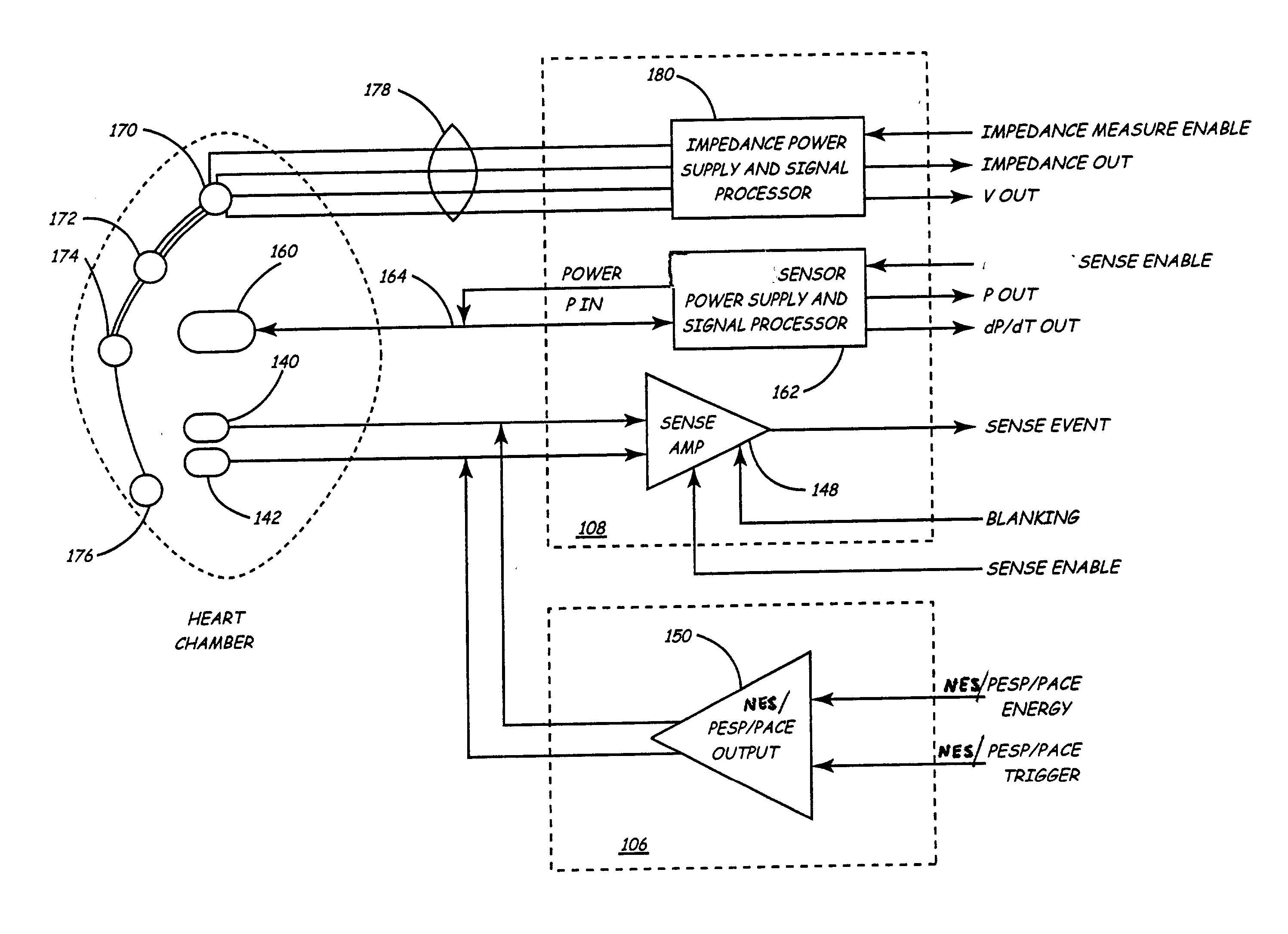
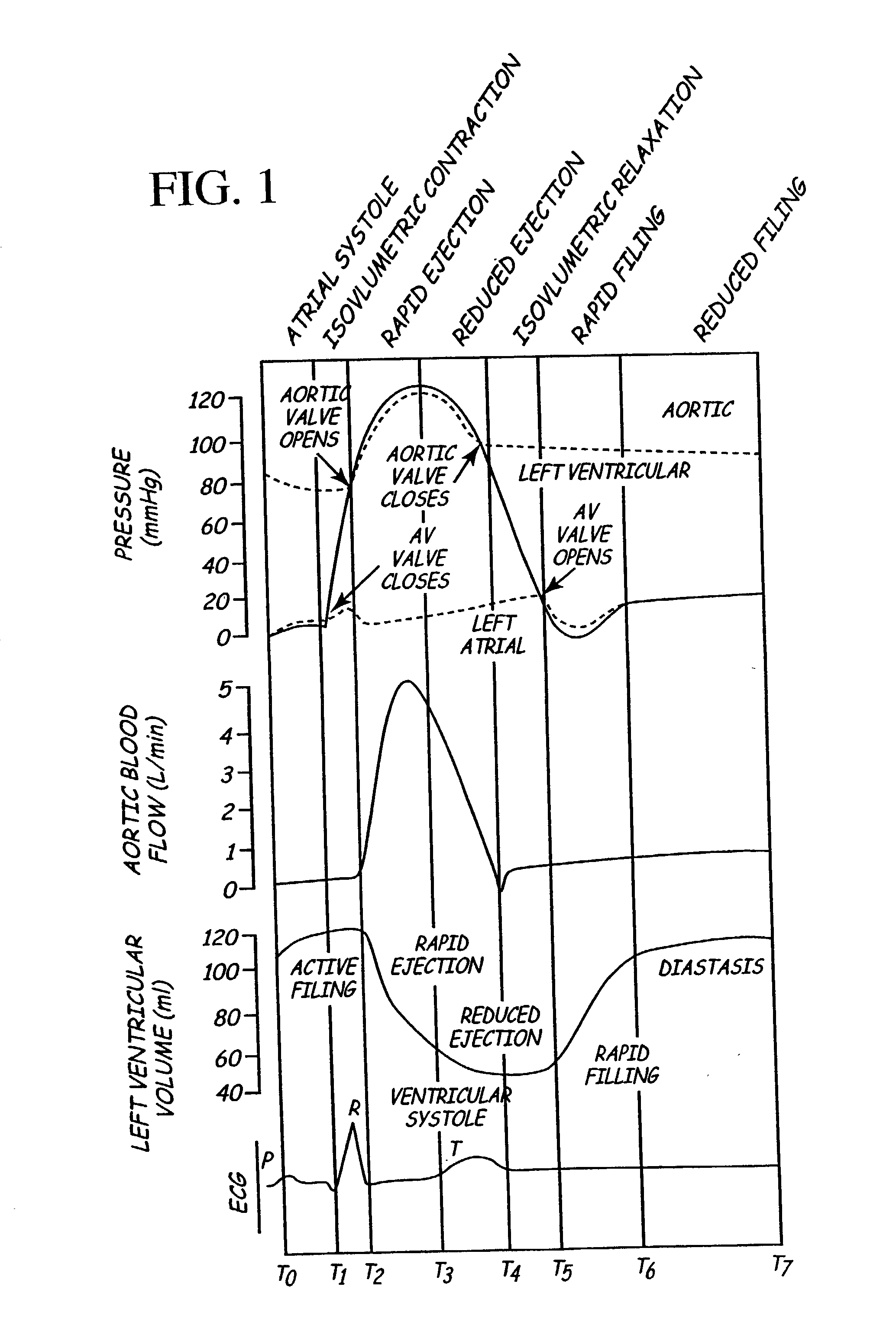
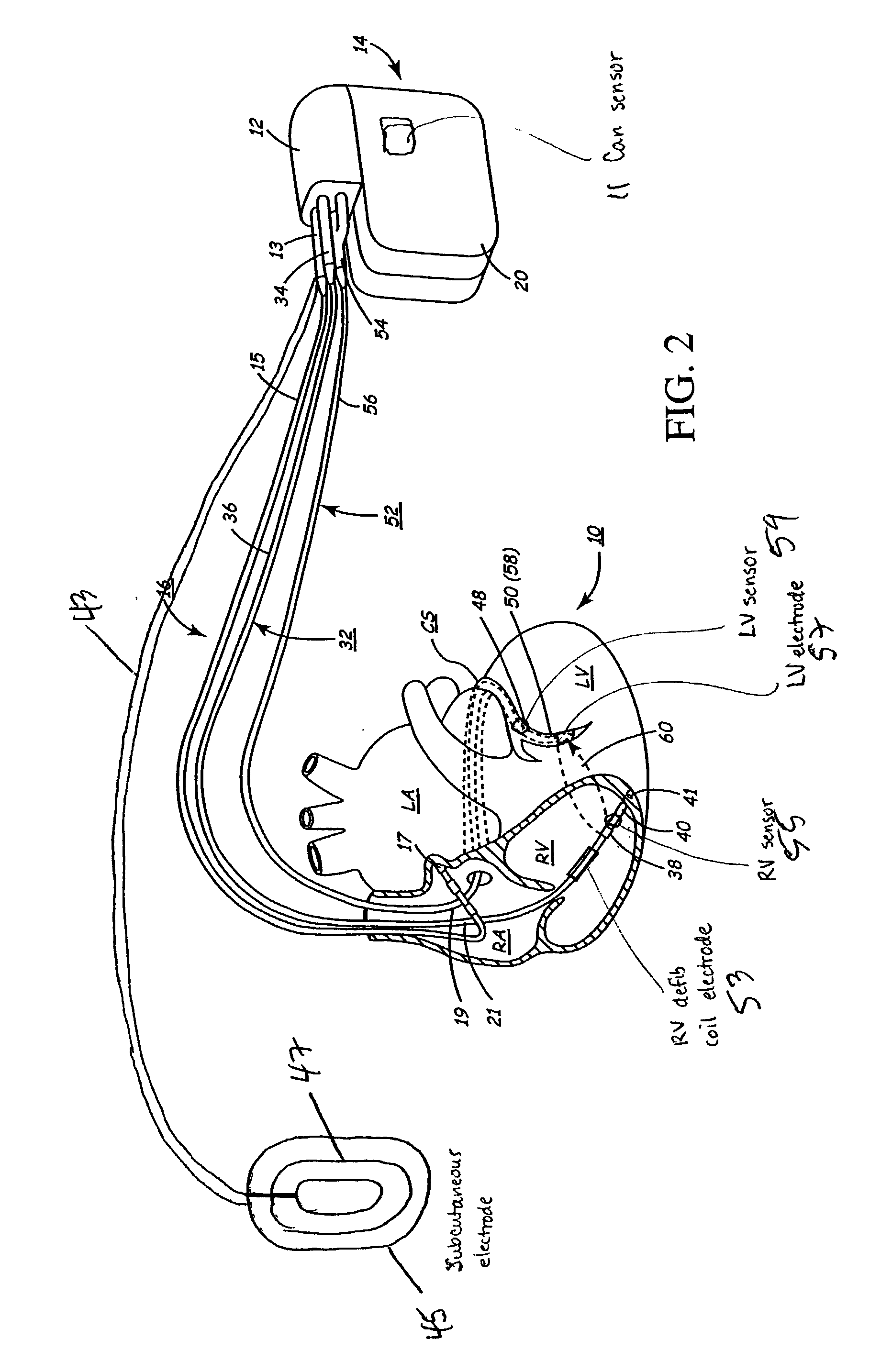
Implantable medical device for treating cardiac mechanical dysfunction by electrical stimulation
a medical device and electrical stimulation technology, applied in the field of implantable medical devices for treating cardiac mechanical dysfunction by electrical stimulation, can solve the problems of cardiac mechanical dysfunction, cardiac arrhythmia, electromechanical dissociation, death, systemic side effects and time-consuming involvement of skilled clinicians, and large release of calcium from the sr. , to achieve the effect of minimizing the potential risk of shock-induced myocardial damage and high detection specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
[0244] ICD Example with Presentation of VT
[0245] ICD systems provides patients with greatly improved survivability from episodes of sudden cardiac arrest when compared to patients treated with AED's mainly because there is minimal time to wait between the onset of the arrhythmia and delivery of therapy when the device is implanted and always ready to detect events. However, some patients, especially those with more pronounced HF, may not tolerate well even the shortest of VF episodes and may have depressed cardiac function long after the arrhythmia is terminated. Additionally, circumstances may arise that lengthen the duration of the tachyarrhythmia before the device delivers a therapy. Some tachyarrhythmias pose detection problems for ICD's, which may postpone delivery of therapy. An arrhythmia could also require several shocks to terminate, further prolonging the episode.
[0246] During a tachyarrhythmia, the coronary blood flow perfusing the heart can become severely impaired, lead...
example 3
[0248] HF Example with Presentation of Acute Decompensation
[0249] Advanced stage HF patients experience sudden worsening of heart failure associated symptoms which require hospitalization. The transition from chronic compensated HF to acute decompensated HF may result from a number of factors including dietary indiscretion, progress of HF disease, and acute myocardial infarction. When severe, symptoms may progress in a few hours to a stage where these patients need to be admitted to a critical care hospital bed, monitored by physiologic sensors, and treated with a variety of drugs including intravenous inotropes. A patient experiencing such a decompensation commonly exhibits low cardiac output at rest, poor contractile function and low dP / dt max, slow relaxation and high tau, elevated diastolic ventricular pressures, and reduced ventricular developed pressures.
[0250] Cardiac resynchronization therapy delivered by an implanted device is an important adjunct to good medical therapy. S...
example 4
[0253] SVT Example with Poor Toleration of High Rate
[0254] Supraventricular tachycardias that result in rapid ventricular rates may be poorly tolerated, particularly in patients with a history of heart failure. In this scenario the patient experiences first symptoms of dizziness and palpitations (a sensation of a fluttering within the chest). Upon evaluation by emergency medical personnel, the heart rate is found to be 220 bpm. Over the next few minutes, the patient's blood pressure drops, and the patient becomes pale, sweaty and confused. An AED device instrumented with NES and PESP therapies as described in this invention is attached to the patient by a pair of adhesive pad electrodes.
[0255] The fast but narrow ECG complexes allow the device to diagnose a serious SVT and the operator is presented with the option of a trial of PESP stimulation or cardioversion. After administering a sedative / analgesic, a 5 minute trial of PESP stimulation is begun by delivering 20 ms pulses of 60 m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com