Patents
Literature
14889 results about "Buffer solution" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A buffer solution (more precisely, pH buffer or hydrogen ion buffer) is an aqueous solution consisting of a mixture of a weak acid and its conjugate base, or vice versa. Its pH changes very little when a small amount of strong acid or base is added to it. Buffer solutions are used as a means of keeping pH at a nearly constant value in a wide variety of chemical applications. In nature, there are many systems that use buffering for pH regulation. For example, the bicarbonate buffering system is used to regulate the pH of blood.
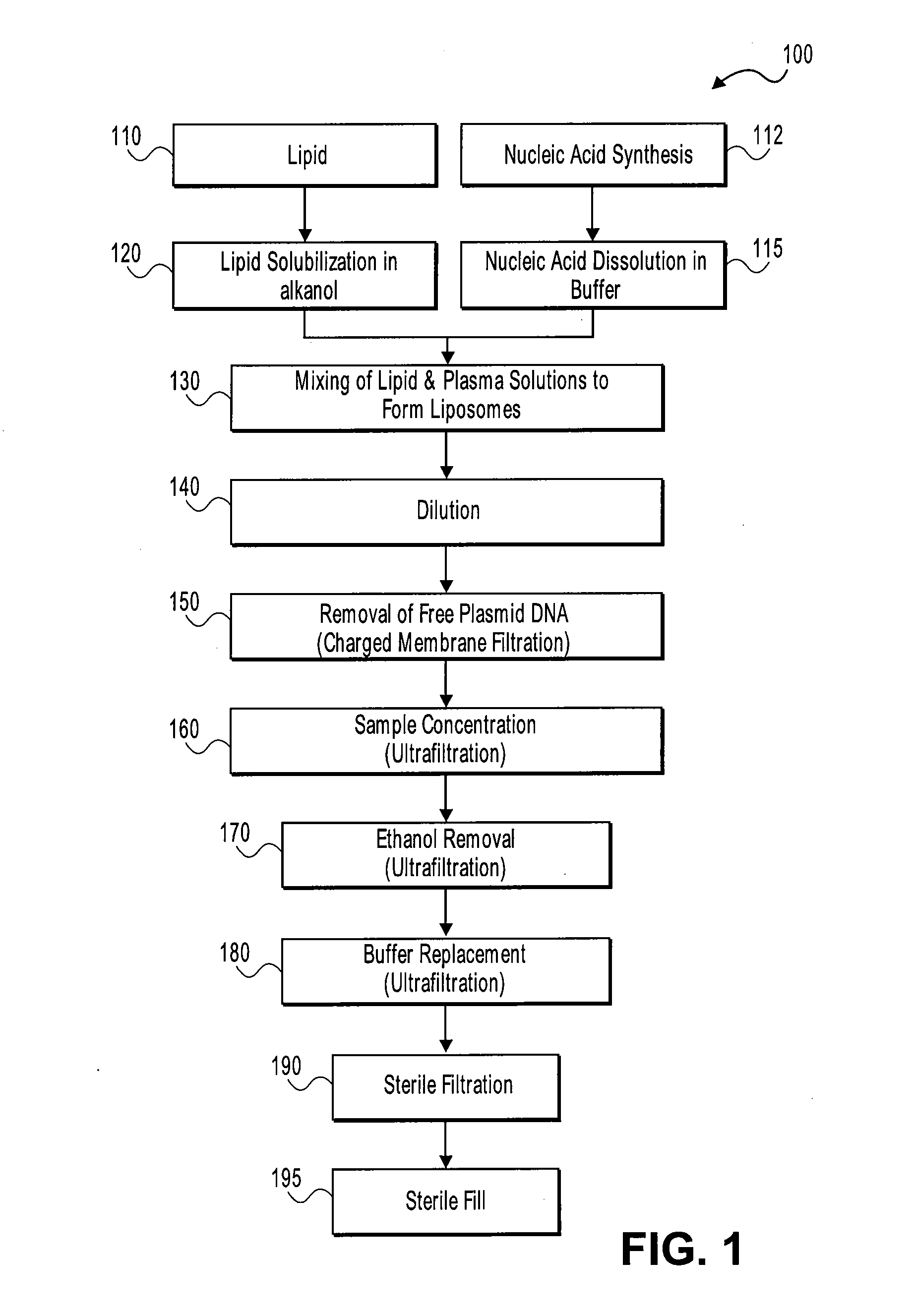
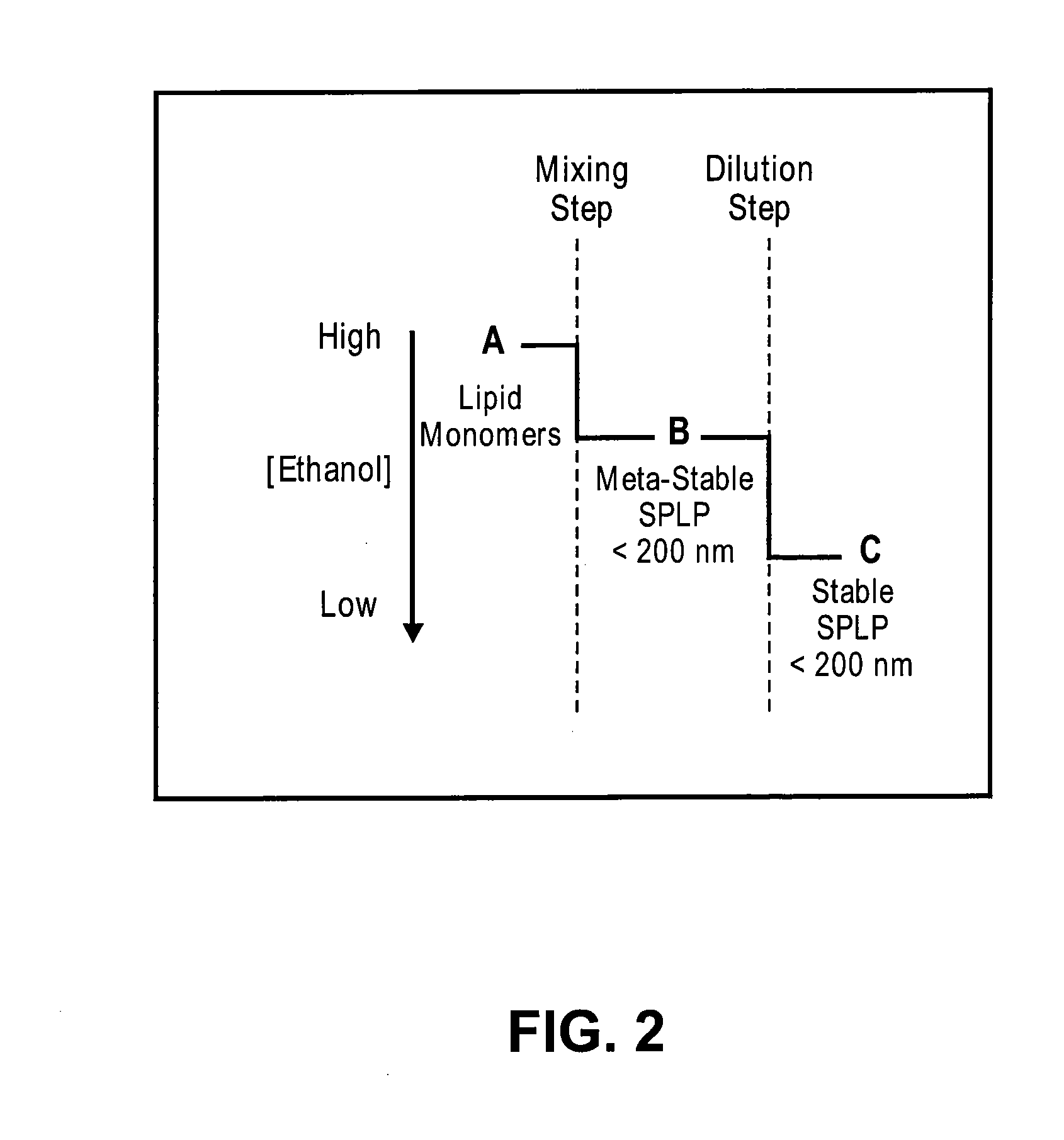
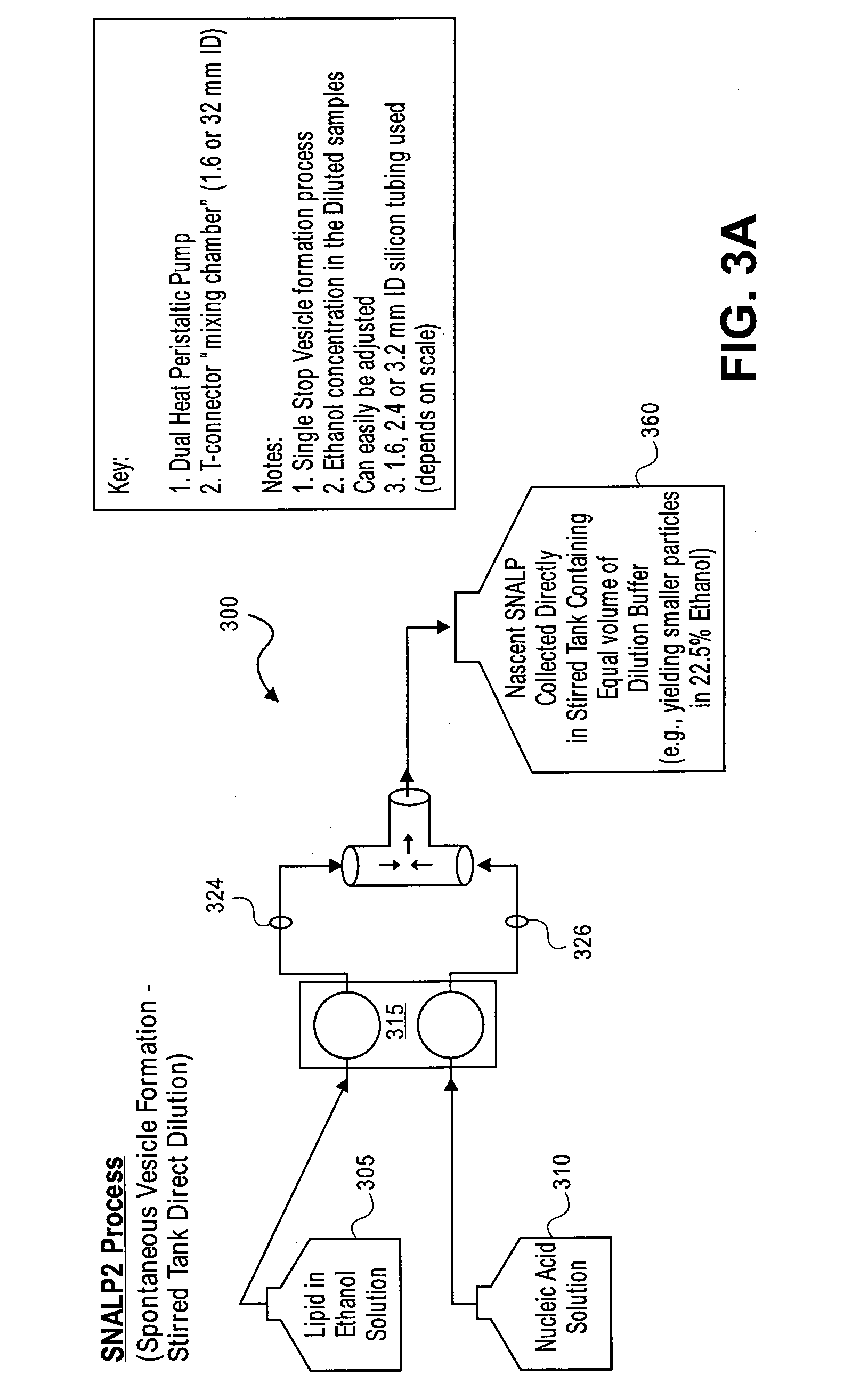
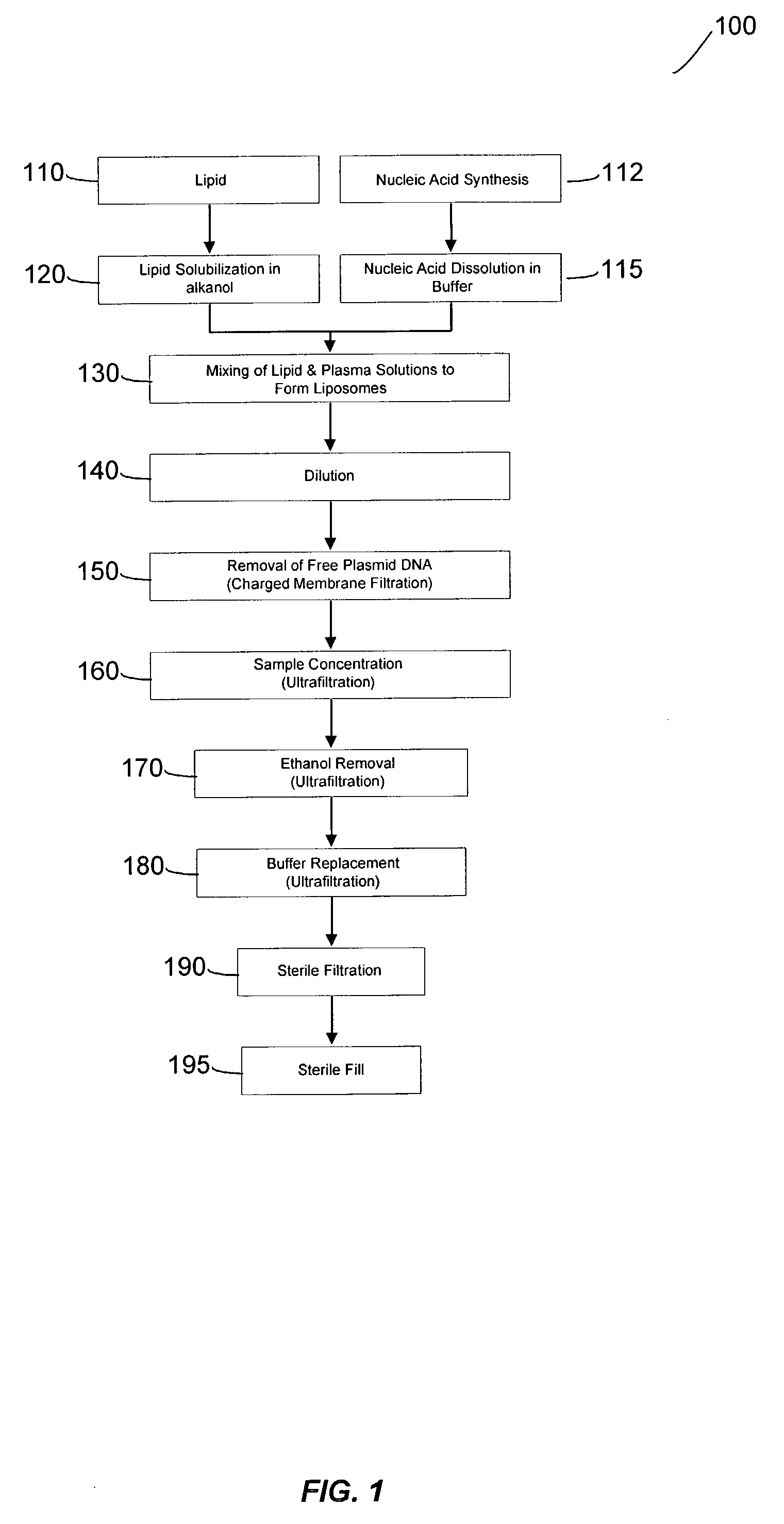
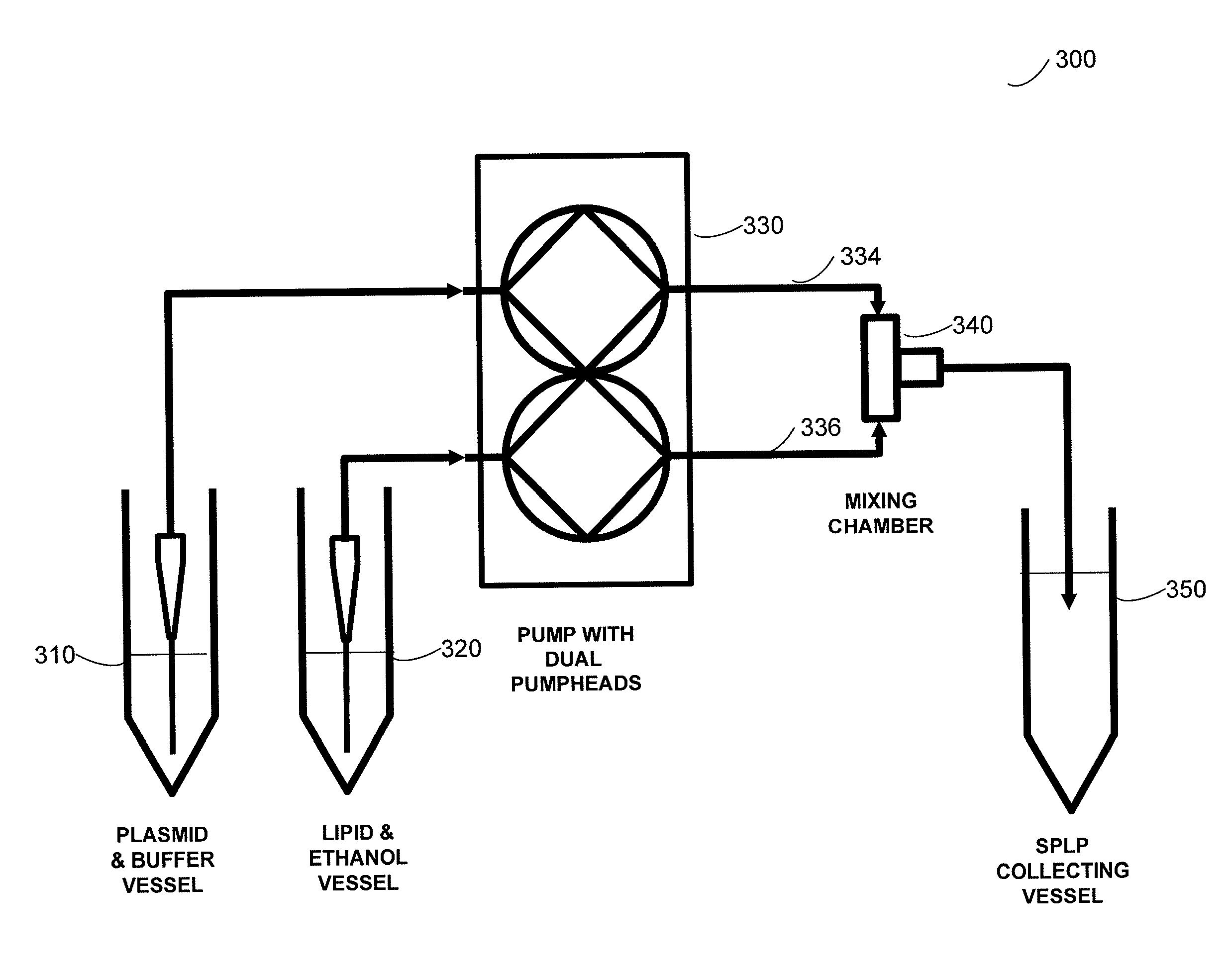
Systems and methods for manufacturing liposomes
ActiveUS20070042031A1Fast preparationOrganic active ingredientsMicroencapsulation basedLipid formationBuffer solution
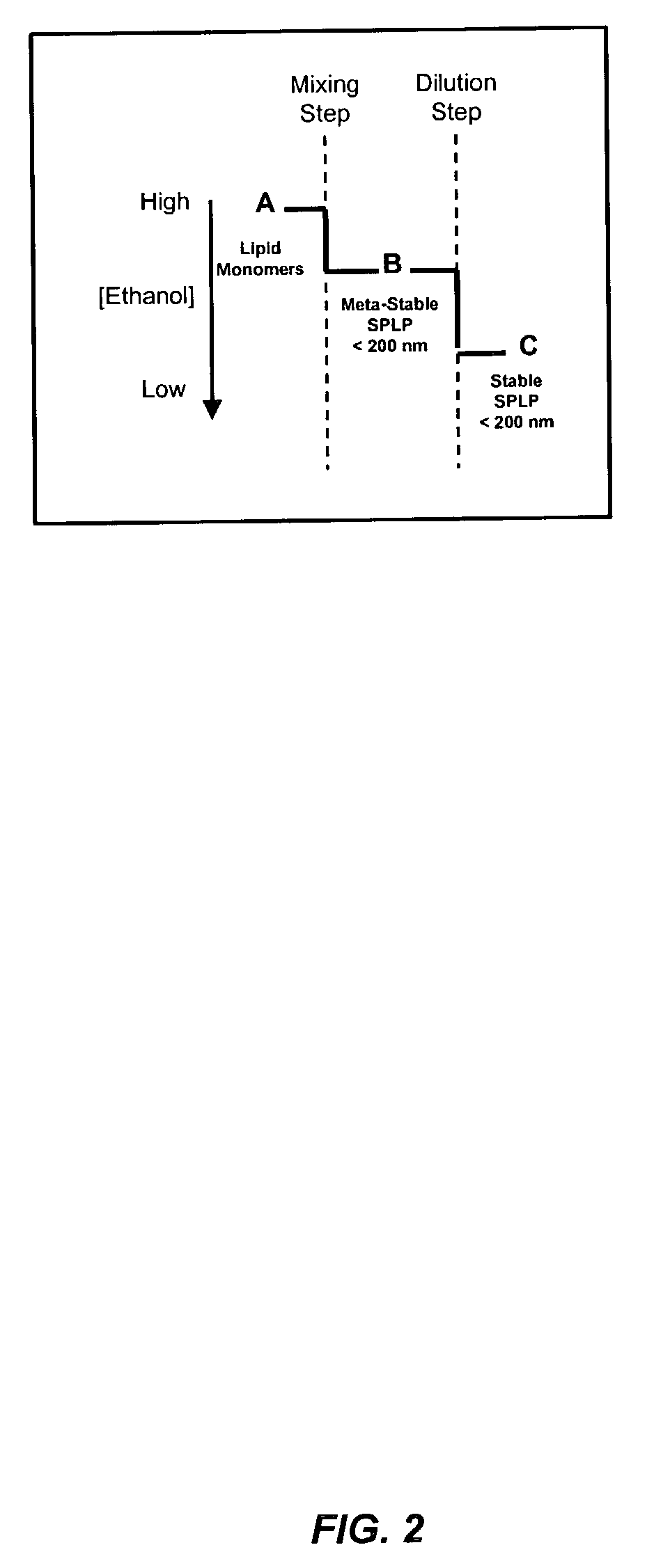
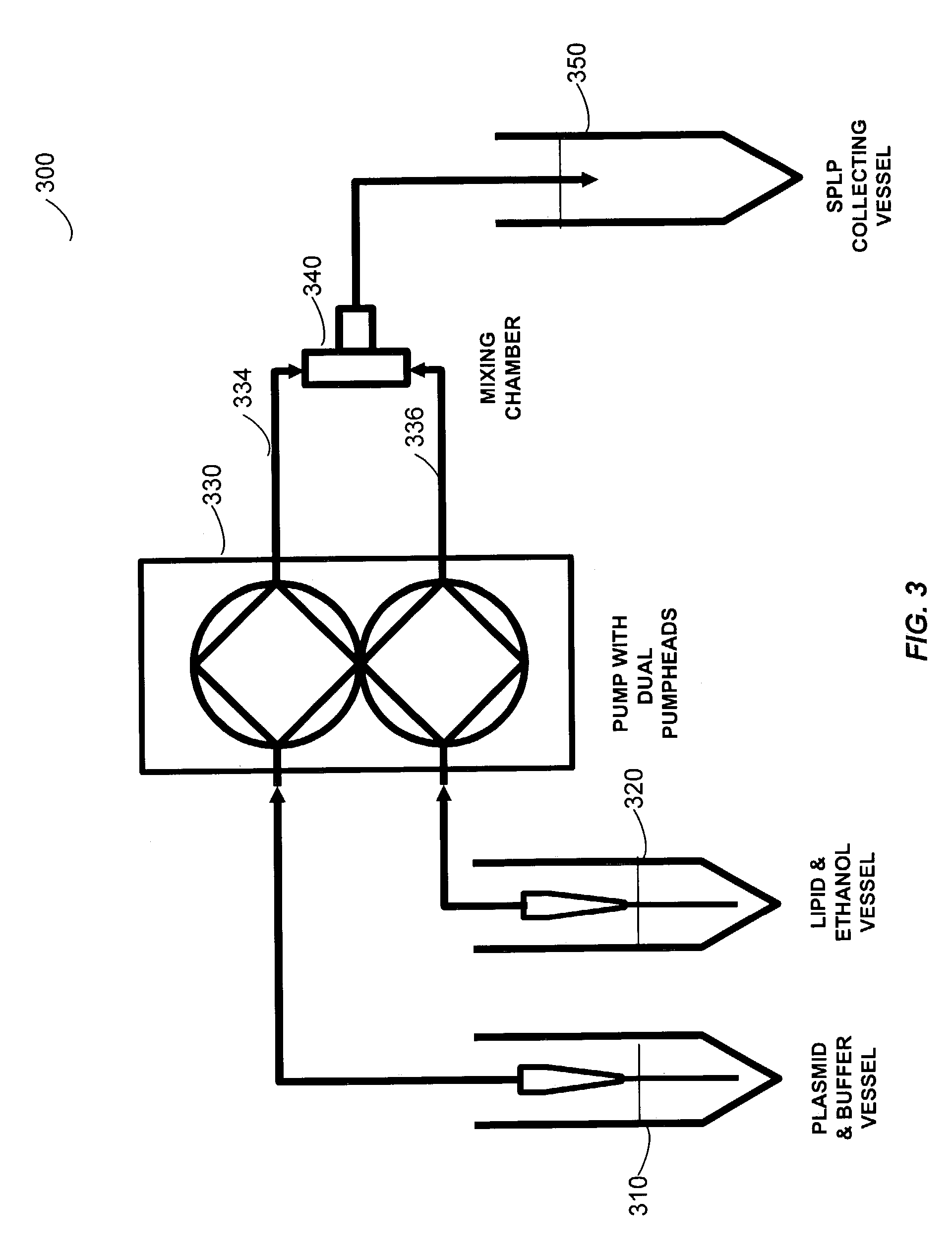
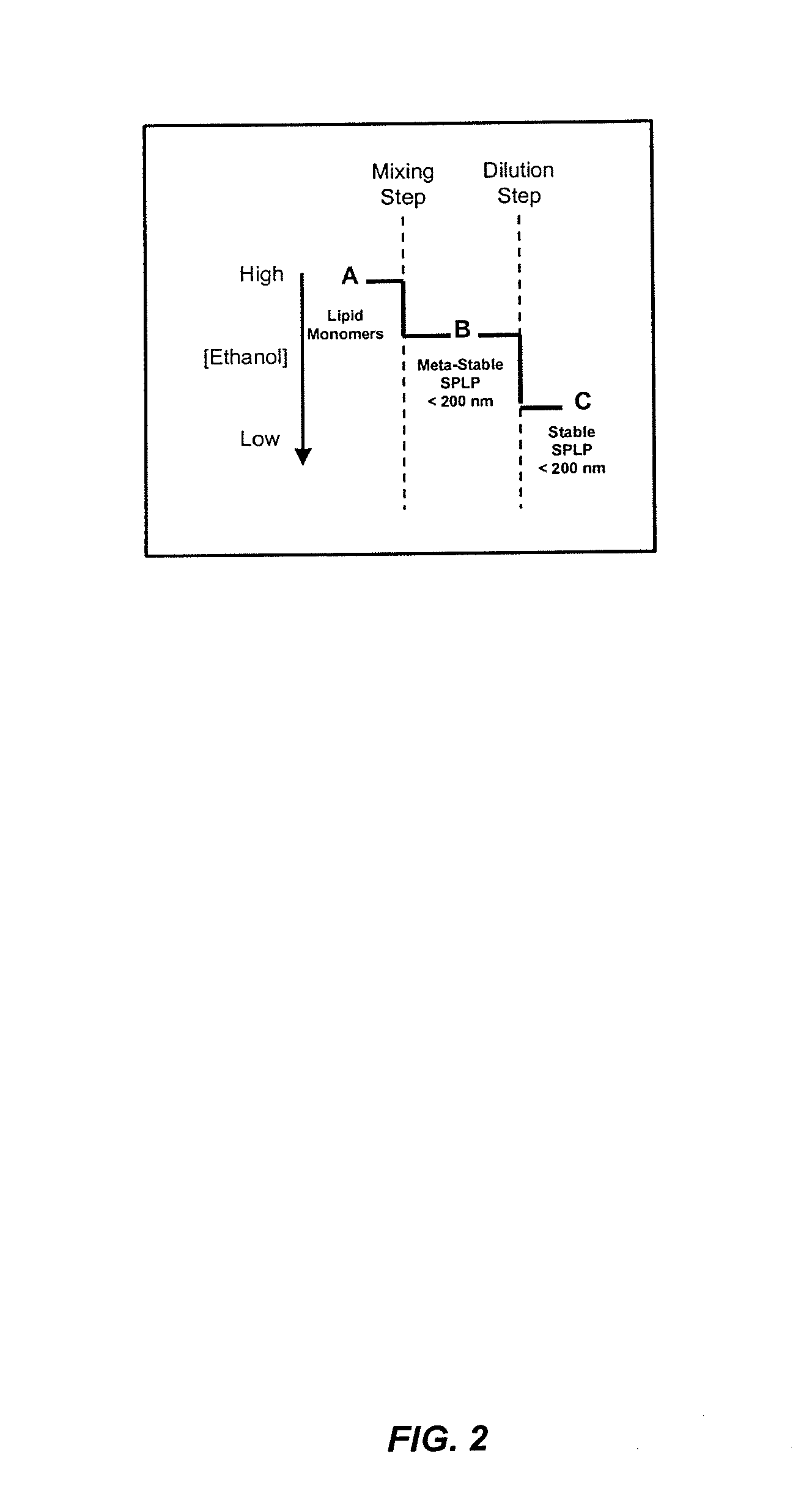
The present invention provides apparatus and processes for producing liposomes. By providing a buffer solution in a first reservoir, and a lipid solution in a second reservoir, continuously diluting the lipid solution with the buffer solution in a mixing chamber produces a liposome. A therapeutic agent, such as nucleic acid, is included in one of the buffer solution or the lipid solution. Upon mixing a liposome encapsulating the therapeutic product is substantially instantaneously formed. Thereafter the liposome solution formed is immediately diluted with buffer solution to enhance homogeneity and maintain small particle size.
Owner:ARBUTUS BIOPHARMA CORPORAT ION
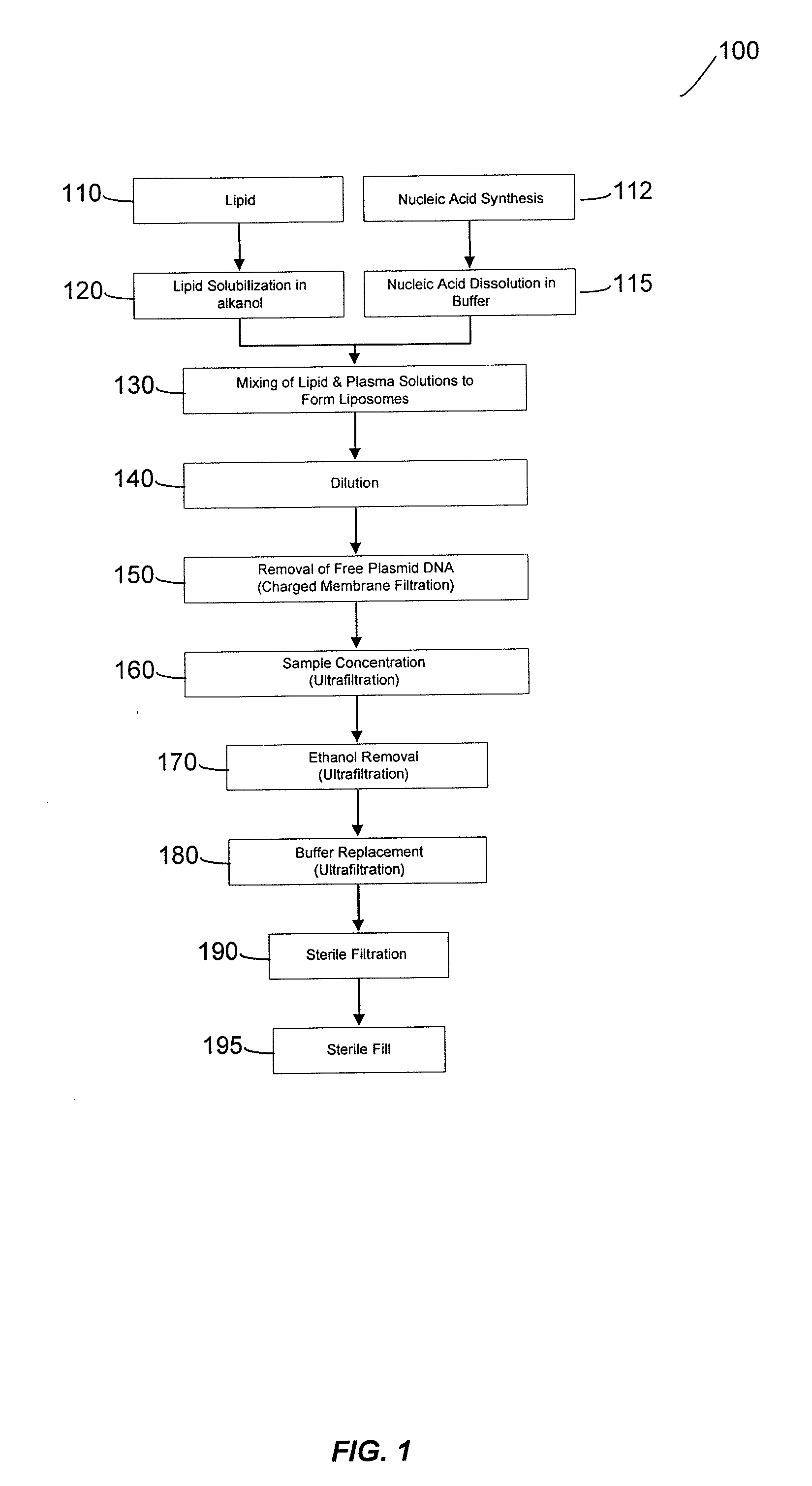
Liposomal apparatus and manufacturing methods
ActiveUS7901708B2High encapsulation efficiencyBiocideSugar derivativesLipid formationOrganic solvent
The present invention provides apparatus and processes for producing liposomes. By providing a buffer solution in a first reservoir, and a lipid solution in a second reservoir, continuously diluting the lipid solution with the buffer solution in a mixing chamber produces a liposome. The lipid solution preferably comprises an organic solvent, such as a lower alkanol.
Owner:ARBUTUS BIOPHARMA CORPORAT ION
Crystallization control method for organic compound and crystallization control solid-state component employed therefor
InactiveUS6123769APolycrystalline material growthFrom normal temperature solutionsValence electronBiopolymer
A method which can control crystallization of a biopolymer such as protein is provided. A silicon crystal (15) whose valence electrons are controlled to be capable of controlling the concentration of holes or electrons of the surface part in response to the environment of a buffer solution (14) containing the biopolymer such as protein is brought into contact with the solution (14), for getting a crystal of the biopolymer deposited on the surface of the silicon crystal (15). Crystallization is controlled by an electrical state which is generated by the controlled valence electrons on the surface of the silicon crystal (15).
Owner:SUMITOMO METAL IND LTD
Separation apparatus and separation method
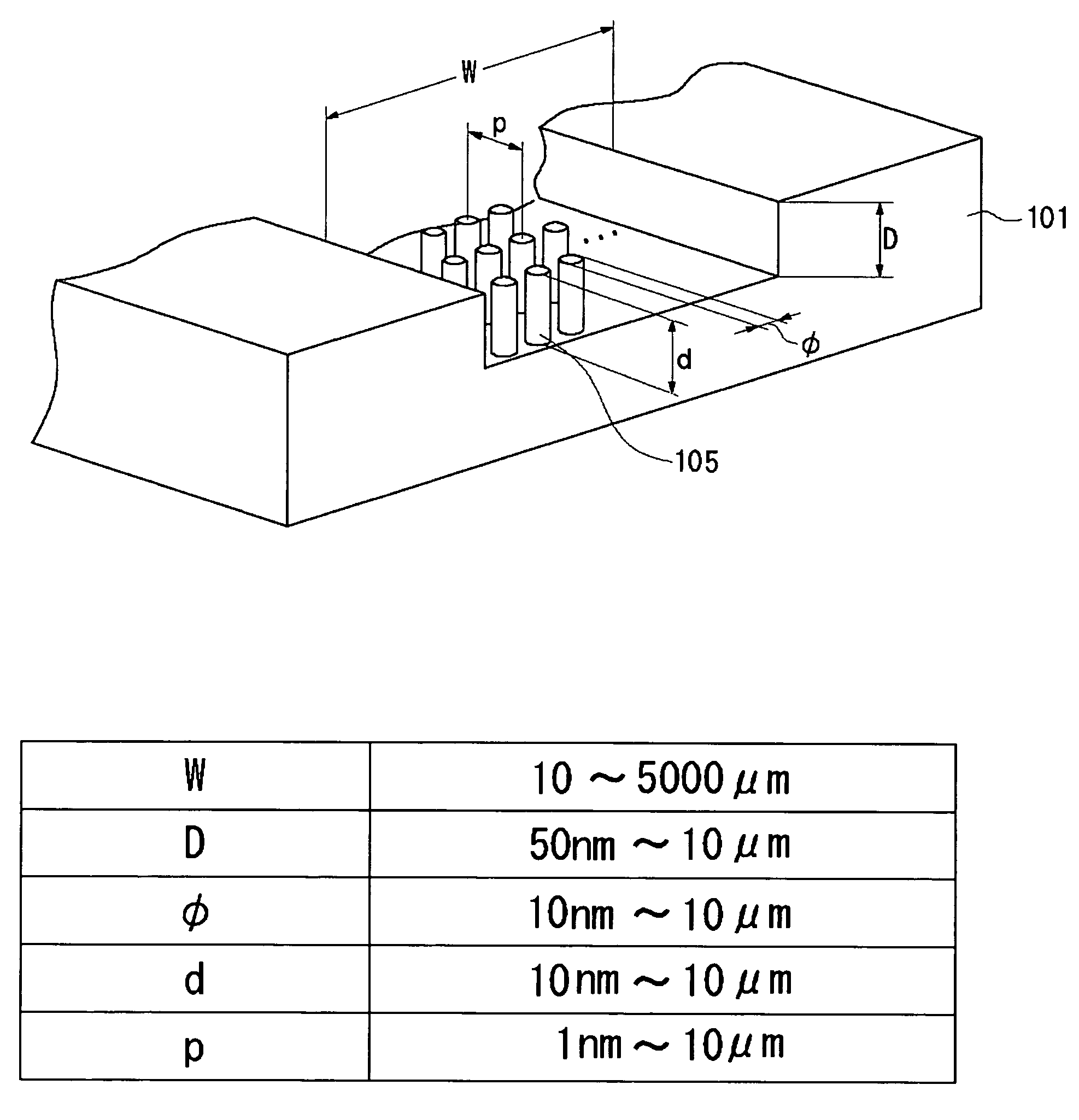
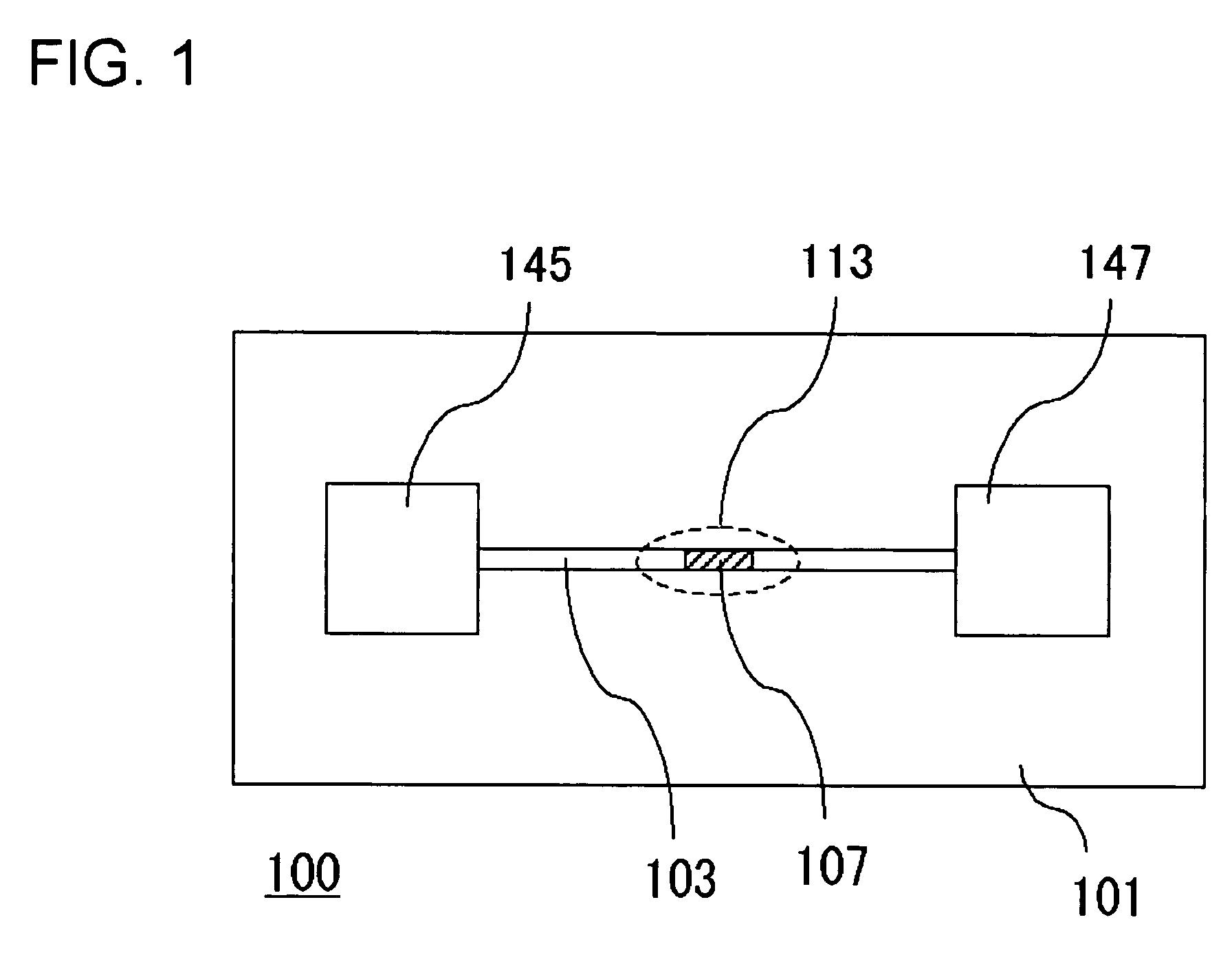
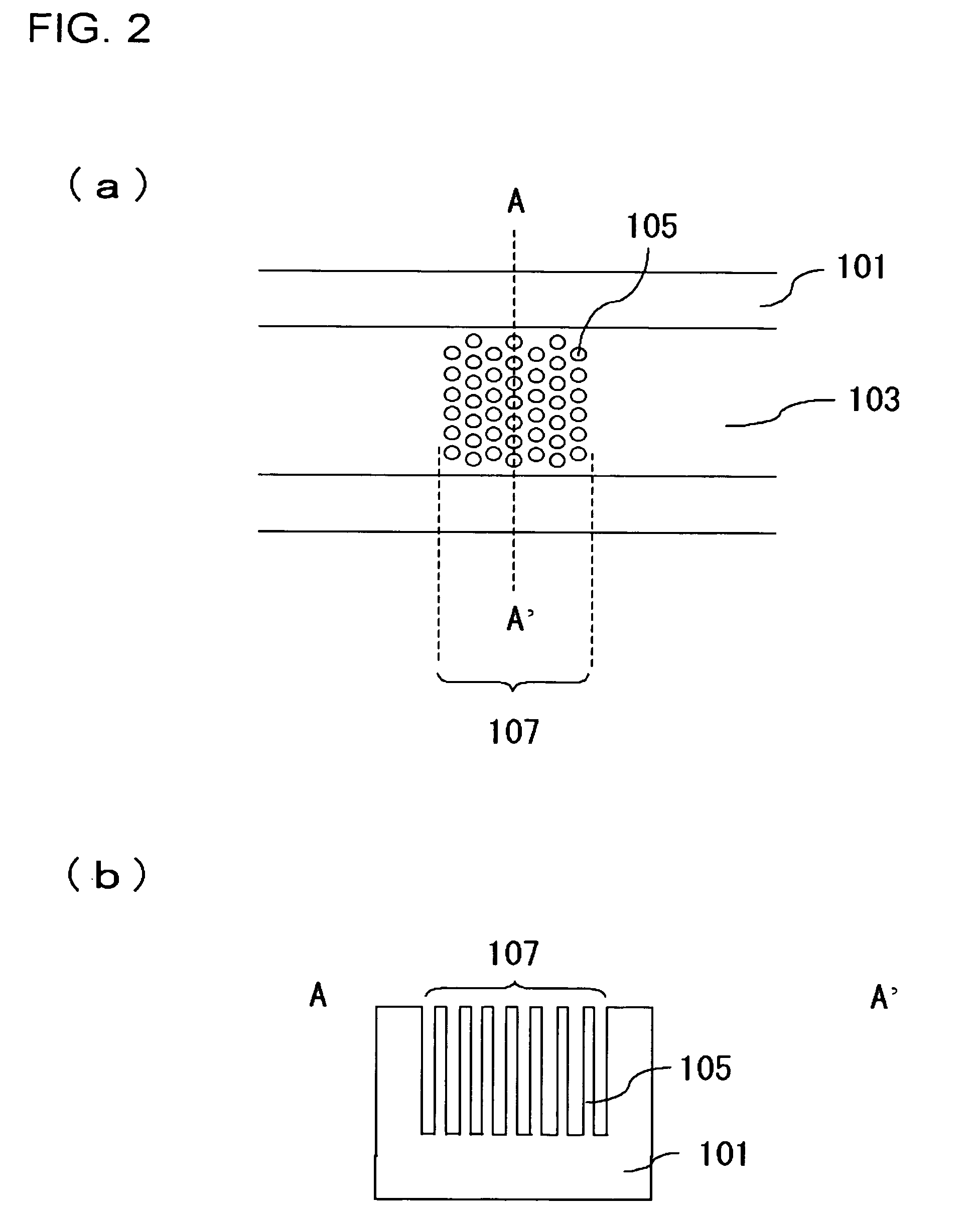
InactiveUS20060000772A1Improve efficiencyHigh activityIon-exchange process apparatusSemi-permeable membranesBuffer solutionAnalytical chemistry
A channel (103) is formed in a substrate (101), and a portion of the channel (103) is provided with a separating portion (107). A number of pillars are formed in the separating portion (107), and an adsorptive substance layer having an adsorptive substance, which exhibits a specific interaction for a specific substance, immobilized on the surface thereof, is formed. Once a sample is introduced into the channel (103), the specific substance is adsorbed on the adsorptive substance layer to be separated from other components. After washing the inside of the channel (103) with a buffer solution, the specific substance is desorbed from the adsorptive substance layer by flowing a eluting solution through the channel (103) and the specific substance is recovered.
Owner:NEC CORP
Apparatus and method for detecting biomolecules
ActiveUS20110165557A1Increase ionic strengthBioreactor/fermenter combinationsBiological substance pretreatmentsBuffer solutionAnalytical chemistry
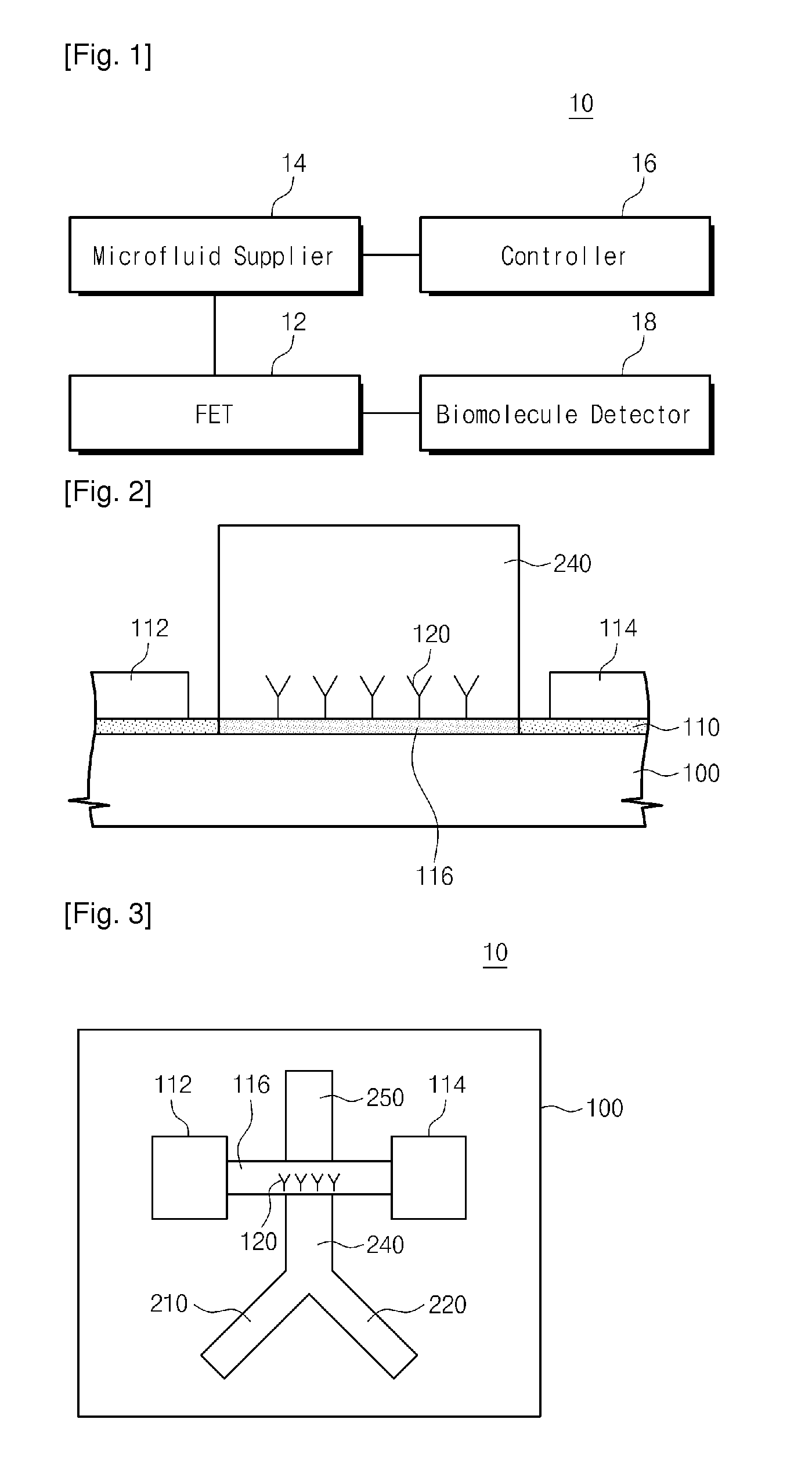
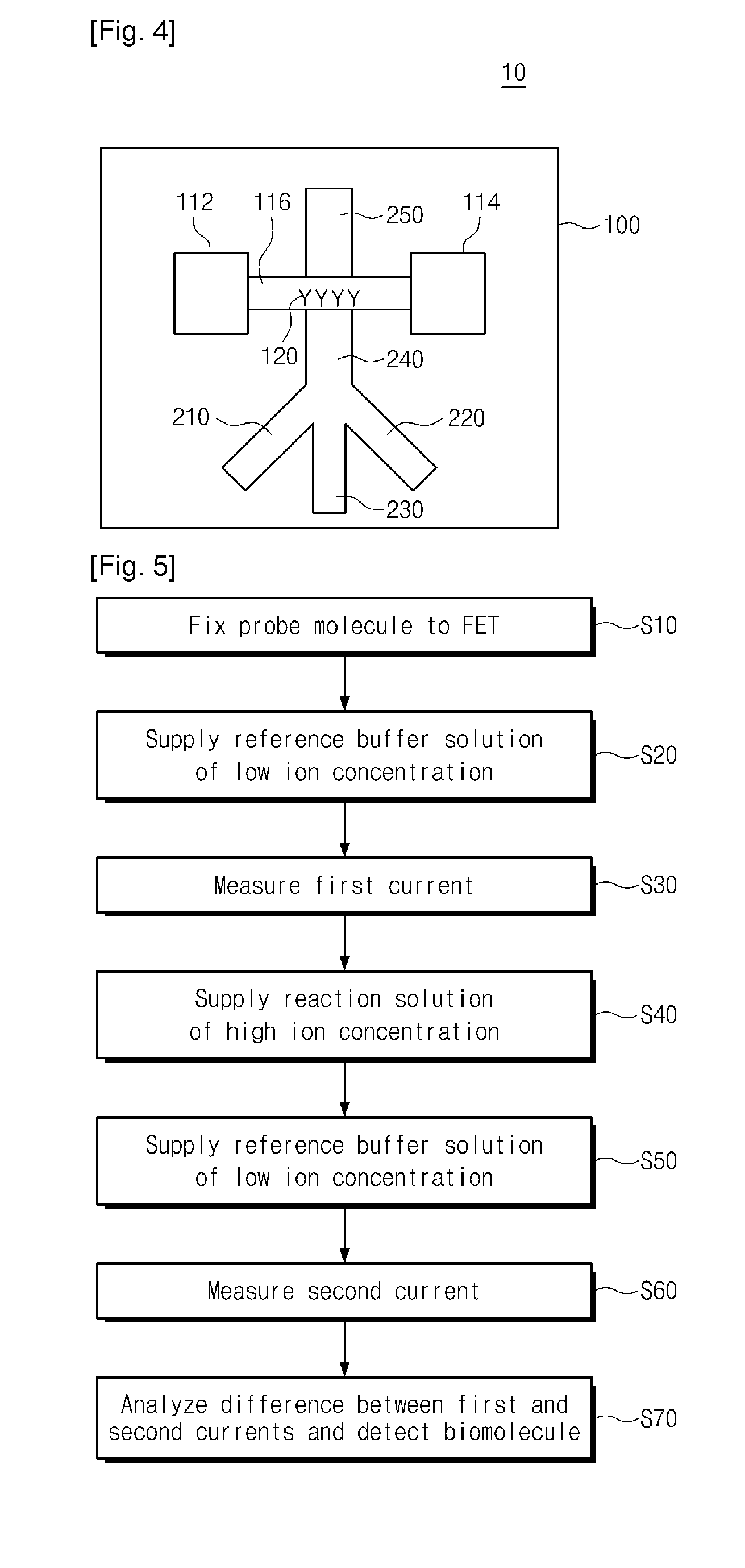
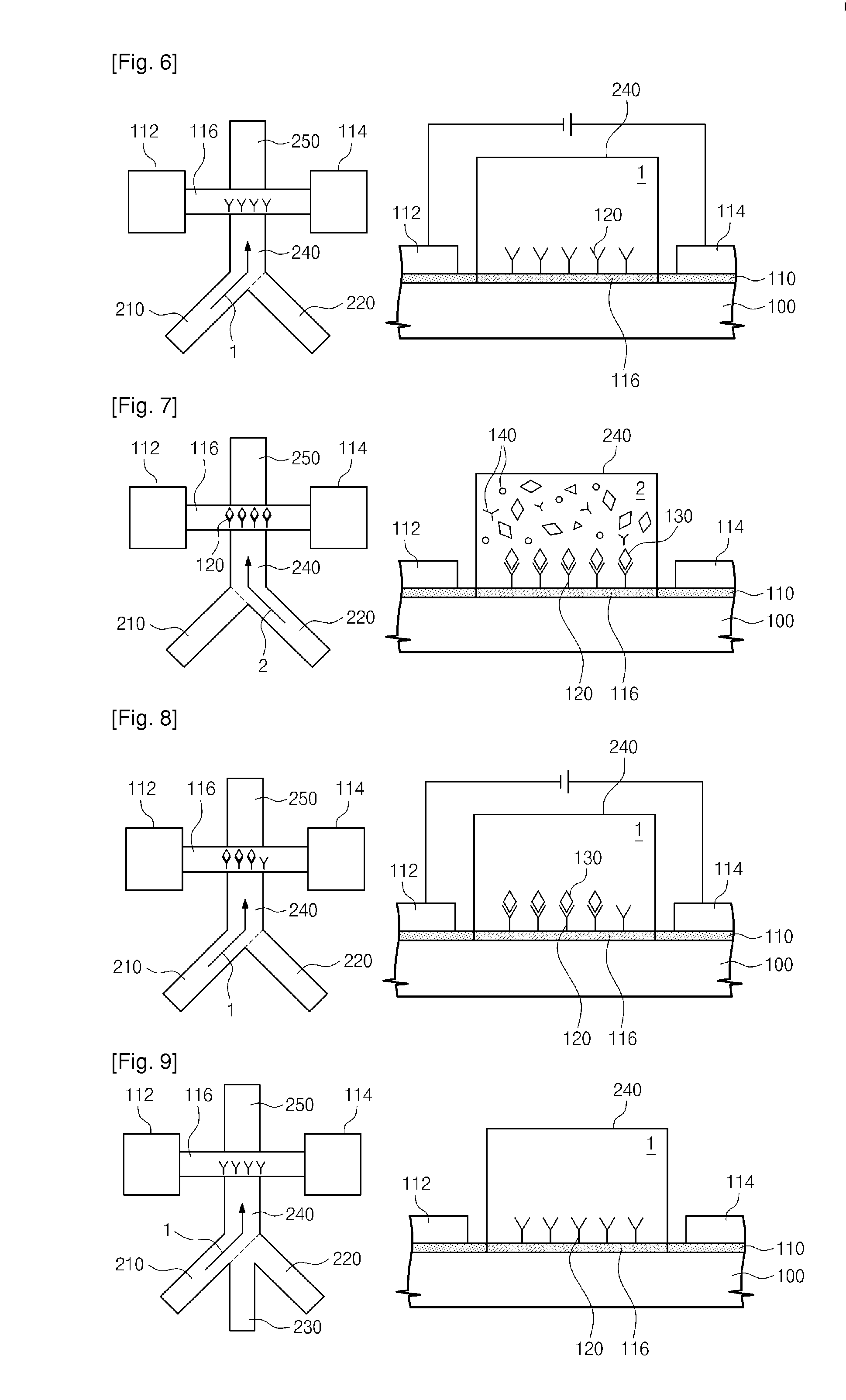
Provided are an apparatus and method for detecting biomolecules. The apparatus includes a FET having a substrate, a source electrode, a drain electrode, a channel region between the source and drain electrodes, and probe molecules fixed to the channel region, wherein the source and drain electrodes are separated on the substrate, a microfluid supplier selectively supplying one of a reference buffer solution of low ionic concentration and a reaction solution of high ionic concentration containing target molecules, to the channel region of the FET to which the probe molecules are fixed, and a biomolecule detector detecting the target molecules by measuring a first current value of the channel region of the FET, and a second current value of the channel region of the FET to which the target molecules and the probe molecules that bind to each other in the reaction solution of high ionic concentration are fixed.
Owner:ELECTRONICS & TELECOMM RES INST
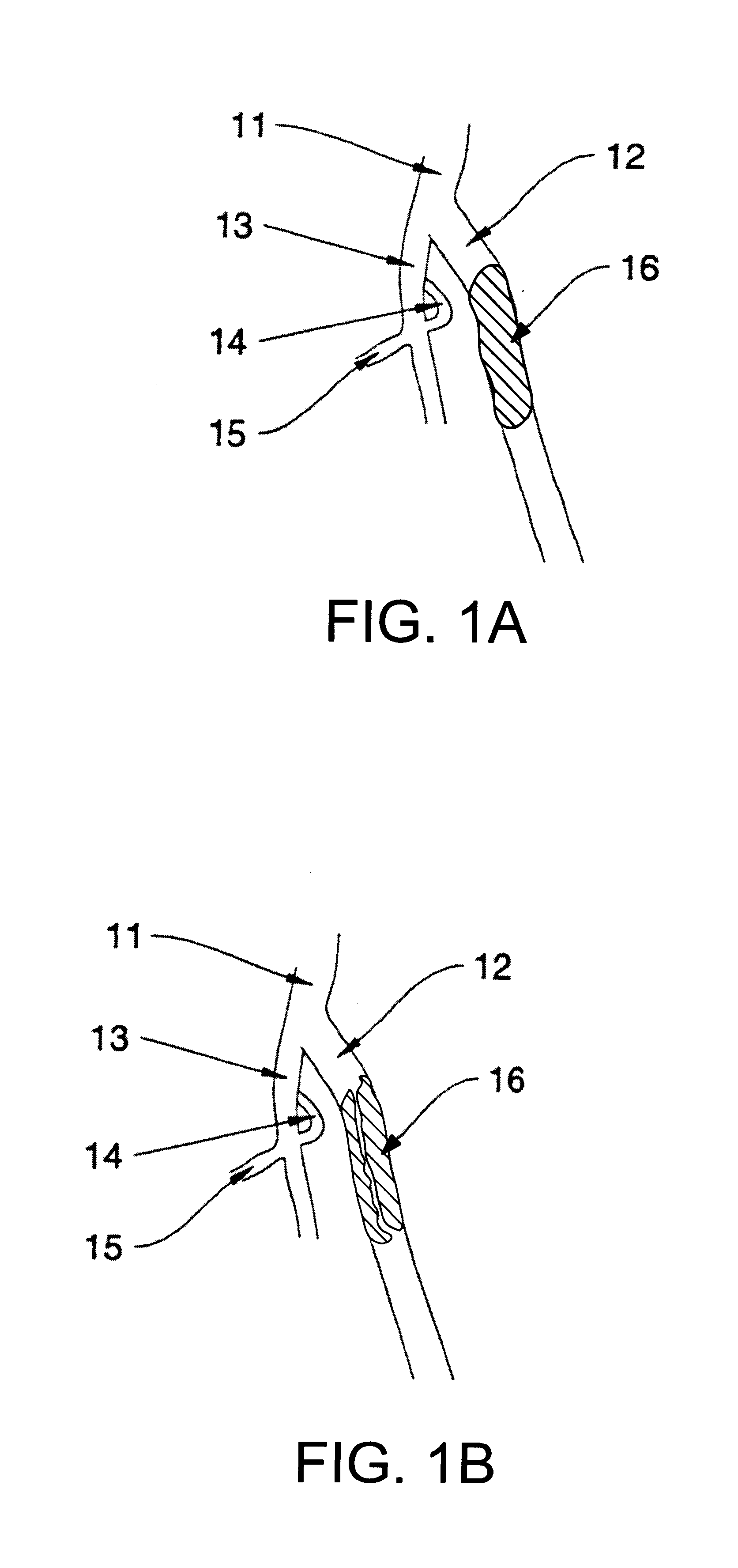
Catheter devices and methods for their use in the treatment of calcified vascular occlusions
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Liposomal apparatus and manufacturing method
The present invention provides apparatus and processes for producing liposomes. By providing a buffer solution in a first reservoir, and a lipid solution in a second reservoir, continuously diluting the lipid solution with the buffer solution in a mixing chamber produces a liposome. The lipid solution preferably comprises an organic solvent, such as a lower alkanol.
Owner:ARBUTUS BIOPHARMA CORPORAT ION
Methods and apparatus for minimizing evaporation of sample materials from multiwell plates
ActiveUS7208125B1Eliminates and reduces of excessive evaporationEliminate the problemBioreactor/fermenter combinationsBiological substance pretreatmentsEvaporationBuffer solution
Methods and devices for reducing evaporation of sample materials from the wells of multiwell plates are disclosed which find particular utility when the plates are placed in a stacked configuration. An example of the methods includes providing at least a first multiwell plate which is configured to be placed in a stacked configuration with at least one second multiwell plate, the at least first multiwell plate having a plurality of wells for receiving sample material therein and opposing side walls which extend around the plate and which define a ridge spaced inwardly of the side walls and extending around the plate between the side walls and the plurality of wells, the method including at least partially filling the ridge with a liquid such as water or buffer solution. The ridge can include one or more ribs which extend upwardly from a lower surface of the ridge to help reduce sloshing of the liquid contained within the ridge and to add structural strength and rigidity to the multiwell plate. The second multiwell plate can be provided with a downwardly extending flange which extends around a lower surface of the plate and which is configured to be removably received by the ridge of the at least first multiwell plate such that when the second multiwell plate is removably positioned on top of the at least first multiwell plate in a stacked configuration, the flange extends at least partially into the ridge and contacts the liquid to thereby create a substantial evaporation barrier to minimize evaporation of sample liquids in the wells of the at least first multiwell plate.
Owner:CALIPER TECH
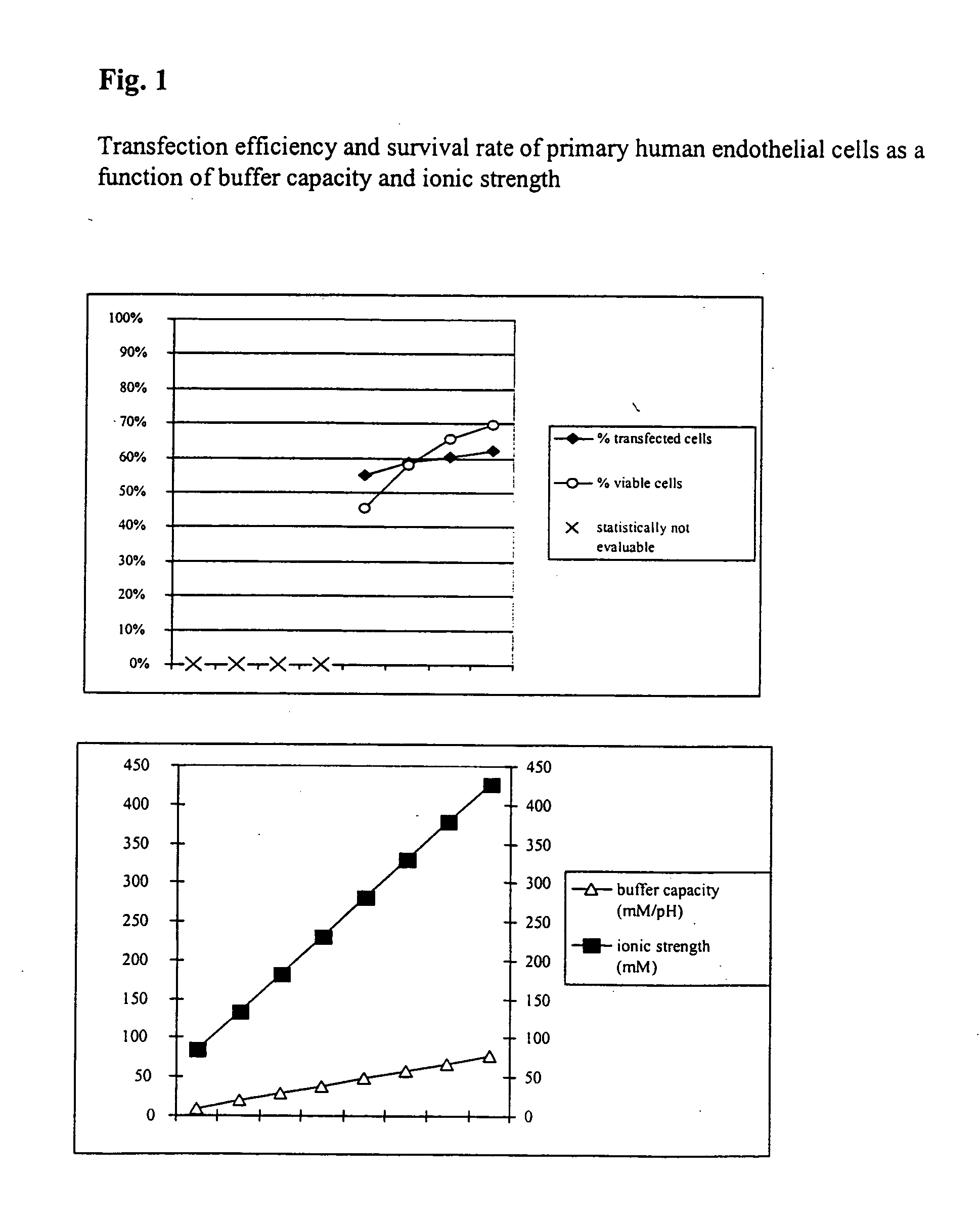
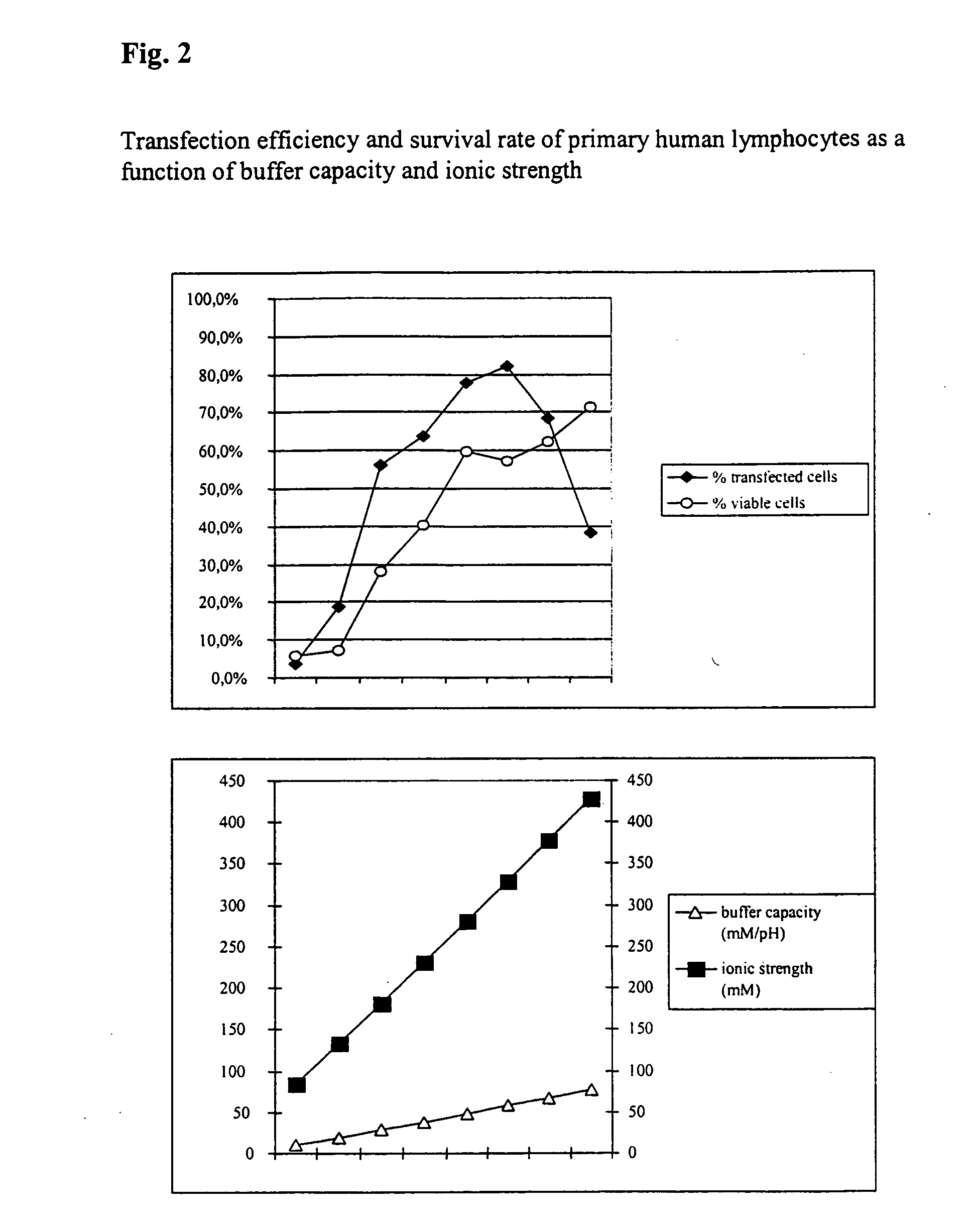
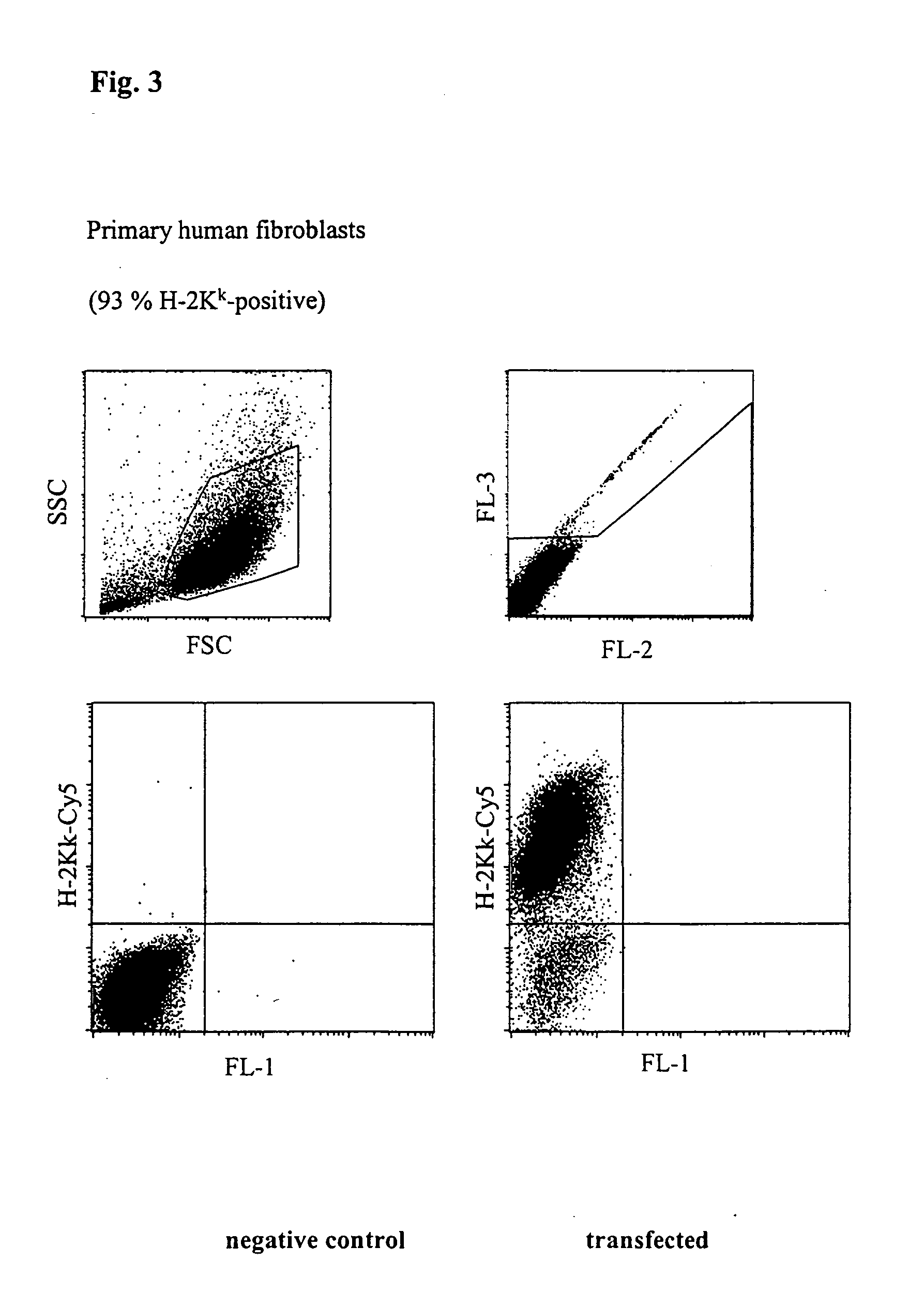
Buffer solution for electroporation and a method comprising the use of the same
InactiveUS20050064596A1High transfection efficiencyReduce cell deathPeptide/protein ingredientsGenetic material ingredientsElectroporationIon
The invention relates to a buffer solution for suspending animal or human cells and for dissolving biologically active molecules in order to introduce said biologically active molecules into the cells using an electric current and to a method for introducing biologically active molecules into animal or human cells using an electric current and a buffer solution. The inventive buffer solution has a buffering capacity of at least 20 mmol*I−1*pH−1 and an ionic strength of at least 200 mmol*I−1 during a change to the pH value from pH 7 to pH 8 and at a temperature of 25° C. The use of a buffer solution of this type in the corresponding method allows biologically active molecules to be introduced into animal and human cells with a high degree of transfection efficiency and at the same time a low cell mortality. Different cell types, in particular dormant and actively dividing cells of low activity, can be successfully transfected in said buffer solution.
Owner:LONZA COLOGNE
Dressing material containing medicine chitoholosida and its preparation method
InactiveCN1579559AHas therapeutic effectSustained releaseAbsorbent padsBandagesSolid componentPhosphate
The invention produces polyethylene alcohol hydrogel dressing containing medicine and chitosan with 60Co gamma-radial or high energy electron beam radial cross linking. Additional, adds in some humectant, plasticizer, medicine, the solvent is the secondary distilled water, physiological saline or phosphate neutral buffer liquid. The product can release medicine slowly and has natural amylose chitosan with biology sterilization activity, it has active sterilization function, at the same time, it has high water quantity, and good water reserving performance, the mechanical intensity is moderate, and the light penetration and air penetration are excellent. It can accord the demands for curing each kind of wound. It can used as the permanent dressing for light skin injuries, and it also can be applied to the temporally close of severe skin organization wound or burn wound.
Owner:CHANGZHOU INST OF ENERGY STORAGE MATERIALS &DEVICES
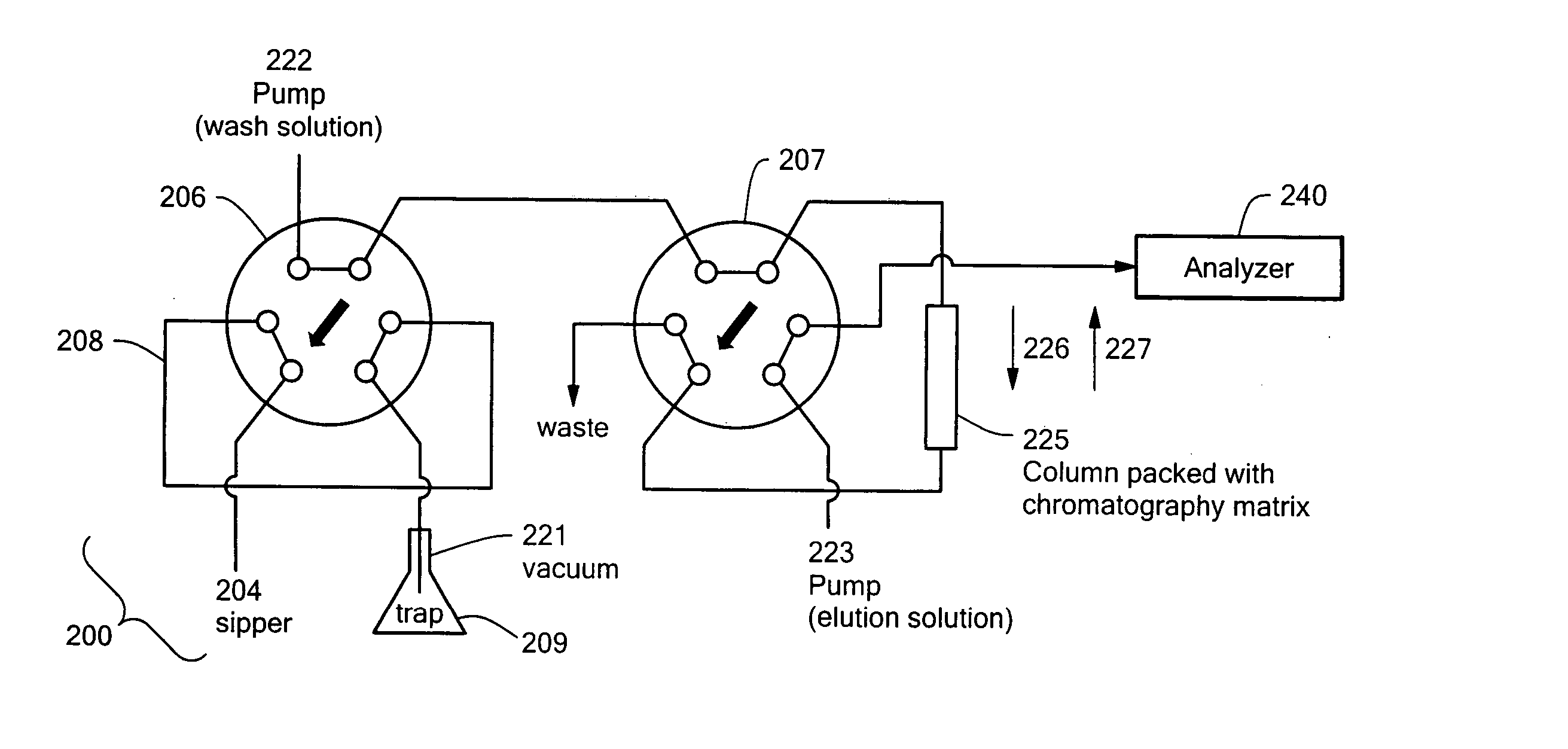
High throughput autosampler
InactiveUS20050194318A1Improve throughputMinimizes sample carryoverSequential/parallel process reactionsComponent separationAutosamplerHigh flux
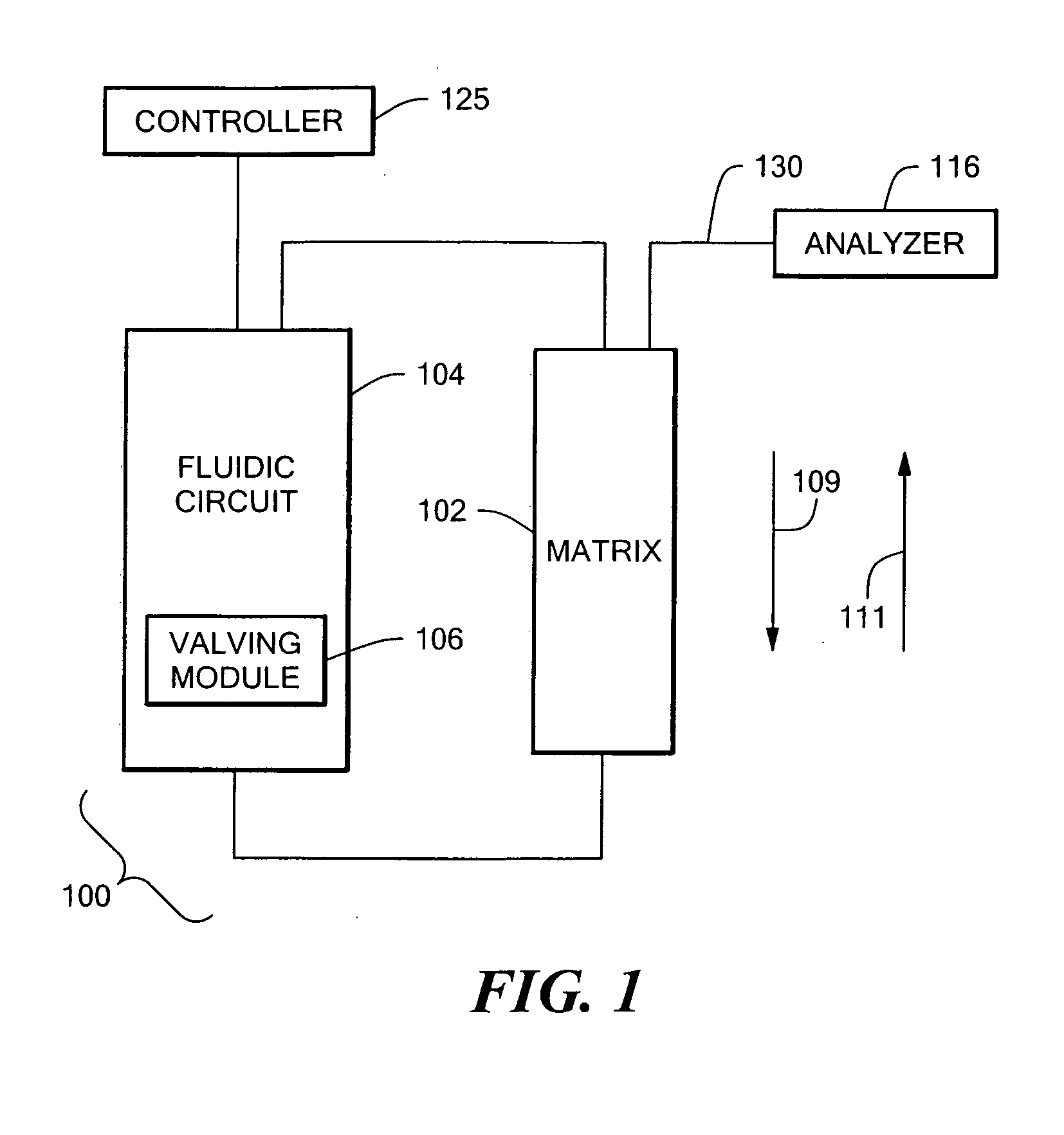
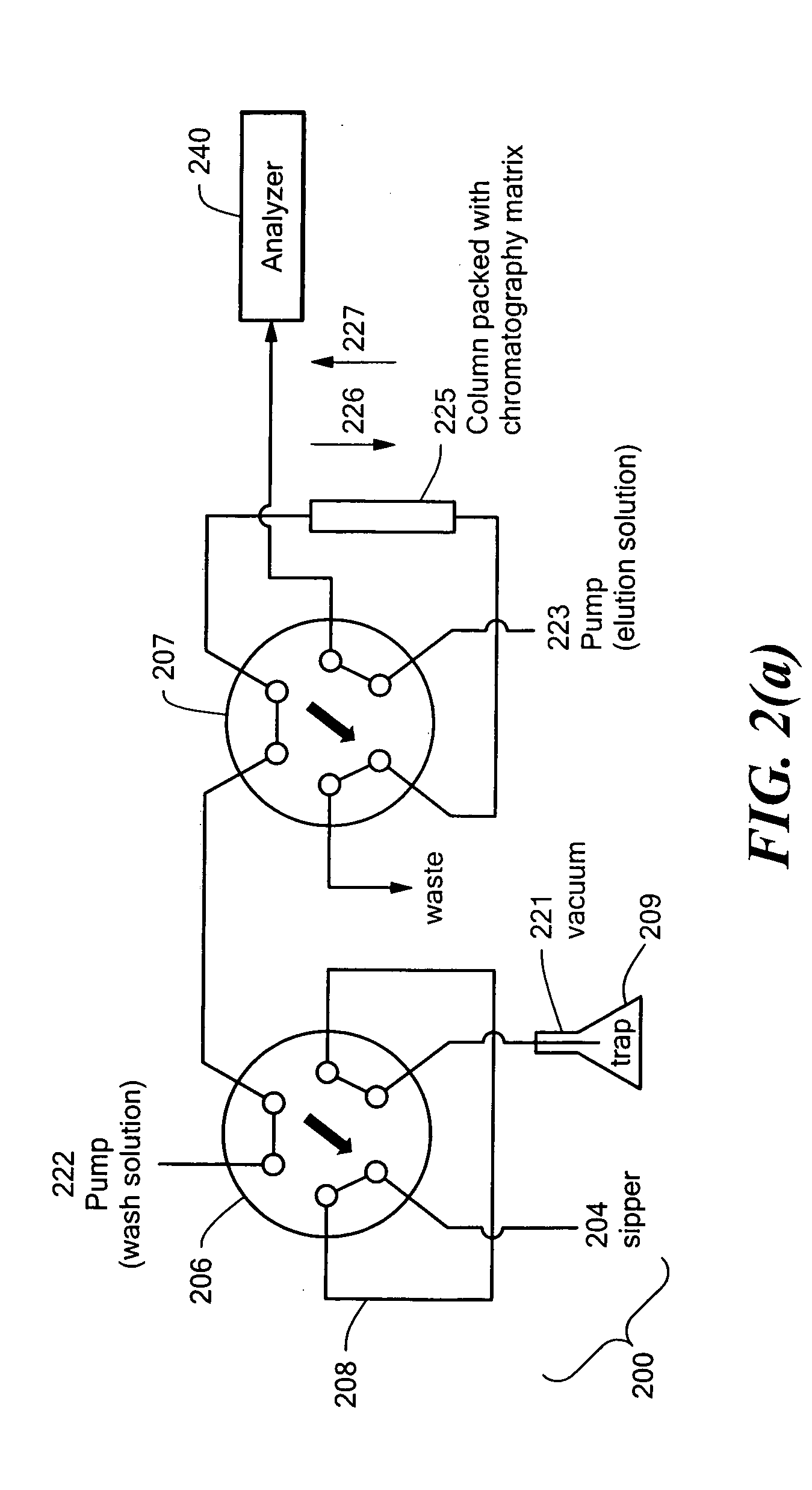
An auto-injection system provides high throughput screening of fluidic samples. A sample injection valve has a first position which applies a reduced pressure to a sample sipper tube for aspirating a fluidic sample into the sample sipper tube, and a second position which delivers the fluidic sample to a sample supply loop. A column control valve has a first position which delivers the fluidic sample from the sample supply loop to a sample chromatography column, and a second position which reverses direction of fluid flow through the sample chromatography column to deliver the fluidic sample to a sample analyzer. A wash control valve has a first position which supplies a wash buffer solution to the sample chromatography column in a forward fluid flow direction, and a second position which supplies elution solvent to flush the sample supply loop.
Owner:AGILENT TECH INC
DNA (Deoxyribose Nucleic Acid) sequencer
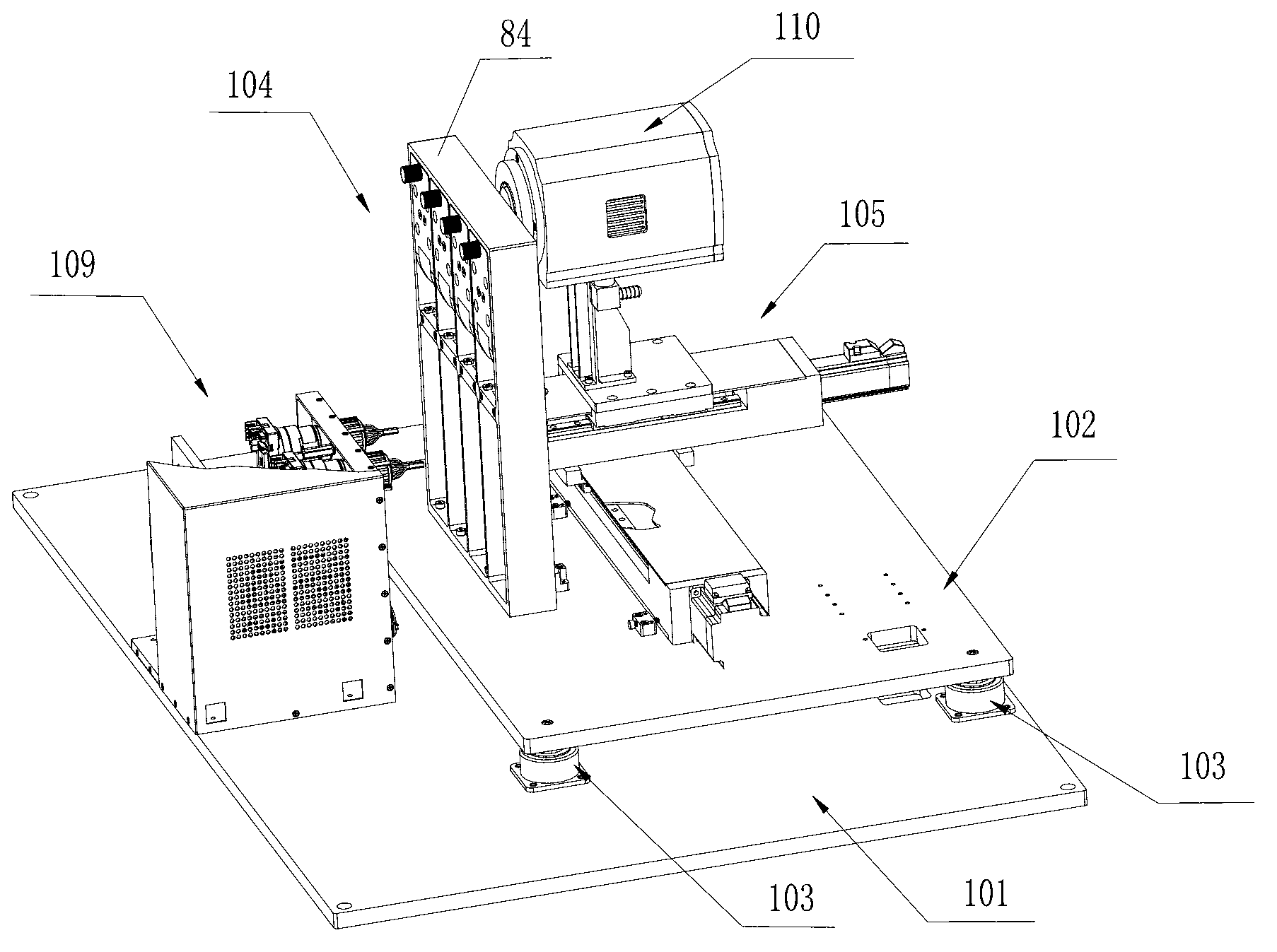
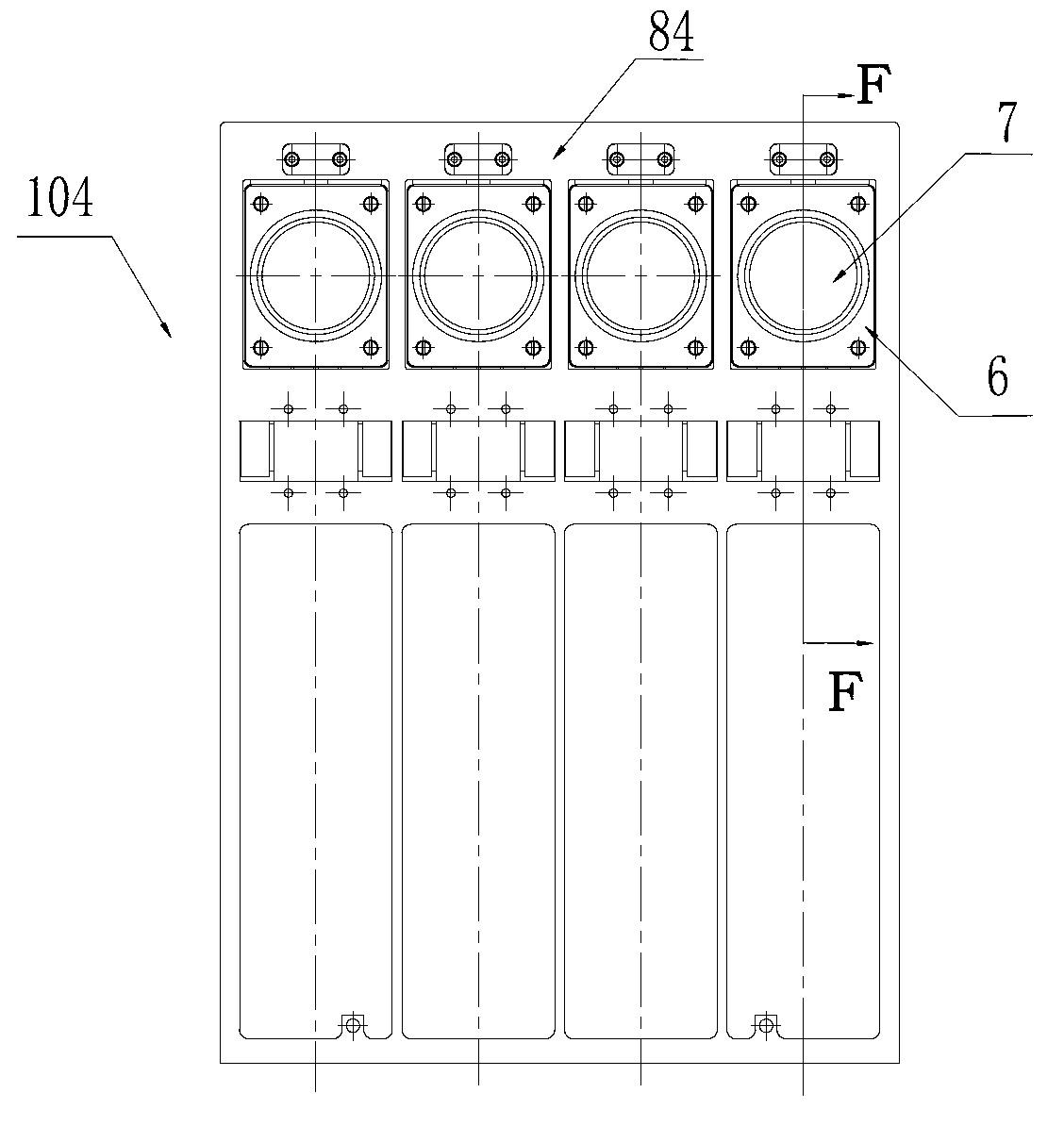
ActiveCN102703312AImprove sequencing efficiencyBioreactor/fermenter combinationsBiological substance pretreatments3-deoxyriboseBiochemical engineering
The invention discloses a DNA (Deoxyribose Nucleic Acid) sequencer which comprises a supporting table, a plurality of vibration dampers, a vibration damping plate, a reaction bin assembly, a CCD (Charge Coupled Device) camera, a two-dimensional regulation supporting device and a medicament supply assembly, wherein the vibration damping plate is connected with the supporting table by a plurality of vibration dampers; the reaction bin assembly is fixedly arranged on the vibration damping plate and is used for performing the DNA sequencing reaction; the CCD camera is used for acquiring an optical signal; the two-dimensional regulation supporting device is used for supporting the CCD camera; and the medicament supply assembly is arranged on the supporting table and is used for providing reagents and buffer solution for the reaction bin assembly. According to the DNA sequencer disclosed by the invention, by the arrangement of a plurality of reaction bins and the matching of the two-dimensional regulation supporting device capable of carrying out two-dimensional regulation and the medicament supply assembly capable of supplying the reagents for a plurality of reaction bins, the aim of simultaneously performing a plurality of reactions is fulfilled, a plurality of samples can be simultaneously sequenced and the DNA sequencing efficiency is greatly improved.
Owner:BEIJING INST OF GENOMICS CHINESE ACAD OF SCI CHINA NAT CENT FOR BIOINFORMATION +1
Chitosan collagen and calcium alginate compounded spongy biological dressing and its preparation process
The composite spongy biological dressing contains chitosan, collagen and calcium alginate with weigh tmixing ratio o f 0.5-8:0.5-8:0.1-8. Its preparation method includes the following steps: selecting chitosan and collagen type I, adding calcium alginate, compounding and cross-linking, using buffer solution to make neutralization, emulsifying, prefreezing and one-step freeze-drying so as to obtain the invented dressing with good biological compatibility and strong adhesion property. Said invented dressing possesses active function of promoting wound healing and hemostatic action, can be combined with anti-bacterial medicine to obtain gene engineeirng dressing for curing wound surface infection, also can be combined with active growth factor or active cell to form gene engineering dressingfor curing intractable ulcer and burn wound surface.
Owner:JIANGXI RUIJI BIOTECH CO LTD
Saliva sample testing device
ActiveUS20060292035A1Easy to operateHigh sensitivityAnalysis using chemical indicatorsVaccination/ovulation diagnosticsSaliva sampleTest flow
In a lateral test flow immunoassay device, a saliva sample and a buffer solution are delivered into a mixing chamber to mix with a second reagent. The resulting test mixture is allowed to incubate for a pre-determined period of time and then selectively delivered to a test strip
Owner:HEALGEN SCI LLC
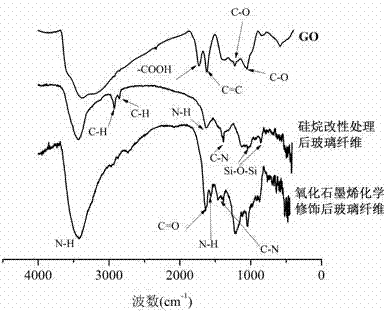
Method for chemically modifying inorganic filler with graphene oxide, product and application
InactiveCN103788413AImprove adhesionHigh mechanical strengthPigment treatment with organosilicon compoundsPigment physical treatmentEpoxySilanes
The invention discloses a method for chemically modifying inorganic filler with graphene oxide and a product. The method comprises the following steps: performing surface hydroxylation treatment and silane coupling agent treatment on the inorganic filler; maintaining the pH of a graphene oxide solution at 5.8-6.0 with an MES buffer solution; sequentially adding EDC and NHS, and performing ultrasonic treatment for 1-3 hours; adding the treated inorganic filler, and performing an amidation reaction at room temperature; and after the reaction, filtering, washing and drying to obtain the graphene oxide modified inorganic filler. The invention also discloses a method for preparing an inorganic filler / epoxy resin composite by use of the product. The process flow of the method disclosed by the invention is simple and environmentally friendly; the GO is connected to the surface of the inorganic filler by a chemical modification process, and the firm covalent bond combination between the GO and inorganic particles is generated, so that the interface adhesiveness and mechanical strength between the inorganic filler and the polymer are enhanced, and a new idea is provided to the modification of an inorganic filler surface and the preparation of a high-performance composite.
Owner:UNIV OF JINAN
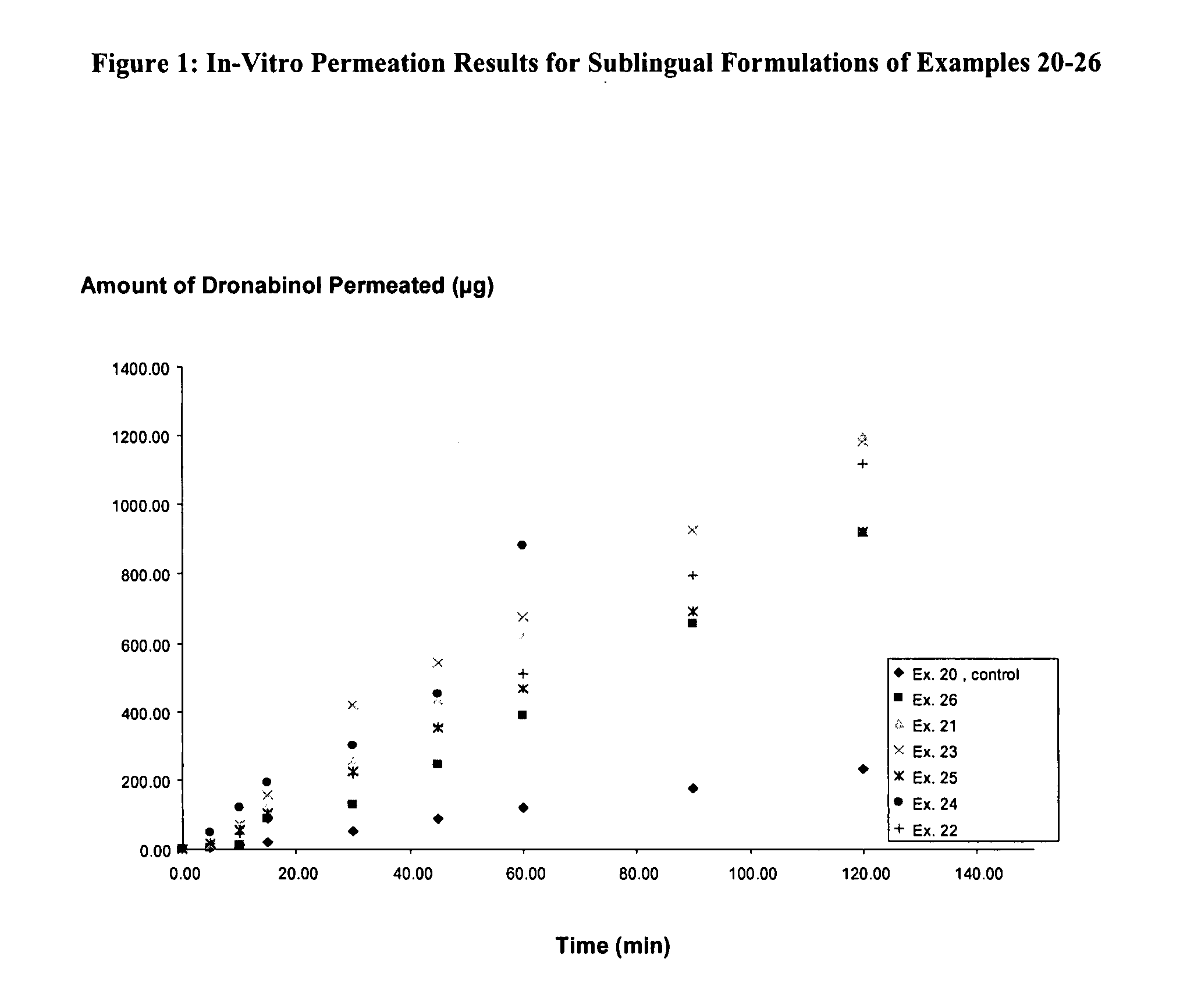
Aqueous dronabinol formulations
InactiveUS20080112895A1Stabilize cannabinoidEffective and stableBiocidePowder deliveryPolyethylene glycolRoom temperature
A room temperature stable aqueous cannabinoid formulation is disclosed. In preferred embodiments, the cannabinoid formulation comprises dronabinol in a mixture of buffer solution, and organic cosolvents such as ethanol, propylene glycol and polyethylene glycol.
Owner:INSYS THERAPEUTICS
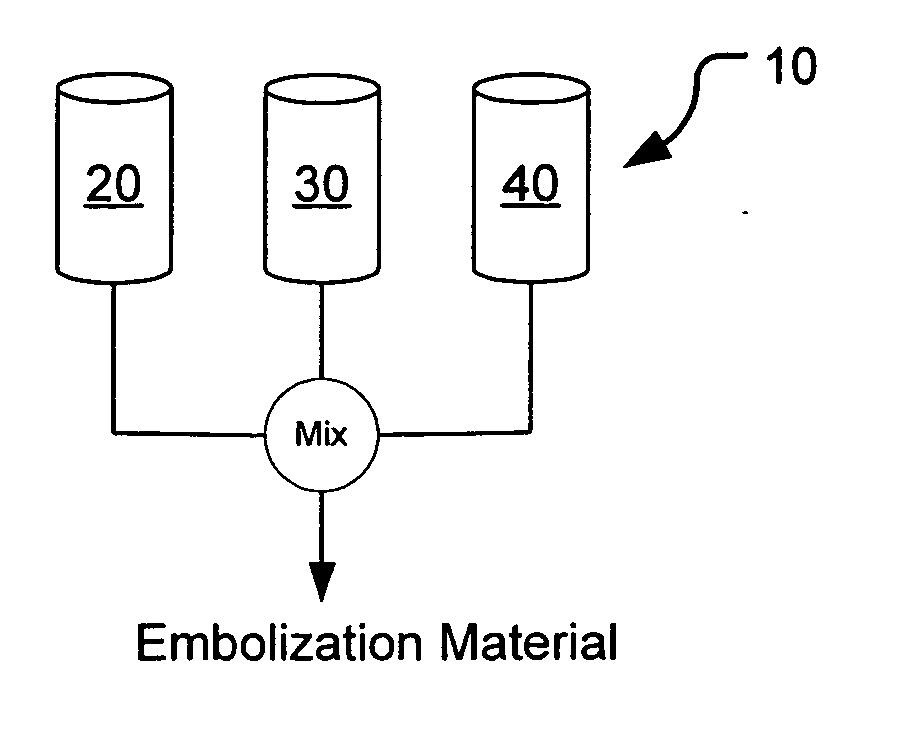
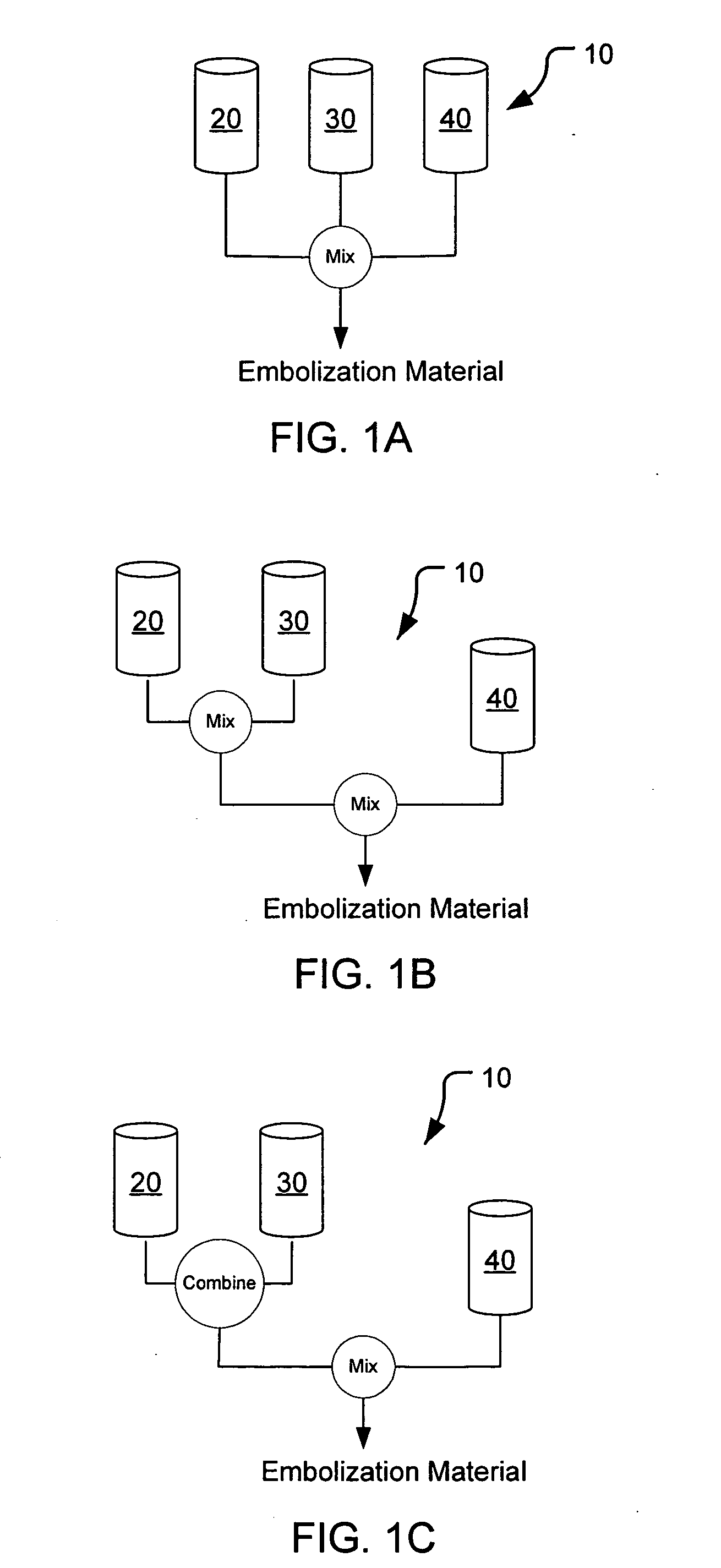
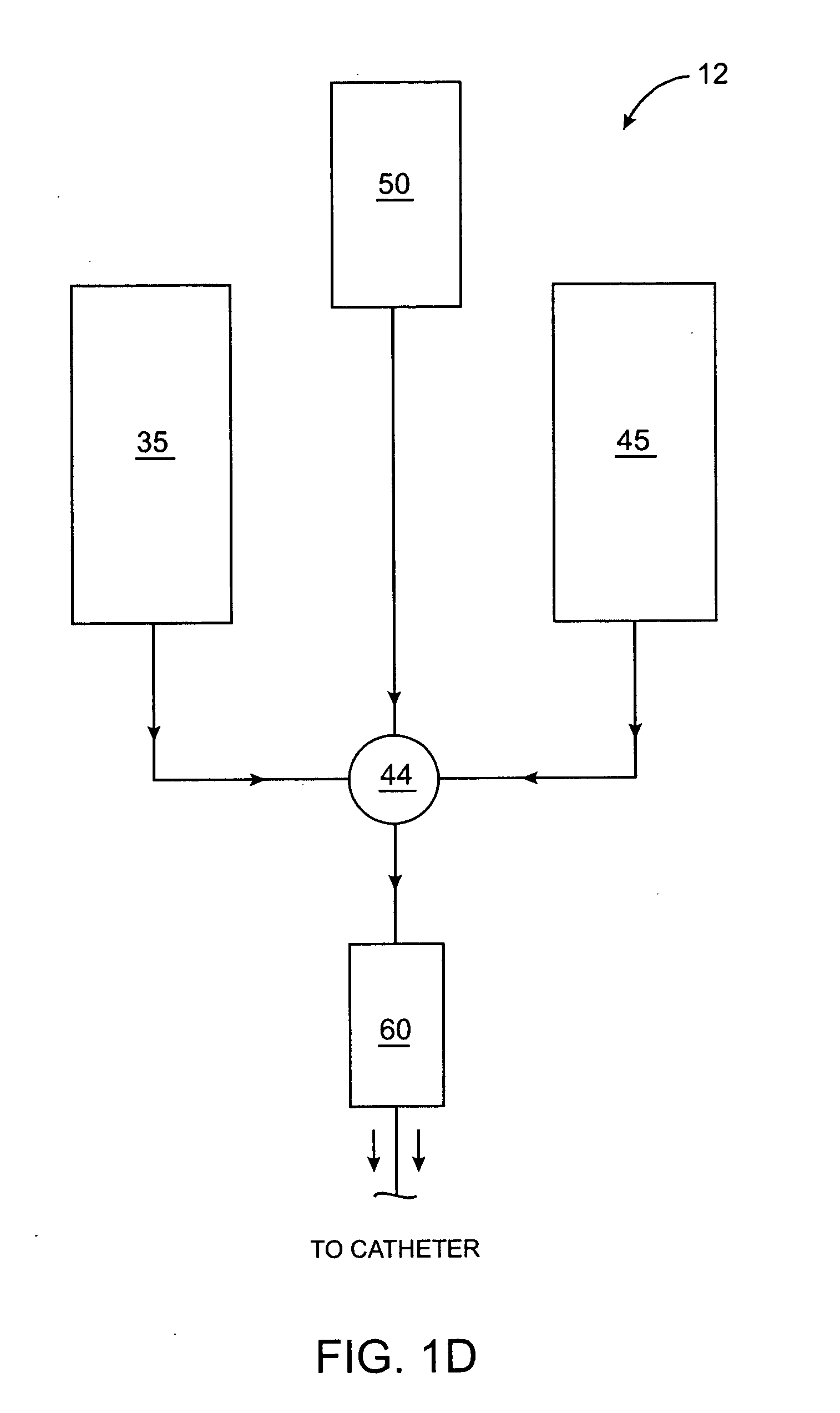
Methods, compositions and devices for embolizing body lumens
InactiveUS20050158272A1Aid in functionalityAid in appearanceSurgical adhesivesX-ray constrast preparationsPentaerythritolMedicine
The present invention provides embolic compositions, methods, and devices for embolizing a body lumen. In one embodiment, the embolic composition comprises a mixture of polyethylene glycol diacrylate (PEGDA), pentaerythritol tetra(3-mercaptopropionate), and a physiologically acceptable buffer solution.
Owner:TRIVASCULAR2
Compositions for the removal of organic and inorganic residues
InactiveUS20050119143A1Soap detergents with organic compounding agentsSurface-active detergent compositionsCarboxylic acidSolvent
A composition and method using same for removing photoresist and / or processing residue from a substrate are described herein. In one aspect, there is provided a composition for removing residue consisting essentially of: an acidic buffer solution having an acid selected from a carboxylic acid or a polybasic acid and an ammonium salt of the acid in a molar ratio of acid to ammonium salt ranging from 10:1 to 1:10; an organic polar solvent that is miscible in all proportions in water; a fluoride, and water wherein the composition has a pH ranging from about 3 to about 7.
Owner:VERSUM MATERIALS US LLC
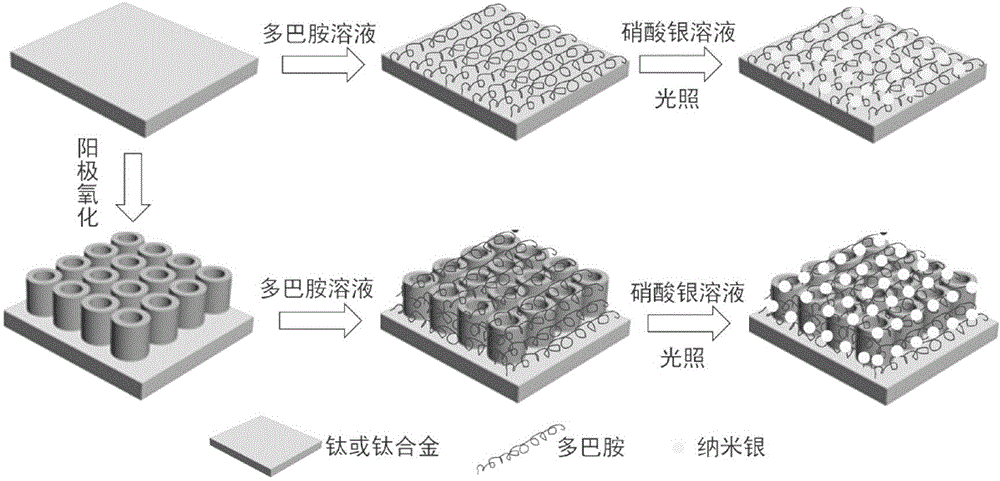
Dopamine-nanosilver composite coating and preparation method thereof
The invention discloses a dopamine-nanosilver composite coating and a preparation method thereof. Firstly, pure titanium or a titanium alloy with titanium dioxide nanotube arrays grown on the surface of the titanium alloy is put in a dopamine buffer solution, then the pure titanium loaded with the dopamine or the titanium alloy with the titanium dioxide nanotube arrays grown on the surface of the titanium alloy is put in a silver nitrate solution, and nanosilver particles are obtained by photoreduction. According to the dopamine-nanosilver composite coating and the preparation method thereof, the chelating property of the dopamine and metal ions and the reducibility in an auto polymerization process are utilized, and the antibacterial performance of material is improved by preparing the nanosilver coating on the surface of the titanium alloy, which has important application value and guiding significance for developing novel functional metal implant material.
Owner:TIANJIN UNIV
Method for detecting residual quantity of multiple alkaline drugs in animal derived food
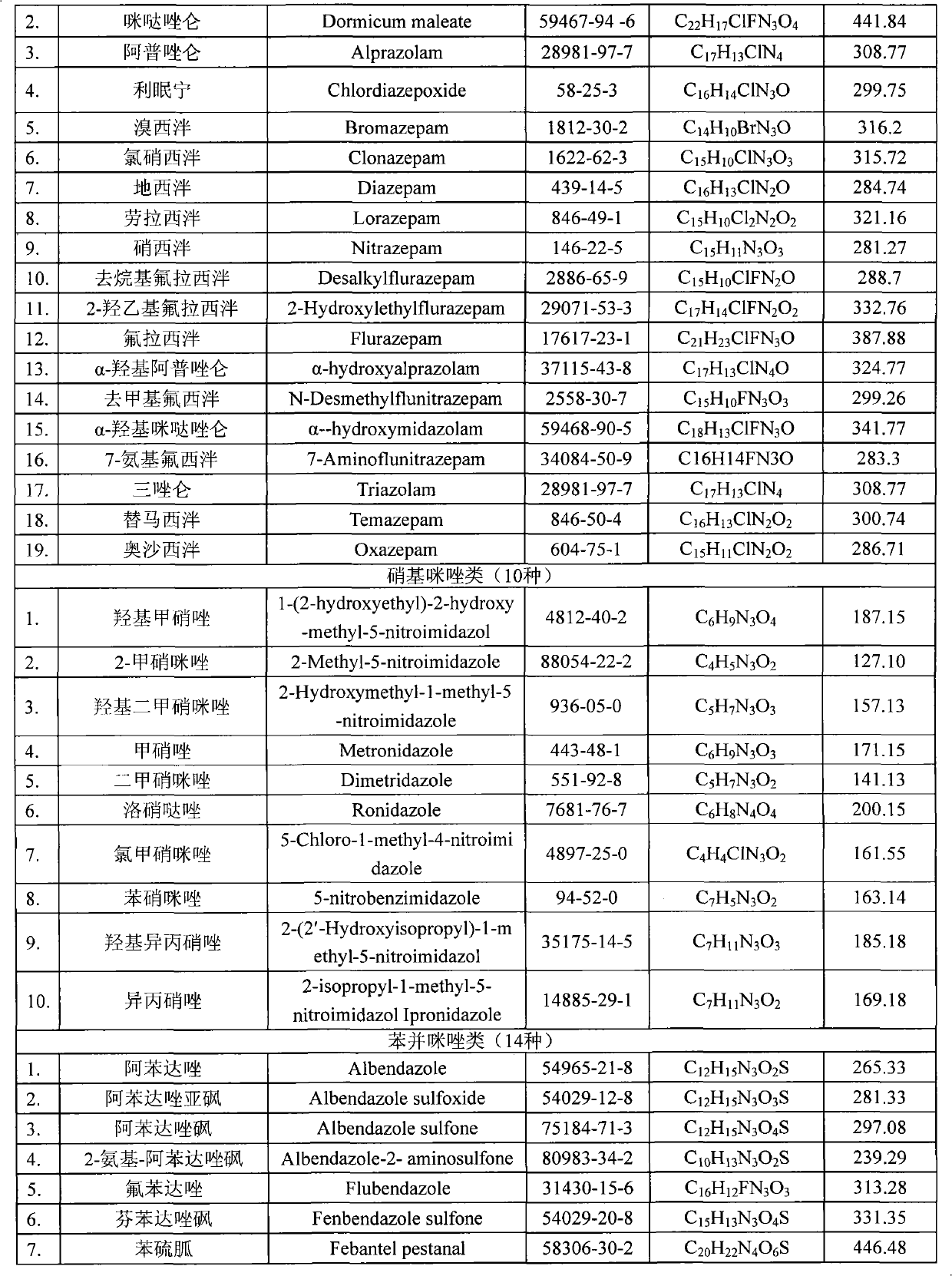
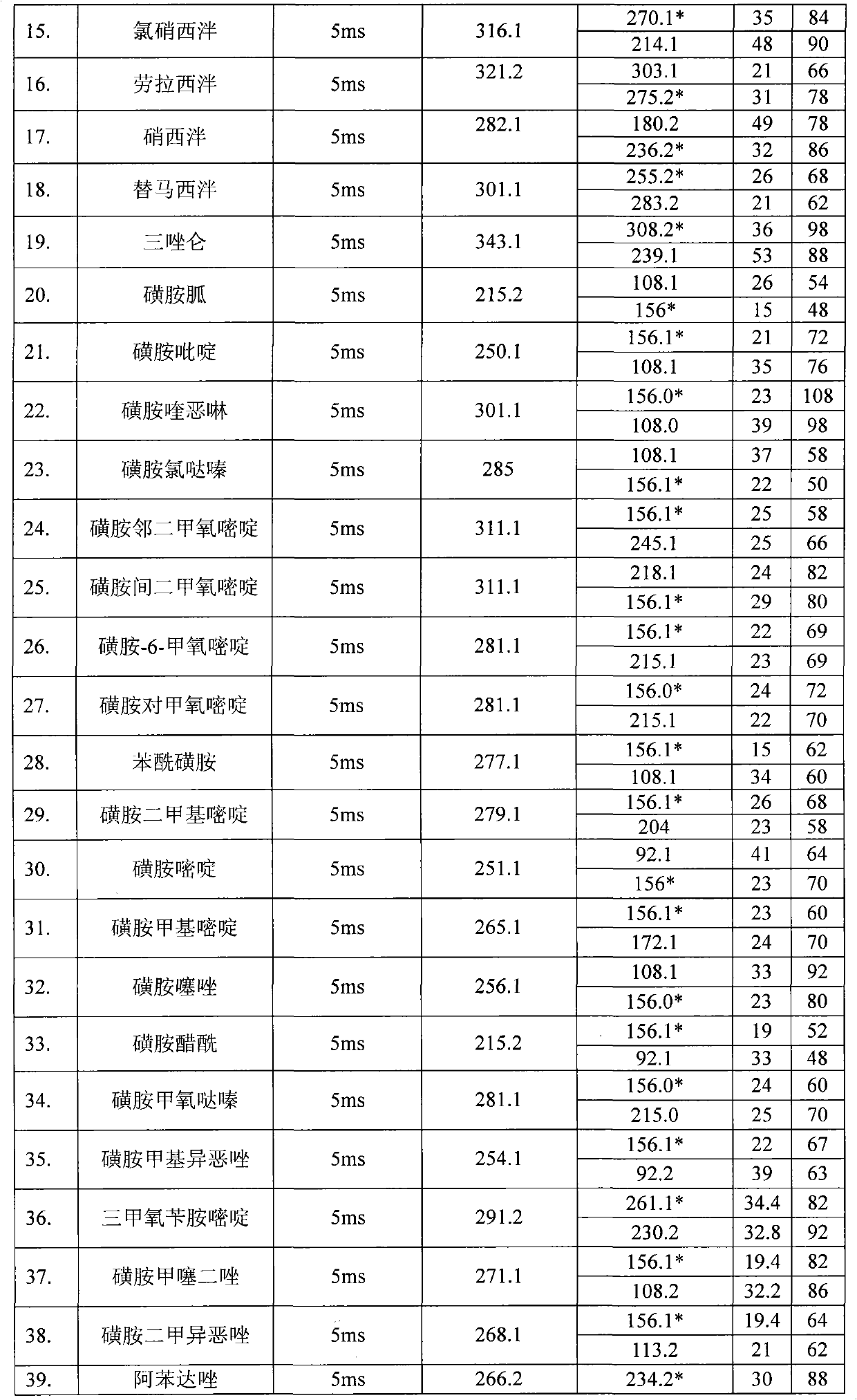
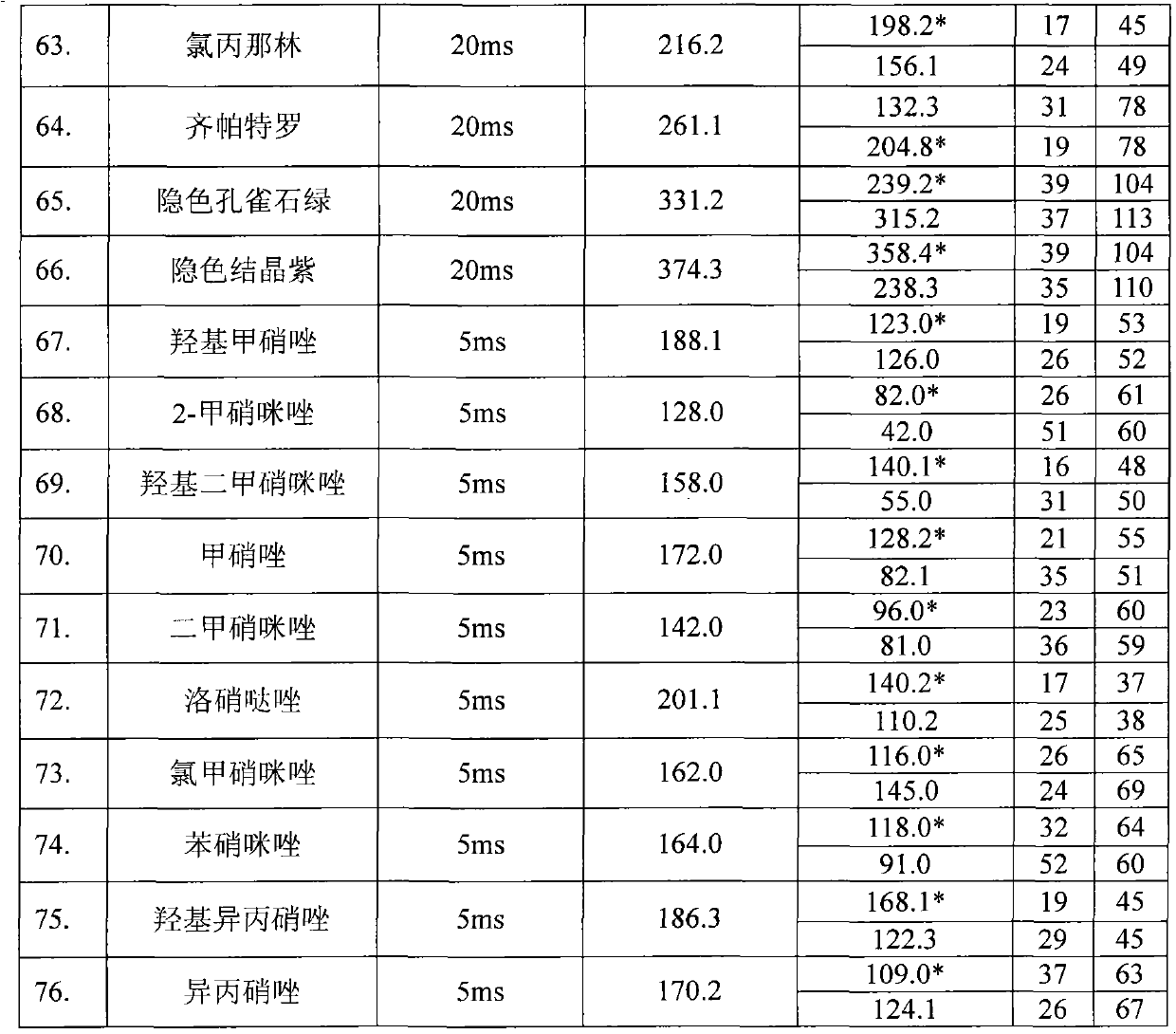
The invention relates to the fields of analytical chemistry and food safety, in particular to a method for detecting the residual quantity of multiple alkaline drugs in animal derived food. Based on the vortex mixed extracting of acetonitrile, isopropanol and citric acid buffer solutions, the purification of a hydrophilic polystyrene-divinylbenzene solid phase extraction column and a cation exchange solid phase extraction column and the liquid phase chromatography-mass spectra determination, the method can detect the residual quantity of multiple alkaline drugs in pork, pork liver, eggs, shrimps and milk, such as beta-receptor agonists,sulfonamides, benzodiazepines, nitroimidazoles, benzimidazoles and triphenylmethanes. The method has the advantages of simple operation, fast and accurate detection and high efficiency.
Owner:SHANGHAI ANPEL SCI INSTR +1
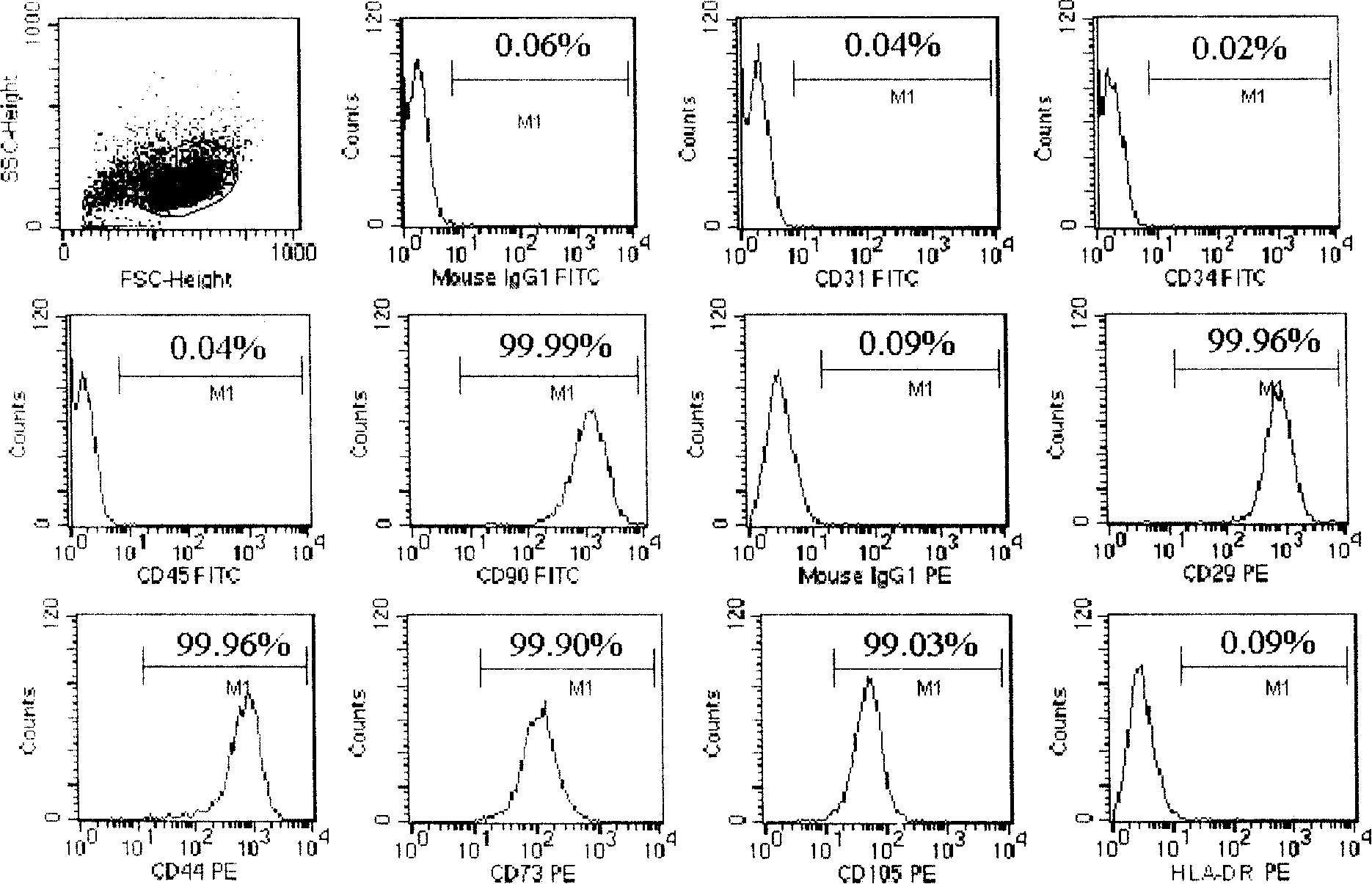
Mesenchyme stem cell preserving fluid and use thereof
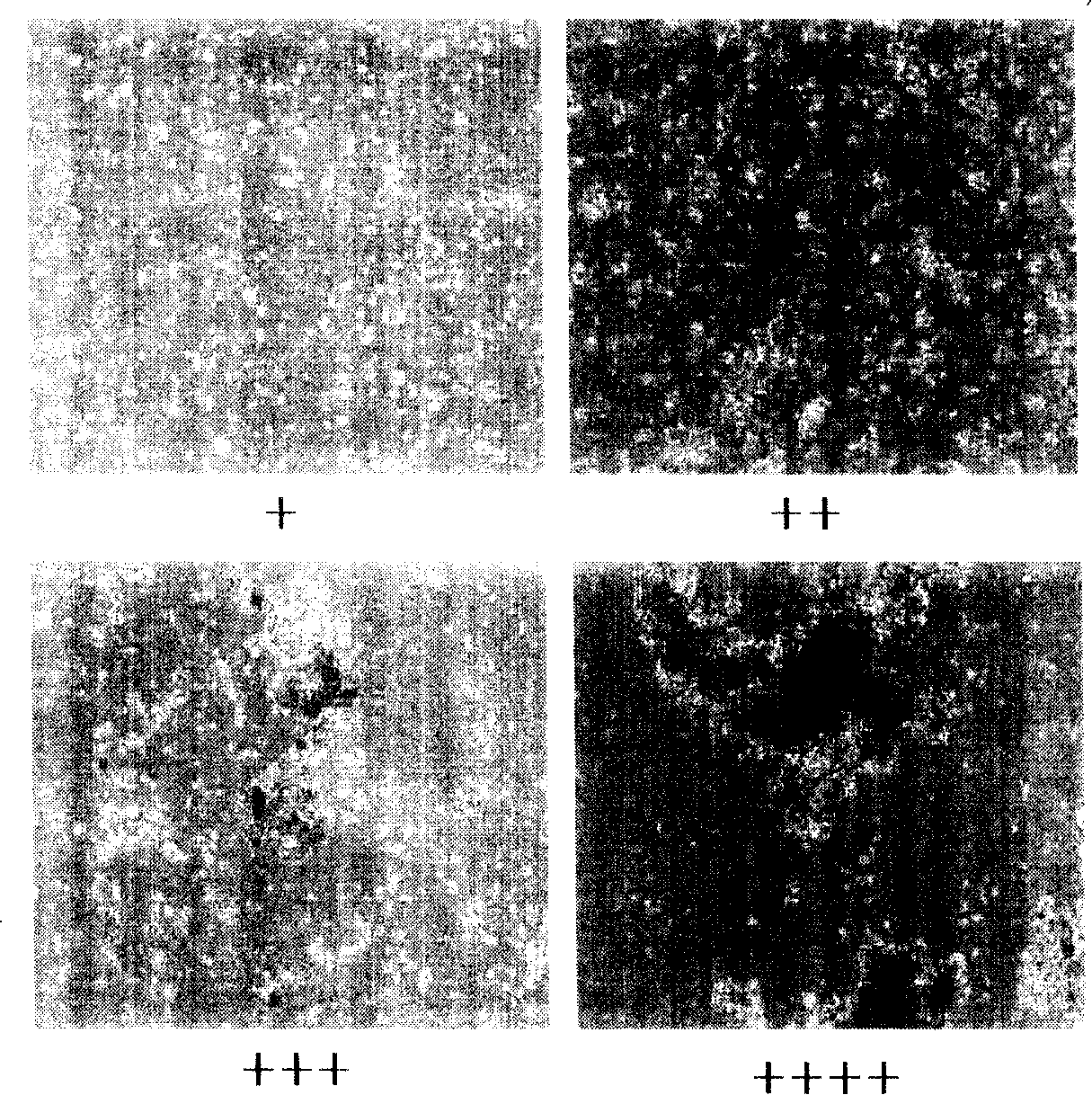
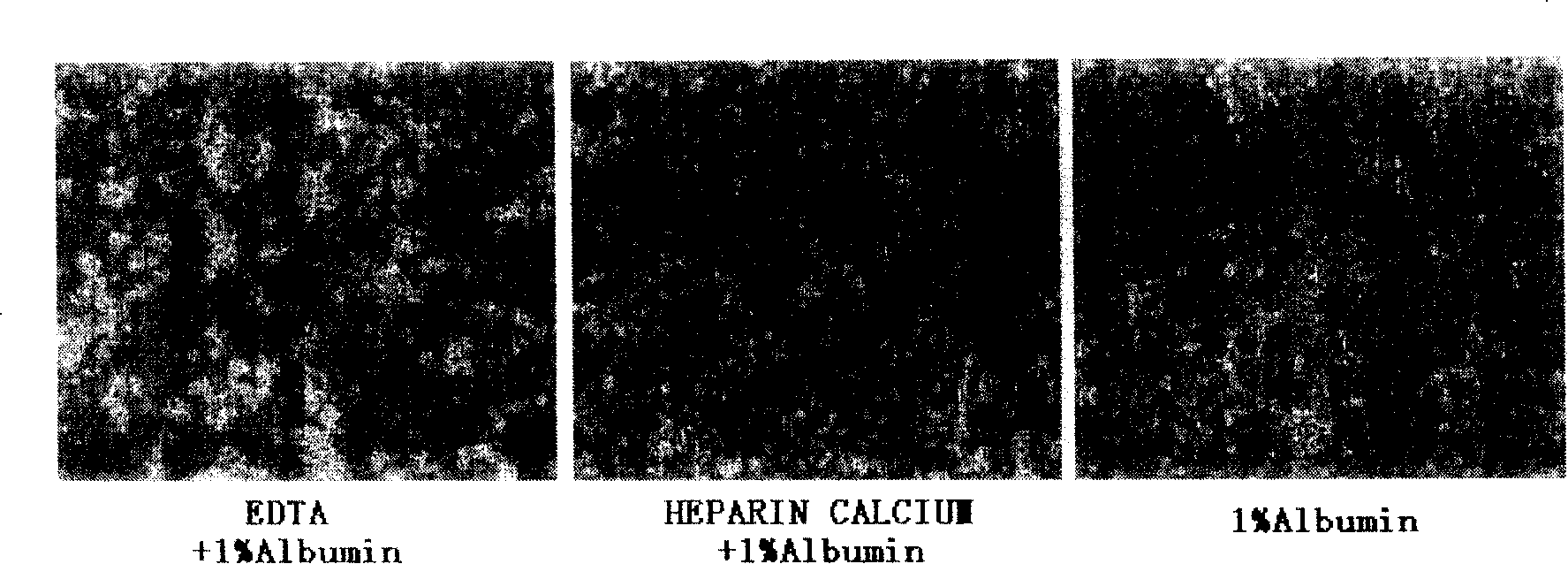
ActiveCN101210232AImprove survival rateReduce adhesionDead animal preservationTissue culturePhosphate ionCell mass
The invention discloses a preservation solution for mesenchymal stem cells and application thereof. The cell preservation solution contains human albumin and heparin as the main components, and other auxiliary reagents such as human cytokine, phosphate ion, metal ions or monosaccharide are contained in a buffer solution for preserving human mesenchymal stem cells. The preservation solution can keep high survival rate of human mesenchymal stem cells in transportation process, reduce adhesion between cells and between the cell and the inner wall of a container, and reduce the possible occurrence of cell mass embolism in blood vessel while clinically infusing human mesenchymal stem cells. The mesenchymal stem cells can be maintained in a state of single-cell suspension at an environment temperature of 4 to 15 DEG C for 24 h, thus greatly enlarging the clinic application range of the human mesenchymal stem cells. The components used in the solution accord with the clinic application, and can meet the requirement for clinic use of the human mesenchymal stem cells.
Owner:TIANJIN AMCELLGENE ENG
Cervical exfoliated cell preservative fluid
InactiveCN101363011AReduce aggregationEfficient killingDead animal preservationTissue cultureRed blood cellCervical cancer screening
The invention relates to a cervical exfoliated cell preservative liquid, the preservative liquid comprises the following components with the contents (by weight): 20 percent to 50 percent of alcohols; 15 percent to 50 percent of anti-aggregation reagent; 5 percent to 10 percent of buffer solution; 1 percent to 20 percent of ion strength maintaining reagent; 0.01 percent to 0.5 percent of anti-microbial reagent; 0.1 to 5 percent of mucus dissolving reagent; and 0 to 0.5 percent of cleaning agent. Compared with the prior art, the preservative liquid can not only lead cells to maintain the shape in an in vitro liquid suspension environment, minimize the protein precipitation, dissolve larger protein substances, such as blood and mucus, and reduce the cell aggregation, but can also selectively eliminate or reduce red cells, effectively kill microbes, prevent the activity of reverse transcriptase and retain the integrity of nucleic acids and proteins for facilitating the later analysis; in addition, the preservative liquid can greatly reduce the costs of consumptive materials for the TCT detection, improve the sensitivity and the specificity of the cervical cancer screening and accelerate the promotion and the popularization of the TCT technology in a medical system.
Owner:SHANGHAI ADICON CLINICAL LAB LNC
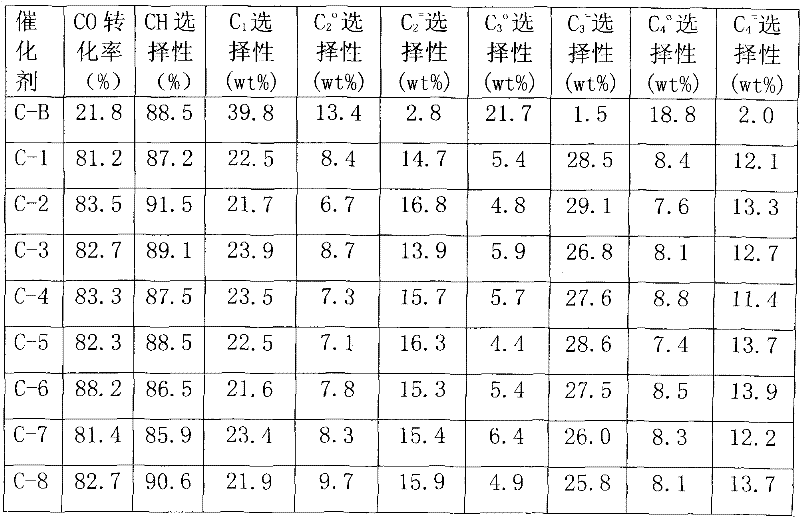
Preparation method of catalyst in process of producing light olefins by high-activity load type iron-based synthesis gas
ActiveCN102441400AOvercoming strong interactionsHigh activityCatalyst carriersHydrocarbon from carbon oxidesActive componentHigh activity
The invention discloses a preparation method of a catalyst in a process of producing light olefins by high-activity load type iron-based synthesis gas. The preparation method comprises the steps of: with silicon gel as a carrier, firstly, carrying out surface modification on the silicon gel carrier, and then loading a metal additive and an active component Fe by adopting an immersion method, wherein an ammonium salt-containing buffer solution is adopted for immersion in the surface modification method of the silicon gel carrier. After the adopted silicon gel carrier is modified, the strong interaction function between the carrier and the active component is overcome, and the activity and the selectivity of the catalyst are improved. The catalyst prepared by adopting the preparation methodis suitable for a reaction process for producing light olefins such as ethylene, propylene, butylene and the like by using the synthesis gas.
Owner:CHINA PETROLEUM & CHEM CORP +1
Mixed gel of polylactic acid microspheres and cross-linked hyaluronic acid for injection and preparation method of mixed gel
The invention relates to a mixed gel of polylactic acid microspheres and cross-linked hyaluronic acid for injection and a preparation method of the mixed gel. The polylactic acid microspheres have the molecular weight of 15000-120000 and the average particle size of 10-150mu m, and the mass fraction of the polylactic acid microspheres in the mixed gel is 5-25%; a salt solution of the cross-linked hyaluronic acid gel is obtained by swelling and balancing the cross-linked hyaluronic acid gel in a sodium chloride solution or a phosphate buffer solution with the osmotic pressure of 250-350mOsmol / L and the pH value of 6.5-7.5; the cross-linked hyaluronic acid gel is a hyaluronic acid gel cross-linked by divinyl sulfone or glycidyl ether. A mixed gel product of polylactic acid and cross-linked hyaluronic acid, obtained by directly mixing the polylactic acid microspheres with the cross-linked hyaluronic acid gel obtained by swelling and balancing in the salt solution is uniform, fine, long in partial retention time, good in plasticity and few in side effects, has an obvious effect on eliminating wrinkles, and has few operation steps and stable product quality.
Owner:河北善远科技有限公司
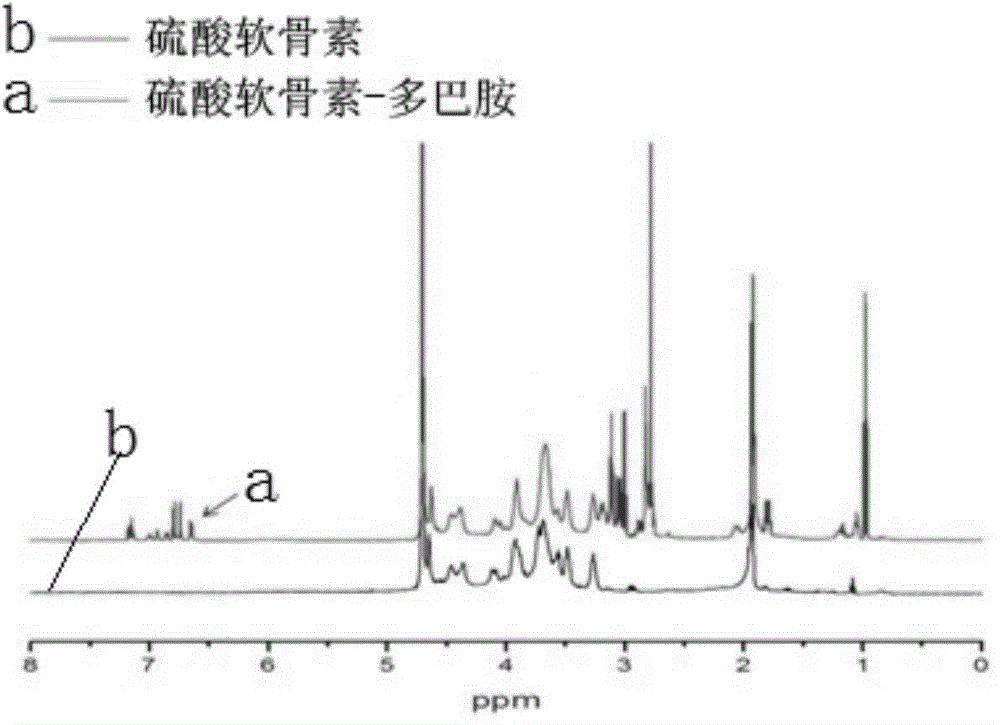
Polysaccharide-dopamine composite biogel and application thereof
The invention discloses a polysaccharide-dopamine composite biogel and application of the biogel. A preparation method of the biogel comprises the following steps: dissolving 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride and N-hydroxy succinimide sodium in an MES (Methyl Ester Sulfonate) buffer solution with a pH value being 5-6 in an inert gas atmosphere so as to prepare an EDC (ethylcarbodiimide) mixed solution, subsequently adding the EDC mixed solution into a polysaccharide solution for reacting at 20-30 DEG C for 0.5-1 hour, then adding a dopamine solution for further reacting at 20-30 DEG C for 1-4 hours, dialyzing a reactant by taking distilled water as a dialyzate, taking trapped fluid for freezing and drying to obtain the polysaccharide-dopamine composite biogel. The polysaccharide-dopamine composite biogel is simple and effective in modification method and capable of increasing the surface viscidity of the cartilage tissue, reducing loss of therapeutic drugs or implanted cells and facilitating the repairing of the cartilage injury.
Owner:ZHEJIANG UNIV
Collection device for a fluid specimen
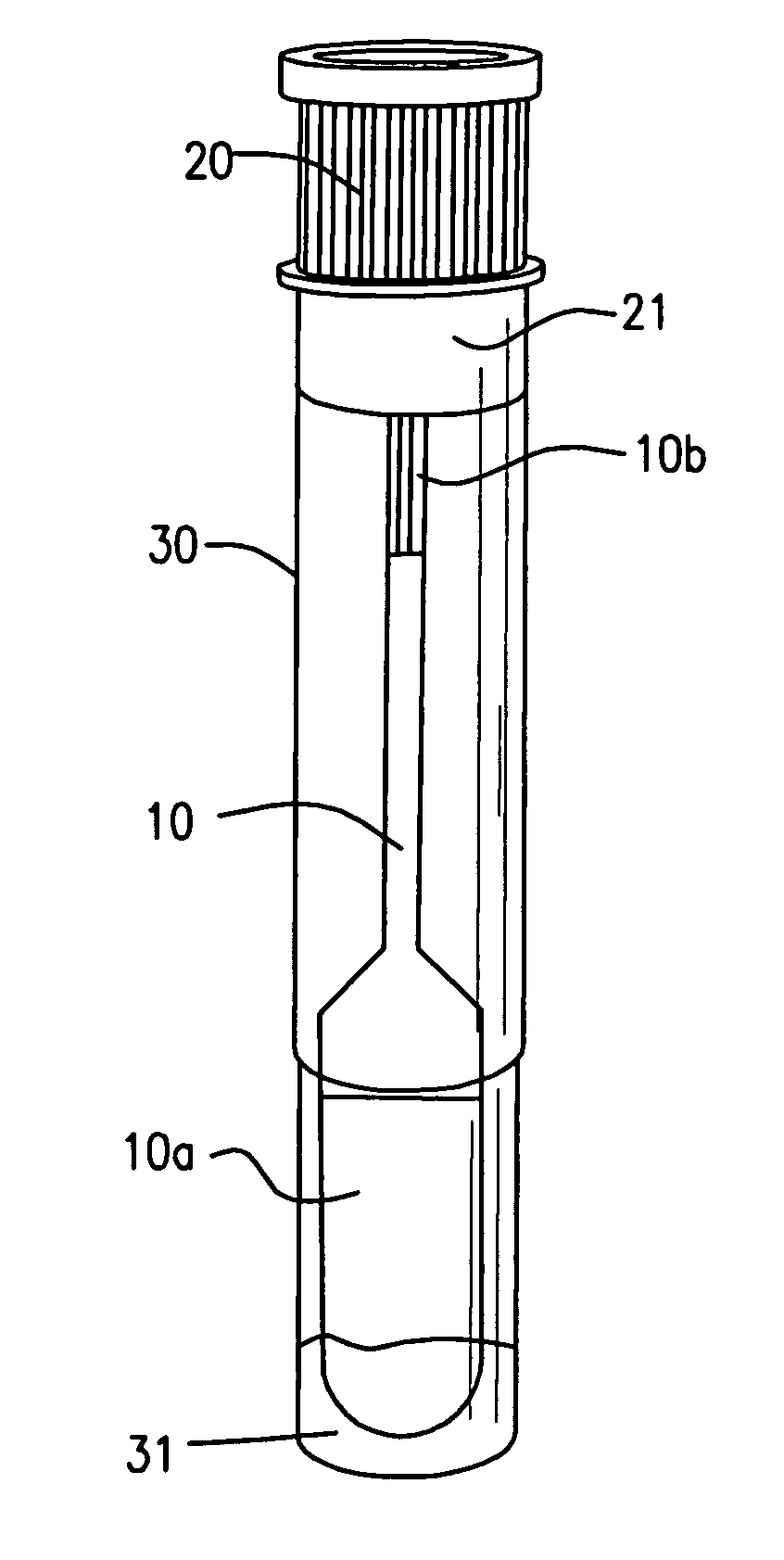
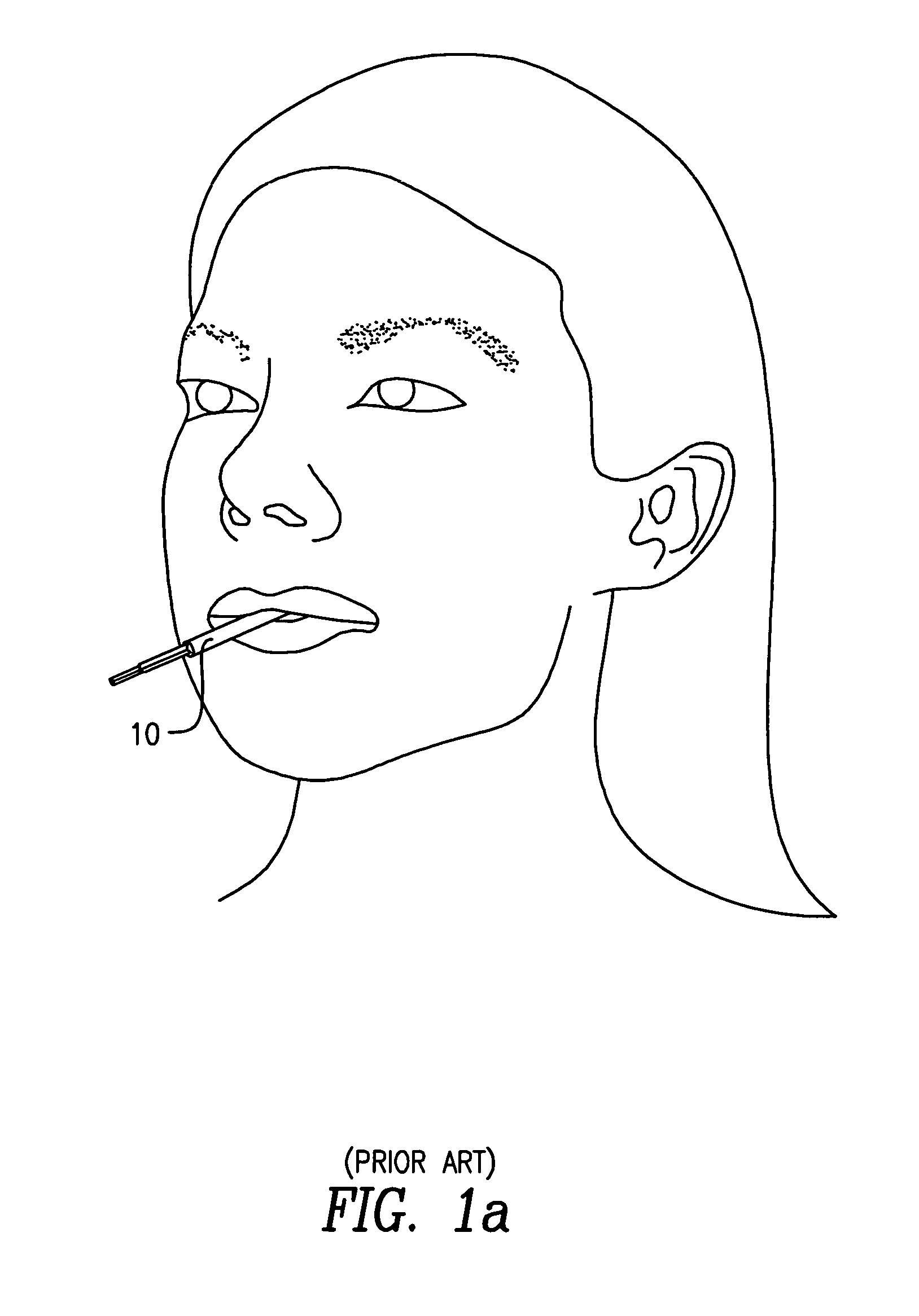
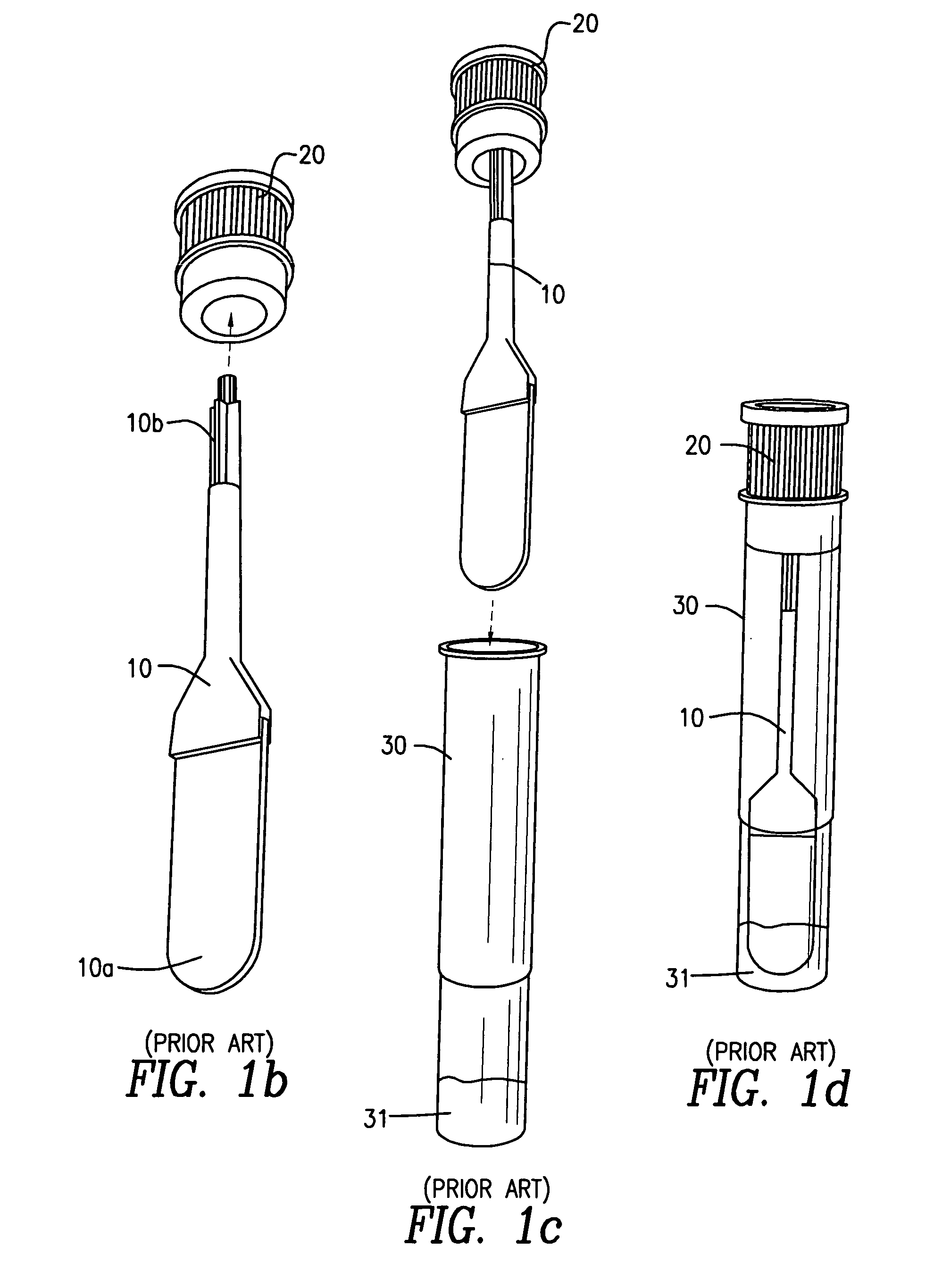
InactiveUS20060245977A1Avoid absorptionAnalysis using chemical indicatorsVaccination/ovulation diagnosticsEngineeringBuffer solution
A device for collecting a fluid specimen absorbed in an absorption pad, comprises a tube for receiving the pad. The pad is selectively secured to one of the two positions. In the first position, the pad is approximate to a bottom of the tube so that the pad can be immersed in a buffer solution for preserving the specimen. In the second position, the pas is distant from the bottom so that the fluid specimen thrown out from the pad under a centrifugal separating force will not be reabsorbed by the pad.
Owner:SALIVA DIAGNOSTIC SYST
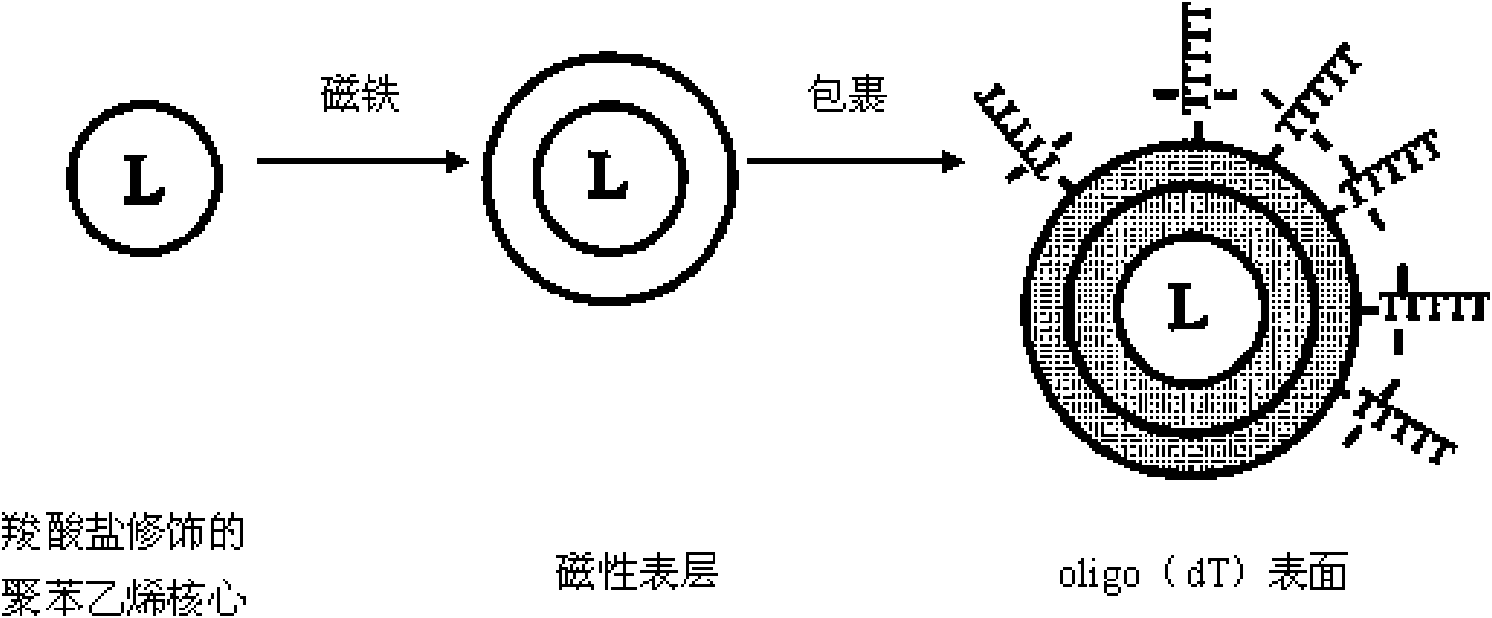
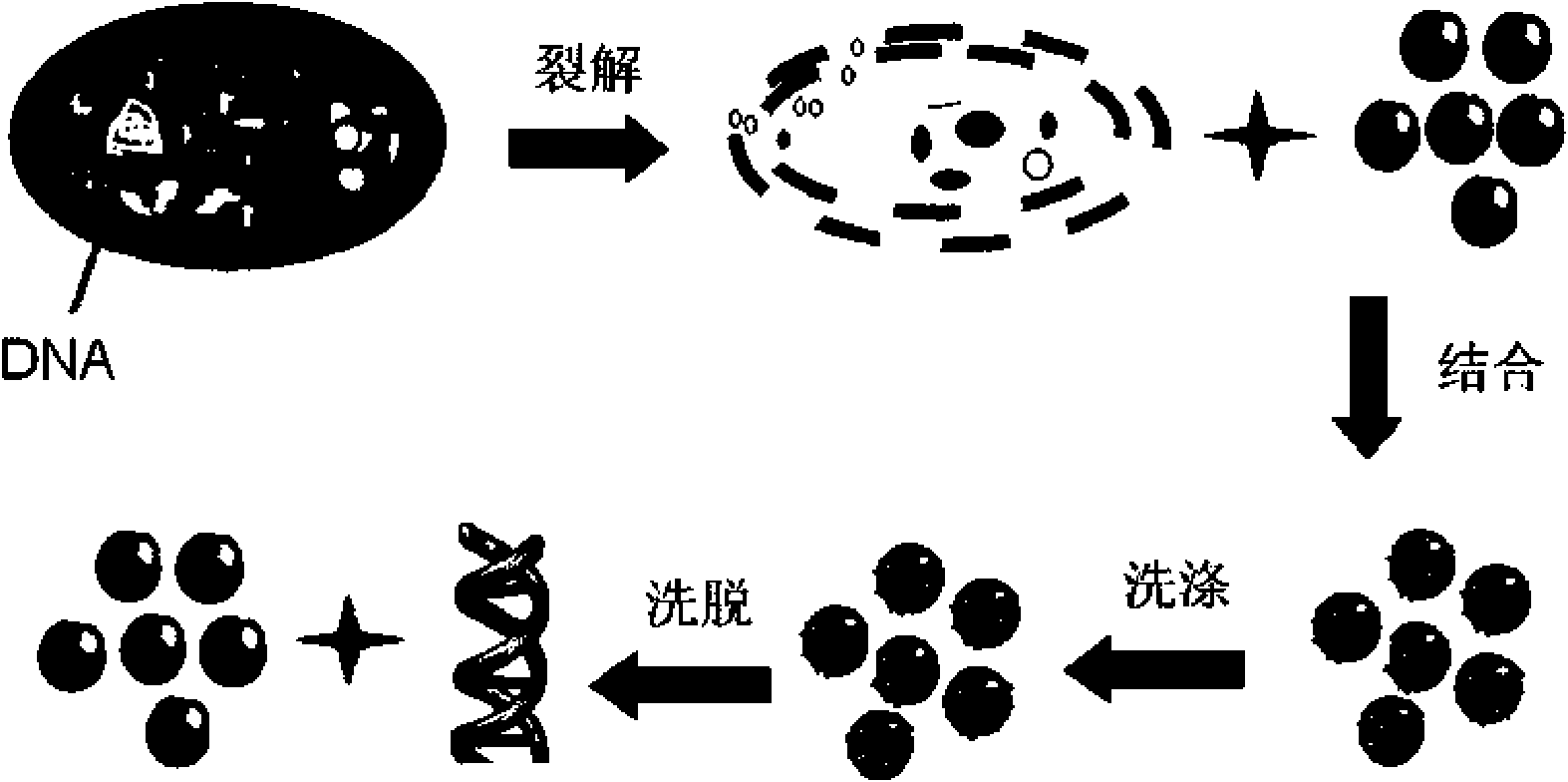
Method for extracting and purifying nucleic acid from samples by magnetic beads
ActiveCN101665785AIncrease productionHigh puritySugar derivativesMicrobiological testing/measurementMagnetic beadElution
The invention relates to a method for extracting and purifying nucleic acid from samples by magnetic beads, which comprises the following steps: (1) magnetic bead treatment: modifying the magnetic beads by peptide-oligonucleotide; (2) cell lysis: adding the sample containing target nucleic acid and needing to be separated into a centrifuge tube, and then adding lysis buffer to lyse the cells; (3)nucleic acid adsorption: adding the magnetic beads modified by the peptide-oligonucleotide into the solution to lead the nucleic acid to be adsorbed on the surfaces of the magnetic beads; placing thecentrifuge tube into a magnetism separation machine to lead the magnetic beads to be adsorbed on the tube side; (4) impurities removal: adding aqueous phase buffer solution to wash the magnetic bead which are adsorbed with the nucleic acid on the surfaces and adsorbed on the tube side so as to separate other impurities on the magnetic bead; and (5) nucleic acid recovery: directly taking the nucleic acid adsorbed on the surfaces of the magnetic beads as the template for PCR amplification, or adding elution buffer to lead the nucleic acid molecules adsorbed on the surfaces of the magnetic beadsto be released into the buffer for downstream experiments. The magnetic beads used by the method can be reserved at the temperature of 4 DEG C or -20 DEG C, and have convenient transport, high adsorption efficient and lower cost. The nucleic acid extracted and purified by the method has wide application range.
Owner:SANSURE BIOTECH INC
Injectable crosslinked hyaluronic acid gel and preparation method thereof
The invention relates to an injectable crosslinked hyaluronic acid gel and a preparation method thereof. The injectable crosslinked hyaluronic acid gel is prepared by blending hyaluronic acid gel granules and chlorinated sodium phosphate physiological buffer solution; the hyaluronic acid gel granules are prepared by comprising the steps of crosslinking treatment, emulsion crosslinking granulation, purification and drying and swelling, filling and sterilization technologies. The hyaluronic acid gel granules are uniform in granule size, the residual of the crosslinking agent is less than 0.2ppm, the injection pushing is proper, and the in-vivo degradation time can last more than 8-12 months. The implant has an excellent esthetical restoration effect, is applicable to being injected to and subcutaneous dermis deep layer to subcutaneous superficial layer, restoration of moderate to severe wrinkles or folds, and can satisfy the restoration demand of wrinkles or folds caused due to skin aging.
Owner:SHAANXI BIO REGENERATIVE MEDICINE CO LTD
Preparation method of polyinosinic-polycytidylic acid dry powder
ActiveCN103599071AEasy to makeEasy to operatePowder deliveryCosmetic preparationsBiological bodyOrganic solvent
The invention provides a preparation method of polyinosinic-polycytidylic acid dry powder. The method comprises the following steps: (1) respectively dissolving polyinosinic acid and polycytidysic acid in a phosphate buffer solution, mixing the solution, adding a stabilizer, uniformly mixing the stabilizer and the solution, and performing heat preservation on the mixture at the temperature of 40-100DEG C; (2) naturally cooling the reaction liquid obtained in the step (1), mixing the reaction liquid with an organic solvent, standing for precipitating, and drying the precipitate, thereby obtaining the polyinosinic-polycytidylic acid dry powder. The preparation method has the beneficial effects that the prepared polyinosinic-polycytidylic acid dry powder has a complete double-helix structural features and physiological activity, the quality and the stability are better than those of the polyinosinic-polycytidylic acid at a solution state, a stronger enzymolysis-resisting performance can be achieved compared with the bare polyinosinic-polycytidylic acid after entering a living body, so that the curative effect can be enhanced. The polyinosinic-polycytidylic acid is purified in the process for making the polyinosinic-polycytidylic acid solution into the dry powder, a plurality of small-molecular-weight substances and impurities can be removed, the toxic and side effects can be reduced, and the quality of the polyinosinic-polycytidylic acid can be improved.
Owner:美亚药业海安有限公司
Full-scale C-reactive protein (CRP) colloidal gold immunoturbidimetric assay kit
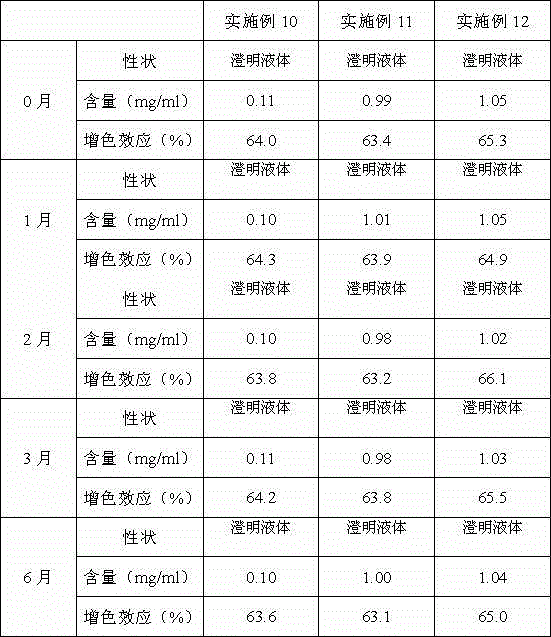
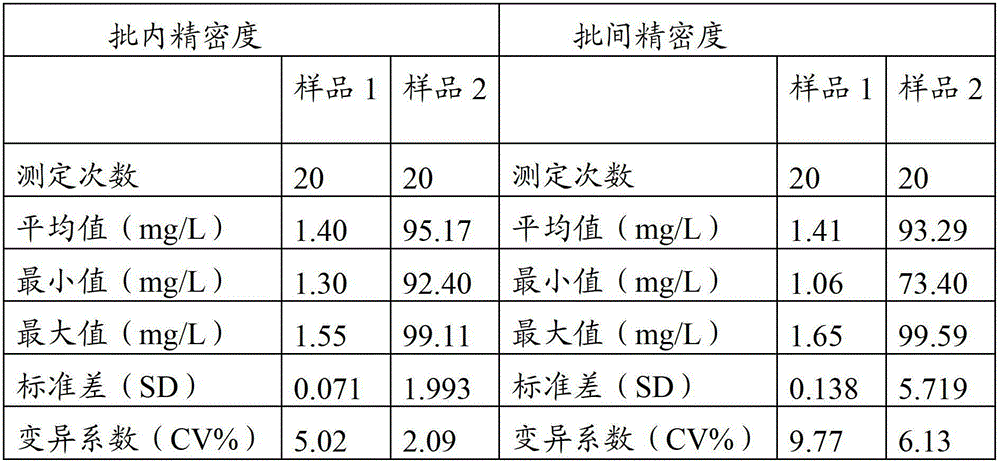
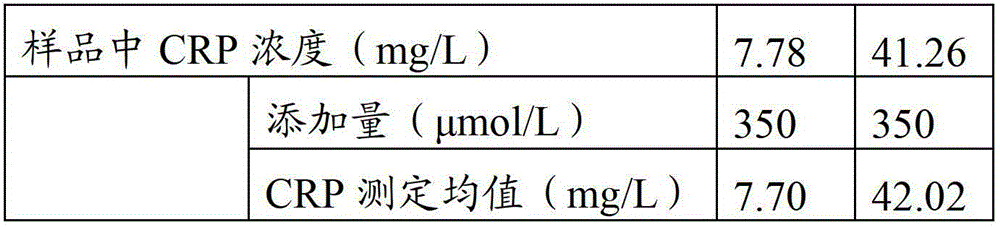
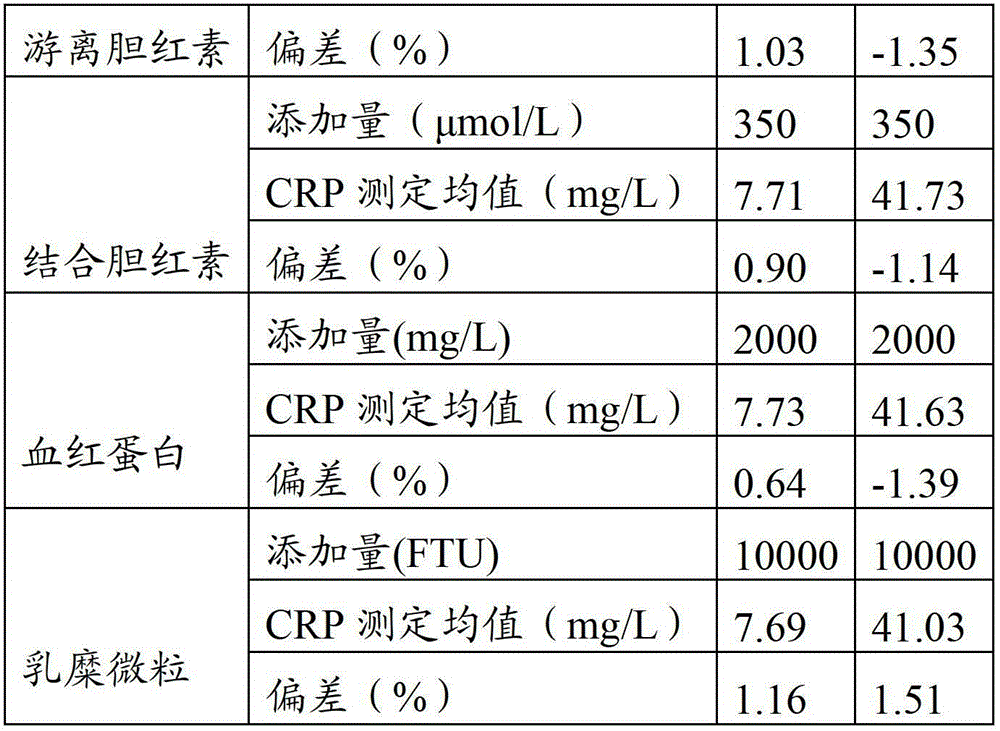
The invention provides a full-scale CRP colloidal gold immunoturbidimetric assay kit. A quantitative assay purpose is reached through enhancing the turbidity by the colloidal gold. The method used by the kit provided by the invention solves a problem that the generation of a precipitate after a latex reinforced immunoturbidimetric reaction goes against biochemical instrument cleaning. The kit provided in the invention comprises a reagent R1 and a reagent R2, wherein the reagent R2 is a proper buffer solution; and the reagent R2 is a buffer solution of the colloidal gold combined with an antihuman CRP antibody. The kit has the characteristics of high sensitivity, strong specificity and good stability, can be used for assaying the content of the CRP in serum or blood plasma, and is suitable for clinical fully-automatic biochemical analyzers. The CRP assay sensitivity of the kit can reach 0.01mg / L, and the upper CRP assay limitation of the kit is 500mg / L.
Owner:重庆沃康生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com