Patents
Literature
1767 results about "Patient data" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
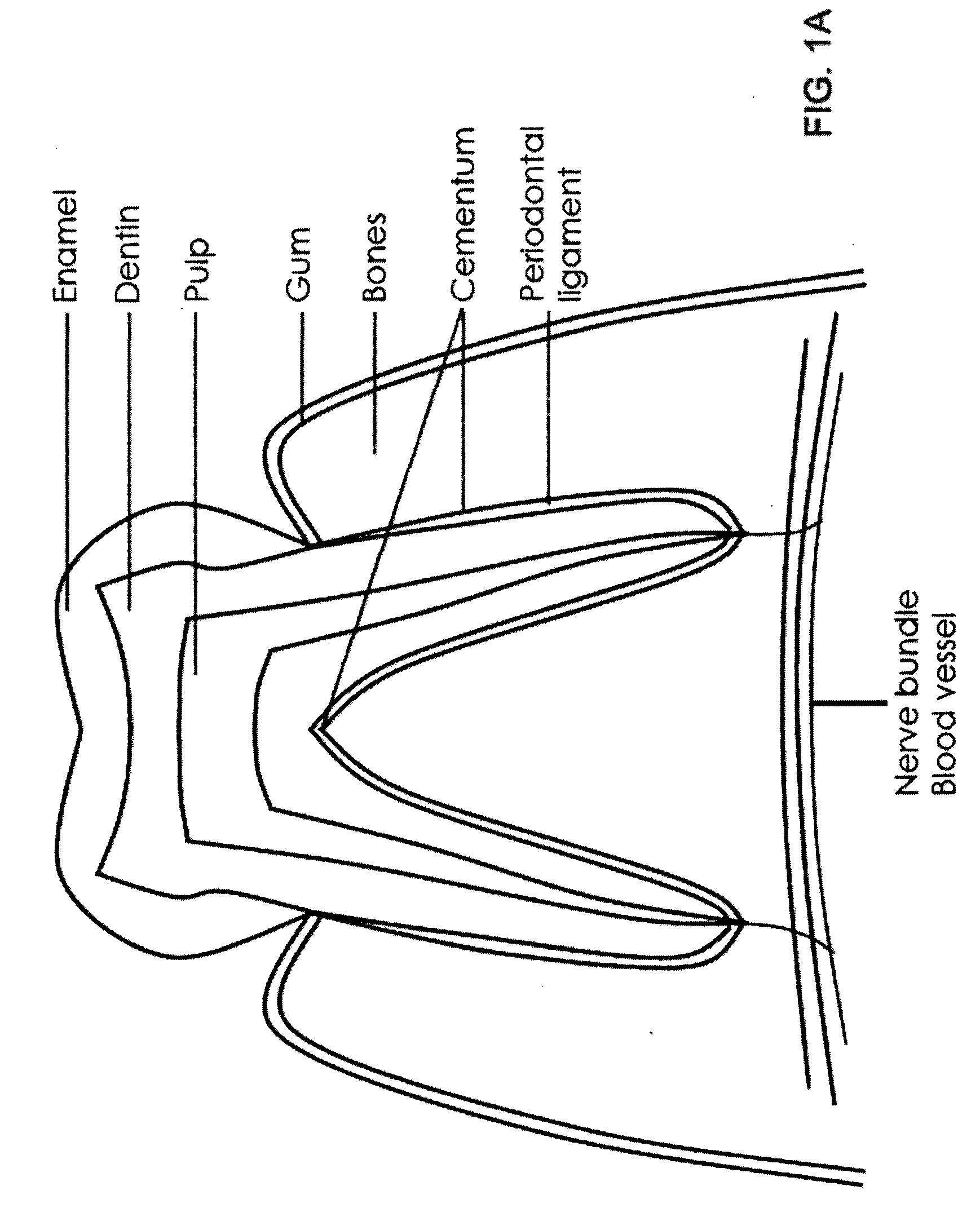
Patient data. Information about an individual patient, which may be relevant to decisions about current or future health or illness. Patient data should be collected using methods that minimise systematic and random error.
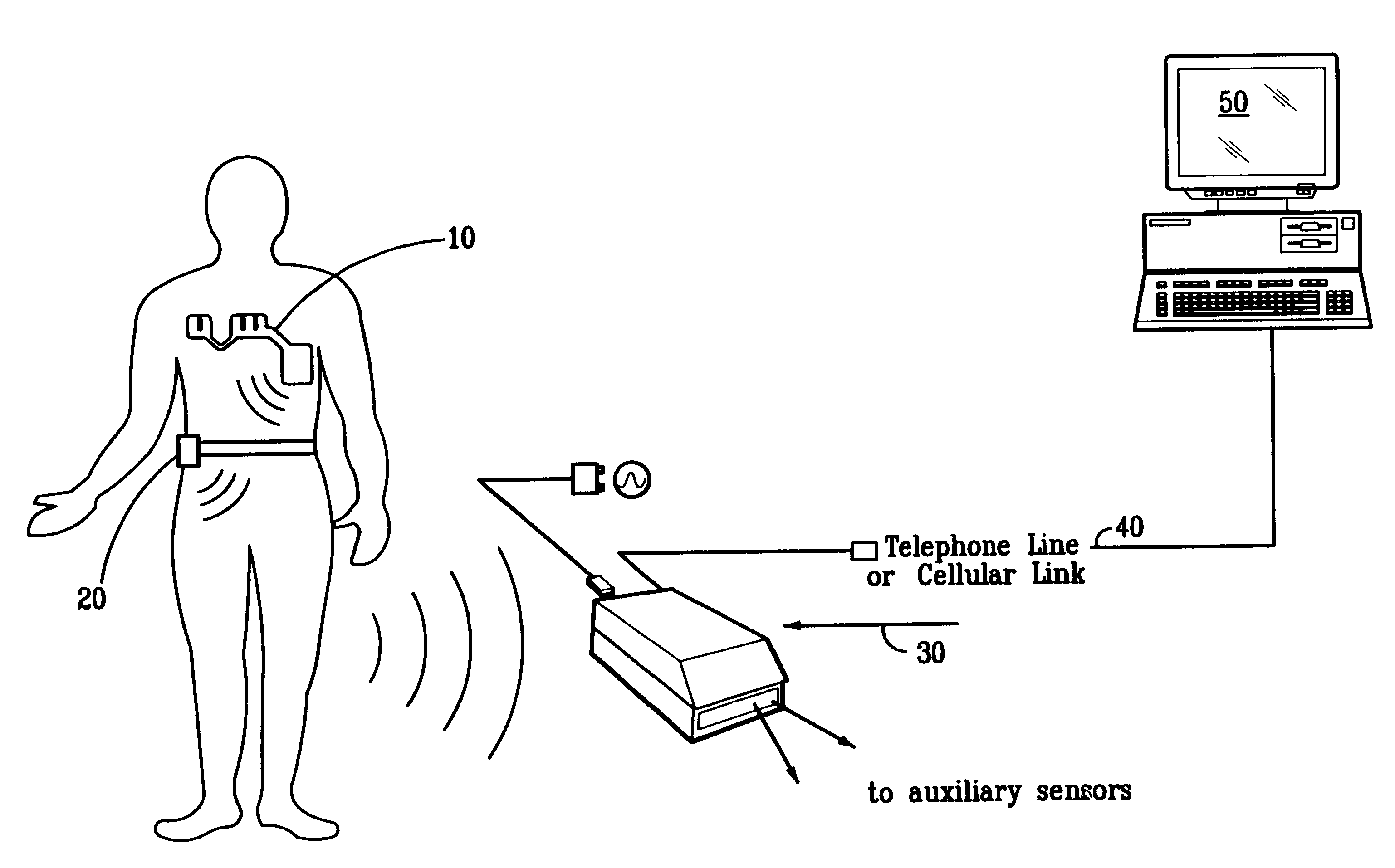
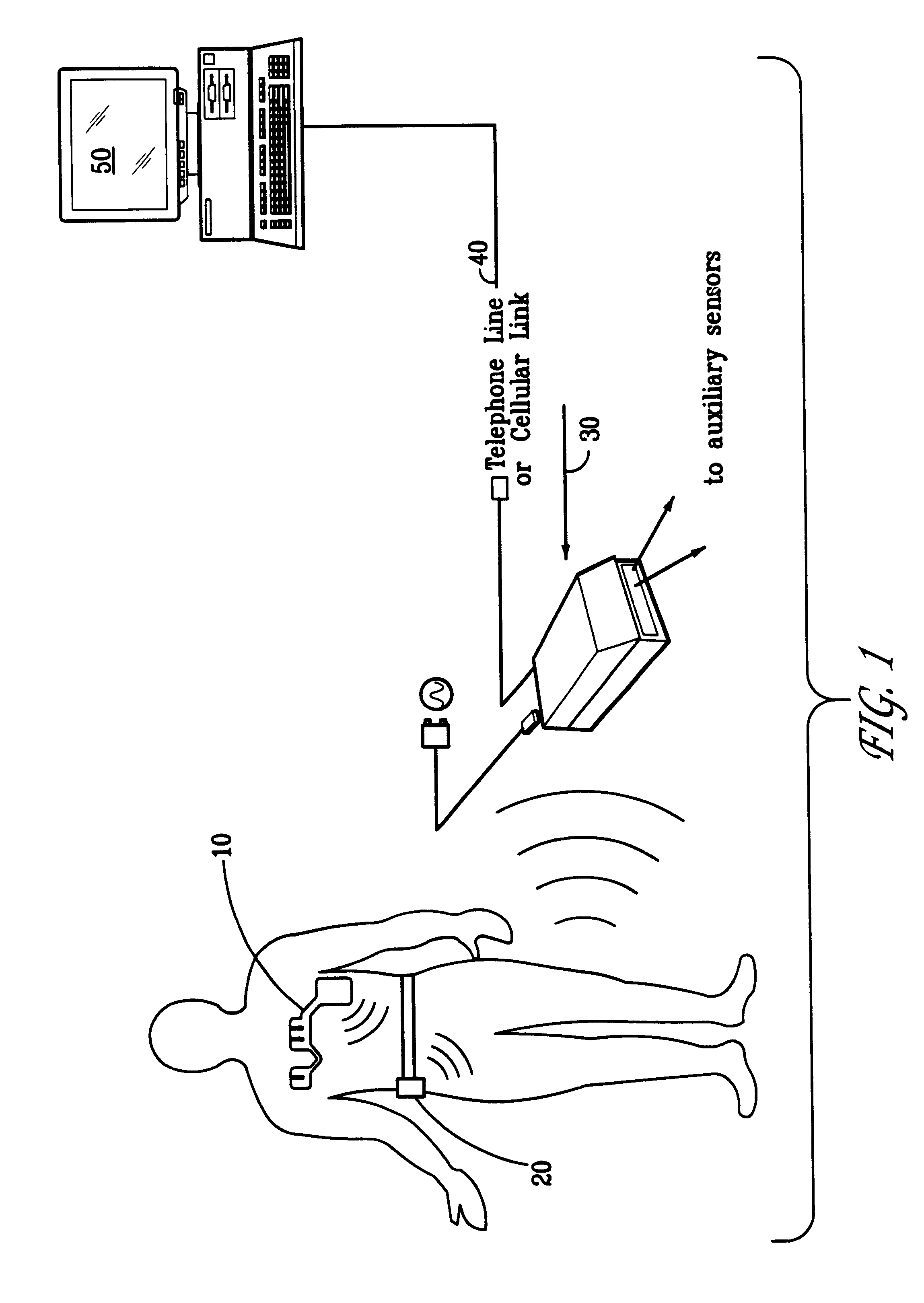
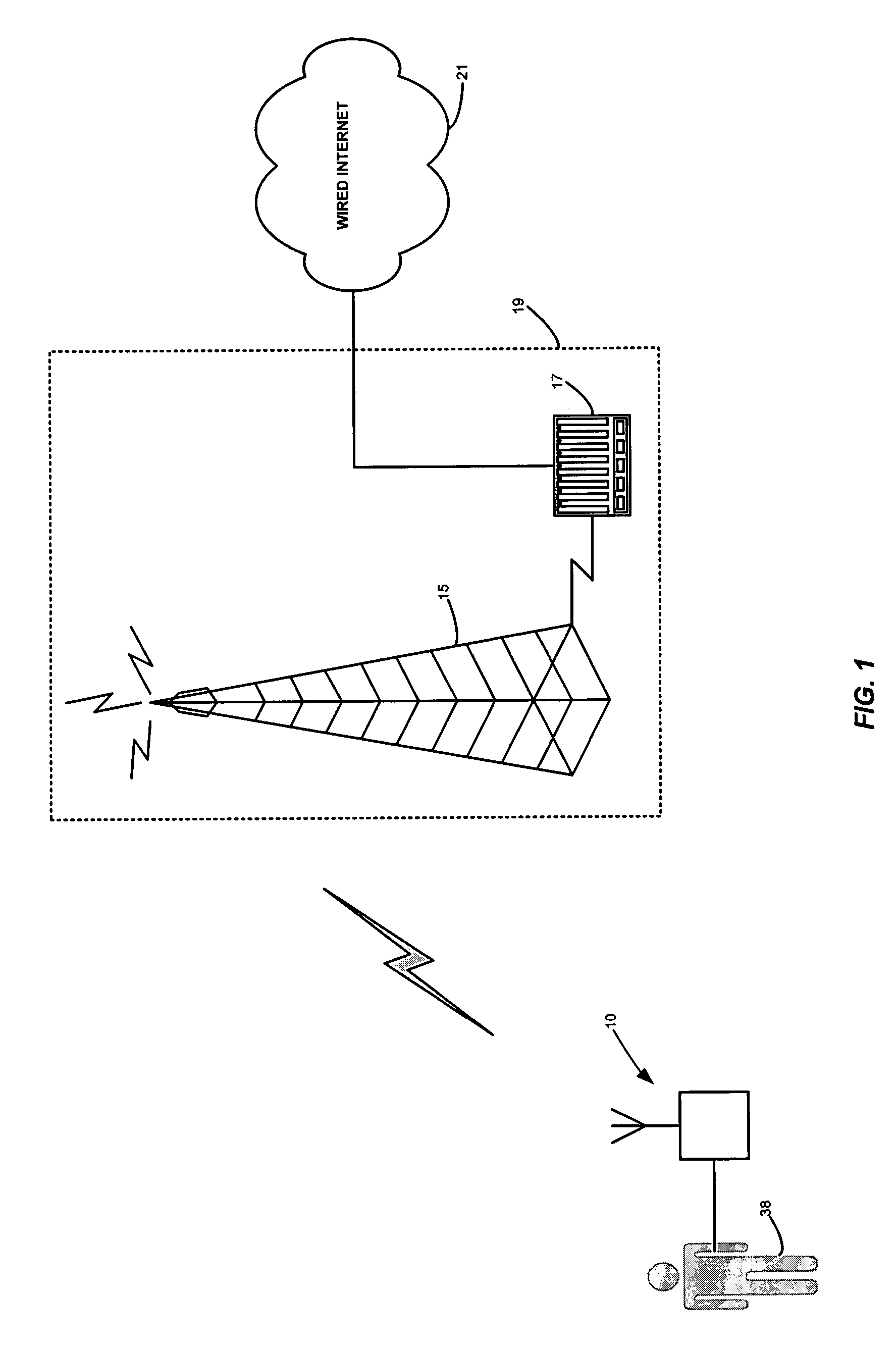
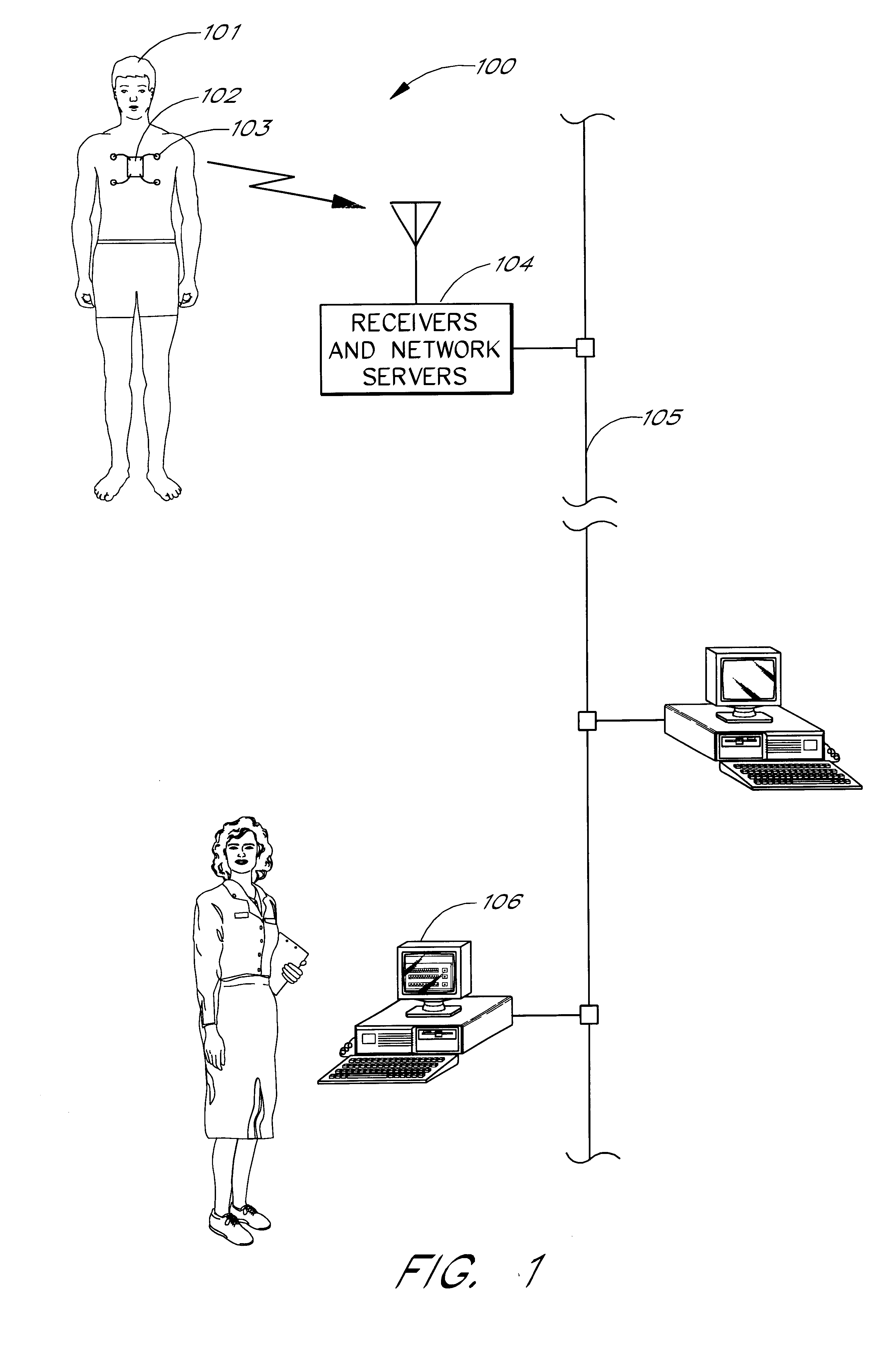
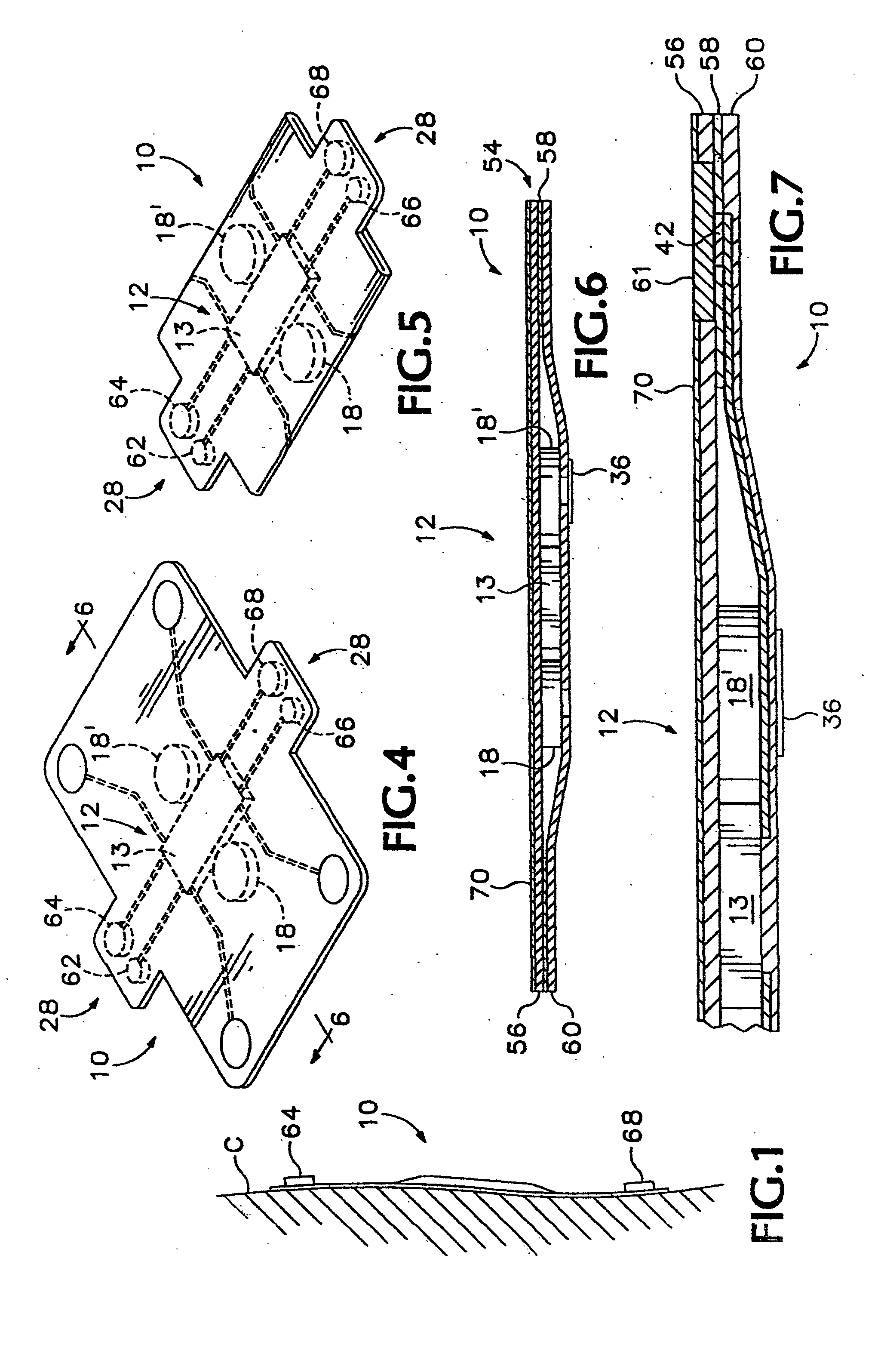
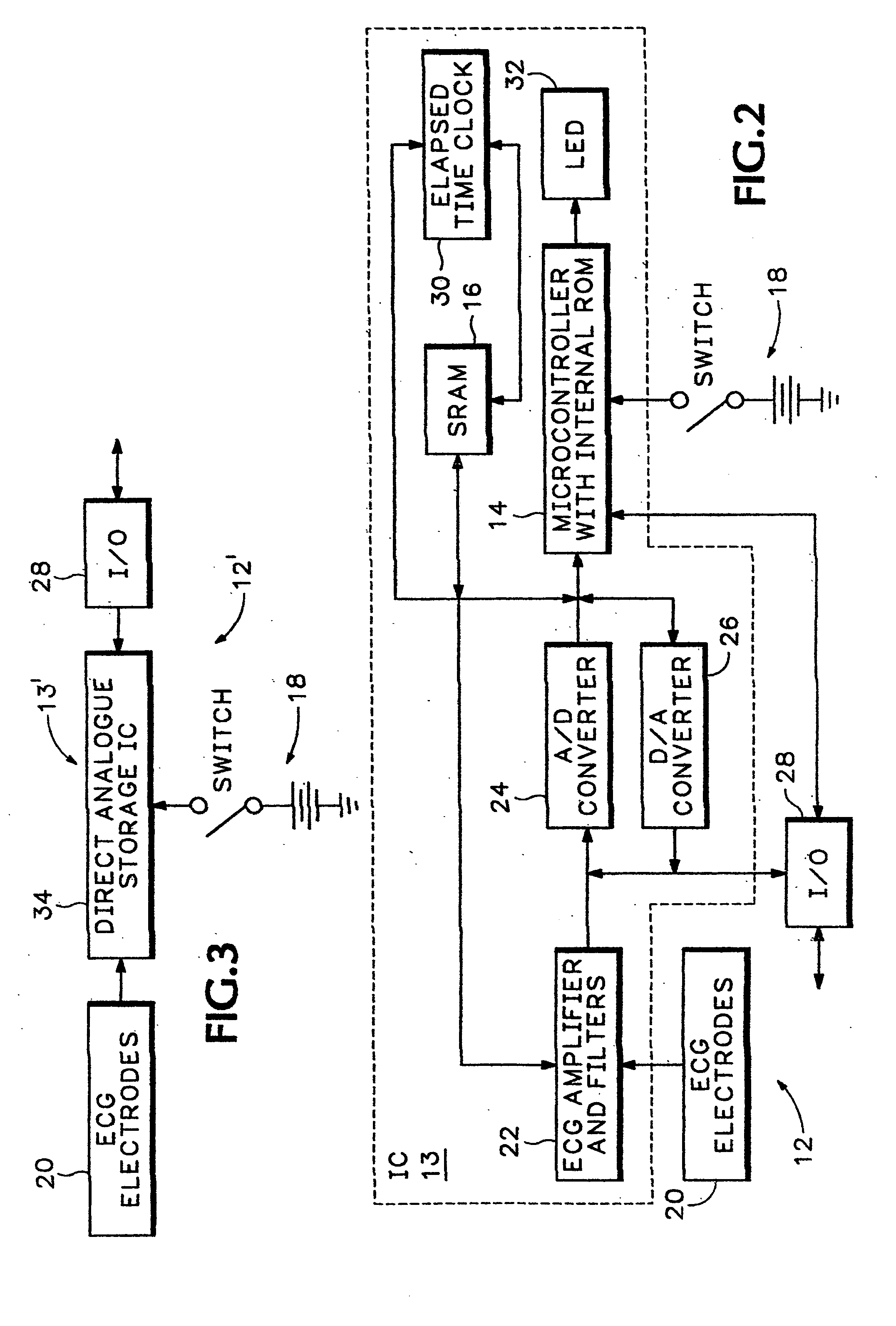
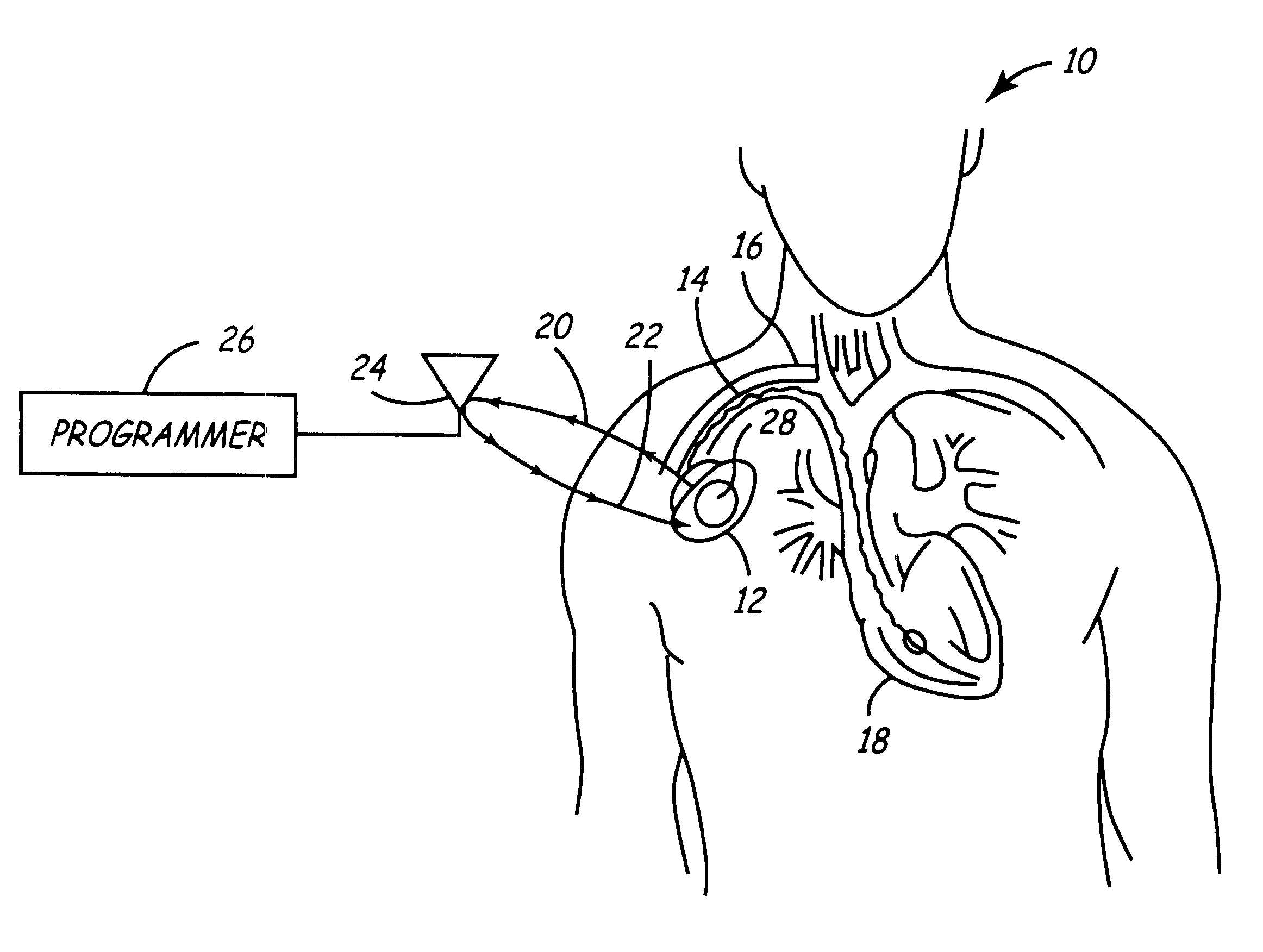
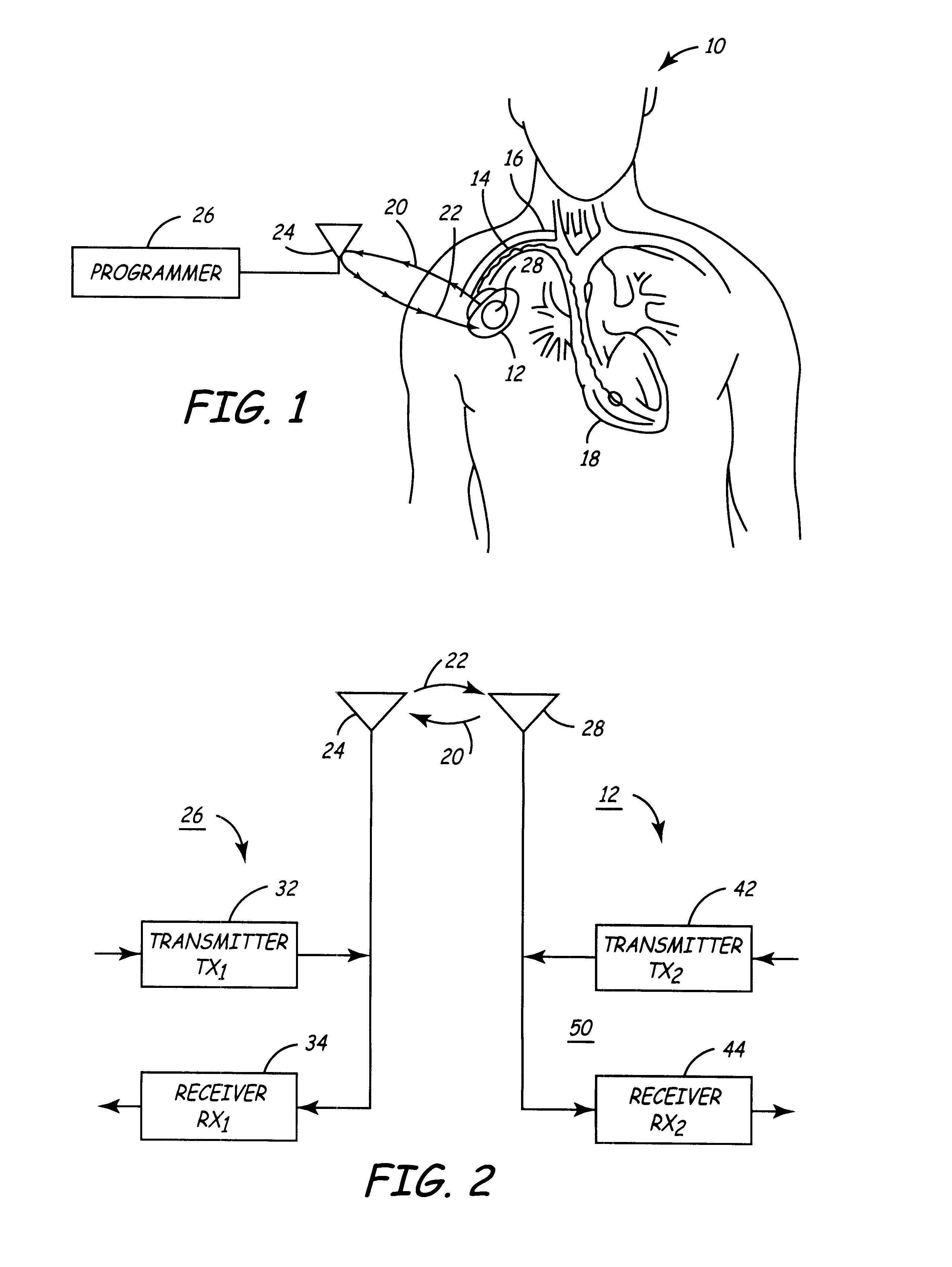
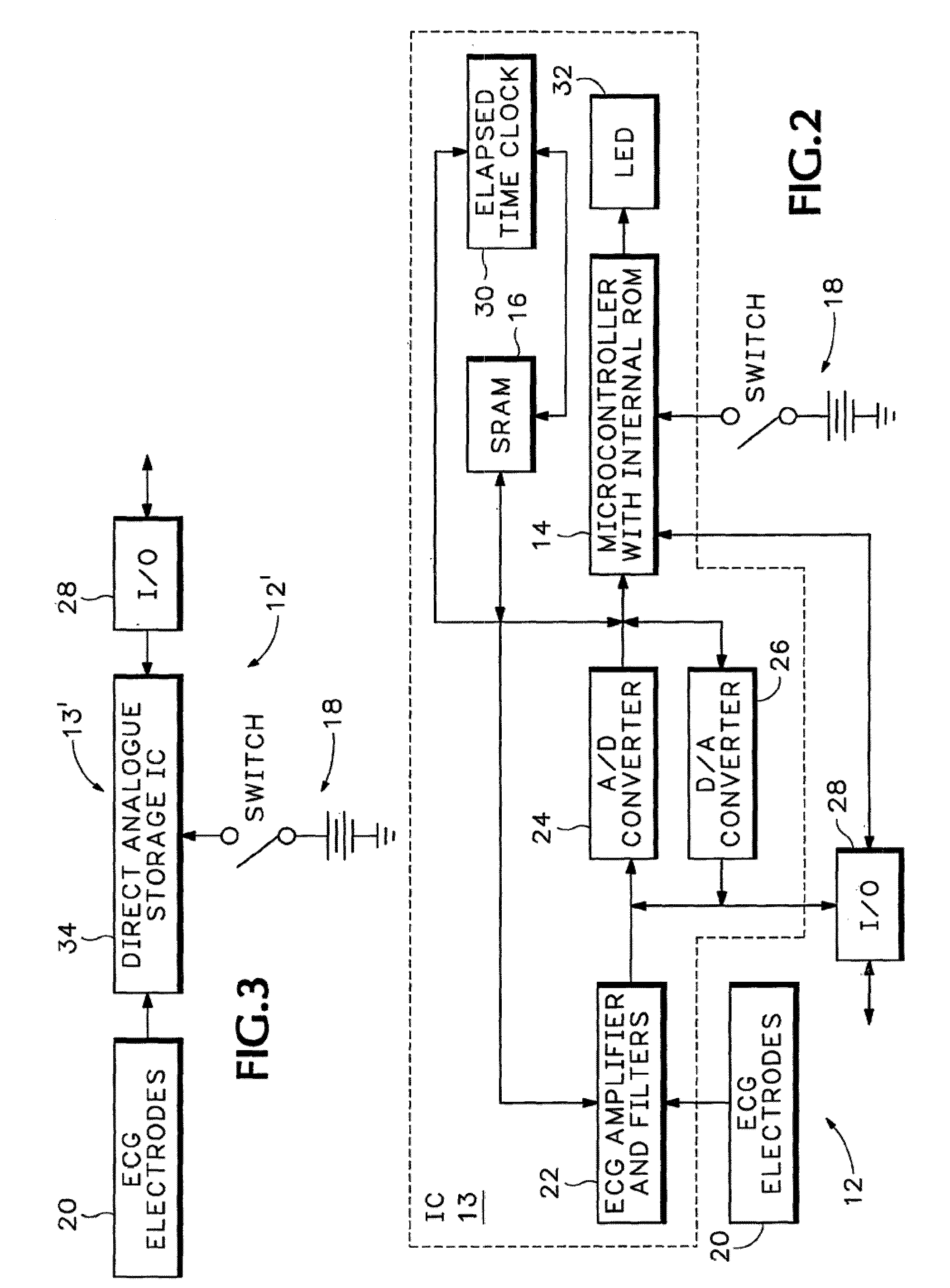
Portable remote patient telemonitoring system
A system and method for monitoring vital signs and capturing data from a patient remotely using radiotelemetry techniques. The system is characterized by a cordless, disposable sensor band with sensors form measuring full waveform ECG, full waveform respiration, skin temperature, and motion, and transmission circuitry for the detection and transmission of vital signs data of the patient. A small signal transfer unit that can either be worn by the patient, e.g., on his or her belt, or positioned nearby receives data from the sensor band, which it then forwards by e.g., radio transmission to a base station that can be located up to 60 meters away. The base station receives data transmissions from the signal transfer unit and is designed to connect to conventional phone lines for transferring the collected data to a remote monitoring station. The base station may also capture additional clinical data, such as blood pressure data, and to perform data checks. Patient safety is enhanced by the ability of the base station to compare clinical data, e.g., ECG, against given profiles and to mark events when appropriate or when the base station is programmed to do so. Such events are indicated to the physician and could be indicated to the patient by reverse transmission to the signal transfer unit. A remote monitoring station allows the presentation and review of data (including events) forwarded by the sensor band. ECG analysis software and a user-friendly graphical user interface are provided to remotely analyze the transmitted data and to permit system maintenance and upkeep. The system of the invention has useful application to the collection of patient clinical data during drug trials and medical testing for regulatory approvals as well as management of patients with chronic diseases.
Owner:CLEARPATH PARTNERS
Method and system for integrated medical tracking
InactiveUS20090099876A1Thorough and integrated and accurate data collectionAccurate data collectionMechanical/radiation/invasive therapiesDiagnostic recording/measuringPatient dataComputer science
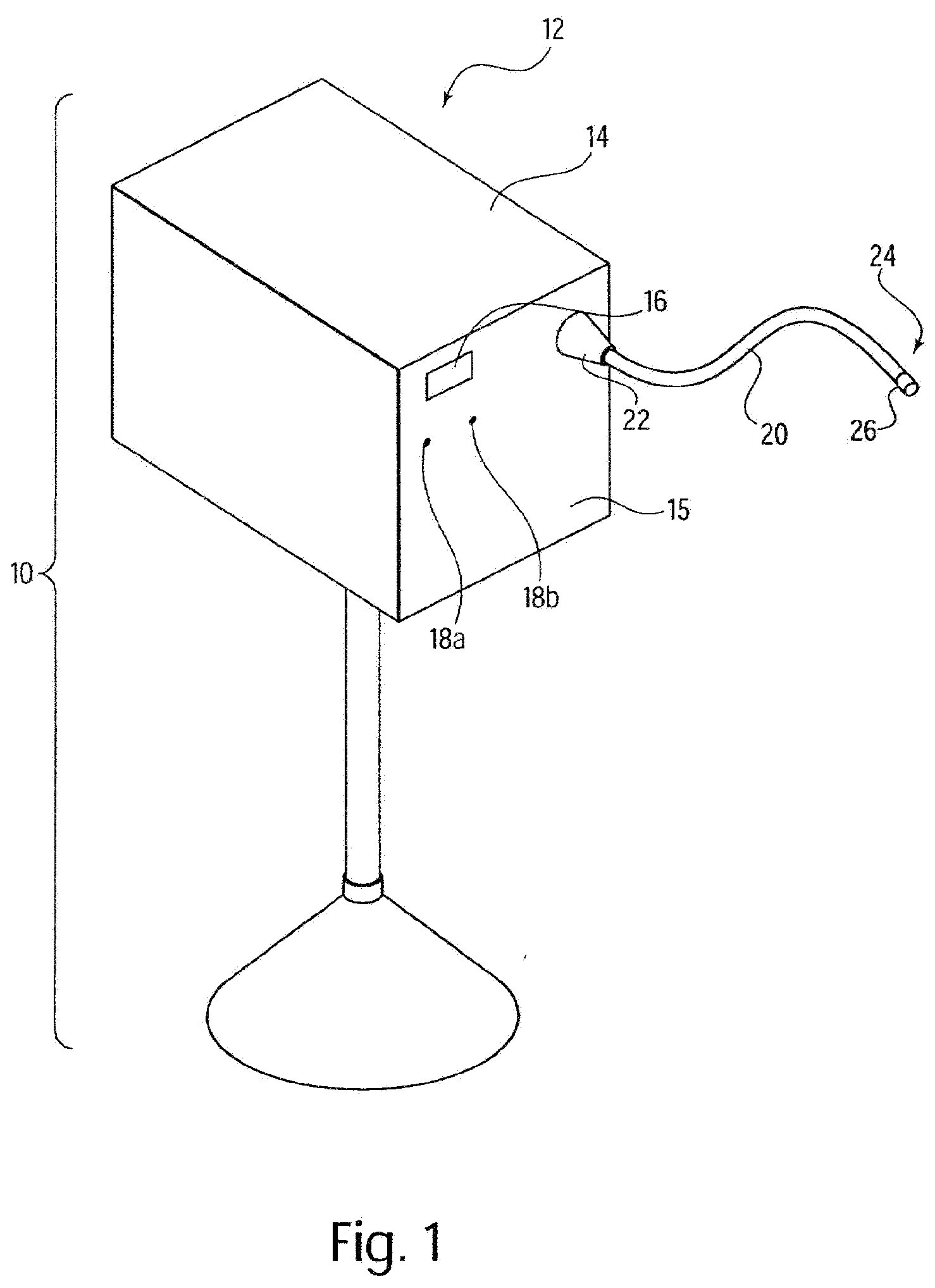
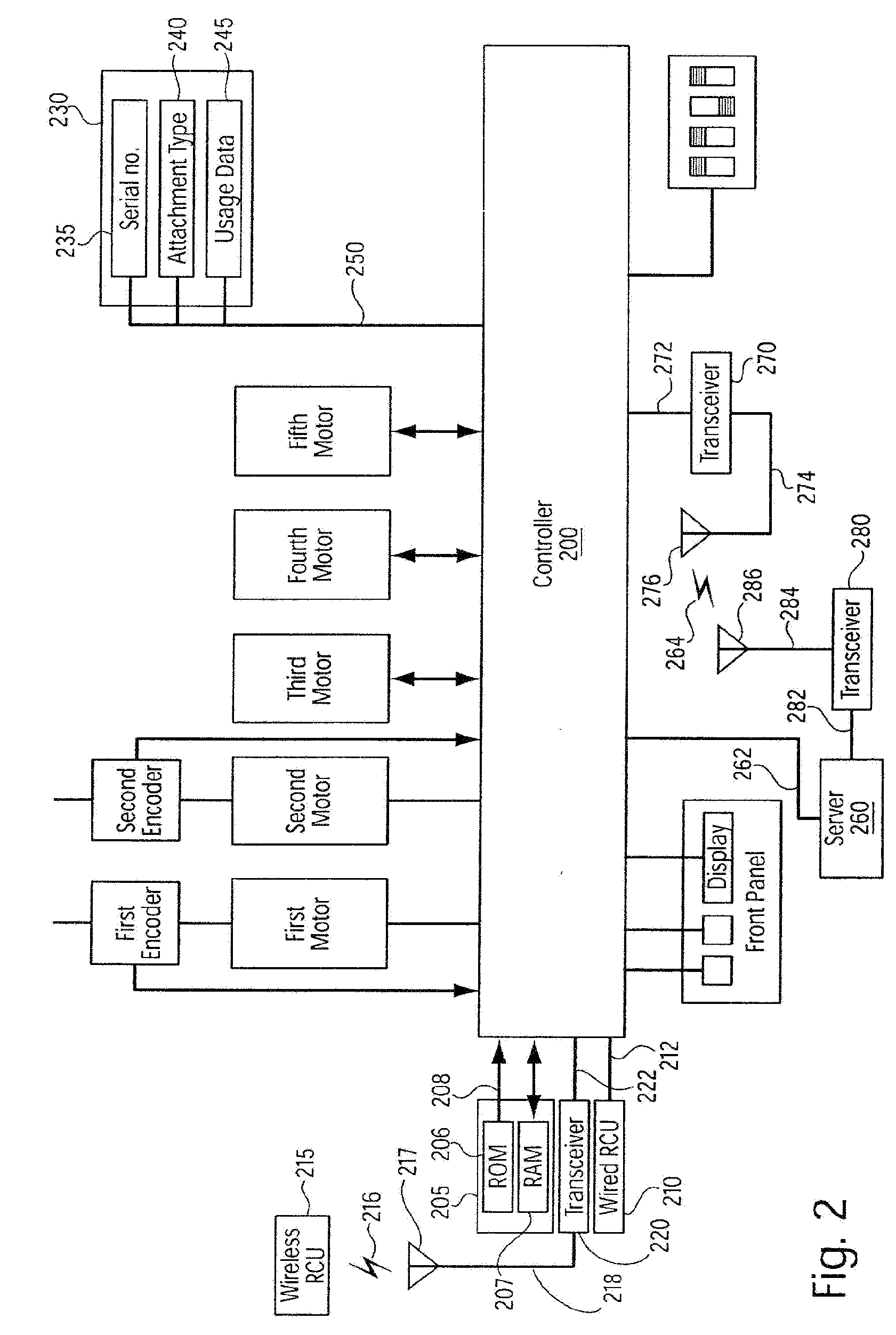
A system for processing data provided by medical devices. The system includes a medical device having a memory device configured to store medical device information. The system also includes an information system connected to the medical device. The information system includes at least a server configured to receive at least a portion of the medical device information from the memory device of the medical device. The server is also configured to employ the medical device information to process at least one of patient data, prescription data and inventory / ordering data. The medical device information may include serial number data, device identifier data, operation data and / or usage data for the medical device. The patient data may include at least one of patient tracking data, patient recordkeeping data and patient billing data. The prescription data may include prescription issuance data, prescription filling data and prescription tracking data. The inventory / ordering data may include at least one of maintenance and replacement schedule data, administrator notification data, and automatically-generated medical device order data.
Owner:TYCO HEALTHCARE GRP LP
System for and method of collecting and populating a database with physician/patient data for processing to improve practice quality and healthcare delivery
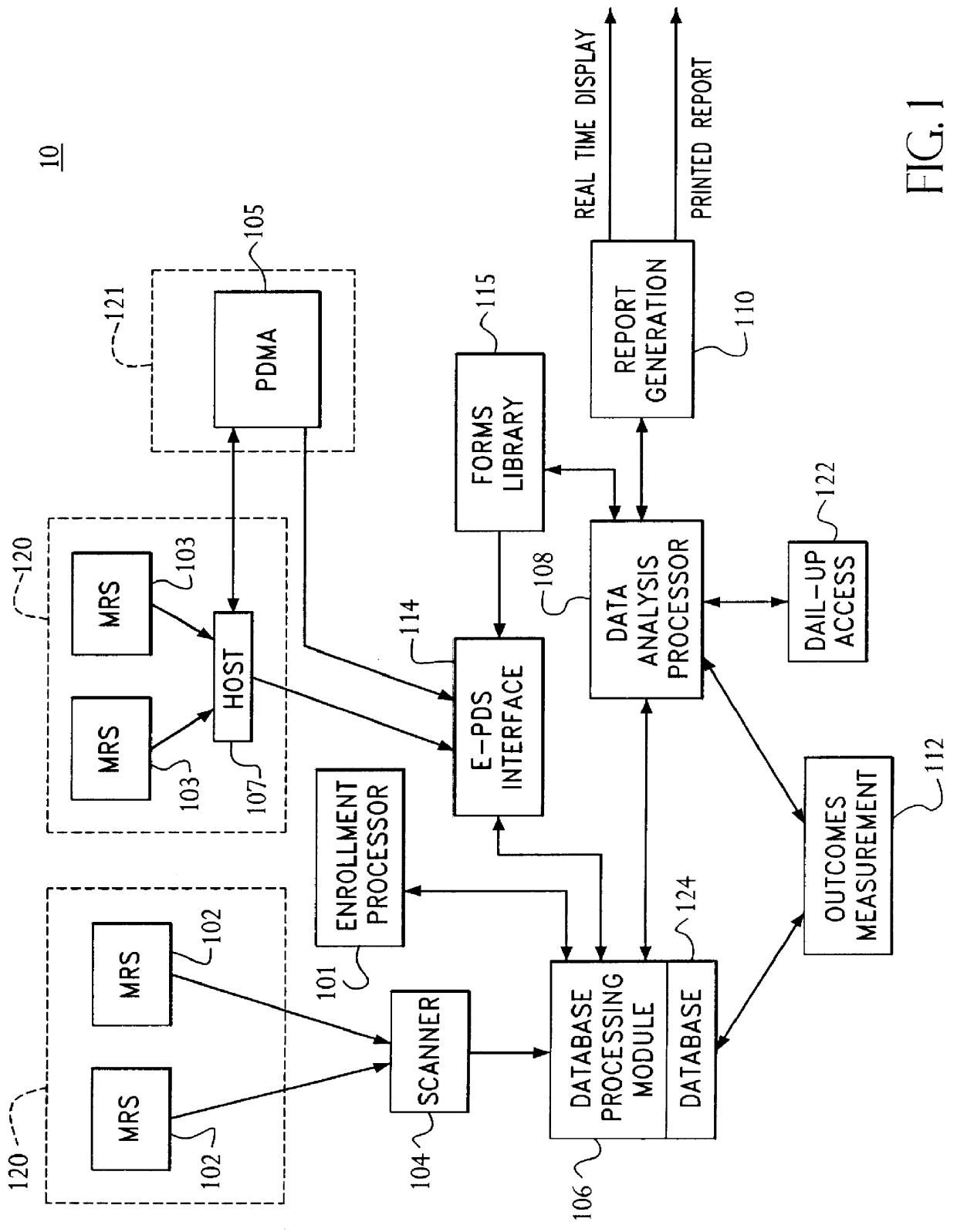
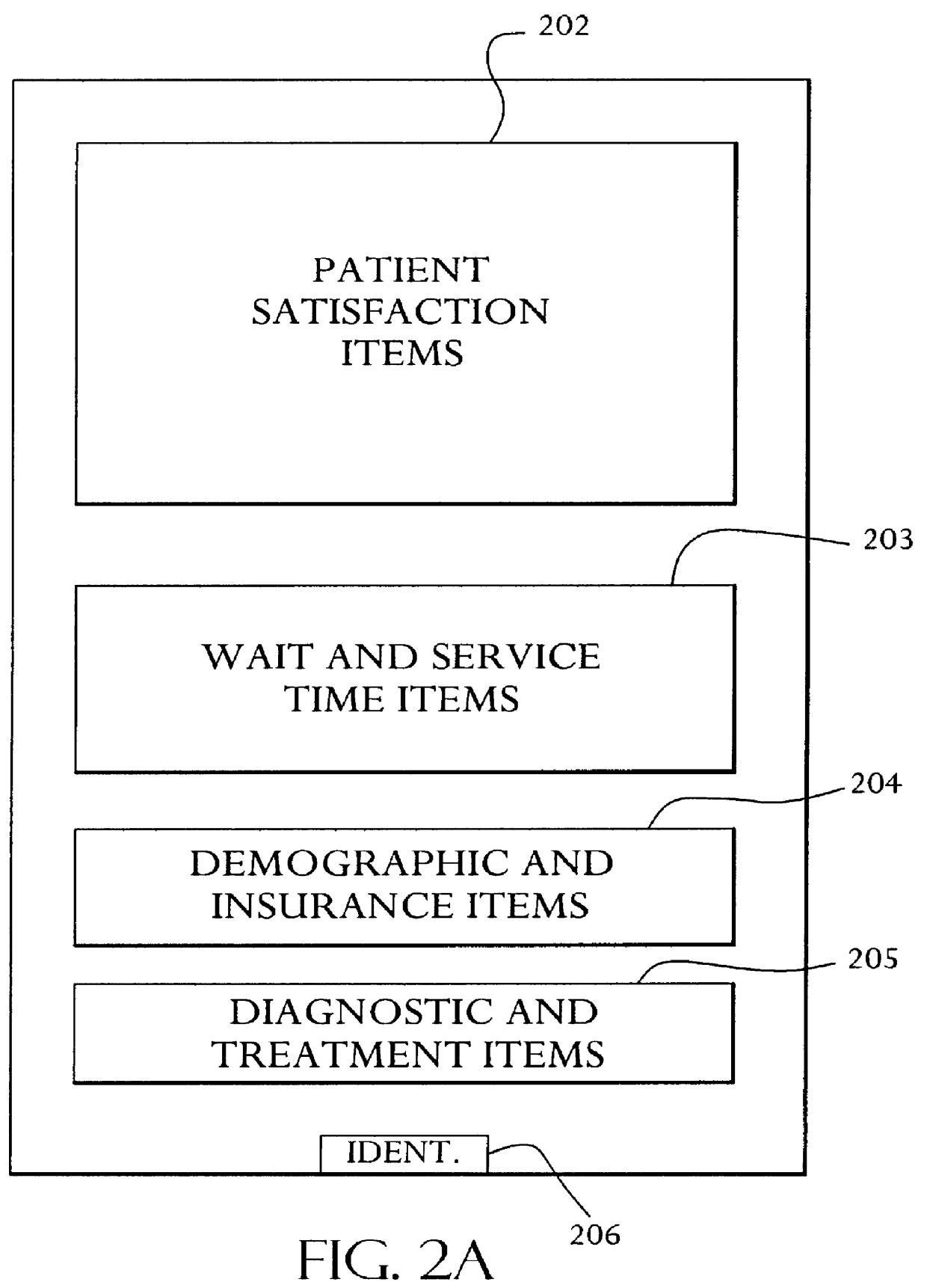
A system and method relates to the field of building and administrating a patient management and health care management database containing data relevant to the clinical care of patients, to the management of the practices to which the patients belong, and to outcomes of that health care and practice management. The disclosed system encompasses (i) designing and administering paper and pen and hand held computer survey instruments; (ii) administering and collecting completed surveys (iii) building and managing a database of information collected from the surveys; (iv) analyzing data collected from the surveys; (v) and providing clinical practices with summary information. Summary information may be used to improve patient care, health outcomes, and the management of physician practices.
Owner:PULSEGROUP
Medication delivery system
InactiveUS6985870B2Reduces potential medication errorDegrade some medicationHand manipulated computer devicesDrug and medicationsEmergency medicinePatient data
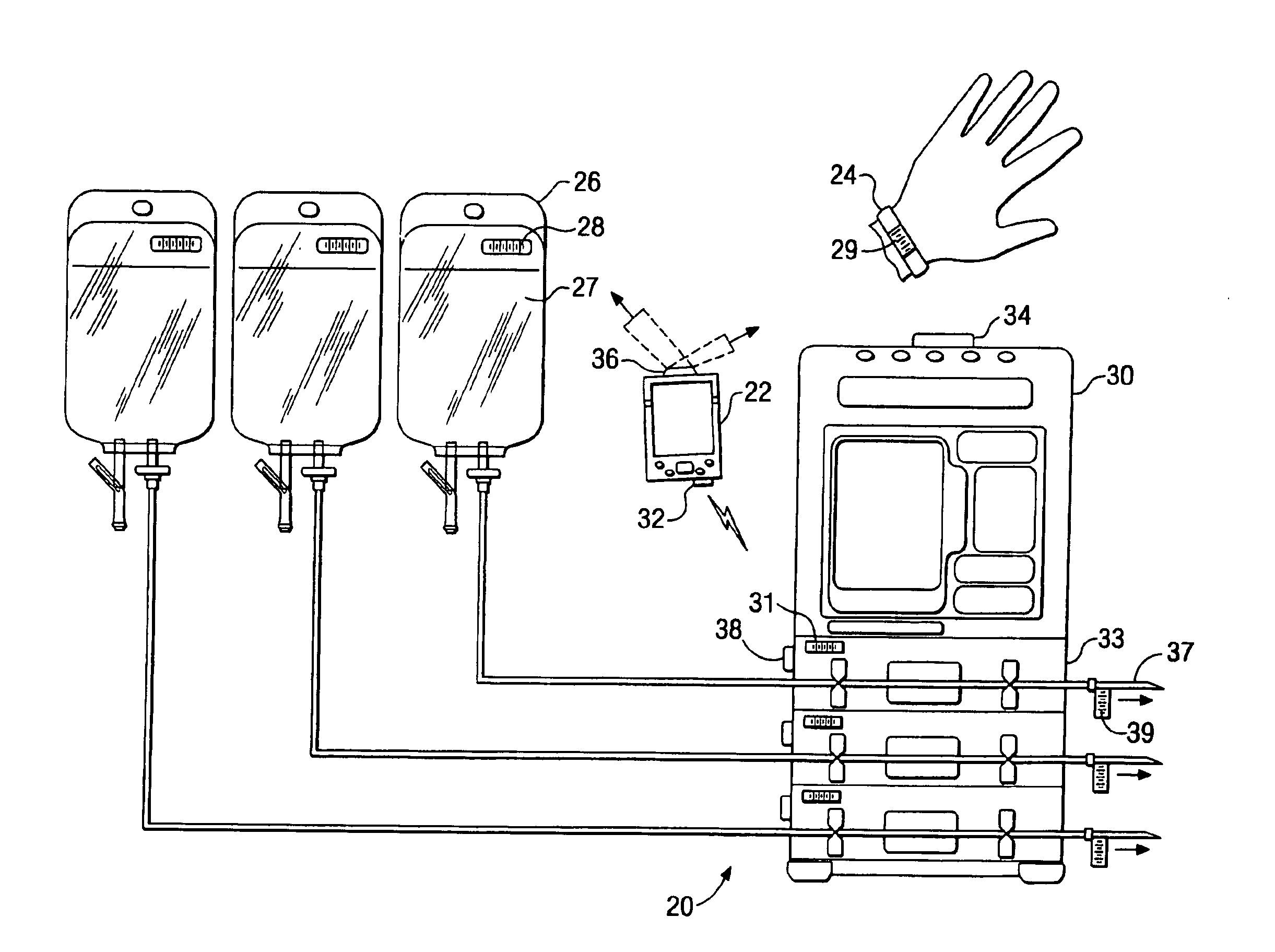
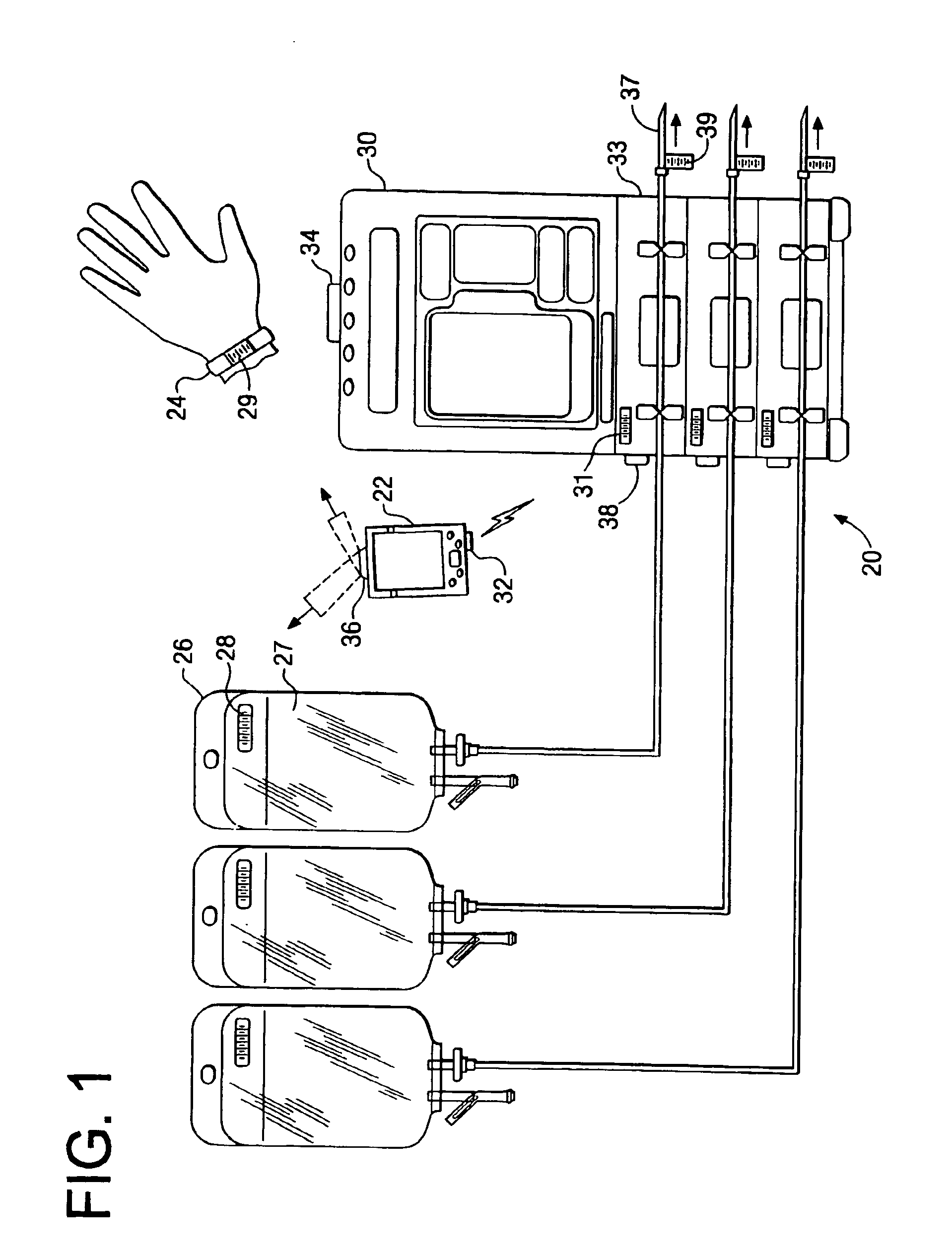
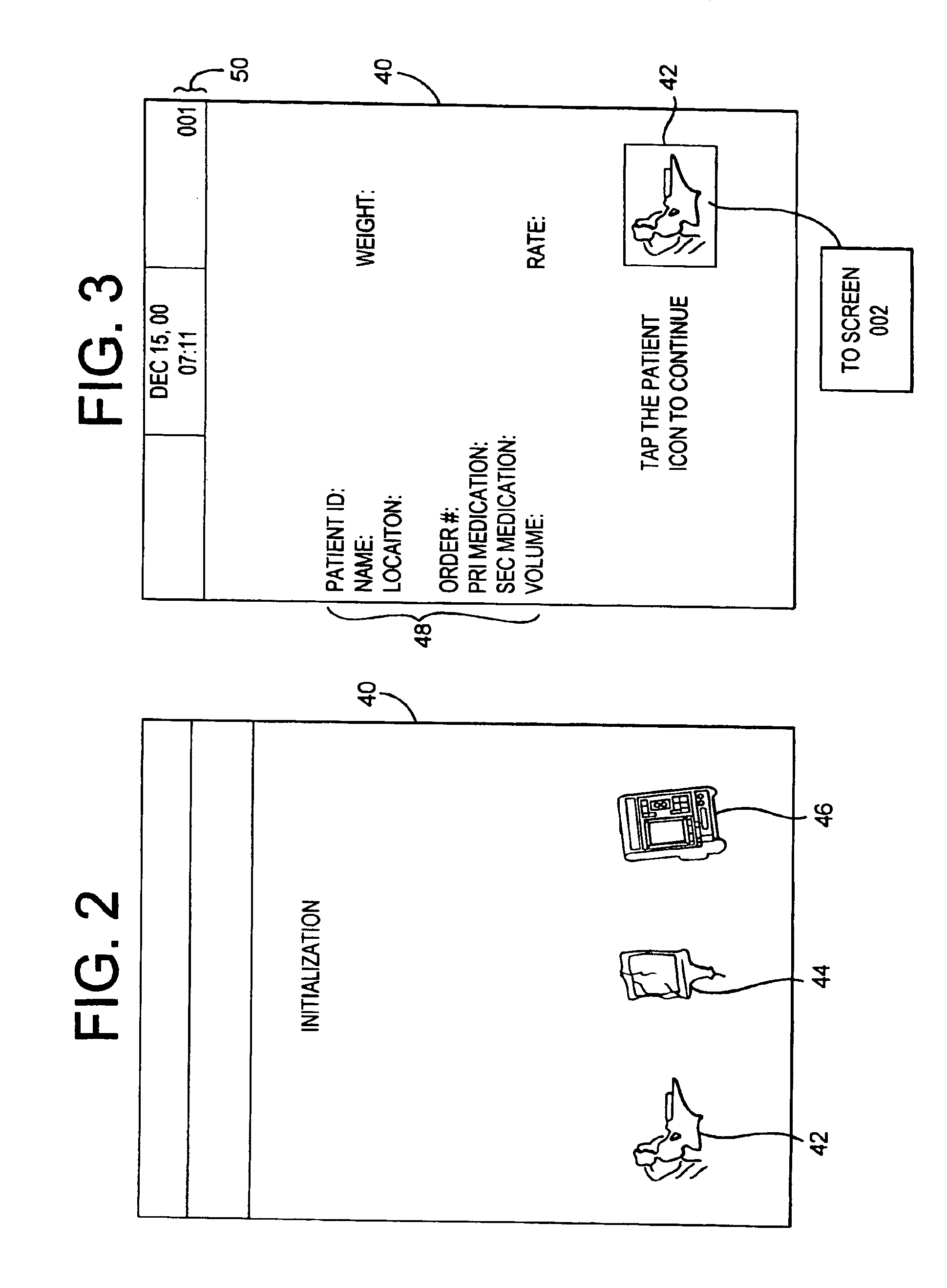
A medication delivery system (20) having features of the present invention comprises a medical container (26) holding a prescribed medication (27) to be delivered to a patient, a tag 24 adapted to be worn by the patient, a handheld computing device (22), and an electronic medication delivery device (30). Data on the medication (27) is contained in a first label (28) on the medication container (27). The first label (28) also contains the instruction on how the medication is delivered to the patient, including the appropriate settings for an electronic medication delivery device for delivering the medication to the patient. Patient data is contained in a second label (29) on the tag (24) worn by the patient. The medication data, medication delivery instruction, and patient data are provided in machine readable formats. The handheld computing device (22) reads the medication data and the medication delivery instruction on the medication container (26) and the patient data on the patient tag (24). The handheld computing device (22) stores the information obtained and performs a matching check to confirm that the medication data matches with the patient data. Upon a confirmed match, it transmits the medication delivery instruction to the electronic medication delivery device (30), which downloads the instruction, programs the delivery device 30, and prompts an operator to begin delivering the medication (27) to the patient according to the downloaded instruction.
Owner:BAXTER INT INC
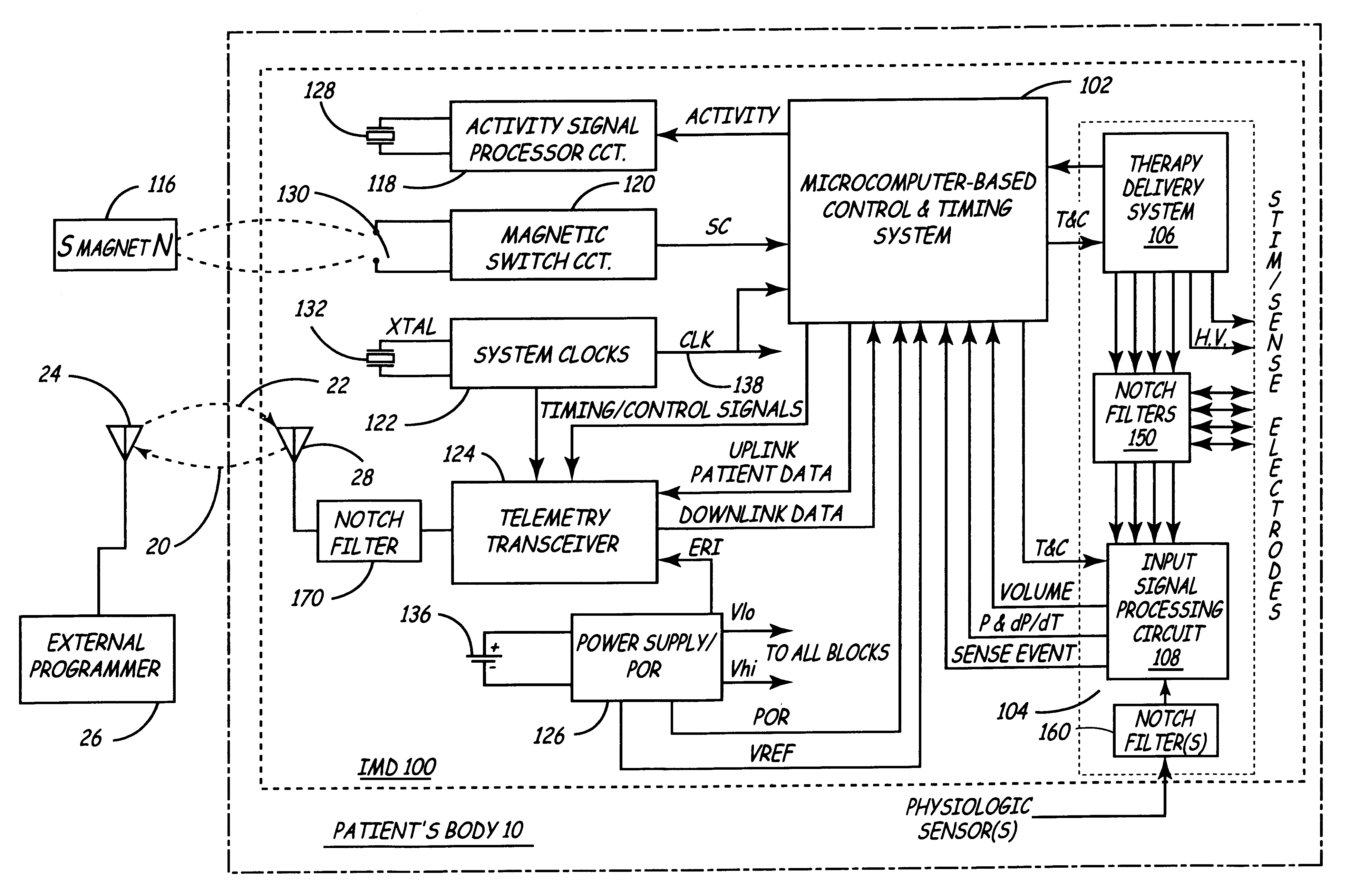
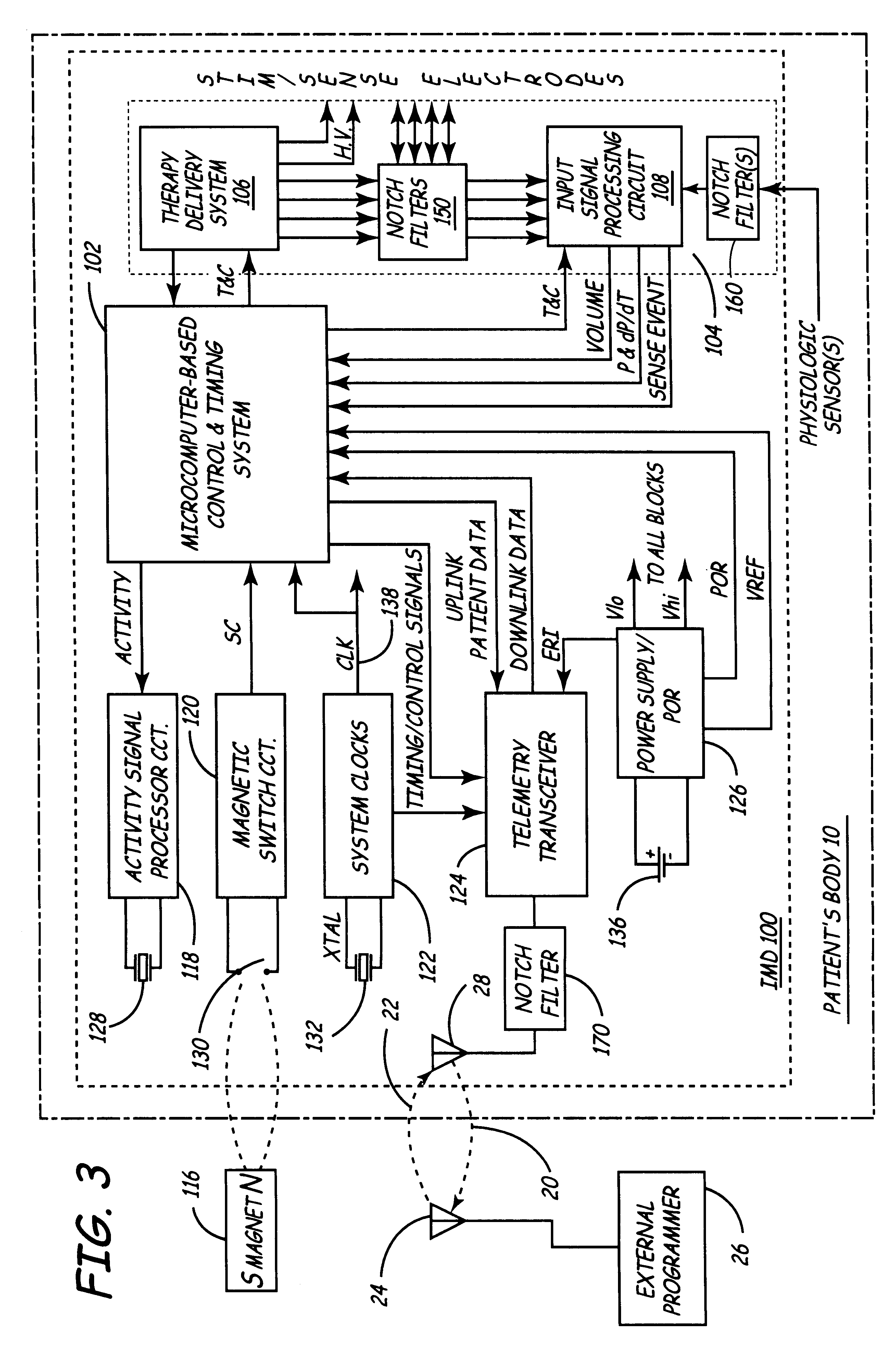
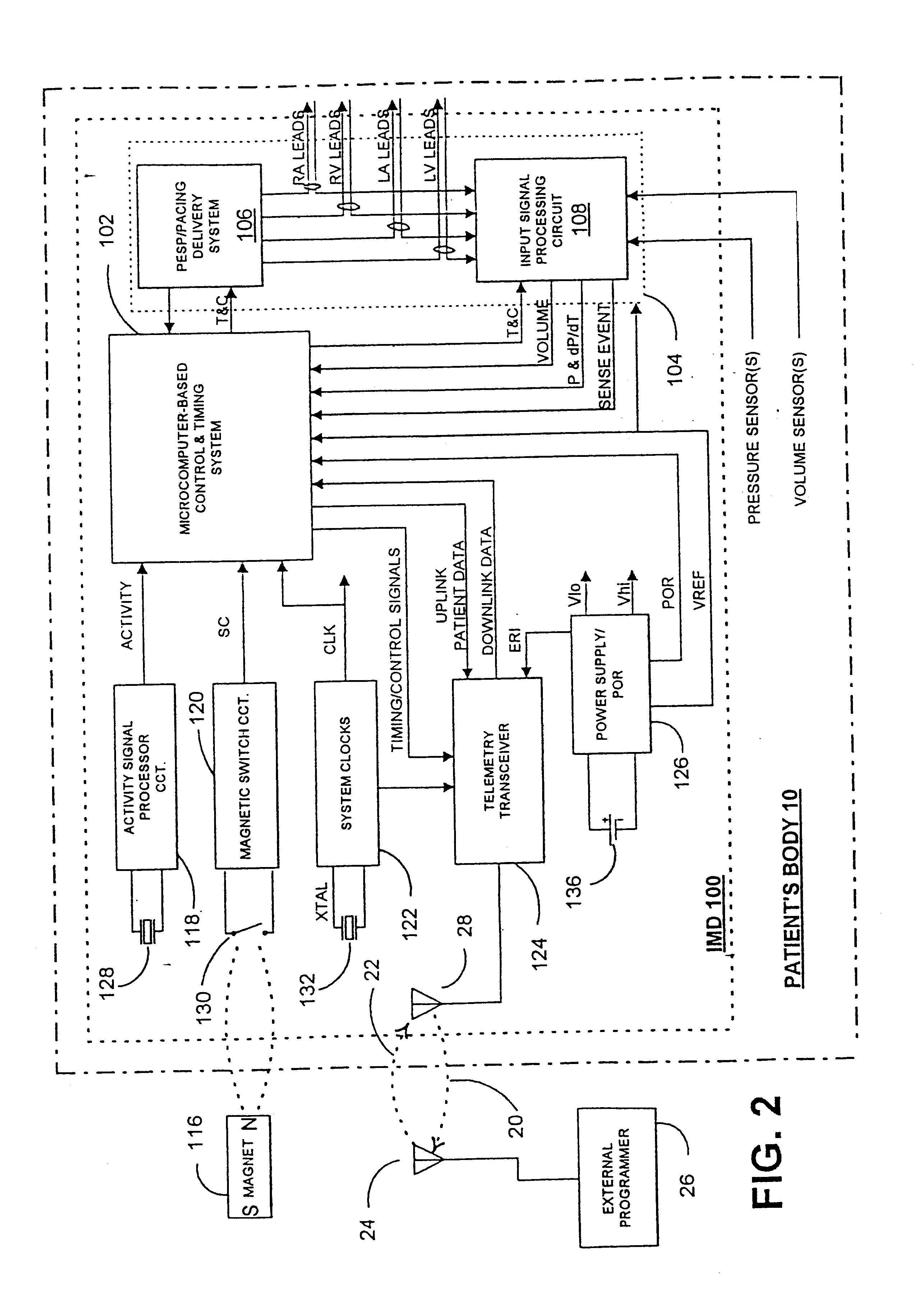
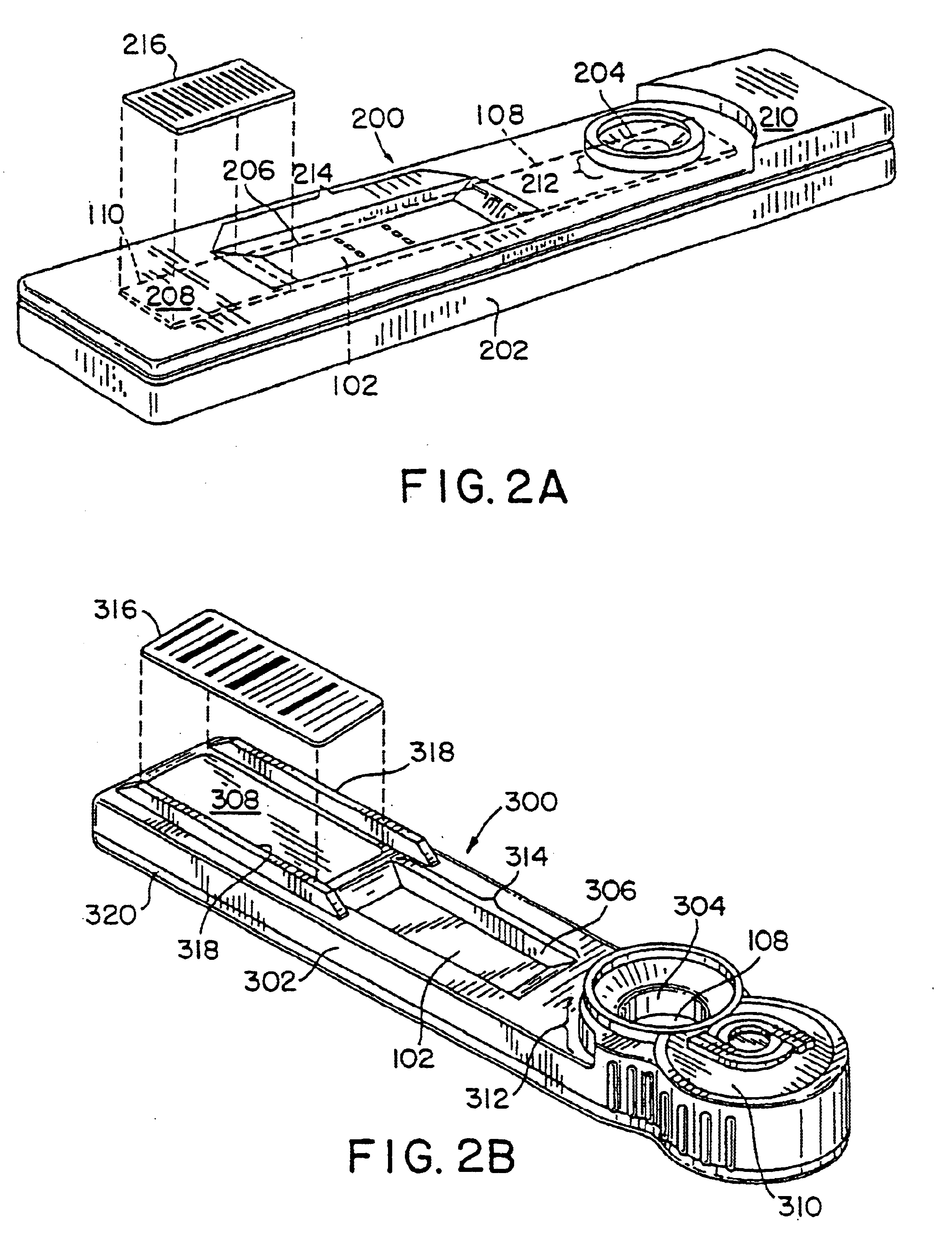
Implantable medical device incorporating integrated circuit notch filters
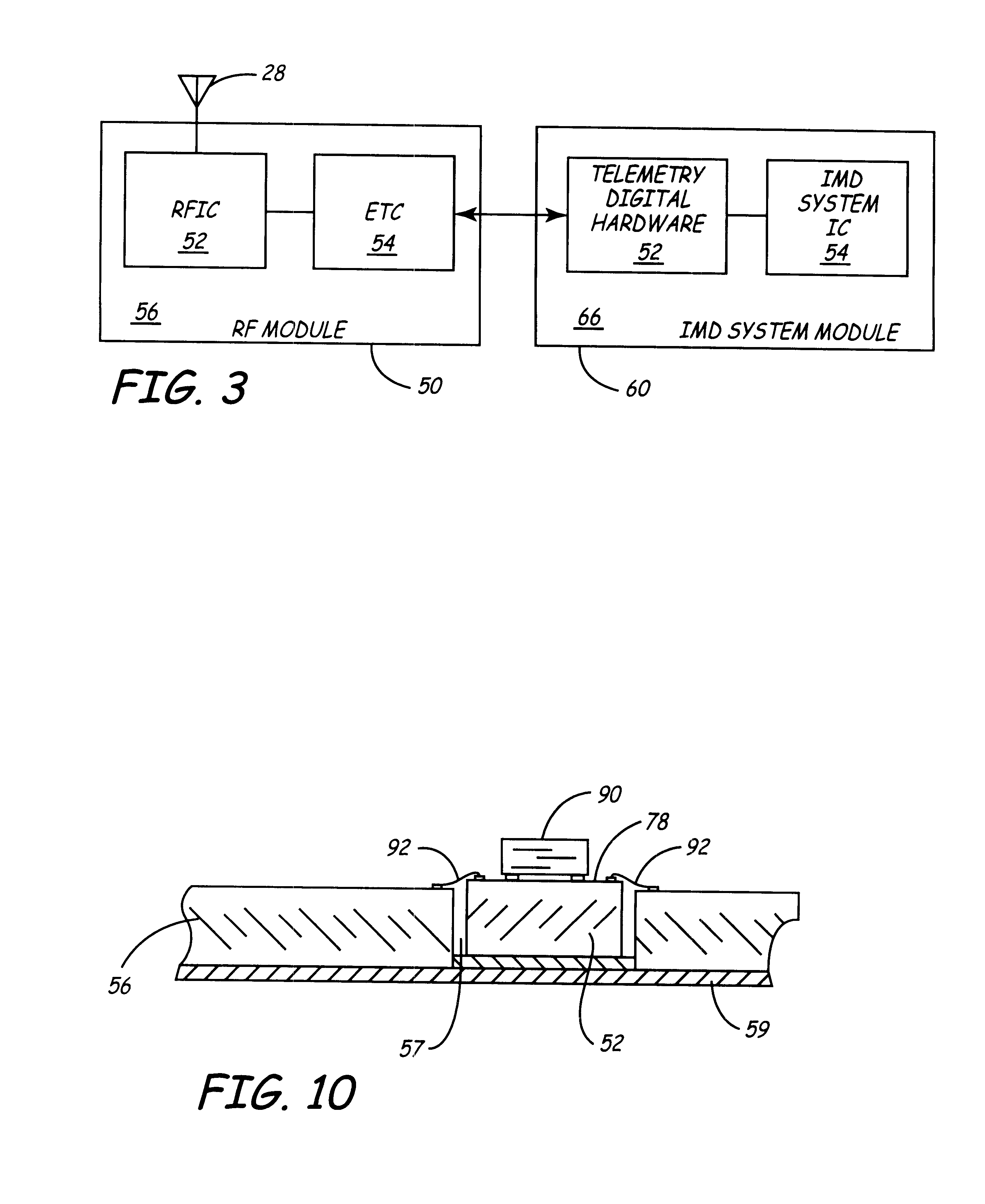
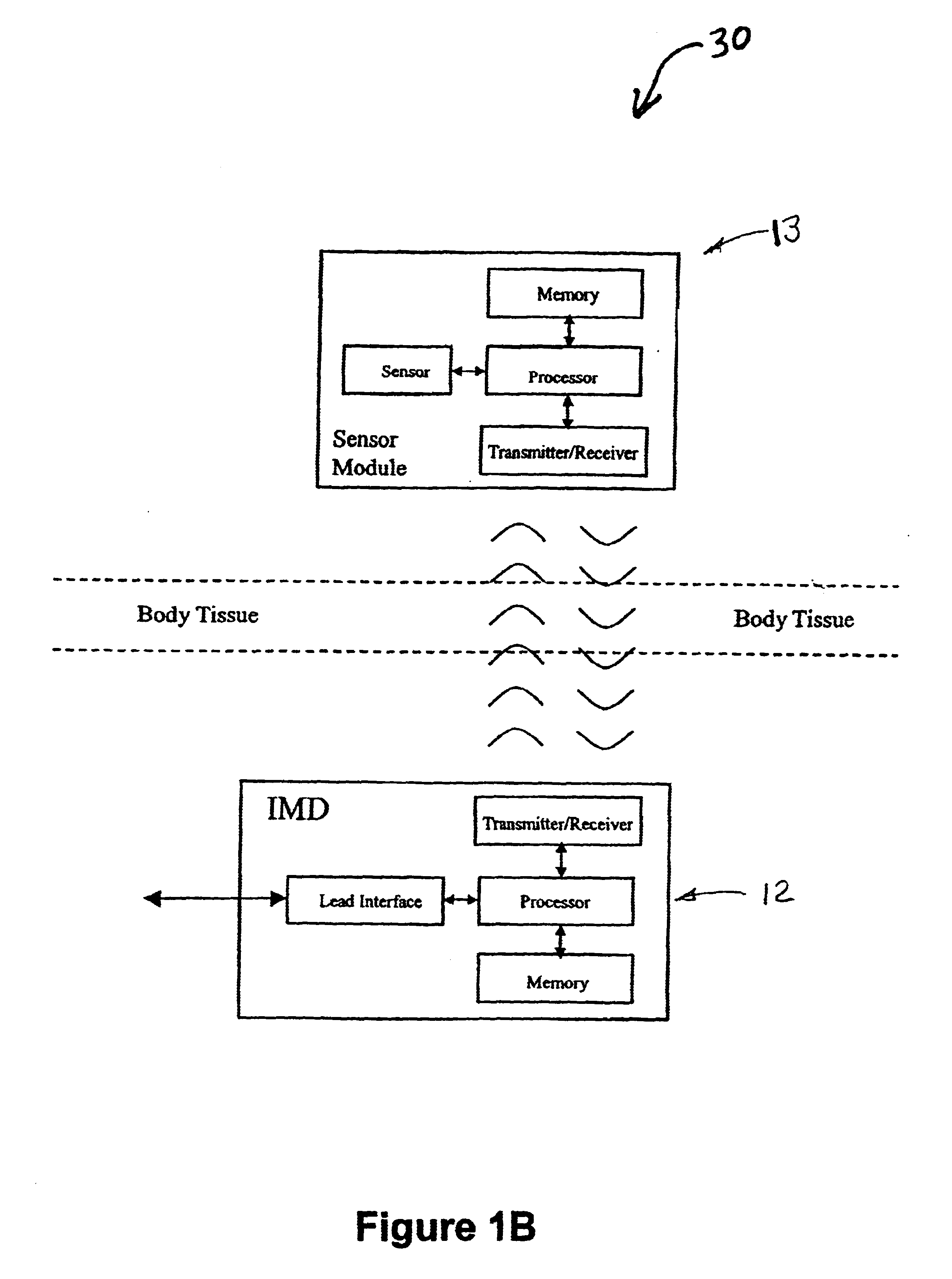
Implantable medical devices (IMDs) having sense amplifiers for sensing physiologic signals and parameters, RF telemetry capabilities for uplink transmitting patient data and downlink receiving programming and interrogation commands to and from an external programmer or other medical device are disclosed. At least one IC chip and discrete components have a volume and dimensions that are optimally minimized to reduce its volumetric form factor. Miniaturization techniques include forming notch filters of MEMS structures or forming discrete circuit notch filters by one or more of: (1) IC fabricating inductors into one or more IC chips mounted to the RF module substrate; (2) mounting each IC chip into a well of the RF module substrate and using short bonding wires to electrically connect bond pads of the RF module substrate and the IC chip; and (3) surface mounting discrete capacitors over IC chips to reduce space taken up on the RF module substrate. The IC fabricated inductors are preferably fabricated as planar spiral wound conductive traces formed of high conductive metals to reduce trace height and width while maintaining low resistance, thereby reducing parasitic capacitances between adjacent trace side walls and with a ground plane of the IC chip. The spiral winding preferably is square or rectangular, but having truncated turns to eliminate 90° angles that cause point-to-point parasitic capacitances. The planar spiral wound conductive traces are further preferably suspended over the ground plane of the IC chip substrate by micromachining underlying substrate material away to thereby reduce parasitic capacitances.
Owner:MEDTRONIC INC
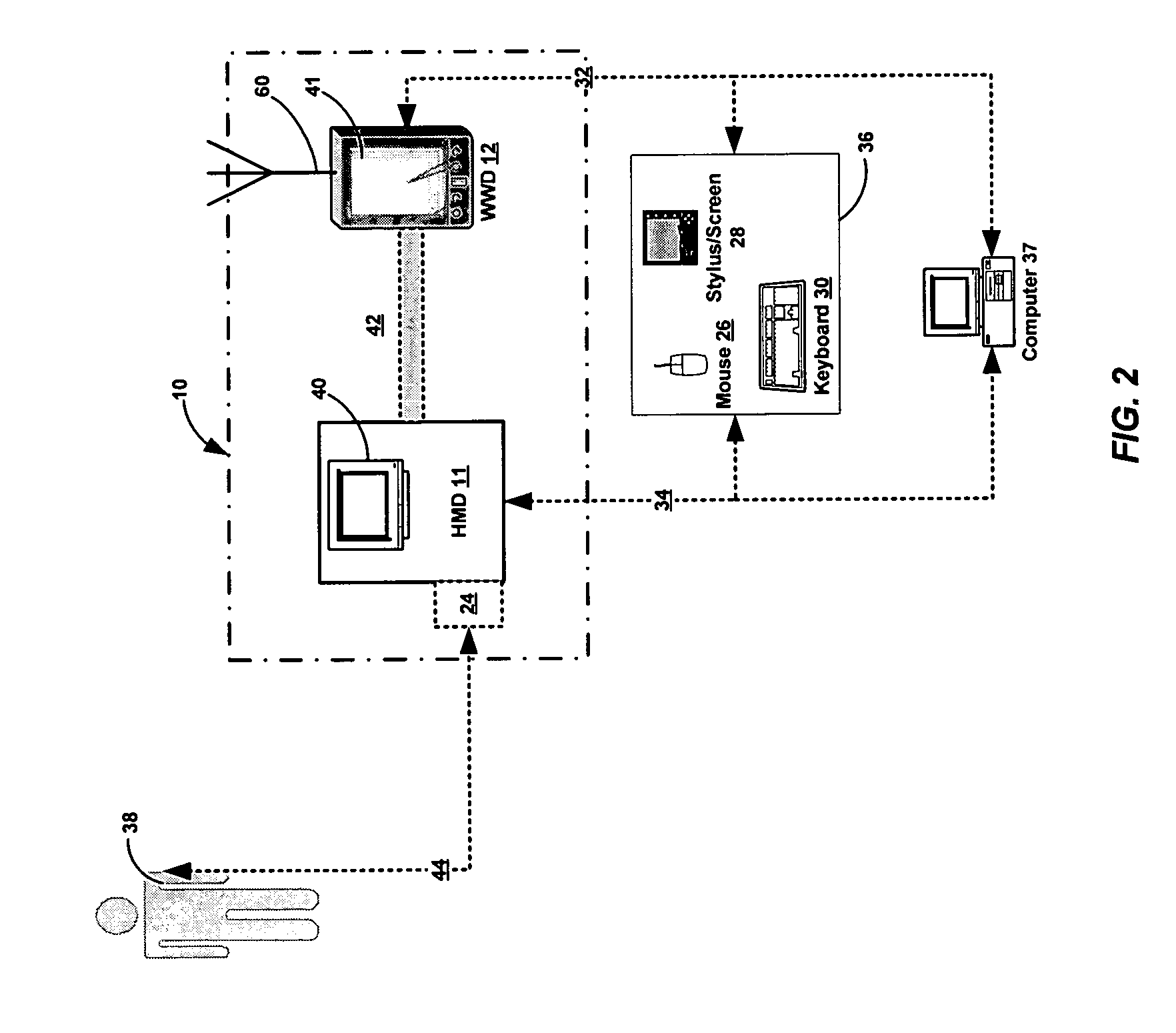
Method and apparatus for health and disease management combining patient data monitoring with wireless internet connectivity
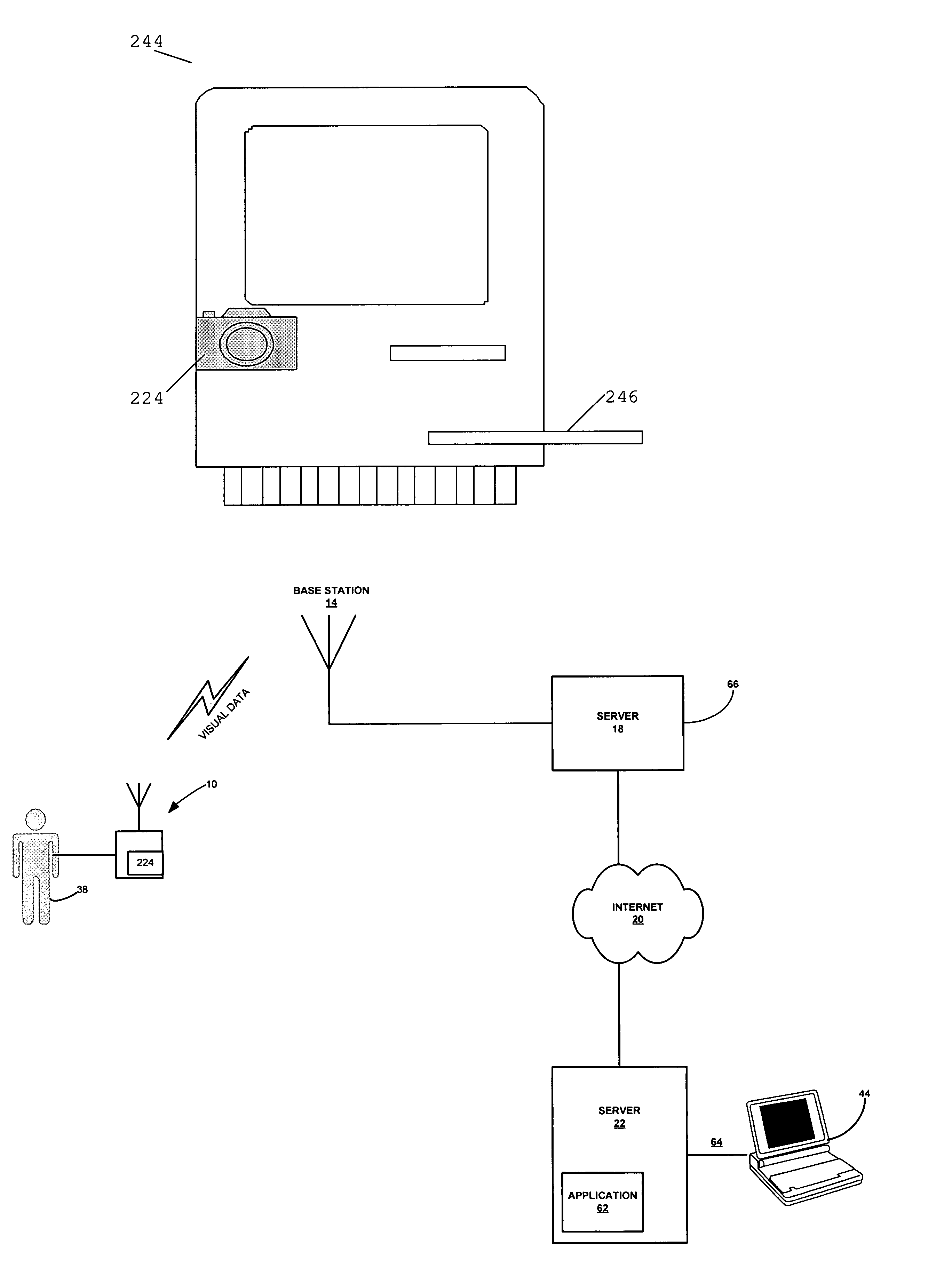
InactiveUS7156809B2Maintain abilityReduced functionalityPhysical therapies and activitiesBioelectric signal measurementOperational systemPatient data
Embodiments of the invention provide a method and apparatus for a wireless health monitoring system for interactively monitoring a disease or health condition of a patient by connecting a mobile phone to or with a digital camera and / or a medical monitoring device. The health related data or visual information from the camera is transmitted to a server using standard internet protocols and may be integrated with various operating systems for handheld or wireless devices, especially those with enhanced capabilities for handing images and visual data.
Owner:KONINK PHILIPS ELECTRONICS NV +1
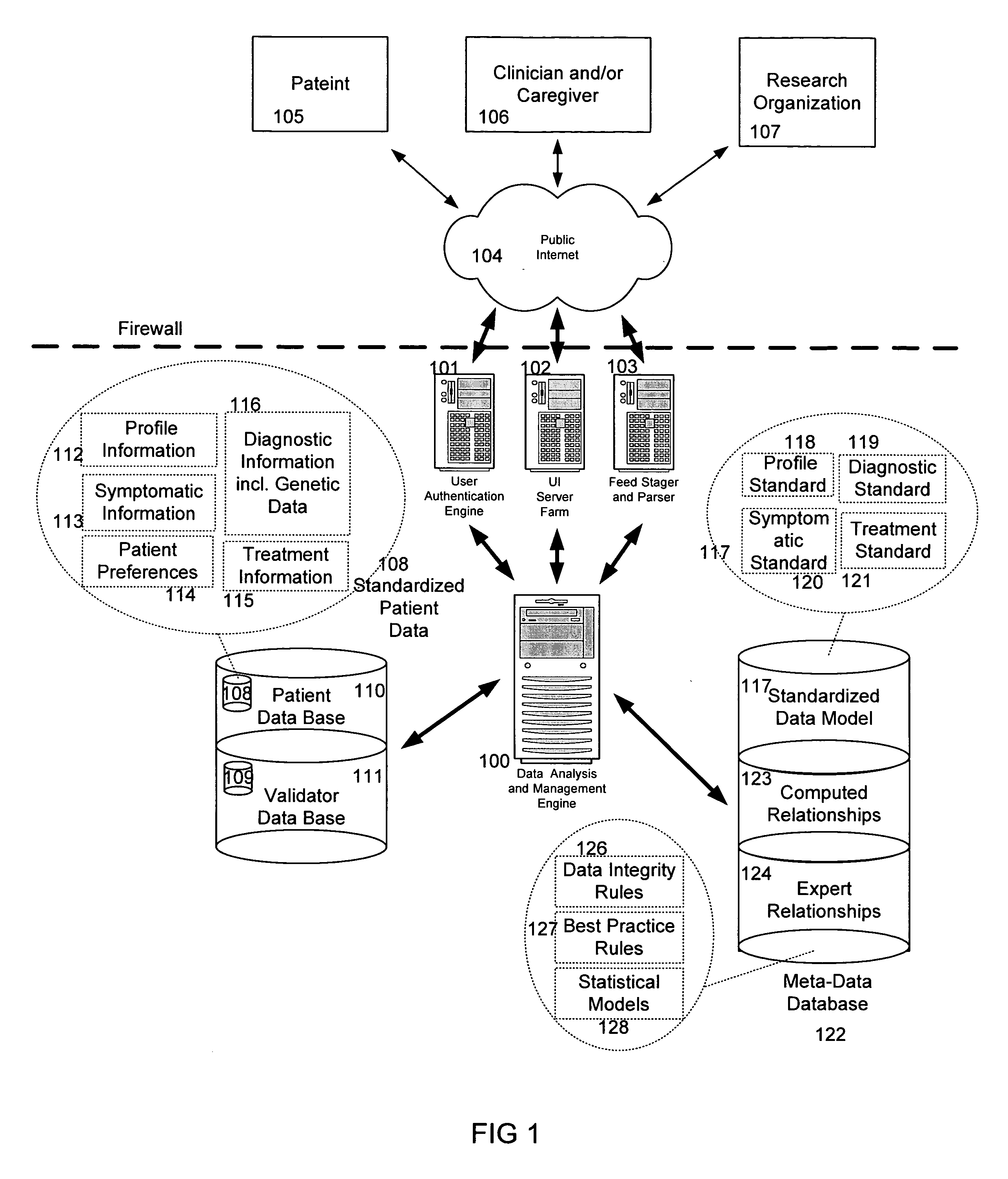
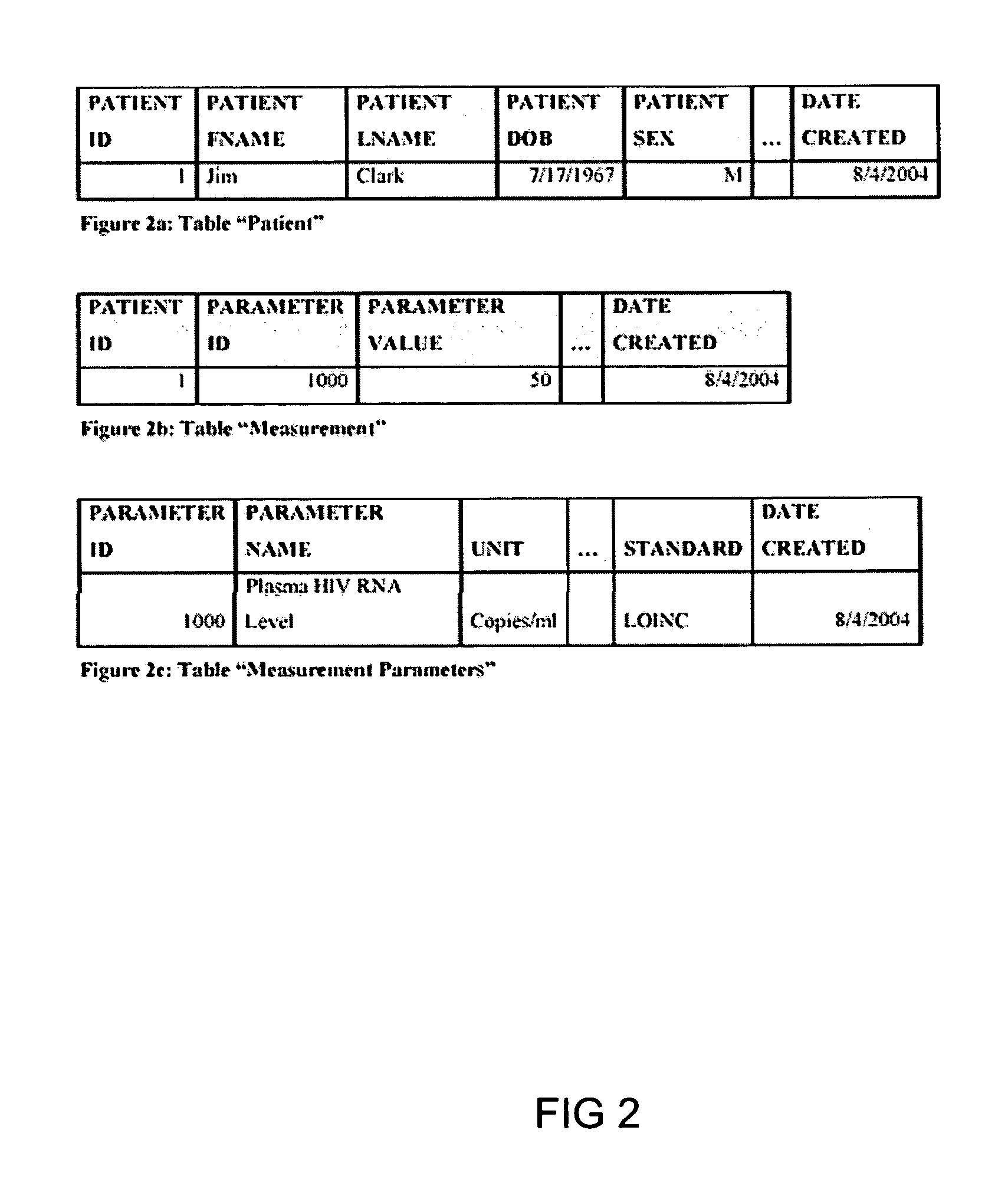
System and method for improving clinical decisions by aggregating, validating and analysing genetic and phenotypic data
The information management system disclosed enables caregivers to make better decisions, faster, using aggregated genetic and phenotypic data. The system enables the integration, validation and analysis of genetic, phenotypic and clinical data from multiple subjects who may be at distributed facilities. A standardized data model stores a range of patient data in standardized data classes that encompass patient profile information, patient symptomatic information, patient treatment information, and patient diagnostic information including genetic information. Data from other systems is converted into the format of the standardized data classes using a data parser, or cartridge, specifically tailored to the source system. Relationships exist between standardized data classes that are based on expert rules and statistical models. The relationships are used both to validate new data, and to predict phenotypic outcomes based on available data. The prediction may relate to a clinical outcome in response to a proposed intervention by a caregiver. The statistical models may be inhaled into the system from electronic publications that define statistical models and methods for training those models, according to a standardized template. Methods are described for selecting, creating and training the statistical models to operate on genetic, phenotypic and clinical data, in particular for underdetermined data sets that are typical of genetic information. The disclosure also describes how security of the data is maintained by means of a robust security architecture, and robust user authentication such as biometric authentication, combined with application-level and data-level access privileges.
Owner:NATERA
Systems, devices and methods for preventing, detecting and treating pressure-induced ischemia, pressure ulcers, and other conditions
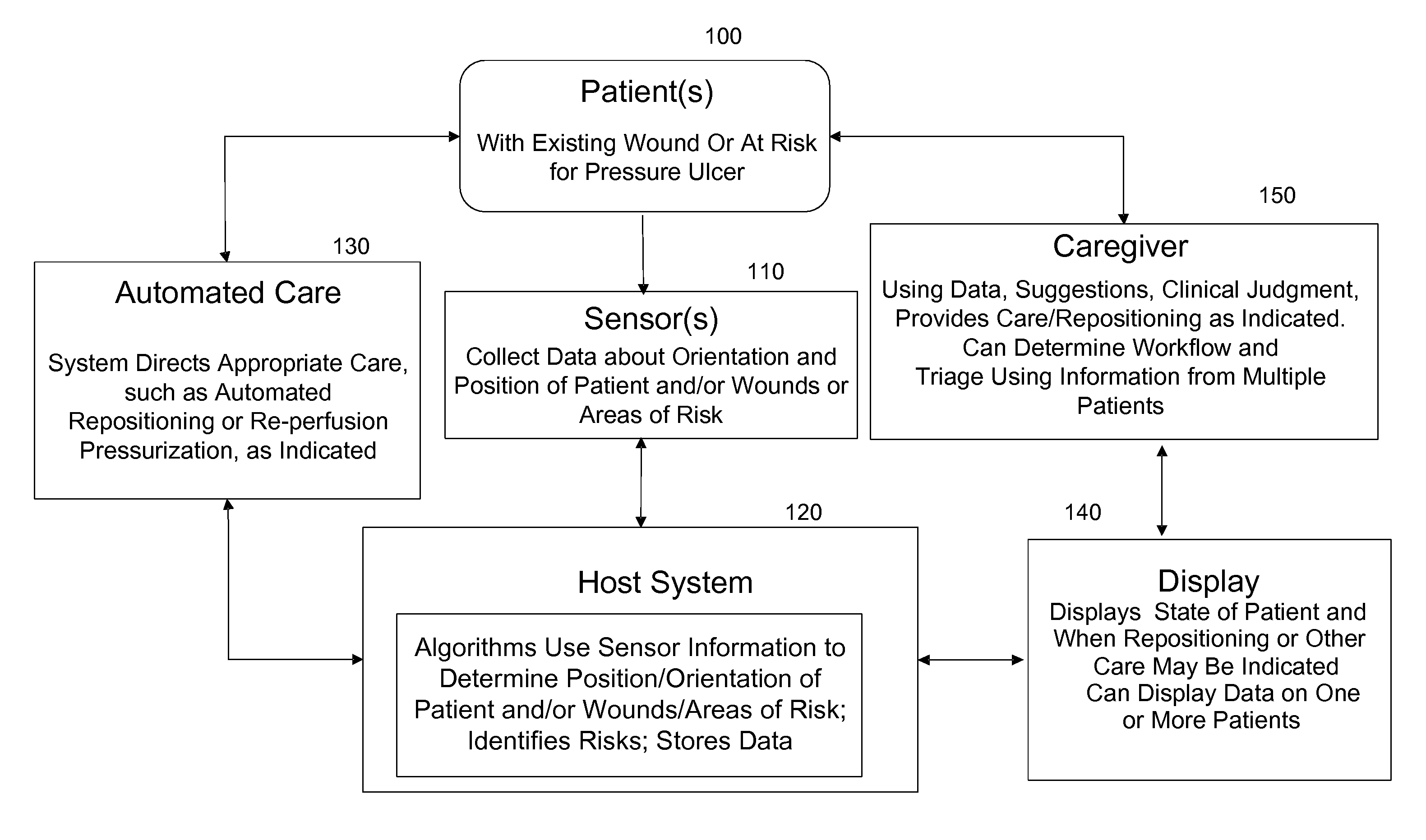
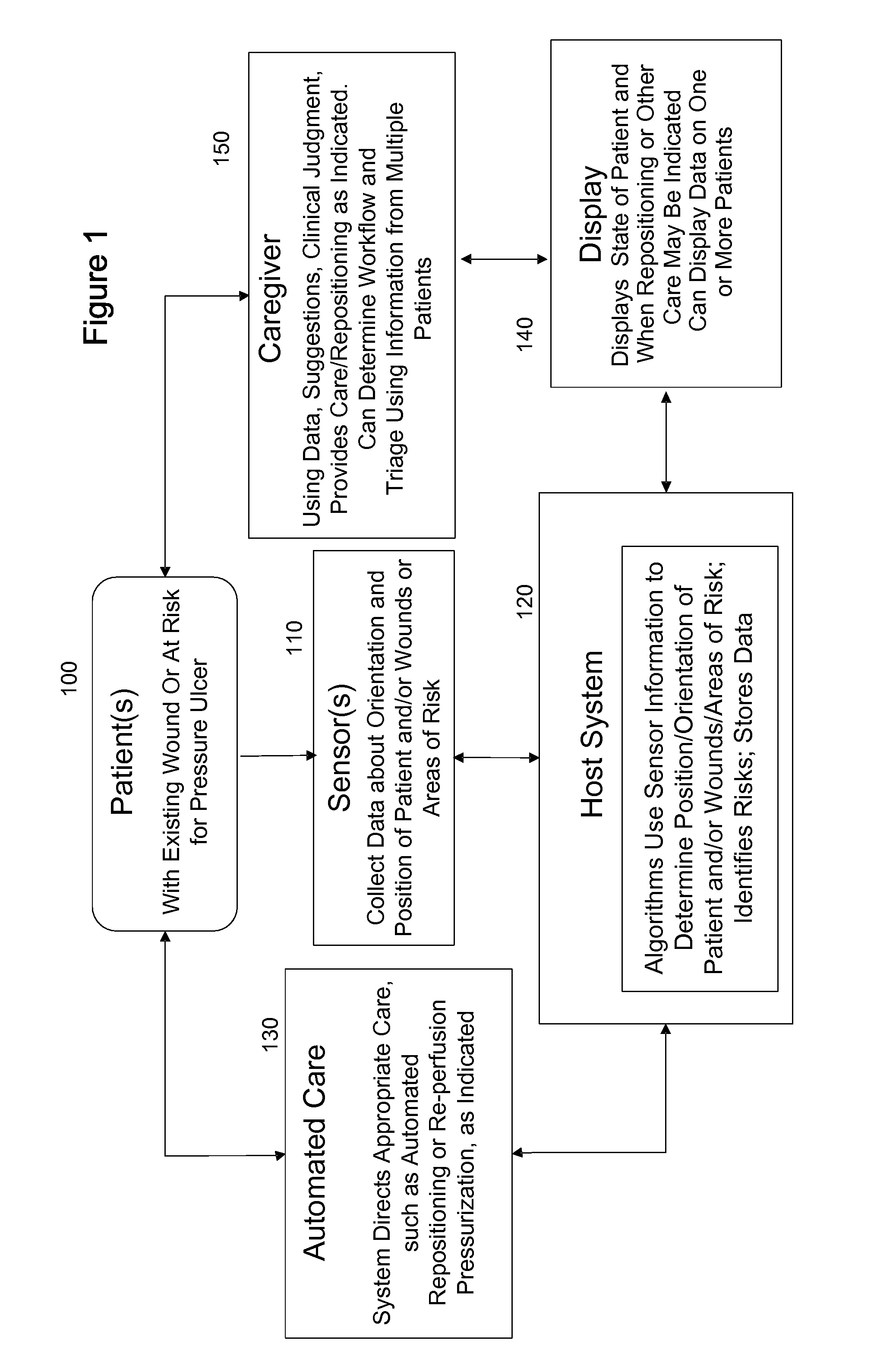
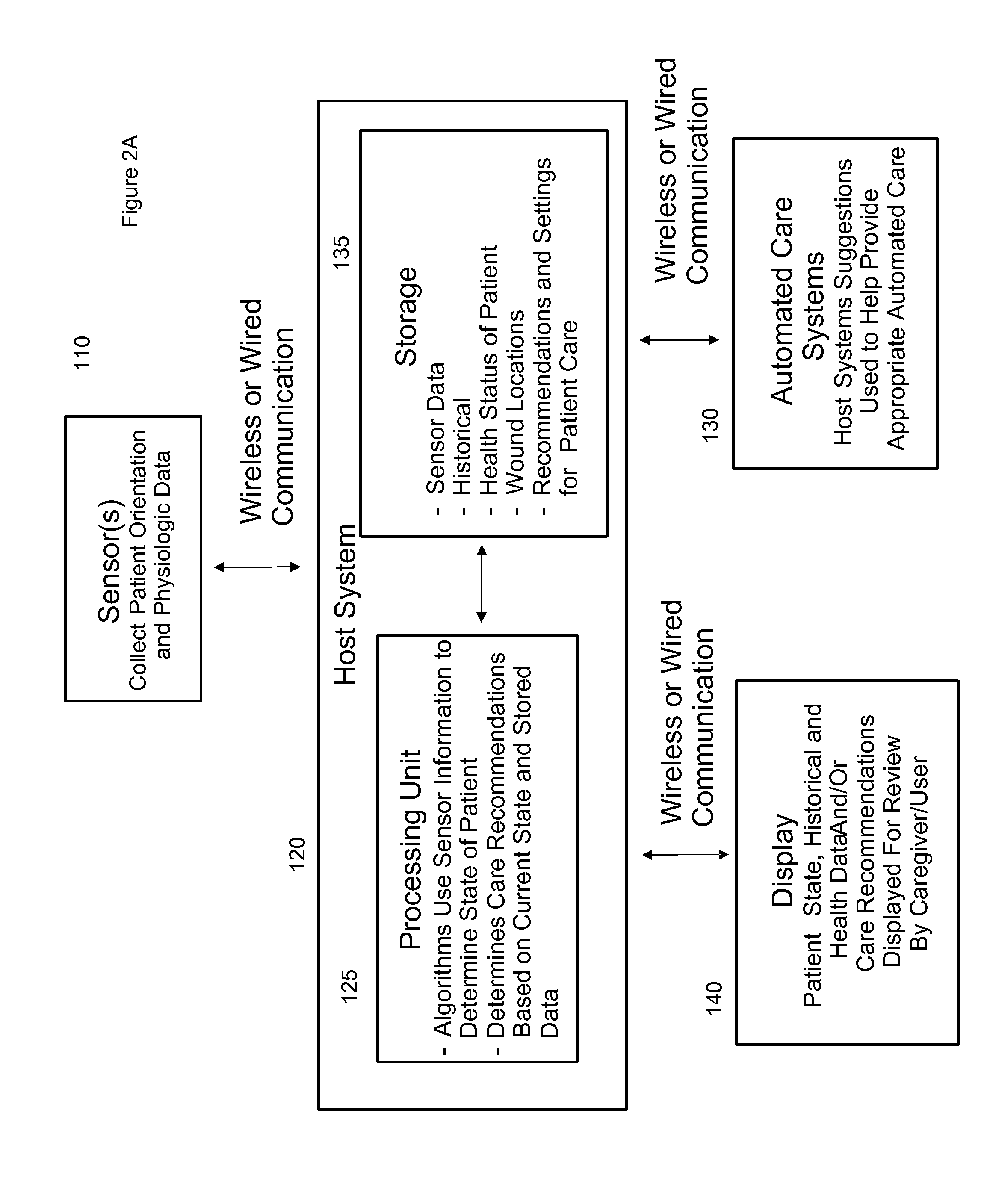
ActiveUS20110263950A1Minimize and eliminate physical contactPromote blood circulationMechanical/radiation/invasive therapiesOperating chairsAccelerometerPatient characteristics
A system for monitoring medical conditions including pressure ulcers, pressure-induced ischemia and related medical conditions comprises at least one sensor adapted to detect one or more patient characteristic including at least position, orientation, temperature, acceleration, moisture, resistance, stress, heart rate, respiration rate, and blood oxygenation, a host for processing the data received from the sensors together with historical patient data to develop an assessment of patient condition and suggested course of treatment. In some embodiments, the system can further include a support surface having one or more sensors incorporated therein either in addition to sensors affixed to the patient or as an alternative thereof. The support surface is, in some embodiments, capable of responding to commands from the host for assisting in implementing a course of action for patient treatment. The sensor can include bi-axial or tri-axial accelerometers, as well as resistive, inductive, capactive, magnetic and other sensing devices, depending on whether the sensor is located on the patient or the support surface, and for what purpose.
Owner:LEAF HEALTHCARE
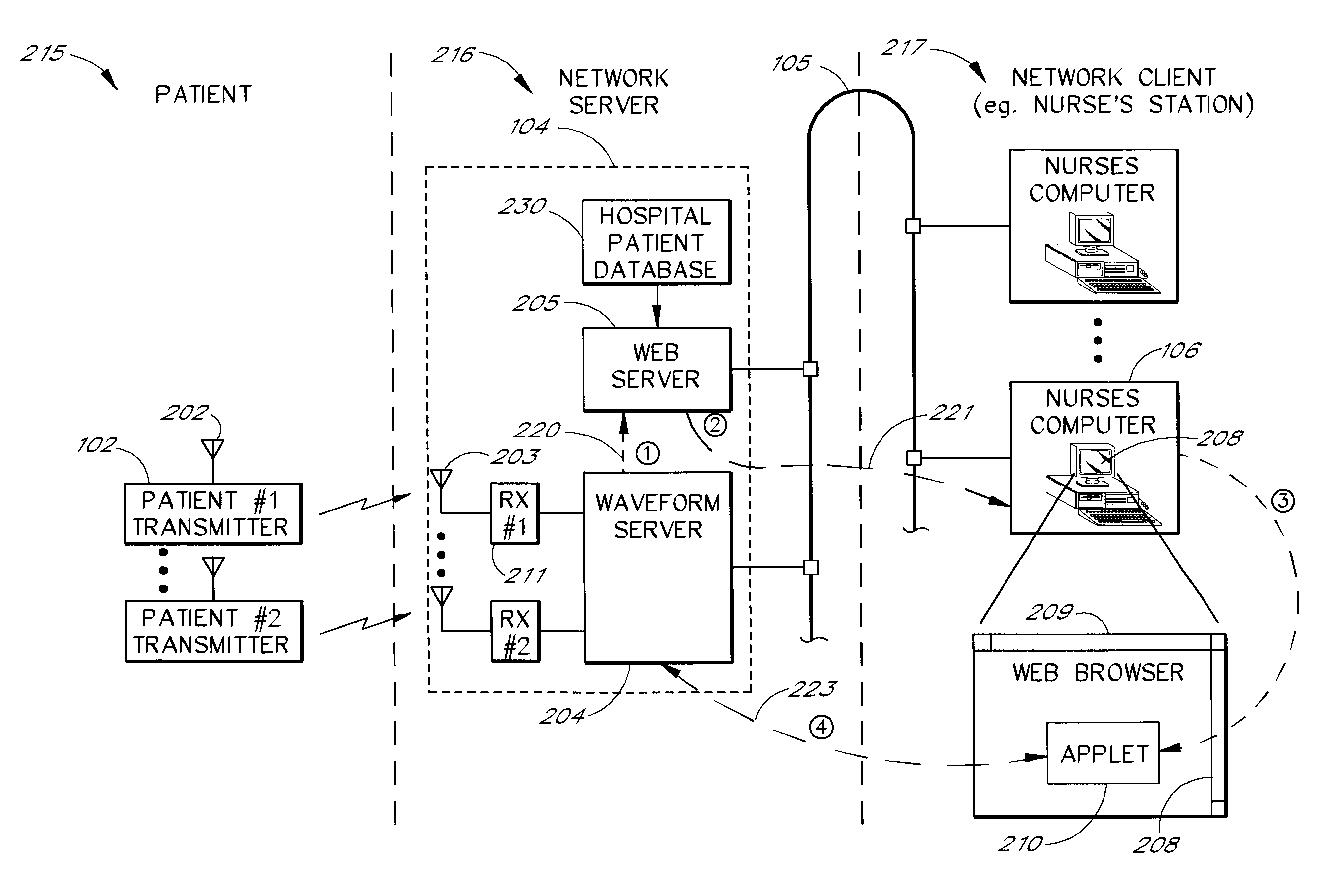
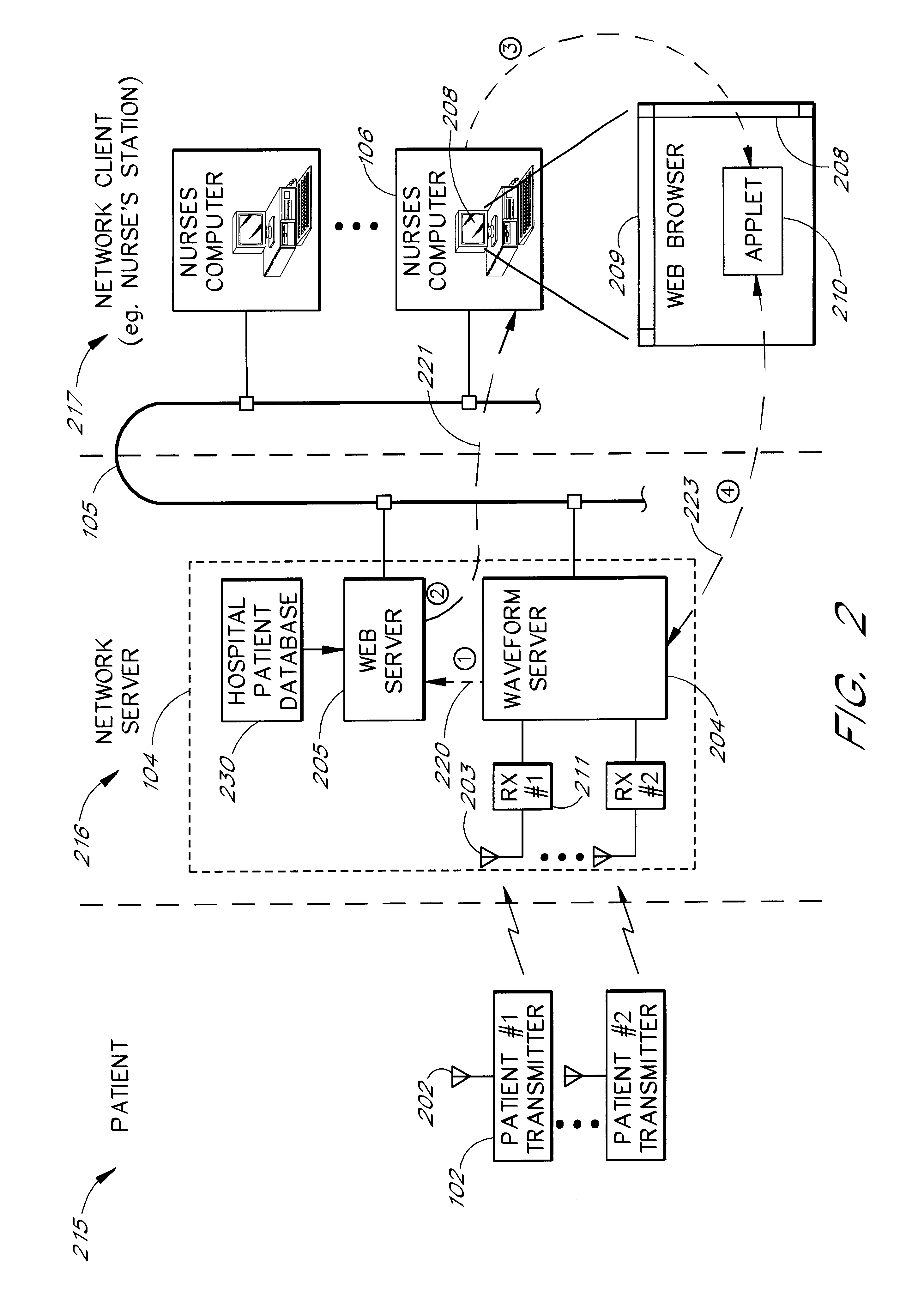
Intranet-based medical data distribution system
InactiveUS6871211B2Low costRapid designMultiple digital computer combinationsAngiographyWeb servicePatient data
A network based medical telemetry system that uses, to the greatest extent possible, standard hardware and software components. The system allows clinicians using computers anywhere in a large hospital to view physiologic data from patients of the hospital. Each patient is fitted with a set of sensors that measure physiologic properties of the patient (e.g., EKG sensors). The sensors are connected to a transmitter that relays the physiologic data to a central server. The central server comprises a WEB server that supplies basic patient data such as the patient's name and medical history. The central server also comprises a waveform server which supplies the physiologic data, in real-time or near real-time. Workstation, used by a clinician receives the basic patient data from the Web server and the physiologic data from the waveform server, and produces a combined display. The combined display is continuously updated, showing, for example, the patient's EKG.
Owner:GE MEDICAL SYST INFORMATION TECH
Method and system for comprehensive evaluation of orthodontic care using unified workstation
A method and system for orthodontic treatment planning, evaluation and quality measurement is provided comprising a workstation having computing platform, a graphical user interface, a processor and a computer storage medium containing digitized records pertaining to a patient. The digitized records include image and other types of data. The computer storage medium further includes a set of software instructions providing graphical user interface tools for providing a user with access to the digitized records for planning orthodontic treatment of a patient. Also provided are reference databases for aiding in the decision process during treatment selection, treatment planning and treatment delivery and progress monitoring and evaluation. Also provided are parameter or criteria measurement techniques and generally acceptable thresholds, which can be updated through learning process and through acquisition of patient data. Once the treatment is planned, the virtual dentition model of the patient in the proposed treatment set-up or the target state is evaluated using several virtual model evaluation features and criteria.
Owner:ORAMETRIX
Method and system for image processing and assessment of a state of a heart
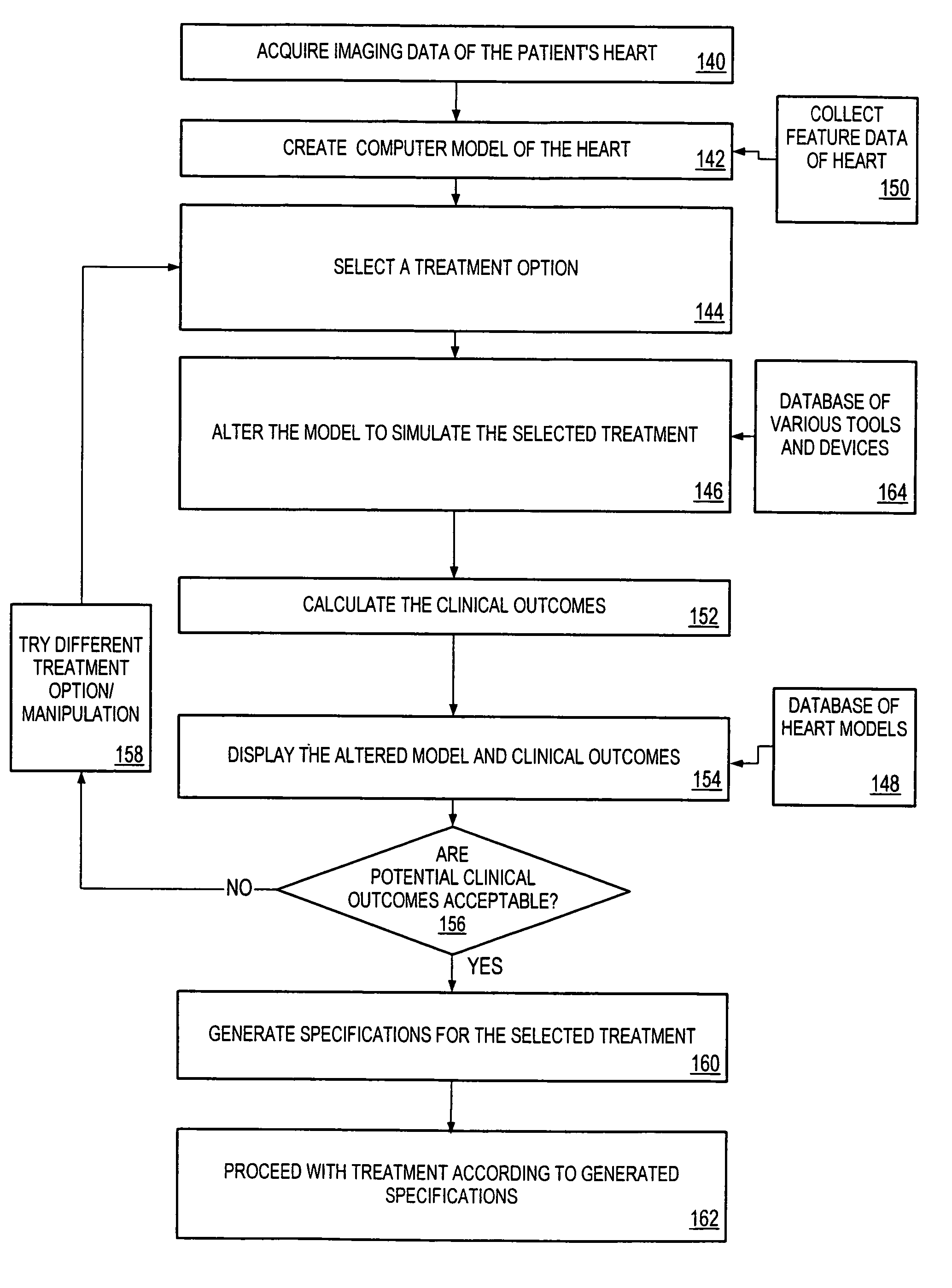
One embodiment discloses a computerized method of facilitating cardiac intervention. The method may include inputting patient data and creating a computerized interactive model of a heart based on the patient data. The model may include features. The model may simulate at least one proposed cardiac intervention by adding or deleting features to the model, and determining the effects of the proposed cardiac simulation upon the entire model. Simulations may be repeated to allow the user to determine an optimal cardiac intervention. A template and / or patient specific instrument may be created from the model to use as a guide during the cardiac intervention.
Owner:CAPITAL SOUTHWEST
Method and system for patient monitoring and respiratory assistance control through mechanical ventilation by the use of deterministic protocols
InactiveUS6148814AReducing ventilator rateRelieve pressureRespiratorsBreathing masksDiseaseClinical staff
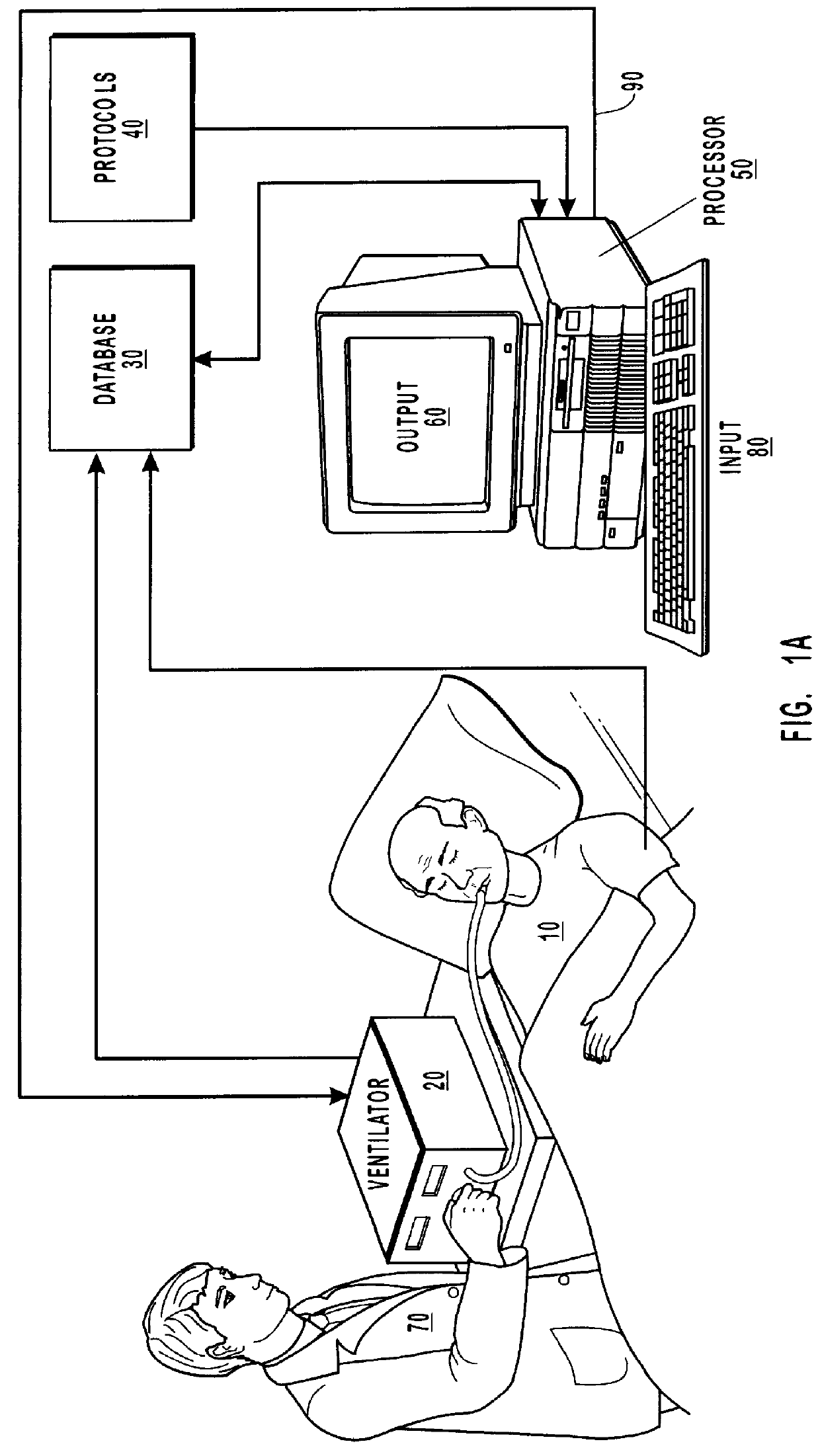
A method and system for managing mechanical ventilation of patients with respiratory disorders is described. The main objective of the system is to generate executable instructions for patient care which take into account a large number of parameters of patient condition and ventilation. Data regarding the state of the patient are stored in a database. Patient data are processed according to a set of protocols which contain rules for patient care decisions arranged in a logical sequence to generate detailed, executable instructions for patient care. Instructions are updated when new data are entered into the database. The data can be acquired in an automated fashion, or the clinician can be instructed to collect and enter new data into the clinical database. Likewise, patient care instructions can be carried out automatically or manually, but it is preferred that instructions are carried out manually as a safety check. The preferred embodiment of the invention includes a computer system, software for processing patient data, and a display device for presenting patient care instructions to the clinician. The system maintains a record of patient data, patient care instructions, whether instructions were followed by the clinical staff, and if not, a reason why.
Owner:INTERMOUNTAIN INTELLECTUAL ASSET MANAGEMENT LLC
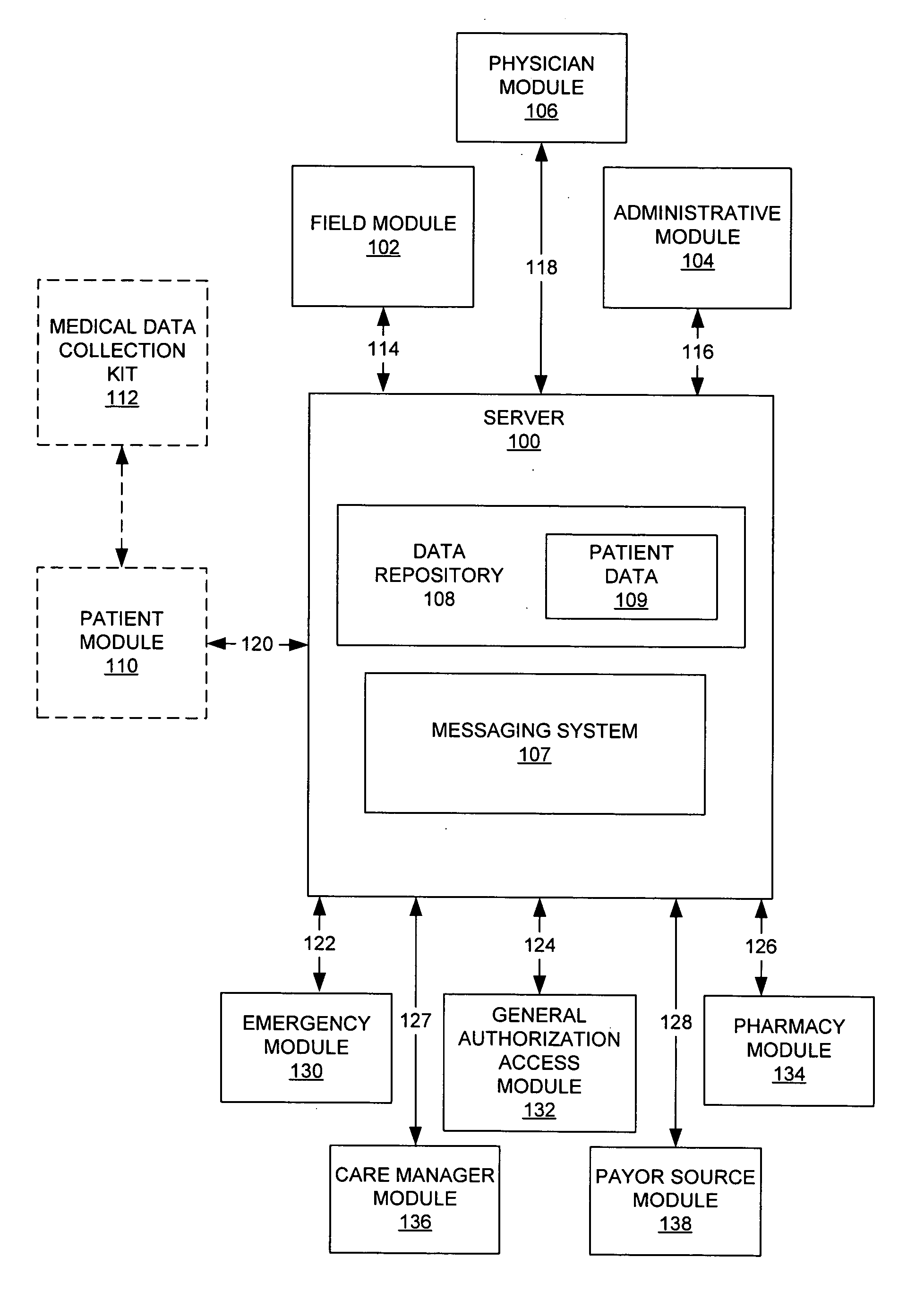
System and method for health care data collection and management
A health care system including a field module configured to gather a first portion of patient data and to send the first portion of patient data to a server, an administrative module configured to perform a plurality of functions on patient data, and a physician module configured to display patient data and perform a patient care function.
Owner:TAHA AMER JAMIL
Heart monitoring body patch and system
InactiveUS20090062670A1Good adhesionEasily attached to patientElectrocardiographySensorsTransceiverHeart monitoring
Provided is a diagnostic patch system for monitoring and storing patient information, which includes sensors, a data storage unit, and a transceiver. Each of the sensors is attached to a skin to detect a patient data. The data storage unit is configured to store stream of the detected patient data from the plurality of sensors. The transceiver, connected with the sensors and the data storage unit, communicates the stream of the patient data with an analyzer, and the analyzer is configured to process and analyze the stream of the patient data. Two or more diagnostic patch systems can communicate with each other.
Owner:BIOSEVEN
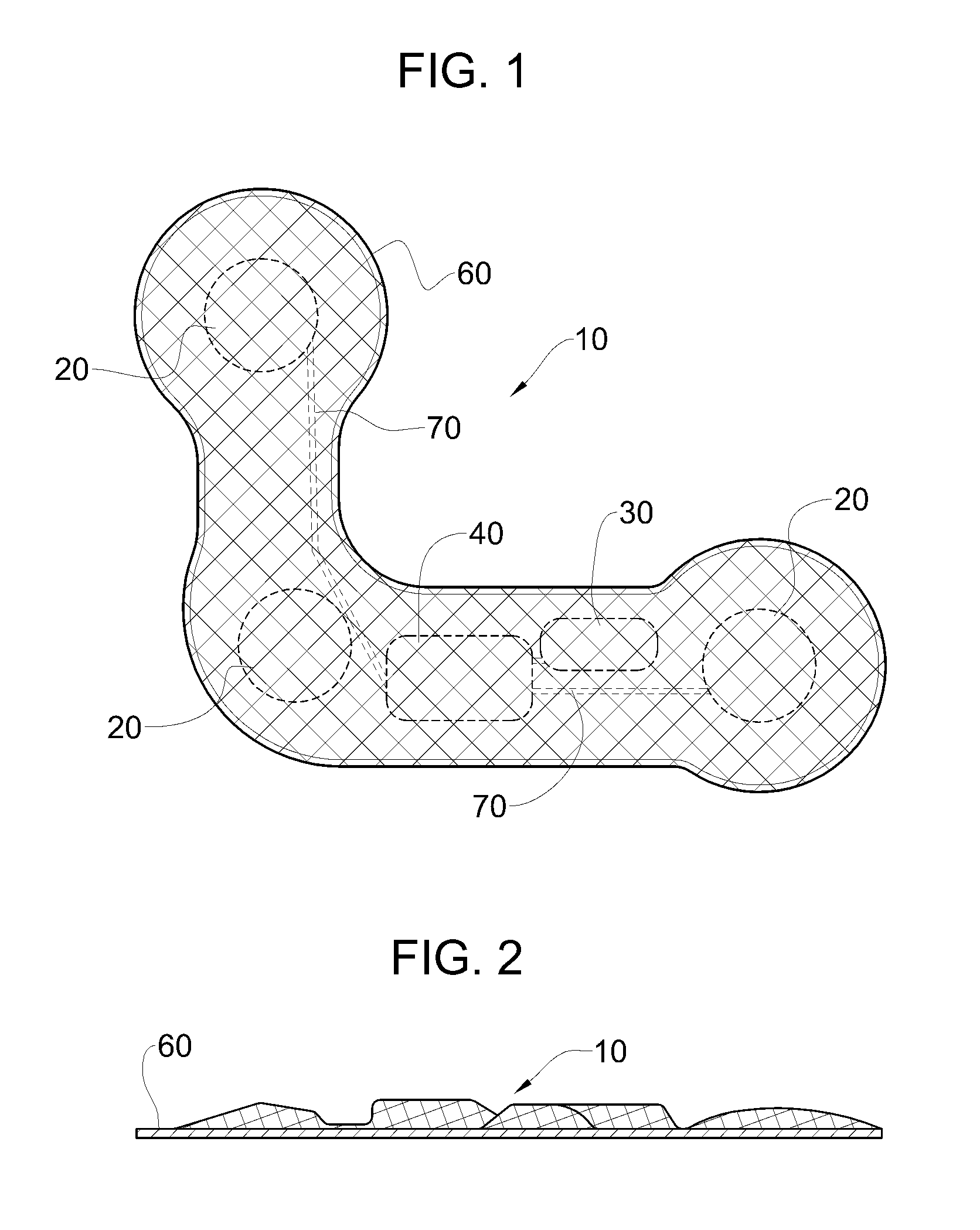
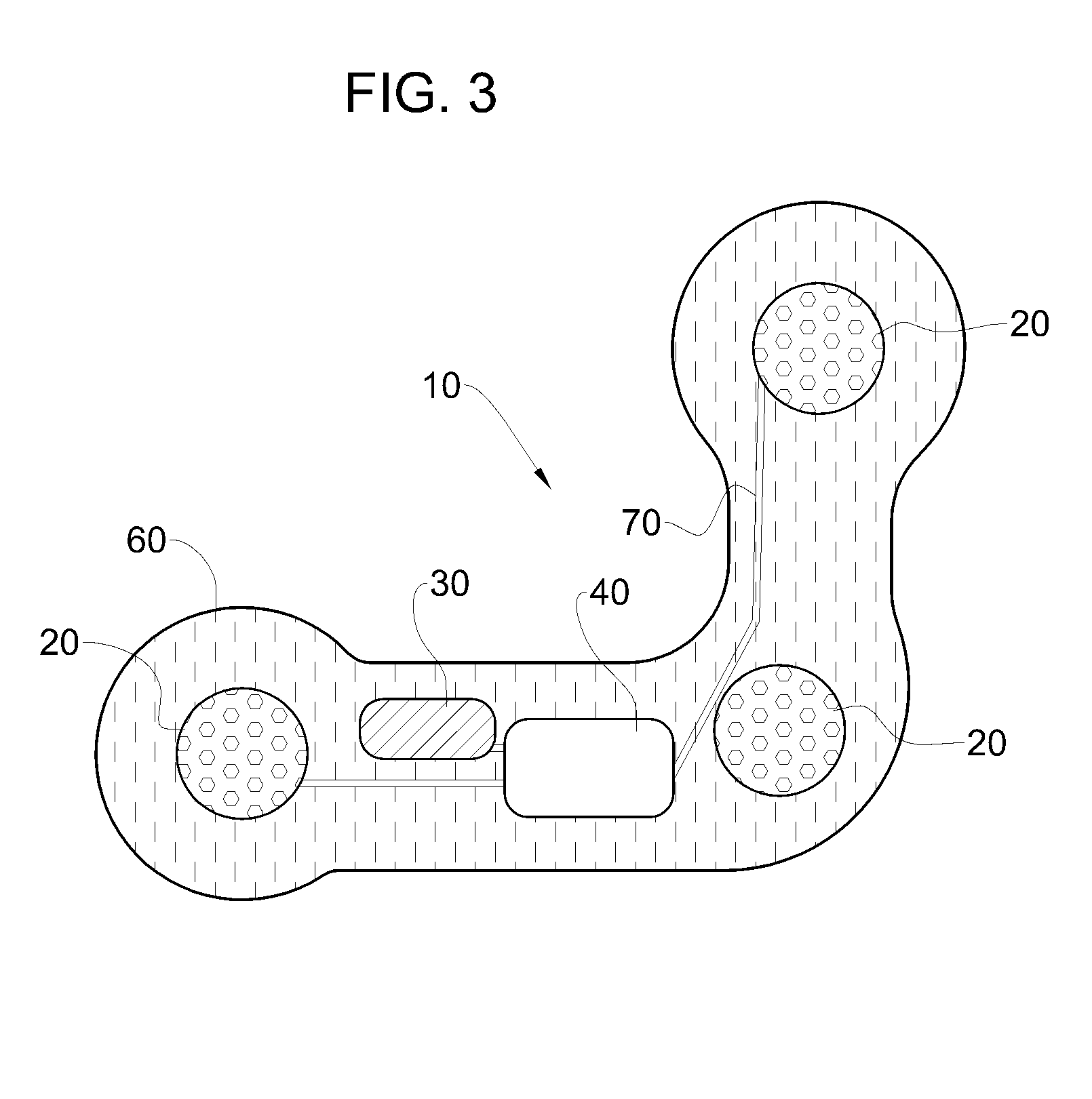
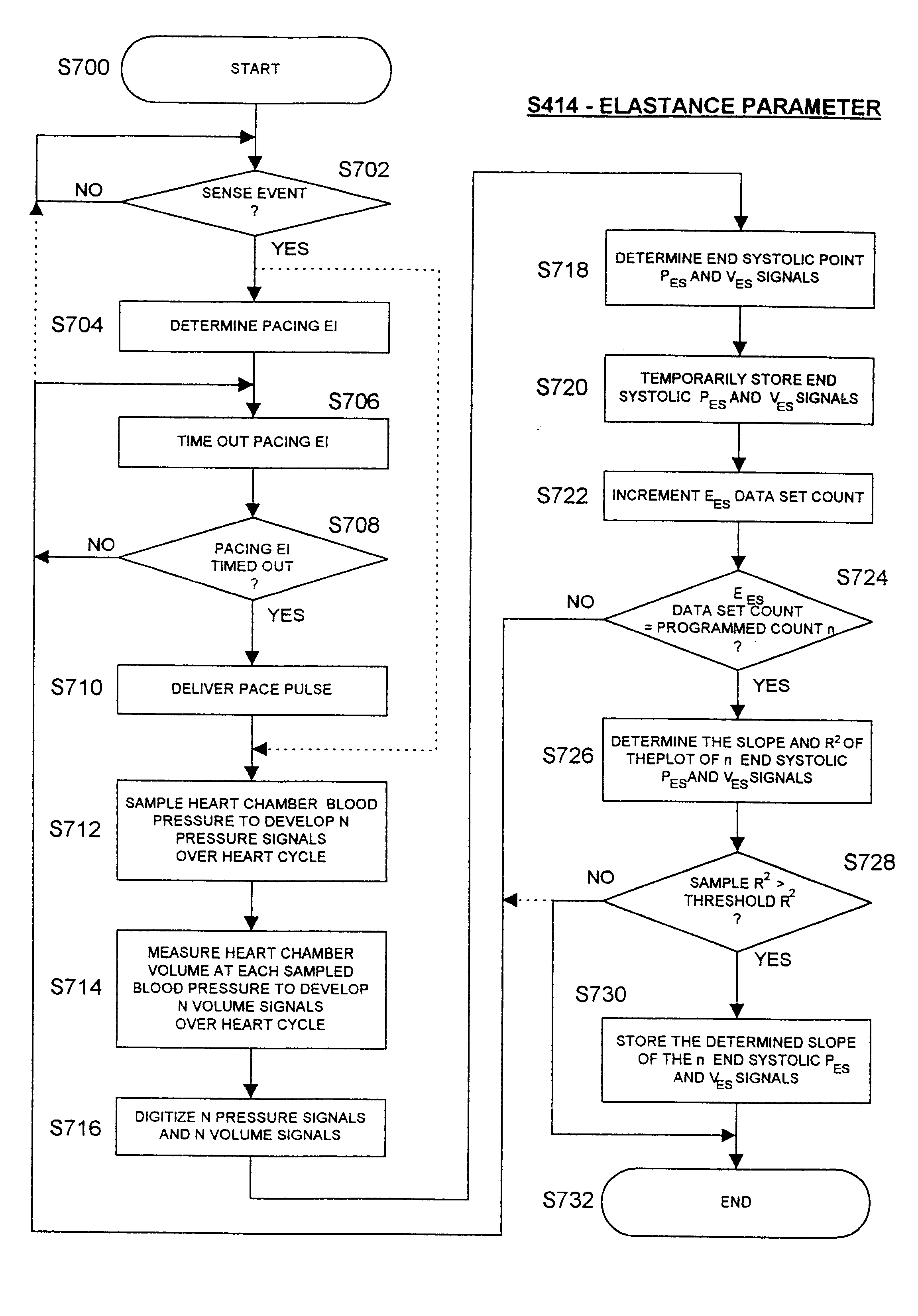
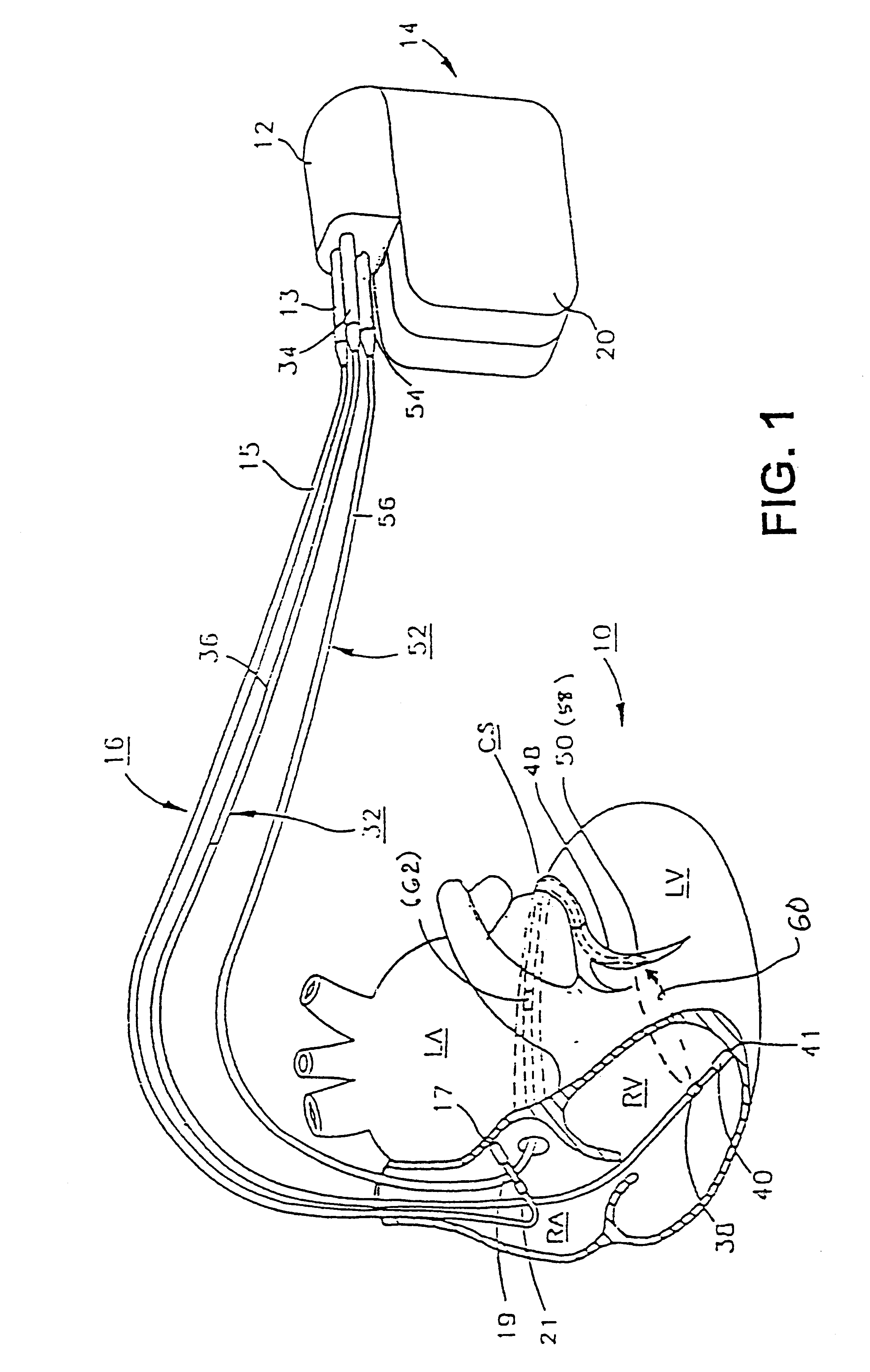
Implantable medical device for treating cardiac mechanical dysfunction by electrical stimulation
InactiveUS6738667B2Increase contractilityEasy to relaxCatheterHeart stimulatorsCardiac cycleHeart chamber
An implantable stimulator and monitor measures a group of heart failure parameters indicative of the state of heart failure employing EGM signals, measures of blood pressure including absolute pressure P, developed pressure (DP=systolic P-diastolic P), and / or dP / dt, and measures of heart chamber volume (V) over one or more cardiac cycles. These parameters include: (1) relaxation or contraction time constant tau (.tau.); (2) mechanical restitution (MR), i.e., the mechanical response of a heart chamber to premature stimuli applied to the heart chamber; (3) recirculation fraction (RF), i.e., the rate of decay of PESP effects over a series of heart cycles; and (4) end systolic elastance (E.sub.ES), i.e., the ratios of end systolic blood pressure P to volume V. These heart failure parameters are determined periodically regardless of patient posture and activity level. The physician can determine whether a particular therapy is appropriate, prescribe the therapy for a period of time while again accumulating the stored patient data for a later review and assessment to determine whether the applied therapy is beneficial or not, thereby enabling periodic changes in therapy, if appropriate. Drug therapies and electrical stimulation therapies, including PESP stimulation, and pacing therapies including single chamber, dual chamber and multi-chamber (bi-atrial and / or bi-ventricular) pacing can be delivered. In patient's prone to malignant tachyarrhythmias, the assessment of heart failure state can be taken into account in setting parameters of detection or classification of tachyarrhythmias and the therapies that are delivered.
Owner:MEDTRONIC INC
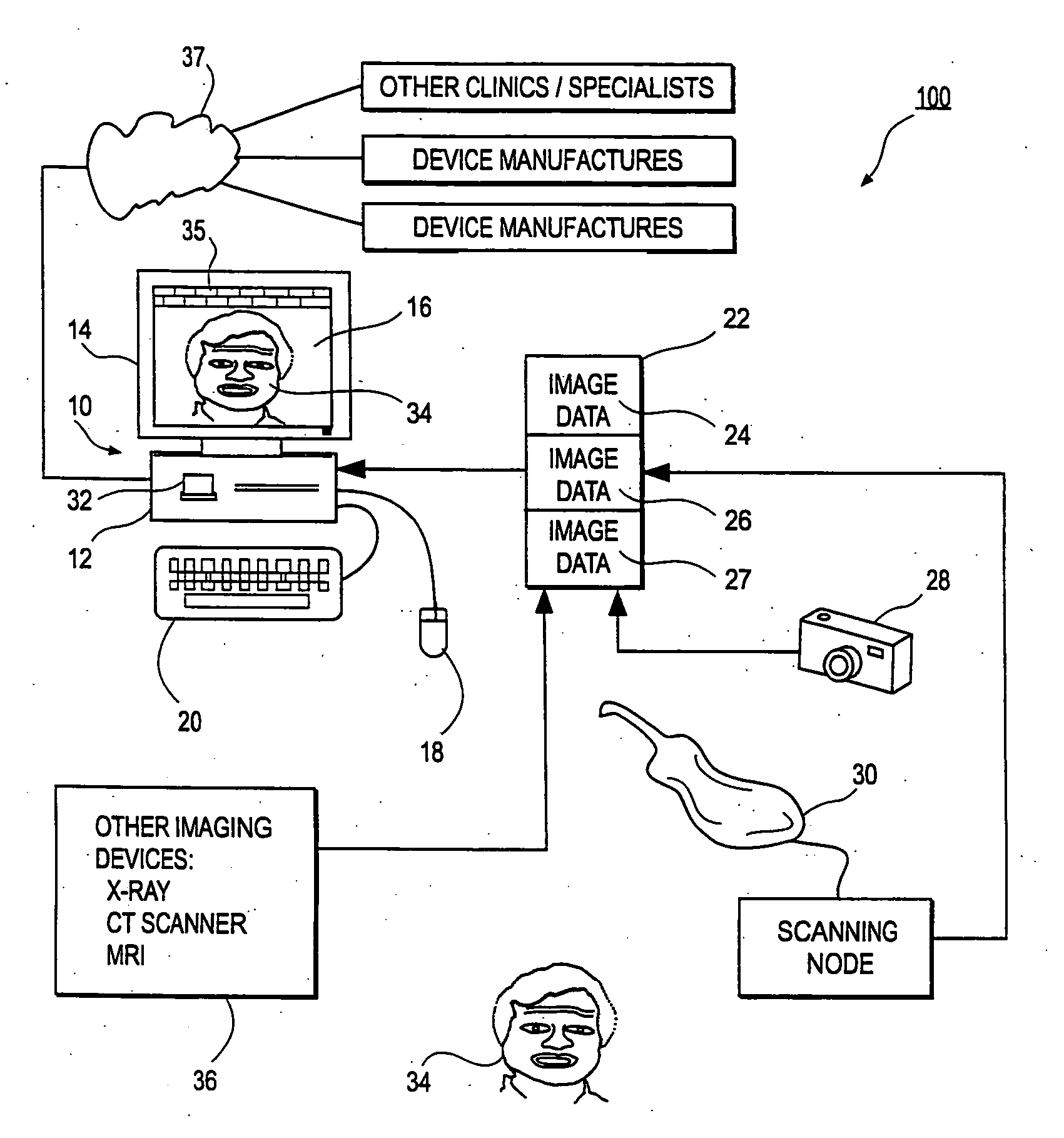
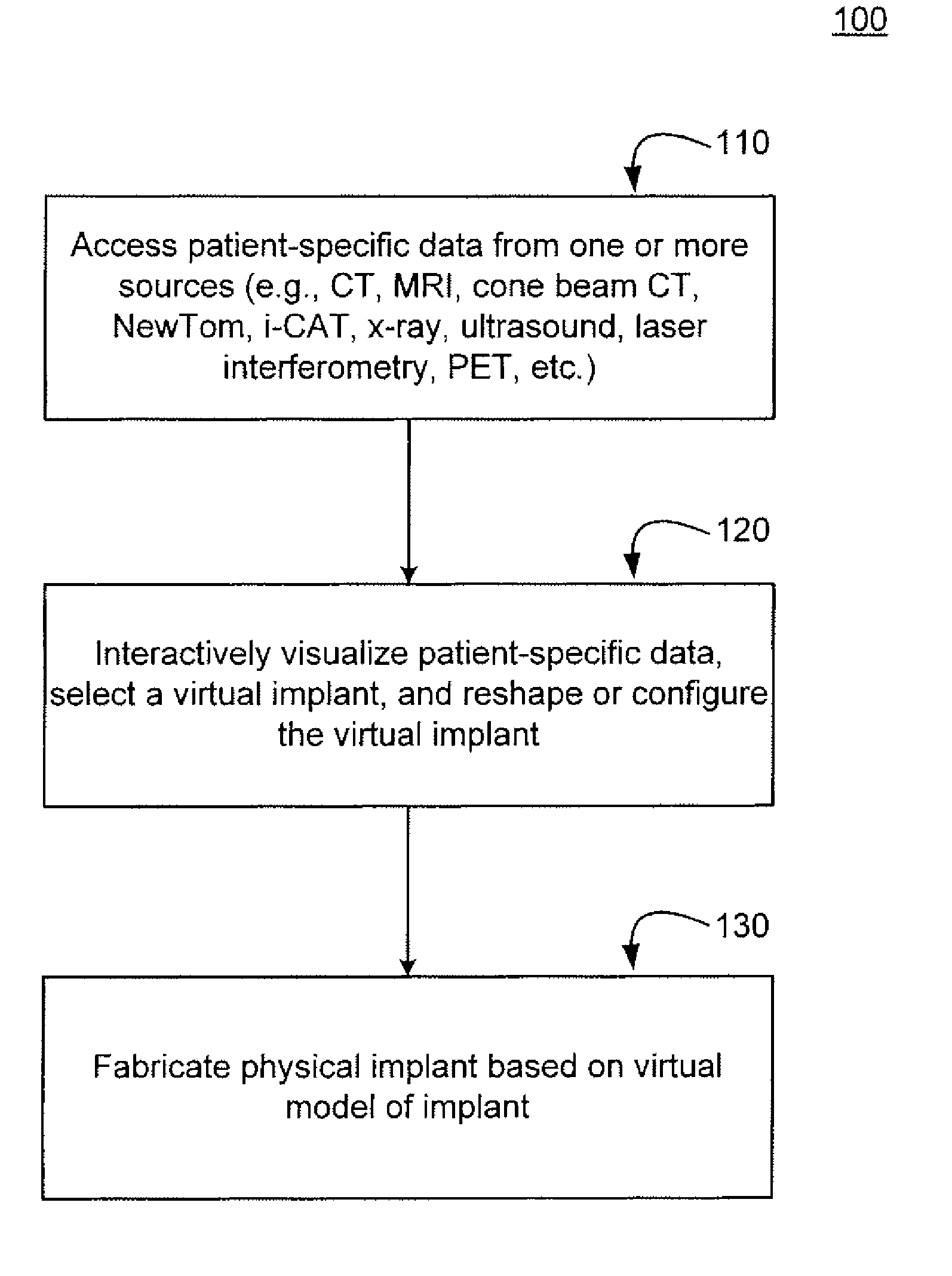
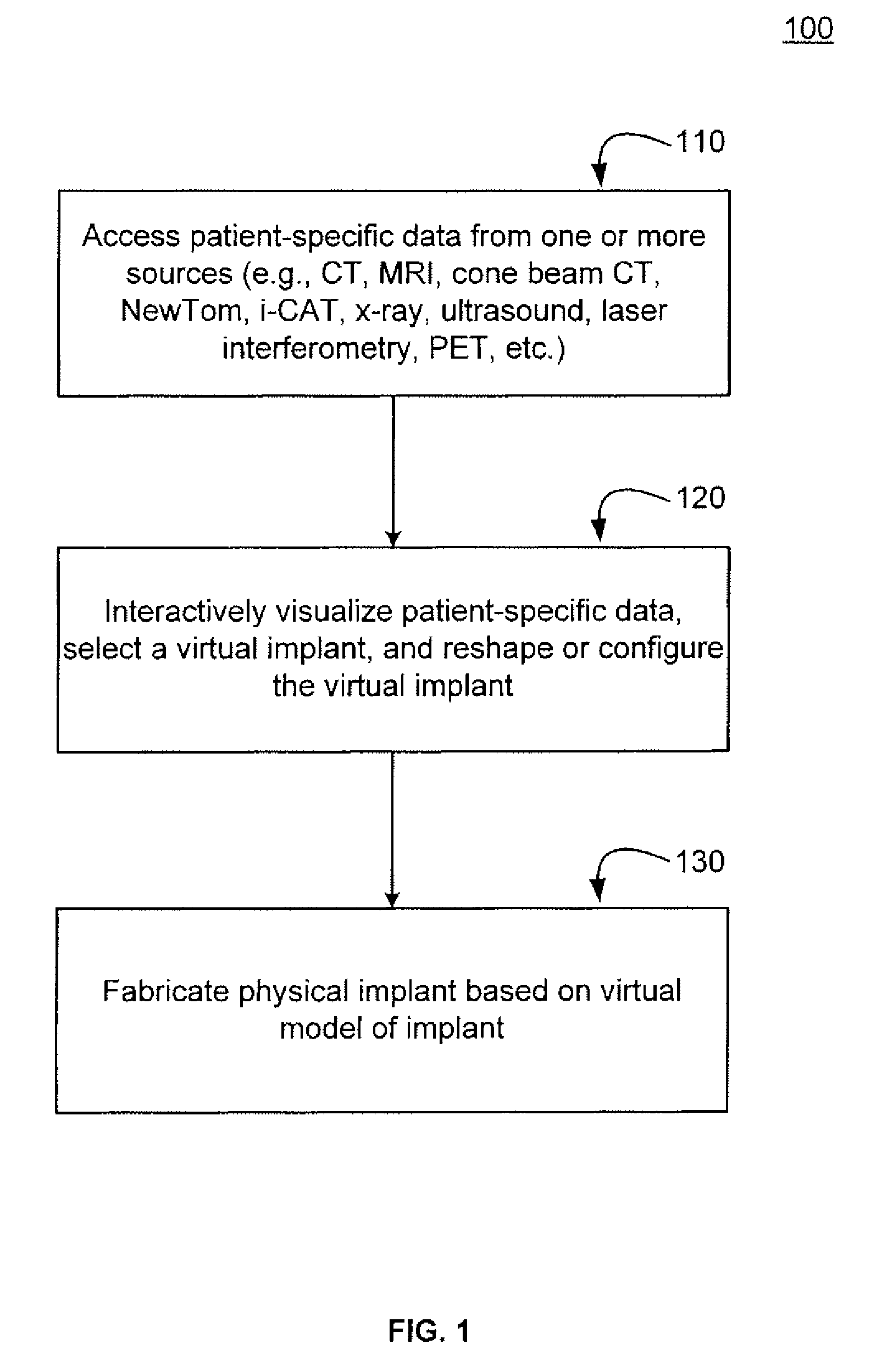
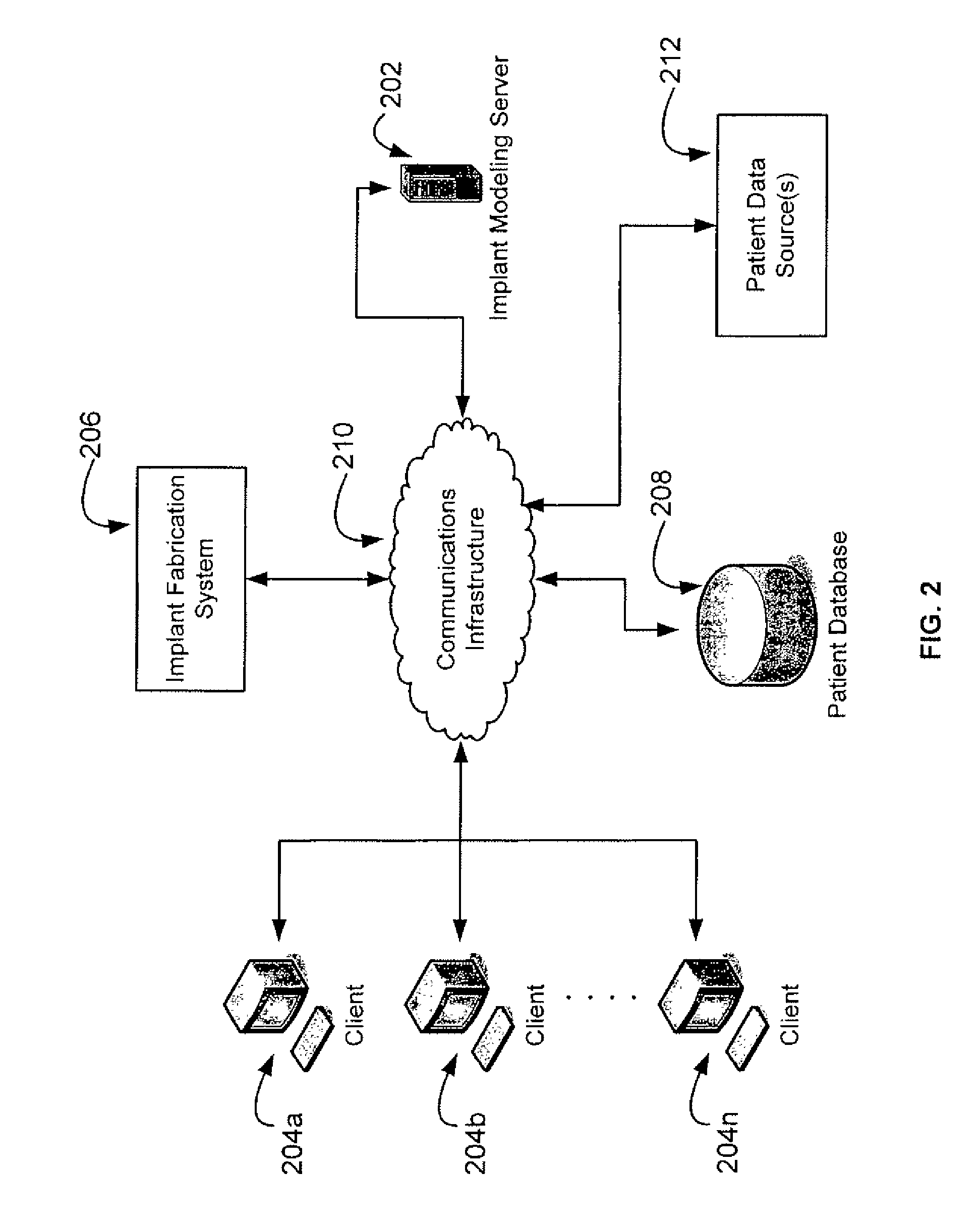
Methods, systems, and computer program products for shaping medical implants directly from virtual reality models
InactiveUS20090149977A1Improve aestheticsReduce probabilityMedical simulationSpecial data processing applicationsAnatomical structuresX-ray
A virtual interactive environment enables a surgeon or other medical professional to manipulate implants, prostheses, or other instruments using patient-specific data from virtual reality models. The patient data includes a combination of volumetric data, surface data, and fused images from various sources (e.g., CT, MRI, x-ray, ultrasound, laser interferometry, PET, etc.). The patient data is visualized to permit a surgeon to manipulate a virtual image of the patient's anatomy, the implant, or both, until the implant is ideally positioned within the virtual model as the surgeon would position a physical implant in actual surgery. Thus, the interactive tools can simulate changes in an anatomical structure (e.g., bones or soft tissue), and their effects on the external, visual appearance of the patient. CAM software is executed to fabricate the implant, such that it is customized for the patient without having to modify the structures during surgery or to produce a better fit.
Owner:SCHENDEL STEPHEN A
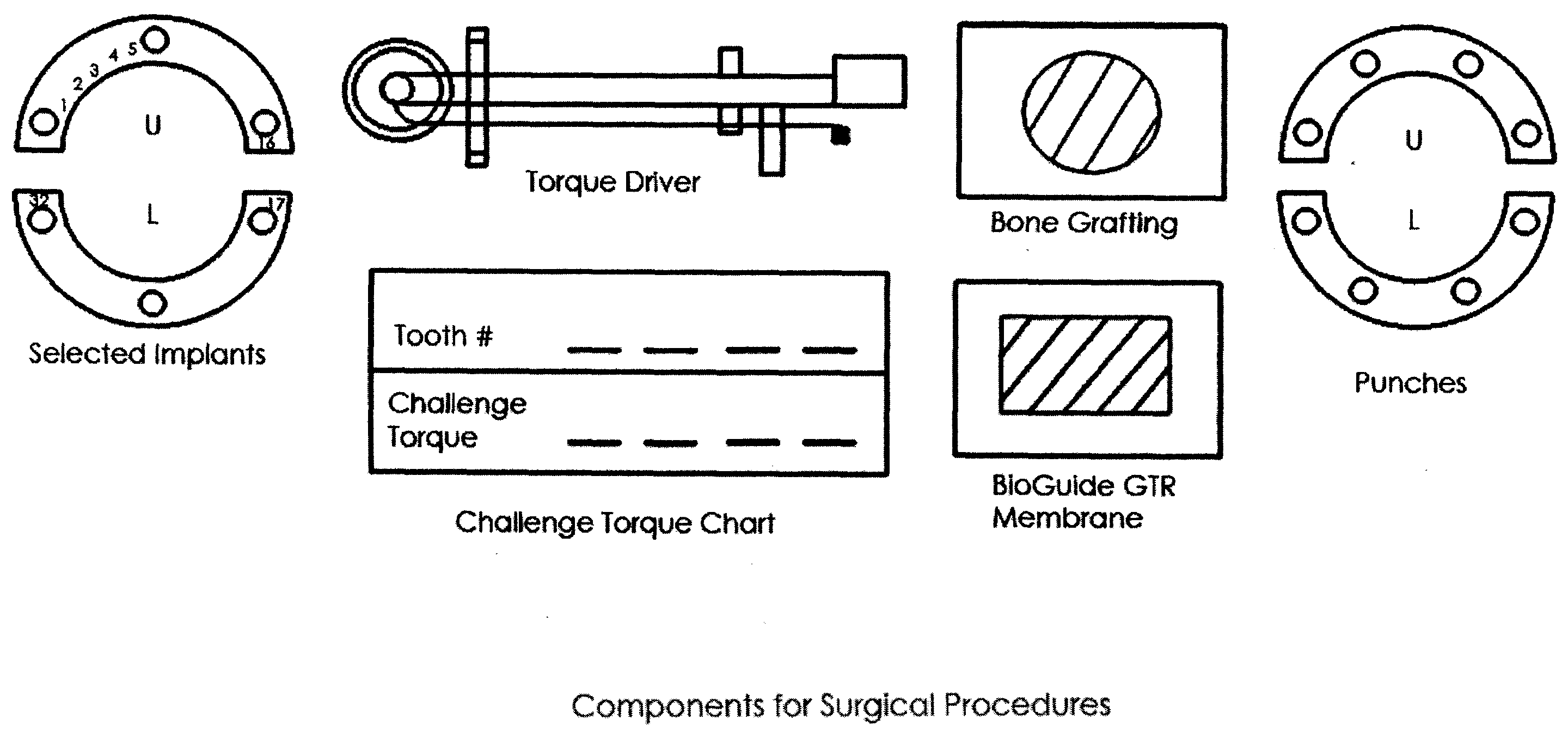
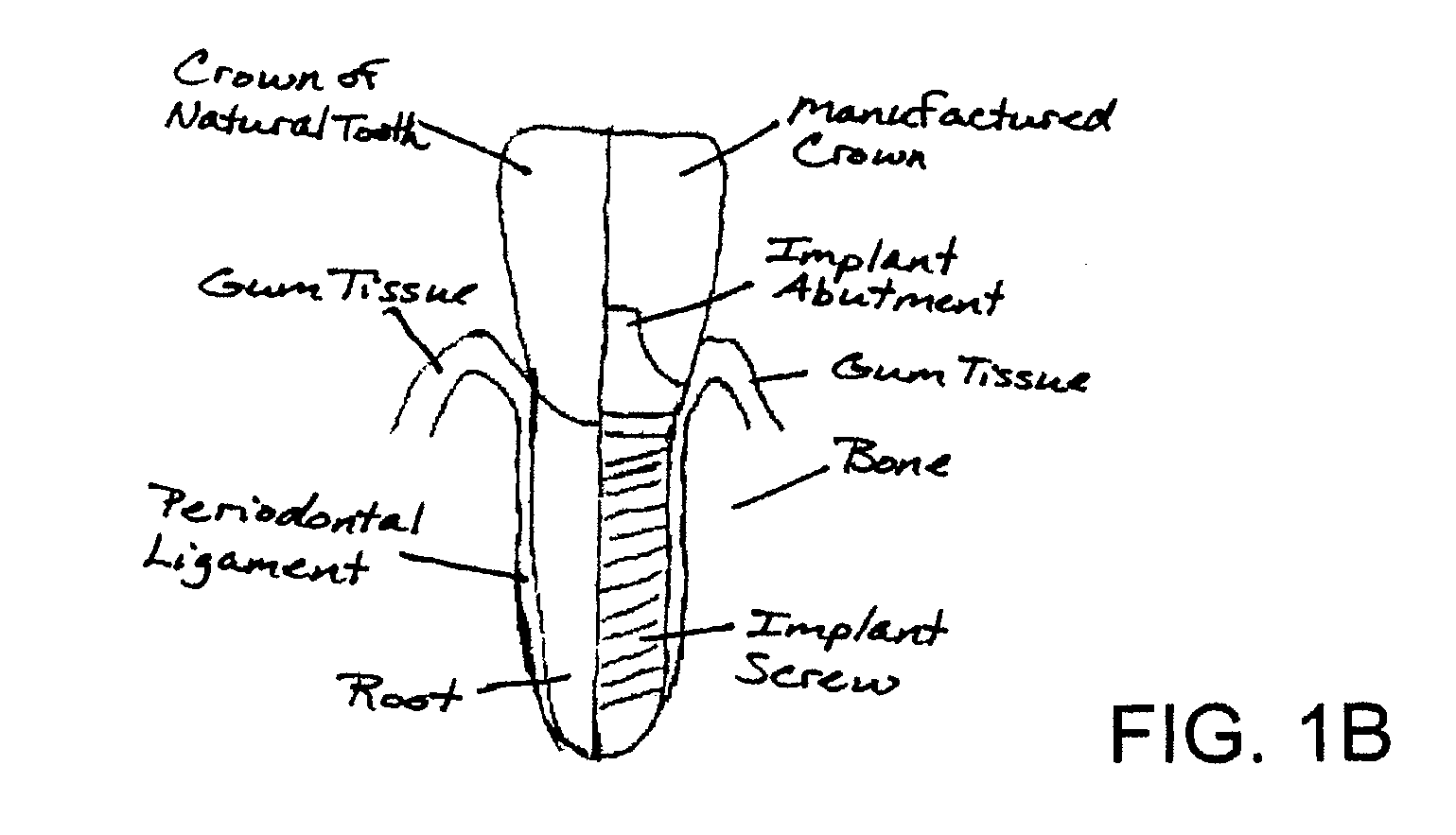
System, Method And Apparatus For Tooth Implant Planning And Tooth Implant Kits
InactiveUS20100105011A1Improve the level ofFacilitate proper fixture placementDental implantsDispensing apparatusPatient dataDental implant
Systems and methods support dental implant patient scheduling and treatment process relating to packaging one or more dental appliances as a kit which is readily used by dental professional during surgery, by communicating manufacturing progress information with a doctor over a network and performing patient scheduling and treatment when the dental appliances reach a certain manufacturing progress. A network-based service may also provide a doctor with a treatment solution including a surgical kit derived from patient data.
Owner:INPRONTO
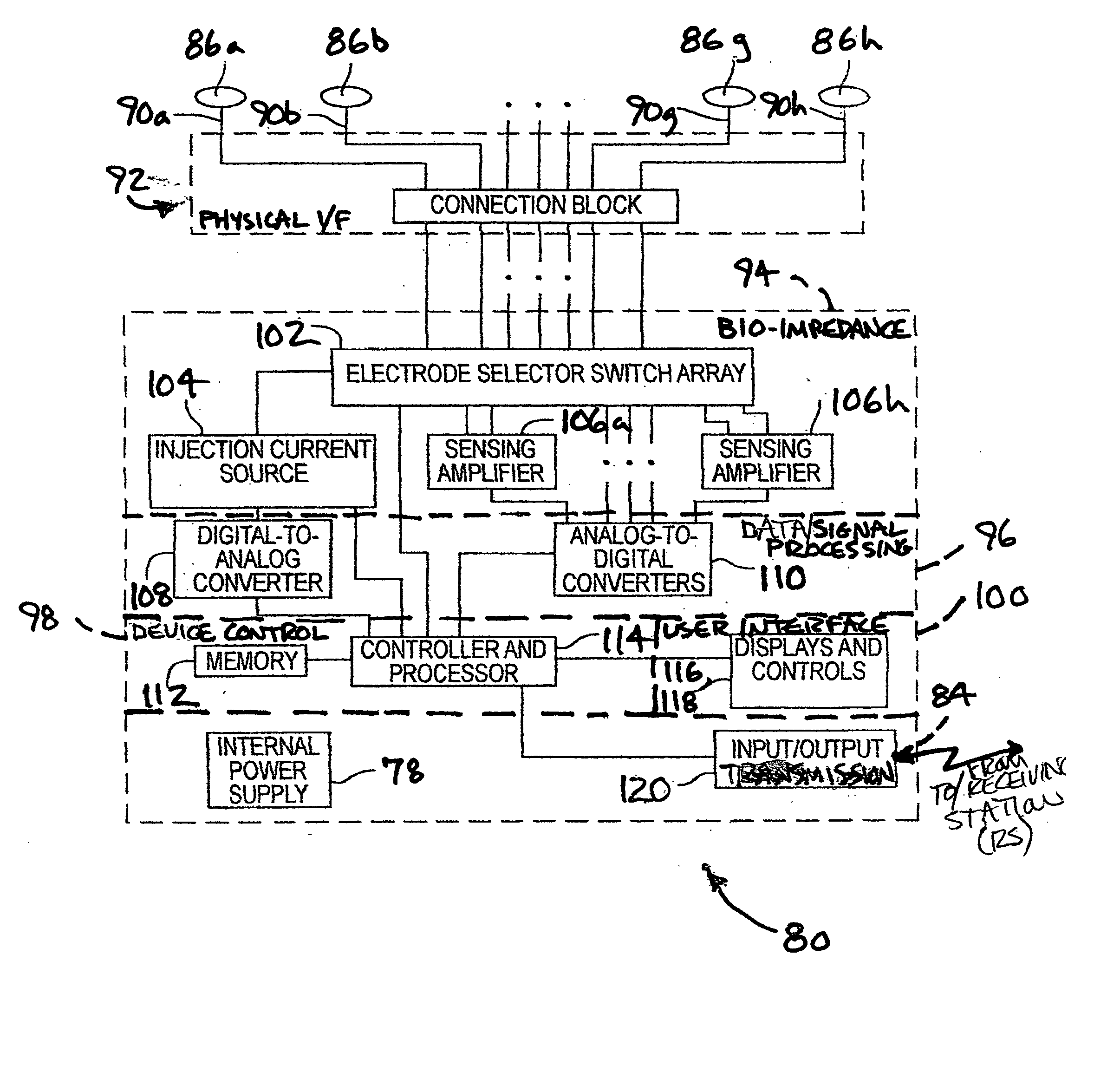
Non-invasive body composition monitor, system and method
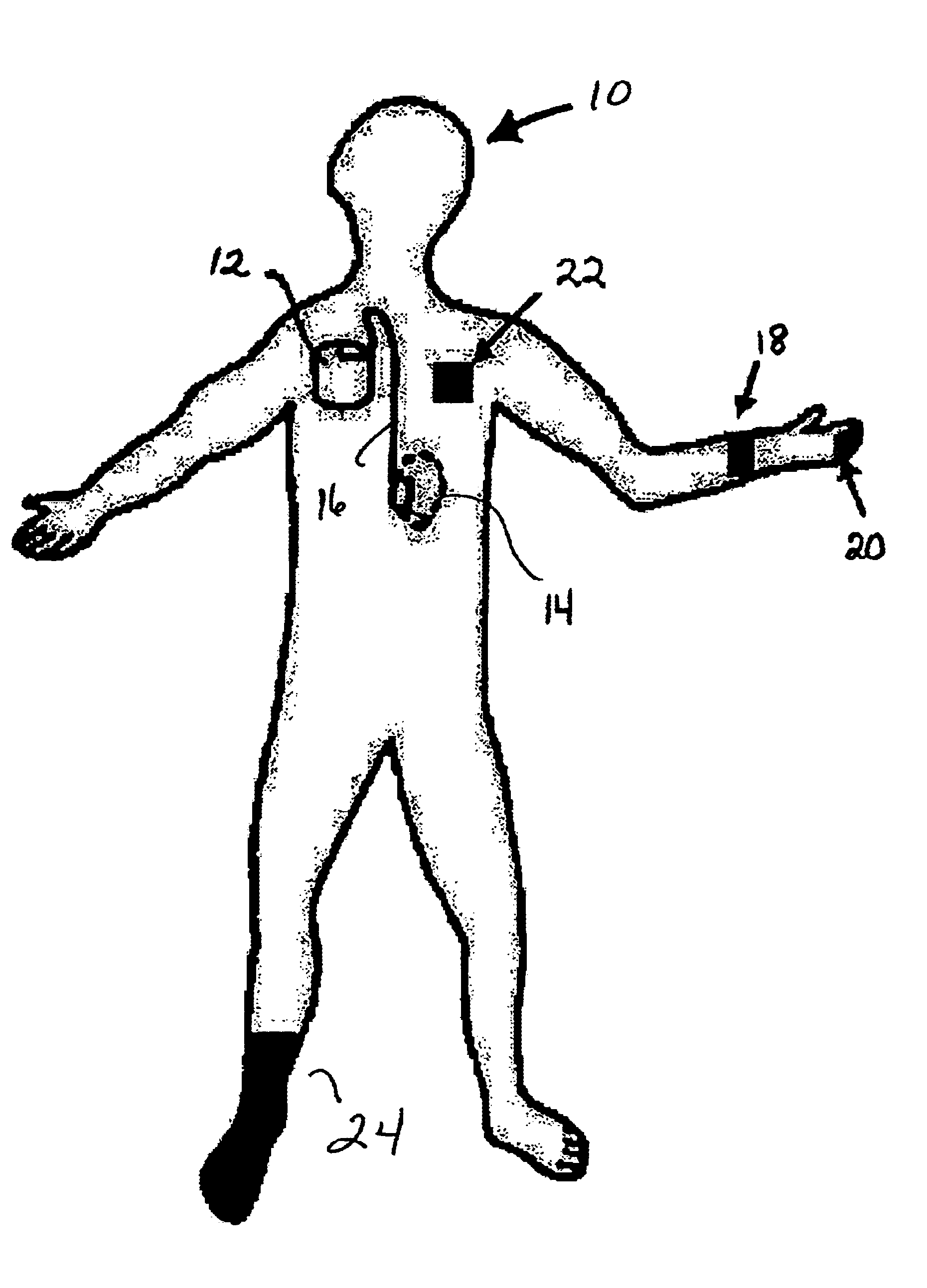
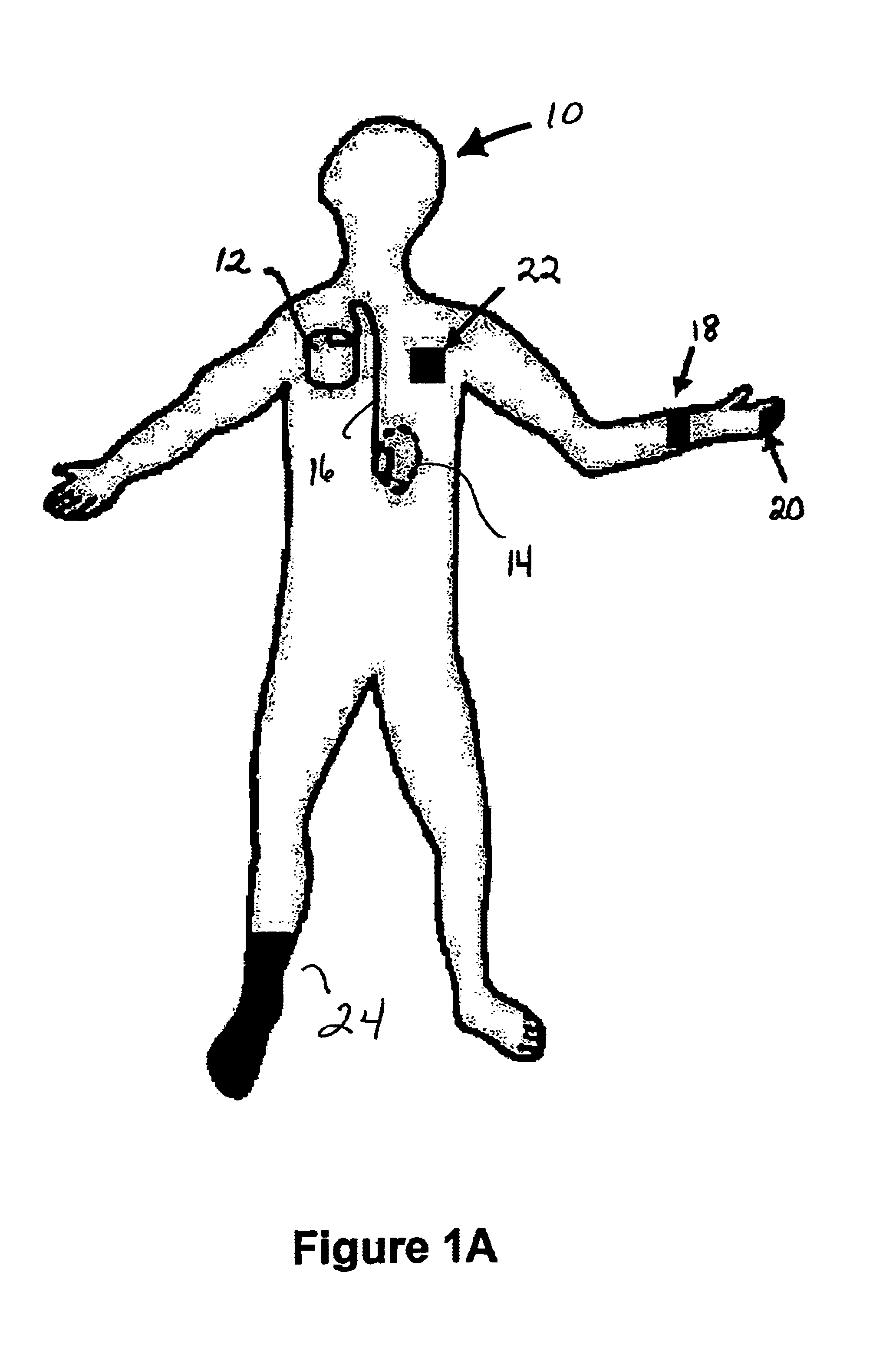
The invented non-invasive vital signs monitor is in a flexible, nominally flat planar form having integral gel electrodes, a sticky-back rear surface, an internal flex circuit capable of sensing, recording and playing out several minutes of the most recently acquired ECG waveform data and a front surface that includes an outplay port. The invented non-invasive body composition ‘risk’ monitor includes a measurement device for monitoring one or more variables including body fluid mass, dehydration, respiratory rate, blood pressure, bio-impedance, cardiography such as cardiac output, and body conformation parameters. The risk monitor may be provided in a lightweight carrying case into which the vital signs monitor plugs. Thus the two monitors may be independent or they may be integrated into one portable, non-invasive device that can convey important patient data to / from a remote patient medical data center via wireless telemetry for oversight, treatment and possible intervention by a physician.
Owner:RIGHT CORP
Point of care diagnostic systems
InactiveUS6867051B1Accurate concentrationAccurately presenceComputer-assisted medical data acquisitionMedical imagesPoint of careDiagnostic test
Systems and methods for medical diagnosis or risk assessment for a patient are provided. These systems and methods are designed to be employed at the point of care, such as in emergency rooms and operating rooms, or in any situation in which a rapid and accurate result is desired. The systems and methods process patient data, particularly data from point of care diagnostic tests or assays, including immunoassays, electrocardiograms, X-rays and other such tests, and provide an indication of a medical condition or risk or absence thereof. The systems include an instrument for reading or evaluating the test data and software for converting the data into diagnostic or risk assessment information.
Owner:CYTYC CORP
Implantable medical device incorporating miniaturized circuit module
Implantable medical devices (IMDS) having RF telemetry capabilities for uplink transmitting patient data and downlink receiving programming commands to and from an external programmer having an improved RF module configured to occupy small spaces within the IMD housing to further effect the miniaturization thereof. An RF module formed of an RF module substrate and at least one IC chip and discrete components has a volume and dimensions that are optimally minimized to reduce its volumetric form factor. Miniaturization techniques include: (1) integrating inductors into one or more IC chips mounted to the RF module substrate; (2) mounting each IC chip into a well of the RF module substrate and using short bonding wires to electrically connect bond pads of the RF module substrate and the IC chip; and (3) surface mounting discrete capacitors over IC chips to reduce space taken up on the RF module substrate. The integrated inductors are preferably fabricated as planar spiral wound conductive traces formed of high conductive metals to reduce trace height and width while maintaining low resistance, thereby reducing parasitic capacitances between adjacent trace side walls and with a ground plane of the IC chip. The spiral winding preferably is square or rectangular, but having truncated turns to eliminate 90° angles that cause point-to-point parasitic capacitances. The planar spiral wound conductive traces are further preferably suspended over the ground plane of the RF module substrate by micromachining underlying substrate material away to thereby reduce parasitic capacitances.
Owner:MEDTRONIC INC
System and method for controlling access and use of patient medical data records
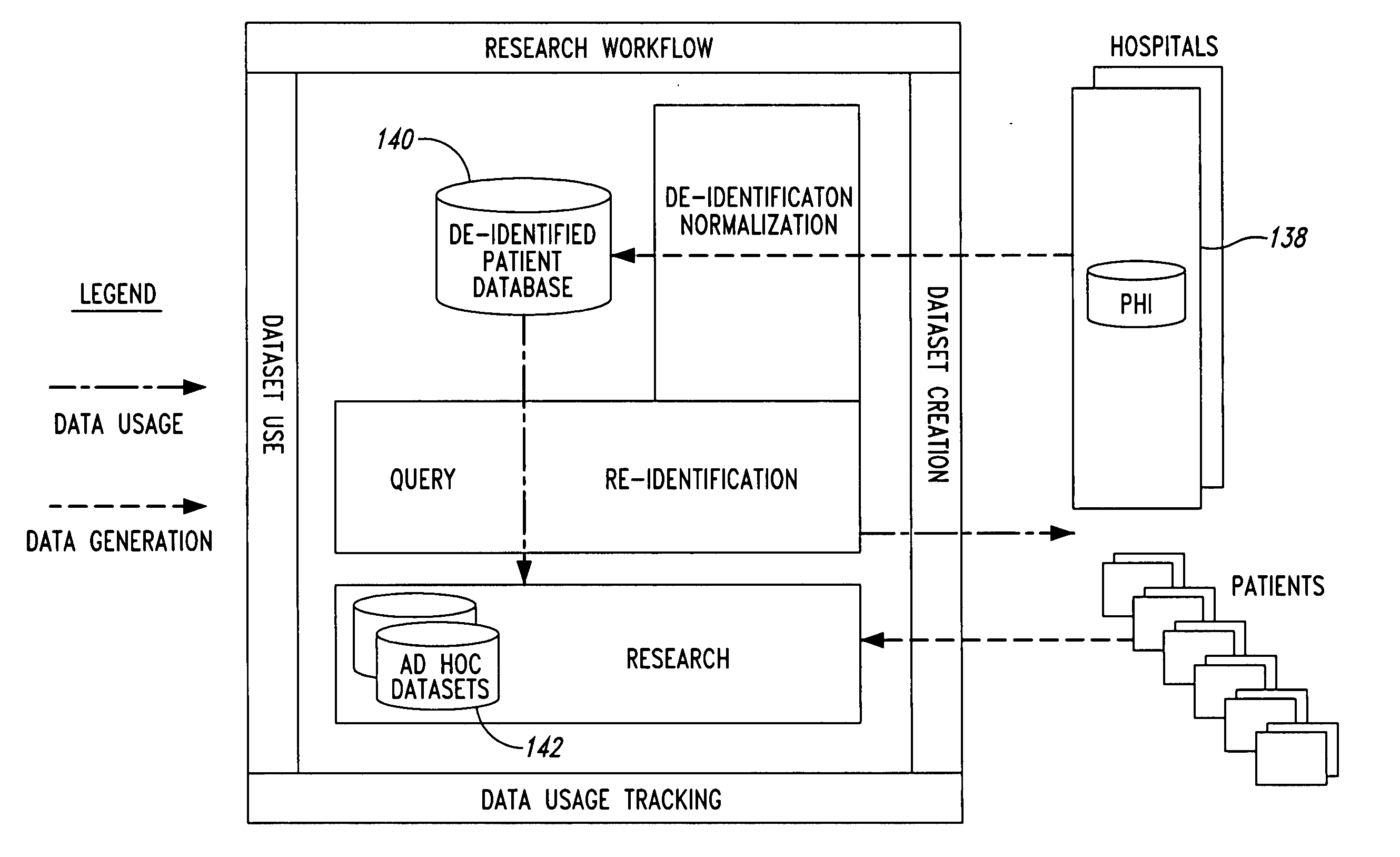
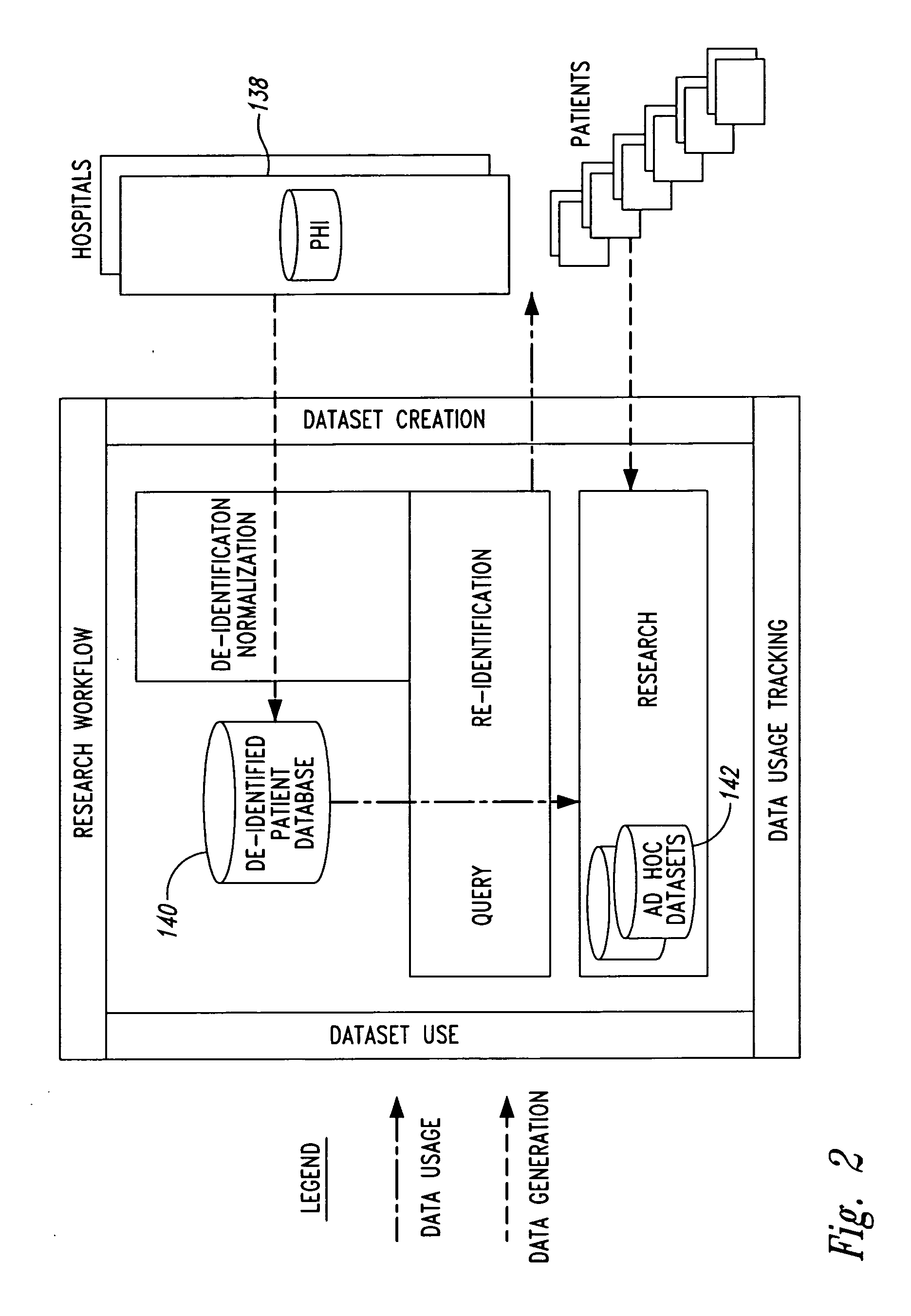
InactiveUS20050236474A1Prohibitive to printComputer security arrangementsPatient personal data managementConfidentialityPatient database
A system for processing patient health information (PHI) protects the confidentiality of PHI to achieve regulatory compliance. The PHI contains patient medical data and associated patient identification data. A de-identification agent extracts patient medical data and separates from all identification data to create de-identified patient data. A key is generated that allows subsequent reassociation of the patient medical data and the patient identification data. The de-identified patient data base may be queried for patient screening purposes. Patient queries are processed only if the study or patient screening has been authorized by appropriate authorities, such as an internal review board. Patients whose medical characteristics conform with the patient query are selected for possible use in a study. If re-identification of the selected patients is necessary, and authorized, the key may be used to provide the necessary reassociation. A data log records all access to patient data.
Owner:CONVERGENCE CT
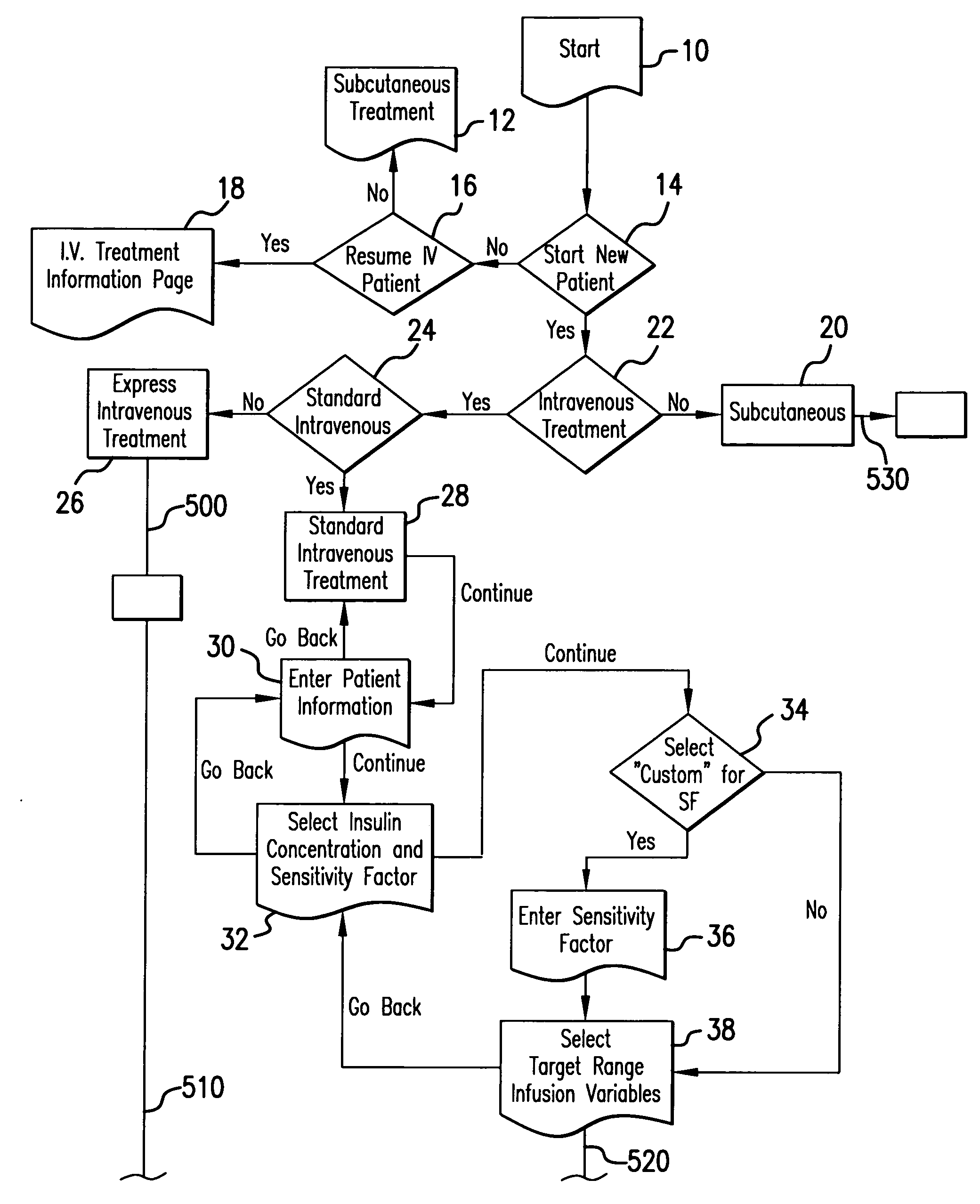
System and method for measuring and predicting insulin dosing rates
InactiveUS20070078314A1Efficient managementDrug and medicationsMedical automated diagnosisPatient dataGlucose polymers
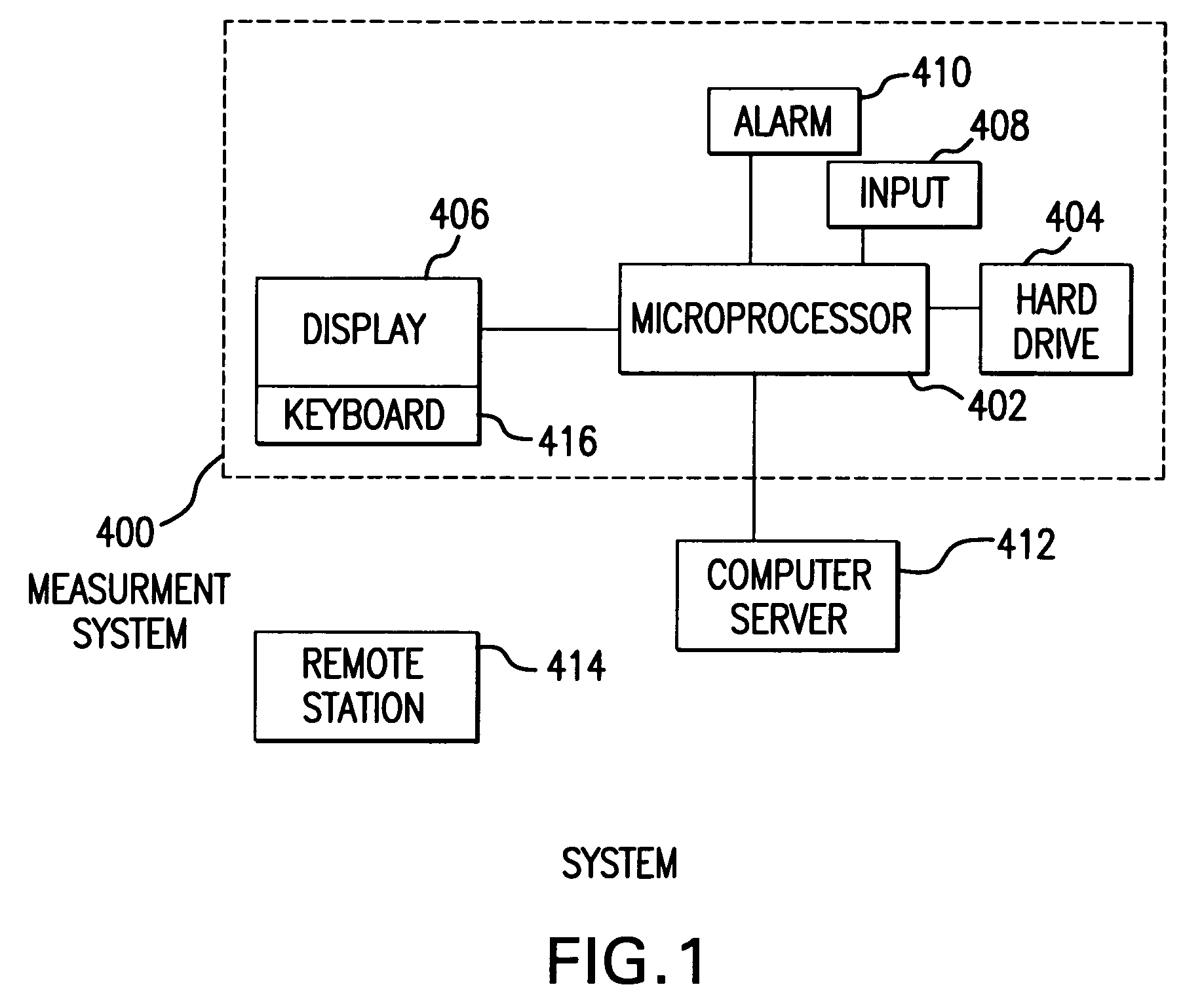
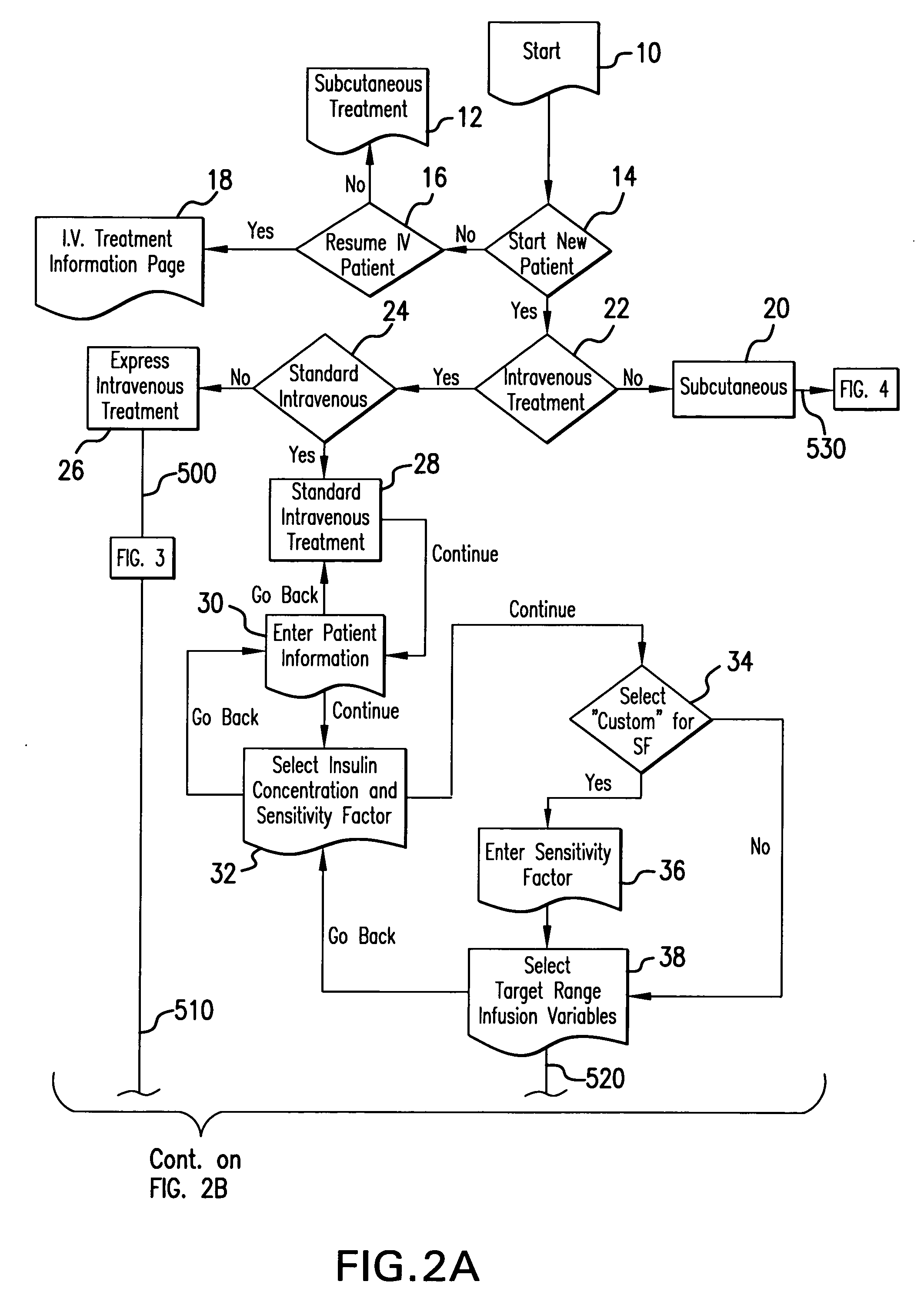
The method and system for managing a patient's blood glucose level predicts an insulin dosing rate to bring a patient's blood glucose level into a preferred target range within a predetermined time interval. The system includes a processor which actuates a blood glucose computer program to measure and predict the patient's blood glucose level. An input mechanism allows for insertion of a preferred target range of the patient's blood glucose level and further permits input of various patient data parameters. The processor calculates the optimum insulin dosing rate for the patient based upon the type of insulin dosing whether it be intravenous dosing and / or subcutaneous dosing. A display mechanism displays the patient dosing parameters and an alarm mechanism alerts a user when the patient's blood glucose level is outside of the preferred patient blood glucose target range.
Owner:GLUCOTEC
Body composition, circulation, and vital signs monitor and method
The invented non-invasive vital signs monitor is in a flexible, nominally flat planar form having integral gel electrodes, a sticky-back rear surface, an internal flex circuit capable of sensing, recording and playing out several minutes of the most recently acquired ECG waveform data and a front surface that includes an outplay port. The invented non-invasive body composition ‘risk’ monitor includes a measurement device for monitoring one or more variables including body fluid mass, dehydration, respiratory rate, blood pressure, bio-impedance, cardiography such as cardiac output, and body conformation parameters. The risk monitor may be provided in a lightweight carrying case into which the vital signs monitor plugs. Finally, a lightweight portable probe or transducer containing a transmissive or reflective electro-optical emitter and receptor in the infrared spectrum is fitted on a subject's finger or toe. Associated electronics energize and monitor the probe, detect cardio-rhythmic fluctuations therefrom, and process digital data over a prescribed window to produce a non-invasive, qualitative or quantitative measure of the subject's circulation. In accordance with one embodiment of the invention, a simple tri-color LED array is used to indicate the subject's circulation as being normal, reduced, or borderline. Thus the vital signs, bio-impedance, and circulation monitors may be independent or they may be integrated into one portable, non-invasive device that can concurrently monitor and locally display or remotely convey important patient data including circulation data to a local subject or physician or to / from a remote patient medical data center via wireless telemetry for oversight, treatment and possible intervention by a remote physician.
Owner:SEMLER SCI
Implantable medical device controlled by a non-invasive physiological data measurement device
The operational and functional aspects of one or more IMDs is controlled by physiological data acquired from an external device. Various externally deployed devices collect vital signals for transmission to the IMD. Upon receipt of the signals the IMD cooperatively modifies therapy and diagnostic procedures to be substantially compliant with the received signals. Further, the IMD may store some of the signals for future follow-up or patient data management as needed.
Owner:MEDTRONIC INC
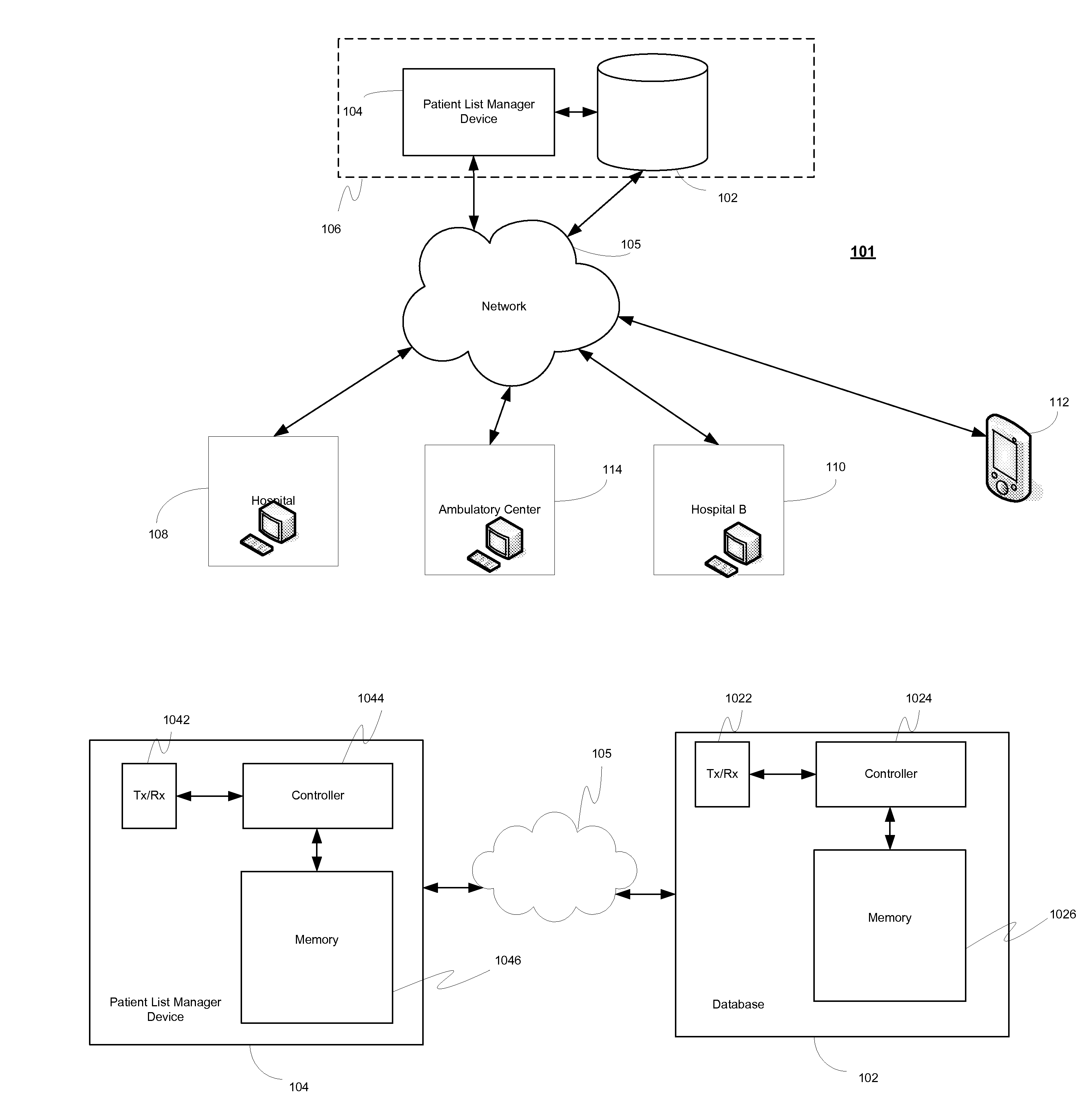
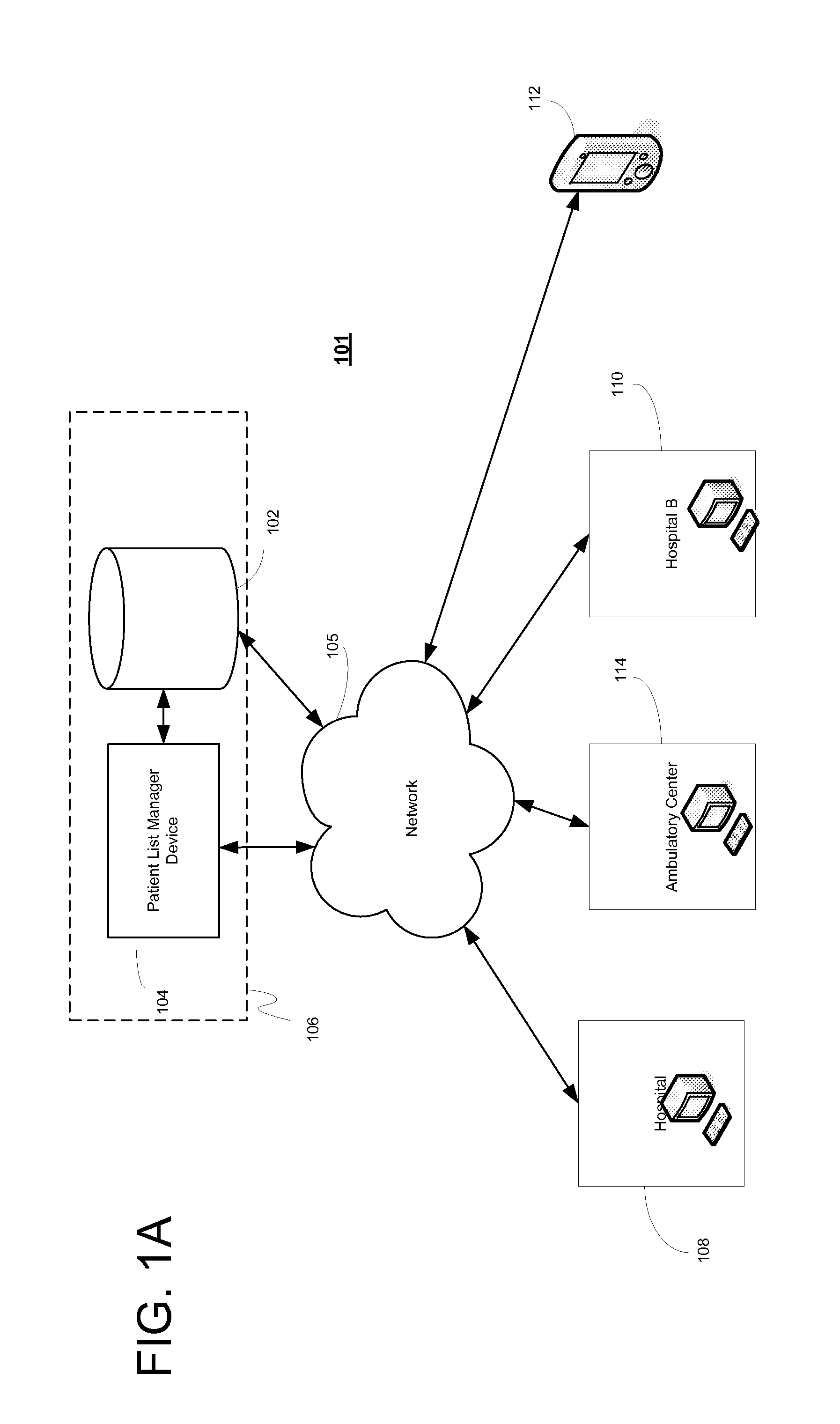
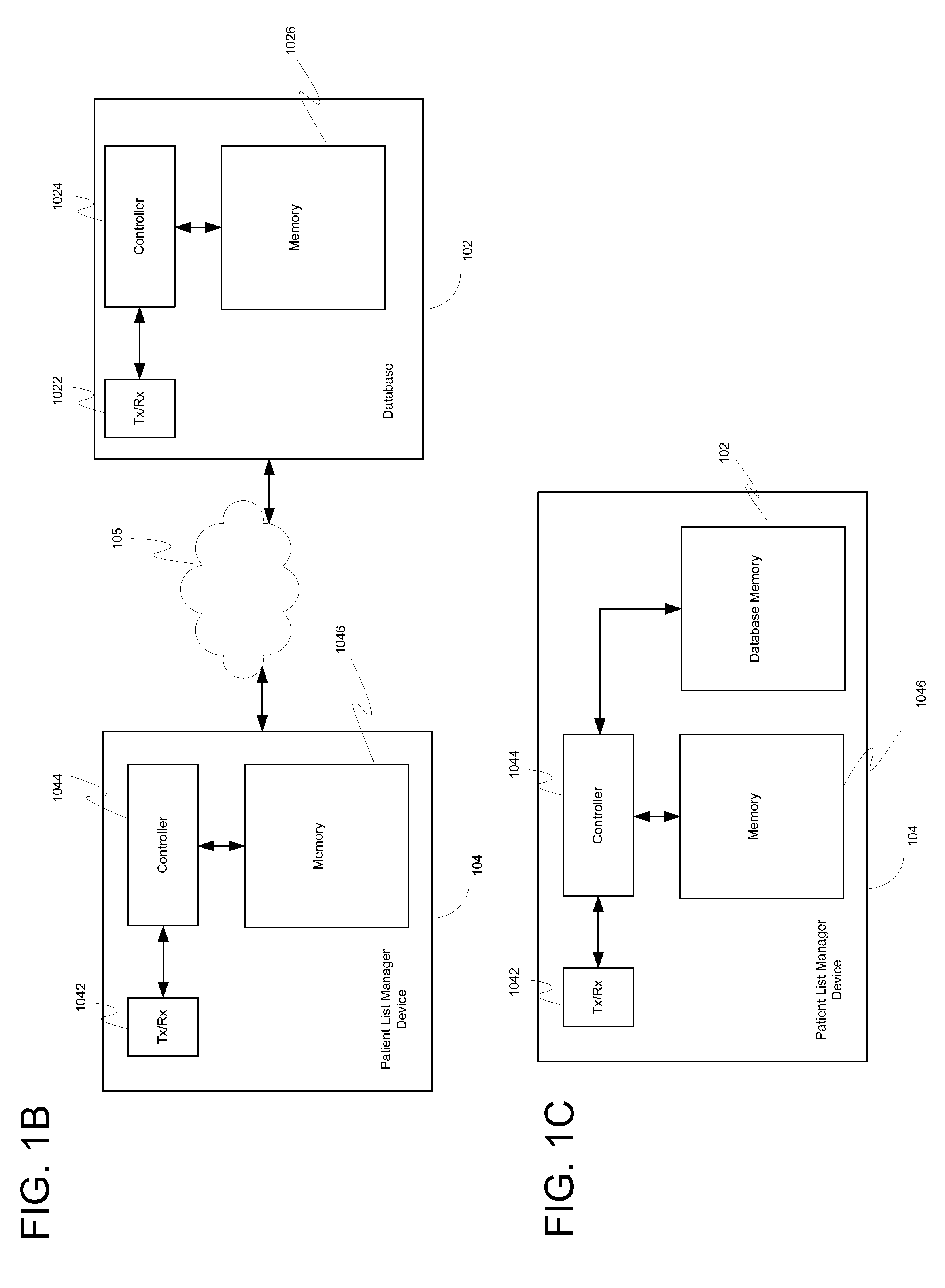
System, client device, server and method for providing a cross-facility patient data management and reporting platform
ActiveUS20150169827A1Readily annotatedAccurate billingKey distribution for secure communicationCryptography processingPoint of carePatient management
A system for providing a patient list manager includes a client device for point of care capture and access of patient data and a server for providing cloud based hosting. One or more modules can leverage the patient data to help users improve and facilitate the efficiency and safety of patient management, diagnostic imaging management, clinical work flows, charge capture, revenue cycle management, accreditation reporting, quality and outcomes monitoring and improvement, among other things.
Owner:ICONIC DATA
System and method for recording patient history data about on-going physician care procedures
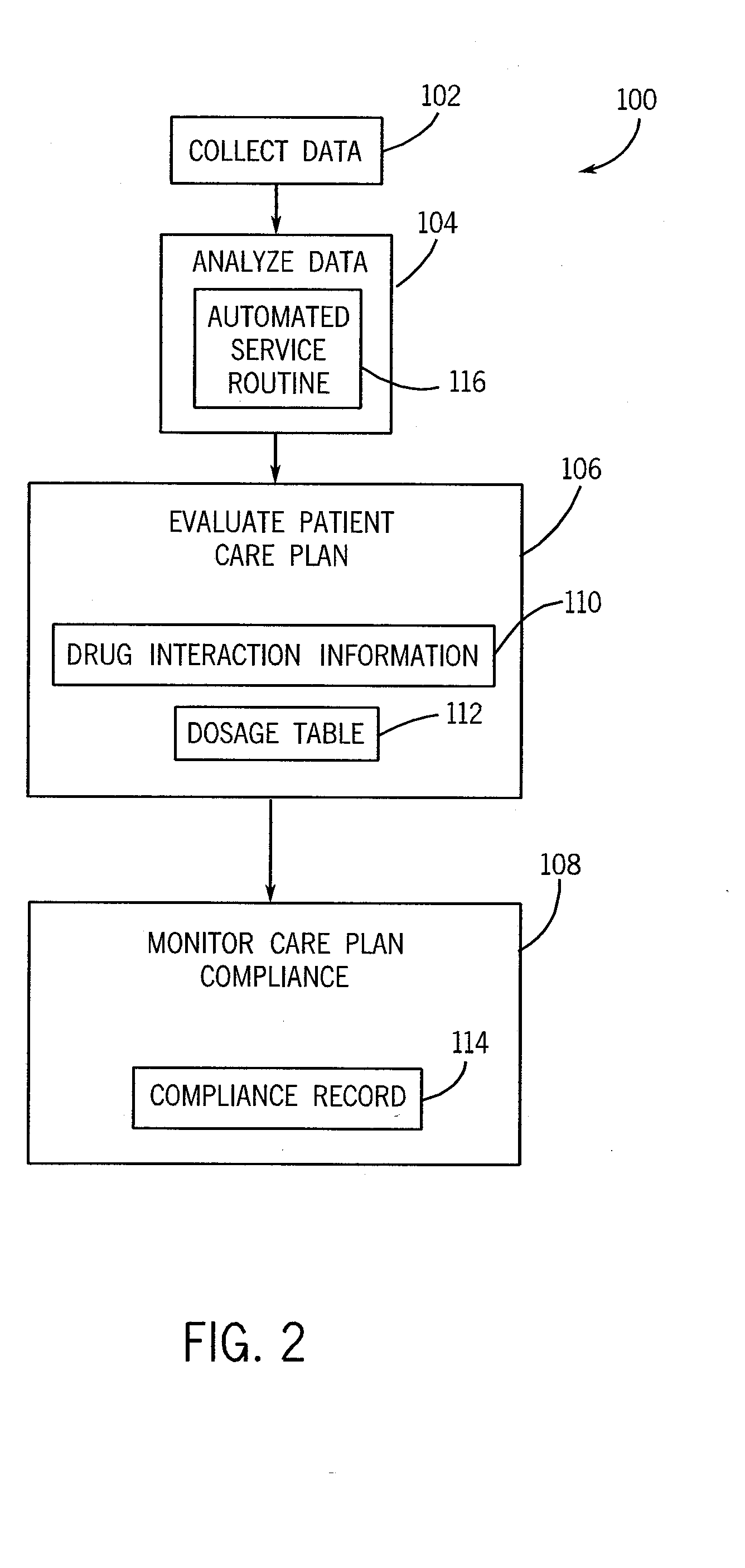
InactiveUS6154726AConvenient recordingOptimize schedulingData processing applicationsComputer-assisted medical data acquisitionStaff timeEfficacy
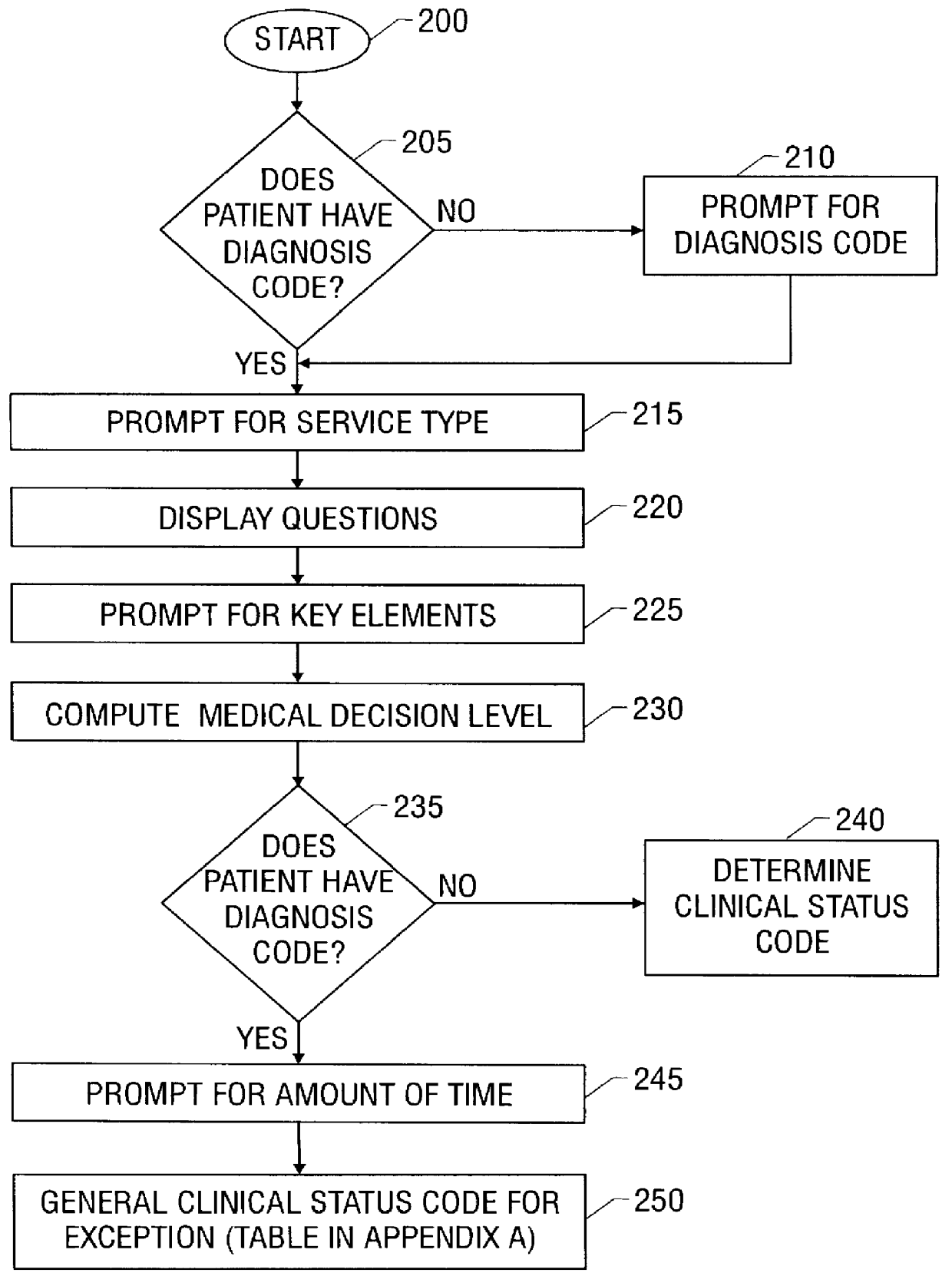
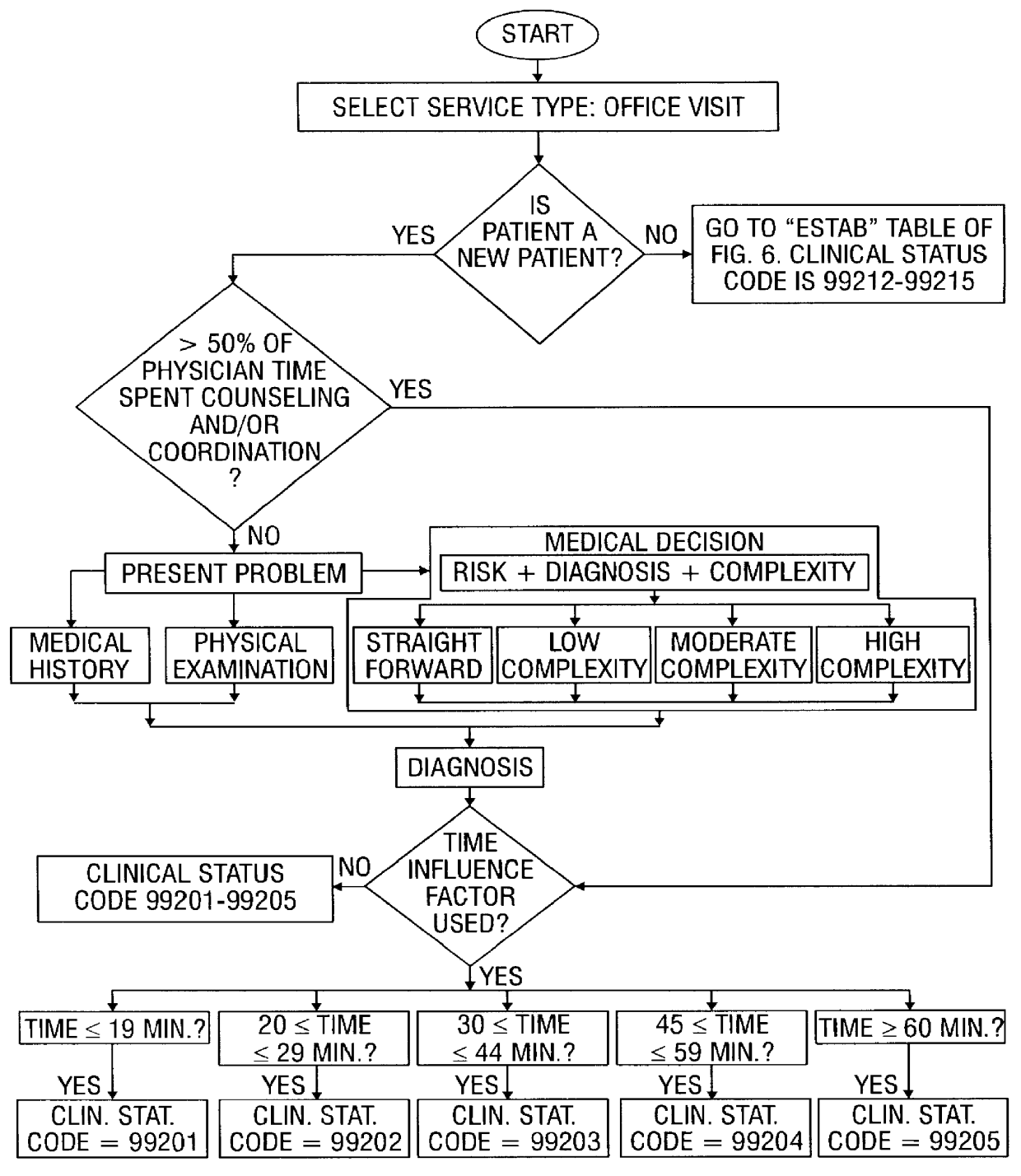
A system and method for processing patient data permits physicians and other medical staff personnel to record, accurately and precisely, historical patient care information. An objective measure of a physician's rendered level of care, as described by a clinical status code, is automatically generated. Data elements used in the determination of the generated clinical status code include a level of history of the patient, a level of examination of the patient, a decision-making process of the physician treating the patient, and a "time influence factor." The quantity and quality of care information for a particular patient is enhanced allowing future care decisions for that patient to be based on a more complete medical history. Enhanced care information can be used in outcome studies to track the efficacy of specific treatment protocols. Archiving of patient information is done in a manner which allows reconstruction of the qualitative aspects of provided medical services. The medical care data can be recorded, saved, and transferred from a portable system to a larger stationary information or database system. Considerable physician and staff time are saved and precision and accuracy are significantly enhanced, by generating these clinical status codes automatically (at the point of service by the care-provider without any intermediary steps) from information recorded simultaneously with the provision of services.
Owner:RENSIMER ENTERPRISES
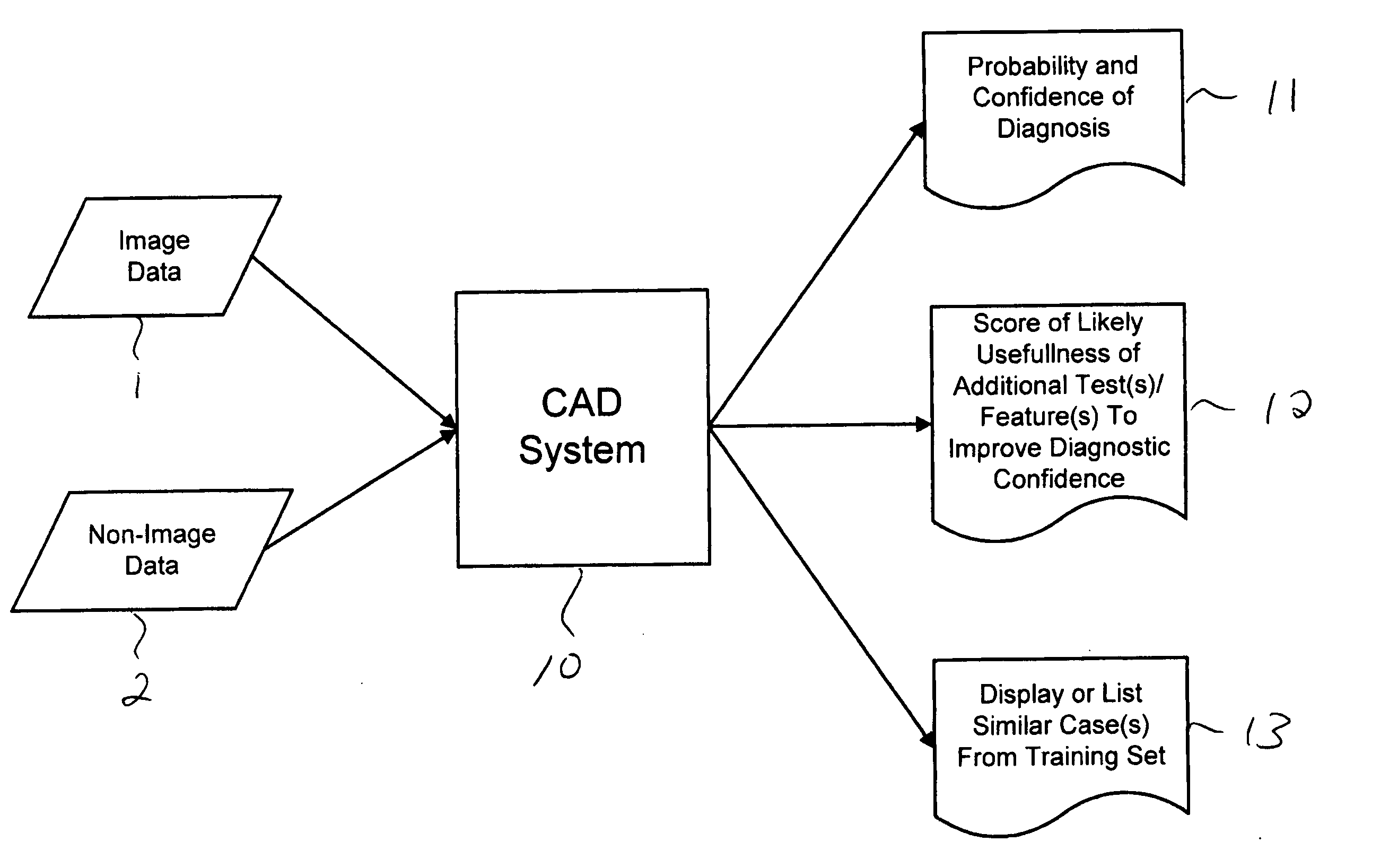
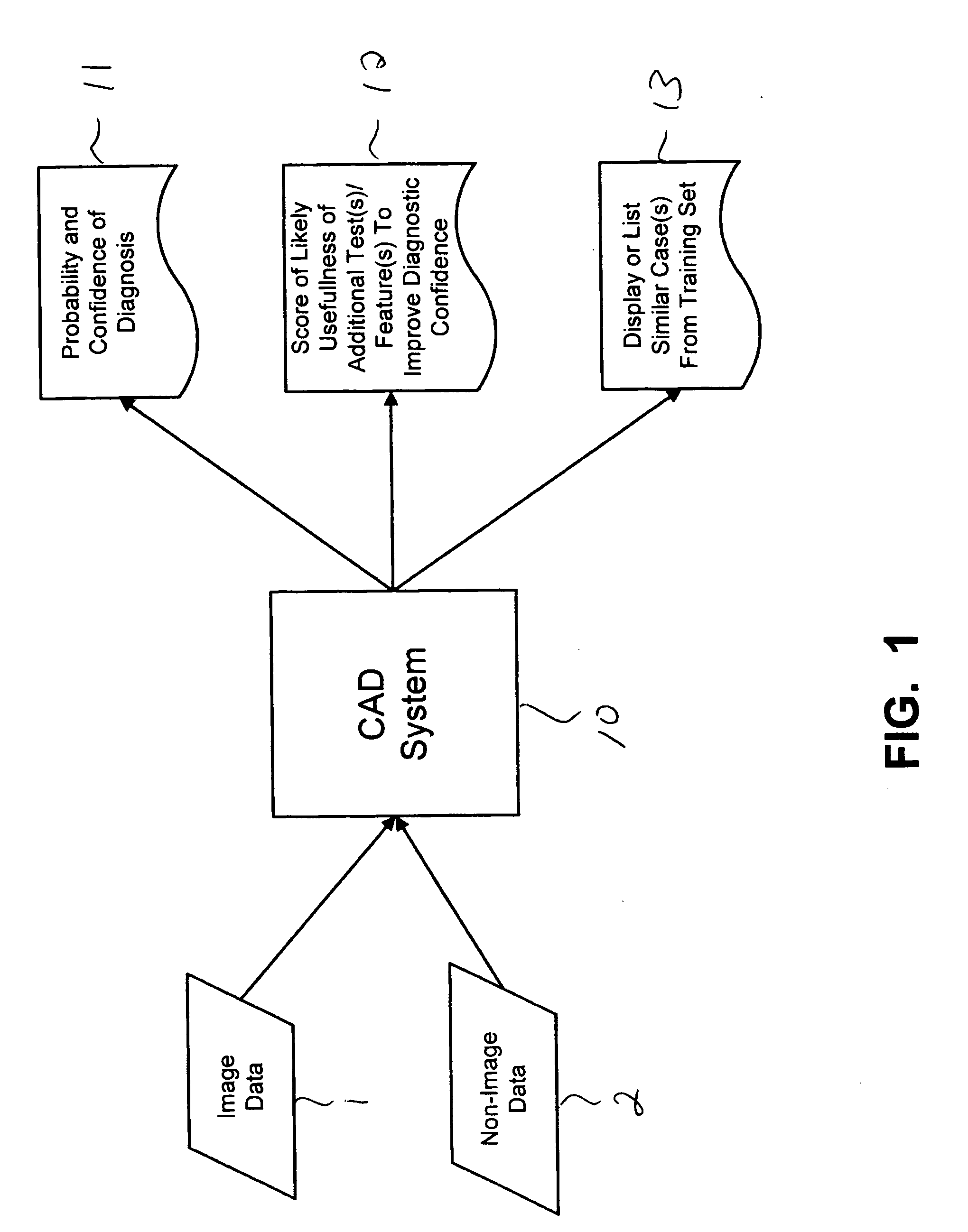
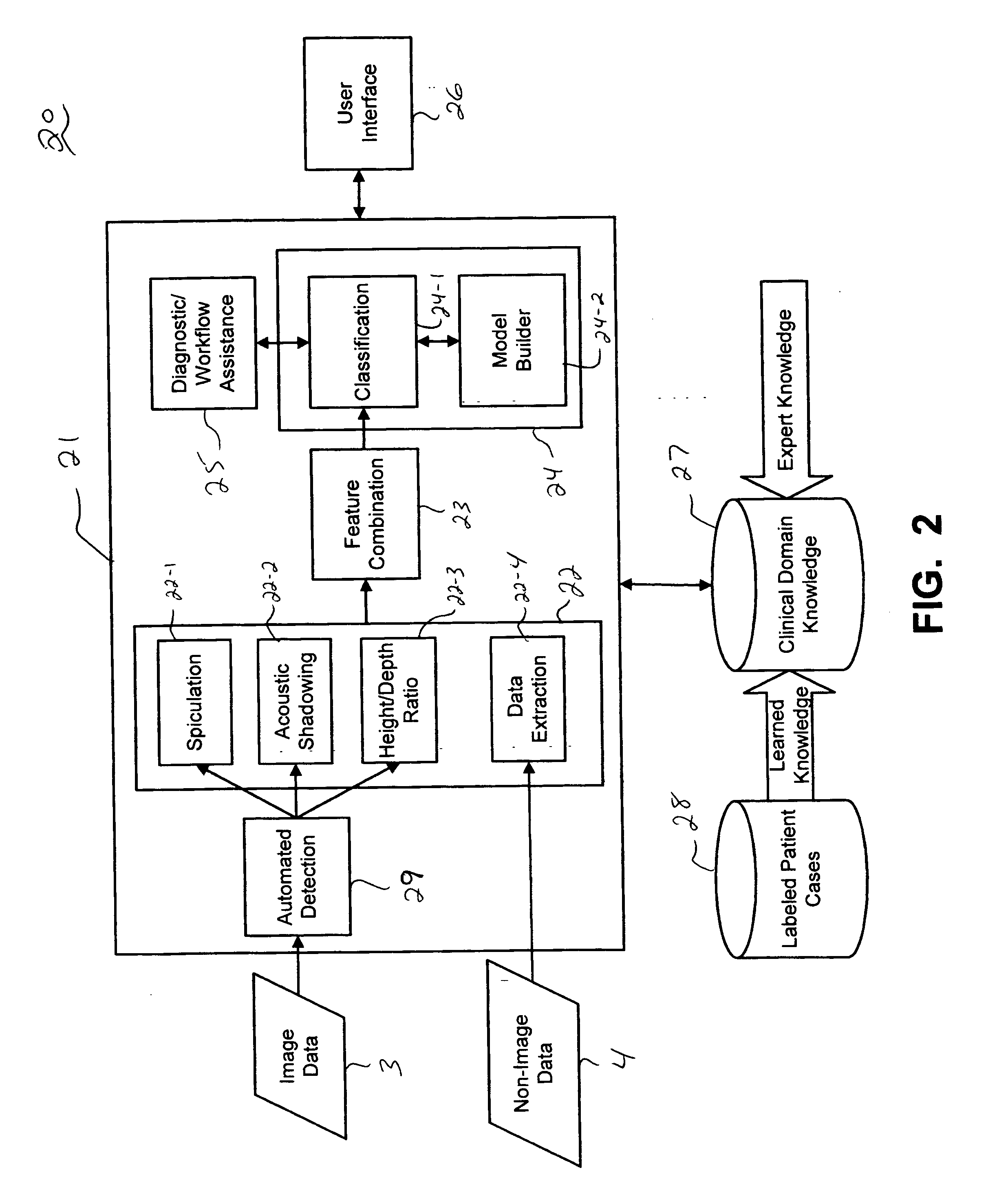
Systems and methods for automated diagnosis and decision support for breast imaging
ActiveUS20050049497A1Ultrasonic/sonic/infrasonic diagnosticsImage enhancementPatient dataDecision taking
CAD (computer-aided diagnosis) systems and applications for breast imaging are provided, which implement methods to automatically extract and analyze features from a collection of patient information (including image data and / or non-image data) of a subject patient, to provide decision support for various aspects of physician workflow including, for example, automated diagnosis of breast cancer other automated decision support functions that enable decision support for, e.g., screening and staging for breast cancer. The CAD systems implement machine-learning techniques that use a set of training data obtained (learned) from a database of labeled patient cases in one or more relevant clinical domains and / or expert interpretations of such data to enable the CAD systems to “learn” to analyze patient data and make proper diagnostic assessments and decisions for assisting physician workflow.
Owner:SIEMENS MEDICAL SOLUTIONS USA INC
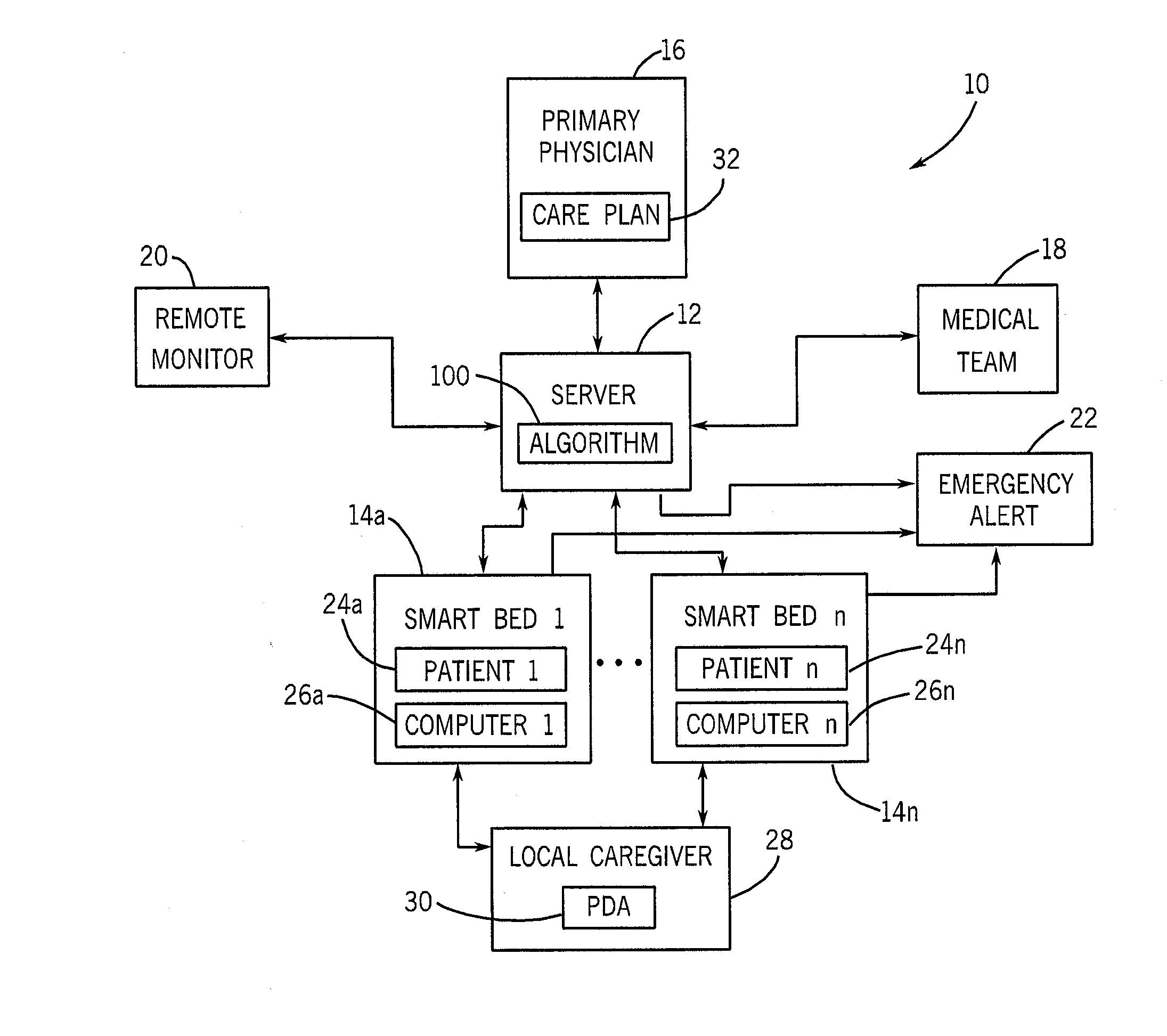
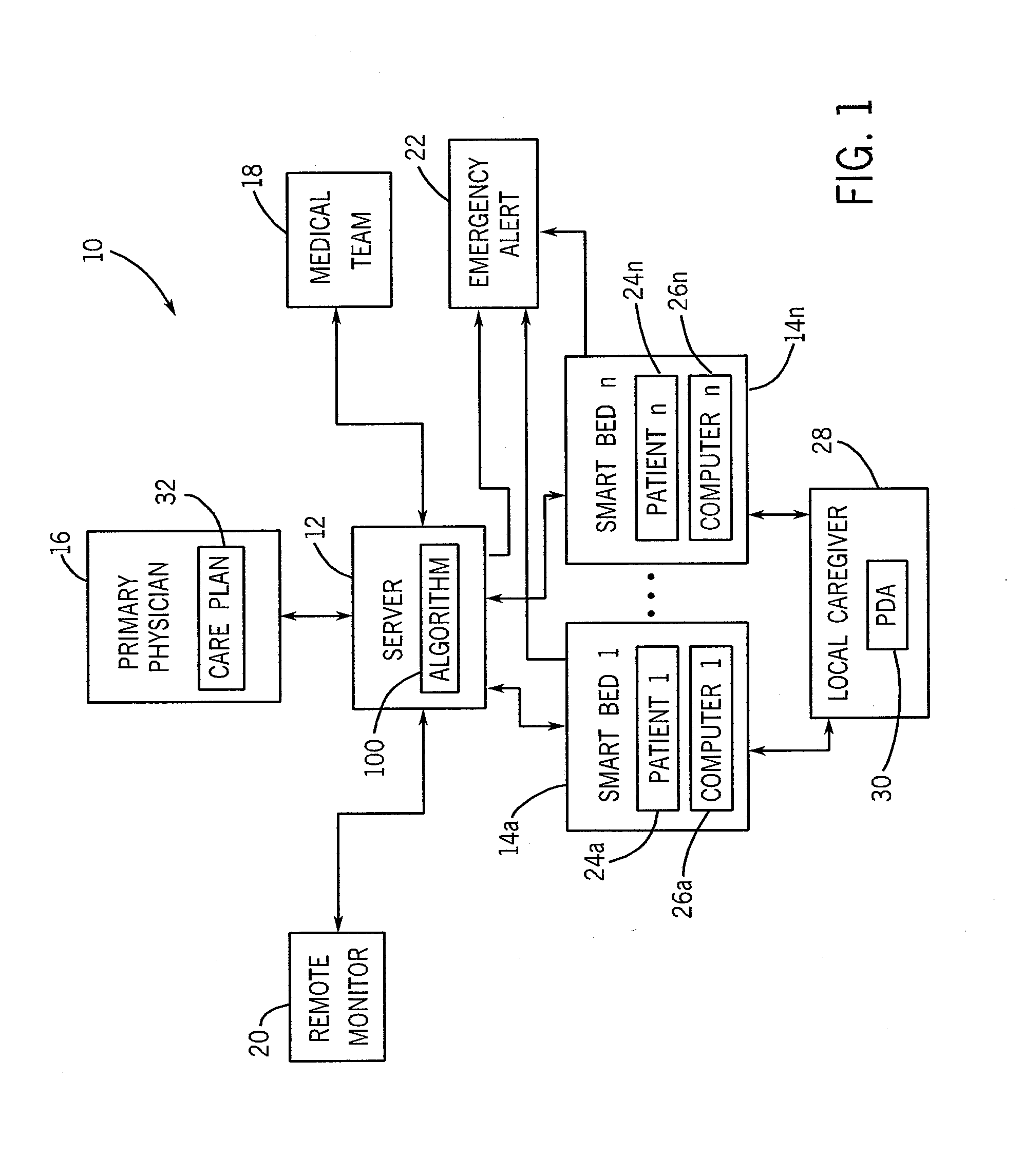
Smart bed system
A smart bed system is disclosed herein. The smart bed system includes a smart bed adapted to accommodate a patient. The smart bed includes a smart bed computer operatively connectable to one or more monitoring devices. The smart bed computer is configured to store any monitored data recorded by the monitoring devices. The smart bed system also includes a server coupled with the smart bed computer. The server is configured to store any patient data pertaining to the patient. The server is configured to receive the monitored data from the smart bed computer and thereafter to store the monitored data as part of the patient data. The patient data can be selectively transferred from the server to the smart bed computer such that the patient data is accessible directly from the smart bed.
Owner:GENERAL ELECTRIC CO
Method for image processing and contour assessment of the heart
One embodiment discloses a computerized method of facilitating cardiac intervention. The method may include inputting patient data and creating a computerized interactive model of a heart based on the patient data. The model may include features. The model may simulate at least one proposed cardiac intervention by adding or deleting features to the model, and determining the effects of the proposed cardiac simulation upon the entire model. Simulations may be repeated to allow the user to determine an optimal cardiac intervention. A template and / or patient specific instrument may be created from the model to use as a guide during the cardiac intervention.
Owner:CHASE MEDICAL +1
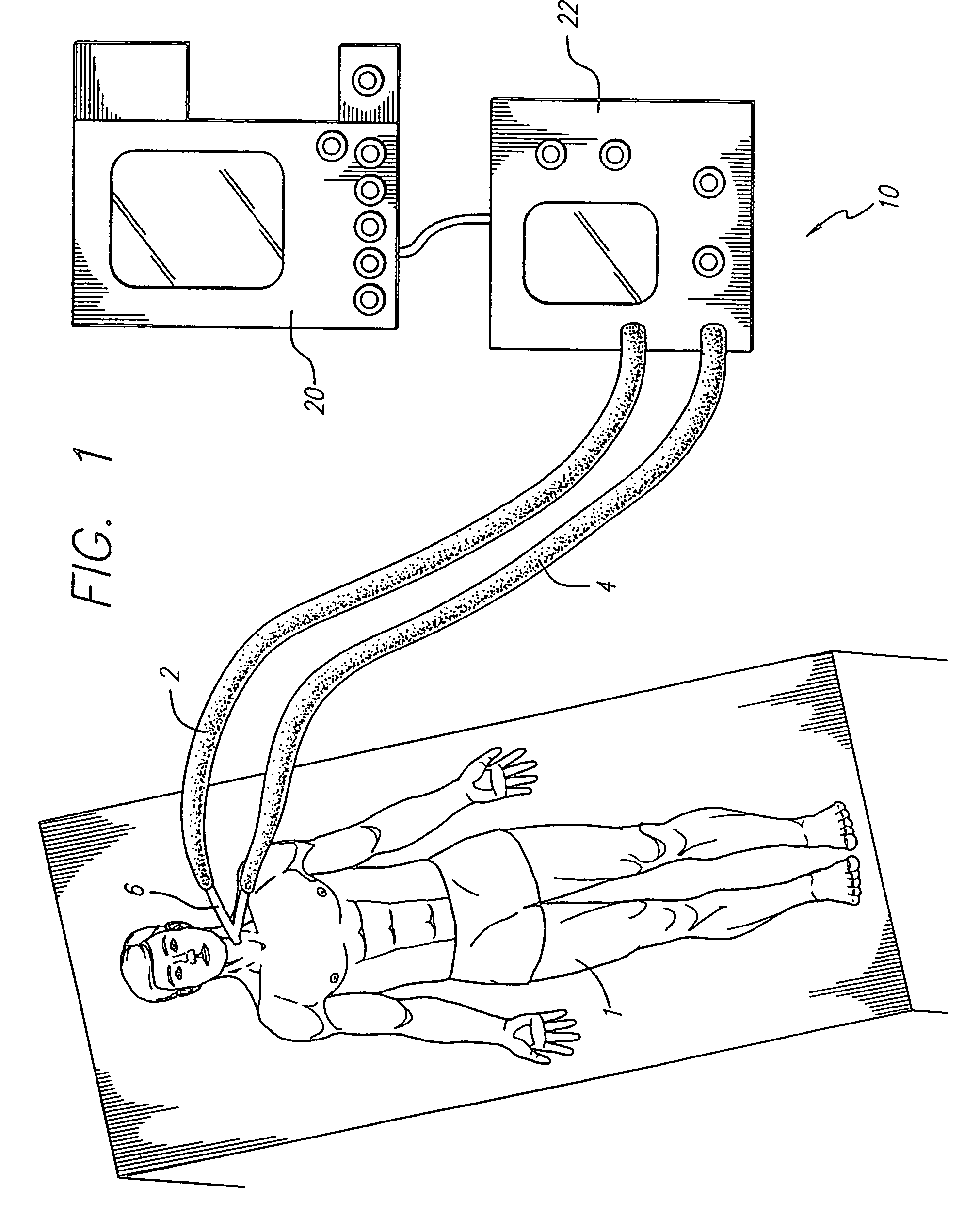
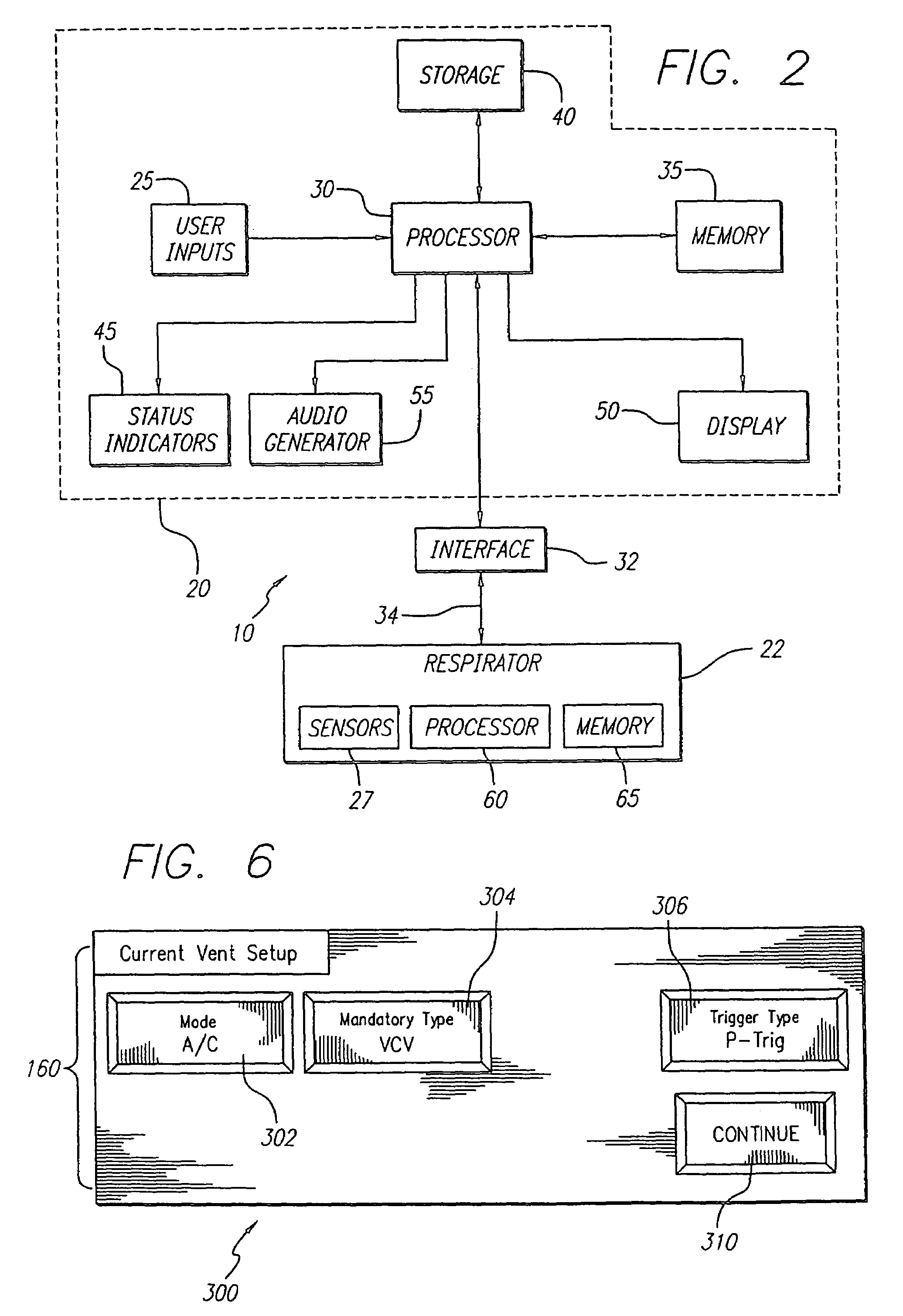
Ventilator breath display and graphic user interface
InactiveUS7036504B2Avoid harmIncrease speedRespiratory device testingMedical devicesGraphicsGraphical user interface
The invention is directed to a ventilation control system for controlling the ventilation of a patient. The ventilation control system utilizes a user-friendly user interface for the display of patient data and ventilator status. The user interface includes a graphic representation of a breath cycle that displays the breath cycle currently being ventilated, and is also responsive to changes in ventilation settings to assist the user in evaluation the effect of those changes on the ventilator strategy before the changes are implemented.
Owner:NELL COR PURITAN BENNETT INC (US)
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com