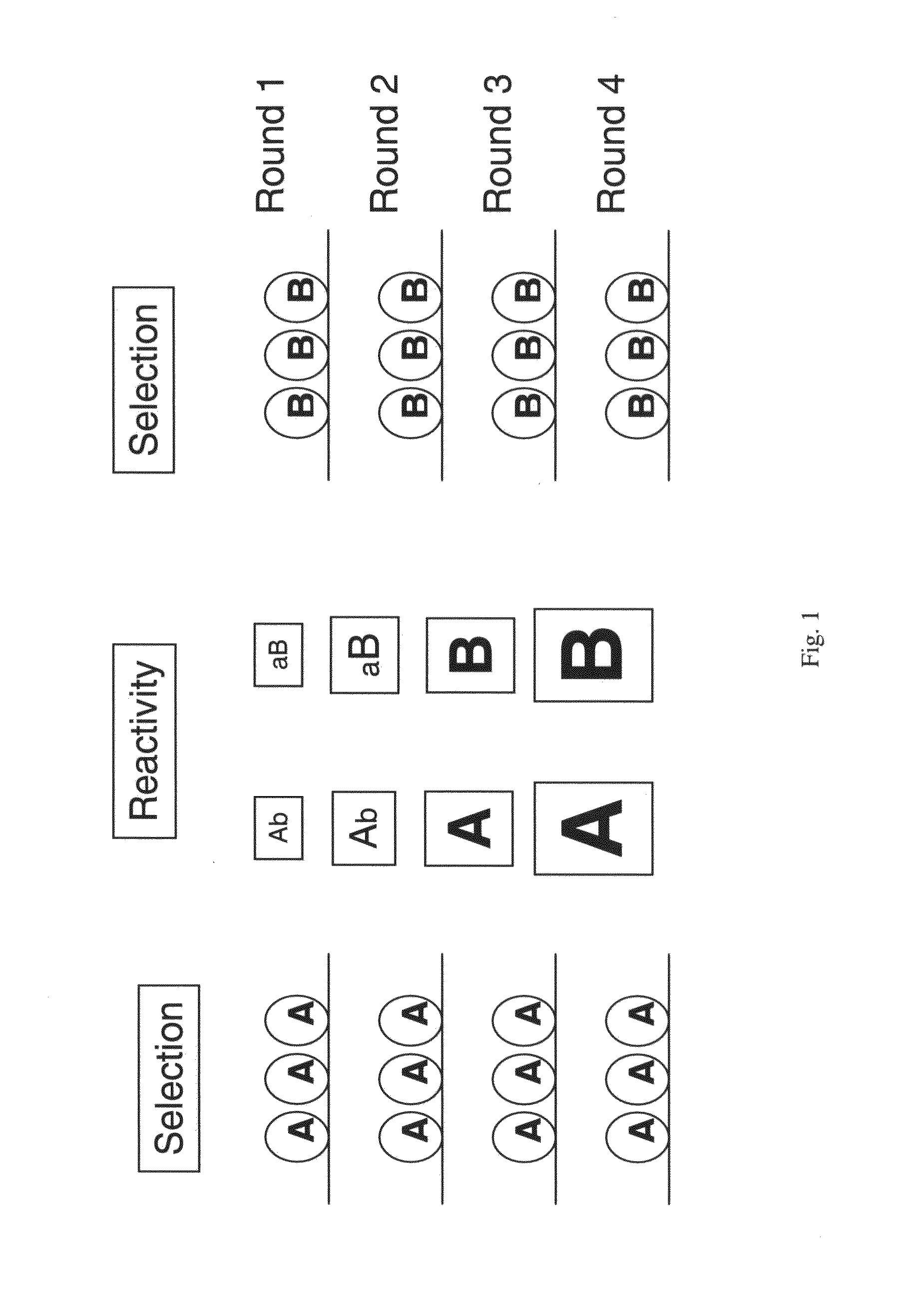
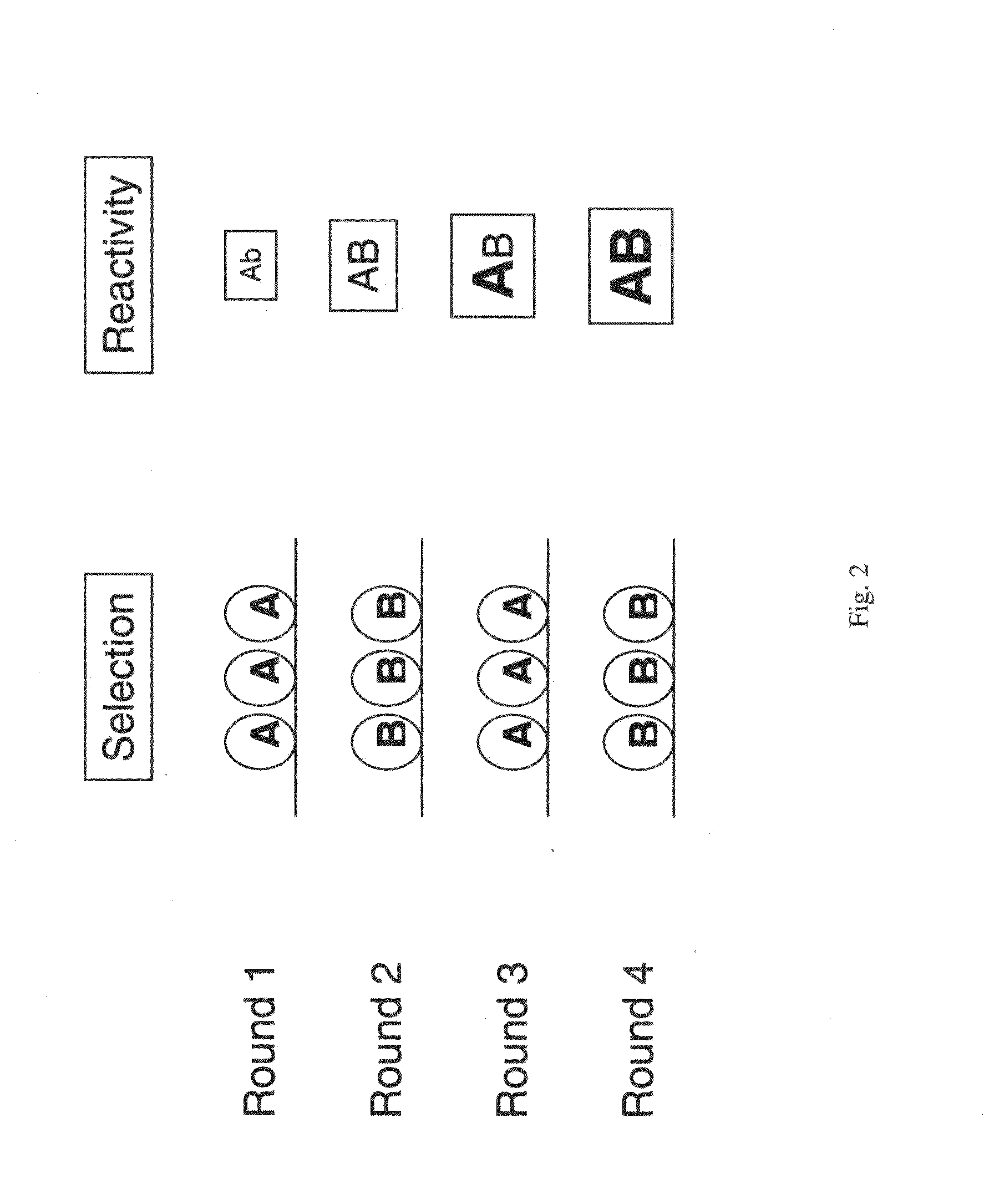
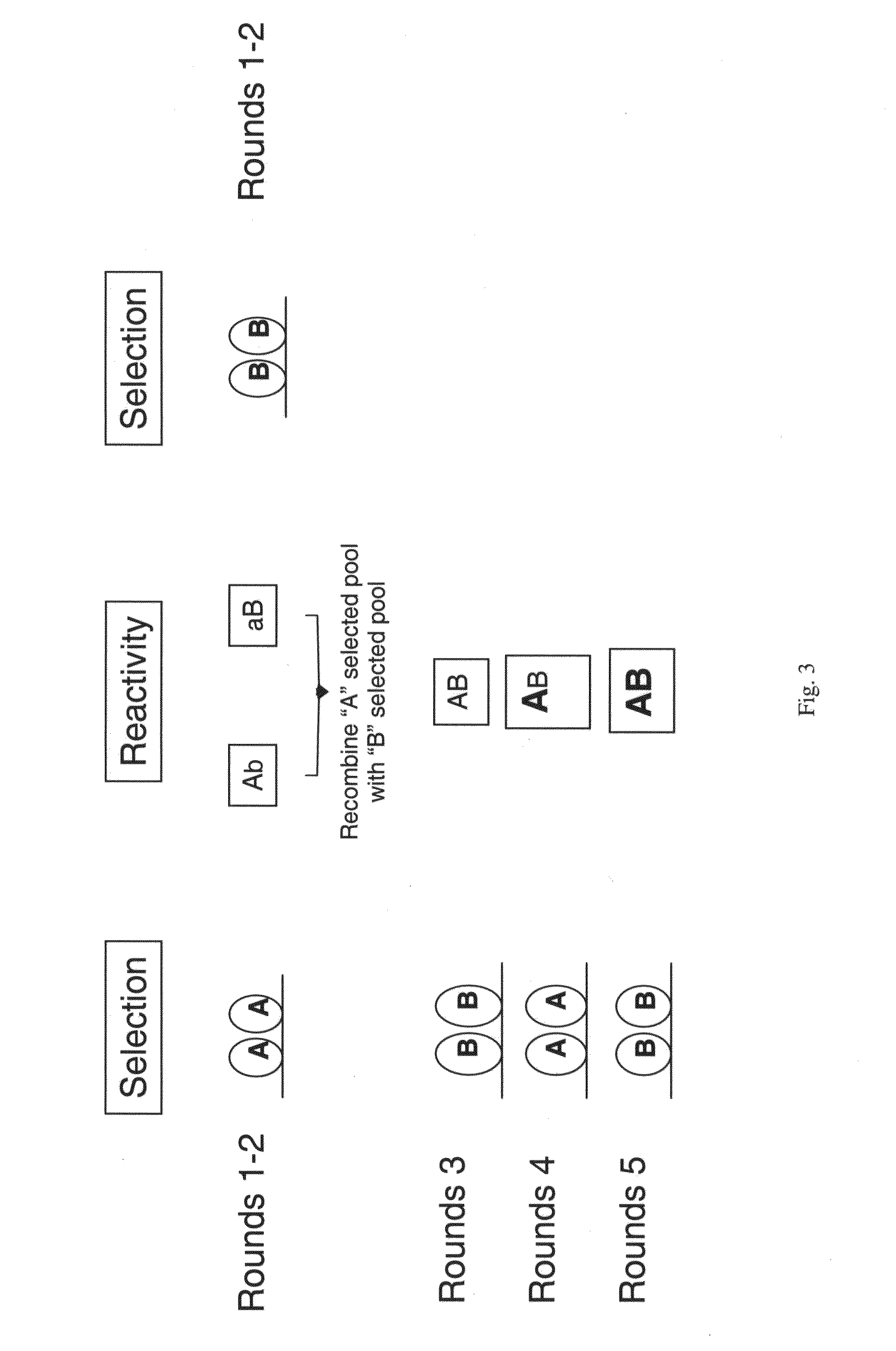
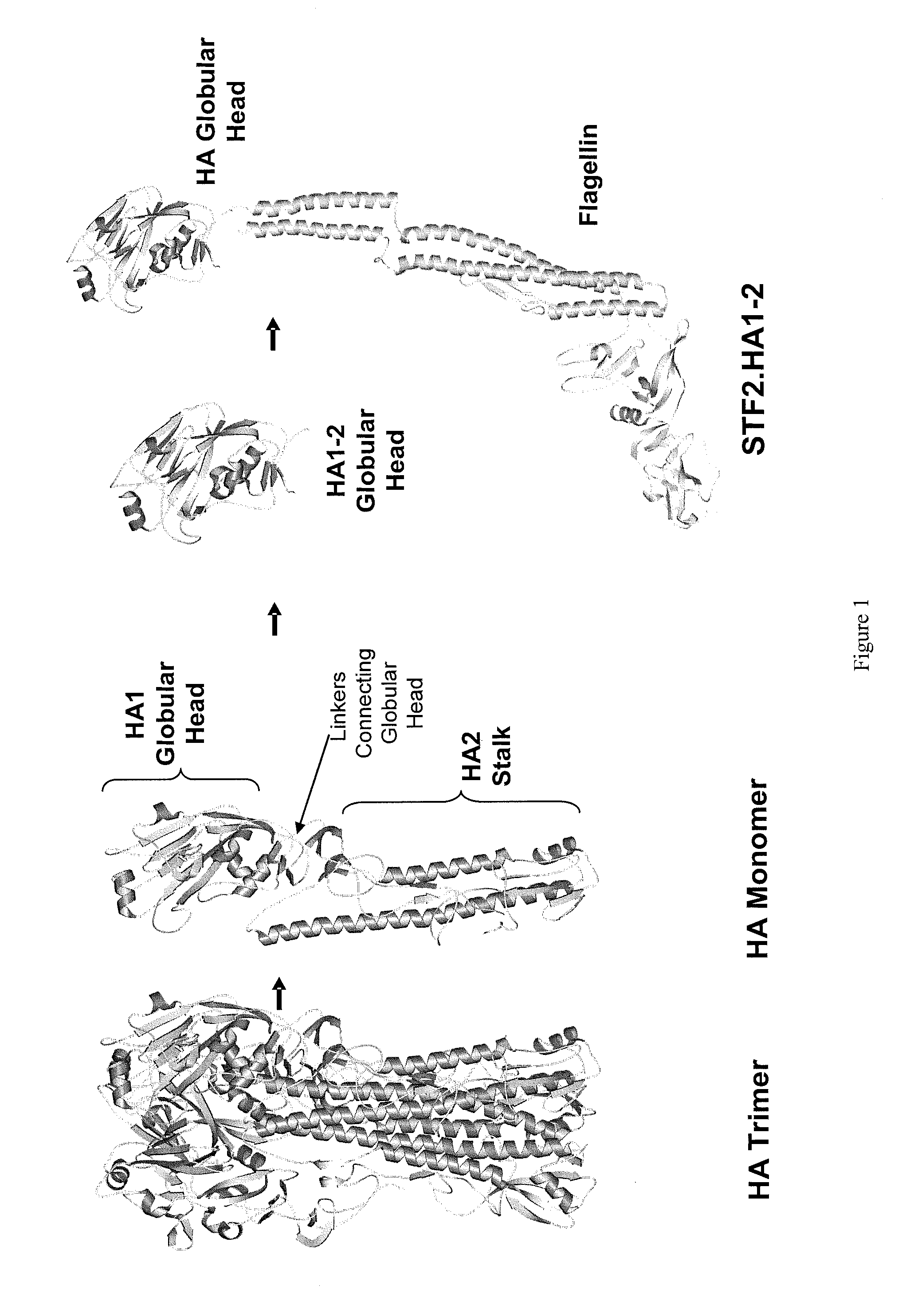
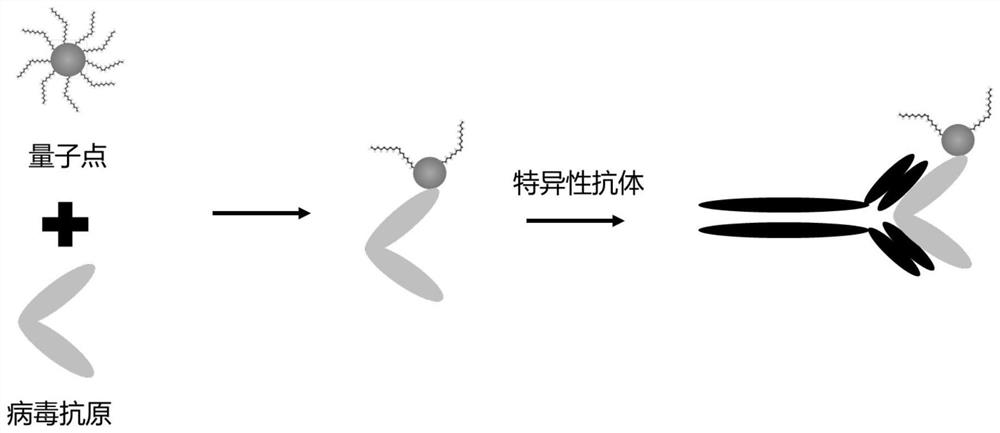
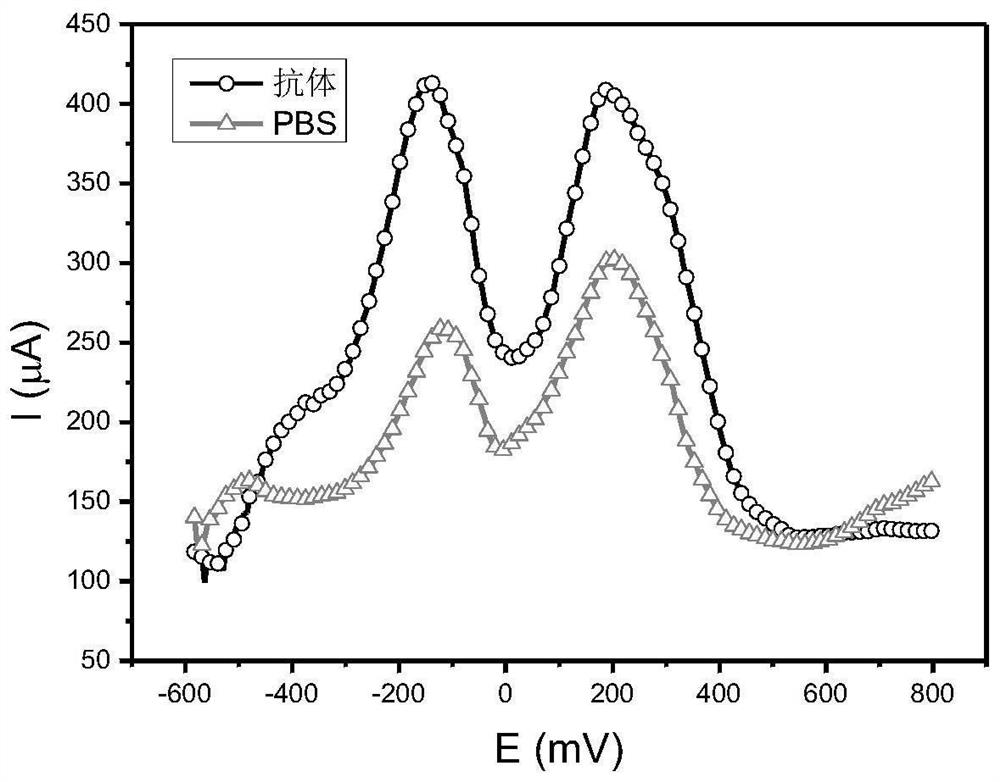
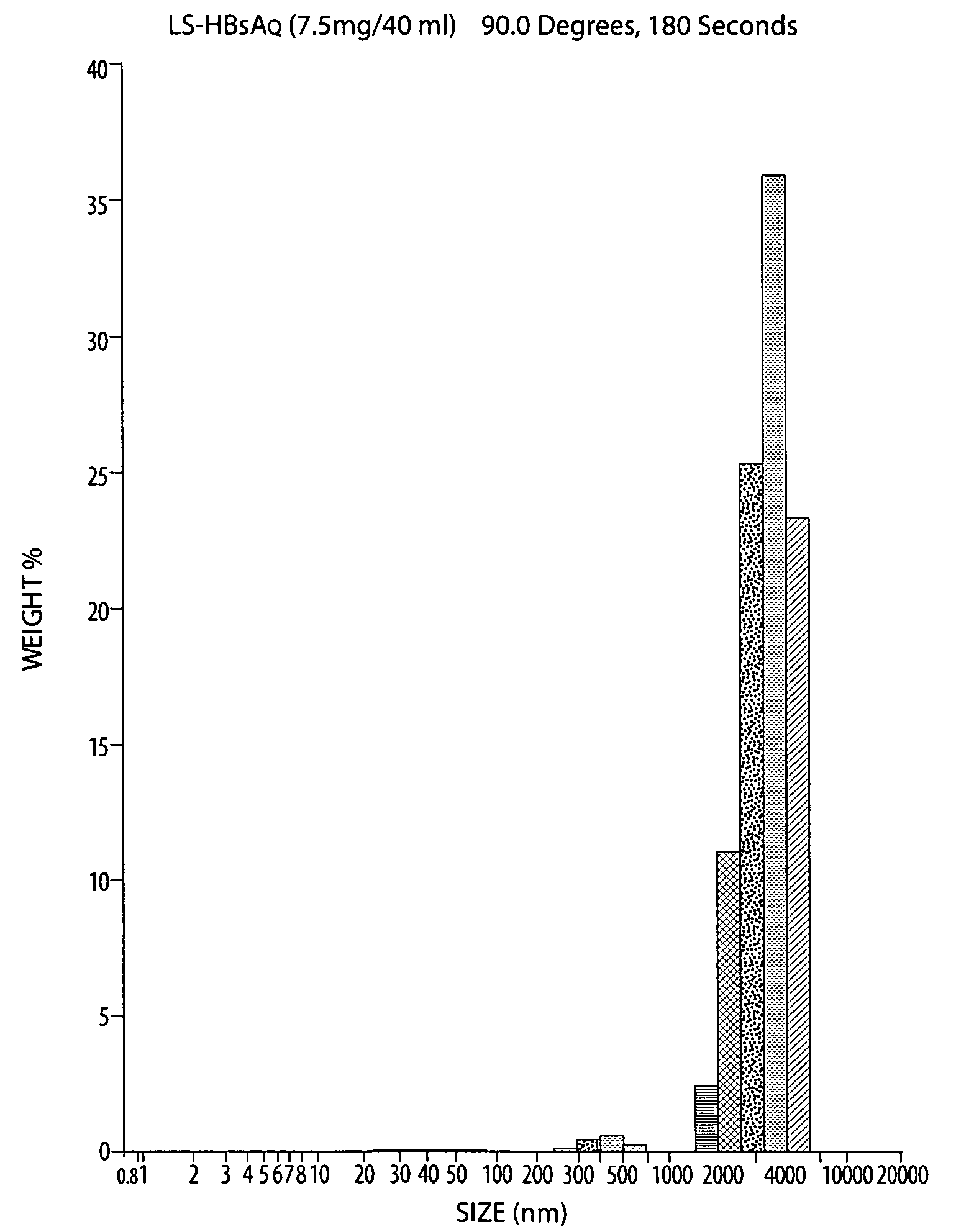
Patents
Literature
259 results about "Viral antigens" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A viral Antigen is an antigen with multiple antigenicities that is protein in nature, strain-specific, and closely associated with the virus particle.
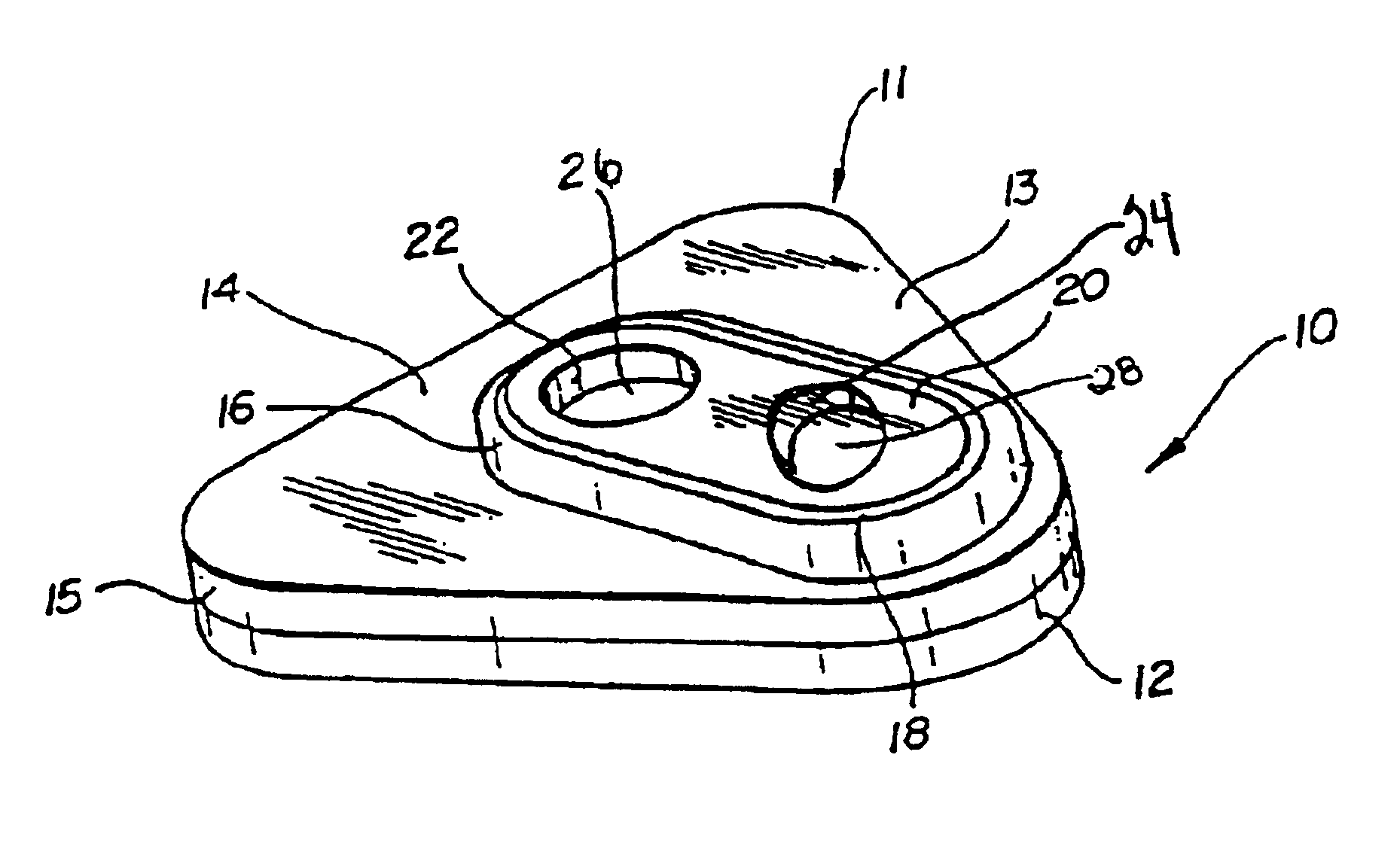
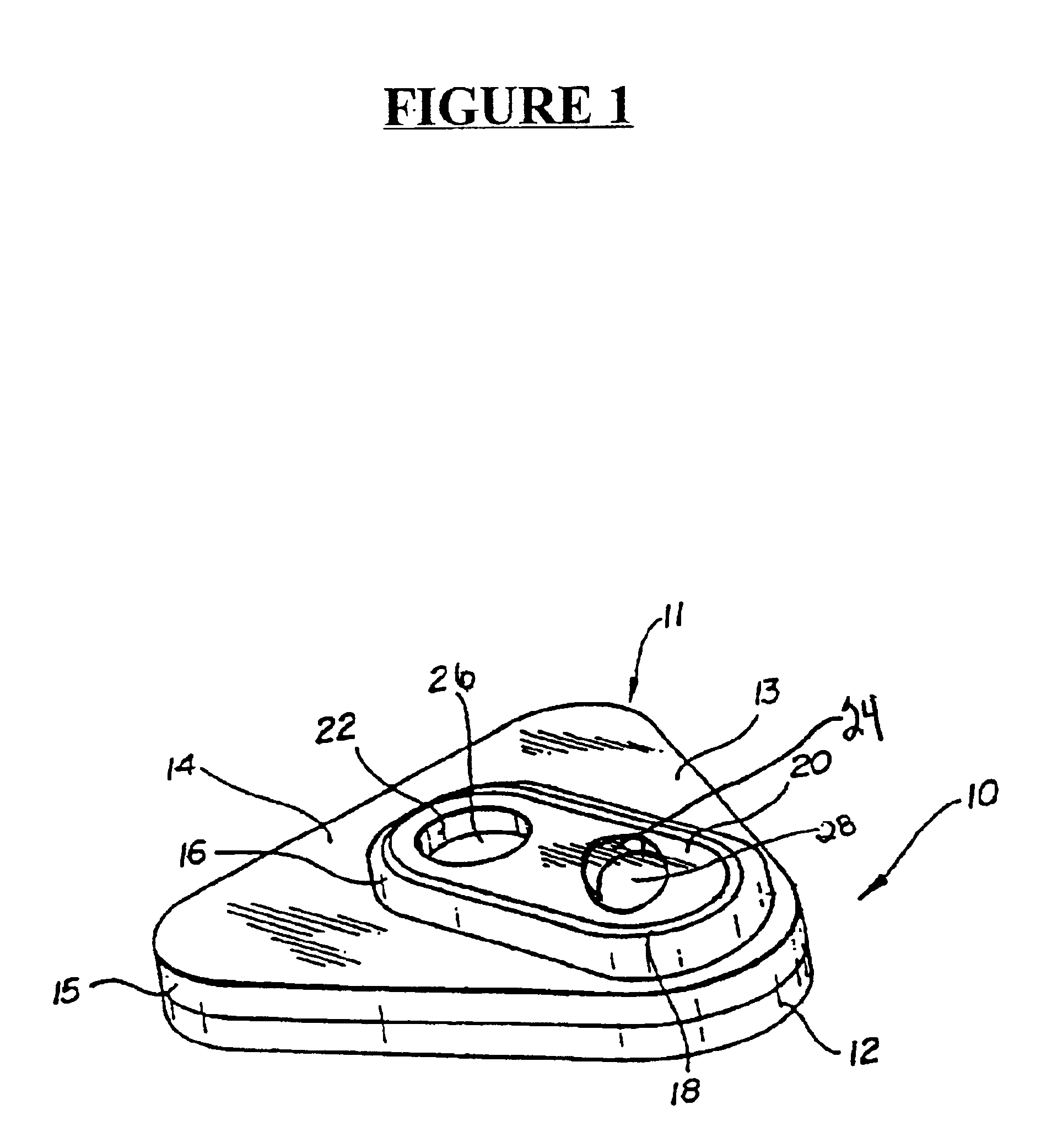
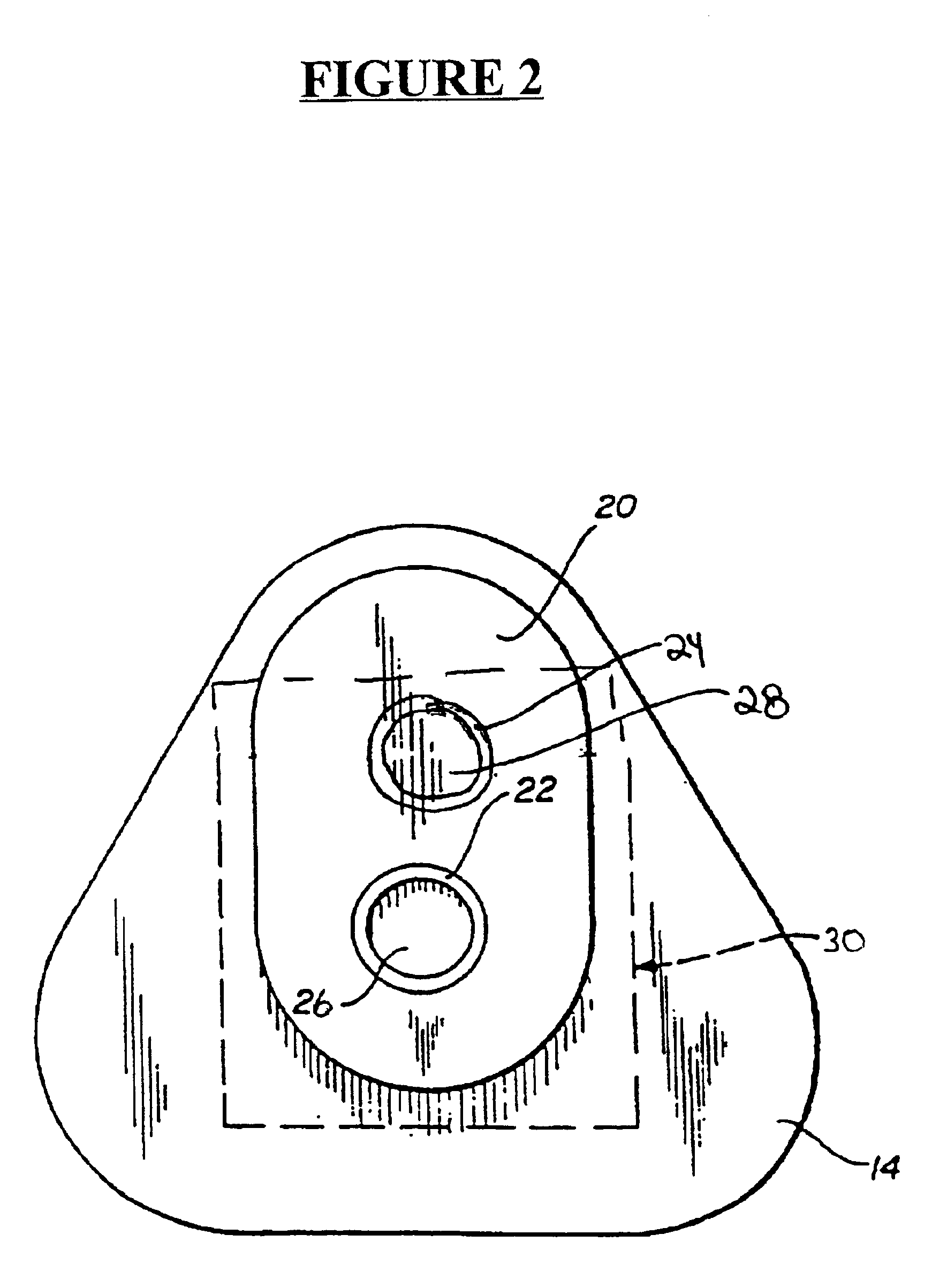
Detection of multiple analytes from a single sample using a multi-well, multi-analyte flow-through diagnostic test device
InactiveUS7052831B2Bioreactor/fermenter combinationsBiological substance pretreatmentsSingle sampleDiagnostic test
The present invention provides a method and device for conducting a rapid in vitro enzyme immunoassay test for the direct and qualitative detection of two or more viral antigens from specimens of symptomatic patients. The method for immunoassay of viral antigens is performed on a membrane. Non-immunological capture of viral antigens takes place by absorption onto the membrane. Captured antigen binds to a detection reagent that includes a label conjugated to a specific antibody. The test is a differentiated test such that two or more viral antigens may be distinguished from each other in a single test. The invention also includes a kit for performing an assay in accordance with the method of the present invention, wherein the kit comprises the device of the present invention.
Owner:BECTON DICKINSON & CO
Assay for porcine circovirus production
ActiveUS7358075B2Microbiological testing/measurementBiological material analysisAssayCell culture media
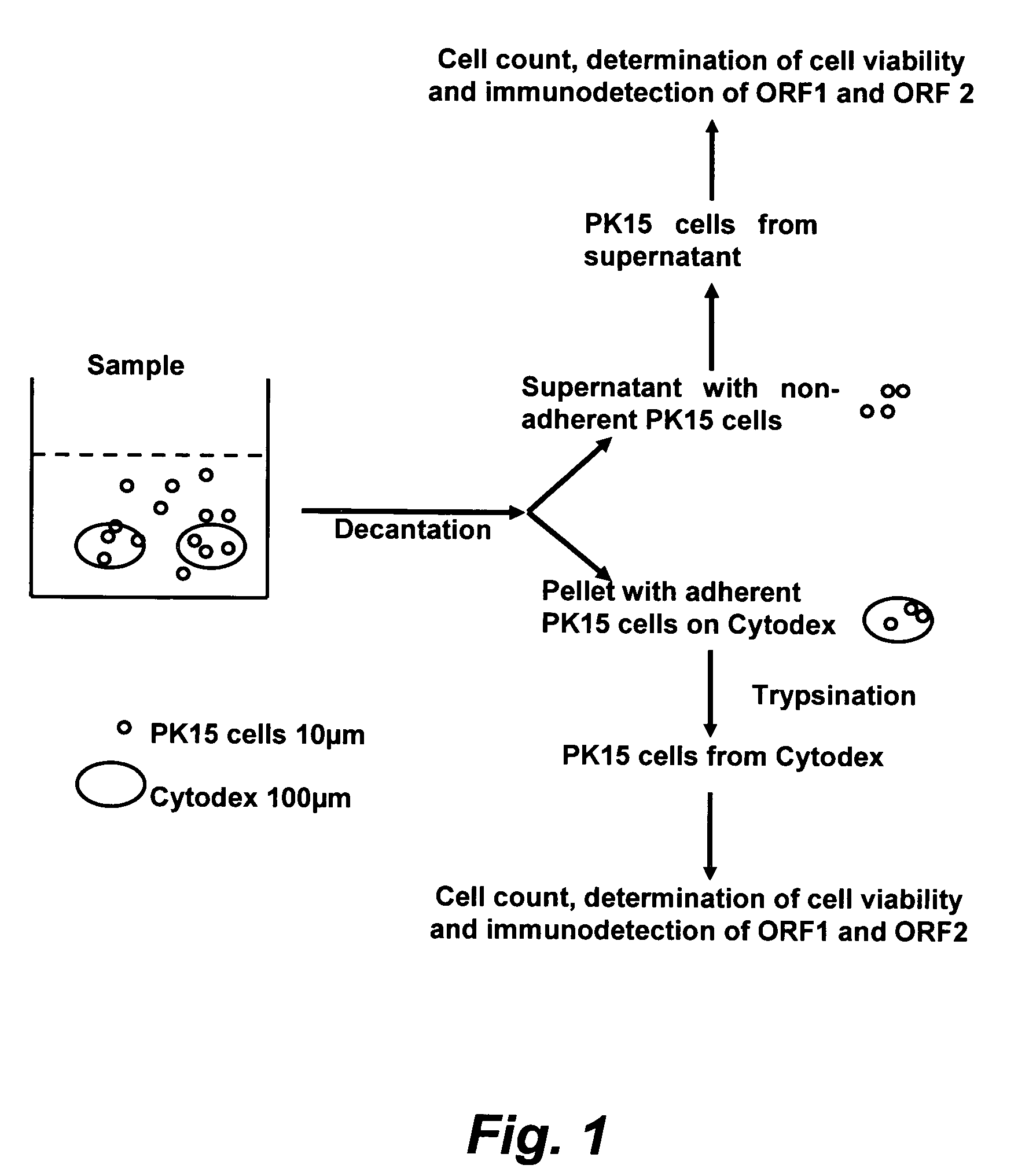
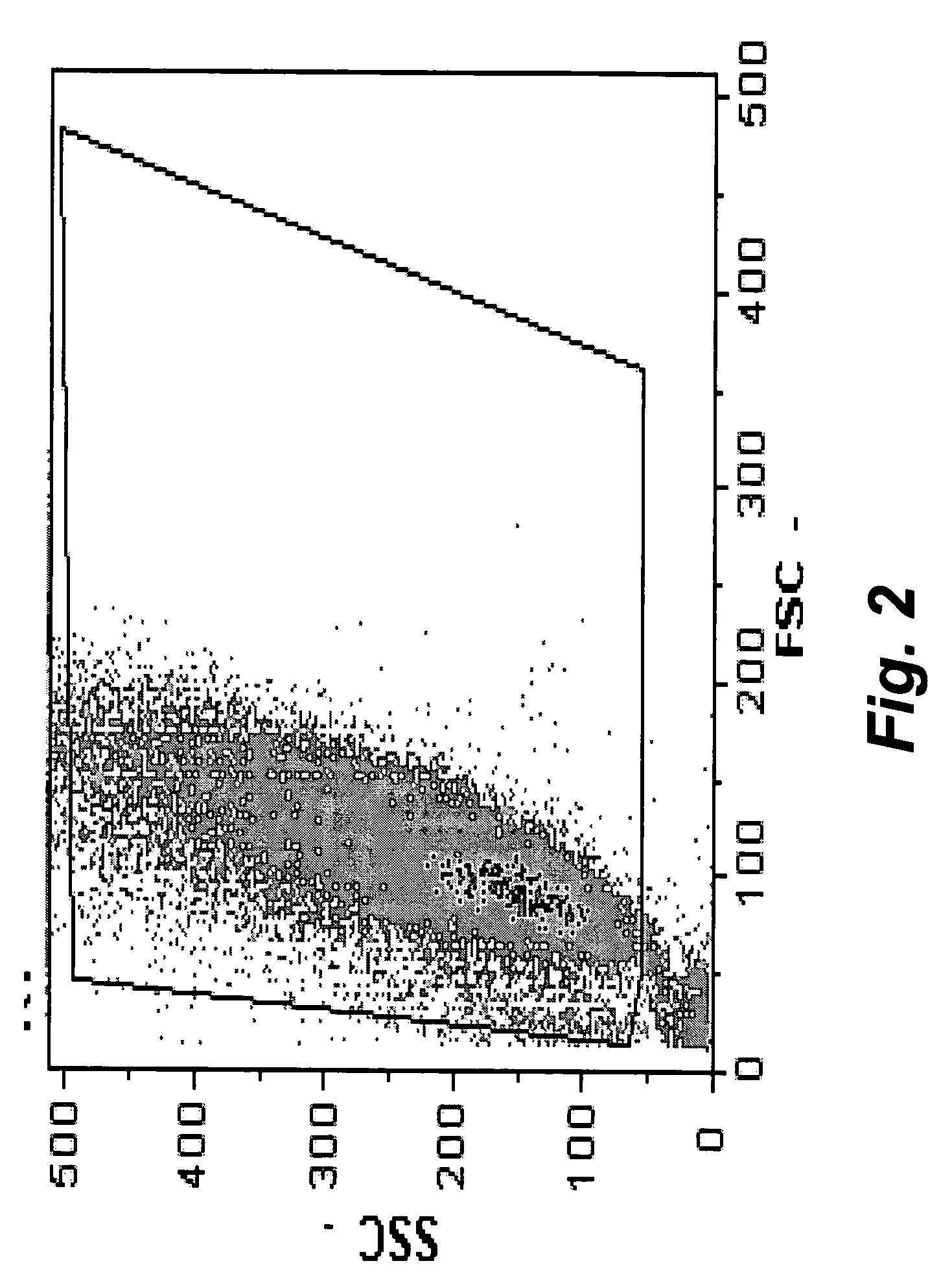
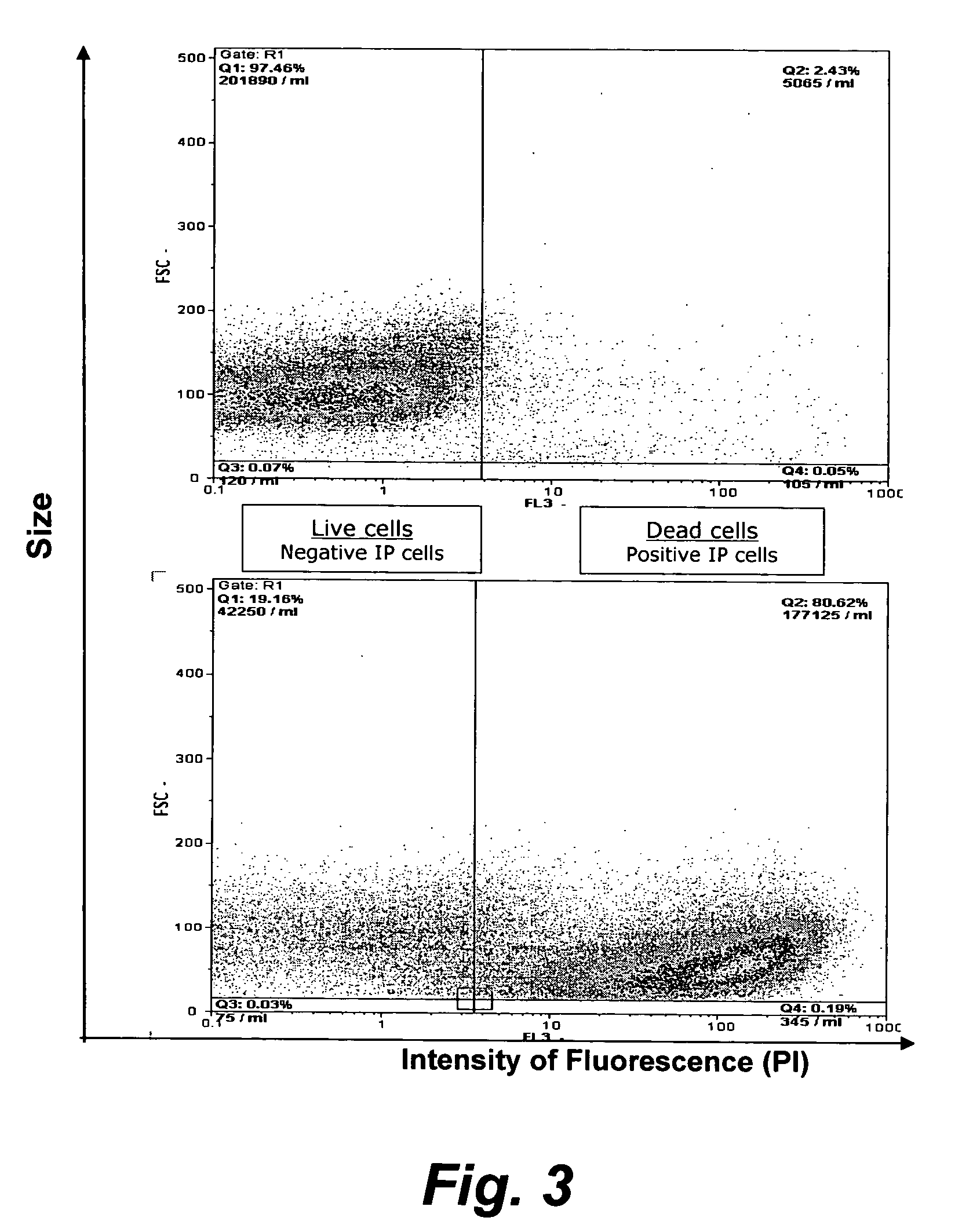
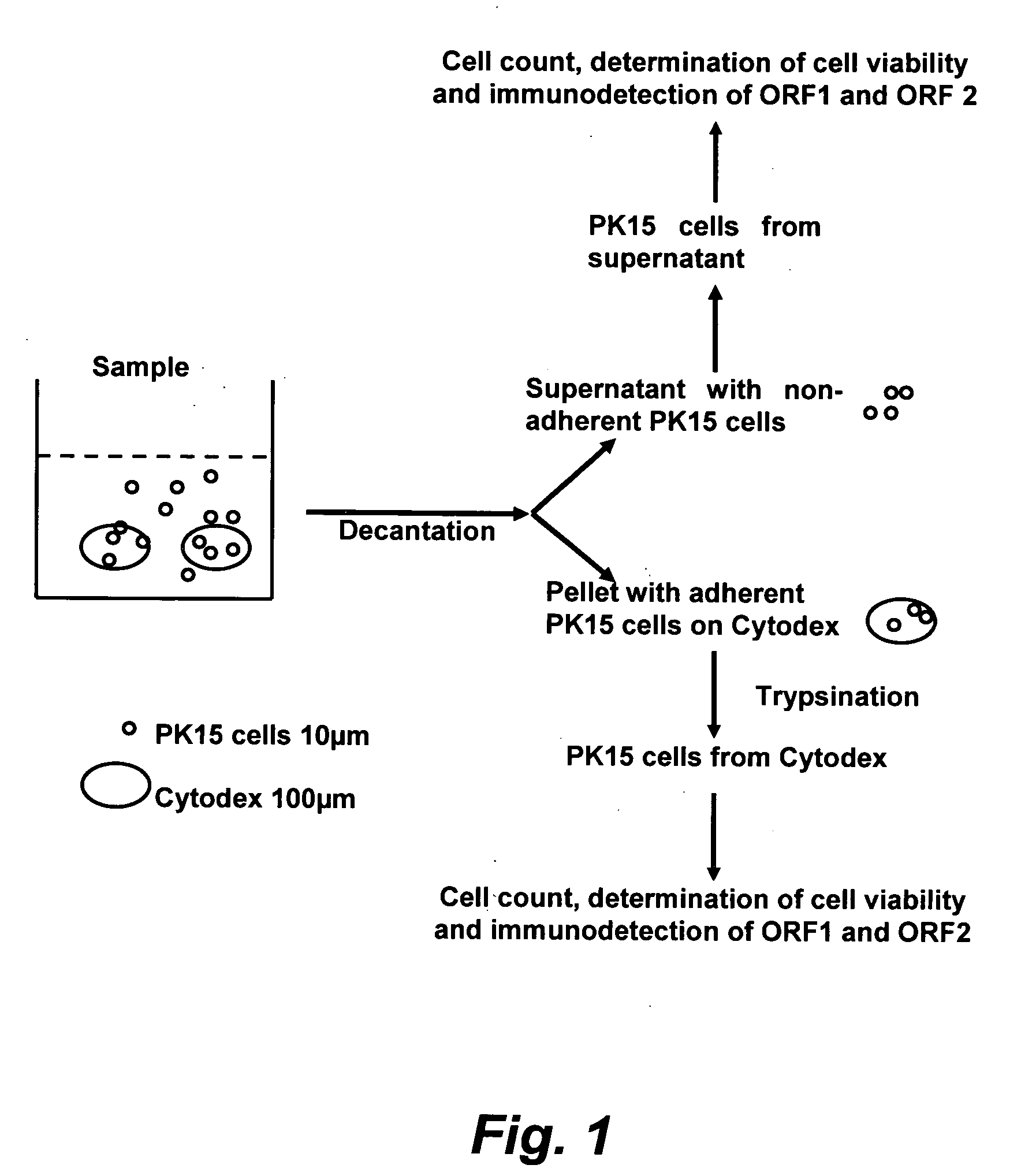
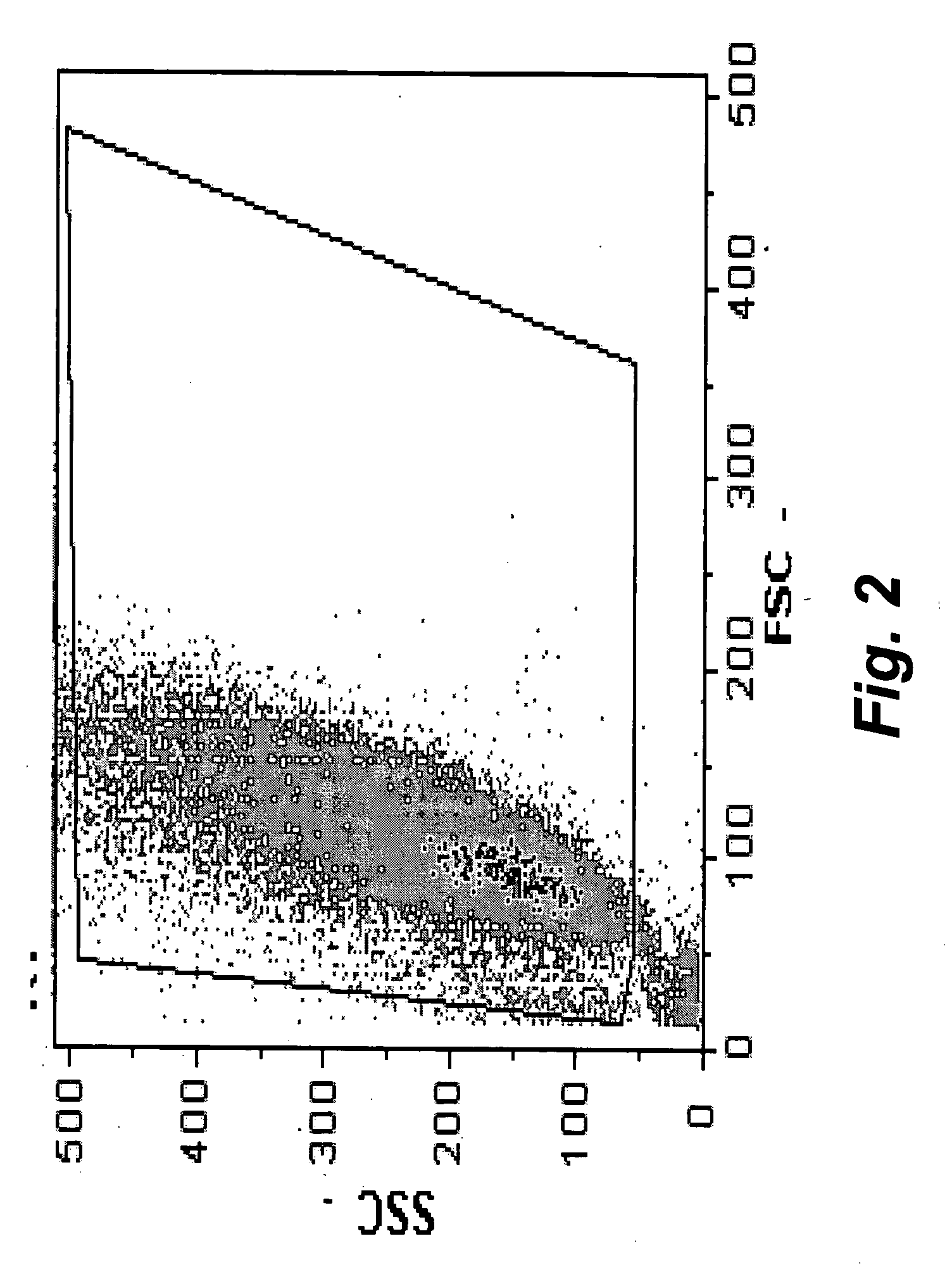
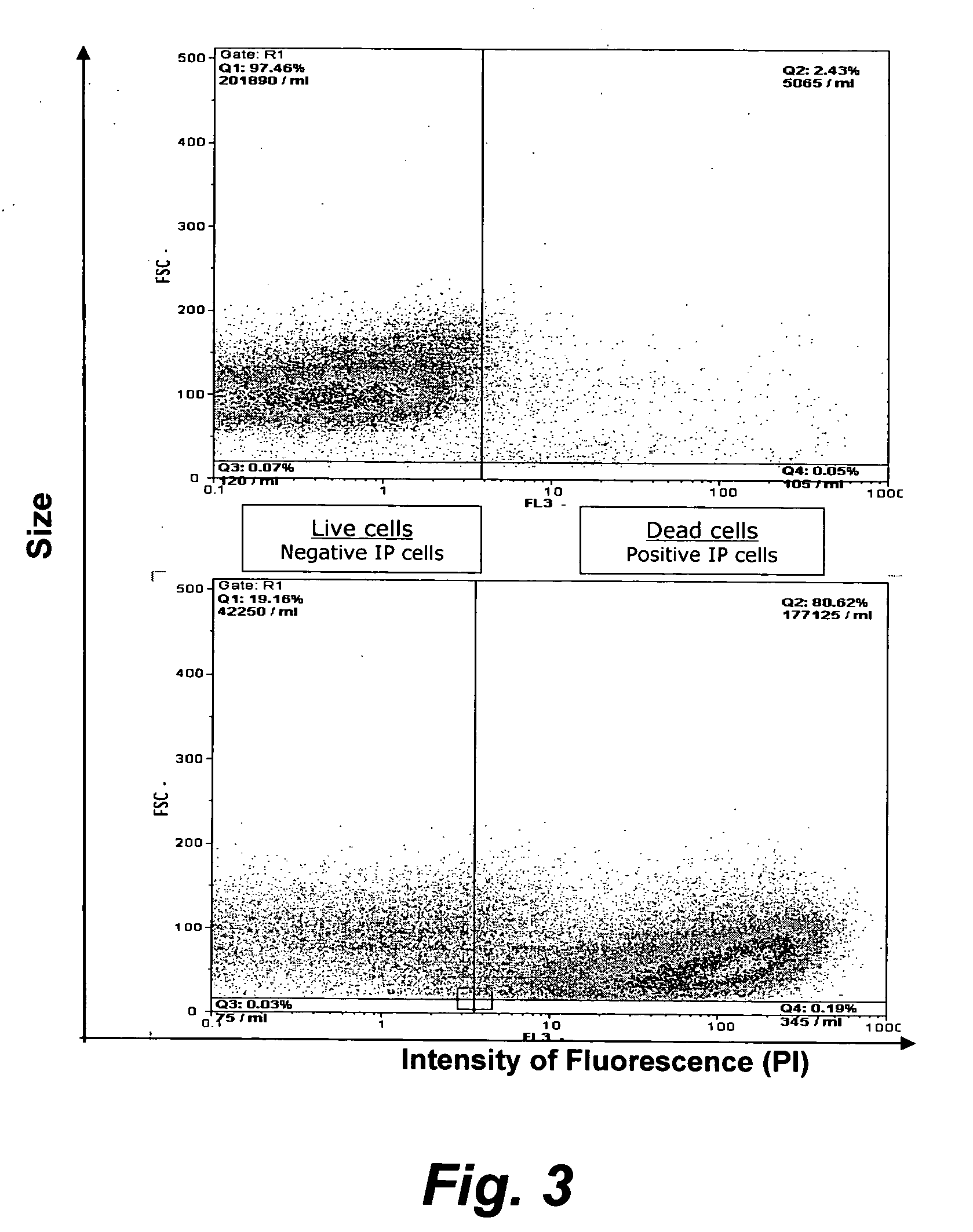
The present invention provides methods for the determination of the viral titer of a culture of host animal host cells infected with a circovirus. The FACS-based methods of the invention may include determining the viability of the host cells in a cell culture medium supernatant and of those cells that remain adherent to a solid support. Detecting and measuring the percentage of cells that expressed the viral antigens ORF1 and ORF2 may determine the viral load of the cultured host cells. The yield of the virus may be established by the detection and measurement of both antigens in supernatant cells, for example 5 to 7 days from when the host cells are transferred to a serum free medium. The methods of the invention may yield rapid quantitative data. This allows the repeated in-process monitoring of the viral production throughout the incubation period, and ready selection of the most appropriate harvesting point.
Owner:MERIAL INC
Assay for porcine circovirus production
ActiveUS20060246425A1Microbiological testing/measurementBiological material analysisIncubation periodViral load
The present invention provides methods for the determination of the viral titer of a culture of host animal host cells infected with a circovirus. The FACS-based methods of the invention may include determining the viability of the host cells in a cell culture medium supernatant and of those cells that remain adherent to a solid support. Detecting and measuring the percentage of cells that expressed the viral antigens ORF1 and ORF2 may determine the viral load of the cultured host cells. The yield of the virus may be established by the detection and measurement of both antigens in supernatant cells, for example 5 to 7 days from when the host cells are transferred to a serum free medium. The methods of the invention may yield rapid quantitative data. This allows the repeated in-process monitoring of the viral production throughout the incubation period, and ready selection of the most appropriate harvesting point.
Owner:MERIAL INC
Vaccines and methods to treat canine influenza
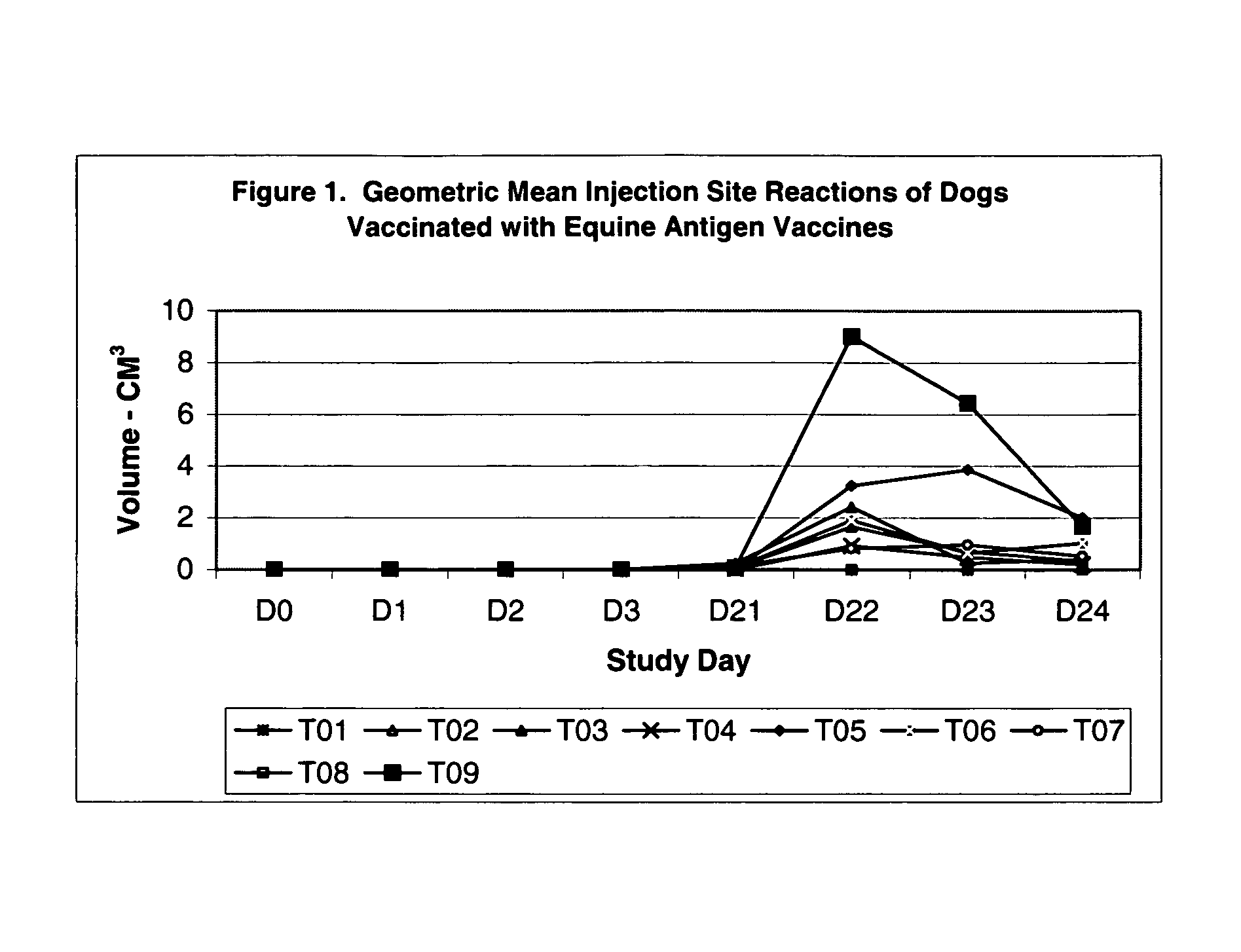
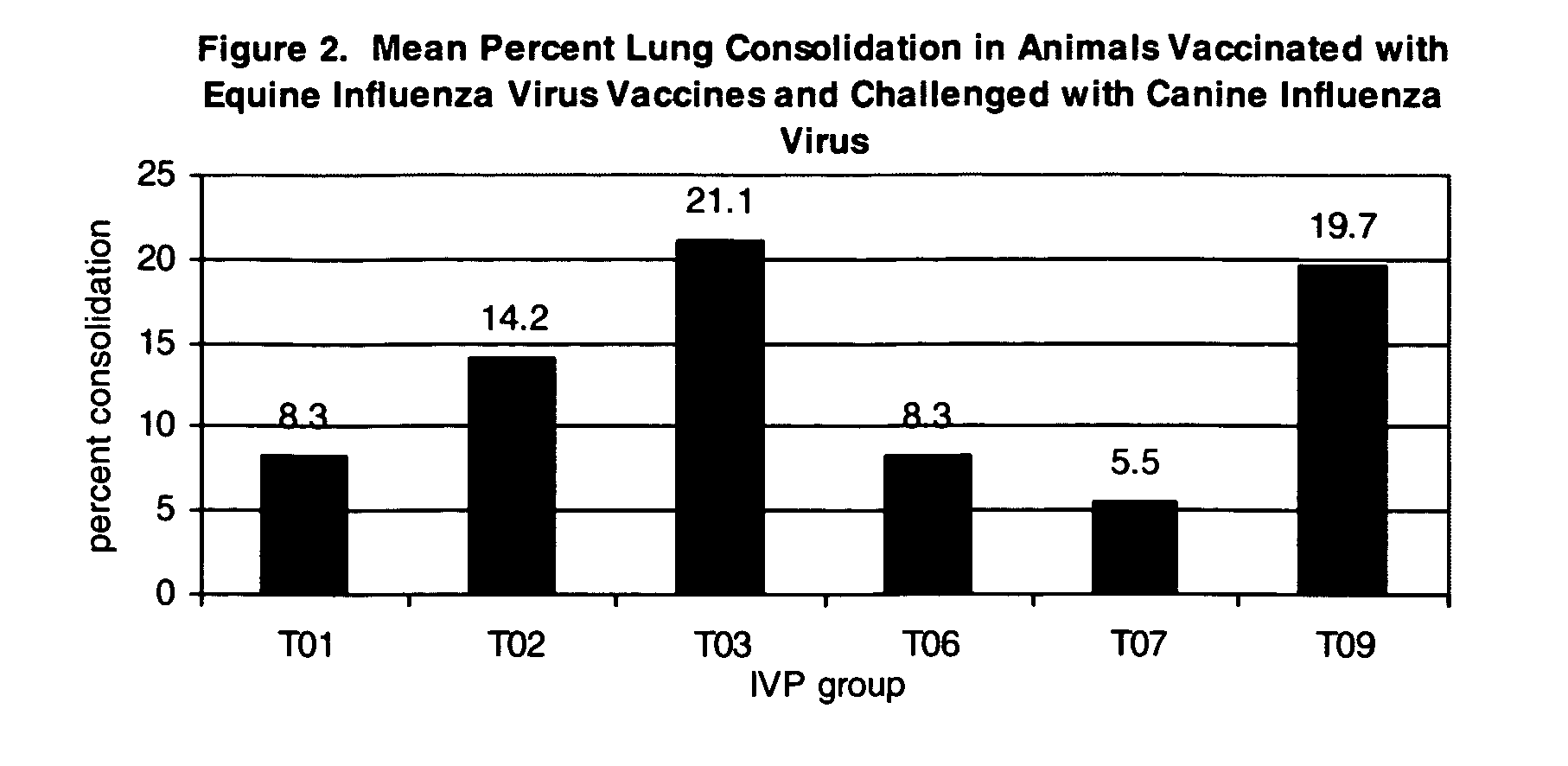
The present invention relates to providing new vaccines and treatments for the diseases related to canine influenza virus. It discloses influenza viral antigens, and methods of presenting these antigens to canines, especially dogs. It relates to attenuated and killed vaccines. The present invention relates to experimentally generated canine and equine influenza viruses. The invention also includes influenza A, including H3, N8, H3N8, H7N7 and viruses which contain at least one genome segment from an canine or equine influenza virus. The present invention also relates to the use of these viruses in therapeutic compositions to protect canines, dogs in particular, from diseases caused by influenza viruses.
Owner:ZOETIS SERVICE LLC
Composition and method for producing an immune response against tumor-related antigens
Disclosed are a novel prostatic acid phosphatase and corresponding coding region derived from mouse. Also disclosed is a method of producing an immune response against an autologous polypeptide tumor antigen by immunizing a subject with a xenogeneic polypeptide antigen, either alone, as part of a viral antigen construct, or as part of a pulsed dendritic cell preparation.
Owner:DENDREON PHARMA LLC
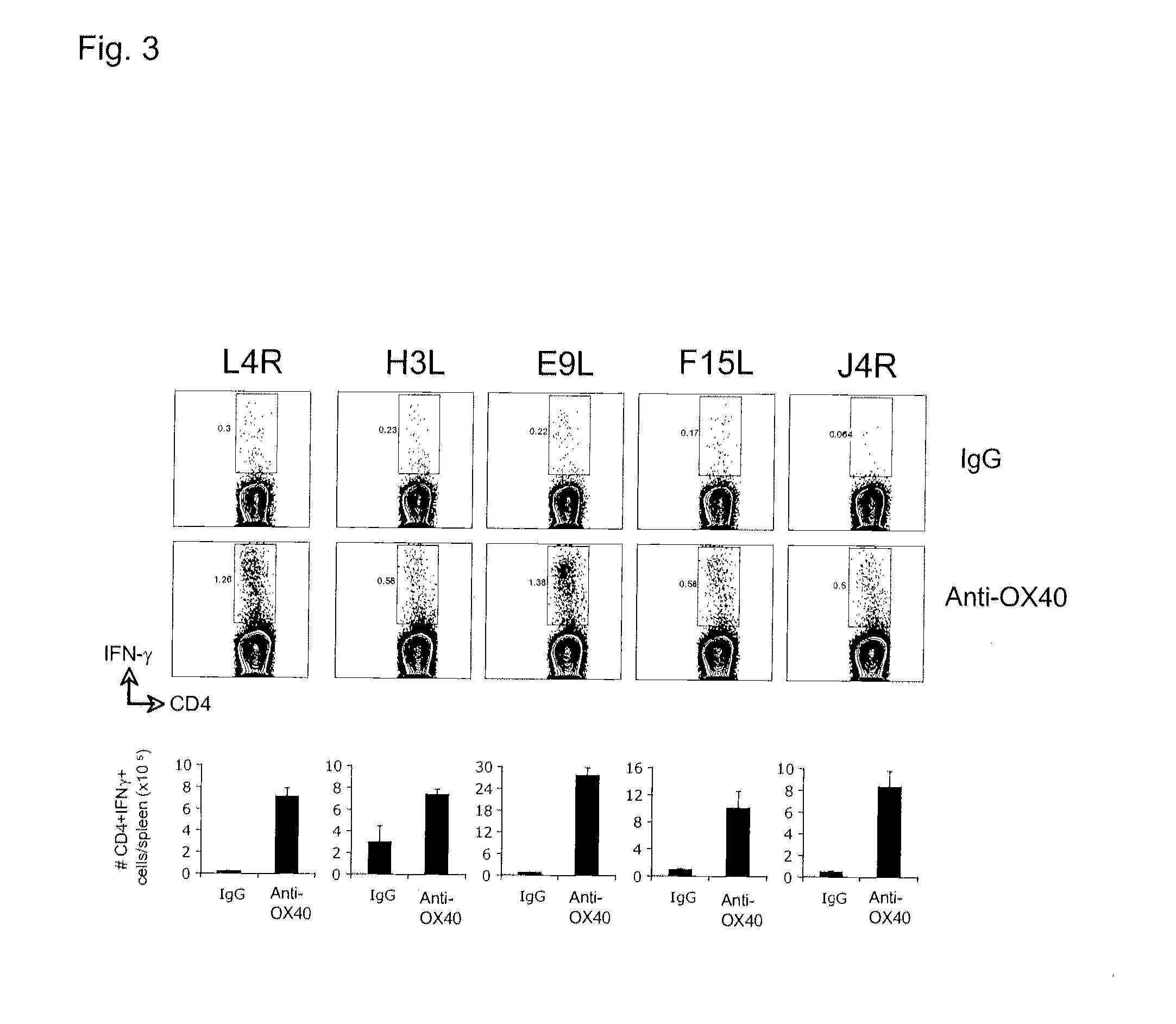
Virus vaccination and treatment methods with ox40 agonist compositions
InactiveUS20120141465A1Reduce sensitivityIncrease and stimulates clearanceBiocideViral antigen ingredientsVaccinationTnfr superfamily
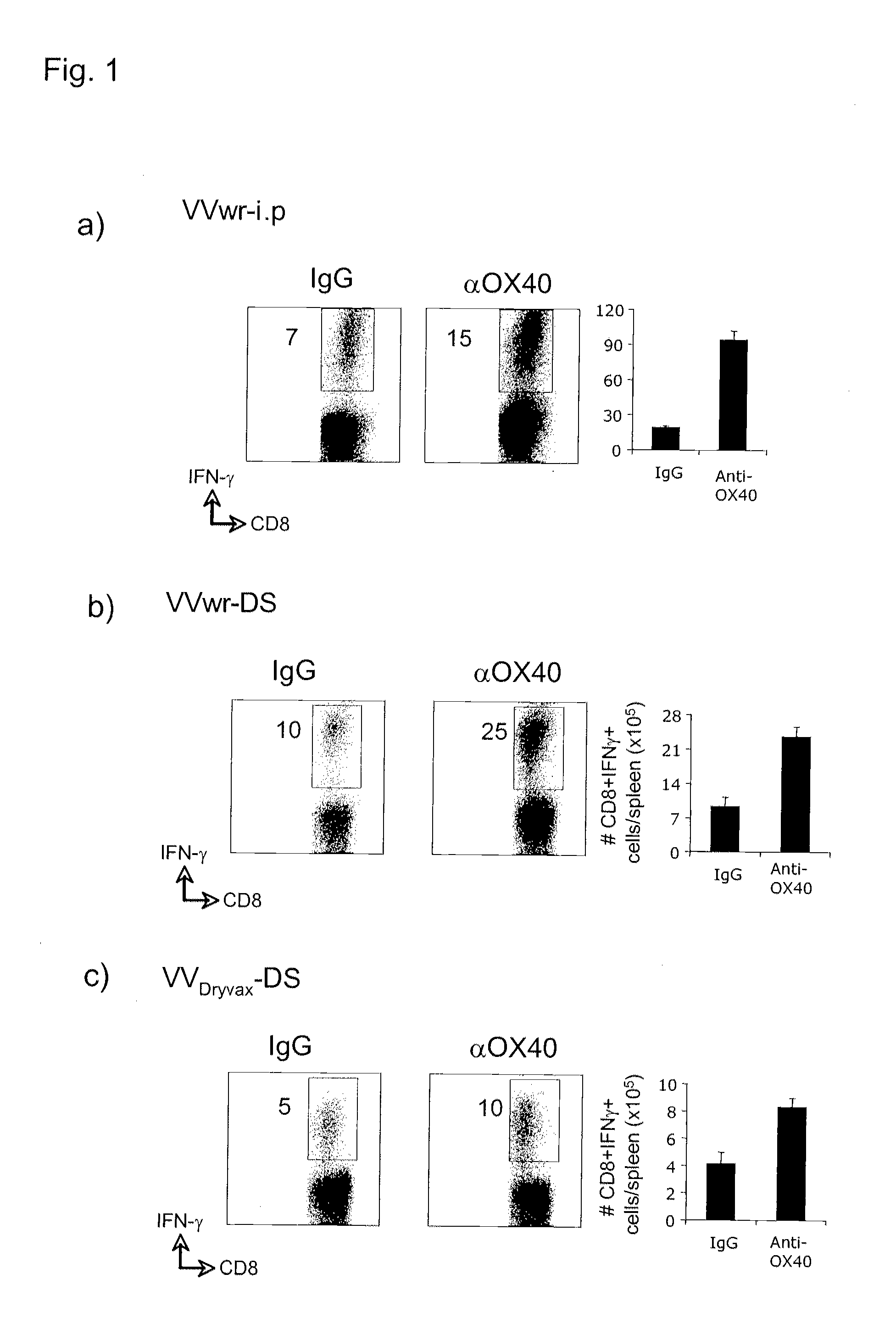
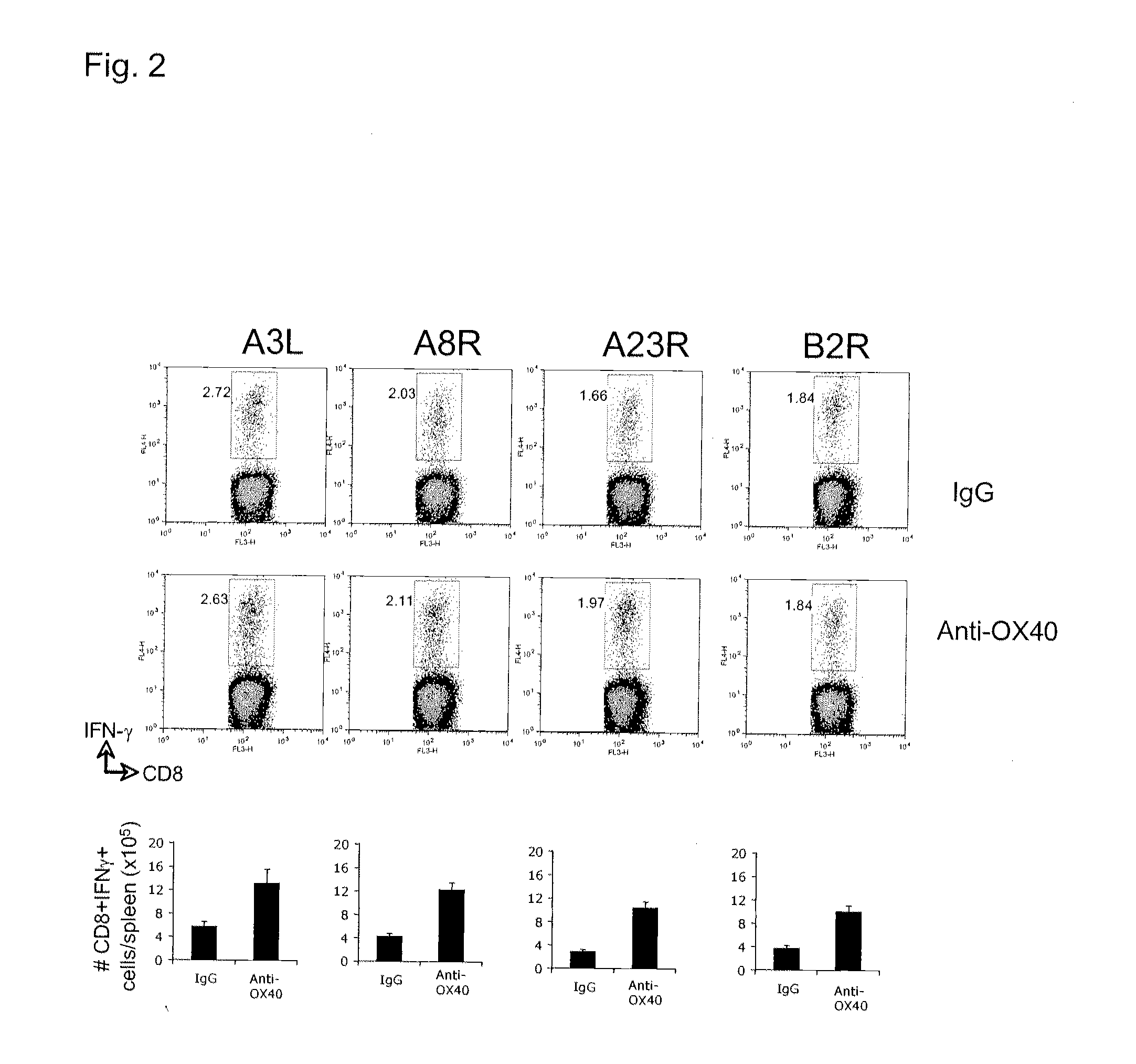
The invention relates to compositions and methods that employ OX40 (CD134), a TNFR superfamily protein, agonists. The invention includes among other things administering an OX40 agonist alone or in combination with a viral antigen, or live or attenuated virus, to treat a viral infection, or for vaccination or immunization.
Owner:LA JOLLA INST FOR ALLERGY & IMMUNOLOGY
Oral smallpox vaccine production and methods to evaluate safety, efficacy, and potency of orally delivered vaccine
InactiveUS20040175398A1Efficient responseSafety and efficacyViral antigen ingredientsMicrobiological testing/measurementHuman useDiagnostic test
This invention relates to methods and systems for generating a safe and effective oral smallpox vaccine for humans using a genetically defective strain of vaccinia virus to confer immunity following oral delivery of the vaccine. This invention is one that expands on current use of vaccinia virus propagation developed for gene therapy applications, and pharmaceuticals and nutraceuticals packaging and formualtion technologies. The vaccine invention can be delivered as a live virus with the ability to express viral proteins but unable to achieve complete, lytic virus replication, or it may be derived from such a virus, contain additional immunogens, or be delivered as viral antigens. Furthermore, the invention establishes innovative methods for formulation and packaging and for preclinical testing of the vaccine invention for safety, efficacy and potency with the use of human intestinal and other test cells and diagnostic test systems and kits.
Owner:INCELLS
Use of polymeric nanoparticles for vaccine delivery
InactiveUS20080044484A1Enhance antigen presentationImprove efficiencyBiocidePowder deliveryCancer cellT lymphocyte
The invention relates generally to the treatment and prevention of human cancer and viral diseases. More specifically, this invention relates to development of a new generation of vaccines that rely on eliciting cellular immune responses, specifically induction of cytotoxic T lymphocytes (CTL), against cancer cells and virus-infected cells via administration of a polymeric nanoparticle containing a vaccine comprising a fusion peptide or a modified peptide. Such a fusion peptide is composed of an insertion signal sequence and a peptide derived from a tumor antigen or a viral antigen, which improves antigen presentation and induces CTL with higher efficiency against cancer cells and virus-infected cells. An exemplary peptide utilized in the invention is Mart-1:27-35 peptide.
Owner:RGT UNIV OF CALIFORNIA
Superior molecular vaccine based on self-replicating rna, suicidal dna or naked dna vector, that links antigen with polypeptide that promotes antigen presentation
InactiveUS20050277605A1Great E7-specific T cell-mediated immunityEfficiently presentedSsRNA viruses positive-senseAntibody mimetics/scaffoldsMHC class IDisease
Improved molecular vaccines comprise nucleic acid vectors that encode a fusion polypeptide that includes polypeptide or peptide physically linked to an antigen. The linked polypeptide is one that (a) promotes processing of the expressed fusion polypeptide via the MHC class I pathway and / or (b) promotes development or activity of antigen presenting cells, primarily dendritic cells. These vaccines employ one of several types of nucleic acid vectors, each with its own relative advantages: naked DNA plasmids, self-replicating RNA replicons and suicidal DNA-based on viral RNA replicons. Administration of such a vaccine results in enhance immune responses, primarily those mediated by CD8+ cytotoxic T lymphocytes, directed against the immunizing antigen part of the fusion polypeptide. Such vaccines are useful against tumor antigens, viral antigens and antigens of other pathogenic microorganisms and can be used in the prevention or treatment of diseases that include cancer and infections.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Composition and method for stimulating immune response to pathogen using complex adenoviral vector
InactiveUS6964762B2Improving immunogenicityStrong immune responseSsRNA viruses negative-senseAntibacterial agentsHeterologousProgenitor
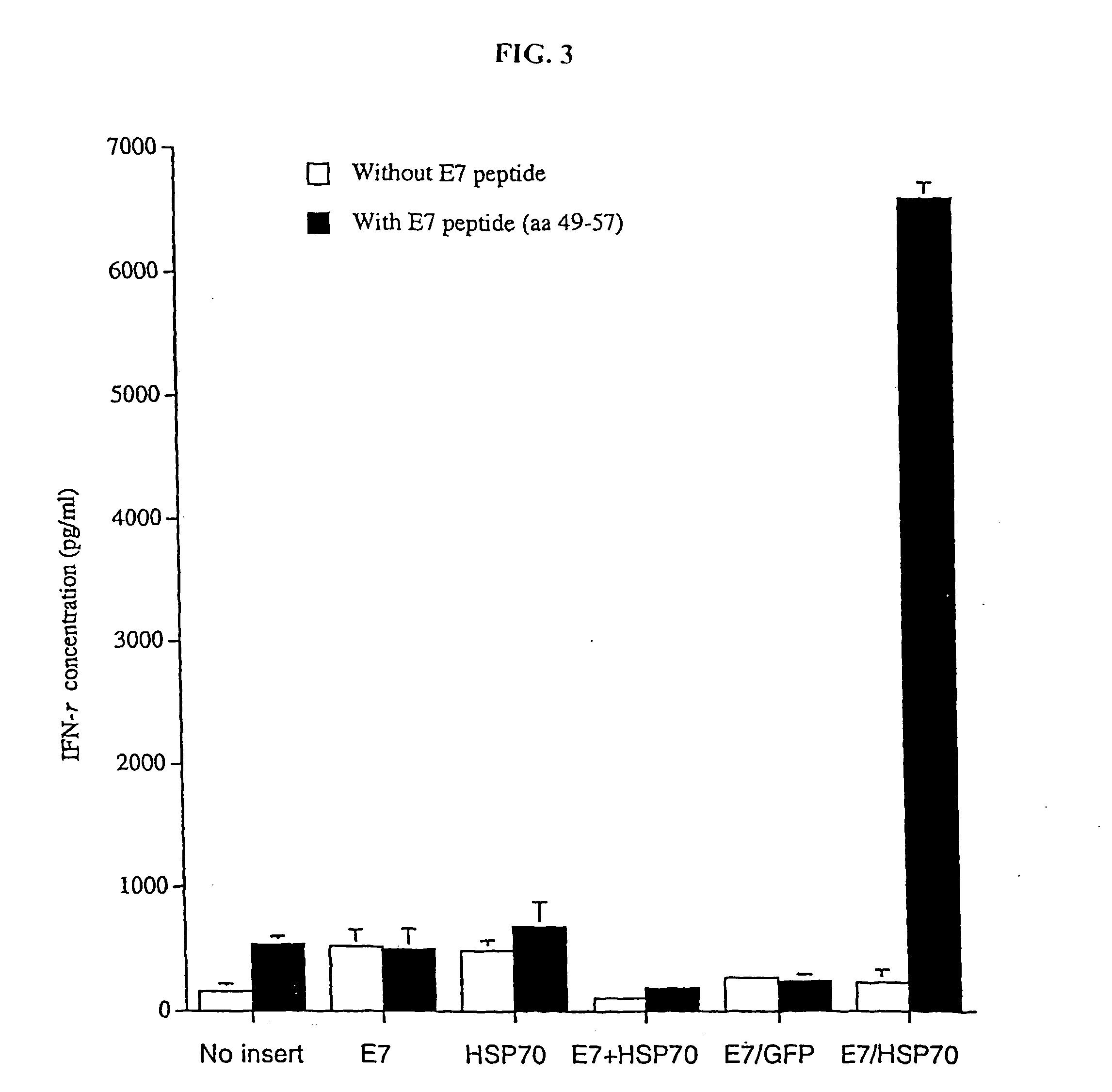
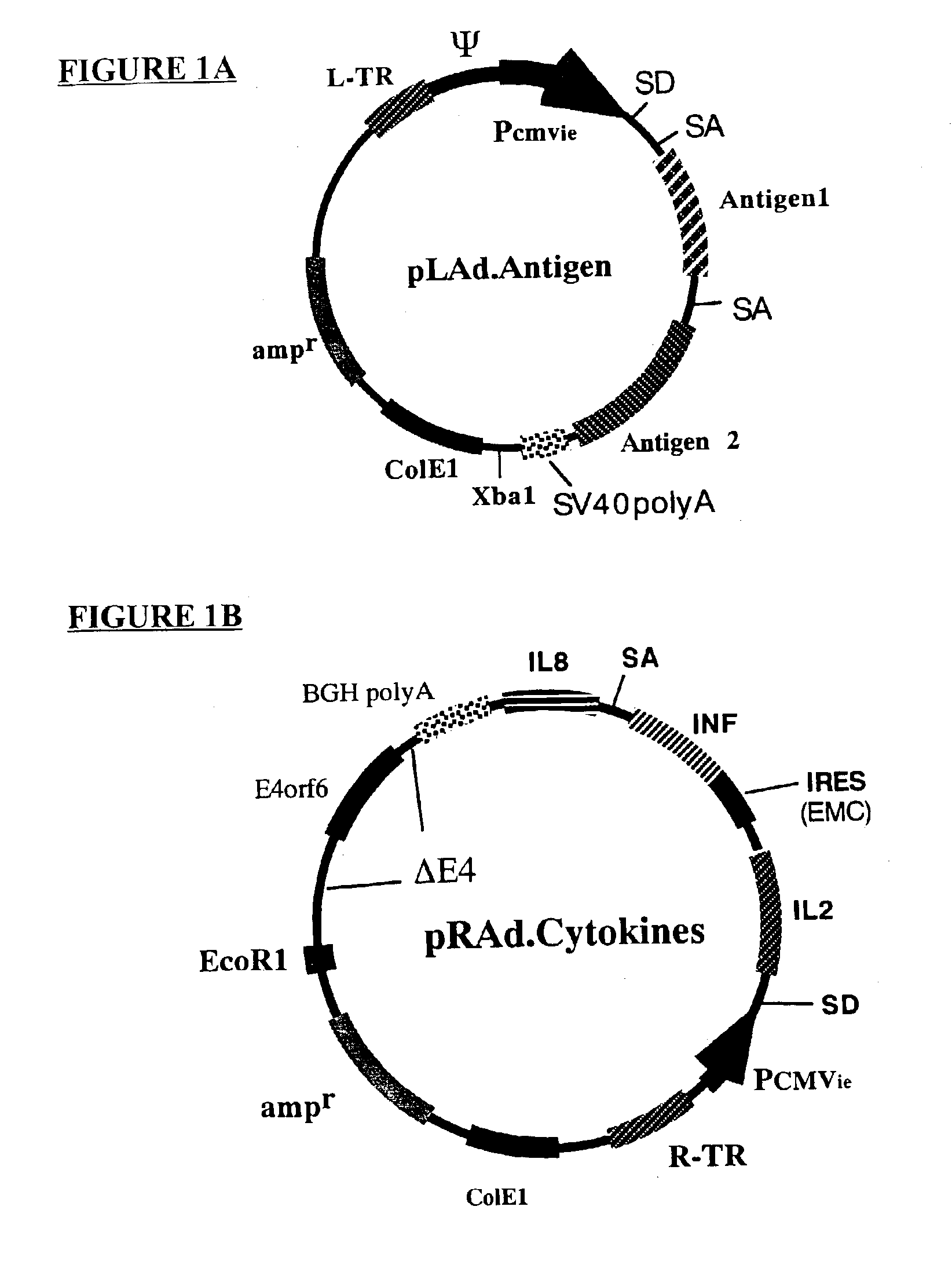
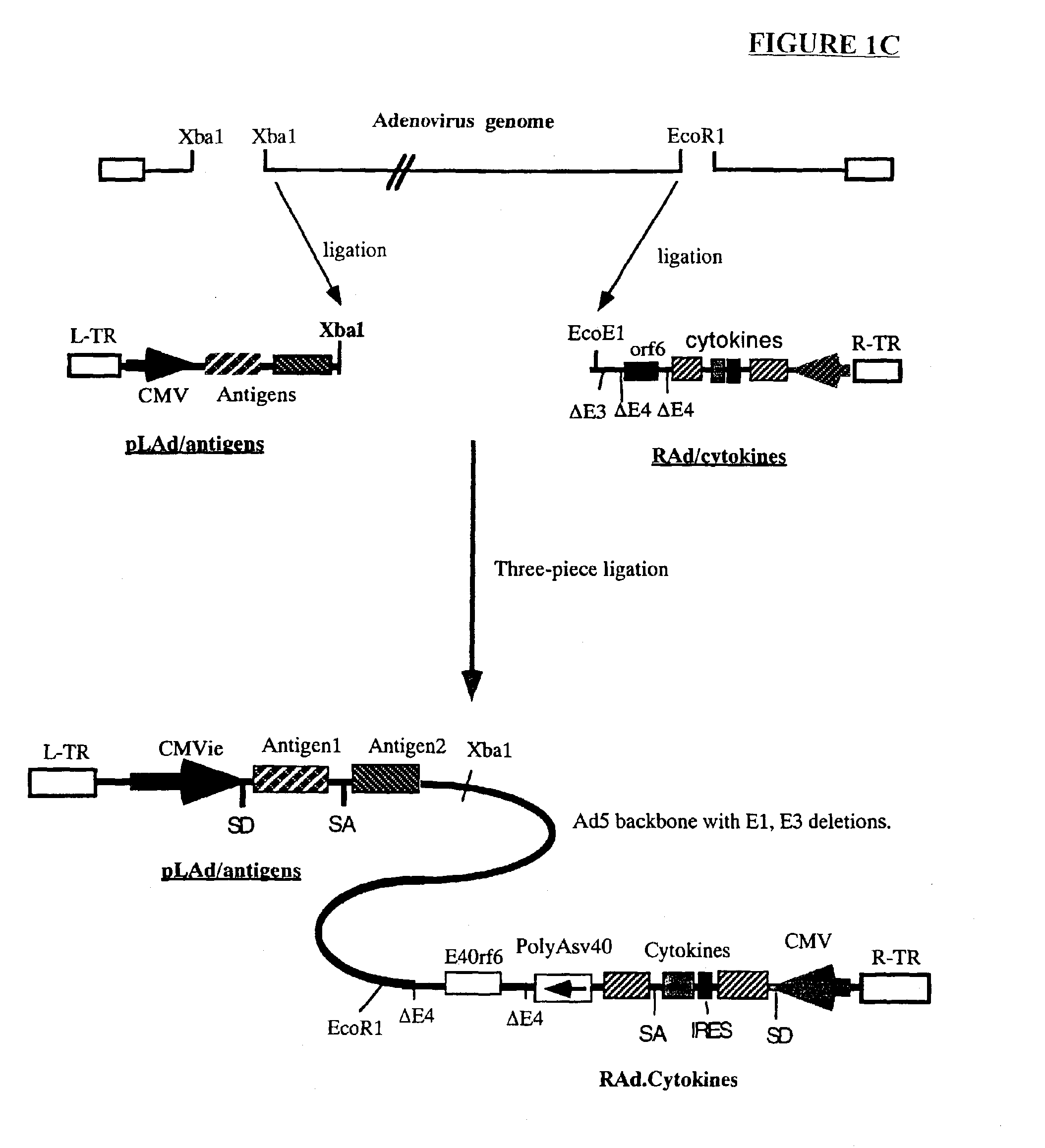
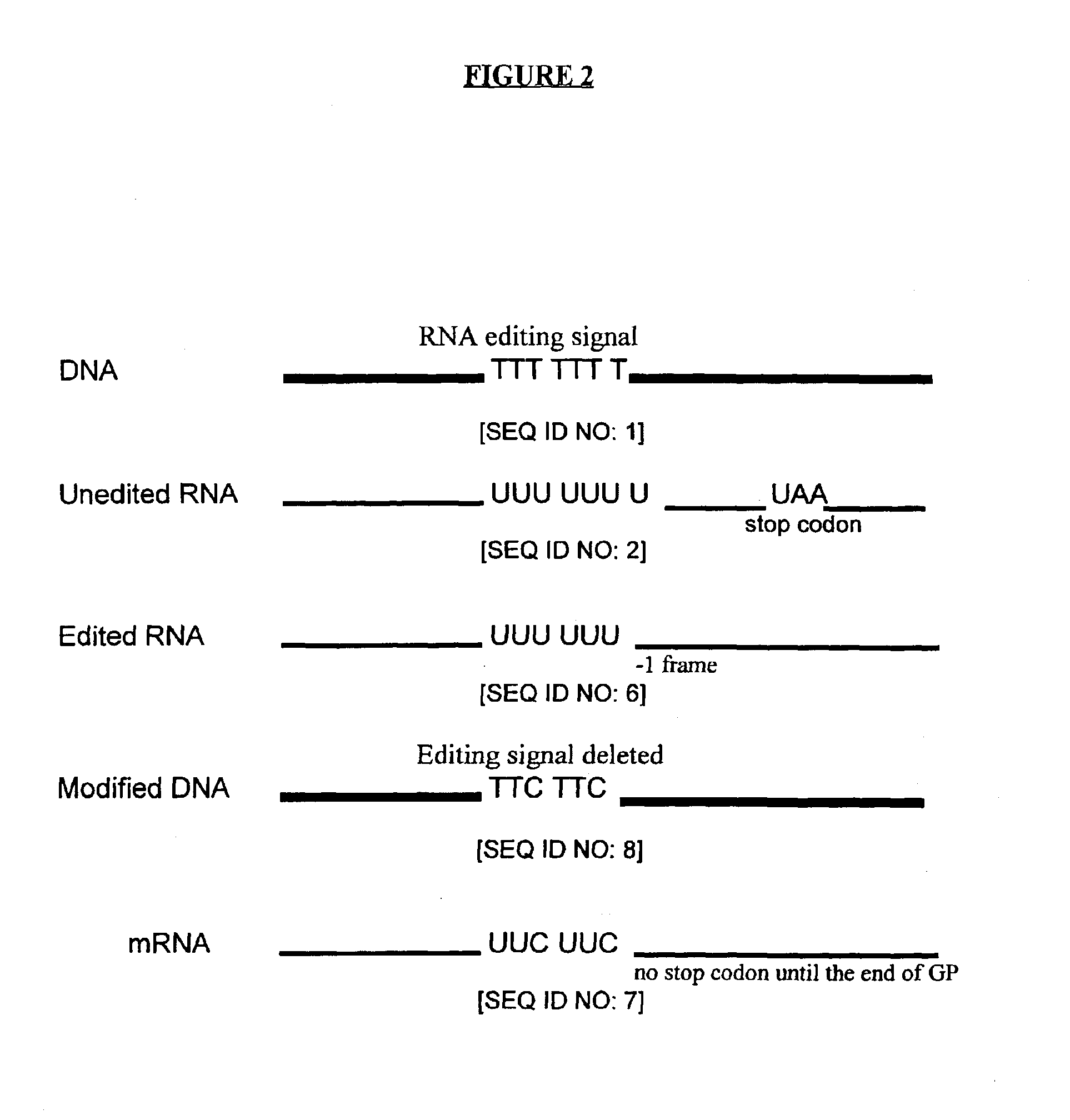
Genetic vaccines and methods are provided for enhancing the immunity of a host such as a human to one or more pathogens. In one aspect, a method of enhancing the immunity of a host to a pathogen is provided. The method comprises administering to the host a recombinant virus comprising an antigen sequence that is heterologous to a native progenitor of the recombinant adenovirus and encodes a viral antigen from a pathogenic virus, expression of which is under the transcriptional control of a first promoter; and a cytokine sequence that is heterologous to the native progenitor of the recombinant adenovirus and encodes a cytokine, expression of which is under the transcriptional control of a second promoter. Expression of the antigen and cytokine sequences elicits an immune response directed against the viral antigen upon infection of the host by the recombinant virus. The method can be used for immunizing a host against a wide variety of pathogen viruses, such as HIV, Ebola virus, Marburg virus, hepatitis B virus, hepatitis C virus, influenza virus, human simplex virus, human papilloma virus and respiratory syncytial virus.
Owner:GENPHAR INC
Changing th1/th2 balance in split influenza vaccines with adjuvants
InactiveUS20090047353A1Eliminate needAvoid the needSsRNA viruses negative-sensePowder deliveryEgg proteinTh2 response
The invention seeks to avoid components in split vaccines that could cause an excessive Th2 response. Thus the invention provides an immunogenic composition comprising a split influenza virus antigen and a Th1 adjuvant, wherein the antigen is preferably prepared from a virus grown in cell culture (e.g., it is free from egg proteins).
Owner:SEQIRUS UK LTD
Cancer immunotherapy with a viral antigen-defined, immunomodulator-secreting cell vaccine
A human cell line, which lacks major histocompatibility class I (MHC-I) antigens and major histocompatibility class II (MHC-II) antigens and which has been modified to comprise and express (i) a nucleotide sequence encoding an immunomodulator and (ii) a nucleotide sequence encoding a viral antigen, and a method of inducing or stimulating an immune response in a human to a viral-associated disease or cancer comprising administering to the human (i) the aforementioned human cell line in an amount sufficient to induce or stimulate an immune response to the viral associated disease or cancer, (ii) a human cell line, which lacks MHC-I and MHC-11 antigens and which has been modified to comprise and express a nucleotide sequence encoding an immunomodulator, and a human cell line, which lacks MHC-I and MHC-II antigens and which has been modified to comprise and express a nucleotide sequence encoding an antigen of EBV, simultaneously or sequentially in either order, by the same or different routes, in amounts sufficient to induce or stimulate an immune response to the viral-associated disease or cancer, or (iii) an immunomodulator and a human cell line, which lacks MHC-I and MHC-II antigens and which has been modified to comprise and express a nucleotide sequence encoding an antigen of EBV, simultaneously or sequentially in either order, by the same or different routes, in amounts sufficient to induce or stimulate an immune response to the viral associated disease or cancer.
Owner:JOHNS HOPKINS UNIV SCHOOL OF MEDICINE
Enhancing Class I Antigen Presentation With Synthetic Sequences
InactiveUS20080206270A1Enhance antigen presentationInduces antitumor and antiviral CTLTumor rejection antigen precursorsCell receptors/surface-antigens/surface-determinantsCancer cellT lymphocyte
The invention relates generally to the treatment and prevention of human cancer and viral diseases. More specifically, this invention relates to development of a new generation of vaccines that rely on eliciting cellular immune responses, specifically induction of cytotoxic T lymphocytes (CTL), against cancer cells and virus-infected cells via administration of a vaccine comprising a fusion peptide or a modified peptide. Such a fusion peptide is composed of an insertion signal sequence and a peptide derived from a tumor antigen or a viral antigen, which improves antigen presentation and induces CTL with higher efficiency against cancer cells and virus-infected cells. An exemplary antigen utilized in the invention is HER2 / neu. The peptides peptide vaccines of the invention are derived from the antigens PRAME, OFA / iLRP, STEAP and SURVIVIN.
Owner:RGT UNIV OF CALIFORNIA
Beta-coronavirus antigen as well as preparation method and application thereof
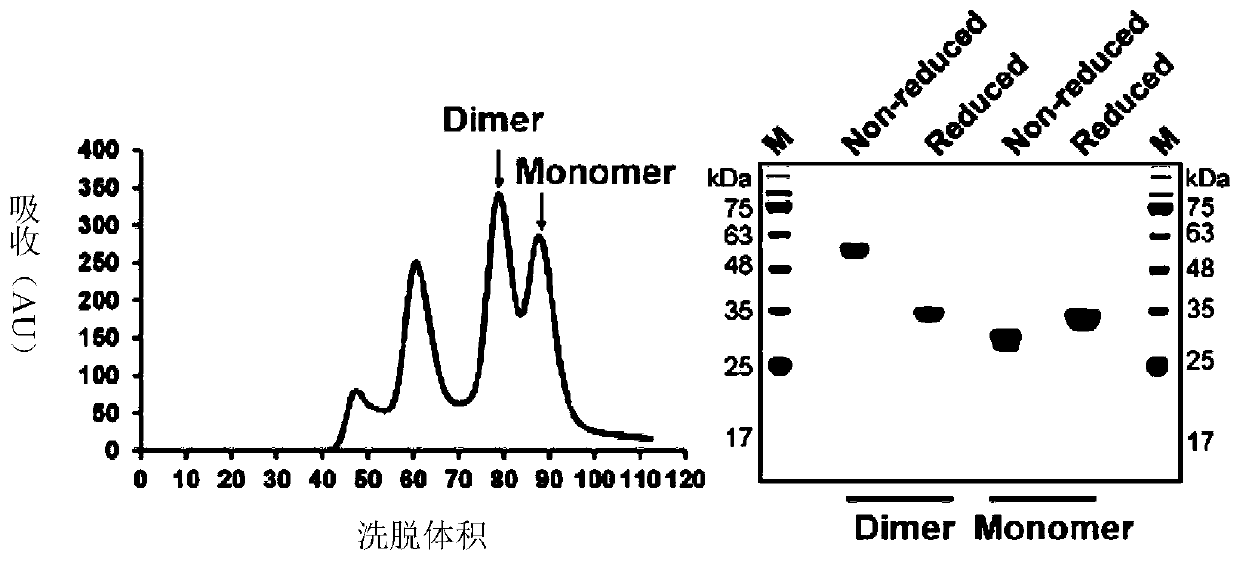
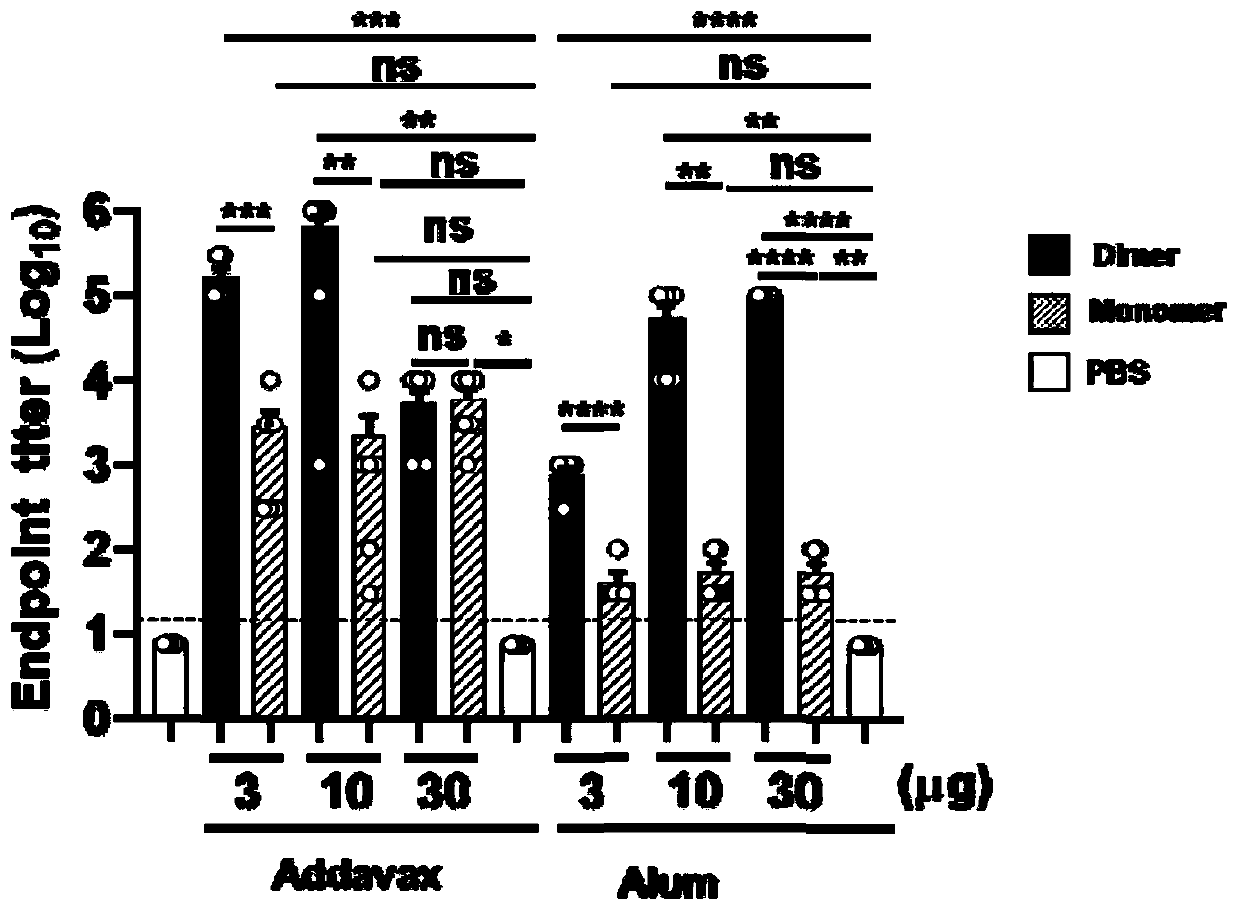
ActiveCN111592602AGood immune effectHigh expressionPolypeptide with localisation/targeting motifSsRNA viruses positive-senseDimerNeutralising antibody
The embodiment of the invention relates to a beta-coronavirus antigen as well as a preparation method and application thereof. Wherein the amino acid sequence of the beta-coronavirus antigen comprisesan amino acid sequence arranged according to a (A-B)-(A-B) style or an amino acid sequence arranged according to a (A-B)-C-(A-B) style or an amino acid sequence arranged according to a (A-B)-(A-B ')style or an amino acid sequence arranged according to a (A-B)-C-(A-B') style, and the beta-coronavirus antigen has a single-chain dimer structure. The single-chain dimer expressed by the embodiment ofthe invention is stable in content and has good immunogenicity as a beta-coronavirus antigen, and a vaccine prepared by using the single-chain dimer as a beta-coronavirus antigen can stimulate mice to generate a neutralizing antibody with very high titer.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
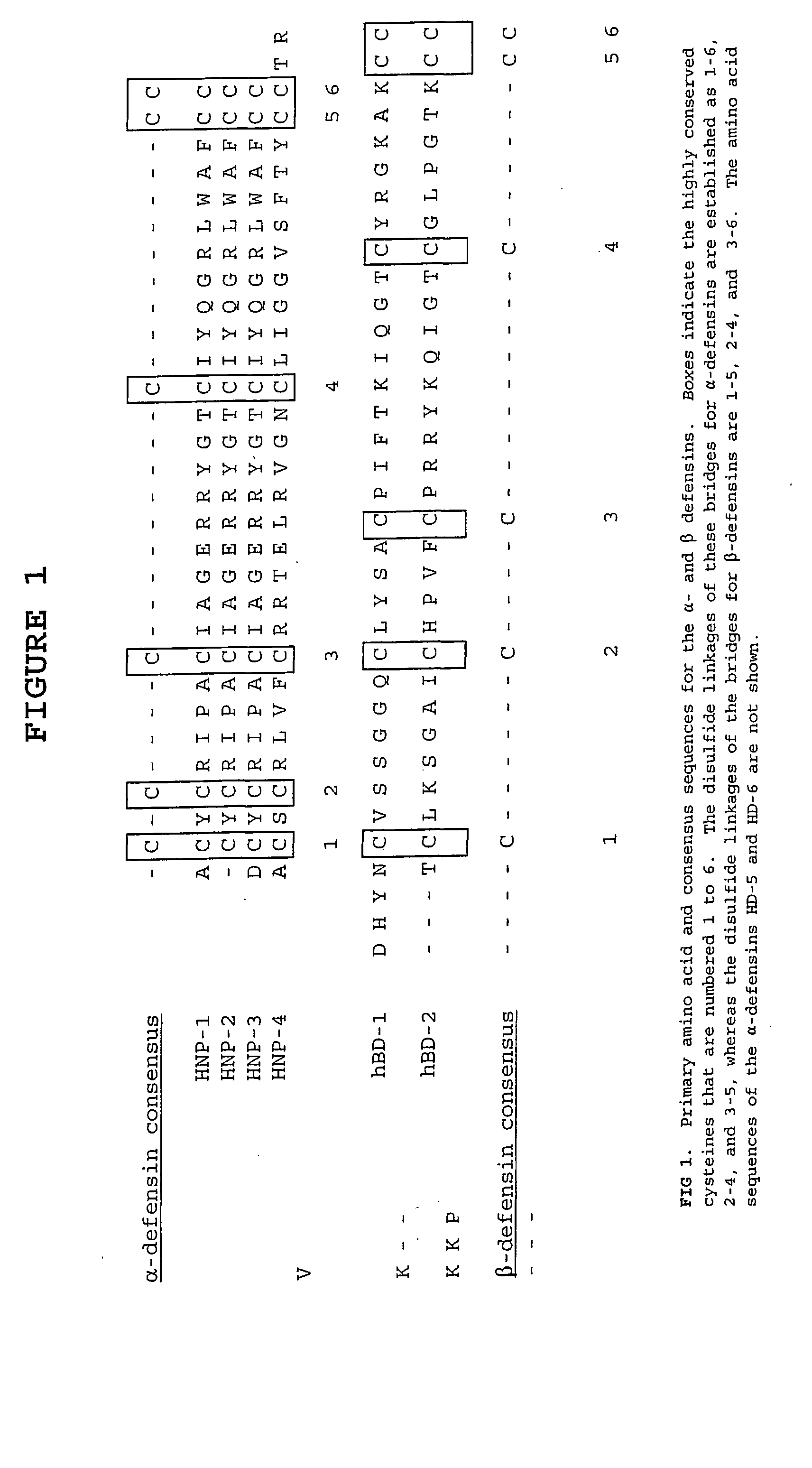
Defensin-antigen fusion proteins
The present invention relates to a vaccine for increasing the immunogenicity of a tumor antigen thus allowing treatment of cancer, as well as a vaccine that increases the immunogenicity of a viral antigen, thus allowing treatment of viral infection, including immunodeficiency virus (HIV) infection. In particular, the present invention provides a fusion protein comprising a defensin fused to either a tumor antigen or viral antigen which is administered as either a protein or nucleic acid vaccine to elicit an immune response effective in treating cancer or effective in treating or preventing viral infection.
Owner:THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC DEPT OF HEALTH & HUMAN SERVICES NAT INST OF HEALTH OFFICE OF TECH TRANSFER
Neutralizing molecules to viral antigens
The present invention concerns methods and means for identifying, producing, and engineering neutralizing molecules against influenza A viruses, and to the neutralizing molecules produced. In particular, the invention concerns neutralizing molecules against various influenza A virus subtypes, including neutralizing antibodies against H5 and / or H3 and / or H1, such as, for example all of H1, H3, and H5 subtypes, and methods and means for making such molecules.
Owner:BIOASSETS LLC
Deletion mutants of flagellin and methods of use
InactiveUS20110135680A1Cost effectiveMaximize immunogenic responseAntibacterial agentsPowder deliveryToll-like receptorProtective immunity
Compositions that include Toll-like Receptor 5 agonists and at least a portion of at least one viral antigen can be employed in methods that stimulate an immune response in a subject, in particular, a protective immune response in a subject. Compositions can be associated with particles and employed in the methods in relatively low doses to provide protective immunity to viral infection.
Owner:VAXINNATE
Detection of multiple analytes from a single sample using a multi-well, multi-analyte flow-through diagnostic test device
InactiveUS20020115062A1Quick distinctionQuick fixBioreactor/fermenter combinationsBiological substance pretreatmentsMulti analyteDiagnostic test
The present invention provides a method and device for conducting a rapid in vitro enzyme immunoassay test for the direct and qualitative detection of two or more viral antigens from specimens of symptomatic patients. The method for immunoassay of viral antigens is performed on a membrane. Non-immunological capture of viral antigens takes place by absorption onto the membrane. Captured antigen binds to a detection reagent that includes a label conjugated to a specific antibody. The test is a differentiated test such that two or more viral antigens may be distinguished from each other in a single test. The invention also includes a kit for performing an assay in accordance with the method of the present invention, wherein the kit comprises the device of the present invention.
Owner:BECTON DICKINSON & CO
Hydrophobia vaccine freezing drying preparations for stable human beings and the preparations thereof
ActiveCN101095950AImprove stabilityNo pollution in the processPowder deliveryAntiviralsFreeze-dryingMedicine
The invention relates to a kind of hydrophobia vaccine for human, which in detail relates to a freeze-drying hydrophobia vaccine for human and its preparation method. It is contained in physiological soluble buffer solution with pH being 7-8, the sodium chloride weight proportion is 0.4-1.0%, one or several from mycose, sucrose, gelatin, maltose and dextran is used as stabilizing agent, the concentration is 0.5-5%; the weight proportion of shaping agent for freeze-drying agent is 1-5%, and the purified hydrophobia viral antigen concentration is 4-20 IU per ml.
Owner:LIAONING CHENGDA BIOTECH
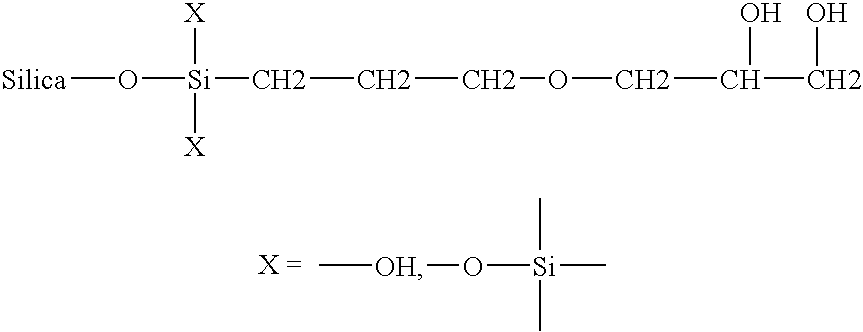
Microparticles with adsorbed polypeptide-containing molecules
ActiveUS7501134B2Easy to produceSsRNA viruses negative-senseAntibacterial agentsHemagglutininLactide
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
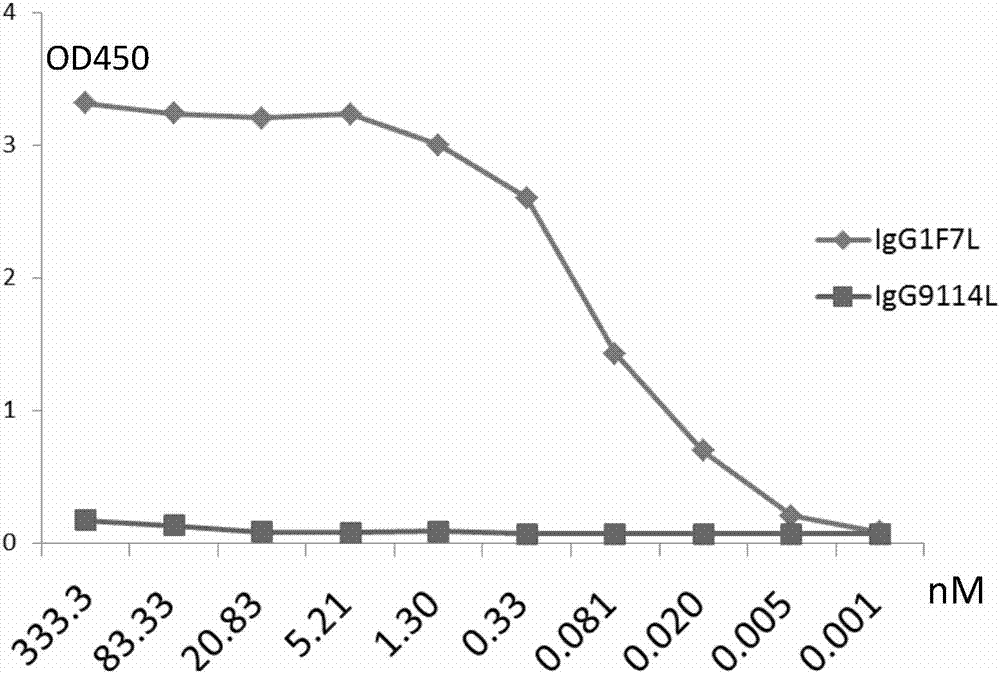
Human anti-H7N9 avian influenza virus neutralizing antibody 1F7L and its use
InactiveCN107226861AEffective neutralizationAvoid infectionImmunoglobulins against virusesAntiviralsNatural Killer Cell Inhibitory ReceptorsNeutralizing antibody
The invention discloses a human anti-H7N9 avian influenza virus neutralizing antibody 1F7L screened by a single cell sorting technology. The amino acid sequences in the light and heavy chain variable regions are shown in the formulas of SEQ ID No. 2 and SEQ ID No. 5. The antibody has the ability to neutralize the H7N9 influenza viruses in vitro and mediates the killing (ADCC) of the H7N9 influenza virus-infected cells with effector cells mainly comprising NK cells. The antibody can be used as a treatment drug for highly pathogenic avian influenza infection and can also be used for the development of H7N9 influenza virus antigen detection reagents.
Owner:THE THIRD PEOPLES HOSPITAL OF SHENZHEN
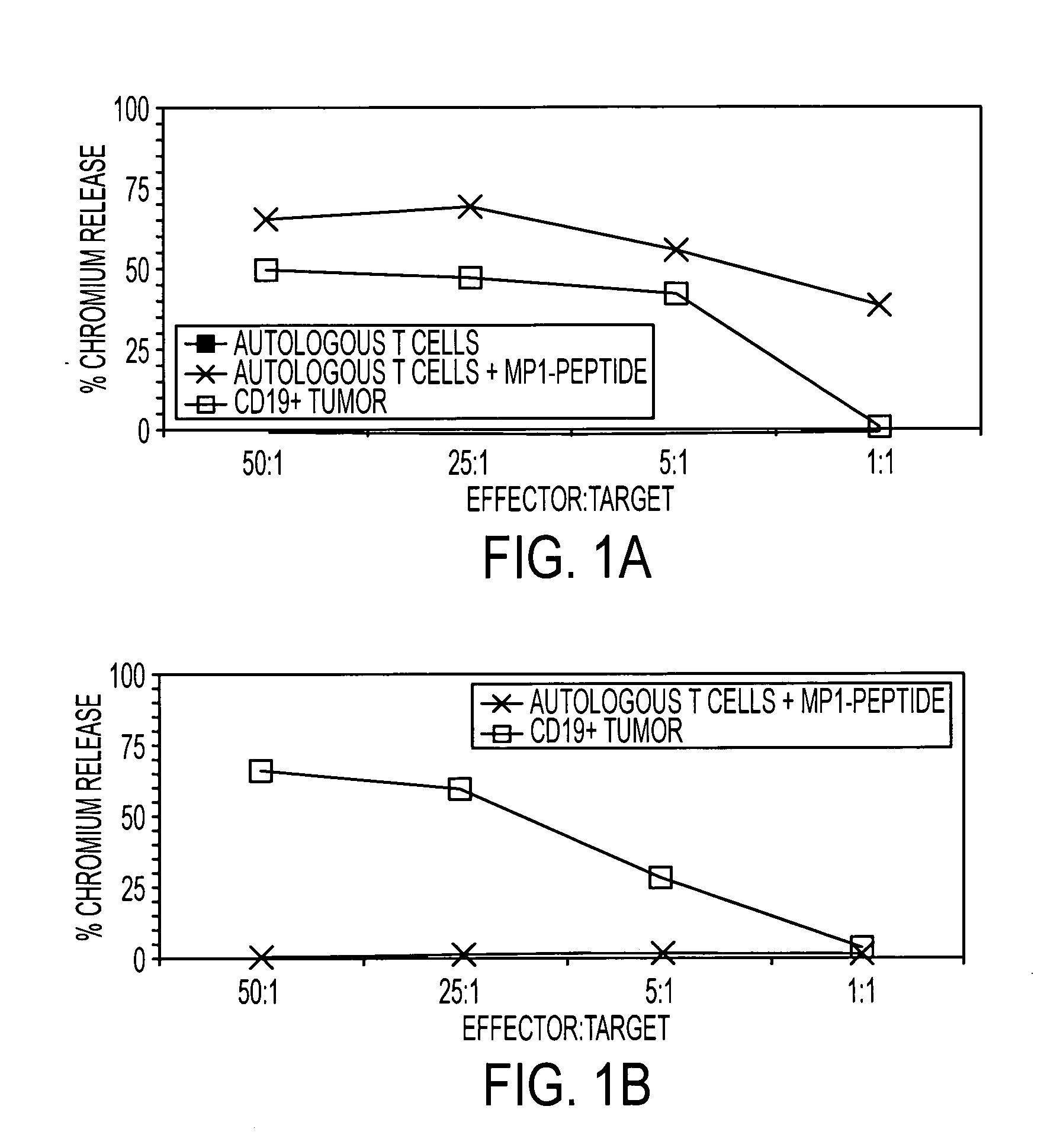
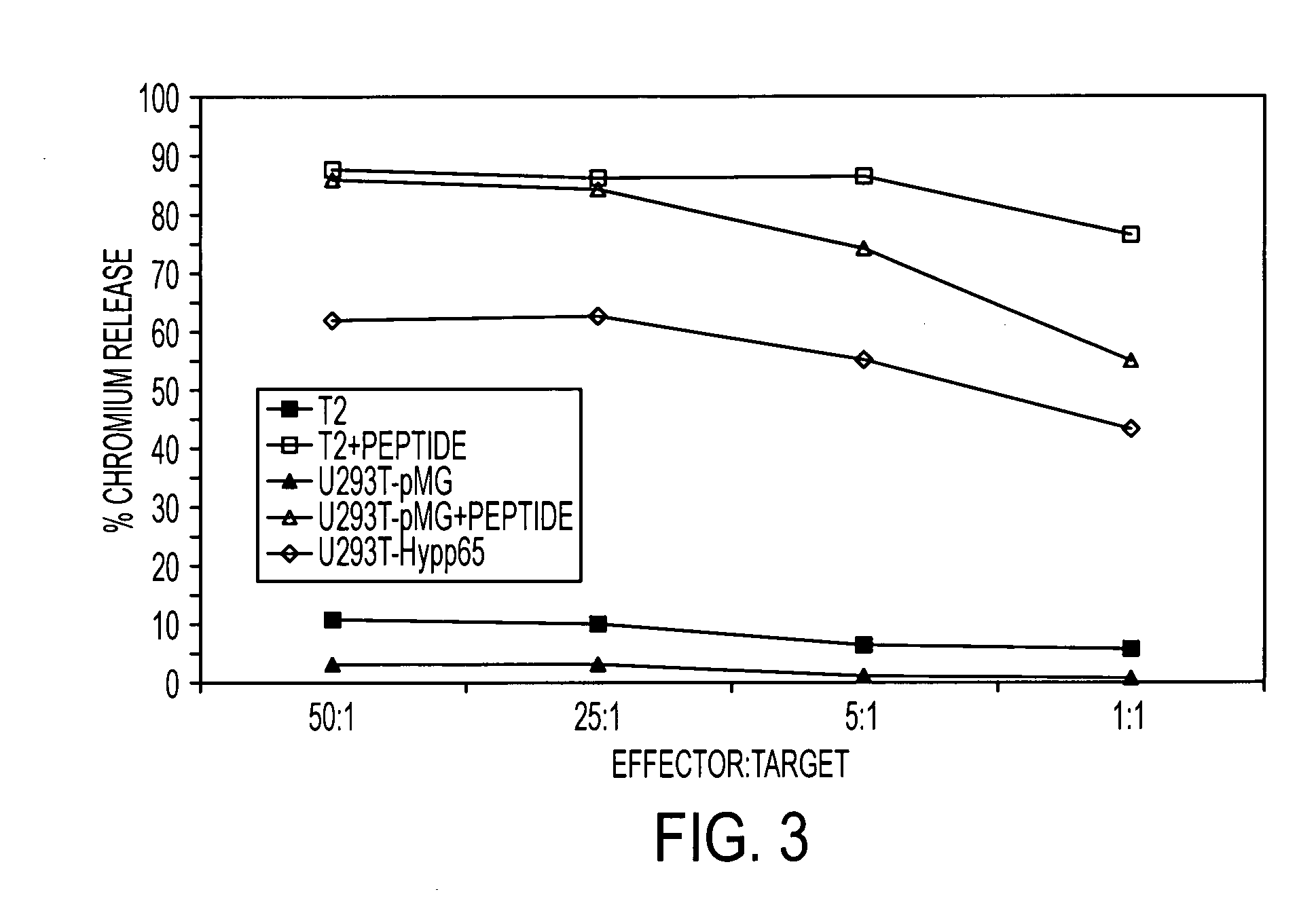
Dual antigen specific T cells with trafficking ability
InactiveUS20060018878A1Maintain in vivo functionEfficient workBiocideGenetic material ingredientsAbnormal tissue growthDisease
The present invention is directed to mammalian T cells and methods for using these T cells. More specifically, the invention relates to viral specific T cells that express an endogenous viral antigen receptor, a chimeric anti-tumor receptor and the chemokine receptor CCR7. These T cells are a source of effector cells that persist in vivo in response to stimulation with viral antigen, leading to long-term function after their transfer to patients with cancer and autoimmune diseases and that are able to traffic to lymph nodes and sites of minimal residual disease.
Owner:CITY OF HOPE
ELISA detection method and reagent kit for bluetongue viral antigen
InactiveCN101403759AImprove featuresIncreased sensitivityBiological testingSerum igeMonoclonal antibody
The invention discloses an ELISA detection method and a kit of blue-tongue virus antigen. The detection method uses the monoclonal antibody of anti-blue-tongue virus VP7 protein as dual-anti sandwich ELISA detection method of a coating and enzyme-marked antibody; and the kit comprises the monoclonal antibody of the anti-blue-tongue virus VP7 protein of the coating and enzyme-marked antibody. The kit and the detection method can be used for detecting the blue-tongue virus antigen, have higher specificity and sensitiveness, can be used in large-scale forserological detection, is suitable for epidemiological investigation and has wide application prospect.
Owner:FIELD OPERATION BLOOD TRANSFUSION INST OF PLA SCI ACAD OF MILITARY
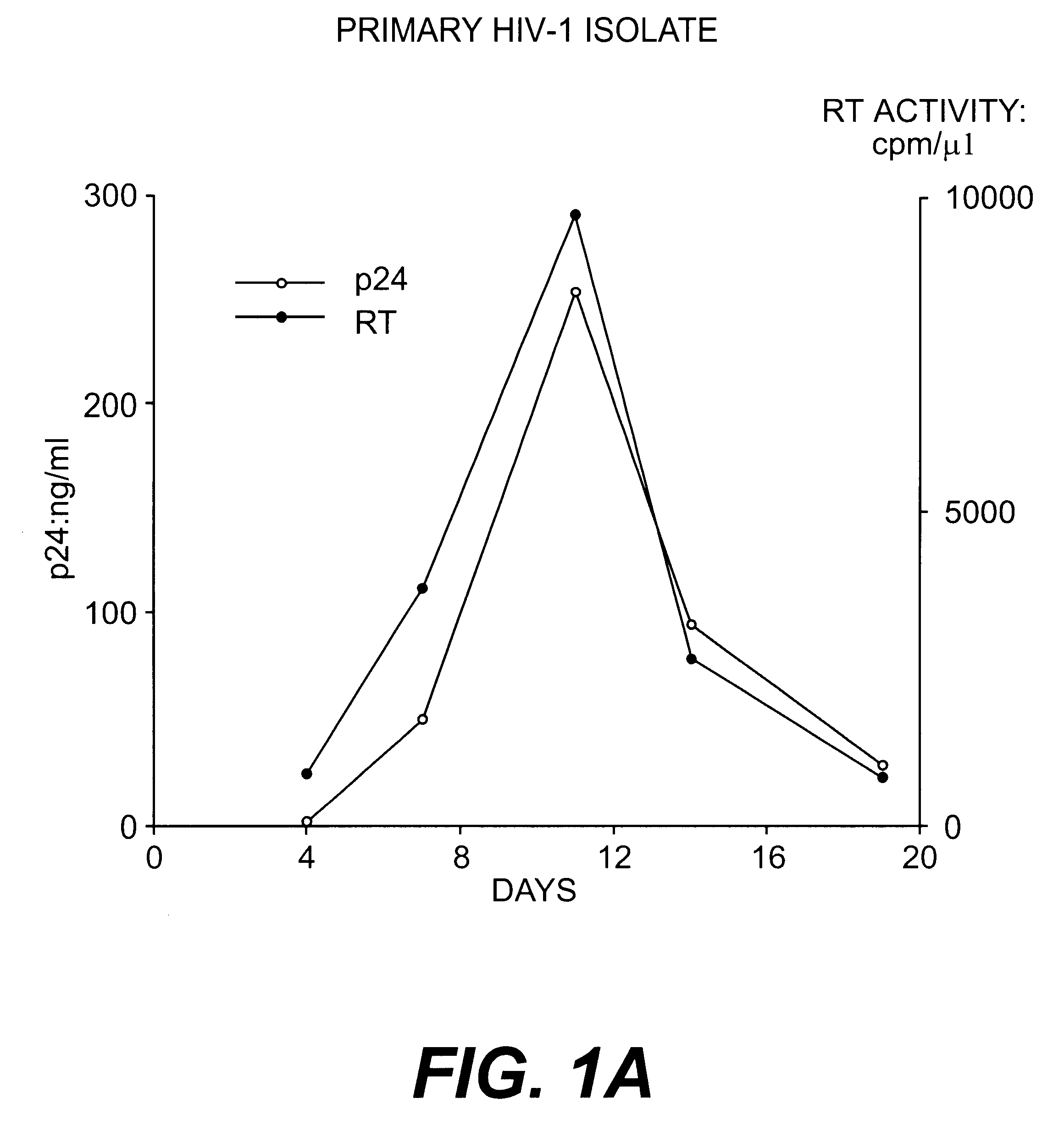
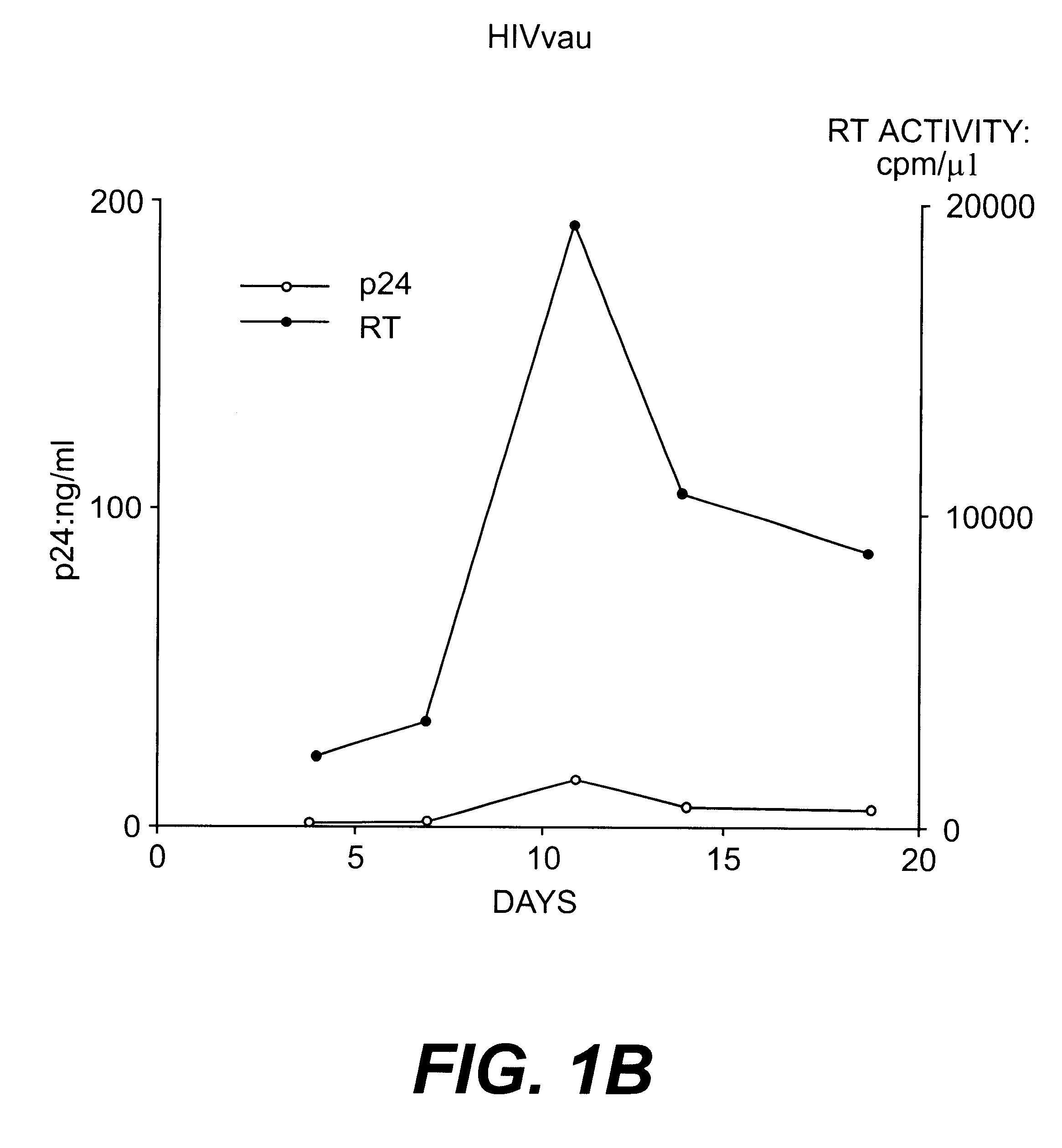
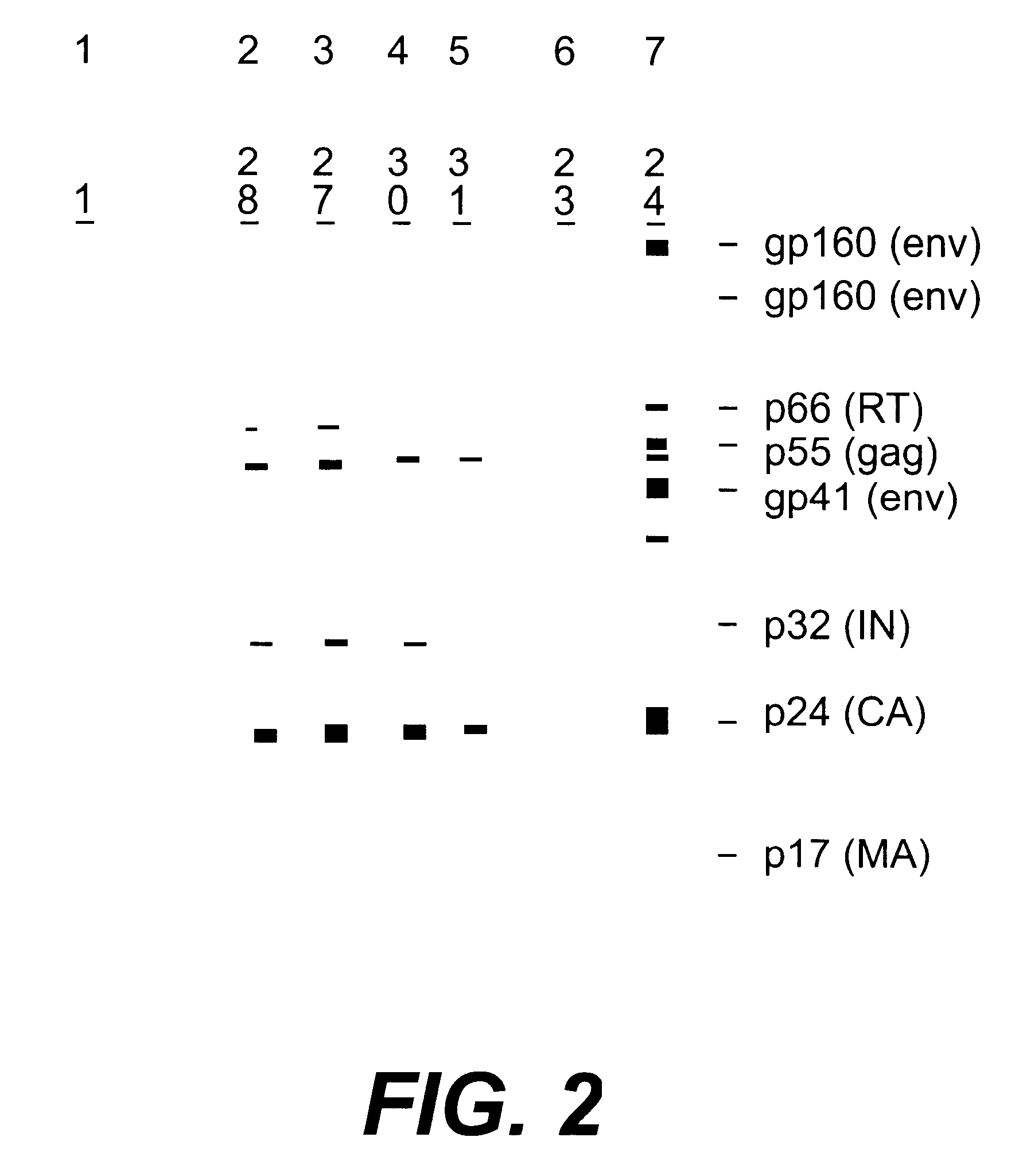
Nucleotide sequences of HIV-1 type (or subtype) O retrovirus antigens
An HIV-1 type (or subtype) O retrovirus protein, or a natural or synthetic polypeptide or peptide including at least a part of said protein, which is capable of being recognised by antibodies isolated from a serum resulting from infection by an HIV-1 type O VAU strain or an HIV-1 type (or subtype) O DUR strain.
Owner:INST PASTEUR
Affinity absorption column for specially removing virions from blood and preparing method thereof
InactiveCN101628135AStrong specificityReduce harmIon-exchange process apparatusOther blood circulation devicesTreatment effectSide effect
The invention discloses an affinity absorption column for specially removing virions from blood and preparing method thereof, in particular relates to an affinity absorption column for blood purification. The affinity absorption column comprises an absorption carrier, an activator and antivirus surface protein antibody; the activator is added into the absorption carrier to obtain an activated carrier; the antivirus surface protein antibody is added into the activated carrier to perform antibody coupling to obtain an immune absorber, and the immune absorber is loaded into the absorption column to obtain the affinity absorption column. The affinity absorption column has the advantages of strong specificity, little toxic side effect, good therapeutic effect, good repeatability and low cost, and is suitable for blood purification; besides, being a separation technology, the affinity absorption column can be widely applied to other fields for the purification and separation of virus antibodies.
Owner:WUHAN UNIV
Microparticles with adsorbed polypeptide-containing molecules
ActiveUS20050220883A1Without usingEasy to produceSsRNA viruses negative-senseAntibacterial agentsHemagglutininLactide
Microparticles with absorbed polypeptide-containing molecules formed without the use of surfactant, methods of making such microparticle compositions, and uses thereof, are disclosed. The microparticles comprise a polymer, such as a poly(α-hydroxy acid), a polyhydroxy butyric acid, a polycaprolactone, a polyorthoester, a polyanhydride, and the like. Preferred polymers are poly(D,L-lactide-co-glycolides), more preferable those having a lactide / glycolide molar ratio ranging from 40:60 to 60:40 and having a molecular weight ranging from 20,000 Daltons to 70,000 Daltons. Preferred polypeptide containing molecules are bacterial and viral antigens (including HIV antigens, meningitis B antigens, streptococcus B antigens, and Influenza A hemagglutinin antigens).
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
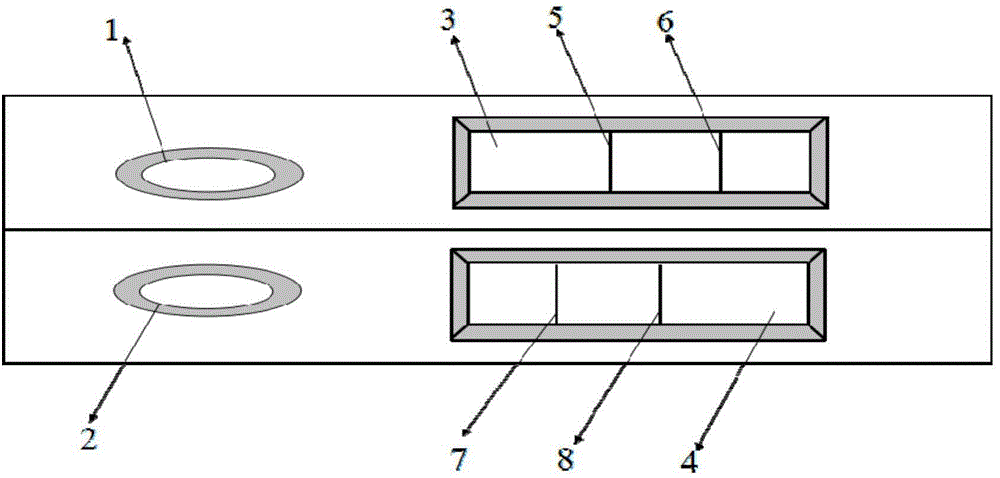
Colloidal gold immunochromatography test strip for simultaneously detecting viral antigens and antibodies
The invention relates to a colloidal gold immunochromatography test strip for simultaneously detecting viral antigens and antibodies. The test strip comprises a first glue strip and a second glue strip which are arranged in parallel, wherein the first glue strip comprises a first sample pad, a first conjugate pad, a first nitrocellulose membrane and first absorbent paper, which are sequentially lapped with one another, the first conjugate pad is coated with colloidal gold marked by the viral antigens, the first nitrocellulose membrane is coated with viral antibodies and antibodies, the viral antibodies are taken as a first detection line, and the antibodies are used for resisting the viral antigens on the colloidal gold and are taken as a quality control line; the second glue strip comprises a second sample pad, a second conjugate pad, a second nitrocellulose membrane and second absorbent paper, which are sequentially lapped with one another, the second conjugate pad is coated with the colloidal gold marked by the viral antigens, the second nitrocellulose membrane is coated with anti-human IgG antibodies and anti-human IgM antibodies, the anti-human IgG antibodies are taken as a second detection line, and the anti-human IgM antibodies are taken as a third detection line. The colloidal gold immunochromatography test strip can be used for simultaneously detecting the viral antigens as well as the IgG antibodies and IgM antibodies of the viral antigens.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
Semiconductor sensor for virus detection as well as preparation method and application thereof
ActiveCN111965231ALarge specific surface areaRich in surface activityBiological material analysisBiological testingEngineeringField effect
The invention belongs to the technical field of biochemical sensing, and discloses a semiconductor sensor for virus detection as well as a preparation method and an application thereof. The preparation method comprises the steps of (1) preparing an oxide or chalcogenide colloid semiconductor nano-material; (2) coating the surface of the colloid semiconductor nano material with a virus specific antigen or antibody to obtain a sensitive material; and (3) based on the sensitive material, preparing the virus detection sensor by adopting device structures such as a chemically modified electrode ora field effect transistor. The virus specific antigen or antibody is introduced to the surface of the colloid semiconductor nano material, charge transfer caused by a specific binding reaction betweenthe virus antigen and antibody is converted into a sensor electric signal to be output through a semiconductor sensitive effect, and the real-time performance and convenience of a virus detection technology are improved.
Owner:HUAZHONG UNIV OF SCI & TECH +1
Methods for tailoring the immune response to an antigen or immunogen
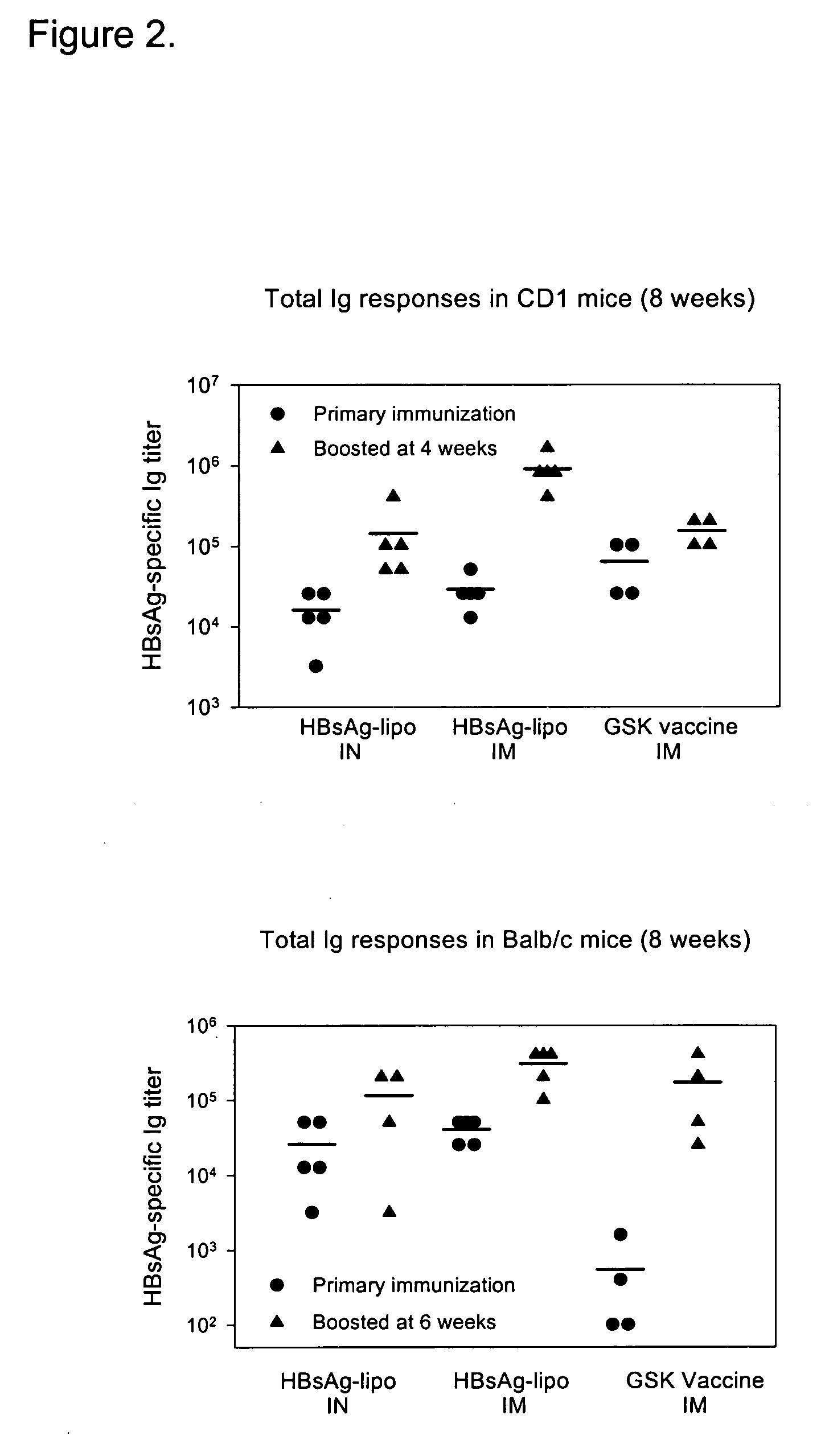
The invention relates to methods and reagents for immunizing animals to elicit specific cellular and humoral immune-responses against specific antigens, such as viral antigens, including HBsAg antigen. The invention provides methods of using specifically prepared immunogen in fresh or lyophilized liposome, proper routes of administration of the immunogen, proper doses of the immunogen, and specific combinations of heterologous immunization including DNA priming in one administration route followed by liposome-mediated protein antigen boost in a different route to tailor the immune responses in respects of enhancing cell mediated immune response, cytokine secretion, humoral immune response, immune protection and selective skewing of T helper responses to be Th1, Th2, or a mixed or balanced Th response.
Owner:MUCOSAL VACCINE TECH LLC
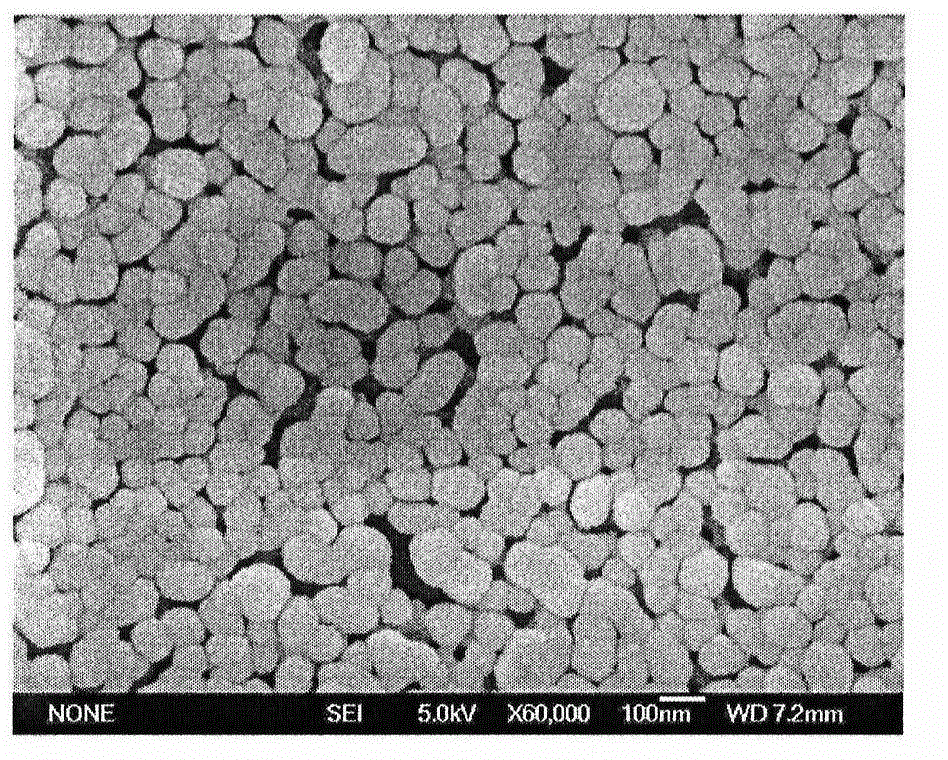
Preparation of antigen-loaded polymer lipid nanosphere and application as vaccine adjuvant
ActiveCN106177974AIncrease intakeProcessing and submission is goodPowder deliveryPharmaceutical non-active ingredientsZeta potentialBiological activation
The invention relates to a polymer lipid nanoparticle, a preparation method and application thereof as a vaccine adjuvant system. The polymer nanoparticle is a solid sphere composed of a macromoleclar polymer, positively charged cationic lipid is inlaid on the surface, the nanoparticle surface can adsorb protein antigen, polypeptide antigen, viral antigen and other active drugs; the polymer nanoparticle has an average particle size of less than 300nm, a dispersion coefficient, i.e. a PDI (polydispersity) value of less than 0.2, and a Zeta potential average value of greater than 20mV. The polymer nanoparticle provided by the invention has the characteristics of uniform size, high antigen carrying rate, and good maintenance of antigen activity, when the polymer nanoparticle carries an antigen and serves as a vaccine adjuvant, the intake of antigen presenting cells (APCs) to the antigen can be increased, the activation level of antigen presenting cells is improved, and the follow-up immune response level is strengthened.
Owner:王连艳
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com