Humanized monoclonal antibody, preparation method thereof, and application thereof
A monoclonal antibody, humanized technology, applied in biochemical equipment and methods, chemical instruments and methods, and botanical equipment and methods, etc., can solve the problems of low production capacity of antibody drugs, ineffective treatment of patients or recurrence, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1 Optimization of Nucleotide Sequence and Evaluation of Transient Expression
[0056] The amino acid sequences of the light chain and heavy chain of the monoclonal antibody of the present invention are from WHO Drug Information, Vol.22, No.2, 2008. The amino acid sequence reported in the above literature was converted into a nucleotide sequence, and a series of parameters that may affect the expression of the antibody in mammalian cells were targeted: codon bias, GC content (that is, guanine G and ratio of cytosine C), CpG island (that is, the region with high density of CpG dinucleotides in the genome), secondary structure of mRNA, splicing site, pre-mature PolyA site, internal Chi site (in the genome A short DNA fragment, the probability of homologous recombination increases near this site) or ribosome binding site, RNA unstable sequence, inverted repeat sequence and restriction enzyme site that may interfere with cloning, etc. At the same time, related seq...
Embodiment 2
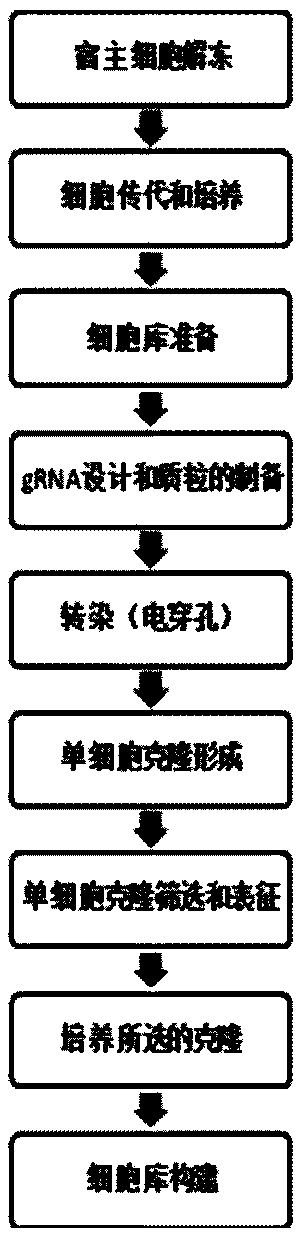
[0065] Example 2 Construction of CHO-DG44 Fut8- / - cell line
[0066] The host cell used is CHO-DG44Fut8- / - cell line, which is the cell line of CHO-DG44 with biallelic fucose knockout (Fut8- / -), provided by Nanjing KingScript Biotechnology Co., Ltd. Company development. The specific method is to transform the expression system through genetic engineering technology, namely CRISPR / Cas9 technology, and knock out the gene responsible for encoding fucose, namely the FUT8 gene, in the antibody expression host cell CHO-DG44. Specific technical routes such as figure 1 shown.
Embodiment 3
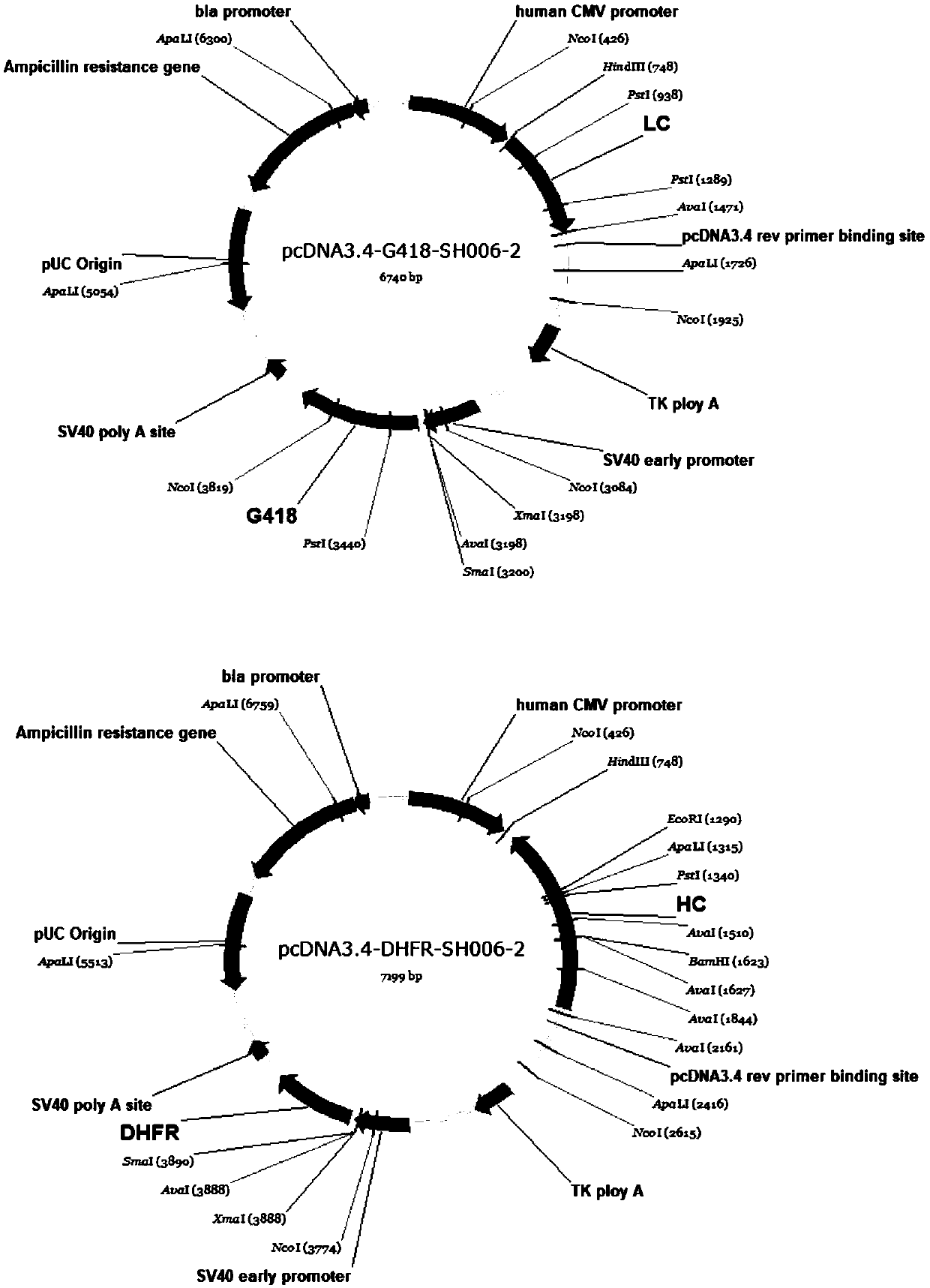
[0067] The construction of embodiment 3 expression vector
[0068] The pcDNA3.4-G418 and pcDNA3.4-DHFR vectors (purchased from invitrogen) were used as special vectors for expressing and transfecting CHO-DG44 cells. pcDNA3.4-G418 contains the promoter CMV Promoter used for the light chain, the eukaryotic screening marker G418 tag and the prokaryotic screening tag Ampicilline. pcDNA3.4-DHFR contains the heavy chain promoter CMVPromoter, the eukaryotic screening marker DHFR tag and the prokaryotic screening tag Ampicilline.
[0069]The nucleotide sequences of SH006-2 antibody expressing light chain and heavy chain were obtained by gene synthesis, and connected to pcDNA3.1(+) and pcDNA3.1-Hygro(+) vectors (synthesized by Nanjing GenScript) respectively. Using the pcDNA3.1(+) vector containing the SH006-2 light chain and the pcDNA3.1-Hygro(+) vector containing the SH006-2 heavy chain as templates, two restriction endonucleases HindIII and XhoI were used for double-enzyme Diges...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com