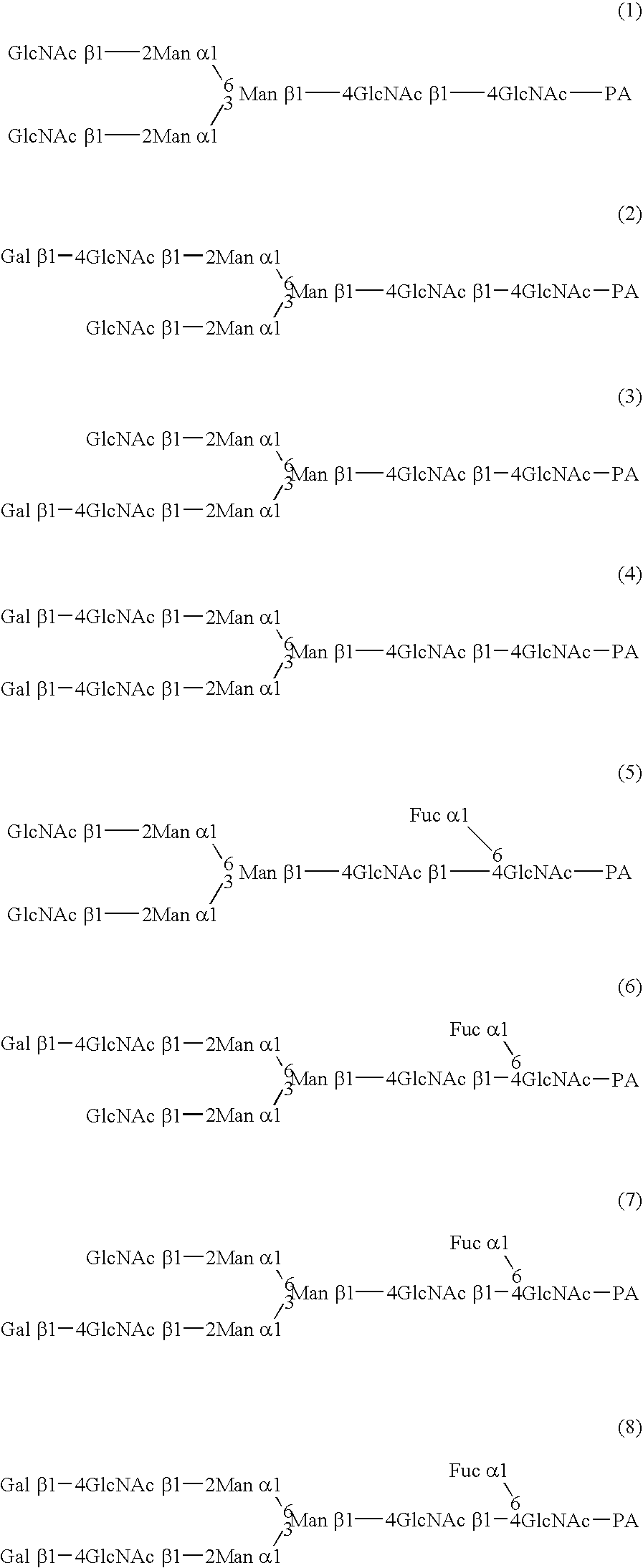Patents
Literature
7867results about "Genetically modified cells" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cells of which genome is modified
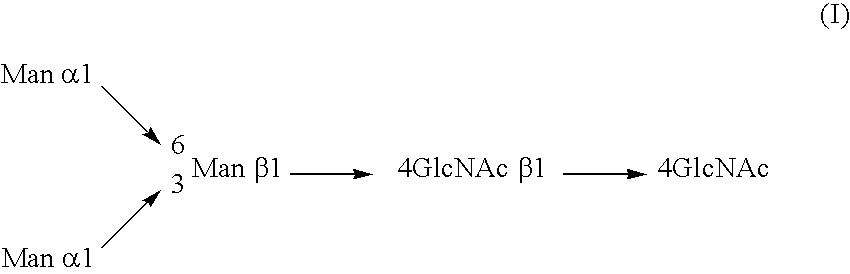
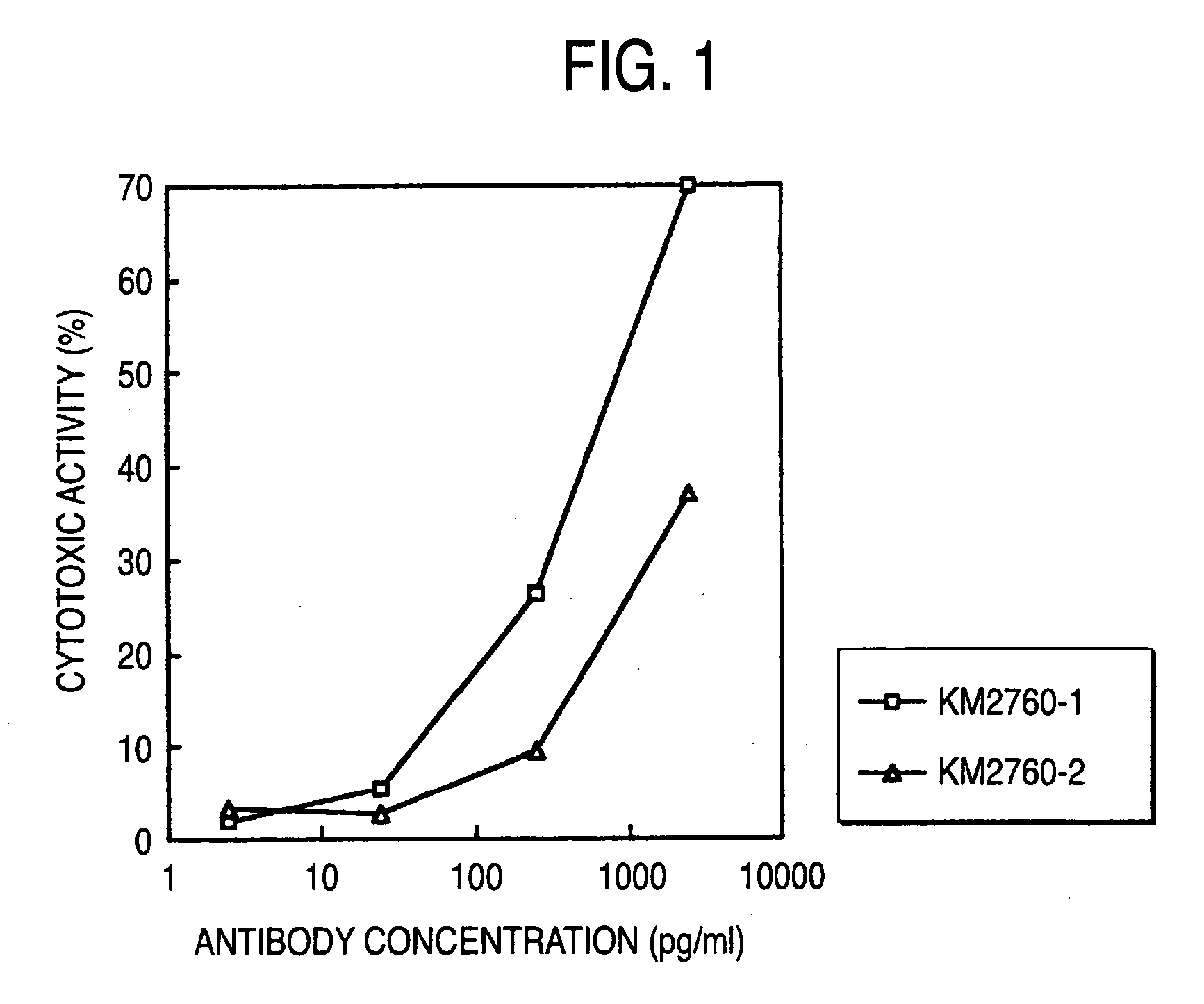
InactiveUS20040110704A1Raise the ratioDecreased and deleted activityAntibacterial agentsAntipyreticGlycosideN-Acetylglucosamine
A cell in which genome is modified so as to have a more decreased or deleted activity of an enzyme relating to modification of a sugar chain in which 1-position of fucose is bound to 6-position of N-acetylglucosamine in the reducing end through alpha-bond in a complex N-glycoside-linked sugar chain than its parent cell, and a process for producing an antibody composition using the cell.
Owner:KYOWA HAKKO KOGYO CO LTD
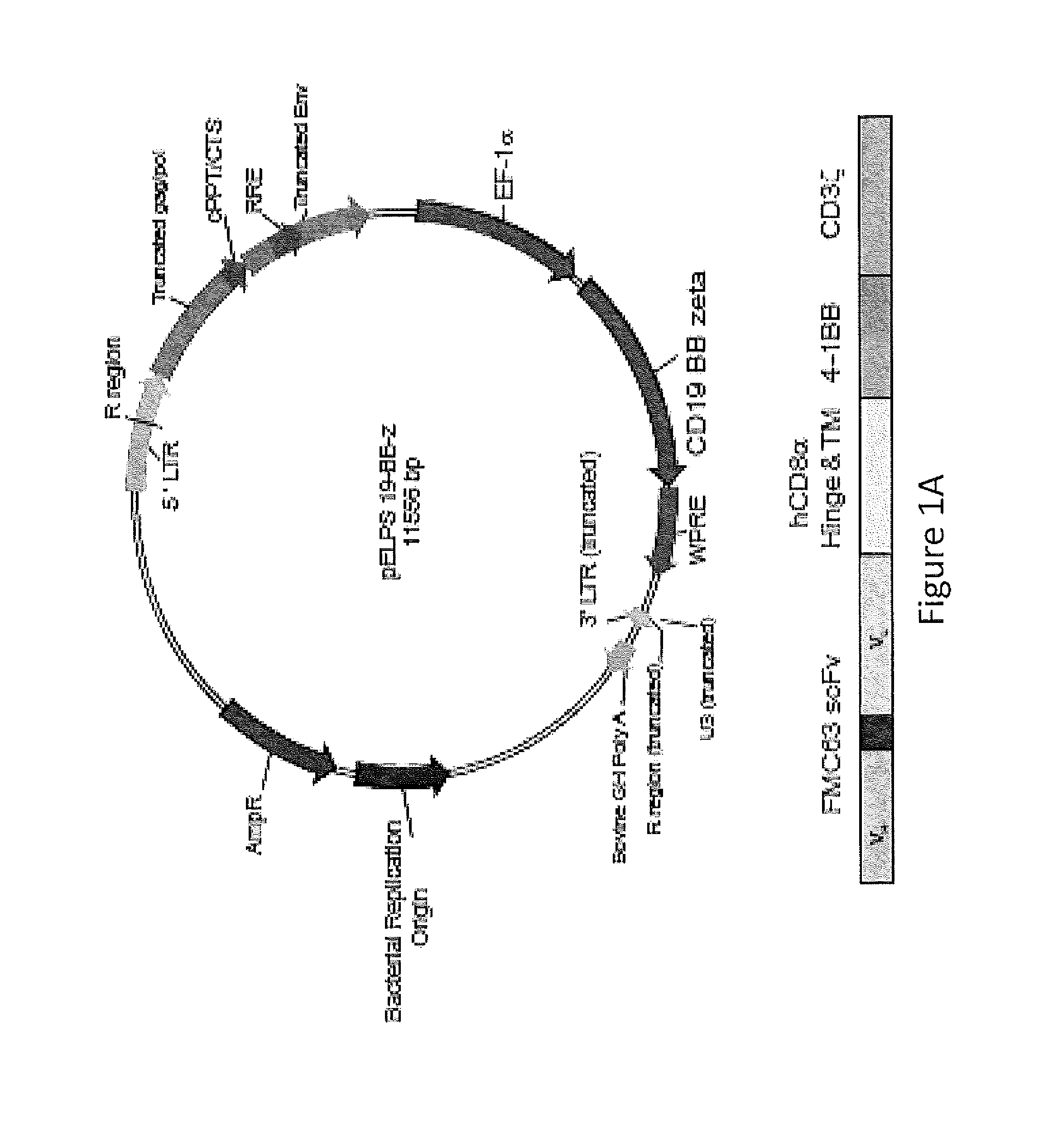
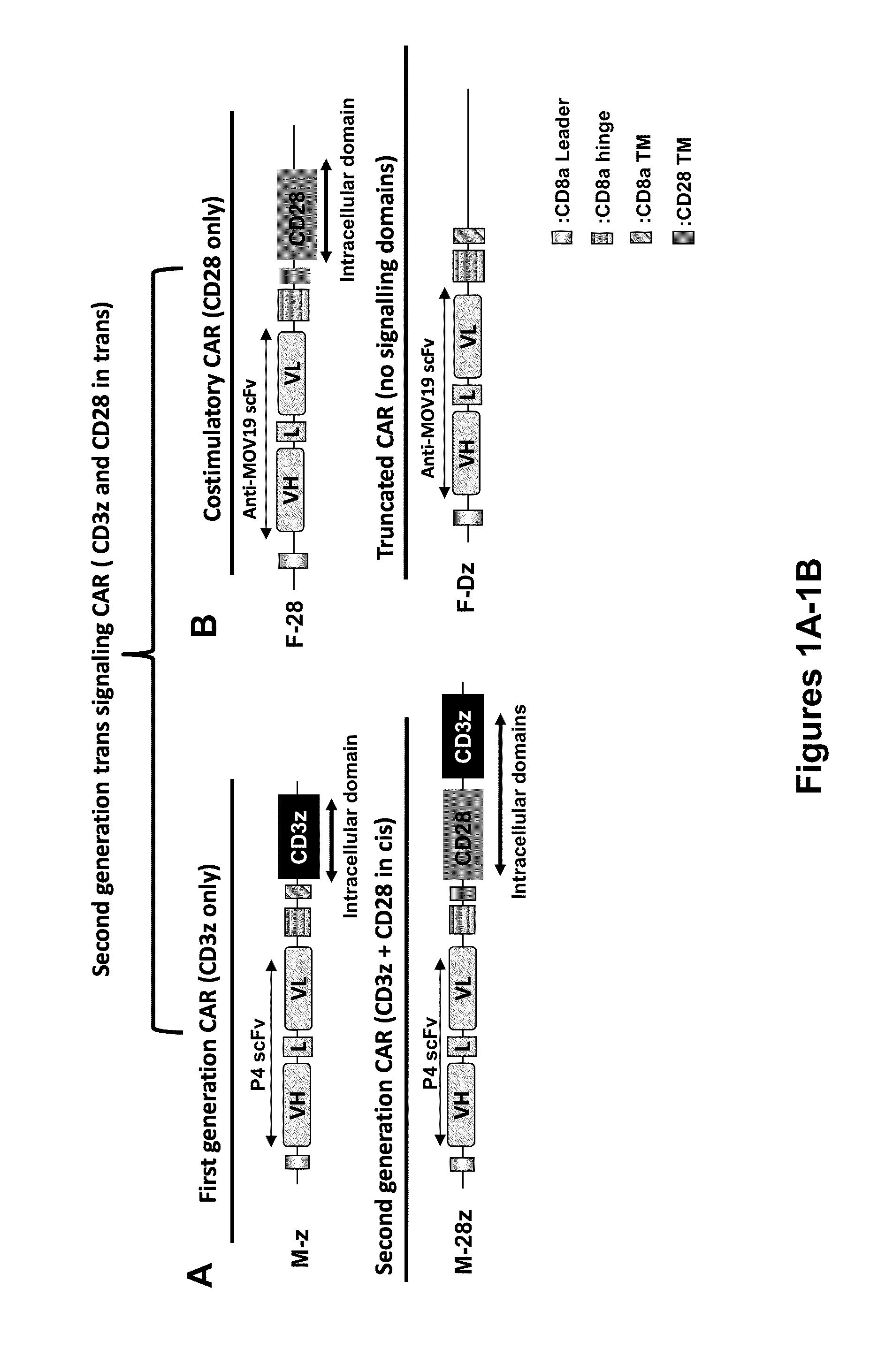
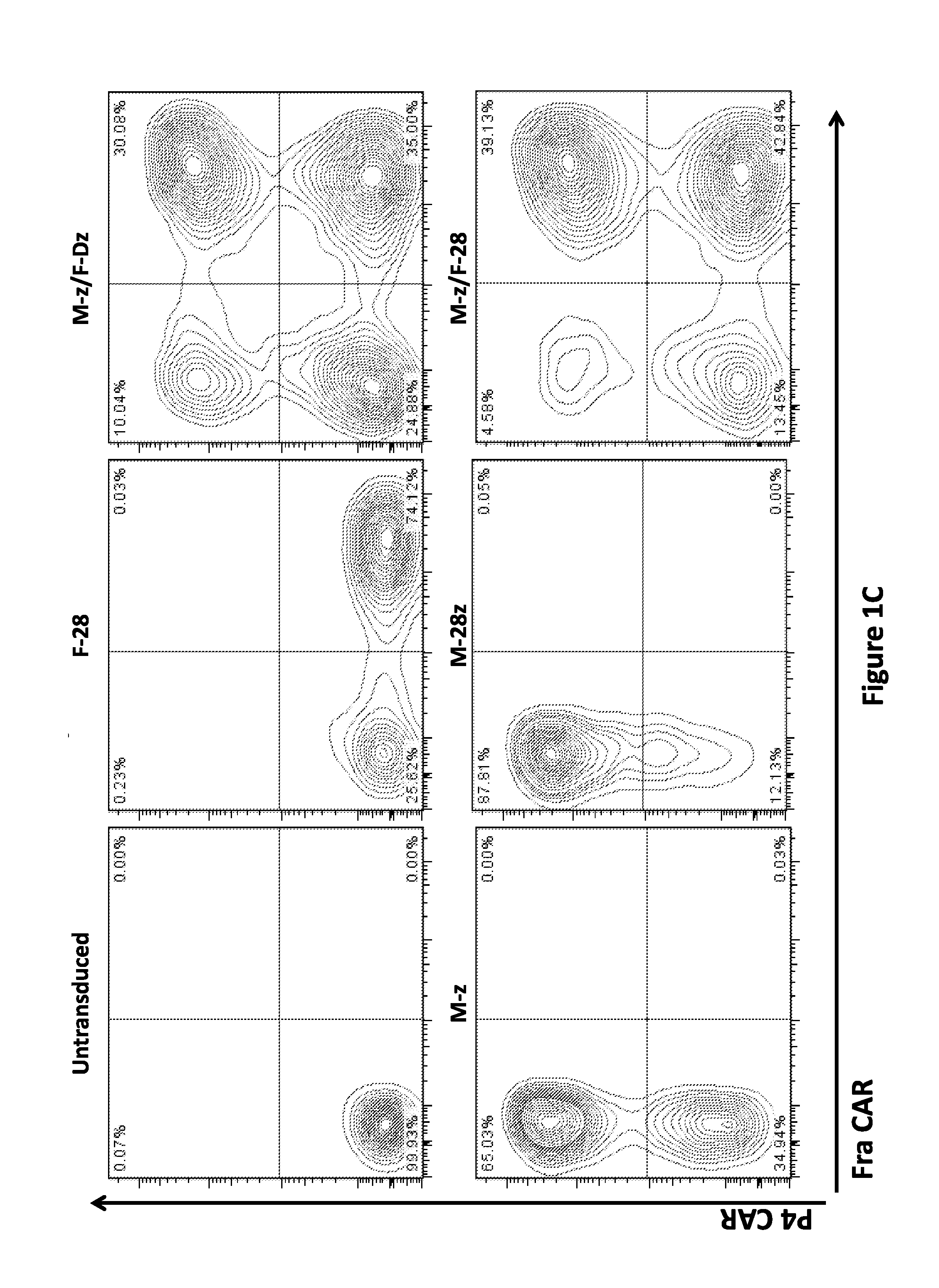
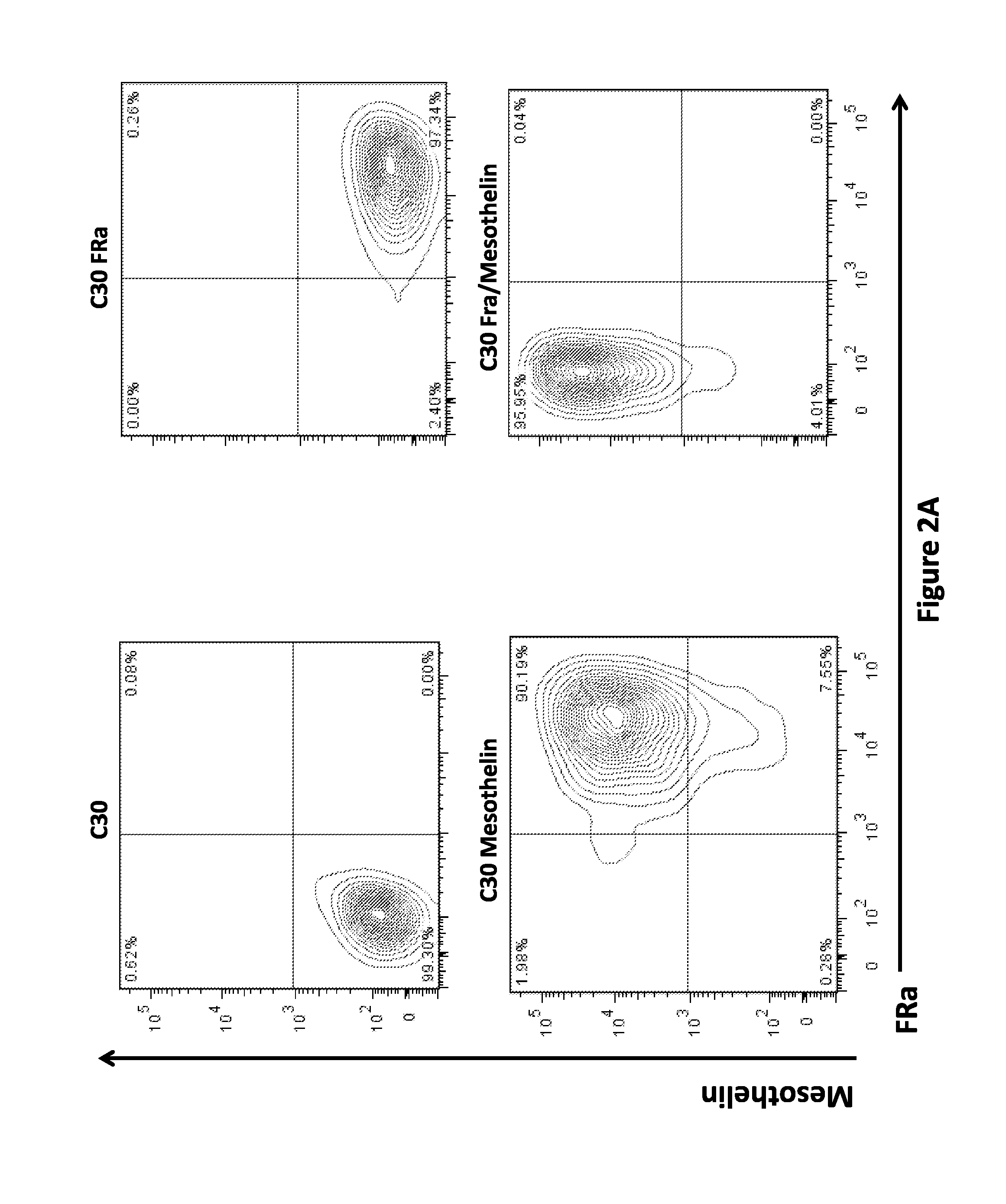
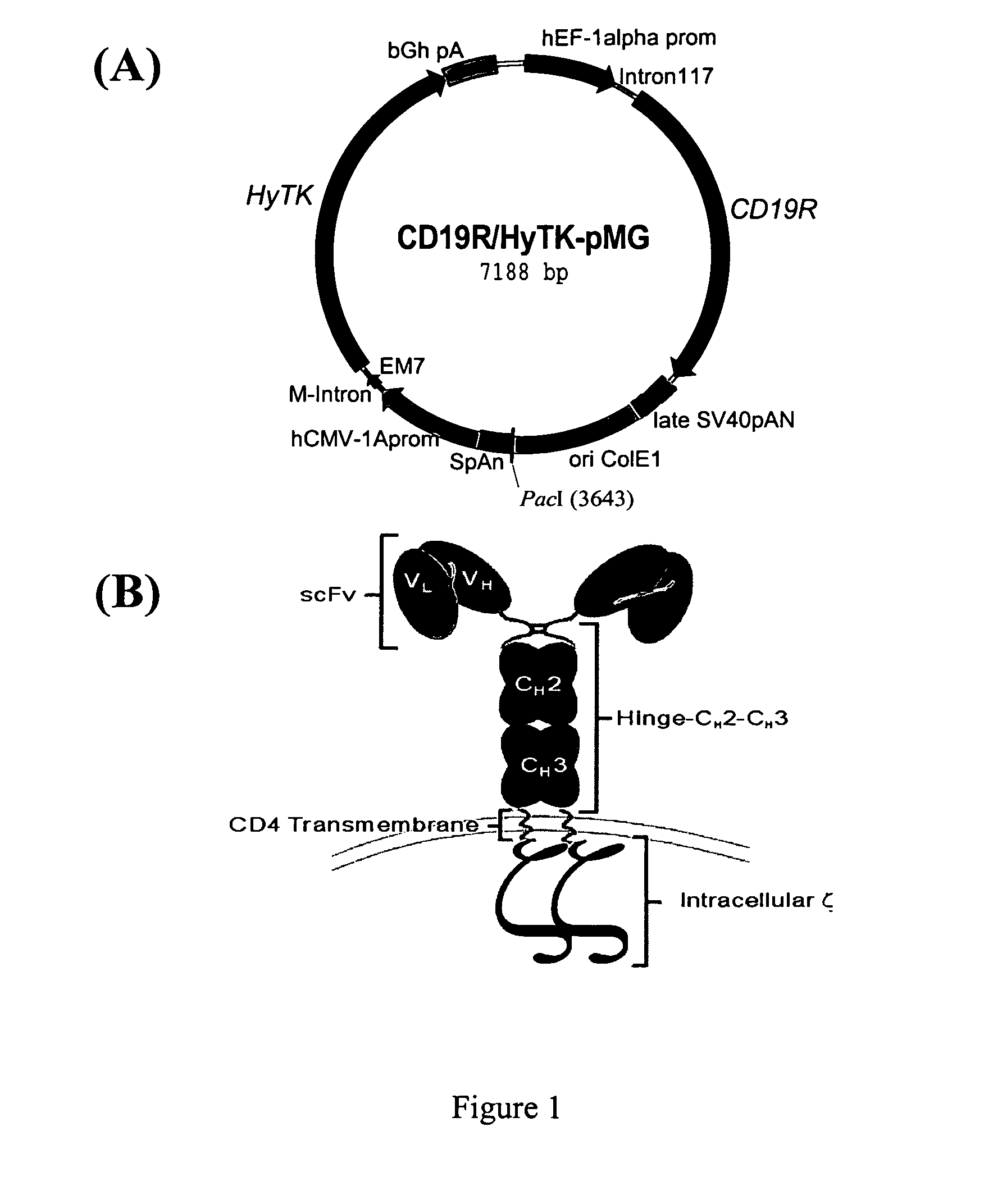
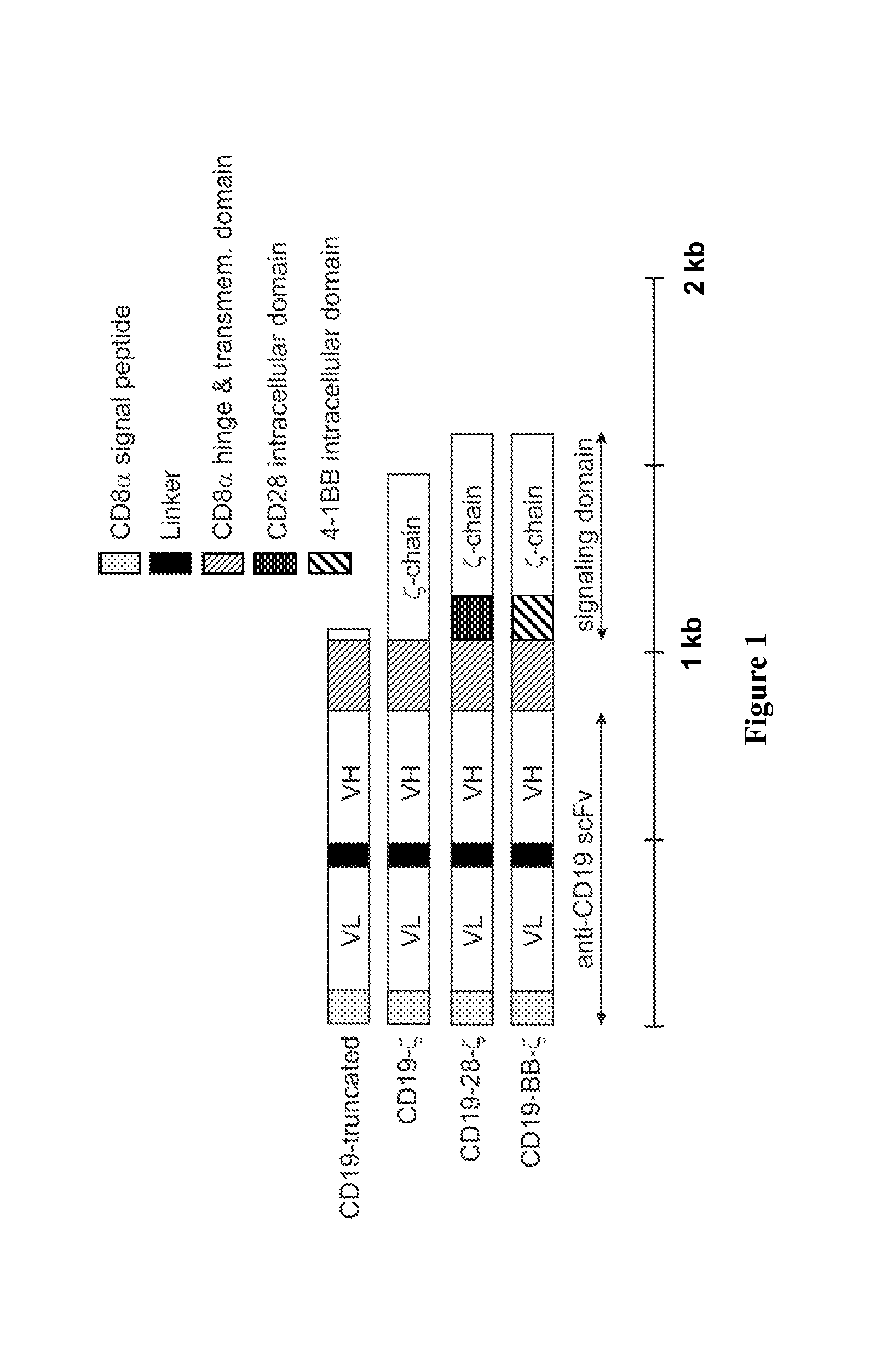
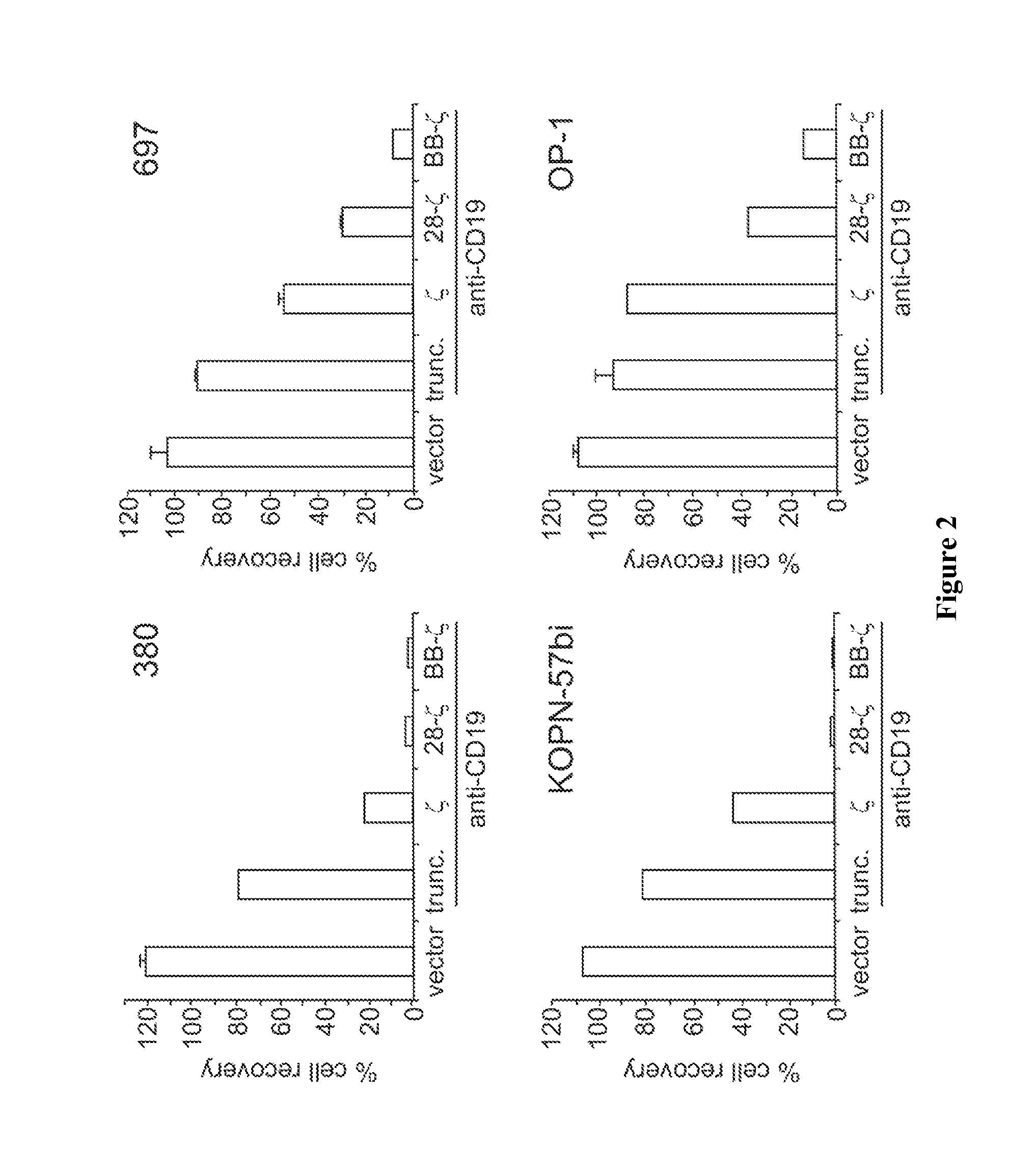
Use of Chimeric Antigen Receptor-Modified T-Cells to Treat Cancer
ActiveUS20130287748A1Stimulate immune responseVirusesPeptide/protein ingredientsBinding domainAntigen binding
The present invention provides compositions and methods for treating cancer in a human. The invention includes relates to administering a genetically modified T cell to express a CAR wherein the CAR comprises an antigen binding domain, a transmembrane domain, a costimulatory signaling region, and a CD3 zeta signaling domain.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
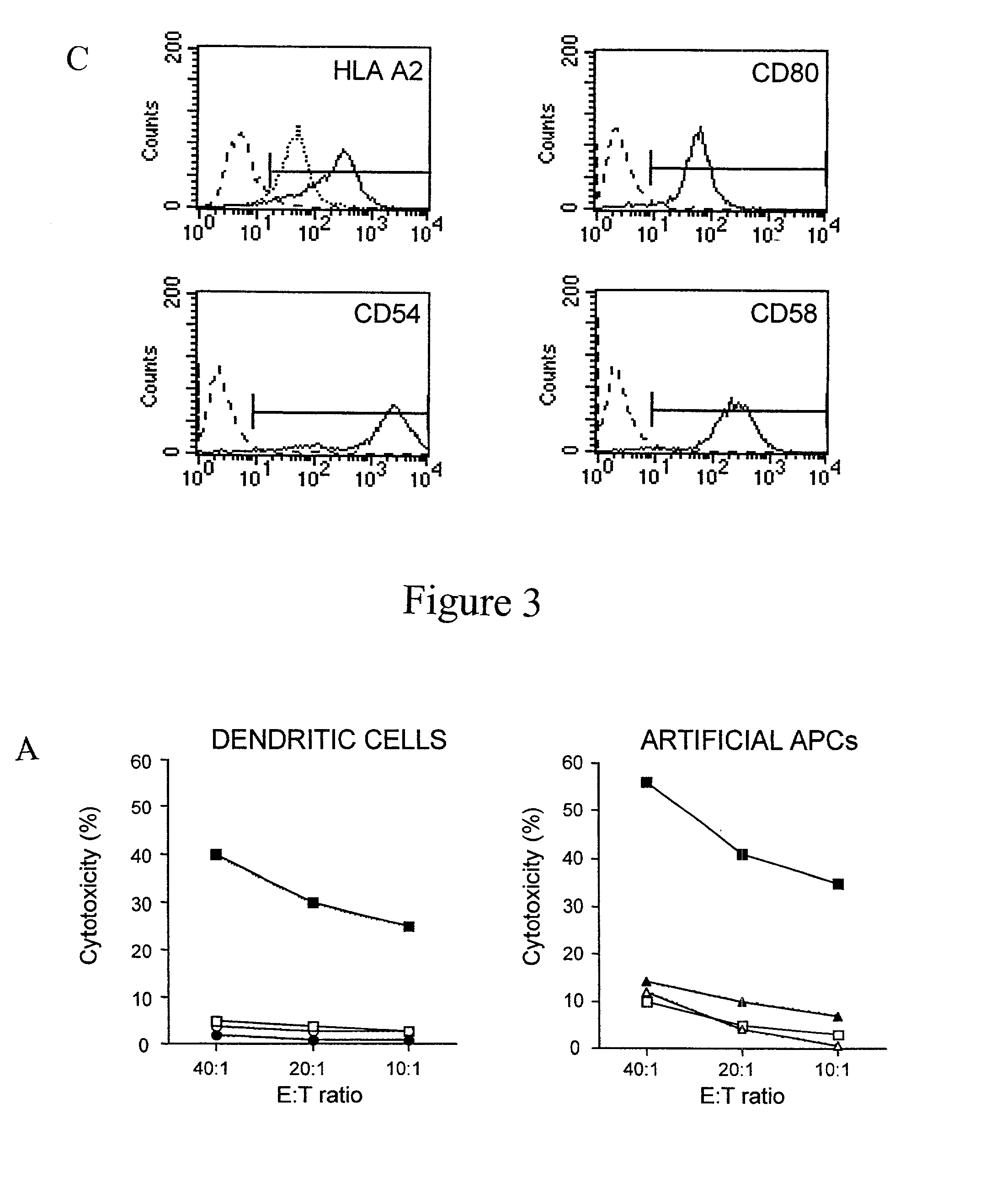
Novel artificial antigen presenting cells and uses therefor
The invention relates to novel artificial antigen presenting cells (aAPCs). The aAPC comprises at least one stimulatory ligand and at least one co-stimulatory ligand where the ligands each specifically bind with a cognate molecule on a T cell of interest, thereby mediating expansion of the T cell. The aAPC of the invention can further comprise additional molecules useful for expanding a T cell of interest. The aAPC of the invention can be used as an “off the shelf” APC that can be readily designed to expand a T cell of interest. Also, the aAPC of the invention can be used identify the stimulatory, co-stimulatory, and any other factors that mediate growth and expansion of a T cell of interest. Thus, the present invention provides powerful tools for development of novel therapeutics where activation and expansion of a T cell can provide a benefit.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Methods and materials for the growth of primate-derived primordial stem cells in feeder-free culture
Methods and materials for culturing primate-derived primordial stem cells are described. In one embodiment, a cell culture medium for growing primate-derived primordial stem cells in a substantially undifferentiated state is provided which includes a low osmotic pressure, low endotoxin basic medium that is effective to support the growth of primate-derived primordial stem cells. The basic medium is combined with a nutrient serum effective to support the growth of primate-derived primordial stem cells and a substrate selected from the group consisting of feeder cells and an extracellular matrix component derived from feeder cells. The medium further includes non-essential amino acids, an anti-oxidant, and a first growth factor selected from the group consisting of nucleosides and a pyruvate salt.
Owner:ASTERIAS BIOTHERAPEUTICS INC
Three-dimensional culture of pancreatic parenchymal cells cultured living stromal tissue prepared in vitro
InactiveUS6022743AIncrease surface areaIncreased proliferationImmobilised enzymesSurgical needlesLigament structureIn vivo
A stromal cell-based three-dimensional cell culture system is prepared which can be used to culture a variety of different cells and tissues in vitro for prolonged periods of time. The stromal cells and connective tissue proteins naturally secreted by the stromal cells attach to and substantially envelope a framework composed of a biocompatible non-living material formed into a three-dimensional structure having interstitial spaces bridged by the stromal cells. The living stromal tissue so formed provides the support, growth factors, and regulatory factors necessary to sustain long-term active proliferation of cells in culture and / or cultures implanted in vivo. When grown in this three-dimensional system, the proliferating cells mature and segregate properly to form components of adult tissues analogous to counterparts in vivo, which can be utilized in the body as a corrective tissue. For example, and not by way of limitation, the three-dimensional cultures can be used to form tubular tissue structures, like those of the gastrointestinal and genitourinary tracts, as well as blood vessels; tissues for hernia repair and / or tendons and ligaments; etc.
Owner:REGENEMED
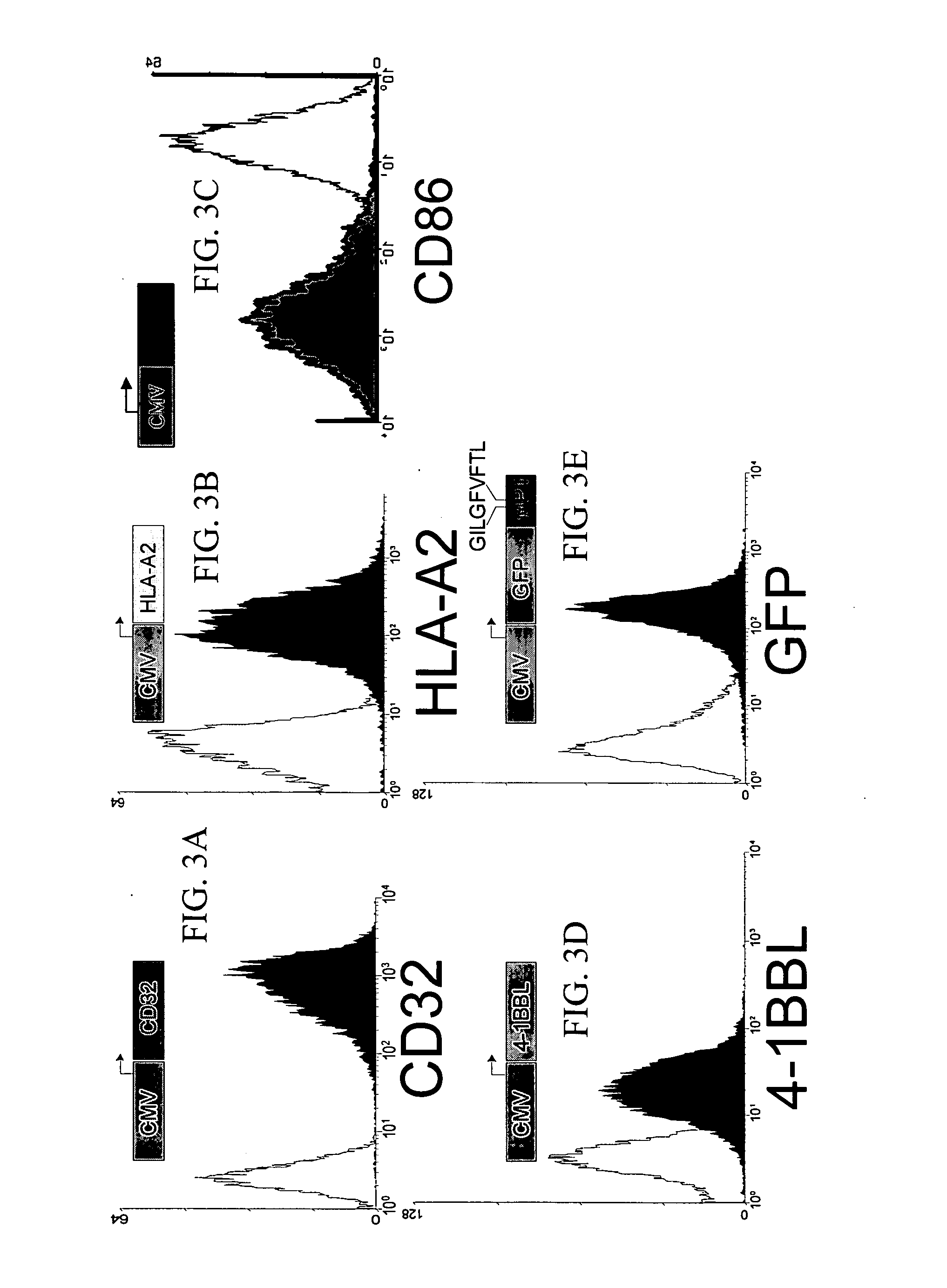
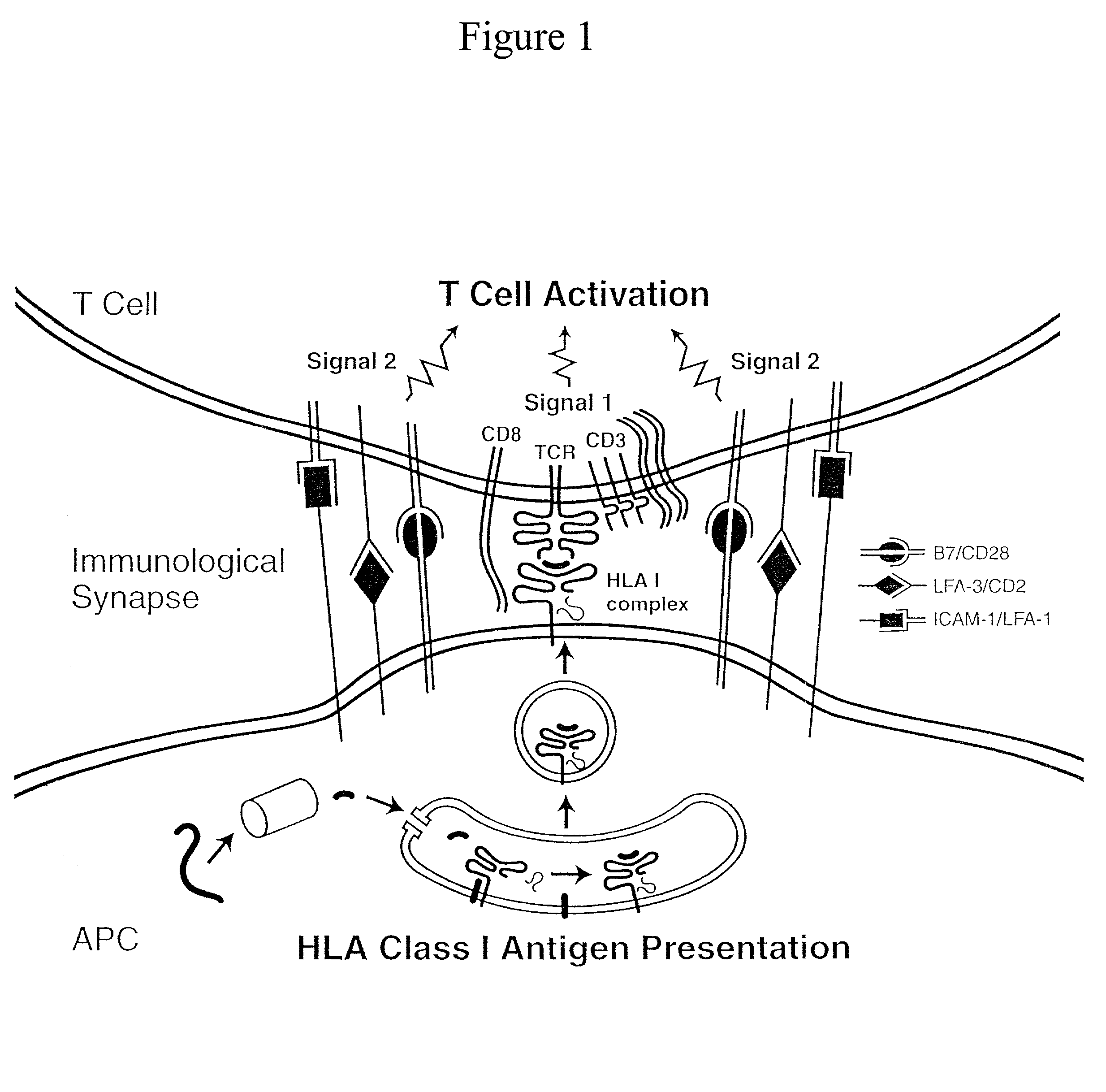
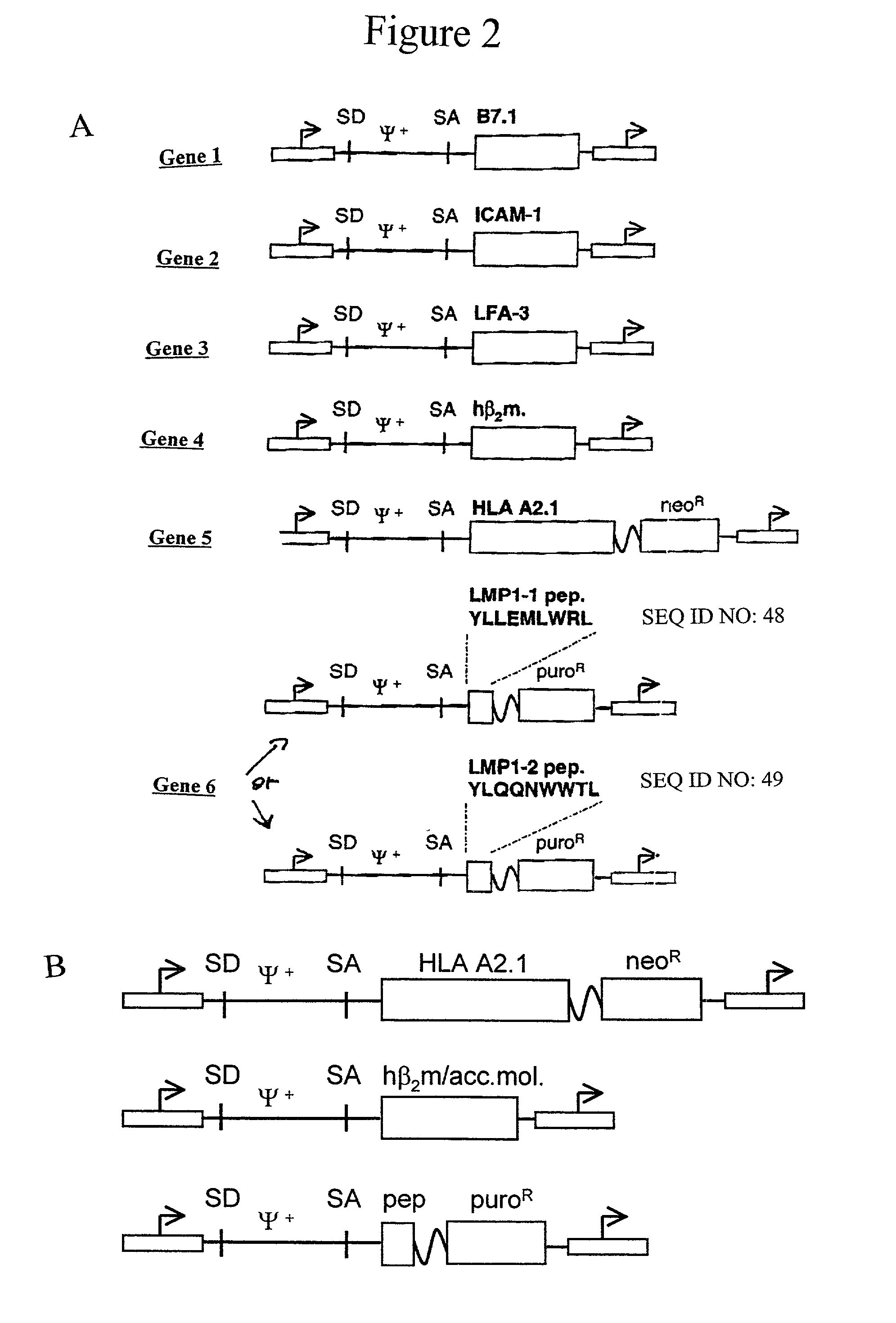
Artificial antigen presenting cells and methods of use thereof
InactiveUS20020131960A1Palliating their conditionReduce riskBiocideCompound screeningEpitopeAccessory molecule
The invention provides an artificial antigen presenting cell (AAPC) comprising a eukaryotic cell expressing an antigen presenting complex comprising a human leukocyte antigen (HLA) molecule of a single type, at least one exogenous accessory molecule and at least one exogenous T cell-specific epitope. Methods of use for activation of T lymphocytes are also provided.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
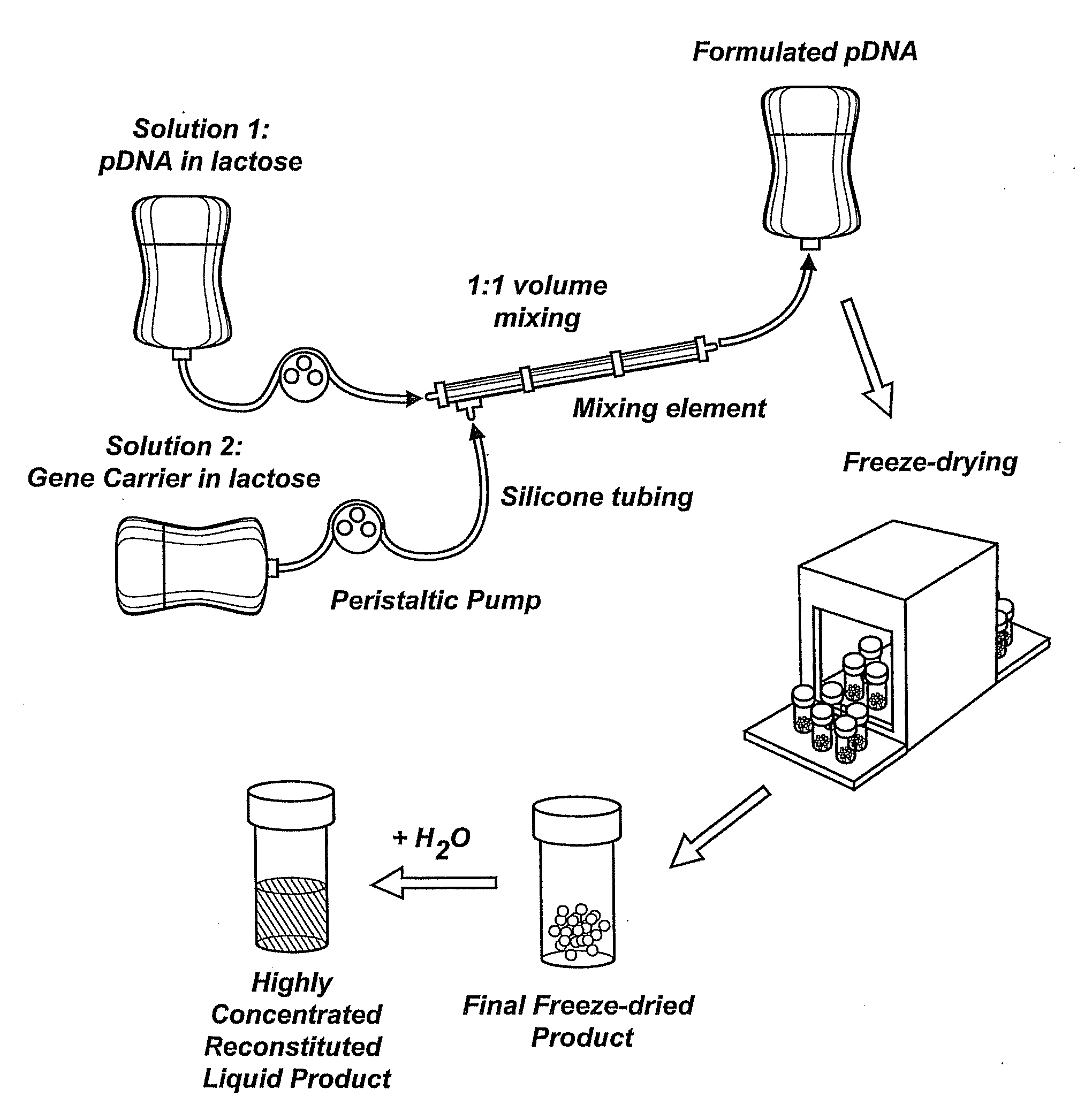
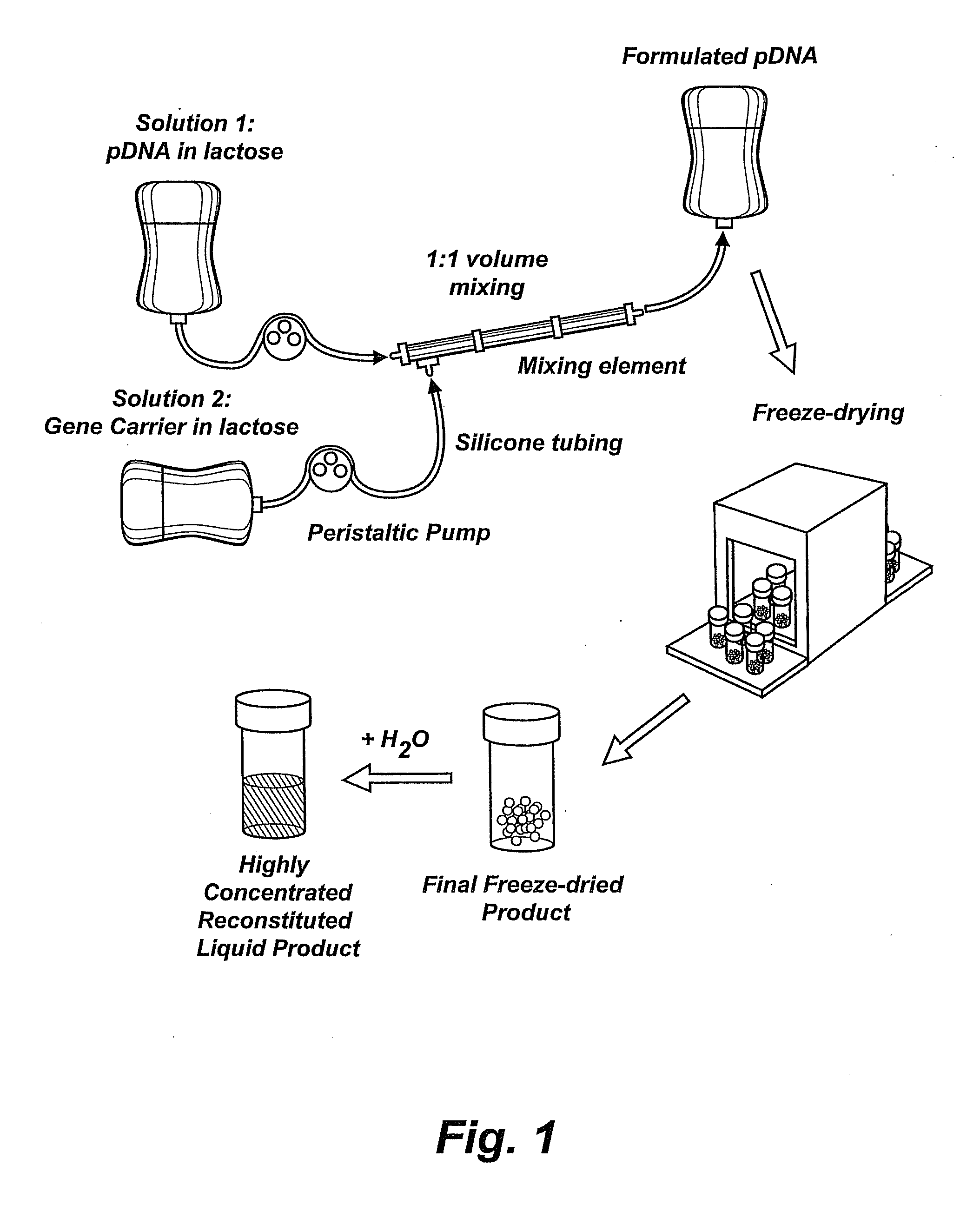
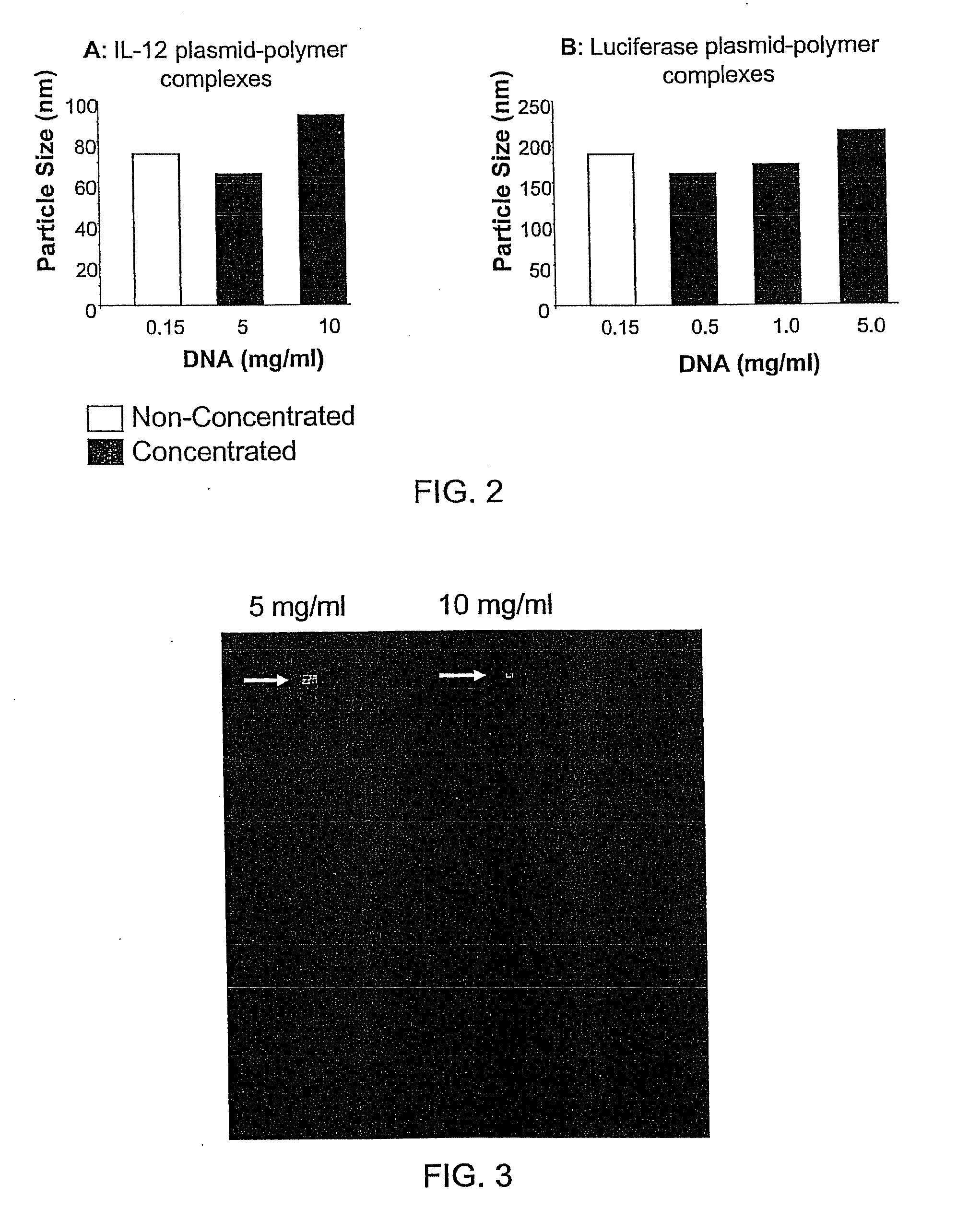
Nucleic Acid-Lipopolymer Compositions
InactiveUS20090042829A1Increase efficiency and dosing flexibilityEfficiently be lyophilizedSpecial deliveryPeptide/protein ingredientsCholesterolFiller Excipient
Compositions, methods, and applications that increase the efficiency of nucleic acid transfection are provided. In one aspect, a pharmaceutical composition may include at least about 0.5 mg / ml concentration of a nucleic acid condensed with a cationic lipopolymer suspended in an isotonic solution, where the cationic lipopolymer includes a cationic polymer backbone having cholesterol and polyethylene glycol covalently attached thereto, and wherein the molar ratio of cholesterol to cationic polymer backbone is within a range of from about 0.1 to about 10, and the molar ratio of polyethylene glycol to cationic polymer backbone is within a range of from about 0.1 to about 10. The composition further may include a filler excipient.
Owner:CLSN LAB
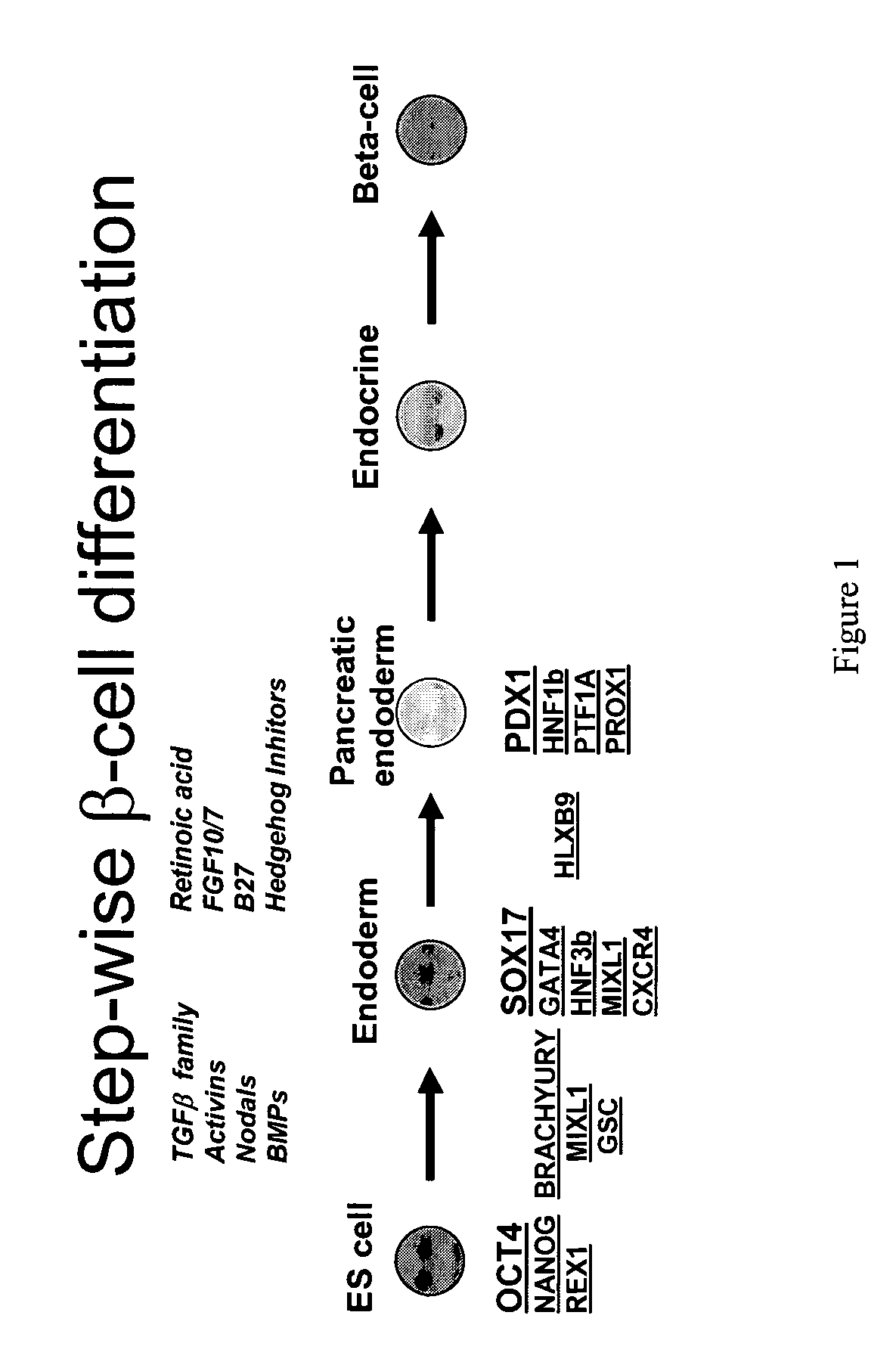
PDX1 expressing endoderm
InactiveUS20050266554A1Increase differentiationIncrease productionGastrointestinal cellsDiagnosticsGerm layerCell type
Disclosed herein are cell cultures comprising PDX1-positive endoderm cells and methods of producing the same. Also disclosed herein are cell populations comprising substantially purified PDX1-positive endoderm cells as well as methods for enriching, isolating and purifying PDX1-positive endoderm cells from other cell types. Methods of identifying differentiation factors capable of promoting the differentiation of endoderm cells, such as PDX1-positive foregut endoderm cells and PDX1-negative definitive endoderm cells, are also disclosed.
Owner:CYTHERA
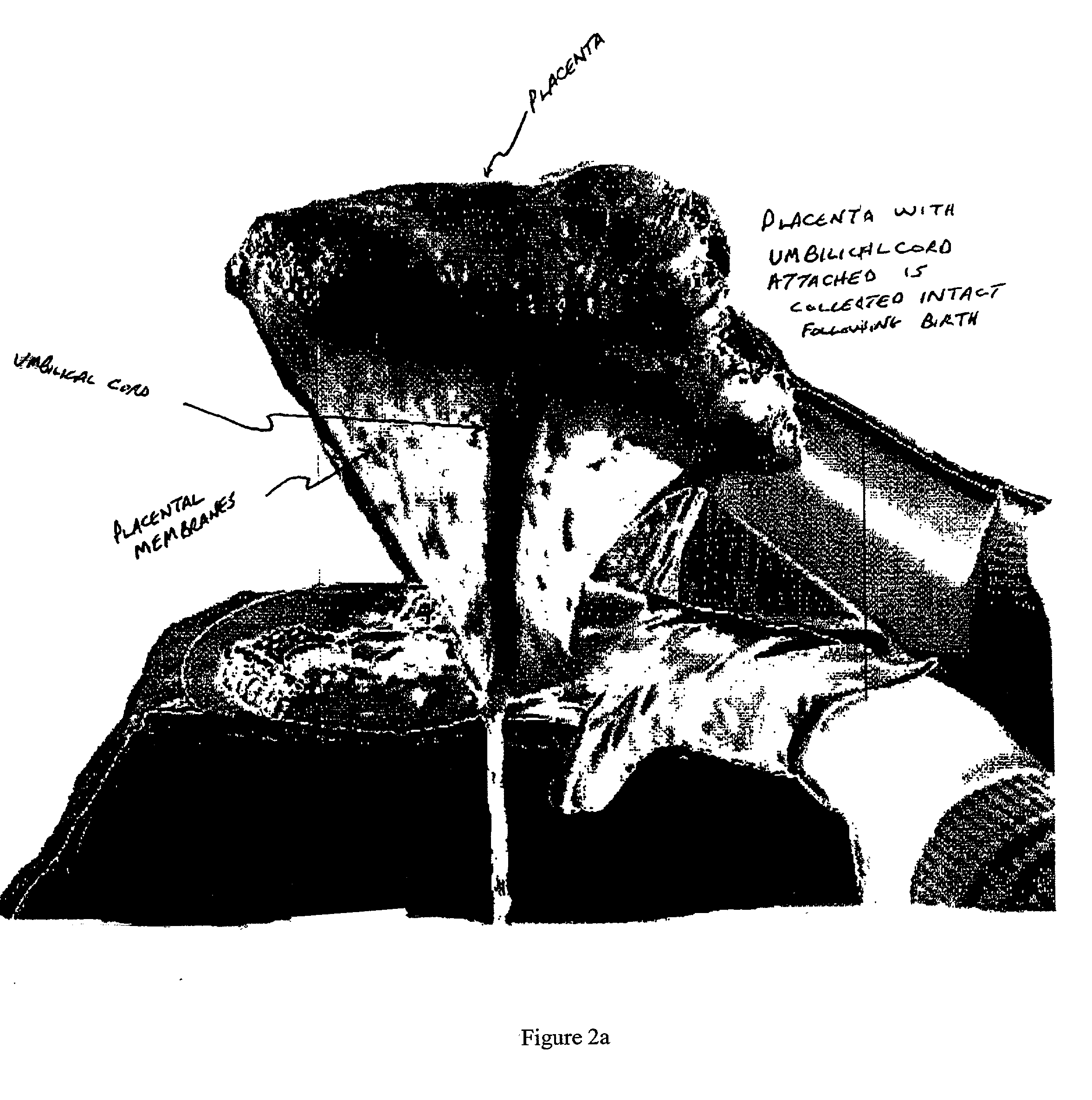
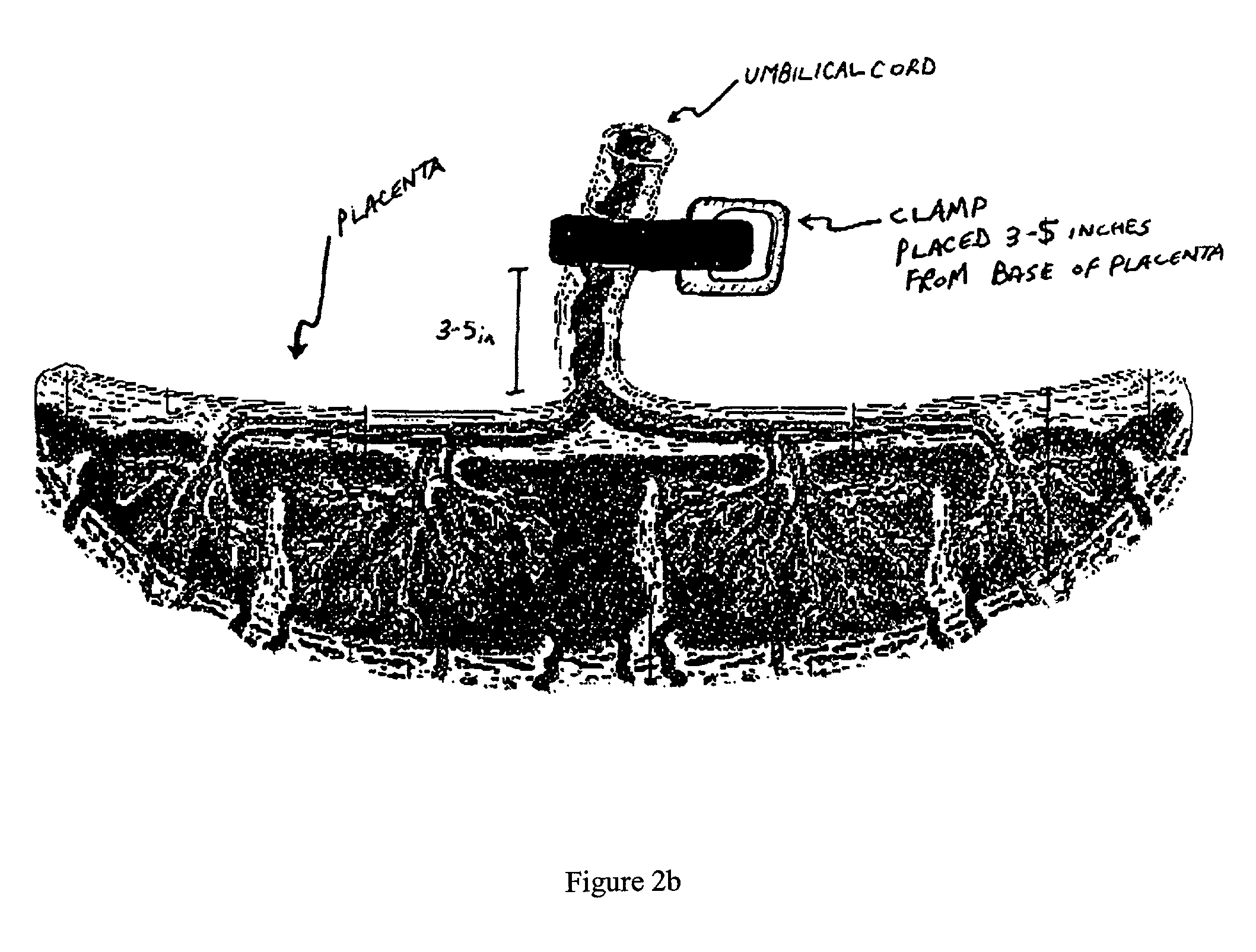
Method of collecting placental stem cells
InactiveUS20020123141A1Increase concentrationImprove the environmentSenses disorderAntipyreticCord blood stem cellEmbryo
A method of collecting embryonic-like stem cells from a placenta which has been treated to remove residual cord blood by perfusing the drained placenta with an anticoagulant solution to flush out residual cells, collecting the residual cells and perfusion liquid from the drained placenta, and separating the embryonic-like cells from the residual cells and perfusion liquid. Exogenous cells can be propagated in the placental bioreactor and bioactive molecules collected therefrom.
Owner:CELULARITY INC
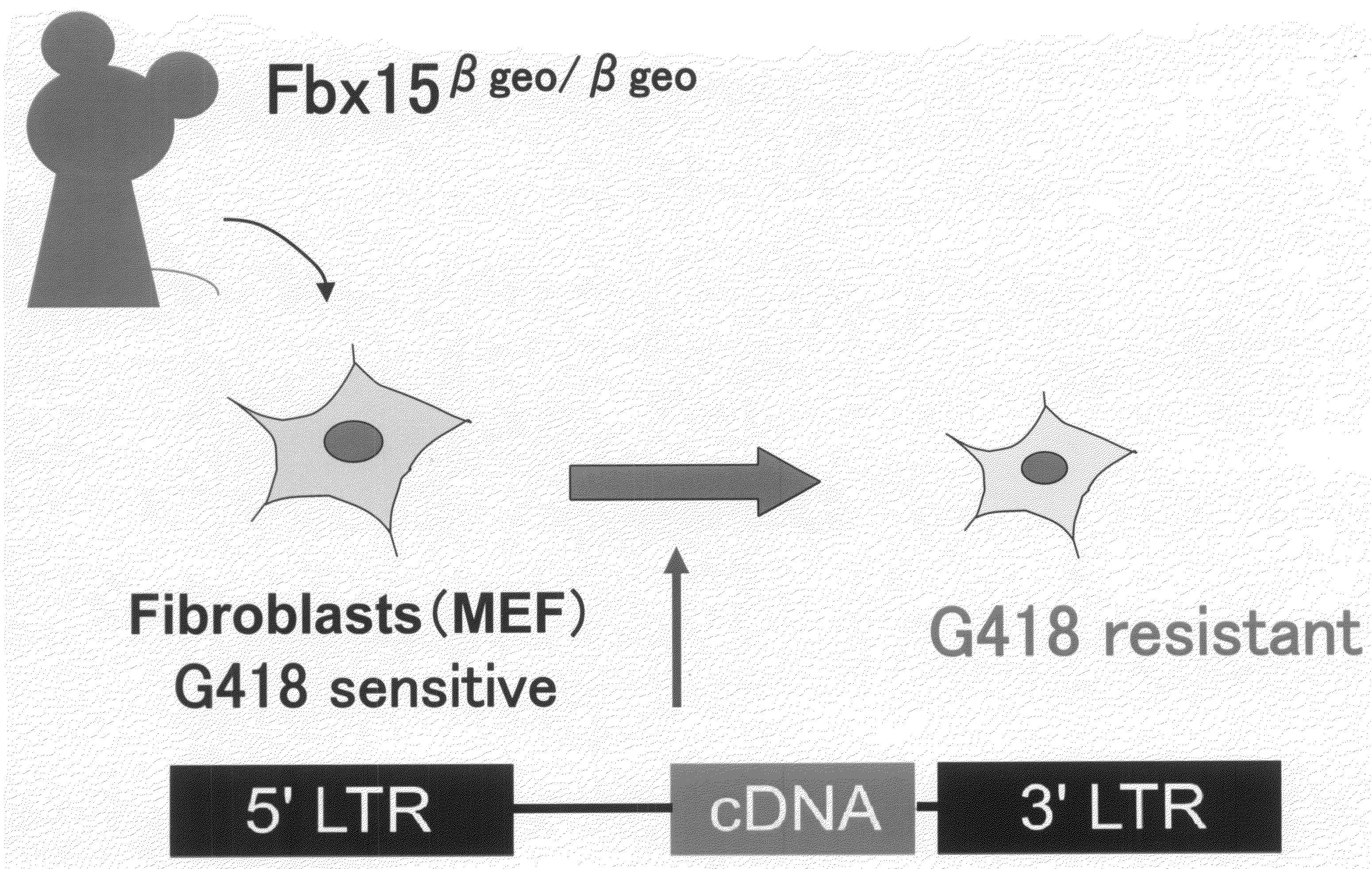
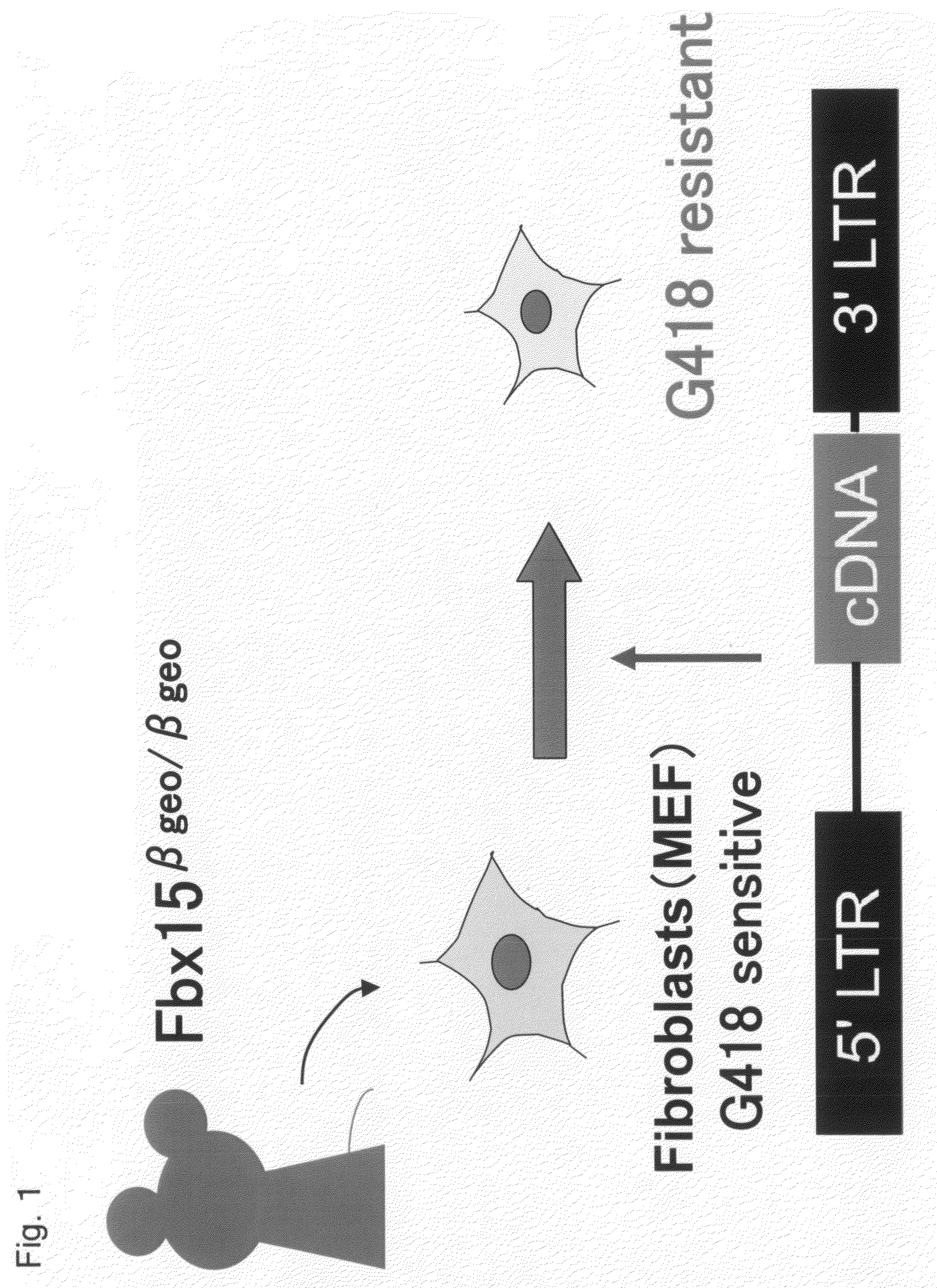
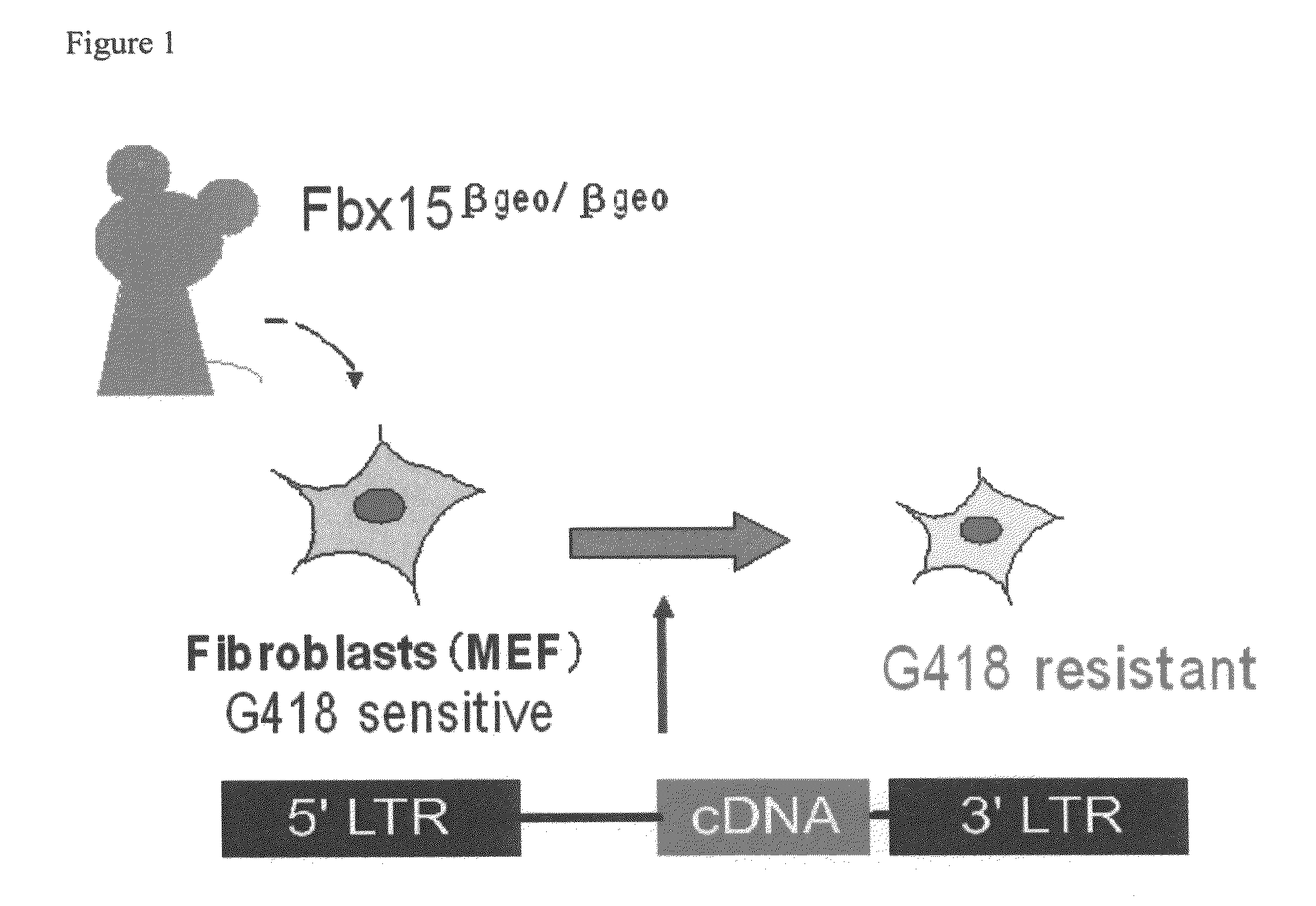
Nuclear Reprogramming Factor
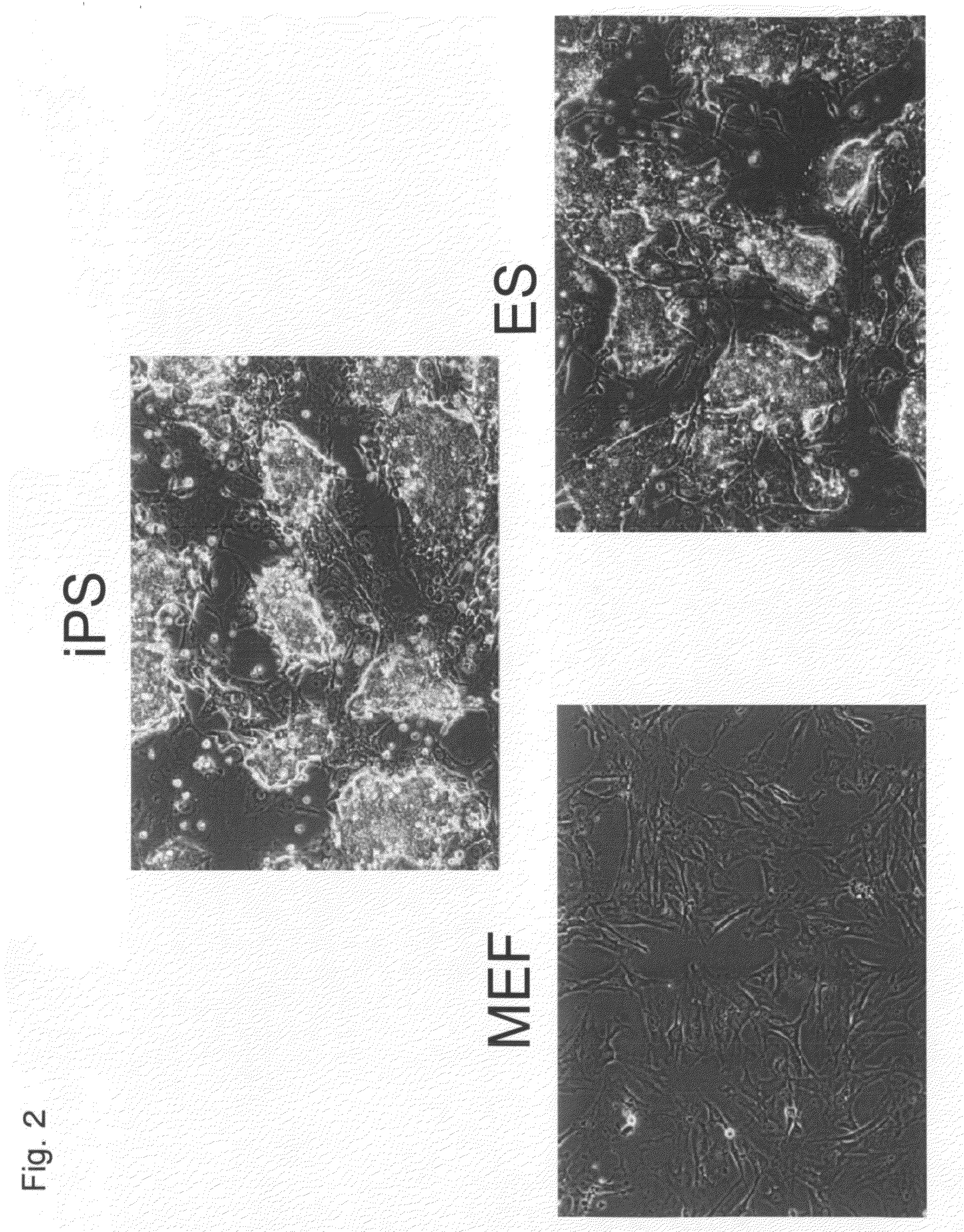
There is provided a nuclear reprogramming factor for a somatic cell, which comprises a gene product of each of the following three kinds of genes: an Oct family gene, a Klf family gene, and a Myc family gene, as a means for inducing reprogramming of a differentiated cell to conveniently and highly reproducibly establish an induced pluripotent stem cell having pluripotency and growth ability similar to those of ES cells without using embryo or ES cell.
Owner:KYOTO UNIV
Use of a Trans-Signaling Approach in Chimeric Antigen Receptors
ActiveUS20140099309A1Heightened tumor specificitySugar derivativesAntibody mimetics/scaffoldsTrans signalingActivation cells
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Method for the generation of antigen-specific lymphocytes
InactiveUS20070116690A1Function increaseEnhancing function of T cellBiocideVirusesAutoimmune conditionAutoimmune disease
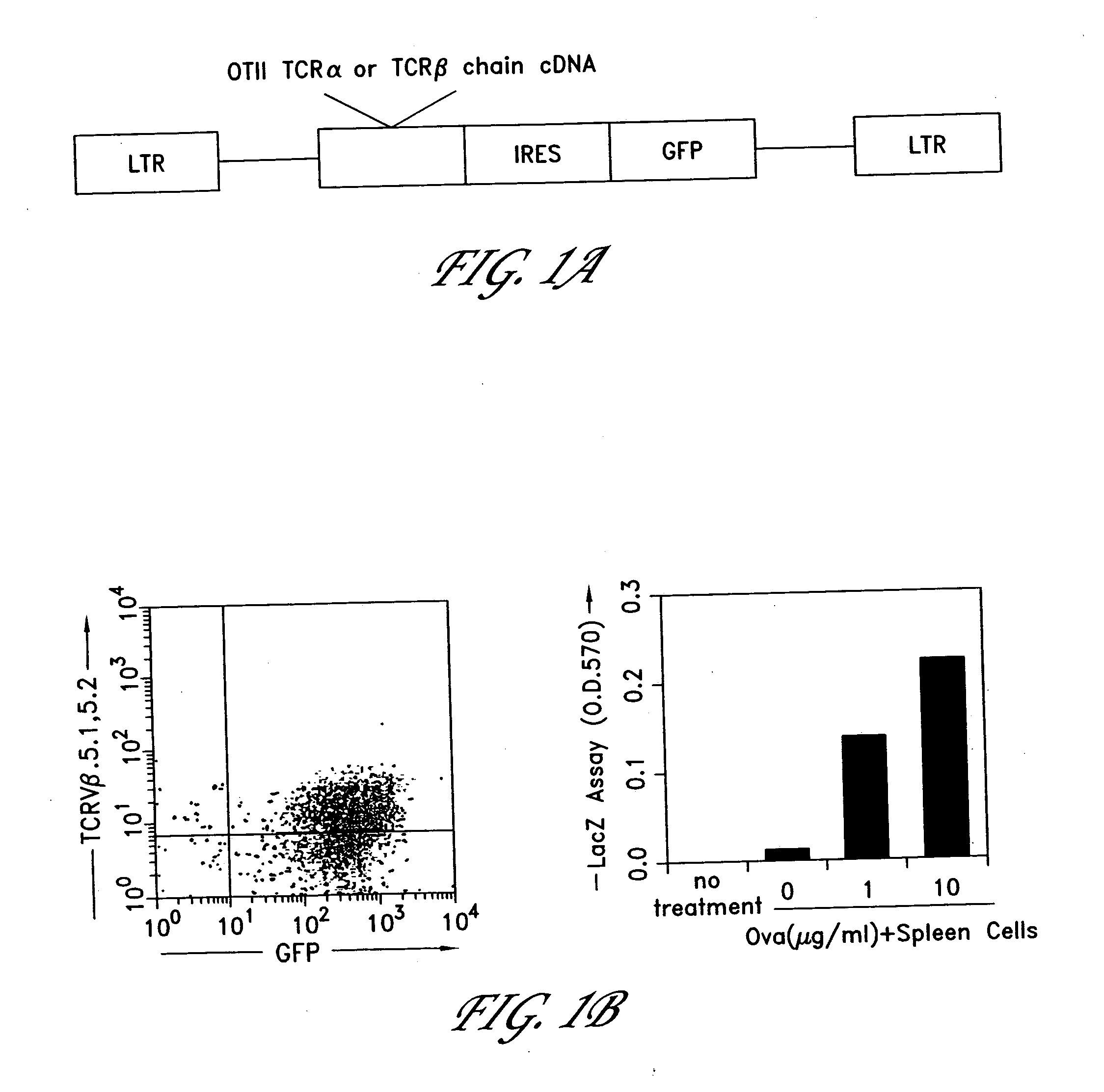
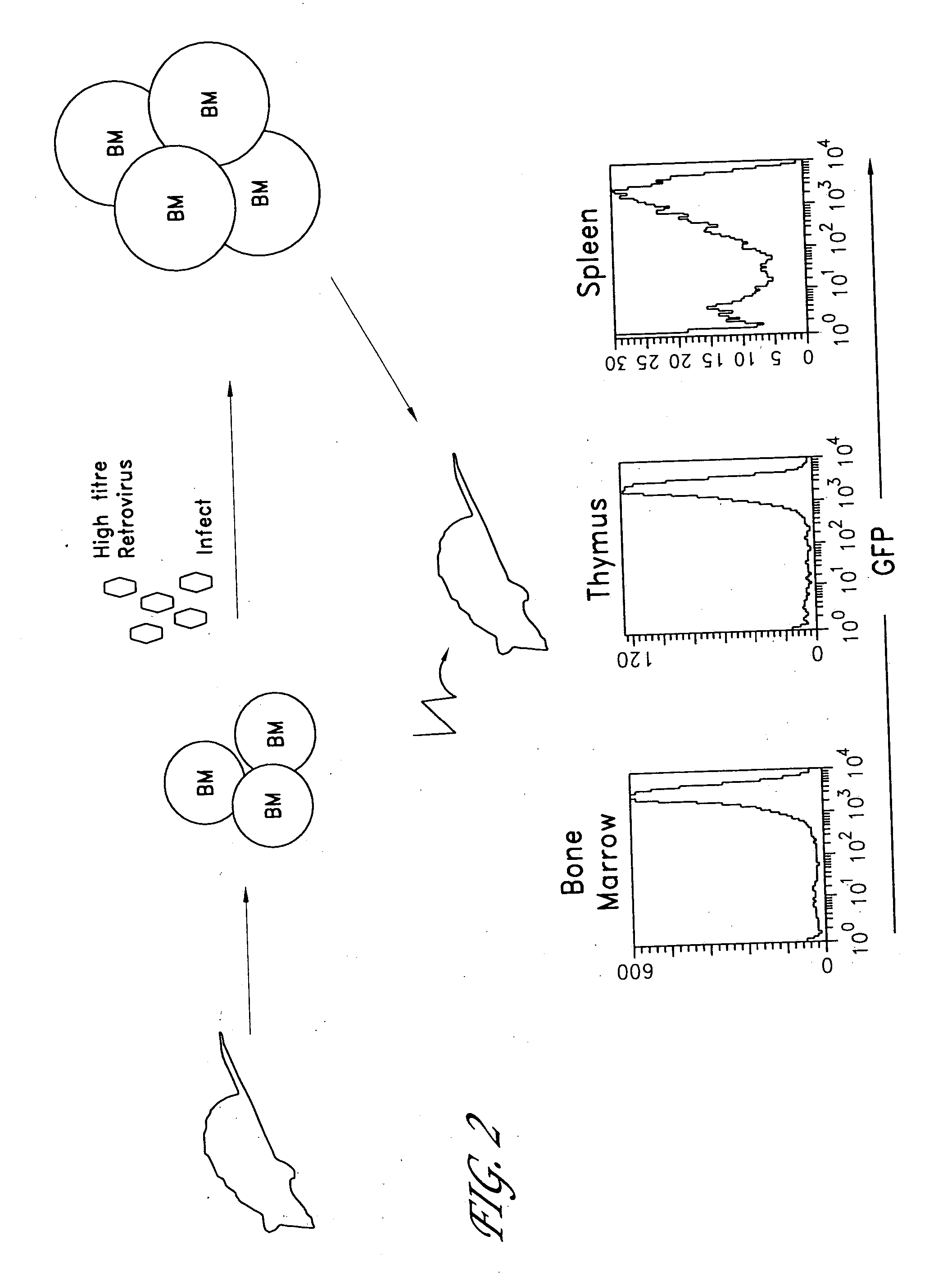
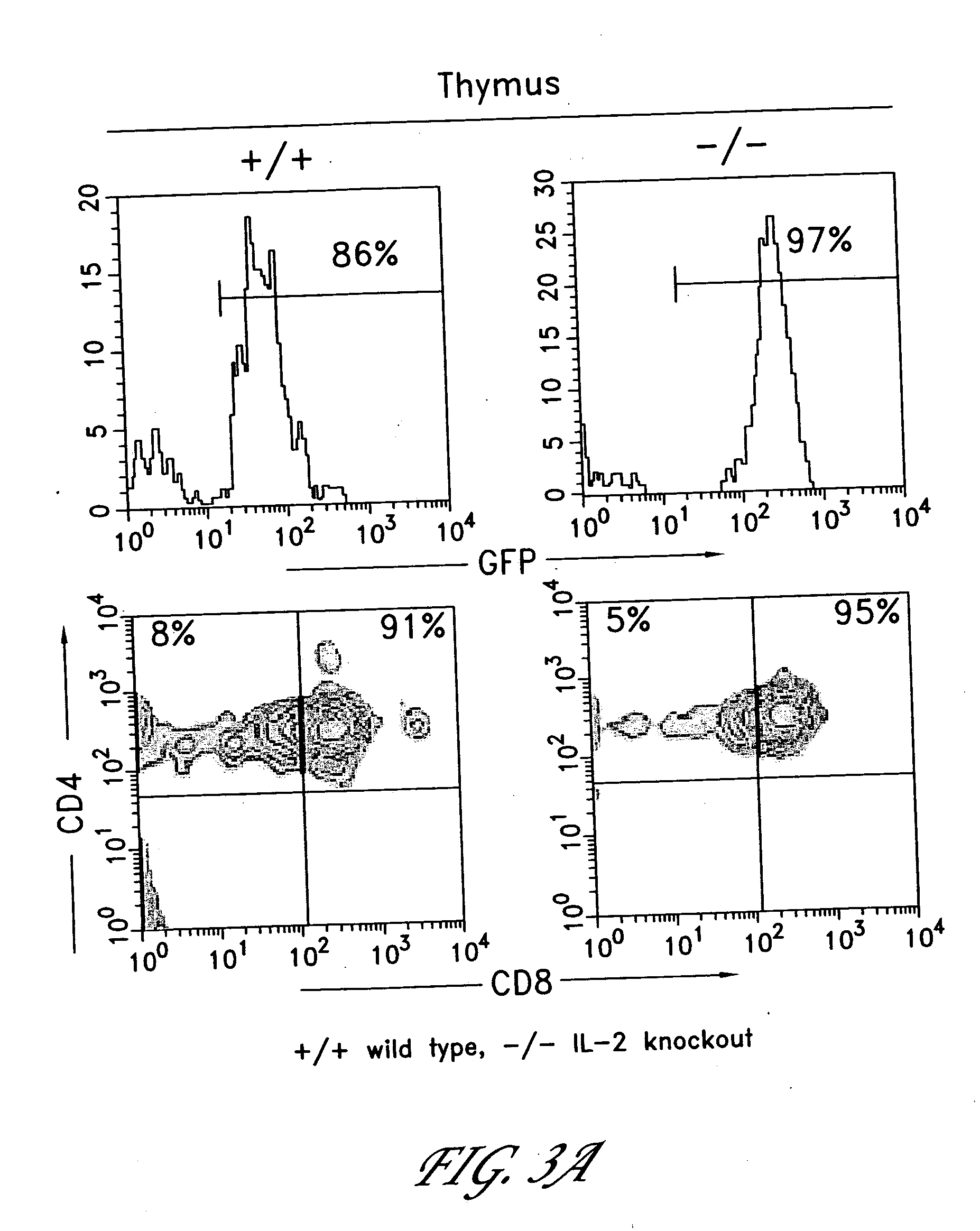
The invention provides systems and methods for the generation of lymphocytes having a unique antigen specificity. In a preferred embodiment, the invention provides methods of virally infecting cells from bone marrow with one or more viral vectors that encode antigen-specific antibodies for the production of, for example B cells and T cells. In some embodiments, the viral vectors include an IRES or 2A element to promote separation of, for example, the α subunit and β subunit of a T cell receptor (TCR) or heavy and light chains of a B-cell antibody. The resulting lymphocytes, express the particular antibody that was introduced in the case of B cells and TCR in the case of T cells. The lymphocytes generated can be used for a variety of therapeutic purposes including the treatment of various cancers and the generation of a desired immune response to viruses and other pathogens. The resulting cells develop normally and respond to antigen both in vitro and in vivo. We also show that it is possible to modify the function of lymphocytes by using stem cells from different genetic backgrounds. Thus our system constitutes a powerful tool to generate desired lymphocyte populations both for research and therapy. Future applications of this technology may include treatments for infectious diseases, such as HIV / AIDS, cancer therapy, allergy, and autoimmune disease.
Owner:CALIFORNIA INST OF TECH
Decellularized extracellular matrix of conditioned body tissues and uses thereof
InactiveUS20050013870A1Increase in sizeHigh strengthGastrointestinal cellsGenetically modified cellsCell-Extracellular MatrixECM Protein
The present invention relates generally to decellularized extracellular matrix of conditioned body tissues. The decellularized extracellular matrix contains a biological material, preferably vascular endothelial growth factor (VEGF), produced by the conditioned body tissue that is in an amount different than the amount of the biological material that the body tissue would produce absent the conditioning. The invention also relates to methods of making and methods of using said decellularized extracellular matrix. Specifically, the invention relates to treating defective, diseased, damaged or ischemic cells, tissues or organs in a subject by administering, injecting or implanting the decellularized extracellular matrix of the invention into a subject in need thereof. The invention is further directed to a tissue regeneration scaffold for implantation into a subject inflicted with a disease or condition that requires tissue or organ repair, regeneration and / or strengthening. Additionally, the invention is directed to a medical device, preferably a stent or an artificial heart, having a surface coated or covered with the decellularized extracellular matrix of the invention or having a component comprising the decellularized extracellular matrix of the invention for implantation into a subject, preferably a human. Methods for making the tissue regeneration scaffold and methods for manufacturing a coated or covered medical device having a component comprising decellularized extracellular matrix of conditioned body tissues are also provided.
Owner:BOSTON SCI SCIMED INC
Generation and application of universal T cells for B-ALL
InactiveUS20070036773A1Hinder recognitionEnhanced siRNA effectBiocideGenetic material ingredientsAntigenNatural Killer Cell Inhibitory Receptors
The present invention is directed to universal T cells and their use in treating diseases and other physiological conditions. More specifically, the present invention is directed to universal T cells and their use in treating treating B-lineage acute lymphoblastic leukemia (B-ALL) in particular and malignancy in general. The universal T cells contain (i) nucleic acid encoding a chimeric antigen receptor (CAR) to redirect their antigen specificity and effector function and (ii) nucleic acids encoding shRNA and / or siRNA molecules to down-regulate cell-surface expression of T cell classical HLA class I and / or II genes to avoid recognition by recipient T cells. The universal T cells may also contain a nucleic acid encoding a non-classical HLA gene, such as an HLA E gene to enforce expression of HLA E genes and / or an HLA G gene to enforce expression of HLA G genes, to avoid recognition by recipient NK cells. The universal T cells may further contain a nucleic acid encoding a selection-suicide gene.
Owner:CITY OF HOPE
T cell receptor-deficient t cell compositions
The invention is directed to modified T cells, methods of making and using isolated, modified T cells, and methods of using these isolated, modified T cells to address diseases and disorders. In one embodiment, this invention broadly relates to TCR-deficient T cells, isolated populations thereof, and compositions comprising the same. In another embodiment of the invention, these TCR-deficient T cells are designed to express a functional non-TCR receptor. The invention also pertains to methods of making said TCR-deficient T cells, and methods of reducing or ameliorating, or preventing or treating, diseases and disorders using said TCR-deficient T cells, populations thereof, or compositions comprising the same.
Owner:TRUSTEES OF DARTMOUTH COLLEGE THE
Therapy of cancer by insect cells containing recombinant baculovirus encoding genes
Provided are compositions and methods of use for insect cells comprising baculovirus encoding non-surface expressed proteins and peptides. The claimed invention particularly relates to compositions comprising insect cells containing baculovirus that express cytokines. Such compositions may be administered by, for example, direct intratumoral injection into tumors in mammals, resulting in tumor reduction or recission. Another aspect of the claimed invention concerns methods of promoting resistance to the reoccurence of tumors in mammals who have undergone such tumor recission. In a specific aspect of the claimed invention, the mammals are human subjects presenting with various forms of cancer.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
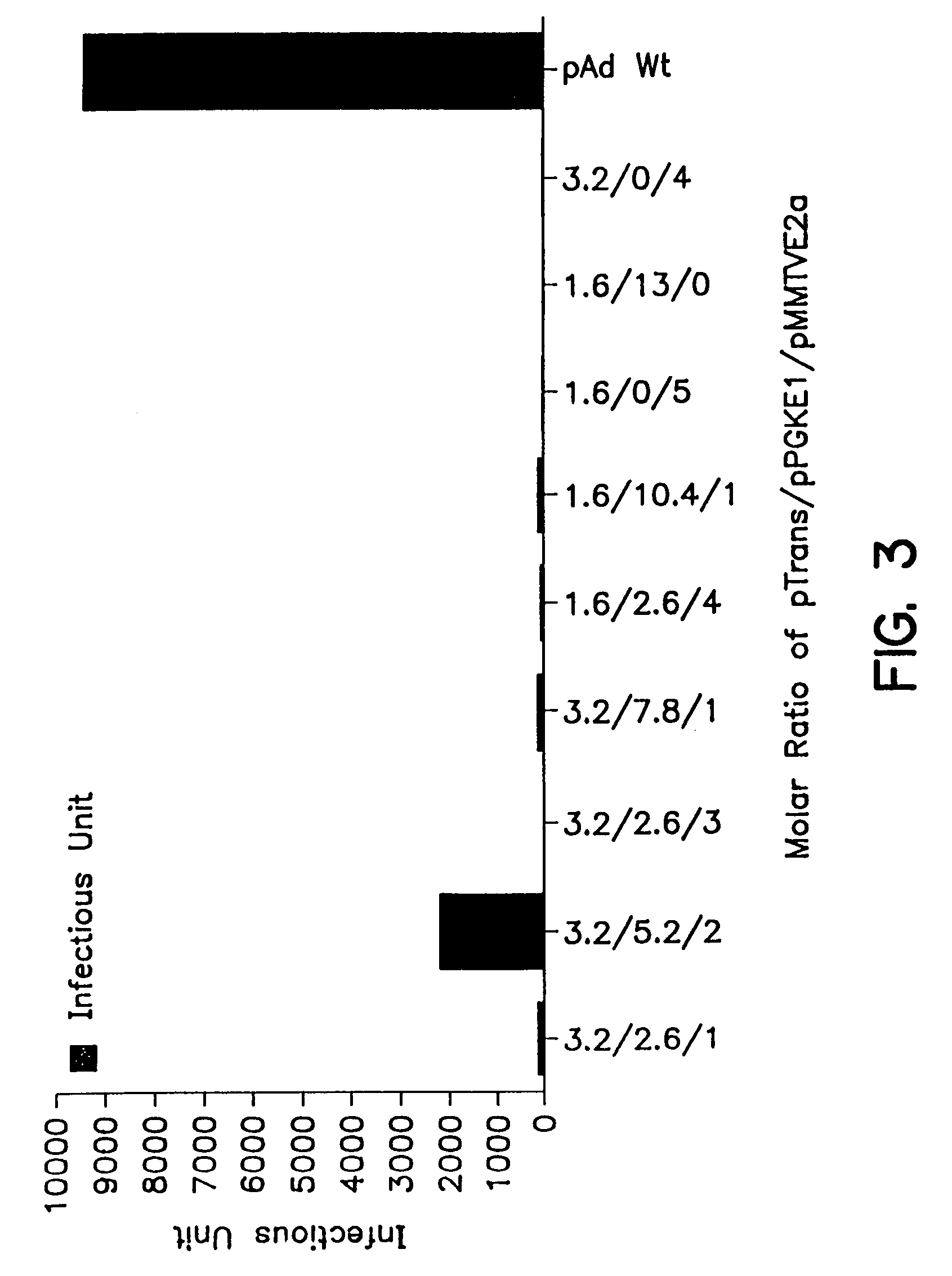
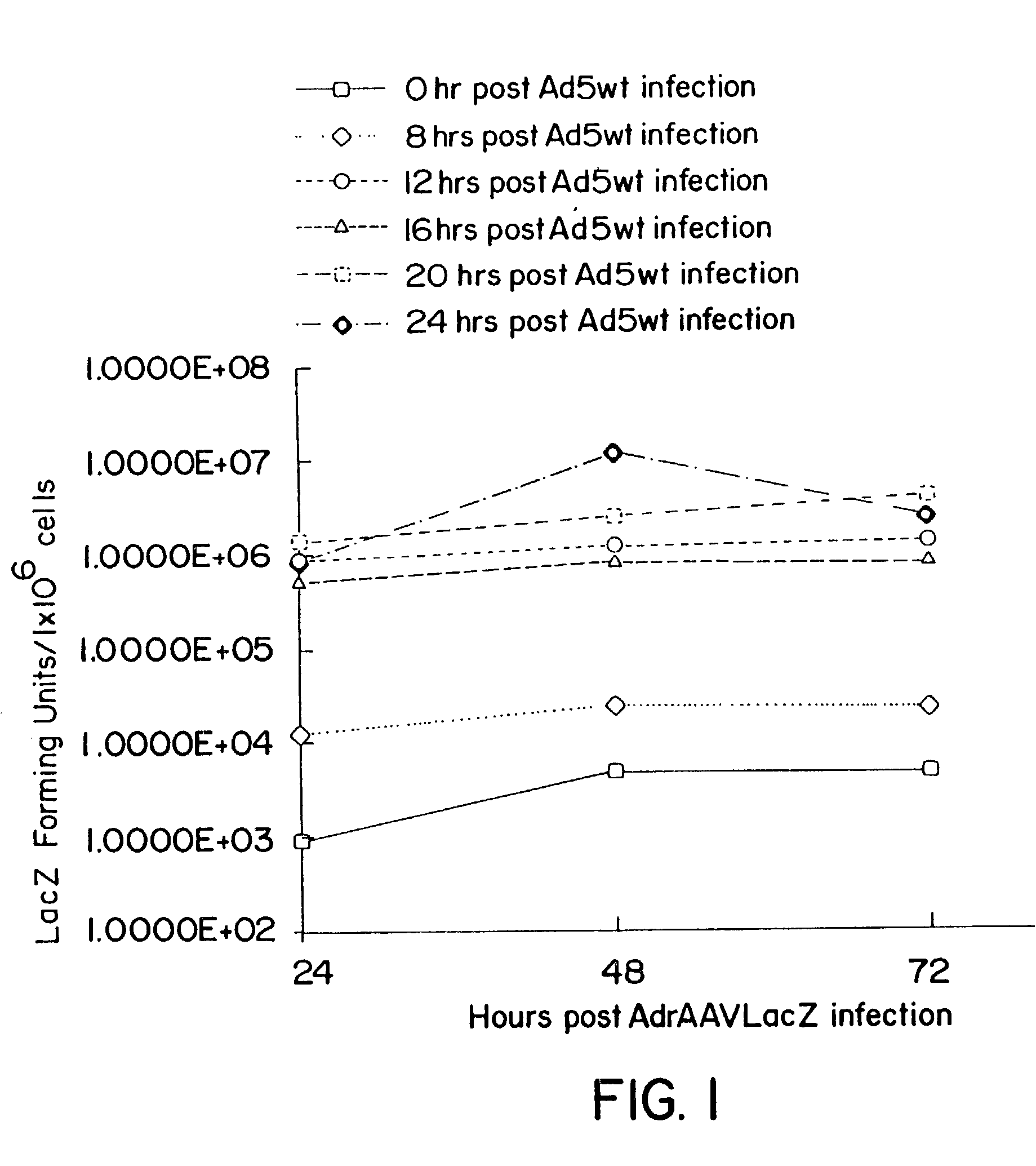
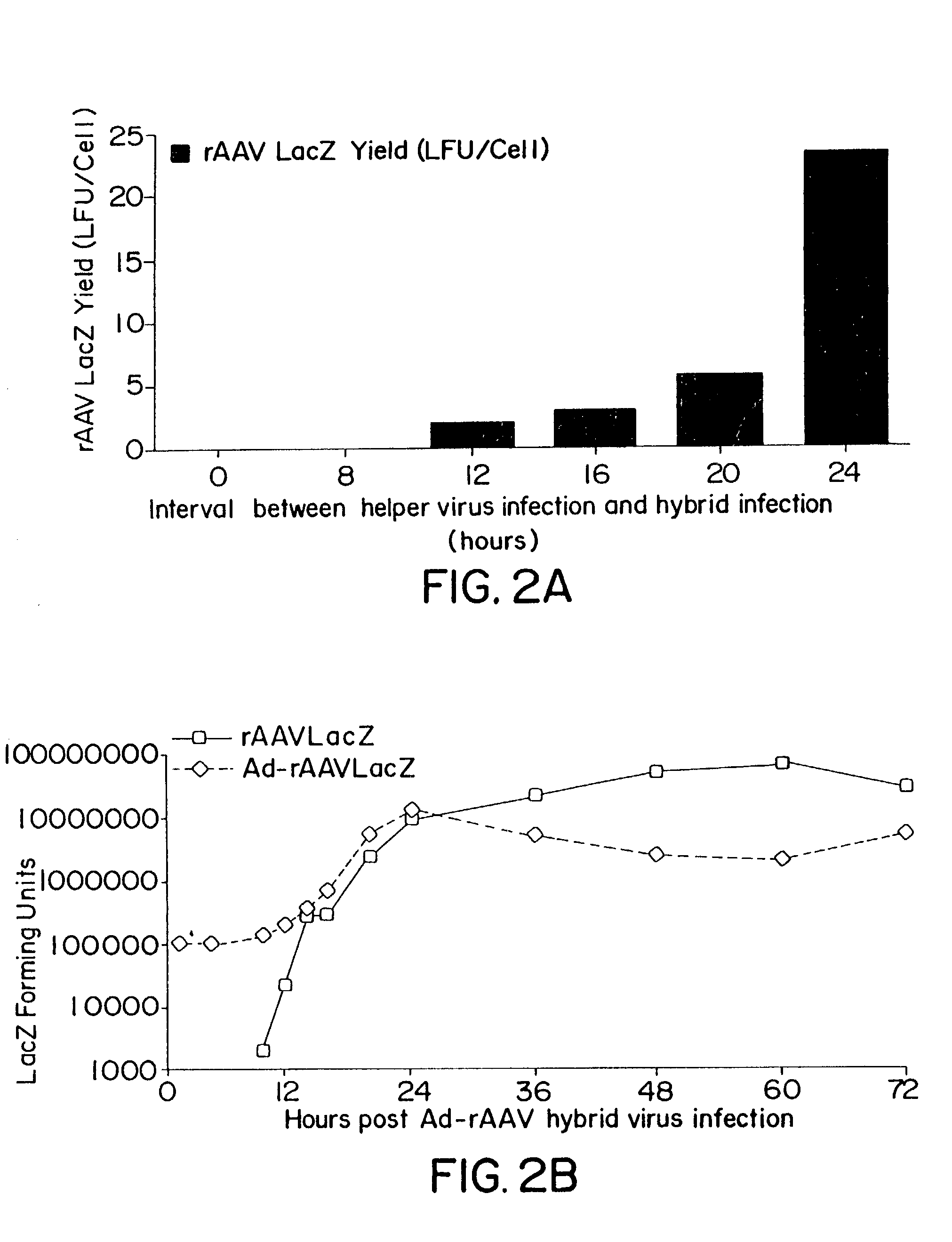
Compositions and methods for helper-free production of recombinant adeno-associated viruses
InactiveUS6953690B1Efficient productionIncrease the number ofBiocideGenetic therapy composition manufactureMammalWild type
A method for producing recombinant adeno-associated virus in the absence of contaminating helper virus or wild-type virus involves culturing a mammalian host cell containing a transgene flanked by adeno-associated virus (AAV) inverse terminal repeats and under the control of regulatory sequences directing expression thereof, an AAV rep sequence and an AAV cap sequence under the control of regulatory sequences directing expression thereof, and the minimum adenovirus DNA required to express an E1a gene product, an E1b gene product and an E2a gene product, and isolating therefrom a recombinant AAV which expresses the transgene in the absence of contaminating helper virus or wildtype AAV. This method obviates a subsequent purification step to purify rAAV from contaminating virus. Also provided are various embodiments of the host cell.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
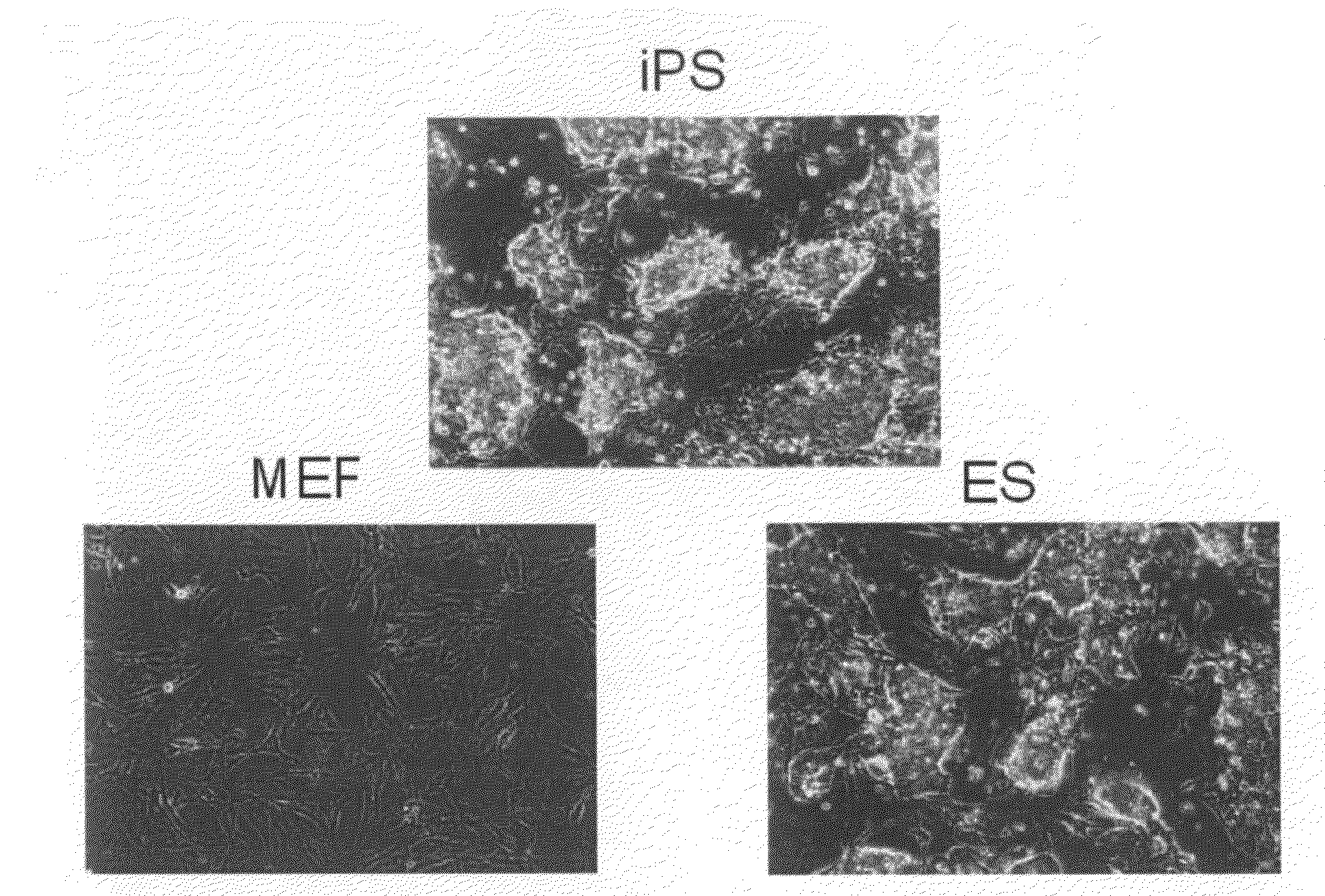
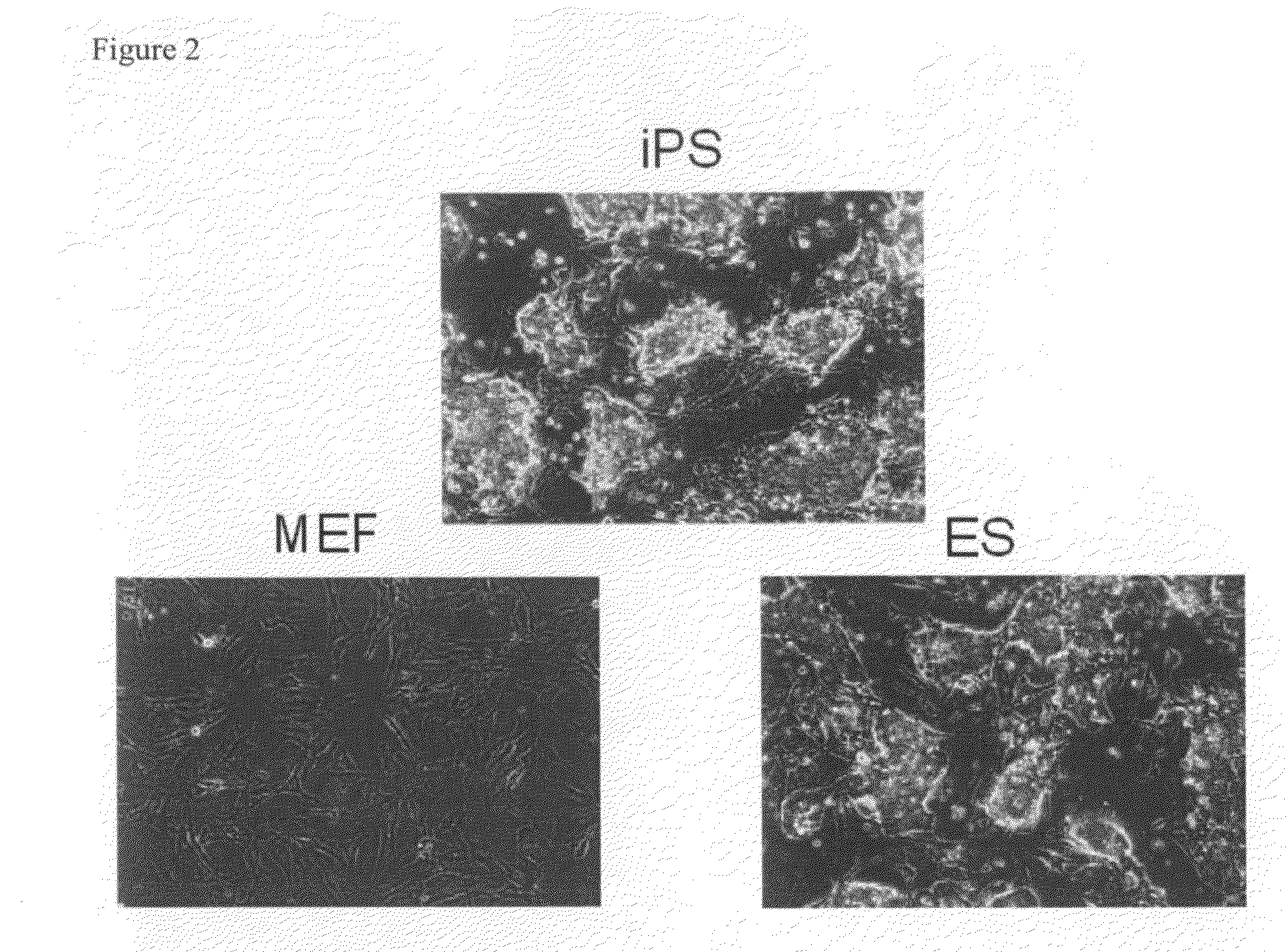
Nuclear reprogramming factor and induced pluripotent stem cells
InactiveUS20090227032A1Easy to prepareEffective isolationGenetically modified cellsPeptidesNuclear reprogrammingCell therapy
The present invention relates to a nuclear reprogramming factor having an action of reprogramming a differentiated somatic cell to derive an induced pluripotent stem (iPS) cell. The present invention also relates to the aforementioned iPS cells, methods of generating and maintaining iPS cells, and methods of using iPS cells, including screening and testing methods as well as methods of stem cell therapy. The present invention also relates to somatic cells derived by inducing differentiation of the aforementioned iPS cells.
Owner:KYOTO UNIV
Methods and cell line useful for production of recombinant adeno-associated viruses
InactiveUS7238526B2High yieldInduce expressionGenetically modified cellsGenetic material ingredientsPlasmid VectorAdeno associate virus
Methods for efficient production of recombinant AAV employ a host cell which comprising AAV rep and cap genes stably integrated within the cell's chromosomes, wherein the AAV rep and cap genes are each operatively linked to regulatory sequences capable of directing the expression of the rep and cap gene products upon infection of the cell with a helper virus, a helper gene, and a helper gene product. A method for producing recombinant adeno-associated virus (rAAV) involves infecting such a host cell with a helper virus, gene or gene product and infecting the infected host cell with a recombinant hybrid virus or plasmid vector containing adenovirus cis-elements necessary for replication and virion encapsidation, AAV sequences comprising the 5′ and 3′ ITRs of an AAV, and a selected gene operatively linked to regulatory sequences directing its expression, which is flanked by the above-mentioned AAV sequences.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
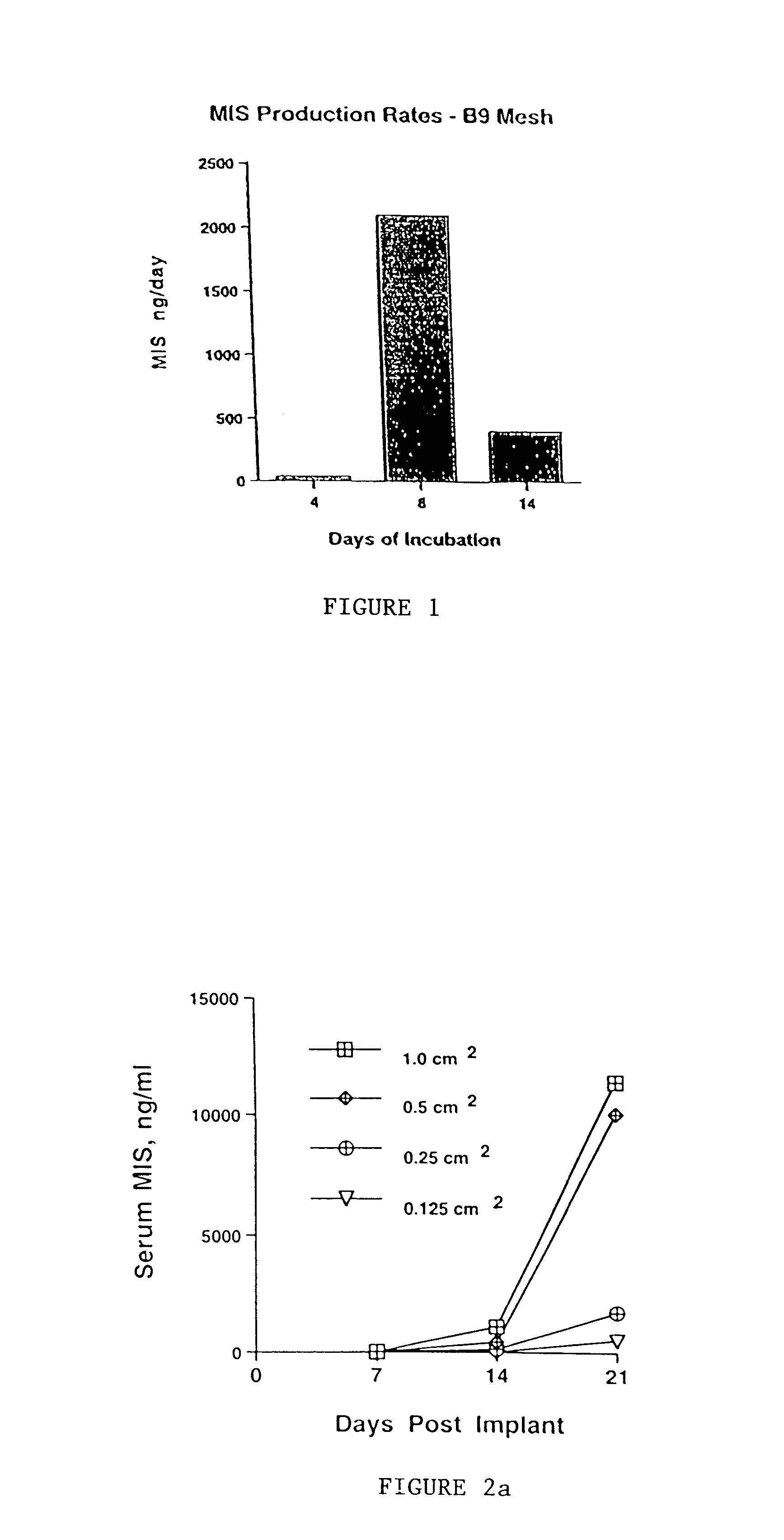
Delivery of therapeutic biologicals from implantable tissue matrices
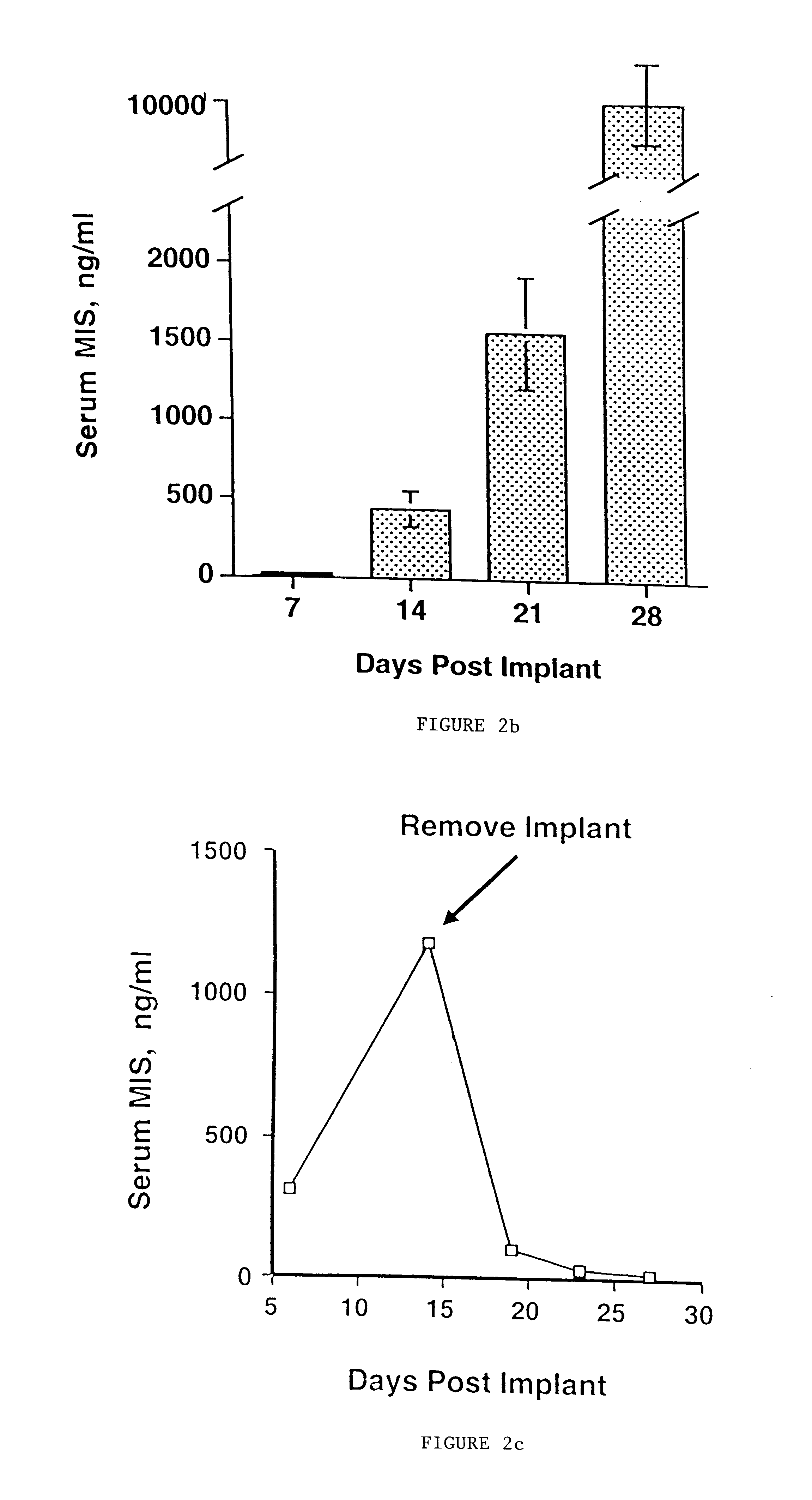
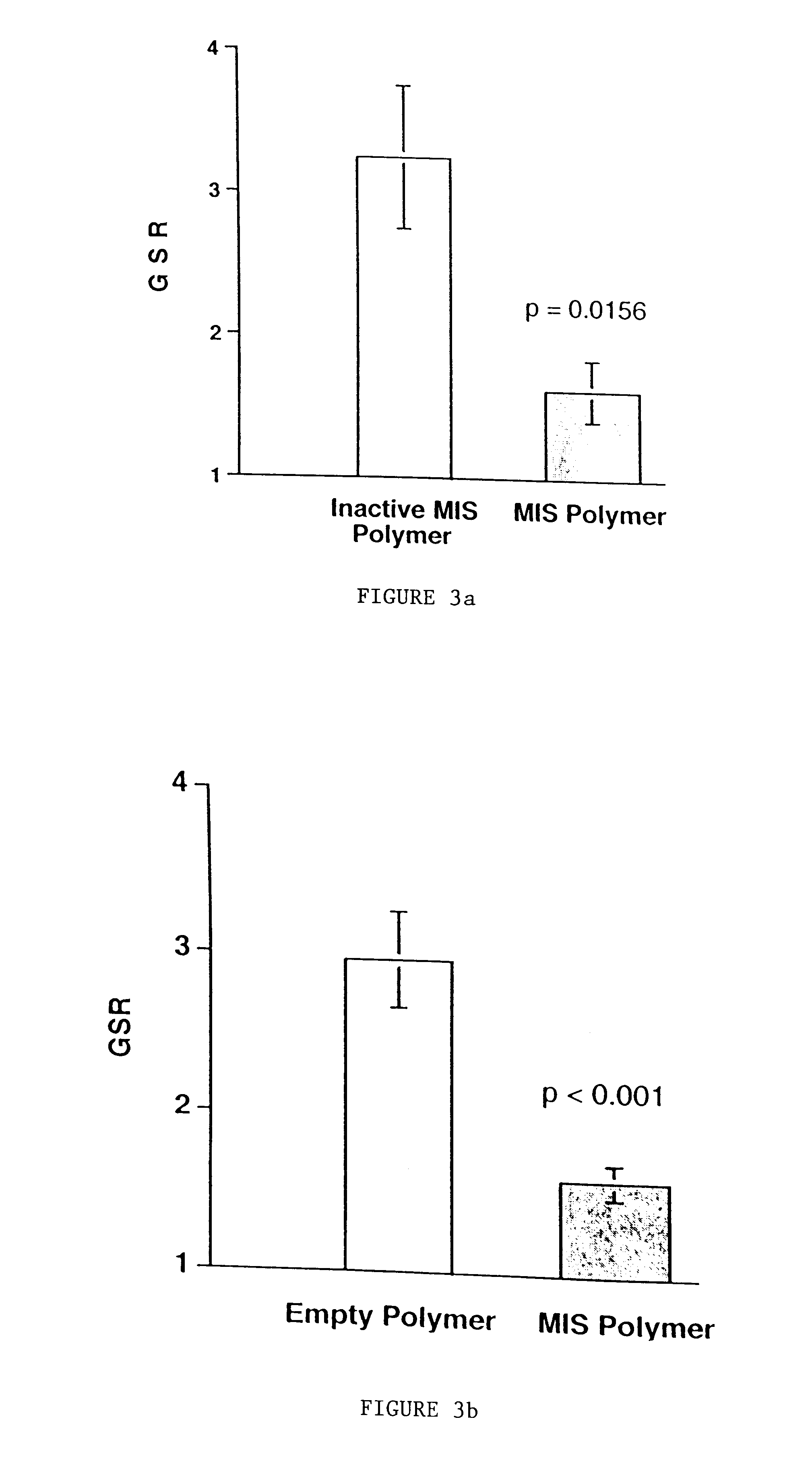
Normal cells, such as fibroblasts or other tissue or organ cell types, are genetically engineered to express biologically active, therapeutic agents, such as proteins that are normally produced in small amounts, for example, MIS, or other members of the TGF-beta family Herceptin(TM), interferons, andanti-angiogenic factors. These cells are seeded into a matrix for implantation into the patient to be treated. Cells may also be engineered to include a lethal gene, so that implanted cells can be destroyed once treatment is completed. Cells can be implanted in a variety of different matrices. In a preferred embodiment, these matrices are implantable and biodegradable over a period of time equal to or less than the expected period of treatment, when cells engraft to form a functional tissue producing the desired biologically active agent. Implantation may be ectopic or in some cases orthotopic. Representative cell types include tissue specific cells, progenitor cells, and stem cells. Matrices can be formed of synthetic or natural materials, by chemical coupling at the time of implantation, using standard techniques for formation of fibrous matrices from polymeric fibers, and using micromachining or microfabrication techniques. These devices and strategies are used as delivery systems via standard or minimally invasive implantation techniques for any number of parenterally deliverable recombinant proteins, particularly those that are difficult to produce in large amounts and / or active forms using conventional methods of purification, for the treatment of a variety of conditions that produce abnormal growth, including treatment of malignant and benign neoplasias, vascular malformations (hemangiomas), inflammatory conditions, keloid formation, abdominal or plural adhesions, endometriosis, congenital or endocrine abnormalities, and other conditions that can produce abnormal growth such as infection. Efficacy of treatment with the therapeutic biologicals is detected by determining specific criteria, for example, cessation of cell proliferation, regression of abnormal tissue, or cell death, or expression of genes or proteins reflecting the above.
Owner:THE GENERAL HOSPITAL CORP
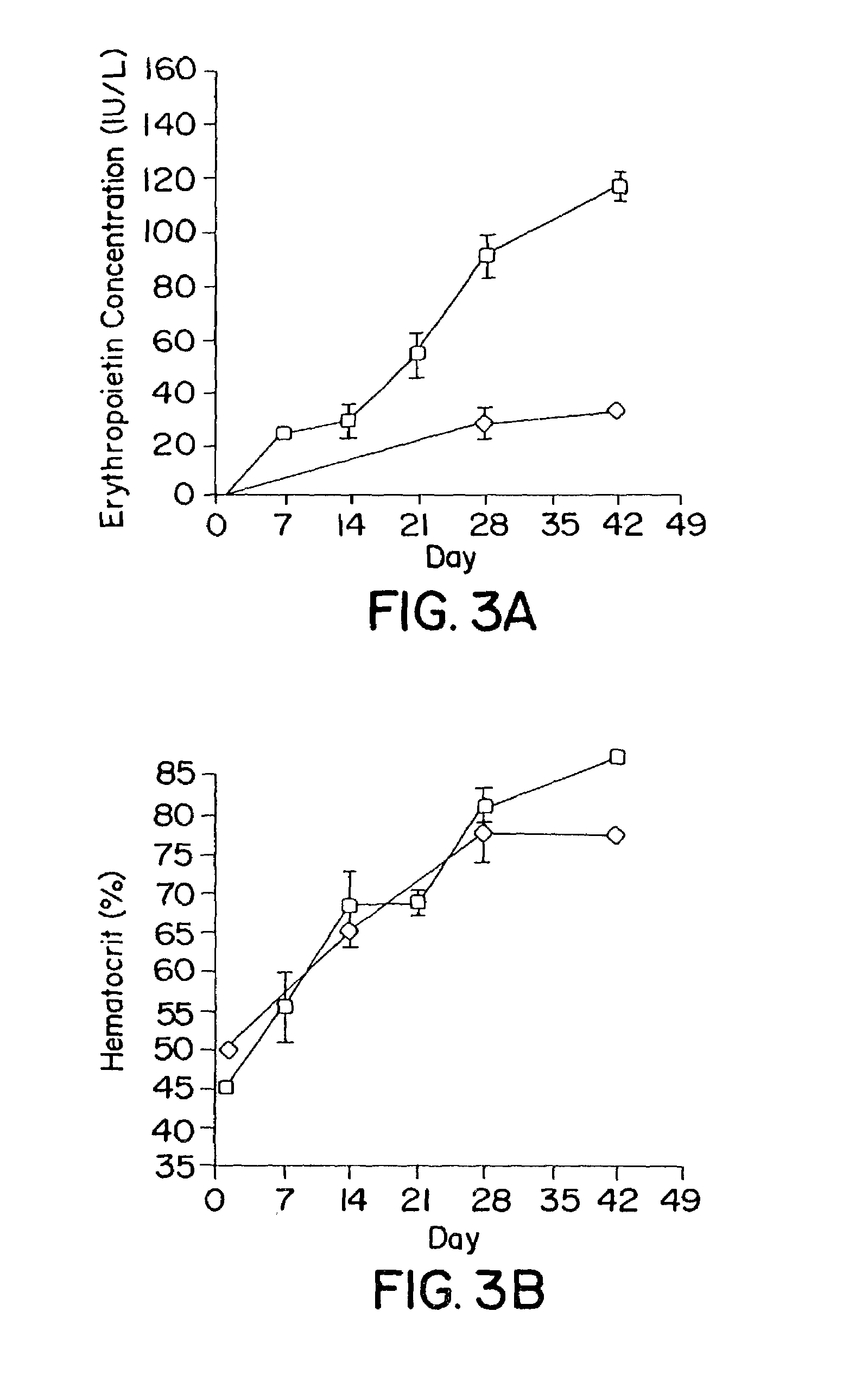
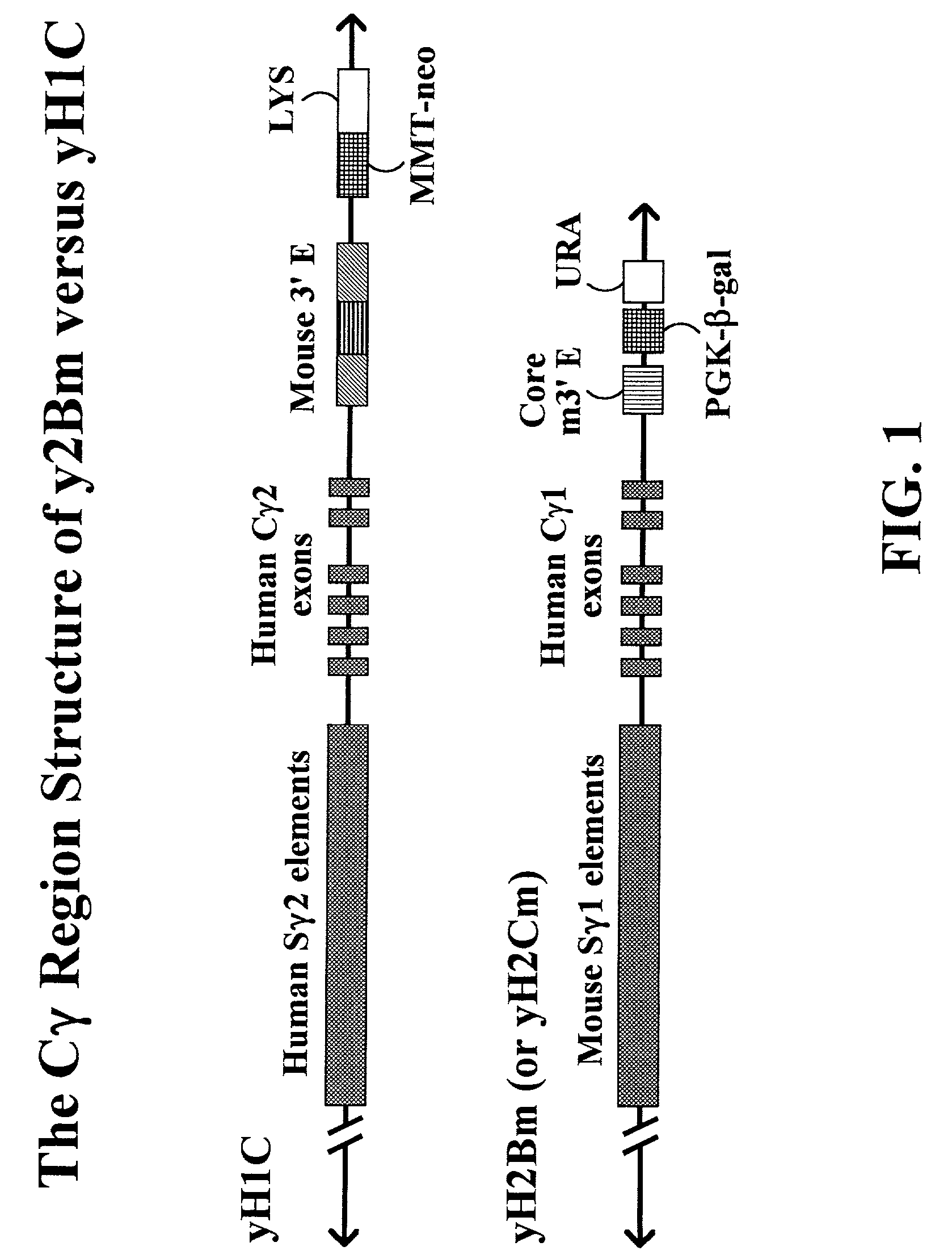
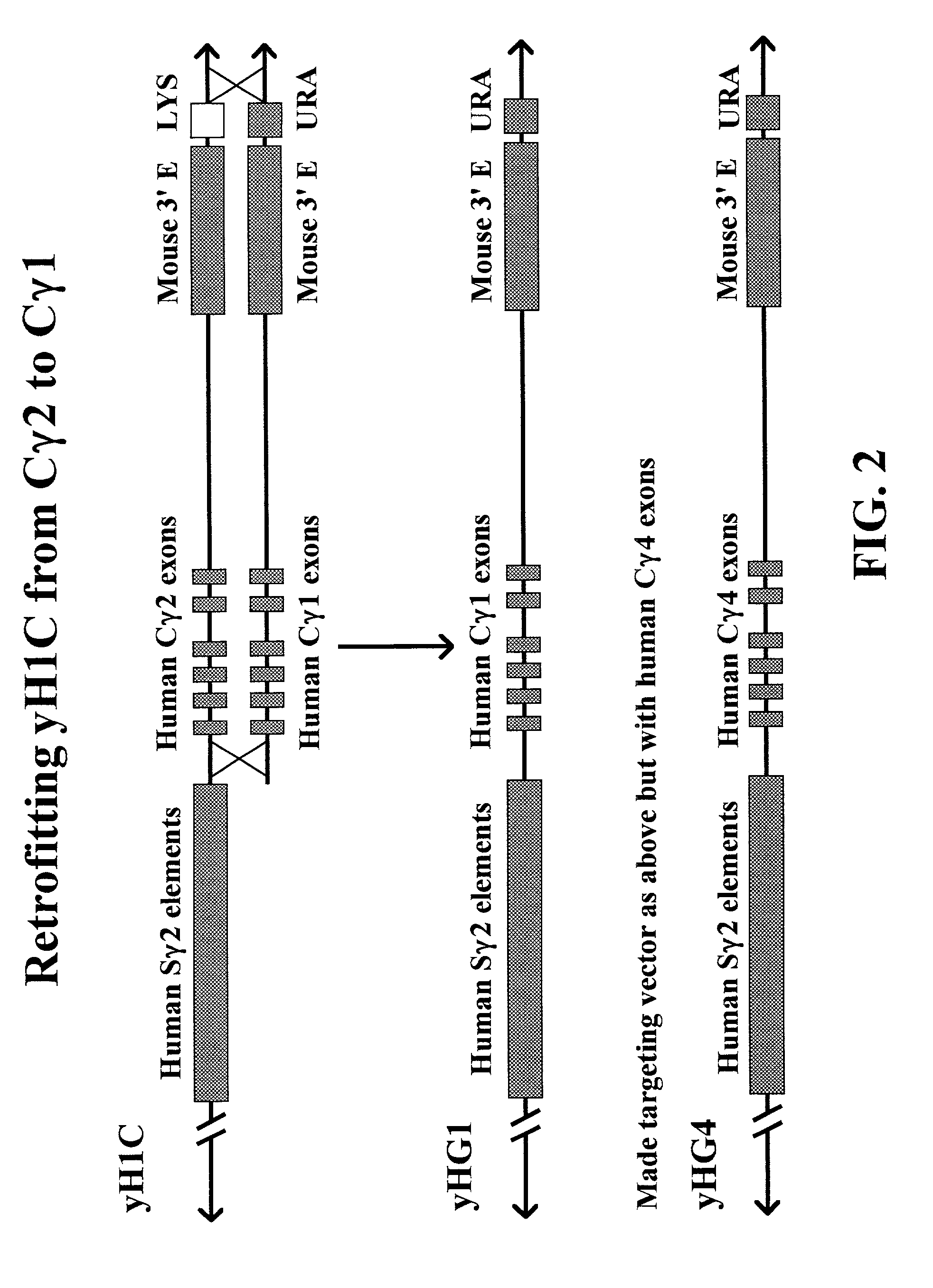
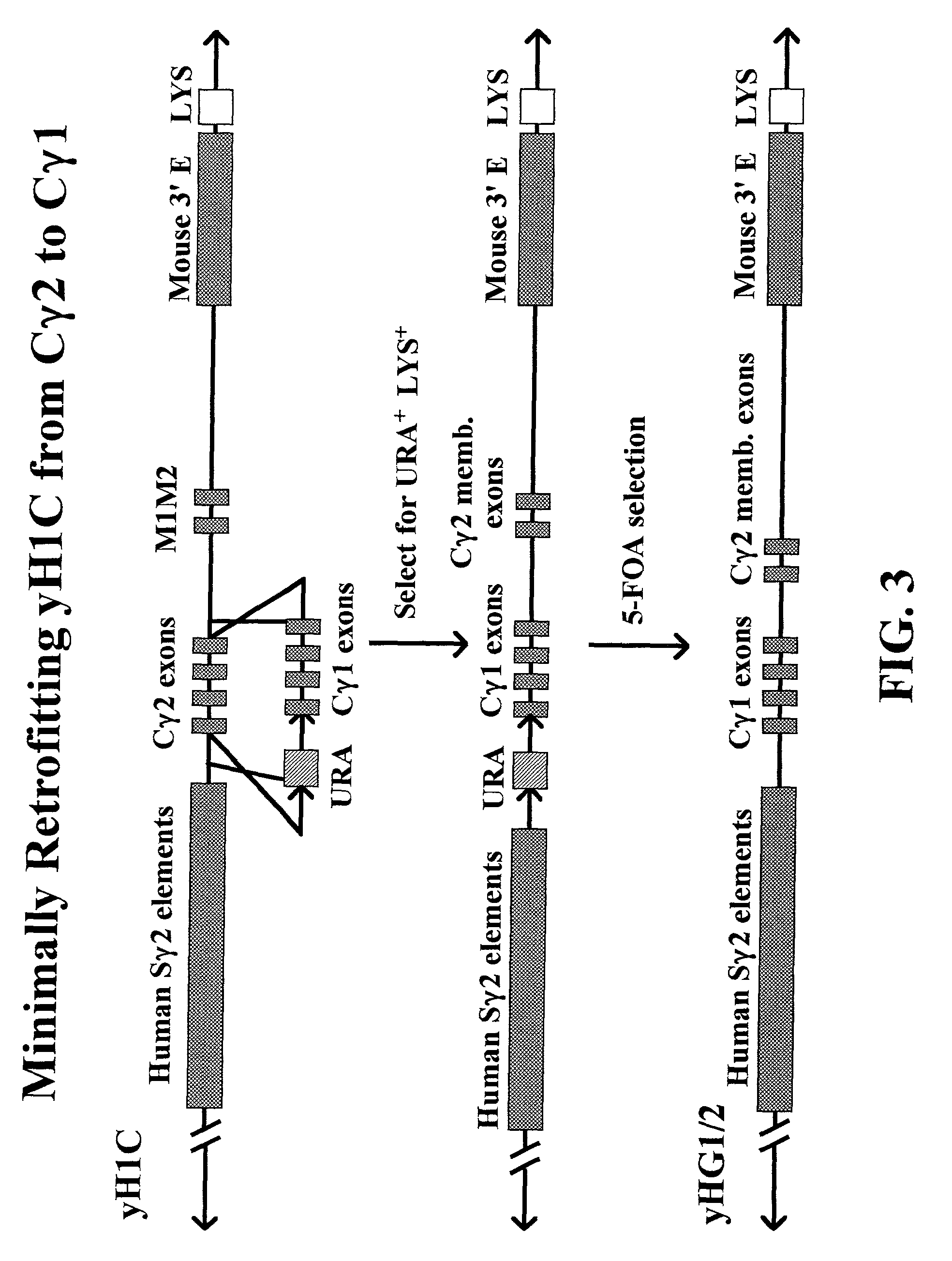
Transgenic animals for producing specific isotypes of human antibodies via non-cognate switch regions
InactiveUS7049426B2Immunoglobulins against cytokines/lymphokines/interferonsImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenExon
The present invention provides fully human antibodies in a transgenic animal of a desired isotype in response to immunization with any virtually any desired antigen. The human immunoglobulin heavy chain transgene in the foregoing animals comprises a human constant region gene segment comprising exons encoding the desired heavy chain isotype, operably linked to switch segments from a constant region of a different heavy chain isotype, i.e., a non-cognate switch region. Said additional constant region segment comprises a switch region and human constant region coding segment, wherein the constant region coding segment is operably linked to a switch region that it is not normally associated with, i.e., a non-cognate switch region. In the transgenes of the invention, the non-cognate switch region may be a switch region from a different species than the constant region coding segment. The switch region and membrane exons of the invention may comprise a human gamma-2 constant region and the secreted constant region exons are from a human gamma-1 or a human gamma-4 constant region.
Owner:ABQENIX INC
Cellular compositions and methods of making and using them
InactiveUS20050074435A1Increase the number ofEasy to collectPeptide/protein ingredientsDrug screeningProgenitorHematopoietic cell
The invention relates to cellular compositions comprising hematopoietic cells with the potential or increased potential to form non-hematopoietic cells; methods for producing such cellular compositions; methods for differeentitation of cells of cellular compositions of the invention into cells that exhibit morphological, physiological, functional, and / or immunological features of non-hematopoietic cells; and uses of the cellular compositions. The invention also relates to a method for the expansion of hemtopoietic stem and progenitor cells.
Owner:MOUNT SINAI HOSPITAL
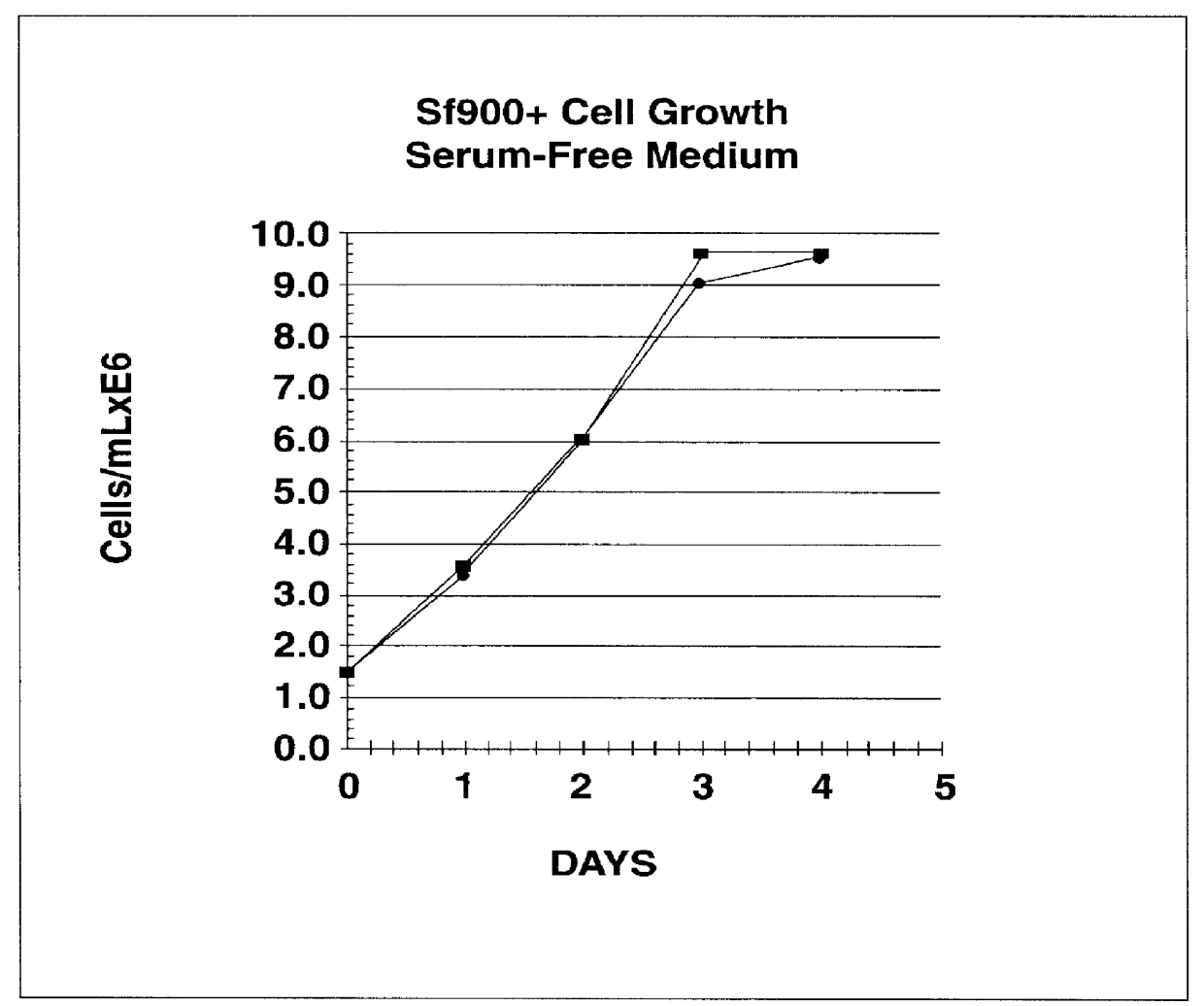
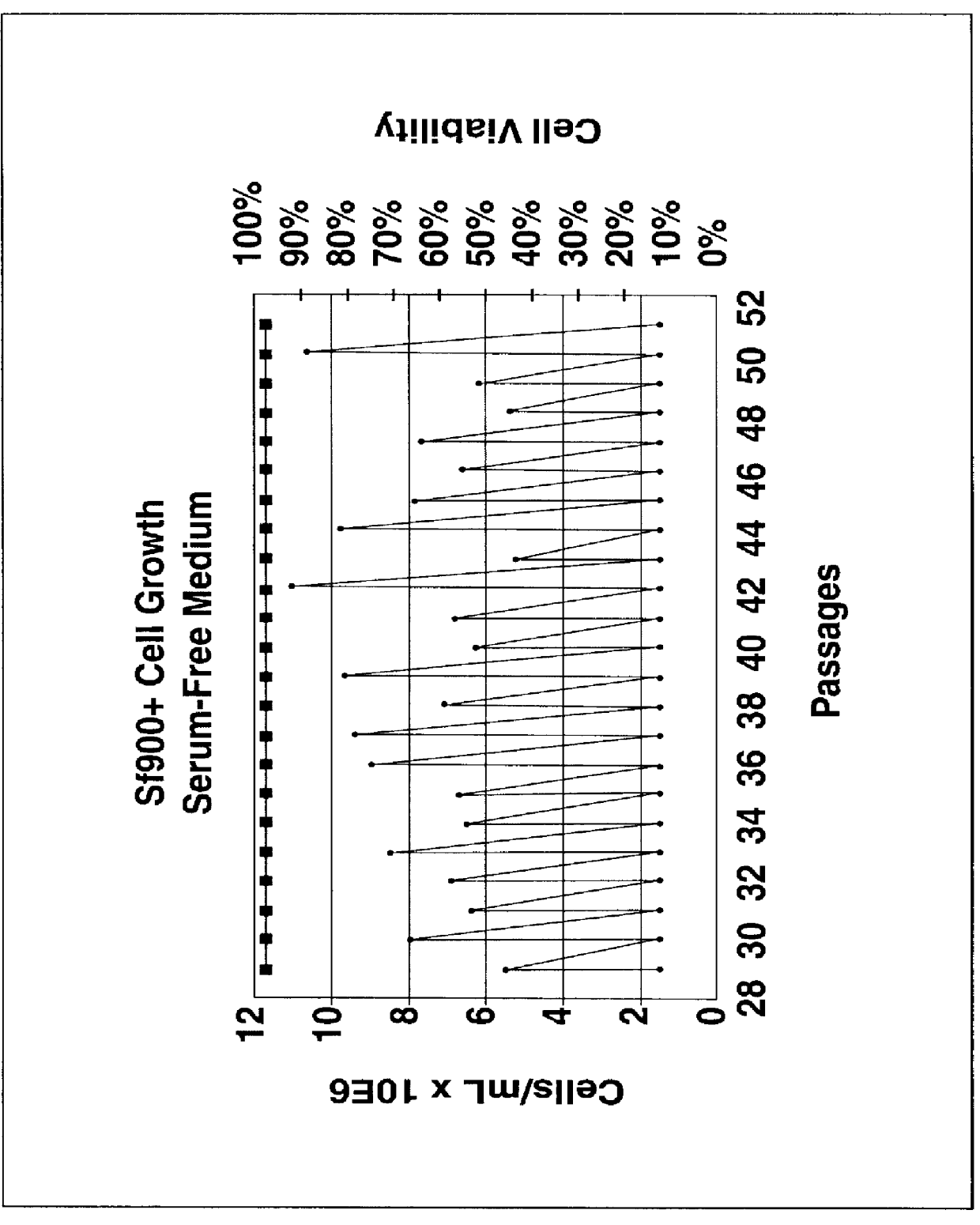
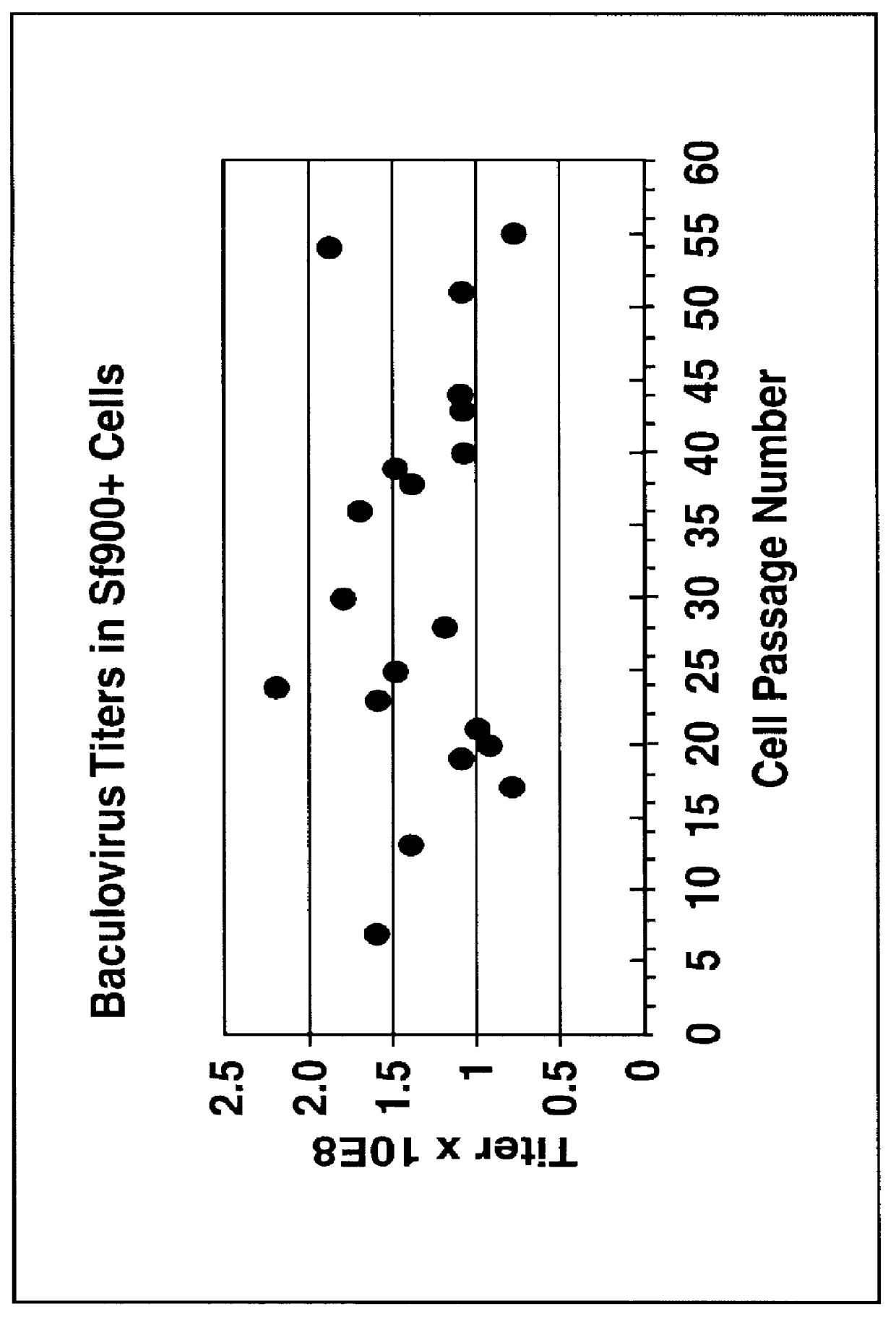
Spodoptera frugiperda single cell suspension cell line in serum-free media, methods of producing and using
InactiveUS6103526AAvoid infectionHigh densityConnective tissue peptidesInvertebrate cellsSerum free mediaAdjuvant
Disclosed and claimed is a new insect cell line, Sf900+, ATCC CRL-12579. The insect cell line was established from Lepidoptera, Noctuidae, Spodoptera frugiperda Sf-9 (ATCC CRL-1711) through multiple rounds of limiting dilution and selection in a serum-free insect medium supplemented with added human insulin. The insect cell line is useful in BEVS or as an adjuvant and has many characteristics and advantages. Also disclosed and claimed are recombinant proteins from recombinant baculovirus expression in insect cells such as Sf900+ cells, for instance, HA, NA, EPO, CD4, CEA, and thrombospondin.
Owner:PROTEIN SCI
CICM cells and non-human mammalian embryos prepared by nuclear transfer of a proliferating differentiated cell or its nucleus
InactiveUS6235970B1Simple procedureSimplifying and facilitating procedureNervous disorderMuscular disorderPresent methodNuclear transfer
An improved method of nuclear transfer involving the transplantation of donor differentiated cell nuclei into enucleated oocytes of the same species as the donor cell is provided. The resultant nuclear transfer units are useful for multiplication of genotypes and transgenic genotypes by the production of fetuses and offspring, and for production of isogenic CICM cells, including human isogenic embryonic or stem cells. Production of genetically engineered or transgenic mammalian embryos, fetuses and offspring is facilitated by the present method since the differentiated cell source of the donor nuclei can be genetically modified and clonally propagated.
Owner:UNIVERSITY OF MASSACHUSETTS AMHERST
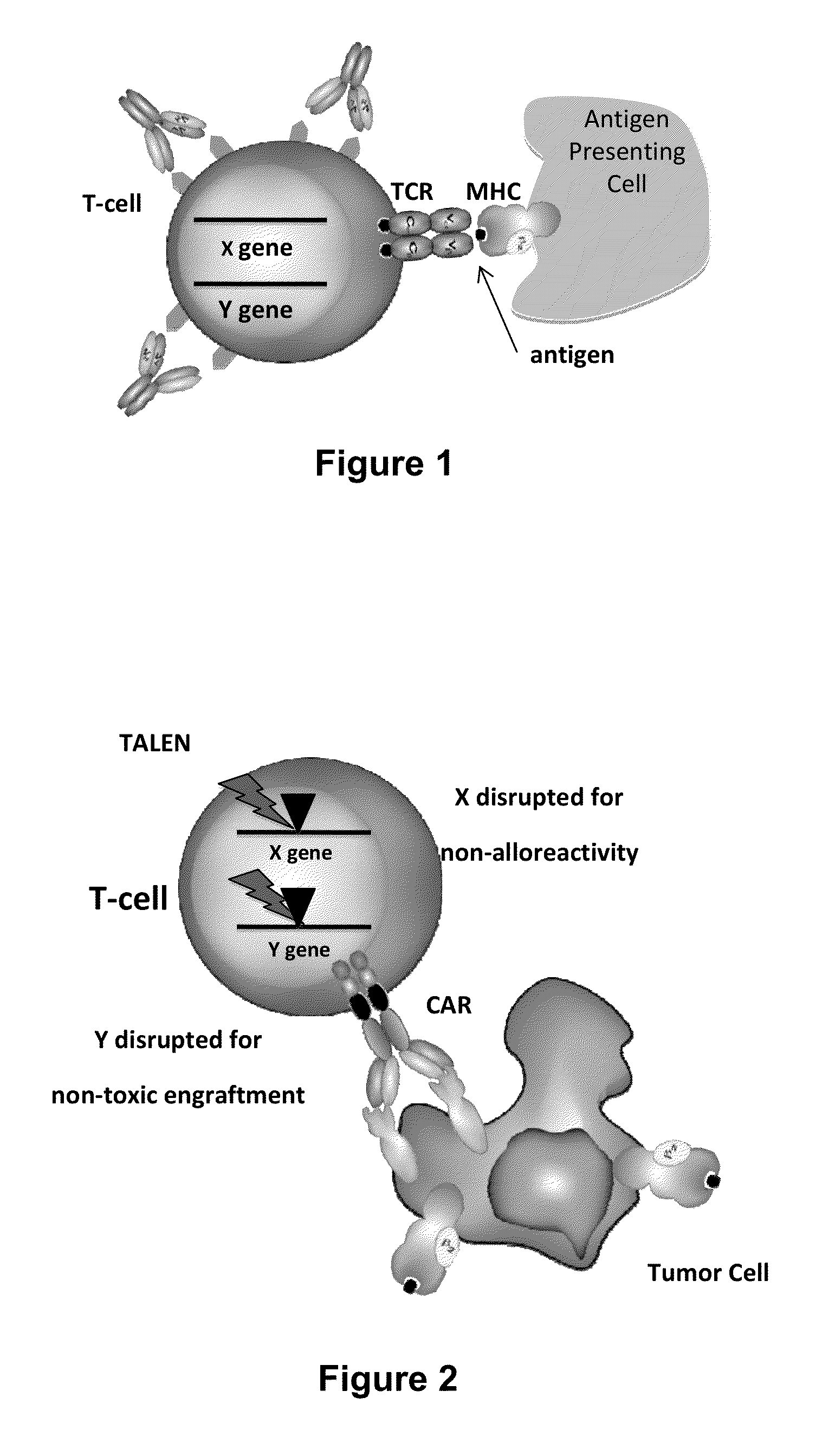
Methods for engineering allogeneic and immunosuppressive resistant t cell for immunotherapy
ActiveUS20130315884A1Precise positioningPeptide/protein ingredientsAntibody mimetics/scaffoldsImmunosuppressive drugPrimary cell
Methods for developing engineered T-cells for immunotherapy that are both non-alloreactive and resistant to immunosuppressive drugs. The present invention relates to methods for modifying T-cells by inactivating both genes encoding target for an immunosuppressive agent and T-cell receptor, in particular genes encoding CD52 and TCR. This method involves the use of specific rare cutting endonucleases, in particular TALE-nucleases (TAL effector endonuclease) and polynucleotides encoding such polypeptides, to precisely target a selection of key genes in T-cells, which are available from donors or from culture of primary cells. The invention opens the way to standard and affordable adoptive immunotherapy strategies for treating cancer and viral infections.
Owner:CELLECTIS SA
Use of RNA for reprogramming somatic cells
InactiveUS20110065103A1Promotes reprogrammingBlood/immune system cellsDrug compositionsReprogrammingSomatic cell
The present invention provides methods for de-differentiating somatic cells into stem-like cells without generating embryos or fetuses. More specifically, the present invention provides methods for effecting the de-differentiation of somatic cells to cells having stem cell characteristics, in particular pluripotency, by introducing RNA encoding factors inducing the de-differentiation of somatic cells into the somatic cells and culturing the somatic cells allowing the cells to de-differentiate.
Owner:BIONTECH AG +2
Somatic cell reprogramming
ActiveUS20080233610A1High nucleus to cytoplasm ratioImprove effectivenessNervous disorderVirusesSomatic cellCell biology
The present invention relates to methods for reprogramming a somatic cell to pluripotency by administering into the somatic cell at least one or a plurality of potency-determining factors. The invention also relates to pluripotent cell populations obtained using a reprogramming method.
Owner:WISCONSIN ALUMNI RES FOUND
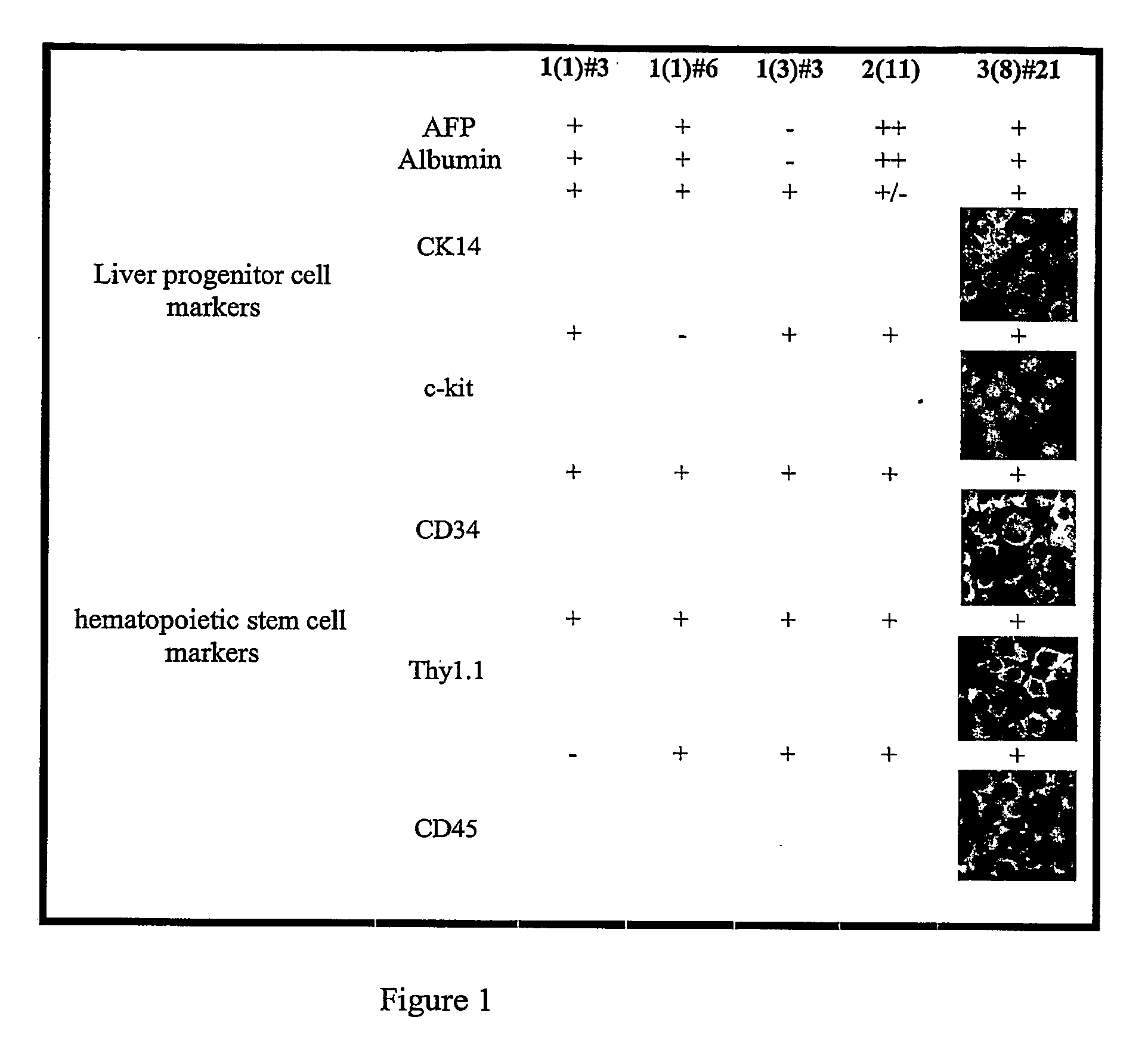
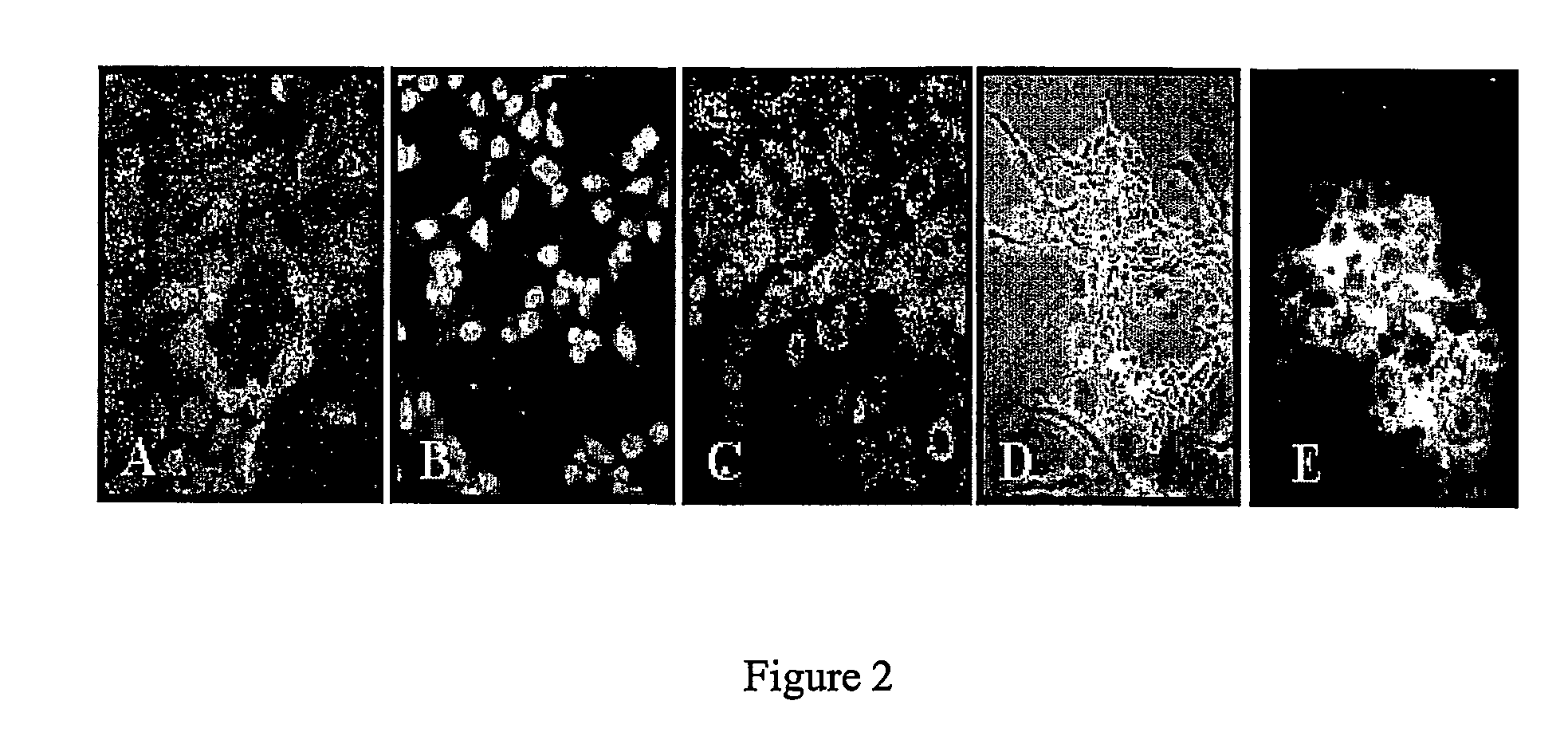
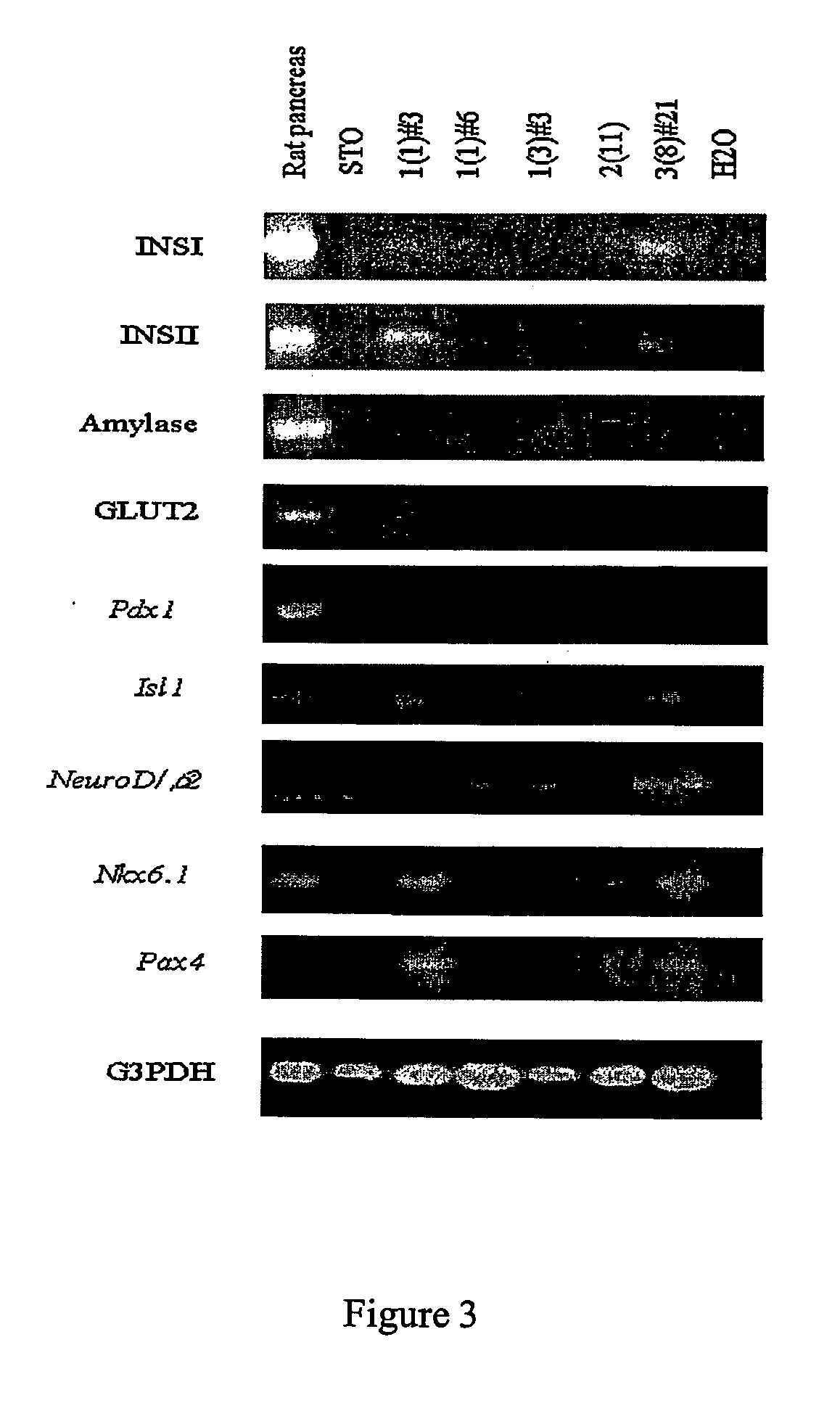
Conversion of liver stem and progenitor cells to pancreatic functional cells
The subject invention a method for converting liver stem / progenitor cells to a pancreatic functional cell by transfecting said liver cells with a pancreatic development gene and / or by culturing with pancreatic differentiation factors. The resulting cells produce and secrete insulin protein in response to glucose stimulation.
Owner:IXION BIOTECH
Chimeric receptors with 4-1bb stimulatory signaling domain
InactiveUS20130266551A1Provide immunitySpecific and vigorous preferential expansionBiocideAntibody mimetics/scaffoldsChimeric antigen receptorBiological activation
The present invention relates to a chimeric receptor capable of signaling both a primary and a co-stimulatory pathway, thus allowing activation of the co-stimulatory pathway without binding to the natural ligand. The cytoplasmic domain of the receptor contains a portion of the 4-1BB signaling domain. Embodiments of the invention relate to polynucleotides that encode the receptor, vectors and host cells encoding a chimeric receptor, particularly including T cells and natural killer (NK) cells and methods of use.
Owner:ST JUDE CHILDRENS RES HOSPITAL INC
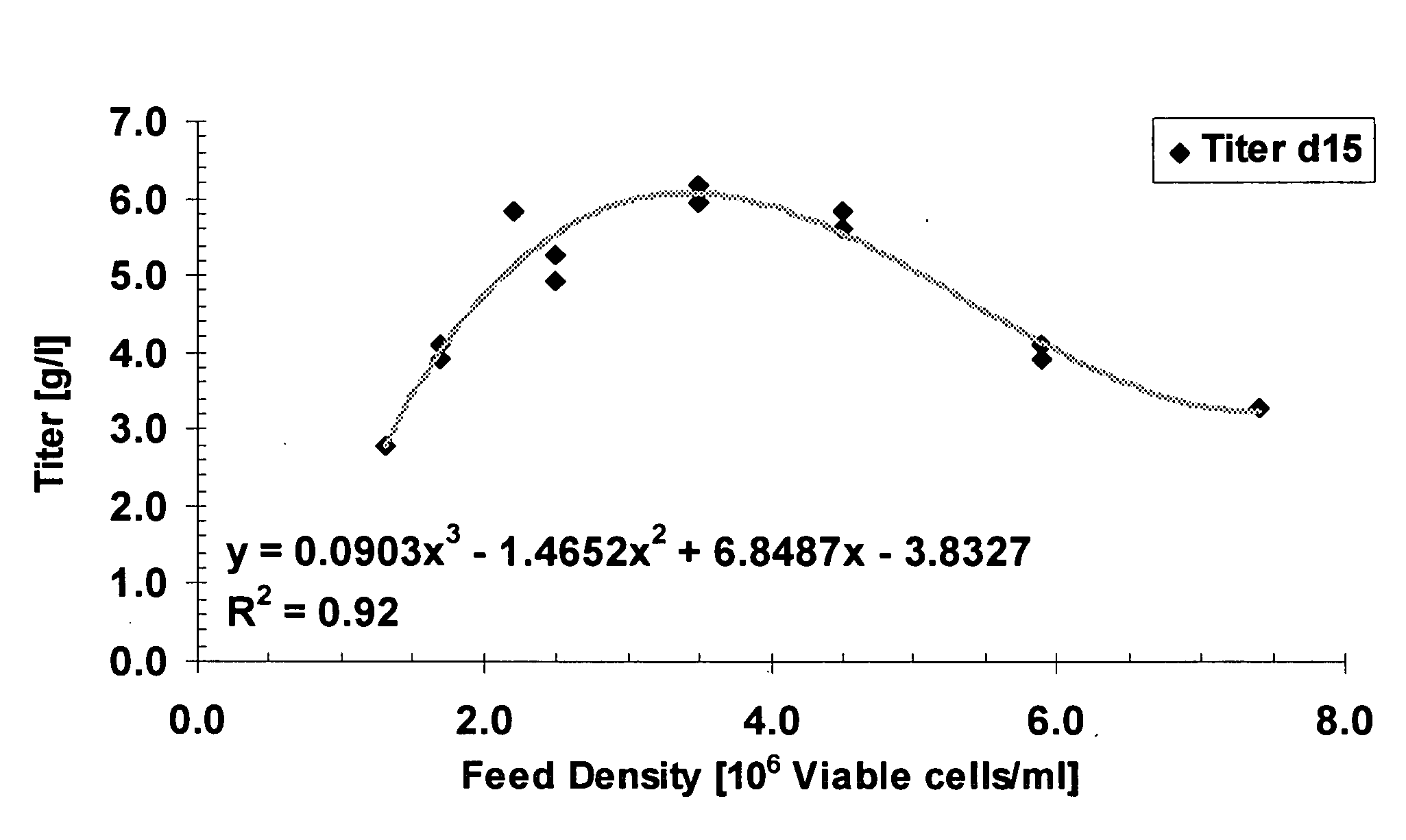
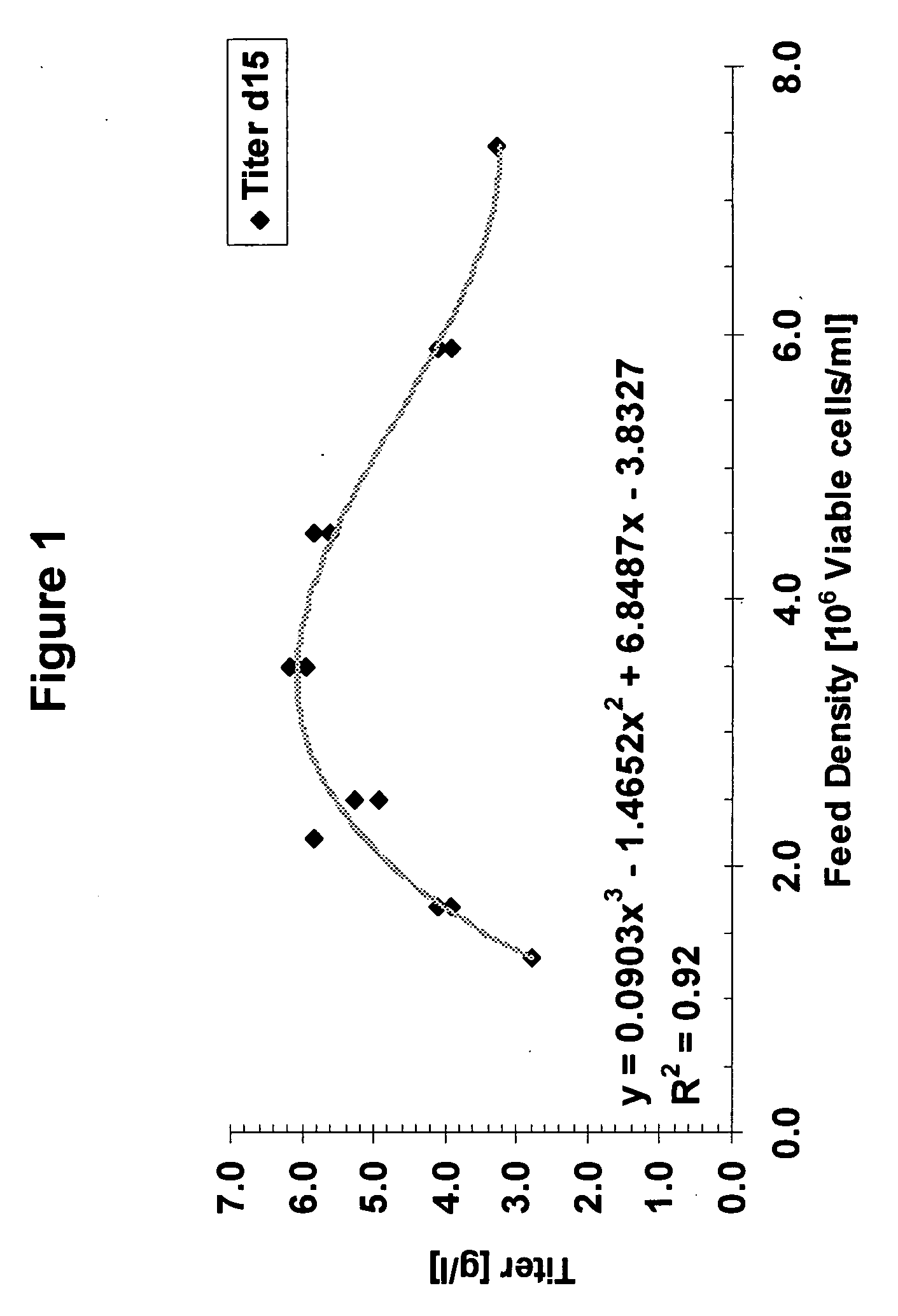
Cell culture improvements
ActiveUS20080227136A1Improve productivityImproved cell culture longevityGenetically modified cellsMicrobiological testing/measurementCell culture mediaImproved method
The invention describes improved methods and compositions for producing a recombinant protein, e.g., an antibody, in mammalian cell culture. In addition, the invention provides improved cell culture media, including improved production media, feed solutions, and combination feeds, which may be used to improve protein productivity in mammalian cell culture.
Owner:ABBVIE INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com