Patents
Literature
65 results about "Ribonucleoprotein" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
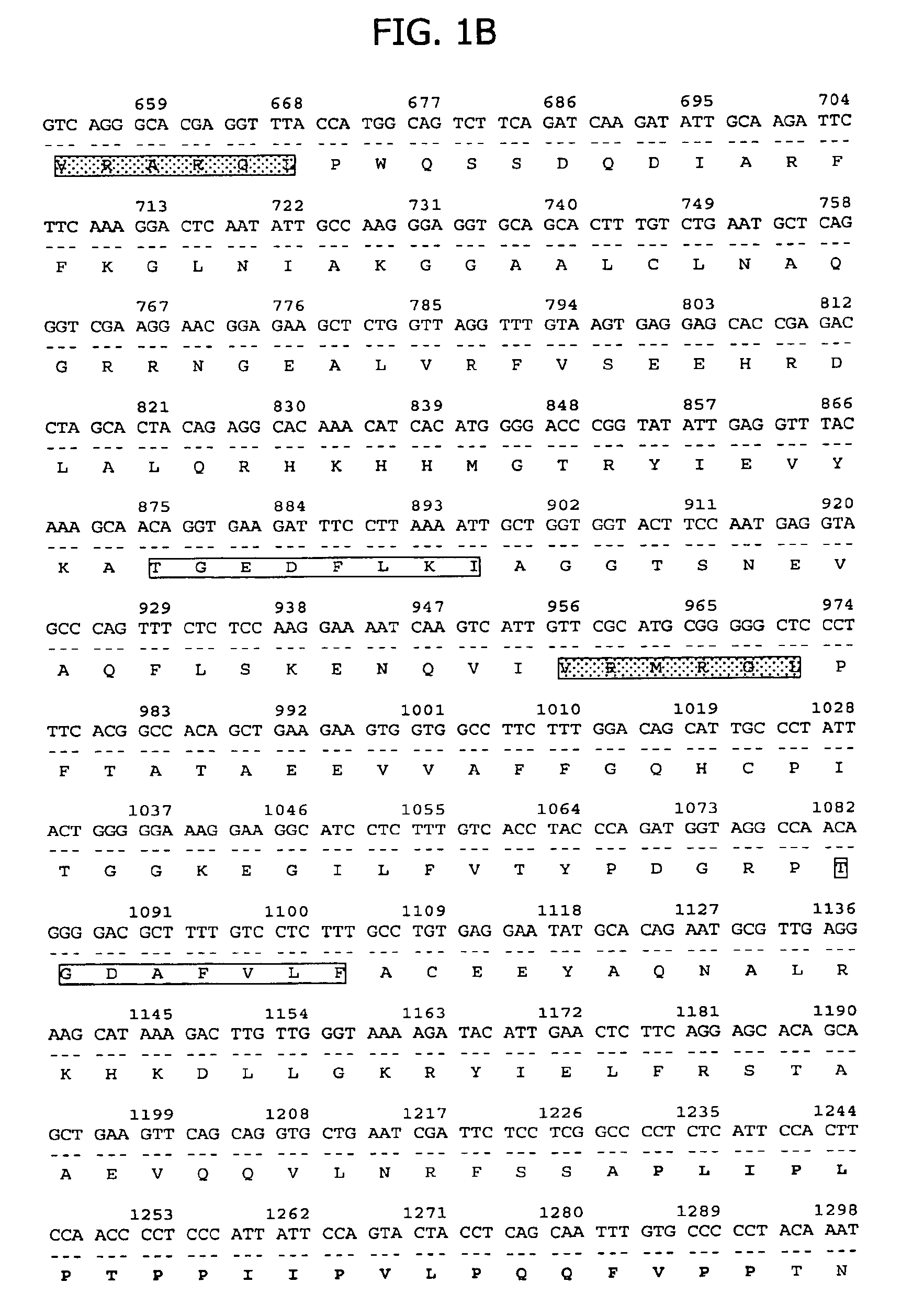
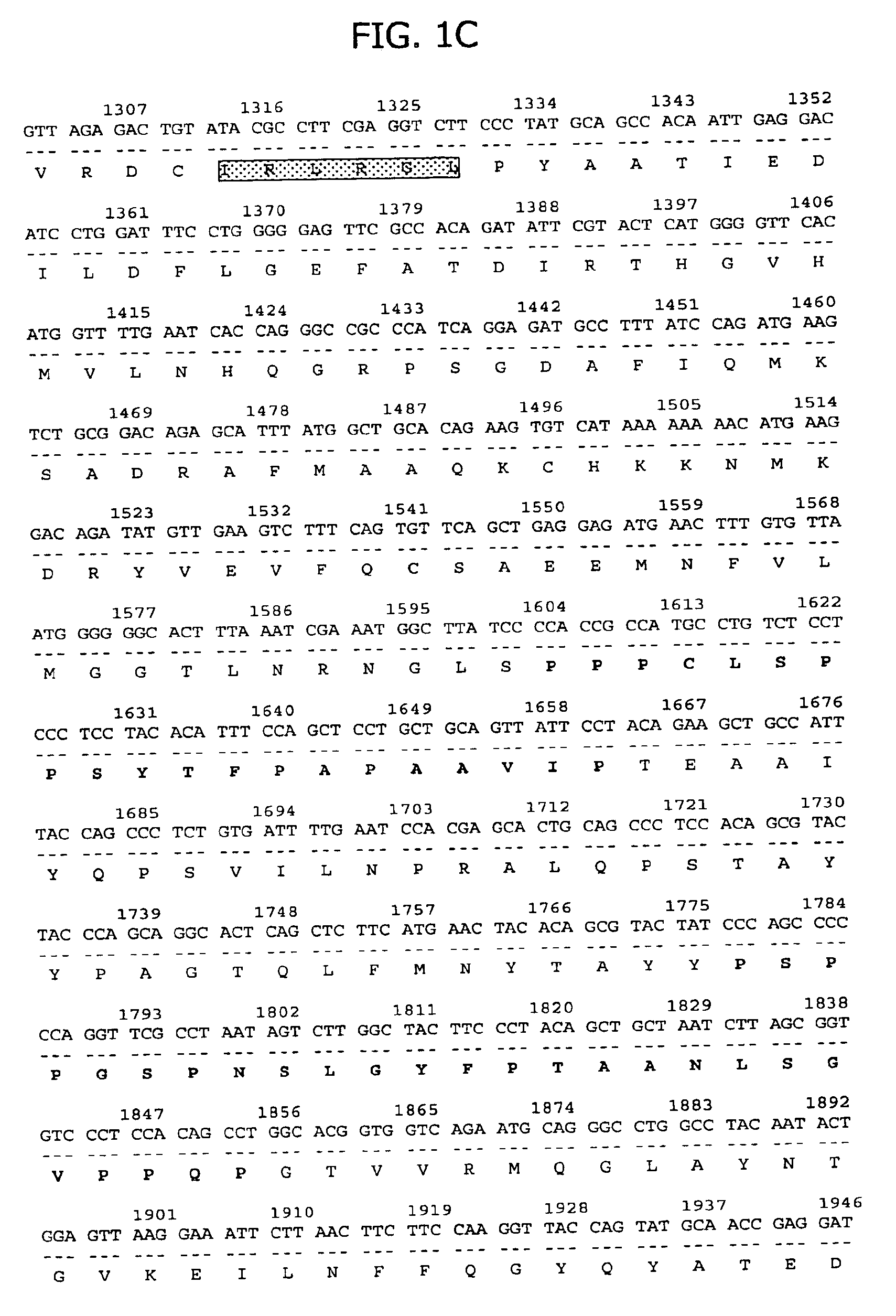
Ribonucleoprotein is a nucleoprotein that contains RNA, i.e. it is an association that combines ribonucleic acid and protein together. A few known examples include the ribosome, the enzyme telomerase, vault ribonucleoproteins, RNase P, hnRNP and small nuclear RNPs, which are implicated in pre-mRNA splicing and are among the main components of the nucleolus. Currently, over 2000 RNPs can be found in PDB database. Based on known structures some common features of protein-RNA interface were deduced. For example, RNP in snRNPs has an RNA-binding motif in its RNA-binding protein. Aromatic amino acid residues in this motif result in stacking interactions with RNA. Lysine residues in the helical portion of RNA-binding proteins help to stabilize interactions with nucleic acids. This nucleic acid binding is strengthened by electrostatic attraction between the positive lysine side chains and the negative nucleic acid phosphate backbones. Additionally, it is possible to model RNPs computationally. RNPs among many can play an important role in influenza A virus replication. The viral RNA is transcribed into mRNAs by the RNA polymerase attached to the RNPs.
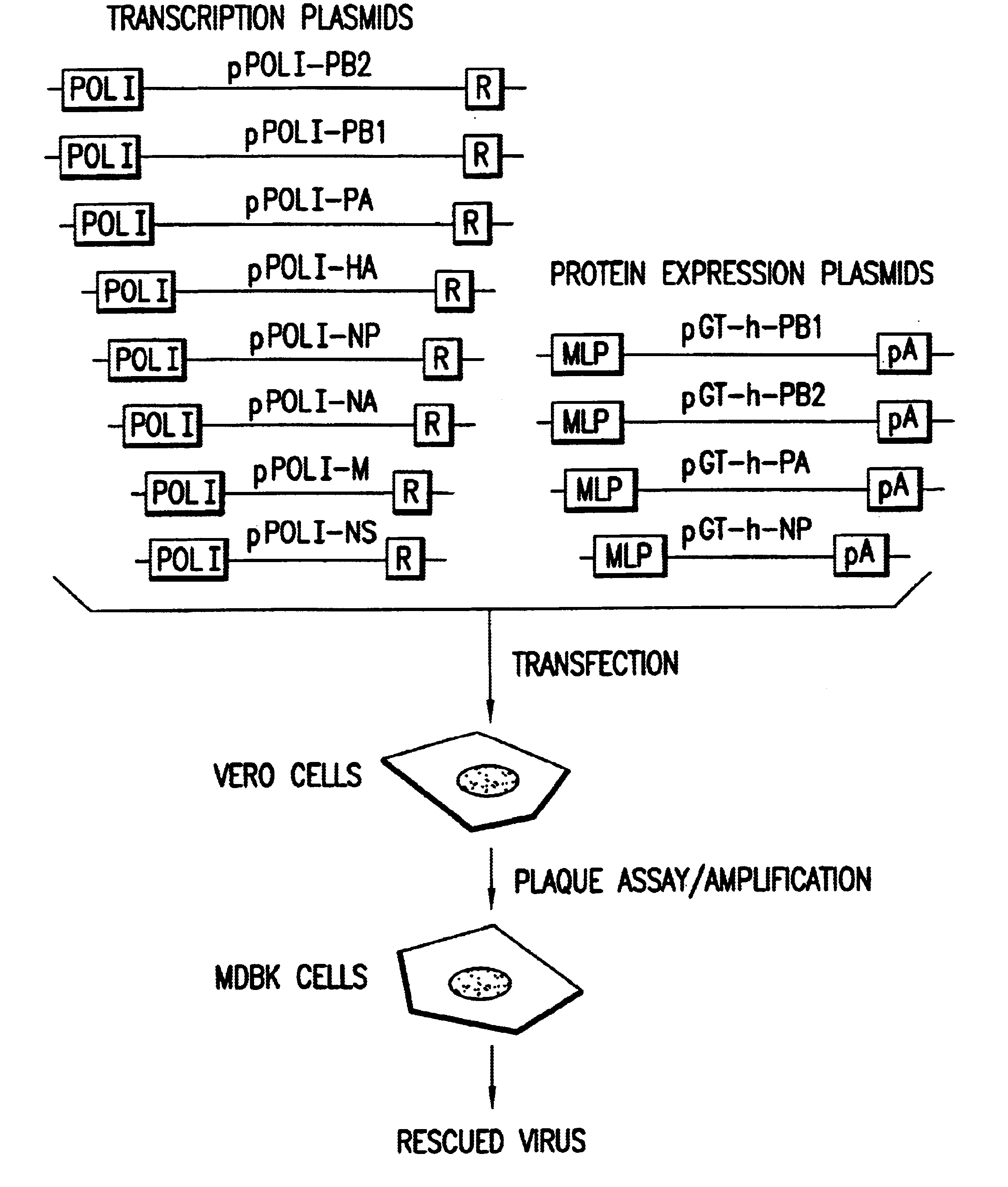
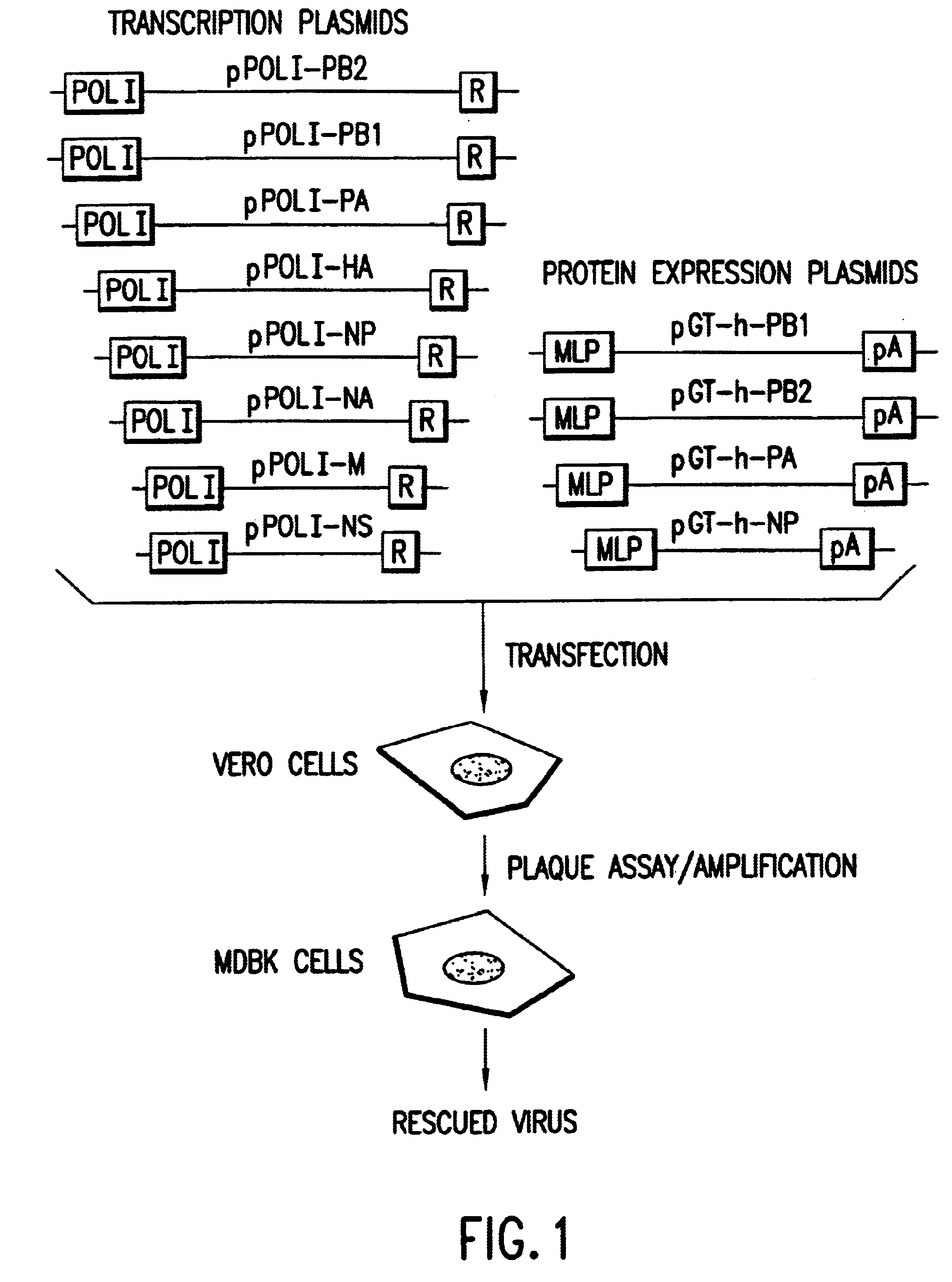
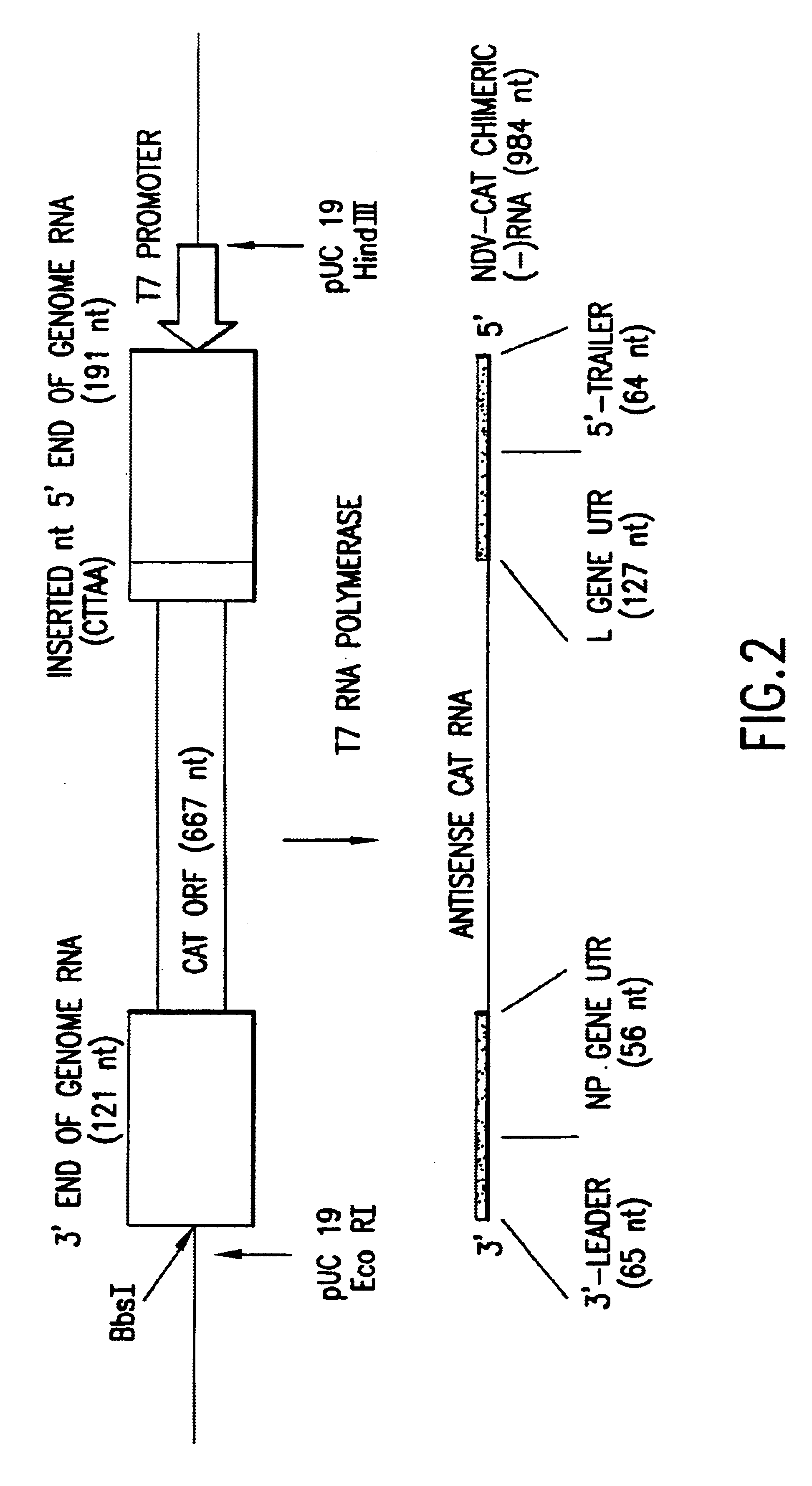
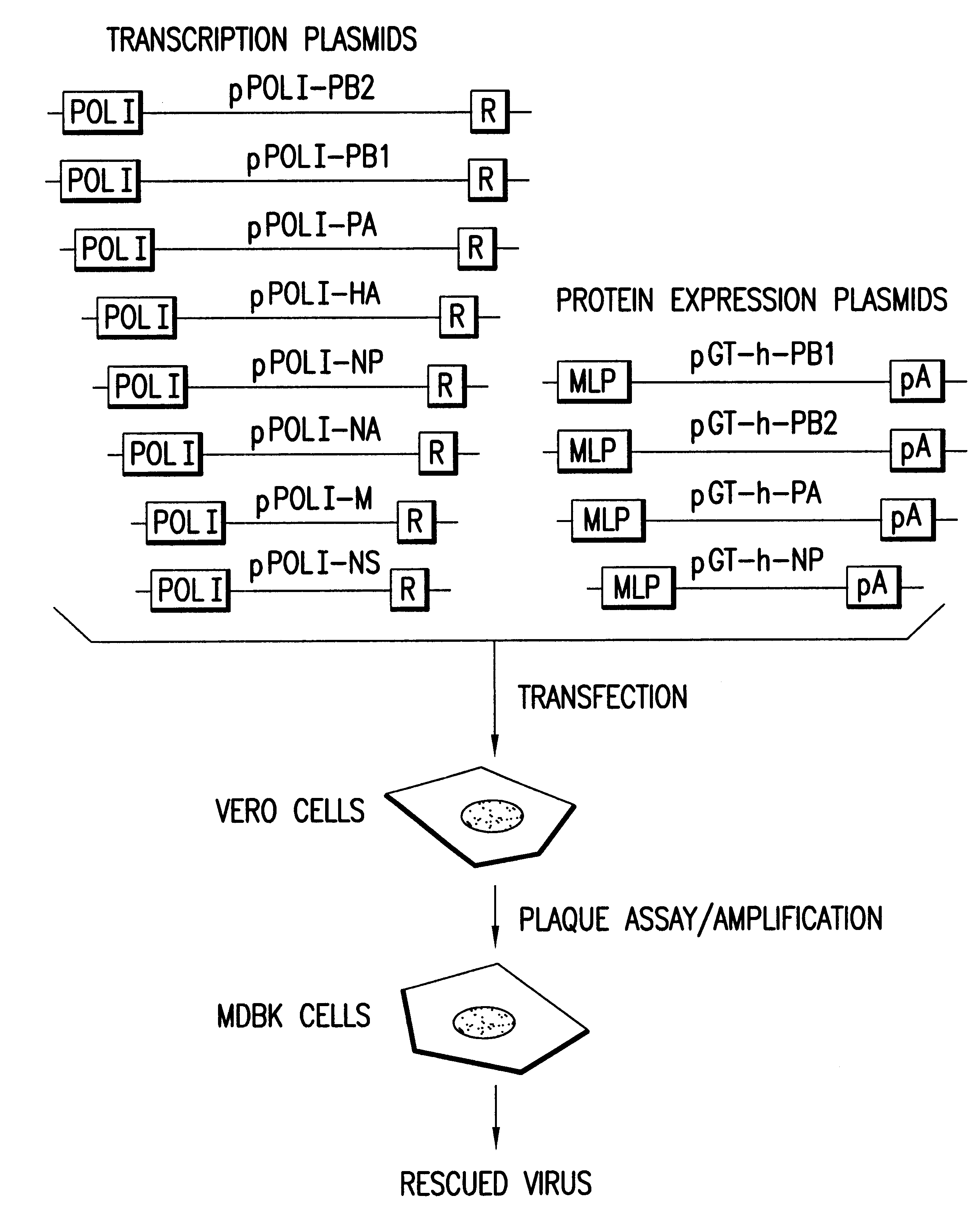
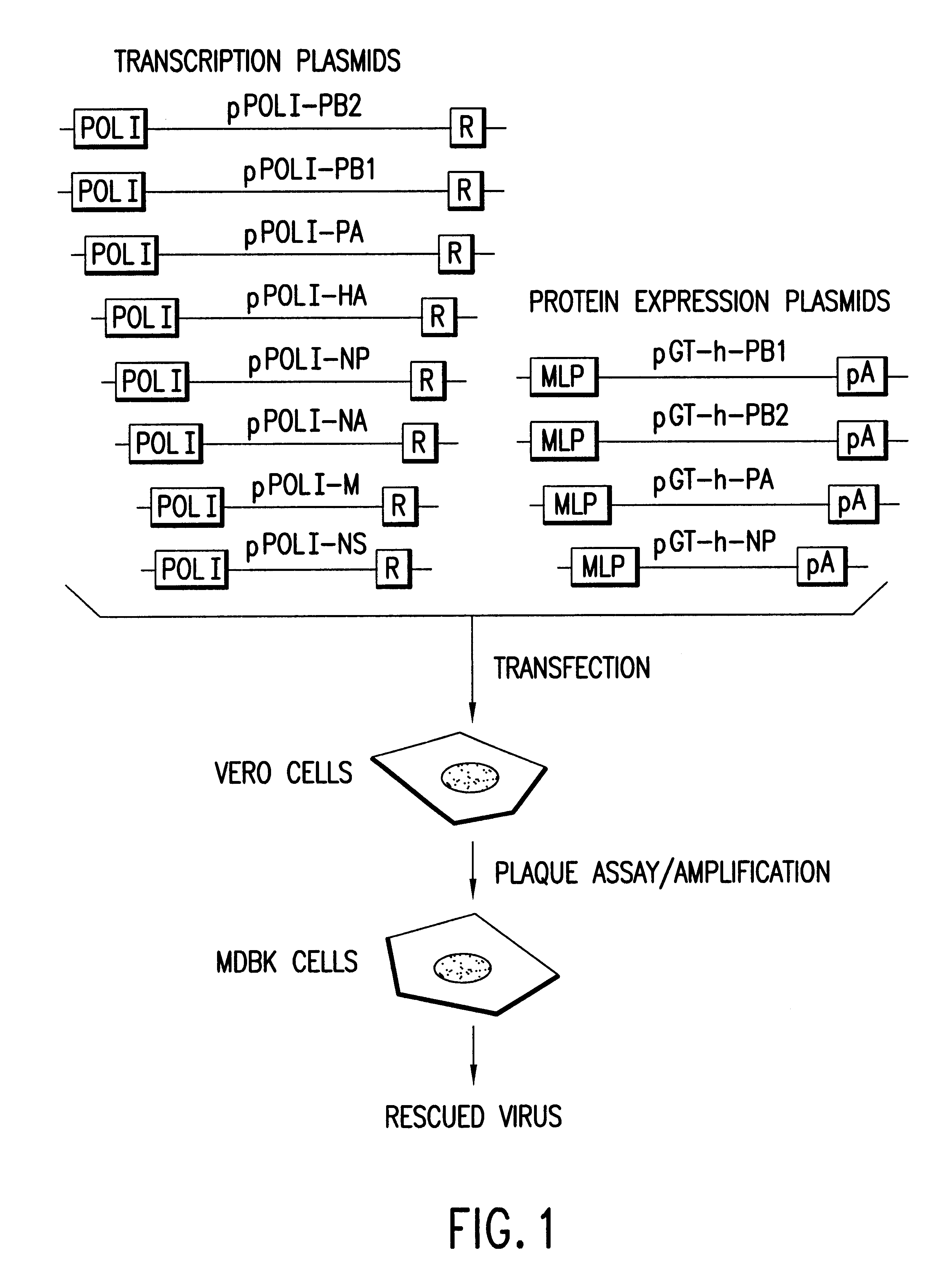
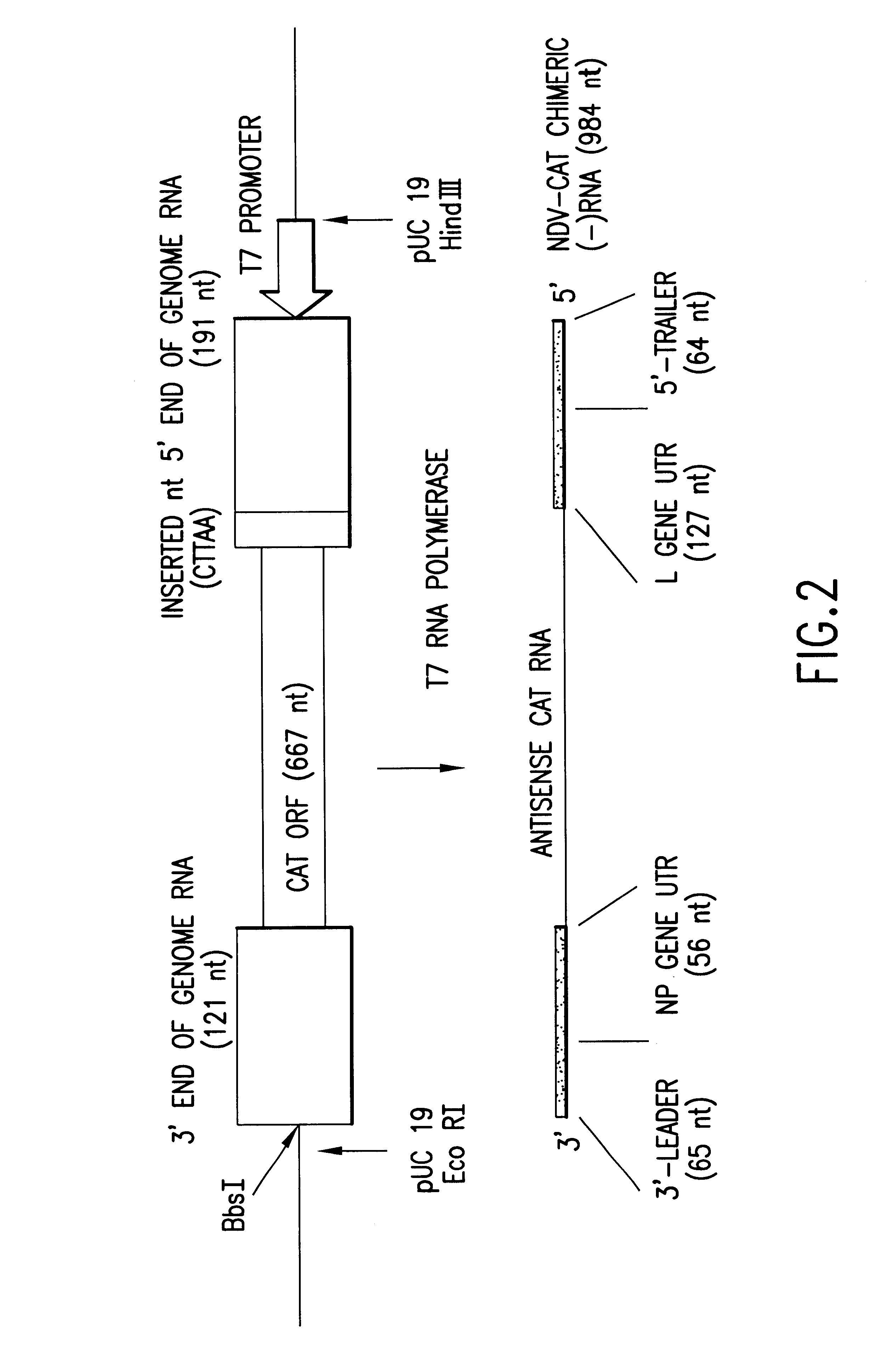
Helper-free rescue of recombinant negative strand RNA virus
Owner:MT SINAI SCHOOL OF MEDICINE
Helper-free rescue of recombinant negative strand RNA viruses
InactiveUS6544785B1SsRNA viruses negative-senseGenetic material ingredientsNegative strandNucleic acid sequence
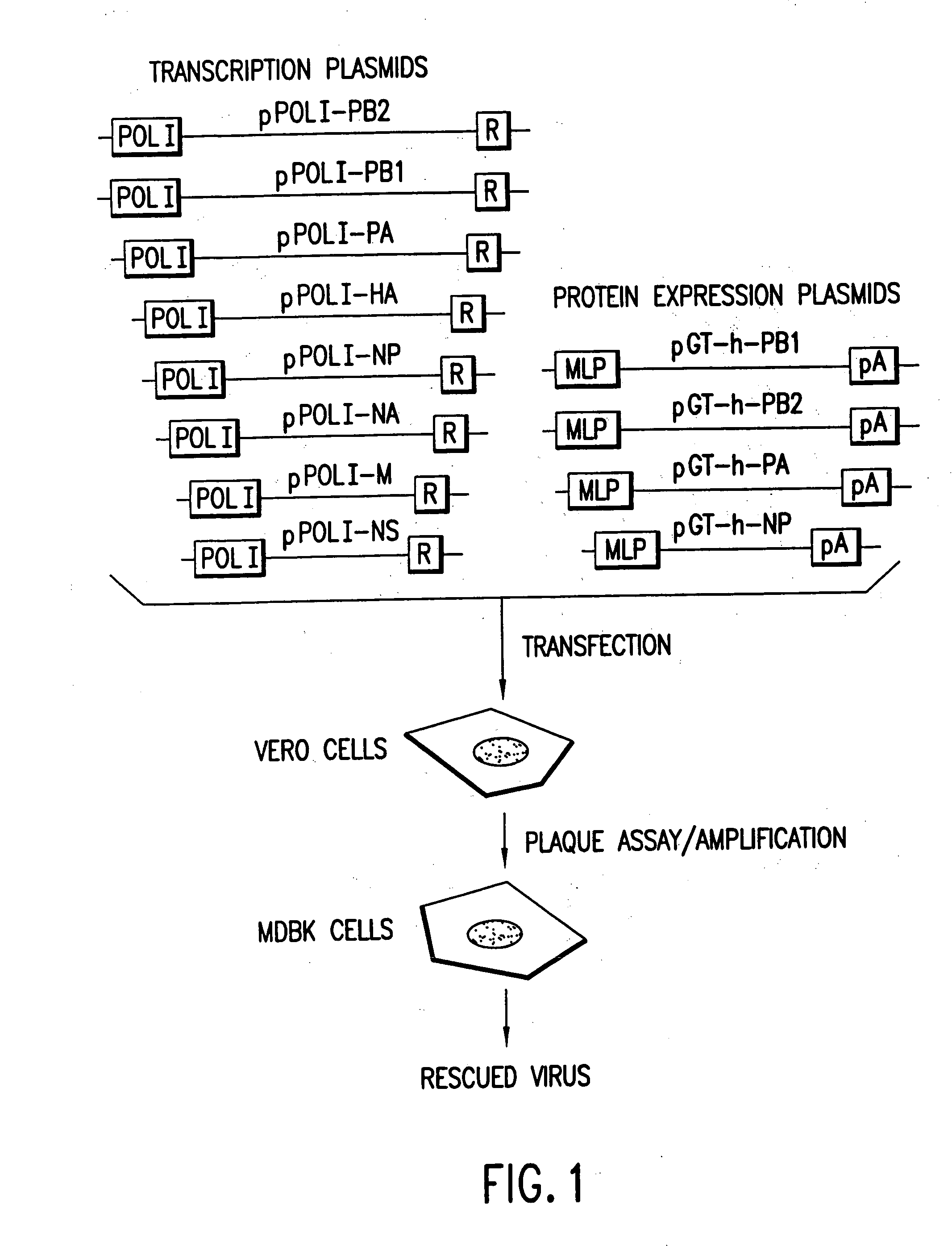
The present invention relates methods of generating infectious negative-strand virus in host cells by an entirely vector-based system without the aid of a helper virus. In particular, the present invention relates methods of generating infectious recombinant negative-strand RNA viruses intracellularly in the absence of helper virus from expression vectors comprising cDNAs encoding the viral proteins necessary to form ribonucleoprotein complexes (RNPs) and expression vectors comprising cDNA for genomic viral RNA(s) (vRNAs) or the corresponding cRNA(s). The present invention also relates to methods of generating infectious recombinant negative-strand RNA viruses which have mutations in viral genes and / or which express, package and / or present peptides or polypeptides encoded by heterologous nucleic acid sequences. The present invention further relates the use of the recombinant negative-strand RNA viruses or chimeric negative-strand RNA viruses of the invention in vaccine formulations and pharmaceutical compositions.
Owner:MT SINAI SCHOOL OF MEDICINE
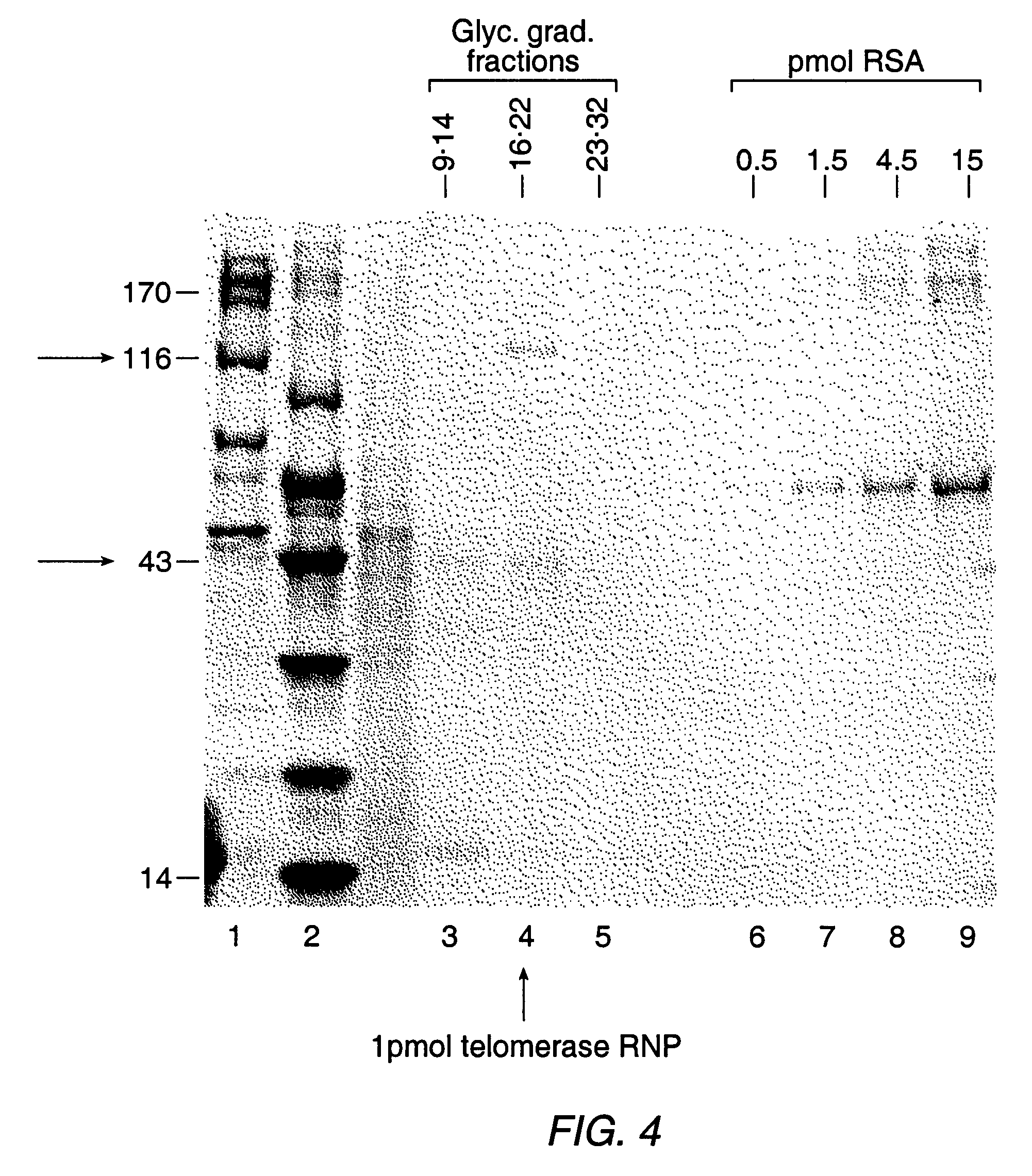
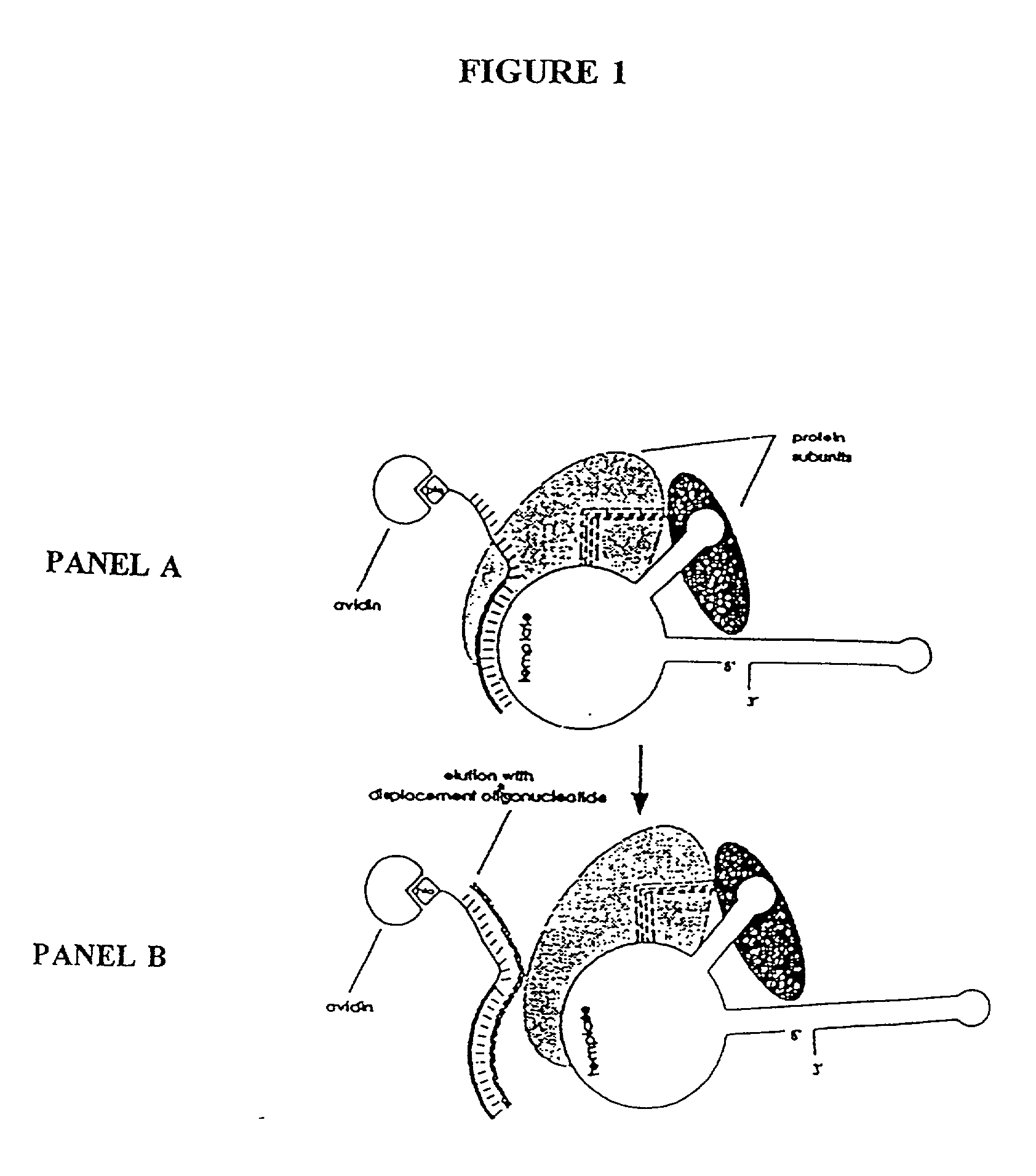
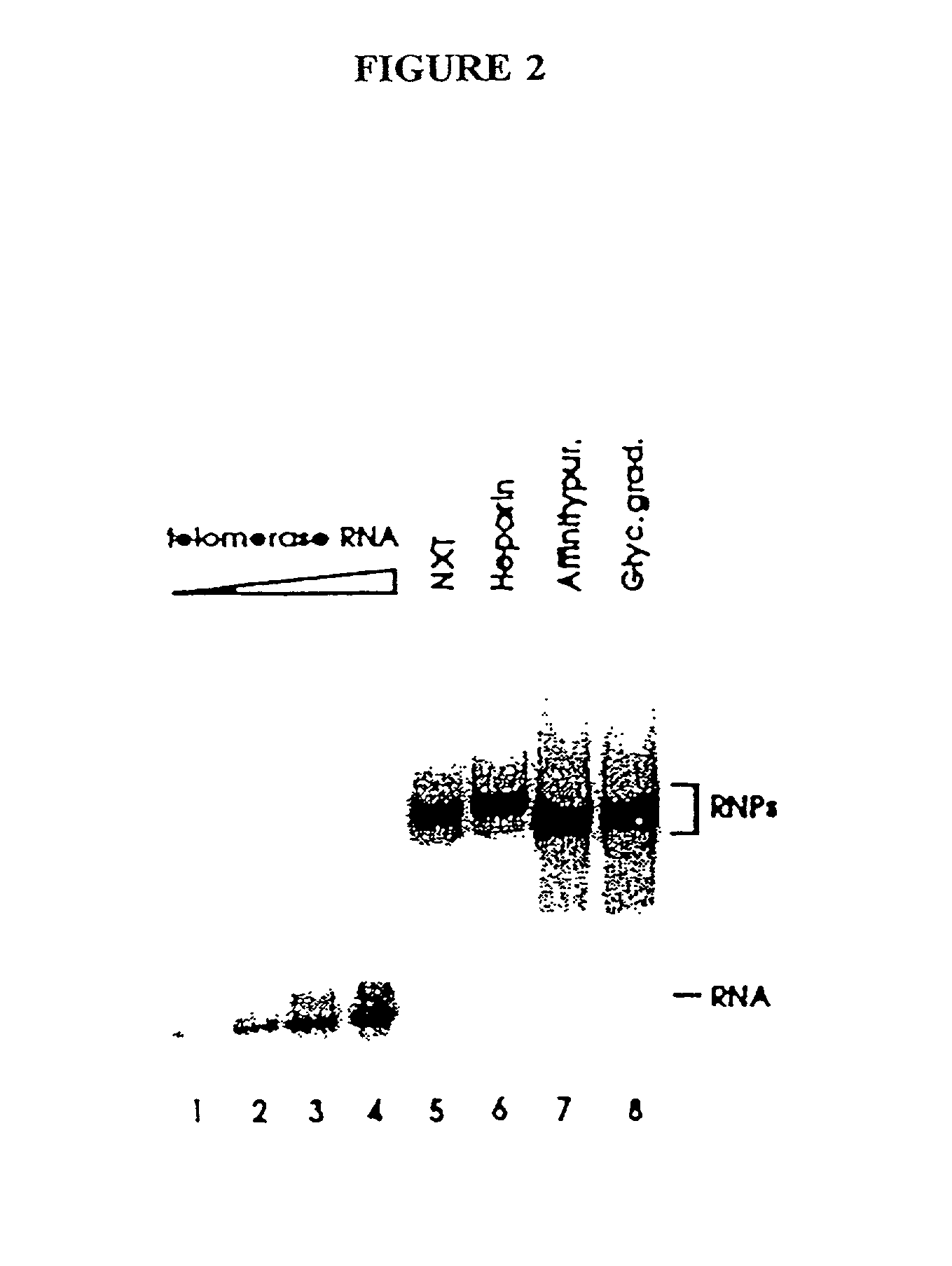
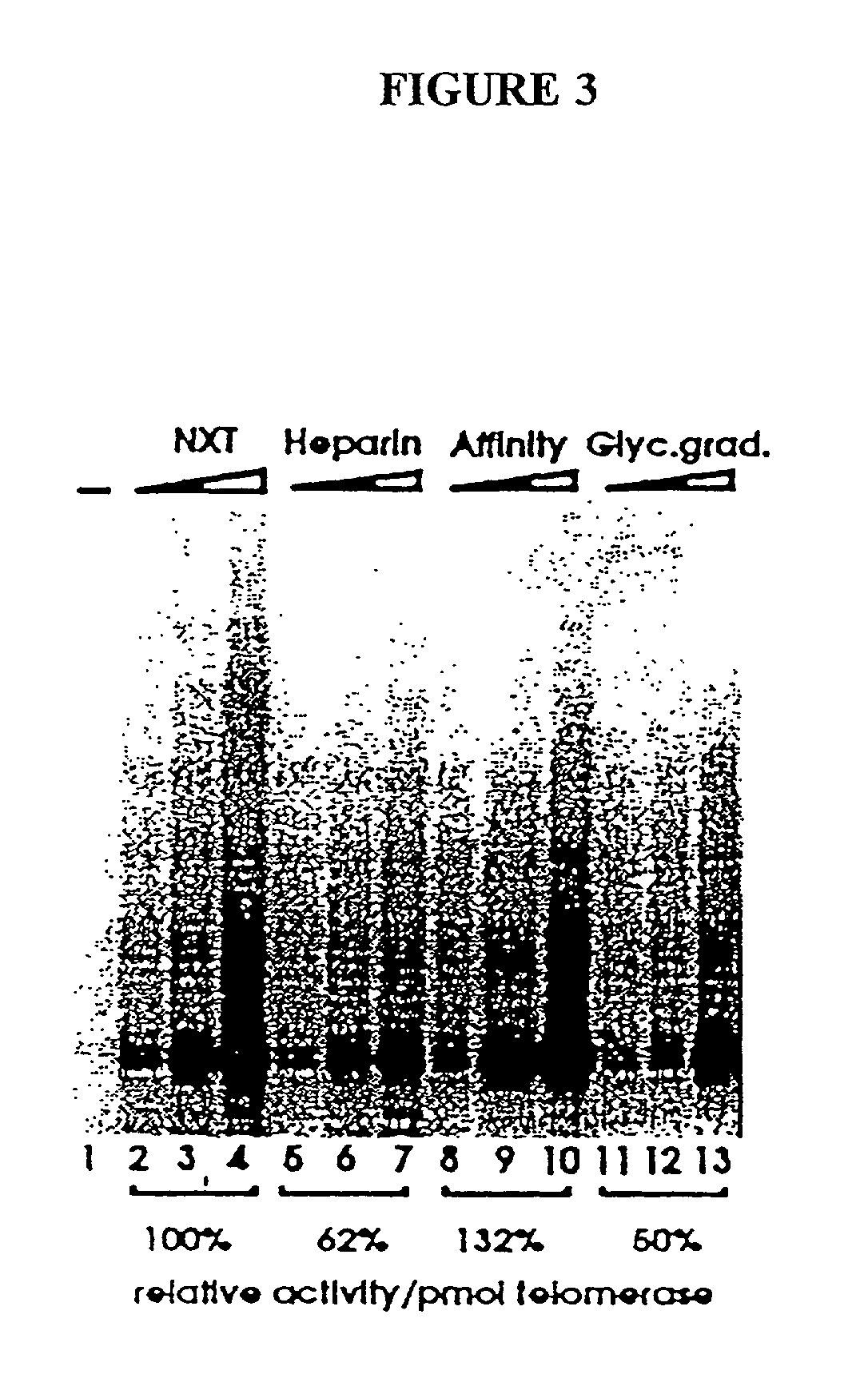
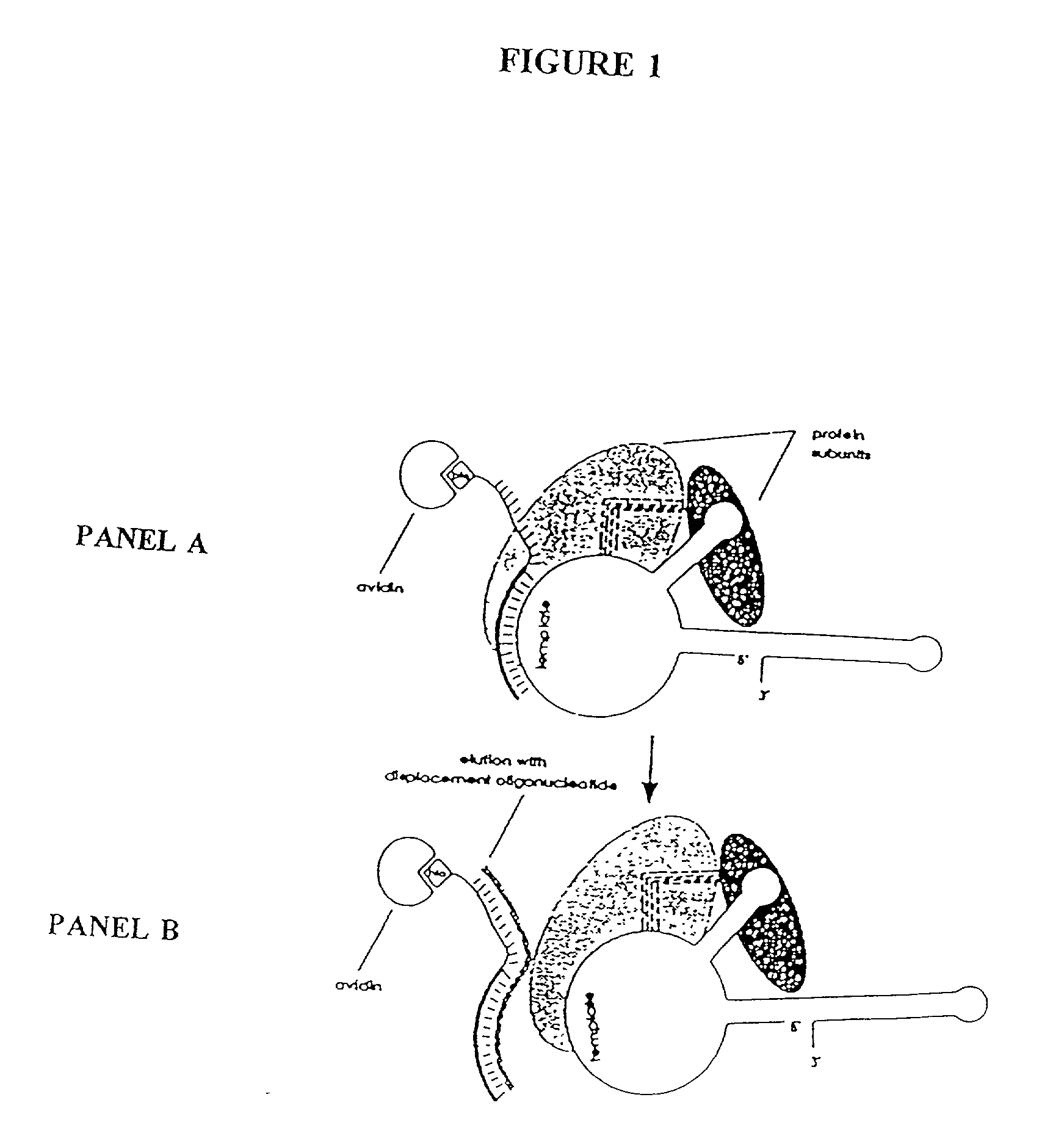
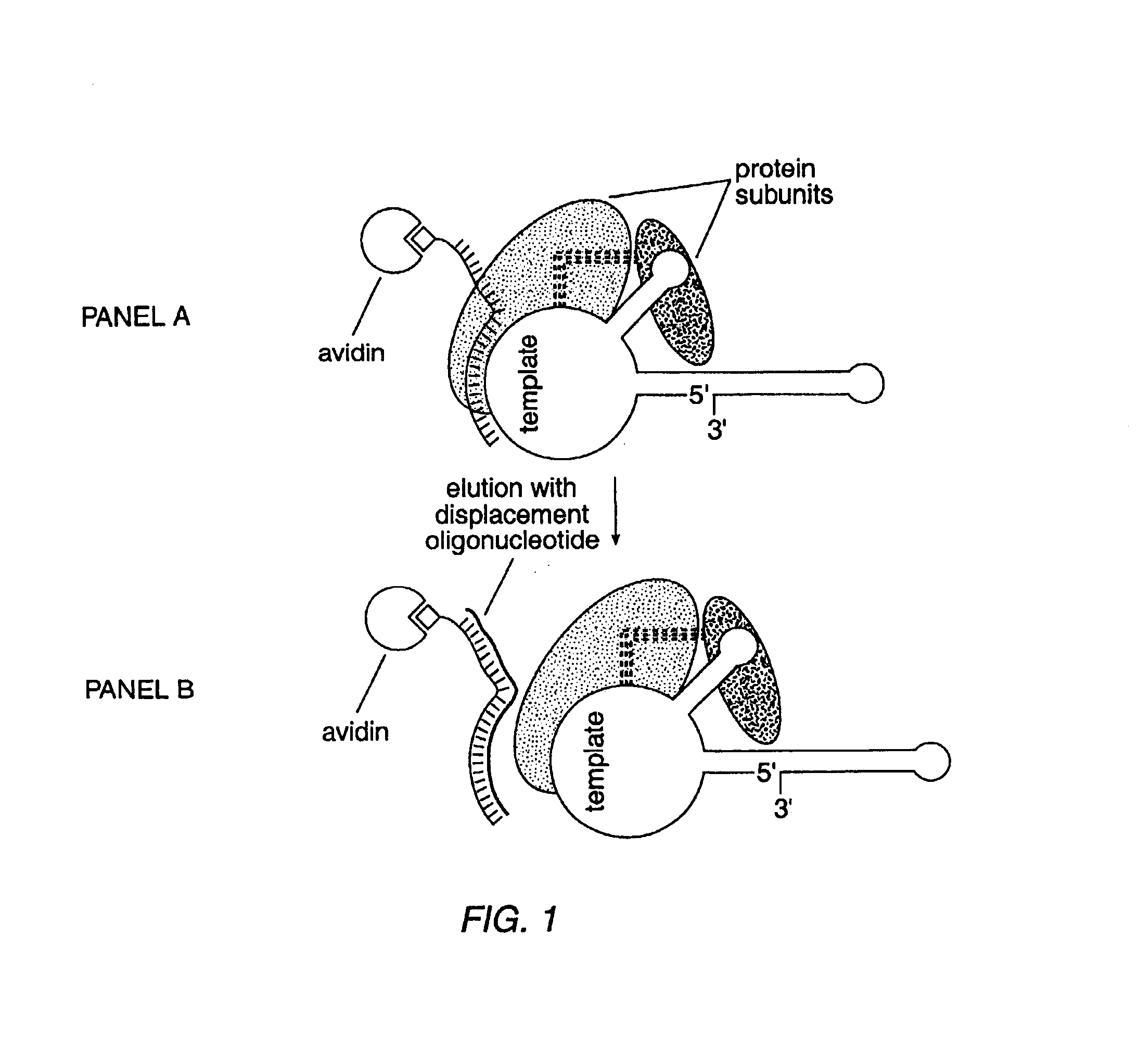
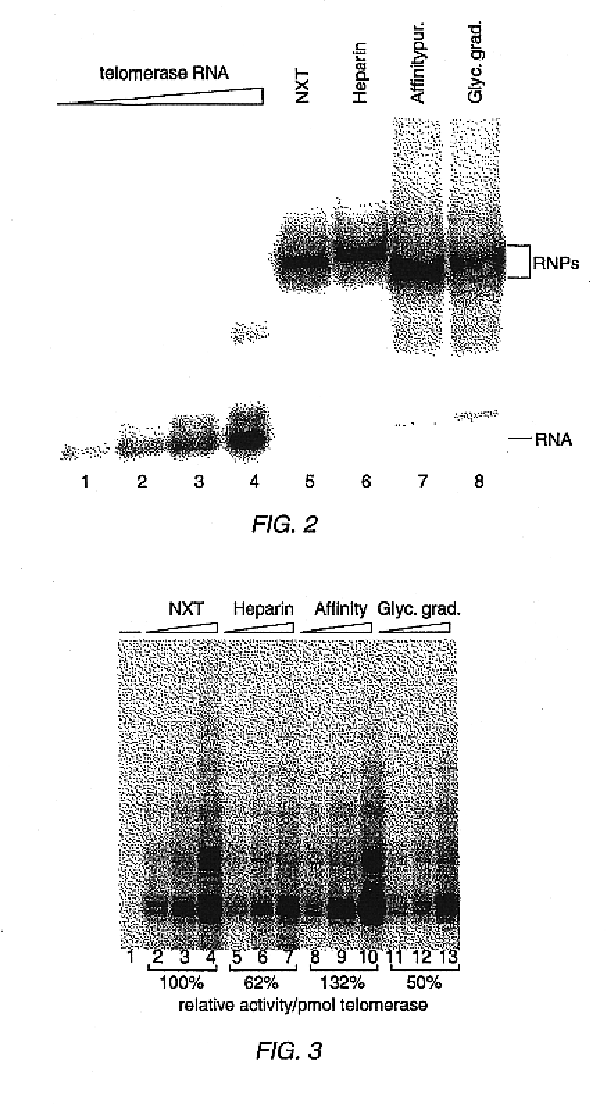
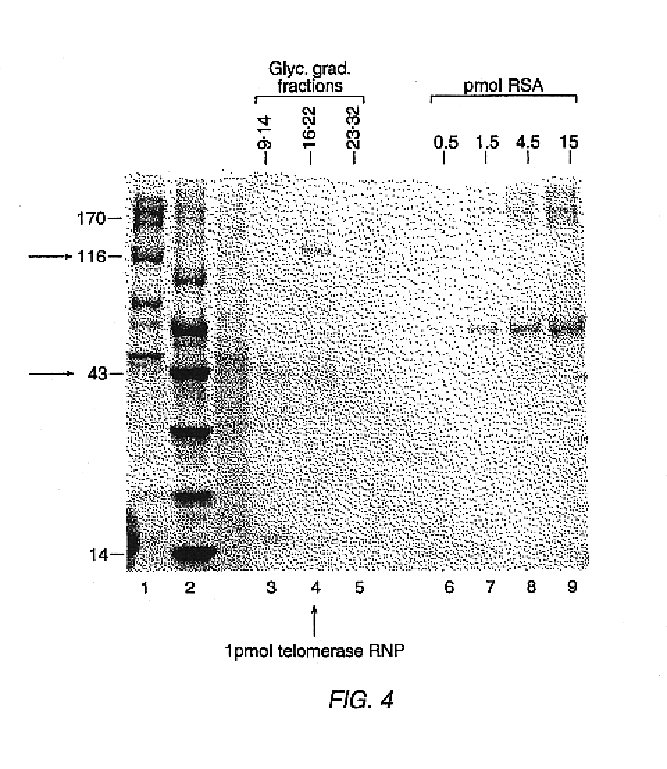
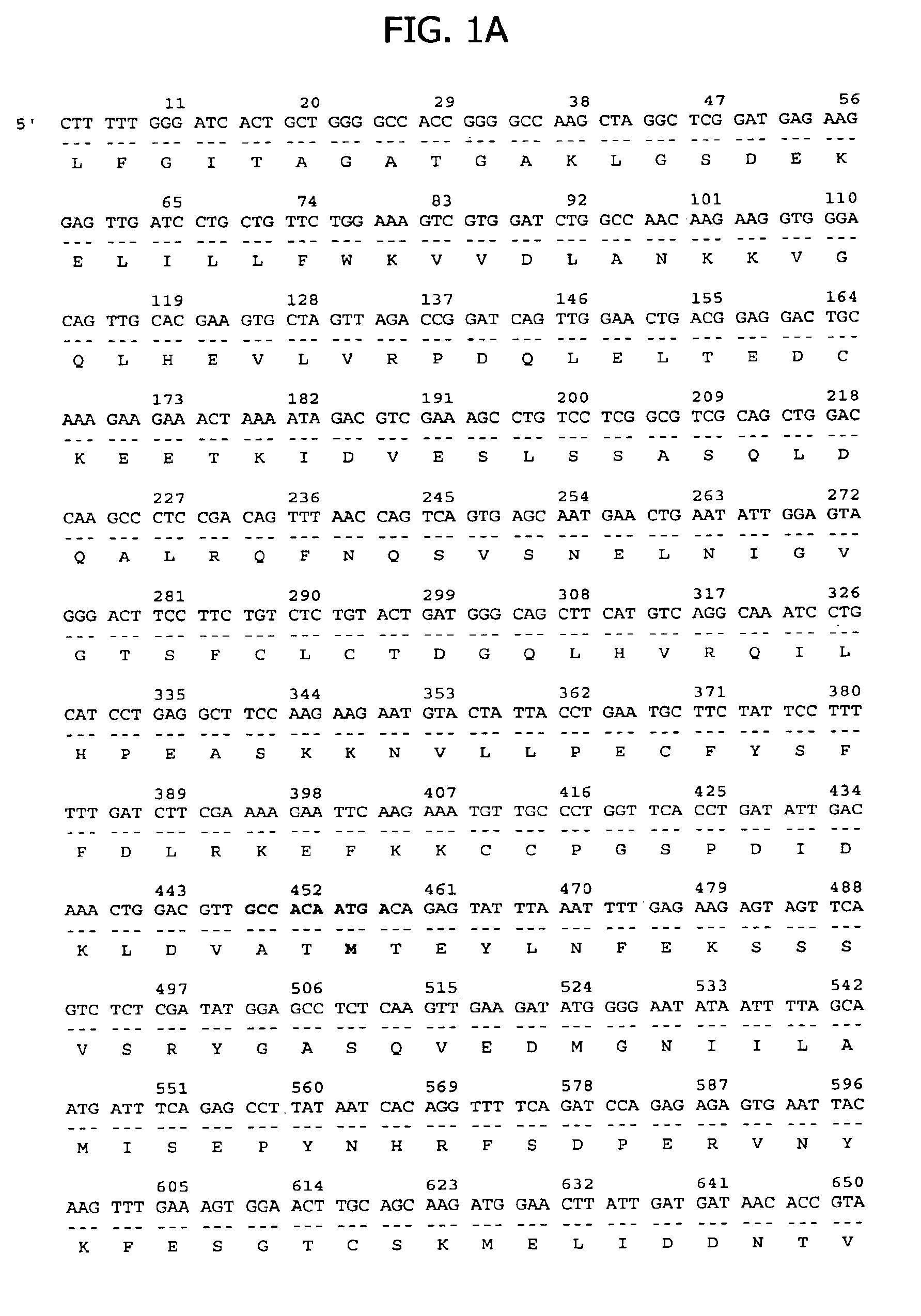
Telomerase
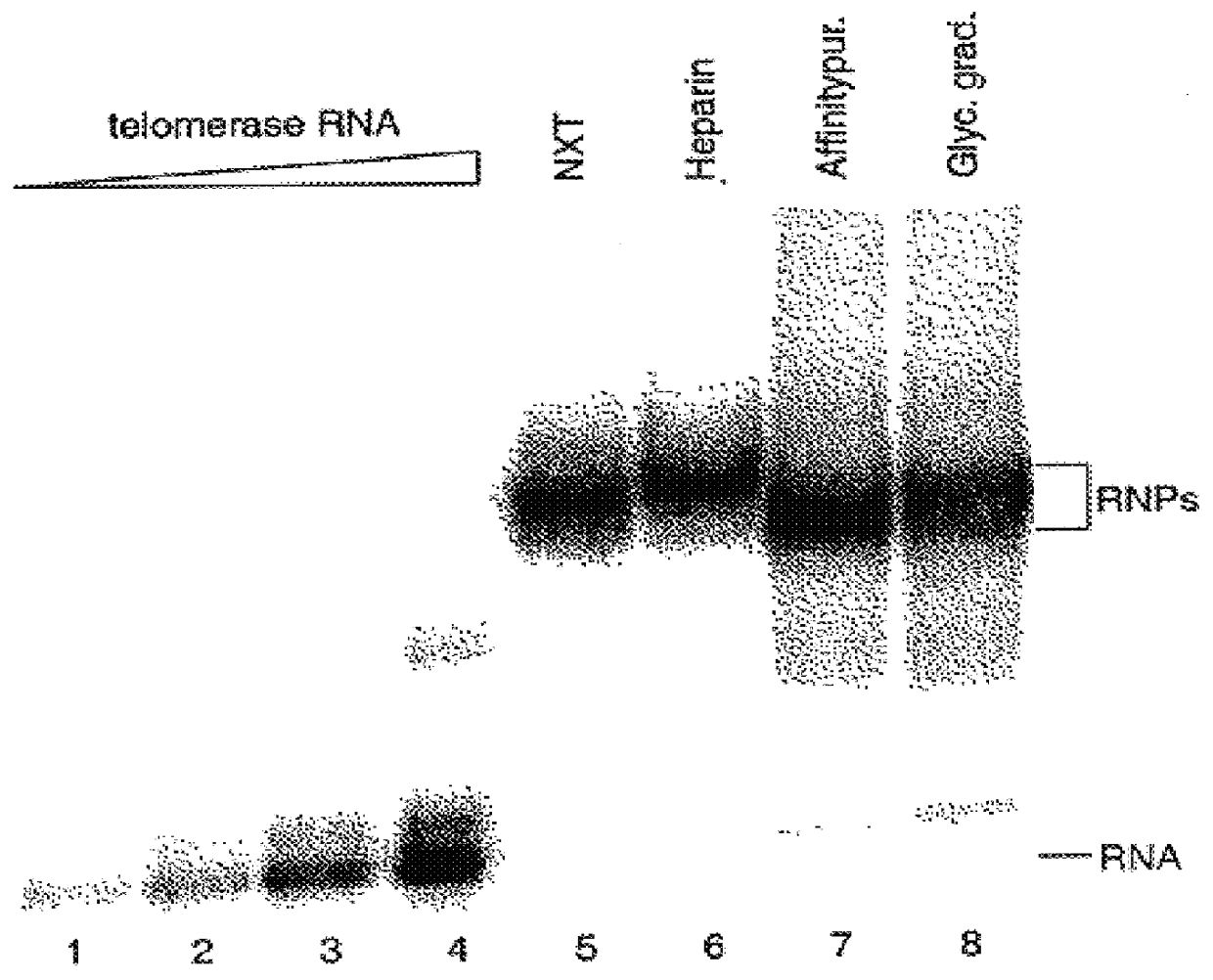
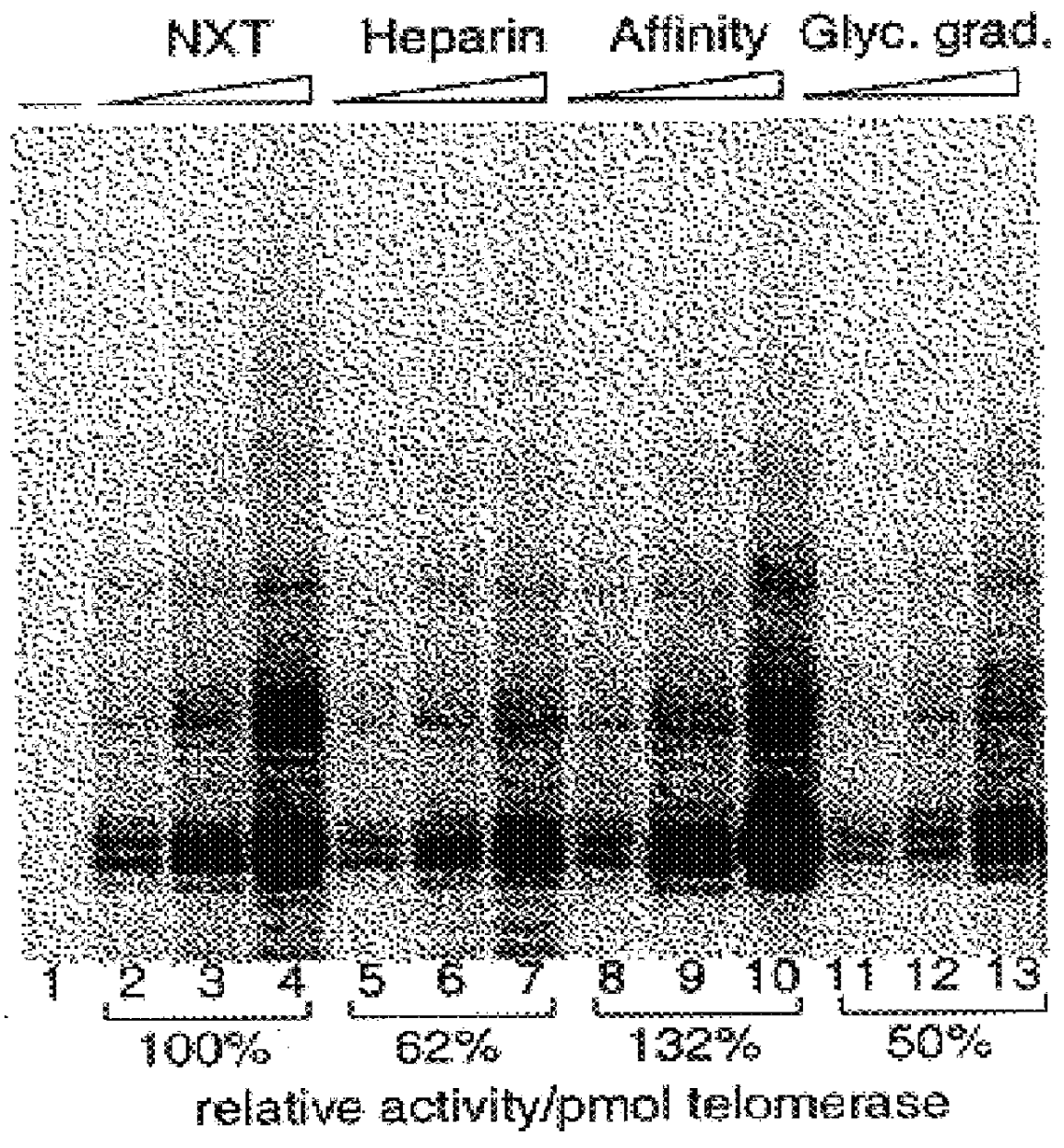
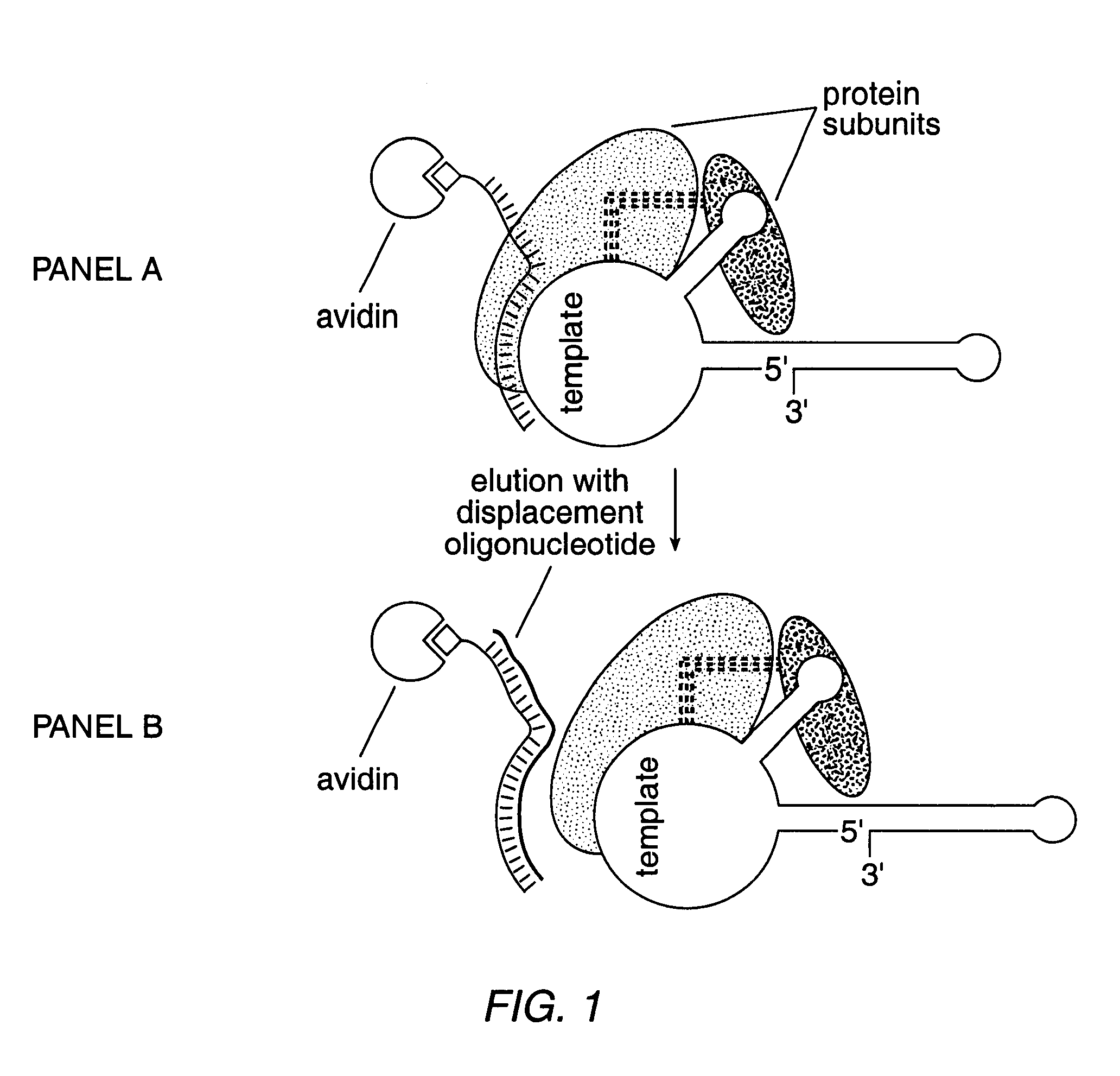
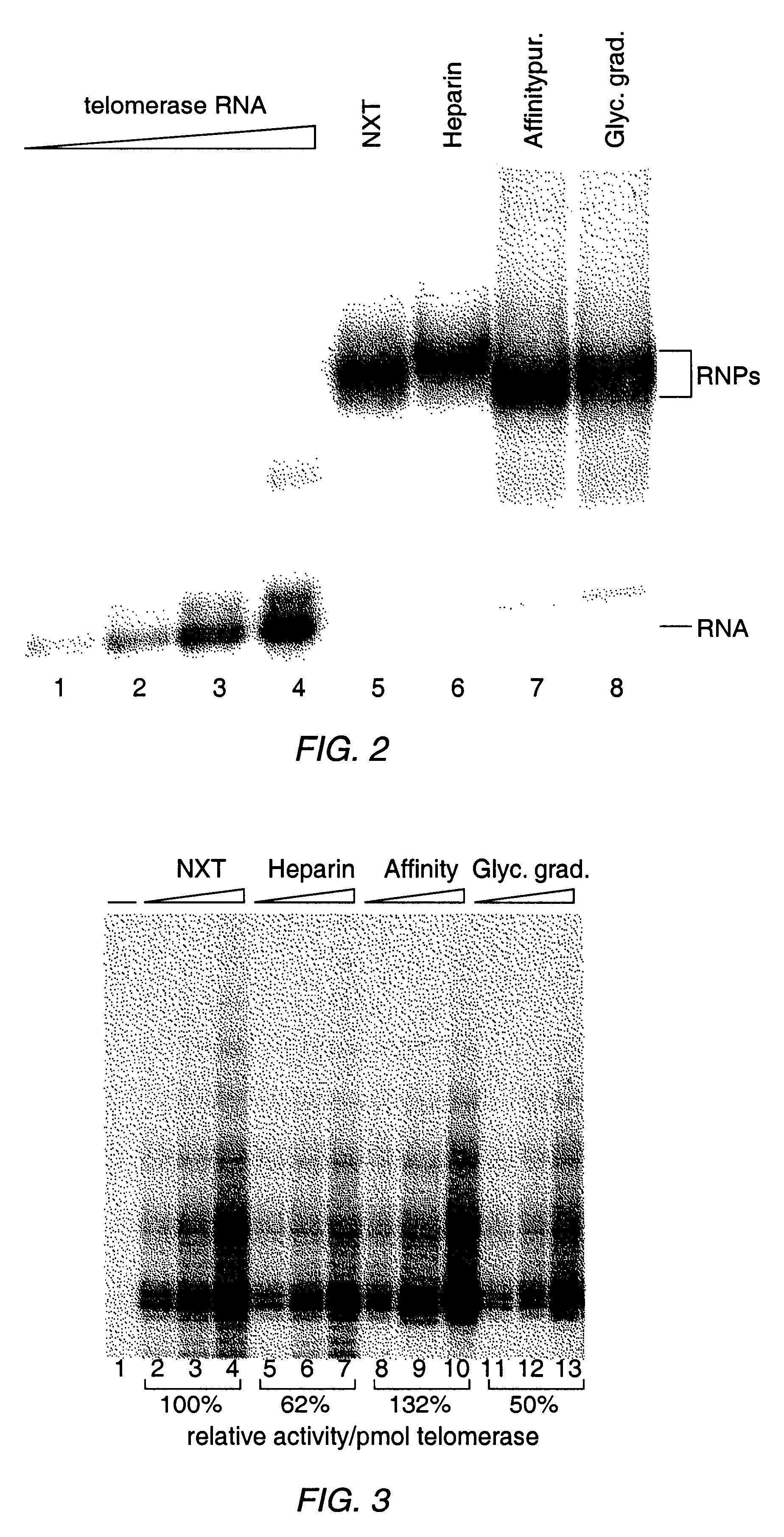
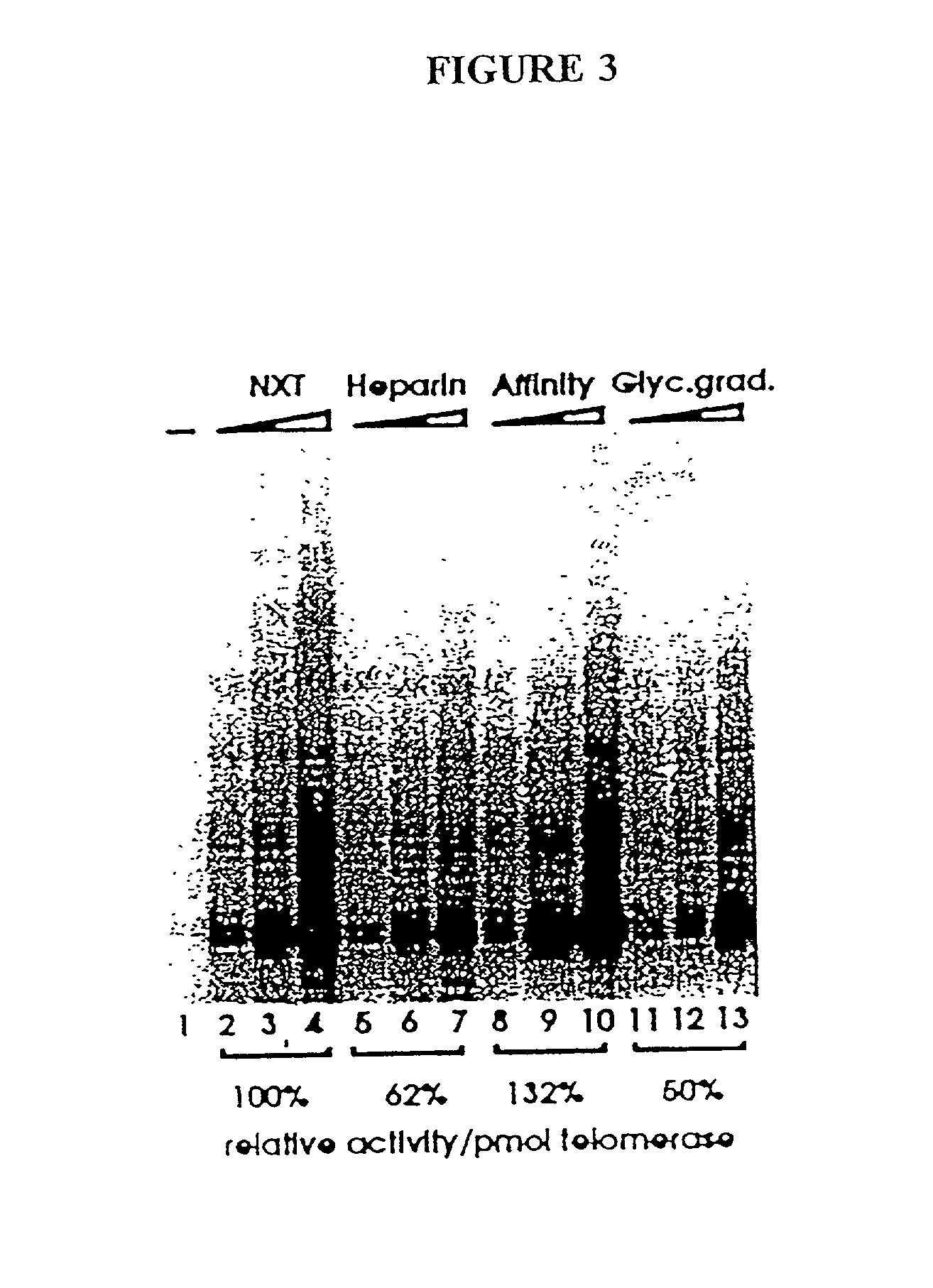
InactiveUS6261836B1Improve purification effectAvoid the needPeptide/protein ingredientsAntibody mimetics/scaffoldsTelomeraseRibonucleoprotein complex
The present invention is directed to telomerase nucleic acids and amino acids. In particular, the present invention is directed to nucleic acid and amino acid sequences encoding various telomerase protein subunits and motifs, including the 123 kDa and 43 kDa telomerase protein subunits of Euplotes aediculatus, and related sequences from Schizosaccharomyces, Saccharomyces sequences, and human telomerase. The present invention is also directed to polypeptides comprising these telomerase protein subunits, as well as functional polypeptides and ribonucleoproteins that contain these subunits.
Owner:GERON CORPORATION +1
Telomerase
InactiveUS6093809AImprove purification effectAvoid the needSugar derivativesPeptide/protein ingredientsTelomeraseRibonucleoprotein complex
The present invention is directed to novel telomerase nucleic acids and amino acids. In particular, the present invention is directed to nucleic acid and amino acid sequences encoding various telomerase protein subunits and motifs, including the 123 kDa and 43 kDa telomerase protein subunits of Euplotes aediculatus, and related sequences from Schizosaccharomyces, Saccharomyces sequences, and human telomerase. The present invention is also directed to polypeptides comprising these telomerase protein subunits, as well as functional polypeptides and ribonucleoproteins that contain these subunits.
Owner:GERON CORPORATION +1
Telomerase
InactiveUS6309867B1Avoid the needEnlarge regionPeptide/protein ingredientsAntibody mimetics/scaffoldsTelomeraseRibonucleoprotein complex
The present invention is directed to novel telomerase nucleic acids and amino acids. In particular, the present invention is directed to nucleic acid and amino acid sequences encoding various telomerase protein subunits and motifs, including the 123 kDa and 43 kDa telomerase protein subunits of Euplotes aediculatus, and related sequences from Schizosaccharomyces, Saccharomyces sequences, and human telomerase. The present invention is also directed to polypeptides comprising these telomerase protein subunits, as well as functional polypeptides and ribonucleoproteins that contain these subunits.
Owner:UNIV OF COLORADO THE REGENTS OF
Ribonucleoprotein transfection agents
Provided herein are compositions and methods useful, inter alia, for the delivery of ribonucleoprotein complexes (e.g., Cas9 / guide RNA complexes) into cells. The compositions and methods provided herein are particularly useful for the delivery of ribonucleoprotein complexes into pluripotent cells and lymphatic cells.
Owner:LIFE TECH CORP
Method Enabling the Use of Extracellular Ribonucleic Acid (RNA) Extracted from Plasma or Serum to Detect, Monitor or Evaluate Cancer or Premalignant Conditions
InactiveUS20080261292A1Assess prognosisPredict prognosisSugar derivativesMicrobiological testing/measurementNeoplasmCirculating RNA
This invention relates to the use of tumor-derived or associated extracellular ribonucleic acid (RNA) found circulating in the plasma or serum fraction of blood for the detection, monitoring, or evaluation of cancer or premalignant conditions. Extracellular RNA may circulate as non-bound RNA, protein-bound RNA, lipid-RNA complexes, lipoprotein (proteolipid)-RNA complexes, protein-RNA complexes including within or in association with ribonucleoprotein complexes, nucleosomes, or within apoptotic bodies. Any intracellular RNA found in plasma or serum can additionally be detected by this invention. Specifically, this invention enables the extraction of circulating RNA from plasma or serum and utilizes nucleic acid amplification assays for the identification, detection, inference, monitoring, or evaluation of any neoplasm, benign, premalignant, or malignant, in humans or other animals, which might be associated with that RNA. Further, this invention allows the qualitative or quantitative detection of tumor-derived or associated extracellular RNA circulating in the plasma or serum of humans or animals with or without any prior knowledge of the presence of cancer or premalignant tissue.
Owner:ONCOMEDX
Novel telomerase
InactiveUS20020187471A1Good choiceEarly diagnosisFungiPeptide/protein ingredientsTelomeraseRibonucleoprotein complex
The present invention is directed to novel telomerase nucleic acids and amino acids. In particular, the present invention is directed to nucleic acid and amino acid sequences encoding various telomerase protein subunits and motifs, including the 123 kDa and 43 kDa telomerase protein subunits of Euplotes aediculatus, and related sequences from Schizosaccharomyces, Saccharomyces sequences, and human telomerase. The present invention is also directed to polypeptides comprising these telomerase protein subunits, as well as functional polypeptides and ribonucleoproteins that contain these subunits.
Owner:UNIV OF COLORADO THE REGENTS OF
Novel telomerase
InactiveUS20030009019A1SpecificallyEfficiently catalyze endonucleolytic cleavageBacteriaPeptide/protein ingredientsTelomeraseRibonucleoprotein complex
Owner:UNIV OF COLORADO THE REGENTS OF
Helper-free rescue of recombinant negative strand RNA virus
The present invention relates methods of generating infectious negative-strand virus in host cells by an entirely vector-based system without the aid of a helper virus. In particular, the present invention relates methods of generating infectious recombinant negative-strand RNA viruses intracellularly in the absence of helper virus from expression vectors comprising cDNAs encoding the viral proteins necessary to form ribonucleoprotein complexes (RNPs) and expression vectors comprising cDNA for genomic viral RNA(s) (vRNAs) or the corresponding cRNA(s). The present invention also relates to methods of generating infectious recombinant negative-strand RNA viruses which have mutations in viral genes and / or which express, package and / or present peptides or polypeptides encoded by heterologous nucleic acid sequences. The present invention further relates the use of the recombinant negative-strand RNA viruses or chimeric negative-strand RNA viruses of the invention in vaccine formulations and pharmaceutical compositions.
Owner:MT SINAI SCHOOL OF MEDICINE
Method for detecting polynucleotides encoding telomerase
InactiveUS6808880B2Avoid the needEnlarge regionPeptide/protein ingredientsAntibody mimetics/scaffoldsTelomeraseNucleotide
The present invention is directed to novel telomerase nucleic acids and amino acids. In particular, the present invention is directed to nucleic acid and amino acid sequences encoding various telomerase protein subunits and motifs, including the 123 kDa and 43 kDa telomerase protein subunits of Euplotes aediculatus, and related sequences from Schizosaccharomyces, Saccharomyces sequences, and human telomerase. The present invention is also directed to polypeptides comprising these telomerase protein subunits, as well as functional polypeptides and ribonucleoproteins that contain these subunits.
Owner:UNIV OF COLORADO THE REGENTS OF
Gene upregulated in cancers of the prostate
InactiveUS6893818B1Improve the level ofVirusesSugar derivativesRibonucleoprotein complexOpen reading frame
The present invention relates to a novel protein designated 20P2H8 which shares homology with several heterogenous nuclear ribonucleoproteins (hnRNPs). A full length approximately 3600 bp 20P2H8 cDNA (SEQ ID NO: 10, encoding a 517 amino acid open reading frame (SEQ ID NO: 2), is provided herein.
Owner:AGENSYS INC
Western blot kit for detecting antibody of autoimmune disease and preparation method thereof
ActiveCN103105489AOvercome the cumbersome operation of individual detection one by oneImprove accuracyMaterial analysisAntigenAnti-mitochondrial antibody
The invention provides a western blot kit for detecting the antibody of autoimmune disease and a preparation method of the western blot kit, and relates to a western blot kit for detecting related antibodies of various autoimmune diseases, aiming at overcoming the technical defect that a western blot product is unavailable for testing and screening various autoimmune diseases in the prior art. The nitrocellulose membrane or the nylon membrane contains at least two parallel detection lines coated by at least two of ten natural antigens or recombinant antigens, i.e. dsDNA (deoxyribonucleic acid), Sm / RNP (ribonucleoprotein), CCP (critical compression pressure), SSA (sulfosalicylic acid), SSB (single-strand binding protein), GAD (glutamic acid decarboxylase), ICA (islet cell antibody), IA-2A (islet cell), TG (triglyceride) and AMA-M2 (anti-mitochondrial antibody), a high-concentration quality control band, a median-concentration quality control band and a low-concentration quality control band. The deficiency of the detection sensitivity and the specificity of the single autoantibody can be overcome, the operating complexity for independently detecting the related autoantibody of various diseases one by one can be overcome, and the detection efficiency and the result judging accuracy degree can be greatly improved.
Owner:SHENZHEN YHLO BIOTECH
Crispr/cpf1 systems and methods
ActiveUS20180187176A1Improve purification effectHydrolasesStable introduction of DNARibonucleoprotein complexCRISPR/Cpf1
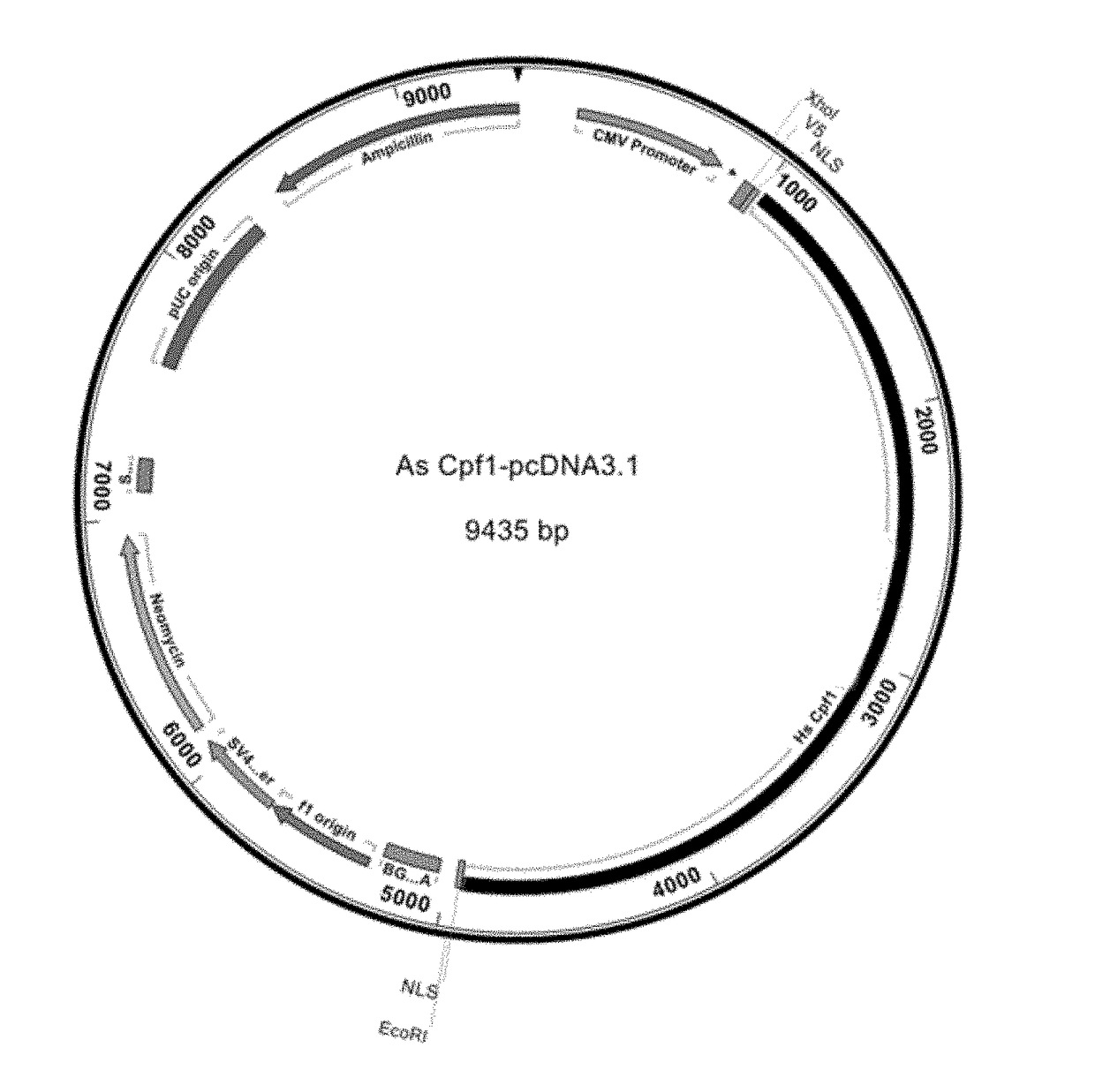
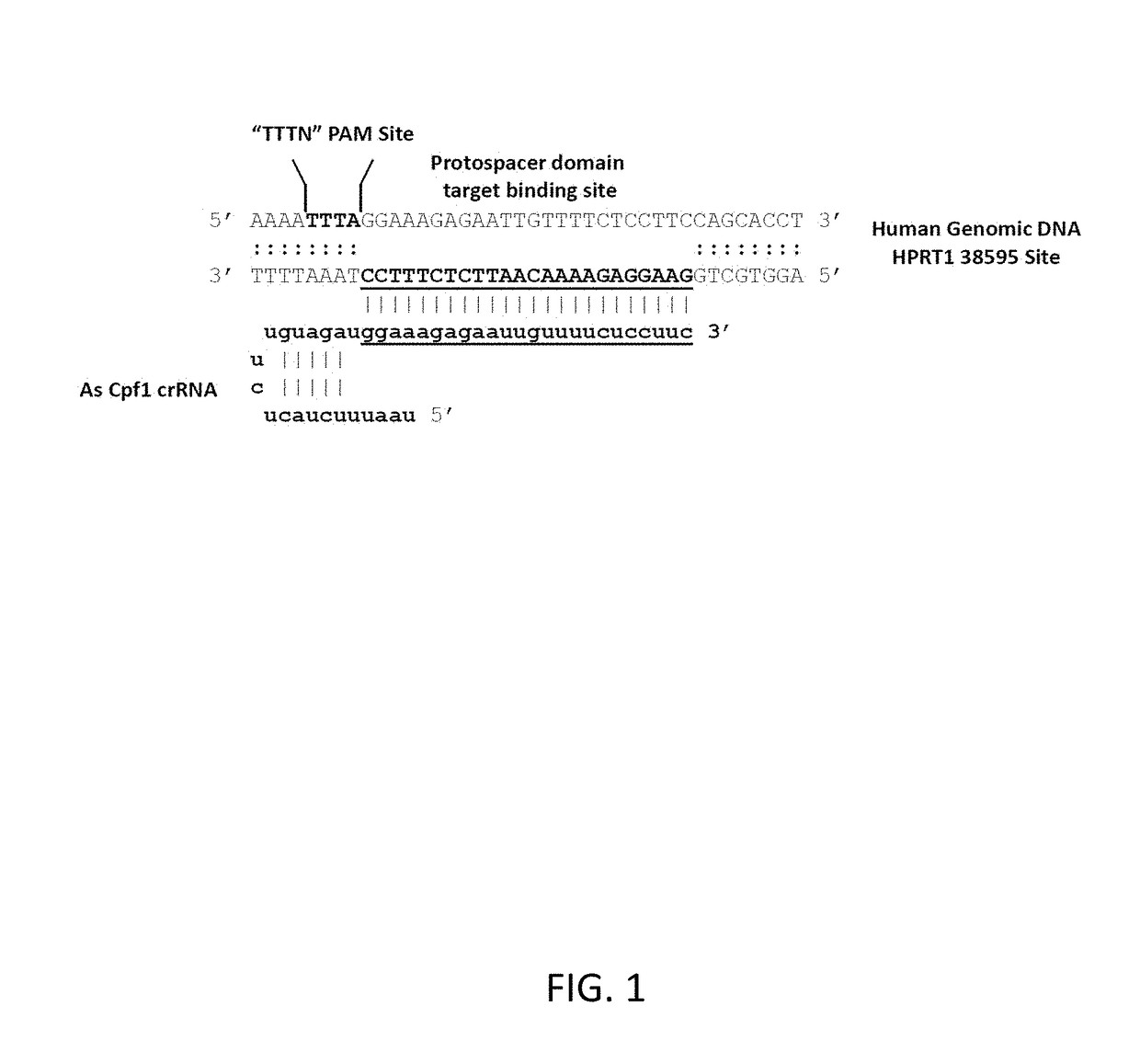
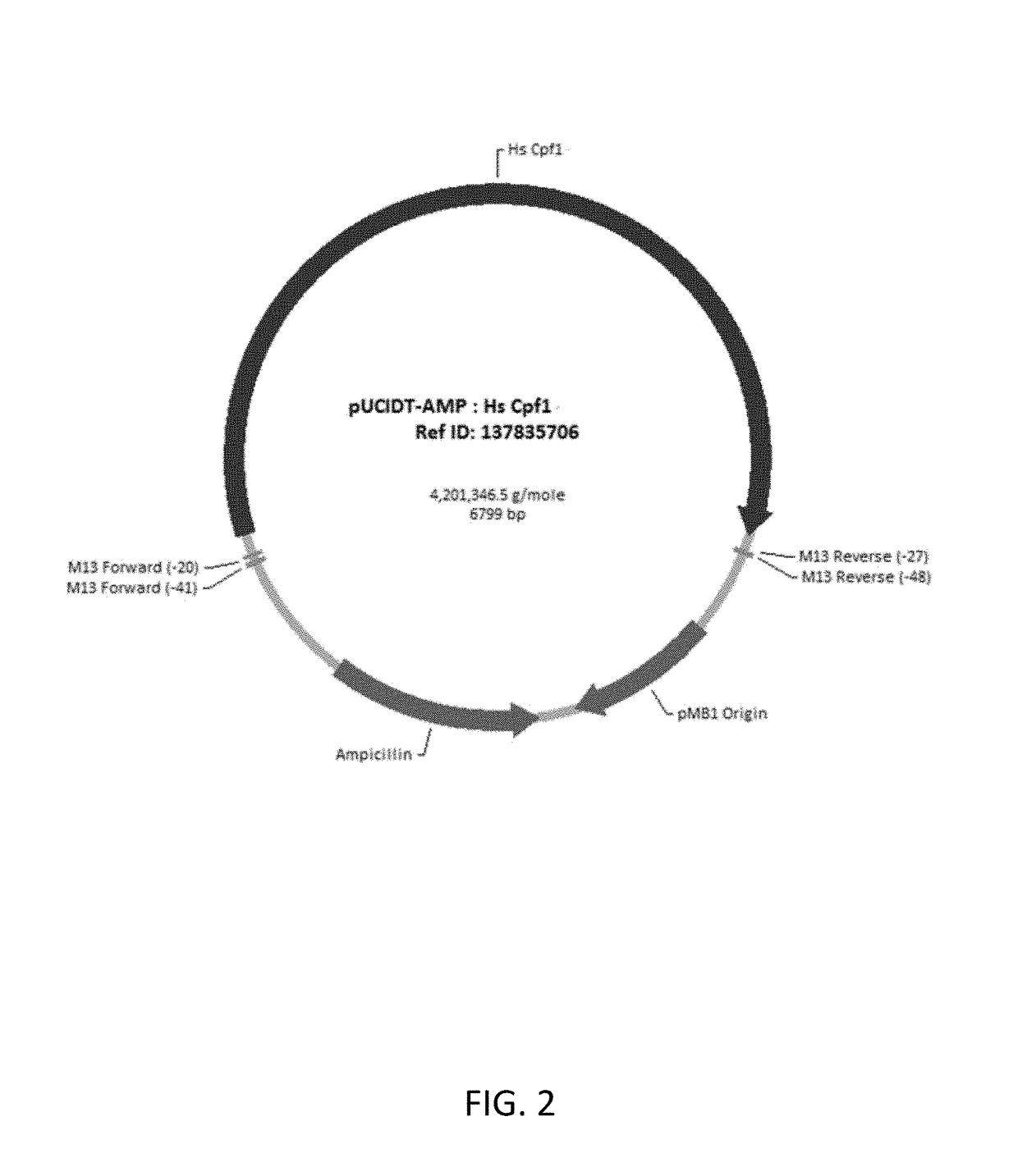
This invention pertains to recombinant AsCpf1 and LbCpf1 nucleic acids and polypeptides for use in CRISPR / Cpf1 endonuclease systems and mammalian cell lines encoding recombinant AsCpf1 or LbCpf1 polypeptides. The invention includes recombinant ribonucleoprotein complexes and CRSPR / Cpf1 endonuclease systems having a suitable AsCpf1 crRNA is selected from a length-truncated AsCpf1 crRNA, a chemically-modified AsCpf1 crRNA, or an AsCpf1 crRNA comprising both length truncations and chemical modifications. Methods of performing gene editing using these systems and reagents are also provided.
Owner:INTEGRATED DNA TECHNOLOGIES
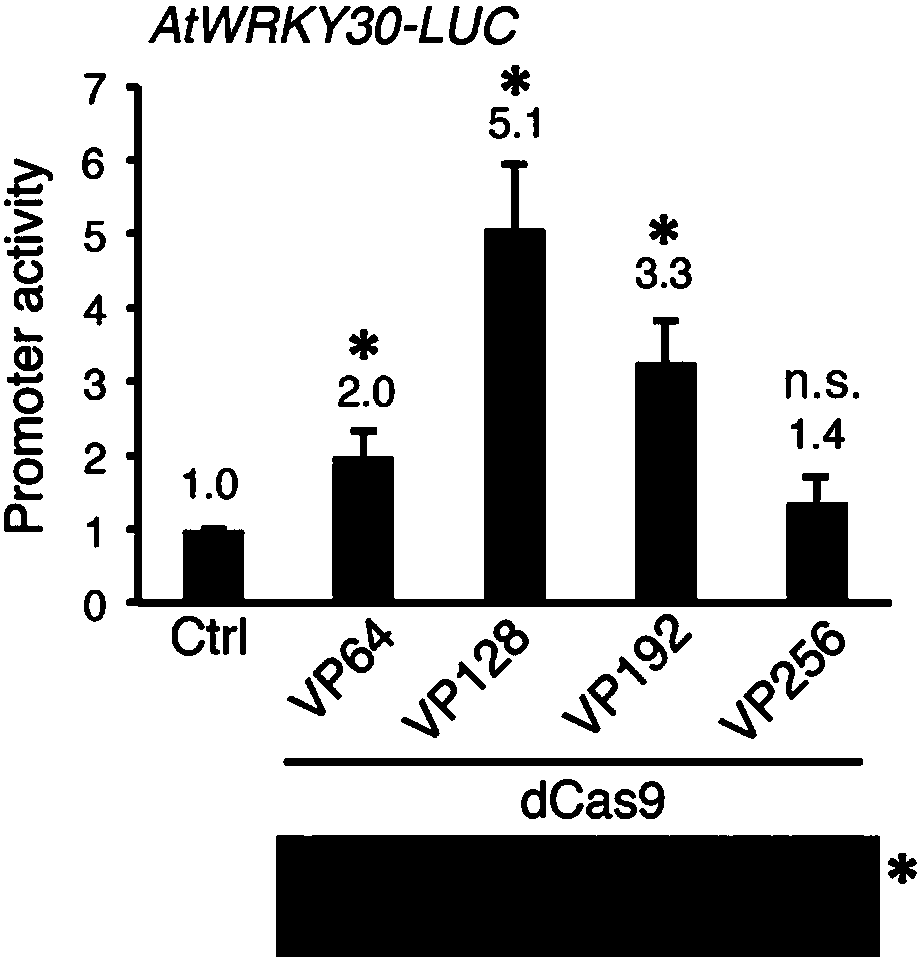
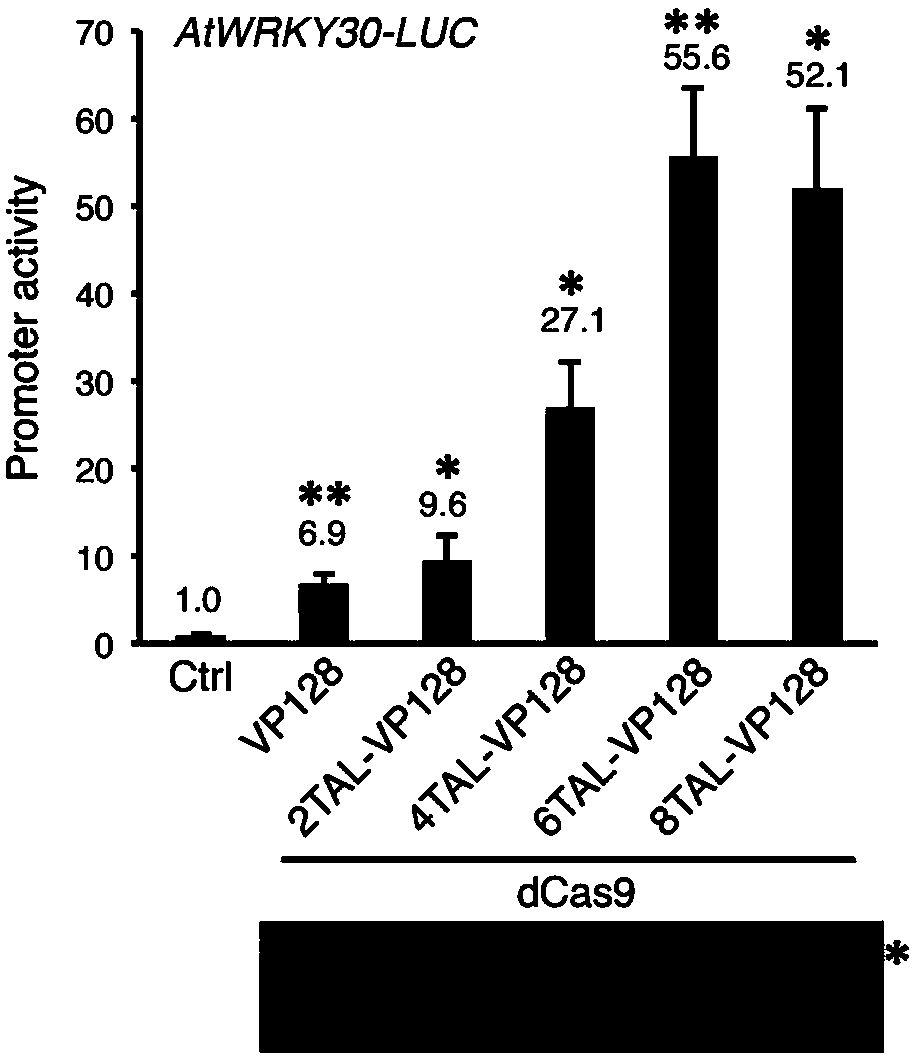
High-efficiency artificial activating transcription factor dCas9-TV, and coding gene and applications thereof
ActiveCN107722125ATranscriptional activationEliminate the need for cloningHydrolasesAntibody mimetics/scaffoldsMetaboliteBiological activation
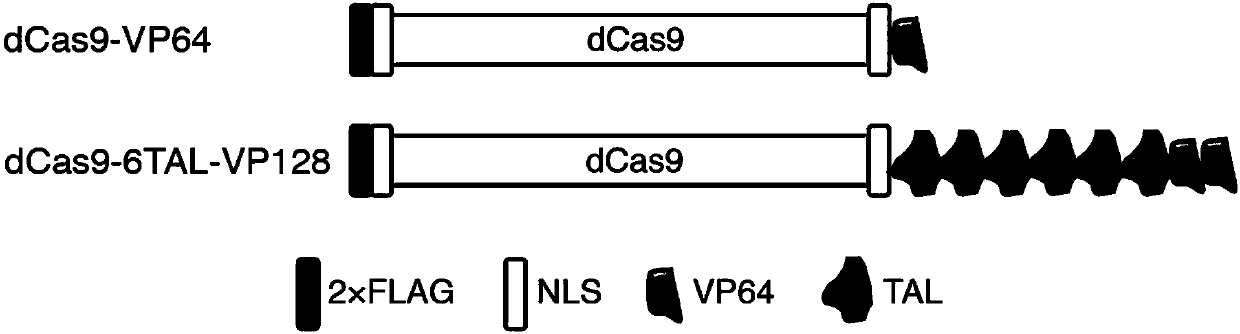
The invention relates to a high-efficiency artificial activating transcription factor dCas9-TV, and a coding gene and applications thereof. According to a construction method, the carboxyl terminal ofnuclease inactivated Cas9 protein (dCas9) is connected with a plurality of copies of VP64 and TAL transcription-activating domains so as to obtain a series of novel artificial activating transcription factors, and obtain dCas9-TV with the best transcriptional activation activity via screening. When only one guide RNA (gRNA) is adopted for targeting a specific gene promoter, dCas9-TV is capable ofrealizing high efficiency activating of transcription of endogenous genes of Arabidopis thaliana and paddy rice; when a plurality of gRNA are adopted for targeting a plurality of target genes, dCas0-TV is capable of realizing transcription activation of a plurality of genes. In addition, it is confirmed that dCas9-TV possesses the same high efficiency targeting transcription activation activity in human cells. An in vitro assembled dCas9-TV / gRNA ribonucleoprotein compound can be adopted for transcription activation of Arabidopis thaliana and paddy rice endogenous genes. The high-efficiency artificial activating transcription factor dCas9-TV can be adopted in the fields such as genome genetic screening, metabolite biosynthesis pathway reconstruction, and crop improvement.
Owner:SUN YAT SEN UNIV
Lupus erythematosus detection protein chip and kit thereof
InactiveCN101726586AThe detection indicators are comprehensive and appropriateImprove detection efficiencyBiological testingDiseaseProtein markers
The invention discloses lupus erythematosus detection protein chip and kit thereof. The chip comprises a substrate, protein markers distributed in an array type and control point coatings, wherein the markers and the control point coatings are seven antigen markers, positive controls and negative controls comprising dsDNA (double-stranded deoxyribonucleic acid), ssDNA (single stranded deoxyribonucleic acid), Histon, SS-A(Ro60), SS-B(La), Sm (smith) and RNP (ribonucleoprotein) / Sm which are uniformly distributed on the substrate in a dot matrix mode. The invention has comprehensive and appropriate detection indexes and consistent applied reaction conditions, can detect a plurality of indexes at a time, is convenient and quick, greatly improves the detection efficiency and reduces the detection cost. The invention can be suitable for aided diagnosis of suspected lupus erythematosus disease people.
Owner:上海裕隆生物科技有限公司
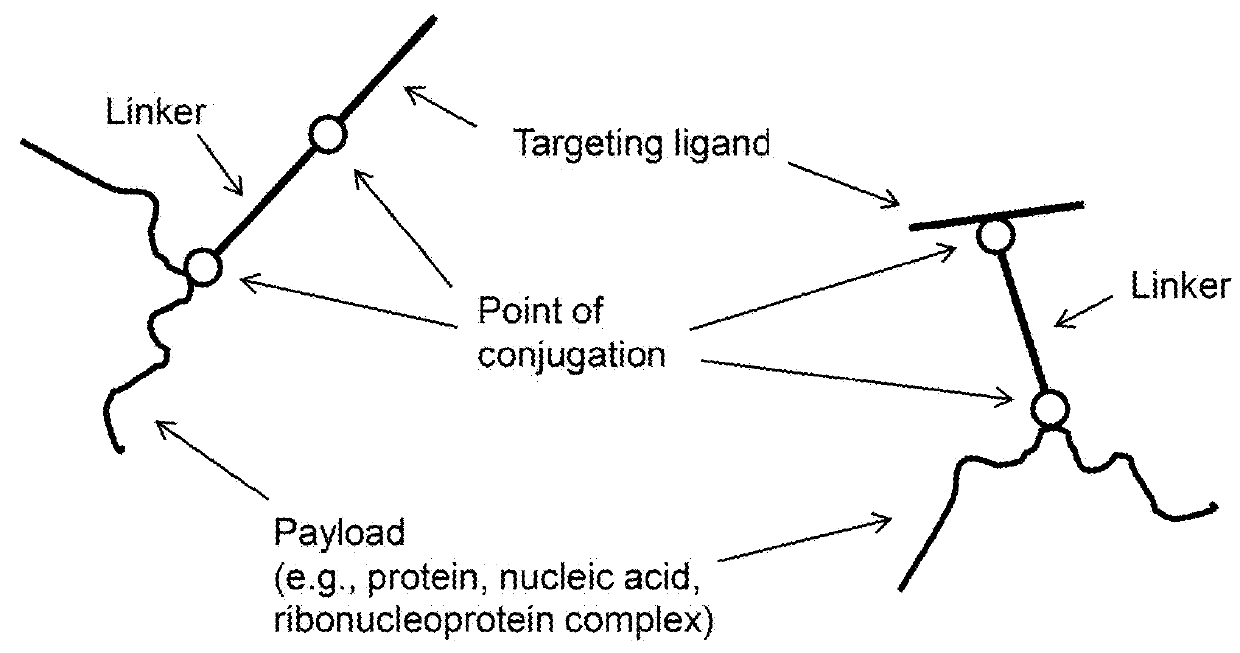
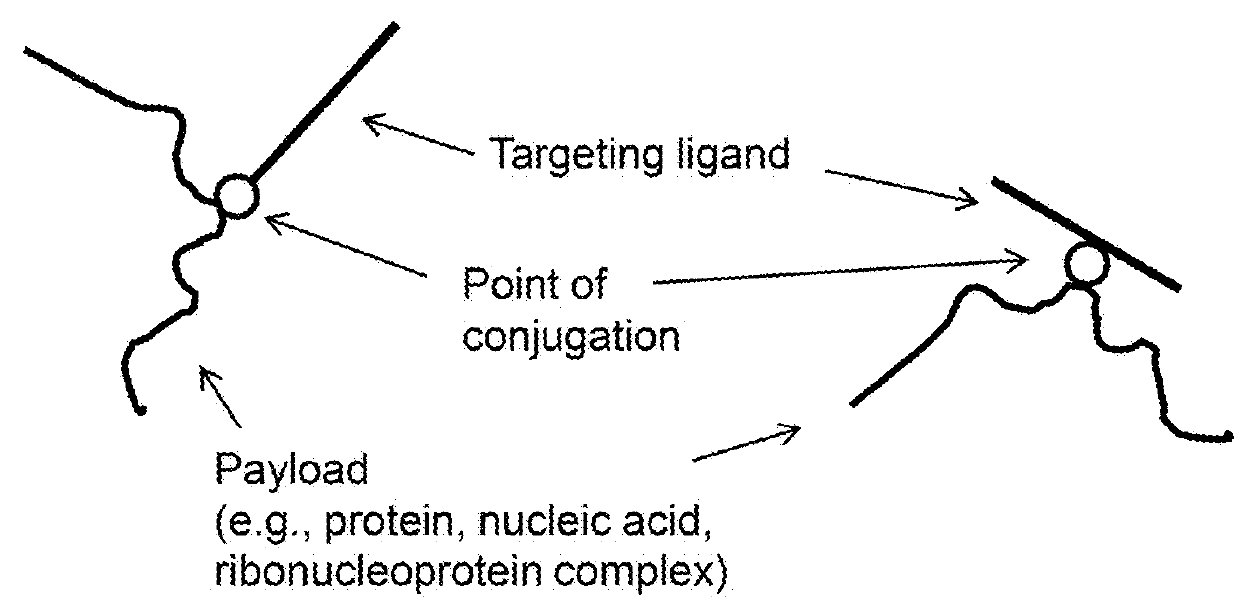
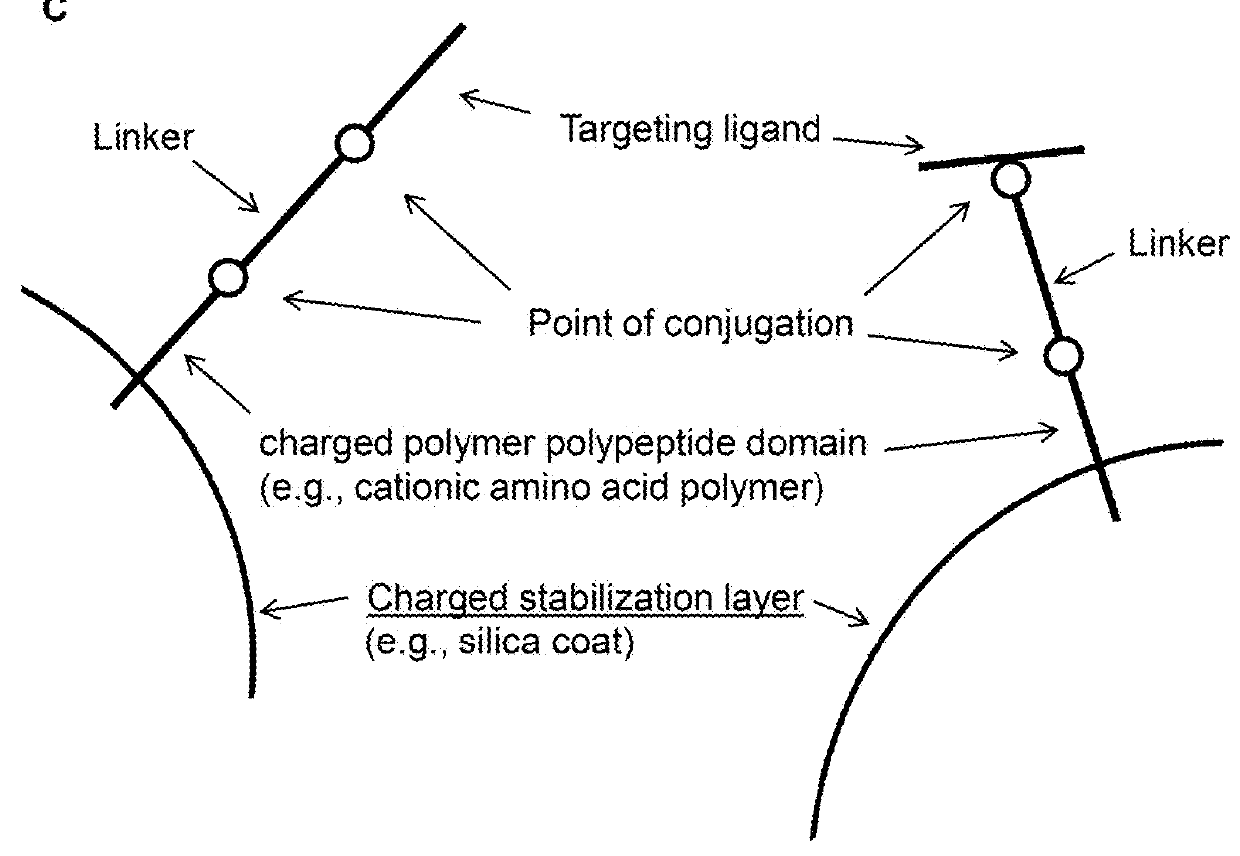
Methods and compositions for nucleic acid and protein payload delivery
Provided are methods and compositions for delivering a nucleic acid, protein, and / or ribonucleoprotein payload to a cell. Also provided are delivery molecules that include a peptide targeting ligand conjugated to a protein or nucleic acid payload (e.g., an siRNA molecule), or conjugated to a charged polymer polypeptide domain (e.g., poly-arginine such as 9R or a poly-histidine such as 6H, and the like). The targeting ligand provides for (i) targeted binding to a cell surface protein, and (ii) engagement of a long endosomal recycling pathway. As such, when the targeting ligand engages the intended cell surface protein, the delivery molecule enters the cell (e.g., via endocytosis) but is preferentially directed away from the lysosomal degradation pathway.
Owner:LIGANDAL INC
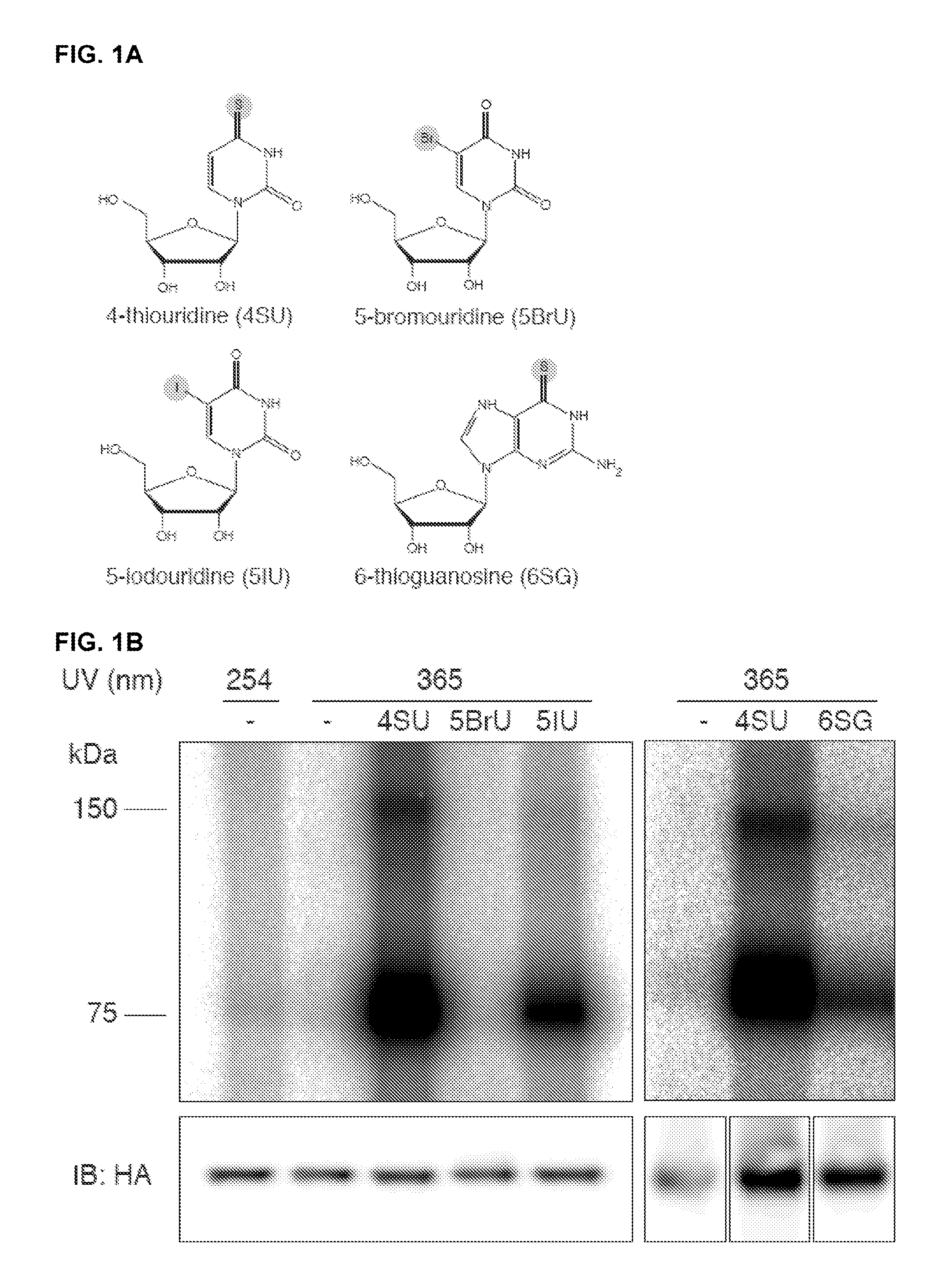
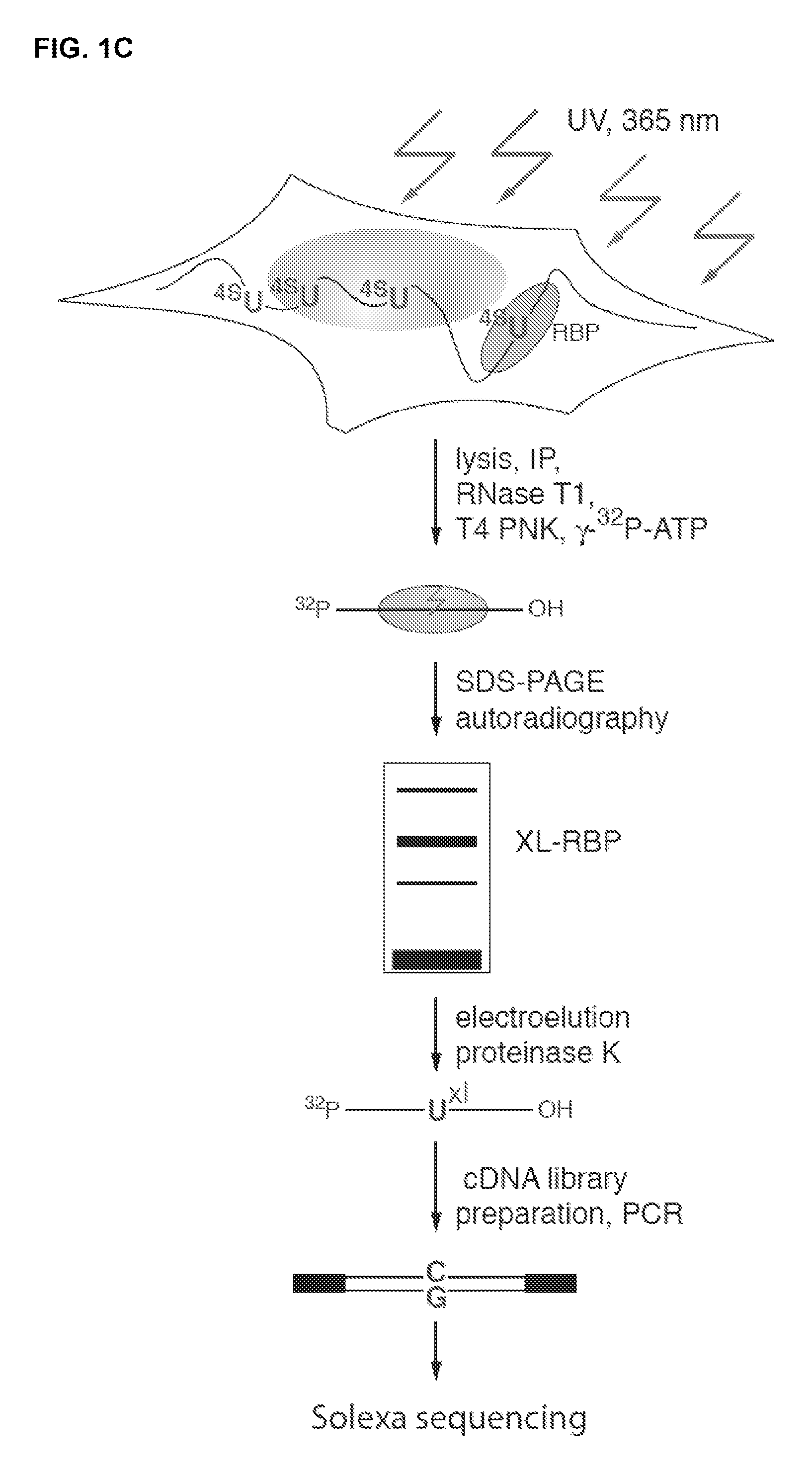
Methods for Identifying RNA Segments Bound by RNA-Binding Proteins or Ribonucleoprotein Complexes
ActiveUS20110287412A1Microbiological testing/measurementDNA preparationCross-linkRibonucleoprotein complex
The present invention relates to a method for identifying a binding site on an RNA transcript, wherein the binding site binds to one or more binding moieties. The method includes, among other things, introducing a photoreactive nucleoside into living cells wherein the living cells incorporate the photoreactive nucleoside into RNA transcripts during transcription thereby producing modified RNA transcripts; reverse transcribing the RNA of isolated cross-linked segments thereby generating cDNA transcripts with one mutation wherein the photoreactive nucleoside is transcribed to a mismatched deoxynucleoside; amplifying the cDNA transcripts thereby generating amplicons; and analyzing the sequences of the amplicons aligned against the reference sequence so as to identify the binding site, wherein the sequences of each amplicon having a mutation resulting from the introduction of the photoreactive nucleoside is considered to be a valid amplicon comprising at least a portion of a binding site on the RNA transcript.
Owner:UNIVERSITY OF BASEL +1
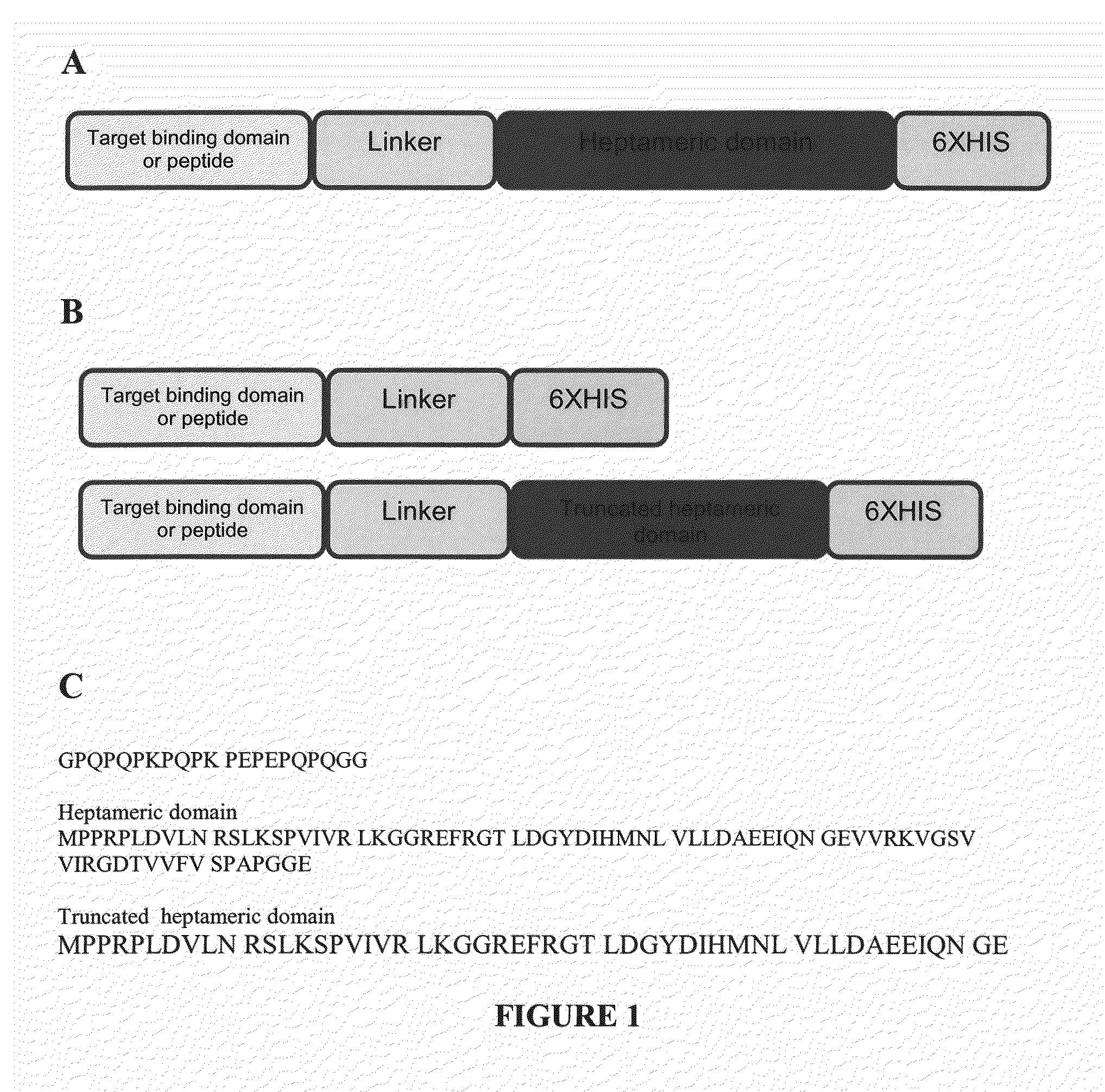
Methods and Compositions for Heptameric Targeting Ligands
ActiveUS20140086835A1Treat cancerUltrasonic/sonic/infrasonic diagnosticsBacteriaCrystallographyRibonucleoprotein complex
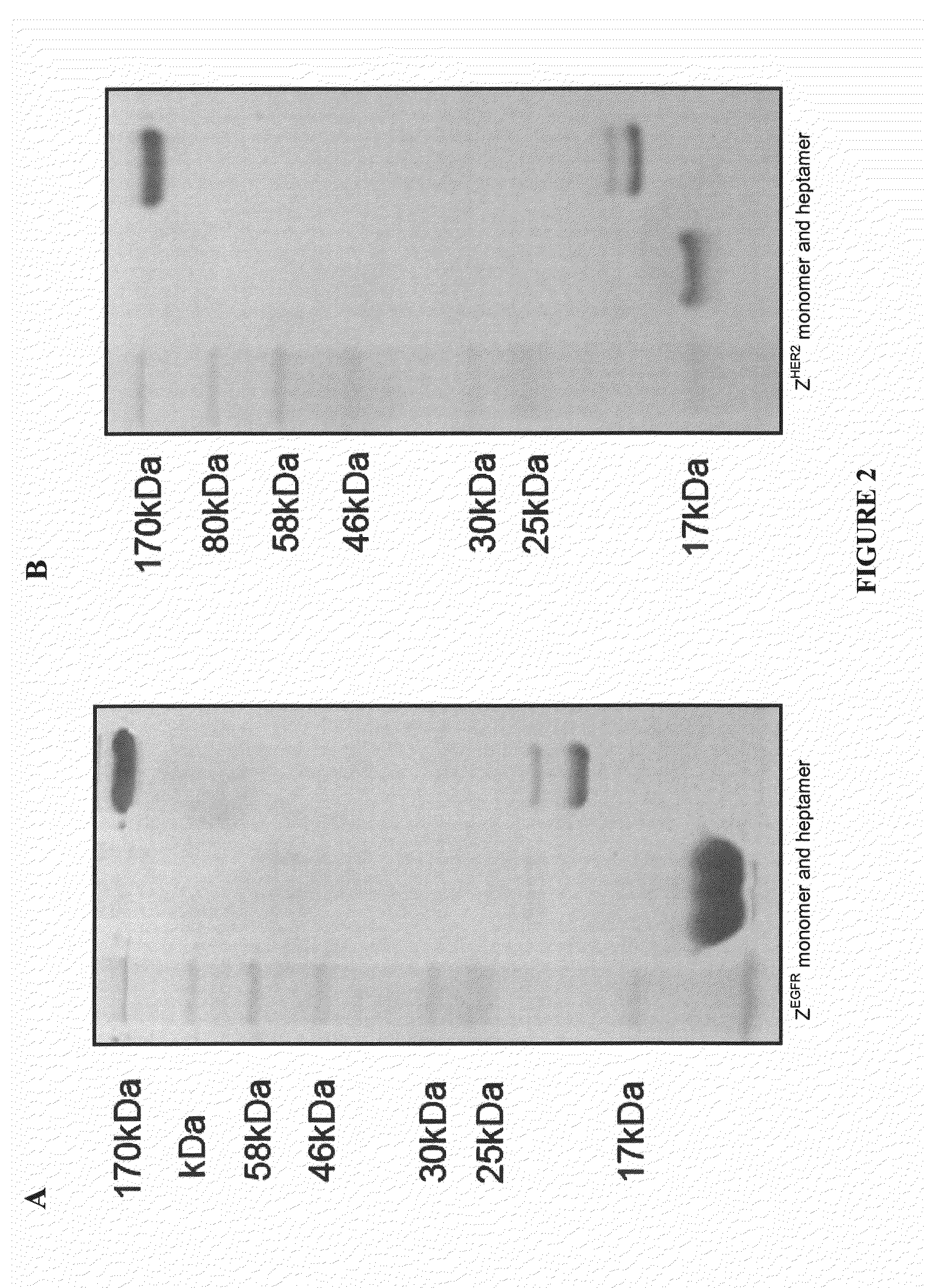
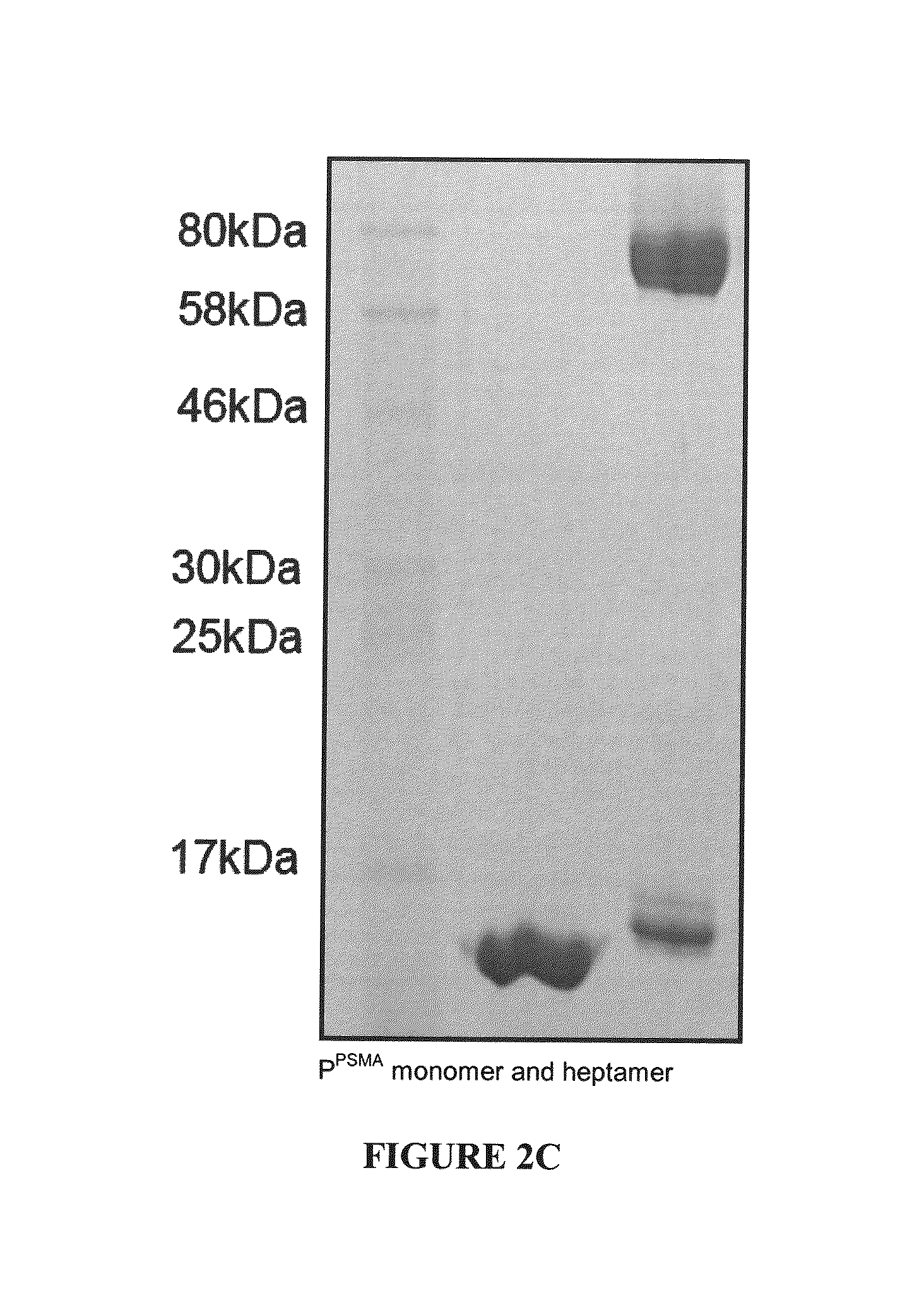
The present invention provides a self assembly molecule having an affinity for one or more target molecules, for use in formation of a heptameric complex, comprising: a) a monomer comprising a multimerization domain of Archaeal Sm1 (AF-Sm1) protein or SM-like ribonucleoprotein from other organisms, able to interact with other molecules of the same monomer comprising a multimerization domain of AF-Sm1 protein or SM-like ribonucleoprotein to self-assemble into a heptamer; and b) a target binding domain or peptide attached directly or via a linker to the monomer of (a). Also provided are heptamers comprising these self assembly molecules and methods for their use in therapy, imaging and diagnostics.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Method enabling use of extracellular RNA extracted from plasma or serum to detect, monitor or evaluate cancer
InactiveUS20020155469A1Predict prognosisHigh sensitivitySugar derivativesMicrobiological testing/measurementLipid formationCirculating RNA
Owner:ONCOMEDX
Method enabling use of extracellular RNA extracted from plasma or serum to detect, monitor or evaluate cancer
InactiveUS20080050783A1Assess prognosisPredict prognosisSugar derivativesMicrobiological testing/measurementLipid formationNeoplasm
This invention relates to the use of tumor-derived or associated extracellular ribonucleic acid (RNA) found circulating in the plasma or serum fraction of blood for the detection, monitoring, or evaluation of cancer or premalignant conditions. Extracellular RNA may circulate as non-bound RNA, protein-bound RNA, lipid-RNA complexes, lipoprotein (proteolipid)-RNA complexes, protein-RNA complexes including within or in association with ribonucleoprotein complexes, nucleosomes, or within apoptotic bodies. Any intracellular RNA found in plasma or serum can additionally be detected by this invention. Specifically, this invention enables the extraction of circulating RNA from plasma or serum and utilizes nucleic acid amplification assays for the identification, detection, inference, monitoring, or evaluation of any neoplasm, benign, premalignant, or malignant, in humans or other animals, which might be associated with that RNA. Further, this invention allows the qualitative or quantitative detection of tumor-derived or associated extracellular RNA circulating in the plasma or serum of humans or animals with or without any prior knowledge of the presence of cancer or premalignant tissue.
Owner:ONCOMEDX
Method enabling use of extracellular RNA extracted from plasma or serum to detect, monitor or evaluate cancer
InactiveUS20070009934A1Assess prognosisMicrobiological testing/measurementFermentationLipid formationCirculating RNA
This invention relates to the use of tumor-derived or associated extracellular ribonucleic acid (RNA) found circulating in the plasma or serum fraction of blood for the detection, monitoring, or evaluation of cancer or premalignant conditions. Extracellular RNA may circulate as non-bound RNA, protein-bound RNA, lipid-RNA complexes, lipoprotein (proteolipid)—RNA complexes, protein-RNA complexes including within or in association with ribonucleoprotein complexes, nucleosomes, or within apoptotic bodies. Any intracellular RNA found in plasma or serum can additionally be detected by this invention. Specifically, this invention enables the extraction of circulating RNA from plasma or serum and utilizes nucleic acid amplification assays for the identification, detection, inference, monitoring, or evaluation of any neoplasm, benign, premalignant, or malignant, in humans or other animals, which might be associated with that RNA. Further, this invention allows the qualitative or quantitative detection of tumor-derived or associated extracellular RNA circulating in the plasma or serum of humans or animals with or without any prior knowledge of the presence of cancer or premalignant tissue.
Owner:KOPRESKI MICHAEL
Antibodies Against a Cancer-Associated Epitope of Variant HNRNPG and Uses Thereof
InactiveUS20100303814A1Reduce capacityIn-vivo radioactive preparationsSugar derivativesComplementarity determining regionSpecific antibody
The present application provides the amino acid and nucleic acid sequences of heavy and light chain complementarity determining regions of a cancer specific antibody directed to an epitope of variant Heterogeneous Ribonucleoprotein G (HnRNPG). In addition, the application provides cancer specific antibodies and immunoconjugates comprising the cancer specific antibody attached to a toxin or label, and methods of uses thereof. The application also relates to diagnostic methods and kits using the cancer specific antibodies disclosed herein. Further, the application provides novel cancer-associated epitopes and antigens of variant HnRNPG, and uses thereof.
Owner:VIVENTIA BIO
Barley gingko milk product and preparation method thereof
The invention discloses a barley gingko milk product and a preparation method thereof. Barley sprout powder, gingko, pure milk or milk powder are used as main raw materials, through the scientific formula and the advanced process, the barley gingko milk product is produced, and has faint scent taste, rich nutrition and higher health function. The product not only has components of crude protein, crude fat, reducing sugar, ribonucleoprotein, mineral substance, crude fiber, multiple vitamins and the like, but also has better health functions of improving acidic constitution and preventing various diseases.
Owner:丁相军
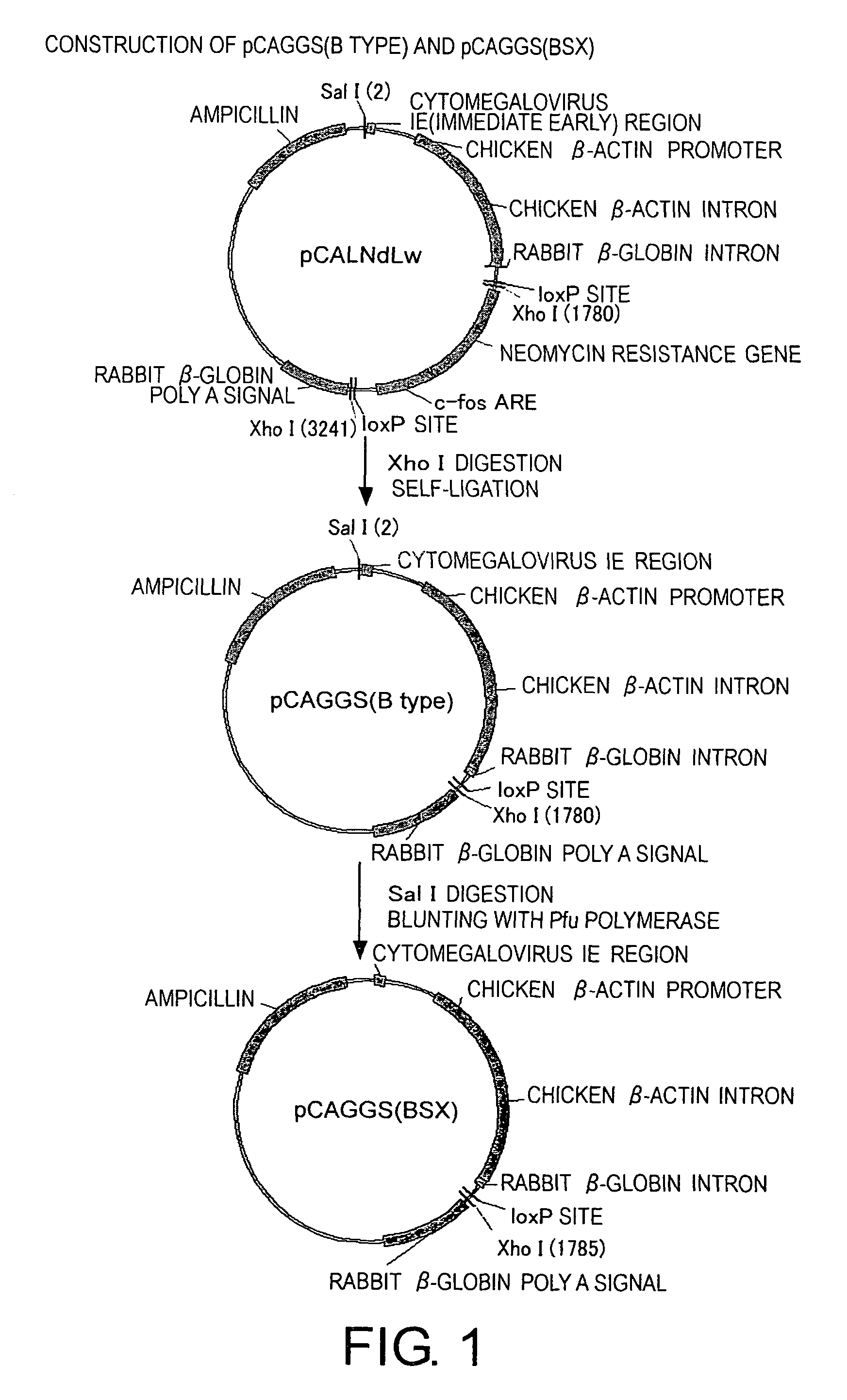
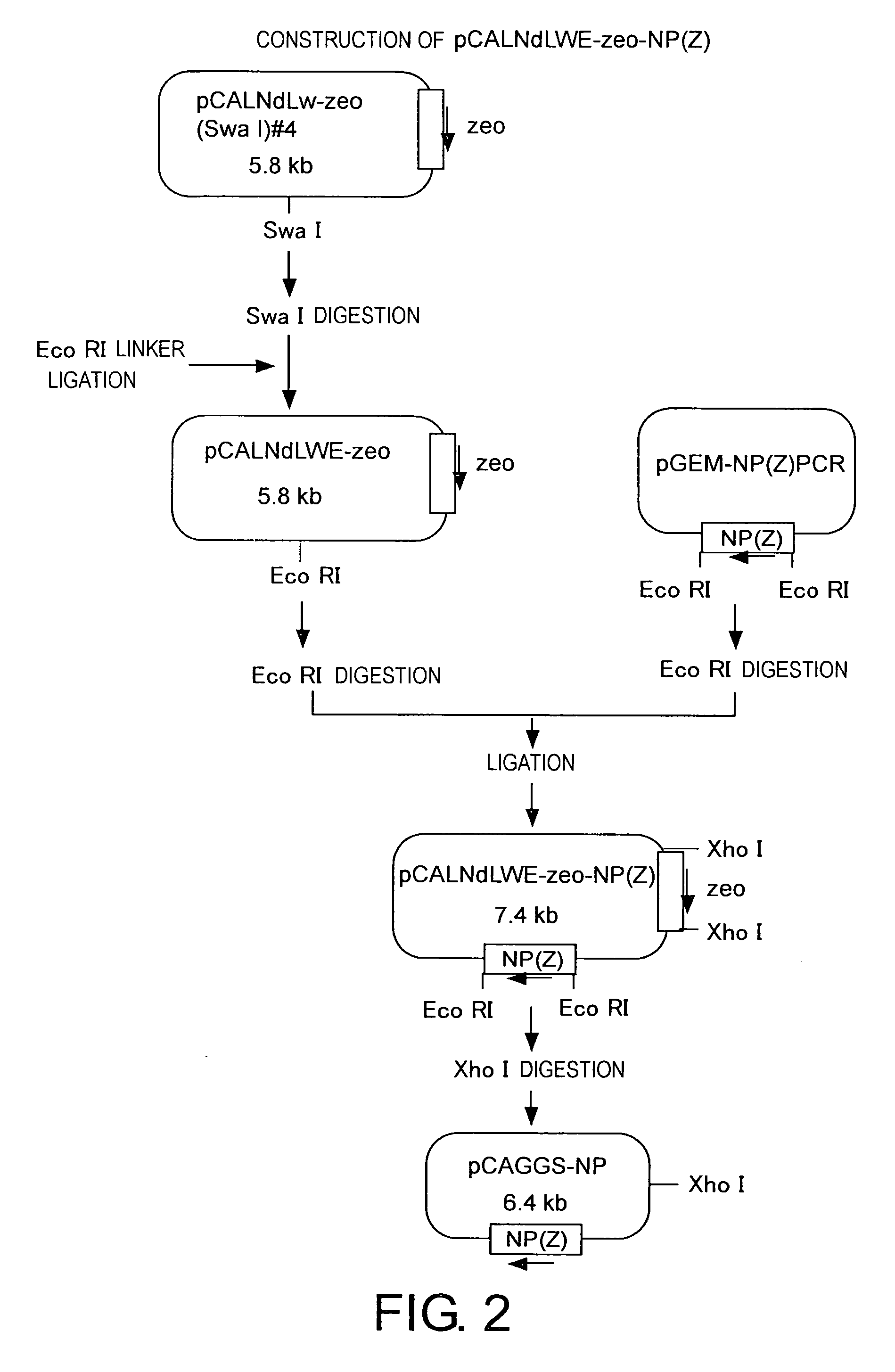
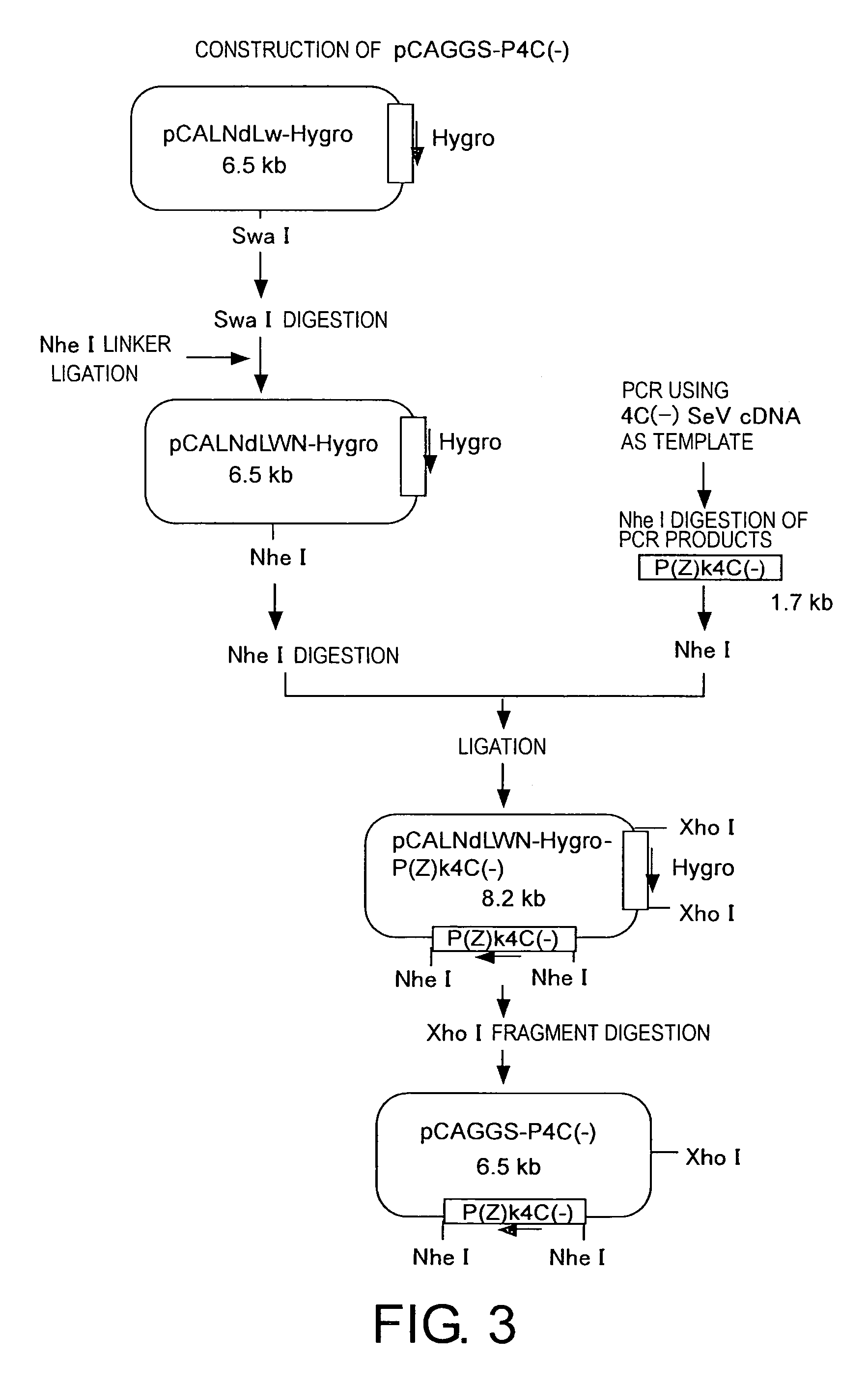
Methods for producing minus-strand RNA viral vectors using hybrid promoter comprising cytomegalovirus enhancer and chicken β-actin promoter
ActiveUS8741650B2Improve securitySsRNA viruses positive-senseSugar derivativesRibonucleoprotein complexActin
The present invention provides methods for producing a minus-strand RNA viral vector, which comprise using a promoter comprising a cytomegalovirus enhancer and a chicken β-actin promoter, to induce the transcription of the genome RNA of a minus-strand RNA viral vector and the expression of minus-strand RNA viral proteins that form a ribonucleoprotein with the genome RNA. The methods of the present invention enable high efficiency production of highly safe minus-strand RNA viral vectors. The methods of the present invention are particularly useful for producing minus-strand RNA viral vectors that are deficient in envelope-constituting protein genes.
Owner:ID PHARMA
Preparation and application of enzyme-linked immuno sorbent assay (ELISA) kit for detecting virus antibody IgM of fever with throbocytopenia associated syndrome
InactiveCN102809649AGood growth characteristicsRaise antibody levelsBiological testingViral antibodyOpen reading frame
The invention relates to preparation and application of an enzyme-linked immuno sorbent assay (ELISA) kit for detecting a virus-specific antibody of fever with throbocytopenia associated syndrome. The kit comprises an anti-human-IgM-enveloped ELISA reaction plate, horse radish peroxidase (HRP)-coupled nucleocapsid protein (NP) and a tetramethylbenzidine (TMB) color development agent. A preparation method for a ribonucleoprotein (rNP)-enveloped reaction plate comprises the following steps of: amplifying an open reading frame sequence of nucleocapsid protein of a virus strain of a novel bunyavirus JS-2007-001 by a reverse transcription polymerase chain reaction (PCR) method, inserting the sequence into pQE30 to construct a pQE30-NP expression plasmid, transfecting an M15 strain by using pQE30-NP, performing inducible expression of 6His-NP recombinant protein by using isopropyl-beta-d-thiogalactoside (IPTG), purifying the 6His-NP recombinant protein by using Hitrap, and marking to obtain HRP-coupled NP(HRP-rNP). The reaction plate can be combined with a novel bunyavirus-resistant antibody (IgM) in a sample when incubated with the sample to be tested conventionally, and the HRP-rNP incubation is used in the subsequent detection, so that an 'anti-human IgM-antibody-enzyme-labeled antigen' capture detection system is formed; the color reaction after an enzyme substrate, namely the TMB color development agent is added is analyzed by an enzyme-labeled instrument; and the content of the specific antibody (IgM) of the novel bunyavirus is obtained according to the color development degree.
Owner:万里明
Nanocapsule delivery system for ribonucleoproteins
ActiveUS20190099381A1Assist in deliveryFacilitate endosomal escapeOrganic active ingredientsSpecial deliveryPolymer scienceRibonucleoprotein complex
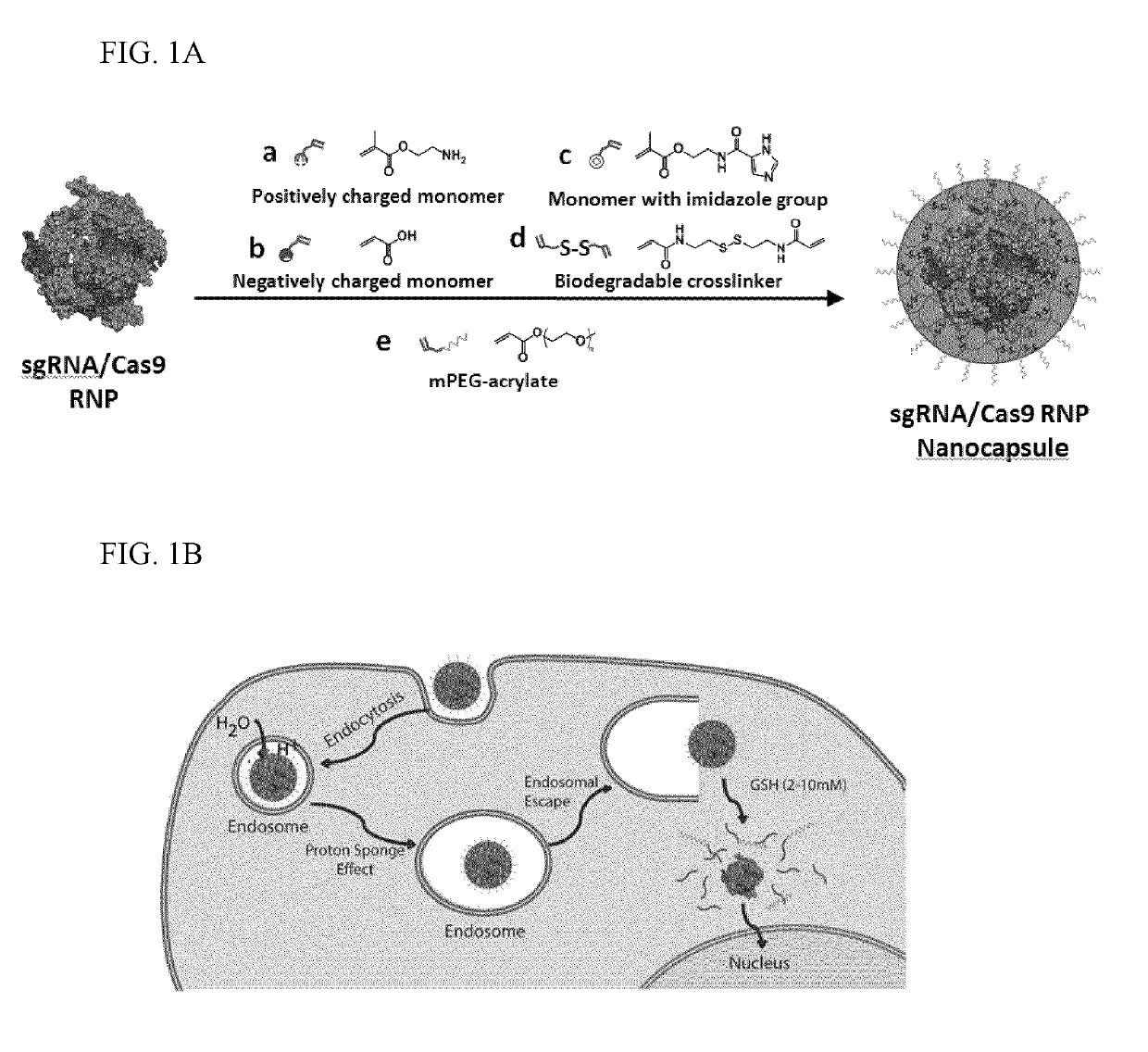
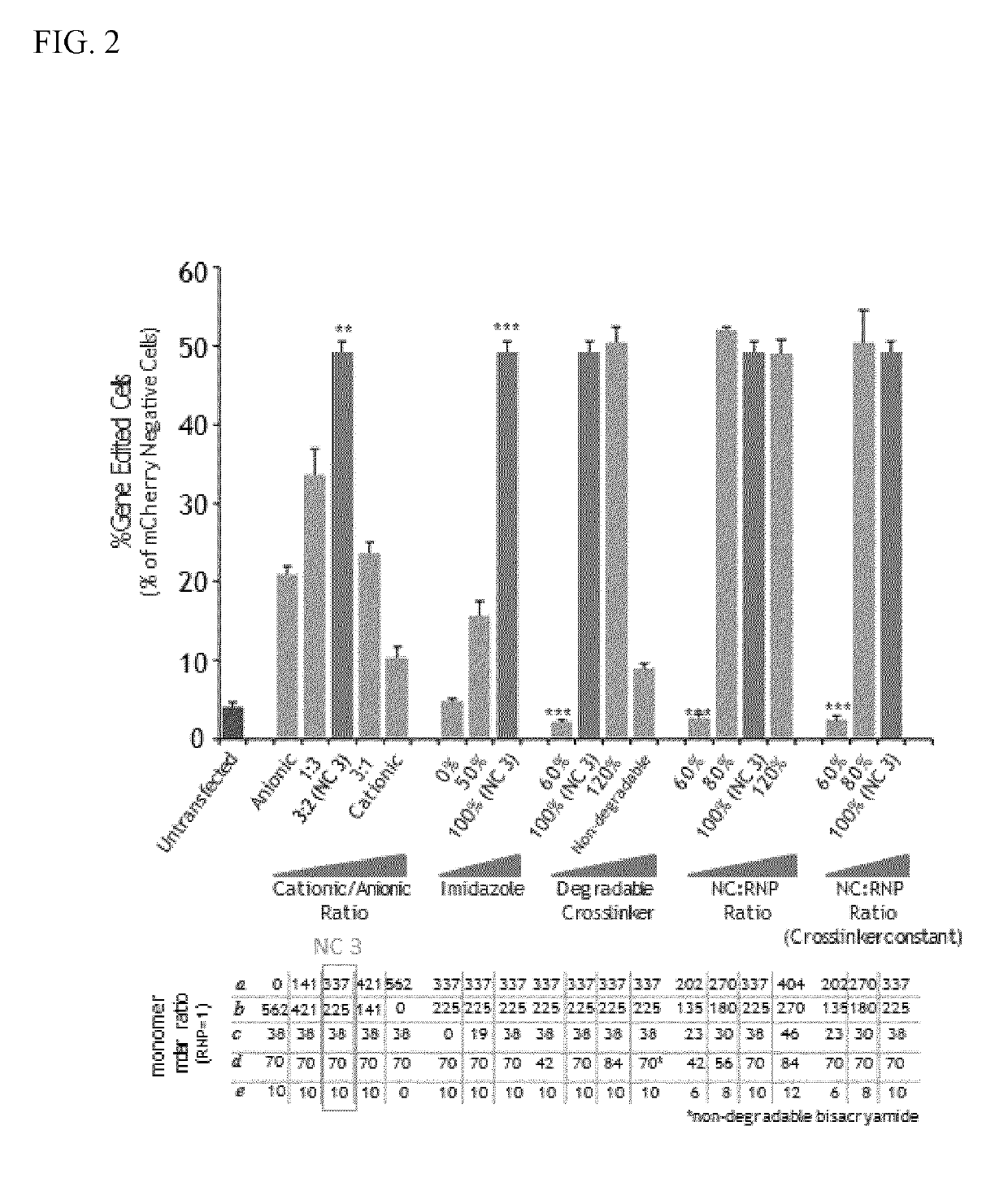
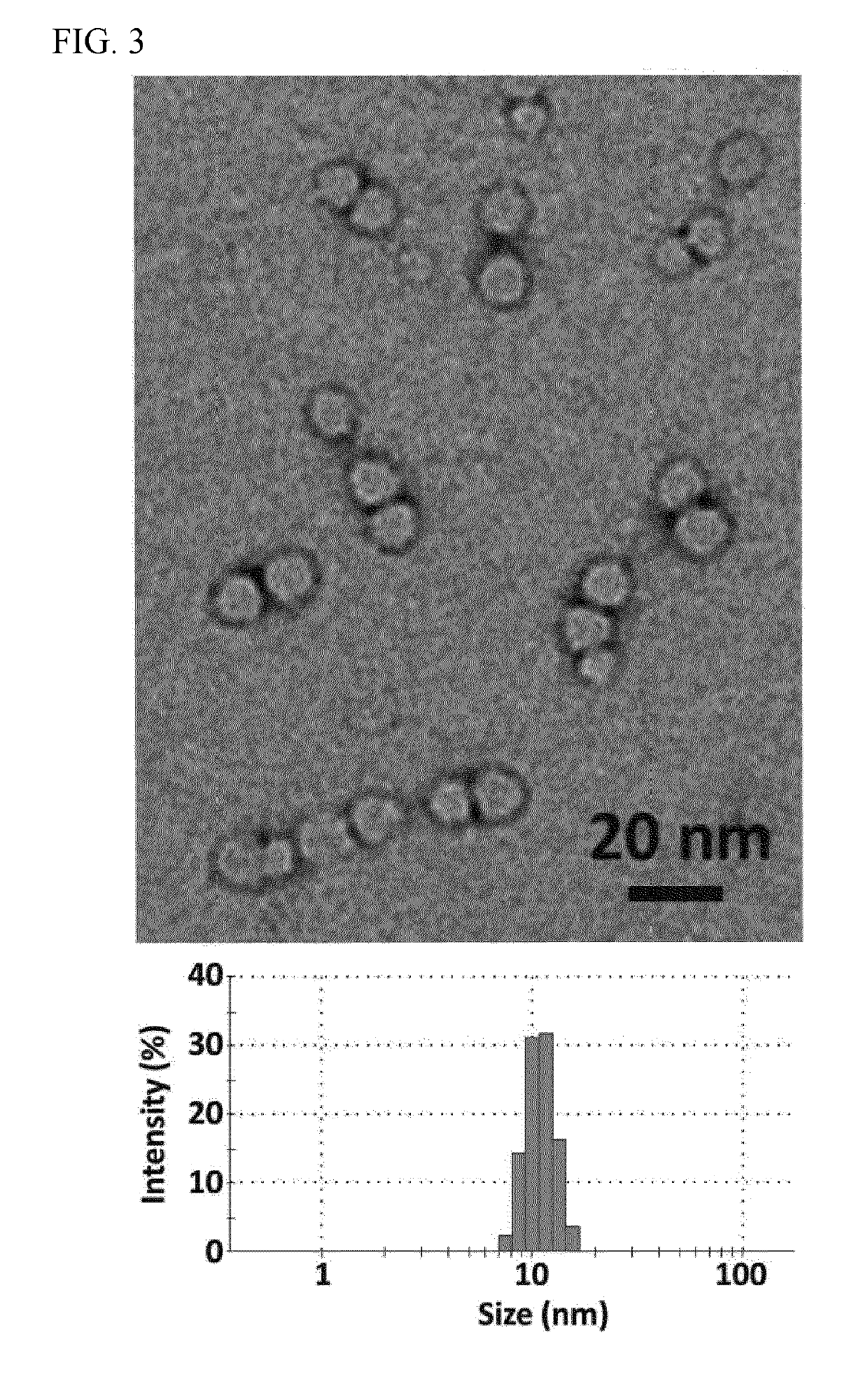
Provided herein are nanocapsules comprising a single ribonucleoprotein (RNP) complex as a core and an biodegradable crosslinked polymer shell that encapsulates the core, wherein the RNP complex comprises a Cas9 polypeptide and a guide RNA, and the biodegradable crosslinked polymer shell comprises polymerized monomers of imidazolyl acryloyl monomers, bisacryloyl disulfide monomers (a biodegradable cross-linker), optionally PEG acryloyl monomers, and either cationic acryloyl monomers, anionic acryloyl monomers, or both cationic and anionic acryloyl monomers (optionally in combination with non-ionic acryloyl monomers) as defined herein. Also provided are methods of making the nanocapsules, kits containing the nanocapsules and methods of delivering the encapsulated RNP to cells.
Owner:WISCONSIN ALUMNI RES FOUND
Efficient clustered regularly interspaced short palindromic repeats ribonucleoprotein (CRISPR RNP) and donor DNA co-location mediated gene insertion or replacement method and application thereof
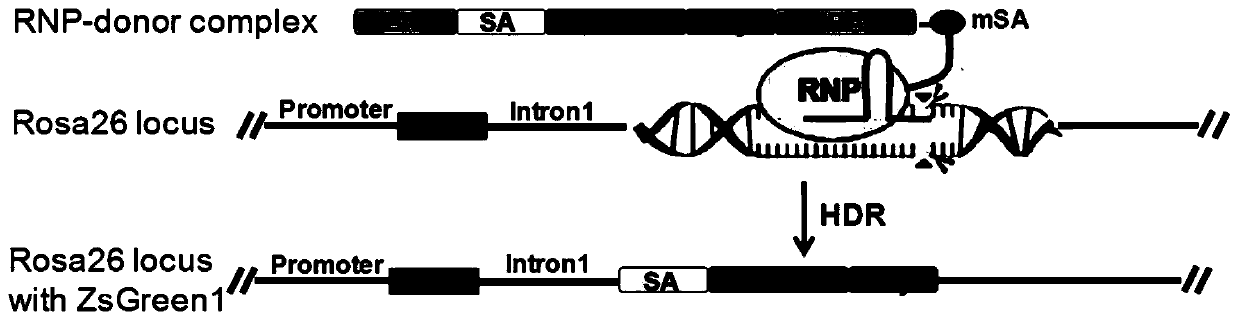
ActiveCN111575319AAccurate replacementHigh homologous recombination efficiencyHydrolasesAntibody mimetics/scaffoldsBiotechnologyLivestock breeding
The invention discloses an efficient clustered regularly interspaced short palindromic repeats ribonucleoprotein (CRISPR RNP) and donor DNA co-location mediated gene insertion or replacement method and application thereof. The method comprises the following steps: (1) biotin labeling is performed on donor DNA to obtain biotin-labeled donor DNA; (2) fusion protein of Cas9 protein and monovalent streptavidin, sgRNA and the biotin-labeled donor DNA are evenly mixed and undergo standing to obtain a CRISPR RNP-donor DNA complex; and (3) cell nucleus transformation is performed on the CRISPR RNP-donor DNA complex to realize gene insertion or replacement. According to the method, by utilizing the characteristic that the fusion protein can be combined with the biotin-labeled donor DNA, the CRISPRRNP and the donor DNA appear at a target locus together, and precise insertion of the donor DNA at the target locus or accurate replacement of a gene at the target locus is realized; and the method issuitable for the aspect of crop and livestock breeding.
Owner:AGRO BIOLOGICAL GENE RES CENT GUANGDONG ACADEMY OF AGRI SCI
Kit (chemiluminiscence method) for determining anti-ribonucleoprotein 70 antibody IgG and manufacturing method thereof
InactiveCN106596919ALow costHigh detection sensitivityChemiluminescene/bioluminescenceAntigenRibonucleoprotein complex
The invention discloses a kit (chemiluminiscence method) for determining an anti-ribonucleoprotein 70 antibody IgG. The kit comprises magnetic particles coated by anti-ribonucleoprotein 70 antigen, acridinium ester marked by mouse-anti-human IgG, a sample diluent, a test diluent, an anti-ribonucleoprotein 70 antibody IgG calibration product, pre-exciting liquid and exciting liquid. The invention further discloses a manufacturing method of the kit (chemiluminiscence method) for determining the anti-ribonucleoprotein 70 antibody IgG. Compared with an existing kit, the kit has the advantages of being easy to operate, high in sensitivity and wide in detection range.
Owner:SHENZHEN YHLO BIOTECH
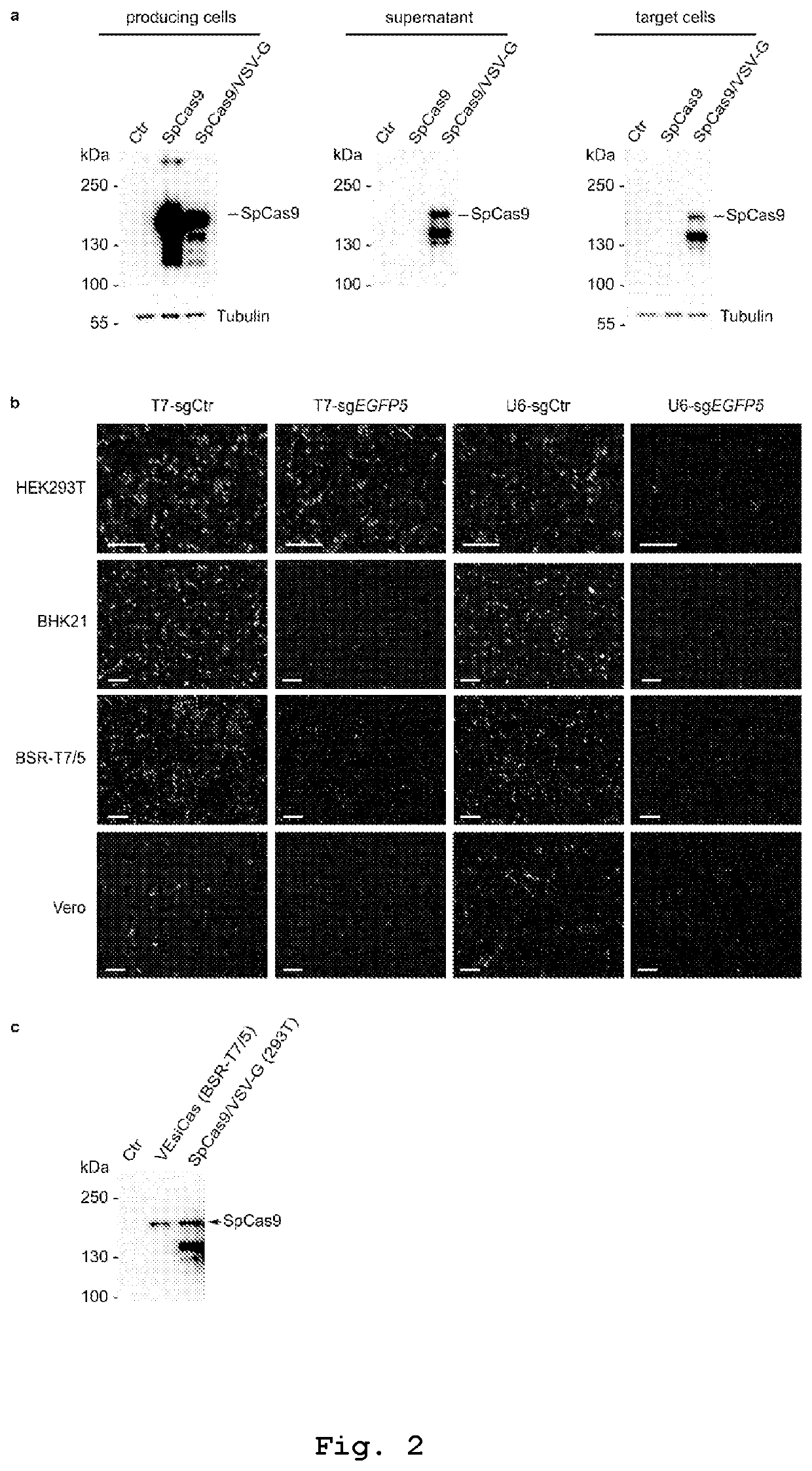
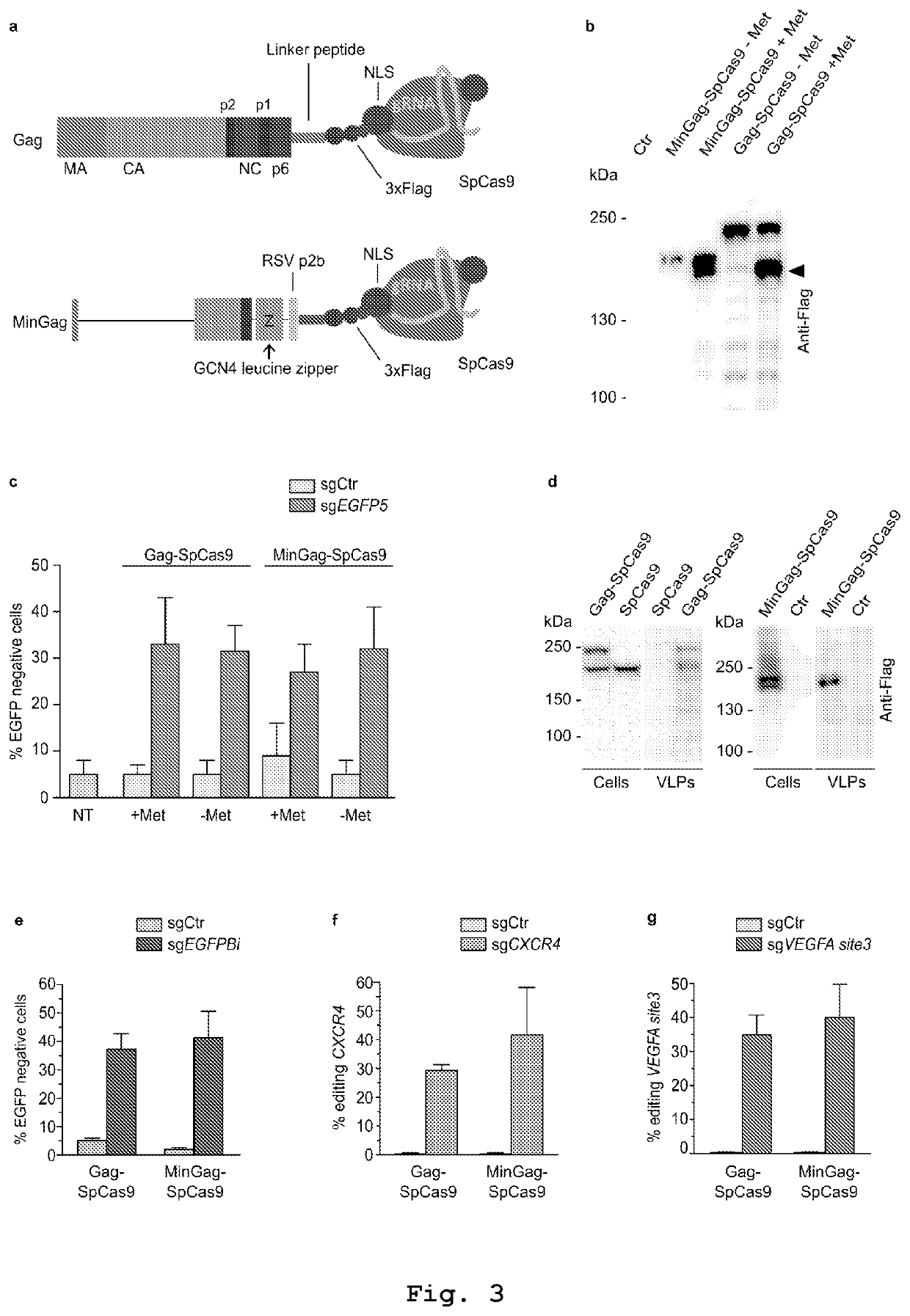
Vesicles for traceless delivery of guide RNA molecules and/or guide RNA molecule/RNA-guided nuclease complex(ES) and a production method thereof
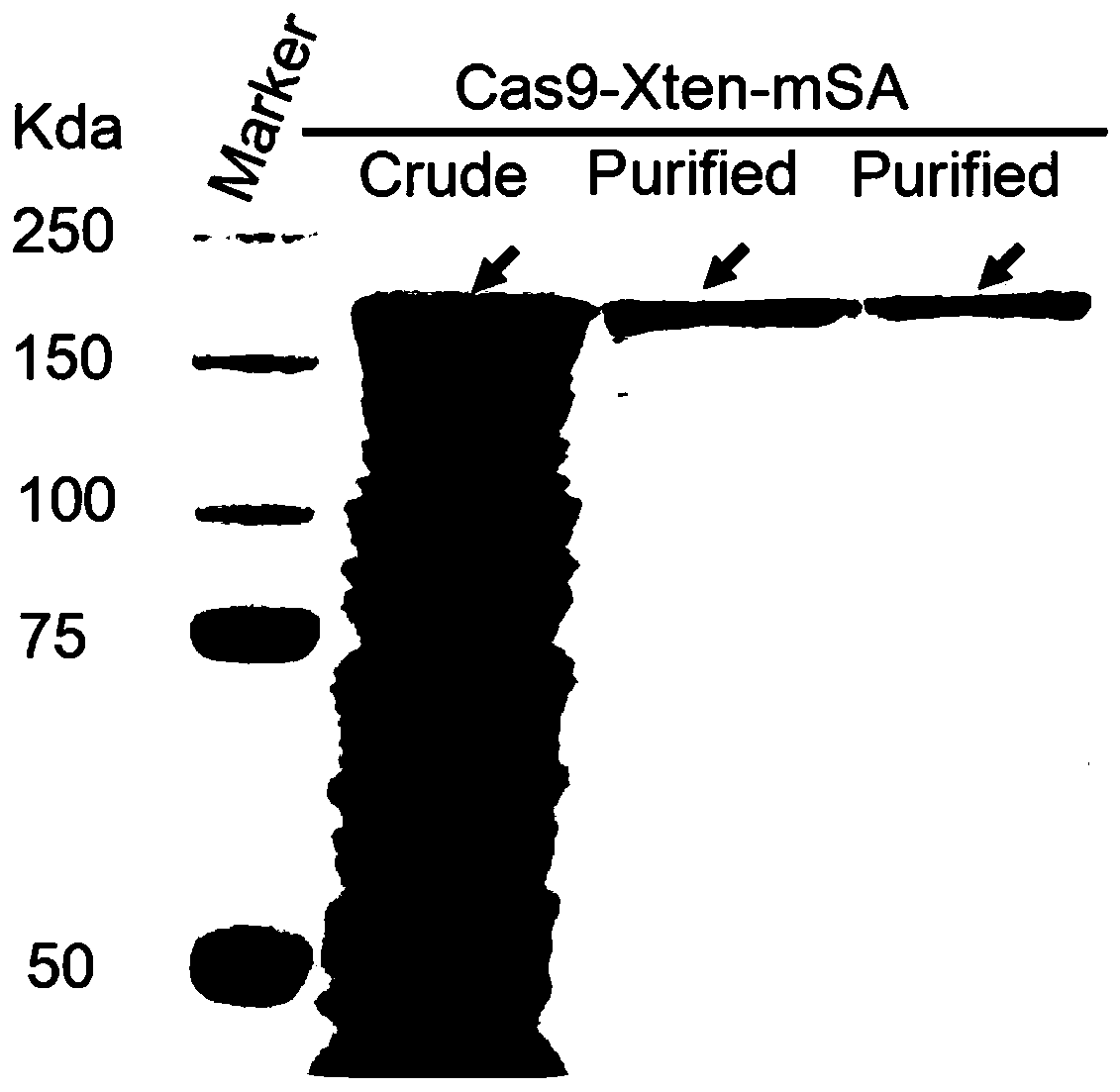
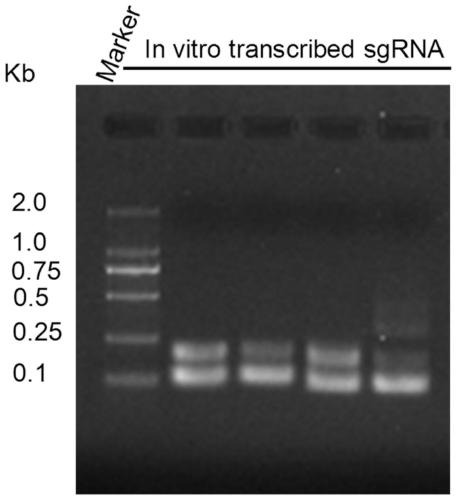
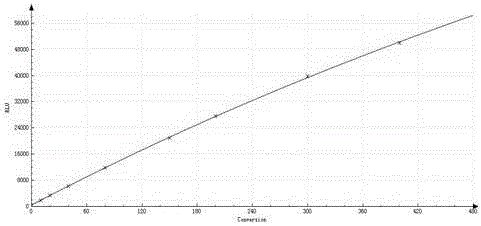
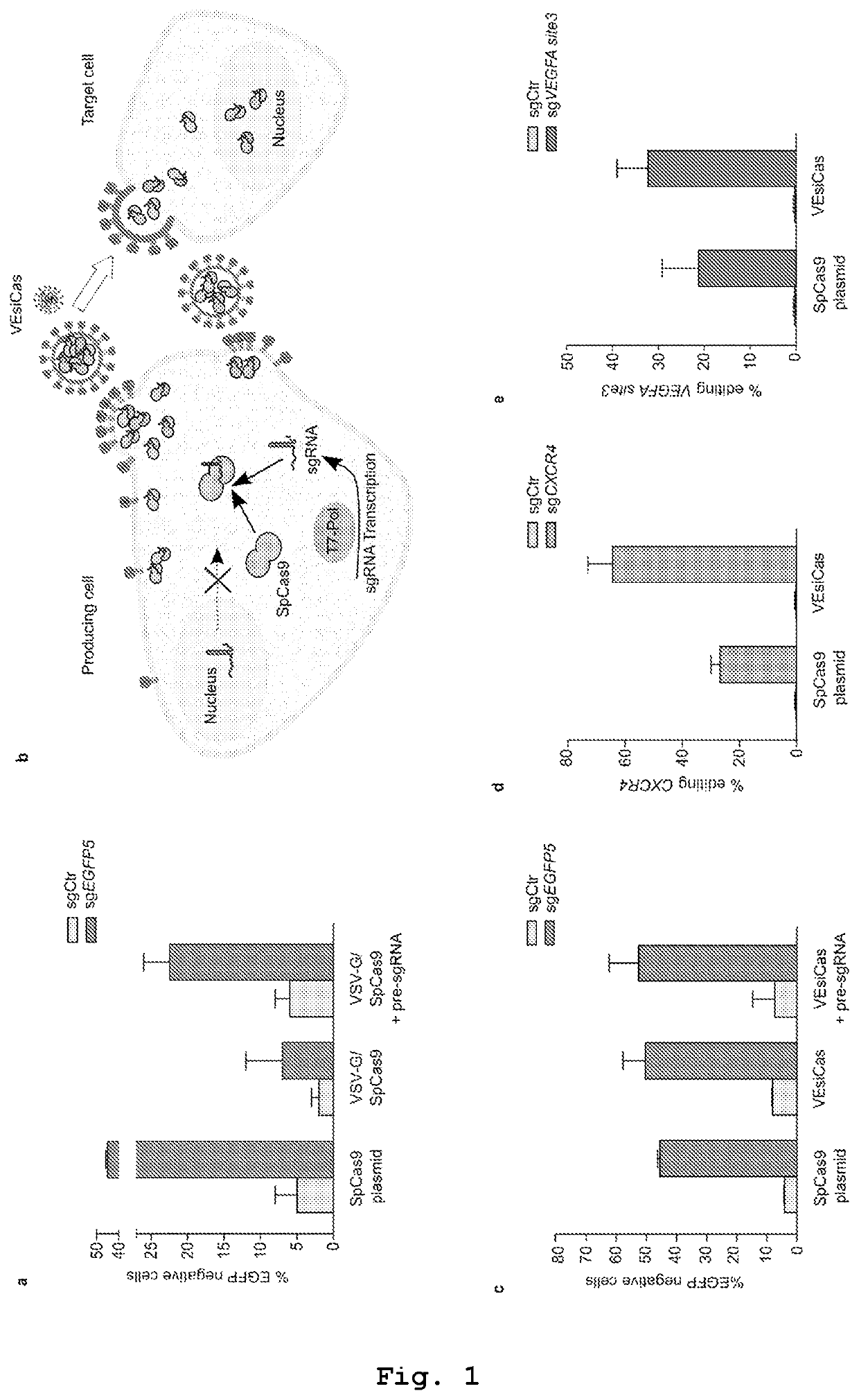
PendingUS20210261957A1SsRNA viruses negative-senseSpecial deliveryRibonucleoprotein complexBiochemistry
The present invention describes vesicles carrying gRNA(s) and / or CRISPR-associated RNA guided-nuclease ribonucleoprotein complexes (RNPs) that are efficiently delivered into target cells through fusogenic envelope proteins.
Owner:ALIA THERAPEUTICS SRL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com