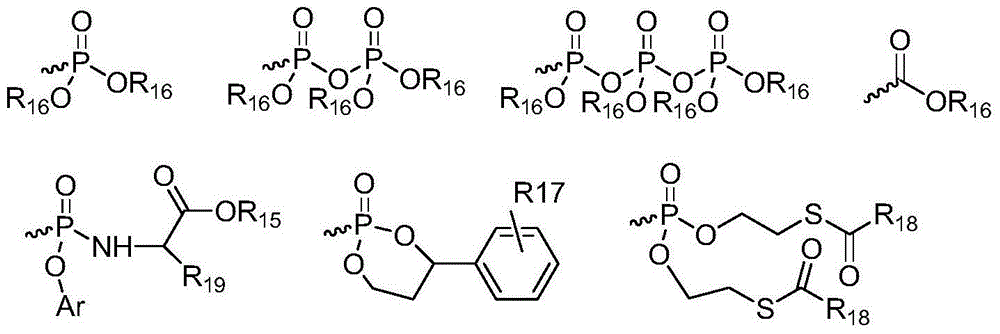
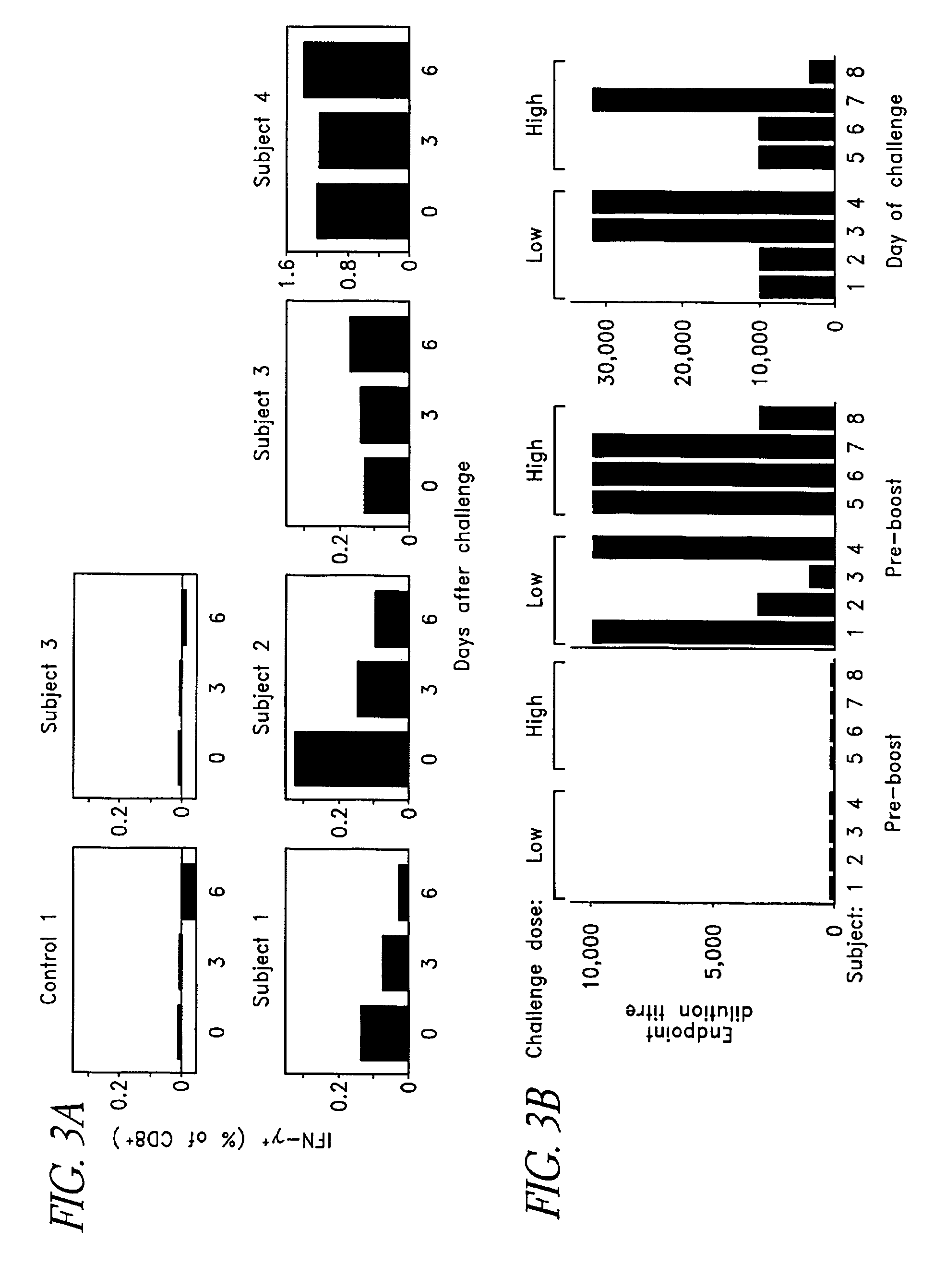
Patents
Literature
166 results about "Ebola virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
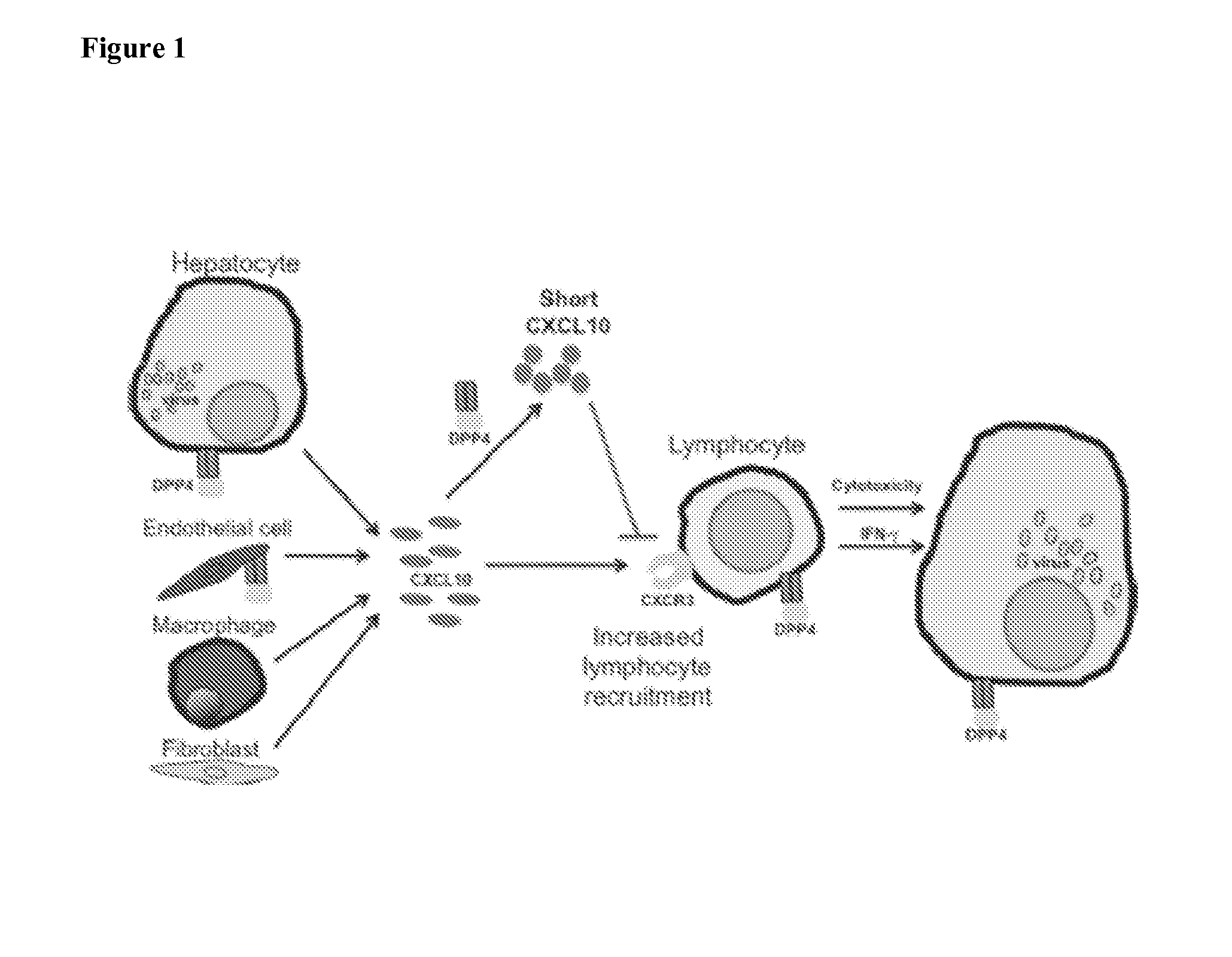
Ebola virus is one of five known viruses within the genus Ebolavirus. Four of the five known ebolaviruses, including EBOV, cause a severe and often fatal hemorrhagic fever in humans and other mammals, known as Ebola virus disease. Ebola virus has caused the majority of human deaths from EVD, and is the cause of the 2013–2014 Ebola virus epidemic in West Africa, which has resulted in at least 17,145 suspected cases and 6,405 confirmed deaths. Ebola virus and its genus were both originally named for Zaire, the country where it was first described, and was at first suspected to be a new "strain" of the closely related Marburg virus. The virus was renamed "Ebola virus" in 2010 to avoid confusion. Ebola virus is the single member of the species Zaire ebolavirus, which is the type species for the genus Ebolavirus, family Filoviridae, order Mononegavirales. The natural reservoir of Ebola virus is believed to be bats, particularly fruit bats, and it is primarily transmitted between humans and from animals to humans through body fluids. The EBOV genome is a single-stranded RNA approximately 19,000 nucleotides long.
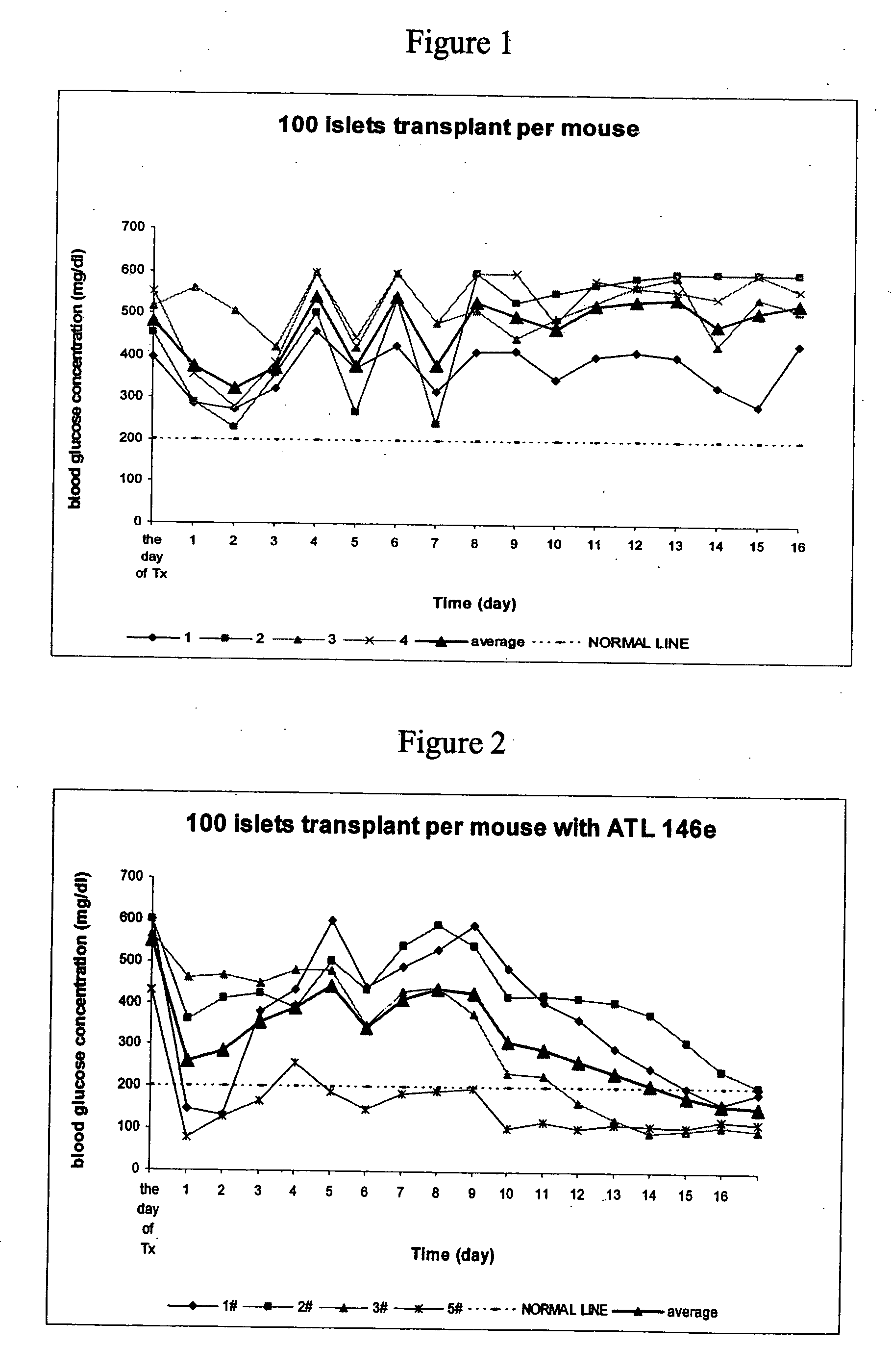
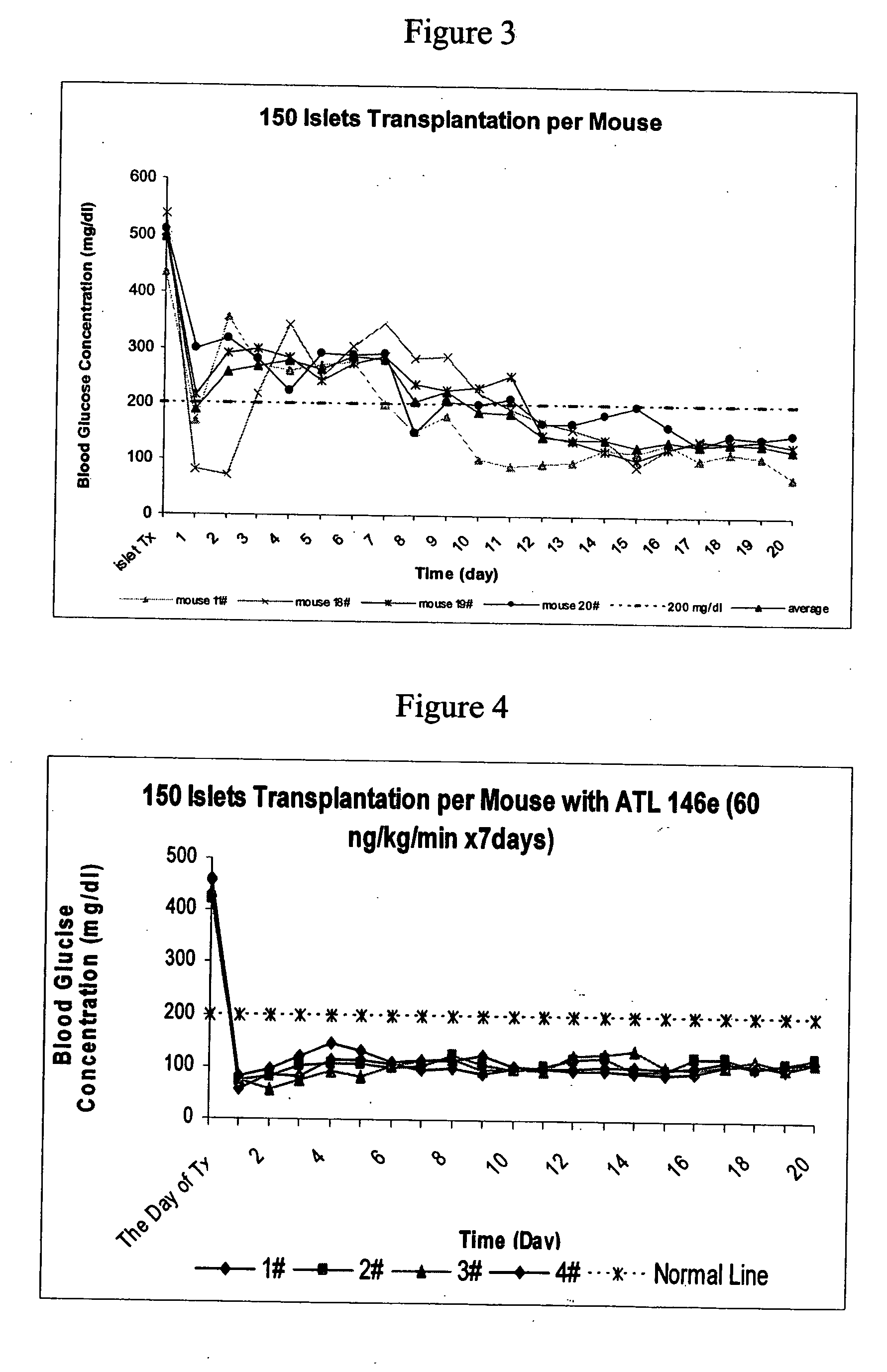
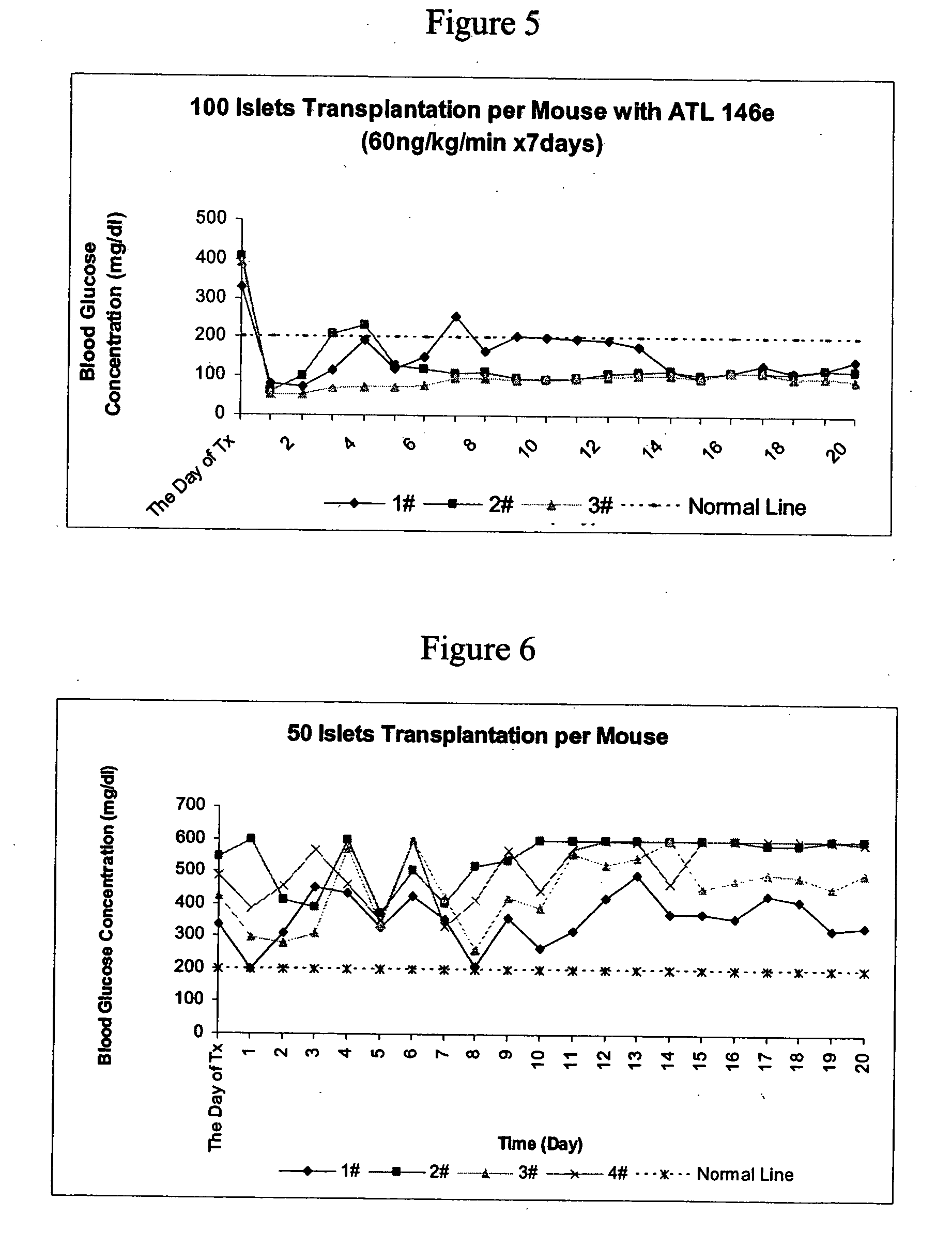
Method to reduce inflammatory response in transplanted tissue
InactiveUS20050182018A1Inhibit inflammationLower immune responseBiocideSugar derivativesPandrug resistant bacteriaDisease cause
The present invention provides a therapeutic method for treating biological diseases that includes the administration of an effective amount of a suitable antibiotic agent, antifungal agent or antiviral agent in conjunction with an A2A adenosine receptor agonist. If no anti-pathogenic agent is known the A2A agonist can be used alone to reduce inflammation, as may occur during infection with antibiotic resistant bacteria, or certain viruses such as those that cause SARS or Ebola. Optionally, the method includes administration of a type IV PDE inhibitor.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
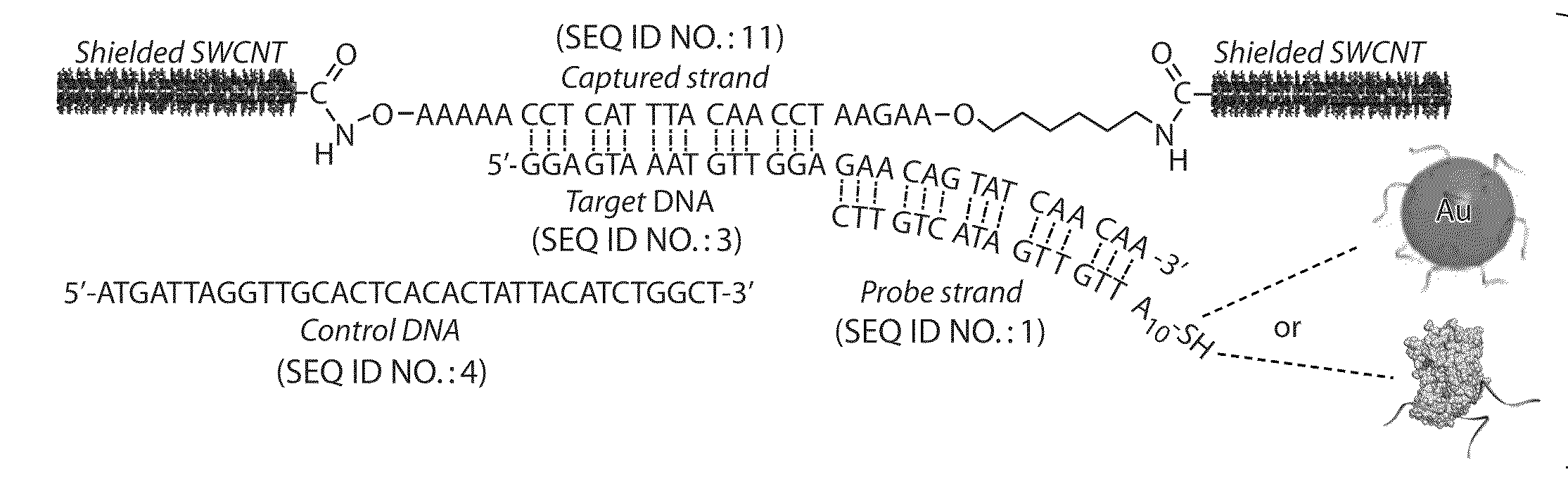
Nanostructured devices including analyte detectors, and related methods
The present invention provides compositions and devices comprising nanostructure networks, and related methods. The compositions may exhibit enhanced interaction between nanostructures, providing improved device performance (e.g., improved conductivity). In some embodiments, the devices are capable of interacting with various species to produce an observable signal from the device. In some cases, the compositions and devices may be useful in the determination of analytes, including—biological analytes (e.g., DNA, ebola virus, other infective agents, etc.), small, organic analytes, and the like. The embodiments described herein may exhibit high sensitivity and specificity to analytes and may be capable of analyte detection at femtomolar concentrations (e.g., 10 fM).
Owner:MASSACHUSETTS INST OF TECH
Antigen fragment and truncation based on ebola virus envelope protein as well as application
ActiveCN103864904AAvoid infectionEasy to manufactureSsRNA viruses negative-senseViral antigen ingredientsAntigenEbola virus
The invention discloses an antigen fragment and truncation based on ebola virus envelope protein as well as an application. The protein or the truncation of the protein is disclosed by the invention; the protein is shown in SEQ ID No. 3 or protein which is obtained by replacing and / or deleting and / or adding one or more amino acid residues in an amino acid sequence shown in the SEQ ID No. 3 and has the identical function as the protein shown in SEQ ID No. 3. The antigen fragment and the truncation thereof disclosed by the invention can induce relatively strong humoral immune response, has good immunogenicity and can be applied to development of ebola virus vaccines and preparation of neutralizing antibodies.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
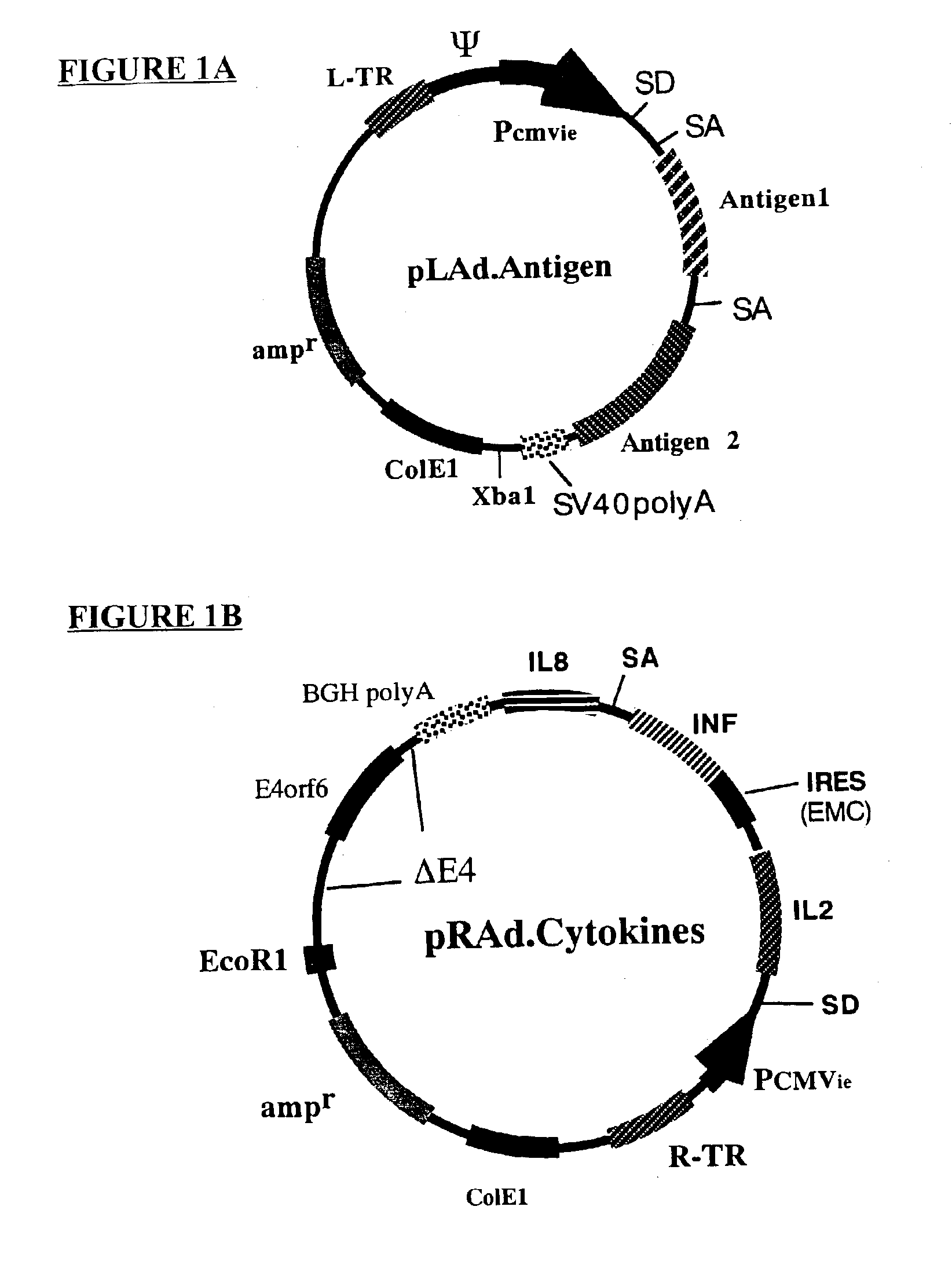
Composition and method for stimulating immune response to pathogen using complex adenoviral vector
InactiveUS6964762B2Improving immunogenicityStrong immune responseSsRNA viruses negative-senseAntibacterial agentsHeterologousProgenitor
Genetic vaccines and methods are provided for enhancing the immunity of a host such as a human to one or more pathogens. In one aspect, a method of enhancing the immunity of a host to a pathogen is provided. The method comprises administering to the host a recombinant virus comprising an antigen sequence that is heterologous to a native progenitor of the recombinant adenovirus and encodes a viral antigen from a pathogenic virus, expression of which is under the transcriptional control of a first promoter; and a cytokine sequence that is heterologous to the native progenitor of the recombinant adenovirus and encodes a cytokine, expression of which is under the transcriptional control of a second promoter. Expression of the antigen and cytokine sequences elicits an immune response directed against the viral antigen upon infection of the host by the recombinant virus. The method can be used for immunizing a host against a wide variety of pathogen viruses, such as HIV, Ebola virus, Marburg virus, hepatitis B virus, hepatitis C virus, influenza virus, human simplex virus, human papilloma virus and respiratory syncytial virus.
Owner:GENPHAR INC
Method to reduce inflammatory response in transplanted tissue
InactiveUS7427606B2Lower immune responseGood effectBiocideSugar derivativesResistant bacteriaAntibiotic Agents
The present invention provides a therapeutic method for treating biological diseases that includes the administration of an effective amount of a suitable antibiotic agent, antifungal agent or antiviral agent in conjunction with an A2A adenosine receptor agonist. If no anti-pathogenic agent is known the A2A agonist can be used alone to reduce inflammation, as may occur during infection with antibiotic resistant bacteria, or certain viruses such as those that cause SARS or Ebola. Optionally, the method includes administration of a type IV PDE inhibitor.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
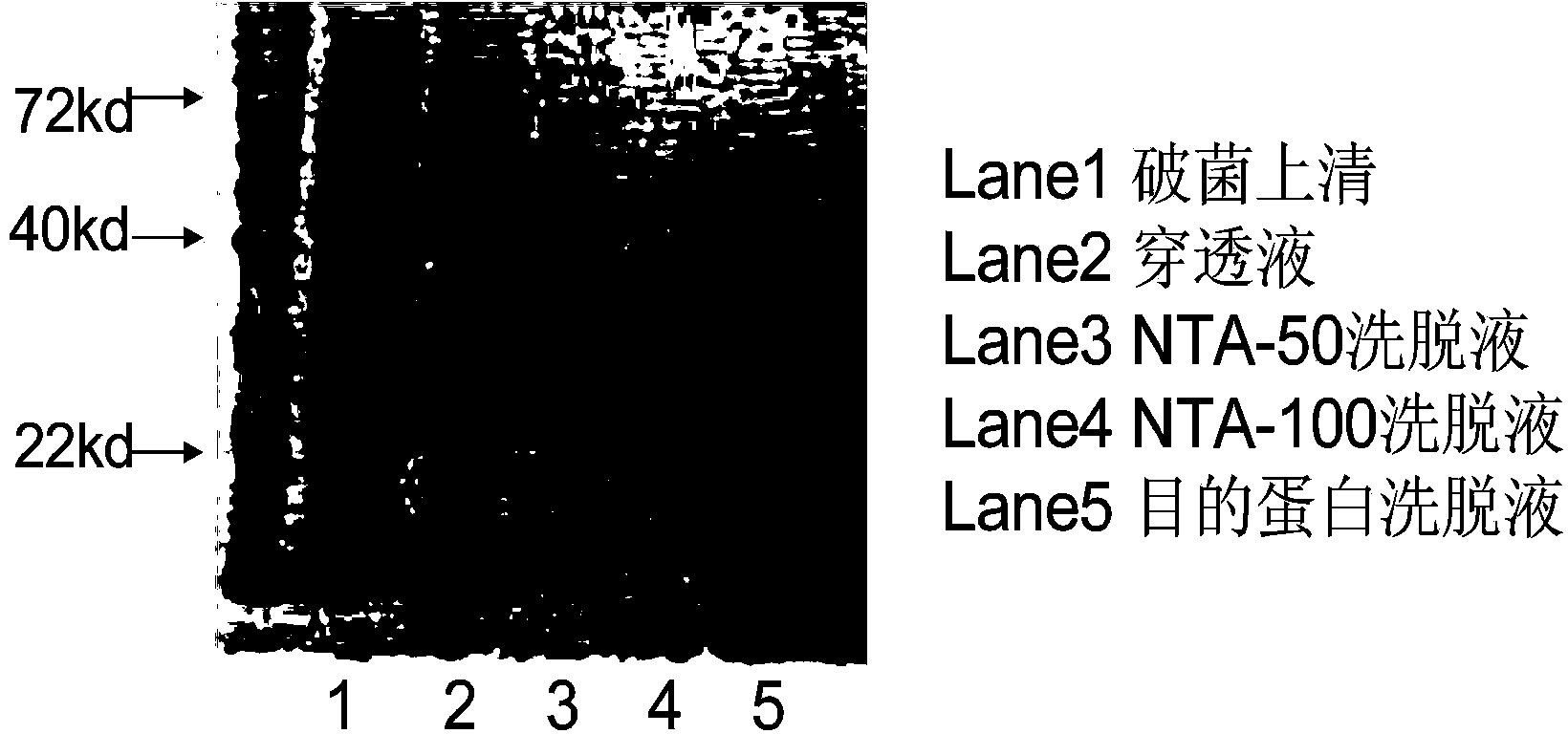
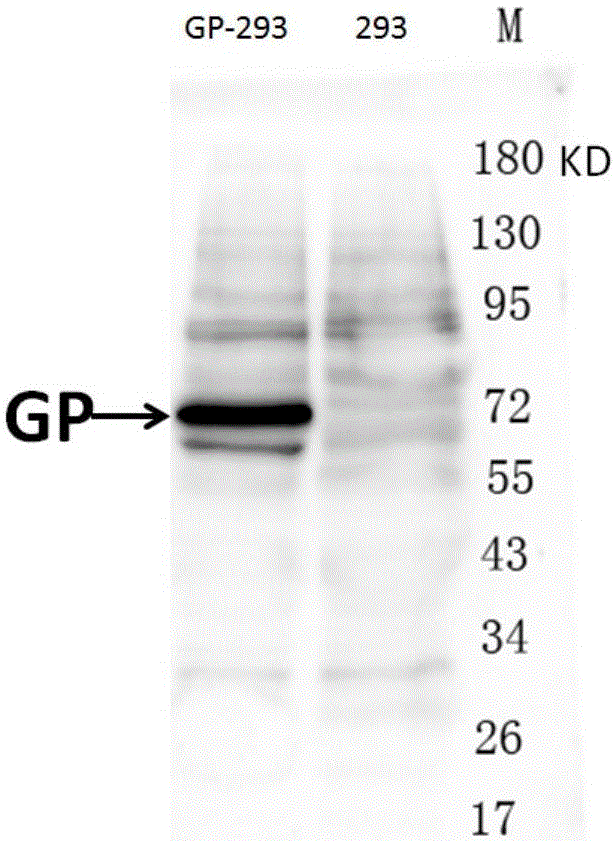
Hybridoma cell strain ZJEB8-01, Ebola-virus GP albumen resistant monoclonal antibody, and preparation and application of Ebola-virus GP albumen resistant monoclonal antibody
ActiveCN105087497AStable secretionNo decline in secretionImmunoglobulins against virusesTissue cultureAntigenEbola virus
The invention relates to a hybridoma cell strain ZJEB8-01, an Ebola-virus GP albumen resistant monoclonal antibody, and preparation and application of the Ebola-virus GP albumen resistant monoclonal antibody. The hybridoma cell strain ZJEB8-01 can be used for secreting the Ebola-virus GP albumen resistant monoclonal antibody. Binding of the monoclonal antibody and the 412th-431st amino acid sequence antigen peptides of Ebola-virus GP albumen has high specificity and sensitiveness, and the monoclonal antibody can be applied to preparation of Ebola-virus GP albumen detection reagents. Sequencing of the monoclonal antibody is completed, and the material basis is laid for humanization transformation of monoclonal antibody sequences, research and development of the neutralizing antibody for neutralizing Ebola viruses and development of the antibody into clinical Ebola virus treatment antibody in later period.
Owner:THE FIRST AFFILIATED HOSPITAL ZHEJIANG UNIV COLLEGE OF MEDICINE
Compositions and methods for treatment of filovirus-mediated diseases
The invention features compositions, methods, and kits useful for the treatment of filovirus-mediated diseases, e.g., hemorrhagic fever caused by Ebola virus, in an animal.
Owner:US ARMY MEDICAL RES MATERIEL COMMAND USAMRMC +1
Nanoparticle vaccines with novel structural components
ActiveUS20200009244A1Treating and preventing HIV- infectionAvoid infectionSsRNA viruses negative-sensePowder deliveryEbola virusNanoparti cles
The present invention provides novel nanoparticle presented vaccine compositions that are stabilized with a locking domain. Various immunogens can be employed in the preparation of the vaccine compositions, including viral immunogens such as HIV-1 and Ebola viral immunogens, and non-viral immunogens such as immunogens derived from bacteria, parasites and mammalian species. The invention also provides methods of using such vaccine compositions in various therapeutic applications, e.g., for preventing or treating viral infections.
Owner:THE SCRIPPS RES INST
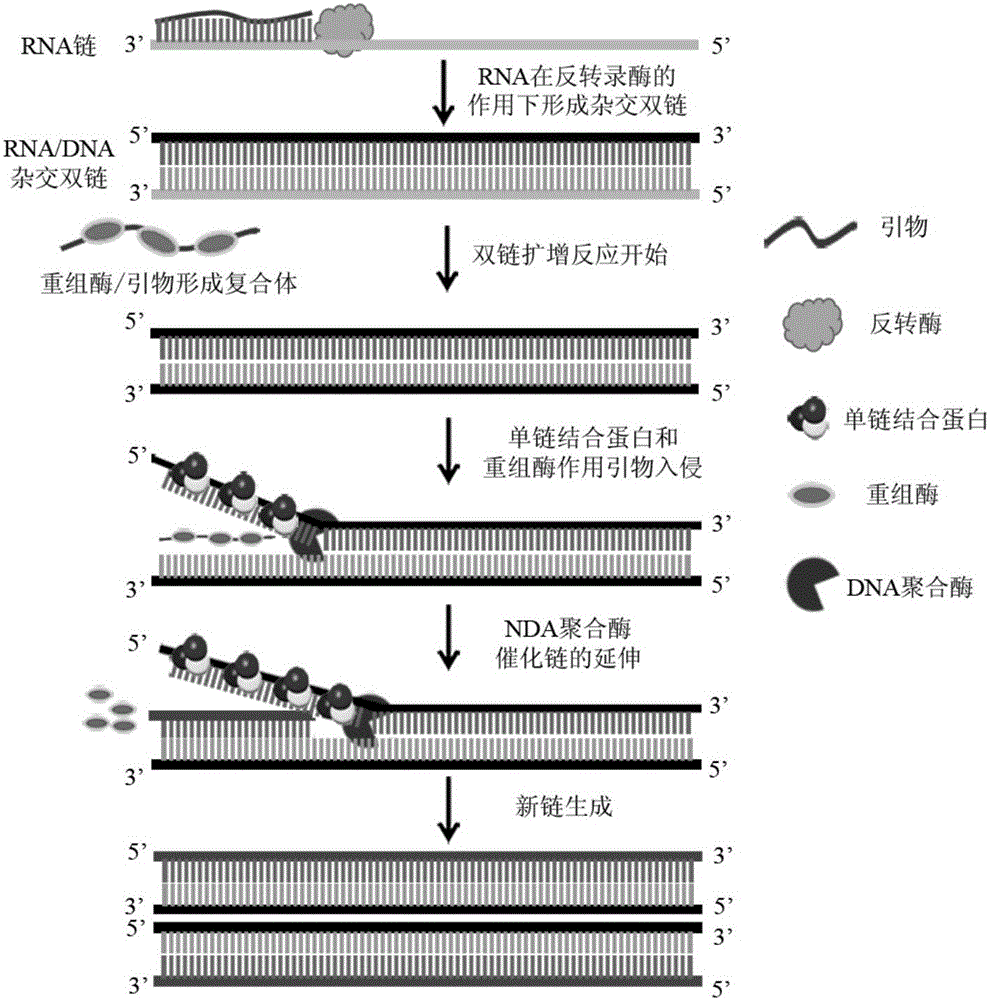
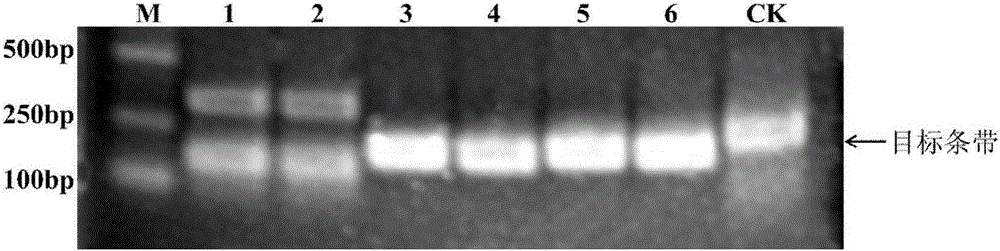
Method, reagent, primer and probe for quickly detecting Ebola viruses under constant-temperature and isothermal conditions
InactiveCN105087825AAvoid pollutionGuaranteed Closed Tube DetectionMicrobiological testing/measurementDNA/RNA fragmentationEbola virusBiology
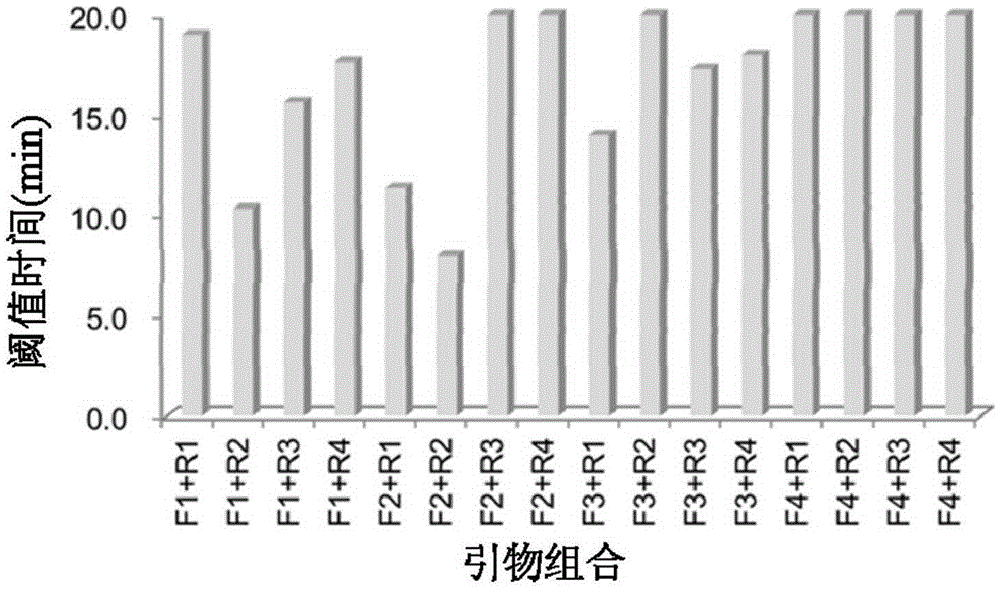
The invention discloses a method, a reagent, a primer and a probe for quickly detecting Ebola viruses under constant-temperature and isothermal conditions. The method, the reagent, the primer and the probe have the advantages that the Ebola viruses can be quickly, sensitively and accurately detected by the aid of the method in real time under the constant-temperature and isothermal conditions, nucleic acid targets can be quickly detected in instruments with fluorescence detection functions under the combined actions of the specific primer, the specific fluorescence probe, six engineering enzymes and other chemical constituents, closed-tube detection can be guaranteed by strict experiment operation steps, and aerosol pollution can be effectively prevented; the method, the reagent, the primer and the probe are applicable to detecting the Ebola viruses by the aid of diversified fluorescence detection devices.
Owner:ZHEJIANG SHANCE HEQISHI BIO SCI& TECH CO LTD
L-nucleoside compounds and application thereof
InactiveCN105646629AInhibitory activityOrganic active ingredientsSugar derivativesEbola virusRNA Virus Infections
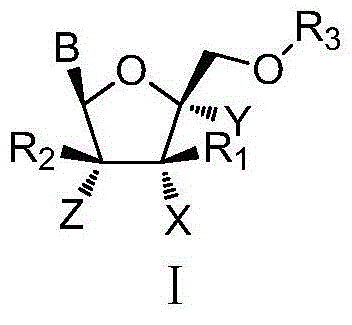
The invention discloses L-nucleoside compounds having the structure characteristic represented by the formula (I) or pharmaceutically acceptable salts thereof, and belongs to the technical field of pharmaceutical chemistry. The compounds can inhibit the activity of RNA viral polymerase, so the compounds can be used as potential drugs for prevention and treatment of infection of RNA viruses such as HCV, influenza virus, HRV (rhinovirus), RSV, Ebola virus, dengue virus, intestinal virus and the like.
Owner:GUANGZHOU HENOVCOM BIOSCI CO LTD
Methods for real-time multiplex isothermal detection and identification of bacterial, viral, and protozoan nucleic acids
ActiveUS20200048722A1Flexible processFacilitate strand displacementMicrobiological testing/measurementAgainst vector-borne diseasesProtozoaLoop-mediated isothermal amplification
Herein disclosed are rapid real-time isothermal multiplex methods of detecting, identifying and quantifying bacterial, viral, and protozoan nucleic acids in a sample. These include contacting the sample with two or more sets of pathogen-specific reverse transcription loop-mediated isothermal amplification primers and novel oligofluorophores specific for the target bacterial, viral, and parasitic nucleic acids of interest such as human immunodeficiency virus, Ebola virus, Marburg virus, Yellow fever virus, hepatitis-B virus, Lassa fever virus, Plasmodium, hepatitis-C virus, hepatitis-E virus, dengue virus, Chikungunya virus, Japanese Encephalitis virus, Middle Eastern Respiratory Syndrome Corona virus, Mycobacterium, West Nile virus, Cytomegalovirus, Parvovirus, Leishmania, Trypanosoma, and Zika virus nucleic acids, under conditions sufficient to produce detectable real-time amplification signals in about 10 to 40 minutes. The amplification signals are produced by pathogen-specific fluorogenic labels included in one or more of the primers. Also, novel reaction and sample lysis buffers, primers, and kits for rapid multiplex detection, quantification, and identification of bacterial, viral, and protozoan nucleic acids by real-time isothermal amplification are herein disclosed.
Owner:NYAN DOUGBEH CHRIS
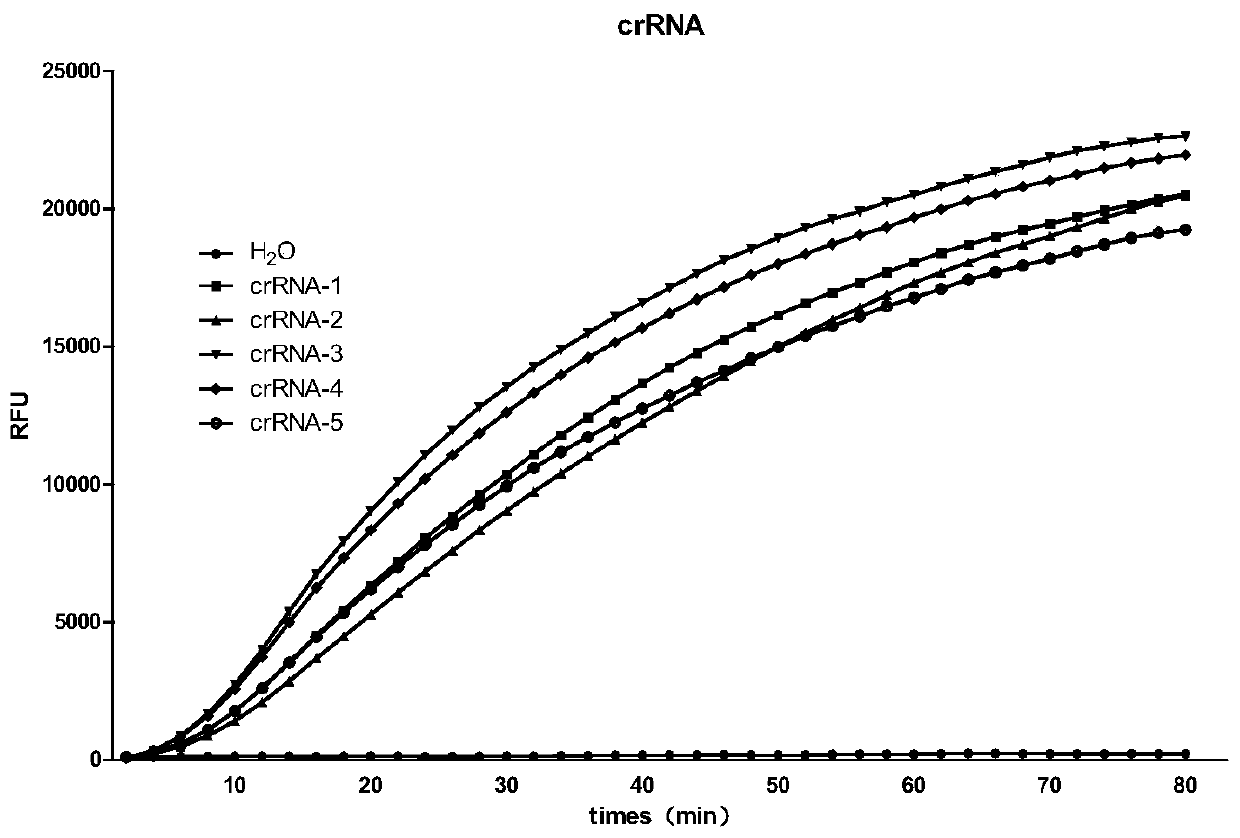
crRNA target point and CRISPR-Cas13a system for detecting Ebola virus
ActiveCN110628955AHighly specific detectionHydrolasesMicrobiological testing/measurementEbola virusGenome
The invention discloses a crRNA target point and CRISPR-Cas13a system for detecting an Ebola virus. The CRISPR-Cas13a system comprises Cas13a protein and crRNA, or a composite formed from the Cas13a protein and the crRNA, and the crRNA comprises an anchoring sequence being combined with the Cas13a protein and a guiding sequence targeted to the sequence of the target point of the Ebola virus; and the sequence of the target point of the Ebola virus is located at the 1001-1028th of the genome of the Ebola virus. The crRNA can realize high-sensitivity and high-specifity detection on the nucleic acid of the Ebola virus by activating Cas13a, and the sensitivity achieves 1 copy / test (1copy / test).
Owner:ACADEMY OF MILITARY MEDICAL SCI
Fluorescent quantitative PCR (polymerase chain reaction) method, primer and kit for detecting EBOV (Ebola virus)
InactiveCN103045755AAnti-pollutionNot easy to polluteMicrobiological testing/measurementMicroorganism based processesComputer-aidedEbola virus
The invention discloses a fluorescent quantitative PCR (polymerase chain reaction) method, primer and kit for detecting the EBOV (Ebola virus). The general method can be used for detecting that the sample to be detected is positive as long as the sample contains one or more of the five types of subtype EBOVs which are Z, S, B, C and R at the same time. The method overcomes the defects of the conventional PCR method for detecting by adopting the advantages of high-efficiency nucleic acid amplification of the PCR technology and the sensitivity of the fluorescence-dye SYBR Green I and the computer-aided fluorescent technology for detecting and improves the detection sensitivity, specificity and operation convenience greatly. In addition, the positive control adopted by the method is a section of RNA molecules transcribed in vitro of a NP gene, and the method is safer than the method for detecting by taking the inactivated virus solution as the positive control. The RNA molecules transcribed in vitro can be prepared in quantity, and the sources of the positive control are stable and reliable.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
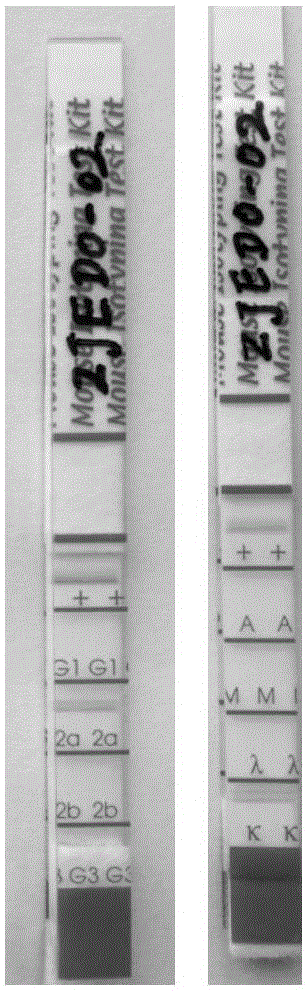
Hybridoma cell strain ZJED0-02, anti-Ebola virus GP (glycoprotein) monoclonal antibody and their preparation and application
ActiveCN105112375AStable secretionNo decline in secretionImmunoglobulins against virusesMicroorganism based processesAntigenEbola virus
The invention relates to a hybridoma cell strain ZJED0-02, an anti-Ebola virus GP (glycoprotein) monoclonal antibody and their preparation and application. The hybridoma cell strain ZJED0-02 is applicable to secreting the anti-Ebola virus GP monoclonal antibody. The monoclonal antibody is highly specific and sensitive to 411th to 490th amino acid sequence antigen peptides of Ebola virus GP and is applicable to the preparation of Ebola virus GP detection reagents. The invention also finishes sequencing of the monoclonal antibody, a neutralizing antibody capable of neutralizing Ebola virus is developed for humanization of monoclonal antibody sequences, and material basis is laid of late development of the neutralizing antibody into antibodies for clinically treating Ebola virus diseases.
Owner:THE FIRST AFFILIATED HOSPITAL ZHEJIANG UNIV COLLEGE OF MEDICINE
Adjunctive treatment of biological diseases
InactiveUS20090170803A1Profound benefitUseful in treatmentAntibacterial agentsBiocideEbola virusLine of therapy
The present invention provides a therapeutic method for treating biological diseases that includes the administration of an effective amount of a suitable antibiotic agent, antifungal agent or antiviral agent in conjunction with an A2A adenosine receptor agonist. If no anti-pathogenic agent is known the A2A agonist can be used alone to reduce inflammation, as may occur during infection with antibiotic resistant bacteria, or certain viruses such as those that cause SARS or Ebola. Optionally, the method includes administration of a type IV PDE inhibitor.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND +1
Compositions and methods for treatment of filovirus-mediated diseases
InactiveUS8475804B2Slow and stop replicationReduce loadBiocideOrganic chemistryEbola virusTreated animal
The invention features compositions, methods, and kits useful for the treatment of filovirus-mediated diseases, e.g., hemorrhagic fever caused by Ebola virus, in an animal.
Owner:US ARMY MEDICAL RES MATERIEL COMMAND USAMRMC +1
Compositions and methods for silencing ebola virus gene expression
InactiveUS20110201667A1Improve effectivenessHigh activitySsRNA viruses negative-senseOrganic active ingredientsEbola virusSilent gene
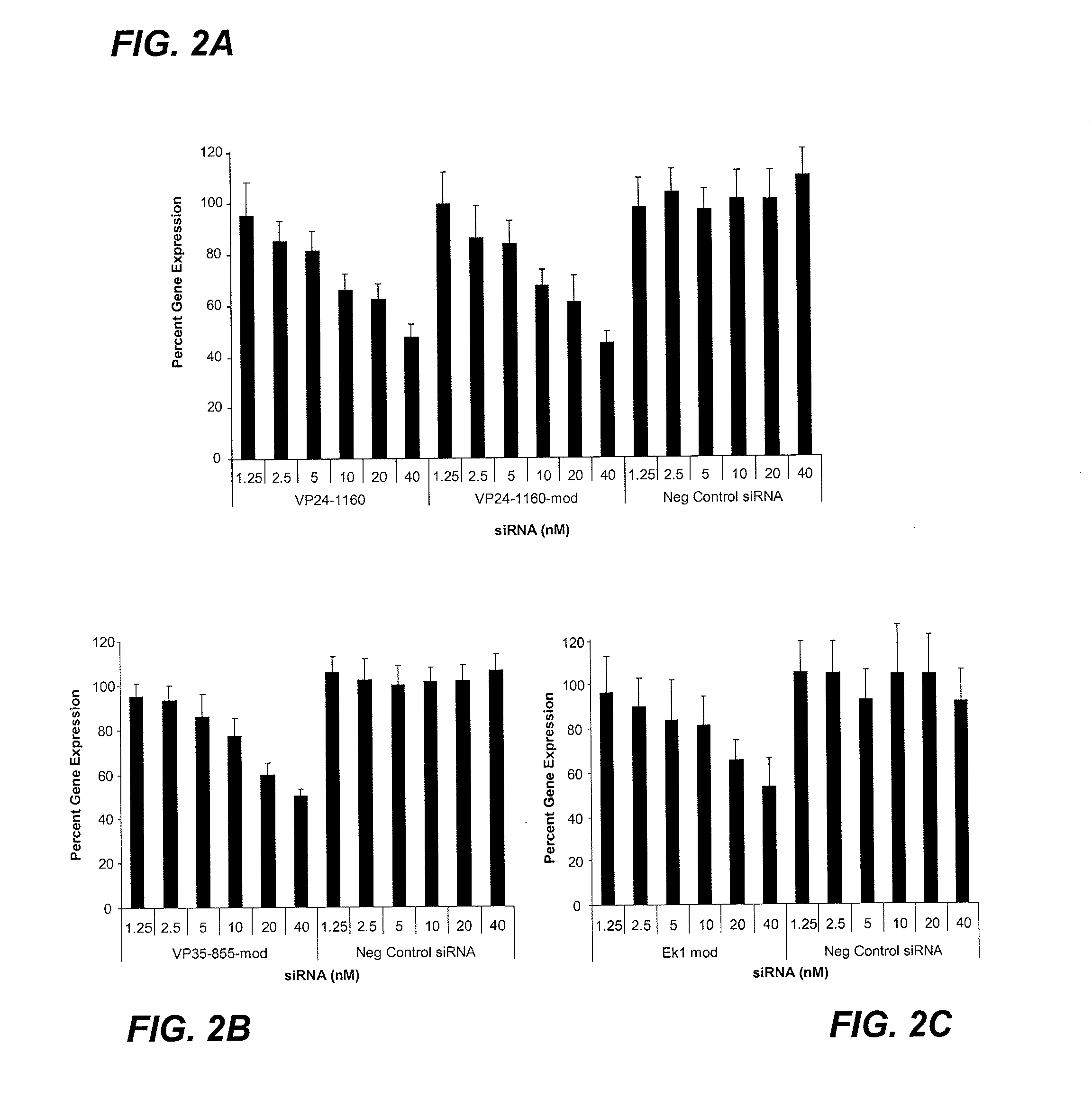
The present invention provides compositions comprising therapeutic nucleic acids (e.g., interfering RNA such as siRNA) that target Ebola virus (EBOV) gene expression and methods of using such compositions to silence EBOV gene expression. More particularly, the invention provides unmodified and chemically modified interfering RNA which silence EBOV gene expression and methods of use thereof, e.g., for preventing or treating EBOV infections caused by one or more EBOV species such as Zaire EBOV. The invention also provides serum-stable nucleic acid-lipid particles comprising one or more interfering RNA molecules, a cationic lipid, and a non-cationic lipid, which can further comprise a conjugated lipid that inhibits aggregation of particles. Methods of silencing EBOV gene expression by administering one or more interfering RNA molecules to a mammalian subject are also provided.
Owner:GEISBERT THOMAS W +6
Azole nucleosides and use as inhibitors of RNA and DNA viral polymerases
InactiveUS20100129317A1Inhibition is effectivePrevent slippingBiocideSugar derivativesCrimean Congo hemorrhagic fever virusPolymerase L
Azole nucleosides represented by the formulae (I) and (II); wherein A=C or N B═C or N X═H; C1-C6 alkyl, cycloalkyl, alkenyl, cycloalkenyl, alkynyl, aryl, heterocyclo, halogen such as F, Cl, Br and I; OH, NH2, NH—(C1-C6 alkyl, cycloalkyl, aryl or heterocyclo); Z═H; C1-C6 alkyl, cycloalkyl, alkynyl, aryl, heterocyclo, halogen such as F, Cl, Br, I; OH NH2, NH—(C1-C6 alkyl, cycloalkyl, aryl or heterocyclo; E=(CH2)HONHR; n is an interger from 0-6 and more typically 0-3; R1= aryl or heterocyclo; each of W, Y, R is individually selected from the group consisting of H; C1-C6 alkyl, cycloalkyl, alkenyl, cycloalkenyl, alkynyl, aryl, heterocyclo, halogen such as F, Cl, Br, and I; O, OH, Oalkyl, Oaryl, NH2, NH(C1-C6 alkyl, cycloalkyl, aryl or heterocyclo); provided that at least one of W, Y, and R is other than H and wherein both W and Y together can be ═O; and each D individually is OH, Oalkyl, Oaryl, FL and H; pharmaceutically acceptable salts thereof, prodrugs thereof and mixtures thereof are provided. Compounds of this disclosure are useful as inhibitors of viral RNA and DNA polymerases such as, but not limited to, Influenza, hantaan Virus, Crimean Congo hemorrhagic fever virus, hepatitis B, hepatitis C, Polio, Coxsackie A and B, Rhino, Echo, orthopoxvirus (small pox), HIV, Ebola, and West Nile virus polymerases; and especially orthopoxvirus, HIV, and hepatitis B.
Owner:SOUTHERN RES INST & IP +1
Marburg and Ebola dual-virus fluorescent quantitative PCR (Polymerase Chain Reaction) detection method and system
ActiveCN102140533AMicrobiological testing/measurementFluorescence/phosphorescenceEbola virusYellow fever
The invention discloses Marburg and Ebola dual-virus fluorescent quantitative PCR (Polymerase Chain Reaction) detection method and system, wherein the detection system comprises primers, probes, a Premix EX Taq reaction solution and sterilizing Tris water. As two pairs of primers and probes have very good specificity, the detection system has high sensitivity and is suitable for simultaneously detecting Marburg and Ebola viruses without having cross reaction with other kinds of hemorrhagic fever arbovirus, such as yellow fever, dengue and rift valley fever.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Compositions and methods for treatment of filovirus-mediated diseases
InactiveUS20130289024A1Slow and stop replicationReduce loadAntiviralsHeterocyclic compound active ingredientsEbola virusPediatrics
The invention features compositions, methods, and kits useful for the treatment of filovirus-mediated diseases, e.g., hemorrhagic fever caused by Ebola virus, in an animal.
Owner:US ARMY MEDICAL RES MATERIEL COMMAND USAMRMC +1
Prepn and application of natural bamboo vinegar disinfectant liquid
The present invention collects filtrate produced during baking bamboo charcoal and utilizes it as natural bamboo vinegar disinfectant liquid. The natural bamboo vinegar liquid is used as main material for preparing bamboo vinegar liquid containing Ag, Zn, Cu and other metal ions, and is compounded with proper amount of stabilizer to prepare spray or concentrated preparation. The disinfectant of the present invention can kill various viruses and pathogenetic bacteria and is non-toxic and non-excitant, and may be used widely in preventing dandy fever, Ebola virus, bird flu and other infectious diseases, household disinfection, and killing fungus.
Owner:徐江 +1
RPA kit used for detecting Ebola virus, and special-purpose primers, probes, and applications of RPA kit
InactiveCN105400904ASimplify testing proceduresShorten detection timeMicrobiological testing/measurementDNA/RNA fragmentationConserved sequenceEbola virus
The invention provides a RPA (recombinase polymerase amplification) kit used for detecting Ebola virus, special-purpose primers and probes of the RPA kit, and applications of the RPA kit in detection of Ebola virus. The RPA kit, the special-purpose primers, and the probes are designed based on Ebola virus NP gene conserved sequence, and the Ebola virus NP gene conserved sequence possesses an oligonucleotide sequence represented by SEQ ID NO.1. It is shown by research results that the RPA kit can be used for rapid detecting of Ebola virus in samples with specificity and sensitivity.
Owner:INST OF PLA FOR DISEASE CONTROL & PREVENTION
Filovirus Consensus Antigens, Nucleic Acid Constructs And Vaccines Made Therefrom, And Methods Of Using Same
ActiveUS20150335726A1Preventing filovirus infectionSsRNA viruses negative-sensePolypeptide with localisation/targeting motifSequence signalAntigen
Nucleic acid molecules and compositions comprising one or more nucleic acid sequences that encode a consensus filovirus immunogen including a consensus Marburgvirus filovirus glycoprotein MARV GP immunogen, a consensus Ebolavirus Sudan filovirus glycoprotein SEBOV GP immunogen and a consensus Ebolavirus Zaire glycoprotein ZEBOV GP immunogen are disclosed. The coding sequences optionally include operable linked coding sequence that encode a signal peptide. Immunomodulatory methods and methods of inducing an immune response against filovirus, particularly Marburgvirus, Ebolavirus Sudan and Ebolavirus Zaire are disclosed. Method of preventing filovirus infection, particularly infection by Marburgvirus, Ebolavirus Sudan and Ebolavirus Zaire and methods of treating individuals infected with filovirus infection, particularly infection by Marburgvirus, Ebolavirus Sudan and Ebolavirus Zaire are disclosed. Consensus filovirus proteins are disclosed.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Compositions and methods for silencing Ebola virus gene expression
InactiveUS8716464B2Improve effectivenessHigh activitySsRNA viruses negative-senseOrganic active ingredientsEbola virusStable nucleic acid lipid particle
The present invention provides compositions comprising therapeutic nucleic acids (e.g., interfering RNA such as siRNA) that target Ebola virus (EBOV) gene expression and methods of using such compositions to silence EBOV gene expression. More particularly, the invention provides unmodified and chemically modified interfering RNA which silence EBOV gene expression and methods of use thereof, e.g., for preventing or treating EBOV infections caused by one or more EBOV species such as Zaire EBOV. The invention also provides serum-stable nucleic acid-lipid particles comprising one or more interfering RNA molecules, a cationic lipid, and a non-cationic lipid, which can further comprise a conjugated lipid that inhibits aggregation of particles. Methods of silencing EBOV gene expression by administering one or more interfering RNA molecules to a mammalian subject are also provided.
Owner:GEISBERT THOMAS W +6
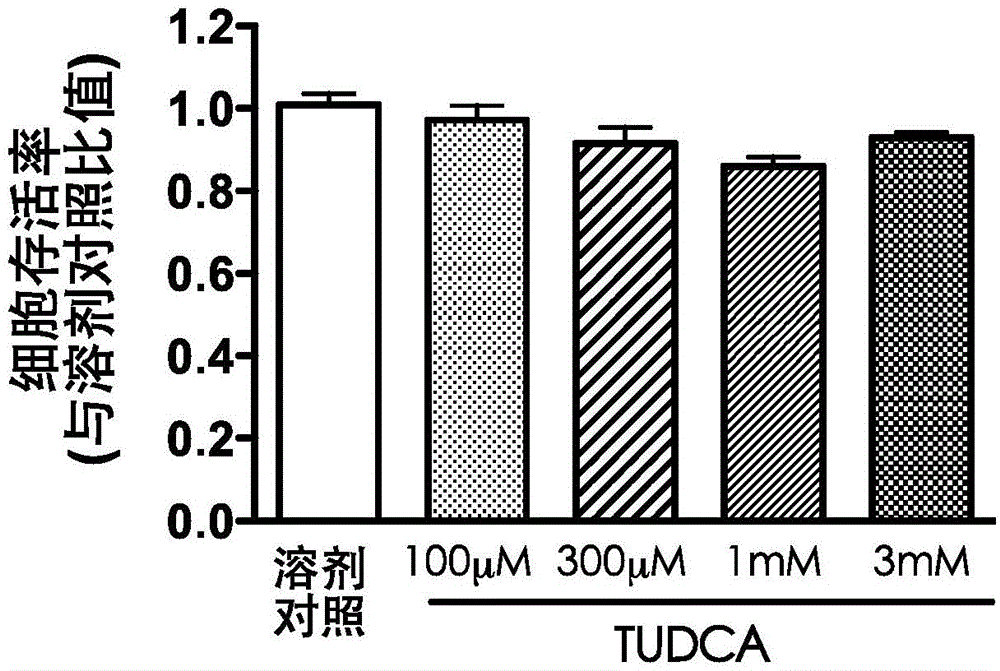
New uses of tauroursodeoxycholic acid
InactiveCN106031731AEffective in preventing pulmonary fibrosisOrganic active ingredientsAntiviralsBiomedicinePulmonary fibrosis
The present invention relates to the technical field of biomedicine, and mainly provides new uses of tauroursodeoxycholic acid, wherein the new uses comprise that the tauroursodeoxycholic acid inhibits the virus-entry-host cells adopting endocytosis so as to achieve prevention and treatment of virus infection, anti-injury, and anti-fibrosis. The present invention provides applications of the tauroursodeoxycholic acid in preparation of drugs for treatment of acute lung injury and pulmonary fibrosis, such that the application range of the tauroursodeoxycholic acid is broadened, and the new drug selection is provided for the virus infection patients. According to the present invention, it is confirmed that the tauroursodeoxycholic acid can effectively prevent and treat H5N1 avian influenza virus, human adenovirus and Zaire-type Ebola virus, and it is cleared that the pulmonary fibrosis prevention and control effect of the tauroursodeoxycholic acid is superior to the pulmonary fibrosis prevention and control effect of the chloroquine.
Owner:THE INST OF BASIC MEDICAL SCI OF CHINESE ACAD OF MEDICAL SCI
SARS and Ebola inhibitors and use thereof, and methods for their discovery
The instant invention is drawn to methods useful for the treatment or the prevention of a viral infection. The methods include administering at least one compound that is an inhibitor of cathepsin L to an individual. The methods are particularly useful in individuals infected with, or at risk of infection with, SARS virus or Ebola virus. The invention also includes methods of identifying potential therapeutics for use in the methods of treatment or prevention of a viral infection.
Owner:DIAMOND SCOTT L +3
Method of accelerated vaccination against Ebola viruses
The present invention relates to genetic vaccines for stimulating cellular and humoral immune responses in humans and other hosts, and, in particular, relates to recombinant viruses that express heterologous antigens of pathogenic viruses, in single dose form.
Owner:UNITED STATES OF AMERICA +1
Fulminating-infectious-disease pathogen detecting primer pair and kit
ActiveCN105483293AReduced Pollution ChancesShorten detection timeMicrobiological testing/measurementAgainst vector-borne diseasesColor changesBiology
The invention discloses a fulminating-infectious-disease pathogen detecting primer pair and a kit. The primer pair comprises at least a pair of RT-LAMP primers of Ebola viruses, Lassa fever viruses, Marburg viruses, rift valley fever viruses, yellow fever viruses and Chikungunya fever viruses. By means of the primer system, the amplification reaction background is reduced, and sensitivity and specificity are quite good. The kit formed by the primer pair further comprises detecting liquid and a micro-fluidic chip; as an independent RT-PCR secondary amplification step of a detecting liquid system is omitted, detecting time is shortened; as the denaturation process and the renaturation process of nucleic acid do not exist, the polluted chance of RNA enzymes and the polluted chance of amplification nucleic acid are reduced, and the sensibility and the safety of detection are improved. By means of the constant-temperature sealed environment provided by a micro-fluidic chip system, rapid and constant-temperature amplification and automation result distinguishing of a nucleic acid extracting template are finally achieved, the requirement for test hardware is reduced, the use level of a reaction reagent is reduced, detection cost is reduced, and the result can be directly determined through color changes.
Owner:INST OF PLA FOR DISEASE CONTROL & PREVENTION
Broad Spectrum Inhibitors of the Post Proline Cleaving Enzymes for Treatment of Hepatitis C Virus Infections
InactiveUS20150202218A1Increase secretionBiocidePeptide/protein ingredientsEcho virusesCOXSACKIE A VIRUS
Disclosed are methods of treating, inhibiting, or preventing a viral infection in a mammal in need thereof by administering a therapeutically or prophylactically effective amount of an inhibitor of FAP, an inhibitor of DPPIV, an inhibitor of DPP8, or an inhibitor of DPP9. The inhibitor may act as both an inhibitor of DPPIV and an inhibitor of DPP8 / 9. The viral infection includes, but is not limited to, hepatitis B virus, hepatitis C virus, human immunodeficiency virus, Polio virus, Coxsackie A virus, Coxsackie B virus, Rhino virus, respiratory syncytial virus, dengue virus, equine infectious anemia virus, Echo virus, small pox virus, Ebola virus, and West Nile virus.
Owner:TRUSTEES OF TUFTS COLLEGE TUFTS UNIV
Anti-Ebola virus monoclonal antibody, preparation method and uses thereof
ActiveCN108570106AHigh ADCC activityHigh activityGenetically modified cellsImmunoglobulins against virusesEbola virusHeavy chain
The invention belongs to the field of immunology and molecular biology, and relates to an anti-Ebola virus monoclonal antibody, a preparation method and uses thereof, specifically to an anti-Ebola virus monoclonal antibody or an antigen-binding part thereof, wherein the amino acid sequences of the light chain variable region and the heavy chain variable region comprise one group selected from thefollowing groups (1)-(3): the group (1): the amino acid sequence of the light chain variable region is represented by SEQ ID NO:4, and the amino acid sequence of the heavy chain variable region is represented by SEQ ID NO:6; the group (2): the amino acid sequence of the light chain variable region is represented by SEQ ID NO:10, and the amino acid sequence of the heavy chain variable region is represented by SEQ ID NO:12; and the group (3): the amino acid sequence of the light chain variable region is represented by SEQ ID NO:16, and the amino acid sequence of the heavy chain variable region is represented by SEQ ID NO:18. According to the present invention, the anti-Ebola virus monoclonal antibody has advantages of enhanced ADCC activity, good antigen-binding activity and good virus-neutralizing activity.
Owner:BEIJING MABWORKS BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com