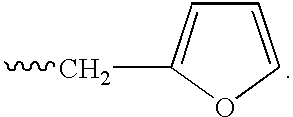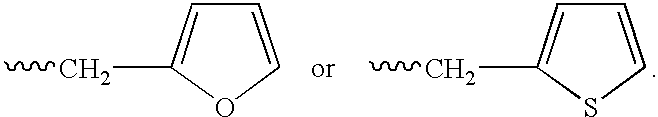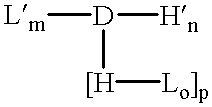Patents
Literature
41647results about "Extracellular fluid disorder" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
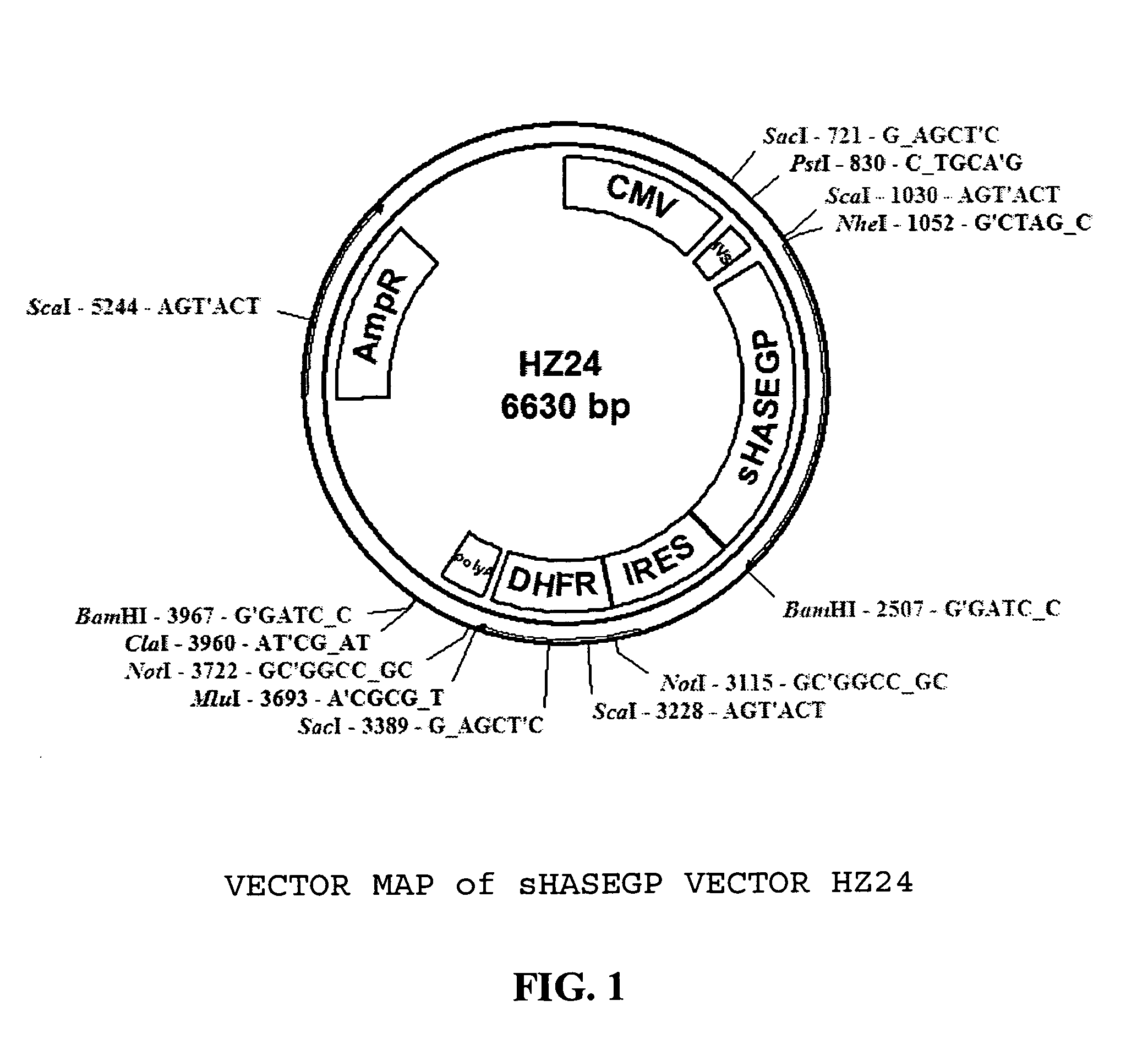
Soluble glycosaminoglycanases and methods of preparing and using soluble glycosaminoglycanases
ActiveUS20050260186A1Improve extentIncrease ratingsAntibacterial agentsSenses disorderHyaluronidasePathology diagnosis
The invention relates to the discovery of novel soluble neutral active Hyaluronidase Glycoproteins (sHASEGPs), methods of manufacture, and their use to facilitate administration of other molecules or to alleviate glycosaminoglycan associated pathologies. Minimally active polypeptide domains of the soluble, neutral active sHASEGP domains are described that include asparagine-linked sugar moieties required for a functional neutral active hyaluronidase domain. Included are modified amino-terminal leader peptides that enhance secretion of sHASEGP. The invention further comprises sialated and pegylated forms of a recombinant sHASEGP to enhance stability and serum pharmacokinetics over naturally occurring slaughterhouse enzymes. Further described are suitable formulations of a substantially purified recombinant sHASEGP glycoprotein derived from a eukaryotic cell that generate the proper glycosylation required for its optimal activity.
Owner:HALOZYME
Drug releasing anastomosis devices and methods for treating anastomotic sites
ActiveUS7108701B2Reduce drug toxicityGood curative effectSuture equipmentsSurgical needlesBiological bodyReady to use
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned.
Owner:WYETH
Nanostructure-enhanced platelet binding and hemostatic structures
InactiveUS8319002B2Enhancing overall rate and strengthInduce platelet binding and efficient hemostasisBiocideSurgical adhesivesPlateletNanofiber
Methods, systems, and apparatuses for nanomaterial-enhanced platelet binding and hemostatic medical devices are provided. Hemostatic materials and structures are provided that induce platelet binding, including platelet binding and the coagulation of blood at a wound / opening caused by trauma, a surgical procedure, ulceration, or other cause. Example embodiments include platelet binding devices, hemostatic bandages, hemostatic plugs, and hemostatic formulations. The hemostatic materials and structures may incorporate nanostructures and / or further hemostatic elements such as polymers, silicon nanofibers, silicon dioxide nanofibers, and / or glass beads into a highly absorbent, gelling scaffold. The hemostatic materials and structures may be resorbable.
Owner:NANOSYS INC
Plasma protein matrices and methods for their preparation
InactiveUS7009039B2Rapid cell growthRapid vascularizationBiocidePeptide/protein ingredientsBiological propertyFreeze-drying
A freeze dried biocompatible matrix comprising plasma proteins, useful as implants for tissue engineering as well as in biotechnology, and methods of producing the matrix are provided. Mechanical and physical parameters can be controlled by use of auxiliary components or additives which may be removed after the matrix is formed in order to improve the biological properties of the matrix. The matrices according to the present invention may be used clinically per se, or as a cell-bearing implant.
Owner:PROCHON BIOTECH
Reinforced foam implants with enhanced integrity for soft tissue repair and regeneration
InactiveUS6852330B2Sufficient structural integritySufficient propertyPowder deliveryOrganic active ingredientsTissue repairSoft tissue repair
A biocompatible tissue repair stimulating implant or “scaffold” device, and methods for making and using such a device, are provided. The implant includes one or more layers of a bioabsorbable polymeric foam having pores with an open cell pore structure. A reinforcement component is also present within the implant to contribute enhanced mechanical and handling properties. The implant houses a biological component that may be released to tissue adjacent the location in which the implant is implanted to faciliate and / or expedite the healing of tissue. This biological component resides primarily within the foam component of the implant, being incorporated within pores formed within the foam.
Owner:DEPUY SYNTHES PROD INC
Hemostatic fibrous material
InactiveUS20120004636A1Promote blood clottingPromoting blood clottingBiocideDiagnosticsFiberMolecular materials
Owner:TELEFLEX LIFE SCI LTD
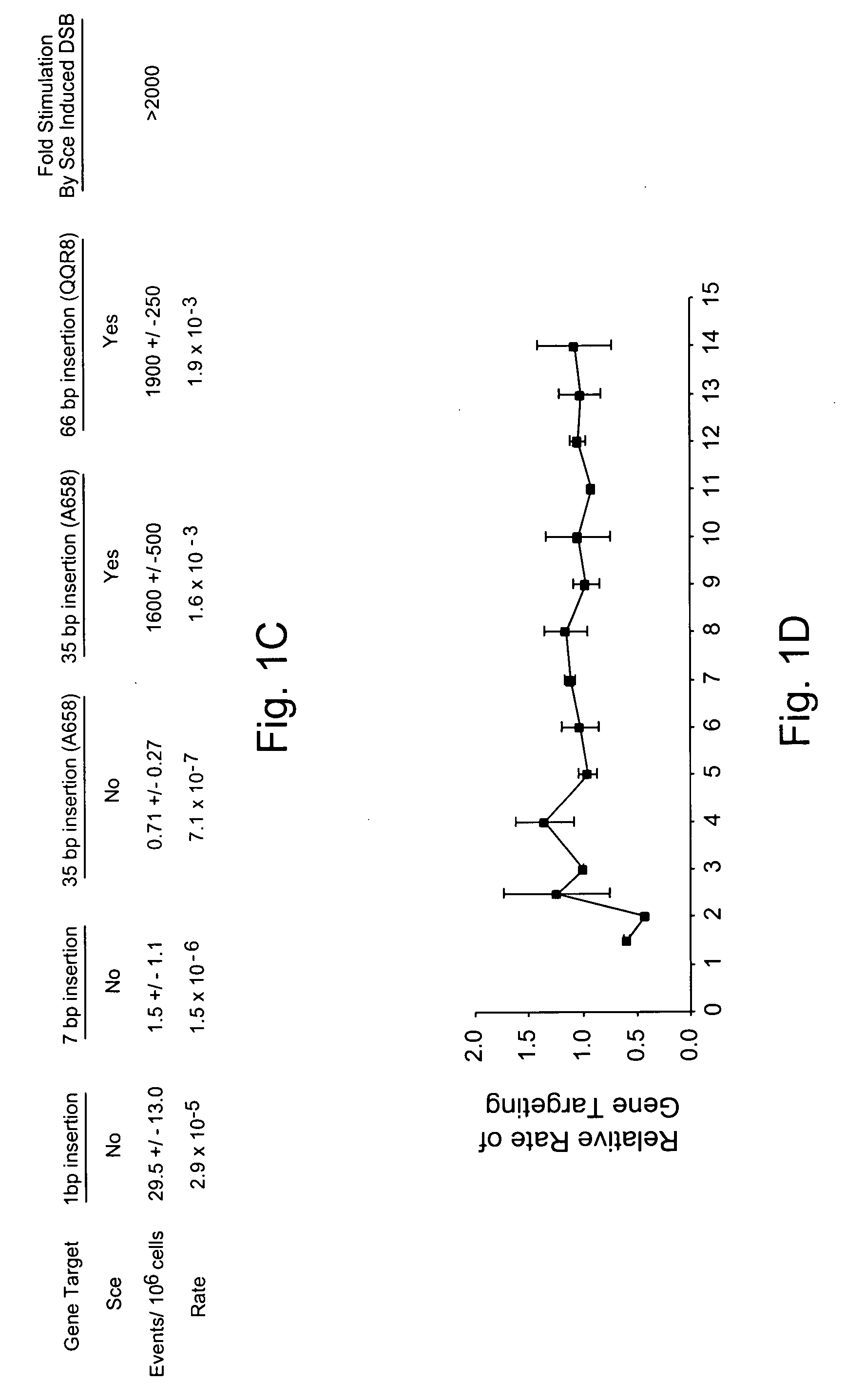
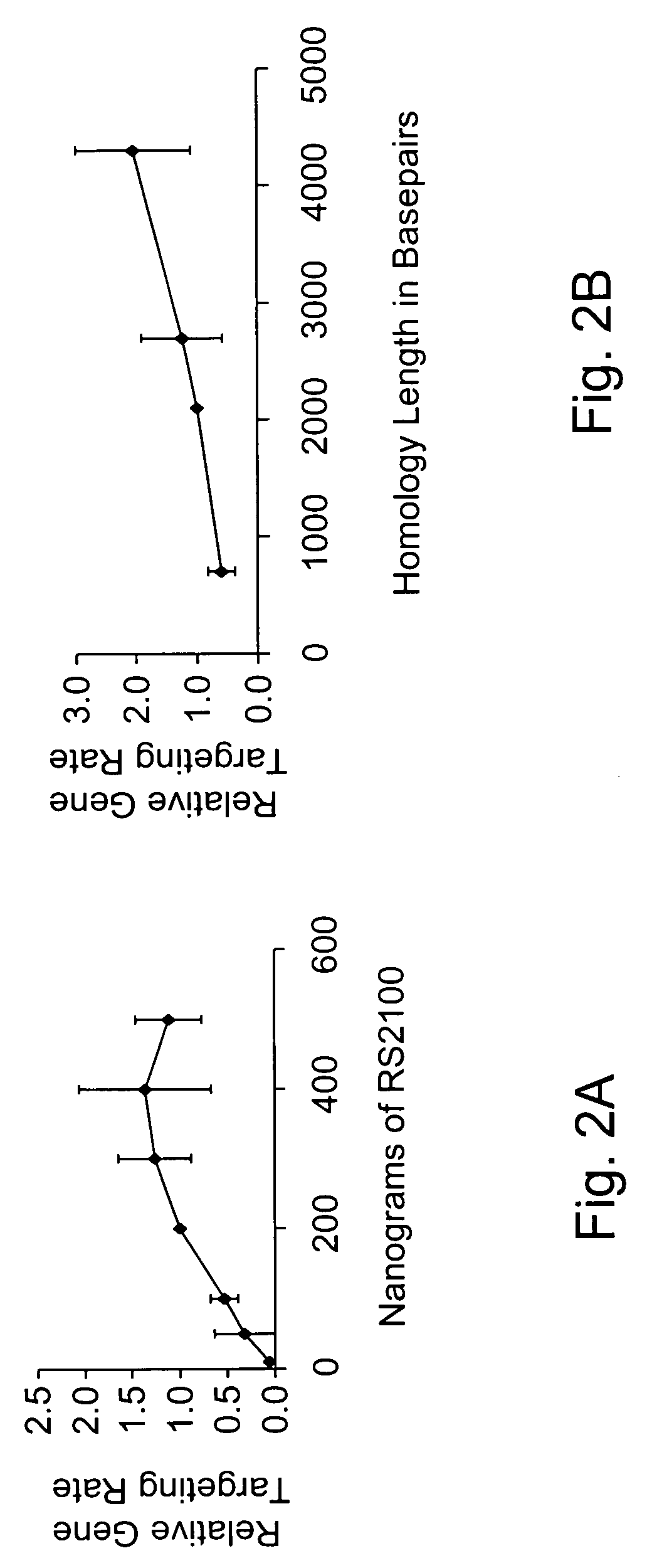
Use of chimeric nucleases to stimulate gene targeting
ActiveUS20050026157A1Ameliorate genetic disorderIncrease productionAntibacterial agentsFusion with DNA-binding domainGene targetsGenetic Change
Gene targeting is a technique to introduce genetic change into one or more specific locations in the genome of a cell. For example, gene targeting can introduce genetic change by modifying, repairing, attenuating or inactivating a target gene or other chromosomal DNA. In one aspect, this disclosure relates to methods and compositions for gene targeting with high efficiency in a cell. This disclosure also relates to methods of treating or preventing a genetic disease in an individual in need thereof. Further disclosed are chimeric nucleases and vectors encoding chimeric nucleases.
Owner:CALIFORNIA INST OF TECH
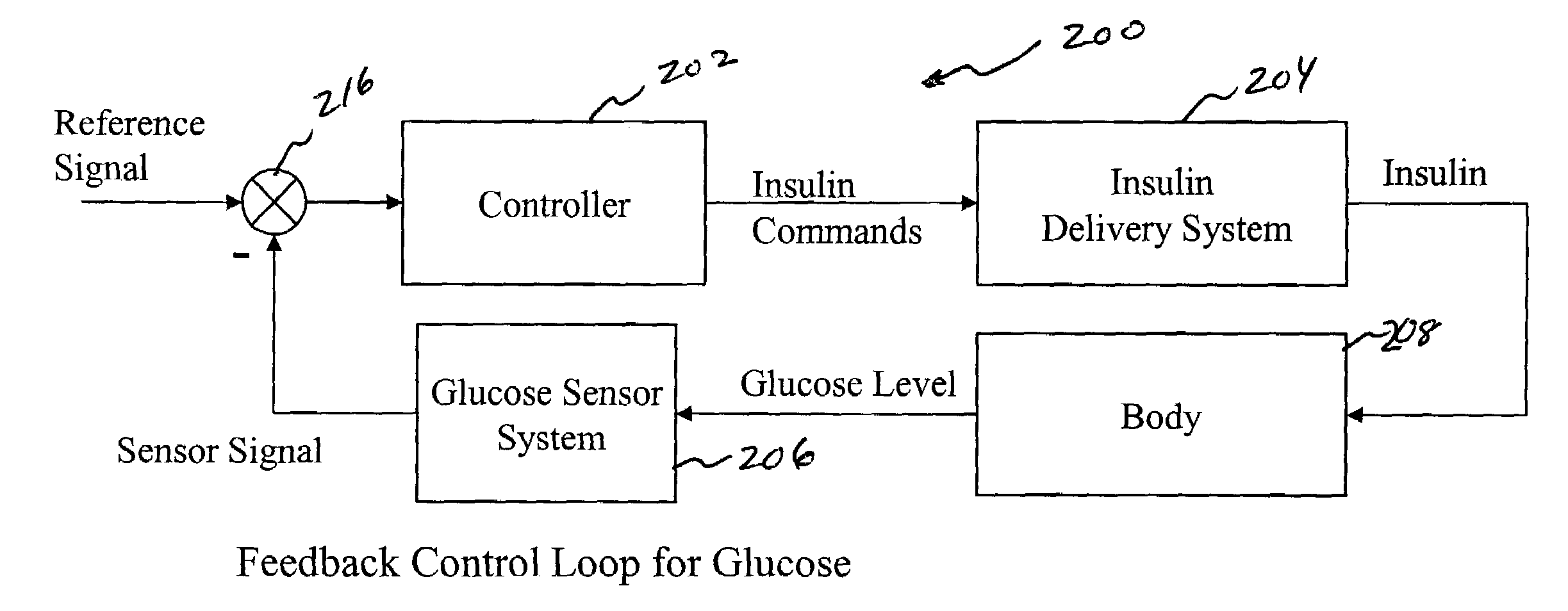
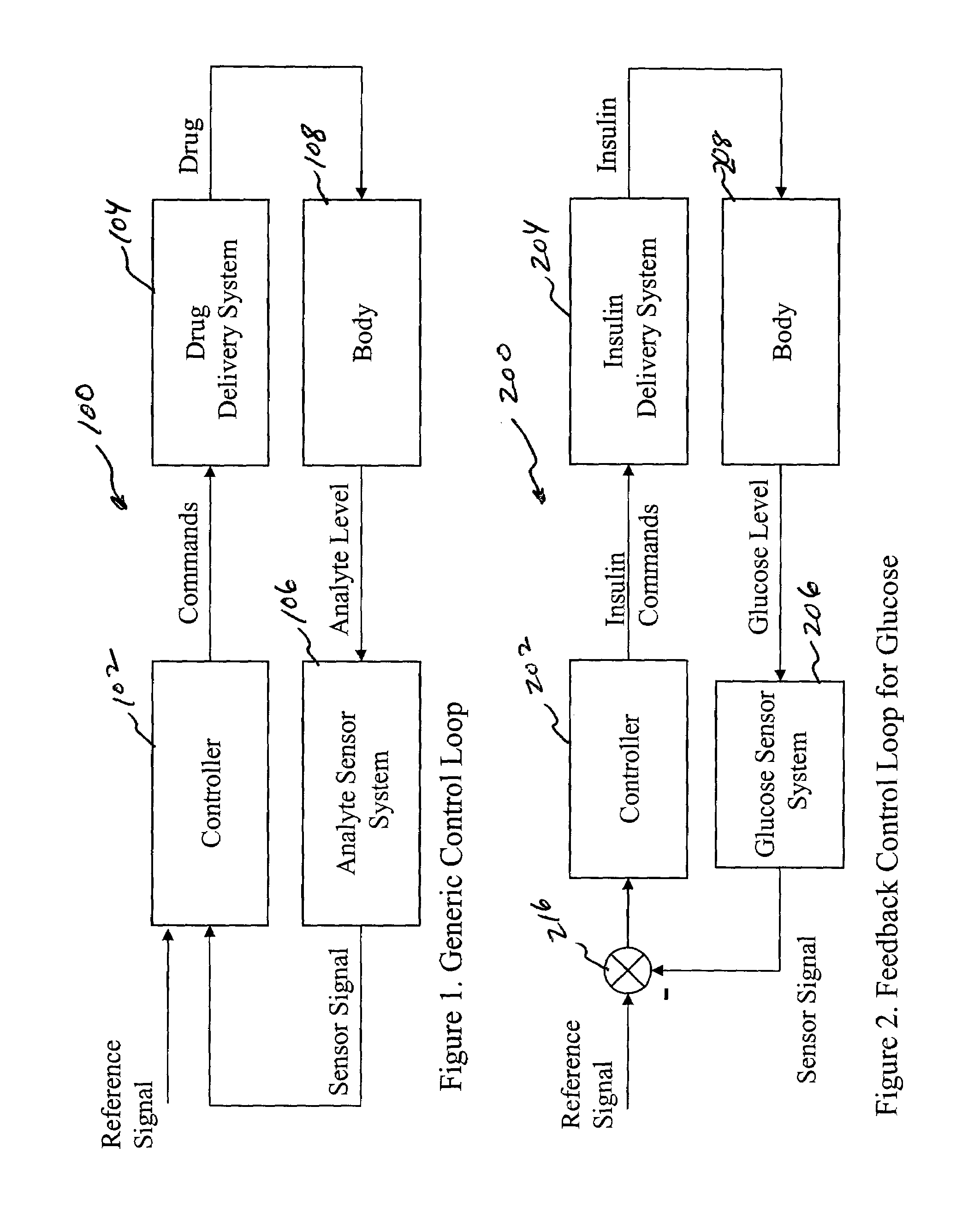
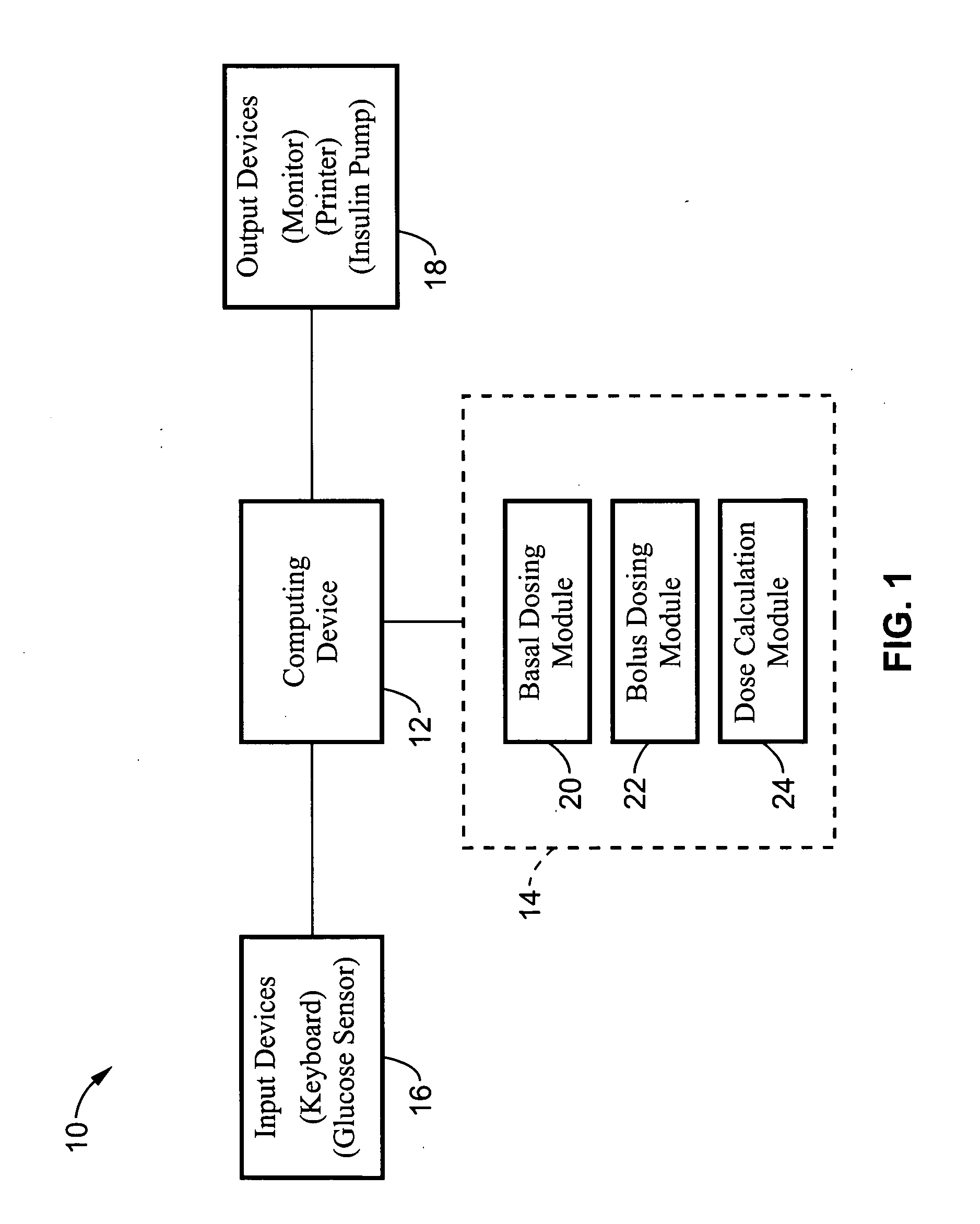
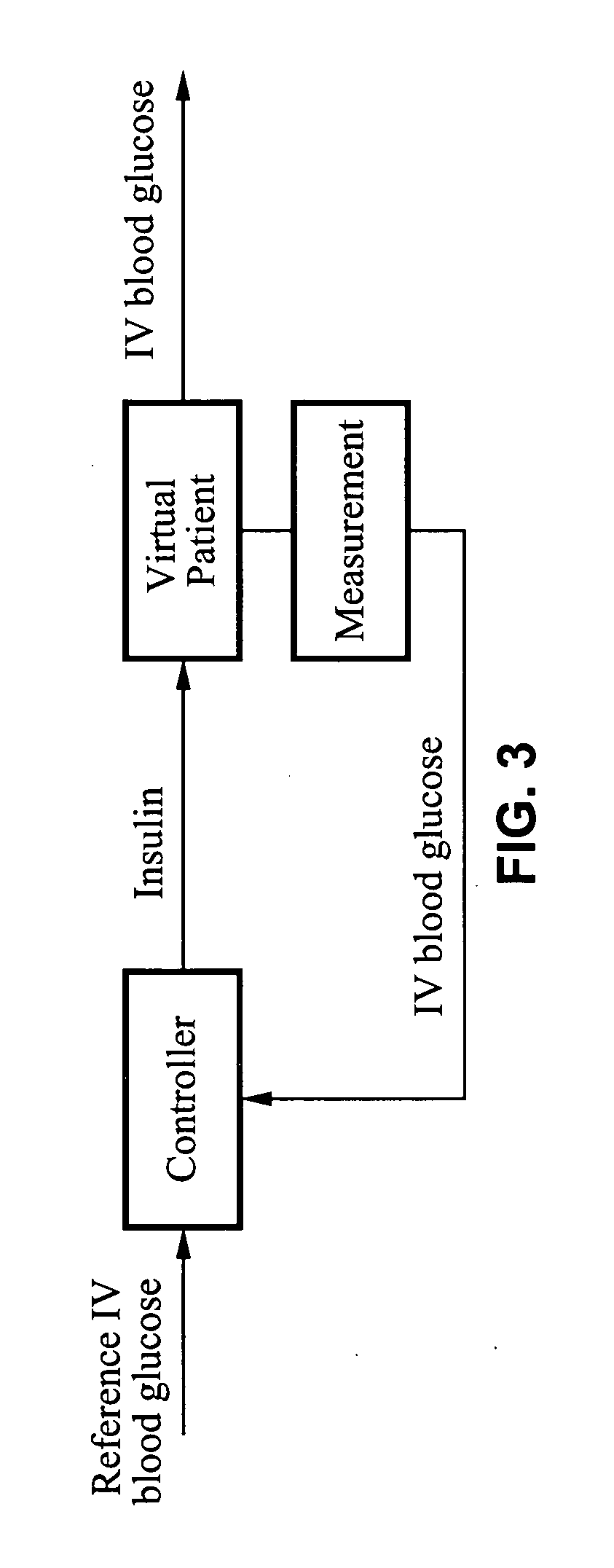
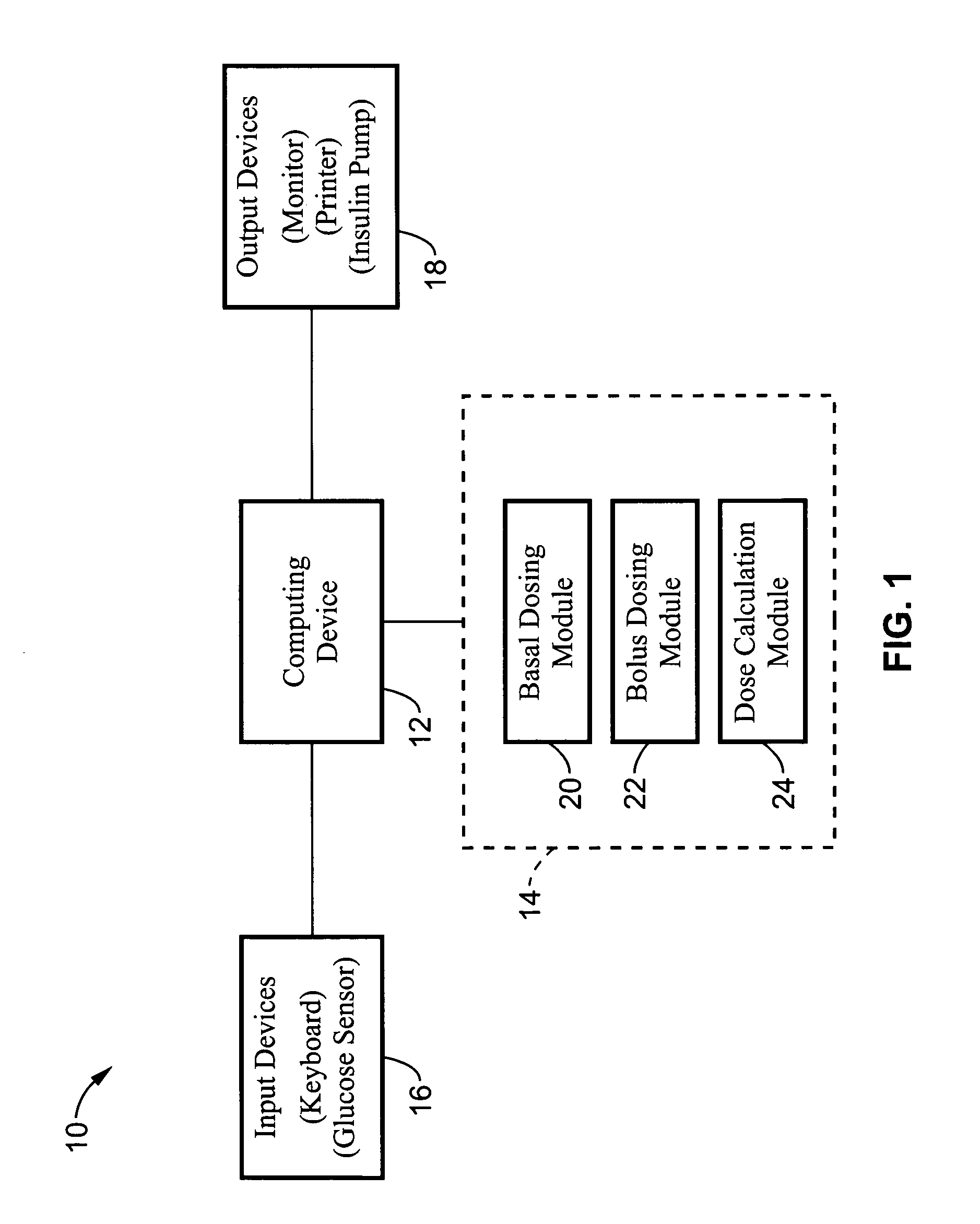
System and method for initiating and maintaining continuous, long-term control of a concentration of a substance in a patient using a feedback or model-based controller coupled to a single-needle or multi-needle intradermal (ID) delivery device
ActiveUS7060059B2Maintaining continuous, long-term control of the blood glucose concentrationsImprove performancePeptide/protein ingredientsDrug and medicationsInsulin infusionClosed loop
A closed loop therapy system for controlling a concentration of a substance, such as blood glucose concentration, in the body of a user. The system and method employ a sensor system that measures a glucose level in the body, a controller that uses the measured glucose levels to generate an output that can be used to automatically or manually control an intradermal insulin infusion system to set a constant or time-varying profile of target blood glucose concentrations in a user, and then infuse an appropriate amount of insulin into the body of the user so as to reach and maintain the target values of the blood glucose concentration.
Owner:BECTON DICKINSON & CO
Anti-PD-L1 antibodies and uses therefor
The present invention is based, in part, on the identification of novel human anti-PD-1, PD-L1, and PD-L2 antibodies. Accordingly, the invention relates to compositions and methods for diagnosing, prognosing, and treating conditions that would benefit from modulating PD-1, PD-L1, and / or PD-L2 activity (e.g., persistent infectious diseases, autoimmune diseases, asthma, transplant rejection, inflammatory disorders and tumors) using the novel human anti-PD-1, PD-L1, and PD-L2 antibodies described herein.
Owner:DANA FARBER CANCER INST INC +2
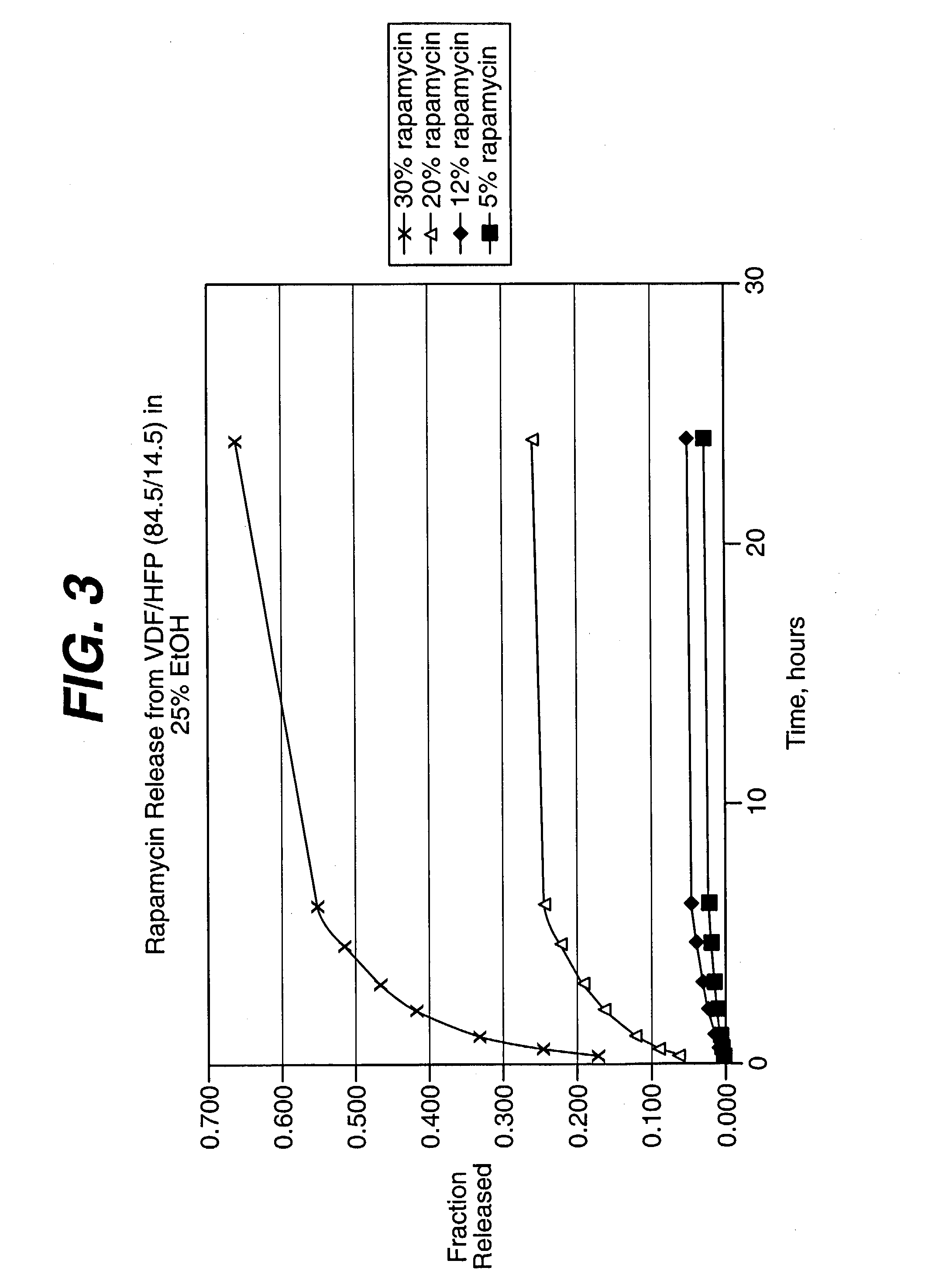
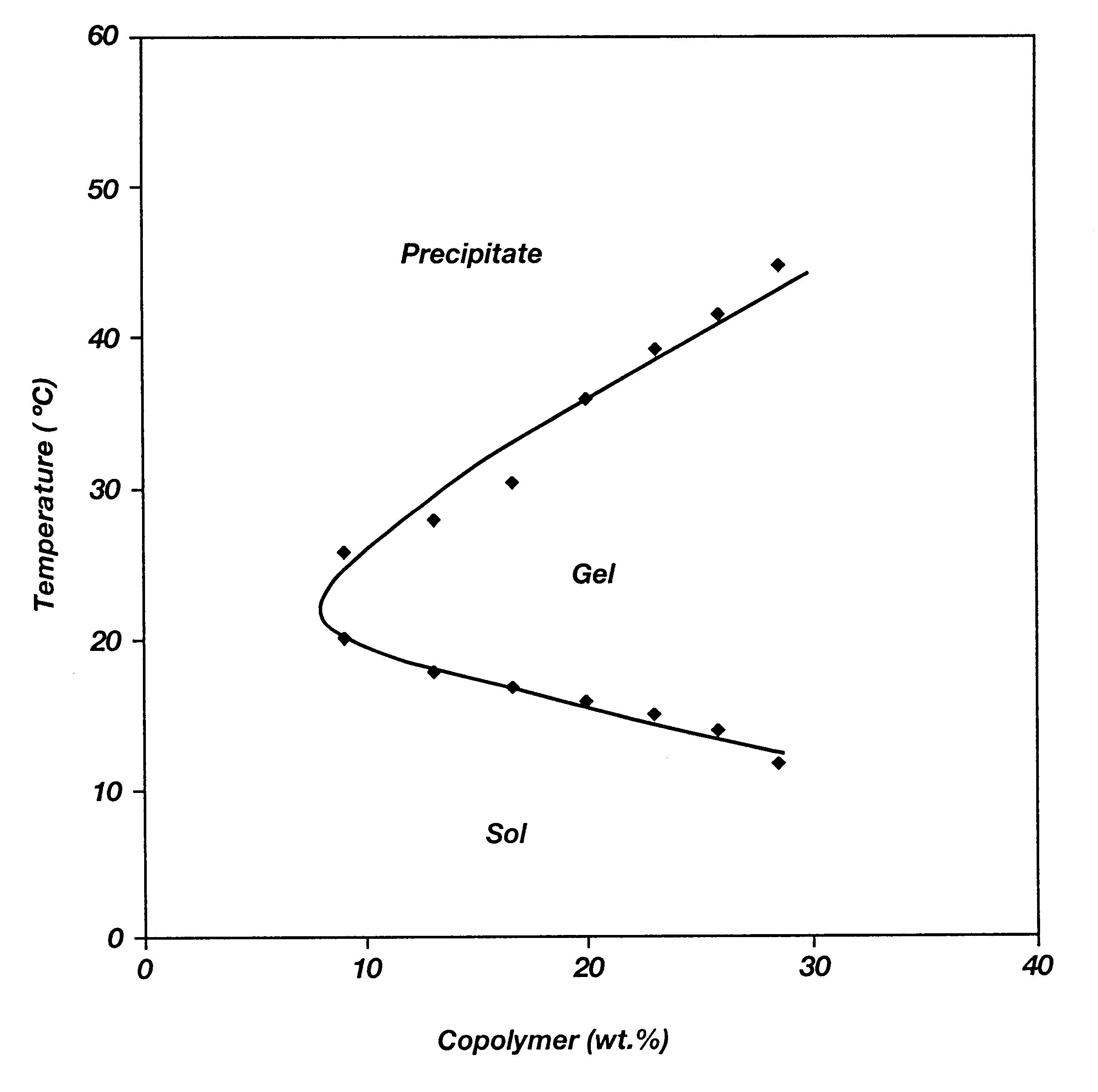
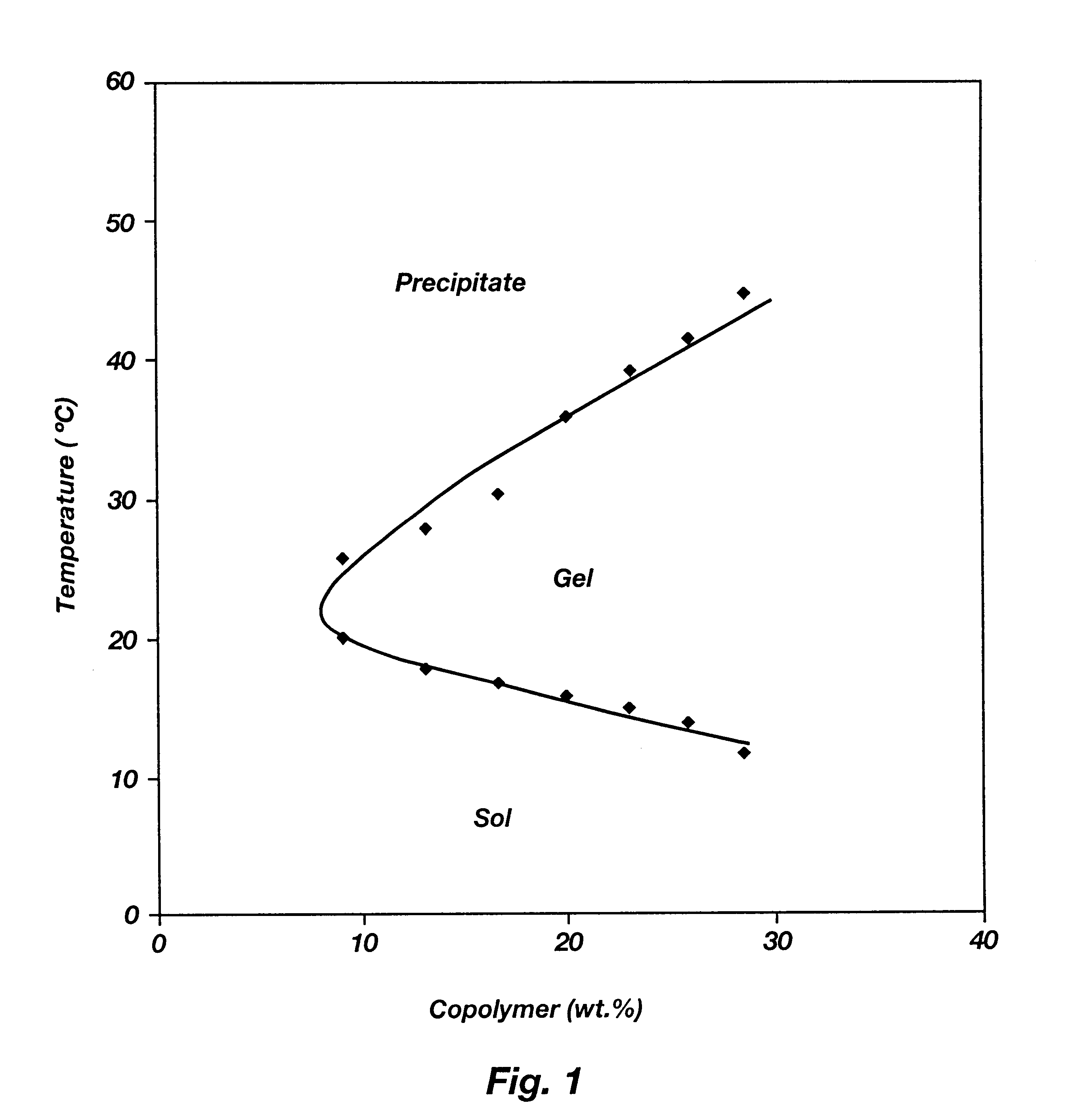
Biodegradable low molecular weight triblock poly(lactide-co- glycolide) polyethylene glycol copolymers having reverse thermal gelation properties
InactiveUS6201072B1Difficult to formulateDifficult to administerOrganic active ingredientsPowder deliverySolubilityPolymer science
A water soluble, biodegradable ABA- or BAB-type tri-block polymer is disclosed that is made up of a major amount of a hydrophobic A polymer block made of a biodegradable polyester and a minor amount of a hydrophilic polyethylene glycol(PEG) B polymer block, having an overall average molecular weight of between about 2000 and 4990, and that possesses reverse thermal gelation properties. Effective concentrations of the tri-block polymer and a drug may be uniformly contained in an aqueous phase to form a drug delivery composition. At temperatures below the gelation temperature of the tri-block polymer the composition is a liquid and at temperatures at or above the gelation temperature the composition is a gel or semi-solid. The composition may be administered to a warm-blooded animal as a liquid by parenteral, ocular, topical, inhalation, transdermal, vaginal, transurethral, rectal, nasal, oral, pulmonary or aural delivery means and is a gel at body temperature. The composition may also be administered as a gel. The drug is released at a controlled rate from the gel which biodegrades into non-toxic products. The release rate of the drug may be adjusted by changing various parameters such as hydrophobic / hydrophilic component content, polymer concentration, molecular weight and polydispersity of the tri-block polymer. Because the tri-block polymer is amphiphilic, it functions to increase the solubility and / or stability of drugs in the composition.
Owner:KIM PH D SUNG WAN +2
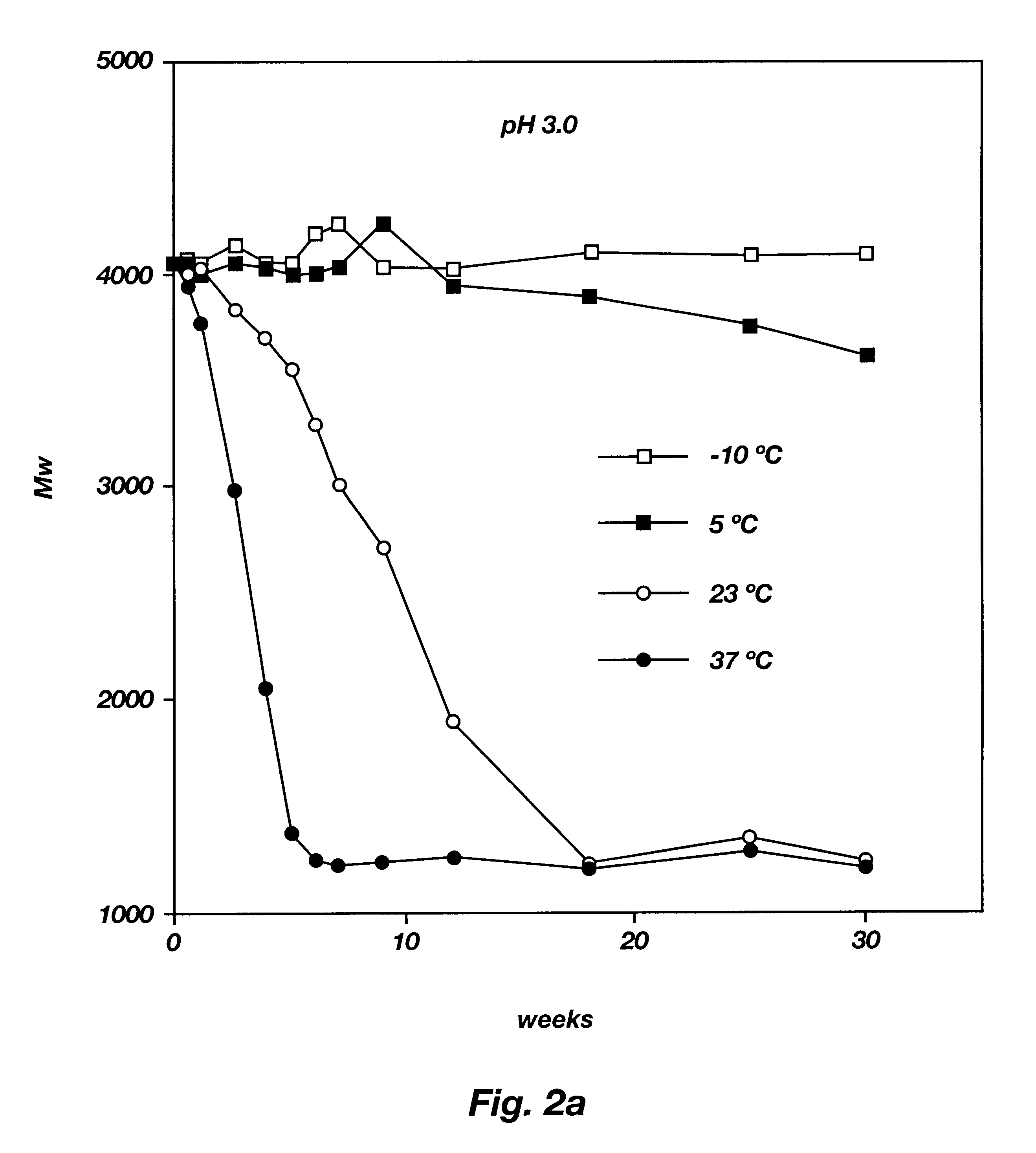
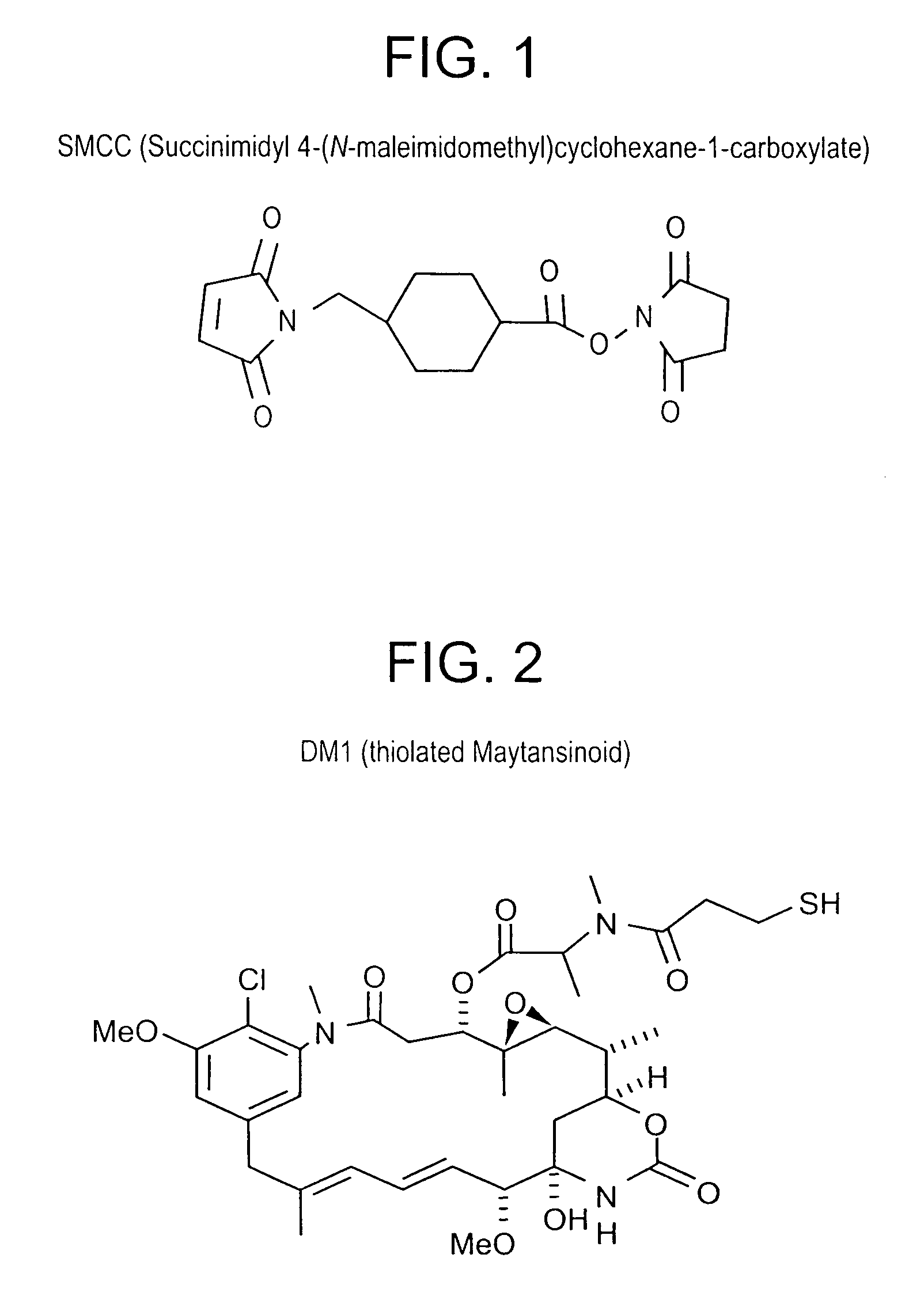
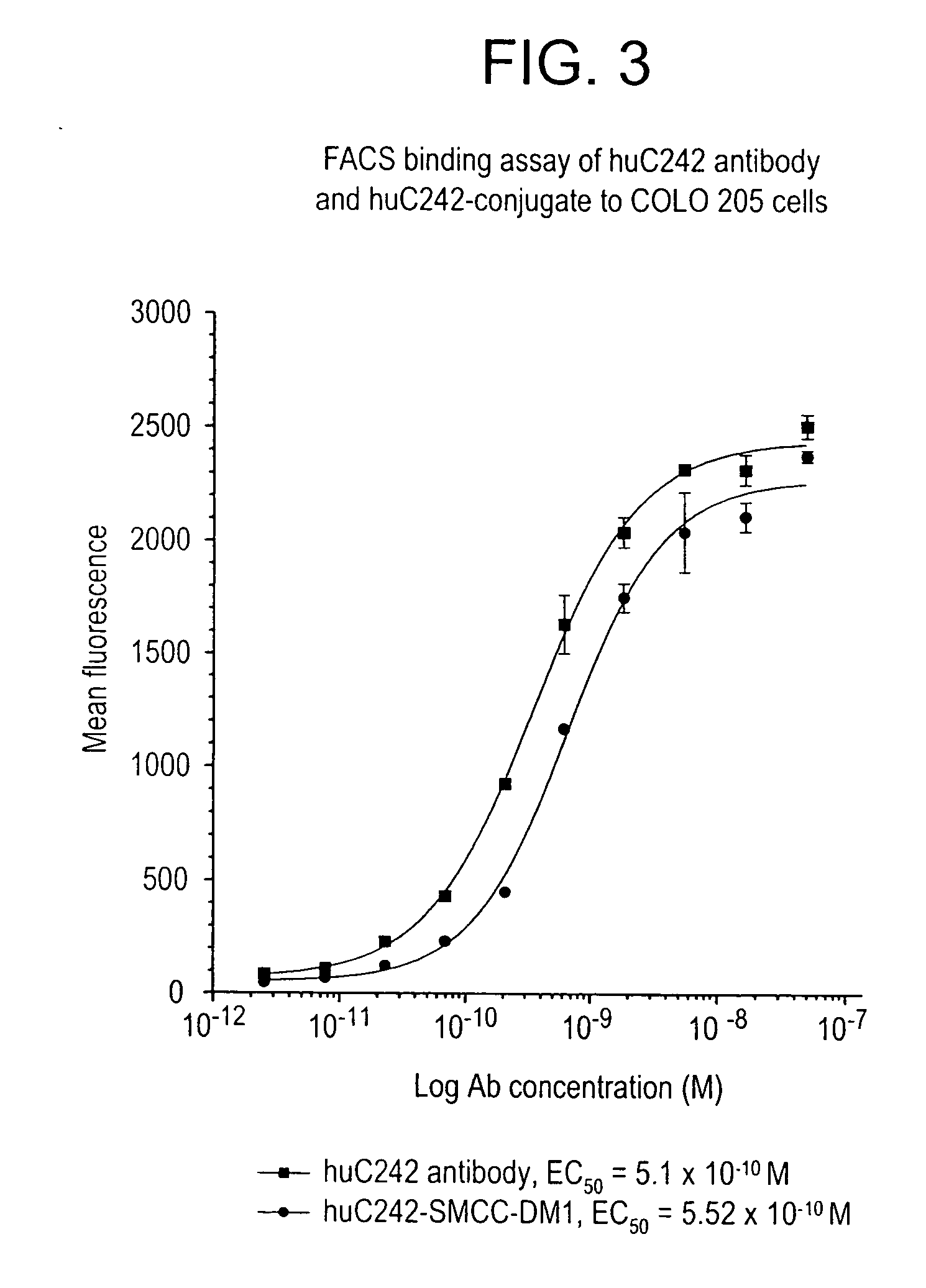
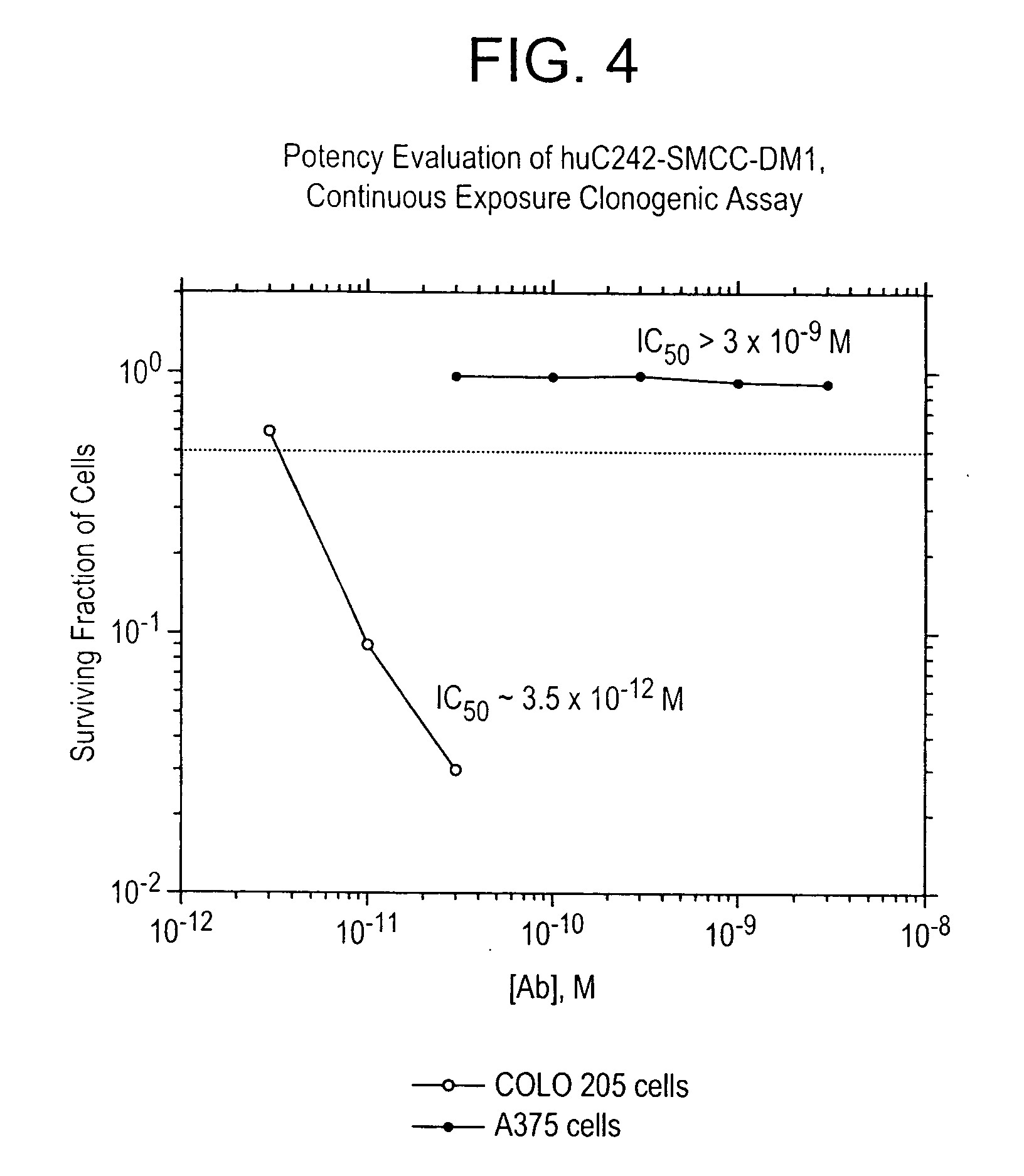
Method of targeting specific cell populations using cell-binding agent maytansinoid conjugates linked via a non-cleavable linker, said conjugates, and methods of making said conjugates
The present invention discloses a method for targeting maytansinoids to a selected cell population, the method comprising contacting a cell population or tissue suspected of containing the selected cell population with a cell-binding agent maytansinoid conjugate, wherein one or more maytansinoids is covalently linked to the cell-binding agent via a non-cleavable linker and the cell-binding agent binds to cells of the selected cell population.
Owner:IMMUNOGEN INC
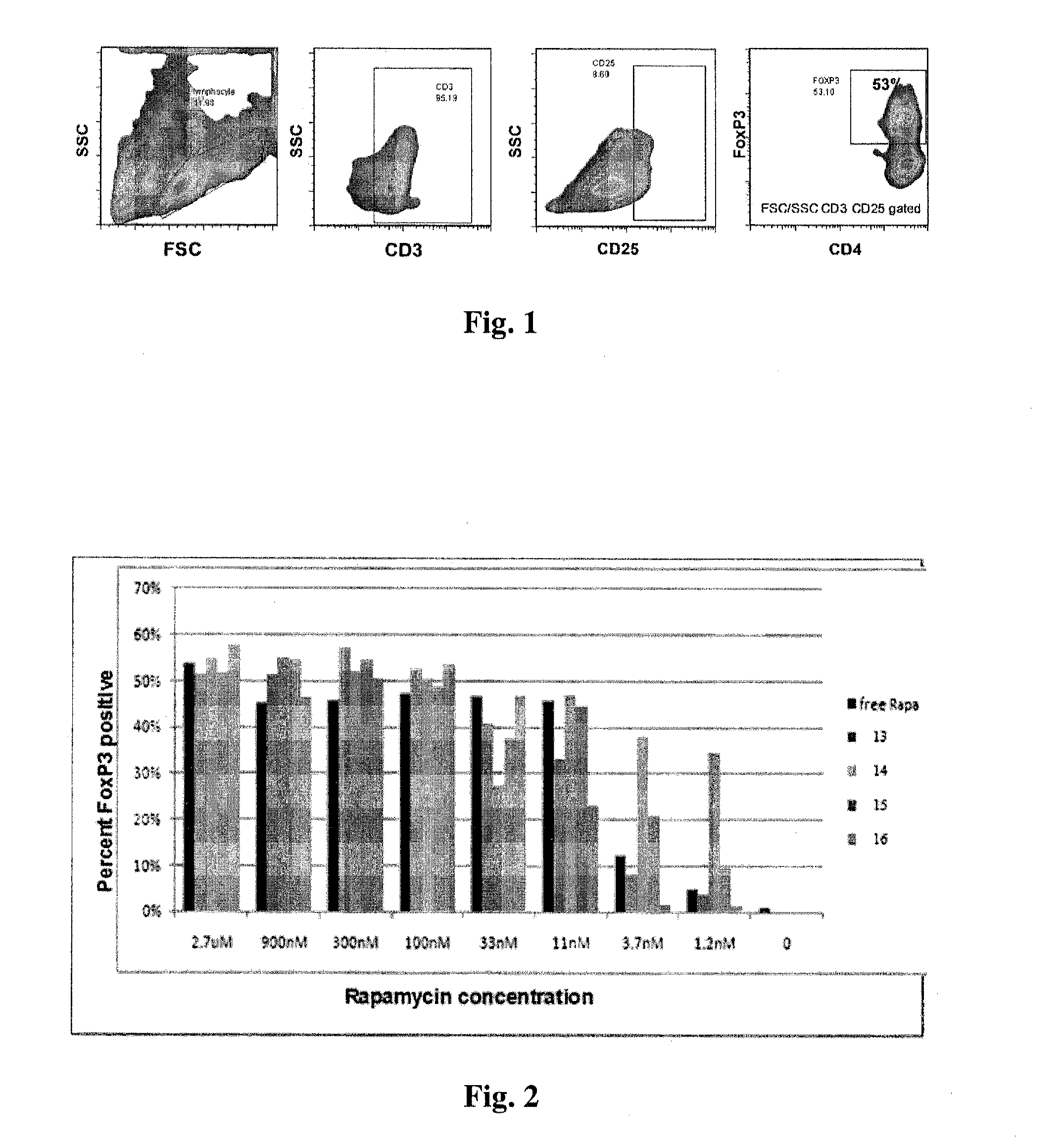
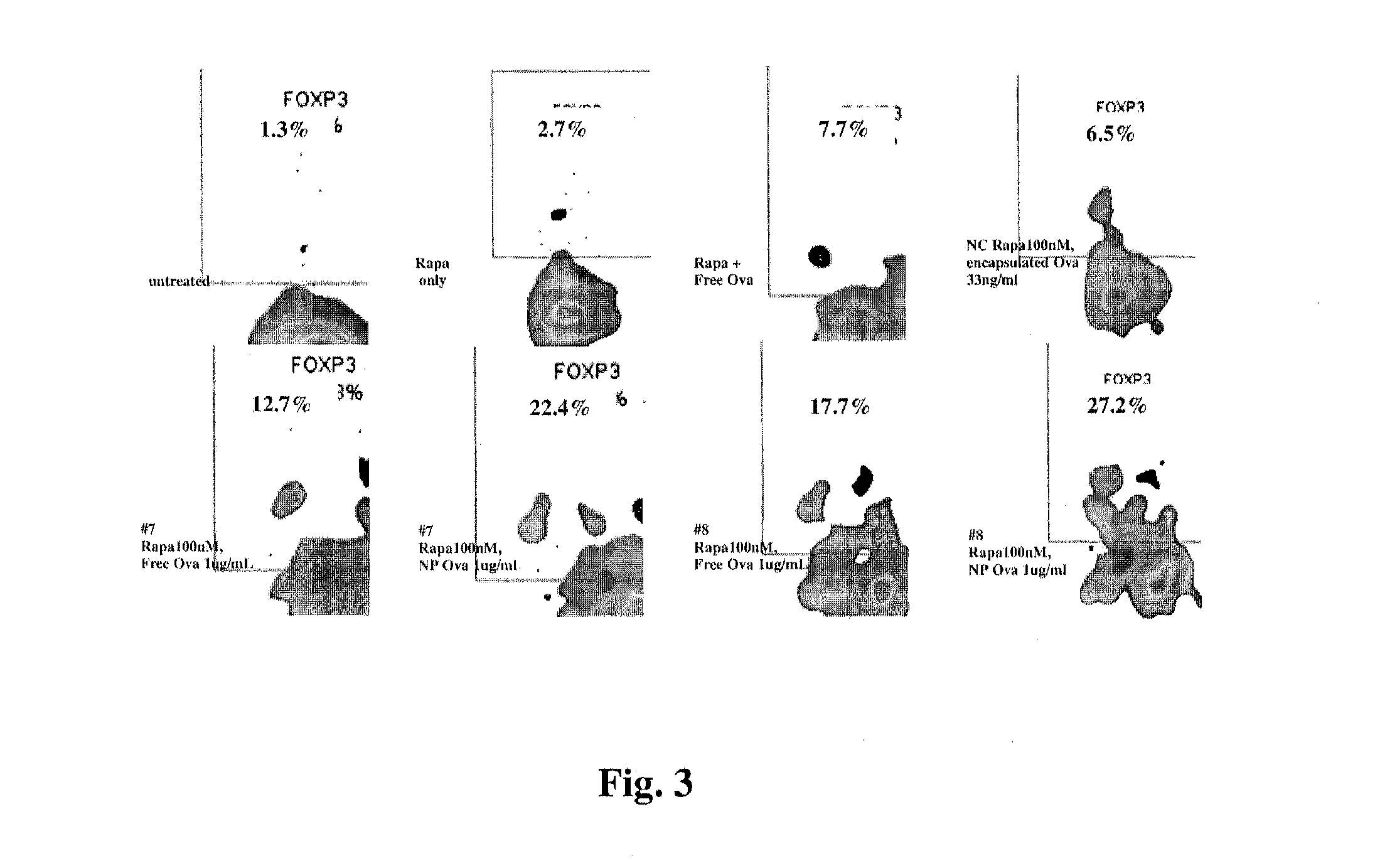
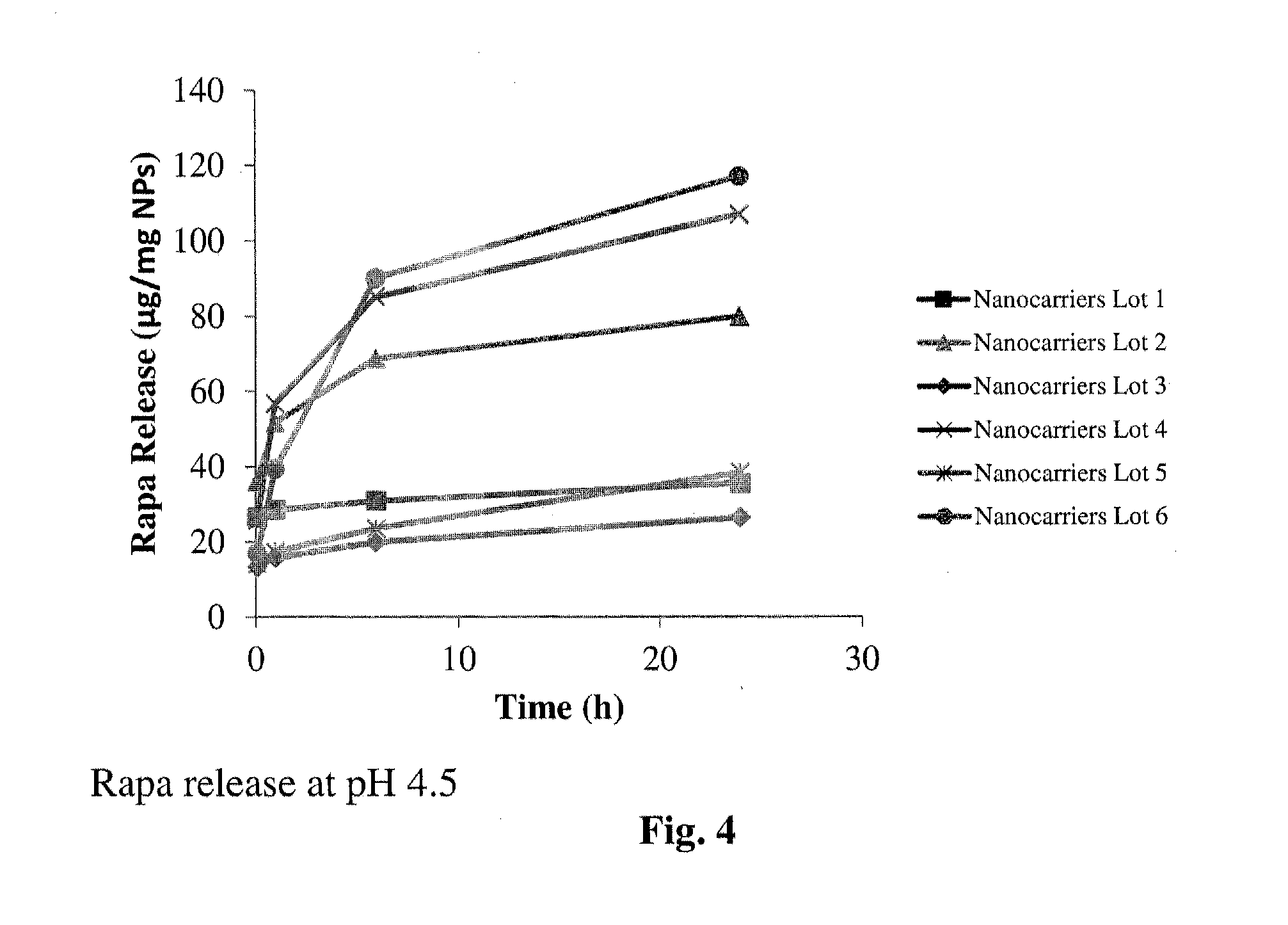
Controlled release of immunosuppressants from synthetic nanocarriers
InactiveUS20120301498A1Reduce in quantityReduce percentagePowder deliveryOrganic active ingredientsControlled releaseAntigen
Disclosed are synthetic nanocarrier compositions that provide controlled release of immunosuppressants as well as related methods. The synthetic nanocarrier compositions may also include antigen in some embodiments.
Owner:SELECTA BIOSCI
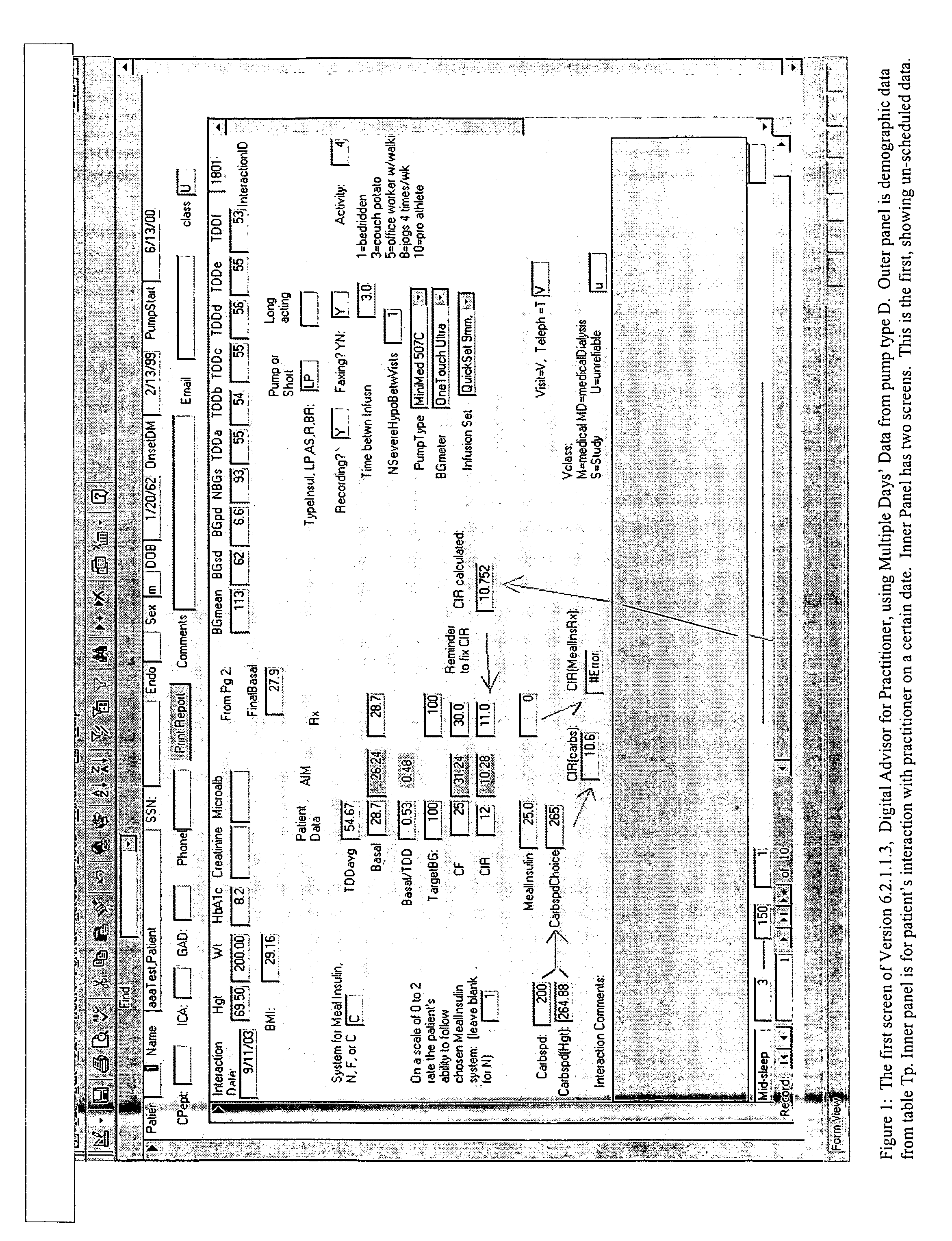
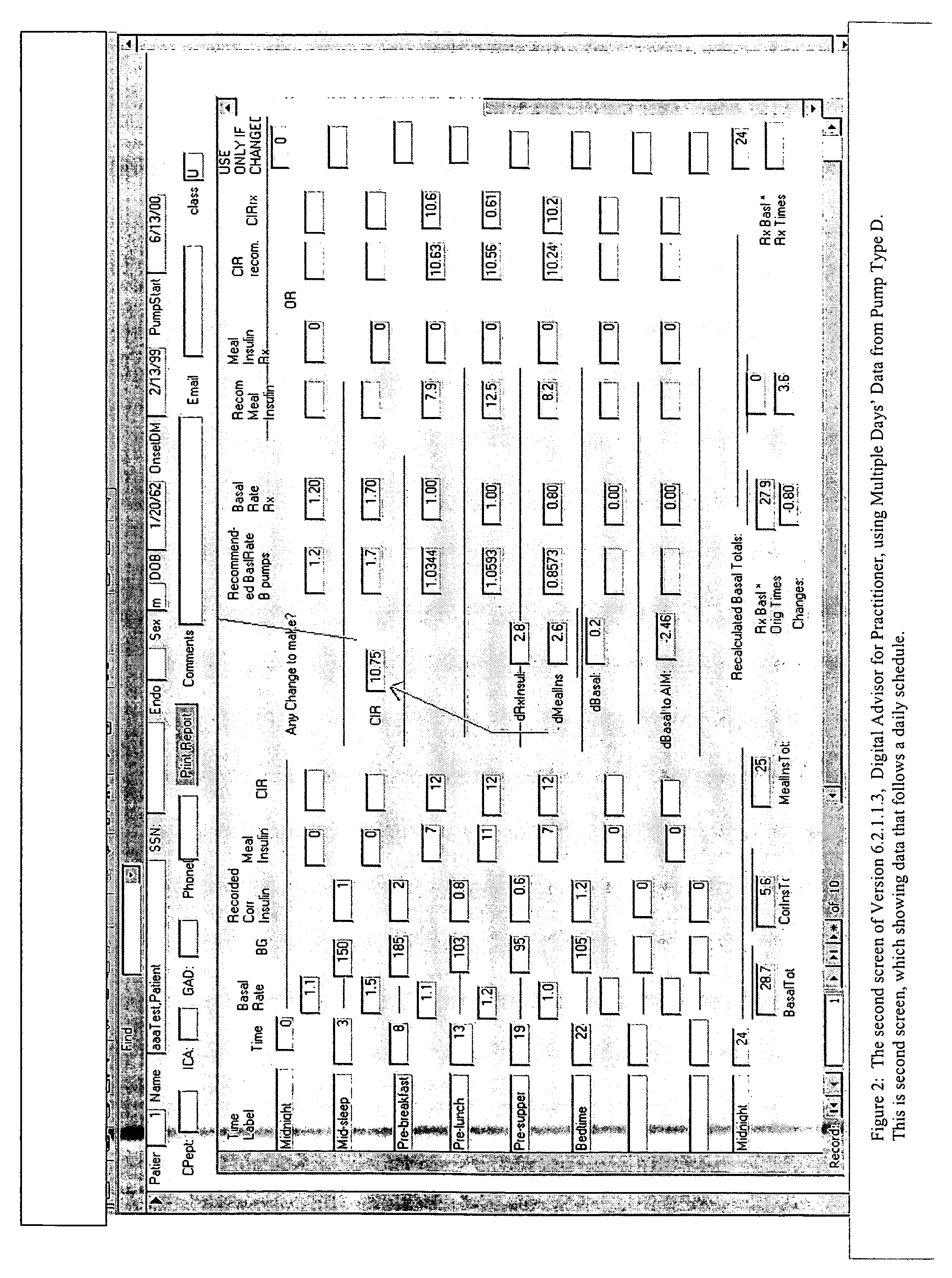
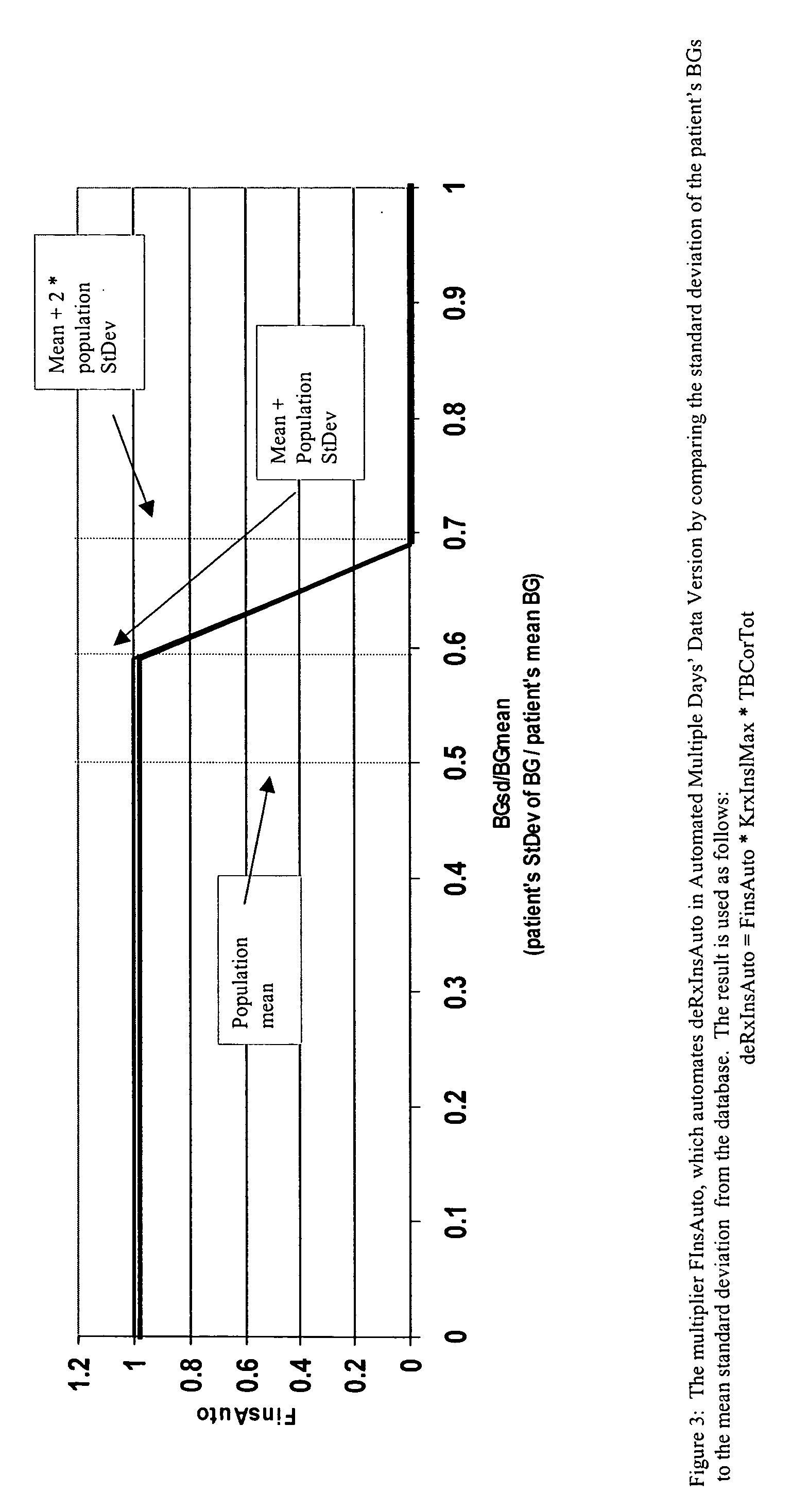
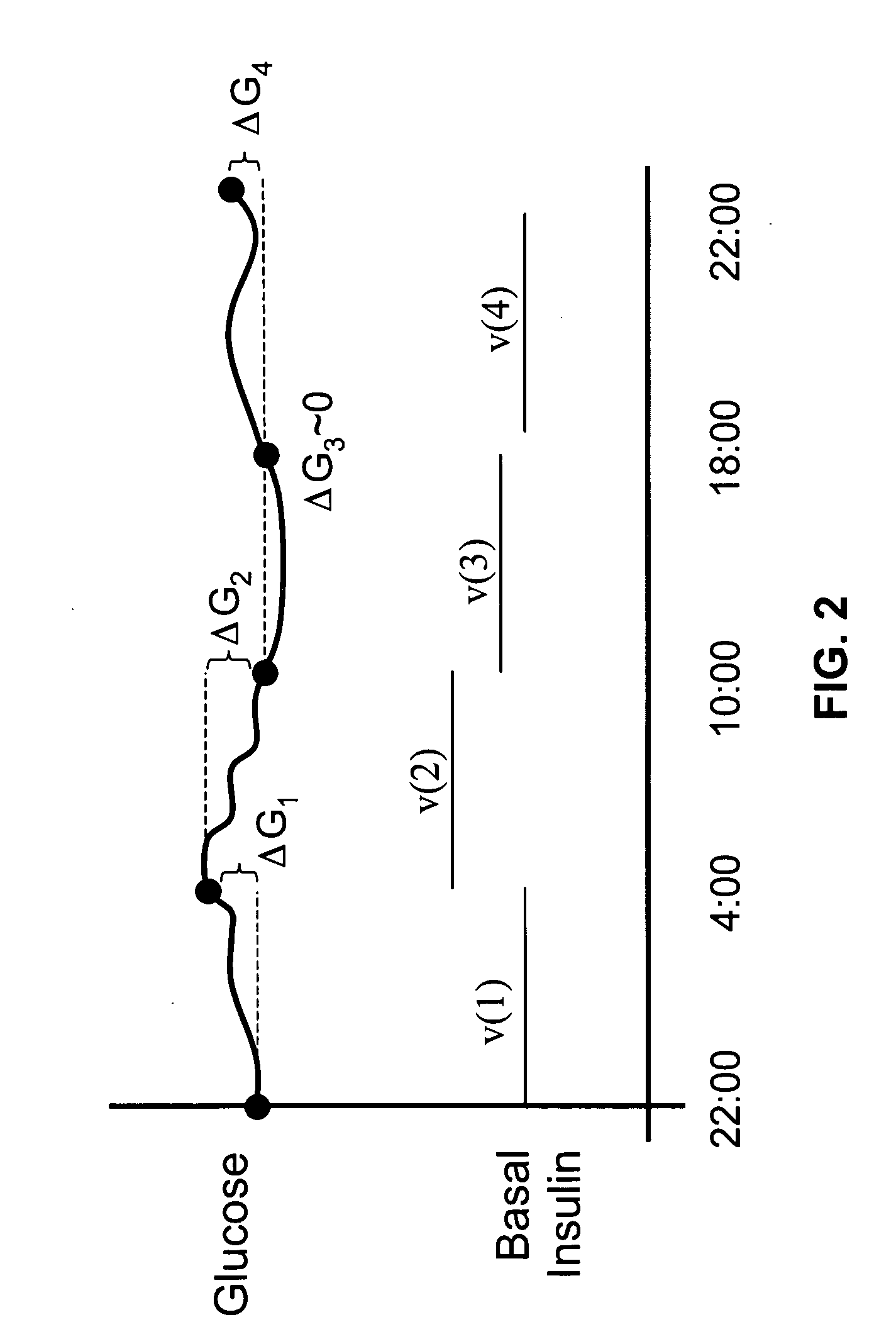
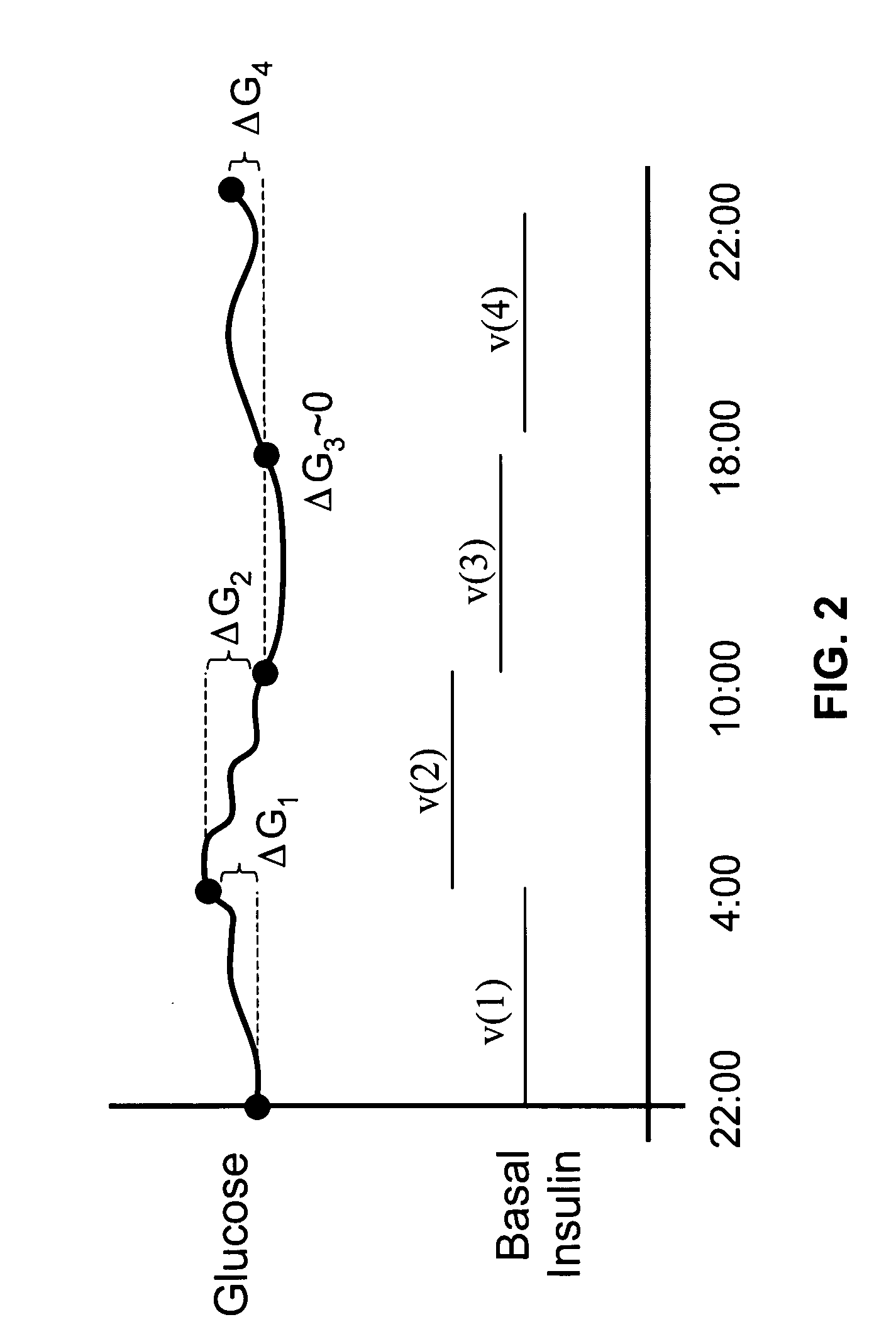
Method and system for determining insulin dosing schedules and carbohydrate-to-insulin ratios in diabetic patients
ActiveUS20050049179A1Prevent overshootPeptide/protein ingredientsAutomatic syringesInsulin regimenCompound (substance)
Method for digitally determining the daily insulin regimen for a diabetic patient. The invention divides the patient's day into adjustable time intervals containing basal insulin dosage rates and Carbohydrate-to-Insulin Ratio(s) (for determining meal insulin doses). The invention identifies the Corrective Insulin doses over a time interval as an “error” in the Prescription Insulin (Basal Insulin+Meal Insulin). Methods involve first estimating the change to one of these two components of Prescription Insulin, and then determining the change to the other by subtracting from the error. One method estimates Change in Meal Insulin distributed among intervals proportional to old Meal Insulin. Another method lumps After-Meal Corrective Insulin together with Meal Insulin. Another method splits the interval at the After-Meal Corrective Dose and determines Basal from Time-Boundary Corrective Dose. Data may be obtained from the previous day, and a small fraction of error applied, leading to asymptotic reduction of error. Data may be obtained from recent history, and a larger fraction of error applied by doctor or automatic method.
Owner:ASEKO
Mixed micellar drug deliver system and method of preparation
Pharmaceutical compositions comprising a macromolecular pharmaceutical agent in micellar form are disclosed. The micelles are formed from an alkali metal alkyl sulfate, and at least one additional micelle-forming compound as described in the specification. An alkali metal salicylate and a pharmaceutically acceptable edetate are also included in the composition. Micelle size ranges between about 1 and 10 nanometers. Methods for making and using the compositions are also disclosed.
Owner:GENEREX PHARMA
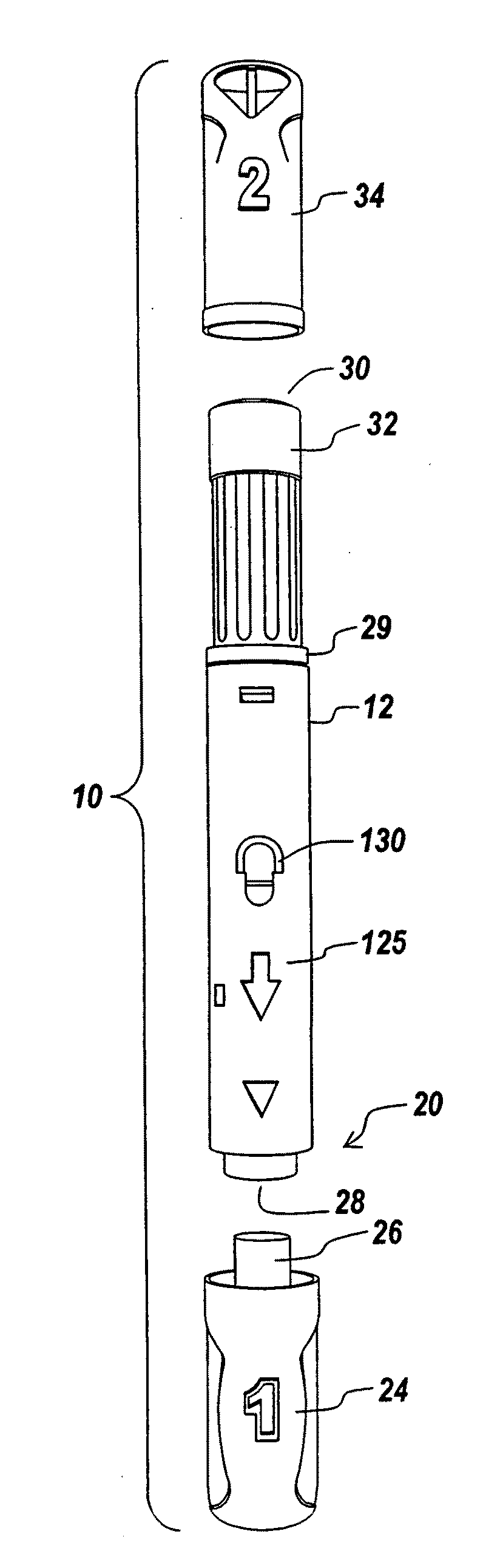
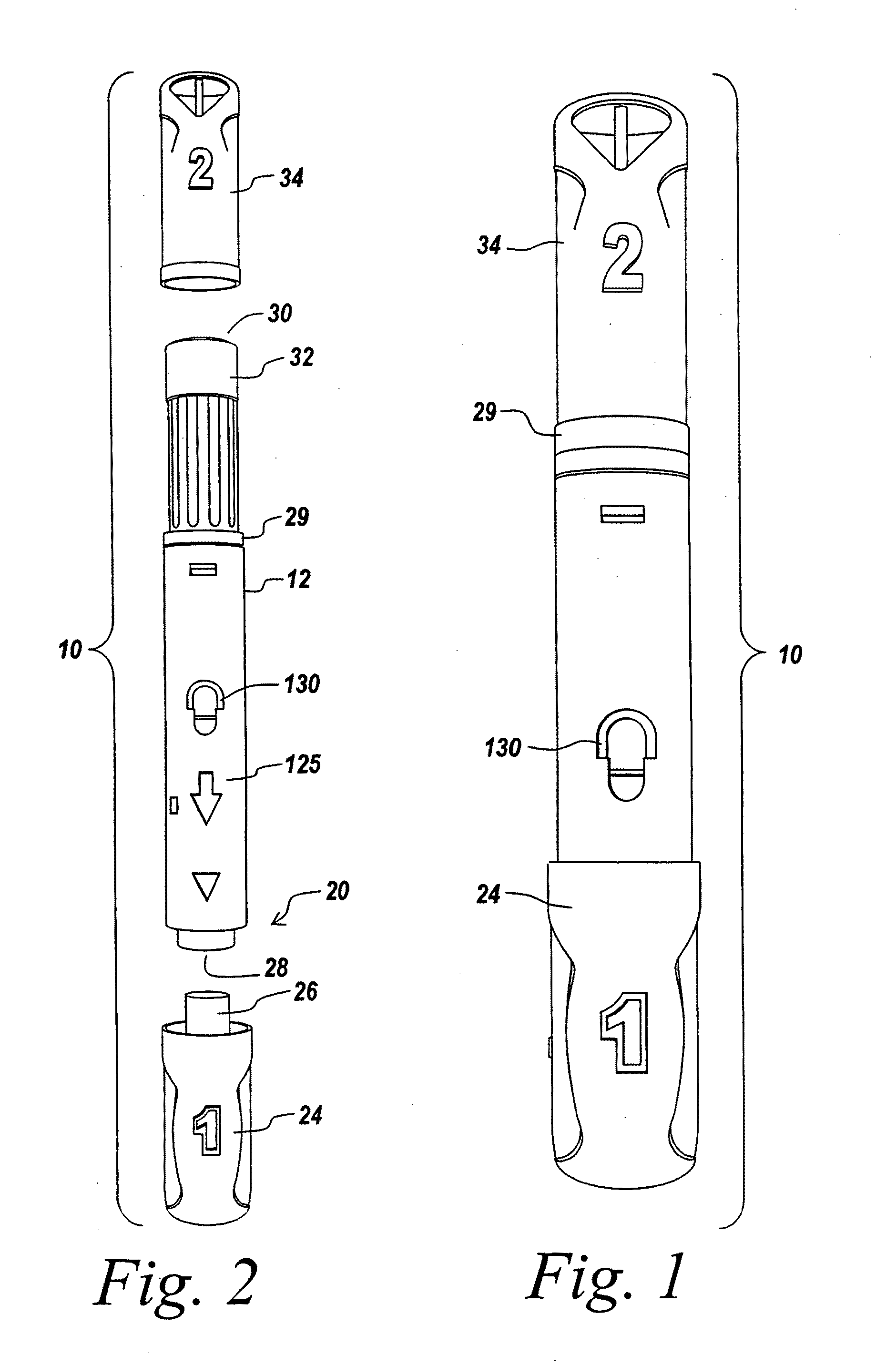
Automatic injection device
ActiveUS20100160894A1Easy to useReduce anxietyPeptide/protein ingredientsAntipyreticHypodermoclysisSubcutaneous injection
The invention provides an automatic injection device for providing a subcutaneous injection of a substance into a user, comprising: a housing having an open first end and a second end; a syringe movably disposed in the housing, the syringe including a barrel portion for holding the substance, a hollow needle in fluid communication with the barrel portion for ejecting the substance from the syringe, and a bung for sealing the barrel portion and selectively applying pressure to the substance to force the substance through the hollow needle; a plunger for first moving the syringe towards the first end such that the needle projects from the first end and subsequently applying pressure to the bung, the plunger including a rod connected at a first end to the bung, a compressible expanded central portion and a flange between a second end of the rod and the compressible expanded central portion; and a biasing mechanism for biasing the plunger towards the first open end of the housing, the biasing mechanism disposed about the second end of the rod between the flange and the second end of the housing. The present invention also provides methods and kits for using an automatic injection device, and methods and kits for promoting an automatic injection device comprising a medication based on advantageous properties of the device as compared to a pre-filled syringe. The invention also provides methods and kits for training a recipient on use of the automatic injection device.
Owner:ABBVIE BIOTECHNOLOGY LTD
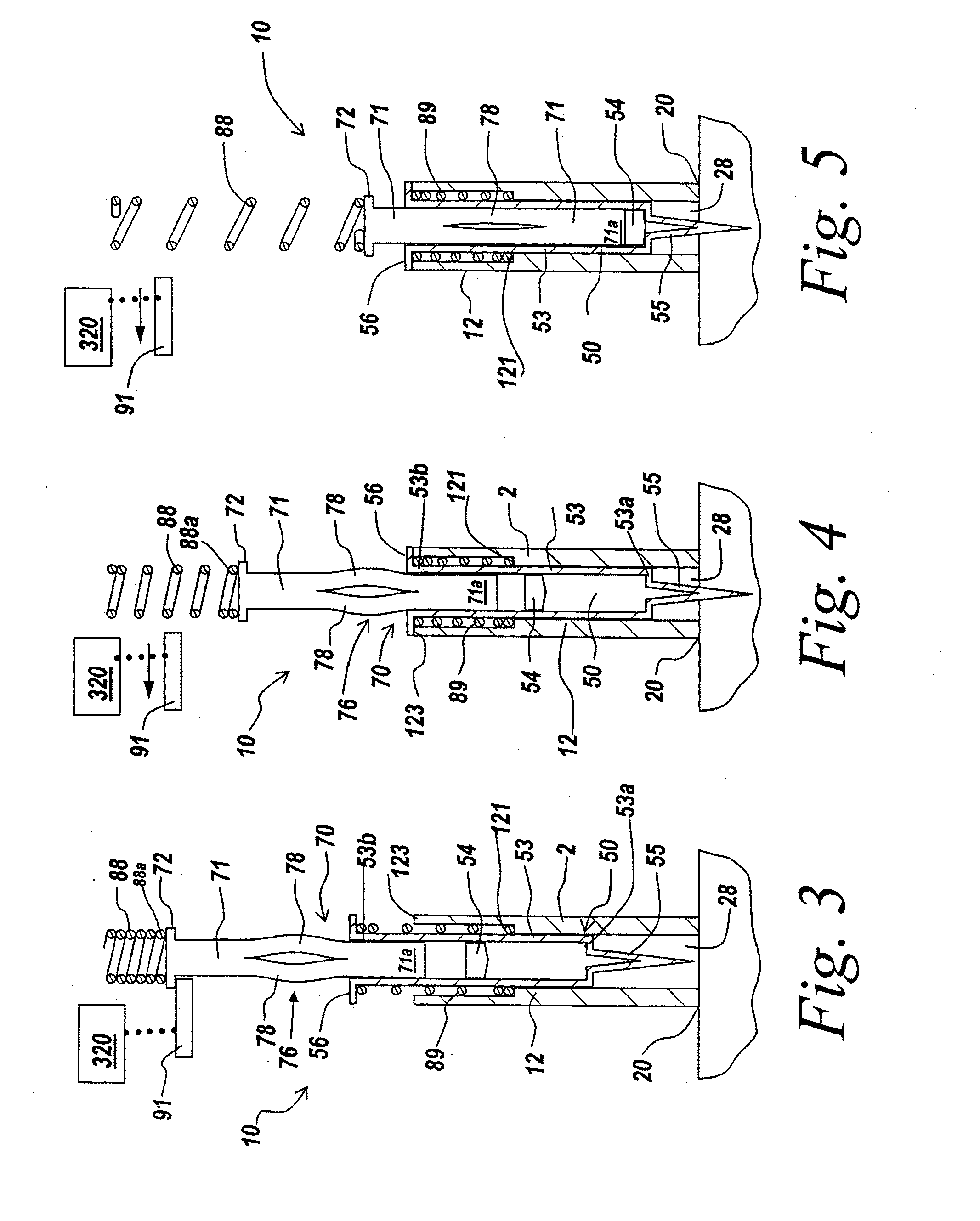
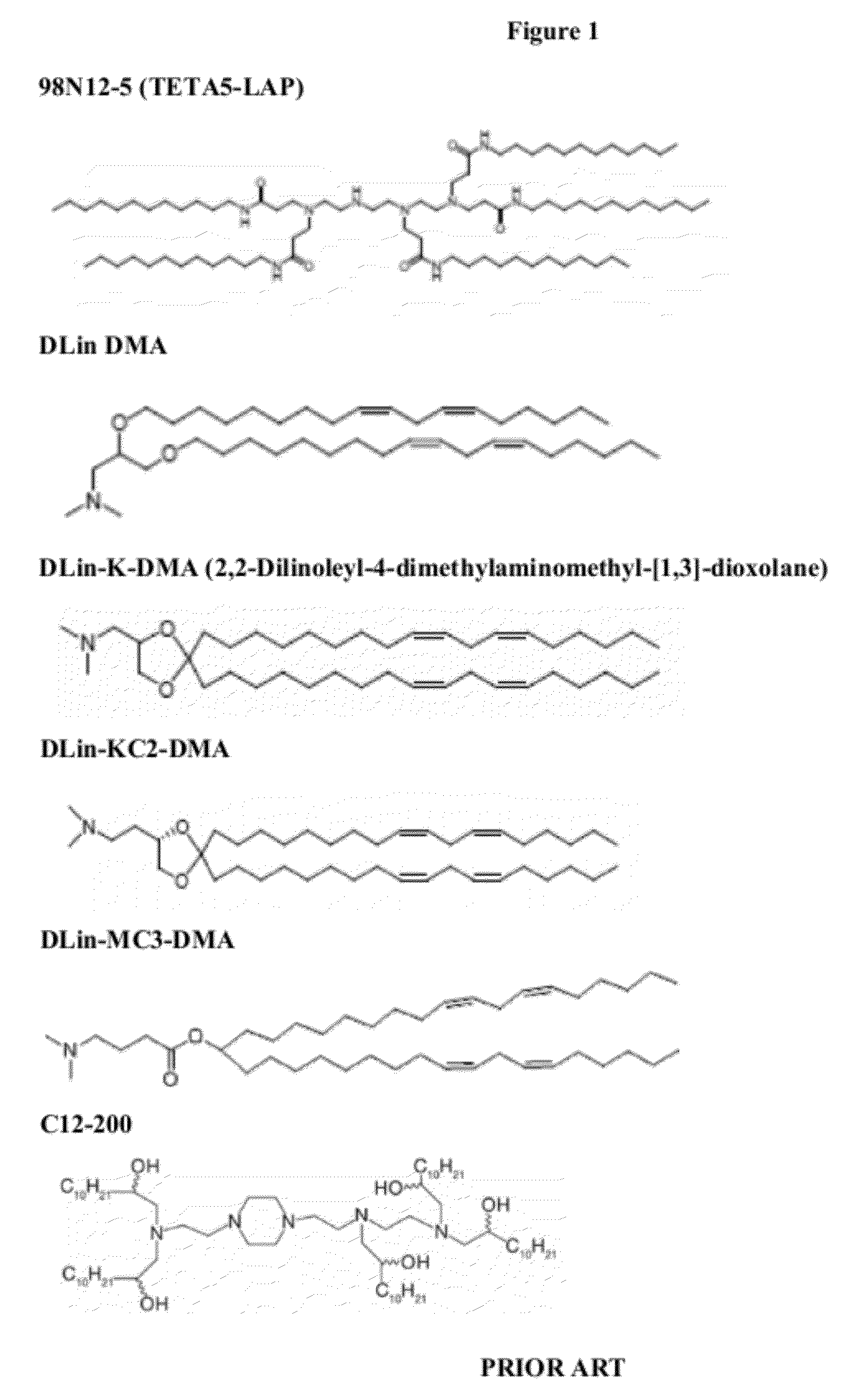
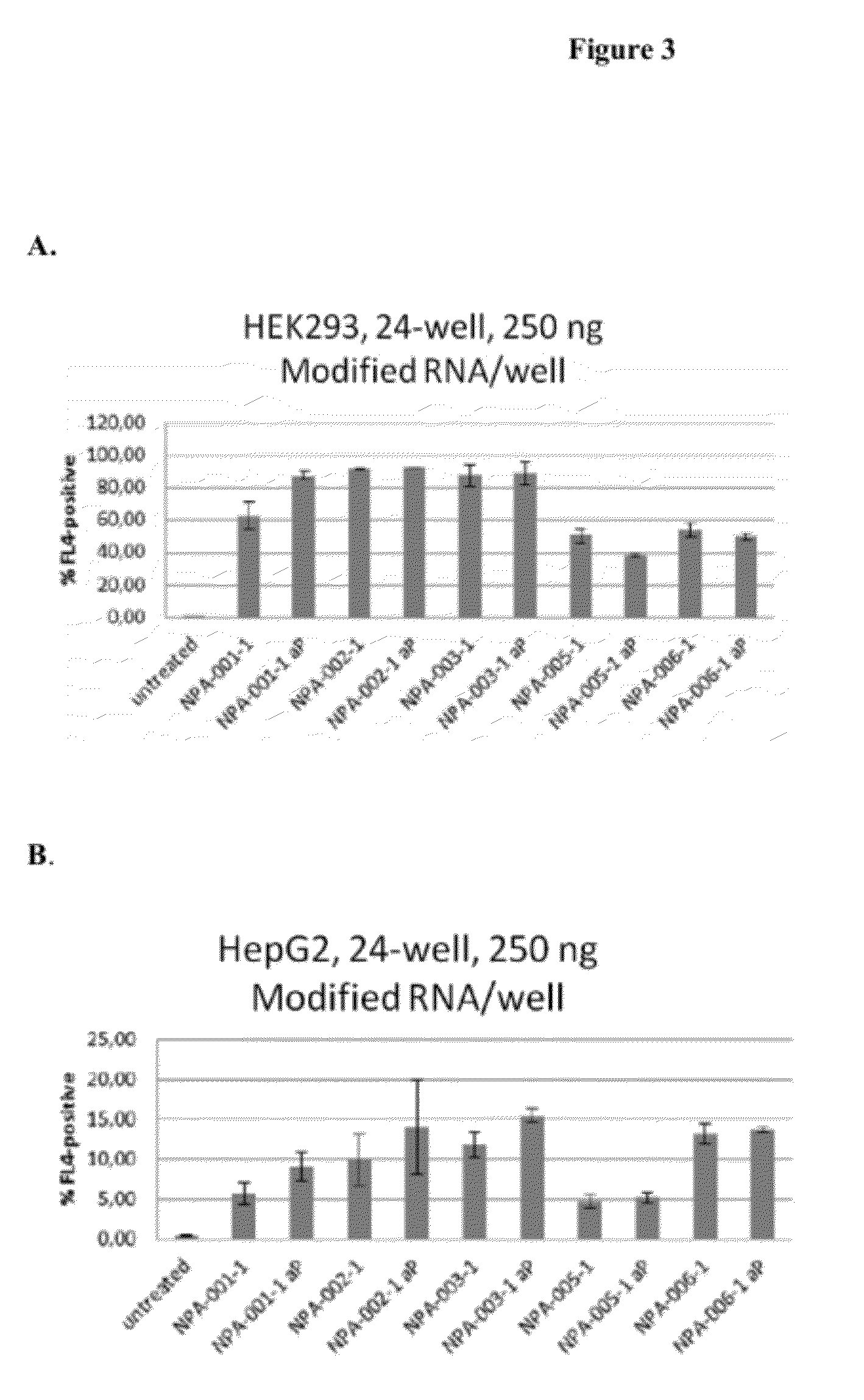
Delivery and formulation of engineered nucleic acids
ActiveUS20120251618A1Improve the level ofIncrease in level of polypeptideNervous disorderAntipyreticNucleic acidProtein expression
Provided are formulations, compositions and methods for delivering biological moieties such as modified nucleic acids into cells to modulate protein expression. Such compositions and methods include the delivery of biological moieties, and are useful for production of proteins.
Owner:MODERNATX INC
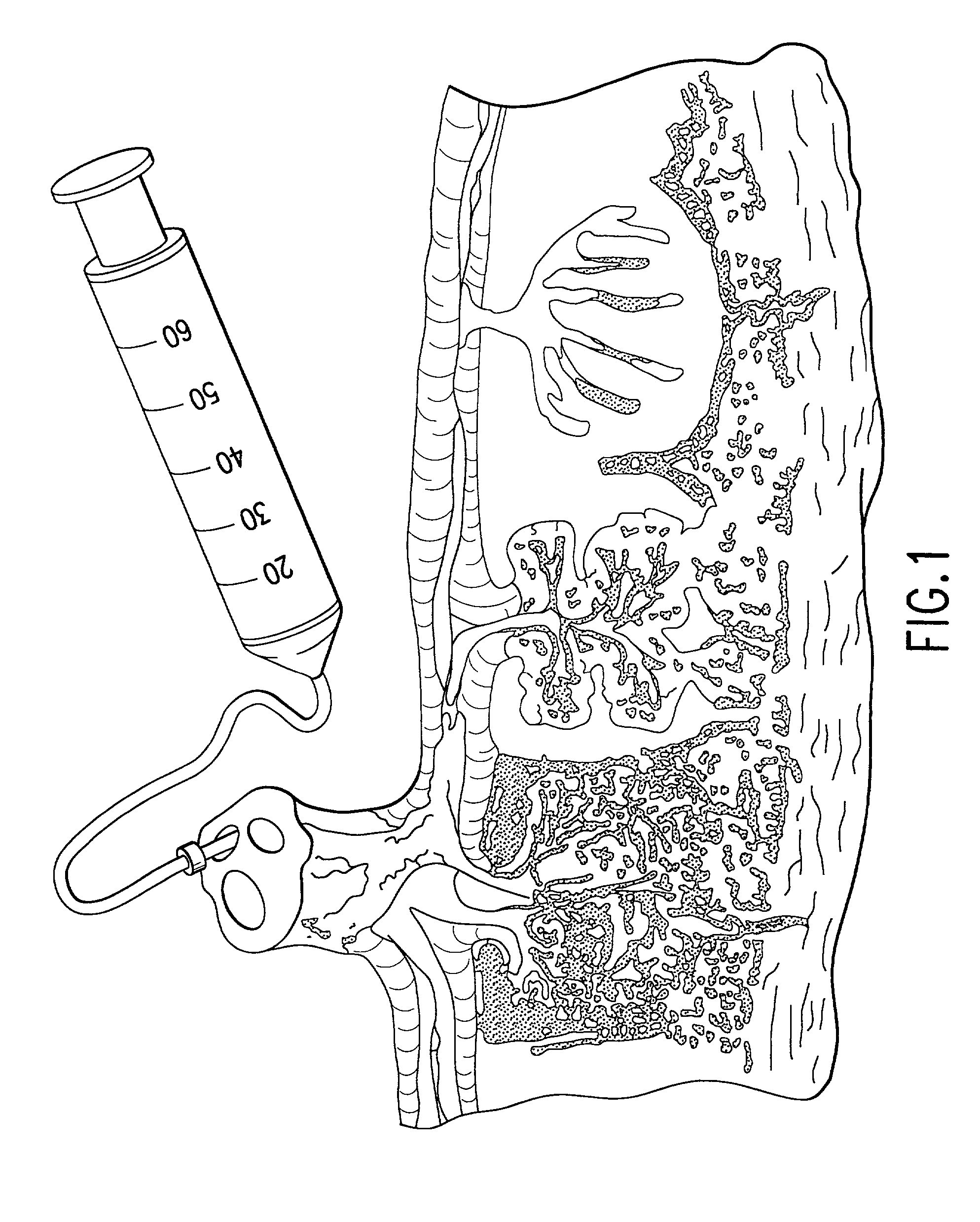
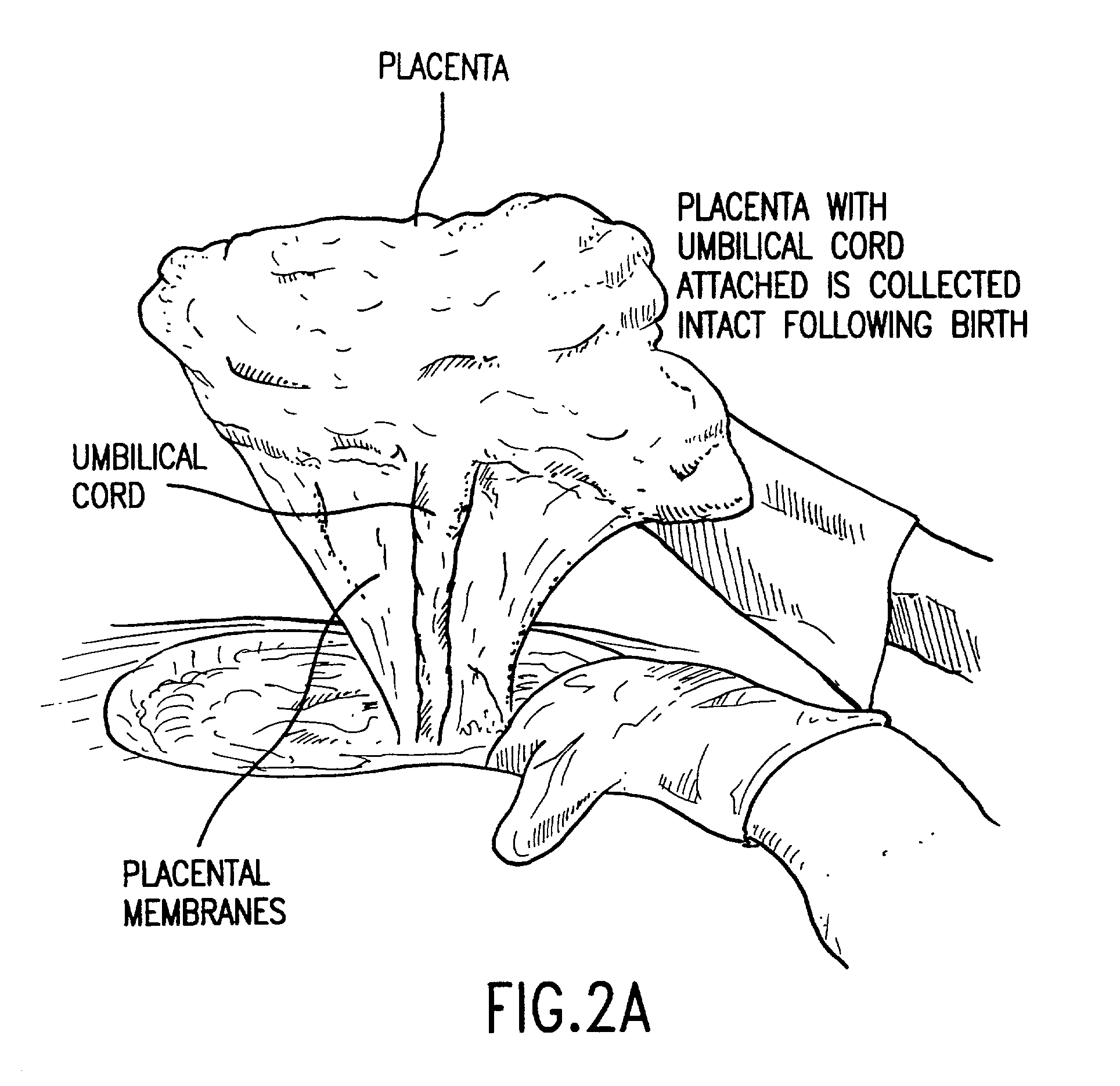
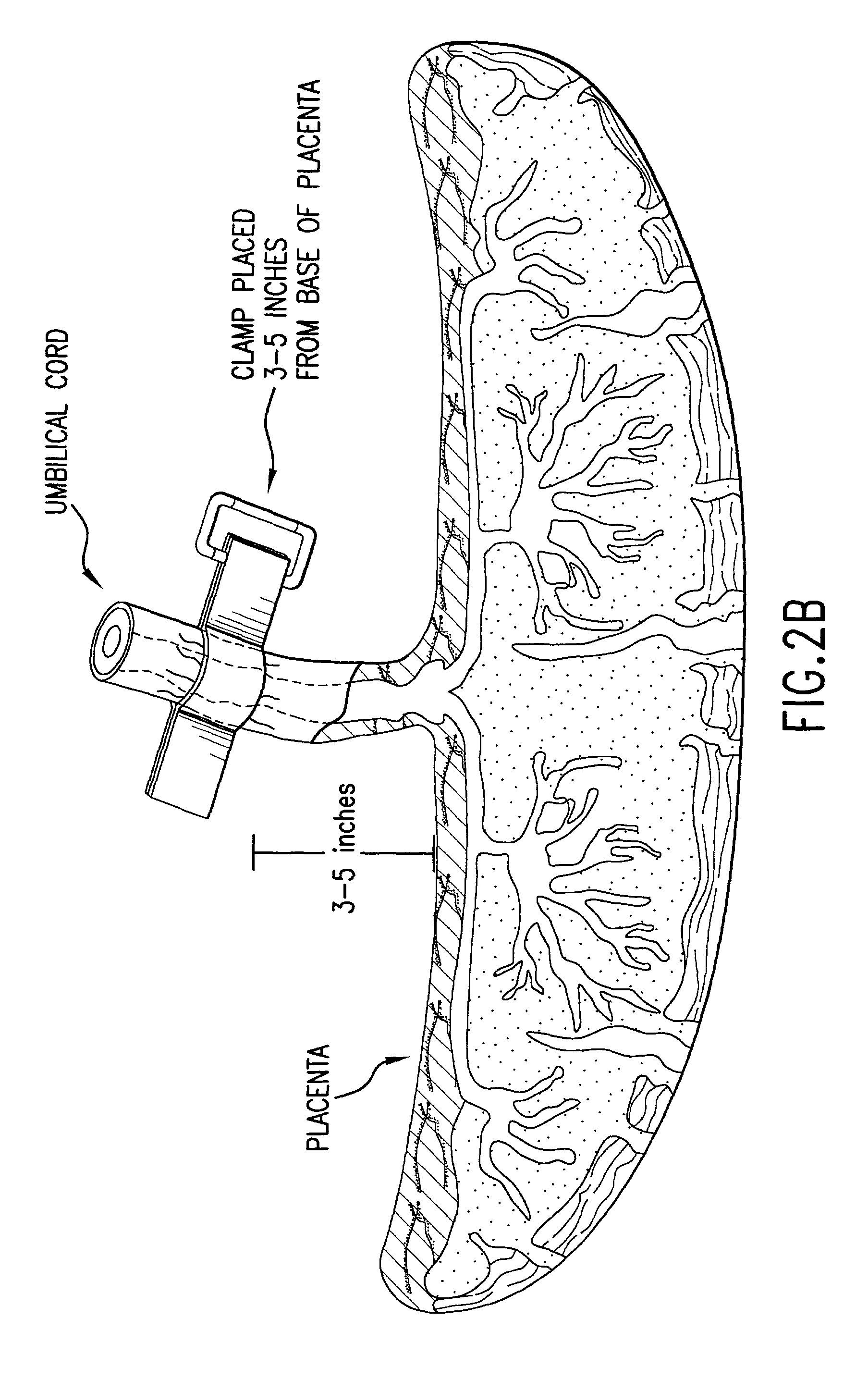
Post-partum mammalian placenta, its use and placental stem cells therefrom
InactiveUS20030032179A1Enhance exsanguinationEnhance sterile conditionSenses disorderAntipyreticAnticoagulant AgentEmbryo
The present invention provides a method of extracting and recovering embryonic-like stem cells, including, but not limited to pluripotent or multipotent stem cells, from an exsanguinated human placenta. A placenta is treated to remove residual umbilical cord blood by perfusing an exsanguinated placenta, preferably with an anticoagulant solution, to flush out residual cells. The residual cells and perfusion liquid from the exsanguinated placenta are collected, and the embryonic-like stem cells are separated from the residual cells and perfusion liquid. The invention also provides a method of utilizing the isolated and perfused placenta as a bioreactor in which to propagate endogenous cells, including, but not limited to, embryonic-like stem cells. The invention also provides methods for propagation of exogenous cells in a placental bioreactor and collecting the propagated exogenous cells and bioactive molecules therefrom.
Owner:CELULARITY INC
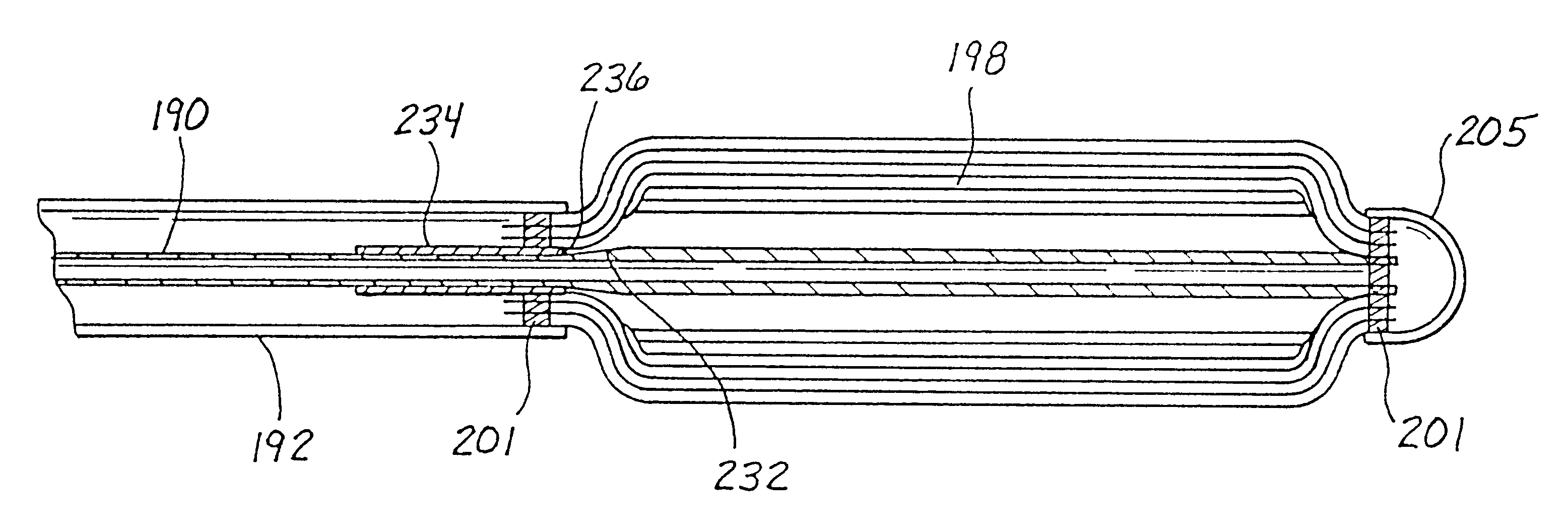
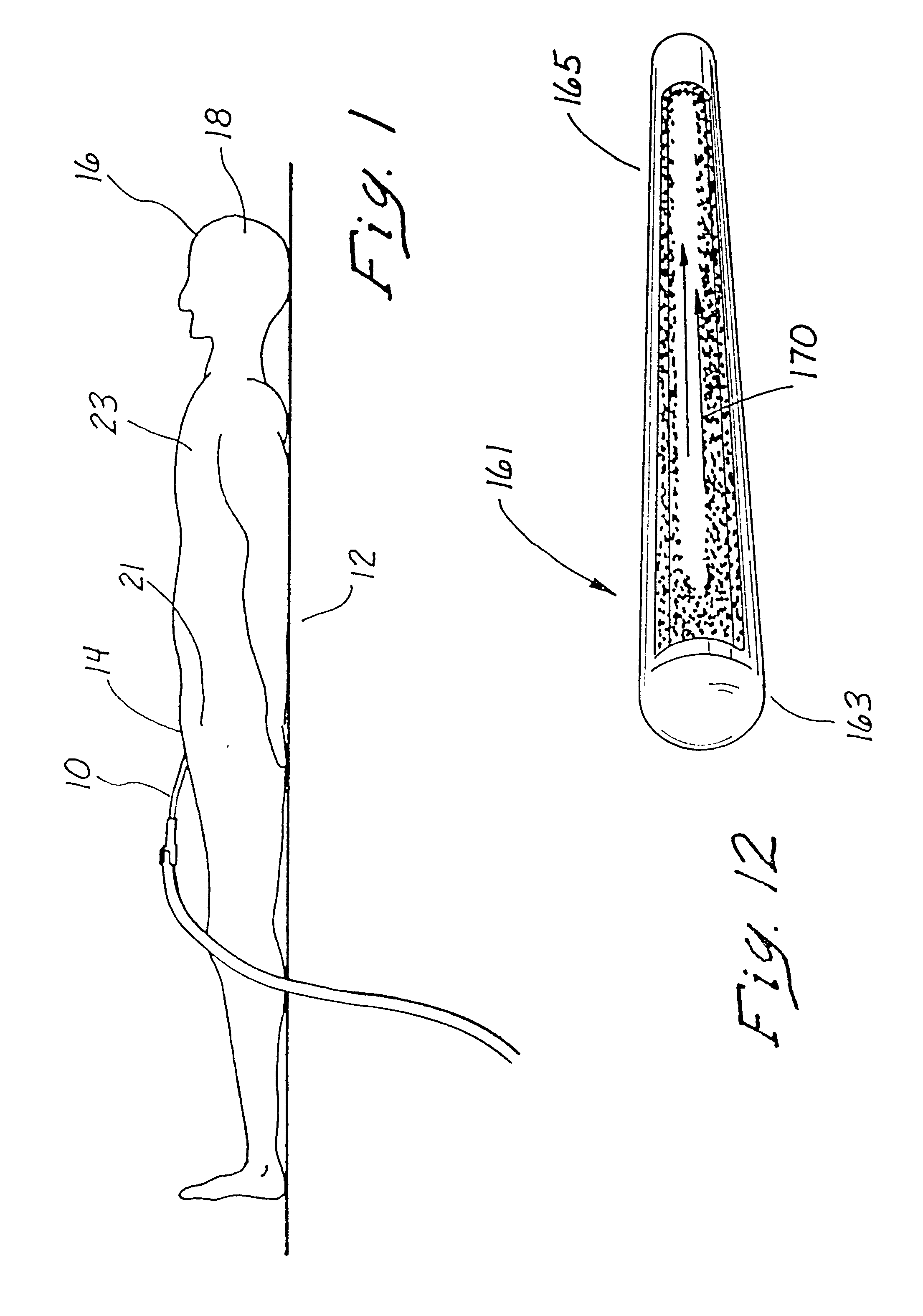
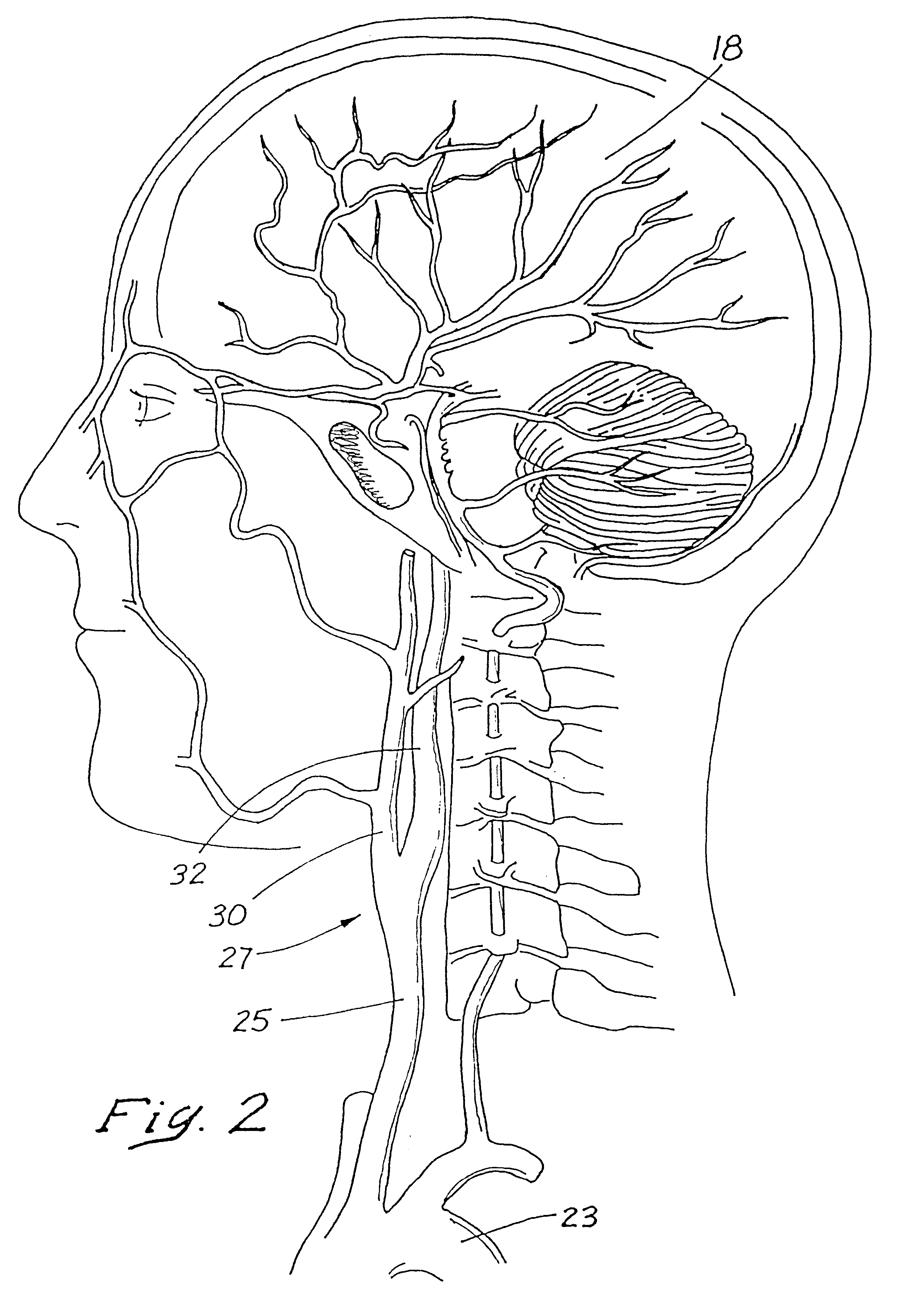
Indwelling heat exchange catheter and method of using same
A catheter is adapted to exchange heat with a body fluid, such as blood, flowing in a body conduit, such as a blood vessel. The catheter includes a shaft with a heat exchange region disposed at its distal end. This region may include hollow fibers which are adapted to receive a remotely cooled heat exchange fluid preferably flowing in a direction counter to that of the body fluid. The hollow fibers enhance the surface area of contact, as well as the mixing of both the heat exchange fluid and the body fluid. The catheter can be positioned to produce hypothermia in a selective area of the body or alternatively positioned to systemically cool the entire body system.
Owner:ZOLL CIRCULATION +1
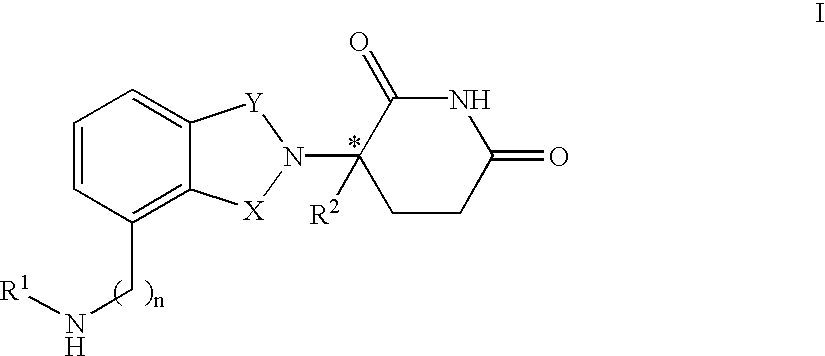
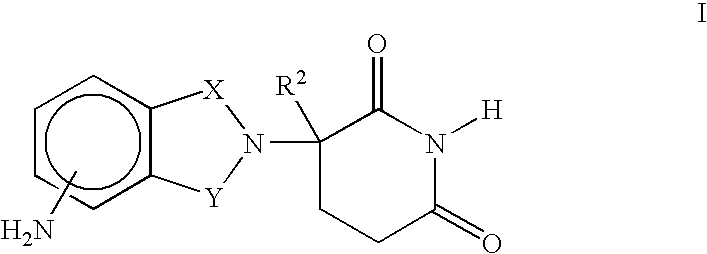
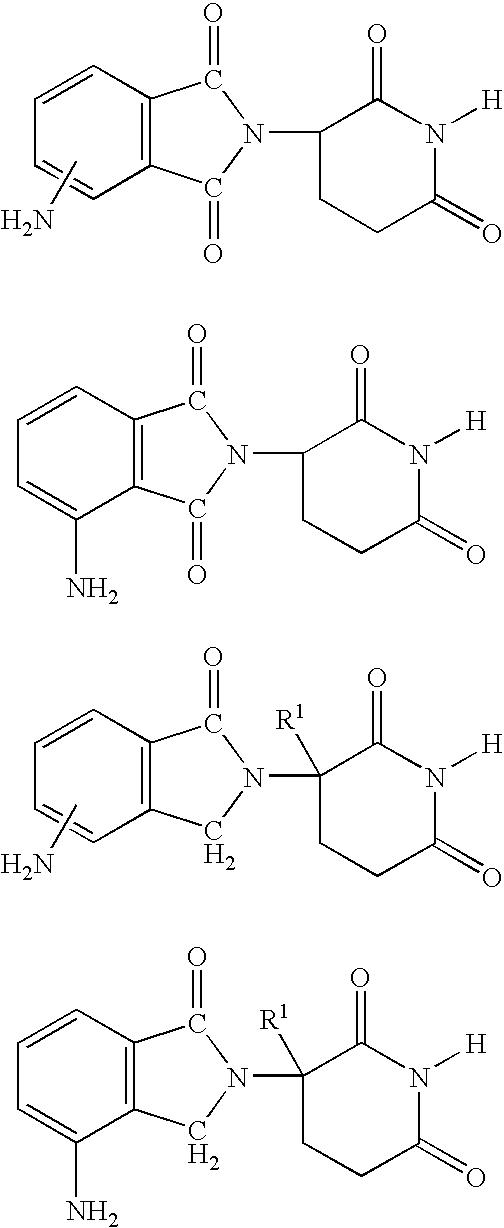
Isoindole-imide compounds, compositions, and uses thereof
The invention relates to isoindole-imide compounds and pharmaceutically acceptable salts, hydrates, solvates, clathrates, enantiomers, diastereomers, racemates, or mixtures of stereoisomers thereof, pharmaceutical compositions comprising these isoindole-imide compounds, and methods for reducing the level of cytokines and their precursors in mammals. In particular, the invention pertains to isoindole-imide compounds that are potent inhibitors of the production of TNF-alpha in mammals. The isoindole-imides described herein are useful for treating or preventing diseases or disorders in mammals, for example, cancers, such as solid tumors and blood-born tumors; heart disease, such as congestive heart failure; osteoporosis; and genetic, inflammatory; allergic; and autoimmune diseases.
Owner:CELGENE CORP
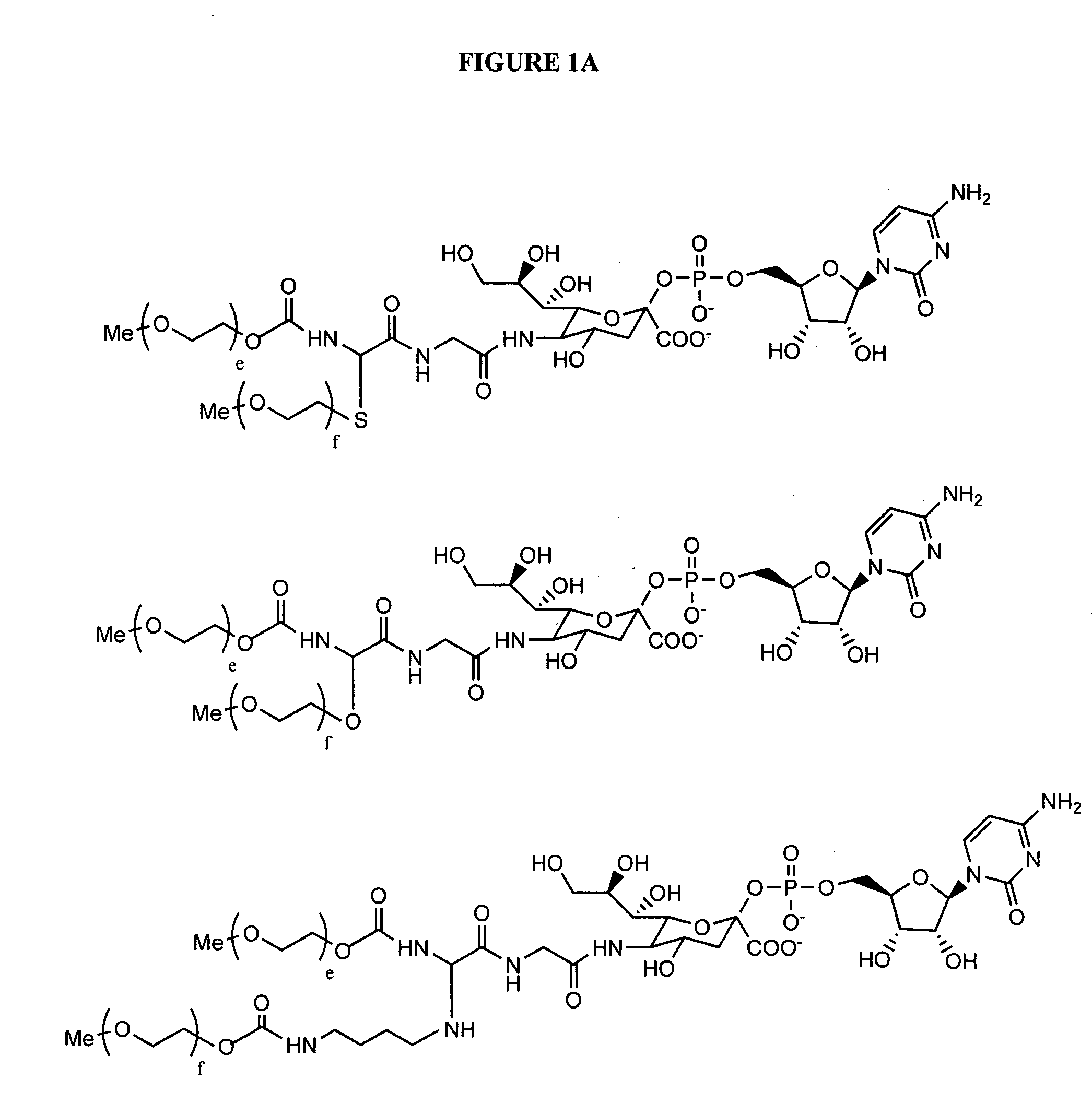
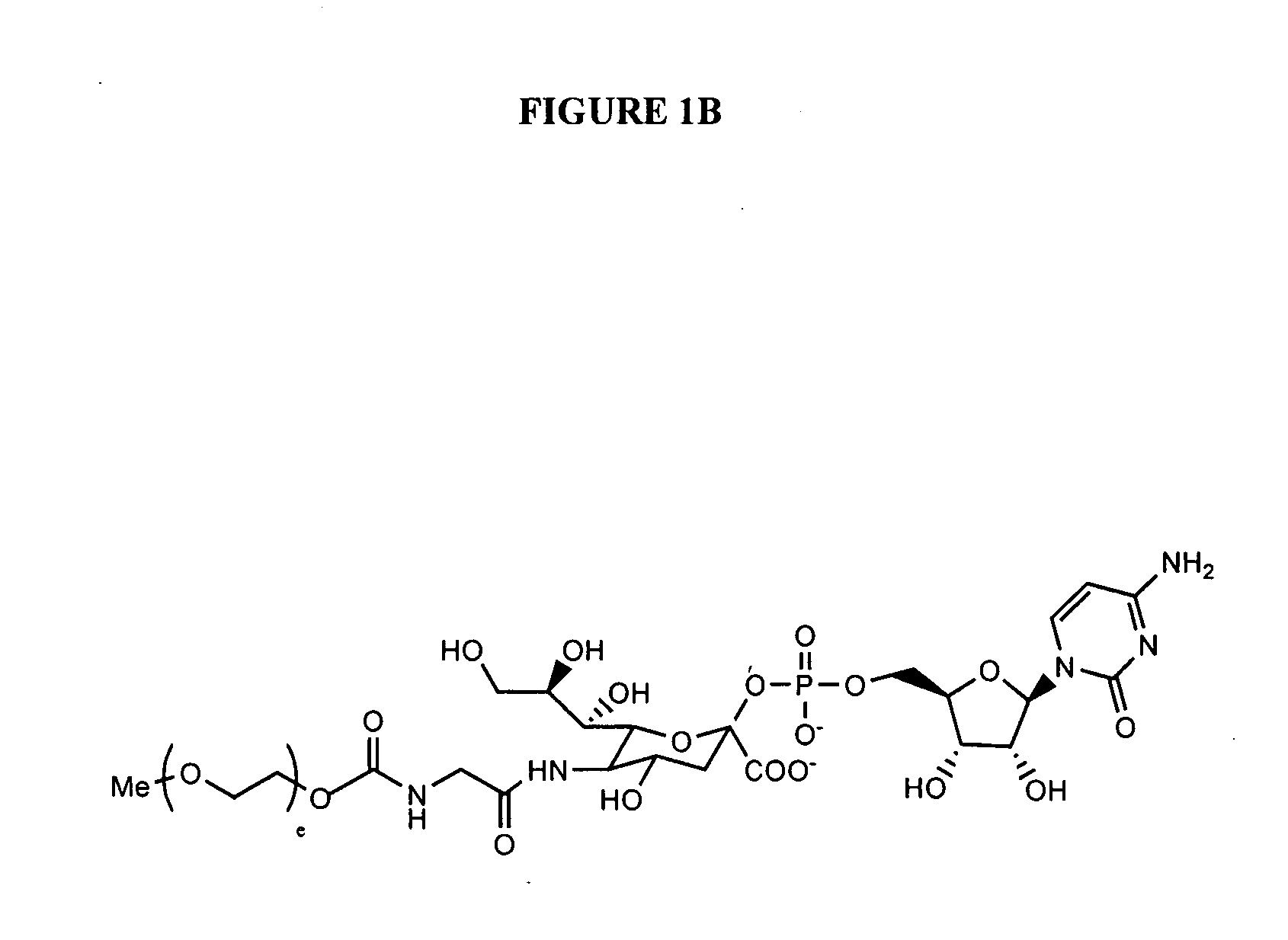
Amphiphilic drug-oligomer conjugates with hydroyzable lipophile components and methods for making and using the same
InactiveUS6309633B1Reduce deliveryExtended durationAntibacterial agentsOrganic active ingredientsTherapeutic proteinCholesterol
The invention provides a drug-oligomer conjugate having the following general formula:wherein D is a therapeutic drug moiety; H and H' are each a hydrophilic moiety, independently selected from the group consisting of straight or branched PEG polymers having from 2 to 130 PEG subunits, and sugars; L is a lipophilic moiety selected from the group consisting of alkyl groups having 2-26 carbon atoms, cholesterol, adamantane and fatty acids; o is a number from 1 to the maximum number of covalent bonding sites on H; m+n+p together have a value of at least one and not exceeding the total number of covalent bonding sites on D for the -H', -L and -H-L substituents; the H-L bond(s) are hydrolyzable and the D-L' bond(s), when present, are hydrolyzable; the conjugate being further characterized by one of the following: (i) m is 0 and p is at least 1; (ii) n is 0 and p is at least 1; (iii) m and n are each 0 and p is at least 1; (iv) p is 0 and m and n are each at least 1. The therapeutic drug moiety is preferably a therapeutic protein or peptide, preferably insulin or a functional equivalent thereof.
Owner:BIOCON LTD
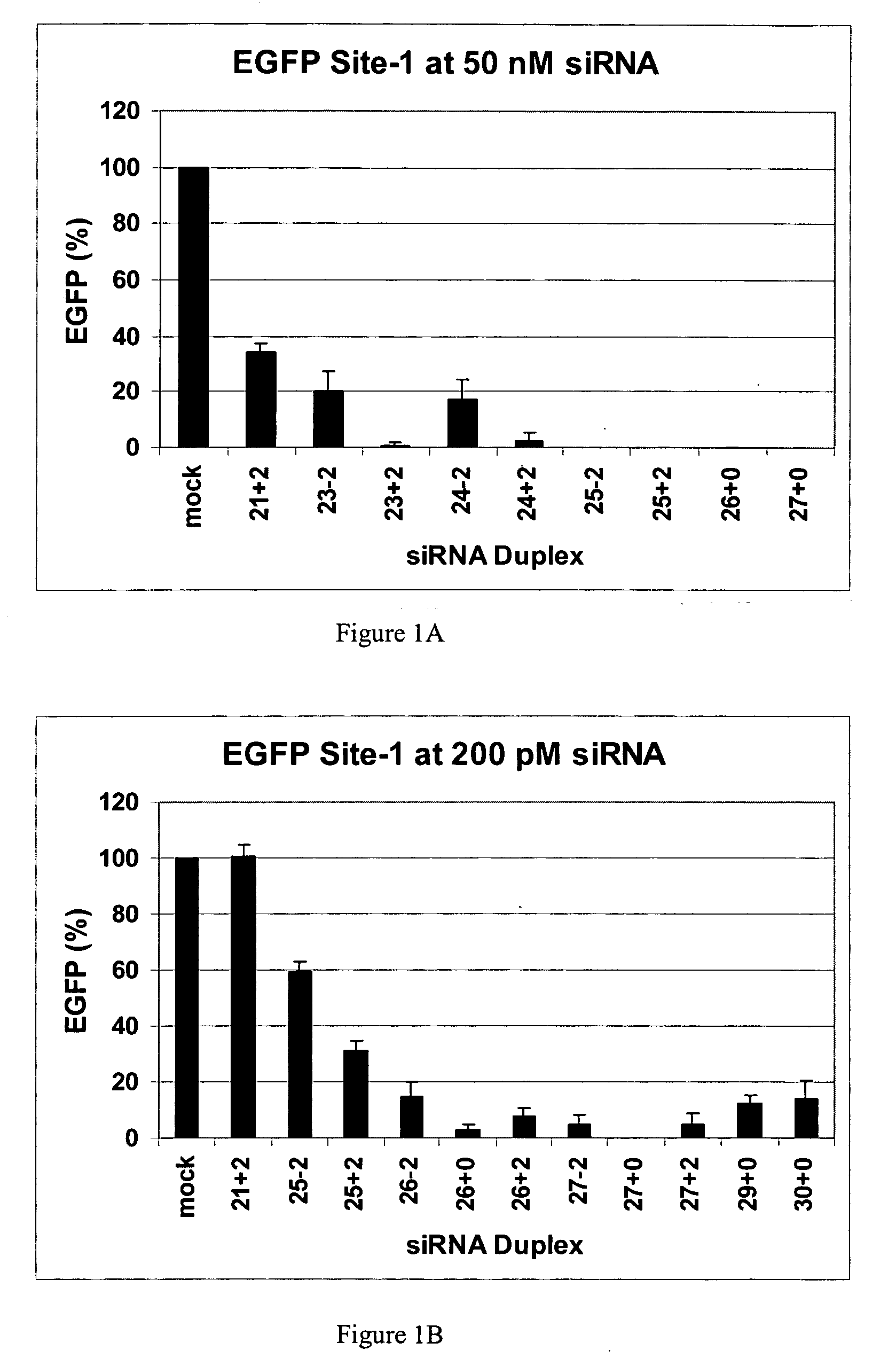
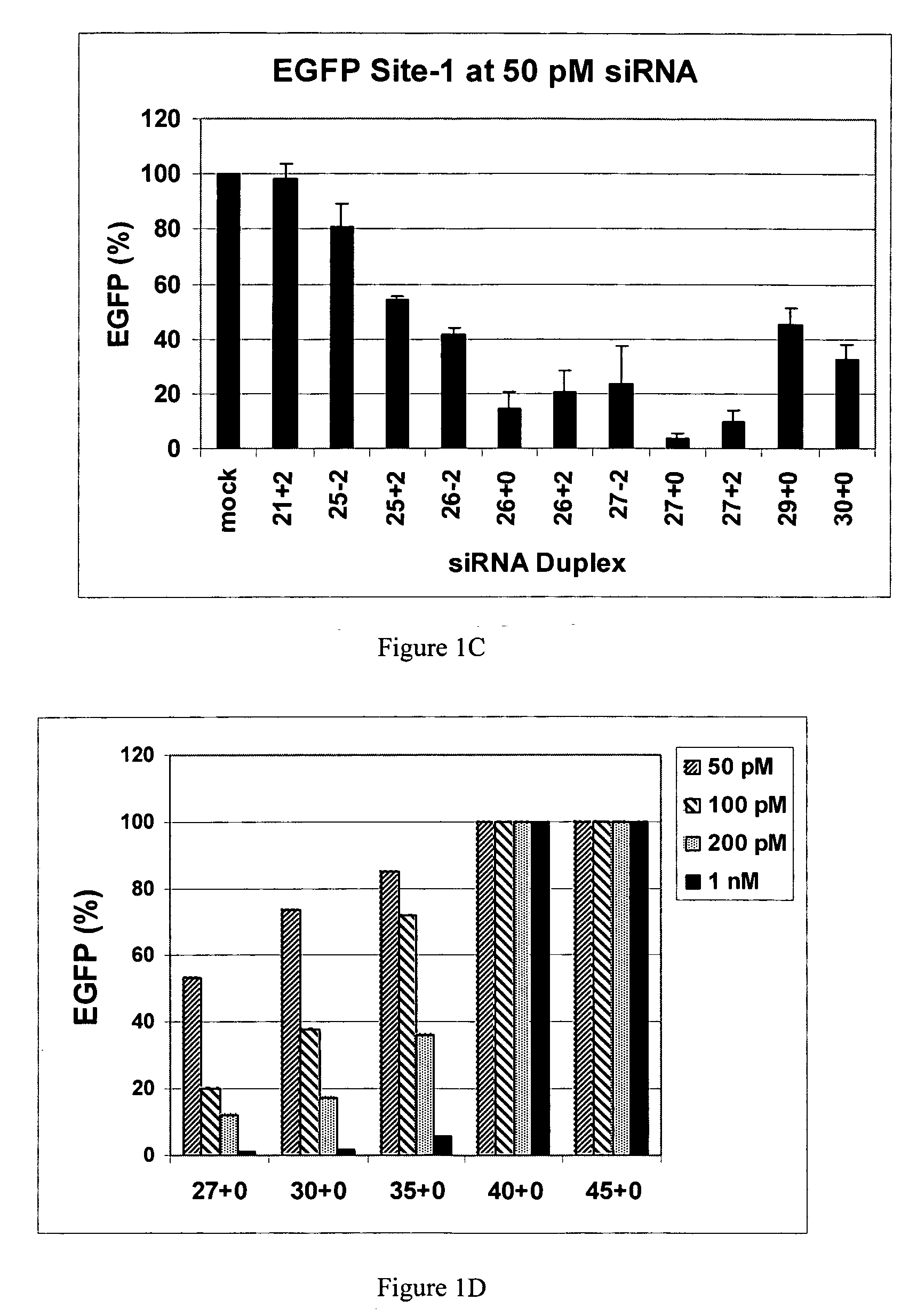
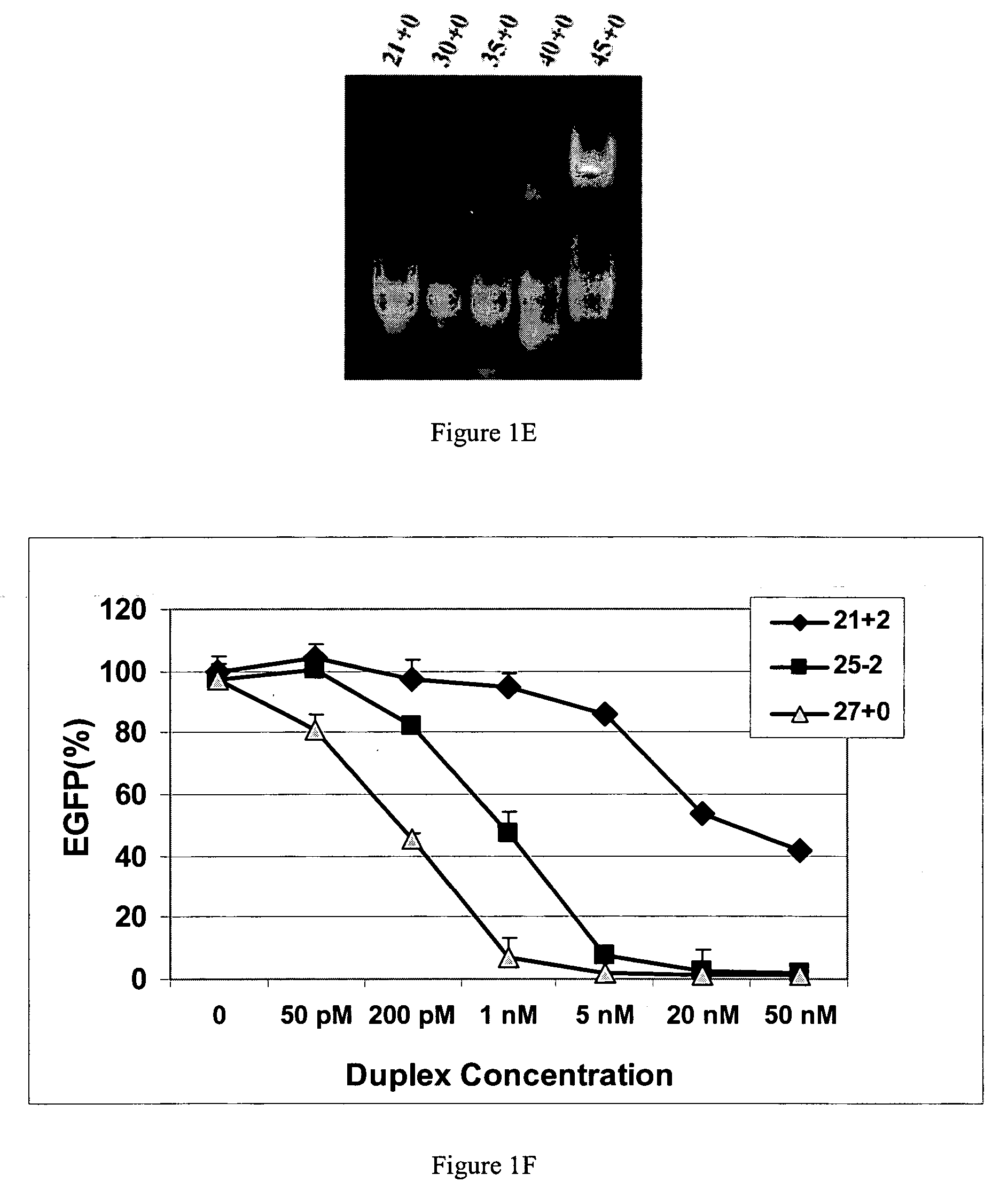
Polynucleotides for causing RNA interference and method for inhibiting gene expression using the same
InactiveUS20080113351A1High RNA interference effectLittle riskOrganic active ingredientsNervous disorderBase JNucleotide
The present invention provides a polynucleotide that not only has a high RNA interference effect on its target gene, but also has a very small risk of causing RNA interference against a gene unrelated to the target gene. A sequence segment conforming to the following rules (a) to (d) is searched from the base sequences of a target gene for RNA interference and, based on the search results, a polynucleotide capable of causing RNAi is designed, synthesized, etc.:(a) The 3′ end base is adenine, thymine, or uracil,(b) The 5′ end base is guanine or cytosine,(c) A 7-base sequence from the 3′ end is rich in one or more types of bases selected from the group consisting of adenine, thymine, and uracil, and(d) The number of bases is within a range that allows RNA interference to occur without causing cytotoxicity.
Owner:ALPHAGEN
Biocompatible crosslinked polymers
InactiveUS7009034B2Improve performanceImprove visibilityUltrasonic/sonic/infrasonic diagnosticsPowder deliveryWound dressingPost operative
Biocompatible crosslinked polymers, and methods for their preparation and use, are disclosed in which the biocompatible crosslinked polymers are formed from water soluble precursors having electrophilic and nucleophilic functional groups capable of reacting and crosslinking in situ. Methods for making the resulting biocompatible crosslinked polymers biodegradable or not are provided, as are methods for controlling the rate of degradation. The crosslinking reactions may be carried out in situ on organs or tissues or outside the body. Applications for such biocompatible crosslinked polymers and their precursors include controlled delivery of drugs, prevention of post-operative adhesions, coating of medical devices such as vascular grafts, wound dressings and surgical sealants. Visualization agents may be included with the crosslinked polymers.
Owner:INCEPT LLC
Fragmented polymeric compositions and methods for their use
InactiveUS6063061AImprove liquidityEasy to controlSurgical adhesivesSurgical drugsCross-linkBreast implant
Molecular cross-linked gels comprise a variety of biologic and non-biologic polymers, such as proteins, polysaccharides, and synthetic polymers. Such molecular gels may be applied to target sites in a patient's body by extruding the gel through an orifice at the target site. Alternatively, the gels may be mechanically disrupted and used in implantable articles, such as breast implants. When used in vivo, the compositions are useful for inhibiting post-surgical spinal and other tissue adhesions, for filling tissue divots, tissue tracts, body cavities, surgical defects, and the like.
Owner:BAXTER INT INC +1
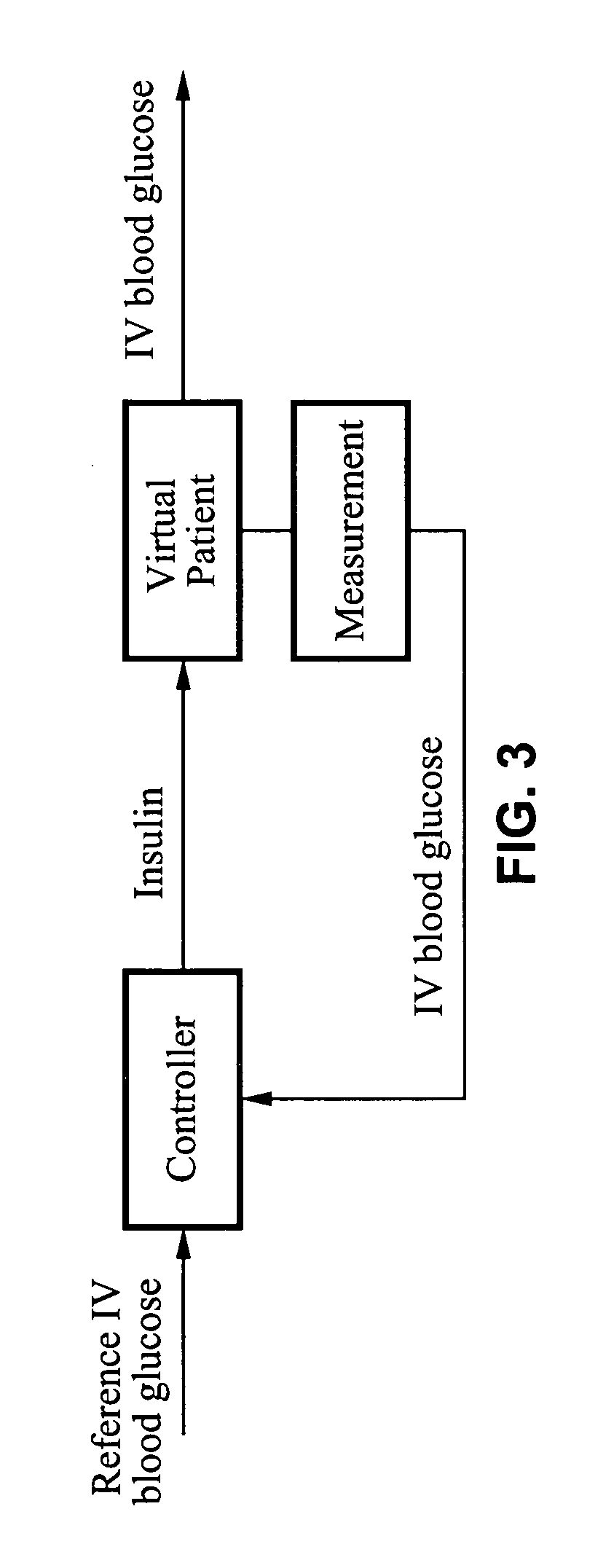
Method and apparatus for glucose control and insulin dosing for diabetics
ActiveUS20050272640A1Ensure robustnessAccurately predicting insulin bolus dosagesPeptide/protein ingredientsDrug and medicationsPhysiologyMonitors blood glucose
A computer implemented method and associated apparatus for the combined control of insulin bolus dosing and basal delivery for the goal of achieving normal glycemic response to meals, exercise, stressors, and other perturbations to blood glucose levels. A run-to-run algorithm is used to monitor blood glucose levels and adjust insulin delivery as conditions are varied.
Owner:RGT UNIV OF CALIFORNIA
Modulation of stem and progenitor cell differentiation, assays, and uses thereof
InactiveUS20030235909A1Modulate their differentiationIncrease speedOrganic active ingredientsSenses disorderAssayPlacenta
The present invention relates to methods of modulating mammalian stem cell and progenitor cell differentiation. The methods of the invention can be employed to regulate and control the differentiation and maturation of mammalian, particularly human stem cells along specific cell and tissue lineages. The methods of the invention relate to the use of certain small organic molecules to modulate the differentiation of stem or progenitor cell populations along specific cell and tissue lineages, and in particular, to the differentiation of embryonic-like stem cells originating from a postpartum placenta or for the differentiation of early progenitor cells to a granulocytic lineage. Finally, the invention relates to the use of such differentiated stem or progenitor cells in transplantation and other medical treatments.
Owner:SIGNAL PHARMA LLC +2
Peptide agonists of GLP-1 activity
InactiveUS6528486B1Lower blood sugar levelsEffective and stablePeptide/protein ingredientsMetabolism disorderD-GlucoseGlycemic
The present invention relates to novel peptide conjugates which have increased stability and are useful in the treatment of excess levels of blood glucose.
Owner:ZP HLDG SPV
Methods and compositions for the specific inhibition of gene expression by double-stranded RNA
The invention is directed to compositions and methods for selectively reducing the expression of a gene product from a desired target gene in a cell, as well as for treating diseases caused by the expression of the gene. More particularly, the invention is directed to compositions that contain double stranded RNA (“dsRNA”), and methods for preparing them, that are capable of reducing the expression of target genes in eukaryotic cells. The dsRNA has a first oligonucleotide sequence that is between 25 and about 30 nucleotides in length and a second oligonucleotide sequence that anneals to the first sequence under biological conditions. In addition, a region of one of the sequences of the dsRNA having a sequence length of at least 19 nucleotides is sufficiently complementary to a nucleotide sequence of the RNA produced from the target gene to trigger the destruction of the target RNA by the RNAi machinery.
Owner:CITY OF HOPE +1
Drug releasing coatings for medical devices
ActiveUS20080118544A1Avoid dependenceAvoid disadvantagesOrganic active ingredientsBiocideControlled releaseDrug release
The invention relates to a medical device for delivering a therapeutic agent to a tissue. The medical device has a layer overlying the exterior surface of the medical device. The layer contains a therapeutic agent and an additive. The additive has a hydrophilic part and a hydrophobic part and the therapeutic agent is not enclosed in micelles or encapsulated in particles or controlled release carriers.
Owner:LUTONIX INC
Glycopegylated erythropoietin
InactiveUS20060111279A1Improved pharmacokinetic propertiesCost effectiveSaccharide peptide ingredientsDepsipeptidesDiseaseSugar moiety
The present invention provides conjugates between erythropoietin and PEG moieties. The conjugates are linked via an intact glycosyl linking group interposed between and covalently attached to the peptide and the modifying group. The conjugates are formed from glycosylated peptides by the action of a glycosyltransferase. The glycosyltransferase ligates a modified sugar moiety onto a glycosyl residue on the peptide. Also provided are methods for preparing the conjugates, methods for treating various disease conditions with the conjugates, and pharmaceutical formulations including the conjugates.
Owner:NOVO NORDISK AS
Method and apparatus for glucose control and insulin dosing for diabetics
ActiveUS7651845B2Accurately predicting insulin bolus dosagesProcess controlPeptide/protein ingredientsDrug and medicationsMonitors blood glucoseGlycemic
Owner:RGT UNIV OF CALIFORNIA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com