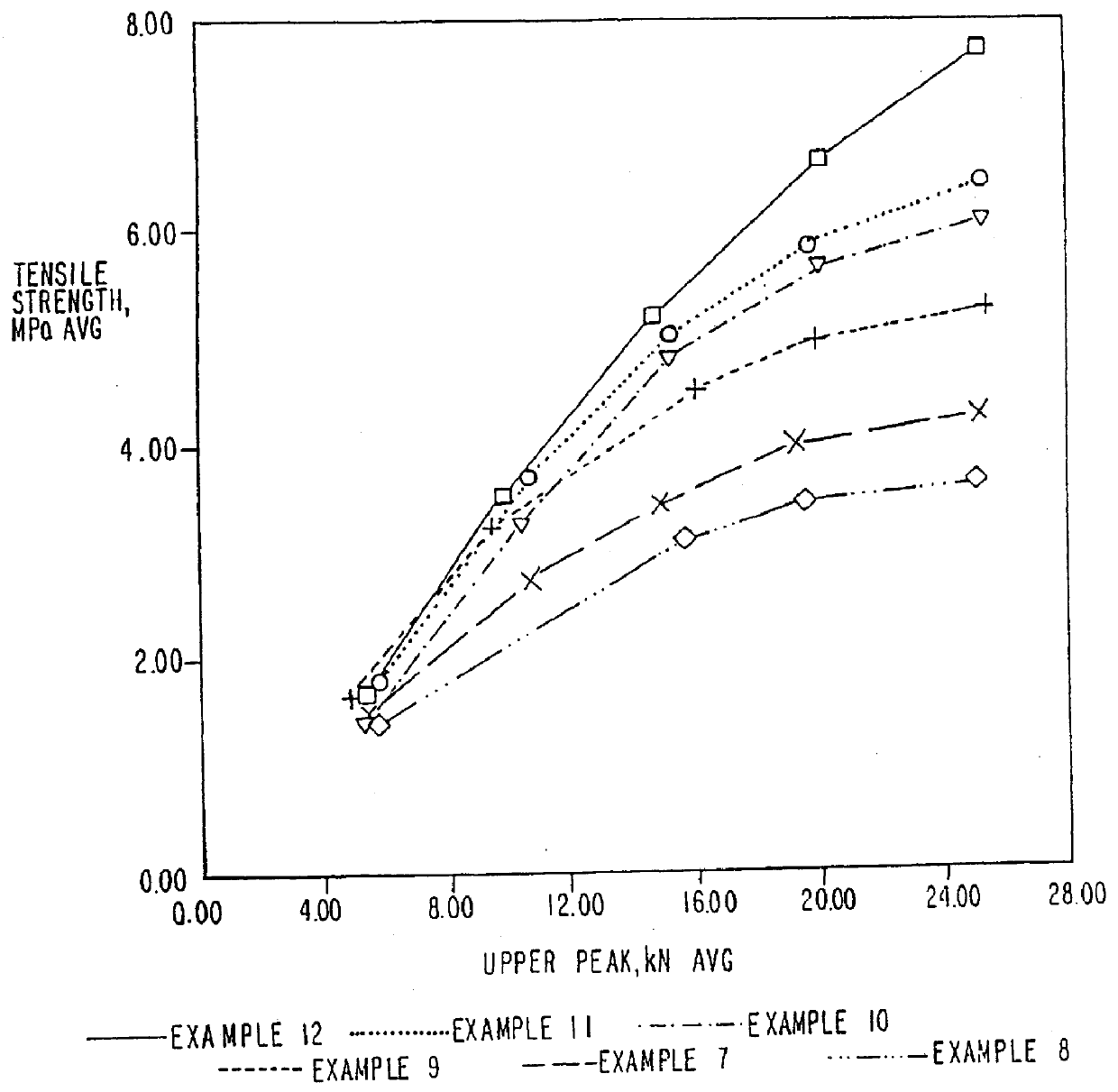
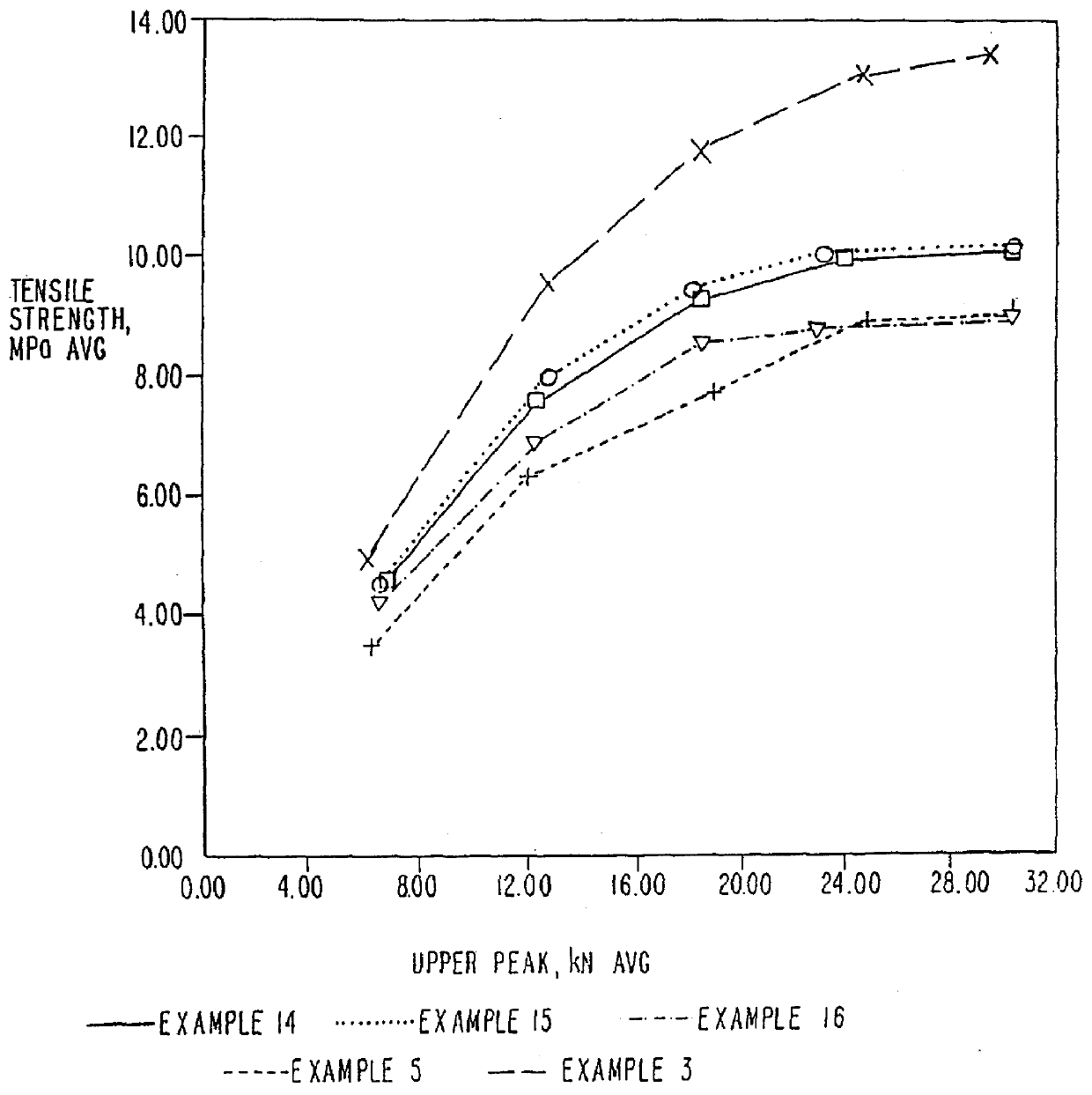
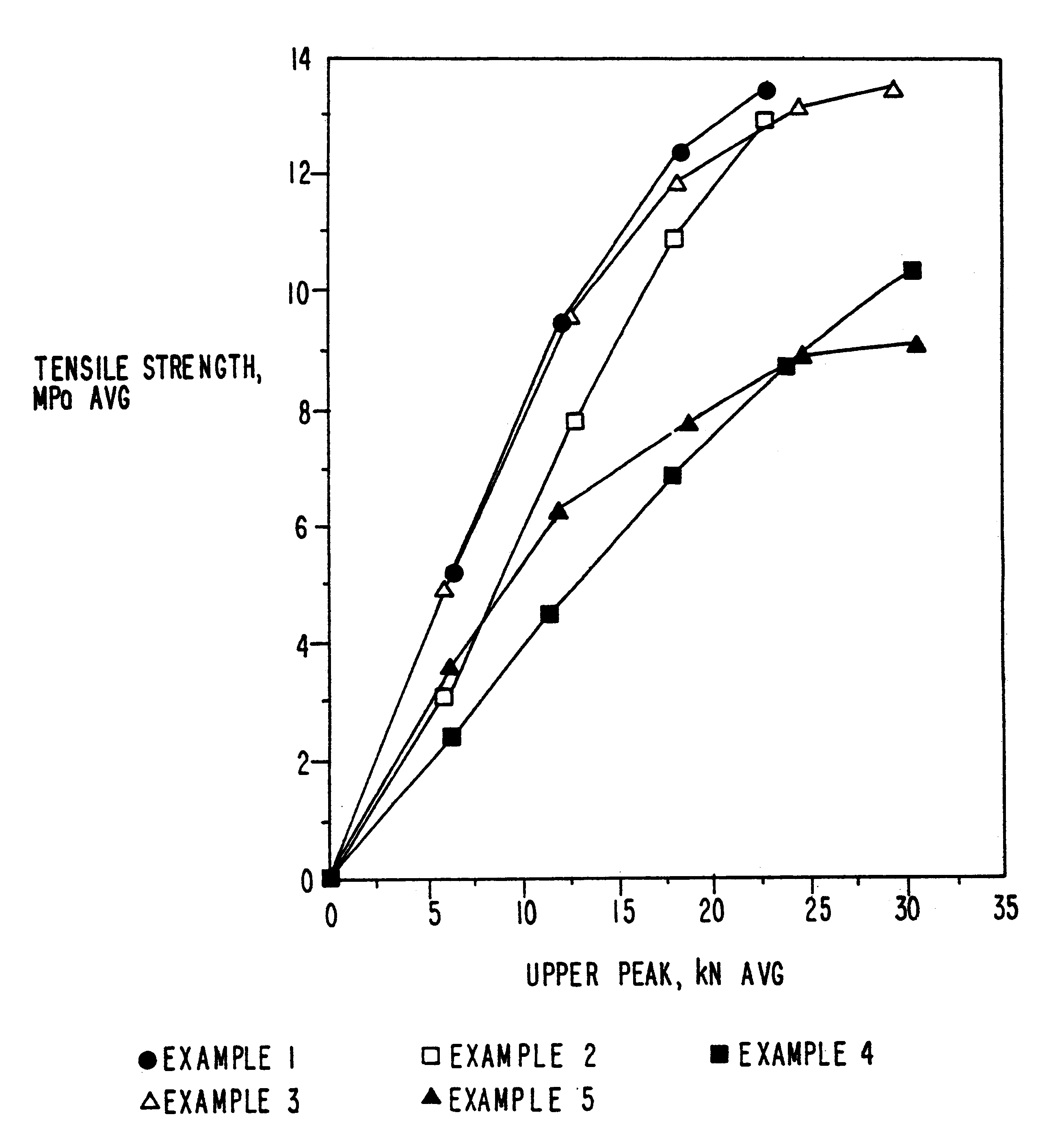
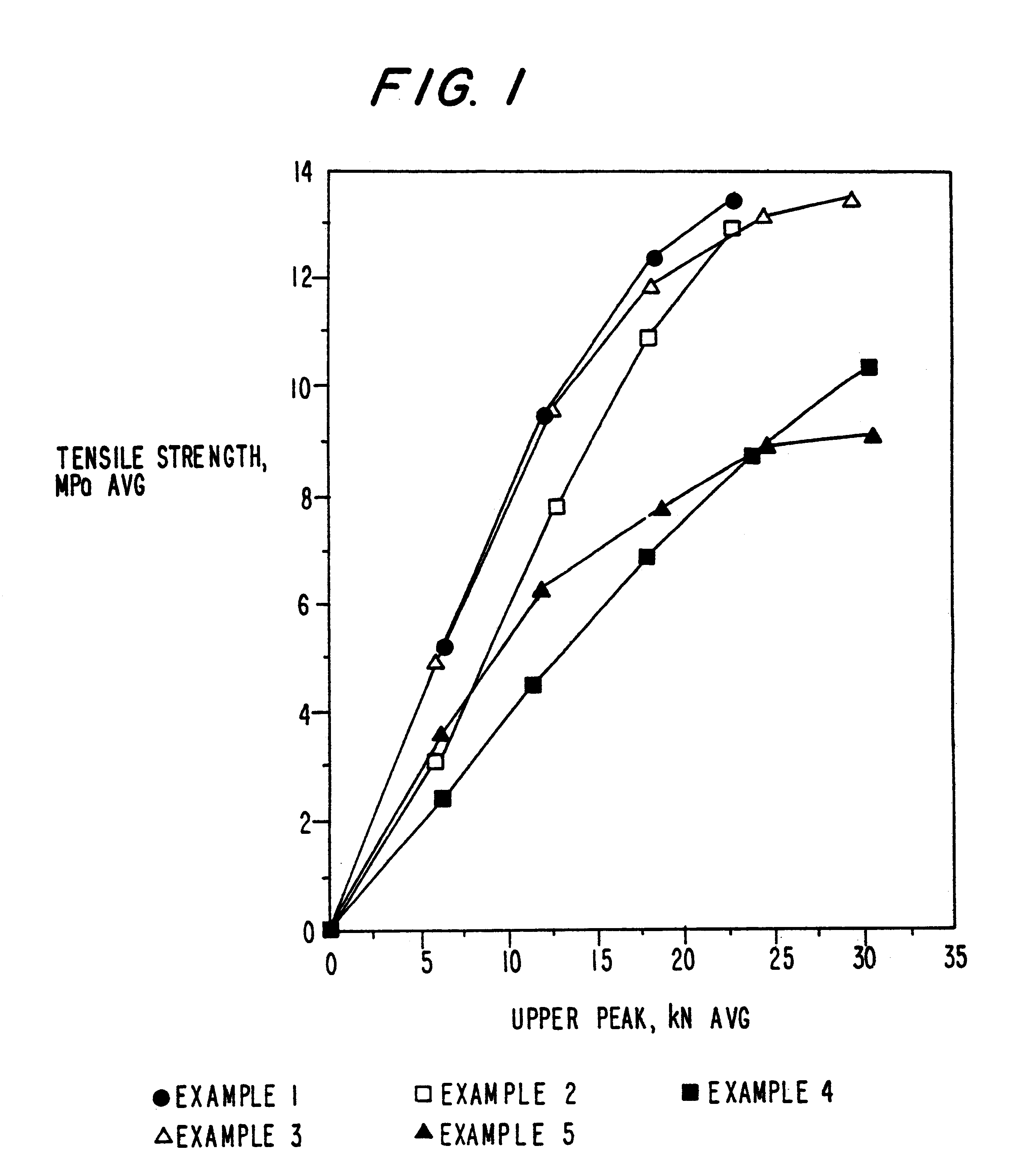
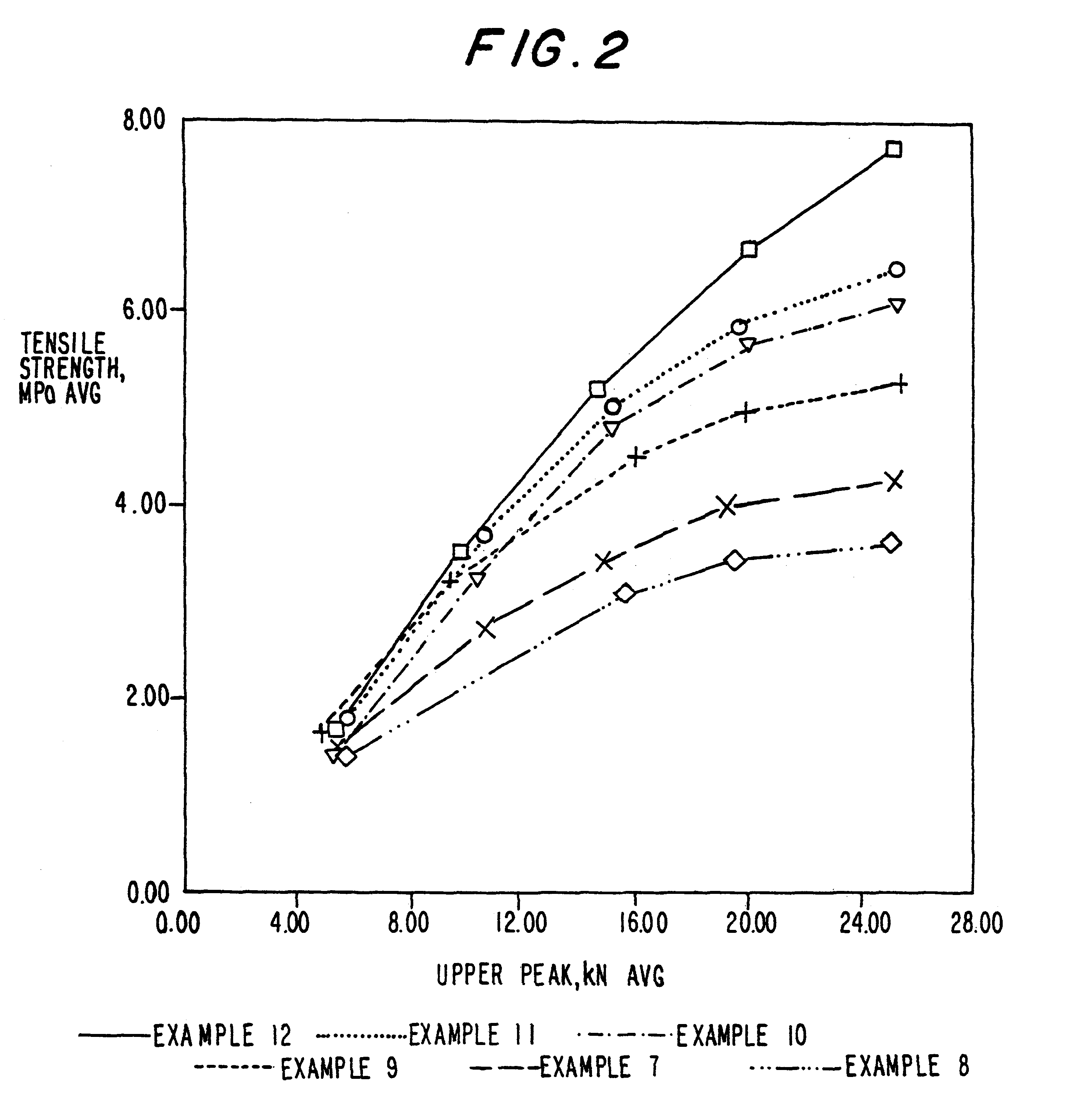
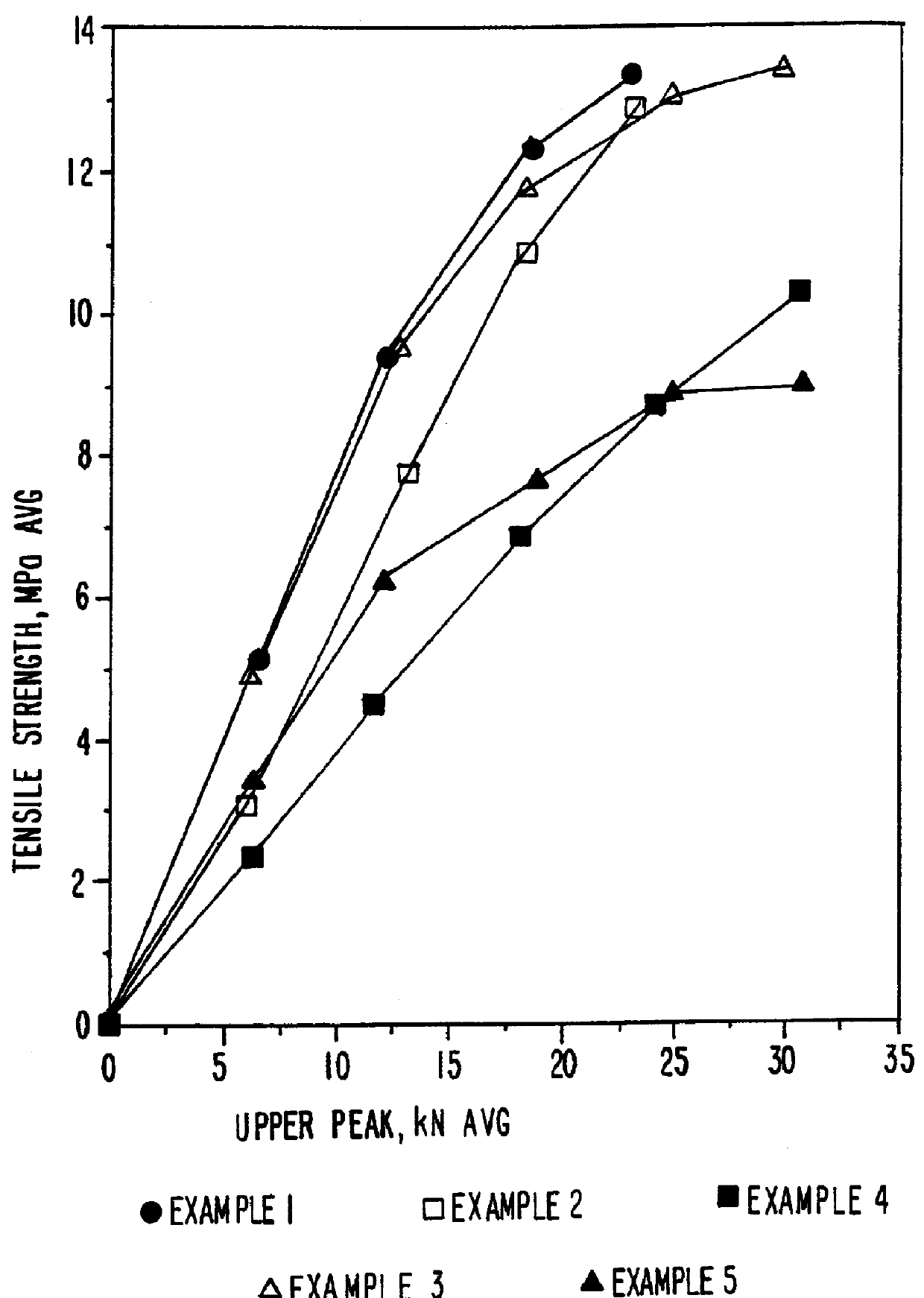
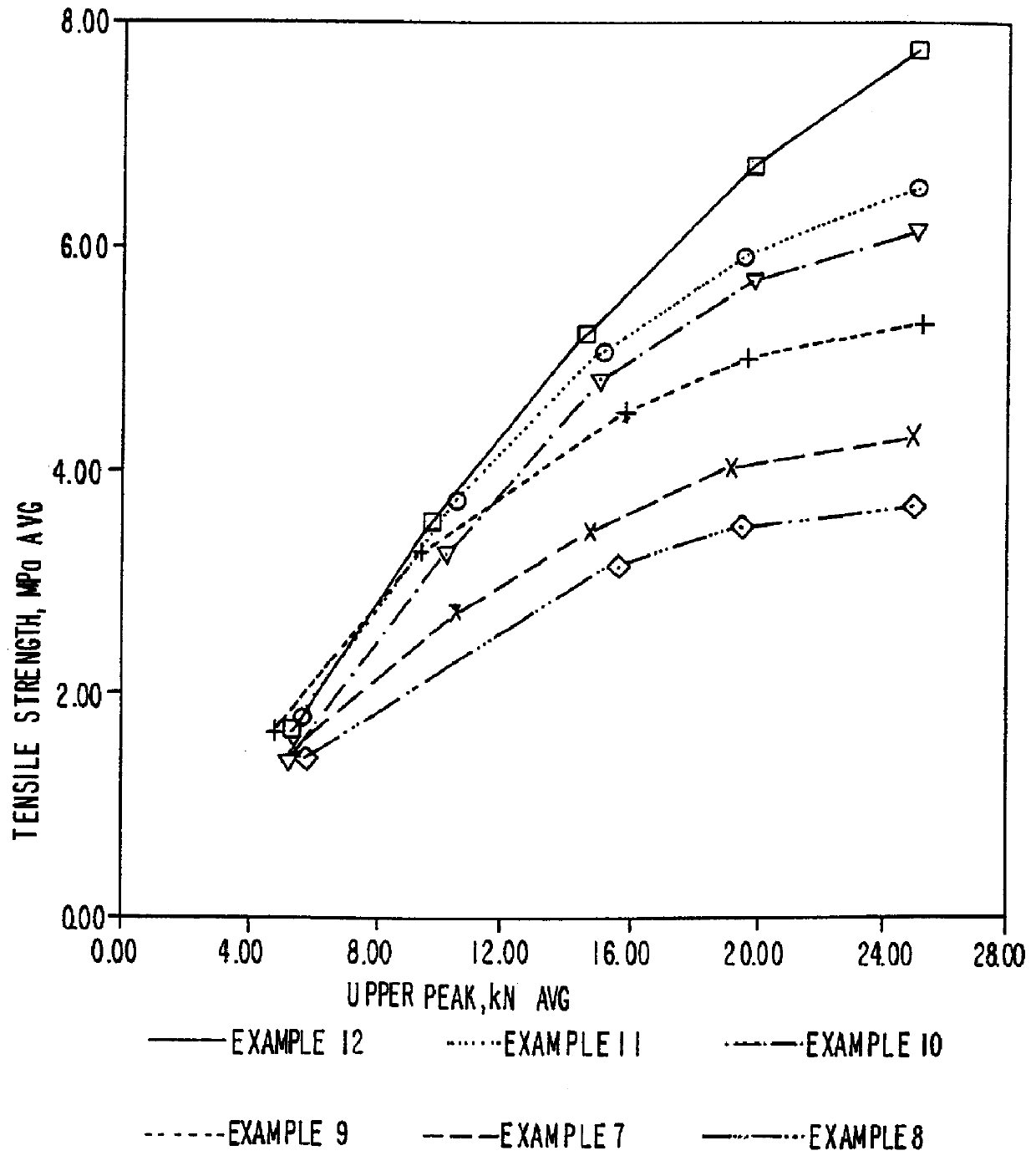
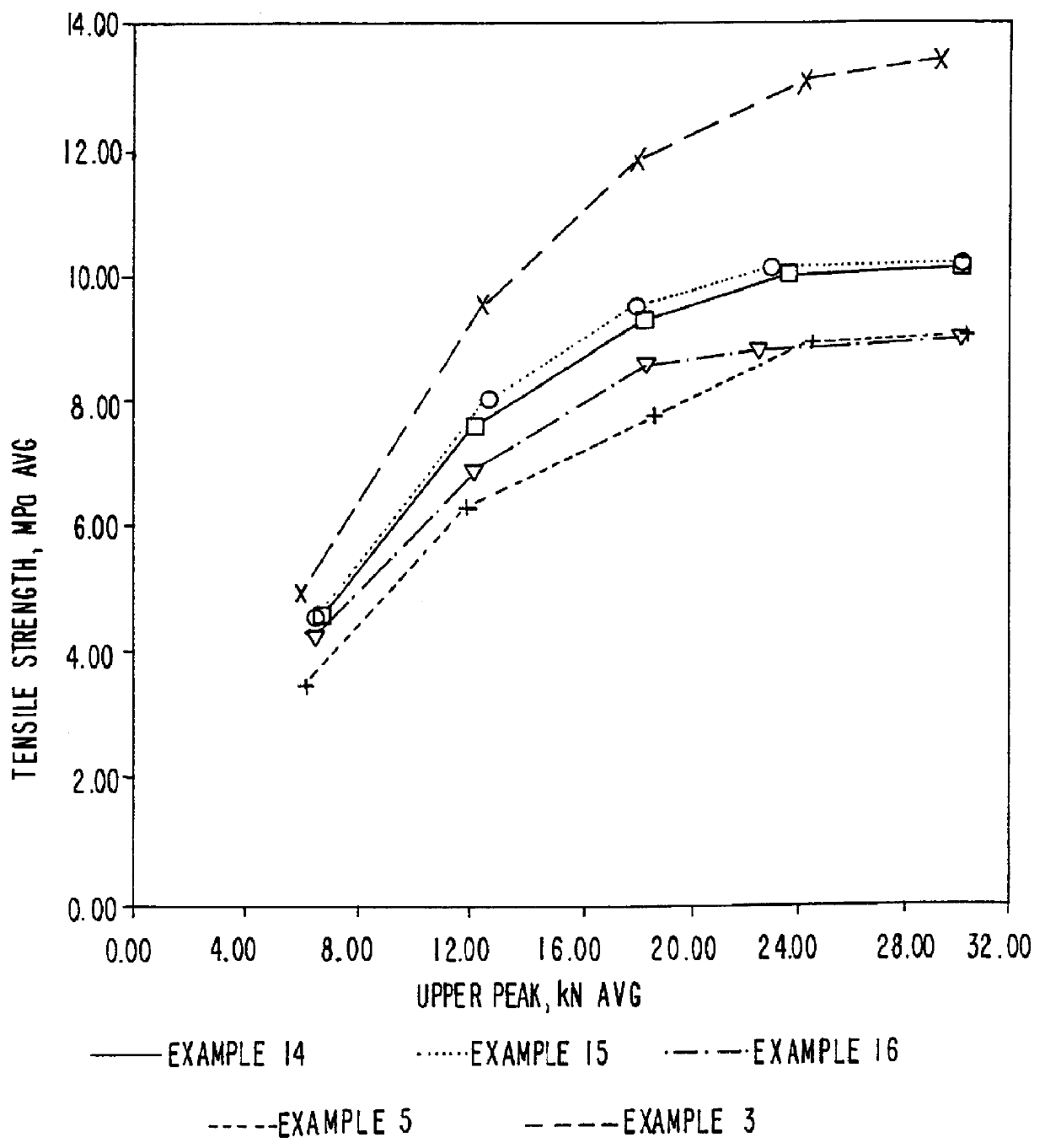
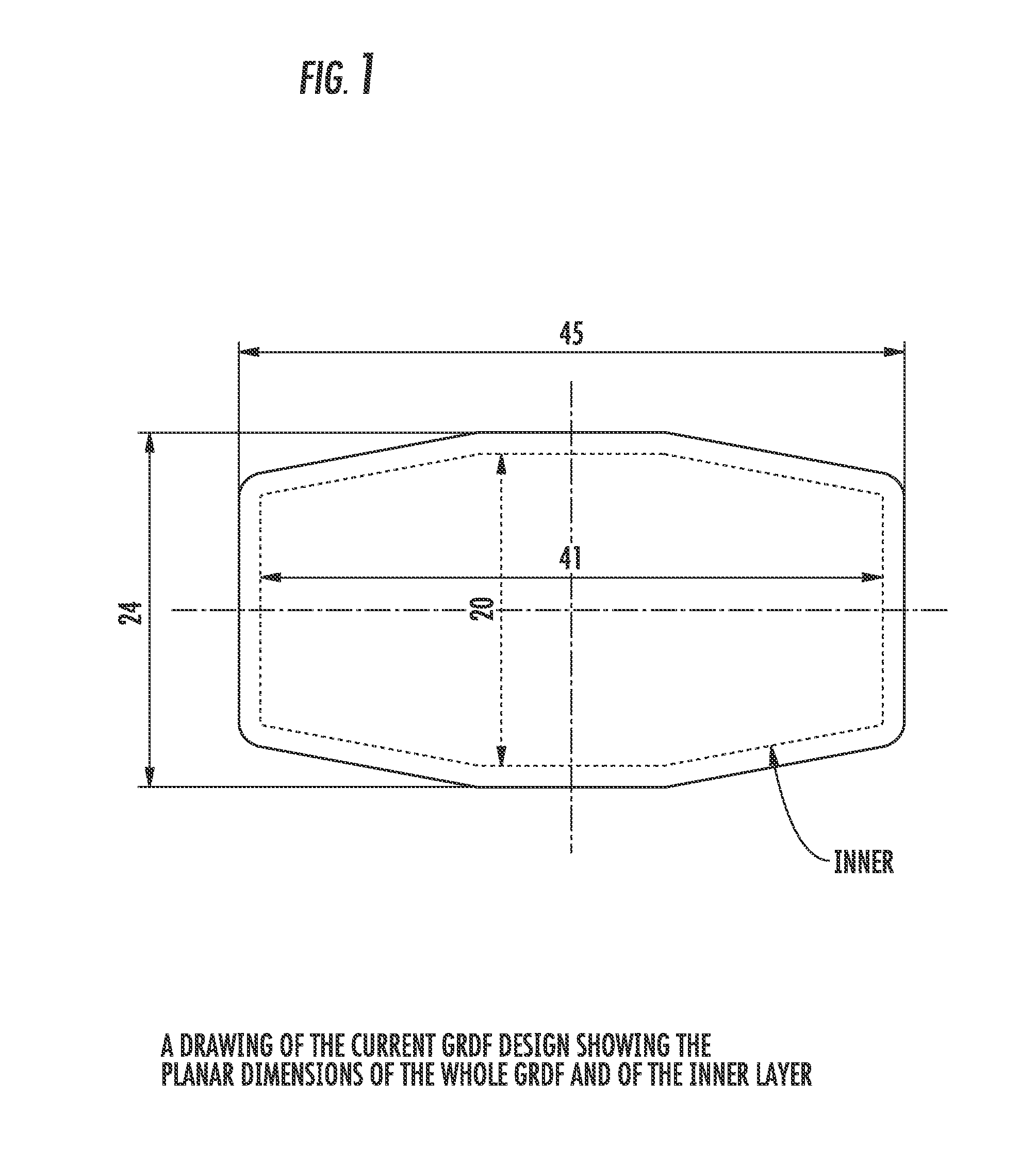
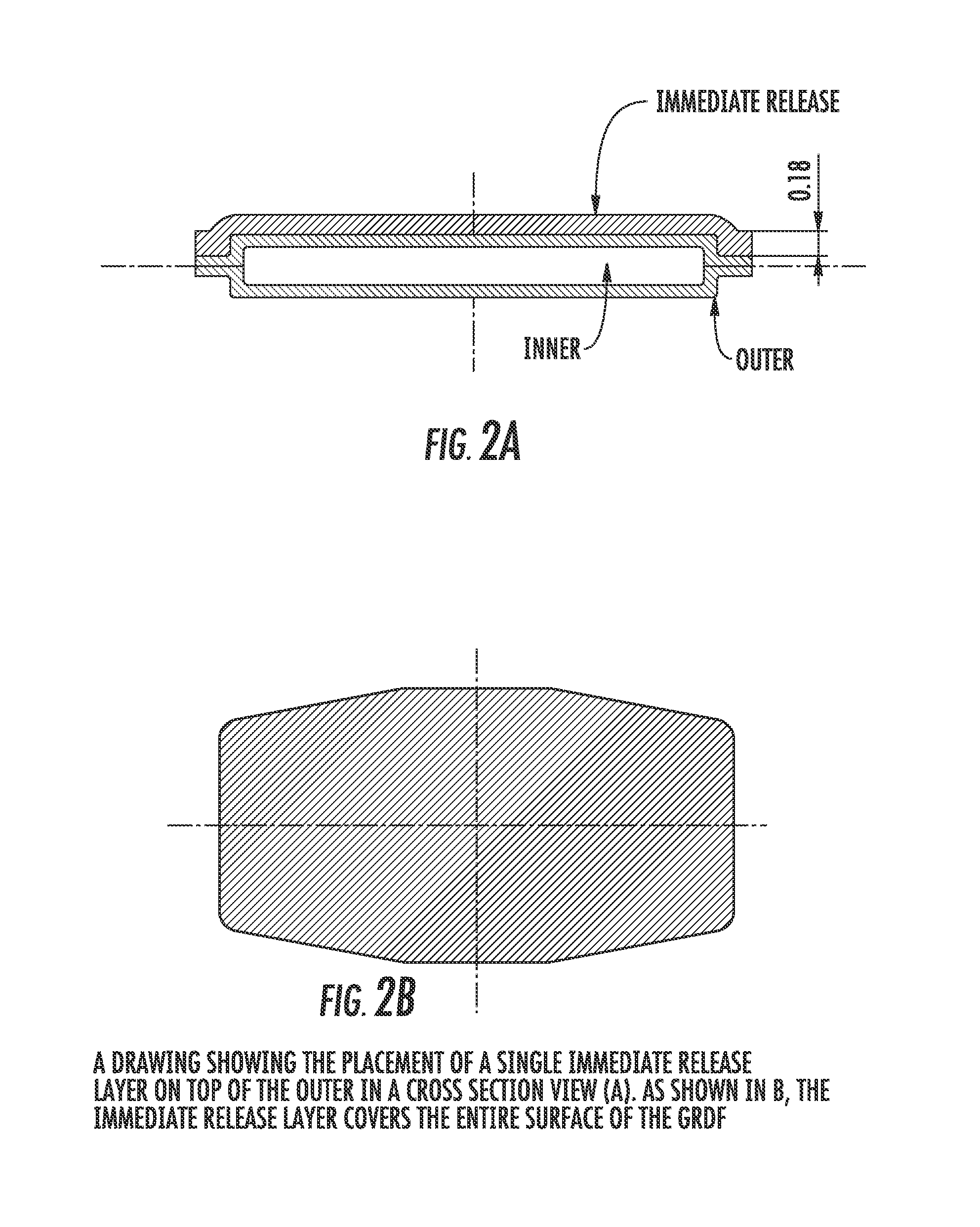
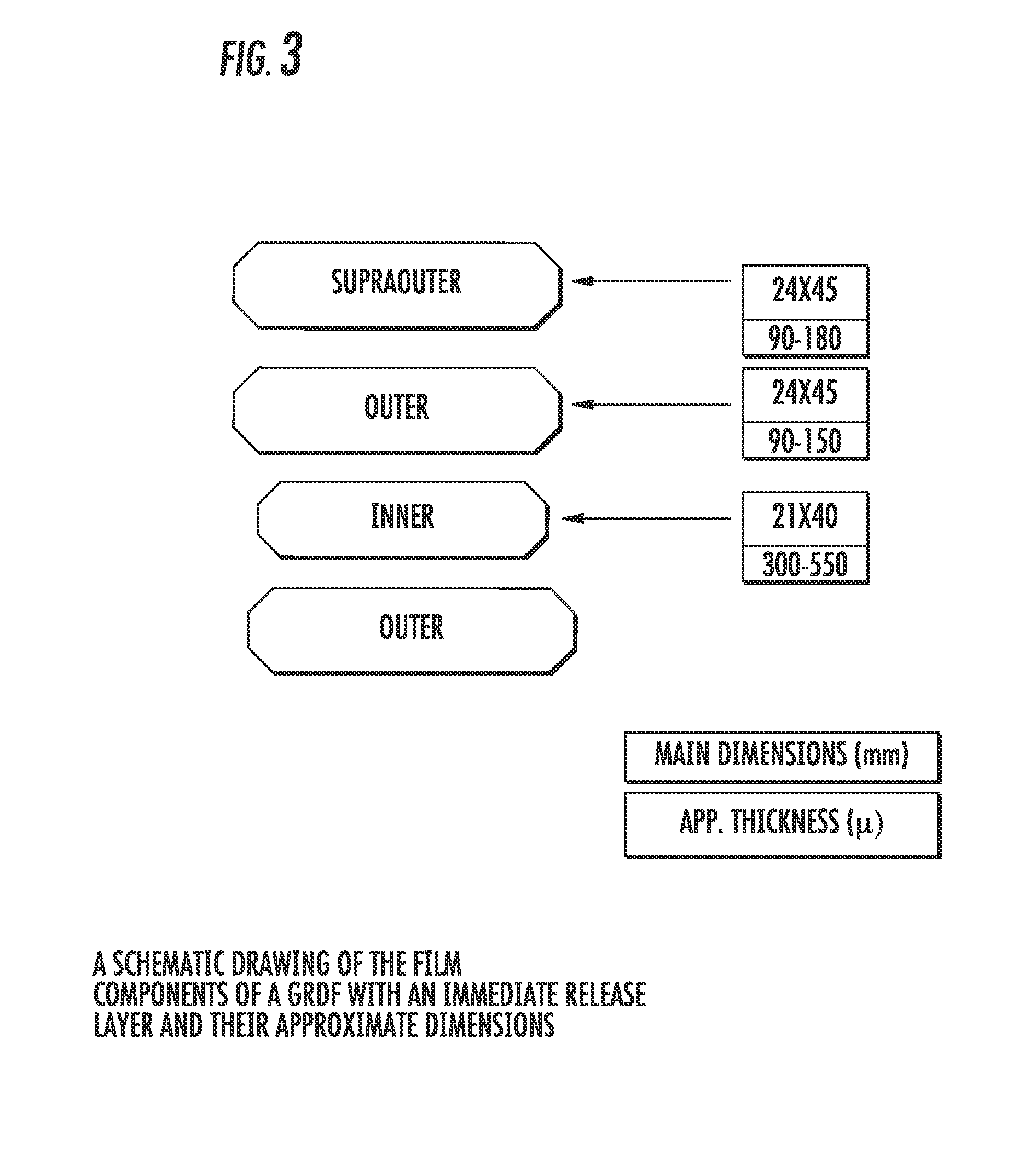
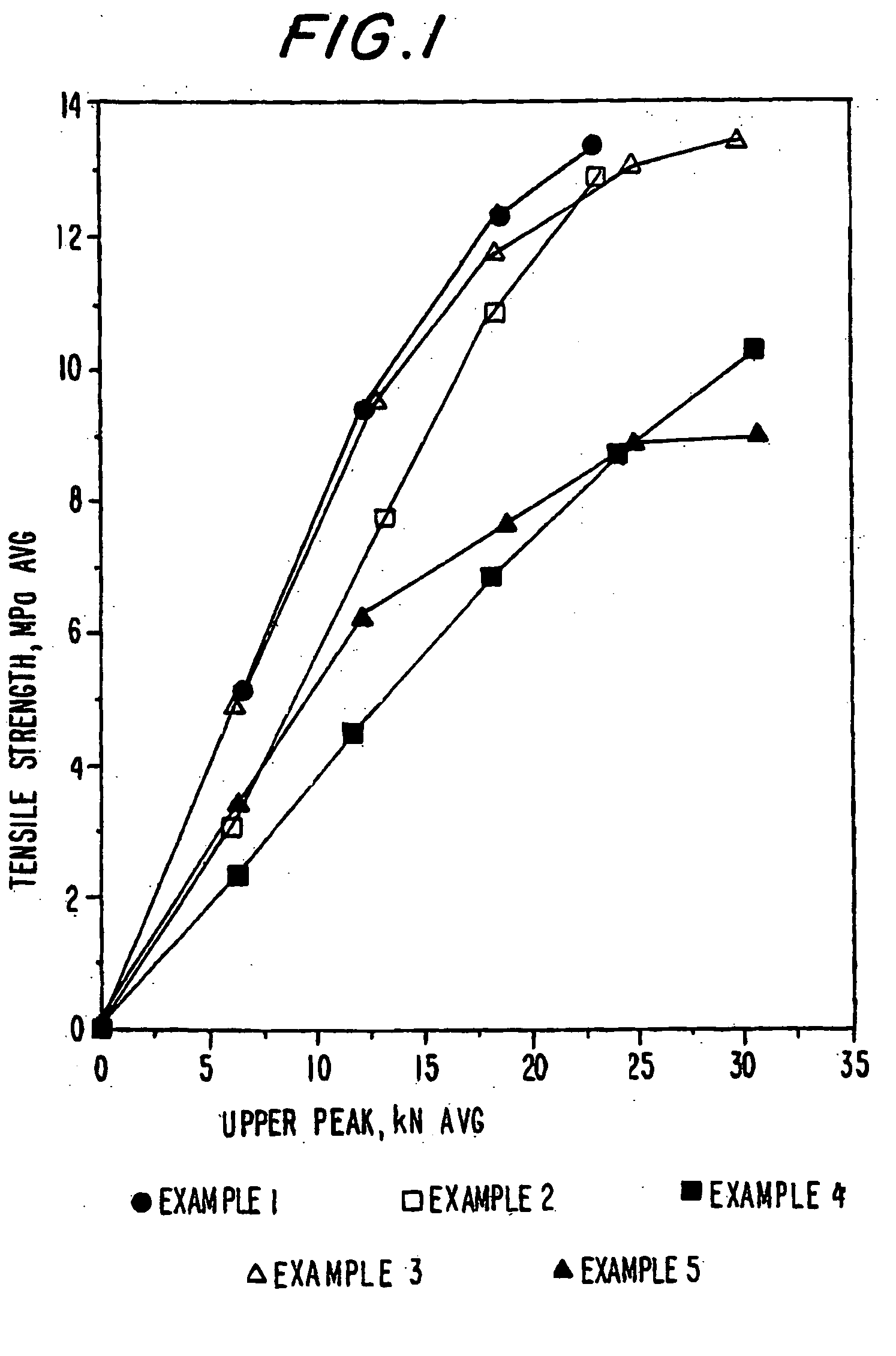
Patents
Literature
811 results about "Pharmaceutical Excipient" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
New compositions containing quinoline compounds
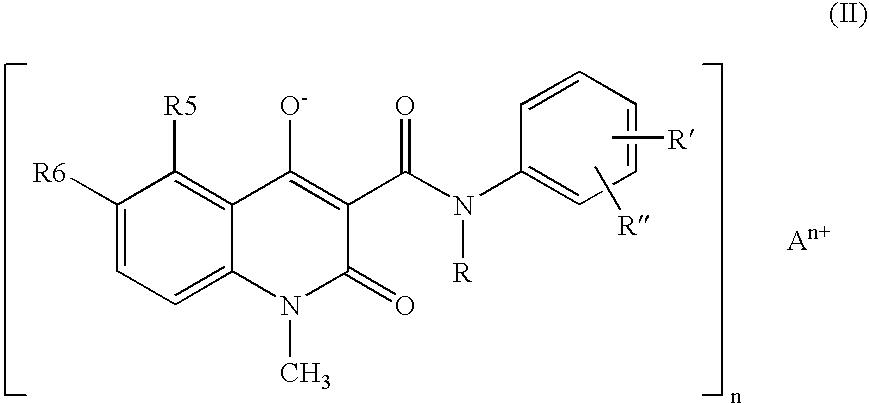
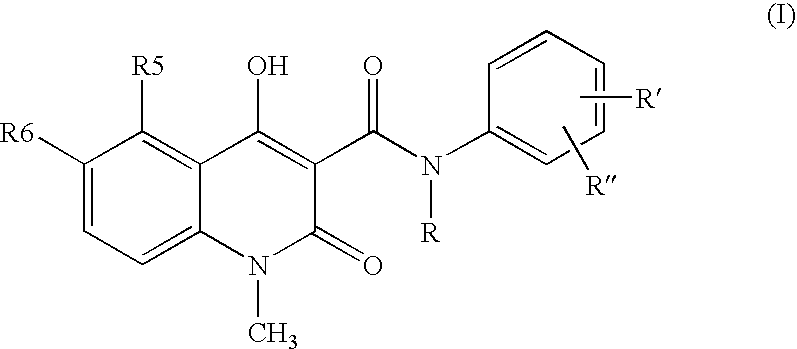
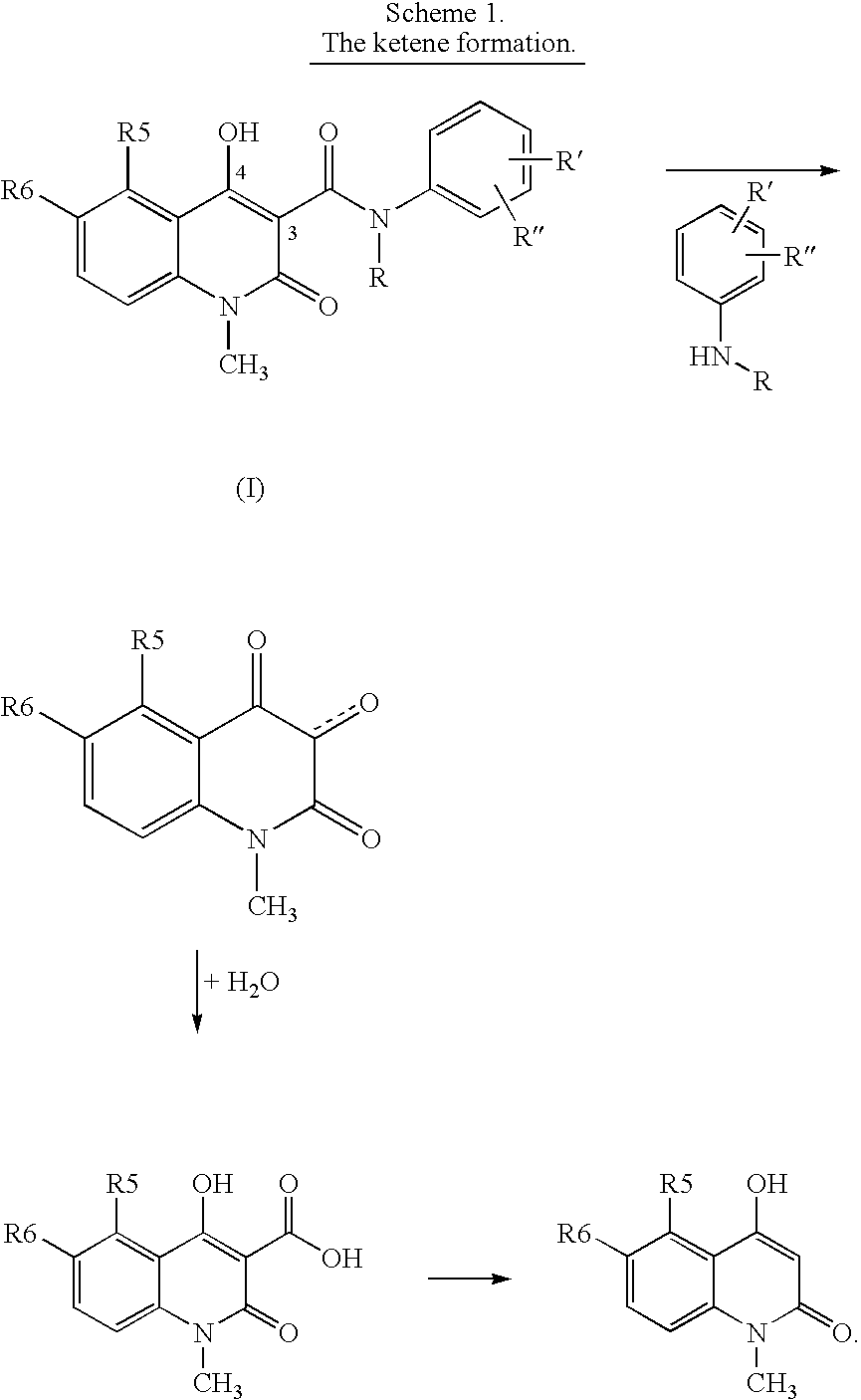
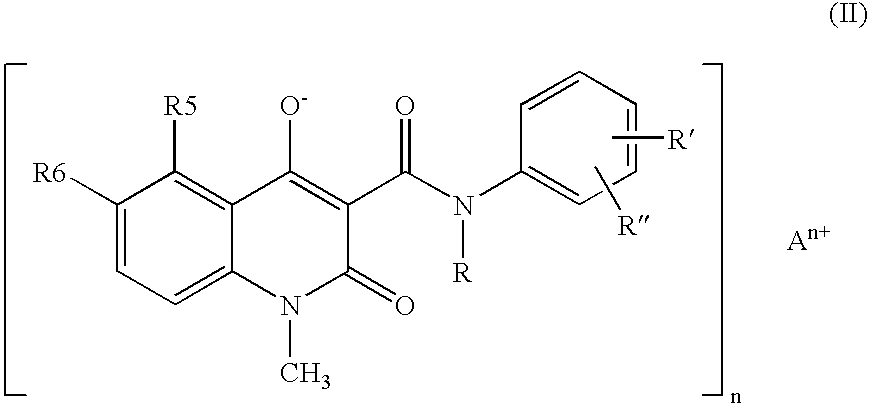
A stable solid pharmaceutical composition consisting essentially of an effective amount of a salt of formula (II) together with an alkaline-reacting component maintaining the pH preferably above 8, or a salt with a divalent metal cation; and at least one pharmaceutical excipient; said salt of formula (II) being essentially stable during storage at room temperature for a period of at least 3 years. A process for stabilizing the salt of formula (II). A crystalline salt of formula (II) and a process for preparing said salt.
Owner:ACTIVE BIOTECH AB
Pharmaceutical excipient having improved compressibility
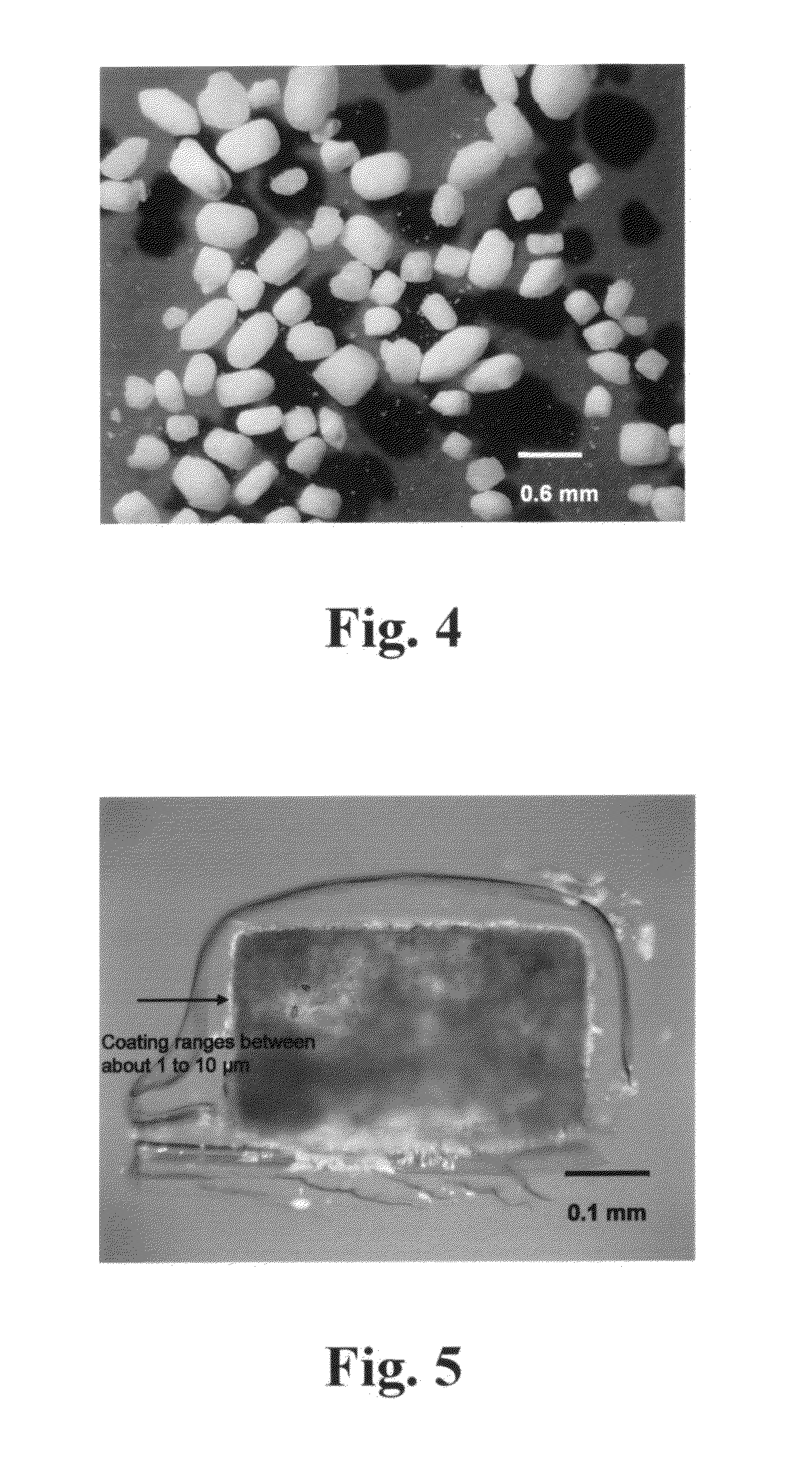
InactiveUS6103219AImprove compression performanceReduce the amount requiredCosmetic preparationsPowder deliverySilica particleColloid
A microcrystalline cellulose-based excipient having improved compressibility, whether utilized in direct compression, dry granulation or wet granulation formulations, is disclosed. The excipient is an agglomerate of microcrystalline cellulose particles and from about 0.1% to about 20% silicon dioxide particles, by weight of the microcrystalline cellulose, wherein the microcrystalline cellulose and silicon dioxide are in intimate association with each other. The silicon dioxide utilized in the novel excipient has a particle size from about 1 nanometer to about 100 microns. Most preferably, the silicon dioxide is a grade of colloidal silicon dioxide.
Owner:J RETTENMAIER & SOEHNE GMBH CO KG ROSENBERG
Compositions and methods of making rapidly dissolving lonically masked formulations
The present invention includes compositions and methods for reduce the taste of the drug in the drug resin complex. The composition may include one or more drug-resin complexes and a highly compressible, free-flowing pharmaceutical excipient. The resin is present in an amount effective to reduce the taste of the drug in the drug resin complex relative to an otherwise identical pharmaceutical composition without the resin; and wherein the highly compressible, free-flowing pharmaceutical excipient causes release of the drug-resin complex in the mouth.
Owner:NEOS THERAPEUTICS LP
Pharmaceutical excipient having improved compressibility
InactiveUS6217909B1Improve compression performanceReduce the amount requiredInorganic/elemental detergent compounding agentsPowder deliverySilica particleSilicon dioxide
Owner:J RETTENMAIER & SOEHNE GMBH CO KG ROSENBERG
Pharmaceutical excipient having improved compressibility
InactiveUS6106865AStrong and robustLess-efficient and costly tablet productionPowder deliveryCosmetic preparationsCompressibilityPharmaceutical preservatives
A composition, comprising (a) microcrystalline cellulose; and (b) a compressibility augmenting agent which (i) physically restricts the proximity of the interface between adjacent cellulose surfaces; or (ii) inhibits interactions between adjacent cellulose surfaces; or (iii) accomplishes both (i) and (ii) above, is disclosed. The composition is in the form of agglomerated particles of microcrystalline cellulose and the compressibility augmenting agent in intimate association with each other.
Owner:J RETTENMAIER & SOEHNE GMBH CO KG ROSENBERG
Compositions containing quinoline compounds
A stable solid pharmaceutical composition consisting essentially of an effective amount of a salt of formula (II)together with an alkaline-reacting component maintaining the pH preferably above 8, or a salt with a divalent metal cation; andat least one pharmaceutical excipient;said salt of formula (II) being essentially stable during storage at room temperature for a period of at least 3 years. A process for stabilizing the salt of formula (II).A crystalline salt of formula (II) and a process for preparing said salt.
Owner:ACTIVE BIOTECH AB
Controlled release formulations using intelligent polymers
InactiveUS6893661B1Promote absorptionMaintenance of therapeutically effective blood levelPowder deliveryOrganic active ingredientsSmart polymerWater contact
An extended release dosage composition of pharmaceutically active substances that have a water contact angle (θ) such that cos θ is between +0.9848 and −0.9848 presented as a matrix tablet containing the said pharmaceutically active substances, with / without suitable pharmaceutical excipients in intimate mixture with two groups of intelligent polymers having opposing wettability characteristics, one demonstrating a stronger tendency towards hydrophobicity and the other a stronger tendency towards hydrophilicity, the polymer combination being between the ratios of 1:50 and 50:1 amounts effective to control the release of said pharmaceutically active substances in a mathematically predictable manner, wherein the polymer demonstrating a stronger tendency towards hydrophobicity is not less than 5% wt / wt and preferably between 5-70% wt / wt of the final formulation composition. The intelligent polymers being ethylcellulose (EC) as a more strongly hydrophobic and hydroxyethylcellulose (HEC) and / or hydroxypropyl methylcellulose (HPMC) as more strongly hydrophilic (the ratio of HEC to HPMC being between 1:100 and 100:1). The matrix tablet is optionally coated with an enteric coat, 0-5%-15% wt / wt to prevent the initial burst effect seen in such systems and to impart gastrointestinal tract (GIT) “stealth” characteristics especially in the presence of food.
Owner:VALEANT INT BERMUDA
Pharmaceutical excipient having improved compressibility
InactiveUS6936277B2Improve compression performanceHigh compression compactibilityPowder deliveryPill deliveryActive agentMedicine
A method of preparing an excipient composition includes forming an aqueous slurry containing a mixture of microcrystalline cellulose in the form of a wet cake and a surfactant, said surfactant being present in an amount from about 0.1% to about 0.5% by weight of the wet-cake microcrystalline cellulose; and drying said slurry to obtain an excipient comprising a plurality of agglomerated particles of microcrystalline cellulose in intimate association with said surfactant. The excipient may be mixed with a therapeutically active agent to form a dosage form. The surfactant provides a hydrophobic boundary at cellulose surfaces, and improves absorptivity of the therapeutically active agent.
Owner:J RETTENMAIER & SOEHNE GMBH CO KG ROSENBERG
Pharmaceutical excipient having improved compressibility
InactiveUS6471994B1Improve compression performanceGood disintegrationPowder deliveryNanotechSilica particleCompressibility
A microcrystalline cellulose-based excipient having improved compressibility, whether utilized in direct compression, dry granulation or wet granulation formulations, is disclosed. The excipient is an agglomerate of microcrystalline cellulose particles and from about 0.1% to about 20% silicon dioxide particles, by weight of microcrystalline cellulose, wherein the microcrystalline cellulose and silicon dioxide are in intimate association with each other. The silicon dioxide utilized in the novel excipient has a particle size from about 1 nanometer to about 100 microns. Most preferably, the silicon dioxide is a grade of colloidal silicon dioxide. An extra low moisture excipient is provided which exhibits improved compressibility as compared to conventional microcrystalline cellulose, while providing a moisture content of from about 0.5 to 2.5% LOD, preferably between about 0.5 and about 1.8%, more preferably between 0.8 and 1.5%, and most preferably between about 0.8 and about 1.2 %.
Owner:J RETTENMAIER & SOEHNE GMBH CO KG ROSENBERG
Preparation and use of Dendrobium nobile polysaccharide extract
InactiveCN101407557AQuality is easy to controlIncrease contentOrganic active ingredientsMetabolism disorderAlcoholMedicine
The invention provides a preparation method of dendrobium nobile polysaccharide extracts, which is realized by steps of degreasing, extracting, alcohol precipitating, protein removing, monose and oligosaccharide removing and purifying gel columns and the like. The polysaccharide content in the polysaccharide extract is more than 93 percent. The polysaccharide extract has remarkable oxidation prevention activity, can be combined with medicament excipients and carriers permitted by preparation agents, and can be applied in preparing medicaments or medicament combinations for treating and preventing cardiovascular diseases. The preparation method of the dendrobium nobile polysaccharide extracts is reasonable in design and convenient in operation.
Owner:ZHEJIANG UNIV
Purified galactomannan as an improved pharmaceutical excipient
InactiveUS6063402AExcellent hardness propertiesOrganic active ingredientsBiocideProtein materialsImpurity
Disclosed is a substantially anhydrous, powdered, galactomannan composition consisting essentially of a galactomannan hydrocolloid exhibiting about 50% to about 90% by weight of anhydromannose residues and about 10% to about 50% by weight anhydrogalactose residues; less than about 1% by weight of protein material and less than about 3% of other nonaqueous impurities. This material is useful for preparing pharmaceutical compositions both in the substantially anhydrous form but preferably in an anhydrated form which includes about 5-15% by weight water. The pharmaceutical compositions comprise a therapeutically effective amount of a drug, the hydrated powered gallactomannan composition and optionally other pharmaceutically-acceptable excipients. When the hydrated powdered purified glactomannan of the invention is used to form a tablet, one sees improved hardness in the tablet formed. The pharmaceutical composition of the invention is particularly valuable for delivering a therapeutically effective drug to the colon without significant release of the drug in the upper GI tract after oral administration of the composition. Unique means to prepare the purified galactomannan in large quantities is provided.
Owner:AMARIN DEV
Combination antihistamine medication
The invention provides a topical pharmaceutical composition for application to the nasal or ocular mucosa which comprises (1) a pharmaceutical excipient suitable for topical administration, (2) a mucosal adjuvant, (3) an antihistamine drug and (4) a mast cell stabilizer, a non-steroidal anti-inflammatory drug, a phosphodiesterase inhibitor, an anti-IgE agent, heparin, a topical steroid or a leukotriene blocker.
Owner:FAIRFIELD CLINICAL TRIALS
Pharmaceutical excipient having improved compressibility
InactiveUS6746693B2Improve compression performanceReduce the amount requiredPowder deliveryNanotechSilica particleCompressibility
Owner:J RETTENMAIER & SOEHNE GMBH CO KG ROSENBERG
PLA/PLGA shell-core microballoons prepared by oil in water-solid in oil method, and preparation method thereof
InactiveCN101461786ASmooth and rounded surfaceDisadvantages of Avoiding PollutionPharmaceutical non-active ingredientsGranular deliveryControlled releaseAcetic acid
The invention relates to a PLA / PLGA shell-core microsphere prepared by solid-in-oil-in-water in the technical field of pharmacy and a preparation method thereof. The microsphere comprises 0.01 to 50 percent of medicine, 20 to 99.99 percent of polylactic acid and / or polylactic acid-glycolic acid, or / and 0 to 30 percent of pharmaceutical excipient (weight percentage). The method comprises the steps of: adding medicine particles into a PLA and / or PLGA organic solution to be emulsified, then selecting a hydrophilic organic solvent to re-emulsify to form unhardened balls, finally hardening in another large oil phase, removing the organic solvent and collecting micropheres. The method overcomes the disadvantages of low envelope rate of the prior W / O and W / O / W, serious burst release of S / O / O, and environmental pollution, controls the grain diameter of the microsphere according to the need, does not pollute the environment, and can be applied to the preparation of slow release or controlled release microspheres of various medicines and adjuvants of vaccines.
Owner:SHANGHAI JIAO TONG UNIV
Combination antihistamine and steroid medication
The invention provides a topical pharmaceutical composition for application to the nasal or ocular mucosa which comprises (1) a pharmaceutical excipient suitable for topical administration, (2) an antihistamine drug and (3) a mast cell stabilizer, a non-steroidal anti-inflammatory drug, a phosphodiesterase inhibitor, an anti-IgE agent, heparin, a topical steroid or a leukotriene blocker.
Owner:FAIRFIELD CLINICAL TRIALS
Compositions and methods of making rapidly dissolving ionically masked formulations
The present invention includes compositions and methods for reduce the taste of the drug in the drug resin complex. The composition may include one or more drug-resin complexes and a highly compressible, free-flowing pharmaceutical excipient. The resin is present in an amount effective to reduce the taste of the drug in the drug resin complex relative to an otherwise identical pharmaceutical composition without the resin; and wherein the highly compressible, free-flowing pharmaceutical excipient causes release of the drug-resin complex in the mouth.
Owner:NEOS THERAPEUTICS LP
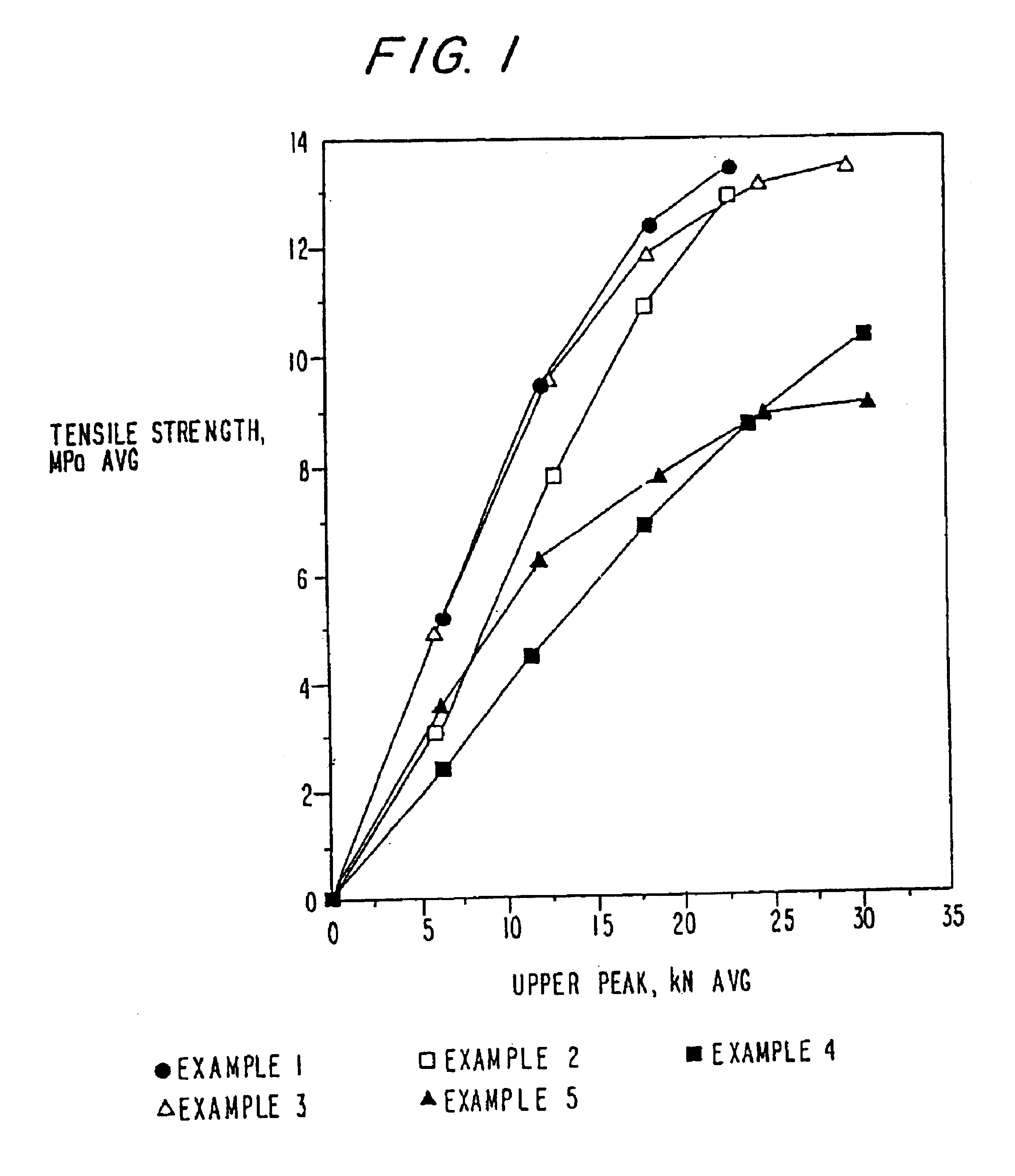
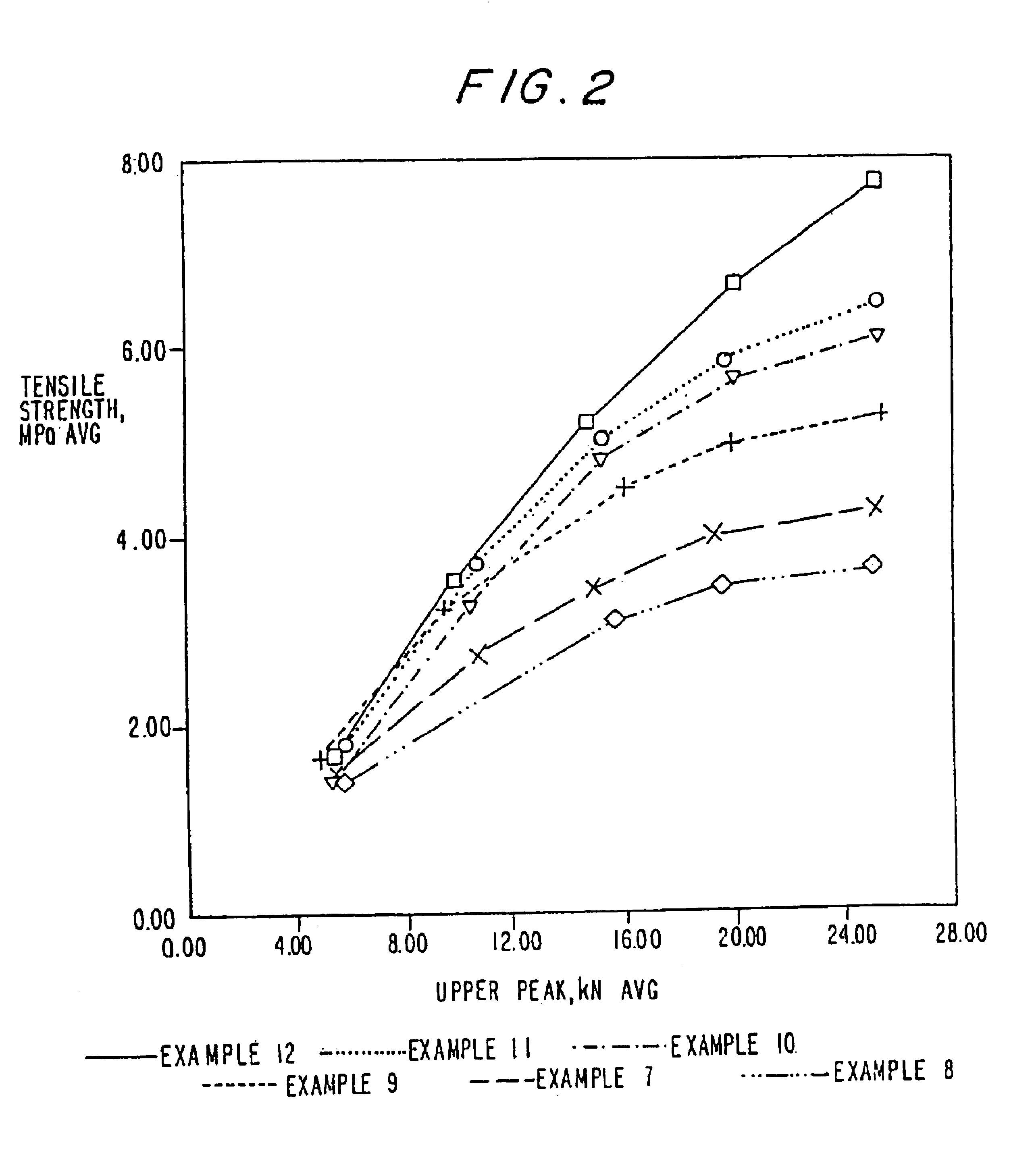
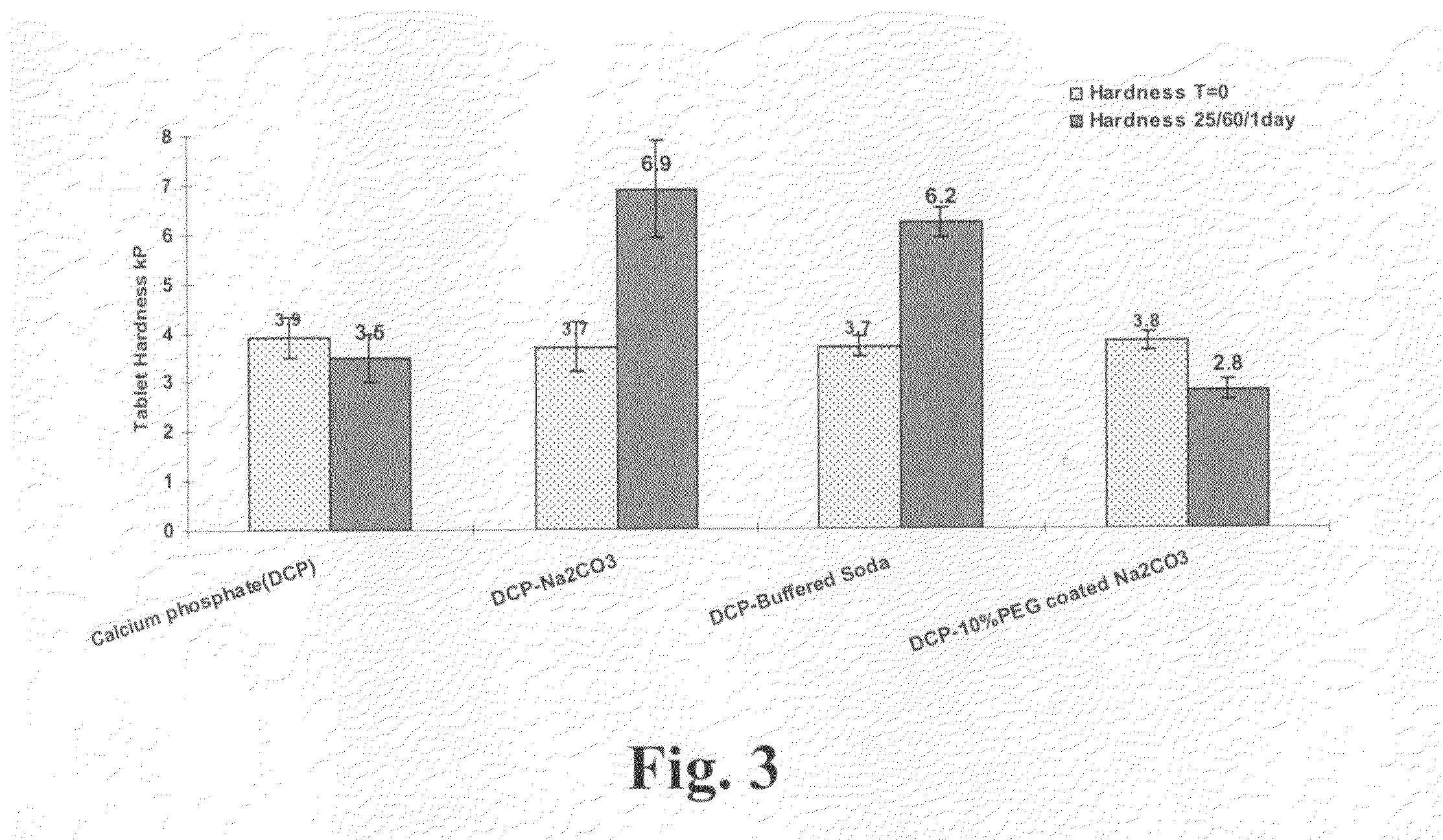
Polyethylene glycol-coated sodium carbonate as a pharmaceutical excipient and compositions produced from the same
Non-effervescent pharmaceutical compositions having at least one particle of carbonate coated by a layer of polyethylene glycol that substantially covers the at least one carbonate particle are described. Compositions are also described where the compositions include a weakly basic therapeutic agent, a first pH-modifying agent having at least one particle of carbonate coated by a layer of polyethylene glycol, and a second pH-modifying agent. The weakly basic therapeutic agent could be, but is not limited to, zolpidem or scopolamine. Compositions including zolpidem and scopolamine are used to treat insomnia and depression, respectively.
Owner:TRANSCEPT PHARMA
Hydroxypropyl- sulfobutyl-beta- cyclodextrin and its preparation method, analytical method and pharmaceutical uses
ActiveCN1800221ALow hemolyticHemolyticOrganic active ingredientsComponent separationPharmaceutical drugCyclodextrin Derivatives
The invention relates to a hydroxypropyl -sulfo butyl -ª‰-cyclodextrin and its preparing method, analysis method and the applying in medicine, wherein the hydroxypropyl -sulfo butyl -ª‰-cyclodextrin is the cyclodextrin derivation displaced by hydroxypropyl and sulfo butyl. The n-(2, 3, 6-O-2-hydroxypropyl )-m-(2, 3, 6-O-sulfo butyl)-ª‰-cyclodextrin, each mole cyclodextrin displaced group number is n hydroxypropyl and m sulfo butyl, wherein n is the average displaced degree of the hydroxypropyl displaced group; m is the average displaced degree of the sulfo butyl displaced group; n+mú¢Z is the total average displaced degree; nú¢any of the 1,2,3,4,5,6, 7,8,9; m=any of the 1,2,3,4,5,6, 7,8,9; z=any of the 1,2,3,4,5,6, 7,8,9,10.
Owner:BIKA BIOTECH GUANGZHOU CO LTD
Carbidopa/Levodopa gastroretentive drug delivery
A gastroretentive drug formulation for the sustained release of an active agent in the gastrointestinal tract comprises an internal layer or compartment comprising an active agent and one or more pharmaceutical excipients, of which at least one is a polymer and two membranes forming together an envelope around the inner membrane, each membrane comprising at least one polymeric combination of an enteric polymer which is not soluble in gastric juice, and an hydrophilic swelling polymer, and at least one plasticizer.
Owner:INTEC PHARMA
Pharmaceutical excipient having improved compressibility
InactiveUS20060008522A1Improve compression performanceHigh compression compactibilityWood working apparatusPill deliveryActive agentSlurry
A method of preparing an excipient composition includes forming an aqueous slurry containing a mixture of microcrystalline cellulose in the form of a wet cake and a surfactant, said surfactant being present in an amount from about 0.1% to about 0.5% by weight of the wet-cake microcrystalline cellulose; and drying said slurry to obtain an excipient comprising a plurality of agglomerated particles of microcrystalline cellulose in intimiate association with said surfactant. The excipient may be mixed with a therapeutically active agent to form a dosage form. The surfactant provides a hydrophobic boundary at cellulose surfaces, and improves absorptivity of the therapeutically active agent.
Owner:J RETTENMAIER & SOEHNE GMBH CO KG ROSENBERG
Rapidly disintegrating tablet comprising an acid-labile active ingredient
InactiveUS7147869B2Improve stabilityUniform deliveryBiocideDigestive systemOral medicationTriglyceride
A rapidly disintegrating tablet for oral administration of acid-labile active ingredients is described. The rapidly disintegrating tablet for oral administration of an acid-labile active ingredient comprises a plurality of individual active ingredient units together with pharmaceutical excipients, where the acid-labile active ingredient is present in the individual active ingredient units in a matrix composed of a mixture comprising at least one solid paraffin and one or more substances from the group of fatty alcohol, triglyceride and fatty acid ester, and where excipients which, on oral intake of the tablet, bring about rapid disintegration of the tablet are present.
Owner:TAKEDA GMBH
Pharmaceutical composition based on agonist of benzodiazepine
The present invention describes the use of pharmaceutical compounds in pharmaceutical compositions for sublingual administration, including as active ingredient thereof, an agonist of the central receptor of benzodiazepinics chosen among diazepam, lorazepam, bromazepam, triazolam, alprazolam, flunitrazepam, nitrazepam and midazolam maleate, in a mixture with a pharmaceutical excipient consisting of, at least, 70% of the weight of the final formulation containing 40-45% by weight of lactose, 15-27% by weight of sorbitol and 12-16% by weight of cellulose.
Owner:SIGMA PHARMA LTDA
Dexlansoprazole compositions
Premixes of dexlansoprazole with pharmaceutical excipients, processes for preparing premixes, pharmaceutical formulations containing the premixes, and their use in treatment of erosive esophagitis and heartburn associated with non-erosive gastroesophageal reflux disease.
Owner:DR REDDYS LAB LTD +1
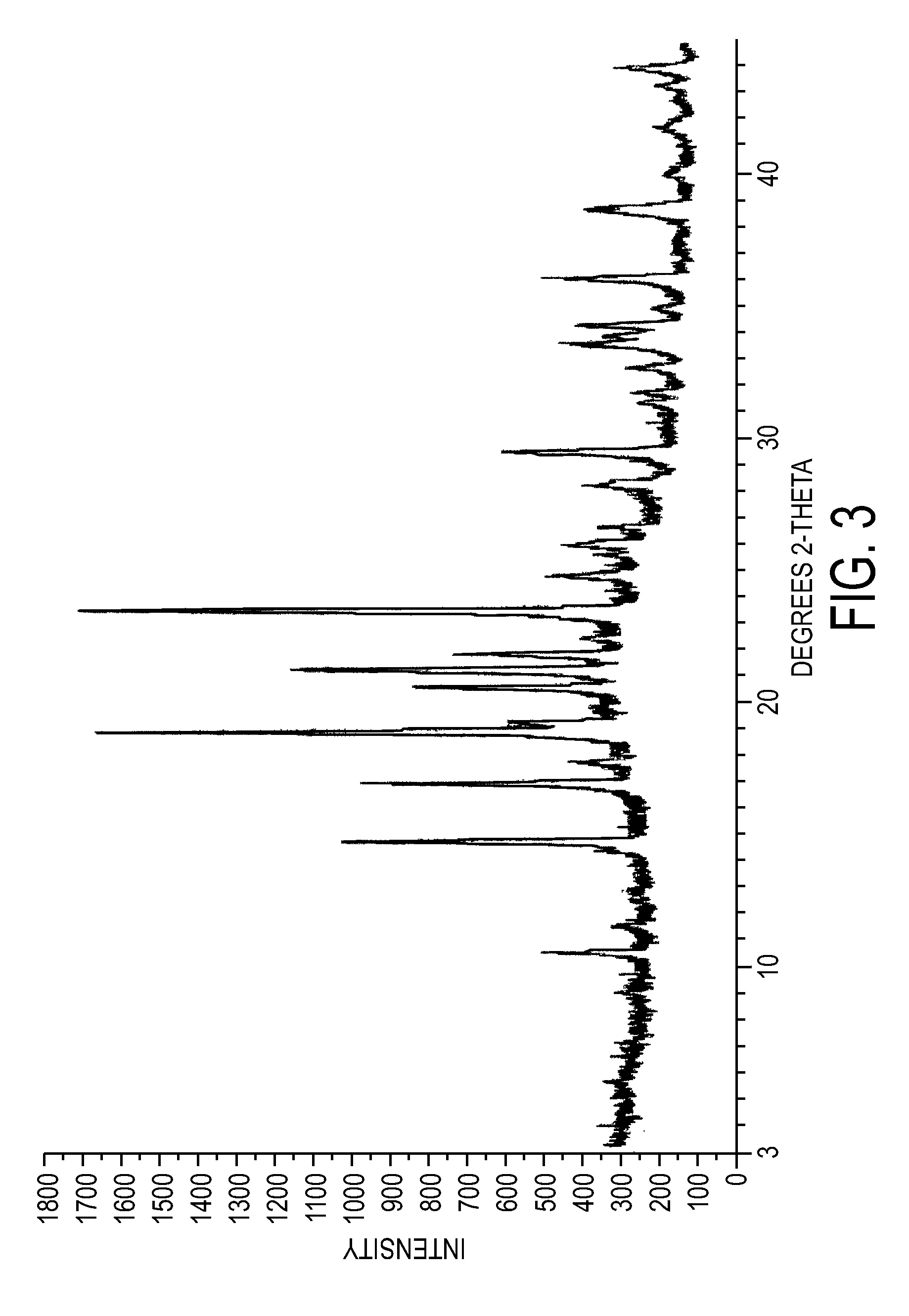
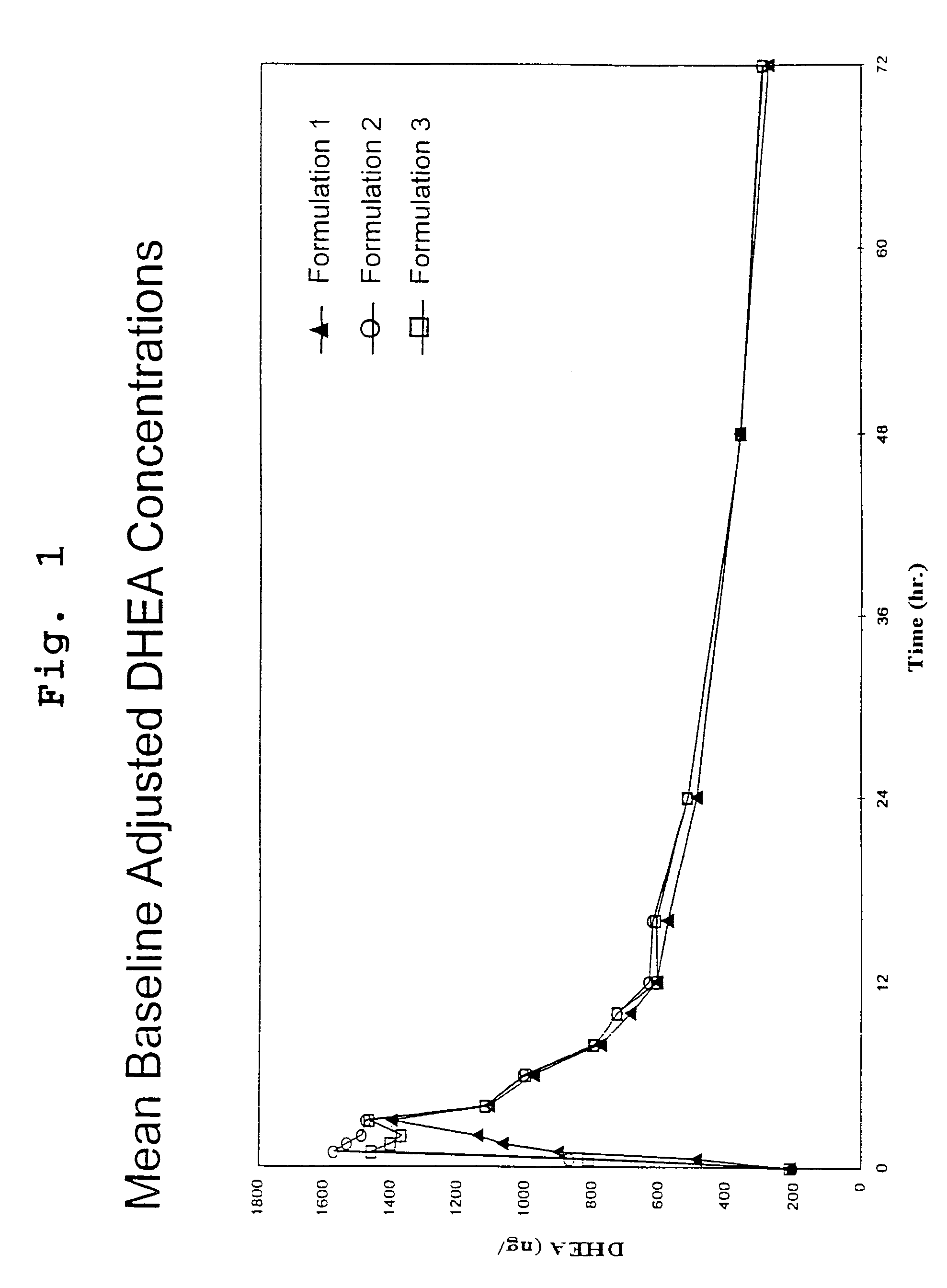
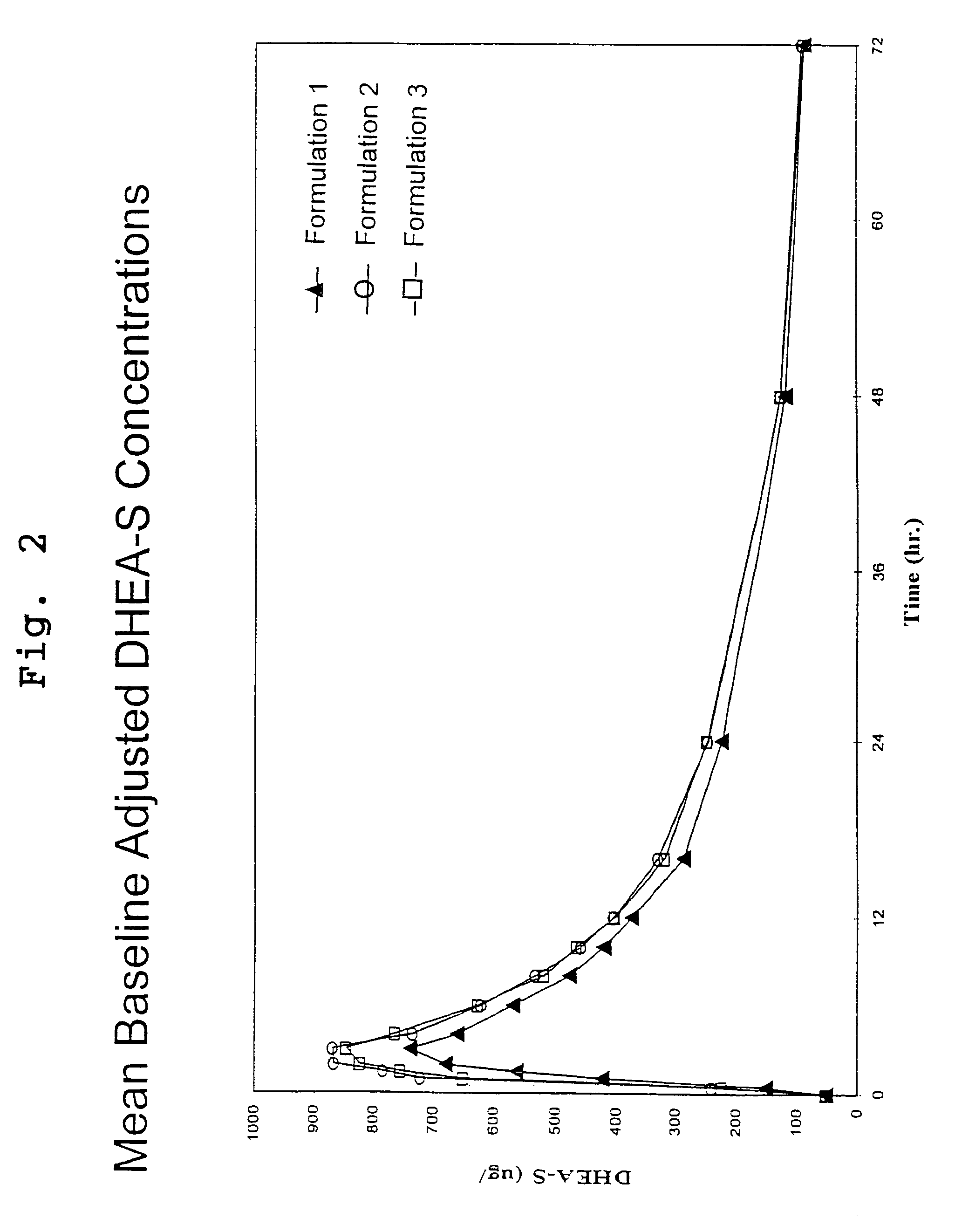
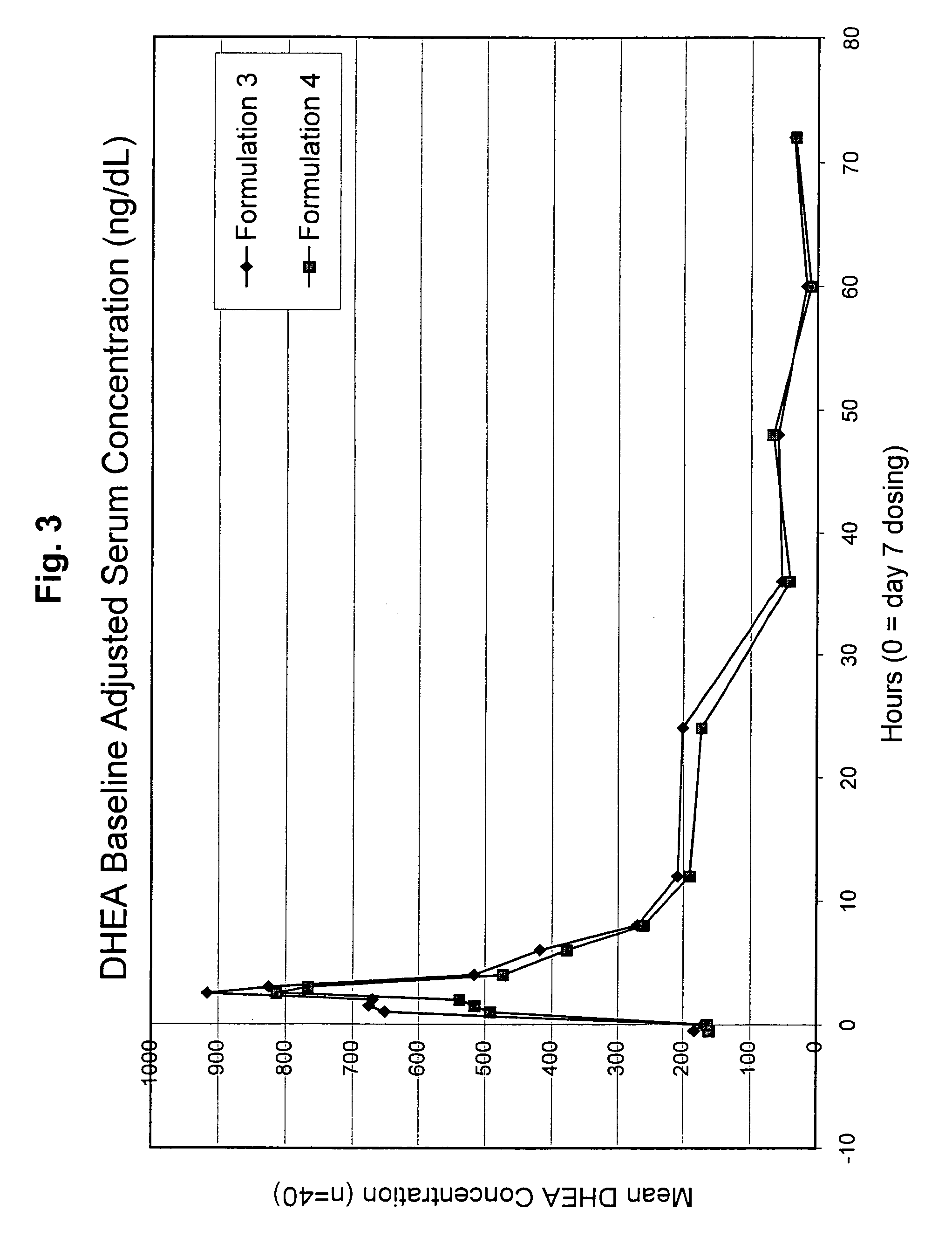
DHEA composition and method
Disclosed are improved pharmaceutical formulations comprising dehydroepiandrosterone (DHEA), enriched in selected polymorphic forms, for therapeutic applications. In one embodiment, the formulation comprises, in solid form, DHEA, at least 85% of which is present as a single polymorph selected from the form I polymorph or the form II polymorph, and at least one pharmaceutical excipient. Methods for making and using such compositions are also disclosed.
Owner:GENELABS TECH INC
Panax notoginseng polysaccharide extract and preparation method, preparations and applications thereof
InactiveCN102961424AContribute to comprehensive utilizationLow costImmunological disordersAntineoplastic agentsPANAX NOTOGINSENG ROOTFiltration
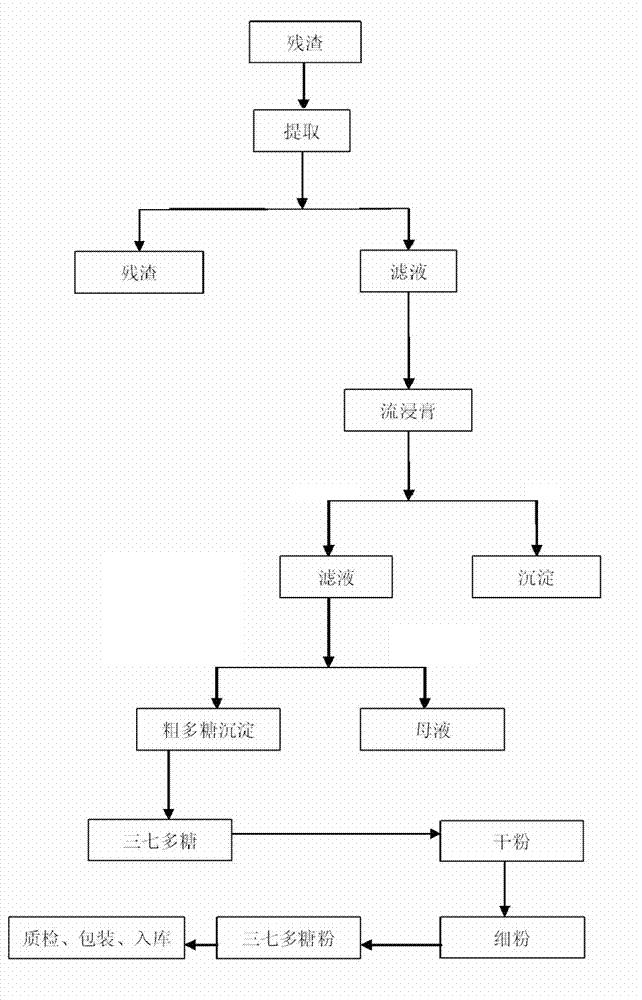
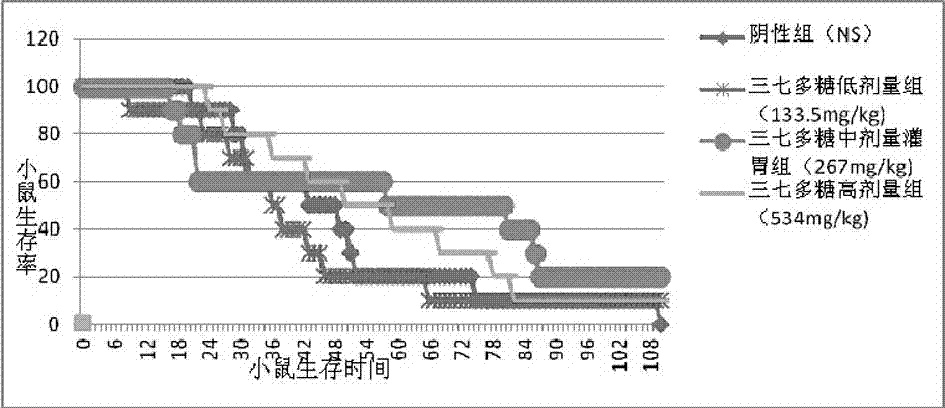
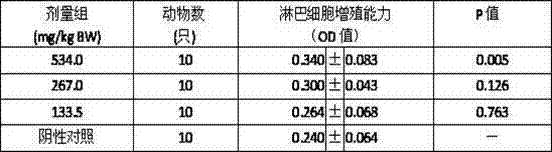
The invention discloses a panax notoginseng polysaccharide extract and a preparation method, preparations and applications thereof. The panax notoginseng polysaccharide extract is prepared by taking waste residues produced after panax notoginseng saponins are extracted as raw materials through the steps of extraction, concentration, purification, drying, crushing and detection, wherein the content of panax notoginseng polysaccharides is not less than 15%. The preparation method of the panax notoginseng polysaccharide extract comprises the steps of extraction, concentration, purification, drying, crushing and detection, and specifically comprises the steps of adding waste residues produced after panax notoginseng saponins are extracted into purified water for extracting, carrying out filtration and concentration on the obtained object, adding ethanol into the obtained object for precipitating, and carrying out filtration, purification and drying on the obtained product so as to obtain a target dried product. Pharmaceutical excipients are added into the panax notoginseng polysaccharide extract to be prepared into powder, capsules, granules, tablets or pills; and the panax notoginseng polysaccharide extract is applied to the preparation of antineoplastic drugs and immunity enhancing drugs. According to the invention, waste residues produced after panax notoginseng saponins are extracted are reused, thereby changing waste material into things of value, facilitating the comprehensive utilization of panax notoginseng resources, reducing the cost and improving the economic benefits.
Owner:YUNNAN YUNKE PHARMA
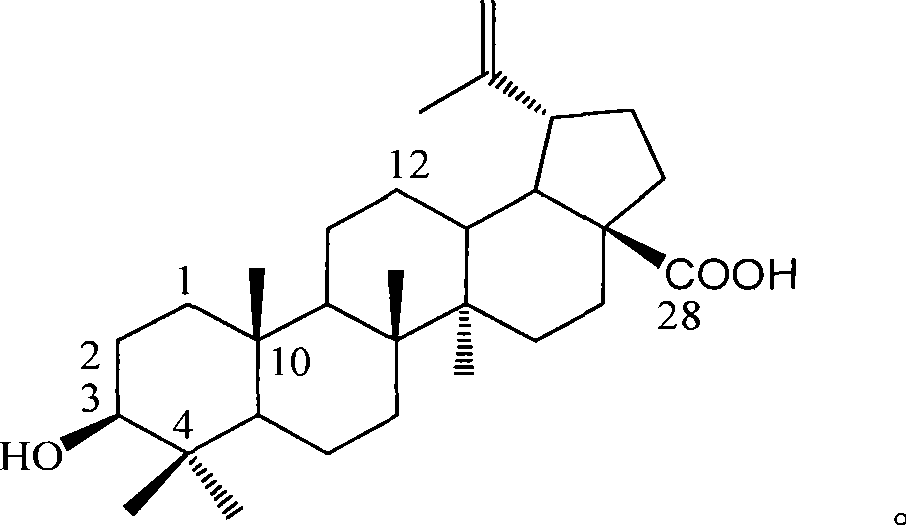
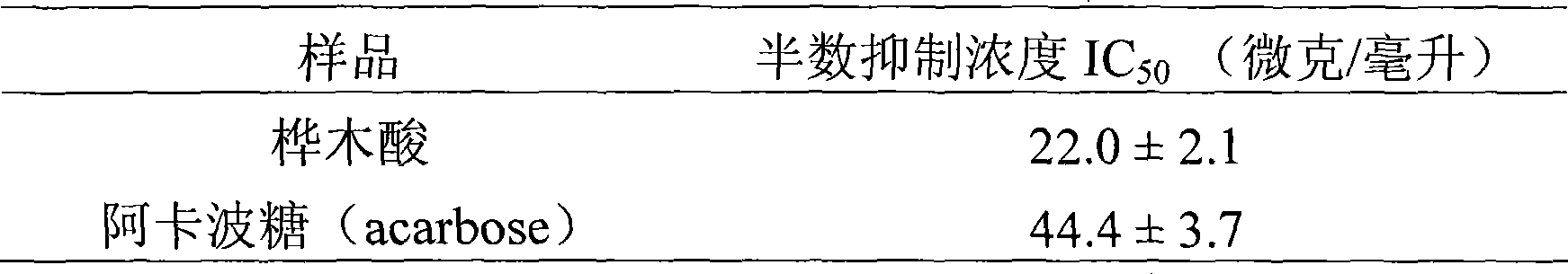
Use of betulinic acid in preparing glycosidase inhibitor medicine
InactiveCN101416971ALow costRich sourcesOrganic active ingredientsMetabolism disorderLupeolDrug excipient
The invention provides the application of betulinic acid and salts thereof suitable for medical purpose in the preparation of a drug of a glycosidase inhibitor. The compound of the invention is the compound of lupeol type pentacyclic triterpene acid obtained by separating lagerstroemia plants. Pharmacological tests prove that the compound has obvious effect in inhibiting Alpha-glucosidase, the inhibitory activity of which exceeds acarbose 100% for initial therapy. Therefore, drug compositions made from the compound or the salts thereof suitable for medical purpose and drug excipients or carriers allowable for preparation can be applied to the preparation of drugs for preventing or treating Type II Diabetes, namely, noninsulin-dependent diabetes mellitus. The constitutional formula shown in formula (1) of the invention is shown as above.
Owner:ZHEJIANG UNIV
Granular pharmaceutical compositions
Pharmaceutical compositions comprising a plurality of formulated particles containing at least one active ingredient and at least one pharmaceutically acceptable excipient, granulated with a granulating composition containing at least one pharmaceutical excipient.
Owner:DR REDDYS LAB LTD +1
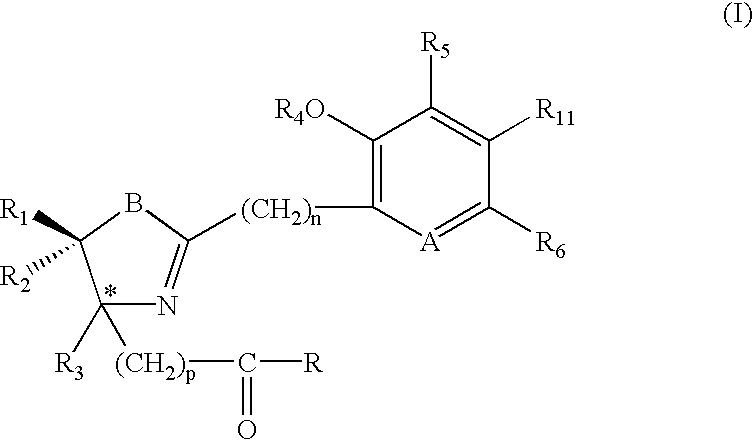
Iron binding agents
Composition, article of manufacture for and method of treating malaria in a human having an infestation of Plasmodium protozoans are described. The method comprises administering a therapeutically-effective amount of a compound of formula (I) or (IV), i.e. sufficient quantity to reduce the population of Plasmodium. The composition of the invention is a compound of formula (I) or (IV) with a pharmaceutical excipient. The article of manufacture is the composition in combination with labeling for treating malaria. The substituents are detailed in the specification.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Pharmaceutical formulations comprising substituted benzimidazole derivatives
InactiveUS20120058194A1Increasing duration of timeImprove stabilityBiocidePowder deliveryBenzimidazole derivativeGastro-esophageal reflux disease
Stabilized substituted benzimidazole modified release pharmaceutical formulations with at least two drug-containing fractions, wherein the release from a first fraction precedes the release from a second fraction, pharmaceutical excipients, processes for preparing the stable formulations, packaging therefor, and their use in treatment of erosive esophagitis and heartburn associated with non-erosive gastroesophageal reflux disease.
Owner:DR REDDYS LAB LTD +1
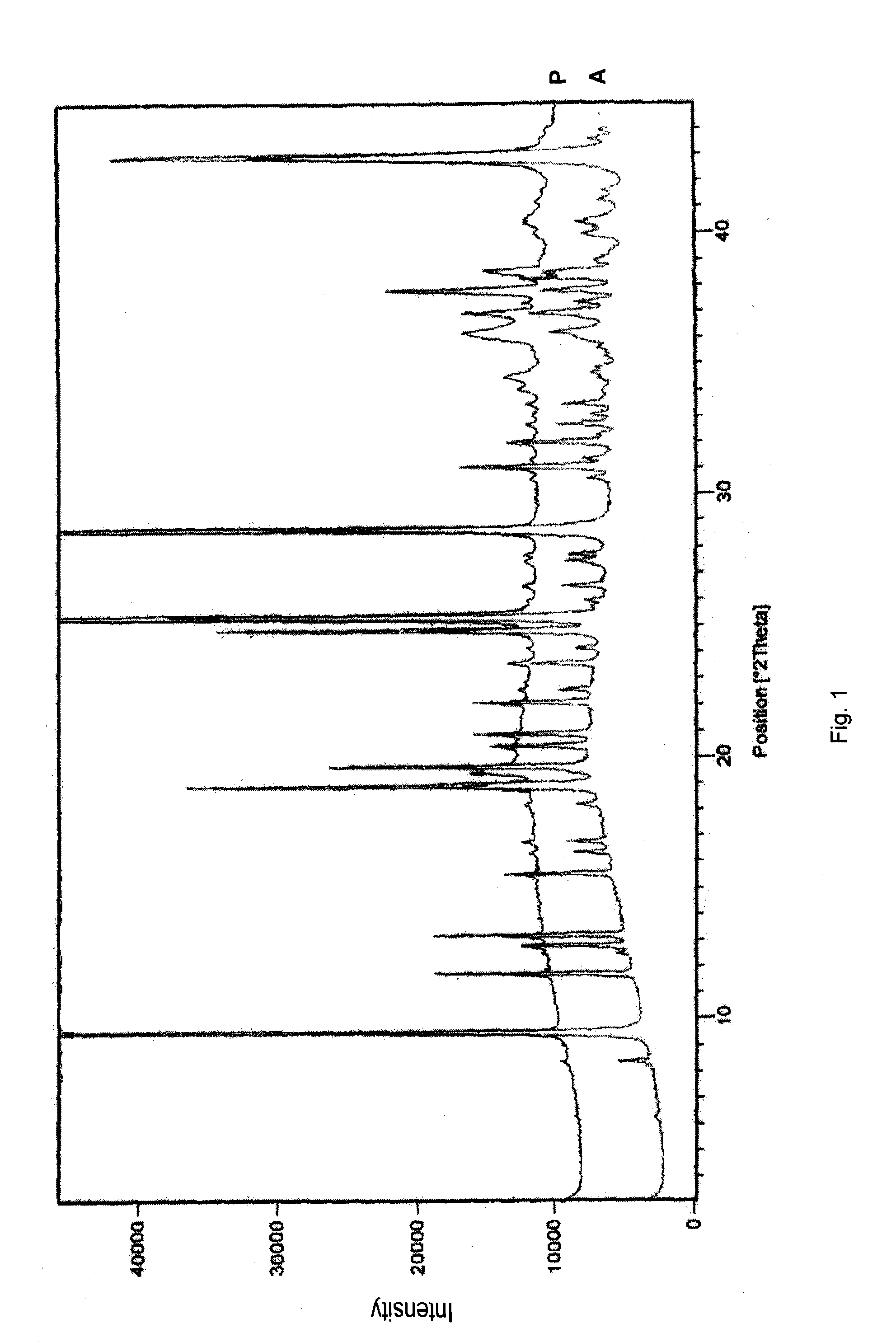
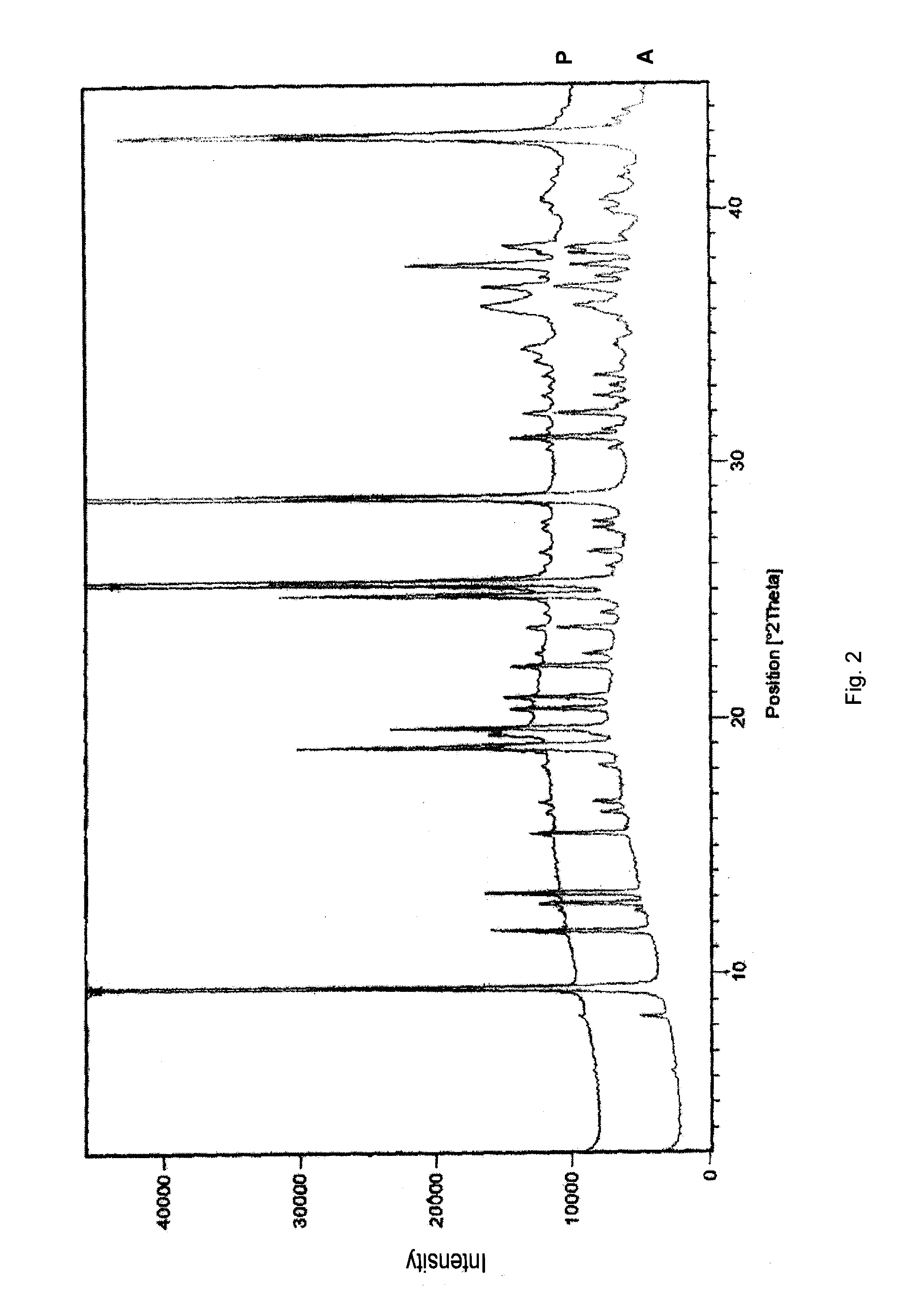
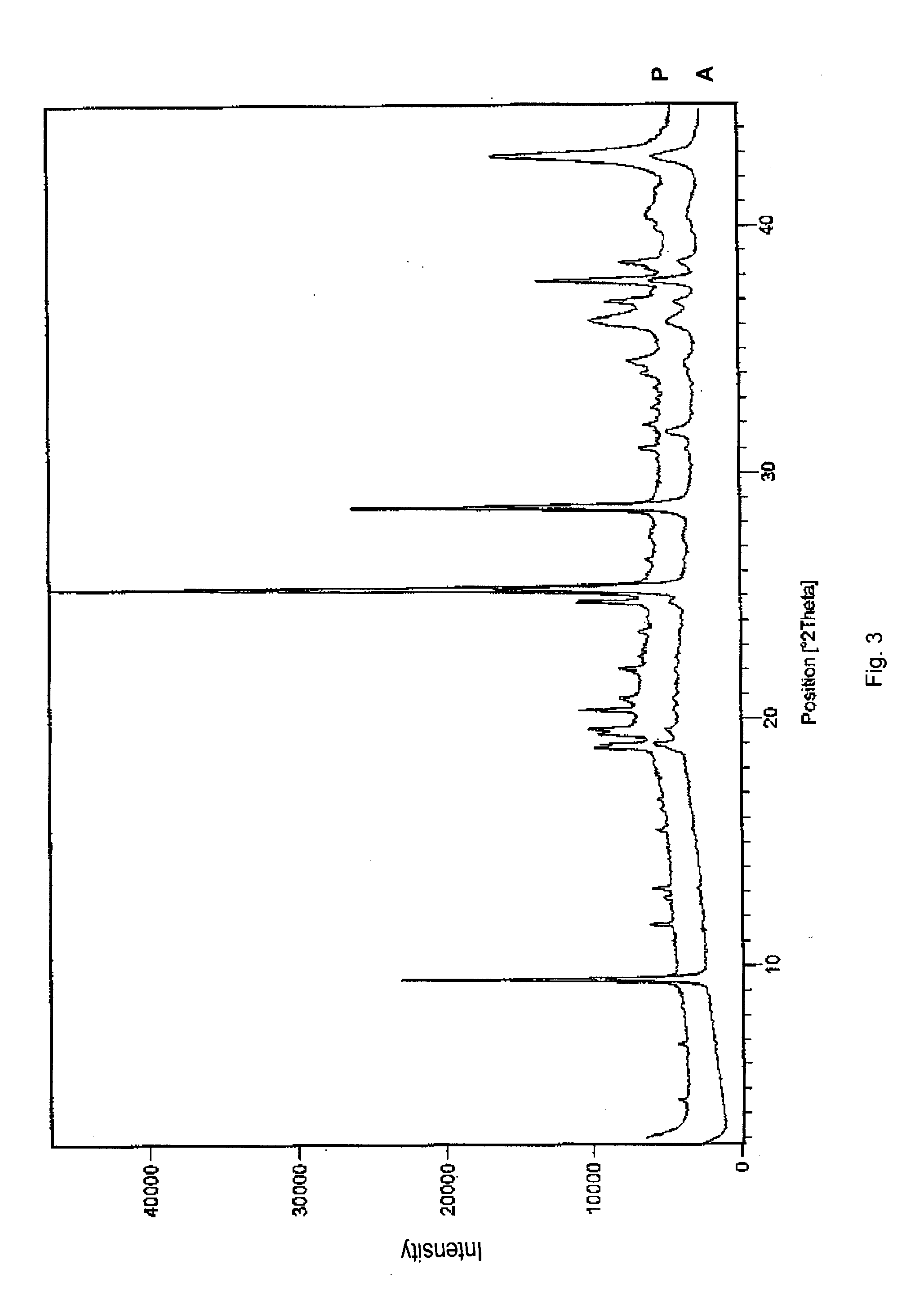
Absorbable and hemostatic multifunctional particle with tissue induction and preparation and application of multifunctional particle
ActiveCN102772821AGood hemostasisImprove liquid absorption performanceAbsorbent padsBandagesHyaluronic acidPharmaceutical Excipient
The invention relates to a multifunctional particle used for stopping human body wounds bleeding, filling, absorbing fluid, preventing adhesion and inducing tissue regeneration and repair. The absorbable particle with multiple functions of stopping bleeding, filling, absorbing the fluid, preventing the adhesion and inducing the tissue regeneration and repair comprises the following raw materials in weight percentage: 30-98.95 percent of polyanionic substance, 1-70 percent of cationic substance and / or polycationic substance, 0.05-2 percent of hyaluronic acid and / or functional factor polypeptide and 0-68.95 percent of pharmaceutical excipient. The particle is 0.01-1.0mm in particle size, and the particle is irregular in shape. The absorbable particle material with the multiple functions of stopping bleeding, filling, absorbing the fluid, preventing the adhesion and inducing the tissue regeneration and repair can be used for stopping the human body wounds bleeding, filling, absorbing fluid, preventing the adhesion and inducing the tissue regeneration and repair and is especially suitable for the wounds and defective tissues during minimally invasive surgery.
Owner:SUZHOU BOCHUANG TONGKANG PHARM TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com