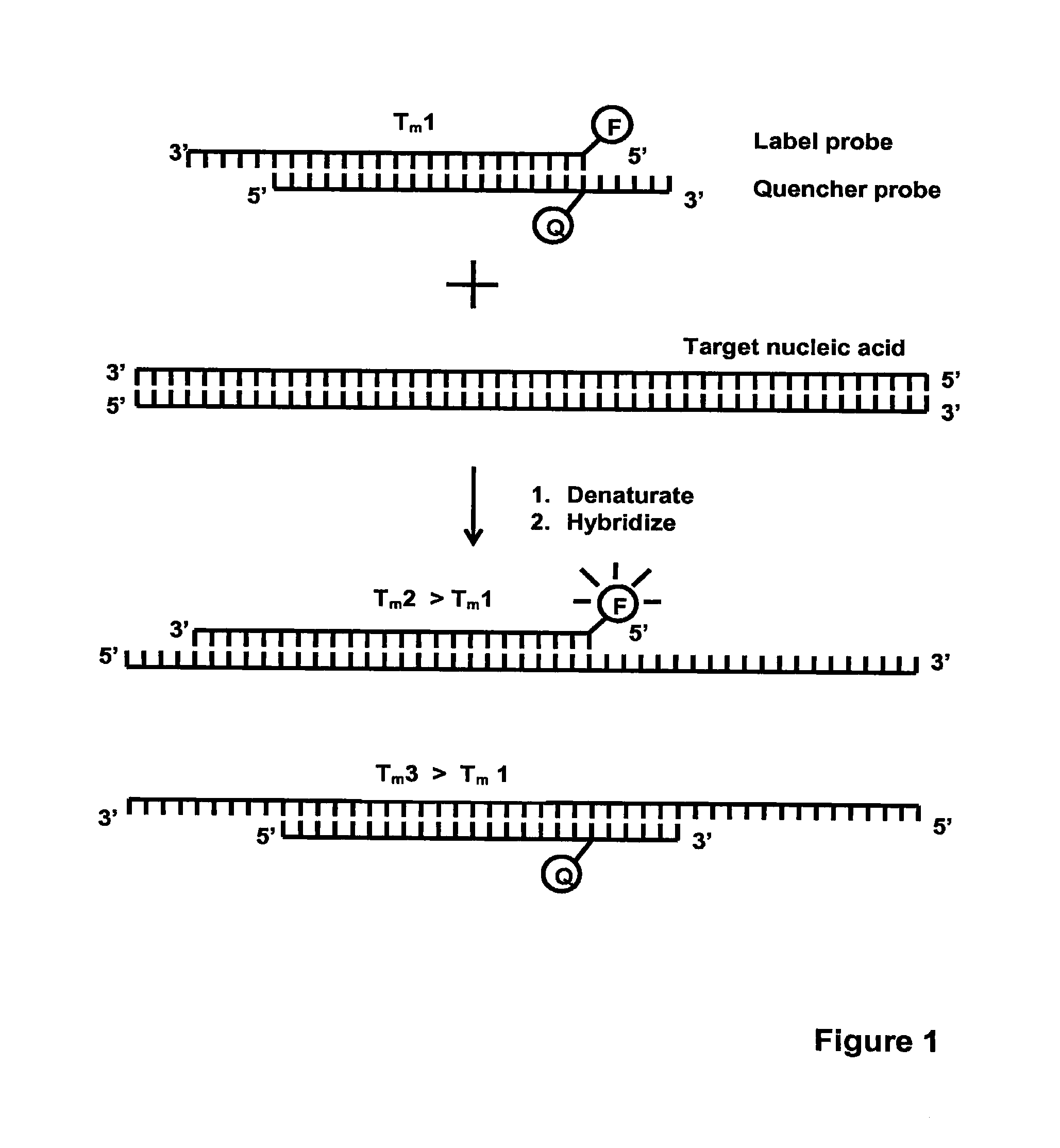
Method for detecting nucleic acids which employs a double-stranded oligonucleotide probe containing i) a first probe including a first label moiety, and ii) a second probe partially complementary with the first probe and including a second label moiety capable of interacting with the first moiety when brought in close proximity with each other, the second moiety being a quencher or acceptor of emission of the first moiety. The first or second probe includes a sequence complementary to that of a target nucleotide, and the second or first probe, respectively, includes a sequence complementary to a complement of the target nucleotide sequence of the nucleic acid to be detected. Oligonucleotides for determining Chlamydia trachomatis are also disclosed.
Owner:ABACUS DIAGNOSTICA OY
Primer, probe and method for detecting human urological genital tract causal agent
InactiveCN101117646AImprove early detection rateSave operating timeMicrobiological testing/measurementDiseaseChlamydia trachomatis
The present invention discloses a guiding object and a probe for testing the human urogenital tract pathogens, and a method for testing the human urogenital tract pathogens using the guiding object and the probe. The present invention can test three different pathogens including Neisseria gonorrhoeae, mycoplasma ureaplasma and chlamydia trachomatis in a same reaction tube simultaneously. The present invention not only can increase the early detection rate of the sexually transmitted diseases, but also can reduce the operating time of the medical personnel, lower the cost, and lighten the economic burden of the patient.
Owner:SHANGHAI SHENYOU JIANHAI BIOLOGICAL TECH LIABILITY
PCR detection reagent kit for sexual disease mixed infection
InactiveCN1865451AStrong specificityMicrobiological testing/measurementImmunodeficiency virusOidiomycin
The invention discloses a synchronizing detection PCR agent box (hepatitis B virus, human immunodeficiency virus, human breast tumor virus, lues helicoid, human cytomegalovirus, simple herpesvirus, white oidiomycin, urea-dissolving mycoplasma, gonotoxin and Chlamydi trachomatis), which consists of blood type and secretion stains type. The user adds predisposed sample in the enlarging pipe to start enlarging reaction, which finishes detecting work simply and rapidly. The invention can detect ten venereal disease causal agents, which is fit for customs quarantine, prevailing monitor and hospital screen examination.
Owner:广州中医药大学热带医学研究所
Triple nucleic acid detection kit for neisseria gonorrhoeae / ureaplasma urealyticum/ chlamydia trachomatis
ActiveCN103409508ASimple and fast operationAvoid pollutionMicrobiological testing/measurementFluorescence/phosphorescenceSynechococcusPositive control
The invention discloses a triple nucleic acid detection kit for neisseria gonorrhoeae / ureaplasma urealyticum / chlamydia trachomatis. The kit comprises an RT-PCR (Reverse Transcription-Polymerase Chain Reaction) reaction liquid, an enzyme mixed liquid, a triple reaction liquid for neisseria gonorrhoeae / ureaplasma urealyticum / chlamydia trachomatis, positive control and negative control. The kit disclosed by the invention overcomes the deficiencies that in the prior art, neisseria gonorrhoeae / ureaplasma urealyticum / chlamydia trachomatis is poor in specificity, lower in sensitivity and the like, effectively prevents pollution, has the advantages of high sensitivity, good specificity, strong repeatability, quick and objective detection result and the like, and has good application prospect for detecting neisseria gonorrhoeae / ureaplasma urealyticum / chlamydia trachomatis.
Owner:JIANGSU BIOPERFECTUS TECH CO LTD
Combination detection gene chip kit for three kinds of genitourinary infection pathogen
InactiveCN101121946AReduce the number of detection reactionsReduce testing costsMicrobiological testing/measurementBiotechnologyPositive control
The invention relates to a combined test gene chip reagent kit for three urogenital tract infection pathogens, pertaining to the field of biotechnology. The gene chip consists of 18 sites, i.e. 5 specific and conservative test-target sequences of each pathogen of chlamydia trachomatis, mycoplasma urealytium and gonorrhoeae are taken as testing sites, and 2 positive control sites and 1 negative control site are added; PCR method is adopted to expand testing-target sites and DNA segments of the control sites to act as a probe and fixed on the peridium slide to achieve a test gene chip. The matrix of the chip is 9X9, each checking site is repeated by 4 times, and arranged evenly. The reagent kit is capable to meet the requirement of all reagents during the testing process. The testing method involves the combination of PCR expansion, molecular hybridization and fluorescent labeling. The invention has the advantages that the testing cost is low, pollution to testing samples is removed, the specificity and flexibility of testing are excellent. The practical clinic application proves that the flexibility of the invention is as high as 97 to 99.7 percent, and 99 to 99.7 percent for specificity.
Owner:昆明云大生化科技有限责任公司
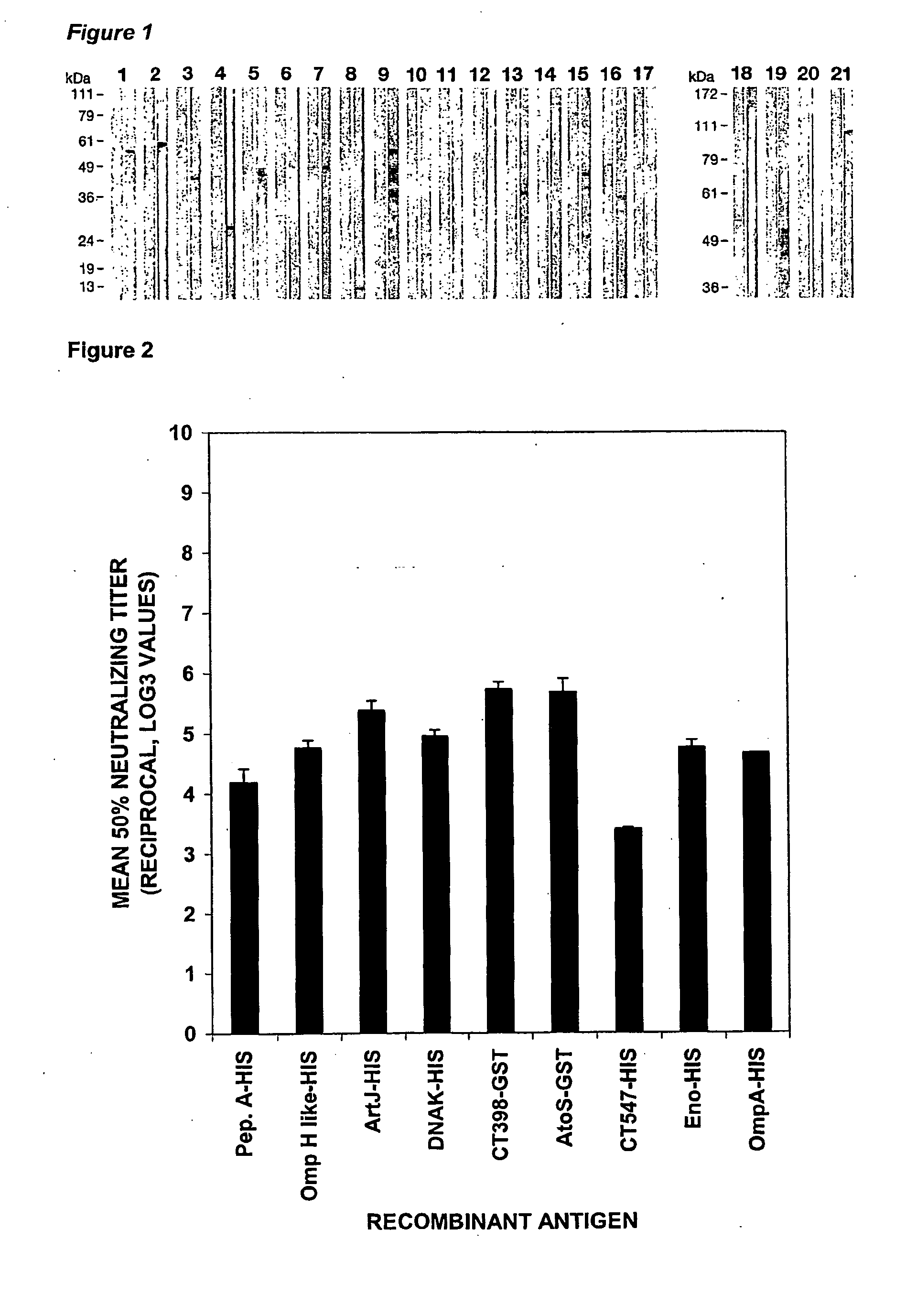
Immunogenic compositions for chlamydia trachomatis
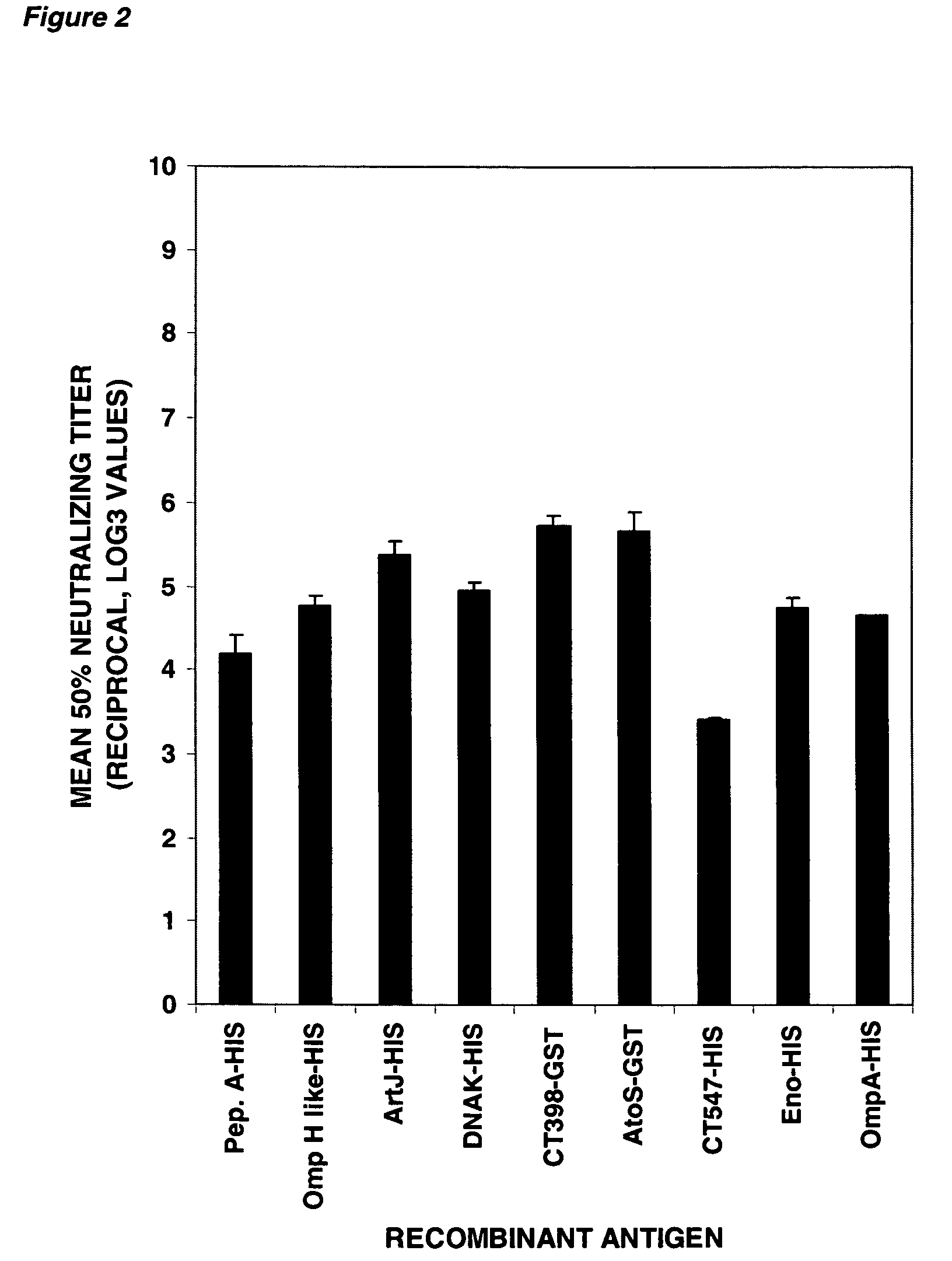
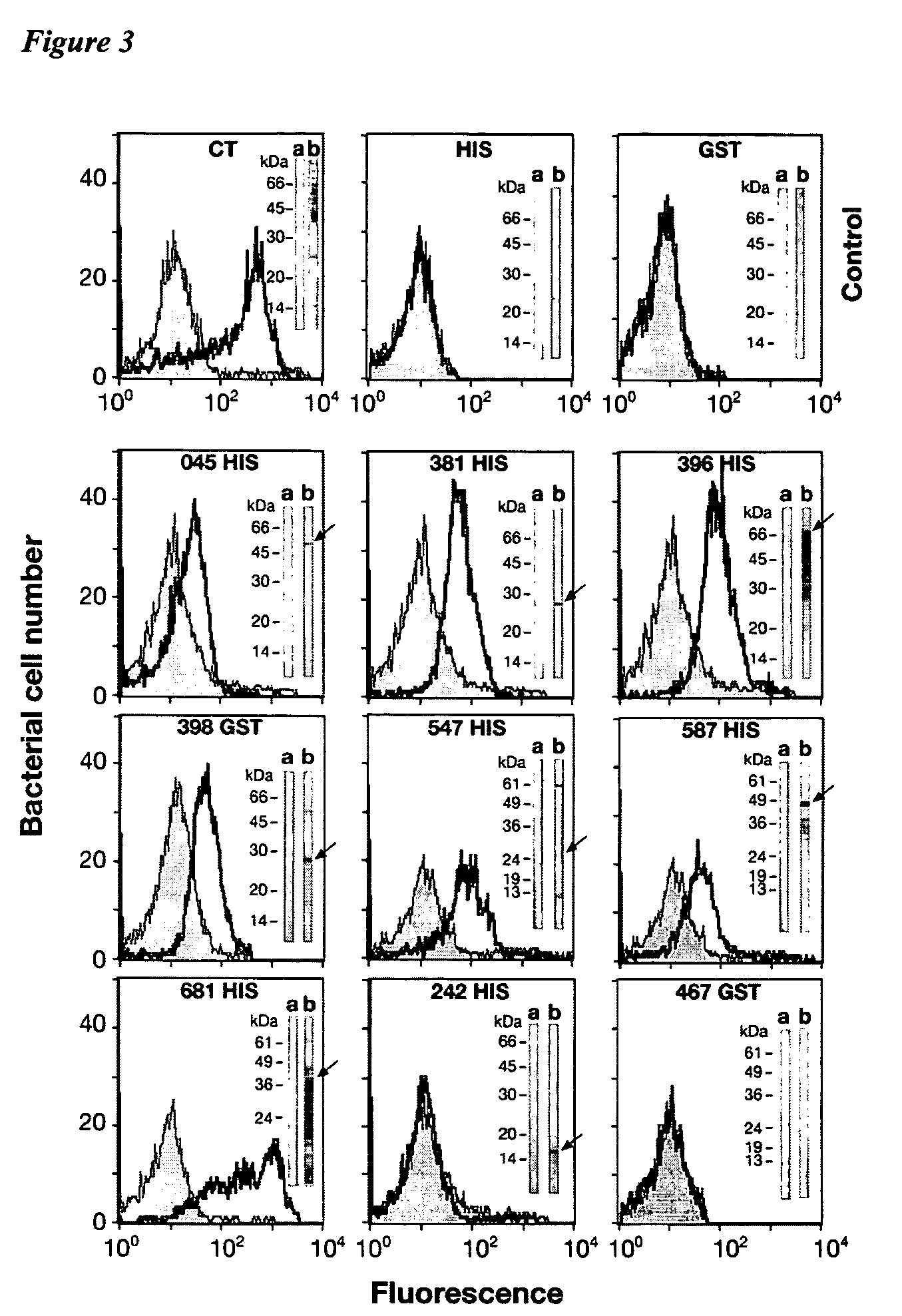
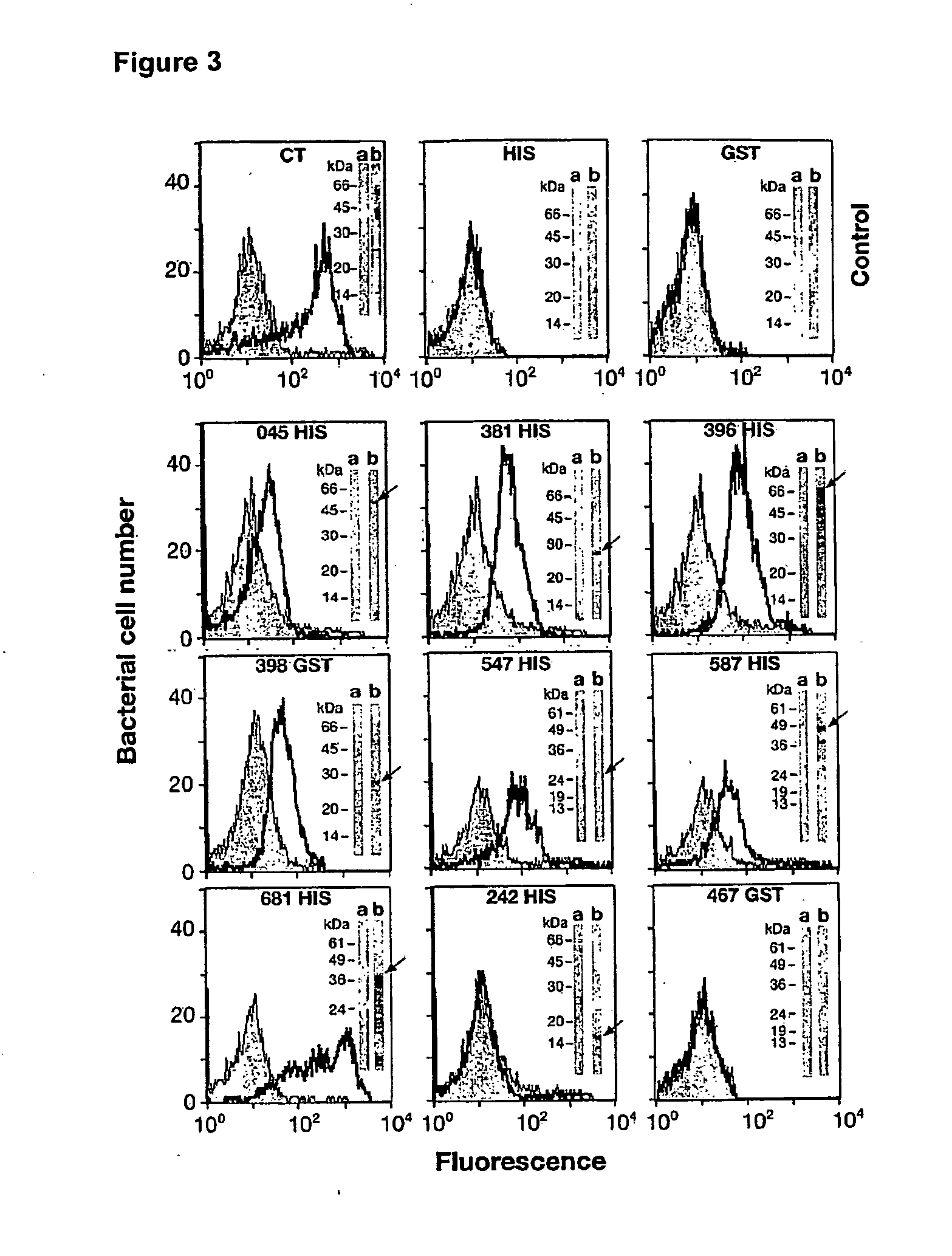
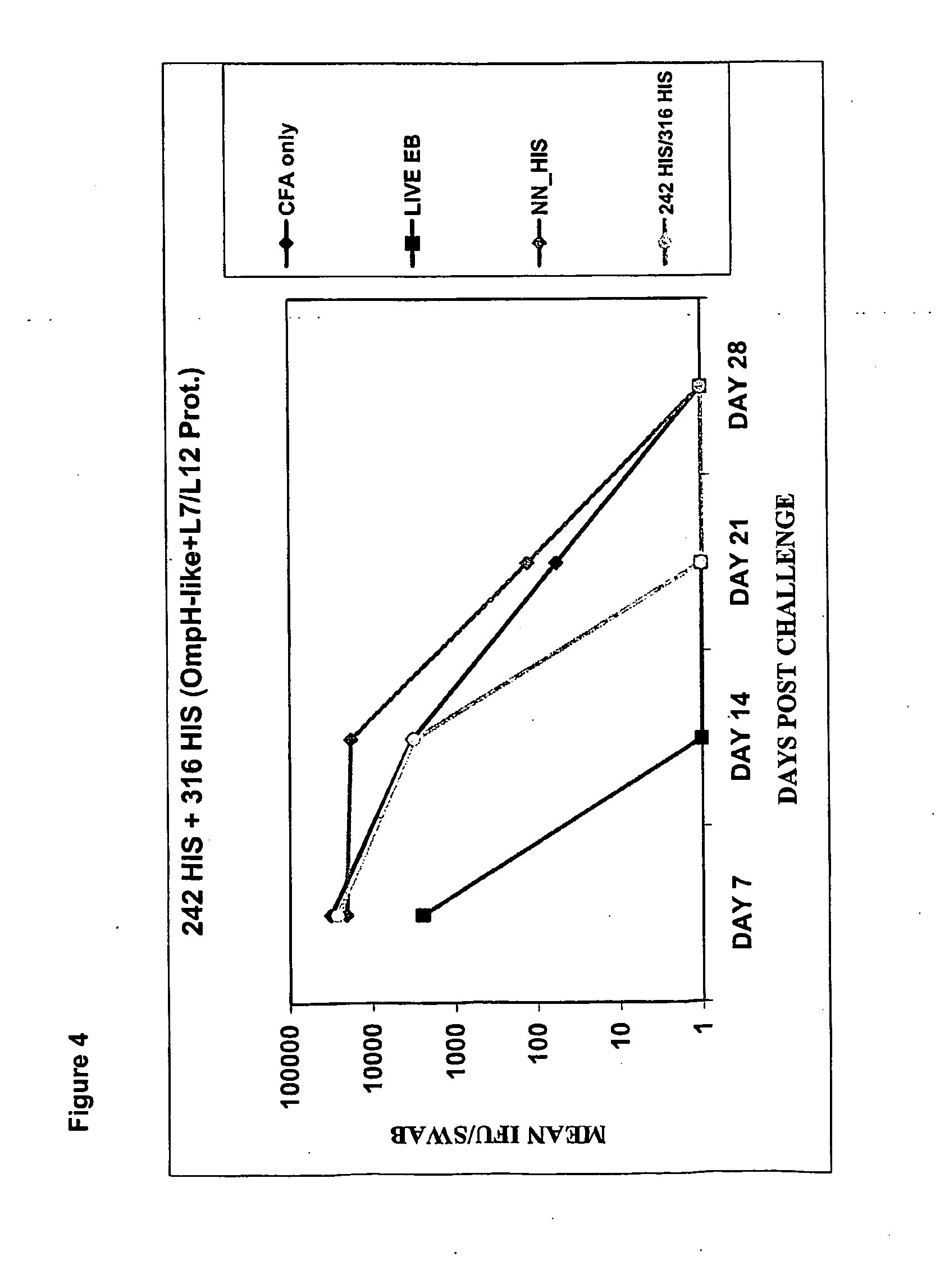
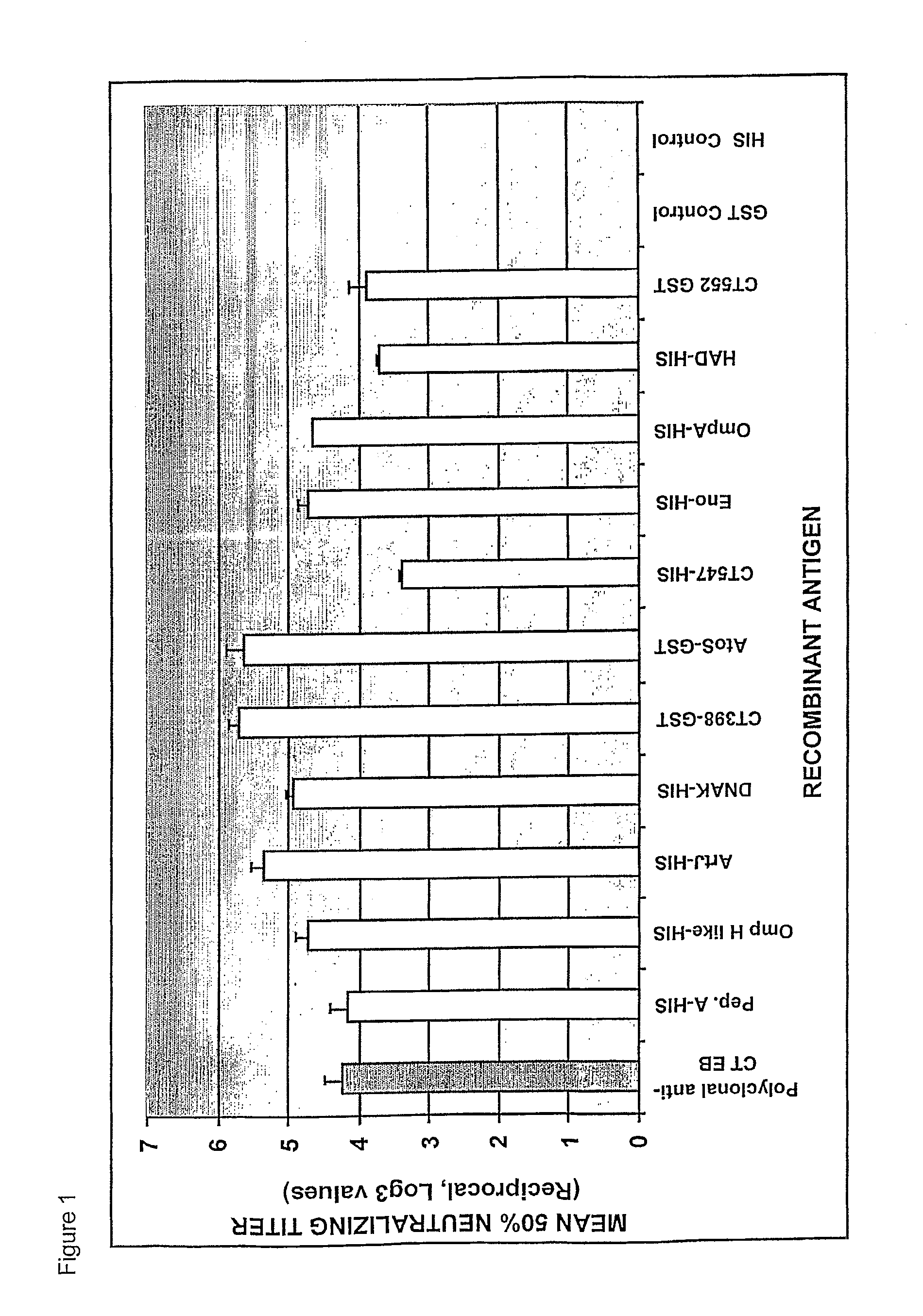
The invention relates to compositions comprising combinations of Chlamydia trachomatis antigens and their use in vaccines. Specific combinations may be selected from a first antigen group of PepA, LcrE, ArtJ, DnaK, and CT398, and a second antigen group of PepA, LcrE, ArtJ, DnaK, CT398, OmpH-like, L7 / L12, OmcA, AtoS, CT547, Eno, HtrA and MurG. The invention further relates to the use of combinations of adjuvants for use with antigens associated with a sexually transmissible disease, such as Chlamydia trachomatis antigens. Preferred adjuvant combinations include mineral salts, such as aluminium salts and oligonucleotides comprising a CpG motif.
Owner:CHIRON CORP
Nano silver clean-keeping sterilized shower cream and manufacture method thereof
InactiveCN101416924AImprove the bactericidal effectImprove repair effectCosmetic preparationsToilet preparationsShower gelChemistry
The invention discloses a nano-silver cleaning sterilizing shower gel which is prepared by the following materials according to weight percentage: 15 percent to 25 percent of surfactant (AES), 8 percent to 12 percent of mild surfactant, 8 percent to 12 percent of amphoteric surfactant, 0.001 percent to 0.01 percent of nano-silver, 0.1 percent to 0.3 percent of chelating agent, 0.1 percent to 0.2 percent of citric acid (Acd), 0.2 percent to 1 percent of flavor, 50 percent to 70 percent of distilled water, 0.1 percent to 0.2 percent of preservative and 1 percent to 3 percent of thickener. The preparation method is as follows: the nano-silver particles are dissolved in the distilled water and uniformly mixed with the chelating agent after floating particles are filtrated; then the mixture is heated to the constant temperature of 80 DEG C and the surfactant is added into the mixture and uniformly mixed; after the mild surfactant is added into the mixture and uniformly mixed, the amphoteric surfactant is added into the mixture and the mixture is cooled to 45 DEG C; the citric acid is added into the mixture and uniformly mixed, and the pH value is adjusted to be between 5.5 and 7.0; during the cooling, the flavor, the preservative, the thickener and pigments are added in sequence and blended while being added until the temperature drops to the room temperature. The shower gel has strong bactericidal function to chlamydia trachomatis and the neisseria gonorrhoeae of sexually transmitted diseases.
Owner:卓鸿思
Gene chips for detecting of pathogens of sexually transmitted diseases and reagent kit for detecting
ActiveCN101407836ATimely diagnosisImprove throughputMicrobiological testing/measurementDiseaseMycoplasma hominis
The invention provides a gene chip used for detecting the common pathogens of sexually transmitted diseases and a kit used for detection, wherein, the gene chip includes a solid phase vector and an oligonucleotide probe fixed on the solid phase vector; the oligonucleotide probe mainly comprises a DNA segment or a complementary DNA segment thereof which is selected from Diplococcus gonorrhoeae, Ureaplasma urealyticum and the 16S rRNA gene of M.hominis, the outer membrane protein gene (ompA gene) of a Chlamydia trachomatis, the glycosidoprotein B gene (gB gene) of a herpes simplex virus (HSV) and the L1 gene of a papilloma virus (HPV). The gene chip and the kit can be utilized for achieving the goal of detecting the common pathogens of sexually transmitted diseases; the gene chip and the kit for detection are simple and convenient to be operated, have high flux, accuracy and repetitiveness; and the gene chip and the kit for detection can be applied to the clinic detection and epidemiology analyzing of medical and health organizations.
Owner:TIANJIN BIOCHIP TECH CO LTD
Chlamydial antigens
InactiveUS8481057B2Sequence differenceAvoid immune responsePeptide/protein ingredientsChlamydiaceae ingredientsAntigenChlamydophila
The invention is in the field of immunology and vaccinology. In particular, it relates to antigens derived from Chlamydia trachomatis that are expressed on the cell surface and so are ideal for use in immunization as well as combinations of these antigens.
Owner:CHIRON CORP
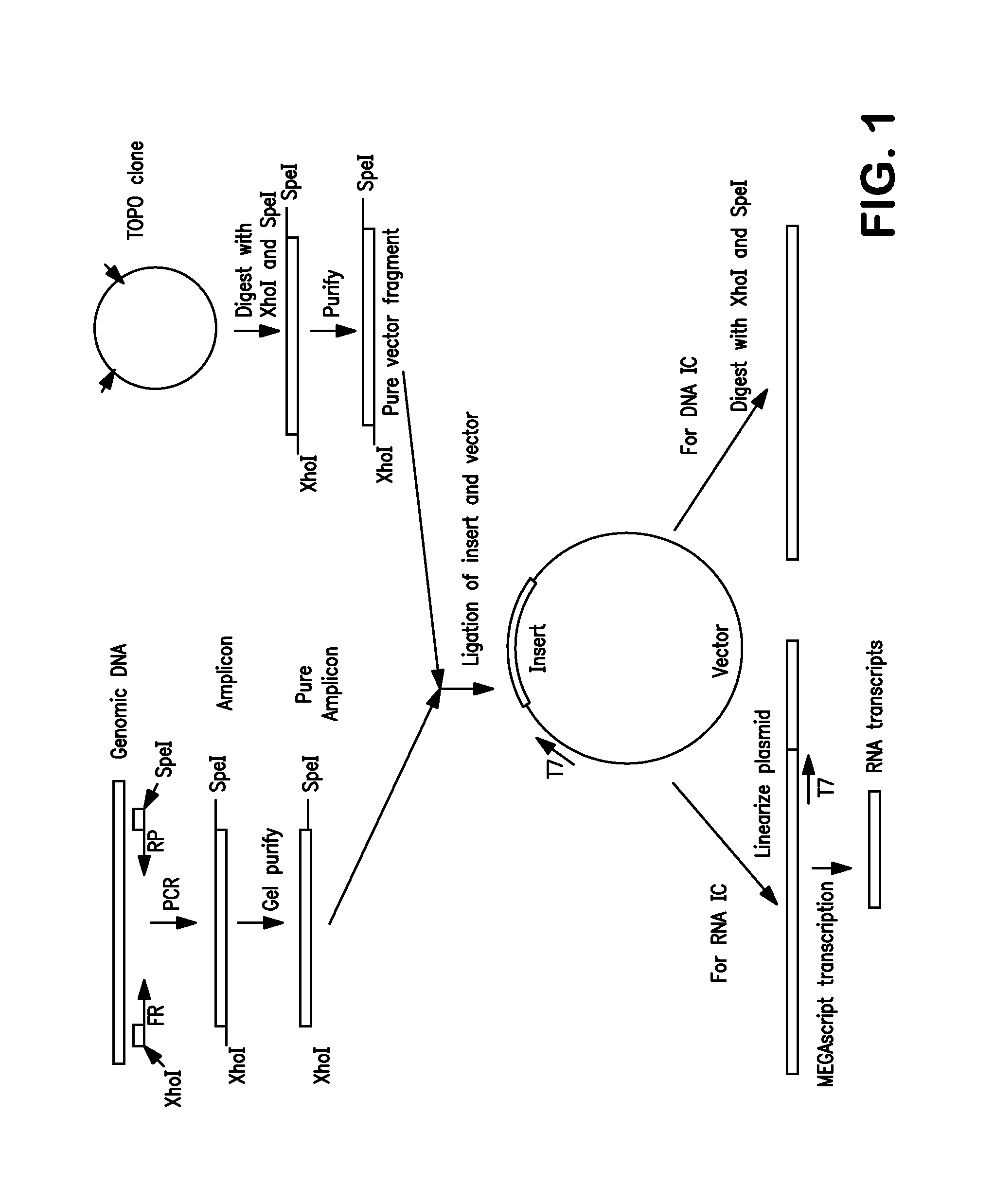
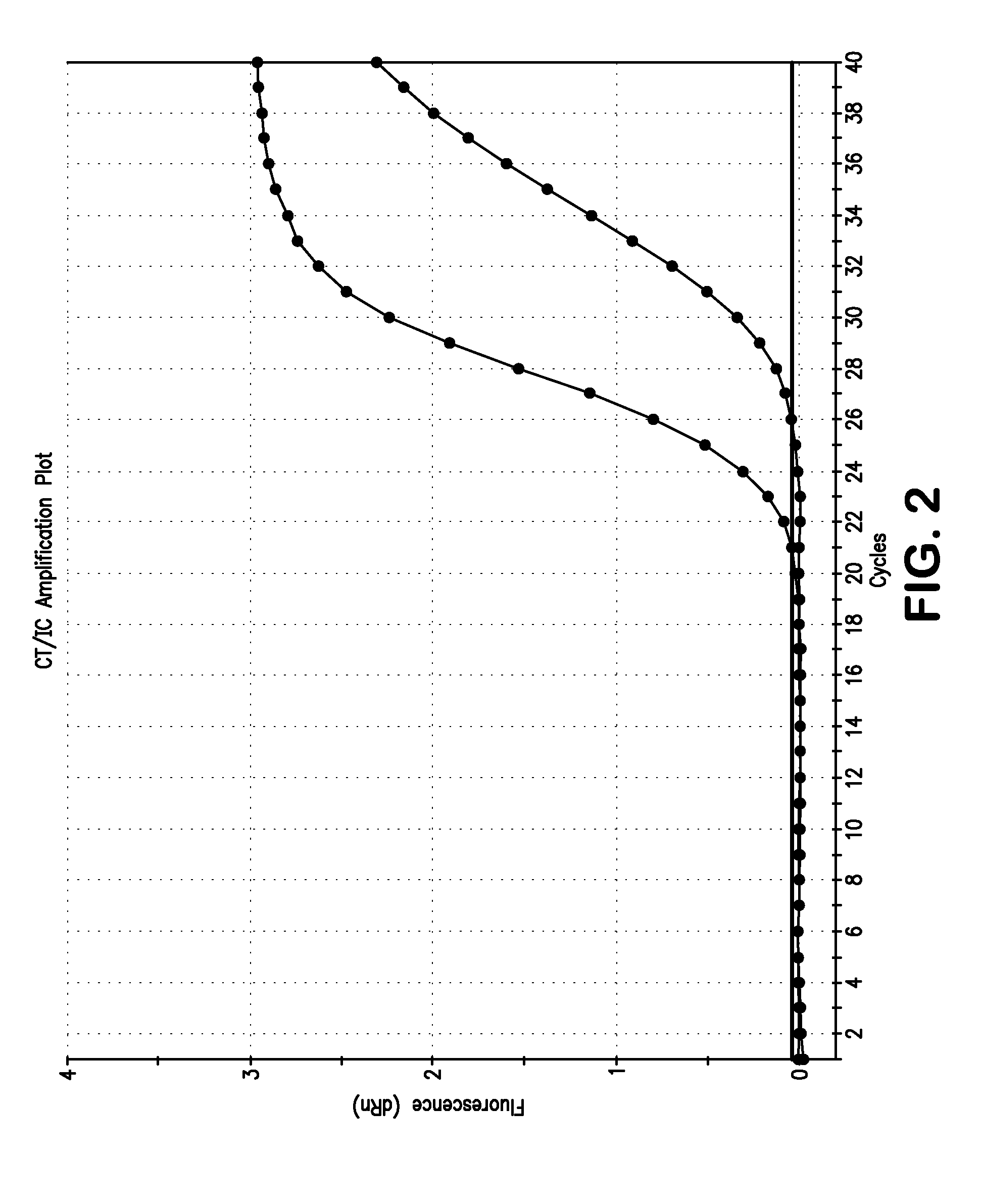
Non-Competitive Internal Controls for Use in Nucleic Acid Tests
InactiveUS20110003309A1Sugar derivativesMicrobiological testing/measurementNucleic acid testHuman immunodeficiency
Provided are non-competitive internal controls for use in nucleic acid tests (NATs), which are obtained from the organisms Methanobacterium thermoautrophicum (MET) and Zea mays (Corn). The non-competitive internal controls have utility in DNA and RNA NATs selected from Influenza A, Influenza B, parainfluenza viruses 1 to 4 (PIV-1 to PIV-4), respiratory syncytial virus type A (RSV A), RSV B, human metapneumovirus (hMPV), Chlamydia trachomatis (CT), and Neisseria gonorrhea (GC), Hepatitis B virus (HBV), Hepatitis C virus (HCV), Human Immunodeficiency Virus I (HIV-1), and Severe Acute Respiratory Syndrome (SARS).
Owner:SIEMENS HEALTHCARE DIAGNOSTICS INC
Neisseria gonorrhoeae, Chlamydia trachomatis and Ureaplasma urealyticum detection kits
ActiveCN102277429AWill not affect the results of the hybridizationGood synchronizationMicrobiological testing/measurementBiotinA-DNA
The invention discloses a gonococcus, chlamydia trachomatis and ureaplasma urealyticum detection kit. The detection kit comprises a gene chip (1) and various primers (2), wherein the gene chip carries (i) nucleotide probes of different pathogens, (ii) a biotin point-labeled DNA (deoxyribonucleic acid) sequence and (iii) a DNA sequence carrying beta-globulin encoding part; the nucleotide probes are a sequence of SEQ ID Nos:1-3 or a sequence complementary to SEQ ID Nos:1-3; the biotin point-labeled DNA sequence is SEQ ID No.4; the DNA sequence carrying beta-globulin encoding part serving as an internal control point is SEQ ID No.5; and various primers are used for amplifying the DNA sequences in a clinical sample, and have DNA sequences of SEQ ID Nos.6-11. The kit can quickly and accuratelydetect infections of gonococcus, chlamydia trachomatis and ureaplasma urealyticum, and has magnificent meaning to pathogen detection.
Owner:GUANGDONG HYBRIBIO BIOTECH CO LTD
Assay for Chlamydia trachomatis by amplification and detection of Chlamydia trachomatis cytotoxin gene
ActiveUS20100055708A1Easy to detectMicrobiological testing/measurementMicroorganismChlamydia trachomatis
A region of the Chlamydia trachomatis cytotoxin gene has been identified which is useful for performing amplification assays to determine specifically whether C. trachomatis is present in the sample being tested. Oligonucleotides useful for performing thermal Strand Displacement Assay (tSDA) reactions on this gene are disclosed. The disclosed oligonucleotides can be used in an assay which is specific for multiple strains of C. trachomatis and which does not show cross reactivity with the genomes of other microorganisms or with human DNA.
Owner:BECTON DICKINSON & CO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com