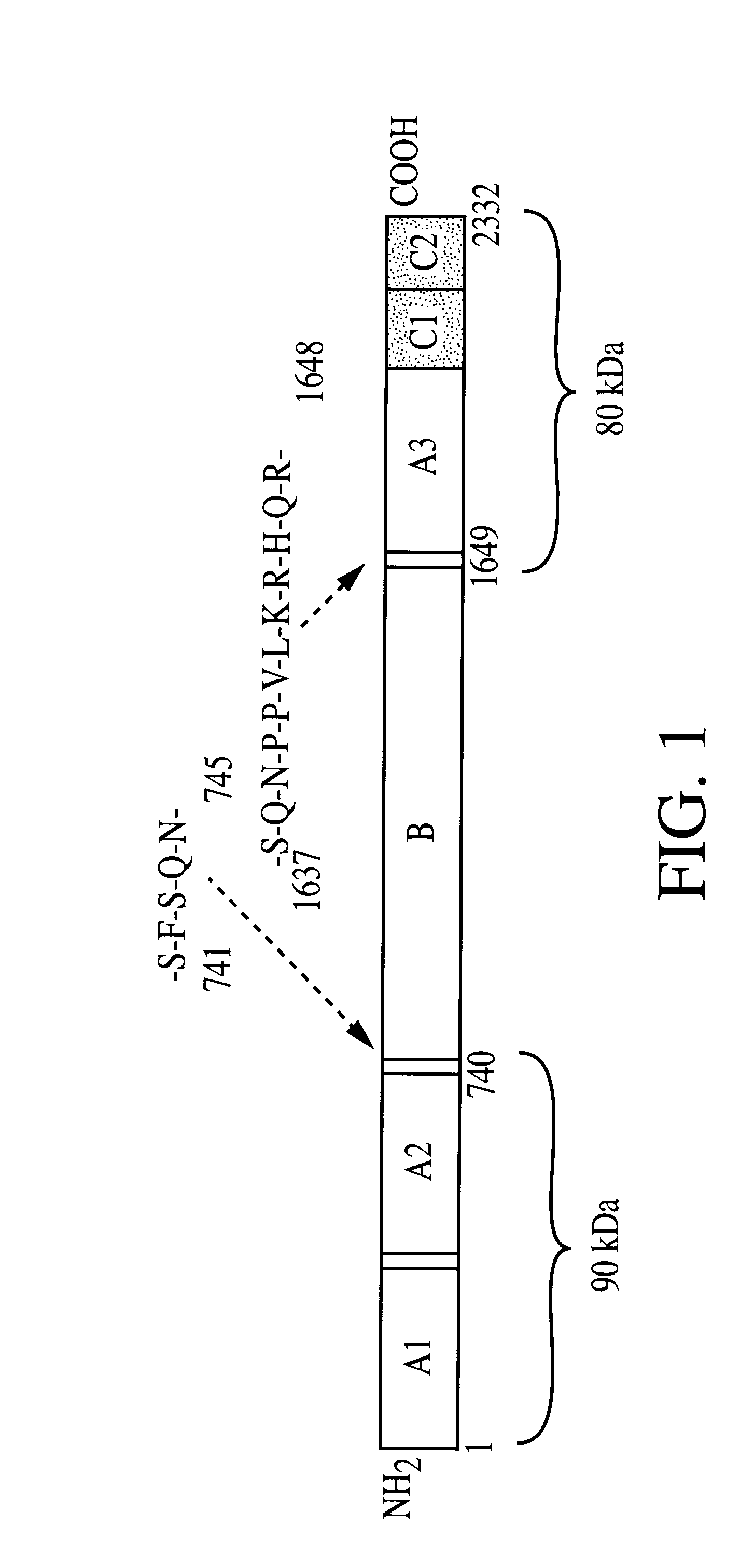
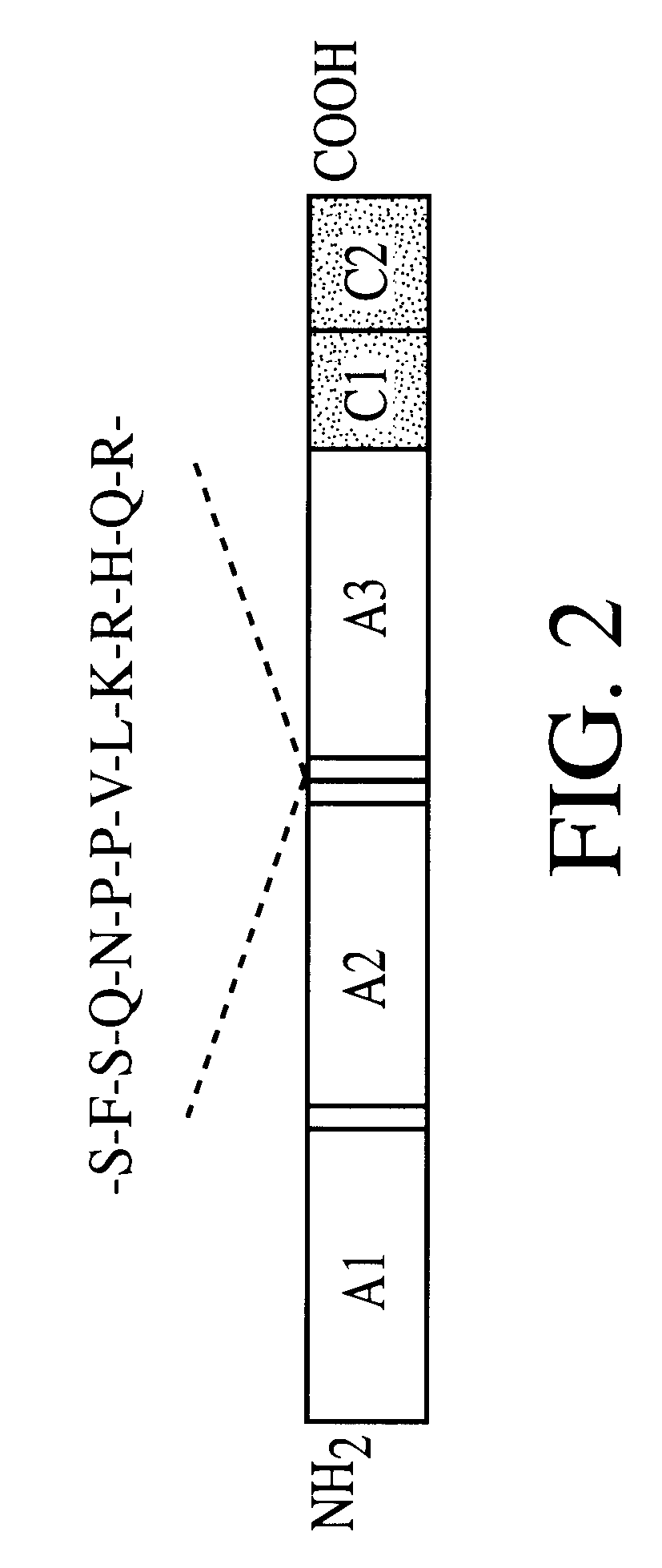
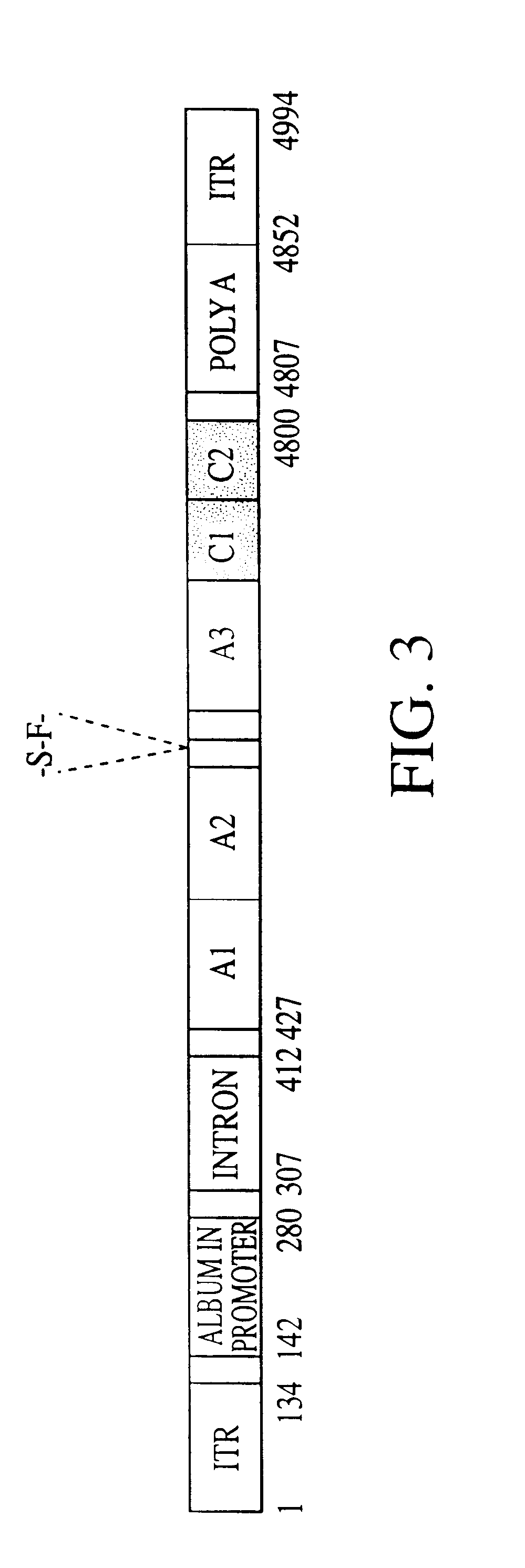
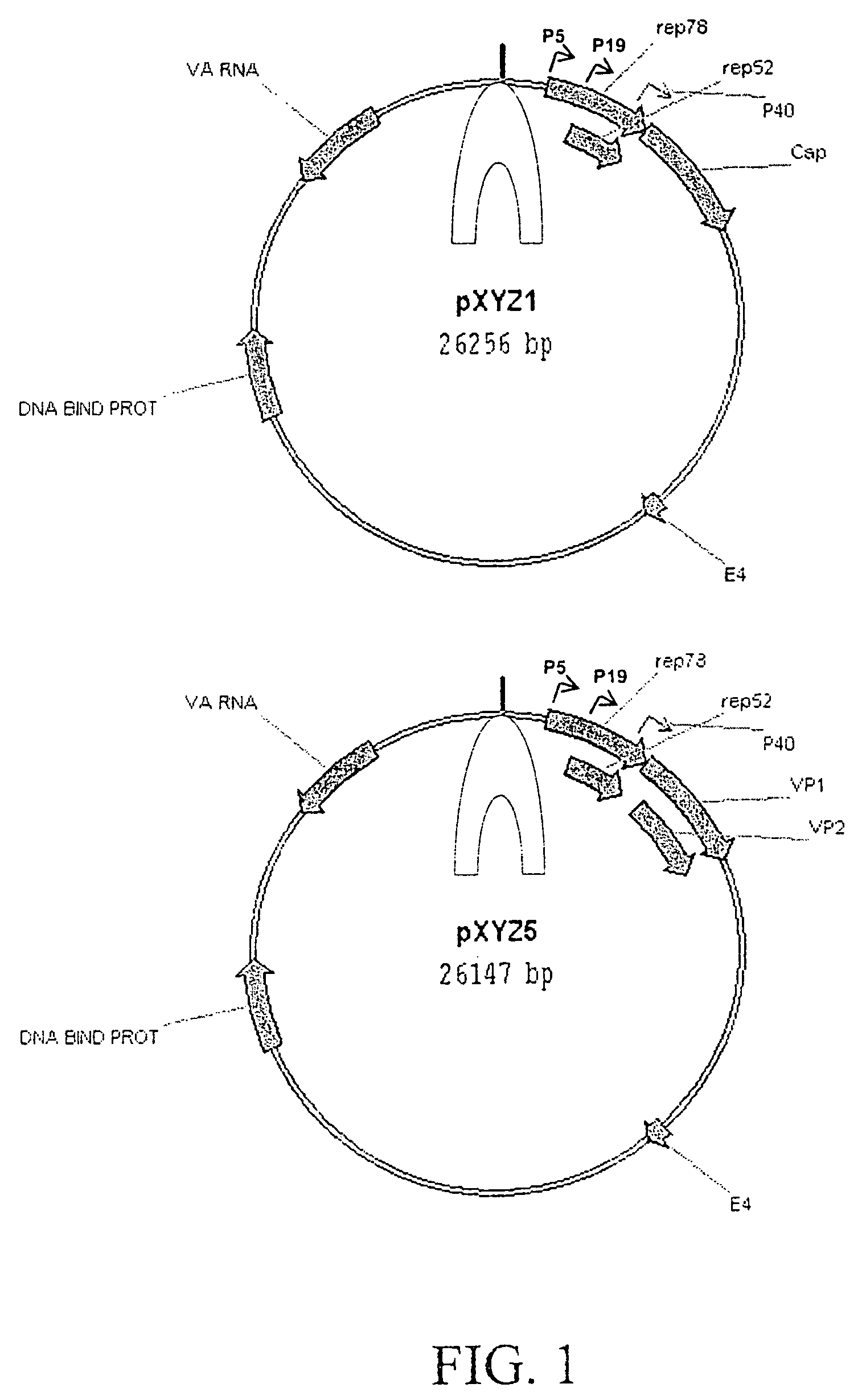
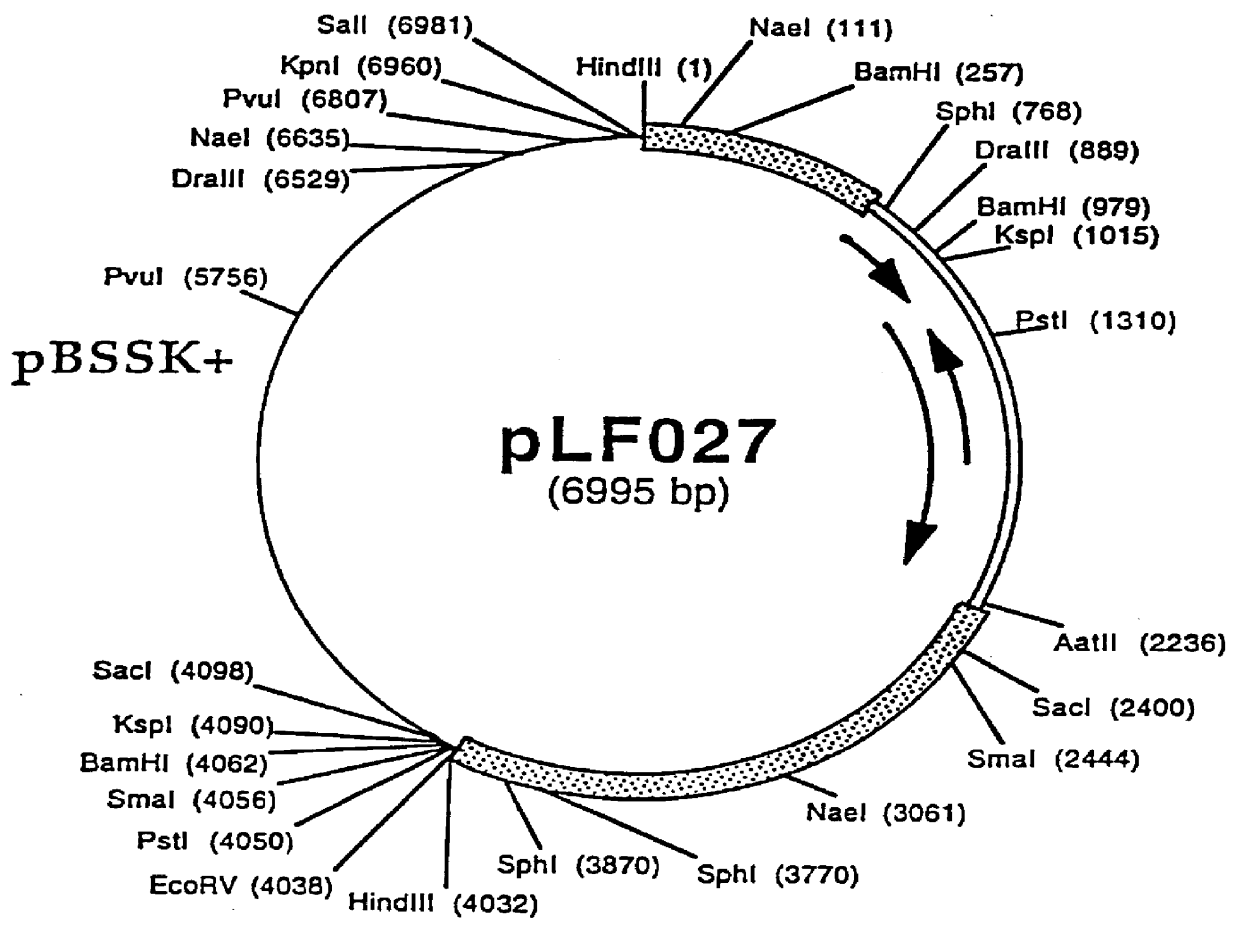
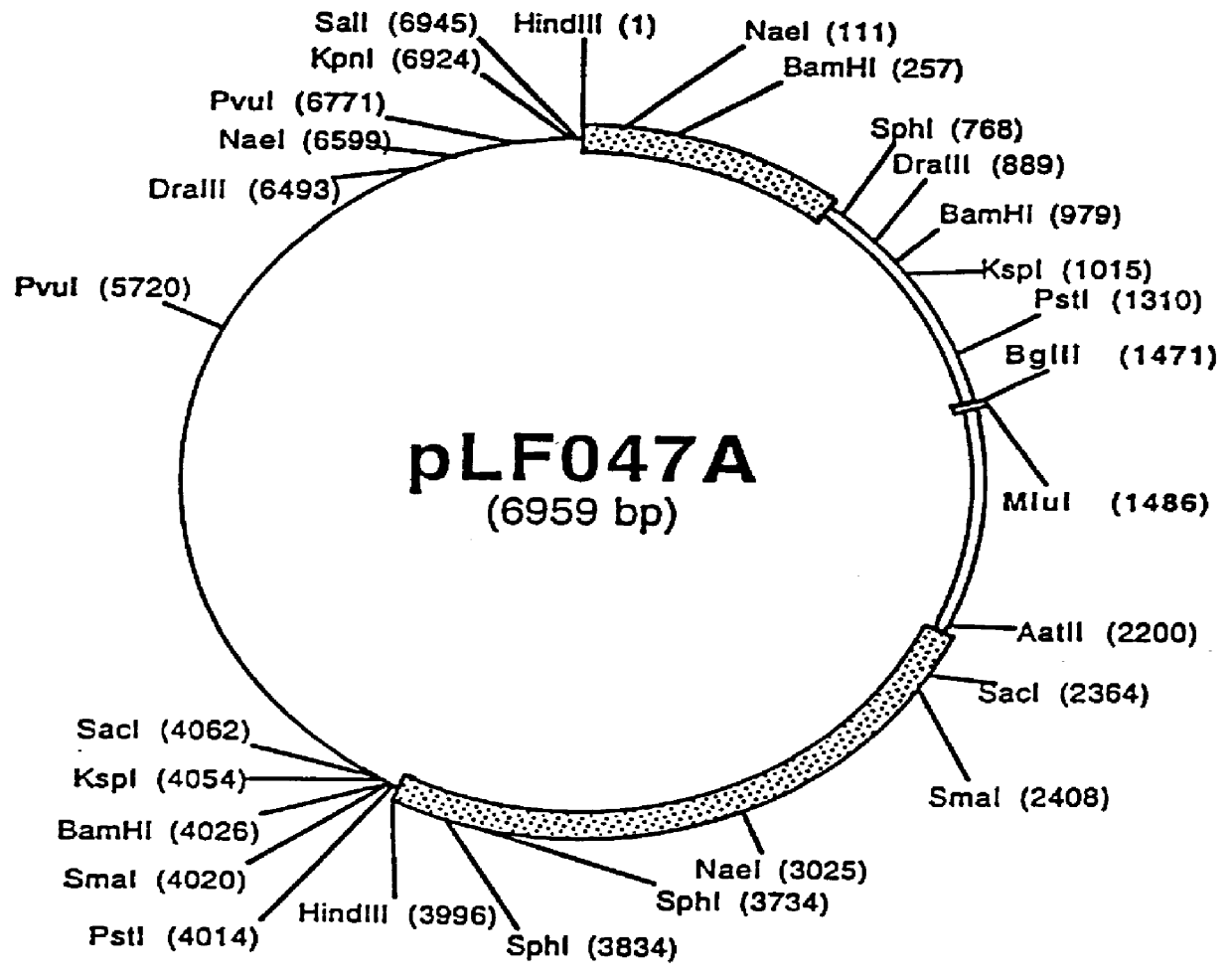
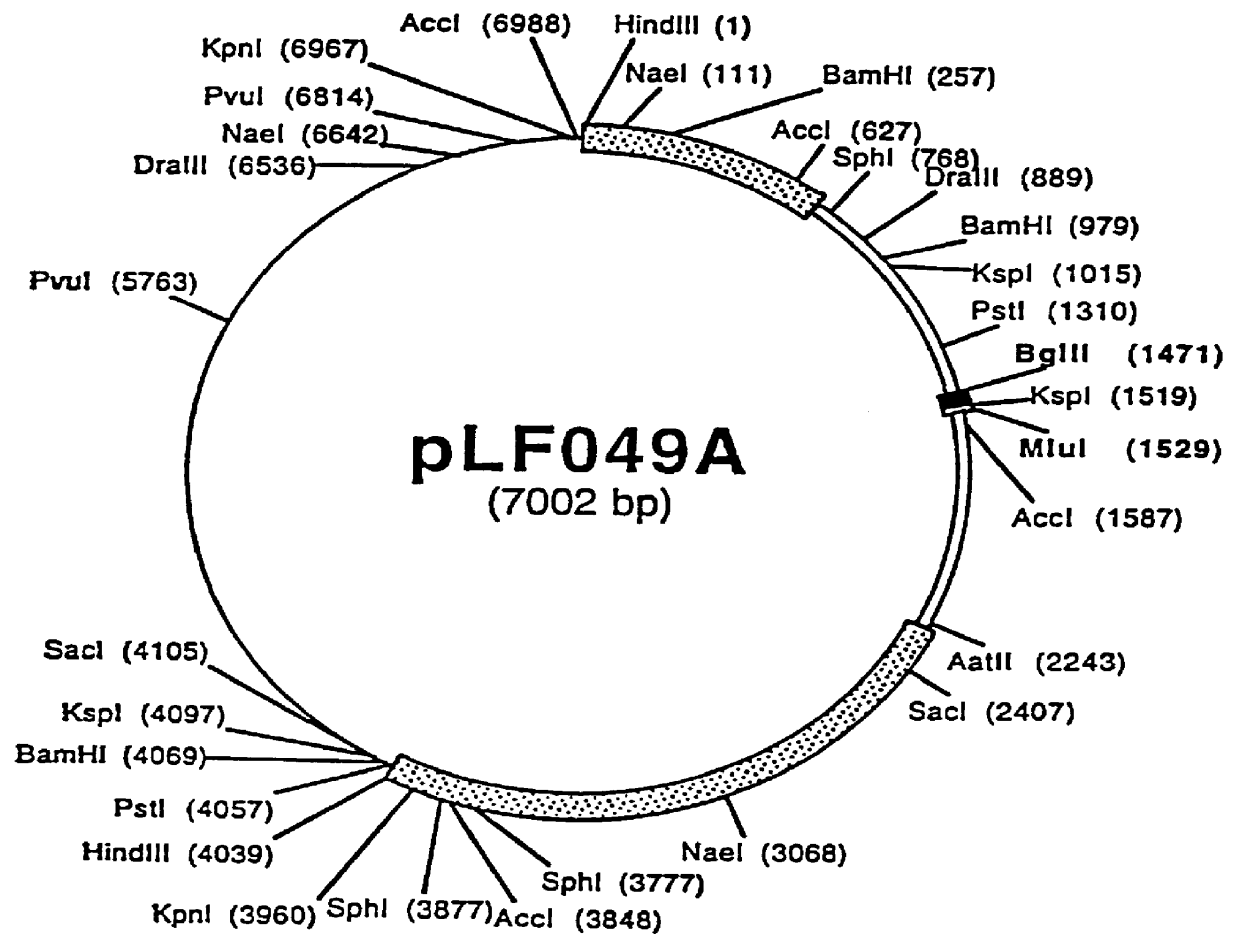
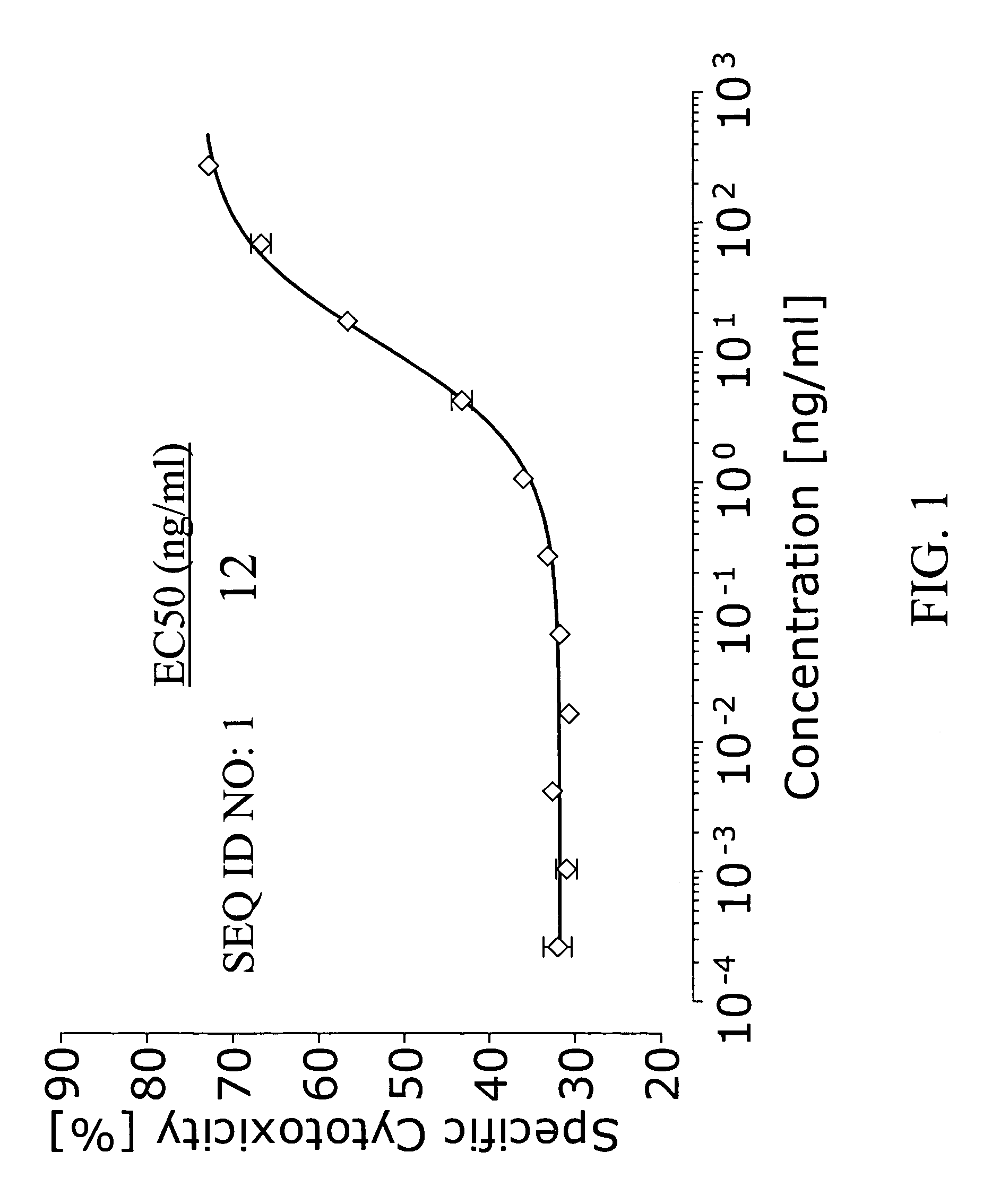
Patents
Literature
3938results about "Vectors" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
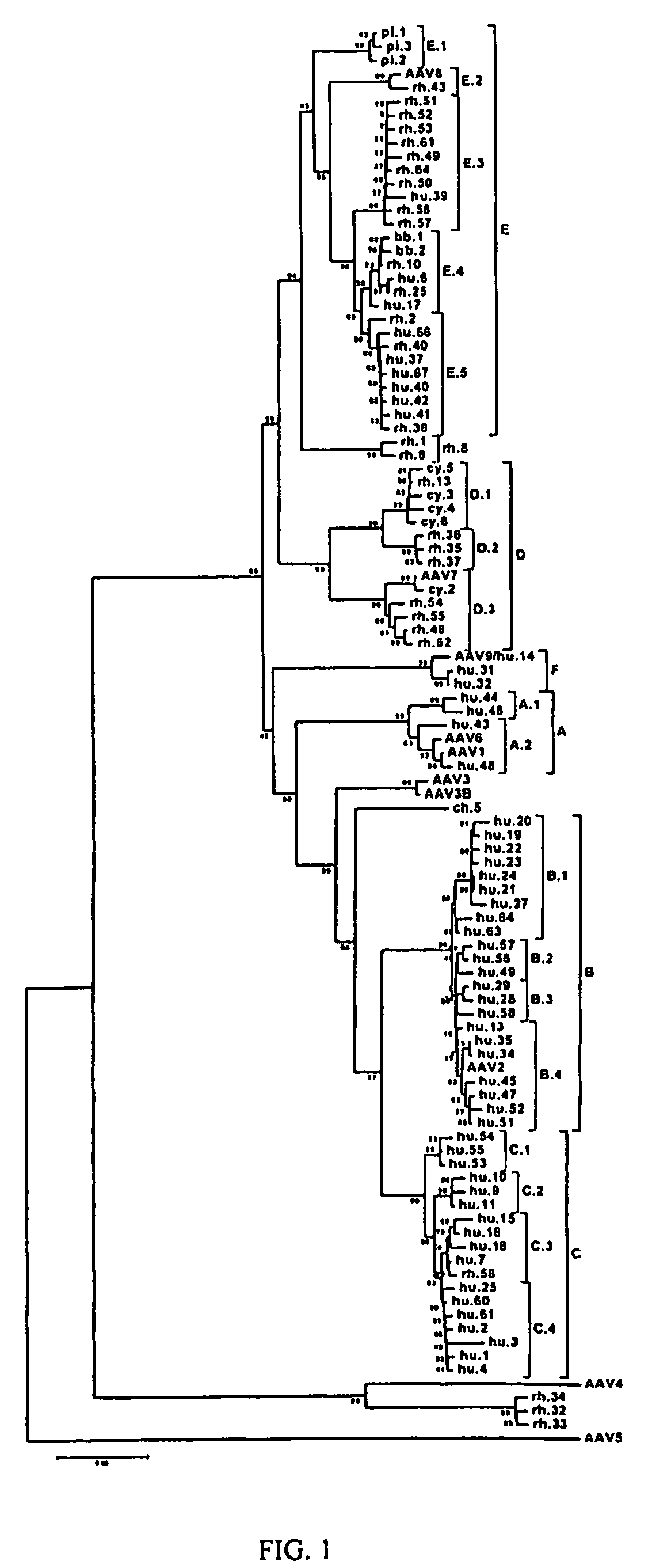
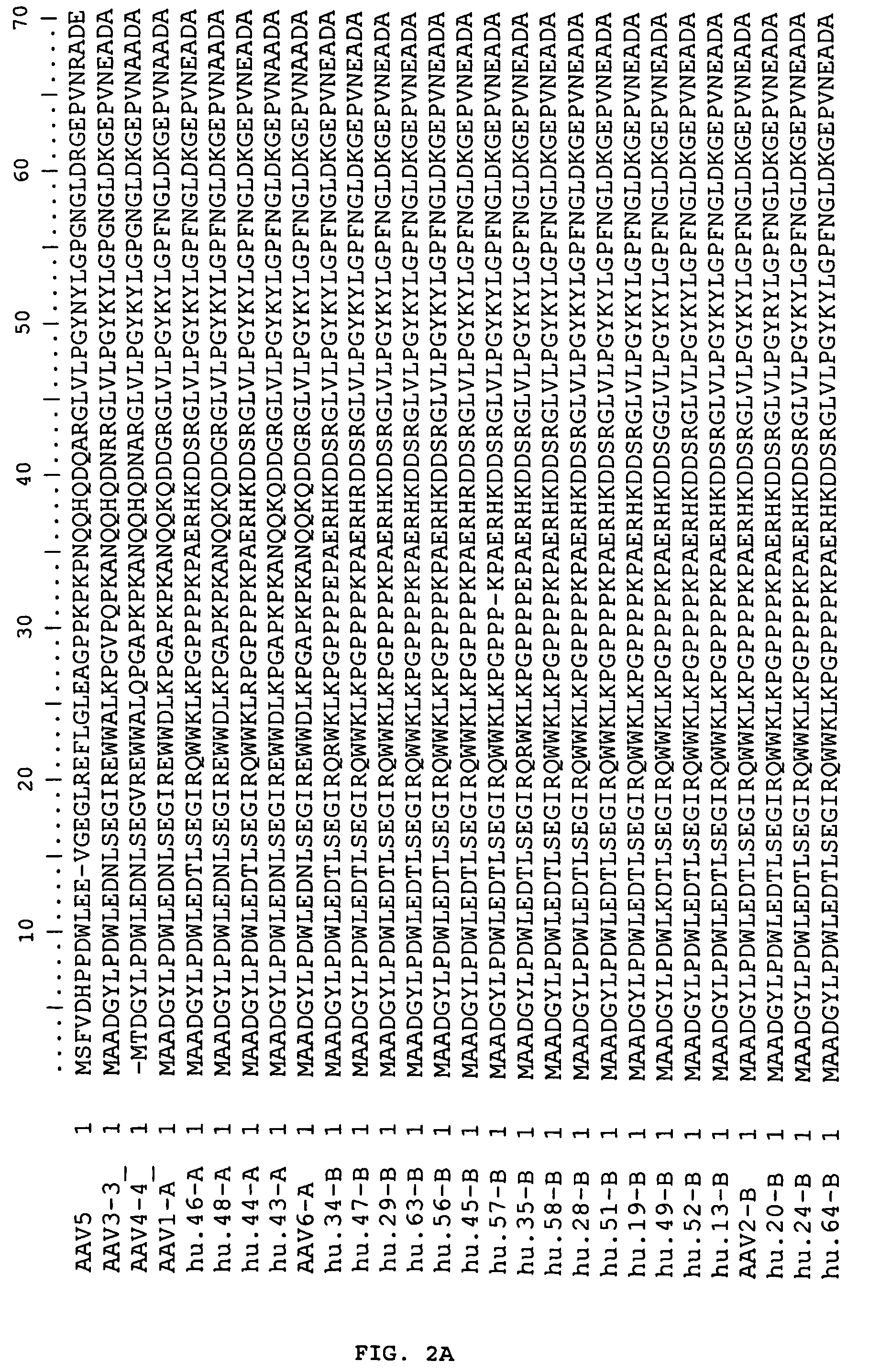
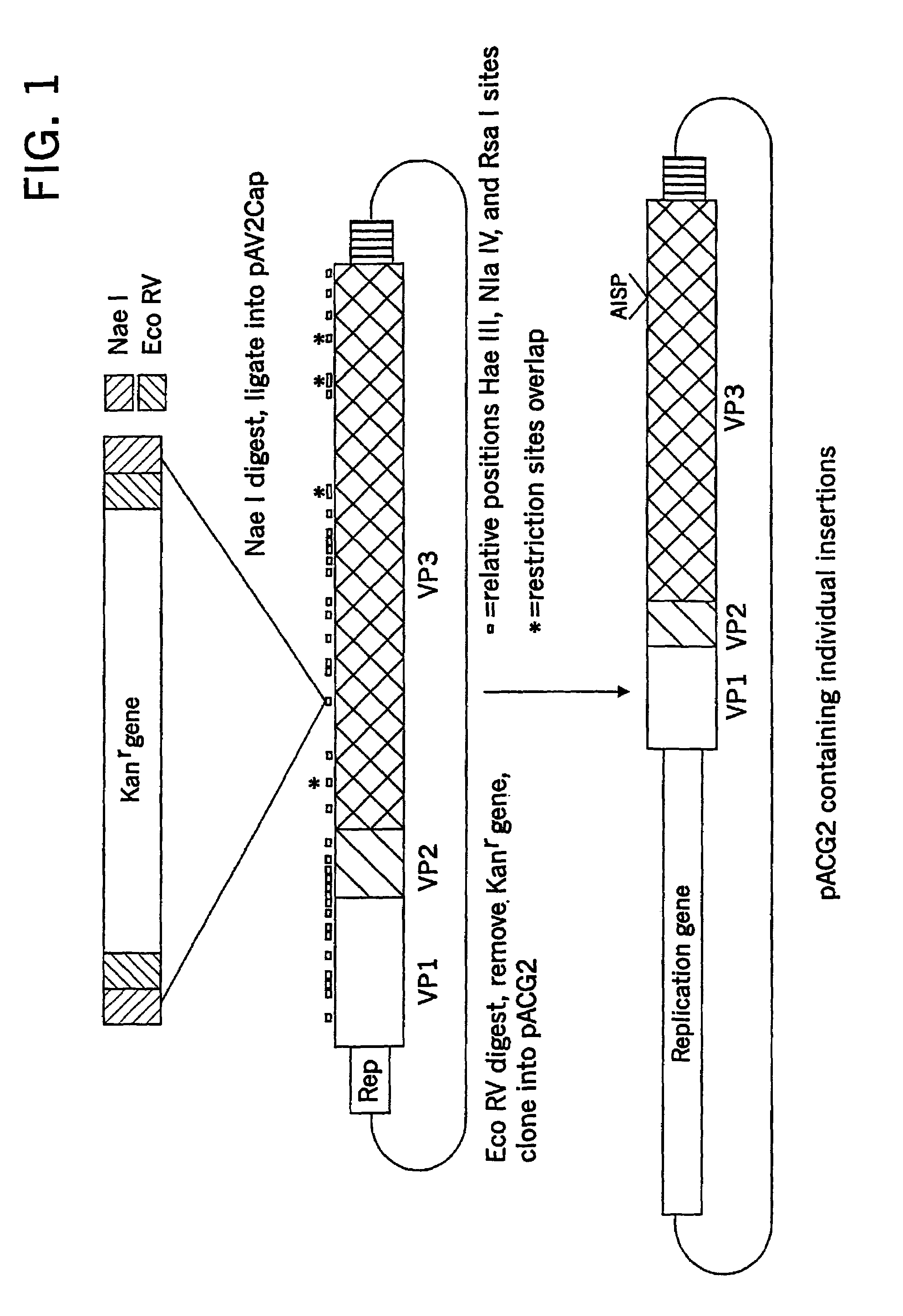
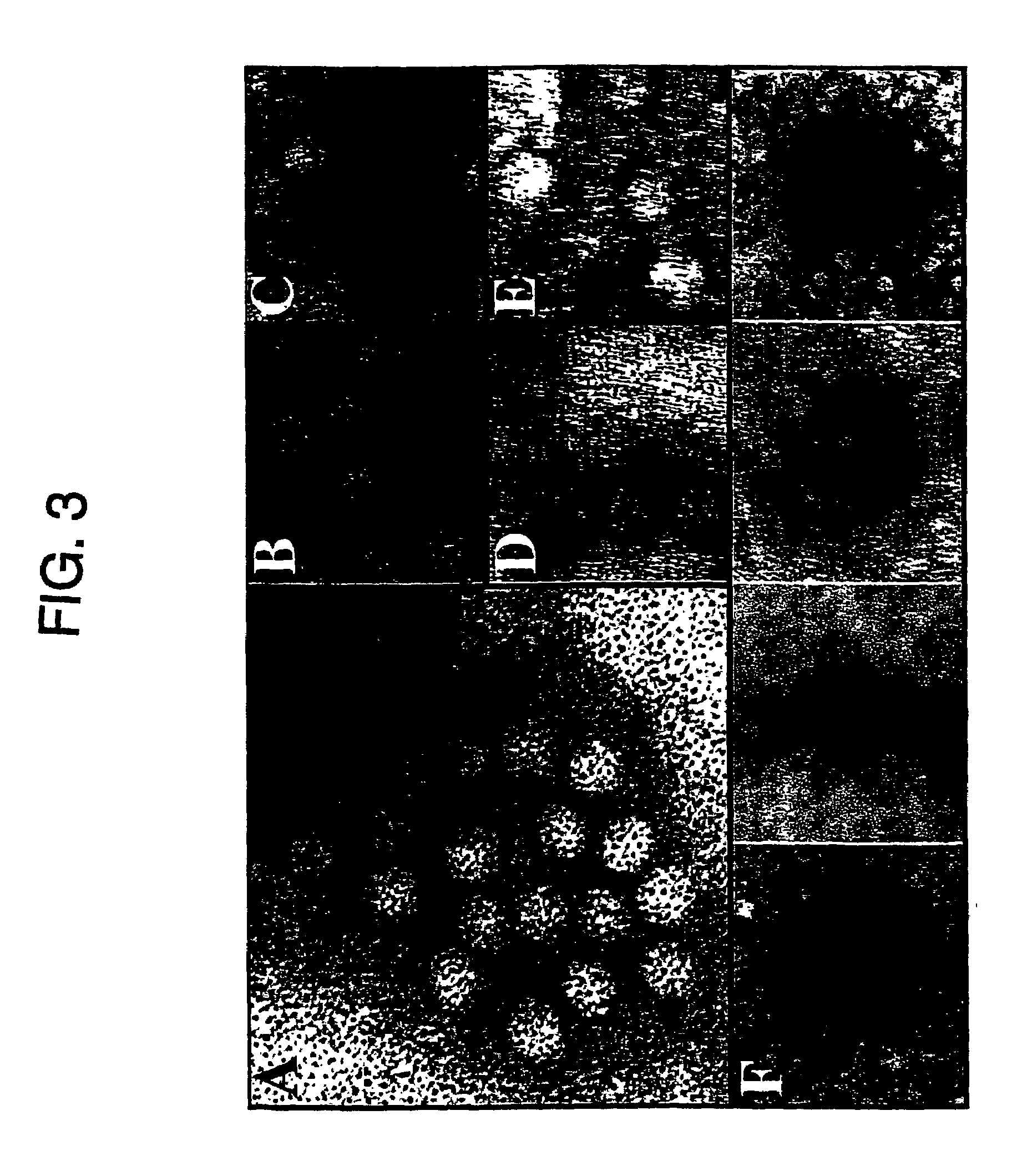
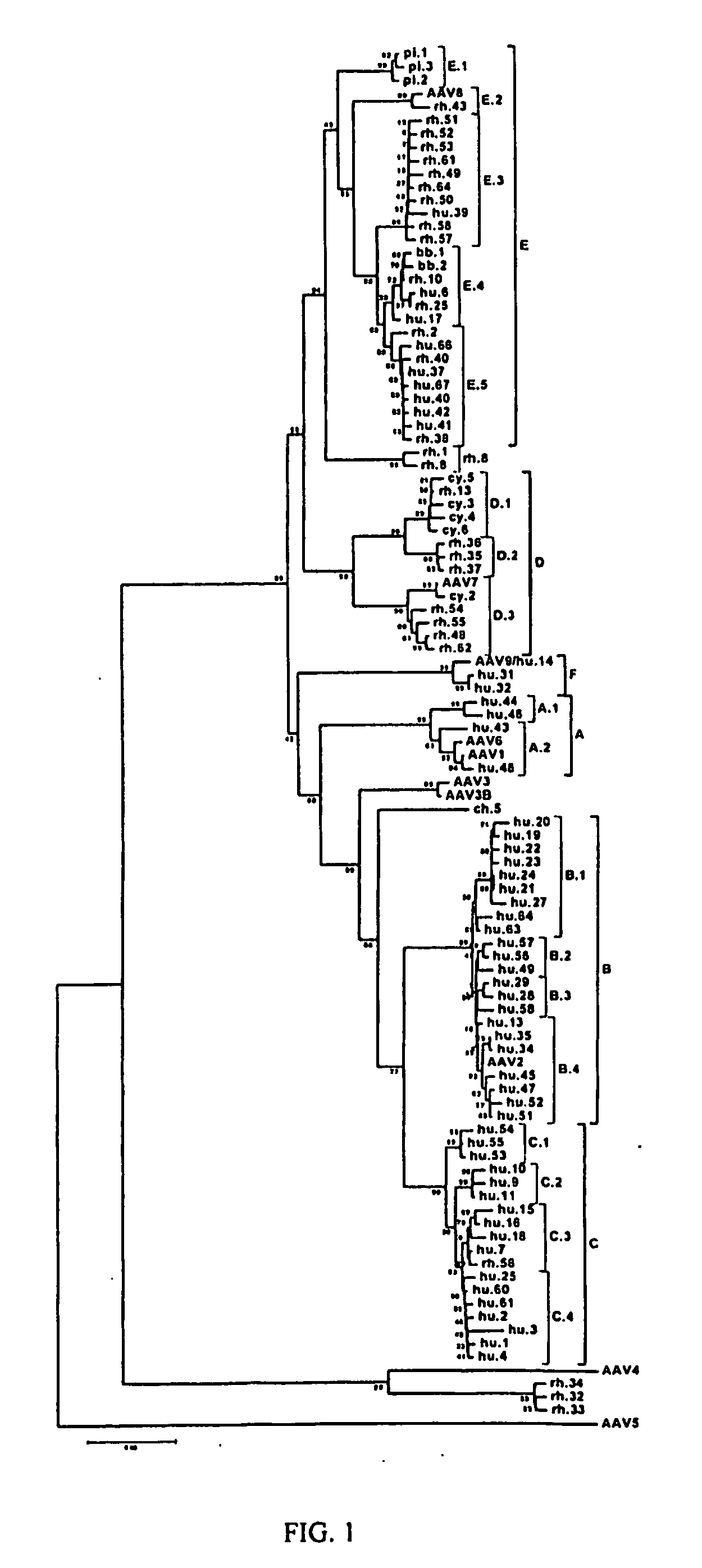
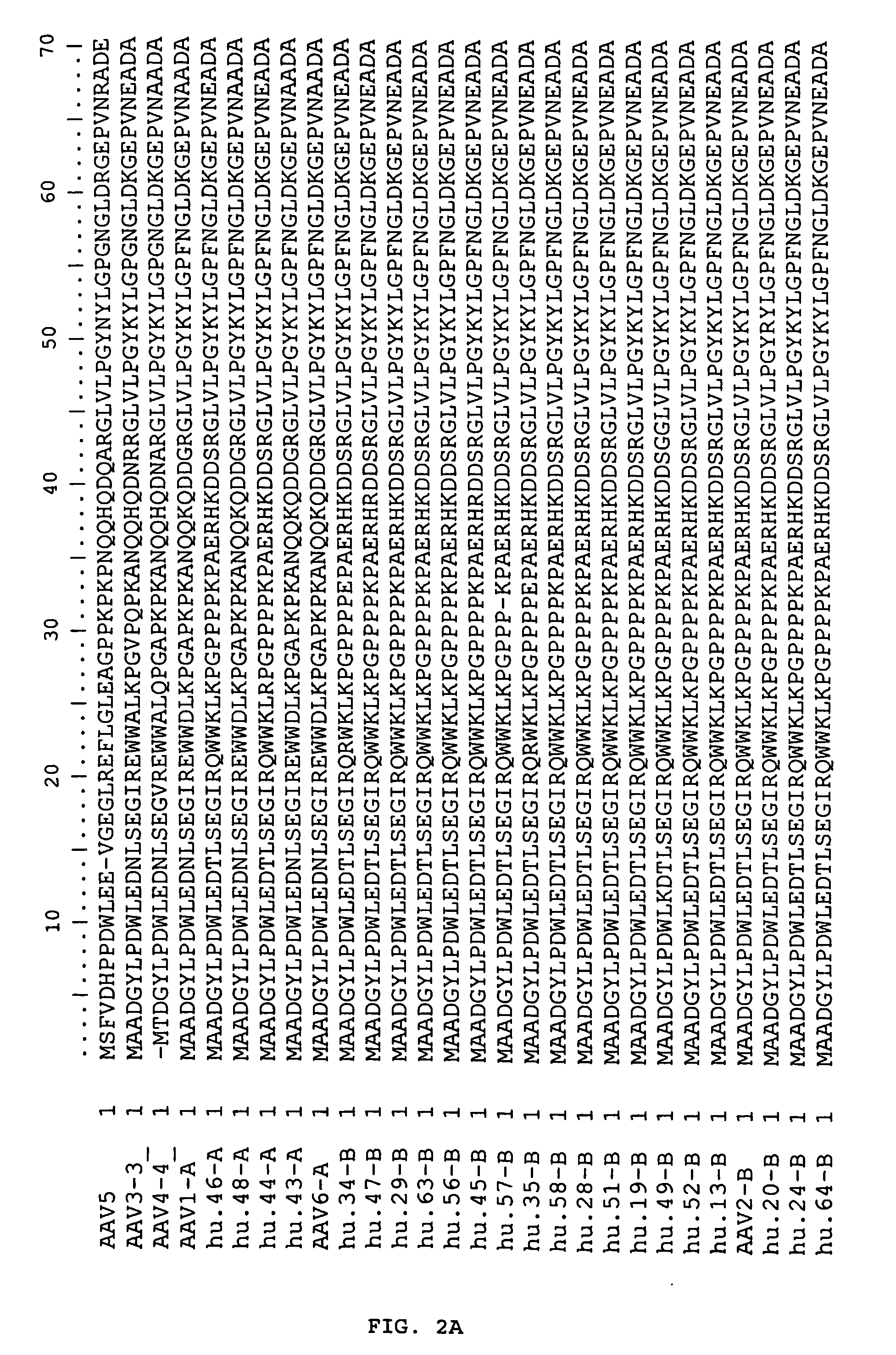
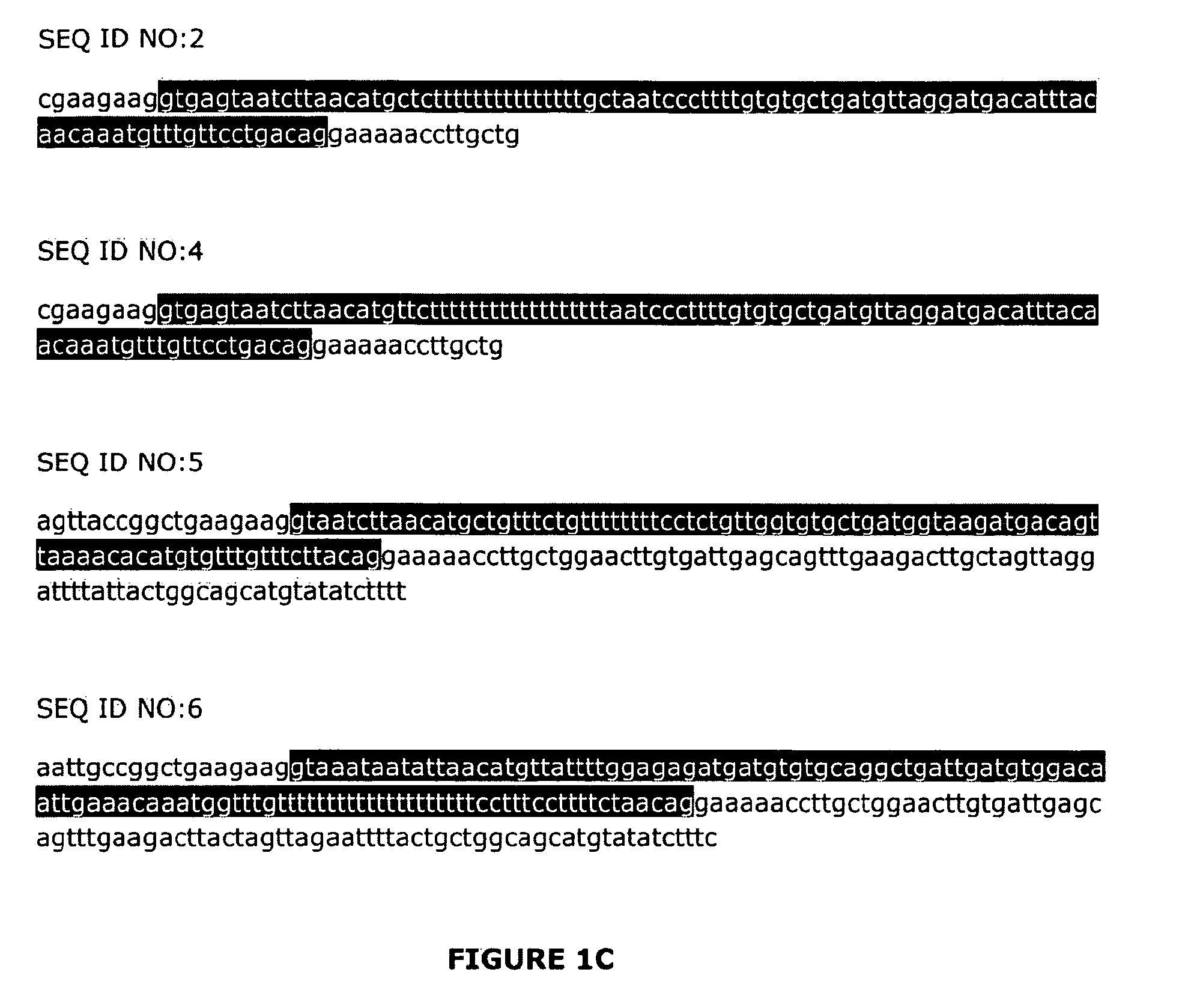
Adeno-associated virus (AAV) clades, sequences, vectors containing same, and uses therefor
Sequences of novel adeno-associated virus capsids and vectors and host cells containing these sequences are provided. Also described are methods of using such host cells and vectors in production of rAAV particles. AAV-mediated delivery of therapeutic and immunogenic genes using the vectors of the invention is also provided.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Virus vectors and methods of making and administering the same
The present invention provides genetically-engineered parvovirus capsids and viruses designed to introduce a heterologous gene into a target cell. The parvoviruses of the invention provide a repertoire of vectors with altered antigenic properties, packaging capabilities, and / or cellular tropisms as compared with current AAV vectors.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Adeno-associated virus (aav) clades, sequences, vectors containing same, and uses therefor
Sequences of novel adeno-associated virus capsids and vectors and host cells containing these sequences are provided. Also described are methods of using such host cells and vectors in production of rAAV particles. AAV-mediated delivery of therapeutic and immunogenic genes using the vectors of the invention is also provided.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
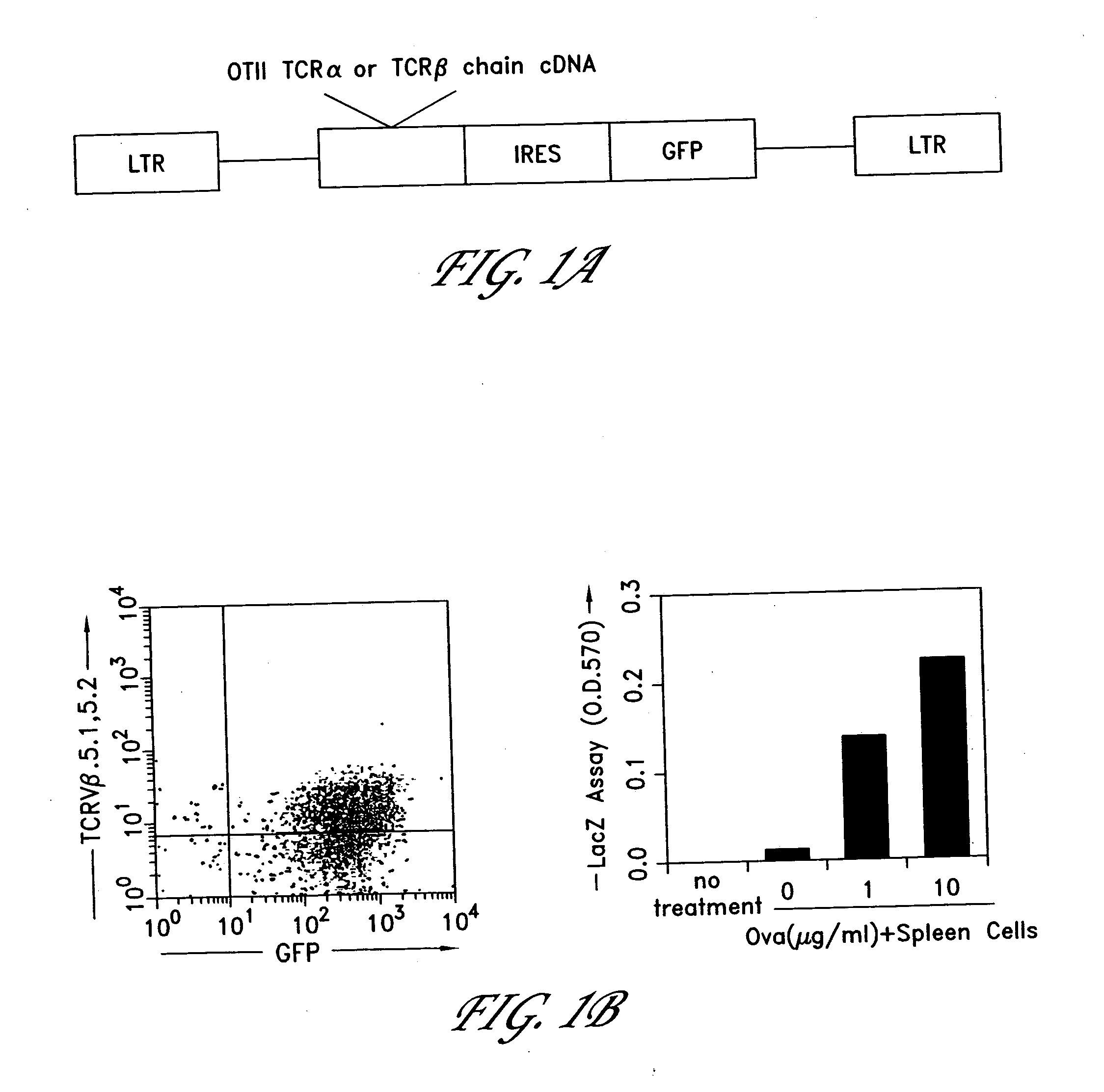
Method for the generation of antigen-specific lymphocytes
InactiveUS20070116690A1Function increaseEnhancing function of T cellBiocideVirusesAutoimmune conditionAutoimmune disease
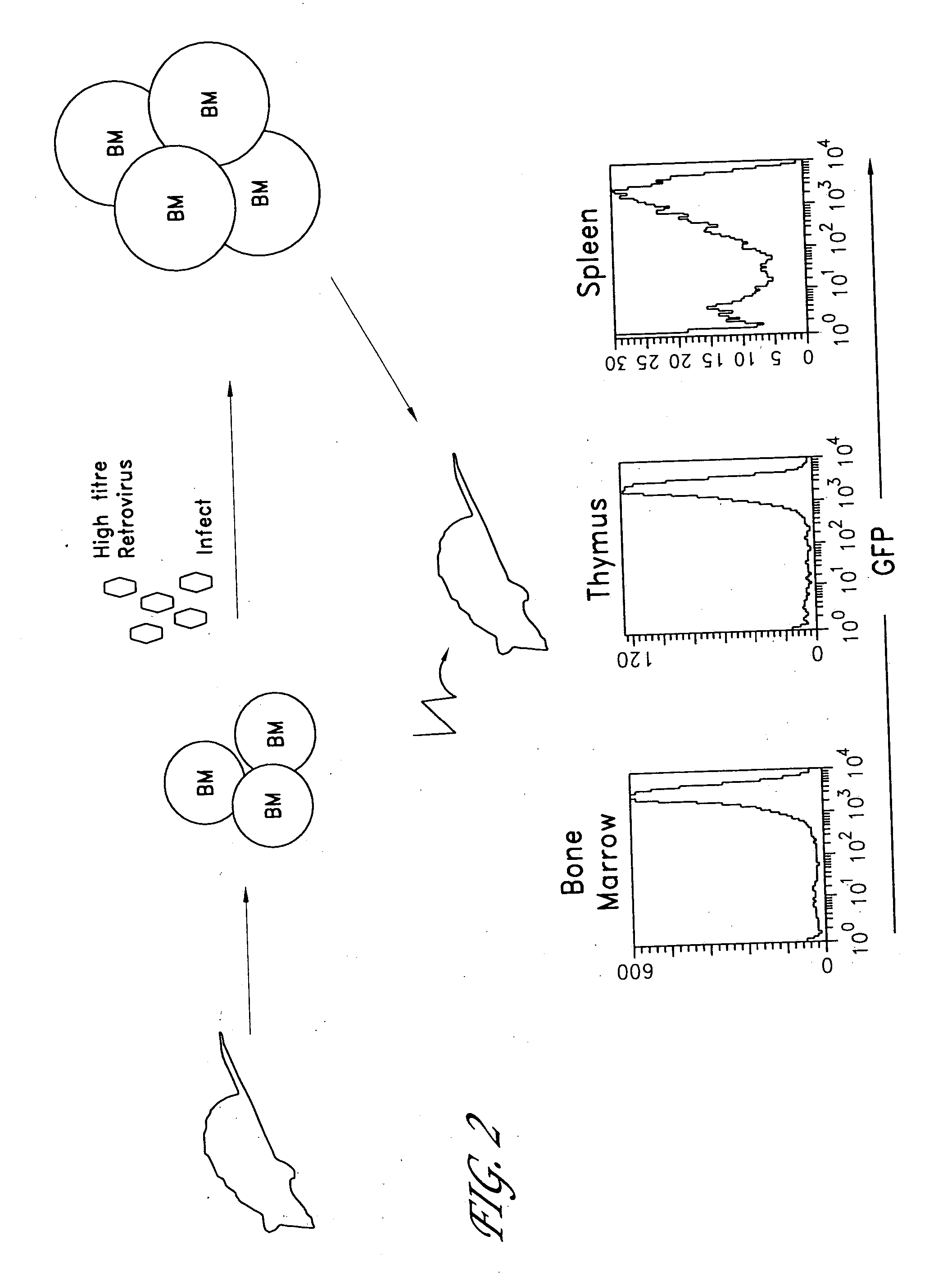
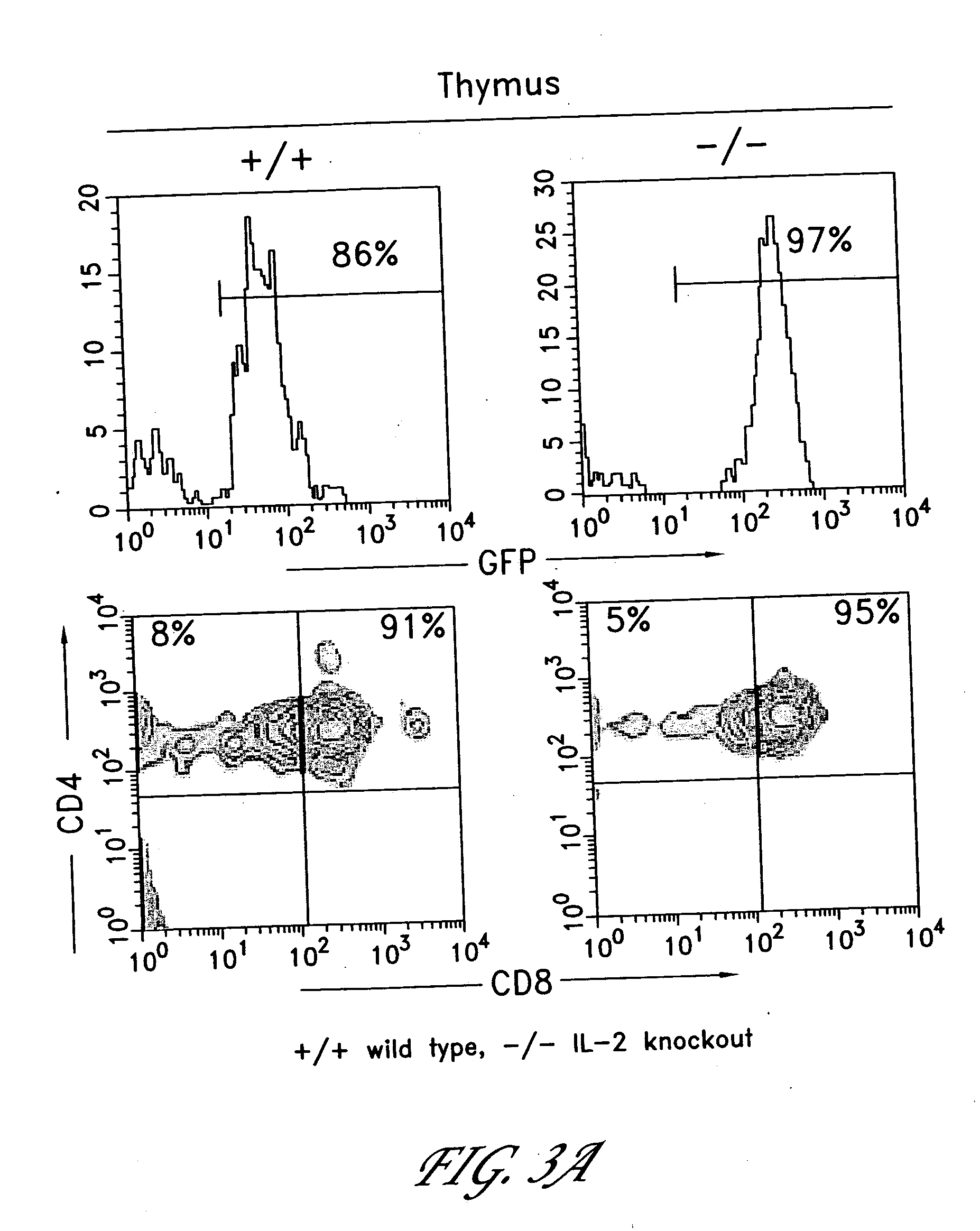
The invention provides systems and methods for the generation of lymphocytes having a unique antigen specificity. In a preferred embodiment, the invention provides methods of virally infecting cells from bone marrow with one or more viral vectors that encode antigen-specific antibodies for the production of, for example B cells and T cells. In some embodiments, the viral vectors include an IRES or 2A element to promote separation of, for example, the α subunit and β subunit of a T cell receptor (TCR) or heavy and light chains of a B-cell antibody. The resulting lymphocytes, express the particular antibody that was introduced in the case of B cells and TCR in the case of T cells. The lymphocytes generated can be used for a variety of therapeutic purposes including the treatment of various cancers and the generation of a desired immune response to viruses and other pathogens. The resulting cells develop normally and respond to antigen both in vitro and in vivo. We also show that it is possible to modify the function of lymphocytes by using stem cells from different genetic backgrounds. Thus our system constitutes a powerful tool to generate desired lymphocyte populations both for research and therapy. Future applications of this technology may include treatments for infectious diseases, such as HIV / AIDS, cancer therapy, allergy, and autoimmune disease.
Owner:CALIFORNIA INST OF TECH
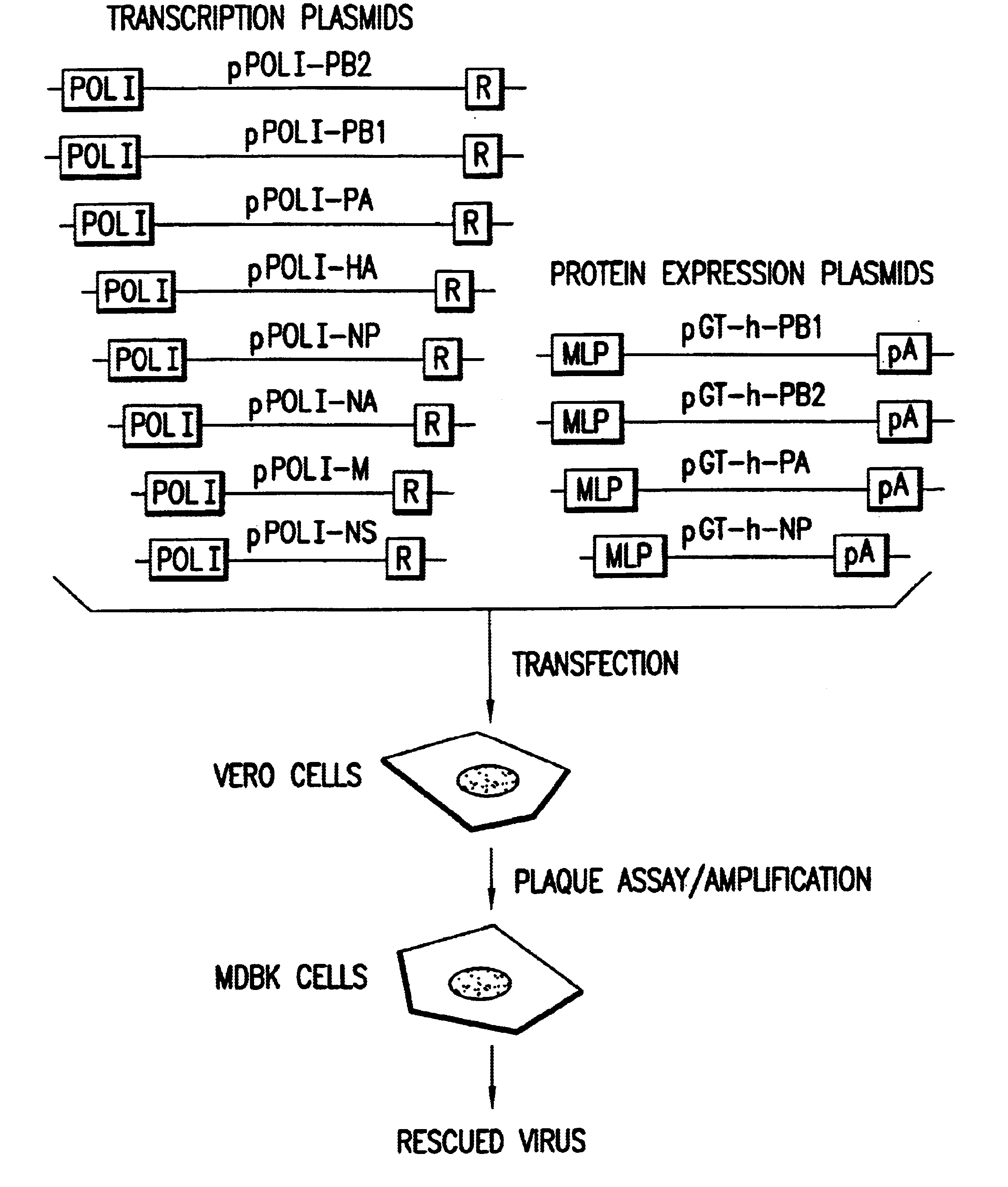
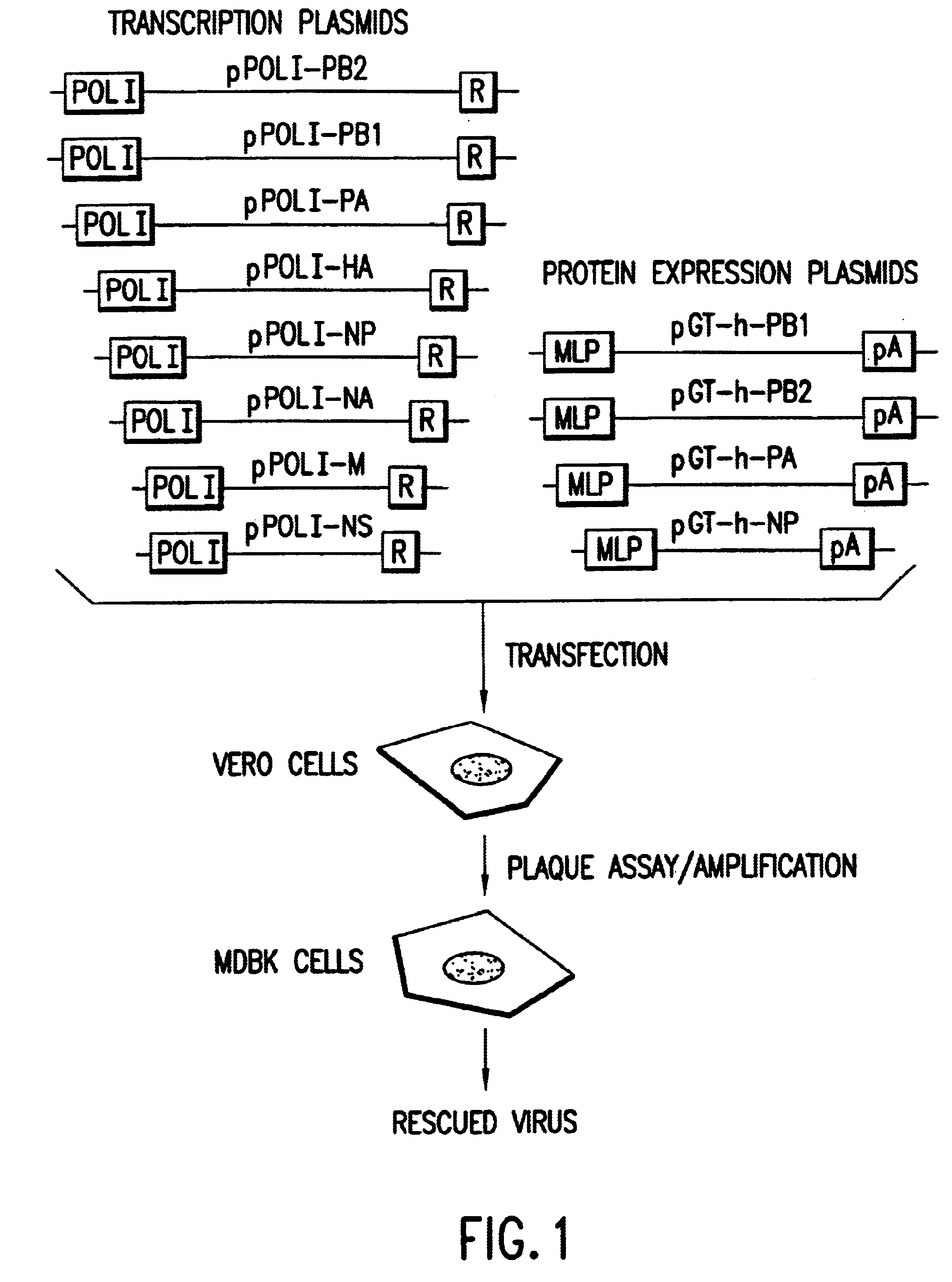
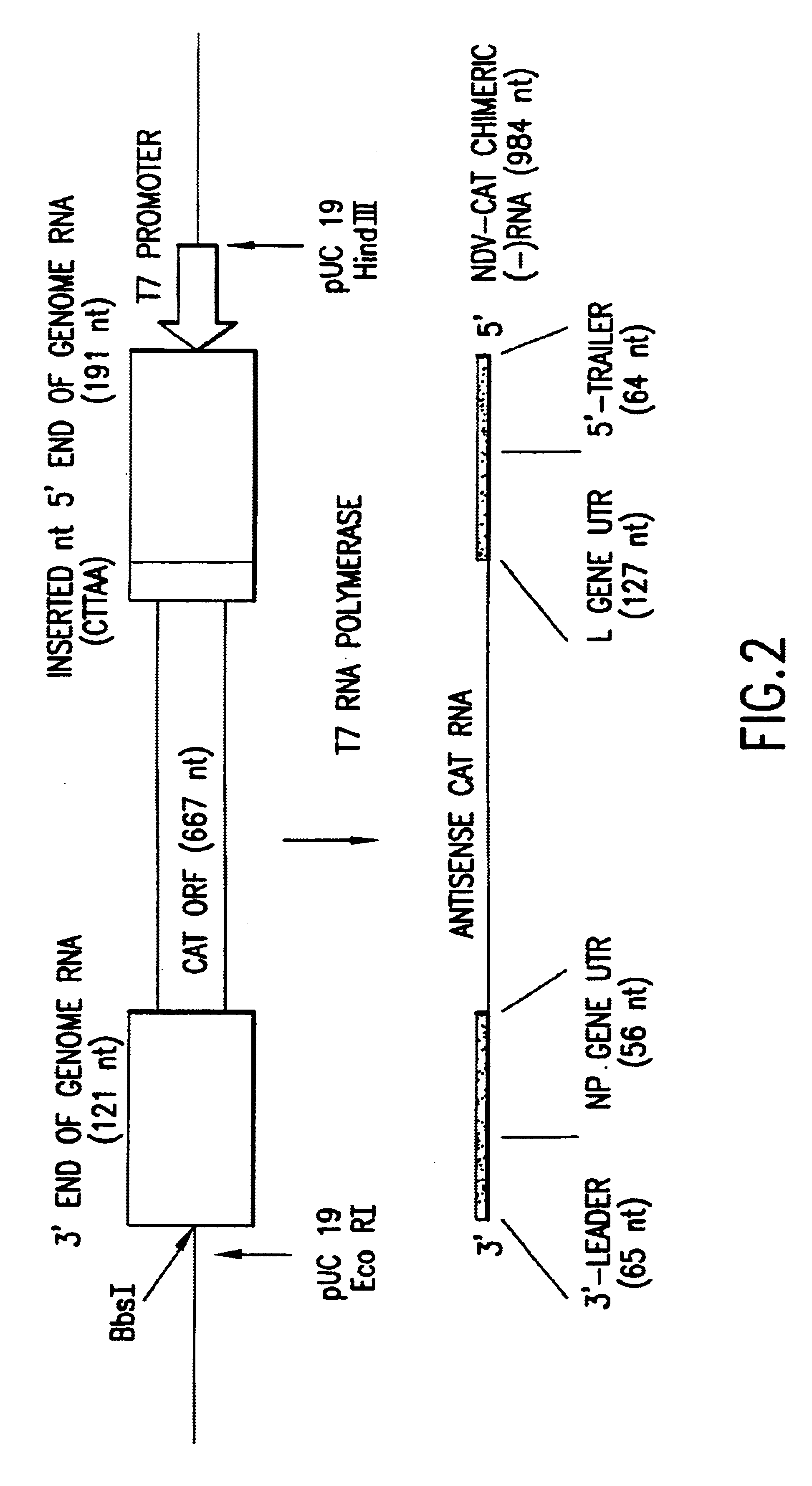
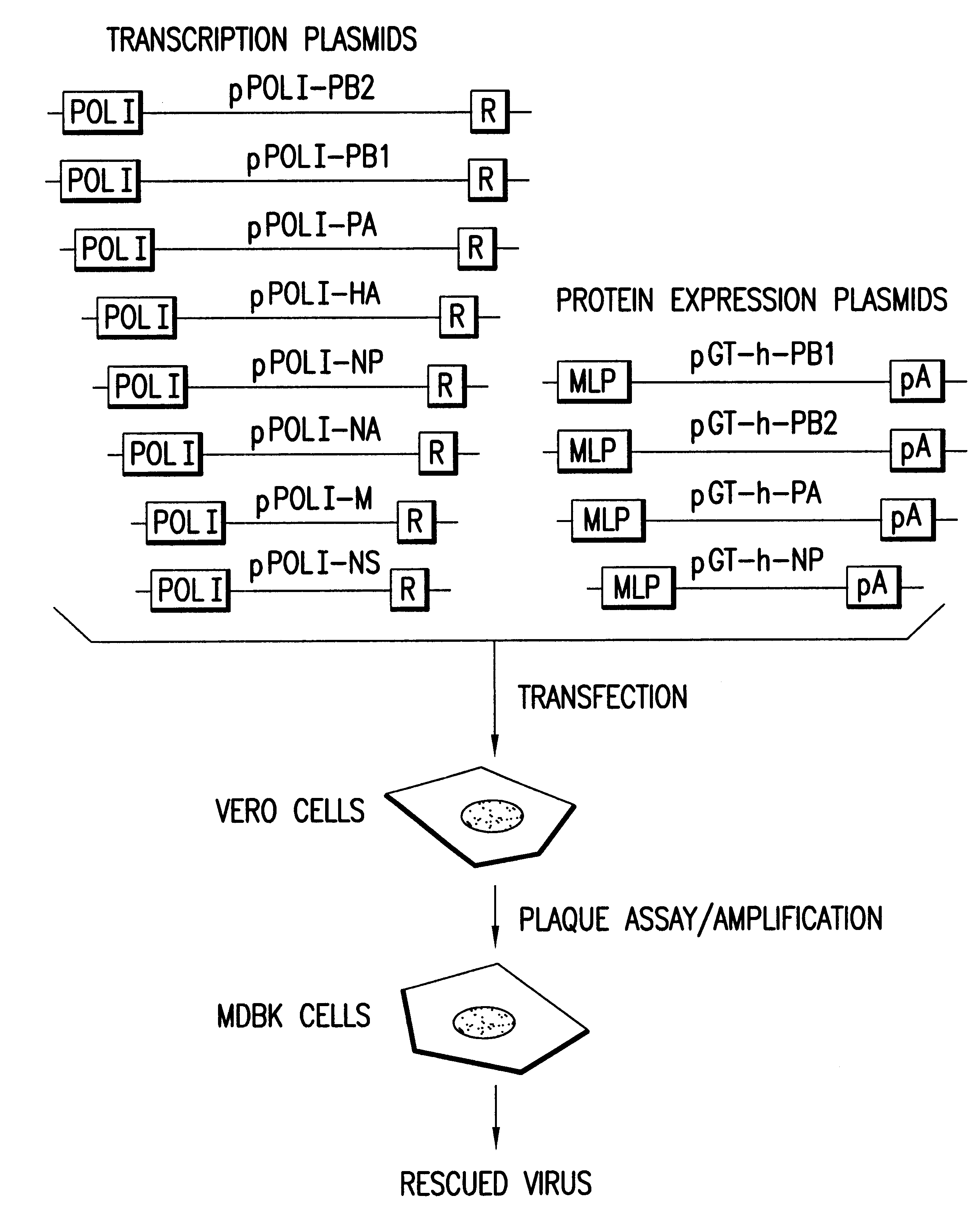
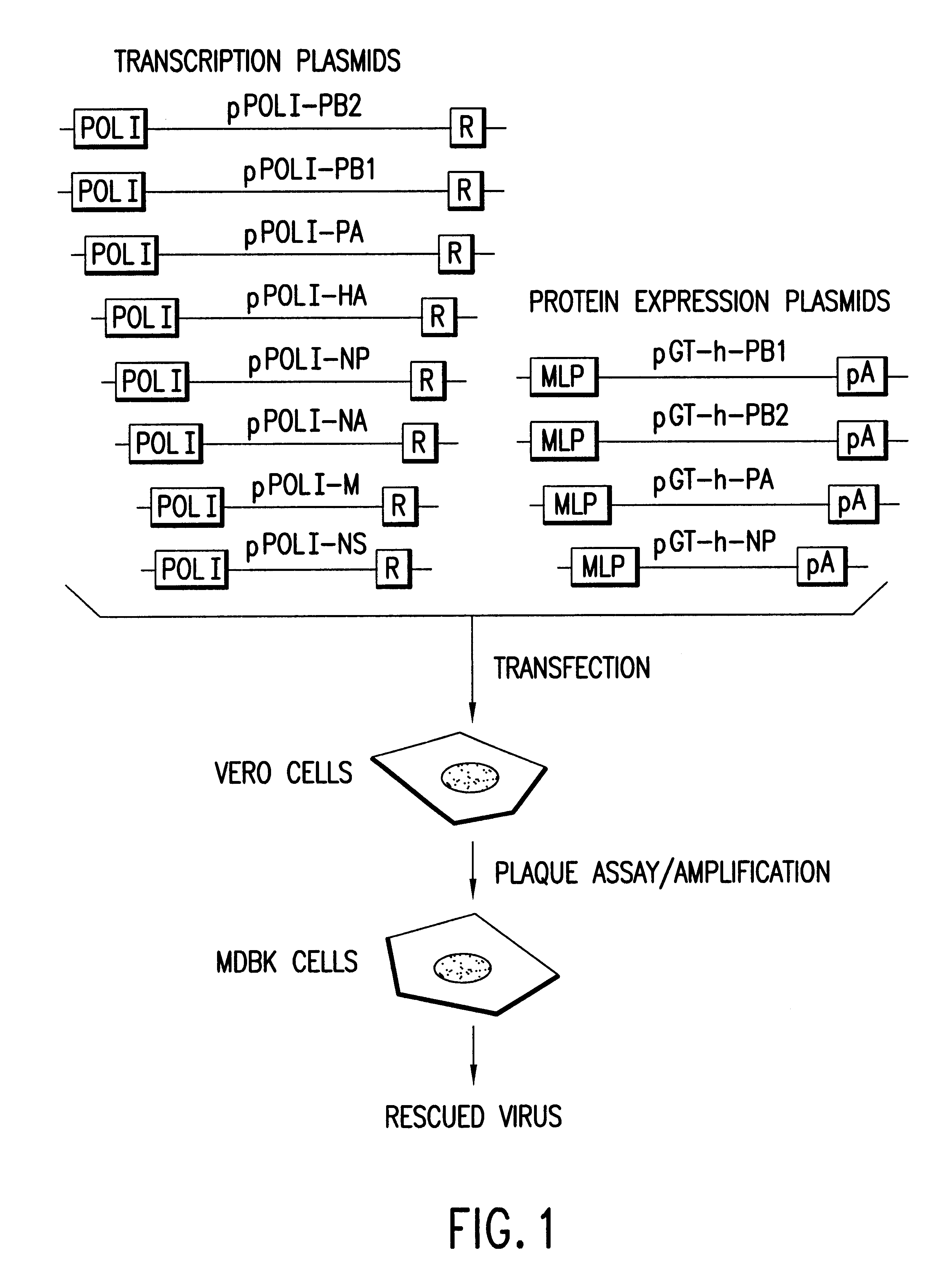
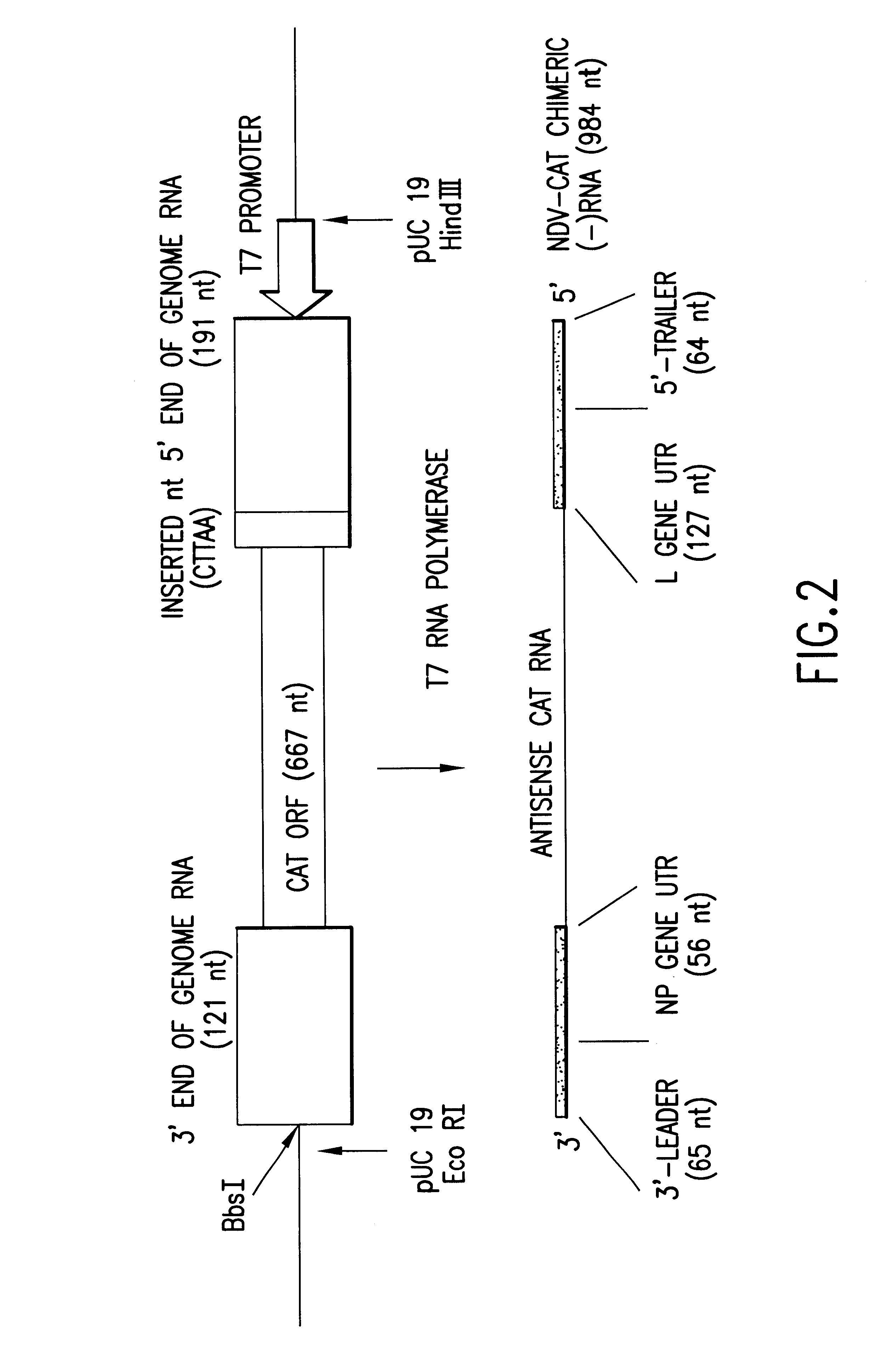
Helper-free rescue of recombinant negative strand RNA virus
Owner:MT SINAI SCHOOL OF MEDICINE
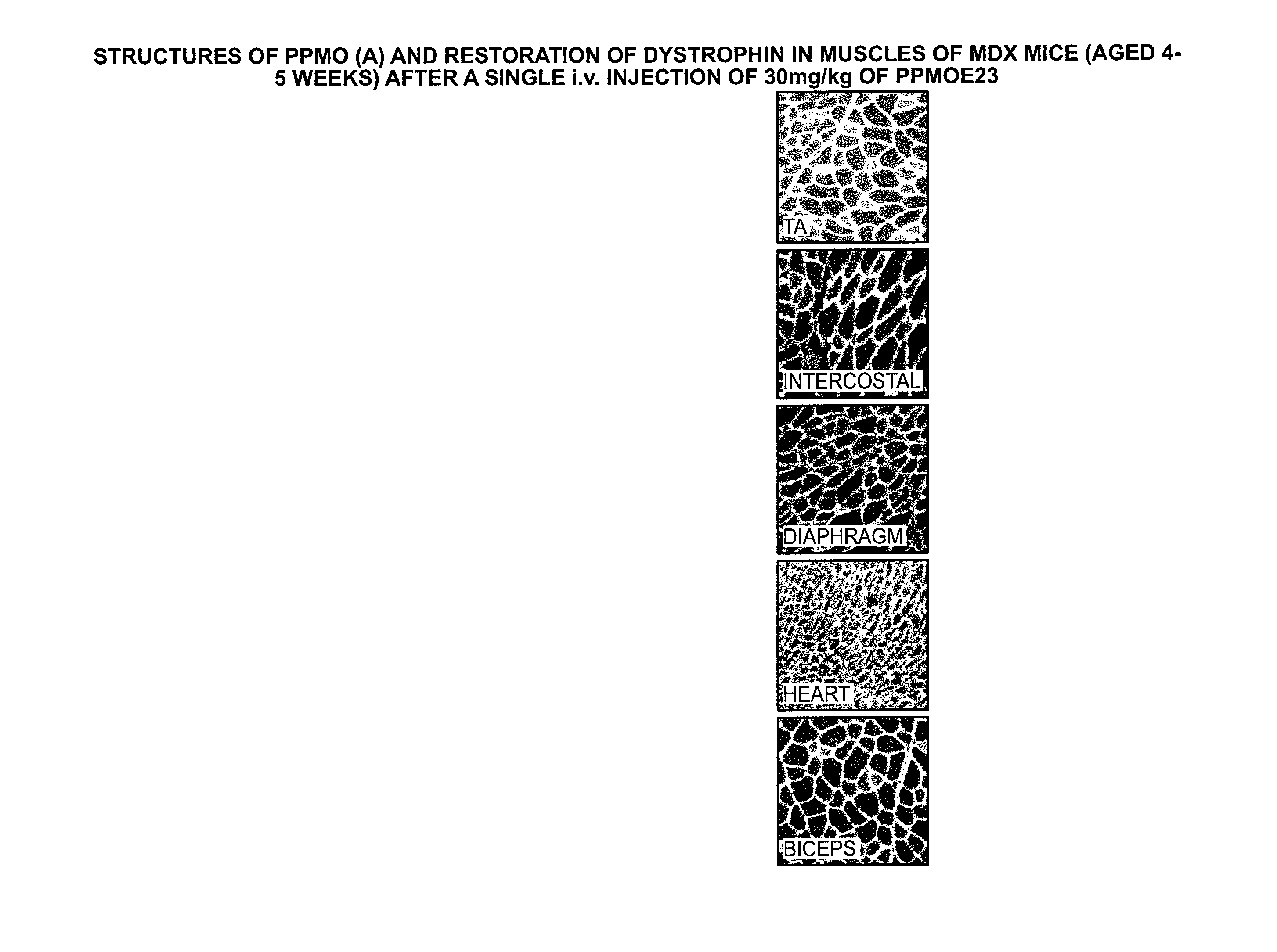
Compound and method for treating myotonic dystrophy
An antisense compound for use in treating myotonic dystrophy DM1 or DM2, a method of enhancing antisense targeting to heart and quadricep muscles, and a method for treating DM1 or DM2 in a mammalian subject are disclosed. The oligonucleotide has 8-30 bases, with at least 8 contiguous bases being complementary to the polyCUG or polyCCUG repeats in the 3′UTR region of dystrophia myotonica protein kinase (DMPK) mRNA in DM1 or DM2, respectively. Conjugated to the oligonucleotide is a cell-penetrating peptide having the sequence (RXRR(B / X)R)2XB, where R is arginine; B is β-alanine; and each X is —C(O)—(CH2)n—NH—, where n is 4-6. The antisense compound is effective to selectively block the sequestration of muscleblind-like 1 protein (MBNL1) and / or CUGBP, in heart and quadricep muscle in a myotonic dystrophy animal model.
Owner:SAREPTA THERAPEUTICS INC
Adeno-associated virus vectors for expression of factor VIII by target cells
InactiveUS6200560B1Easily transfectedConvenient platformBiocideFactor VIIHigh level expressionHuman cell
The present invention provides improved viral vectors useful for the expression of genes at high levels in human cells. In particular, the present invention provides recombinant adeno-associated vectors (AAV) suitable for gene therapy. These vectors are capable of delivering nucleic acid containing constructs which result in the production of full-length therapeutic levels of biologically active Factor VIII in the recipient individual in vivo. The present invention also provides pharmaceutical compositions comprising such AAV vectors, as well as methods for making and using these constructs.
Owner:GENZYME CORP
Production of pseudotyped recombinant AAV virions
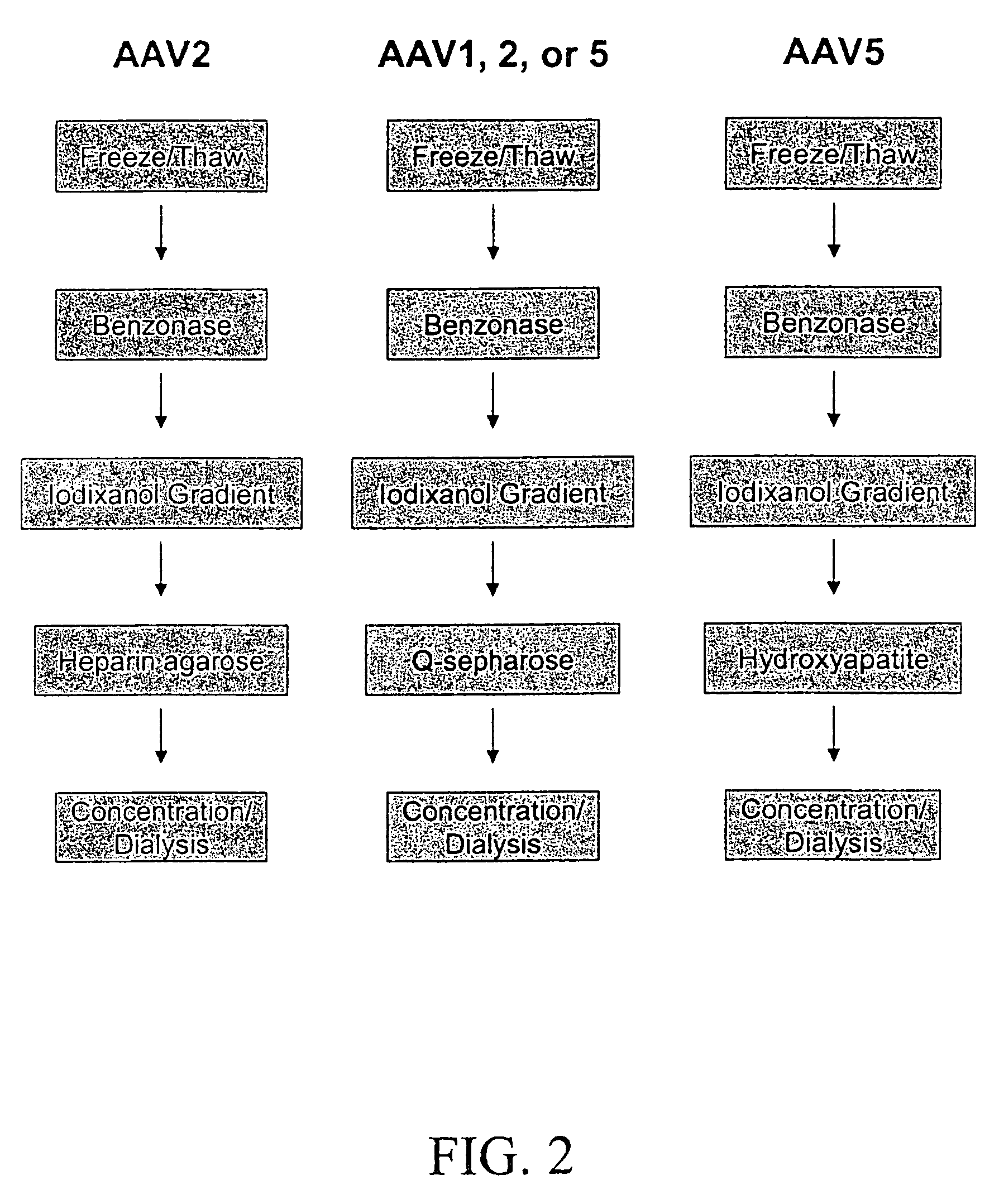
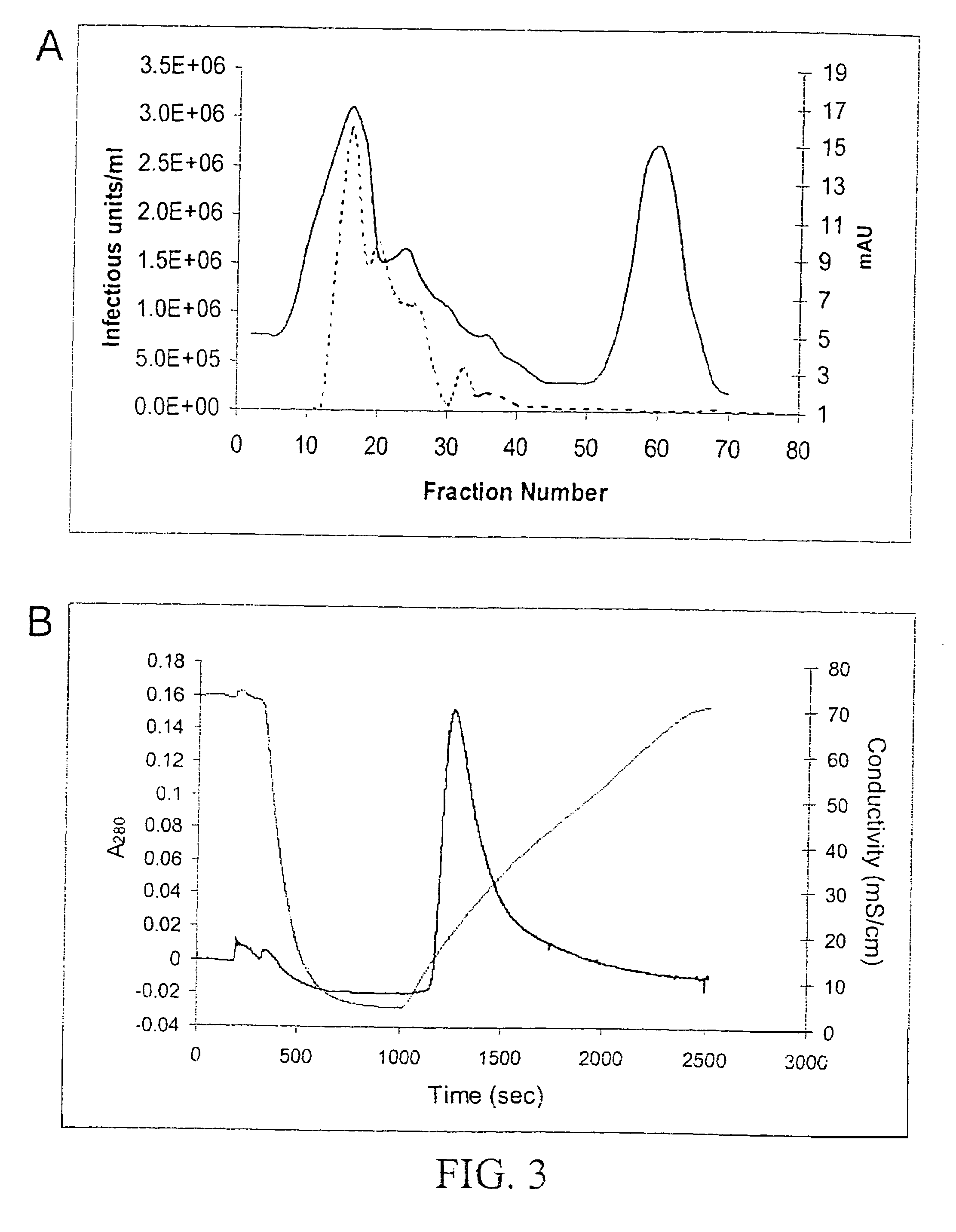
InactiveUS7094604B2Highly purified and concentratedEfficient and large-scale productionVectorsSugar derivativesPurification methodsSerotype
Vectors that encode Adeno-Associated Virus (AAV) Rep and Cap proteins of different serotypes and Adenovirus transcription products that provide helper functions were used to produce pseudotyped recombinant AAV (rAAV) virions. Purification methods generated pseudotyped rAAV virion stocks that were 99% pure with titers of 1×1012–1×1013 vector genomes / ml.
Owner:UNIVIRSITY OF FLORIDA RES FOUND INC
Helper-free rescue of recombinant negative strand RNA viruses
InactiveUS6544785B1SsRNA viruses negative-senseGenetic material ingredientsNegative strandNucleic acid sequence
The present invention relates methods of generating infectious negative-strand virus in host cells by an entirely vector-based system without the aid of a helper virus. In particular, the present invention relates methods of generating infectious recombinant negative-strand RNA viruses intracellularly in the absence of helper virus from expression vectors comprising cDNAs encoding the viral proteins necessary to form ribonucleoprotein complexes (RNPs) and expression vectors comprising cDNA for genomic viral RNA(s) (vRNAs) or the corresponding cRNA(s). The present invention also relates to methods of generating infectious recombinant negative-strand RNA viruses which have mutations in viral genes and / or which express, package and / or present peptides or polypeptides encoded by heterologous nucleic acid sequences. The present invention further relates the use of the recombinant negative-strand RNA viruses or chimeric negative-strand RNA viruses of the invention in vaccine formulations and pharmaceutical compositions.
Owner:MT SINAI SCHOOL OF MEDICINE
Recombinant canine adenoviruses, method for making and uses thereof
InactiveUS6090393AProvide securityIncrease capacitySsRNA viruses negative-senseVectorsBiotechnologyImmunogenicity
Disclosed and claimed are recombinant adenoviruses, methods of making them, uses for them (including in immunological, immunogenic, vaccine or therapeutic compositions, or, as a vector for cloning, replicating or expressing DNA and methods of using the compositions and vector), expression products from them, and uses for the expression products. More particularly, disclosed and claimed are recombinant canine adenoviruses (CAV) and methods of making them, uses for them, expression products from them, and uses for the expression products, including recombinant CAV2 viruses. Additionally, disclosed and claimed are truncated promoters, expression cassettes containing the promoters, and recombinant viruses and plasmids containing the promoters or expression cassettes.
Owner:VIROGENETICS +1
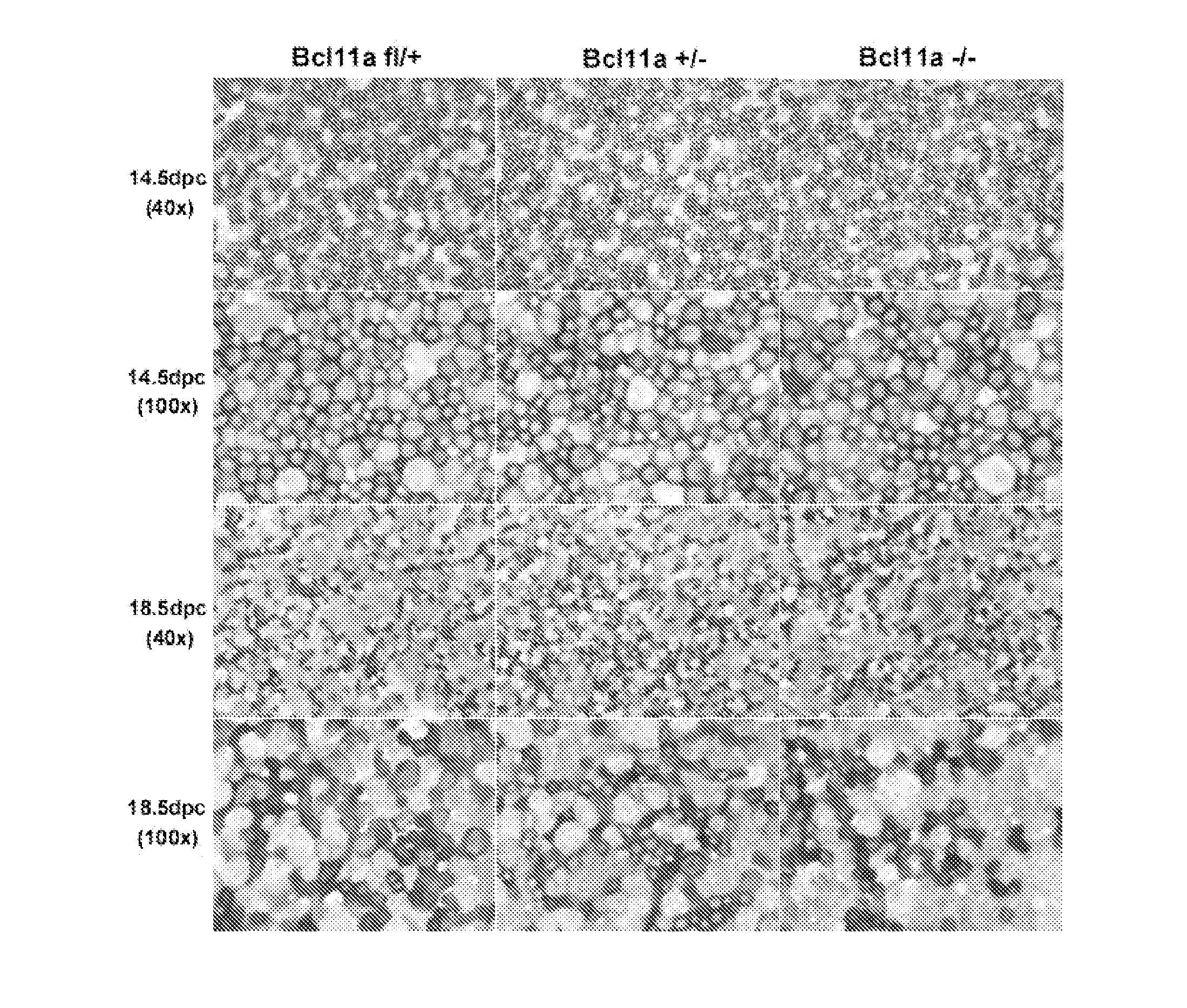
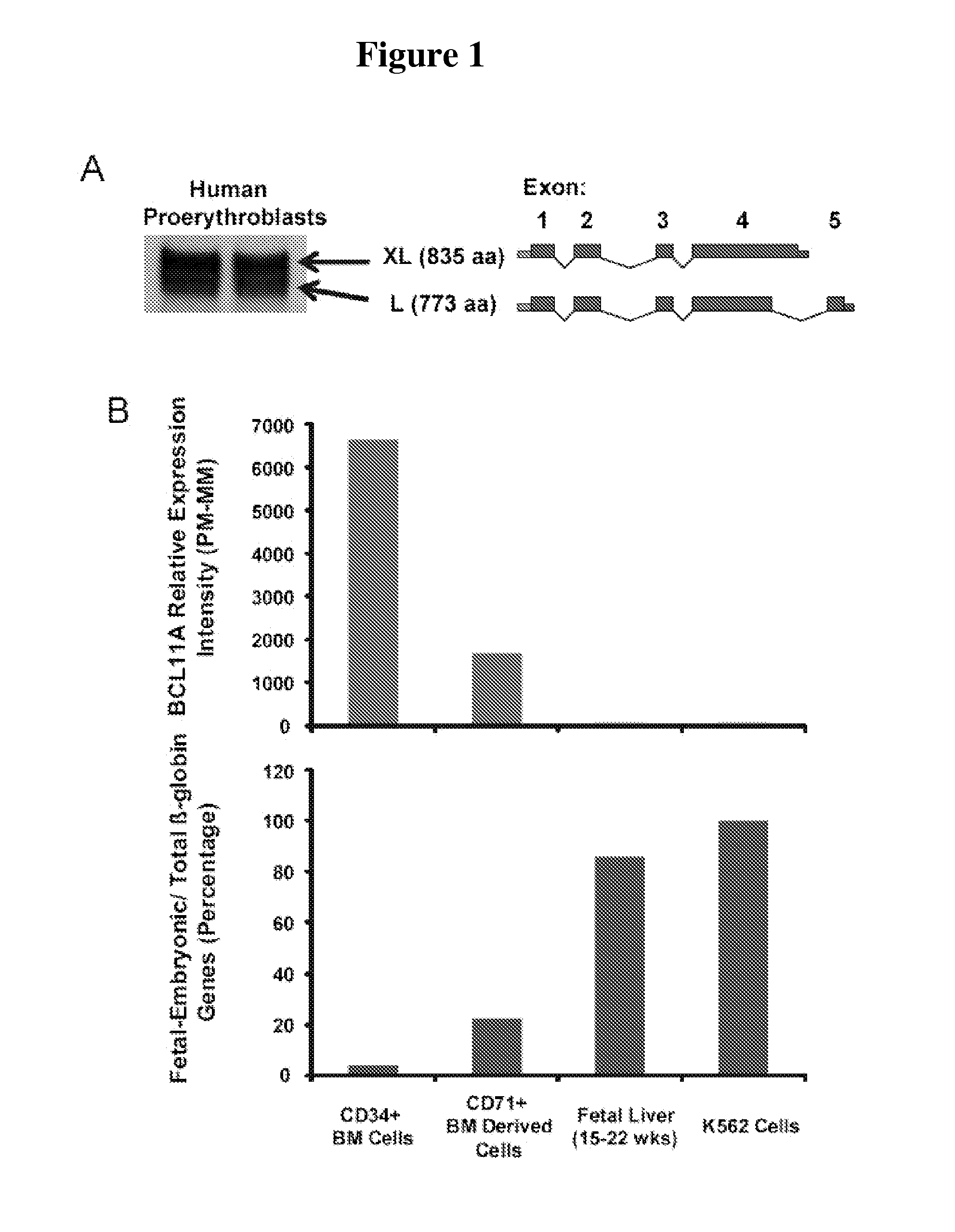
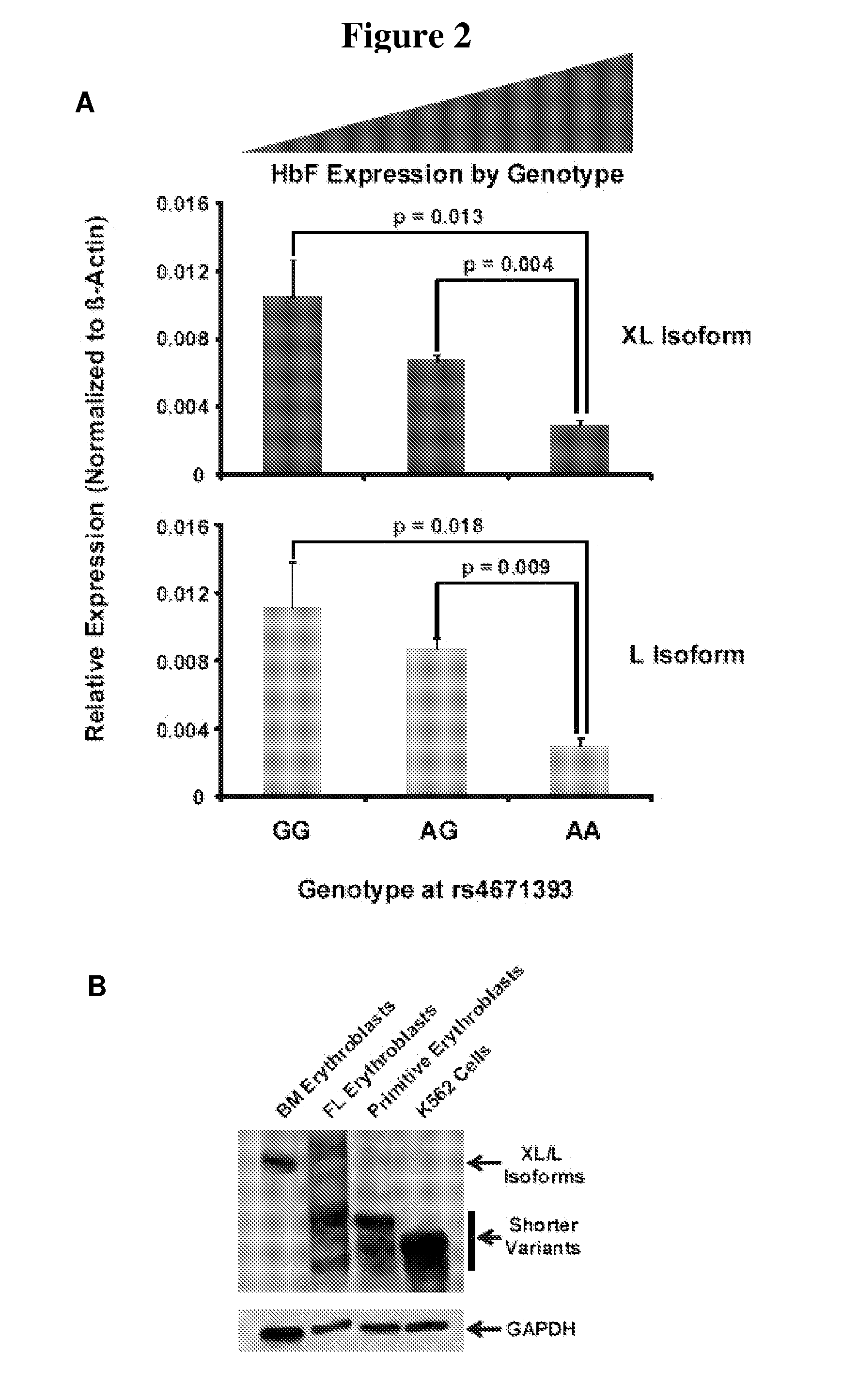
Modulation of bcl11a for treatment of hemoglobinopathies
ActiveUS20110182867A1Increasing fetal hemoglobin levelHigh expressionOrganic active ingredientsVirusesHemoglobinopathyFetal hemoglobin
The invention relates to methods and uses of modulating fetal hemoglobin expression (HbF) in a hematopoietic progenitor cells via inhibitors of BCL11A expression or activity, such as RNAi and antibodies.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
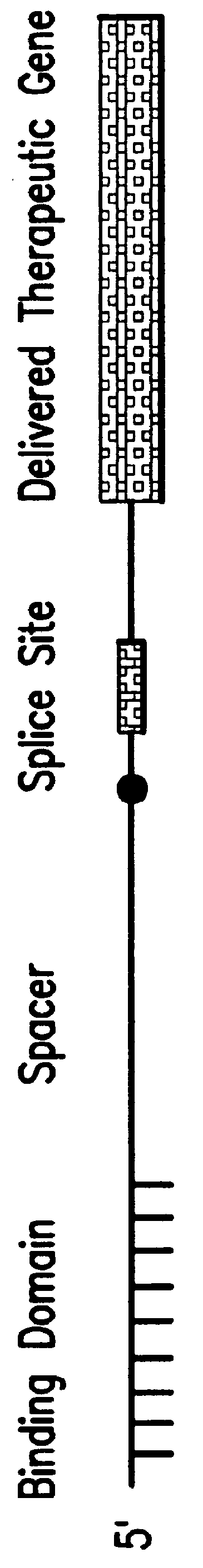
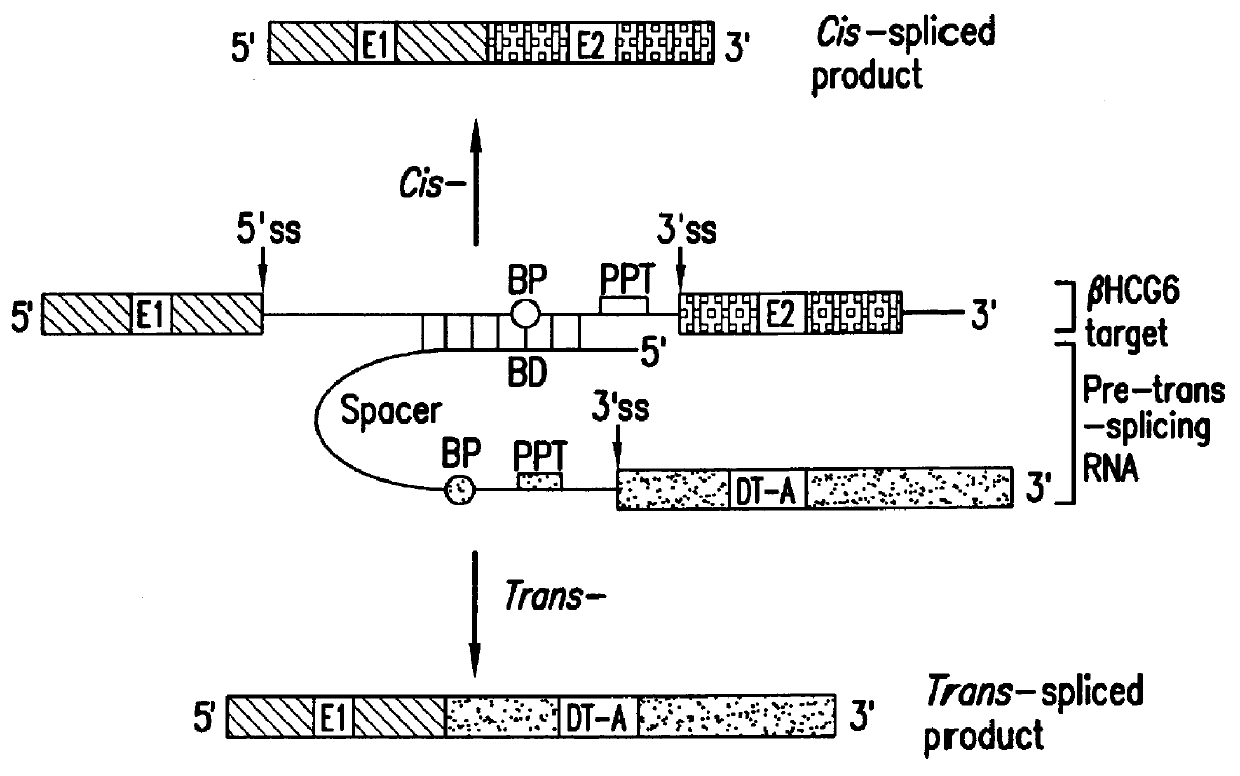
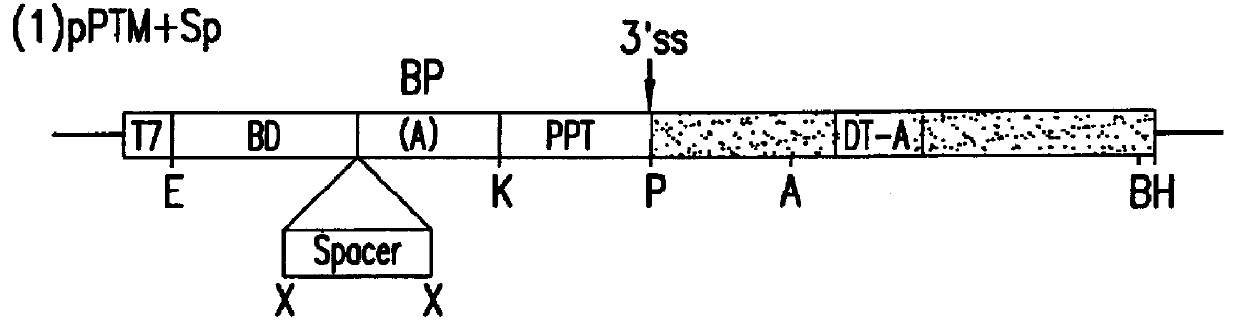
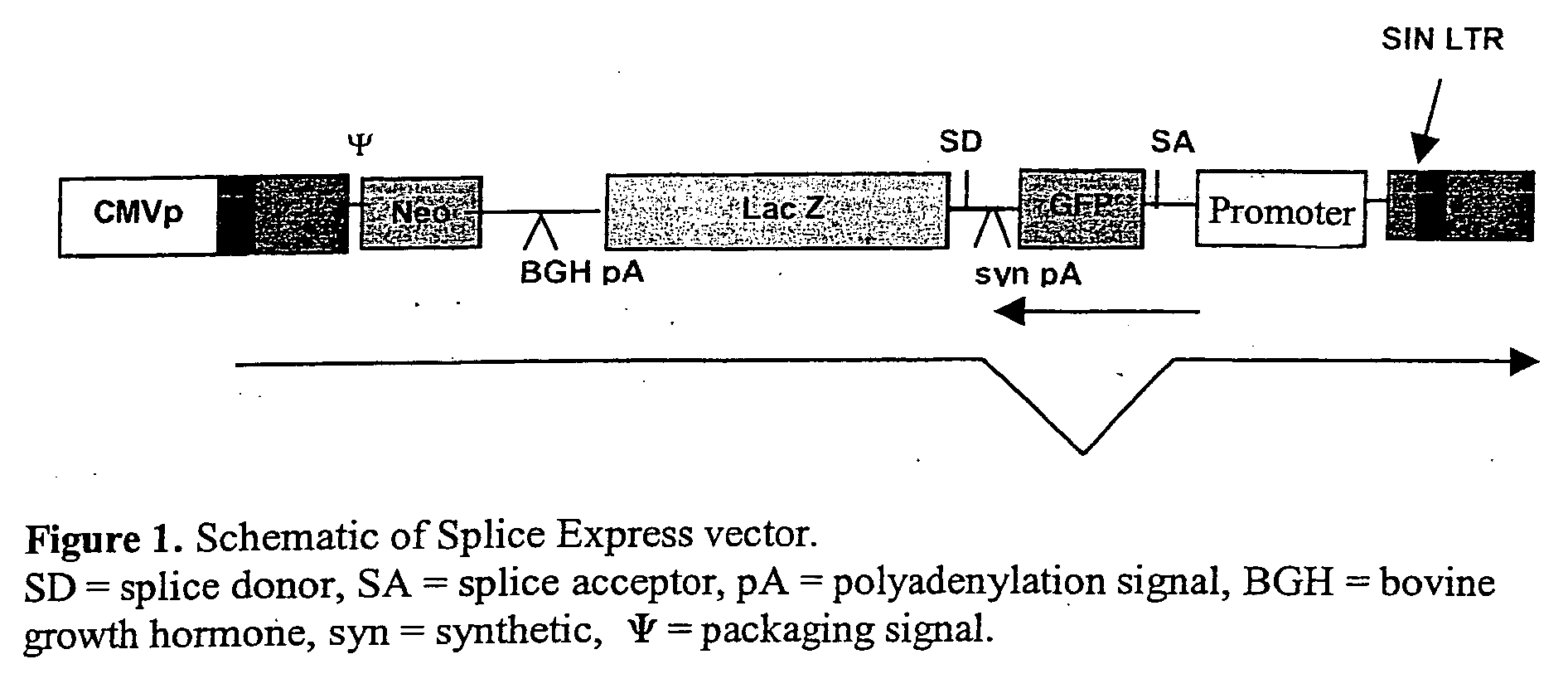
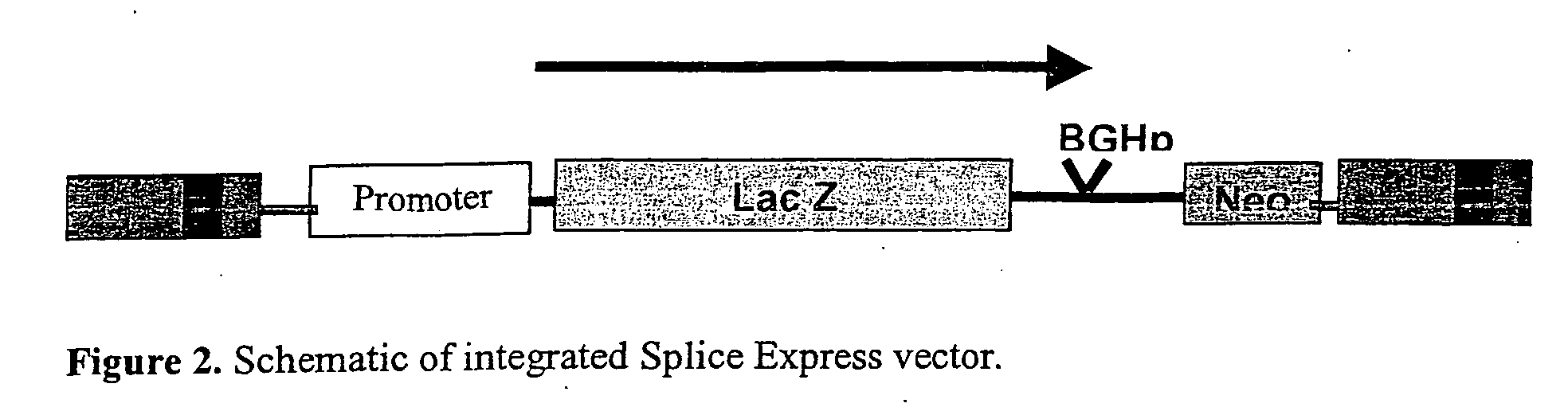
Methods and compositions for use in spliceosome mediated RNA trans-splicing
The molecules and methods of the present invention provide a means for in vivo production of a trans-spliced molecule in a selected subset of cells. The pre-trans-splicing molecules of the invention are substrates for a trans-splicing reaction between the pre-trans-splicing molecules and a pre-mRNA which is uniquely expressed in the specific target cells. The in vivo trans-splicing reaction provides a novel mRNA which is functional as mRNA or encodes a protein to be expressed in the target cells. The expression product of the mRNA is a protein of therapeutic value to the cell or host organism a toxin which causes killing of the specific cells or a novel protein not normally present in such cells. The invention further provides PTMs that have been genetically engineered for the identification of exon / intron boundaries of pre-mRNA molecules using an exon tagging method. The PTMs of the invention can also be designed to result in the production of chimeric RNA encoding for peptide affinity purification tags which can be used to purify and identify proteins expressed in a specific cell type.
Owner:INTRONN HLDG +1
Compositions and methods for helper-free production of recombinant adeno-associated viruses
InactiveUS6953690B1Efficient productionIncrease the number ofBiocideGenetic therapy composition manufactureMammalWild type
A method for producing recombinant adeno-associated virus in the absence of contaminating helper virus or wild-type virus involves culturing a mammalian host cell containing a transgene flanked by adeno-associated virus (AAV) inverse terminal repeats and under the control of regulatory sequences directing expression thereof, an AAV rep sequence and an AAV cap sequence under the control of regulatory sequences directing expression thereof, and the minimum adenovirus DNA required to express an E1a gene product, an E1b gene product and an E2a gene product, and isolating therefrom a recombinant AAV which expresses the transgene in the absence of contaminating helper virus or wildtype AAV. This method obviates a subsequent purification step to purify rAAV from contaminating virus. Also provided are various embodiments of the host cell.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Vector system
InactiveUS7259015B2Eliminate the effects ofInhibition of activationFungiNervous disorderVector systemTyrosine
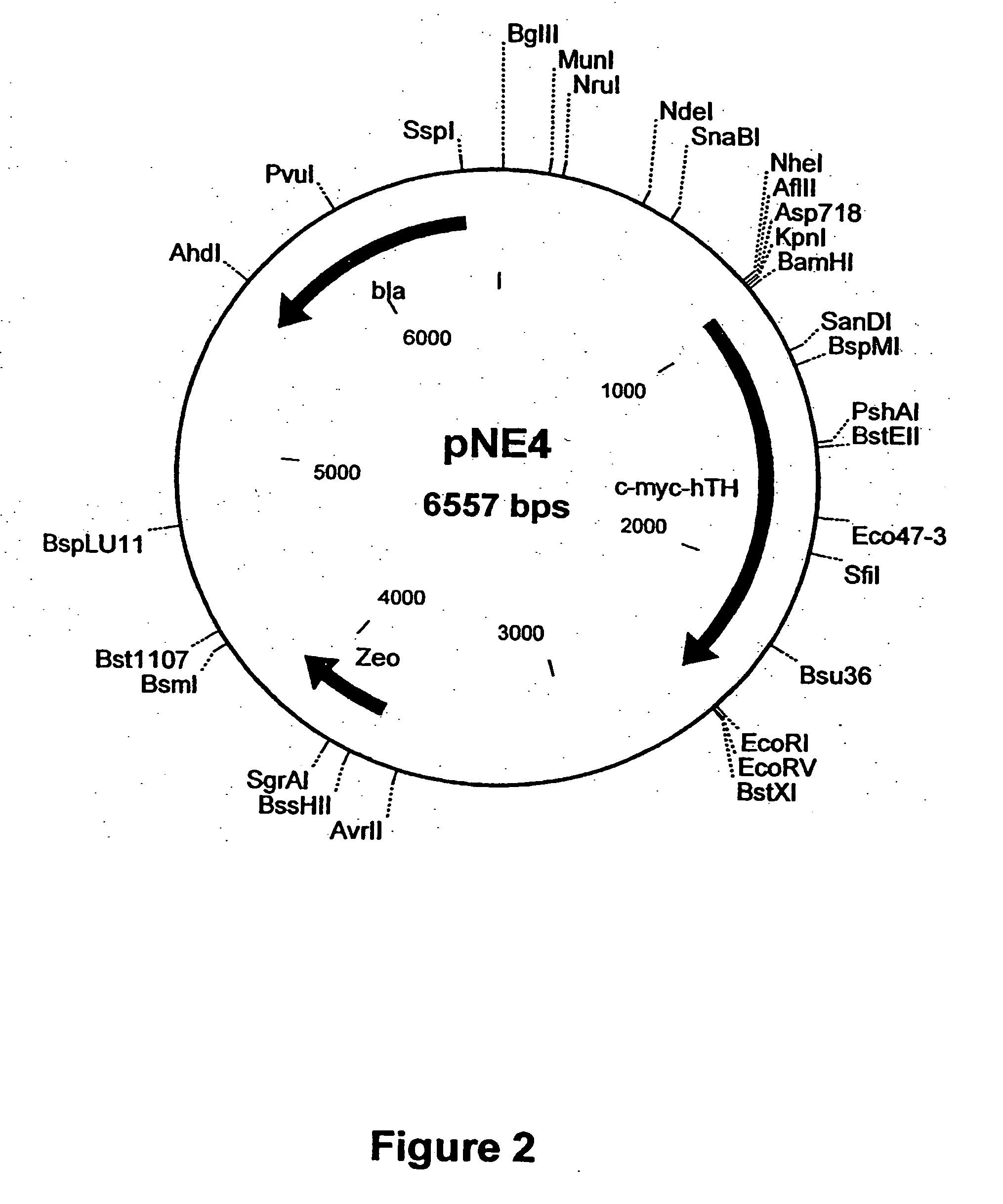
Provided are retroviral vector genomes and vector systems comprising the genomes. In particular, a retroviral vector genome comprising two or more NOIs, operably linked by one or more Internal Ribosome Entry Site(s); a lentiviral vector genome comprising two or more NOIs suitable for treating a neurodegenerative disorder; and a lentiviral vector genome which encodes tyrosine hydroxylase, GTP-cyclohydrolase I and, optionally, Aromatic Amino Acid Dopa Decarboxylase are provided.
Owner:OXFORD BIOMEDICA (UK) LTD
Truncated transcriptionally active cytomegalovirus promoters
InactiveUS6156567AProvide securityIncrease capacitySsRNA viruses negative-senseGenetic material ingredientsBiotechnologyEukaryotic plasmids
Recombinant adenoviruses, methods of making them, uses for them, including in immunological, immunogenic, vaccine or therapeutic compositions, or, as a vector for cloning, replicating or expressing DNA and methods of using the compositions and vector, expression products from them, and uses for the expression products are provided. More particularly, recombinant canine adenoviruses (CAV) and methods of making them, uses for them, expression products from them, and uses for the expression products, including recombinant CAV2 viruses are provided. Additionally, truncated promoters, expression cassettes containing the promoters, and recombinant viruses and plasmids containing the promoters or expression cassettes are provided.
Owner:VIROGENETICS
Bispecific antibodies
ActiveUS7235641B2Improve productivityImprove efficiencyAntipyreticAntibody mimetics/scaffoldsBispecific antibodyImmune effector cell
Owner:AMGEN RES (MUNICH) GMBH
Adeno-associated vectors for expression of factor VIII by target cells
InactiveUS6221349B1Easily transfectedConvenient platformBiocidePeptide/protein ingredientsHigh level expressionHuman cell
The present invention provides improved viral vectors useful for the expression of genes at high levels in human cells. In particular, the present invention provides adeno-associated vectors (AAV) suitable for gene therapy. These vectors are capable of delivering nucleic acid containing constructs which result in the production of full-length therapeutic levels of biologically active Factor VIII in the recipient individual in vivo. The present invention also provides pharmaceutical compositions comprising such AAV vectors, as well as methods for making and using these constructs.
Owner:GENZYME CORP
Vectors having enhanced expression and methods of making and uses thereof
InactiveUS6130066AHigh expressionIncrease transcriptionVectorsSugar derivativesOpen reading frameVaccinia
Disclosed and claimed are vectors having enhanced expression and methods for making and using them. Enhancement of expression is from substantially co-temporal expression of at least one first nucleic acid molecule and at least one second nucleic acid molecule. The second nucleic acid molecule encodes a transcription factor or a translation factor or a transcription factor and a translation factor. The contemporaneous expression can be from operably linking the first and second nucleic molecules to a single promoter, or from operably linking the first nucleic acid molecule to a first promoter and the second nucleic molecule to a second promoter wherein the first and second promoters function substantially contemporaneously. Thus, the first and second nucleic acid molecules can be at the same locus in the vector, or at different loci. The second nucleic acid molecule can encode: one transcription factor or more than one transcription factor; or one translation factor or more than one translation factor; or at least one transcription factor and at least one translation factor. The transcription factor can be from vaccinia H4L, D6, A7, G8R, A1L, A2L, H5R, or combinations thereof. The translation factor can be from a K3L open reading frame, an E3L open reading frame, a VAI RNA, an EBER RNA, a sigma 3 open reading frame, a TRBP open reading frame, or combinations thereof. The vector can be a poxvirus such as an attenuated poxvirus, e.g., NYVAC, or ALVAC.
Owner:VIROGENETICS
Vector system
InactiveUS20070025970A1Eliminate the effects ofInhibition of activationBiocideFungiVector systemTyrosine hydroxylase
The present invention relates to retroviral vector genomes and to vector systems comprising such genomes. In particular the present invention relates to a retroviral vector genome comprising two or more NOIs operably linked by one or more Internal Ribosome Entry Site(s); a lentiviral vector genome comprising two or more NOIs suitable for treating a neurodegenerative disorder; and a lentiviral vector genome which encodes tyrosine hydroxylase, GTP-cyclohydrolase I and optionally Aromatic Amino Acid Dopa Decarboxylase.
Owner:OXFORD BIOMEDICA (UK) LTD
Synthetic 5'UTRs, Expression Vectors, and Methods for Increasing Transgene Expression
ActiveUS20100293625A1Increase transgene expressionImprove stabilityVectorsSugar derivativesReticulum cellIntein
The present invention provides synthetic 5′UTRs comprising a first polynucleotide fragment and a second polynucleotide fragment, wherein the first polynucleotide fragment comprises at least one splice site of a first eukaryotic gene, the second polynucleotide fragment comprises at least a portion of 5′ untranslated region of a second eukaryotic gene, and the first polynucleotide fragment is located 5′ of the second polynucleotide fragment. In one embodiment, the first polynucleotide fragment comprises the second intron of a sarcoplasmic / endoplasmic reticulum calcium ATPase gene and the second polynucleotide fragment comprises at least a portion of the 5′ untranslated region (5′UTR) of a eukaryotic casein gene. The synthetic 5′UTRs are useful for increasing the expression of a transgene when positioned between a promoter and a transgene within an expression vector. The present invention also provides vectors comprising synthetic 5′UTRs and methods for increasing the expression of a transgene using synthetic 5′UTRs.
Owner:PRECIGEN INC
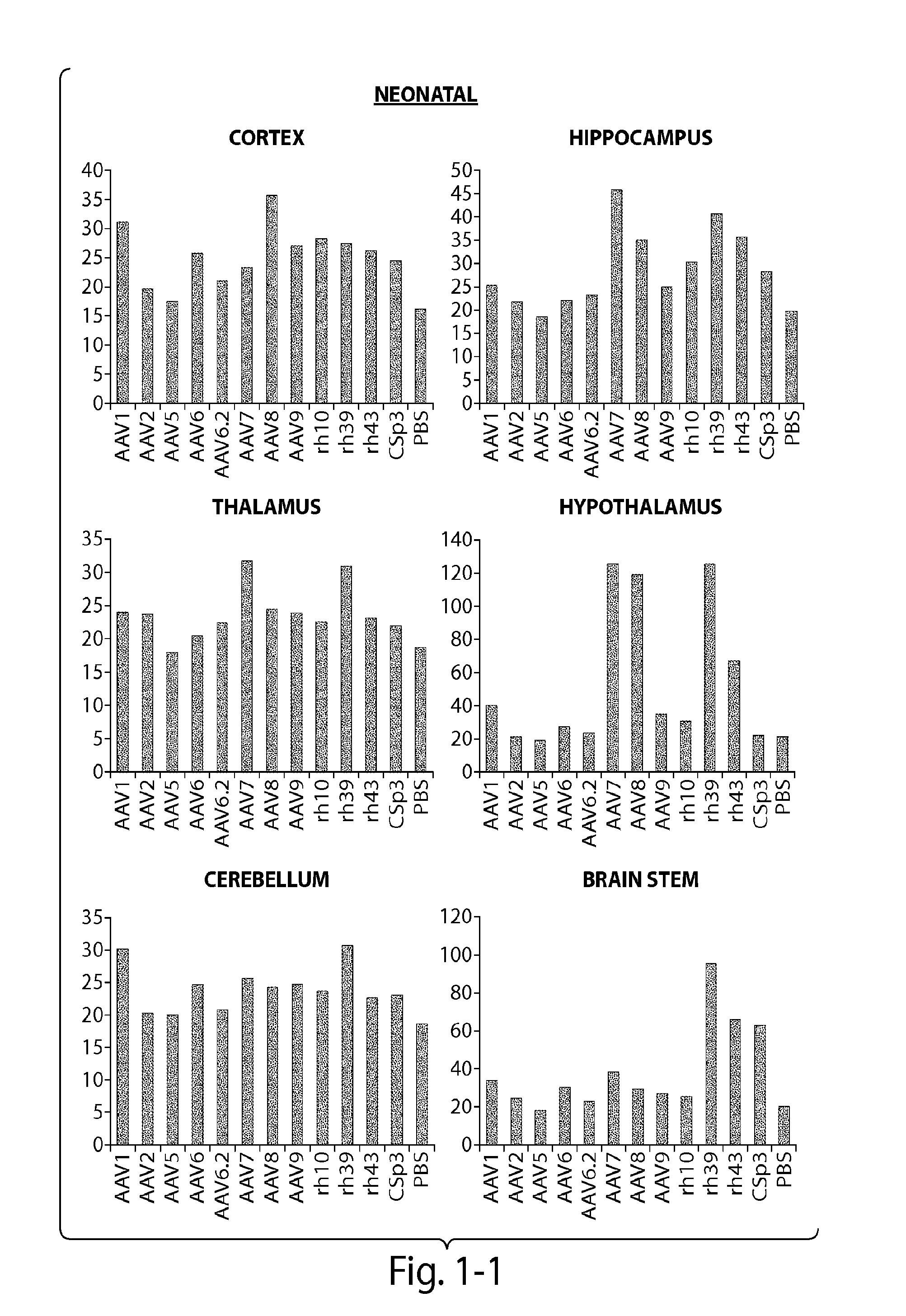
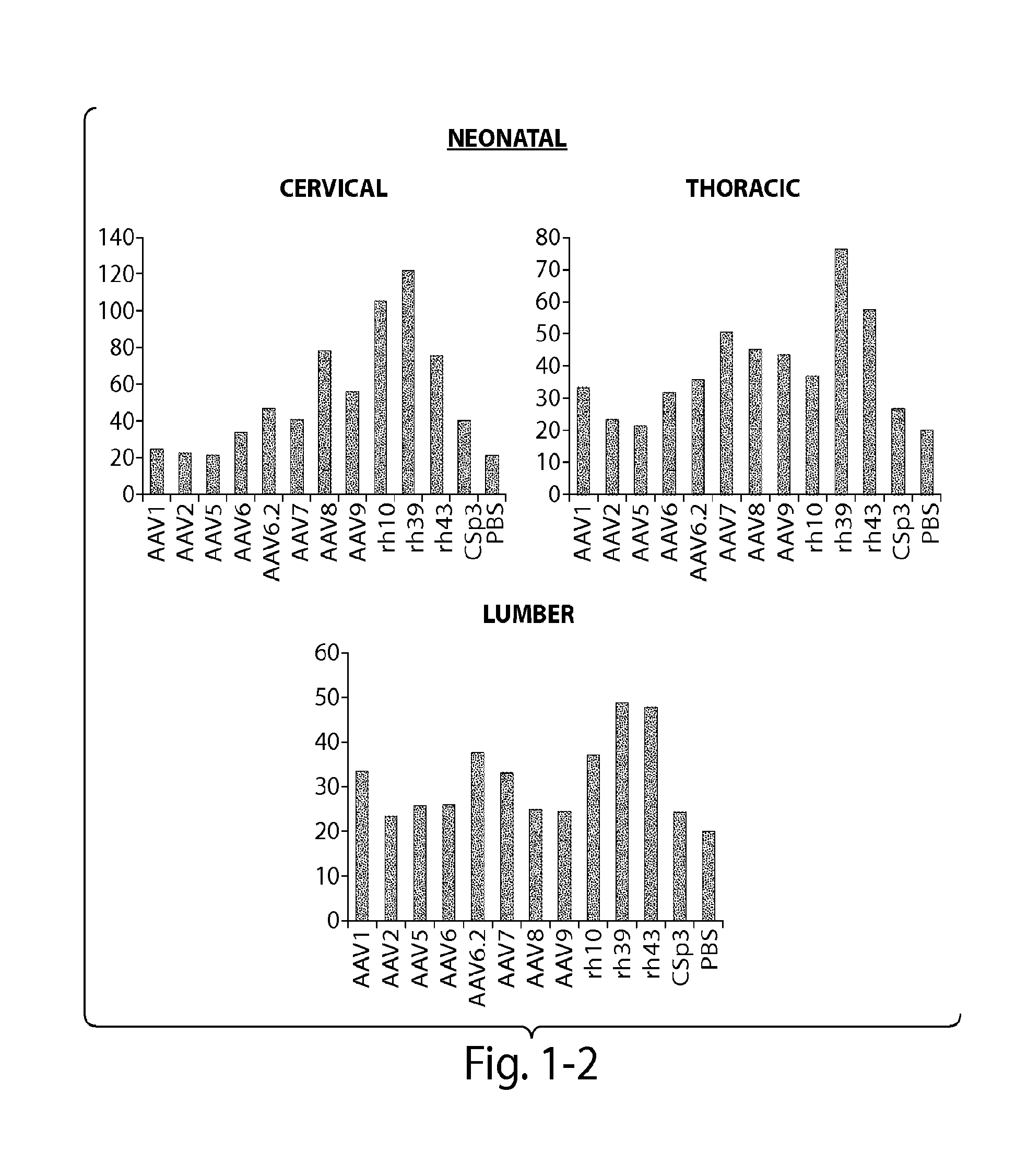
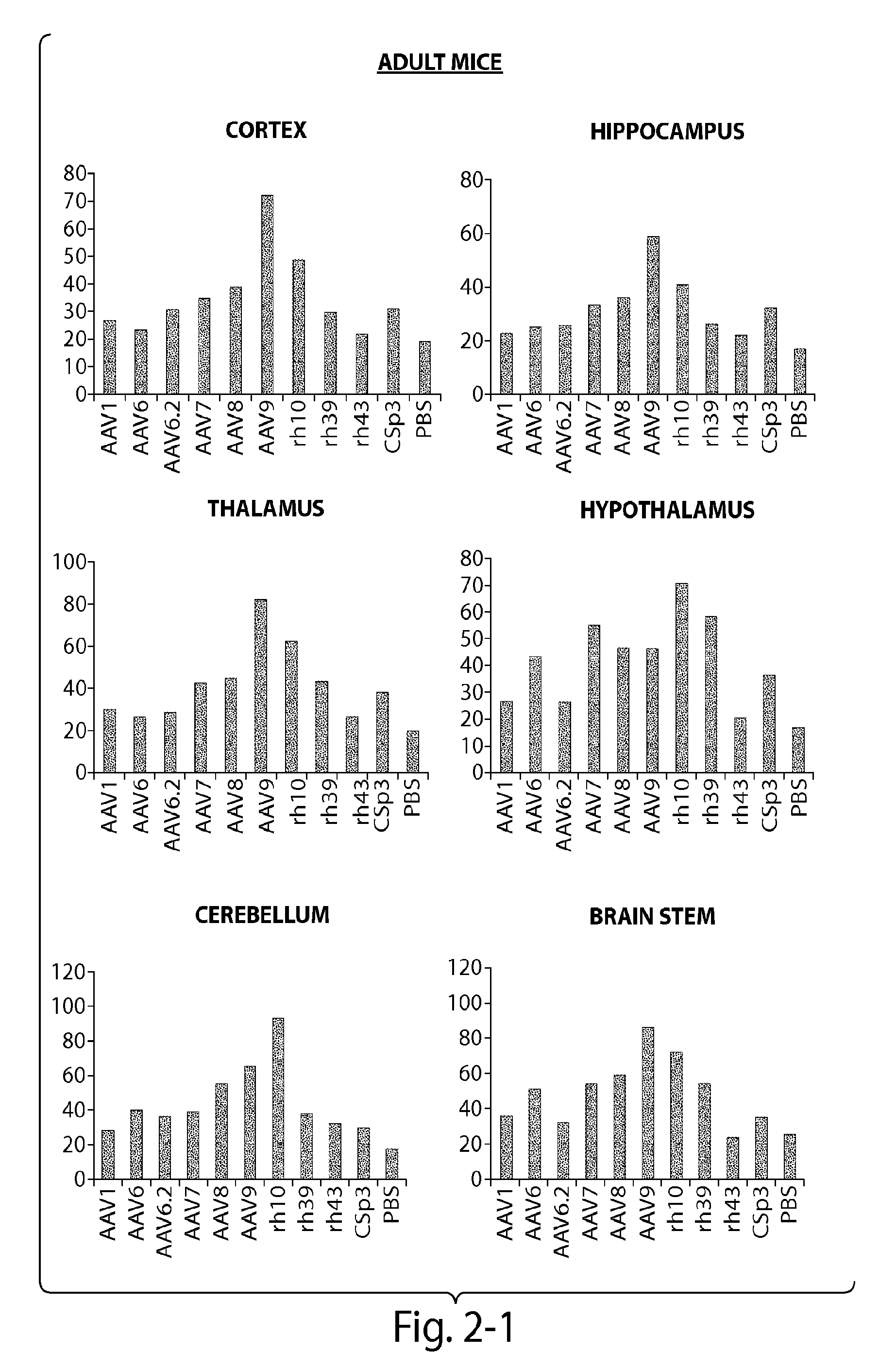
CNS targeting aav vectors and methods of use thereof
ActiveUS20130195801A1Widely distributedStable and nontoxic gene transferOrganic active ingredientsBiocideAdeno associate virusTransgene
Owner:UNIV OF MASSACHUSETTS
Mutant adeno-associated virus virions and methods of use thereof
ActiveUS20090202490A1Reduce the binding forceAltered infectivityOrganic active ingredientsBiocideCell type specificNeutralizing antibody
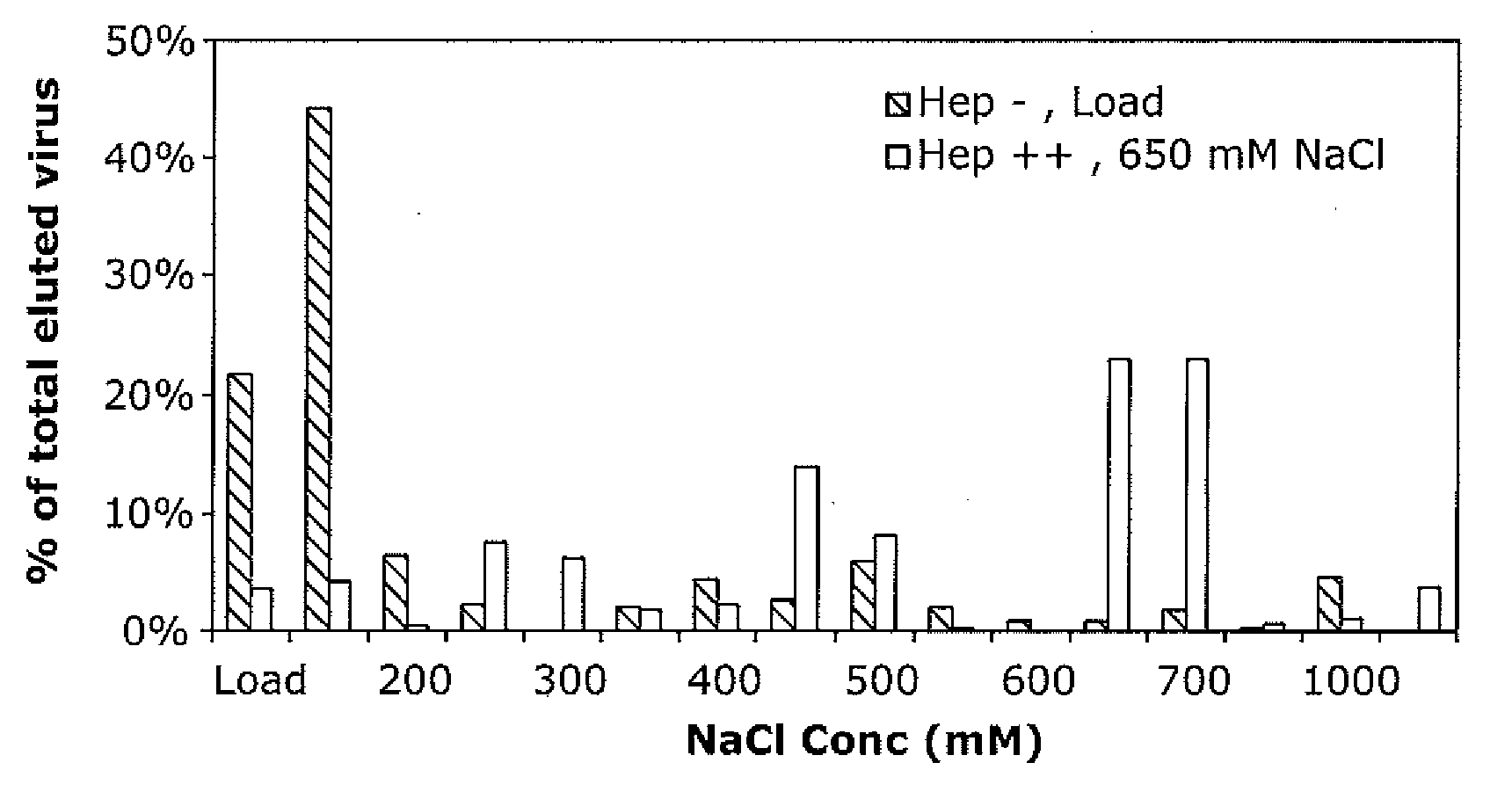
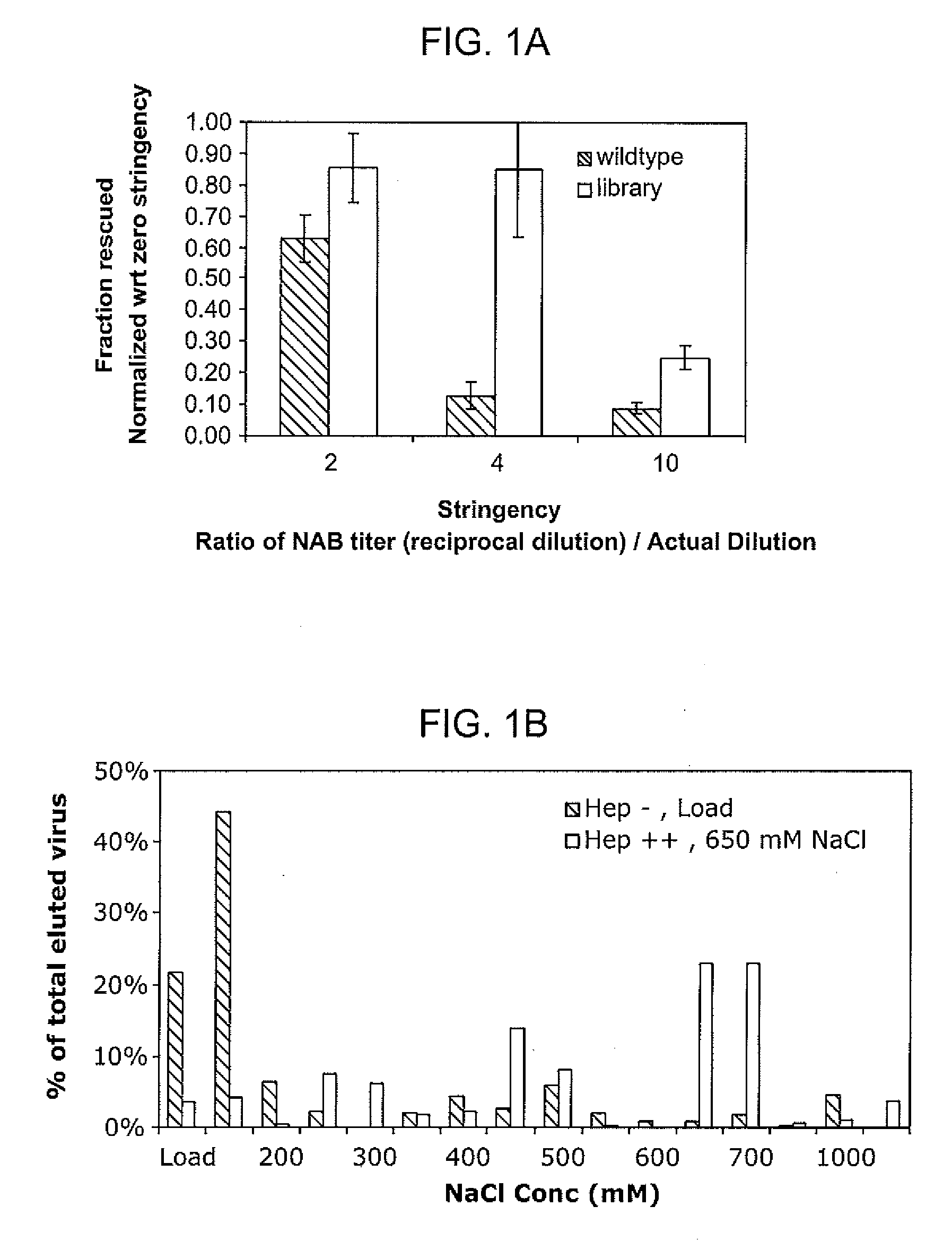
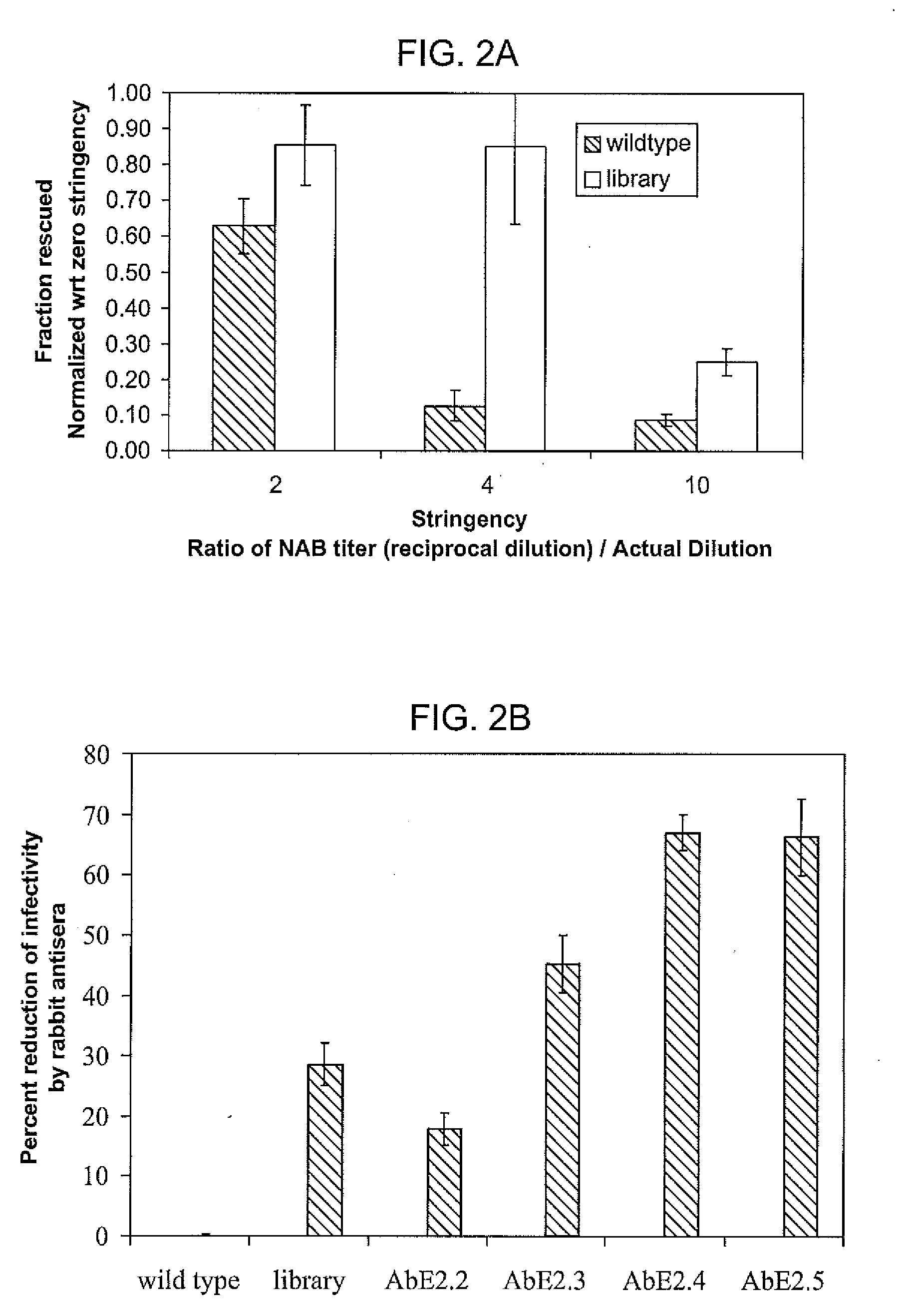
The present invention provides mutant adeno-associated virus (AAV) that exhibit altered capsid properties, e.g., reduced binding to neutralizing antibodies in serum and / or altered heparin binding and / or altered infectivity of particular cell types. The present invention further provides libraries of mutant AAV comprising one or more mutations in a capsid gene. The present invention further provides methods of generating the mutant AAV and mutant AAV libraries, and compositions comprising the mutant AAV. The present invention further provides recombinant AAV (rAAV) virions that comprise a mutant capsid protein. The present invention further provides nucleic acids comprising nucleotide sequences that encode mutant capsid proteins, and host cells comprising the nucleic acids. The present invention further provides methods of delivering a gene product to an individual, the methods generally involving administering an effective amount of a subject rAAV virion to an individual in need thereof.
Owner:RGT UNIV OF CALIFORNIA +1
Method for expression of small antiviral RNA molecules within a cell
ActiveUS20030059944A1Prevents sequenceHigh expressionOrganic active ingredientsPeptide/protein ingredientsGeneticsViral life cycle
In one aspect, the invention provides methods and compositions for the expression of small RNA molecules within a cell using a retroviral vector. The methods can be used to express double stranded RNA complexes. Small interfering RNA (siRNA) can be expressed using the methods of the invention within a cell, that interfere with a viral life cycle by down regulating either the viral genome, a viral genome transcript, or a host cell that. In another aspect the invention provides methods for treating patients having suffering from infection, particularly infection with HIV.
Owner:CALIFORNIA INST OF TECH
Human antibodies derived from immunized xenomice
Antibodies with fully human variable regions against a specific antigen can be prepared by administering the antigen to a transgenic animal which has been modified to produce such antibodies in response to antigenic challenge, but whose endogenous loci have been disabled. Various subsequent manipulations can be performed to obtain either antibodies per se or analogs thereof.
Owner:KUCHERLAPATI RAJU +4
Methods of generating chimeric adenoviruses and uses for such chimeric adenoviruses
A method for providing an adenovirus from a serotype which does not grow efficiently in a desired cell line with the ability to grow in that cell line is described. The method involves replacing the left and right termini of the adenovirus with the corresponding termini from an adenovirus which grow efficiently in the desired cell line. At a minimum, the left terminus spans the 5′ inverted terminal repeat, the left terminus spans the E4 region and the 3′ inverted terminal repeat. The resulting chimeric adenovirus contains the internal regions spanning the genes encoding the penton, hexon and fiber from the serotype which does not grow efficiently in the desired cell. Also provided are vectors constructed from novel simian adenovirus sequences and proteins, host cells containing same, and uses thereof.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
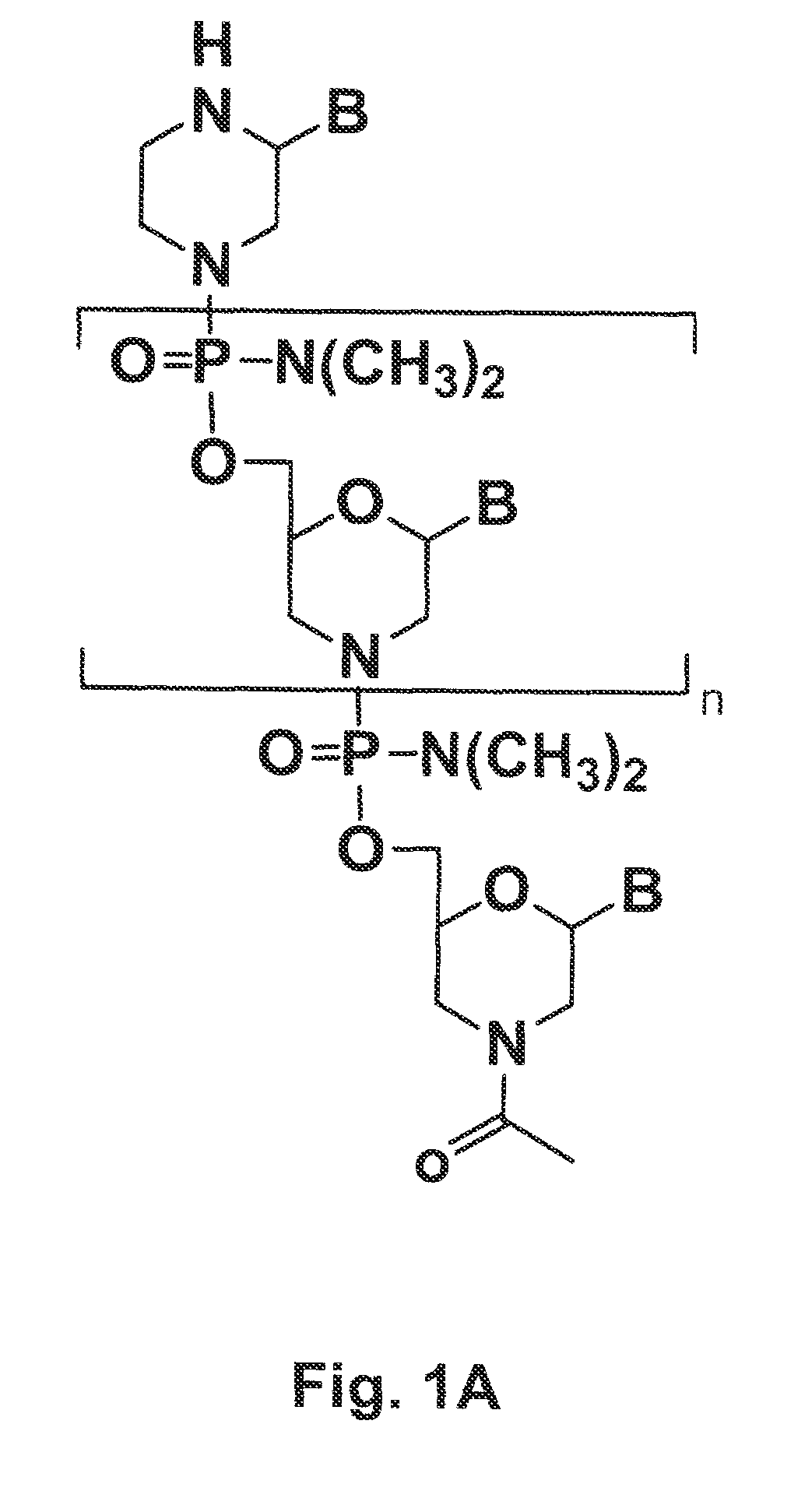
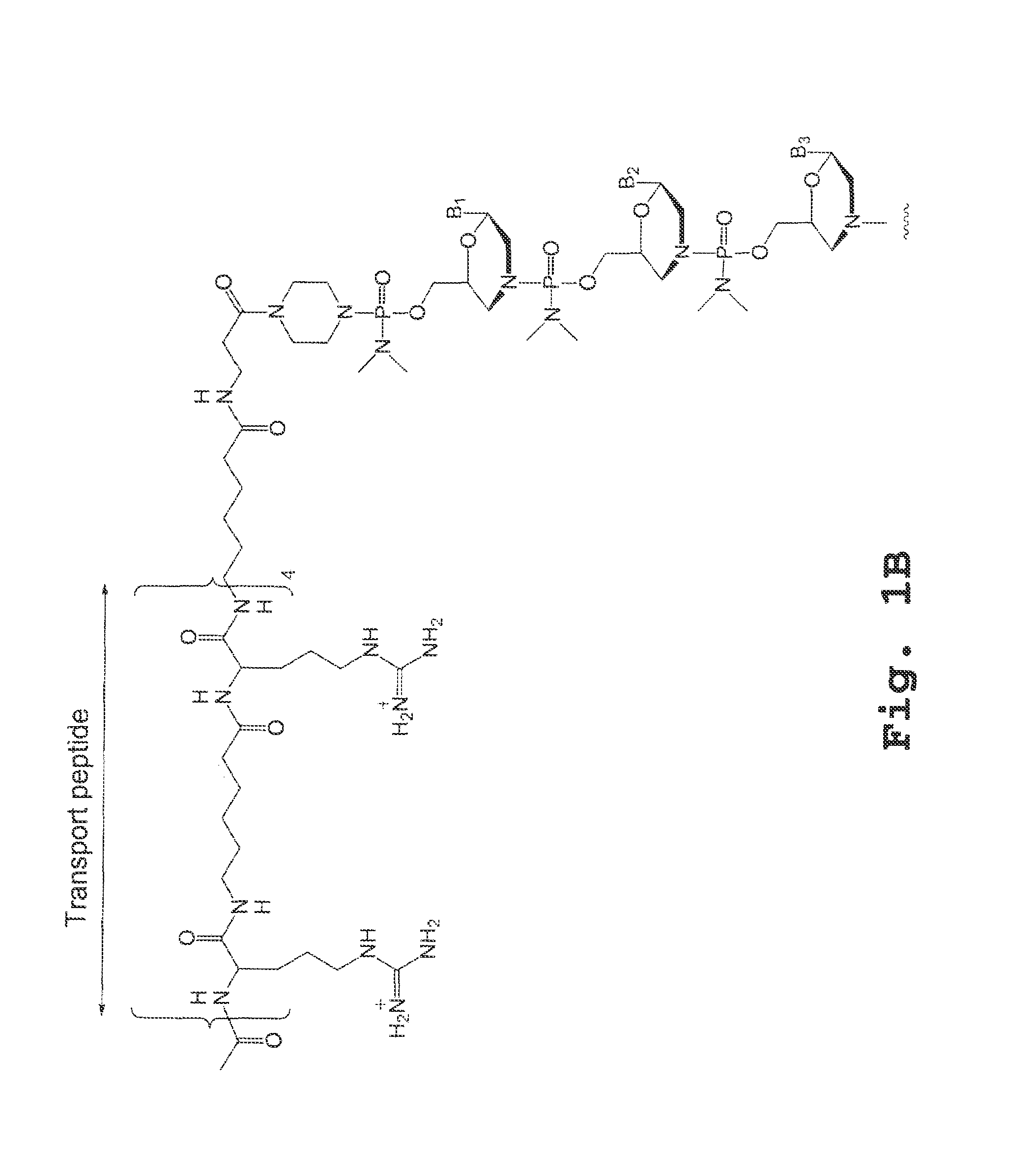
Tissue specific peptide conjugates and methods
Cell-penetrating peptides useful for targeting a therapeutic compound to a selected mammalian tissue, methods for their identification, methods of forming conjugate compounds containing such peptides, and conjugates formed thereby are disclosed. The cell-penetrating peptides are 8 to 30 amino acid residues in length and consist of subsequences selected from the group consisting of RXR, RX, RB, and RBR; where R is arginine, B is β-alanine, and each X is independently —C(O)—(CHR1)n—NH—, where n is 4-6 and each R1 is independently H or methyl, such that at most two R1's are methyl. In one embodiment, X is a 6-aminohexanoic acid residue.
Owner:AVI BIOPHARMA
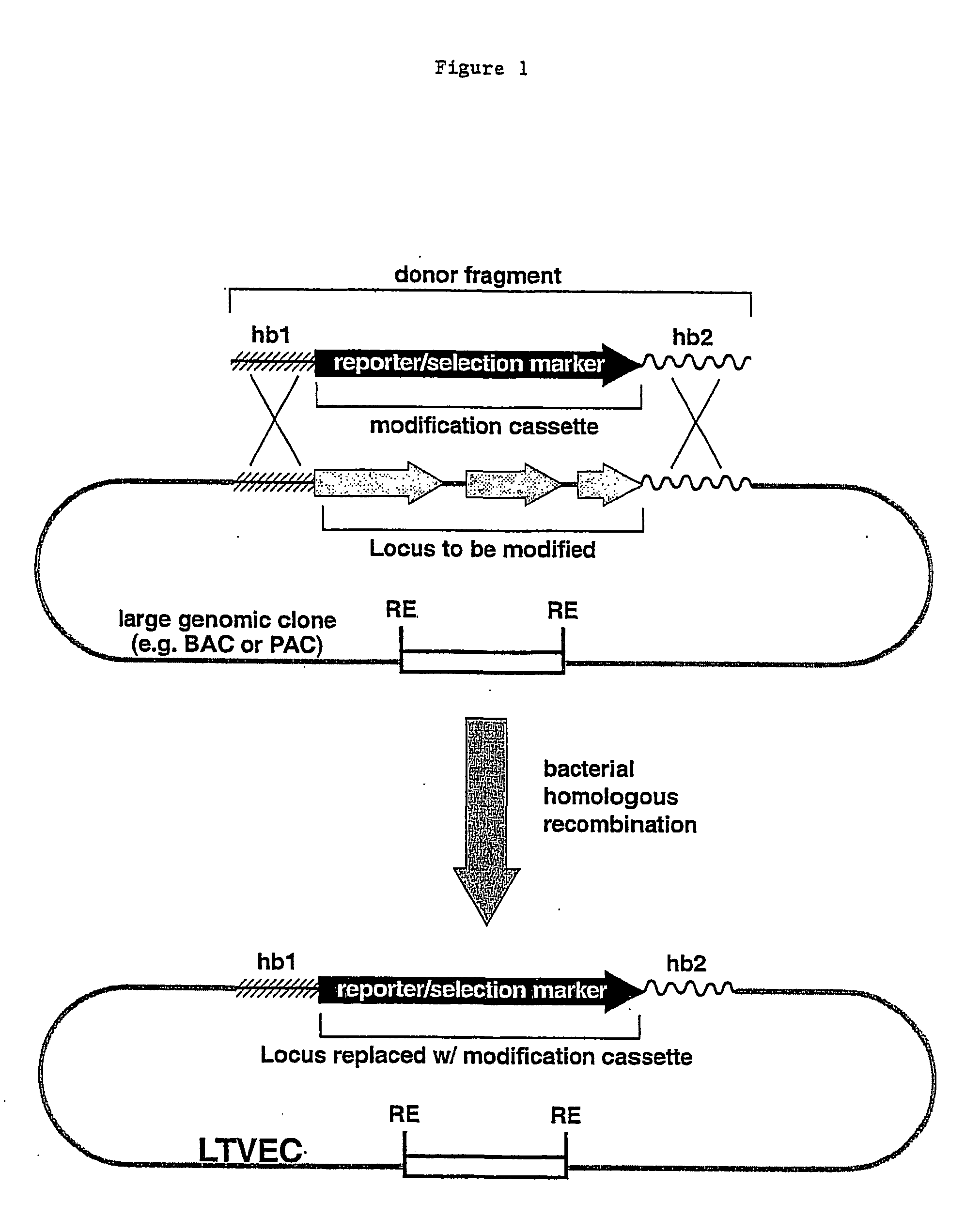
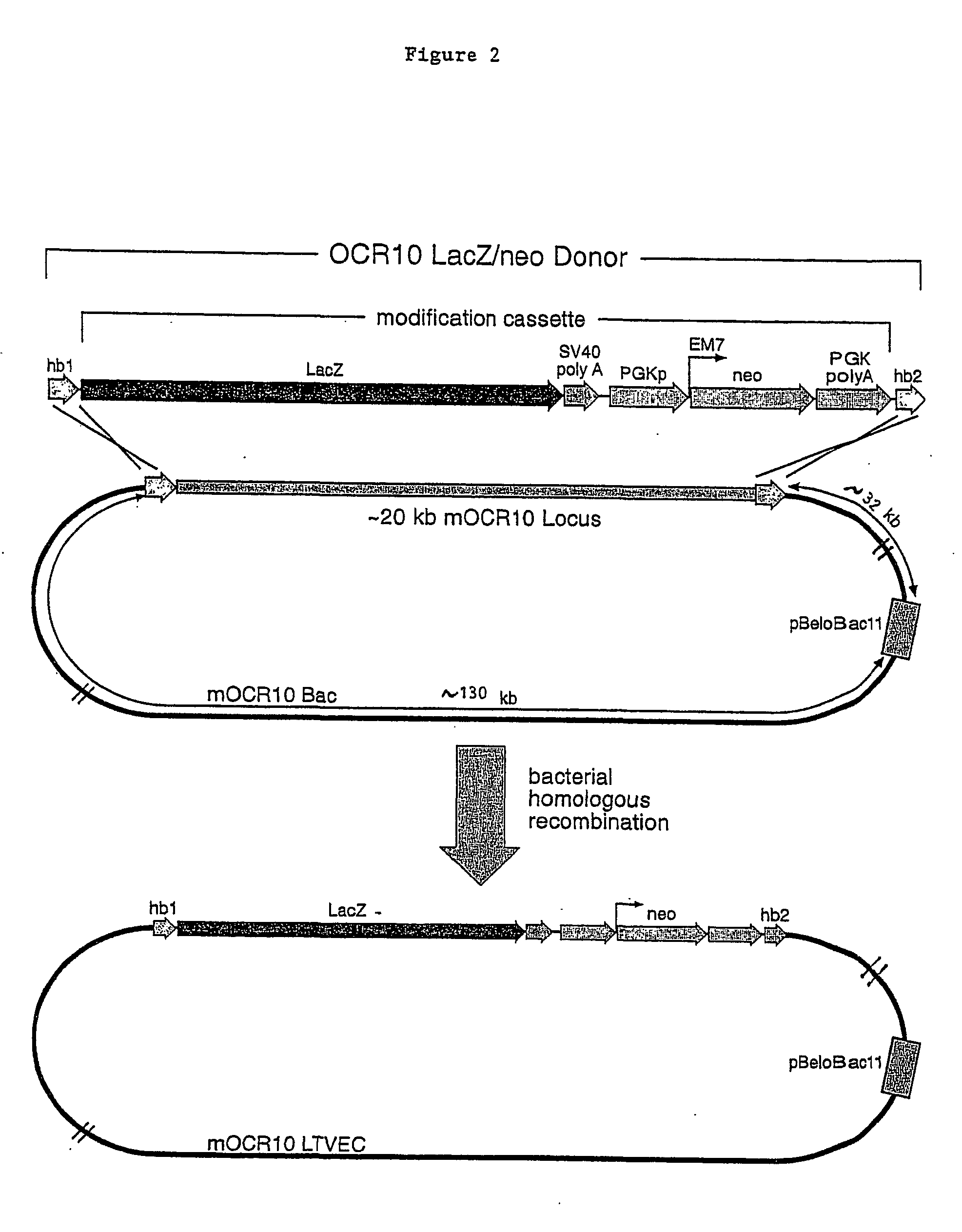
Methods of modifying eukaryotic cells
A method for engineering and utilizing large DNA vectors to target, via homologous recombination, and modify, in any desirable fashion, endogenous genes and chromosomal loci in eukaryotic cells. These large DNA targeting vectors for eukaryotic cells, termed LTVECs, are derived from fragments of cloned genomic DNA larger than those typically used by other approaches intended to perform homologous targeting in eukaryotic cells. Also provided is a rapid and convenient method of detecting eukaryotic cells in which the LTVEC has correctly targeted and modified the desired endogenous genes(s) or chromosomal locus (loci) as well as the use of these cells to generate organisms bearing the genetic modification.
Owner:REGENERON PHARM INC
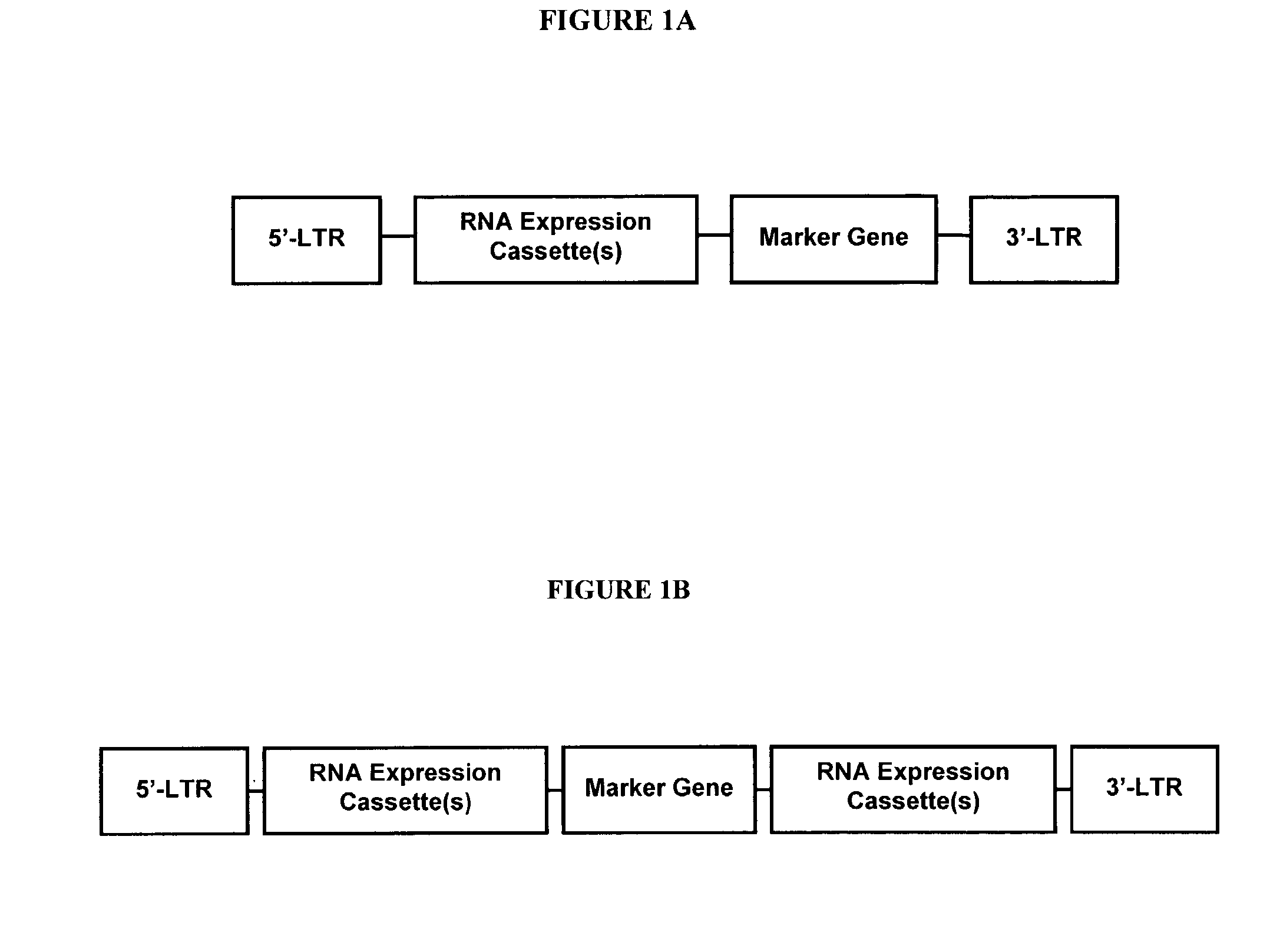
Vectors
InactiveUS20060281180A1Stable long-term expressionEfficient expressionAntibacterial agentsFactor VIIGeneticsViral vector
Provided is a lentiviral vector capable of delivering a nucleotide of interest (NOI) to a desired target site and wherein the NOI encodes the Factor VIII and the Factor VIII is expressed following delivery of the NOI to the desired target site.
Owner:OXFORD BIOMEDICA (UK) LTD
Transgenic organism
InactiveUS20090007284A1Efficient productionEfficiency advantageAnimal cellsVectorsNucleotide sequencingPolynucleotide
A method of producing a transgenic cell comprising introducing into a cell a non-primate lentiviral expression vector comprising a nucleotide of interest (NOI). Also described is a method of producing a transgenic cell comprising introducing into a cell a lentiviral expression vector comprising a NOI capable of generating an antisense oligonucleotide, a ribozyme, an siRNA, a short hairpin RNA, a micro-RNA or a group 1 intron. Also described is a viral vector comprising a first nucleotide sequence, wherein said first nucleotide sequence comprises: (a) a second nucleotide sequence comprising an aptazyme; and (b) a third nucleotide sequence capable of generating a polynucleotide; wherein (a) and (b) are operably linked and wherein the aptazyme is activatable to cleave a transcript of the first nucleotide sequence such that said polynucleotide is generated.
Owner:RADCLIFFE PHILIPPA +2
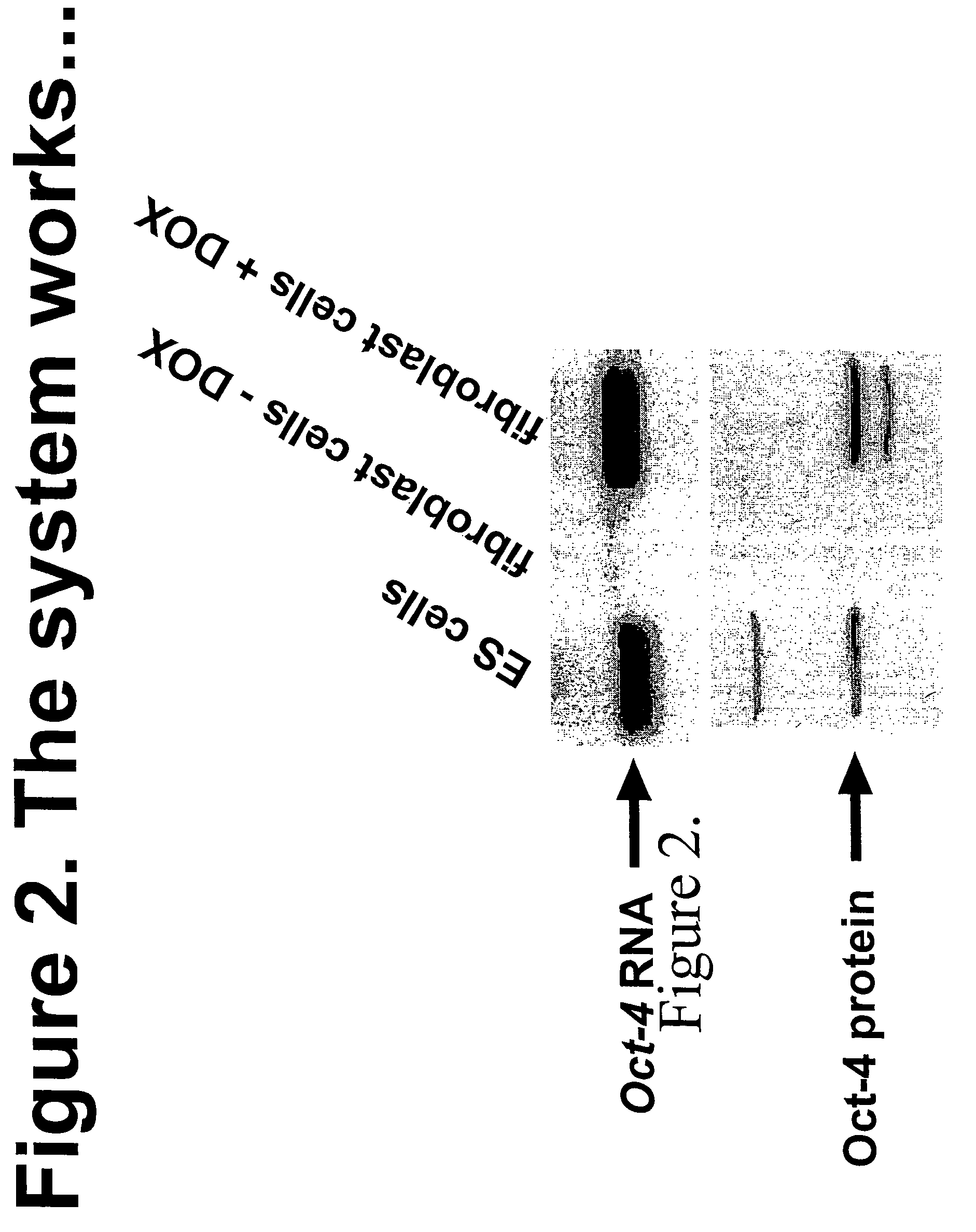
Methods for reprogramming somatic cells
Owner:WHITEHEAD INST FOR BIOMEDICAL RES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com