Quality control substance for detecting chlamydi trachomatis
A technology for Chlamydia trachomatis and quality control substances, which is applied in the fields of clinical laboratory science and biology, can solve the problem of restricting the quality assurance system of pathogen detection, being unsuitable for large-scale clinical detection quality control work, increasing operational complexity and manufacturing costs. The risk of biological infection and other problems, to achieve the effect of practicality, wide applicability, and reduction of the generation of enzyme cleavage sites.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
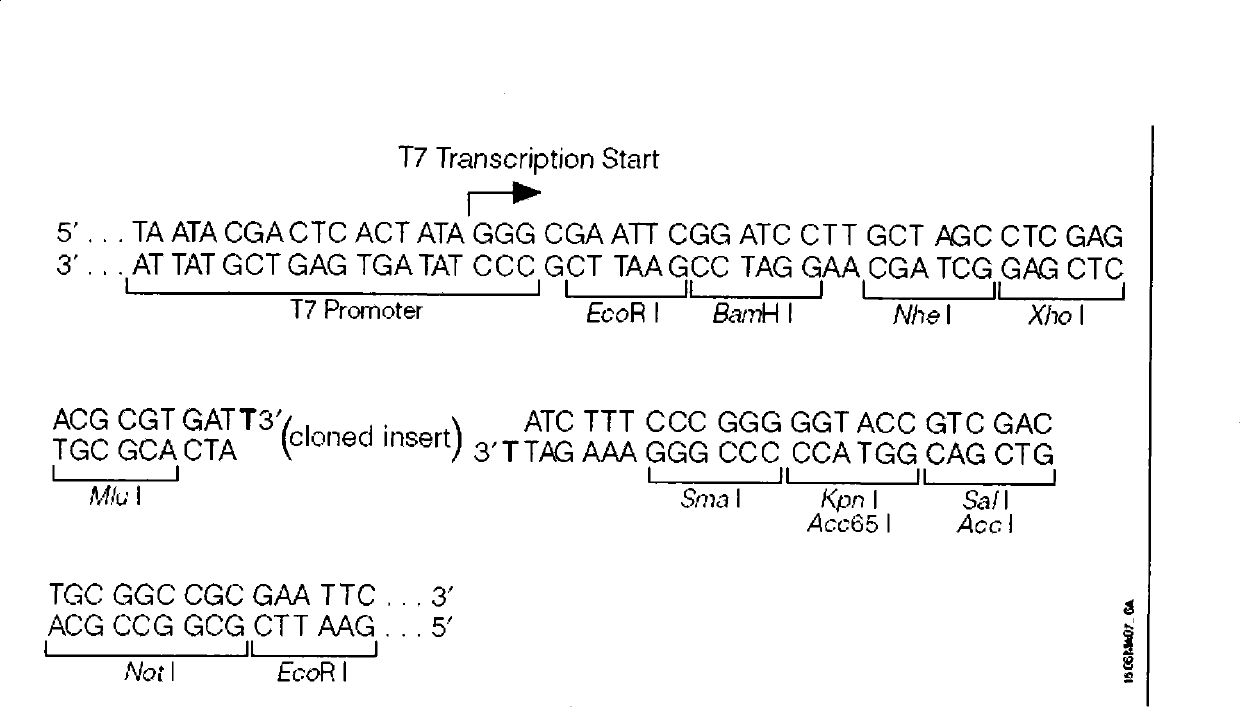
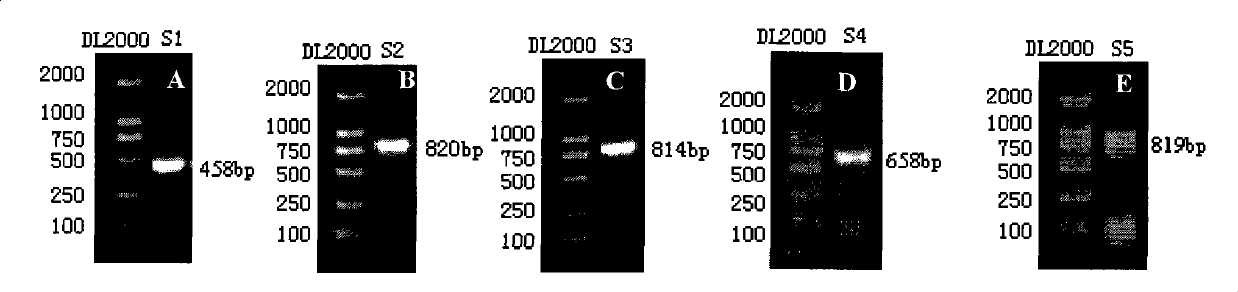
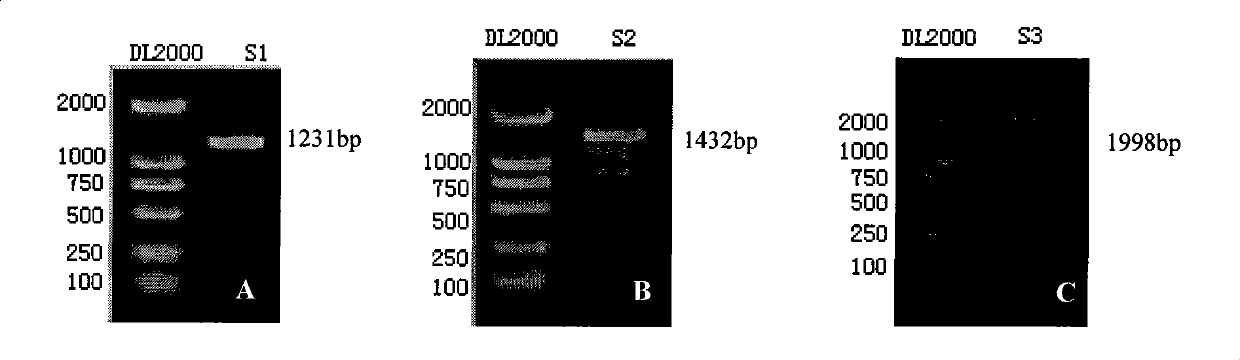
[0053] Example 1: Construction of recombinant vectors containing CT cryptic plasmid fragments
[0054] 1. Experimental materials:
[0055] 1. Source of specimens: Chlamydia trachomatis cell cultures were preserved in our laboratory, and cervical epithelial cells (HTB-Siha cells) were purchased from the Shanghai Cell Bank of the Chinese Academy of Sciences.
[0056] 2. Main reagents:
[0057] Bacterial DNA Extraction Kit Tiangen Biochemical China
[0058] Spin Column Type Ordinary Gel DNA Recovery Kit Tiangen Biochemical China
[0059] Taq polymerase Promega Inc. USA
[0060] DNA Electrophoresis Marker: DL2000 Takara Japan
[0061] DNA Electrophoresis Marker: DL15000 Takara Japan
[0062] TA Cloning Kit (T-Easy Vector) Promega, Inc. USA
[0063] Plasmid (miniature) extraction kit Promega USA
[0064] Restriction enzyme Sal I New England Biology USA
[0065] Restriction enzyme Not I New England Biology USA
[0066] T4DNA Ligase Promega, Inc., USA
[0067] Strain: DH5 α...
Embodiment 2
[0374] Example 2: Stability study of quality control substances and external quality evaluation of CT PCR detection applied in clinical laboratories
[0375] 1. Experimental materials
[0376] 1. Specimen source: Chlamydia trachomatis PCR detection quality control material constructed in Example 1
[0377] 2. Main reagents:
[0378] Cell culture medium DMEM (high glucose) Hyclone Corporation USA
[0379] Fluorescent quantitative RT-PCR detection reagent for Chlamydia trachomatis Guangzhou Daan Company China
[0380] Chlamydia trachomatis Fluorescent Quantitative RT-PCR Detection Reagent Shenzhen Piji Company China
[0381] 3. Main instruments:
[0382] Real-time fluorescence PCR instrument (7500Real Time PCR System) American biological application system United States
[0383] Real-time fluorescent PCR instrument (Lightcycler) Roche Company USA
[0384] Real-time fluorescent PCR instrument (Line-gene PCR instrument) Hangzhou Bioer Technology Co., Ltd. China
[0385] Des...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com