Anti-Ebola virus monoclonal antibody, preparation method and uses thereof
A technology of monoclonal antibody and Ebola virus, applied in the fields of immunology and molecular biology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0127] Example 1: Obtaining the Nucleotide Sequence of Antibody MIL77
[0128] The antibody amino acid sequence is a recombinant antibody sequence from patents WO2009 / 094755 A1 and US2004 / 0053865A1. The nucleotide sequence of the antibody MIL77 of the present invention was obtained by selecting codons with high frequency used in mammalian cells for reverse translation, rationally optimizing through bioinformatics technology, and using molecular modeling and molecular biology mutation experiments.
[0129] Wherein, the light chain nucleotide sequence of the antibody MIL77-1 is:
[0130] gacatccagatgactcagtctccagcctccctatctgtatctgtgggagaaactgtctccatcacatgtcgagcaag tgagaatatttcagtagtttagcatggtatcagcagaaacagggaaaatctcctcagctcctggtctattctgcaa caatcttagcagatggtgtgccatcaaggttcagtggcagtggatcaggcactcagtattccctcaagatcaacagc ctgcagtctgaagattttggggacttattactgtcaacatttttggggtactccgtacacgttcggagggggggaccaa gctggaaataaaa cgtacggtggctgcaccatctgtcttcatcttcccgccatctgatgagcagttgaaatc...
Embodiment 2
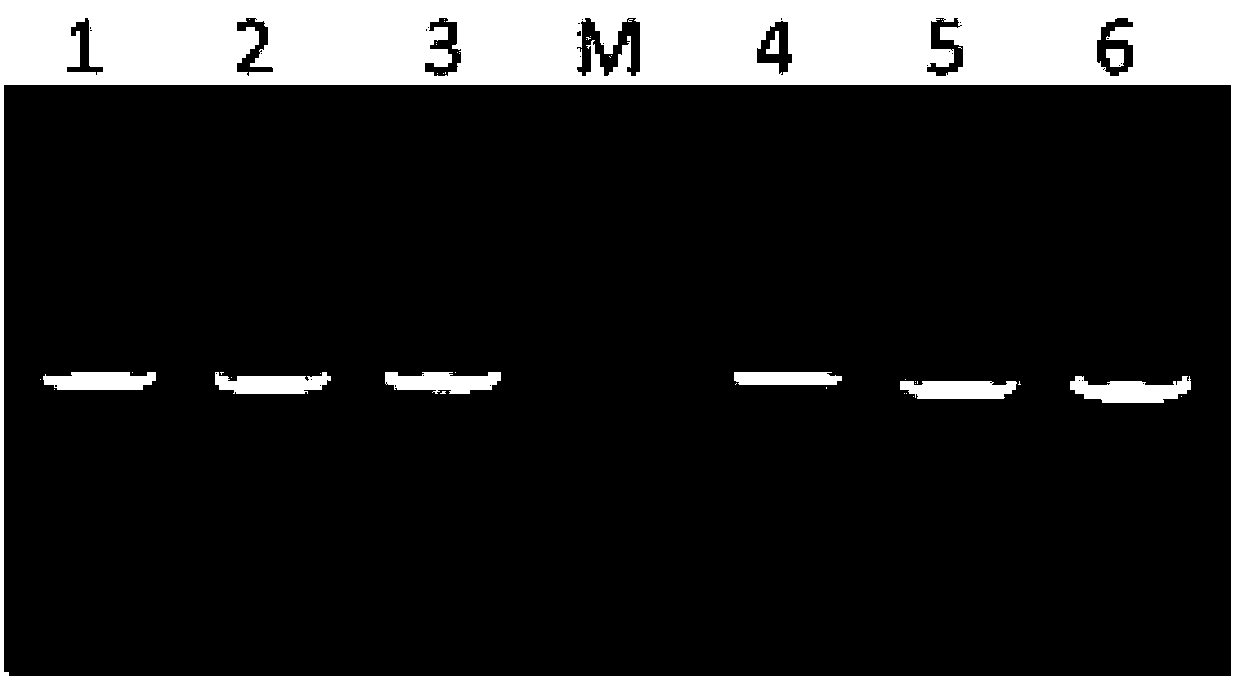
[0153] Example 2: Cloning of Antibody MIL77-1, MIL77-2 and MIL77-3 Genes
[0154] 1. Experimental materials
[0155] Phusion polymerase, Taq DNA polymerase, restriction endonuclease, pGEM-T-easy vector, and Pyrobest DNA polymerase are all products of NEB Company;
[0156] dNTP and DNA Marker standards are TaKaRa products;
[0157] The DNA recovery kit is a product of Qiagen;
[0158] Trans2-Blue competent state is the product of Quanshijin Company;
[0159]Small-scale plasmid kit (DP107-02) is a product of Tiangen Biochemical Company;
[0160] The large extraction plasmid kit (DP117) is a product of Tiangen Biochemical Company;
[0161] OPD is a product of Sigma;
[0162] FITC-labeled goat anti-human antibody: a product of Pierce;
[0163] Primers were designed with Biosun software;
[0164] Primer synthesis (Genewiz), Genewiz Biotechnology (Beijing) Co., Ltd.;
[0165] Gene sequencing was completed by Beijing Nuosai Genome Research Center Co., Ltd.
[0166] 2. Expe...
Embodiment 3
[0193] Example 3: Preparation of MIL77-1, MIL77-2 and MIL77-3 antibodies
[0194] 1. Experimental materials
[0195] The dry powder medium commissioned by Hyclone is formulated for host cell acclimatization, cell line screening and antibody preparation to cultivate cells. During cell screening, methionine sulfoximine (Methionine sulfoximine) purchased from Sigma is added to the prepared medium. , MSX), using trypan blue dry powder purchased from sigma to prepare a solution for cell counting.
[0196] 2. Experimental methods and results
[0197] 2.1 Construction of antibody eukaryotic expression vector
[0198] Select the expression vector pTGS-FRT-DHFR (patent authorization number: ZL200510064335.0) obtained by the applicant, remove the hygromycin selection tag, and add GS (glutamine synthetase, glutamine synthetase) through the PshA1 and Xho1 restriction sites Expression cassette, as a selection marker; wherein GS cDNA was obtained by RT-PCR from the cell line CHO expres...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Equilibrium dissociation constant | aaaaa | aaaaa |
| Equilibrium dissociation constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com