Humanized monoclonal antibody, its preparation method and its use
A monoclonal antibody, humanized technology, applied in biochemical equipment and methods, chemical instruments and methods, botanical equipment and methods, etc., can solve the problems of low production capacity of antibody drugs, ineffective treatment of patients or recurrence, etc., to make up for Drug resistance and ineffectiveness, improving the effect of tumor treatment, and enhancing the effect of affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1 Optimization of Nucleotide Sequences and Evaluation of Transient Expression
[0056] The amino acid sequences of the light chain and heavy chain of the monoclonal antibody of the present invention are from WHO Drug Information, Vol. 22, No. 2, 2008. The amino acid sequences reported in the above literature were converted into nucleotide sequences and targeted for a series of parameters that may affect the expression of antibodies in mammalian cells: codon preference, GC content (i.e., guanine G and 4 bases in DNA). Cytosine C ratio), CpG islands (i.e., regions with high density of CpG dinucleotides in the genome), mRNA secondary structure, splice sites, pre-mature PolyA sites, internal Chi sites (in the genome) A short DNA fragment with an increased chance of homologous recombination near this site) or optimization of ribosome binding sites, RNA instability sequences, inverted repeats, and restriction sites that may interfere with cloning; At the same time,...
Embodiment 2
[0065] Example 2 Construction of CHO-DG44 Fut8- / - cell line
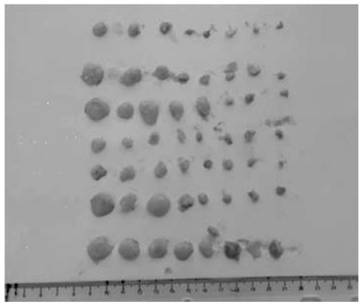
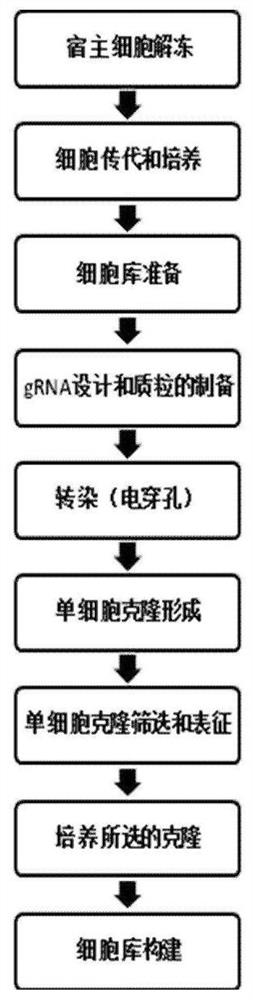
[0066] The host cell used is CHO-DG44Fut8- / - cell line, which is a fucose biallelic knockout (Fut8- / -) CHO-DG44 cell line, which was developed by Nanjing GenScript Biotechnology Co., Ltd. Company development. The specific method is to transform the expression system through genetic engineering technology, namely CRISPR / Cas9 technology, and knock out the gene responsible for encoding fucose, that is, the FUT8 gene, in the antibody expression host cell CHO-DG44. Specific technical routes such as figure 1 shown.
Embodiment 3
[0067] The construction of embodiment 3 expression vector
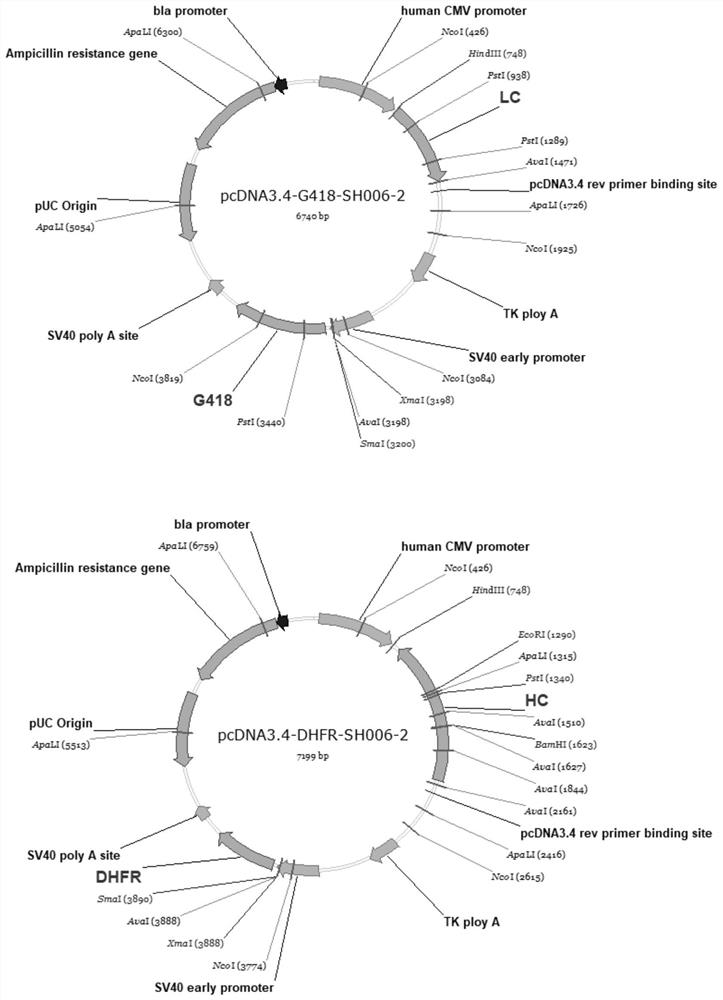
[0068] The pcDNA3.4-G418 and pcDNA3.4-DHFR vectors (purchased from invitrogen) were used as special vectors for expression and transfection of CHO-DG44 cells. pcDNA3.4-G418 contains the promoter CMV Promoter used for the light chain, the eukaryotic selection marker G418 tag and the prokaryotic selection tag Ampicilline. pcDNA3.4-DHFR contains the promoter CMVPromoter used for the heavy chain, the eukaryotic selection marker DHFR tag and the prokaryotic selection tag Ampicilline.
[0069]The nucleotide sequences of SH006-2 antibody expressing light chain and heavy chain were obtained by gene synthesis, and were respectively connected to pcDNA3.1(+) and pcDNA3.1-Hygro(+) vectors (synthesized by Nanjing GenScript). The pcDNA3.1(+) vector containing SH006-2 light chain and the pcDNA3.1-Hygro(+) vector containing SH006-2 heavy chain were used as templates, and two restriction enzymes HindIII and XhoI were used to carry ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com