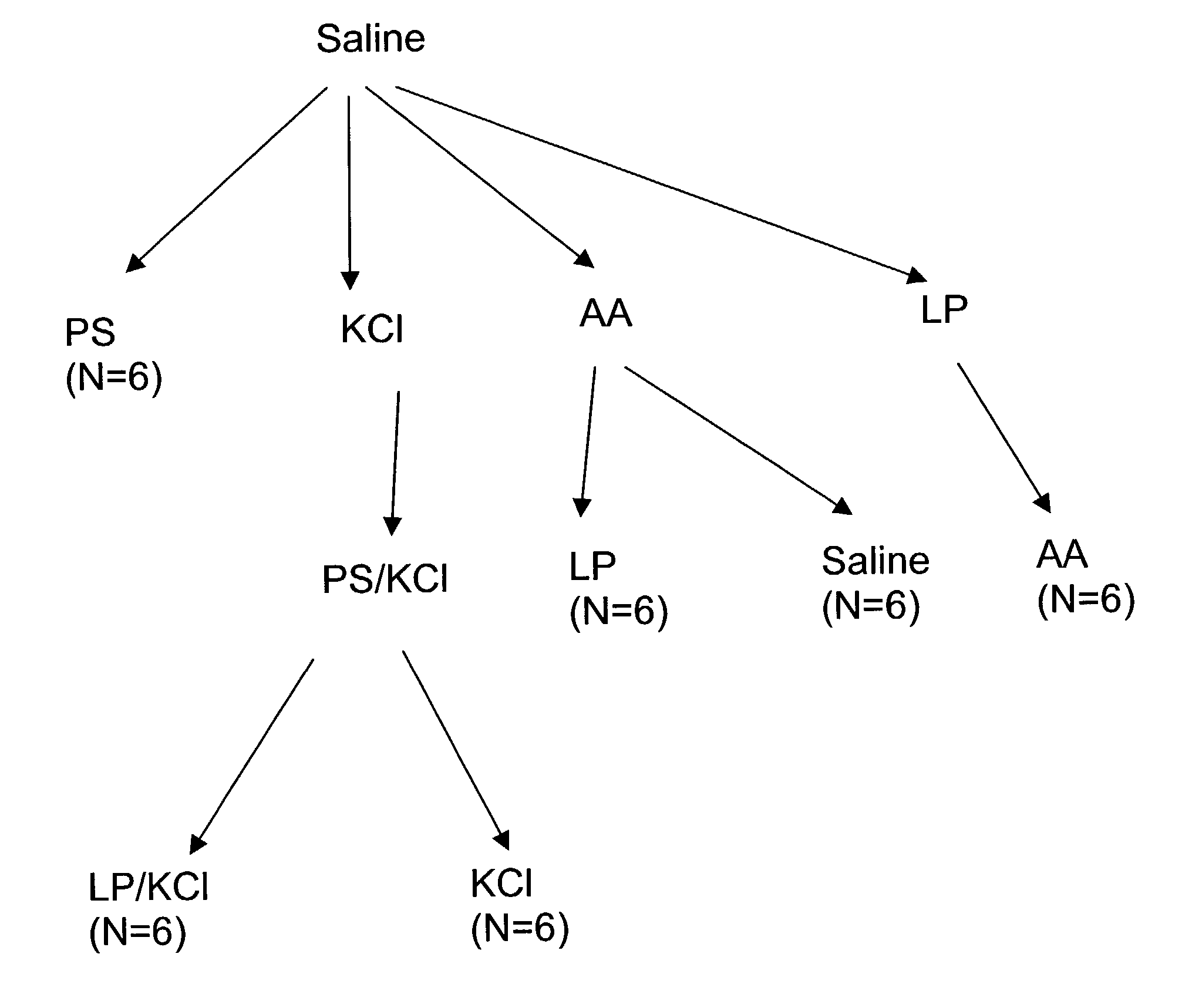
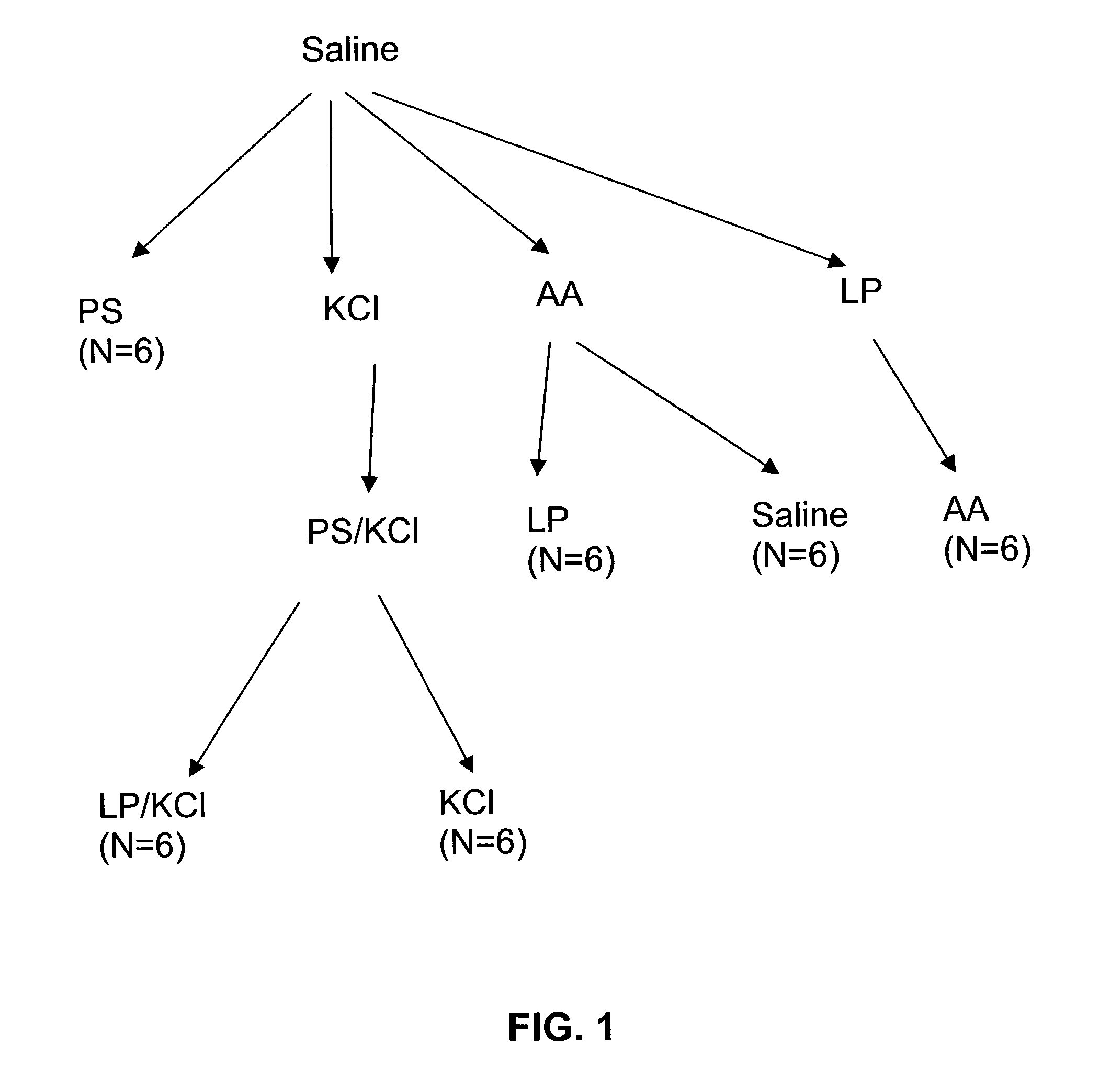
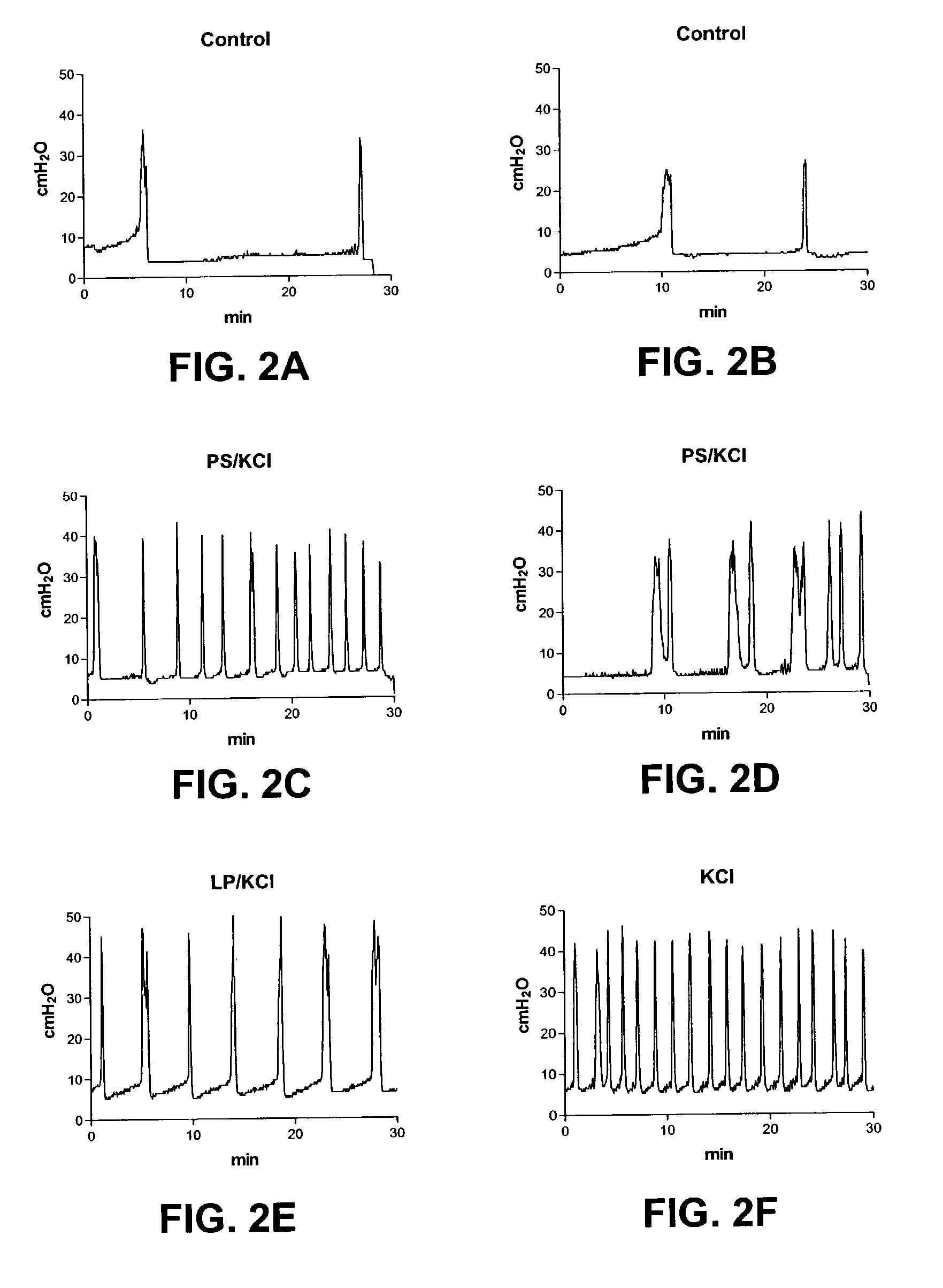
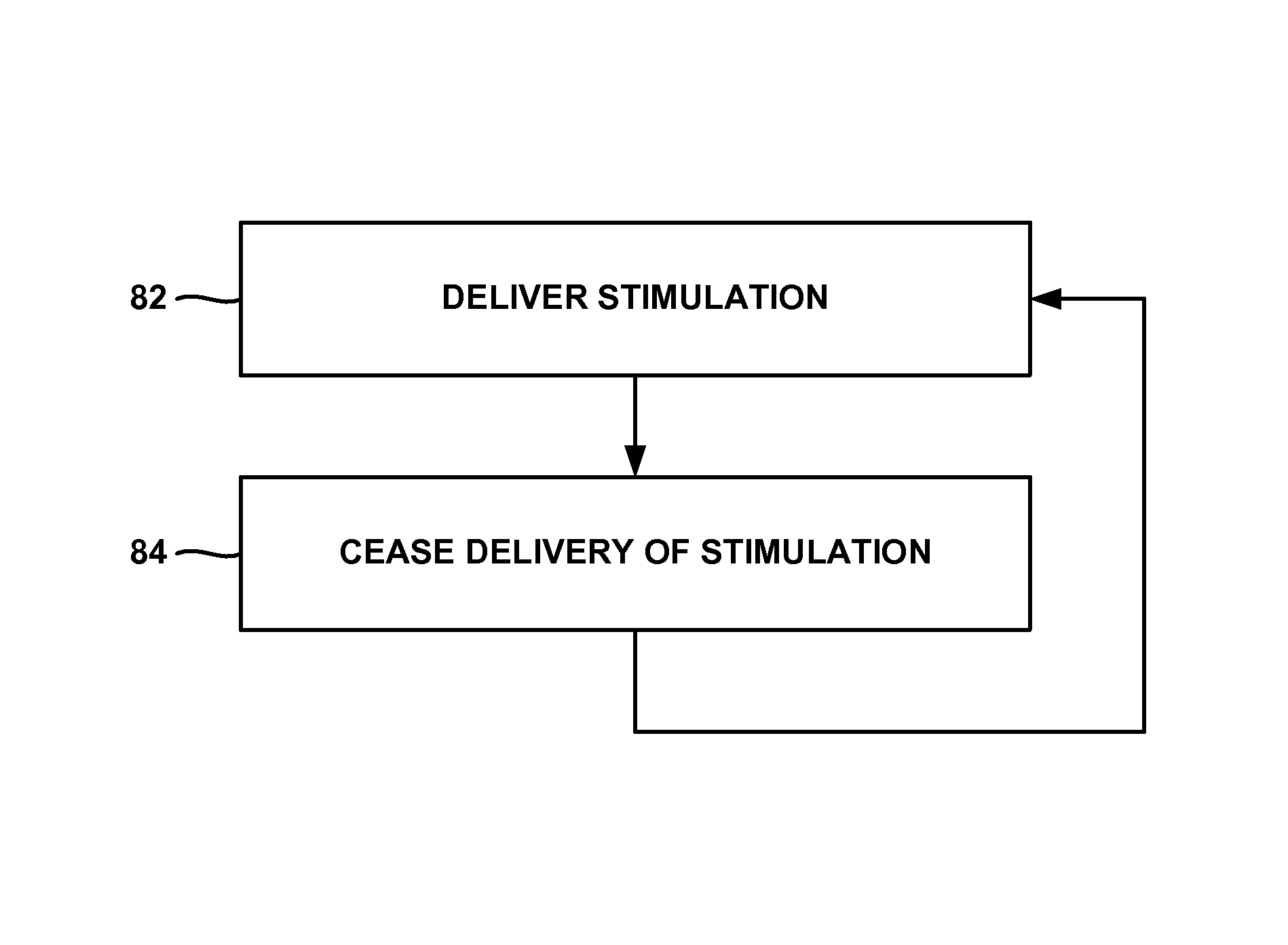
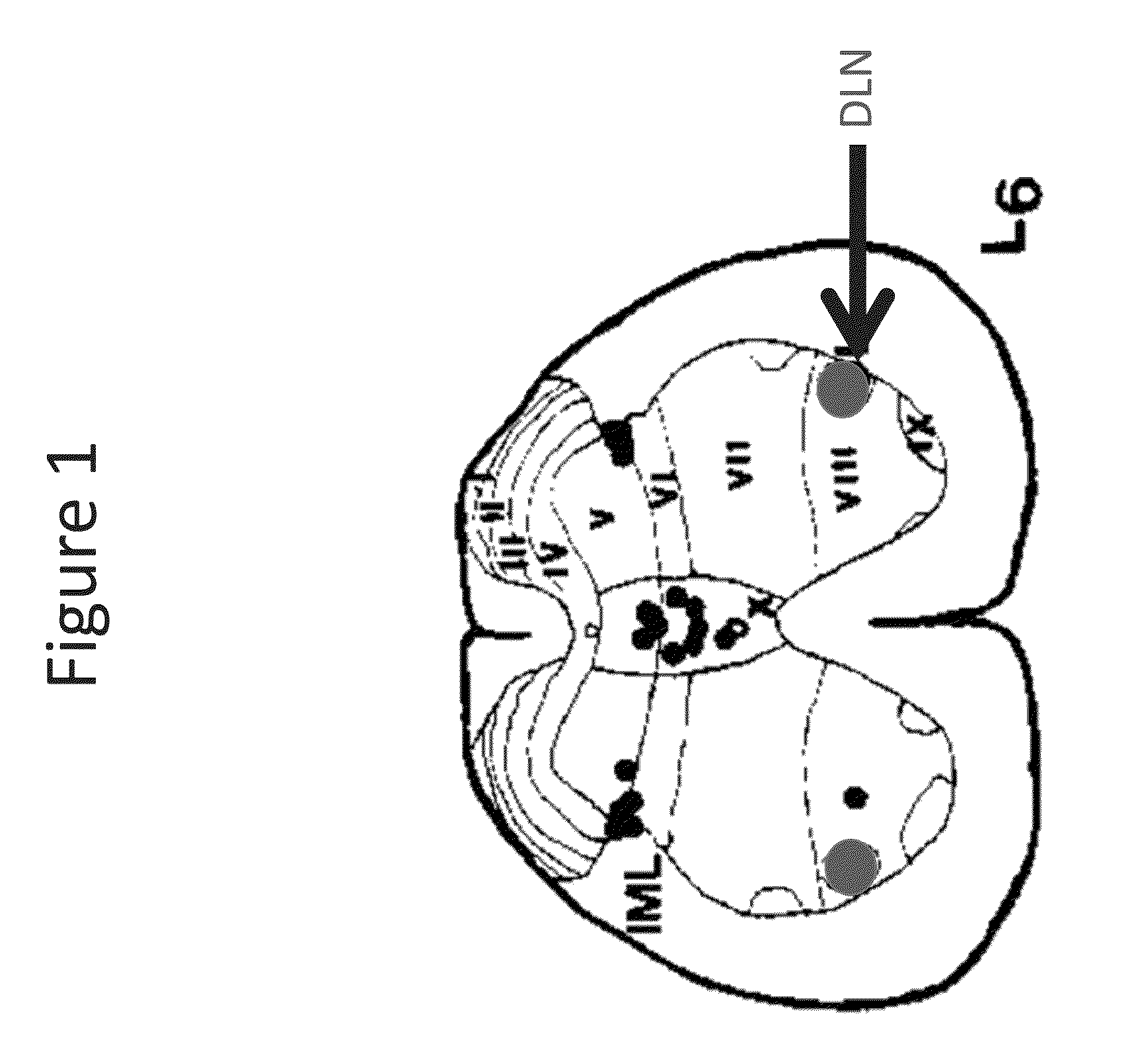
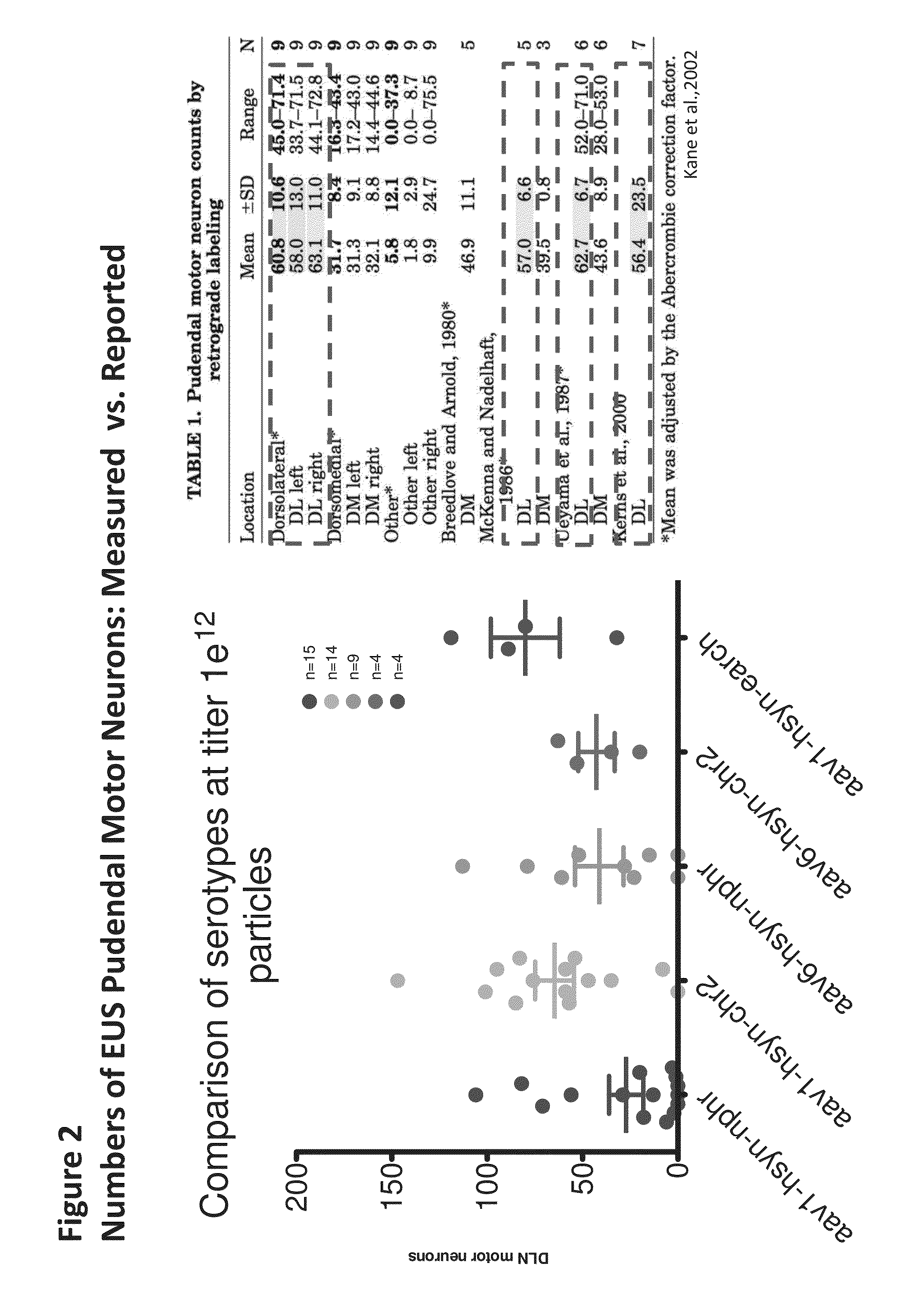
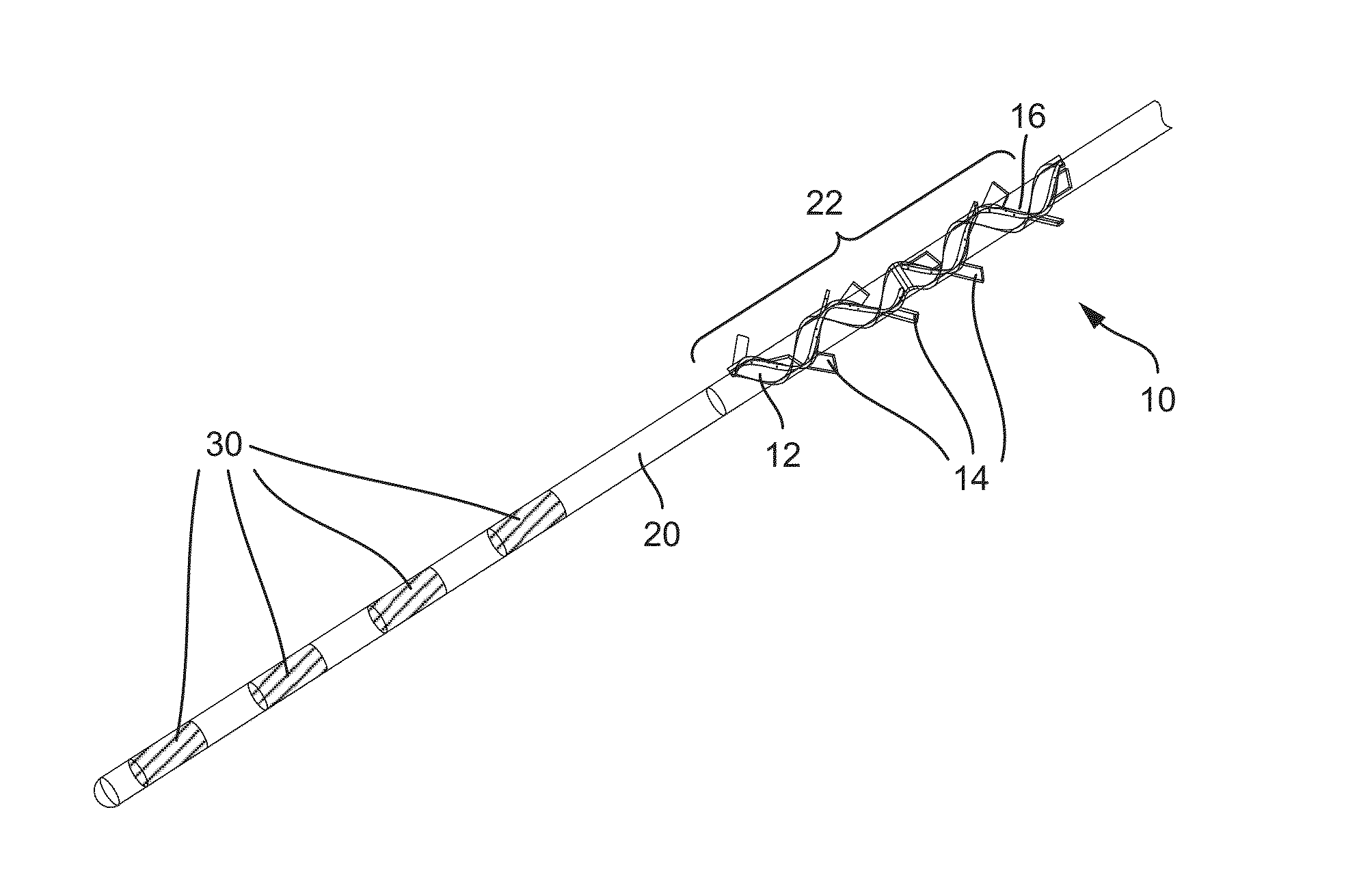
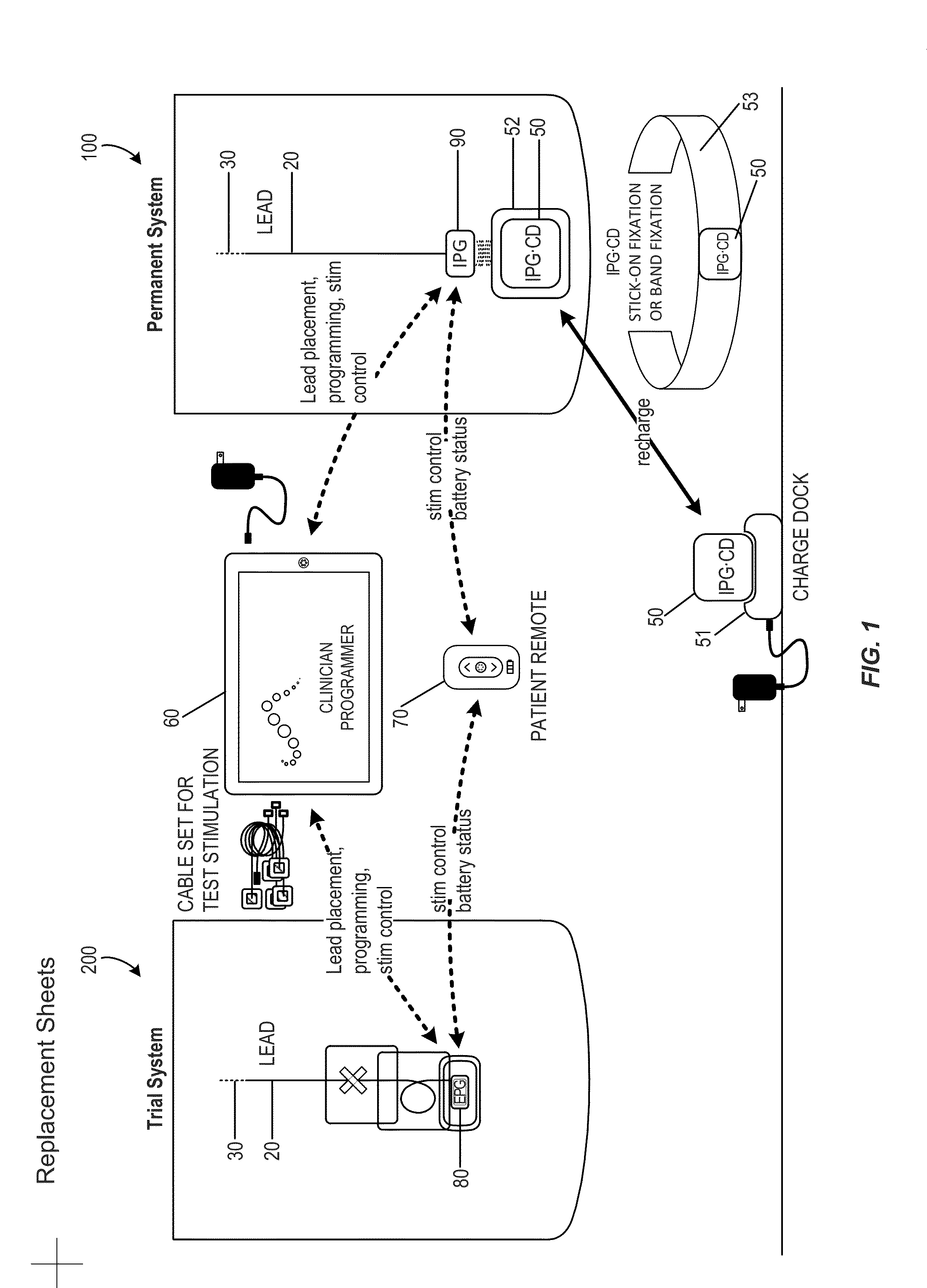
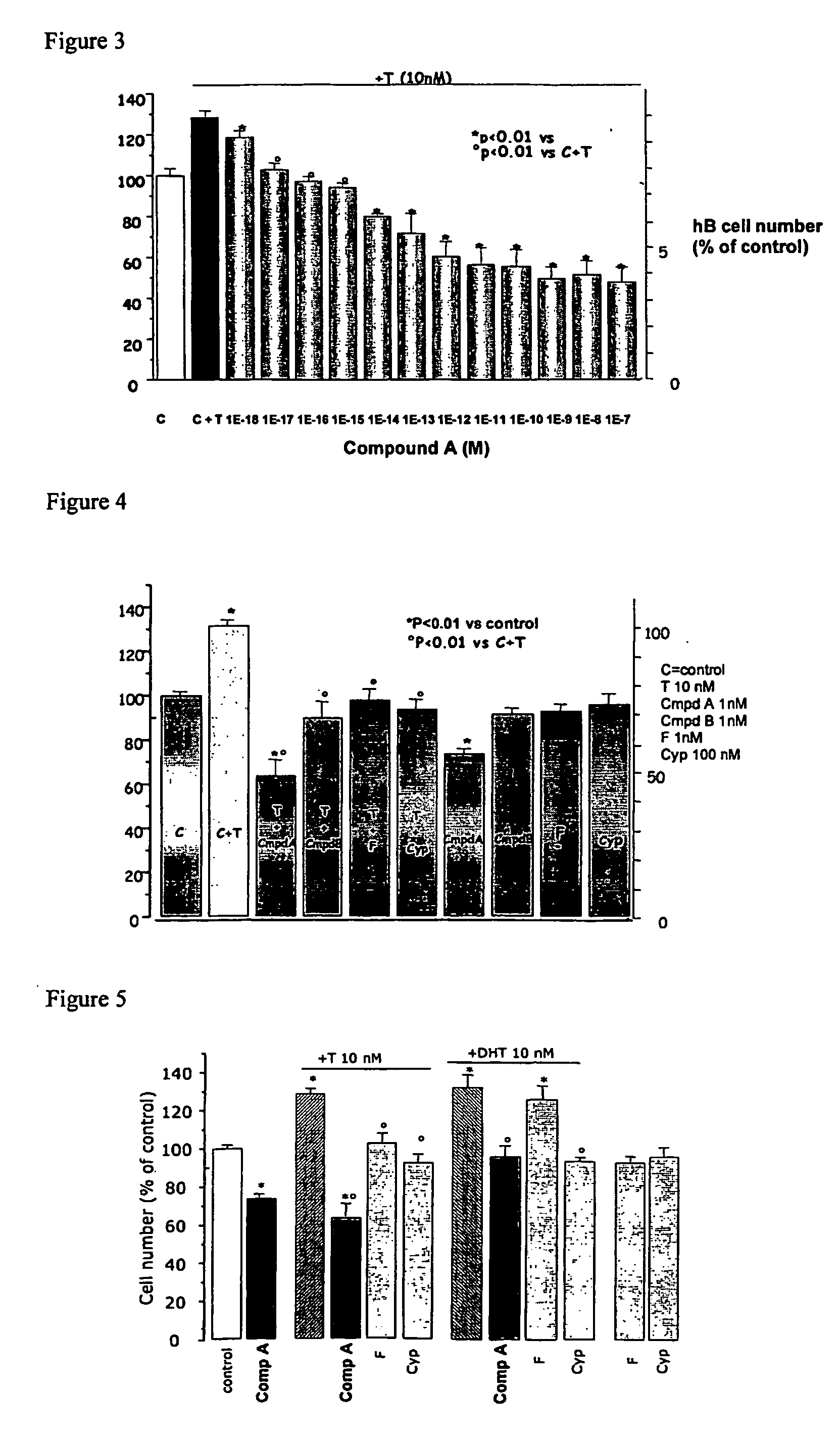
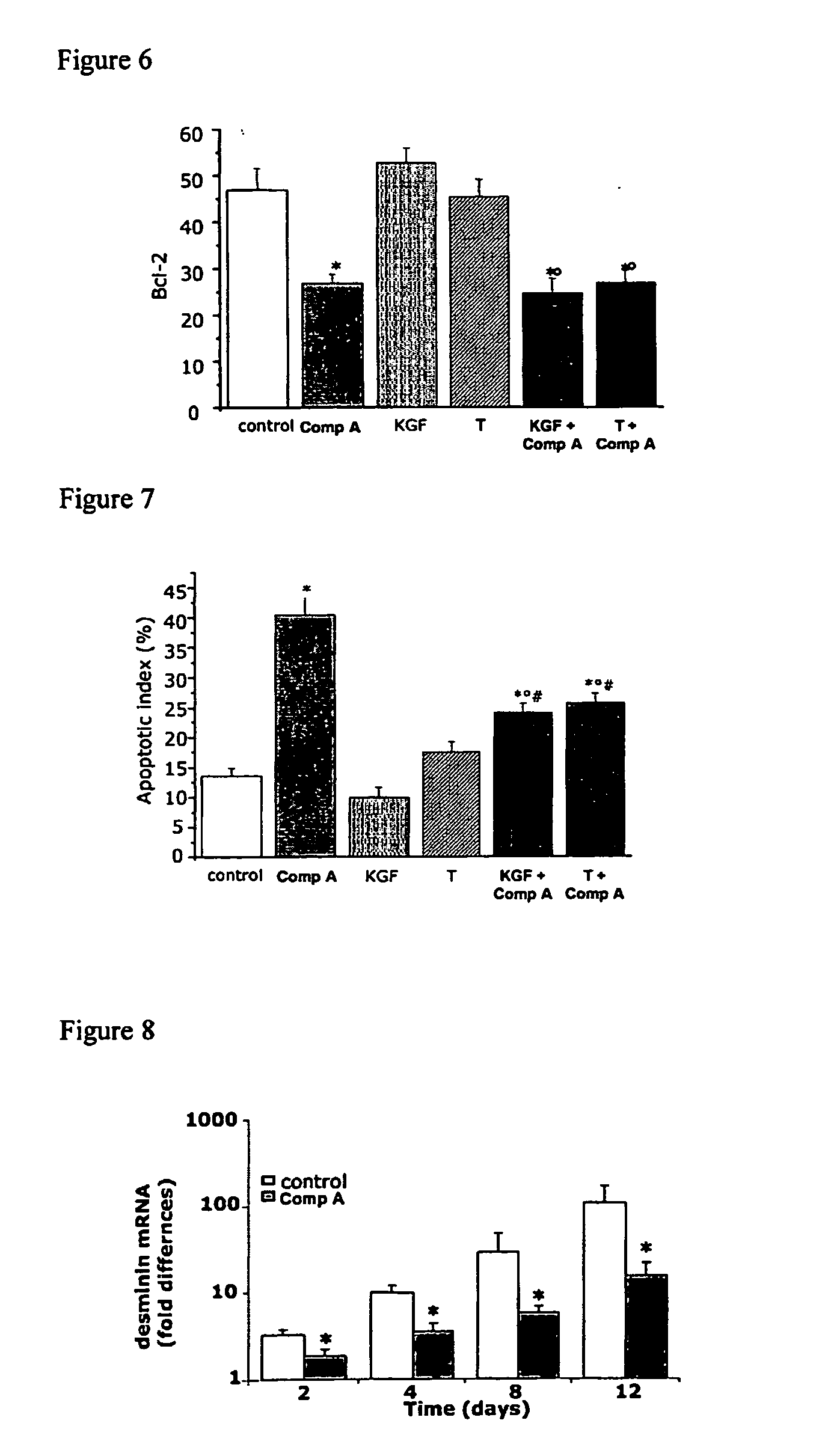
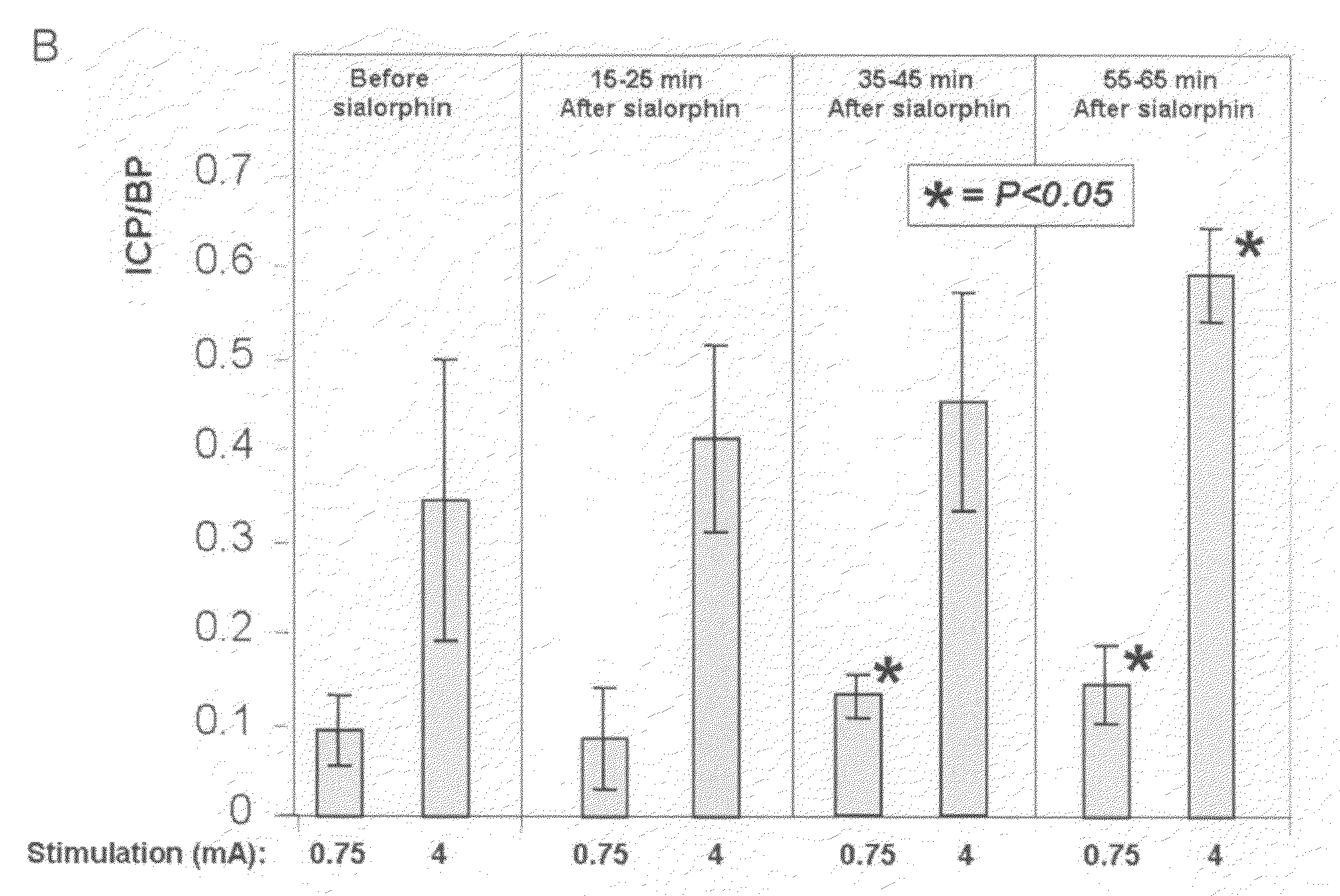
Patents
Literature
62 results about "Bladder dysfunctions" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
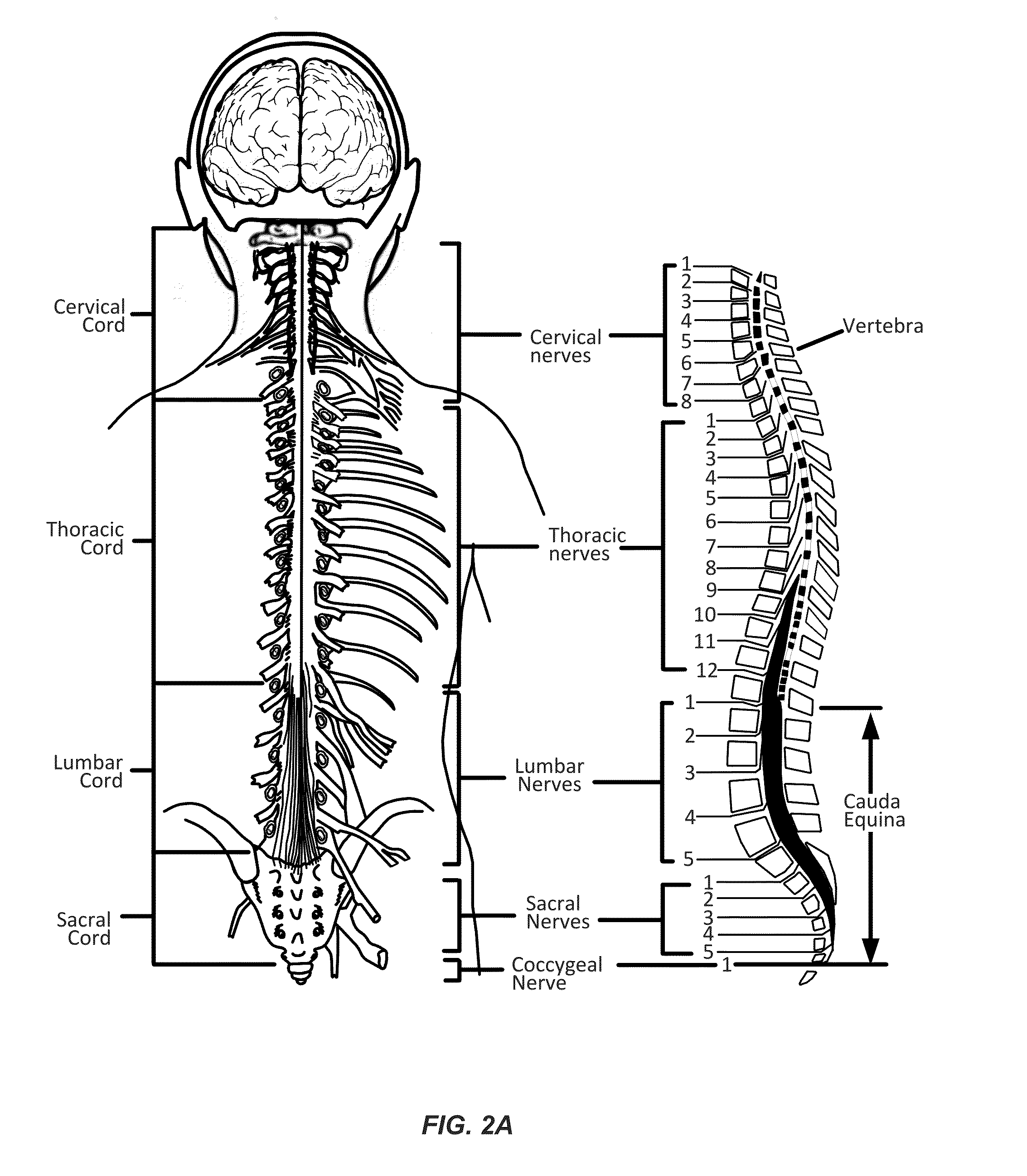
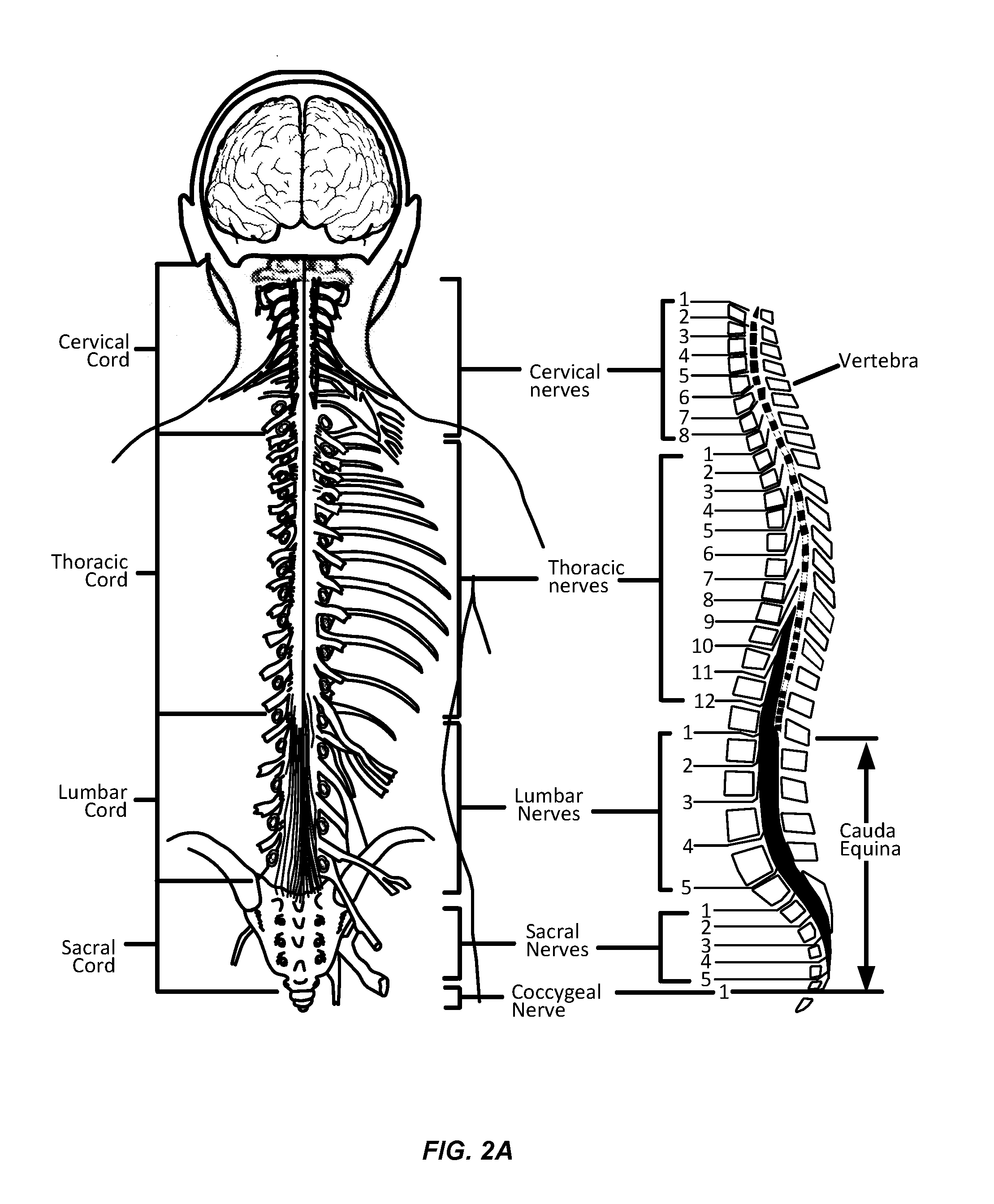
Neurogenic bladder dysfunction, sometimes simply referred to as neurogenic bladder, is a dysfunction of the urinary bladder due to disease of the central nervous system or peripheral nerves involved in the control of micturition (urination).
Application of lipid vehicles and use for drug delivery
InactiveUS7063860B2Reduce and prevent antibody-mediated resistanceIncrease stimulationBiocideAntipyreticAnticarcinogenCapsaicin
The present invention relates to compositions and methods for the administration of lipid-based vehicles to treat various disorders, including bladder inflammation, infection, dysfunction, and cancer. In various aspects, the compositions and methods of the invention are useful for prolonged delivery of drugs, e.g., antibiotics, pain treatments, and anticancer agents, to the bladder, genitourinary tract, gastrointestinal system, pulmonary system, and other organs or body systems. In particular, the present invention relates to liposome-based delivery of vanilloid compounds, such as resiniferatoxin, capsaicin, or tinyatoxin, and toxins, such as botulinum toxin, for the treatment of bladder conditions, including pain, inflammation, incontinence, and voiding dysfunction. Further related are methods of using these vehicles alone or in conjunction with antibodies, e.g., uroplakin antibodies, to improve duration of liposome attachment, and provide a long-term intravesical drug delivery platform. The present invention specifically relates to antibody-coated liposomes that are useful for targeting specific receptors for drug, peptide, polypeptide, or nucleic acid delivery. In one particular aspect, the present invention relates to liposomes coated with antibodies against nerve growth factor (NGF) receptor and containing NGF antisense nucleic acids, which are used as a treatment for neurogenic bladder dysfunction.
Owner:UNIVERSITY OF PITTSBURGH
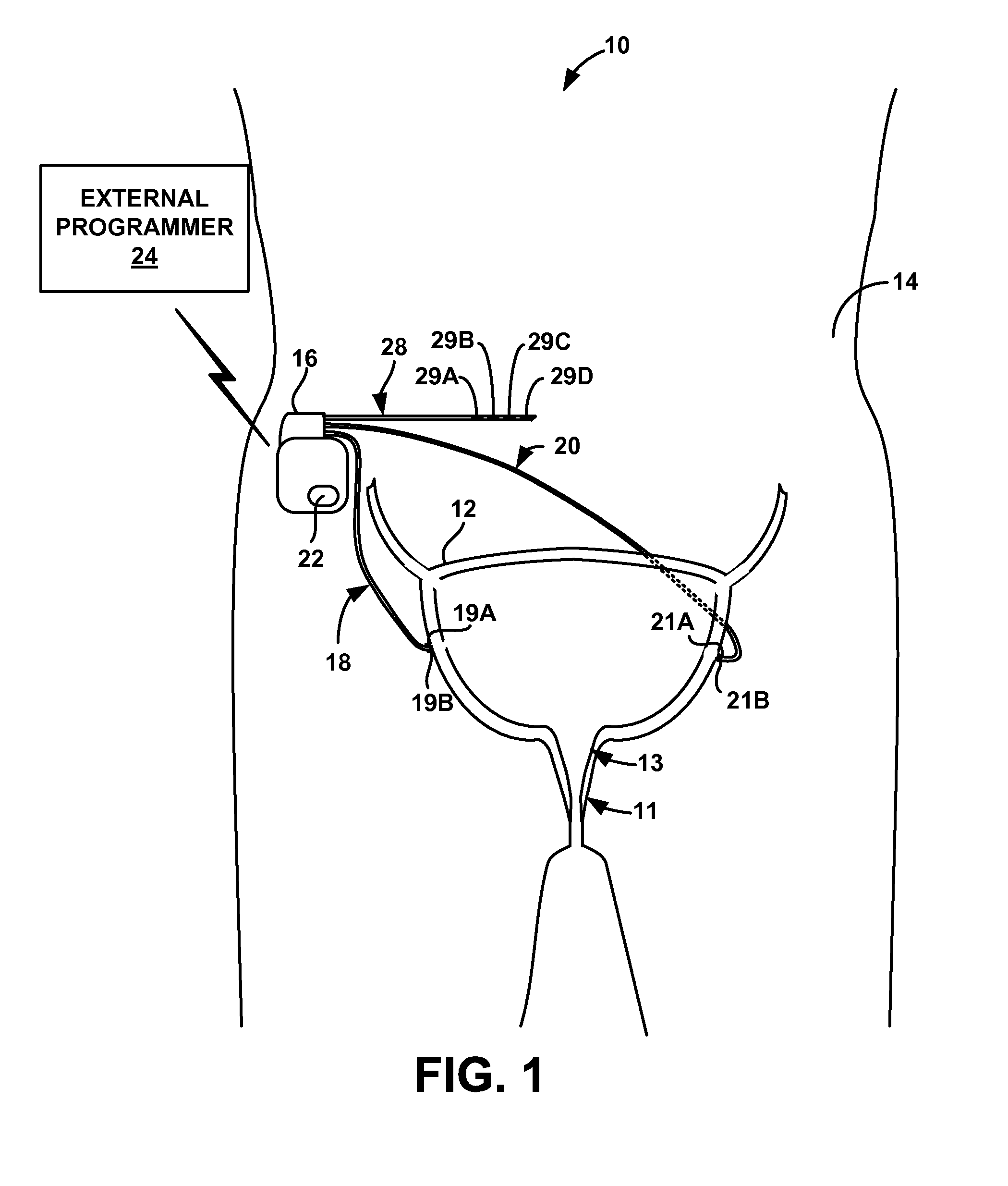
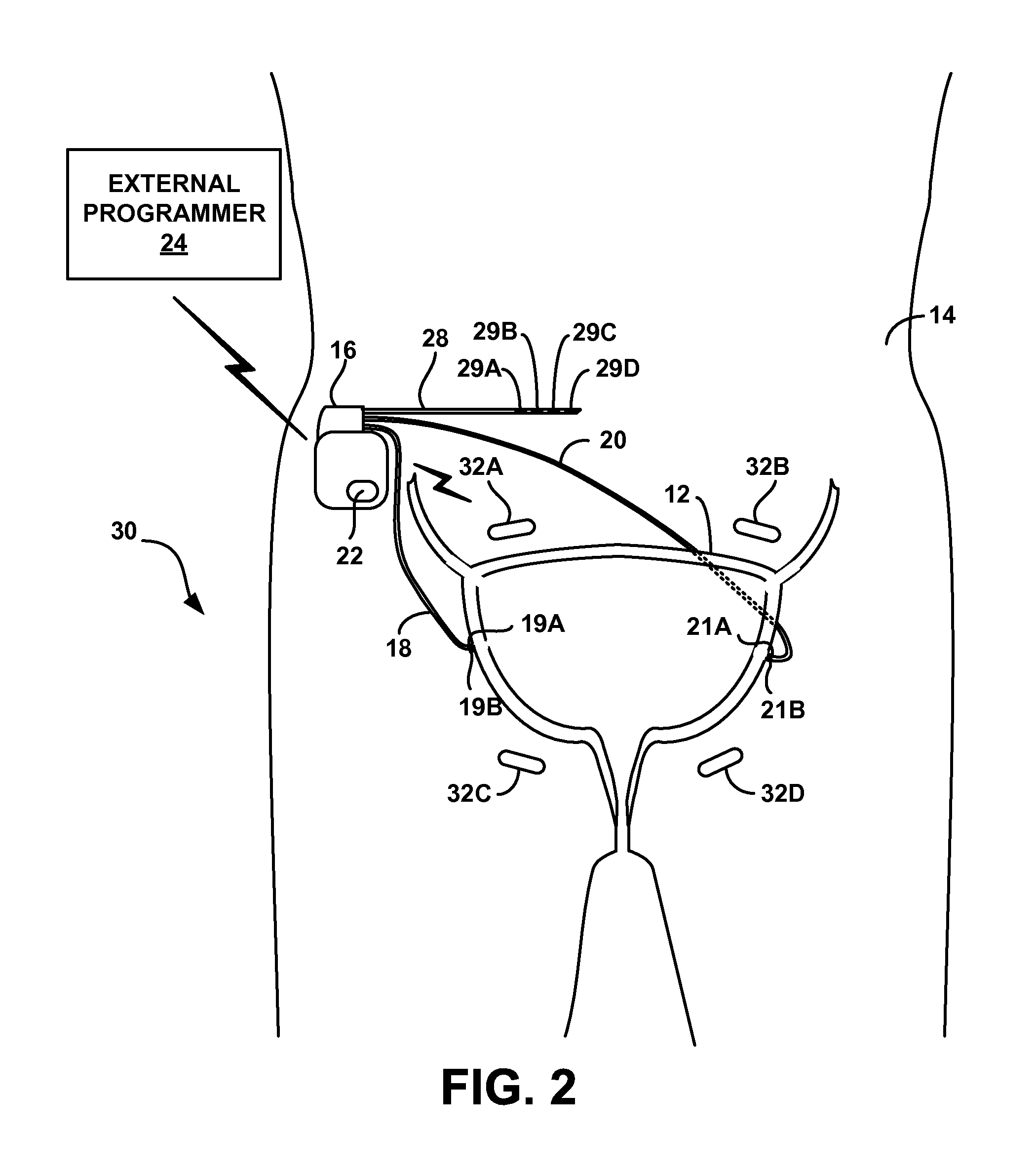
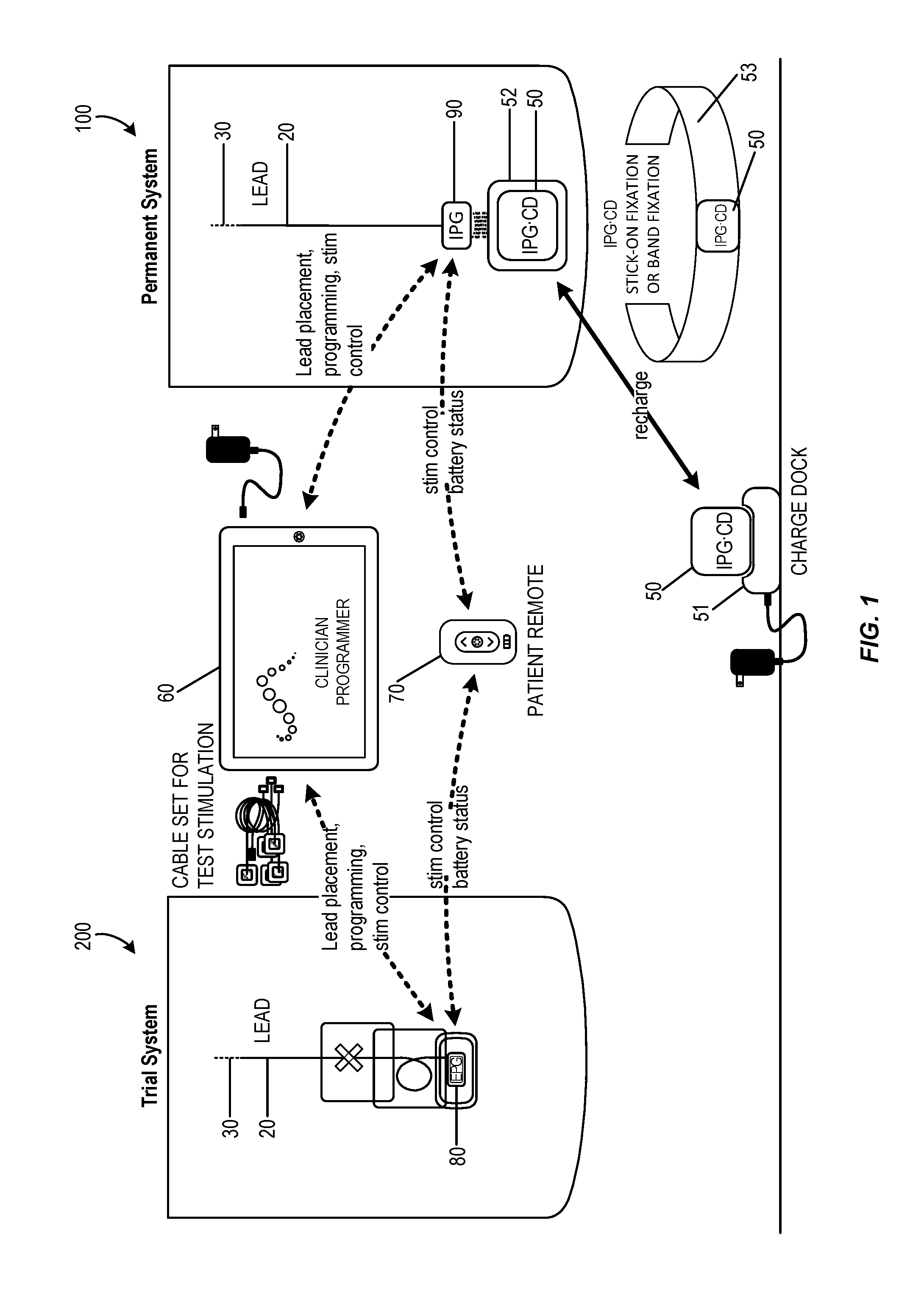
Stimulation therapy for bladder dysfunction
ActiveUS8989861B2Reduce stimulus intensityDecrease in frequency of bladder contractionElectromyographySensorsPhysical therapyElectrical stimulations
A medical system may include a control module and a therapy delivery module configured to generate and deliver electrical stimulation therapy to a patient. The control module may be configured to control the therapy delivery module to deliver electrical stimulation at a first stimulation intensity for a first time period, to deliver electrical stimulation at a second stimulation intensity for a second time period immediately following the first time period, and to deliver electrical stimulation at the first stimulation intensity for a third time period immediately following the second time period. The second stimulation intensity may be less than the first stimulation intensity. The electrical stimulation may elicit a first inhibitory physiological response during the first time period and a second inhibitory physiological response during the second time period. The second inhibitory physiological response may be greater than the first inhibitory physiological response.
Owner:MEDTRONIC INC
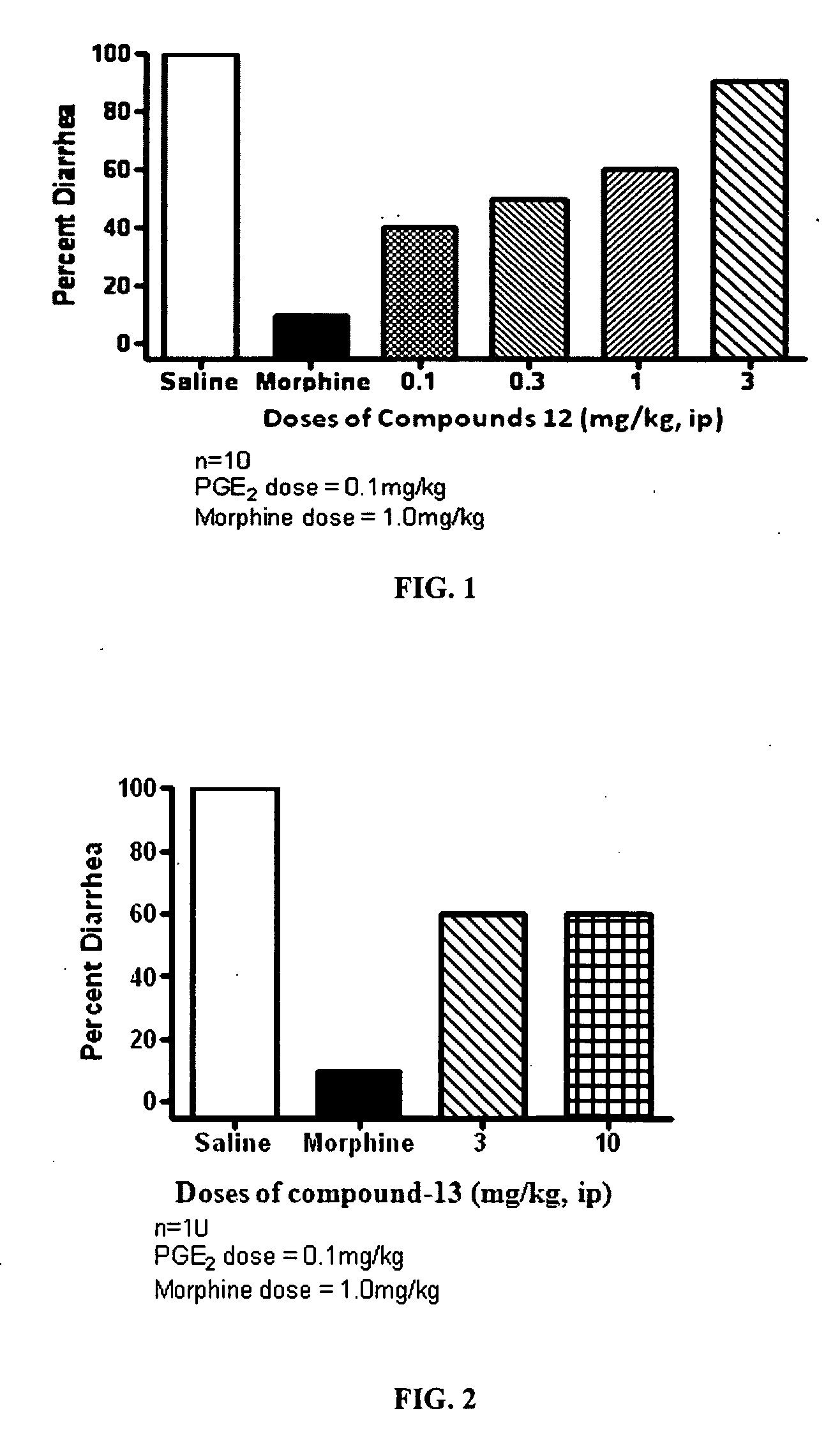
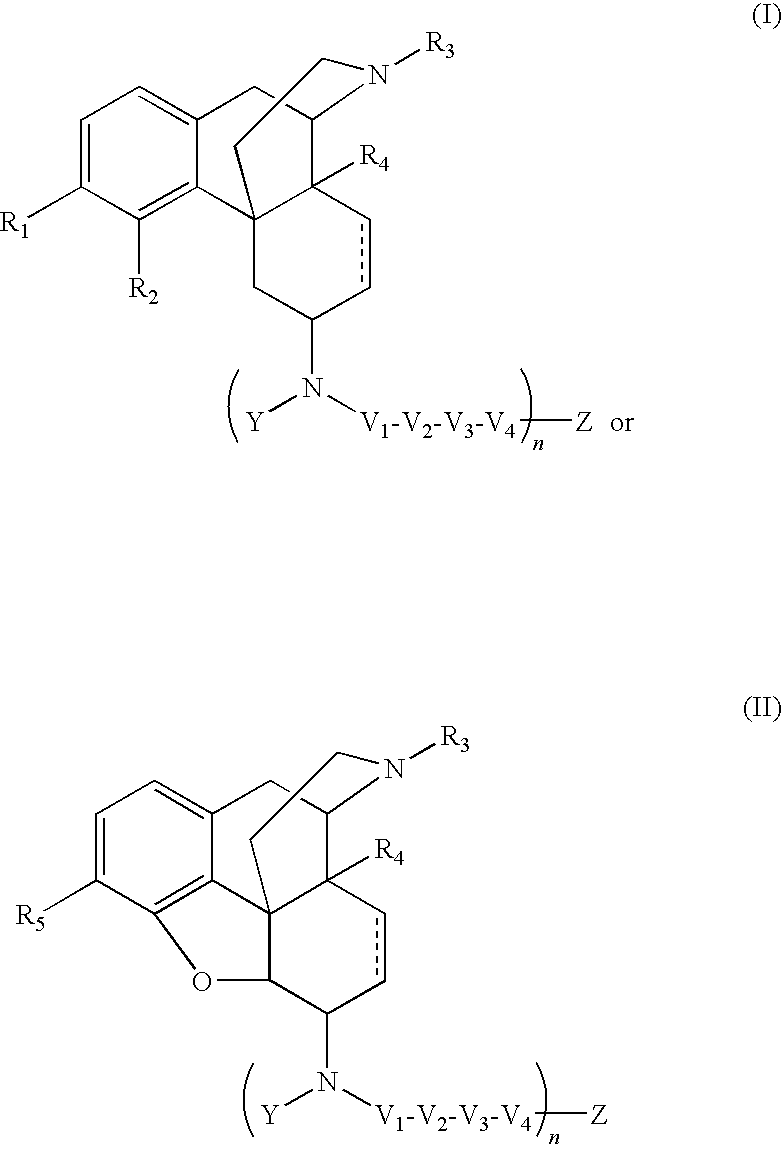
Selective opioid compounds
ActiveUS20090209569A1Reducing lipid permeability of drugReduce penetrationAntibacterial agentsBiocideDiseaseInterstitial cystitis
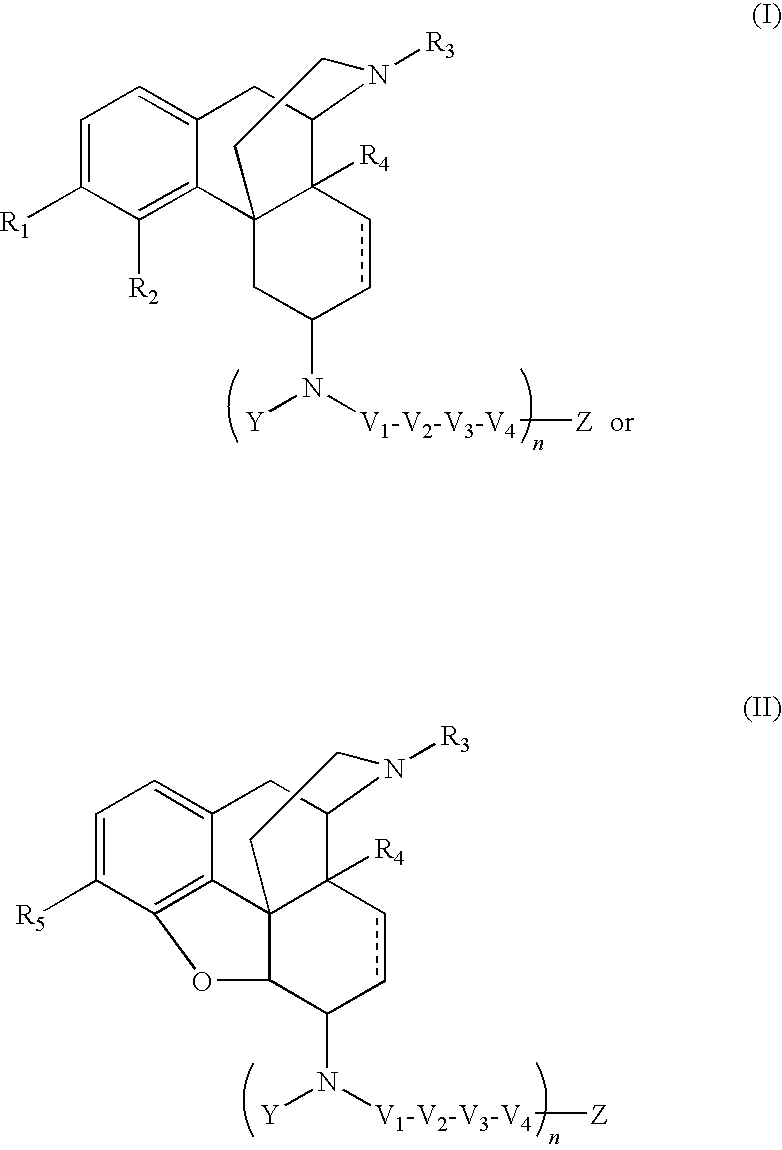
The present invention relates to compounds of Formula I or II, or pharmaceutically acceptable salts, esters, or prodrugs thereof:which relates to morphinan compounds useful as μ, δ, and / or κ receptor opioid compounds and pharmaceuticals containing same that may be useful for mediating analgesia, combating drug addiction, alcohol addiction, drug overdose, mental illness, bladder dysfunctions, neurogenic bladder, interstitial cystitis, urinary incontinence, premature ejaculation, inflammatory pain, peripherally mediated and neuropathic pain, cough, lung edema, diarrhea, cardiac disorders, cardioprotection, depression, and cognitive, respiratory, diarrhea, irritable bowel syndrome and gastro-intestinal disorders, immunomodulation, and anti-tumor agents.
Owner:ALKERMES INC
Treatment of bladder dysfunction
InactiveUS20080177398A1Prevent bladder overdistensionPreventing bladder overdistensionElectrotherapyElectric controllersFunctional disturbanceBladder dysfunctions
A method is provided for treatment of bladder dysfunction of a patient. The method includes, at a site within a patient, measuring an indication of fill-level of a bladder of the patient, and in response to an indication of a high bladder fill-level, conveying a signal indicative of the high bladder fill-level to the patient in a humanly-perceptible manner. Other embodiments are also described.
Owner:GROSS +1
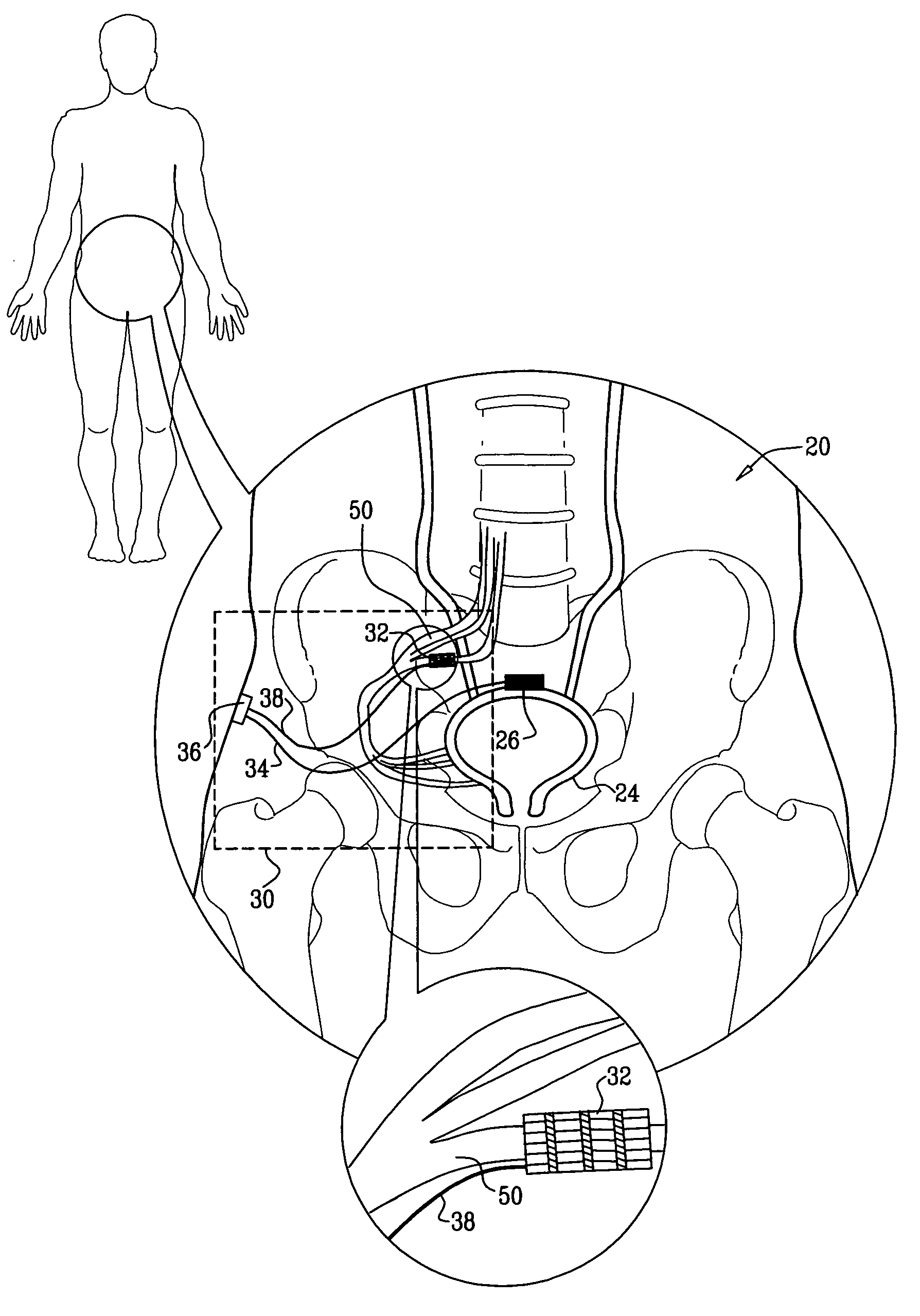
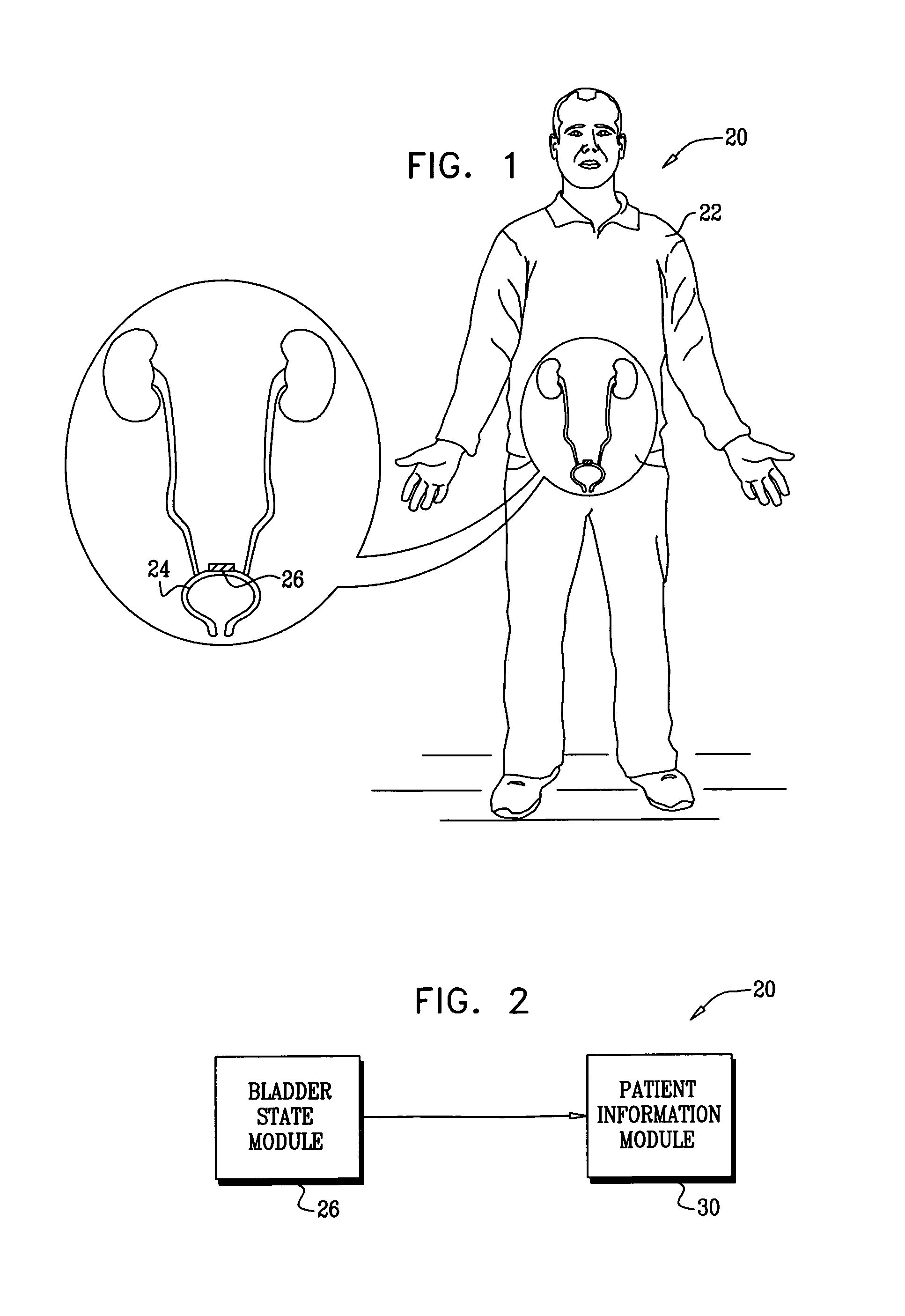
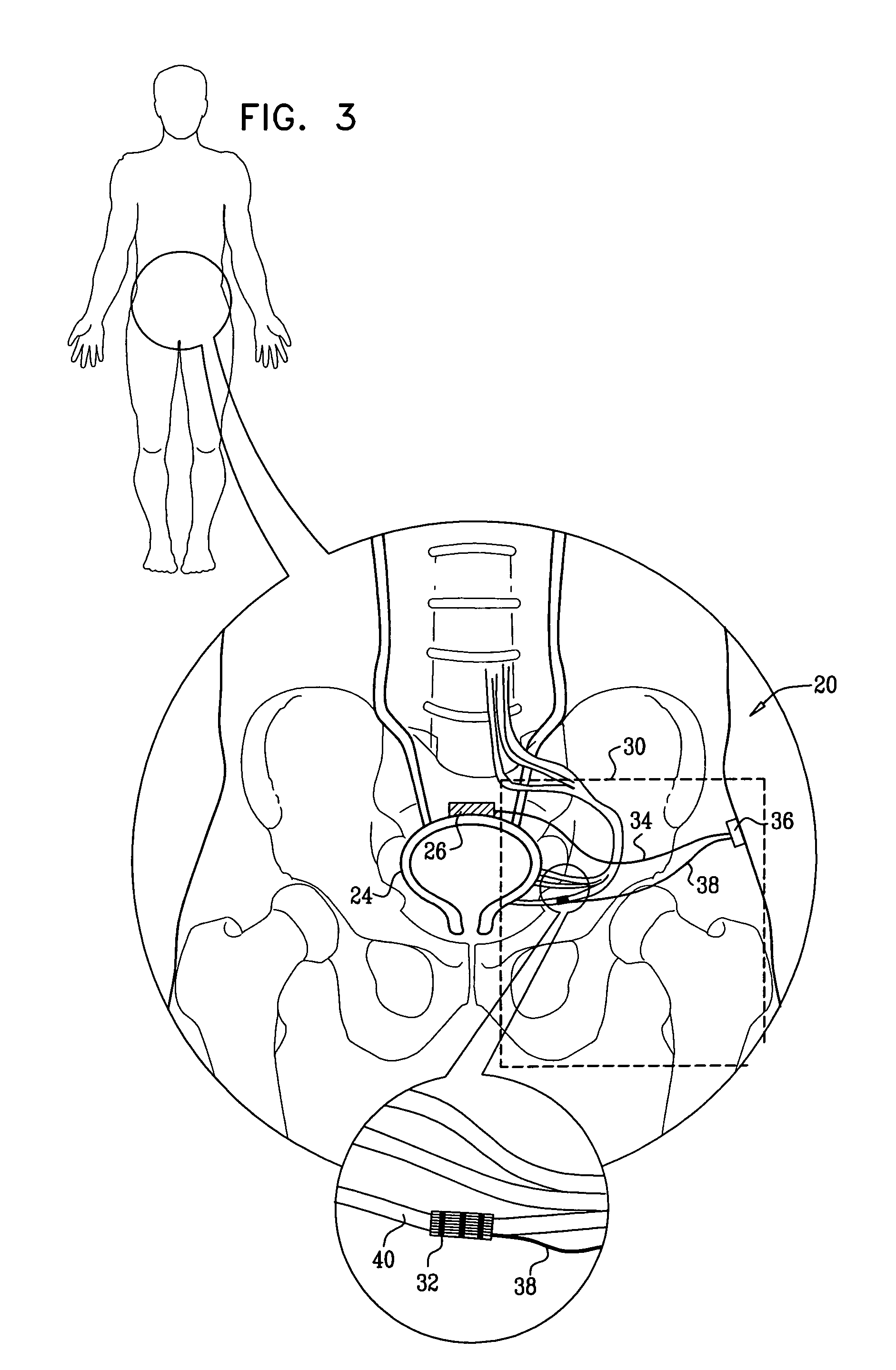
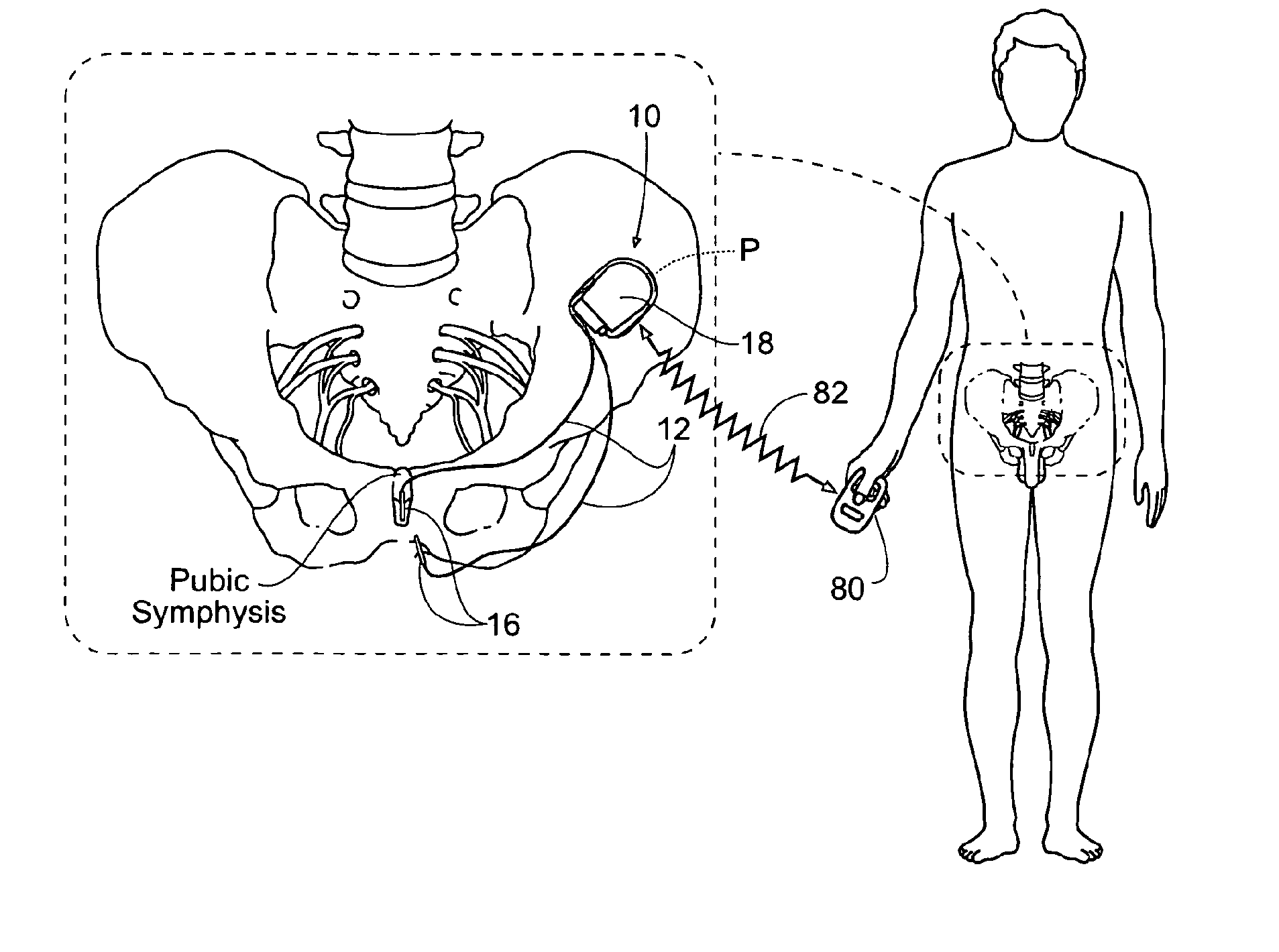
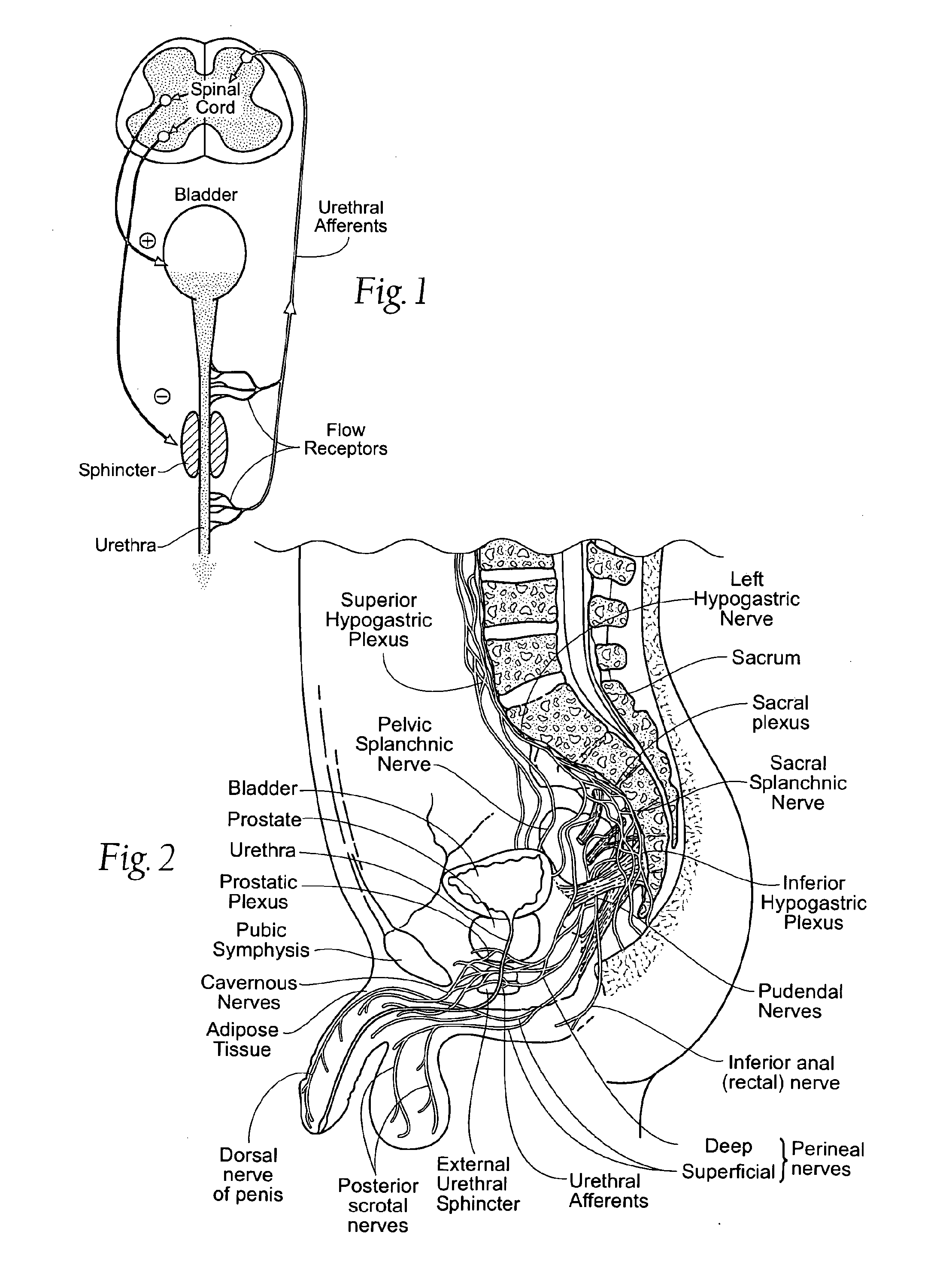
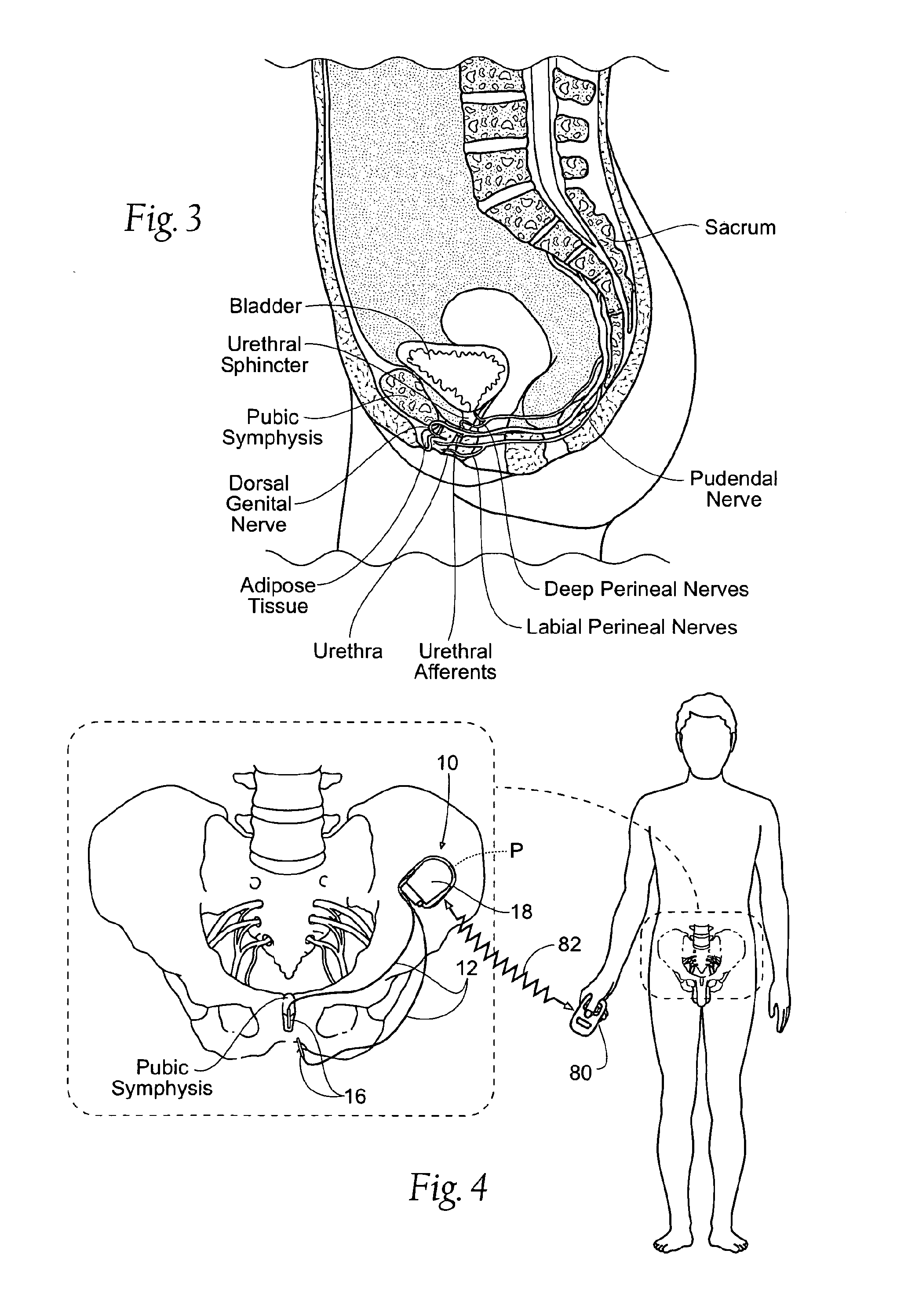
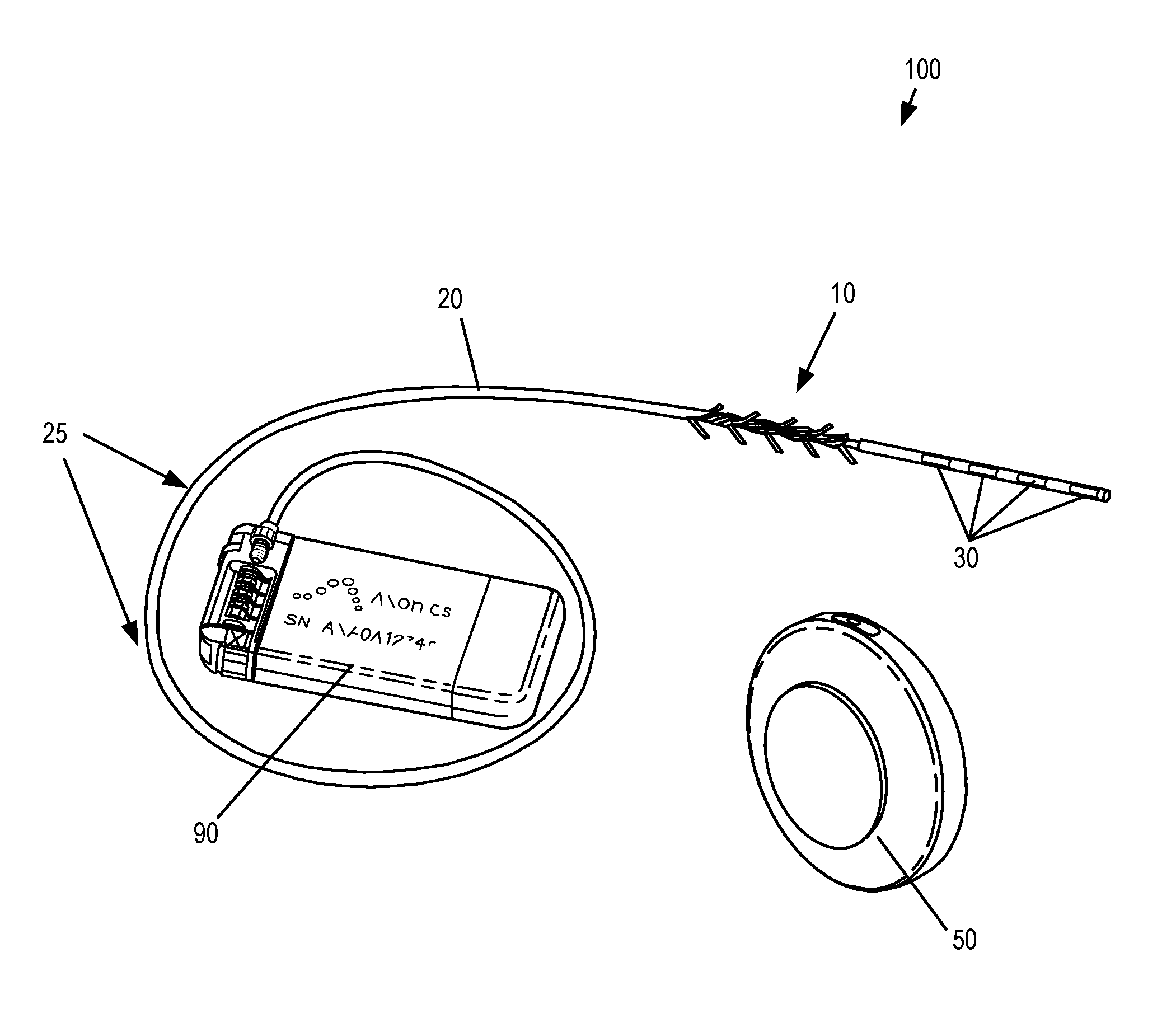
Systems and methods for the treatment of bladder dysfunctions using neuromodulation
Systems and Methods treat bladder dysfunctions using neuromodulation stimulation. Bladder emptying through stimulation of urethral afferents, and continence through stimulation of the dorsal genital nerve, is provided with one or more implanted pulse generators and one or more leads. A simple surgical procedure preserves all existing functions. A stimulating catheter is provided to be used as a clinical screening tool. The stimulating catheter is used to measure bladder pressures and stimulate the urethra at the same time.
Owner:MEDTRONIC URINARY SOLUTIONS
Compositions and methods for treating neurogenic disorders of the pelvic floor
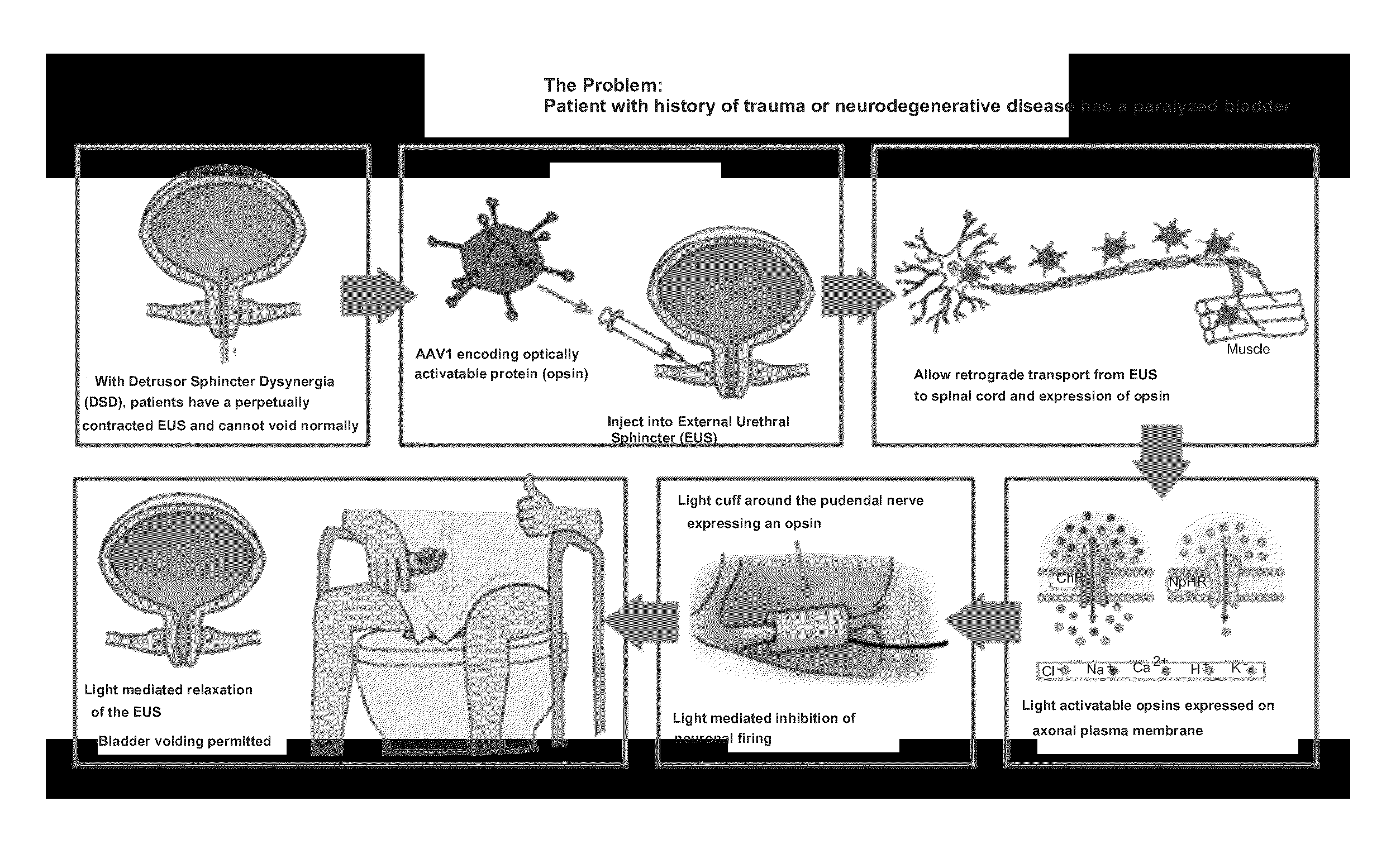
ActiveUS20160038761A1Induce depolarizationCompounds screening/testingElectrotherapyDetrusor hyperreflexiaPelvic diaphragm muscle
Provided herein are methods for the treatment of bladder dysfunction, including detrusor hyperreflexia and detrusor external sphincter dyssynergia, fecal incontinence, and / or sexual dysfunction in an individual via the use of stably expressed light-responsive opsin proteins capable of selective hyperpolarization or depolarization of the neural cells that innervate the muscles responsible for physiologic functioning of urinary bladder, external urinary sphincter, external anal sphincter, and the male and female genitalia.
Owner:CIRCUIT THERAPEUTICS +1
Use of neurotoxin therapy for treatment of urologic and related disorders related to neurogenic bladder dysfunction
InactiveUS7449192B2Inexpensive and safeIncrease capacityBacterial antigen ingredientsPeptide/protein ingredientsDiseaseUrethra
The present invention related to methods for treating neurological-urological conditions, including neurogenic bladder dysfunction. This is accomplished by administration of a botulinum toxin into the lower urinary tract of a patient with a neurogenic bladder dysfunction, including the bladder or urinary sphincter and the bladder wall.
Owner:ALLERGAN INC
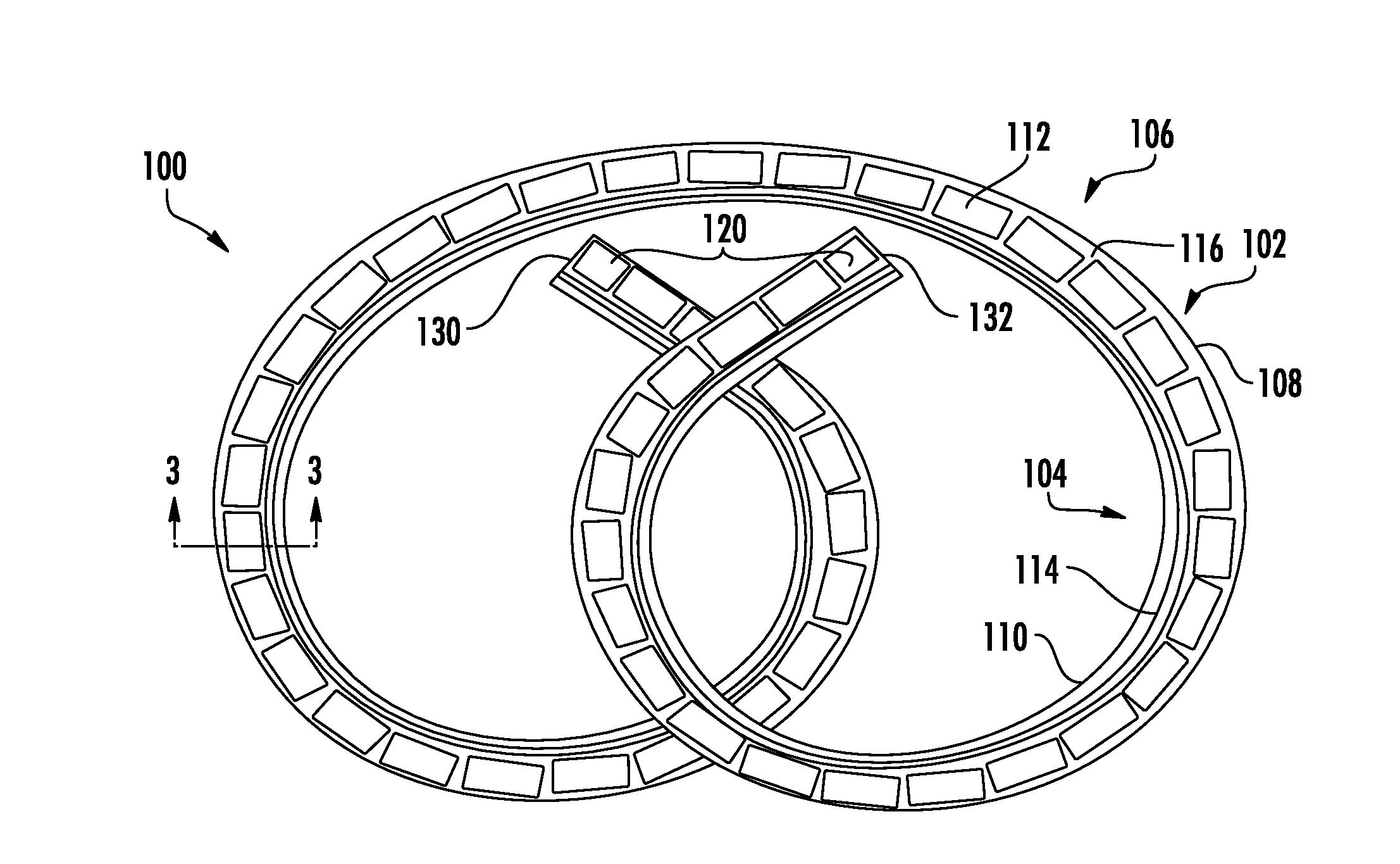
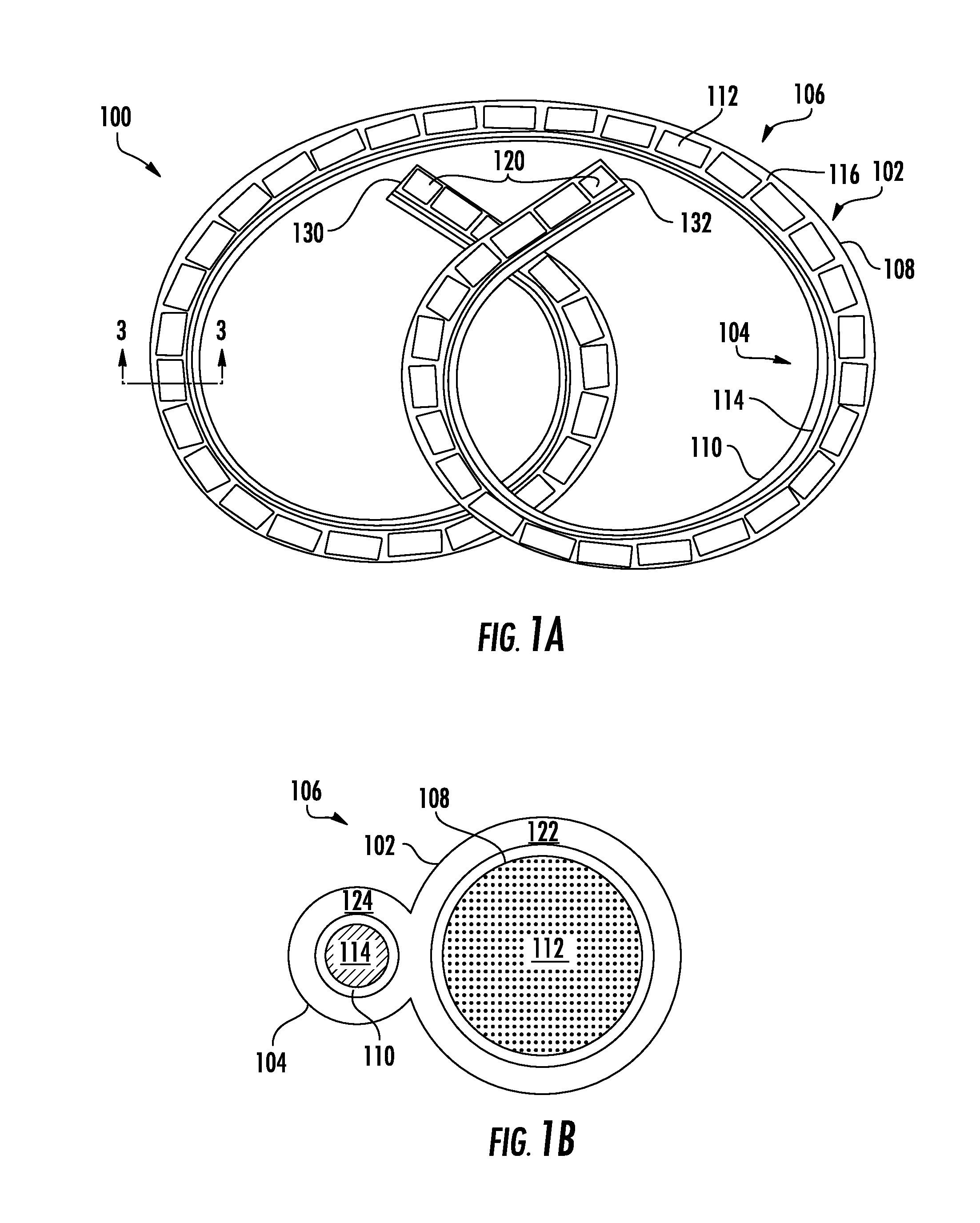
Implantable Lead Affixation Structure for Nerve Stimulation to Alleviate Bladder Dysfunction and Other Indication
ActiveUS20160045724A1Avoid tissue damageLow profileSpinal electrodesPaper/cardboard wound articlesBladder functionLaser cutting
Anchoring devices and methods for affixing an implanted lead of a neurostimulation system at a target location in a patient are provided herein. Such anchoring devices includes a helical body having a plurality of tines extending laterally outward from the lead when deployed that engage tissue to inhibit axial movement of the implanted lead. The plurality of tines are biased towards the laterally extended deployed configuration and fold inward towards the lead to a delivery configuration to facilitate delivery of the lead through a sheath. The tines may be angled in a proximal direction or in both proximal and distal directions and may include various features to assist in visualization and delivery of the lead. The anchor may be formed according to various methods, including laser cutting of a tubular section along with heat or reflow to set the material with the anchor in the deployed configuration and injection molding.
Owner:AXONICS
Application of lipid vehicles and use for drug delivery
InactiveUS20070003610A1Reduce and prevent antibody-mediated resistanceIncrease stimulationBiocideAntipyreticDiseaseAnticarcinogen
The present invention relates to compositions and methods for the administration of lipid-based vehicles to treat various disorders, including bladder inflammation, infection, dysfunction, and cancer. In various aspects, the compositions and methods of the invention are useful for prolonged delivery of drugs, e.g., antibiotics, pain treatments, and anticancer agents, to the bladder, genitourinary tract, gastrointestinal system, pulmonary system, and other organs or body systems. In particular, the present invention relates to liposome-based delivery of vanilloid compounds, such as resiniferatoxin, capsaicin, or tinyatoxin, and toxins, such as botulinum toxin, for the treatment of bladder conditions, including pain, inflammation, incontinence, and voiding dysfunction. Further related are methods of using these vehicles alone or in conjunction with antibodies, e.g., uroplakin antibodies, to improve duration of liposome attachment, and provide a long-term intravesical drug delivery platform. The present invention specifically relates to antibody-coated liposomes that are useful for targeting specific receptors for drug, peptide, polypeptide, or nucleic acid delivery. In one particular aspect, the present invention relates to liposomes coated with antibodies against nerve growth factor (NGF) receptor and containing NGF antisense nucleic acids, which are used as a treatment for neurogenic bladder dysfunction.
Owner:UNIVERSITY OF PITTSBURGH
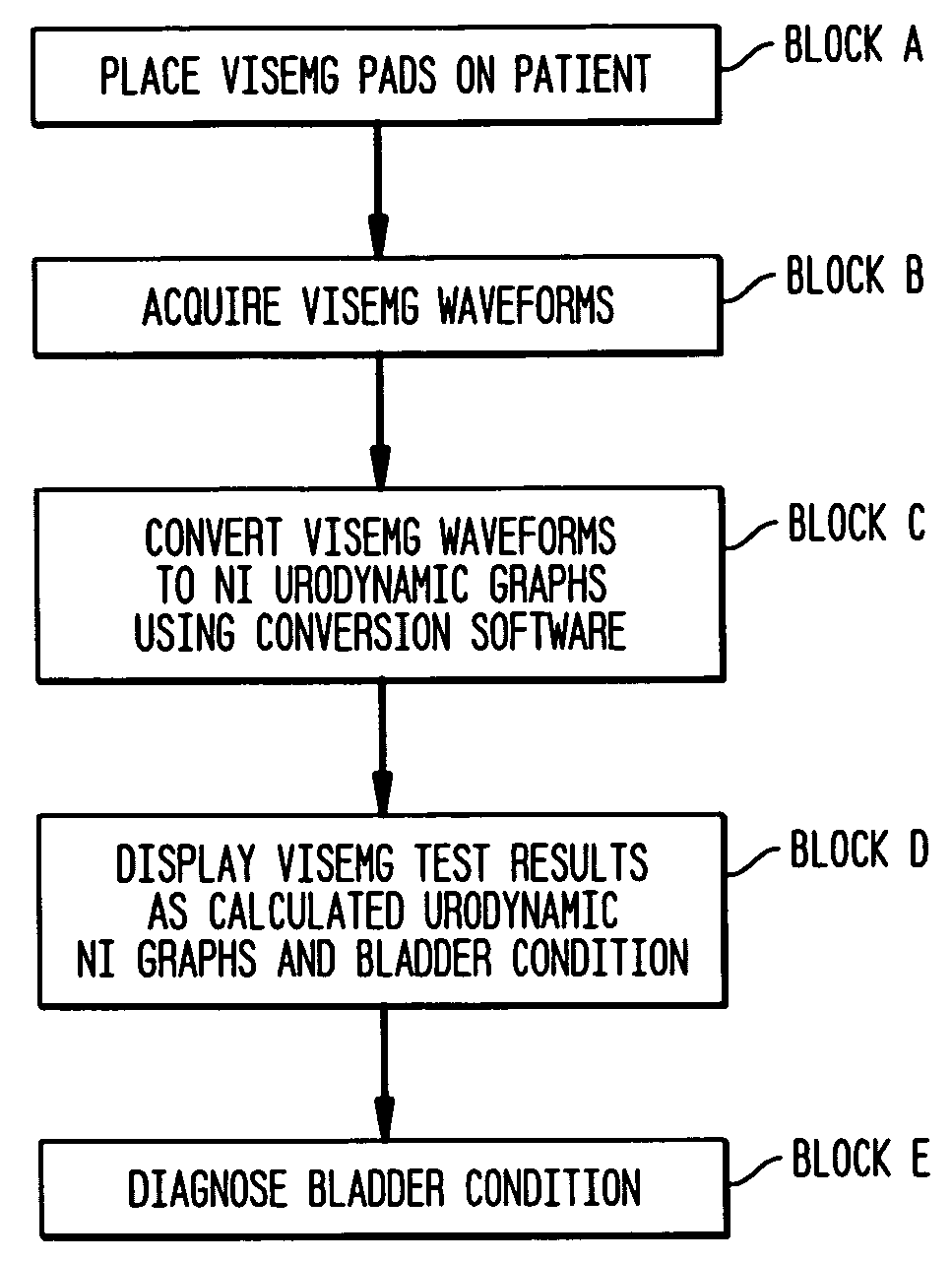
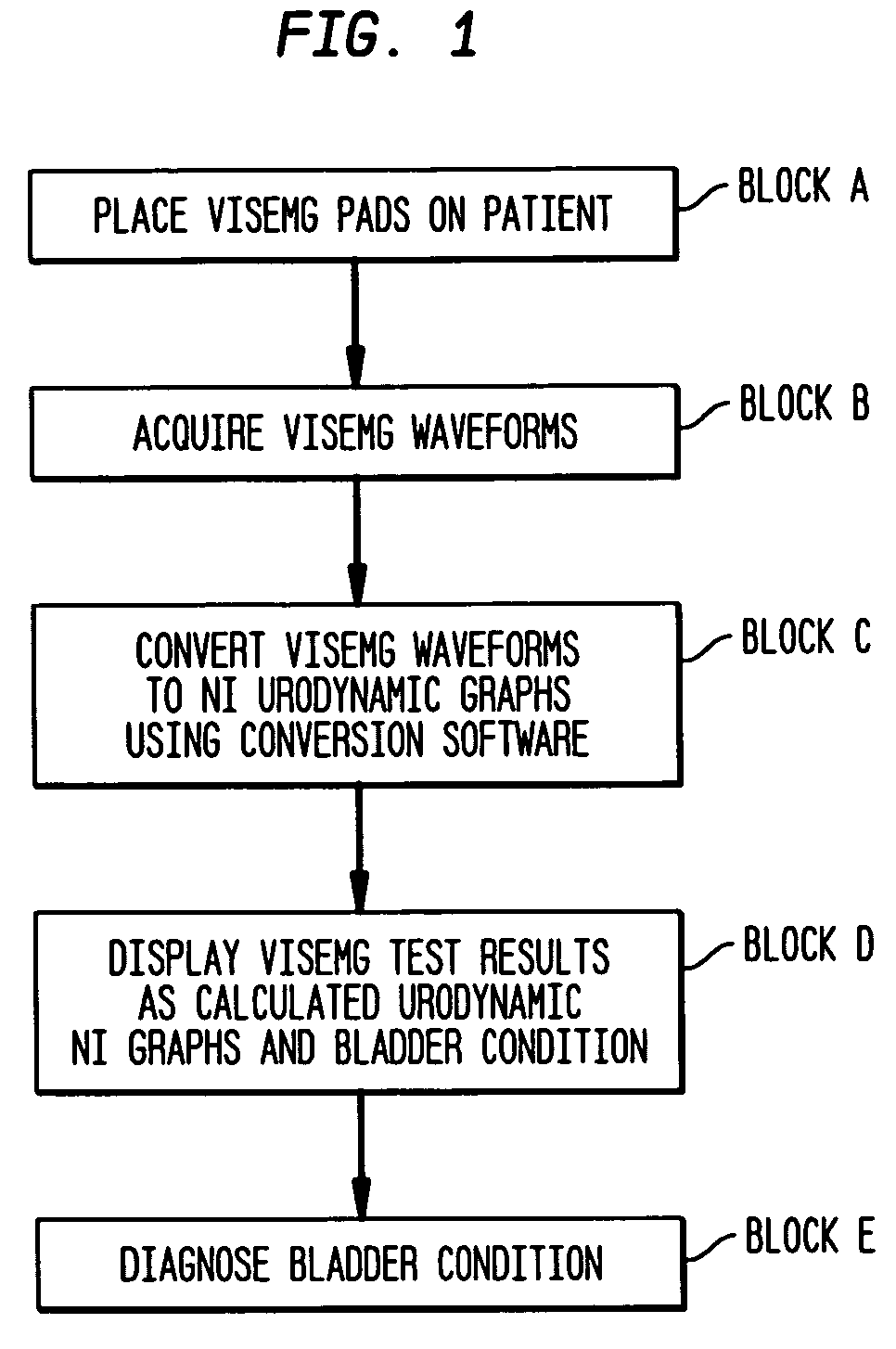
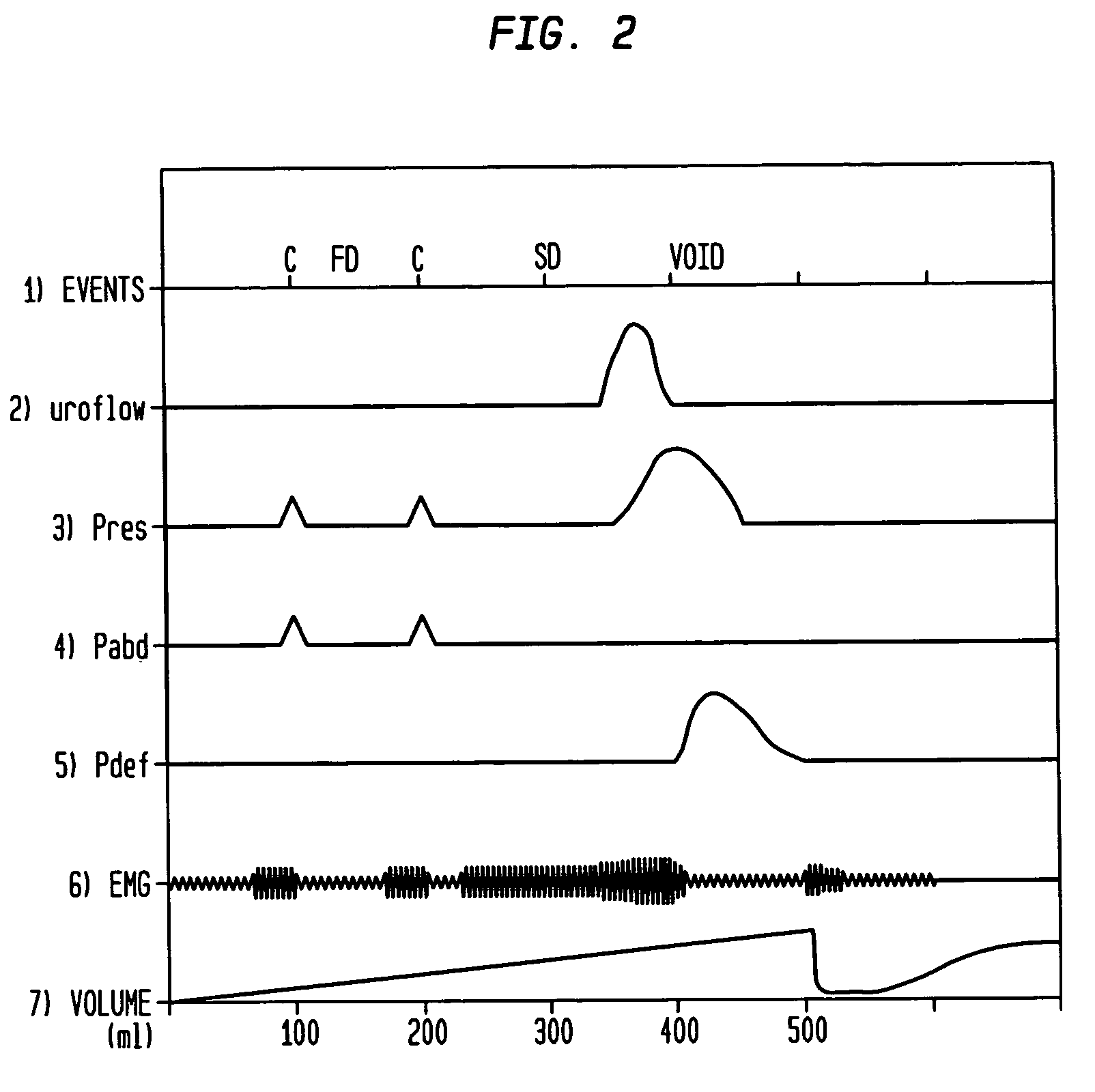
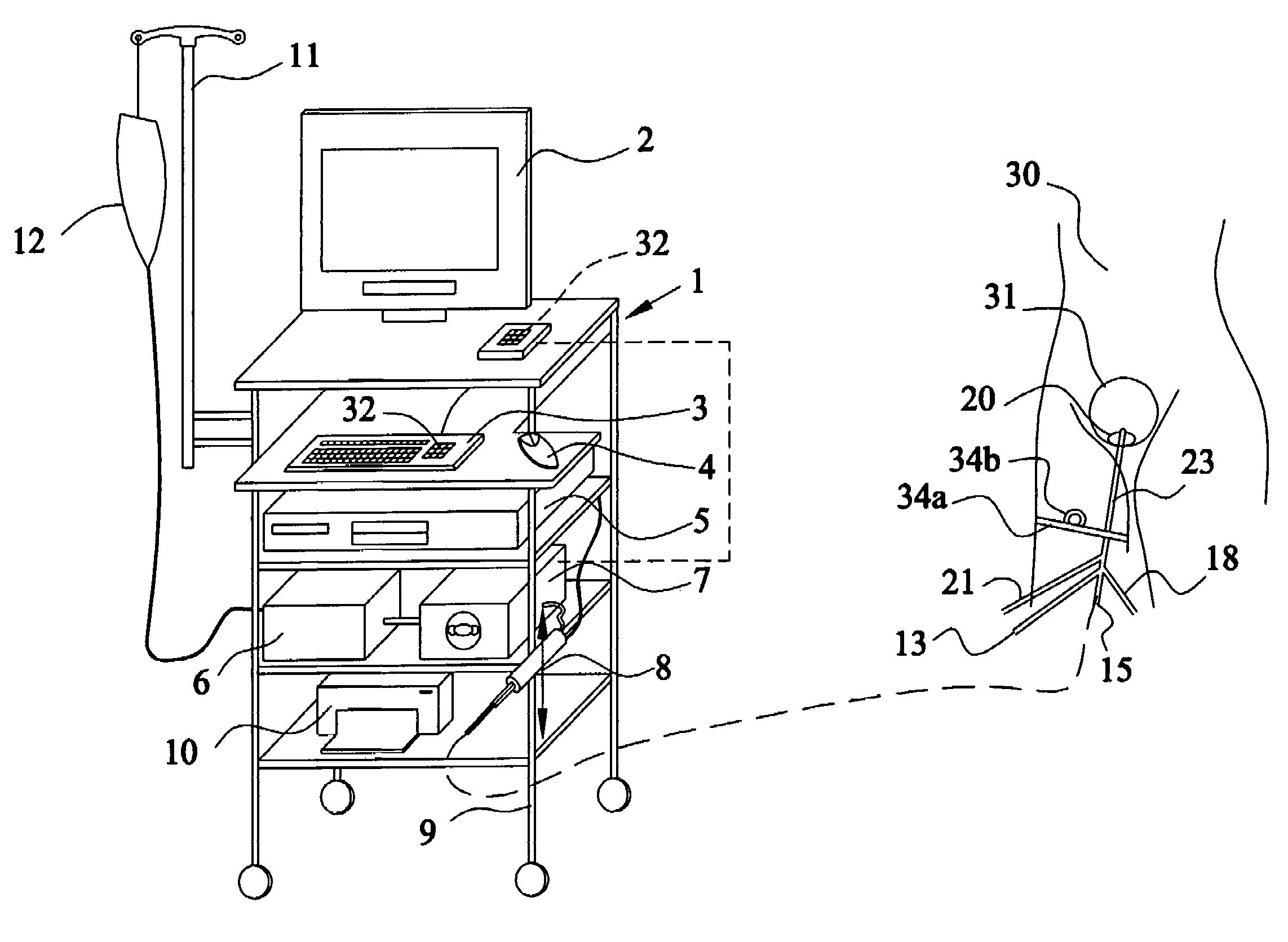
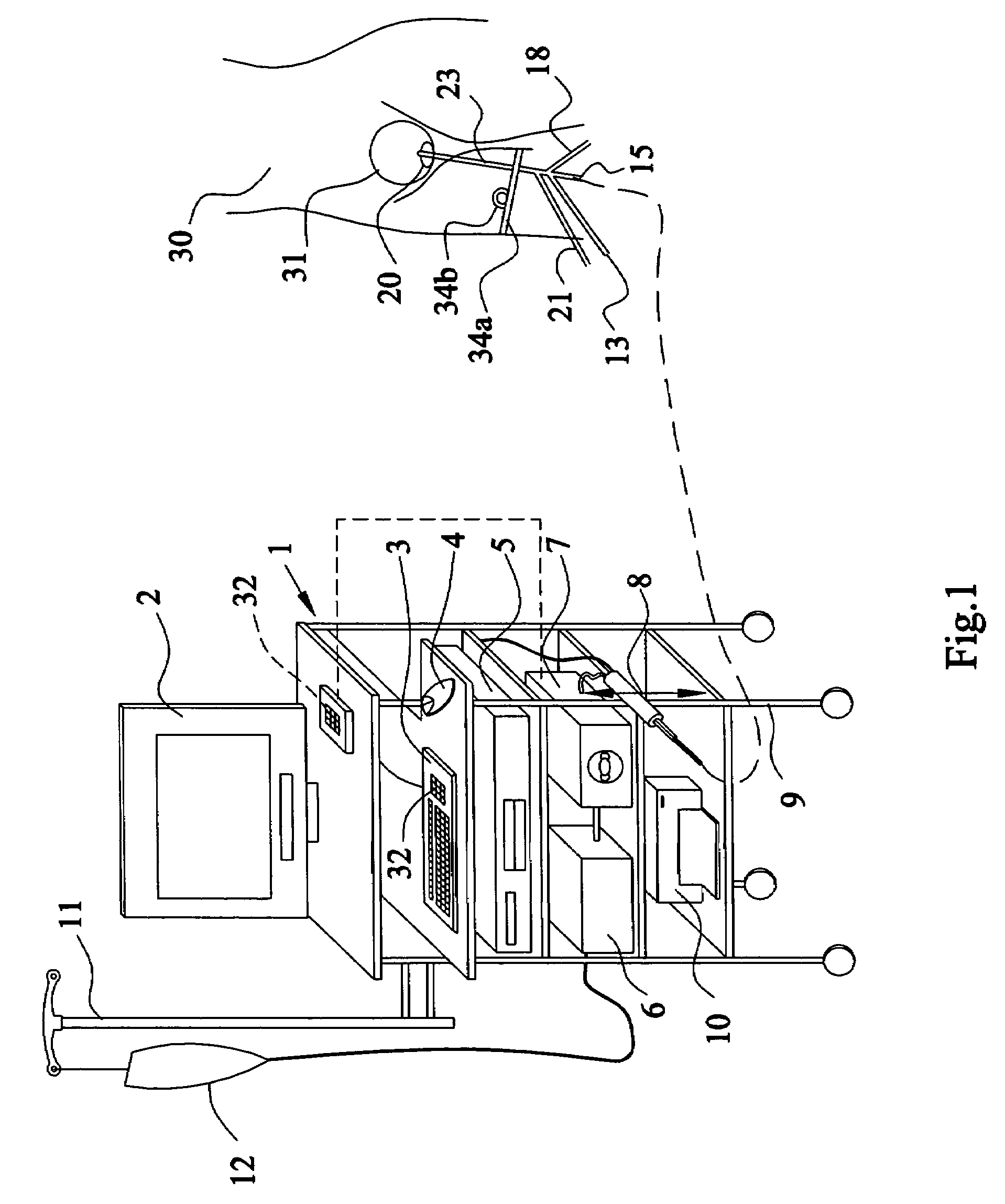
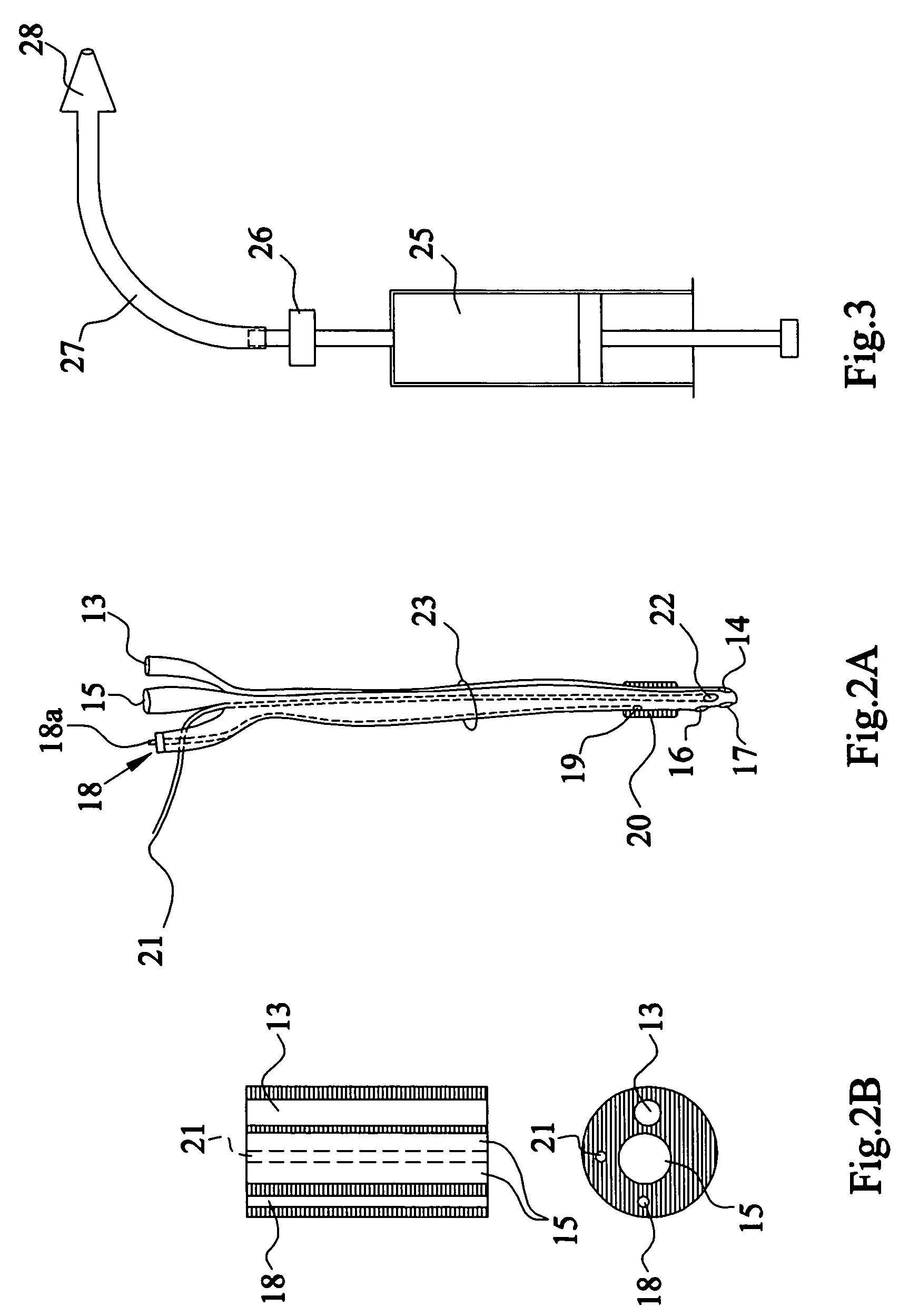
Method and apparatus for measuring bladder electrical activity to diagnose bladder dysfunction
A method of diagnosing bladder pathology uses Vesico Internal Sphincter ElectroMyogram (VISEMG) waveforms obtained from a plurality of VISEMG electrodes disposed on a patient. The VISEMG waveforms are converted to noninvasive (NI) urodynamic graphs. Further, a programmed computer assesses the condition of the bladder based on the VISEMG waveforms. The method does not require traditional urodynamics or any other invasive procedure. An apparatus to perform the inventive method uses a plurality of VISEMG electrodes placed on the patient. Each electrode signal is amplified and filtered and converted to a digital signal. A computer converts the digital signals (the VISEMG waveforms) to NI urodynamic graphs. Any combination of the VISEMG waveforms, the NI urodynamic graphs, and the condition of the bladder based on the VISEMG waveforms can then be shown on a display screen.
Owner:BERGER YITZHAK +1
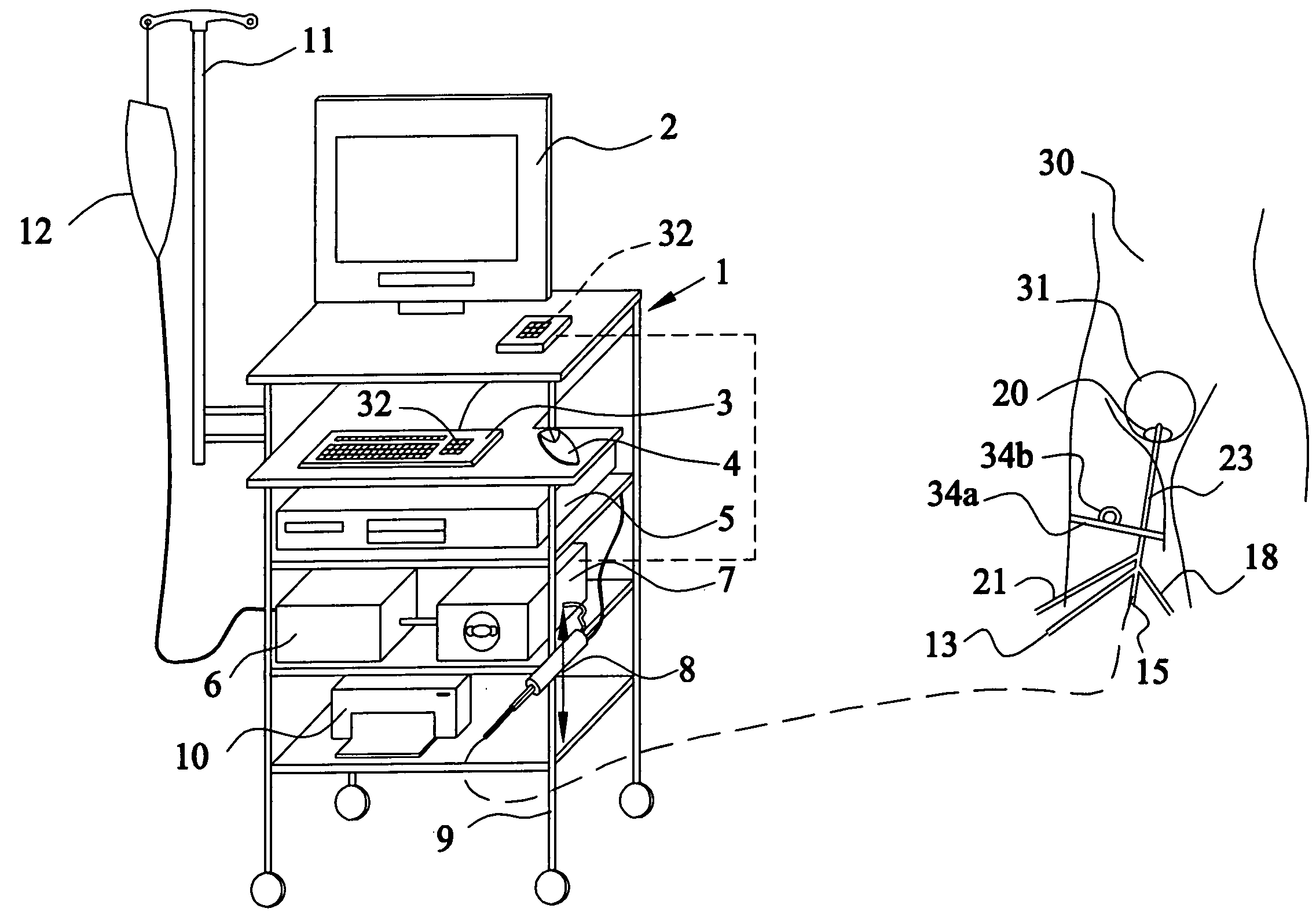
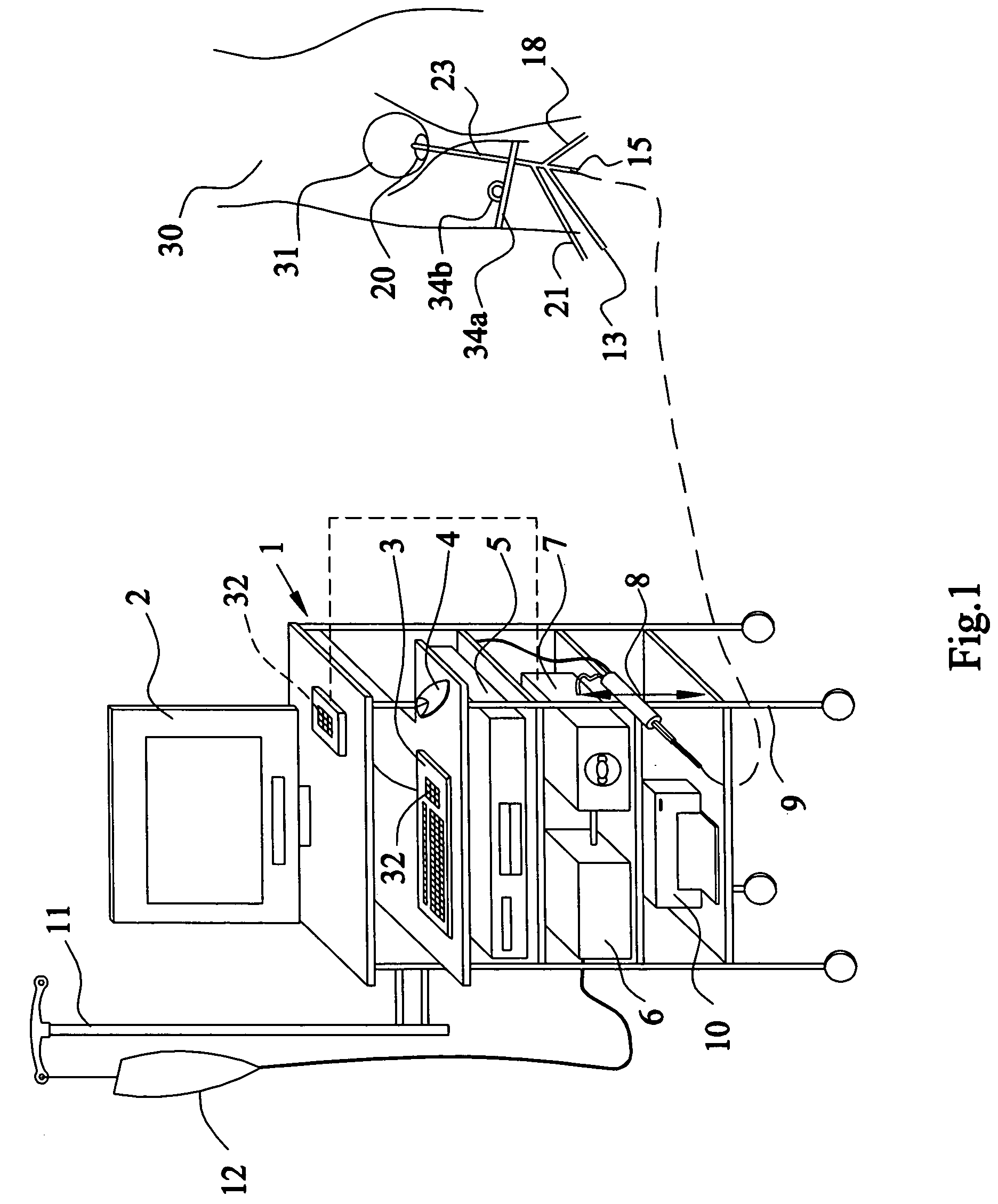
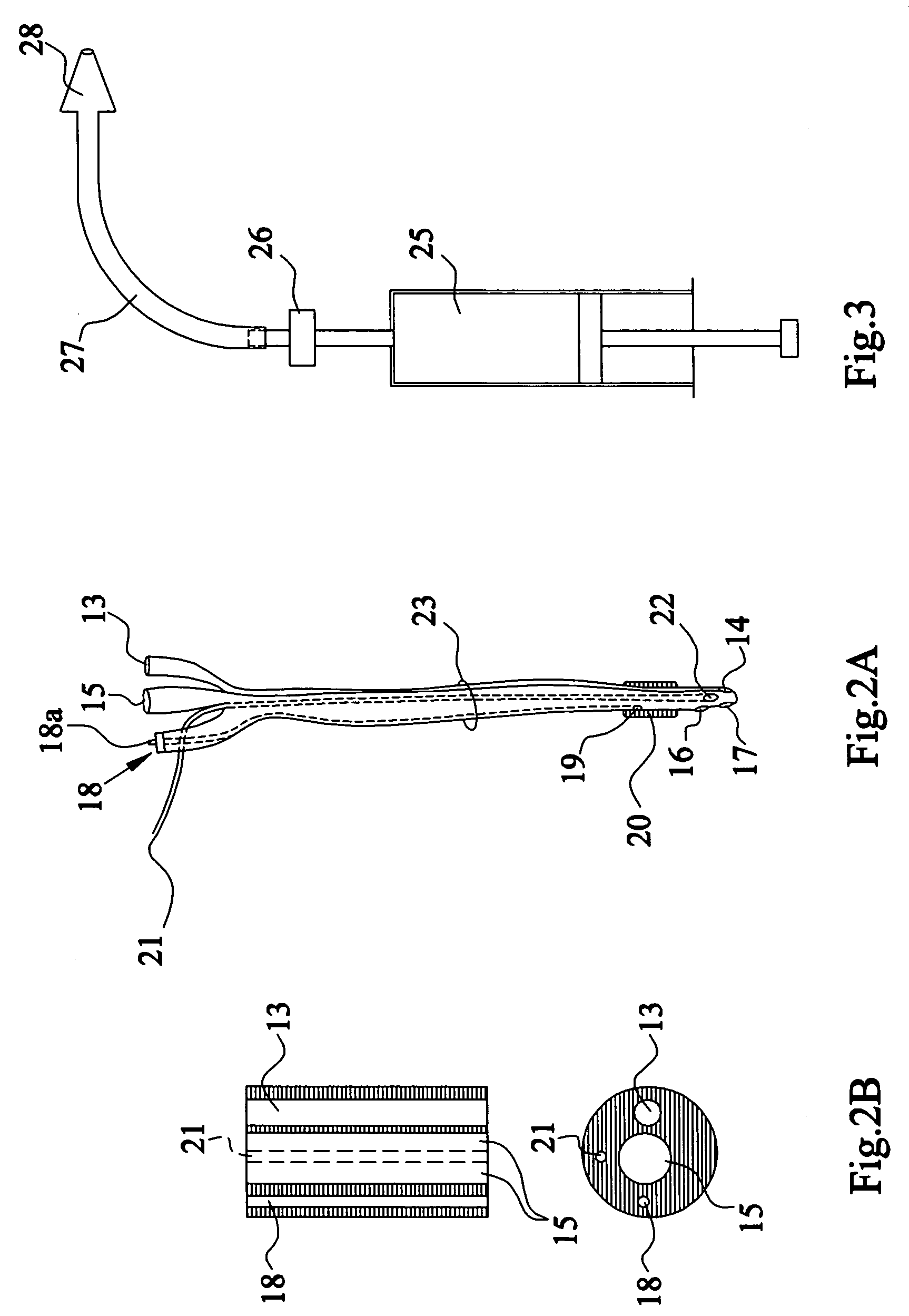
Apparatus and method for the controlled hydrodistention of the urinary bladder
InactiveUS7615046B2Stop progressionAvoid debilitating effectAnti-incontinence devicesWound drainsFunctional disturbanceAutomatic control
A urological medical system for performing a controlled hydrodistention of the urinary bladder for treating a bladder dysfunction problem comprised of a programmed CPU with associated computer equipment for facilitating an automatic control of a treatment procedure to be performed on a patient, a saline solution warmer, a saline solution pump, a four-way catheter wherein a channel is provided for monitoring the pressure within the bladder during the procedure, a syringe for infusing a medicine through the catheter and a strapping device to secure the syringe and to slidingly engage the catheter to a leg of the patient. Desired parameters are monitored during the procedure and a desired pressure is maintained in the bladder for a predetermined time to perform the controlled hydrodistention of the urinary bladder.
Owner:SHEHATA SOBHY
Urinary bladder power pump driven by external electromagnetism
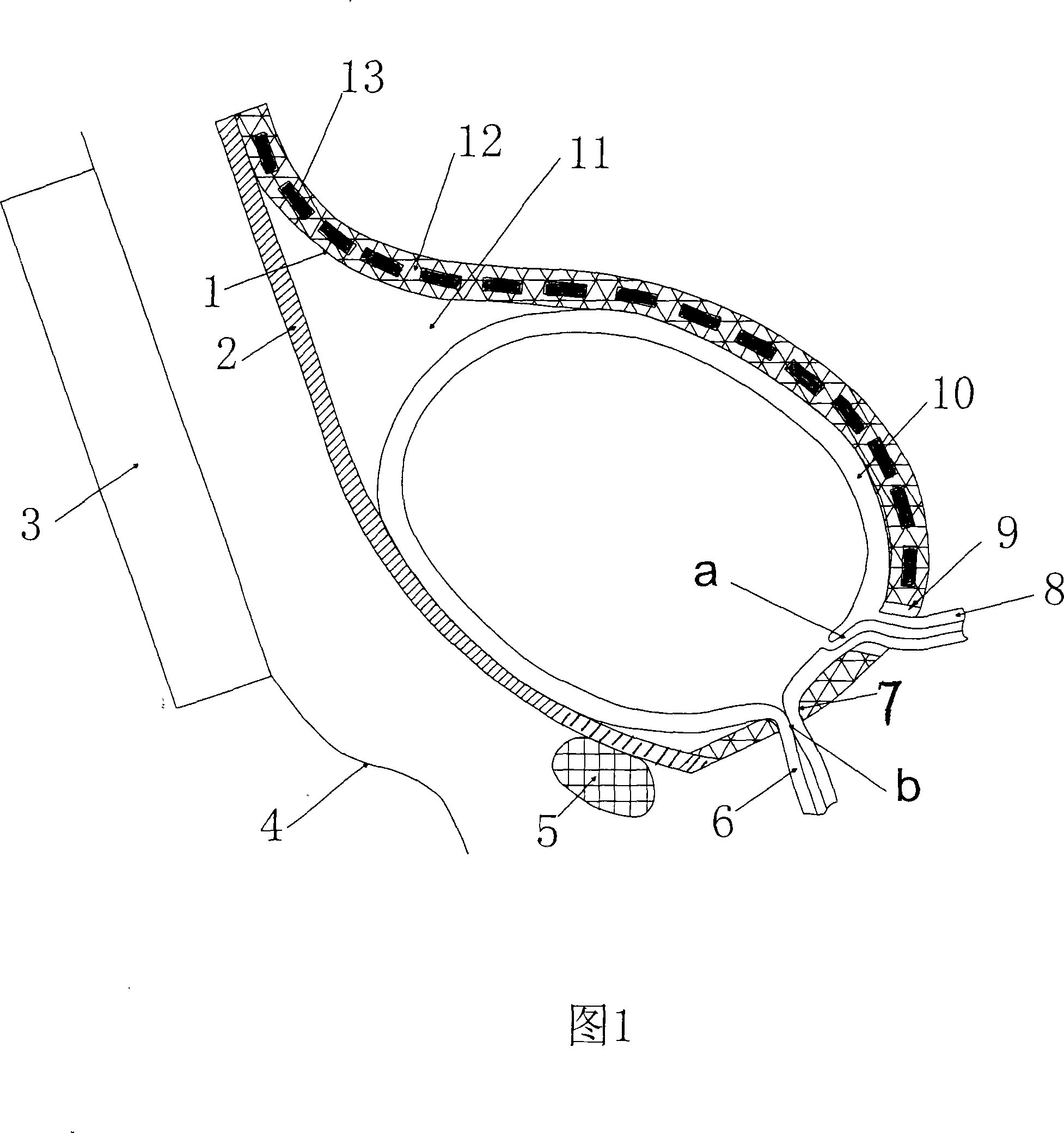
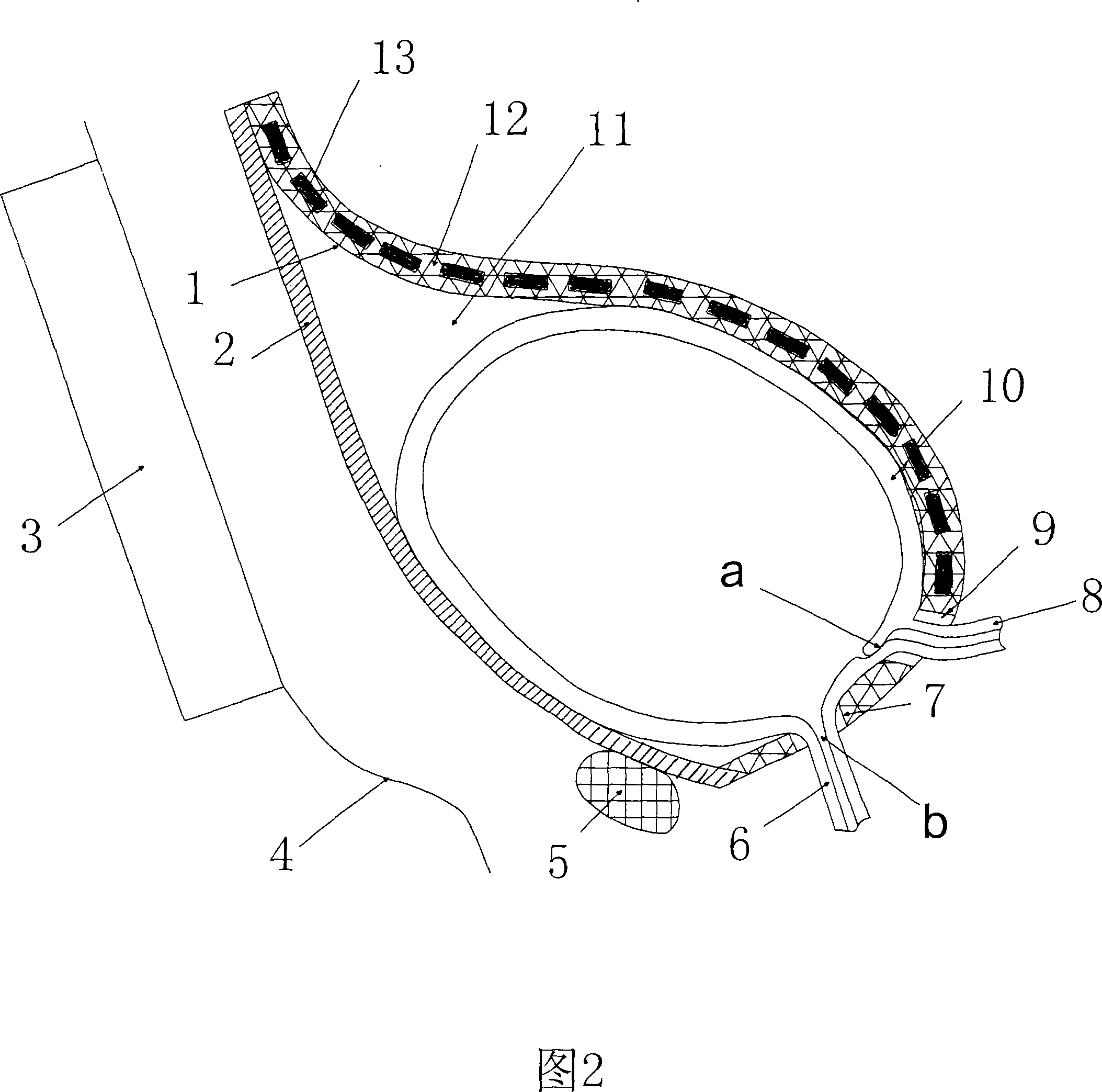
InactiveCN101176689ANot cause infectionCause infectionFlexible member pumpsPositive-displacement liquid enginesMagnetic tension forceEngineering
The invention relates to a bladder power pump utilizing the magnetic force to assist the bladder to urinate, which comprises a magnetic active cell (1), a stator (2) and a magnetic controller (3), wherein, the magnetic controller (3) generates magnetic field if switched on and enables the magnetic force to work on the magnetic active cell (1); the magnetic active cell (1) comprises a flexible sheet (12) and a plurality of permanent magnetic sheets (13), and the permanent magnetic sheets (13) are enwrapped with the flexible sheet (12); the flexible sheet (12) enwrapping the permanent magnetic sheets (13) surrounds a gourd-shaped structure with the stator (2) to circle the outside of the bladder (10) to build a variable working cavity (11); the upper end of the flexible sheet (12) enwrapping the permanent magnetic sheets (13) is contacted and adhered with the stator (2); a through hole (9) of ureter communicating a ureter (8) with a ureter port (a) inside the bladder wall, and a urethra bayonet (7) held at a urethral meatus (b) of a urethra (8) are respectively arranged at the lower end of the flexible sheet (12) where the permanent magnetic sheets (13) are not enwrapped; and the lower end of the flexible sheet (12) where the permanent magnetic sheets (13) are not enwrapped is contacted and adhered with the stator (2). The invention can effectively assist the neurogenic bladder dysfunction sufferer to realize emiction and has the advantages of ingenious design, simple structure, safe use, convenience and practicality.
Owner:GUANGDONG UNIV OF TECH
Use of neurotoxin therapy for treatment of urologic and related disorders related to neurogenic bladder dysfunction
InactiveUS20050048084A1Simple methodHigh selectivityBacterial antigen ingredientsPeptide/protein ingredientsUrethraSphincter
The present invention related to methods for treating neurological-urological conditions, including neurogenic bladder dysfunction. This is accomplished by administration of a botulinum toxin into the lower urinary tract of a patient with a neurogenic bladder dysfunction, including the bladder or urinary sphincter and the bladder wall.
Owner:ALLERGAN INC
Implantable Lead Affixation Structure for Nerve Stimulation to Alleviate Bladder Dysfunction and Other Indication
ActiveUS20160121105A1Reduce deliveryRigid enoughSpinal electrodesDomestic articlesLaser cuttingBiomedical engineering
Anchoring devices and methods for affixing an implanted lead of a neurostimulation system at a target location in a patient are provided herein. Such anchoring devices includes a helical body having a plurality of tines extending laterally outward from the lead when deployed that engage tissue to inhibit axial movement of the implanted lead. The plurality of tines are biased towards the laterally extended deployed configuration and fold inward towards the lead to a delivery configuration to facilitate delivery of the lead through a sheath. The tines may be angled in a proximal direction or in both proximal and distal directions and may include various features to assist in visualization and delivery of the lead. The anchor may be formed according to various methods, including laser cutting of a tubular section along with heat or reflow to set the material with the anchor in the deployed configuration and injection molding.
Owner:AXONICS
Apparatus and method for the controlled hydrodistention of the urinary bladder
InactiveUS20080172041A1Stop progressionAvoid debilitating effectAnti-incontinence devicesWound drainsFunctional disturbanceAutomatic control
A urological medical system for performing a controlled hydrodistention of the urinary bladder for treating a bladder dysfunction problem comprised of a programmed CPU with associated computer equipment for facilitating an automatic control of a treatment procedure to be performed on a patient, a saline solution warmer, a saline solution pump, a four-way catheter wherein a channel is provided for monitoring the pressure within the bladder during the procedure, a syringe for infusing a medicine through the catheter and a strapping device to secure the syringe and to slidingly engage the catheter to a leg of the patient. Desired parameters are monitored during the procedure and a desired pressure is maintained in the bladder for a predetermined time to perform the controlled hydrodistention of the urinary bladder.
Owner:SHEHATA SOBHY
Drug delivery systems and methods for treatment of bladder dysfunction or disorder using trospium
Methods, devices, and medicaments that include trospium are provided for use in the treatment of bladder dysfunction by locally administering the trospium into the bladder to achieve a sustained concentration of trospium in urine in the bladder sufficient to produce a therapeutic concentration of trospium in bladder tissue. The drug may be delivered into the bladder from an intravesical drug delivery device inserted into the bladder, wherein the device continuously releases the drug into the urine in the bladder over an extended period of hours or days.
Owner:TARIS BIOMEDICAL
Compositions and Methods for Treating Neurogenic Disorders of the Pelvic Floor
InactiveUS20140024701A1Induce depolarizationCompounds screening/testingNervous disorderDetrusor hyperreflexiaNeural cell
Provided herein are methods for the treatment of bladder dysfunction, including detrusor hyperreflexia and detrusor external sphincter dyssynergia, fecal incontinence, and / or sexual dysfunction in an individual via the use of stably expressed light-responsive opsin proteins capable of selective hyperpolarization or depolarization of the neural cells that innervate the muscles responsible for physiologic functioning of urinary bladder, external urinary sphincter, external anal sphincter, and the male and female genitalia.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV +1
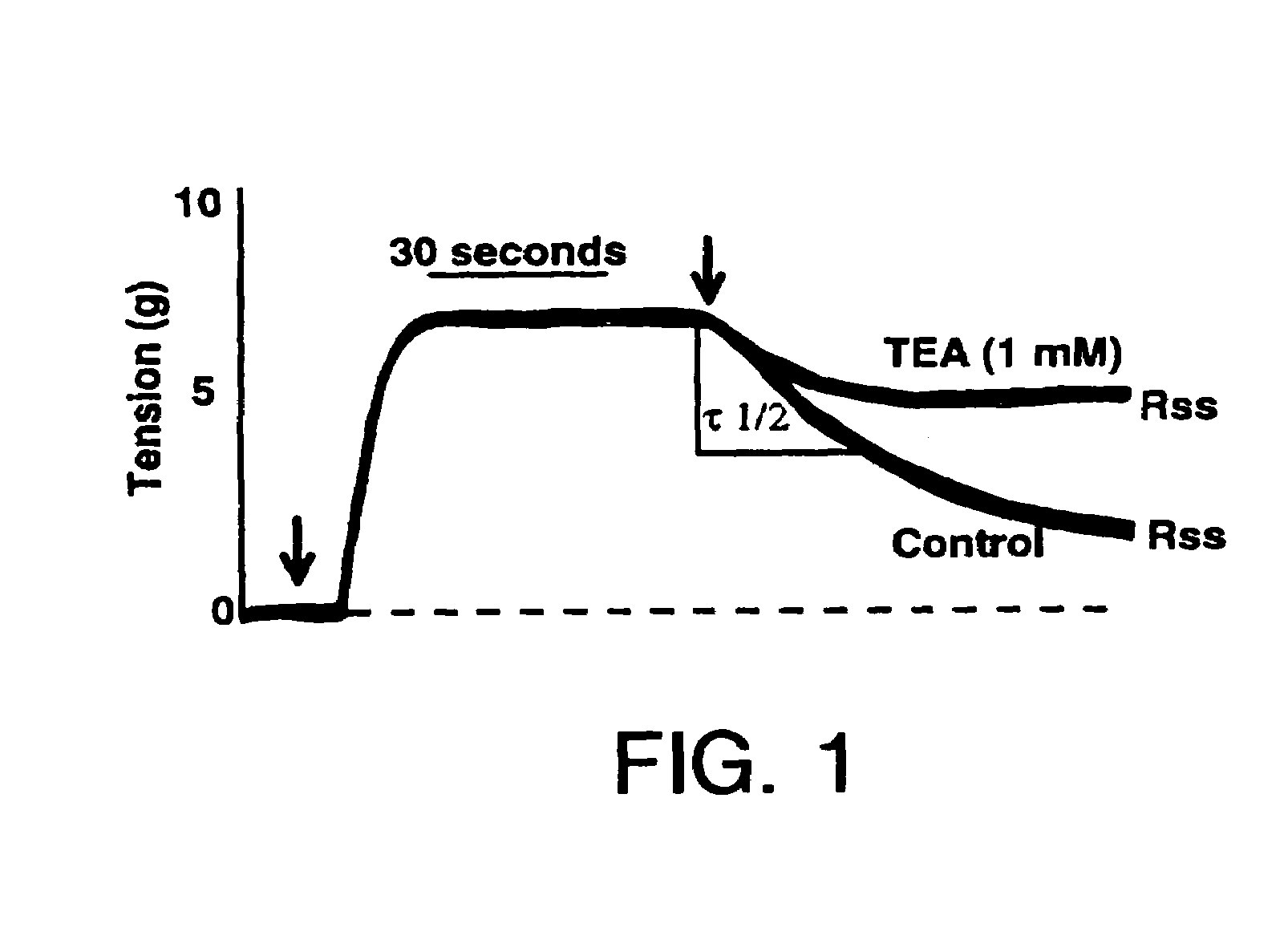
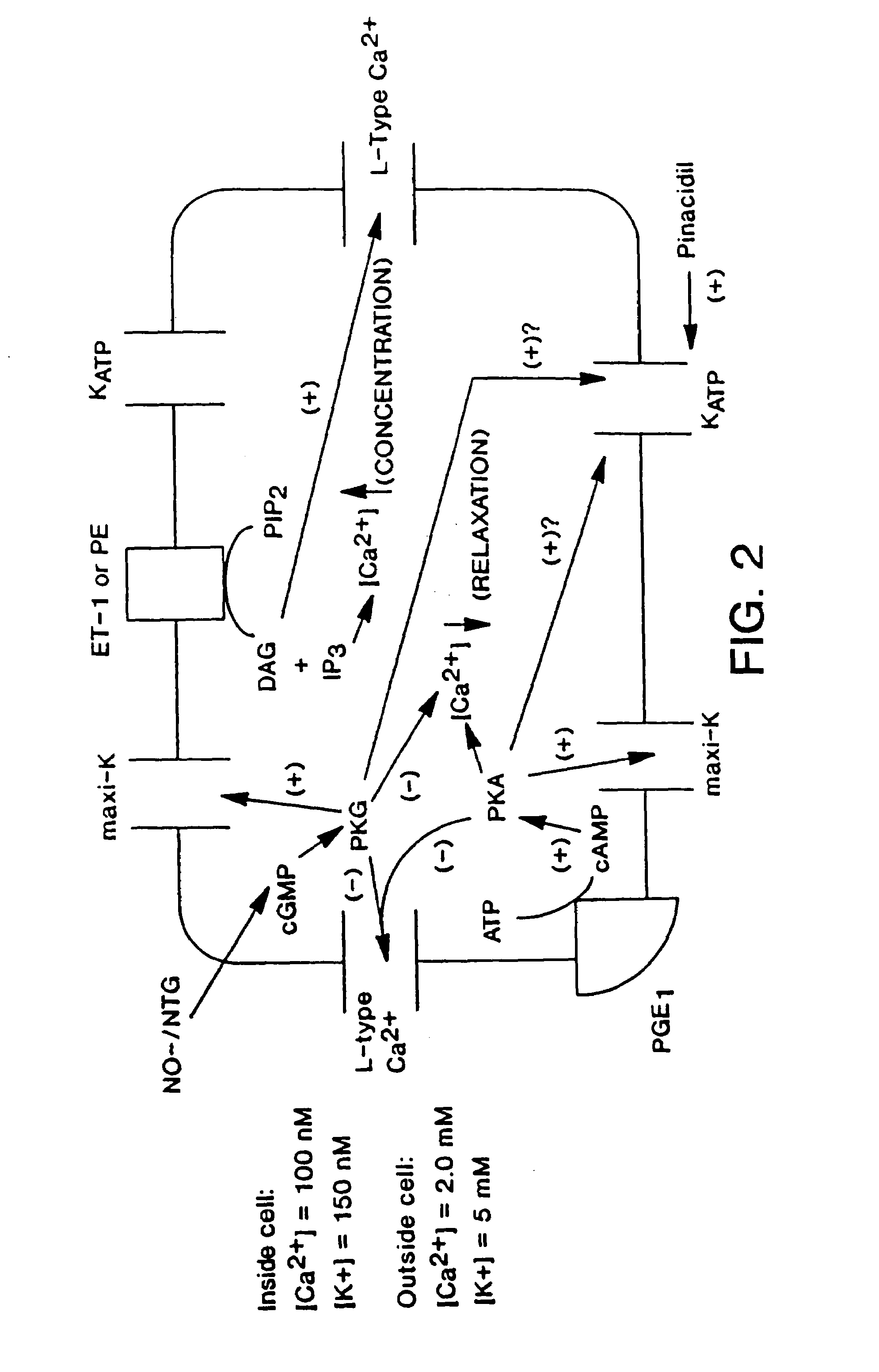
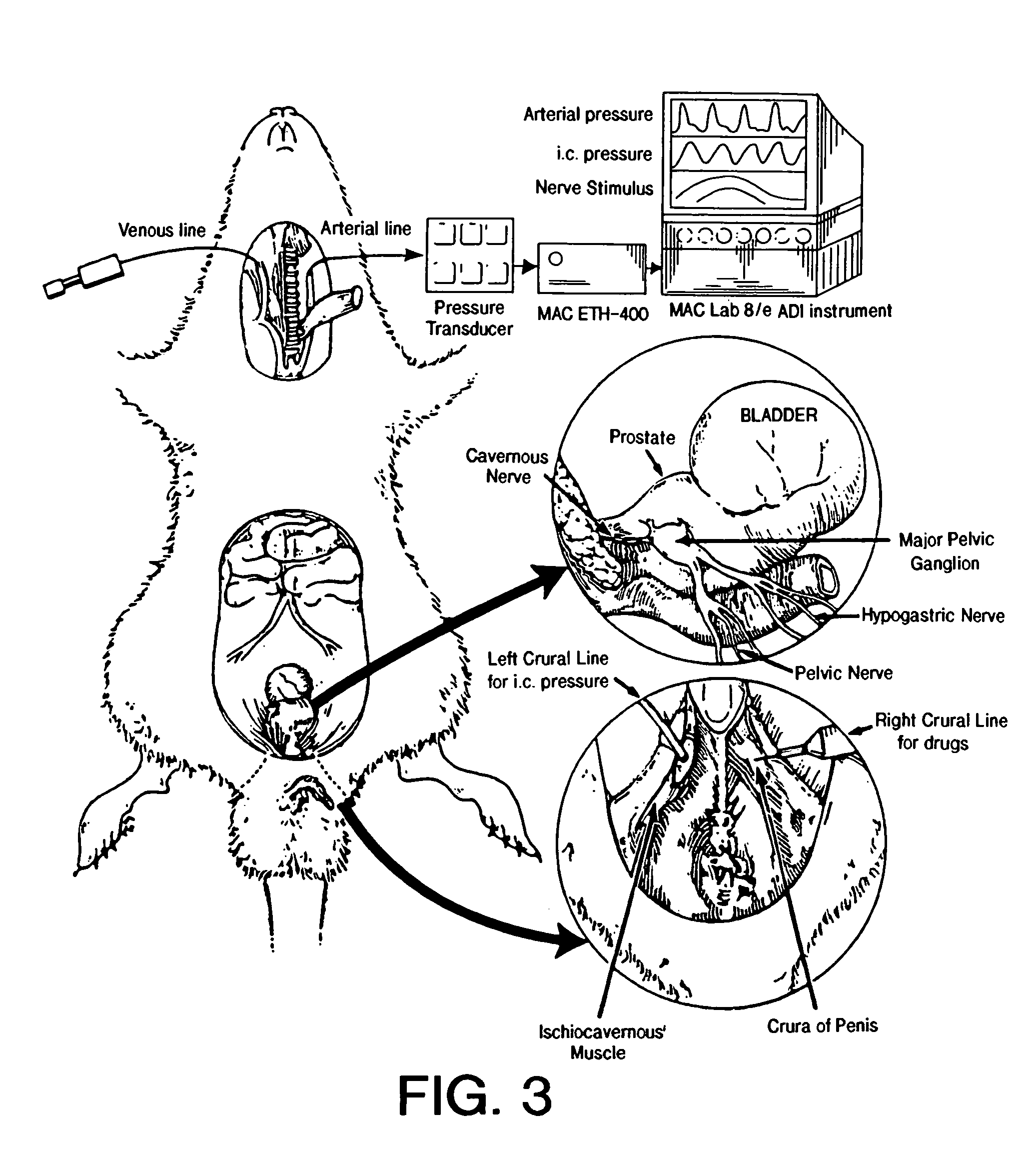
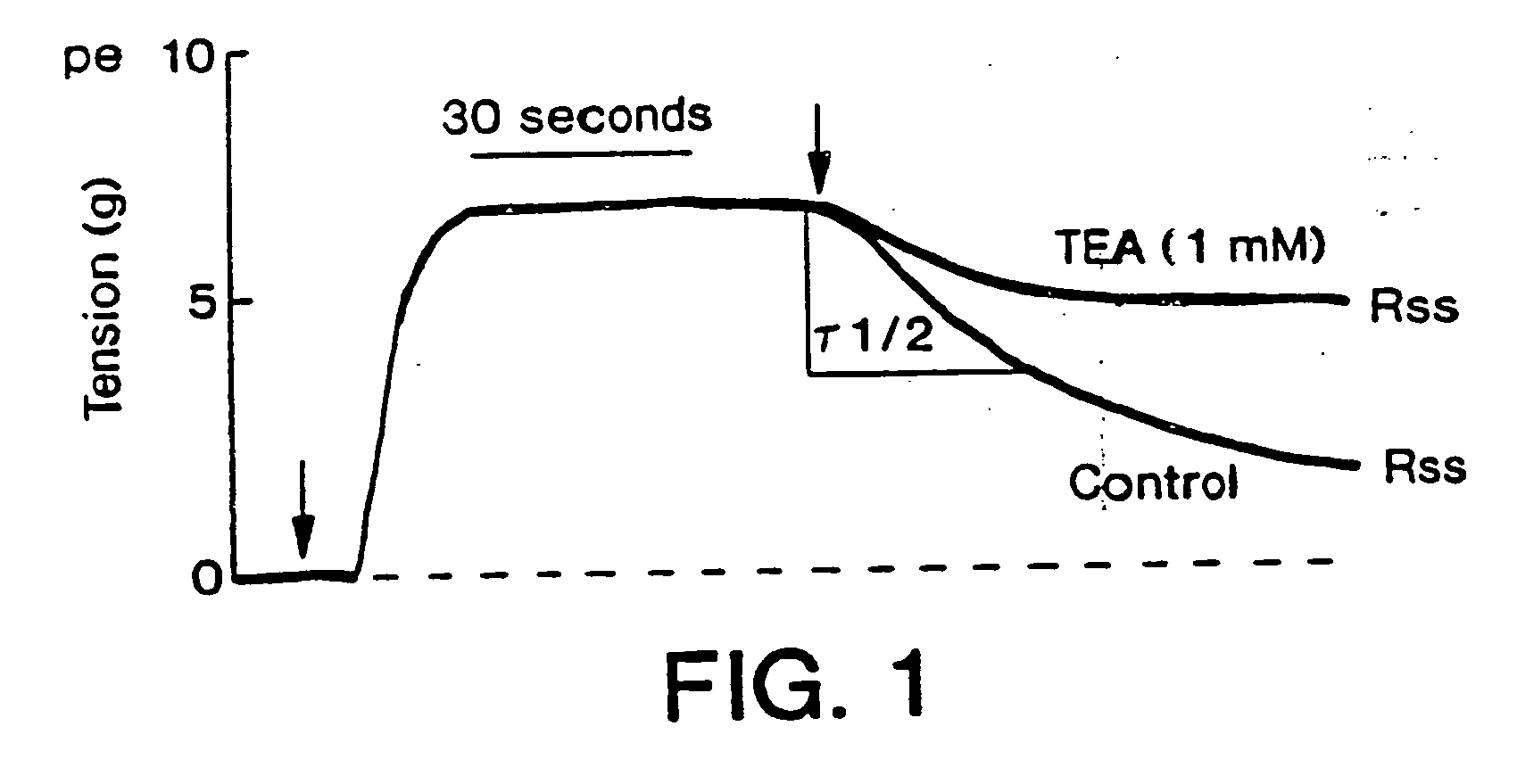
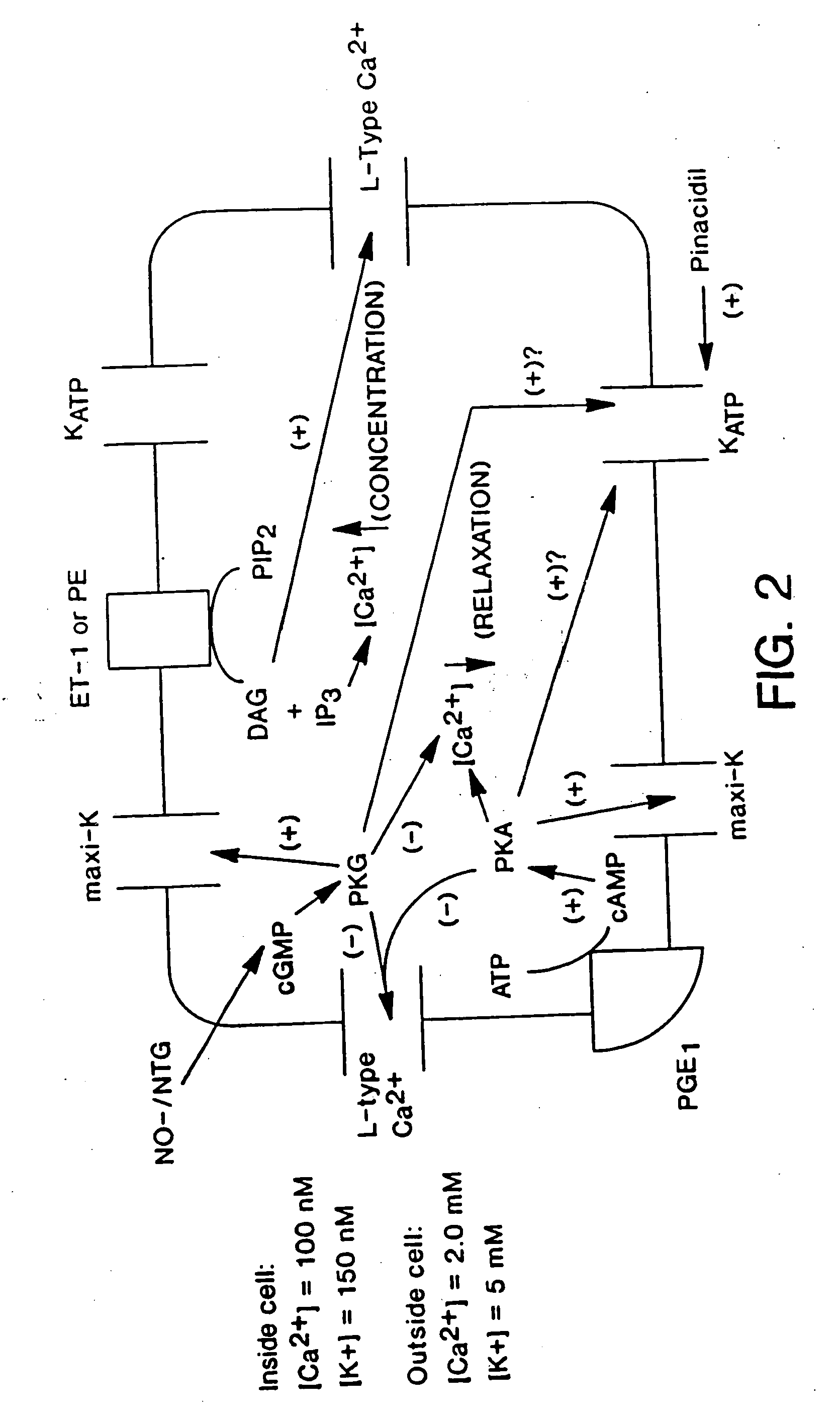
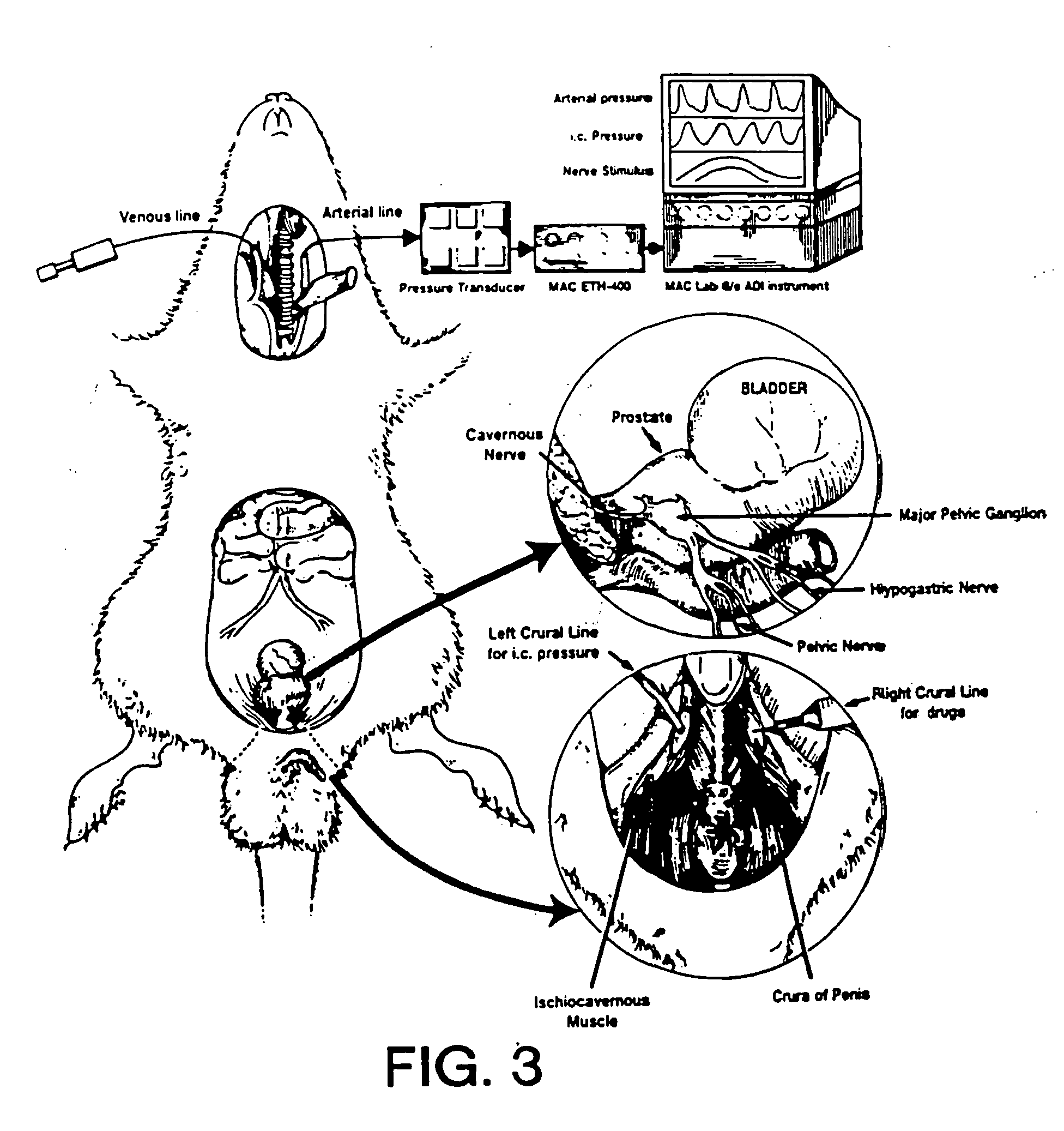
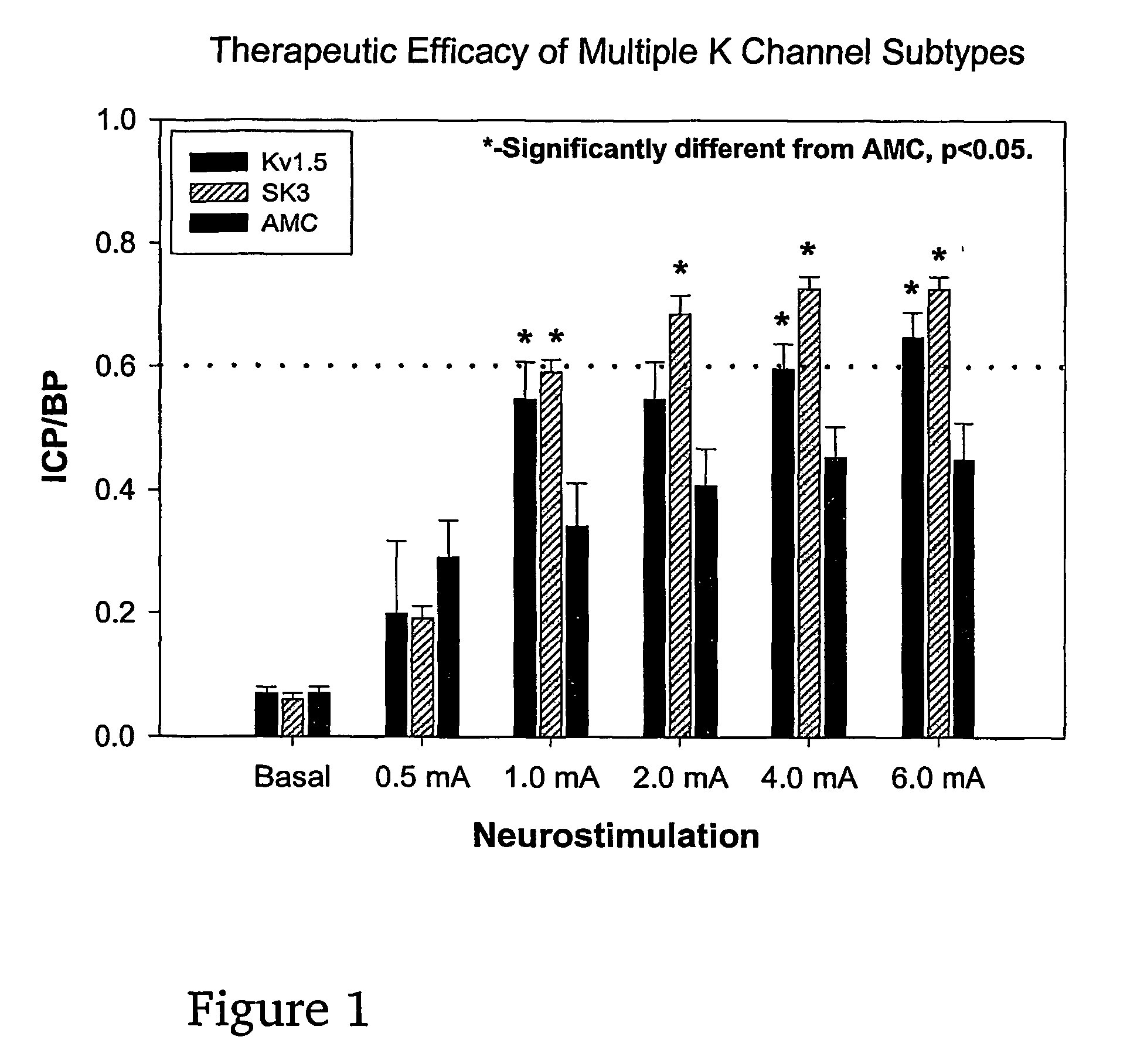
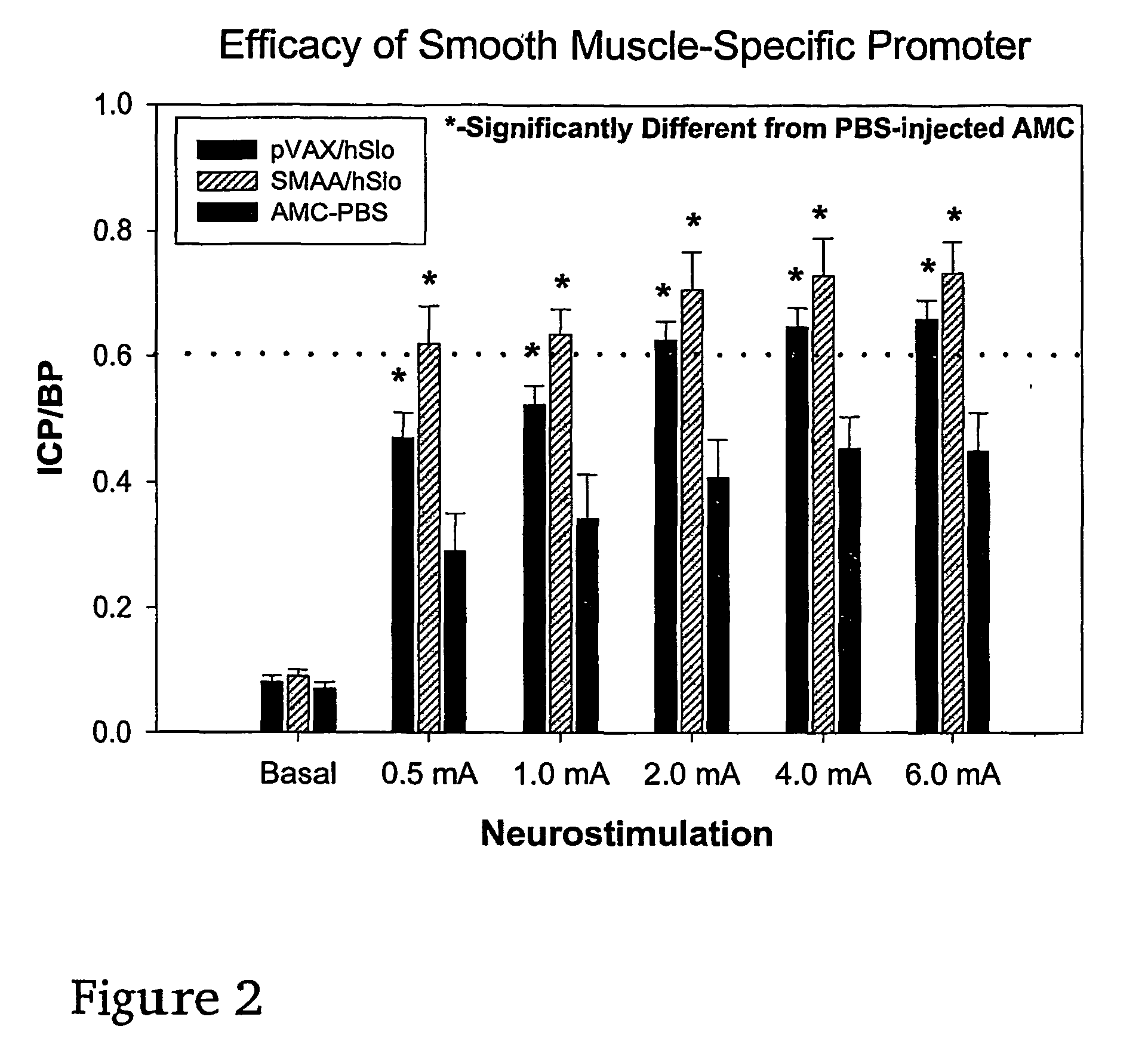
Method of enhancing relaxation of penile smooth muscle by introduction of DNA encoding maxi-K potassium channel protein
InactiveUS7030096B1Enhance smooth muscle contractionRestoring smooth muscle toneBiocidePeptide/protein ingredientsDiseaseProtein insertion
The present invention is directed towards a method of regulating smooth muscle tone, comprising the introduction, into smooth muscle cells of a subject, of a DNA sequence encoding a protein involved in the regulation of smooth muscle tone, and expression of the DNA sequence in a sufficient number of smooth muscle cells of the subject to regulate smooth muscle tone in the subject. Specifically, the invention provides methods of gene therapy for treating erectile dysfunction, bladder dysfunction, and other smooth muscle disorders. The present invention also provides recombinant viral and non-viral vectors comprising DNA encoding a protein involved in the regulation of smooth muscle tone. Further provided by the present invention is a smooth muscle cell which expresses a gene encoding a protein involved in the regulation of smooth muscle tone.
Owner:ALBERT EINSTEIN COLLEGE OF MEDICINE OF YESHIVA UNIV
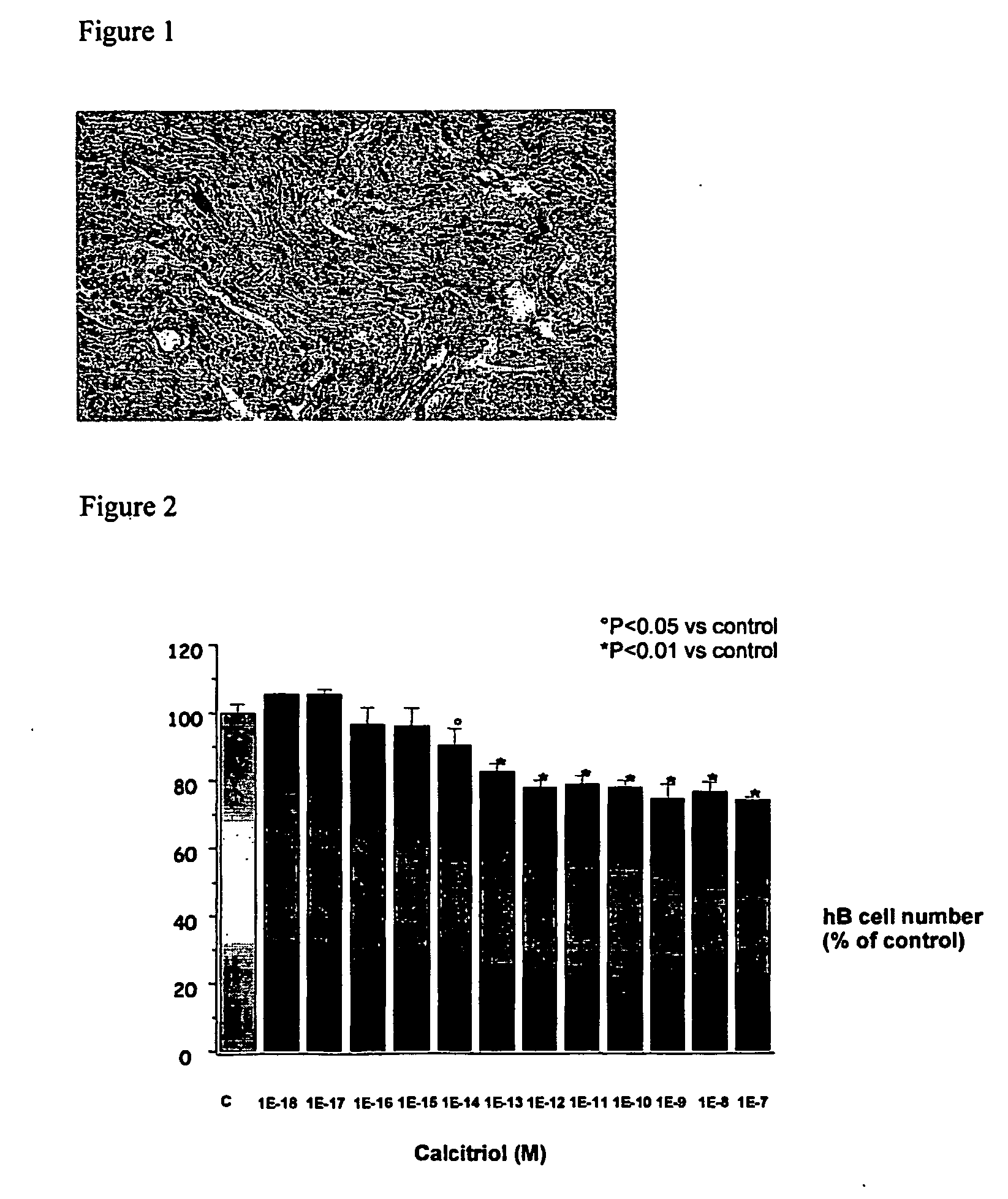
Methods for treating bladder dysfunction
InactiveUS20070054887A1Preventing and/or treatingEffective amountBiocideOrganic active ingredientsVitamin D+MetabolitesCholecalciferol
There is provided according to the invention the use of Vitamin D compounds such as 1-alpha-fluoro-25-hydroxy-16,23e-diene-26,27-bishomo-20-epi-cholecalciferol in the prevention or treatment of bladder dysfunction.
Owner:BIOXELL
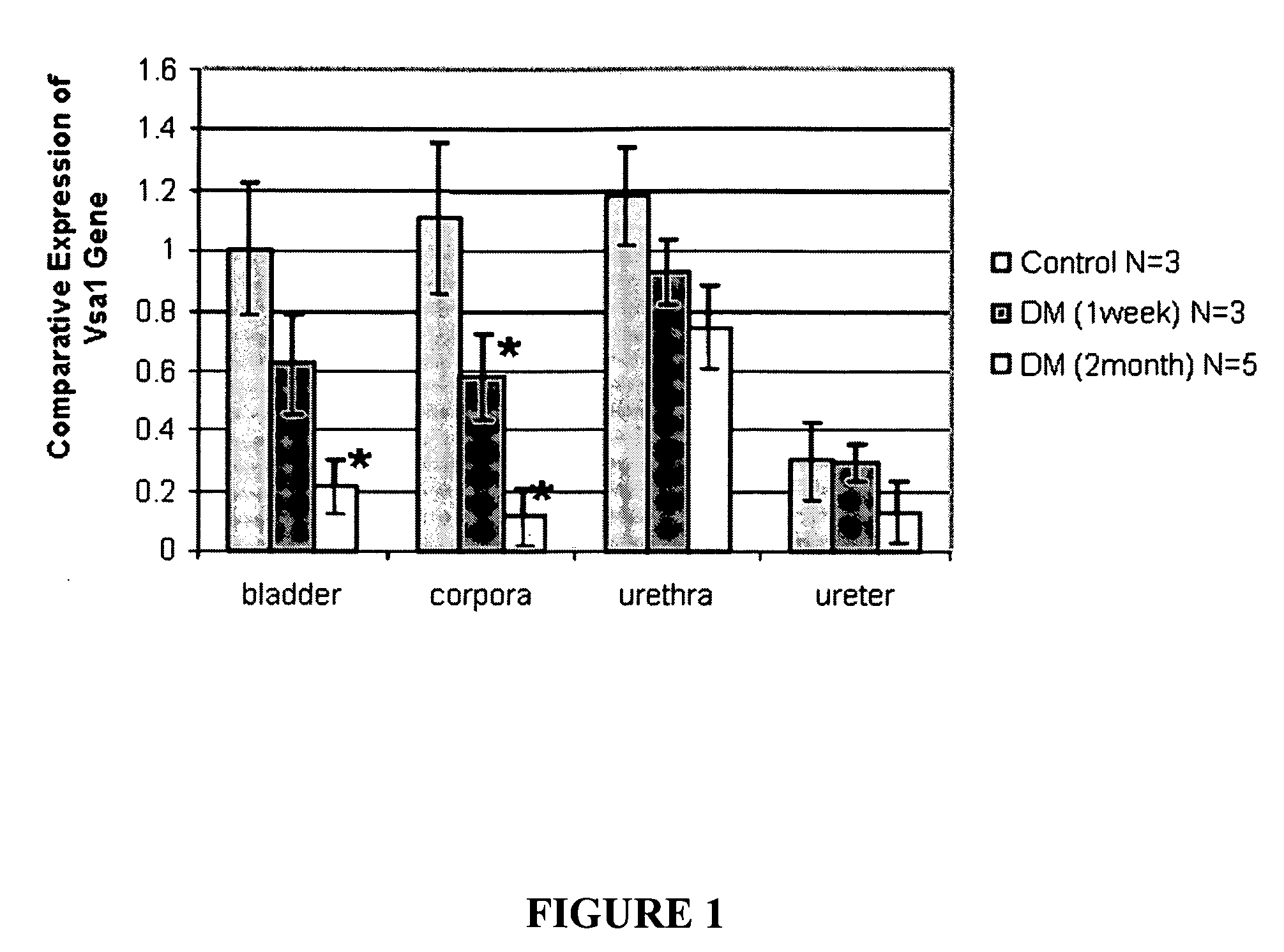
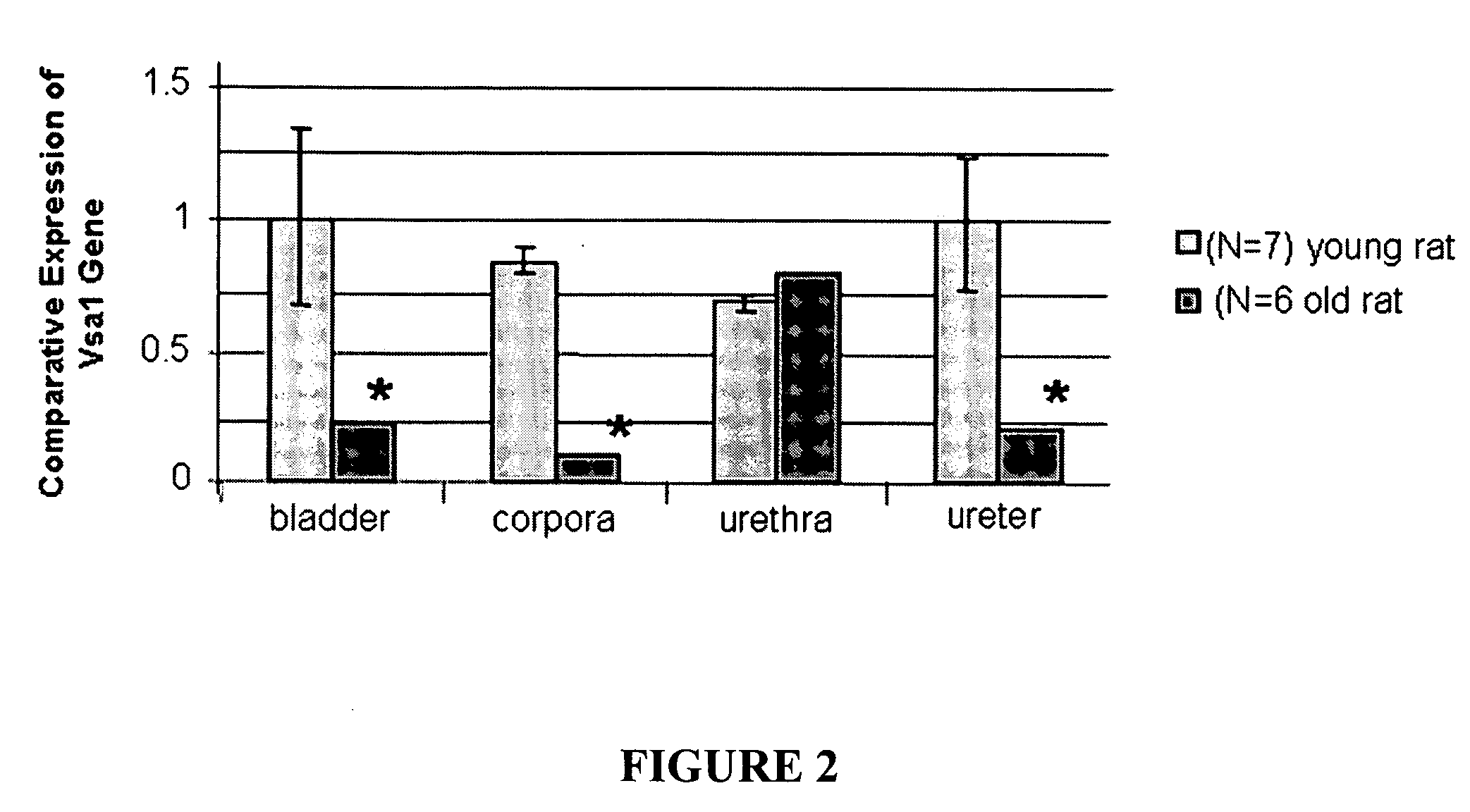
Assays for erectile and bladder dysfunction and vascular health
InactiveUS20090042208A1Microbiological testing/measurementDisease diagnosisBladder functionTherapeutic effect
Assays are provided for erectile and bladder dysfunction and vascular health (e.g., cardiovascular disease, hypertension), where the assays detect the expression of one or more of the human Vcsa1 gene family. The assays are also useful for monitoring the efficacy of treatment of these disorders.
Owner:NAT INST OF HEALTH REPRESENTED BY THE SEC OF THE DEPT OF HEALTH & HUMAN SERVICES NAT INST OF HEALTH
Method of treatment for bladder dysfunction
InactiveUS20100104631A1Improve urinary tract symptomDecreasing bladder irritationPeptide/protein ingredientsUrinary disorderLower riskLiposome
Liposomes are used for intravesical drug delivery, especially delivery of botulinum toxin (BoNT) to help improve lower urinary tract symptoms by decreasing bladder irritation and frequency. The system uses a lower and safer dose of BoNT with lower risk of urinary retention. A simple instillation of liposome-BoNT as a liquid formulation into the bladder, in a typical volume of 30-60 ml, will achieve efficacy similar to that currently achieved with cystscopic needle injection of BoNT. The dose may be lower than that done by injection, thereby causing significantly less risk of urinary retention. Liposome-BoNT can protect the BoNT from bladder and urine breakdown. Liposome-BoNT instillation is more comfortable for the patients and allows many more doctors' offices to offer this form of treatment that would otherwise be restricted to doctors skilled and certified in cystoscopic BoNT injection.
Owner:LIPELLA PHARMA
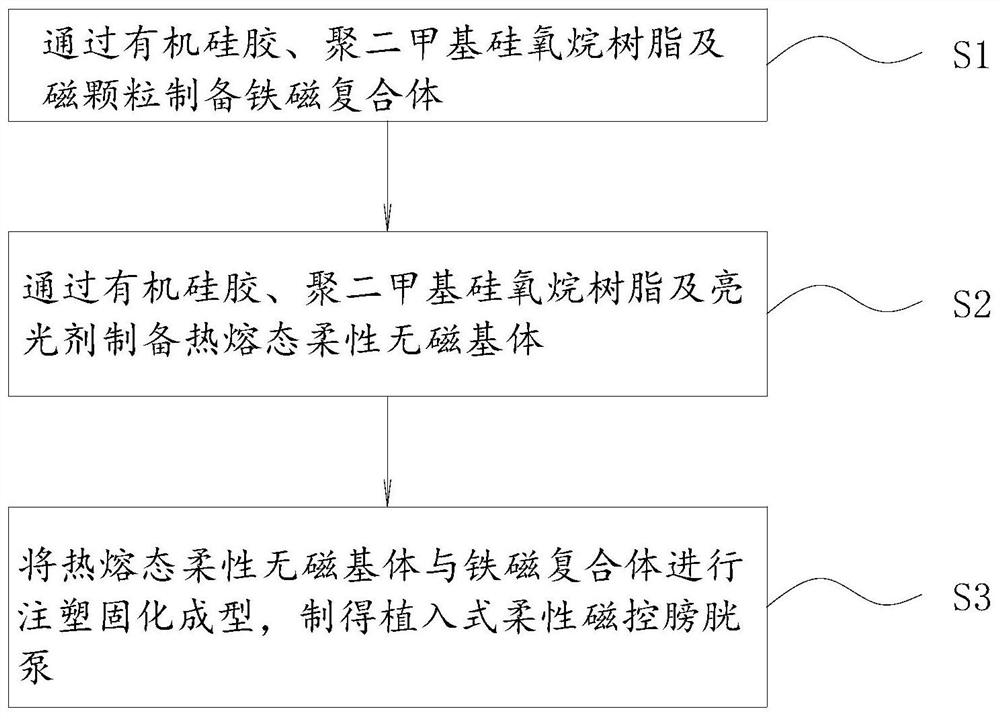
Thermoplastic forming method of implantable flexible magnetic control bladder pump
ActiveCN112936732AGood biocompatibilityHigh modulusNon-surgical orthopedic devicesPolymer scienceBladder function
The invention relates to a thermoplastic forming method of an implantable flexible magnetic control bladder pump. The thermoplastic forming method comprises the following steps of preparing a ferromagnetic complex from organic silica gel, polydimethylsiloxane resin and magnetic particles; preparing a hot-melt-state flexible non-magnetic substrate from organic silica gel, polydimethylsiloxane resin and a brightener; and carrying out injection molding, curing and forming on the hot-melt-state flexible non-magnetic matrix and the ferromagnetic complex, so that the implantable flexible magnetic control bladder pump is manufactured. The implantable flexible magnetic control bladder pump prepared through the thermoplastic forming method can effectively and permanently solve dysuria diseases such as nerve type bladder dysfunction and the like, and after the flexible magnetic control bladder pump is implanted through an operation, the normal urination function can be realized through high torque of magnetic field force applied by an external magnetic field under the condition of not connecting any additional pipeline.
Owner:TONGJI HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI TECH
Thermoplastic forming mold of implantable flexible magnetic control bladder pump
PendingCN112810046ARebuild urination functionImprove production efficiencyDomestic articlesUrethraBladder function
The invention relates to a thermoplastic forming mold of an implantable flexible magnetic control bladder pump. The bladder pump comprises a mold core, a front mold box, a rear mold box, a front mold box insertion block group and a rear mold box insertion block, wherein the mold core is composed of a mold core main body and a mold core handle; the shape of the mold core main body is matched with the shape of a filled bladder; the shape of the mold core handle is matched with the shape of a urethra connected with the bladder; the top of the front mold box is provided with a plurality of insertion grooves matched with the front mold box insertion block group; a front mold containing cavity is formed in the front mold box; an insertion hole matched with the rear mold box insertion block is formed in the top of the rear mold box; a rear mold containing cavity is formed in the rear mold box; and the mold core handle is arranged in the rear mold containing cavity. The implantable flexible magnetic control bladder pump prepared by the thermoplastic forming mold can effectively and permanently solve dysuria diseases such as nerve type bladder dysfunction; and after the flexible magnetic control bladder pump is implanted through an operation, the normal urination function can be realized through high torque of magnetic force applied by an external magnetic field under the condition that no additional pipeline is connected.
Owner:武汉磁济科技有限公司
Bladder injeciton paradigm for administration of botulinum toxins
InactiveUS20170258878A1Reducing and preventing risk for urinary retentionReduce needPeptide/protein ingredientsMuscular disorderSaxitoxinBotulinum toxin a
Methods for treating a bladder dysfunction by injecting a clostridial derivative to a target site below the bladder midline are described.
Owner:ALLERGAN INC
Method of treatment for bladder dysfunction
InactiveUS20120093920A1Inhibits norepinephrine releaseLower Level RequirementsPeptide/protein ingredientsAntiinfectivesLower riskLiposome
Liposomes are used for intravesical drug delivery, especially delivery of botulinum toxin (BoNT) to help improve lower urinary tract symptoms by decreasing bladder irritation and frequency. The system uses a lower and safer dose of BoNT with lower risk of urinary retention. A simple instillation of liposome-BoNT as a liquid formulation into the bladder, in a typical volume of 30-60 ml, will achieve efficacy similar to that currently achieved with cystscopic needle injection of BoNT. The dose may be lower than that done by injection, thereby causing significantly less risk of urinary retention. Liposome-BoNT can protect the BoNT from bladder and urine breakdown. Liposome-BoNT instillation is more comfortable for the patients and allows many more doctors' offices to offer this form of treatment that would otherwise be restricted to doctors skilled and certified in cystoscopic BoNT injection.
Owner:LIPELLA PHARMA
State-dependent peripheral neuromodulation to treat bladder dysfunction
ActiveUS20180296833A1Reduced bladder capacityReduced voiding efficiencySpinal electrodesUrinary disorderState dependentPeripheral neuron
The present invention relates to a neuromodulation apparatus and methods of using the neuromodulation apparatus for treating bladder dysfunction.
Owner:DUKE UNIV
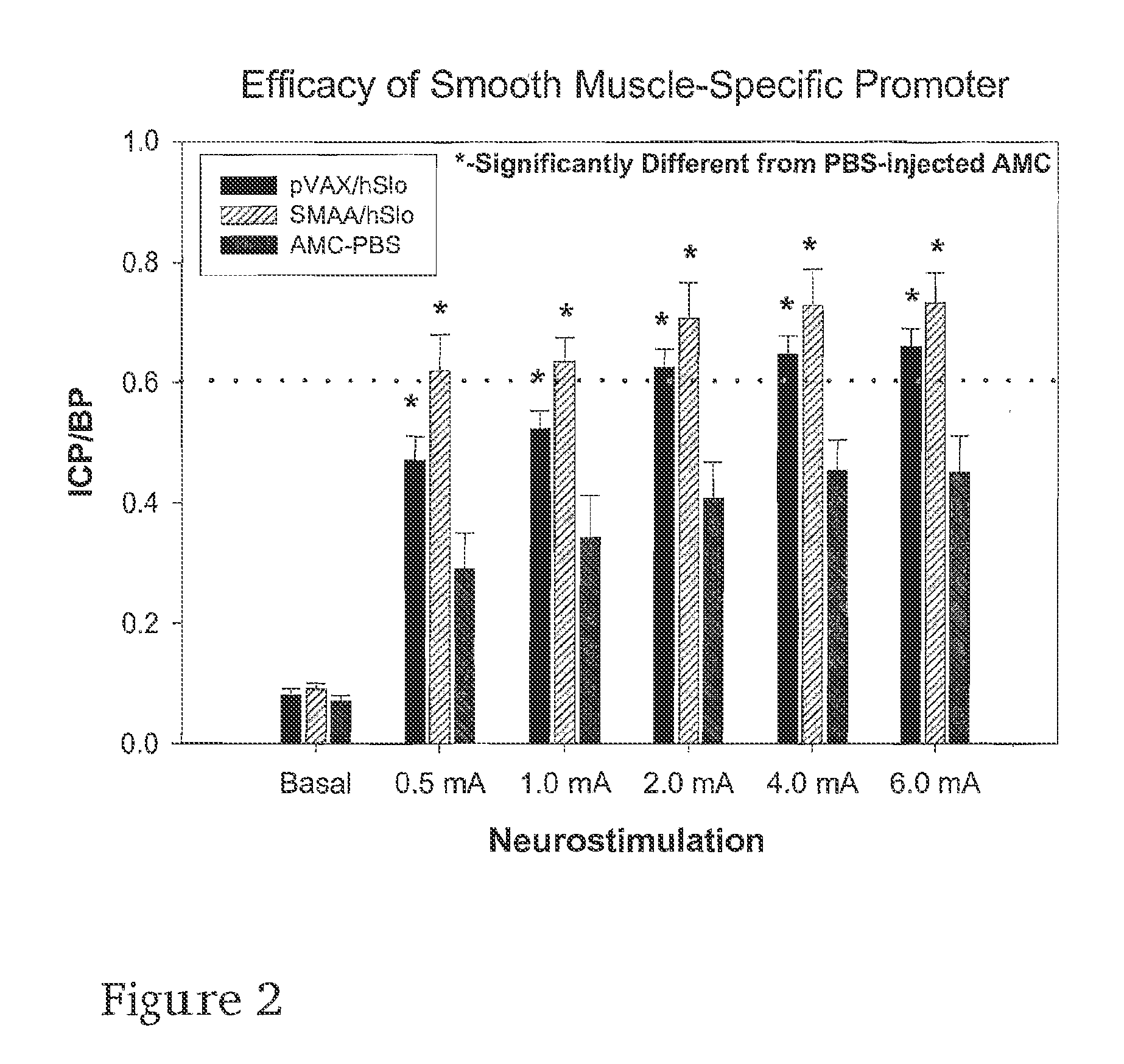
Gene therapy for regulating smooth muscle tone
InactiveUS20070086982A1Enhance smooth muscle contractionModulates contraction of smooth muscleOrganic active ingredientsBiocideDiseaseSmooth muscle
The present invention is directed towards a method of regulating smooth muscle tone, comprising the introduction, into smooth muscle cells of a subject, of a DNA sequence encoding a protein involved in the regulation of smooth muscle tone, and expression of the DNA sequence in a sufficient number of smooth muscle cells of the subject to regulate smooth muscle tone in the subject. Specifically, the invention provides methods of gene therapy for treating erectile dysfunction, bladder dysfunction, and other smooth muscle disorders. The present invention also provides recombinant viral and non-viral vectors comprising DNA encoding a protein involved in the regulation of smooth muscle tone. Further provided by the present invention is a smooth muscle cell which expresses a gene encoding a protein involved in the regulation of smooth muscle tone.
Owner:GELIEBTER JAN +3
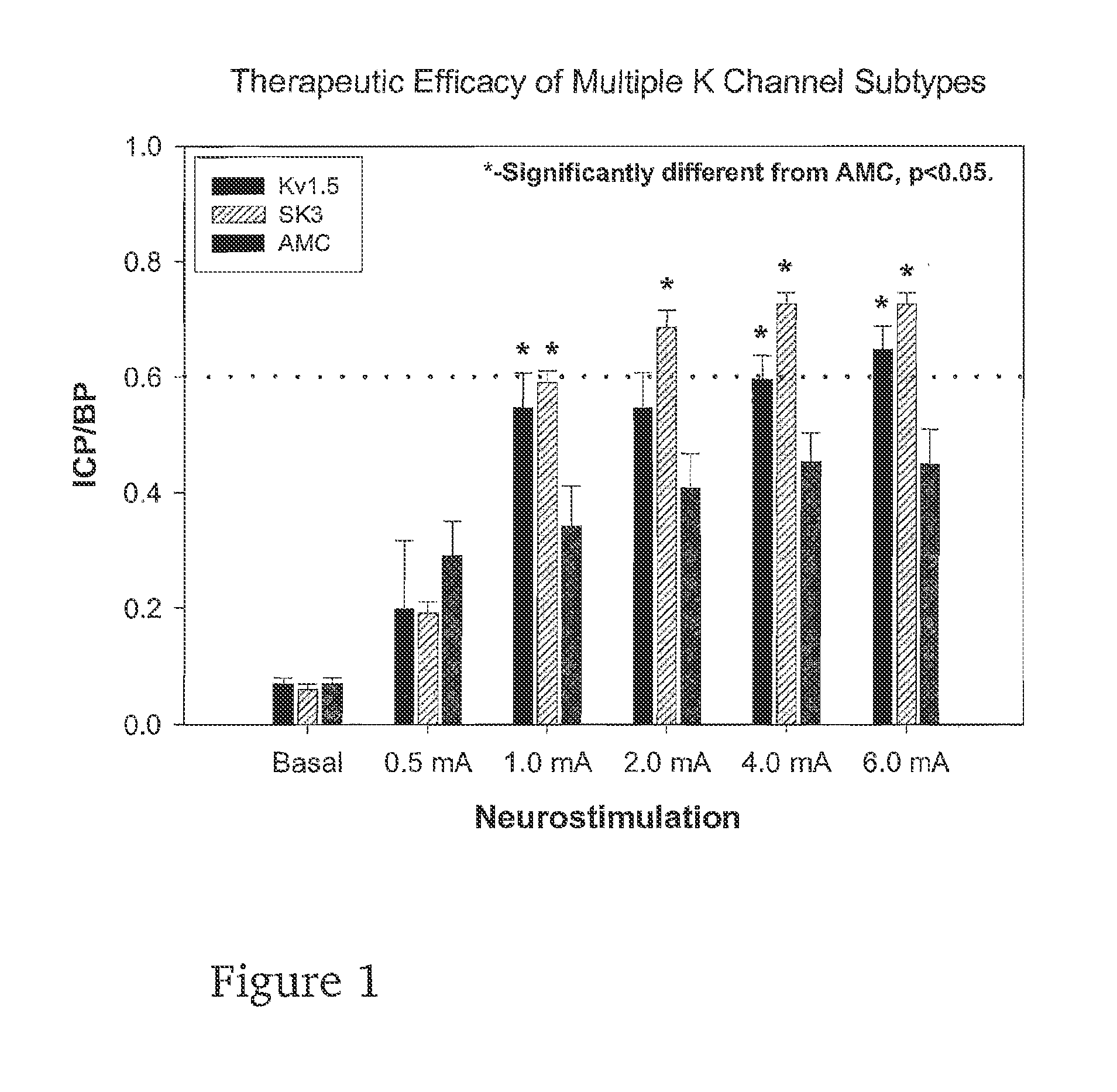
Gene transfer for regulating smooth muscle tone
The invention provides methods of regulating smooth muscle tone in a subject, comprising the introduction, into smooth muscle cells of the subject, of a DNA sequence encoding a potassium channel protein involved in the regulation of smooth muscle tone, and expression of the DNA sequence in a sufficient number of smooth muscle cells of the subject to regulate smooth muscle tone in the subject. The invention provides methods of gene transfer for treating erectile dysfunction, bladder dysfunction, and other smooth muscle disorders.
Owner:ALBERT EINSTEIN COLLEGE OF MEDICINE OF YESHIVA UNIV
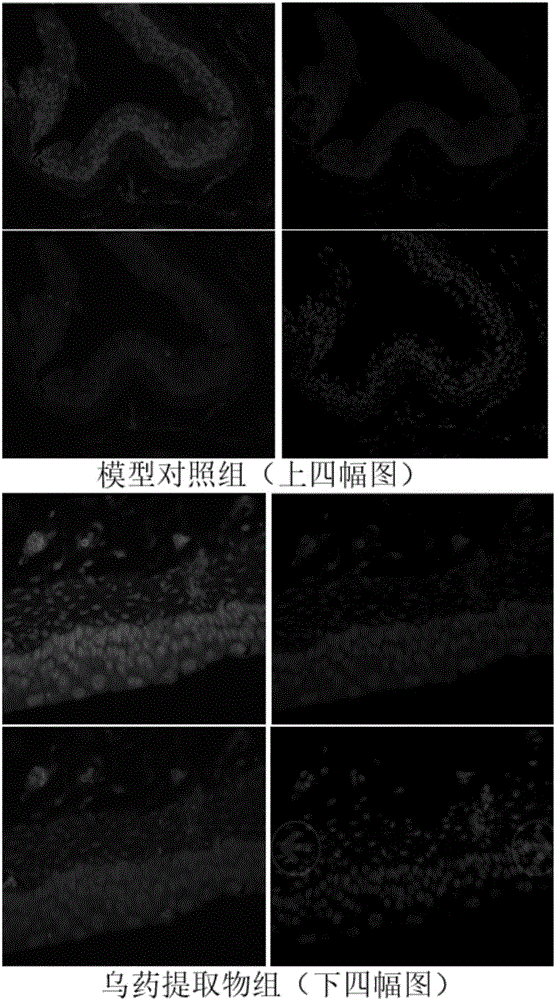
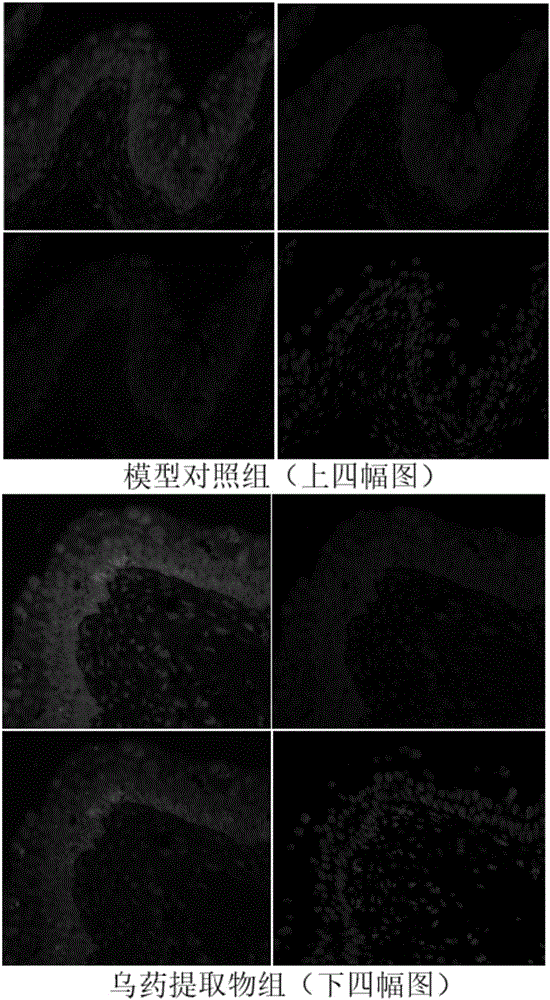
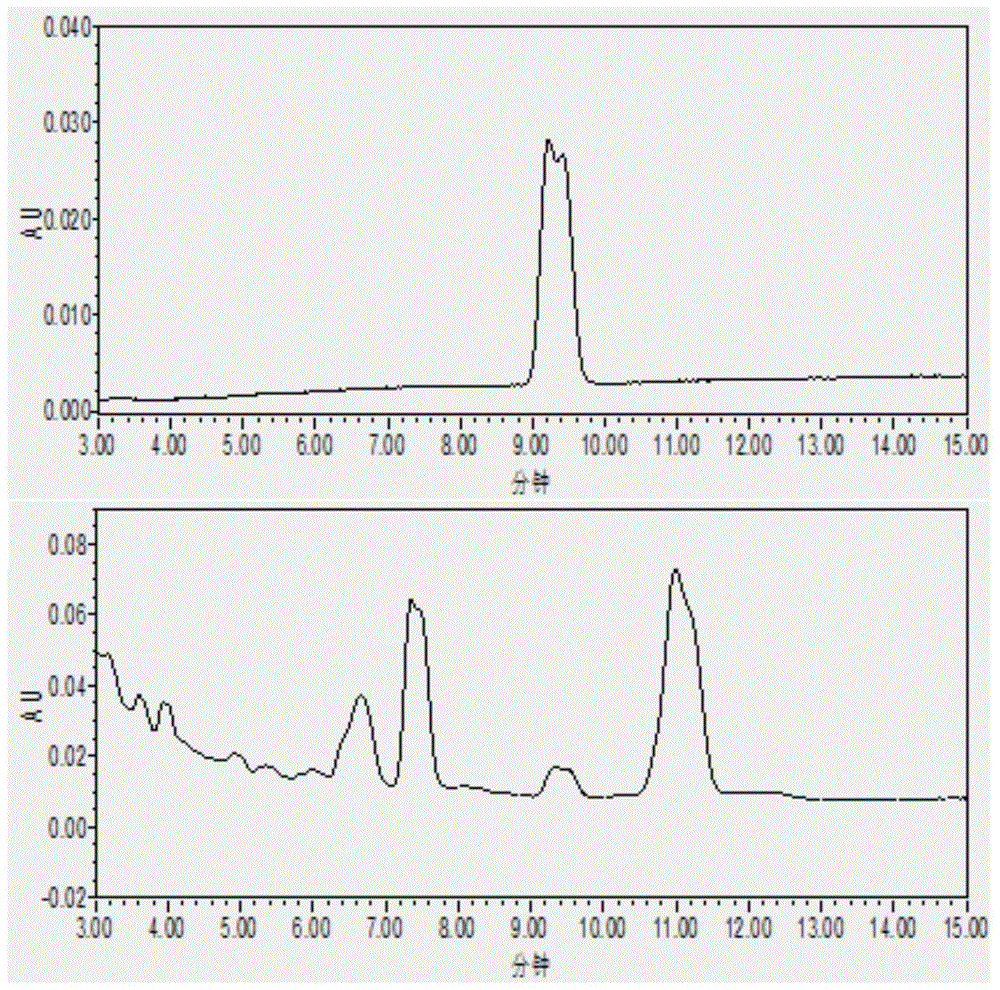
Radix linderae extract for treating diabetic bladder dysfunction disease and preparation method thereof
InactiveCN105796642AGood curative effectAdjust diastolic tensionPowder deliverySpray deliveryDiseaseCholinergic Nerves
The invention discloses a radix linderae extract for treating diabetic bladder dysfunction disease and a preparation method thereof. The radix linderae extract prepared by the preparation method has a perfect curative effect on bladder dysfunction disease and particularly diabetic bladder dysfunction disease and can be used for preparing a related medicine; and the medicine consists of the radix linderae extract and medically acceptable accessories, wherein 500-5,000mg of radix linderae extract is contained in daily dosage. The radix linderae extract and medicine thereof can regulate the diastolic and systolic tension of the bladder smooth muscle so as to regulate the bladder function; and the mechanism is related to a cholinergic nerve receptor (M receptor), a beta-adrenergic receptor (beta-AR) and a non-cholinergic non-adrenergic TRPV1 passage. Therefore, the radix linderae extract can be used for preventing and treating bladder dysfunction disease and has wide application prospects in preparing a medicine for preventing and / or treating diabetic bladder dysfunction disease.
Owner:GUANGZHOU UNIVERSITY OF CHINESE MEDICINE
Gene transfer for regulating smooth muscle tone
The invention provides methods of regulating smooth muscle tone in a subject, comprising the introduction, into smooth muscle cells of the subject, of a DNA sequence encoding a potassium channel protein involved in the regulation of smooth muscle tone, and expression of the DNA sequence in a sufficient number of smooth muscle cells of the subject to regulate smooth muscle tone in the subject. The invention provides methods of gene transfer for treating erectile dysfunction, bladder dysfunction, and other smooth muscle disorders.
Owner:COM AFFILIATION
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com