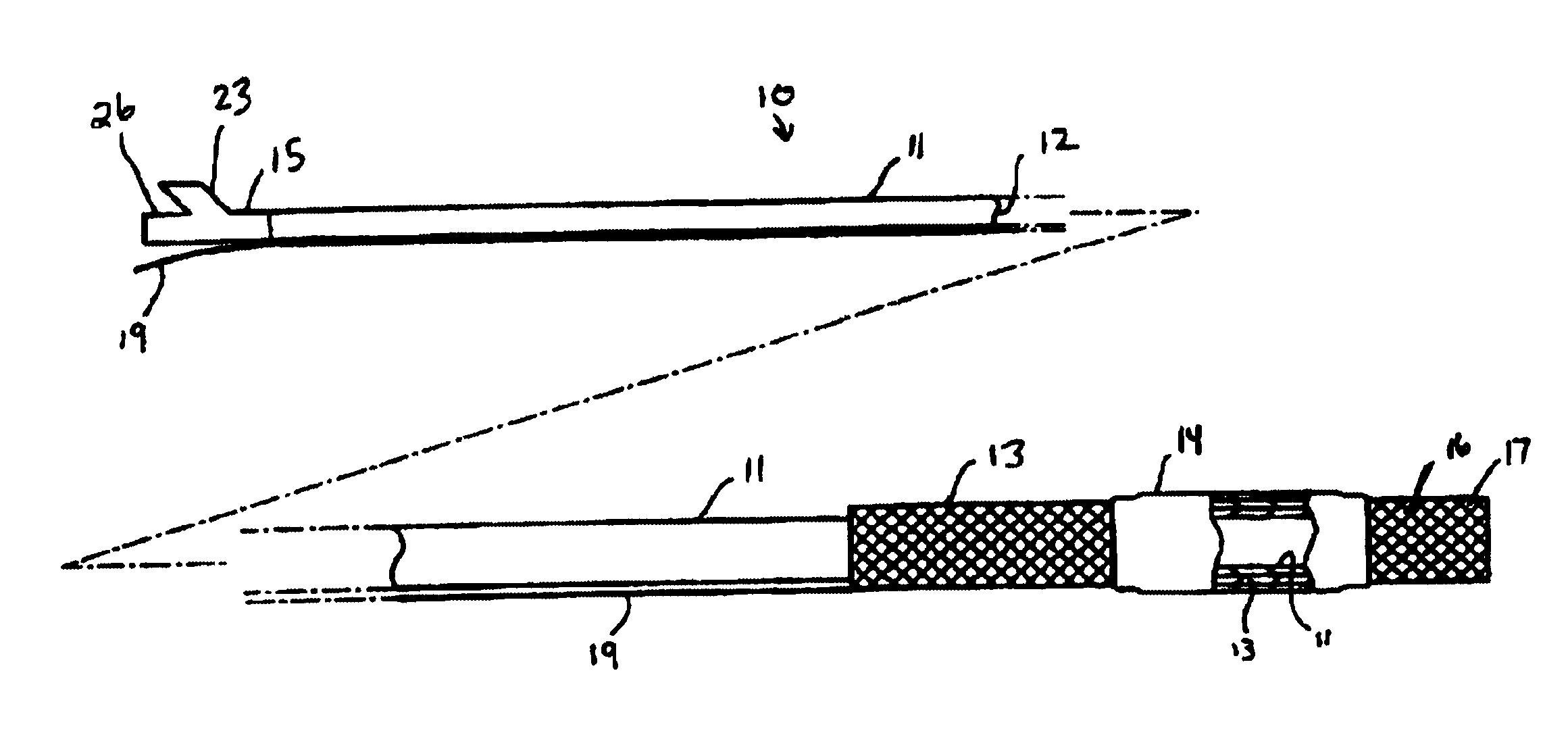
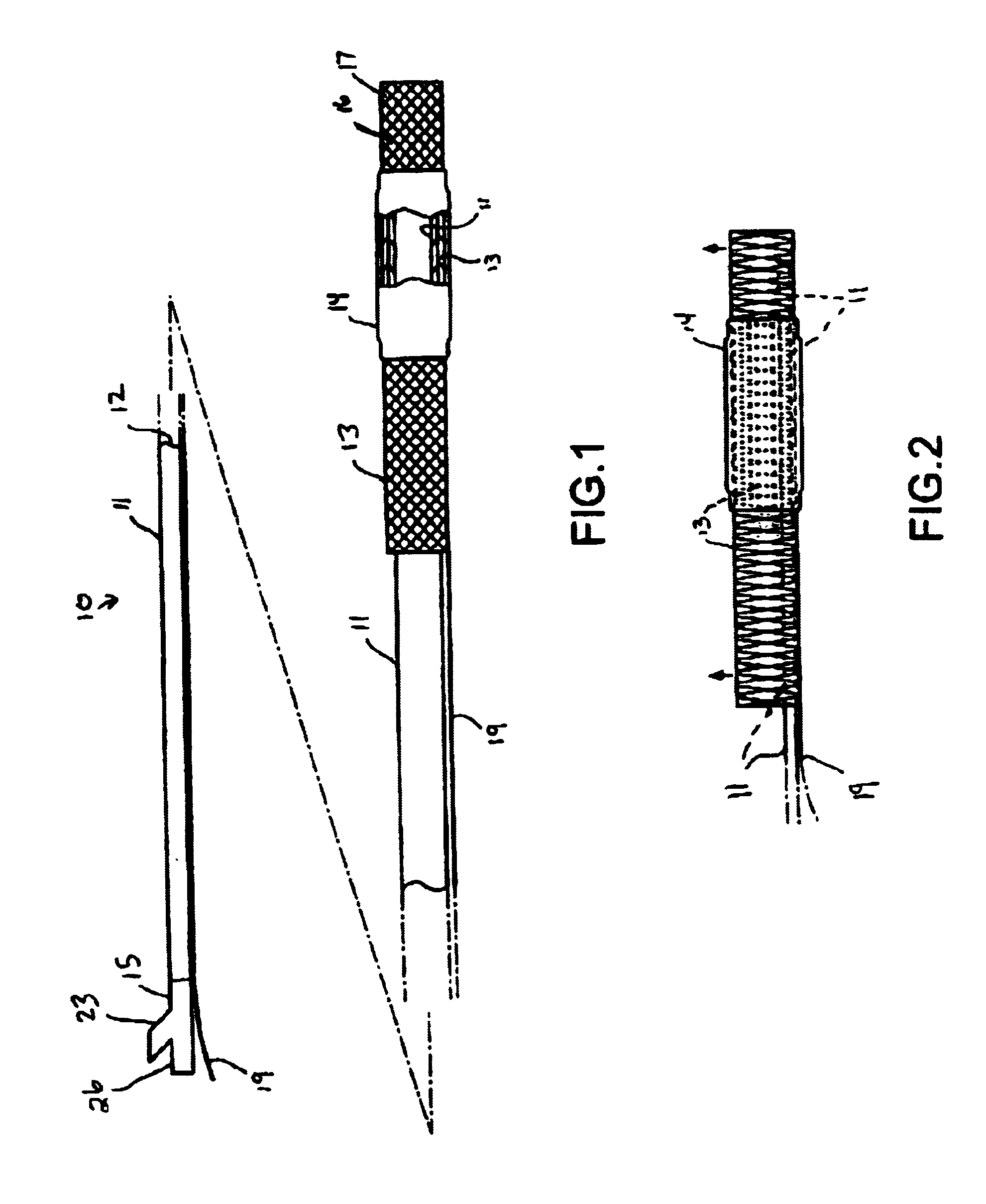
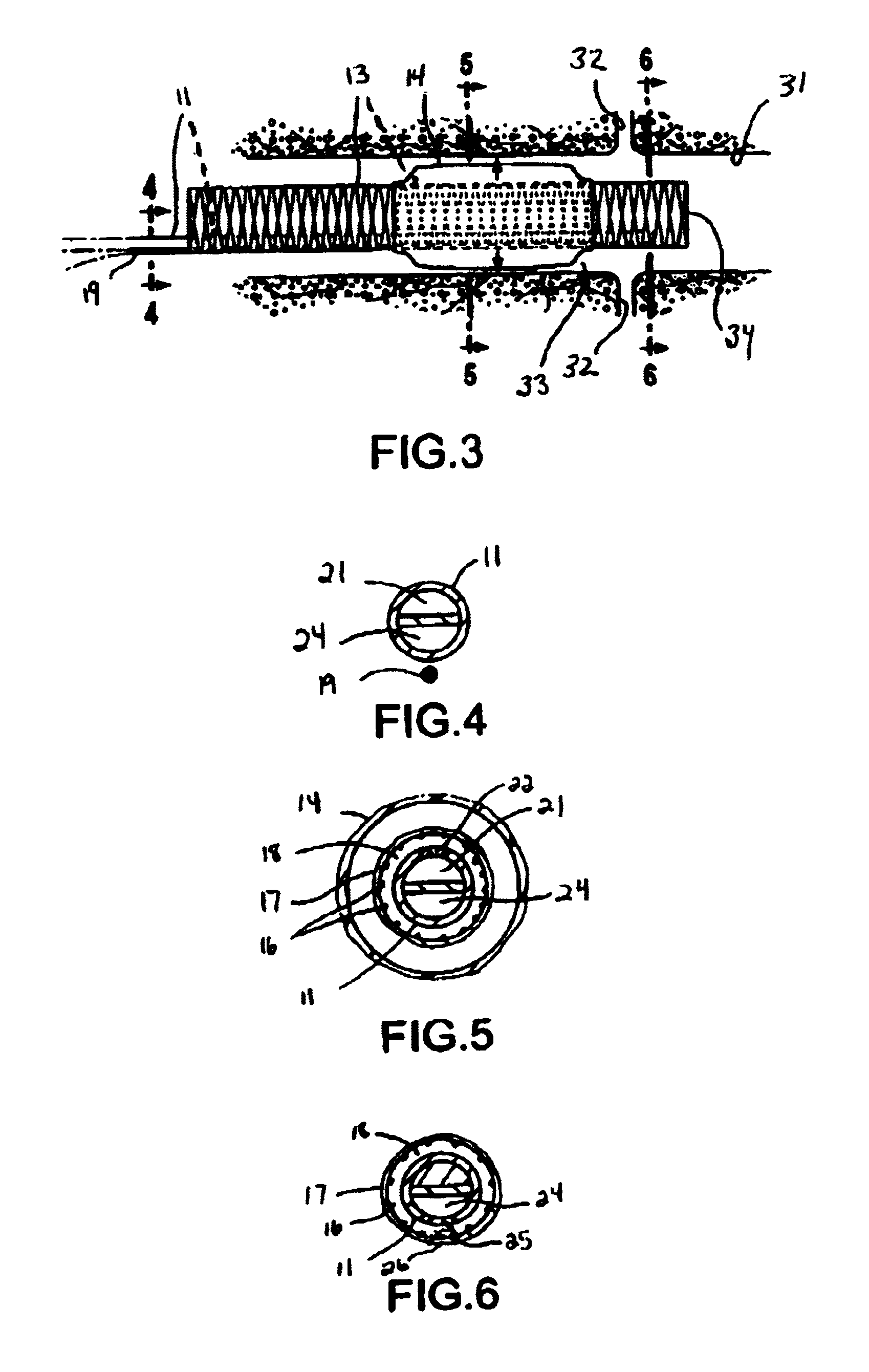
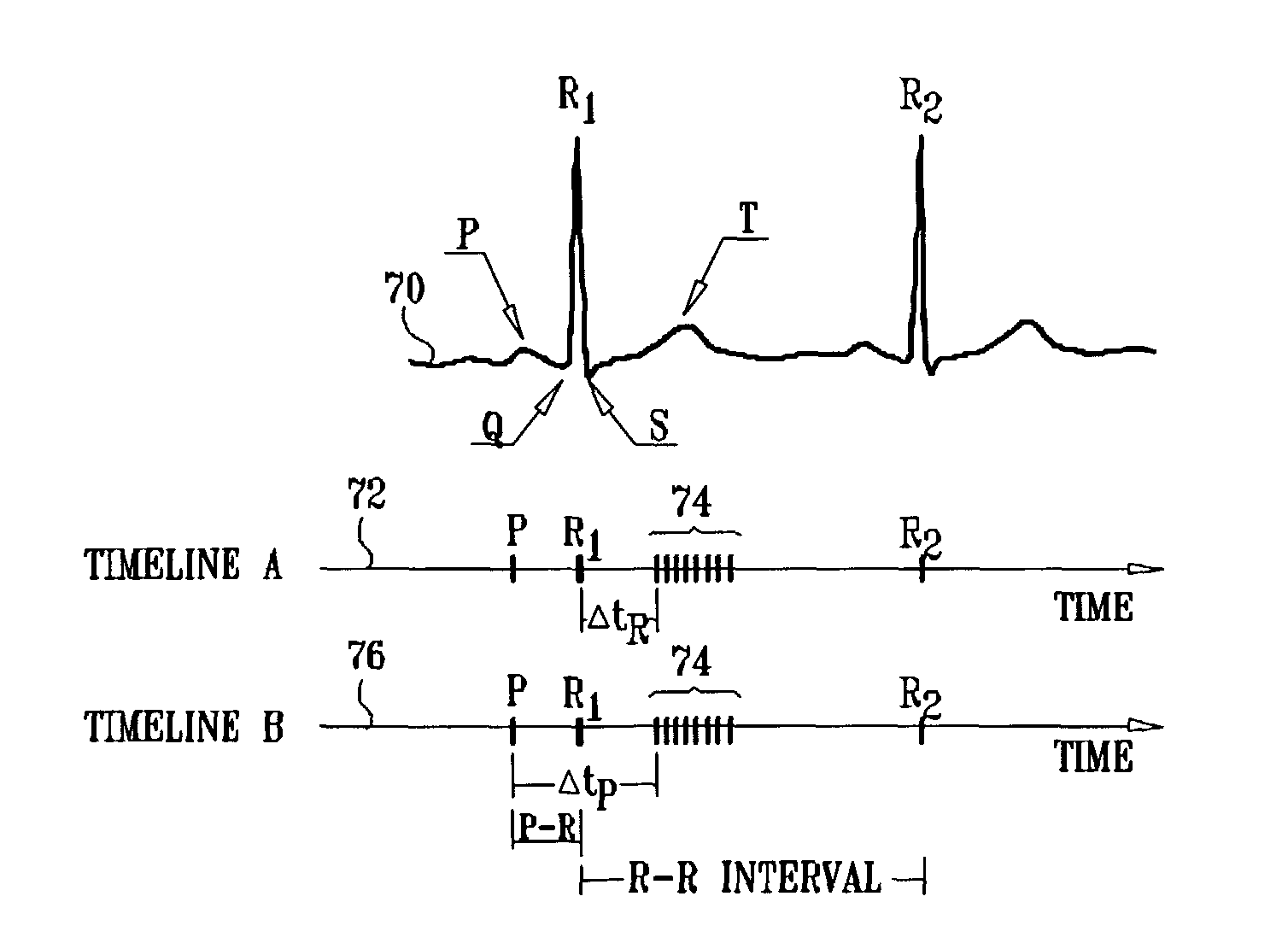
Patents
Literature
4873results about "Heart stimulators" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
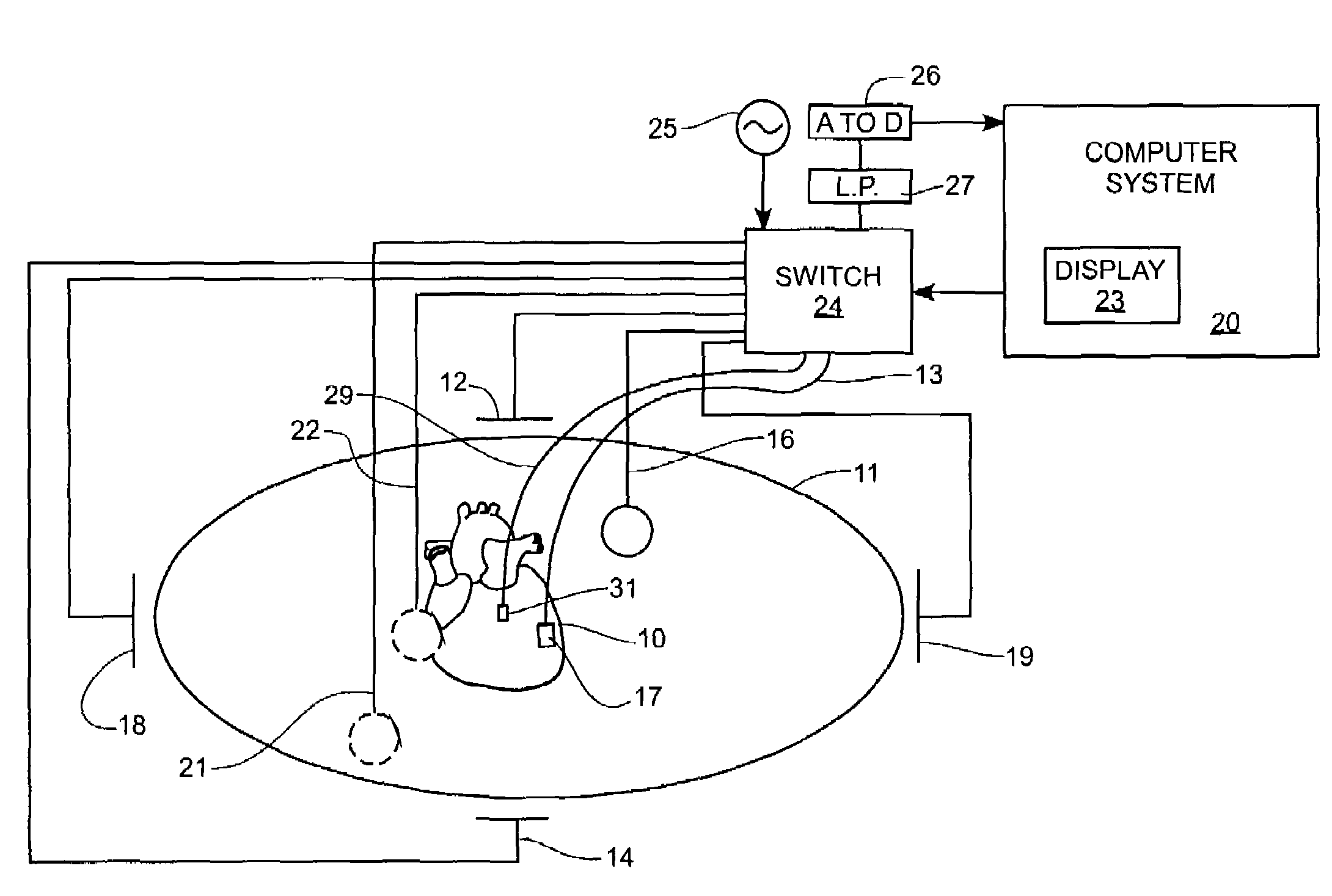
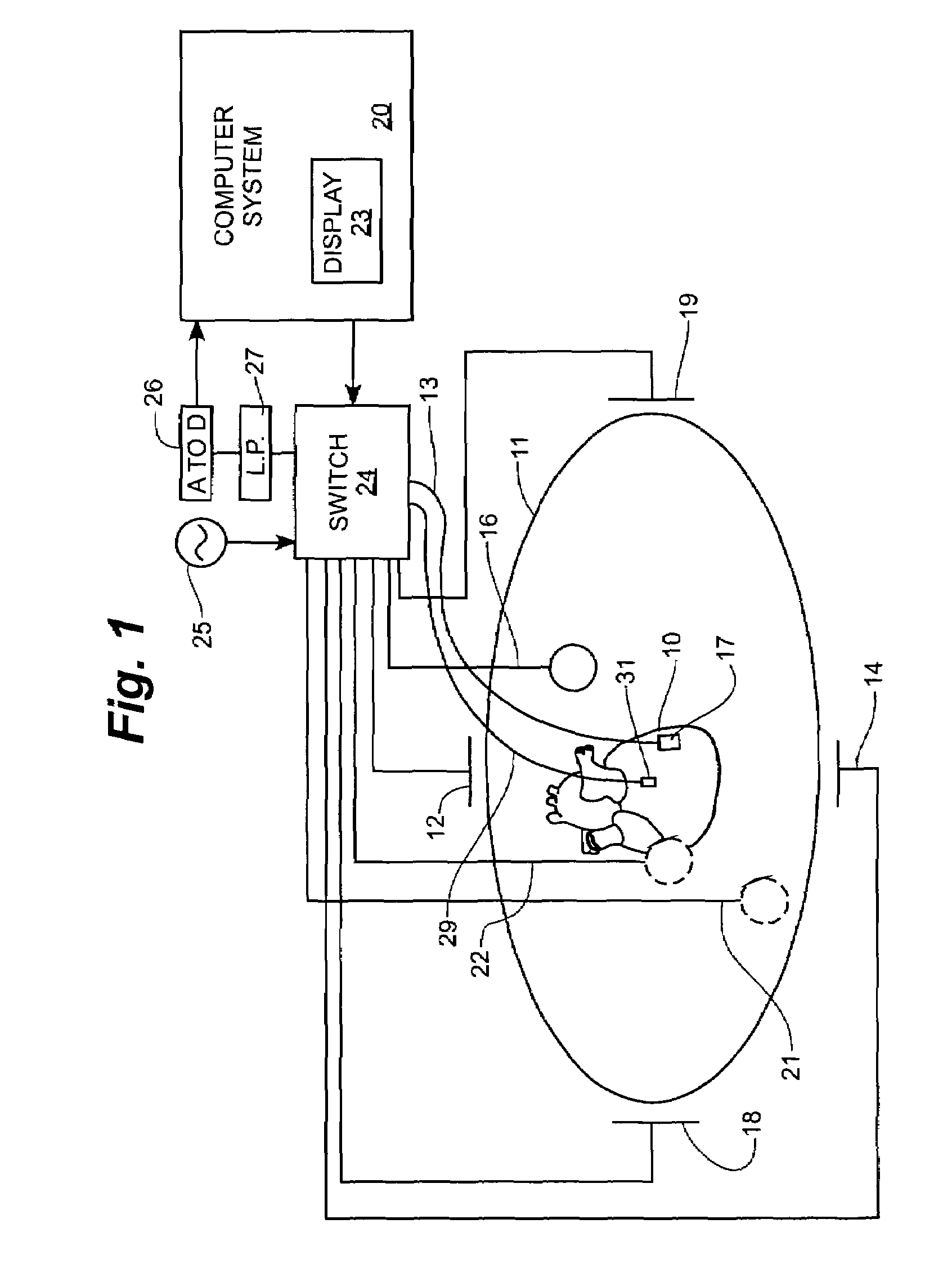
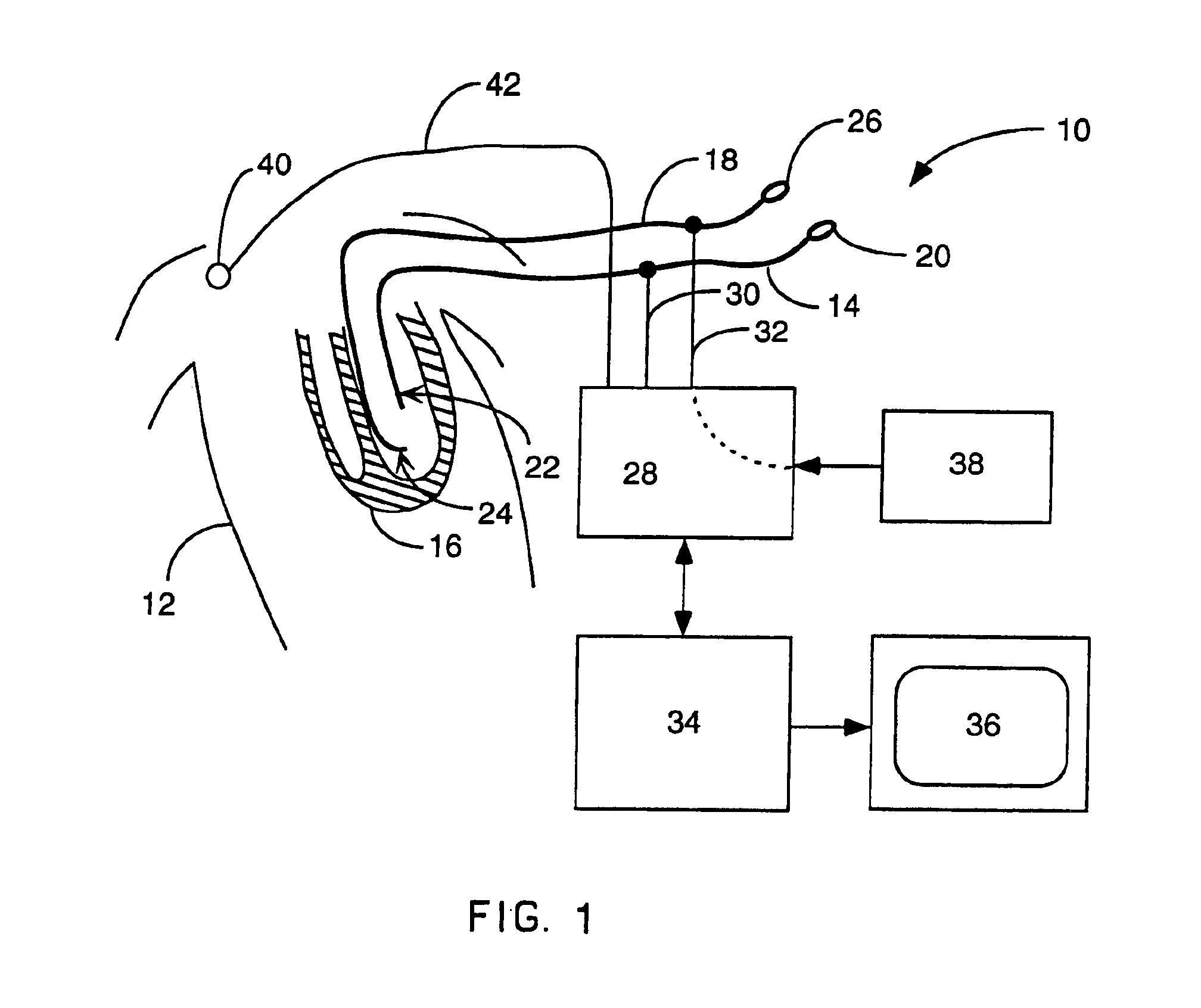
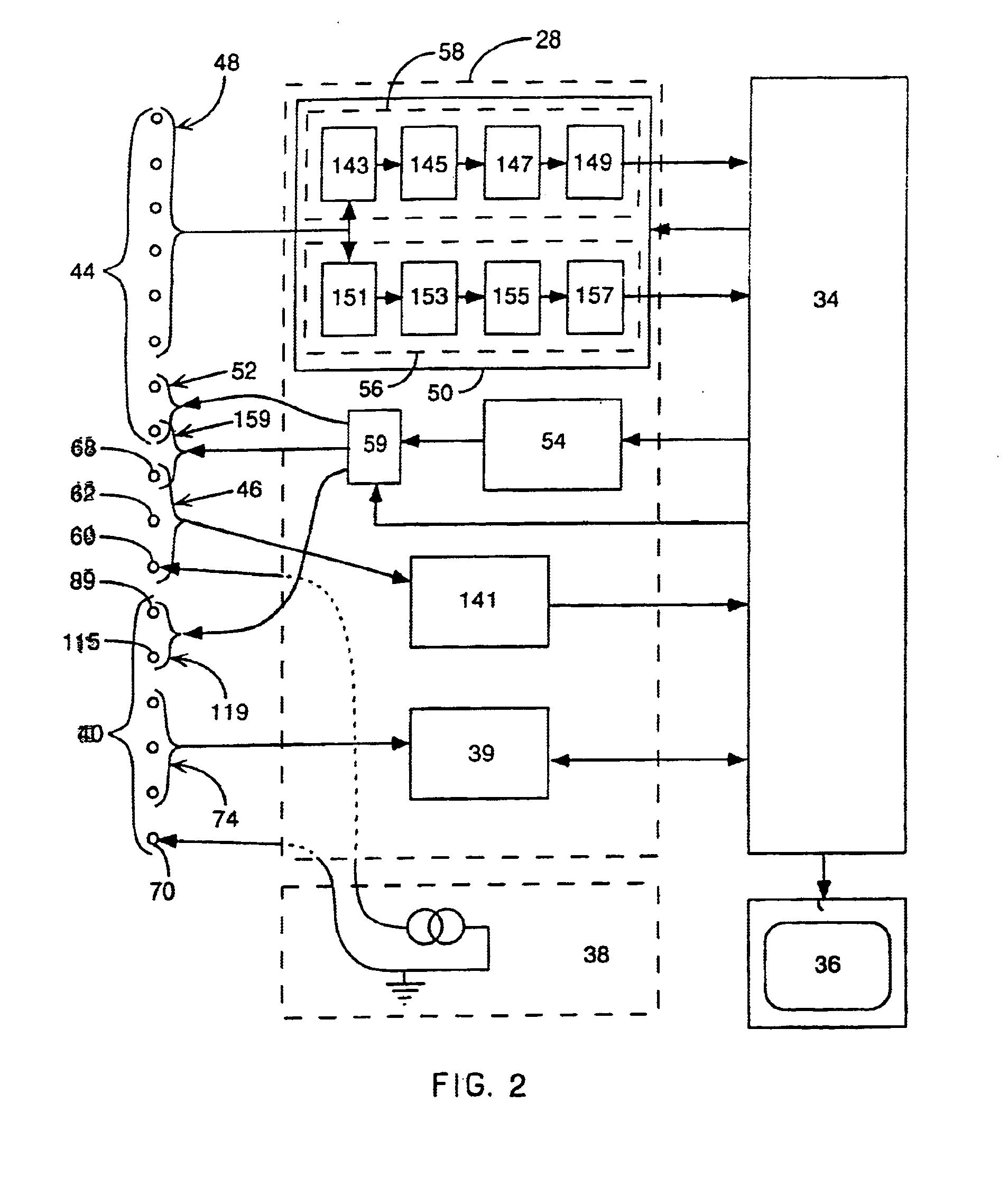
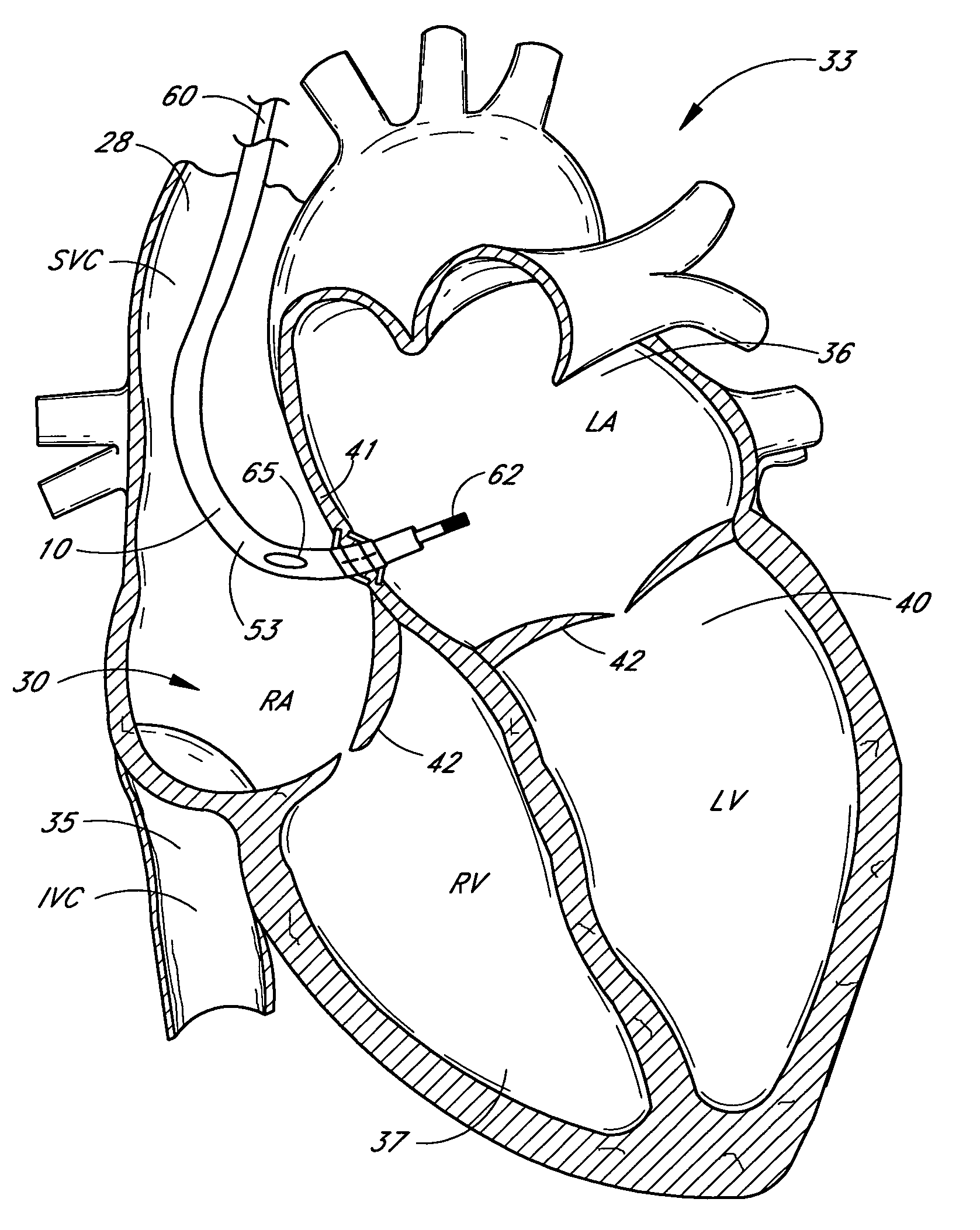
Method and apparatus for catheter navigation and location and mapping in the heart
InactiveUS7263397B2Enhance system functionsAccurate locationElectrocardiographyCatheterHeart chamberBiological activation
A medical system for finding and displaying the location of electrodes within the body. The electrodes may be used to measure the voltage on the heart wall and display this as an activation map on a geometry representing the heart chamber.
Owner:ST JUDE MEDICAL ATRIAL FIBRILLATION DIV
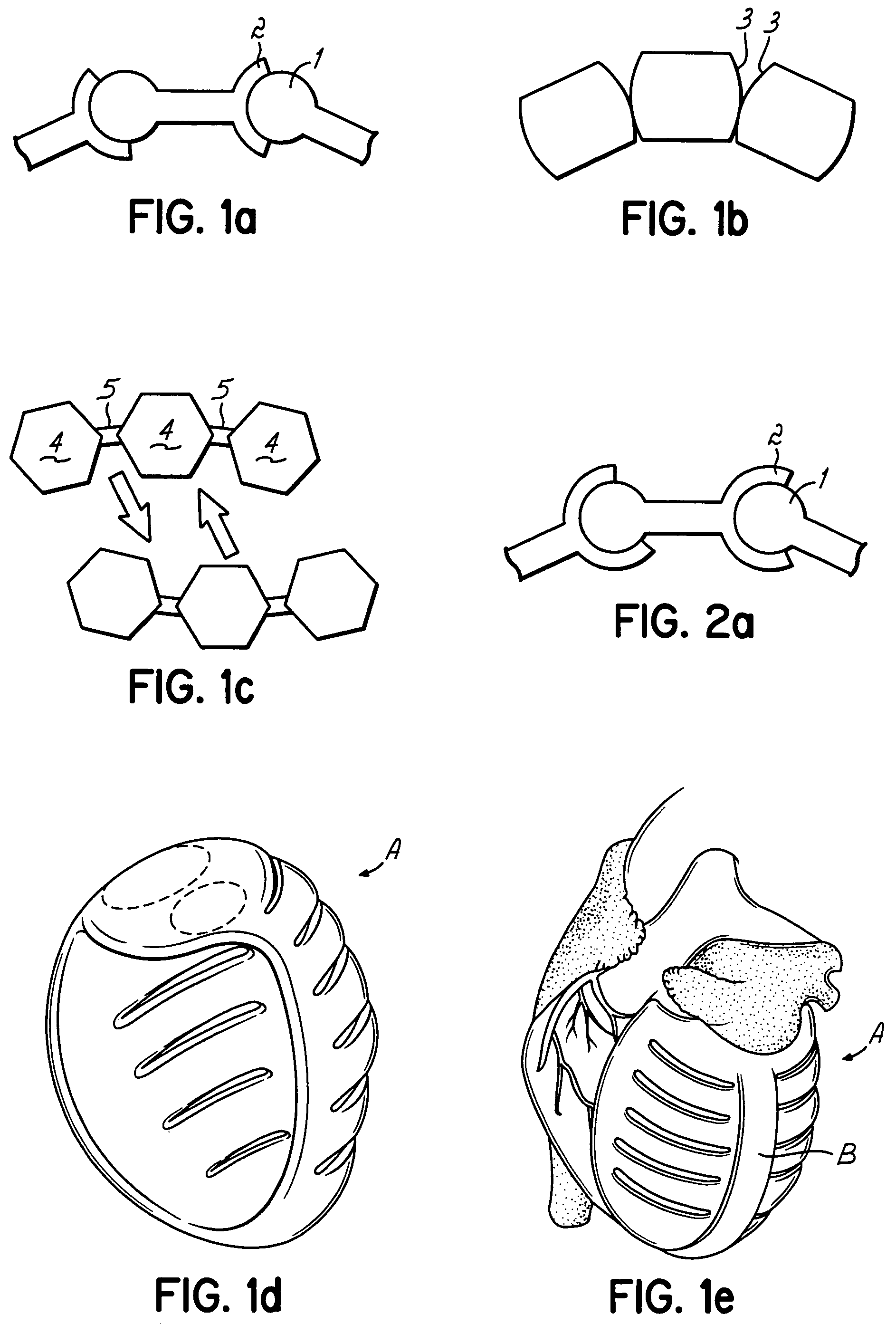
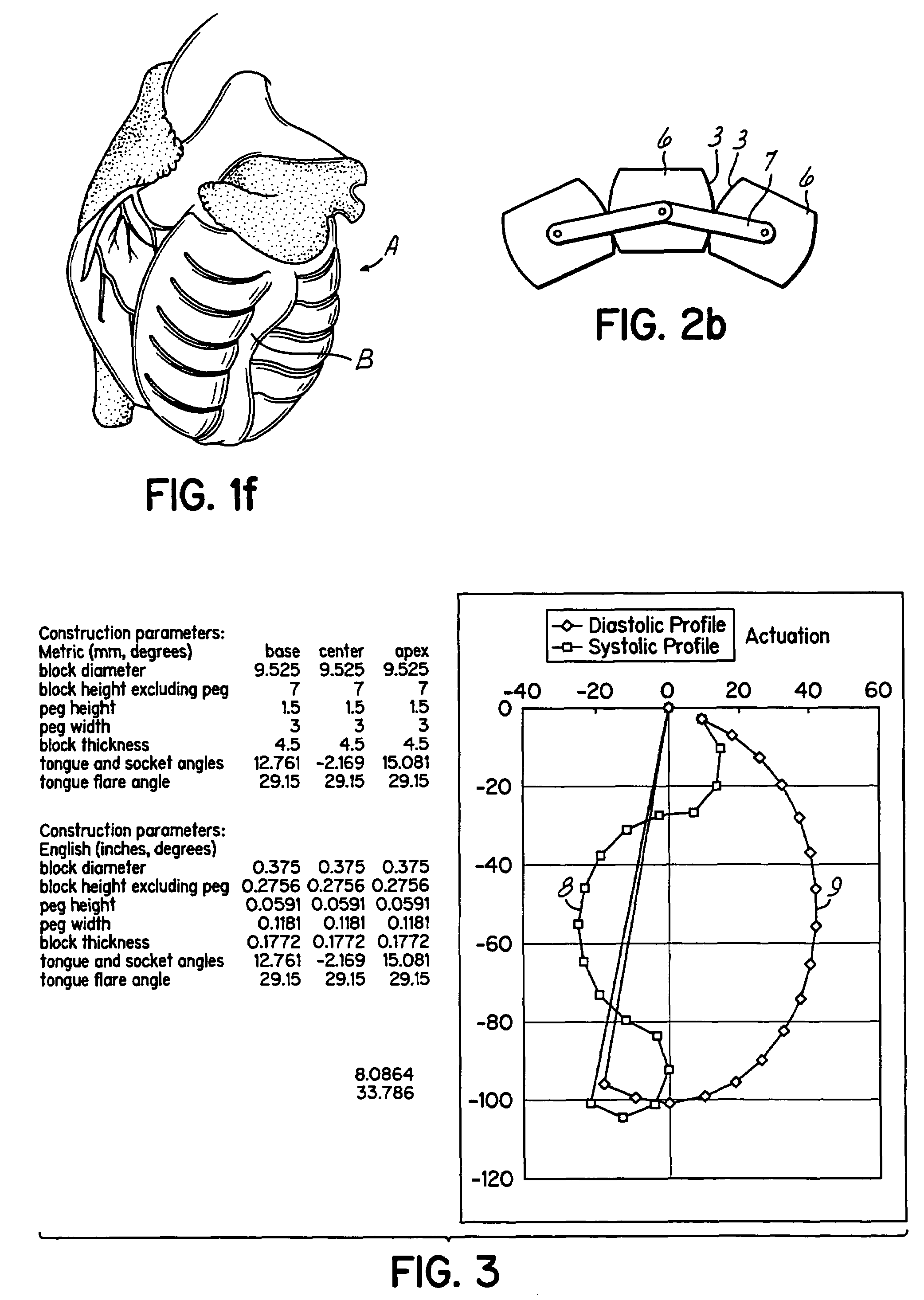
Actuation mechanisms for a heart actuation device
An actuation mechanism for assisting the operation of the natural heart has a varying shape for deforming the heart. In one embodiment, a plurality of links articulates with respect to each other for varying the shape of the actuation mechanism. The plurality of links is configured for being positioned proximate to an outer surface of the heart for deforming the heart by varying the shape of the actuation mechanism. In another embodiment, a jacket for coupling with an outer surface of the heart has a tether coupled to successive sections of the jacket. The tether is operable to be translated with respect to the jacket sections to vary the shape of the jacket for deforming the heart. In another embodiment, a plurality of concentric ring structures are coupled together to move with respect to each other in a concentric fashion. A movement mechanism coupled to the rings is operable to vary their positions with respect to each other to vary the overall shape for deforming the heart.
Owner:UNIVERSITY OF CINCINNATI +1
Method and apparatus for electrically stimulating the nervous system to improve ventricular dysfunction, heart failure, and other cardiac conditions
A method and apparatus are used to provide therapy to a patient experiencing ventricular dysfunction or heart failure. At least one electrode is located in a region associated with nervous tissue, such as nerve bundles T1-T4, in a patient's body. Electrical stimulation is applied to the at least one electrode to improve the cardiac efficiency of the patient's heart. One or more predetermined physiologic parameters of the patient are monitored, and the electrical stimulation is adjusted based on the one or more predetermined physiologic parameters.
Owner:MEDTRONIC INC
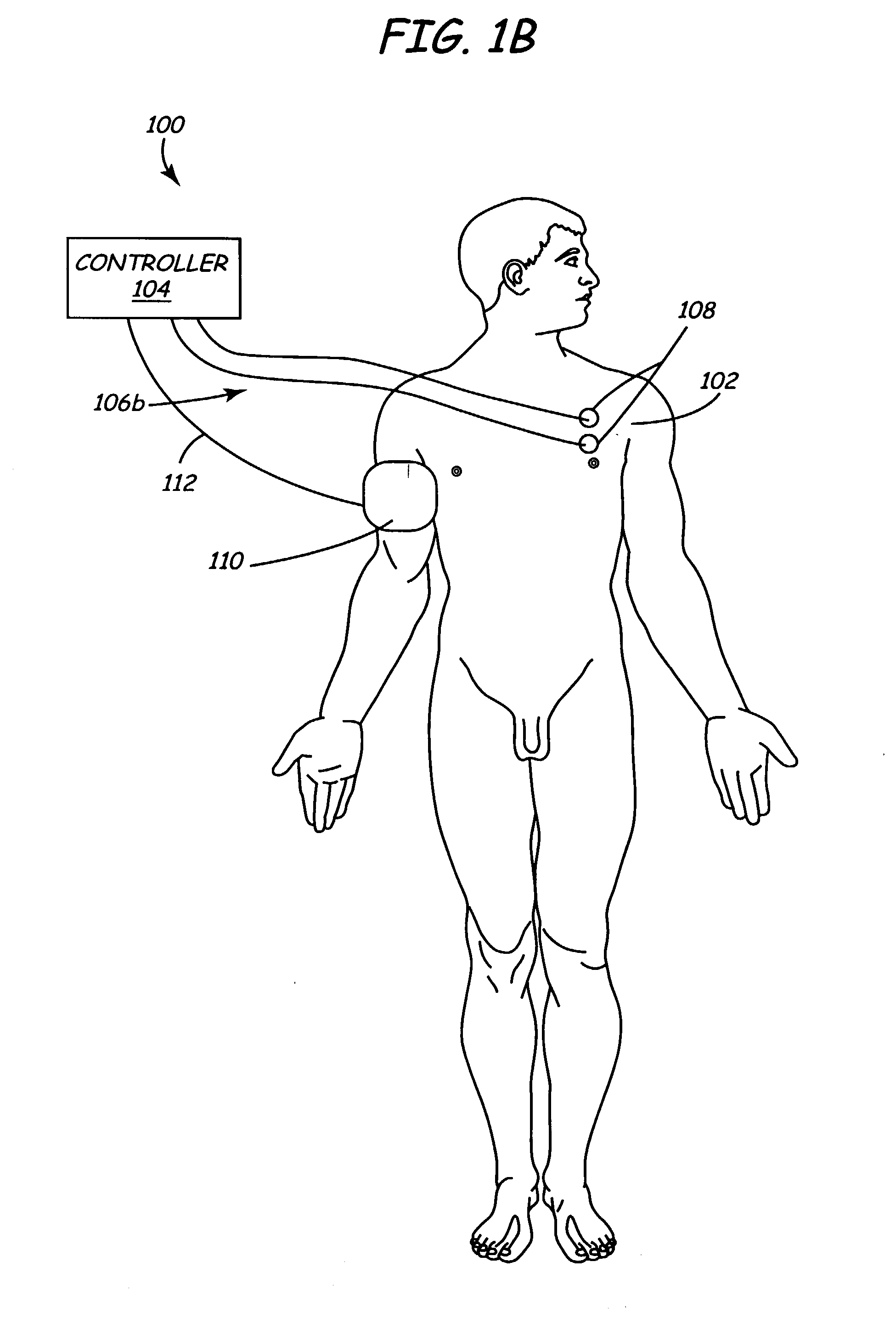
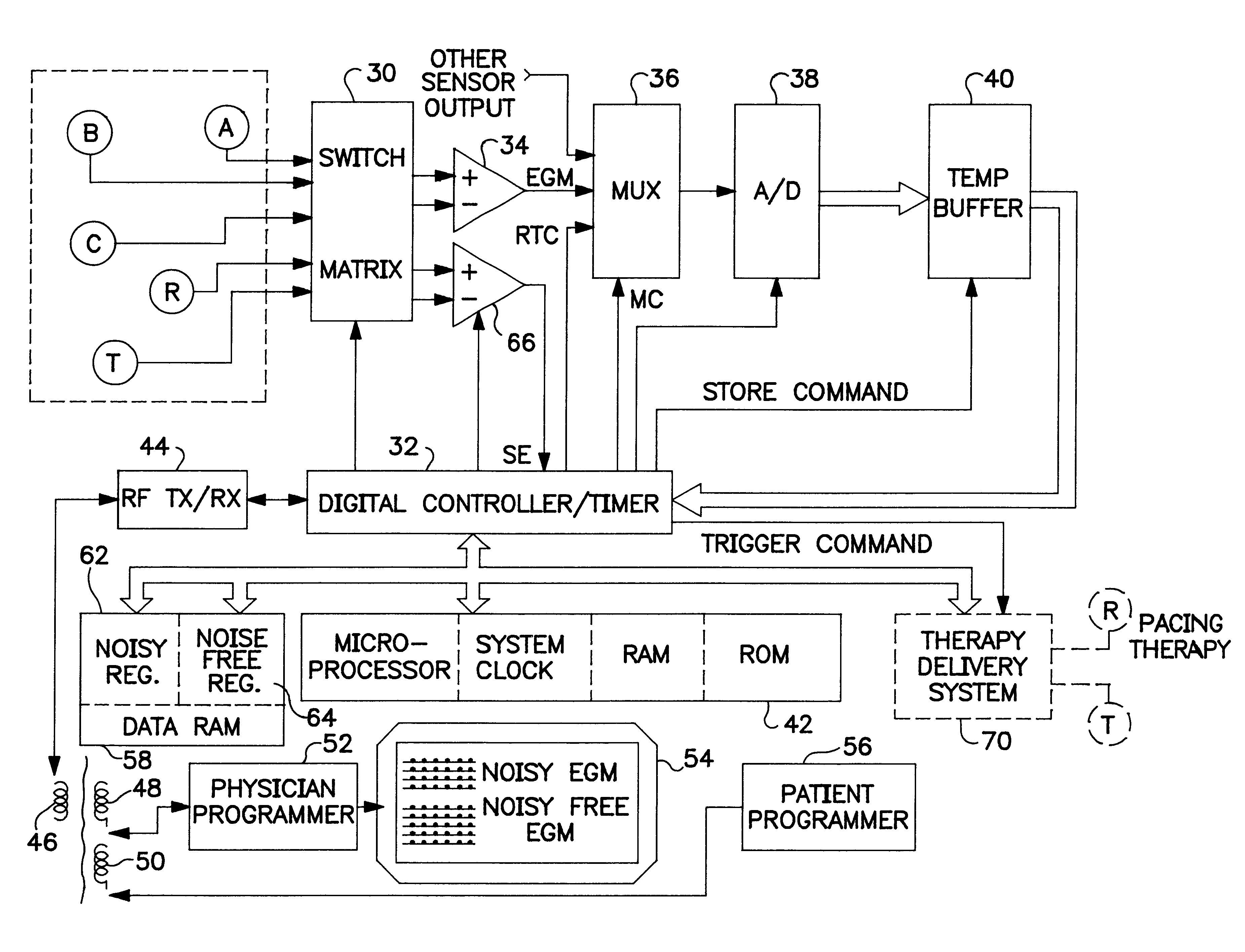
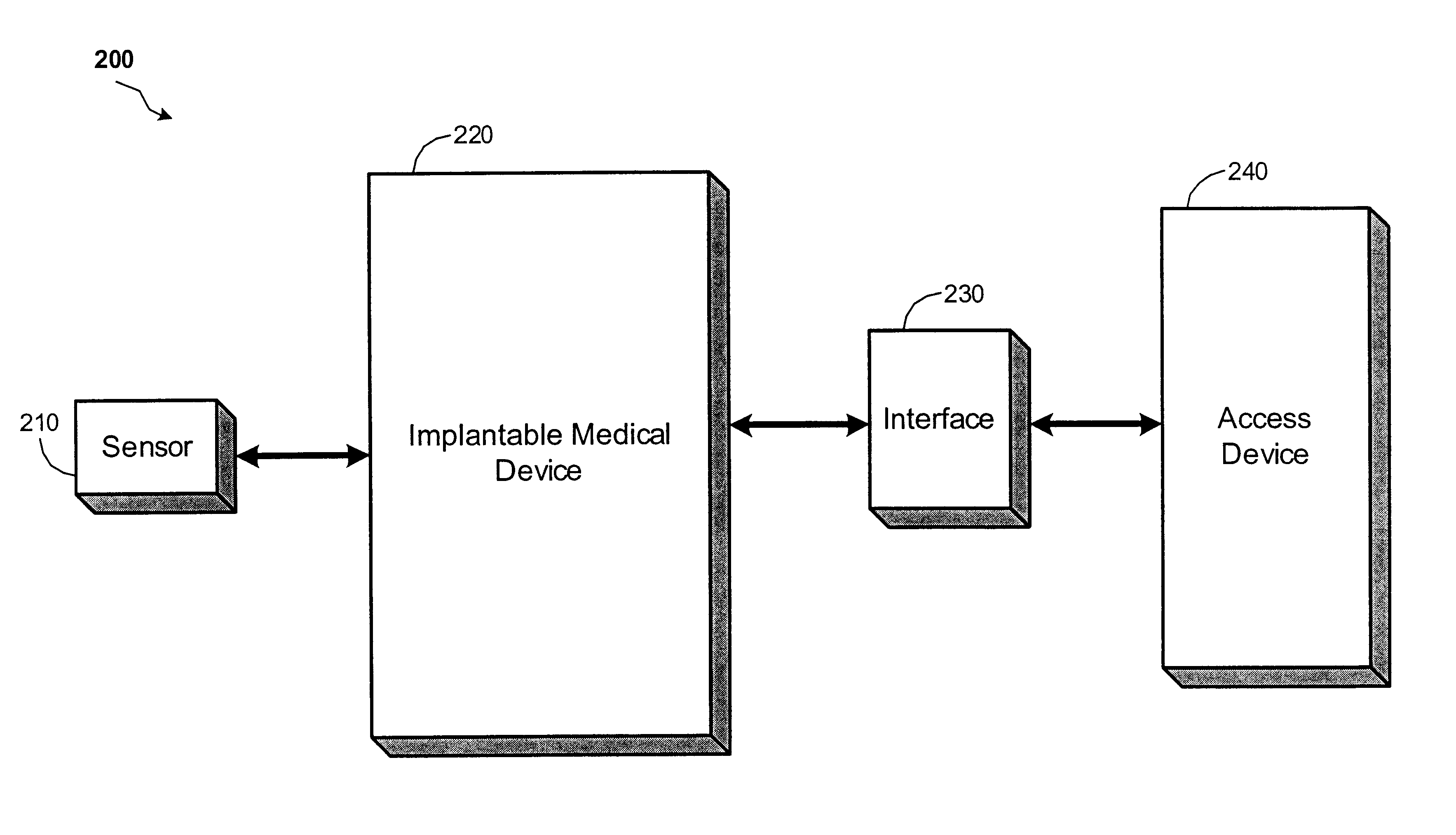
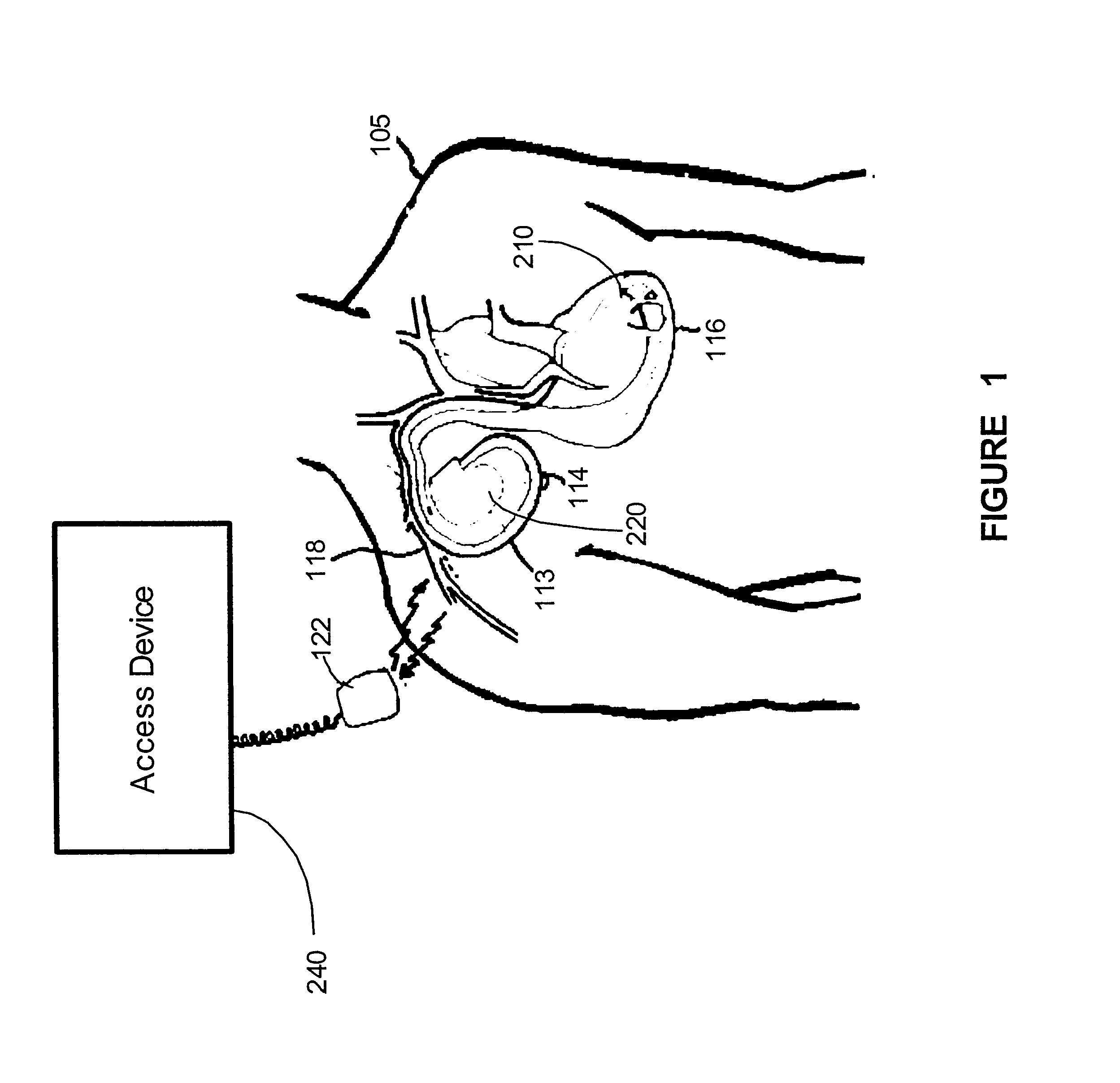
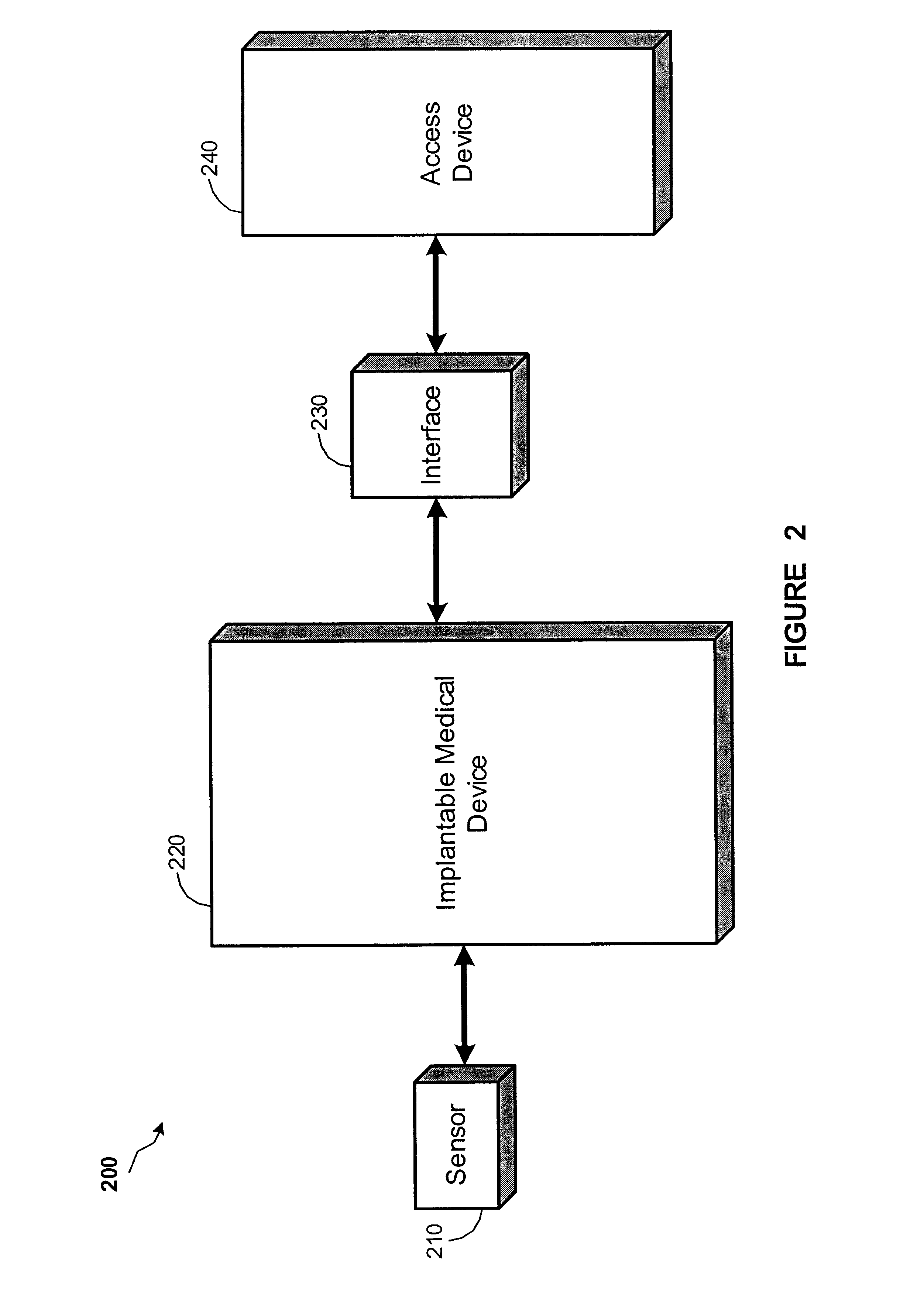
Implantable monitor
An implantable medical device (IMD) capable of monitoring physiologic data, distinguishing relatively noisy and noise free physiologic data, and recording noisy and relatively noise free segments of physiologic data in separate memory registers of a limited memory for retrieval and analysis at a later time. Preferably the physiologic data comprises the sampled EGM of the heart detected from sense electrode pairs that are implanted in the patient at sites where extraneous electrical noise, e.g., electromyographic signals, are also capable of being detected. The sense electrode pairs can constitute one or both sense electrodes located on or adjacent to the atrial and / or ventricular heart chambers and coupled to the IMD by a lead body or sense electrode pairs that are located remotely from the heart, e.g. at a subcutaneous implantation site of the IMD. A plurality of noisy EGM episode data registers store a corresponding plurality of noisy EGM episode data sets on a FIFO basis and another plurality of noise free EGM episode data registers to store a corresponding plurality of relatively noise free EGM episode data sets on a FIFO basis. Any form of discrimination of noisy data from relatively noise free data can be employed at the time of recording, but because the stored EGM episode data sets are subsequently viewed and analyzed by a physician, discrimination with absolute certainty is not required, and the physician can alter the detection criteria to fine tune it.
Owner:MEDTRONIC INC
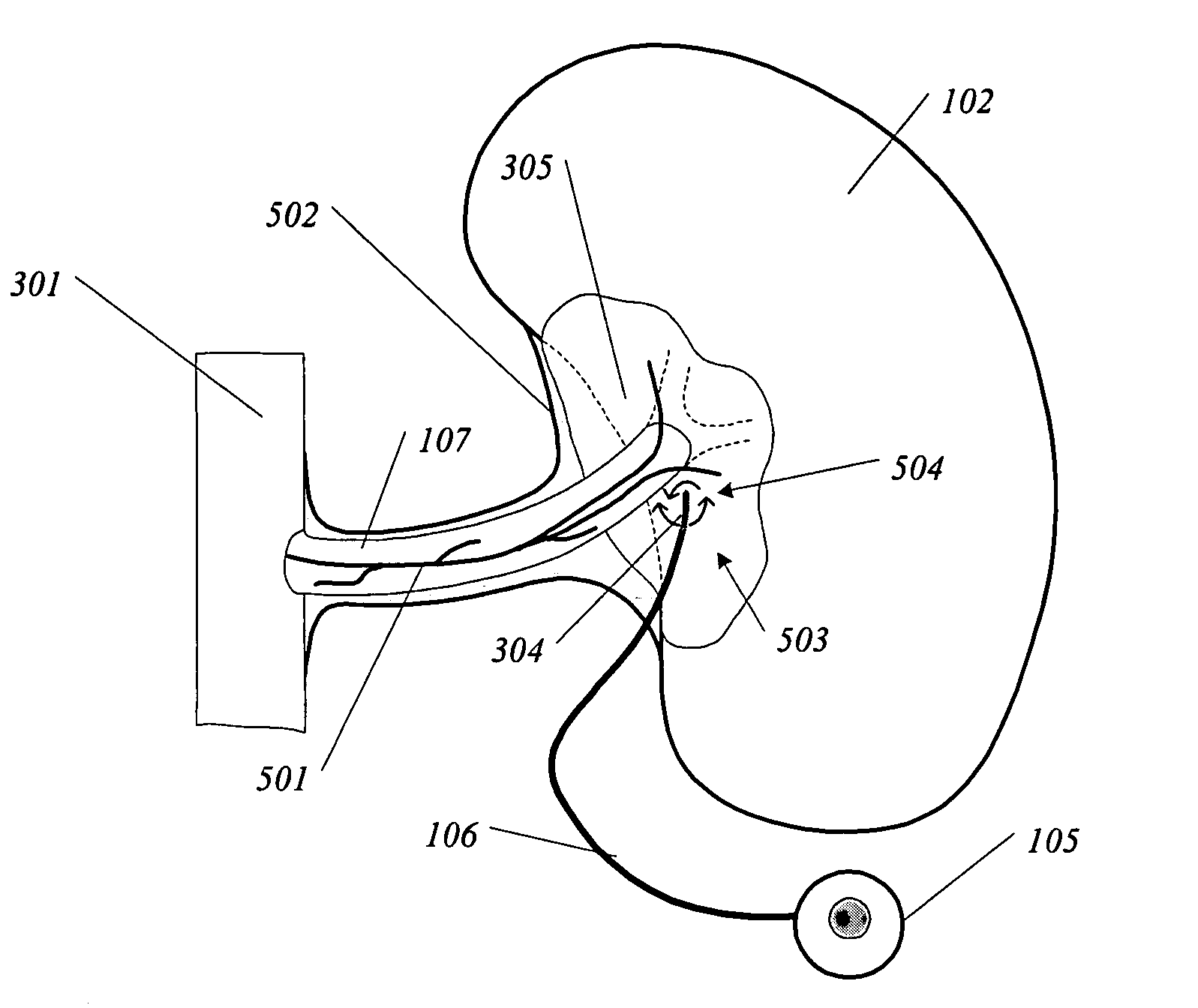
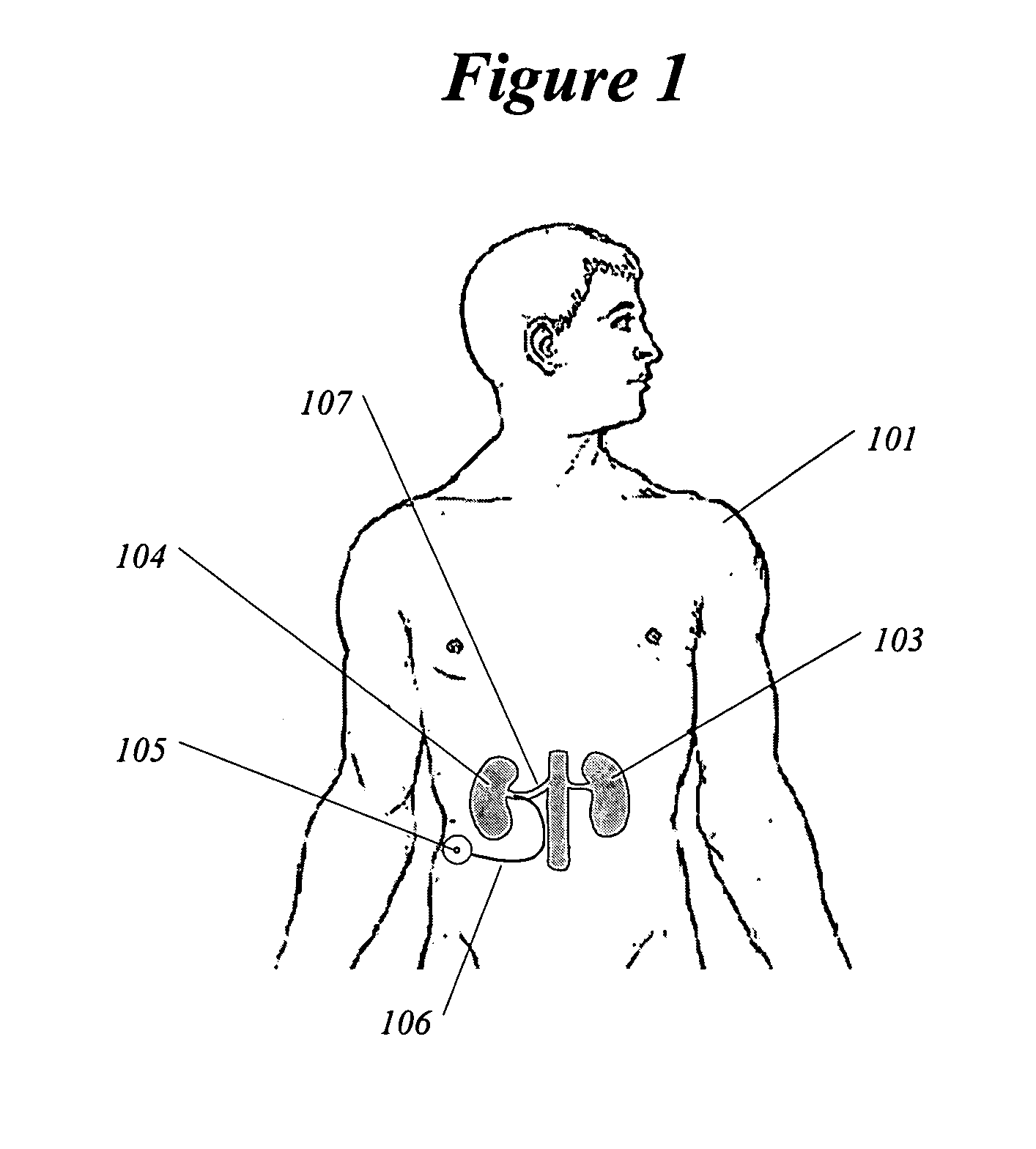
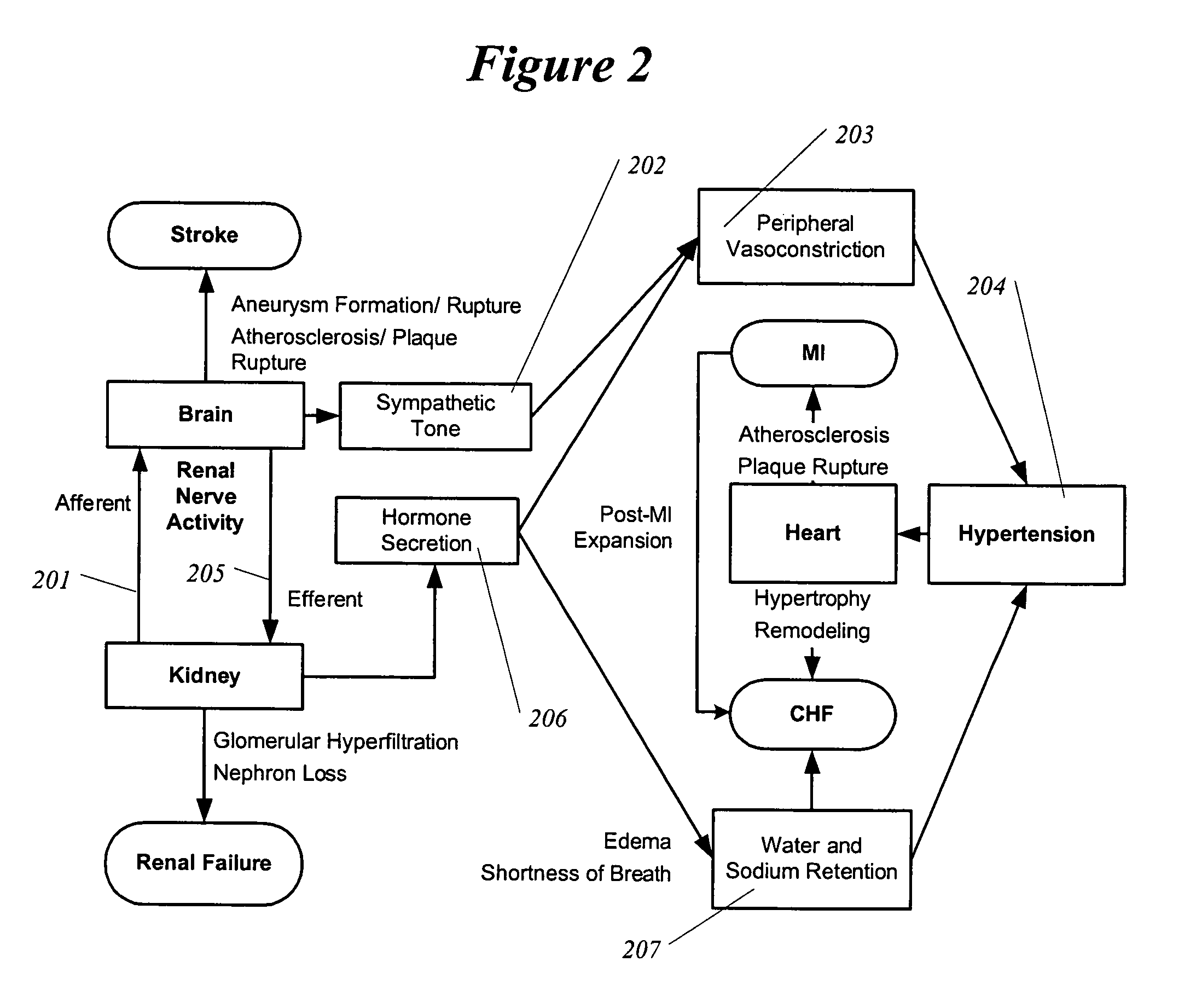
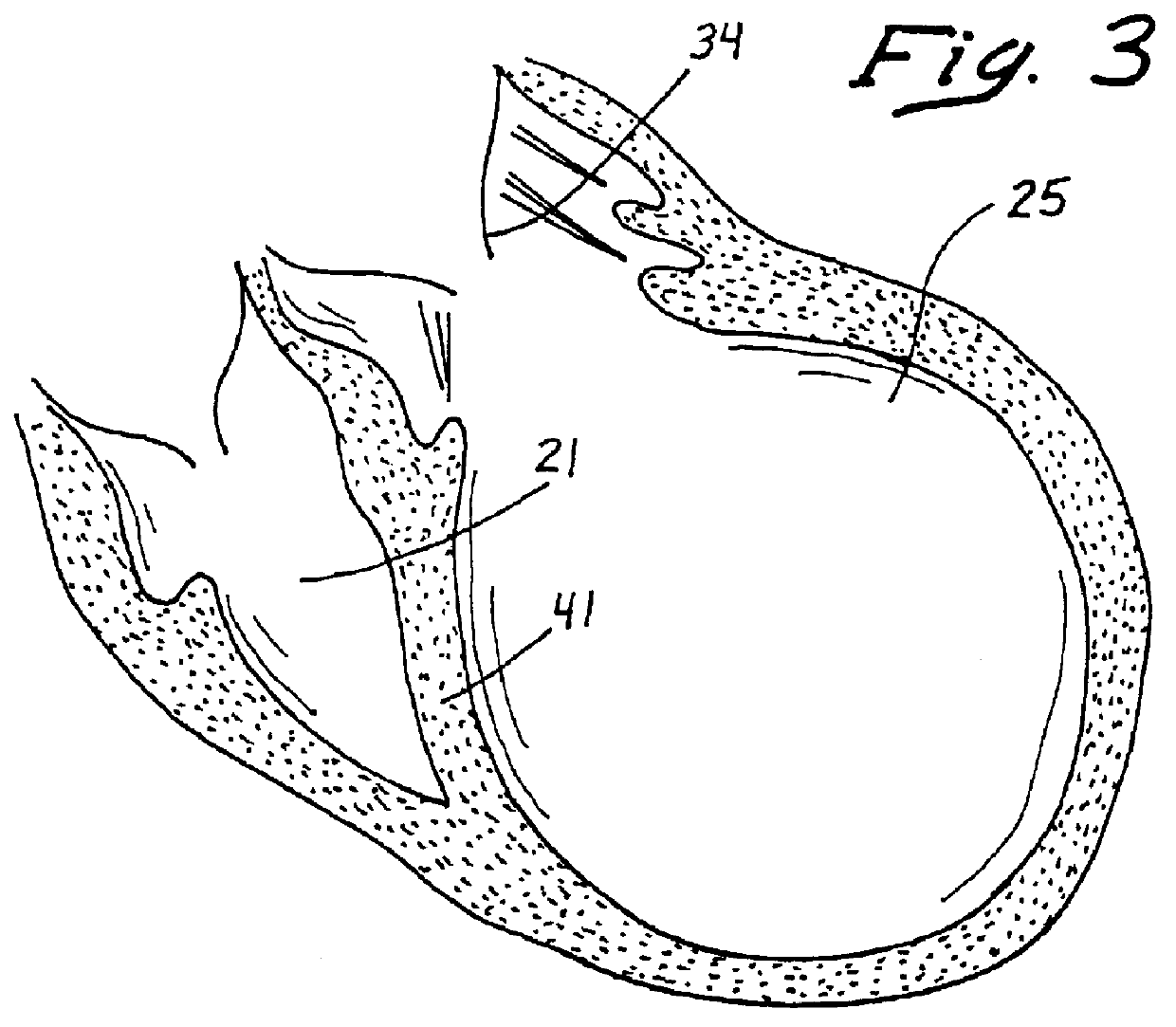
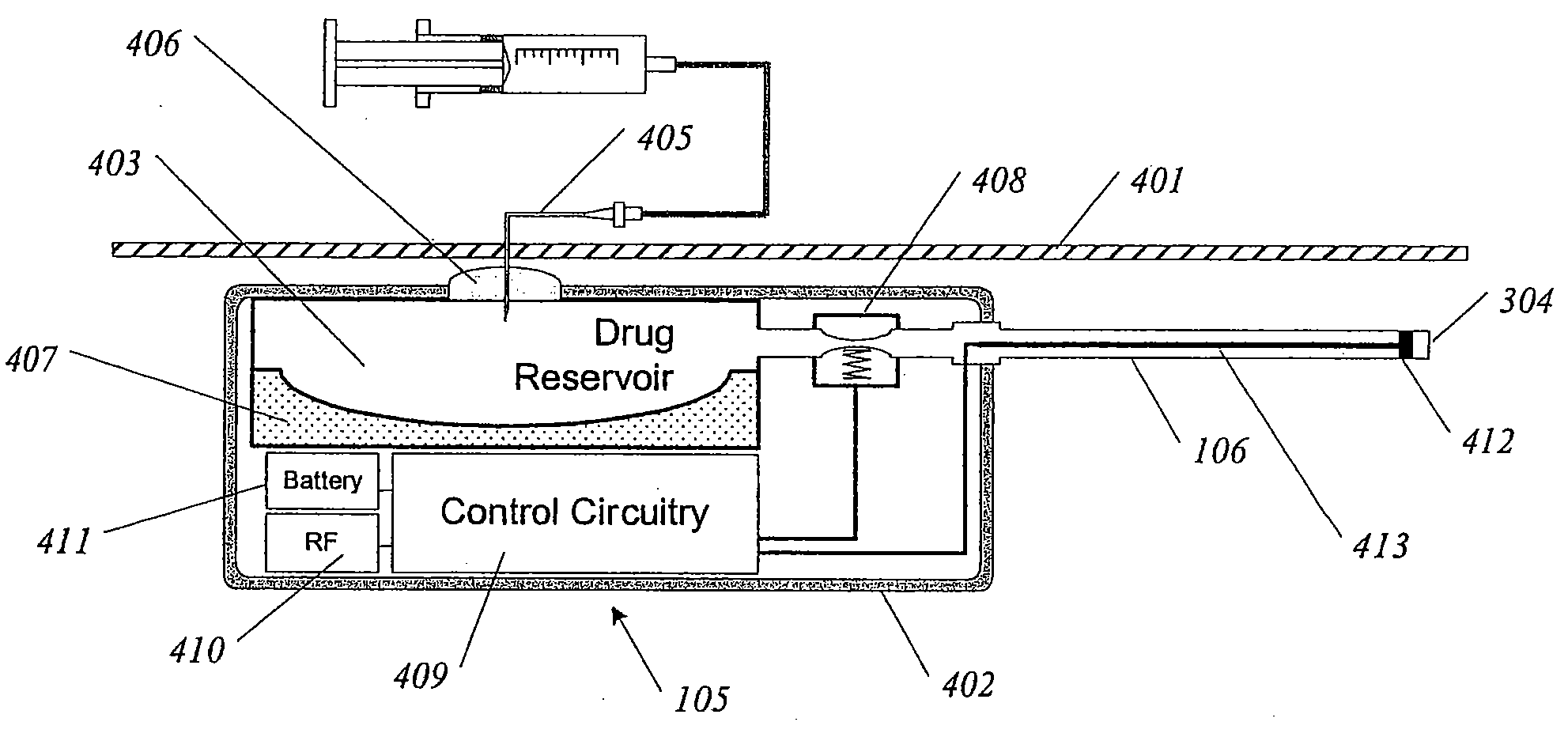
Methods and devices for renal nerve blocking
InactiveUS6978174B2Shorten the progressResolution of overloadPharmaceutical delivery mechanismImplantable neurostimulatorsDiseaseNephropathy
A method and apparatus for treatment of cardiac and renal diseases associated with the elevated sympathetic renal nerve activity by implanting a device to block the renal nerve signals to and from the kidney. The device can be a drug pump eluting implant for targeted delivery of a nerve-blocking agent to the periarterial space of the renal artery.
Owner:MEDTRONIC ARDIAN LUXEMBOURG SARL
Systems and methods used to reserve a constant battery capacity
ActiveUS7450991B2Constant amount of time to recharge the batteryBatteries circuit arrangementsHeart stimulatorsElectrical batteryControl system
Techniques are disclosed which provide a substantially constant battery reserve capacity with respect to therapeutic medical devices. Accordingly, a battery control system may be operable to maintain a substantially constant reserve capacity throughout the life of the battery. The battery reserve capacity activation threshold may be set and continuously or periodically updated so that a battery's remaining capacity equals the predetermined reserve capacity when the measured parameter reaches the activation threshold, thereby allowing a maximum amount of a battery's total capacity to be employed for therapeutic use and reserve only that portion of that capacity determined to provide for a desired level and / or period of device function after reaching the reserve threshold.
Owner:ADVANCED NEUROMODULATION SYST INC
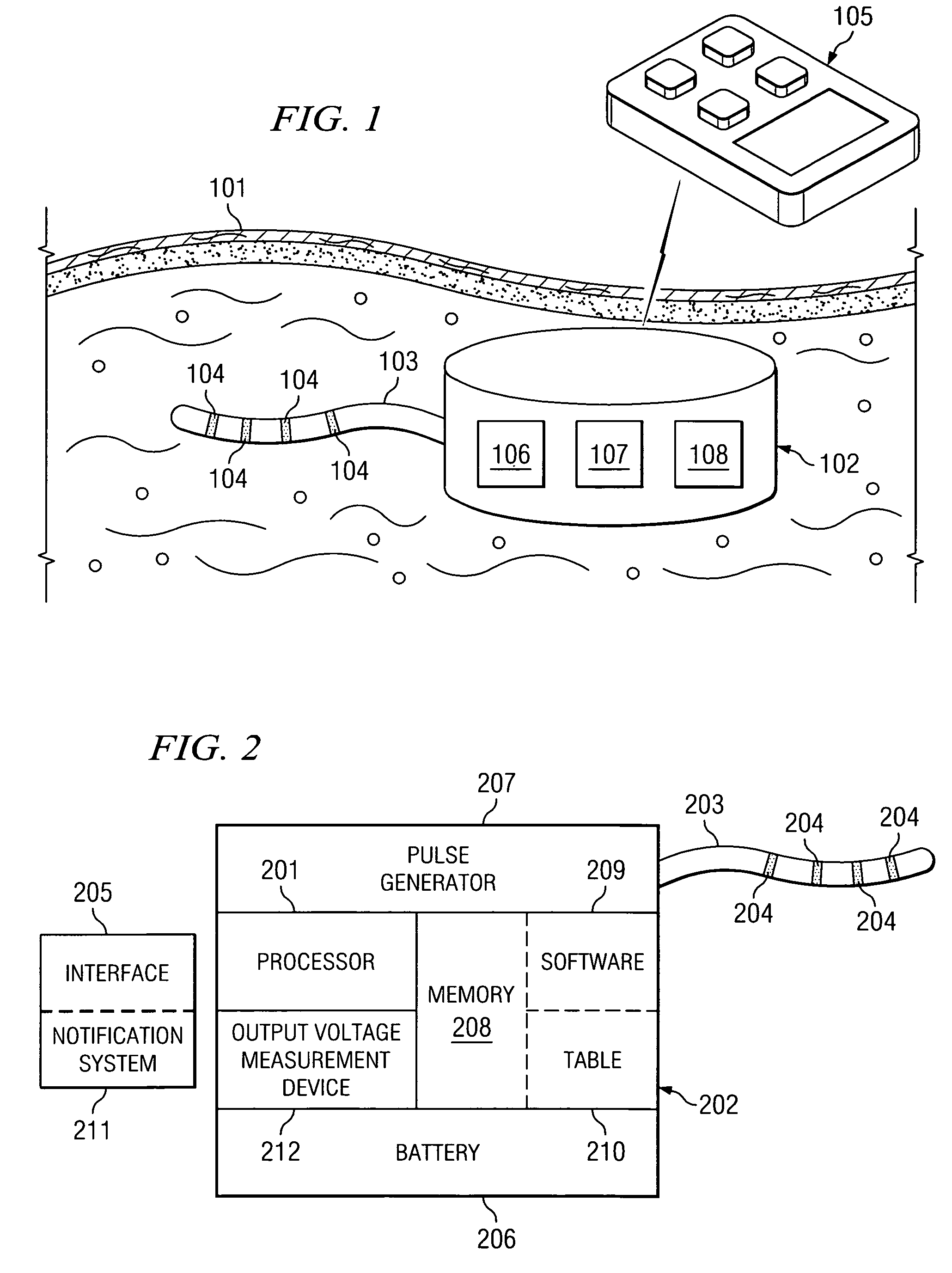
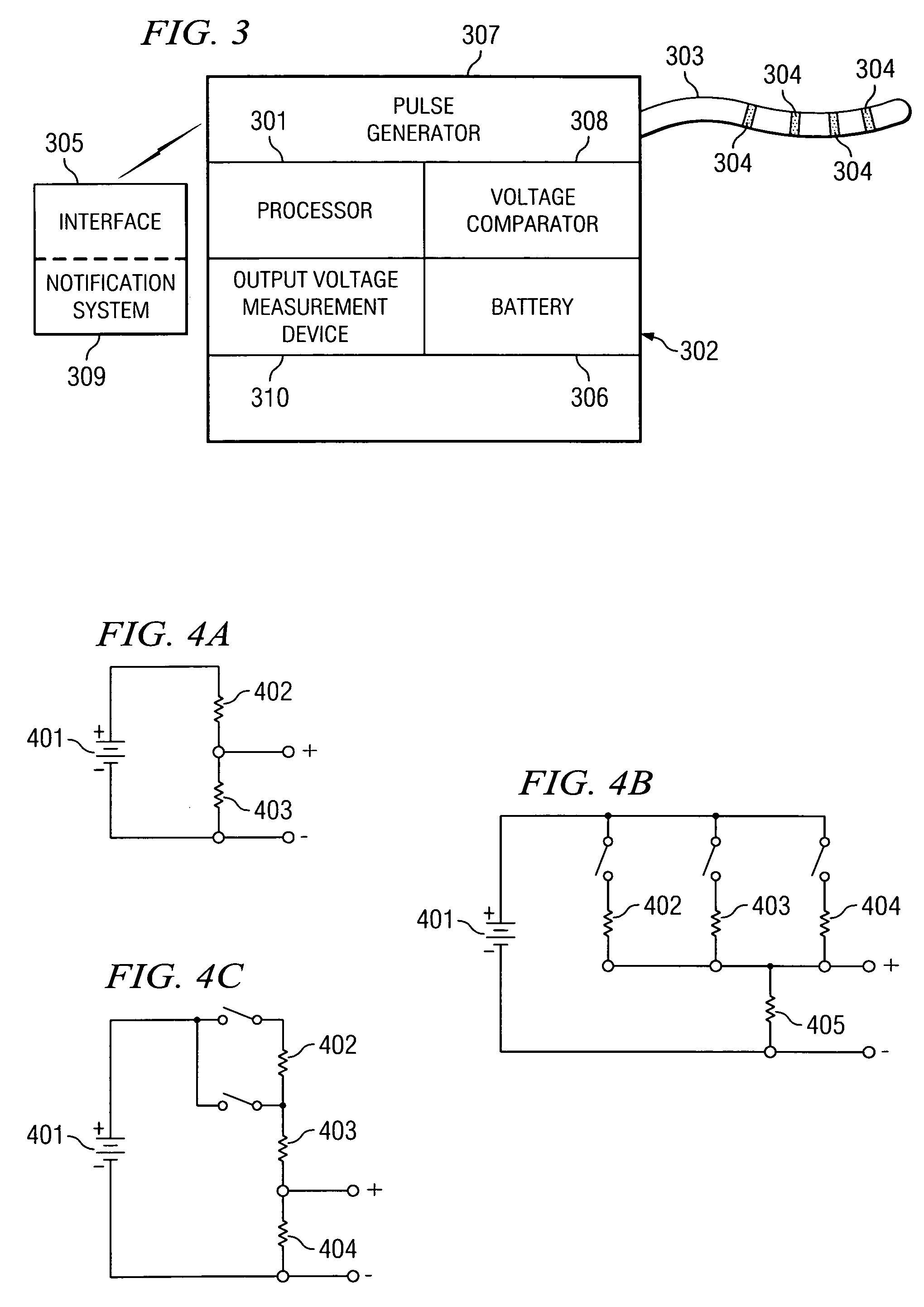
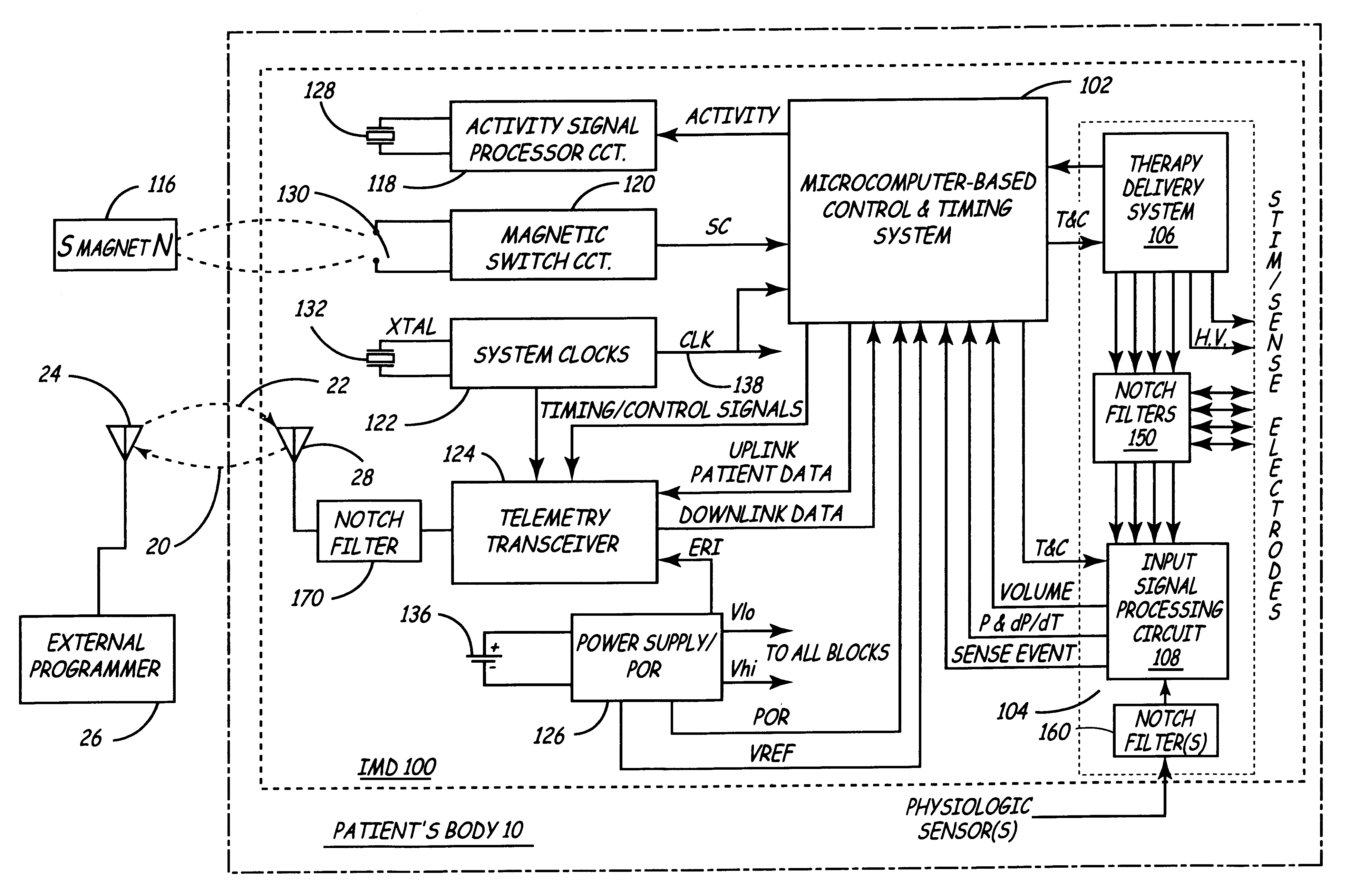
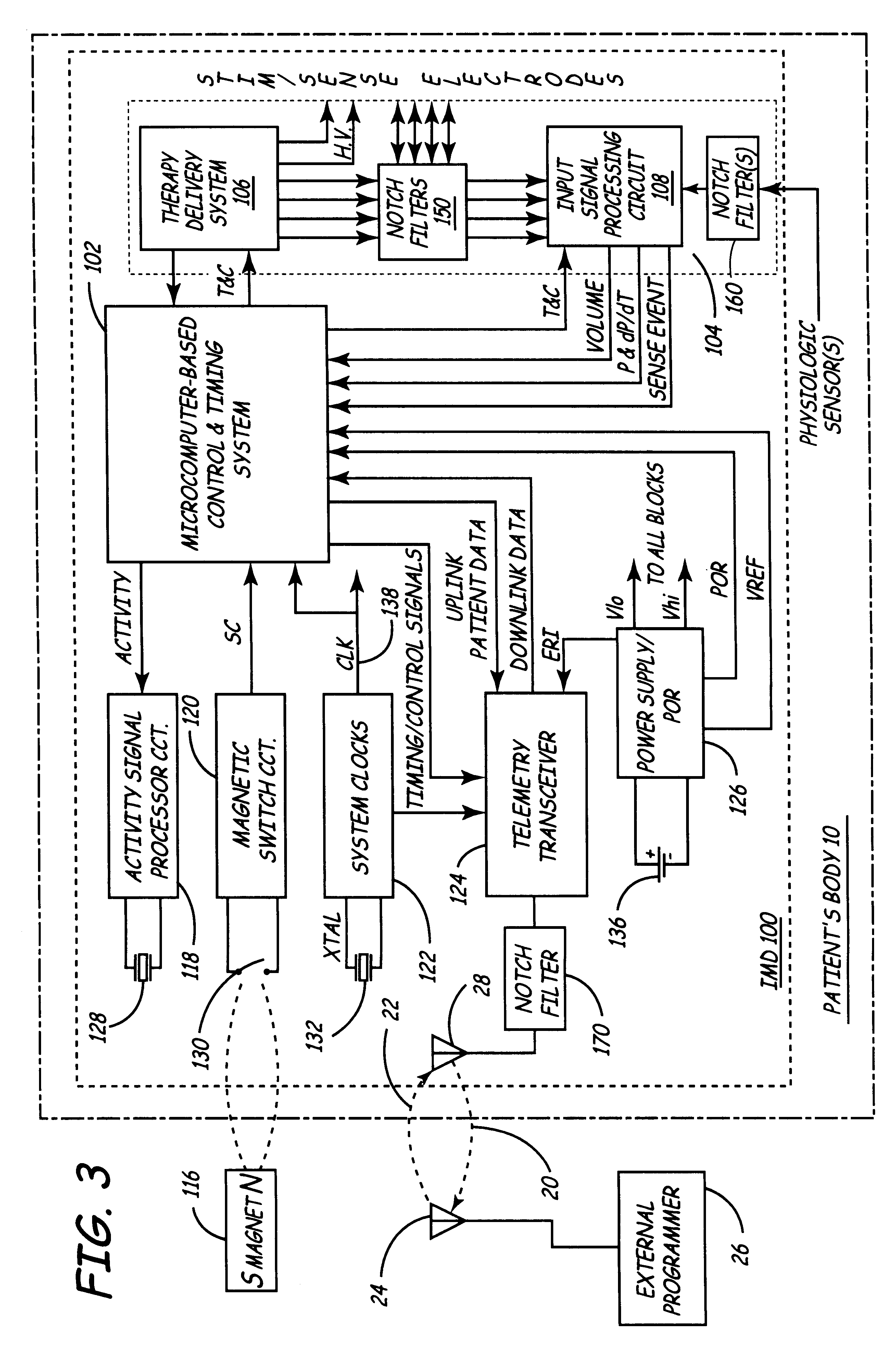
Implantable medical device incorporating integrated circuit notch filters
Implantable medical devices (IMDs) having sense amplifiers for sensing physiologic signals and parameters, RF telemetry capabilities for uplink transmitting patient data and downlink receiving programming and interrogation commands to and from an external programmer or other medical device are disclosed. At least one IC chip and discrete components have a volume and dimensions that are optimally minimized to reduce its volumetric form factor. Miniaturization techniques include forming notch filters of MEMS structures or forming discrete circuit notch filters by one or more of: (1) IC fabricating inductors into one or more IC chips mounted to the RF module substrate; (2) mounting each IC chip into a well of the RF module substrate and using short bonding wires to electrically connect bond pads of the RF module substrate and the IC chip; and (3) surface mounting discrete capacitors over IC chips to reduce space taken up on the RF module substrate. The IC fabricated inductors are preferably fabricated as planar spiral wound conductive traces formed of high conductive metals to reduce trace height and width while maintaining low resistance, thereby reducing parasitic capacitances between adjacent trace side walls and with a ground plane of the IC chip. The spiral winding preferably is square or rectangular, but having truncated turns to eliminate 90° angles that cause point-to-point parasitic capacitances. The planar spiral wound conductive traces are further preferably suspended over the ground plane of the IC chip substrate by micromachining underlying substrate material away to thereby reduce parasitic capacitances.
Owner:MEDTRONIC INC
Method, apparatus, and surgical technique for autonomic neuromodulation for the treatment of disease
InactiveUS20060167498A1Reduce or prevent conditionReducing and preventing symptomSpinal electrodesSurgical needlesSplanchnic nervesDisease
The present invention teaches a method and apparatus for physiological modulation, including neural and gastrointestinal modulation, for the purposes of treating several disorders, including obesity, depression, epilepsy, and diabetes. This includes chronically implanted neural and neuromuscular modulators, used to modulate the afferent neurons of the sympathetic nervous system to induce satiety. Furthermore, this includes neuromuscular stimulation of the stomach to effect baseline and intermittent smooth muscle contraction to increase gastric intraluminal pressure, which induces satiety, and stimulate sympathetic afferent fibers, including those in the sympathetic trunk, splanchnic nerves, and greater curvature of the stomach, to augment the perception of satiety.
Owner:DILORENZO BIOMEDICAL
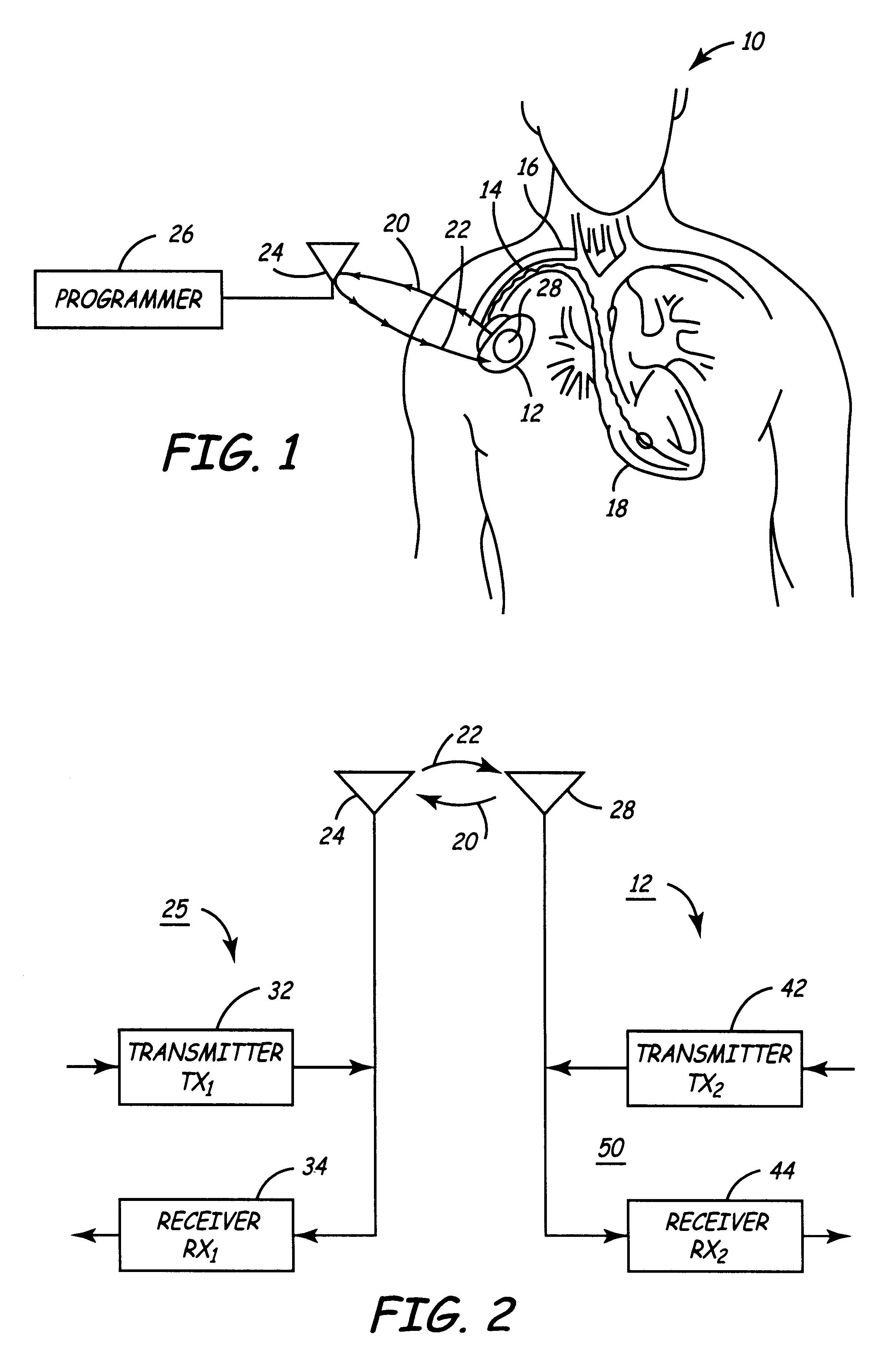
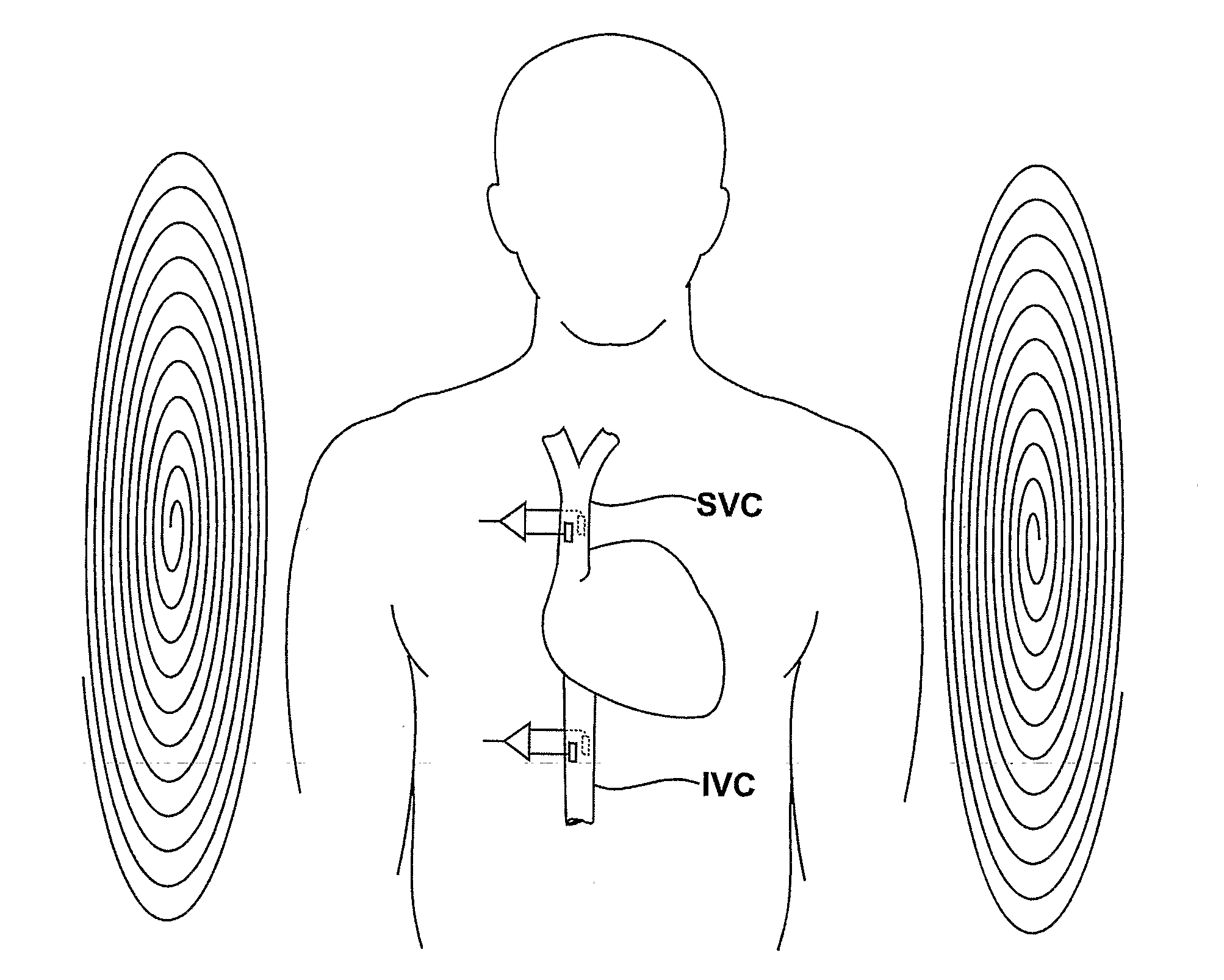
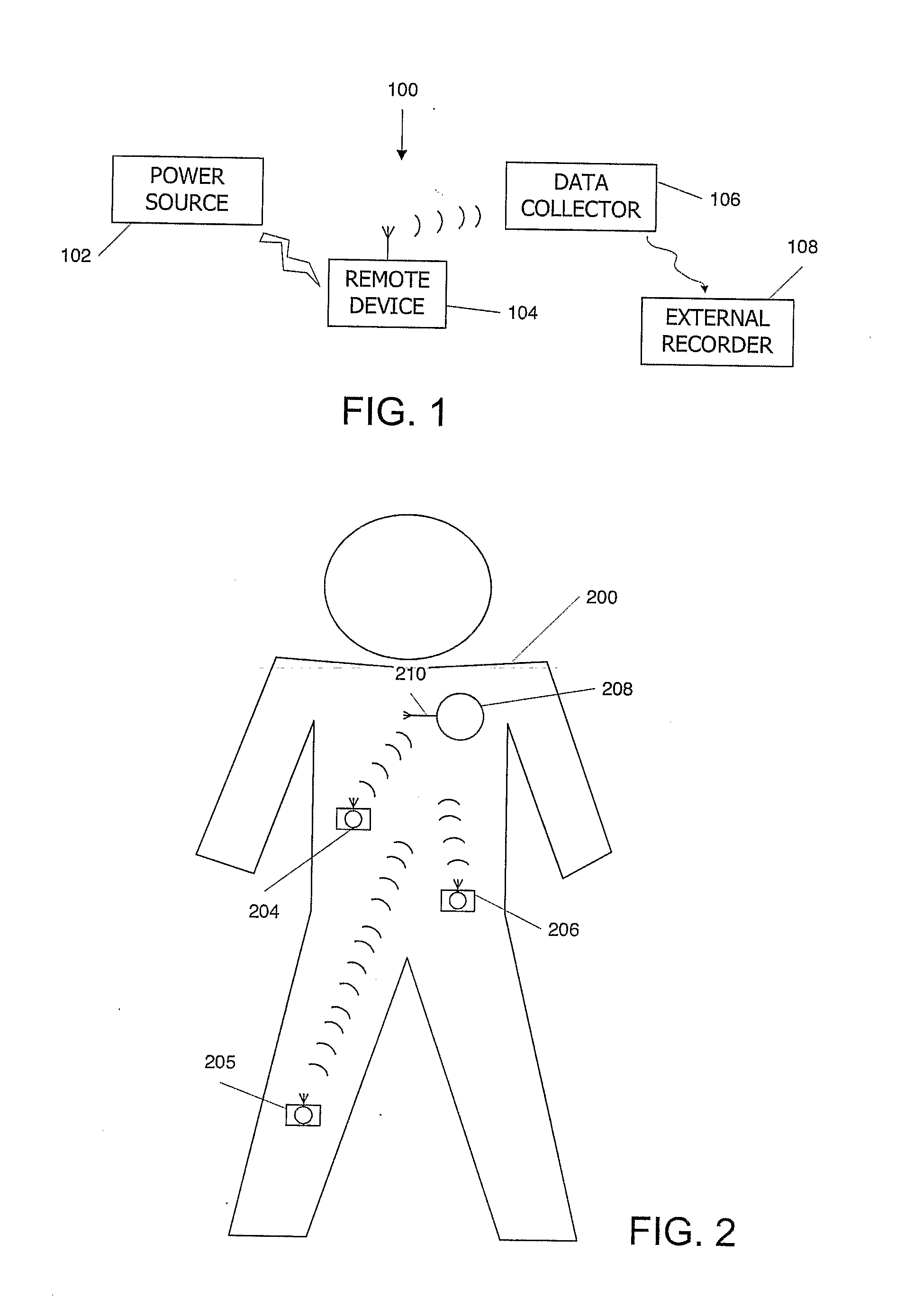
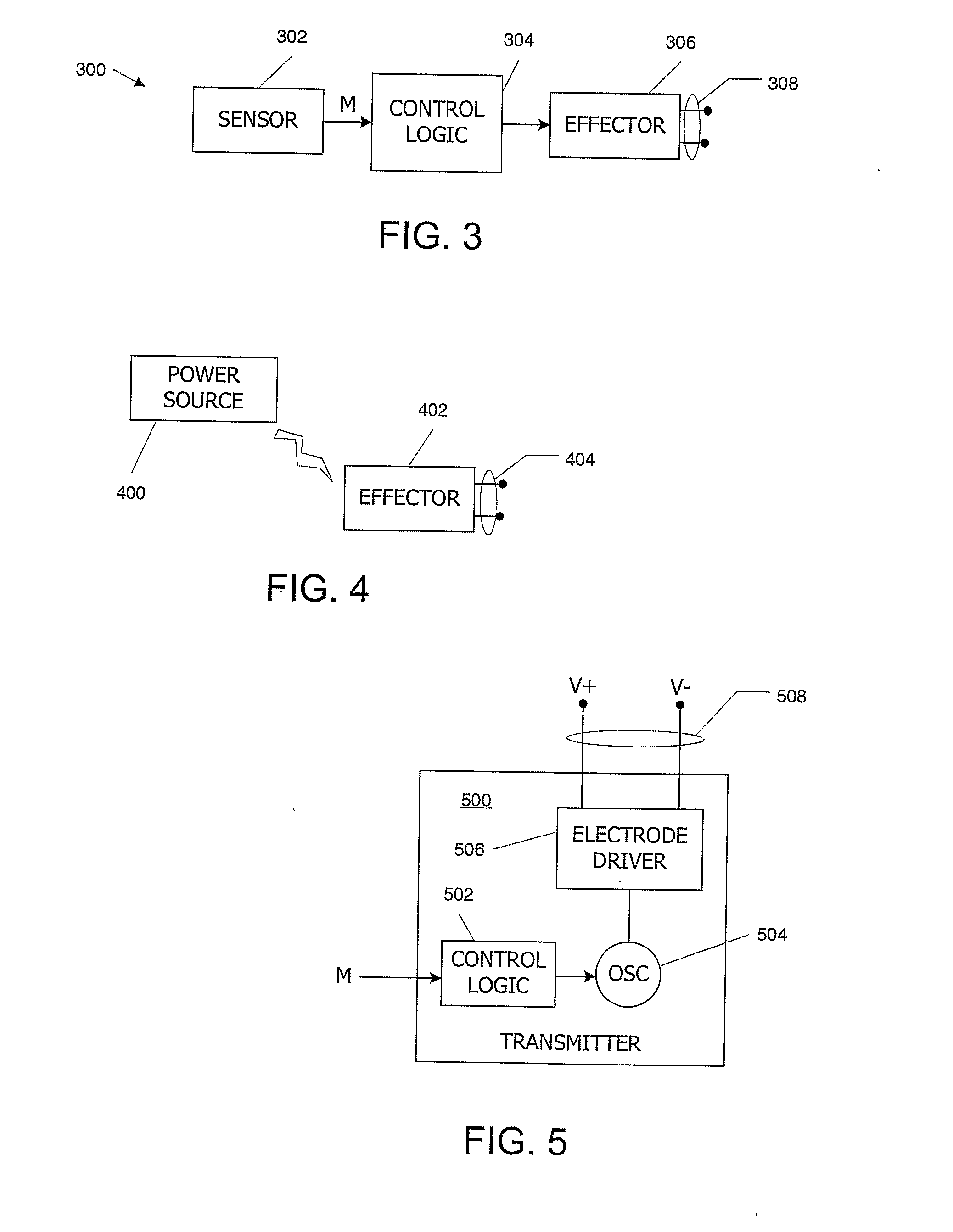
Medical Diagnostic and Treatment Platform Using Near-Field Wireless Communication of Information Within a Patient's Body
ActiveUS20080306359A1Efficient communicationSmall sizeElectric signal transmission systemsCircuit arrangementsMedical deviceBiomedical engineering
The present invention provides implantable systems that communicate wirelessly with each other using a unique format that enables devices configurations and applications heretofore not possible. Embodiments of the present invention provide communication apparatuses and methods for exchanging information with implantable medical devices. In some embodiments, two implantable devices communicate with each other using quasi-electrostatic signal transmission in a long wavelength / low frequency electromagnetic band, with the patient's body acting as a conductive medium.
Owner:OTSUKA PHARM CO LTD
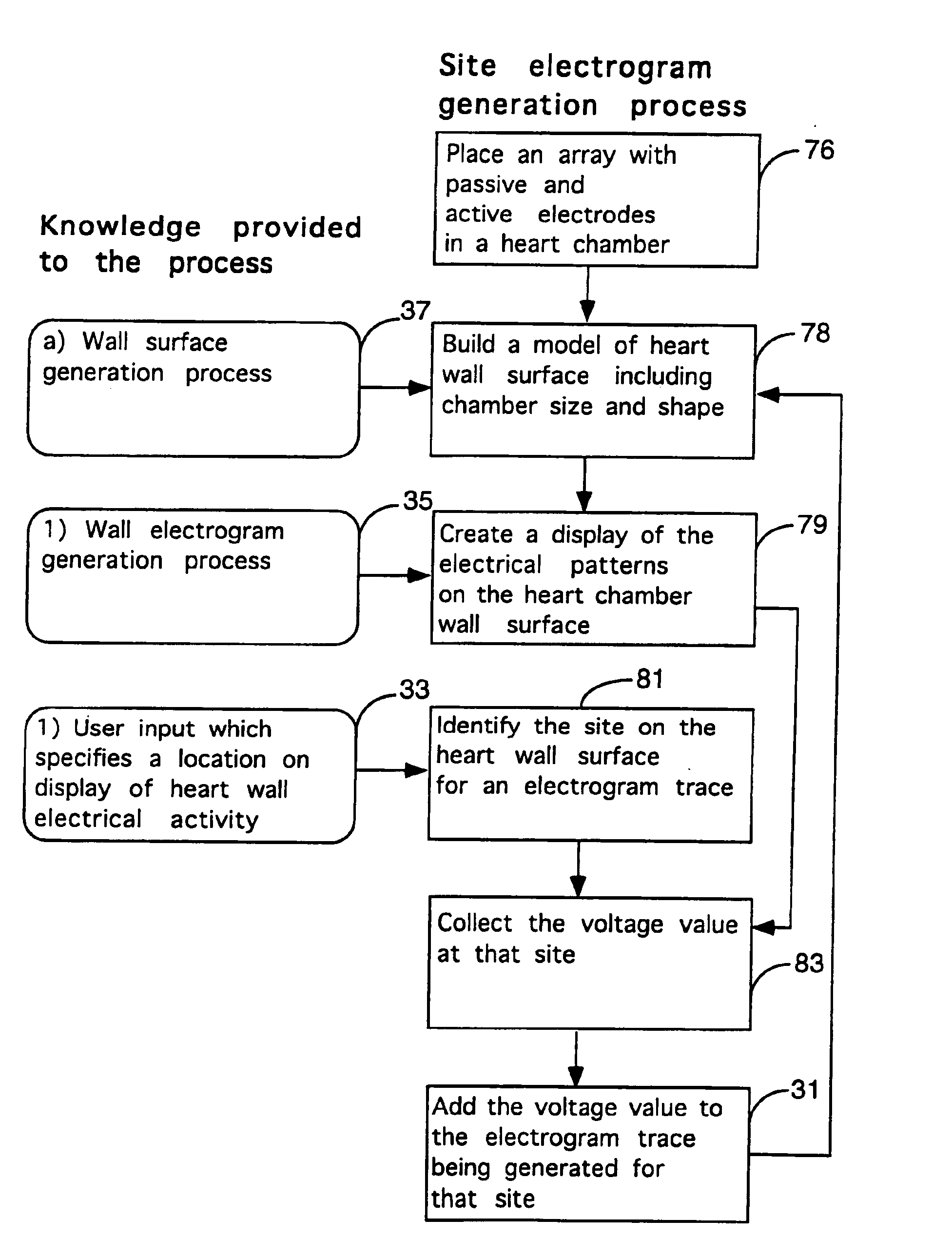
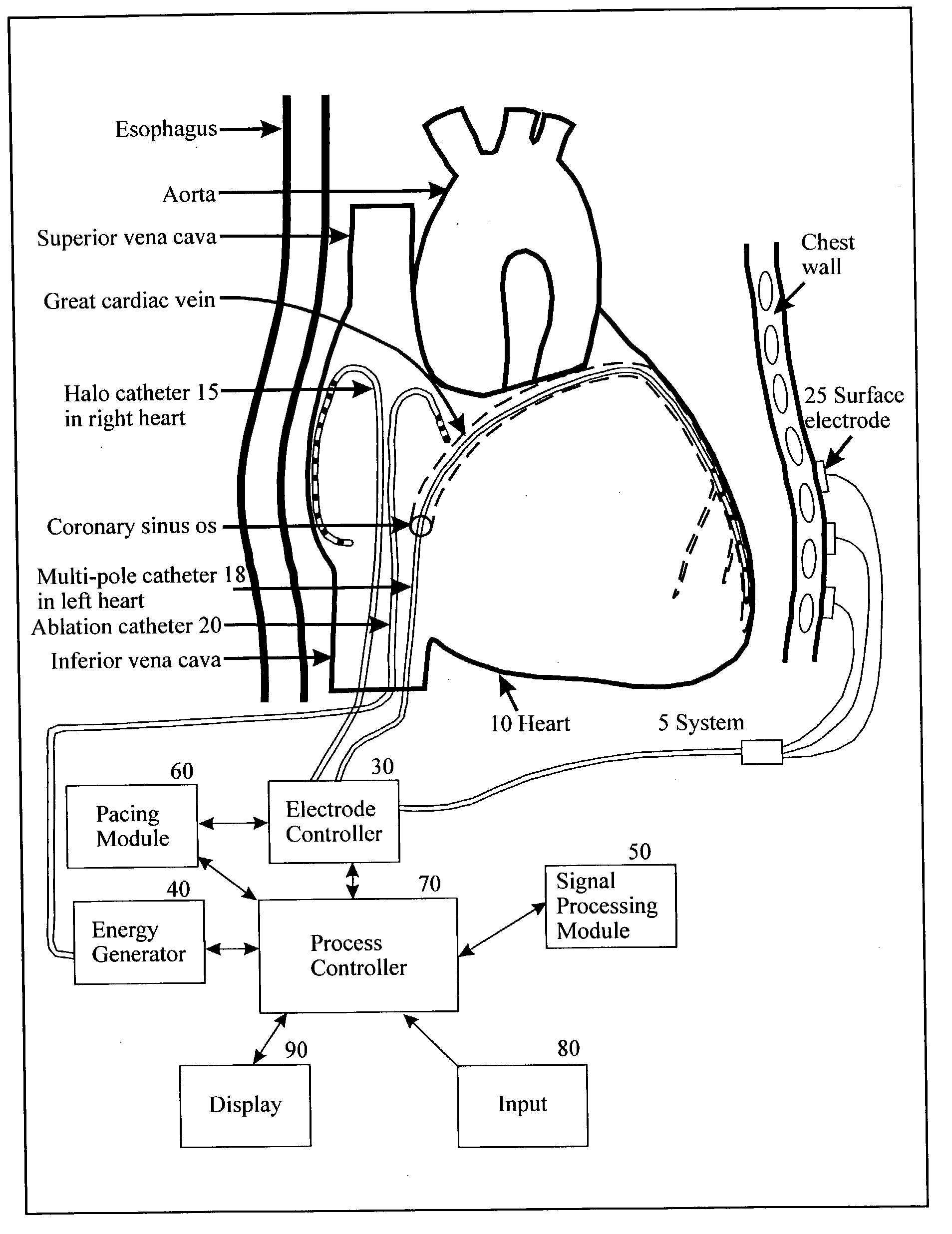
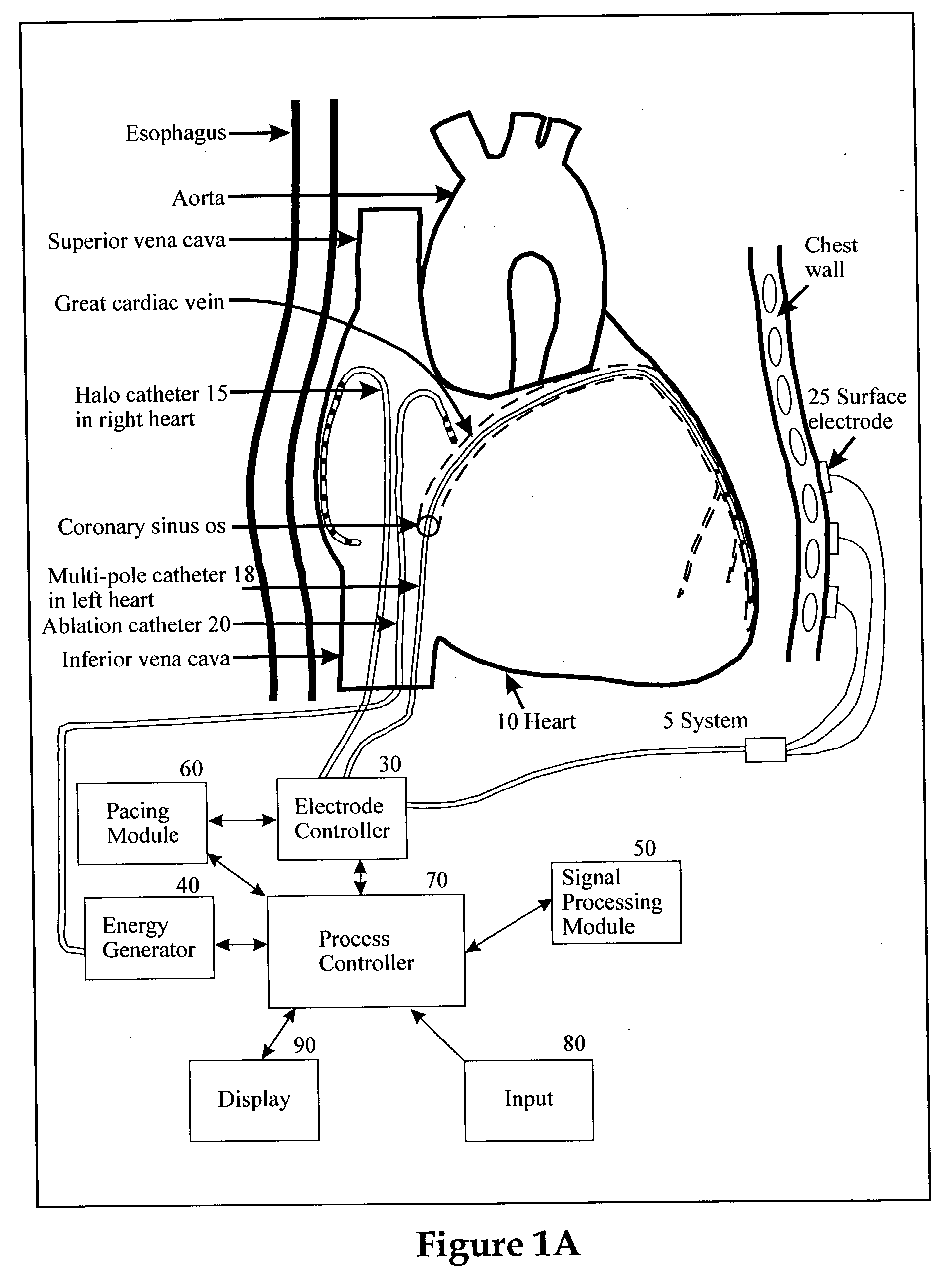
Method for mapping heart electrophysiology
A mapping catheter is positioned in a heart chamber, and active electrode sites are activated to impose an electric field within the chamber. The blood volume and wall motion modulates the electric field, which is detected by passive electrode sites on the preferred catheter. Electrophysiology measurements, as well as geometry measurements, are taken from the passive electrodes and used to display a map of intrinsic heart activity.
Owner:ST JUDE MEDICAL ATRIAL FIBRILLATION DIV
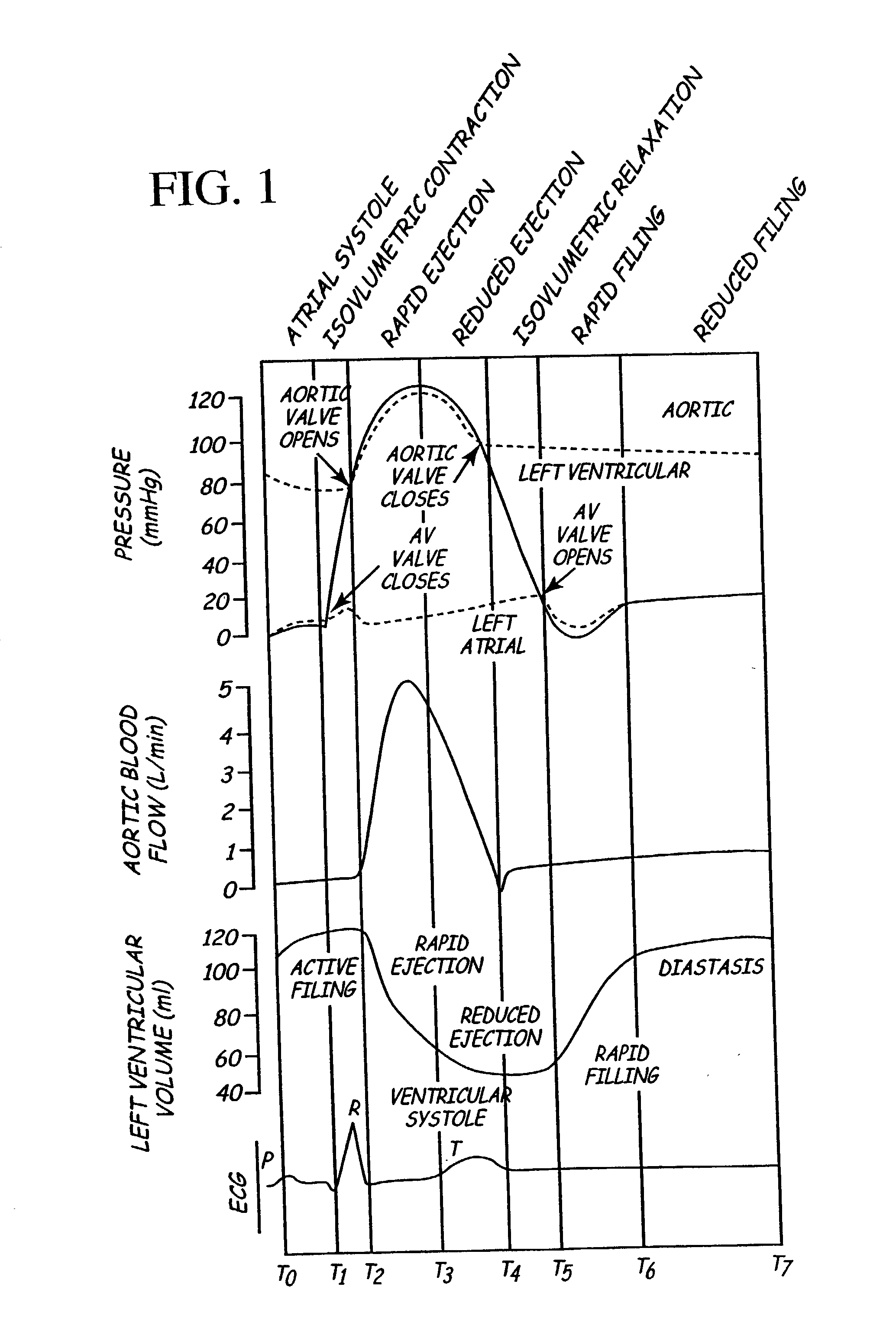
Method for detecting, diagnosing, and treating cardiovascular disease
InactiveUS6970742B2Therapy is simpleGood informationPhysical therapies and activitiesLocal control/monitoringVascular diseaseTherapeutic treatment
A method of treating cardiovascular disease in a medical patient is provided. The method includes the steps of generating a sensor signal indicative of a fluid pressure within the left atrium of the patient's heart, and delivering an electrical stimulus to a location in the heart. The electrical stimulus is delivered based at least in part on the sensor signal. The method also includes the steps of generating a proccessor output indicative of a treatment to a signaling device. The processor output is based at least in part on the sensor signal. At least two treatment signals are provided to the medical patient. The treatment signals are distinguishable from one another by the patient, and are indicative of a therapeutic treatment. The treatment signals are based at least in part on the processor output.
Owner:CEDARS SINAI MEDICAL CENT
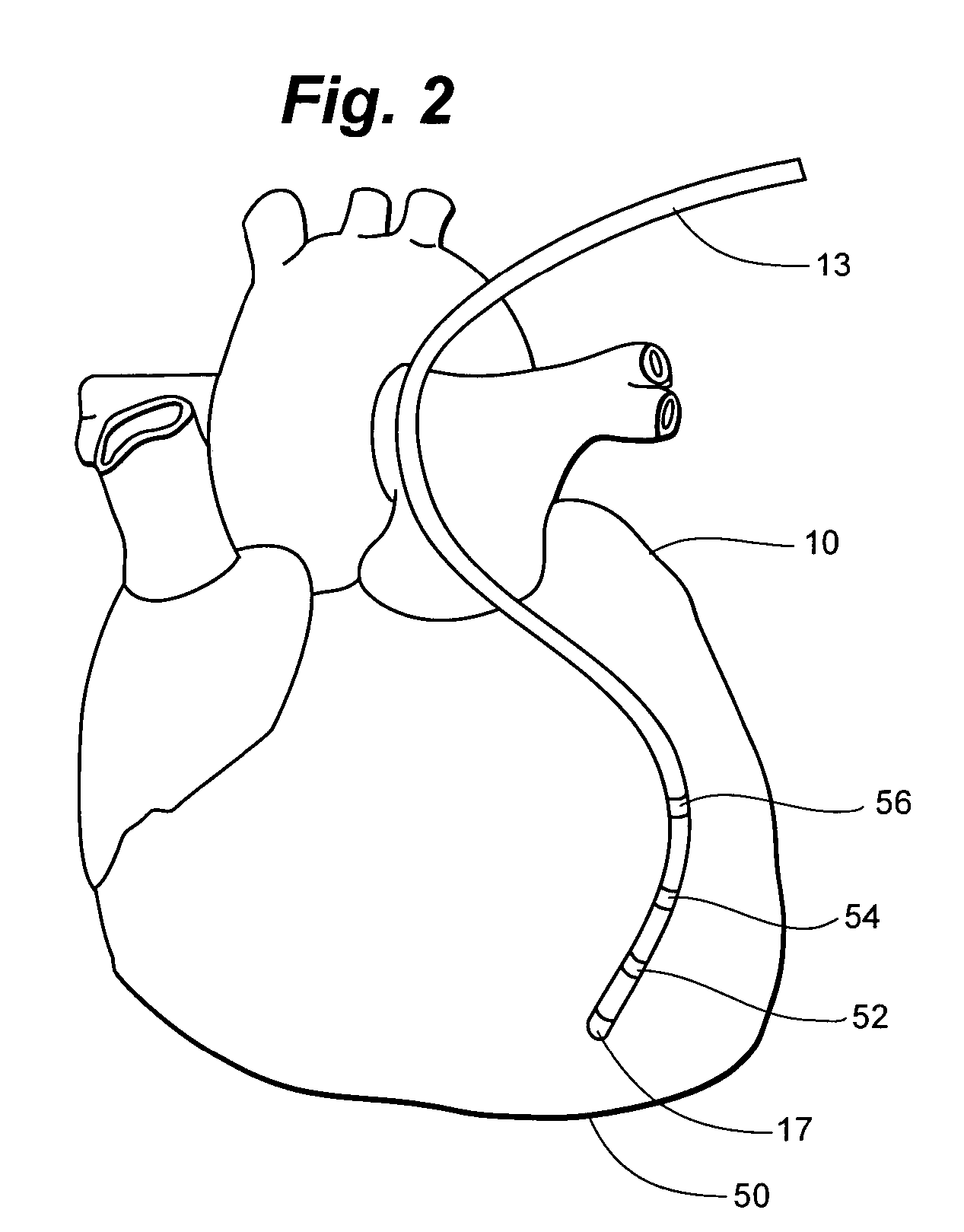
Devices and methods for transluminal or transthoracic interstitial electrode placement
ActiveUS7191015B2Epicardial electrodesTransvascular endocardial electrodesElectrode placementDevice implant
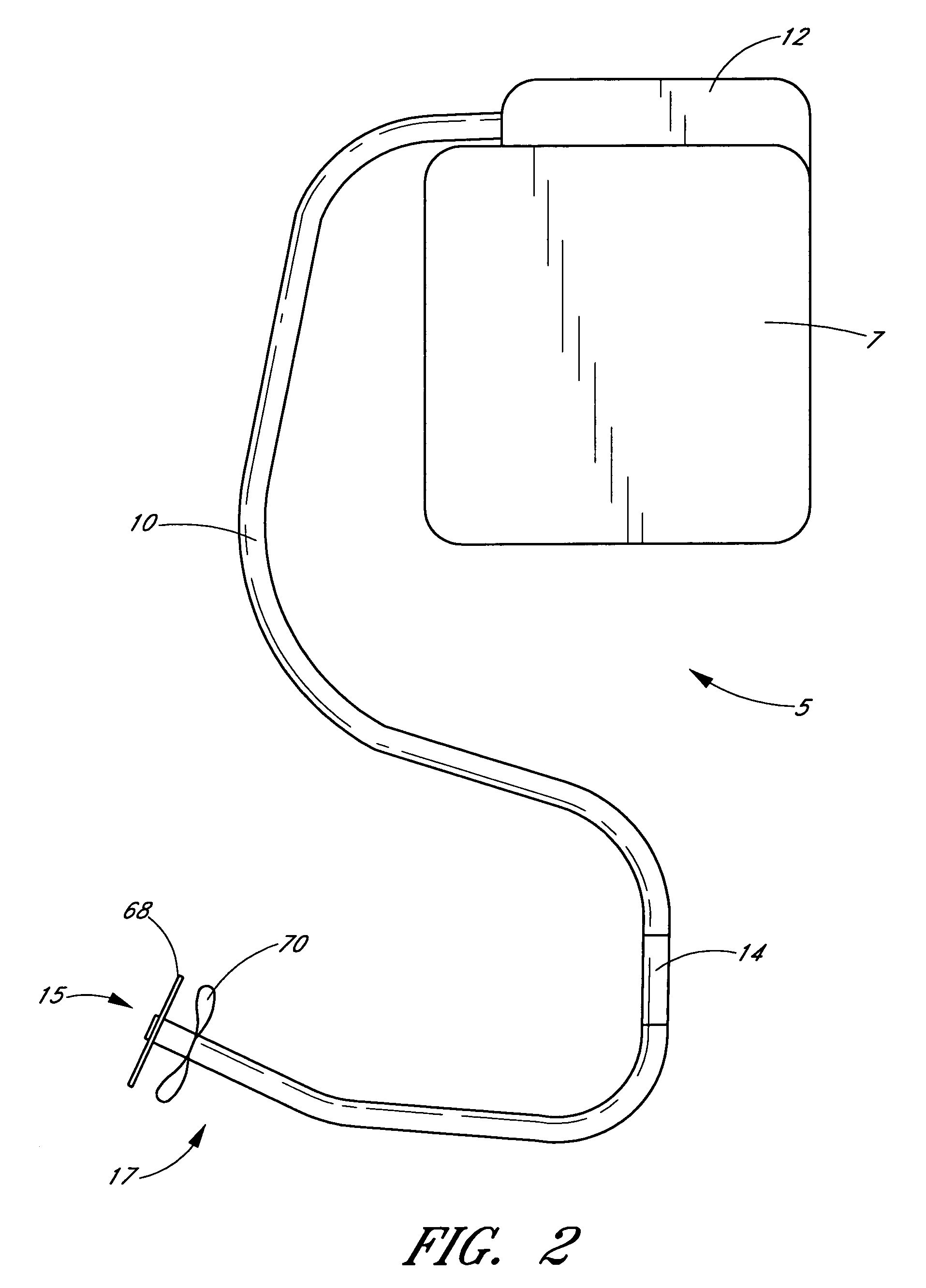
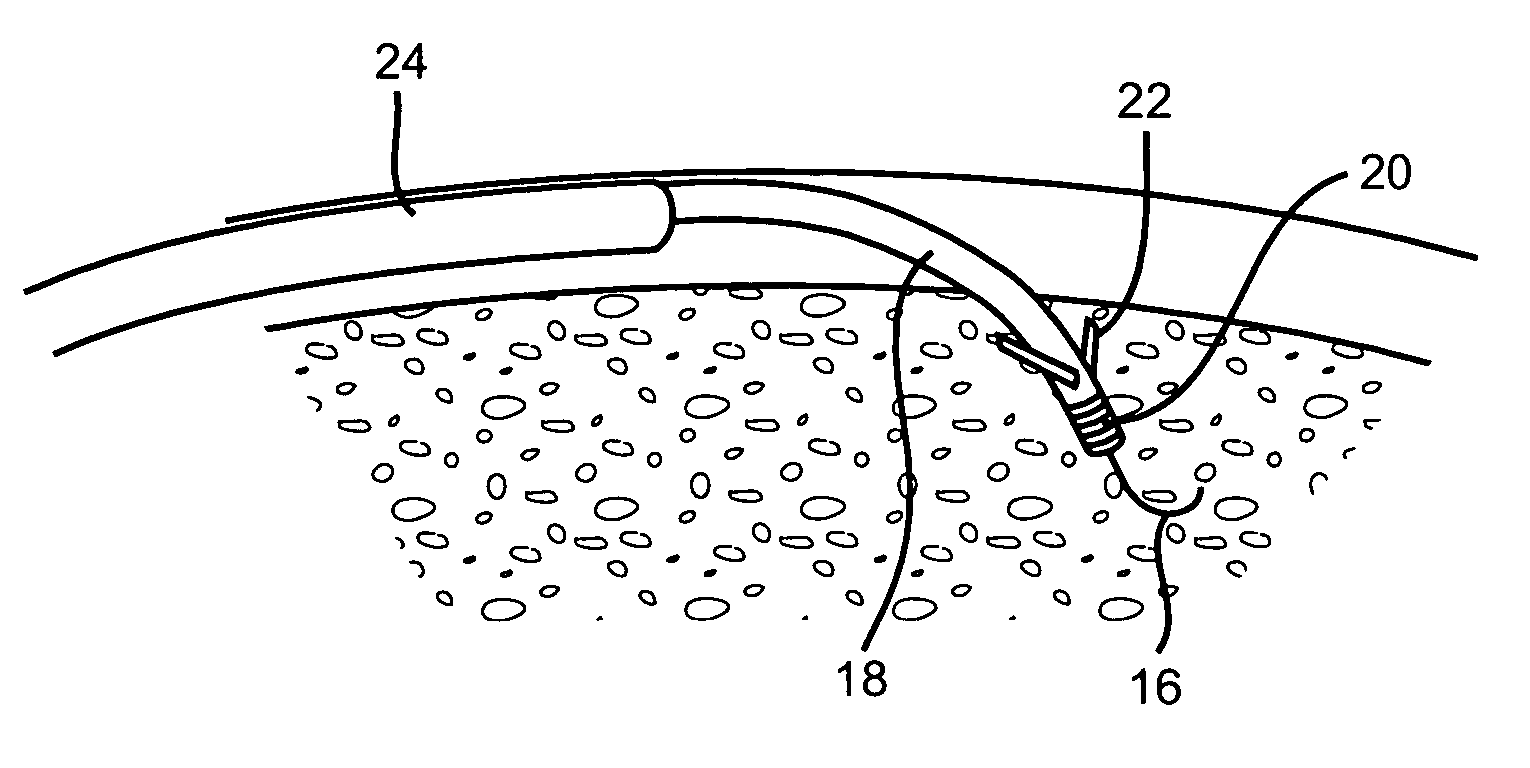
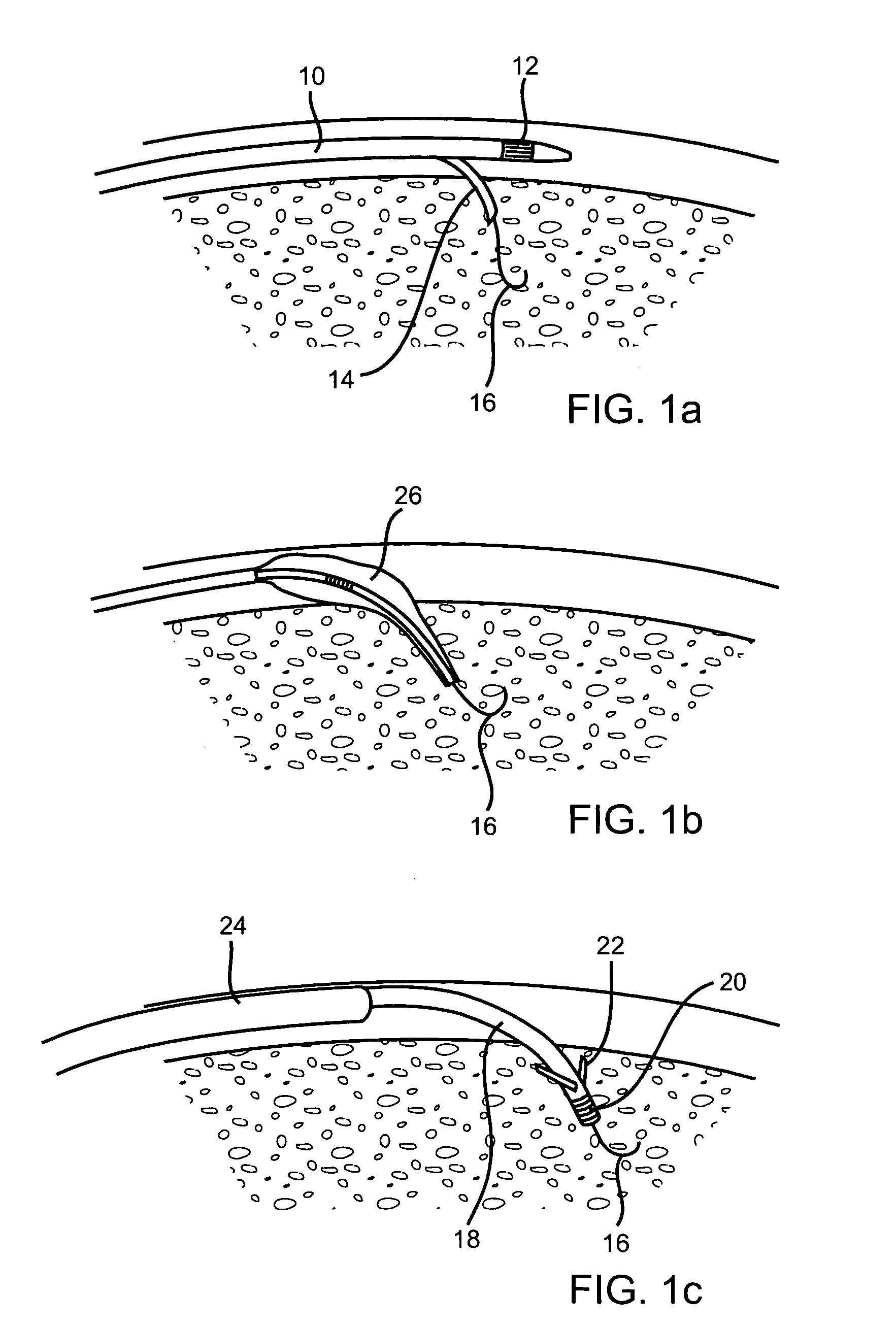
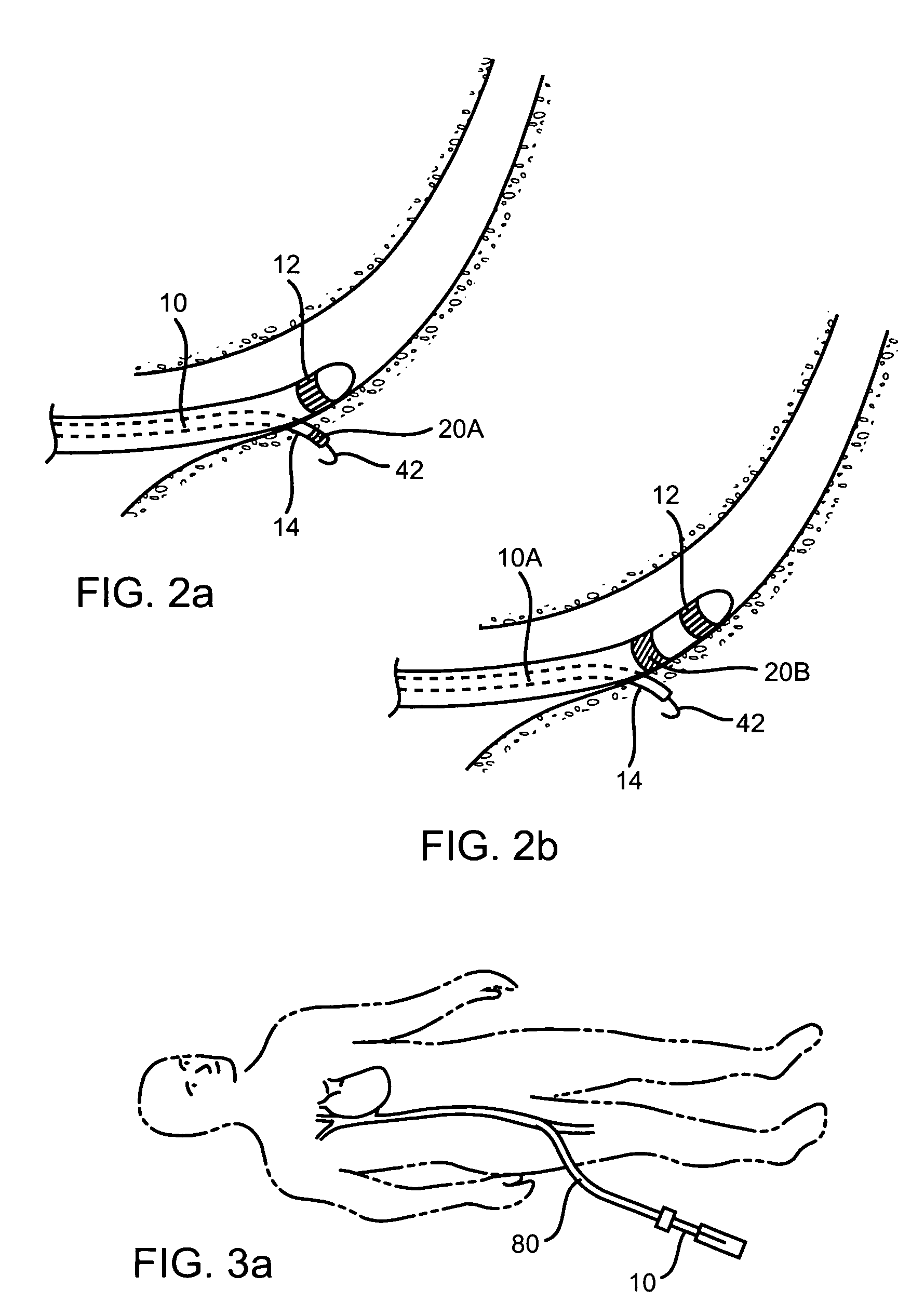
Methods and devices for implanting pacing electrodes or other apparatus, or for delivering substances, to the heart of other tissues within the body. A guided tissue penetrating catheter is inserted into a body lumen (e.g., blood vessel) or into a body cavity or space (e.g., the pericardial space) and a penetrator is advanced from the catheter to a target location. In some embodiments, a substance or an apparatus (such as an electrode) may be delivered through a lumen in the penetrator. In other embodiments, a guidewire may be advanced through the penetrator, the penetrating catheter may then be removed and an apparatus (e.g., electrode) may then be advanced over that guidewire. Also disclosed are various implantable electrodes and electrode anchoring apparatus.
Owner:MEDTRONIC VASCULAR INC
Closed-loop neuromodulation for prevention and treatment of cardiac conditions
A method and apparatus to provide therapy to a patient for protecting cardiac tissue from insult is disclosed. The method comprises delivering electrical stimulation to one or more predetermined portions of the nervous system in a patient's body; and monitoring one or more physiologic indices of the body to determine whether the delivered therapy is effective. That is, a closed-loop feedback controller is used to apply electrical stimulation to preselected regions of the patient's body, and then the physiologic response of the patient is monitored to determine the efficacy of the stimulation.
Owner:MEDTRONIC INC
Electrode assembly for nerve control
InactiveUS6907295B2Minimize cathode effectWeakening rangeSpinal electrodesHeart stimulatorsPower flowAnatomy
Apparatus is provided for applying current to a nerve. A cathode is adapted to be placed in a vicinity of a cathodic longitudinal site of the nerve and to apply, a cathodic current to the nerve. A primary inhibiting anode is adapted to be placed in a vicinity of a primary anodal longitudinal site of the nerve and to apply a primary anodal current to the nerve. A secondary inhibiting anode is adapted to be placed in a vicinity of a secondary anodal longitudinal site of the nerve and to apply a secondary anodal current to the nerve, the secondary anodal longitudinal site being closer to the primary anodal longitudinal site than to the cathodic longitudinal site.
Owner:MEDTRONIC INC
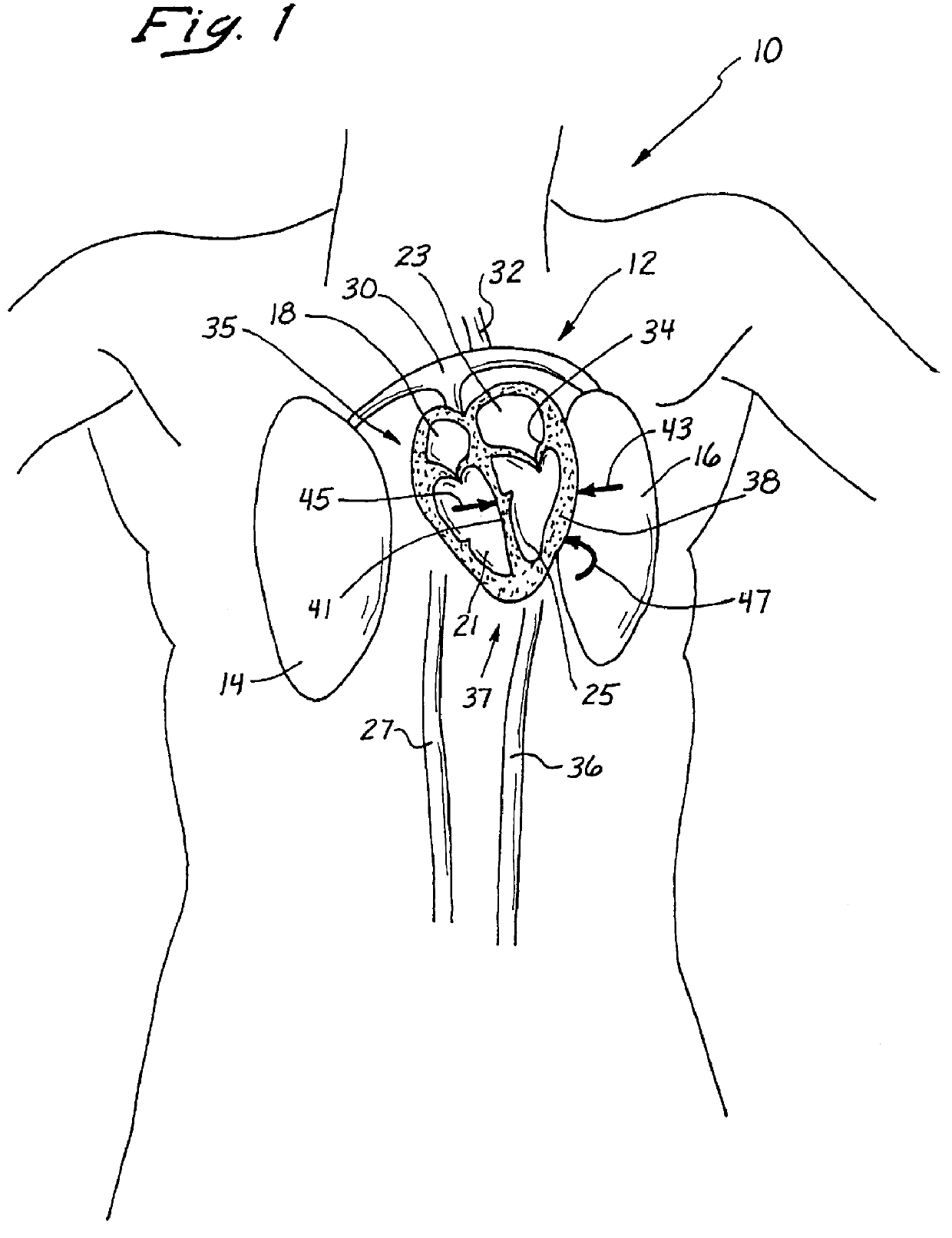
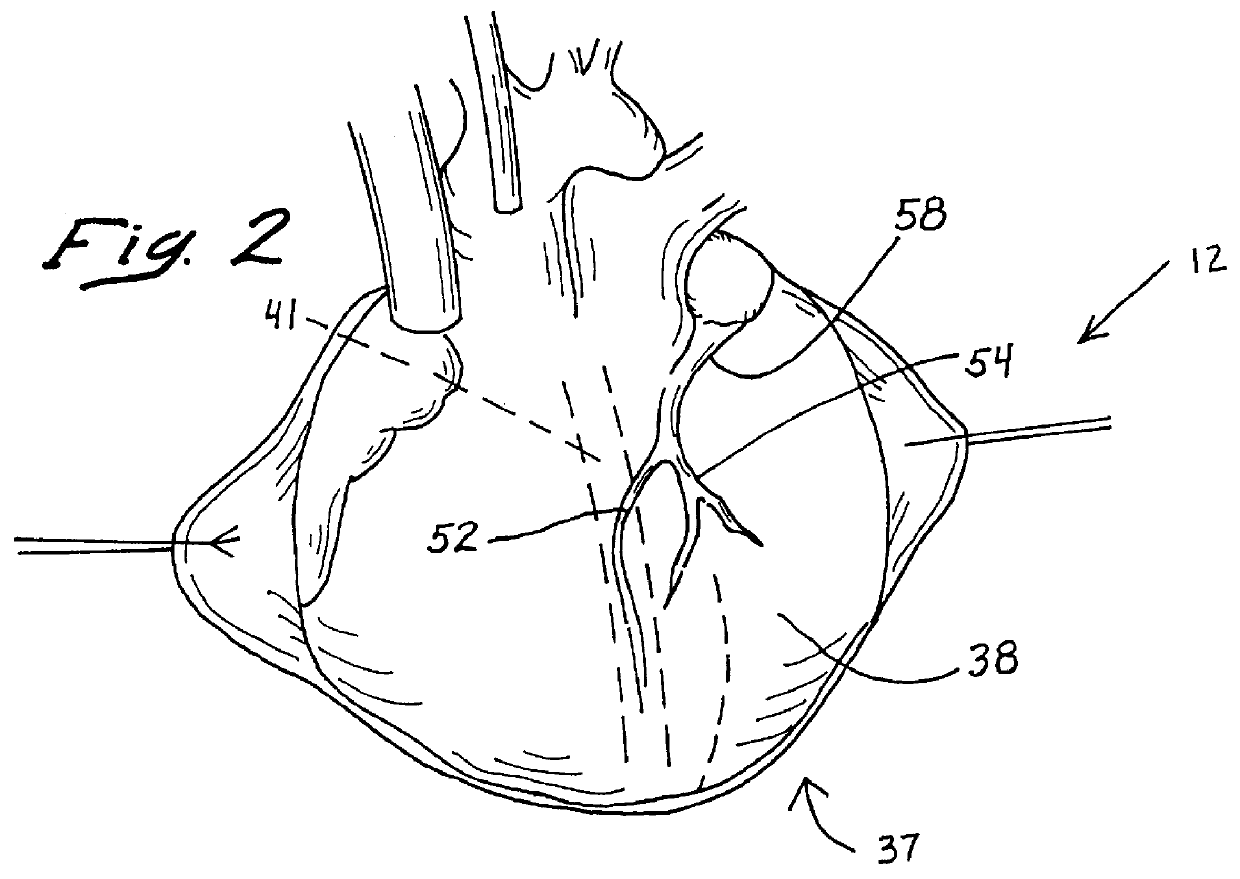
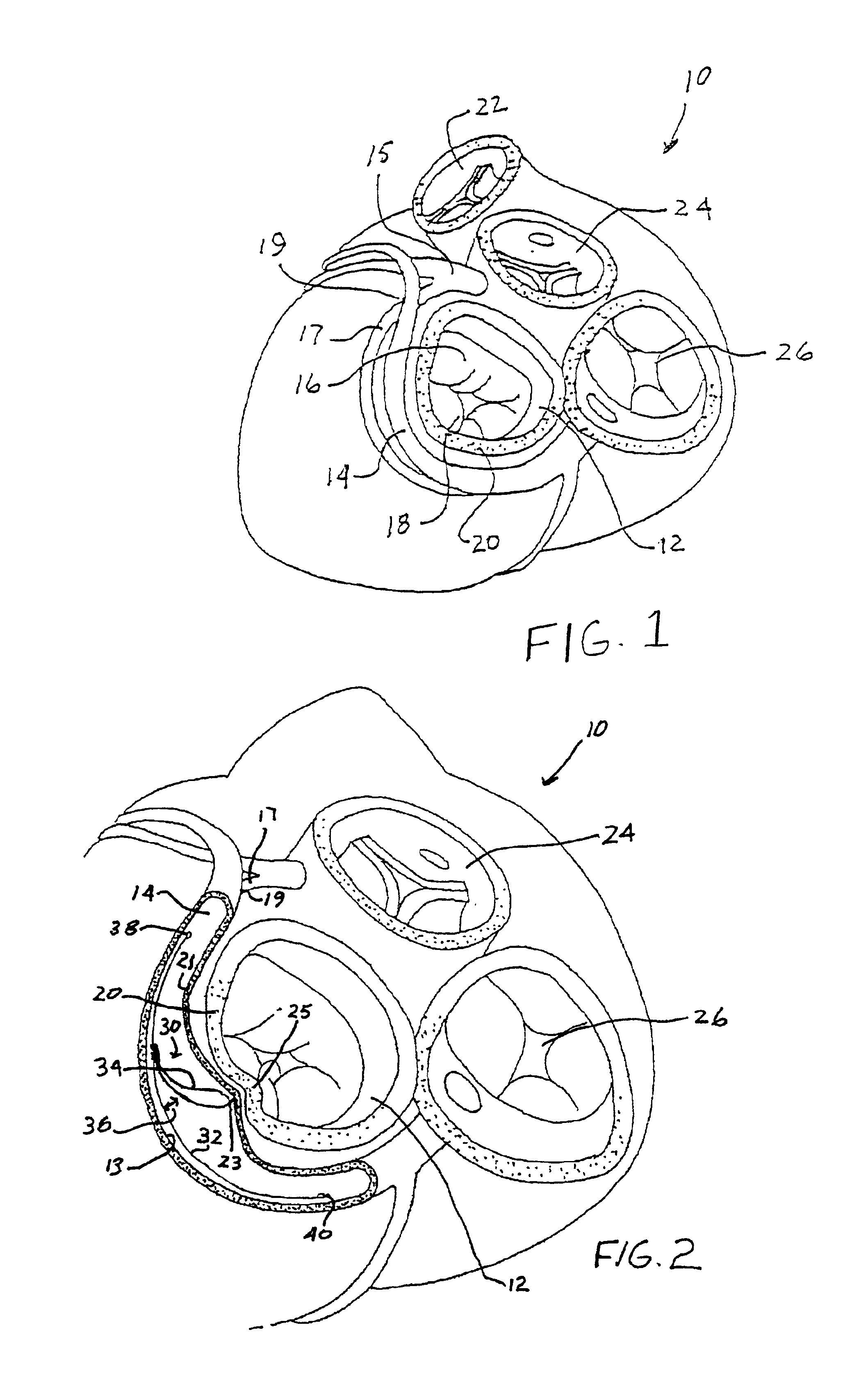
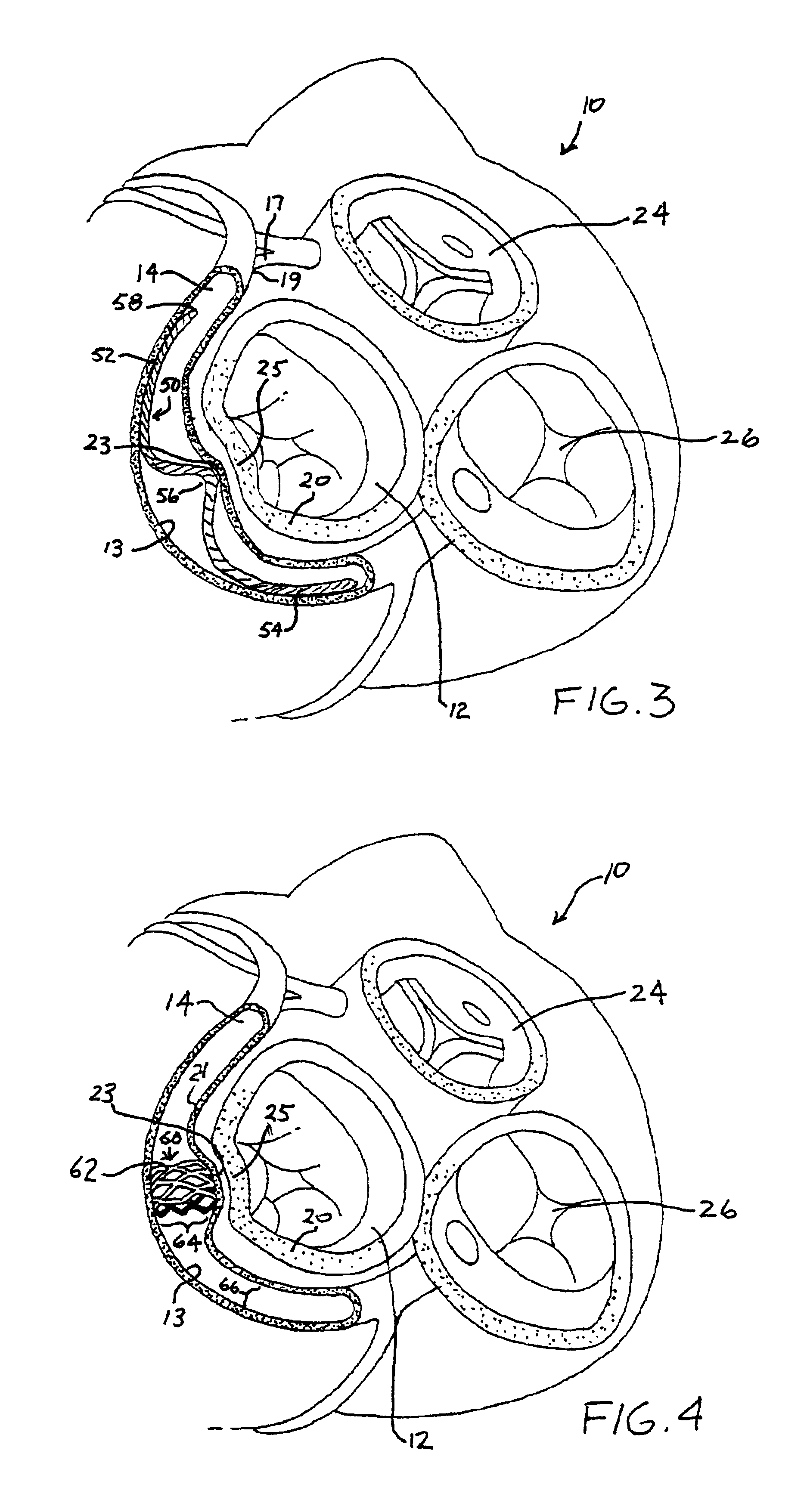
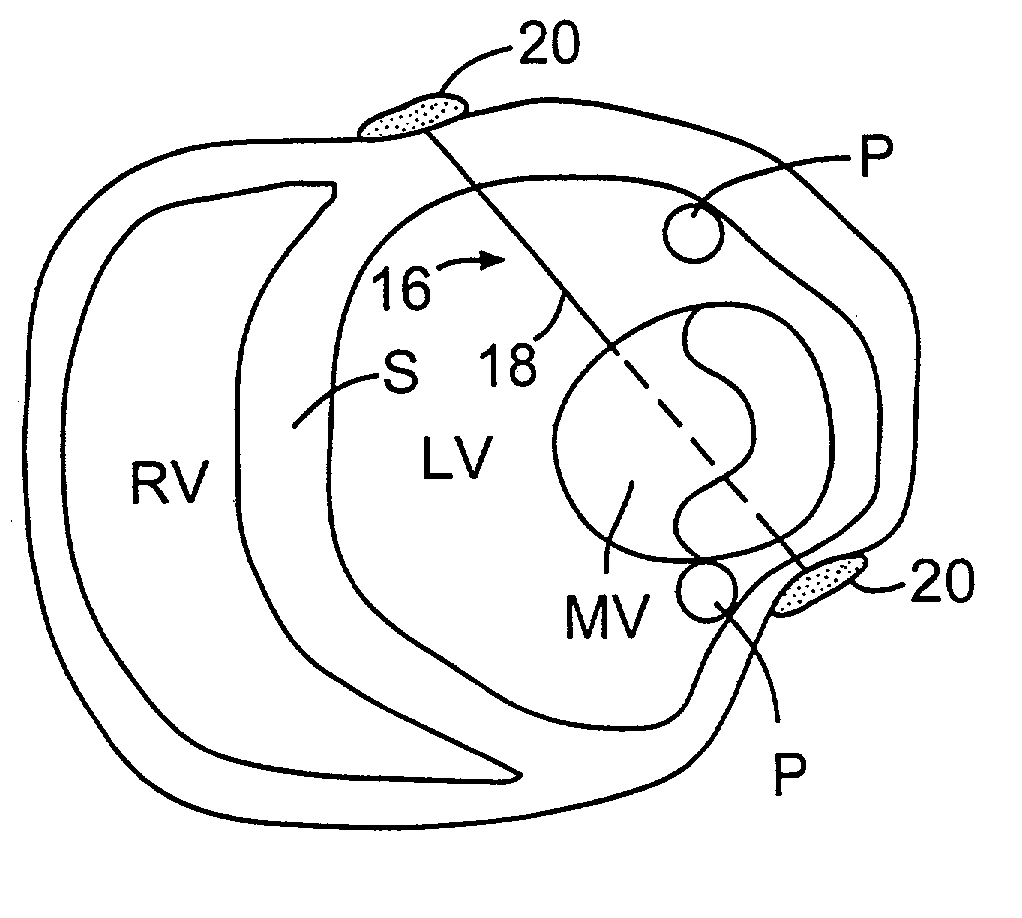
Anterior segment ventricular restoration apparatus and method
The symptoms of congenital heart failure are addressed in this surgical procedure for mounting a patch in the ventricle of the heart to reduce ventricular volume. Placement of the patch is facilitated by palpating a beating heart to identify akinetic, although normal appearing, tissue. The patch has an oval configuration facilitating return of the heart to a normal apical shape which enhances muscle fiber efficiency and a normal writhing pumping action. The patch includes a semi-rigid ring, and a circumferential rim to address bleeding. Patch placement is further enhanced by creating a Fontan neck and use of pledged sutures. Intraoperative vascularization and valve replacement is easily accommodated. Increased injection fraction, reduced muscle stress, improved myocardial protection, and ease of accurate patch placement are all achieved with this procedure.
Owner:CORRESTORE
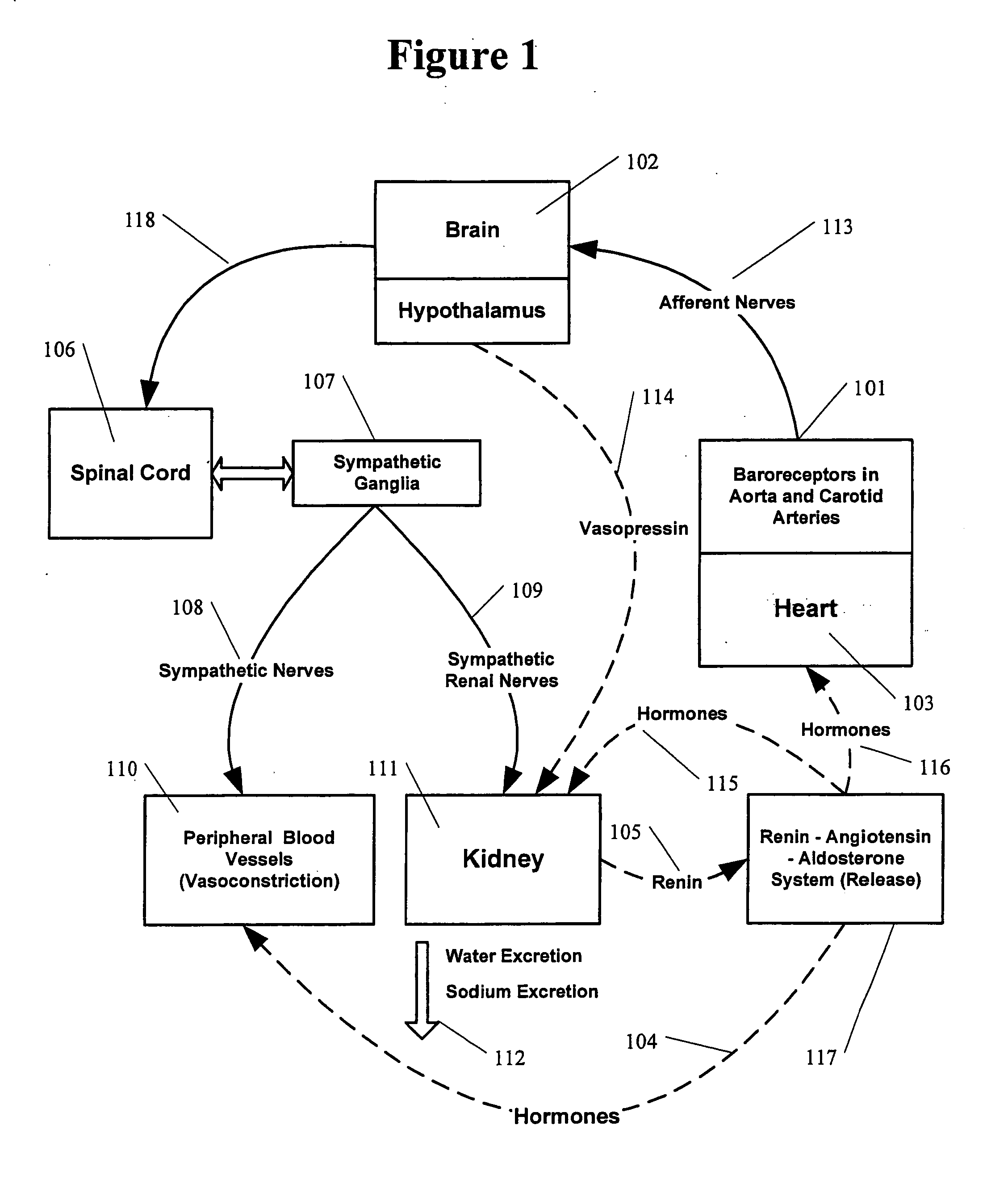
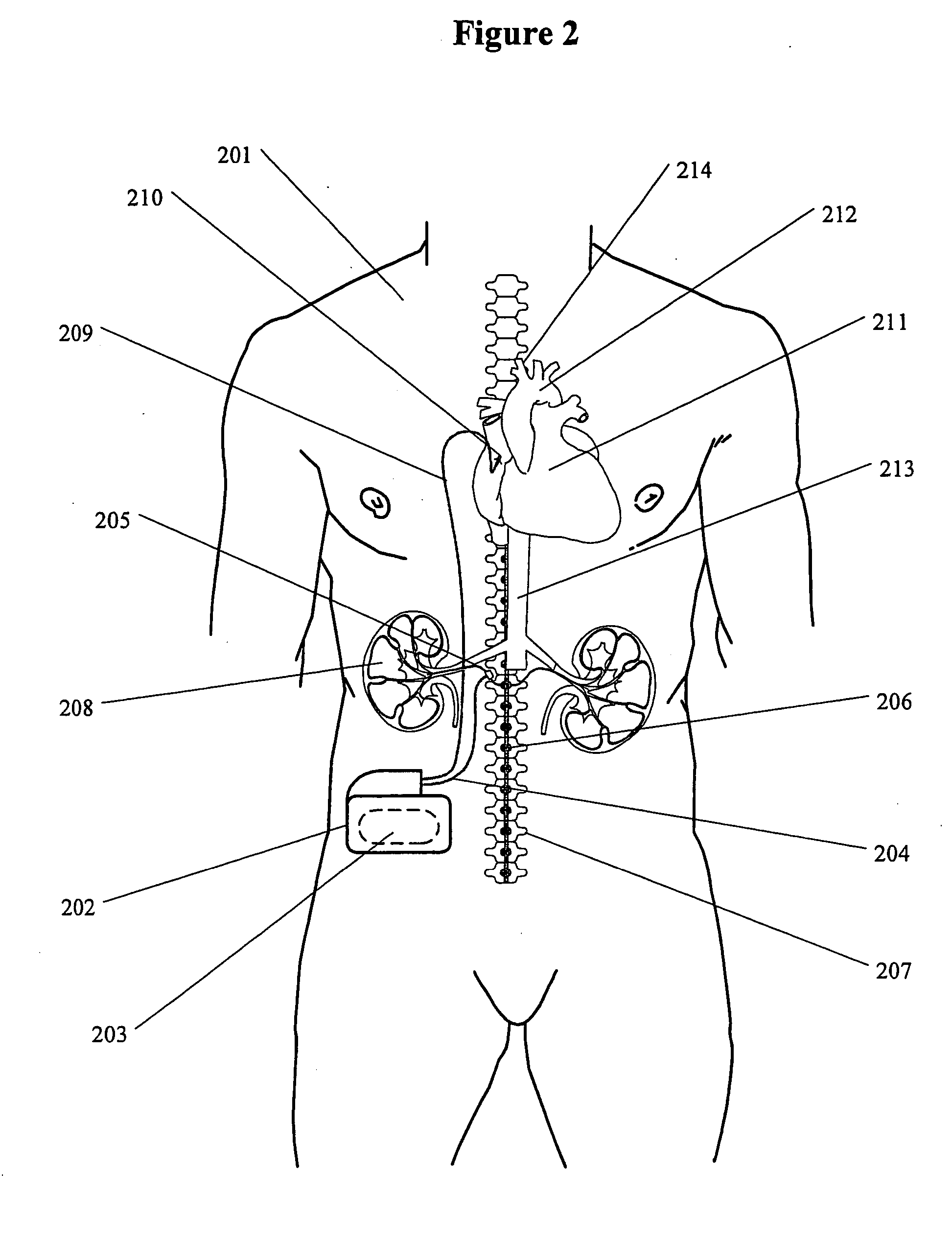
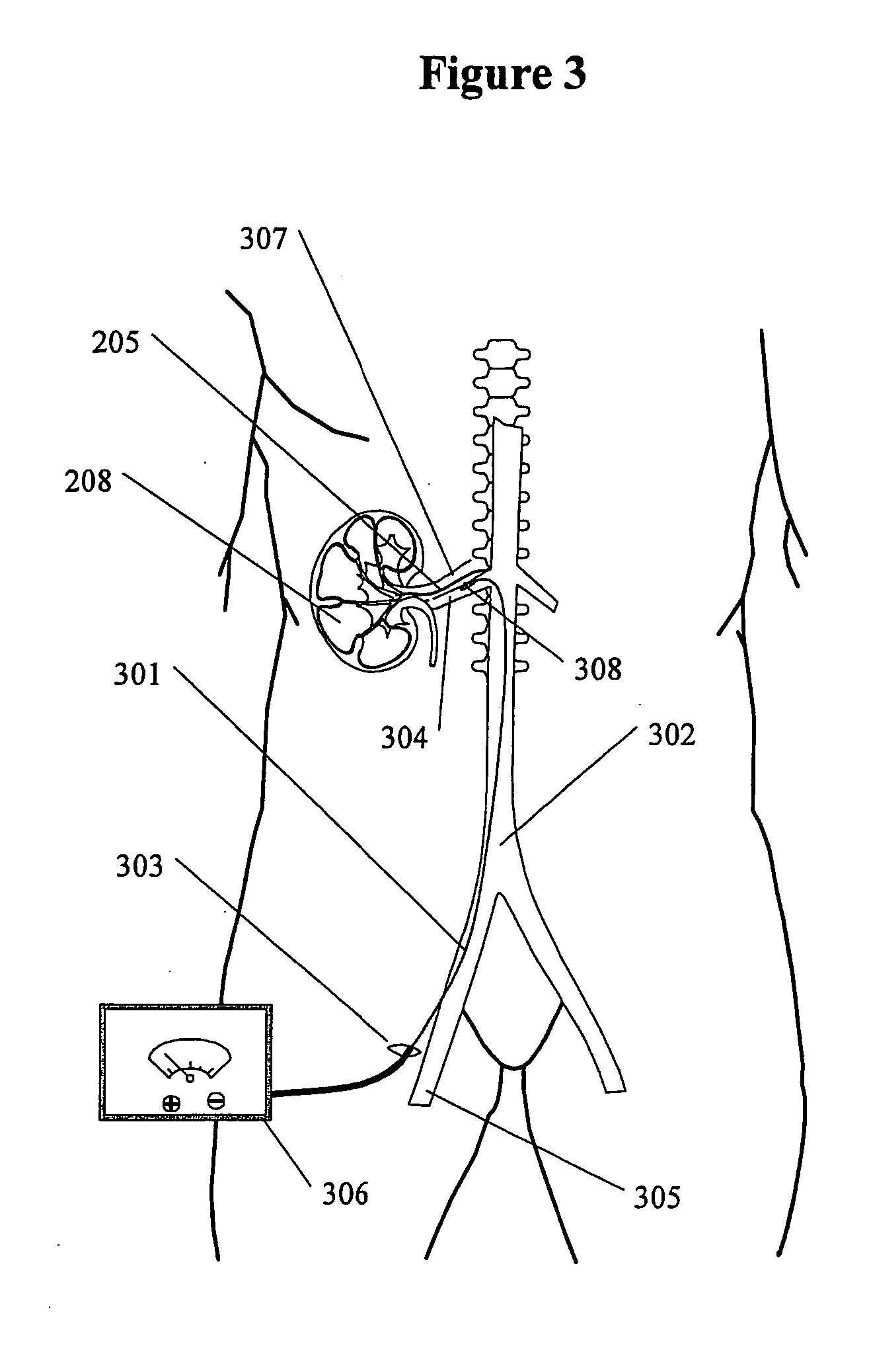
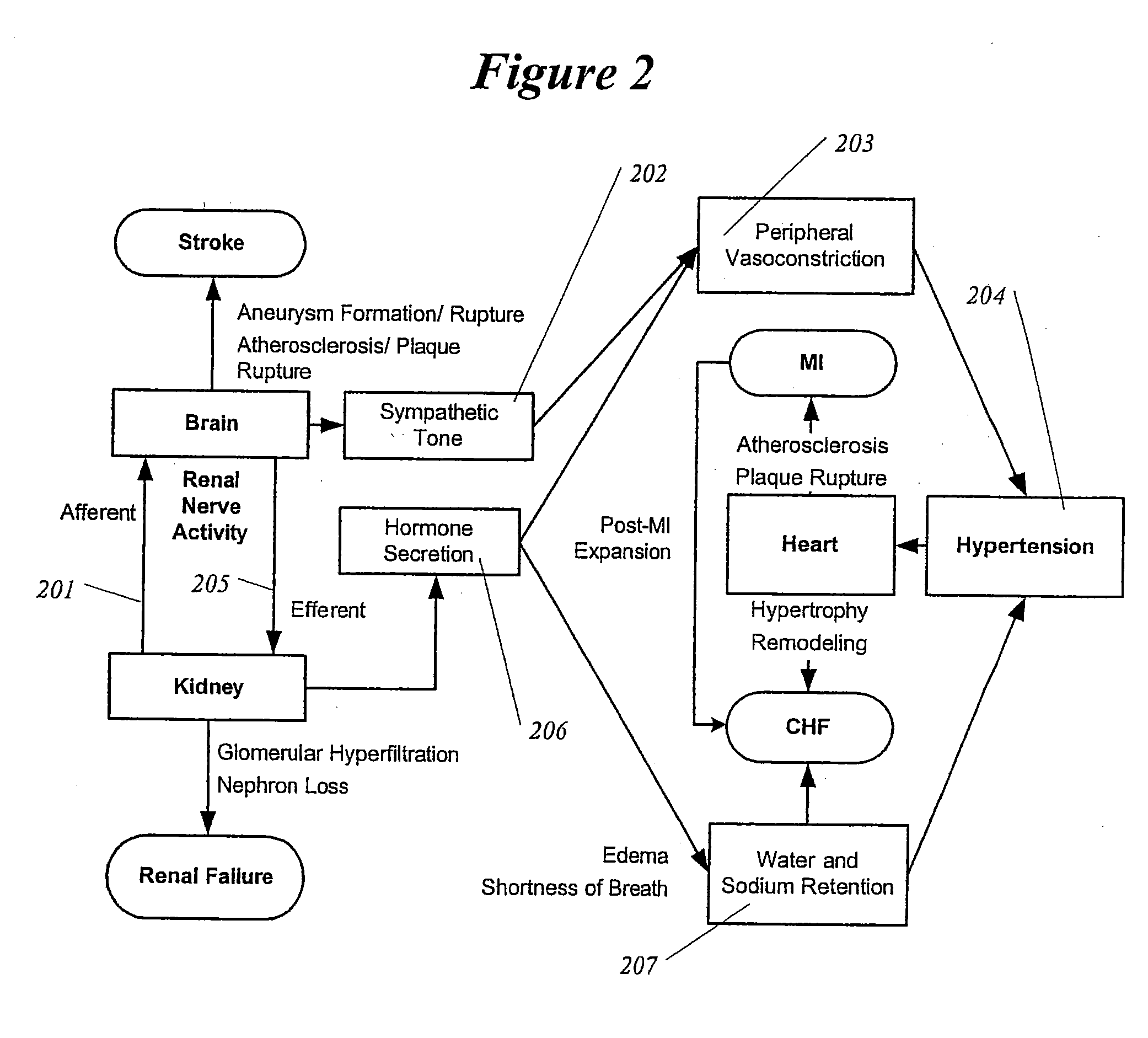
Renal nerve stimulation method and apparatus for treatment of patients
InactiveUS20050228460A1Arrest and slow down progression of diseaseEase of conditionsSpinal electrodesMedical devicesRenal nerveFAILURE KIDNEY
A method and apparatus for treatment of heart failure, hypertension and renal failure by stimulating the renal nerve. The goal of therapy is to reduce sympathetic activity of the renal nerve. Therapy is accomplished by at least partially blocking the nerve with drug infusion or electrostimulation. Apparatus can be permanently implanted or catheter based.
Owner:LEVIN HOWARD R +1
Method and apparatus to detect and treat sleep respiratory events
InactiveUS6641542B2CatheterRespiratory organ evaluationCheyne–Stokes respirationCheyne-stokes breathing
The present invention provides a method and apparatus for detecting and treating sleep respiratory events that includes a plurality of sensors gathering physiological data related to sleep respiratory events. A processor extracts an average cycle length and a frequency of at least one of Cheyne-Stokes respiration and periodic breathing based upon the physiological data, and determines whether therapy is required based on the average cycle length and the frequency.
Owner:MEDTRONIC INC
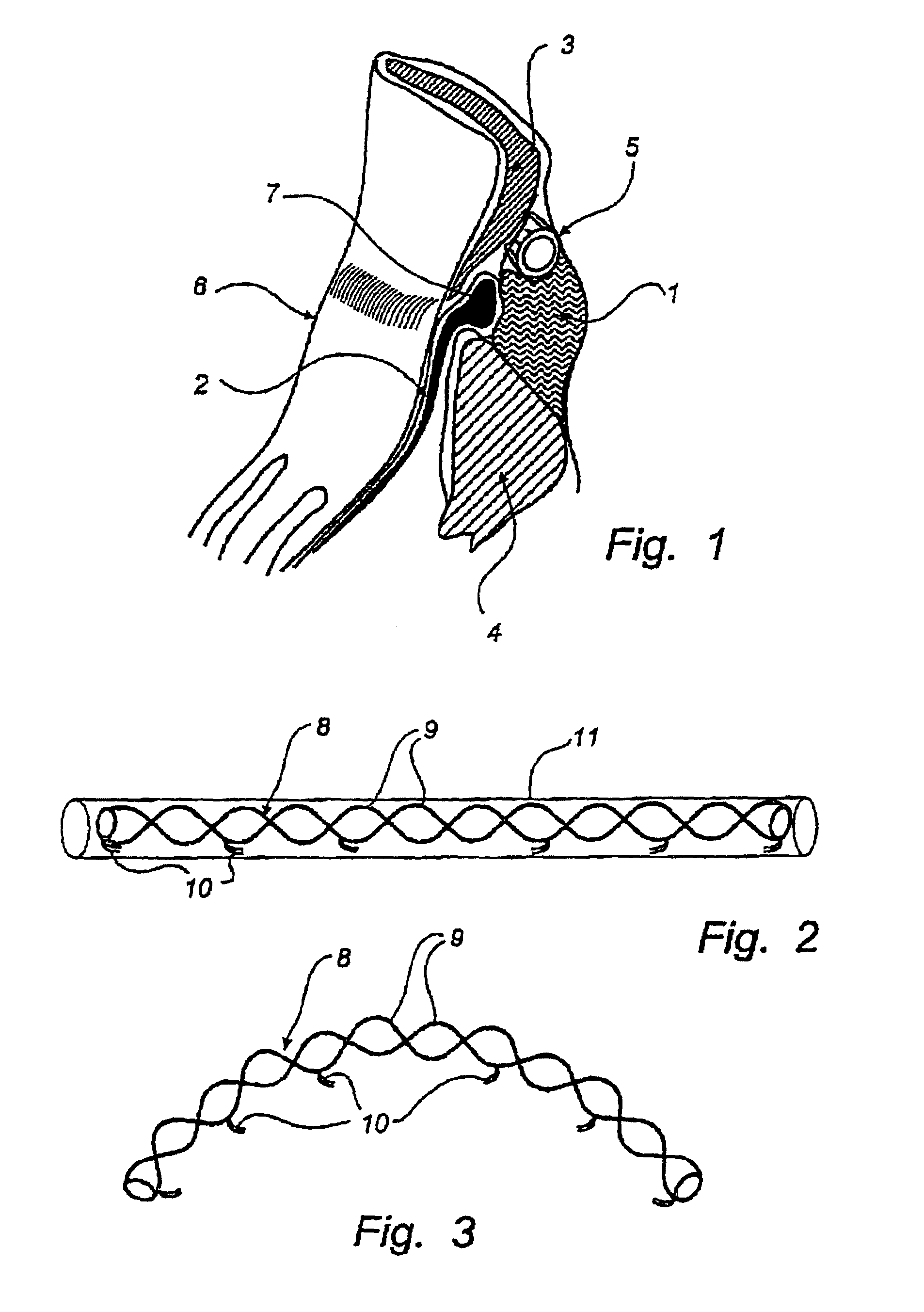
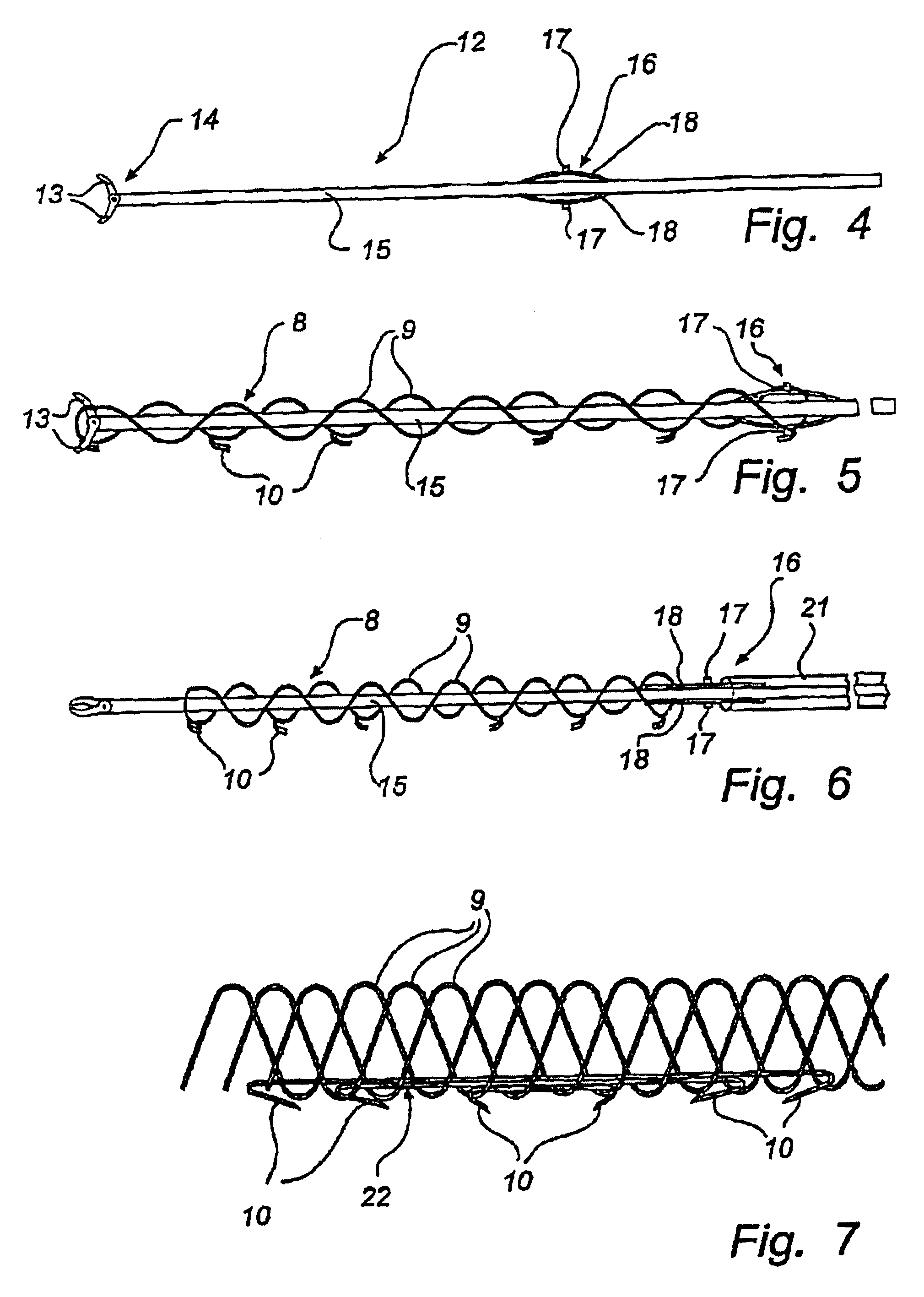
Method and device for treatment of mitral insufficiency
InactiveUS6997951B2Length of device can be decreasedShorten the lengthStentsBone implantCoronary sinusMitral annulus
A device for treatment of mitral annulus dilation is disclosed, wherein the device comprises two states. In a first of these states the device is insertable into the coronary sinus and has a shape of the coronary sinus. When positioned in the coronary sinus, the device is transferable to the second state assuming a reduced radius of curvature, whereby the radius of curvature of the coronary sinus and the radius of curvature as well as the circumference of the mitral annulus is reduced.
Owner:EDWARDS LIFESCIENCES AG +1
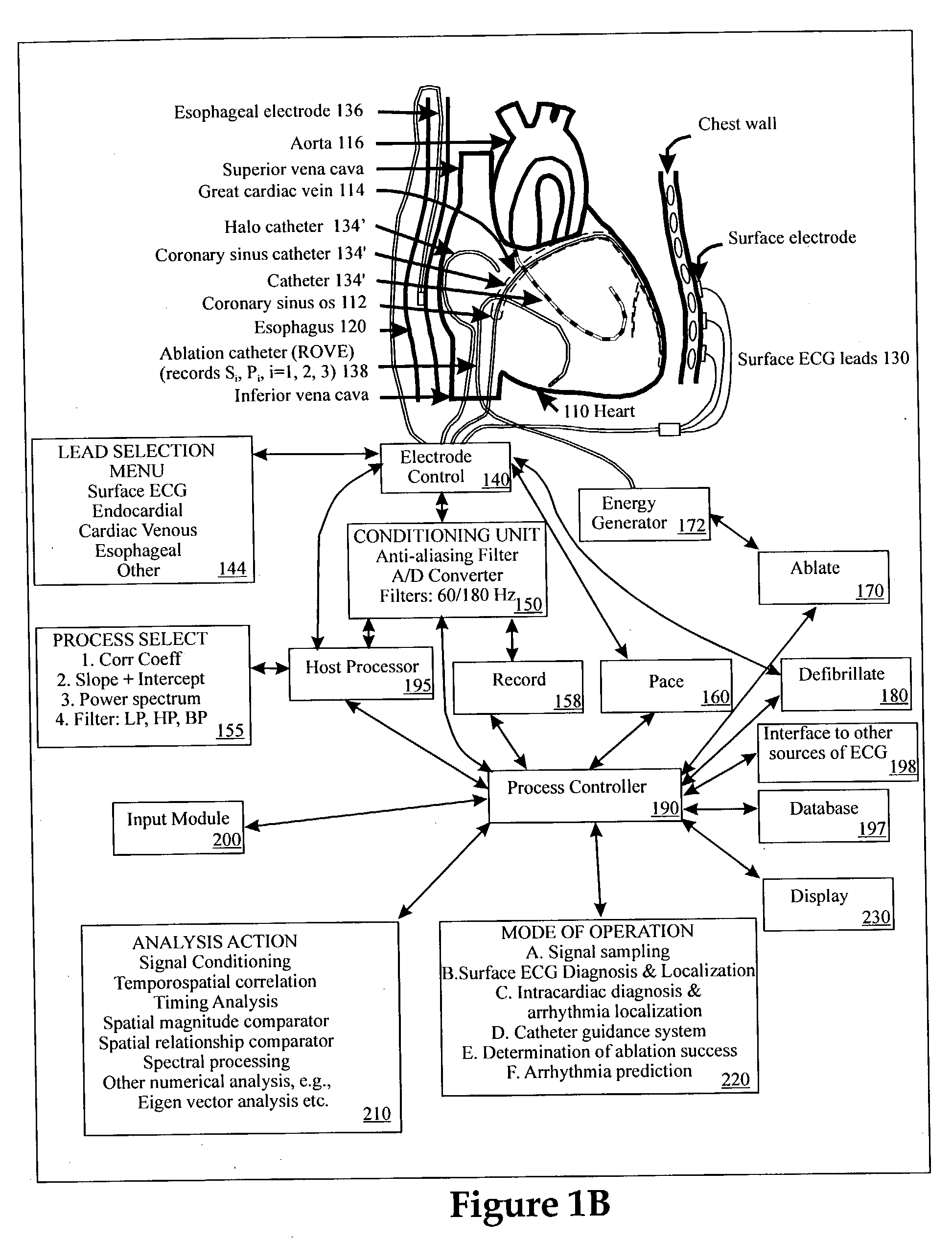
Method and apparatus for classifying and localizing heart arrhythmias
Analyzes surface electrocardiographic and intracardiac signals to identify and separate electrical activity corresponding to distinct but superimposed events in the heart. Assesses the spatial phase, temporal phase, rate, spectrum and reproducibility of each event to determine uniformity of activation in all spatial dimensions. Uses numerical indices derived from these analyses to diagnose arrhythmias. Uses these indices to determine the location of an arrhythmia circuit, and to direct the movement of an electrode catheter to this location for ablation or permanent catheter positioning. Subsequently, uses these indices to determine whether ablation has successfully eliminated the circuit. Uses variability in these indices from the surface electrocardiogram to indicate subtle beat-to-beat fluctuations which reflect the tendency towards atrial and ventricular arrhythmias.
Owner:RGT UNIV OF CALIFORNIA
Methods and devices for renal nerve blocking
InactiveUS20080213331A1Shorten the progressResolution of overloadSpinal electrodesMedical devicesDiseaseRenal nerve
A method and apparatus for treatment of cardiac and renal diseases associated with the elevated sympathetic renal nerve activity by implanting a device to block the renal nerve signals to and from the kidney. The device can be a drug pump or a drug eluding implant for targeted delivery of a nerve-blocking agent to the periarterial space of the renal artery.
Owner:ARDIAN
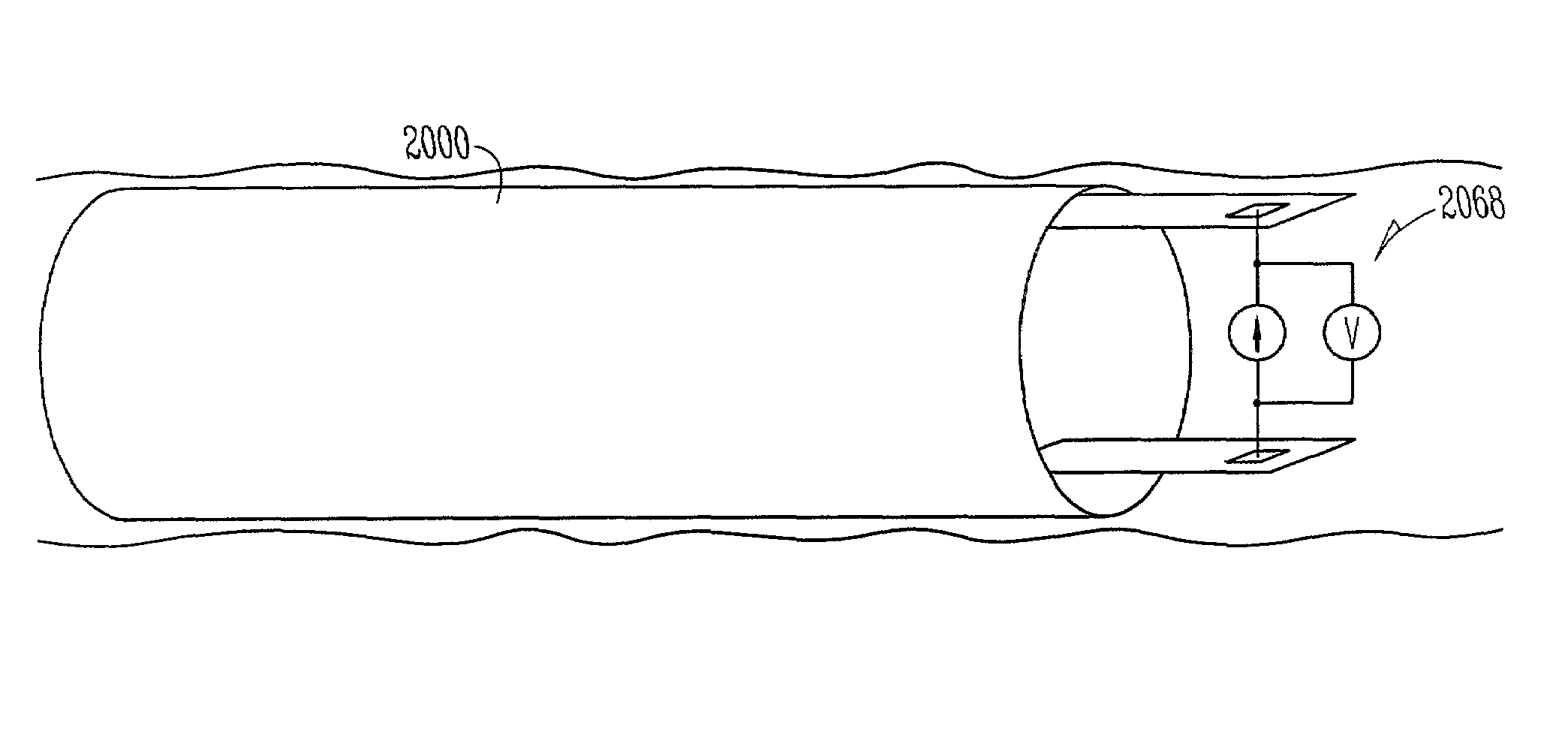
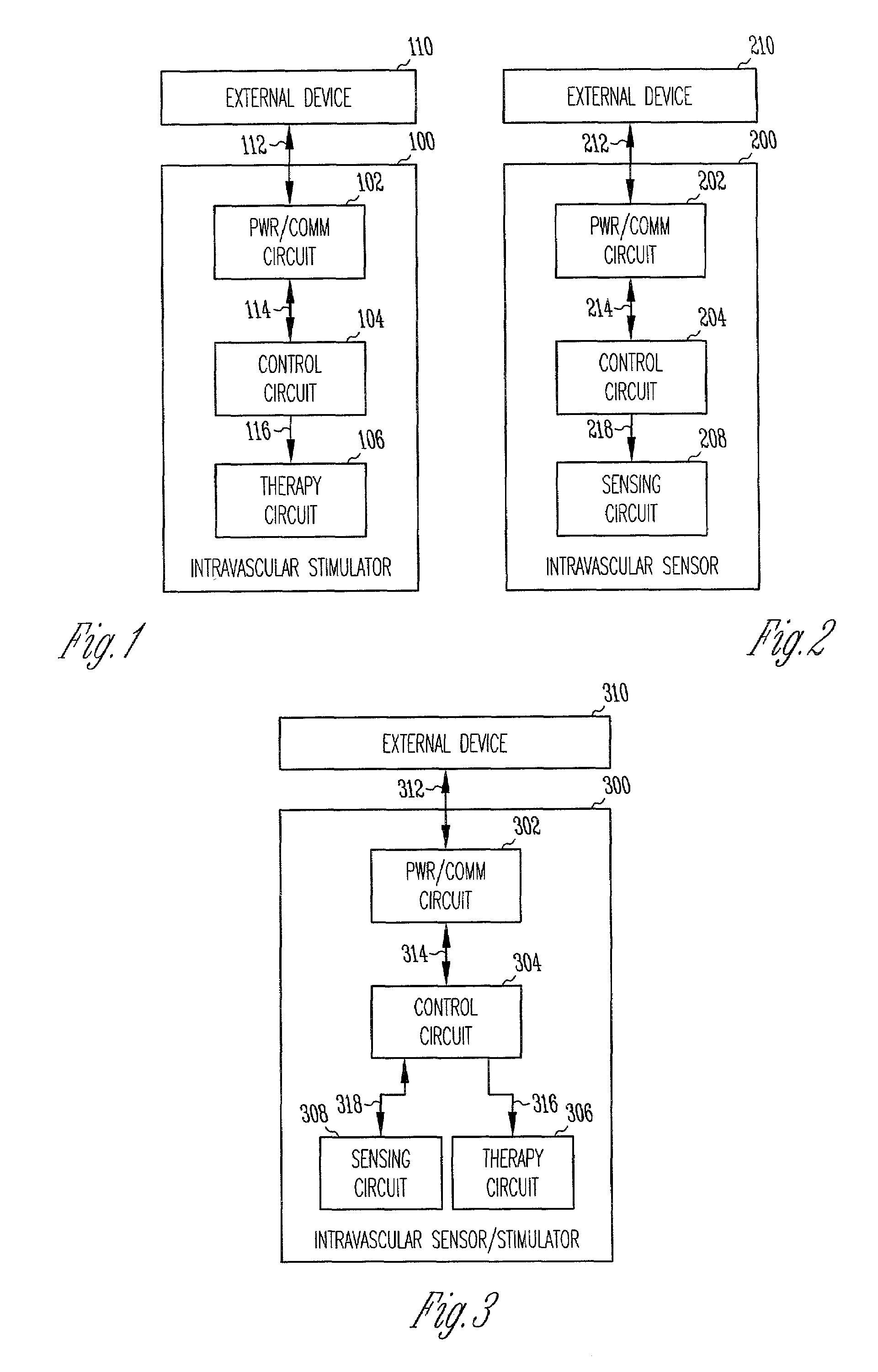
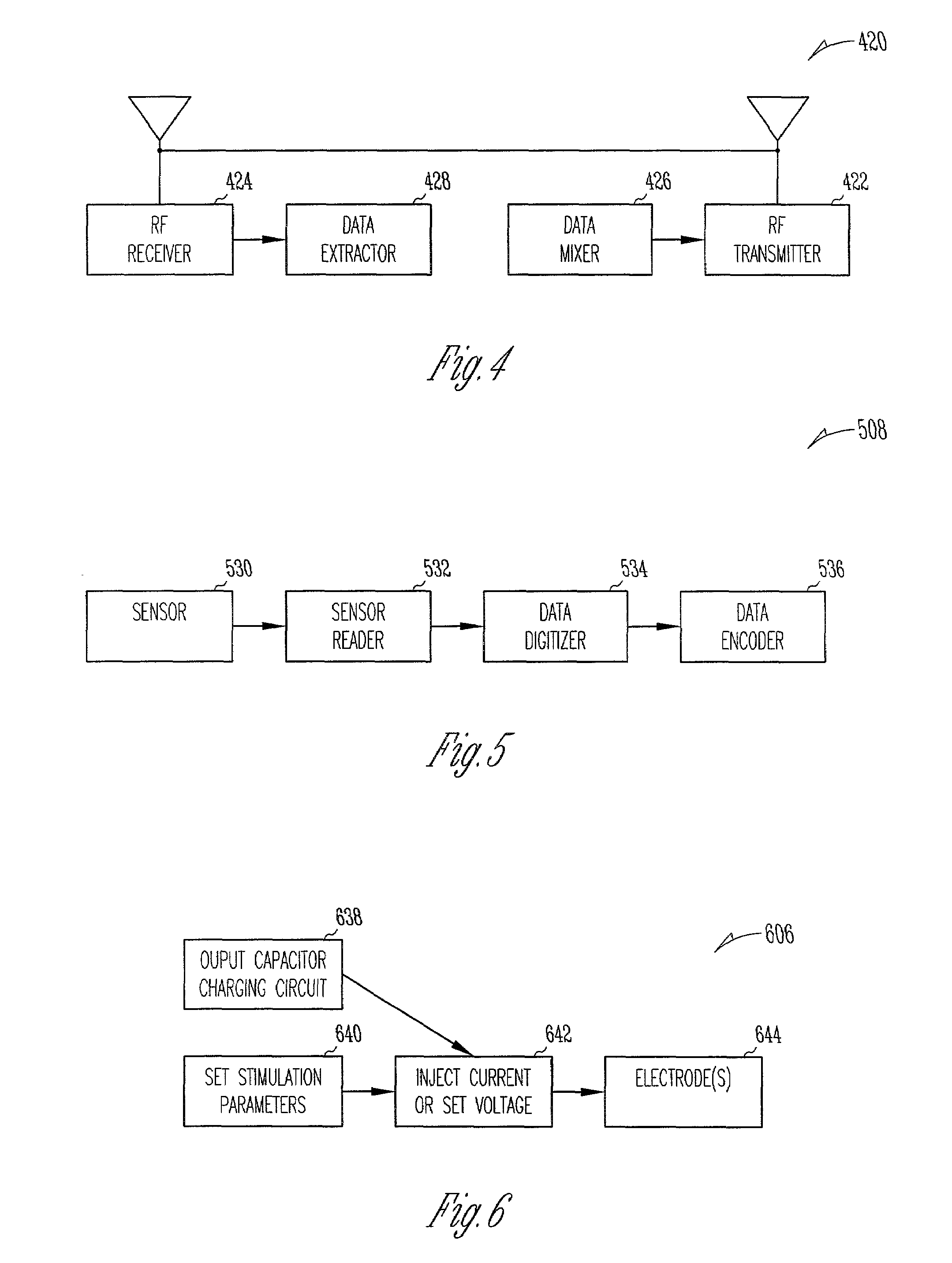
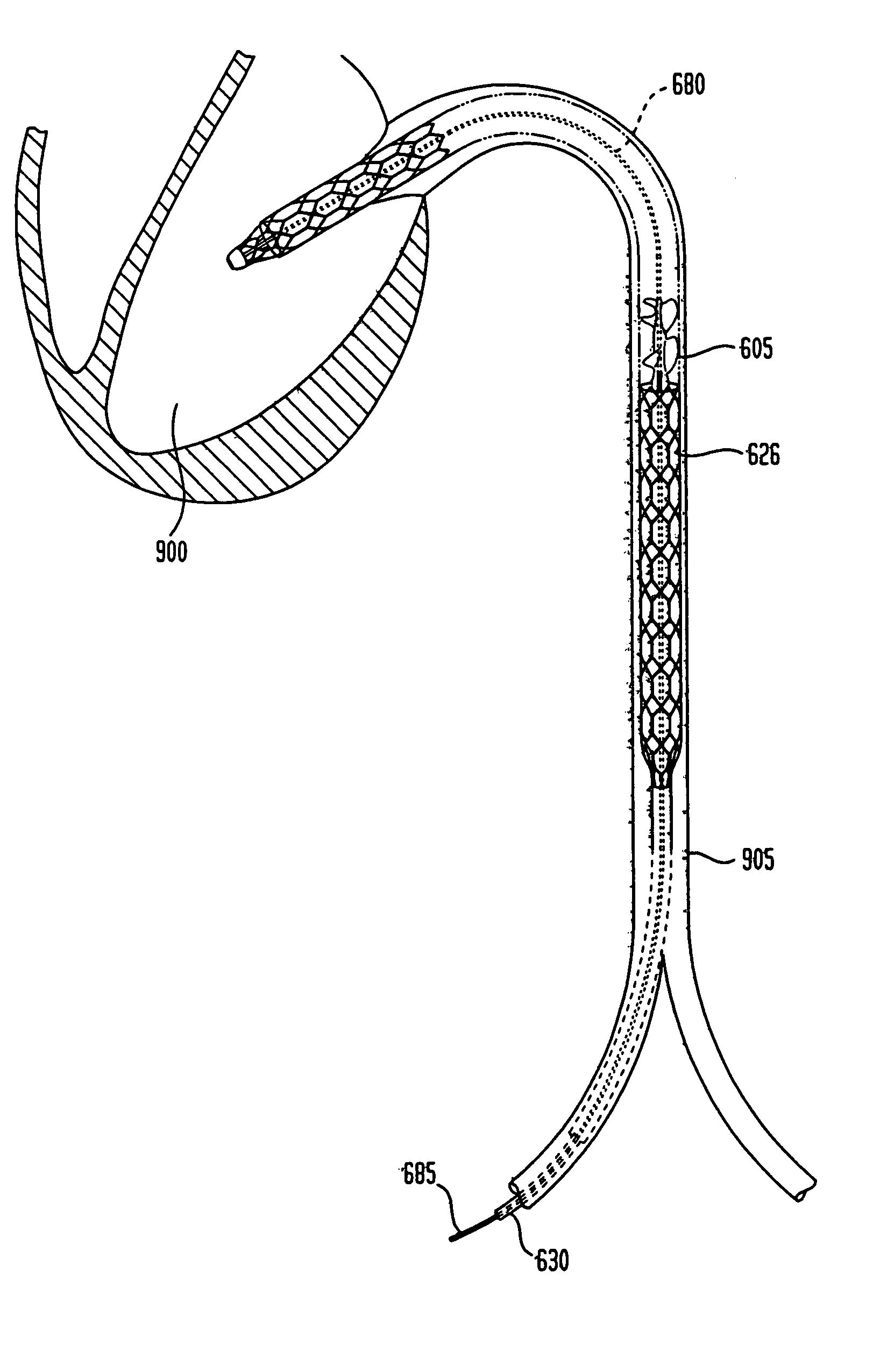
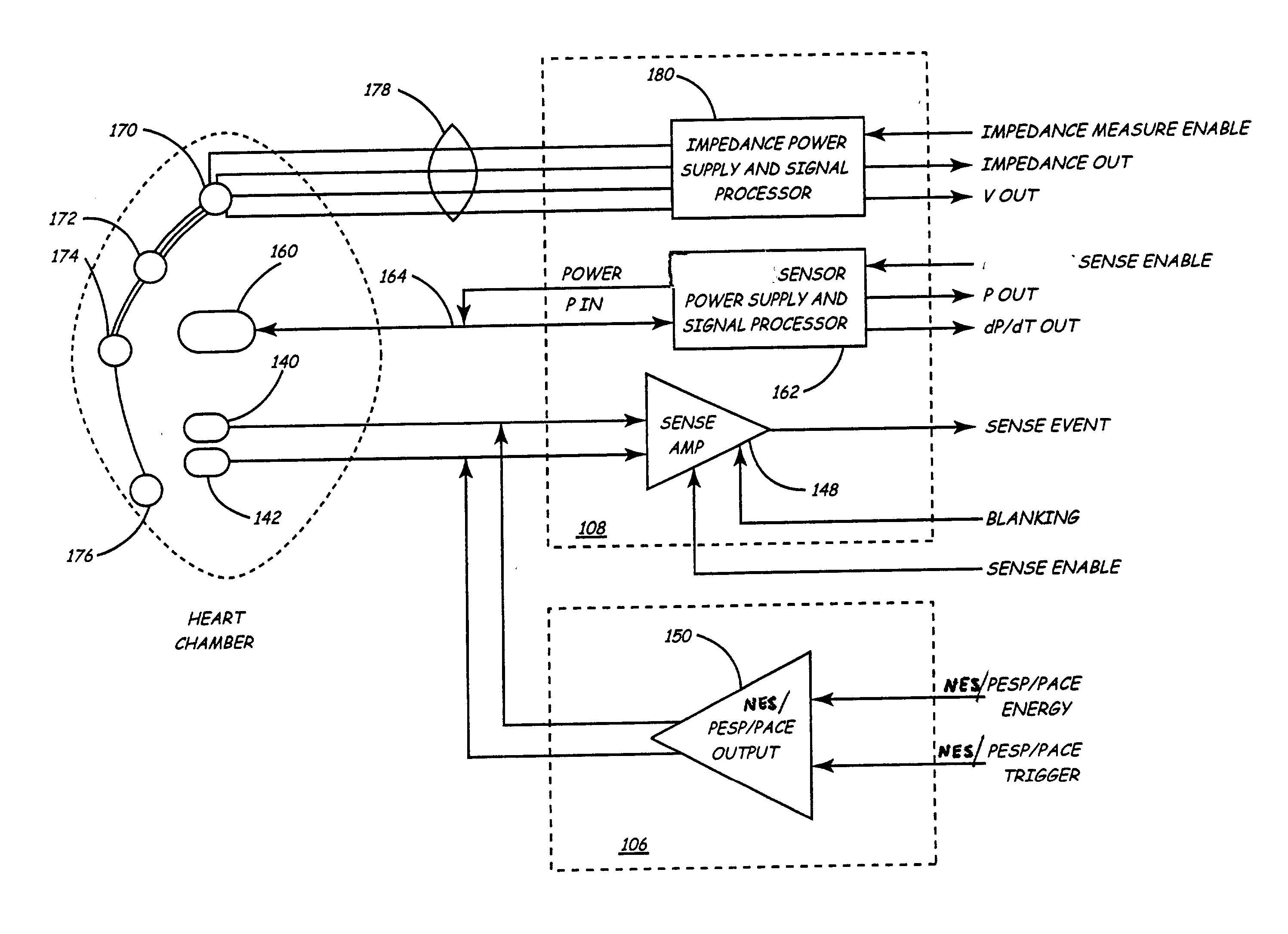
Chronically-implanted device for sensing and therapy
InactiveUS7236821B2Easy to solvePrevent restenosisStentsEndoradiosondesElectricityTherapeutic Devices
Systems, devices and methods are provided for providing sensing and therapy functions using a chronically-implanted device. According to one embodiment, the device includes a structure adapted to be chronically placed within a biosystem, and further includes sensing circuitry and therapy-providing circuitry attached to the structure. The sensing circuitry is adapted to sense mechanical parameters in the biosystem. The therapy-providing circuitry is adapted to provide therapy to the biosystem. According to various embodiments of the device, the sensing circuitry is further adapted to sense electrical and / or chemical parameters in the biosystem, and / or is adapted to provide electrical therapy and / or to provide drug-eluting therapy. One embodiment of the device includes a stent-like structure adapted to be chronically placed intravascularly in the biosystem.
Owner:CARDIAC PACEMAKERS INC
Heart assist device with expandable impeller pump
An impeller includes a hub and at least one blade supported by the hub. The impeller has a stored configuration in which the blade is compressed so that its distal end moves towards the hub, and a deployed configuration in which the blade extends away from the hub. The impeller may be part of a pump for pumping fluids, such as pumping blood within a patient. A blood pump may include a cannula having a proximal portion with a fixed diameter, and a distal portion with an expandable diameter. The impeller may reside in the expandable portion of the cannula. The cannula may have a compressed diameter which allows it to be inserted percutaneously into a patient. Once at a desired location, the expandable portion of the cannula may be expanded and the impeller expanded to the deployed configuration. A flexible drive shaft may extend through the cannula for rotationally driving the impeller within the patient's body.
Owner:PENN STATE RES FOUND +2
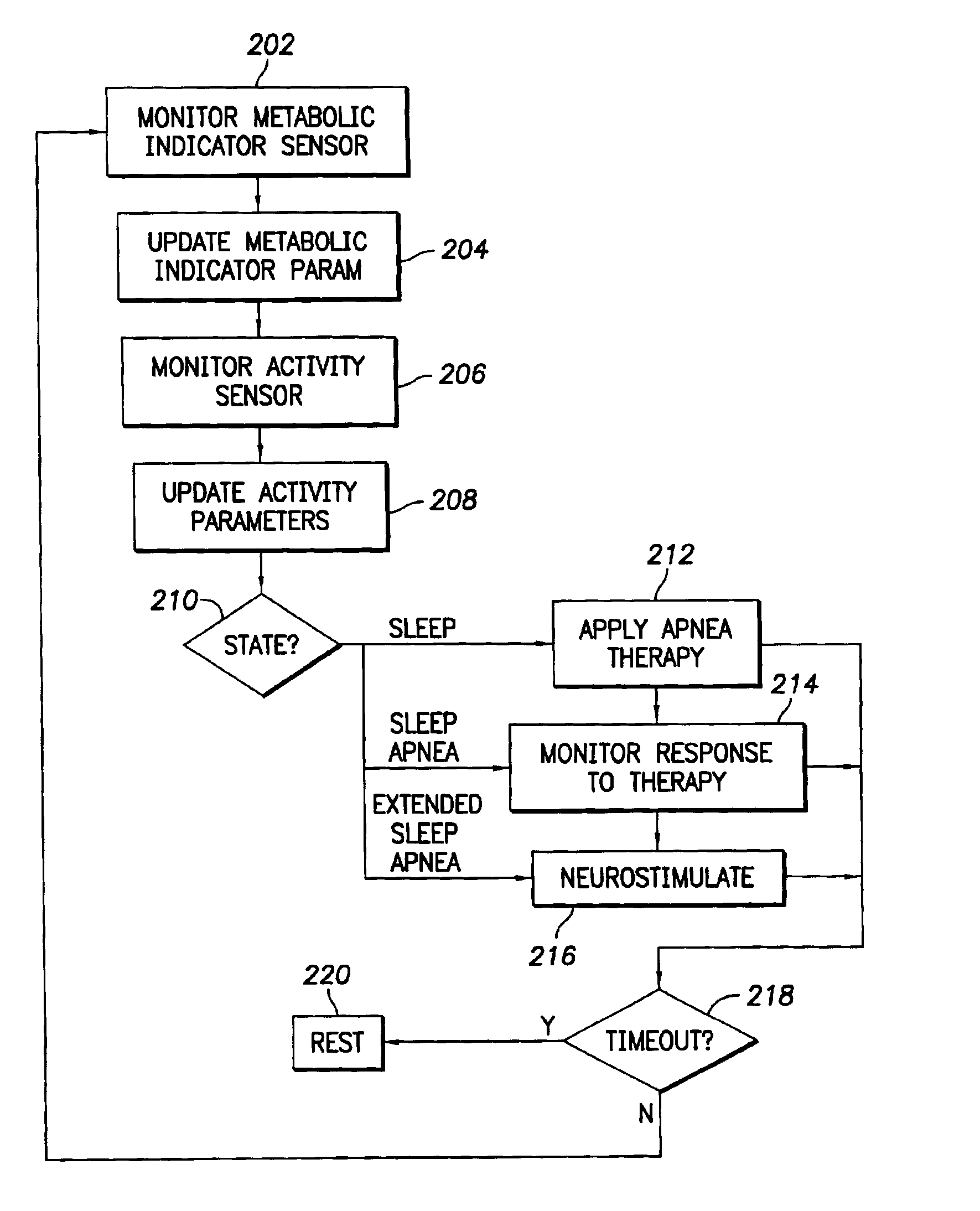
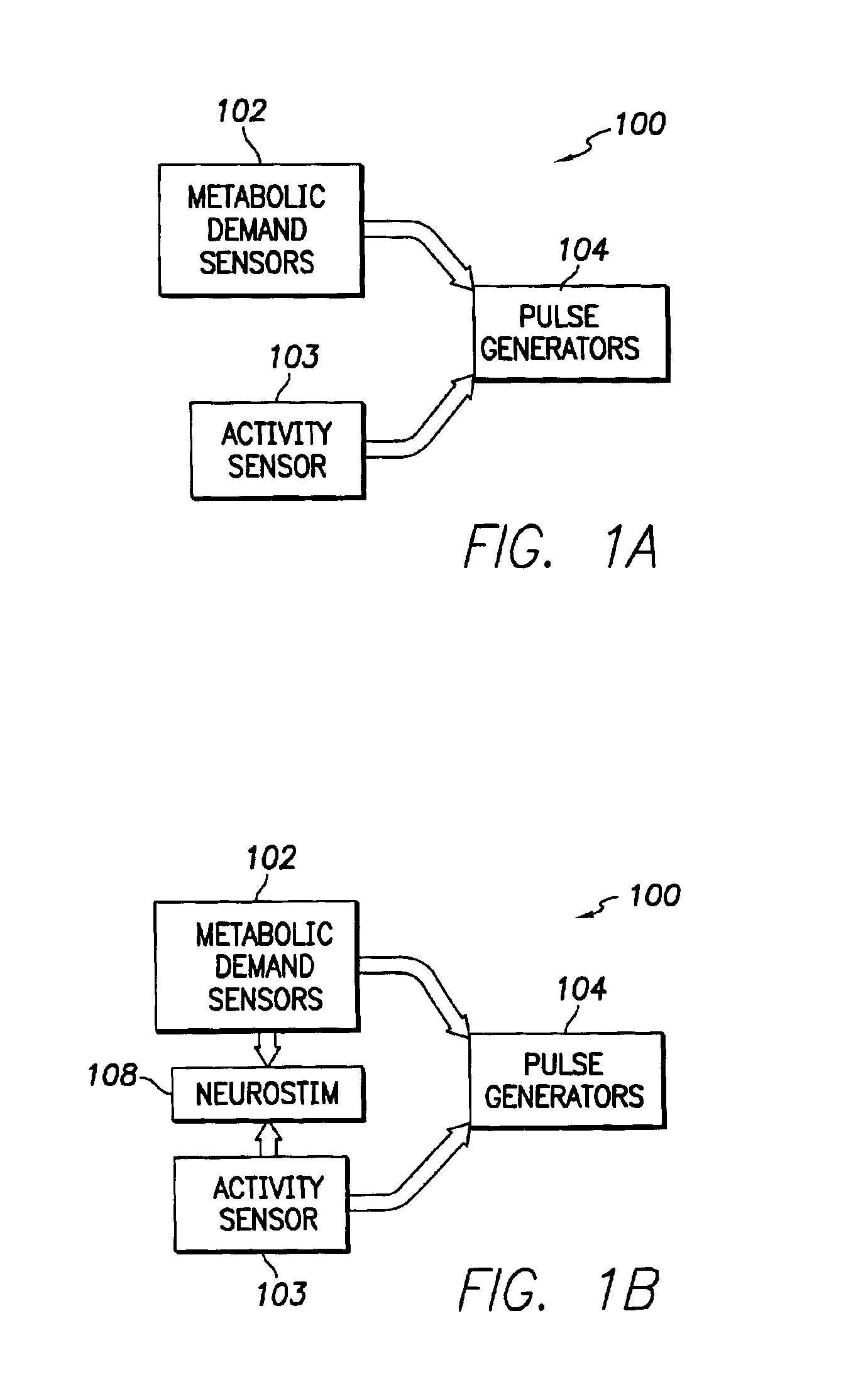
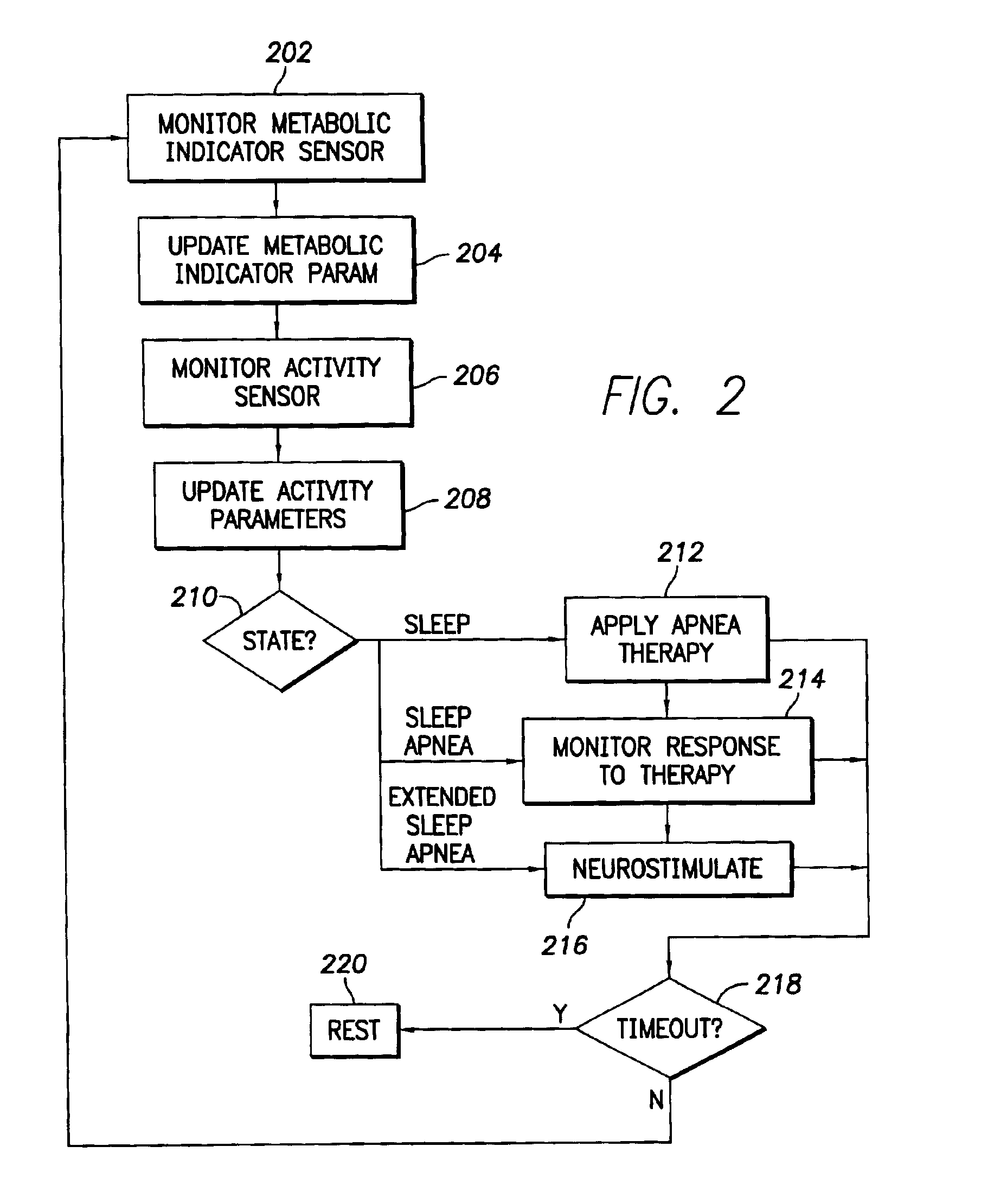
Stimulation device for sleep apnea prevention, detection and treatment
InactiveUS6928324B2Increase oxygen concentrationReduce carbon dioxide concentrationHeart stimulatorsArtificial respirationSleep apneaMetabolic demand
An implantable cardiac stimulation device comprises a metabolic demand sensor, an activity sensor, and one or more pulse generators. The metabolic demand sensor and activity sensor can sense metabolic demand and physical activity parameters, respectively. The pulse generators can generate cardiac pacing pulses with timing based on a comparison of the metabolic demand and physical activity parameters. The timed cardiac pacing pulses can prevent a sleep apnea condition.
Owner:PACESETTER INC
Implantable medical device for treating cardiac mechanical dysfunction by electrical stimulation
InactiveUS20040049235A1Increase the number ofHigh detection specificityHeart defibrillatorsHeart stimulatorsCardiac dysfunctionElectrical stimulations
The above-described methods and apparatus are believed to be of particular benefit for patients suffering heart failure including cardiac dysfunction, chronic HF, and the like and all variants as described herein and including those known to those of skill in the art to which the invention is directed. It will understood that the present invention offers the possibility of monitoring and therapy of a wide variety of acute and chronic cardiac dysfunctions. The current invention provides systems and methods for delivering therapy for cardiac hemodynamic dysfunction.
Owner:MEDTRONIC INC
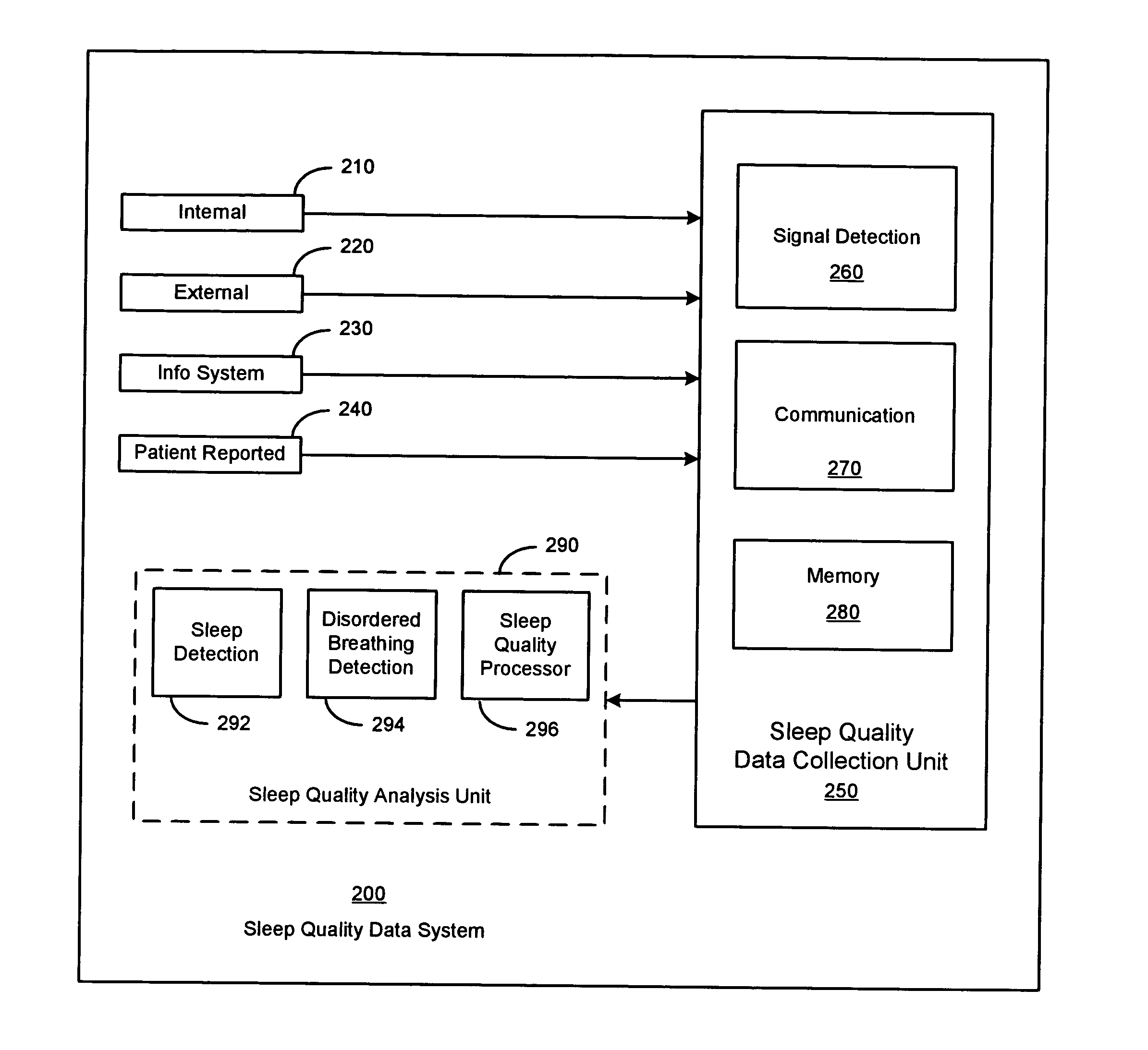
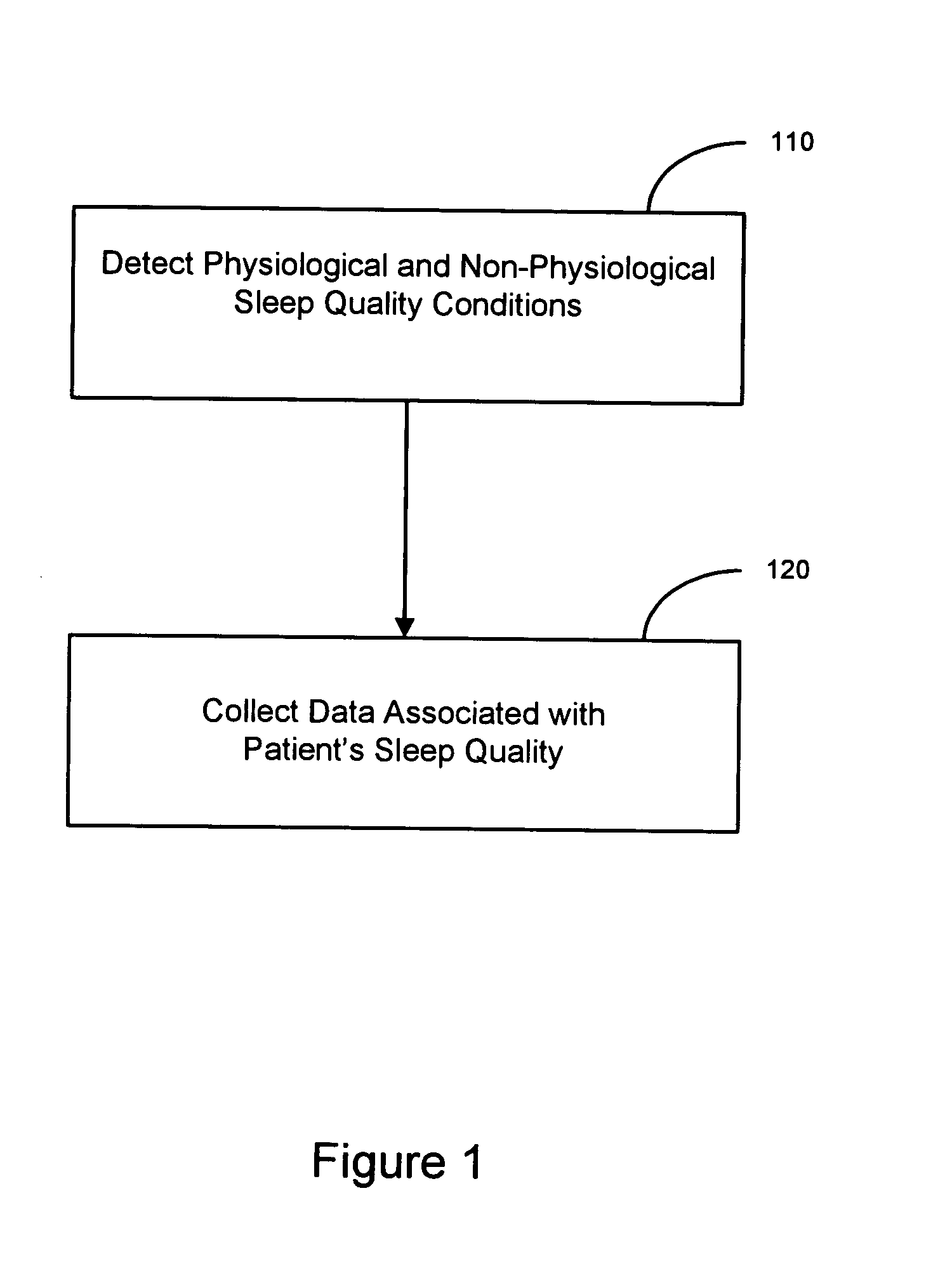
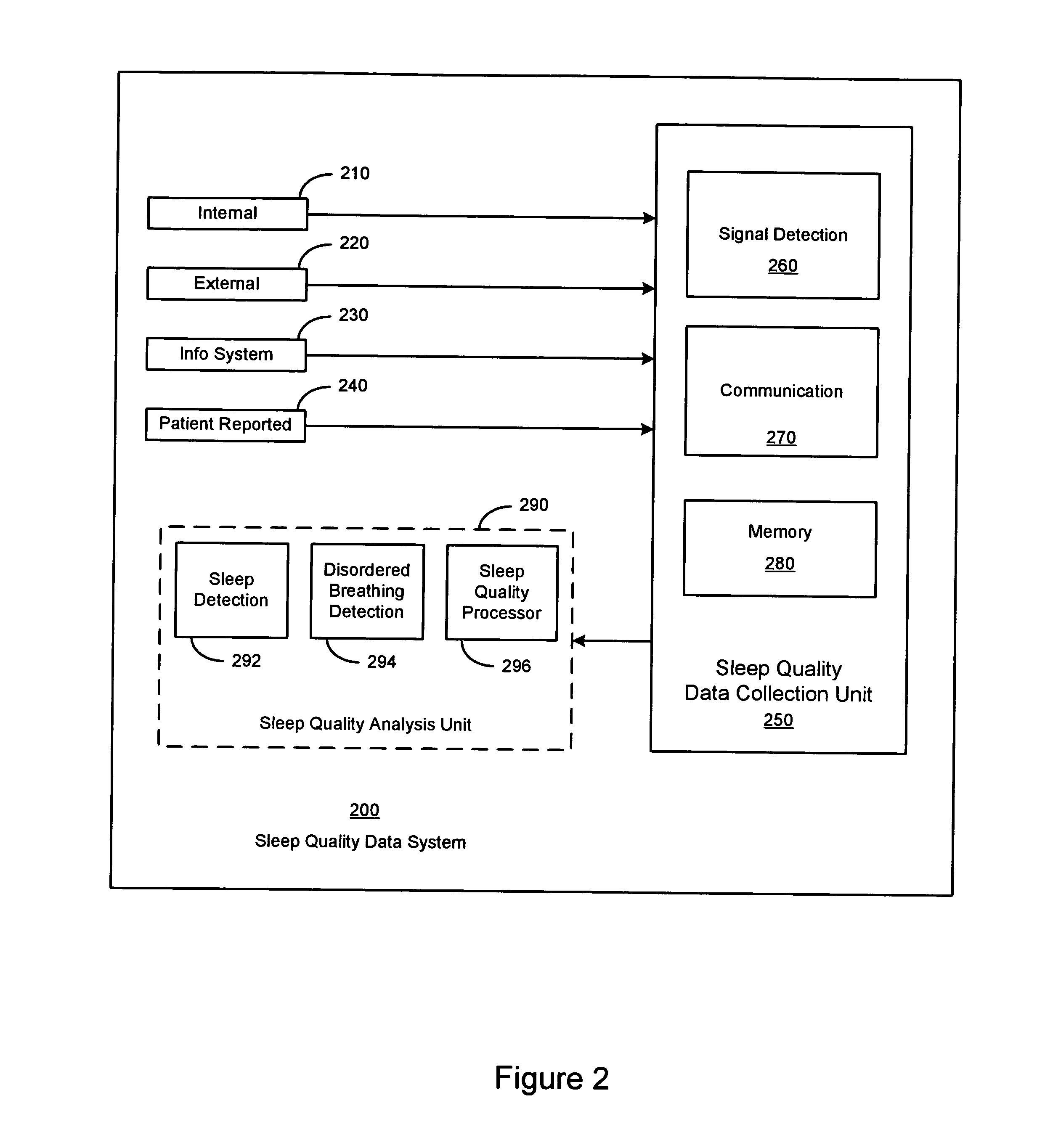
Sleep quality data collection and evaluation
A sleep quality assessment approach involves collecting data based on detected physiological or non-physiological patient conditions. At least one of detecting patient conditions and collecting data is performed using an implantable device. Sleep quality may be evaluated using the collected data by an imlantable or patient-external sleep quality processor. One approach to sleep quality evaluation involves computing one or more summary metrics based on occurrences of movement disorders or breathing disorders during sleep.
Owner:CARDIAC PACEMAKERS INC
Focused compression mitral valve device and method
A mitral valve therapy device and method treats dilated cardiomyopathy. The device is configured to be placed in the coronary sinus of a heart adjacent to the mitral valve annulus. The device includes a force distributor that distributes an applied force along a pericardial wall of the coronary sinus, and a force applier that applies the applied force to one or more discrete portions of a wall of the coronary sinus adjacent to the mitral valve annulus to reshape the mitral valve annulus in a localized manner.
Owner:CARDIAC DIMENSIONS
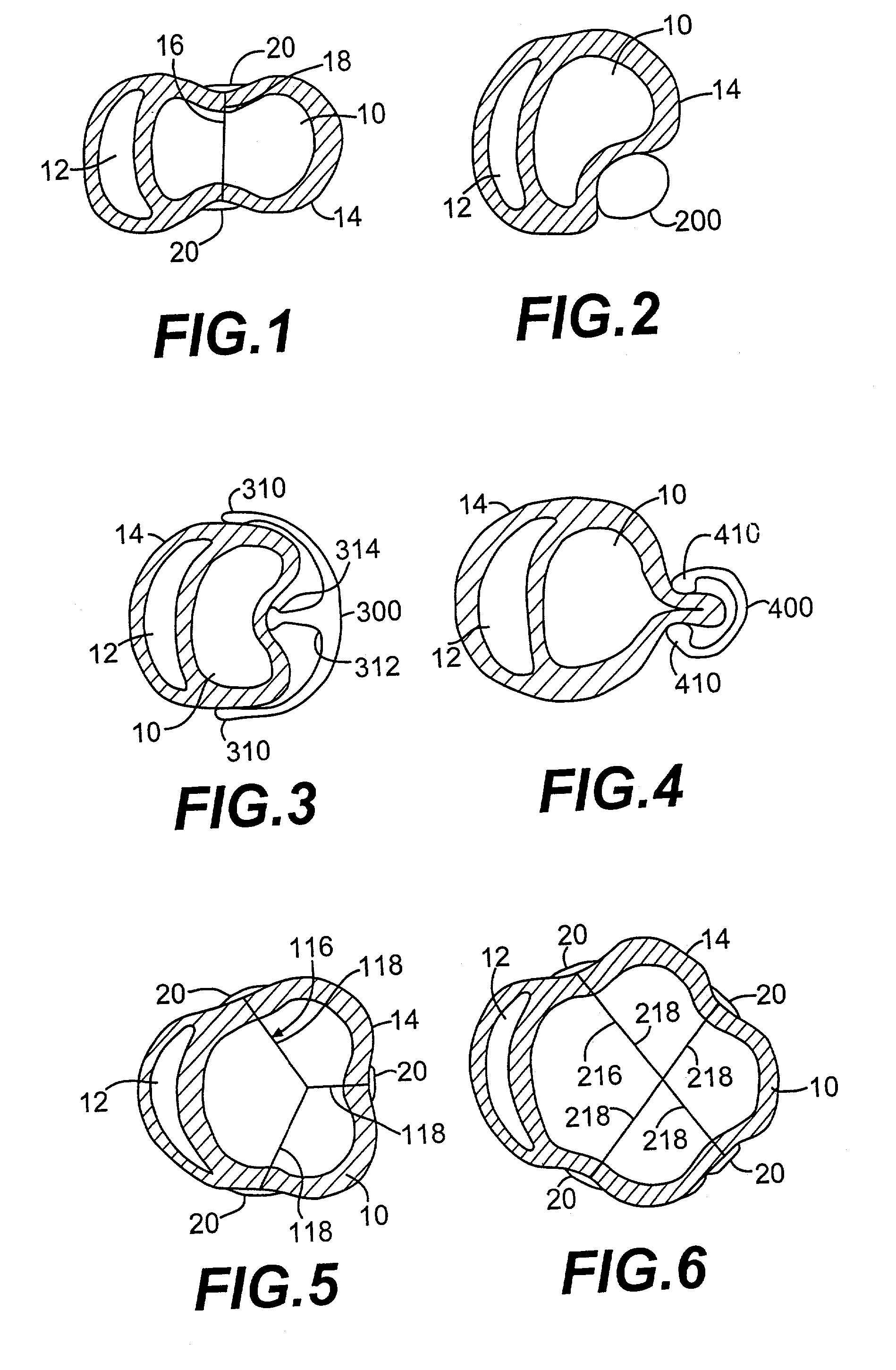
Methods and devices for improving cardiac function in hearts
InactiveUS7189199B2Reduce tensionReduce energy consumptionSuture equipmentsHeart valvesIliac AneurysmWall stress
Various methods and devices are disclosed for improving cardiac function in hearts having zones of infarcted (akinetic) and aneurysmal (dyskinetic) tissue regions. The methods and devices reduce the radius of curvature in walls of the heart proximal infarcted and aneurysmal regions to reduce wall stress and improve pumping efficiency. The inventive methods and related devices include splinting of the chamber wall proximal the infarcted region and various other devices and methods including suture and patch techniques.
Owner:EDWARDS LIFESCIENCES LLC
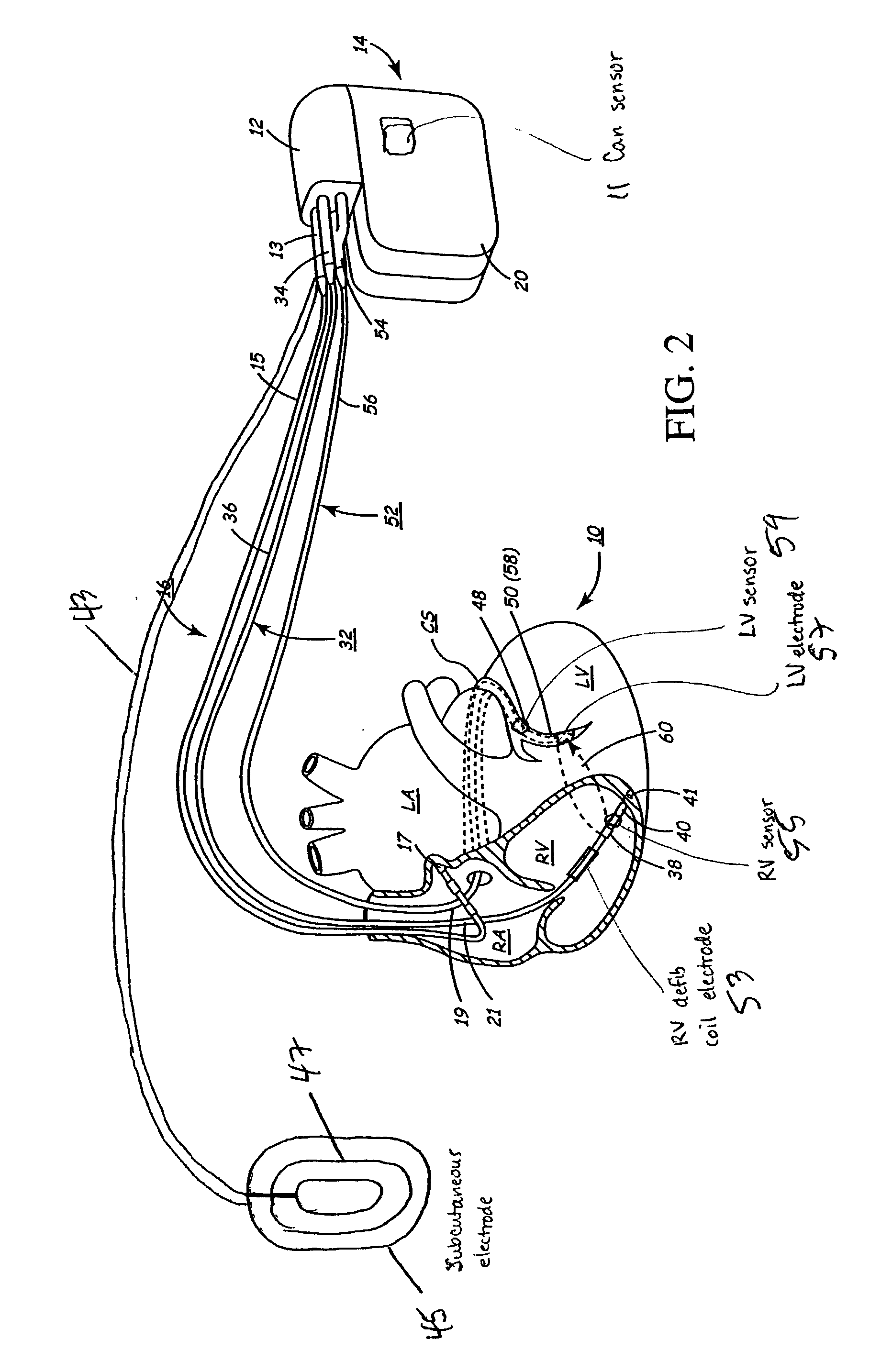
Leadless Cardiac Pacemaker with Secondary Fixation Capability
The invention relates to leadless cardiac pacemakers (LBS), and elements and methods by which they affix to the heart. The invention relates particularly to a secondary fixation of leadless pacemakers which also include a primary fixation. Secondary fixation elements for LBS's may either actively engage an attachment site, or more passively engage structures within a heart chamber. Active secondary fixation elements include a tether extending from the LBS to an anchor at another site. Such sites may be either intracardial or extracardial, as on a vein through which the LBS was conveyed to the heart, the internal or external surface thereof. Passive secondary fixation elements entangle within intraventricular structure such as trabeculae carneae, thereby contributing to fixation of the LBS at the implant site.
Owner:NANOSTIM
Intra-aortic renal drug delivery catheter
InactiveUS7122019B1Reduce the amount requiredDecreased blood flowBalloon catheterMulti-lumen catheterContinuous perfusionBoth kidneys
A catheter for delivering a therapeutic or diagnostic agent to a branch blood vessel of a major blood vessel, generally comprising an elongated shaft having at least one lumen in fluid communication with an agent delivery port in a distal section of the shaft, an expandable tubular member on the distal section of the shaft, and a radially expandable member on the tubular member. The tubular member is configured to extend within the blood vessel up-stream and down-stream of a branch vessel, and has an interior passageway which is radially expandable within the blood vessel to separate blood flow through the blood vessel into an outer blood flow stream exterior to the tubular member and an inner blood flow stream within the interior passageway of the tubular member. The radially expandable member is located down-stream of the shaft agent delivery port, and has an expanded configuration with an outer diameter larger than an outer diameter of the tubular member. The expanded radially expandable member is configured to decrease the blood flow in the outer blood flow stream down-stream of the branch vessel. The catheter provides for delivery of an agent to a branch vessel of a major vessel, and continuous perfusion of the major blood vessel. Another aspect of the invention is directed to methods of delivering a therapeutic or diagnostic agent to one or both kidney's of a patient.
Owner:ANGIODYNAMICS INC
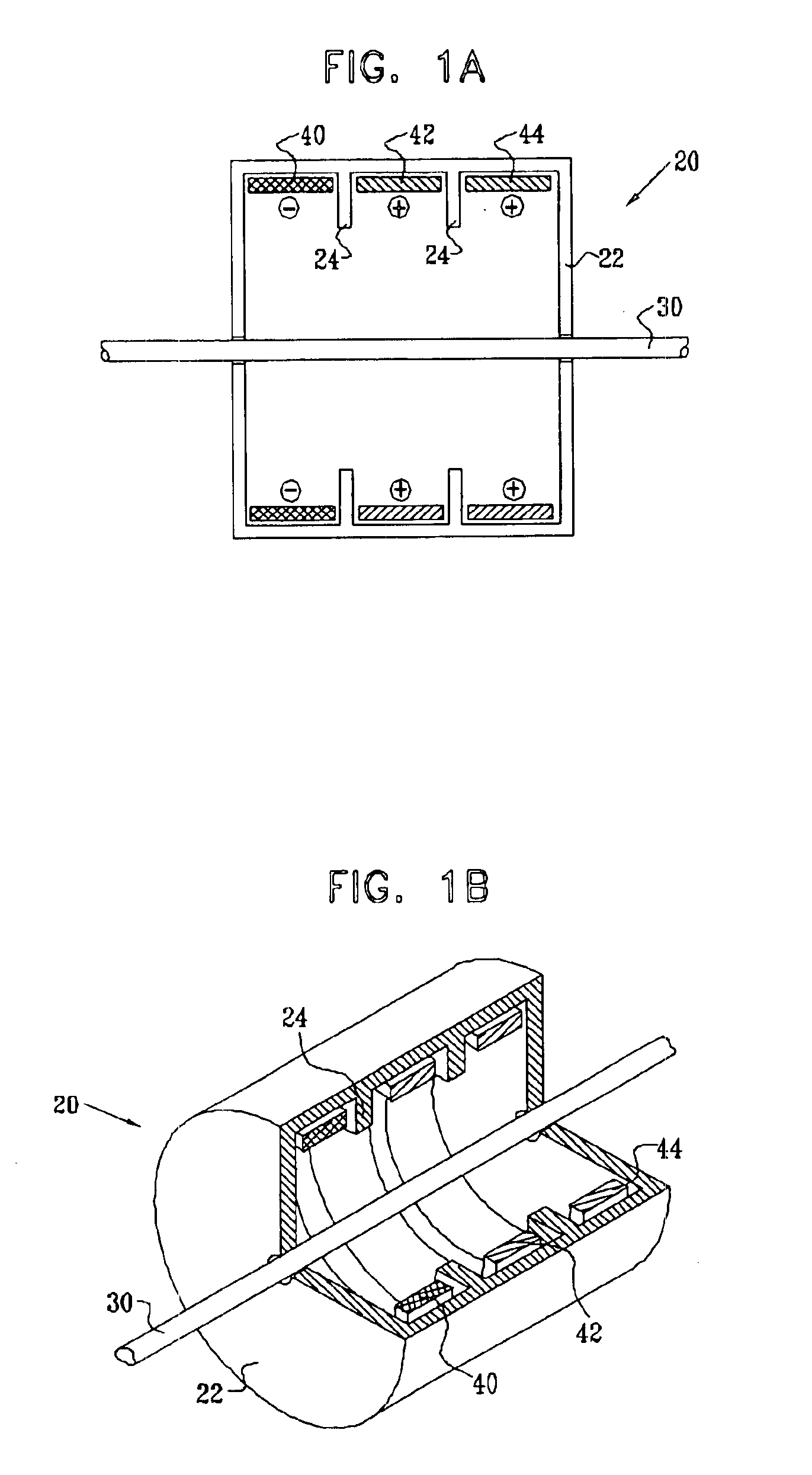
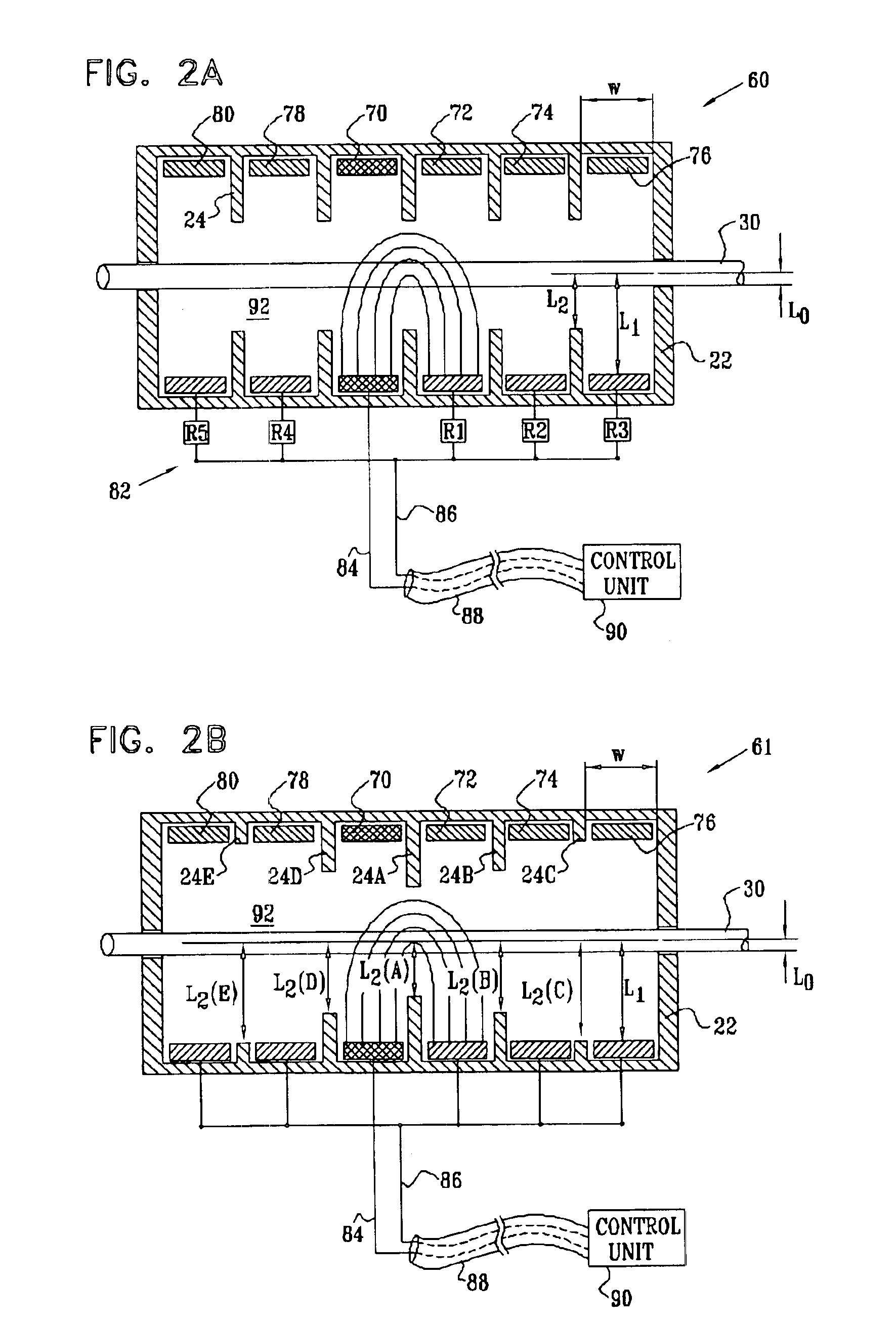
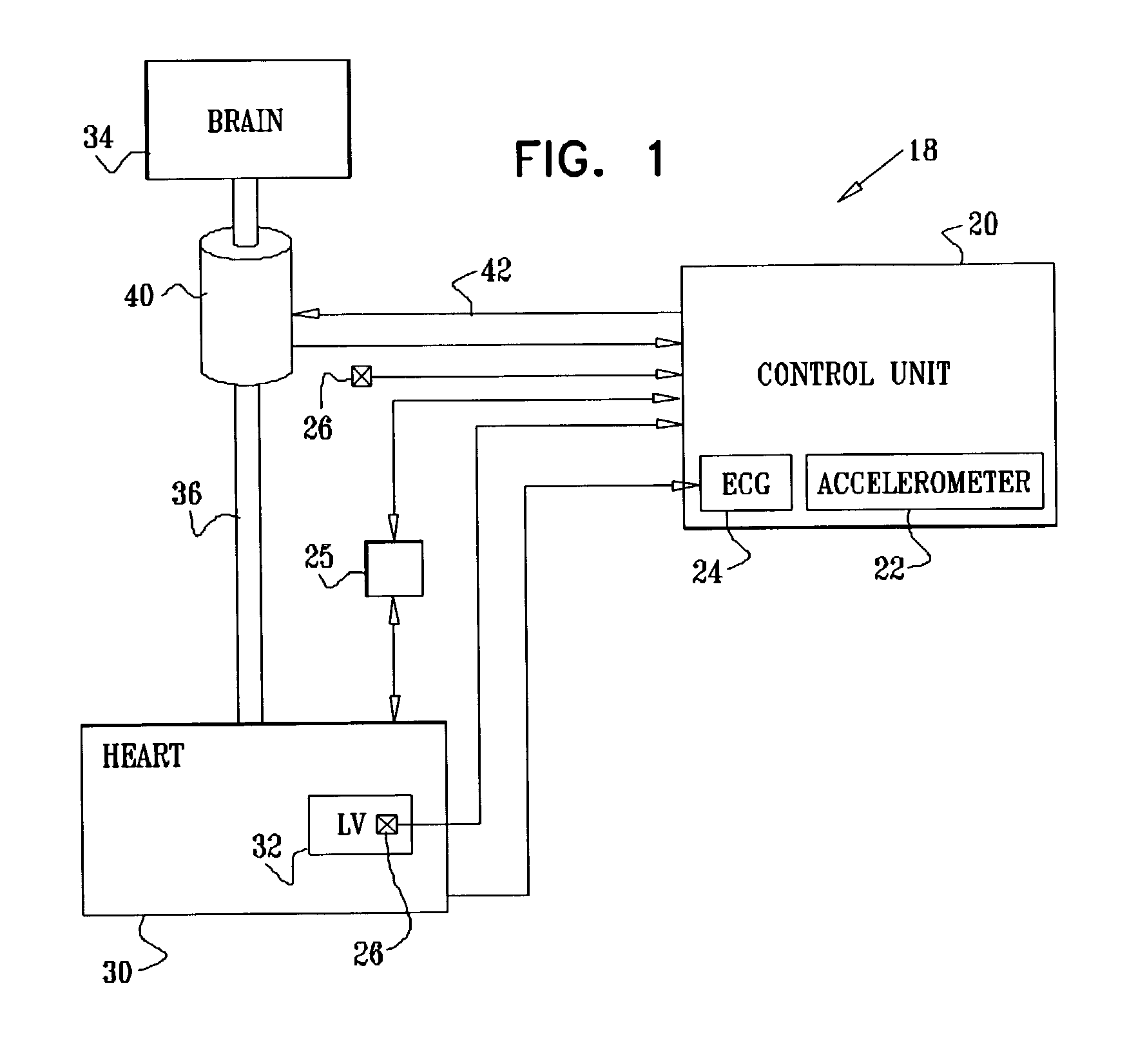
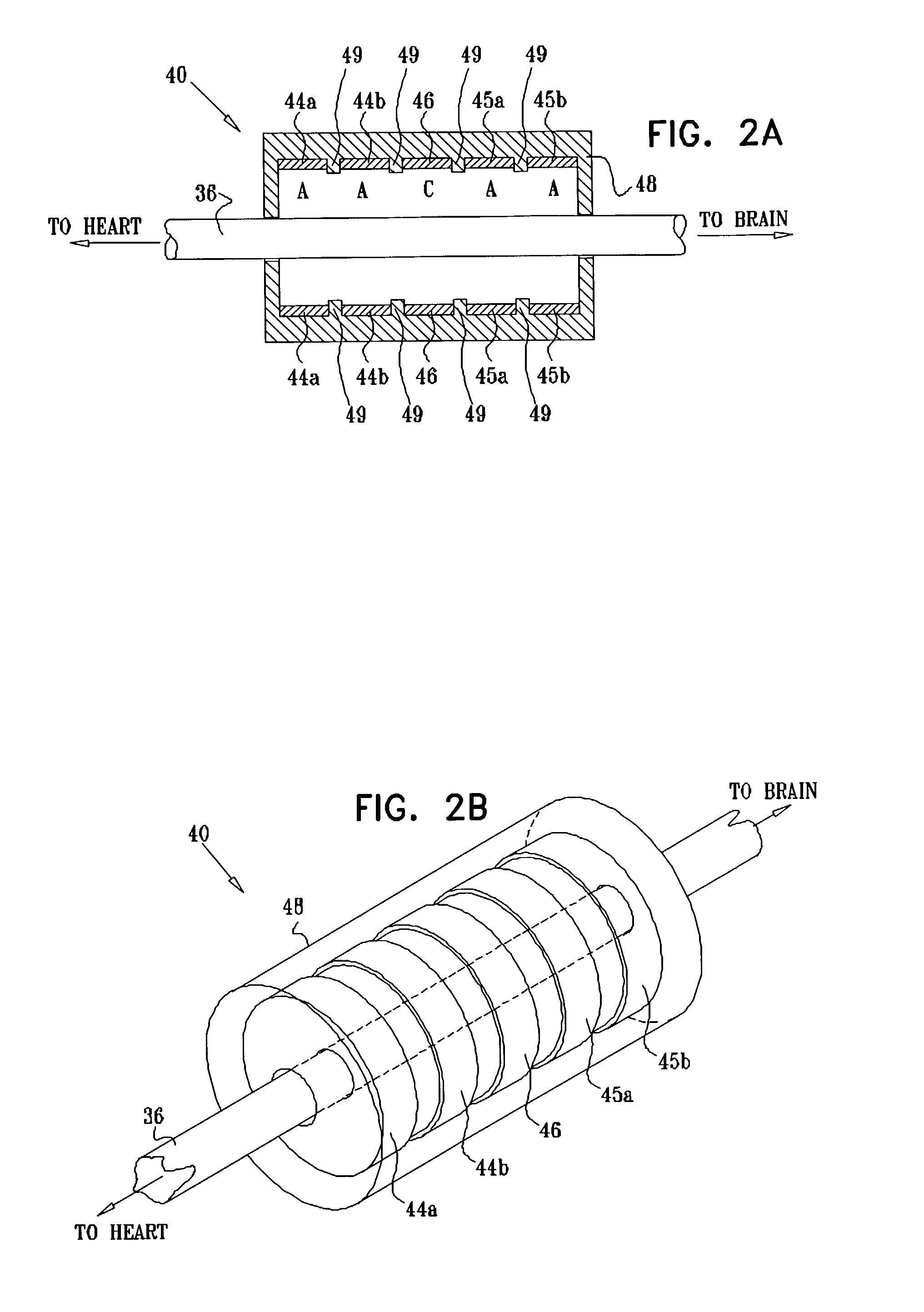
Selective nerve fiber stimulation for treating heart conditions
InactiveUS7778703B2Minimizing adverse side effectRegulate heart rateSpinal electrodesHeart stimulatorsNerve fiber bundleStimulus current
Apparatus for treating a heart condition of a subject is provided, including an electrode device, which is adapted to be coupled to a vagus nerve of the subject. A control unit is adapted to drive the electrode device to apply to the vagus nerve a stimulating current, which is capable of inducing action potentials in a therapeutic direction in a first set and a second set of nerve fibers of the vagus nerve. The control unit is also adapted to drive the electrode device to apply to the vagus nerve an inhibiting current, which is capable of inhibiting the induced action potentials traveling in the therapeutic direction in the second set of nerve fibers, the nerve fibers in the second set having generally larger diameters than the nerve fibers in the first set.
Owner:MEDTRONIC INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com