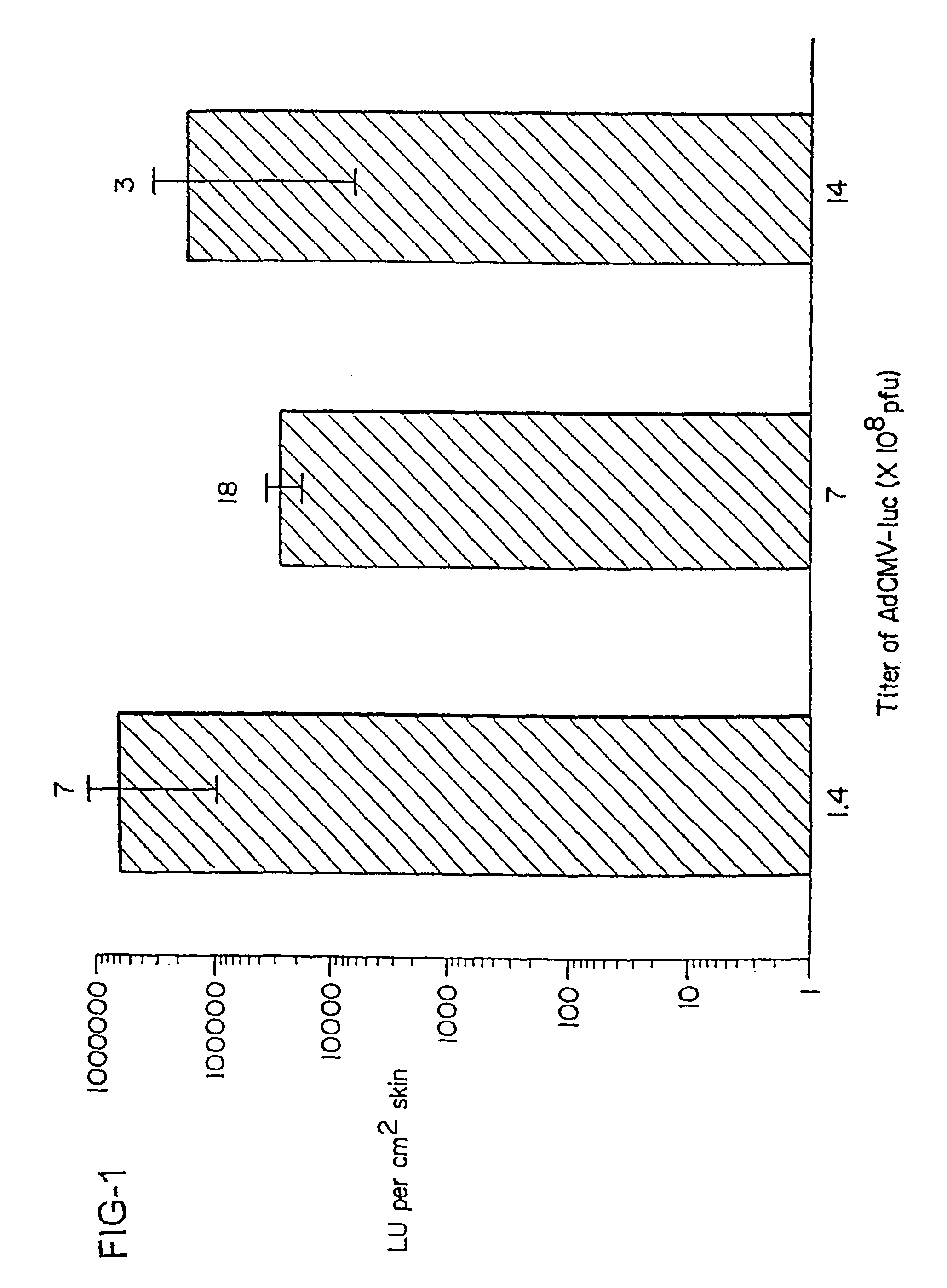
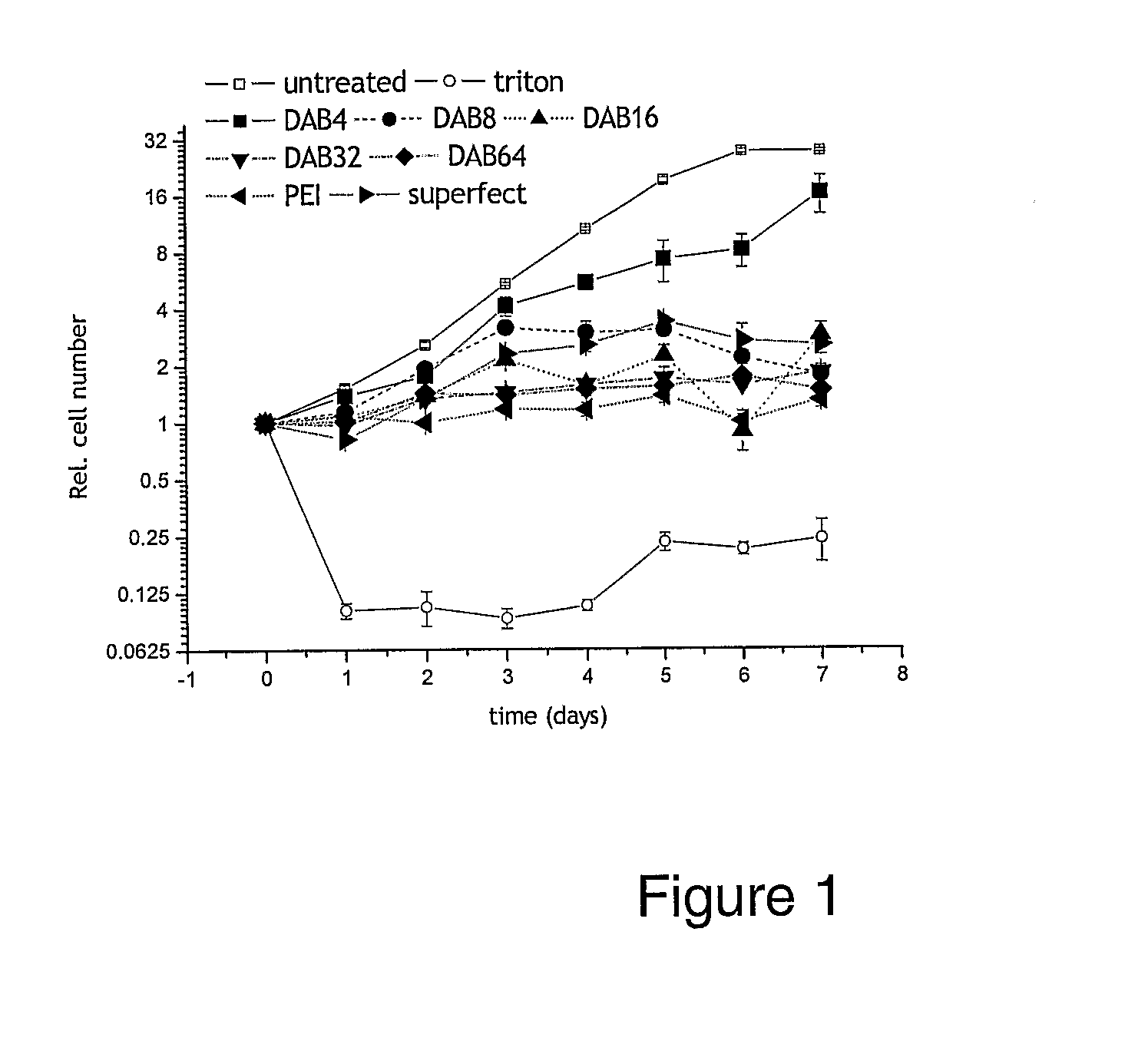
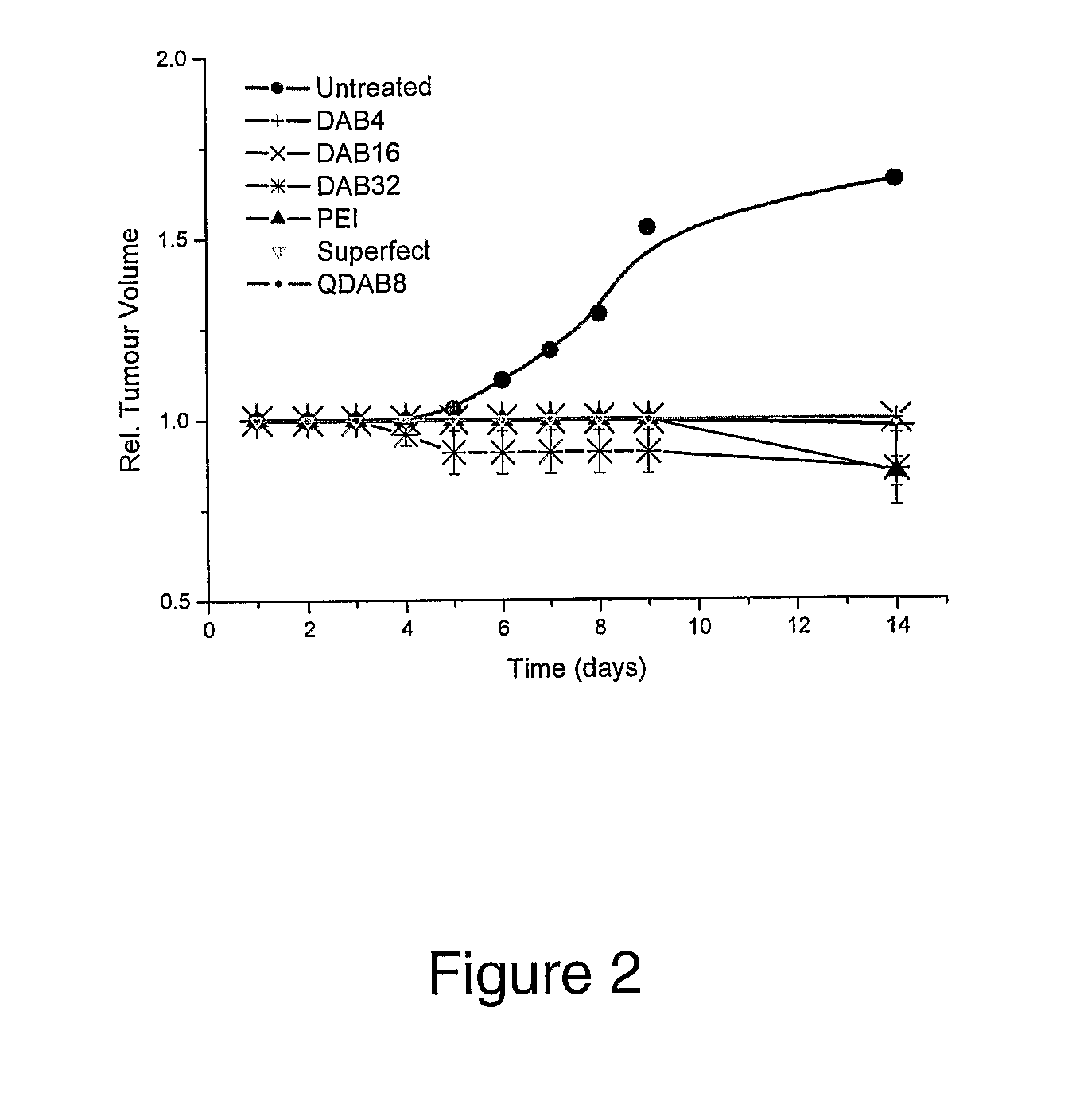
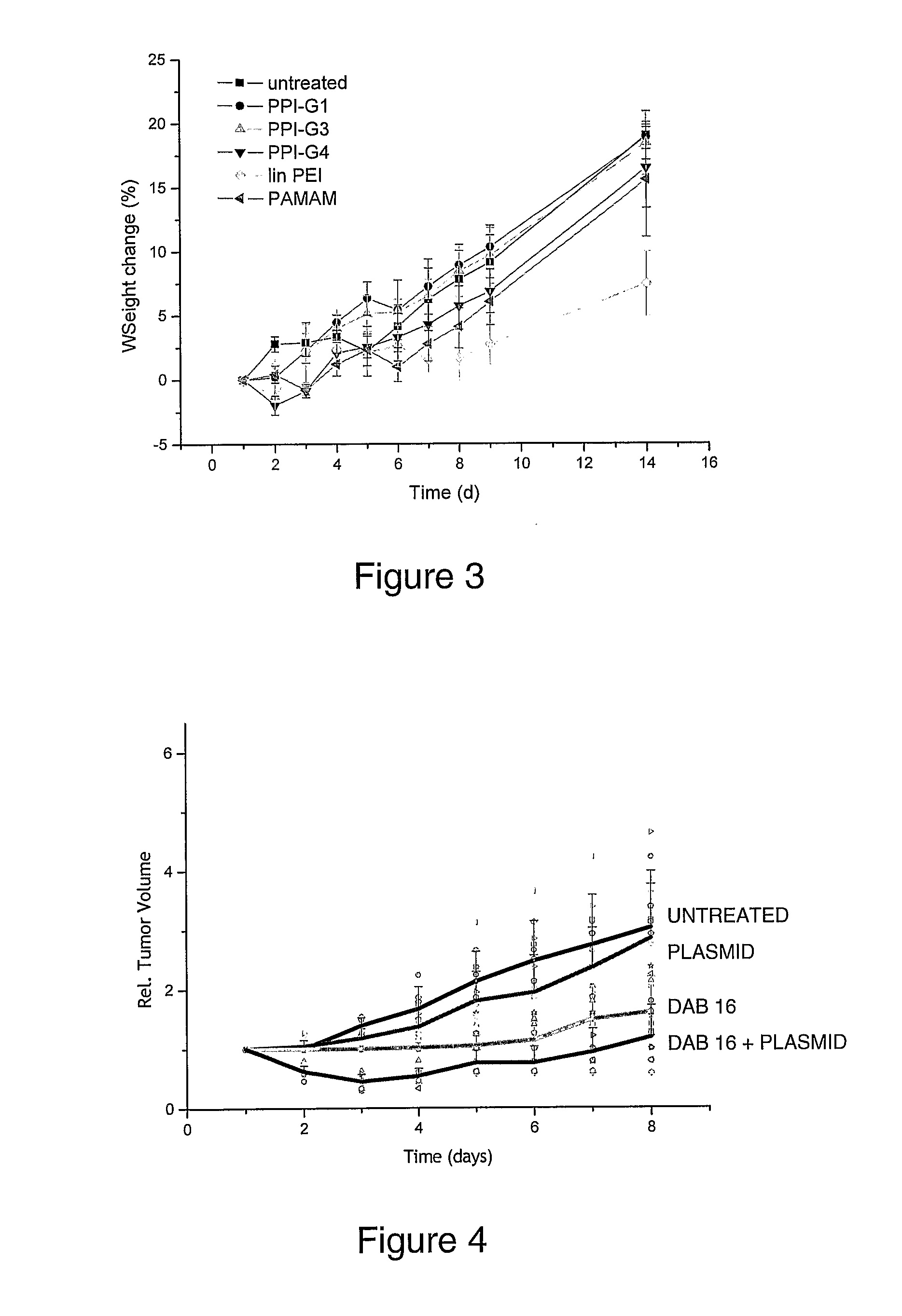
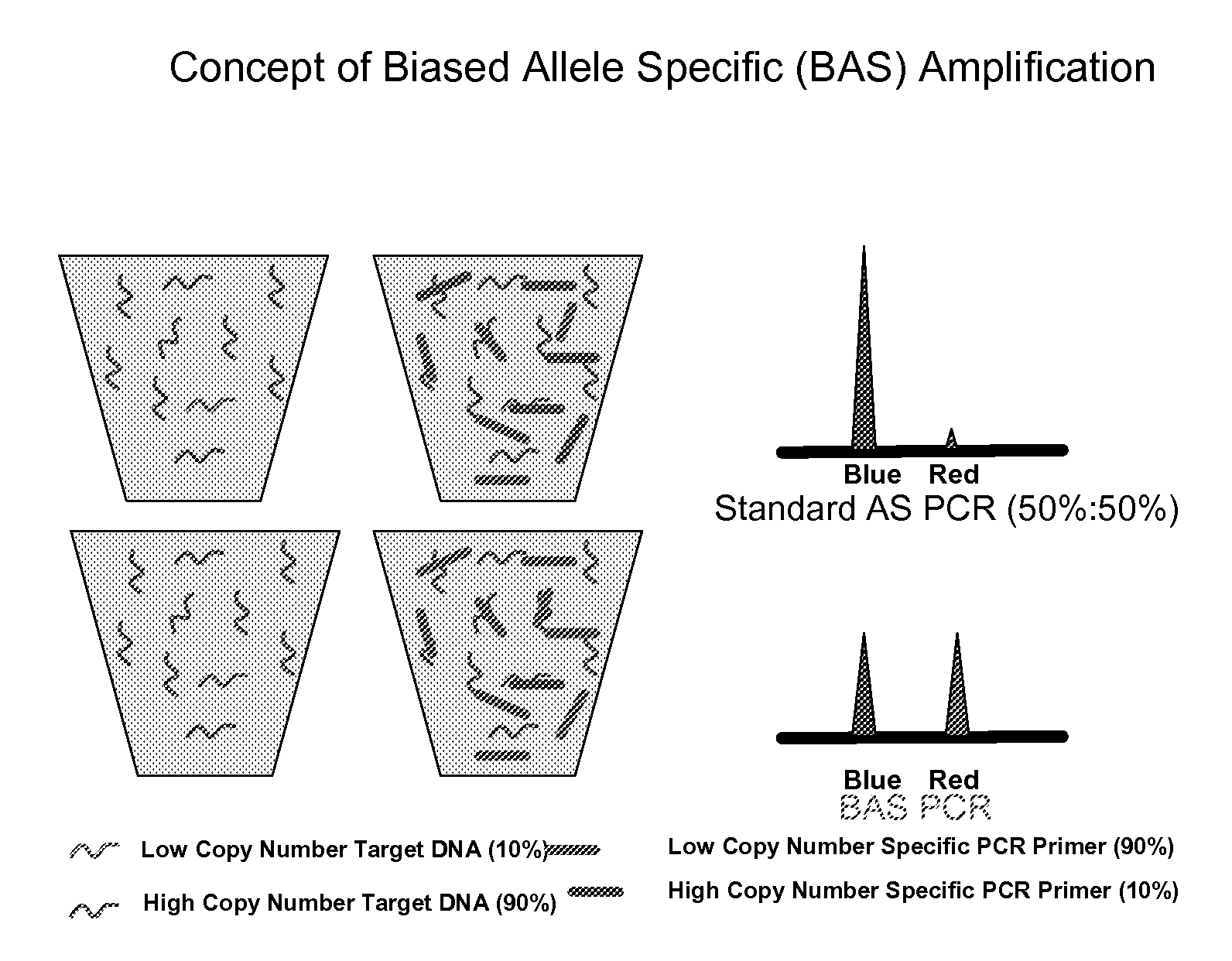
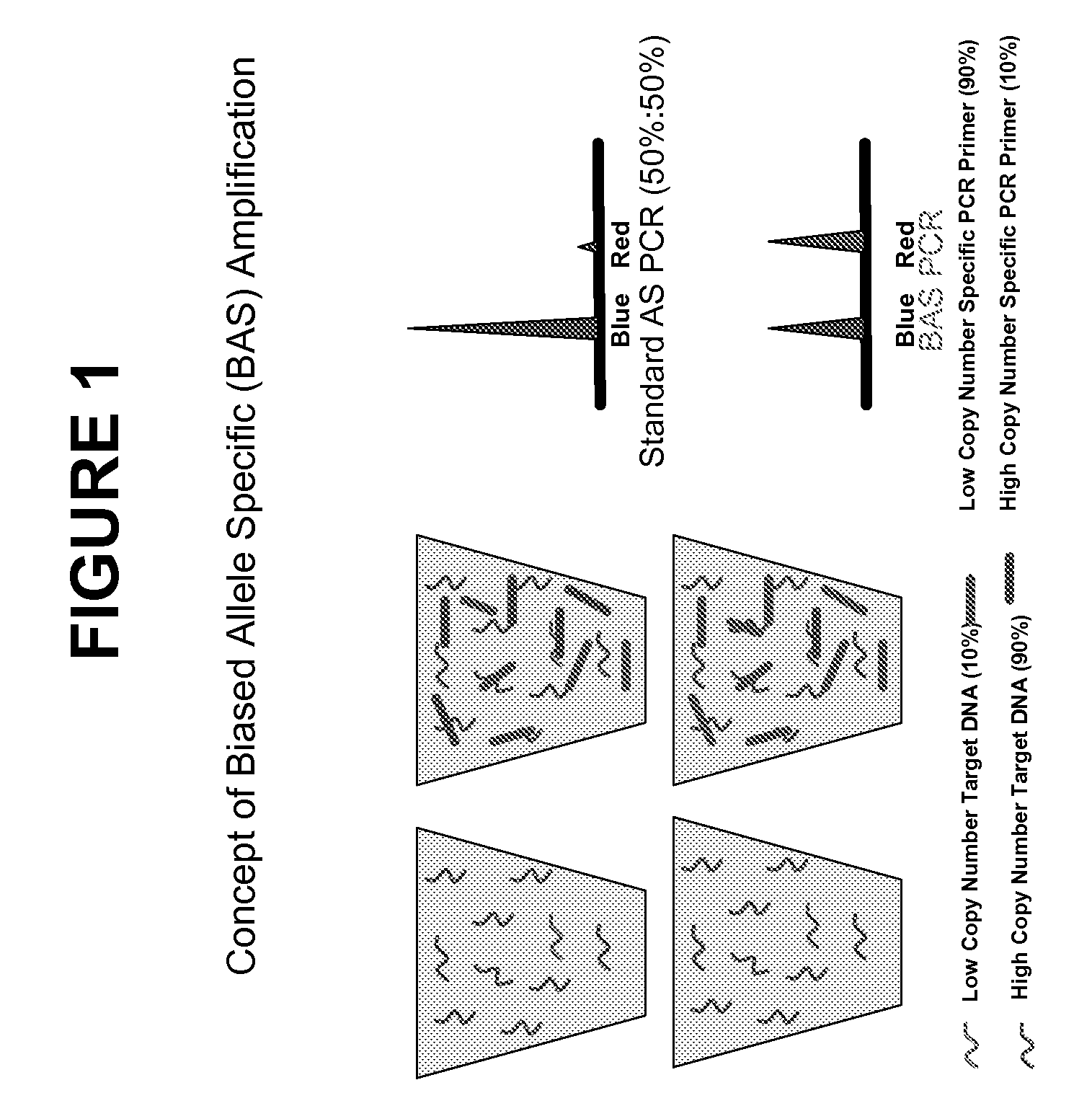
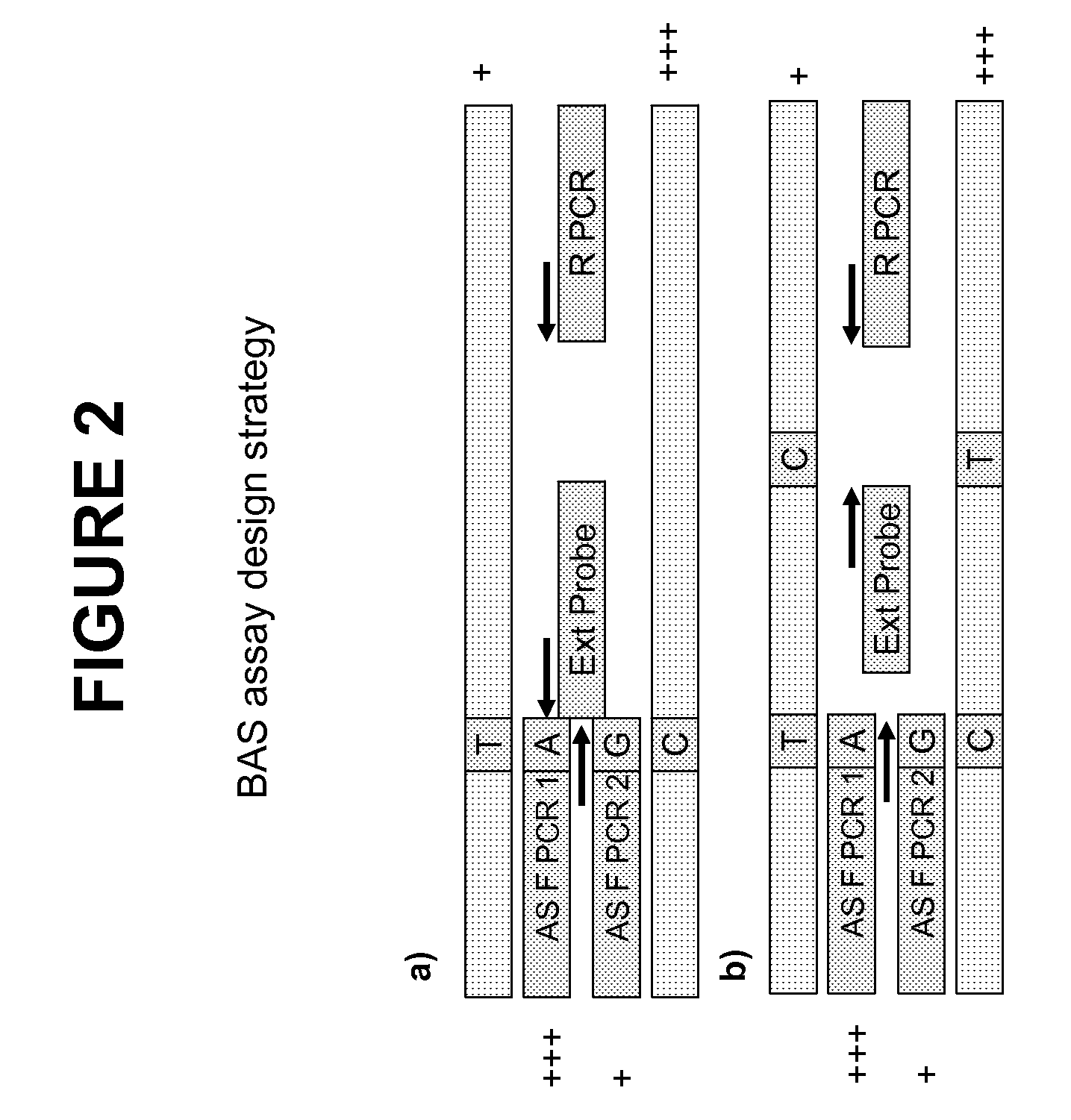
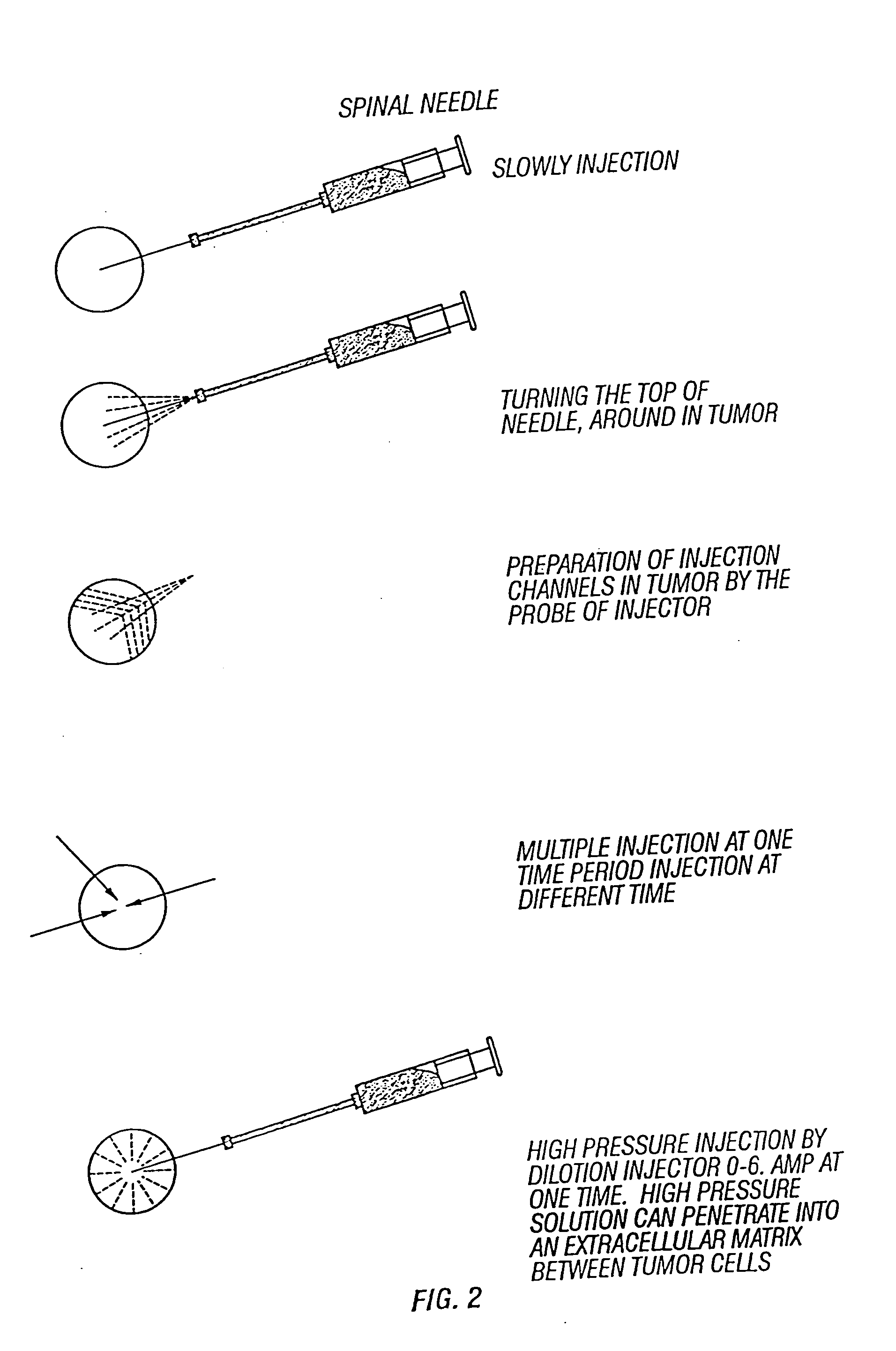
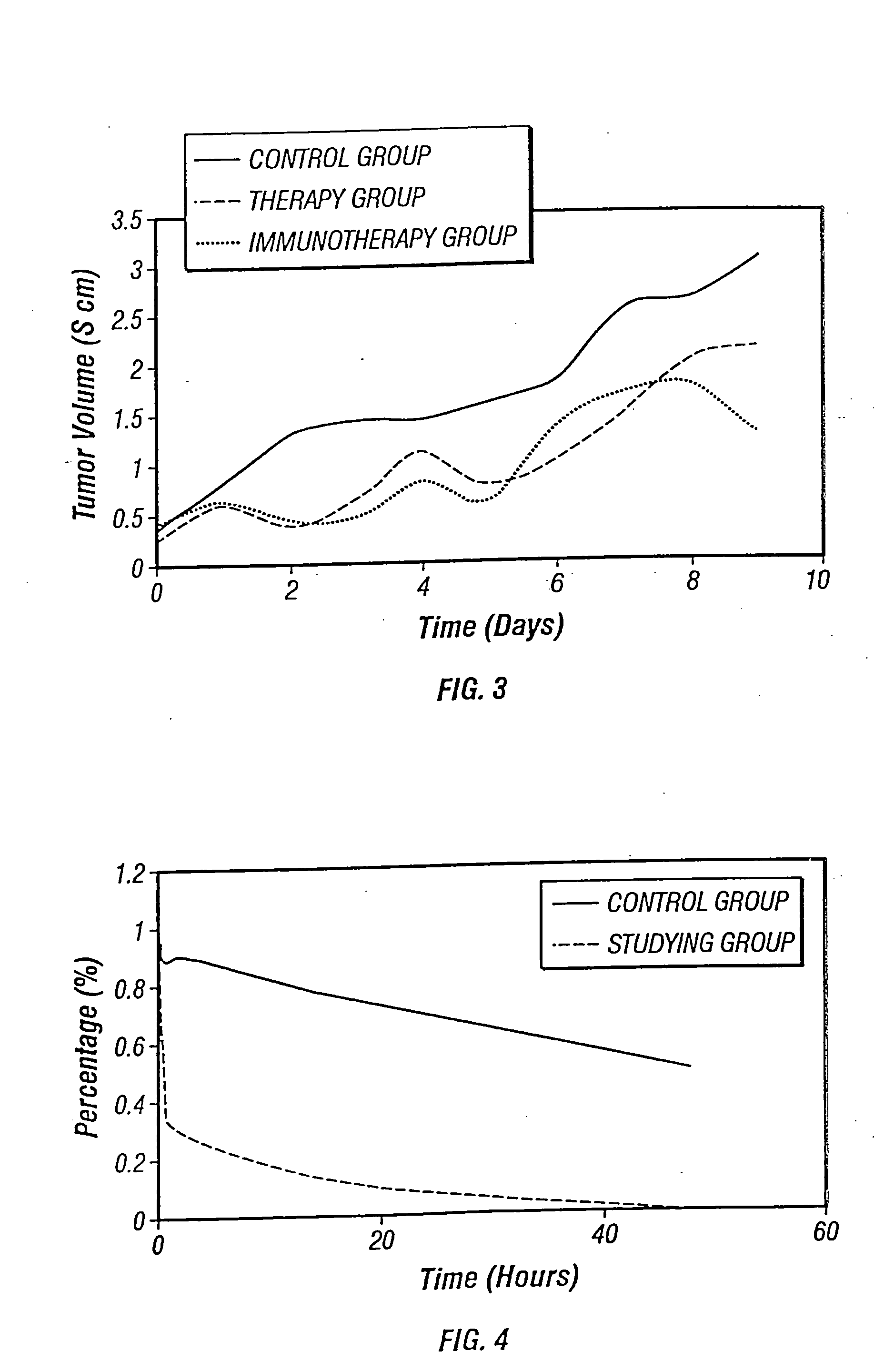
Patents
Literature
800 results about "Neoplasm" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Abnormal growth of tissues that may or may not be cancerous.
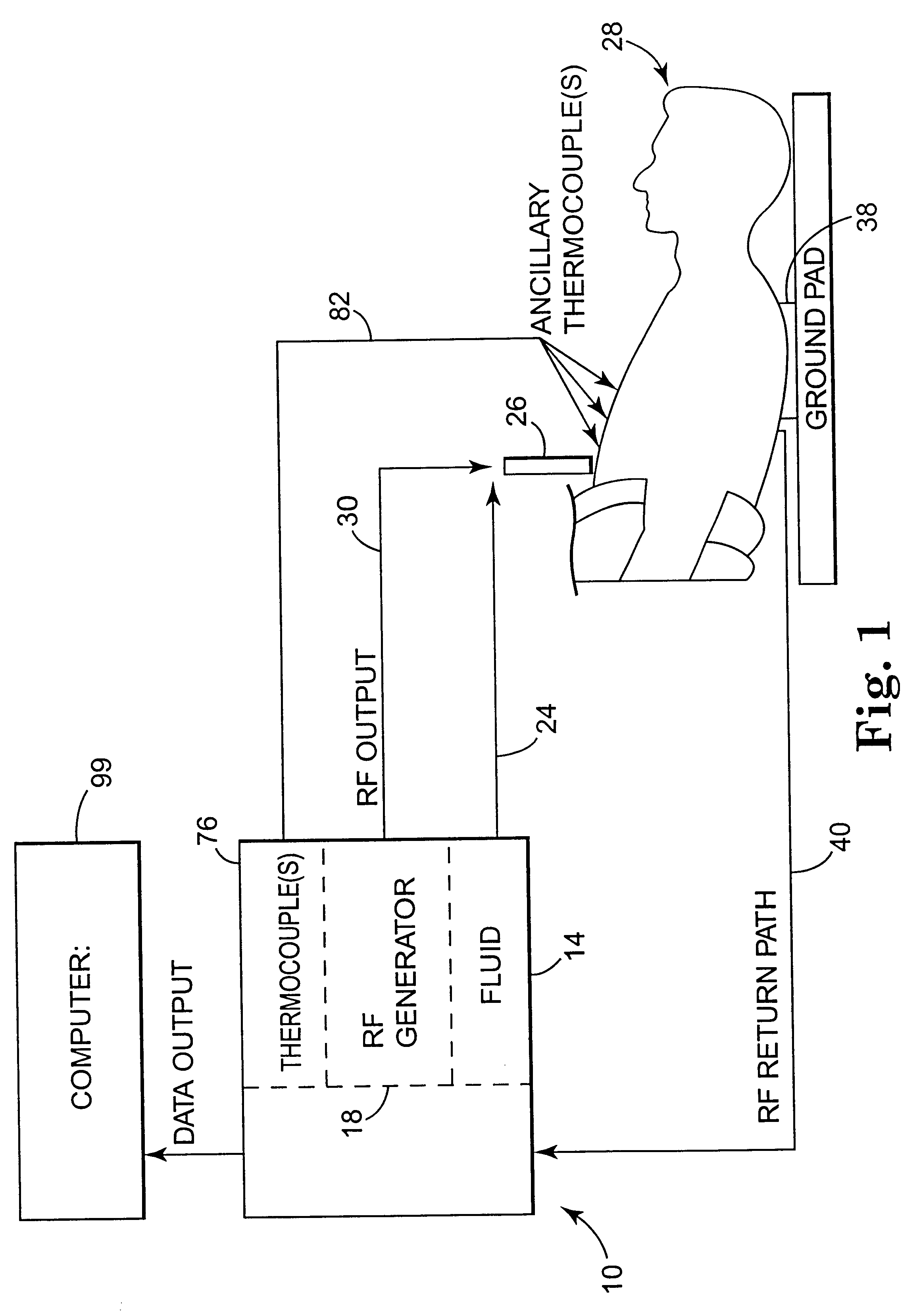
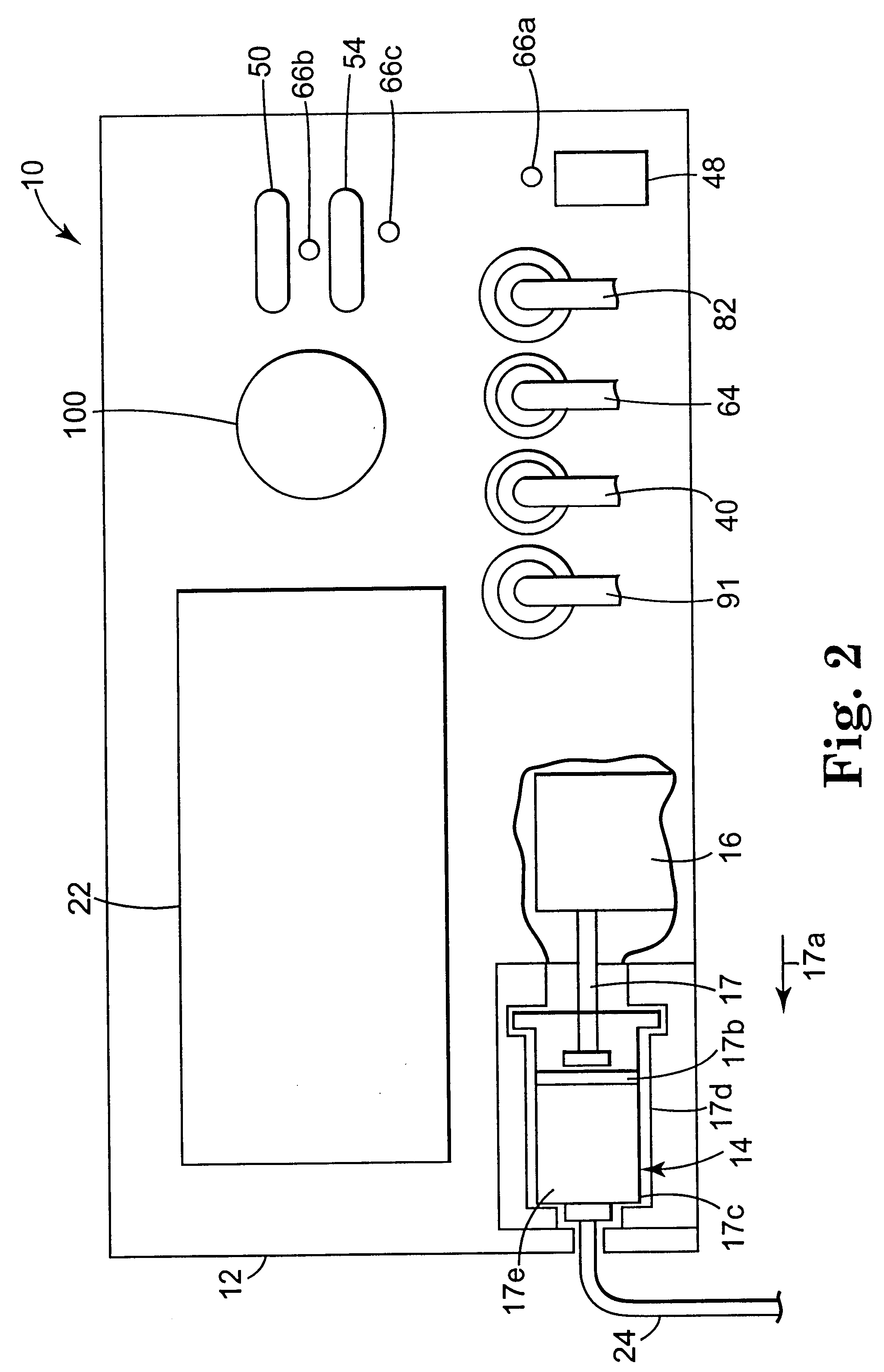
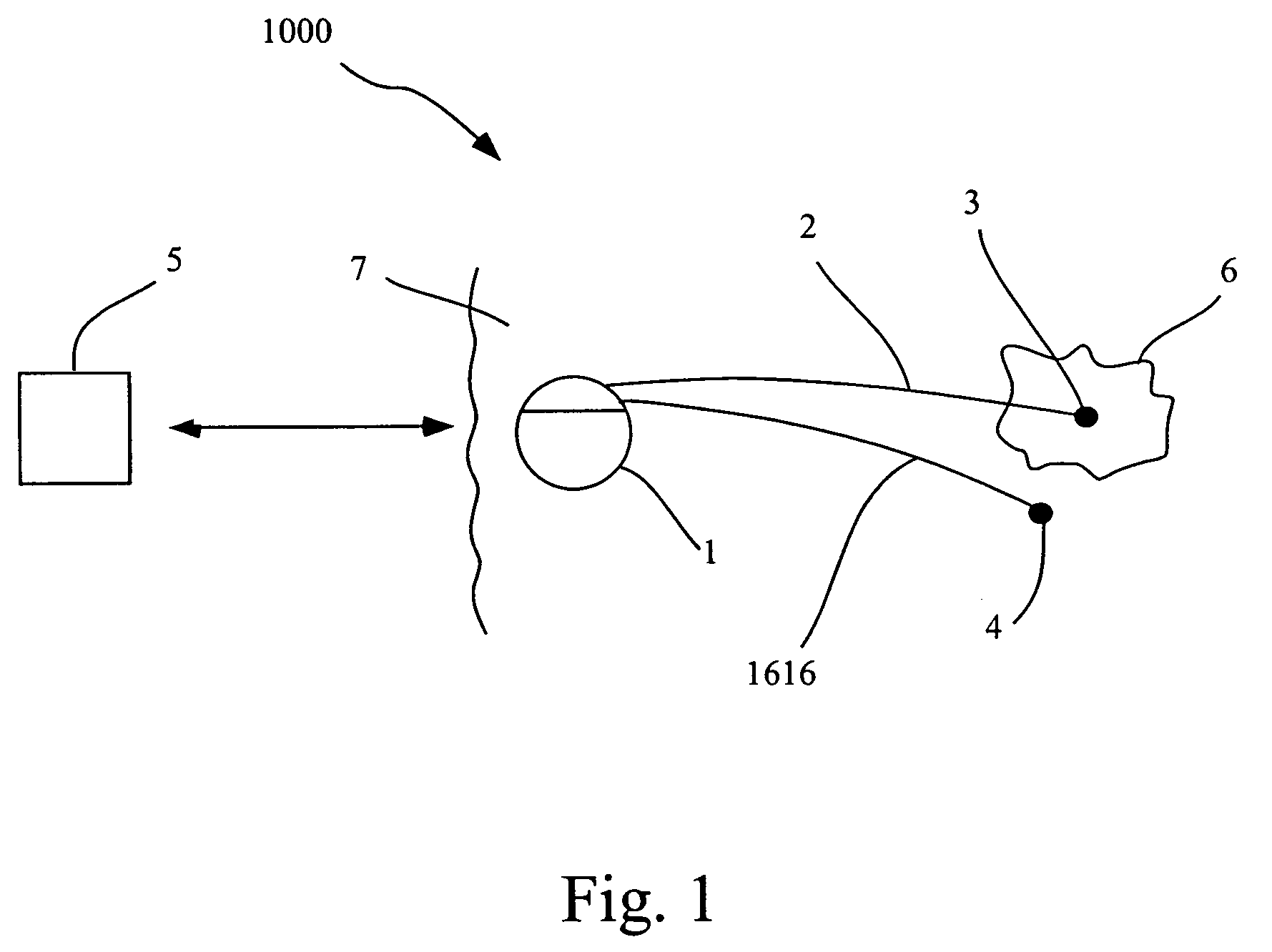
Apparatus and method for creating, maintaining, and controlling a virtual electrode used for the ablation of tissue
InactiveUS6537272B2Improving impedanceReduce the possibilitySurgical instruments for heatingTherapeutic coolingBlood Vessel TissueVascular tissue
The present invention provides an apparatus and a method for producing a virtual electrode within or upon a tissue to be treated with radio frequency alternating electric current, such tissues including but not limited to brain, liver, cardiac, prostate, breast, and vascular tissues and neoplasms. An apparatus in accordance with the present invention includes a source of super-cooled fluid for selectively providing super-cooled fluid to the target tissue to cause a temporary cessation of cellular or electrical activity, a supply of conductive or electrolytic fluid to be provided to the target tissue, and alternating current generator, and a processor for creating, maintaining, and controlling the ablation process by the interstitial or surficial delivery of the fluid to a tissue and the delivery of electric power to the tissue via the virtual electrode. A method in accord with the present invention includes delivering super-cooled fluid to the target tissue to cause a temporary cessation of cellular or electrical activity, evaluating whether the temporary cessation of cellular or electrical activity is the desired cessation of cellular or electrical activity, and if so, delivering a conductive fluid to the predetermined tissue ablation site for a predetermined time period, applying a predetermined power level of radio frequency current to the tissue, monitoring at least one of several parameters, and adjusting either the applied power and / or the fluid flow in response to the measured parameters.
Owner:MEDTRONIC INC
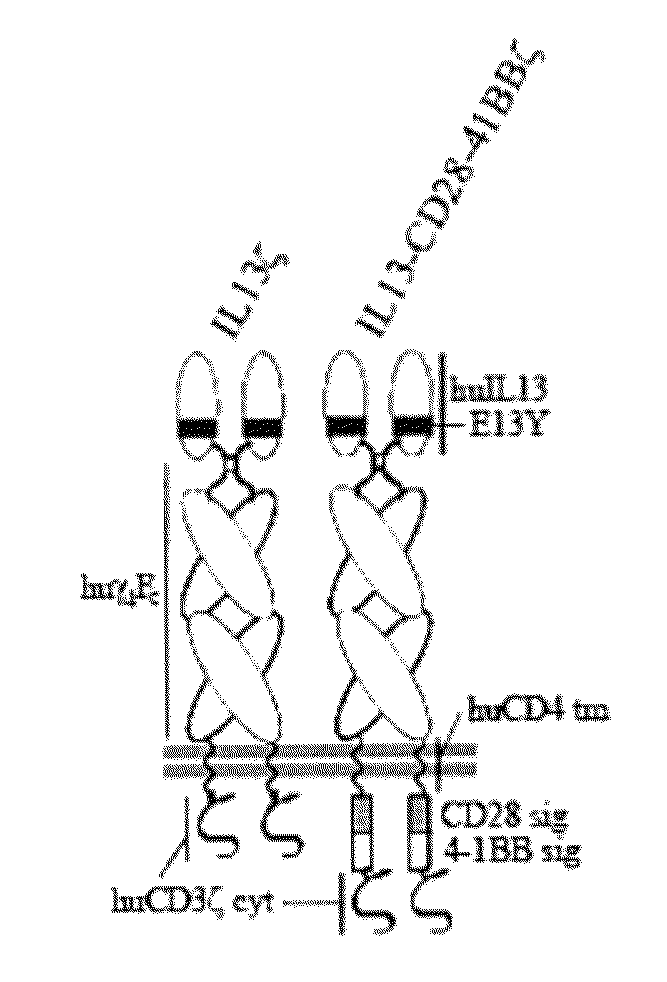
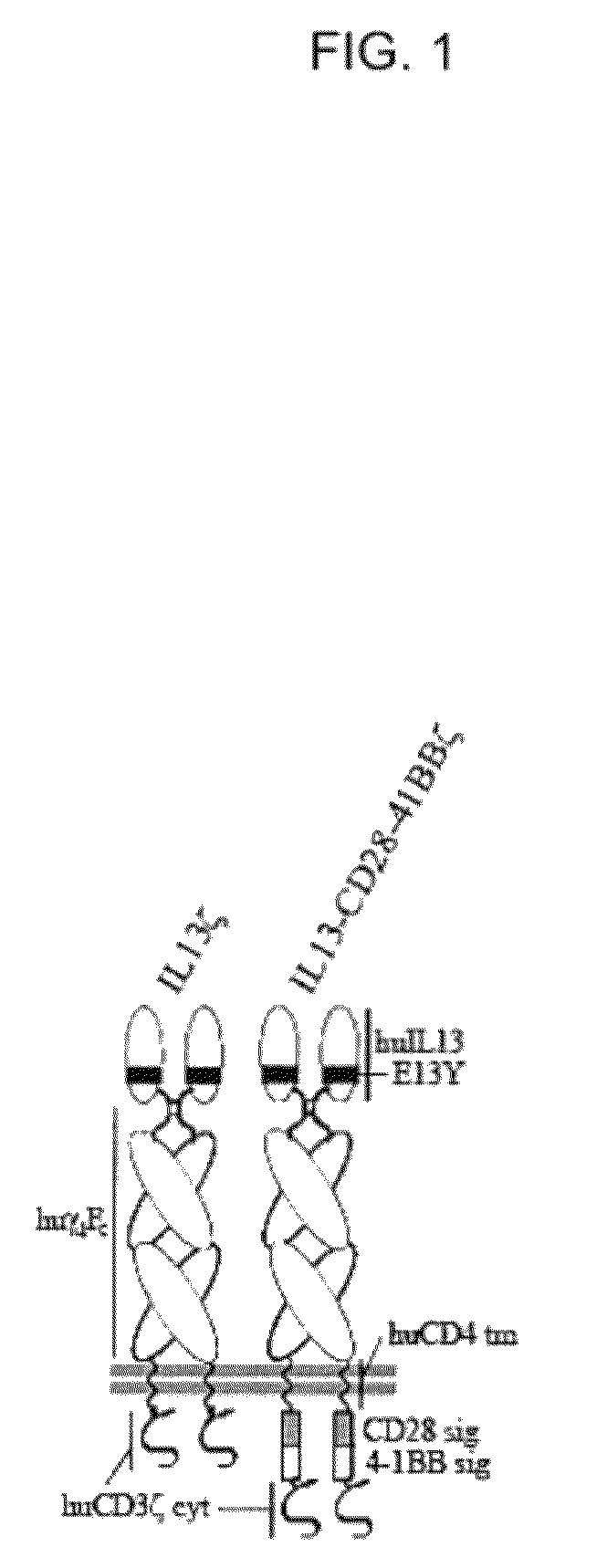
Method and compositions using a chimeric antigen receptor for enhanced anti-tumor effector functioning of T cells
Integration of costimulatory signaling domains within a tumor targeting chimeric antigen receptor (CAR), such as the IL13Rα2 specific IL13-zetakine (IL13ζ), enhances T cell-mediated responses against tumors even in the absence of expressed ligands for costimulatory receptors.
Owner:CITY OF HOPE
Noninvasive genetic immunization, expression products therefrom and uses thereof
InactiveUS6348450B1Improve vaccination schemeEfficient methodSsRNA viruses negative-senseBiocideHemagglutininWhole body
Disclosed and claimed are methods of non-invasive genetic immunization in an animal and / or methods of inducing a systemic immune or therapeutic response in an animal, products therefrom and uses for the methods and products therefrom. The methods can include contacting skin of the animal with a vector in an amount effective to induce the systemic immune or therapeutic response in the animal. The vector can include and express an exogenous nucleic acid molecule encoding an epitope or gene product of interest. The systemic immune response can be to or from the epitope or gene product. The nucleic acid molecule can encode an epitope of interest and / or an antigen of interest and / or a nucleic acid molecule that stimulates and / or modulates an immunological response and / or stimulates and / or modulates expression, e.g., transcription and / or translation, such as transcription and / or translation of an endogenous and / or exogenous nucleic acid molecule; e.g., one or more of influenza hemagglutinin, influenza nuclear protein, tetanus toxin C-fragment, anthrax protective antigen, HIV gp 120, human carcinoembryonic antigen, and / or a therapeutic, an immunomodulatory gene, such as co-stimulatory gene and / or a cytokine gene. The immune response can be induced by the vector expressing the nucleic acid molecule in the animal's cells. The immune response can be against a pathogen or a neoplasm. A prophylactic vaccine or a therapeutic vaccine or an immunological composition can include the vector.
Owner:UAB RES FOUND
Bioactive Polymers
InactiveUS20080267903A1Strong specificityEnhanced interactionSynthetic polymeric active ingredientsAntineoplastic agentsDelivery vehicleActive agent
Various polymers, including cationic polyamine polymers and dendrimeric polymers, are shown to possess anti-proliferative activity, and may therefore be useful for treatment of disorders characterised by undesirable cellular proliferation such as neoplasms and tumours, inflammatory disorders (including autoimmune disorders), psoriasis and atherosclerosis. The polymers may be used alone as active agents, or as delivery vehicles for other therapeutic agents, such as drug molecules or nucleic acids for gene therapy. In such cases, the polymers' own intrinsic anti-tumour activity may complement the activity of the agent to be delivered.
Owner:UNIV COLLEGE OF LONDON
Methods and compositions for the amplification, detection and quantification of nucleic acid from a sample
InactiveUS20080096766A1Microbiological testing/measurementLibrary member identificationNon malignantNeoplasm
The invention relates to methods and kits for the amplification, detection and quantification of a nucleic acid from a sample. The methods of the invention may be used in a wide range of applications, including, but not limited to, the detection and quantification of fetal nucleic acid from maternal plasma, the detection and quantification of circulating nucleic acids from neoplasms (malignant or non-malignant), accurate pooling analysis for low frequency alleles, or any other application requiring sensitive quantitative analysis of nucleic acids.
Owner:SEQUENOM INC
Methods of producing enriched populations of tumor-reactive t cells from tumor
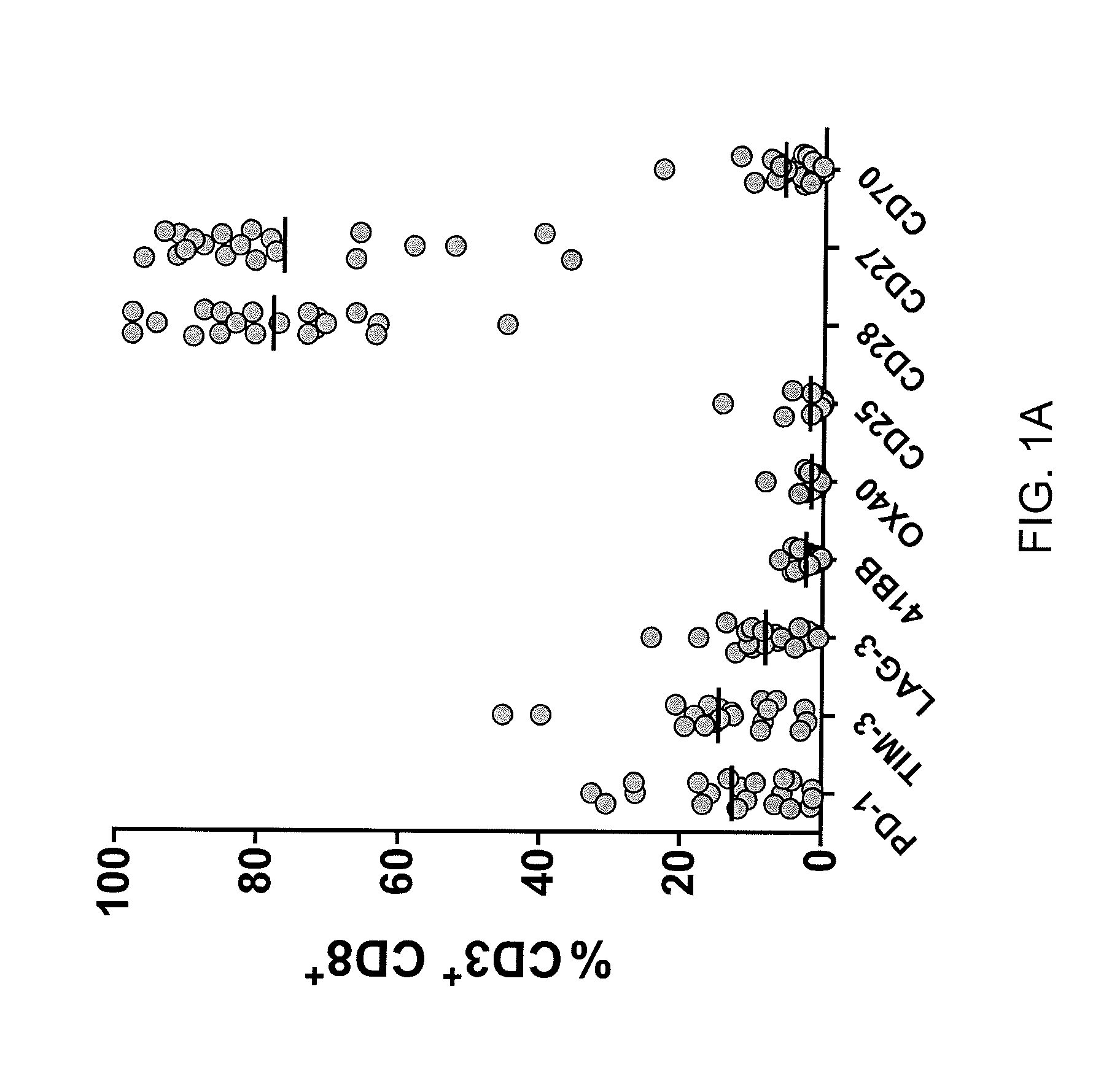
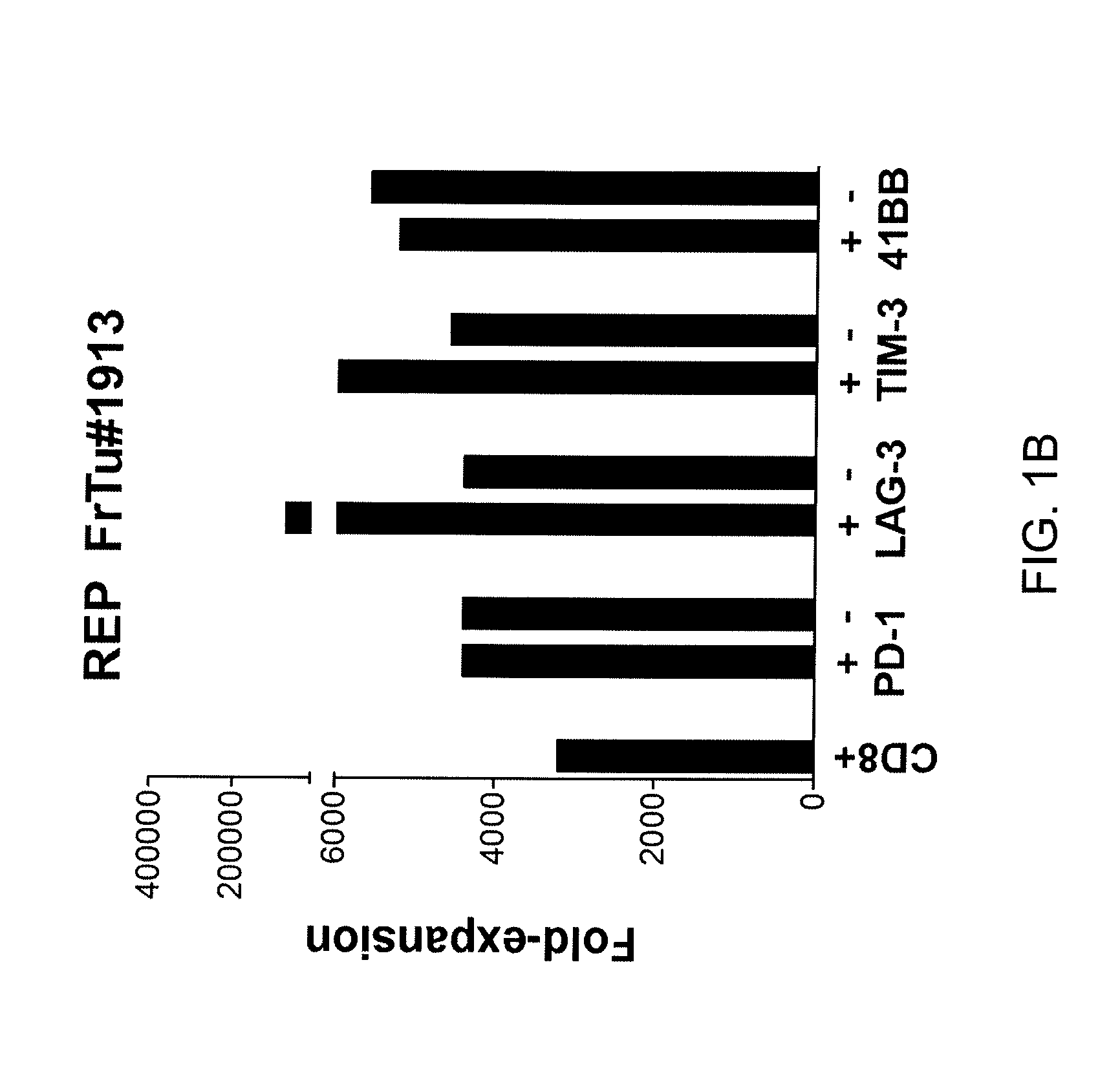
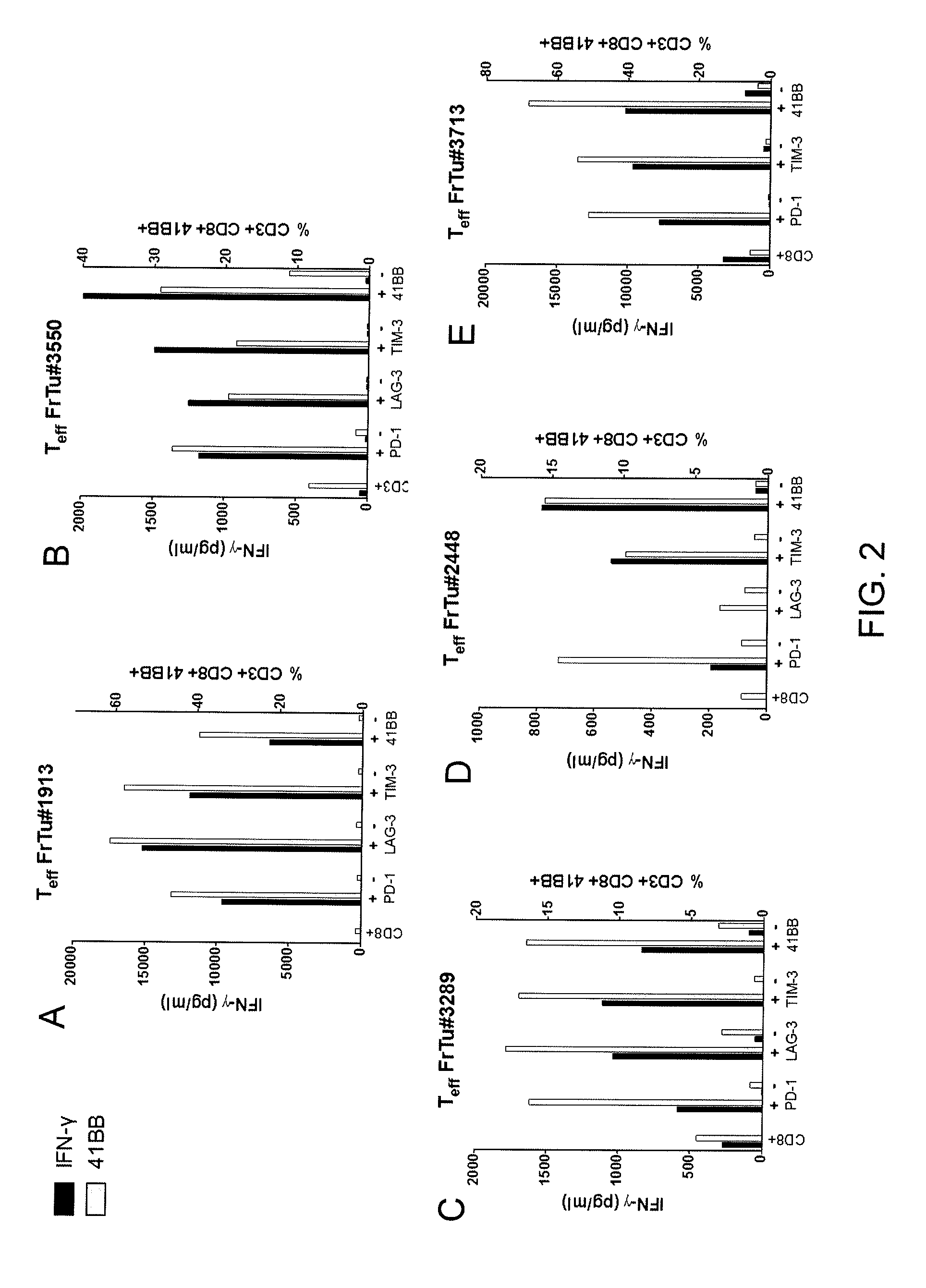
Methods of obtaining a cell population enriched for tumor-reactive T cells, the method comprising: (a) obtaining a bulk population of T cells from a tumor sample; (b) specifically selecting CD8+ T cells that express any one or more of TIM-3, LAG-3, 4-1BB, and PD-1 from the bulk population; and (c) separating the cells selected in (b) from unselected cells to obtain a cell population enriched for tumor-reactive T cells are disclosed. Related methods of administering a cell population enriched for tumor-reactive T cells to a mammal, methods of obtaining a pharmaceutical composition comprising a cell population enriched for tumor-reactive T cells, and isolated or purified cell populations are also disclosed.
Owner:UNITED STATES OF AMERICA
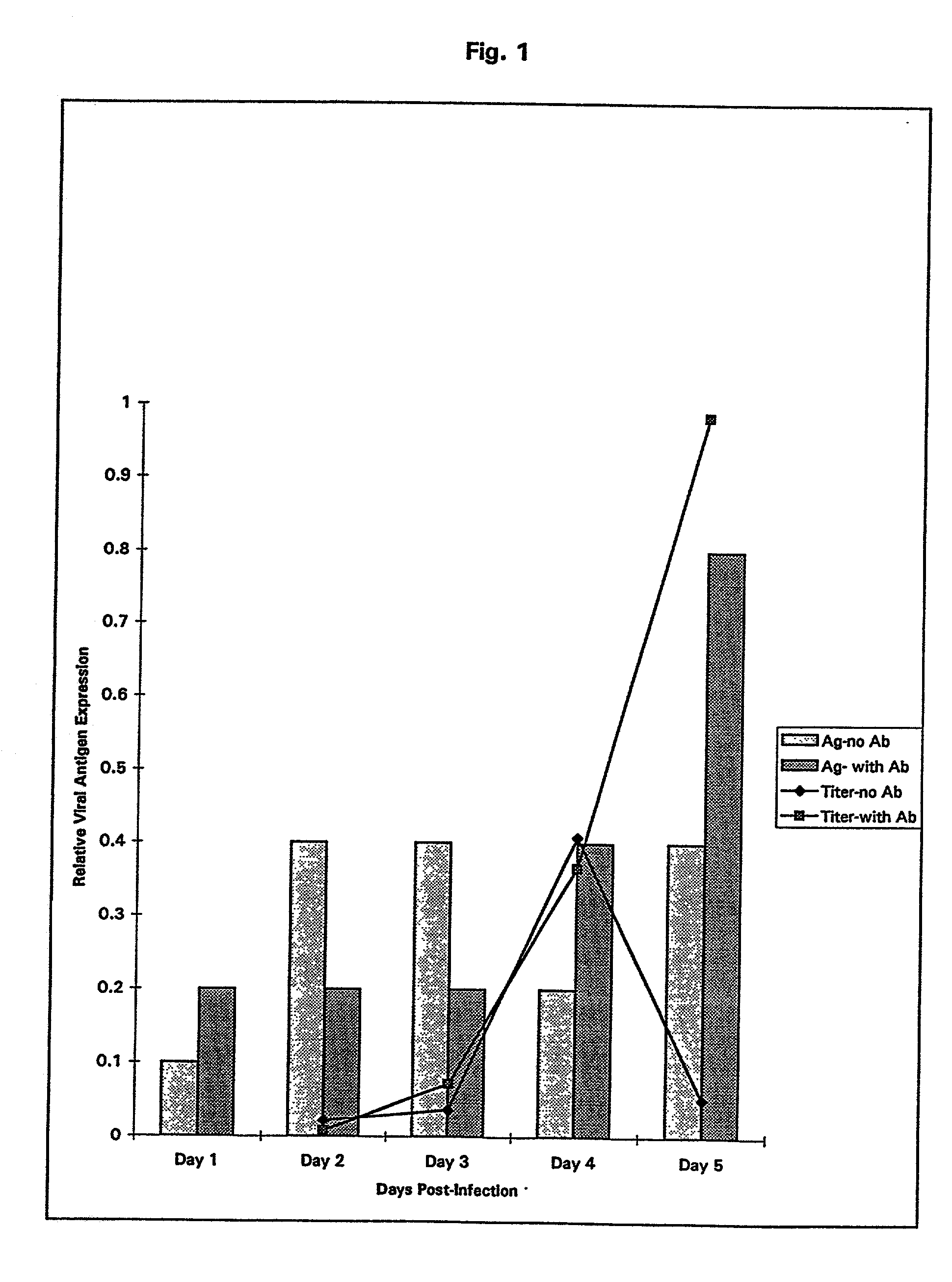
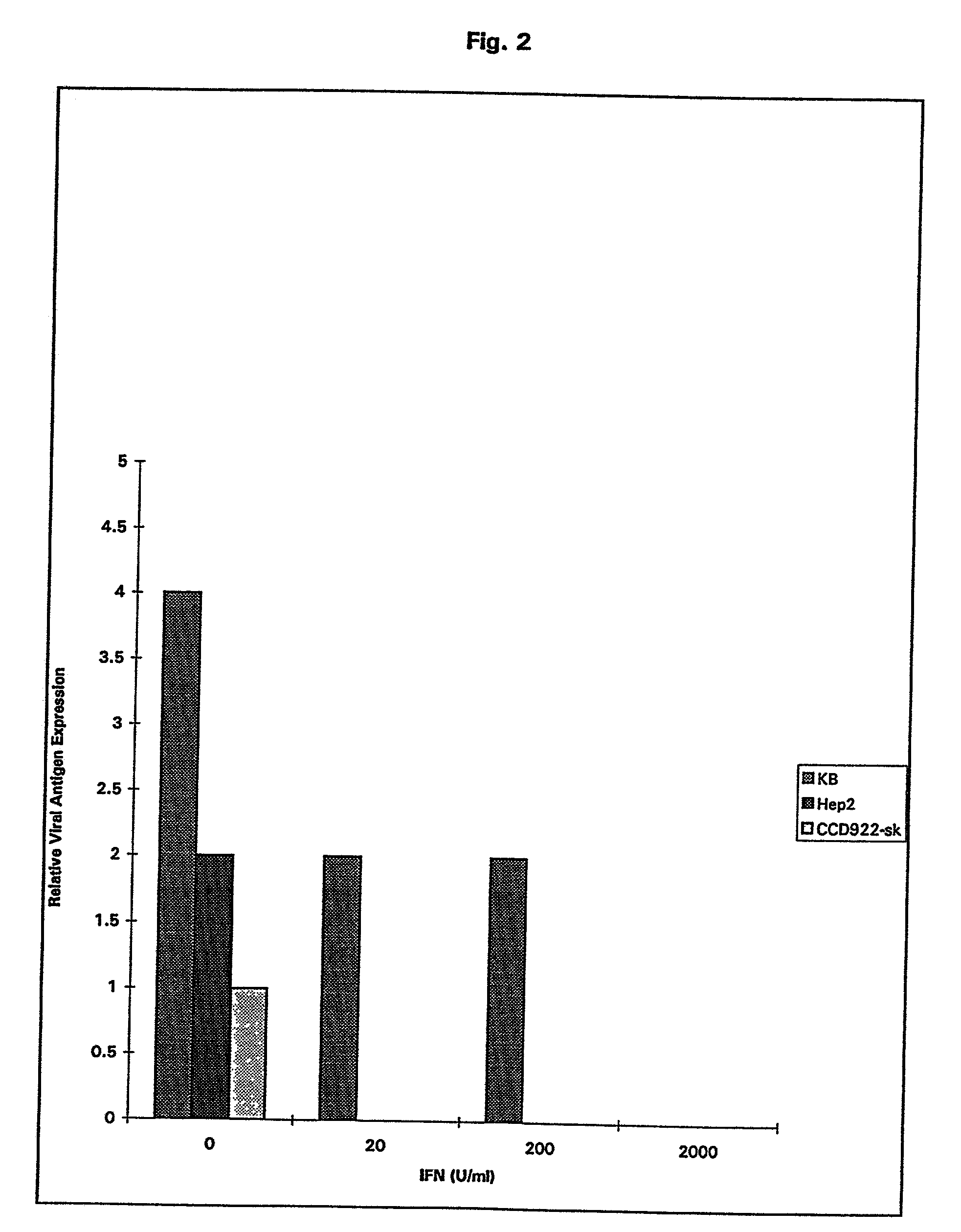
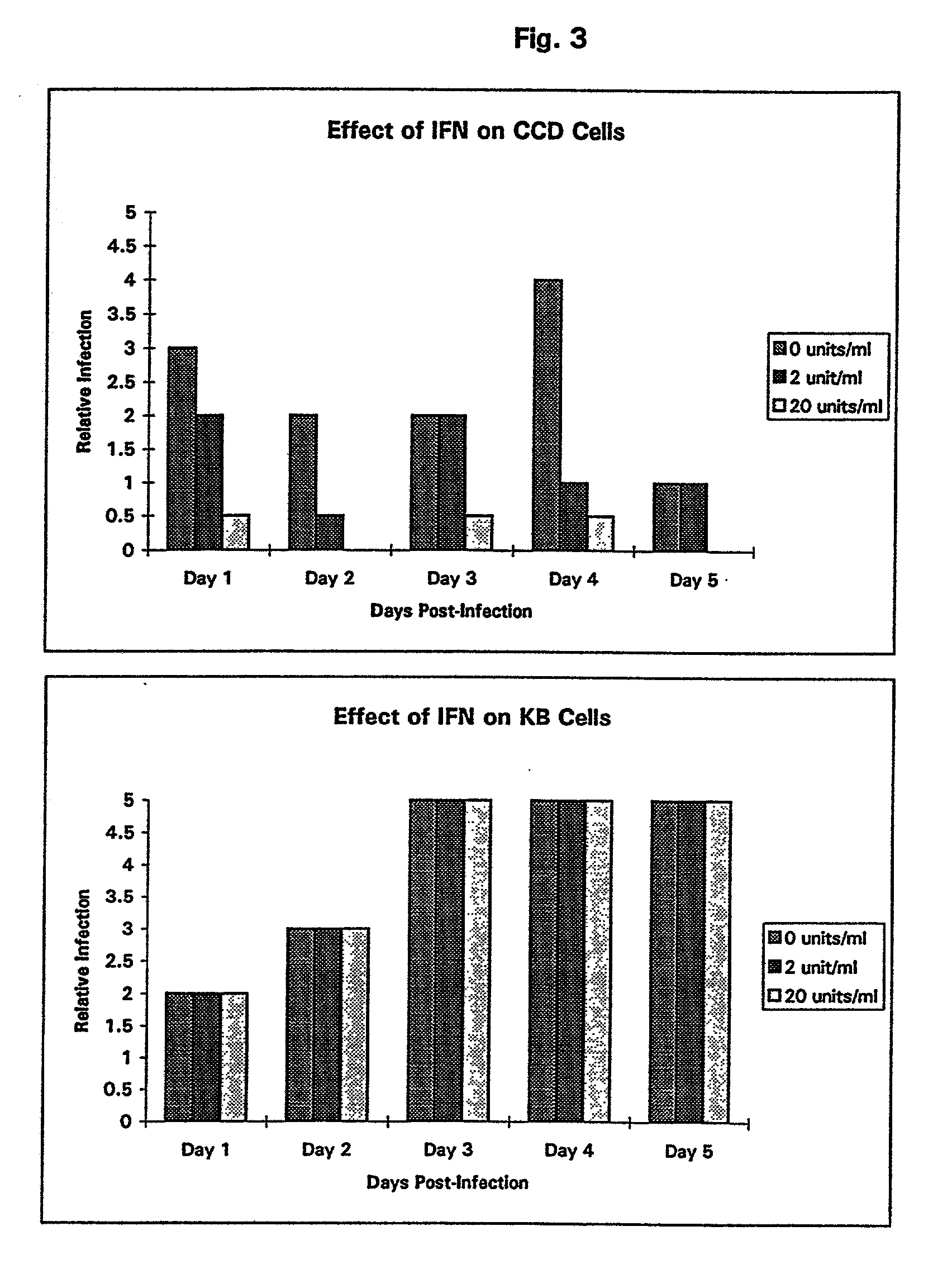
Treatment of neoplasms with viruses
InactiveUS20030044384A1Convenient treatmentReduce productionSsRNA viruses negative-senseBiocideDiseaseAnti viral response
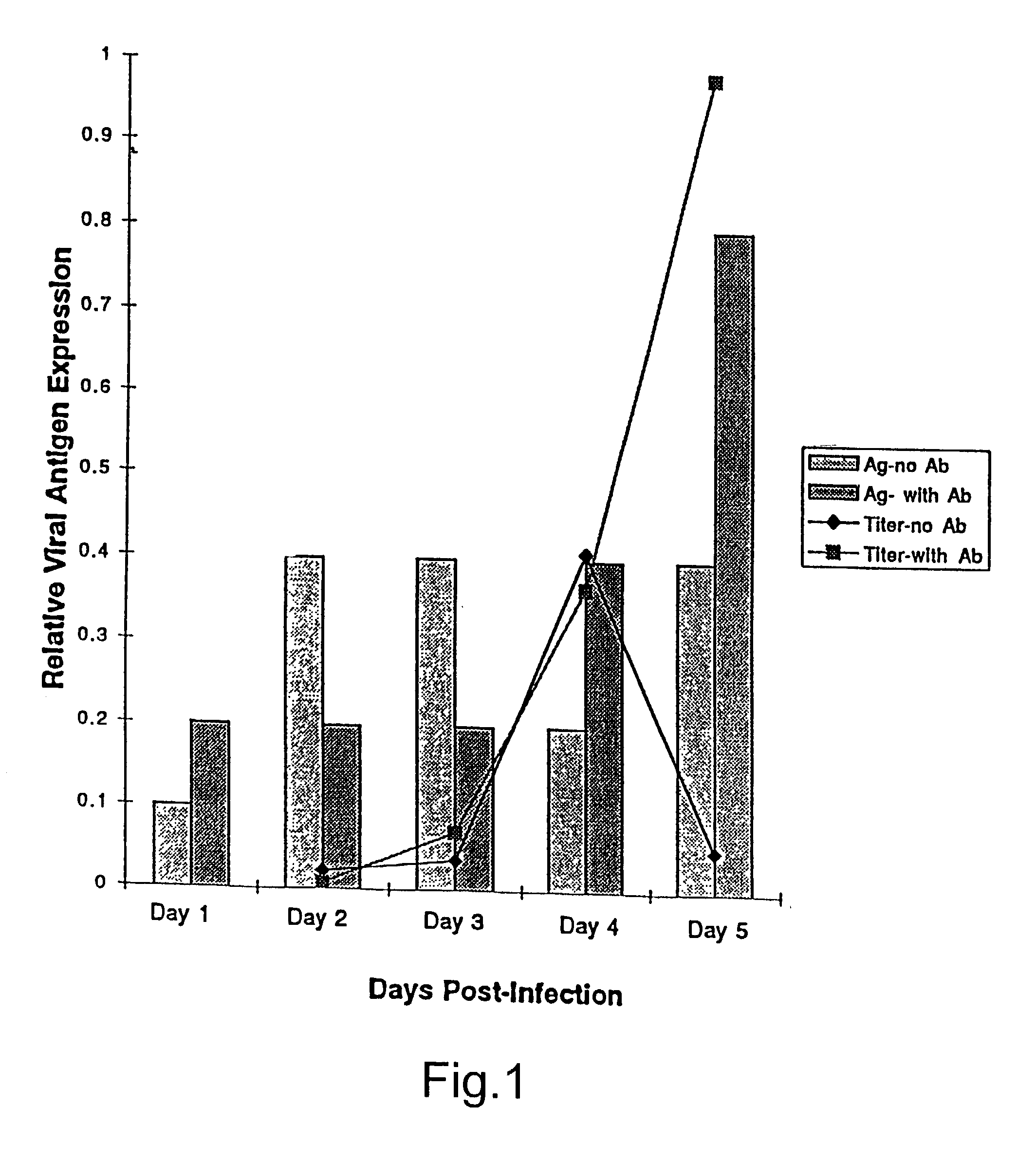
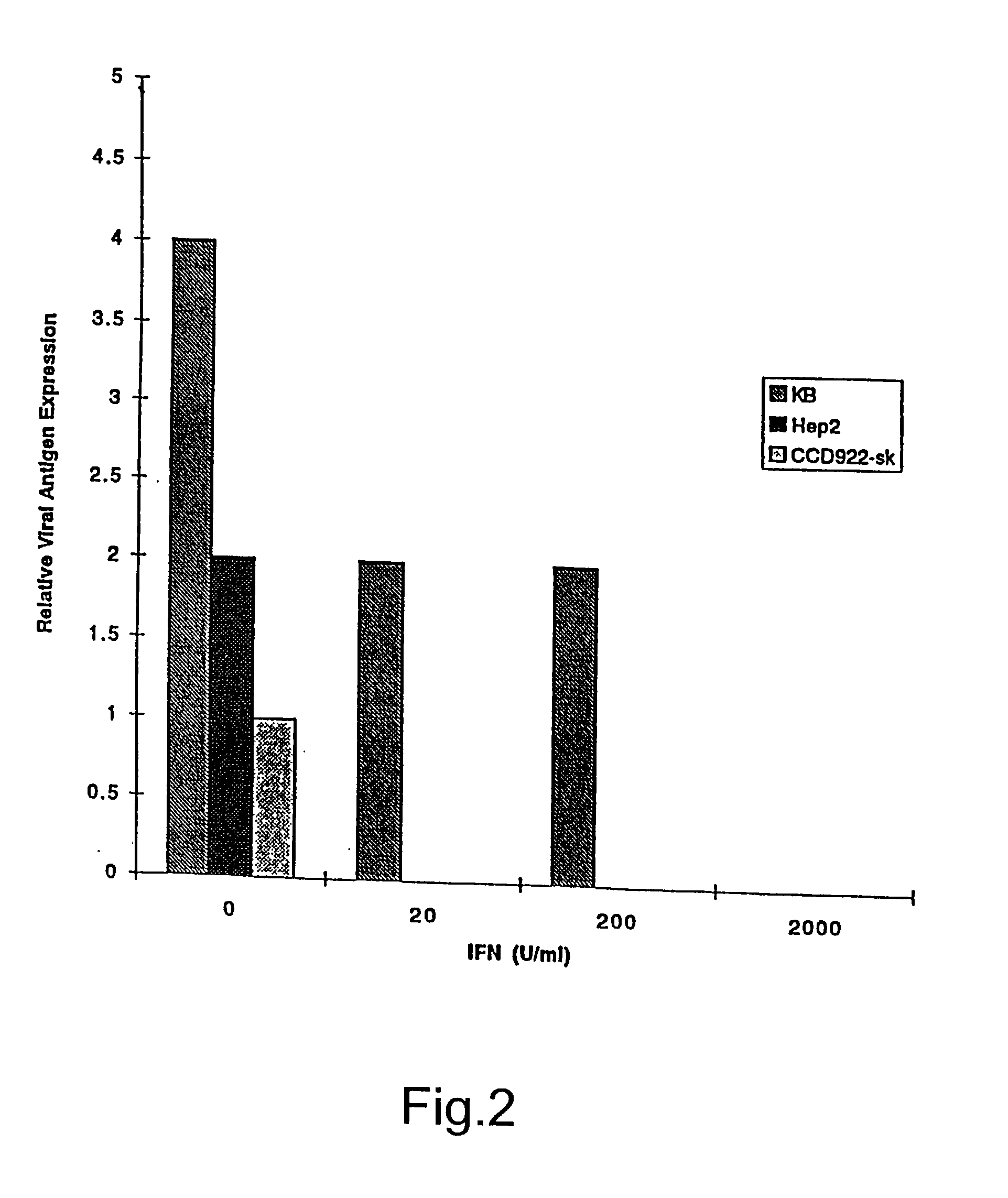
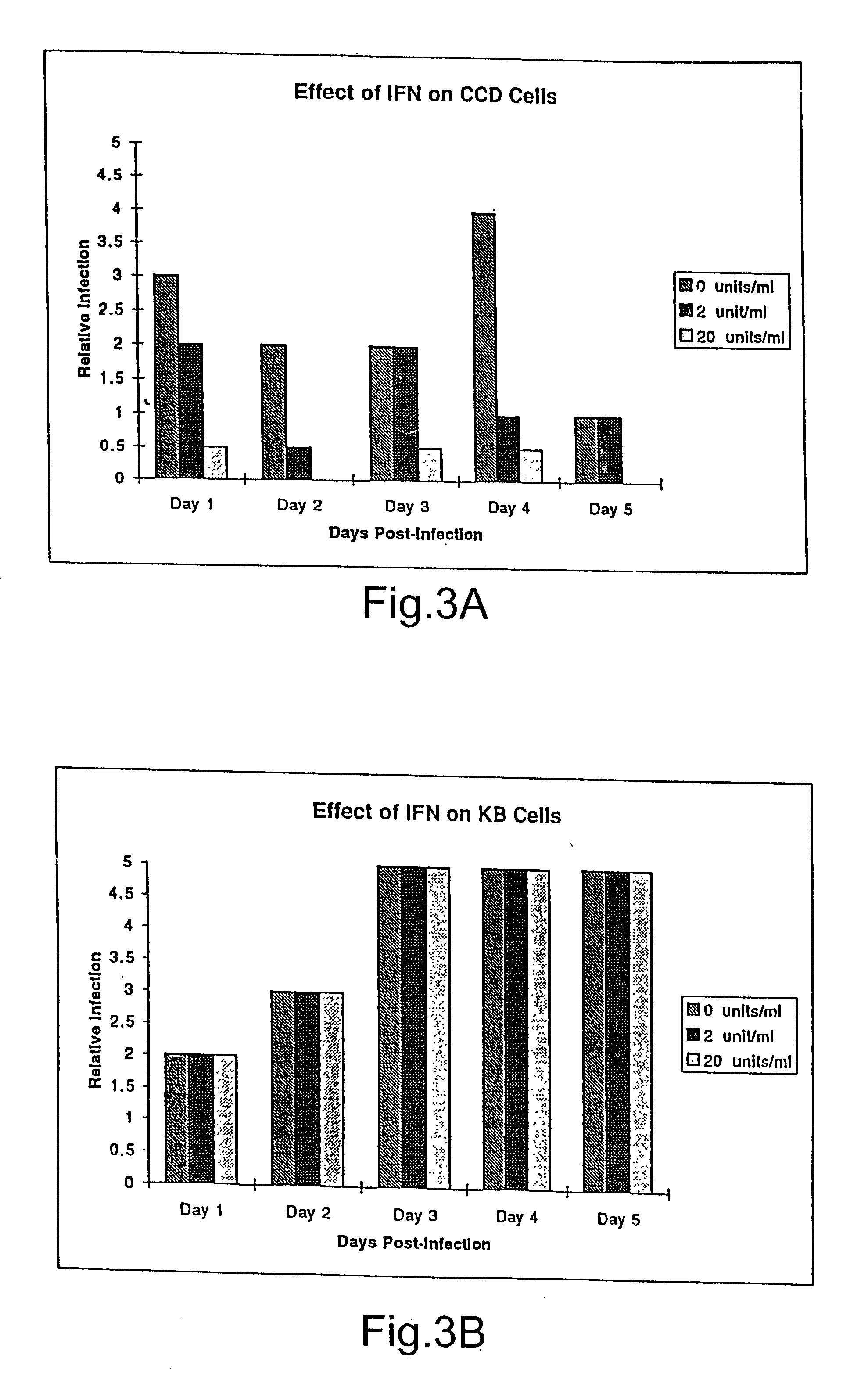
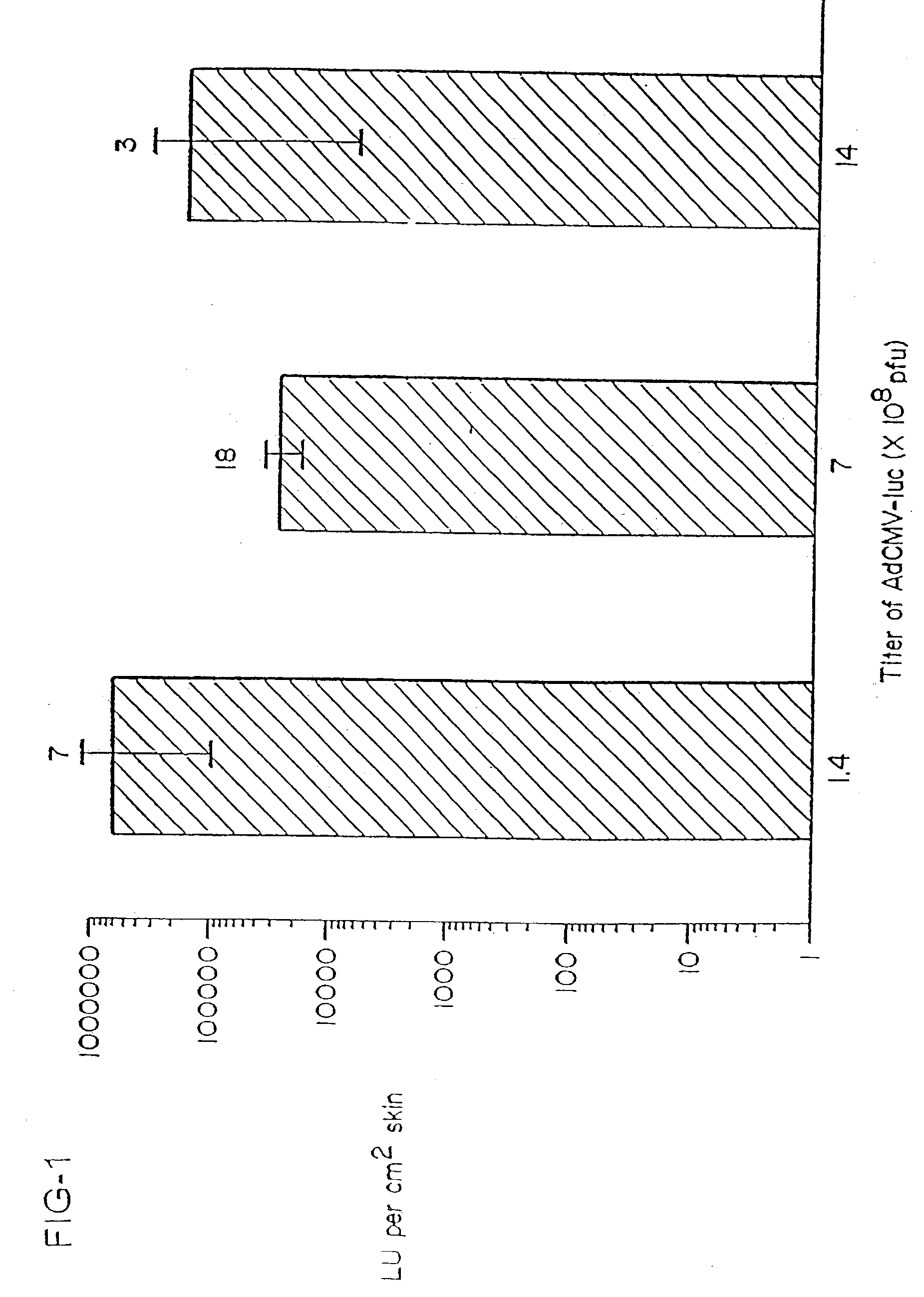
The subject invention relates to viruses that are able to replicate and thereby kill neoplastic cells with a deficiency in the IFN-mediated antiviral response, and their use in treating neoplastic disease including cancer and large tumors. RNA and DNA viruses are useful in this regard. The invention also relates to methods for the selection, design, purification and use of such viruses for cancer therapy.
Owner:PRO VIRUS
Vaccine and drug delivery by topical application of vectors and vector extracts
InactiveUS20040009936A1Improve vaccination schemeEfficient deliverySsRNA viruses negative-senseBiocideNeoplasmGlycoprotein
Disclosed and claimed are methods of non-invasive immunization and drug delivery in an animal and / or methods of inducing a systemic immune or therapeutic response in an animal following topical application of non-replicative vectors, products therefrom and uses for the methods and products therefrom. Also disclosed and claimed are methods of non-invasive immunization and drug delivery in an animal and / or a method of inducing a systemic immune response or systemic therapeutic response to a gene product comprising contacting skin of the animal with cell-free extracts in an amount effective to induce the response, wherein the extracts are prepared by filtration of disrupted cells, wherein the cell comprises and expresses a nucleic acid molecule. Preferably, the cell is temporarily disrupted by sonication, remaining intact and viable after the sonication. Also, methods are disclosed and claimed for enhancing the immunogenicity and efficacy of an epicutaneous vaccine for inducing a systemic immune response to an antigen, in an animal comprising contacting skin of the animal with vaccines admixed with heat-shock protein 27, in an amount effective to induce the response. The methods include contacting skin of the animal with a vector in an amount effective to induce the systemic immune or therapeutic response. The vector can include and express an exogenous nucleic acid molecule encoding an epitope or gene product of interest. The systemic immune response can be to or from the epitope or gene product. The nucleic acid molecule can encode an epitope or antigen of interest and / or a nucleic acid molecule that stimulates and / or modulates an immunological response and / or stimulates and / or modulates expression, e.g., transcription and / or translation, such as transcription and / or translation of an endogenous and / or exogenous nucleic acid molecule; e.g., one or more of influenza hemagglutinin, influenza nuclear protein, influenza M2, tetanus toxin C-fragment, anthrax protective antigen, anthrax lethal factor, anthrax germination factors, rabies glycoprotein, HBV surface antigen, HIV gp120, HIV gp160, human carcinoembryonic antigen, malaria CSP, malaria SSP, malaria MSP, malaria pfg, botulinum toxin A, and mycobacterium tuberculosis HSP; and / or a therapeutic, an immunomodulatory gene, such as co- stimulatory gene and / or a cytokine gene. The immune response can be induced by the vector expressing the nucleic acid molecule in the animal's cells including epidermal cells. The immune response can also be induced by antigens expressed from the nucleic acid molecule within the vector. The immune response can be against a pathogen or a neoplasm. A prophylactic vaccine or a therapeutic vaccine or an immunological composition can include the vector. The animal can be a vertebrate, e.g., a mammal, such as human, a cow, a horse, a dog, a cat, a goat, a sheep or a pig; or fowl such as turkey, chicken or duck. The vector can be one or more of a viral vector, including viral coat, e.g., with some or all viral genes deleted therefrom, bacterial, protozoan, transposon, retrotransposon, and DNA vector, e.g., a recombinant vector; for instance, an adenovirus, such as an adenovirus defective in its E1 and / or E3 and / or E4 region(s) and / or all adenoviral genes.
Owner:UAB RES FOUND
Noninvasive genetic immunization, expression products therefrom, and uses thereof
InactiveUS6716823B1Improve vaccination schemeEfficient methodSsRNA viruses negative-senseBiocideMalariaNon invasive
Disclosed and claimed are methods of non-invasive genetic immunization in an animal and / or methods of inducing a systemic immune or therapeutic response in an animal, products therefrom and uses for the methods and products therefrom. The methods can include contacting skin of the animal with a vector in an amount effective to induce the systemic immune or therapeutic response in the animal. The vector can include and express an exogenous nucleic acid molecule encoding an epitope or gene product of interest. The systemic immune response can be to or from the epitope or gene product. The nucleic acid molecule can encode an epitope of interest and / or an antigen of interest and / or a nucleic acid molecule that stimulates and / or modulates an immunological response and / or stimulates and / or modulates expression, e.g., transcription and / or translation, such as transcription and / or translation of an endogenous and / or exogenous nucleic acid molecule; e.g., one or more of influenza hemagglutinin, influenza nuclear protein, influenza M2, tetanus toxin C-fragment, anthrax protective antigen, anthrax lethal factor, rabies glycoprotein, HBV surface antigen, HIV gp 120, HIV gp 160, human carcinoembryonic antigen, malaria CSP, malaria SSP, malaria MSP, malaria pfg, and mycobacterium tuberculosis HSP; and / or a therapeutic, an immunomodulatory gene, such as co-stimulatory gene and / or a cytokine gene. The immune response can be induced by the vector expressing the nucleic acid molecule in the animal's cells. The animal's cells can be epidermal cells. The immune response can be against a pathogen or a neoplasm. A prophylactic vaccine or a therapeutic vaccine or an immunological composition can include the vector. The animal can be a vertebrate, e.g., a mammal, such as human, a cow, a horse, a dog, a cat, a goat, a sheep or a pig; or fowl such as turkey, chicken or duck. The vector can be one or more of a viral vector, including viral coat, e.g., with some or all viral genes deleted therefrom, bacterial, protozoan, transposon, retrotransposon, and DNA vector, e.g., a recombinant vector; for instance, an adenovirus, such as an adenovirus defective in its E1 and / or E3 and / or E4 region(s). The method can encompass applying a delivery device including the vector to the skin of the animal, as well as such a method further including disposing the vector in and / or on the delivery device. The vector can have all viral genes deleted therefrom. The vector can induce a therapeutic and / or an anti-tumor effect in the animal, e.g., by expressing an oncogene, a tumor-suppressor gene, or a tumor-associated gene. Immunological products generated by the expression, e.g., antibodies, cells from the methods, and the expression products, are likewise useful in in vitro and ex vivo applications, and such immunological and expression products and cells and applications are disclosed and claimed. Methods for expressing a gene product in vivo and products therefor and therefrom including mucosal and / or intranasal administration of an adenovirus, advantageously an E1 and / or E3 and / or E4 defective or deleted adenovirus, such as a human adenovirus or canine adenovirus, are also disclosed and claimed.
Owner:UAB RES FOUND
Method and device for treating cancer with modified output electrical therapy
This invention relates generally to the electrical treatment of malignant tumors and neoplasms by applying a voltage to affected tissue. Devices and various adaptations therein are described for use in electrical therapy. In one embodiment, electrical therapy output is modified to deliver a duty cycle less than 100 percent.
Owner:IONIX MEDICAL
Methods and systems for direct electrical current stimulation as a therapy for cancer and other neoplastic diseases
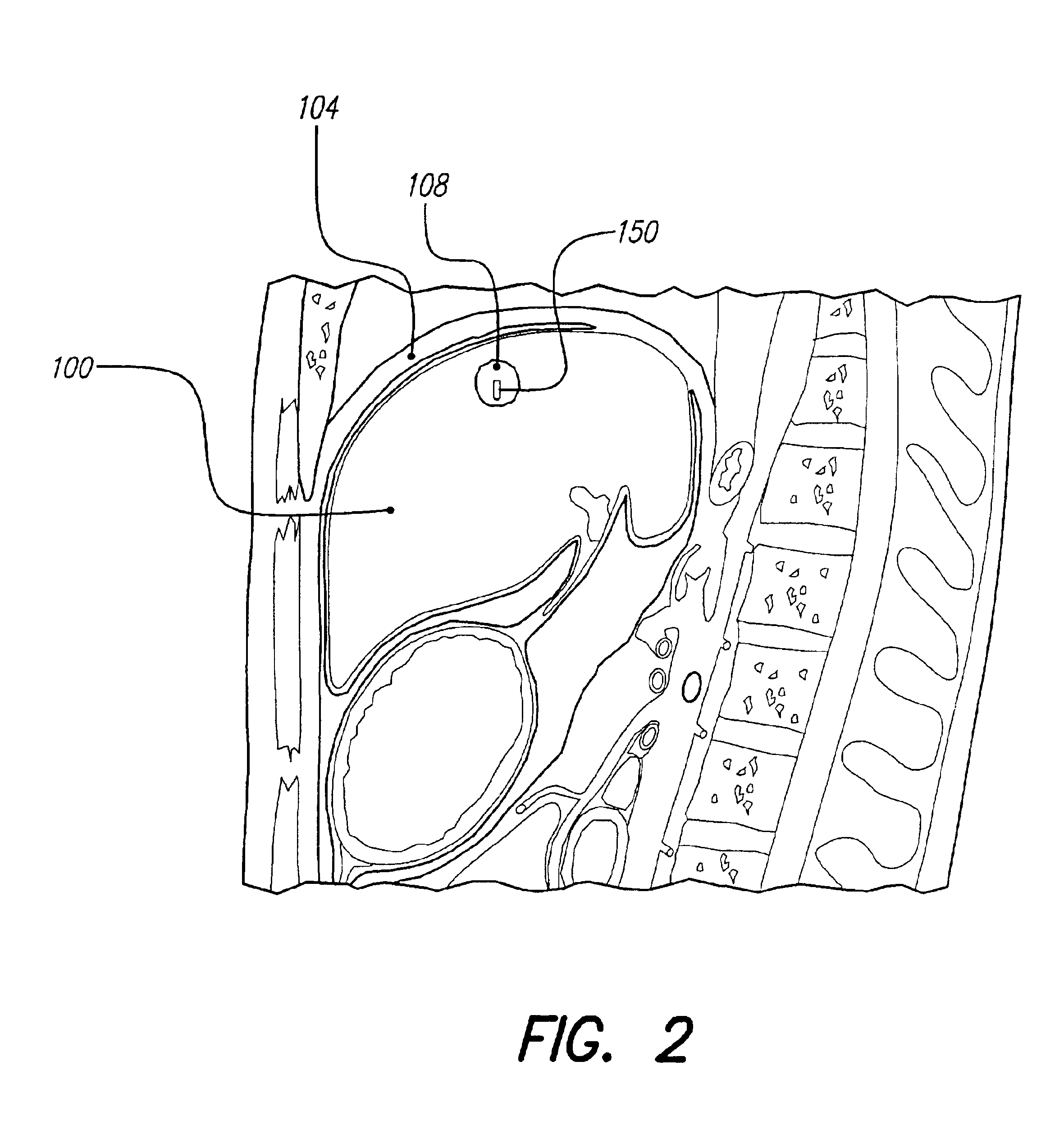
A small implantable stimulator(s) includes at least two electrodes for delivering electrical stimulation to surrounding tissue. The small stimulator provides means of stimulating a neoplasm with direct electrical current, such as relatively low-level direct current, without the need for external appliances during the stimulation session. The stimulator may be configured to be small enough to be implanted entirely within a neoplasm. Open- and closed-loop systems are disclosed.
Owner:BOSTON SCI NEUROMODULATION CORP
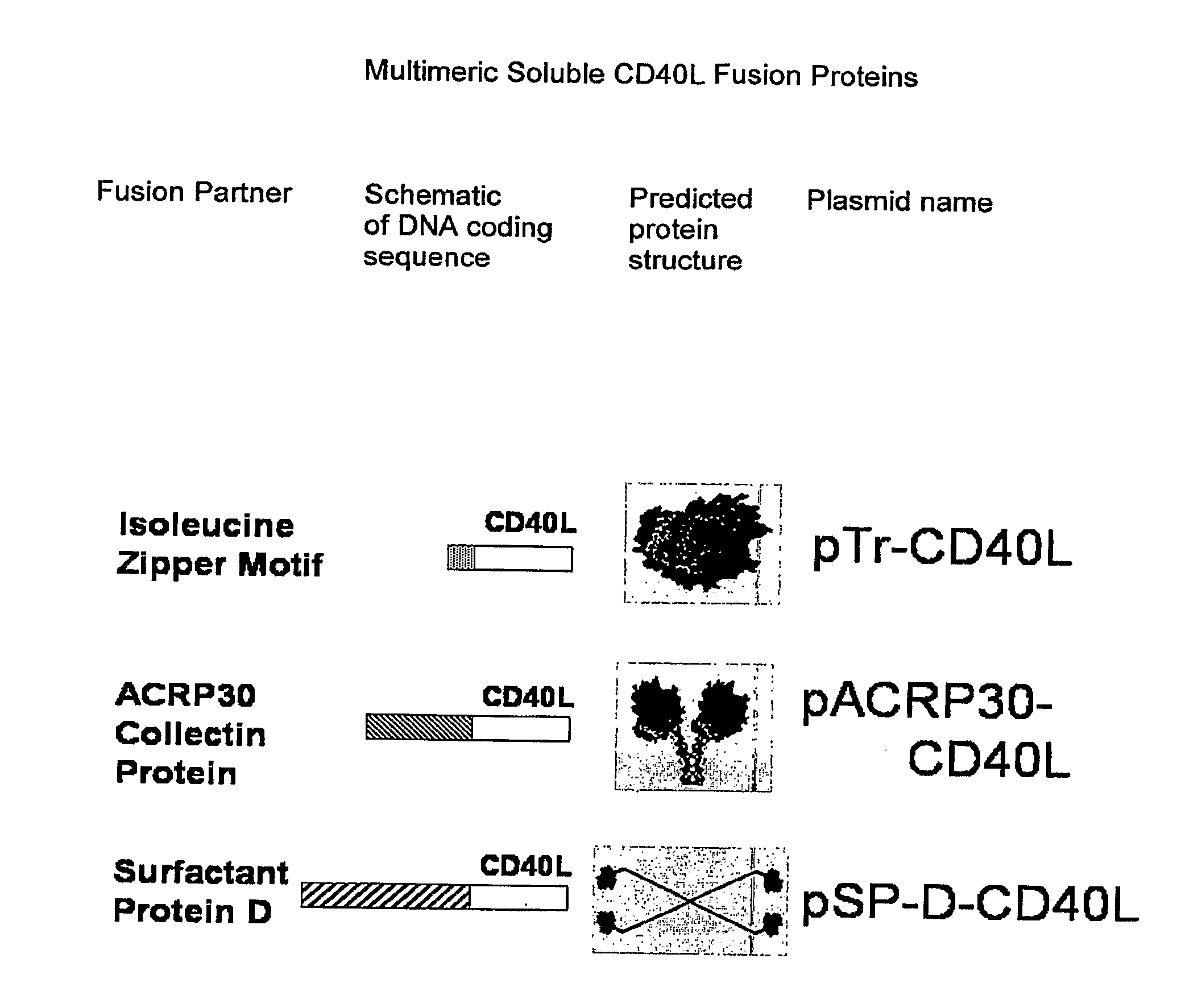
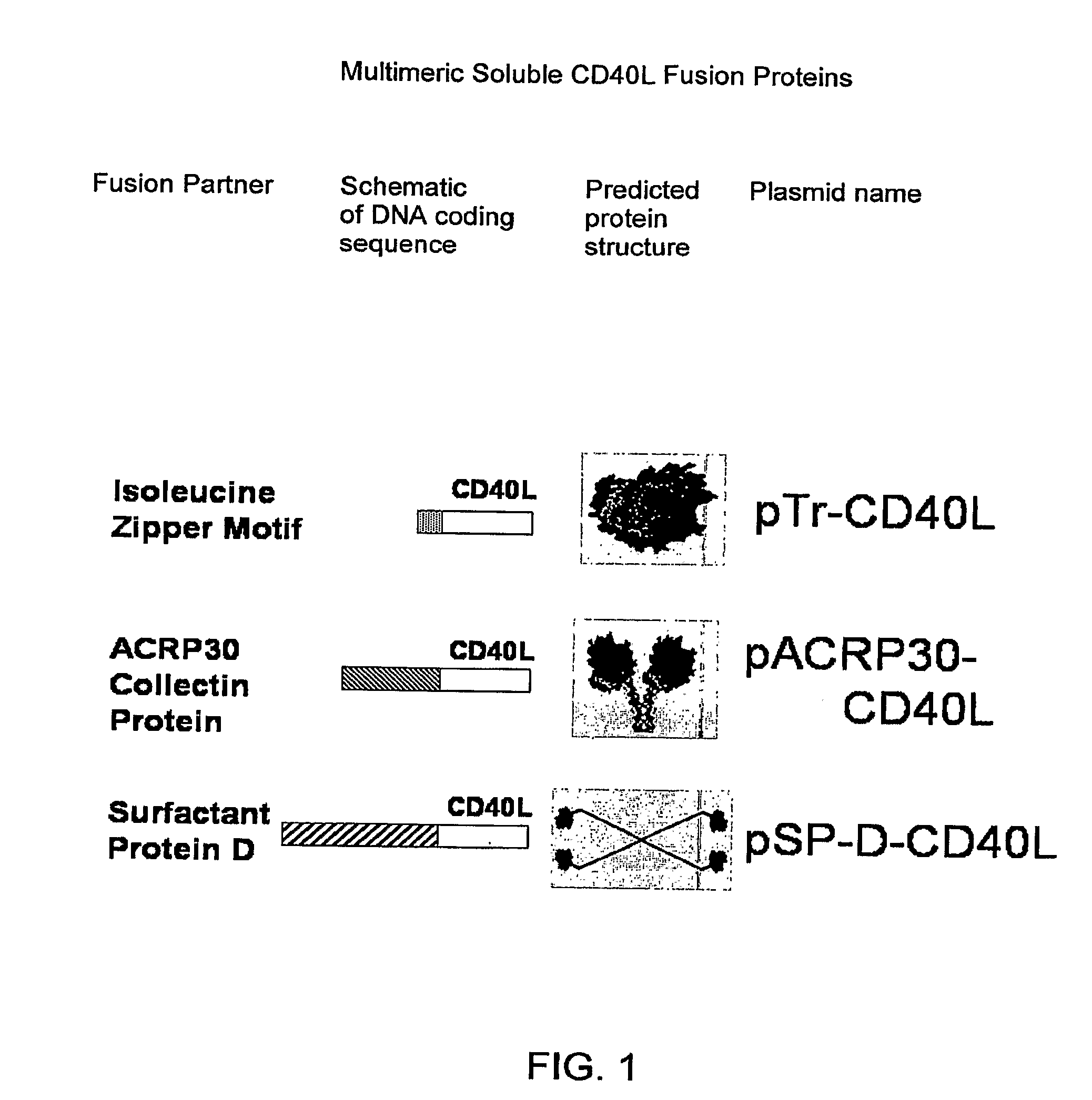
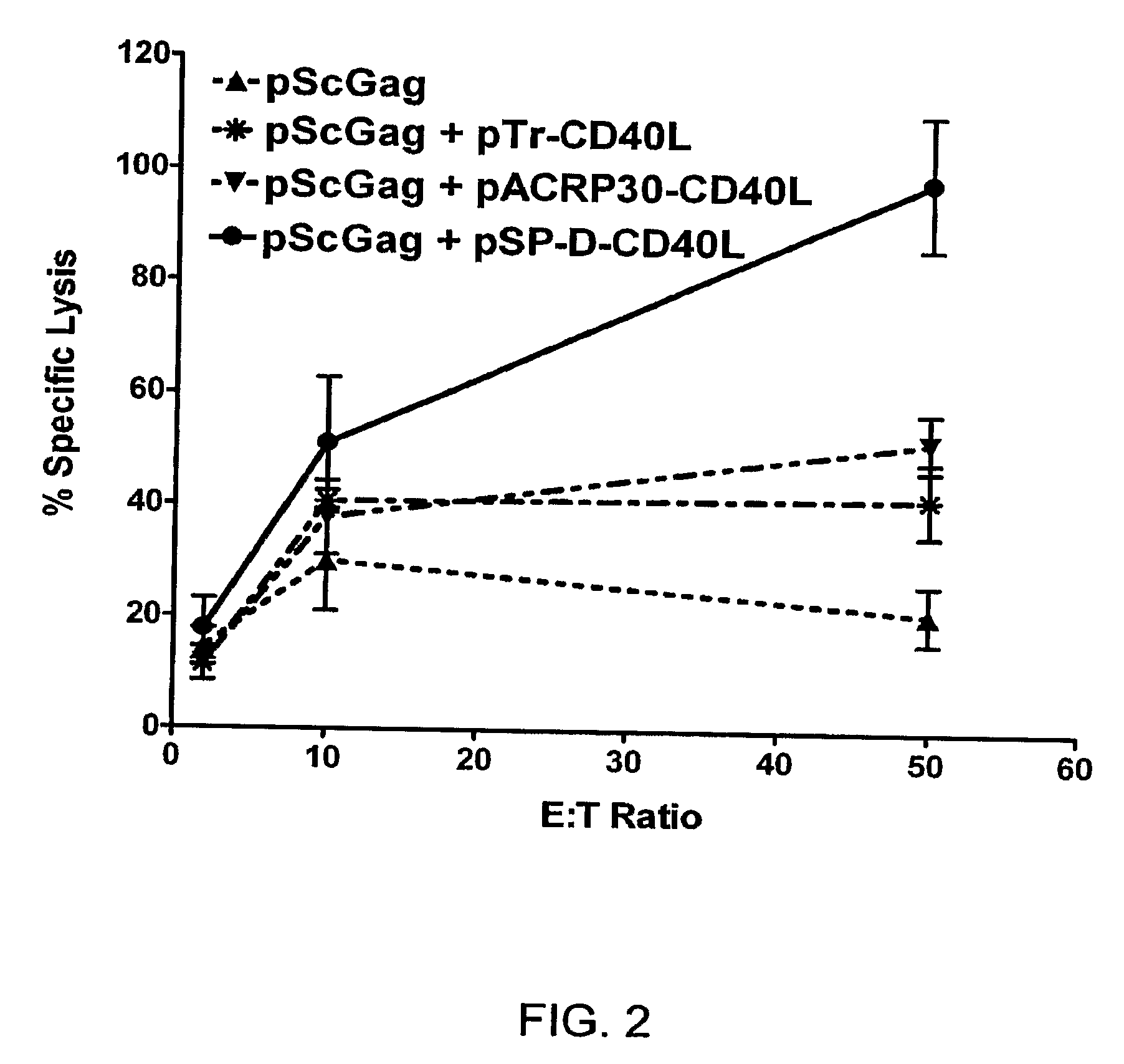
Immunostimulatory Combinations for Vaccine Adjuvants
This invention discloses immunostimulatory combinations of Tumor Necrosis Factor Receptor Superfamily (TN-FRSF) agonists, Toll-Like Receptor (TLR) agonists, “domain present in NAIP, CIITA, HET-E, TP-I (NACHT)-Leucine Rich Repeat (LRR)” or “NLR” agonists, RIG-I-Like Helicase or “RLH” agonists, purinergic receptor agonists and cytokine / chemokine receptor agonists, together with delivery methods. The combinations, when used alone at the site of pathology, provide immunostimulation that induces host humoral and cellular immunologic responses to eliminate pathogens or neoplasms. Alternatively, when the combinations are used with a defined antigens, these combinations can induce focused humoral and cellular immunologic responses useful as prophylactic and / or ameliorative therapeutic modalities for infections and the treatment of neoplastic disorders.
Owner:RGT UNIV OF CALIFORNIA
Formulations, conjugates, and combinations of drugs for the treatment of neoplasms
InactiveUS20050080075A1Reduce generationInhibiting passage across the blood-brain barrierOrganic chemistryPharmaceutical non-active ingredientsNeoplasmBiodistribution
The invention provides formulations and structural modifications for phenothiazine compounds which result in altered biodistributions, thereby reducing the occurrence of adverse reactions associated with this class of drug.
Owner:COMBINATORX
Long-chain fatty acyl elongase inhibitor comprising arylsulfonyl derivative as active ingredient
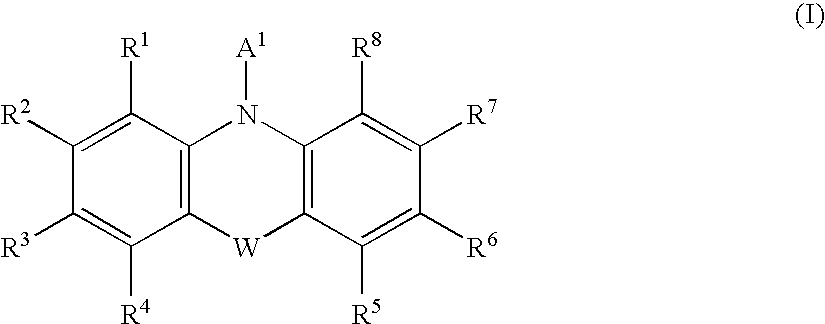
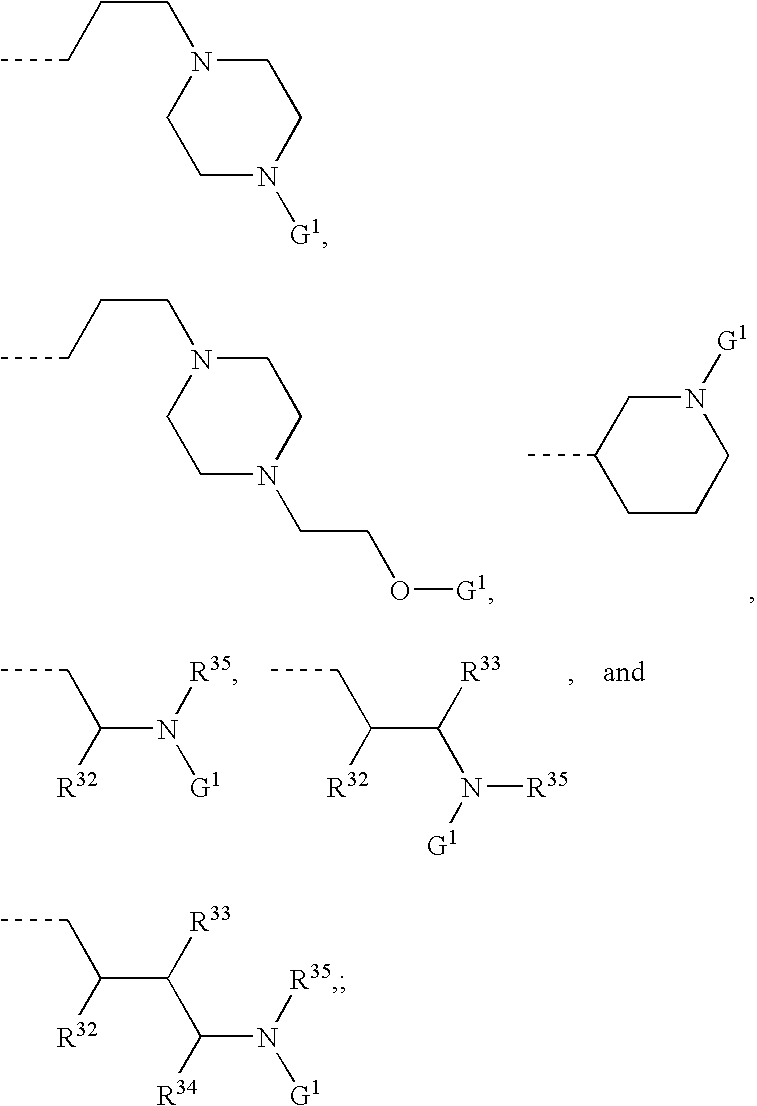
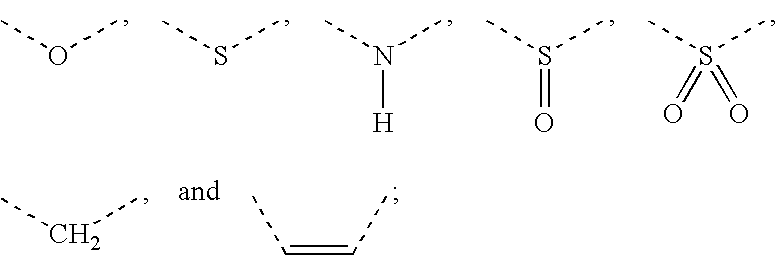
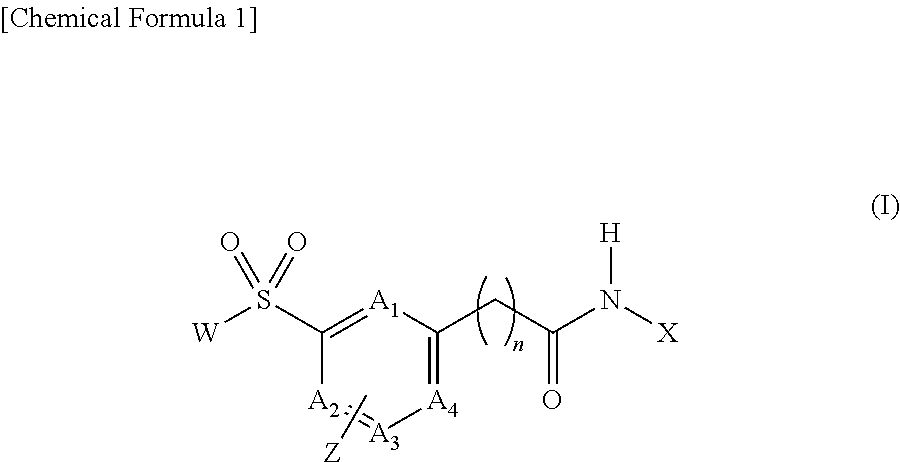
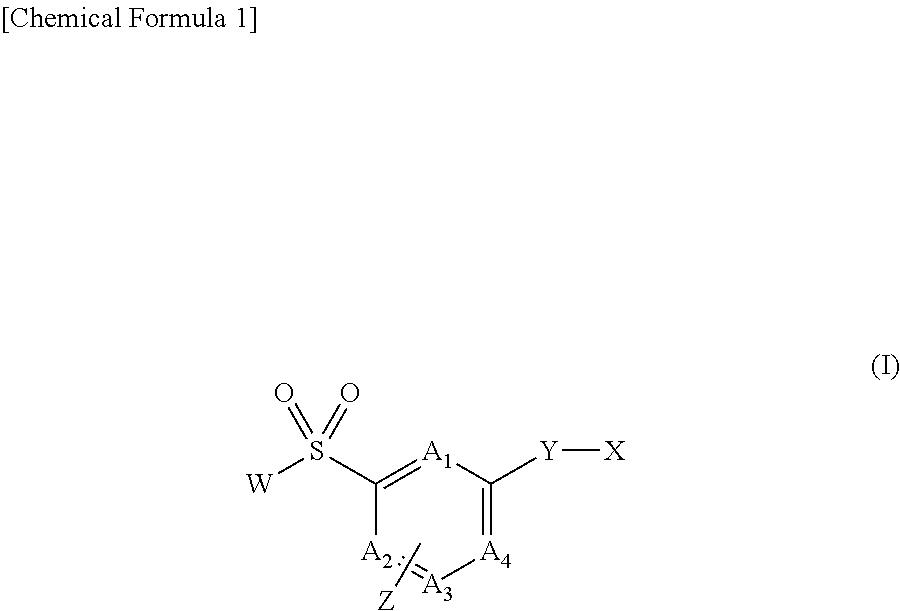
[Problem] To provide compounds useful as preventives or remedies for circular system disorders, nervous system disorders, metabolic disorders, reproduction system disorders, digestive system disorders, neoplasm, infectious diseases, etc., or as herbicides.[Means for Solution] A long-chain fatty acyl elongase inhibitor comprising, as the active ingredient thereof, a compound or a pharmaceutically-active salt thereof of a formula (I):[wherein W represents a hydrogen atom, a C1-6 alkyl, etc.; X represents an aryl, a heteroaryl, etc.; n indicates 0 or 1; Z represents a hydrogen atom, a C1-6 alkyl, etc.; A1, A2, A3 and A4 each independently represent CH or N].
Owner:MSD KK
Detecting mutations for cancer screening and fetal analysis
ActiveUS20170073774A1Accurate detectionMicrobiological testing/measurementProteomicsCell freeEmbolization Therapy
Embodiments are related to the accurate detection of somatic mutations in the plasma (or other samples containing cell-free DNA) of cancer patients and for subjects being screened for cancer. The detection of these molecular markers would be useful for the screening, detection, monitoring, management, and prognostication of cancer patients. For example, a mutational load can be determined from the identified somatic mutations, and the mutational load can be used to screen for any or various types of cancers, where no prior knowledge about a tumor or possible cancer of the subject may be required. Embodiments can be useful for guiding the use of therapies (e.g. targeted therapy, immunotherapy, genome editing, surgery, chemotherapy, embolization therapy, anti-angiogenesis therapy) for cancers. Embodiments are also directed to identifying de novo mutations in a fetus by analyzing a maternal sample having cell-free DNA from the fetus.
Owner:THE CHINESE UNIVERSITY OF HONG KONG
Treatment of neoplasms with viruses
InactiveUS20030165465A1Less weight lossConvenient treatmentSsRNA viruses negative-senseBiocideDiseaseAnti viral response
The subject invention relates to viruses that are able to replicate and thereby kill neoplastic cells with a deficiency in the IFN-mediated antiviral response, and their use in treating neoplastic disease including cancer and large tumors. RNA and DNA viruses are useful in this regard. The invention also relates to methods for the selection, design, purification and use of such viruses for cancer therapy.
Owner:WELLSTAT BIOLOGICS CORP
Method and Device for Treating Abnormal Tissue Growth With Electrical Therapy
InactiveUS20090024075A1ElectrotherapyPharmaceutical non-active ingredientsAbnormal tissue growthRadical radiotherapy
This invention relates generally to the electrical treatment of malignant tumors and neoplasms by applying a voltage to affected tissue. Devices and various adaptations therein are described for use in electrical therapy. Additionally, various chemotherapeutic agent and radiation therapies are described which may be advantageously used in conjunction with electrical therapy to ameliorate cancer.
Owner:IONIX MEDICAL
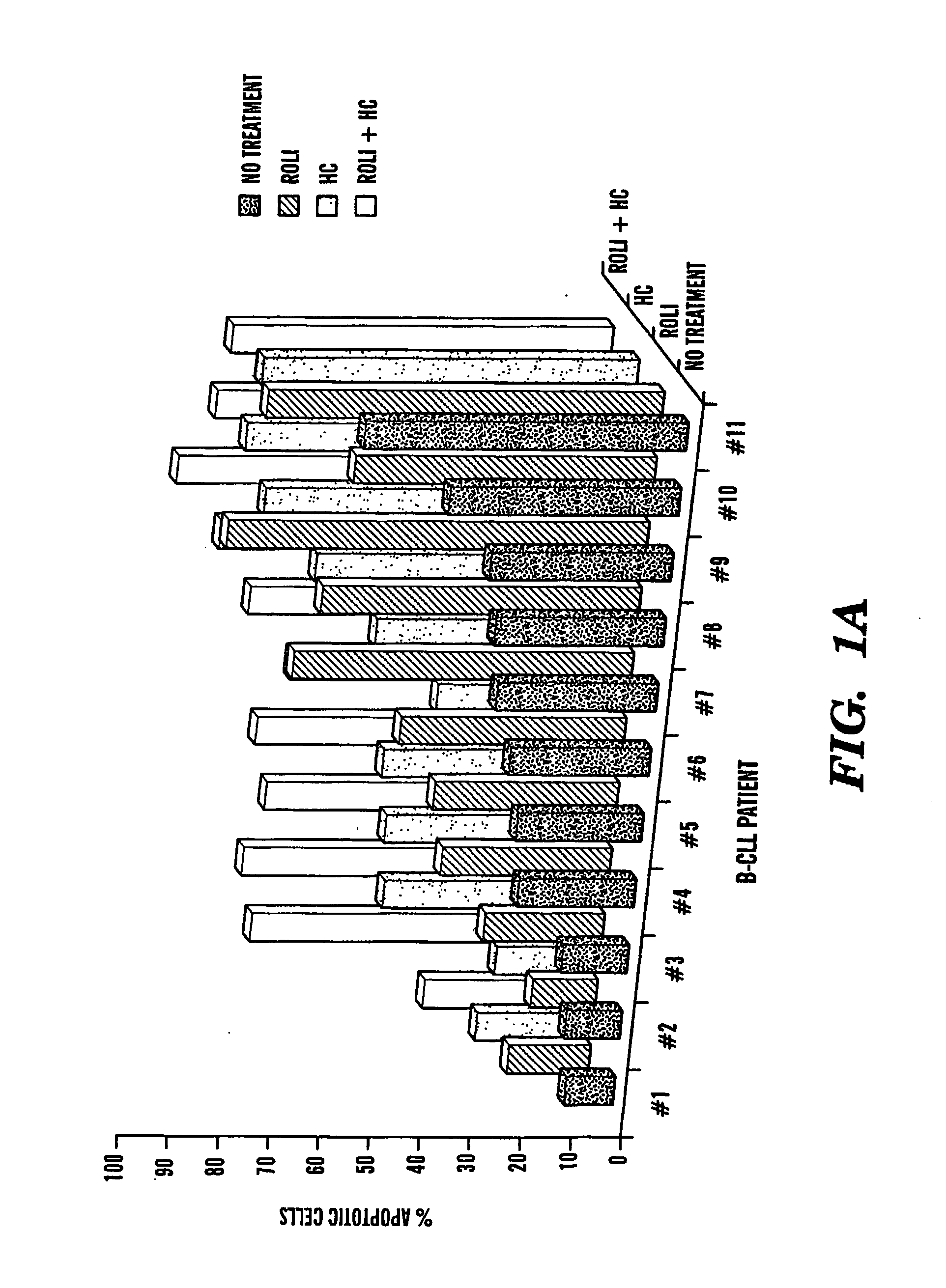
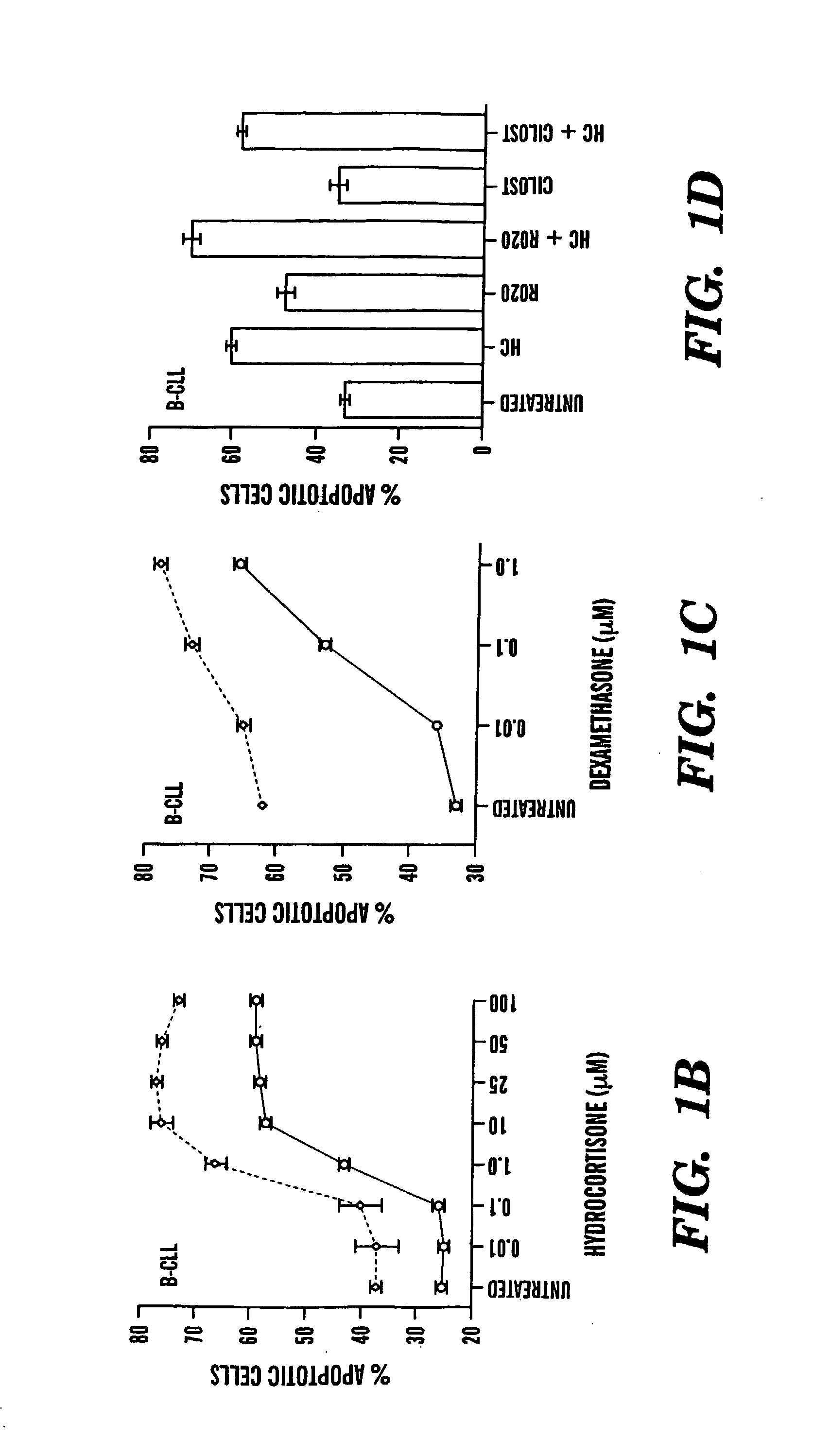
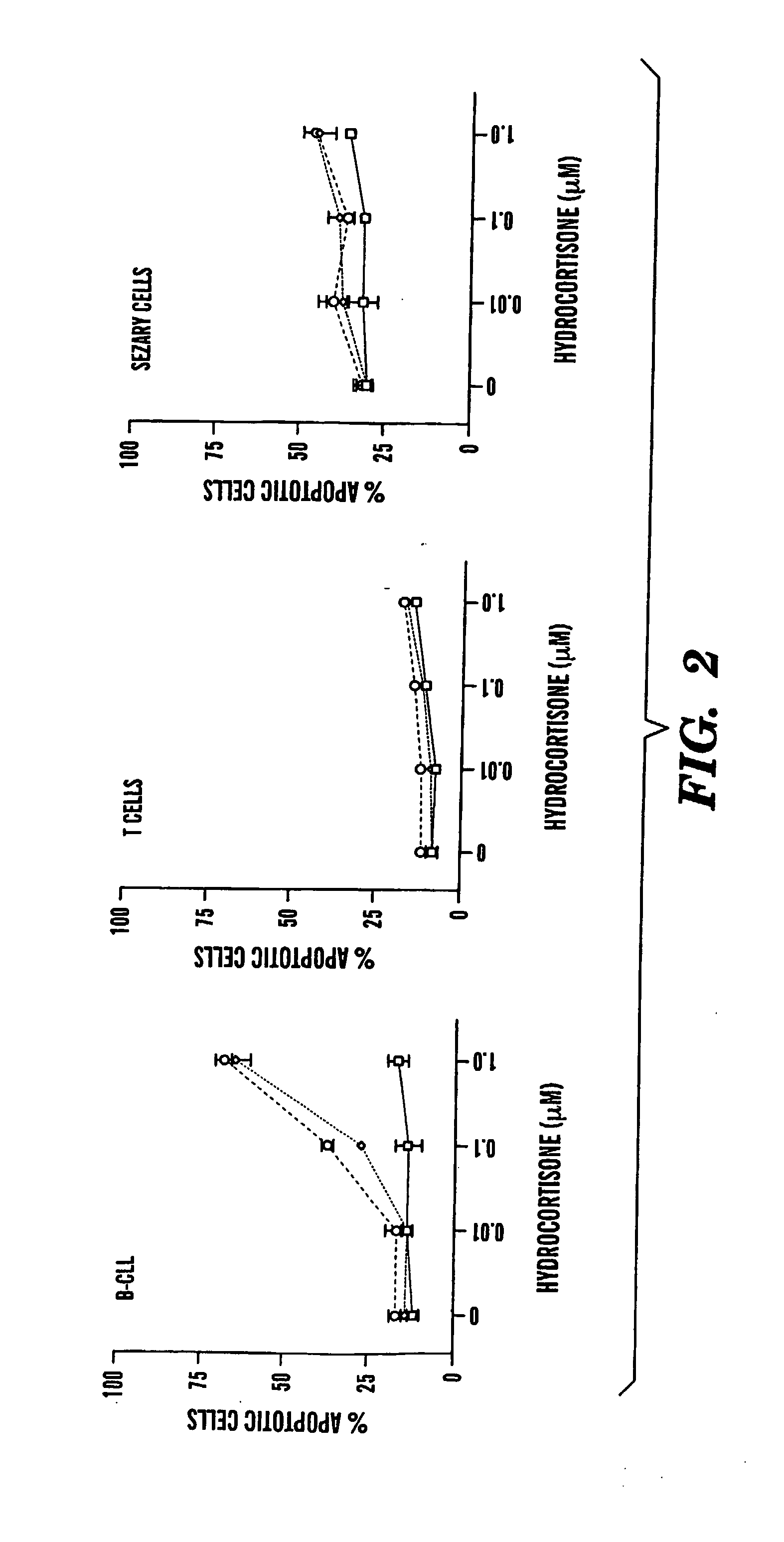
Compositions and Methods for the Treatment of Peripheral B-Cell Neoplasms
InactiveUS20080051379A1High level of apoptosisLevel of apoptosis inducedOrganic active ingredientsBiocidePDE4 InhibitorsAdenosine
The present invention is directed to the use of a PDE4 inhibitor and a glucocorticoid to treat peripheral B-cell neoplasms. In particular, the present invention provides a method of treating individuals (e.g. patients) diagnosed with peripheral B-cell leukemias by administering pharmaceutical compositions comprising Type 4 cyclic adenosine monophosphate phosphodiesterase inhibitors and a glucocorticoid. Preferably, the combination of the PDE4 inhibitor and the glucocorticoid has a synergistic effect on apoptosis such that the level of apoptosis induced is greater than the level that would be expected by simply adding a PDE4 inhibitor to a glucocorticoid.
Owner:BOSTON MEDICAL CENTER INC
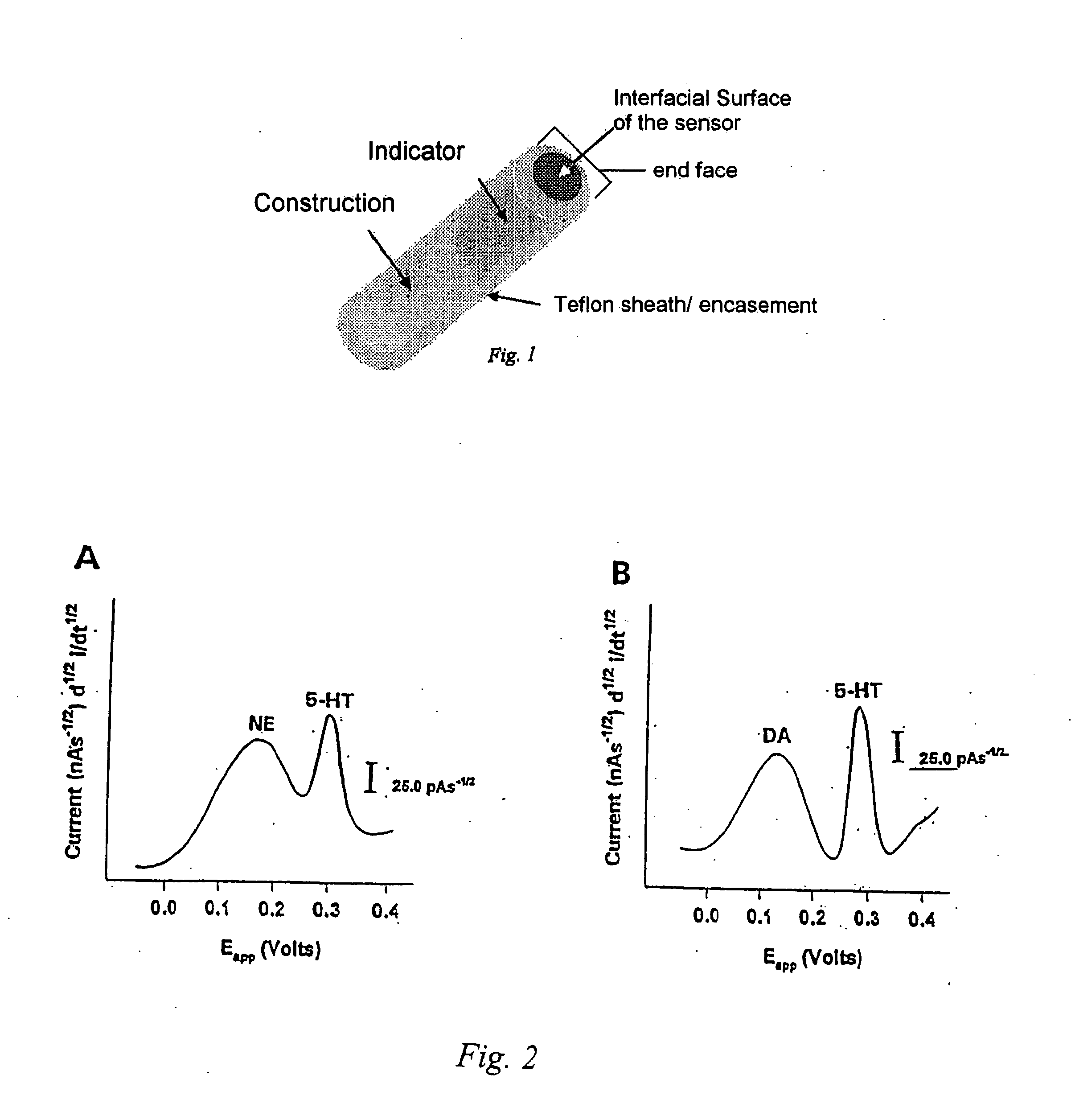
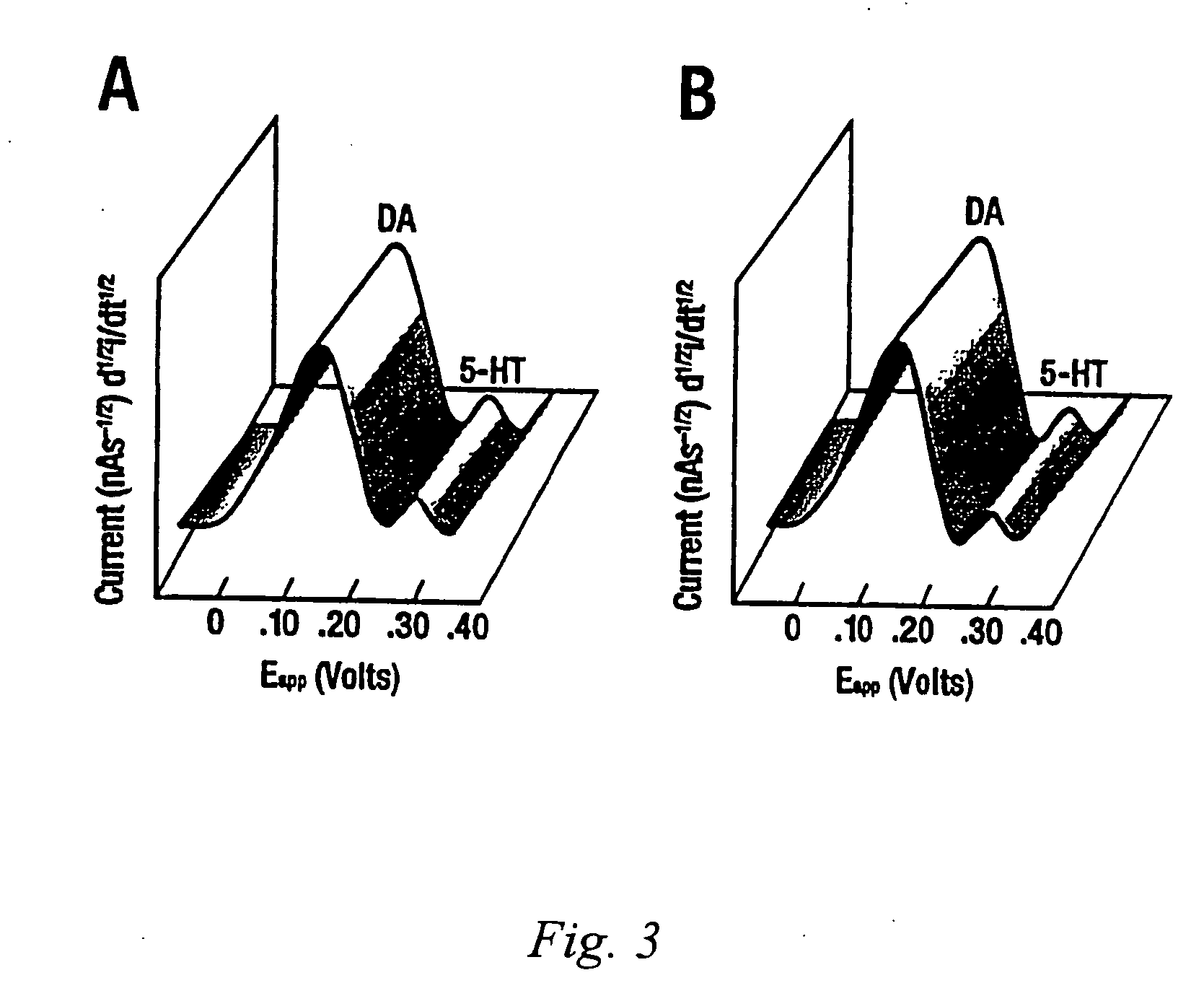
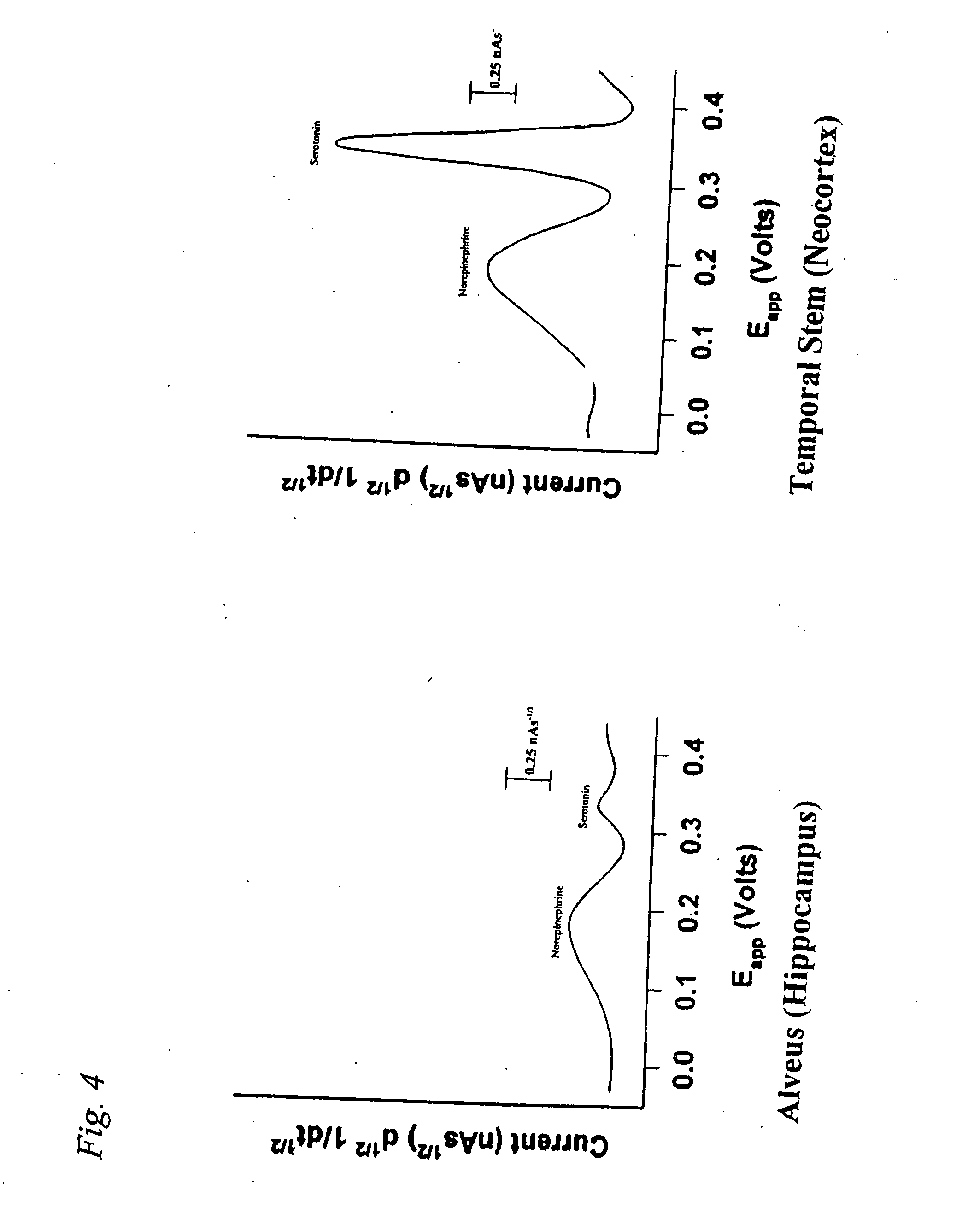
Identification, diagnosis, and treatment of neuropathologies, neurotoxicities, tumors, and brain and spinal cord injuries using electrodes with microvoltammetry
InactiveUS20070026440A1Microbiological testing/measurementVolume/mass flow measurementAbnormal tissue growthInjury brain
The present invention relates to devices and methods of use thereof for detection of biomolecules, in vitro, in vivo, or in situ. The invention relates to methods of diagnosing and / or treating a subject as having or being at risk of developing a disease or condition that is associated with abnormal levels of one or more biomolecules including, but not limited to, inter alia, epilepsy, diseases of the basal ganglia, athetoid, dystonic diseases, neoplasms, Parkinson's disease, brain injuries, spinal cord injuries, and cancer. The invention also provides methods of differentiating white matter from gray matter. In some embodiments, regions of the brain to be resected or targeted for pharmaceutical therapy are identified using sensors. The invention further provides methods of measuring the neurotoxicity of a material by comparing microvoltammograms of a neural tissue in the presence and absence of the material using the inventive sensors.
Owner:RES FOUND THE CITY UNIV OF NEW YORK +1
Method enabling use of extracellular RNA extracted from plasma or serum to detect, monitor or evaluate cancer
InactiveUS20020106684A1Low tumor burdenImmunologic function is relatively intactSugar derivativesMicrobiological testing/measurementA lipoproteinNeoplasm
This invention relates to the use of tumor-derived or associated extracellular ribonucleic acid (RNA) found circulating in the plasma or serum fraction of blood for the detection, monitoring, or evaluation of cancer or premalignant conditions. Extracellular RNA may circulate as non-bound RNA, protein-bound RNA, lipid-RNA complexes, lipoprotein (proteolipid)-RNA complexes, protein-RNA complexes including within or in association with ribonucleoprotein complexes, nucleosomes, or within apoptotic bodies. Any intracellular RNA found in plasma or serum can additionally be detected by this invention. Specifically, this invention enables the extraction of circulating RNA from plasma or serum and utilizes nucleic acid amplification assays for the identification, detection, inference, monitoring, or evaluation of any neoplasm, benign, premalignant, or malignant, in humans or other animals, which might be associated with that RNA. Further, this invention allows the qualitative or quantitative detection of tumor-derived or associated extracellular RNA circulating in the plasma or serum of humans or animals with or without any prior knowledge of the presence of cancer or premalignant tissue.
Owner:ONCOMEDX
Method and device for treating cancer with electrical therapy in conjunction with chemotherapeutic agents and radiation therapy
This invention relates generally to the electrical treatment of malignant tumors and neoplasms by applying a voltage to affected tissue. Devices and various adaptations therein are described for use in electrical therapy. Additionally, various chemotherapeutic agent and radiation therapies are described which may be advantageously used in conjunction with electrical therapy to ameliorate cancer.
Owner:IONIX MEDICAL
Combination of mtor inhibitor and a tyrosine kinase inhibitor for the treatment of neoplasms
InactiveUS20060094674A1Reduce spreadIncreased apoptosisBiocideGenetic material ingredientsMEK inhibitorTyrosine-kinase inhibitor
The invention features methods and compositions including an mTOR inhibitor and a tyrosine kinase inhibitor for reducing the proliferation of and enhancing the apoptosis of neoplastic cells. The addition of an MEK inhibitor to this combination further enhances the effectiveness of this therapeutic method.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC
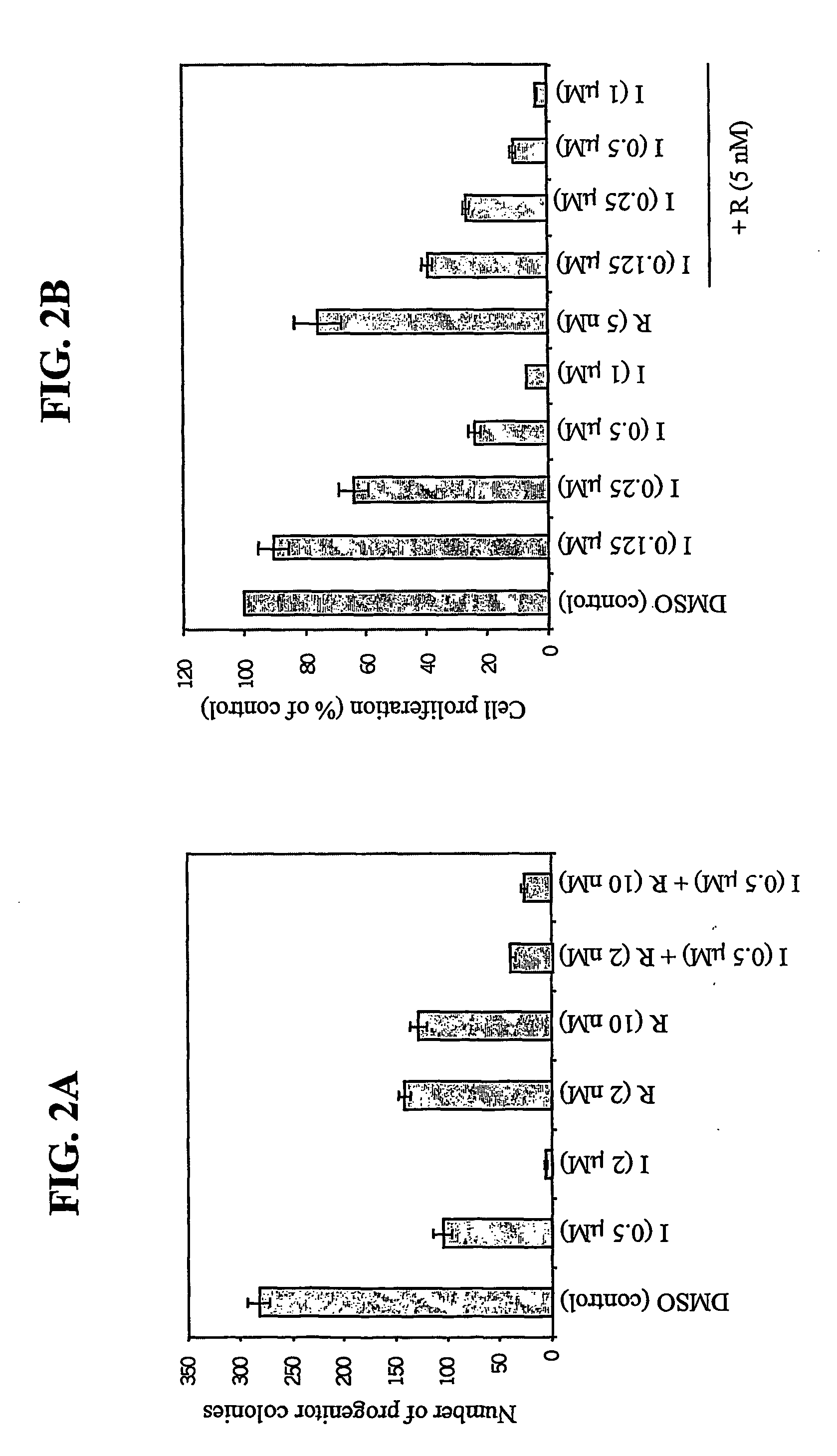
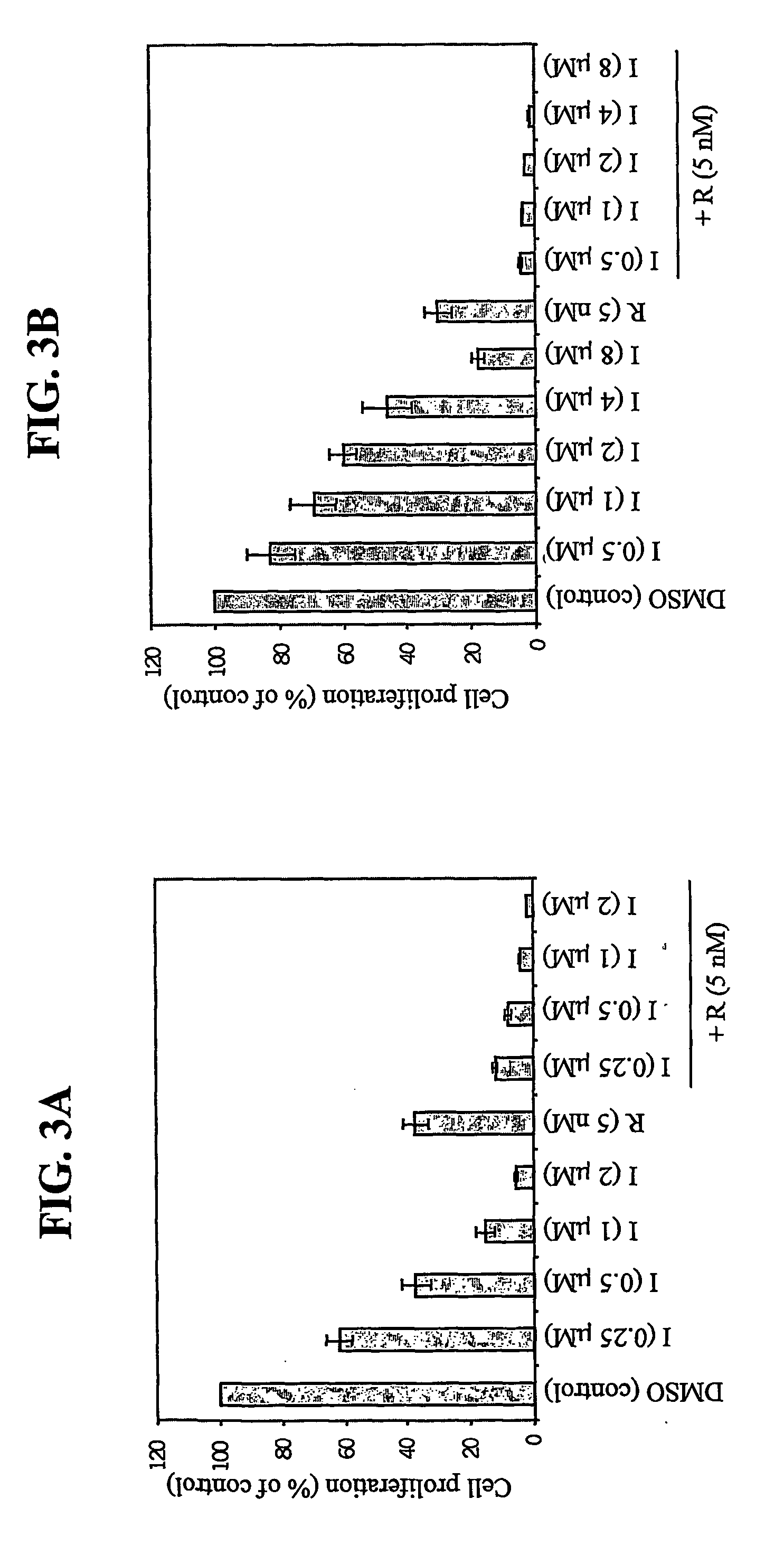
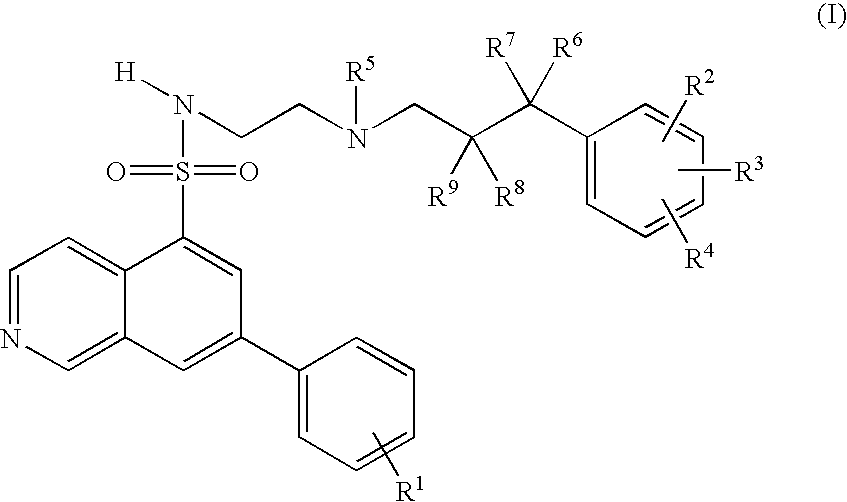
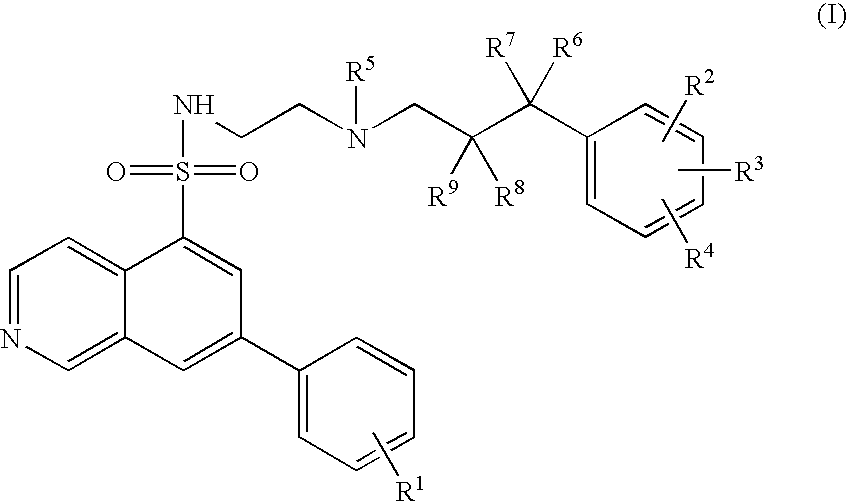
7-phenyl-isoquinoline-5-sulfonylamino derivatives as inhibitors of akt (protein kinase B)
Owner:ELI LILLY & CO
Method for the early detection of pancreatic cancer and other gastrointestinal disease conditions
InactiveUS20080248484A1Early diagnosisSpecific and focused early diagnosisMicrobiological testing/measurementOrgan systemNeoplasm
The present invention uses peripheral blood monocyte-lymphocyte for the early diagnosis of pancreatic cancer, as well as other conditions of the pancreas and other organs. The peripheral blood lymphocytes recognize the new neoplasm in the pancreas, as well as disease processes in other organ systems. The evaluation of this specific recognition of the disease process by the peripheral blood monocyte-lymphocyte through gene microarray expression patterns constitute a successful method for the early detection of pancreatic cancer and other organ disease processes. This document describes the process used in this method of early diagnosis.
Owner:BAUER A ROBERT
Kinase Inhibitors And Uses Thereof
The present invention relates to kinase inhibiting compositions and uses thereof. The invention further provides isolated kinase inhibiting peptides and uses thereof for inhibiting hyperplasia, for inhibiting the growth of neoplasms, and for inducing programmed cell death in a cell population.
Owner:PURDUE RES FOUND INC
Antigenic polypeptide usable as therapeutic agent for malignant neoplasm
ActiveUS8323657B2Promote productionTumor rejection antigen precursorsPeptide/protein ingredientsEpitopeNeoplasm
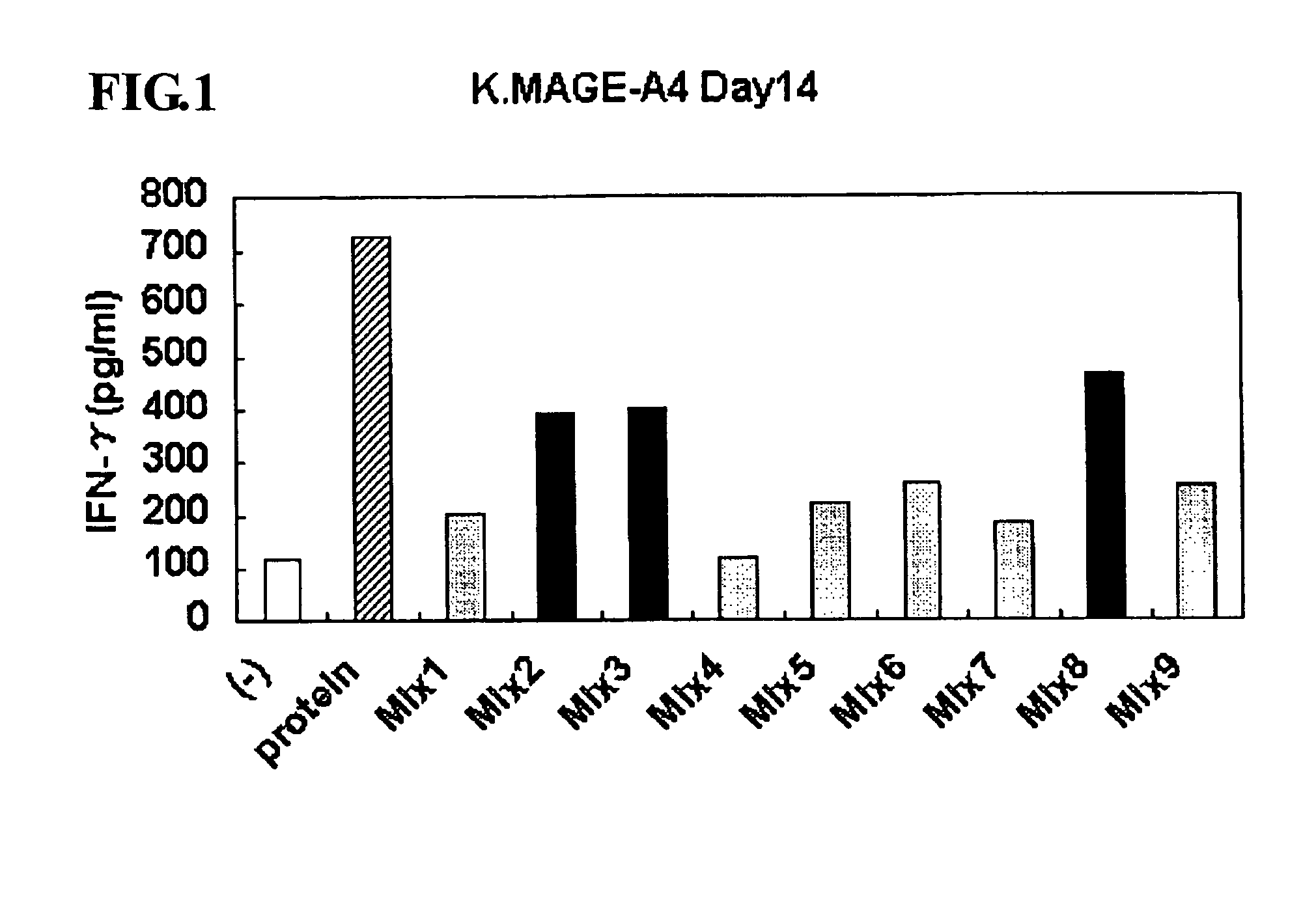
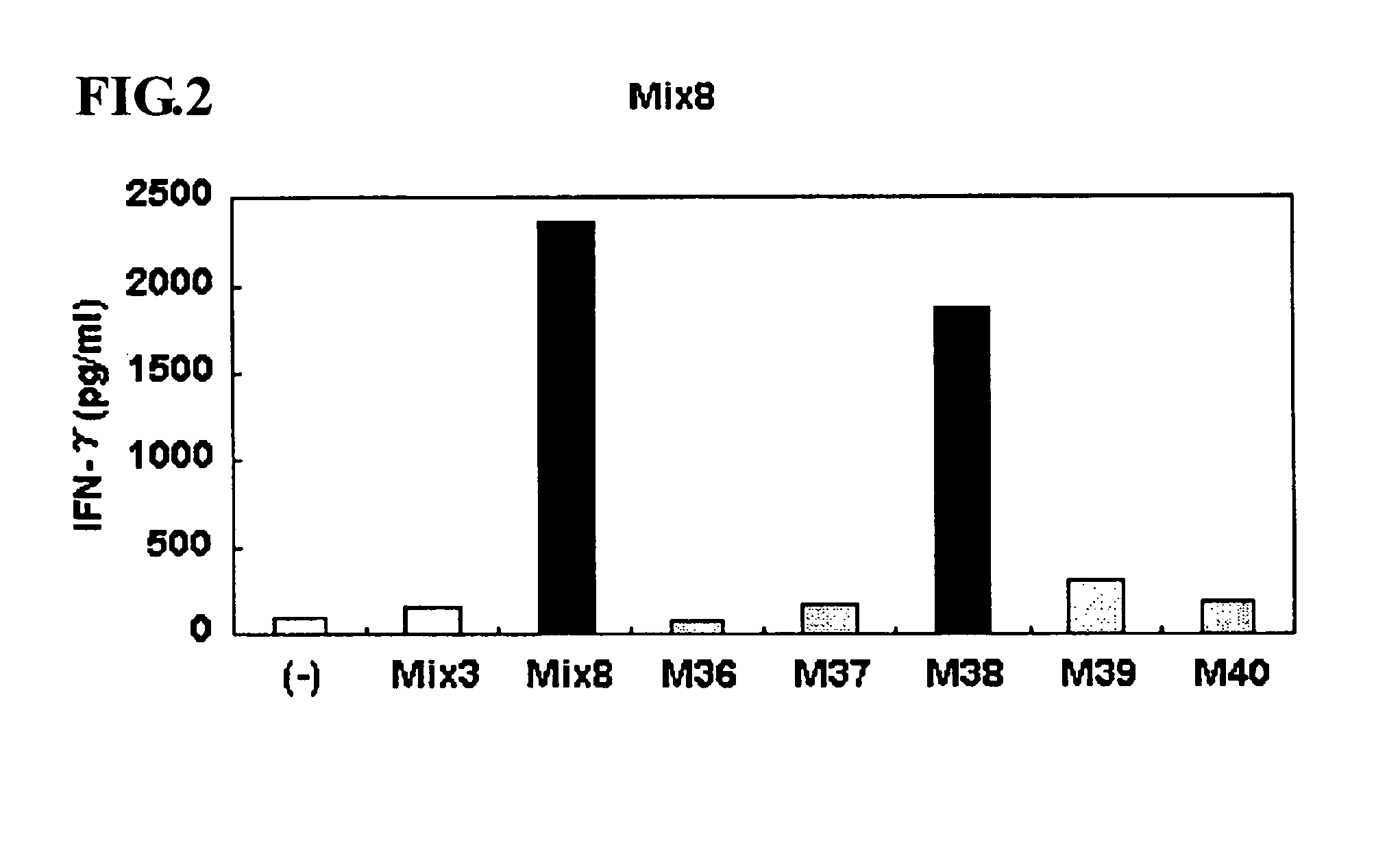
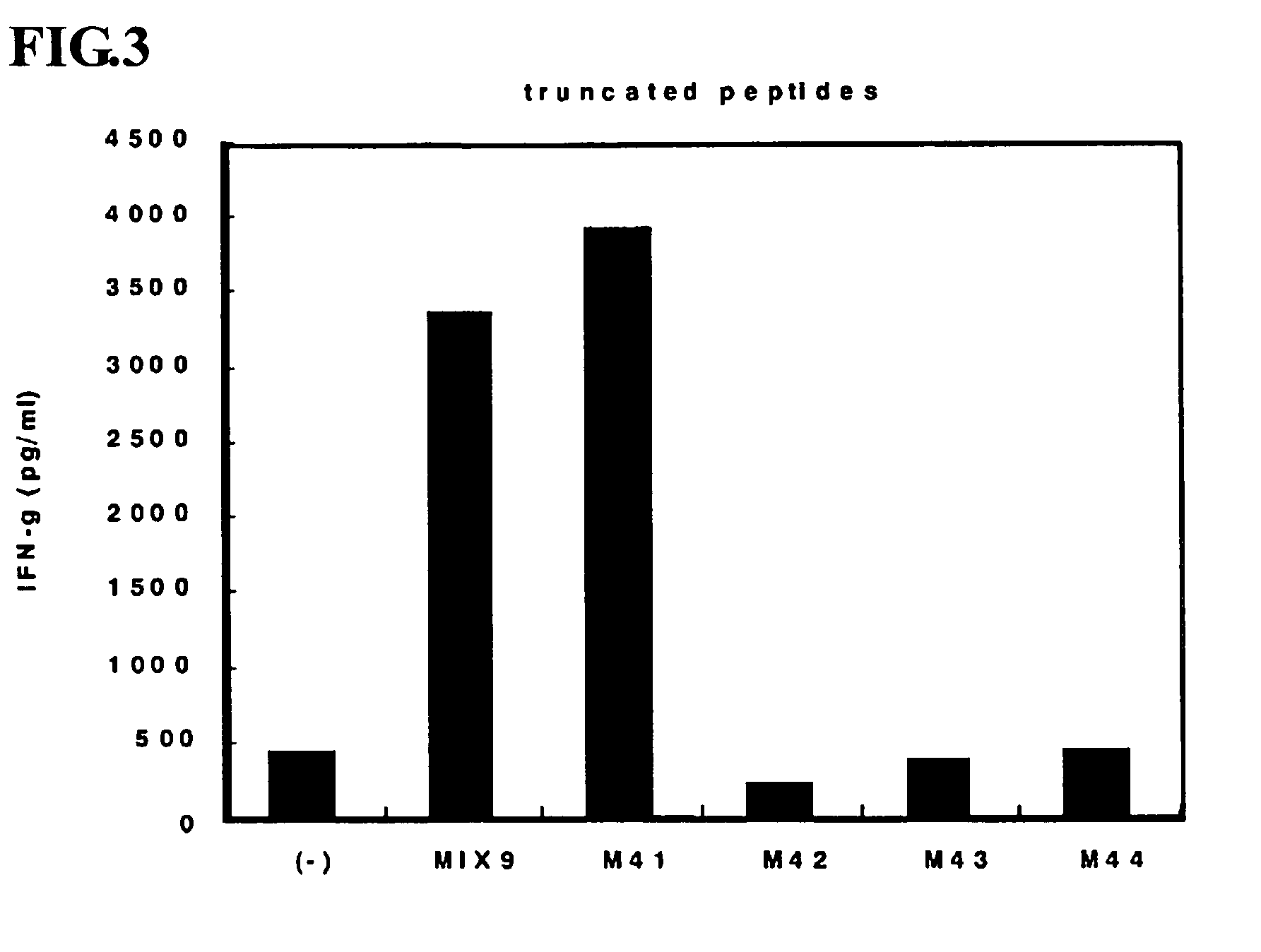
This invention provides a new tumor antigen having an epitope that induces a Th1 cell (a CD4-positive T cell specific to MAGE-A4), and a method ot application thereof.
Owner:HOKKAIDO UNIVERSITY
Combinations and methods for treating neoplasms
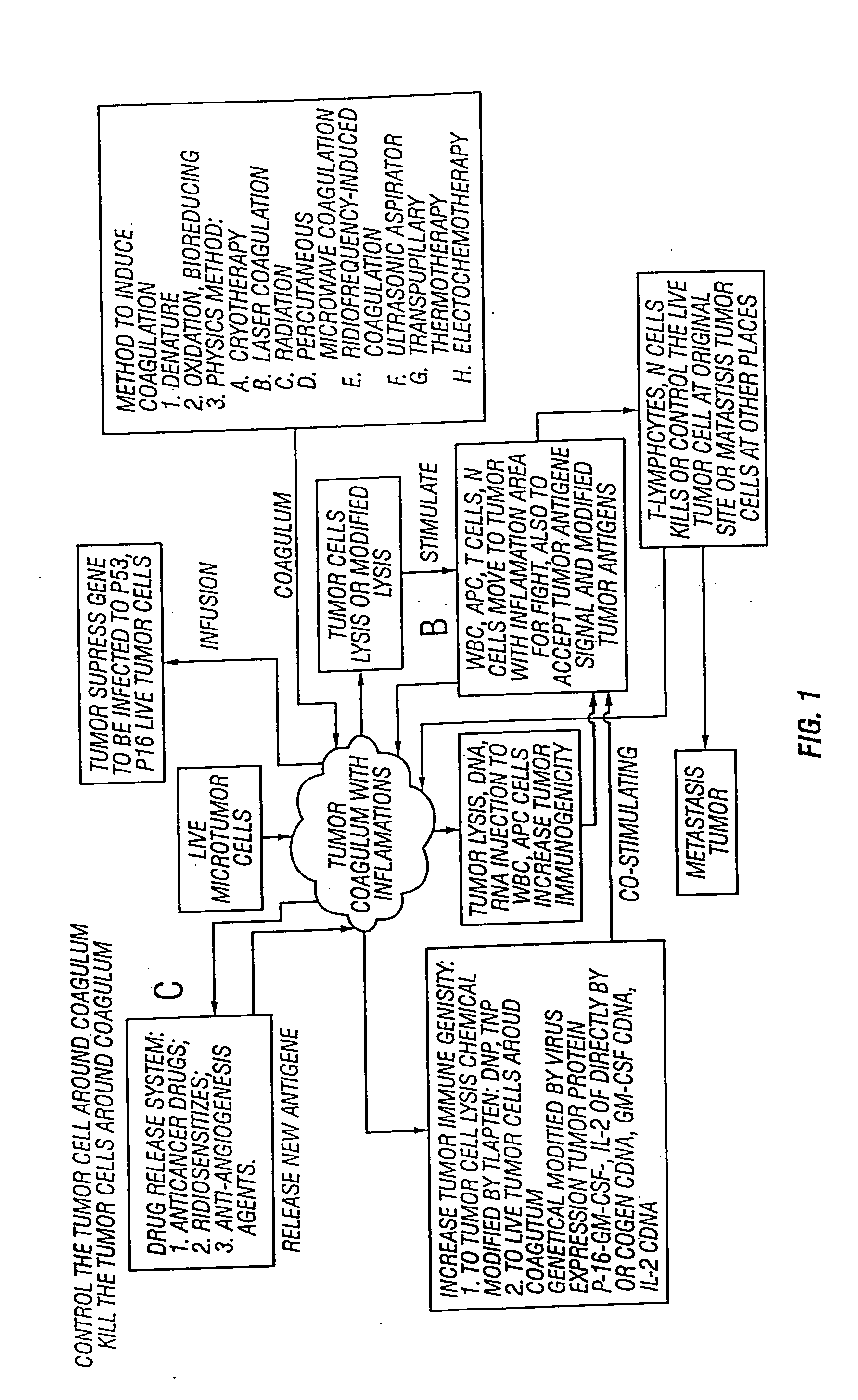
InactiveUS20050118187A1Improve treatment efficiencyInduce coagulationBiocideDipeptide ingredientsAbnormal tissue growthNeoplasm
Owner:YU BAOFA
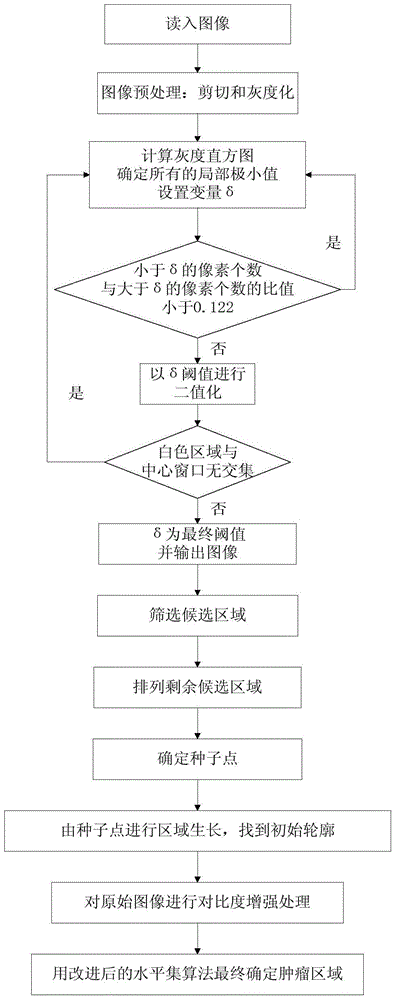
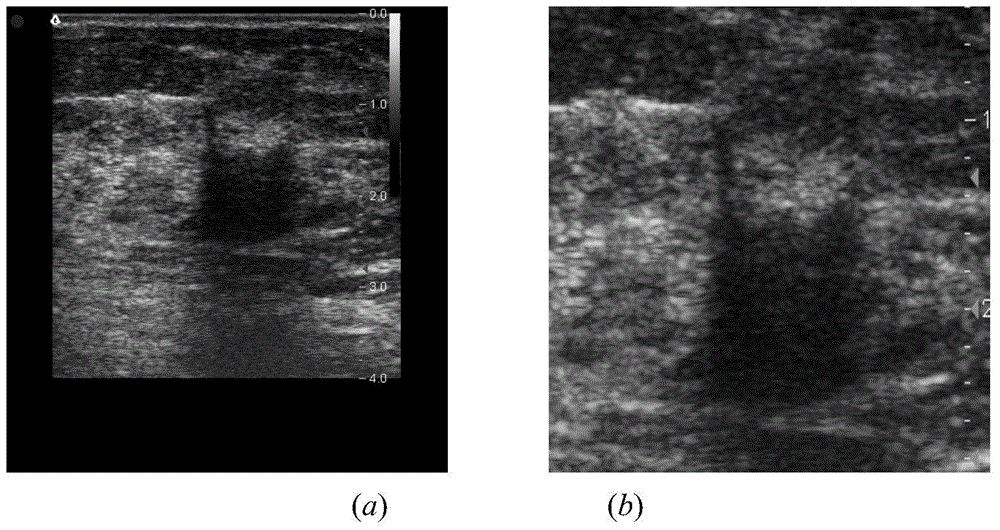
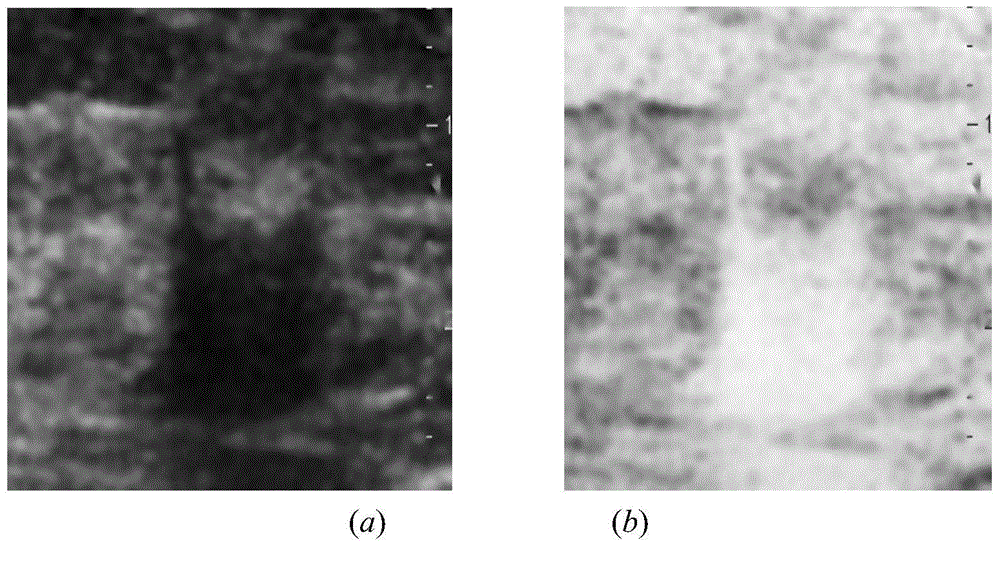
Breast neoplasms ultrasonic image segmentation method based on improved level set algorithm
InactiveCN104599270AGuaranteed positioningReduce over-segmentationImage enhancementImage analysisSonificationAlgorithm
The invention belongs to the field of treatment of medical images, and relates to a breast neoplasms ultrasonic image segmentation method based on improved level set algorithm. The method is characterized in that the original image is pre-treated and remains the effective area, the speckle noise is removed, so as to achieve the purpose of protecting the boundary; the image is adaptively segmented according to the threshold. The method comprises the steps of (1) inversing color for the image; (2) determining the threshold; (3) screening candidate areas; (4) arranging the rest candidate areas; (5) determining seed points. With the adoption of the method, the seed points can be quickly found, the seed points can be kept in a neoplasms area, and the accurate determination of the seed points ensures the accuracy of area growth and level set; in addition, the seed points grow in the area, and the initial contour can be found; the classic Chan-Vese (CV) algorithm is improved; the global statistics information of the curved line of the contour in the development process is considered during calculating the global statistics information; the accuracy of the segmentation result is ensured, and moreover, the automation level of the segmentation method can be further improved.
Owner:BEIJING UNIV OF TECH
Monitoring treatment-resistant clones in lymphoid and myeloid neoplasms by relative levels of evolved clonotypes
The invention is directed to a method of monitoring or detecting treatment-resistant clones in a patient being treated for a lymphoid or myeloid neoplasm from which patient-specific correlating clonotypes have been identified. In some embodiments, such method includes the steps of obtaining a sample from the patient comprising T-cells and / or B-cells; amplifying molecules of nucleic acid from the T-cells and / or B-cells of the sample, the molecules of nucleic acid comprising recombined DNA sequences from T-cell receptor genes or immunoglobulin genes; sequencing the amplified molecules of nucleic acid to form a clonotype profile; determining from the clonotype profile a level of each correlating clonotype and clonotypes clonally evolved therefrom; and correlating a presence of a treatment-resistant clone of the neoplasm with a change in relative levels of the correlating clonotypes and clonotypes clonally evolved therefrom. In part, the invention permits one to distinguish between cases where treatment is effective but insufficiently intense and cases where a cancer clone arises that is resistant to a current treatment approach.
Owner:ADAPTIVE BIOTECH
Method and compositions for treating hematological malignancies
InactiveUS20080039427A1Improve distributionImprove stabilityBiocideOrganic active ingredientsOrganic acidMedicine
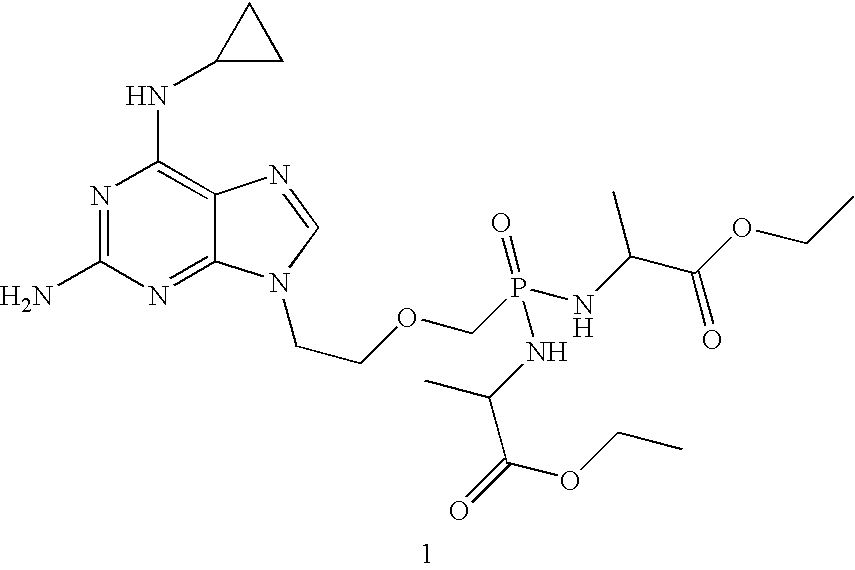
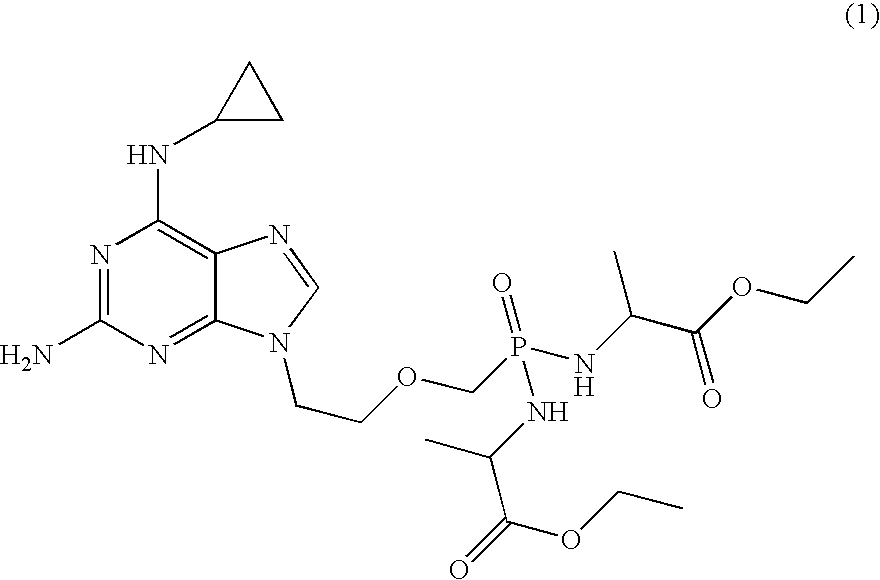
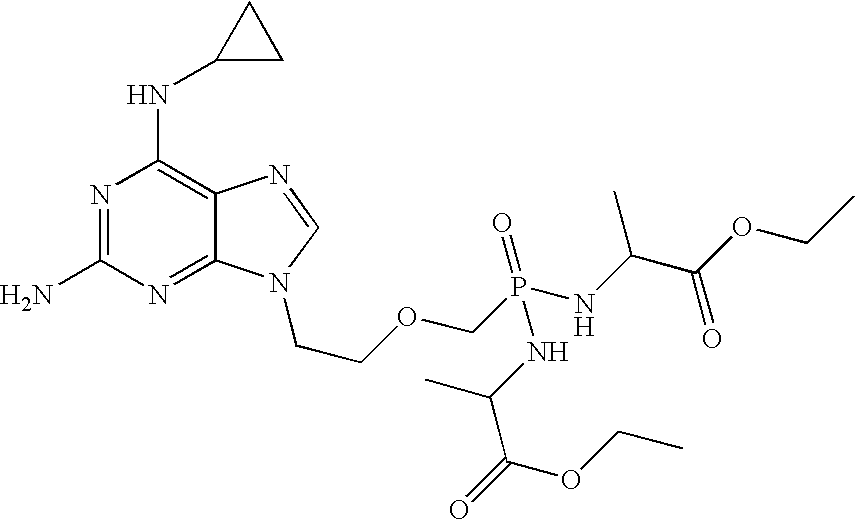
A compound of formula 1 and / or its salts, tautomers or solvates is used to treat hematological malignancies. In an embodiment, an organic acid salt of compound 1 is provided for general use in treatment of neoplasms, and in a further embodiment the salt is stabilized with carbohydrate.
Owner:GILEAD SCI INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com