Patents
Literature
870 results about "Functional disorder" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A functional disorder is a medical condition that impairs normal functioning of bodily processes that remains largely undetected under examination, dissection or even under a microscope. At the exterior, there is no appearance of abnormality. This stands in contrast to a structural disorder (in which some part of the body can be seen to be abnormal) or a psychosomatic disorder (in which symptoms are caused by psychological or psychiatric illness). Definitions vary somewhat between fields of medicine.
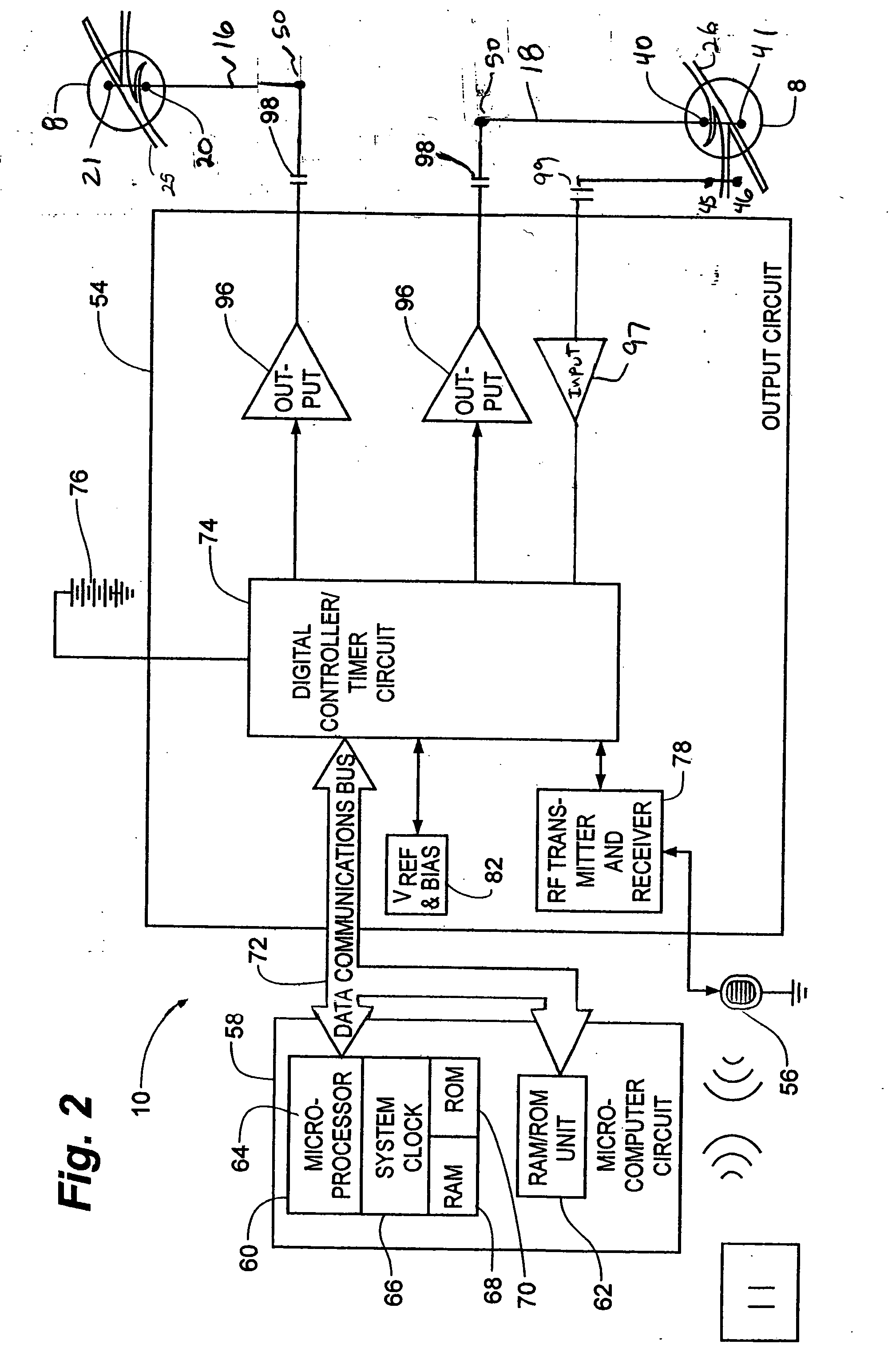
Implantable medical device for treating cardiac mechanical dysfunction by electrical stimulation
InactiveUS20040049235A1Increase the number ofHigh detection specificityHeart defibrillatorsHeart stimulatorsCardiac dysfunctionElectrical stimulations
The above-described methods and apparatus are believed to be of particular benefit for patients suffering heart failure including cardiac dysfunction, chronic HF, and the like and all variants as described herein and including those known to those of skill in the art to which the invention is directed. It will understood that the present invention offers the possibility of monitoring and therapy of a wide variety of acute and chronic cardiac dysfunctions. The current invention provides systems and methods for delivering therapy for cardiac hemodynamic dysfunction.
Owner:MEDTRONIC INC
Application of lipid vehicles and use for drug delivery
InactiveUS7063860B2Reduce and prevent antibody-mediated resistanceIncrease stimulationBiocideAntipyreticAnticarcinogenCapsaicin
The present invention relates to compositions and methods for the administration of lipid-based vehicles to treat various disorders, including bladder inflammation, infection, dysfunction, and cancer. In various aspects, the compositions and methods of the invention are useful for prolonged delivery of drugs, e.g., antibiotics, pain treatments, and anticancer agents, to the bladder, genitourinary tract, gastrointestinal system, pulmonary system, and other organs or body systems. In particular, the present invention relates to liposome-based delivery of vanilloid compounds, such as resiniferatoxin, capsaicin, or tinyatoxin, and toxins, such as botulinum toxin, for the treatment of bladder conditions, including pain, inflammation, incontinence, and voiding dysfunction. Further related are methods of using these vehicles alone or in conjunction with antibodies, e.g., uroplakin antibodies, to improve duration of liposome attachment, and provide a long-term intravesical drug delivery platform. The present invention specifically relates to antibody-coated liposomes that are useful for targeting specific receptors for drug, peptide, polypeptide, or nucleic acid delivery. In one particular aspect, the present invention relates to liposomes coated with antibodies against nerve growth factor (NGF) receptor and containing NGF antisense nucleic acids, which are used as a treatment for neurogenic bladder dysfunction.
Owner:UNIVERSITY OF PITTSBURGH
Implantable medical device for treating cardiac mechanical dysfunction by electrical stimulation
InactiveUS7096064B2Improve toleranceExtension of timeHeart defibrillatorsHeart stimulatorsCardiac dysfunctionElectrical stimulations
The disclosure provides methods and apparatus of particular benefit for patients suffering heart failure including cardiac dysfunction, chronic HF, and the like and all variants thereof. According to the disclosure monitoring and therapy delivery for a wide variety of acute and chronic cardiac dysfunctions are described and depicted. Various forms of paired or coupled pacing therapy delivery provided alone or in combination with neurostimulation therapy delivered by both implantable and external apparatus, including defibrillation therapy are also provided herein.
Owner:MEDTRONIC INC
Methods of using and compositions comprising (+) sibutramine optionally in combination with other pharmacologically active compounds
This invention encompasses methods for the treatment and prevention of disorders that include, but are not limited to, eating disorders; weight gain; obesity; irritable bowel syndrome; obsessive-compulsive disorders; platelet adhesion; apnea; affective disorders such as attention deficit disorders, depression, and anxiety; male and female sexual function disorders; restless leg syndrome; osteoarthritis; substance abuse including nicotine and cocaine addiction; narcolepsy; pain such as neuropathic pain, diabetic neuropathy, and chronic pain; migraines; cerebral function disorders; chronic disorders such as premenstrual syndrome; and incontinence. The invention further encompasses pharmaceutical compositions and dosage forms which comprise optically pure (+) sibutramine, optionally in combination with a phosphodiesterase inhibitor or a lipase inhibitor.
Owner:SEPACOR INC
Implantable medical device for treating cardiac mechanical dysfunction by electrical stimulation
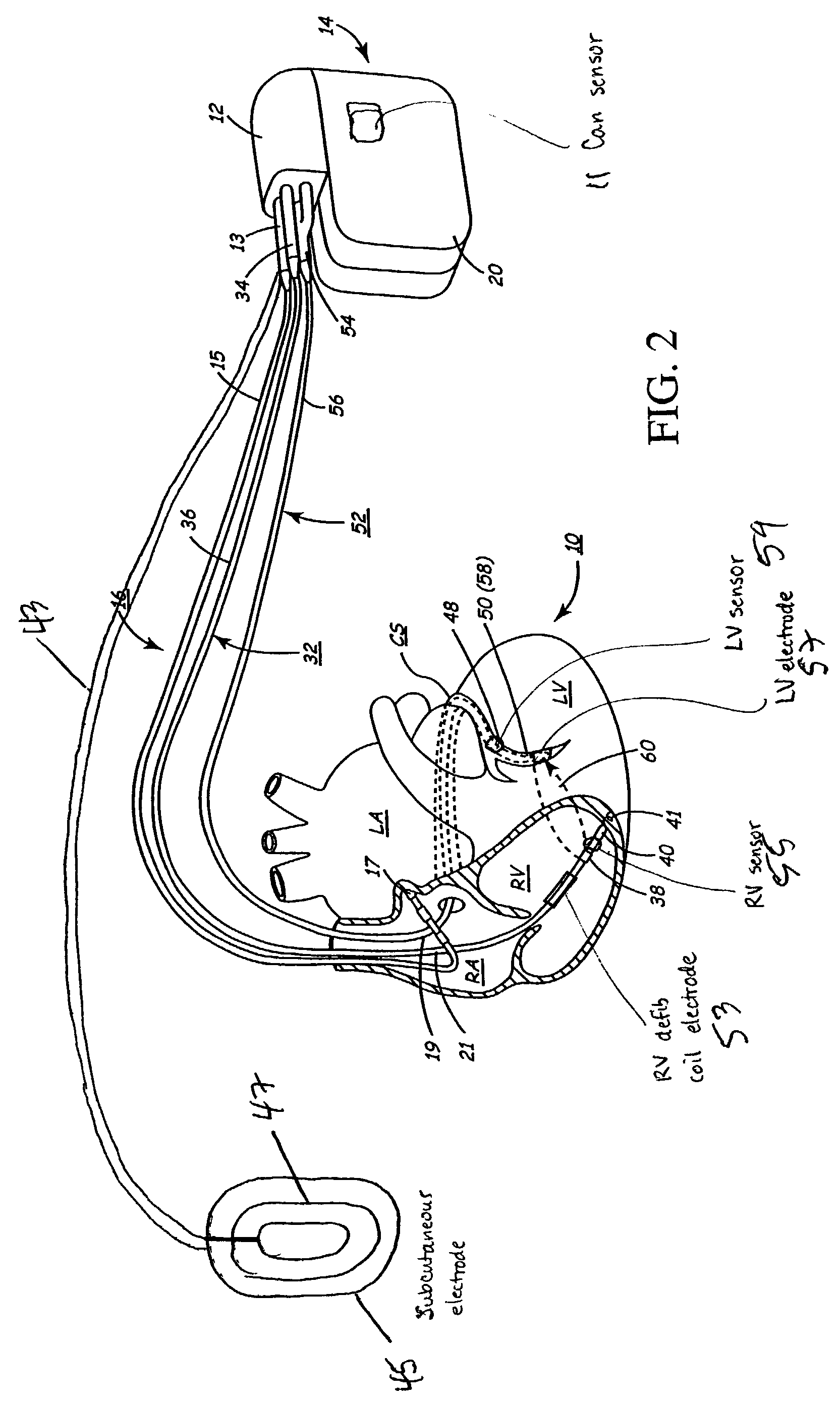
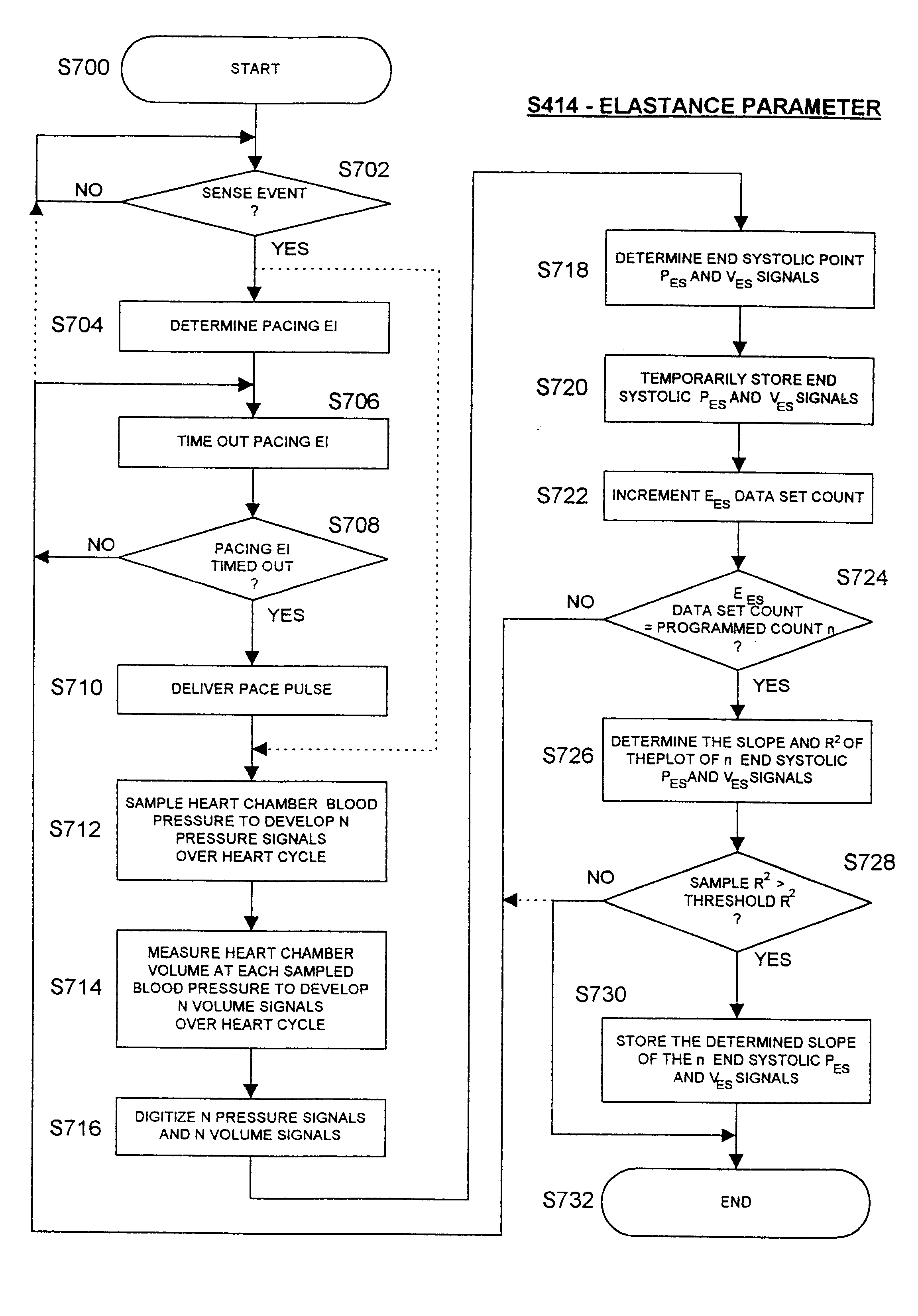
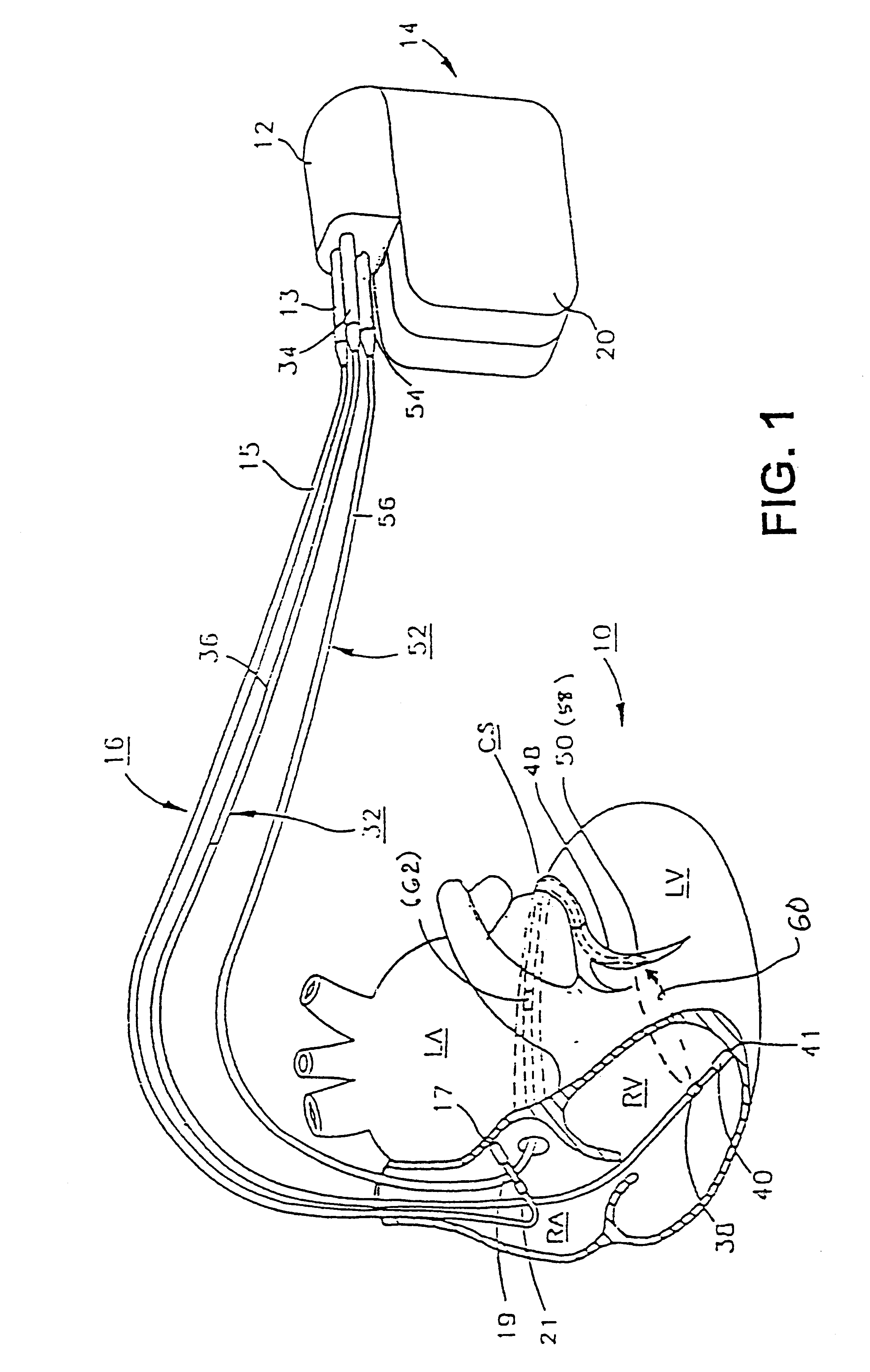
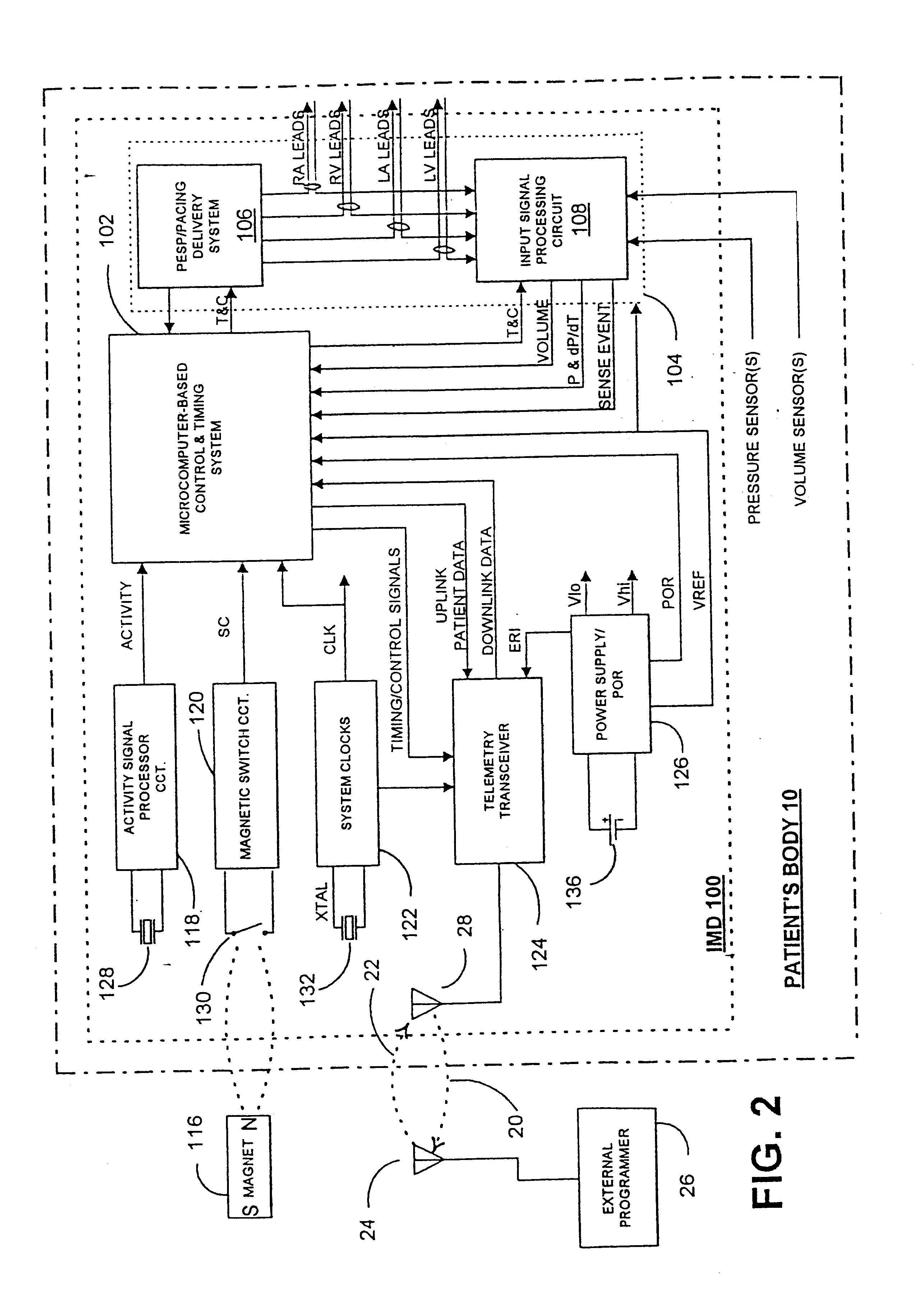
InactiveUS6738667B2Increase contractilityEasy to relaxCatheterHeart stimulatorsCardiac cycleHeart chamber
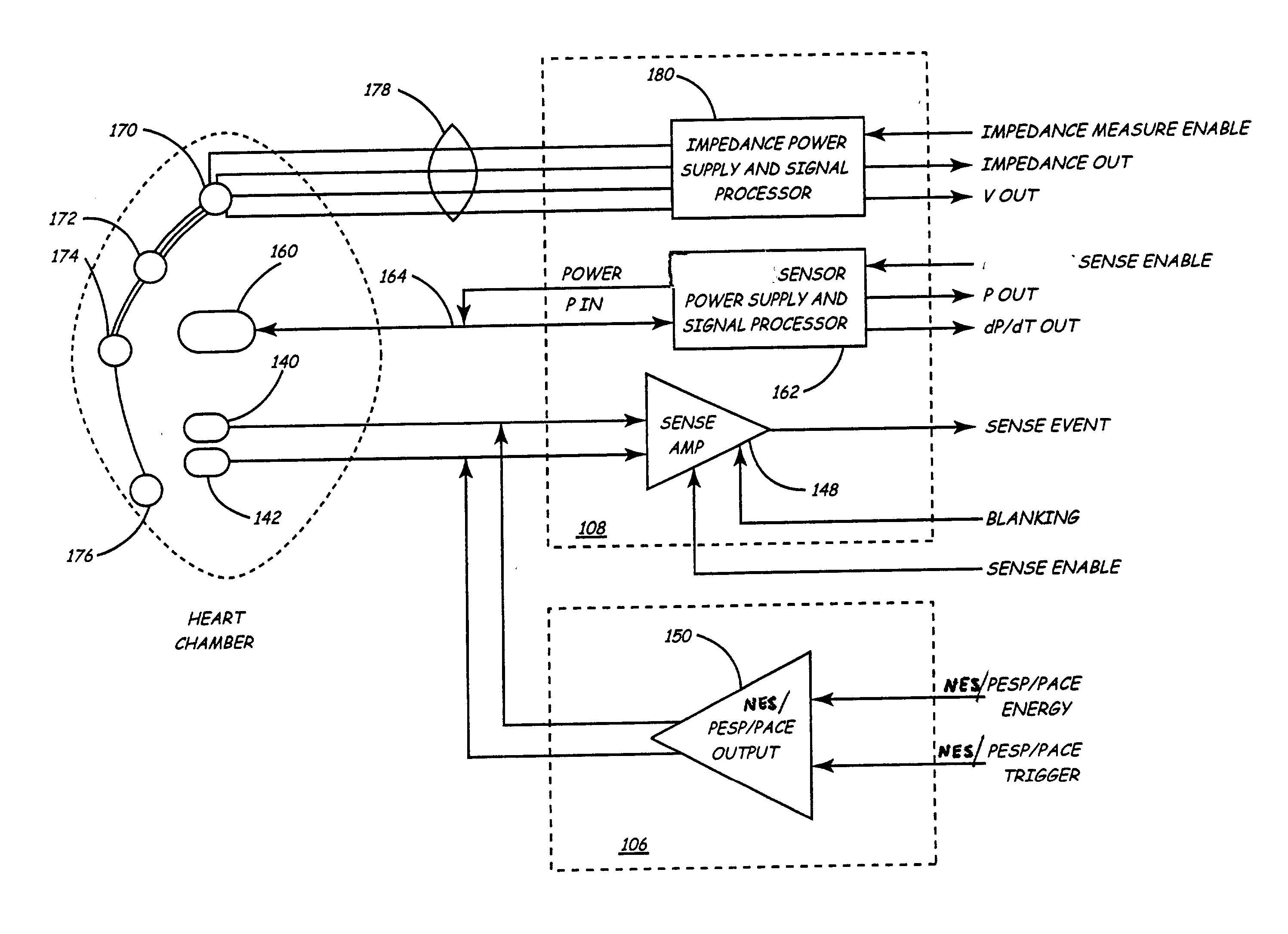
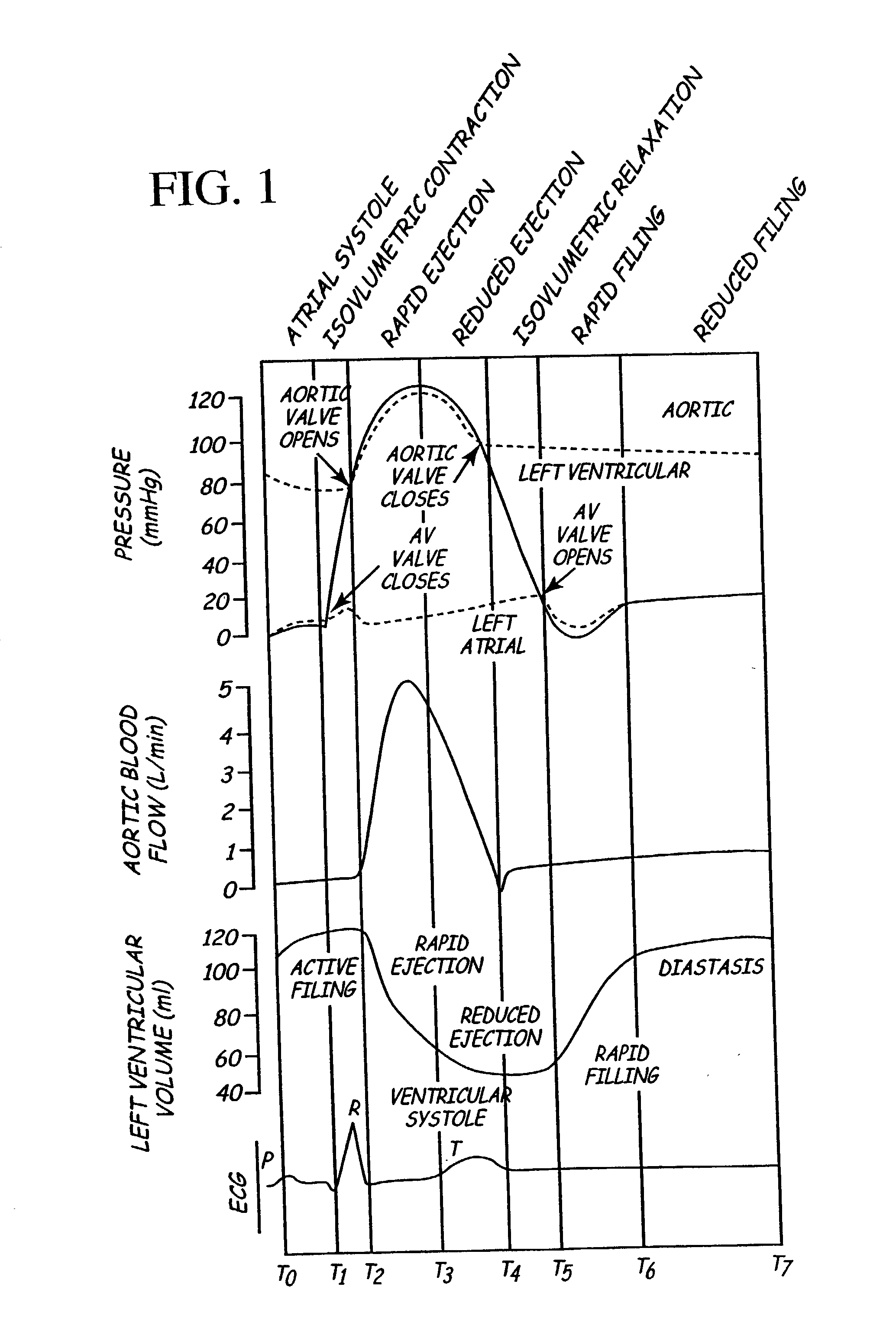
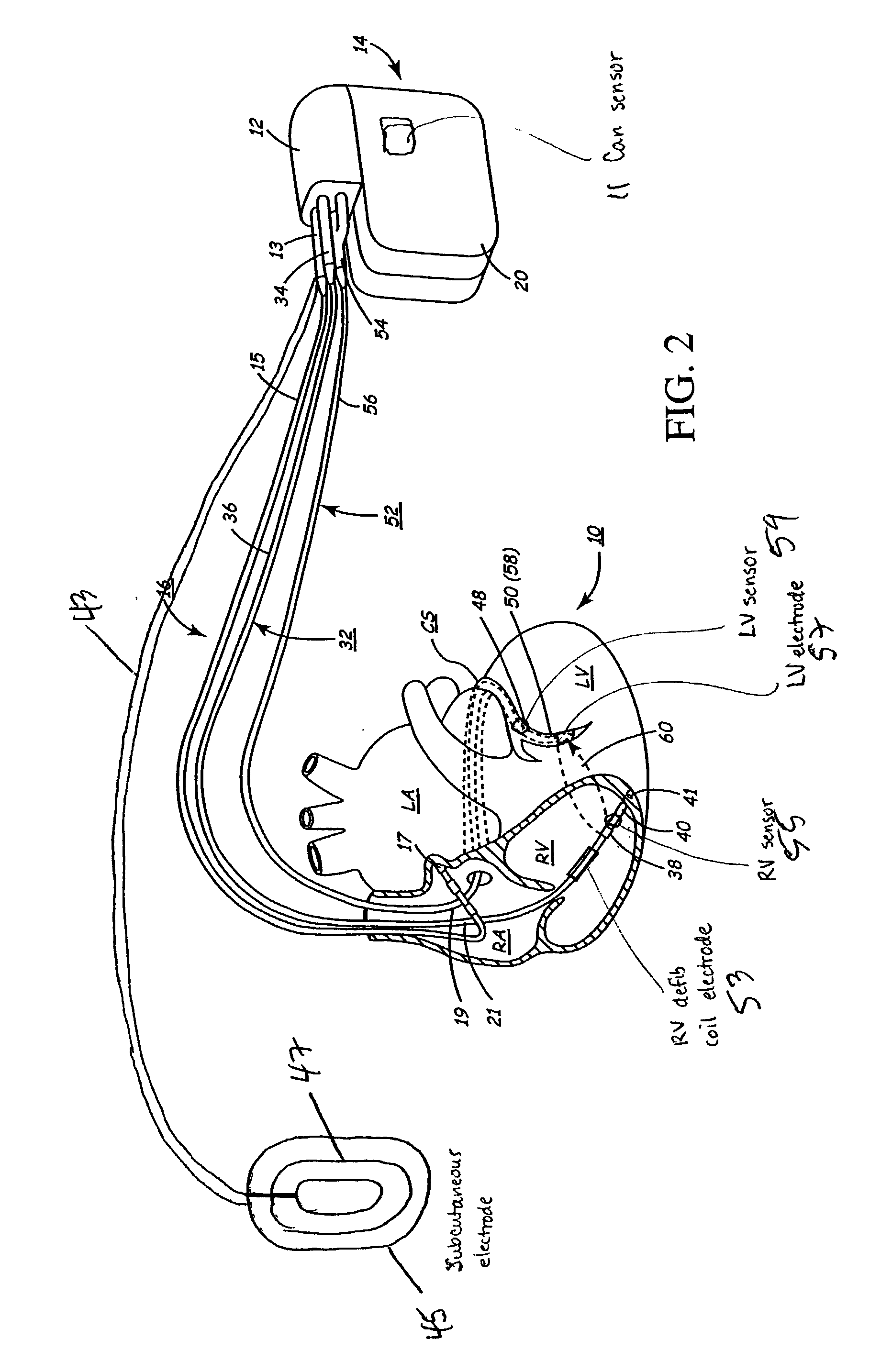
An implantable stimulator and monitor measures a group of heart failure parameters indicative of the state of heart failure employing EGM signals, measures of blood pressure including absolute pressure P, developed pressure (DP=systolic P-diastolic P), and / or dP / dt, and measures of heart chamber volume (V) over one or more cardiac cycles. These parameters include: (1) relaxation or contraction time constant tau (.tau.); (2) mechanical restitution (MR), i.e., the mechanical response of a heart chamber to premature stimuli applied to the heart chamber; (3) recirculation fraction (RF), i.e., the rate of decay of PESP effects over a series of heart cycles; and (4) end systolic elastance (E.sub.ES), i.e., the ratios of end systolic blood pressure P to volume V. These heart failure parameters are determined periodically regardless of patient posture and activity level. The physician can determine whether a particular therapy is appropriate, prescribe the therapy for a period of time while again accumulating the stored patient data for a later review and assessment to determine whether the applied therapy is beneficial or not, thereby enabling periodic changes in therapy, if appropriate. Drug therapies and electrical stimulation therapies, including PESP stimulation, and pacing therapies including single chamber, dual chamber and multi-chamber (bi-atrial and / or bi-ventricular) pacing can be delivered. In patient's prone to malignant tachyarrhythmias, the assessment of heart failure state can be taken into account in setting parameters of detection or classification of tachyarrhythmias and the therapies that are delivered.
Owner:MEDTRONIC INC
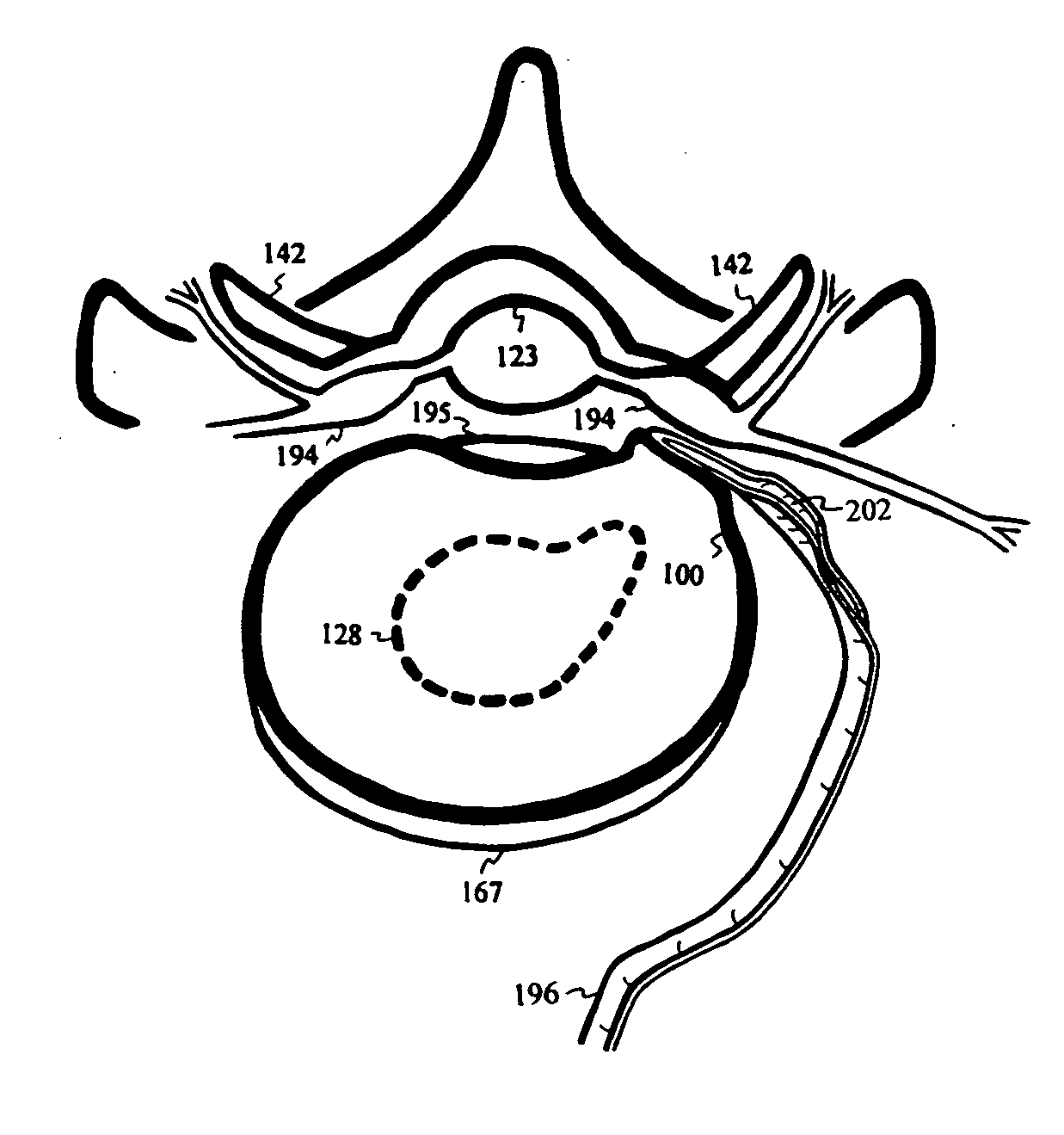
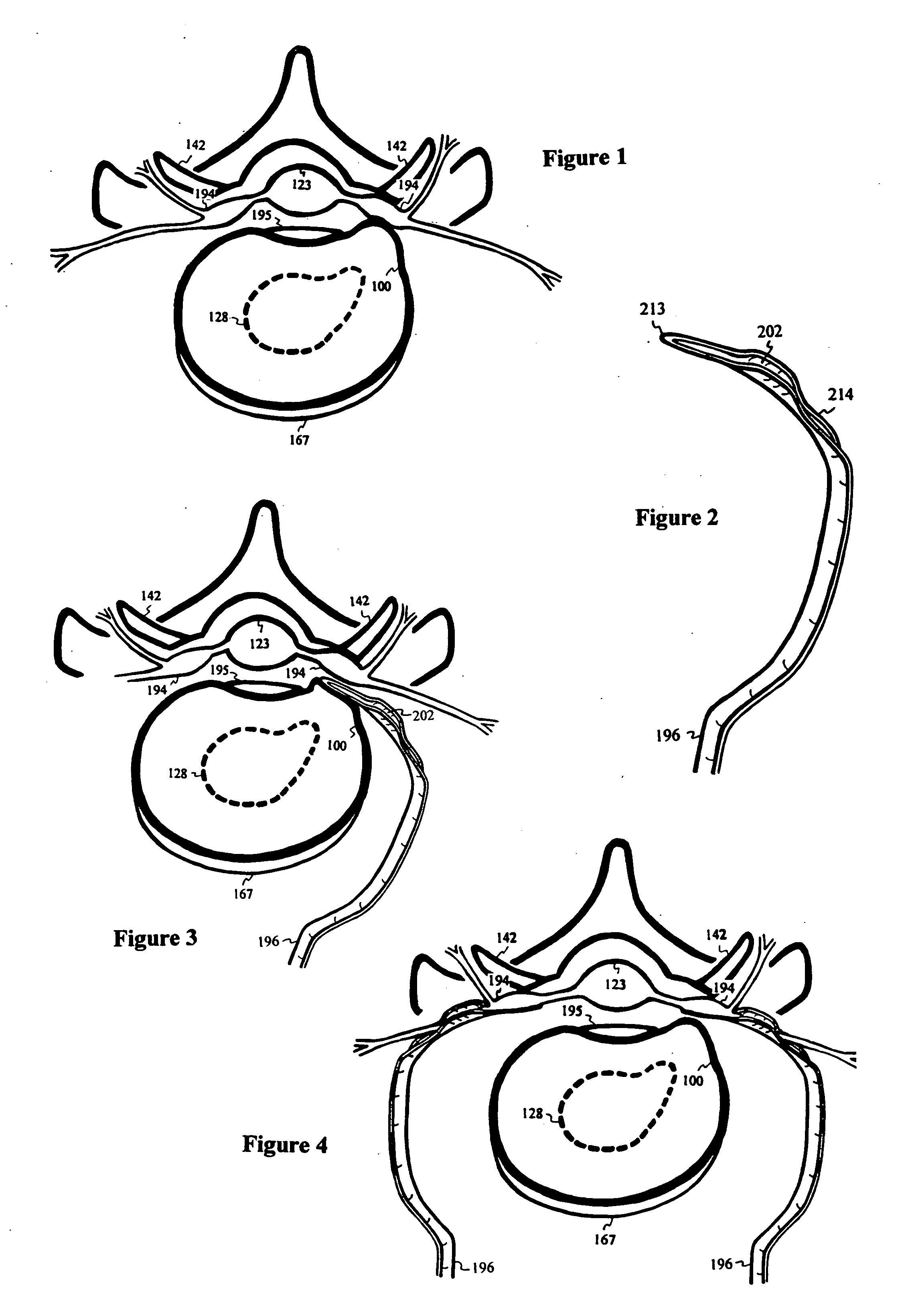
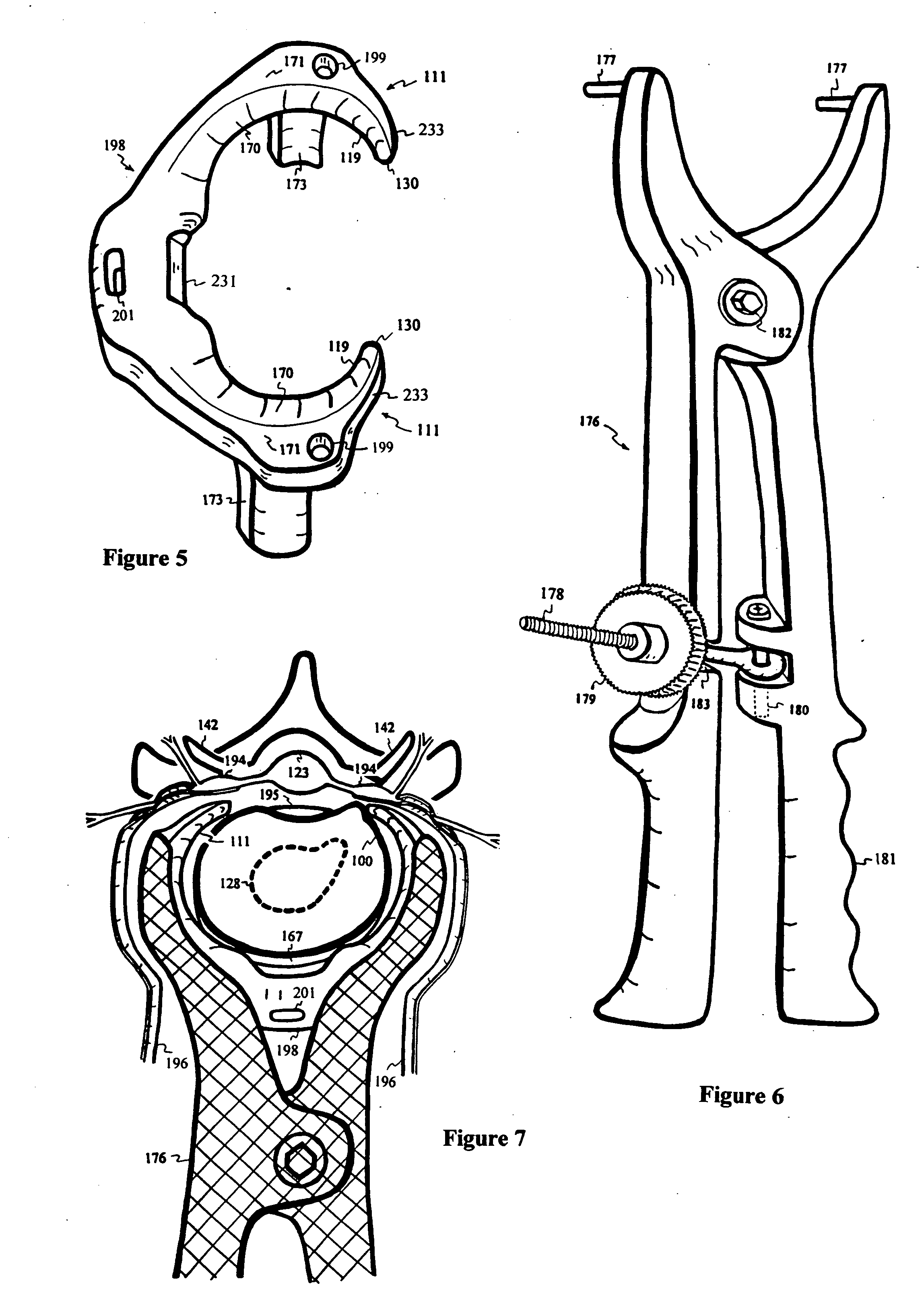
Intervertebral disc repair
InactiveUS20040097927A1Alleviate nerve impingementMinimize segmental instabilitySuture equipmentsInternal osteosythesisAtrophySpinal stenosis
A saddle-shaped compression device and methods of fastening a dysfunctional intervertebral disc are used to (1) compress a protrusion to alleviate nerve impingement, (2) fortify the annulus to stabilize a motion segment, (3) minimize the inward / outward bulging and delamination of the annulus, (4) atrophy the nerve to treat discogenic pain, (5) correct the curvature of spinal deformities, (6) elevate the disc space to treat spinal stenosis, and (7) seal the seepage of nucleus pulposus from a herniated disc.
Owner:YEUNG JEFFREY E +1
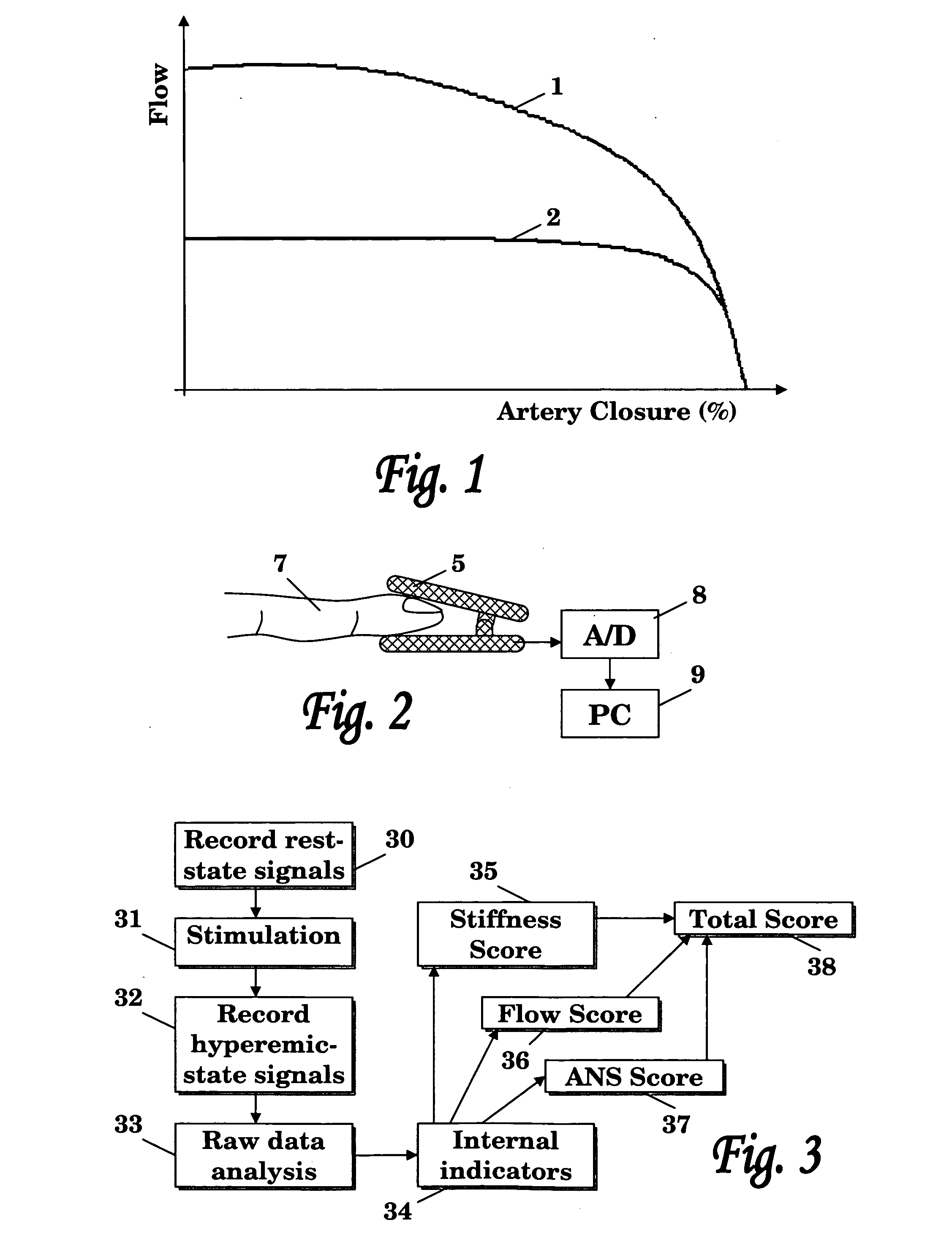
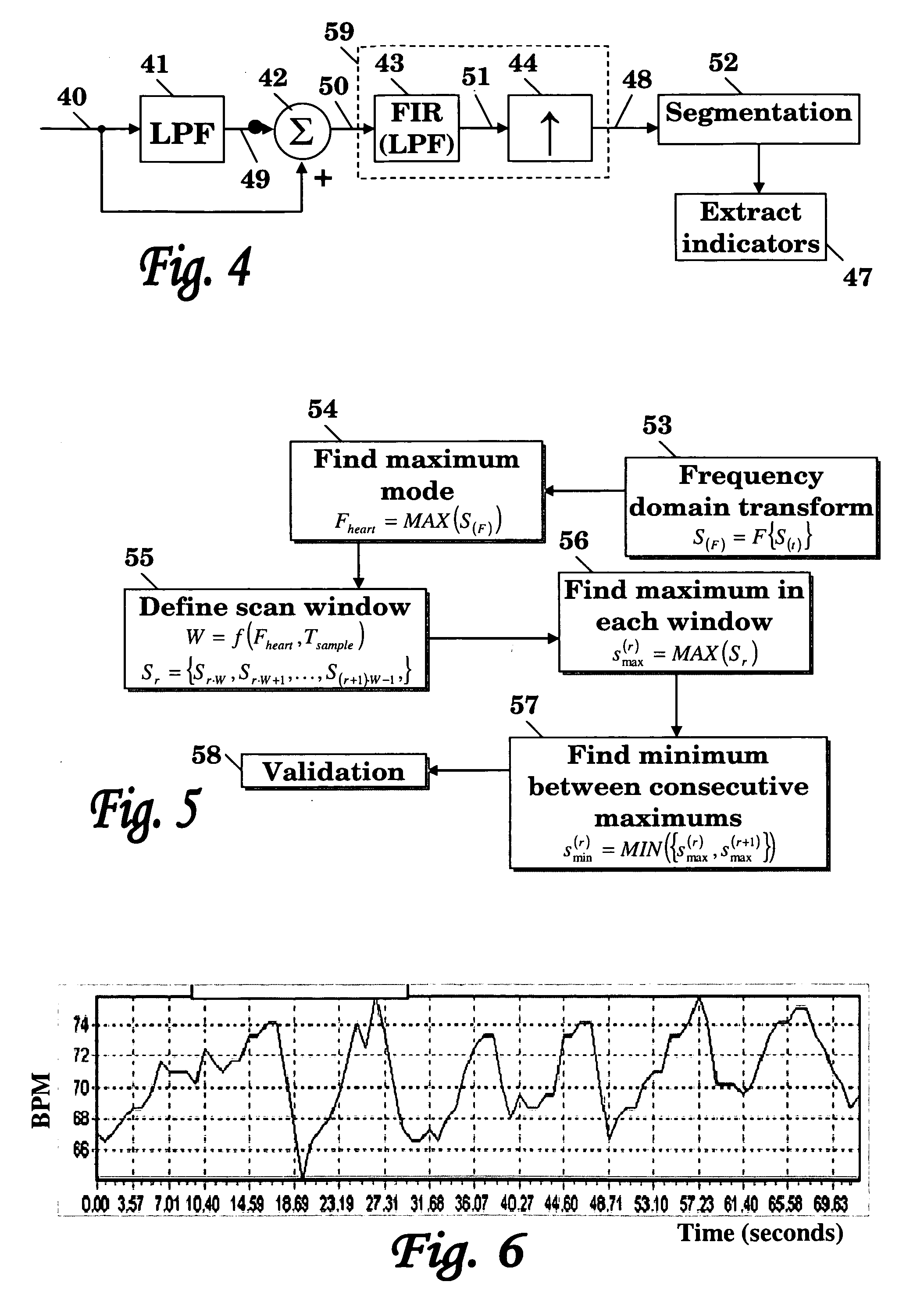
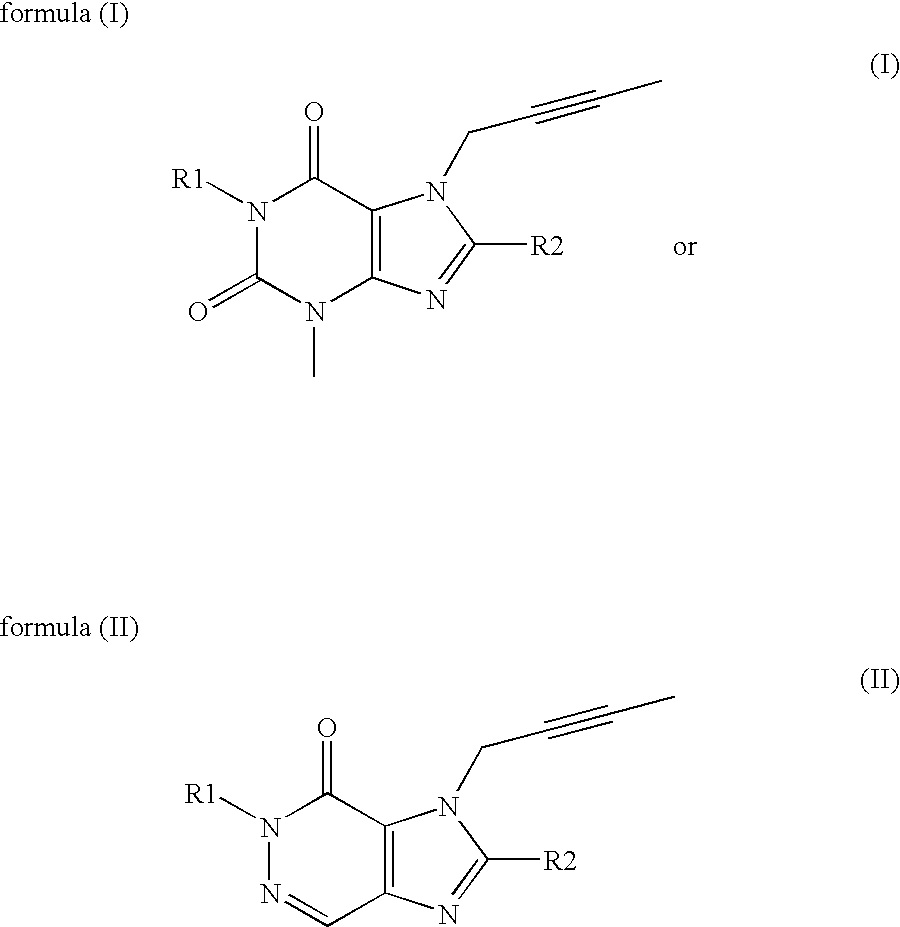
Method and system for cardiovascular system diagnosis
InactiveUS20070021673A1Monitoring function and/or diagnosing dysfunction of the cardiovascular systemCatheterDiagnostic recording/measuringControlled breathingPulse wave
The present invention is directed to a method and system for monitoring function and / or diagnosing dysfunction of the cardiovascular system of a human subject. The method comprise measuring pulse wave signals of the subject during rapid excitation of the cardiovascular system, analyzing the measured signals and computing indicators reflecting a response to said excitation. The cardiovascular excitation preferably comprise a controlled breathing protocol characterized by a predefined frequency of breaths (e.g., about 0.1 Hz).
Owner:SPIROCOR LTD
Uses of dpp-iv inhibitors
ActiveUS20070281940A1Reduced ejection outputReduced ejection volumeBiocideAntipyreticCombined usePatient group
The specification describes the use of selected DPP IV inhibitors for the treatment of physiological functional disorders and for reducing the risk of the occurrence of such functional disorders in at-risk patient groups. In addition, the use of the above-mentioned DPP IV inhibitors in conjunction with other active substances is described, by means of which improved treatment outcomes can be achieved. These applications may be used to prepare corresponding medicaments.
Owner:BOEHRINGER INGELHEIM INT GMBH
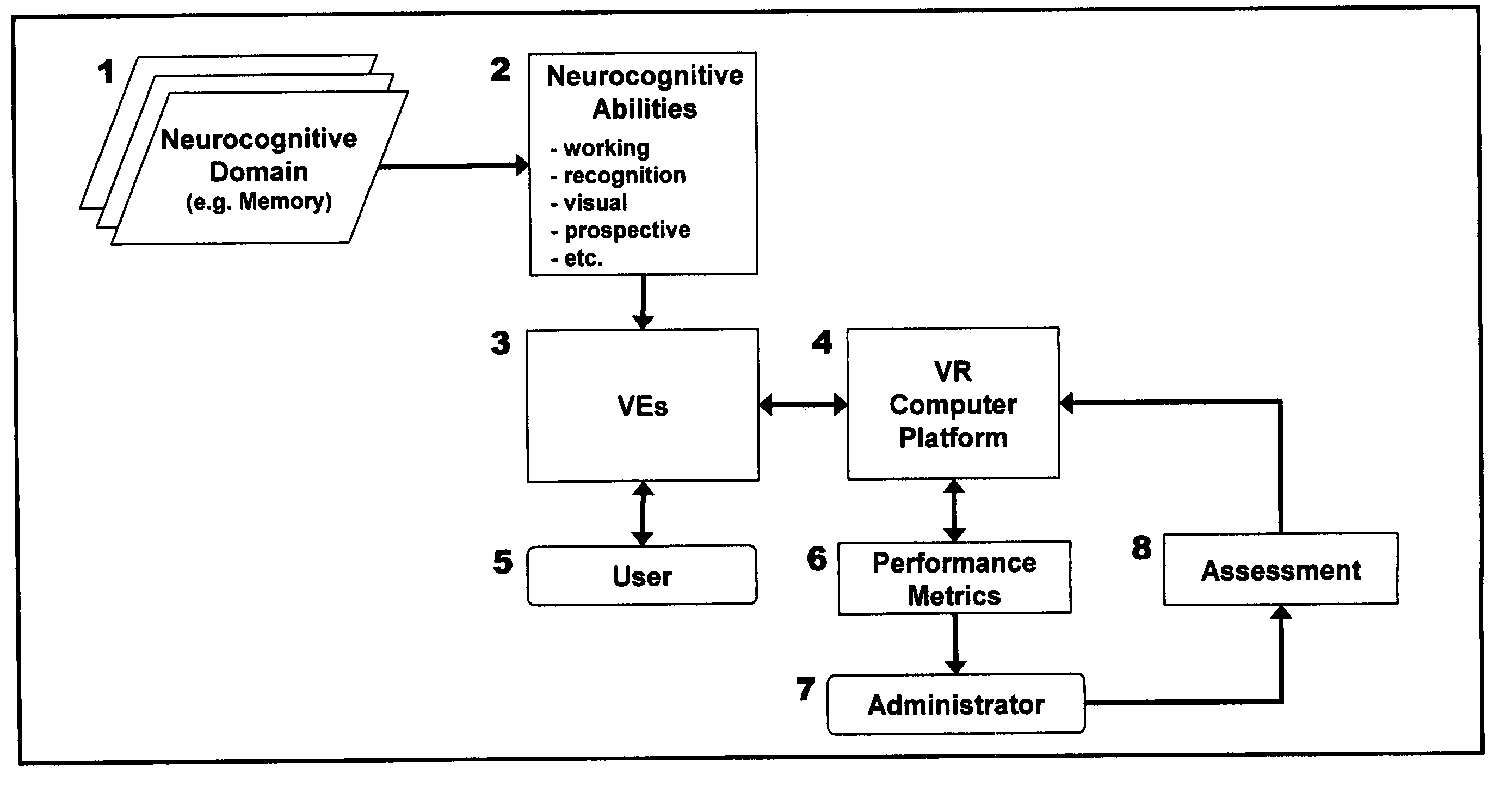
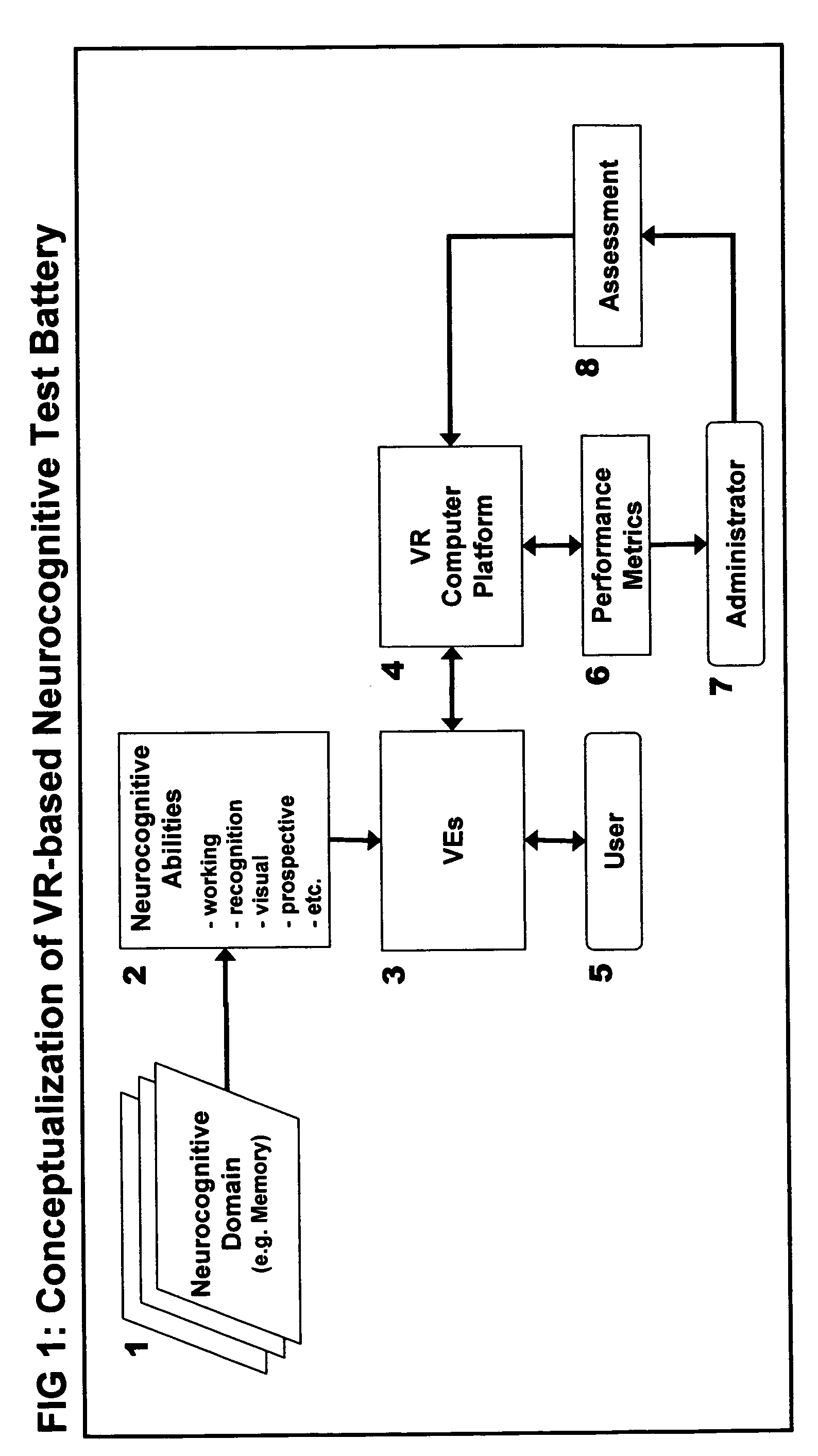
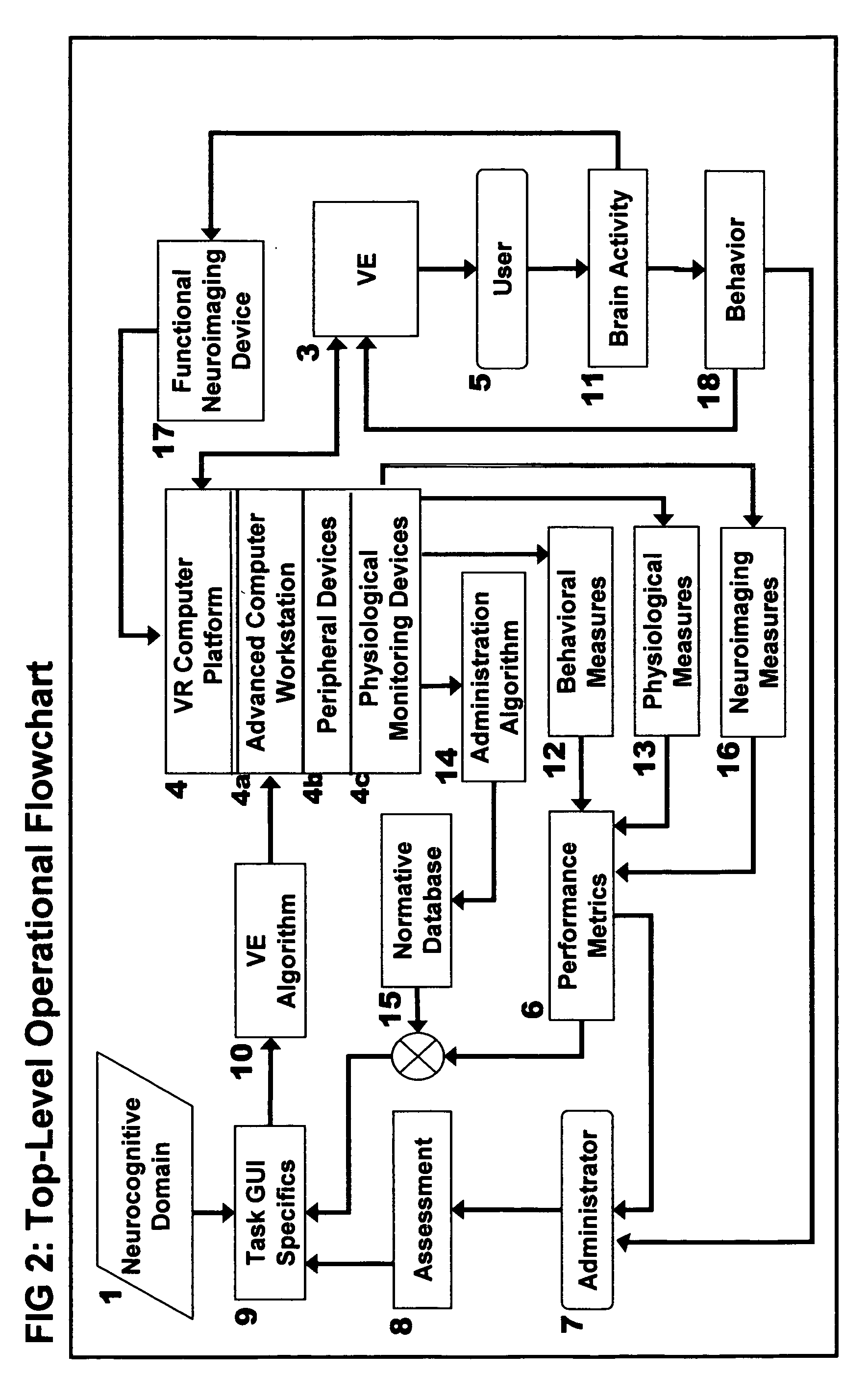
Computer-simulated virtual reality environments for evaluation of neurobehavioral performance
InactiveUS20050216243A1Increase and decrease difficultyStrong specificityMedical simulationTelemedicineTest performanceNervous system
A virtual reality (VR)-based test battery wherein various neurobehavioral performance skills, including motor skills, sensory-perceptual skills, attention, and decision-making can be measured in human subjects. The invention can be used as a screening method within a virtual environment to provide an overall measure of general brain function relating to behavioral ability. In addition, the invention provides comprehensive VR-based neurobehavioral examinations tailored to individual subjects which can automatically self-adjust during operation in accordance with the specific purpose of the assessment, or for forms of cognitive or physical rehabilitation. According to the invention, patients with neurological and psychiatric dysfunctions can be assessed with physiologic monitoring as well as with anatomical and functional neuroimaging to non-invasively map the functional neuroanatomic correlates of VR-based test performance. In a preferred embodiment, the VR-based neurobehavioral testing system is portable allowing computerized tests to be administered in a desk-top or lap-top configuration, or via the Internet for tele-assessment of human subjects who are physically inaccessible to the test administrator. In a particularly preferred embodiment, the method of the invention is used for vocational assessment and training, wherein individual test scores are combined into a final metric useful for assessing a candidate's qualifications for employment, or certification in a particular skill.
Owner:GRAHAM SIMON +3
Administration of growth factors for the treatment of CNS disorders
ActiveUS20070254842A1Prevents and delay onsetReduce severityHeavy metal active ingredientsSenses disorderDiseaseNervous system
A method and system that is directed to the local delivery of growth factors to the mammalian CNS to treat CNS disorders associated with neuronal death and / or dysfunction is described.
Owner:RGT UNIV OF CALIFORNIA
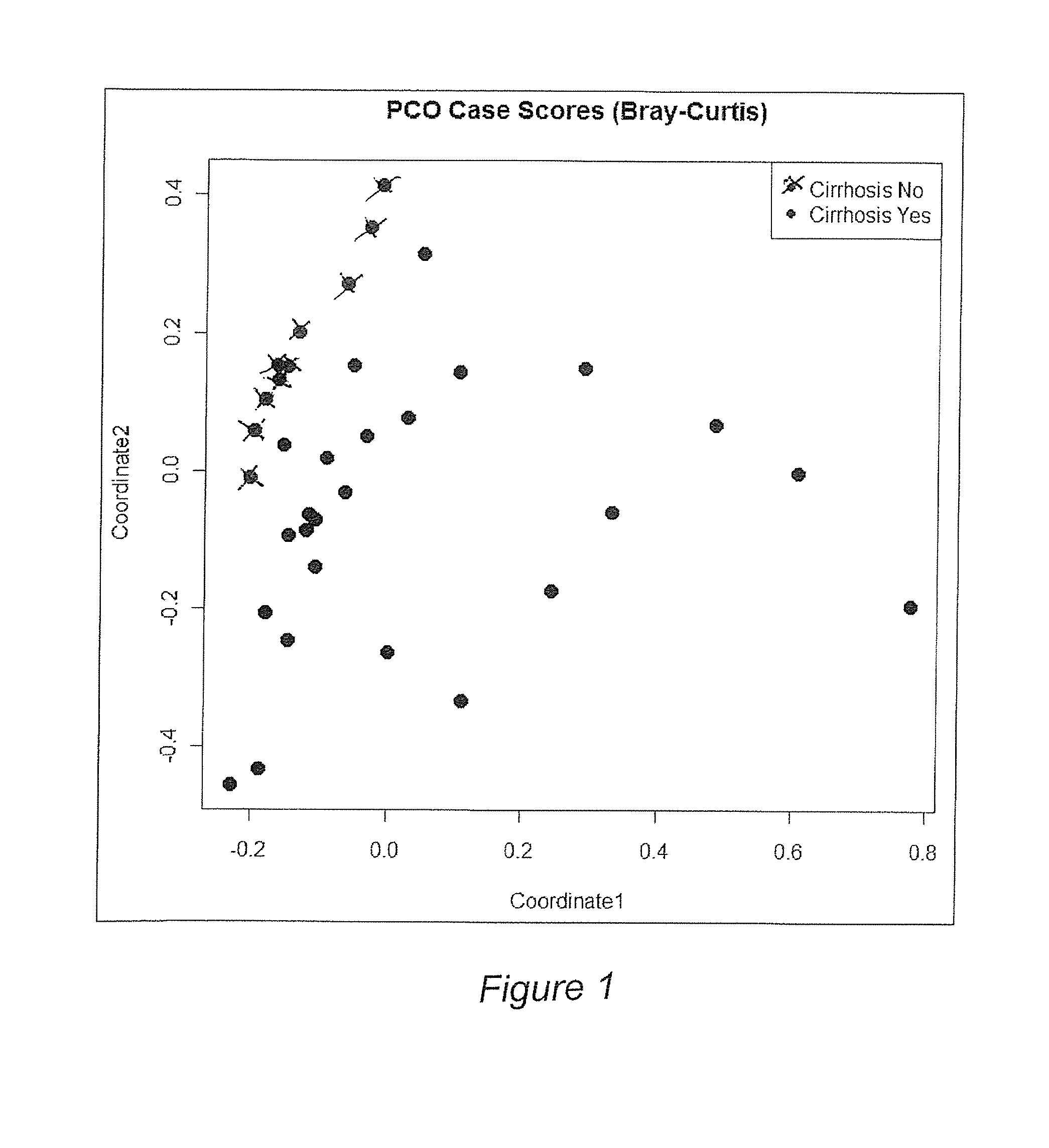
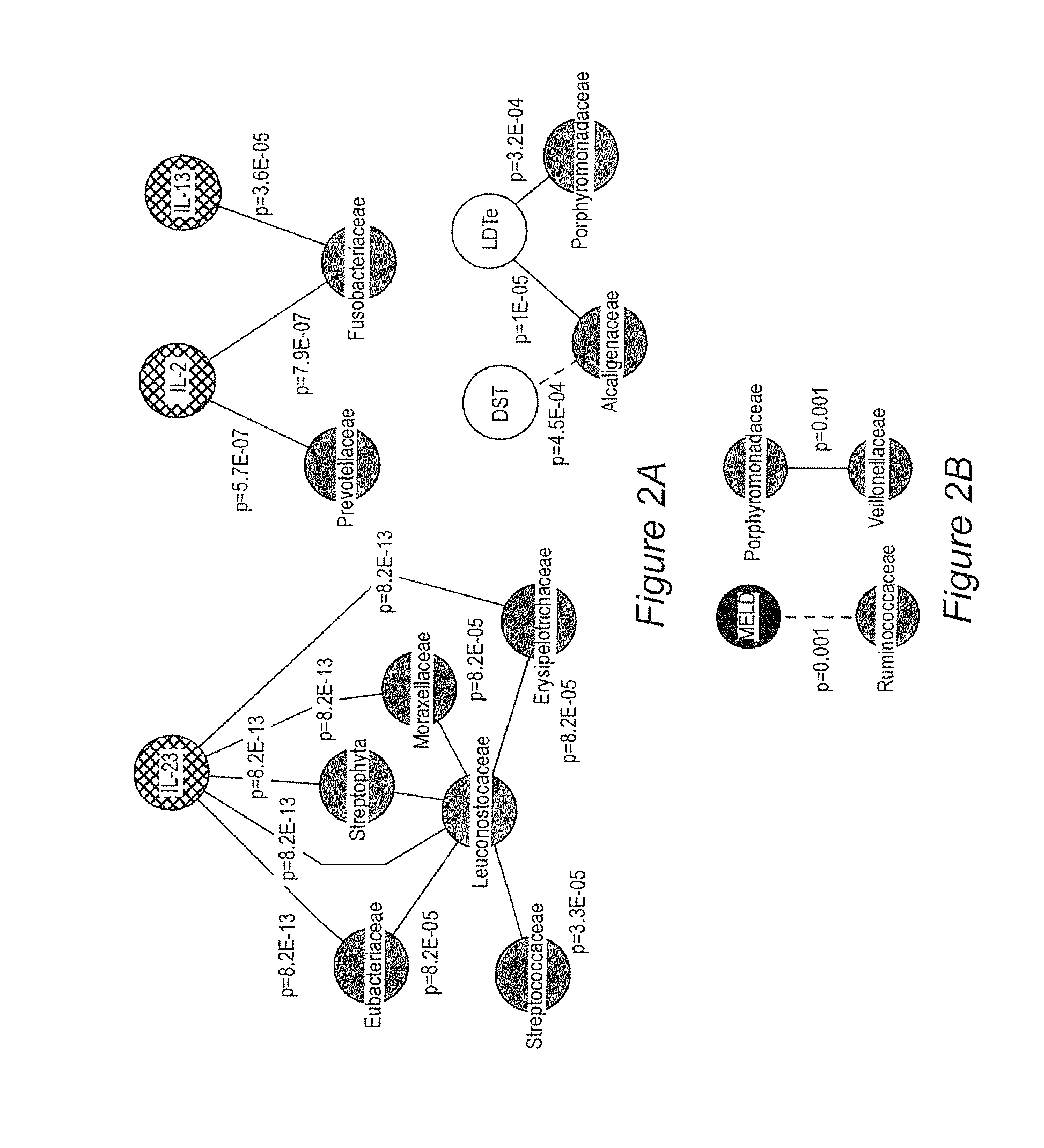
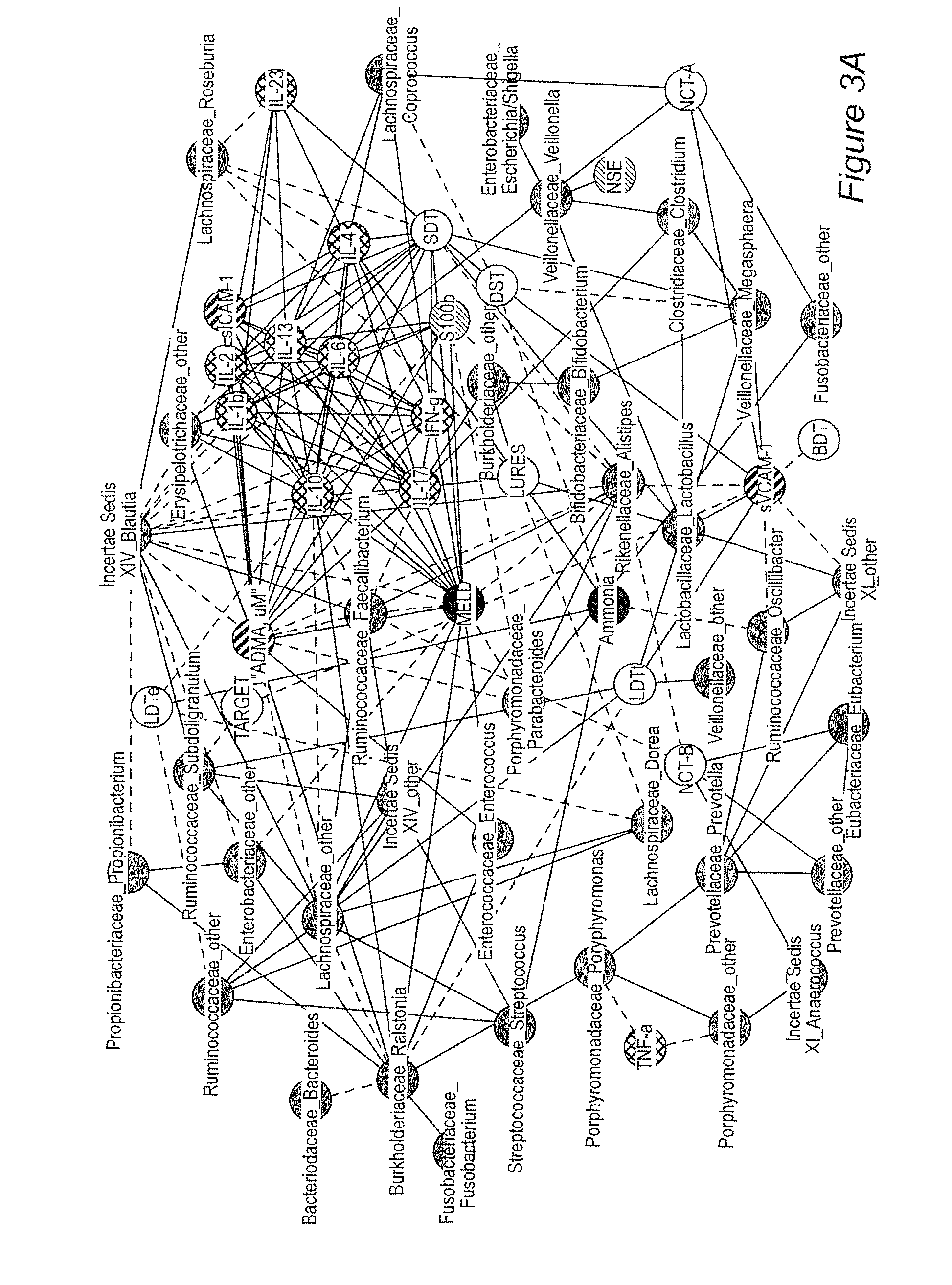
Gut microflora as biomarkers for the prognosis of cirrhosis and brain dysfunction
InactiveUS20140179726A1Efficacy of treatmentReduce the amount requiredBiocideMicrobiological testing/measurementDiseaseTreatment targets
A systems biology approach is used to characterize and relate the intestinal (gut) microbiome of a host organism (e.g. a human) to physiological processes within the host. Information regarding the types and relative amounts of gut microflora is correlated with physiological processes indicative of e.g., a patient's risk of developing a disease or condition, likelihood of responding to a particular treatment, for adjusting treatment protocols, etc. The information is also used to identify novel suitable therapeutic targets and / or to develop and monitor the outcome of therapeutic treatments. An exemplary disease / condition is the development of hepatic encephalopathy (HE), particularly in patients with liver cirrhosis.
Owner:VIRGINIA COMMONWEALTH UNIV +1
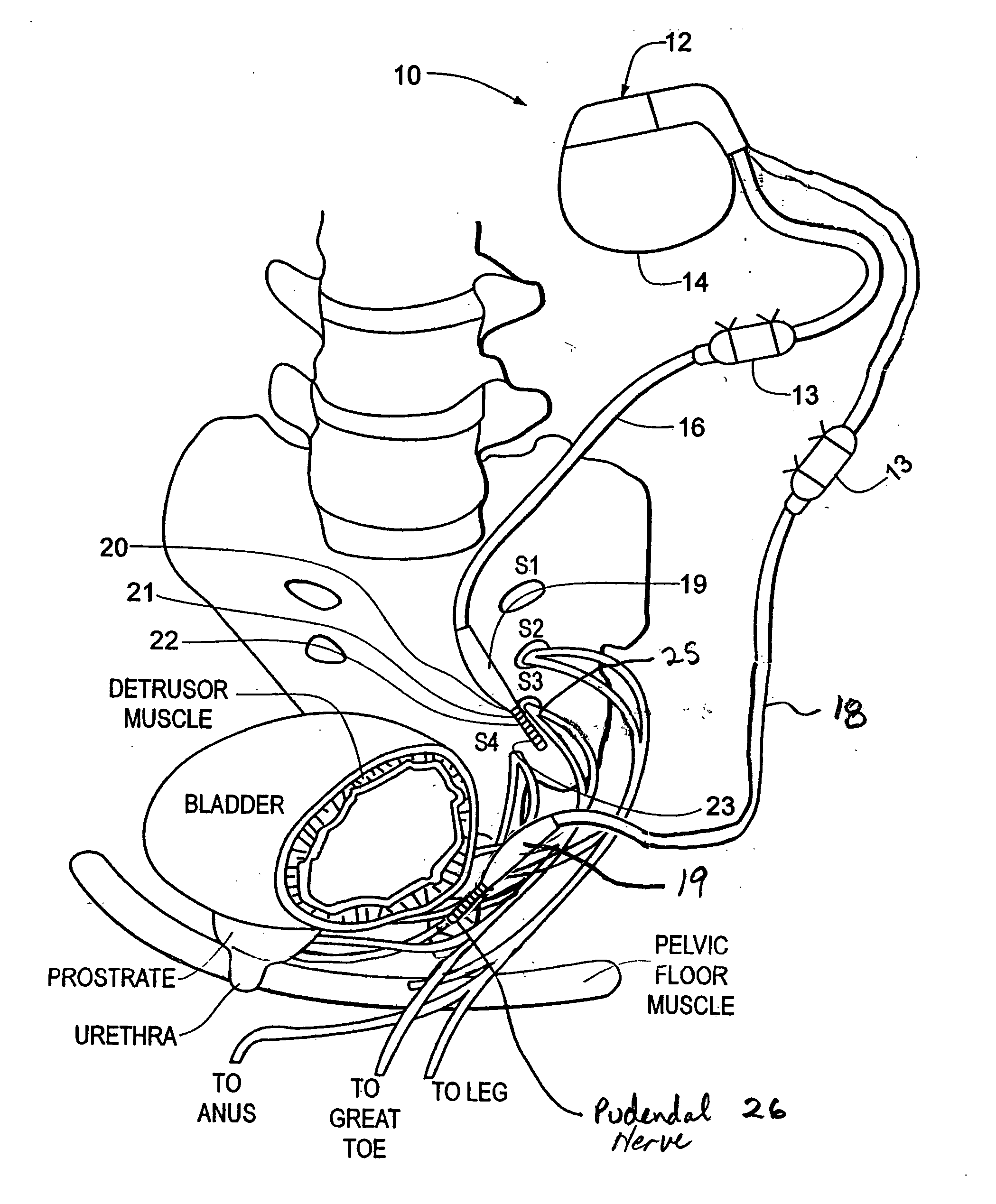
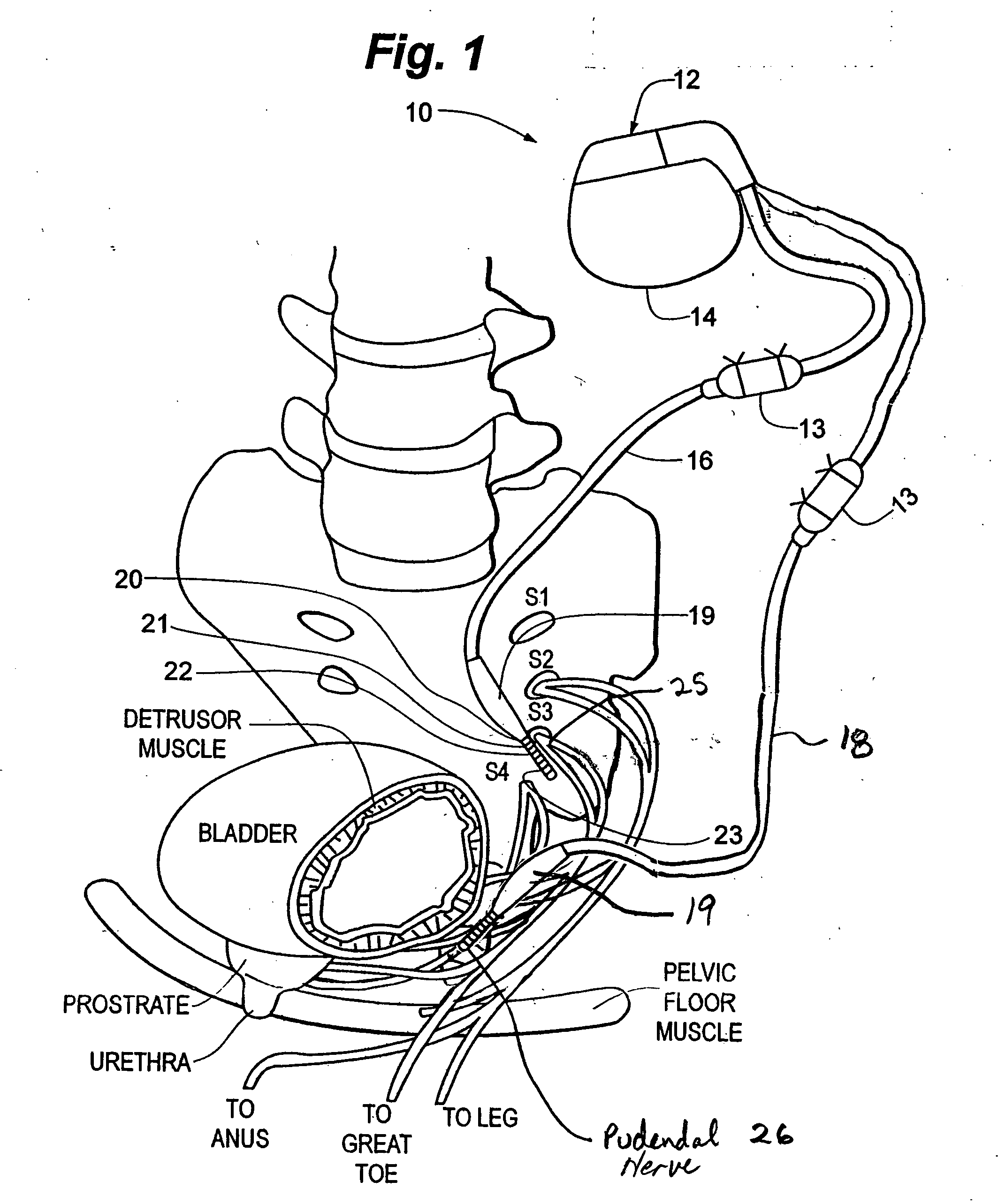
Method, system and device for treating various disorders of the pelvic floor by electrical stimulation of the pudendal nerves and the sacral nerves at different sites
Described are implantable devices and methods for treating various disorders of the pelvic floor by means of electrical stimulation of the pudendal and sacral nerves, or portions thereof, and optional means for delivering drugs in association therewith. Two or more electrical stimulation regimes are applied on a continuous, alternating, intermittent or other basis to the sacral and pudendal nerves, and optionally one or more drugs are infused, injected or otherwise administered, to appropriate portions of a patient's pelvic floor and pudendal nerve and / or sacral nerve, or portions thereof, in an amount and manner effective to treat a number of disorders, including, but not limited to, urinary and / or fecal voiding dysfunctions such as constipation, incontinence disorders such as urge frequency and urinary retention disorders, sexual dysfunctions such as orgasmic and erectile dysfunction, pelvic pain, prostatitis, prostatalgia and prostatodynia.
Owner:MEDTRONIC INC
Uses of dpp-iv inhibitors
Owner:BOEHRINGER INGELHEIM INT GMBH
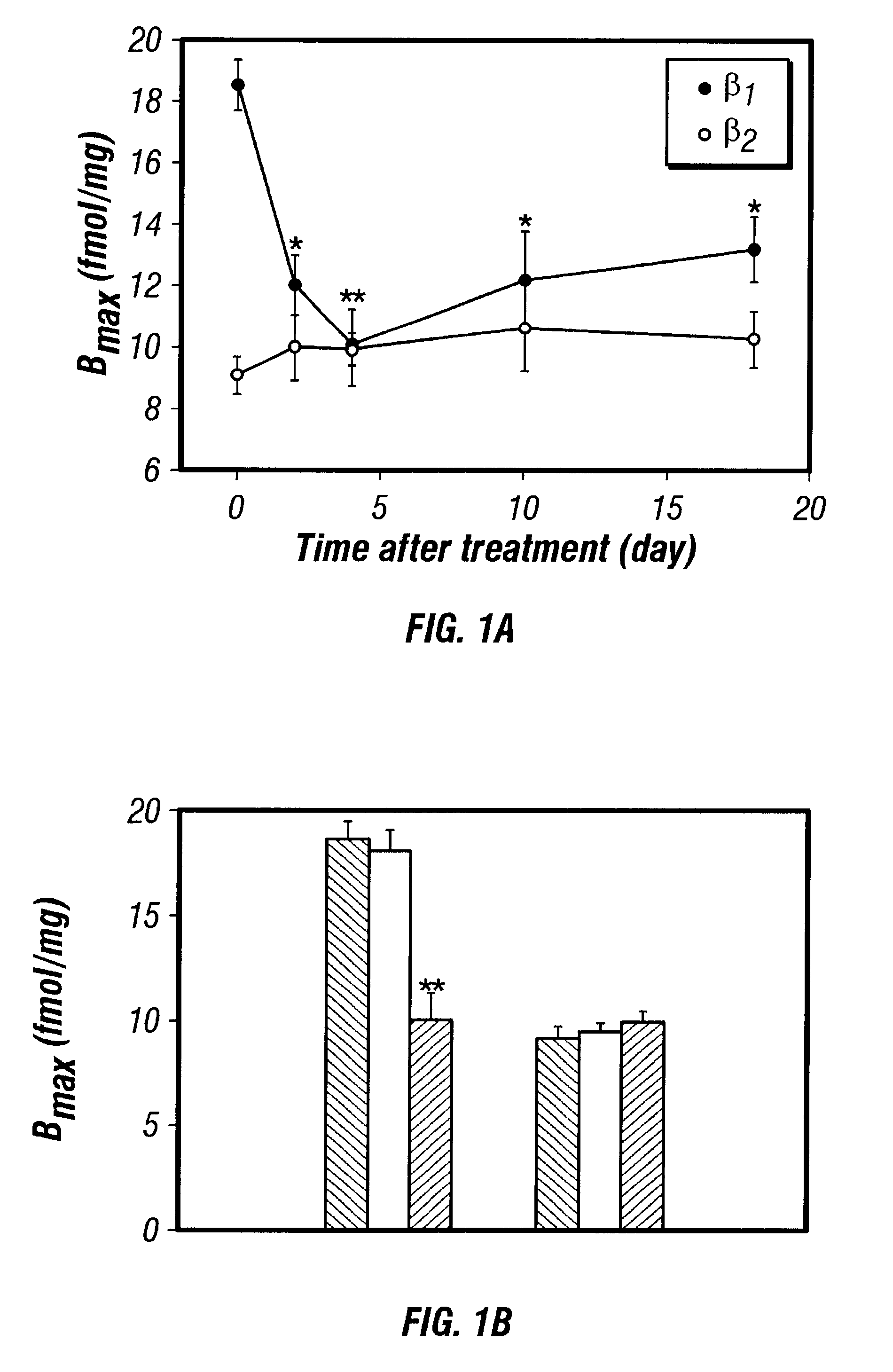
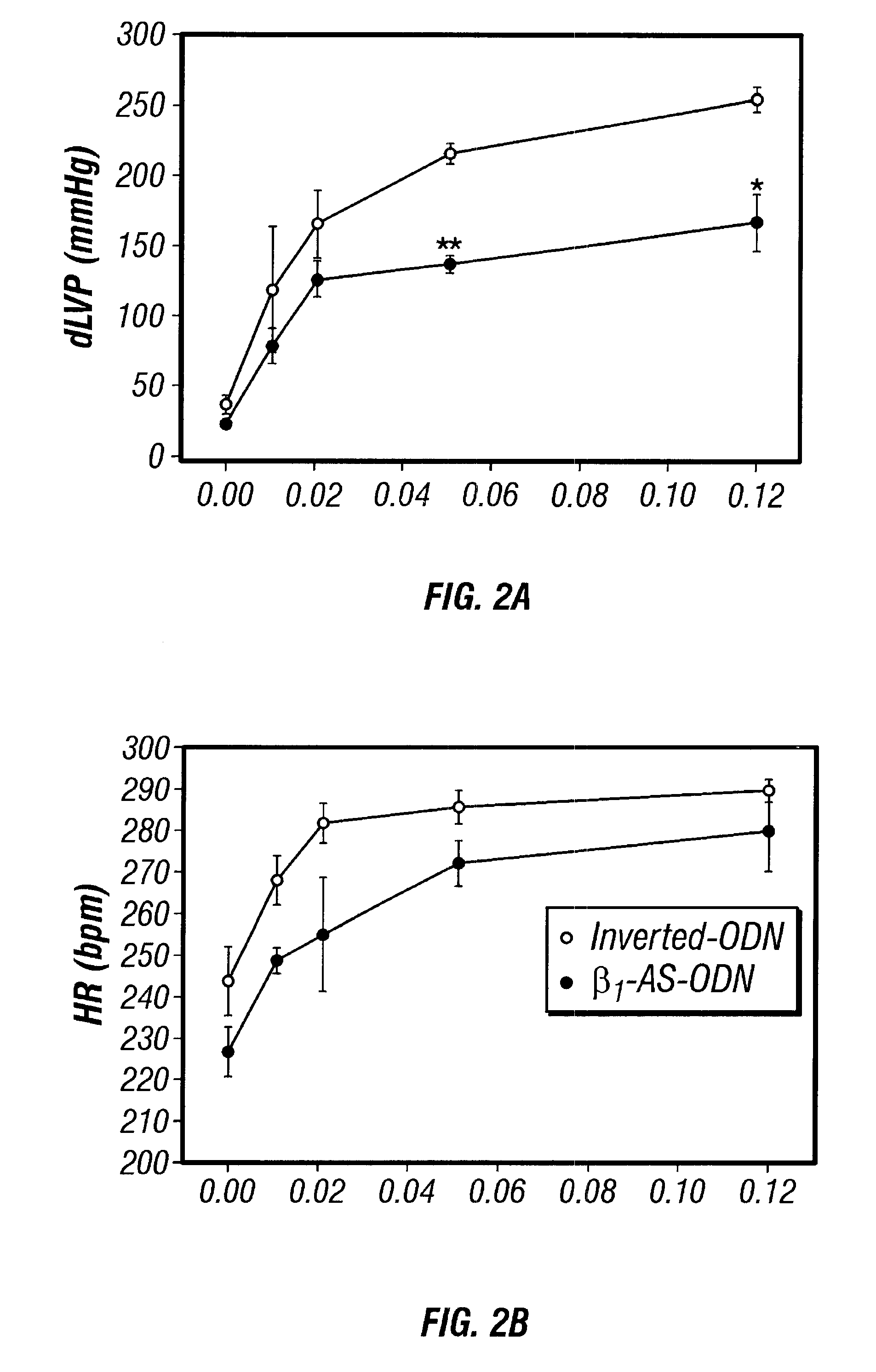
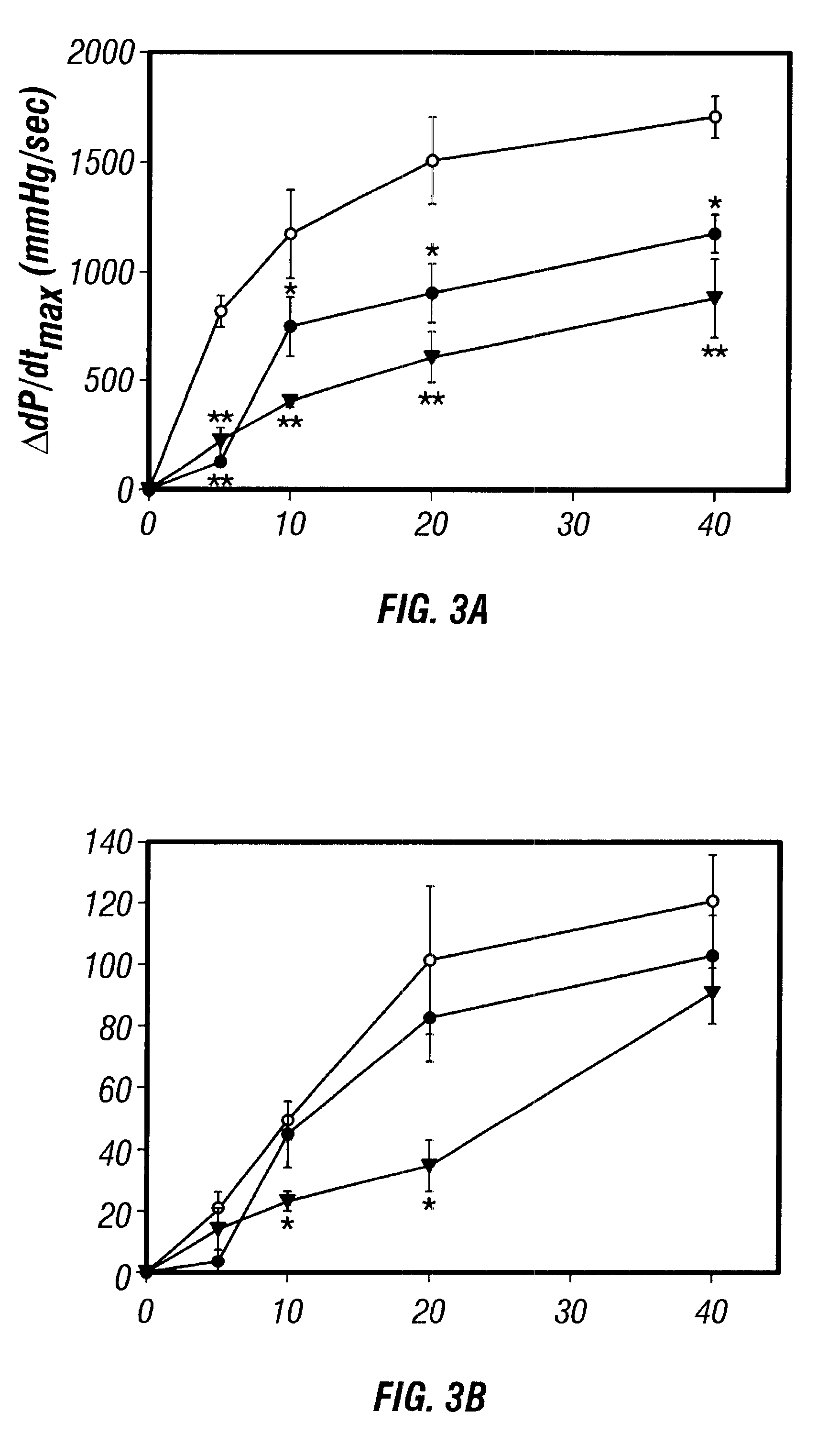
Antisense compositions targeted to beta1-adrenoceptor-specific mRNA and methods of use
InactiveUS6489307B1Reduce inhibitionInhibit and reduce expressionBiocideOrganic active ingredientsEccentric hypertrophyMammal
Disclosed are antisense oligonucleotide, polynucleotide, and peptide nucleic acid compounds that specifically bind to mammalian mRNA encoding a beta1-adrenoceptor polypeptide and that are useful in the control and / or treatment of cardiac dysfunction, hypertension, hypertrophy, myocardial ischemia, and other cardiovascular diseases in an affected mammal, and preferably, in a human subject. The antisense compounds disclosed herein, and pharmaceutical formulations thereof, provide sustained control of beta1-adrenoceptor expression over prolonged periods, and achieve therapeutic effects from as little as a single dose. Administration of these antisense compositions to approved animal models resulted in a decrease in blood pressure, but no significant change in heart rate. Use of such antisense compositions in the reduction of beta1-adrenoceptor polypeptides in a host cell expressing beta1-adrenoceptor-specific mRNA, and in the preparation of medicaments for treating human and animal diseases, and in particular, hypertension and other cardiac dysfunction is also disclosed.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
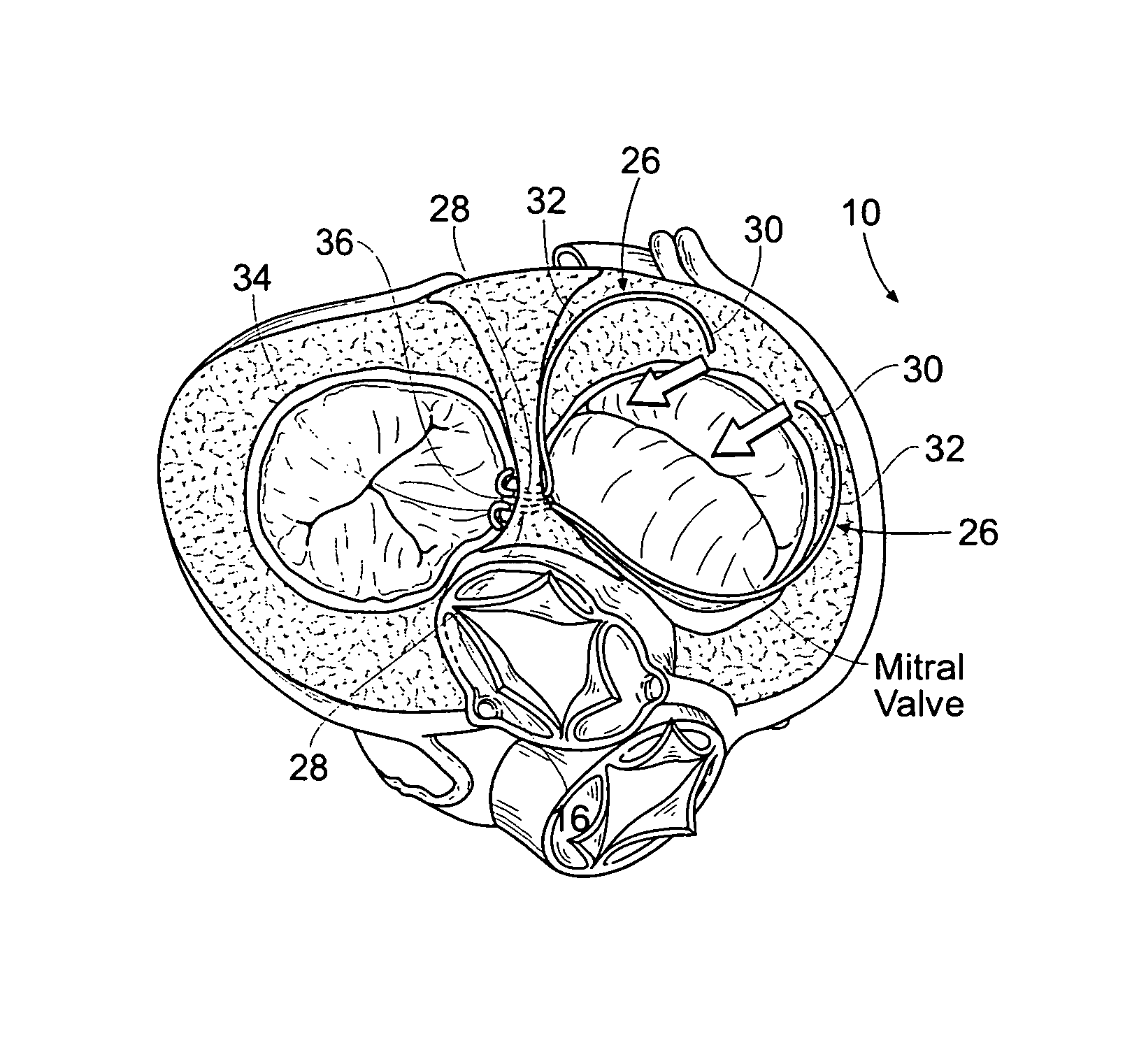
Method of reshaping a heart valve annulus using an intravascular device
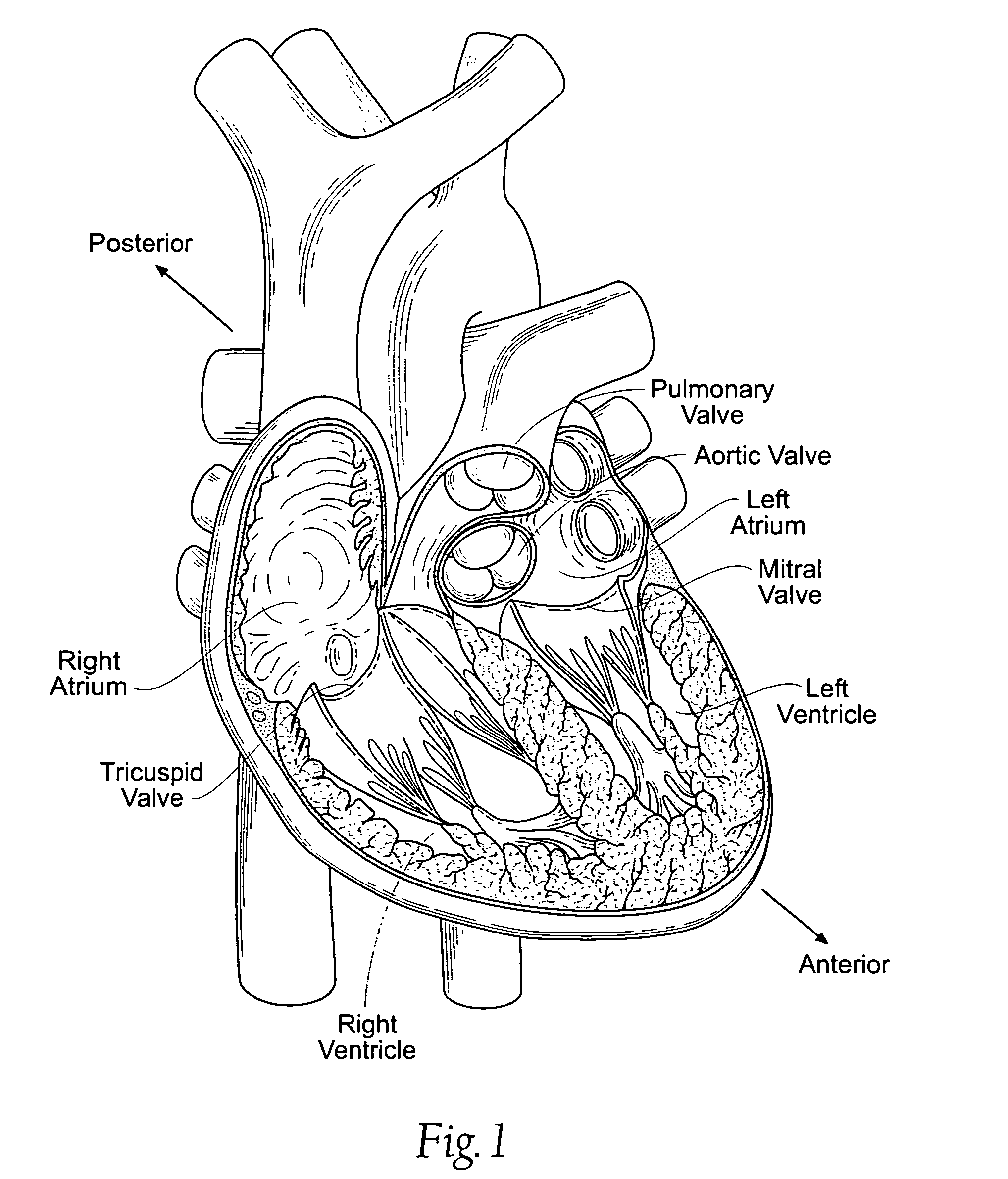
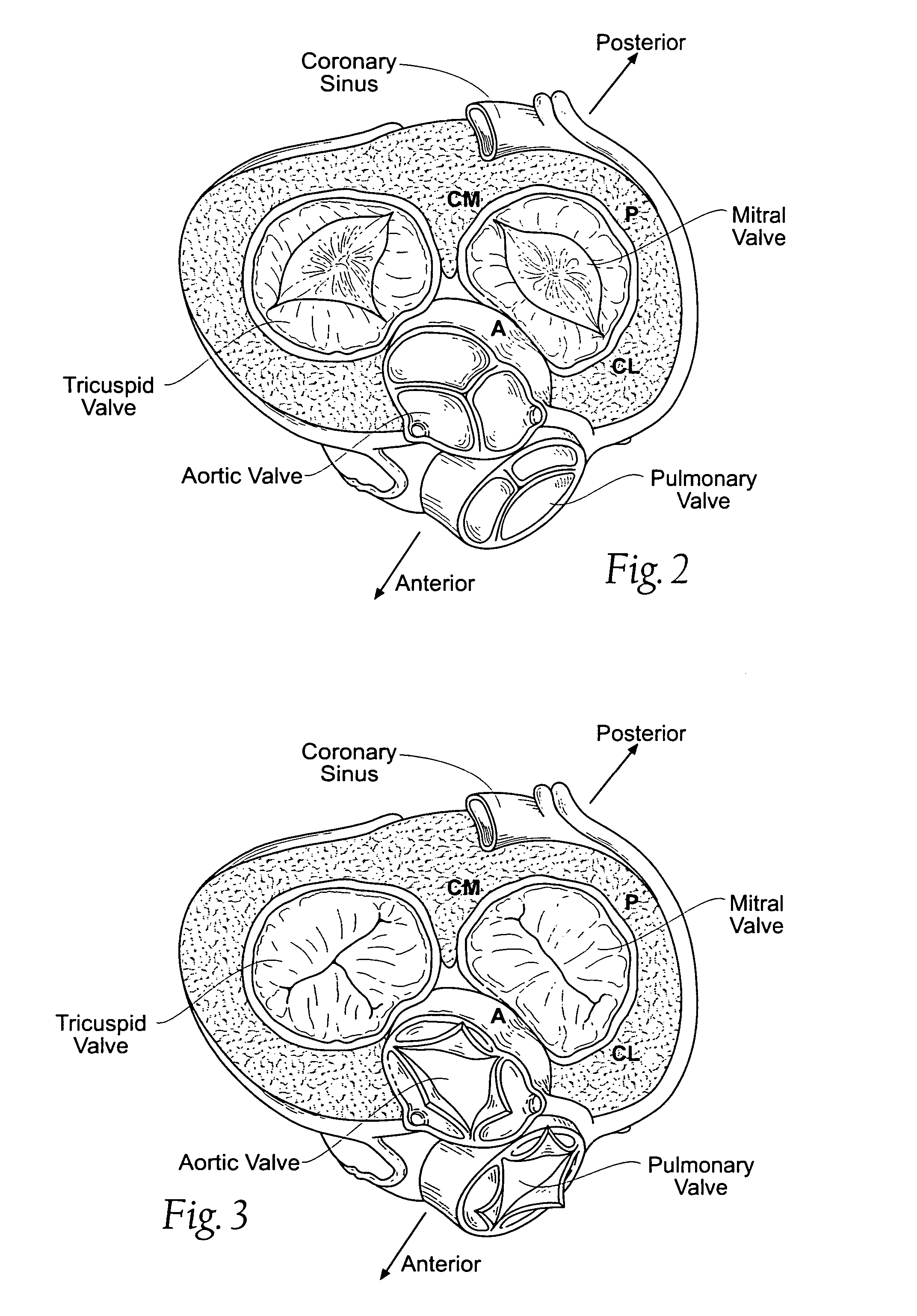
Devices, systems, and methods employ an implant that is sized and configured to attach in, on, or near the annulus of a dysfunctional heart valve. In use, the implant extends either across the minor axis of the annulus, or across the major axis of the annulus, or both. The implant restores to the heart valve annulus and leaflets a more functional anatomic shape and tension. The more functional anatomic shape and tension are conducive to coaptation of the leaflets, which, in turn, reduces retrograde flow or regurgitation.
Owner:VENTURE LENDING & LEASING IV
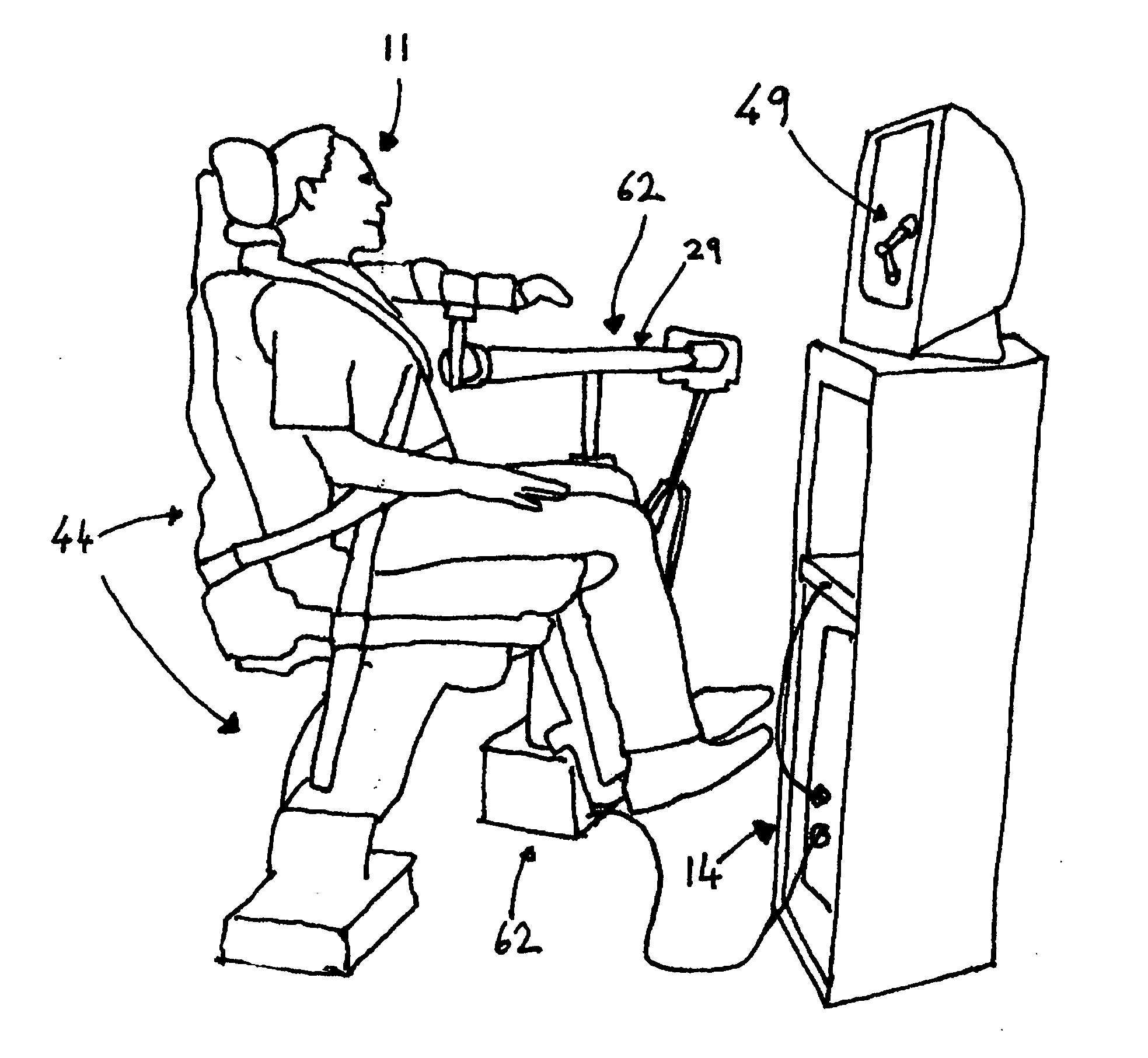
System and methods to overcome gravity-induced dysfunction in extremity paresis
The present invention relates to a system for use in rehabilitation and / or physical therapy for the treatment of injury or disease. The system can overcome gravity-induced dysfunction in extremity paresis following stroke or other neurological disorders.
Owner:NORTHWESTERN UNIV +2
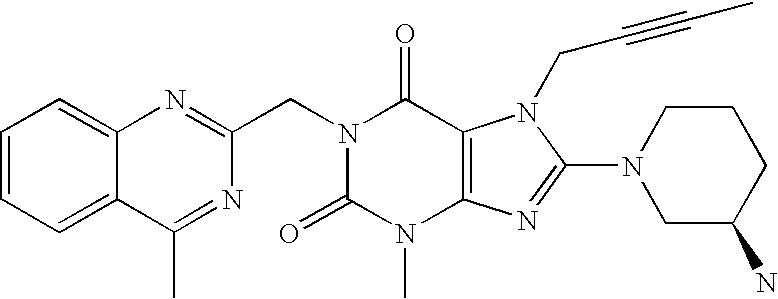
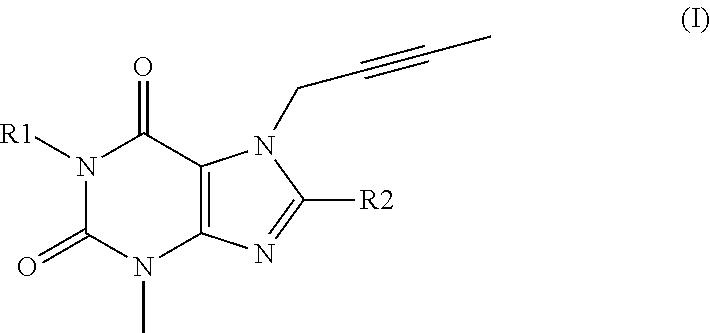
Trk-inhibiting compound
ActiveUS9242977B2Safe prophylactic and therapeutic agentHigh selectivityNervous disorderOrganic chemistryChagas diseaseInflammatory bowel disease
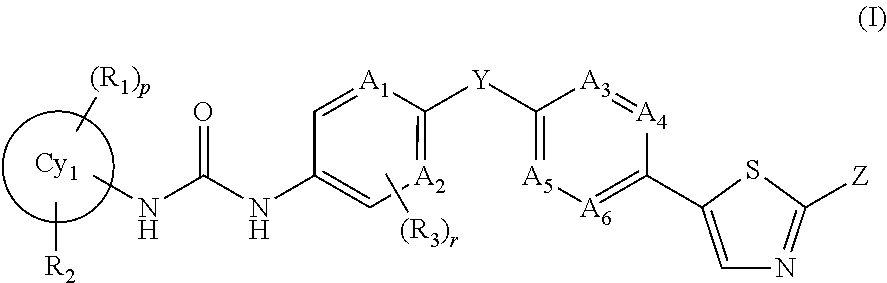
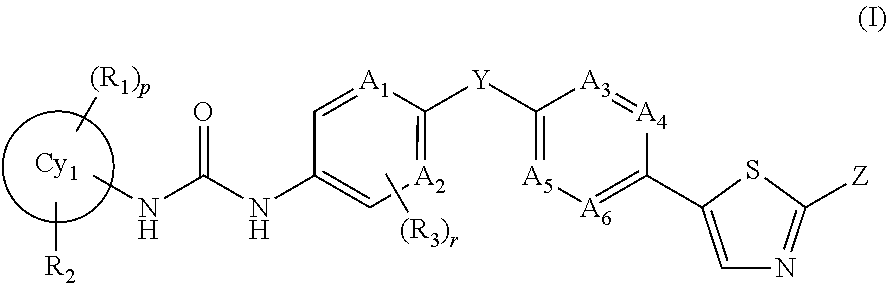
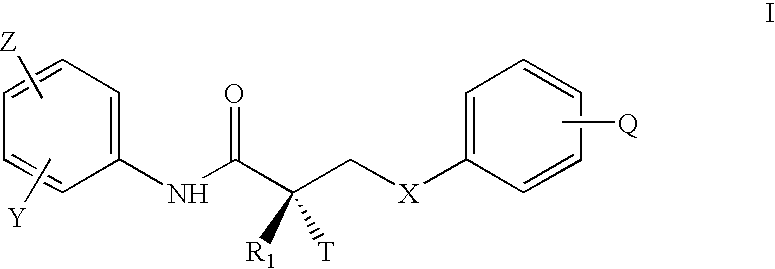
An object of the present invention is to provide a drug containing a compound having Trk-inhibiting activity as an active ingredient in prophylaxis and / or therapy of diseases such as pain, pruritus, lower urinary tract dysfunction, asthma, allergic rhinitis, inflammatory bowel disease or Chagas disease. A compound represented by the general formula (I):(wherein all symbols represent the same meanings as described in the specification), a salt thereof, an N-oxide thereof, a solvate thereof or a prodrug thereof is useful as a drug component having Trk-inhibiting activity in prophylaxis and / or therapy of diseases such as pain, pruritus, lower urinary tract dysfunction, asthma, allergic rhinitis, inflammatory bowel disease or Chagas disease.
Owner:ONO PHARMA CO LTD
Sustained release intraocular implants and methods for preventing retinal dysfunction
InactiveUS20050244506A1Few and no negative side effectFacilitate obtaining successful treatment resultsPowder deliveryBiocideMicrosphereRetinal dysfunction
Biocompatible intraocular microspheres and implants include an alpha-2 adrenergic receptor agonist and a polymer associated with the alpha-2 adrenergic receptor agonist to facilitate release of the alpha-2 adrenergic receptor agonist into an eye for an extended period of time. The alpha-2 adrenergic receptor agonist may be associated with a biodegradable polymer matrix, such as a matrix of a two biodegradable polymers. The implants may be placed in an eye to treat or to prevent the occurrence of one or more ocular conditions, to reduce one or more symptoms of an ocular condition, such as an ocular neurosensory disorder and the like, to enhance normal retinal function and / or to lower intraocular pressure.
Owner:ALLERGAN INC
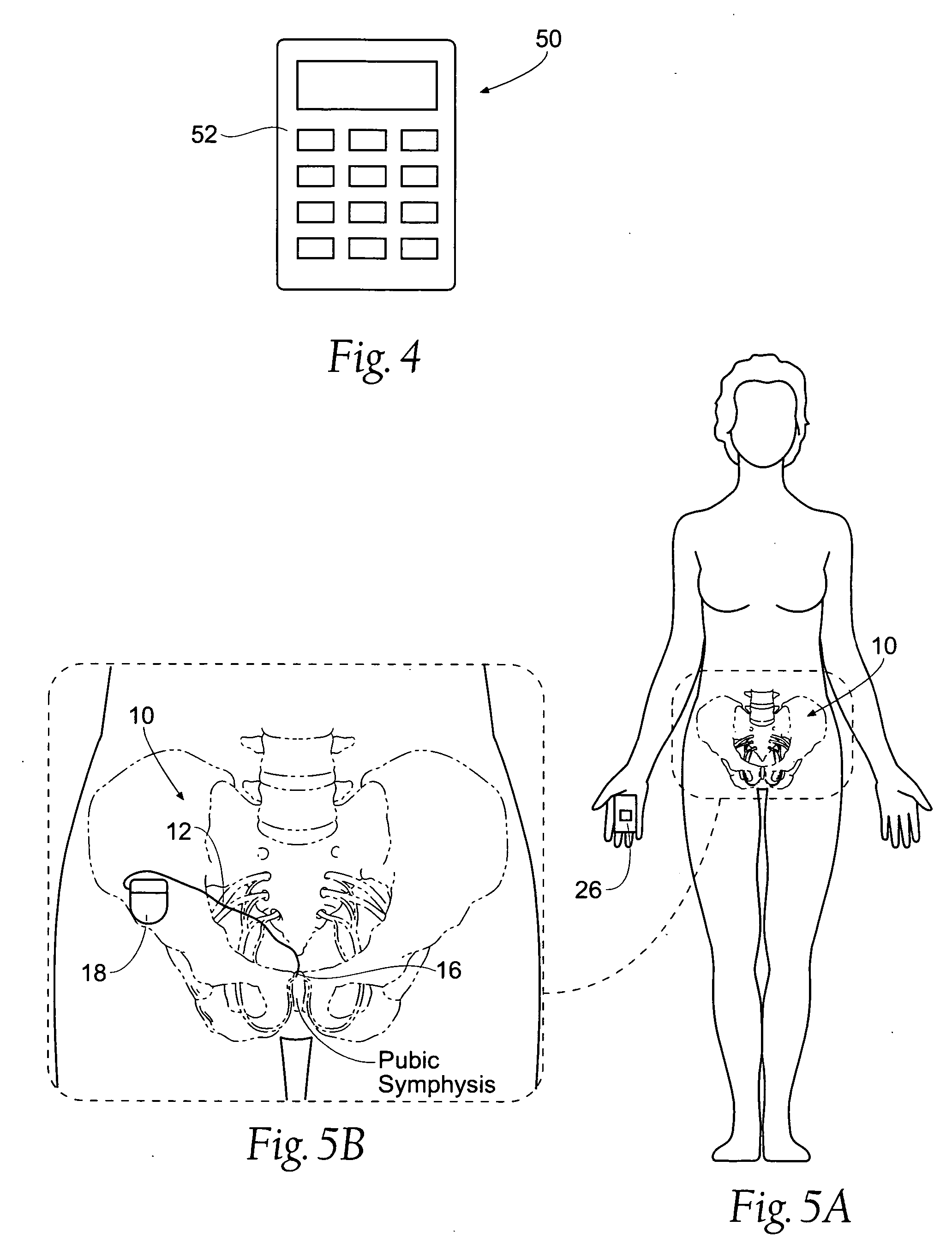
Systems and methods for bilateral stimulation of left and right branches of the dorsal genital nerves to treat dysfunctions, such as urinary incontinence
Systems and methods treat urinary incontinence by the bilateral stimulation of the left and / or right branches of the dorsal genital nerves using a single lead implanted in adipose or other tissue in the region at or near the pubic symphysis.
Owner:MEDTRONIC URINARY SOLUTIONS
Compositions and methods for transient receptor potential vanilloid (TRPV) channel mediated treatments
InactiveUS20080051454A1Increasing TRPV1-responsesCompound screeningBiocideScreening methodArachidonic acid supplementation
The present inventions relate to therapeutic compositions comprising, and methods utilizing, arachidonic acid derivatives and analogs for treatment of patients demonstrating symptoms of pathological conditions. Specifically, the inventions relate to therapeutic compositions for activating transient receptor potential vanilloid-1 channels (TRPV1). Additionally, therapeutic compositions are provided for increasing TRPV1-type responses. These pathological conditions include, but are limited to, hypertension, in particular salt induced hypertension, and cardiovascular complications, including myocardial infarction, kidney dysfunction, diabetes, and inflammation. Further, the inventions relate to drug screening methods for providing additional therapeutic compounds.
Owner:MICHIGAN STATE UNIV
Drug preparations for treating sexual dysfunction
Topical gelled compositions comprising a drug for treating sexual dysfunction dispersed within a polymer matrix and methods and treatments using said compositions.
Owner:GLYCOBIOSCI +1
Method and composition for the treatment of lung surfactant deficiency or dysfunction
ActiveUS20050229926A1Quantity minimizationReduce the amount requiredRespiratorsMedical devicesSurfactant deficiencyAssisted breathing
A method of treating a disease involving surfactant deficiency or dysfunction in a patient's lungs is disclosed comprising the steps of providing a liquid lung surfactant composition; aerosolizing the lung surfactant composition with a vibrating aperture-type aerosol generator to form a surfactant aerosol; and introducing the surfactant aerosol into the gas flow within a circuit of a pressure-assisted breathing system coupled to the patient's respiratory system, whereby a therapeutically effective amount of said surfactant is delivered to the patient's lungs. Apparatus is also disclosed that increases the efficiency of delivery of aerosolized medicaments such as aerosolized lung surfactant.
Owner:NOVARTIS AG
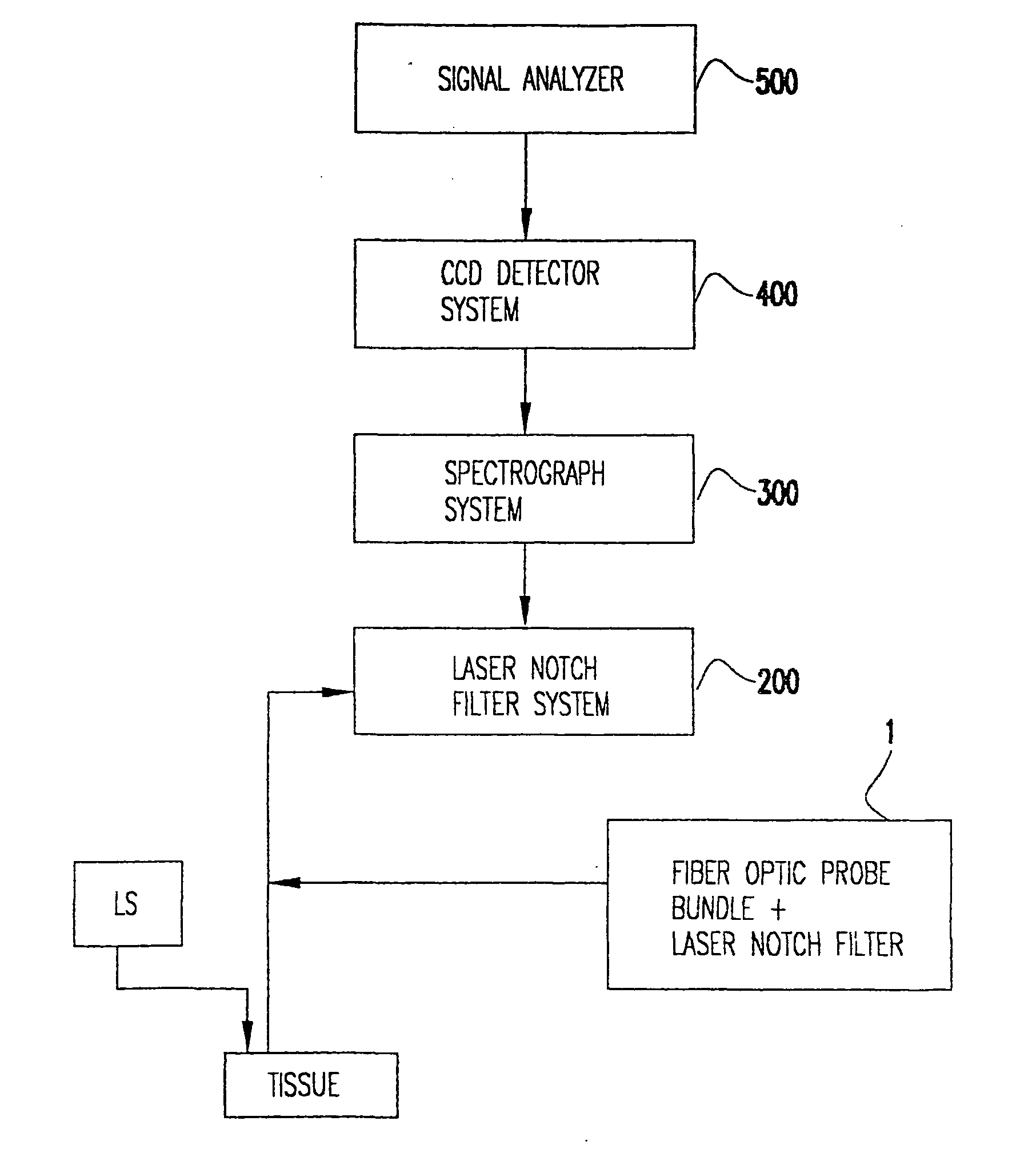
Nitric-oxide detection using Raman spectroscopy
InactiveUS20060074282A1Accurate conditionHigh precisionRadiation pyrometryDiagnostics using spectroscopyDiseaseResonance Raman spectroscopy
In an emergency medicine patient, accurate measurement of change or lack thereof from non-shock, non-ischemimc, non-inflammation, non-tissue injury, non-immune dysfunction conditions is important and is provided, as practical, real-time approaches for accurately characterizing a patient's condition, using Raman spectroscopy with a high degree of accuracy, Resonance Raman spectroscopy is used to monitor tissue nitric oxide activity either in vivo or in vitro, especially as a function of its interaction with hemoglobin or other metalloproteins. Measurement times are on the order of seconds. High-accuracy measurement is achieved with Raman spectroscopy interrogation of tissue. Measurements may be non-invasive to minimally invasive. The invention may be used to monitor the effect of instituting therapies using nitric oxide or disease processes that produce nitric oxide.
Owner:VIRGINIA COMMONWEALTH UNIV
System and methods to overcome gravity-induced dysfunction in extremity paresis
The present invention relates to a system for use in rehabilitation and / or physical therapy for the treatment of injury or disease. The system can overcome gravity-induced dysfunction in extremity paresis following stroke or other neurological disorders.
Owner:DEWALD JULIUS P A +1
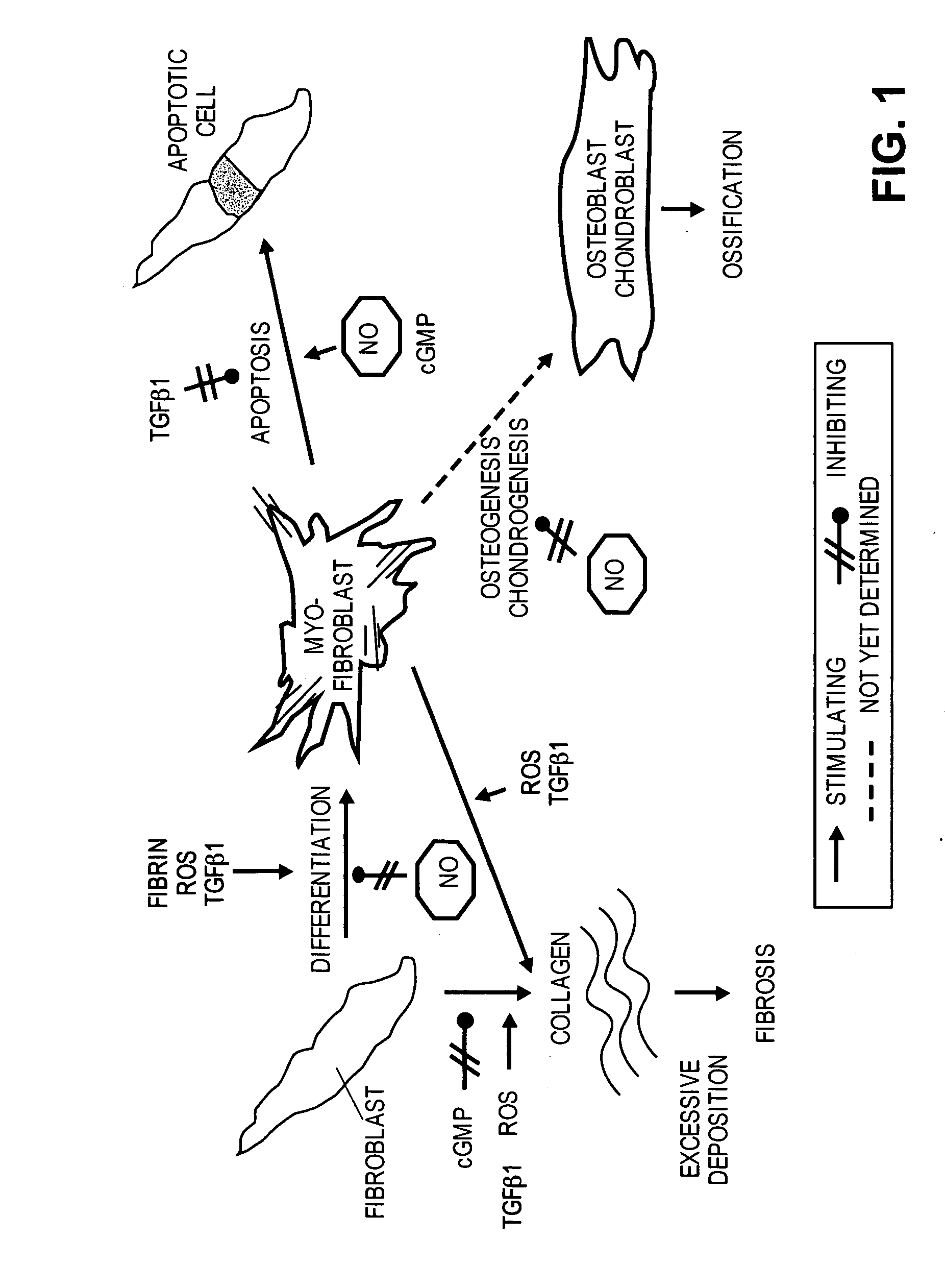
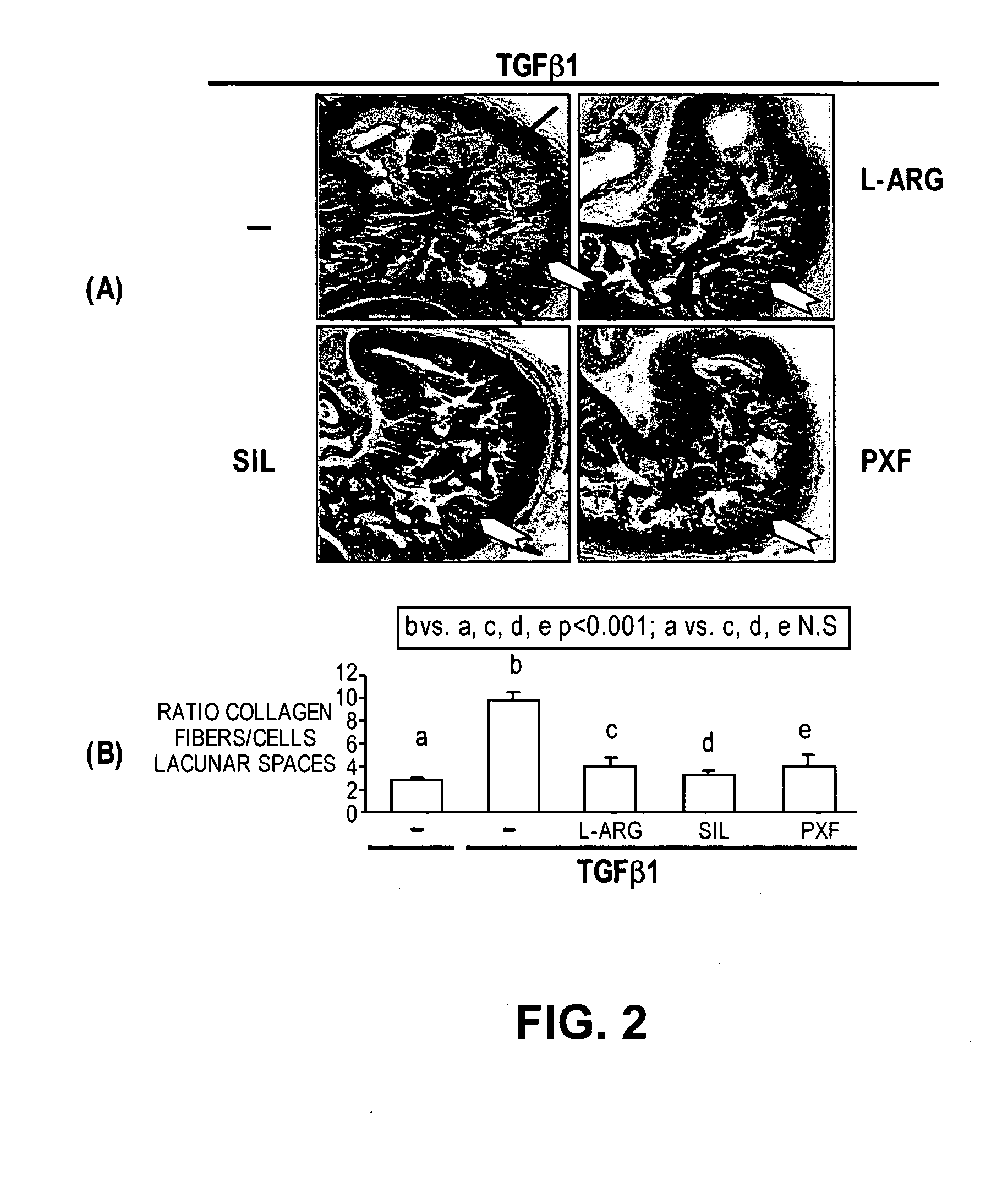
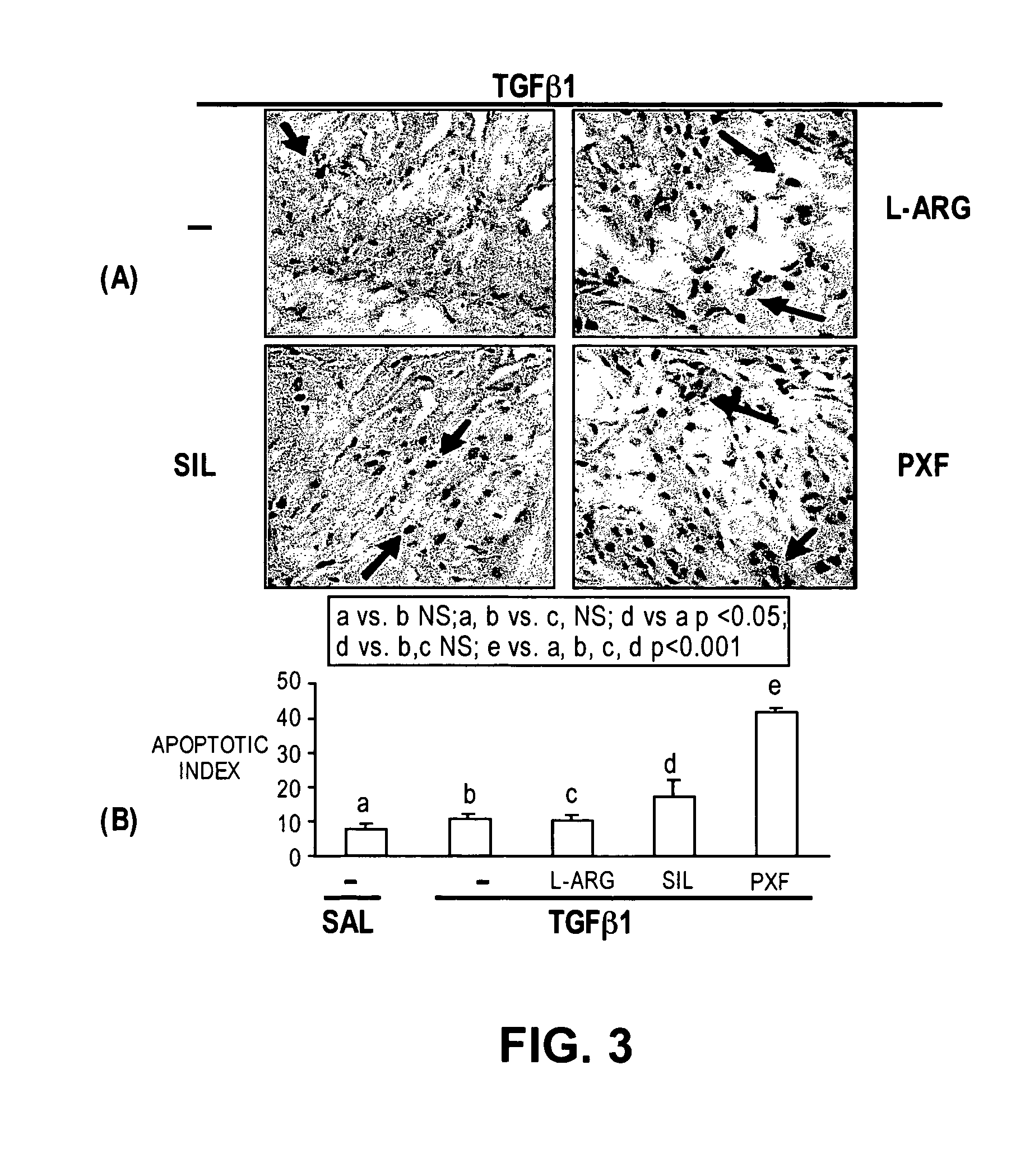
Methods of use of inhibitors of phosphodiesterases and modulators of nitric oxide, reactive oxygen species, and metalloproteinases in the treatment of peyronie's disease, arteriosclerosis and other fibrotic diseases
ActiveUS20050085486A1Increasing NO levelReduce expressionBiocidePharmaceutical delivery mechanismFemale Sexual Arousal DisorderCyclase
The present methods and compositions are of use for treatment of conditions involving fibrosis, such as Peyronie's disease plaque, penile corporal fibrosis, penile veno-occlusive dysfunction, Dupuytren's disease nodules, vaginal fibrosis, clitoral fibrosis, female sexual arousal disorder, abnormal wound healing, keloid formation, general fibrosis of the kidney, bladder, prostate, skin, liver, lung, heart, intestines or any other localized or generalized fibrotic condition, vascular fibrosis, arterial intima hyperplasia, atherosclerosis, arteriosclerosis, restenosis, cardiac hypertrophy, hypertension or any condition characterized by excessive fibroblast or smooth muscle cell proliferation or deposition of collagen and extracellular matrix in the blood vessels and / or heart. In certain embodiments, the compositions may comprise a PDE-4 inhibitor, a PDE-5 inhibitor, a compound that elevates cGMP and / or PKG, a stimulator of guanylyl cyclase and / or PKG, a combination of a compound that elevates cGMP, PKG or NO with an antioxidant that decreases ROS, or a compound that increases MMP activity.
Owner:LOS ANGELES BIOMEDICAL RES INST AT HARBOR UCLA MEDICAL CENT
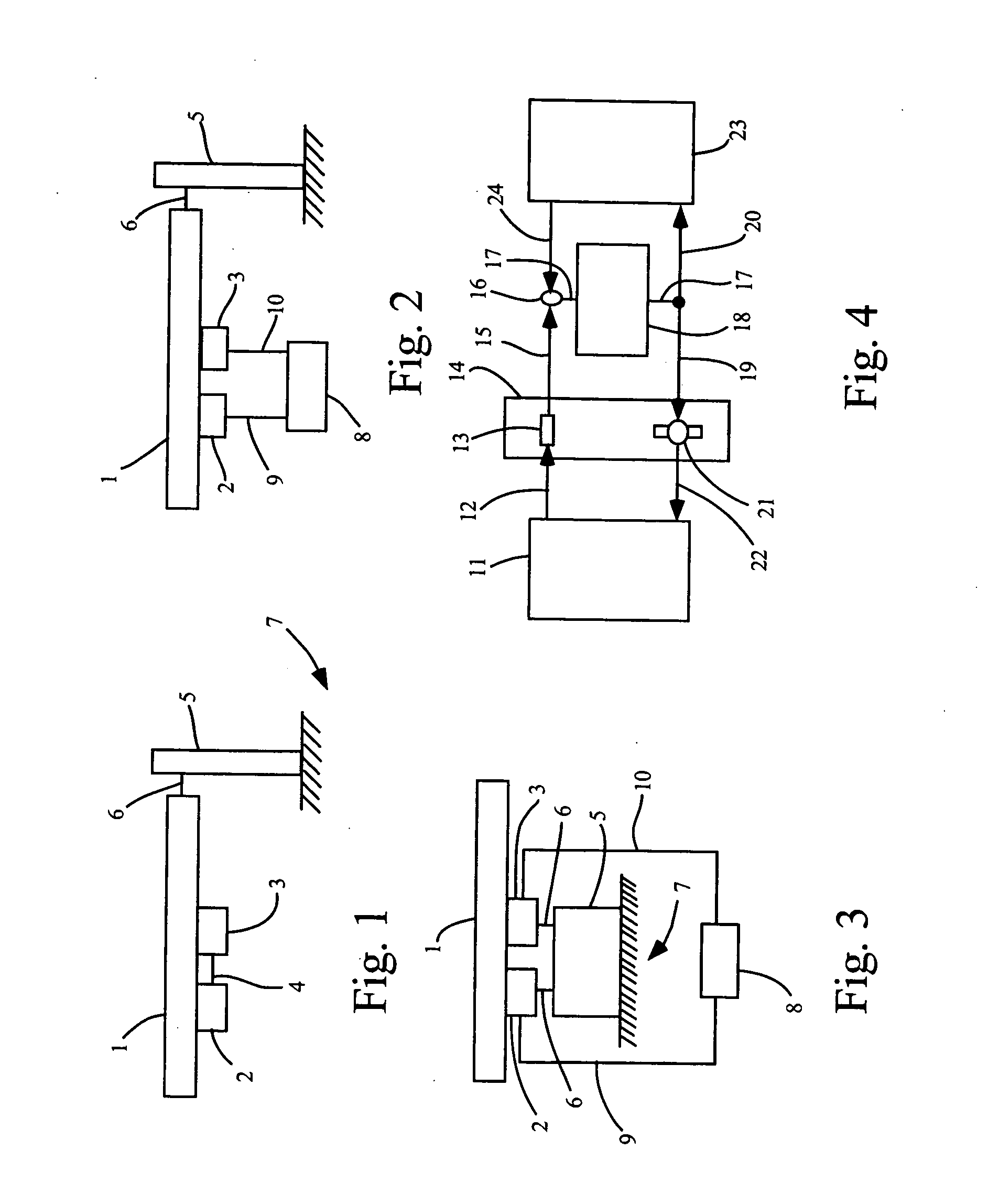
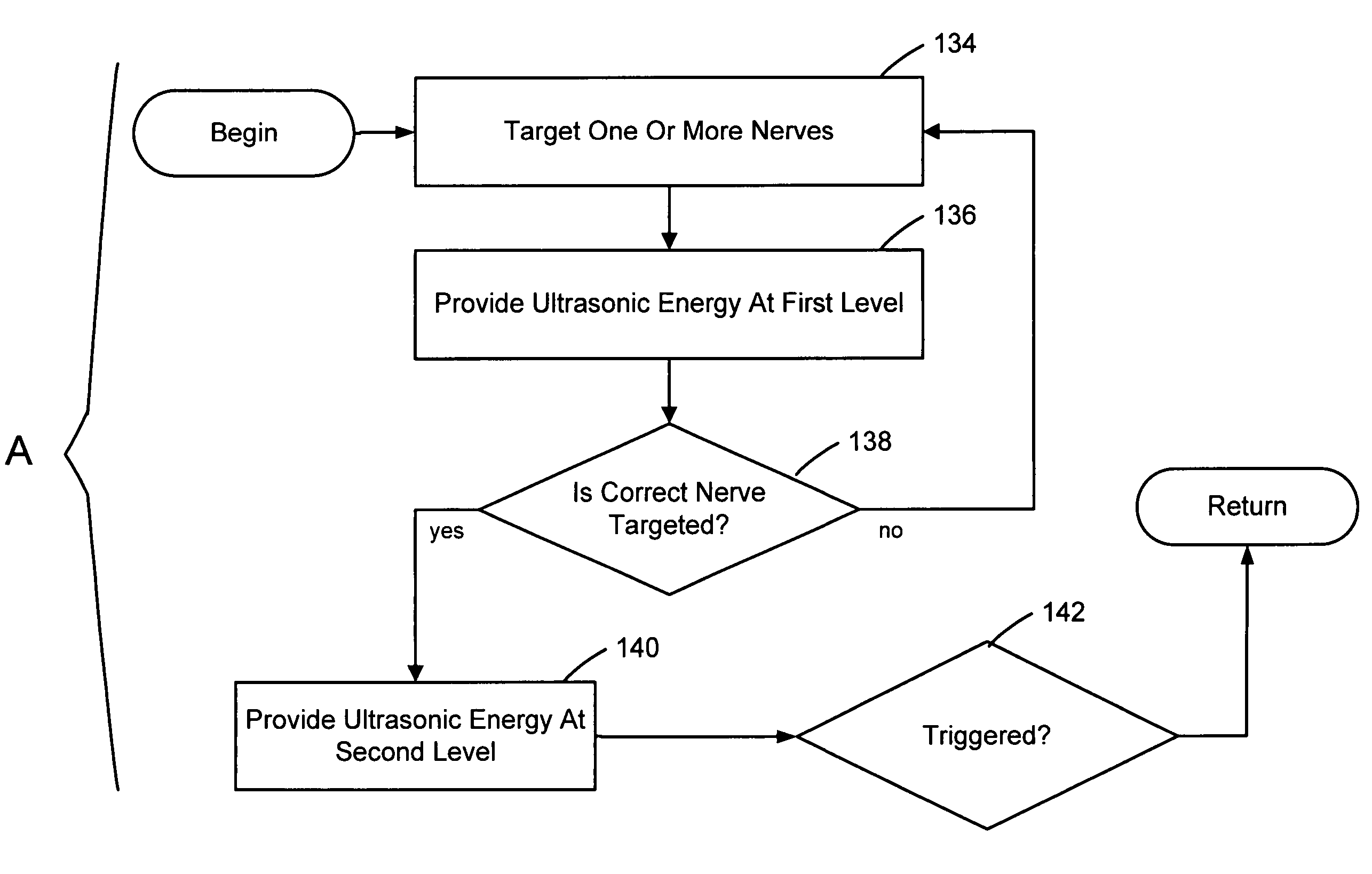
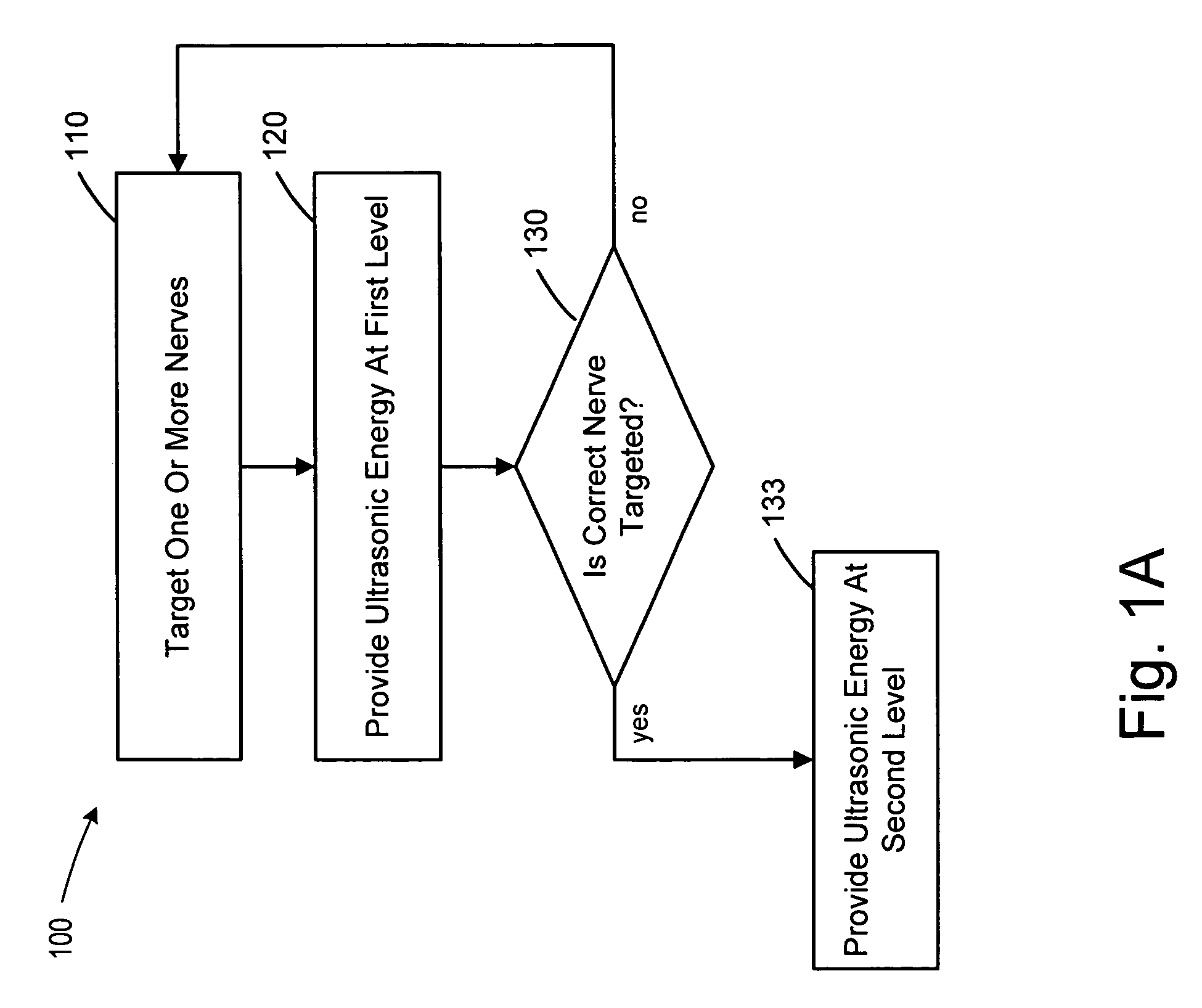
Focused ultrasound for pain reduction
Methods and devices that provide ultrasonic energy used to cause one or more nerves to become dysfunctional. A nerve to be treated is placed in the focal zone of ultrasonic energy emitted by ultrasound transducer. A first level of ultrasonic energy is provided to the nerve using the ultrasound transducer, the first level sufficient to stimulate the nerve. A verification is made that the desired nerve is being stimulated by the first level of ultrasonic energy. For example, the patient may be asked to confirm that the ultrasonically stimulated nerve corresponds to the pain that is affecting the patient. Subsequent to verifying the stimulated nerve is the nerve desired for the reduction of pain, a second level of ultrasonic energy is delivered to the nerve using the ultrasound transducer, the second level of ultrasonic energy sufficient to cause nerve dysfunction.
Owner:VAITEKUNAS JEFFREY J
Drug preparations for treating sexual dysfunction
Topical gelled compositions comprising a drug which causes vasodilation, and optionally prostaglandin E1, dispersed within a polymer matrix, and methods of treating sexual dysfunction, including both male and female sexual dysfunction, using said compositions.
Owner:L A M PHARMA +1
Method and apparatus for treating gland dysfunction
A method and apparatus for treating gland dysfunction caused by gland obstruction in order to restore the natural flow of secretion from the gland comprises the application of a combination of energy, suction, vibration, heat, aspiration, chemical agents and pharmacological agents to loosen and thereafter remove the obstructive material.
Owner:TEARSCIENCE INC
Formulations comprising selective androgen receptor modulators
InactiveUS20060004042A1Reduce incidenceDecreasing regressionBiocideAmide active ingredientsDiseaseProstate cancer incidence
The present invention relates to pharmaceutical compositions and formulations comprising a novel class of androgen receptor targeting agents (ARTA) which demonstrate androgenic and anabolic activity of a nonsteroidal ligand for the androgen receptor. The agents define a new subclass of compounds which are selective androgen receptor modulators (SARM) which are useful for a) male contraception; b) treatment of a variety of hormone-related conditions, for example conditions associated with Androgen Decline in Aging Male (ADAM), such as fatigue, depression, decreased libido, sexual dysfunction, erectile dysfunction, hypogonadism, osteoporosis, hair loss, anemia, obesity, sarcopenia, osteopenia, osteoporosis, benign prostate hyperplasia, alterations in mood and cognition and prostate cancer; c) treatment of conditions associated with Androgen Decline in Female (ADIF), such as sexual dysfunction, decreased sexual libido, hypogonadism, sarcopenia, osteopenia, osteoporosis, alterations in cognition and mood, depression, anemia, hair loss, obesity, endometriosis, breast cancer, uterine cancer and ovarian cancer; d) treatment and / or prevention of chronic muscular wasting; and / or e) decreasing the incidence of, halting or causing a regression of prostate cancer. The present invention provides pharmaceutical compositions comprising the selective androgen receptor modulator compounds, together with pharmaceutically acceptable excipients.
Owner:UNIV OF TENNESSEE RES FOUND
Multi-substituted selective androgen receptor modulators and methods of use thereof
InactiveUS20060183931A1Unexpected anabolicUnexpected androgenicSenses disorderTin organic compoundsDiseaseProstate cancer incidence
This invention provides androgen receptor targeting agents (ARTA). The agents define a new subclass of compounds, which are selective androgen receptor modulators (SARM). Several of the SARM compounds have been found to have an unexpected androgenic and anabolic activity of a nonsteroidal ligand for the androgen receptor. Other SARM compounds have been found to have an unexpected antiandrogenic activity of a nonsteroidal ligand for the androgen receptor. The SARM compounds, either alone or as a composition, are useful for a) male contraception; b) treatment of a variety of hormone-related conditions, for example conditions associated with Androgen Decline in Aging Male (ADAM), such as fatigue, depression, decreased libido, sexual dysfunction, erectile dysfunction, hypogonadism, osteoporosis, hair loss, anemia, obesity, sarcopenia, osteopenia, osteoporosis, benign prostate hyperplasia, alterations in mood and cognition and prostate cancer; c) treatment of conditions associated with Androgen Decline in Female (ADIF), such as sexual dysfunction, decreased sexual libido, hypogonadism, sarcopenia, osteopenia, osteoporosis, alterations in cognition and mood, depression, anemia, hair loss, obesity, endometriosis, breast cancer, uterine cancer and ovarian cancer; d) treatment and / or prevention of acute and / or chronic muscular wasting conditions; e) preventing and / or treating dry eye conditions; f) oral androgen replacement therapy; and / or g) decreasing the incidence of, halting or causing a regression of prostate cancer
Owner:UNIV OF TENNESSEE RES FOUND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com