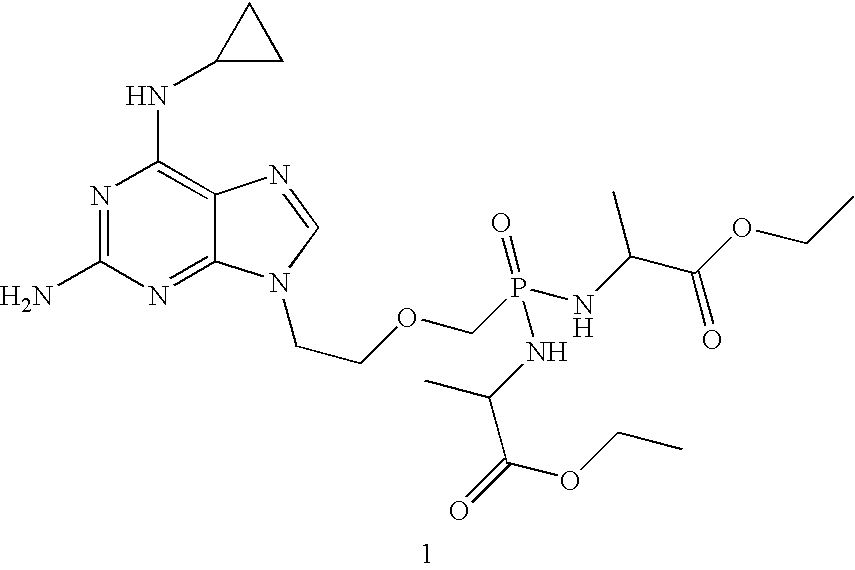
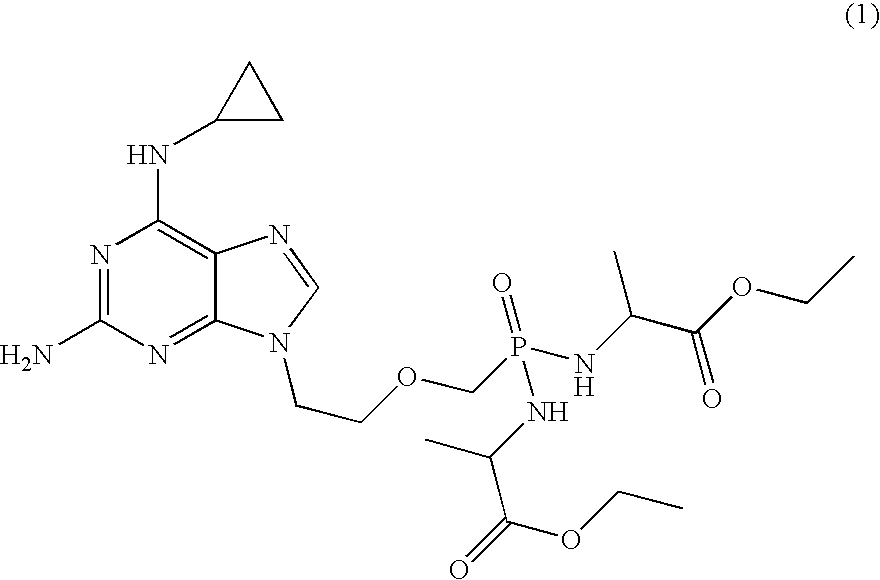
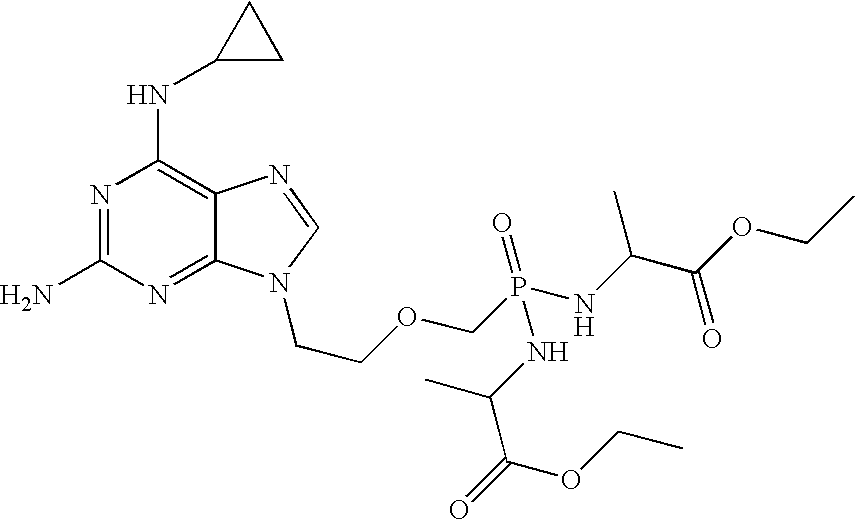
Method and compositions for treating hematological malignancies
a hematological malignancy and composition technology, applied in the direction of drug compositions, biocide, group 5/15 element organic compounds, etc., can solve the problems of substantial unmet medical needs, unmet medical needs for novel, efficacious therapeutics, and lack of promising drugs, and achieve the effect of high preference distribution of cpr-pmedap and enhanced stability of these salts (particularly in parenteral formulations)
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
[0039] Table 1 shows anti-proliferation EC50 of compound 1 and its metabolites, cpr-PMEDAP (9-(2-phosphonylmethoxyethyl)-N6-cyclopropyl-2,6-diaminopurine), PMEG (9-(2-phosphonylmethoxyethyl)guanine), and PMEDAP (9-(2-phosphonylmethoxyethyl)-2,6-diaminopurine). A variety of compounds that are used for treatment of hematologic malignancies were also tested, including a DNA polymerase inhibitor (ara-C), DNA polymerase / ribonucleotide reductase inhibitors (cladribine, clofarabine, fludarabine, gemcitabine), an adenosine deaminase inhibitor (deoxycoformycin), a DNA methylation inhibitor (decitabine), a DNA alkylator (doxorubicin), and a mitosis inhibitor (vincristine). All compounds, with the exception of doxorubicin and vincristine, are nucleoside analogs; ara-C, gemcitabine, and decitabine are cytosine analogs and the rest are adenosine analogs. cpr-PMEDAP and PMEG can be considered adenosine and guanosine analogs, respectively. Compounds were tested using human and canine lymphoblasts ...
example 3
Distribution of Prodrugs between PBMCs and Plasma in Dogs
[0046] Dogs were administered various prodrugs of cpr-PMEDAP as 0.2 mg / kg 30 minute IV infusions. The prodrugs 1-4 were monoamidates (phosphorus was also substituted with phenoxy), whereas the last two compounds were bis(amidates). The A and B compounds were substantially isolated enantiomers at the phosphorus atom chiral center whereas the monoAlatBU was the racemate at this site. Alanine was the L isomer. [0047] Blood was collected into potassium EDTA and separated by centrifugation. Primary blood mononuclear cells (PBMCs) were collected using CPT tubes (sodium citrate). [0048] For analysis, plasma samples were precipitated by addition of 100 μL acetonitirile containing internal standards (D4AP and TDF) to 100 μL of plasma for prodrug analysis. After protein precipitation by centrifugation, 100 μL was transferred into another tube to be dried and then reconstituted in 100 μL water with 0.2% formic acid. An aliquot of 20 μL ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com