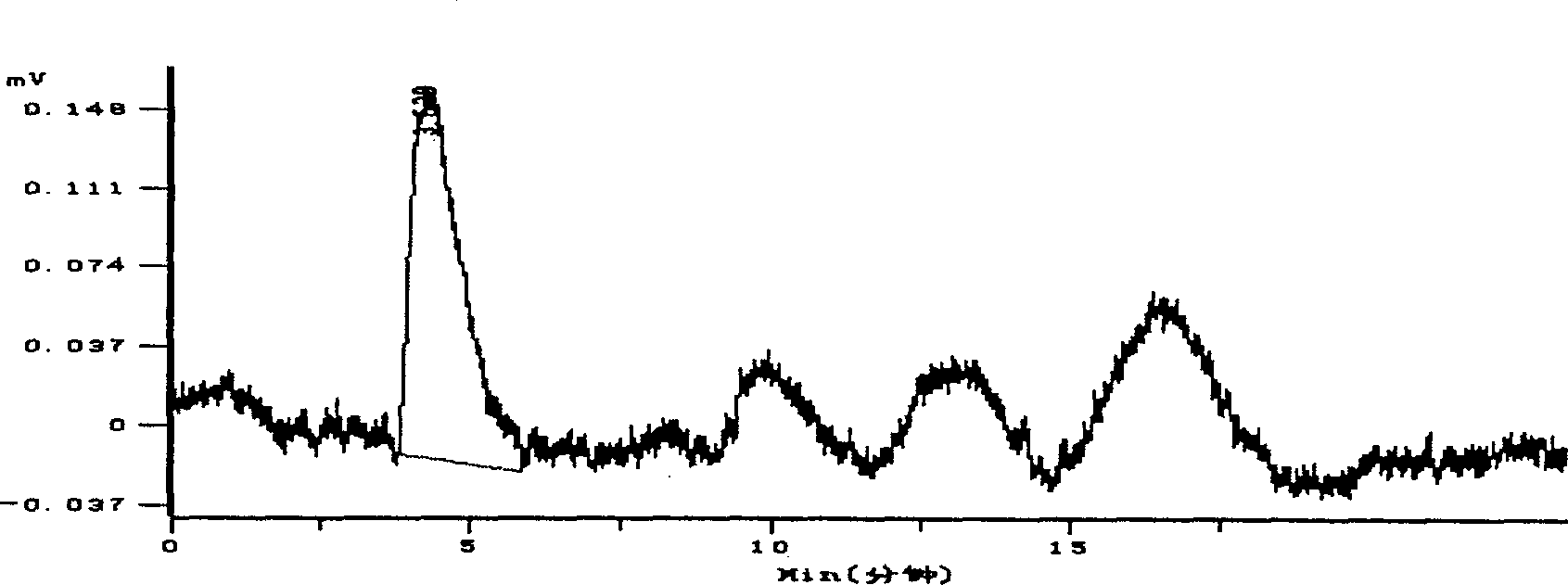
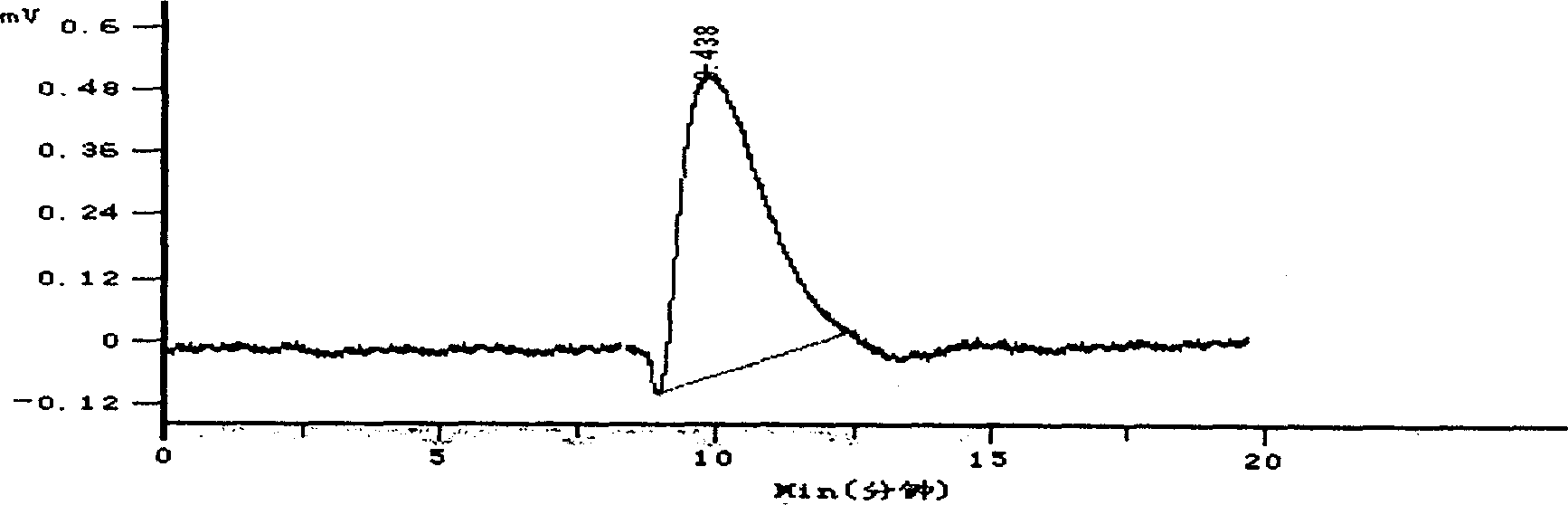
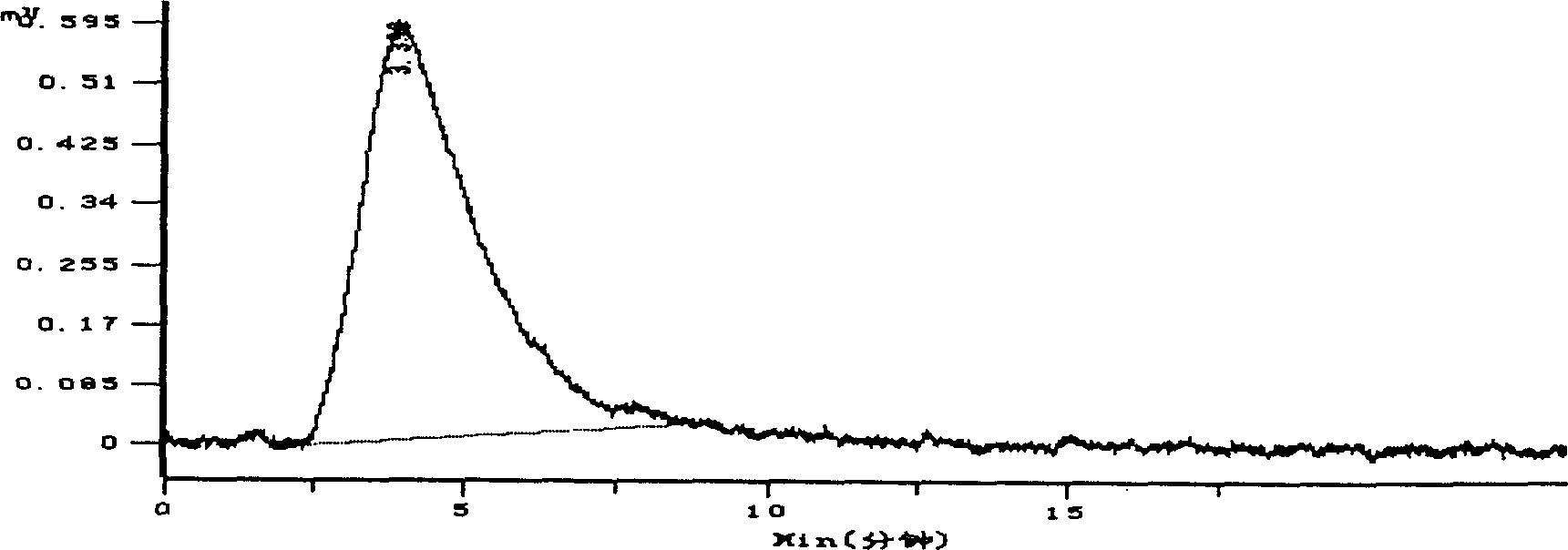
Hydroxypropyl- sulfobutyl-beta- cyclodextrin and its preparation method, analytical method and pharmaceutical uses
一种分析方法、环糊精的技术,应用在药物载体,羟丙基-磺丁基-β-环糊精领域,能够解决赋形剂配比高、影响环糊精作用功能等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0156] Example 1, respectively add 0.02molβ-cyclodextrin, 0.11molNaOH, 45mlH 2 O, Stir to dissolve and heat to 75°C ~ 80°C, then add 0.08mol 1,4-butyl sultone dropwise in a heat-retaining manner, finish adding in about 3 hours, continue to stir for 2 hours, then cool to room temperature 20°C, slowly drop while stirring Add 0.08mol 1,2-propylene oxide, add about 3 hours, continue stirring for 5 hours, adjust pH to neutral with hydrochloric acid, filter, and dialyze the filtrate to remove residual β-cyclodextrin and 1,2-propanediol and Sodium gamma-hydroxybutanesulfonate. After dialysis for 8-10 times, it was concentrated and dried under reduced pressure to obtain 25.7 g of off-white solid with a weight yield of 111.7%. 1 HNMR spectrum (attached Figure 12 ) values are analyzed in the following table:
[0157]
ppm
proton attribution
peak characteristics
proton group
1.05...
Embodiment 2
[0163] Example 2, respectively add 0.02mol of β-cyclodextrin, 0.04mol of NaOH, 90mlH 2 O, stir to dissolve, slowly add 0.03mol of 1,2-epoxypropylene dropwise under stirring at 20°C, and finish adding in about 2 hours. Add 0.3 mol of 1,4-butyl sultone dropwise, finish adding in about 3 hours, continue to stir for 5 hours, then cool to room temperature, adjust the pH to neutral with hydrochloric acid, filter, and dialyze the filtrate to remove residual β-cyclodextrin and 1,2-propanediol and sodium γ-hydroxybutanesulfonate produced by the reaction. After dialysis for 8-10 times, concentrated and dried under reduced pressure to obtain 25 g of off-white solid with a yield of 108.7%. 1 HNMR verification shows that the average substitution number of hydroxypropyl is 1.2, and the average substitution degree of sulfobutyl is 8.9, abbreviated as HP 1 -SBE 9 -β-CD.
Embodiment 3
[0164] Embodiment 3, is substantially the same as embodiment 1, but adds 70mlH 2 O, where adding NaOH, 1,4-butyl sultone, and 1,2-propylene oxide are charged at 0.20, 0.16, and 0.10 mol. Finally, the product yield is 105.5%. 1 HNMR (with Figure 15) verified that the average substitution number of hydroxypropyl is 5.6, and the average substitution degree of sulfobutyl is 2.6, abbreviated as HP 3 -SBE 6 -β-CD.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com