Patents
Literature
12935 results about "Laboratory facility" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A laboratory (UK: /ləˈbɒrətəri/, US: /ˈlæbərətɔːri/; informally, lab) is a facility that provides controlled conditions in which scientific or technological research, experiments, and measurement may be performed.
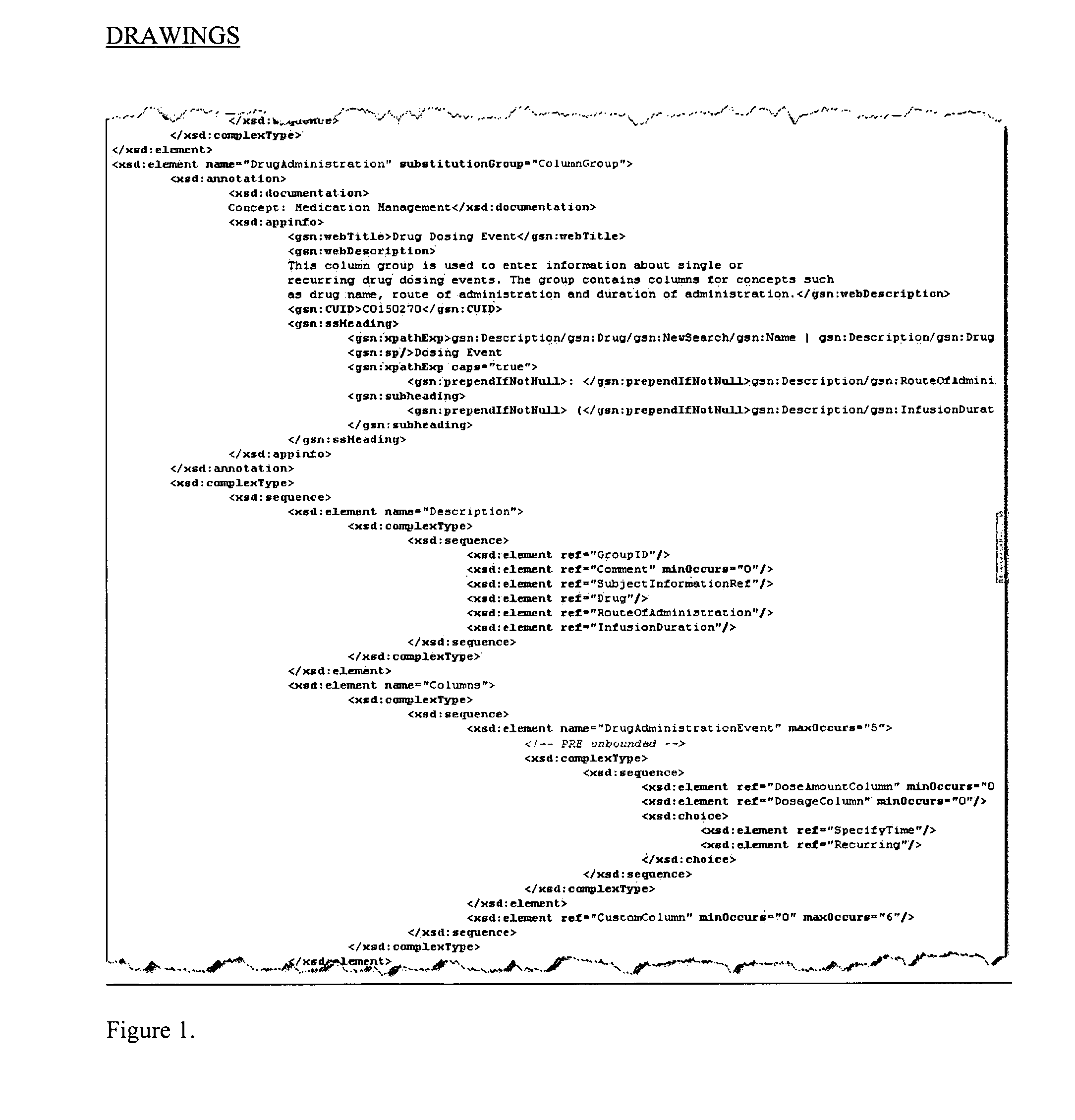
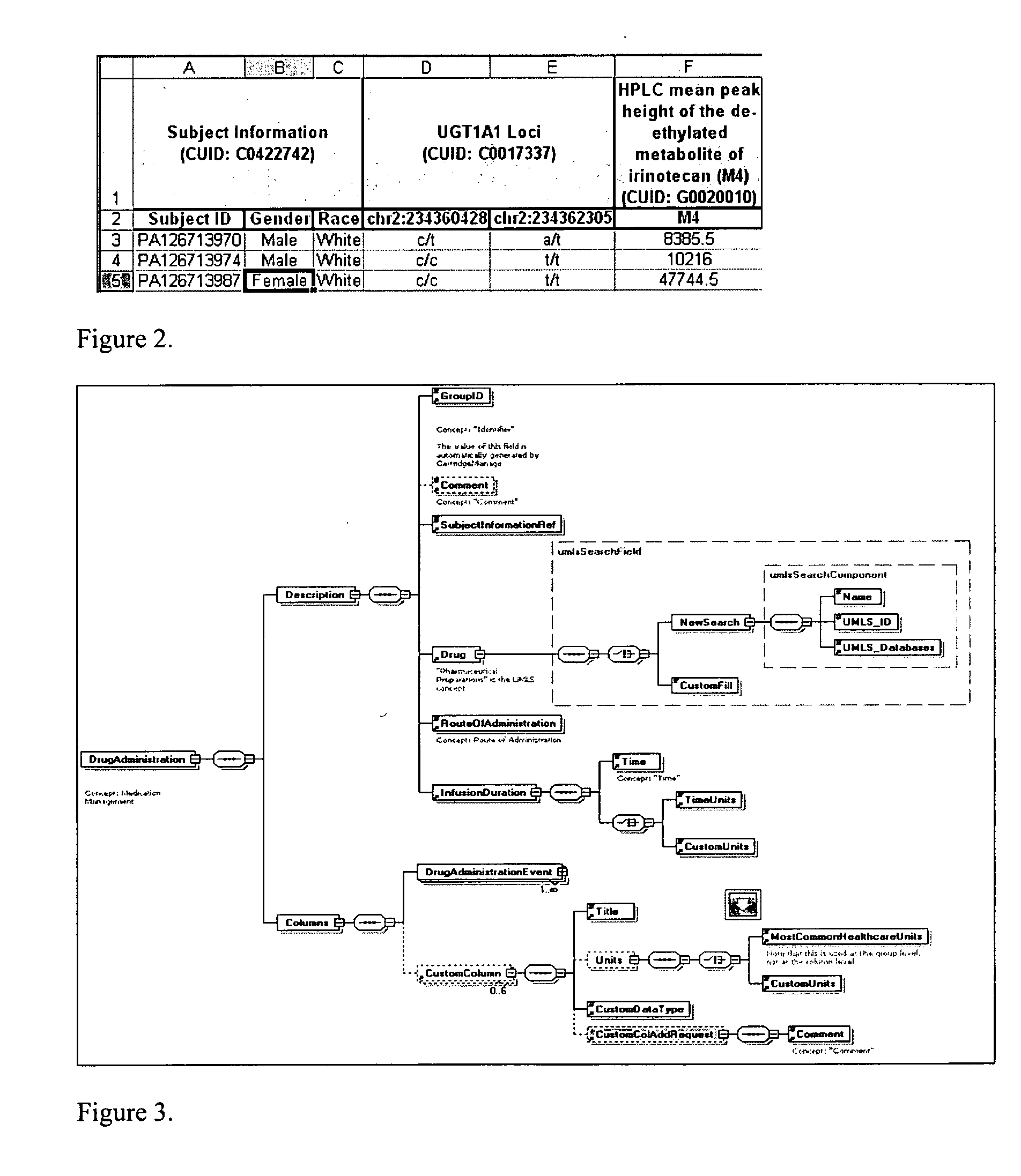
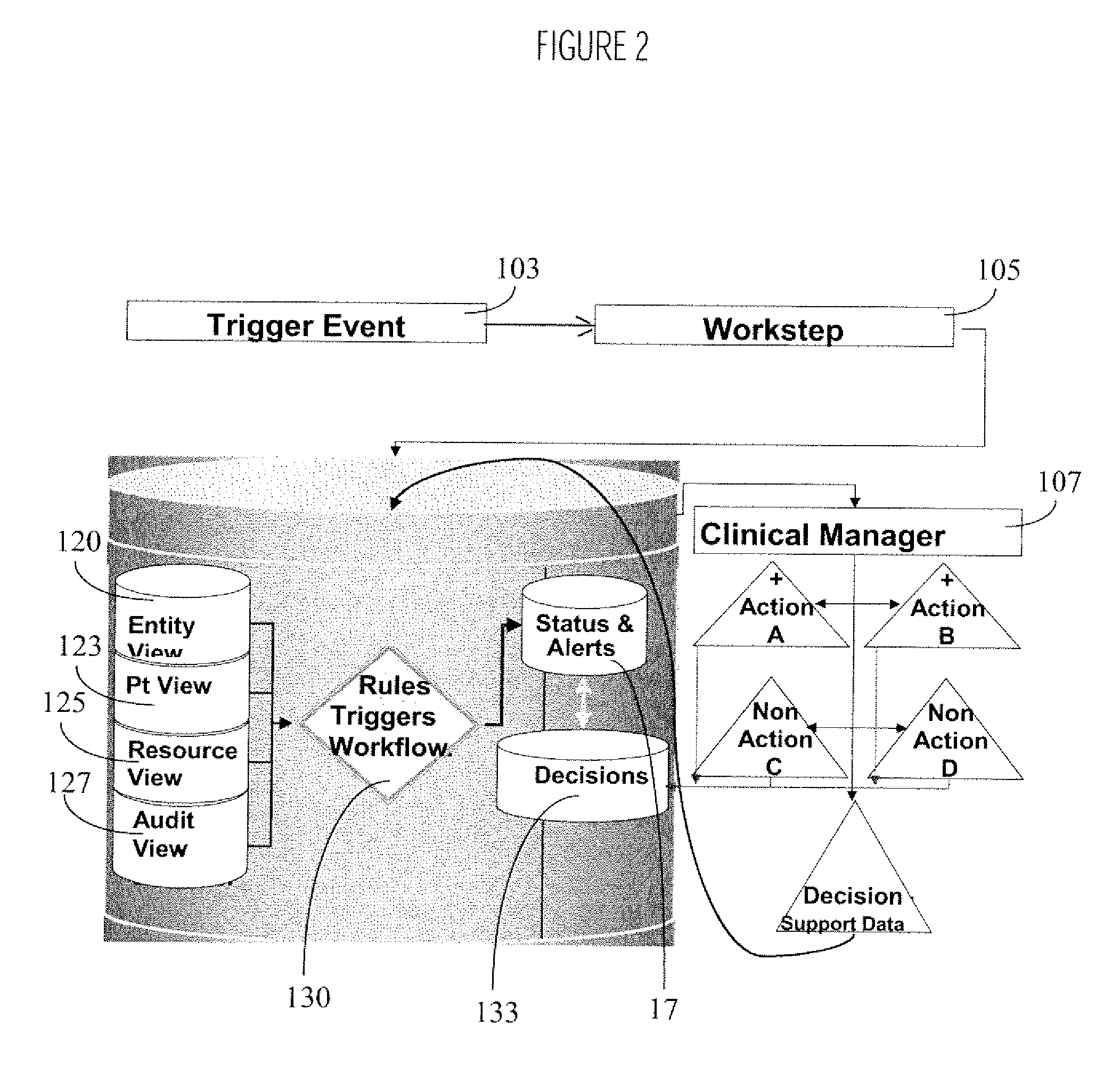
System and method for integrating and validating genotypic, phenotypic and medical information into a database according to a standardized ontology
InactiveUS20070178501A1Safest and most effective treatmentGood decisionData processing applicationsMicrobiological testing/measurementData validationMedical record
The system described herein enables clinicians and researchers to use aggregated genetic and phenotypic data from clinical trials and medical records to make the safest, most effective treatment decisions for each patient. This involves (i) the creation of a standardized ontology for genetic, phenotypic, clinical, pharmacokinetic, pharmacodynamic and other data sets, (ii) the creation of a translation engine to integrate heterogeneous data sets into a database using the standardized ontology, and (iii) the development of statistical methods to perform data validation and outcome prediction with the integrated data. The system is designed to interface with patient electronic medical records (EMRs) in hospitals and laboratories to extract a particular patient's relevant data. The system may also be used in the context of generating phenotypic predictions and enhanced medical laboratory reports for treating clinicians. The system may also be used in the context of leveraging the huge amount of data created in medical and pharmaceutical clinical trials. The ontology and validation rules are designed to be flexible so as to accommodate a disparate set of clients. The system is also designed to be flexible so that it can change to accommodate scientific progress and remain optimally configured.
Owner:NATERA
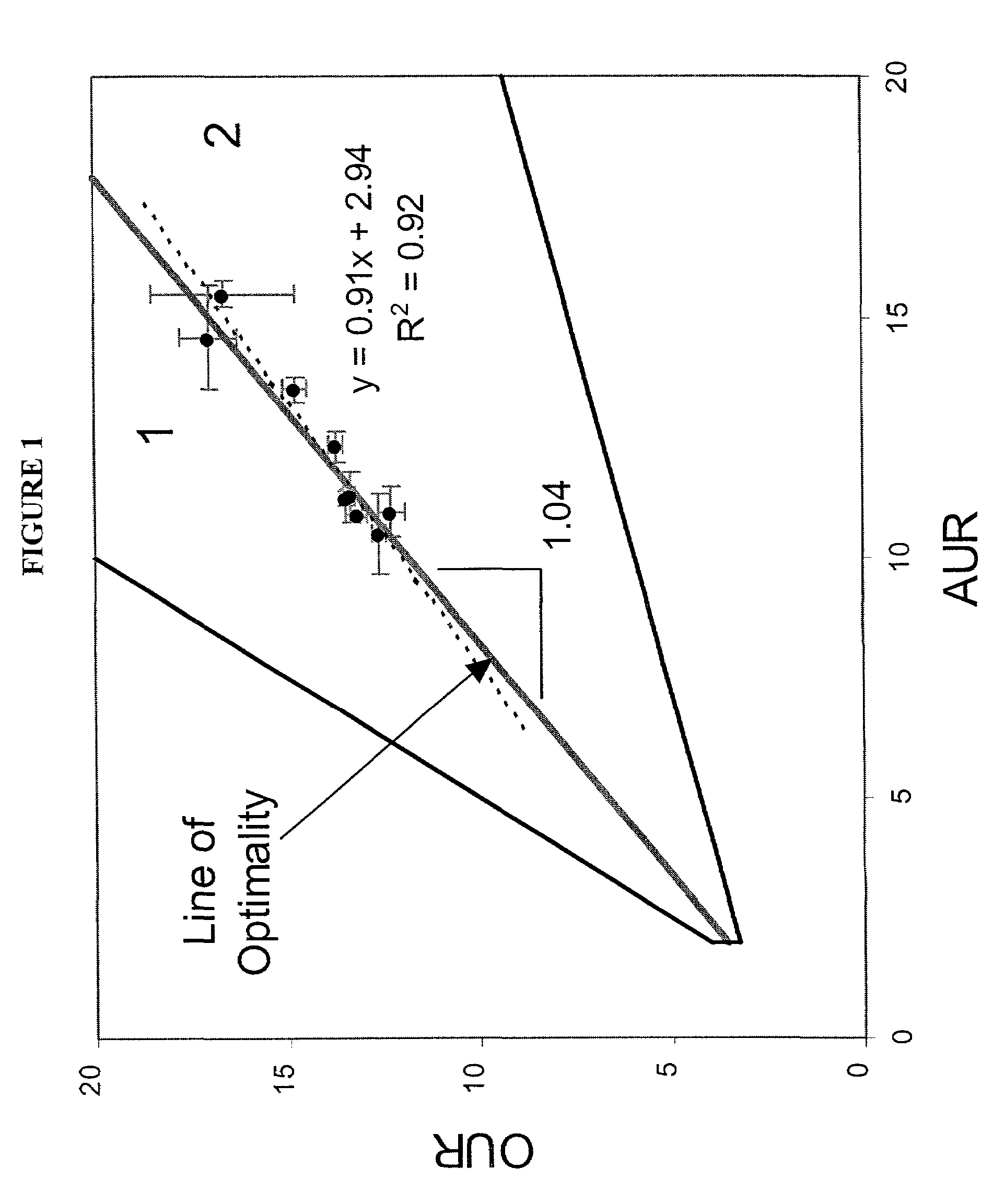
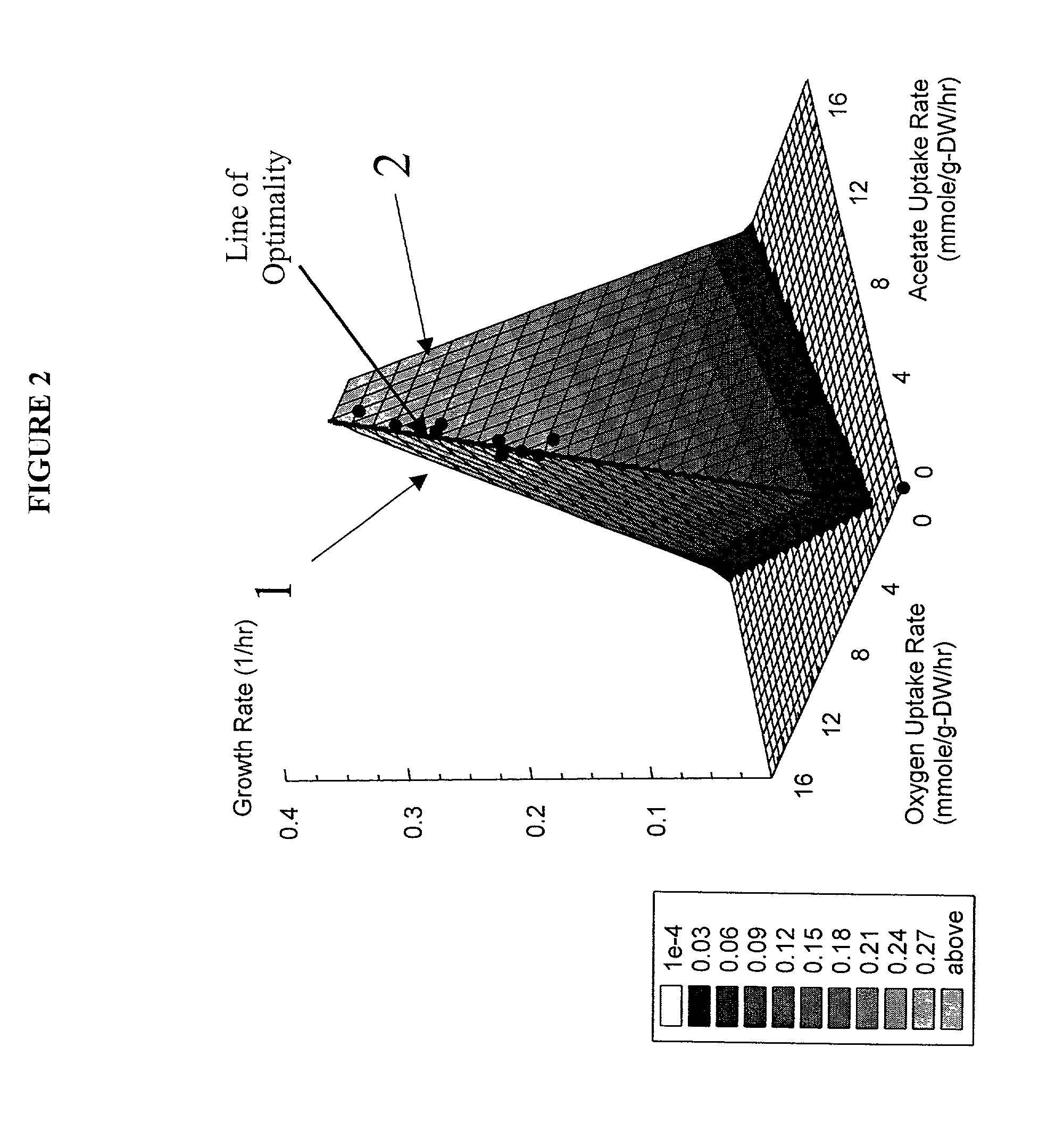
Method for the evolutionary design of biochemical reaction networks
The present invention relates to methods for achieving an optimal function of a biochemical reaction network. The methods can be performed in silico using a reconstruction of a biochemical reaction network of a cell and iterative optimization procedures. The methods can further include laboratory culturing steps to confirm and possibly expand the determinations made using the in silico methods, and to produce a cultured cell, or population of cells, with optimal functions. The current invention includes computer systems and computer products including computer-readable program code for performing the in silico steps of the invention.
Owner:RGT UNIV OF CALIFORNIA
Immunoassay that provides for both collection of saliva and assay of saliva for one or more analytes with visual readout
InactiveUS6248598B1Eliminate riskBioreactor/fermenter combinationsBiological substance pretreatmentsAnalyteSaliva sample
A device that provides for both the collection of saliva and detection of at least one analyte therein, e.g., a drug, is provided. This device provides for rapid analysis of saliva samples, while also providing a convenient assay method that does not require the addition of extraneous reagents, or other materials. Thereby, this device can be used by non-laboratory personnel without risk of user introduced errors.
Owner:BOGEMA STUART C
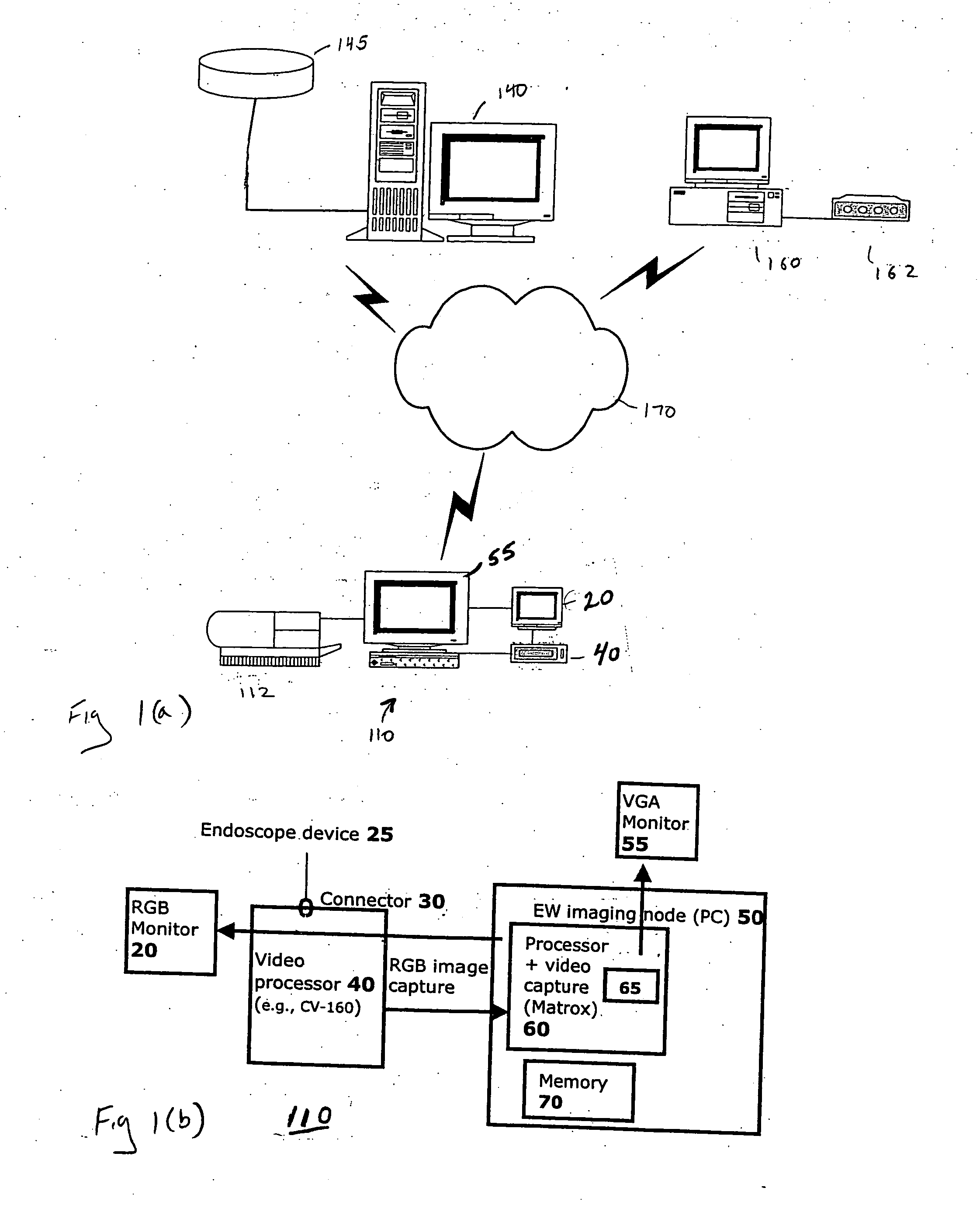
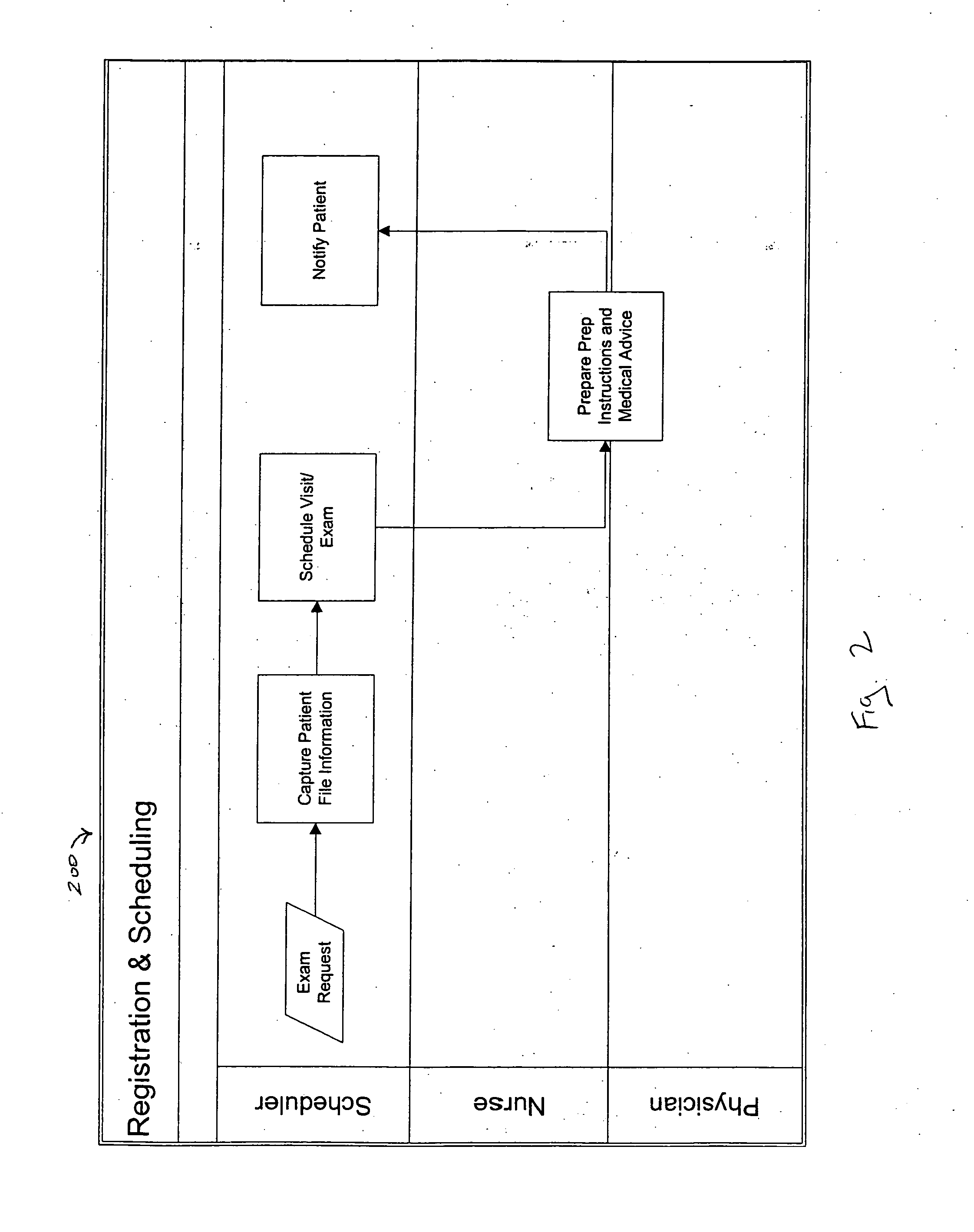
System and method for managing an endoscopic lab
A system designed to support and manage the workflow for all user roles pertaining to an endoscopic laboratory, from registration and scheduling of patient information through pre-procedure, procedure and post-procedure phases of an endoscopic examination, including support for the entry, by various users associated with an endoscopic laboratory, of information and data including the processing and storage of endoscopic images captured during an endoscopic exam of a patient, for association with a patient record stored in a database, and including the entry of procedure notes and generation of reports that include the stored images, all via an integrated user interface.
Owner:OLYMPUS AMERICA +1
Systems and methods for sample use maximization
InactiveUS20120309636A1Increase in sizeImprove accuracyImage enhancementBioreactor/fermenter combinationsPoint of careAnalyte
The present invention provides systems, devices, and methods for point-of-care and / or distributed testing services. The methods and devices of the invention are directed toward automatic detection of analytes in a bodily fluid. The components of the device can be modified to allow for more flexible and robust use with the disclosed methods for a variety of medical, laboratory, and other applications. The systems, devices, and methods of the present invention can allow for effective use of samples by improved sample preparation and analysis.
Owner:THERANOS
Smart disposable plastic lab-on-a-chip for point-of-care testing
InactiveUS20050130292A1None of measures has been particularly successfulRelieve painBioreactor/fermenter combinationsCombination devicesVenous bloodLab-on-a-chip
Disclosed herein is a fully-integrated, disposable biochip for point-of-care testing of clinically relevant parameters. Specifically, in accordance with an embodiment of the present invention, the biochip is designed for POCT (point-of-care-testing) of an array of metabolic parameters including partial pressure of oxygen, Glucose, and Lactate concentration from venous blood samples. The biochip is fabricated on a low-cost plastic substrate using mass manufacturing compatible fabrication processes. Furthermore, the biochip contains a fully-integrated metallic micro-needle for blood sampling. The biochip also uses smart passive microfluidics in conjunction with low-power functional on-chip pressure generators for microfluidic sequencing. The design, configuration, assembly and operation of the biochip are ideally suited for a disposable biochip specifically targeted towards POCT applications.
Owner:UNIVERSITY OF CINCINNATI
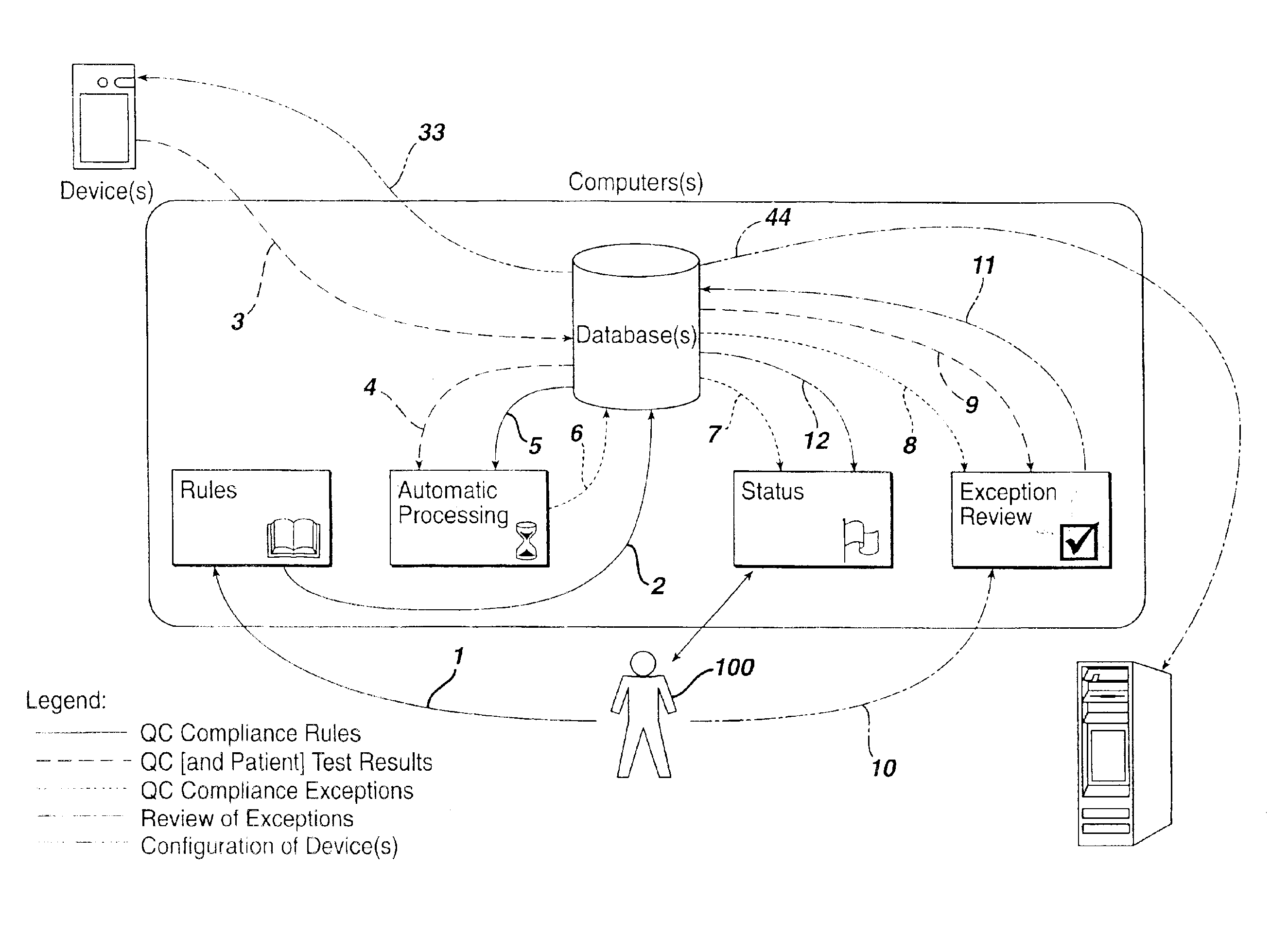
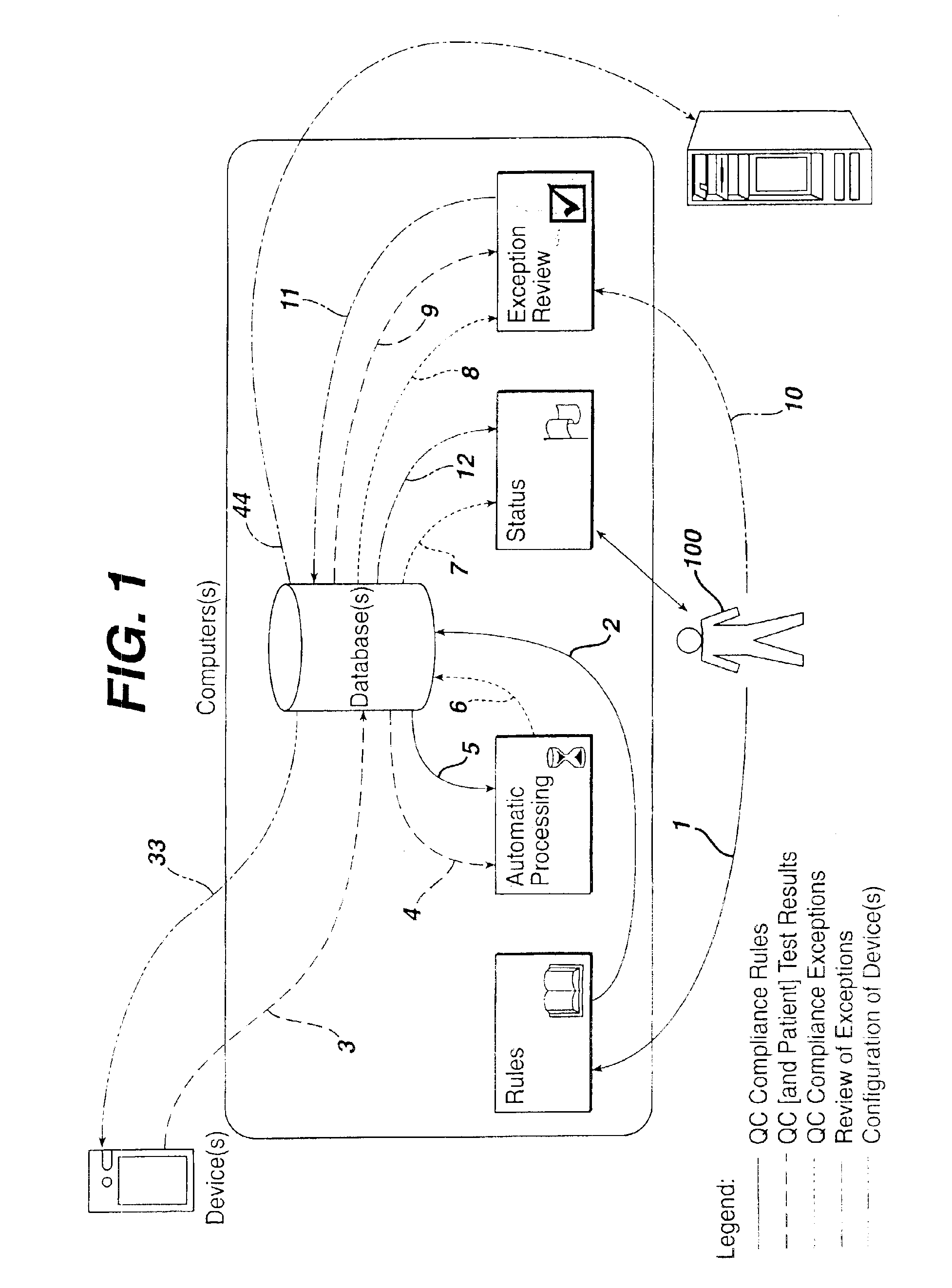
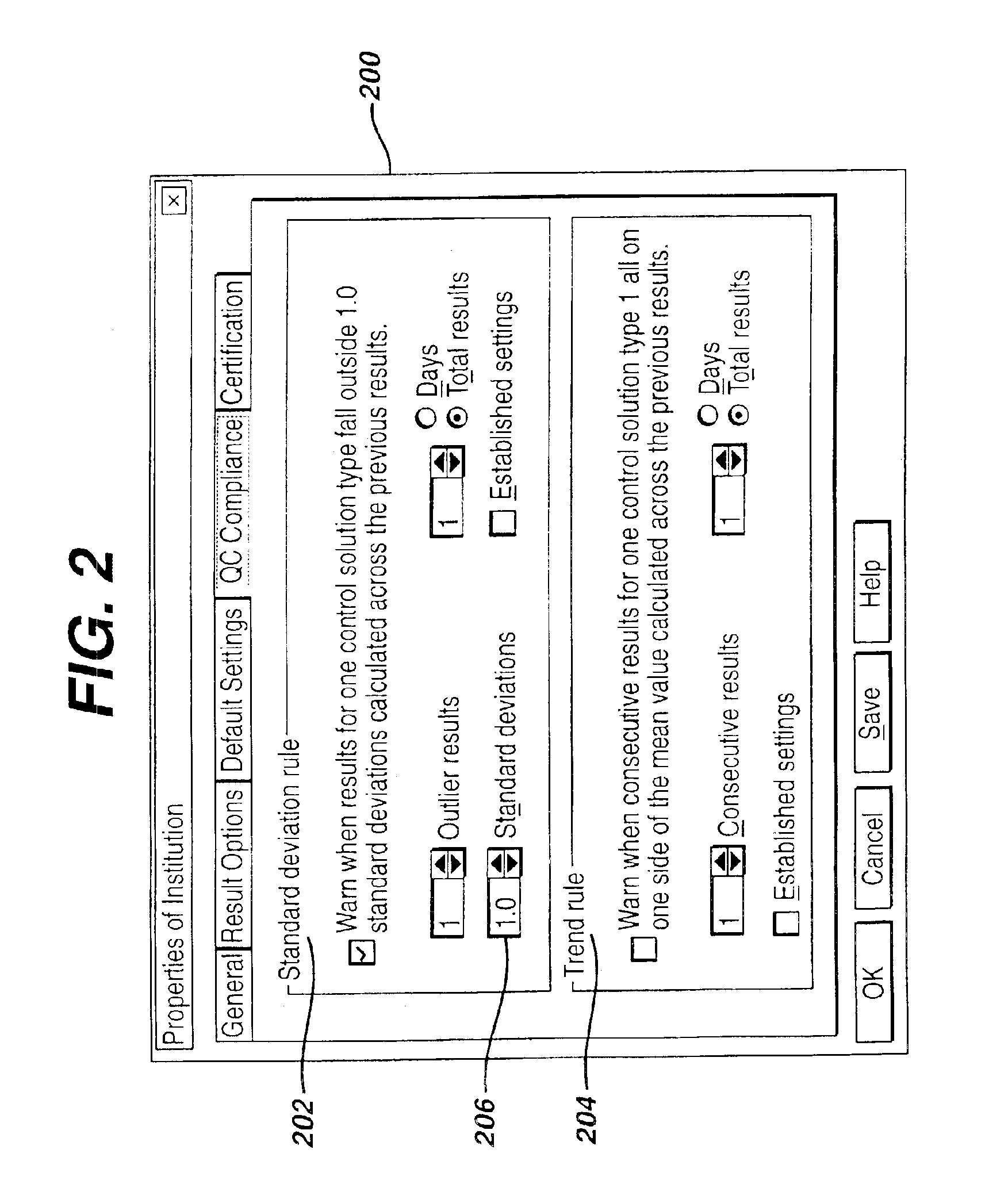
Method for automated exception-based quality control compliance for point-of-care devices
InactiveUS6856928B2Improving patient careQuality improvementDigital computer detailsNuclear monitoringAnalyteQuality control
A computer-implemented method to process POC information for potential QC compliance issues. A system and method for implementation of traditional laboratory analyzer based QC compliance in point-of-care (POC) environments is disclosed. A specific system and method to analyze data from POC testing to identify when the testing exceeds the variation expected under stable operation (i.e., the testing is “out of control”) is disclosed. This system and method is characterized by solving the QC compliance problem for POC devices by individuals not trained in traditional laboratory practices. This also provides the capability in real-time or near real-time to analyze POC testing information regarding the performance of each POC device, reagent kit (i.e., one kit per analyte tested) and / or lot, and operator so one can respond quickly to a particular device, reagent kit and / or lot, or operator that is not performing properly.
Owner:LIFESCAN IP HLDG LLC
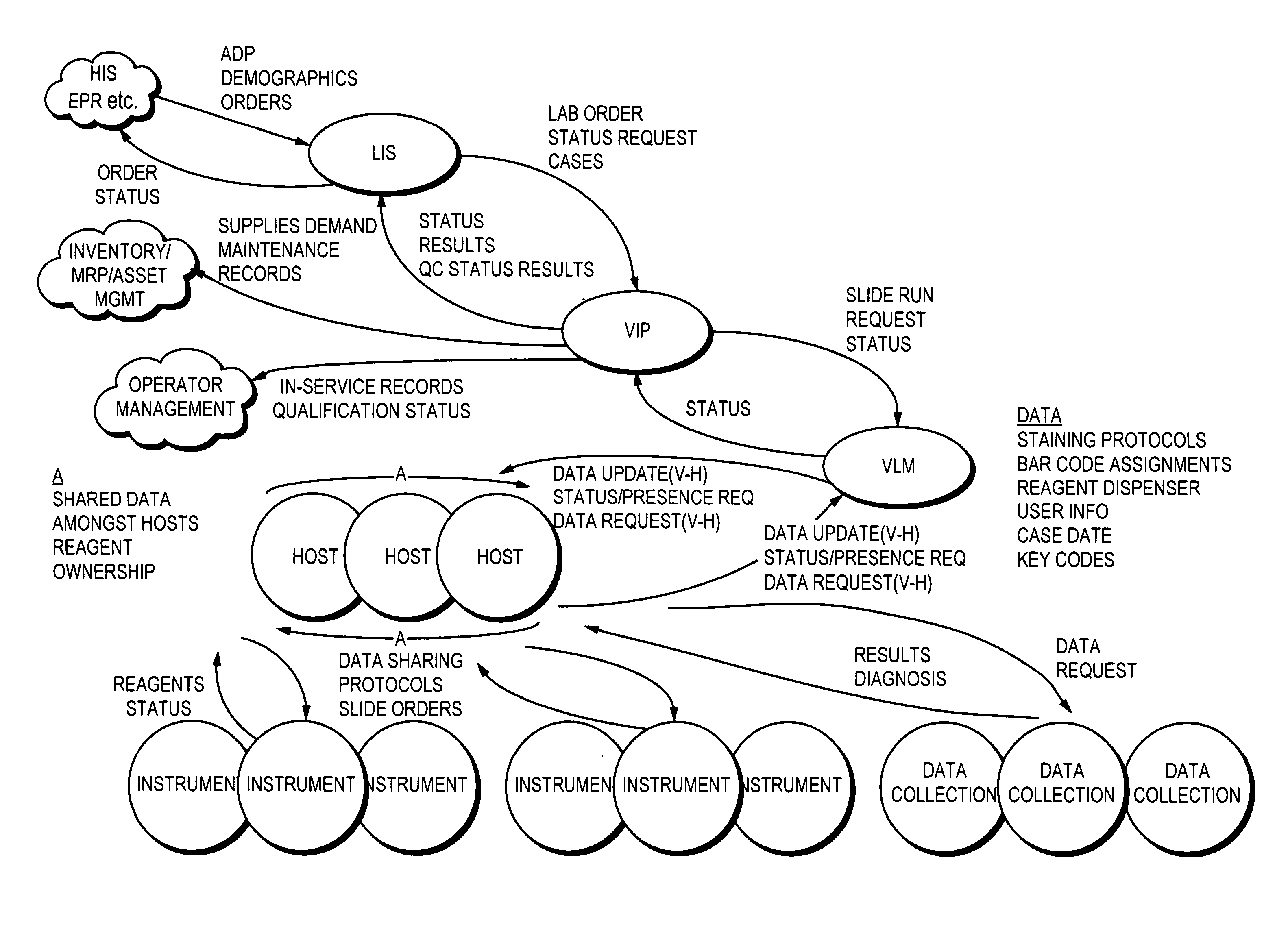
Laboratory instrumentation information management and control network
ActiveUS20050159982A1Maximize throughputComputer-assisted medical data acquisitionOffice automationCommunication interfaceInterface point
An interface point network (IPN) and a method for communication with a laboratory information system using an IPN, wherein the IPN includes at least one host computer in communication with at least one laboratory instrument, the laboratory information system and an interface point server in communication with the host computer and the laboratory information system, the interface point server being configured to function as a communication interface between the host computer and the laboratory information system in a manner responsive to a predetermined communication protocol. Use of bar code and RFID labels for tracking samples and in maintaining sample data is described.
Owner:VENTANA MEDICAL SYST INC
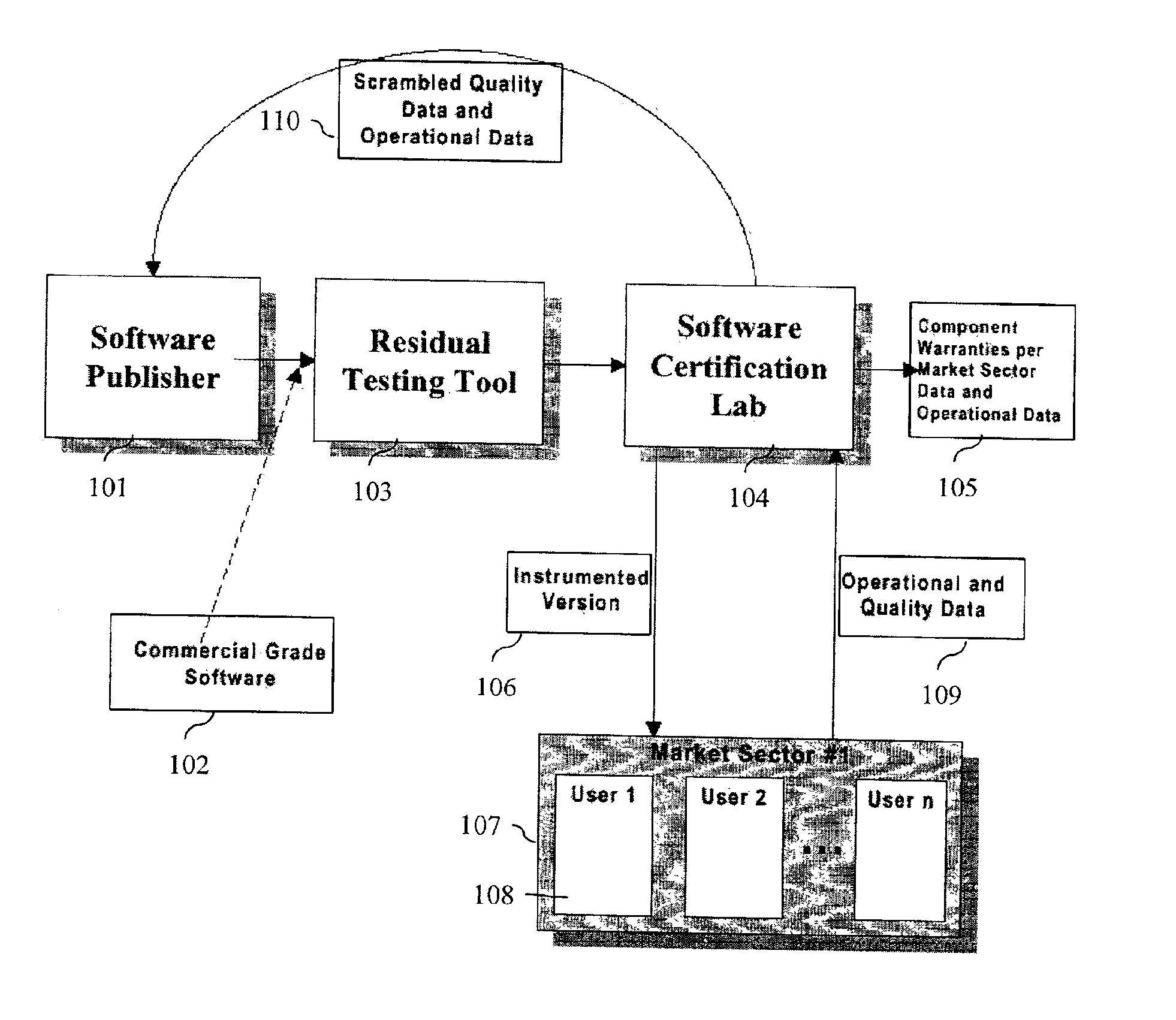
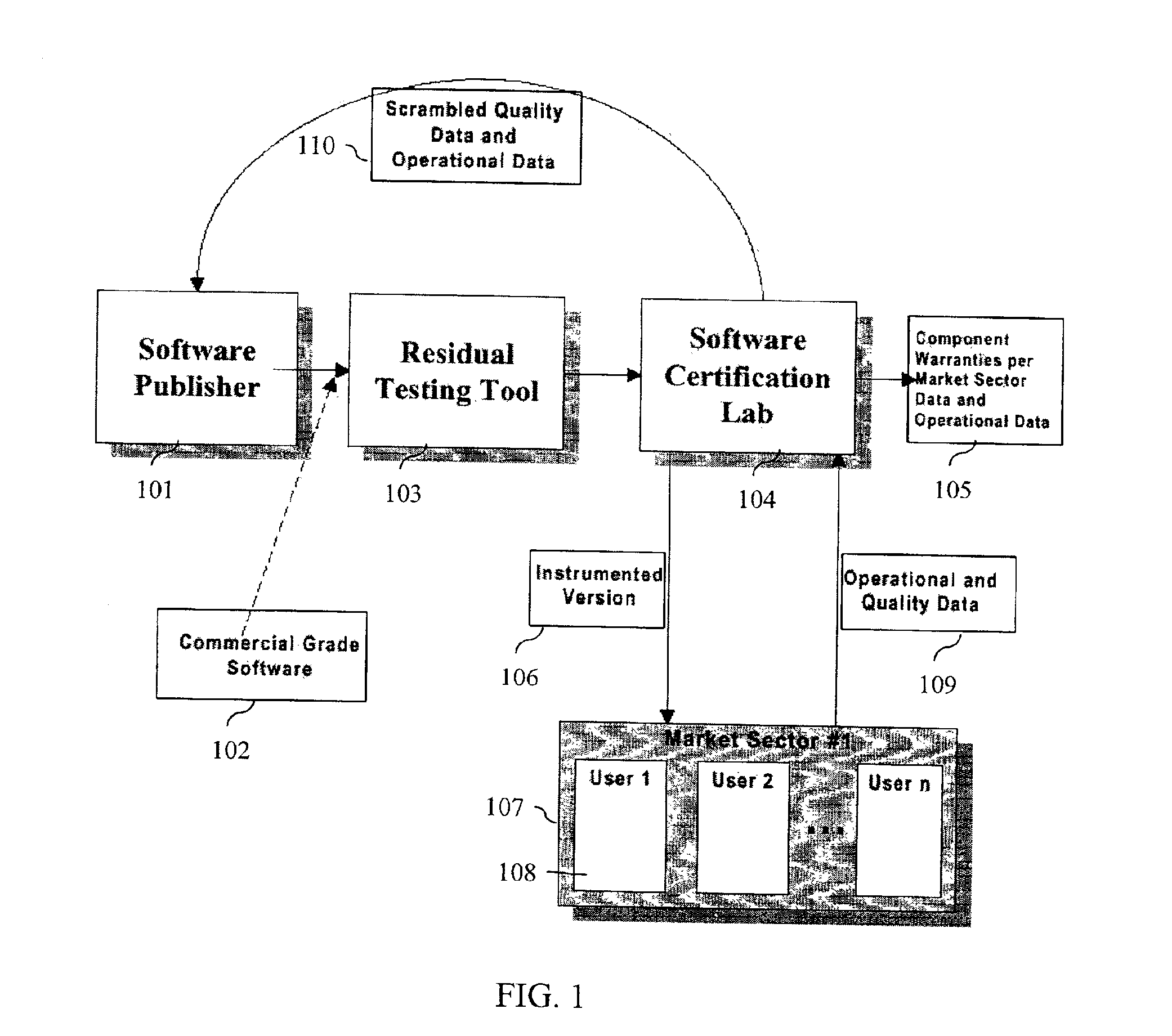
System and method for software certification
InactiveUS6862696B1Improve product reliabilityImprove reliabilityHardware monitoringSpecific program execution arrangementsSoftware engineeringHabit
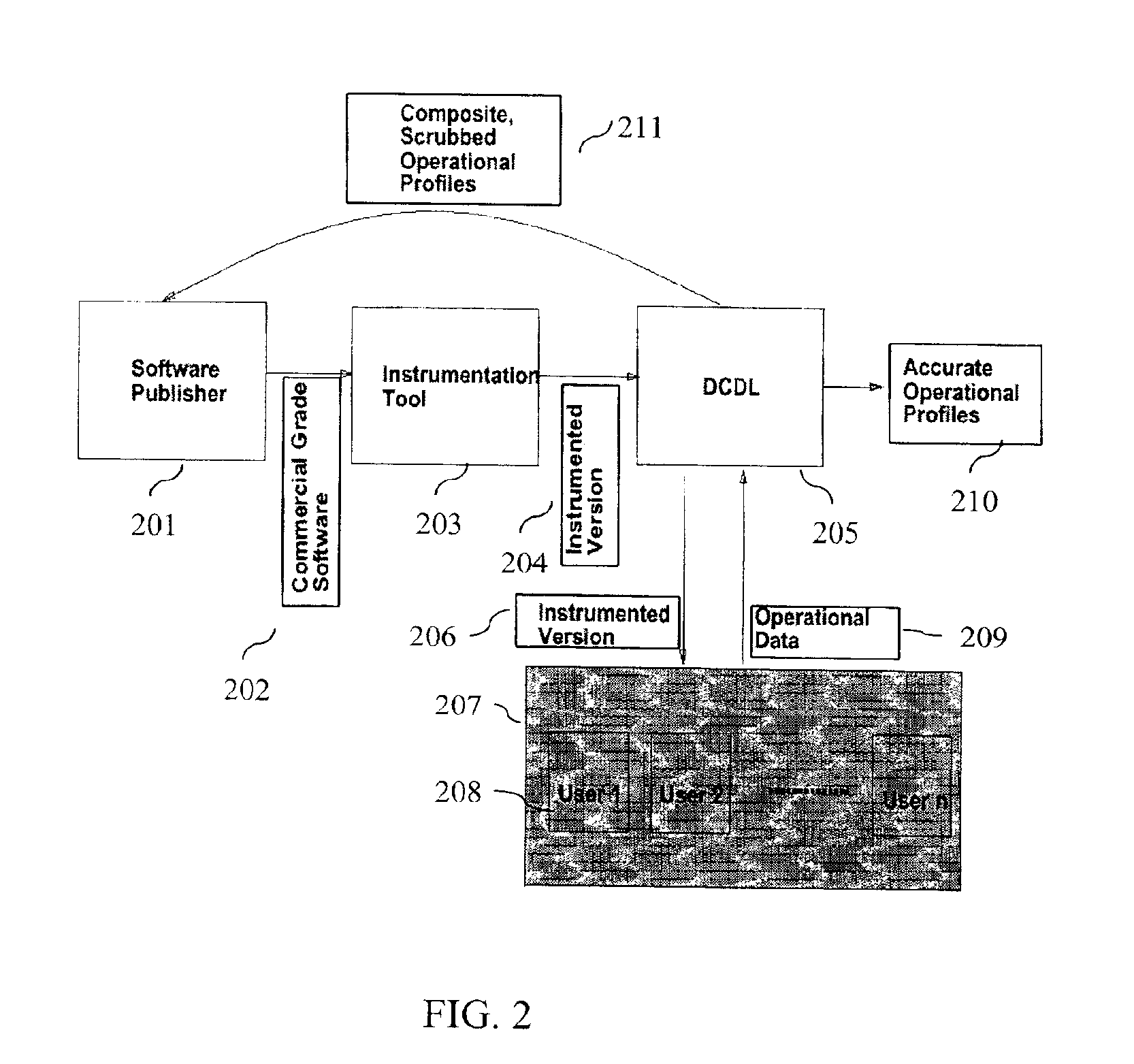
A method and method that builds accurate operational profiles for COTS software. The systems and methods disclosed allow software vendors to detect misused and unused features; identify common machine configurations for a given piece of software or software component; monitor changing user habits as new software version are released; derive more accurate testing methods for in-house testing purposes; and create user manuals which focus on those features most frequently used, or misused, by users. The disclosed system and method provides the tools enabling a software certification laboratory (SCL) to gather detailed usage data and failure data for a software application as it is used in the field. With this data the SCL can confidently issue certificates of reliability for software products.
Owner:SYNOPSYS INC
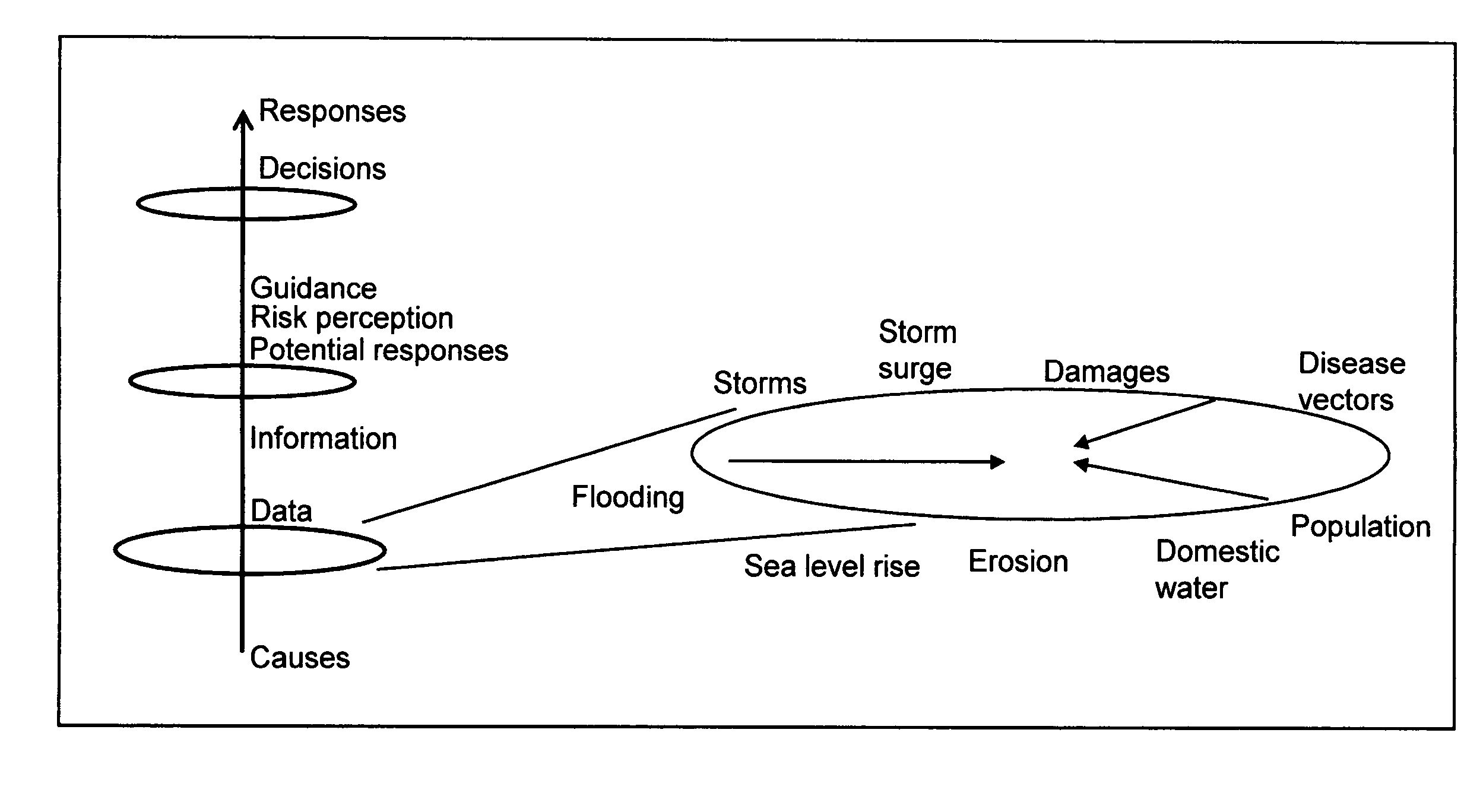
System and method for significant dust detection and enhancement of dust images over land and ocean
InactiveUS20050012035A1Enhanced signalHigh sensitivityRadiation pyrometryMaterial analysis by optical meansWater useDust detection
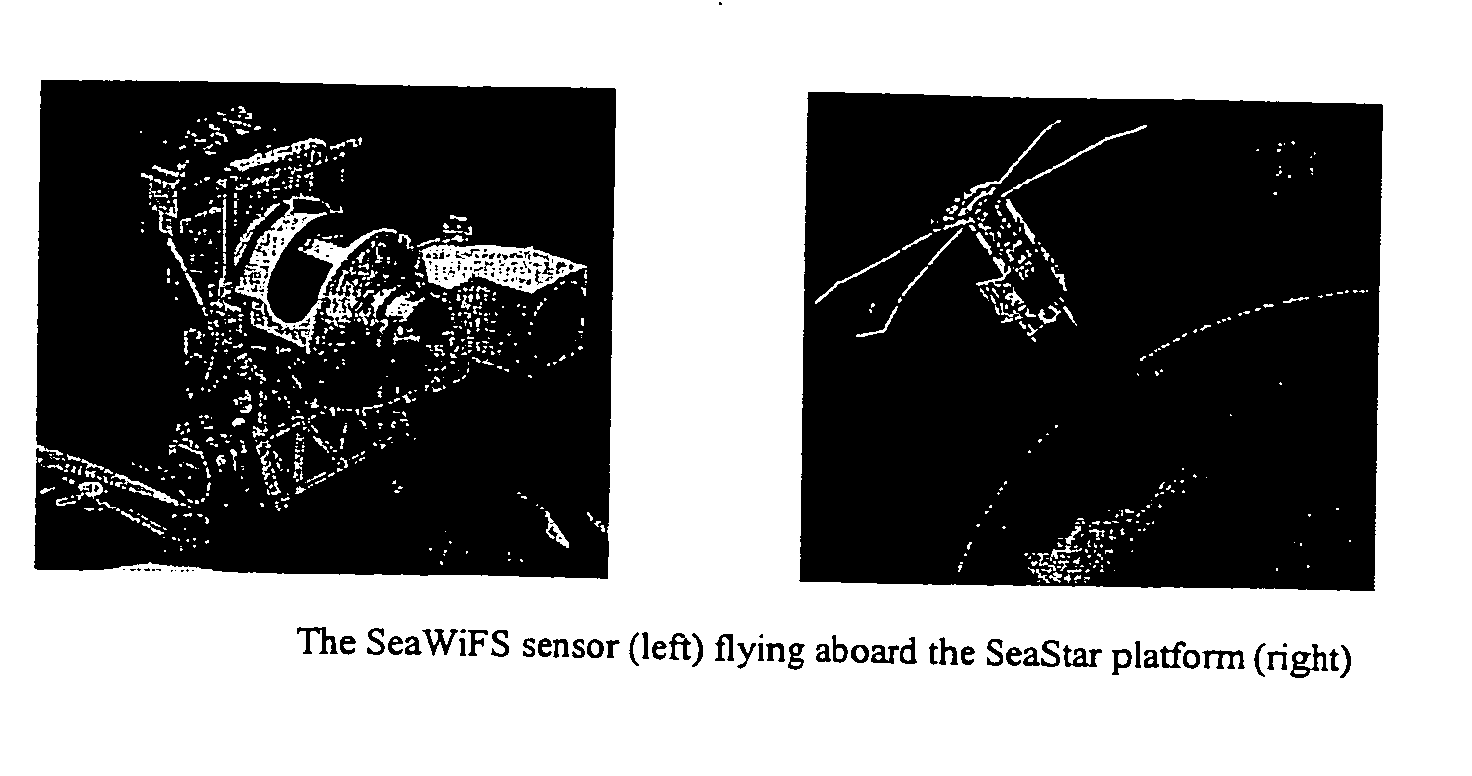
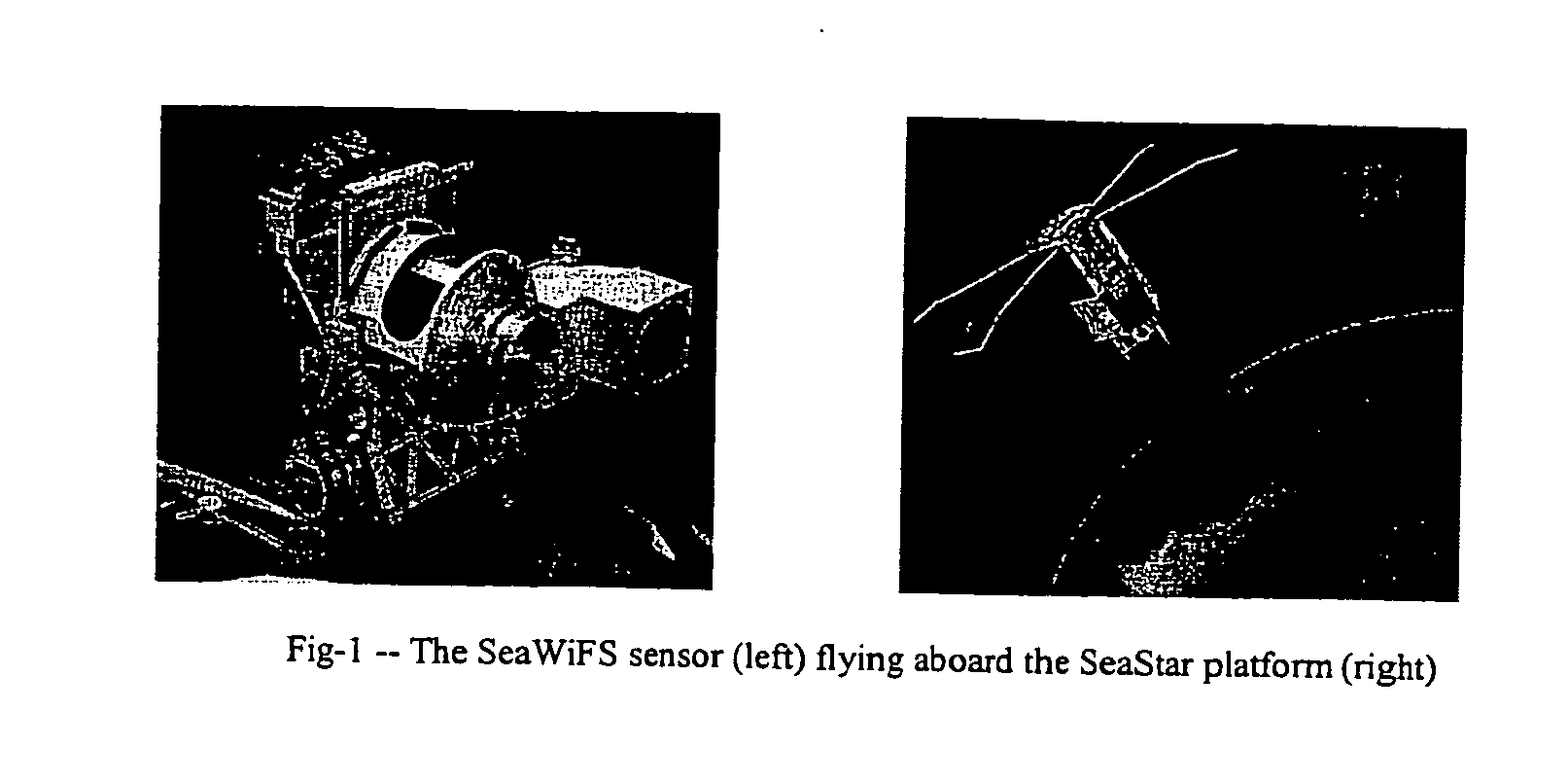
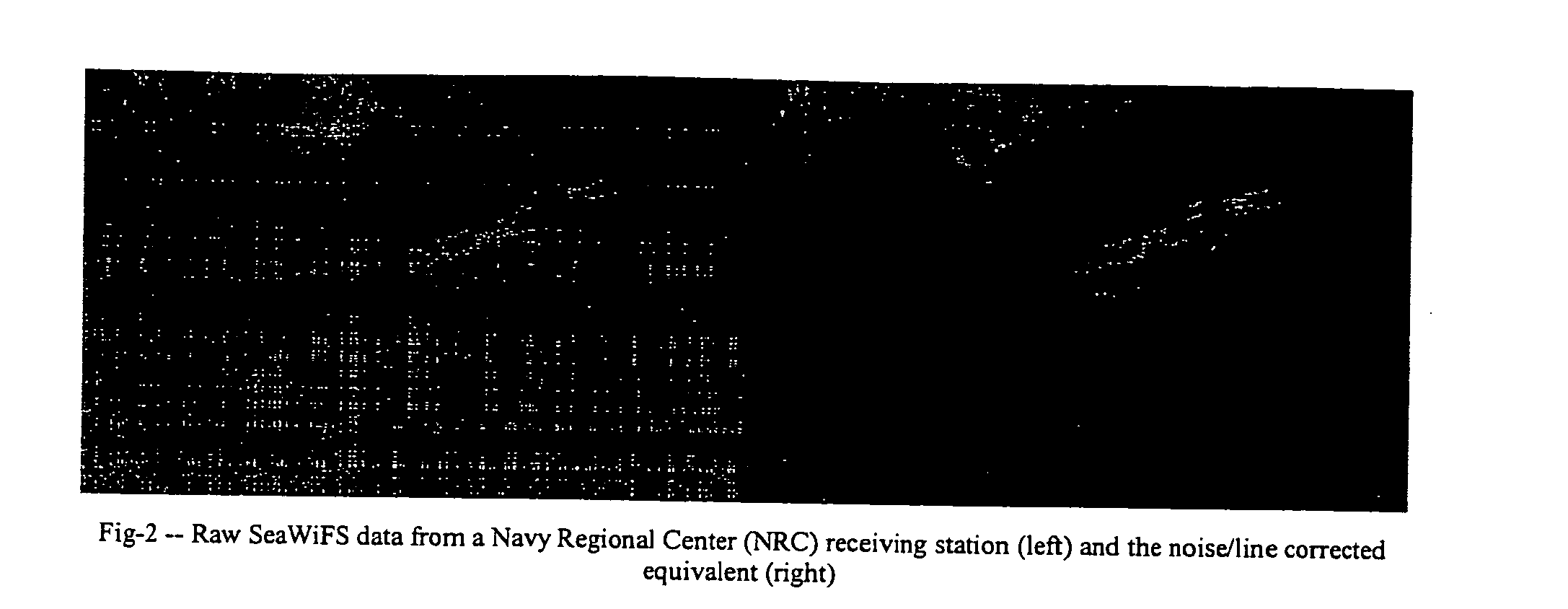
A new processing capability for dust enhancement over land or water using image data from the Sea-viewing Wide Field of View Sensor (SeaWiFS) has been developed for Naval meteorology / oceanography (MetOc) operations support. The data are captured via direct broadcast high-resolution picture transmission (HRPT) at Navy Regional Centers in Rota, Bahrain, and Yokosuka, and processed at the Naval Research Laboratory in Monterey. The raw data are calibrated, corrected for missing lines and clutter, corrected for molecular scatter contamination, and enhanced through multispectral combination to yield value added products. The processing has been automated completely such that products, generated upon receipt of data, are hosted upon a password protected website typically 60 to 90 minutes from time of initial capture. This invention summarizes the SeaWiFS instrument capabilities, the protocol followed for automated near real-time processing, a physical basis for the NRL enhancements, and specific examples of the products with extension to over-land dust enhancement as enabled by MODIS. It closes with a glimpse of the potential utility of these products from the perspective of the warfighter.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY
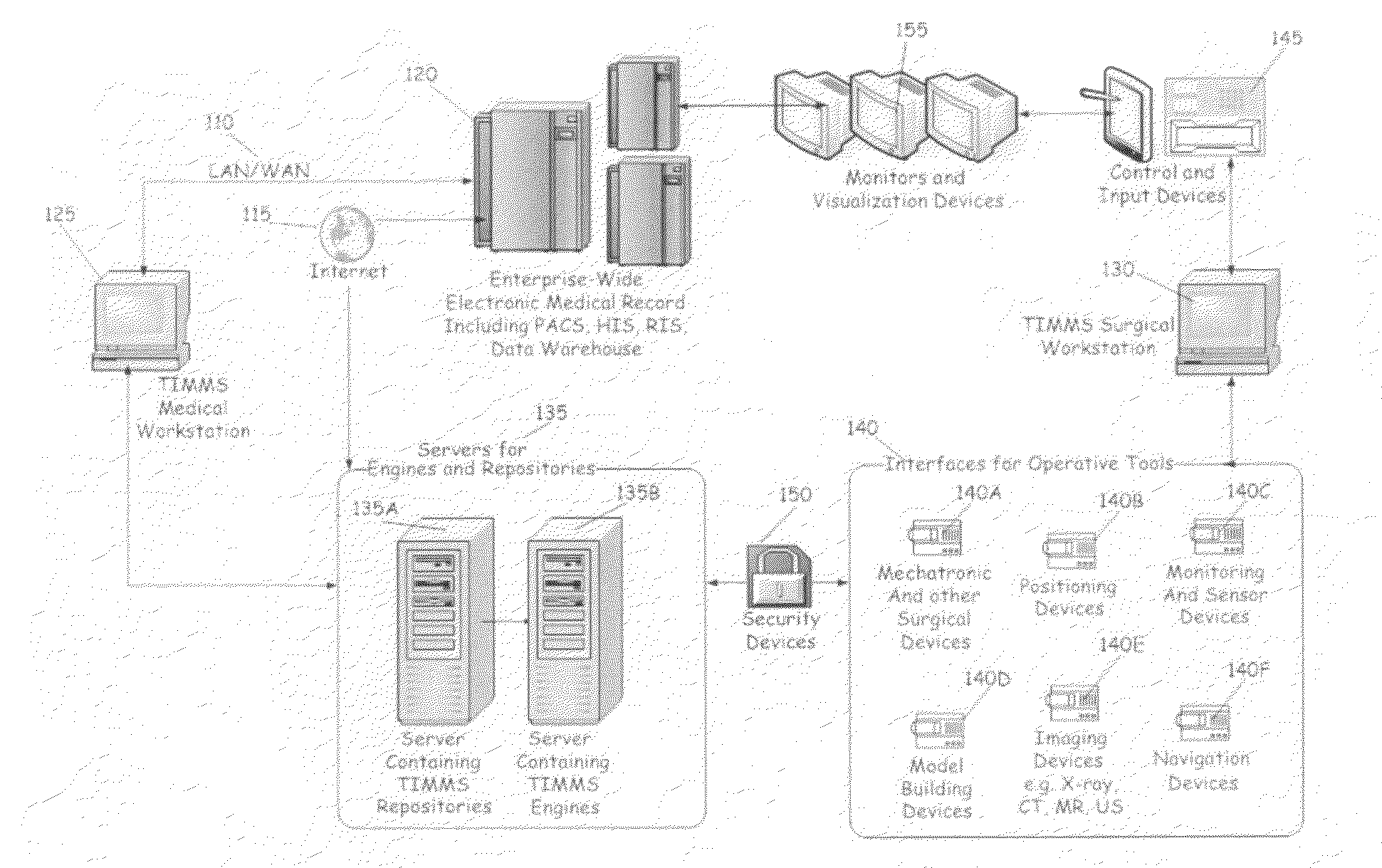
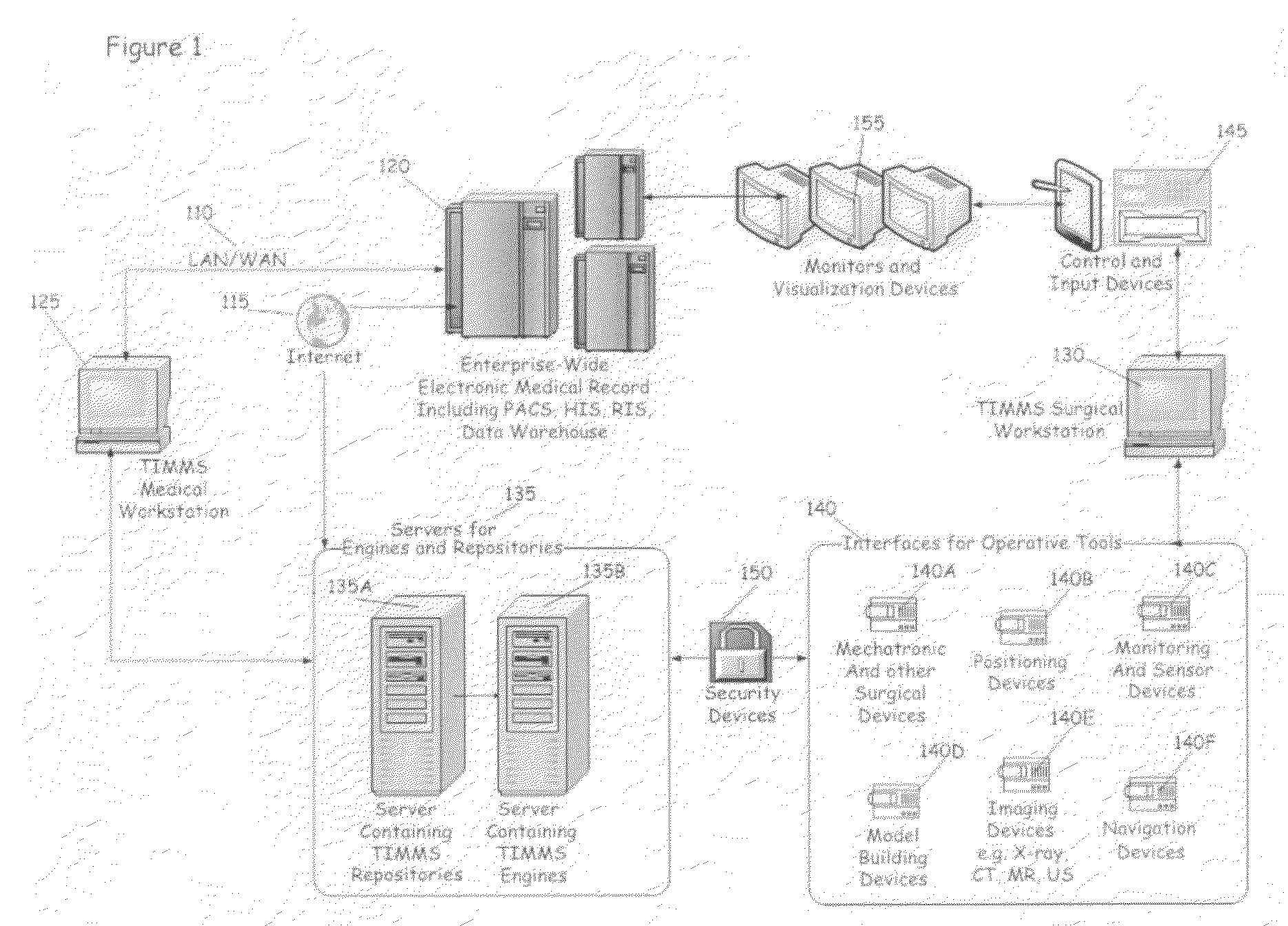
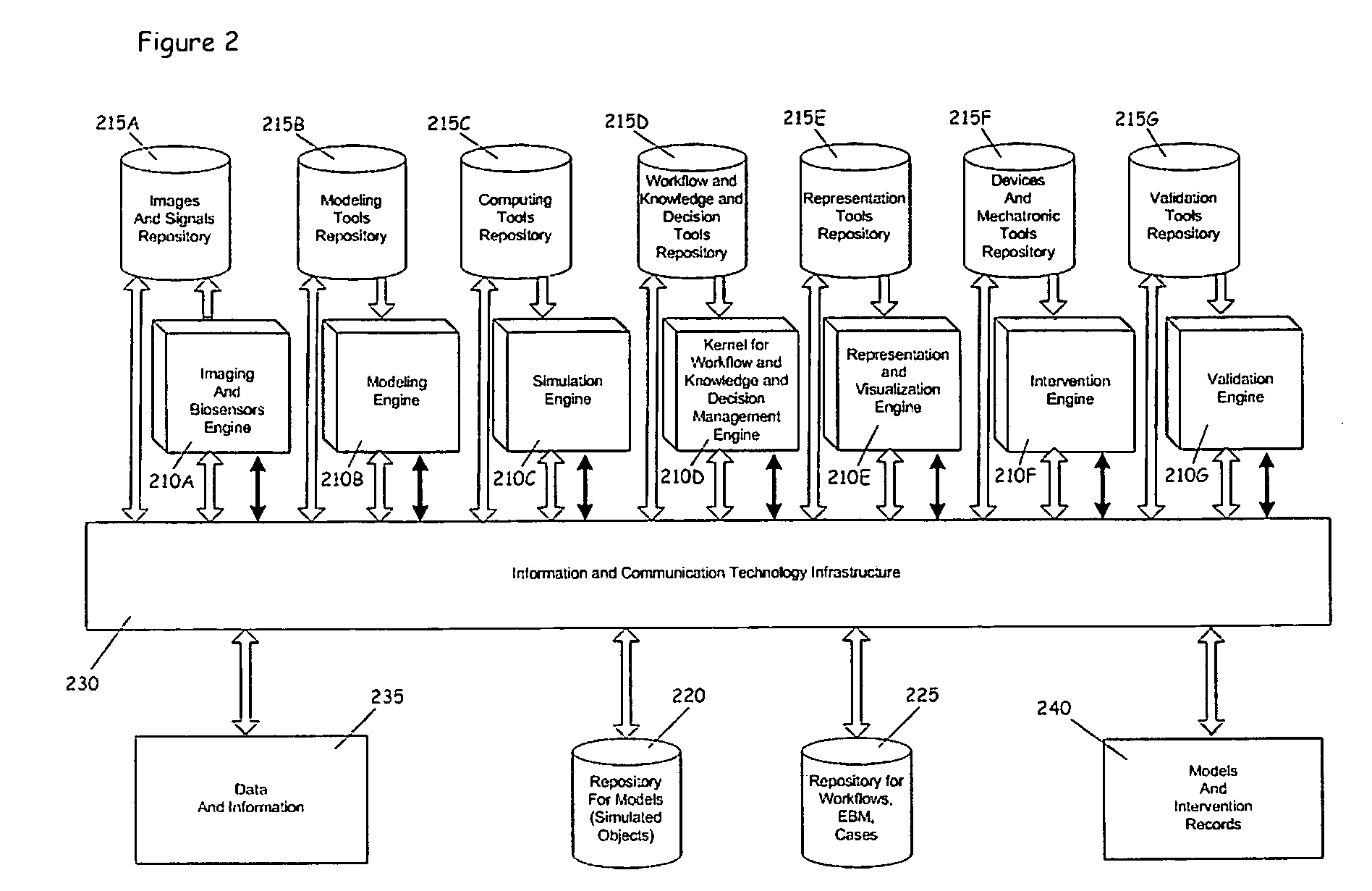
Process for comprehensive surgical assist system by means of a therapy imaging and model management system (TIMMS)
InactiveUS20090326336A1Increase successMechanical/radiation/invasive therapiesDiagnostic recording/measuringModel managementMedical record
This invention provides a process and system for a comprehensive surgical assist system, called a Therapy Imaging and Model Management System (TIMMS), which combines and integrates all of the necessary information and communication technology; workflow analysis, data processing and data synthesis; interactive interfaces between surgeon and mechatronic devices; and, cognitive agents; to provide comprehensive assistance and guidance throughout complex medical and surgical therapies, such as image guided surgery. The components of this invention, which are modular, scalable and may be distributed in location, act synergistically to provide functionality and utility that exceeds the sum of its individual parts.A method of performing surgery on a patient comprising the step of comparing a chosen patient's data to statistical data in a repository of patient data to develop a patient specific model, wherein the data comprises information from two or more sub databases selected from the group consisting of workflow data, electronic medical records, diagnostic data, biological data, measurement data, anatomical data, physiological data, genetic data, molecular data, imaging data, chemical data, clinical laboratory data, simulated data, coordinate data and surgical result and wherein the patient specific model aids in the preoperative, operative or post operative phase of surgery performed in real time on the patient.
Owner:LEMKE HEINZ ULRICH +1
Systems and methods for monitoring behavior informatics
InactiveUS20030100998A2Improve statistics performanceExpand selectionDrug and medicationsBiostatisticsMulti dimensionalOrganism
Abstract of Disclosure A system and method used to assess animal behavior includes a module having sensors that collects a variety of physical and biological data from a test subject. Interpretation of the data is provided to assess the test subject's behavior, neurology, biochemistry and physiology. The module is useful in observing the effects of a drug on the test animal and providing information on the drug's signature. Another advantage is module's portability that allows it to be used in standard laboratory cages. This portability allows the animal to be tested in its own habitat, that can reduce any erroneous data due to stressing the animal when removed to a test cage. Additionally, the module's design allows for parallel data collection and interpretation from several laboratory animals undergoing different experiments. Multi-dimensional modeling of the test subject based the system's interpretation of the data allows pattern recognition of the drug signature, and predictive drug analysis.
Owner:CARNEGIE MELLON UNIV +1
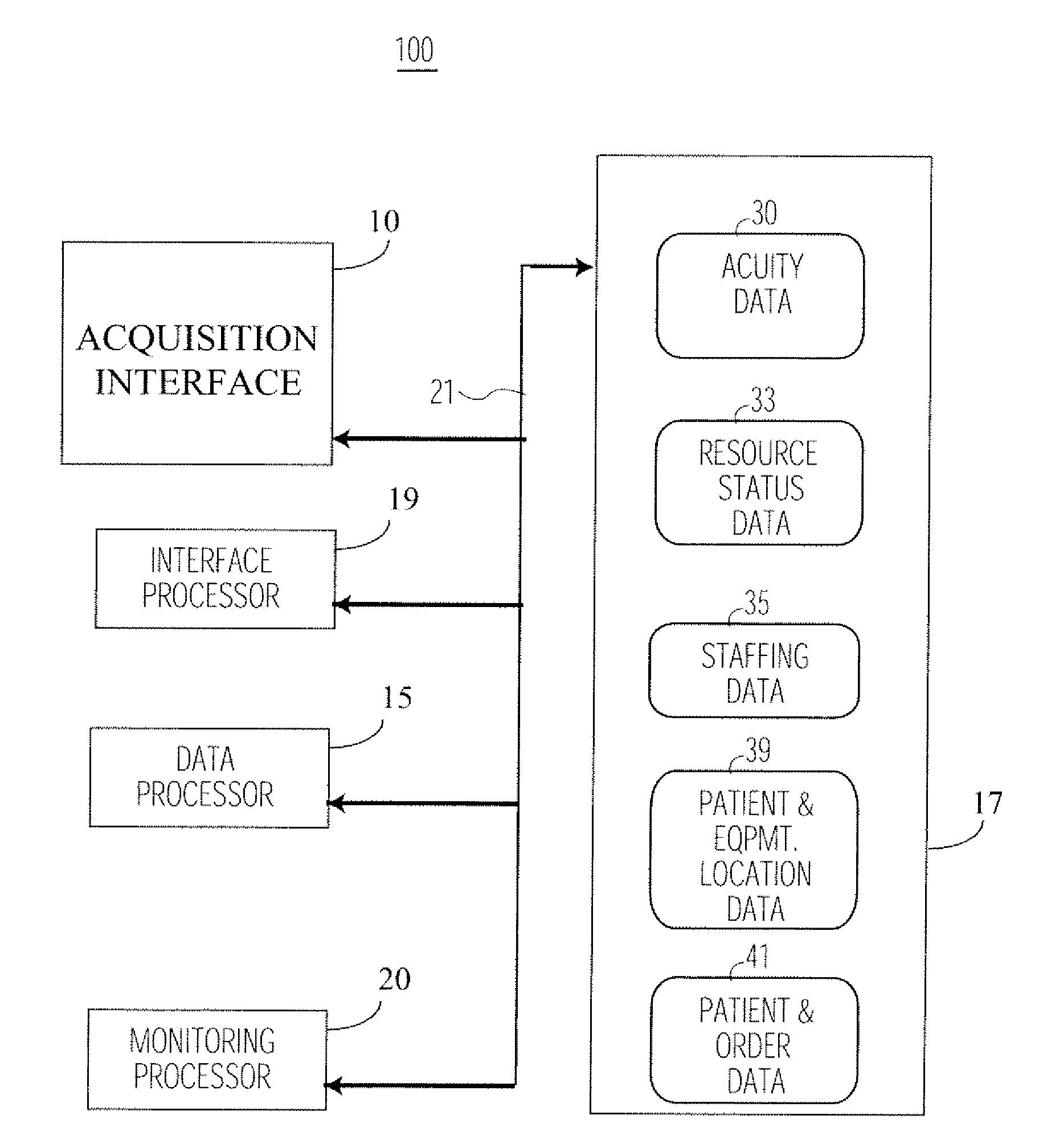
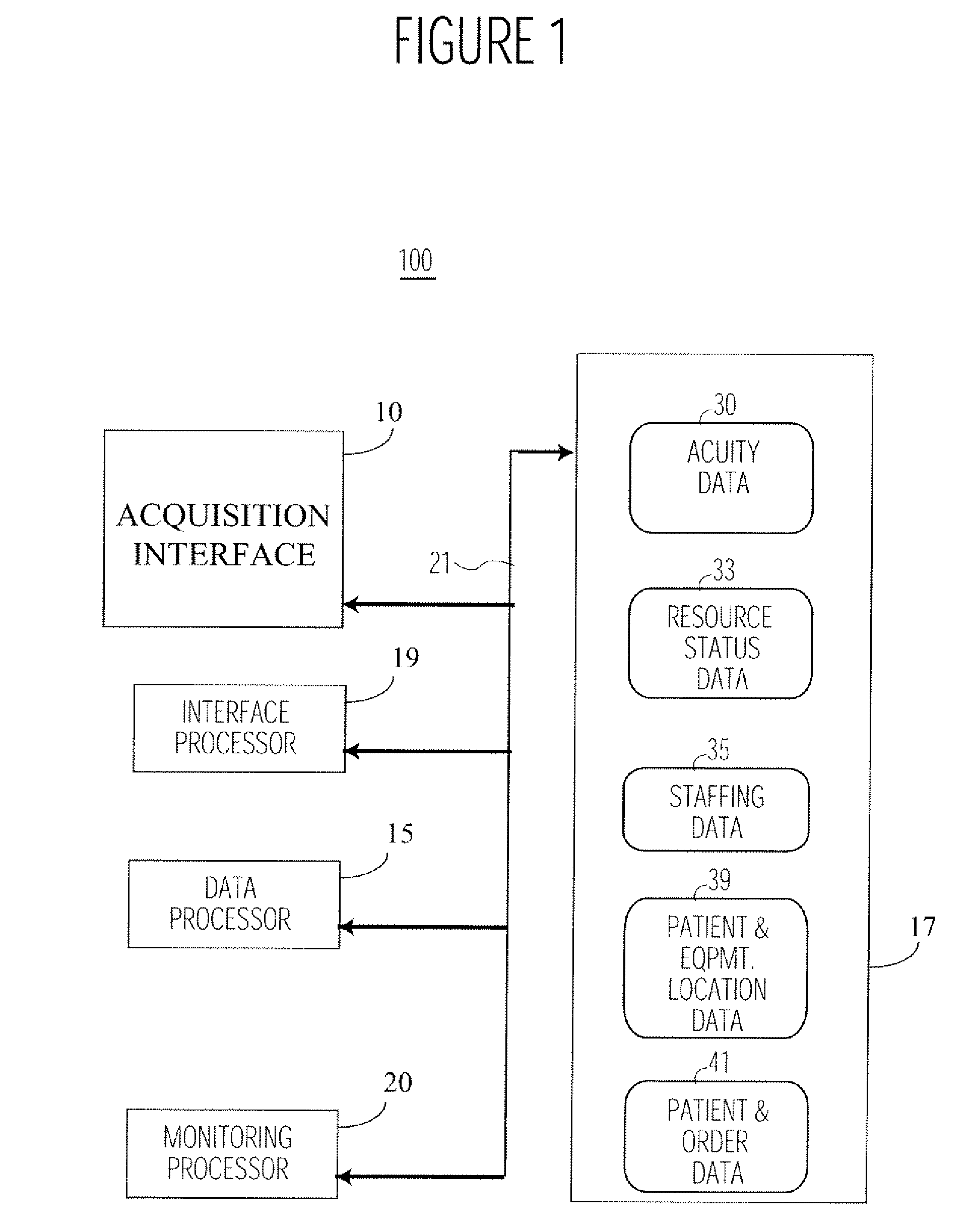
System for Monitoring Healthcare Related Activity In A Healthcare Enterprise
A system provides comprehensive patient and resource status information (manpower and equipment required to maintain optimal patient care) in an organization (e.g., a hospital) for use in adjusting resources to meet existing and future conditions. A system for monitoring activity in a healthcare enterprise includes an acquisition interface for acquiring acuity data representative of severity of medical condition of an individual patient, for multiple different patients. A monitoring processor monitors data identifying orders initiated for treatment to be provided to an individual patient and data identifying laboratory test results received for an individual patient, for multiple different patients. A data processor generates data representing status of healthcare activity for multiple patients in response to the data identifying orders and laboratory test results and the acuity data, for multiple different patients. An interface processor provides the data representing status of healthcare activity to a healthcare worker.
Owner:SIEMENS MEDICAL SOLUTIONS HEALTH SERVICES CORPORAT
Automated analyzer for clinical laboratory
InactiveUS20090117620A1Manual loadingBioreactor/fermenter combinationsBiological substance pretreatmentsClinical chemistryMicro perforated plate
A laboratory automation system that is capable of carrying out clinical chemistry assays, immunoassays, amplification of nucleic acid assays, and any combination of the foregoing, said laboratory automation system employing at least one of micro-well plates and deep multi-well plates as reaction vessels. The use of micro-well plates as reaction vessels enables the laboratory automation system to assume a variety of arrangements, i.e., the laboratory automation system can comprise a variety of functional modules that can be arranged in various ways. In order to effectively carry out immunoassays by means of micro-well plates, a technique known as inverse magnetic particle processing can be used to transfer the product(s) of immunoassays from one micro-well of a micro-well plate to another.
Owner:ABBOTT LAB INC
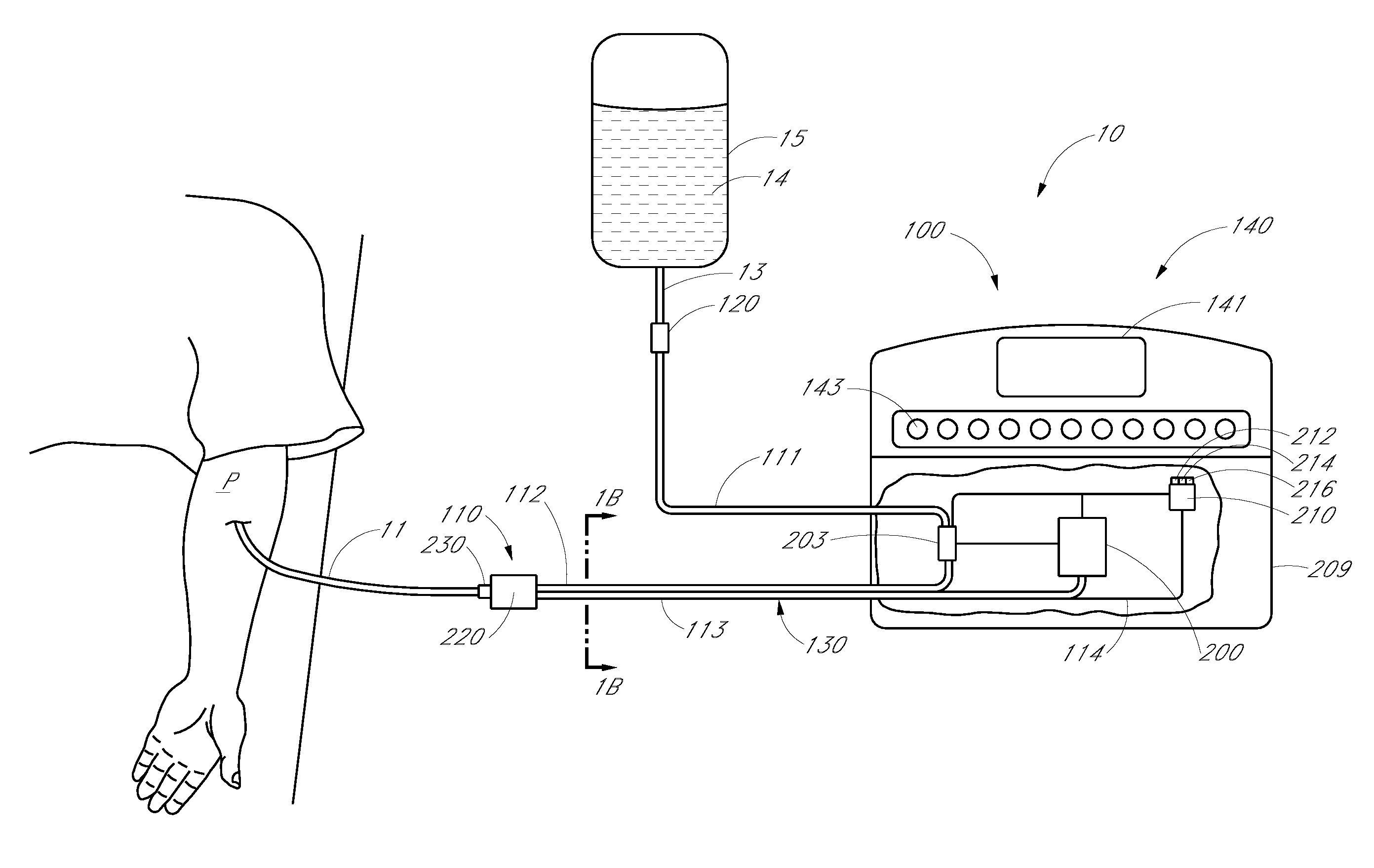
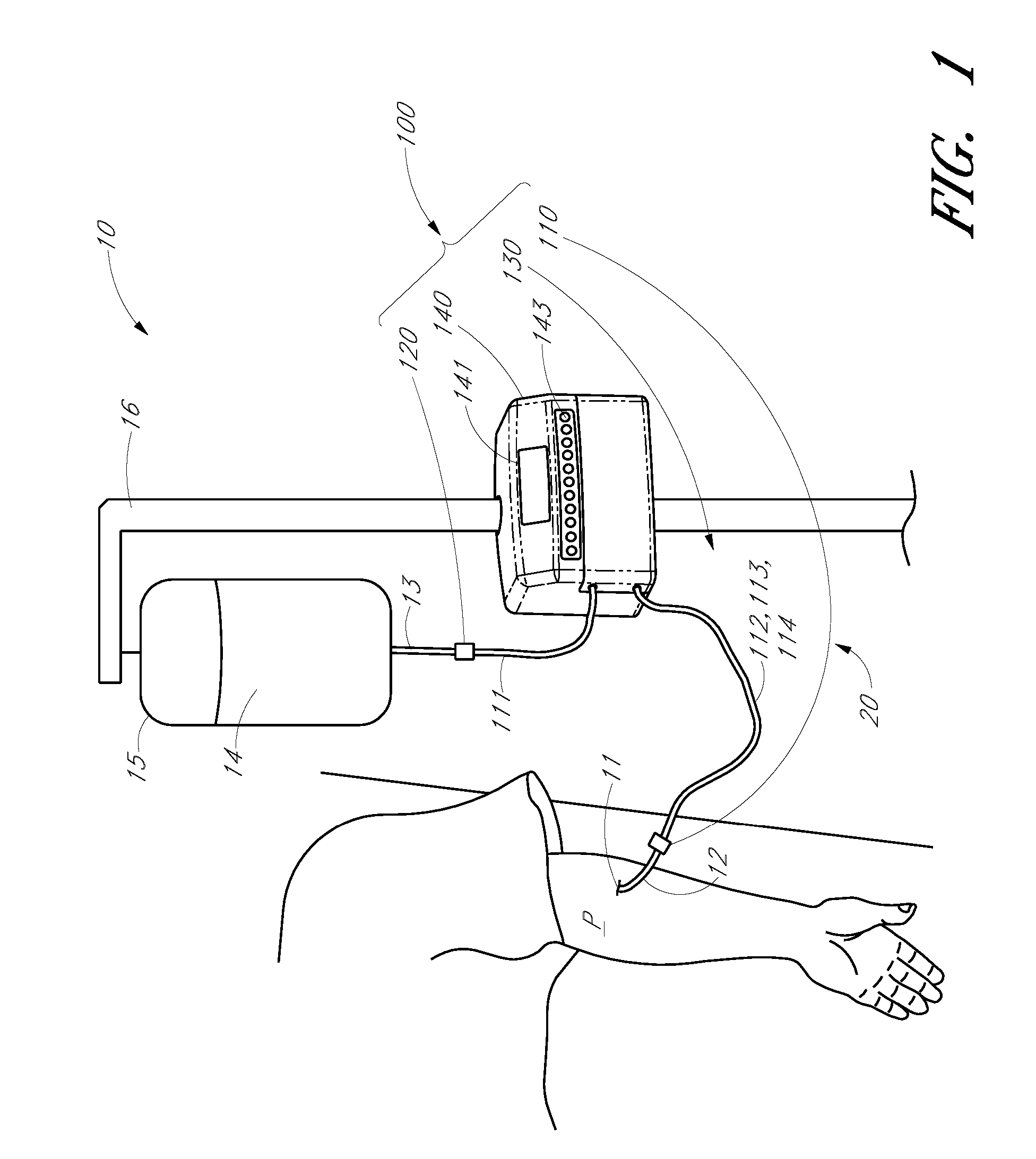
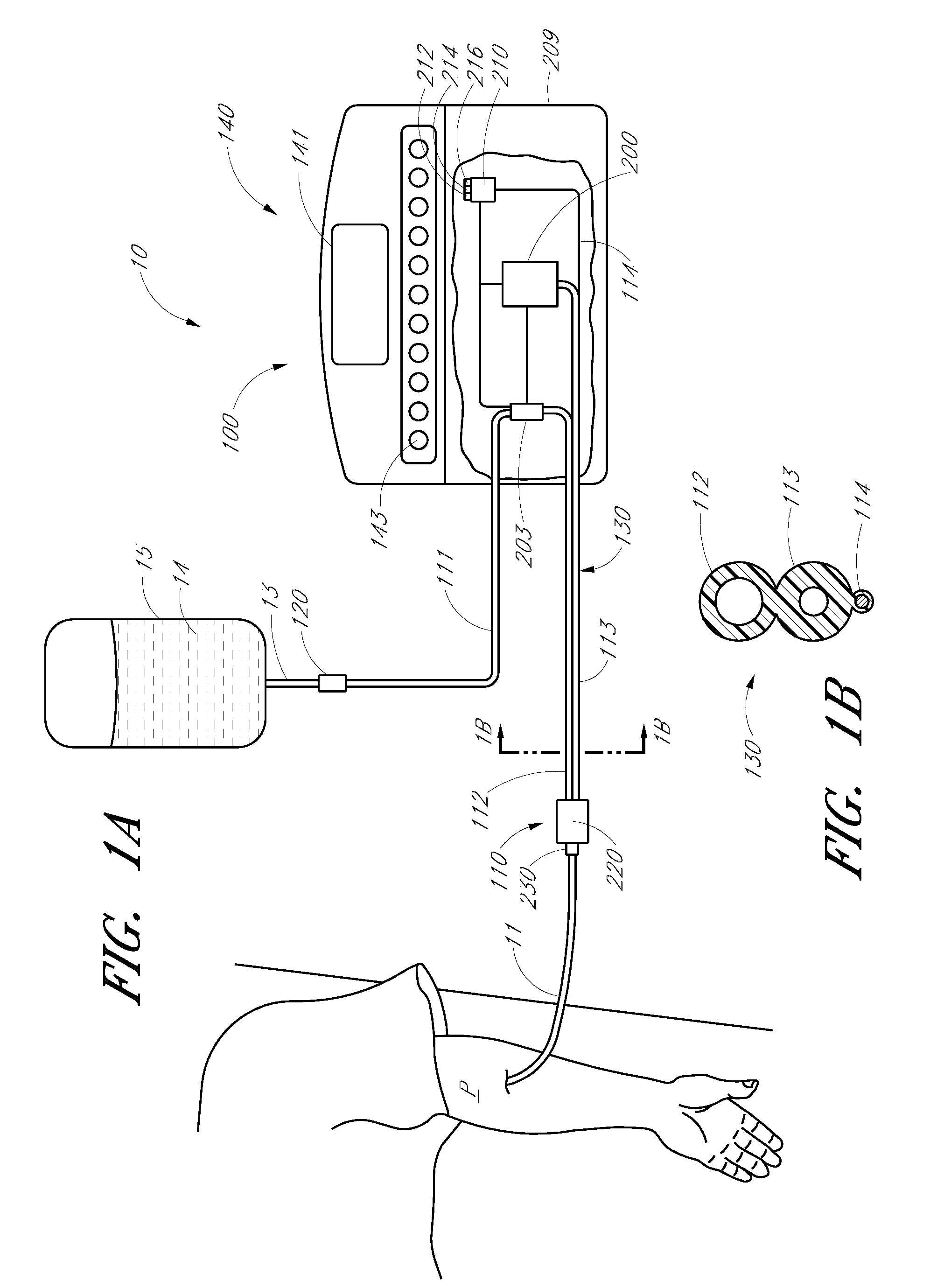
Analyte detection system with periodic sample draw and laboratory-grade analyzer
An embodiment of a system for analyzing a body fluid of a patient comprises a fluid transport network that has a patient end configured to provide fluid communication with the body fluid in the patient and a fluid delivery point spaced from the patient end. A pump system is coupled to the fluid transport network. The pump system has an infusion mode in which the pump system is operable to pump an infusion fluid toward the patient end of the fluid transport network. The pump system has a draw mode in which the pump system is operable to draw the body fluid from the patient into the fluid transport network through the patient end. The system further comprises at least one fluid holder located near the fluid delivery point of the fluid transport network. The at least one fluid holder is positioned to receive a portion of the body fluid delivered to the delivery point by the fluid transport network. A laboratory-grade fluid analyzer is configured to receive the fluid holder and to measure at least one analyte in the portion of the body fluid.
Owner:OPTISCAN BIOMEDICAL
Automated research systems and methods for researching systems
InactiveUS20090138415A1Improve understandingEasy to controlKnowledge representationInference methodsResearch softwareUser input
Systems and methods that provide for automated research into the workings of one or more studied systems include automated research software modules that communicate with domain knowledge bases, research professionals, automated laboratories experiment objects, and data analysis processes, wherein automatically selected experiment objects can be run at an automated laboratory to produce experimental results, and the subsequent data-processing providing automated guidance to a next round of experiment choice and automated research. An Experiment Director rules engine chooses Experiment Objects based on user input through a Query Manager.
Owner:HYDROJOULE LLC
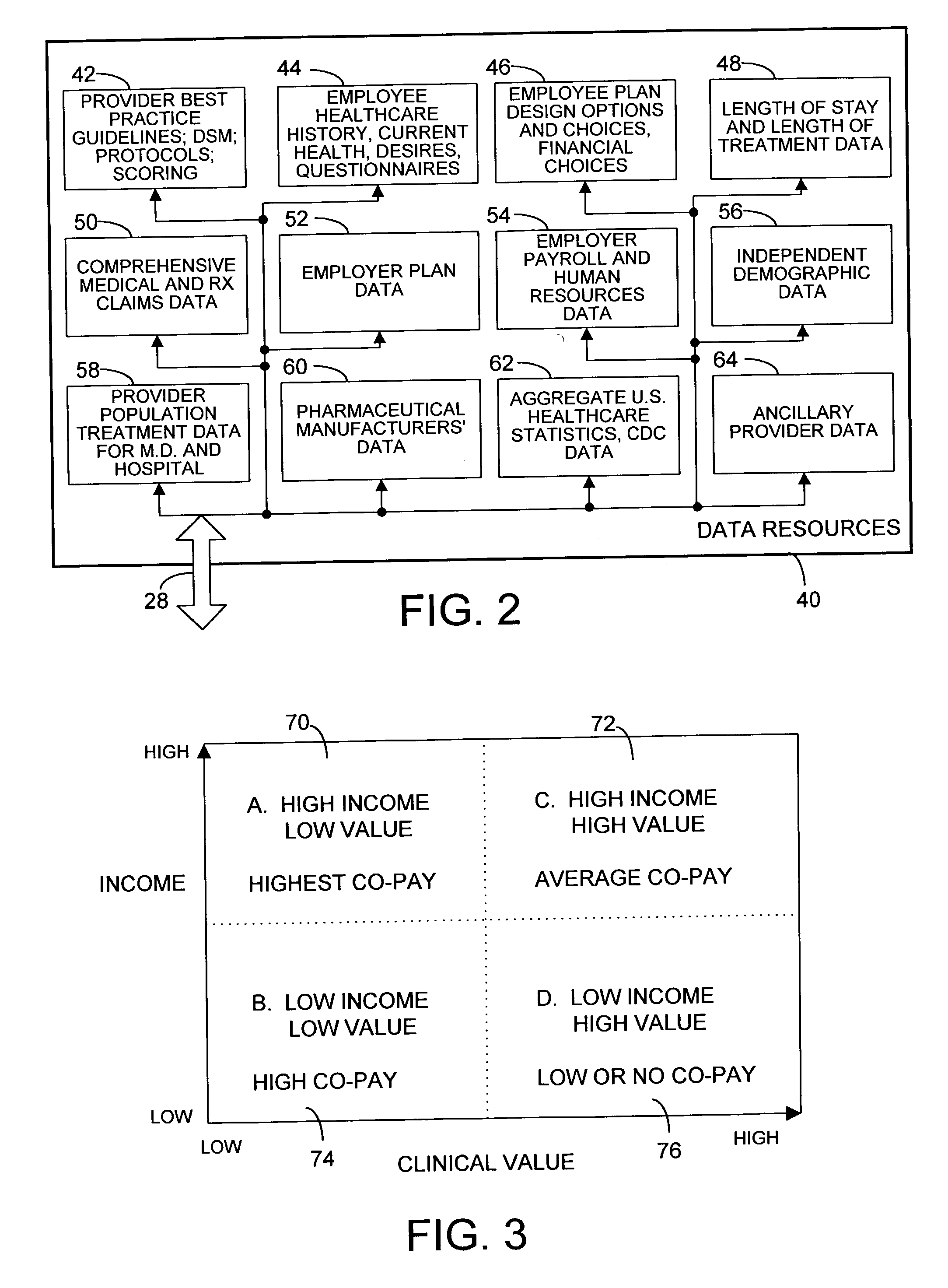
Mass customization for management of healthcare
ActiveUS20060178915A1Deter unnecessary utilizationCost optimizationDiscounts/incentivesDrug and medicationsMass customizationProgram planning
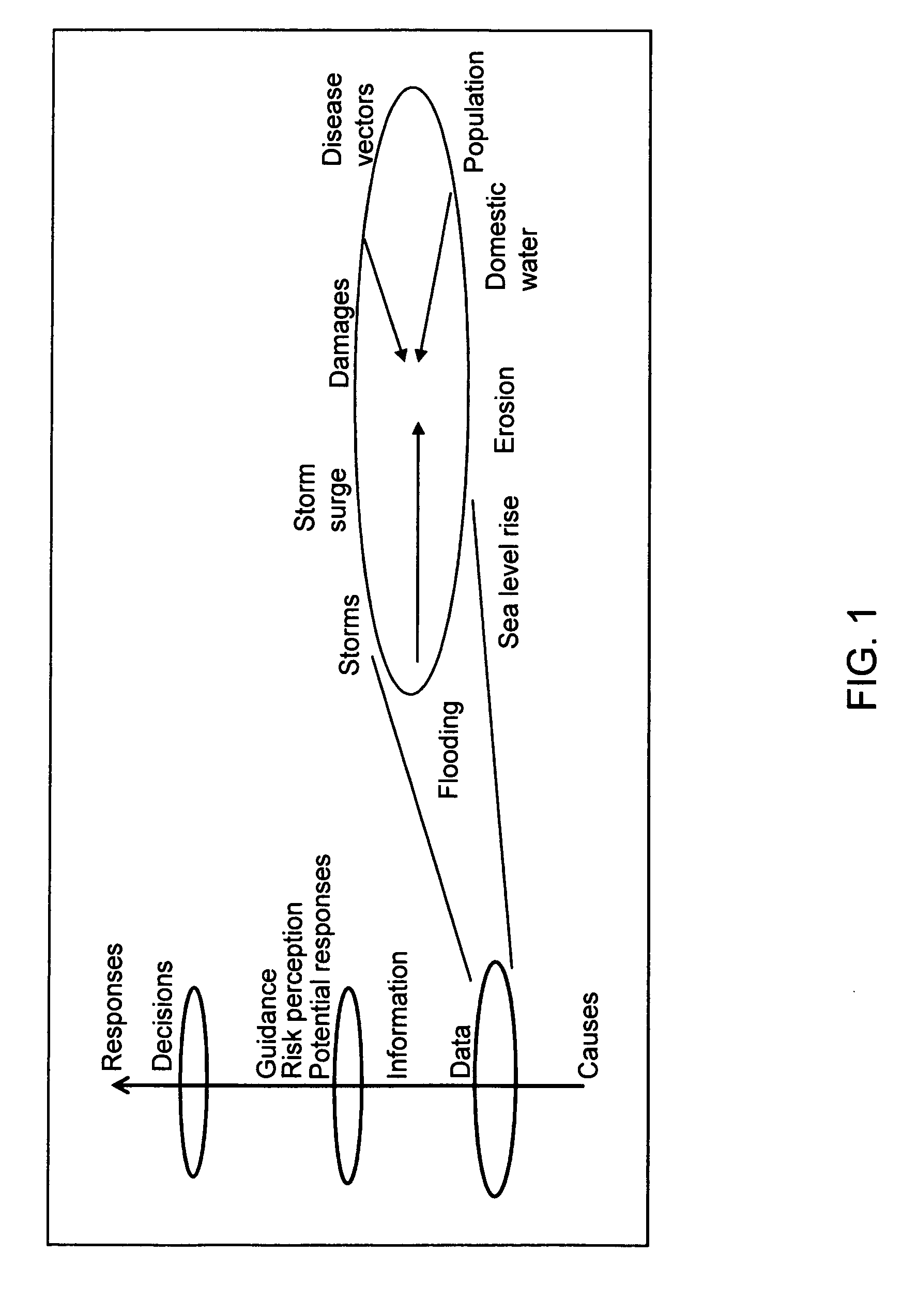
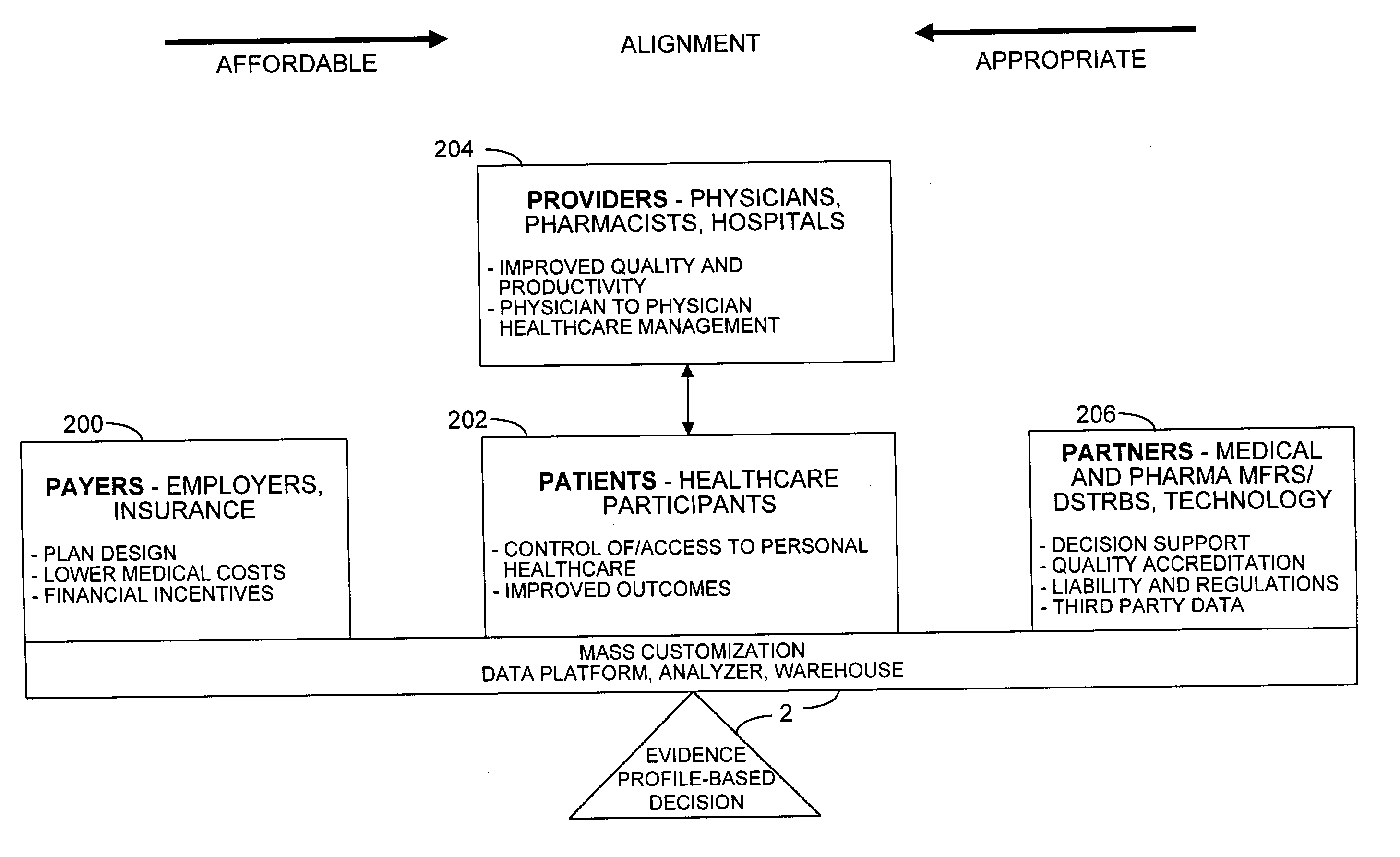
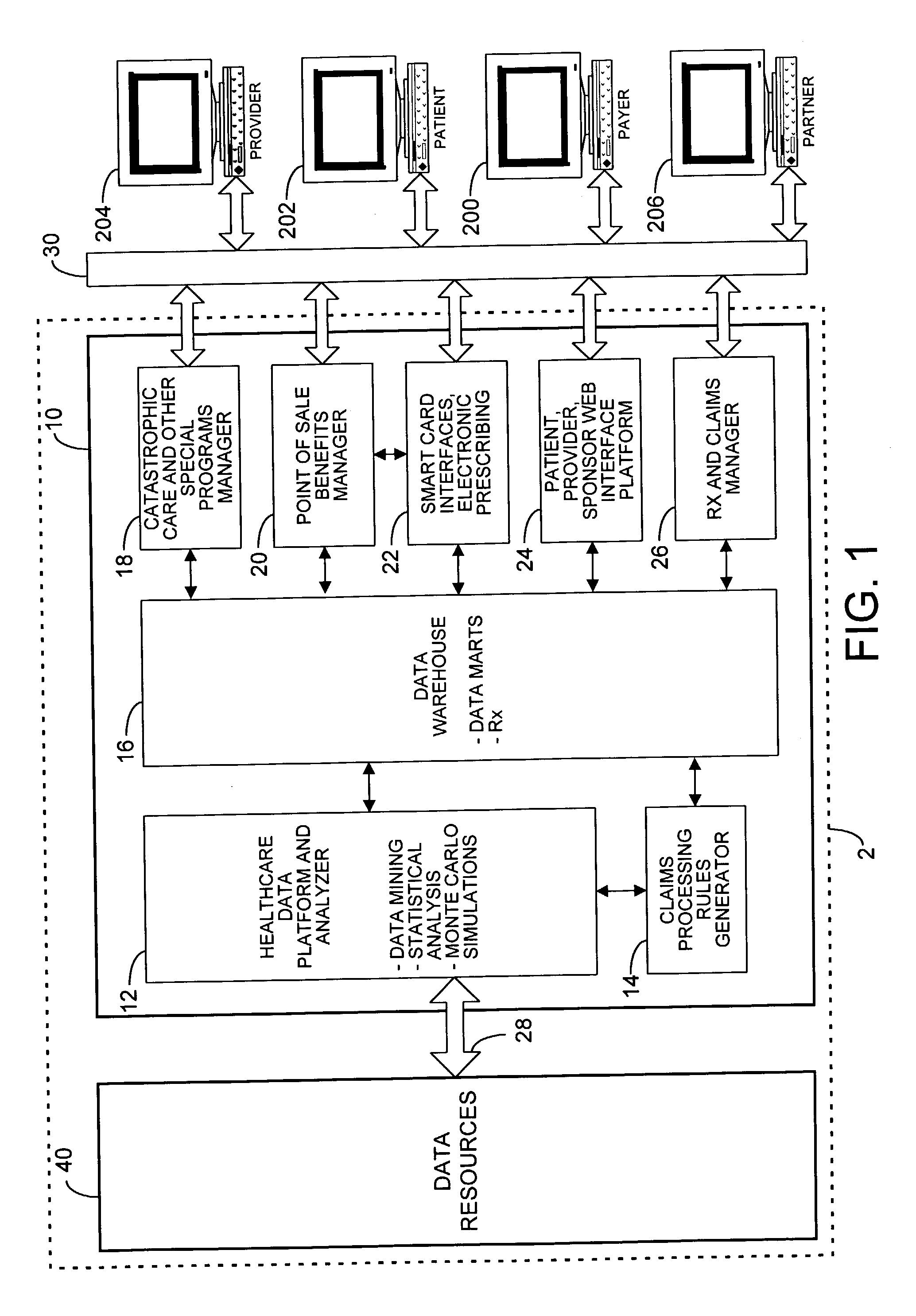
A healthcare mass customization infrastructure individualizes plan designs by incorporating demographics, income, drug history, medical history, lab values, and future genomic information for appropriate and affordable access to medications. The mass customization infrastructure results in quality outcomes for the patients, improved care and productivity for the providers, and lower medical costs for the payers.
Owner:MEDIMPACT HEALTHCARE SYST
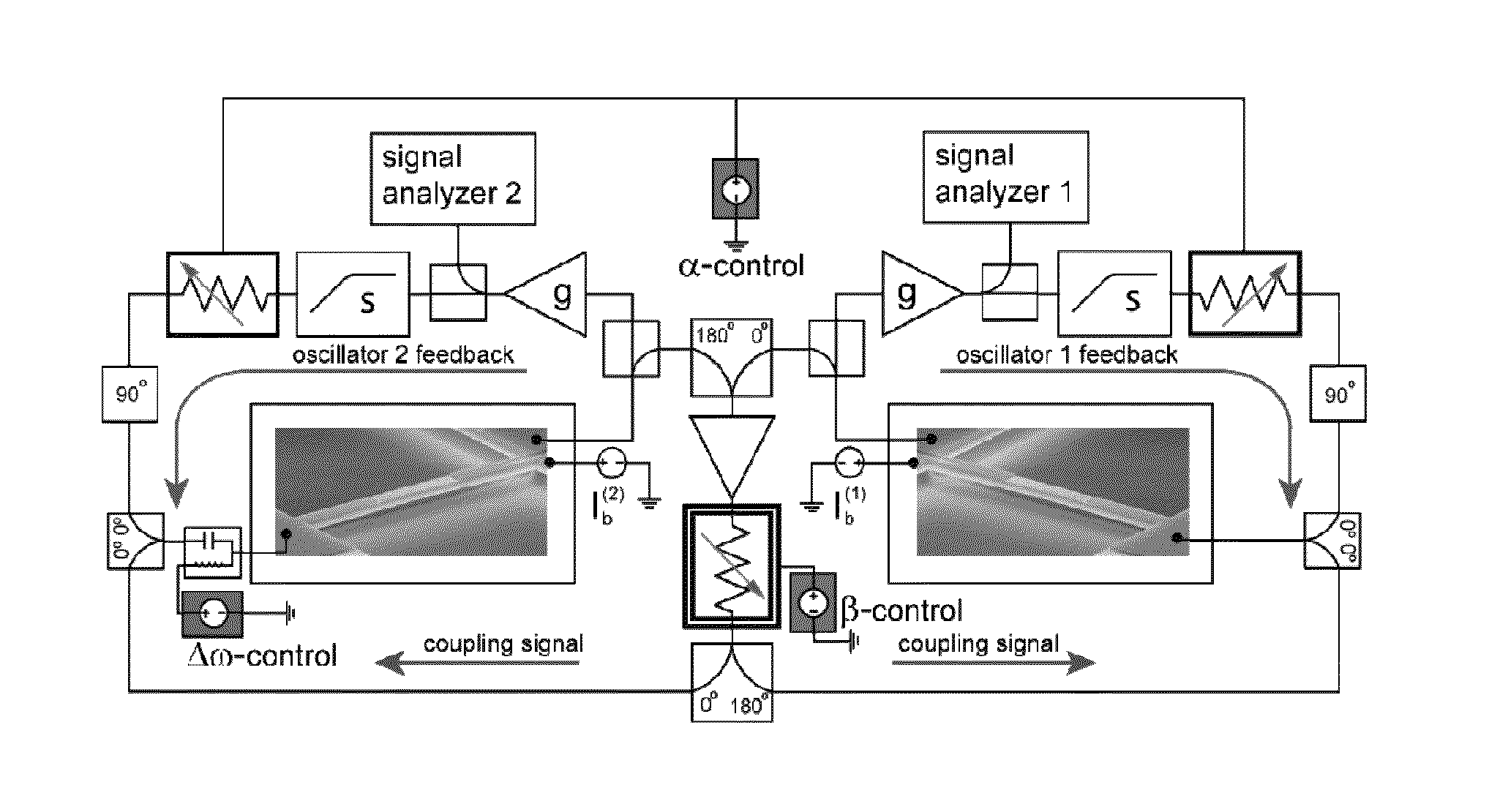
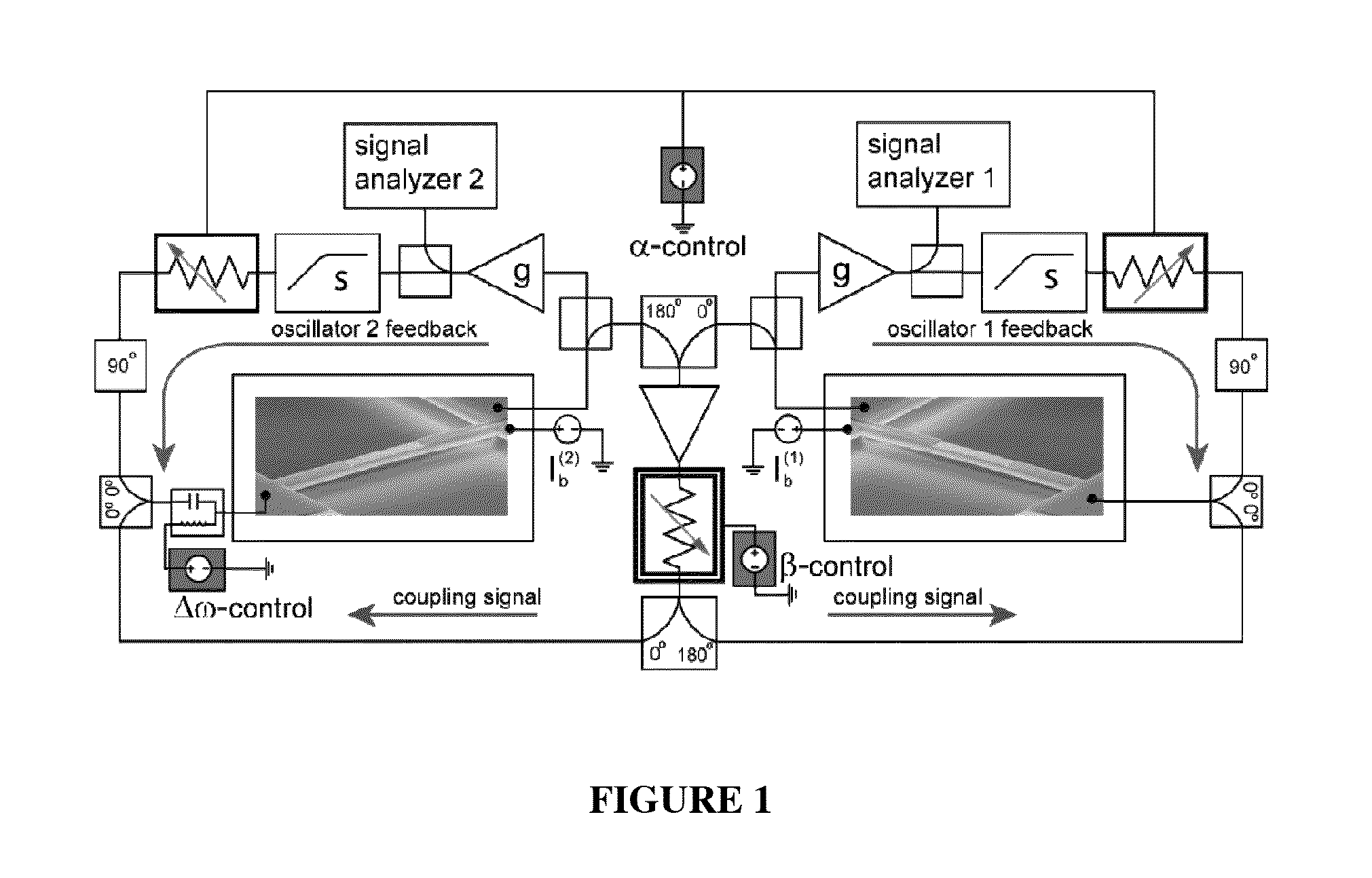
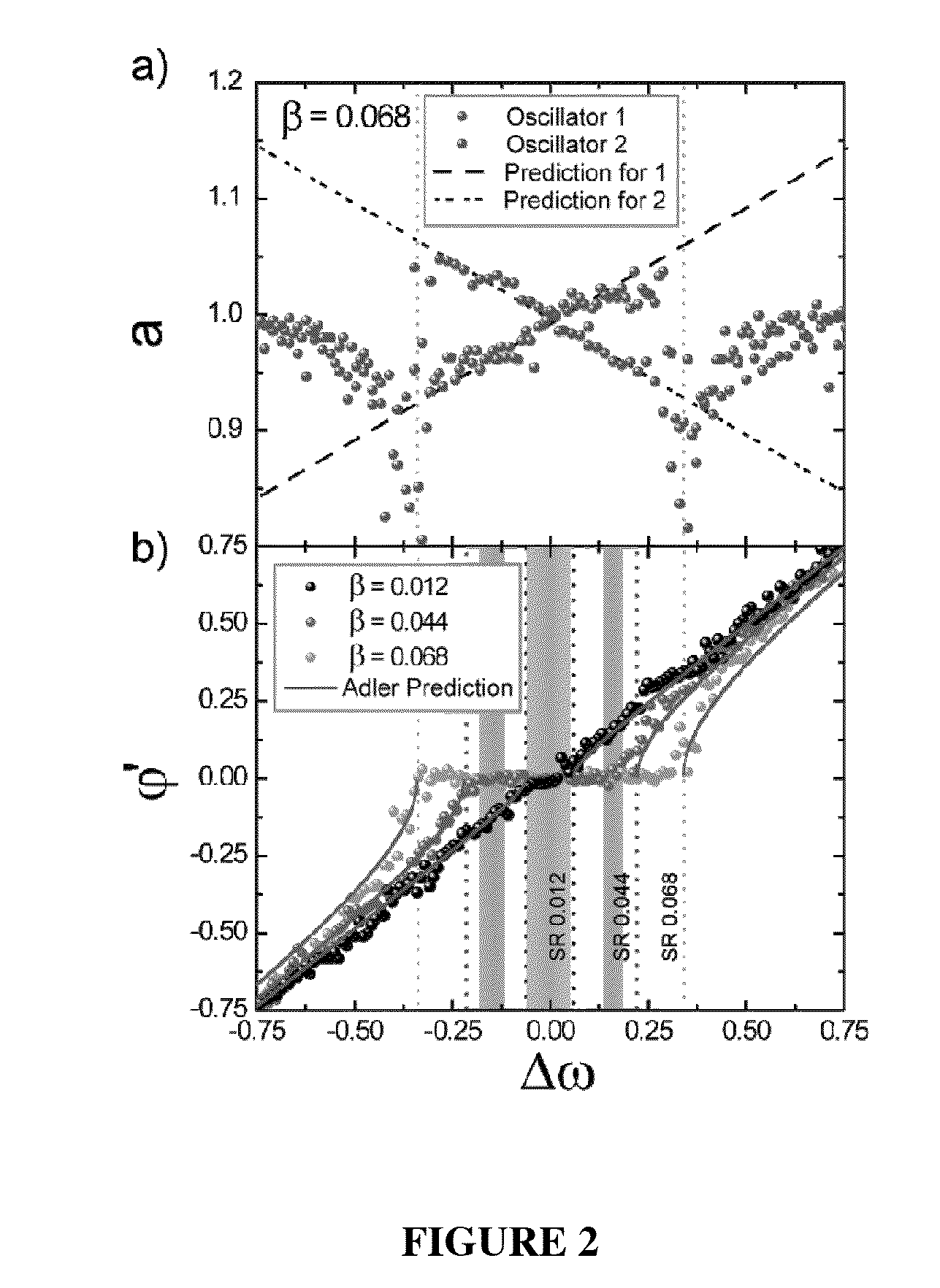
Synchronization of nanomechanical oscillators
ActiveUS20140176203A1Good for observationEasy to controlAnalysing solids using sonic/ultrasonic/infrasonic wavesPulse automatic controlPhase noiseOscillator network
Synchronization of oscillators based on anharmonic nanoelectromechanical resonators. Experimental implimentation allows for unprecedented observation and control of parameters governing the dynamics of synchronization. Close quantitative agreement is found between experimental data and theory describing reactively coupled Duffing resonators with fully saturated feedback gain. In the synchonized state, a significant reduction in the phase noise of the oscillators is demonstrated, which is key for applications such as sensors and clocks. Oscillator networks constructed from nanomechanical resonators form an important laboratory to commercialize and study synchronization—given their high-quality factors, small footprint, and ease of co-integration with modern electronic signal processing technologies. Networks can be made including one-, two-, and three-dimensional networks. Triangular and square lattices can be made.
Owner:CALIFORNIA INST OF TECH
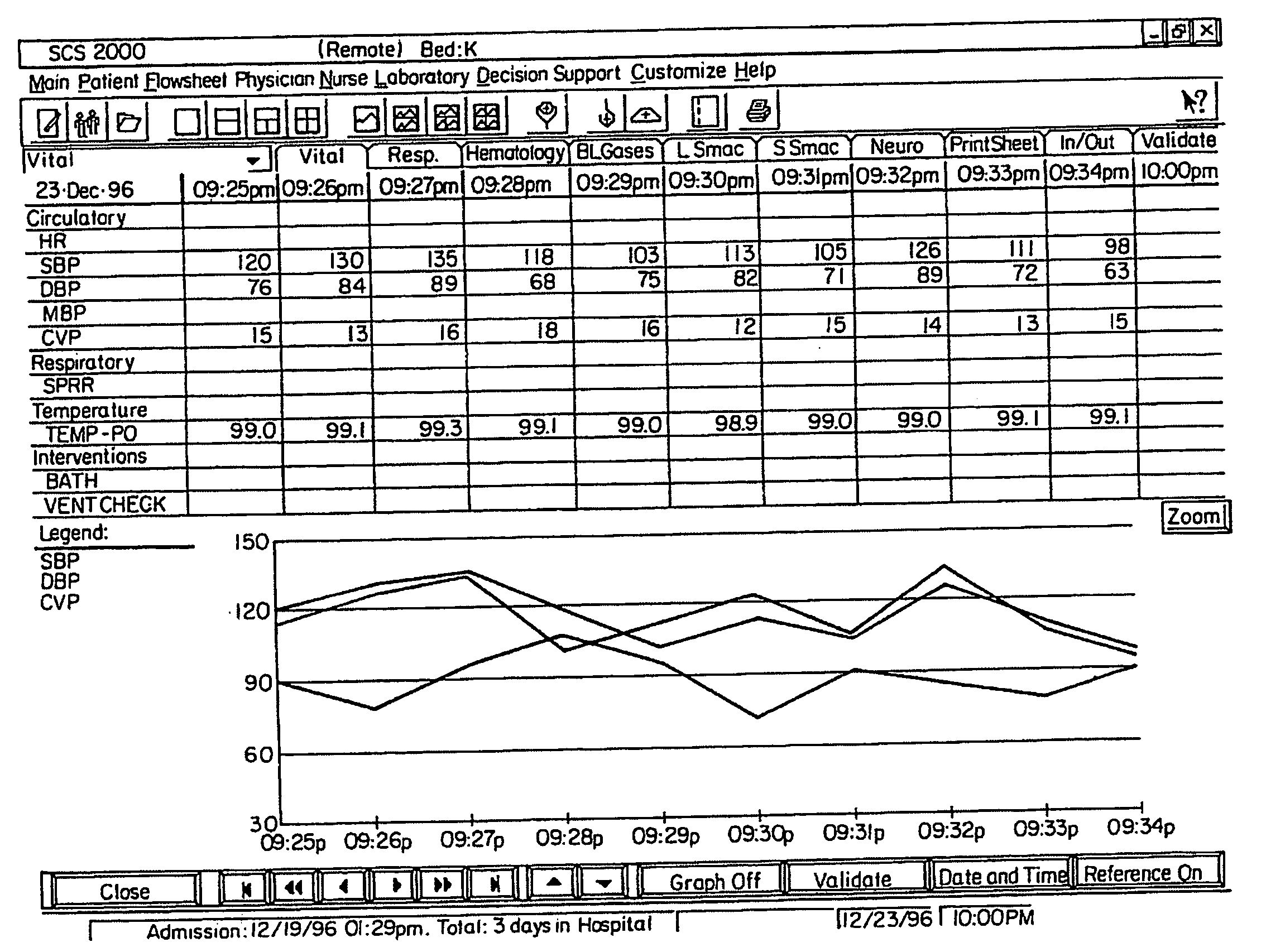
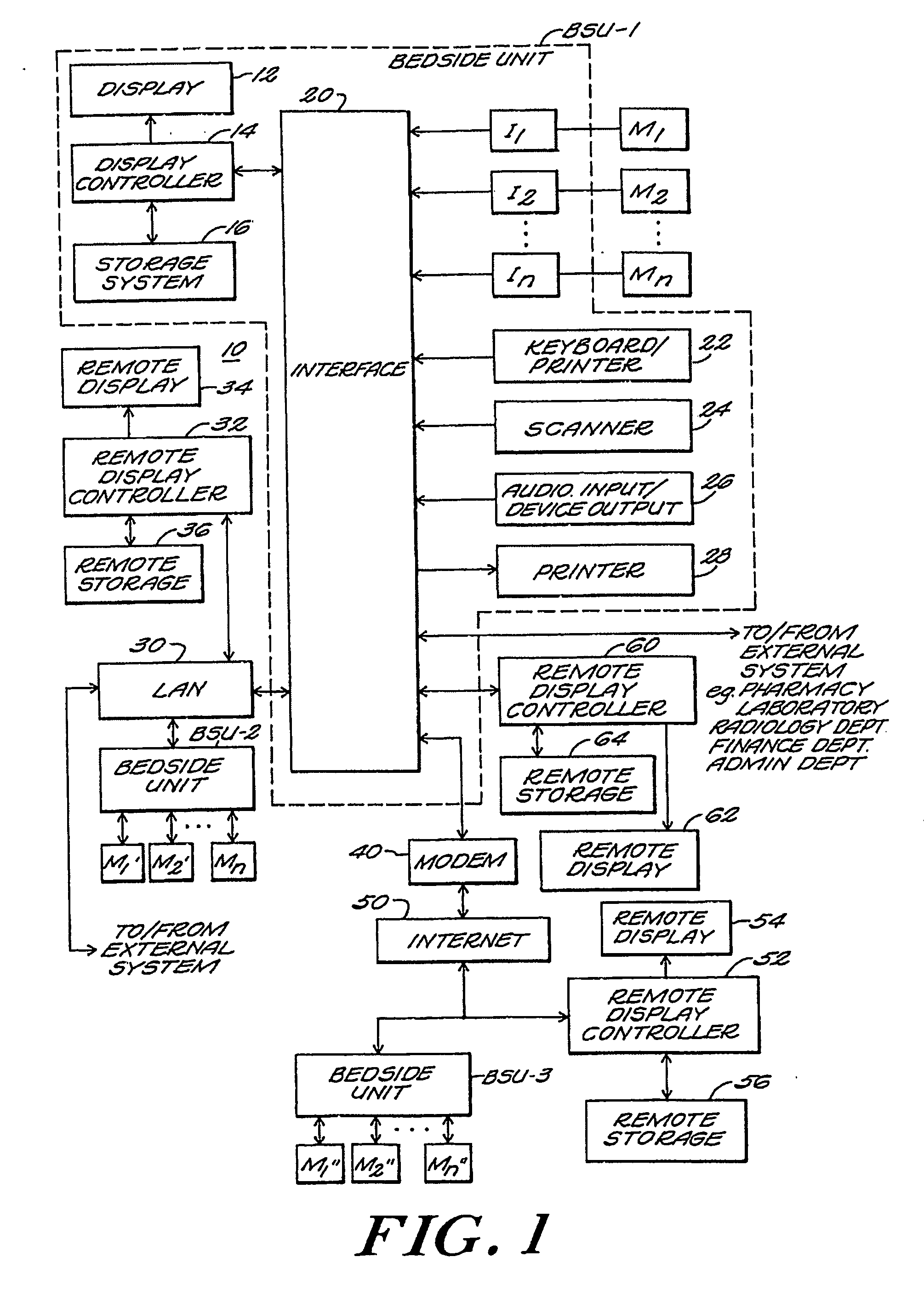
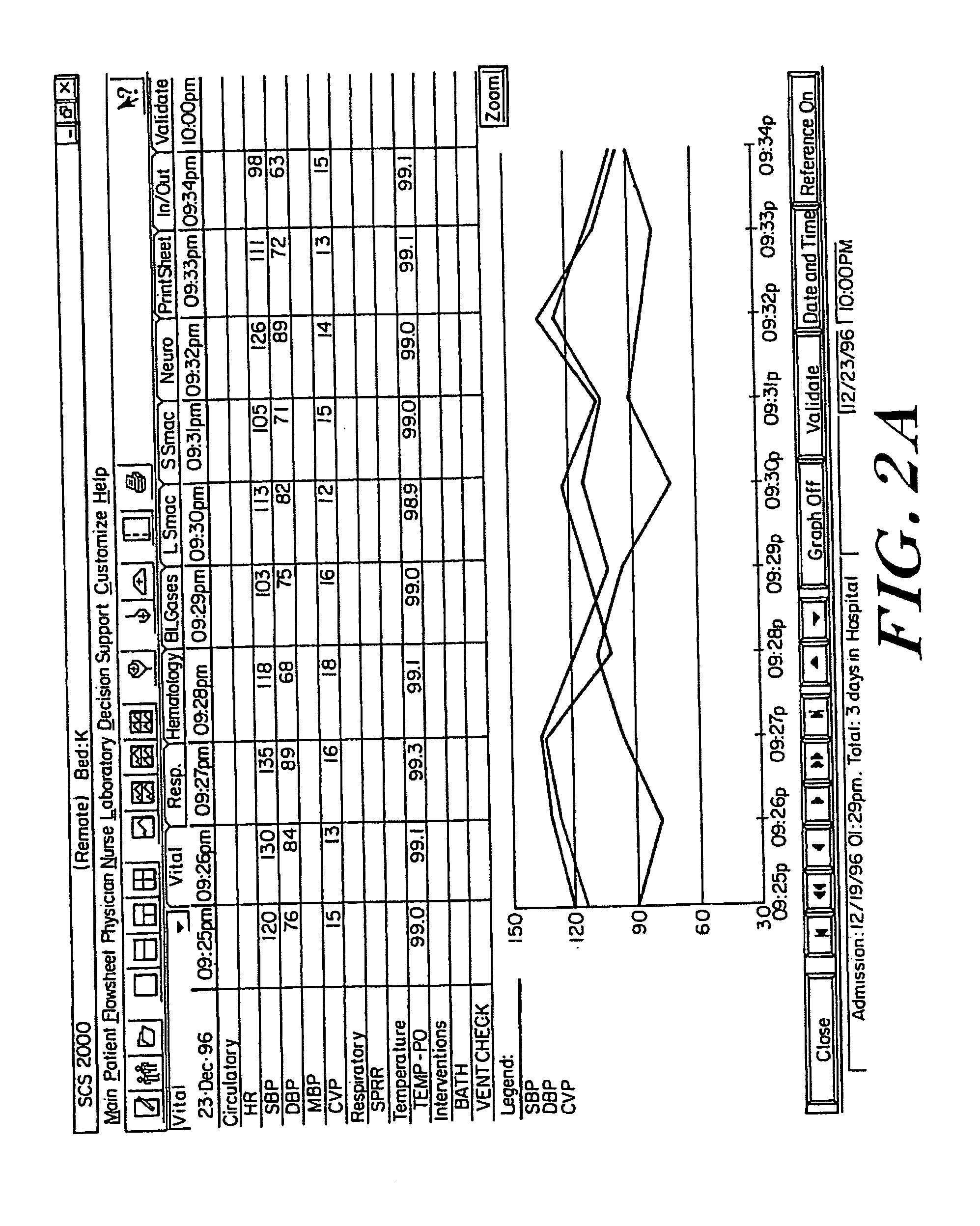
Medical information system
A medical information system receives patient data and information from various sources and displays such information in a variety of formats for use by members of a medical team in a hospital, clinic, or office. The system receives patient information from doctors, pharmacists, patient monitoring equipment, testing laboratories, and / or computer databases. Access to selected subsets of patient information is provided by user selection of specific data sets identified by job function selection icons. A member of the medical team can record observations about a patient using key words and phrases which can be supplemented with additional text for customized notation. Multiple types of patient data are selectively displayed simultaneously, and to multiple remote users. The system can access stored data according to user-specified formulae to compute a score or metric which reflects a relationship between various factors, each factor being weighted appropriately according to its significance as defined in the formula. A user can selectively display data in graphic form by “clicking” on a row of tabular data in a tabular region of the display and “dragging and dropping” that row to a graphic display region of the display.
Owner:IMDSOFT
Automated scheduling of emergency procedure based on identification of high-risk patient
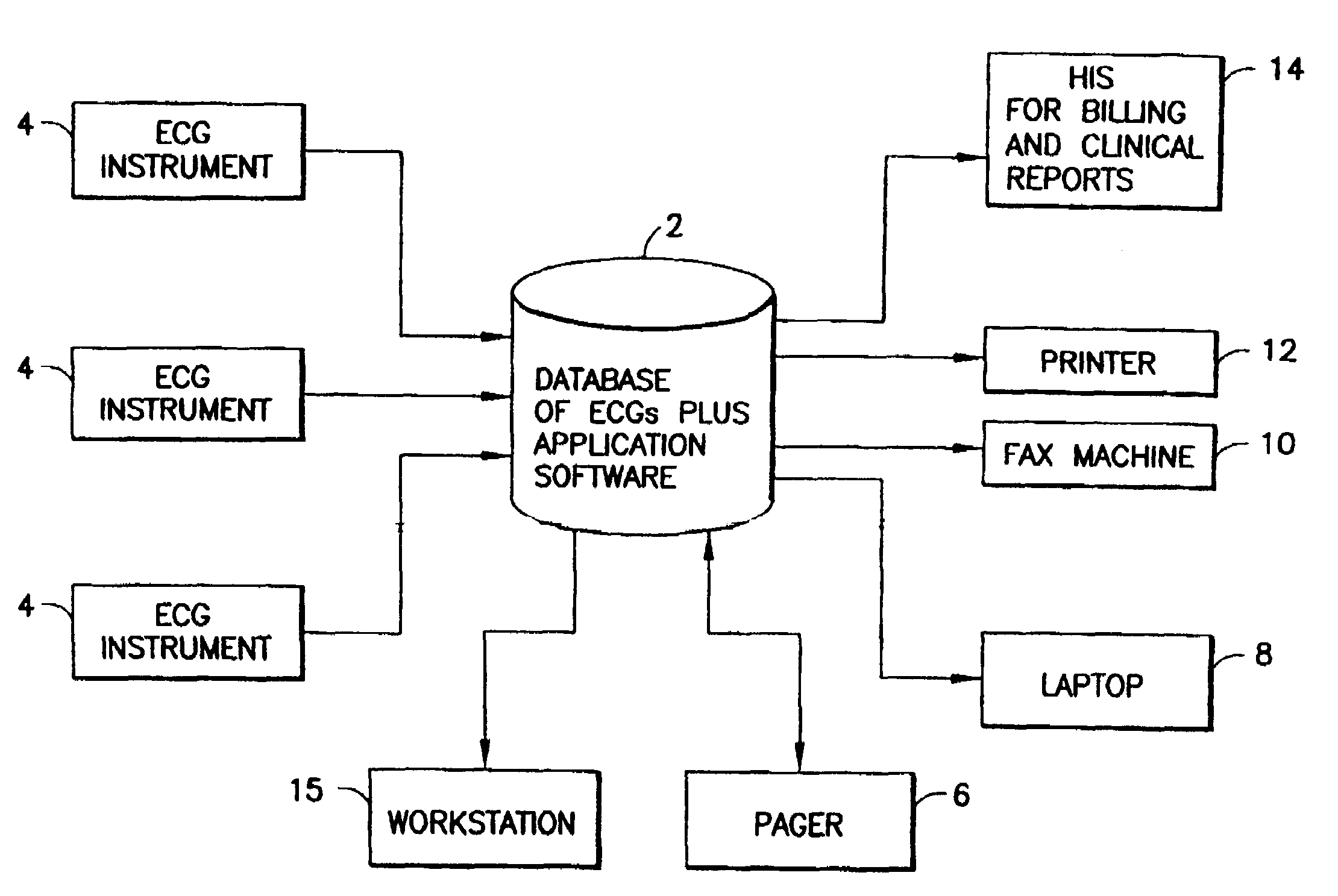
A system and a method for scheduling an emergency procedure in response to detecting that a patient has a high probability of acute myocardial infarction. The system is able to identify patients that are suspected of having acute myocardial infarction (or acute ischemia). The system uses one or more expert software tools or algorithms to analyze received ECG records. Each software tool has logic (e.g., thresholds and / or settings) for automatic routing which is configurable by the customer via a graphical user interface. If any sufficient condition for automatic routing is satisfied, the system routes the data (including the underlying ECG record) and an alert to an electronic device which is accessible by the cardiologist “on call” via a bidirectional pager. If the cardiologist decides that the requested emergency treatment or procedure should be performed, the system accesses the schedules of all associated catheterization labs across multiple hospitals to identify a lab having optimum time-to-treatment. Then the system automatically contacts the selected catheterization lab via a network to schedule the PTCA procedure.
Owner:GE MEDICAL SYST INFORMATION TECH
Pipetting station apparatus
InactiveUS6325114B1Withdrawing sample devicesMaterial analysis by optical meansPipetteMicrowell Plate
A pipette station is described for use in the field of sample analysis. The pipette station increases the rate and ease with which a liquid may be manipulated into and out of sample carriers such as microwell plates. The pipette station includes shafts in the X, Y, and Z direction which possess ball screws which are integrated with motor shafts thus improving accuracy and eliminating the need for a coupling apparatus thereby reducing the space required for the pipette station. The pipette station may be interfaced with an automated laboratory system.
Owner:VELOCITY11 +1
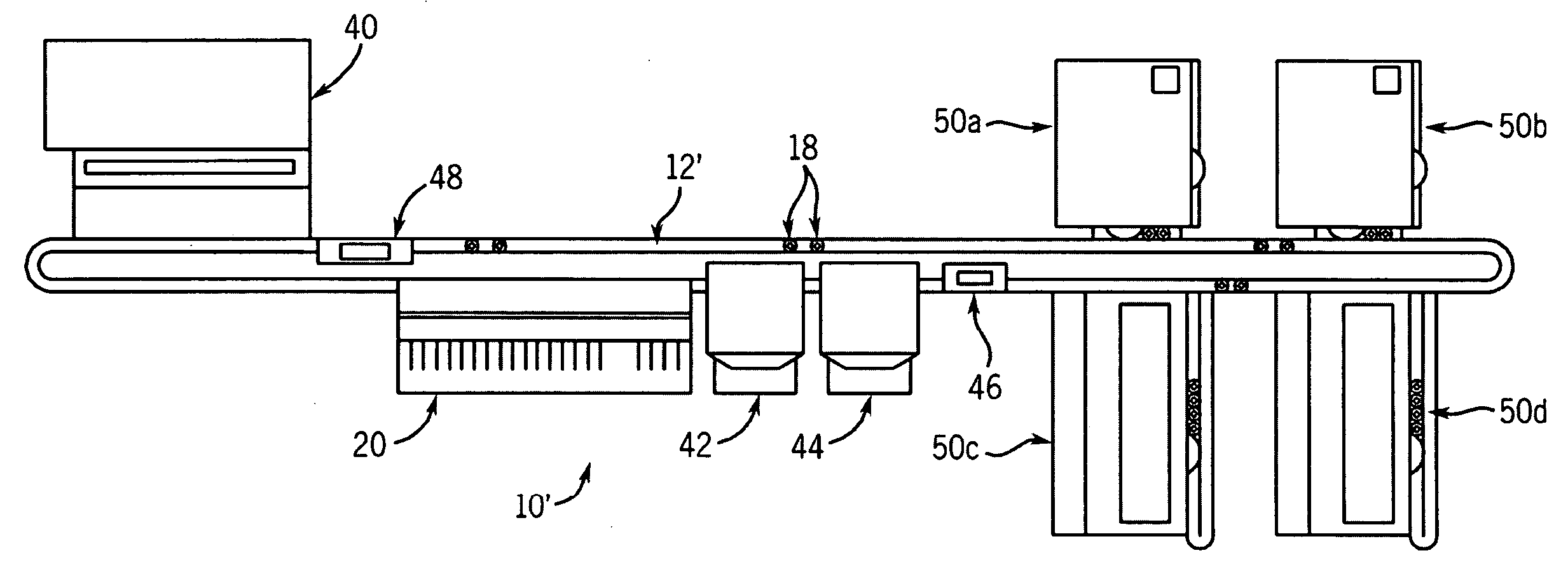
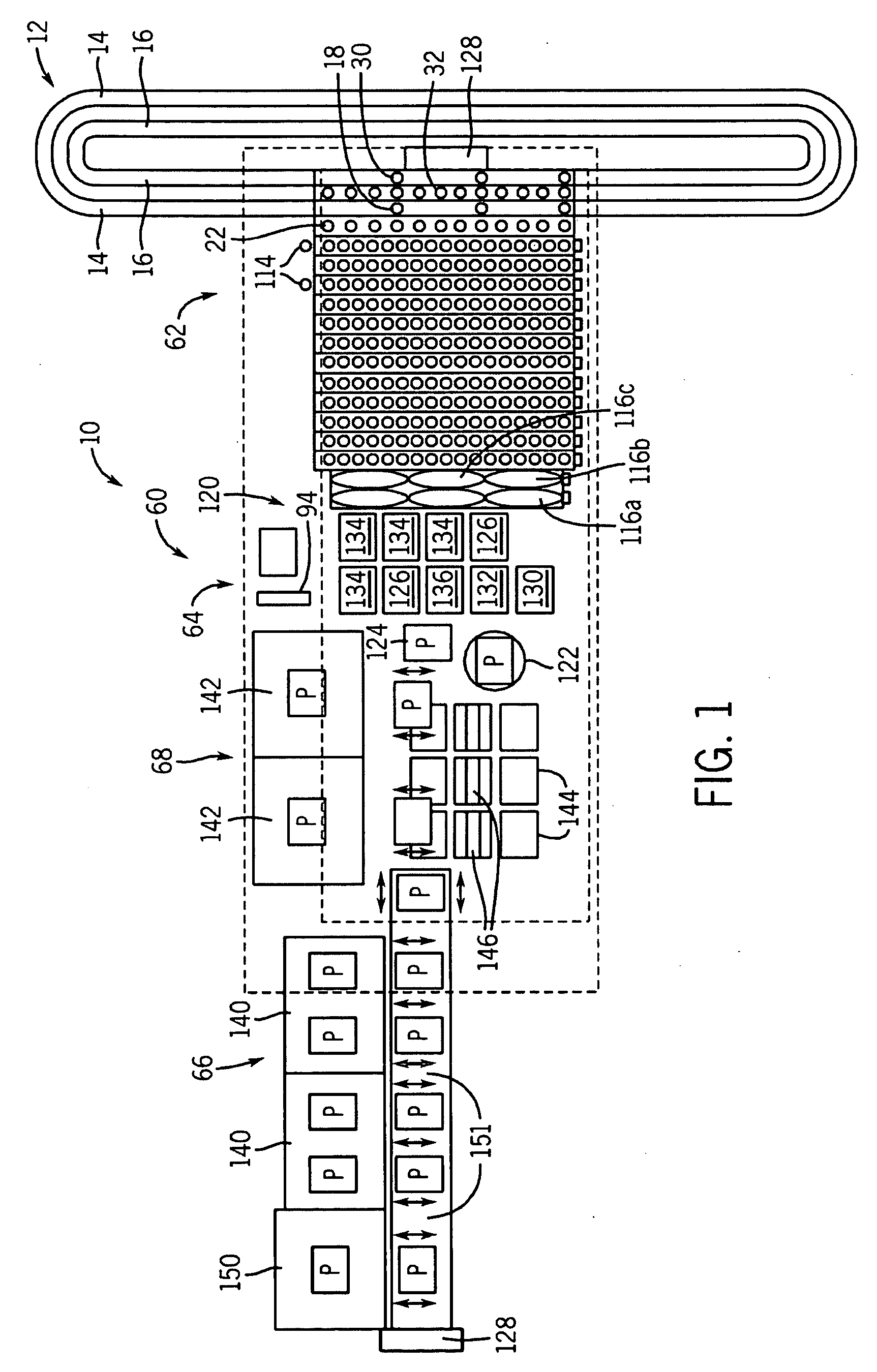
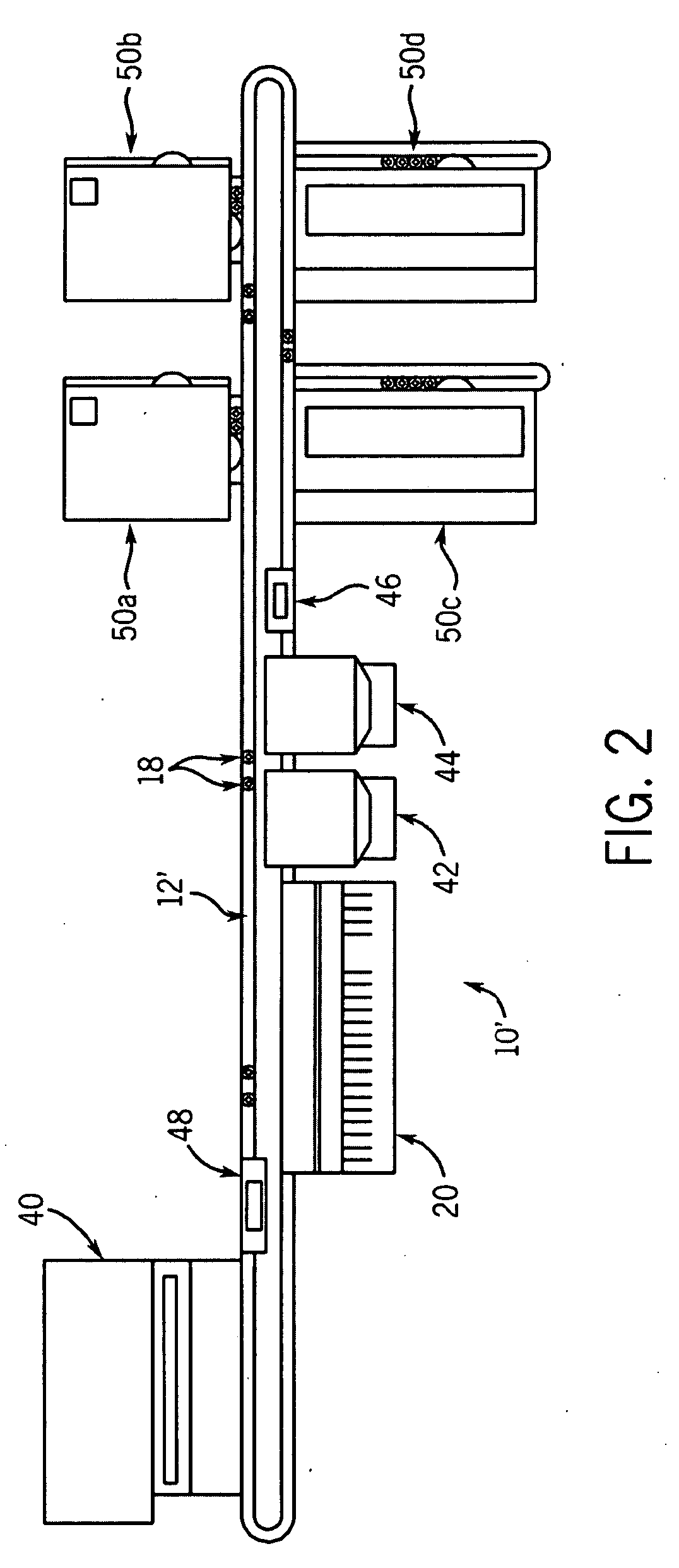
Automated processing system and method of using same
An automated processing system, particularly for use in biotechnology, which is modular in construction and allows for a wide variety of instruments to be inserted and / or removed without having to reprogram the system and methods of using such a system. This may be called "plug and play" functionality. The system may be portable without disassembly and have a generally vertical arrangement so as to utilize less useful lab space than a horizontally arranged system.
Owner:SCINOMIX
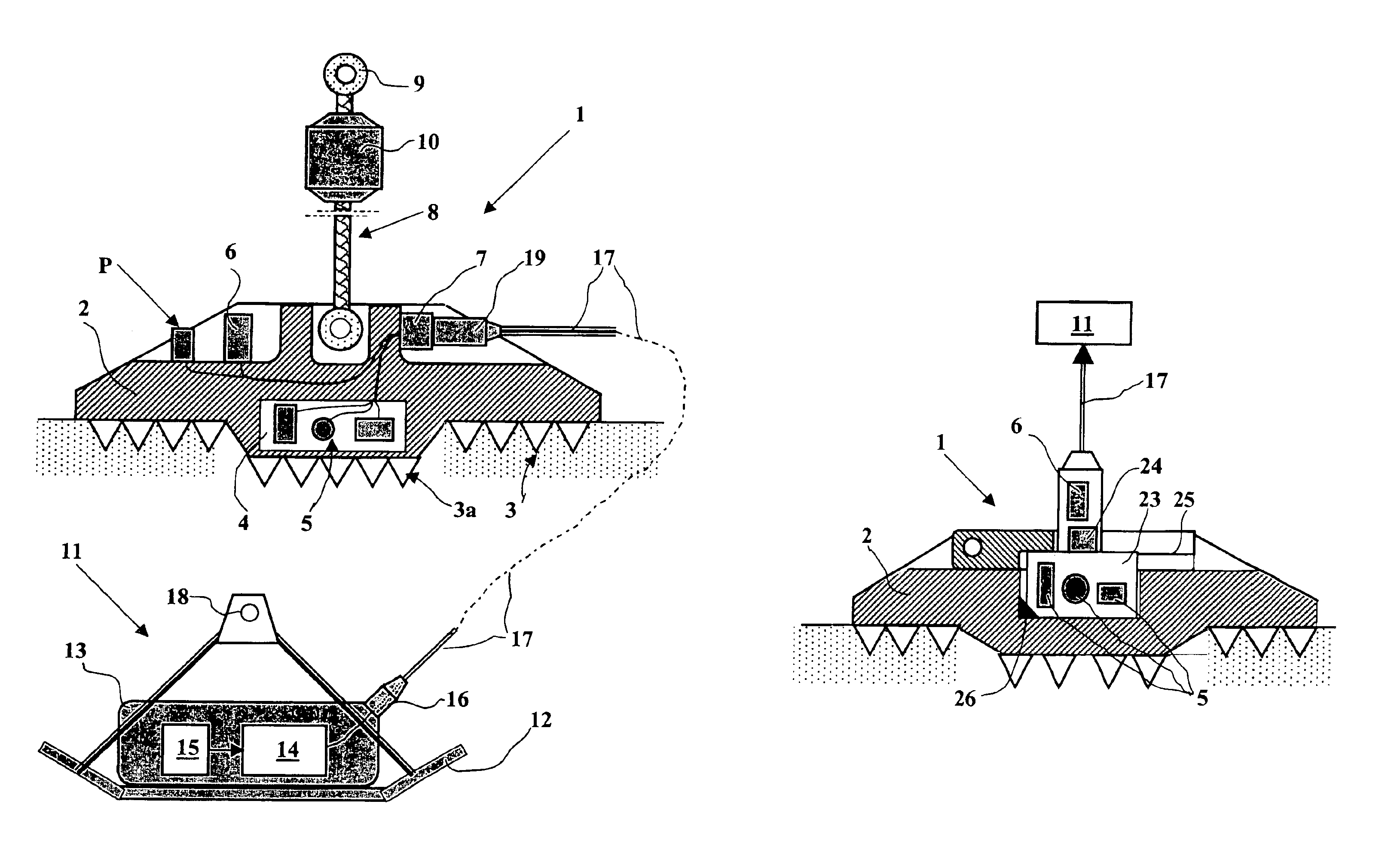
Acquisition method and device for seismic exploration of a geologic formation by permanent receivers set on the sea bottom
InactiveUS6932185B2Good flexibilityFine divisionSeismic energy generationSeismic signal receiversGeophoneHydrophone
A method and device for seismic exploration of a subsea geologic formation by pickups set on the sea bottom and intermittently connectable to active data acquisition stations (11) brought nearby. Permanent passive reception stations (1) comprising a heavy pedestal provided with housings for seismic pickups (geophones (6), hydrophone (7) which receive acoustic or seismic signals from the underlying formation are arranged at the bottom of the water body. When collection sessions for the signals received by the pickups are scheduled, mobile active acquisition stations (11) connected to permanent passive reception stations (1) are positioned at the bottom of the water body. The signals picked up are then recorded, for the time required to carry out at least one session of acquisition and recording of the acoustic or seismic signals received by the passive stations in response to the emission of seismic waves by one or more seismic sources. The mobile active acquisition stations (11) are thereafter recovered at the surface and the records acquired by each one are transferred to a central collection laboratory.
Owner:INST FR DU PETROLE
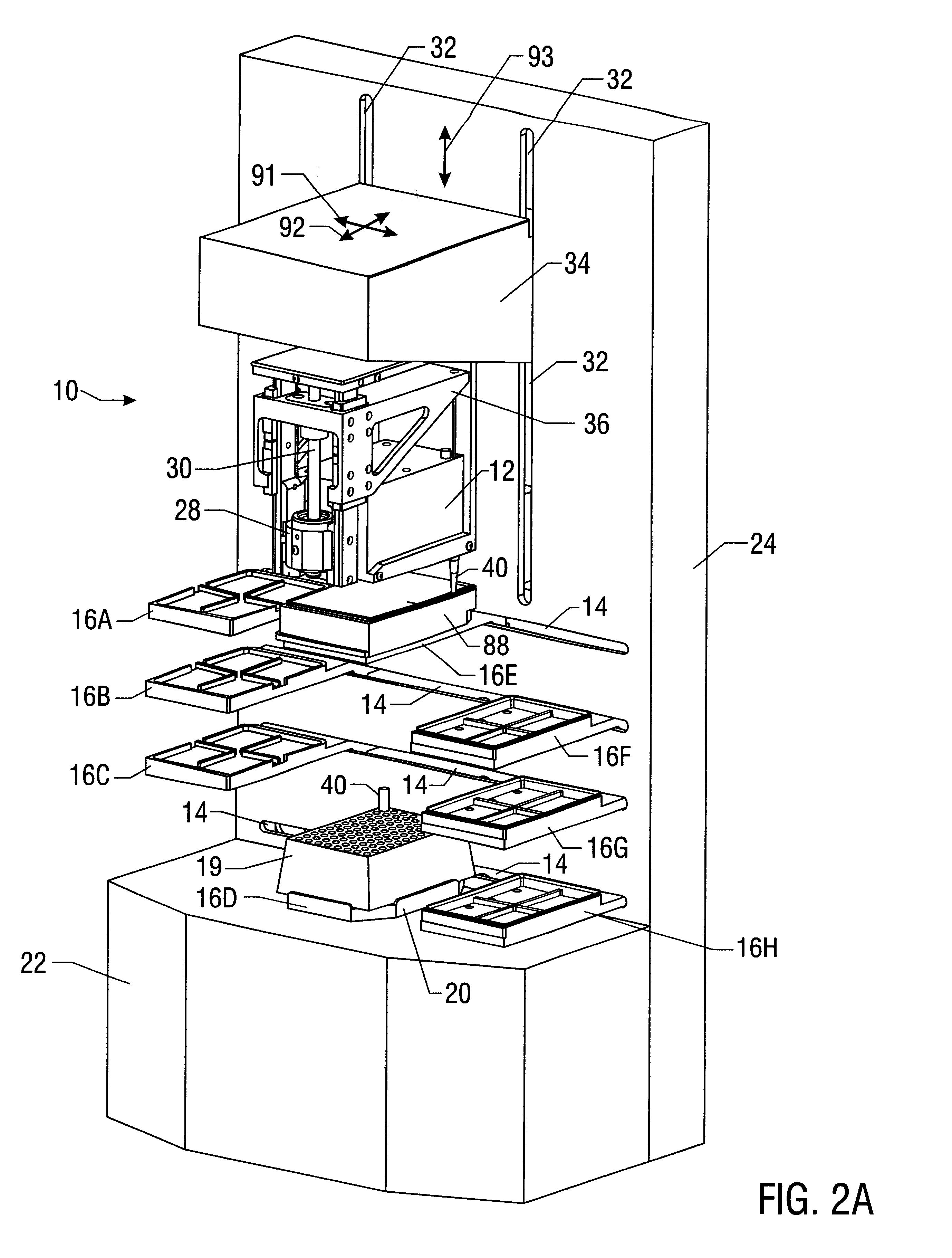
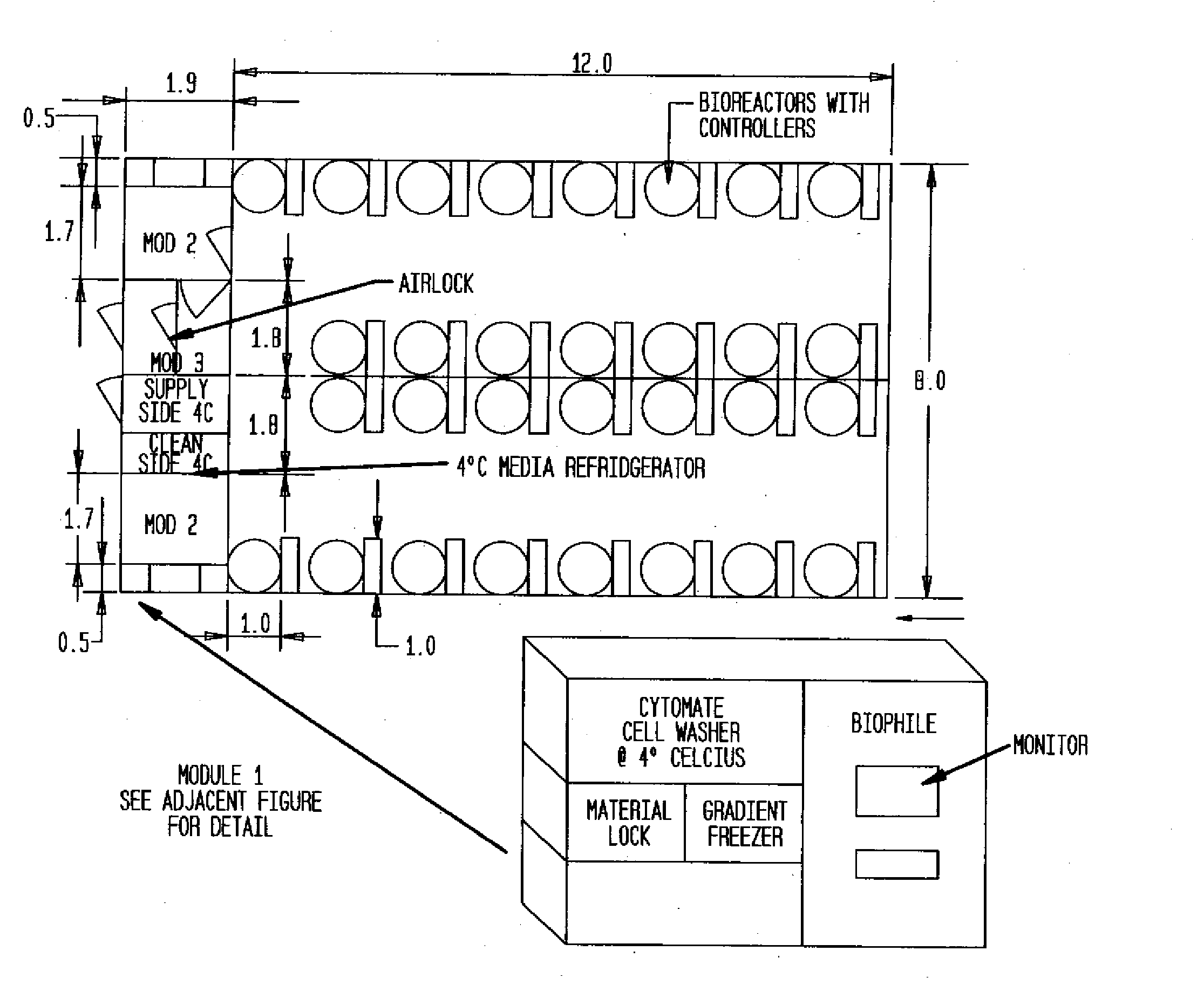
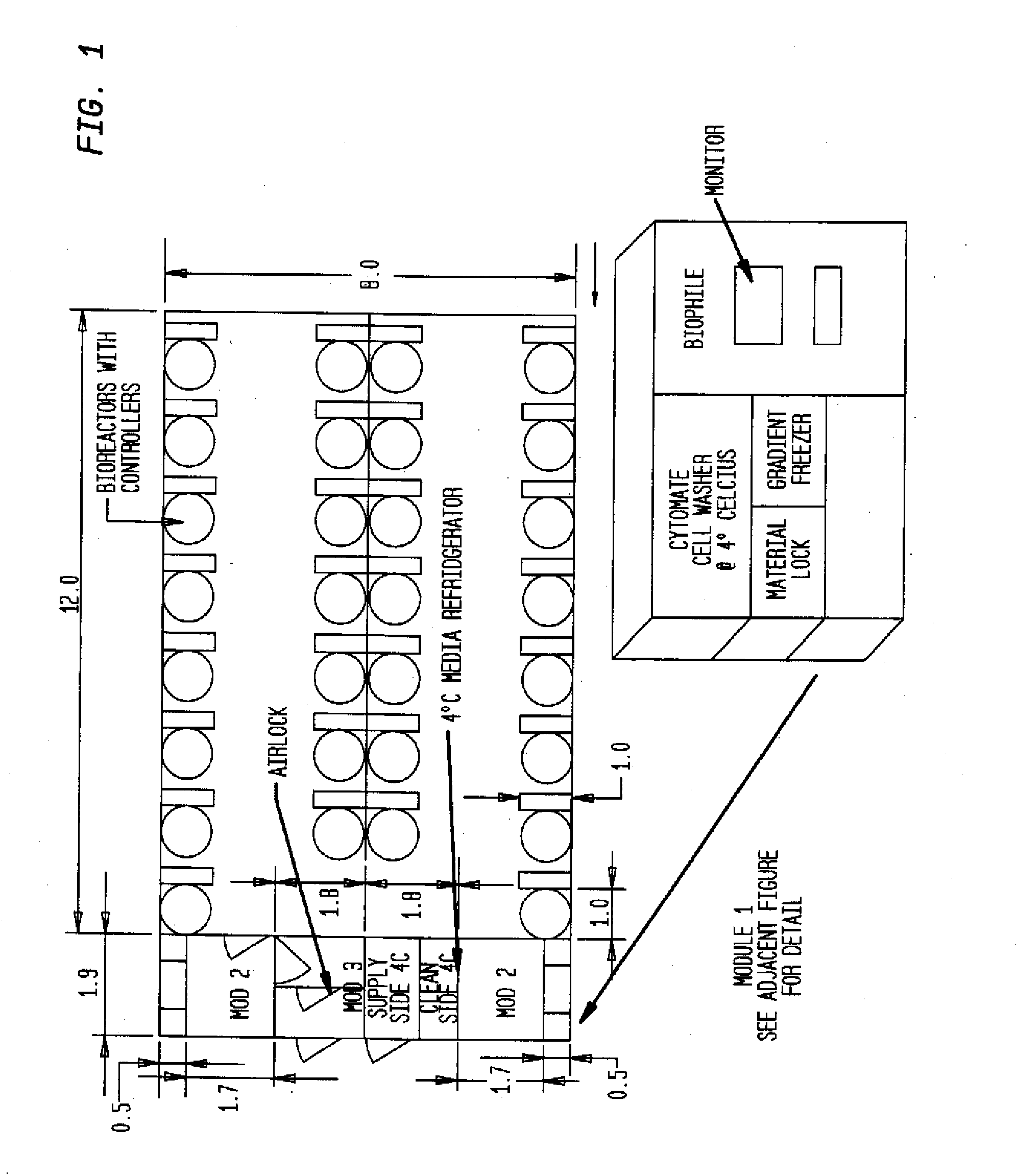
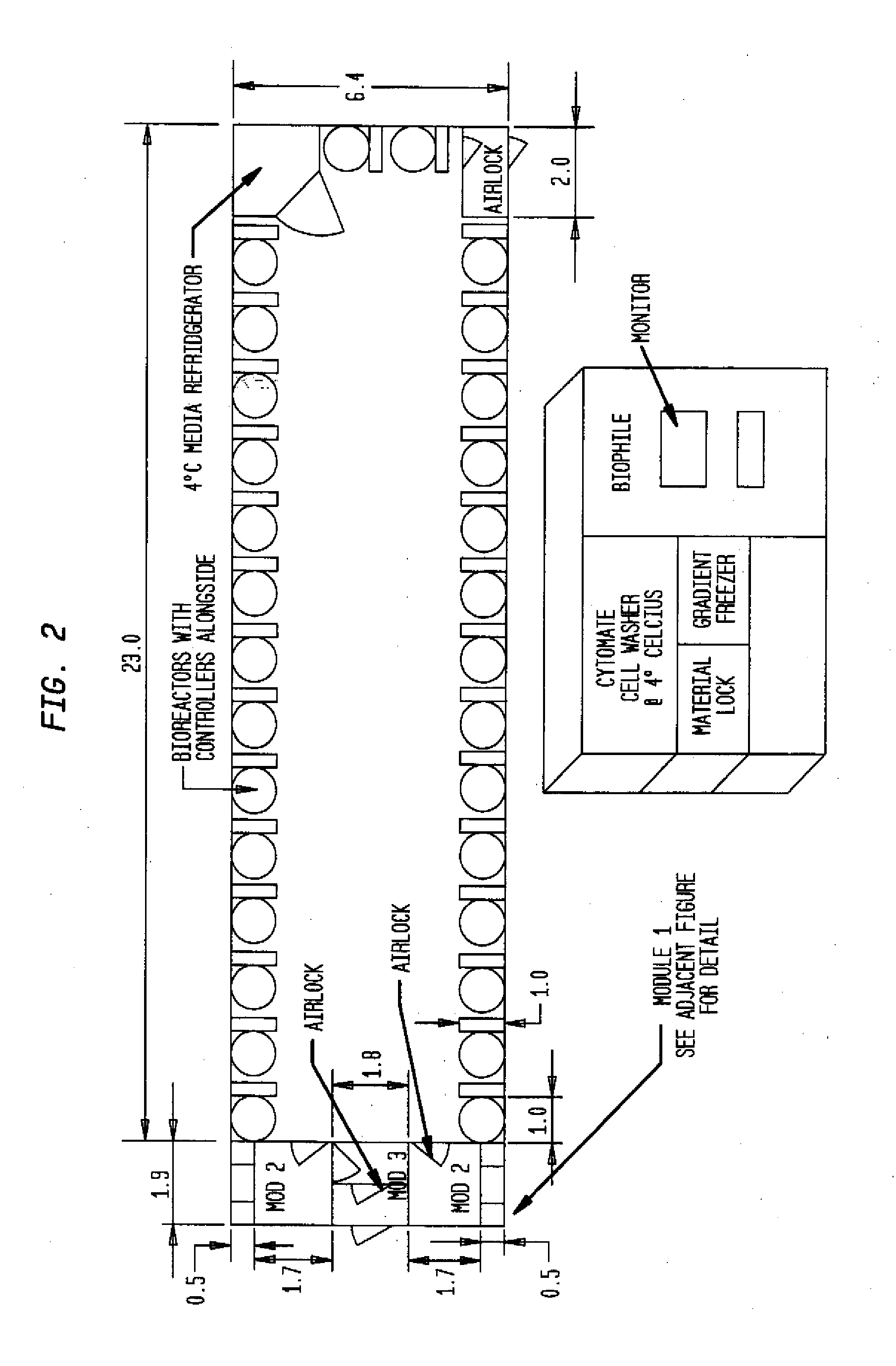
Prevalidated, modular good manufacturing practice-compliant facility
InactiveUS20090305626A1Precious timeShorten the timeApparatus sterilizationDust-free enclosuresInterior spaceGood laboratory practice
The invention is directed to a ready-to-use modular cleanroom and facility, in particular for the production of drugs and biological substances, which is equipped with pre-approved manufacturing equipment cores. The modular cleanroom is implemented in the interior space of a container, such as a standard shipping container, and includes at least one bioreactor station. The modular facility can be installed on-site from pre-approved cleanroom modules without further regulatory approval. The cleanroom and facility comply with FDA-approved good manufacturing practices (GMP) and good laboratory practices (GLP).
Owner:HOPE ERNEST G
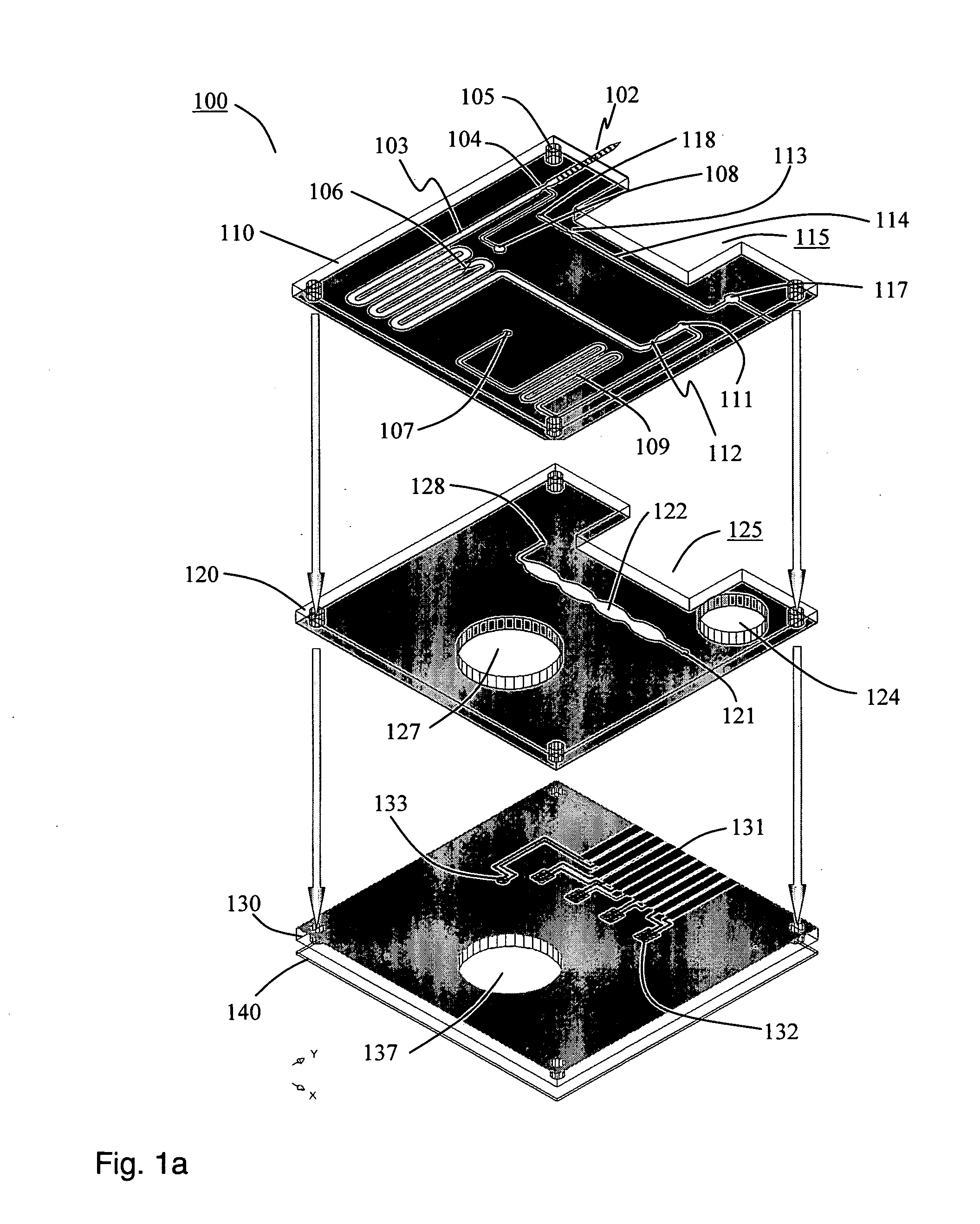
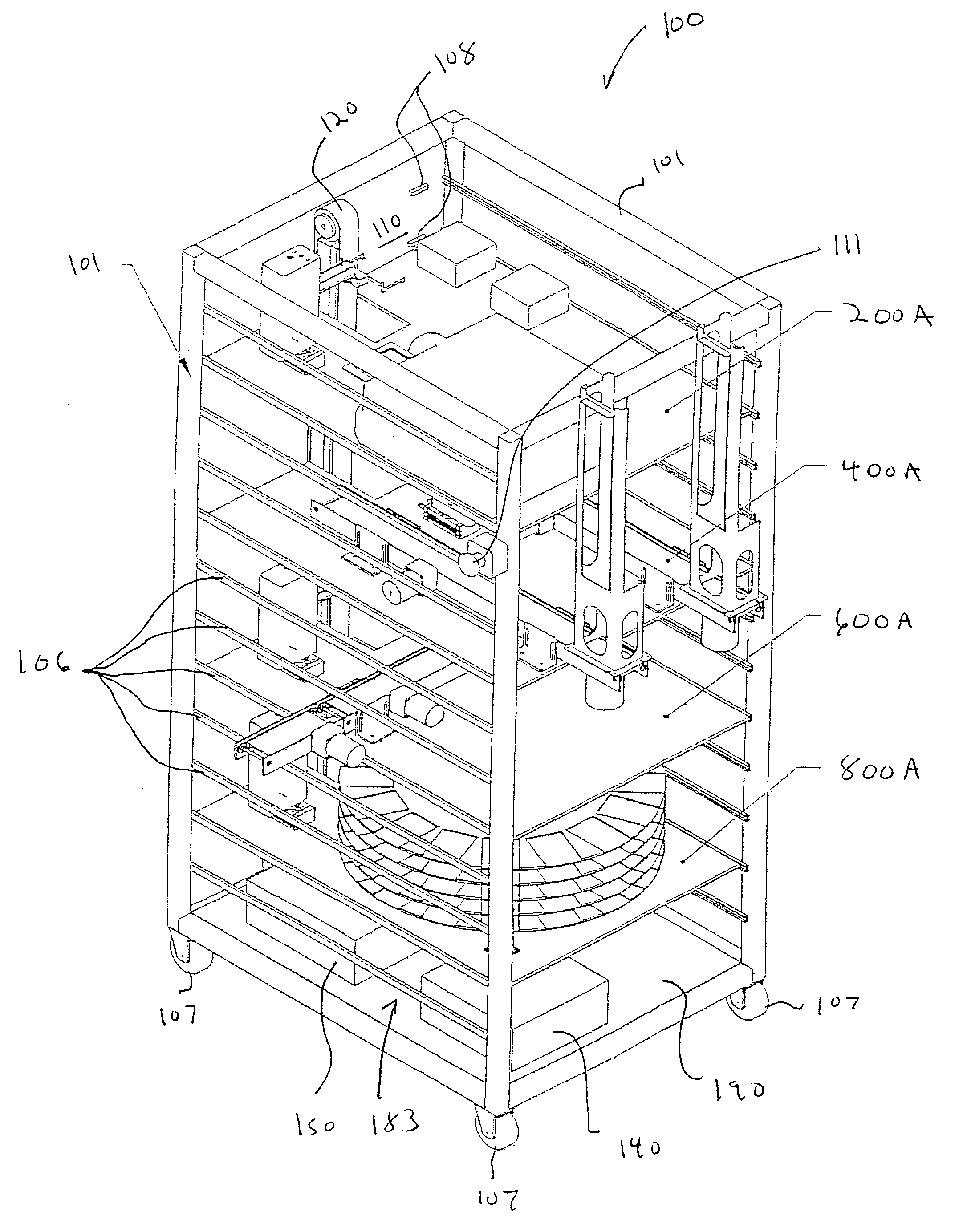
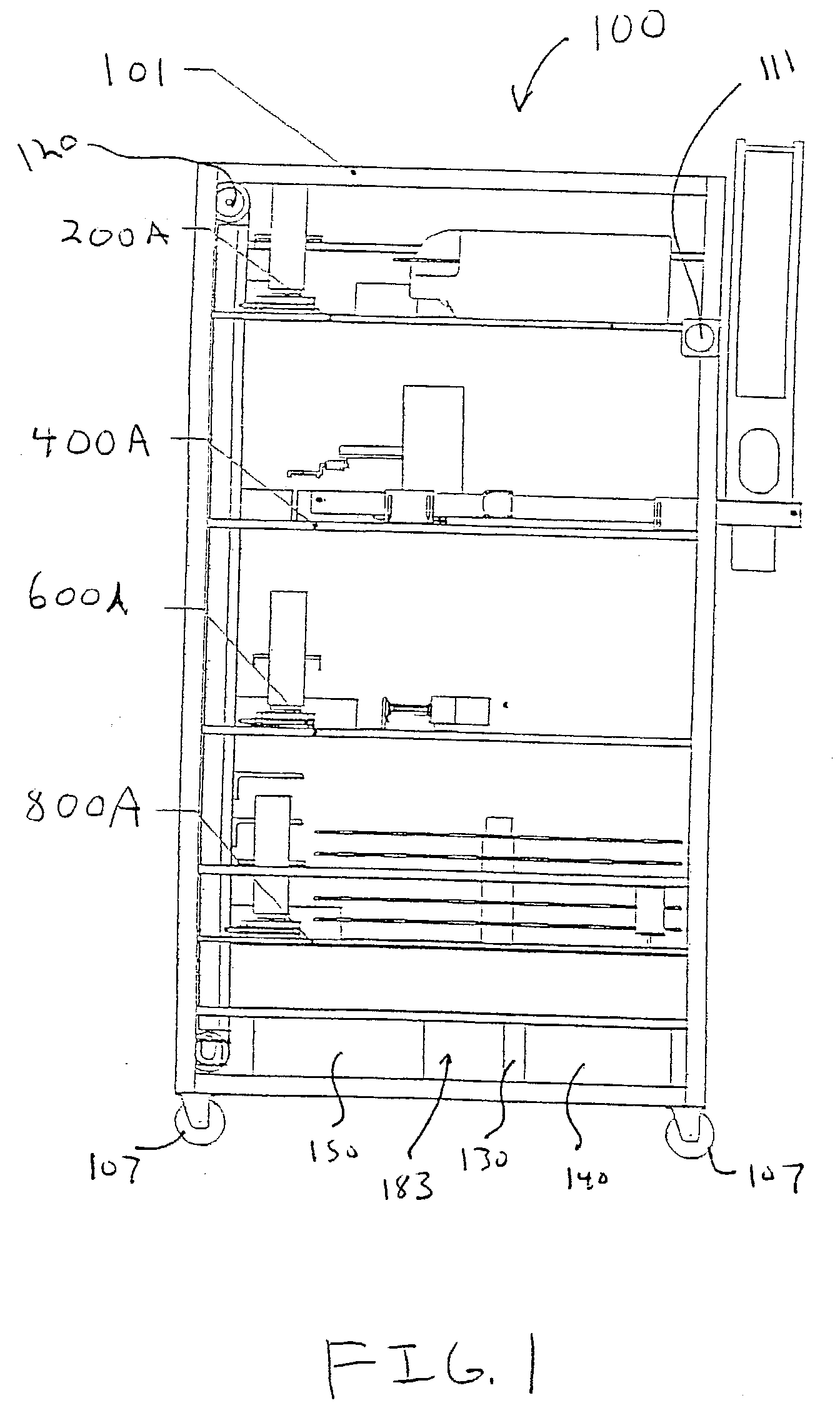
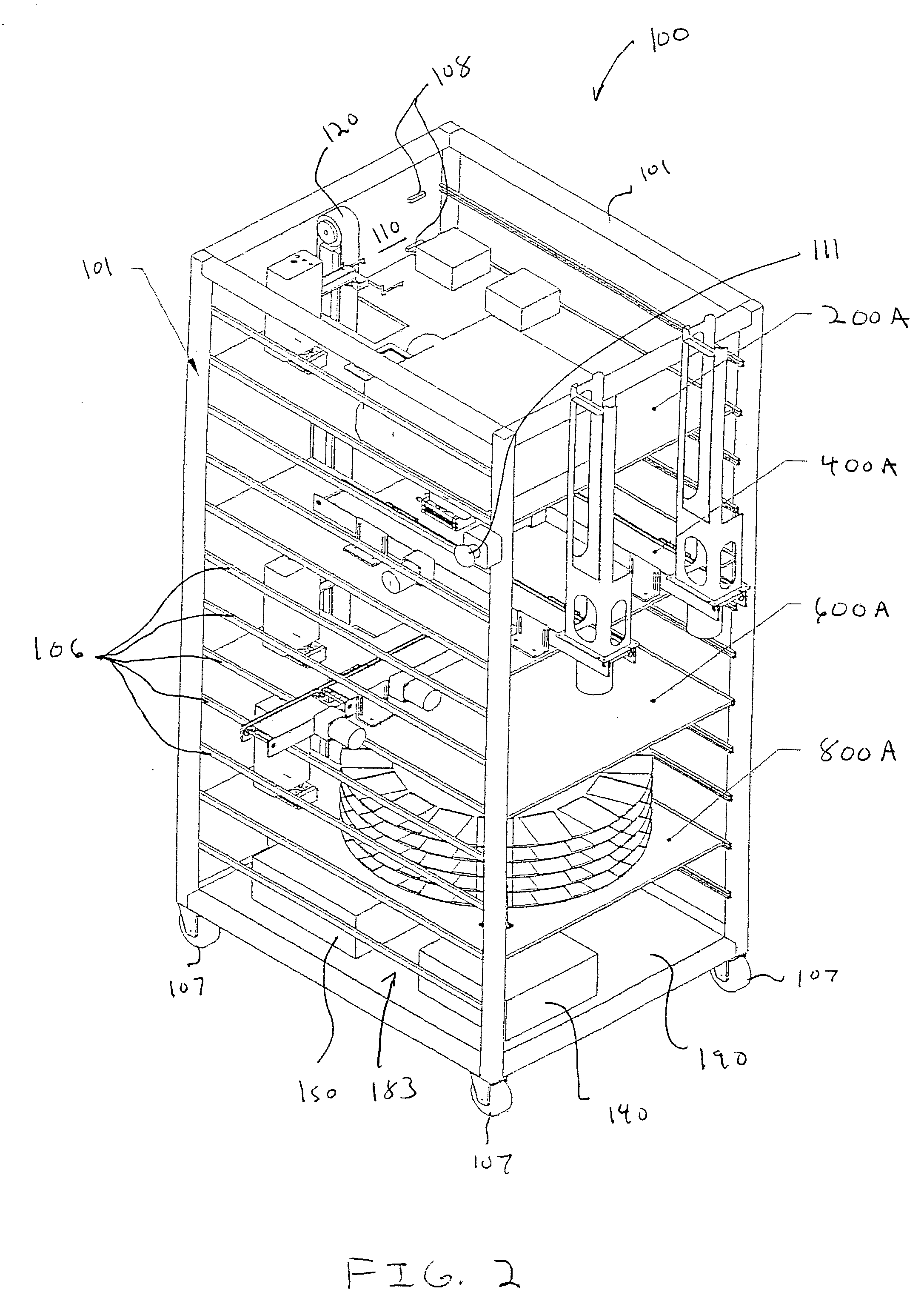
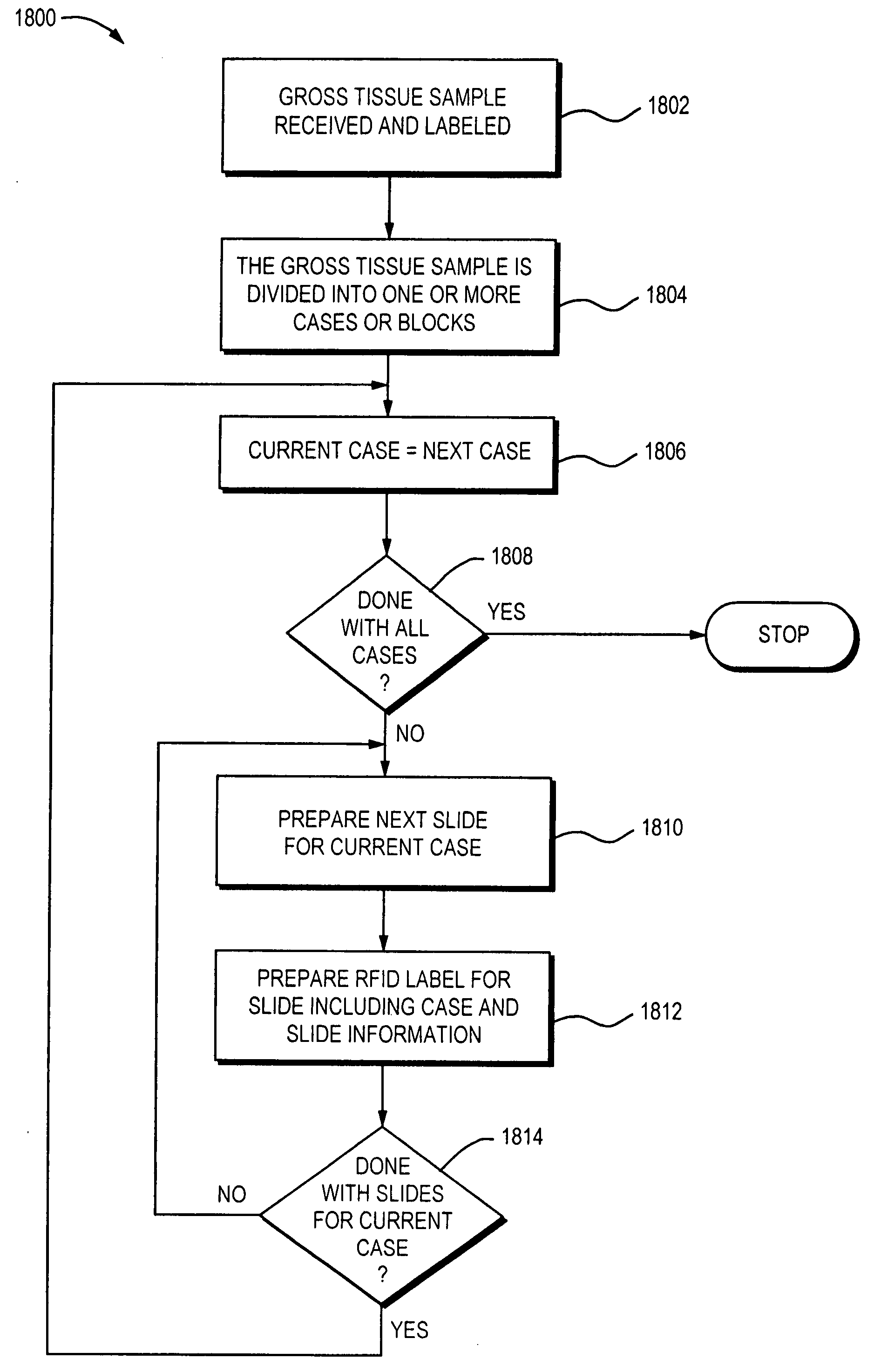
Laboratory instrumentation information management and control network
InactiveUS20080235055A1Computer-assisted medical data acquisitionOffice automationData memoryData store
Described are techniques for identifying samples processed in a laboratory using harmonized identifier. A case identifier identifying a patient from whom a specimen is collected is determined. A specimen identifier associated with the specimen is determined. An entry for the specimen is recorded in a data store where the entry being associated with the case identifier and the specimen identifier. A harmonized specimen identifier including the case identifier and the specimen identifier is formed. The specimen is labeled with the harmonized specimen identifier.
Owner:VENTANA MEDICAL SYST INC
Multiple purpose, portable apparatus for measurement, analysis and diagnosis
InactiveUS20050203353A1Satisfies needAccurately detecting colorBioreactor/fermenter combinationsBiological substance pretreatmentsData displayPoint of care
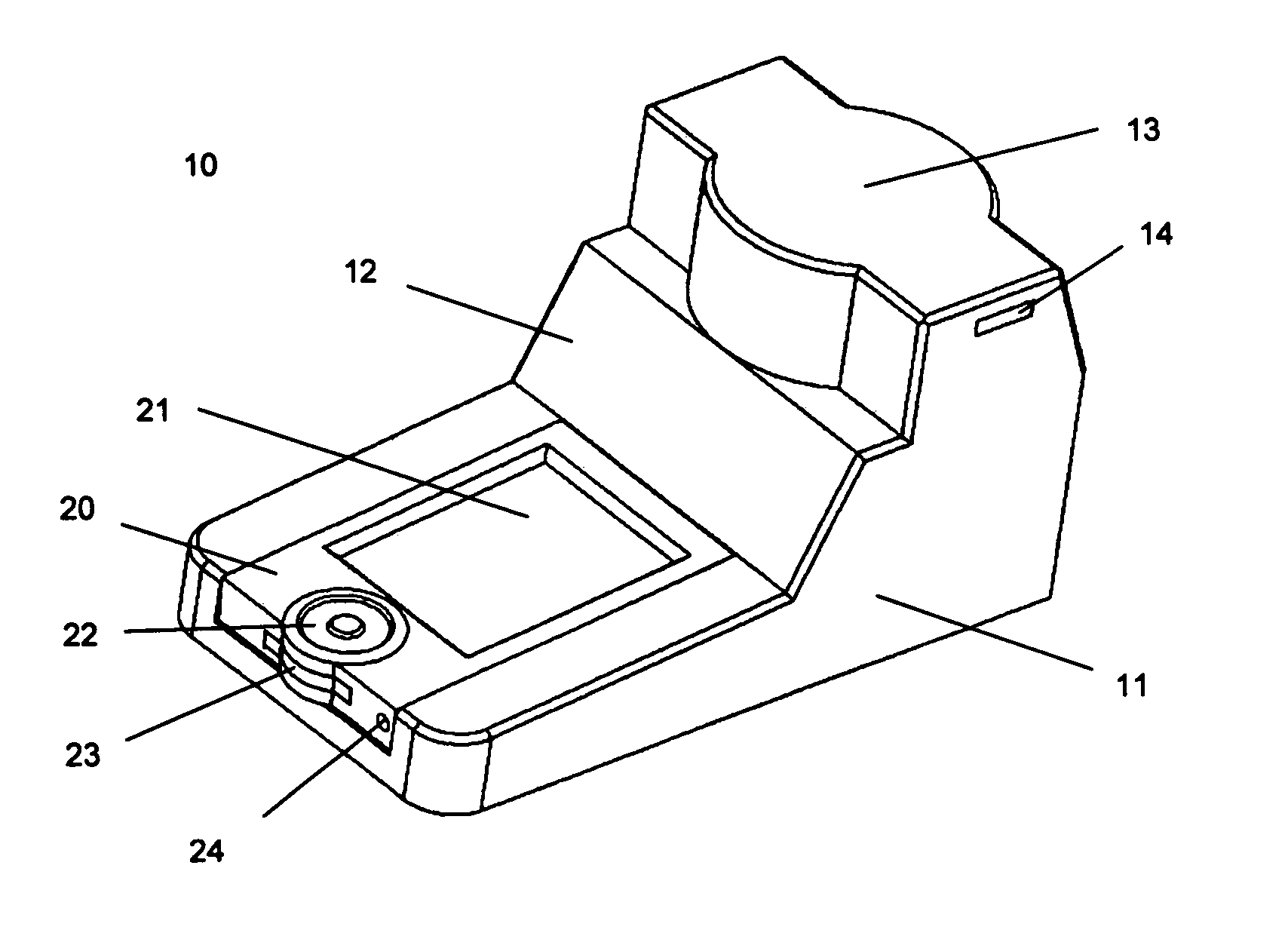
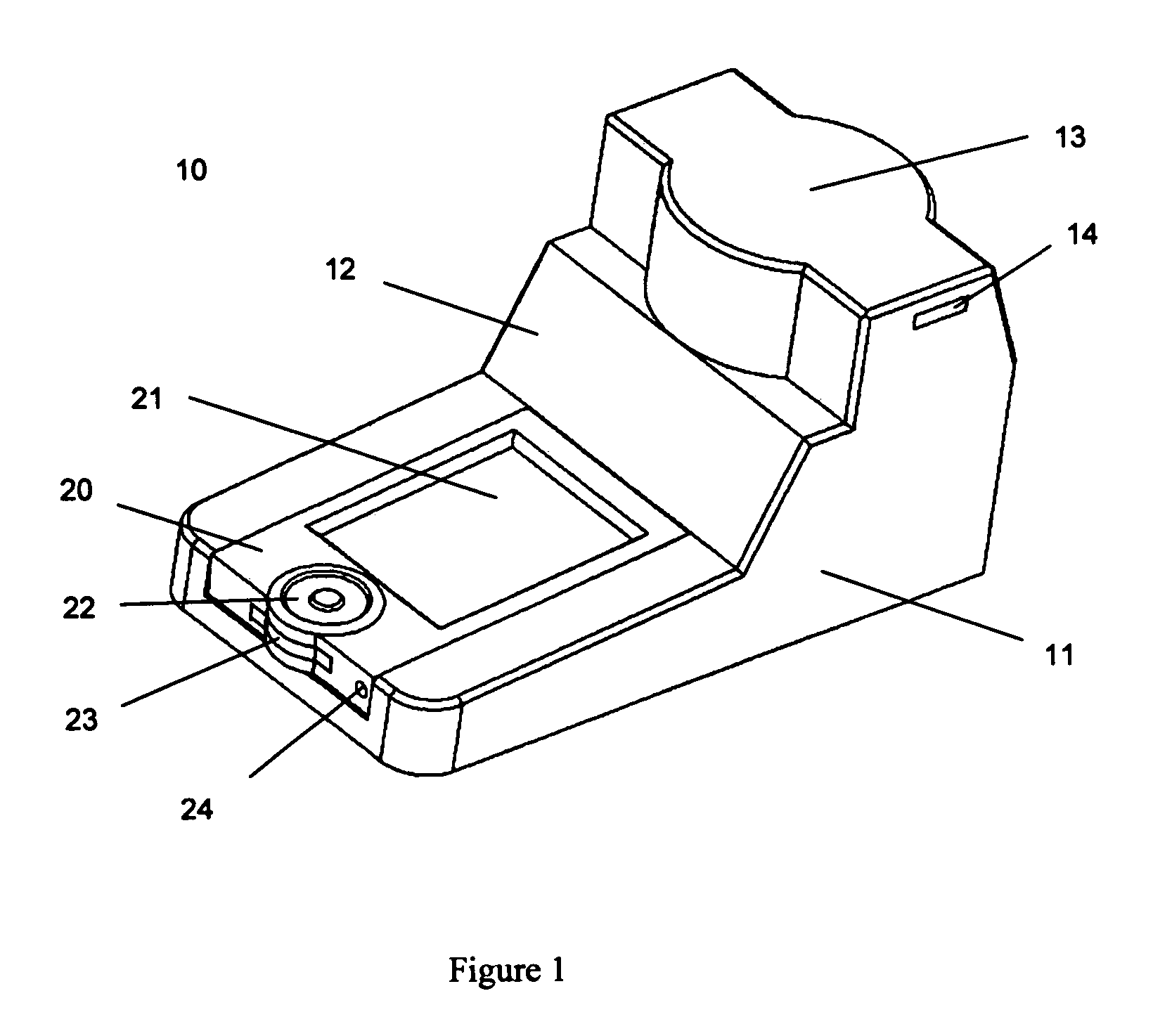
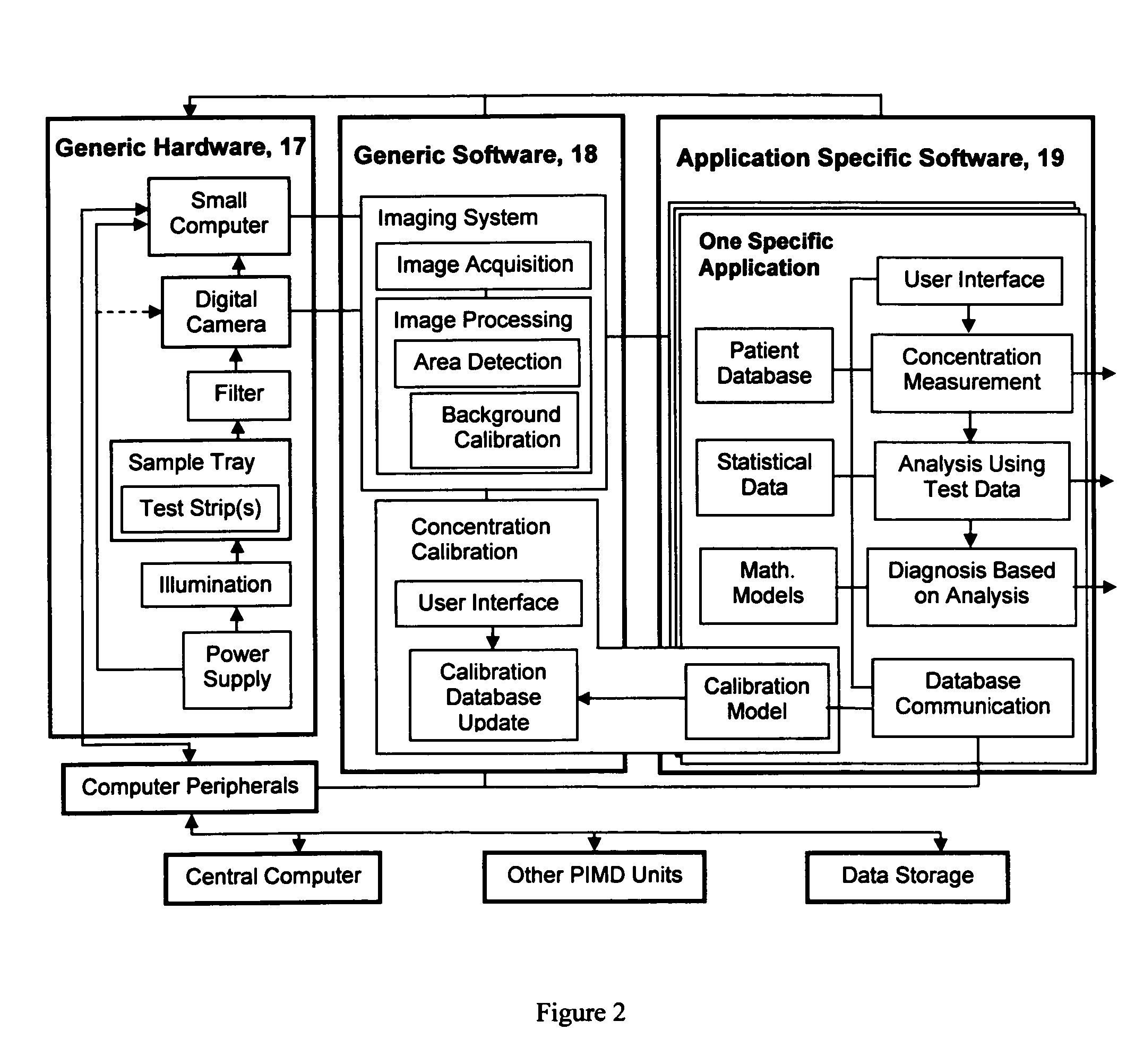
The present invention pertains to a portable apparatus for quantitatively measuring the concentration of specific substances in test samples of a lateral flow or microplate assay in medical, biomedical and chemical applications, and for making subsequent analysis and diagnosis. The portable apparatus includes a sample tray for carrying and aligning the test sample in the apparatus; a enclosure that may also serves as the frame of the apparatus; a digital image acquisition system that is used to obtain the digital image of the test sample on the sample tray; and a data display, processing, and analysis unit that is a general purpose or dedicated computer, such as a handheld computer (HHC), a pocket personal computer (PPC), a personal digital assistant (PDA), a palm-top computer, a laptop computer, or a dedicated microprocessor and associated hardware, for measuring the concentration of specific substances in the test sample, and making subsequent analysis and diagnosis, based on the measurement, statistical data, prior knowledge and mathematical model. The stated enclosure and frame, the digital image acquisition system, and the data display, processing and analysis unit are integrated to form the portable apparatus for various applications. The integrated apparatus of this invention, with a possible name—Portable Intelligent Multi-Diagnoser (PIMD), thus forms a portable and multiple-purpose tool for measuring the concentration of specific substances in test samples, and making subsequent analysis and diagnosis in a variety of settings, such as a mobile site, point of care or near patient care, and small laboratories.
Owner:MA JIE +1
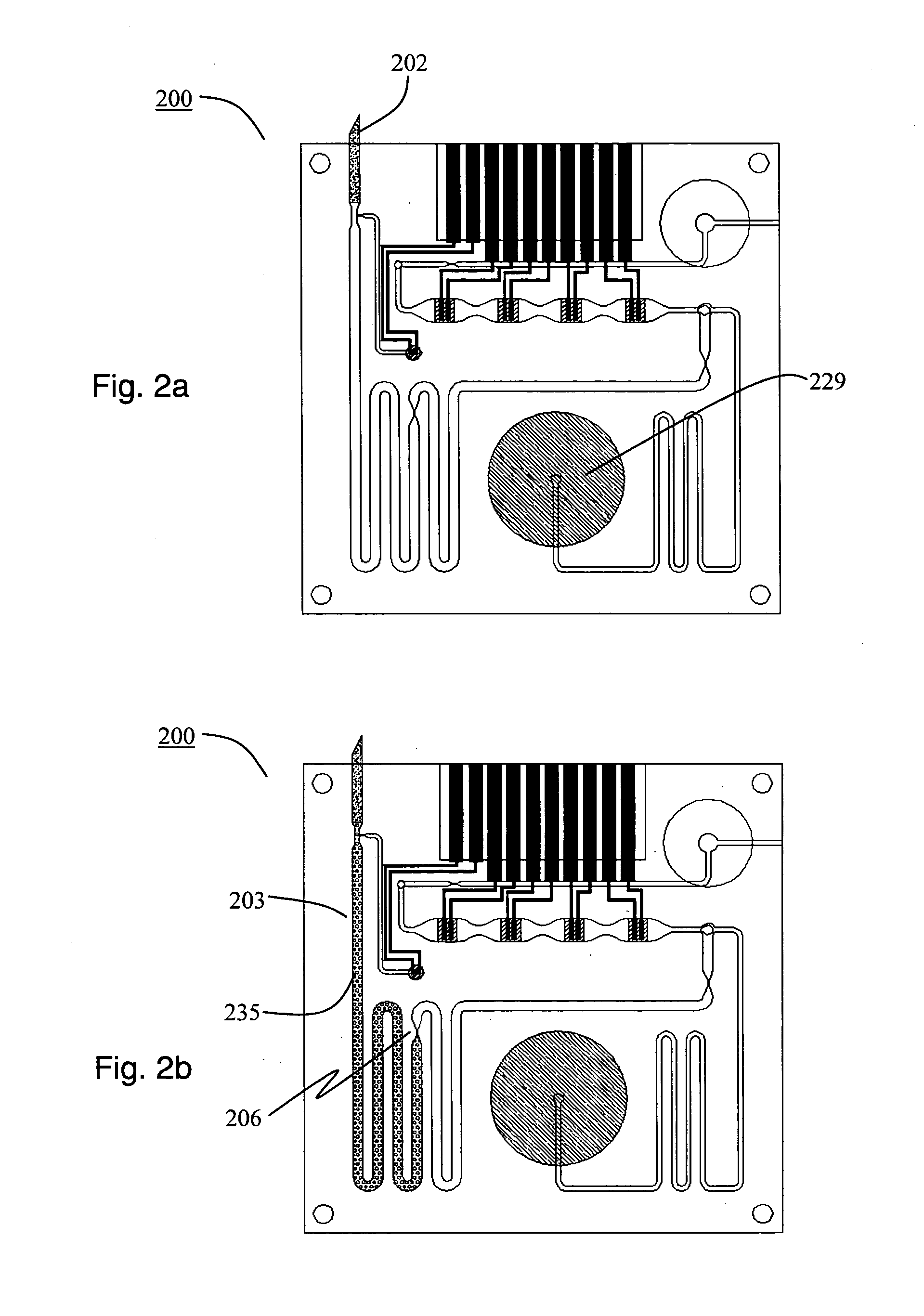
Microprocessor-controlled microfluidic platform for pathogen, toxin, biomarker, and chemical detection with removable updatable sensor array for food and water safety, medical, and laboratory applications
InactiveUS20130217598A1Rich abilityConvenient statisticsBioreactor/fermenter combinationsPeptide librariesSensor arrayDisease monitoring
The invention provides a platform technology with rich ability to flexibly perform, create, deploy, maintain, and update a wide range of panels, assay, array, and / or sequence of tests for a wide range of substances and pathogens. The invention provides a unifying framework for widely-ranging miniature sensor implementation, fluidic / gas interfacing, electrical interfaces and optical interfaces, and further by collocating, allowing the integration a large number highly-selective sensors and chemical sensors—together as needed with appropriately selected supplemental sensors (for example temperature, pH, selective ions, etc.), into a common readily-manufacturable framework. The diverse sensor arrays give rise to statistical enhancing through novel statistical processing approaches. The invention is deployable and useable in a wide range of situations previously unavailable, and addresses many otherwise problematic aspects of field testing for food safety, water safety, epidemic outbreaks, routine diagnosis, and disease monitoring.
Owner:NRI R&D PATENT LICENSING LLC
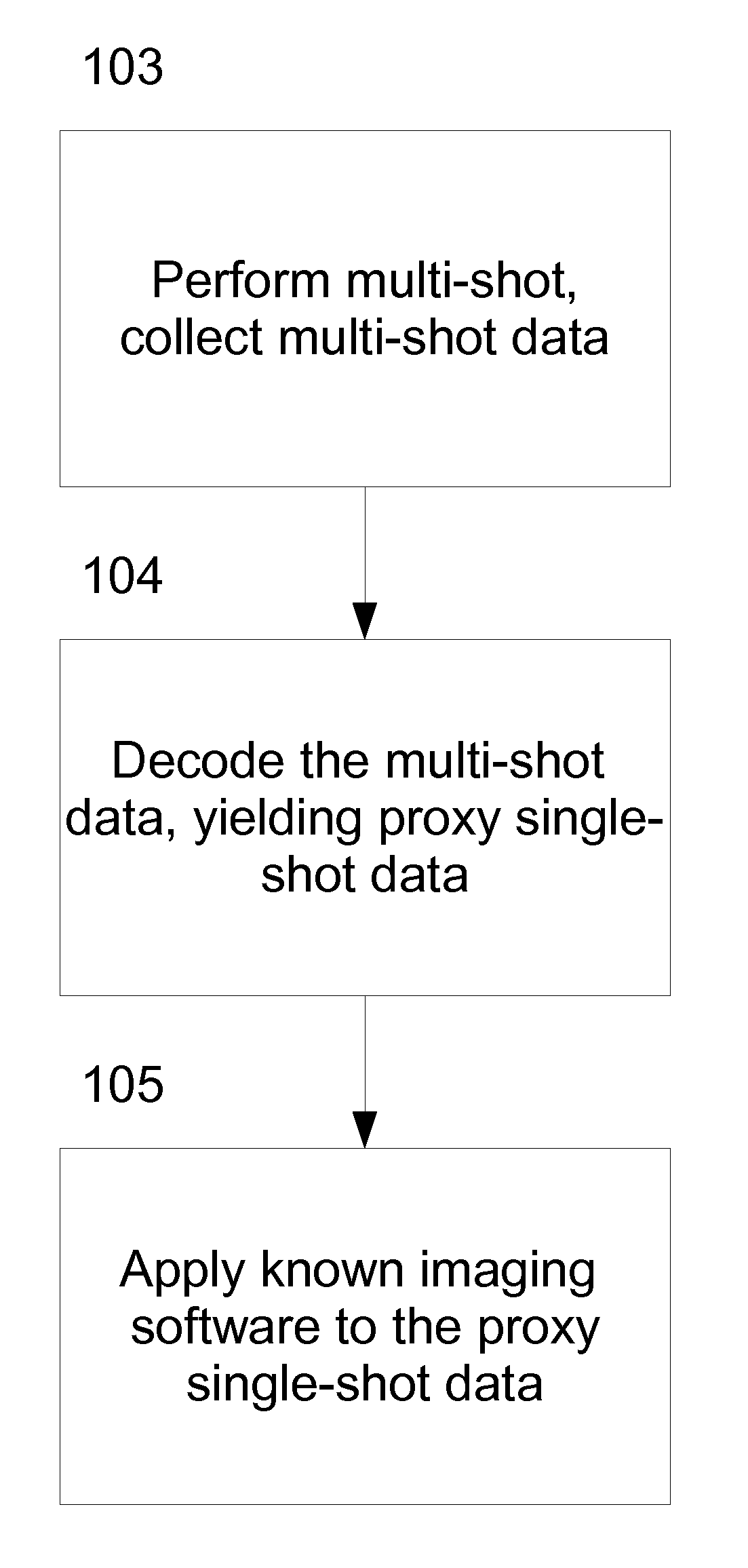
Coding and Decoding: Seismic Data Modeling, Acquisition and Processing
InactiveUS20070274155A1Seismic data acquisitionSeismic signal processingSource encodingData modeling
A method for coding and decoding seismic data acquired, based on the concept of multishooting, is disclosed. In this concept, waves generated simultaneously from several locations at the surface of the earth, near the sea surface, at the sea floor, or inside a borehole propagate in the subsurface before being recorded at sensor locations as mixtures of various signals. The coding and decoding method for seismic data described here works with both instantaneous mixtures and convolutive mixtures. Furthermore, the mixtures can be underdetemined [i.e., the number of mixtures (K) is smaller than the number of seismic sources (I) associated with a multishot] or determined [i.e., the number of mixtures is equal to or greater than the number of sources). When mixtures are determined, we can reorganize our seismic data as zero-mean random variables and use the independent component analysis (ICA) or, alternatively, the principal component analysis (PCA) to decode. We can also alternatively take advantage of the sparsity of seismic data in our decoding process. When mixtures are underdetermined and the number of mixtures is at least two, we utilize higher-order statistics to overcome the underdeterminacy. Alternatively, we can use the constraint that seismic data are sparse to overcome the underdeterminacy. When mixtures are underdetermined and limited to single mixtures, we use a priori knowledge about seismic acquisition to computationally generate additional mixtures from the actual recorded mixtures. Then we organize our data as zero-mean random variables and use ICA or PCA to decode the data. The a priori knowledge includes source encoding, seismic acquisition geometries, and reference data collected for the purpose of aiding the decoding processing.The coding and decoding processes described can be used to acquire and process real seismic data in the field or in laboratories, and to model and process synthetic data.
Owner:IKELLE LUC T
Apparatus and method for analyzing multiple samples
InactiveUS20140251836A1Immobilised enzymesBioreactor/fermenter combinationsHospital networkComputer science
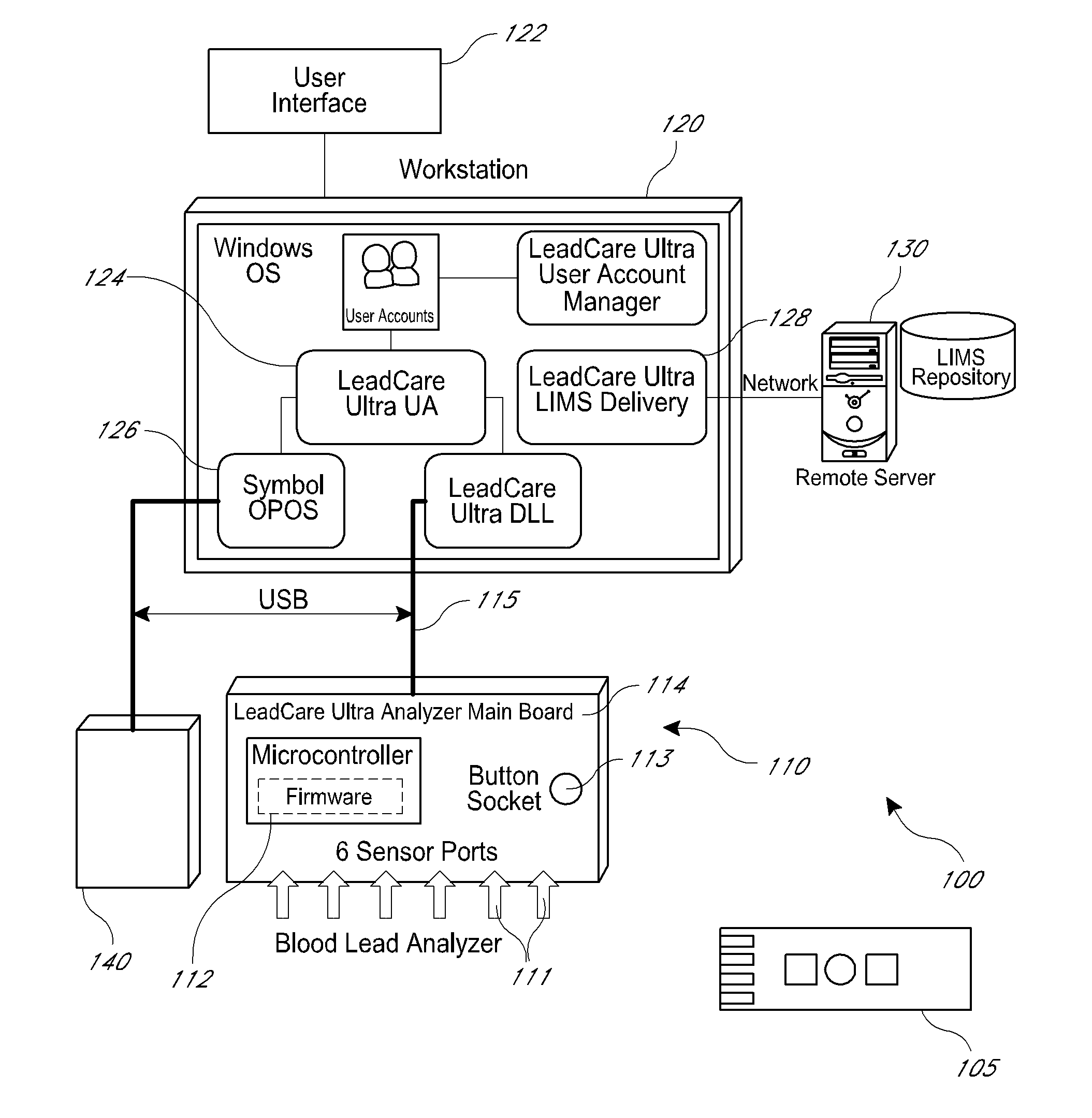
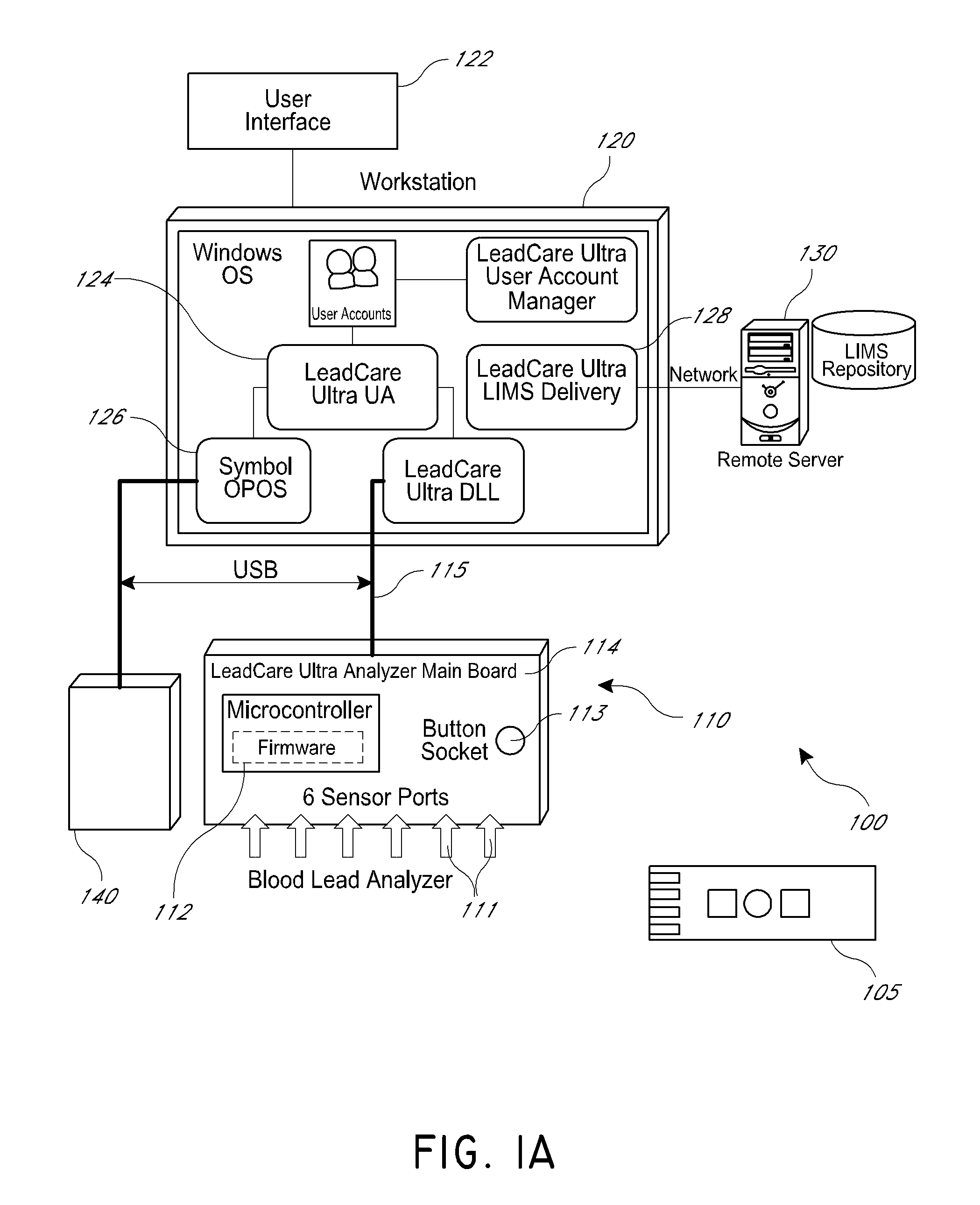
An apparatus and method for analyzing multiple samples is disclosed. In some embodiments, the apparatus and method use a multi-channel analyzer configured to simultaneously process results from a plurality of sample ports. The multi-channel analyzer further comprises a system for storing and transmitting the sample results from the plurality of samples, including unique sample identifiers. In some embodiments, the sample results may be transmitted to a laboratory information management system, a hospital network, or other similar location.
Owner:MAGELLAN DIAGNOSTICS
Devices and methods for obtaining mammary fluid samples for evaluating breast diseases, including cancer
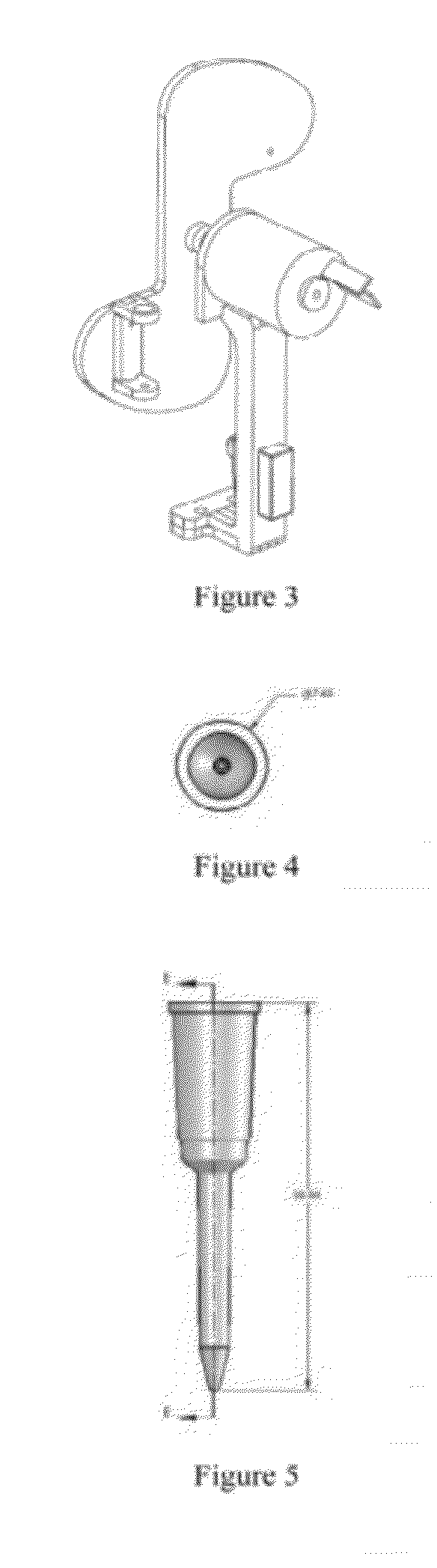
InactiveUS6887210B2Reduce morbidityLow costMicrobiological testing/measurementSurgical needlesCellular componentBreast pump (device)
Biological samples of mammary fluid or components thereof are obtained using a breast pump device coupled with a solid phase sample collection medium, alternatively facilitated by administering oxytocin to the subject. The breast pump device stimulates expression of mammary fluid and provides for collection of diagnostic samples to evaluate breast disease, including cancer. The biological sample may include whole cells or cellular components, purified or bulk proteins, glycoproteins, peptides, nucleotides or other desired constituents comprising a breast disease marker. Methods, kits and adapter devices relating to the breast pump device are also provided. Yet additional methods, devices, accessories, and materials are provided for laboratory handling and processing of breast fluid samples and for related diagnostic methods.
Owner:ATOSSA THERAPEUTICS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com