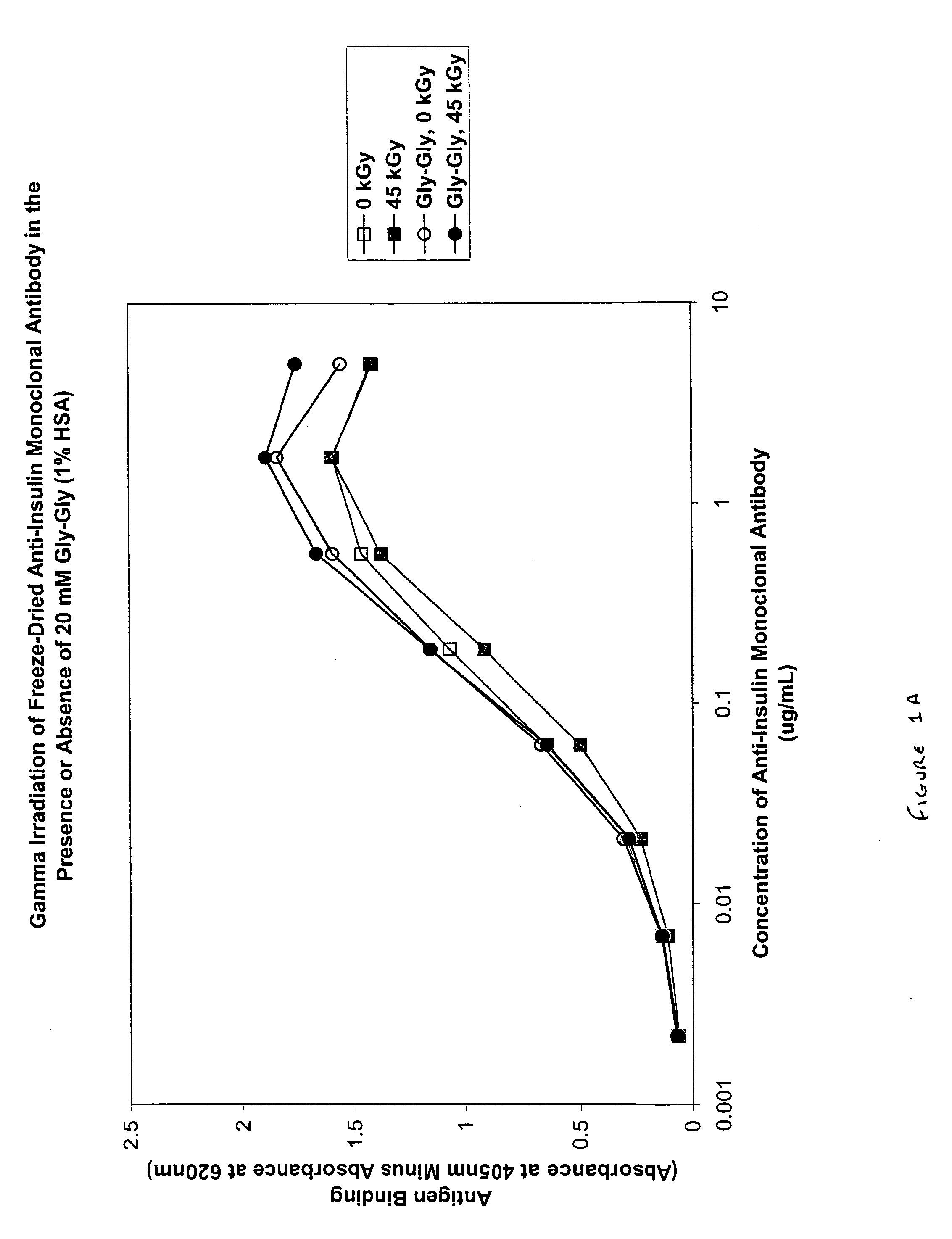
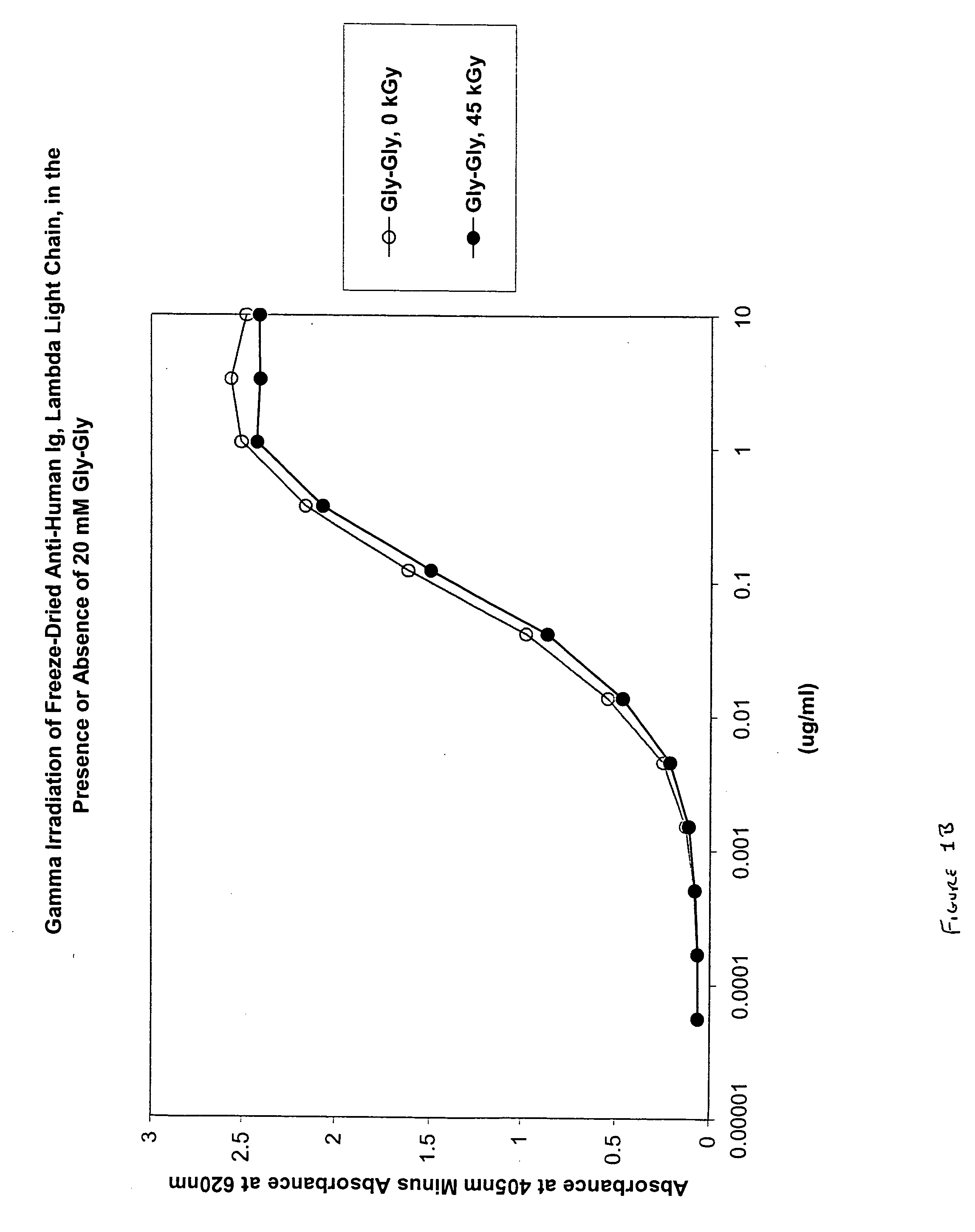
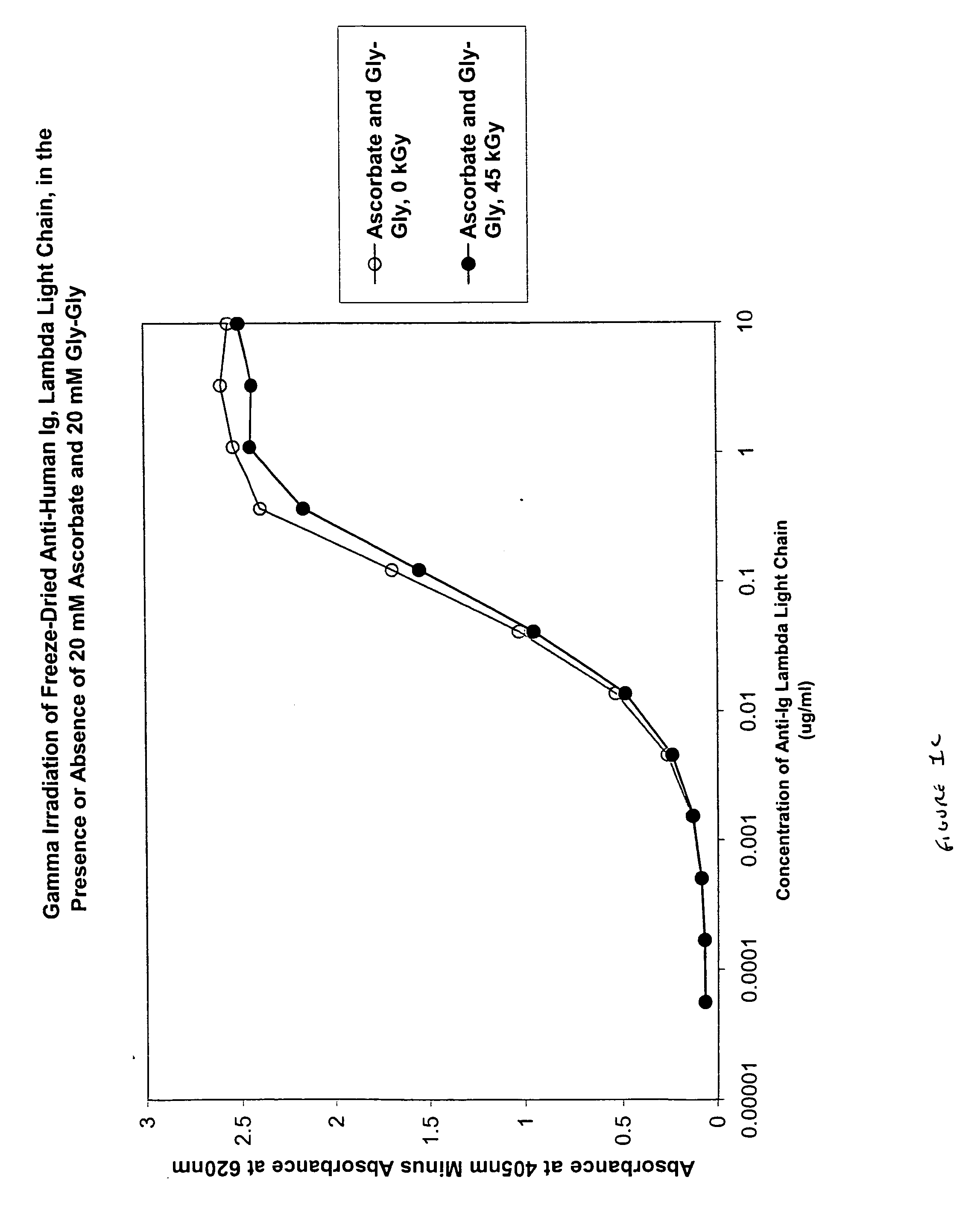
Patents
Literature
609 results about "The Mycoplasmas" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
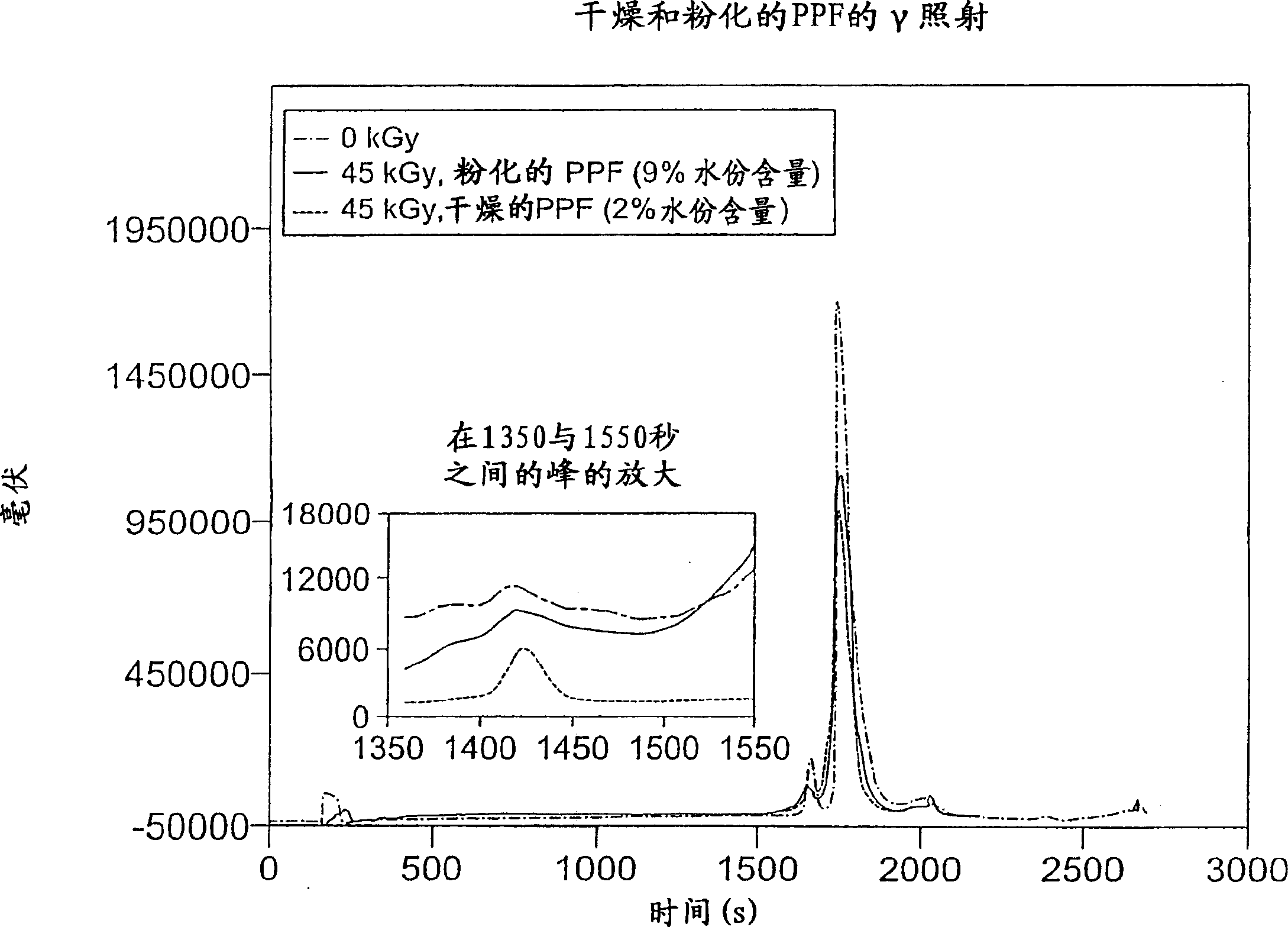
Methods for sterilizing biological materials by irradiation over a temperature gradient
InactiveUS6908591B2Effective sterilizationImprove permeabilityDead animal preservationLavatory sanitoryBiological materialsBiology
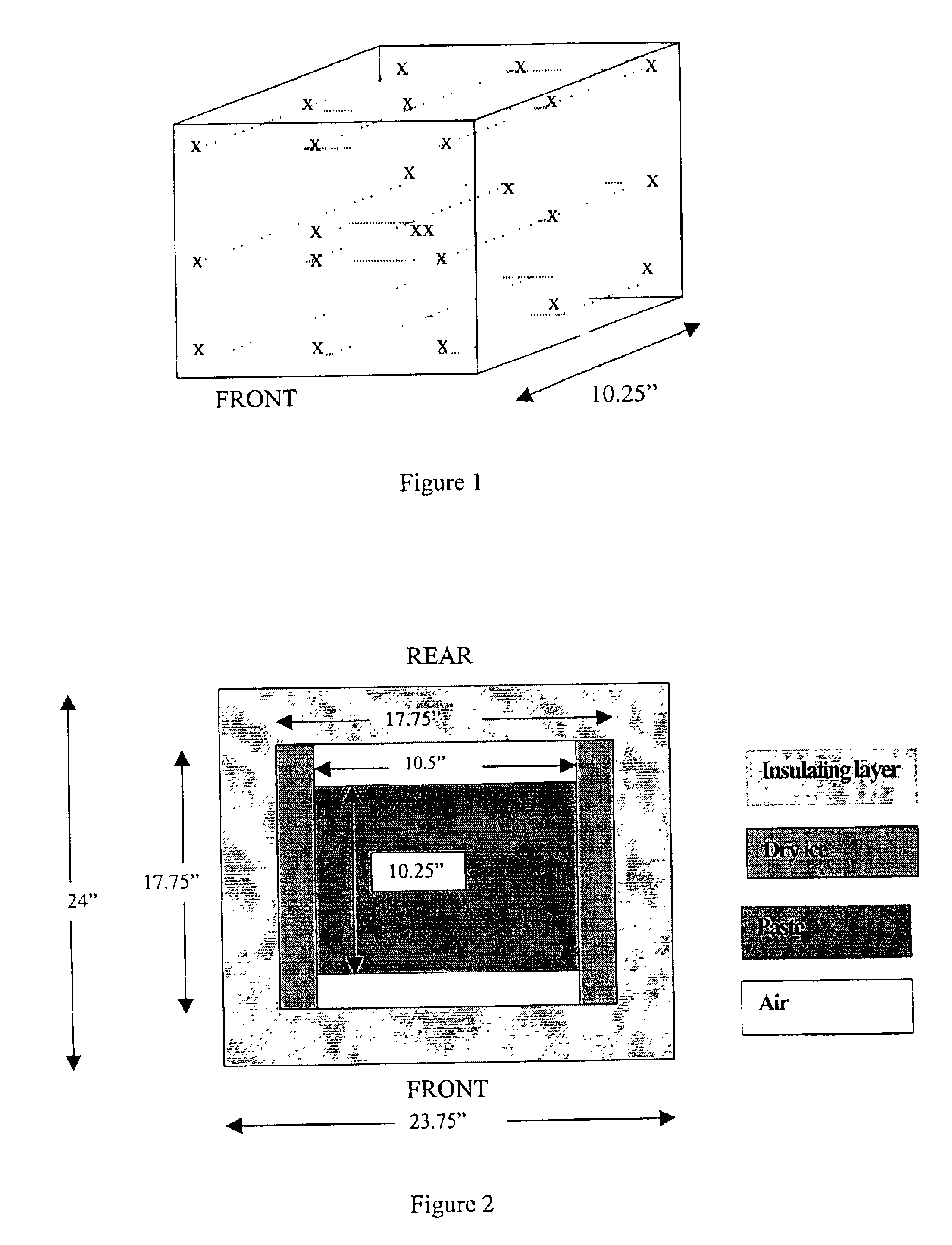
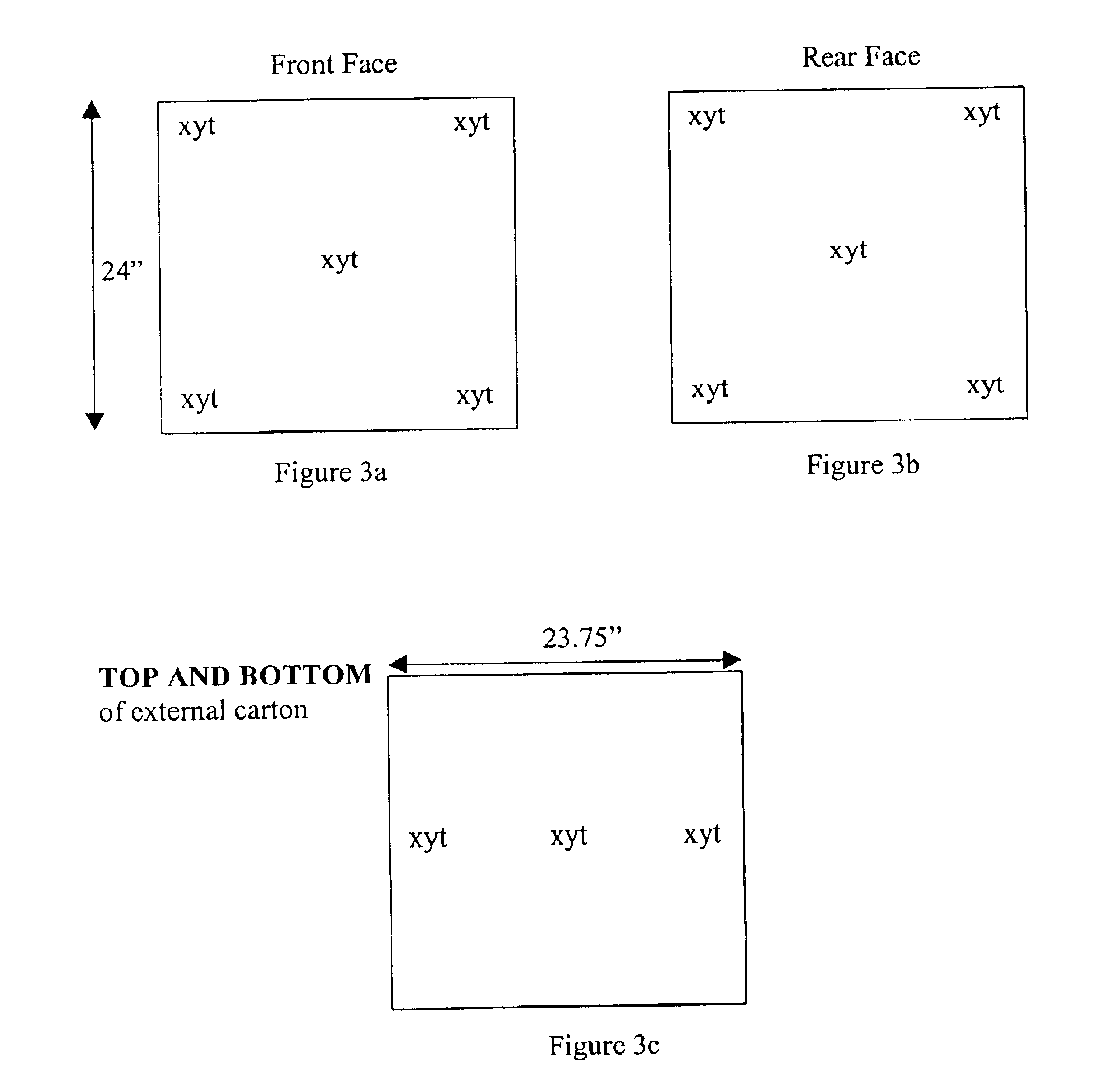
Methods are disclosed for sterilizing tissue to reduce the level of one or more active biological contaminants or pathogens therein, such as viruses, bacteria, (including inter- and intracellular bacteria, such as mycoplasmas, ureaplasmas, nanobacteria, chlamydia, rickettsias), yeasts, molds, fungi, spores, prions or similar agents responsible, alone or in combination, for TSEs and / or single or multicellular parasites. The methods involve sterilizing one or more tissues with irradiation.
Owner:CLEARANT
Mycoplasma Hyopneumoniae Avirulent Adjuvanted Live Vaccine
ActiveUS20090117152A1Preventing and minimize severityElicit immune responseAntibacterial agentsBacterial antigen ingredientsViral antigensImmunogenicity
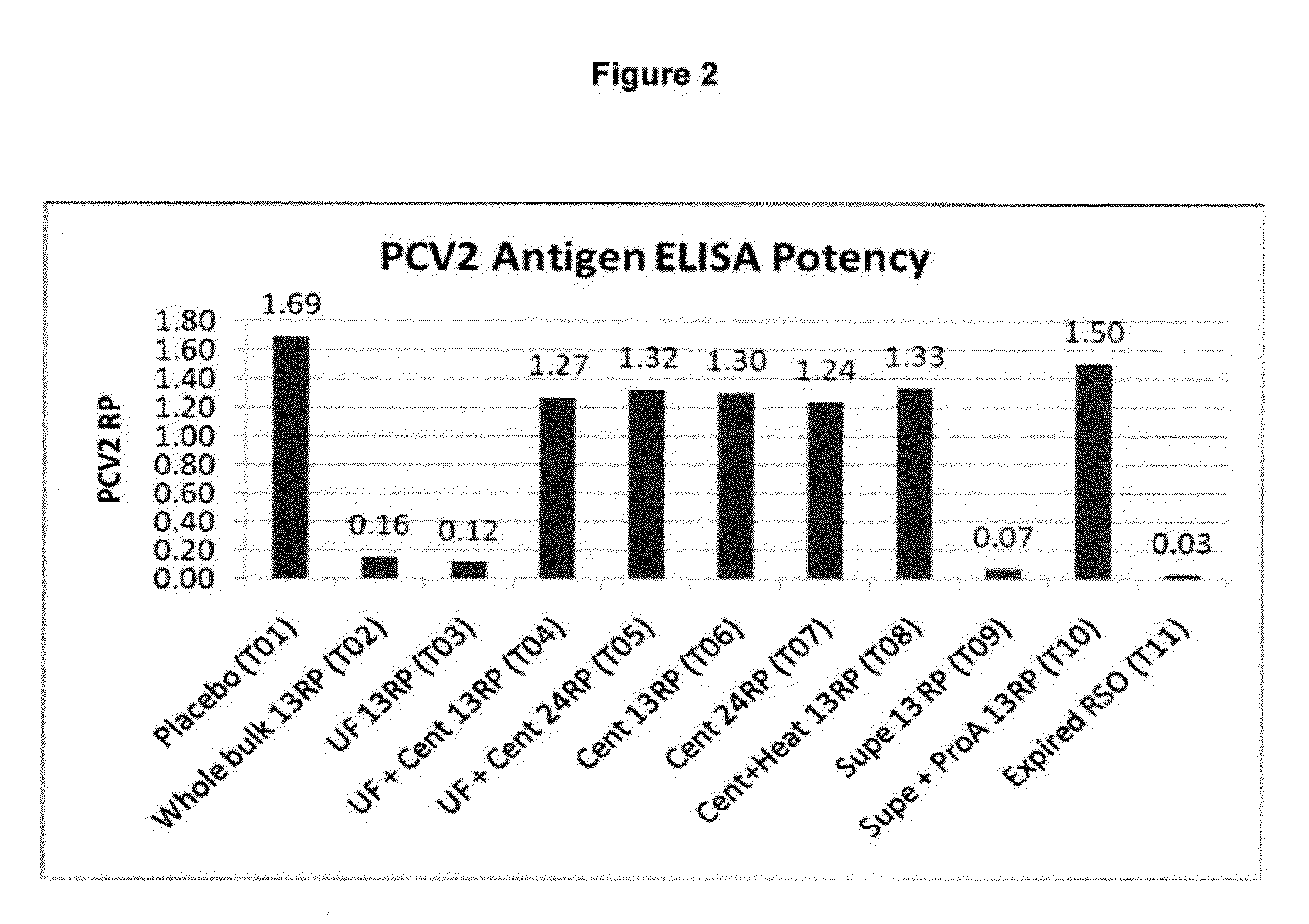
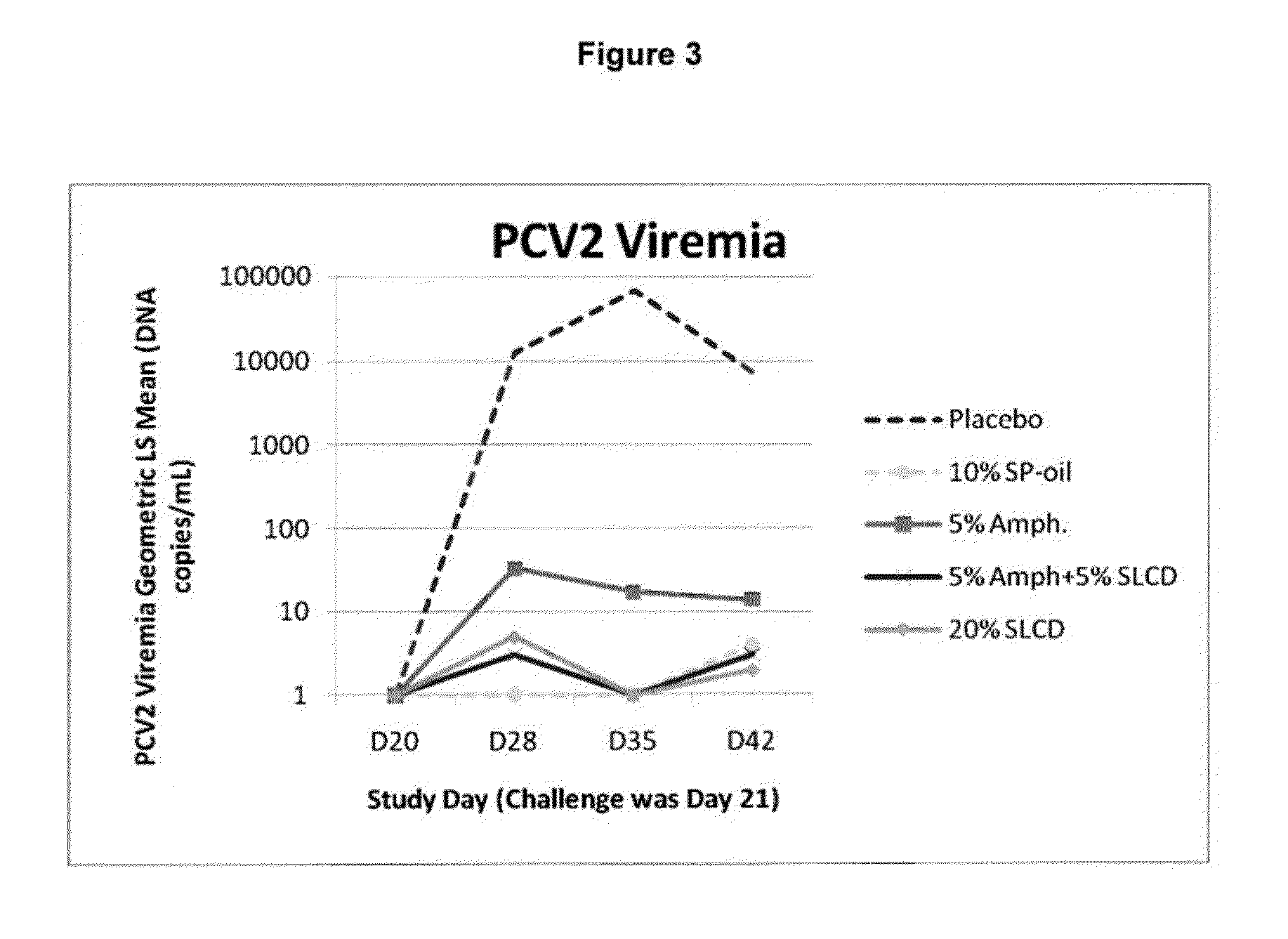
Provided are immunogenic and vaccine compositions and methods for their preparation and use, which compositions are effective in protecting against, minimizing the severity of, preventing, and / or ameliorating M. hyopneumoniae infection. Administration to an animal of one or two doses of an adjuvanted live avirulent M. hyopneumoniae composition disclosed herein is effective in providing immunity to the animal and protection from infection with a virulent strain of M. hyopneumoniae thereby reducing the severity of and / or preventing disease caused by one or more virulent strain of M. hyopneumoniae. Also provided are compositions, which further comprise one or more antigen such as, for example, one or more live bacteria, bacterin, toxoid, and / or virus and / or viral antigen. Exemplified are immunogenic compositions, comprising an adjuvanted live avirulent M. hyopneumoniae and compositions, comprising Porcine Circovirus Type 1-Type 2 chimera modified live vaccine (cPCV1-2) in further combination with an adjuvanted live avirulent M. hyopneumoniae.
Owner:ZOETIS SERVICE LLC
Kit for detecting 10 respiratory tract infection pathogens and application method thereof
ActiveCN107365876AStrong specificityReduce pollution interferenceMicrobiological testing/measurementAgainst vector-borne diseasesRespiratory tract infectionsPathogen
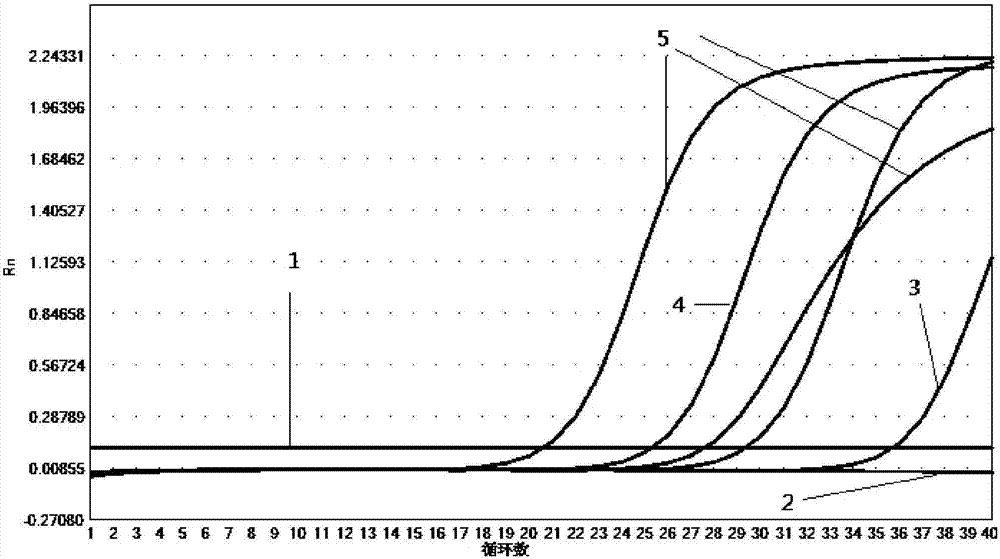
The invention provides a kit for detecting 10 respiratory tract infection pathogens and an application method thereof. The kit comprises primers and a TaqMan probe, wherein the primers are used for amplifying the 10 respiratory tract infection pathogens which include mycoplasma pneumoniae, chlamydia pneumoniae, legionella pneumophila, bordetella pertussis, rhinovirus, respiratory tract adenovirus, influenza A virus, influenza B virus, respiratory syncytial virus and parainfluenza virus. The application method includes: mixing 2 microliters of sample DNA / RNA co-extraction template, 20 microliters of RT-PCR buffer, 1 microliter of mixed enzyme liquid and 2 microliter of primer-probe mixed liquid to perform PCR amplification reaction. The kit uses the Taqman probe fluorescent PCR technology to detect the 10 common respiratory tract infection pathogens, a detection result can be obtained within 2 hours, the kit is high in specificity, and the sensitivity of the kit can reach 100copies / microliter.
Owner:NANJING LANSION BIOTECH CO LTD
Antipathogenic synthetic piptides and compositions comprising them
Non-hemolytic cytolytic agents selected from peptides, complexes of bundled peptides, mixtures of peptides or random peptide copolymers have a selective cytolytic activity manifested in that they have a cytolytic activity on pathogenic cells, being cells which are non-naturally occurring within the body consisting of microbial pathogenic organisms and malignant cells; and are non-hemolytic, having no cytolytic effect on red blood cells. The peptides may be cyclic derivatives of natural peptides such as pardaxin and mellitin and fragments thereof in which L-amino acid residues are replaced by corresponding D-amino acid residues, or are diastereomers of linear peptides composed of varying ratios of at least one positively charged amino acid and at least one hydrophobic amino acid, and in which at least one of the amino acid residues is a D-amino acid. Pharmaceutical compositions comprising the non-hemolytic cytolytic agents can be used for the treatment of several diseases caused by pathogens including antibacterial, fungal, viral, mycoplasma and protozoan infections and for the treatment of cancer.
Owner:YEDA RES & DEV CO LTD
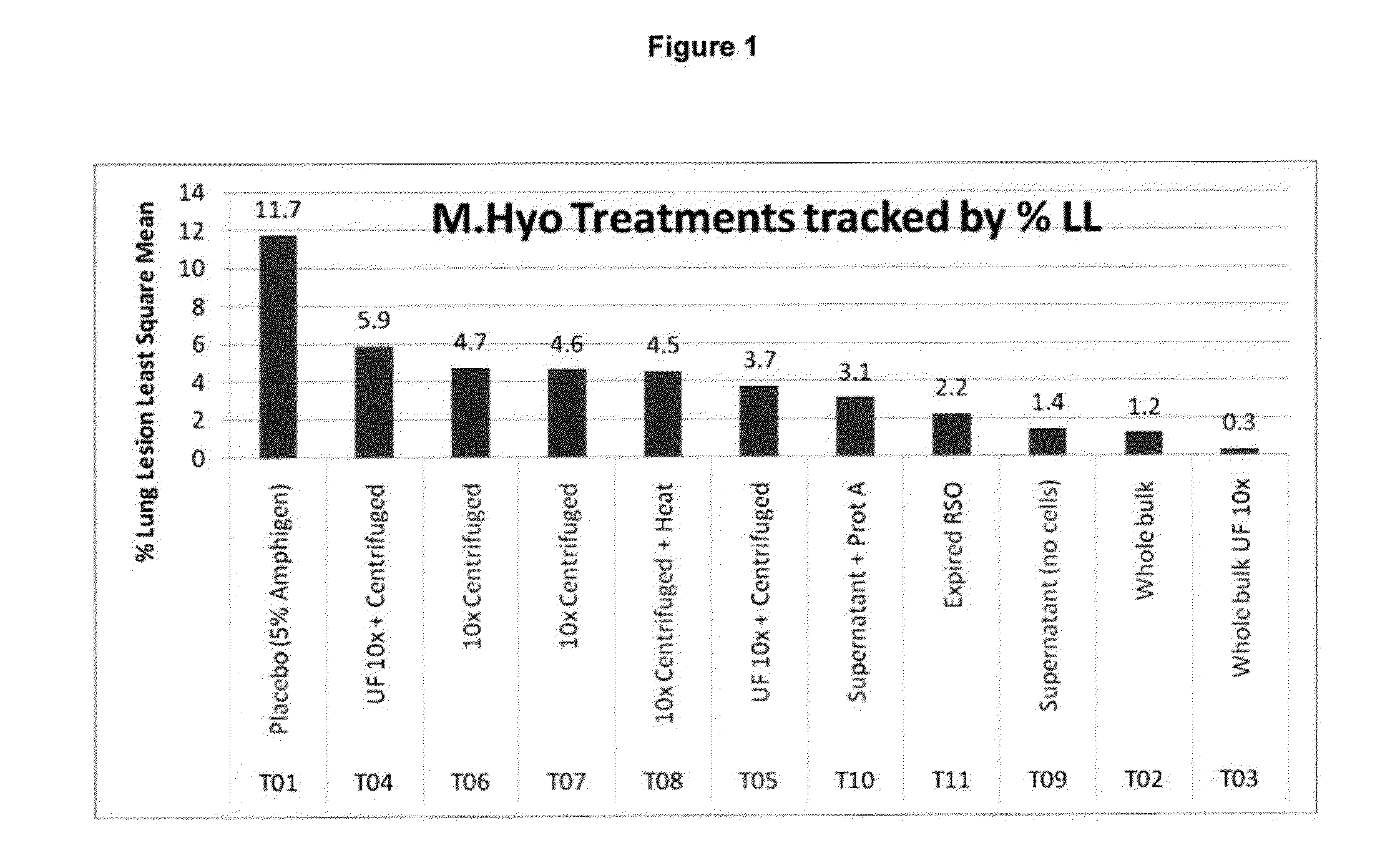
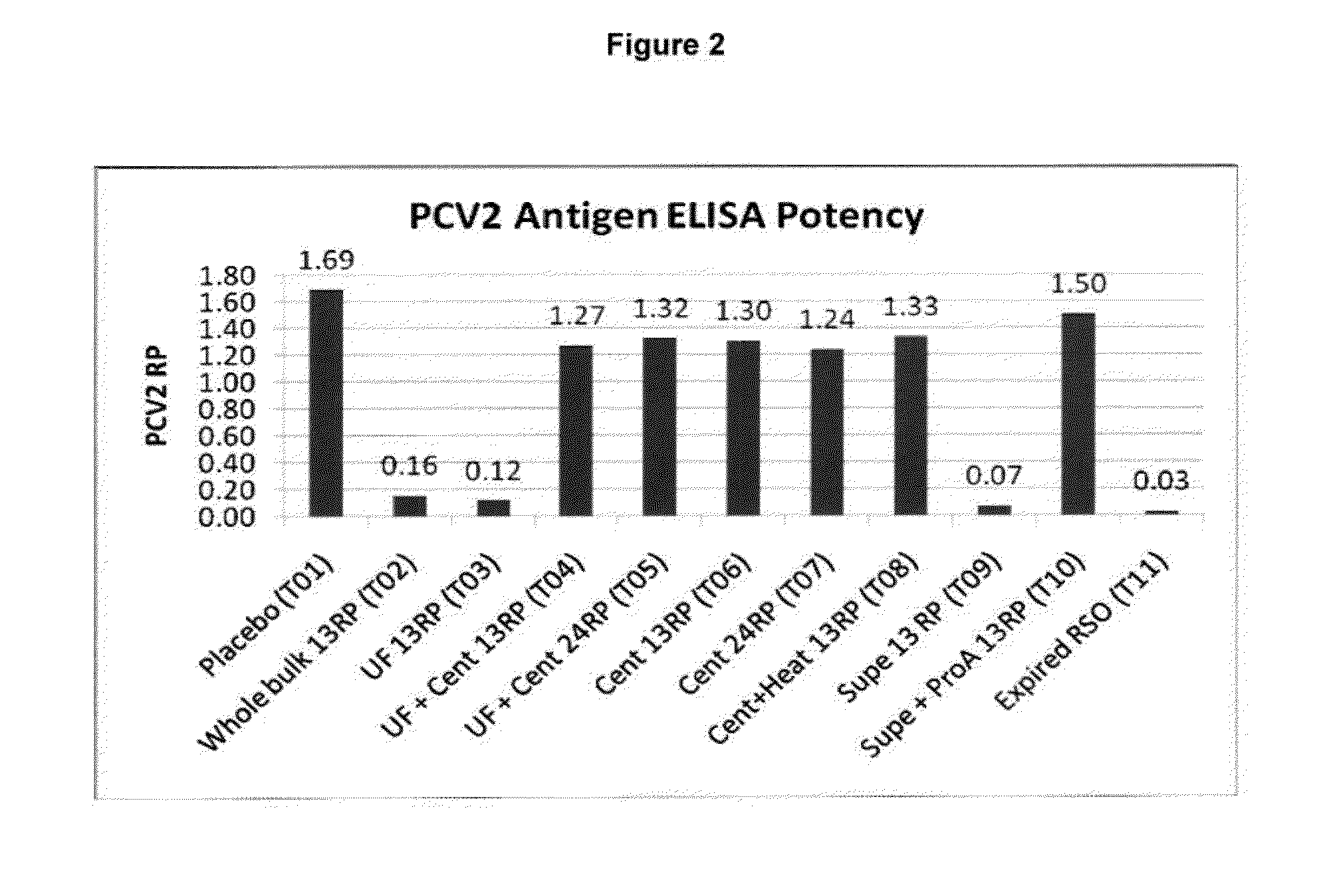
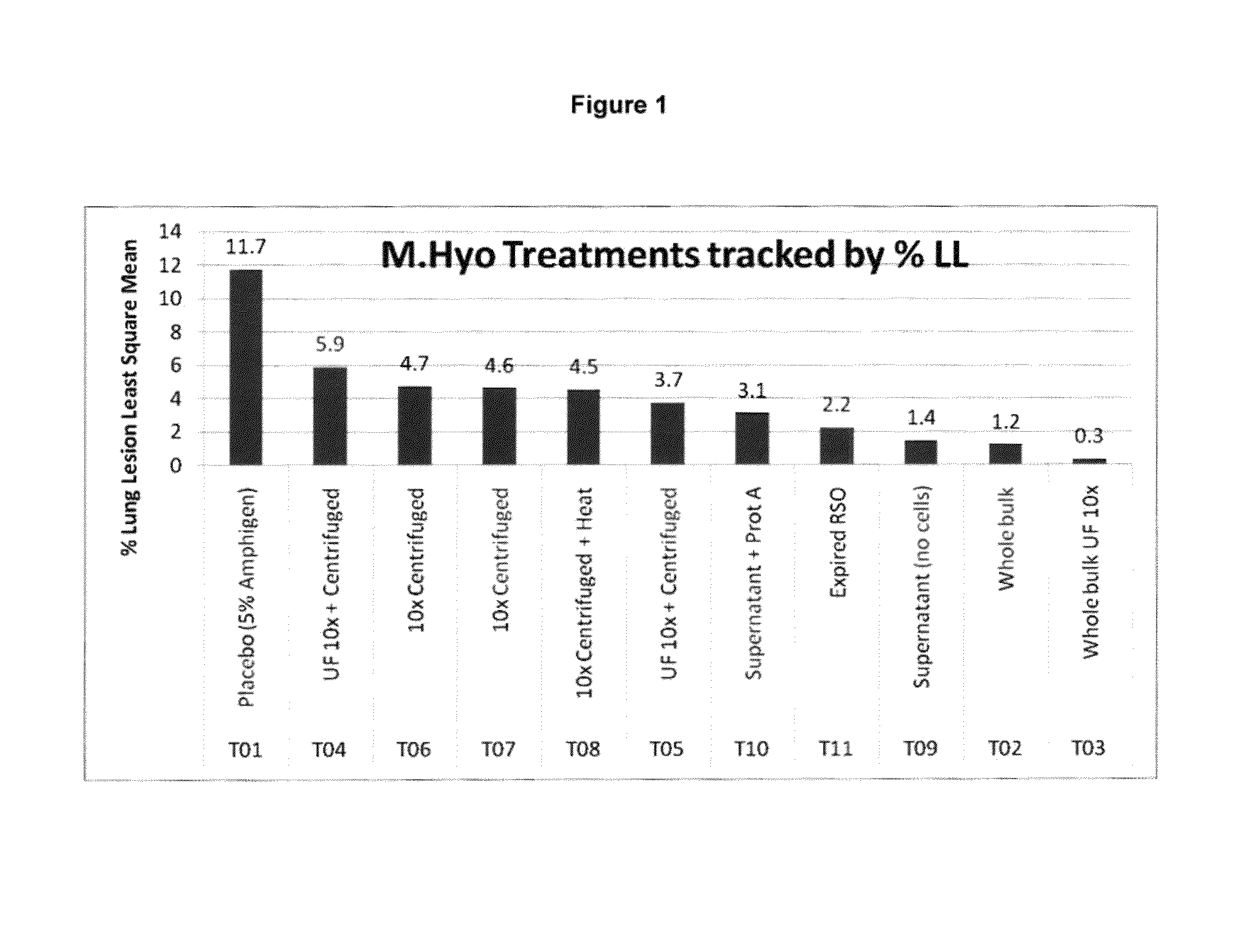
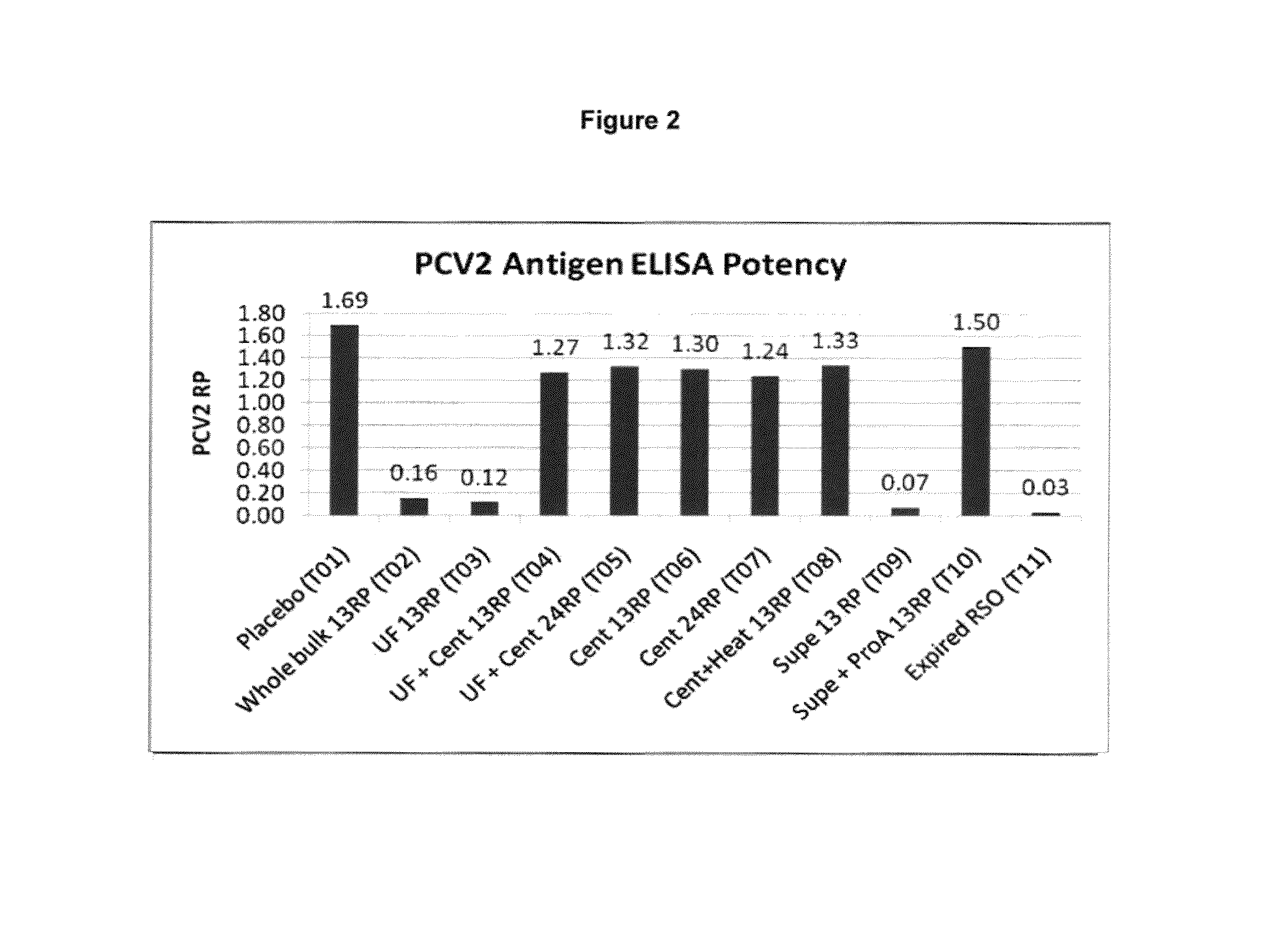
PCV/Mycoplasma Hyopneumoniae/PRRS Combination Vaccine
ActiveUS20130266603A1Protective immune responseAntibacterial agentsBacterial antigen ingredientsImmune complexVirus antigen
This invention provides a trivalent immunogenic composition including a soluble portion of a Mycoplasma hyopneumoniae (M. hyo) whole cell preparation; a porcine circovirus type 2 (PCV2) antigen; and a PRRS virus antigen, wherein the soluble portion of the M. hyo preparation is substantially free of both (i) IgG and (ii) immunocomplexes comprised of antigen bound to immunoglobulin.
Owner:ZOETIS SERVICE LLC
Medicinal composite preparation containing quinolones, preparation method thereof and application thereof
ActiveCN102397552ASolve the problem of food refusalAvoiding Inadequate Intake ProblemsAntibacterial agentsOrganic active ingredientsCross-resistanceIn vivo
The invention discloses a medicinal composite preparation containing quinolones, a preparation method thereof and an application thereof. The medicinal composite preparation containing quinolones comprises the following components, by mass, 0.5-95% of a quinolone medicine or a salt compound thereof, 1-97% of an organic acid or a salt compound thereof, 1-98.5% of a sweetener, 0-45% of a flavor enhancer, 0-10% of a flavoring agent, and the balance excipient. Finished products prepared from the medicinal composite preparation containing quinolones, which have the characteristics of stability, excellent dispersibility, good palatability and easy absorption, can be used as a broad-spectrum antiseptic, have the advantages of strong bactericidal activity, wide in vivo distribution and no cross drug resistance with other antibiotic medicines, and are effective to bacteria and mycoplasmas, can be used to treat various infectious diseases of the alimentary system, the respiratory system, the urinary system or the skin soft tissue of animals, wherein the diseases are caused by the sensitive bacterial or the mycoplasmas.
Owner:GENIFARM LAB INC
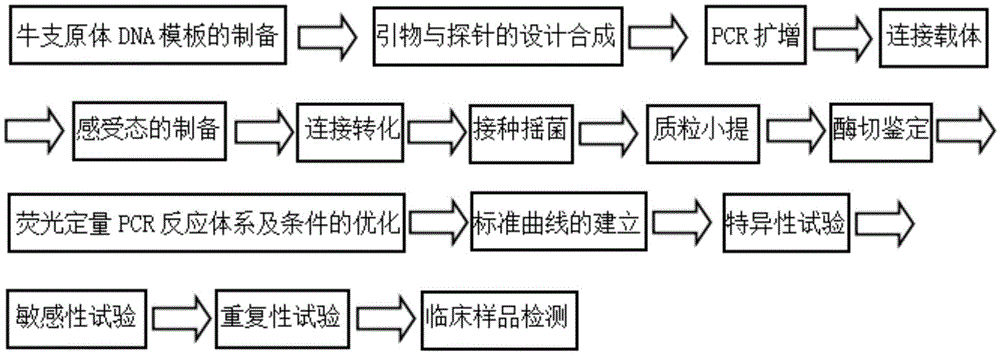
Real-time fluorescence quantification PCR detecting kit for cow mycoplasma and special primers and TaqMan probe thereof
InactiveCN105420379ANo follow-up work requiredReduce workloadMicrobiological testing/measurementDNA/RNA fragmentationFluorescenceMycoplasma bovis
The invention discloses a real-time fluorescence quantification PCR detecting kit for cow mycoplasma, special primers and a TaqMan probe thereof and application of the detecting kit in detection of cow mycoplasma. The primers and the TaqMan probe for real-time fluorescence quantification PCR detecting of the cow mycoplasma are designed according to a specific conserved sequence of an OPPD / F gene of the cow mycoplasma and are used for detecting the cow mycoplasma of a sample to be detected qualitatively and quantitatively. The nucleotide sequence of the upstream primer (OF-A) is shown as SED ID NO:1 in a sequence table, the nucleotide sequence of the downstream primer (OF-B) is shown as SED ID NO:2 in the sequence table, and the nucleotide sequence of the TaqMan probe (OF-P) is shown as SEQ IDNO:3 in the sequence table. By means of the detecting kit, the special primers and the TaqMan probe thereof, the cow mycoplasma can be detected quickly, conveniently, efficiently, highly specifically and highly sensitively, and a novel technical platform is provided for detection of the cow mycoplasma.
Owner:JINYUBAOLING BIO PHARM CO LTD +1
PCV/Mycoplasma Hyopneumoniae Combination Vaccine
This invention provides a multivalent immunogenic composition including a soluble portion of a Mycoplasma hyopneumoniae (M. hyo) whole cell preparation; and a porcine circovirus type 2 (PCV2) antigen, wherein the soluble portion of the M. hyo preparation is substantially free of both (i) IgG and (ii) immunocomplexes comprised of antigen bound to immunoglobulin.
Owner:ZOETIS SERVICE LLC
Mycoplasma Hyopneumoniae Vaccine
ActiveUS20130266601A1Antibacterial agentsSsRNA viruses positive-senseImmunoglobulin IgEMycoplasma hyopneumoniae
This invention provides an immunogenic composition including a soluble portion of a Mycoplasma hyopneumoniae (M.hyo) whole cell preparation, wherein the soluble portion of the M.hyo preparation is substantially free of both (i) IgG and (ii) immunocomplexes comprised of antigen bound to immunoglobulin.
Owner:ZOETIS SERVICE LLC
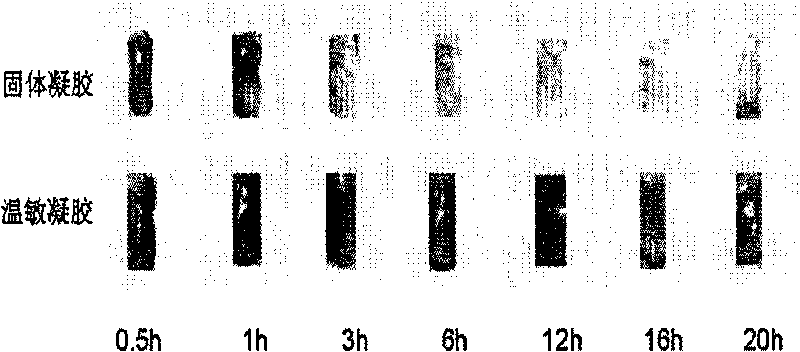
Use and preparation method of vaginal thermosensitive gel
InactiveCN101695473AImprove toleranceImprove complianceAntimycoticsPharmaceutical delivery mechanismSide effectTolerability
The invention provides use and a preparation method of vaginal thermosensitive gel. The use is the use of the vaginal thermosensitive gel in the preparation of medicaments for treating vaginitis including bacterial vaginitis, mycotic vaginitis, mycoplasmal vaginitis, trichomonas vaginitis and senile vaginitis. The vaginal thermosensitive gel of the invention has a pH value in accordance with a vaginal environment, high patient tolerance and long in-vivo duration of more than 20 hours and therefore can reduce dosing frequency, improve patient compliance, strengthen the treatment effect of the medicaments and reduce toxic and side effects. In addition, the temperature of the gel is mild, so the gel has high liquidity before entering bodies and becomes solid after entering the bodies and the administration is convenient and sanitary.
Owner:WUHAN WORLDNER UNITED PHARMA
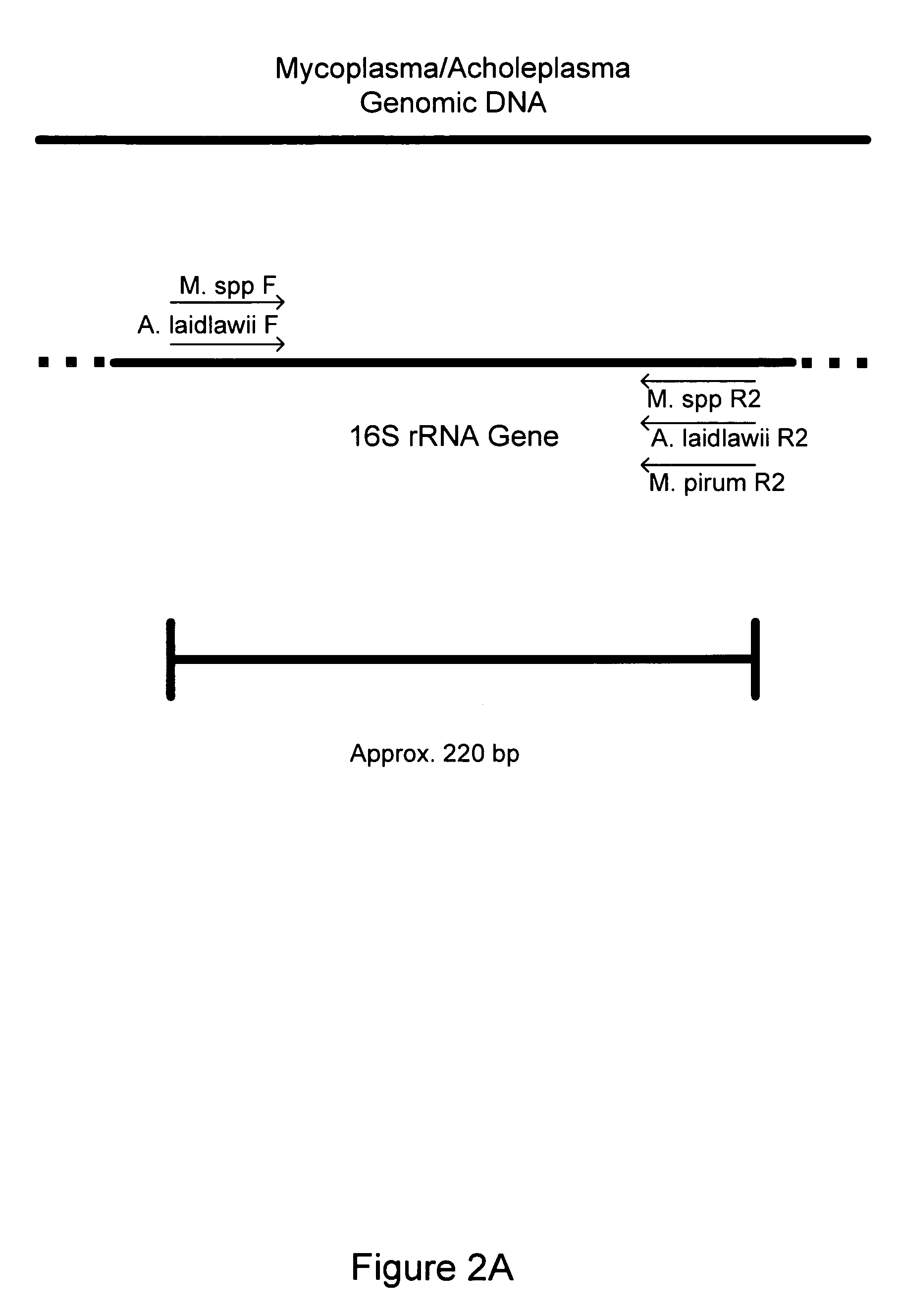
Nucleic acids, compositions, methods, and kits for detecting mycoplasma and acholeplasma species
The invention relates to nucleic acids, methods, compositions, and kits for the PCR-based detection of Mycoplasma and Acholeplasma bacterial species. The nucleic acids, methods, compositions, and kits provide for increased specificity and sensitivity of PCR-based Mycoplasma bacterial assays. Primer sets and PCR-based assays are provided that amplify and detect conserved 16S rRNA gene sequences from multiple Mycoplasma and Acholeplasma species while avoiding amplification and detection of non-target sequences.
Owner:STRATAGENE INC US
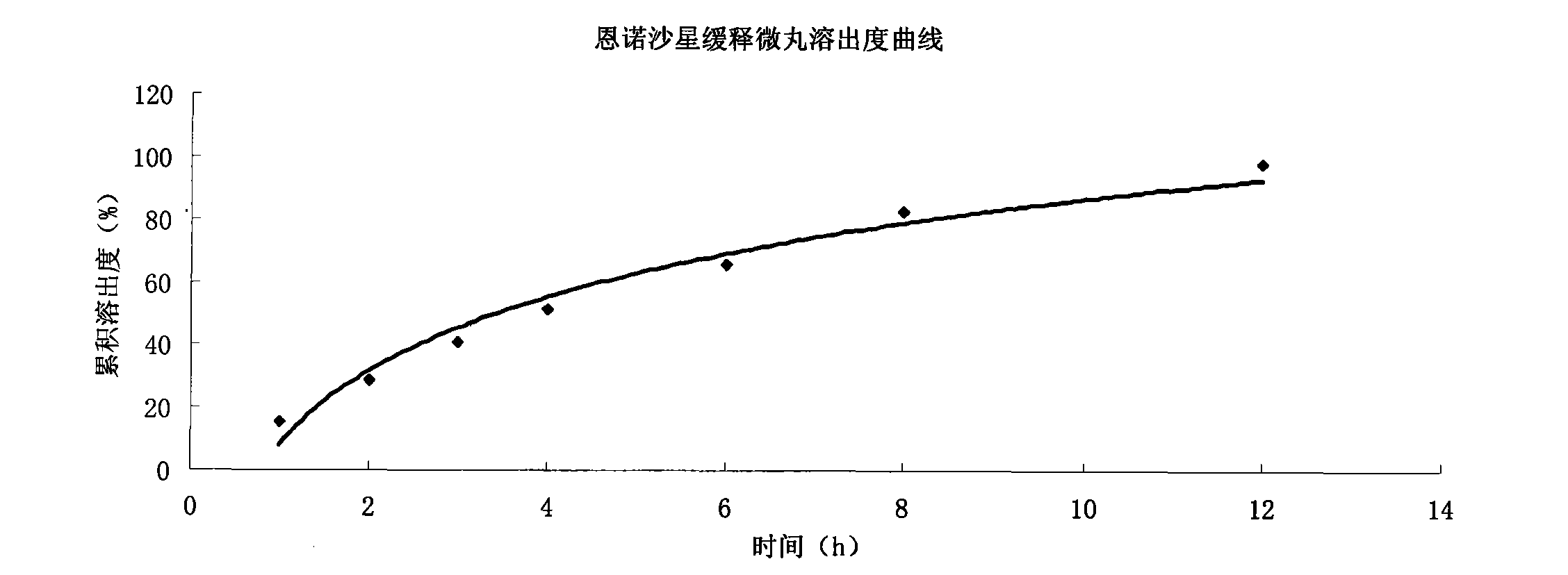
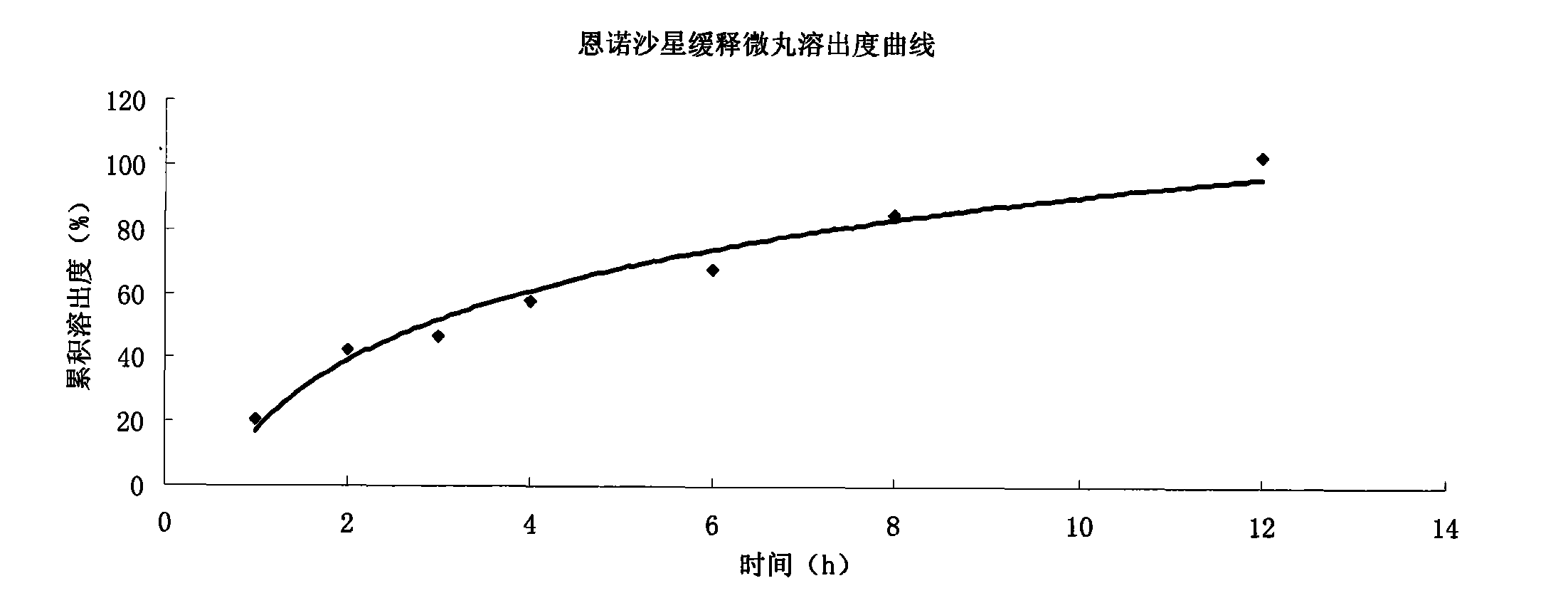
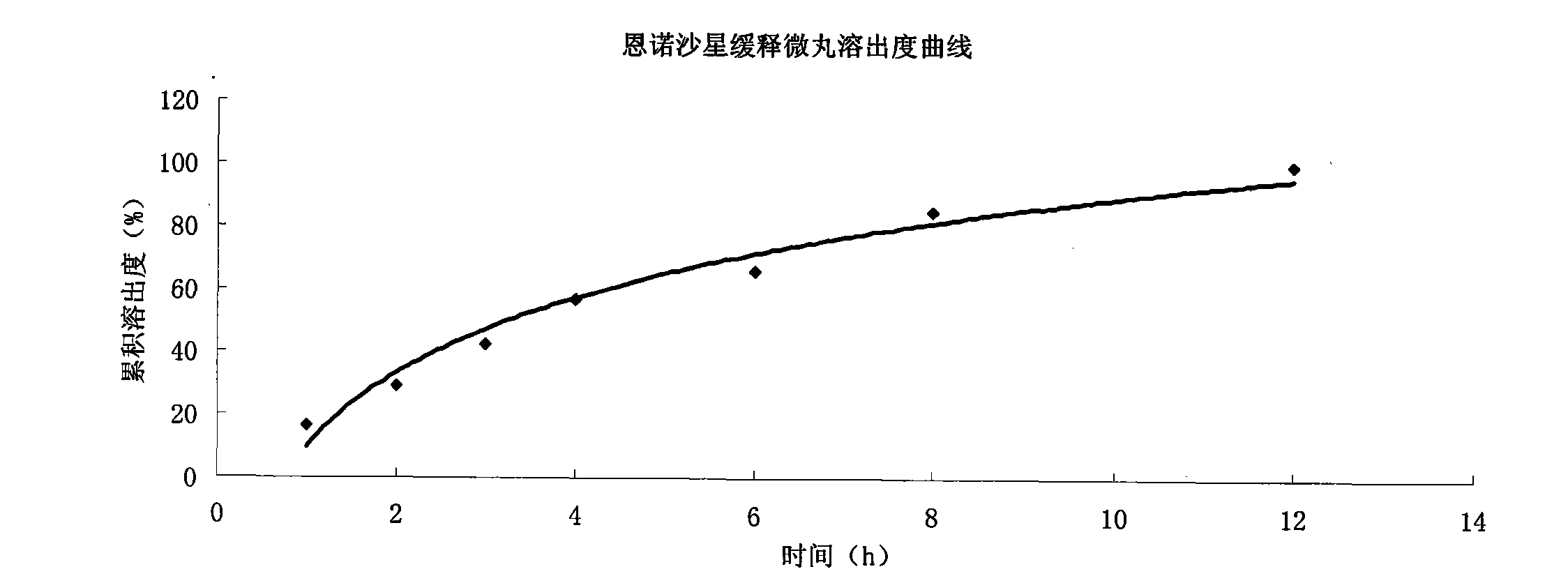
Enrofloxacin slow-release micropill for livestocks, and preparation method of same
InactiveCN102648896AExpand the scope of clinical applicationImprove the characteristics of easy color change when exposed to lightAntibacterial agentsOrganic active ingredientsPharmaceutical formulationSodium carboxymethyl starch
The invention relates to the field of pharmaceutical preparations and particularly relates to a slow-release micropill preparation containing enrofloxacin and a preparation method of the preparation. The slow-release micropill provided by the invention is formed by coating an enrofloxacin micropill; the enrofloxacin micropill comprises enrofloxacin and auxiliary material and is formed through extruding and rounding; the auxiliary material is any one or more of microcrystalline cellulose, starch, cane sugar, artificial gum, lactose and sodium carboxymethyl starch; according to weight percent, the auxiliary material in the micropill accounts for 70% to 95%; and coating is made of high-molecular enteric material, film forming material, opaquer and the like. The slow-release micropill preparation has the characteristics of slow release and high bioavailability, can be used for treating bacteria and mycoplasma infection of livestocks, and has better curative effect on chronic respiratory diseases, colibacillosis and salmonellosis, and the frequency of medicine taking can be reduced; and in addition, the slow-release micropill provided by the invention has the advantages that the stability of medicine is improved, and peculiar bitter of enrofloxacin can be covered completely, so that feeding intake of the animals is not influenced, and the recovery rate is improved.
Owner:ZHENGZHOU FUYUAN ANIMAL PHARMA
Composition for detecting and typing pathogens causing respiratory tract infection, kit, method and application
ActiveCN111321251AReduce wasteReduce psychological burdenMicrobiological testing/measurementMicroorganism based processesRespiratory syncytial virus (RSV)Pneumonitis
The invention relates to the field of molecular biology detection, and particularly relates to detection of novel coronavirus 2019- nCoV, influenza A virus, influenza B virus, respiratory adenovirus,respiratory syncytial virus and mycoplasma pneumoniae. The invention provides a composition for detecting the pathogens. Meanwhile, the invention further provides a kit containing the composition, application of the composition and a method for detecting and typing the pathogens causing respiratory tract infection. The composition is combined with a fluorescent probe method, six pathogens causingrespiratory tract infection can be detected and typed at the same time in two tubes, and the advantages that the cost is low, the flux is high, the influence of interference substances is avoided, operation is easy and convenient, and false positive and environmental pollution caused by sample crossing are avoided are achieved.
Owner:SANSURE BIOTECH INC
Method for preparing veterinary oxytetracycline-artemisinin injection and clinical application of veterinary oxytetracycline-artemisinin injection
InactiveCN102228462AReduce stimulationLeading technologyAntibacterial agentsAntipyreticFLUNIXIN MEGLUMINECompound organic
The invention relates to a method for preparing a veterinary oxytetracycline-artemisinin injection and clinical application of the veterinary oxytetracycline-artemisinin injection. Raw materials of the injection mainly comprise oxytetracycline, artemisinin, trimethoprim, flunixin meglumine, magnesium oxide or magnesium chloride, an antioxidant, a pH regulator, a compound organic solvent and water. As a veterinary compound preparation, the veterinary oxytetracycline-artemisinin injection is mainly used for treating infectious diseases and secondary infection which are caused by sensitive gram positive bacteria and negative bacteria, eperythrozoon, rickettsia and mycoplasma. The method for preparing the veterinary compound oxytetracycline-artemisinin injection is simple, the production process is mild, the chelating temperature is low, the stability is high, and the injection is convenient to use and is suitable for industrialized production.
Owner:SHANDONG DEZHOU SHENNIU ANIMAL HEALTH PROD CO LTD
Diagnosis of systemic lupus erythematosus
Diagnostic methods for the detection of SLE in a human body sample are disclosed. Nucleic acid hybridization and antibody-based methods derived from identification of Mycoplasma haemosapiens or its 16S sequence are described.
Owner:SPHINGOMONAS RES PARTNERS
Method of expressing protein having unnatural amino acid integrated thereinto
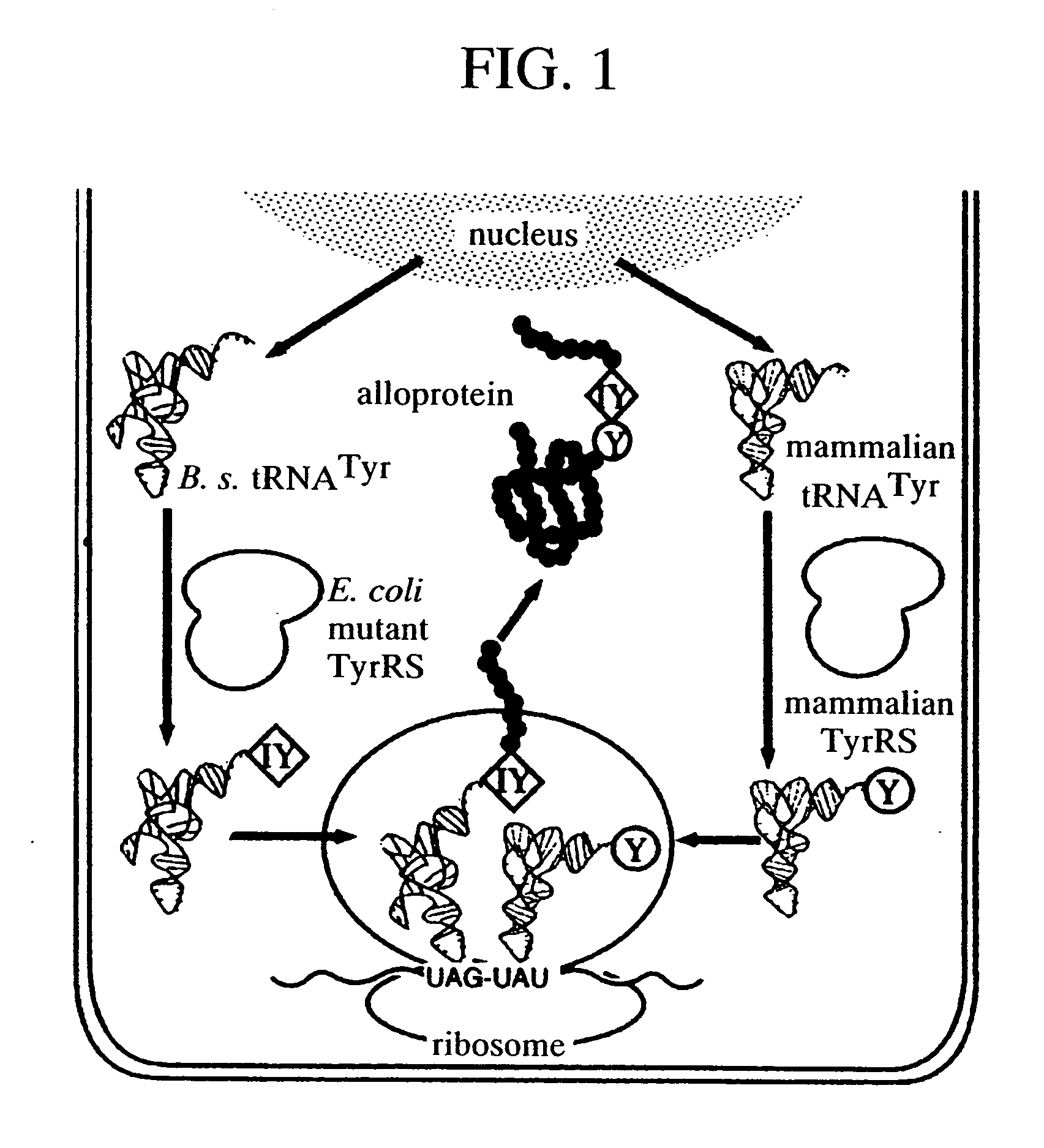
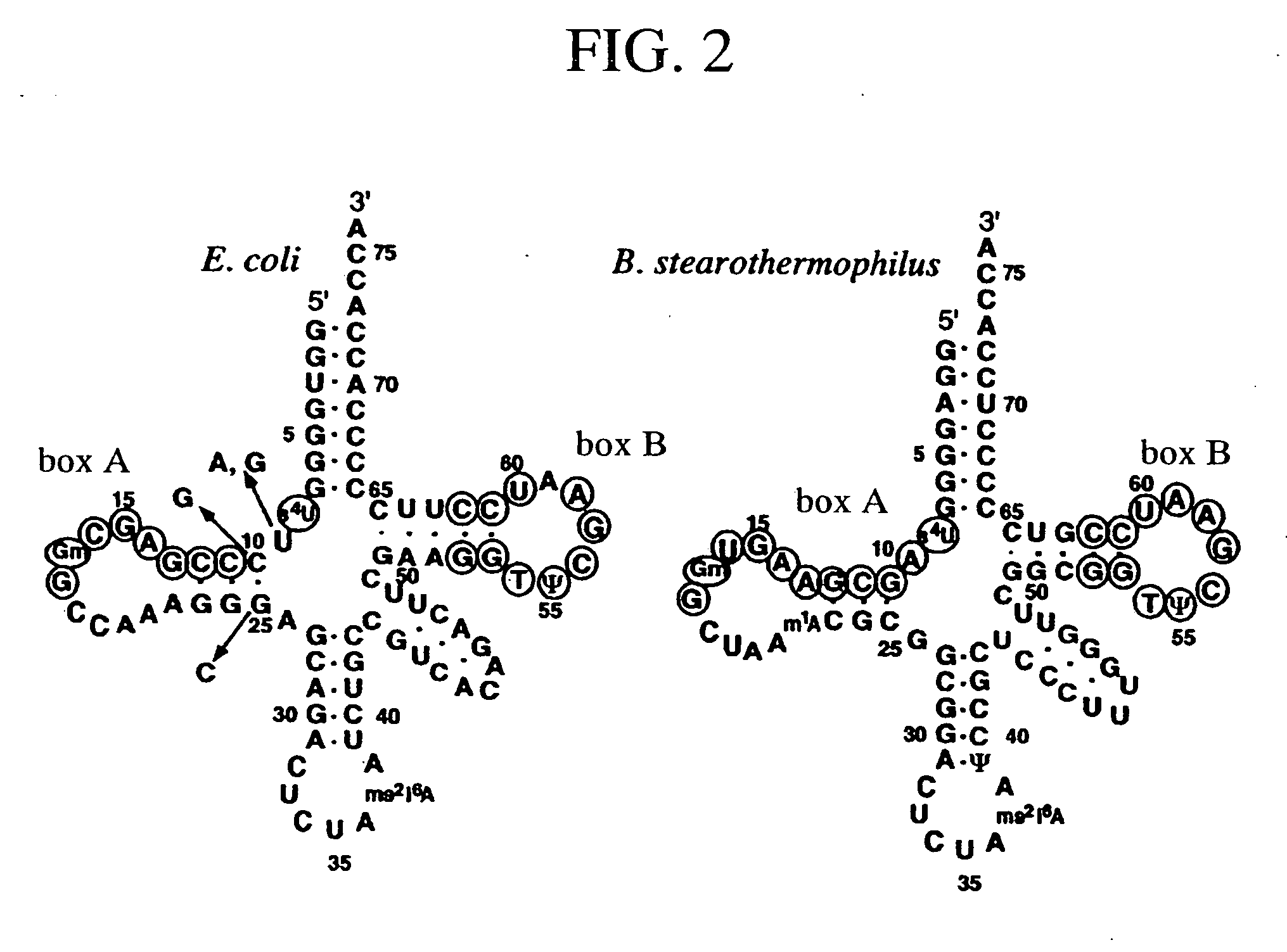
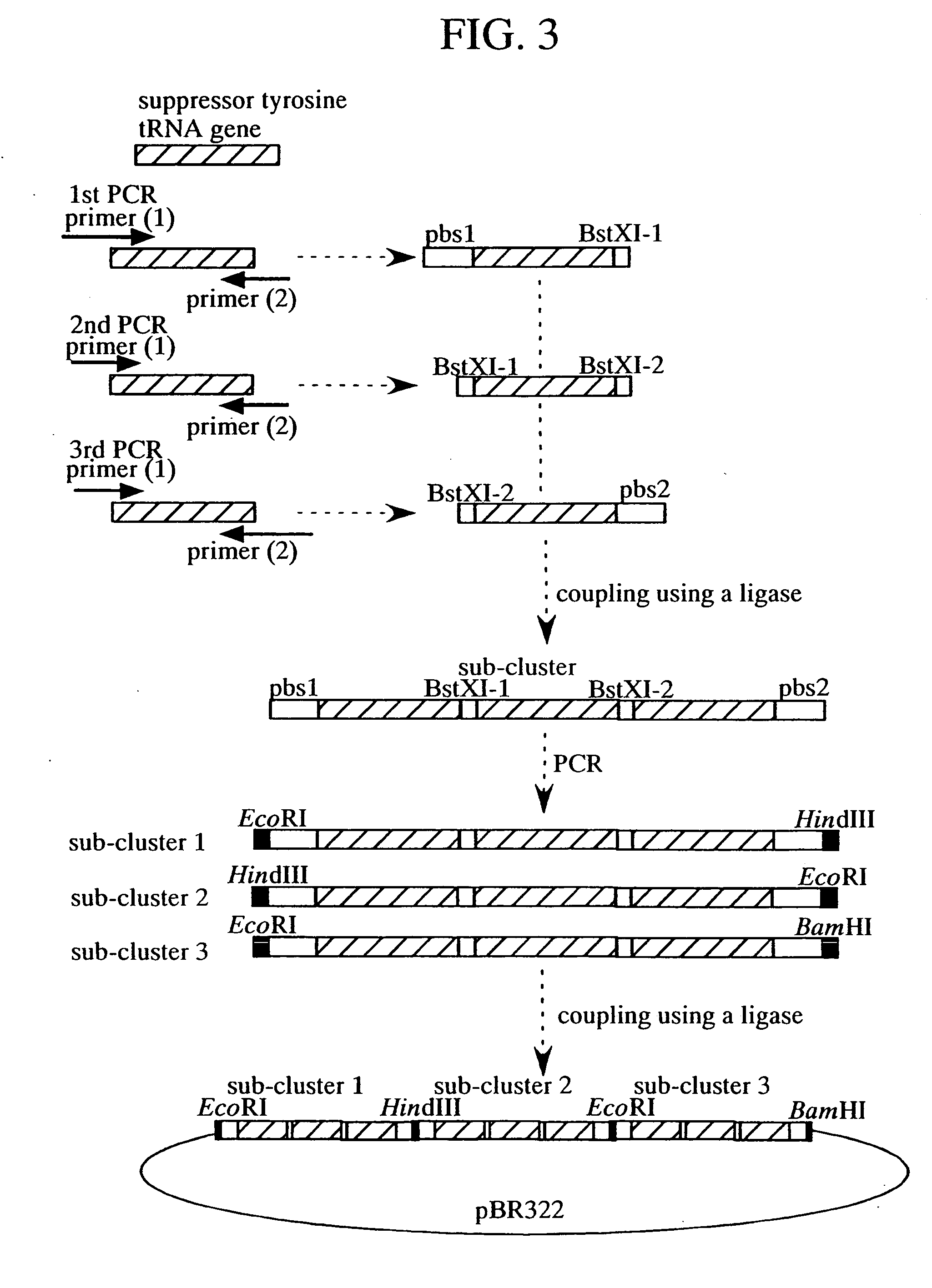
A method of expressing a protein having an unnatural amino acid integrated thereinto characterized by comprising expressing (A) a mutant tyrosyl tRNA synthase which is a mutant of Escherichia coli-origin tyrosyl tRNA synthase and has an elevated specificity for a unnatural tyrosine derivative compared with the specificity for tyrosine, (B) a suppressor tRNA originating in an eubacterium belonging to the genus Bacillus, Mycoplasma or Staphylococcus which is capable of binding to the above tyrosine derivative in the presence of the above mutant TyrRS, and (C) a desired protein gene having a nonsense mutation at a desired site in animal cells to thereby incorporate the above tyrosine derivative into the nonsense mutation site in the above protein.
Owner:RIKEN
Kit and method for detecting mycoplasma pollution in CHO cultured cells
InactiveCN103627781AMicrobiological testing/measurementMicroorganism based processesMicrobiologyMycoplasma contamination
The invention relates to a kit and a method for detecting mycoplasma pollution in CHO cultured cells, and belongs to the field of biological technology detection. The kit comprises a primer pair of the DNA sequence of a specific amplified CHO cell glyceraldehyde-3-phosphatedehydrogenase and a primer pair of the DNA sequence of a specific amplified mycoplasma 16srRNA conserved domain, and the two above primer pairs are placed in a same PCR system. According to the kit, when mycoplasma pollution is detected by employing the PCR technology, the determination of reliability of a detection result can be finished at the same time. The kit and the method help to substantially improve the reliability of the detection result on mycoplasma pollution in CHO cultured cells, the operation is simple, the detection period is short, and the sample detection operationality is good.
Owner:NCPC NEW DRUG RES & DEV
Mycoplasma hyopneumoniae vaccine
Owner:ZOETIS SERVICE LLC
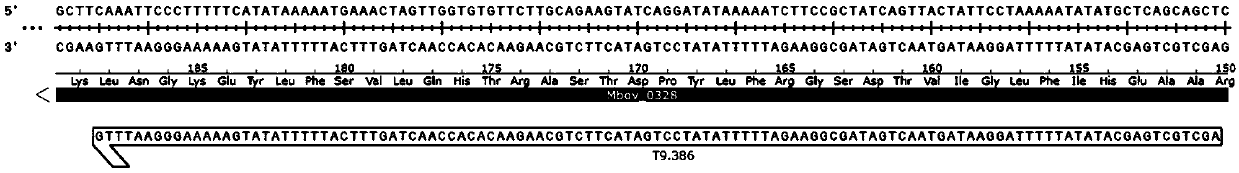
Mycoplasma bovis mutant strain with growth defect under cell coculture and application
ActiveCN109652357AMarked growth defectSignificantly small colony phenotypeBacteriaHydrolasesPhosphodiesteraseBovine embryo
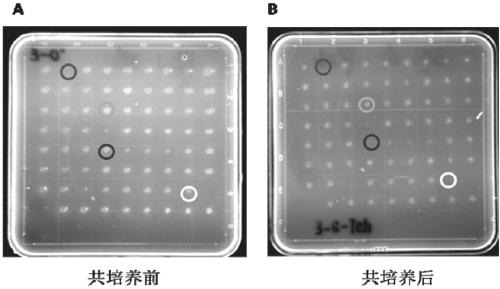
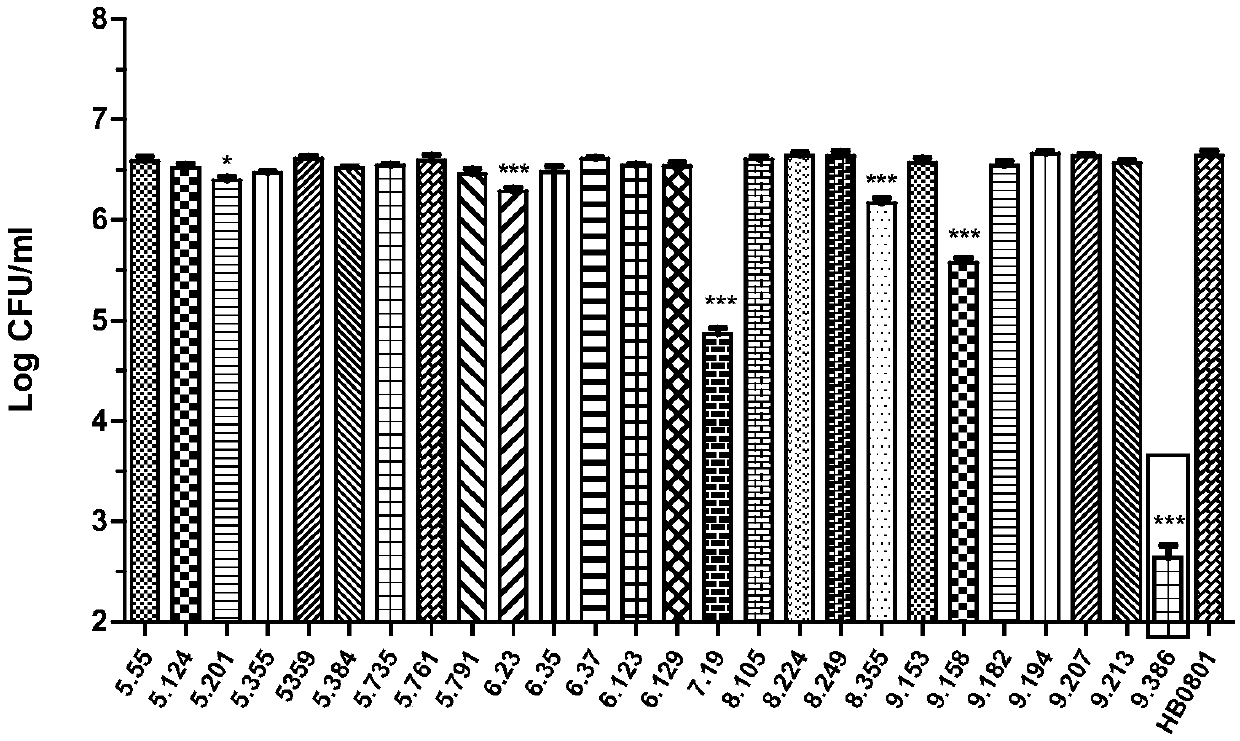
The invention belongs to the technical field of zoonosis prevention and treatment, and particularly relates to a mycoplasma bovis mutant strain with a growth defect under cell coculture and application. The mycoplasma bovis Mbov-0328 gene deletion mutant strain T9.386 is sifted out from a mycoplasma bovis gene mutant library, and the gene is coded to form cyclic dinucleotide phosphodiesterase. When the mutant strain and a bovine embryo pneumonocyte are co-cultured, the remarkable growth defect phenotype is shown; the mutant strain shows small colonial morphology on a PPLO solid culture medium.Proteomics between the mutant strain and a wild strain shows a remarkable difference expression spectrum. The mutant strain has 38 differential expression proteins, wherein 30 proteins are in up-regulated expression, and 8 proteins are in down-regulated expression. The mutant strain can be applied to the field of mycoplasma bovis metabolic physiology, pathopoiesia and immune prevention.
Owner:HUAZHONG AGRI UNIV
Primers, probes and kit for rapidly detecting mycoplasma bovis on site
InactiveCN106868167AExcellent detection timeStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationForward primerBacteroides
The invention discloses primers, probes and a kit for rapidly detecting mycoplasma bovis on site. The kit comprises a forward primer sequence shown as SEQ No.1, a reverse primer sequence shown as SEQ No.2, and a probe sequence shown as SEQ No.3, wherein the 5'-terminal of the reverse primer sequence is labeled by biotin; the 5'-terminal of the probe sequence is labeled by FAM, dSpacer is arranged at the position away from the 5'-terminal by 30 basic groups, and the 3'-terminal is blocked by C3-spacer. The primers, the probe assembly and the kit for detecting the mycoplasma bovis RPA-nfo are high in sensitivity and strong in specificity, the mycoplasma bovis DNA of 10 copies / reaction can be detected at the minimum, and the primers and the probes for detecting RPA-nfo respectively have no cross reaction with other mycoplasmas, as well as the bacteria including pasteurella multocida, mannheimia haemolytica, arcanobacterium pyogenes, histophilus somni, and streptococcus pneumoniae.
Owner:SHANDONG NORMAL UNIV
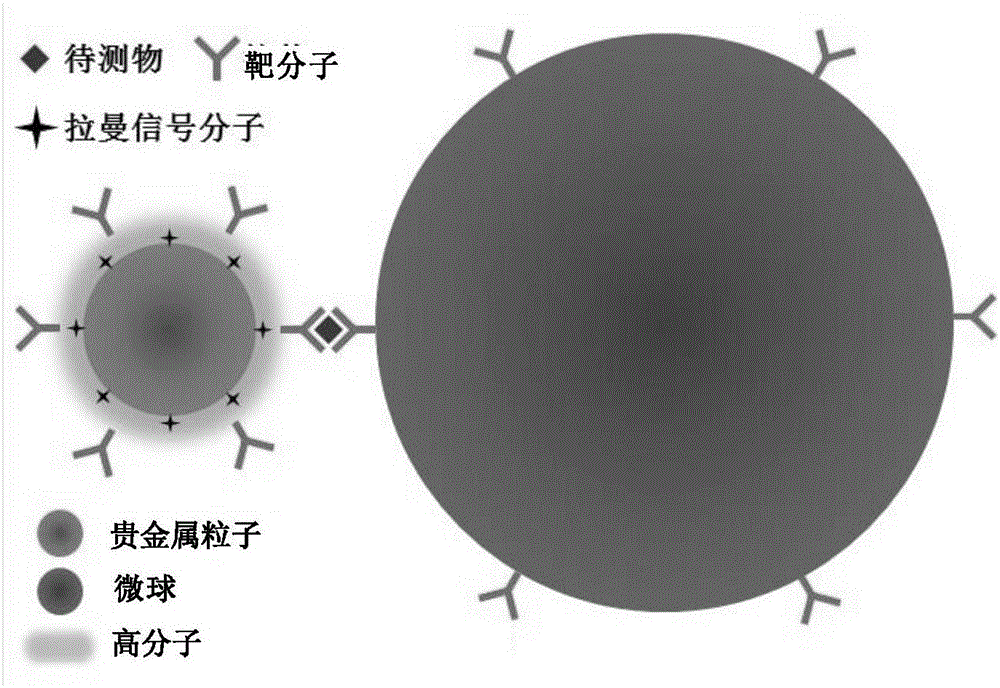
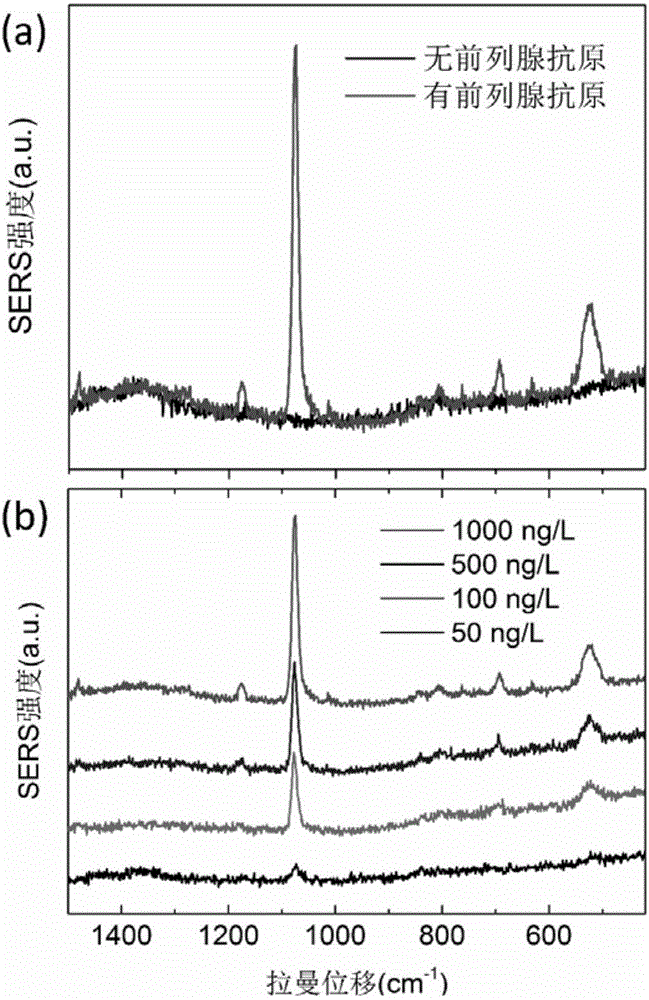
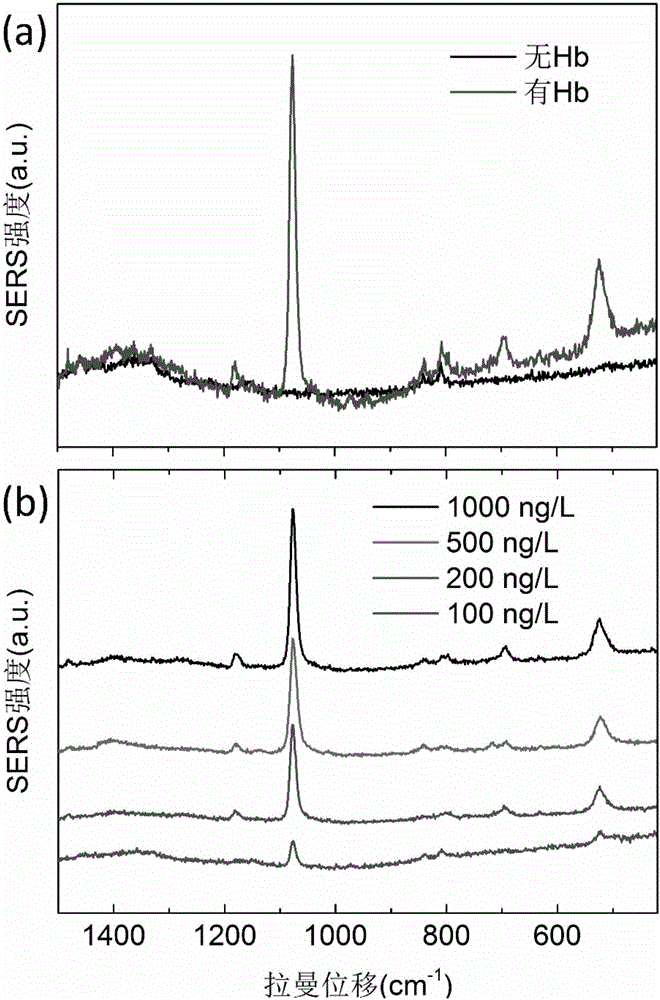
Surface enhanced Raman scattering technology-based composite material and preparation method thereof
The invention relates to a surface enhanced Raman scattering technology-based composite material and a preparation method thereof. The composite material comprises microspheres modified with first target molecules on the surfaces and a second component modified with Raman signal molecules and second target molecule-modified polymers on the surface. In a solution containing a substance to be detected, the substance to be detected, the microspheres and the second component are bonded to the second target molecules through the first target molecules so that the composite material which can be easily precipitated through centrifugation is obtained. The invention also discloses the preparation method and a use of the composite material. The composite material can be used for detecting a human prostate-specific antigen (PSA), a human ferrohemoglobin (Hb), a procalcitonin (PCT) or disease markers (such as tumor markers, cardiovascular disease markers, senile dementia markers, mycoplasma and chlamydia), can effectively reduce false positive and false negative effects and has advantages of high sensitivity, simpleness, fastness and low cost.
Owner:NINGBO INST OF MATERIALS TECH & ENG CHINESE ACADEMY OF SCI
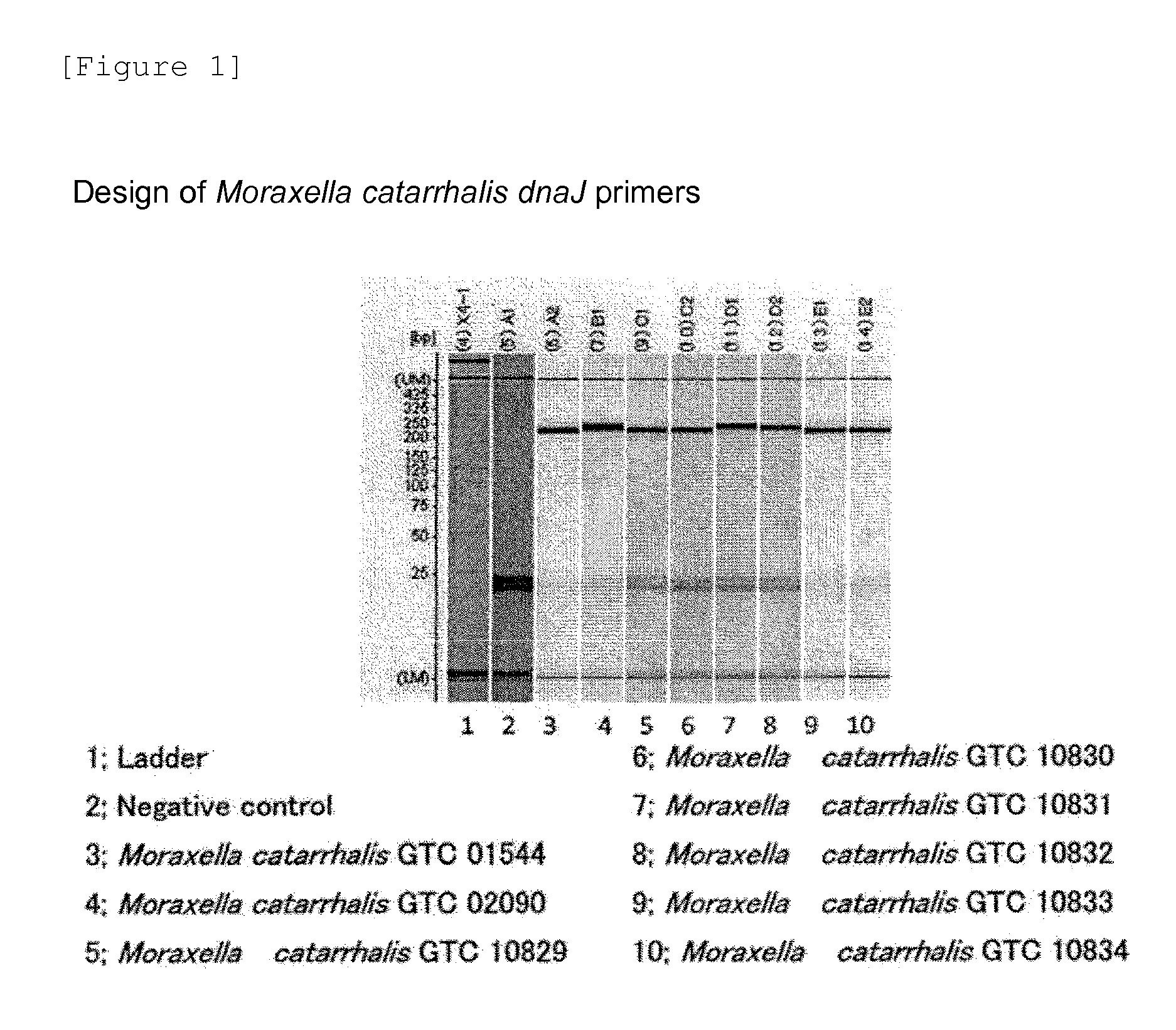
Method for detecting pneumonia causative bacteria using nucleic acid chromatography
InactiveUS20130023443A1Quick and accurate identificationNucleotide librariesMicrobiological testing/measurementBacteroidesStaphylococcus aureus
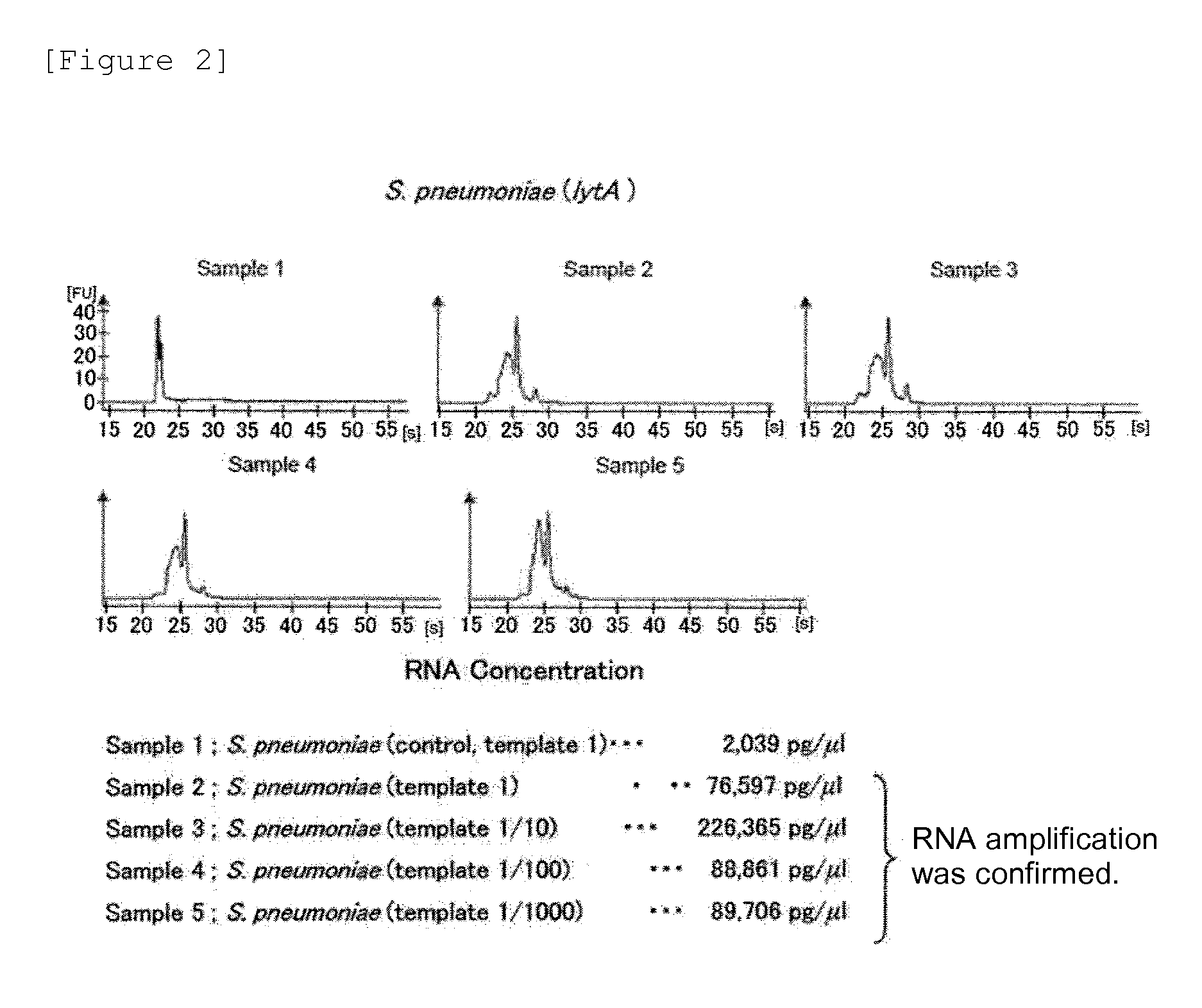
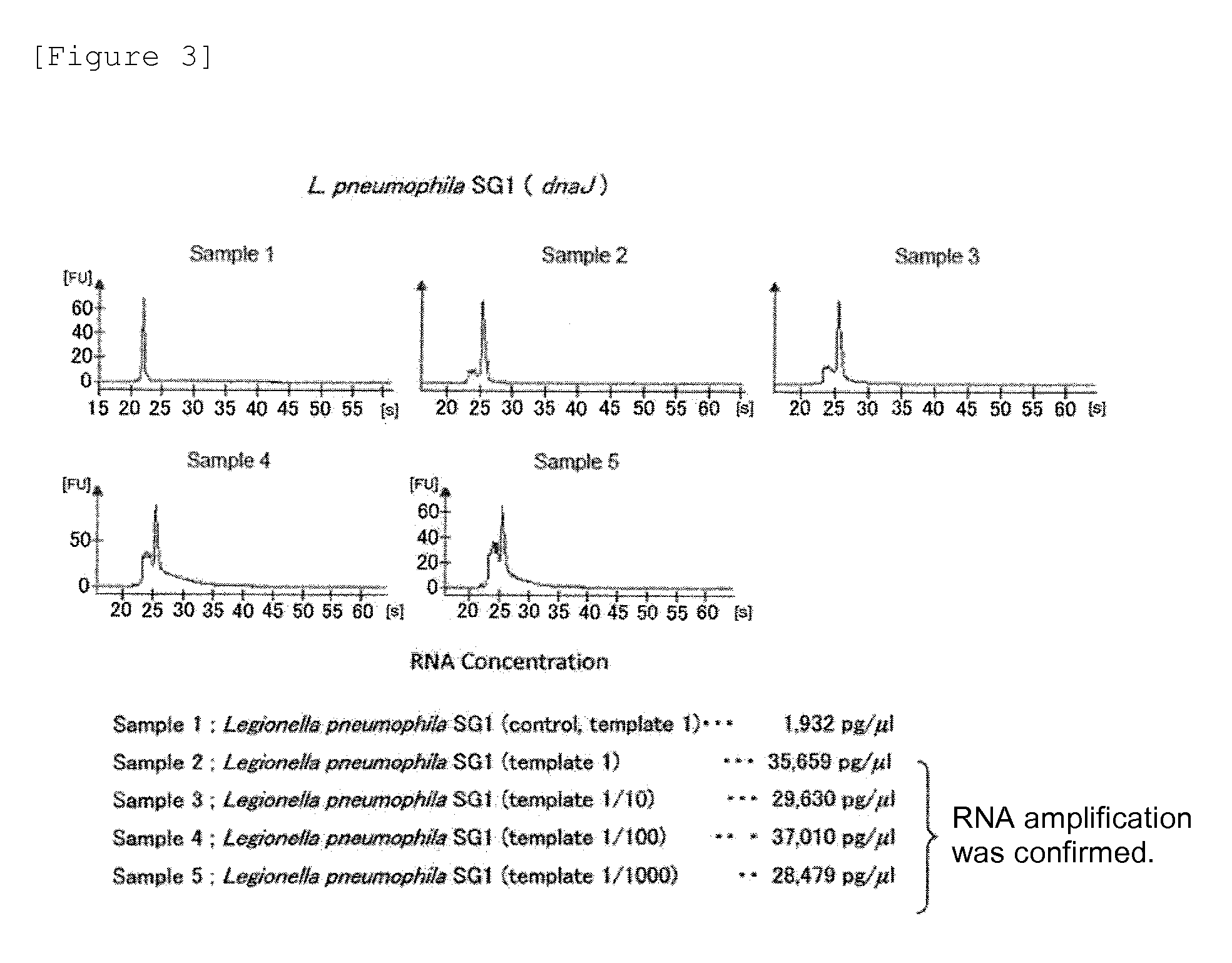
Provided are a method and a kit for accurately and rapidly detecting ten types of targeting pneumonia bacteria: Streptococcus pneumoniae, Haemophilus influenzae, Mycoplasma pneumoniae, Chlamydophila pneumoniae, Legionella pneumophila, Klebsiella pneumoniae, Pseudomonas aeruginosa, Moraxella catarrhalis, methicillin-resistant Staphylococcus aureus (MRSA), and Staphylococcus aureus. A set of primer pairs directed to their respective target regions contained in the DnaJ gene, etc., of the ten types of pneumonia causative bacteria is designed for the ten bacterial strains and used to amplify gene products. A set of bacterial strain-specific probe pairs is further designed for the ten bacterial strains such that the probe pairs hybridize with the amplification products via sequences in the respective target regions differing from the sequences hybridized by the set of primer pairs. A first probe-bound labeled high molecular carrier in which plural types of first probes for the pneumonia bacteria are bound to a labeled high molecular carrier and a solid-phase second probe-carrying developing support are used as the set of probe pairs to perform nucleic acid chromatography.
Owner:YAMAGUCHI TECH LICENSING ORG
Fusion protein of mycoplasma pneumonia protein epitope and preparation and application thereof
ActiveCN105884902AEasy to purifyRefolding is not requiredAntibacterial agentsBacterial antigen ingredientsEpitopeGlycine
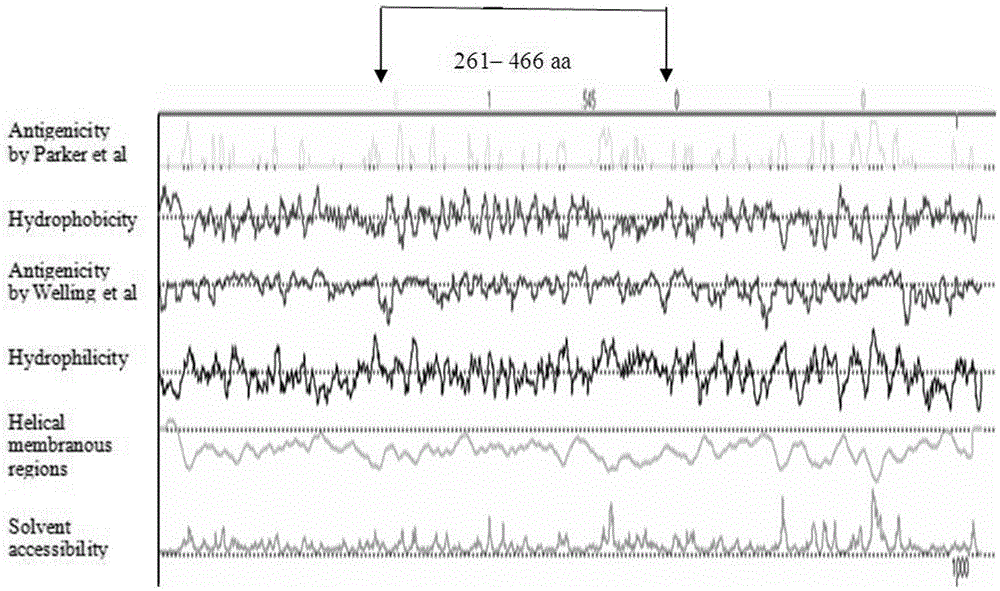
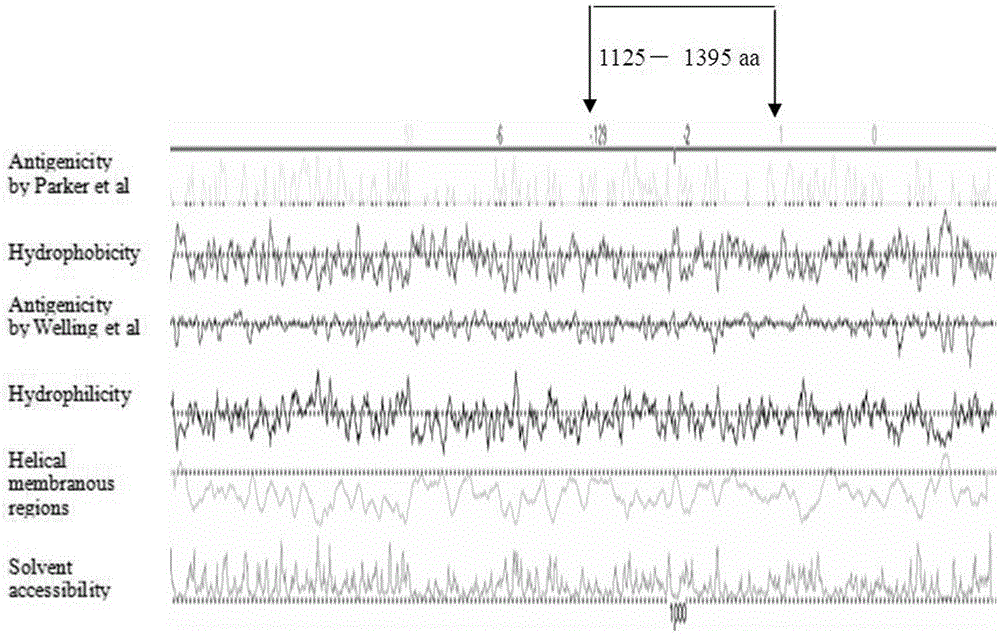
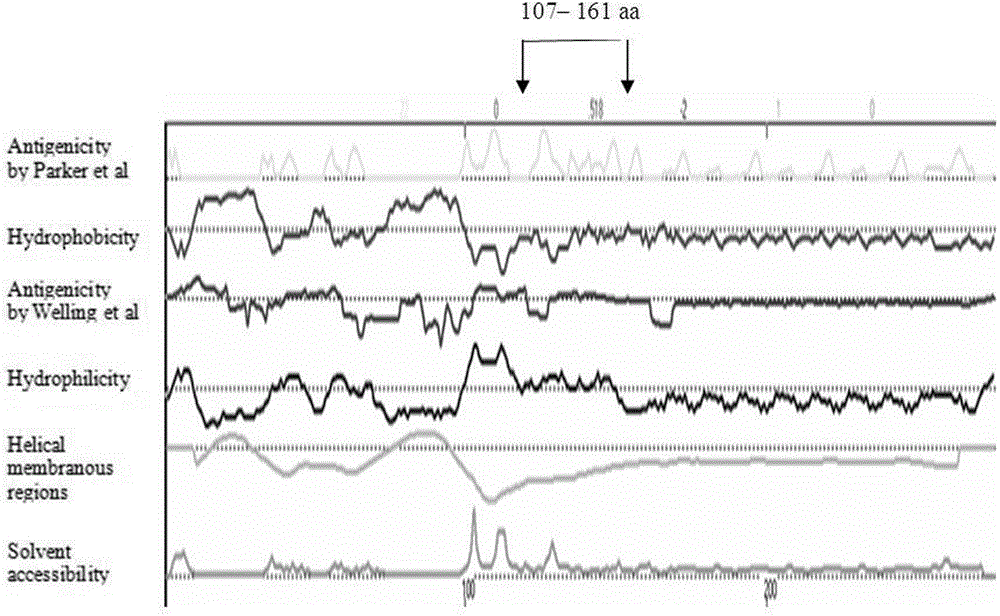
The invention discloses a fusion protein of a mycoplasma pneumonia protein epitope and preparation and application thereof, and relates to the fields of genetic engineering technologies, vaccines and diagnosis reagents. Amino acid sequences of a mycoplasma pneumoniae P116 protein, a P1 protein and a P30 protein are analyzed through a computer, mycoplasma pneumoniae P116 protein fragments, namely from the 261st amino acid to the 466th amino acid containing the strong epitope, mycoplasma pneumoniae P1 protein fragments, namely from the 1125th amino acid to the 1395th amino acid containing the strong epitope and mycoplasma pneumoniae P30 protein fragments, namely from the 107th amino acid to the 161st amino acid containing the strong epitope are screened out. Series connection gene sequences of novel mycoplasma pneumoniae P116 protein fragments, mycoplasma pneumoniae P1 protein fragments and mycoplasma pneumoniae P30 protein fragments are synthesized chemically, three gene fragments are connected in series, the fusion protein of P116 protein fragments, P1 protein fragments and P30 protein fragments is expressed by means of the genetic engineering technology, the three protein fragments are connected through glycine-glycine-serine, and 538 amino acids are formed in the overall length of the fusion protein.
Owner:李越希
Mycoplasma hyopneumoniae fusion gene and application
ActiveCN104293816AImproving immunogenicityHigh expressionAntibacterial agentsBacterial antigen ingredientsEscherichia coliNucleotide
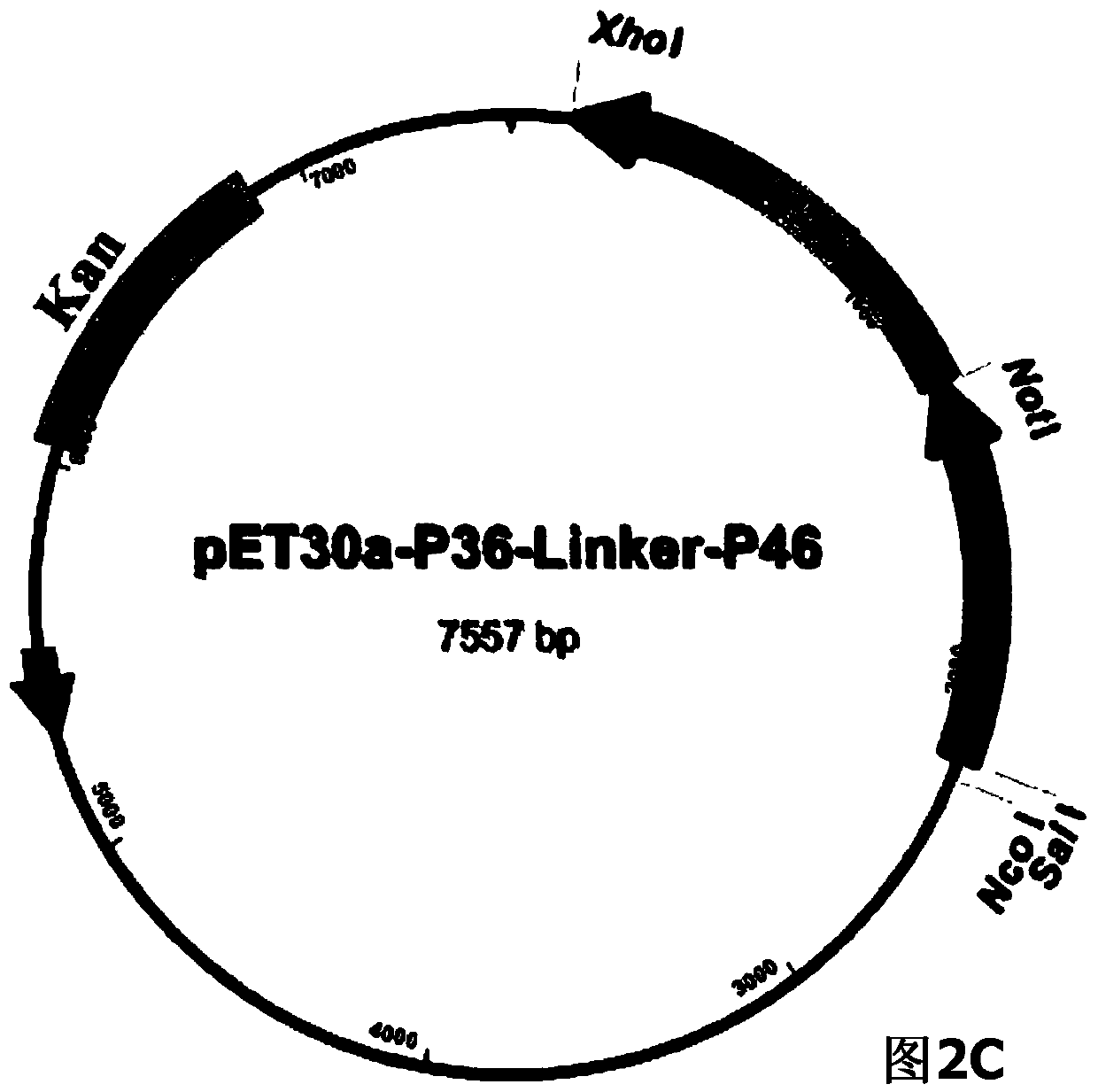
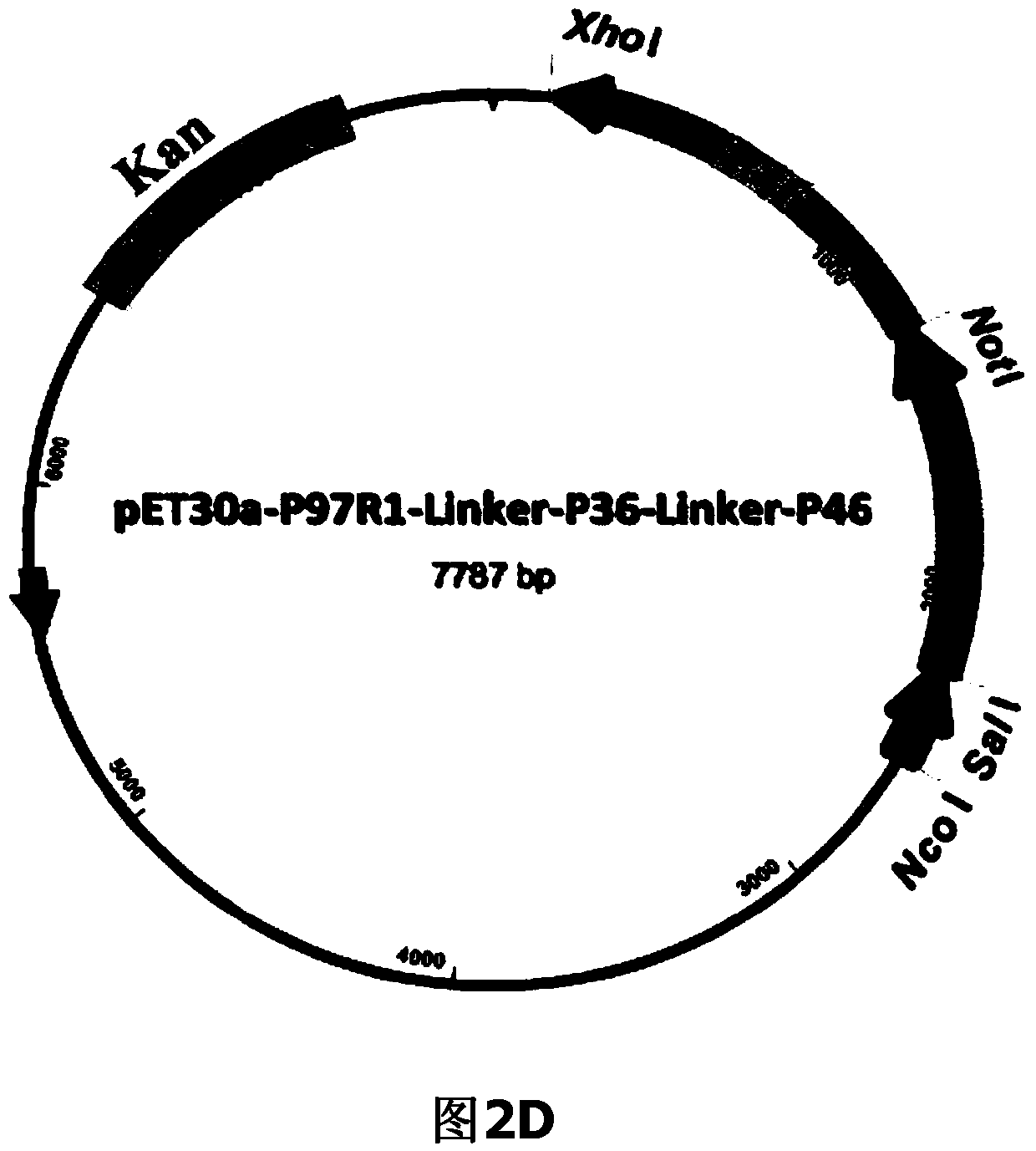
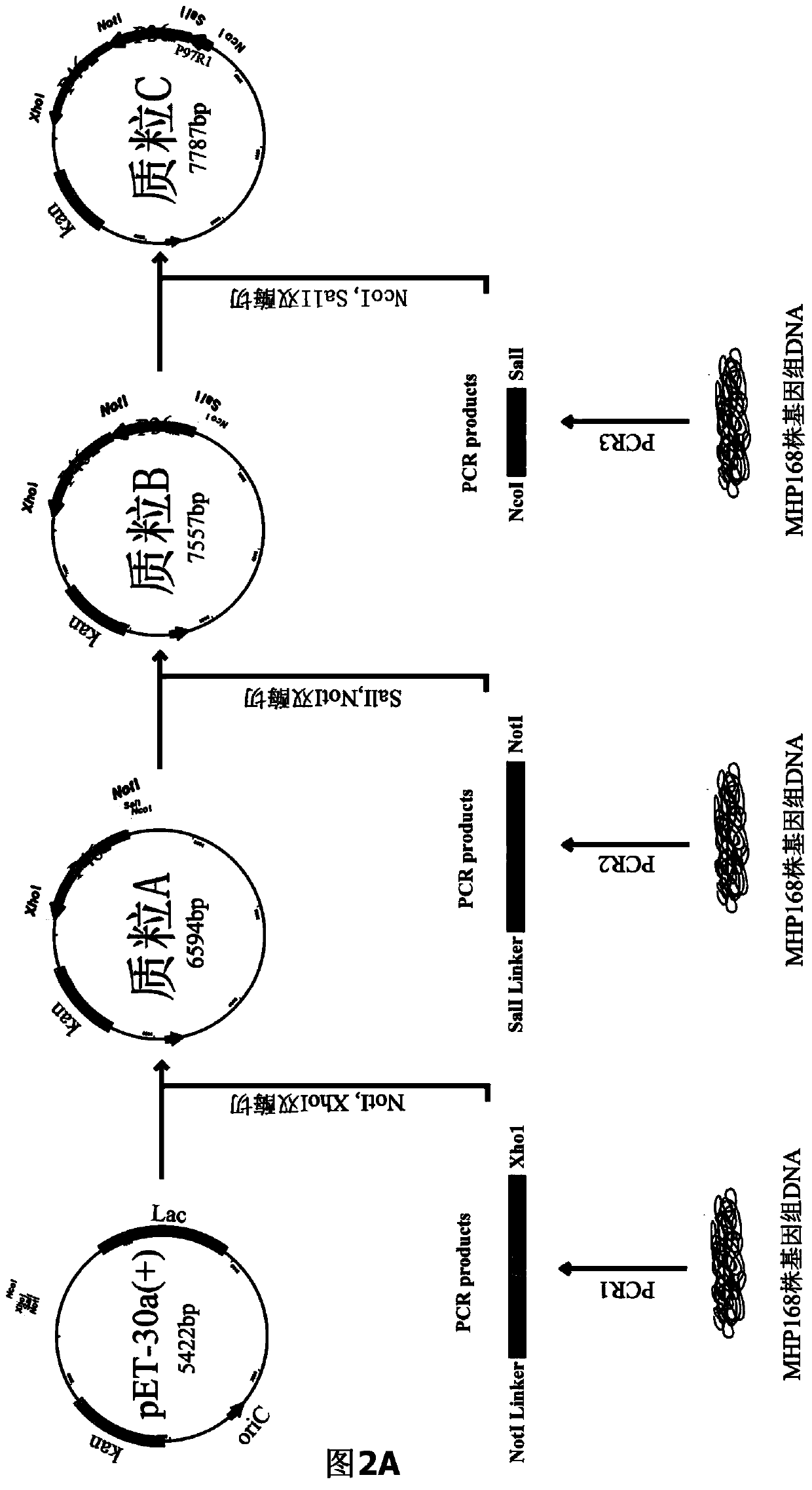
The invention belongs to the field of animal gene engineering, and in particular relates to a synthetic fusion gene for expressing mycoplasma hyopneumoniae and application. The fusion gene is characterized in that a mycoplasma hyopneumoniae P97R1 gene is connected in series with a P36 gene through a Linker, the termination codon of the P36 gene is deleted, the P46 gene with signal peptide removed is fused at the C end of the P36 gene through Linker, and then the fusion gene P97R1-P36-P46 is obtained, wherein the nucleotide sequence of the fusion gene is shown as SEQ ID NO: 1. The fusion gene is included in a prokaryotic expression plasmid, and escherichia coli BL21 / pET30a-P97R1-Linker-P36-Linker-P46 containing the plasmid is collected in CCTCC with the collection number CCTCC NO: M2014269. The invention further discloses immune efficacy evaluation of the fusion gene and application of the fusion gene in a novel vaccine.
Owner:HUAZHONG AGRI UNIV
Kit for loop-mediated isothermal amplification detection of Mycoplasma ovipneumoniae and preparation and usage methods thereof
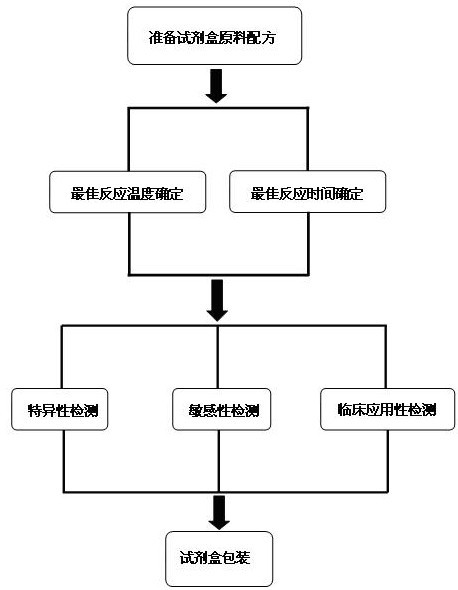
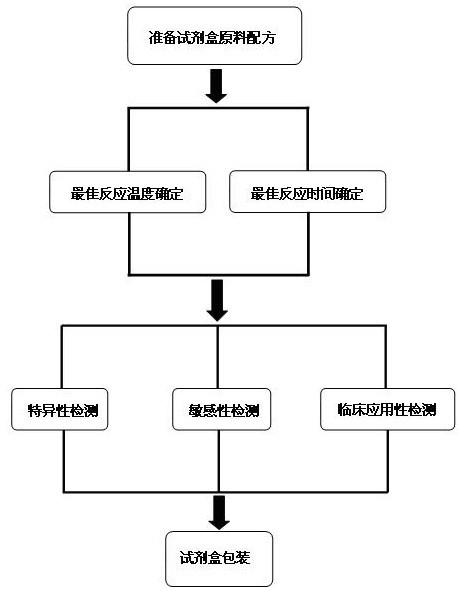
ActiveCN102634602AQuick checkReduce high costMicrobiological testing/measurementMicroorganism based processesPositive controlSpecific detection
The invention discloses a raw material composition of a kit for loop-mediated isothermal amplification (LAMP) detection of Mycoplasma ovipneumoniae and a preparation method and a usage method of the kit. The raw material composition comprises 1000-2000muL of reaction solution, 100-200muL of Bst (Bacillus stearothermophilus) DNA polymerase, 50-100muL of positive control, 50-100muL of negative control, 1-2mL of liquid paraffin and 1-2mL of ultrapure water. The reaction solution comprises an inner primer mixture solution, an outer primer mixture solution, an LAMP reaction buffer, Mg<2+> and dNTPs (deoxyribonucleotide triphosphates), wherein the volume ratio of the five liquids in the reaction solution is 2:2:2.5:2:1. The preparation method comprises the following steps: 1) determination of an optimum reaction temperature and an optimum reaction time; 2) specific detection, sensibility test and clinical application detection and 3) kit packaging. The kit disclosed by the invention has rapid, simple and accurate characteristics for pathogen detection of goat suspected cases of mycoplasma ovipneumoniae infection in goat farms.
Owner:GUIZHOU UNIV
PCV/mycoplasma hyopneumoniae/PRRS combination vaccine
Owner:ZOETIS SERVICE LLC
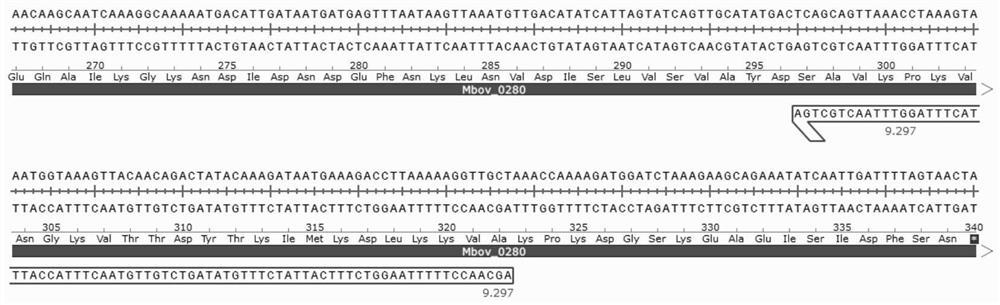
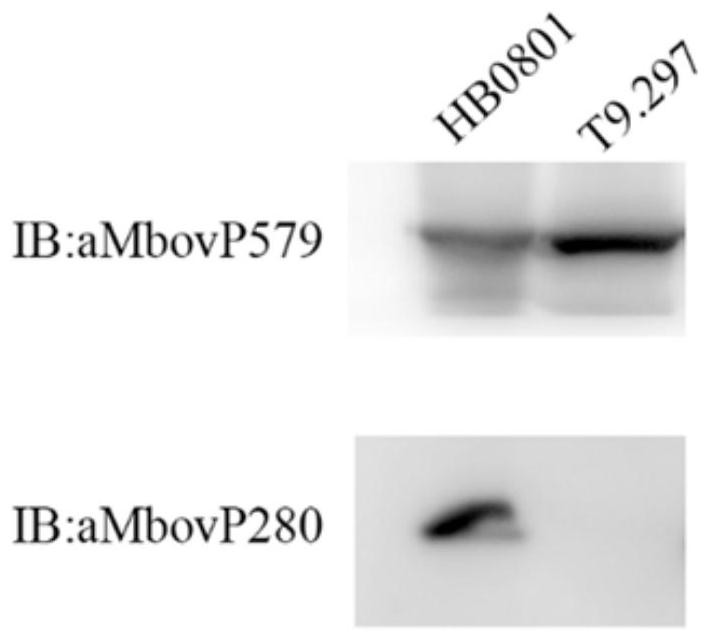
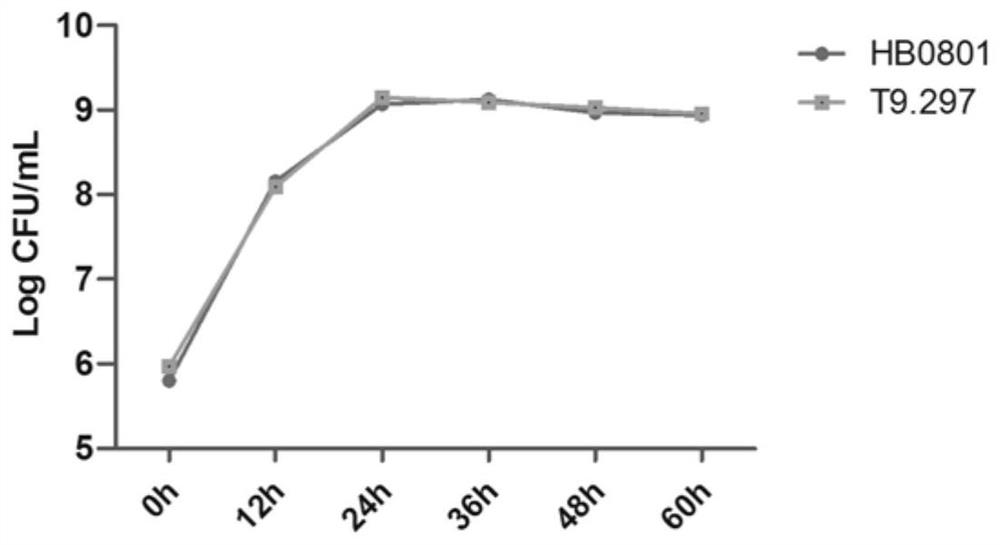
Mycoplasma bovis Mbov_0280 gene mutant and application thereof
ActiveCN111705026AReduce apoptosisAntibacterial agentsBacterial antigen ingredientsInfectious DisorderNucleotide
The invention discloses a mycoplasma bovis Mbov_0280 gene mutant and belongs to the technical field of animal infectious disease prevention and treatment. The mutant is named Mycoplasma bovis T9.297 is preserved in the China Center for Type Culture Collection and has a preservation number of CCTCC NO:M 2020085. The Mbov_0280 gene has a nucleotide sequence as shown in SEQ ID NO:1. The strain has the performance for inducing host macrophages apoptosis is weakened in comparison with a wild strain HB0801; the mutant has low toxicity since the bovine macrophages serve as a critical immune cell forresisting adventitious infection of a host; and the mutant favors a body to resisting mycoplasma bovis infection, and is expected to achieve a vital function in the field of mycoplasma bovis immunological control.
Owner:HUAZHONG AGRI UNIV
Methods for sterilizing biological materials using dipeptide stabilizers
InactiveUS20060182652A1Reducing residual solvent contentEffective sterilizationOther blood circulation devicesDead animal preservationYeastDipeptide
Methods are disclosed for sterilizing biological materials to reduce the level of active biological contaminants or pathogens such as viruses, bacteria, nanobacteria, yeasts, molds, mycoplasmas, ureaplasmas, prions and parasites. These methods involve the use of dipeptide stabilizers in methods of sterilizing biological materials with irradiation.
Owner:CLEARANT
Primer, probe and method for detecting human urological genital tract causal agent
InactiveCN101117646AImprove early detection rateSave operating timeMicrobiological testing/measurementDiseaseChlamydia trachomatis
The present invention discloses a guiding object and a probe for testing the human urogenital tract pathogens, and a method for testing the human urogenital tract pathogens using the guiding object and the probe. The present invention can test three different pathogens including Neisseria gonorrhoeae, mycoplasma ureaplasma and chlamydia trachomatis in a same reaction tube simultaneously. The present invention not only can increase the early detection rate of the sexually transmitted diseases, but also can reduce the operating time of the medical personnel, lower the cost, and lighten the economic burden of the patient.
Owner:SHANGHAI SHENYOU JIANHAI BIOLOGICAL TECH LIABILITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com