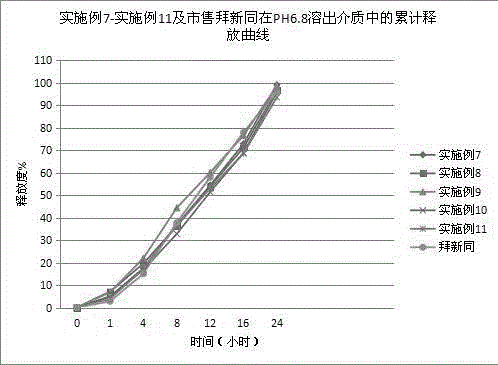
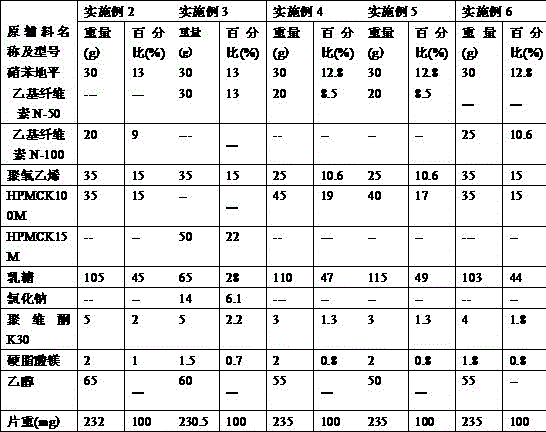
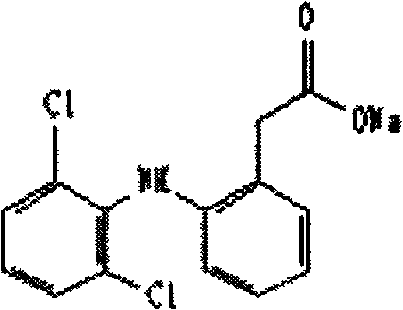
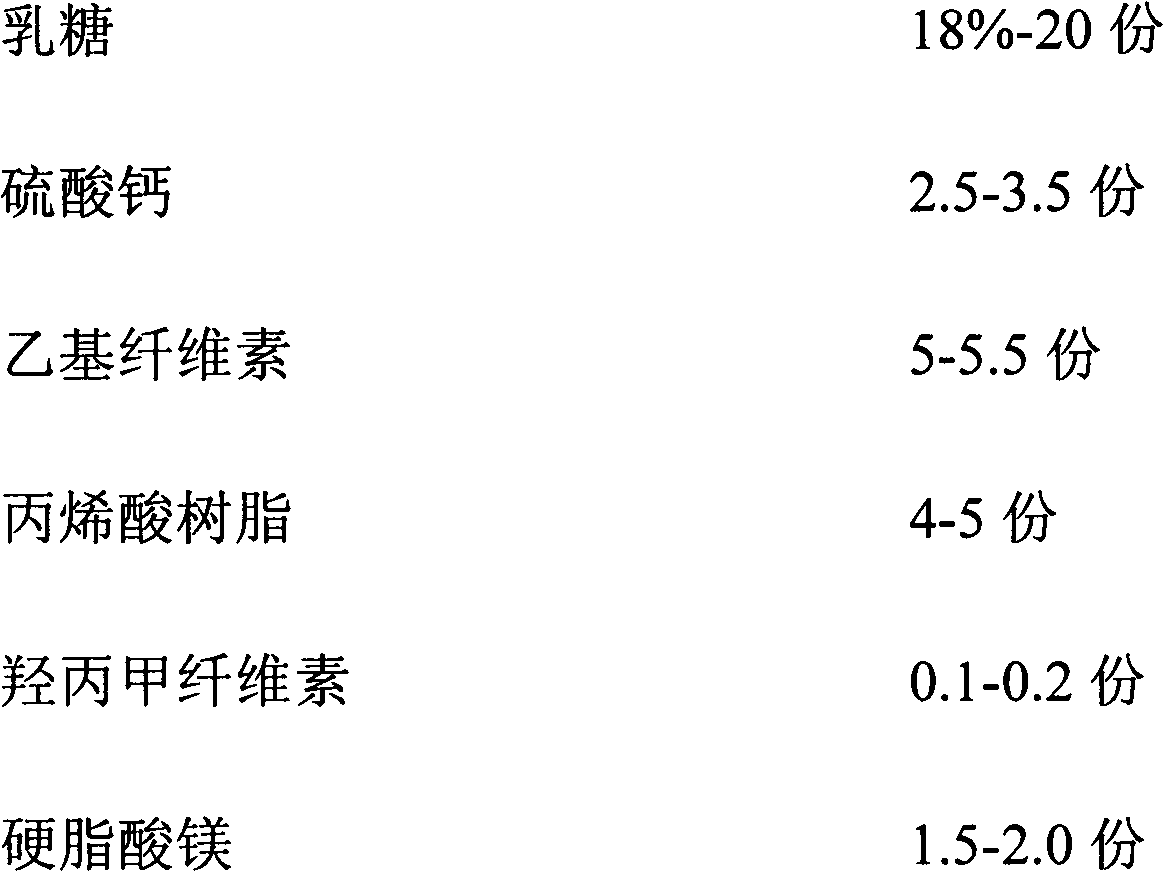
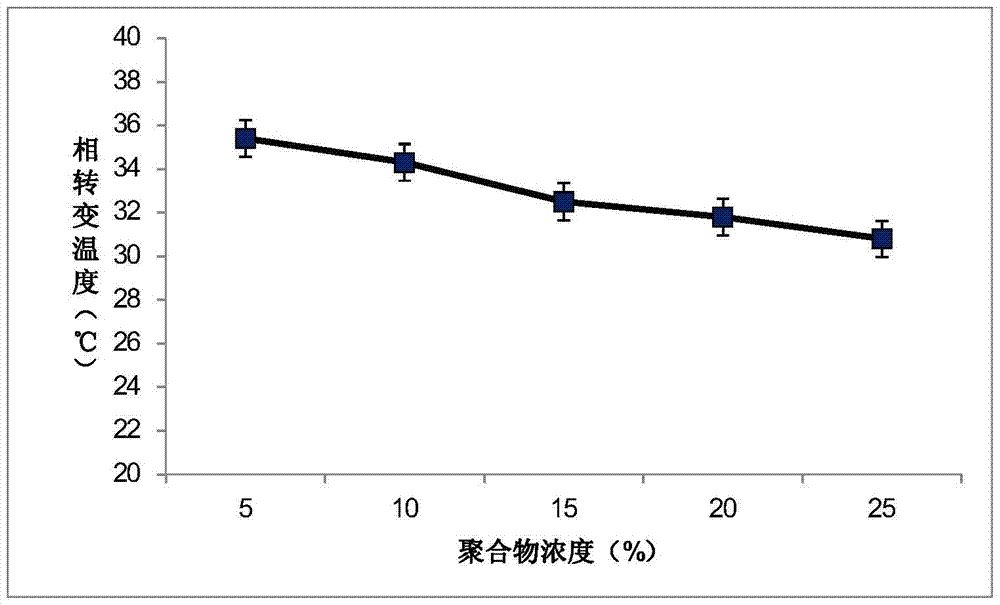
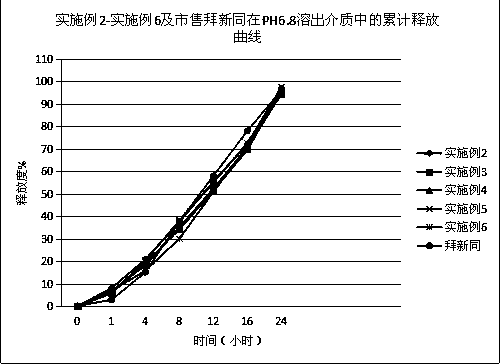
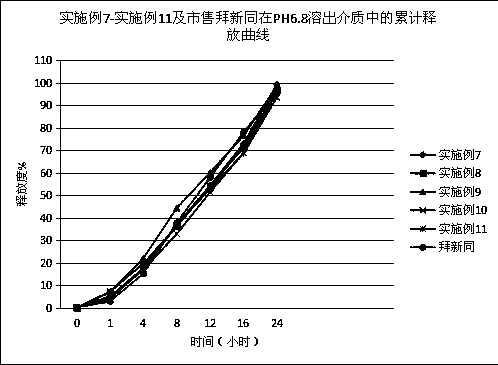
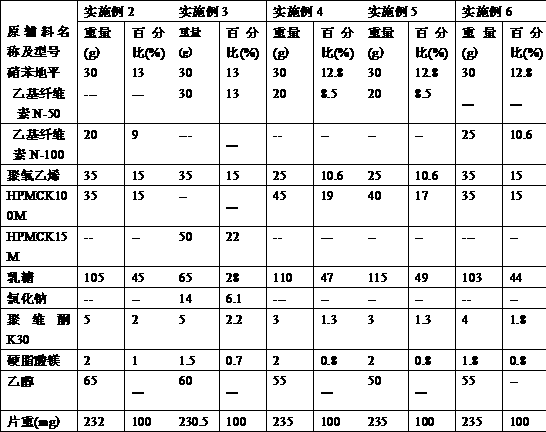
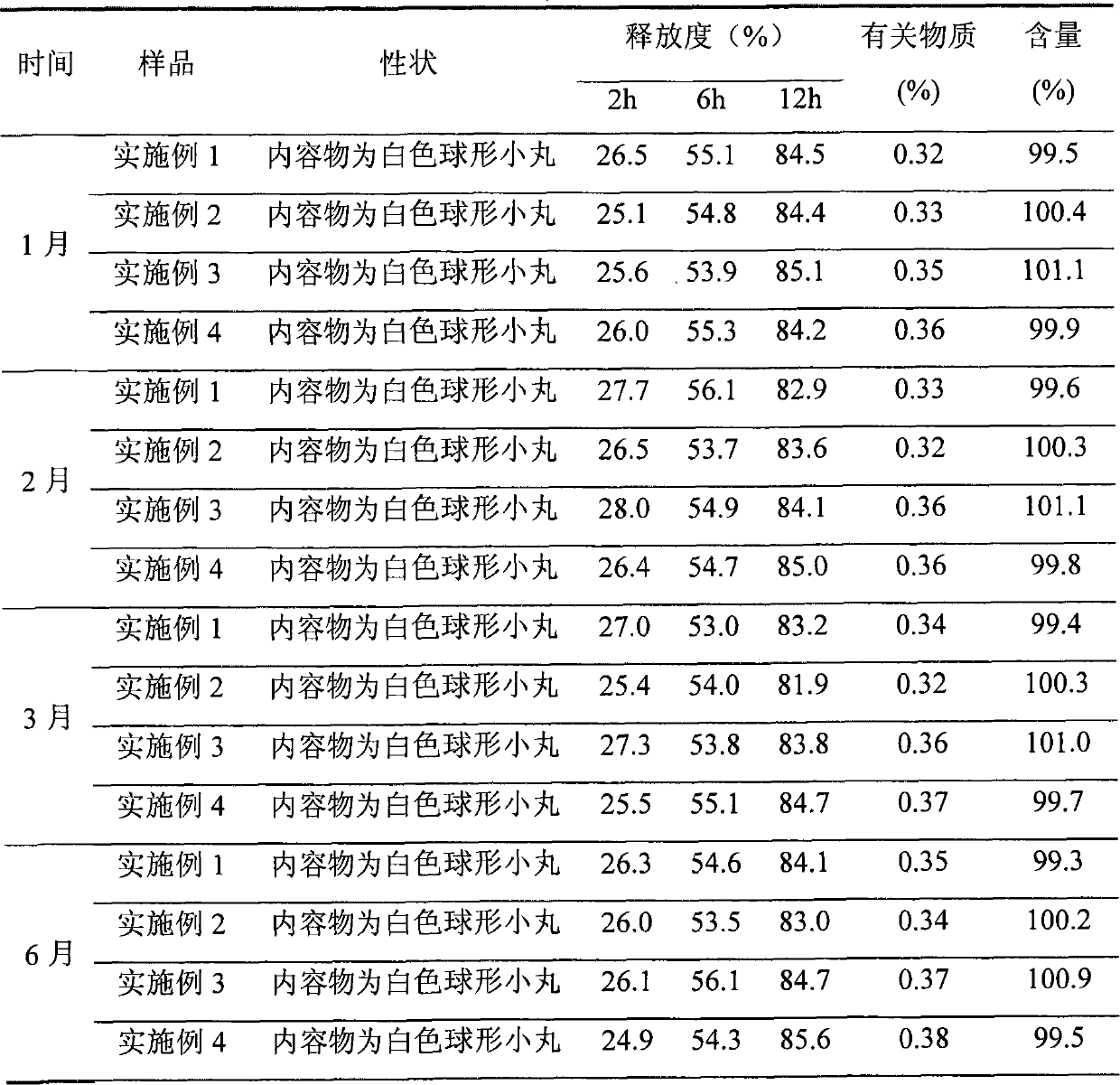
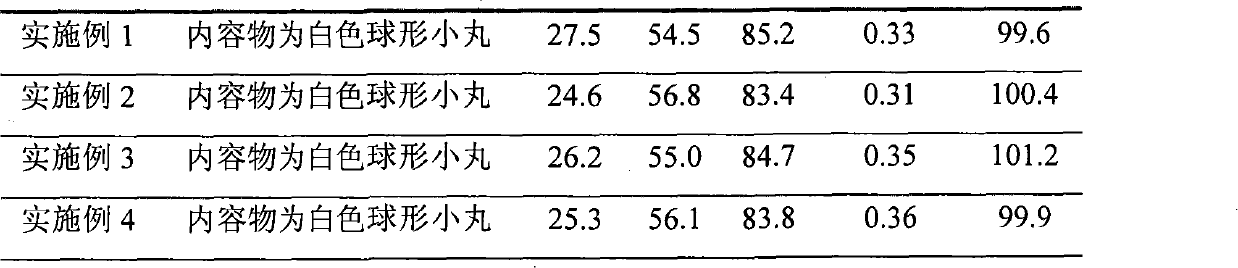
Patents
Literature
42results about How to "Clear release curve" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Temperature-sensitive gel containing ropivacaine and used for long-acting injection and preparation method of temperature-sensitive gel
ActiveCN104606129AEasy to administerSimple preparation processAntipyreticAerosol deliveryLactidePh regulation
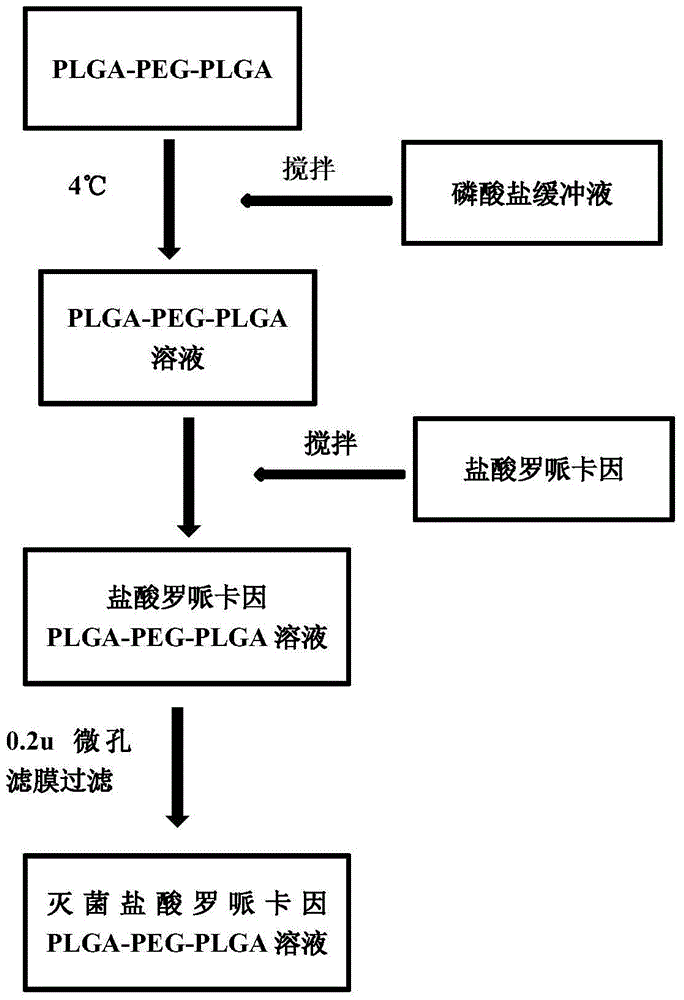
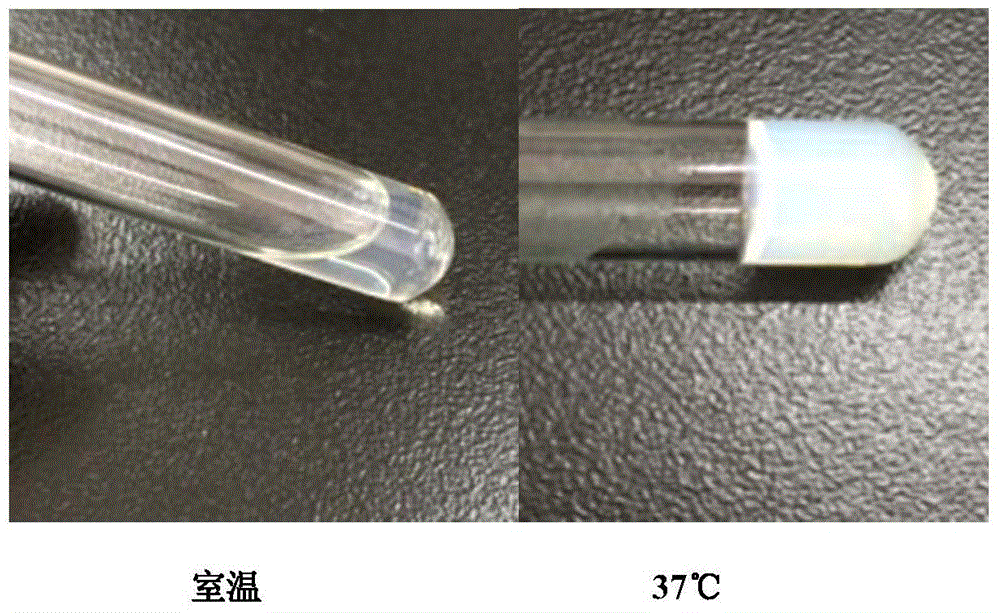
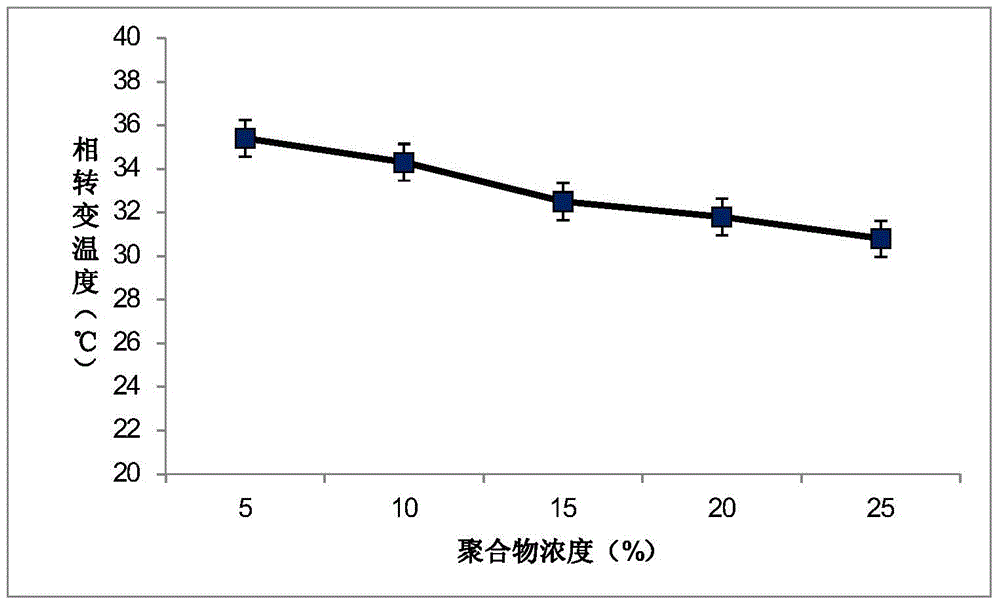
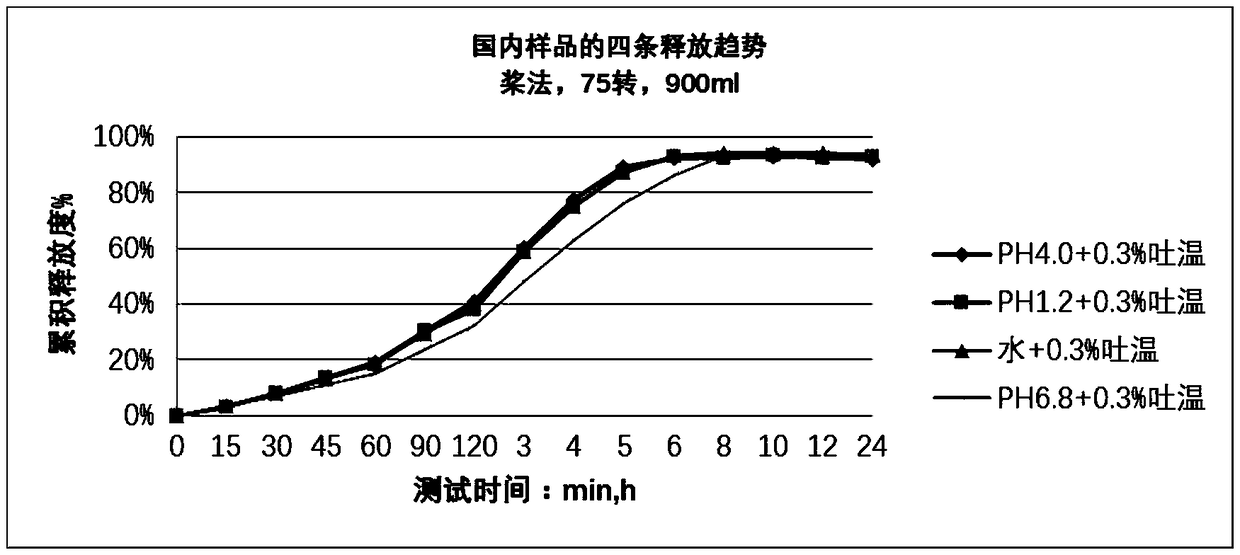
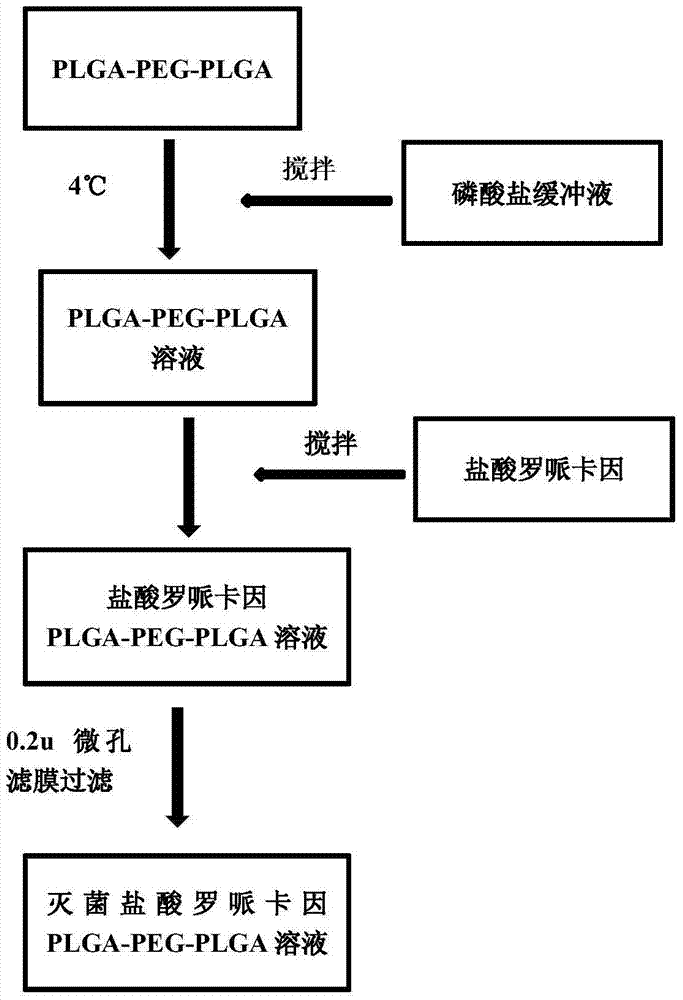
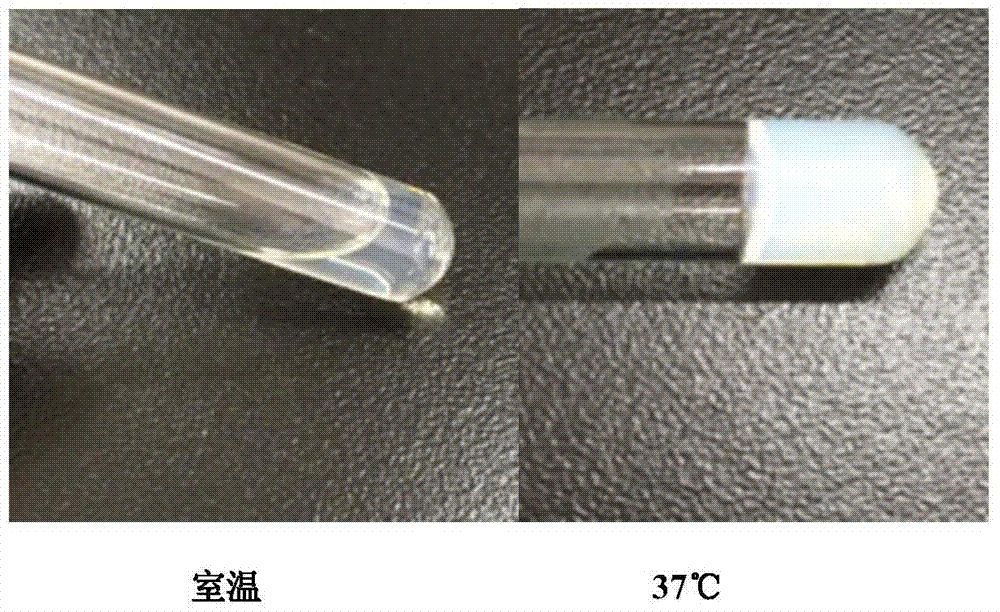
The invention discloses temperature-sensitive gel containing ropivacaine and used for long-acting injection and a preparation method of the temperature-sensitive gel. The gel comprises a main drug ropivacaine, ropivacaine hydrochloride or ropivacaine mesylate, a PLGA (poly (lactic-co-glycolic acid)) -PEG (polyethylene glycol)-PLGA copolymer as well as water, normal saline or pH regulation solution for injection, wherein the mole ratio of lactide to glycolide is (2-3): 1, and the PEG molecular weight of the PLGA-PEG-PLGA copolymer is 1,000. The preparation method comprises the following steps: the copolymer is sufficiently swelled in a solvent firstly, then the main drug is added, and sterilization is performed through a filter membrane. The temperature-sensitive gel facilitates drug delivery and can gel rapidly at human body temperature so as to play a slow release role, smaller than or equal to 35%-45% of the drug is released in vitro after 12 h, larger than or equal to 65%-75% of the drug is released after 48 h, larger than or equal to 80% of the drug is released after 72 h, and the design requirement for postoperative continuous analgesia for 48 h through single injection of a local anesthesia drug is met. Pharmacodynamics study shows that a temperature-sensitive gel group containing ropivacaine can prolong the efficacy maintaining time remarkably and continuously play 48 h analgesic effect.
Owner:WUHAN GENERAL HOSPITAL OF GUANGZHOU MILITARY
Nifedipine sustained release tablet and preparation method thereof
InactiveCN107569465AImprove stabilityClear release curveOrganic active ingredientsPharmaceutical non-active ingredientsPharmacologyLong acting
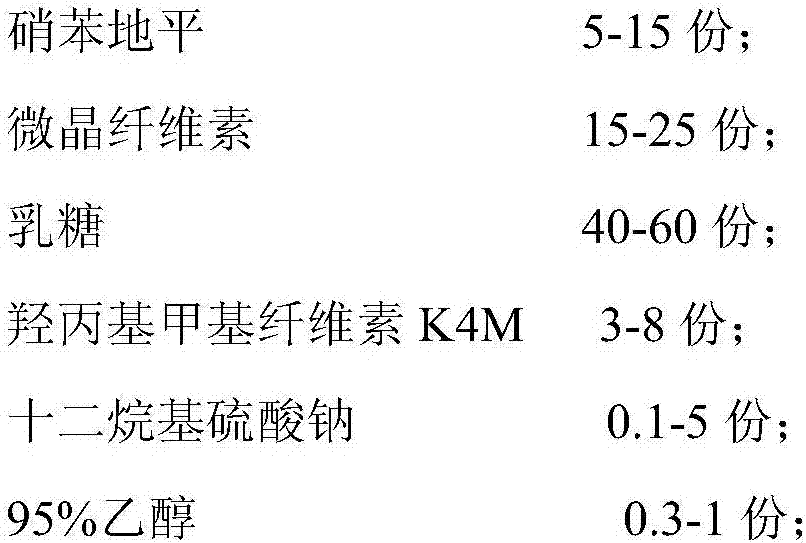
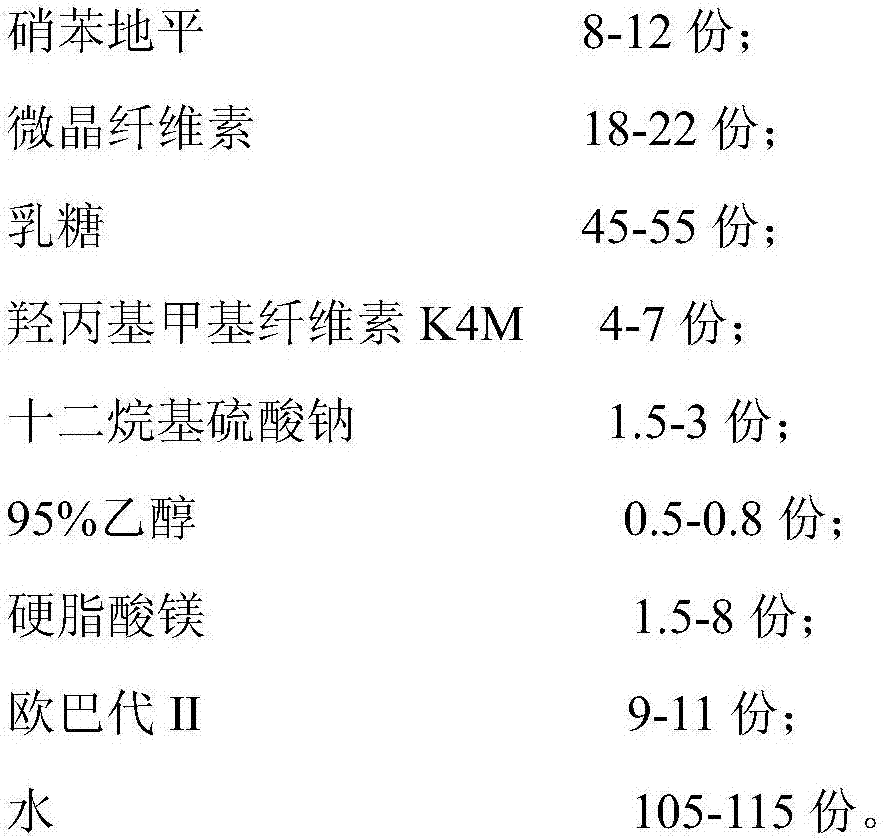
The invention relates to a nifedipine sustained release tablet and a preparation method of the nifedipine sustained release tablet. The nifedipine sustained release tablet is prepared from the following raw materials in parts by weight: 5-15 parts of nifedipine, 15-25 parts of microcrystalline cellulose, 40-60 parts of lactose, 3-8 parts of hydroxypropyl methyl cellulose K4M, 0.1-5 parts of sodiumdodecyl sulfate, 0.3-1 part of 95% alcohol, 0.5-10 parts of magnesium stearate, 8-12 parts of Opadry II, and 100-120 parts of water. For the nifedipine sustained release tablet and the preparation method provided by the invention, nitrobenzene and a high polymer material are mixed to prepare a hydrophilic gel matrix tablet, so that the purpose of slow release is achieved. Compared with a common tablet, the sustained release tablet provided by the invention has the advantages of long acting duration, mild action, slow release, and few and slight adverse reactions.
Owner:ANHUI YONSENT PHARMA
Nifedipine controlled release composition and preparation method thereof
ActiveCN102846574AImprove stabilitySimple preparation processOrganic active ingredientsPill deliveryNifedipineControlled release
The invention relates to a controlled release pharmaceutical formulation, and in particular relates to a nifedipine controlled release composition and a preparation method thereof. The nifedipine controlled release composition is simple in preparation process, low in cost, good in formulation stability and excellent release rate, can be used for effectively preventing product ageing, and is stable in release after being stored for a long time.
Owner:DEZHOU DEYAO PHARMA
Zinc gluconate pellets and preparation method thereof
InactiveCN101703478AGood dispersionQuick effectOrganic active ingredientsMetabolism disorderGluconic acidDissolution
The invention provides a zinc gluconate pellet preparation, which is prepared from zinc gluconate and pharmaceutic adjuvant, and is characterized in that: the pharmaceutic adjuvant is an excipient and an adhesive, wherein in the pellet preparation, the weight percent of the zinc gluconate is 10 to 80 percent, the weight percent of the excipient is 12 to 89 percent and the weight percent of the adhesive is 1 to 8 percent. The pellet preparation can be prepared into a slow release preparation or an enteric-coated preparation. The zinc gluconate pellet preparation has high dissolution rate, and high bioavailability; and the method is simple, convenient, and easy to operate.
Owner:JF PHARMALAND TECH DEV
Nifedipine controlled-release tablet composition and preparation method thereof
ActiveCN106344531AReduce usageAvoid peaks and valleys in blood concentrationOrganic active ingredientsPill deliveryPOLYOXYETHYLENE ETHERFineness
The invention relates to a nifedipine controlled-release tablet composition, comprising the following components in percentage by weight: 4.0-35.0% of nifedipine, 5.0-20.0% of polyoxyethylene, 5.0-30.0% of high-viscosity hydrophilic matrix material, 5.0-20.0% of a hydrophobic retarder, 20.0-60.0% of a filling agent, 0-10.0% of an adhesive solution, and 0.5-1.0% of a lubricant. A preparation method of the composition comprises the following steps: micronizing raw materials under a dark condition until the fineness ensures the powder to pass through a 200-mesh sieve, and screening an auxiliary material through a sieve with 80-100 meshes; adding the medicinal raw materials into the adhesive solution, and stirring uniformly for later use; and weighing a formula amount of polyoxyethylene, hydroxypropyl methylcellulose, the retarder namely ethyl cellulose and the filling agent, mixing uniformly, adding the prepared adhesive solution to prepare a soft material, and performing granulation, drying, straightening, addition of the lubricant, total blending, tabletting and package to obtain the composition. The nifedipine controlled-release tablet composition has the advantages that the preparation process is simple and easy, the production cost is low, and the production cycle is short.
Owner:浙江为康制药有限公司
Chondroitin sulfate pellet and preparation method thereof
InactiveCN101703475AGood dispersionQuick effectOrganic active ingredientsAntipyreticDissolutionBioavailability
The invention provides a chondroitin sulfate pellet preparation, which is prepared from chondroitin sulfate and pharmaceutic adjuvant. The pellet preparation is characterized in that the pharmaceutic adjuvant is excipient and bonding agent, wherein the pellet preparation comprises the following components in percentage by weight: 10 to 90 percent of the chondroitin sulfate, 3 to 89 percent of the excipient, and 1 to 7 percent of the bonding agent. The pellet preparation can be prepared into a slow-release preparation or an enteric-coated preparation as requested. The chondroitin sulfate pellet preparation has high dissolution rate and high bioavailability, and the method is simple and convenient and is easy to operate.
Owner:JF PHARMALAND TECH DEV
Astragalus polyose pellet and preparation method thereof
InactiveCN101444489AGood dispersionQuick effectOrganic active ingredientsMetabolism disorderAstragalus polysaccharideBioavailability
The invention provides an astragalus polyose pellet preparation composed of astragalus polyose and medical supplementary materials. The astragalus polyose pellet preparation is characterized in that the medical supplementary materials are excipients and binders; and in the pellet preparation, the content of astragalus polyose 0.5-70 percent by weight, the content of excipients is 30-99.5 percent by weight, and the content of binders is 1-5 percent by weight. The pellet preparation has high dissolution rate and high bioavailability, and the preparation method is simple, convenient and easy for operation.
Owner:JF PHARMALAND TECH DEV
Beta-carotene pellet and method for preparing the same
InactiveCN101219126AGood dispersionQuick effectOrganic active ingredientsAntinoxious agentsBeta-CaroteneAdjuvant
The invention provides a beta-carotene micro-pill prepared from beta-carotene and medicinal adjuvant, characterized in that the medicinal adjuvant comprises an excipient and a bonding agent; the content of the beta-carotene in the beta-carotene micro-pill is 1-10 weight percent, the excipient 70-95 weight percent and the bonding agent 1-5 weight percent. The beta-carotene micro-pill of the invention has the advantages of high dissolution rate, high bioavailability, easy preparation method and convenient and easy operation.
Owner:JF PHARMALAND TECH DEV
Sea buckthorn extract pellet and preparation method thereof
InactiveCN101444547AQuick effectImprove bioavailabilityMetabolism disorderDigestive systemHippophae rhamnoidesPharmaceutical preservatives
The invention provides a sea buckthorn extract pellet preparation composed of sea buckthorn and medical supplementary materials. The sea buckthorn extract pellet preparation is characterized in that the medical supplementary materials are excipients and binders; and in the pellet preparation, the content of sea buckthorn extract 2-75 percent by weight, the content of excipients is 20-97 percent by weight, and the content of binders is 1-5 percent by weight. The pellet preparation has high dissolution rate and high bioavailability, and the preparation method is simple, convenient and easy for operation.
Owner:JF PHARMALAND TECH DEV
Donkey-hide gelatin micro-pills and preparation method thereof
The invention discloses a pellet formulation of donkey-hide gelatin which is prepared by donkey-hide gelatin and pharmaceutical excipients, wherein the pharmaceutical excipients are excipient and adhesive. In the pellet formulation, the weight percentage of the donkey-hide gelatin is 20-80 %, the weight percentage of the excipient is 20-80%, and the weight percentage of the adhesive is 1-5%. The pellet formulation can be made into sustained release formulation or enteric-coated formulation according to the requirements. The inventive pellet formulation has high dissolution rate and bioavailability, easy and convenient preparation method, and easy operation.
Owner:JF PHARMALAND TECH DEV
Diclofenac sodium sustained release tablet and preparation method thereof
ActiveCN102846572AImprove stabilityClear release curveOrganic active ingredientsAntipyreticSustained Release TabletAcrylic resin
The invention relates to a sustained release pharmaceutical formulation, and in particular relates to a diclofenac sodium sustained release tablet and a preparation method thereof. The diclofenac sodium sustained release tablet is prepared from diclofenac sodium, lactose, calcium sulfate, ethylcellulose, acrylic resin, hydroxypropyl methylcellulose, magnesium stearate and 95% ethanol in certain proportions. The diclofenac sodium sustained release tablet is simple in preparation process, low in cost, good in formulation stability and excellent in release rate.
Owner:DEZHOU DEYAO PHARMA
Pearl powder micro-pills and preparation method thereof
The invention discloses a pellet formulation of pearl powder which is prepared by pearl powder and pharmaceutical excipients, wherein the pharmaceutical excipients are excipient and adhesive. In the pellet formulation, the weight percentage of the pearl powder is 5-80%, the weight percentage of the excipient is 20-95%, and the weight percentage of the adhesive is 1-5%. The inventive pellet formulation has high dissolution rate and bioavailability, easy and convenient preparation method, and easy operation.
Owner:JF PHARMALAND TECH DEV
Levorotation carnitine pellet and method for preparing the same
InactiveCN101219133AGood dispersionQuick effectOrganic active ingredientsNervous disorderMedicineDissolution
The invention provides a levocarnitine micro-pill which is prepared from levocarnitine and pharmaceutic adjuvant, characterized in that the pharmaceutic adjuvant is an excipient and a bonding agent; the content of the levocarnitine in the levocarnitine micro-pill is 10 to 90 weight percent; the excipient 20-80 weight percent and the bonding agent 1-5 weight percent. The levocarnitine micro-pill of the invention has the advantages of high dissolution rate, high bioavailability, easy preparation method and convenient and easy operation.
Owner:JF PHARMALAND TECH DEV
Sea cucumber peptide micro-pills and preparation method thereof
InactiveCN101455680AQuick effectImprove bioavailabilityPeptide/protein ingredientsDigestive systemPeptidePharmaceutical Excipient
The invention discloses a pellet formulation of sea cucumber peptide which is prepared by sea cucumber peptide and pharmaceutical excipients, wherein the pharmaceutical excipients are excipient and adhesive. In the pellet formulation, the weight percentage of the sea cucumber peptide is 2-85%, the weight percentage of the excipient is 20-80%, and the weight percentage of the adhesive is 1-5%. The inventive pellet preparation has high dissolution rate and bioavailability, easy and convenient preparation method, and easy operation.
Owner:JF PHARMALAND TECH DEV
Vitamin E micropill and preparation method thereof
InactiveCN101444487AGood dispersionQuick effectOrganic active ingredientsAntinoxious agentsMedicineBioavailability
The invention provides a preparation of a vitamin E micropill which is made of vitamin E and medical accessories and is characterized in that the medical accessories comprise excipient and bond. The preparation of the micropill is made of the following components by the weight percentage: 1-85 percent of vitamin E, 15-99 percent of excipient and 1-5 percent of bond. The preparation of the micropill has the advantages of high stripping rate and high bioavailability, and the preparation method is simple and convenient and can be operated easily.
Owner:JF PHARMALAND TECH DEV
Recombined human vascellum esoderma inhibin durative action preparation, preparation method and application thereof
ActiveCN101292951ARelieve painReduce the burden onPeptide/protein ingredientsPharmaceutical delivery mechanismMicrosphereRelease time
The invention relates to the field of medical technology and discloses a long-acting preparation of lactic-glycolic acid copolymer (PLGA) for the recombination of endostatin of vein and the application to preparing a drug for treating tumor. The invention uses four methods of implanting a pump in vivo, implanting a microsphere in vivo, a W / O / W solvent volatilization method and a W / O / O solvent volatilization method to prepare the long-acting preparation. The sustained-release time of the long-acting preparation prepared is 7 to 28 days in accordance with the needs.
Owner:SHANDONG SIMCERE BIO PHARMA CO LTD
Nifedipine sustained release tablet and preparation method thereof
ActiveCN108853044ASimple preparation processLow costOrganic active ingredientsDrageesNifedipineSustained Release Tablet
The invention discloses a nifedipine sustained release tablet and a preparation method thereof. The nifedipine sustained release tablet consists of a rapid released component A, a supplemented released component B, a sustained release component C and an auxiliary material magnesium stearate; the component A consists of nifedipine, microcrystal cellulose, lactose hydrate, a tween-80 and purified water; the component B consists of nifedipine, microcrystal cellulose, lactose hydrate, starch and purified water; and the component C consists of nifedipine, microcrystal cellulose and ethyl cellulose.The preparation method comprises the following steps: firstly separately preparing the component A, the component B and the component C, wherein the prepared three components and the auxiliary material magnesium stearate are mixed to form a mixed material; tabletting the mixed material to obtain medicine tablets; and coating the medicine tablets, and obtaining the nifedipine sustained release tablet. The nifedipine sustained release tablet is prepared by virtue of the preparation method of the invention; and the preparation method is simple, the cost is low, the preparation stability is good,the release curve is better, and the defects in the prior art can be overcome.
Owner:ZHENGZHOU MINGZE MEDICAL TECH
Ganoderan polysaccharide pellet and preparation method thereof
InactiveCN101703532AGood dispersionQuick effectMetabolism disorderAntinoxious agentsBioavailabilityPharmaceutical Adjuvants
The invention provides a ganoderan polysaccharide pellet preparation, which is prepared from ganoderan polysaccharides and pharmaceutical adjuvants. The ganoderan polysaccharide pellet preparation is characterized in that: the pharmaceutical adjuvants are an excipient and a binding agent, wherein the pellet preparation comprises the following components in percentage by weight: 10 to 20 percent of ganoderan polysaccharides, 3 to 89 percent of excipient and 1 to 7 percent of bonding agent. The pellet preparation can be prepared into a slow-release or enteric-coated preparation as required. The ganoderan polysaccharide pellet preparation of the invention has high dissolving rate and high bioavailability. The method of the invention is simple, convenient and easy to operate.
Owner:JF PHARMALAND TECH DEV
Lutein pellet and preparation method thereof
InactiveCN103845293AGood dispersionQuick effectSenses disorderHydroxy compound active ingredientsLuteinAdhesive
The invention provides a lutein pellet, which is prepared from lutein and a pharmaceutic adjuvant. The lutein pellet is characterized in that the pharmaceutic adjuvant is an excipient and an adhesive, wherein the pellet preparation contains 5-20% of lutein, 70-85% of excipient and 1-5% of adhesive. The lutein pellet disclosed by the invention is high in dissolution rate, and high in bioavailability, and the preparation method is simple, convenient and easy to operate.
Owner:JF PHARMALAND TECH DEV
Diclofenac sodium sustained release tablet and preparation method thereof
ActiveCN102846572BImprove stabilityClear release curveOrganic active ingredientsAntipyreticSustained Release TabletAcrylic resin
The invention relates to a sustained release pharmaceutical formulation, and in particular relates to a diclofenac sodium sustained release tablet and a preparation method thereof. The diclofenac sodium sustained release tablet is prepared from diclofenac sodium, lactose, calcium sulfate, ethylcellulose, acrylic resin, hydroxypropyl methylcellulose, magnesium stearate and 95% ethanol in certain proportions. The diclofenac sodium sustained release tablet is simple in preparation process, low in cost, good in formulation stability and excellent in release rate.
Owner:DEZHOU DEYAO PHARMA
Thermosensitive gel for ropivacaine long-acting injection and preparation method thereof
ActiveCN104606129BEasy to administerSimple preparation processAntipyreticAerosol deliveryLactideSingle injection
The invention discloses a temperature-sensitive gel for ropivacaine long-acting injection and a preparation method thereof. The gel comprises main ingredients ropivacaine, ropivacaine hydrochloride or ropivacaine mesylate; Glycolide molar ratio 2-3:1, PLGA-PEG-PLGA copolymer with PEG molecular weight of 1000, PEG accounts for 18-30% of the total mass of the copolymer; water for injection, normal saline or pH adjustment solution. The preparation method is to fully swell the copolymer in a solvent, add the main drug, and sterilize through a filter membrane. The temperature-sensitive gel is convenient for administration, gels rapidly at body temperature and exerts a slow-release effect. The drug release in vitro is ≤35-45%, 48 hours ≥65%-75%, and 72 hours ≥80%, which is in line with local anesthetics. After a single injection of continuous analgesia 48h design requirements. Pharmacodynamic studies have shown that the ropivacaine temperature-sensitive gel group can significantly prolong the maintenance time of the drug effect, and can continuously exert an analgesic effect for 48 hours.
Owner:WUHAN GENERAL HOSPITAL OF GUANGZHOU MILITARY
Collagen protein micro-pill and preparation method thereof
InactiveCN101455837AGood dispersionQuick effectPeptide/protein ingredientsMetabolism disorderMedicineAdhesive
The invention provides a collagen pellet preparation, which is prepared from a collagen and a pharmaceutic adjuvant. The collagen pellet preparation is characterized in that the pharmaceutic adjuvant is an excipient and an adhesive; the weight percentage of the collagen in the pellet preparation is between 10 and 90 percent; the weight percentage of the excipient is between 20 and 80 percent; and the weight percentage of the adhesive is between 1 and 5 percent. The pellet preparation of the invention has the advantages of high dissolution rate, high bioavailability, simple and convenient preparation method, and easy operation.
Owner:JF PHARMALAND TECH DEV
A kind of nifedipine controlled-release tablet composition and preparation method thereof
ActiveCN106344531BReduce usageAvoid peaks and valleys in blood concentrationOrganic active ingredientsPill deliveryPOLYOXYETHYLENE ETHERFineness
The invention relates to a nifedipine controlled-release tablet composition, comprising the following components in percentage by weight: 4.0-35.0% of nifedipine, 5.0-20.0% of polyoxyethylene, 5.0-30.0% of high-viscosity hydrophilic matrix material, 5.0-20.0% of a hydrophobic retarder, 20.0-60.0% of a filling agent, 0-10.0% of an adhesive solution, and 0.5-1.0% of a lubricant. A preparation method of the composition comprises the following steps: micronizing raw materials under a dark condition until the fineness ensures the powder to pass through a 200-mesh sieve, and screening an auxiliary material through a sieve with 80-100 meshes; adding the medicinal raw materials into the adhesive solution, and stirring uniformly for later use; and weighing a formula amount of polyoxyethylene, hydroxypropyl methylcellulose, the retarder namely ethyl cellulose and the filling agent, mixing uniformly, adding the prepared adhesive solution to prepare a soft material, and performing granulation, drying, straightening, addition of the lubricant, total blending, tabletting and package to obtain the composition. The nifedipine controlled-release tablet composition has the advantages that the preparation process is simple and easy, the production cost is low, and the production cycle is short.
Owner:浙江为康制药有限公司
Poria extract pellet and preparation method thereof
InactiveCN102462707ALow incidence of adverse reactionsIncrease the areaDigestive systemGranular deliveryMedicineAdhesive
The invention provides a poria extract pellet preparation, which is prepared from a poria extract and pharmaceutical auxiliary materials and is characterized in that the auxiliary materials comprise an excipient and an adhesive; and in the pellet preparation, the weight percentage of the poria extract is 5-80%, the weight percentage of the excipient is 19-85% and the percentage of the adhesive is 1-10%. The pellet preparation can be prepared into a sustained-release or intestine-soluble preparation as required. Pharmacological experiments show that the pellet provided by the invention has good actions of enhancing immune function, protecting liver and the like.
Owner:JF PHARMALAND TECH DEV
Compound lozenge controlling childe tooth decay
InactiveCN105169375AEvenly dispersedHigh encapsulation efficiencyAntibacterial agentsHydroxy compound active ingredientsSolventAnaphylactic reaction
The invention discloses a compound lozenge controlling childe tooth decay, and belongs to the technical field of medicines. The lozenge comprises medicinal compositions and auxiliary material compositions. The medicinal compositions comprise, in parts by weight, 1-3 parts of lysyl oxidase, 1.5-2.5 parts of D-galactose, 5-8 parts of nanometer diatomite, 0.2-0.6 part of calcium dihydrogen phosphate, 0.11-0.13 part of chromium picolinate, 0.08-0.1 part of tretinoin, 0.02-0.03 part of tiamcinolone acetonide. The auxiliary material compositions comprise, in parts by weight, 70-100 parts of a medicine carrier, 0.12-0.25 part of a solvent, 0.01-0.03 part of an aseptic, 0.14-0.26 part of compound vitamin, and 2-5 parts of a corrigent. The lozenge contains multiple effective compositions, is reasonable in matching ratio, is capable of resisting bacteria, diminishing inflammation, removing dental plaque and harmful bacteria, preventing and treating decayed teeth and preventing carious teeth, does not cause anaphylactic reaction, and enhances oral cavity health-care effect.
Owner:吕欢
Agaric extract pellet and preparation method thereof
InactiveCN102462706AControl releaseReduce the frequency of takingMetabolism disorderFungi medical ingredientsMedicinePharmaceutic Adjuvant
The invention provides an agaric extract pellet preparation which is prepared from an agaric extract and pharmaceutic adjuvants and is characterized in that the pharmaceutic adjuvants comprise an excipient and a binding agent; and the pellet preparation comprises 15-70wt% of the agaric extract, 25-84wt% of the excipient and 1-5wt% of the binding agent. The pellet preparation can be prepared into a sustained-release preparation or an enteric preparation according to requirements. Pharmacology experiments show that the pellet provided by the invention has the effects of improving the immunity, resisting cancers and the like.
Owner:JF PHARMALAND TECH DEV
Folium ginkgo extract micropill and preparation method thereof
InactiveCN101444544AQuick effectImprove bioavailabilityNervous disorderAntinoxious agentsPharmaceutical preservativesExcipient
The invention provides a preparation of a folium ginkgo extract micropill which is made of a folium ginkgo extract and medical accessories and is characterized in that the medical accessories comprise excipient and bond. The preparation of the folium ginkgo extract micropill is made of the following components by the weight percentage of 0.5-85 percent of folium ginkgo extract, 15-99.5 percent of excipient and 1-5 percent of bond. The preparation of the micropill has the advantages of high stripping rate and high bioavailability, and the preparation method is simple and convenient and can be operated easily.
Owner:JF PHARMALAND TECH DEV
Piclofenac potassium sustained release capsule and preparing technique thereof
InactiveCN101322695BSmall toxicityGood formulation stabilityOrganic active ingredientsAntipyreticSustained release pelletsDiclofenac Acid
The invention provides a diclofenac potassium sustained-release capsule which is anti-inflammation and analgesic drug, and a production method thereof. The diclofenac potassium sustained-release capsule is formed by preparing diclofenac potassium into sustained-release pellets and then filling the pellets into the capsule; the diclofenac potassium sustained-release pellet consists of a blank pellet core taken as mother nucleus, a main drug layer which is coated outside of the pellet core and contains diclofenac potassium and a sustained-release coating layer covered on the main drug layer. The diclofenac potassium sustained-release capsule of the invention has fine preparation stability and prominent release effect.
Owner:海南华旗药业销售有限公司
Lutein micropills and preparation thereof
InactiveCN101214232AGood dispersionQuick effectSenses disorderHydroxy compound active ingredientsLuteinBioavailability
The present invention provides a xanthin pellet preparation which is made from the xanthin and medical auxiliary materials and is characterized in that the medical auxiliary materials are excipient and bond. In the pellet preparation, the weight percentages of the materials are: 1 percent to 10 percent of the xanthin, 75 percent to 95 percent of the excipient and 1 percent to 5 percent of the bond. The pellet preparation of the present invention has high leaching rate and high biological utilization degree, and the preparation method is simple and convenient and is easy for operation.
Owner:JF PHARMALAND TECH DEV
Medlar extract pellet and preparation method thereof
InactiveCN102462781AIncrease the areaReduce dosageMetabolism disorderDigestive systemAdhesiveMedicine
The invention provides a medlar extract pellet preparation, which is prepared with medlar extracts and pharmaceutical excipients. The medlar extract pellet preparation is characterized in that the pharmaceutical excipients adopt excipients and adhesives, the weight percentage of the medlar extracts in the pellet preparation is 10-60 percent, the weight percentage of the excipients is 35-89 percent, and the weight percentage of the adhesives is 1-5 percent. The pellet preparation can be made into sustained-release or enteric-coated preparations in accordance with requirements. Pharmacological experiments show that the pellet has good functions of resisting against aging and improving the immunity and the like.
Owner:JF PHARMALAND TECH DEV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com