Patents
Literature
163 results about "Urinary retention" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Inability to voluntarily empty the bladder (pass urine) completely or partially.
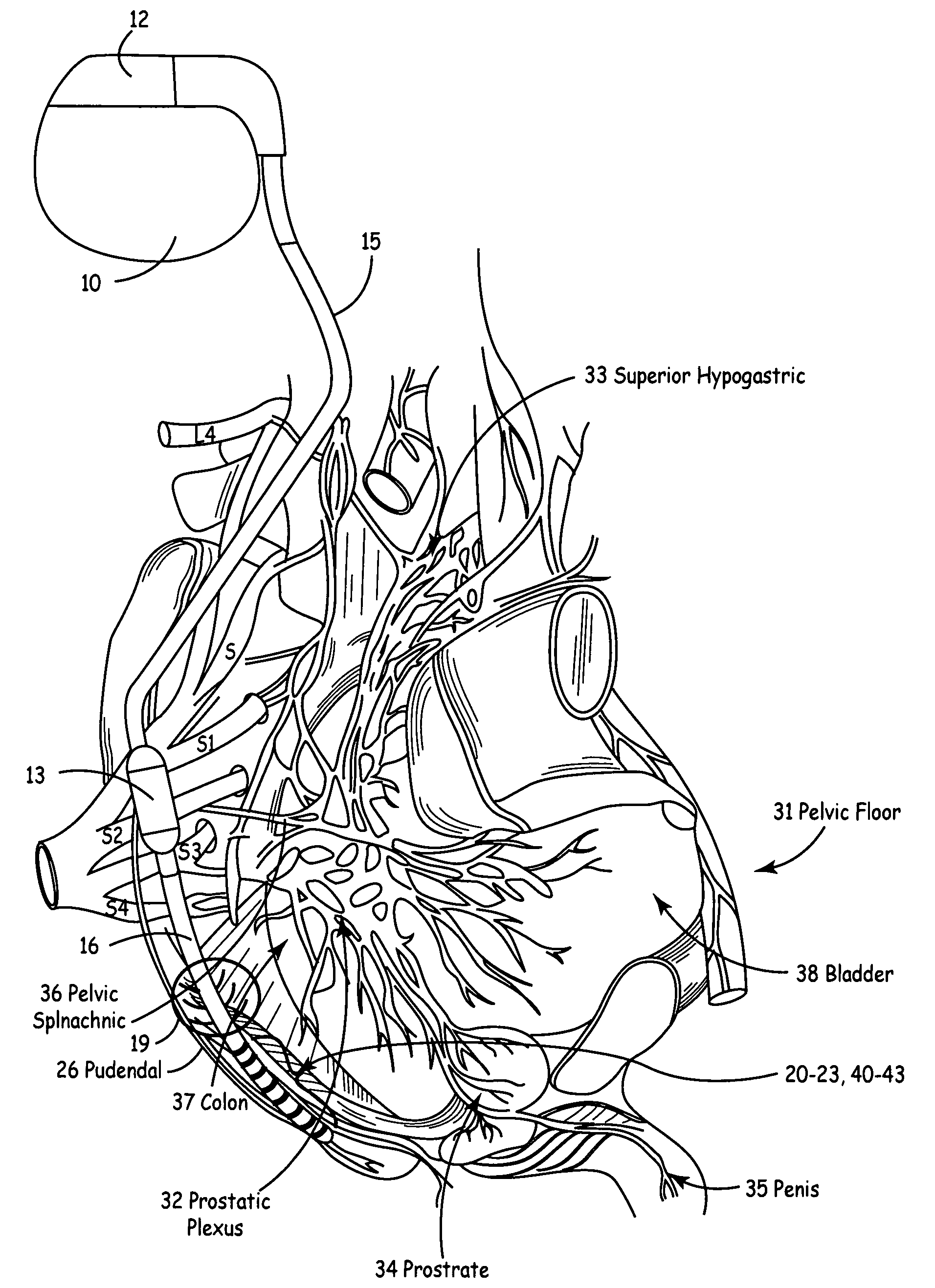
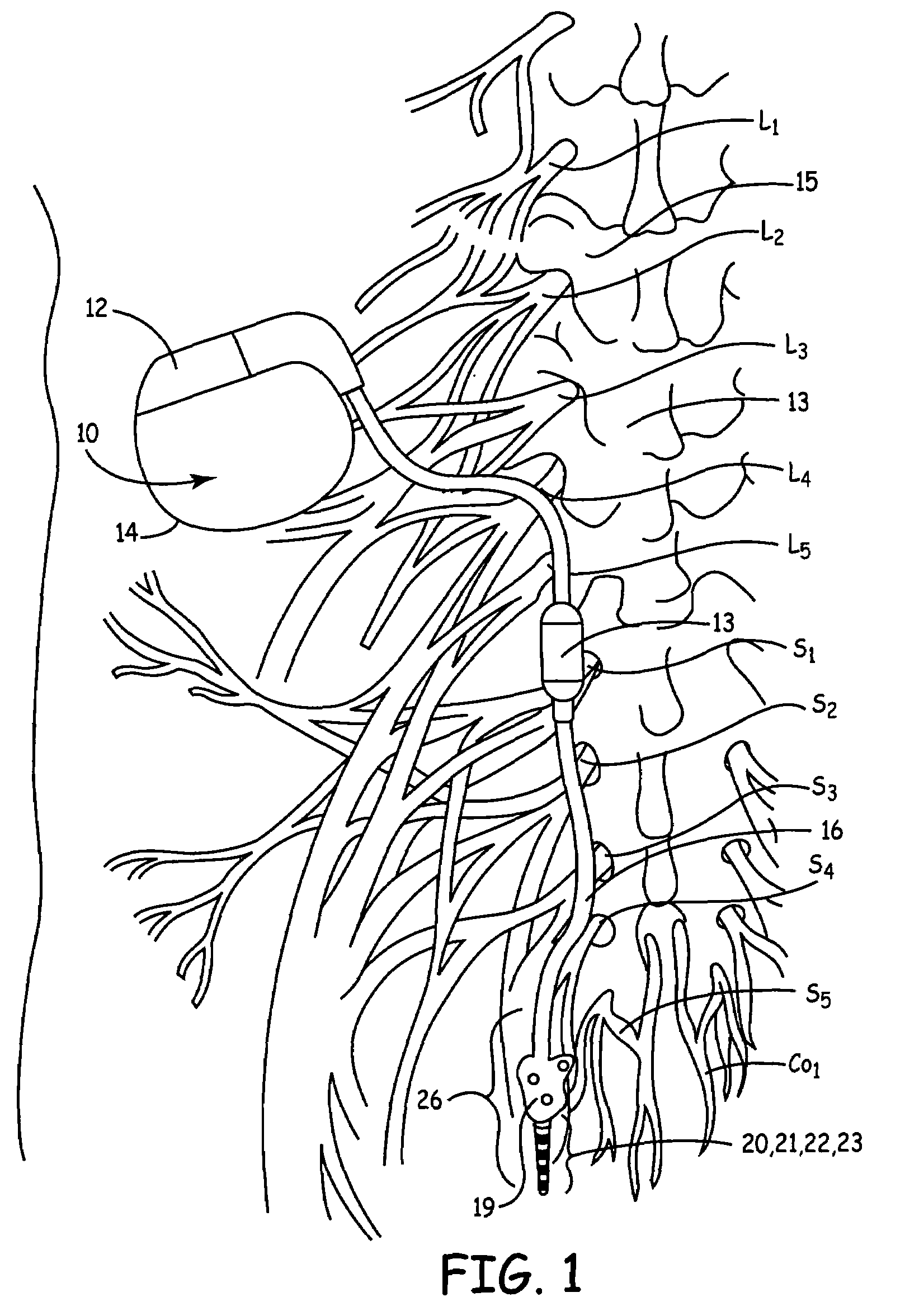
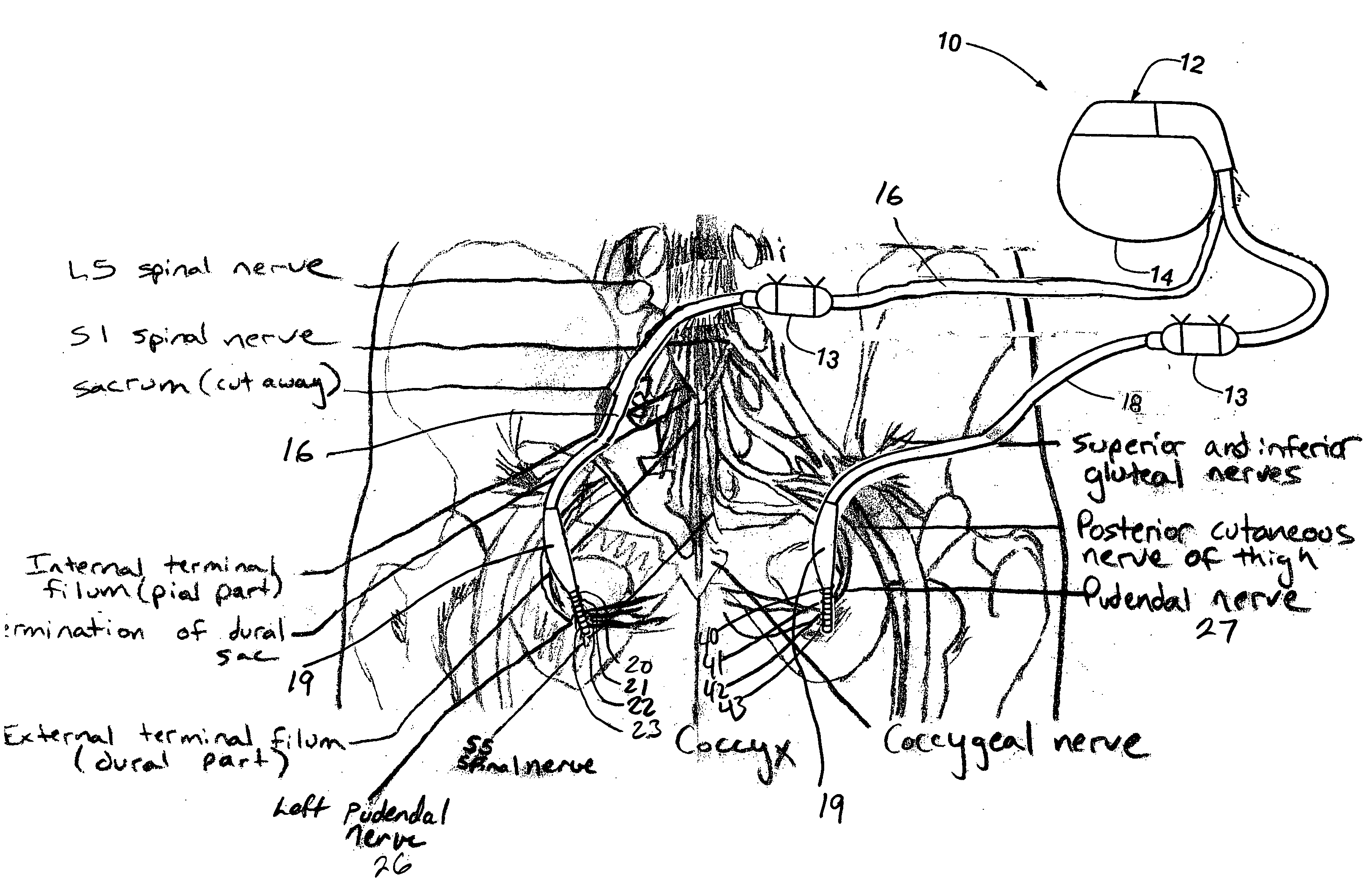
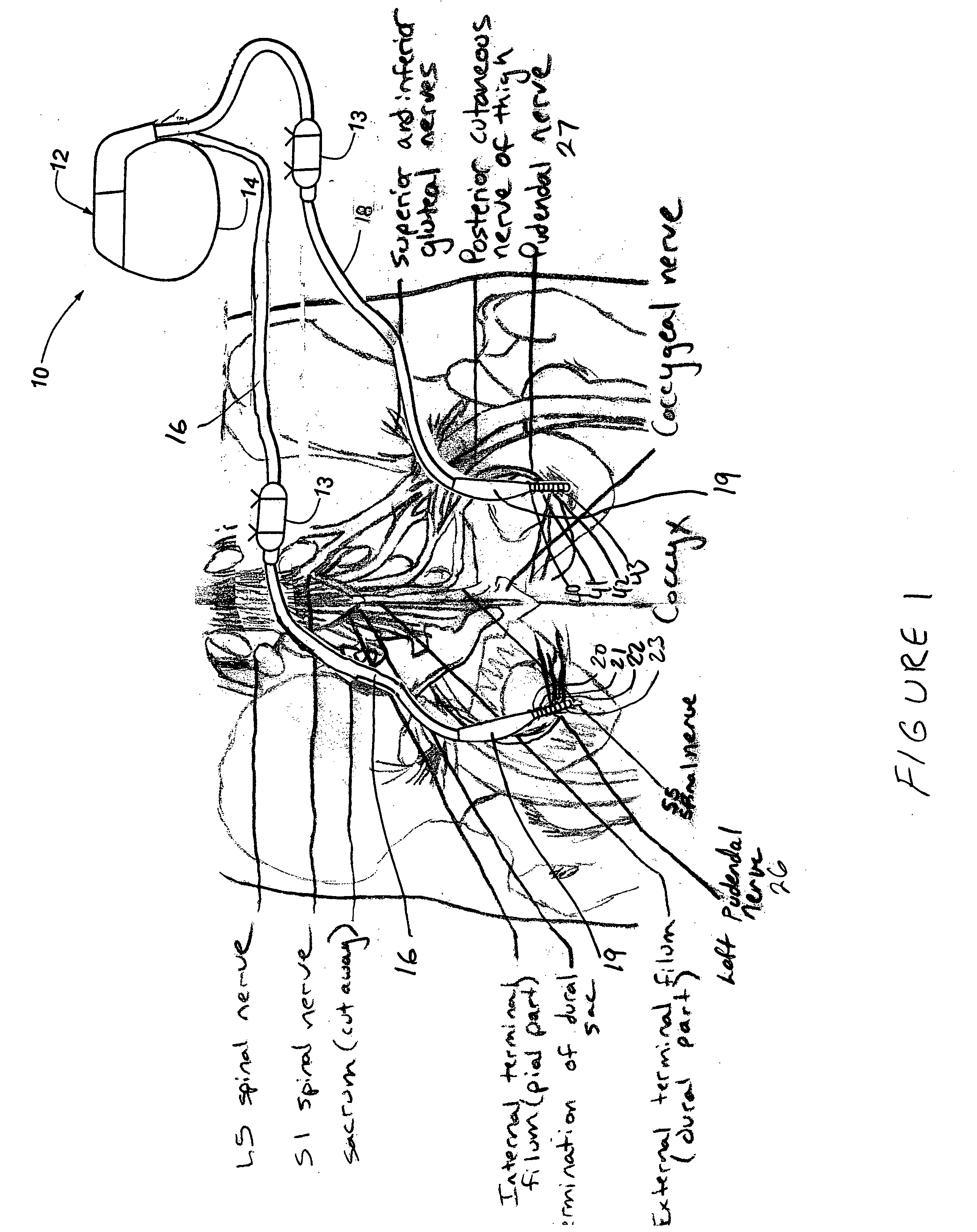
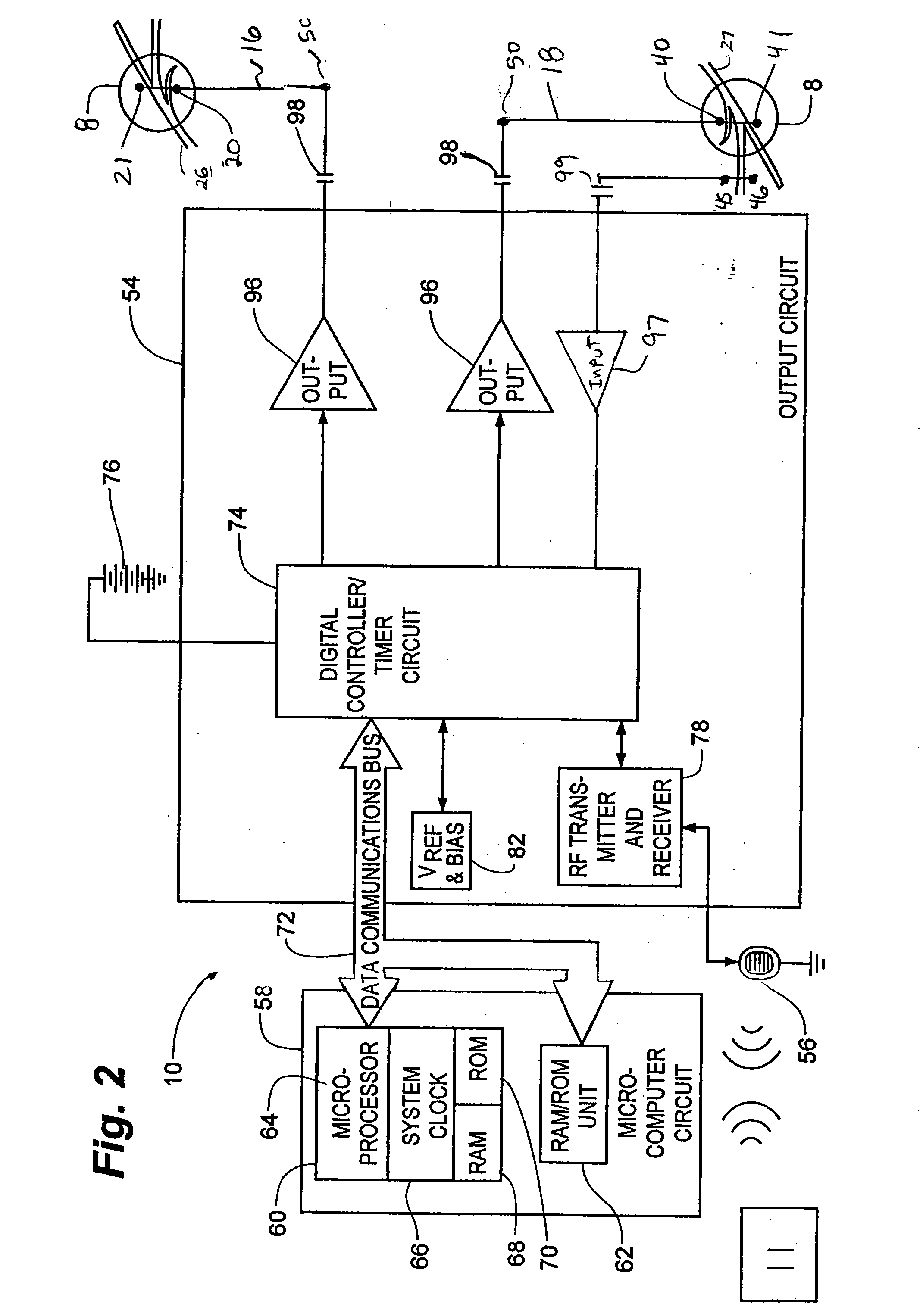
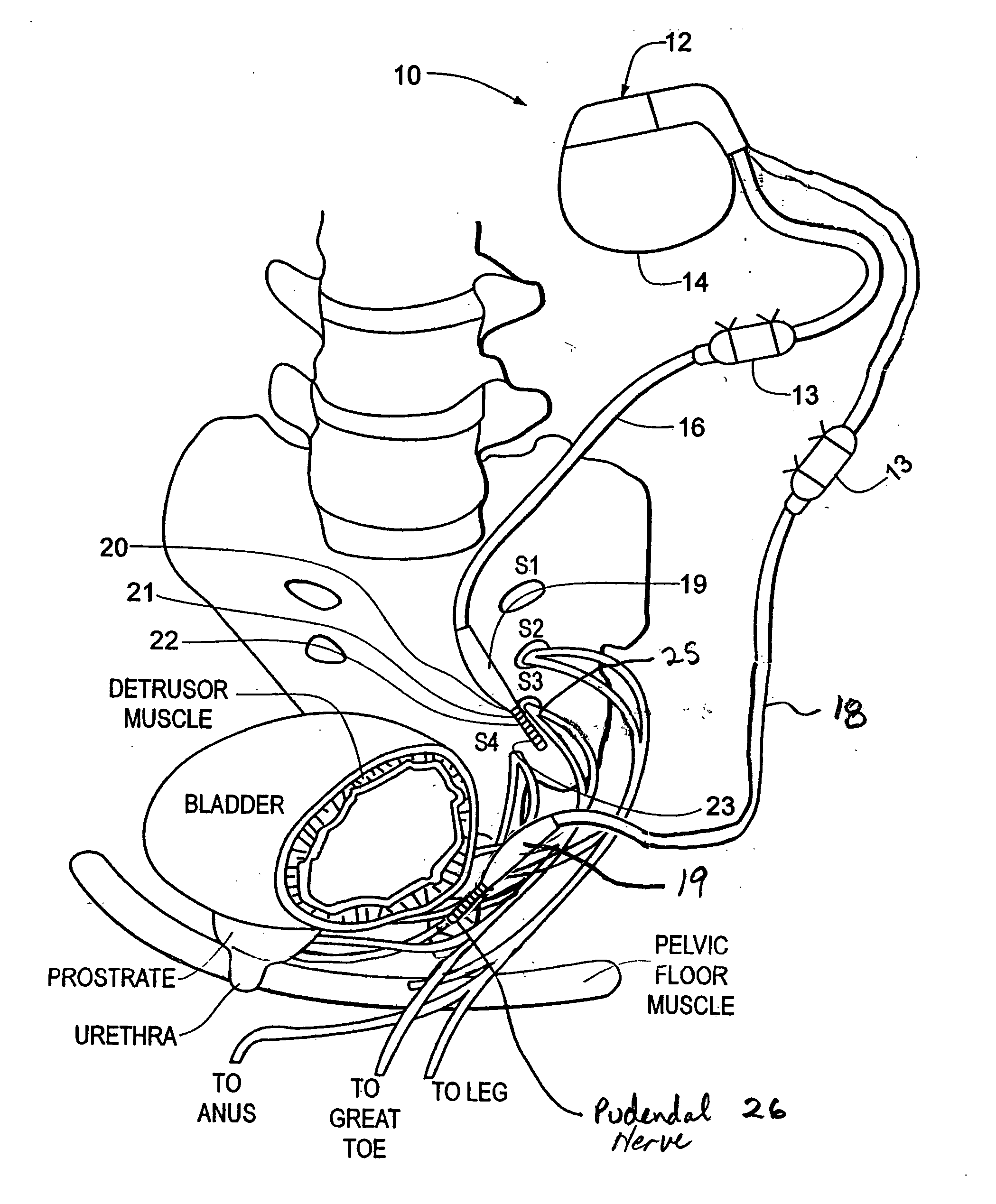
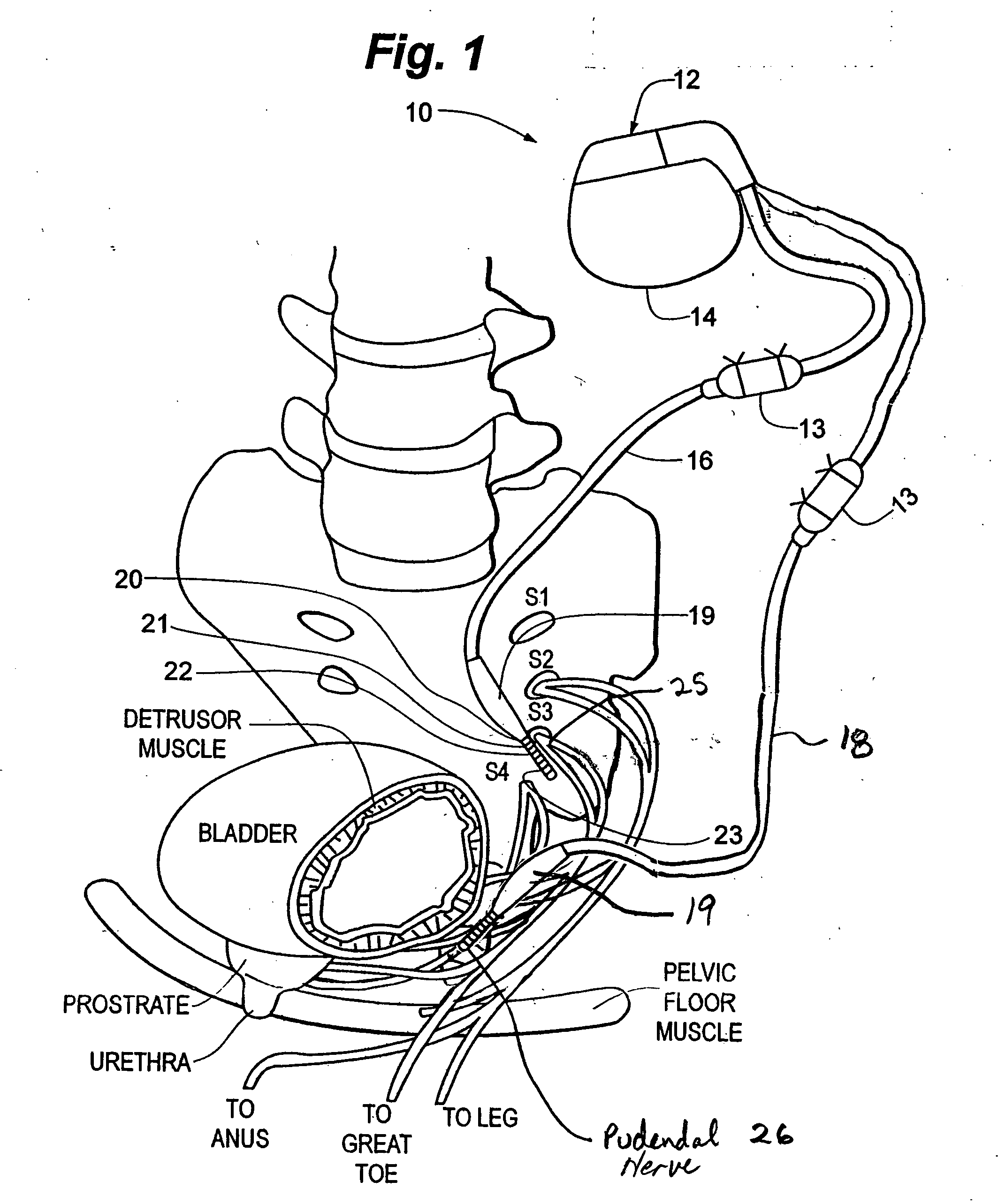
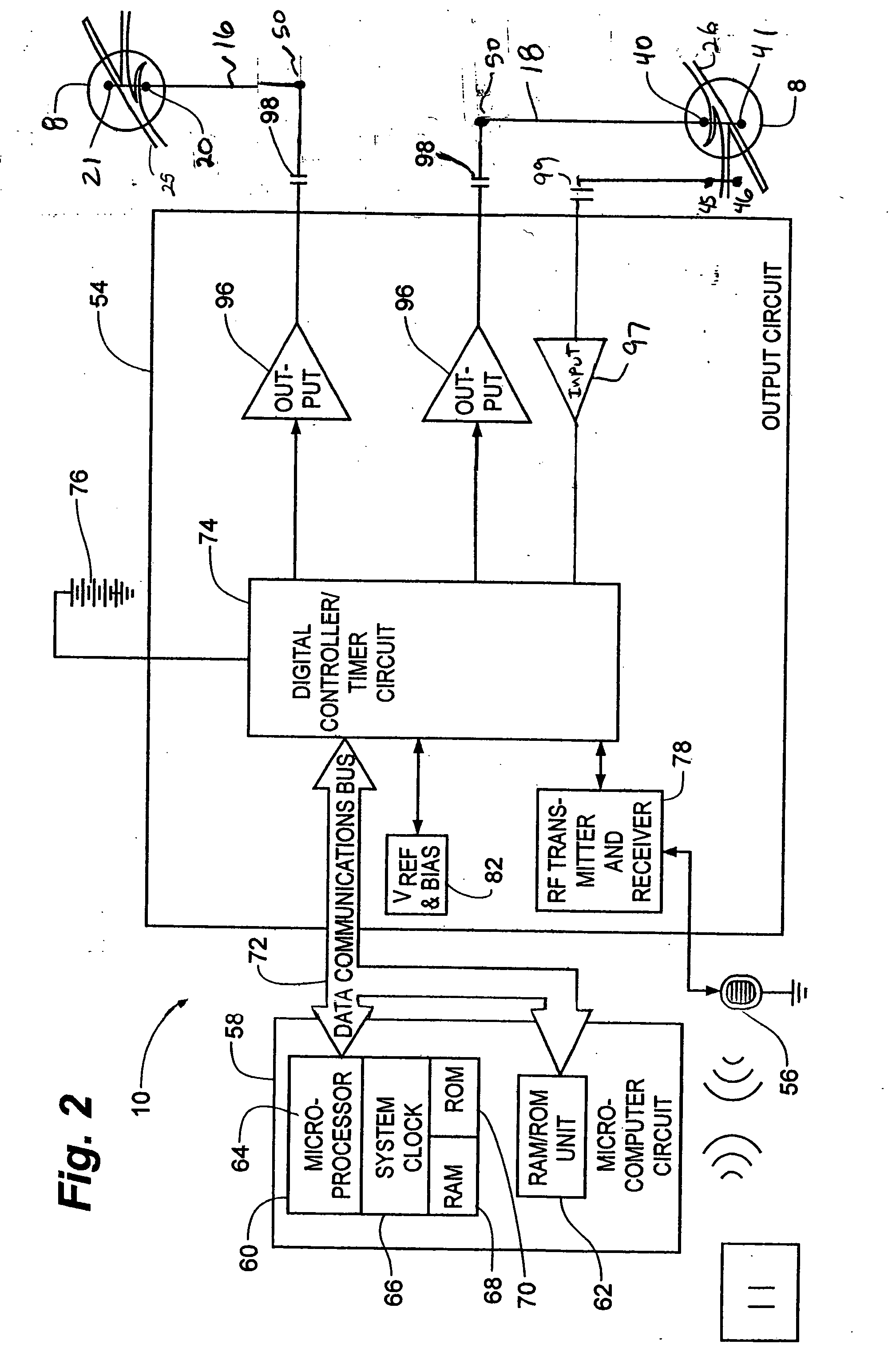
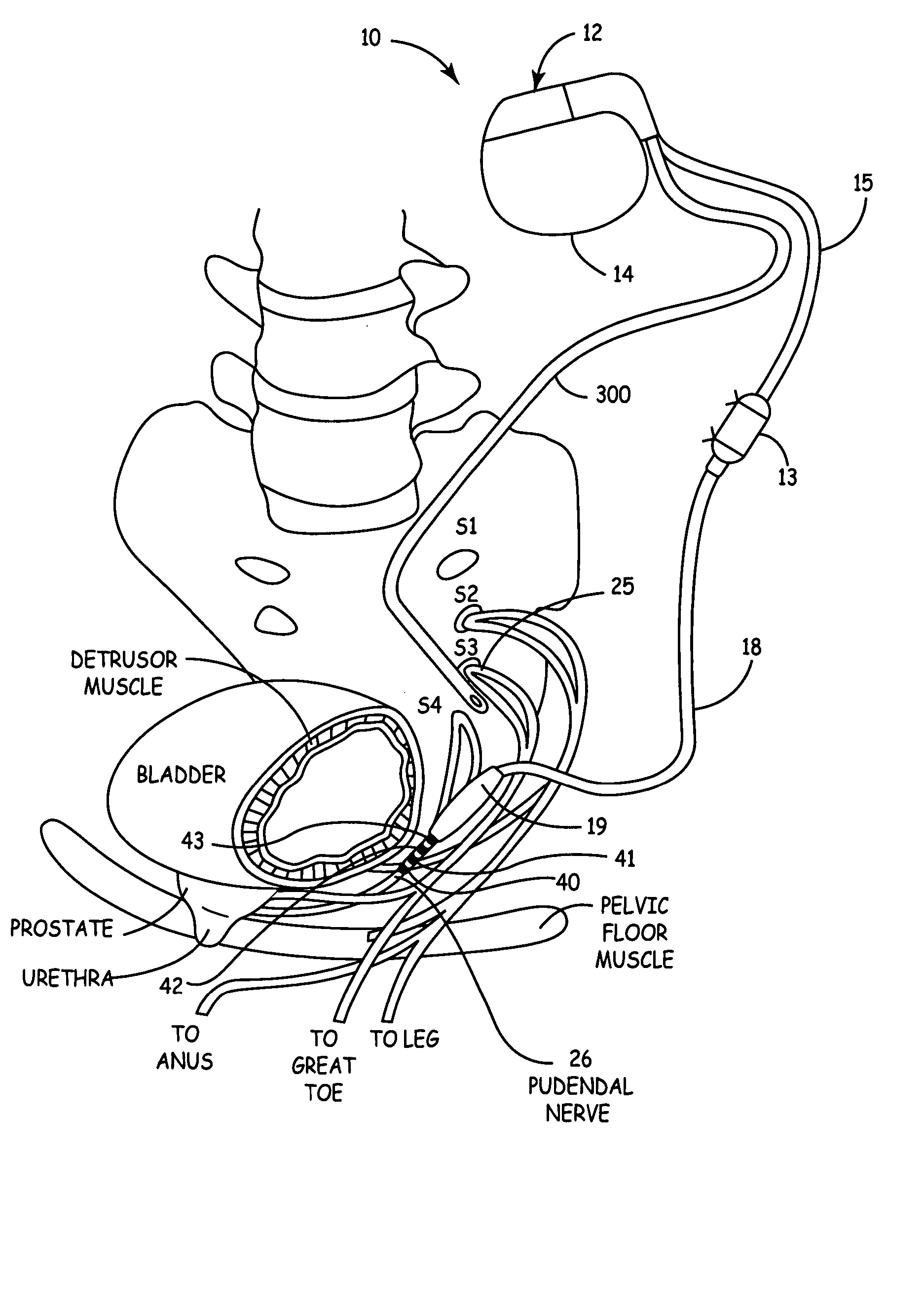
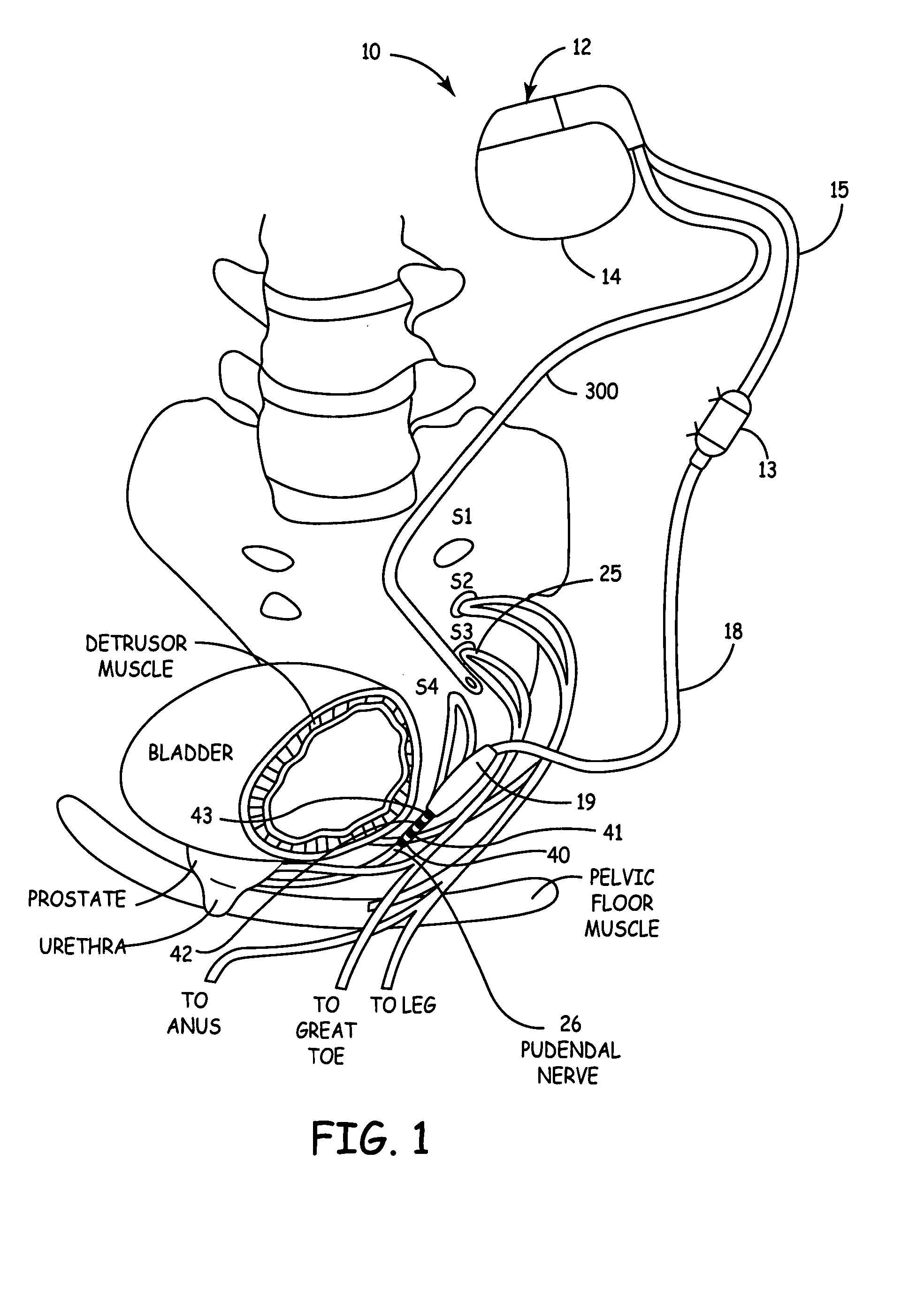
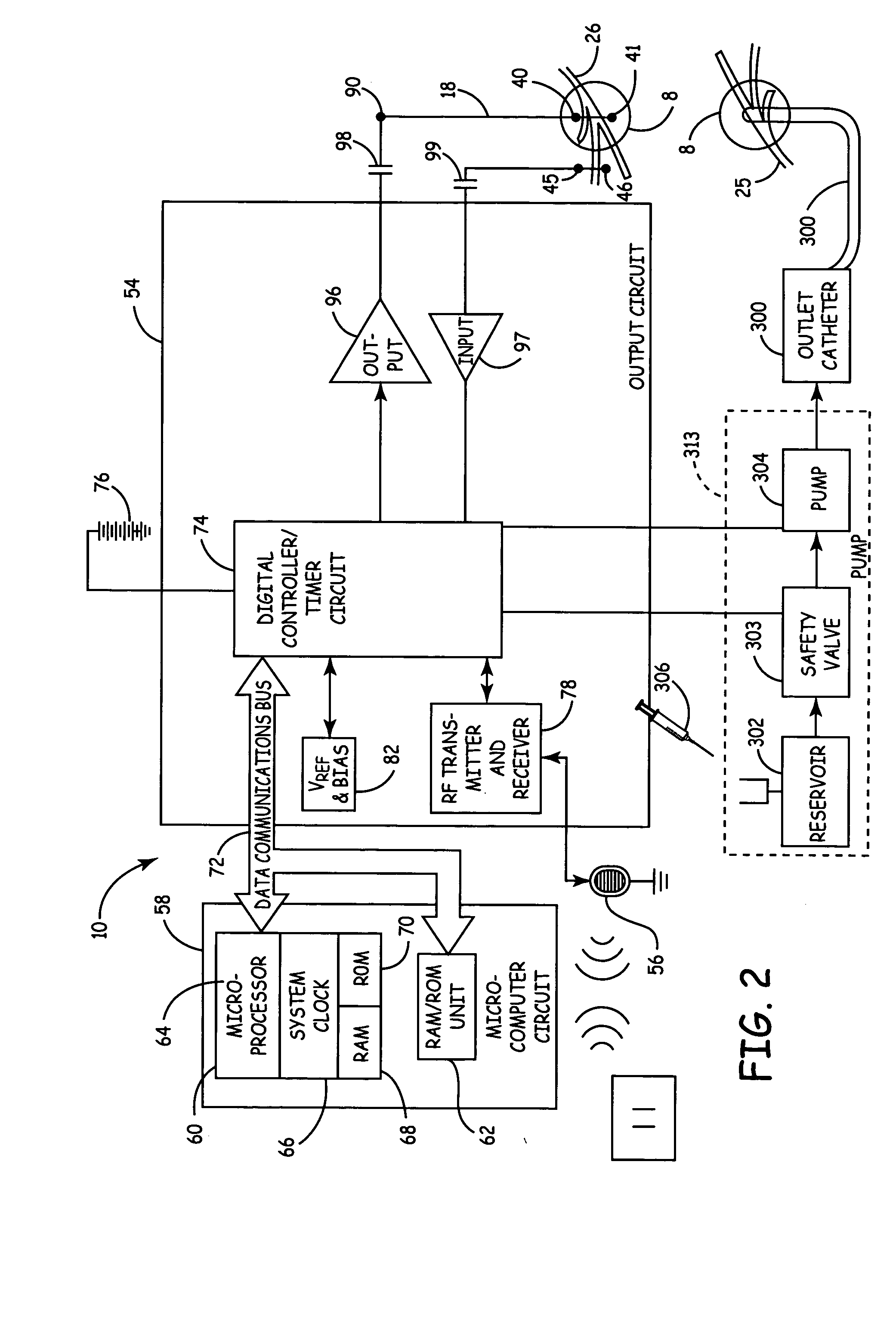
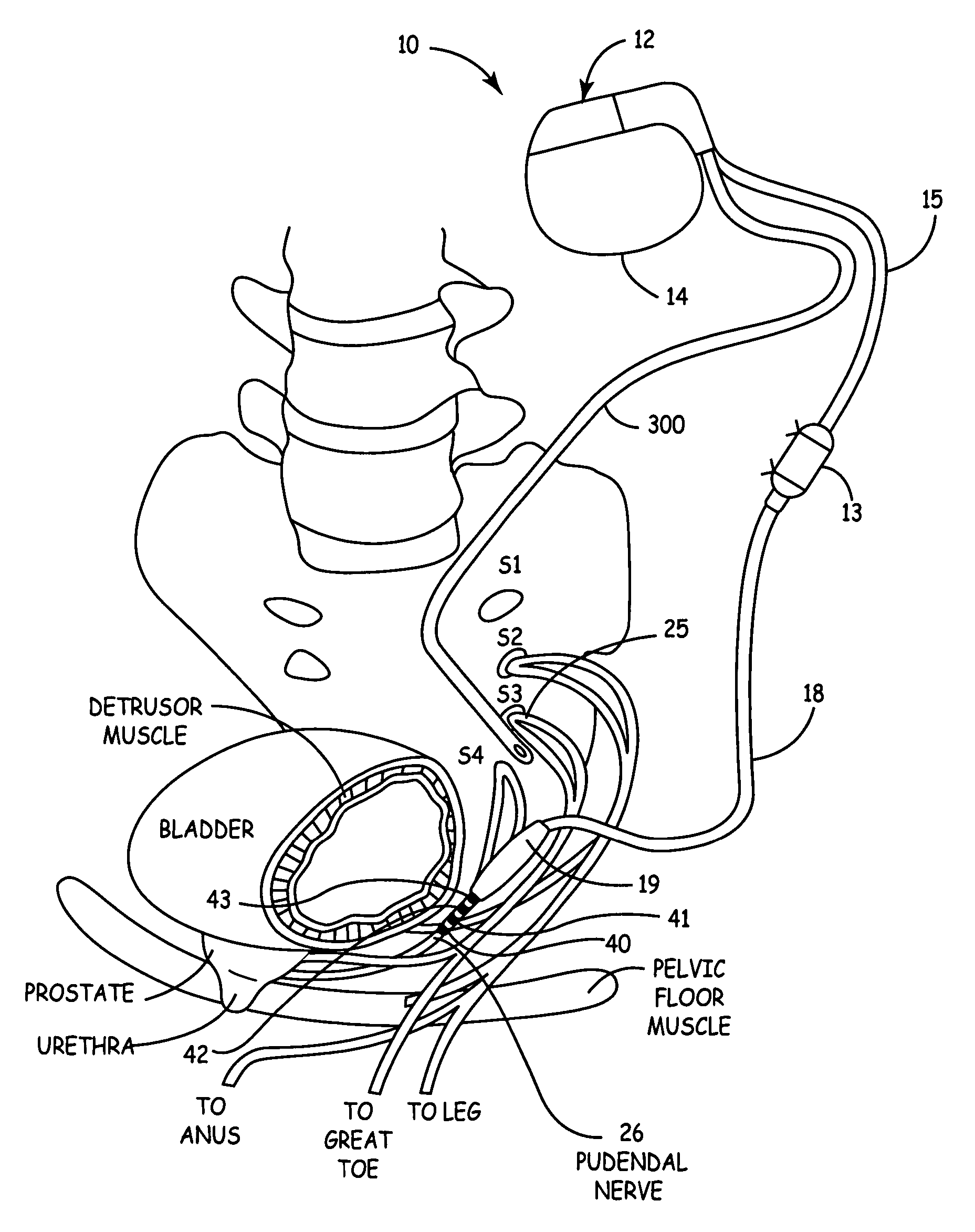
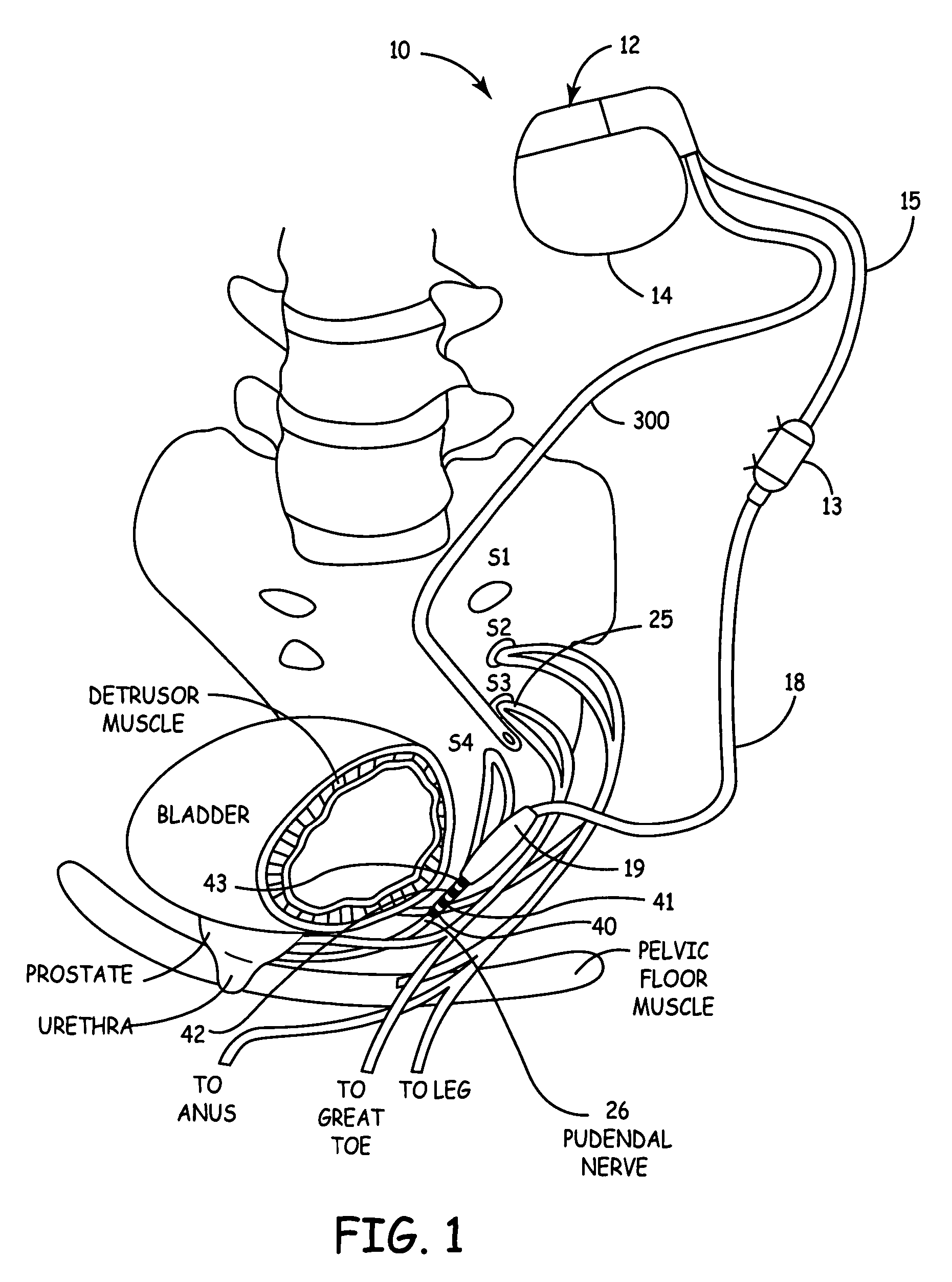
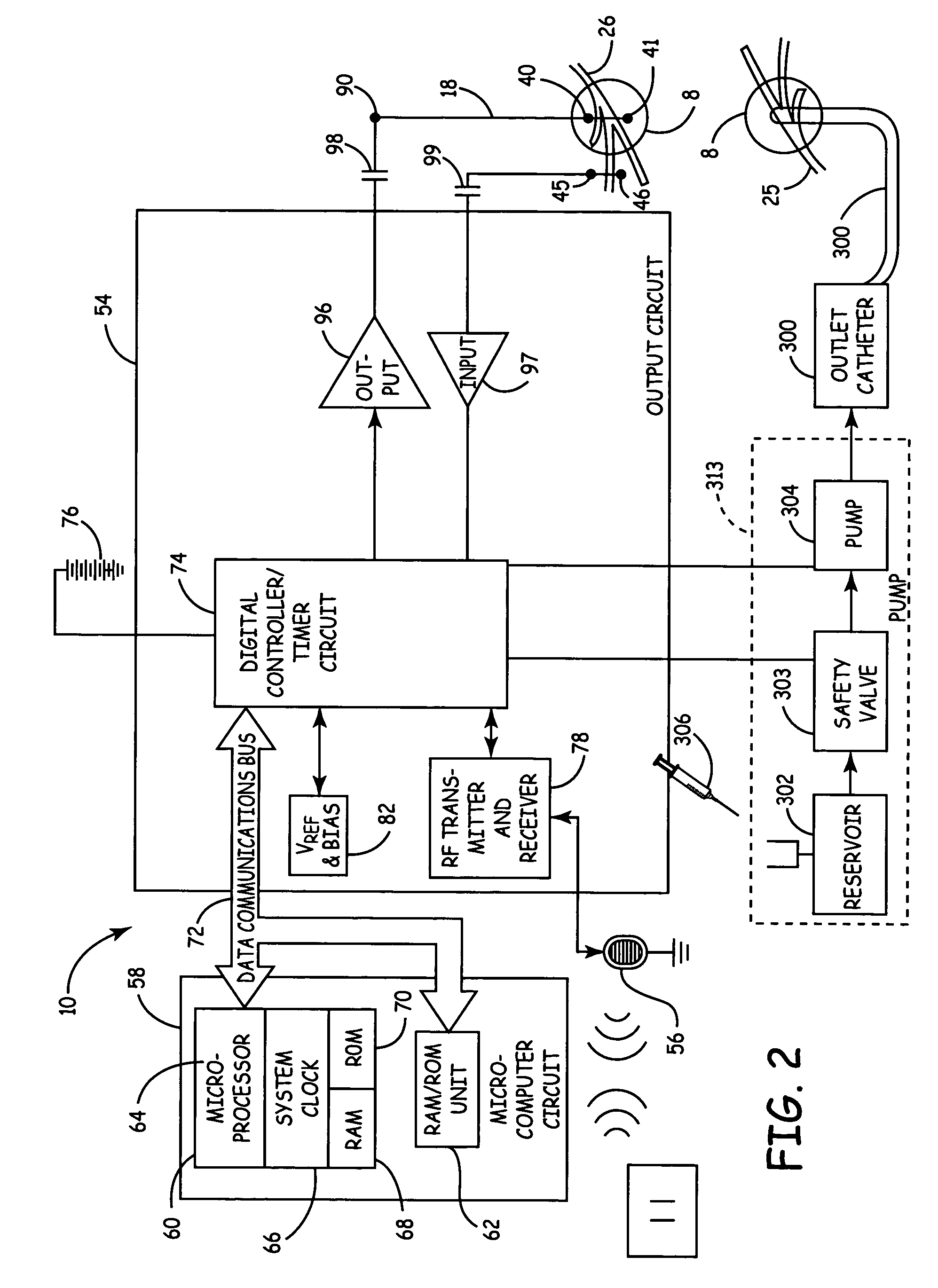
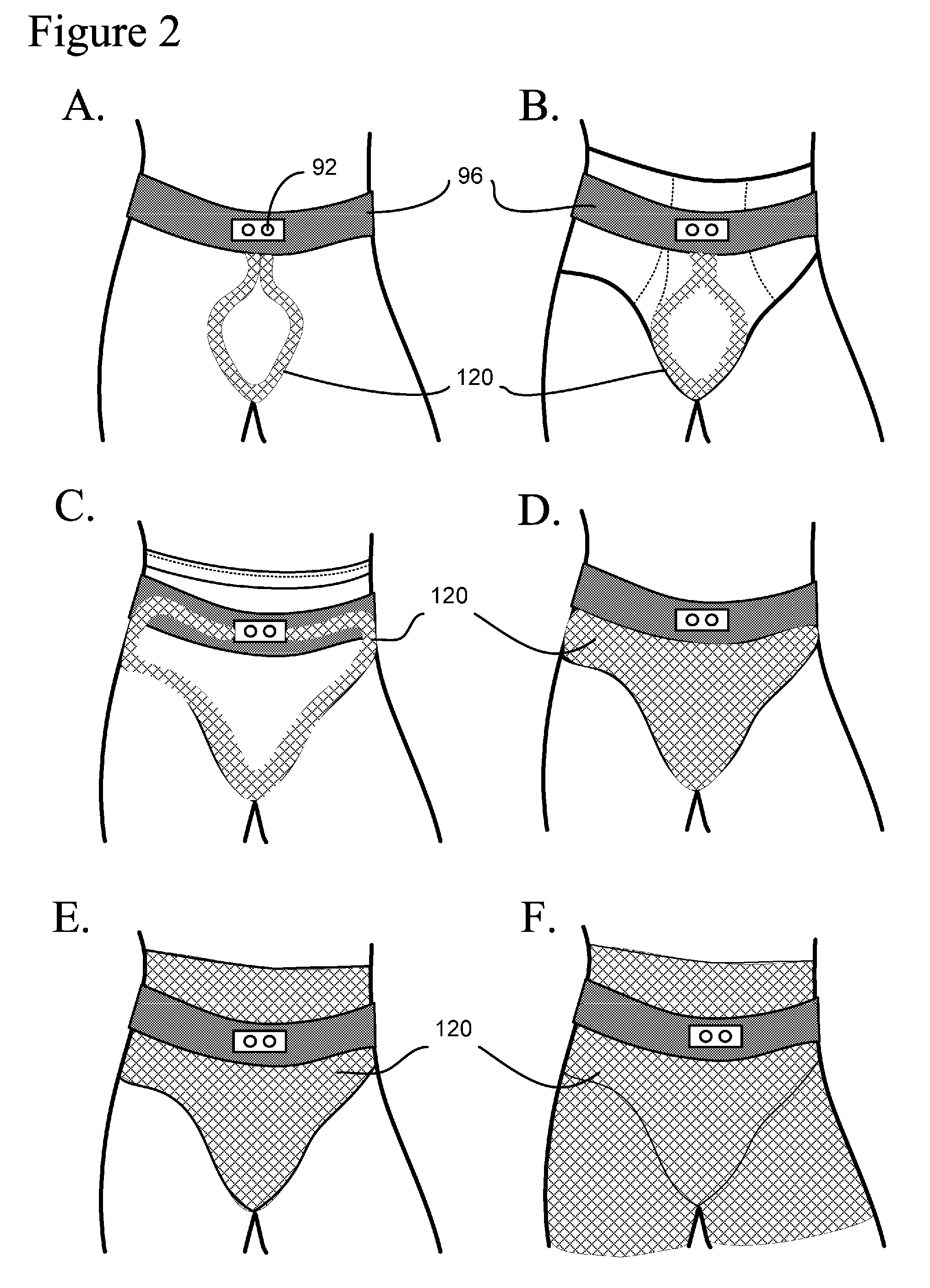
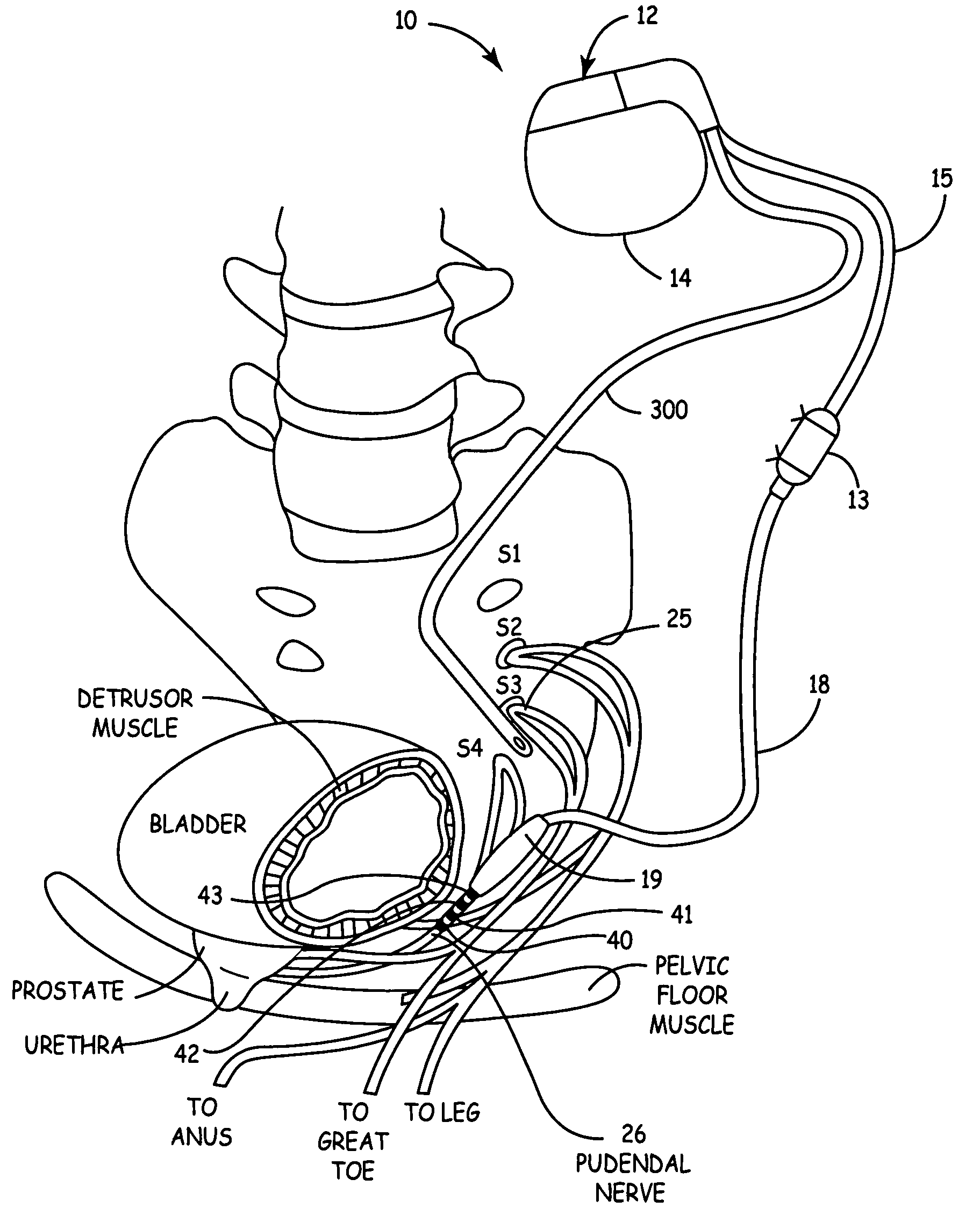
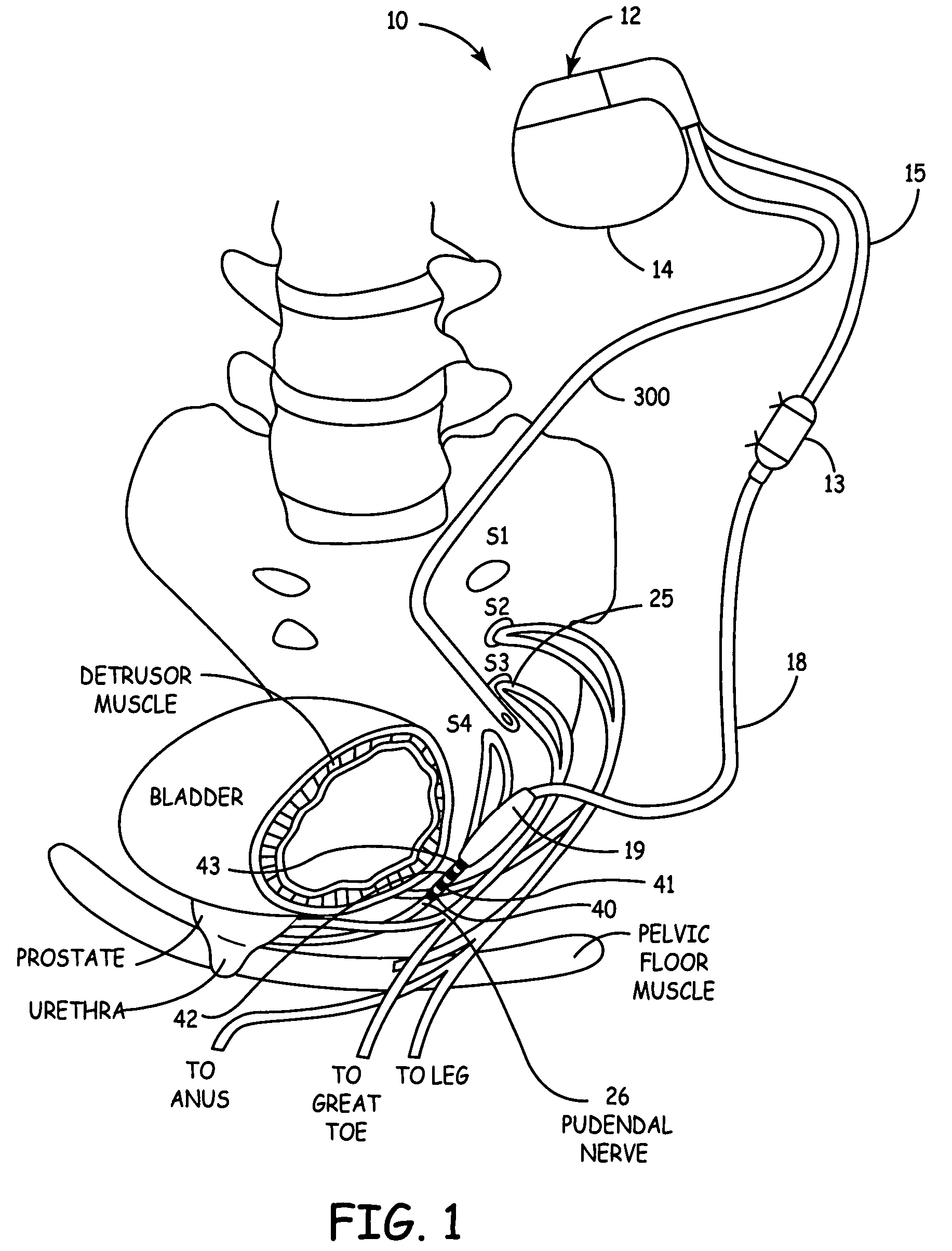
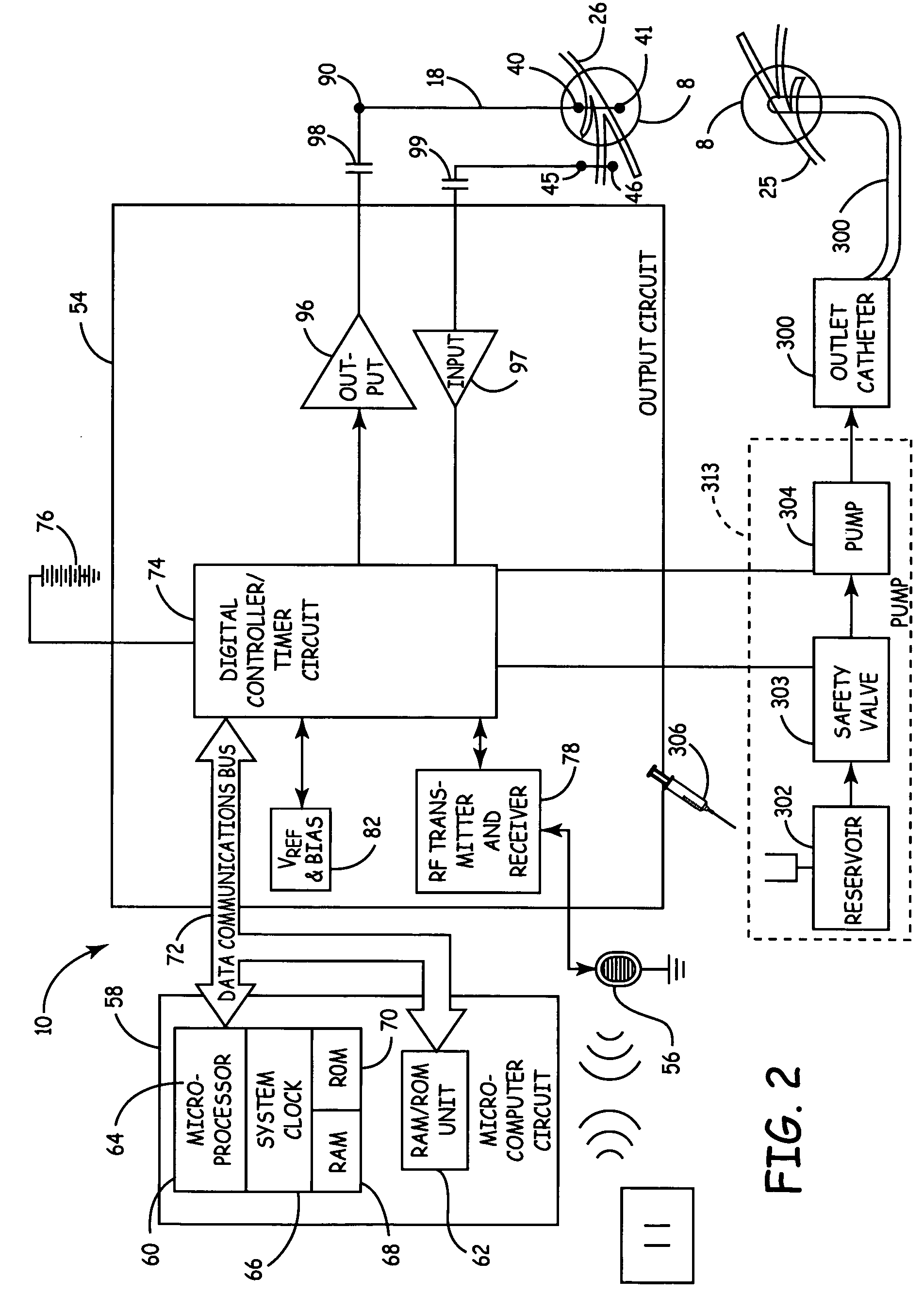
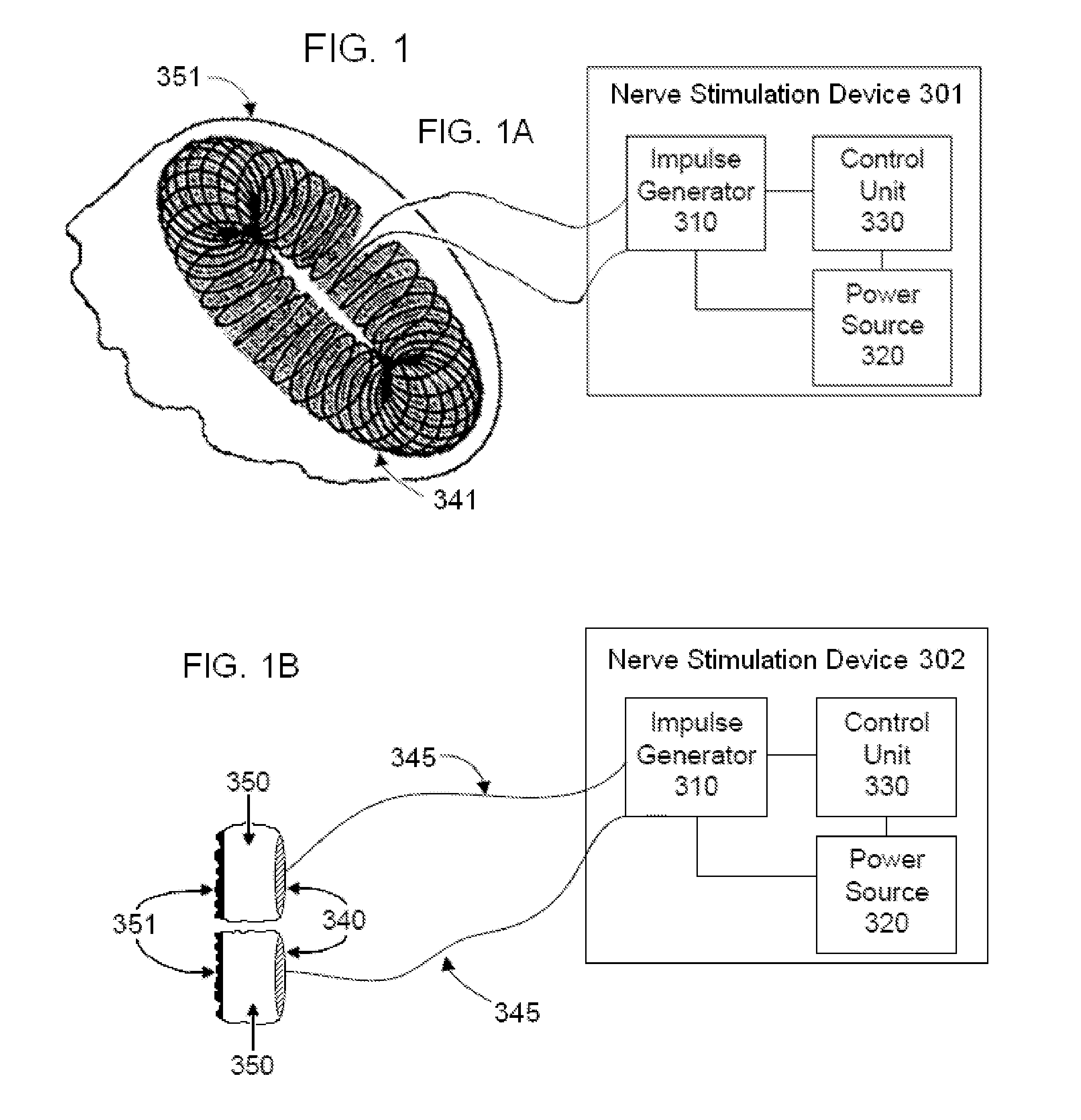
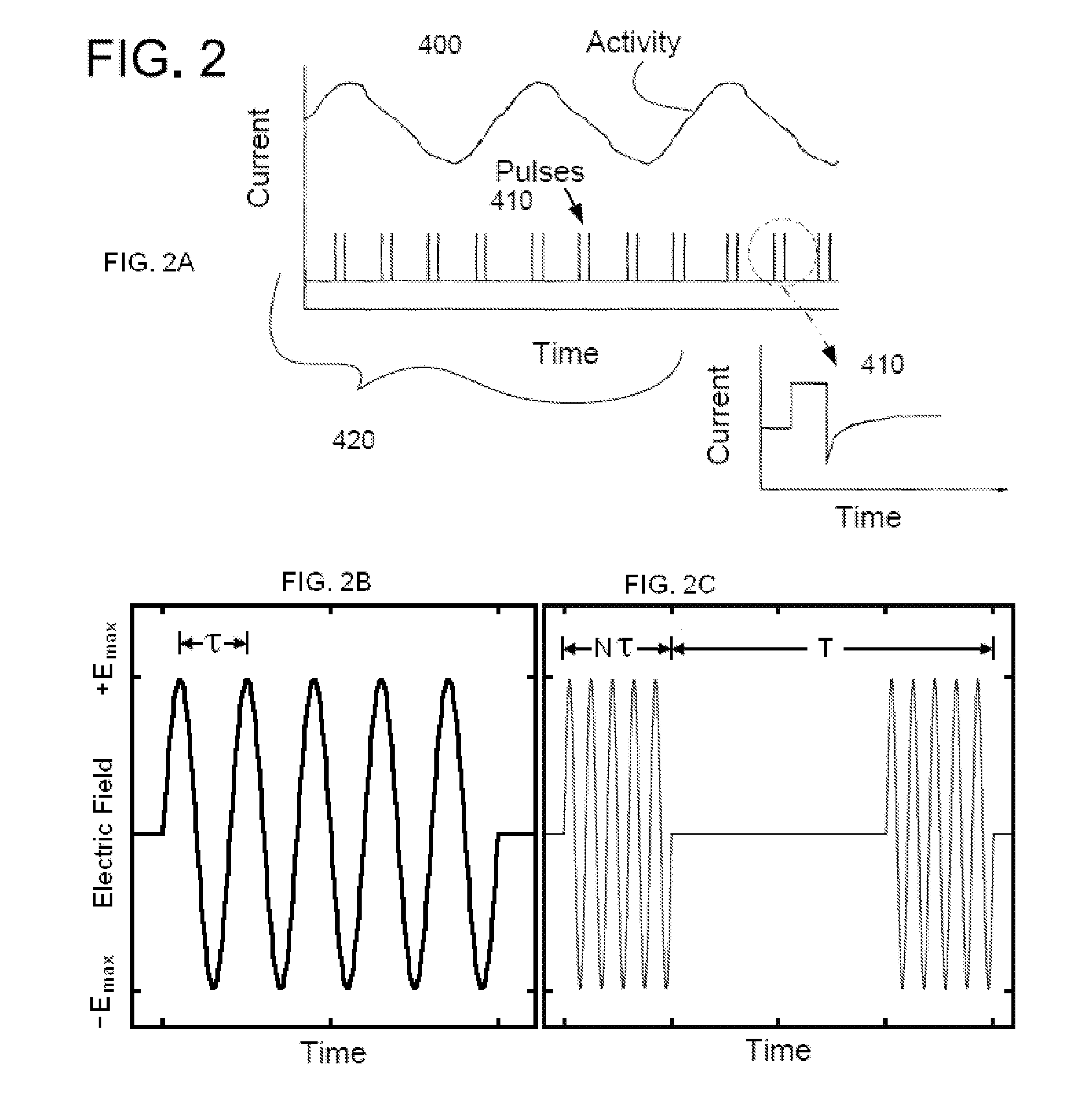
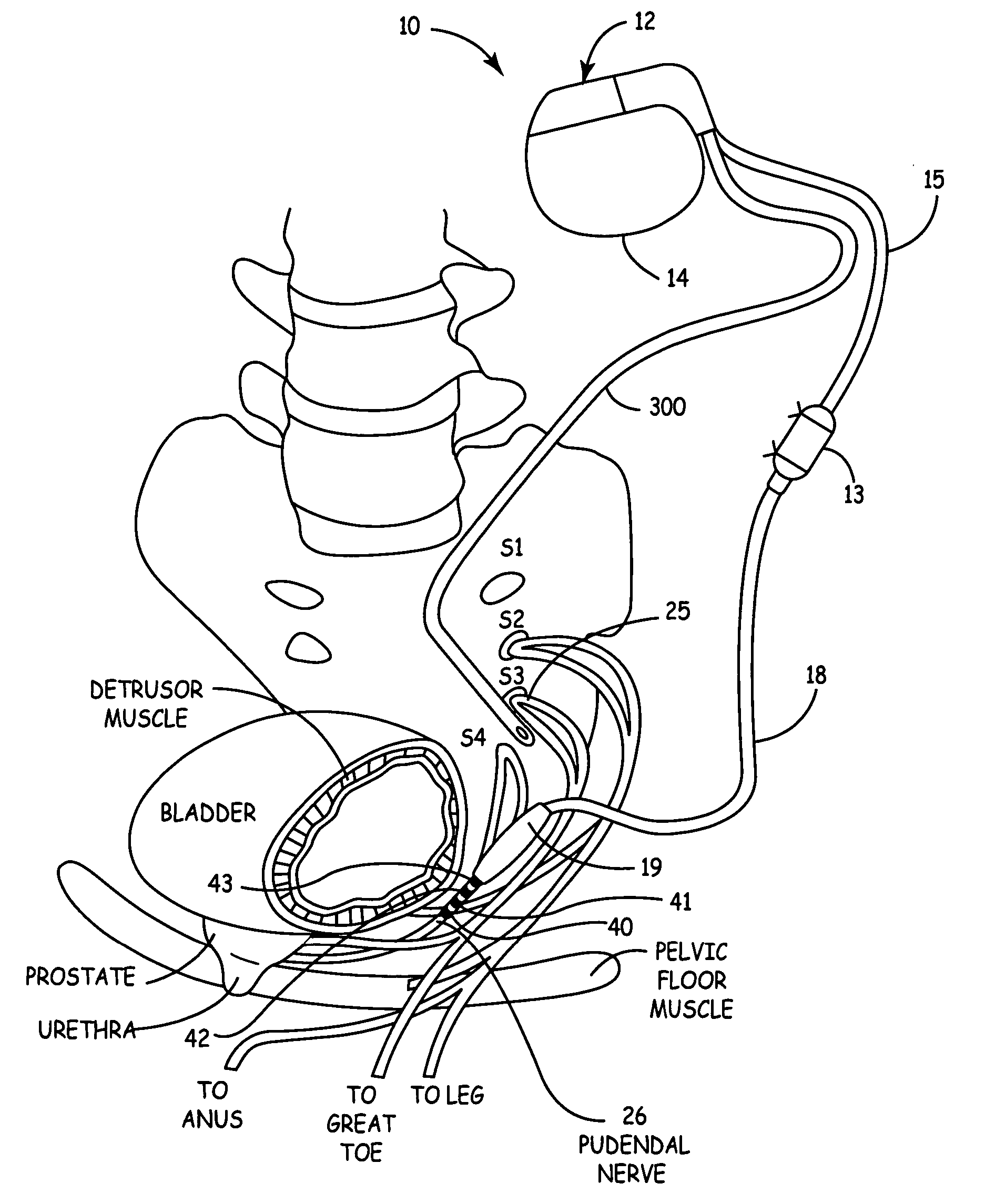
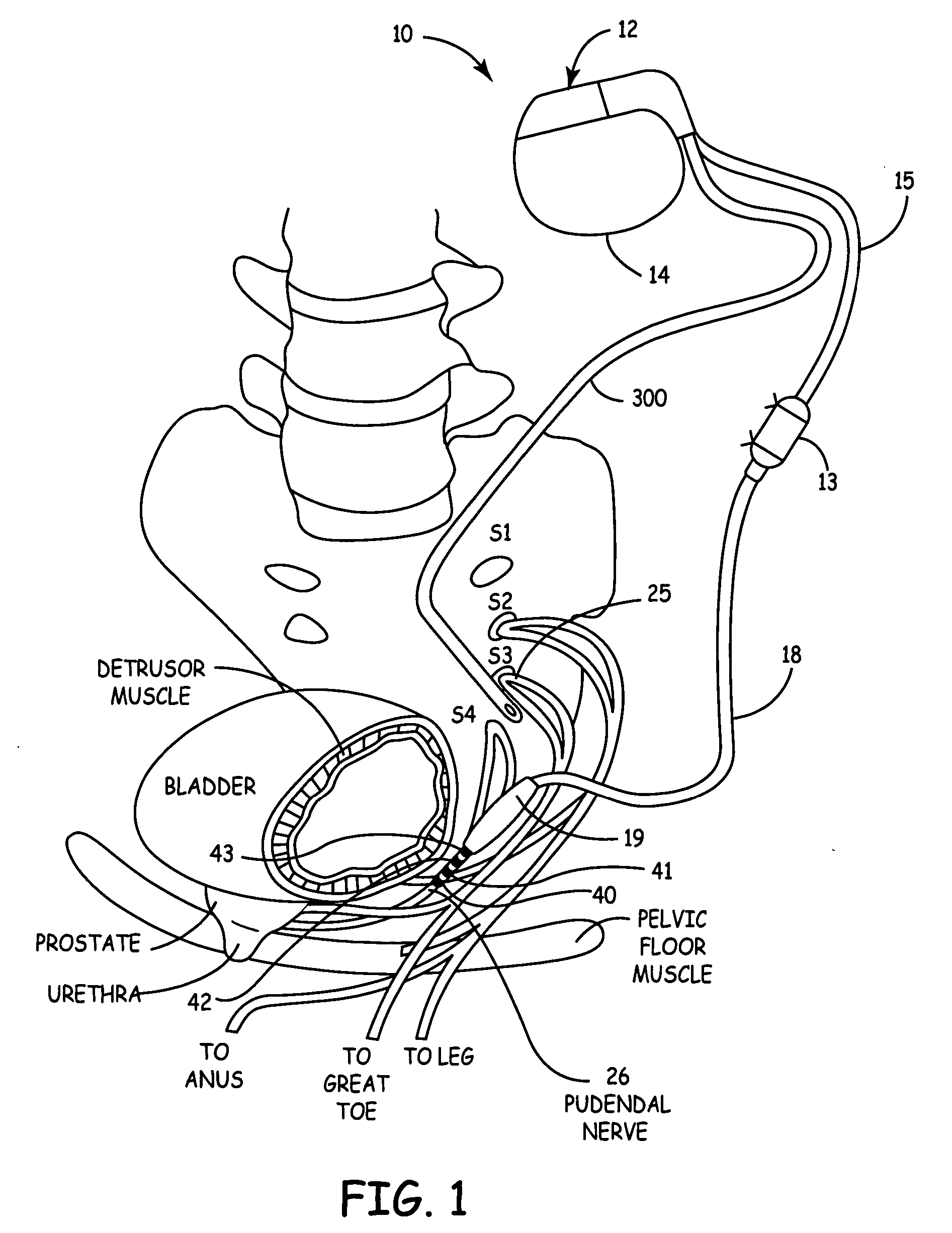
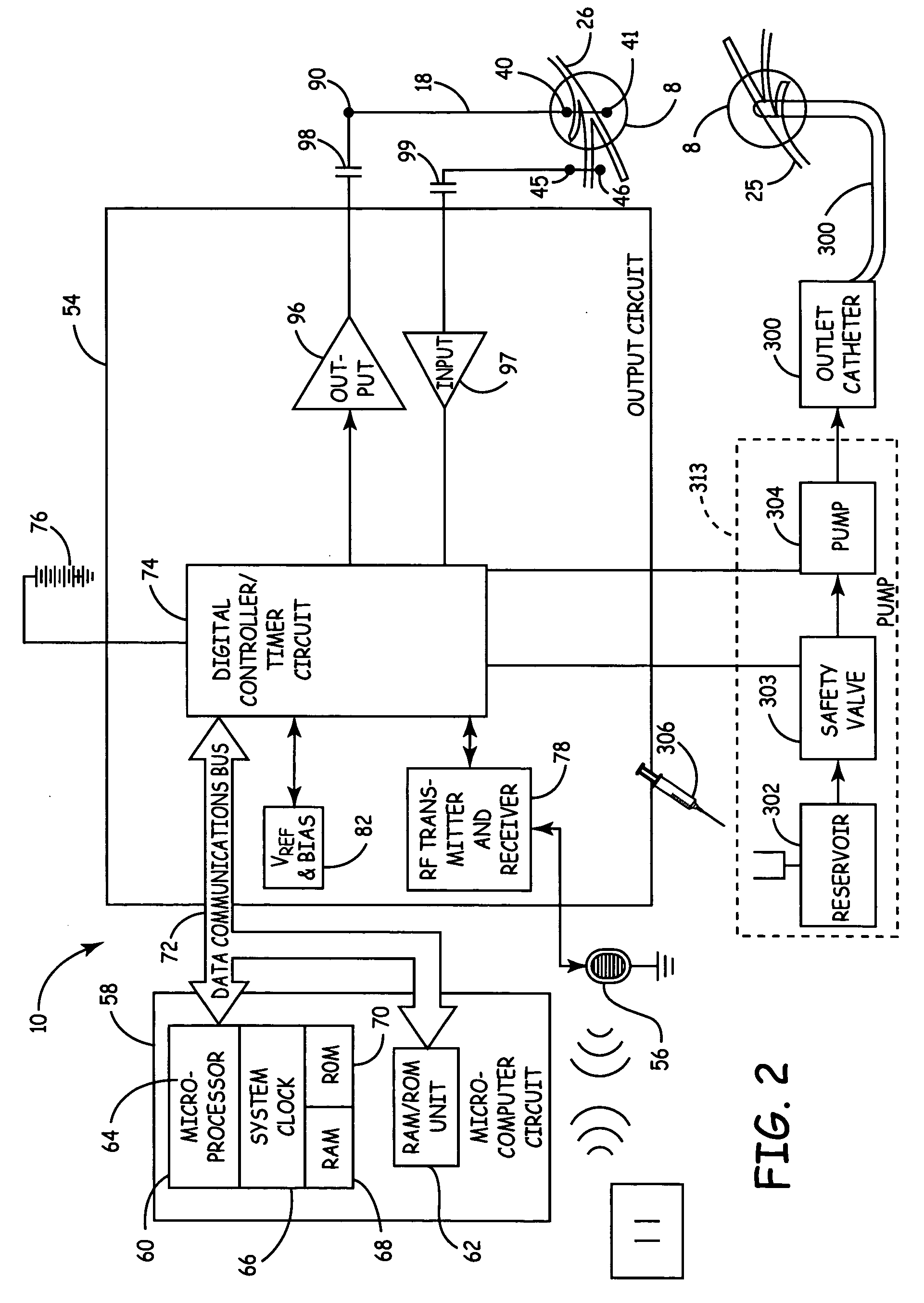
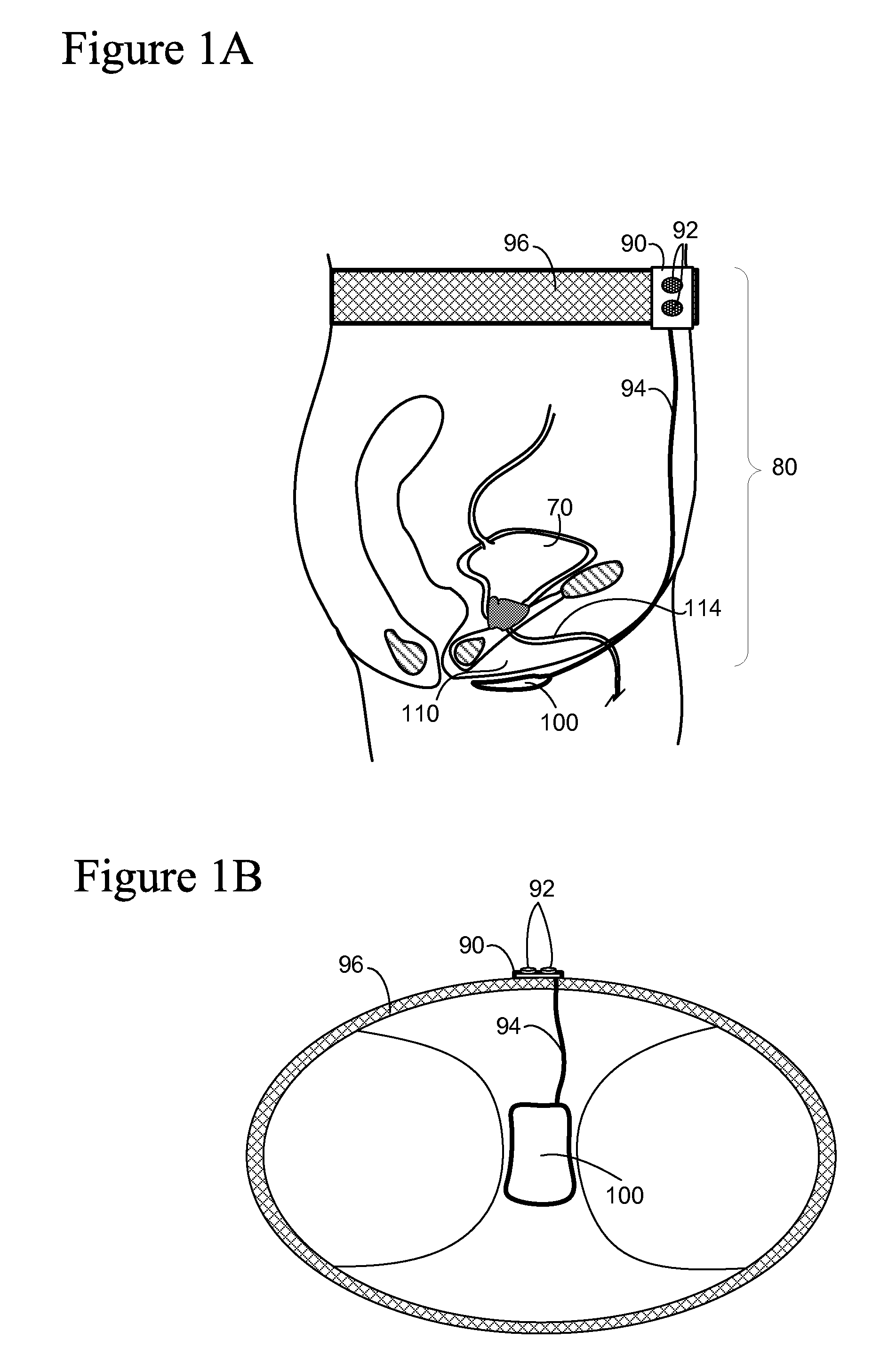
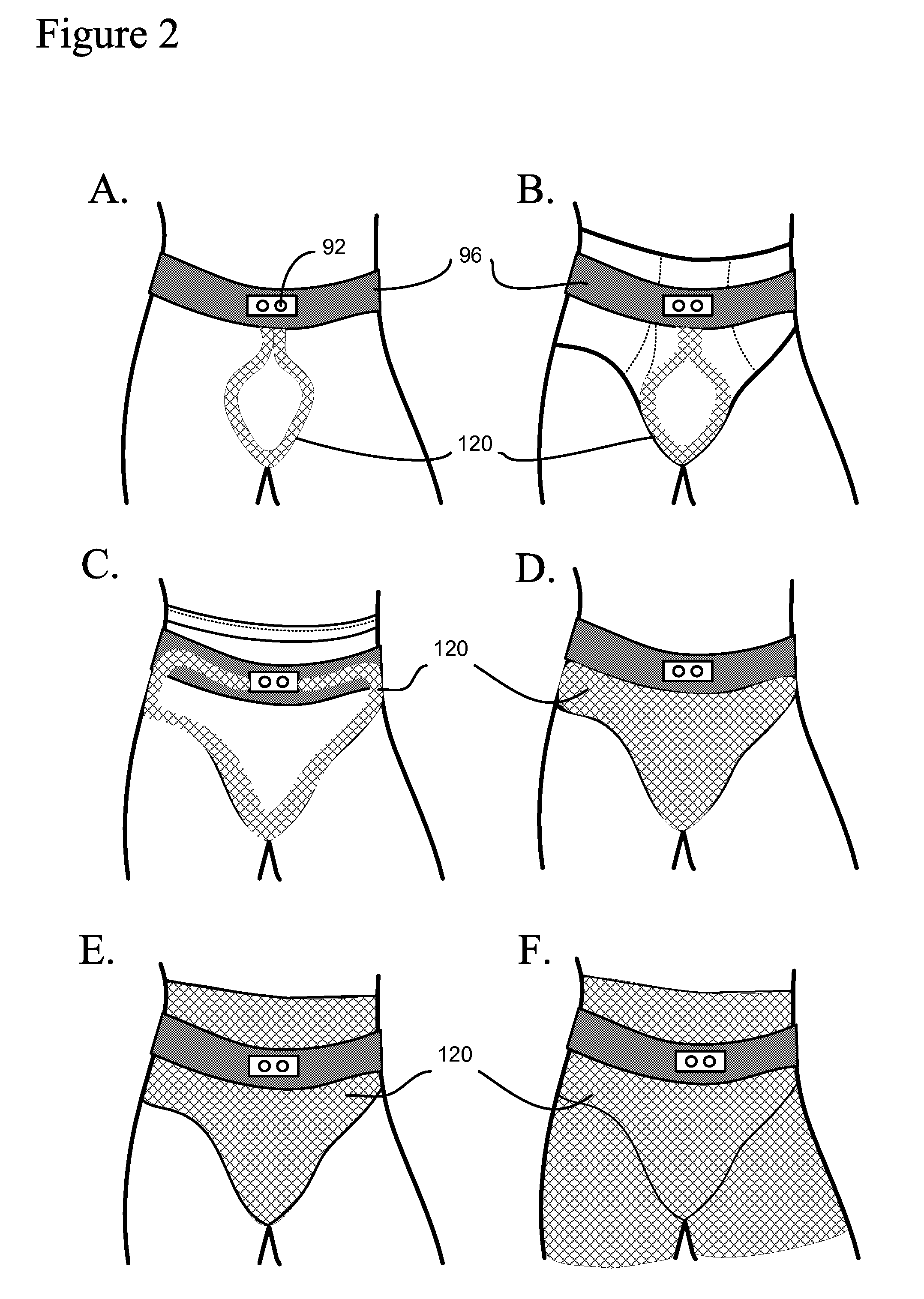
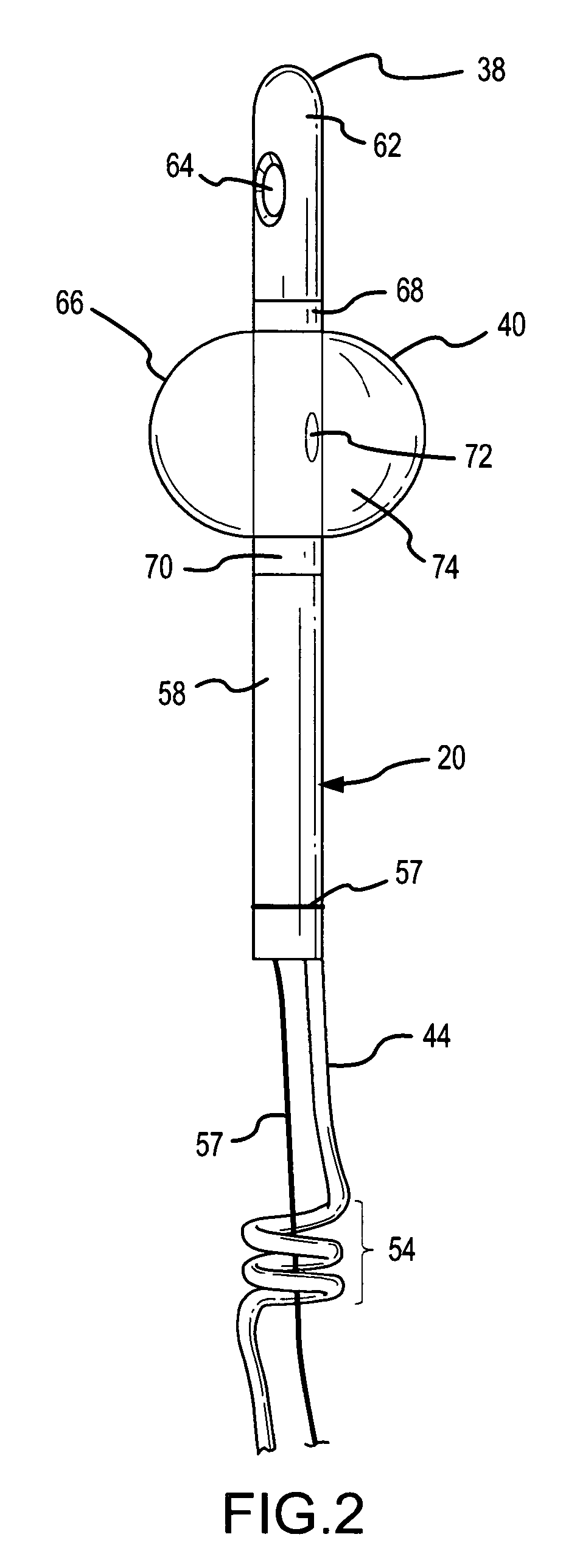
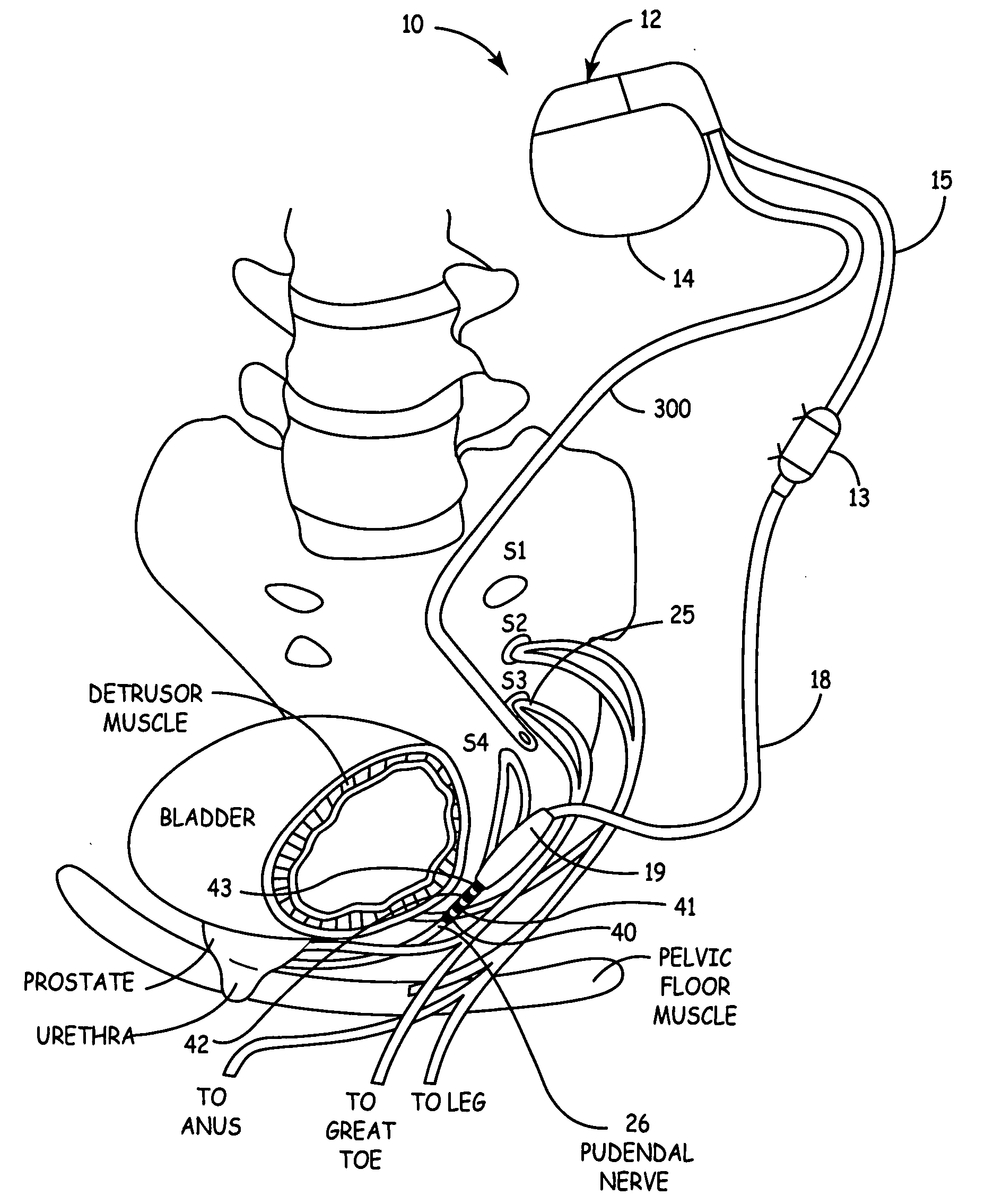
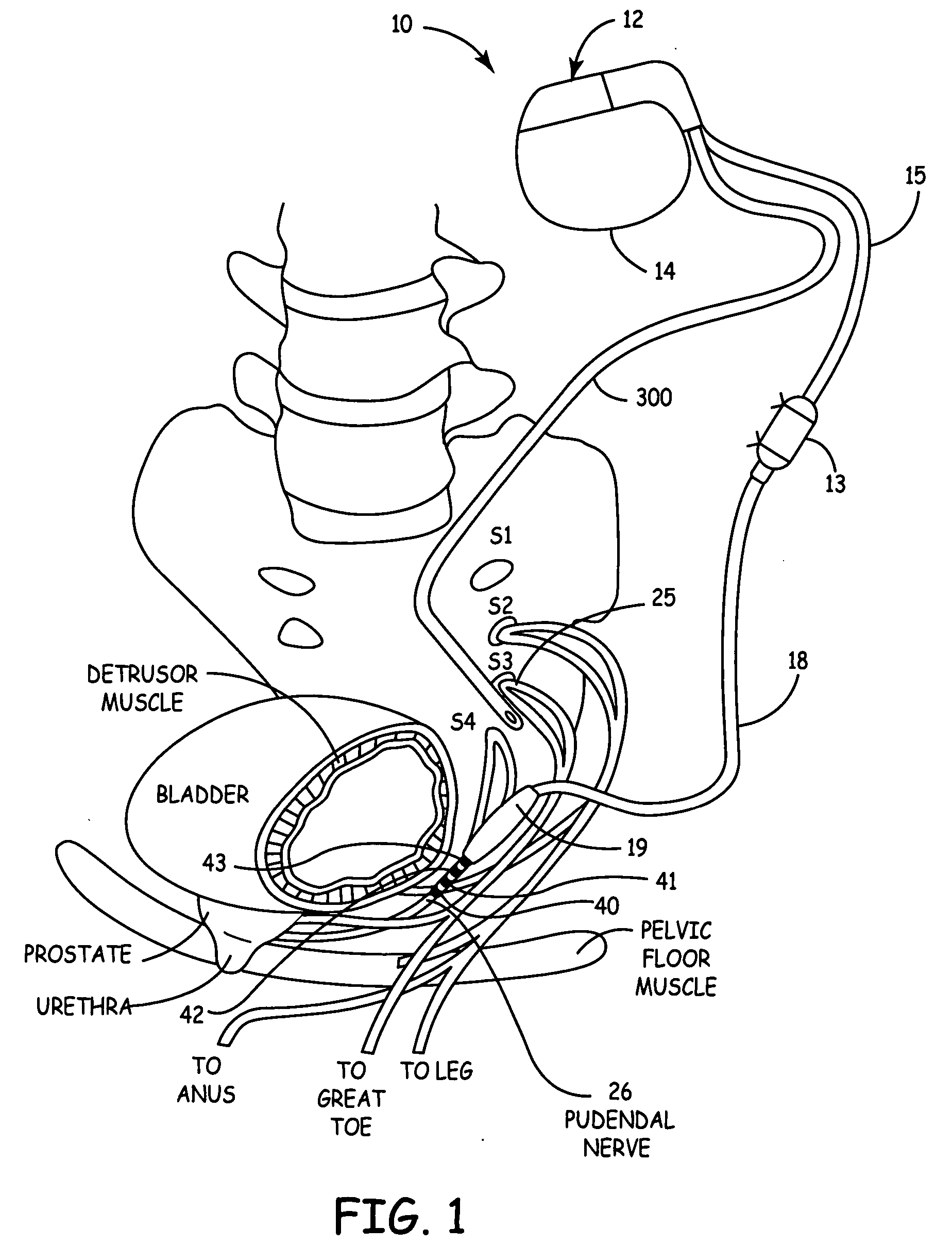
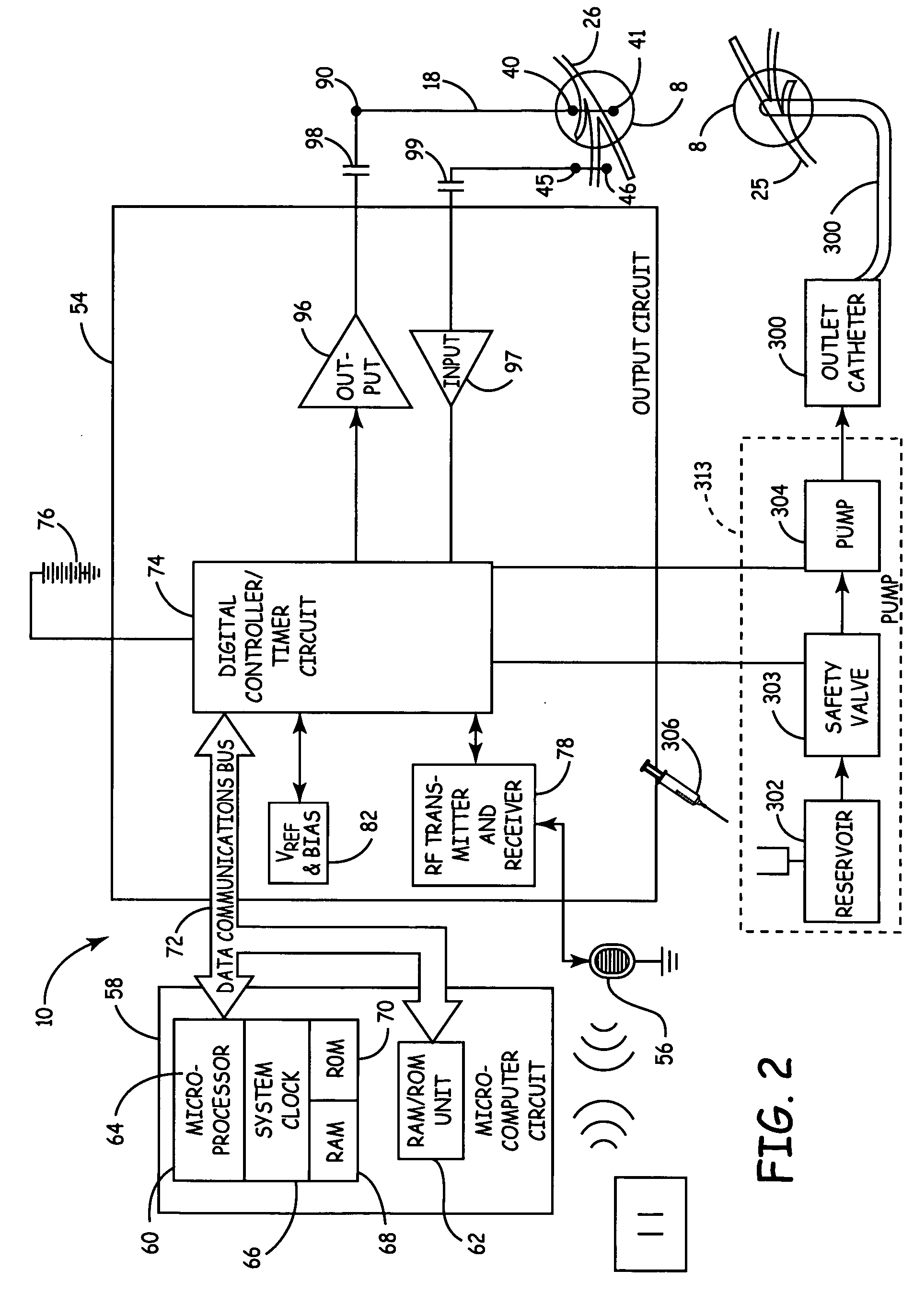
Method, system and device for treating disorders of the pelvic floor by means of electrical stimulation of the pudendal and associated nerves, and the optional delivery of drugs in association therewith
ActiveUS7328068B2Undesirable side effects of sacral nerve stimulation may be avoided or minimizedUndesirable side-effectDigestive electrodesGenital electrodesDiseaseProstatalgia
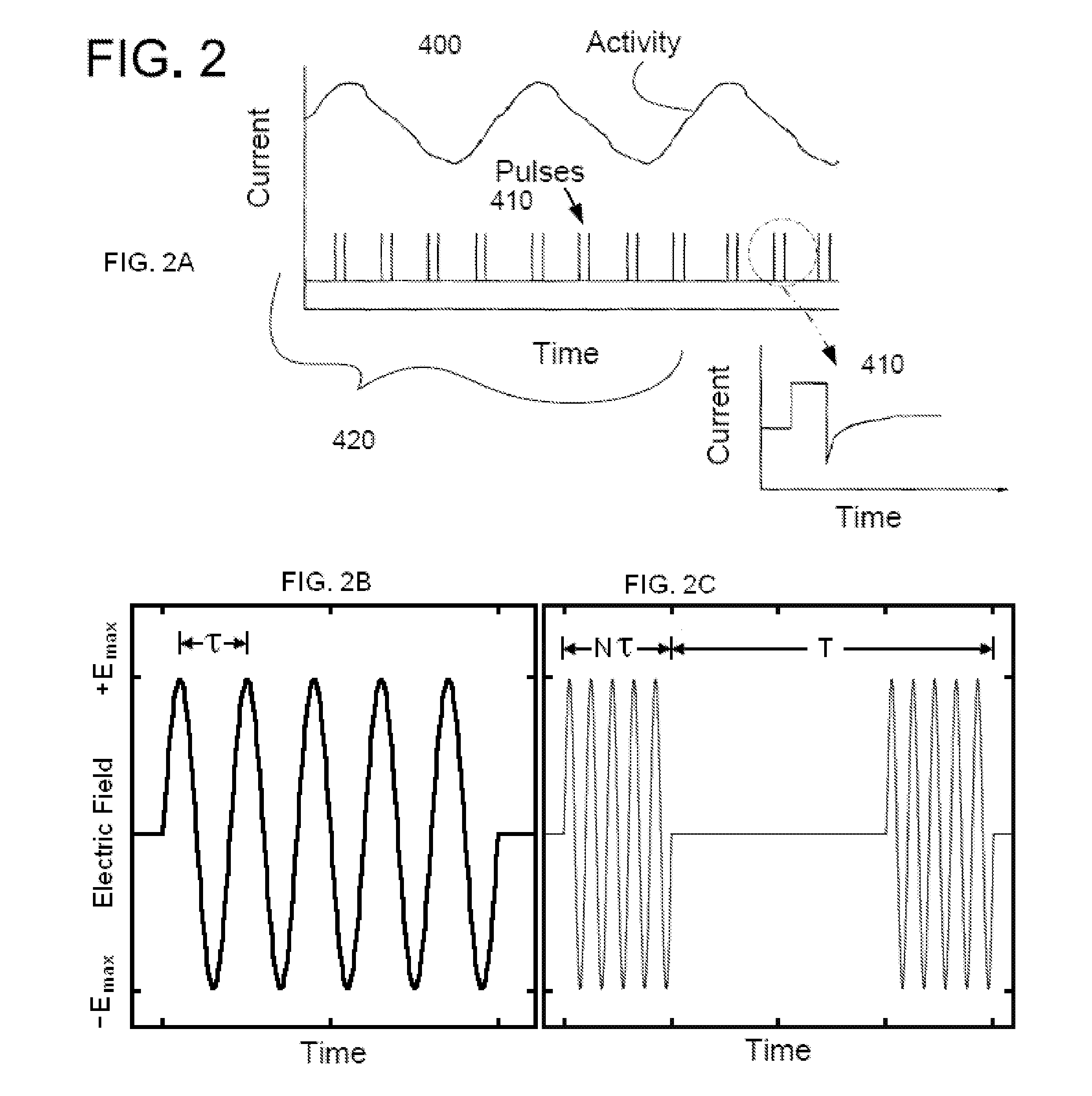
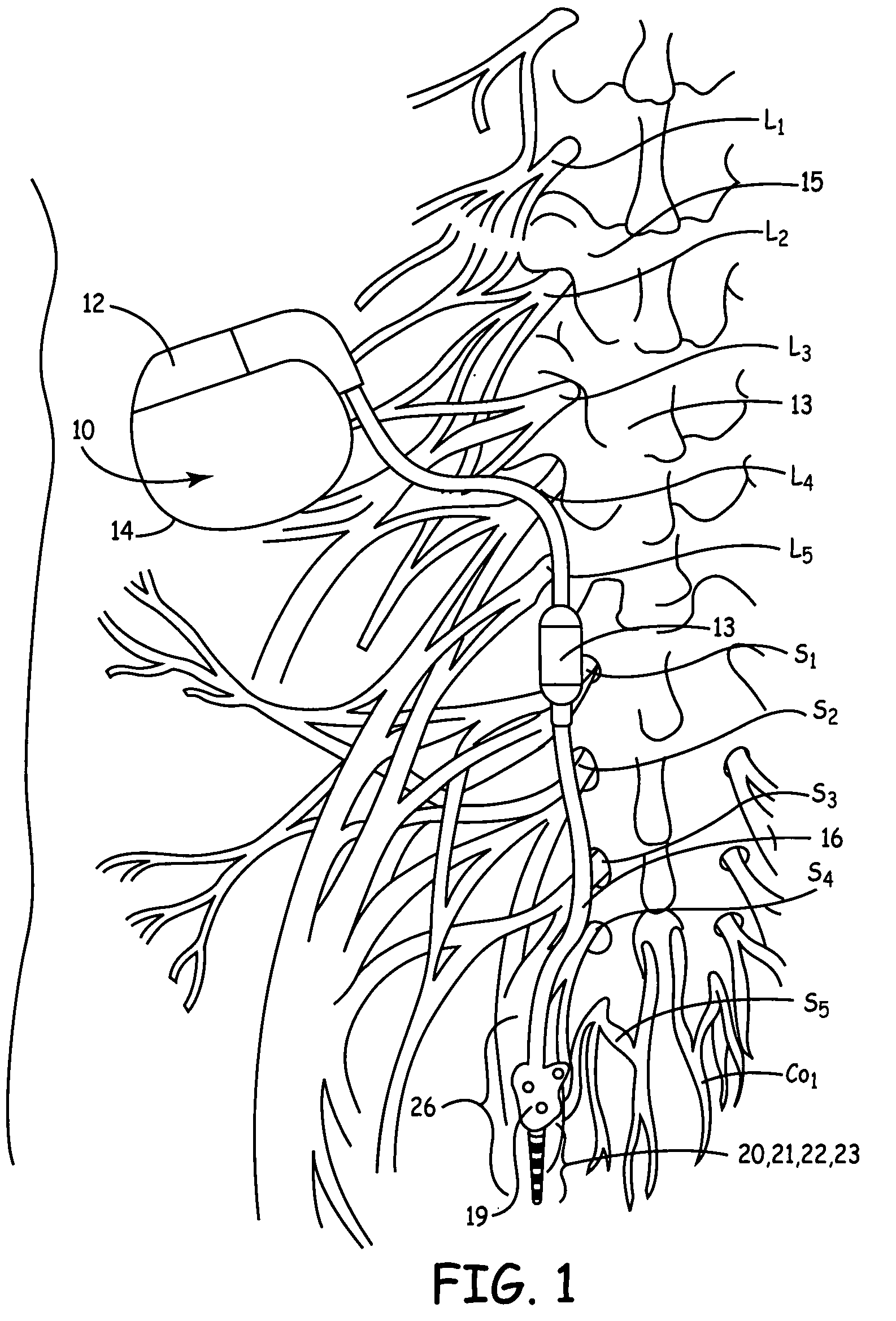
Described are implantable devices and methods for treating various disorders of the pelvic floor by means of electrical stimulation of the pudendal or other nerves, and optional means for delivering drugs in association therewith. A method of precisely positioning and implanting a medical electrical lead so as to provide optimal stimulation of the pudendal nerve or a portion thereof is also described. Placement of a stimulation lead next to or on the pudendal nerve may be performed using conventional prior art techniques through gross anatomical positioning, but usually does not result in truly optimal lead placement. One method of the present invention utilizes neurophysiological monitoring to assess the evoked responses of the pudendal nerve, and thereby provide a method for determining the optimal stimulation site. Additionally, one or more electrical stimulation signals are applied, and optionally one or more drugs are infused, injected or otherwise administered, to appropriate portions of a patient's pelvic floor and pudendal nerve or portions thereof in an amount and manner effective to treat a number of disorders, including, but not limited to, urinary and / or fecal voiding dysfunctions such as constipation, incontinence disorders such as urge frequency and urinary retention disorders, sexual dysfunctions such as orgasmic and erectile dysfunction, pelvic pain, prostatitis, prostatalgia and prostatodynia.
Owner:MEDTRONIC INC
Method, system and device for treating various disorders of the pelvic floor by electrical stimulation of the left and right pudendal nerves
InactiveUS20040193228A1Directly and effectively stimulatingUndesirable side effects of sacral nerve stimulation may be avoided or minimizedElectrotherapyDiseaseProstatalgia
Described are implantable devices and methods for treating various disorders of the pelvic floor by means of electrical stimulation of the pudendal and sacral nerves, or portions thereof, and optional means for delivering drugs in association therewith. Two or more electrical stimulation regimes are applied on a continuous, alternating, intermittent or other basis to the sacral and pudendal nerves, and optionally one or more drugs are infused, injected or otherwise administered, to appropriate portions of a patient's pelvic floor and pudendal nerve and / or sacral nerve, or portions thereof, in an amount and manner effective to treat a number of disorders, including, but not limited to, urinary and / or fecal voiding dysfunctions such as constipation, incontinence disorders such as urge frequency and urinary retention disorders, sexual dysfunctions such as orgasmic and erectile dysfunction, pelvic pain, prostatitis, prostatalgia and prostatodynia.
Owner:GERBER MARTIN T
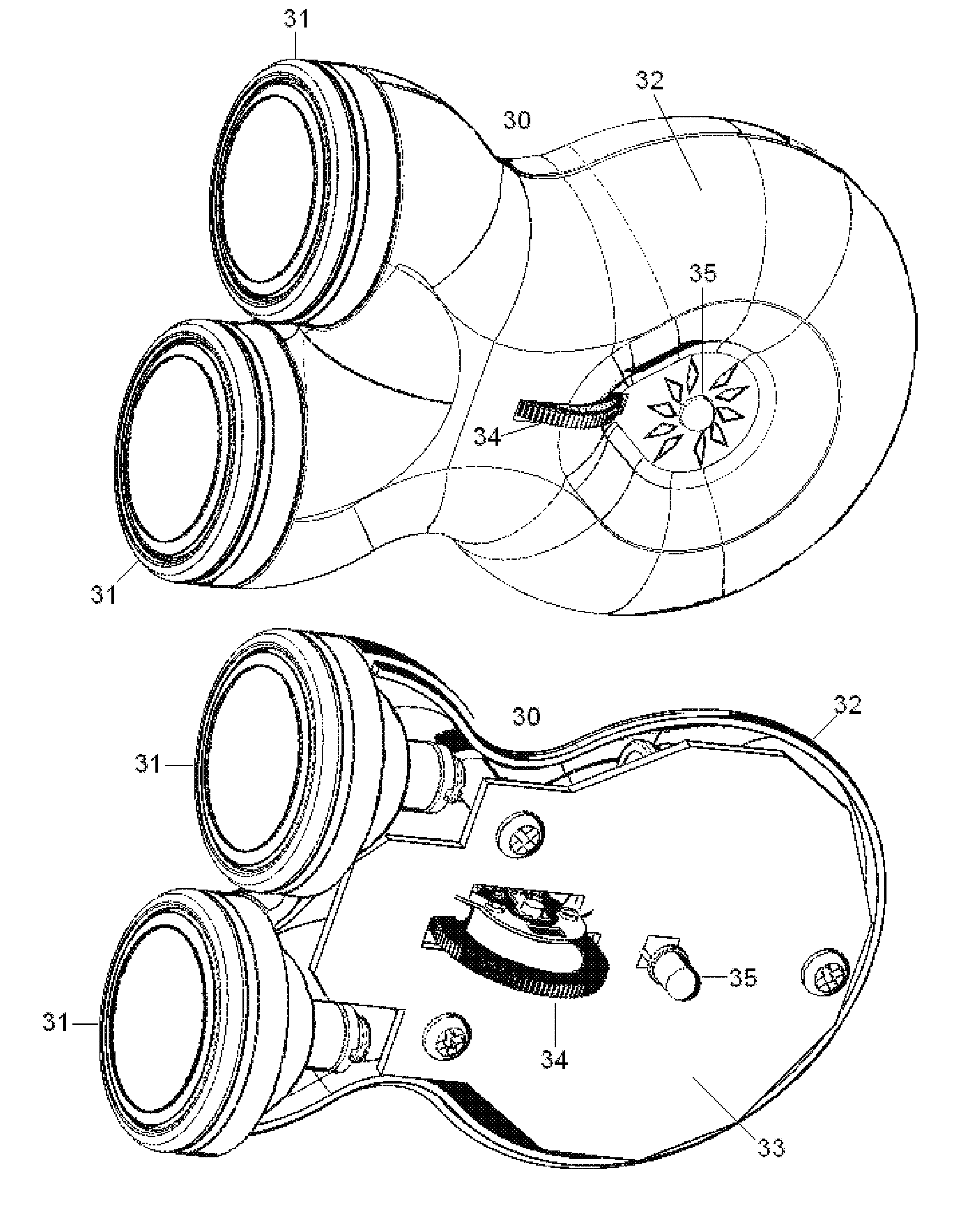
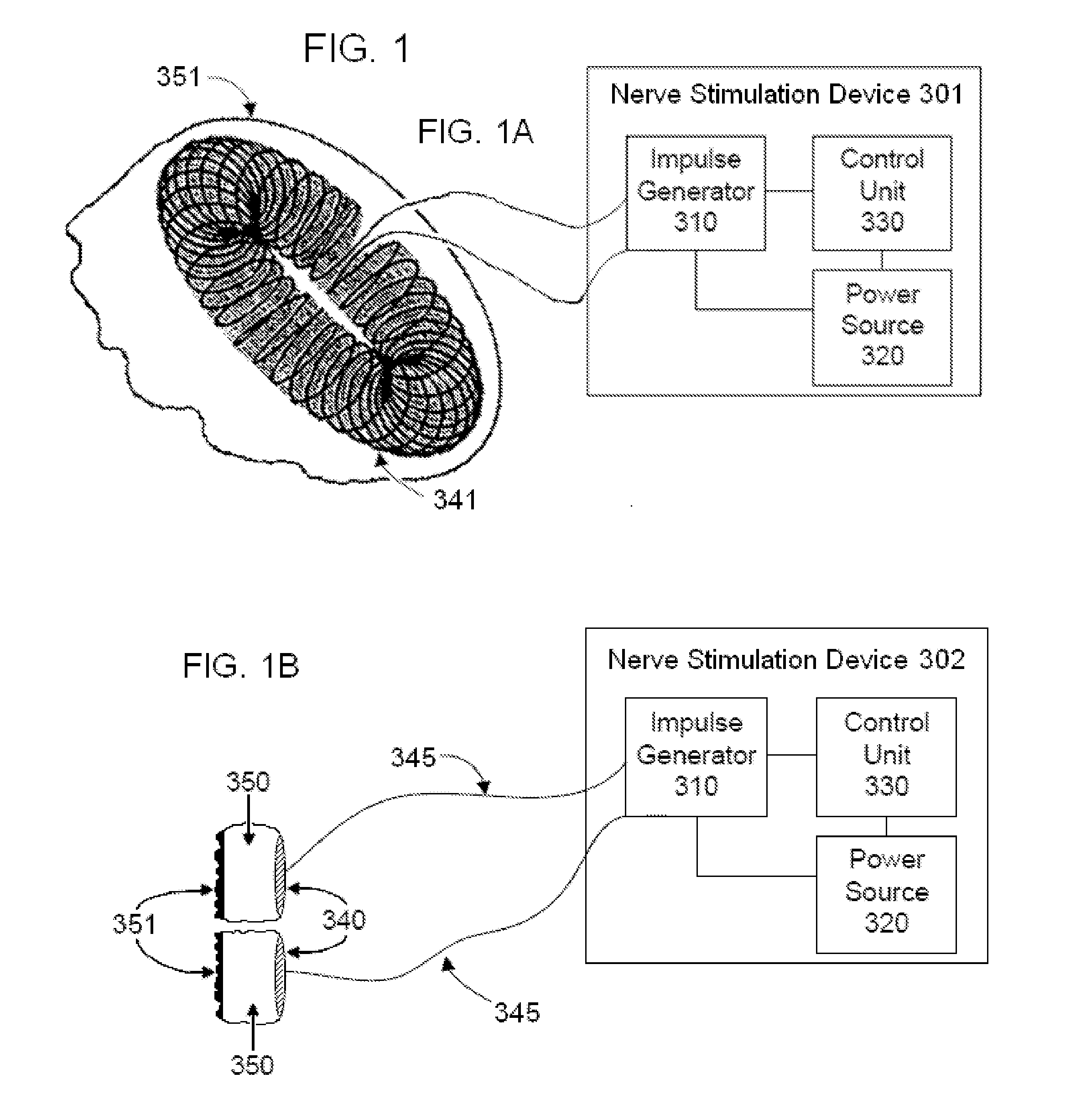
Non-invasive electrical and magnetic nerve stimulators used to treat overactive bladder and urinary incontinence
Transcutaneous electrical and magnetic nerve stimulation devices are disclosed, along with methods of treating lower urinary tract disorders using energy that is delivered noninvasively by the devices. The disorders comprise overactive bladder, urge incontinence, stress, incontinence, urge frequency, non-obstructive urinary retention and interstitial cystitis / painful bladder syndrome. In preferred embodiments of the disclosed methods, a posterior tibial nerve of a patient is stimulated non-invasively. Methods are disclosed for selecting protocol parameters for a nerve stimulation session for treating each individual patient, wherein modules of the bladder of the patient are represented as coupled non-linear oscillators.
Owner:ELECTROCORE
Method, system and device for treating disorders of the pelvic floor by means of electrical stimulation of the pudenal and associated nerves, and the optional delivery of drugs in association therewith
ActiveUS20050113877A1Reduce traumaAvoid damageDigestive electrodesArtificial respirationDiseaseProstatalgia
Described are implantable devices and methods for treating various disorders of the pelvic floor by means of electrical stimulation of the pudendal or other nerves, and optional means for delivering drugs in association therewith. A method of precisely positioning and implanting a medical electrical lead so as to provide optimal stimulation of the pudendal nerve or a portion thereof is also described. Placement of a stimulation lead next to or on the pudendal nerve may be performed using conventional prior art techniques through gross anatomical positioning, but usually does not result in truly optimal lead placement. One method of the present invention utilizes neurophysiological monitoring to assess the evoked responses of the pudendal nerve, and thereby provide a method for determining the optimal stimulation site. Additionally, one or more electrical stimulation signals are applied, and optionally one or more drugs are infused, injected or otherwise administered, to appropriate portions of a patient's pelvic floor and pudendal nerve or portions thereof in an amount and manner effective to treat a number of disorders, including, but not limited to, urinary and / or fecal voiding dysfunctions such as constipation, incontinence disorders such as urge frequency and urinary retention disorders, sexual dysfunctions such as orgasmic and erectile dysfunction, pelvic pain, prostatitis, prostatalgia and prostatodynia.
Owner:MEDTRONIC INC
Method, system and device for treating various disorders of the pelvic floor by electrical stimulation of the pudendal nerves and the sacral nerves at different sites
Described are implantable devices and methods for treating various disorders of the pelvic floor by means of electrical stimulation of the pudendal and sacral nerves, or portions thereof, and optional means for delivering drugs in association therewith. Two or more electrical stimulation regimes are applied on a continuous, alternating, intermittent or other basis to the sacral and pudendal nerves, and optionally one or more drugs are infused, injected or otherwise administered, to appropriate portions of a patient's pelvic floor and pudendal nerve and / or sacral nerve, or portions thereof, in an amount and manner effective to treat a number of disorders, including, but not limited to, urinary and / or fecal voiding dysfunctions such as constipation, incontinence disorders such as urge frequency and urinary retention disorders, sexual dysfunctions such as orgasmic and erectile dysfunction, pelvic pain, prostatitis, prostatalgia and prostatodynia.
Owner:MEDTRONIC INC
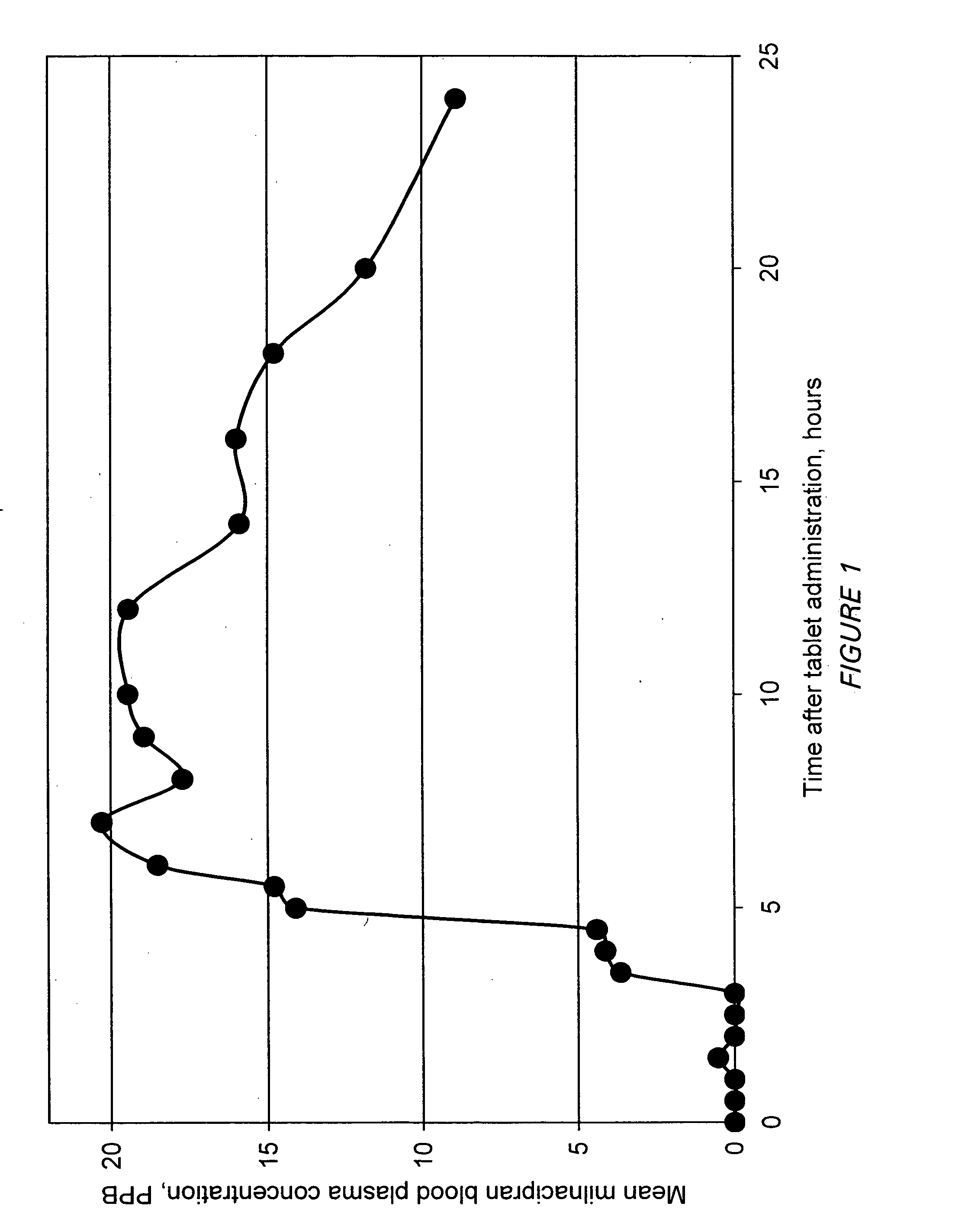
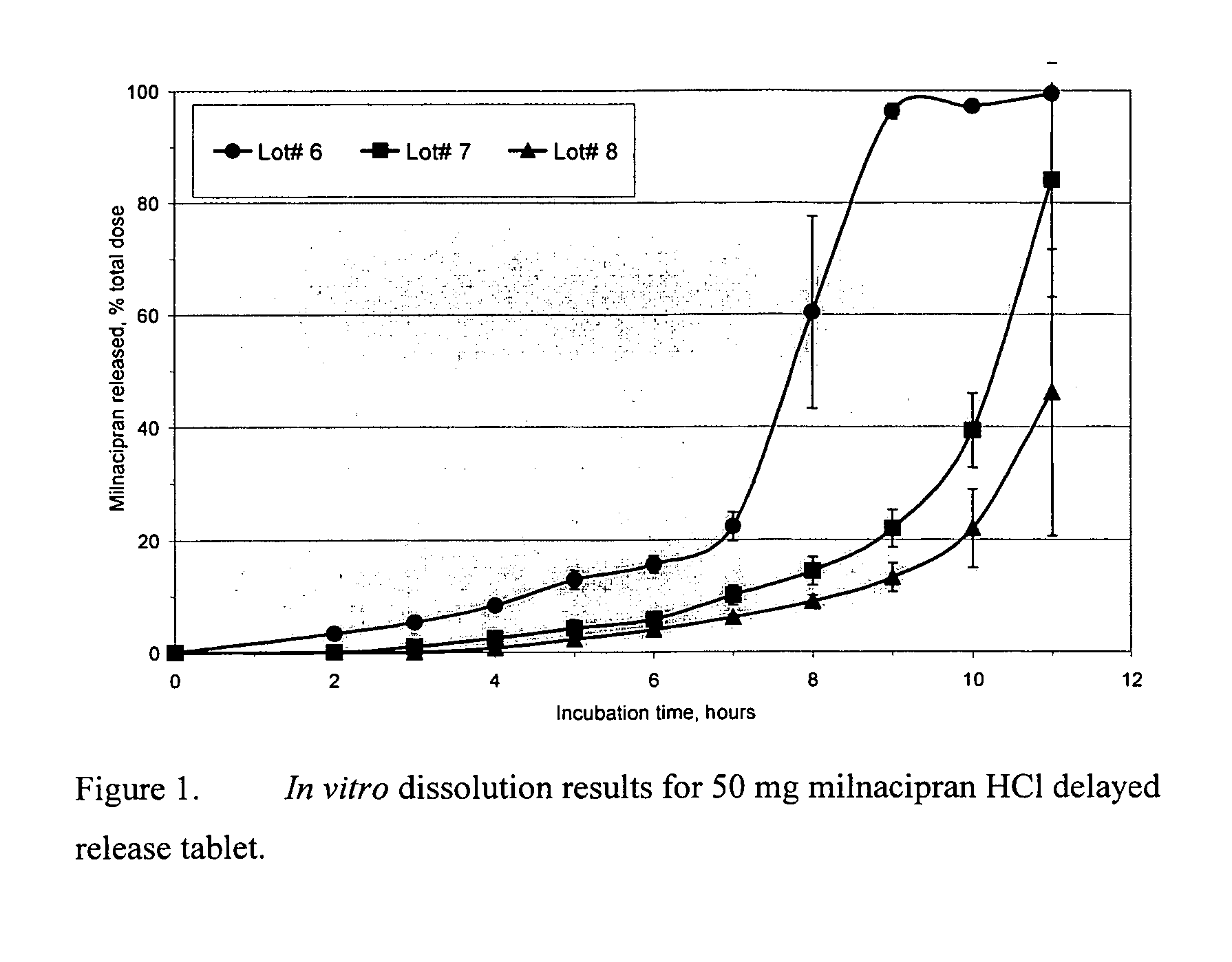
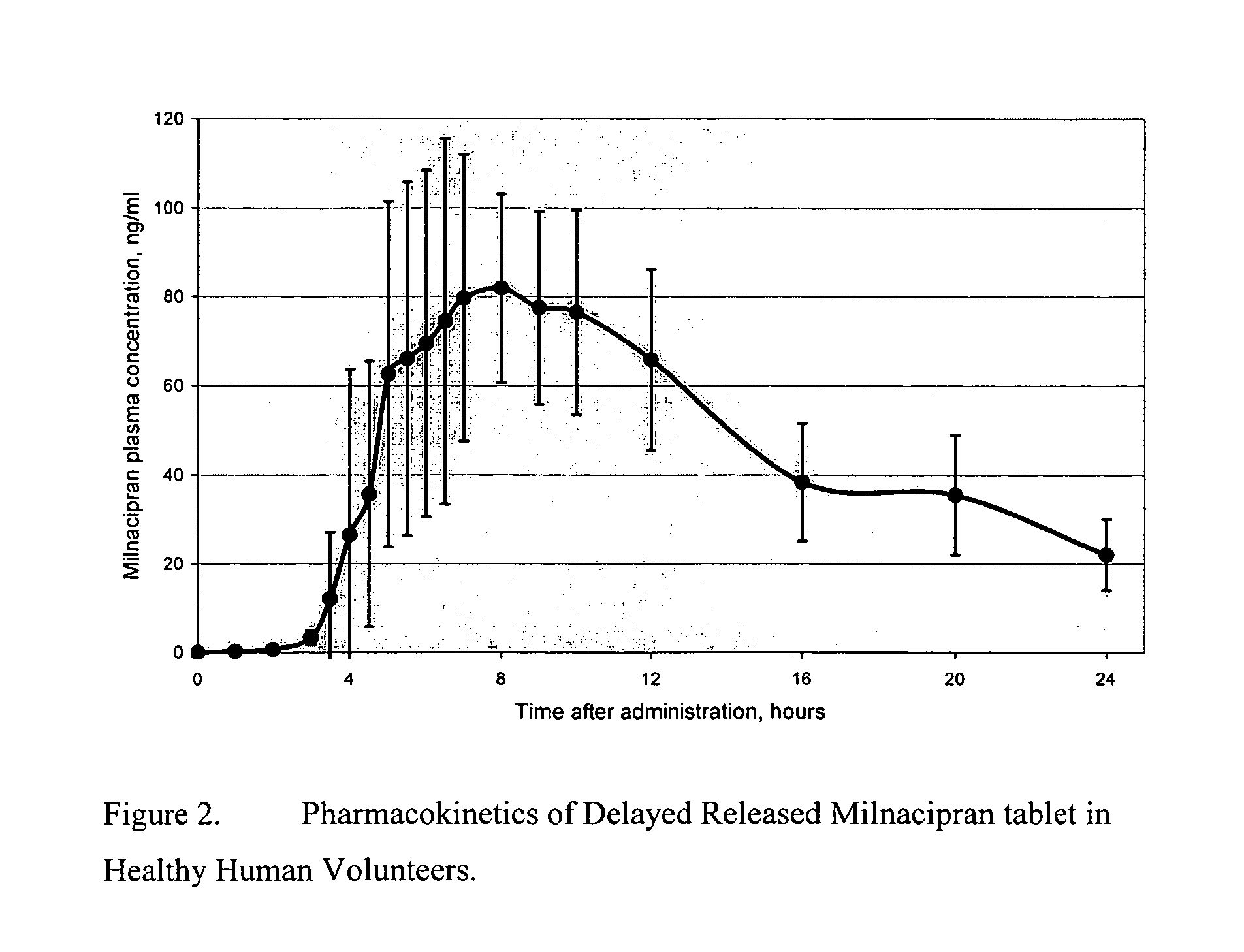
Modified release compositions of milnacipran
A once-a-day oral milnacipran modified release formulation has been developed. The formulation comprises an extended release dosage unit (optionally containing the immediate release portion) coated with delayed release coating. The milnacipran composition, when administered orally, first passes through the stomach releasing from zero to less than 10% of the total milnacipran dose and then enters the intestines where drug is released slowly over an extended period of time. The release profile is characterized by a 0.05-4 hours lag time period during which less than 10% of the total milnacipran dose is released followed by a slow or extended release of the remaining drug over a defined period of time. The composition provides in vivo drug plasma levels characterized by Tmax at 4-10 hours and an approximately linear drop-off thereafter and Cmax below 3000 ng / ml, preferably below 2000 ng / ml, and most preferably below 1000 ng / ml. The composition allows milnacipran to be delivered over approximately 24 hours, when administered to a patient in need, resulting in diminished incidence or decreased intensity of common milnacipran side effects such as sleep disturbance, nausea, vomiting, headache, tremulousness, anxiety, panic attacks, palpitations, urinary retention, orthostatic hypotension, diaphoresis, chest pain, rash, weight gain, back pain, constipation, vertigo, increased sweating, agitation, hot flushes, tremors, fatigue, somnolence, dyspepsia, dysoria, nervousness, dry mouth, abdominal pain, irritability, and insomnia.
Owner:COLLEGIUM PHARMA INC
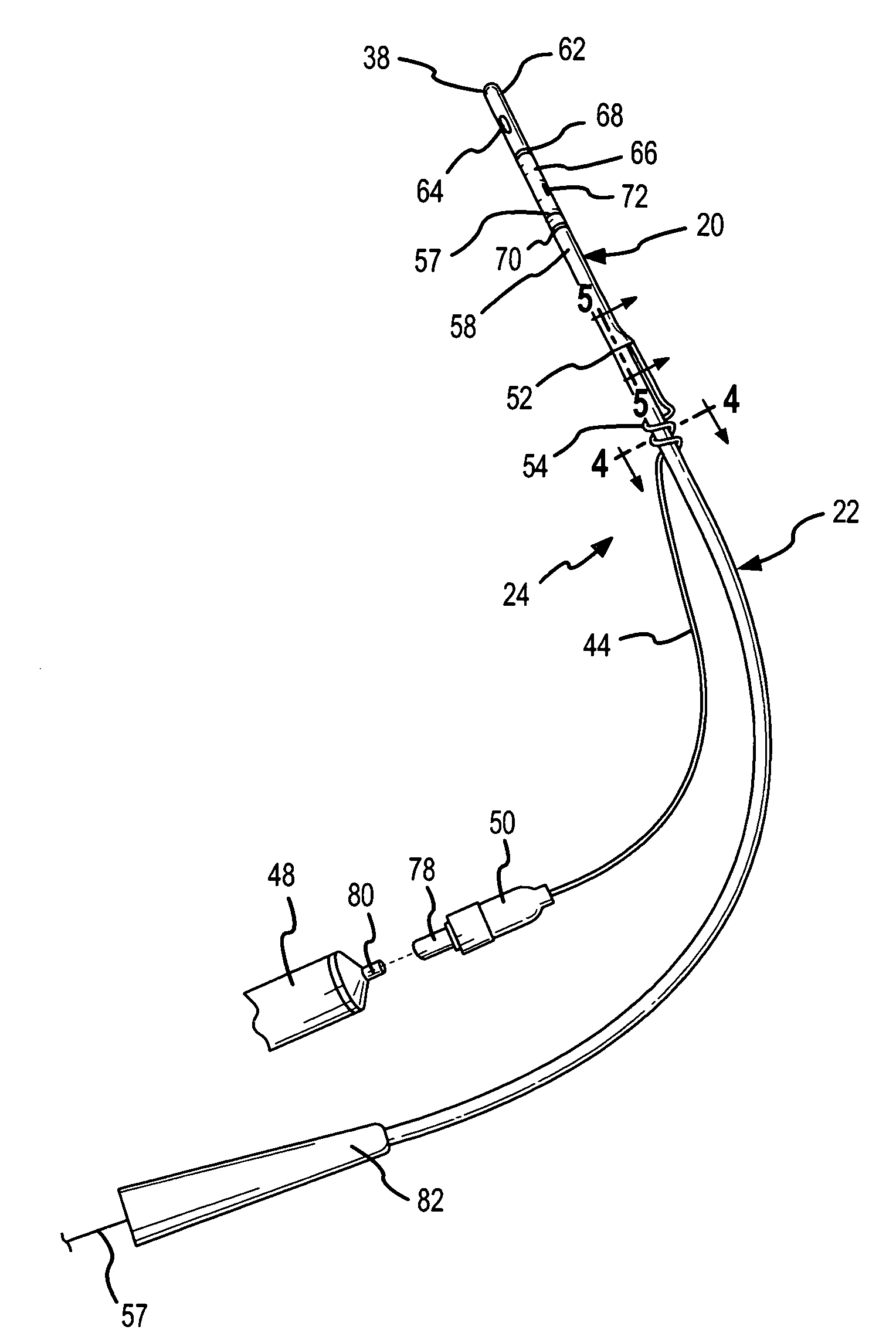
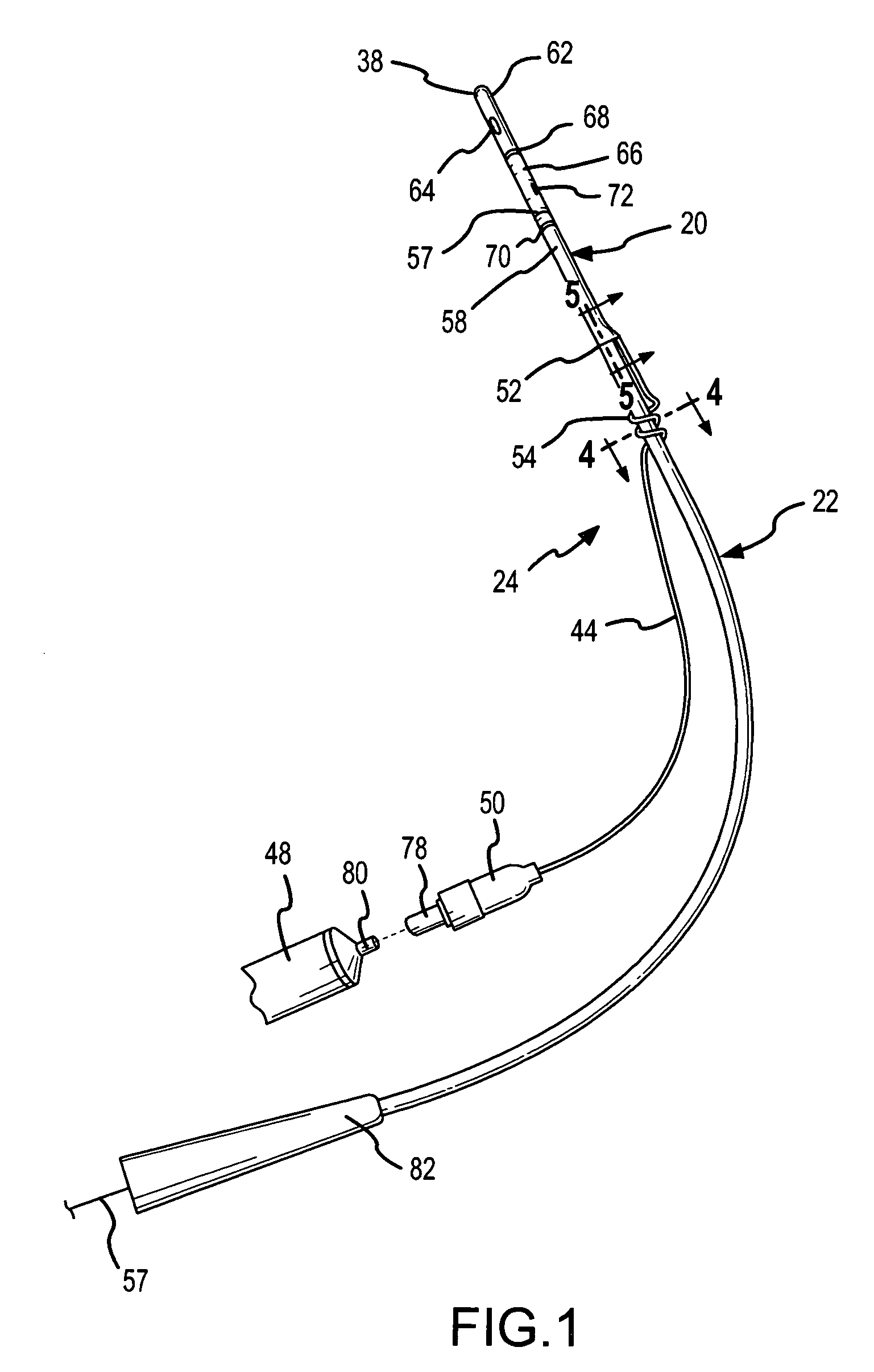
Method, system and device for treating disorders of the pelvic floor by delivering drugs to various nerves or tissues
InactiveUS20050010259A1Strong specificityLower medical costsElectrotherapyMedical devicesDiseaseLigament structure
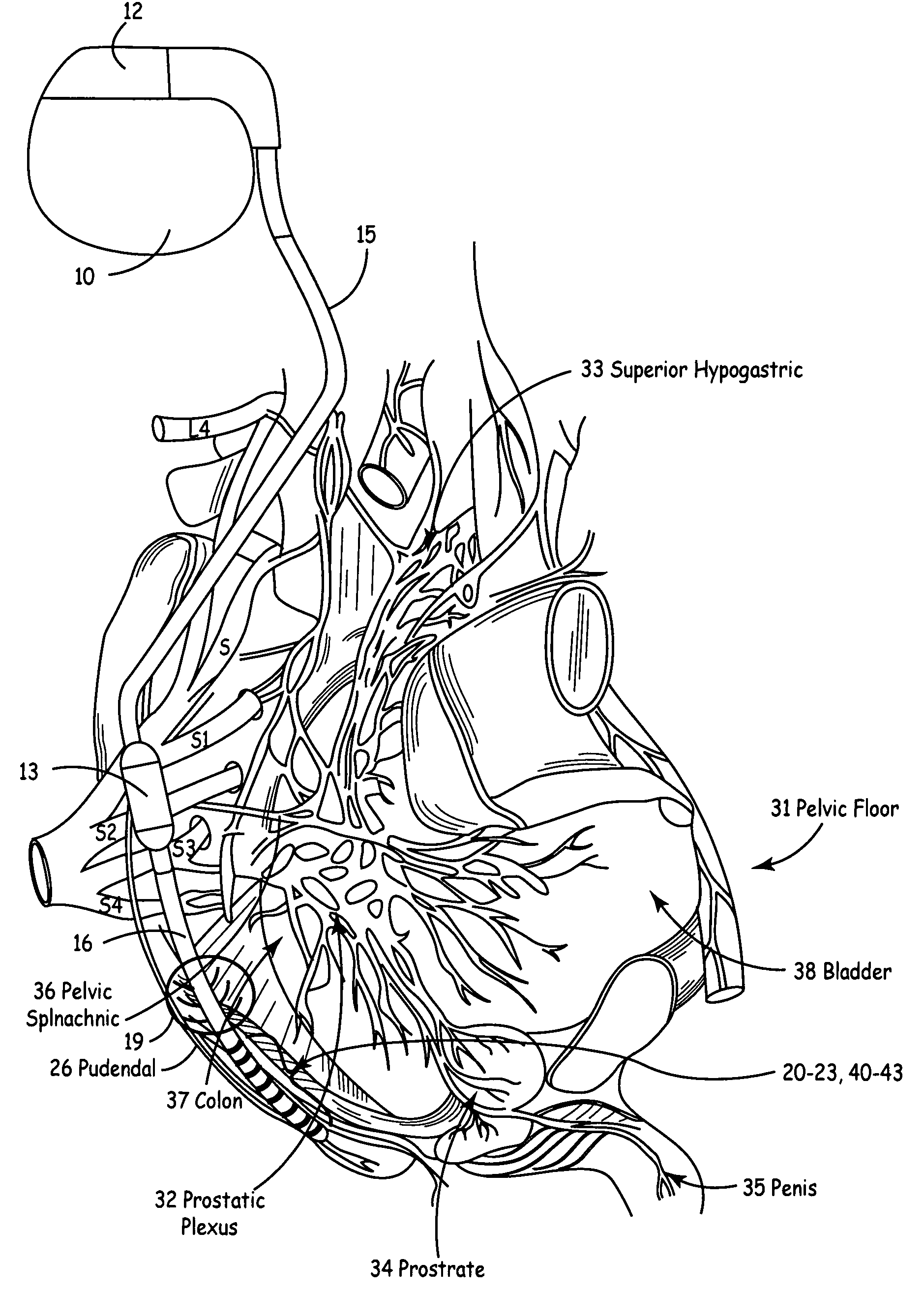
Described are implantable devices and methods for treating various disorders of the pelvic floor by delivering one or more drugs to a patient's hypogastric nerve or branches or portions thereof, prostatic plexus nerve or branches or portions thereof, sacral splanchnic nerve or branches or portions thereof, pelvic splanchnic nerve or branches or portions thereof, prostate or branches or portions thereof, pelvic floor, colon or branches or portions thereof, bladder or portions thereof, vagina or portions thereof, anus or portions thereof, external anal sphincter or portions thereof, urethra or portions thereof, penile dorsal nerve or portions thereof, inferior rectal nerves or branches or portions thereof, perineal nerves or branches or portions thereof, scrotal nerves or branches or portions thereof, scrotum or portions thereof, Alcock's Canal or branches or portions thereof, sacro-tuberous ligament or branches or portions thereof, ischial tuberosity or branches or portions thereof, greater sciatic foramen or branches or portions thereof, or lesser sciatic foramen or branches or portions thereof. One, two or more drug delivery regimes are provided on a continuous, alternating, intermittent or other basis to one or more selected nerves, nerve portions or tissues in an amount and manner effective to treat a number of disorders, including, but not limited to, urinary and / or fecal voiding dysfunctions such as constipation, incontinence disorders such as urge frequency and urinary retention disorders, sexual dysfunctions such as orgasmic and erectile dysfunction, pelvic pain, prostatitis, prostatalgia and prostatodynia.
Owner:MEDTRONIC INC
Method, system and device for treating disorders of the pelvic floor by delivering drugs to various nerves or tissues
InactiveUS7427280B2EfficaciousMore delivery of drugElectrotherapyMedical devicesDiseaseLigament structure
Described are implantable devices and methods for treating various disorders of the pelvic floor by delivering one or more drugs to a patient's hypogastric nerve or branches or portions thereof, prostatic plexus nerve or branches or portions thereof, sacral splanchnic nerve or branches or portions thereof, pelvic splanchnic nerve or branches or portions thereof, prostate or branches or portions thereof, pelvic floor, colon or branches or portions thereof, bladder or portions thereof, vagina or portions thereof, anus or portions thereof, external anal sphincter or portions thereof, urethra or portions thereof, penile dorsal nerve or portions thereof, inferior rectal nerves or branches or portions thereof, perineal nerves or branches or portions thereof, scrotal nerves or branches or portions thereof, scrotum or portions thereof, Alcock's Canal or branches or portions thereof, sacro-tuberous ligament or branches or portions thereof, ischial tuberosity or branches or portions thereof, greater sciatic foramen or branches or portions thereof, or lesser sciatic foramen or branches or portions thereof. One, two or more drug delivery regimes are provided on a continuous, alternating, intermittent or other basis to one or more selected nerves, nerve portions or tissues in an amount and manner effective to treat a number of disorders, including, but not limited to, urinary and / or fecal voiding dysfunctions such as constipation, incontinence disorders such as urge frequency and urinary retention disorders, sexual dysfunctions such as orgasmic and erectile dysfunction, pelvic pain, prostatitis, prostatalgia and prostatodynia.
Owner:MEDTRONIC INC
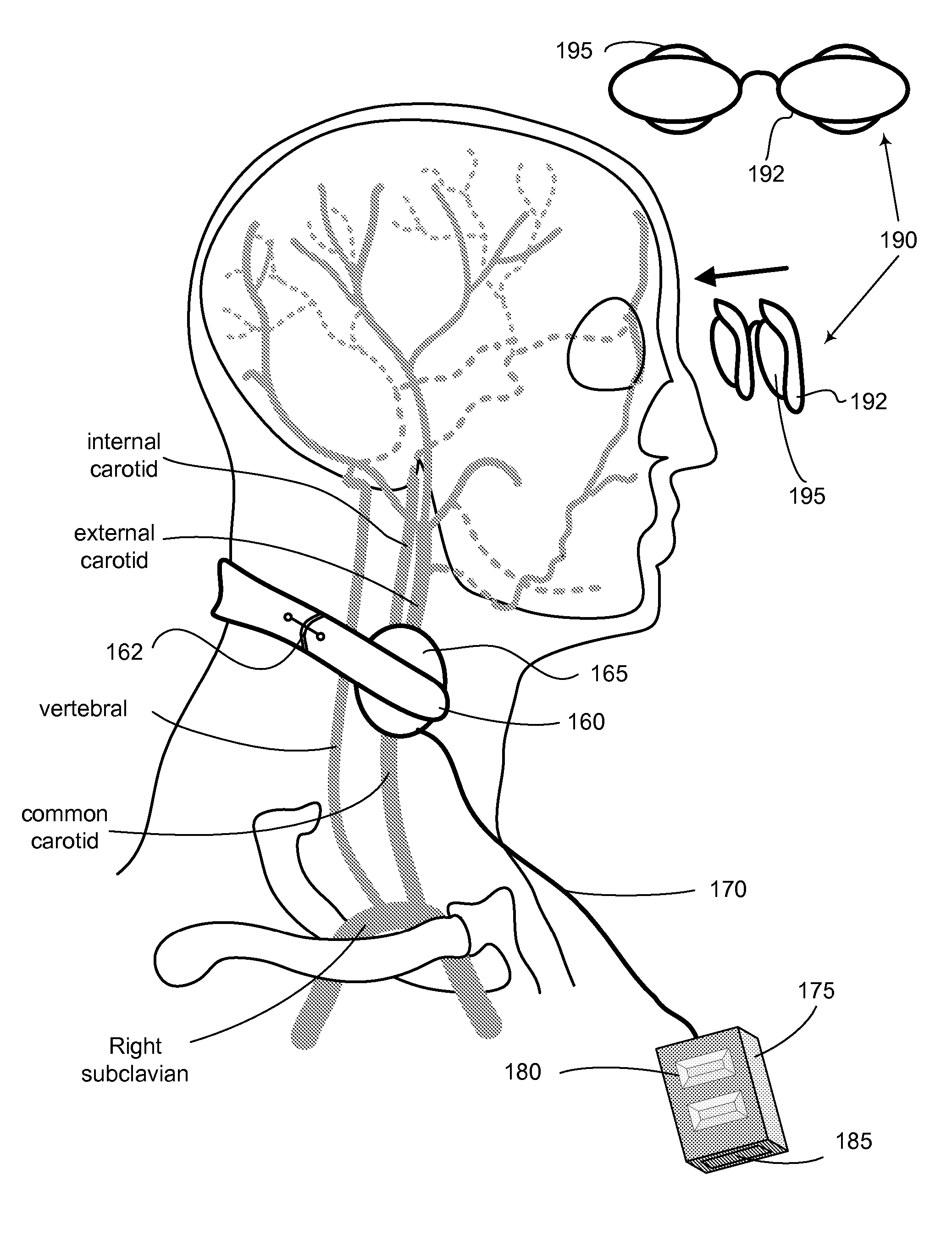
Non-invasive modulation of the autonomic nervous system
InactiveUS20060293719A1Easy accessReduction of urinary hesitancyUltrasound therapyElectrotherapyLaryngospasmDisease
The present invention is directed to methods and apparatus for modulation of the sympathetic-parasympathetic balance by application of heat, carotid and / or ocular message to reduce sympathetic tone or increase parasympathetic tone in a target muscle system to relieve a symptom of urinary hesitancy, shy bladder syndrome, DESD, urinary retention, or laryngeal spasm, as well as to monitor the efficacy of treatments for bladder conditions and to assist in the passage of medical devices through bodily sphincters as well as to treat congestive heart failure.
Owner:THERMARX
Method, system and device for treating disorders of the pelvic floor by drug delivery to the pudendal and sacral nerves
InactiveUS7276057B2EfficaciousMore delivery of drugElectrotherapyPharmaceutical delivery mechanismDiseaseFunctional disturbance
Described are implantable devices and methods for treating various disorders of the pelvic floor by delivering one or more drugs to a sacral nerve or nerve portion, as well as to a pudendal nerve or nerve portion. Two or more drug delivery regimes are provided on a continuous, alternating, intermittent or other basis to the sacral and pudendal nerves in an amount and manner effective to treat a number of disorders, including, but not limited to, urinary and / or fecal voiding dysfunctions such as constipation, incontinence disorders such as urge frequency and urinary retention disorders, sexual dysfunctions such as orgasmic and erectile dysfunction, pelvic pain, prostatitis, prostatalgia and prostatodynia.
Owner:MEDTRONIC INC
Pulsatile release compositions of milnacipran
InactiveUS20060003004A1Minimize exposureReduces milnacipran gastrointestinal side effectCapsule deliveryCoatingsPalpitationsPanic
A once-a-day oral milnacipran pulsatile release composition has been developed that releases the drug in spaced apart “pulses”. The dosage forms are comprised of first, second and optional third dosage units, with each dosage unit having a different drug release profile. This dosage form provides in vivo drug plasma levels characterized by Cmax below 3000 ng / ml, preferably below 2000 ng / ml, and most preferably below 1000 ng / ml. The composition provides pulsatile release of milnacipran to produce a therapeutic effect over approximately 24 hours, when administered to a patient in need, resulting in diminished incidence or decreased intensity of common milnacipran side effects such as sleep disturbance, nausea, vomiting, headache, tremulousness, anxiety, panic attacks, palpitations, urinary retention, orthostatic hypotension, diaphoresis, chest pain, rash, weight gain, back pain, constipation, vertigo, increased sweating, agitation, hot flushes, tremors, fatigue, somnolence, dyspepsia, dysoria, nervousness, dry mouth, abdominal pain, irritability, and insomnia.
Owner:COLLEGIUM PHARMA INC
Partial-length indwelling urinary catheter and method permitting selective urine discharge
A partial-length catheter, or an extendable tube or sleeve member of the catheter, is selectively movable within the prostatic urethra to open a urine drainage passageway through and obstructed portion of the prostatic urethra or to open the external urinary sphincter muscle and thereby discharge urine from the bladder. A control element is manipulated at a position exterior of the urinary canal to selectively move the catheter or the extendable tube or sleeve member, thereby selectively controlling urine discharge. The catheter may also be used to diagnose urinary retention problems caused by a weak bladder or a prostatic obstruction.
Owner:PROSTALUND
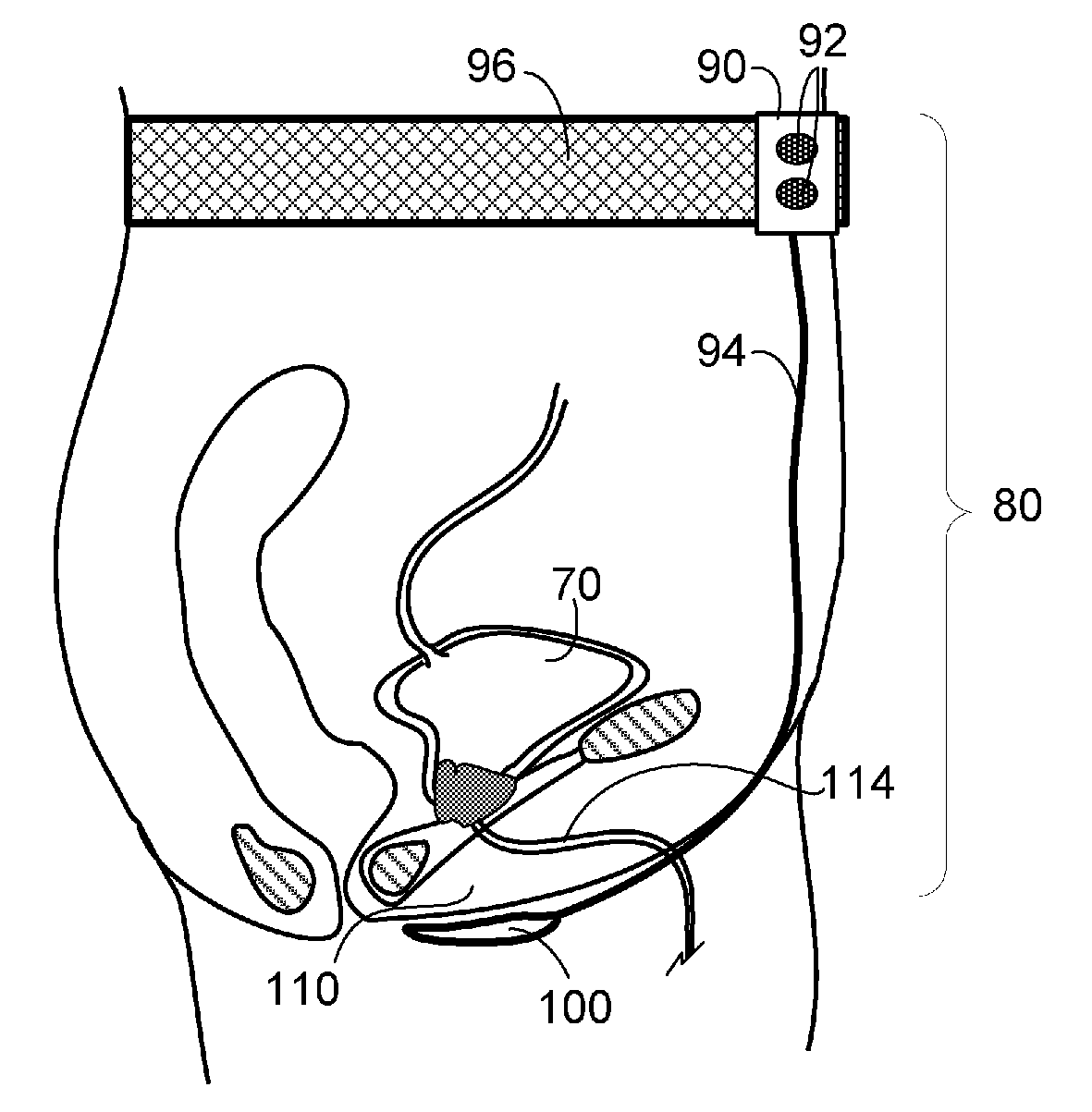
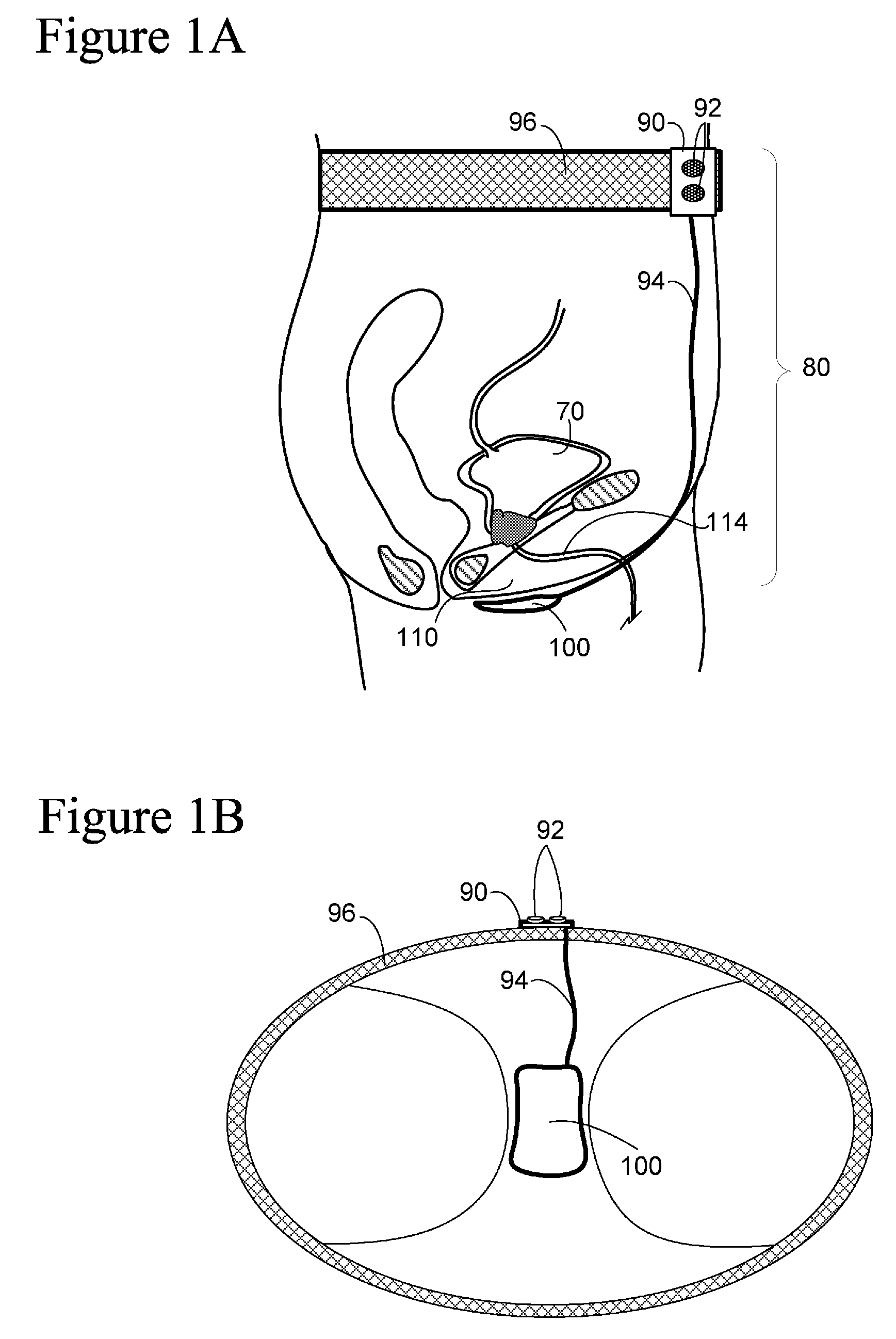
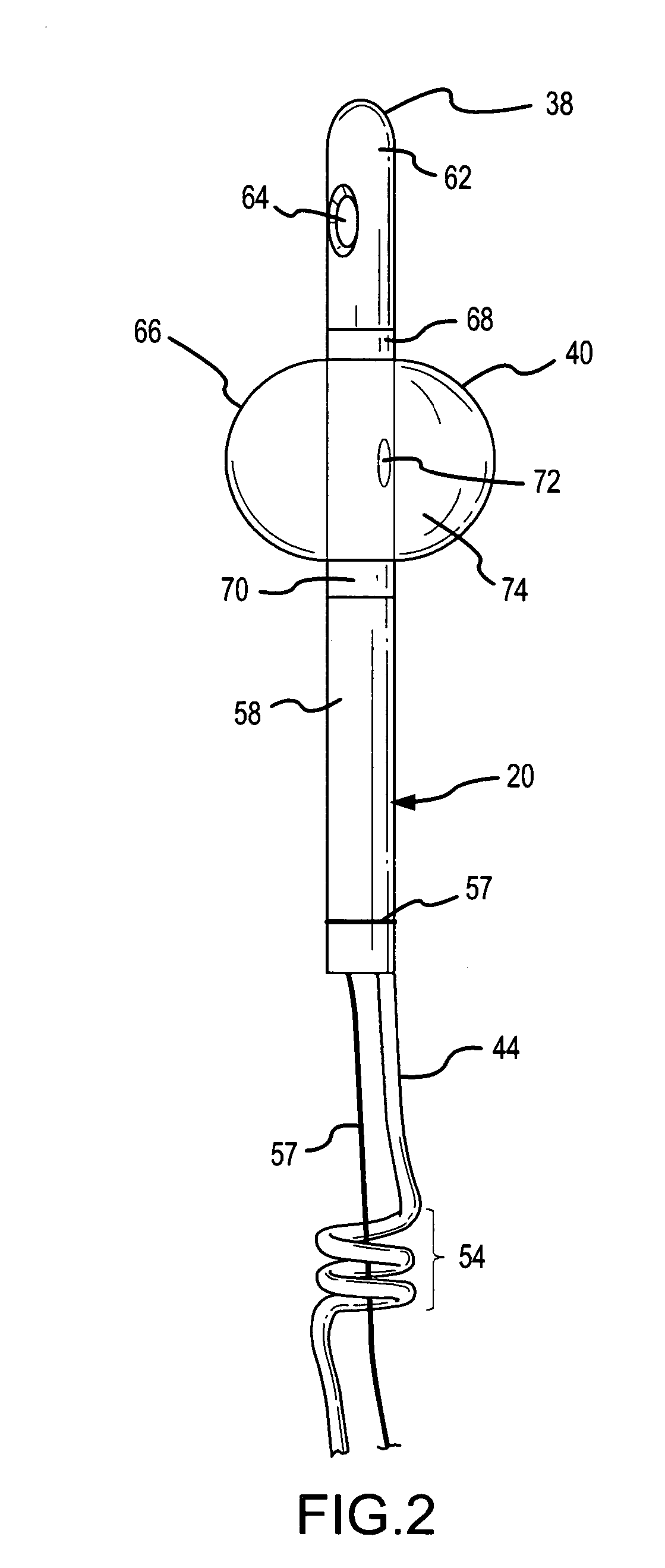
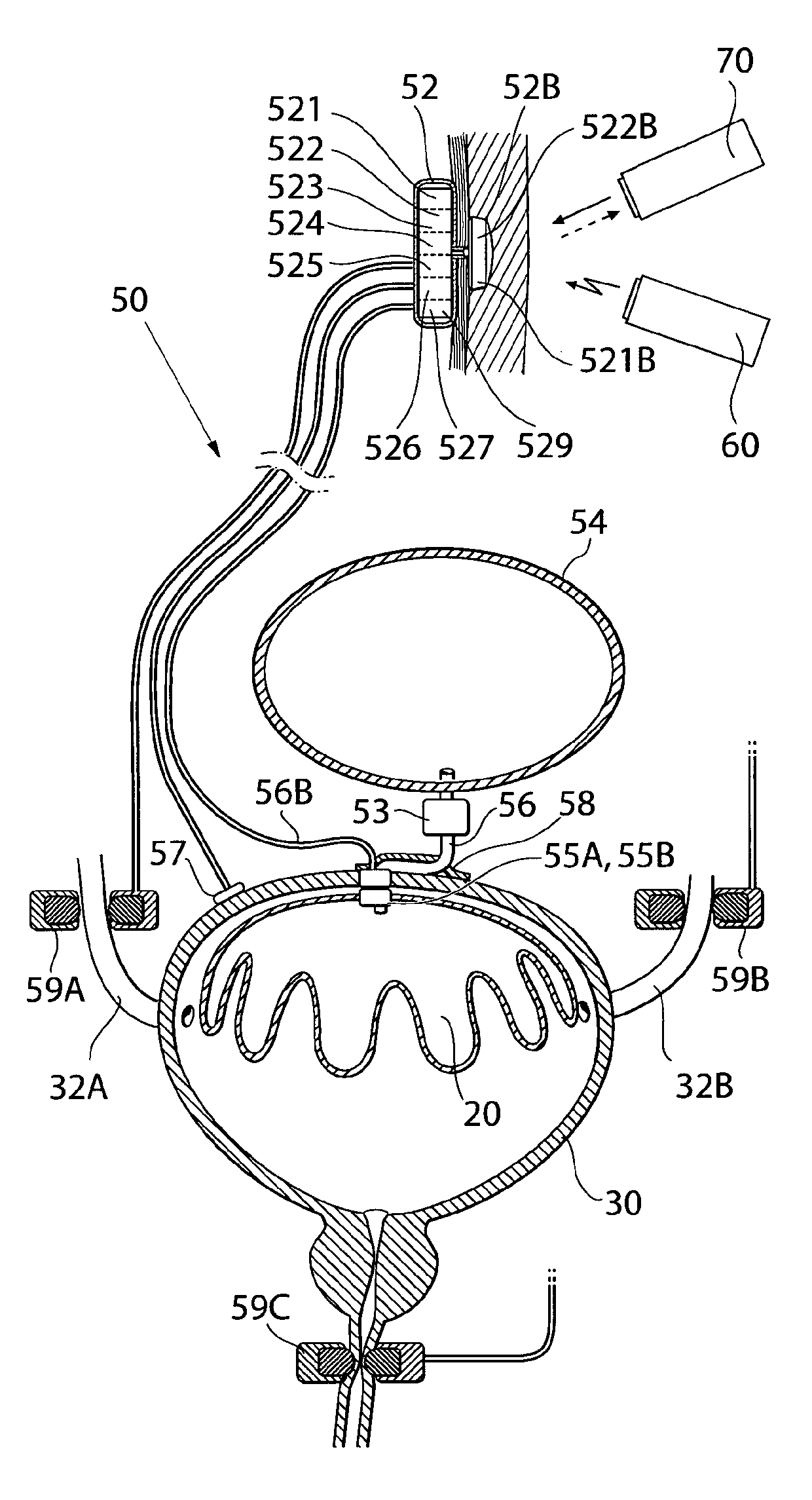
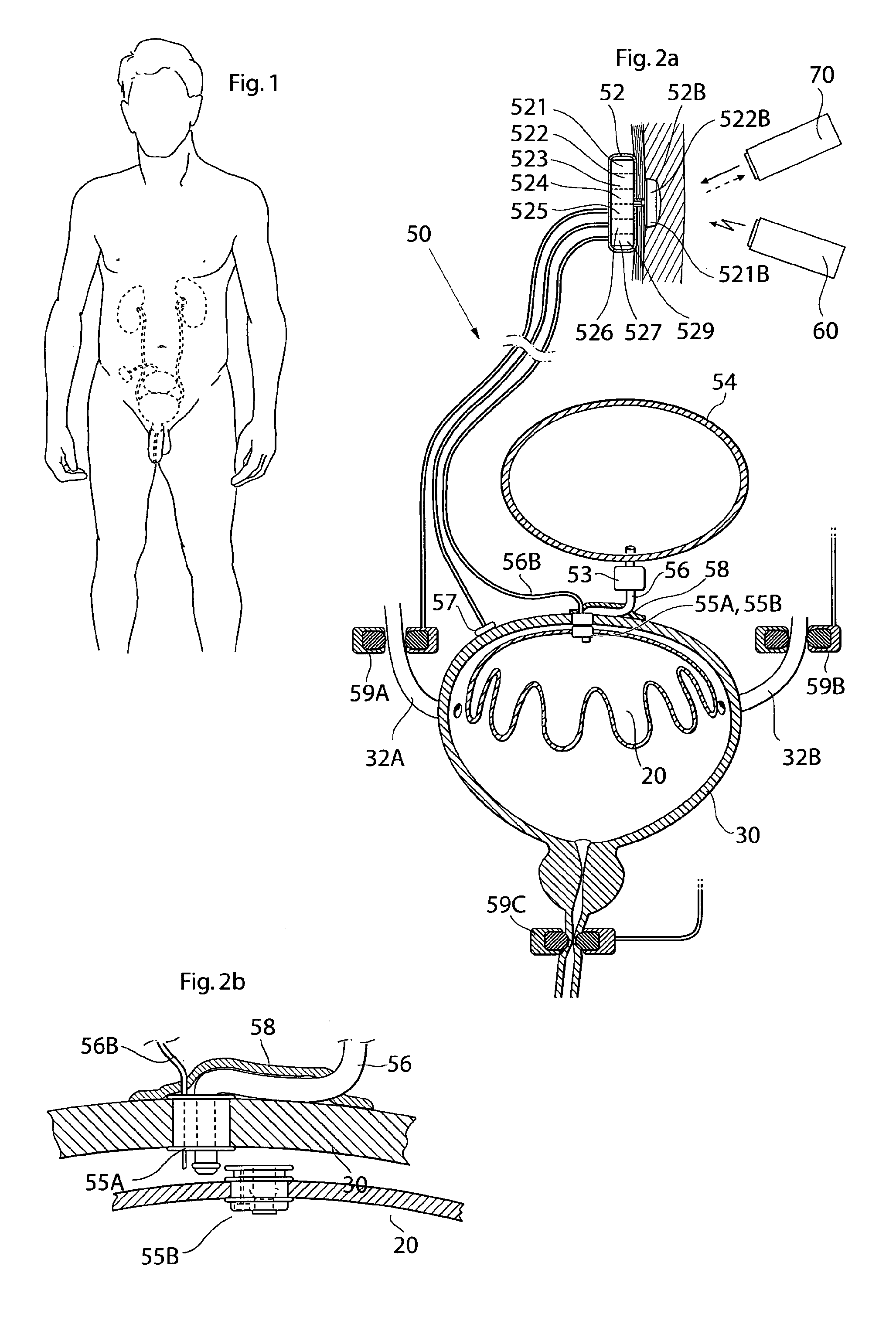
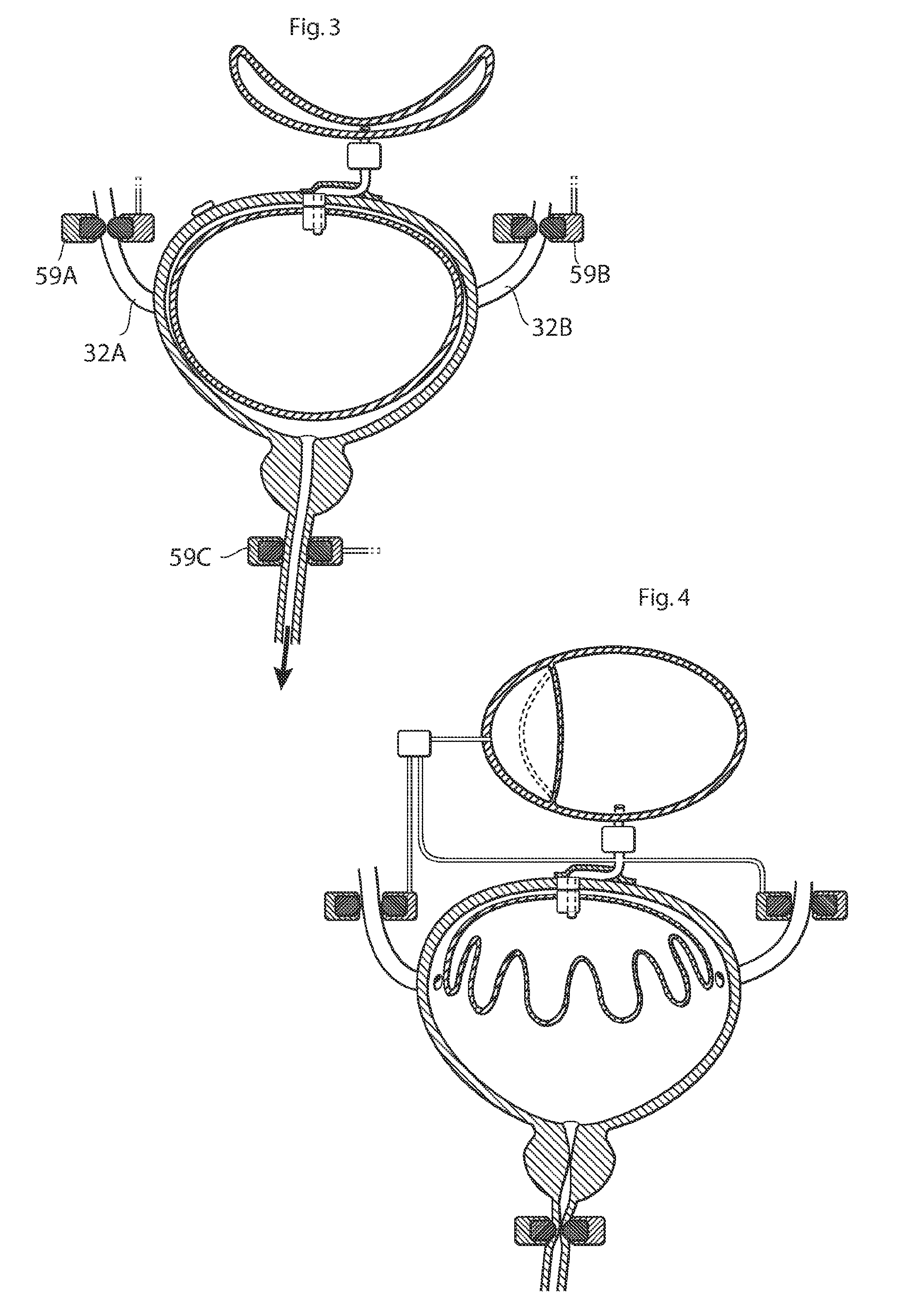
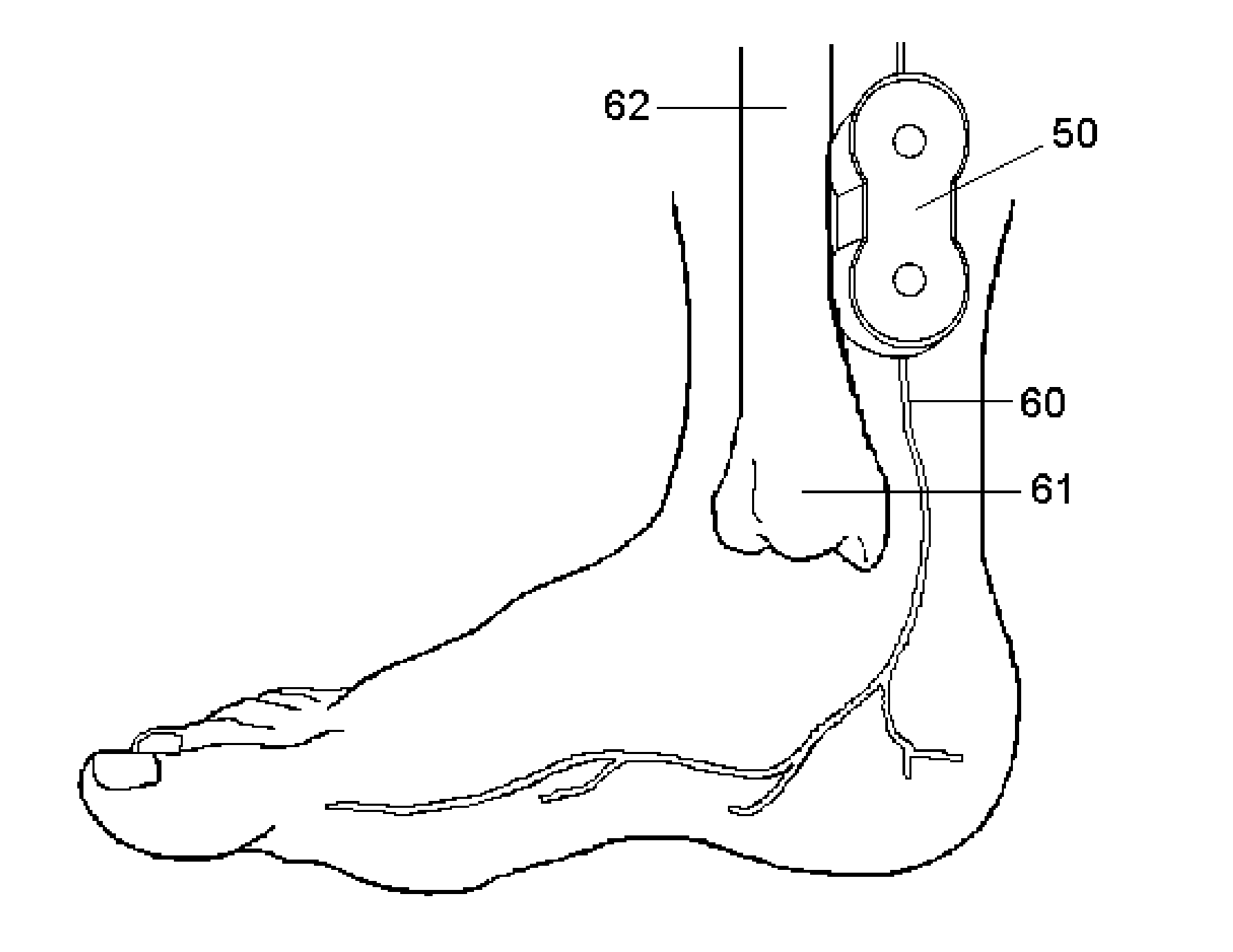
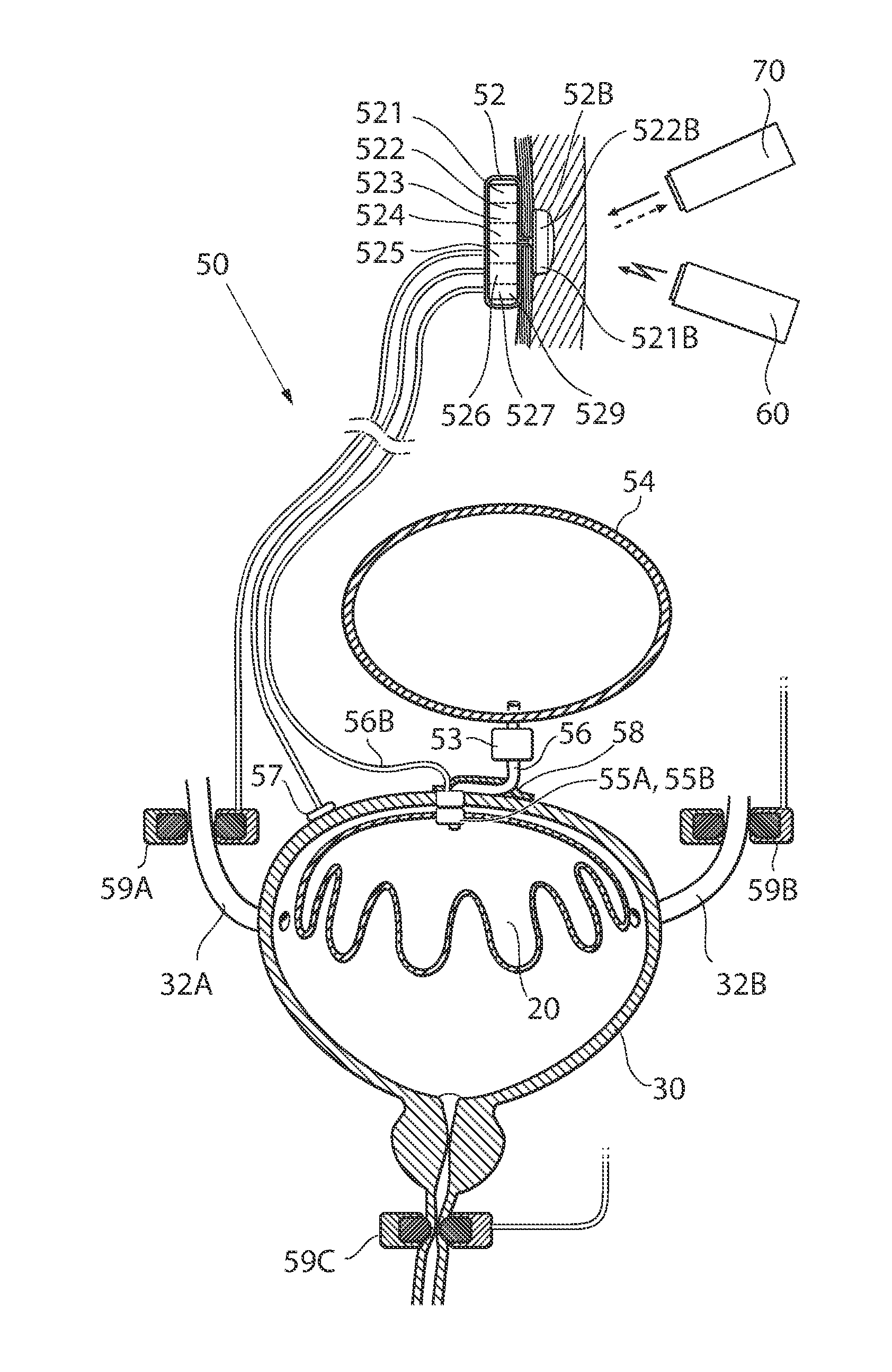
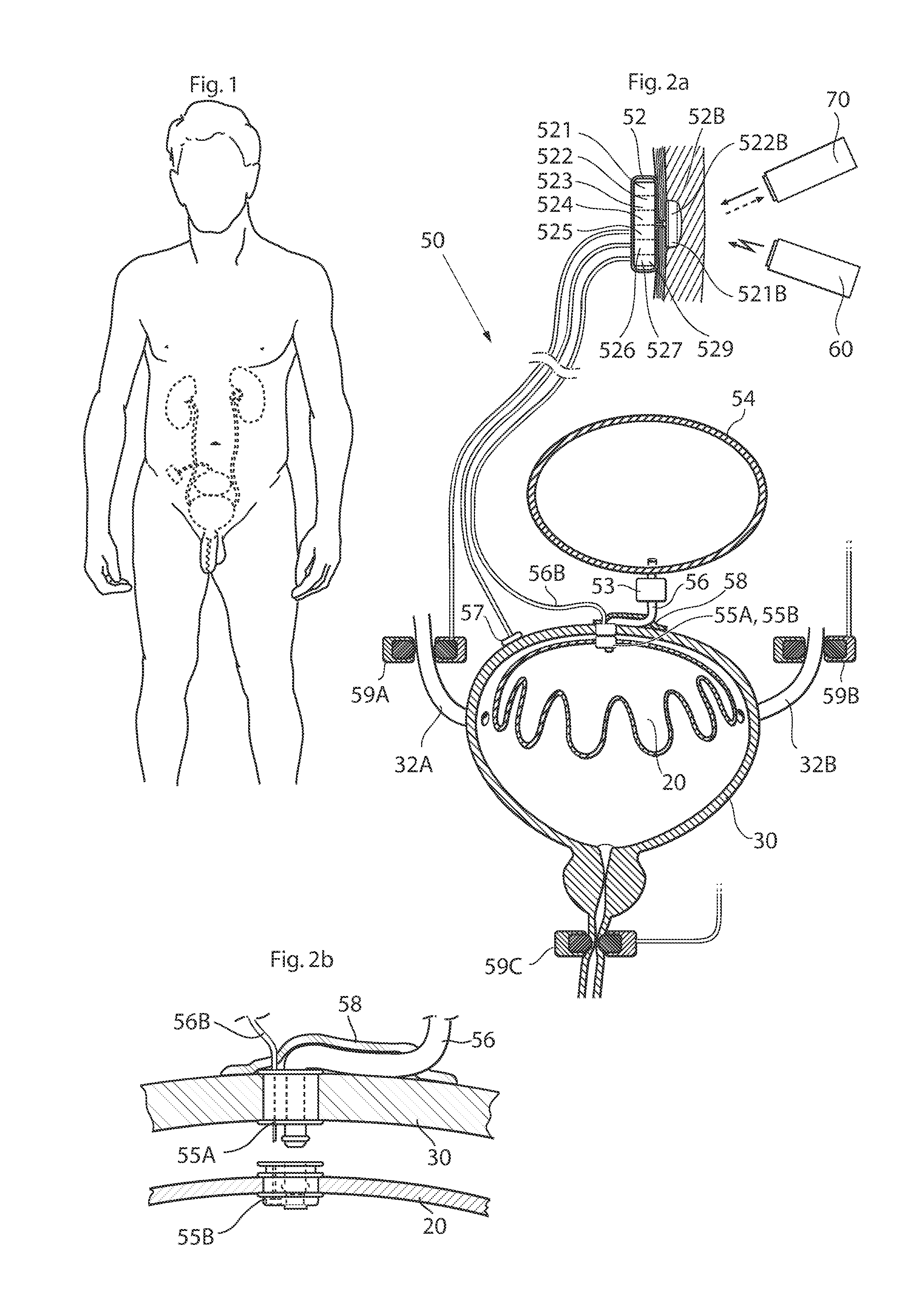
Implantable device for internal urinary control
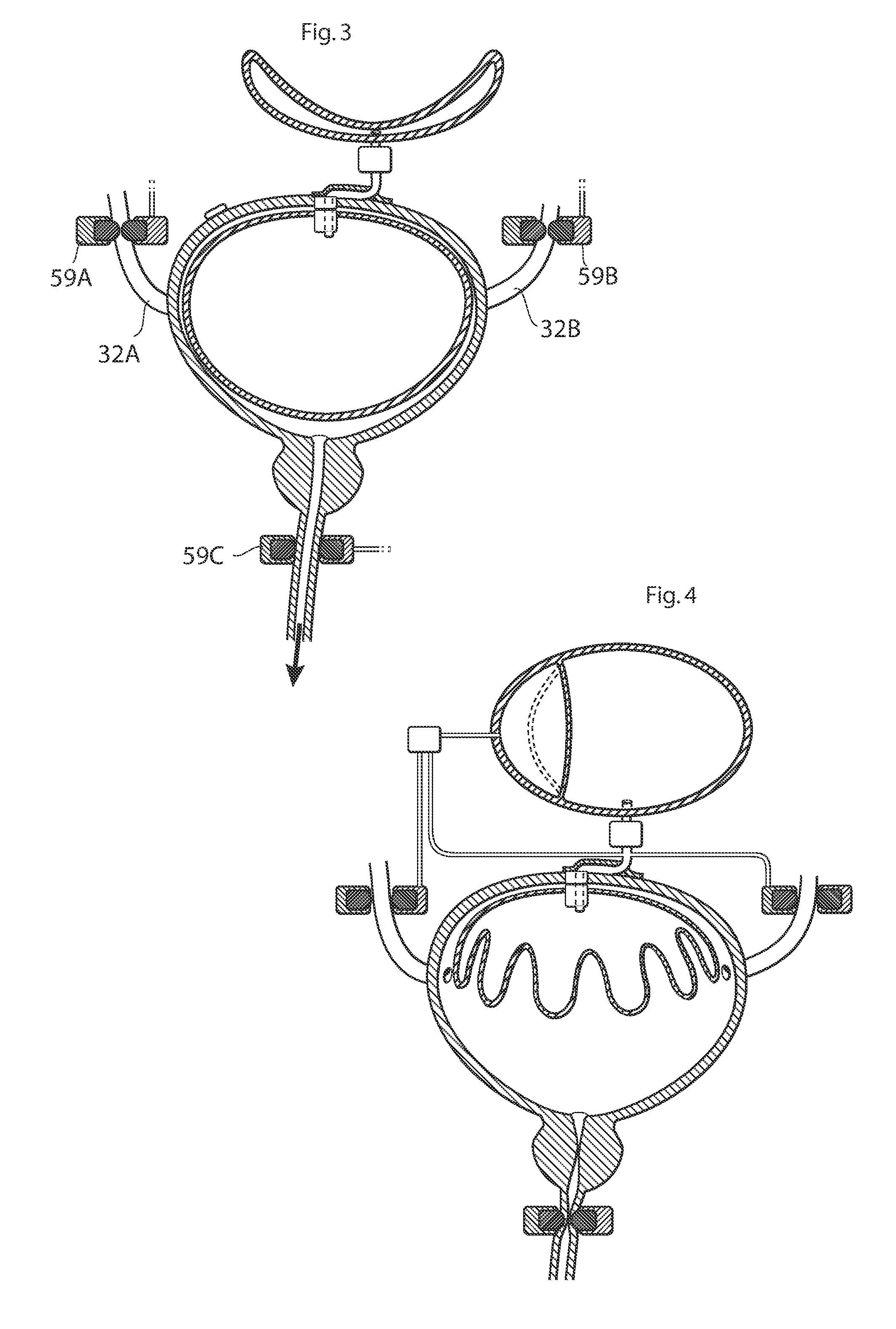
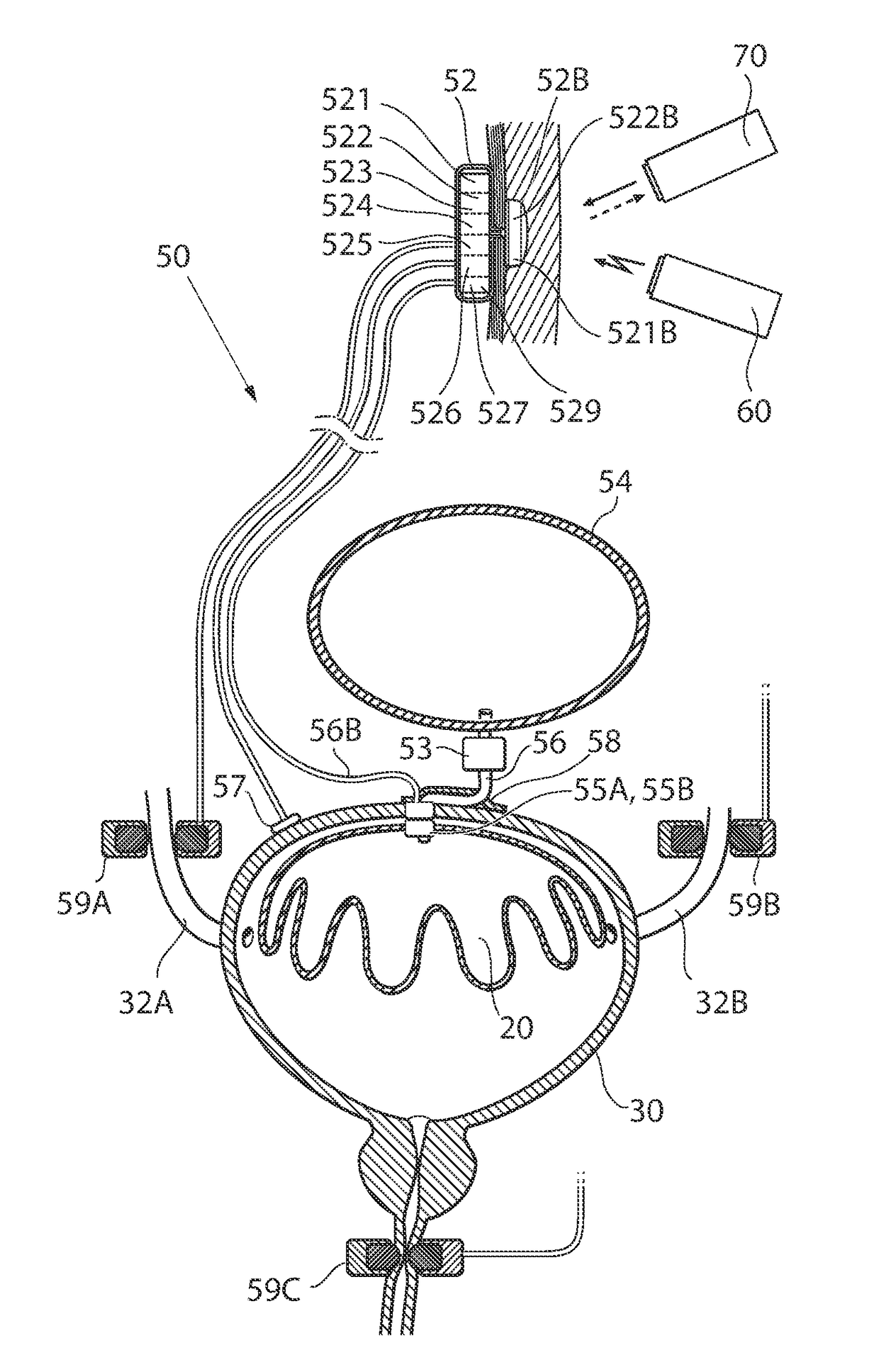
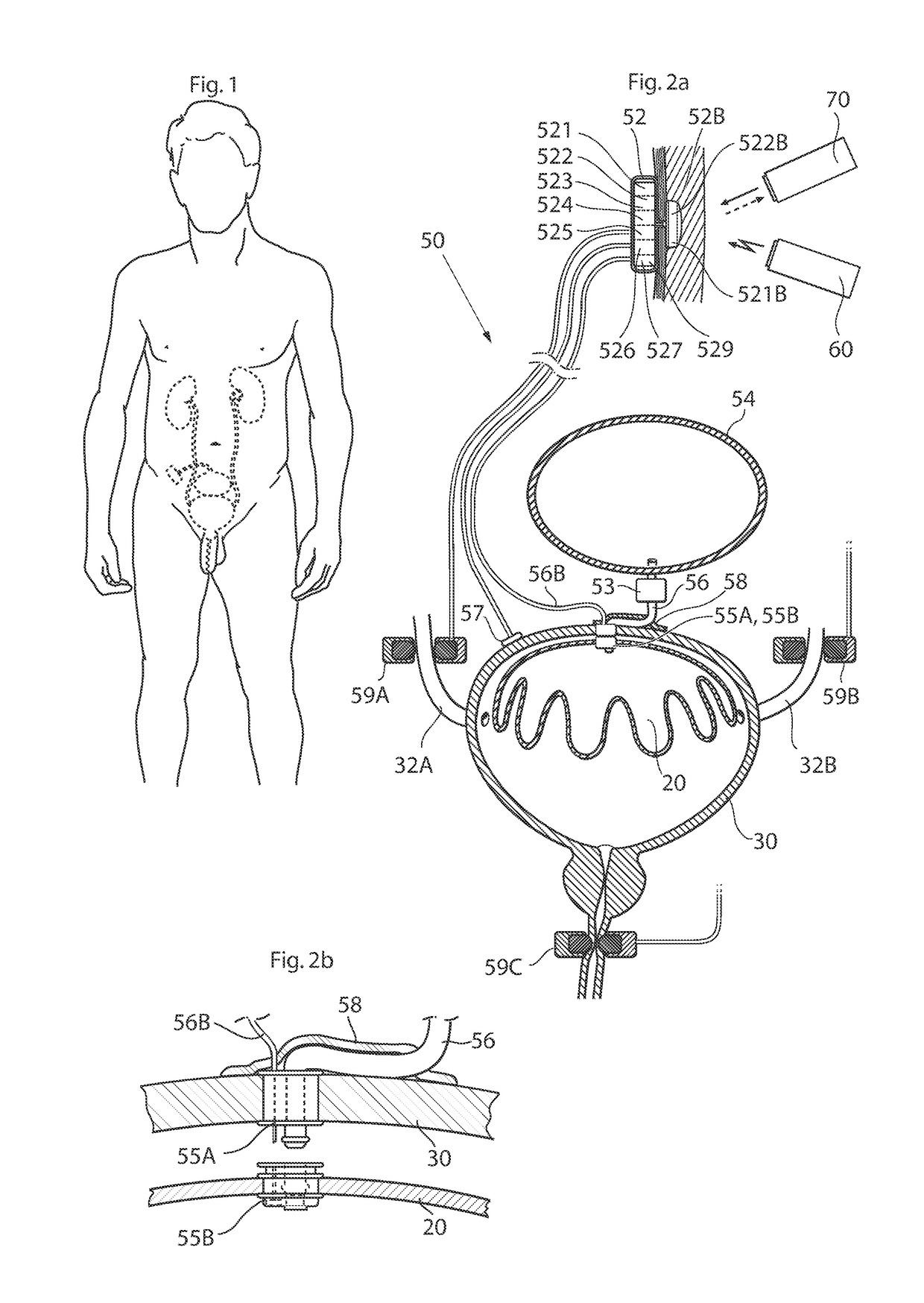
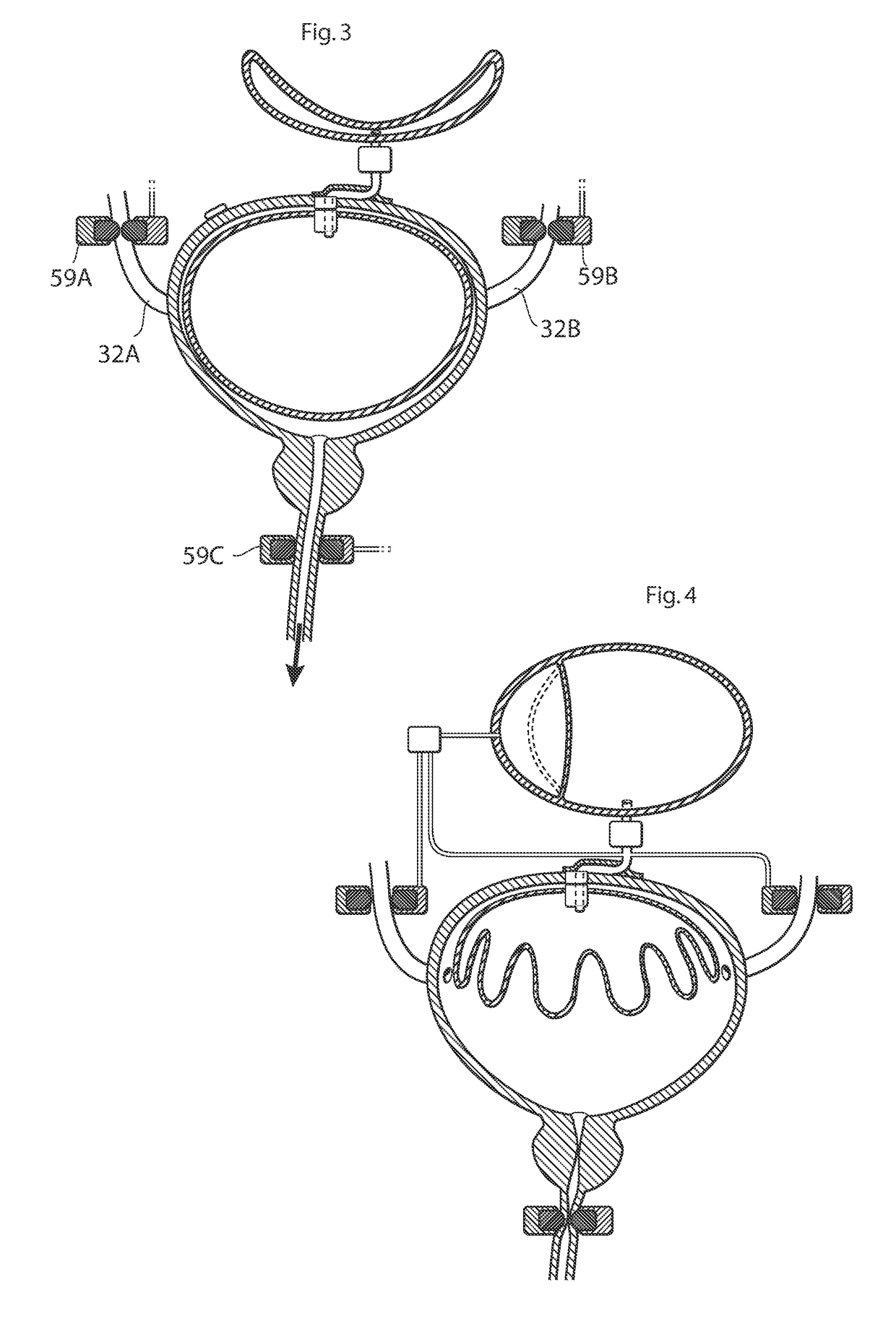
ActiveUS20110196194A1Easy to replaceReliable and goodUrinary bladderAnti-incontinence devicesImplanted deviceUrinary control
The present invention relates to an implantable apparatus for obtaining urinary control and emptying of the urinary bladder, thereby preventing from or treating involuntary urinary retention. In general terms, the apparatus comprises an expandable member adapted to be implanted inside the urinary bladder of the patient for discharging urine, and a control device for controlling the volume of the expandable member. The control device is adapted to be connected to the expandable member through the wall of the urinary bladder.
Owner:FORSELL PETER
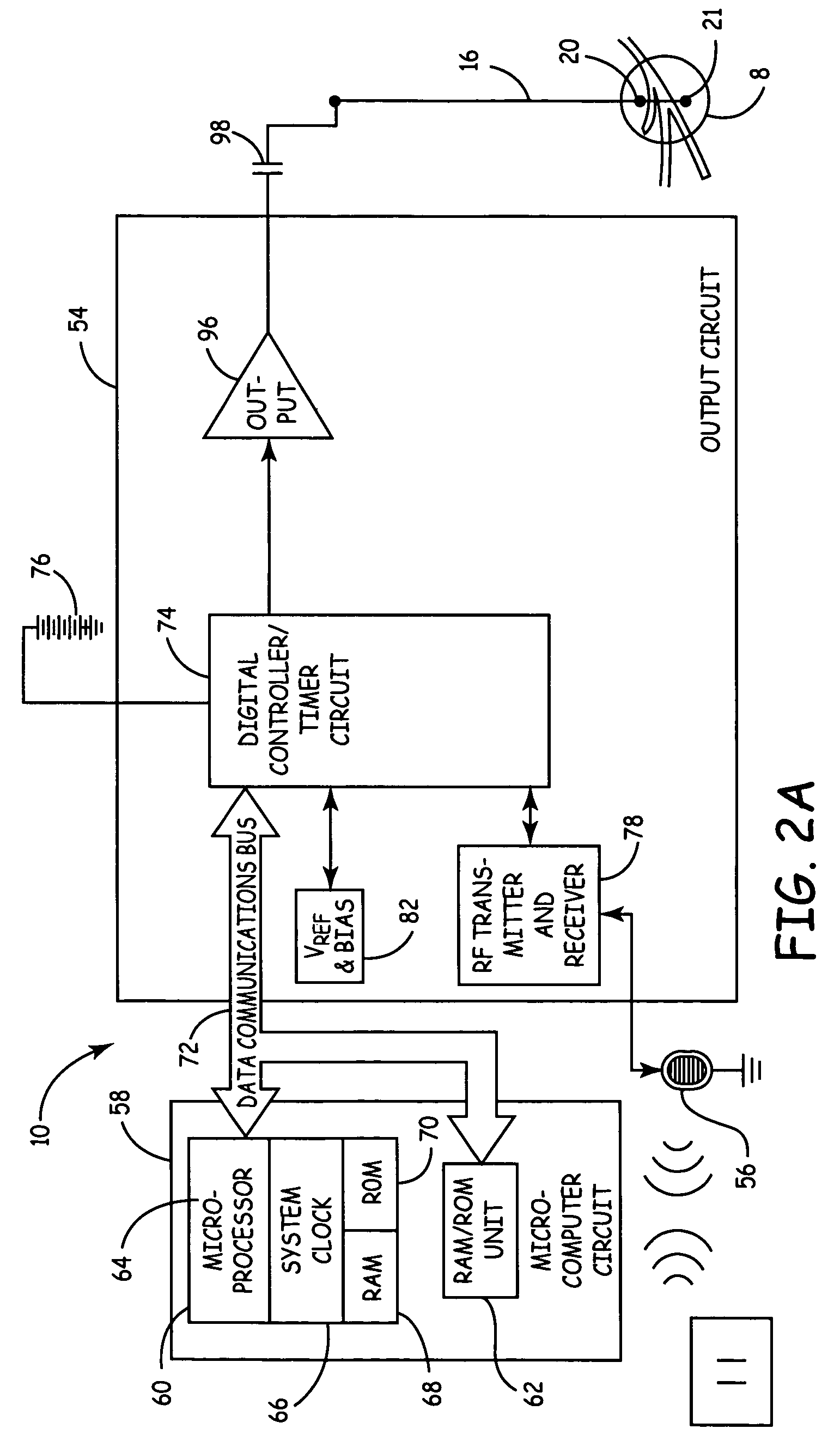
Sacral nerve stimulation system
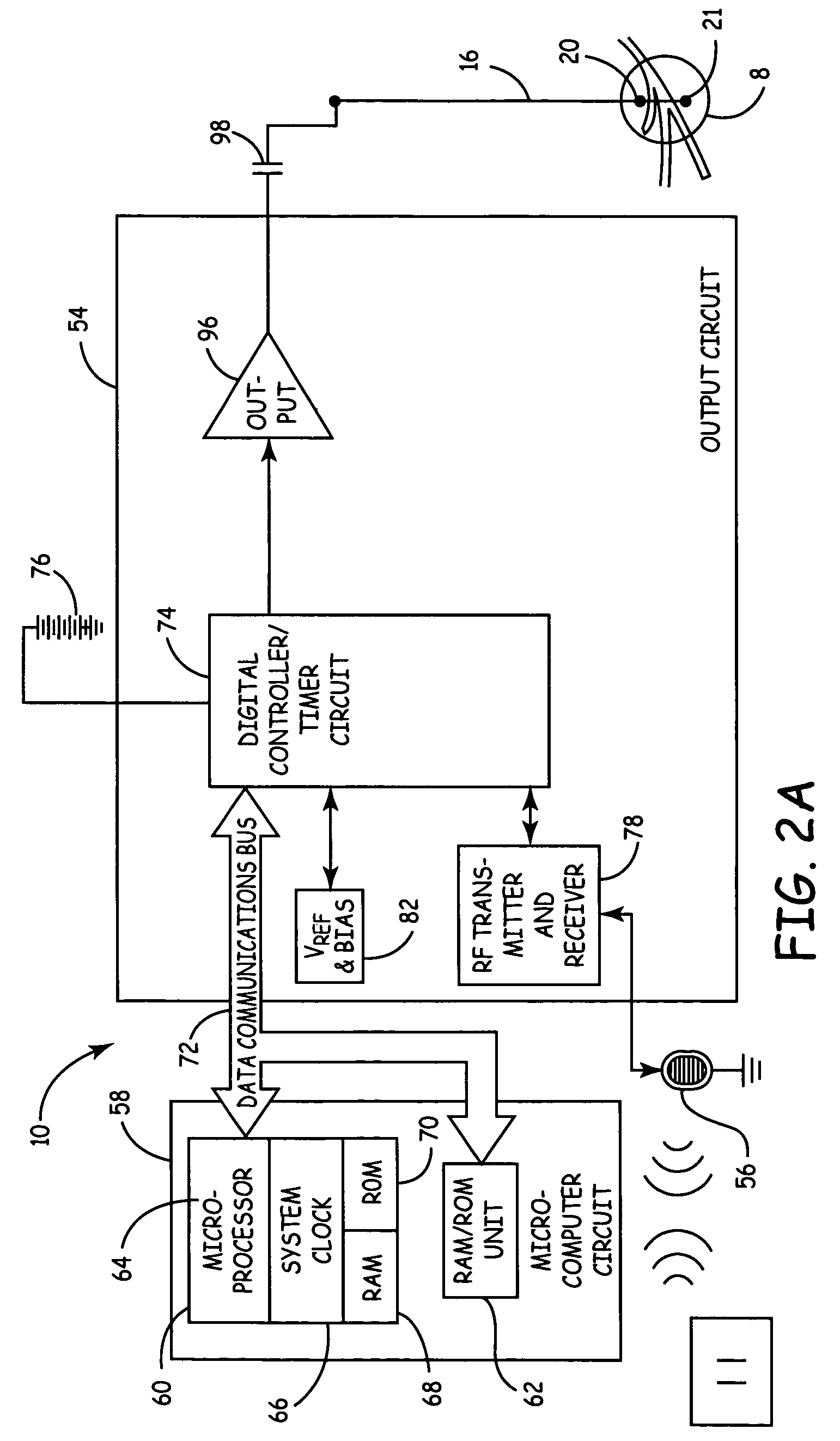
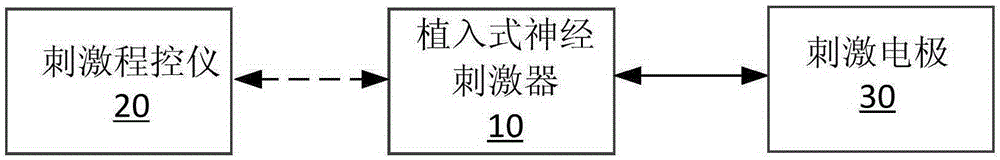
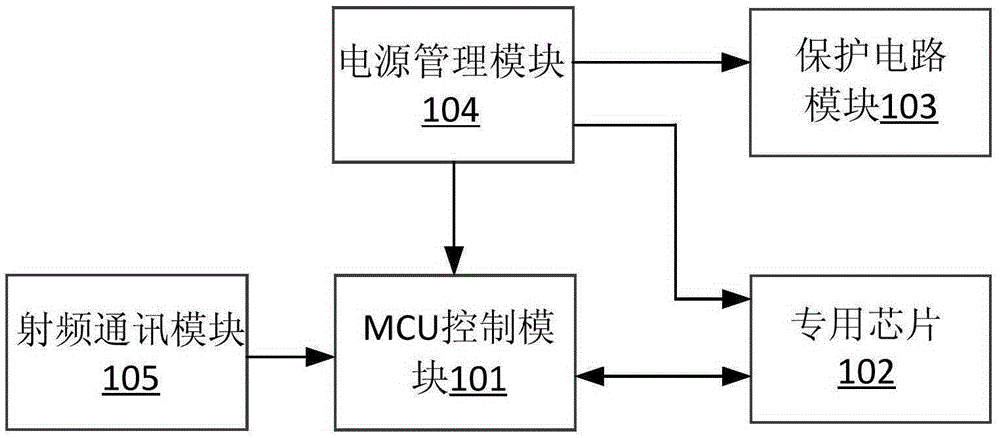
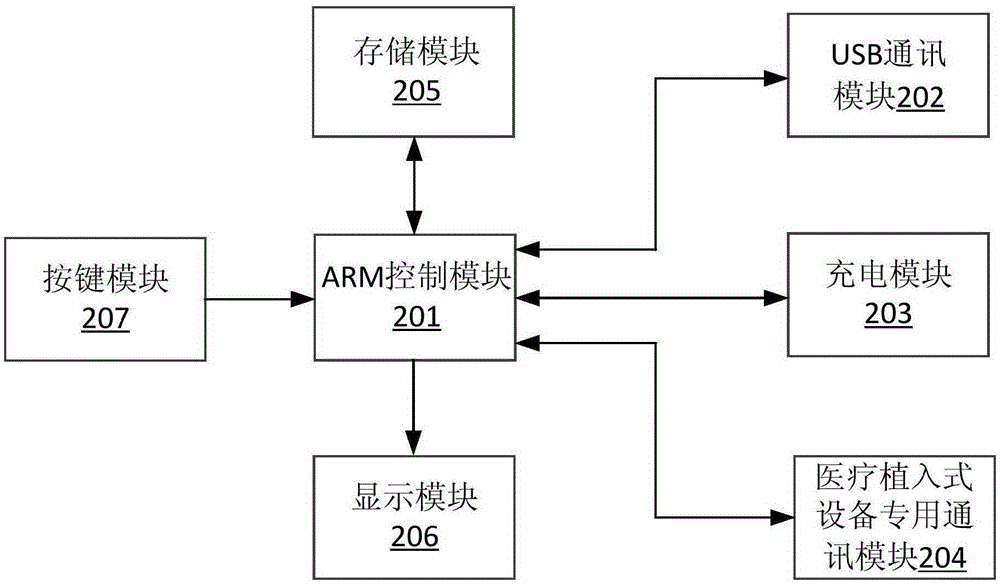
The invention discloses a sacral nerve stimulation system, which comprises an implanted nerve stimulator, a stimulator programmed controller and a stimulating electrode, wherein the implanted nerve stimulator comprises a MCU (micro-programmed control unit) control module, a special chip, a protection circuit module, a power management module and a radio frequency communication module; the stimulator programmed controller comprises an ARM control module, a USB communication module, a charger module, a medical implanted equipment special communication module, a storage module, a display module and a key module; the stimulating electrode is connected to the special chip; and sacral nerves are directly stimulated after a stimulating pulse is received. The implant stimulator disclosed by the invention, by generating lasting electric pulse, can conduct electric stimulation on target nerves in a plurality of parts; the implant stimulator is controlled by the stimulator programmed controller in vitro; and the stimulation system achieves a satisfying effect when applied to such fields as uracratia, urinary retention, Parkinson, spinal neuralgia, peripheral electrical stimulation therapy and the like.
Owner:HANGZHOU CHENGNUO MEDICAL SCI & TECH CO LTD
Non-invasive electrical and magnetic nerve stimulators used to treat overactive bladder and urinary incontinence
Transcutaneous electrical and magnetic nerve stimulation devices and methods generate energy that is delivered noninvasively to selected nerve fibers within the patient to treat lower urinary tract disorders. The disorders comprise overactive bladder, urge incontinence, stress, incontinence, urge frequency, non-obstructive urinary retention and interstitial cystitis / painful bladder syndrome. In preferred embodiments, a posterior tibial nerve of a patient is stimulated non-invasively to treat bladder disorders.
Owner:ELECTROCORE
Implantable device for internal urinary control
ActiveUS20110263928A1Easy to replaceGood adhesionUrinary bladderAnti-incontinence devicesImplanted deviceUrinary control
The present invention relates to an implantable apparatus for obtaining urinary control and emptying of the urinary bladder, thereby preventing from or treating involuntary urinary retention. In general terms, the apparatus comprises an expandable member adapted to be implanted inside the urinary bladder of the patient for discharging urine, and a control device for controlling the volume of the expandable member. The control device is adapted to be connected to the expandable member through the wall of the urinary bladder.
Owner:FORSELL PETER
Implantable device for internal urinary control
ActiveUS9788928B2Easy to replaceGood adhesionUrinary bladderAnti-incontinence devicesUrinary controlUrinary retention
The present invention relates to an implantable apparatus for obtaining urinary control and emptying of the urinary bladder, thereby preventing from or treating involuntary urinary retention. In general terms, the apparatus comprises an expandable member adapted to be implanted inside the urinary bladder of the patient for discharging urine, and a control device for controlling the volume of the expandable member. The control device is adapted to be connected to the expandable member through the wall of the urinary bladder.
Owner:FORSELL PETER
Use of neurotoxin therapy for treatment of urologic and related disorders related to urinary retention
InactiveUS20050049175A1Relieving urinary retentionRelieve symptomsBiocideBacterial antigen ingredientsDiseaseUrethra
The present invention related to methods for treating neurological-urological conditions, including urinary retention. This is accomplished by administration of a botulinum toxin into the lower urinary tract of a patient with urinary retention, including the bladder or urinary sphincter and the bladder wall.
Owner:ALLERGAN INC
Method, system and device for treating disorders of the pelvic floor by electrical stimulation of and the delivery of drugs to the left and right pudendal nerves
InactiveUS20060122659A9Strong specificityLower medical costsSpinal electrodesPharmaceutical delivery mechanismDiseaseProstatalgia
Described are devices and methods for treating various disorders of the pelvic floor by means of electrical stimulation of the pudendal nerves, and means for delivering one or more drugs in association with such electrical stimulation therapies. One or more electrical stimulation signals are applied, and one or more drugs are infused, injected or otherwise administered, to appropriate portions of a patient's pelvic floor and the pudendal nerves or portions thereof in an amount and manner effective to treat a number of disorders, including, but not limited to, urinary voiding dysfunction, fecal voiding dysfunction, constipation, stress incontinence, urge incontinence, urinary retention disorder, sexual dysfunction, orgasmic dysfunction, erectile dysfunction, pelvic pain, prostatitis, prostatalgia and prostatodynia.
Owner:MEDTRONIC INC
Use of neurotoxin therapy for treatment of urologic and related disorders related to lowering elevated bladder pressure
InactiveUS7455845B2Inexpensive and safeSimple methodBacterial antigen ingredientsPeptide/protein ingredientsDiseaseUrethra
Owner:ALLERGAN INC
Non-invasive modulation of the autonomic nervous system
InactiveUS20080004679A1Facilitate comfortFacilitate successUltrasound therapySurgical instruments for heatingDiseaseNervous system
The present invention is directed to methods and apparatus for modulation of the sympathetic-parasympathetic balance by application of heat, carotid and / or ocular message to reduce sympathetic tone or increase parasympathetic tone in a target muscle system to relieve a symptom of urinary hesitancy, shy bladder syndrome, DESD, urinary retention, or laryngeal spasm, as well as to monitor the efficacy of treatments for bladder conditions and to assist in the passage of medical devices through bodily sphincters as well as to treat congestive heart failure.
Owner:THERMARX
Partial-length indwelling urinary catheter and method permitting selective urine discharge
Owner:PROSTALUND
Method, system and device for treating disorders of the pelvic floor by drug delivery to the pudendal and sacral nerves
InactiveUS20050021008A1EfficaciousMore delivery of drugElectrotherapyPharmaceutical delivery mechanismDiseaseSexual impotence
Described are implantable devices and methods for treating various disorders of the pelvic floor by delivering one or more drugs to a sacral nerve or nerve portion, as well as to a pudendal nerve or nerve portion. Two or more drug delivery regimes are provided on a continuous, alternating, intermittent or other basis to the sacral and pudendal nerves in an amount and manner effective to treat a number of disorders, including, but not limited to, urinary and / or fecal voiding dysfunctions such as constipation, incontinence disorders such as urge frequency and urinary retention disorders, sexual dysfunctions such as orgasmic and erectile dysfunction, pelvic pain, prostatitis, prostatalgia and prostatodynia.
Owner:MEDTRONIC INC
Treating microvasculature diseases with acetyl cholinesterase inhibitors
There is disclosed a method of treating various diseases caused by micro-vasculature circulation problems, including, but not limited to, vascular insufficiency, phantom pain, diabetic neuropathy, neuropathic pain, autoimmune / inflammatory diseases (e.g., multiple sclerosis, Parkinson's disease, Crohn's Disease, lupus, rheumatoid arthritis, polymyalgia rheumatica, polymyositis, dermatomyositis, sarcoidosis), urinary retention, lymphoedema, and chronic renal insufficiency. Specifically, there is disclosed a treatment providing an effective amount of an acetyl cholinesterase inhibitor compound (or combination of compounds) to treat one or a plurality of microvasculature diseases.
Owner:WILLS STEPHEN
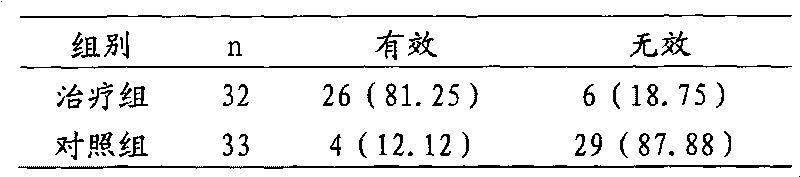
Traditional Chinese medicine composition for treating uroschesis and prostatitis and preparation method thereof
The invention discloses a traditional Chinese medicine composition for treating uroschesis and prostatitis as well as a preparation method and an application thereof. The traditional Chinese medicine composition is prepared by using platycodon root, rhizoma cimicifugae, almond with peel, semen plantaginis, rhizome of oriental water plantain, poria, lightred tuckahoe, phellodendron chinense schneid., rhizoma anemarrhenae and cortex cinnamomi as raw materials. The platycodon root, the rhizoma cimicifugae and the almond with peel are acted into the lung channels and play a role of releasing the lung and stretching, thereby regulating the urinary passages and dredging the bladder; the semen plantaginis and the rhizome of oriental water plantain have the function of promoting urination; and the phellodendron chinense schneid., the rhizoma anemarrhenae and the cortex cinnamomi are pills for tonifying the kidney and have the function of clearing downward flow of damp and heat, promoting the functioning of bladder, and treating heat stagnating in bladder, urodialysis, abdominal distension and wet and pain urinary passages. The composition has no toxic or side effects, convenient taking, free urine, remarkable effect, high cure rate and worth of popularization and application in clinic.
Owner:CHANGZHOU SIMM DRUG RES & DEV CENT +1
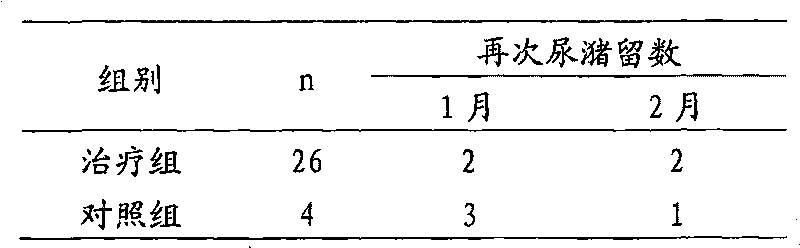
Traditional Chinese medical composition for treating postpartum urinary retention
InactiveCN103110766ANo side effectsSignificant effectBlood disorderExtracellular fluid disorderLicorice rootsAstragalus mongholicus
The invention discloses a traditional Chinese medical composition for treating postpartum urinary retention. The traditional Chinese medical composition for treating postpartum urinary retention consists of astragalus mongholicus, angelica sinensis, codonopsis pilosula, poria cocos, fried rhizoma atractylodis macrocephalae, rhizoma alismatis, cassia twig, fructus psoraleae, rattletop, radix bupleuri, almond, ligusticum wallichii, flowers carthami and honey-fried licorice root by certain weigh percent. The traditional Chinese medical composition has the function of tonifying middle-Jiao and Qi, eliminating dampness and diuresis and clearing heat and dispersing blood stasis, and can be effectively used for treating postpartum urinary retention.
Owner:徐姝一
Neostigmine bromide sustained-release tablet and preparation method thereof
InactiveCN102258492AImprove stabilityAvoid instabilityDigestive systemMuscular disorderSustained Release TabletSide effect
The present invention belongs to the technical field of medicinal preparation. The invention discloses a formula of a neostigmine bromide slow release preparation which can be taken once every day for treating myasthemia gravis, functional flatulence after being performed an operation and urinary retention, as well as its preparation method. The preparation is disclosed as a slow release tablet form composed of a skeleton core containing neostigmine bromide and the slow release preparation and a coating. The neostigmine bromide slow release preparation of the present invention is capable of overcoming the disadvantage of present medicament common tablets in the market, slowly releasing to keep stable blood and medicine concentration, acting for a longer period, possessing low toxicity andside effect and conveniently taking the preparation, and the slow release preparation keeps effective blood and medicine concentration for a long time, reduces the medicine taking frequency, raises the compliance of the patients and reduces the side-effect due to over peak concentration. The invention has the advantages of simple preparation technology, low cost, easy control and easy industrial production.
Owner:CHONGQING MEDICAL UNIVERSITY
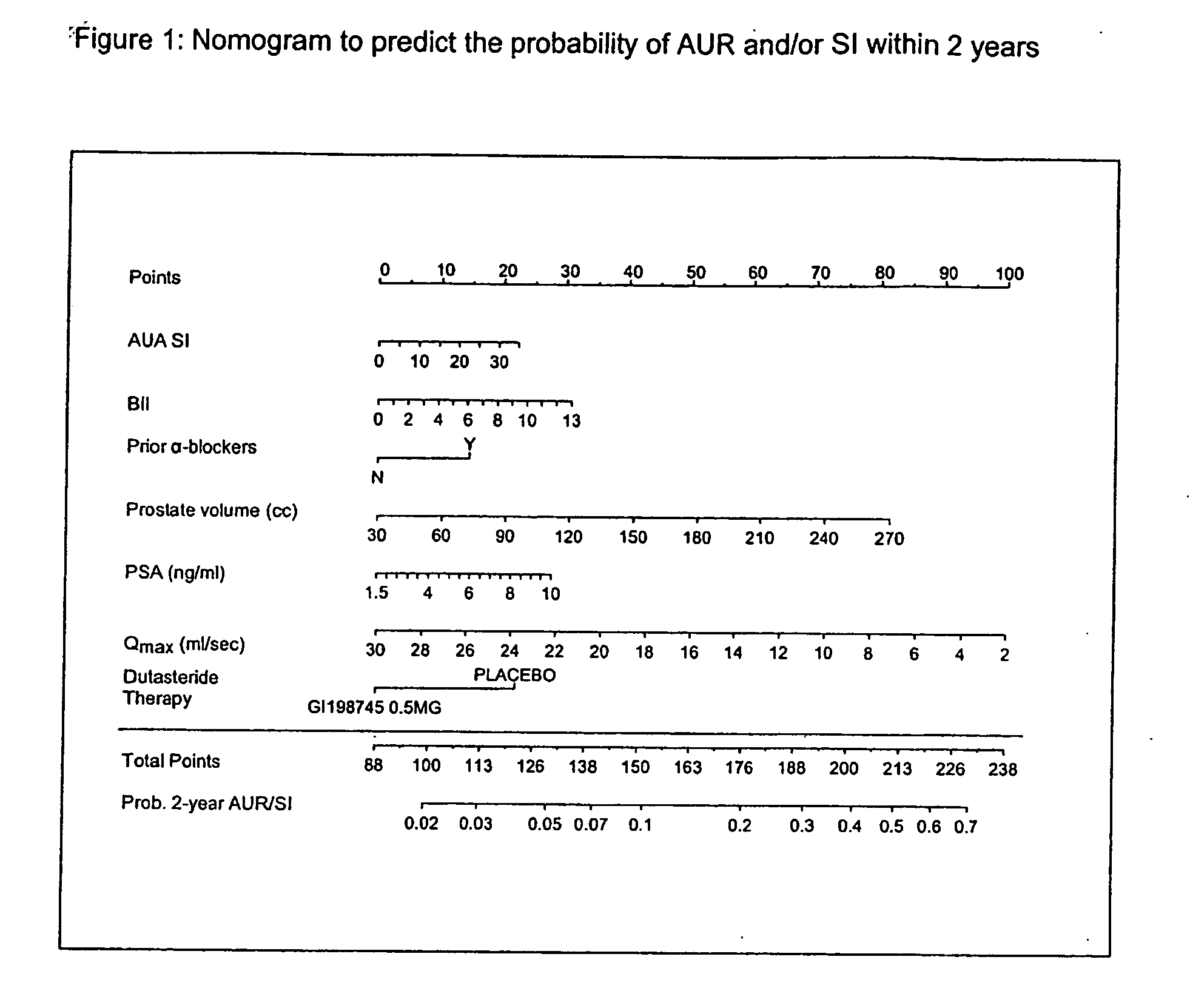
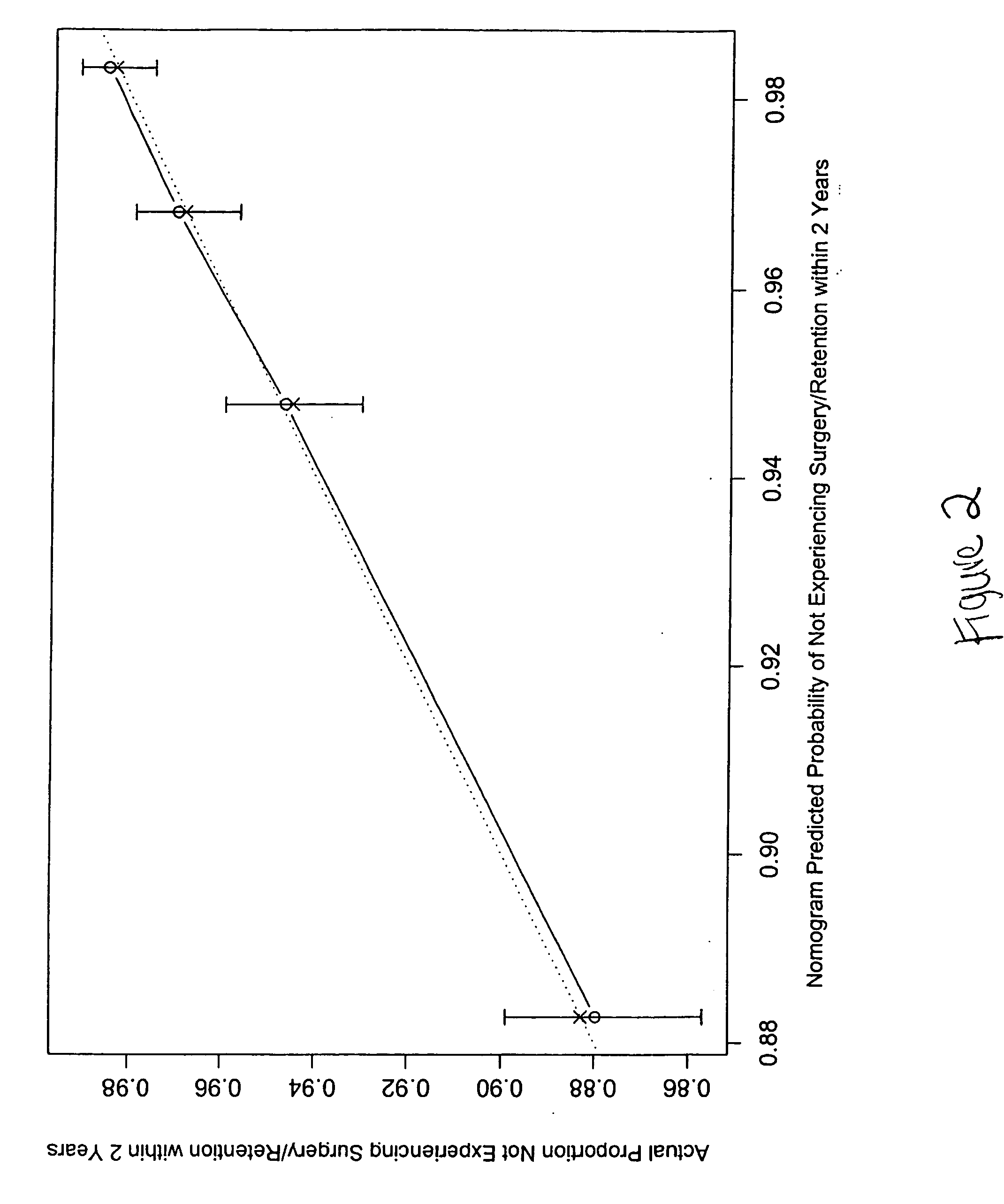
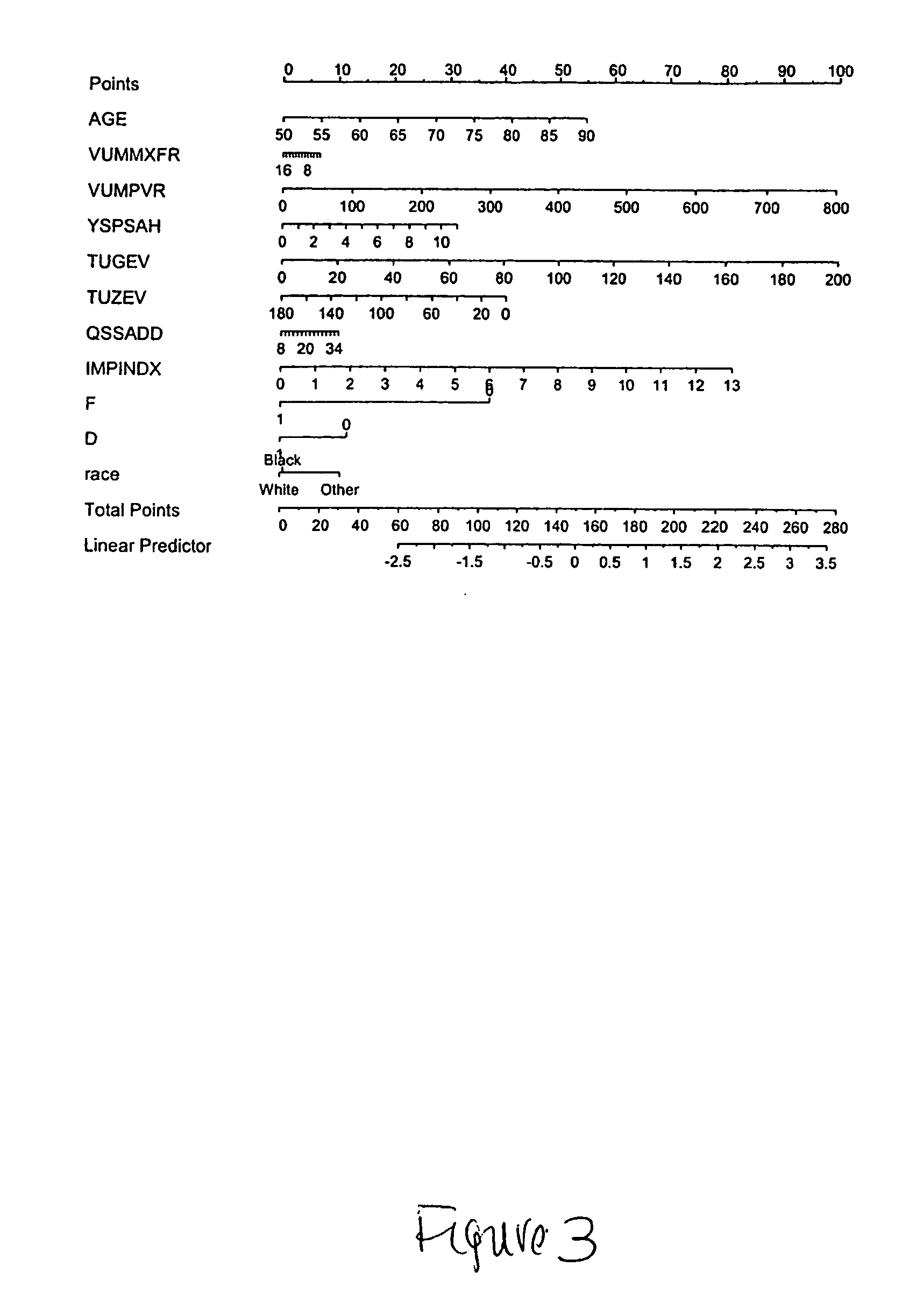
Method to predict risk of bph progression
InactiveUS20070134725A1Health-index calculationDrug and medicationsProstate hyperplasiaAcute urine retention
A method to predict benign prostatic hyperplasia symptom progression, acute urinary retention, need for surgical intervention and / or prostate cancer development in patients is provided.
Owner:BAYLOR COLLEGE OF MEDICINE +1
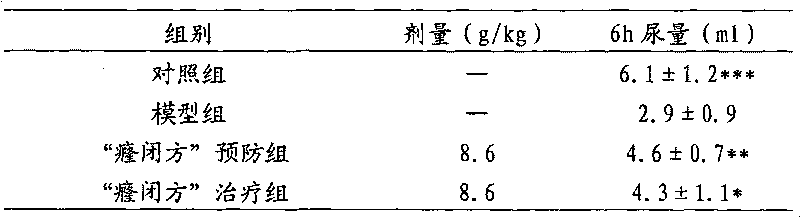
Traditional Chinese medicinal composition for curing hyperuricacidemia, gout and urinary retention
InactiveCN102579658AGood effectAbundant resourcesSkeletal disorderBlood disorderFormularySide effect
The invention relates to a traditional Chinese medicinal composition for curing hyperuricacidemia, gout and urinary retention. The traditional Chinese medicinal composition comprises fruit of austral akebia, Digua fig stem, China fir and weeping willow, and specific ingredients and dosage of a prescription are modified according to symptoms of patients. The composition is capable of fully utilizing traditional Chinese medicinal resources, reducing cost, improving curative effects, clearing heat, promoting diuresis, promoting blood circulation to arrest pain, promoting urination, obviously reducing blood uric acid levels of patients to the range of normal values, and markedly eliminating gout symptoms in different parts of patient bodies. Besides, the traditional Chinese medicinal composition can be taken for a long term without any toxic and side effects and has a certain preventive effect on the hyperuricacidemia and the gout.
Owner:刘继勇 +2
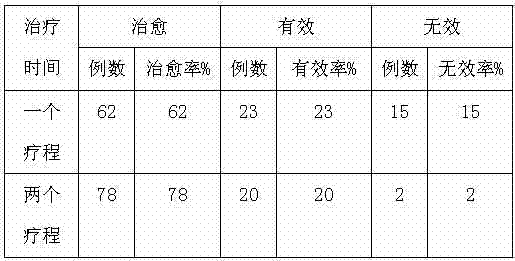
Feed for treating urinary retention of sow and preparation method of feed
ActiveCN103750024AImprove disease resistanceHigh cure rateOrganic active ingredientsPeptide/protein ingredientsDiseaseVitamin C
The invention discloses a feed for treating the urinary retention of a sow and a preparation method of the feed. The feed comprises the following raw materials: corn, millet, soybean meal, peanut meal, fish meal, wax gourd powder, calcium hydrophosphate, plasma protein powder, vitamin C, vitamin D, salt and traditional Chinese medicine additives. The feed and the preparation method have the beneficial effects that the feed contains the traditional Chinese medicine additives for treating the urinary retention of the sow; according to the main treatment principles of clearing away heat, eliminating dampness, softening hardness, dissipating stagnation, tonifying Qi and nourishing kidney, the traditional Chinese medicine additives and other feed raw materials are matched with each other and co-act; the feed has the advantages of high cure rate, quick efficiency, no medicine residue and low cost for treating the urinary retention of the sow, and is capable of improving the self disease resistance of an organism.
Owner:SHANDONG NEW HOPE LIUHE GROUP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com