Patents
Literature
315 results about "Saliva sample" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
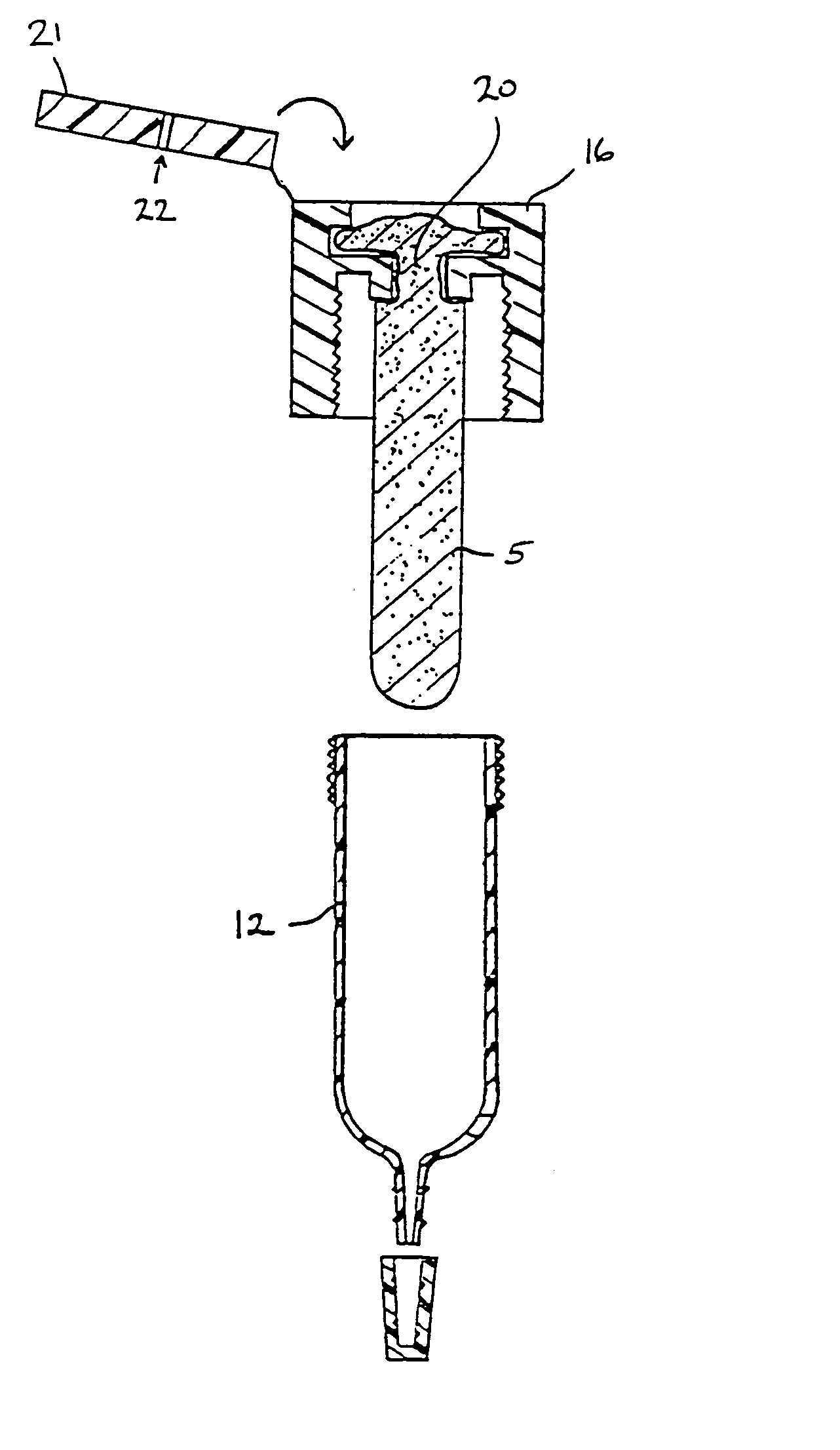
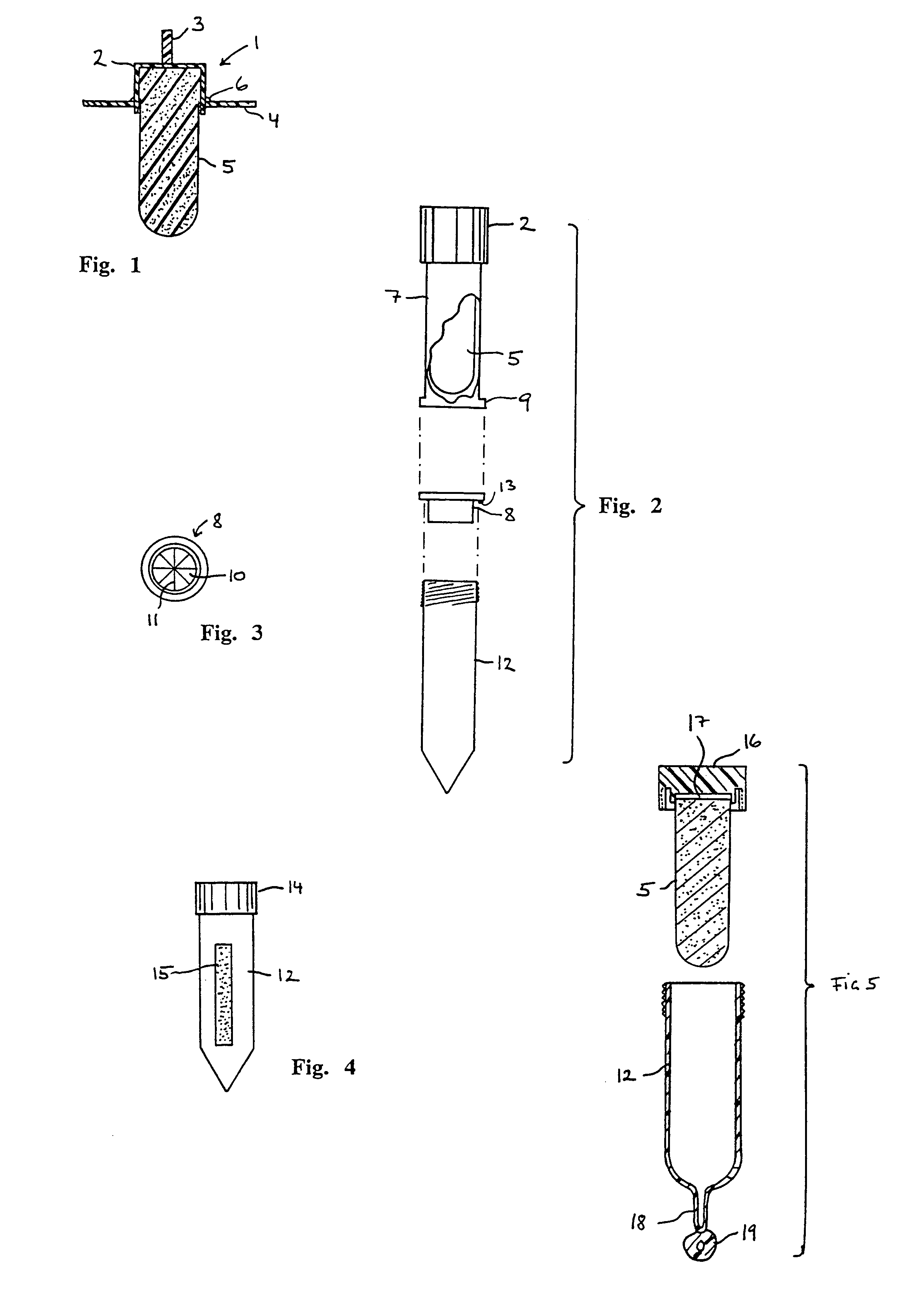
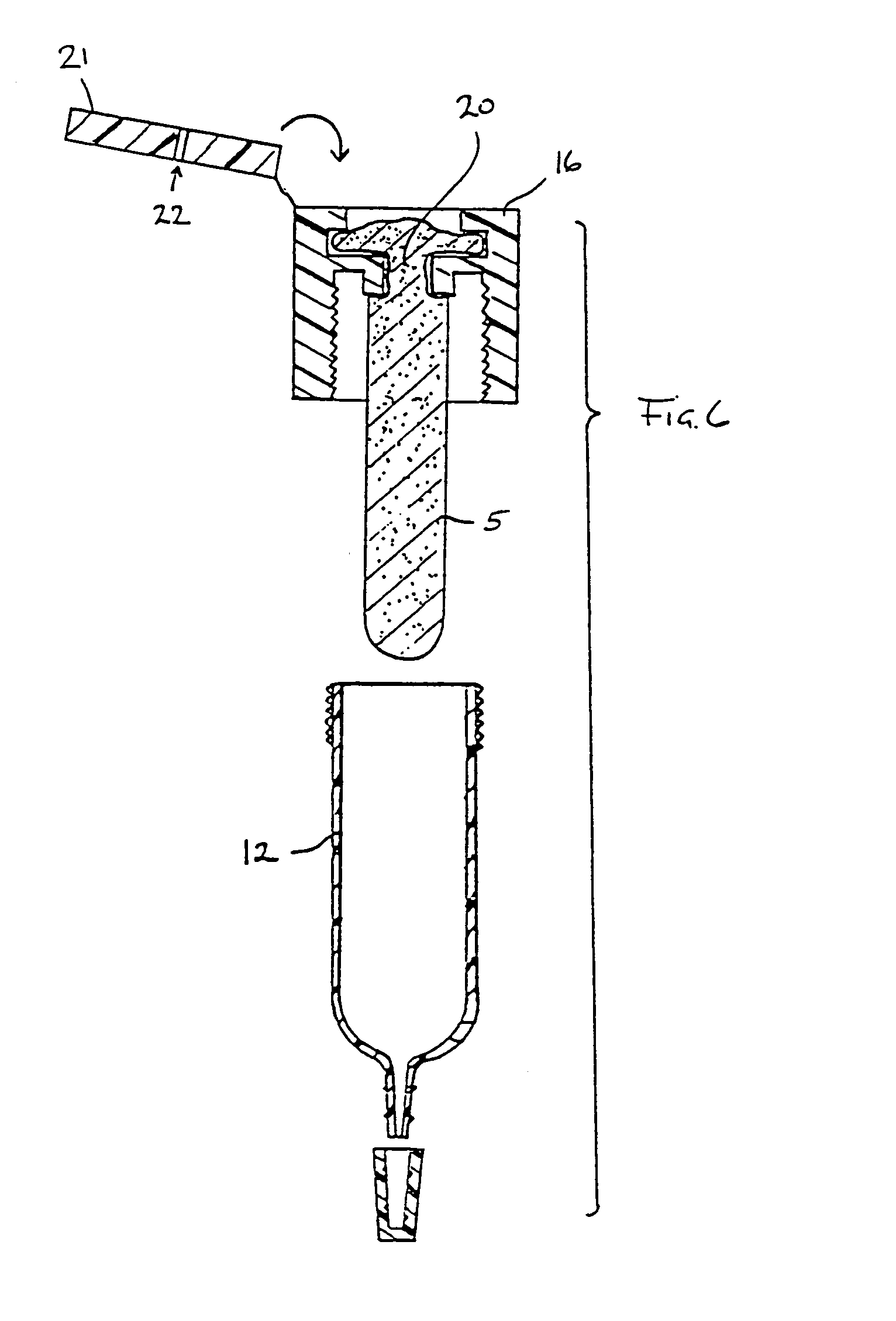
Immunoassay that provides for both collection of saliva and assay of saliva for one or more analytes with visual readout
InactiveUS6248598B1Eliminate riskBioreactor/fermenter combinationsBiological substance pretreatmentsAnalyteSaliva sample
A device that provides for both the collection of saliva and detection of at least one analyte therein, e.g., a drug, is provided. This device provides for rapid analysis of saliva samples, while also providing a convenient assay method that does not require the addition of extraneous reagents, or other materials. Thereby, this device can be used by non-laboratory personnel without risk of user introduced errors.
Owner:BOGEMA STUART C
Saliva sample testing device
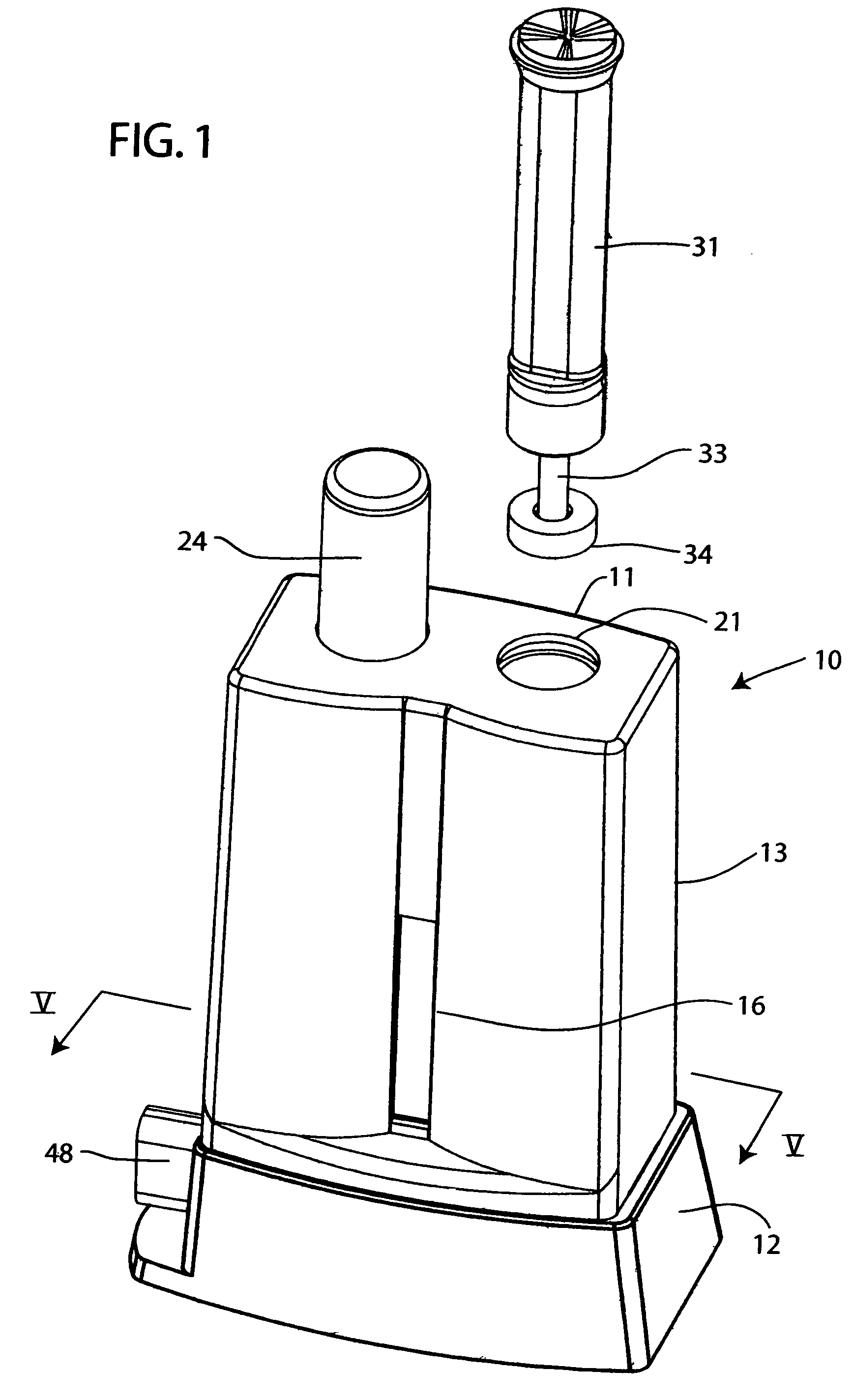
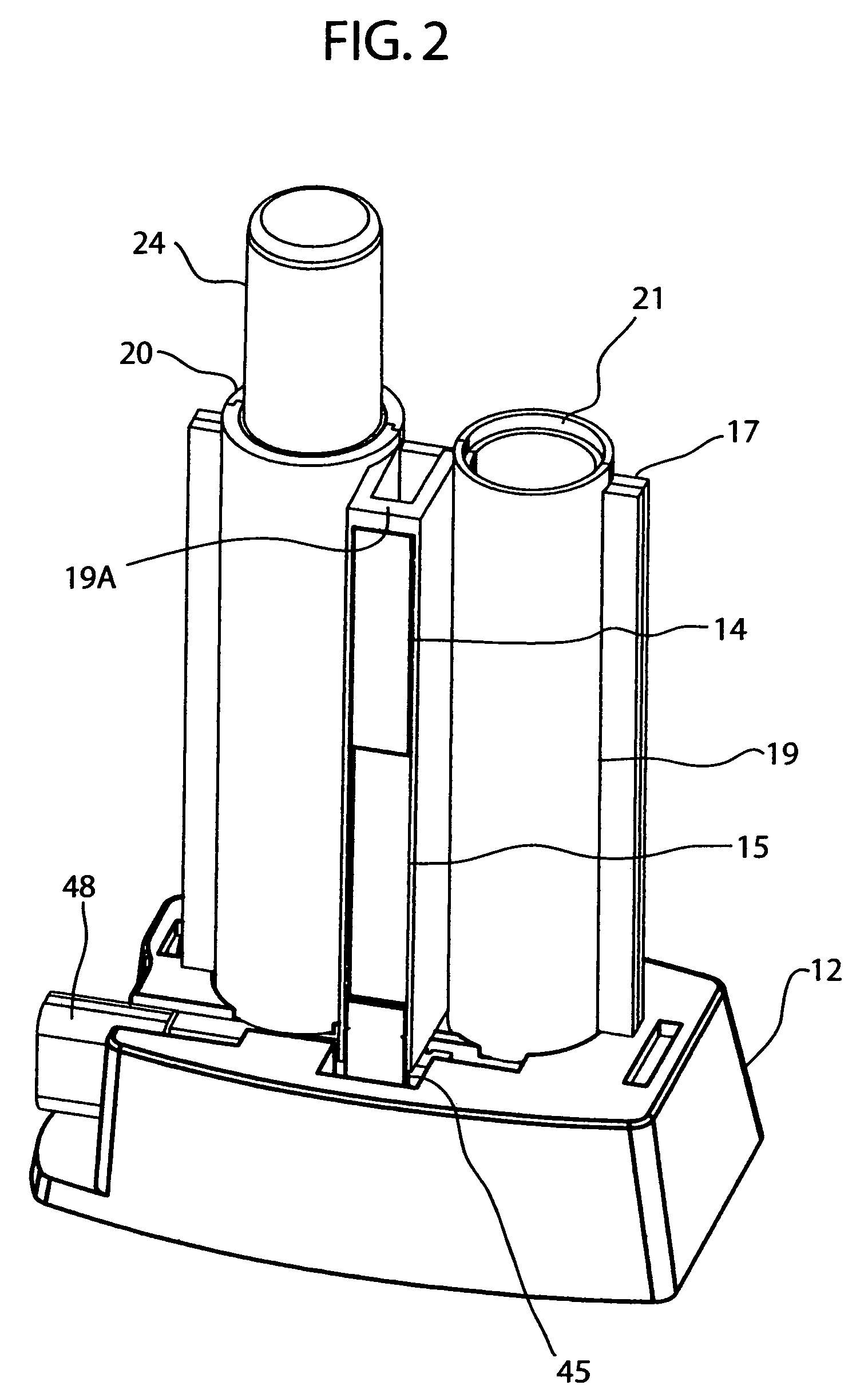
ActiveUS20060292035A1Easy to operateHigh sensitivityAnalysis using chemical indicatorsVaccination/ovulation diagnosticsSaliva sampleTest flow
In a lateral test flow immunoassay device, a saliva sample and a buffer solution are delivered into a mixing chamber to mix with a second reagent. The resulting test mixture is allowed to incubate for a pre-determined period of time and then selectively delivered to a test strip
Owner:HEALGEN SCI LLC
Image processing system for predicting ovulation
InactiveUS6960170B2Stimulates saliva productionAnalysis using chemical indicatorsSurgerySaliva sampleBristle
An image processing system for predicting ovulation using a test channel for collecting saliva sample and a miniature camera for capturing the image of the saliva at dried state for analyzing the crystalline patterns for ovulation prediction. A rotary bristle element is attached to the drive head and a notch-like test channel traverses the width of the drive head. A conductivity sensor is mounted on a wall of the test channel for detecting filling and drying of the saliva sample. An algorithm in the microprocessor analyzes the image of the dried saliva and calculates the characteristic line length of line segments of connected saliva dots. A ferning index is also defined and calculated based on the percentage of area coverage of line segments which are exceeding the threshold line length. Trend curves are established based on the daily saliva analysis in a woman's menstrual cycle for predicting days from the ovulation.
Owner:KUO YOUTI
Compositions for stabilizing DNA, RNA and proteins in saliva and other biological samples during shipping and storage at ambient temperatures
ActiveUS20130209997A1Prevent degradationSugar derivativesMicrobiological testing/measurementSaliva sampleColloid
Compositions and methods are disclosed for substantially liquid, gel, suspension, slurry, semisolid and / or colloid storage of biological samples following admixture with the herein disclosed storage composition, permitting substantial recovery of biological activity following storage without refrigeration. In certain embodiments, unfractionated saliva samples may be stored without refrigeration for weeks, months or years in a form that permits recovery of intact DNA following the storage period.
Owner:BIOMATRICA INC
Saliva sample testing device
InactiveUS20060292034A1Easy to operateHigh sensitivityAnalysis using chemical indicatorsVaccination/ovulation diagnosticsSaliva sampleMedicine
In this testing device, a saliva sample and a buffer solution are delivered from separate chambers into a mixing chamber to mix with a second reagent. The resulting test mixture is allowed to incubate for a pre-determined period of time and then selectively delivered to a test strip
Owner:AMERICAN BIO MEDICA
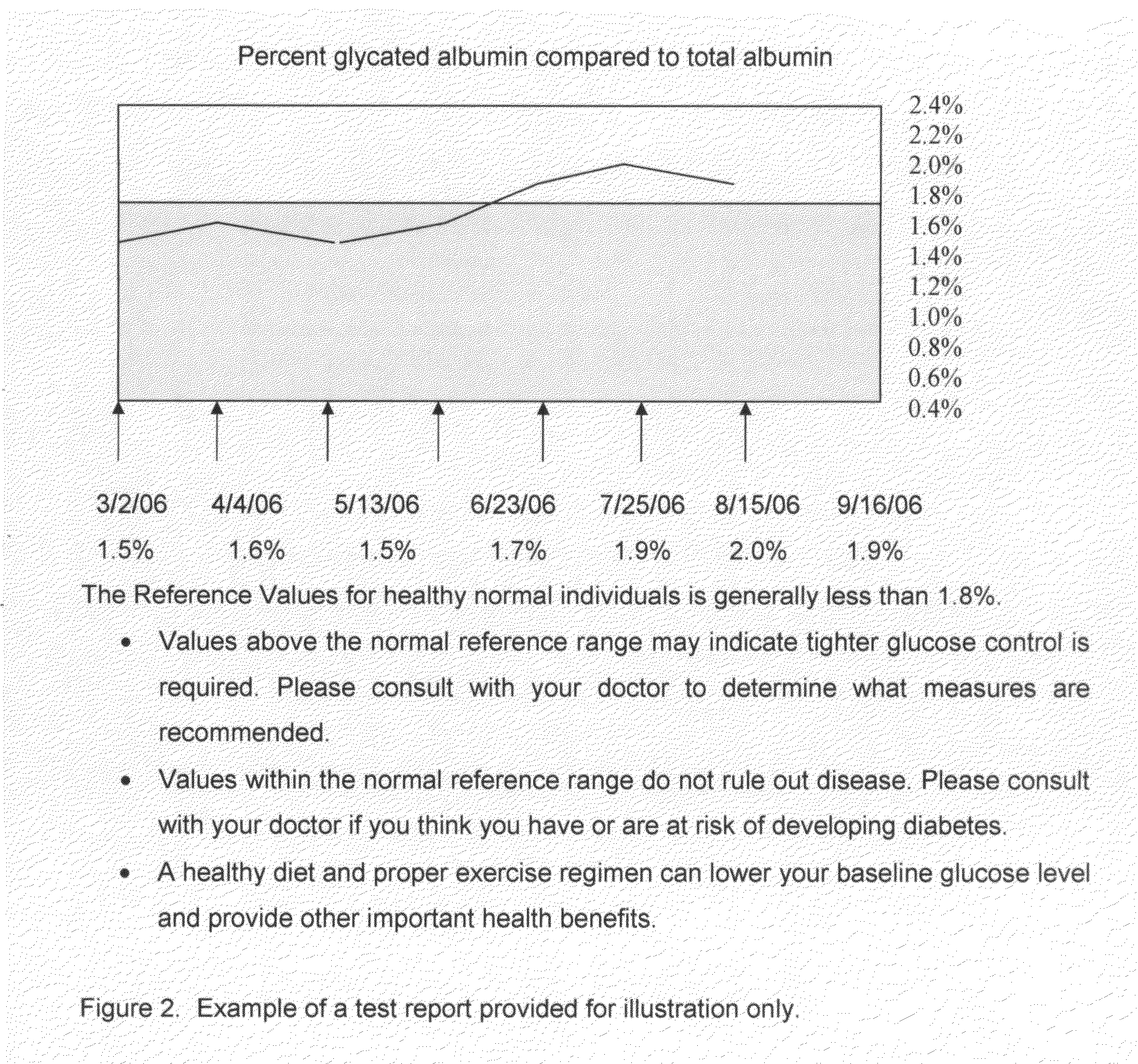
Home test for glycated albumin in saliva
InactiveUS20080227210A1Withdrawing sample devicesVaccination/ovulation diagnosticsSaliva sampleSaliva collection
A home test for measuring glycated albumin levels in saliva. The saliva sample is collected at home using a standardized saliva collection kit and mailed to a testing laboratory that performs the test and reports the result directly back to the customer via the internet. The home test can be used to monitor glucose control in diabetics and in healthy individuals. It may also be used as a diagnostic aid in identifying individuals with diabetes, or who are at risk of developing diabetes.
Owner:SMITH HENRY
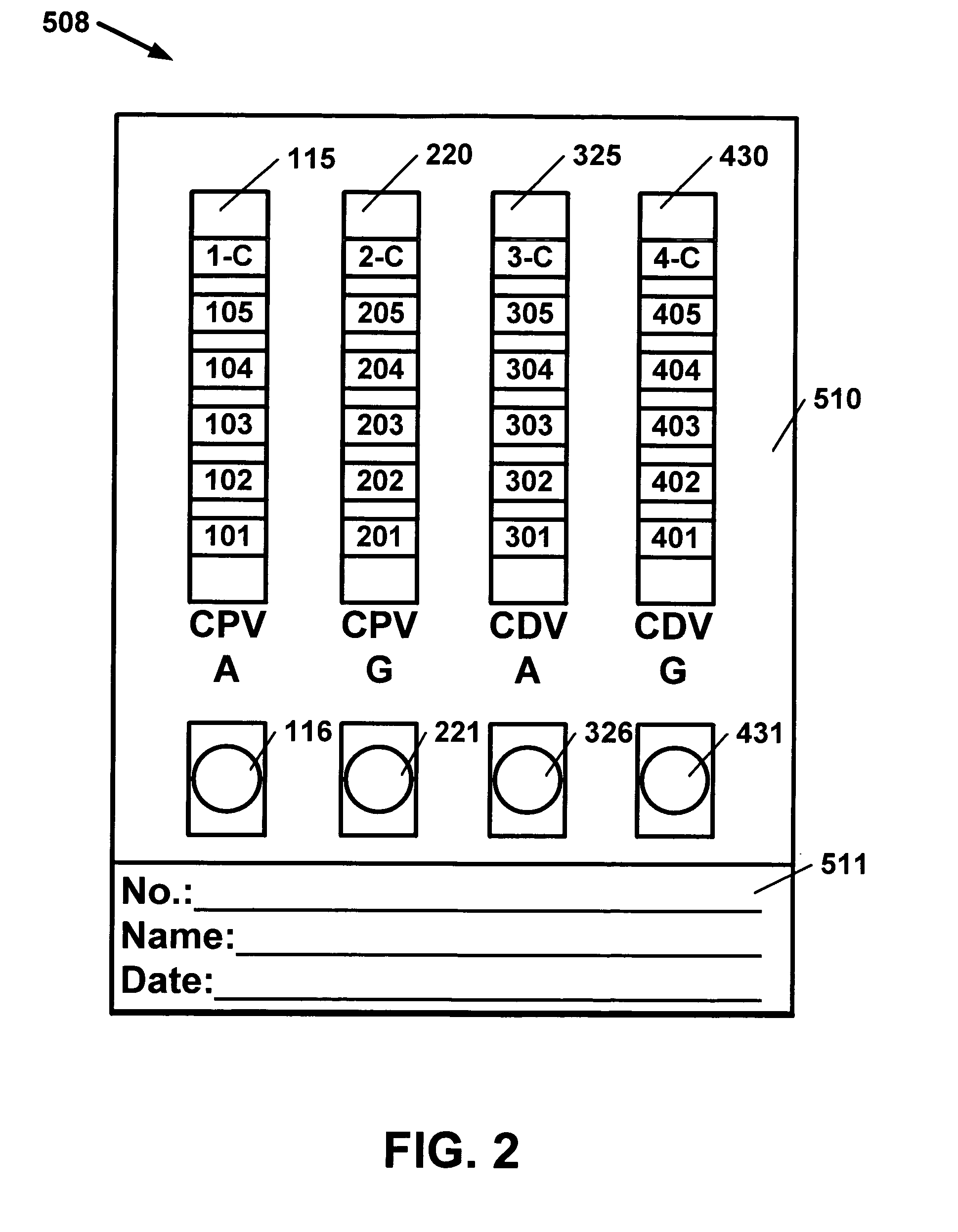
Antibody detection method and device for a saliva sample from a non-human animal
InactiveUS20130309656A1Bioreactor/fermenter combinationsBiological substance pretreatmentsSaliva sampleCanine distemper virus CDV
A rapid test apparatus, system, and method of use utilizing lateral flow immunoassay (LFIA) detection of a selected ligand in a liquid sample from a body fluid such as saliva in a pet in which antibodies and their complimentary antigens are used with detection-nanoparticles to provide a visual or measurable end point indicator in which the method measures the exposure to viruses in the canine from Canine Parvovirus (CPV) and / or Canine Distemper virus (CDV).
Owner:DAVIS DAVID C
Saliva sample testing device
ActiveUS7507374B2Easy to operateHigh sensitivityAnalysis using chemical indicatorsVaccination/ovulation diagnosticsSaliva sampleTest flow
In a lateral test flow immunoassay device, a saliva sample and a buffer solution are delivered into a mixing chamber to mix with a second reagent. The resulting test mixture is allowed to incubate for a pre-determined period of time and then selectively delivered to a test strip.
Owner:HEALGEN SCI LLC
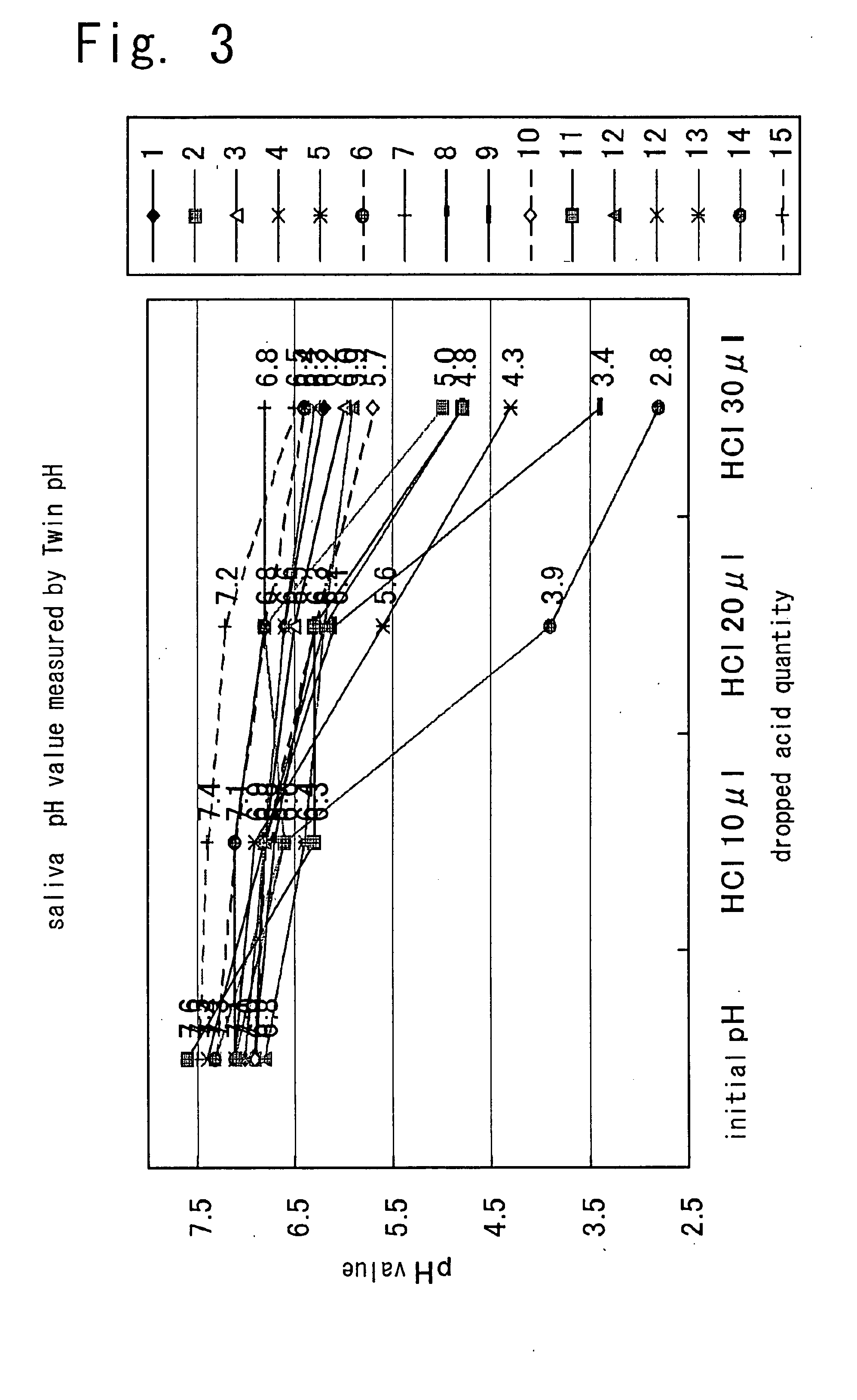
Method of estimating risk of dental decay, apparatus of estimating risk of dental decay, system of estimating risk of dental decay and program of estimating risk of dental decay
InactiveUS20050221401A1Accurate identificationImprove convenienceTeeth fillingSurgeryCementum cariesSaliva sample
The present invention provides a method of evaluating the risk of possible caries and the device, system, and program of the same, which is useful for the subjects to keep their mouth conditions best by measuring the chemical properties of saliva samples collected from the mouths of subjects to allow for easy and sanitary evaluation of the risk of possible caries in a short time and to help the subjects accurately recognize their risk of possible caries and closely consult their dentist. In the present invention, saliva from the mouth of subject is collected, a given ratio of acid is added to the saliva, and the chemical property of the saliva is measured to evaluate the risk of possible caries base on the measured value.
Owner:HORIBA LTD
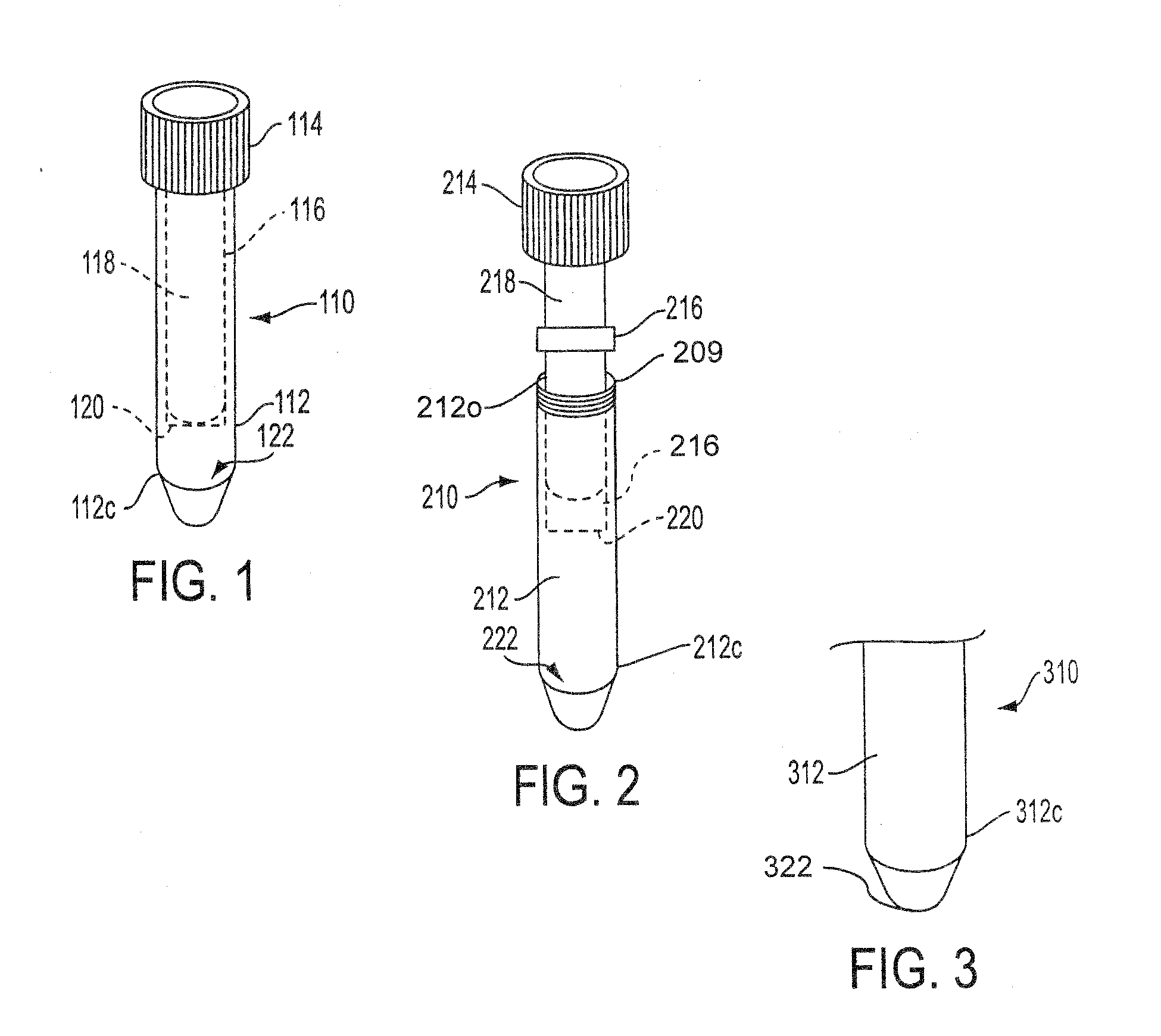
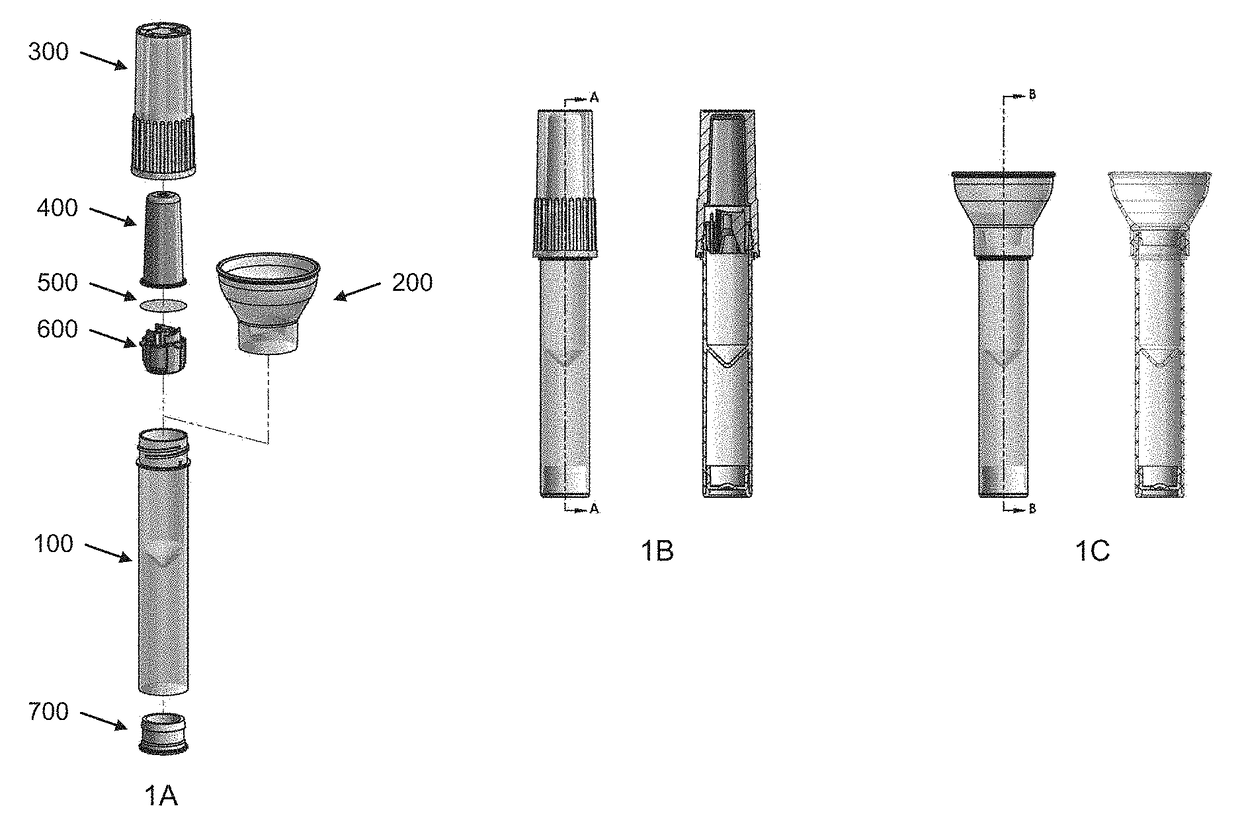
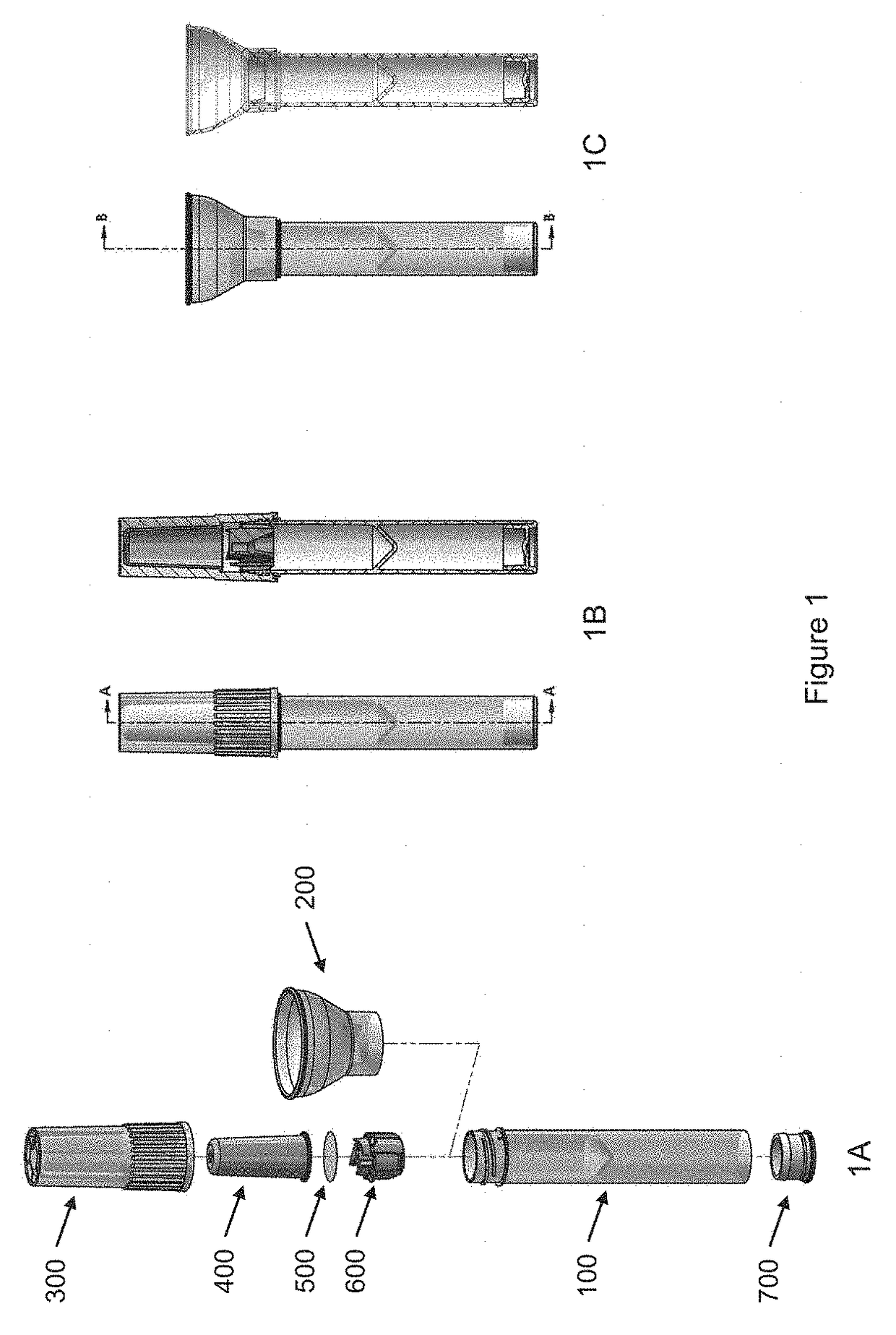
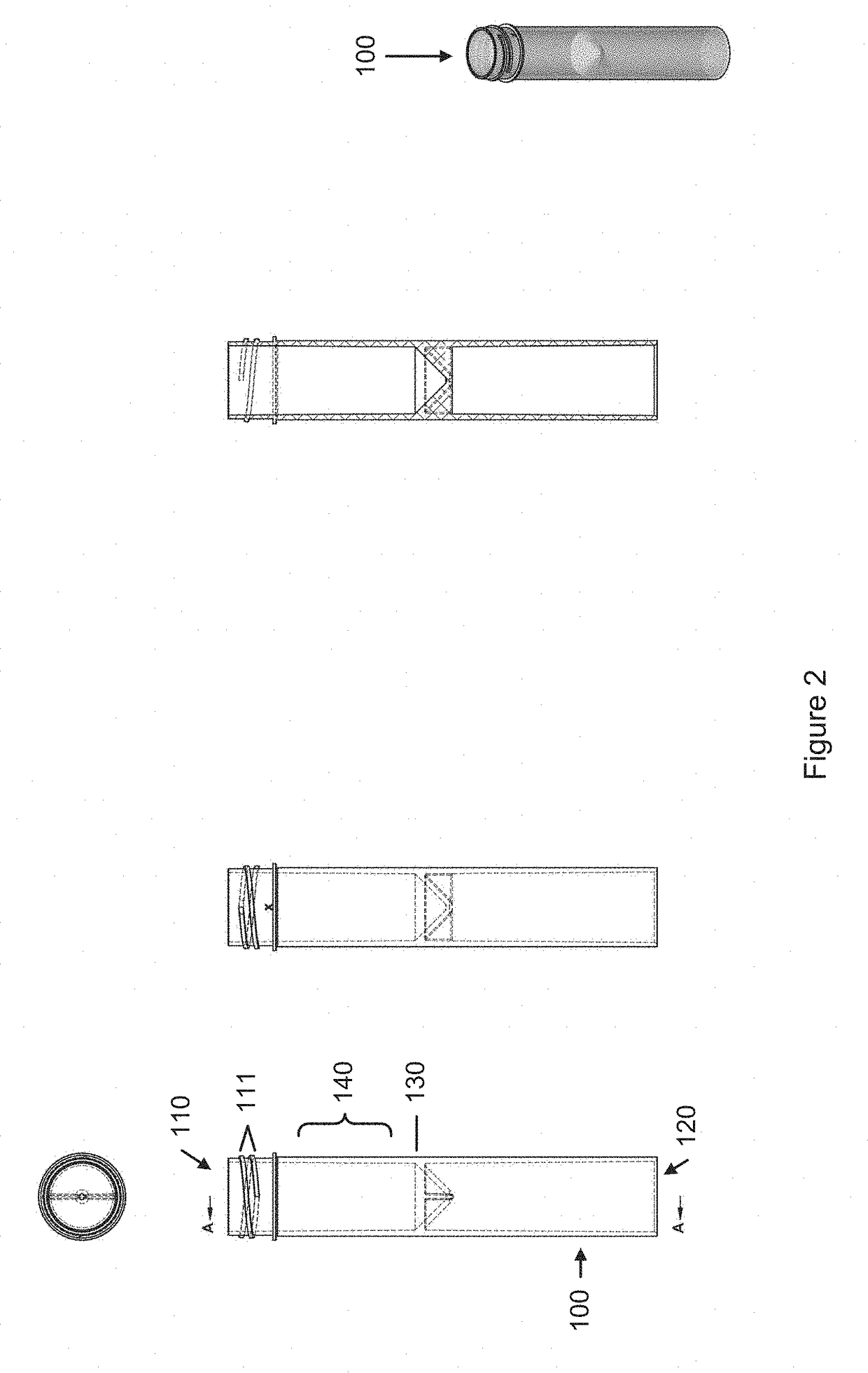
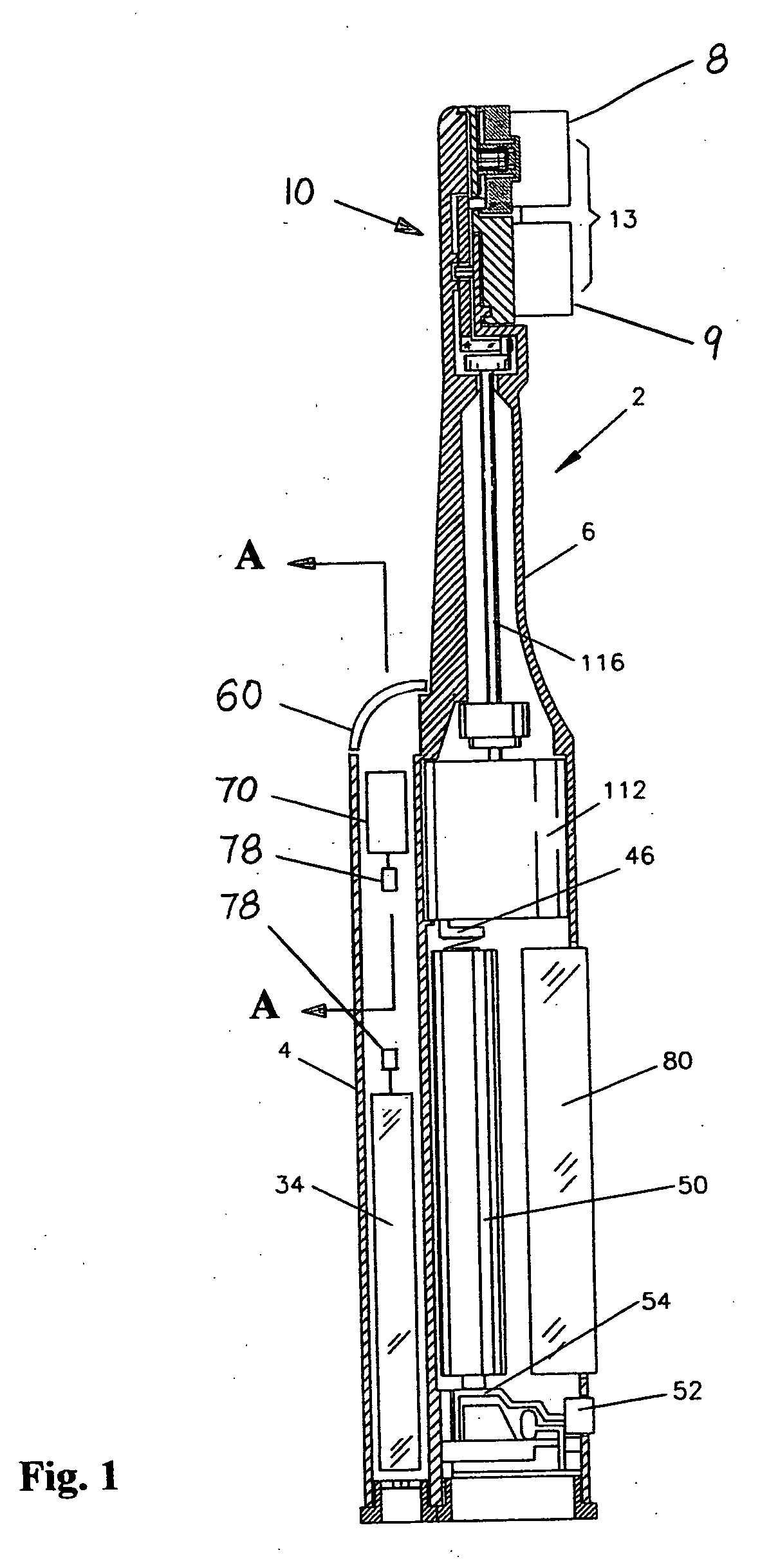
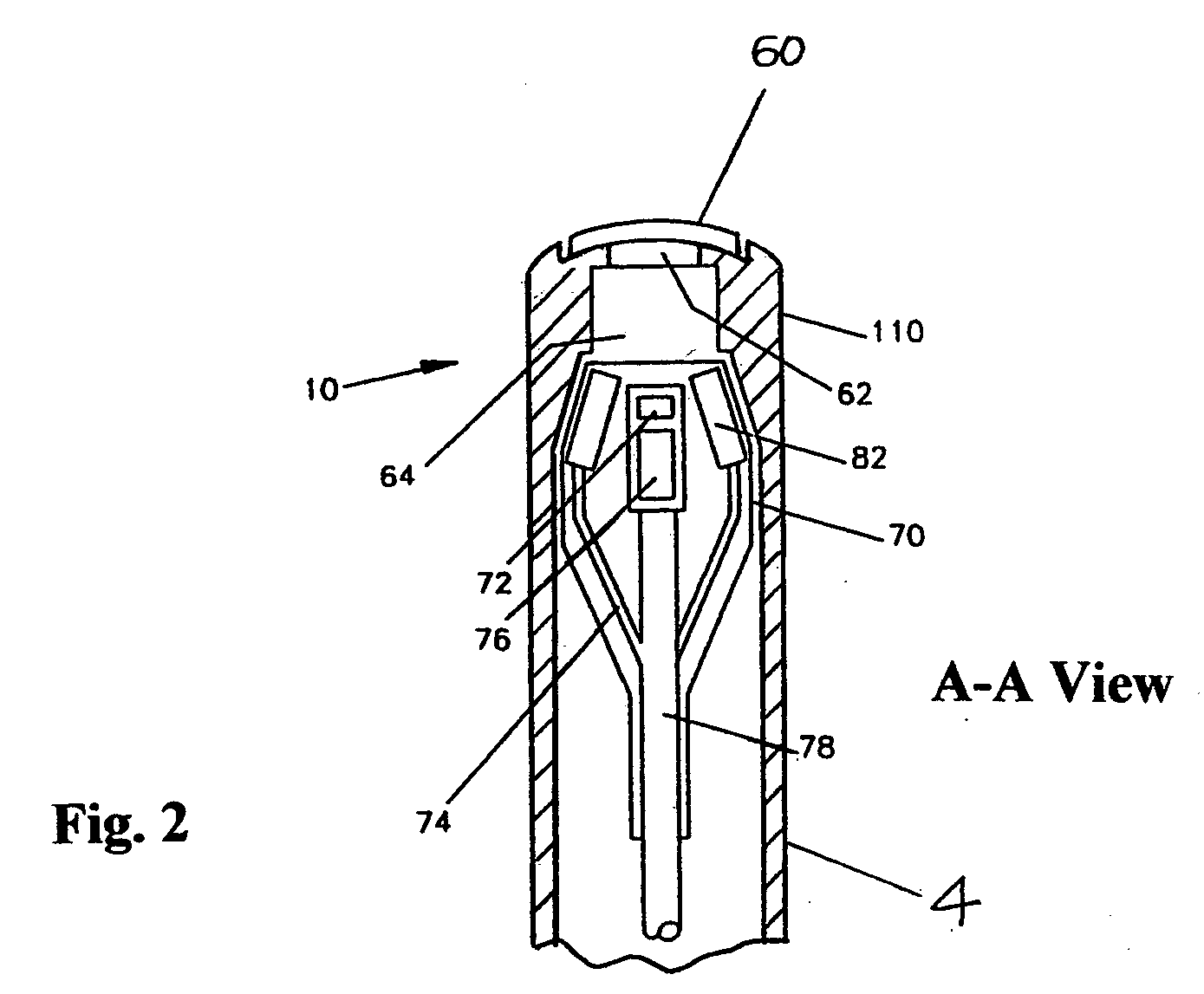
Sample collection device
A sample collection device having a sample tube, funnel, and cap having a capsule and a piercing insert, the capsule having a stabilization solution. After depositing the sample into the tube via the funnel, the cap is screwed onto the tube, piercing the capsule and releasing the stabilization fluid into the tube. The device can be used at home without clinicial supervision for collecting a saliva sample and transporting the sample to an analysis location for DNA analysis.
Owner:ANCESTRY COM DNA
Saliva sample collection system
InactiveUS7387899B1Reliable and convenient methodExposure to dangerBioreactor/fermenter combinationsBiological substance pretreatmentsSaliva sampleMedicine
Test kit whereby the sponge portion of kit is used to swab saliva. The saliva is then extracted from sponge by either squeezing it out or through the use of a centrifuge. The sponge portion of the kit is attached to a collection container when used in the centrifuge. A filter can be placed between these two portions of the kit to filter out only substances of certain molecular weights and to clean saliva. When the saliva is collected by squeezing, the sponge is placed in soft walled vial that is squeezed to extract saliva. After extracted from the sponge, the collected saliva can be removed from kit by twisting off cap. This test kit can be used for, but not limited to, testing for HIV antibodies, hepatitis and drugs.
Owner:DR JOSEPH P DANGELO FOUND
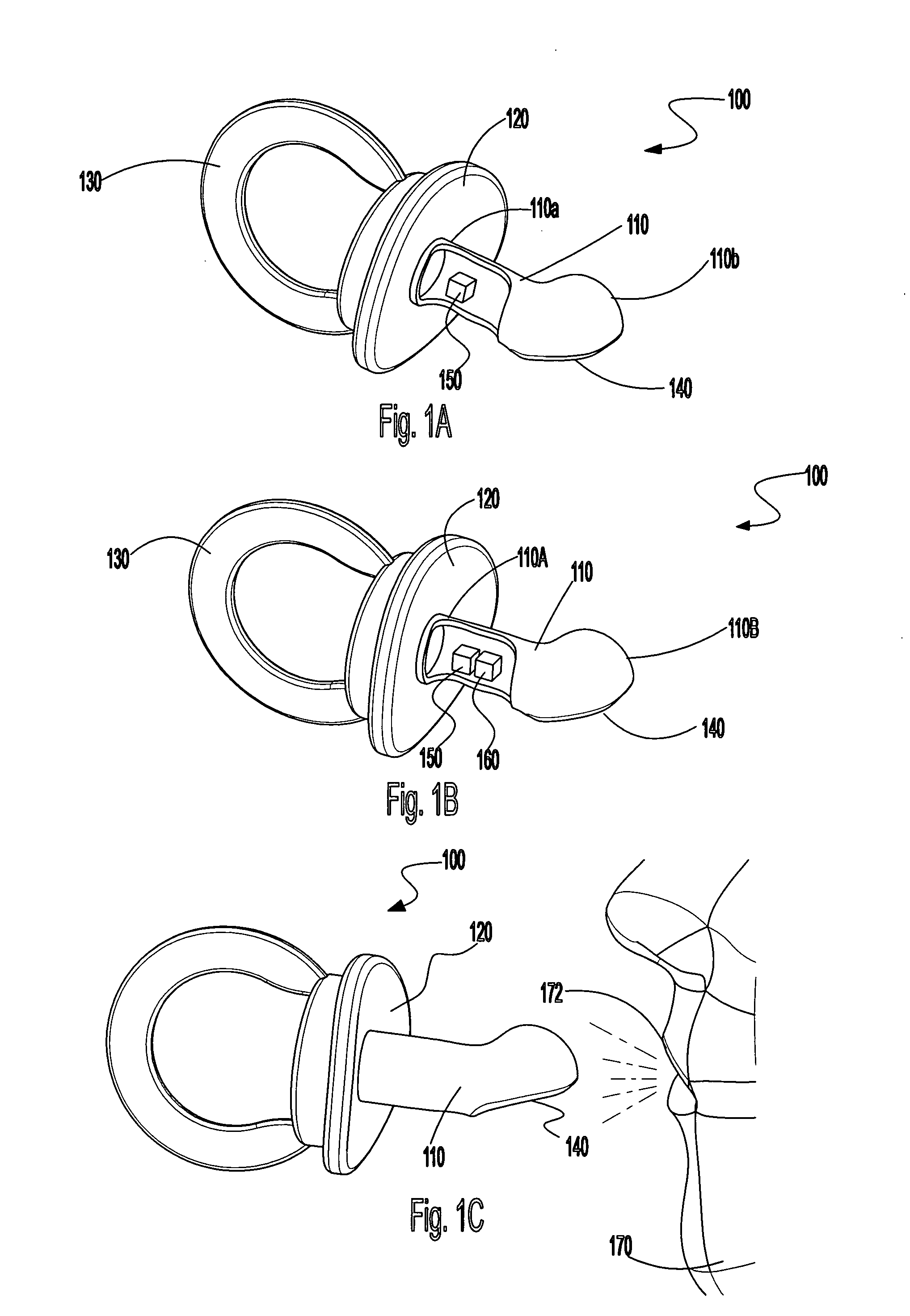
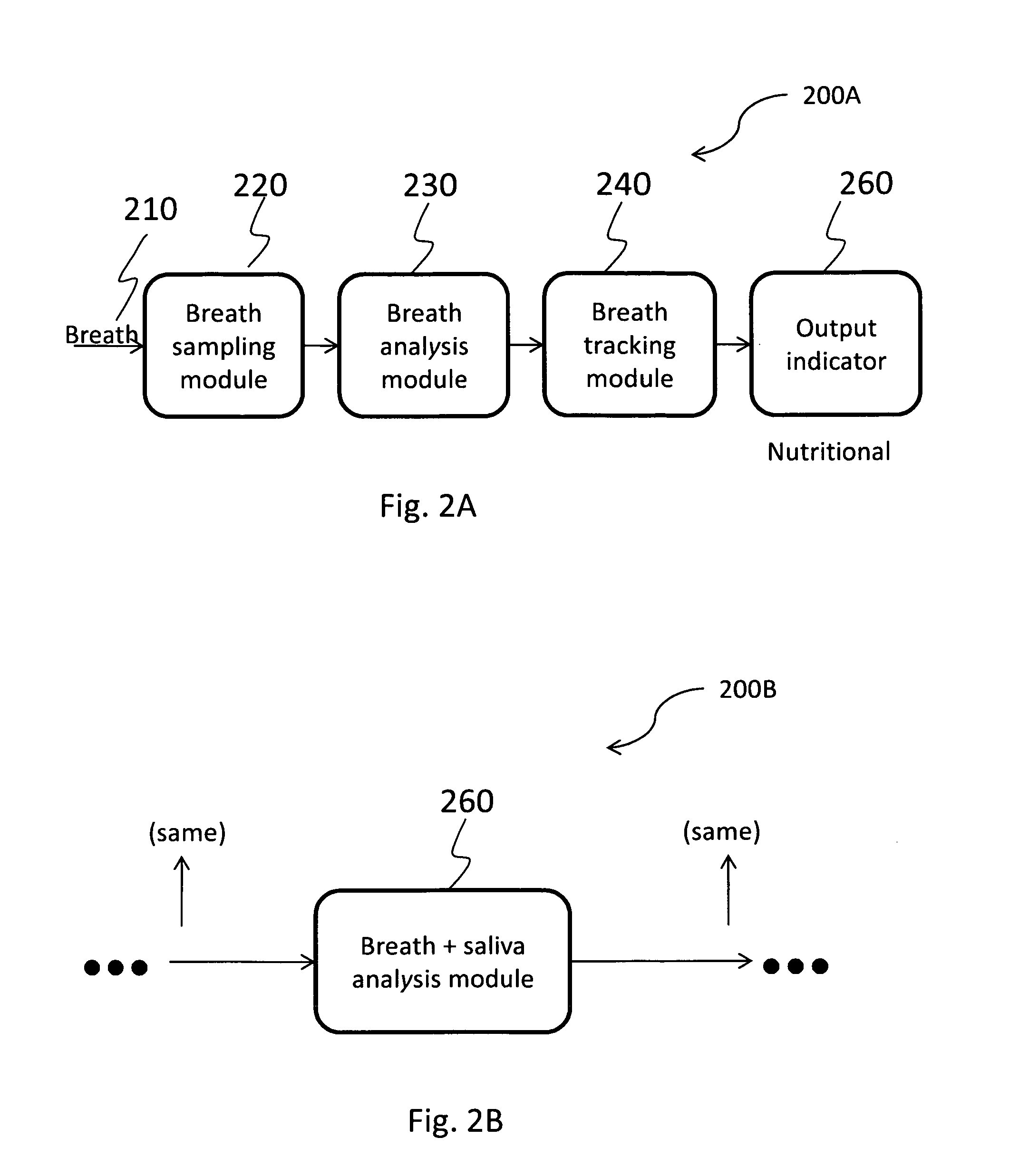
Pacifier receiving breath and saliva samples for providing nutritional information
A pacifier is presented including a nipple configured to receive a plurality of breath and saliva samples from a user, a base portion configured for attachment to the nipple, and an output mechanism attached to the base portion and configured for displaying different nutritional information values. The output mechanism may include one or more removable strips. The plurality of breath and saliva samples received from the user via the nipple are analyzed for nutritional information, preferably, continuously and in real-time, and the nutritional information includes at least one of carbohydrates, fats, minerals, protein, fiber, vitamins, water, salt, sugar, sodium, antioxidants, and phytochemicals.
Owner:KOUNTOTSIS THEODOSIOS +1
Fluid specimen testing device
InactiveUS20060292036A1Eliminate disadvantagesAnalysis using chemical indicatorsVaccination/ovulation diagnosticsSaliva sampleFluid specimen
A saliva sample testing device has a base housing upon which is mounted an upper housing. The base housing has a drawer structure in which are formed reaction wells for receiving fluid specimens. The drawer may be slidable or pivotable out of this base housing to provide access to the reaction wells. In a modification the reaction wells are formed in the top surface of the base housing and the upper housing is tiltable upon or detached from the base housing to provide access to the reaction wells.
Owner:AMERICAN BIO MEDICA CORP
Caries risk test for predicting and assessing the risk of disease
Provided are methods, test devices, and diagnostic kits for predicting, assessing, and diagnosing the risk of a disease using salivary analysis. The method comprises providing a whole (unfractionated) saliva sample from a subject; contacting an aliquot of said saliva with one or more lectins under conditions that allow said one or more lectins to bind to a lectin-binding component of said saliva; detecting the amount of bound lectin; and comparing the amount of bound lectin to the amount known to bind a saliva sample from a control patient, to predict the risk of a disease in the subject. Also provided are methods for reducing the risk of a disease and a method for assessing the risk of the disease at a defined level.
Owner:PROACTIVE ORAL SOLUTIONS +1
Lectin test chip for saliva sample, and treatment method thereof
InactiveCN103336126AEasy to prepareEasy to handlePeptide librariesBiological testingSaliva sampleGlycoprotein i
The invention provides a lectin test chip for a saliva sample, wherein a production cost is low, test targeting is strong, inherent advantages of a lectin chip are combined, and rapid, simple and efficient detection can be performed so as to obtain intermediate result information (saliva whole protein N-glycosylation and O-glycosylation carbohydrate chain structure information) adopted as type 2 diabetes mellitus evaluation. The lectin test chip adopts an epoxidation film base, and is characterized in that at least one of lectins such as PTL-I, LCA and SBA is immobilized on the epoxidation film base in a spotting immobilization manner. According to the present invention, the preparation method and the treatment method of the lectin test chip are optimized so as to establish the optimal reaction system for production of whole protein N-glycosylation and O-glycosylation information for saliva sample detection, and provide convenience for rapid and efficient detection of saliva glycoprotein carbohydrate chain structures of type 2 diabetes mellitus patients.
Owner:NORTHWEST UNIV(CN)
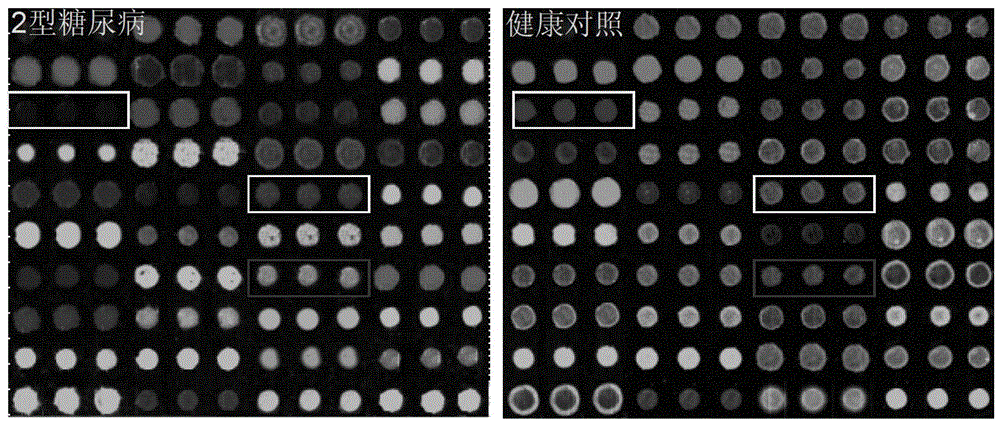
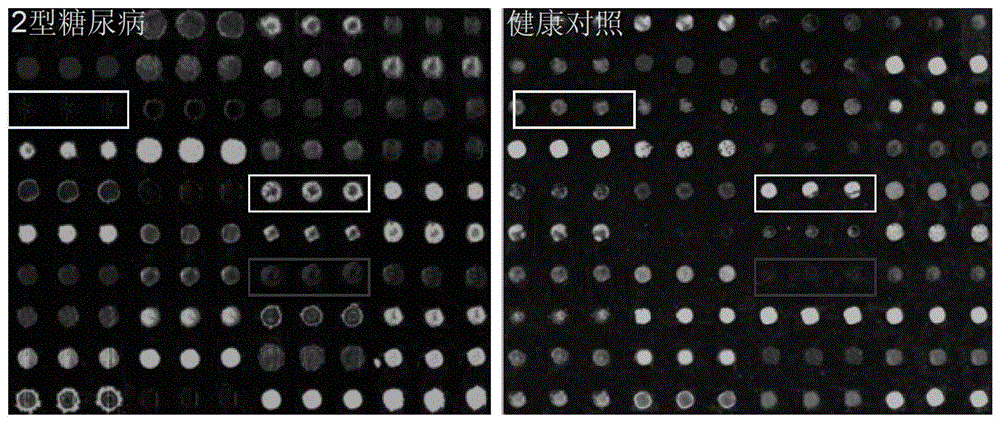
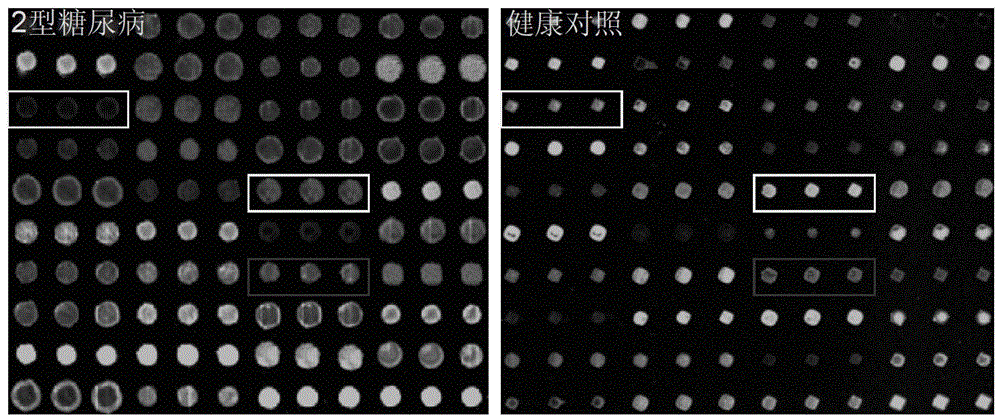
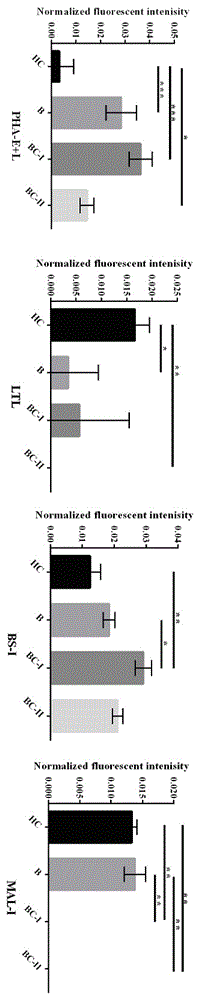
Agglutinin chip for identifying breast cancer based on sialoprotein, reagent kit and application of reagent kit
The relates to an agglutinin chip used for identifying cancer, in particular to an agglutinin chip for identifying breast cancer based on sialoprotein, a reagent kit and application of the reagent kit, and aims at providing an agglutinin chip for identifying breast cancer in a non-destructive mode through the change of carbohydrate chains in saliva, a reagent kit and application of the reagent kit. The agglutinin chip for identifying breast cancer based on sialoprotein comprises an agglutinin testing probe set. The agglutinin testing probe set at least comprises a combination of PHA-E+L, LTL, BS-I, MAL-I, LCA, BPL, PTL-II, NPA and GSL-I, or at least comprises a combination of LTL, BS-I, MAL-I, LCA, BPL, NPA and GSL-I. By means of the agglutinin chip, differentially-expressed glycoprotein carbohydrate chain structures in a patient saliva sample can be rapidly detected, whether a corresponding person suffers from a breast tumor or not is detected, and whether the breast tumor is breast cancer or not is determined; besides, saliva is adopted as an object to be detected and is easy and convenient to collect, and health risks existing when a serum sample is collected for detection in the prior art are avoided.
Owner:深圳格道糖生物技术有限公司
Swine saliva sampling method
InactiveUS20070062460A1Conveniently takenVaccination/ovulation diagnosticsOther apparatusSaliva sampleAbsorbent material
A method and device for obtaining livestock saliva samples is described. An absorbent material is mounted in an animal pen such that the animal voluntarily mouths the material, thereby depositing saliva in the material. The saliva can then be extracted from the absorbent material and tested as desired.
Owner:SIMER ROBERT
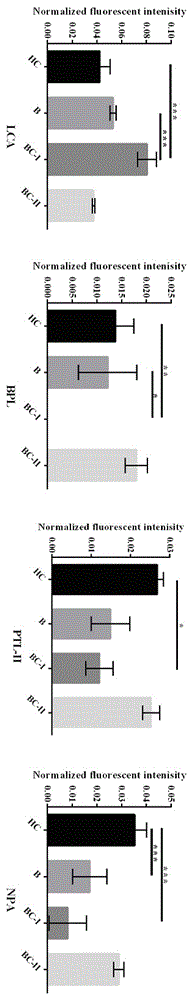
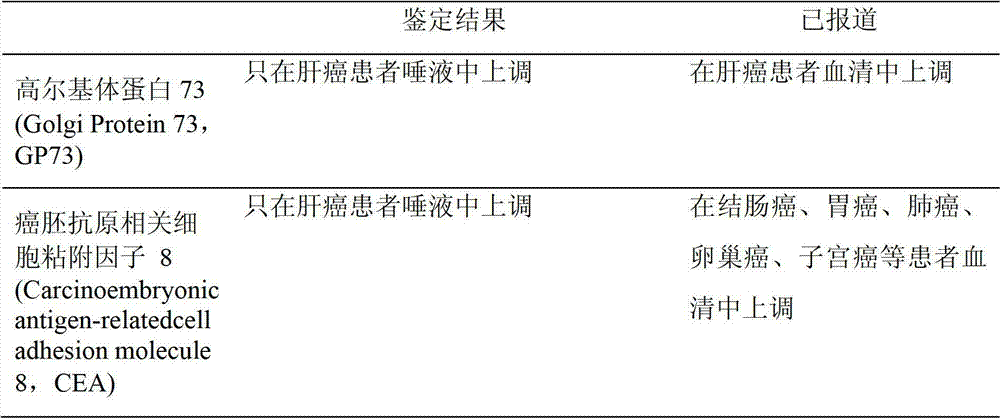
Method for rapid nondestructive detection of liver tumor marker and test paper strip adopted by the method
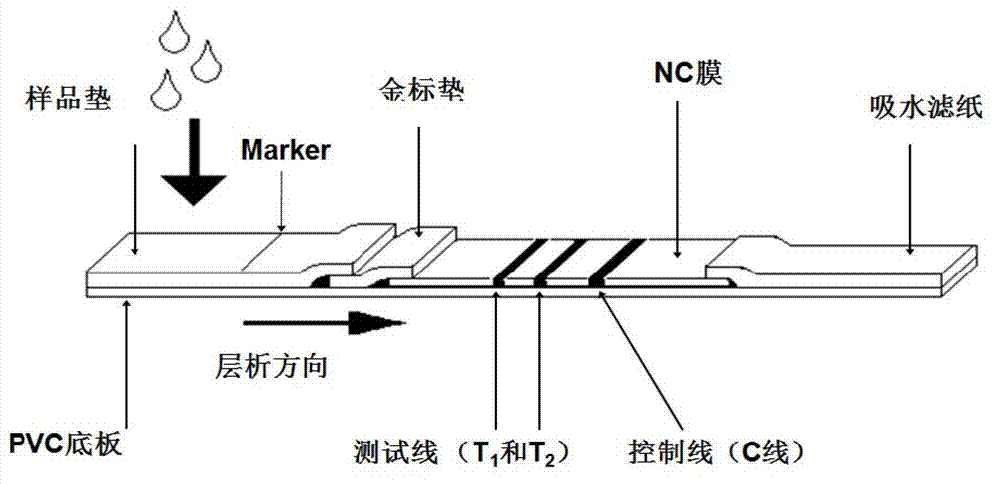
The invention provides a method for rapid nondestructive detection of a liver tumor marker and a test paper strip adopted by the method. A gold maker pad of the test paper strip adopted by the method comprises glass fibers adsorbed with colloidal gold-marked monoclonal antibodies GP73-1. A nitrocellulose film is orderly marked with a detection band T1 and a quality control line C, wherein monoclonal antibodies GP73-2 are fixed to the detection band T1 and rabbit anti-mouse polyclonal antibodies Ig are fixed to the quality control line C. The method for rapid nondestructive detection of a liver tumor marker comprises the following steps of taking a saliva sample, inserting an end of a sample pad of the test paper strip which is an immunochromotographic test paper strip into the saliva sample for 10 to 15min so that the saliva sample appears on a NC film, taking out the test paper strip, and observing coloring states of the detection band and the quality control line to obtain a detection result. The method utilizes a saliva sample as a detection object and compares the saliva sample with a serum sample and other body fluid samples. The method has the advantages of easy sample acquisition, nondestructive detection, accurate detection, safety, convenience, low cost and easy preservation.
Owner:NORTHWEST UNIV(CN)
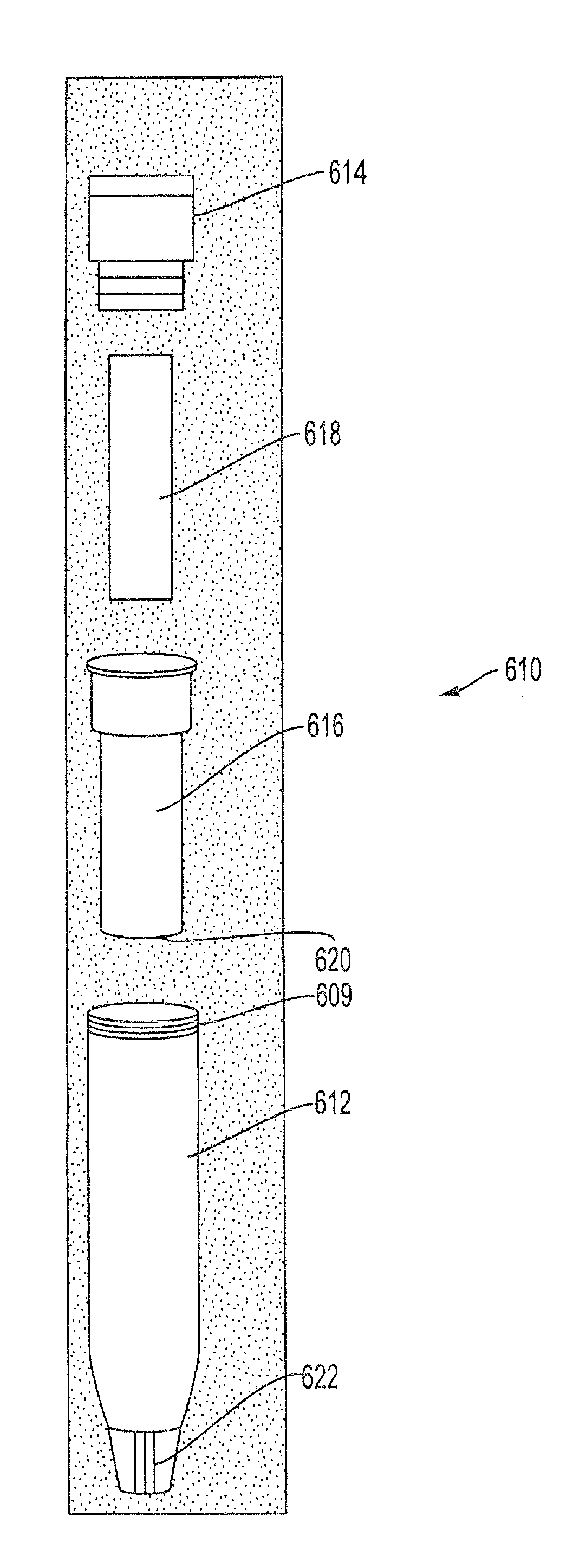
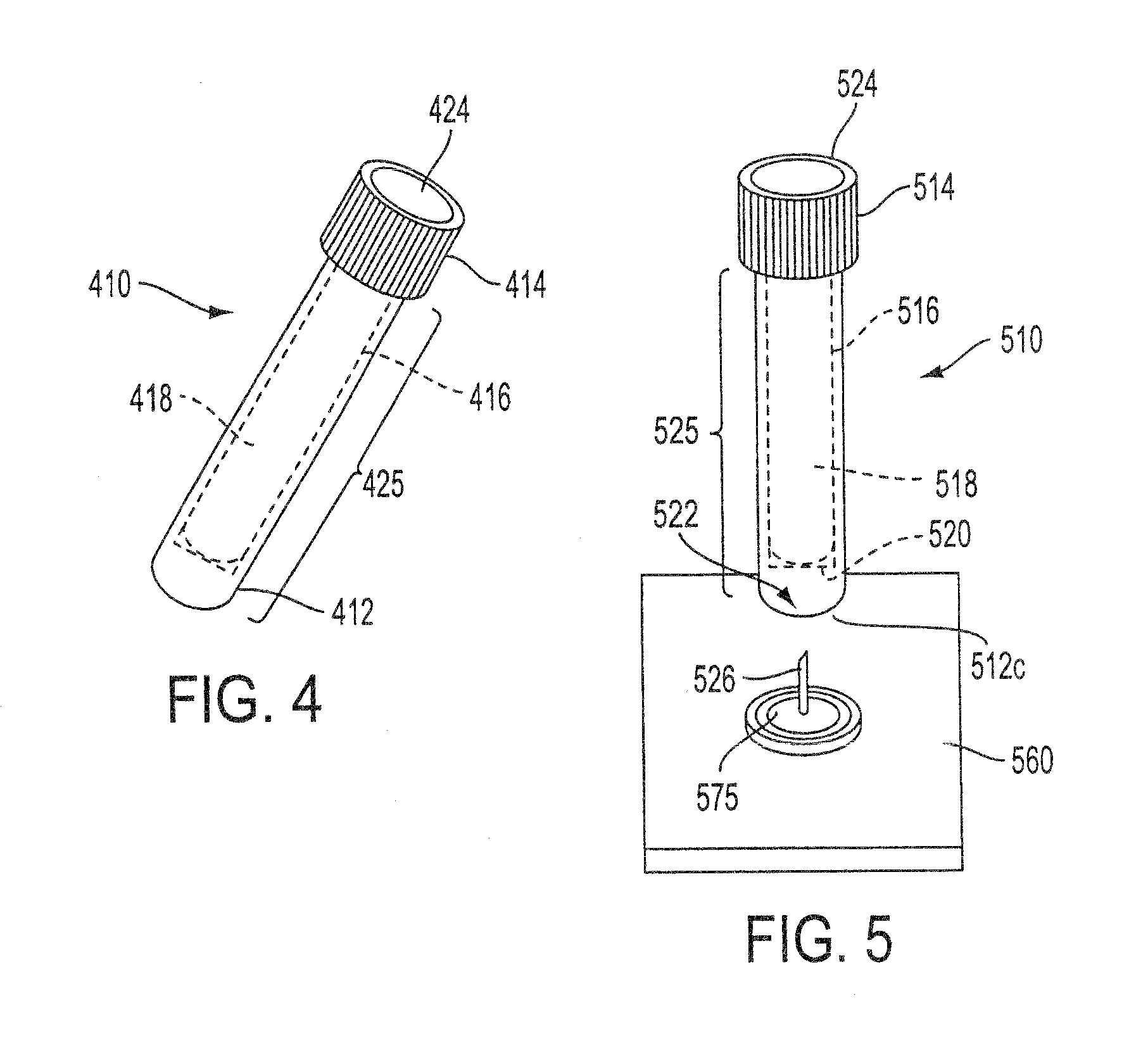
Sample Collection System and Method for Use Thereof
A sample collection system capable of collecting, storing and dispensing a liquid sample is disclosed. The collection system includes a collector composed of a material which has the unique ability to express constituents of interest at levels which are much more concentrated than their levels in the fluid samples from which they are expressed, where the expressed highly concentrated sample can then be used with modern rapid screening / testing protocols, such as solid phase assays, to test for the constituents of interest. Thus, it is now possible to obtain analytes of interest, such as the HIV protein antibodies, from saliva samples at concentrations that are detectable with systems and / or devices that are typically utilized only for blood serum or plasma testing. The collector is sized and shaped to fit within a recovery container, which, in turn, is sized and shaped to fit within a collection tube. The recovery container includes an aperture which does not permit passage of fluid under ambient conditions, but facilitates transfer thereof when subjected to pressure. An optional channel within the collection tube facilitates dispensing of the sample for further processing.
Owner:ARONOWITZ JACK L
Kit for detecting novel coronavirus N protein and application thereof
InactiveCN111398583AEasy to operateThe detection process is fastImmunoassaysAlveolar lavage fluidSaliva sample
The invention relates to a kit for detecting novel coronavirus N protein and an application of the kit. The kit comprises (1) one antibody capable of being bound to the novel coronavirus N protein, and (2) the other antibody marked by a marker and capable of being bound to the novel coronavirus N protein when the novel coronavirus N protein can be bound to (1) to limit the antibody. The using method of the kit comprises the step of determining whether a patient suffers from the novel coronavirus pneumonia or not by detecting the level of the novel coronavirus N protein in an individual sample.The kit for detecting the novel coronavirus N protein provided by the invention can be used for detecting the coronavirus N protein in nasopharynx swabs, oropharynx swabs, alveolar lavage fluids, serum, plasma, whole blood, sputum, oral swabs or saliva samples so as to be used for diagnosing novel coronavirus pneumonia.
Owner:BEIJING ELCOTEQ BIO TECH
Method of evaluating oral cancer risk in human
InactiveUS20110143962A1Bioreactor/fermenter combinationsBiological substance pretreatmentsParticulatesSaliva sample
A method of providing a risk evaluation and diagnosis of human oral cancer, by examining at the presence in human saliva sample of a combination of particulate nucleic acids from bacteria, virus, as well as human, and / or the presence of particulate biochemical volatile organic compounds, which are indicative of an increased risk of oral cancer.
Owner:INSTITUT CLINIDENT
Immunochromatographic kit for rapidly detecting novel coronavirus N protein as well as preparation method and application of immunochromatographic kit
PendingCN111398589AThe detection process is fastEasy to operateImmunoassaysAlveolar lavage fluidSaliva sample
The invention relates to an immunochromatographic kit for rapidly detecting novel coronavirus N protein as well as a preparation method and application of the immunochromatographic kit. The immunochromatographic kit comprises a test strip, the test strip comprises a detection line and a quality control line, the detection line is coated with an anti-novel coronavirus N protein antibody, and the quality control line is coated with a goat anti-rabbit polyclonal antibody. The immunochromatographic kit for rapidly detecting the novel coronavirus N protein provided by the invention can be used fordetecting the novel coronavirus N protein in a nasopharynx swab, an oropharynx swab, an alveolar lavage fluid, an oral swab or a saliva sample so as to be used for diagnosing novel coronavirus pneumonia.
Owner:BEIJING ELCOTEQ BIO TECH
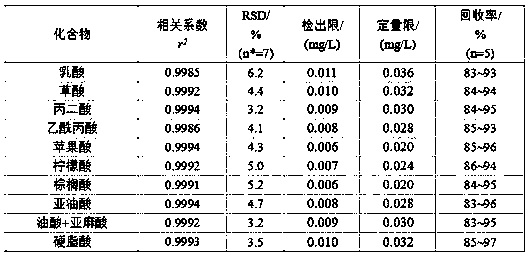
Method for separating and assaying organic acid and fatty acid substances in saliva
ActiveCN105954454AMethod is feasibleThe pre-processing process is simpleComponent separationSaliva sampleGas chromatography–mass spectrometry
The invention relates to a method for separating and assaying organic acid and fatty acid substances in saliva, belongs to the technical field of analytical chemistry and particularly relates to the method dedicated to separating and assaying the organic acid and fatty acid substances in the saliva. The method comprises the steps of collecting a saliva sample by adopting absorbent cotton, then, putting the absorbent cotton into a needle cylinder of a syringe, carrying out pushing squeezing by using a push rod of the syringe so as to obtain the saliva sample, carrying out high-speed centrifugation, then, extracting supernatant, then, carrying out salting-out extraction, a methyl esterification reaction and solvent extraction separation sequentially, then, concentrating an extract until the extract is approximately dry, then, carrying out redissolving, carrying out filtrating by a microporous filtrating membrane, assaying the organic acid and fatty acid substances in the saliva by adopting a gas chromatography-mass spectrometry method by using filtrate, making a standard curve, and carrying out quantification by an external standard method. The method provided by the invention can be effectively used for assaying the organic acid and fatty acid substances in the saliva, is feasible, accurate and reliable, is low in detection limit and good in accuracy and has popularization and application values.
Owner:CHINA TOBACCO YUNNAN IND
Preservative agent for DNA of saliva
The invention discloses a preservative agent for DNA (Deoxyribose Nucleic Acid) of saliva. The preservative agent comprises the following components in percentage by volume: 5-10 g / 100mL of trihytdroxy methyl-aminomethane, 10-25 g / 100mL of ethylene diamine tetraacetic acid, 7-17 g / 100mL of sugar, 1-10 g / 100mL of sodium chloride and 0.5-5 g / 100mL of surfactant. The solvent of the preservative agent is water; the pH value of the preservative agent is 7.0-9.5; the DNA in a saliva sample can be effectively preserved at room temperature for a long time; and the defects in the prior art that the preservative time of the DNA acquired from the saliva sample is short and the DNA is required to be preserved at low temperature are effectively overcome. The preservative agent disclosed by the invention is low in cost, simple in preparation method and suitable for industrial production.
Owner:XIAMEN ZEESAN BIOTECH
Sample collection device
A sample collection device having a sample tube, funnel, and cap having a capsule and a piercing insert, the capsule having a stabilization solution. After depositing the sample into the tube via the funnel, the cap is screwed onto the tube, piercing the capsule and releasing the stabilization fluid into the tube. The device can be used at home without clinicial supervision for collecting a saliva sample and transporting the sample to an analysis location for DNA analysis.
Owner:ANCESTRY COM DNA
Apparatus and methods for steroid hormone testing
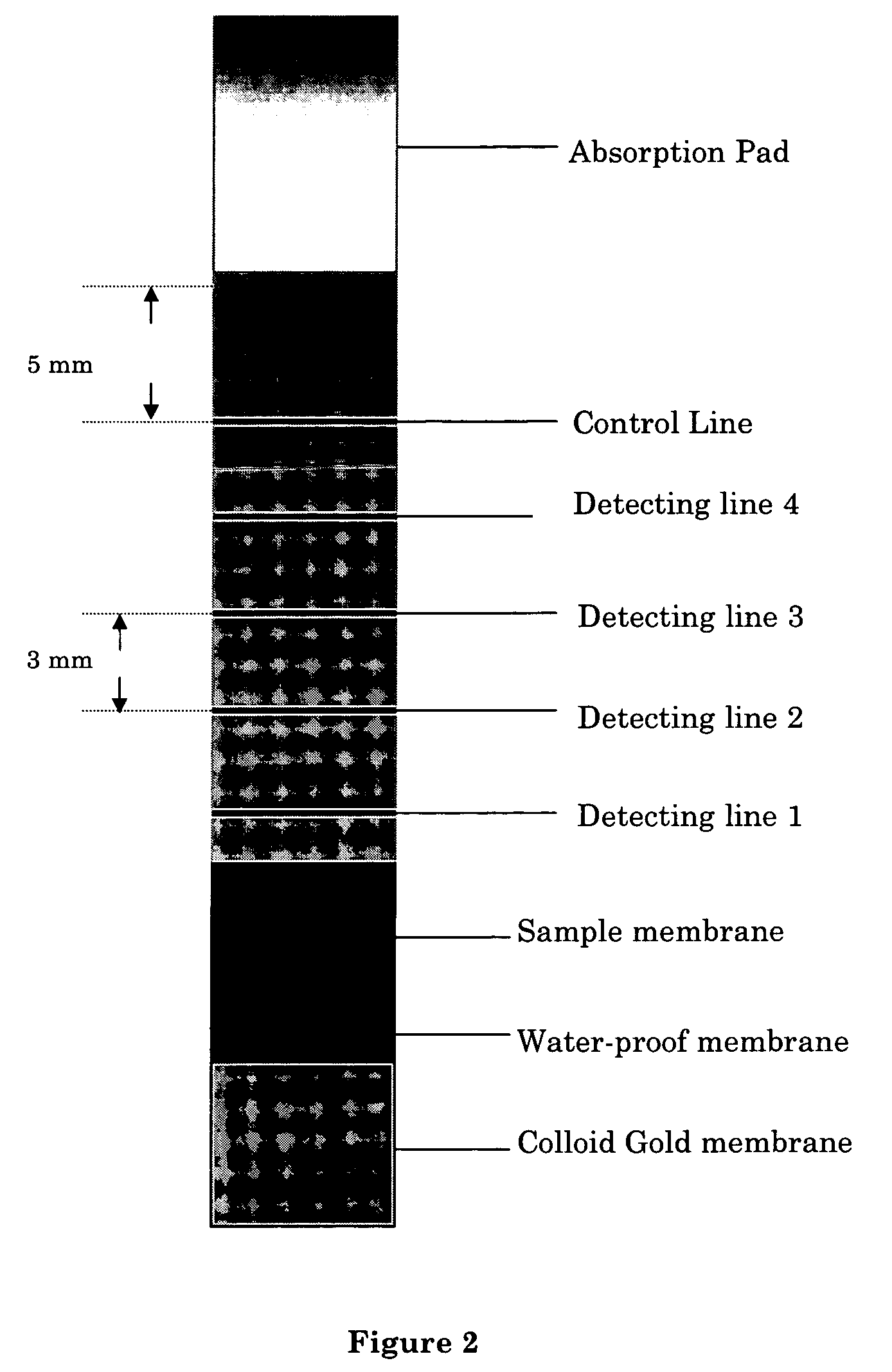
An immuno-chromatographic detection device for detecting an analyte in sample, such as estrogen in a urine or saliva sample, the device comprising (a) a binding membrane having immobilized thereon (i) an test antibody against said analyte in at least one detection region, and (ii) a control antibody against a control antigen known to be present in the sample in a control region, (b) a sample membrane located at a first end of the binding membrane for receiving the sample, wherein the sample membrane is in chromatographic connection with the binding membrane, and (c) a label membrane containing (iii) a labeled antigen that is capable of binding to the test antibody and upon binding with the test antibody exhibits an observable change at the at least one detection region, and (iv) a labeled control antigen that is capable of binding to the control antibody and upon binding with the control antibody exhibits an observable change at the control region, wherein the sample membrane is separated from the label membrane by a waterproof membrane which is removable to allow the sample membrane and label membrane to be connected chromatographically. Also provided are kits comprising the device, method for detecting the analyte, and methods for manufacturing the device and kit.
Owner:NJ INT
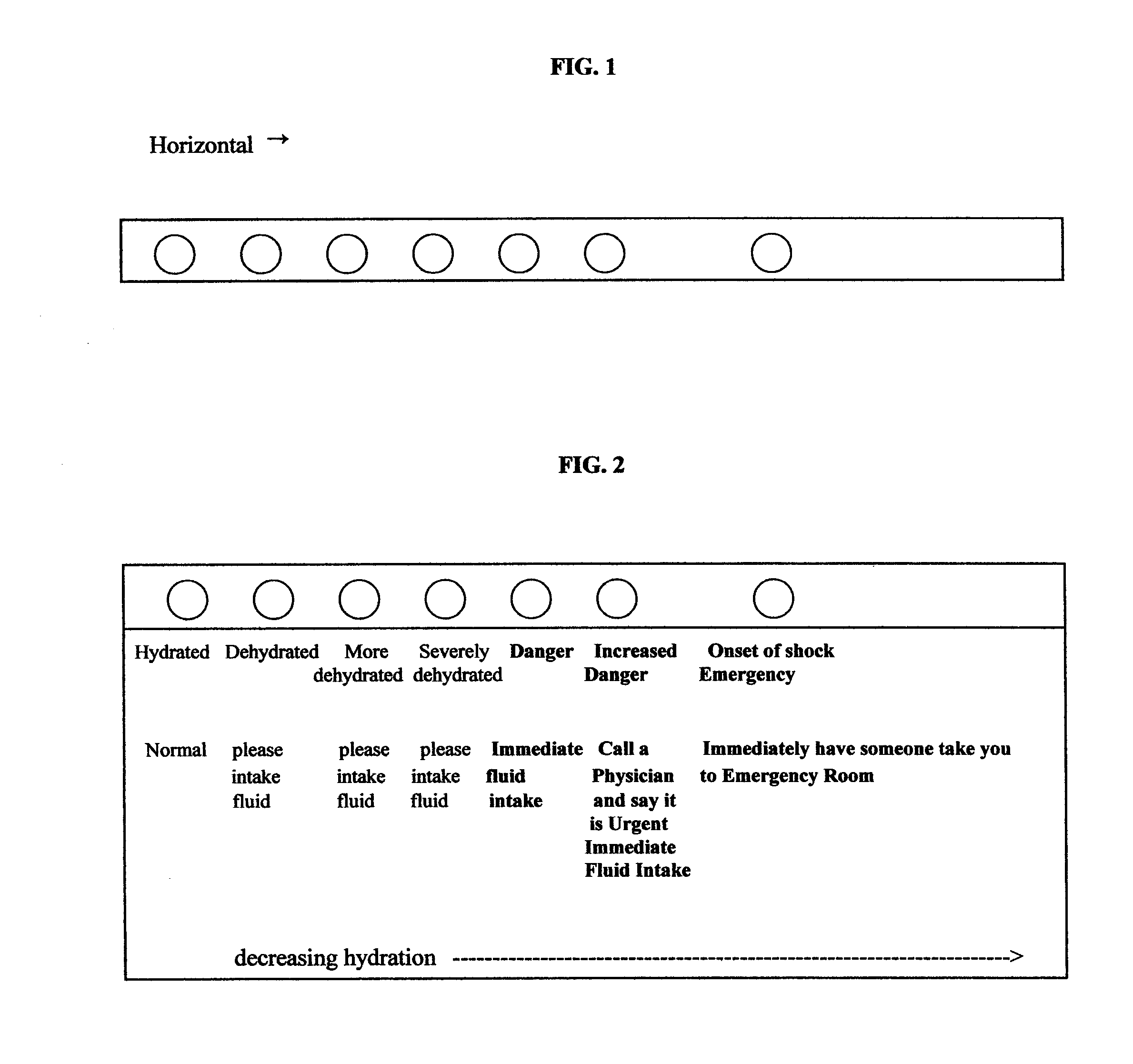
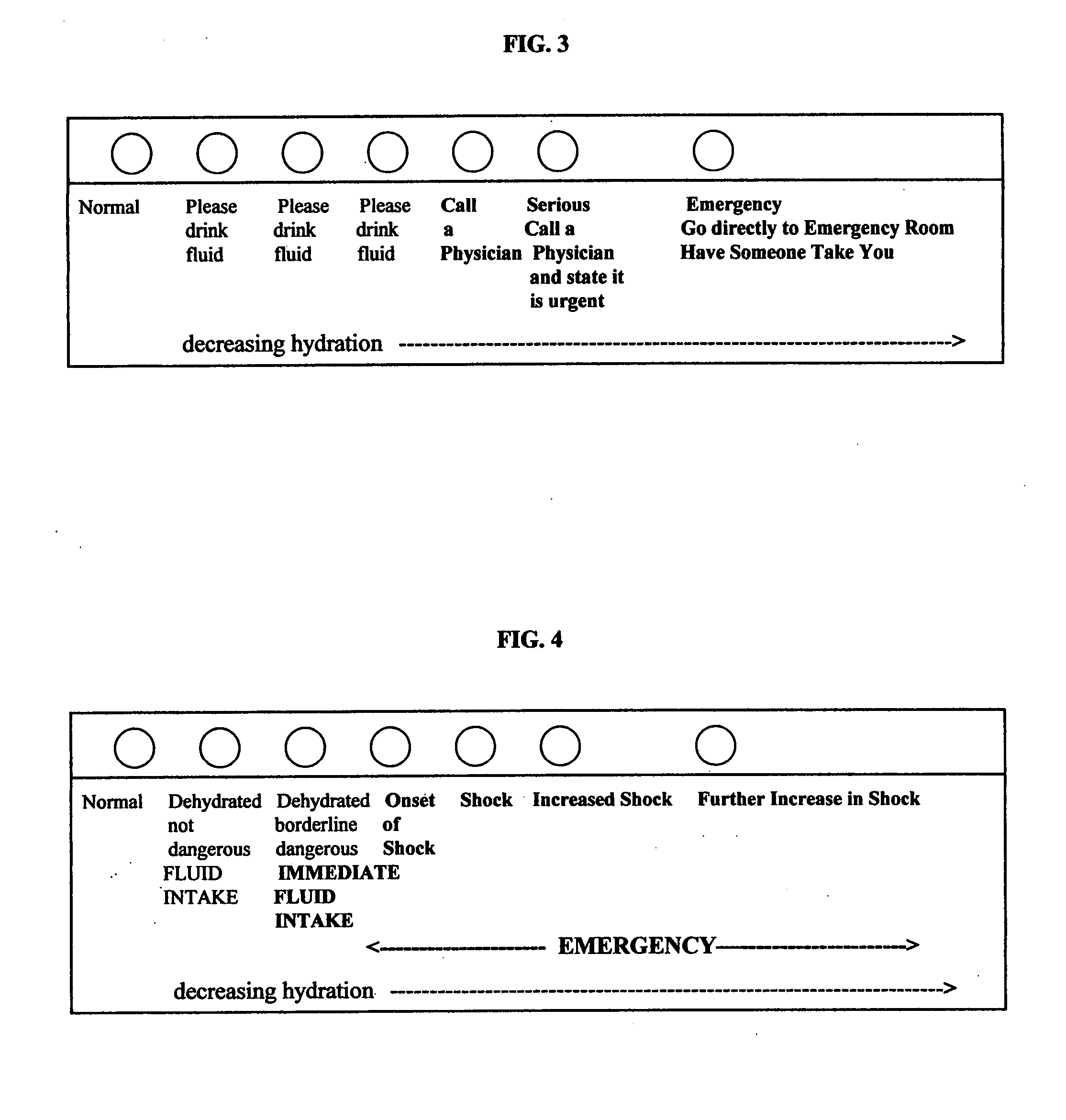
Methods for assessing dehydration and shock, assays and kits for the methods
InactiveUS20080050451A1Prevent dehydrationBiocideBioreactor/fermenter combinationsAssaySaliva sample
The present invention is in the medical and consumer arenas. The invention presents the use of a salivary amylase assay to diagnose, quantify, and monitor the hydration status, the onset and progress of shock in an animal that produces salivary amylase, such as a human. The assay tests the saliva sample from the animal. Test kits and assays for such use are also presented.
Owner:HYDRADX
Method for Detecting Anti-Transglutaminase Antibodies
InactiveUS20080038760A1Easy to detectEnhanced signalBiological material analysisBiological testingSaliva sampleGlutaminase
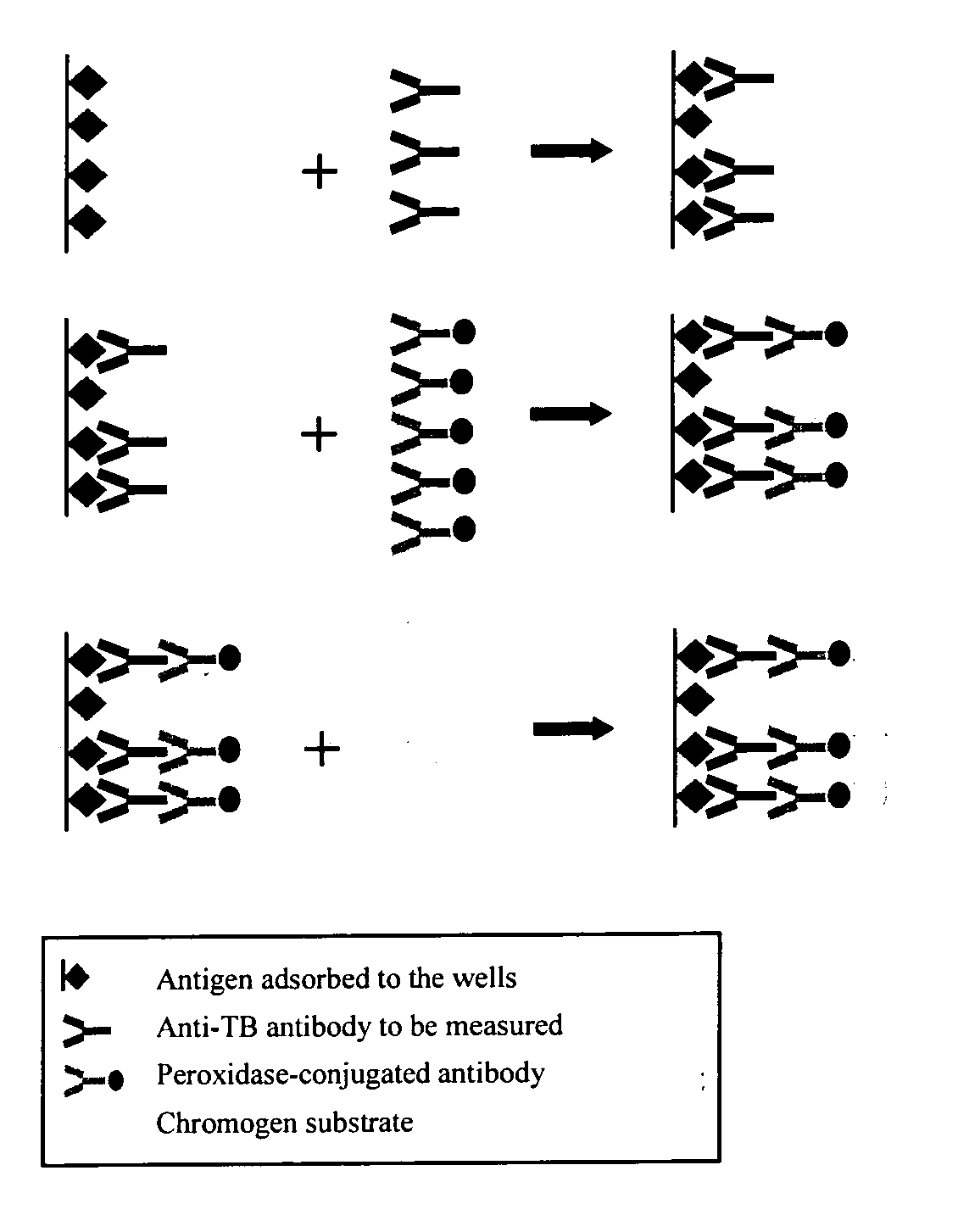
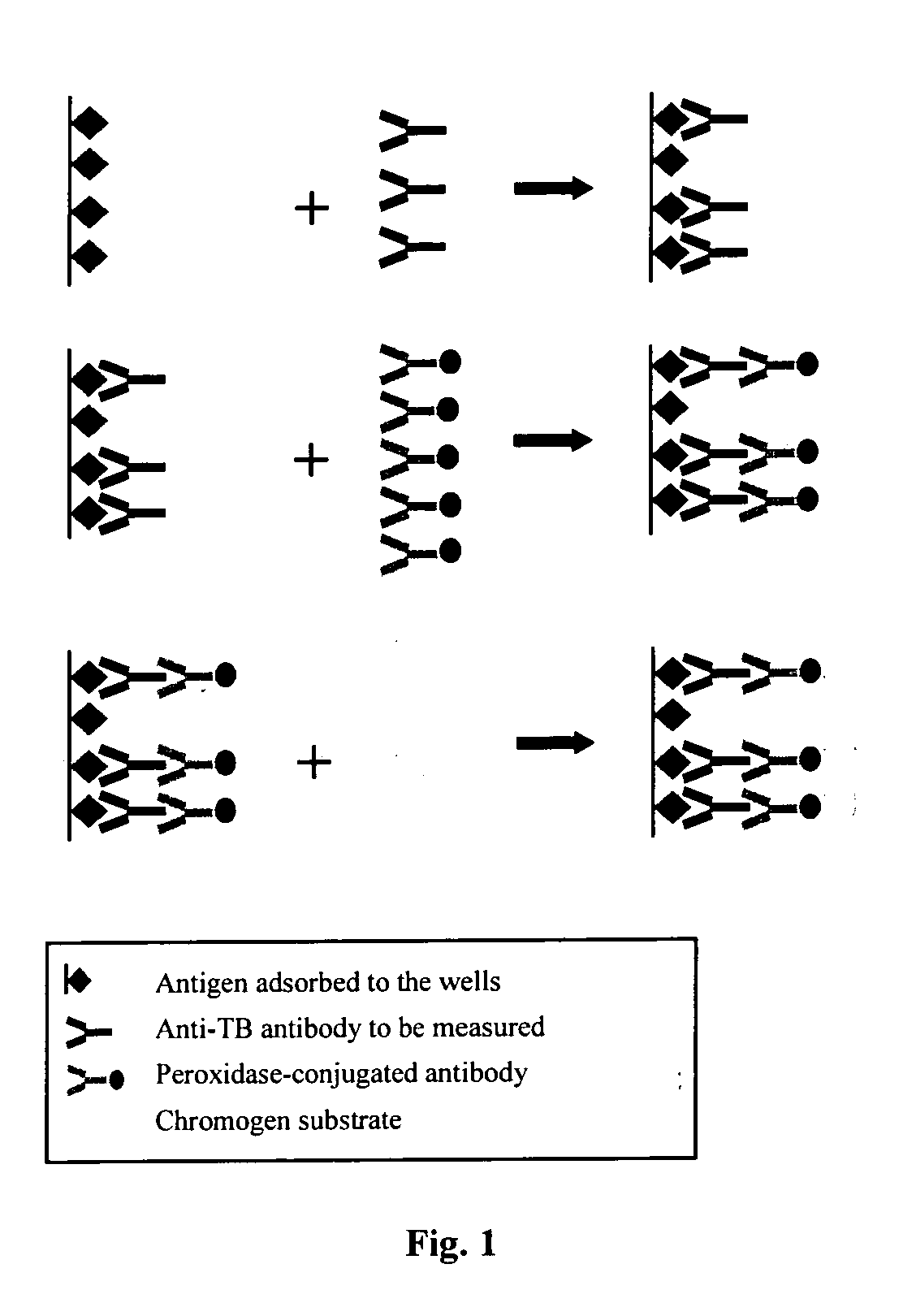
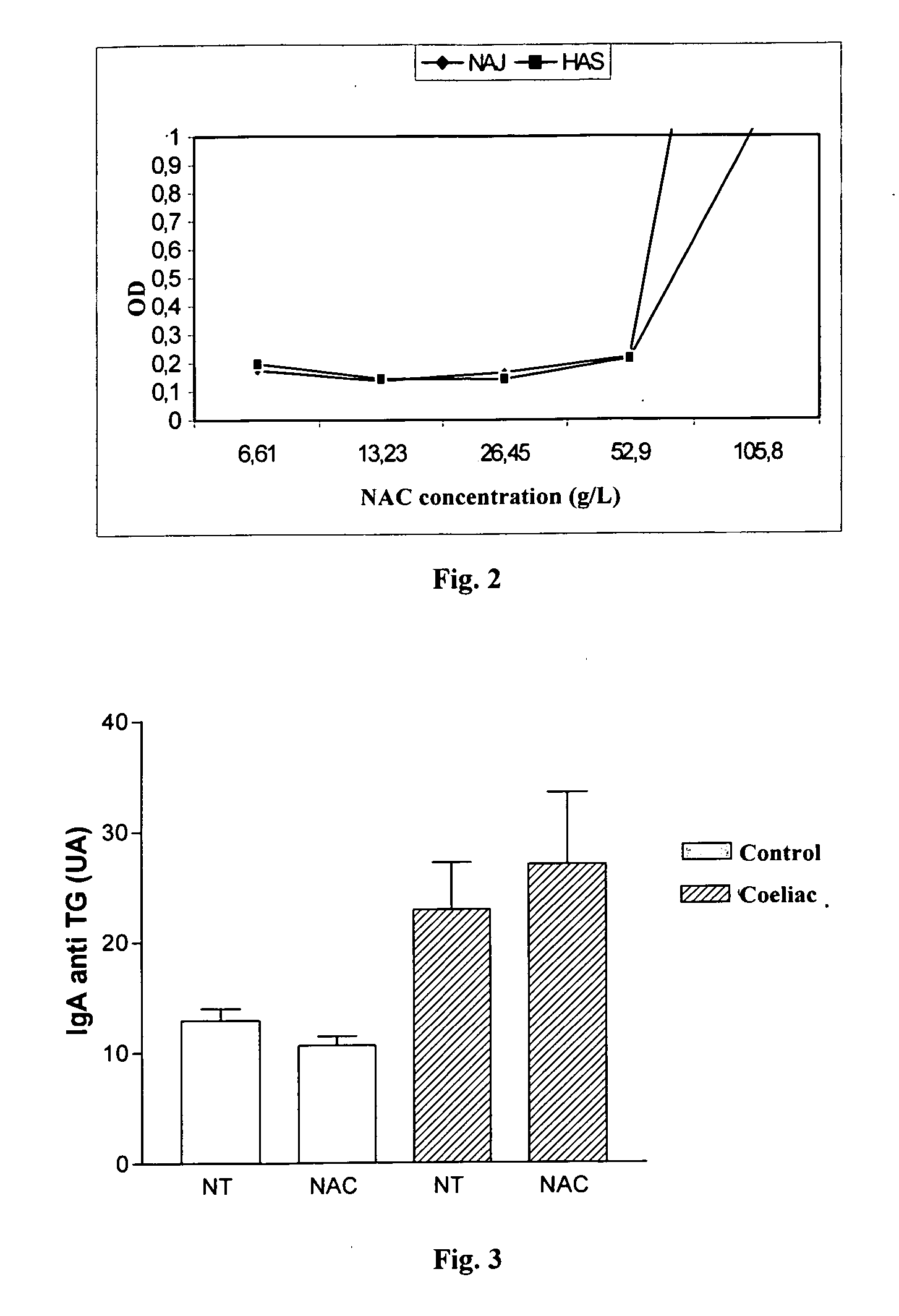
Methods for detecting anti-transglutaminase antibodies in a saliva sample containing the antibodies are disclosed. Saliva samples are pre-treated. The antibodies are subsequently detected in the pre-treated saliva by an immune reaction with a transglutaminase under conditions suitable for forming immuno-complexes with the antibodies. The method is useful for diagnosing and / or therapeutically controlling celiac disease.
Owner:UNIV LIBRE DE BRUXELIES
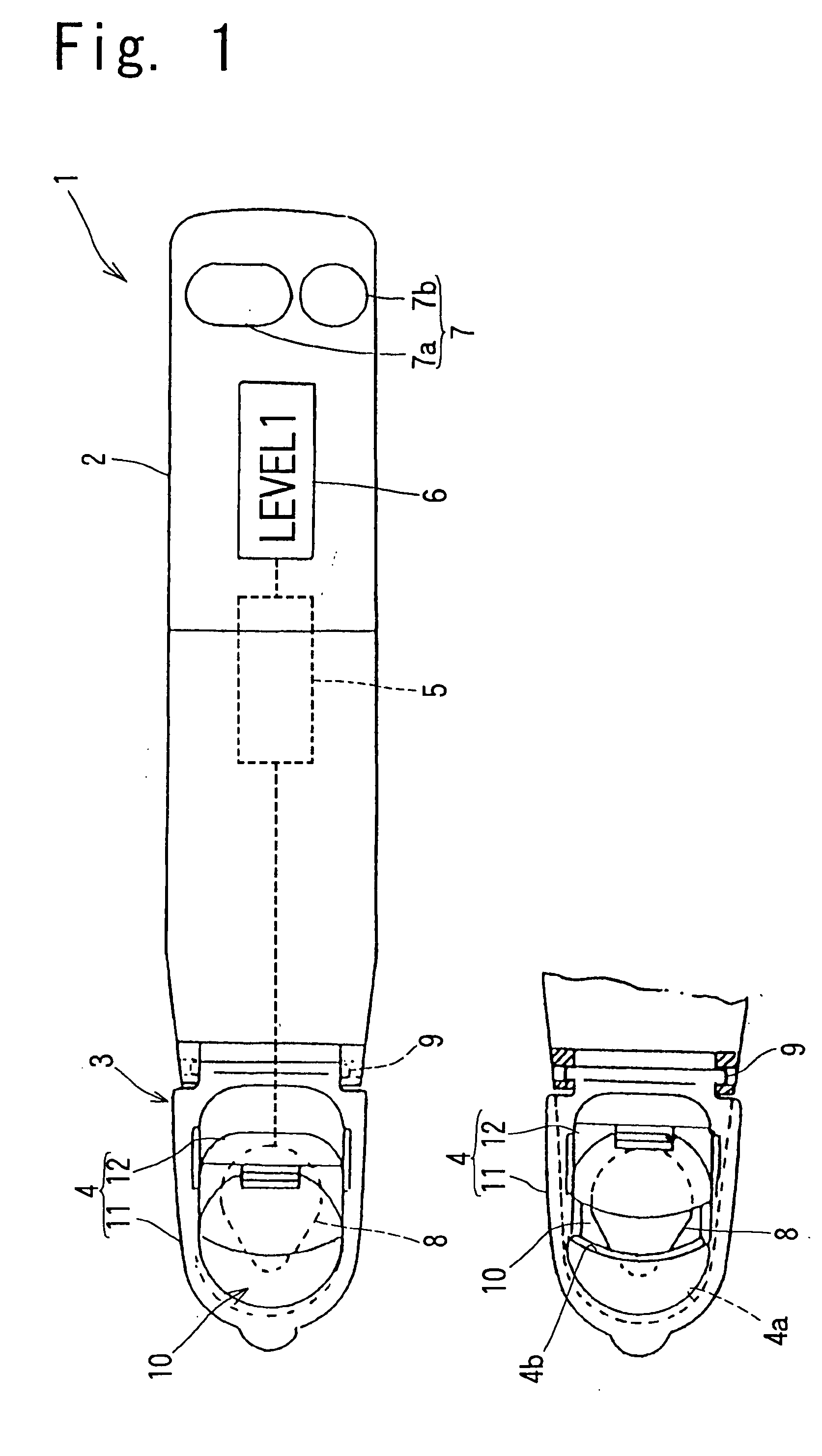
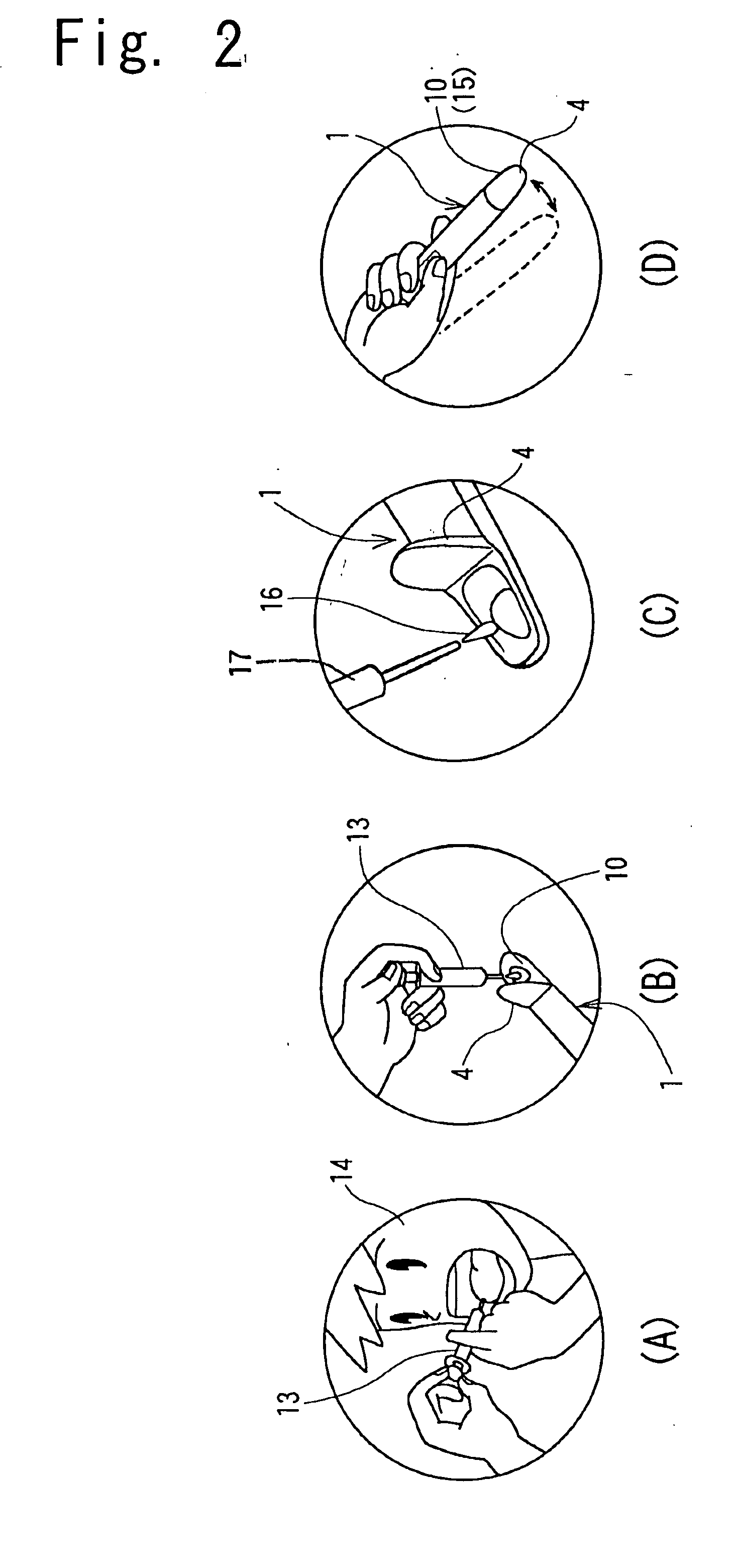
Ovulation-prediction devices with image processing system
InactiveUS20080255472A1Simple algorithmIntuitive imageSurgeryVaccination/ovulation diagnosticsSaliva sampleImaging processing
An ovulation-prediction device with an image processing system containing a transmissive test platen for placing saliva samples and a miniature camera for capturing the image of saliva sample at dried state for analyzing the increase of salt content for predicting ovulation. An image processing method with an algorithm described for removing noise in a saliva sample for calculating the density of dark pixels which represents salt content in the sample. A Density Index is defined. Trend curve of Density Index vs. day is established based on the daily saliva analysis in a woman's menstrual cycle for predicting days from the ovulation. Also illustrated are applications of the image processing system in an electrical toothbrush, a dental massager and a portable compact device using a disposable tape cassette for testing saliva samples.
Owner:KUO YOUTI
Composition for preserving DNA in saliva
InactiveCN105695447AEffective preservationReduce degradationDNA preparationSaliva sampleNucleic acid detection
The invention relates to a composition for preserving DNA in saliva, which includes a denaturant, a buffer, a chelating agent, sodium chloride and polyethylene glycol. The composition of the present invention can effectively preserve DNA in saliva for a long time under room temperature conditions, and can effectively reduce the degradation of DNA in isolated saliva, ensure the integrity of DNA, facilitate subsequent nucleic acid detection research, and is simple to use and operate. Convenient and greatly saves the processing time of saliva samples.
Owner:智海生物工程(北京)股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com