Slow-release film agent of isegenan
A technology of Estherglam and slow-release film, which is applied in the field of slow-release film preparations to achieve long-acting antibacterial effects, increase bioavailability, and prolong the effect of drug action time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1 Preparation of slow-release film of the present invention
[0039] Extended-release film prescription
[0040] The protective layer:
[0041] PVA17-88 80% Glycerin 0.8% Xylitol 0.4% Propylene Glycol 3.2% Tween 80 1% Purified Water
[0042] Drug film layer:
[0043] Estherglam 0.03% Gum Arabic 50% CMC-Na10% Glycerin 0.8% Propylene Glycol 3.2% Xylitol 0.4% Tween 80 1% Starch 3.2% Sorbic Acid 0.2% Phosphate buffer (pH6.8) added to the balance
[0044] Preparation method:
[0045] 1. Fabrication of protective layer
[0046] Wash and purify PVA17-88 repeatedly with an appropriate amount of 85% ethanol, soak in sufficient pure water overnight to fully swell, heat at 75°C to dissolve, filter, and slowly add glycerin, propylene glycol, Tween, xylitol and pure water in sequence And fully stir to make the mixture uniform, remove air bubbles, and form a film
[0047] 2. Preparation of drug film layer
[0048] ①Soak and swell CMC-Na with buffer solution at a rat...
Embodiment 2
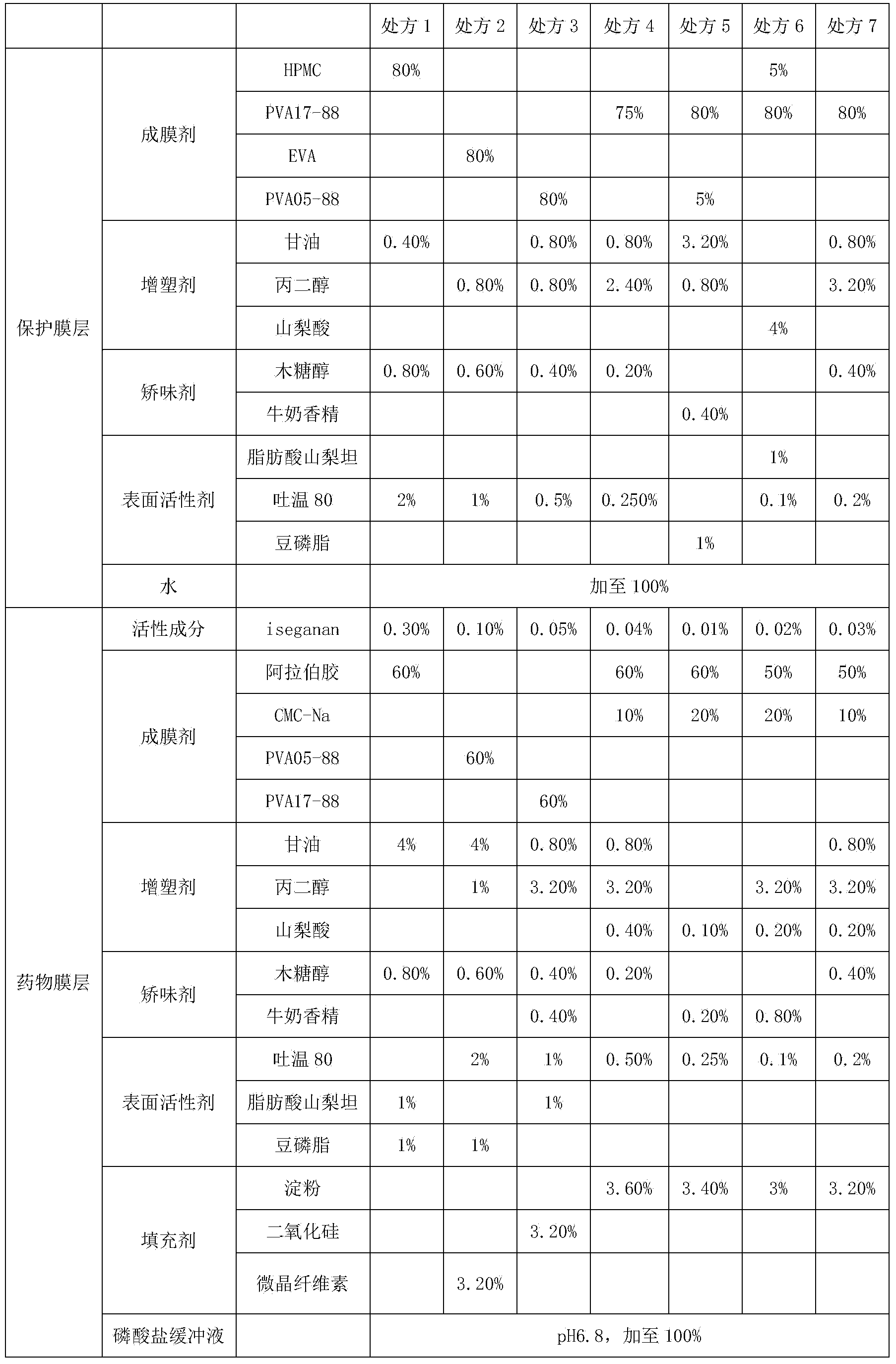
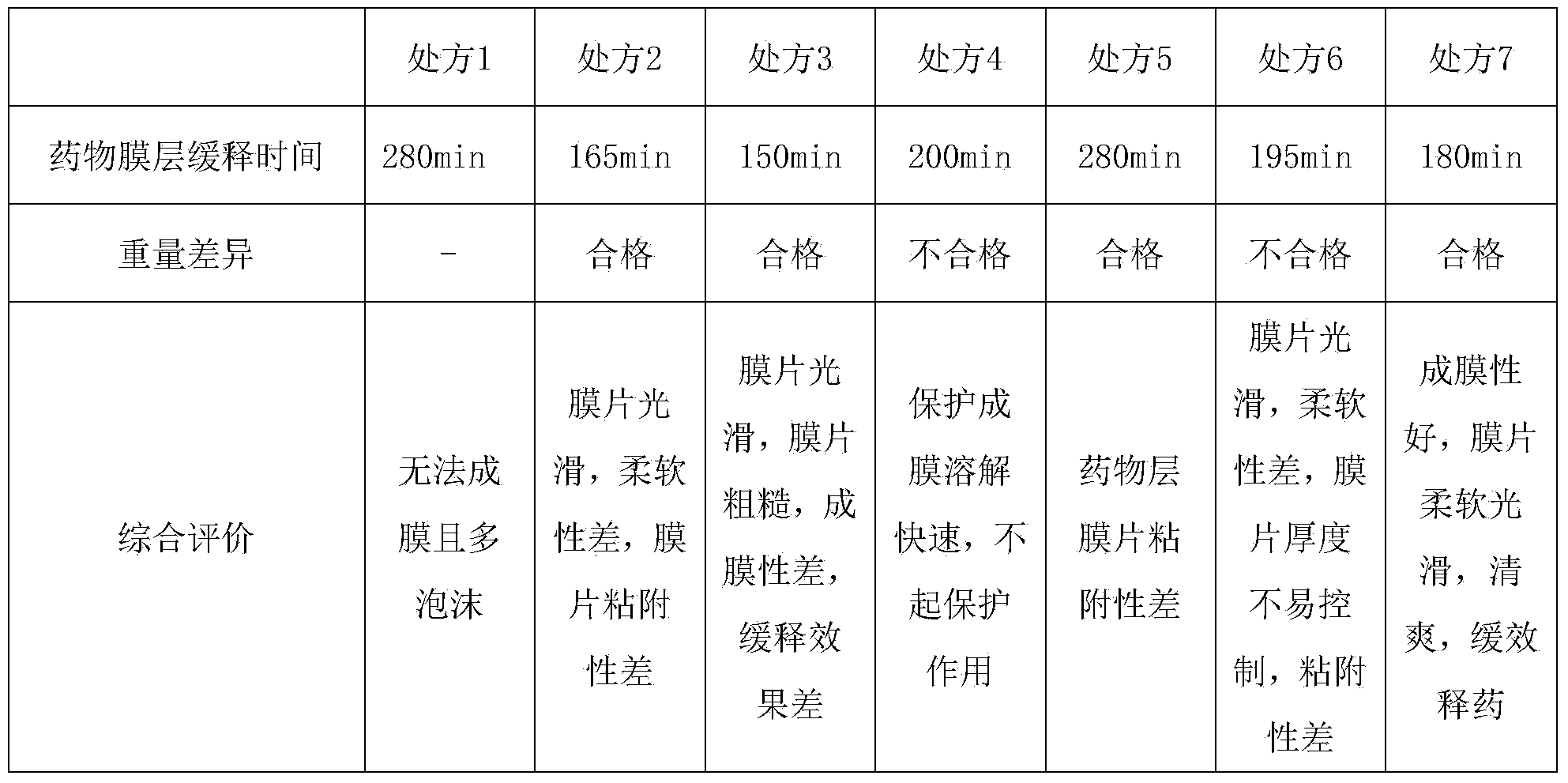
[0055] Example 2 Screening of sustained-release film formulations
[0056] According to the ratio in Table 1, the sustained-release film formulation was investigated, and the results are shown in Table 2
[0057] Table 1
[0058]
[0059] Table 2
[0060]
[0061] According to the content in the above table, only the film prepared by prescription 7 can meet the requirements, not only the sustained release time reaches 3 hours, but also the best film-forming performance, appearance and taste.
Embodiment 3
[0062] Example 3 Inspection of Weight Difference of Sustained-release Film
[0063] 1. Inspection method: Take 20 pieces of the test product, weigh them accurately, obtain the average weight, and then weigh each piece accurately. Weight per piece compared to average weight
[0064] 2. The test results are as follows
[0065] table 3
[0066]
[0067] Inspection and judgment result: qualified
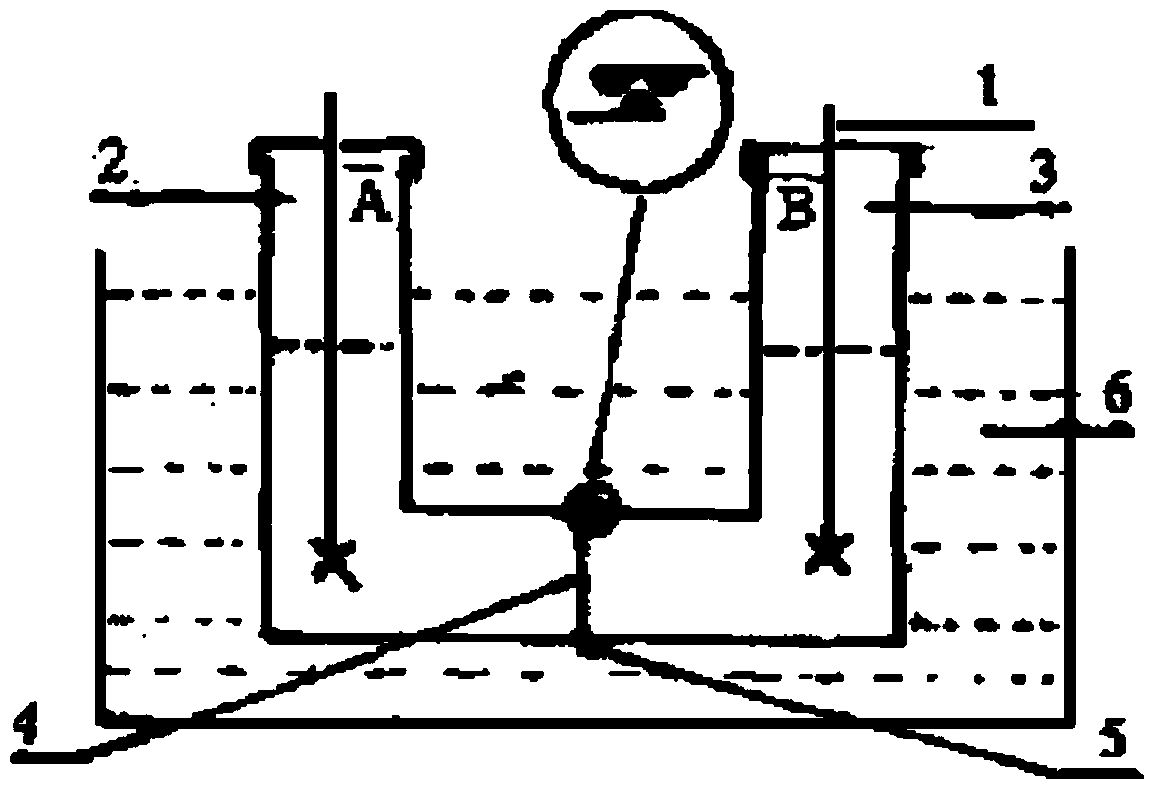
[0068] 3. Release rate test of sustained-release film
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com