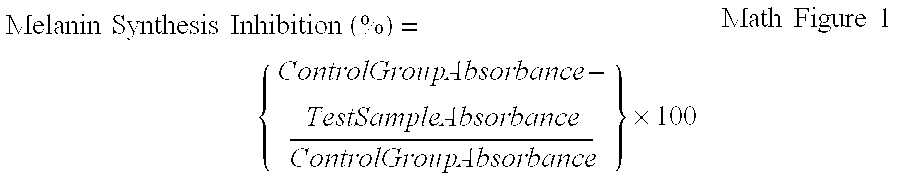Patents
Literature
111 results about "Formulation stability" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
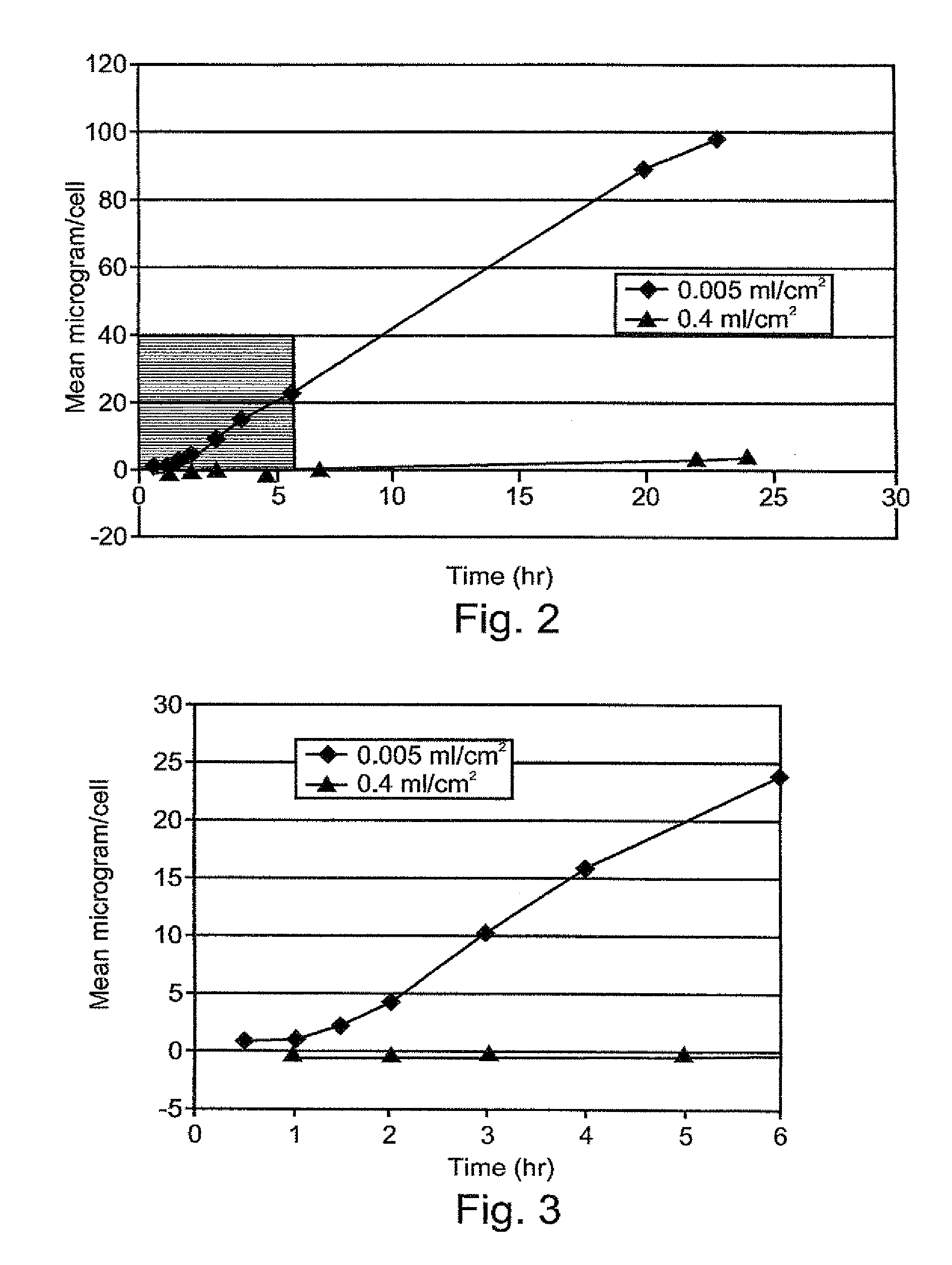
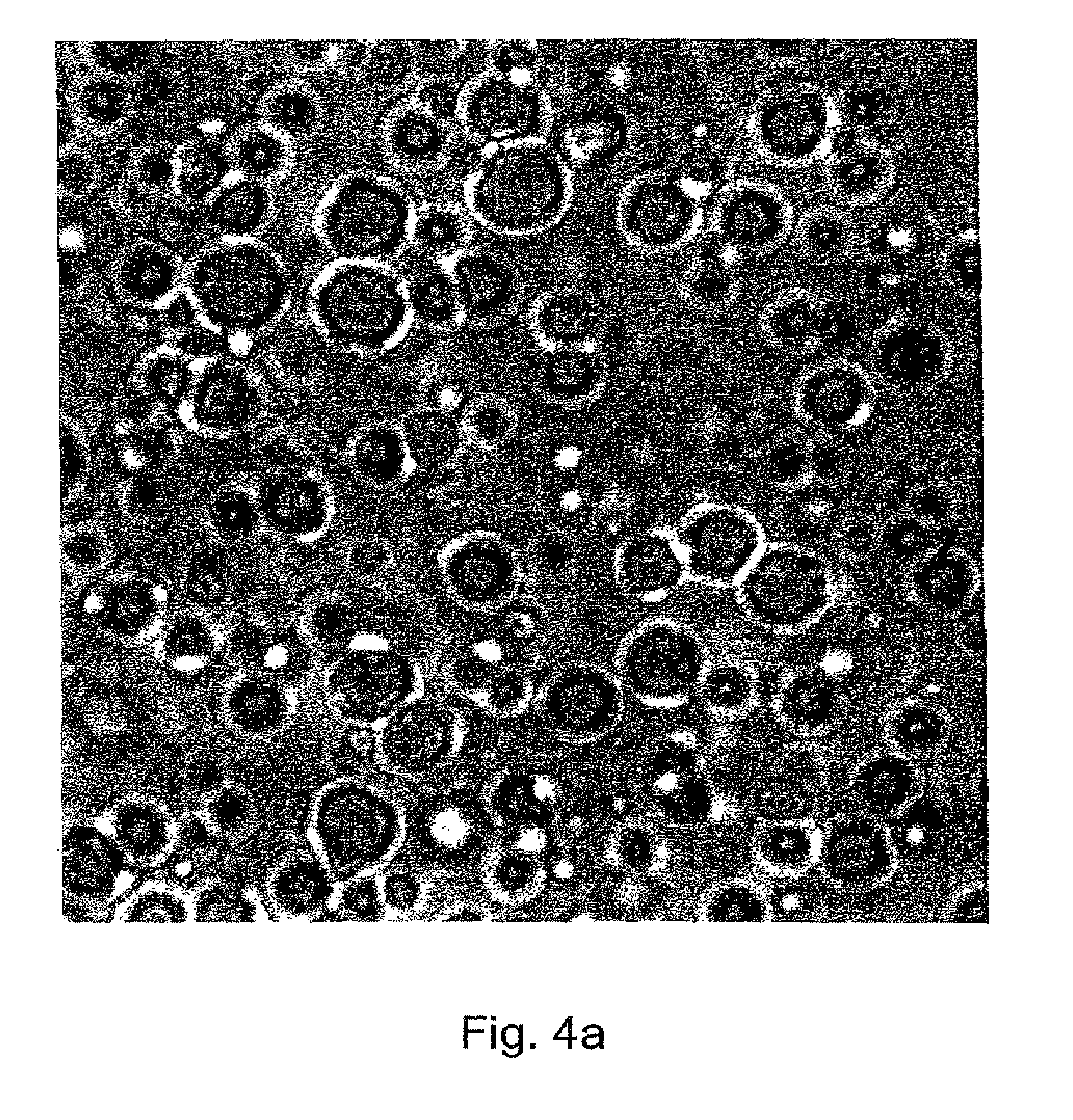
Methods of evaluating protein formulation stability and surfactant-stabilized insulin formulations derived therefrom
InactiveUS6737401B2Improve physical stabilityReliable timePeptide/protein ingredientsMicrobiological testing/measurementCell AggregationsProtein aggregation
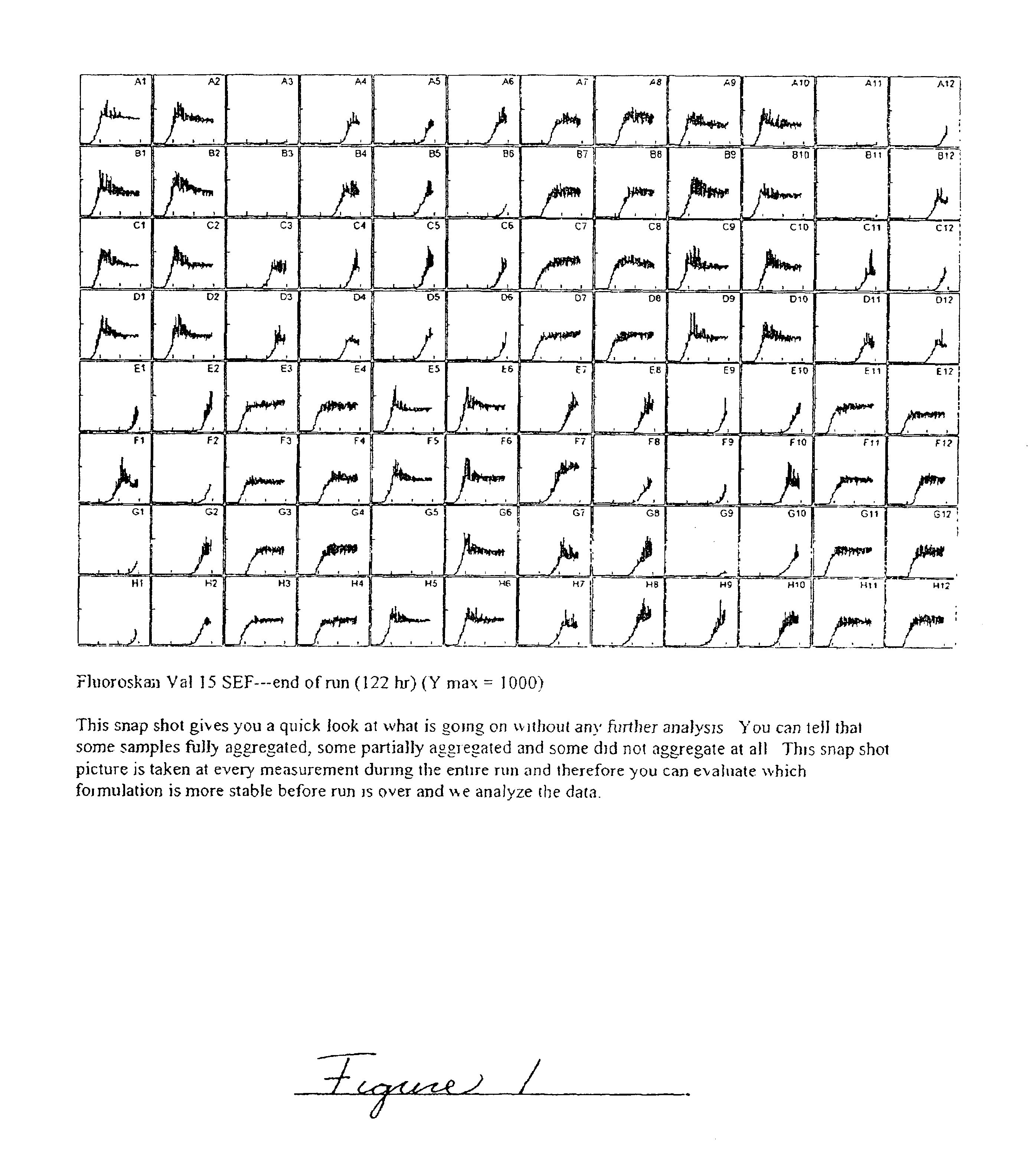
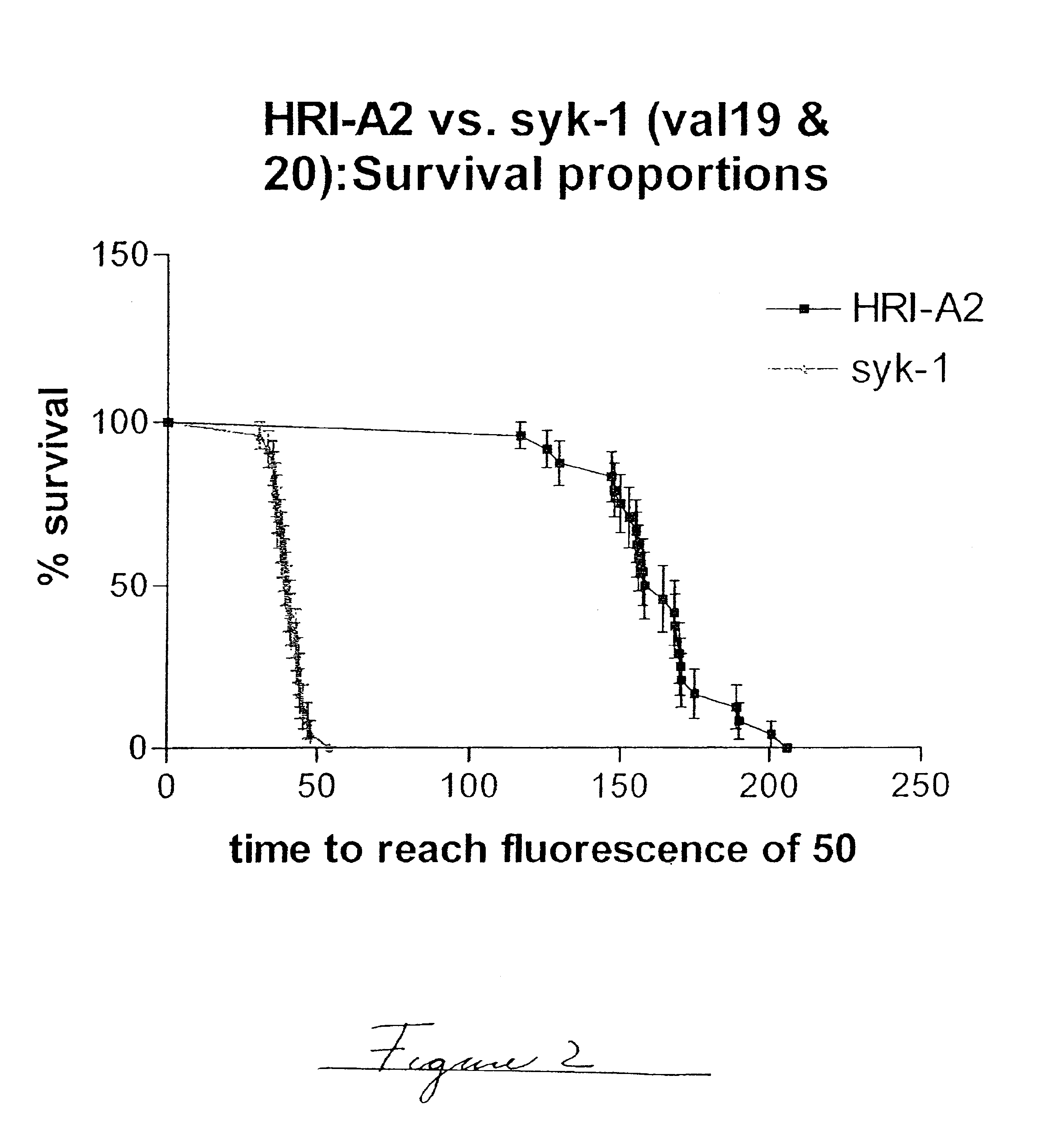
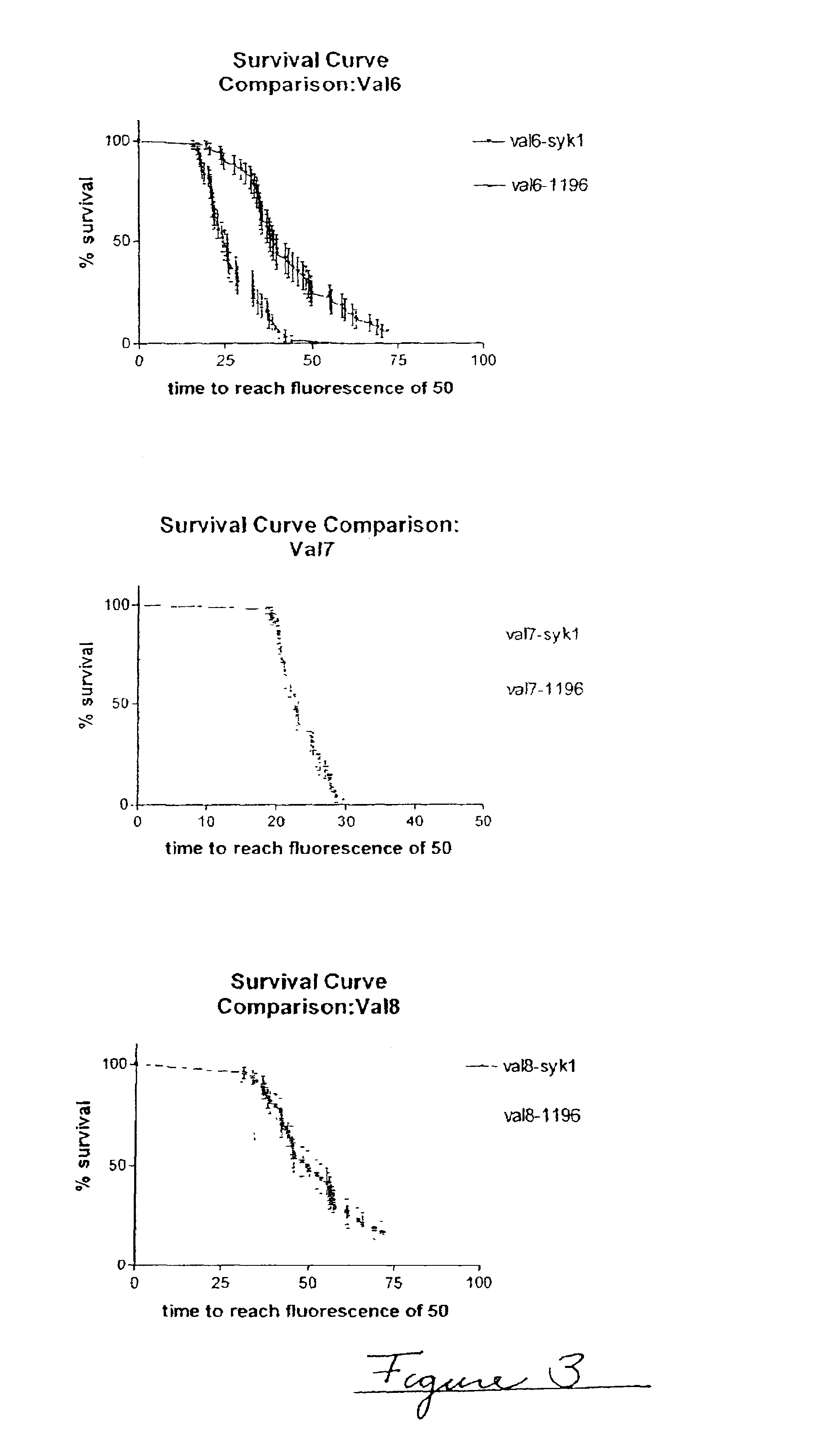
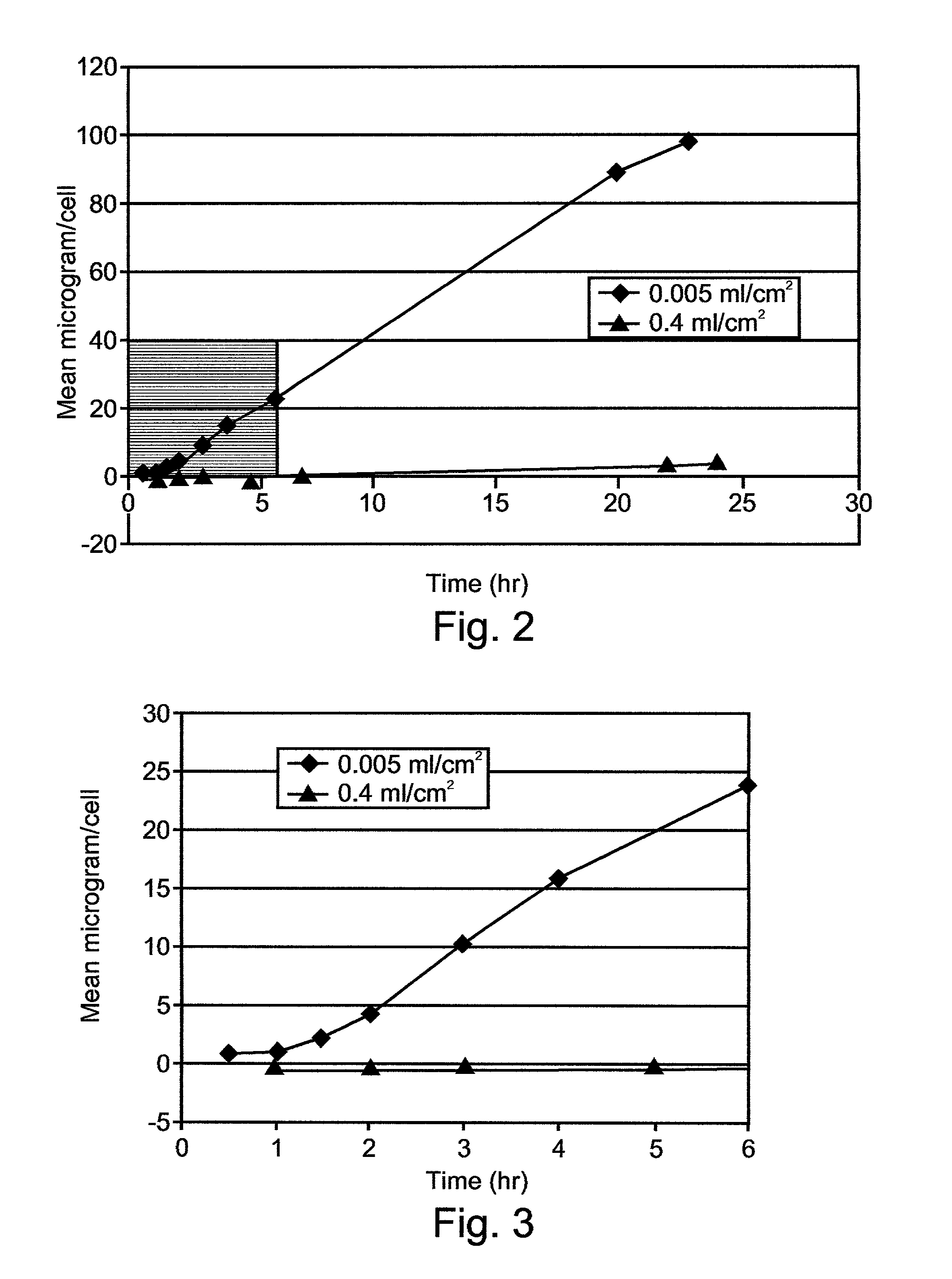
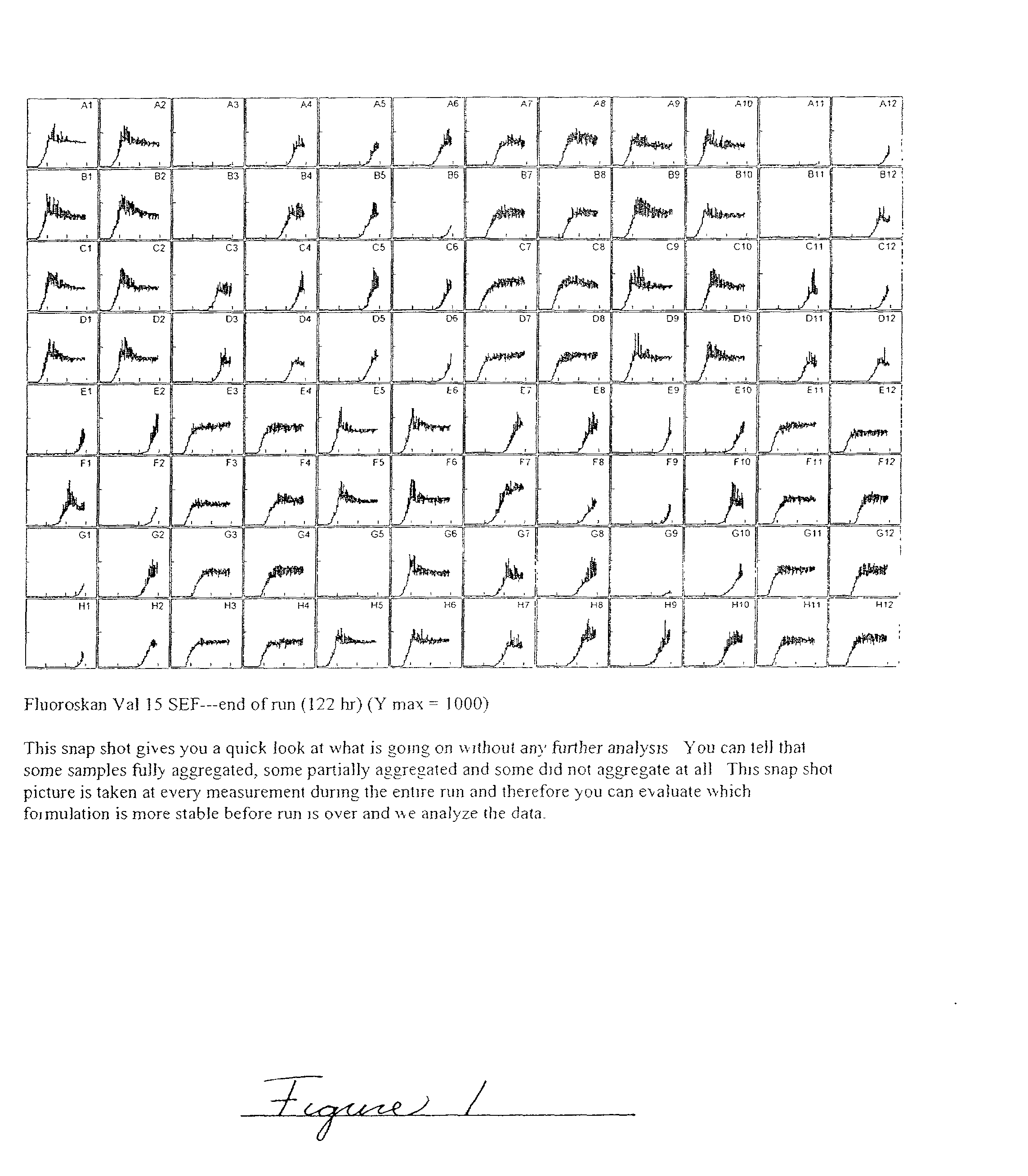
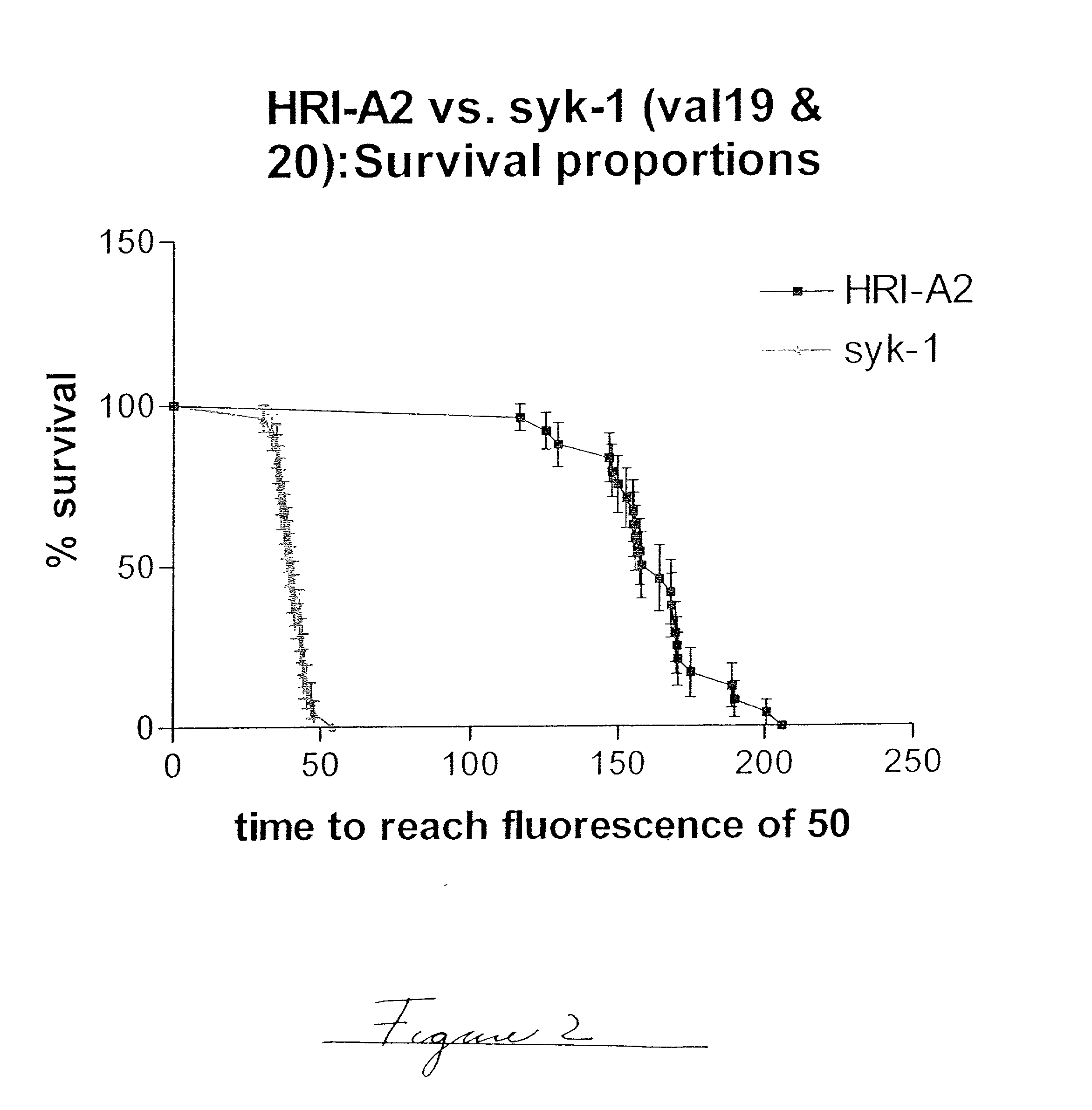
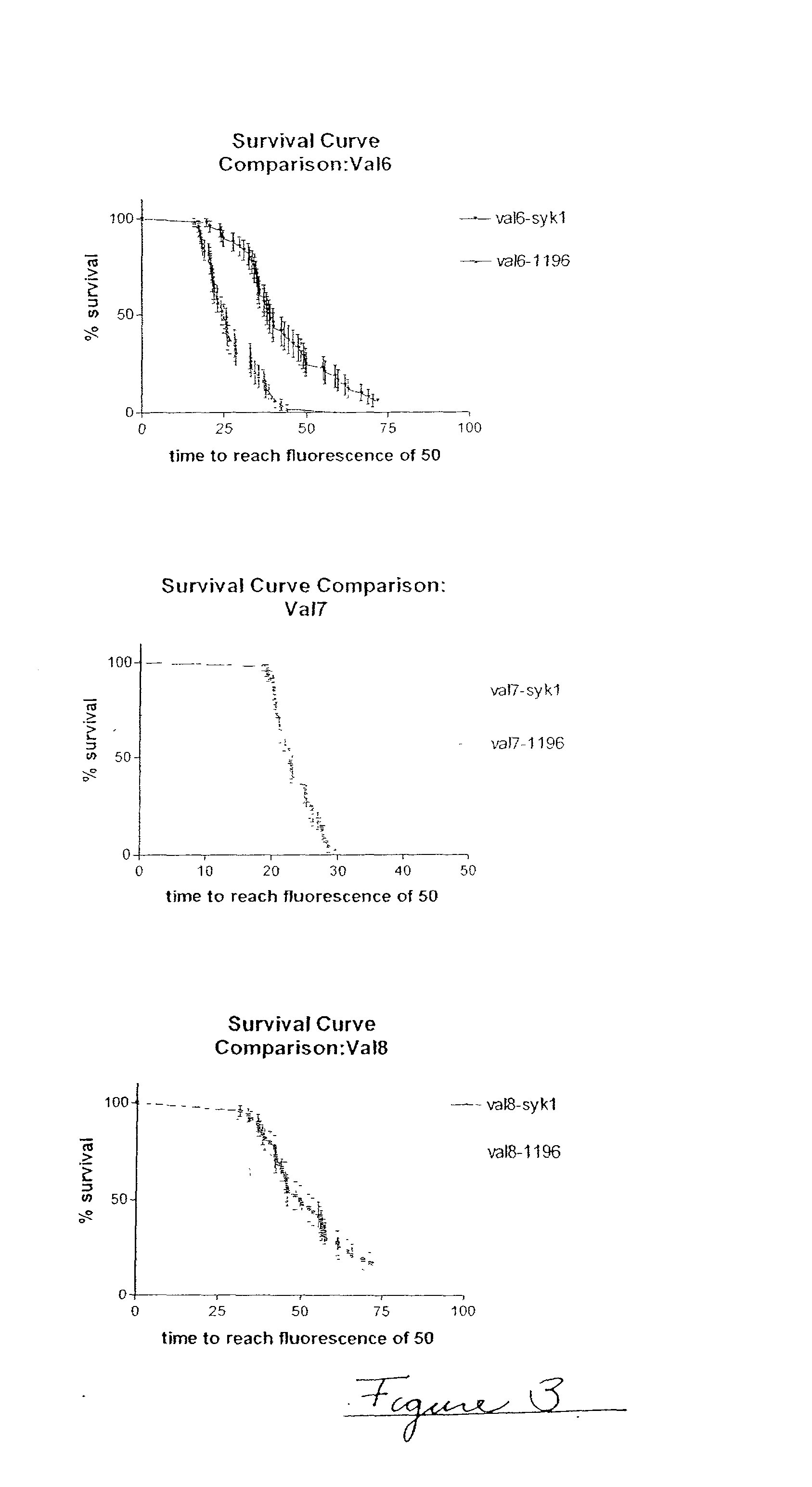
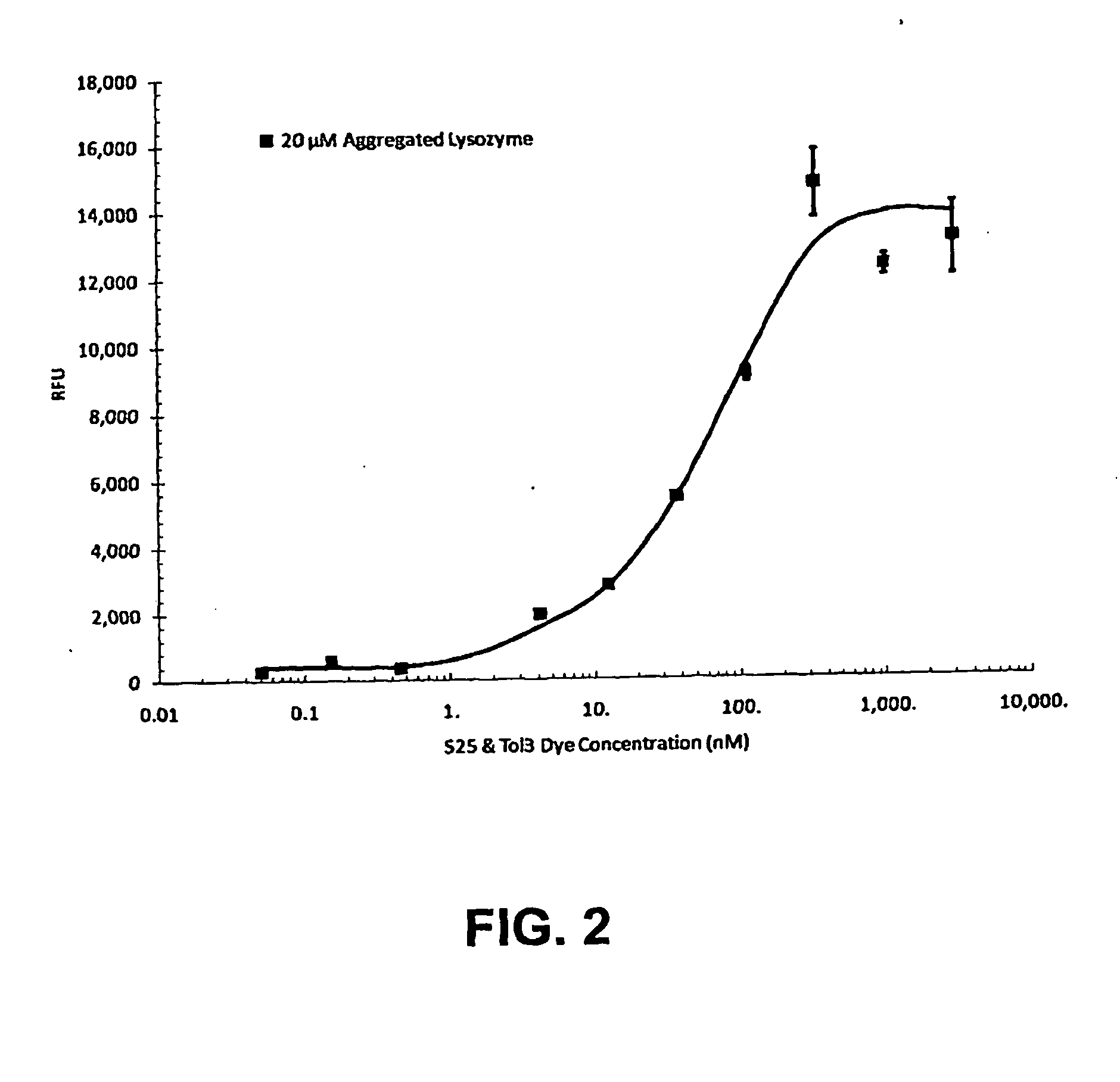
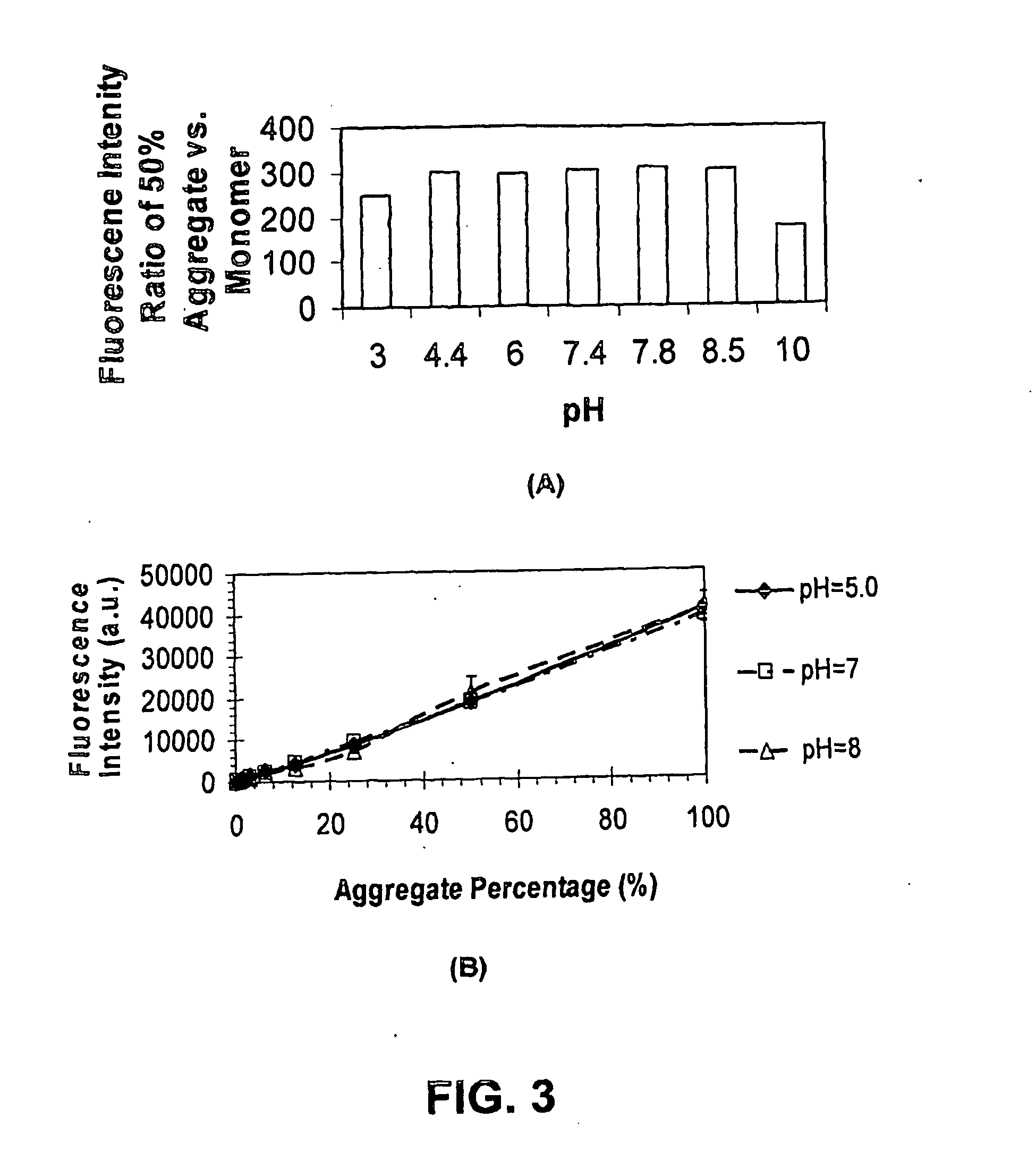
Embodiments of the invention are directed to a method of estimating the physical stability of a protein formulation. A particular embodiment of the invention places the protein formulation under an agitational stress that causes the protein to aggregate at an accelerated rate. In one embodiment, the change in protein aggregation is monitored spectroscopically using Thioflavin-T. Embodiments of the invention then utilize a survival curve analysis to ascertain the relative physical stability of the different protein formulations under study. This method was used to develop novel surfactant-stabilized insulin formulations in a rapid, cost efficient manner, thus illustrating the utility of the inventive method to the discovery and development of pharmaceutical protein formulations.
Owner:MEDTRONIC MIMIMED INC
Composition exhibiting enhanced formulation stability and delivery of topical active ingredients
InactiveUS7758888B2Improve stabilityExtended shelf lifeAntibacterial agentsCosmetic preparationsBenzoyl peroxideMedicine
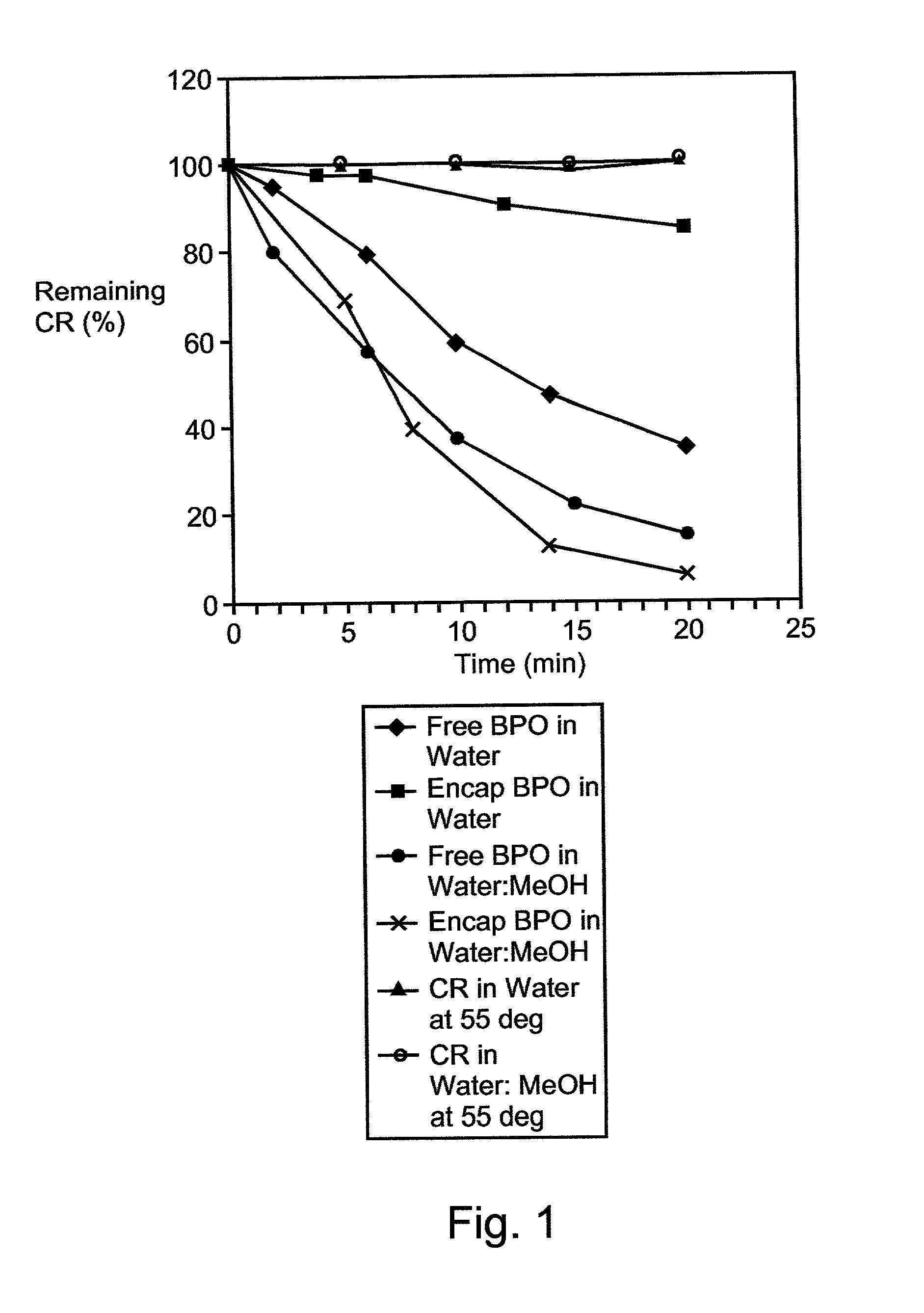
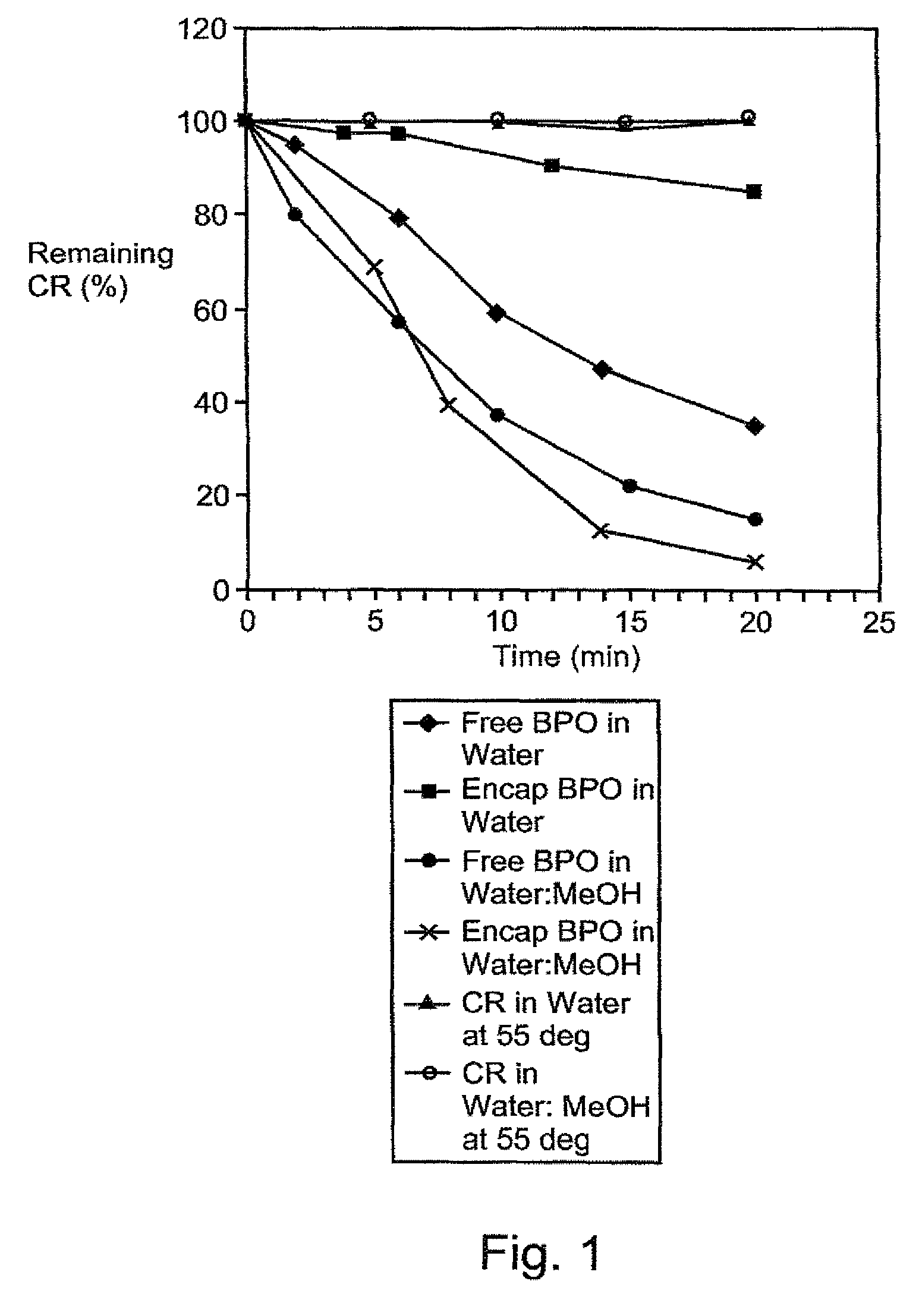
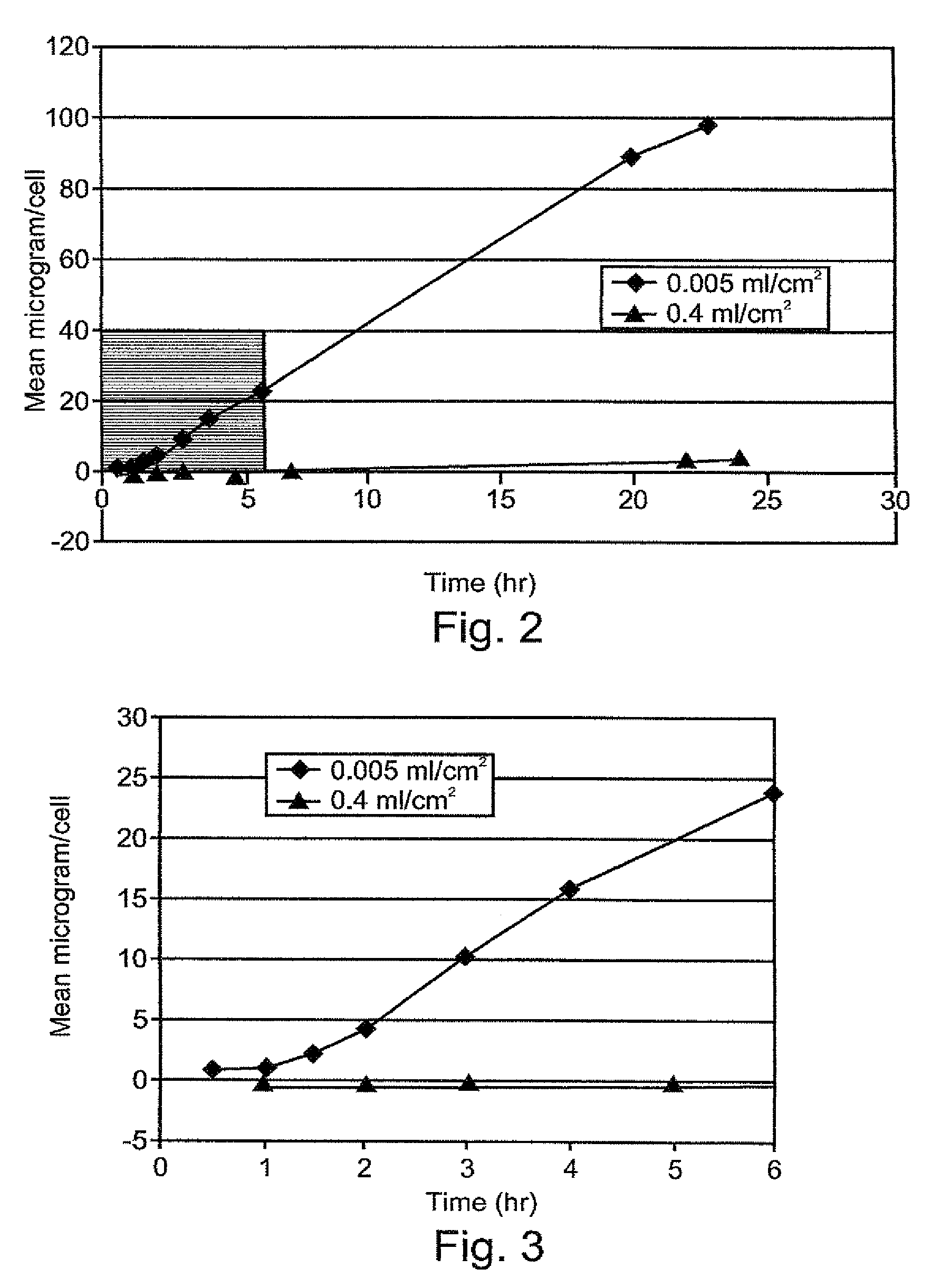
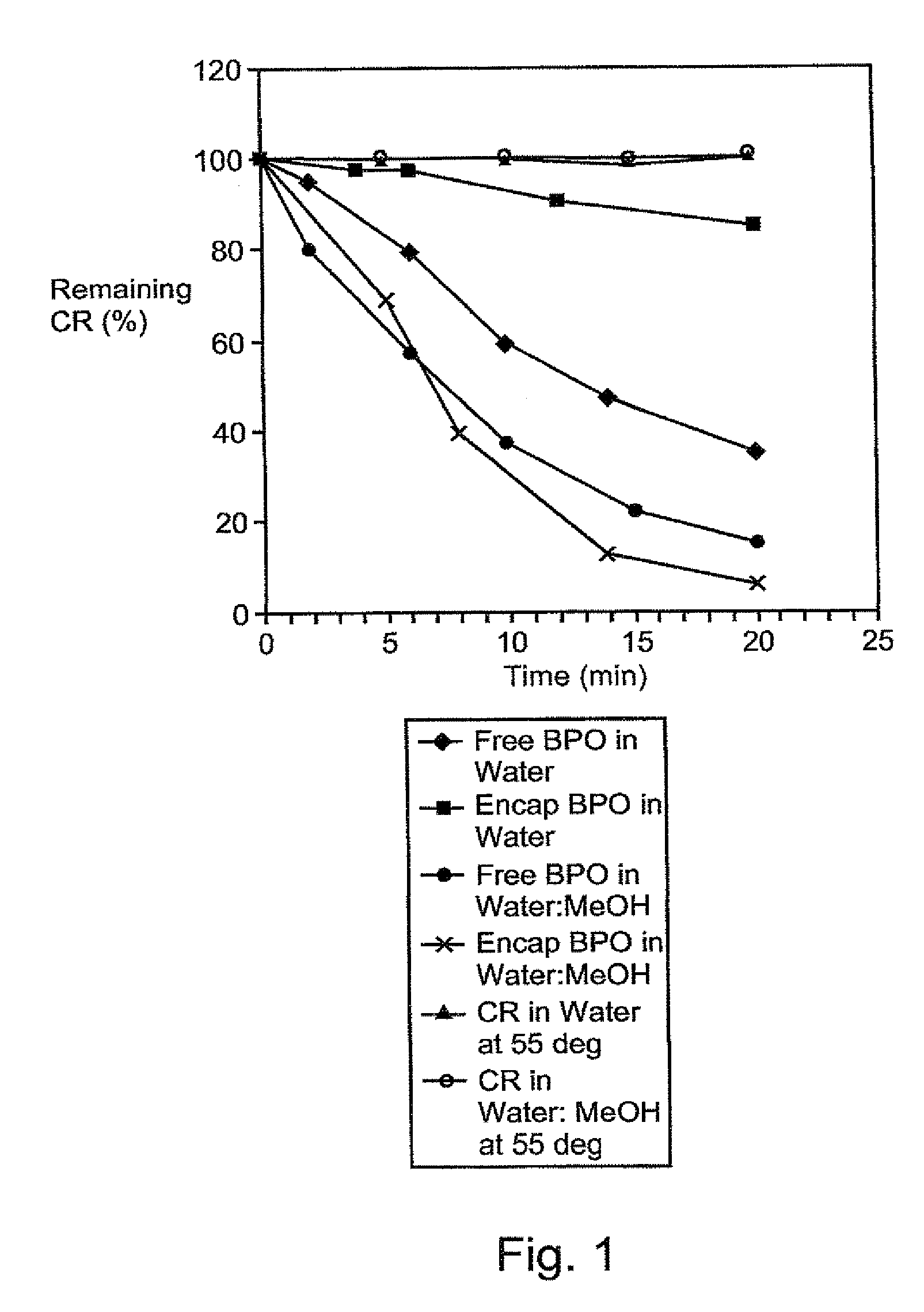
A therapeutic, cosmetic or cosmeceutic composition for topical application, capable of stabilizing an active ingredient and delivering the active ingredient, comprising a plurality of microcapsules having a core-shell structure. The microcapsules have a diameter of approximately 0.1 to 100 micron. The core of each microcapsule includes at least one active ingredient and is encapsulated within a microcapsular shell. The shell is comprised of at least one inorganic polymer obtained by a sol-gel process, and the shell protects the active ingredient before topical application and is designed to release the active ingredient from the microcapsules following application. The composition is useful in encapsulating active ingredients, such as benzoyl peroxide, that are unstable in other formulation, or are irritating to the skin.
Owner:SOL GEL TECH
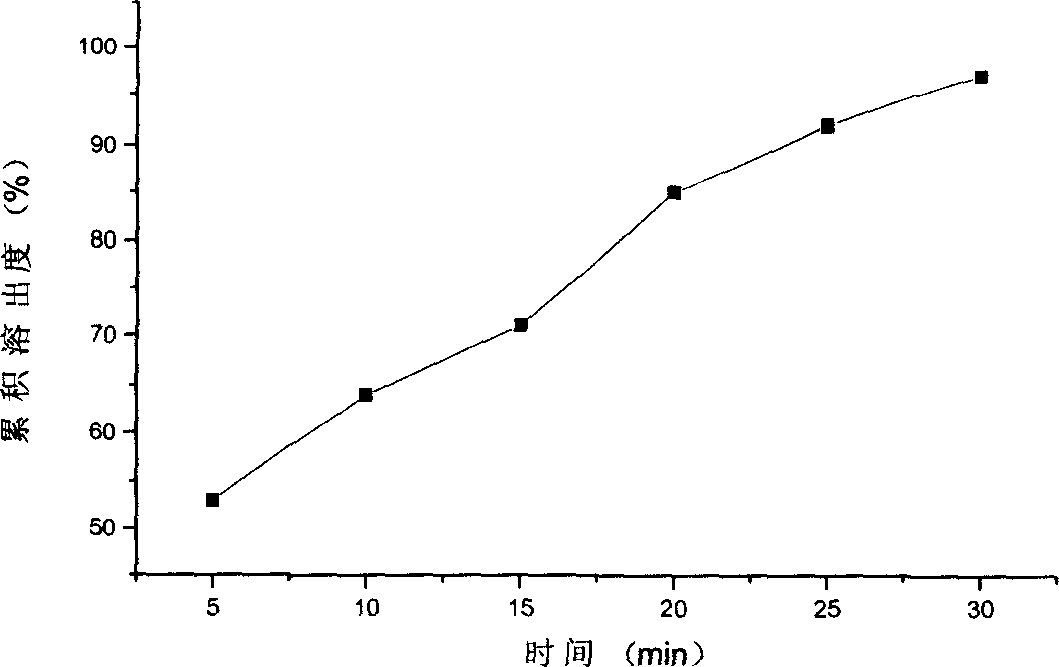
Dyer's woad root vitamin C fizzy formulation
InactiveCN101181320AHigh clarityGreat tasteOrganic active ingredientsAntiinfectivesSolubilityVitamin C
The invention belongs to the field of chemical pharmacy, and in particular relates to an effervescent preparation of isatidis vitamin C and a preparation method thereof. The invention is prepared by adding Radix Radix Radix Extract Powder and vitamin C with auxiliary materials such as acid, alkali, filler, flavoring agent and lubricant. Through optimization and screening, the present invention determines the reasonable ratio of the main drug, auxiliary materials and disintegrants. The preparation has strong stability, high effective content, short effervescent time, and the liquid medicine after effervescence has good clarity and taste. Well, the preparation process of the present invention is simple, the method is controllable, and the quality is stable. The effervescent tablet of the present invention can be compressed into a large round plain tablet or coated with a film, or compressed into a special-shaped tablet or a slow-controlled effervescent preparation. It is proved by experiments that the preparation of the invention can significantly increase the solubility of the drug, has good solubility, can be better absorbed by the human body, and has good market development prospects.
Owner:FUDAN UNIV
Composition exhibiting enhanced formulation stability and delivery of topical active ingredients
InactiveUS8039020B2Improve stabilityExtended shelf lifeCosmetic preparationsBiocideBenzoyl peroxideMedicine
A therapeutic, cosmetic or cosmeceutic composition for topical application, capable of stabilizing an active ingredient and delivering the active ingredient, comprising a plurality of microcapsules having a core-shell structure. The microcapsules have a diameter of approximately 0.1 to 100 micron. The core of each microcapsule includes at least one active ingredient, and is encapsulated within a microcapsular shell. The shell is comprised of at least one inorganic polymer obtained by a sol-gel process, and the shell protects the active ingredient before topical application and is designed to release the active ingredient from the microcapsules following application. The composition is useful in encapsulating active ingredients, such as benzoyl peroxide, that are unstable in other formulation, or are irritating to the skin.
Owner:SOL GEL TECH
Skin preparations for external use
InactiveUS20060099161A1Superb effectAvoid decompositionBiocideCosmetic preparationsStainingSkin treatments
A skin treatment composition comprising anti-bacterial zeolite and trisalt ethylenediaminehydroxyethyl triacetate. A skin treatment composition comprising anti-bacterial zeolite and alum and / or dried alum. A skin treatment composition comprising anti-bacterial zeolite and polyoxyethylene polyoxypropylene 2-decyltetradecyl ether. The object is to provide a skin treatment composition containing anti-bacterial zeolite that exhibits the effect of preventing discoloration of the skin treatment compositions and / or reducing the degree of discoloration. Another object is to provide a skin treatment composition that is superior in terms of formulation stability and tactile sensation during use. Yet another object is to provide a skin treatment composition having the effect of preventing the skin treatment composition from staining clothing and / or reducing the degree of such staining.
Owner:SHISEIDO CO LTD
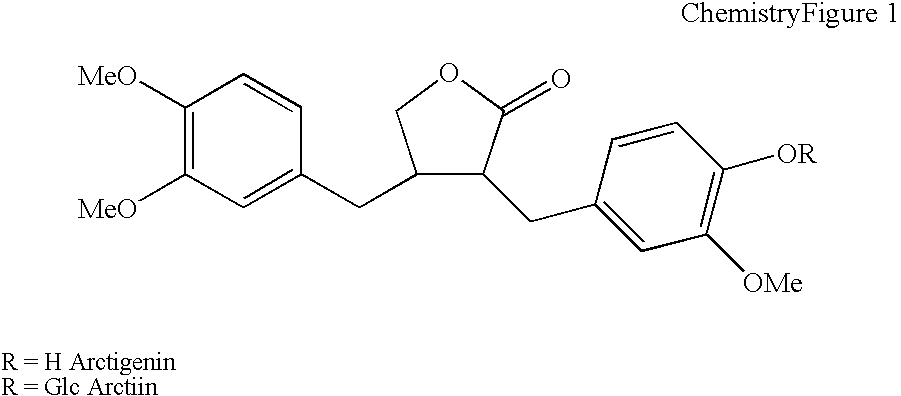
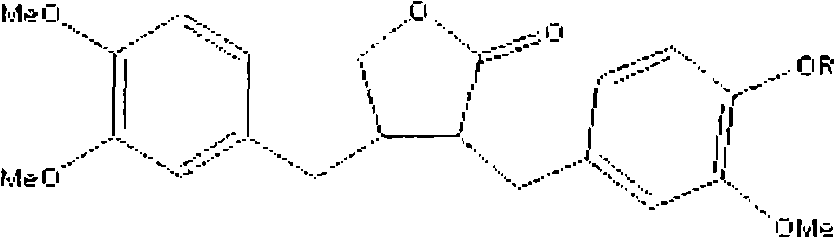
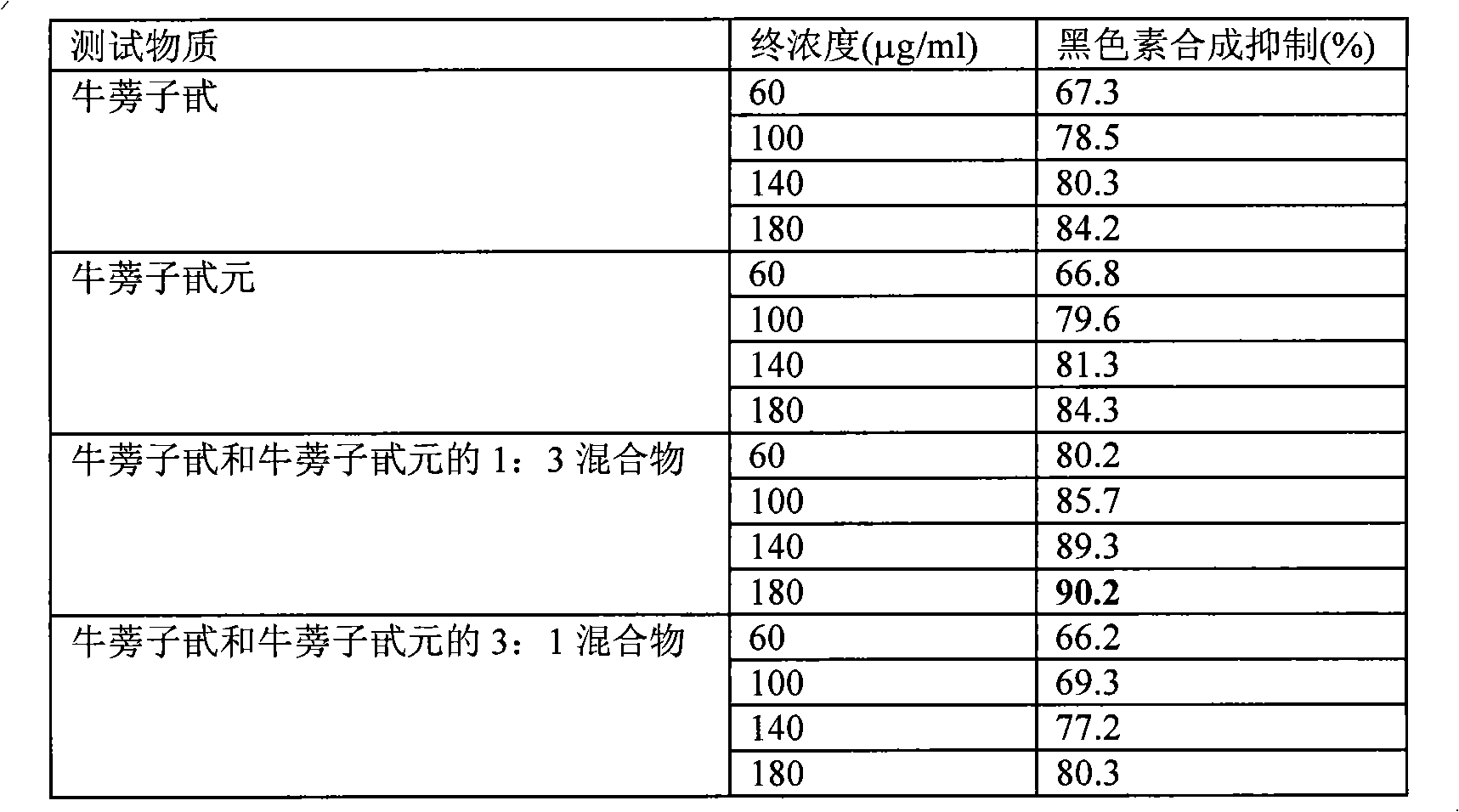
Cosmetic composition for skin whitening comprising arctiin, arctigenin or the mixture thereof as active
Present Invention relates to a skin whitening cosmetic composition comprising arctiin, arctigenin or a mixture thereof as an active ingredient. The disclosed composition can control or inhibit the synthesis of melanin by acting on a melanin synthesis route of α-MSH secreted by UV, and thus show an excellent whitening effect compared to a conventional skin whitening cosmetic. Also, the composition has high formulation stability and low skin irritation.
Owner:COREANA COSMETICS
Composition exhibiting enhanced formulation stability and delivery of topical active ingredients
InactiveUS20100255107A1Improve stabilityExtended shelf lifeCosmetic preparationsBiocideBenzoyl peroxideMedicine
A therapeutic, cosmetic or cosmeceutic composition for topical application, capable of stabilizing an active ingredient and delivering the active ingredient, comprising a plurality of microcapsules having a core-shell structure. The microcapsules have a diameter of approximately 0.1 to 100 micron. The core of each microcapsule includes at least one active ingredient, and is encapsulated within a microcapsular shell. The shell is comprised of at least one inorganic polymer obtained by a sol-gel process, and the shell protects the active ingredient before topical application and is designed to release the active ingredient from the microcapsules following application. The composition is useful in encapsulating active ingredients, such as benzoyl peroxide, that are unstable in other formulation, or are irritating to the skin.
Owner:SOL GEL TECH
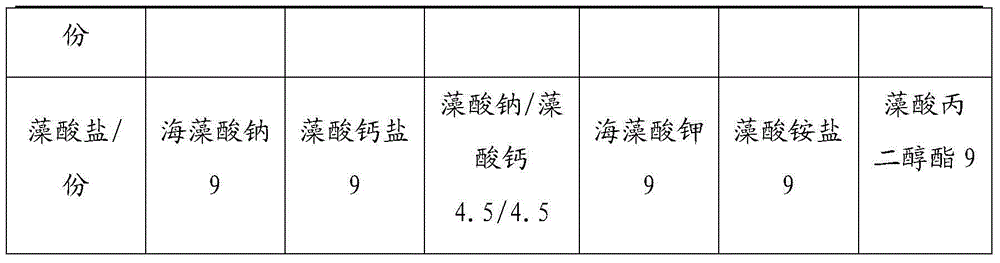
Alginate dressing containing composite silver-zinc antibacterial agents and preparation method thereof
ActiveCN104940980ALow toxicityGood biocompatibilityArtificial thread manufacturing machinesAbsorbent padsTreatment effectBiocompatibility Testing
The invention discloses an alginate dressing containing composite silver-zinc antibacterial agents. The alginate dressing is prepared by the following raw materials through spinning, wherein the raw materials comprise, by weight, 0.4-1.4 part composite silver-zinc antibacterial agents, 84.6-95.6 parts of purified water and 4-14 parts of alginate. The composite silver-zinc antibacterial agents applied to the alginate dressing has the advantages that the dispensation is good in stability, and the alginate dressing is low in toxicity and free of stimulation and sensitization, has good biocompatibility and high moisture absorption performance, and is easy to remove, high in oxygen permeation, biodegradability and biocompatibility, high in antibacterial sterilization efficiency, and good in treatment effect. The composite silver-zinc antibacterial agents are added to the alginate dressing, the composite silver-zinc antibacterial agents contain two antimicrobial components of nano-silver and nano-zinc, the preparation of the two antimicrobial components is in a certain range, the antibacterial sterilization efficiency is high, and the treatment effect is good.
Owner:SHENZHEN TSINGHUA YUANXING NANO MATERIAL CO LTD
Methods of evaluating protein formulation stability and surfactant-stabilized insulin formulations derived therefrom
InactiveUS20030054979A1Improve physical stabilityReliable timePeptide/protein ingredientsMicrobiological testing/measurementCell AggregationsProtein aggregation
Embodiments of the invention are directed to a method of estimating the physical stability of a protein formulation. A particular embodiment of the invention places the protein formulation under an agitational stress that causes the protein to aggregate at an accelerated rate. In one embodiment, the change in protein aggregation is monitored spectroscopically using Thioflavin-T. Embodiments of the invention then utilize a survival curve analysis to ascertain the relative physical stability of the different protein formulations under study. This method was used to develop novel surfactant-stabilized insulin formulations in a rapid, cost efficient manner, thus illustrating the utility of the inventive method to the discovery and development of pharmaceutical protein formulations.
Owner:MEDTRONIC MIMIMED INC
Crystallized coating
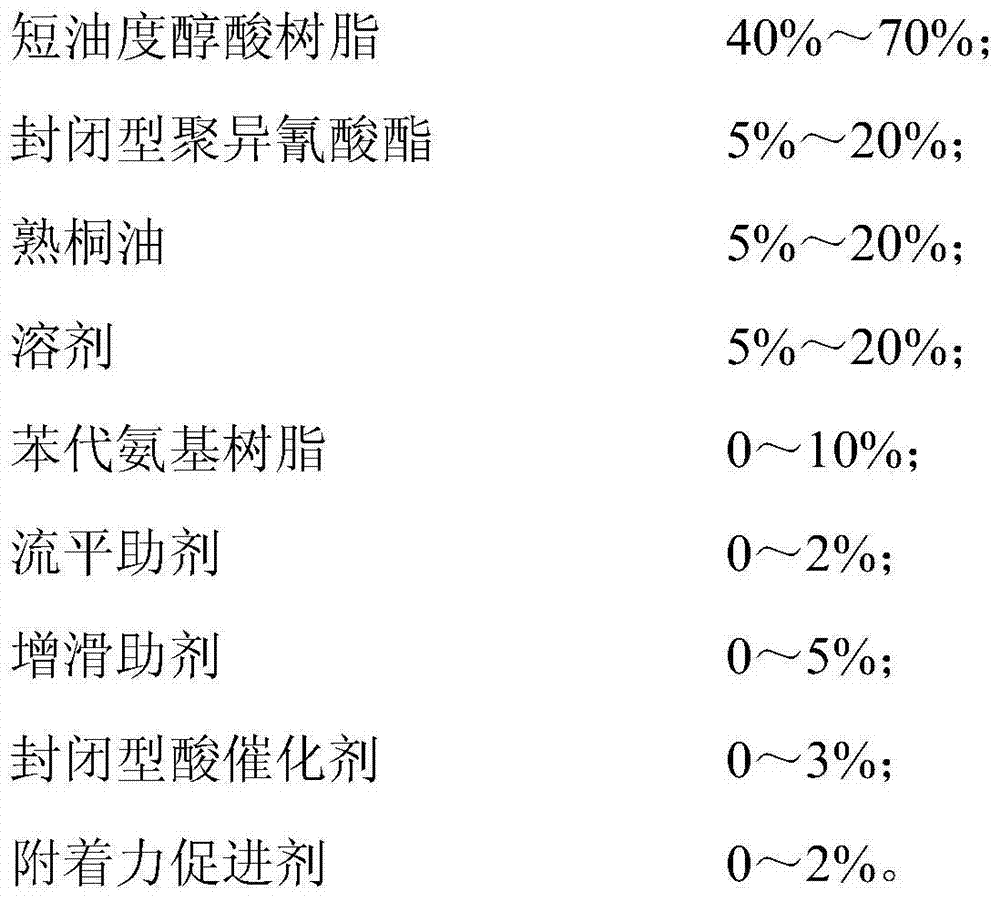
The invention belongs to the technical field of coatings and in particular relates to an environment-friendly high-performance crystallized coating. The crystallized coating comprises the following components in percentage by mass: 40-70 percent of a short oil alkyd resin, 5-20 percent of closed polyisocyanate, 5-20 percent of boiled tung oil, 5-20 percent of a solvent, 0-10 percent of a phenylated amino resin, 0-2 percent of a leveling agent, 0-5 percent of a slip additive, 0-3 percent of a closed acid catalyst and 0-2 percent of an adhesion promoter. Compared with the prior art, the crystallized coating disclosed by the invention has the advantages that the formula stability is high, and the storage period is long; the coating does not yellow when baked at a high temperature; the coating is environment-friendly and does not contain cobalt, manganese or other limited driers, the odor is small in the construction process, environment friendliness is realized, and the environment-friendly requirement is met; and moreover, coating in a roller painting mode can be realized, the loss is low, and the production efficiency can be greatly improved.
Owner:DONGGUAN CITY XINWEI ENVIRONMENTAL PROTECTION TECH CO LTD
Skin external preparations and cosmetics
InactiveUS20100280111A1Improve homogeneityImprove stabilityOrganic active ingredientsCosmetic preparationsMedicineAcyl group
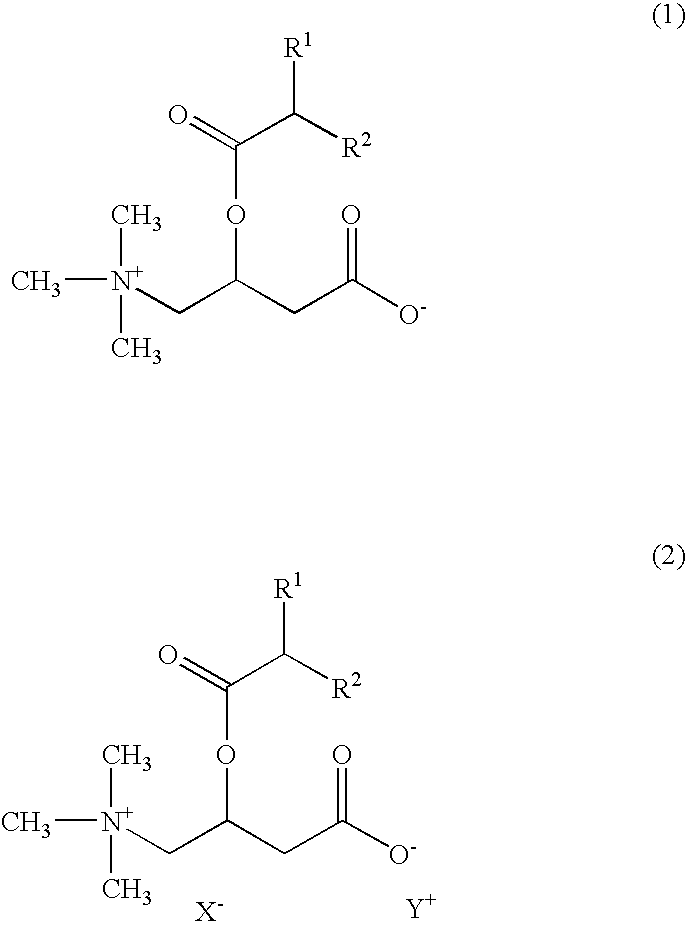
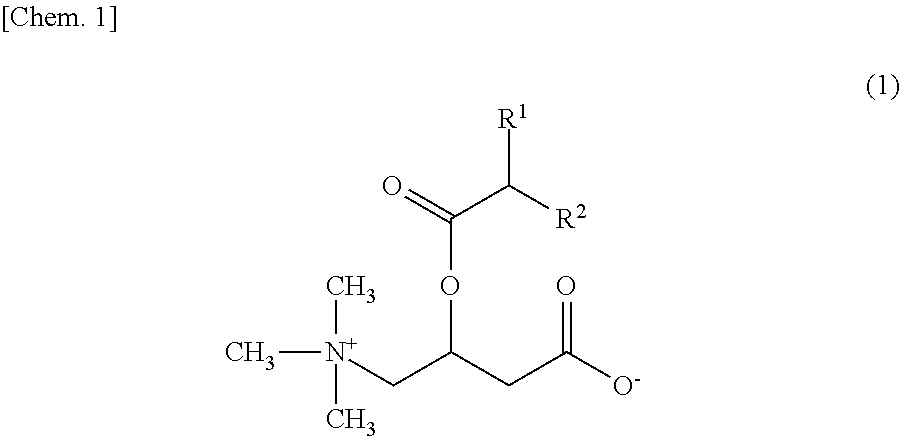
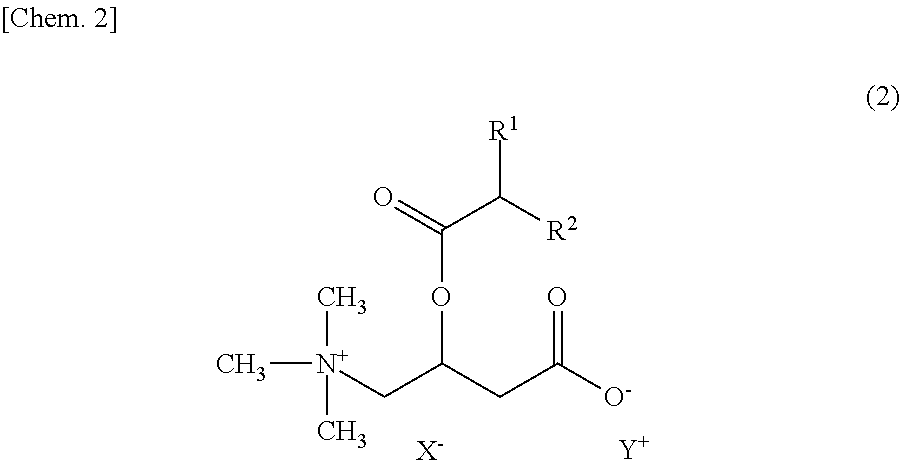
An object of the present invention is to provide skin external preparations and cosmetics which contain a branched acyl carnitine and have excellent formulation stability. A skin external preparation of the present invention includes a carnitine derivative represented by the following Formula (1) and / or a carnitine derivative salt represented by the following Formula (2), and an amphoteric surfactant.In Formula (1), R1 and R2 are each independently a C1-18 optionally branched, saturated or unsaturated aliphatic hydrocarbon group. In Formula (2), R1 and R2 are the same as in Formula (1), X− is a specific anion and Y+ is a specific cation.
Owner:SHOWA DENKO KK
Low streak degreasing composition
ActiveUS8980818B2Inorganic/elemental detergent compounding agentsMaterial nanotechnologyInorganic particleInorganic particles
The present invention relates to an aqueous composition comprising three components. The first component is a primary detergent, non-ionic surfactant with a critical packing parameters (CPP) of ≧0.95. The second component is a inorganic particle whose surface has been modified to improve stability. The third necessary component is a secondary surfactant with a CPP of ≦0.85. The secondary surfactant can function to improve the overall cleaning performance, the streaking performance and provide overall formulation stability.
Owner:AKZO NOBEL CHEM INT BV
Surfactant formulation for release of underground fossil fluids
The present disclosure provides a surfactant formulation for use in treating and recovering fossil fluid from a subterranean formation. The surfactant formulation includes a primary surfactant, a formulation stability agent and injection water. The surfactant formulation may be injected into one or more injection wells located within the subterranean formation and fossil fluids can then be subsequently recovered from one or more producing wells.
Owner:INDORAMA VENTURES OXIDES LLC
Dyes for analysis of protein aggregation
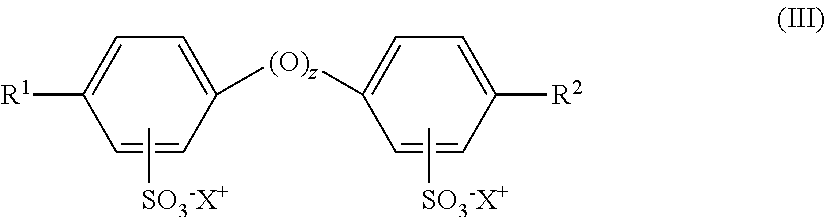
InactiveUS20130137112A1Methine/polymethine dyesPeptide preparation methodsCell AggregationsProtein aggregation
Provided are dyes and compositions which are useful in a number of applications, such as the detection and monitoring protein aggregation, kinetic studies of protein aggregation, neurofibrillary plaques analysis, evaluation of protein formulation stability, and analysis of molecular chaperone activity.
Owner:ENZO LIFE SCI INC
Composite single-agent-type hair dye
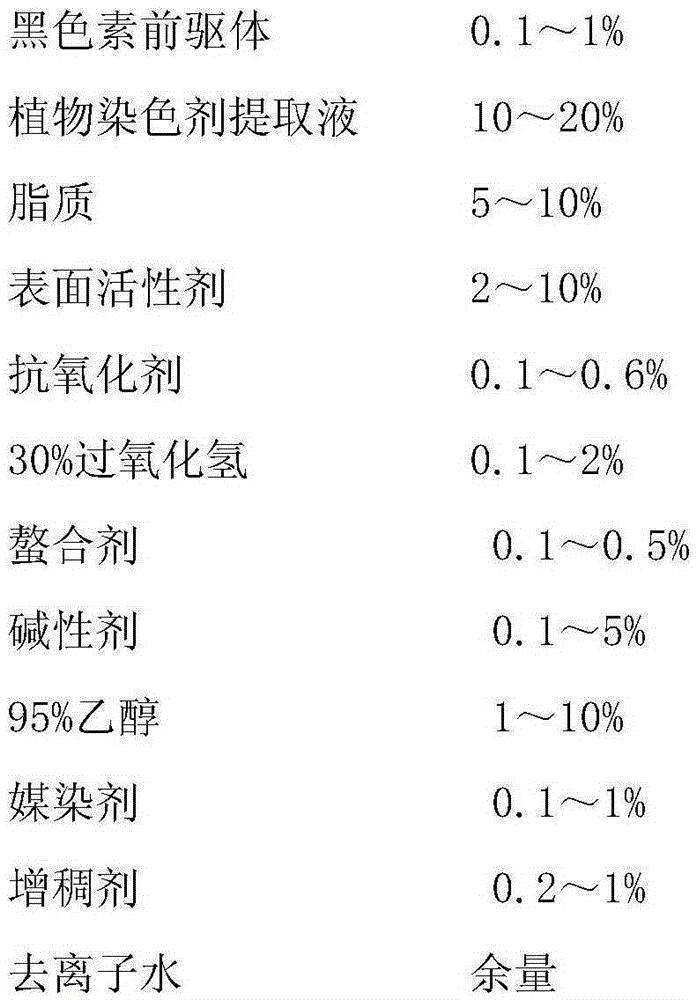
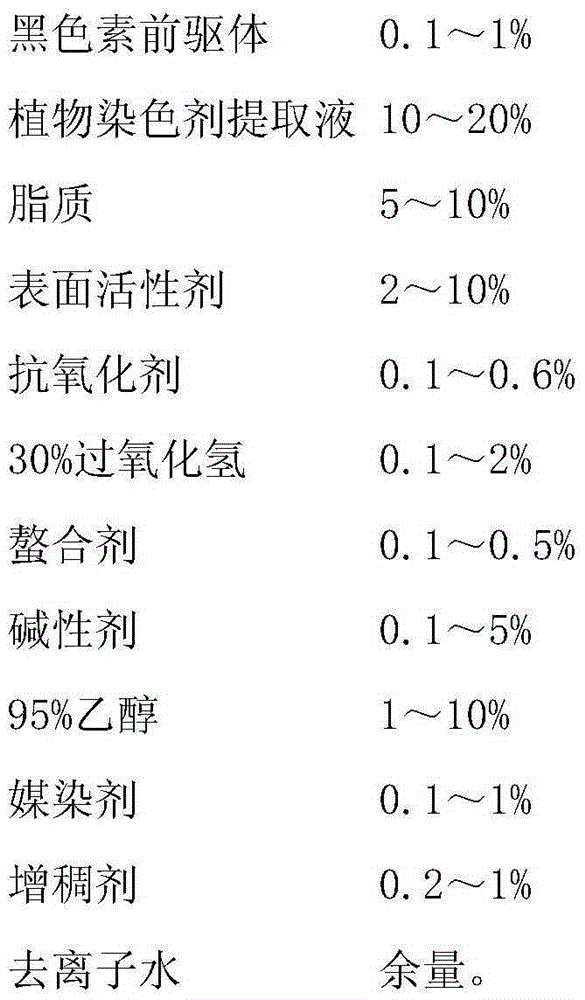
InactiveCN105380892ASimple recipeRaw material safetyCosmetic preparationsHair cosmeticsHair dyesCarboxylic acid
The invention relates to the field of special cosmetics, in particular to a single-agent-type hair dye. An adopted dyeing agent is prepared from a plant dyeing agent and a melanin precursor. The melanin precursor is prepared from 5,6-dihydroxyindole, 5,6-dihydroxyindole-2-carboxylic acid, 5,6-dihydroxyindoline and 5,6-dihydroxyindoline-2-carboxylic acid. The plant dyeing agent is an extracting solution of gallnuts, mulberries, black sesame, polygonum multiflorum, Chinese herbaceous peonies and lotus roots. According to the prepared composite single-agent-type hair dye, the formula is simple, the raw materials are safe, the stability of the formula is high, and the composite single-agent-type hair dye has the hair caring effect and the hair protection effect, and also has the advantages of being good in hair dyeing effect and high in dyeing performance.
Owner:济南益豪环保技术有限公司
High-Purity Monoalkenyl-Containing Glycerin Derivative, And Method For Producing Same
ActiveUS20160052849A1Reduce contentStable productionCosmetic preparationsOrganic compound preparationGlycerol DerivativesDistillation
The present invention relates to a monoalkenyl-containing glycerin derivative with purity of not less than 92% and electrical conductivity of not greater than 50 μS / cm. The monoalkenyl-containing glycerin derivative can be manufactured by a manufacturing method comprising a step (A): a step of reacting a ketalized glycerin derivative and a monoalkenyl glycidyl ether in the presence of an inorganic base to obtain a ketal of monoalkenyl-containing glycerin derivative; a step (B): a step of purifying the ketal of monoalkenyl-containing glycerin derivative obtained in the step (A) by distillation; and a step (C): a step of hydrolyzing the ketal of monoalkenyl-containing glycerin derivative obtained in the step (B). The present invention is able to provide a high-purity monoalkenyl-containing glycerin derivative that was difficult in the past. It is further able to provide a glycerin derivative-modified silicone, and applications therefor, that is chemically stable, and further has excellent utility for its emulsifiability, and the like, and excellent formulation stability.
Owner:DOW TORAY CO LTD
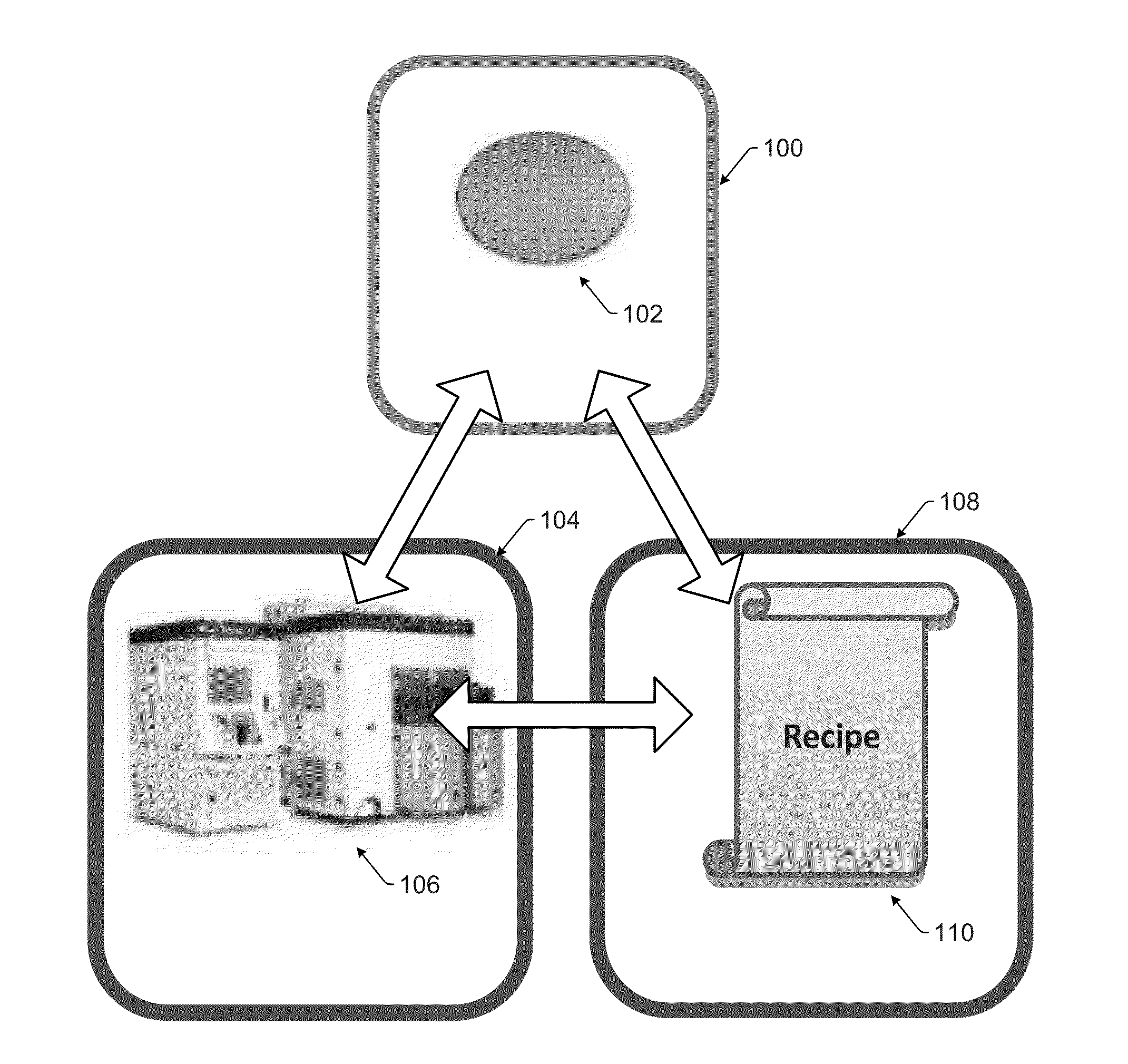
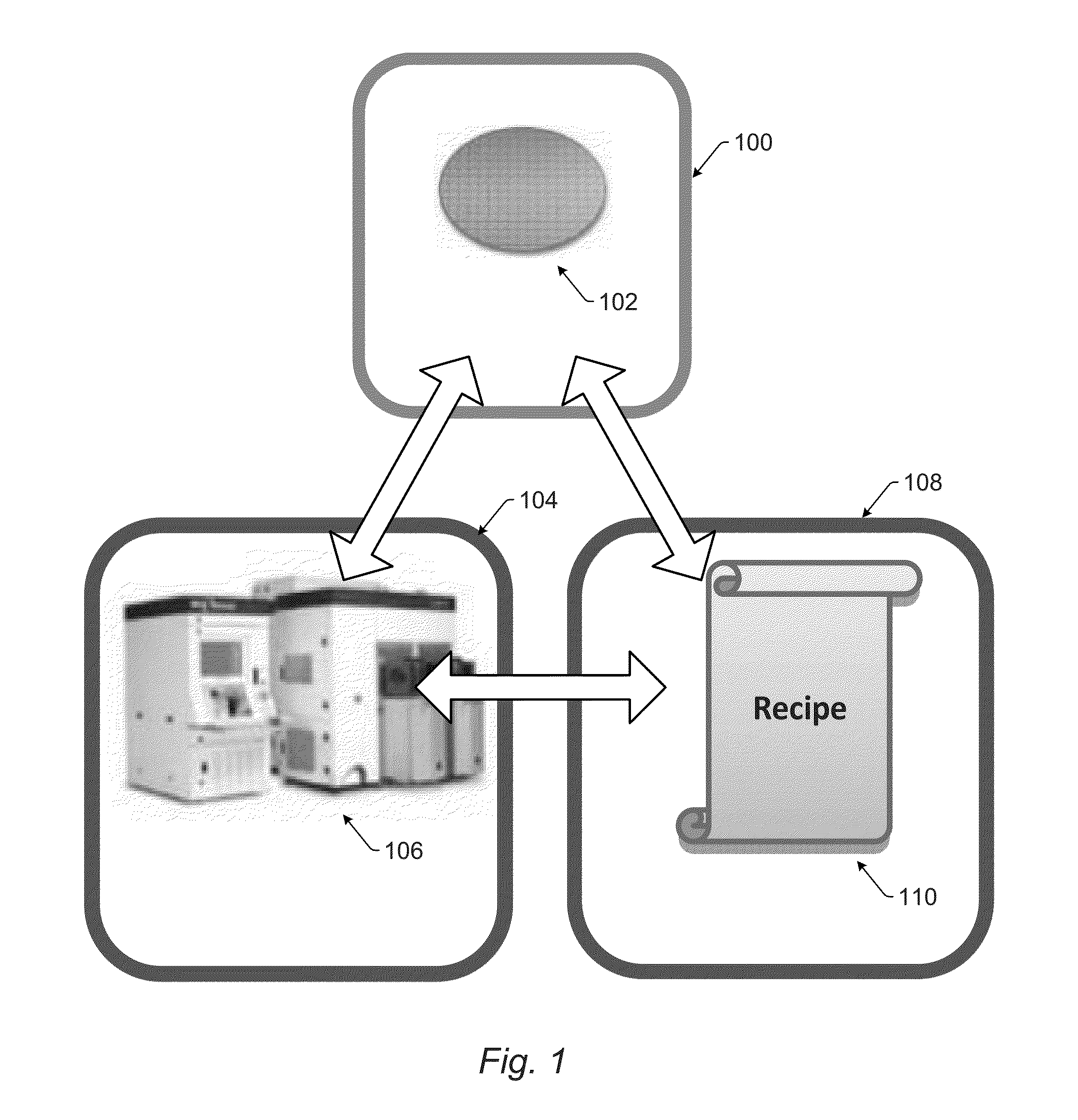
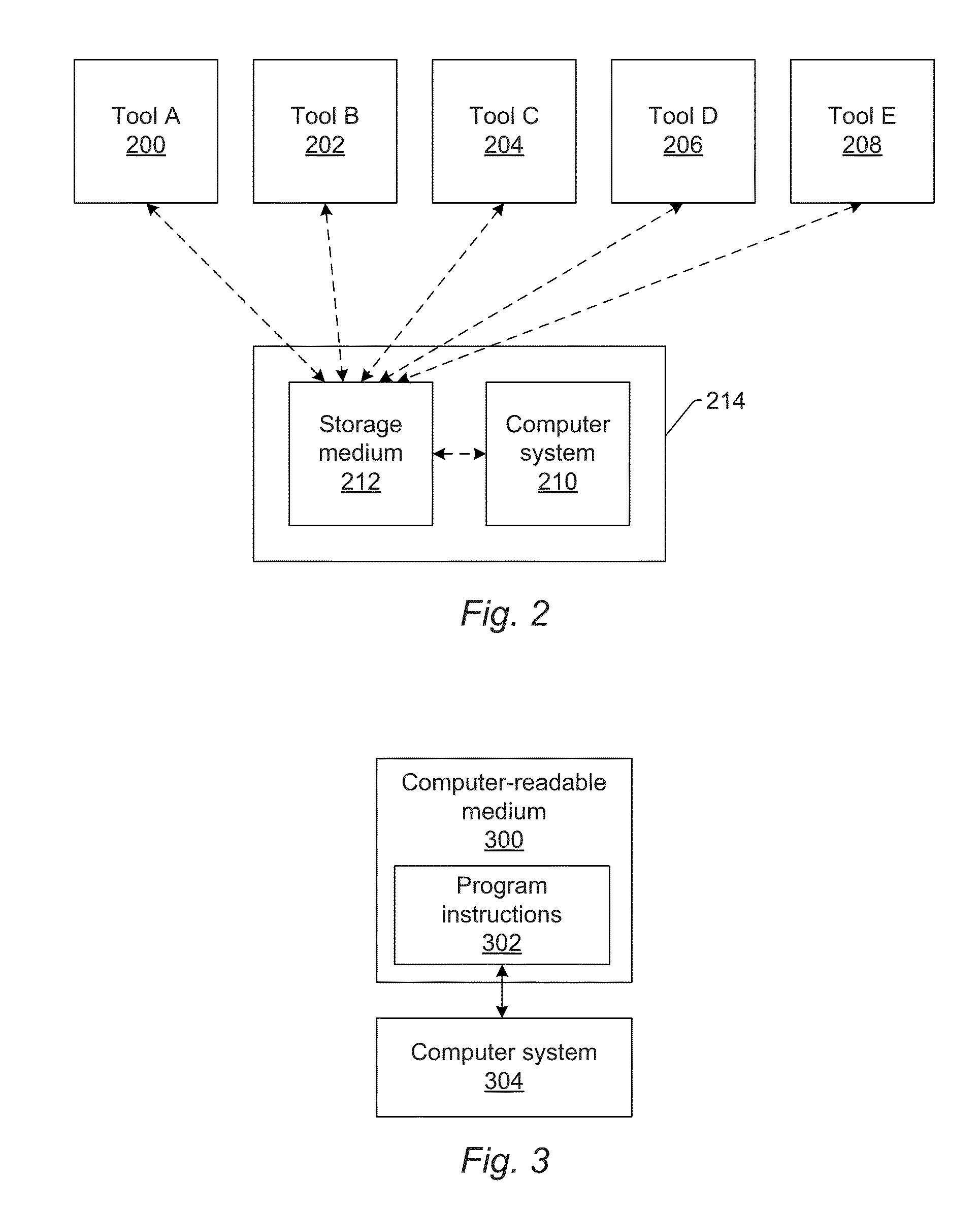
Automatic Recipe Stability Monitoring and Reporting
Systems and methods for monitoring stability of a wafer inspection recipe over time are provided. One method includes collecting inspection results over time. The inspection results are generated by at least one wafer inspection tool while performing the wafer inspection recipe on wafers at different points in time. The method also includes identifying abnormal variation in the inspection results by comparing the inspection results generated at different times to each other. In addition, the method includes determining if the abnormal variation is attributable to the wafers, the wafer inspection recipe, or one or more of the at least one wafer inspection tool thereby determining if the wafer inspection recipe is stable over time.
Owner:KLA TENCOR TECH CORP
Cosmetic composition for skin whitening comprising arctiin, arctigenin or the mixture thereof as active ingredient
Present Invention relates to a skin whitening cosmetic composition comprising arctiin, arctigenin or a mixture thereof as an active ingredient. The disclosed composition can control or inhibit the synthesis of melanin by acting on a melanin synthesis route of alpha-MSH secreted by UV, and thus show an excellent whitening effect compared to a conventional skin whitening cosmetic. Also, the composition has high formulation stability and low skin irritation.
Owner:COREANA COSMETICS
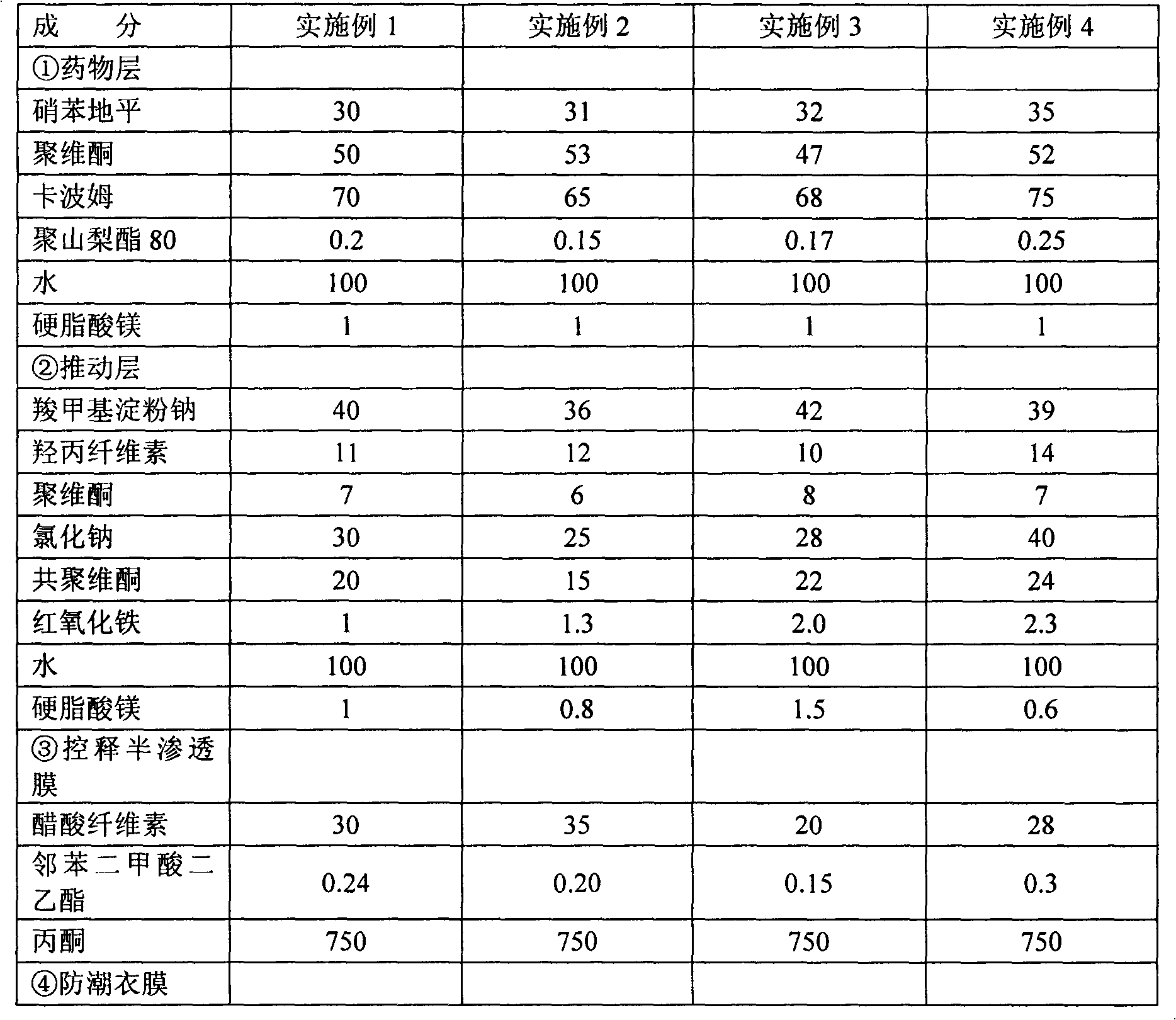
Anastrozole dispersed tablet formulation
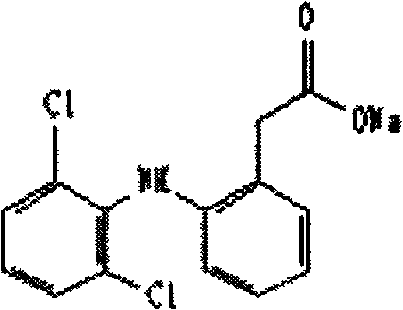
InactiveCN1634042APromote absorptionImprove Medication AdherenceOrganic active ingredientsPill deliveryClinical efficacyClinical trial
The invention belongs to medical technology, in particular to a dispersible tablet form of anastrozole. Through a large number of clinical investigations, we have overcome the technical prejudice that anastrozole does not need to be developed into dispersible tablets in the prior art, and obtained the present invention. Clinical experiments have confirmed that the adverse reactions of the present invention are significantly reduced, and the compliance of medication is significantly improved. Obtained unexpected clinical efficacy. It has the characteristics of low production cost, stable preparation, fast absorption and high bioavailability. The invention is suitable for treating advanced breast cancer of postmenopausal women, and provides a good therapeutic drug dosage form for breast cancer patients.
Owner:LUNAN PHARMA GROUP CORPORATION
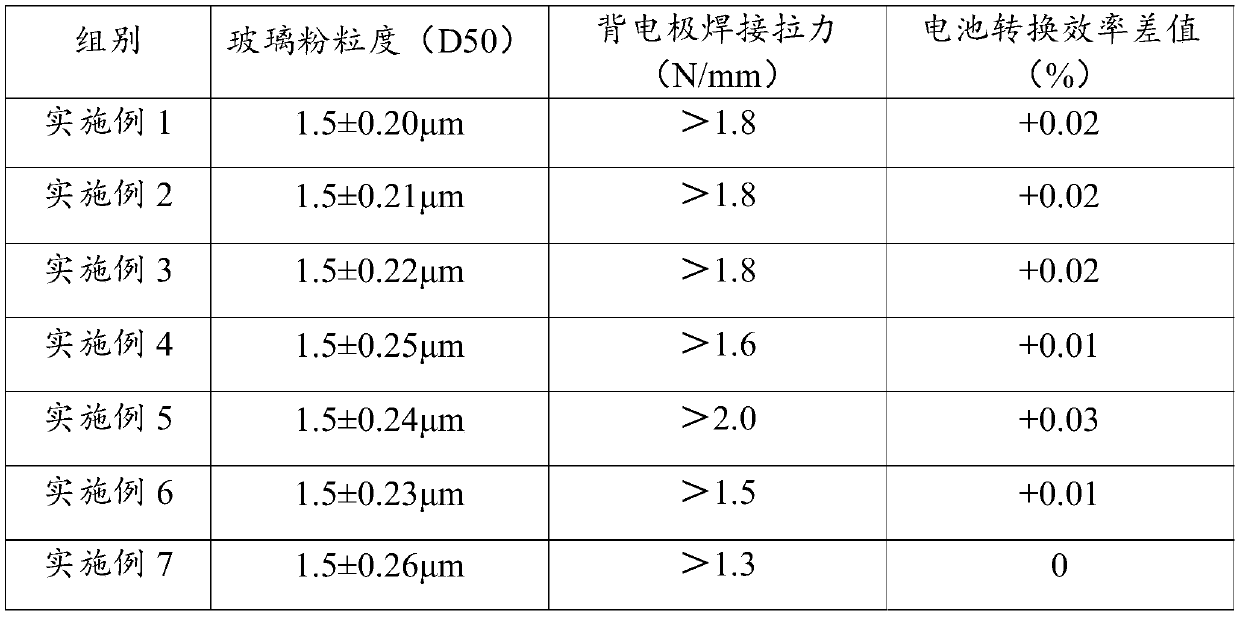
PERC battery back pole slurry and preparation method thereof
ActiveCN109785994AImprove stabilityImprove particle size uniformityNon-conductive material with dispersed conductive materialSemiconductor devicesFiller ExcipientSlurry
The invention discloses a preparation of PERC battery back pole slurry. The preparation method comprises the steps of: 1) putting tetraethyl orthosilicate, triethyl phosphate, boric acid and organic metal salt in a reaction still in proportion, adding absolute ethyl alcohol as a reaction medium, uniformly stirring the materials, adjusting the PH value of the solution to 3.5-6.5, carrying out reaction and ageing at room temperature, separating the supernatant liquid to obtain glass slurry, and drying and grinding the glass slurry to obtain glass powder; 2) adding a solvent, a thickener and an additive in the reaction still in proportion, and carrying out stirring and fusion to form a uniform organic bond; and 3) adding silver powder, a conductive filling agent, the glass powder and the organic bond in a ball milling tank in proportion; adding a ball milling medium, carrying out grinding and dispersion on a ball mill for 6-7 hours, and separating the ball milling medium to obtain the PERC battery back pole slurry. The synthesized glass powder has favorable ingredient stability and granularity uniformity. According to the preparation method, the ingredient stability of the slurry is improved and the bad influences, on the workshop environment, of organic volatile matters generated in the production process are reduced.
Owner:GUANGZHOU RUXING TECH DEV +1
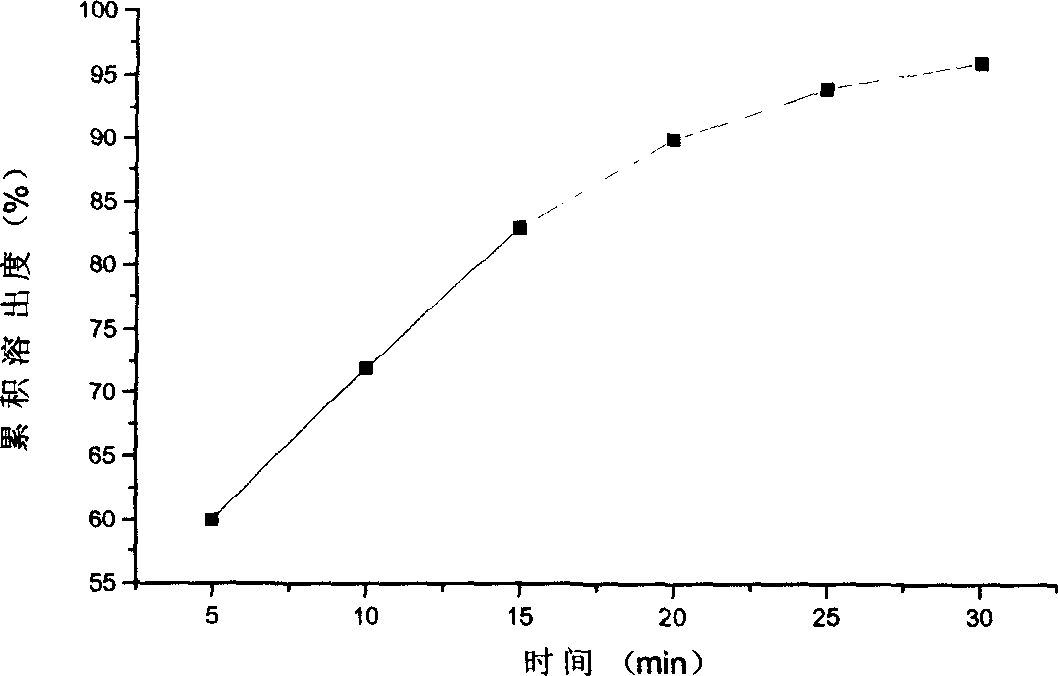
Nifedipine controlled release composition and preparation method thereof
ActiveCN102846574AImprove stabilitySimple preparation processOrganic active ingredientsPill deliveryNifedipineControlled release
The invention relates to a controlled release pharmaceutical formulation, and in particular relates to a nifedipine controlled release composition and a preparation method thereof. The nifedipine controlled release composition is simple in preparation process, low in cost, good in formulation stability and excellent release rate, can be used for effectively preventing product ageing, and is stable in release after being stored for a long time.
Owner:DEZHOU DEYAO PHARMA
A kind of skin barrier repair preparation and preparation method thereof
ActiveCN104173207BGuaranteed stabilityCosmetic preparationsToilet preparationsAdditive ingredientSkin repair
The invention discloses a skin barrier repairing preparation, which comprises the following components: deionized water, sucrose stearate, phytosterol isostearate, vitamin E, glycerin, butylene glycol, and 1,2-pentanediol , Octyldodecanol, Dimethicone, Hydrogenated Polyisobutylene, Oleic Acid, Linoleic Acid, Ceramide 2, Ceramide 3, Ceramide 6II, Sodium Hyaluronate, Xanthan Gum, Disodium EDTA , Acrylates / C10‑30 Alkyl Acrylate Crosspolymer, Allantoin, Sorbitan Caprylate. At the same time, a preparation method of the above-mentioned skin barrier repairing preparation is provided. The invention solves the dissolution problem of the compound ceramide, reduces the influence of ceramide crystallization on stability under low temperature conditions, improves the stability performance of the preparation, and can add other skin repairing ingredients and compound the compound ceramide. The present invention has good product formula stability performance, and simultaneously has multiple repairing effects on the skin barrier.
Owner:上海贝泰妮生物科技有限公司
Preparation method of modified resin-based engineering plastic
InactiveCN108440874AImprove flame retardant performanceHigh thermal conductivityHeat-exchange elementsCoatingsFiberAcetic anhydride
The invention discloses a preparation method of modified resin-based engineering plastic and belongs to the technical field of preparation of plastics. The preparation method of the modified resin-based engineering plastic, disclosed by the invention, has the benefits that polyvinyl chloride is mixed with polyamide resin to obtain mixed resin, the mixed resin is modified with antimony trioxide andred phosphorus, and then graphite powder is modified with a concentrated sulfuric acid solution, and is blended with acetic anhydride and potassium permanganate, and thus, expanded graphite powder isprepared at high temperature; the expanded graphite powder is a carbon source in an expansion system, also is a heat insulating layer and can exert an obvious heat insulating effect; steel fibers areadded in a resin base material, interlaced resin base material molecular chains are connected among the steel fibers, and the steel fibers are taken as a filler to modify engineering plastic; a layerof coconut oil is continuously smeared in an engineering plastic blank, so that the further corrosion is prevented, and the service life of the engineering plastic is prolonged; and a formula is goodin stability and has a long-lasting corrosion inhibition ability, thereby improving the corrosion resistance of the engineering plastic and having a wide application prospect.
Owner:FOSHAN JIUBAI TECH INFORMATION CONSULTATION CO LTD
Hair dye
InactiveUS20130232701A1Improve dyeing effectLess-uneven dyeingCosmetic preparationsHair cosmeticsHair dyesIron salts
It is an object of the present invention to provide a safe hair dye, which has a good dyeing property and is easy-to-use, and which has less uneven dyeing and has high stability of the formulation. The present invention provides a hair dye which comprises a combination of (1) a first gel agent containing tannic acid, benzyl alcohol and xanthane gum, and (2) a second agent containing an iron salt.
Owner:FUJIFILM CORP
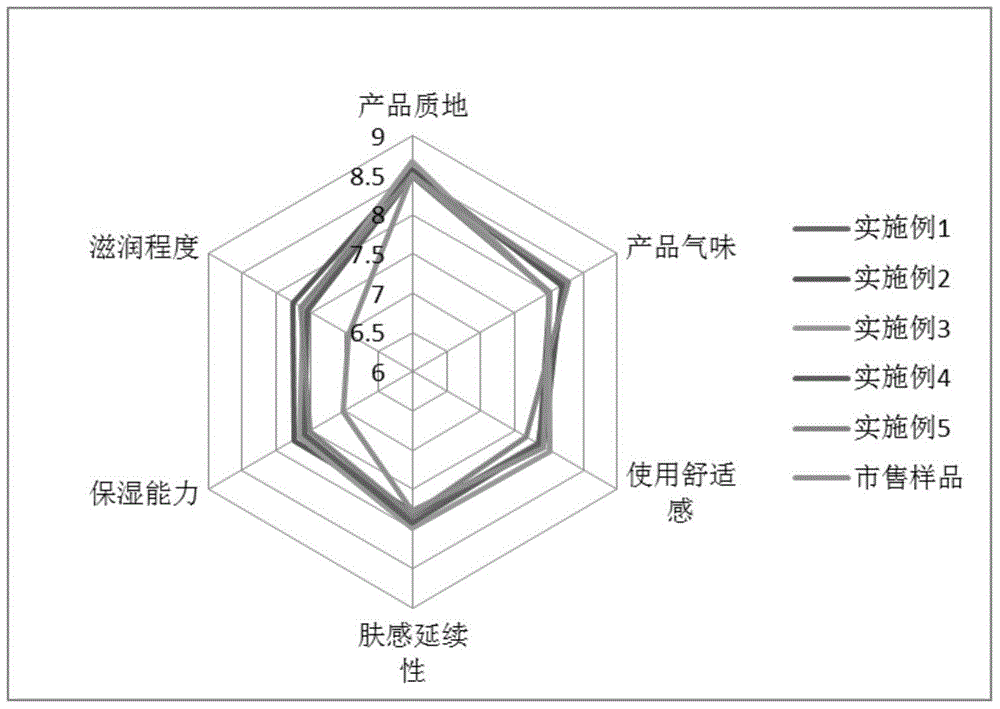
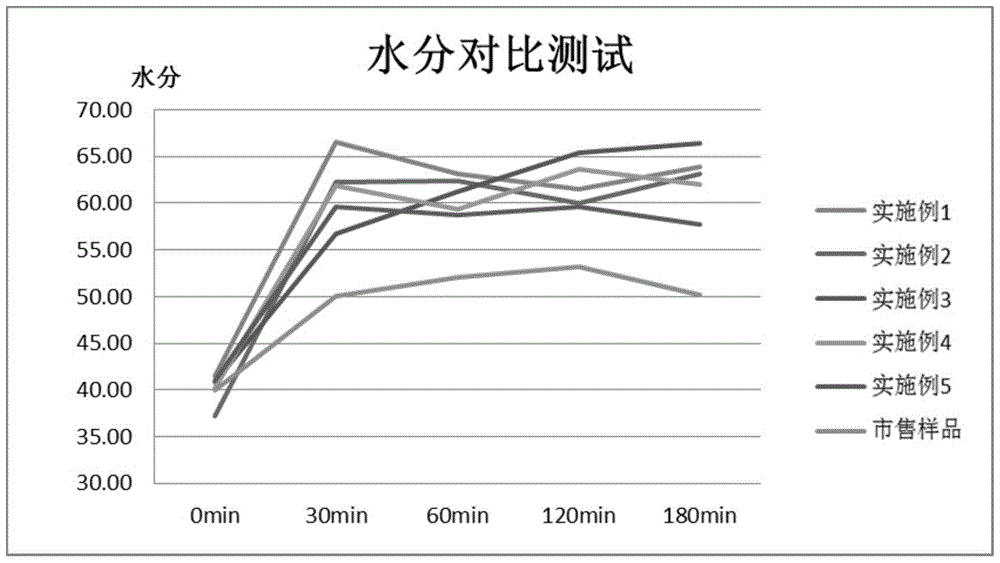
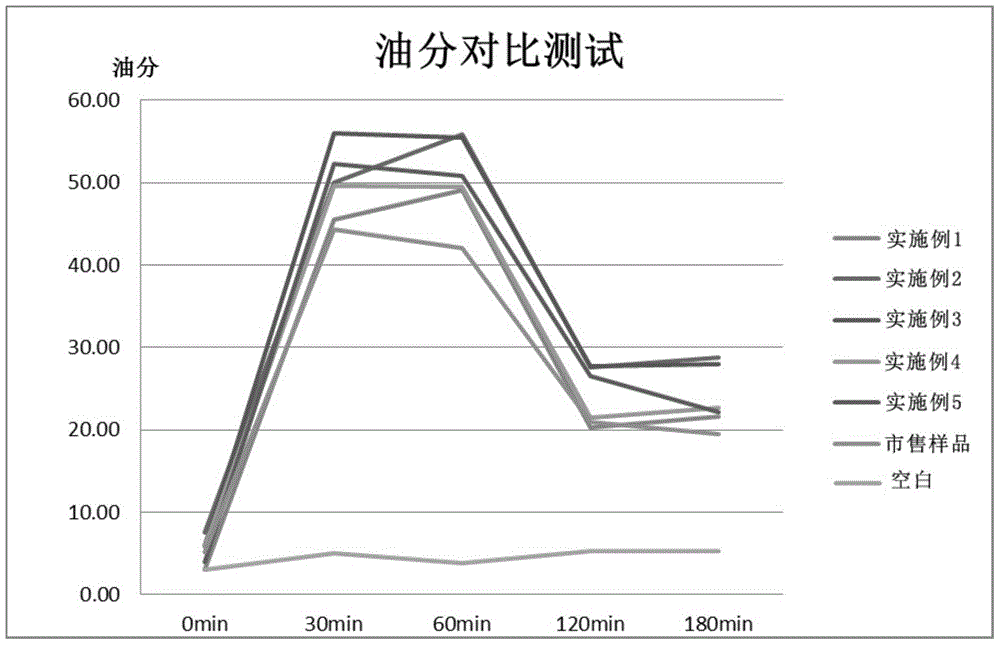
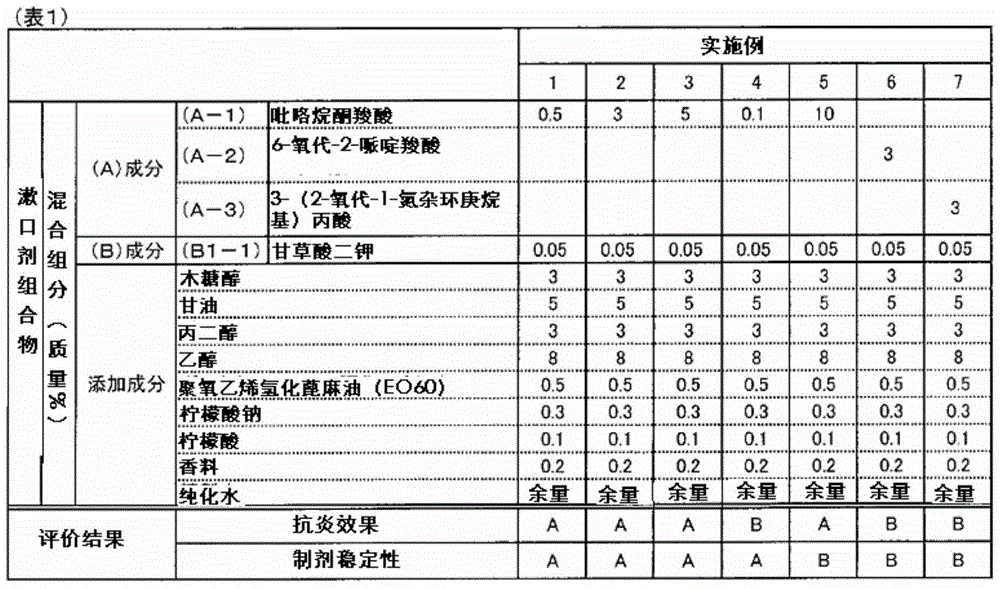
Composition for oral cavity
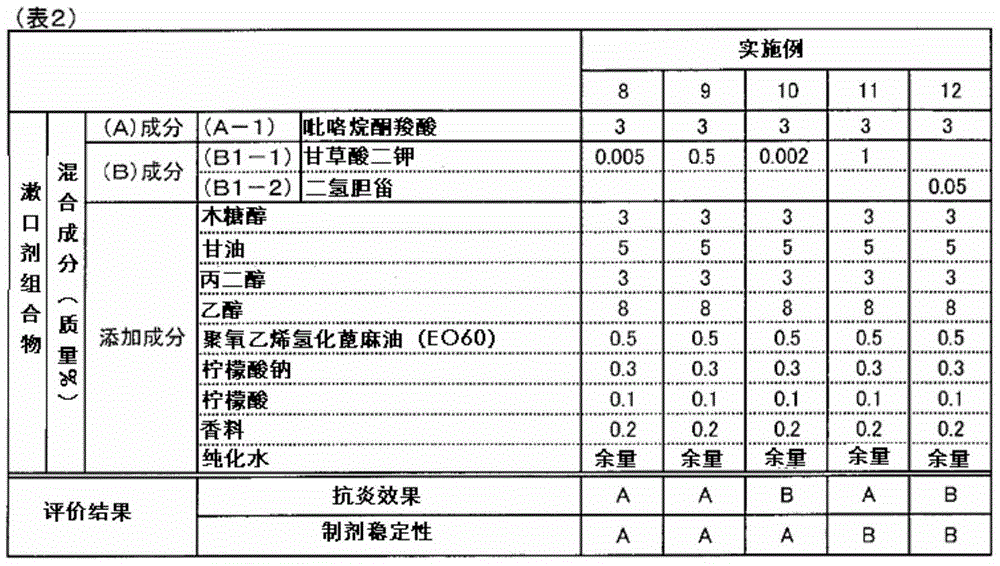
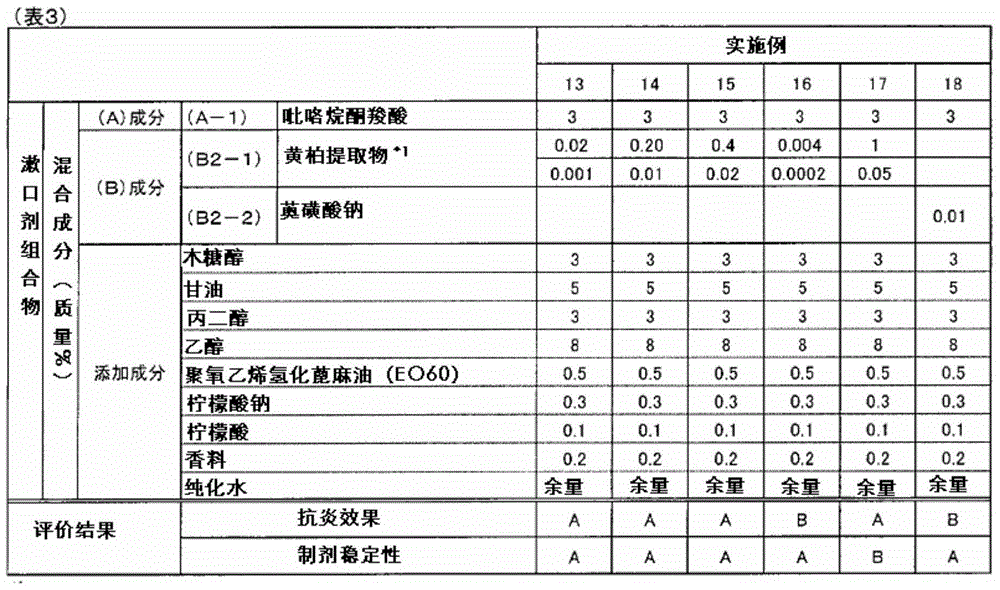
ActiveCN105142607AGood anti-inflammatory effectImprove stabilityCosmetic preparationsToilet preparationsAnti-inflammatoryFormulation stability
Owner:LION CORP
Metal cooling fluid
InactiveCN106833548AGood formulation stabilityImprove cooling effectHeat-exchange elementsHazardous substanceCooling effect
The invention discloses a metal cooling fluid, which is prepared from the following raw materials in parts by weight: 20 to 30 parts of propylene glycol, 20 to 30 parts of sorbitol, 8 to 12 parts of a sebacic acid, 4 to 8 parts of a fatty acid, 4 to 6 parts of a boric acid, 4 to 6 parts of borax, 1 to 2 parts of an azole compound, 0.8 to 1.8 parts of styrene, 1 to 1.5 parts of sodium sulfate and a proper amount of water. The metal cooling fluid does not contain any substance that being toxic and harmful to a human body, is high in formula stability and is recyclable, and has a fast cooling effect.
Owner:安徽省东至县东鑫冲压件有限责任公司
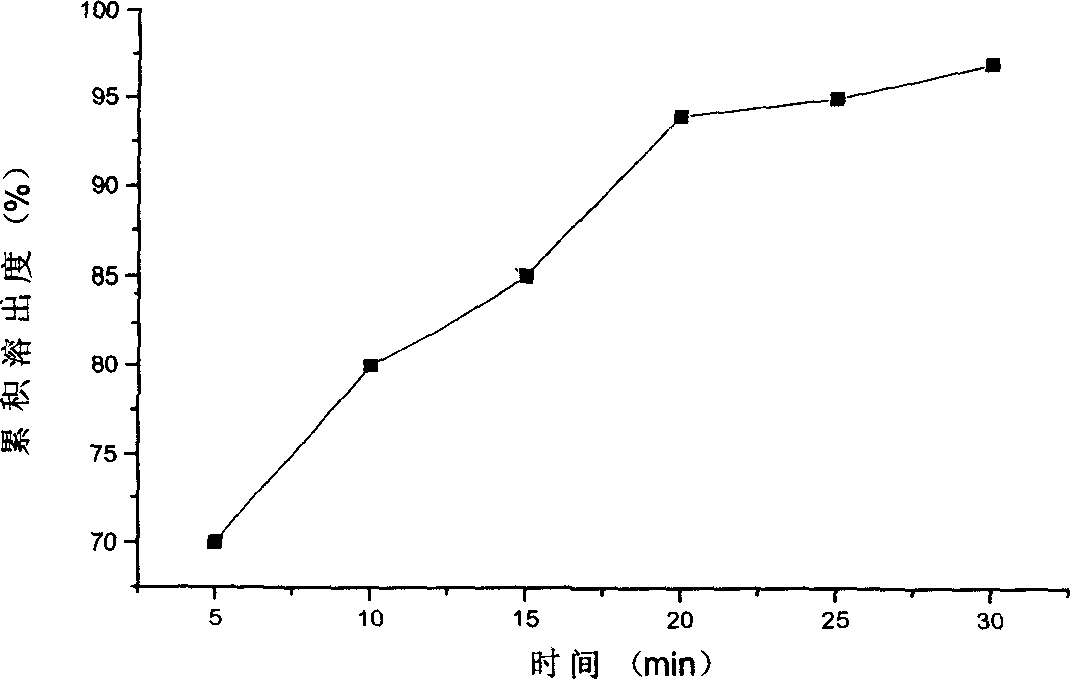
Diclofenac sodium sustained release tablet and preparation method thereof
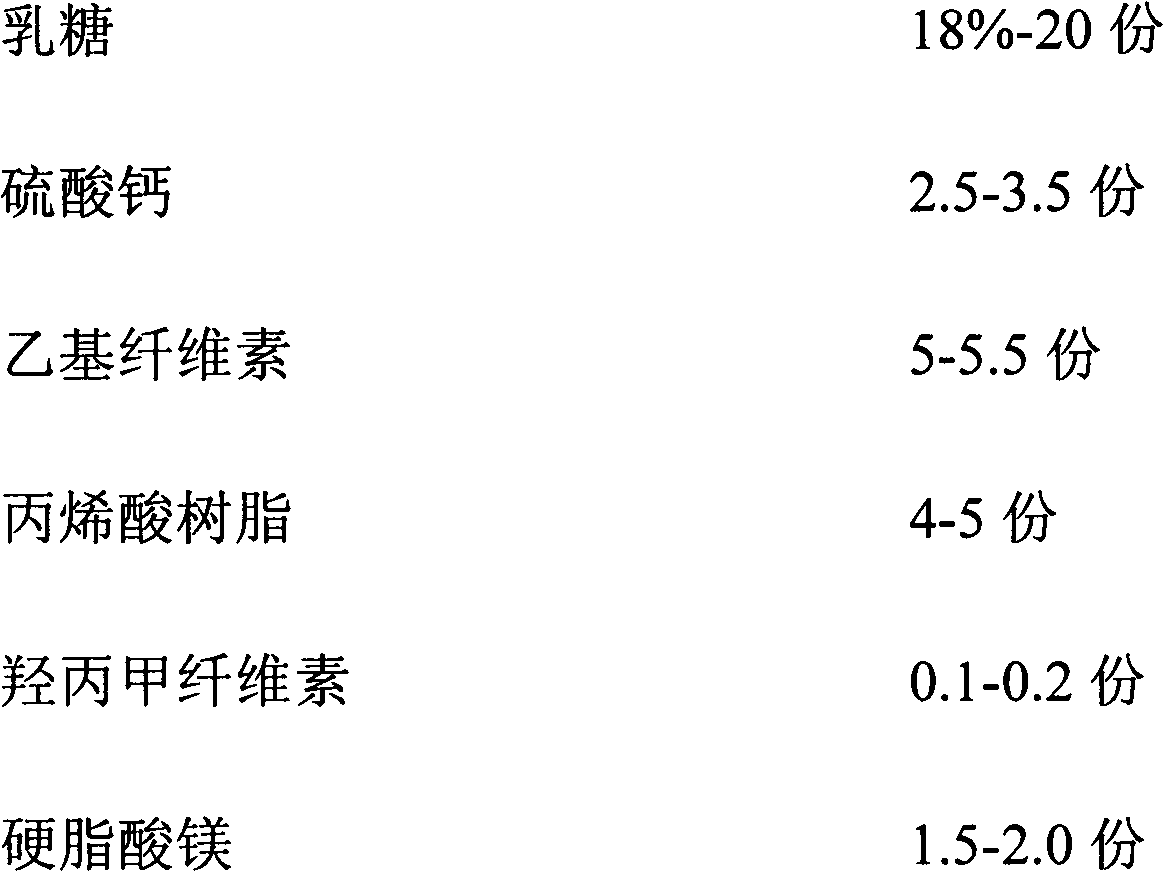
ActiveCN102846572AImprove stabilityClear release curveOrganic active ingredientsAntipyreticSustained Release TabletAcrylic resin
The invention relates to a sustained release pharmaceutical formulation, and in particular relates to a diclofenac sodium sustained release tablet and a preparation method thereof. The diclofenac sodium sustained release tablet is prepared from diclofenac sodium, lactose, calcium sulfate, ethylcellulose, acrylic resin, hydroxypropyl methylcellulose, magnesium stearate and 95% ethanol in certain proportions. The diclofenac sodium sustained release tablet is simple in preparation process, low in cost, good in formulation stability and excellent in release rate.
Owner:DEZHOU DEYAO PHARMA
Oil-in-water form organogel cosmetic composition having variable transparency
InactiveUS20150305990A1Improve stabilitySure easyCosmetic preparationsBiocideRefractive indexOil phase
There is provided an oil-in-water type organogel cosmetic composition having variable transparency, including 0.05% by weight to 50.00% by weight of an oil phase portion, and 50% by weight to 99.95% by weight of a water phase portion on the basis of total weight, wherein the oil phase portion and the water phase portion has a difference in refractive indices within a range of 0.03 to 0.16. The organogel cosmetic composition may be usefully utilized because the composition not only has high formulation stability according to temperature and time, but also enables an optimal application time to be easily determined with the naked eye by controlling the contents of components contained in the water phase portion and the oil phase portion in such a manner that the color of the composition may be changed from opaque ivory white color to transparent color when the composition is applied to skin.
Owner:GENIC
Composition containing extract derived from green tea leafstalk for skin whitening
InactiveCN106456521AGood whitening effectImprove dimCosmetic preparationsToilet preparationsMedicineSkin color
The present invention relates to a composition containing an extract derived from green tea leafstalks for skin whitening, wherein the composition is safe for the skin and simultaneously has excellent skin-whitening efficacy and high stability. Since the present invention uses green tea leafstalks, which are typically not used and discarded, the present invention has excellent value in terms of using waste resources. In addition, by containing the extract derived from green tea leafstalks, the composition of the present invention is safe for the skin, has high formulation stability, shows an excellent skin-whitening effect which reduces symptoms of pigmentation such as melasma and freckles, and reduces skin darkness and therefore leads to even and bright skin color.
Owner:AMOREPACIFIC CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com