Primer, probe and method for detecting human urological genital tract causal agent
A technology for urogenital tract and pathogens, applied in the field of primers and probes for detecting human urogenital tract pathogens, can solve the problems of high cost, unfavorable mixed infection detection, and time-consuming operation process, so as to reduce operation time, cost and Effects of Patient Financial Burden
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1 detects the primers, probe sequences and methods of Neisseria gonorrhoeae (NG), ureaplasma urealyticum (UU), Chlamydia trachomatis (CT)
[0045] 1. Primers and Probes for Neisseria gonorrhoeae
[0046] According to the target nucleic acid sequence of Neisseria gonorrhoeae (Genbank Accession: M10316), suitable regions were selected to design primers and probes. Two sets of primers and probes were designed. After experimental comparison, one set was selected. The sequence is as follows:
[0047] NG-F1: 5'-GCT ACG CAT ACC CGC GTT GC-3' (SEQ ID NO: 1, located at target sequence 3141-3160bp)
[0048] NG-R1: 5'-CGA AGA CCT TCG AGC AGA CA-3' (SEQ ID NO: 2, located at target sequence 3531-3512bp)
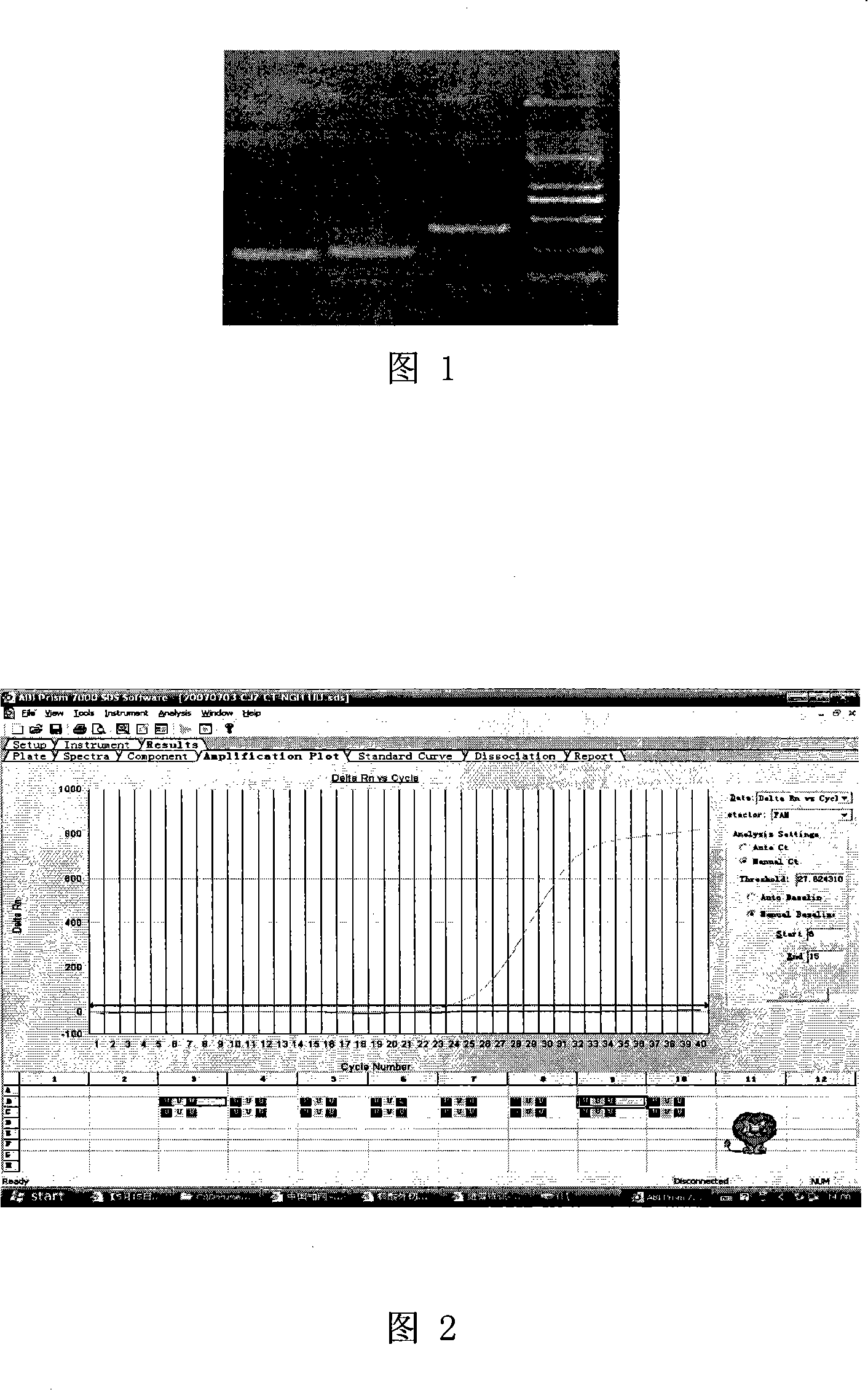
[0049] The amplified products of NG-F1 and NG-R1 are 391bp long (see Figure 1), corresponding to the cryptic plasmid region of the target sequence.
[0050] Hybridization probe:
[0051] NG-P1: FAM-5'-CTG TTT AAG TCG TCC AGC TCG TTC-3'-BHQ1 (SEQ ID NO: 7, located at ...
Embodiment 2
[0087] Embodiment 2 detects the preparation of the kit of Neisseria gonorrhoeae (NG), Ureaplasma urealyticum (UU), Chlamydia trachomatis (CT)
[0088] 1. The raw material that kit of the present invention adopts
[0089] (1) Primers and probes
[0090] The primers and probes of the present invention were synthesized by Shanghai Sangon Bioengineering Technology Service Co., Ltd.
[0091] (2) Other materials
[0092]The purified water complies with the requirements of the "Quality Control Standards for Main Raw and Auxiliary Materials of Biological Products in China". Supplied by Lomag Corporation. All chemical reagents are analytically pure or above, and all reagents have passed the inspection.
[0093] (3) Reference substance
[0094] 1) Negative control substance
[0095] purified water. The alternative test is qualified.
[0096] 2) Positive control substance
[0097] Known concentration of NG, UU, CT pathogenic microorganism bacterial liquid, known concentration of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com