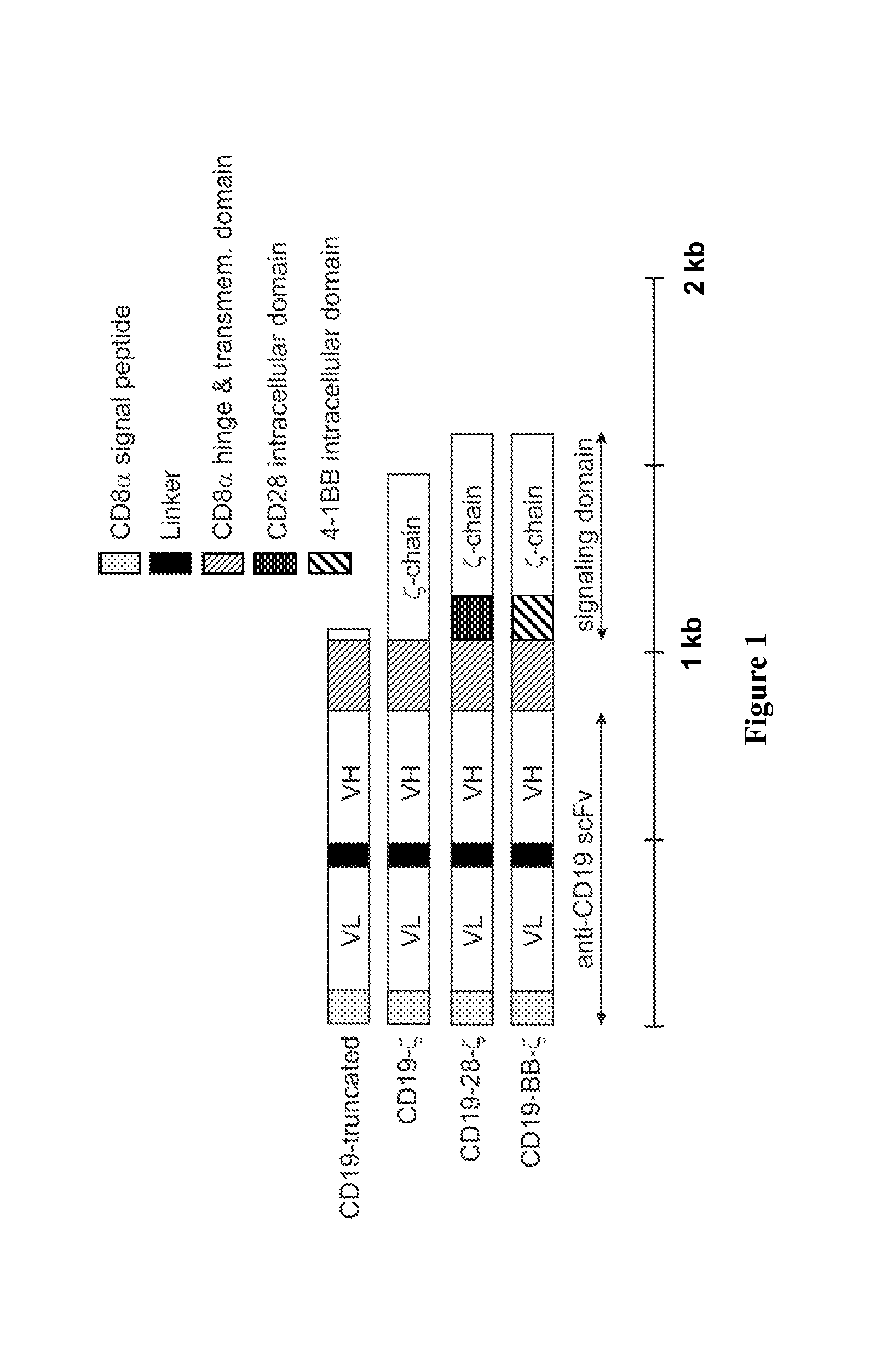
Chimeric receptors with 4-1bb stimulatory signaling domain
a chimeric receptor and stimulatory signaling technology, applied in the field of chimeric cell membrane receptors, can solve the problems of insufficient engagement of the t-cell receptor (tcr) alone to induce persistent activation of resting naive or memory t cells, failure to activate cells, and state of non-responsiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
9.1 Example 1
Introduction
[0101]In approximately 20% of children and 65% of adults with acute lymphoblastic leukemia (ALL), drug-resistant leukemic cells survive intensive chemotherapy and cause disease recurrence. [Pui C H et al, Childhood acute lymphoblastic leukemia—Current status and future perspectives. Lancet Oncology 2:597-607 (2001); Verma A, Stock W. Management of adult acute lymphoblastic leukemia: moving toward a risk-adapted approach. Curr Opin Oncol 13:14-20T (2001)] lymphocyte-based cell therapy should bypass cellular mechanisms of drug resistance. Its potential clinical value for leukemia is demonstrated by the association between T-cell-mediated graft-versus-host disease (GvHD) and delay or suppression of leukemia recurrence after allogeneic stem cell transplantation. [Champlin R. T-cell depletion to prevent graft-versus-host disease after bone marrow transplantation. Hematol Oncol Clin North Am 4:687-698 (1990); Porter D L, Antin J H. The graft-versus-leukemia effect...
example 2
9.2 Example 2
[0139]T lymphocytes transduced with anti-CD19 chimeric receptors have remarkable anti-ALL capacity in vitro and in vivo, suggesting the clinical testing of receptor-modified autologous T cells in patients with persistent minimal residual disease. However, the use of allogeneic receptor-modified T lymphocytes after hematopoietic cell transplantation (HCT) might carry the risk of severe graft-versus-host disease (GvHD). In this setting, the use of CD3-negative natural killer (NK) cells is attractive because they should not cause GvHD.
[0140]Spontaneous cytotoxicity of NK cells against ALL is weak, if measurable at all. To test whether anti-CD 19 chimeric receptors could enhance it, we developed methods to specifically expand human primary NK cells and induce high levels of receptor expression. Specific NK cell expansion has been problematic to achieve with established methods which favor CD3+ T cell expansion. Even after T-cell depletion, residual T cells typically become ...
example 3
9.3 Example 3
Artificial Antigen Producing Cells (APCs) Pave The Way For Clinical Application By Potent Primary In Vitro Induction
Materials And Methods
Cells
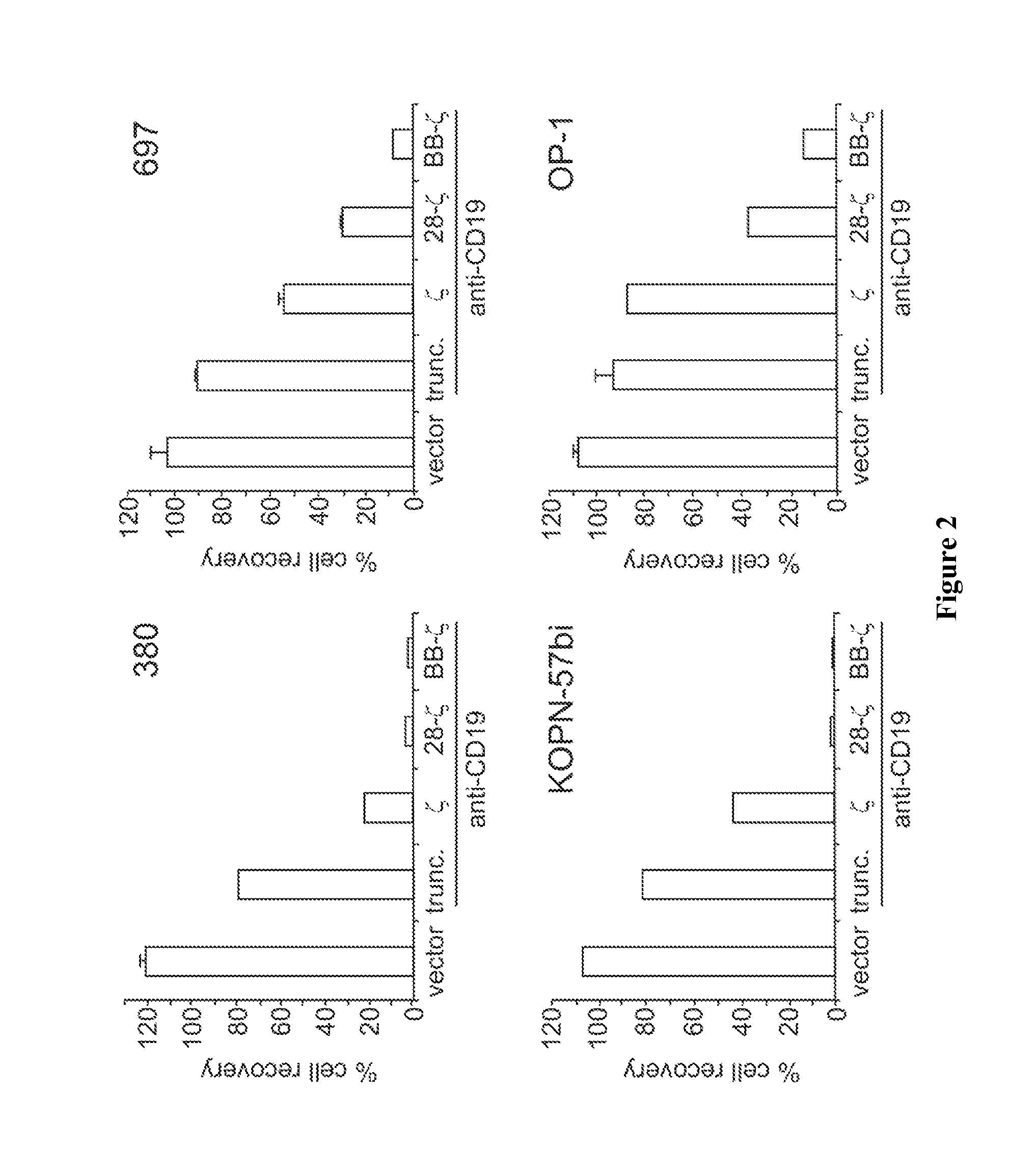
[0147]The CD 19 human B-lineage ALL cell lines RS4;11, OP-1, 380, 697, and KOPN57bi; the T-cell line GEM-C7; and the myeloid cell lines K562 and U-937 were available in our laboratory. Cells were maintained in RPMI-1640 (Gibco, Grand Island, N.Y.) supplemented with 10% fetal calf serum (FCS; BioWhittaker, Walkersville, Md.) and antibiotics.
[0148]Primary leukemia cells were obtained with appropriate informed consent and Institutional Review Board (M) approval from nine patients with B-lineage ALL; from four of these patients, we also studied (with IRB approval) cryopreserved peripheral blood samples obtained during clinical remission. An unequivocal diagnosis of B-lineage ALL was established by morphologic, cytochemical, and immunophenotypic criteria; in each case, more than 95% of the cells were positive for CD19. Peripheral blood...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Nucleic acid sequence | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com