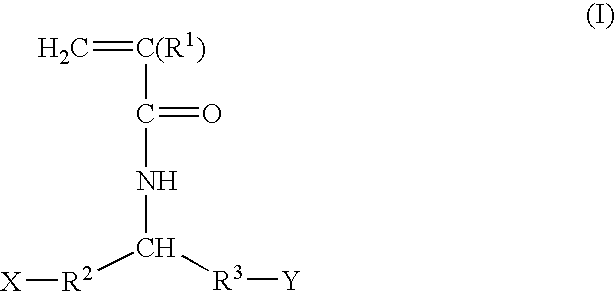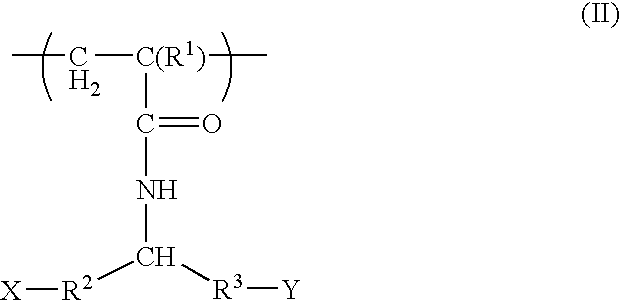Patents
Literature
2092 results about "Tissue repair" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Tissue healing (or tissue repair) refers to the body's replacement of destroyed tissue by living tissue (Walter and Israel 1987) and comprises two essential components - Regeneration and Repair.
Medical devices and applications of polyhydroxyalkanoate polymers
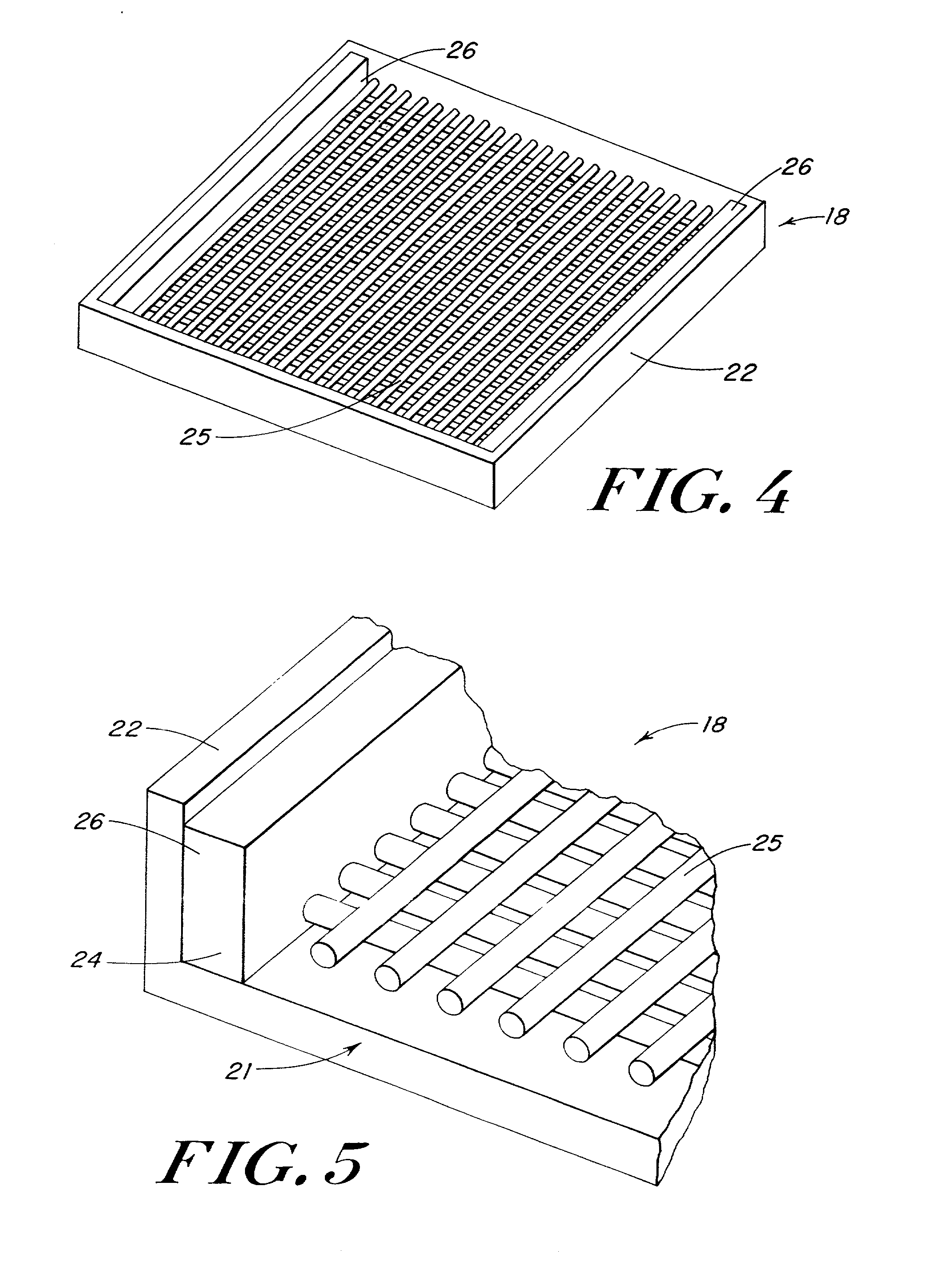
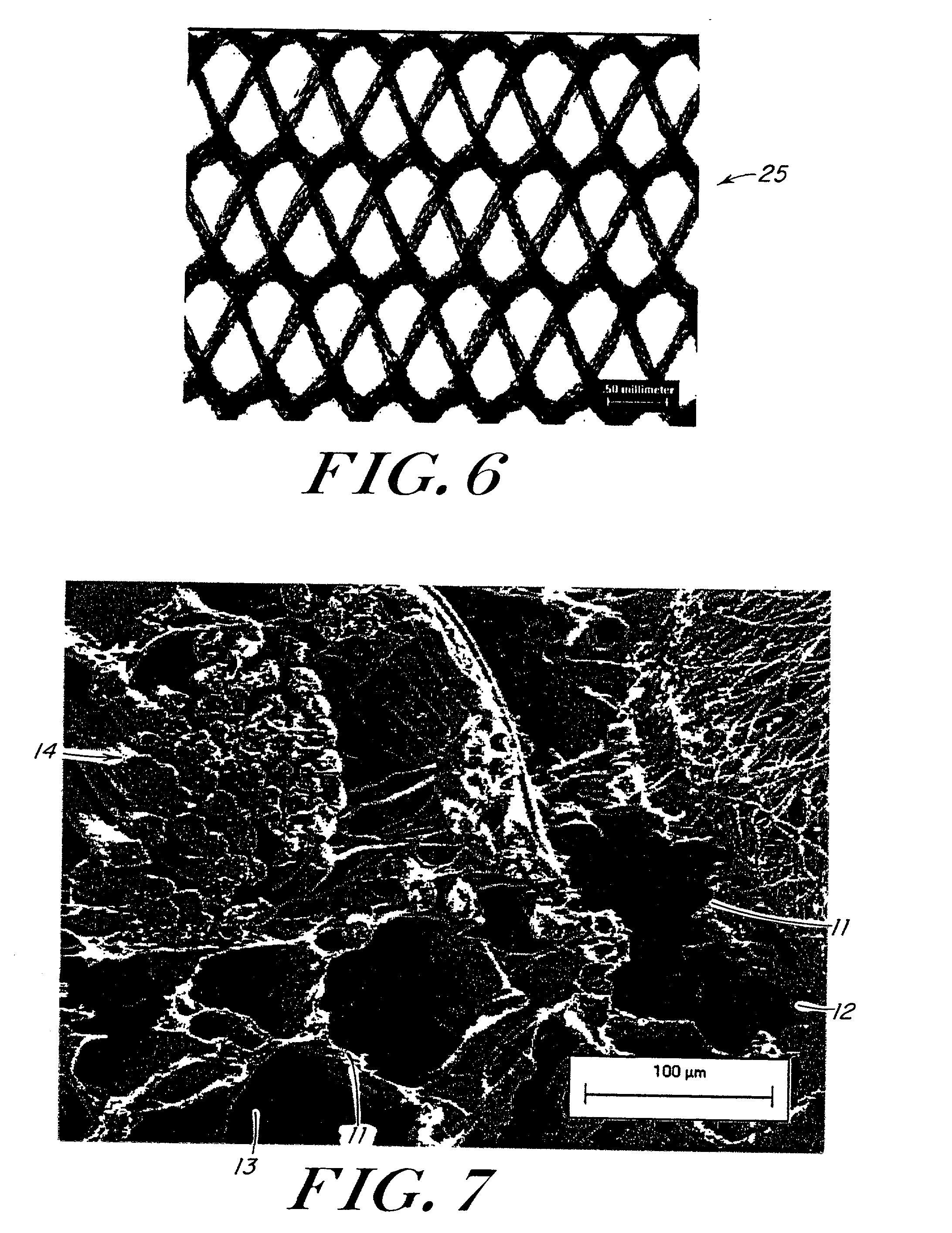
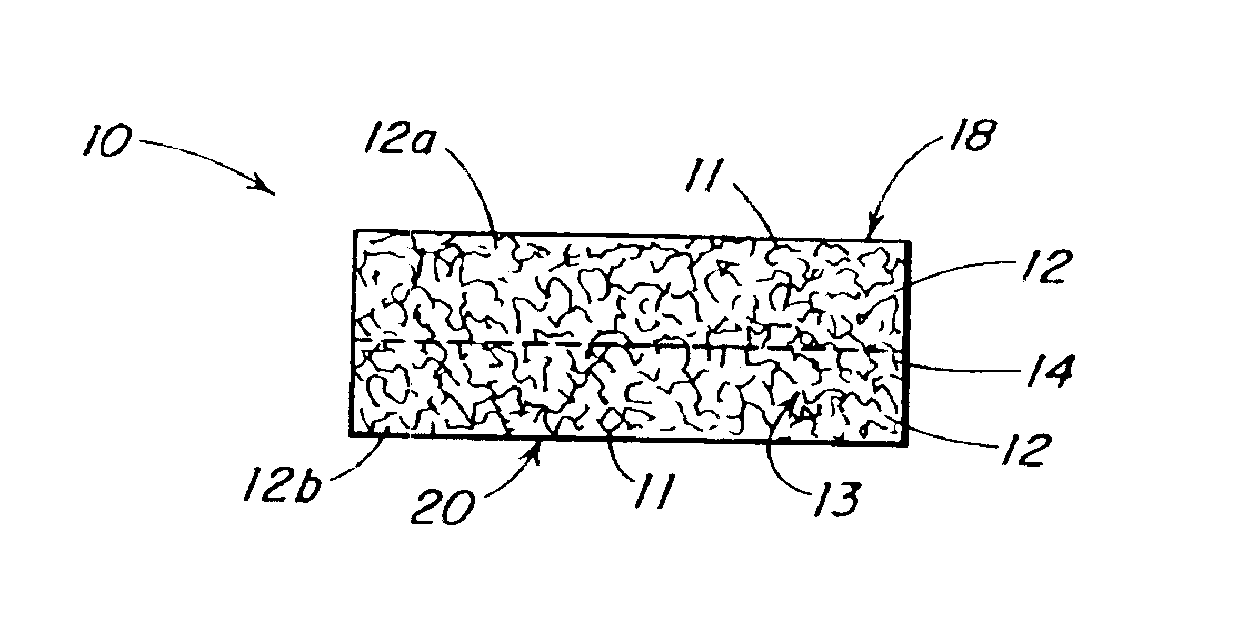
InactiveUS6838493B2High porosityReduce probabilitySuture equipmentsOrganic active ingredientsTissue repairBiocompatibility Testing
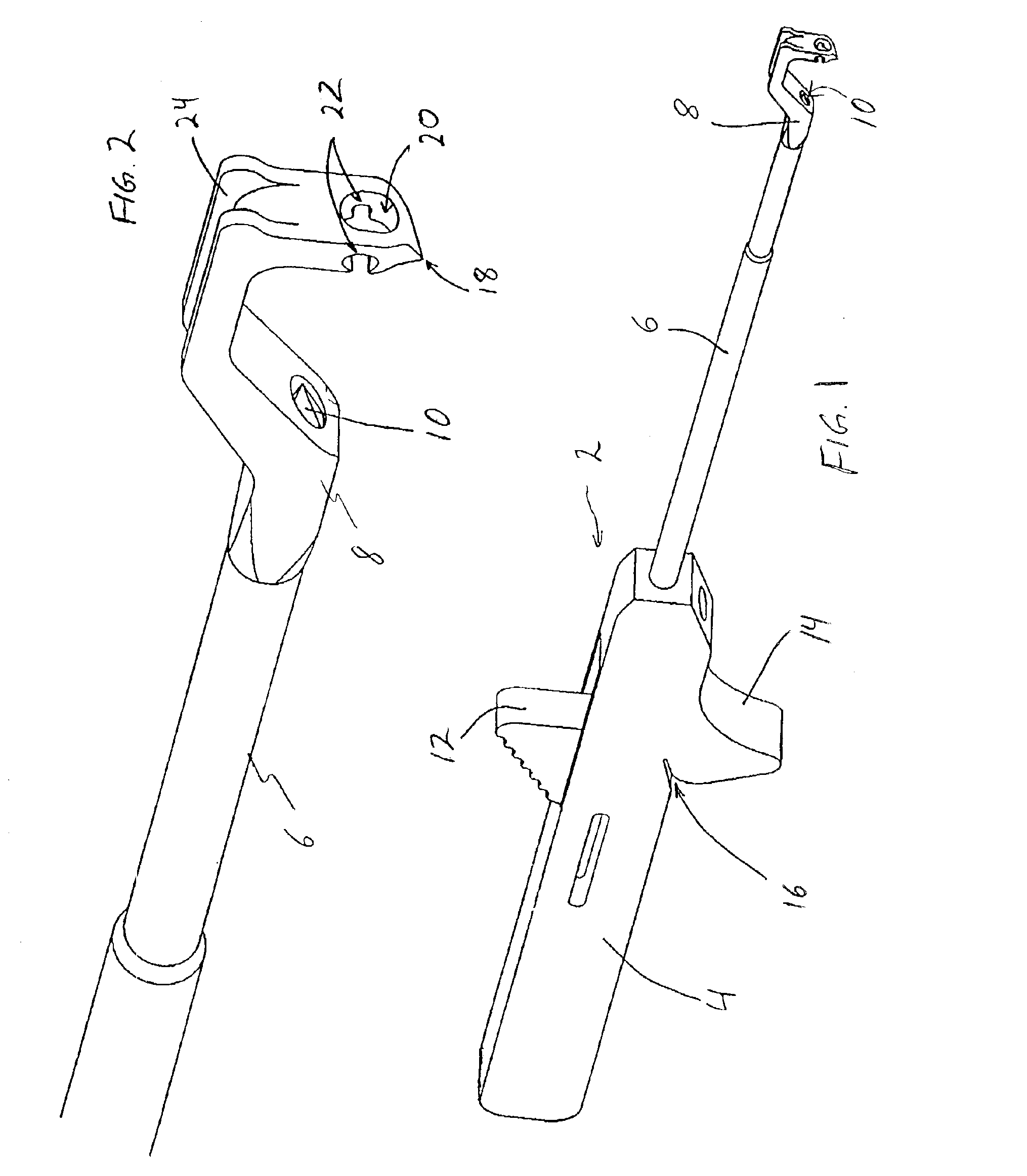
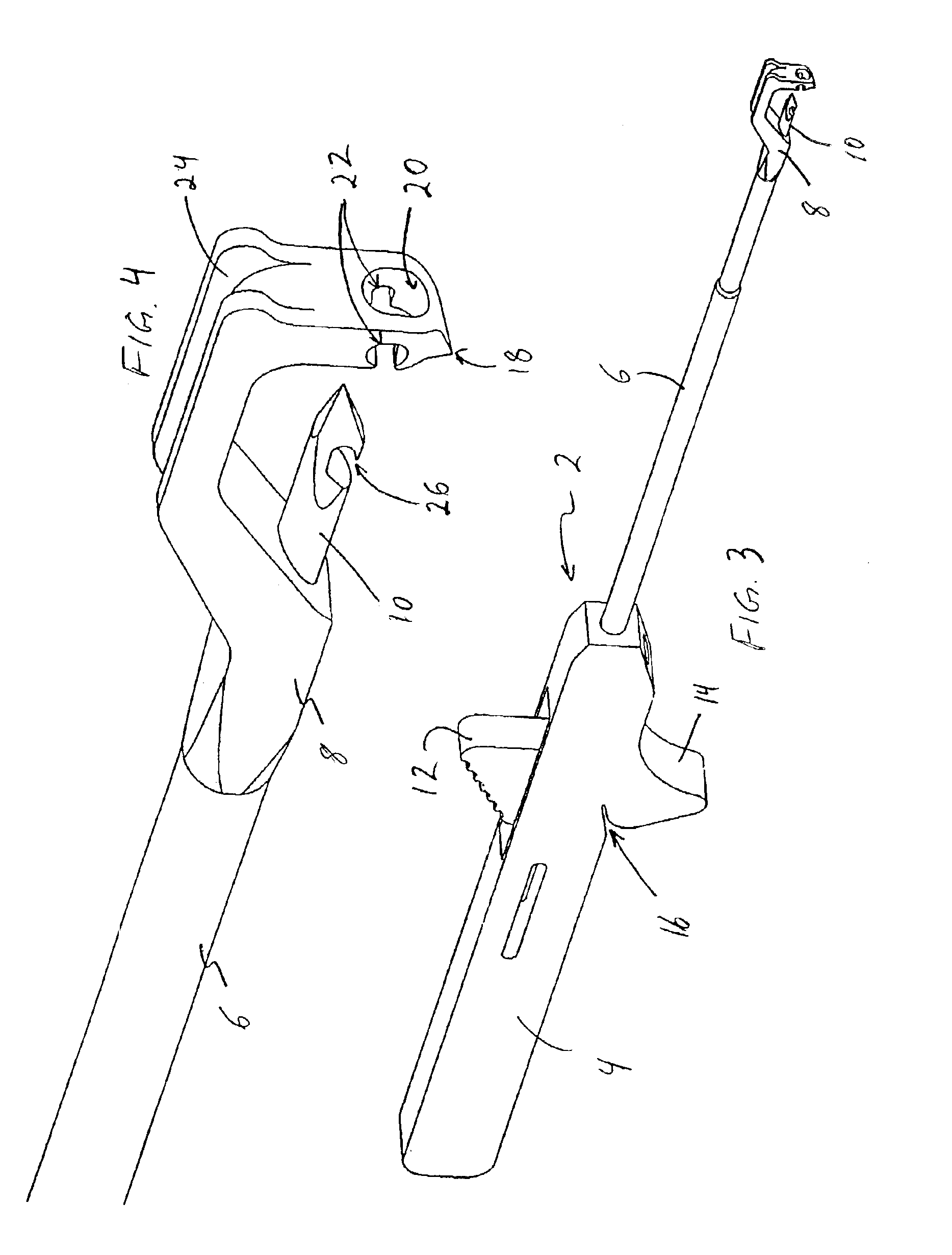
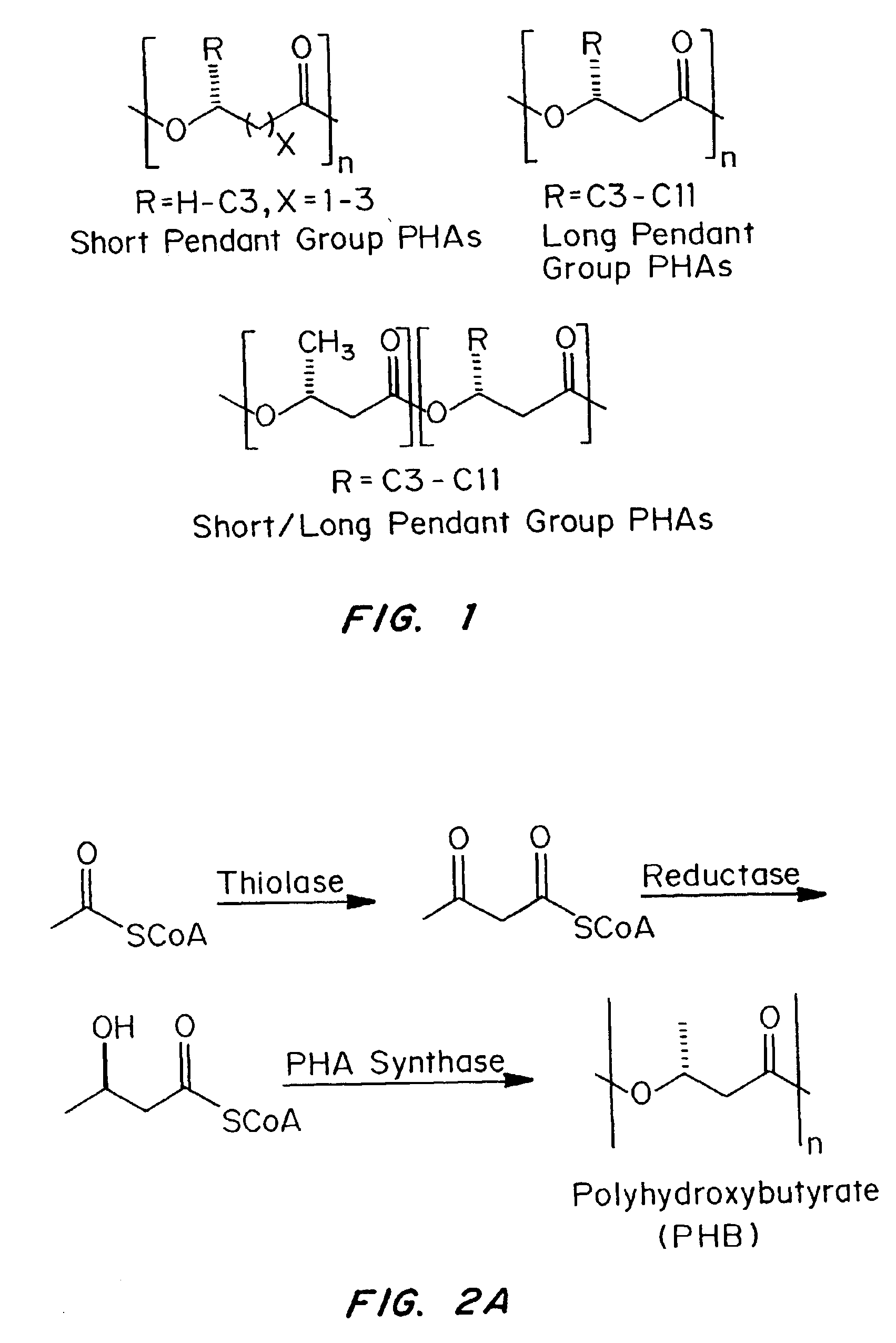
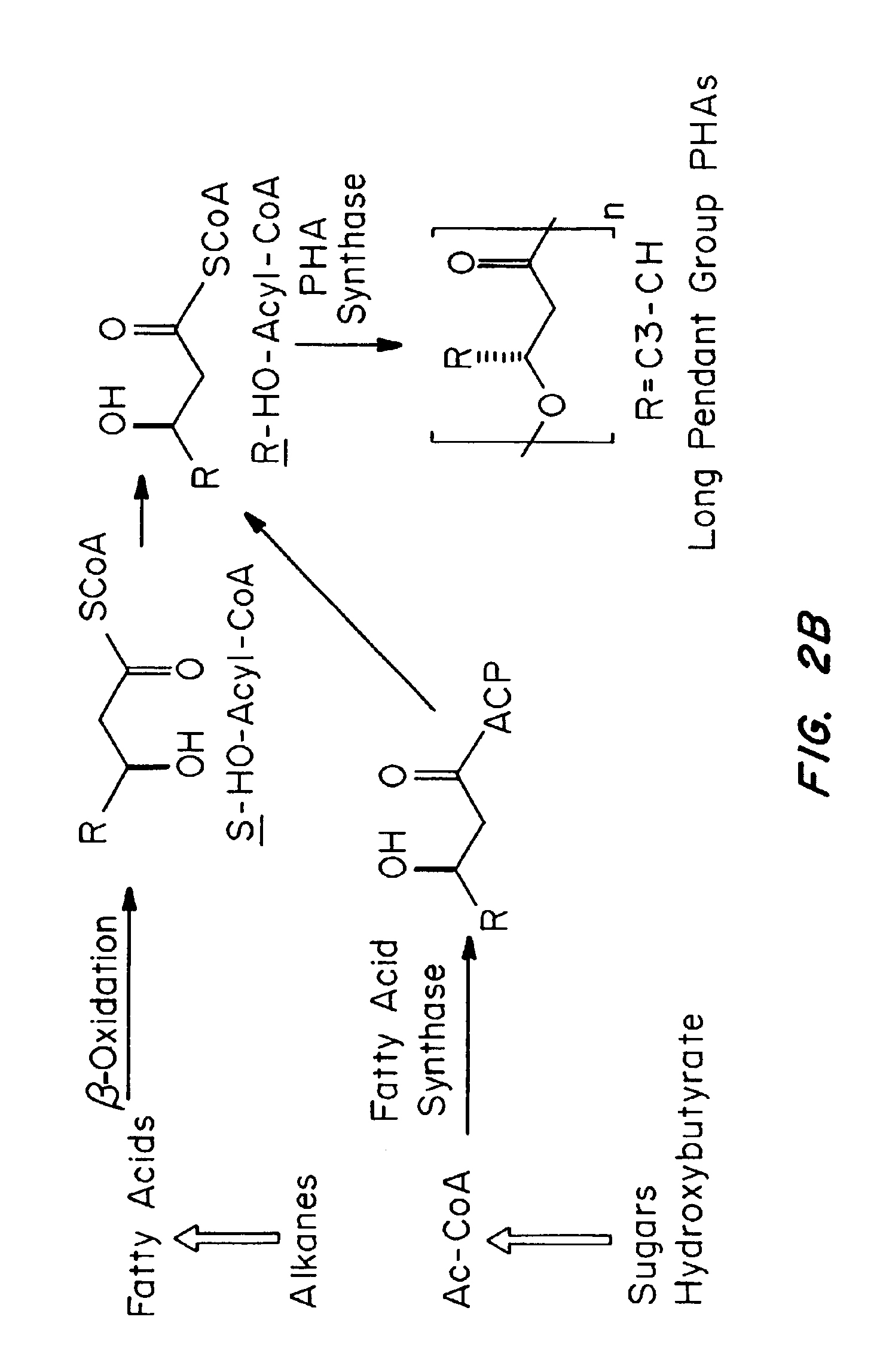
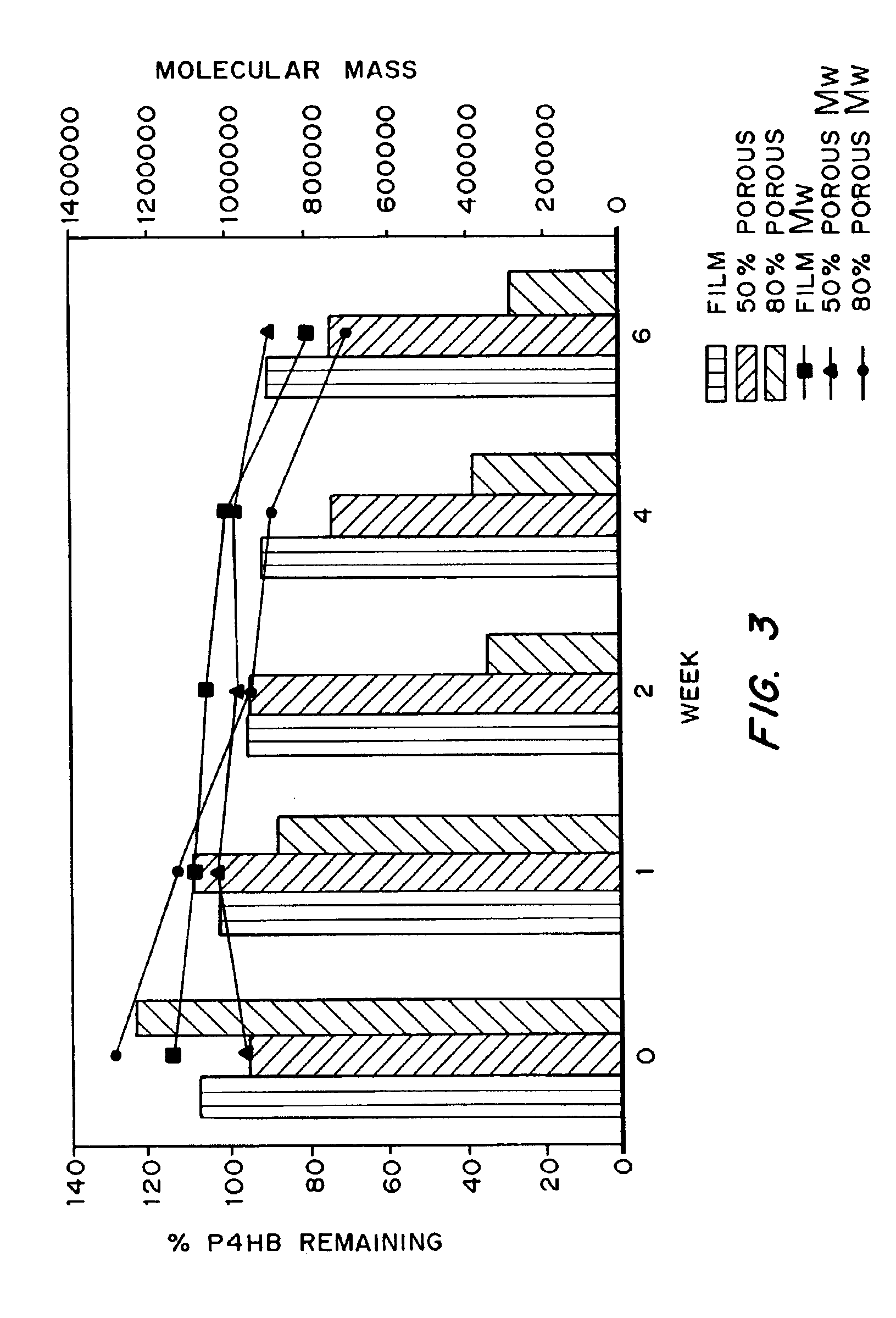
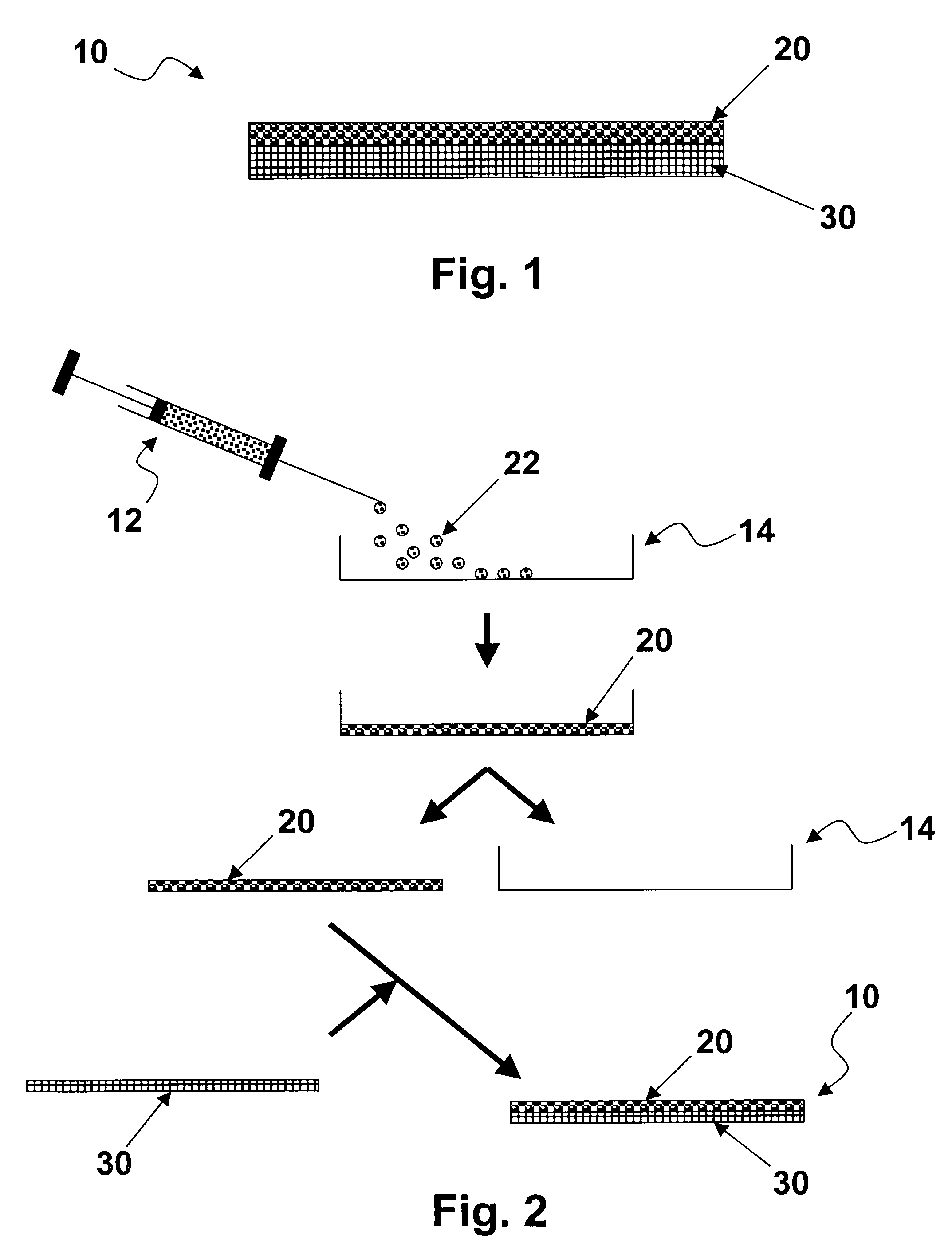
Devices formed of or including biocompatible polyhydroxyalkanoates are provided with controlled degradation rates, preferably less than one year under physiological conditions. Preferred devices include sutures, suture fasteners, meniscus repair devices, rivets, tacks, staples, screws (including interference screws), bone plates and bone plating systems, surgical mesh, repair patches, slings, cardiovascular patches, orthopedic pins (including bone filling augmentation material), adhesion barriers, stents, guided tissue repair / regeneration devices, articular cartilage repair devices, nerve guides, tendon repair devices, atrial septal defect repair devices, pericardial patches, bulking and filling agents, vein valves, bone marrow scaffolds, meniscus regeneration devices, ligament and tendon grafts, ocular cell implants, spinal fusion cages, skin substitutes, dural substitutes, bone graft substitutes, bone dowels, wound dressings, and hemostats. The polyhydroxyalkanoates can contain additives, be formed of mixtures of monomers or include pendant groups or modifications in their backbones, or can be chemically modified, all to alter the degradation rates. The polyhydroxyalkanoate compositions also provide favorable mechanical properties, biocompatibility, and degradation times within desirable time frames under physiological conditions.
Owner:TEPHA INC
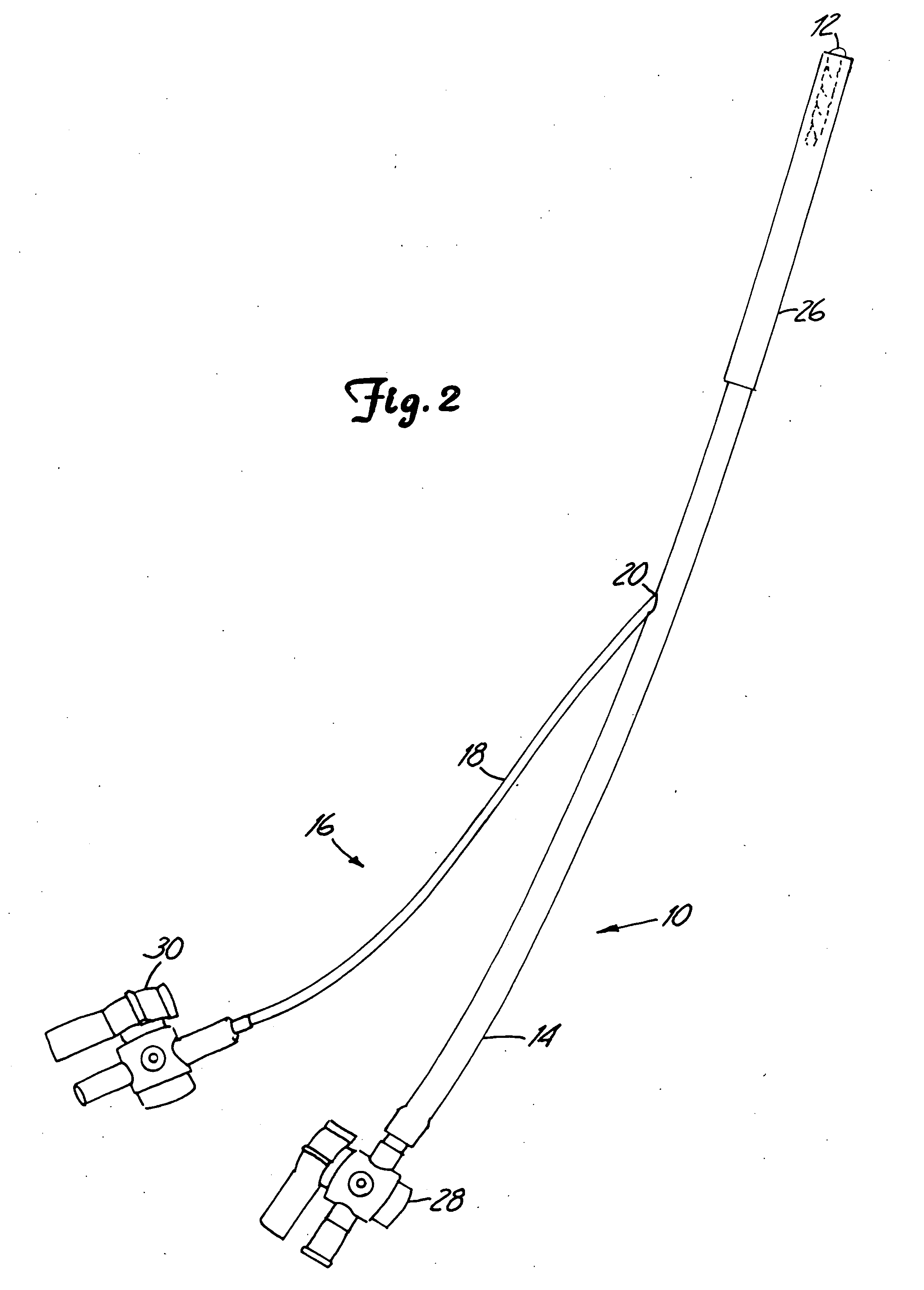
Attachment device and methods of using the same
Owner:MEDTRONIC INC
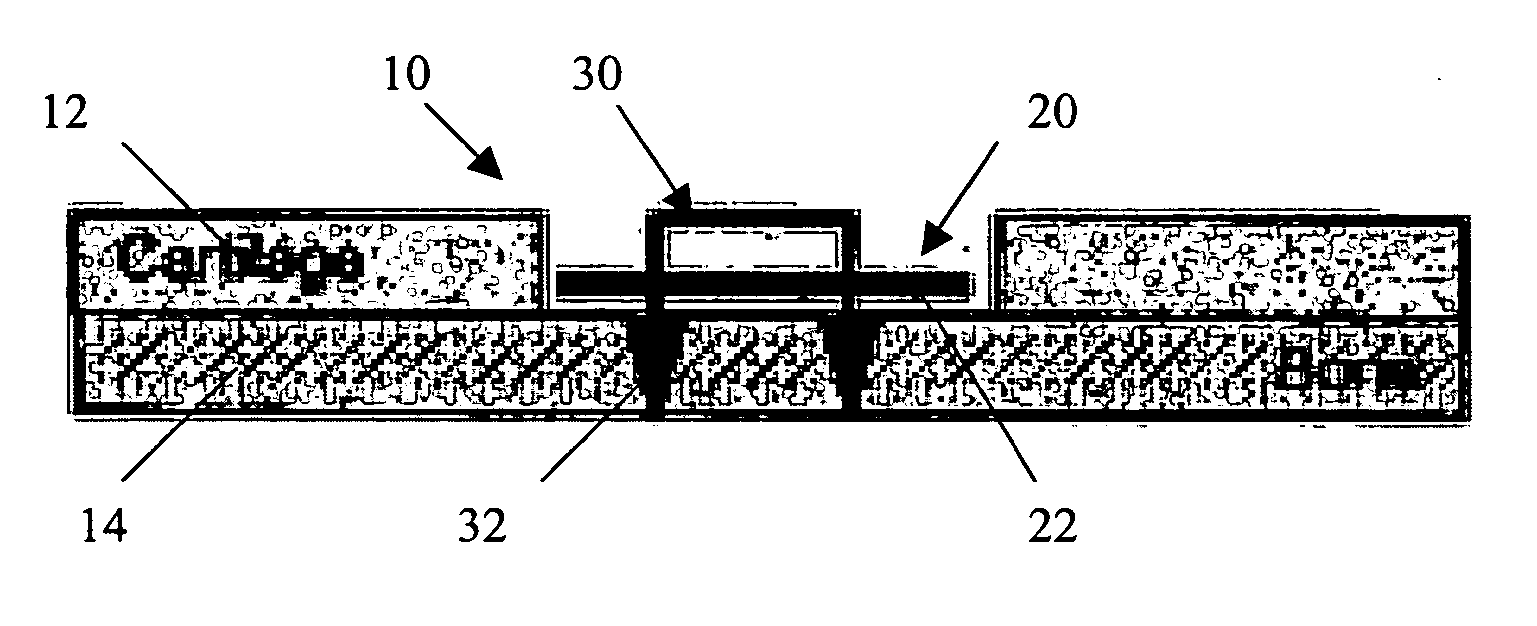
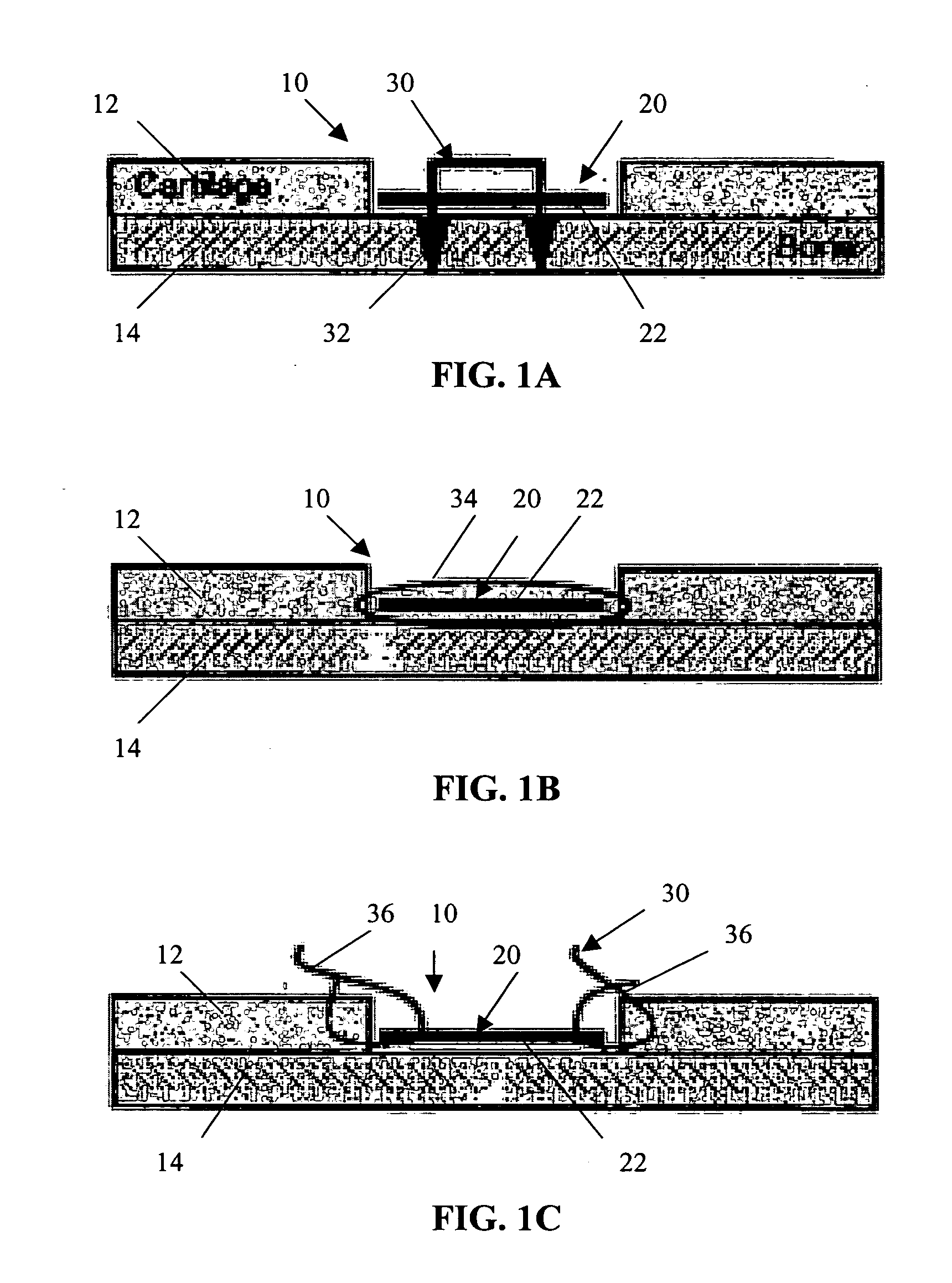
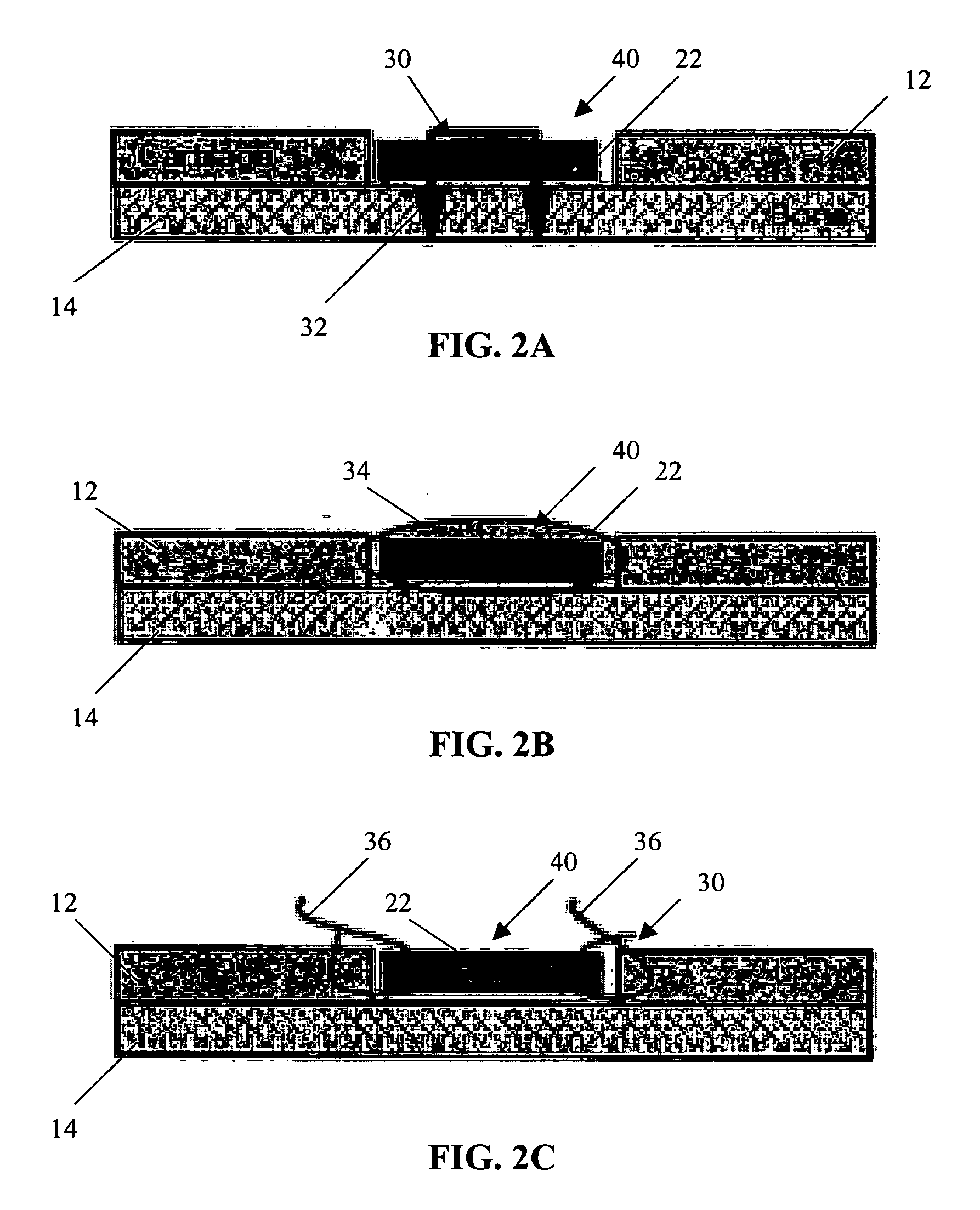
Use of reinforced foam implants with enhanced integrity for soft tissue repair and regeneration
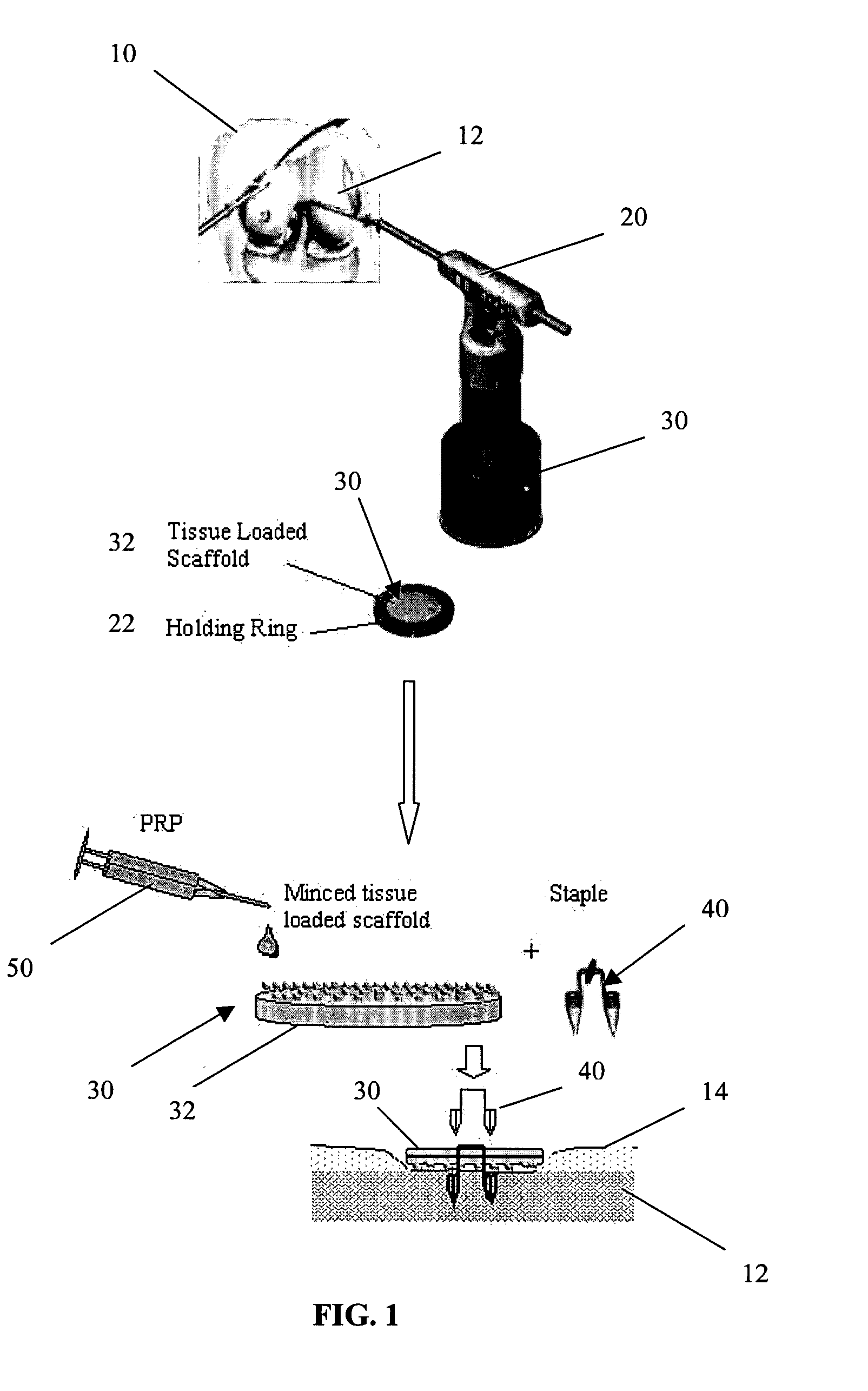
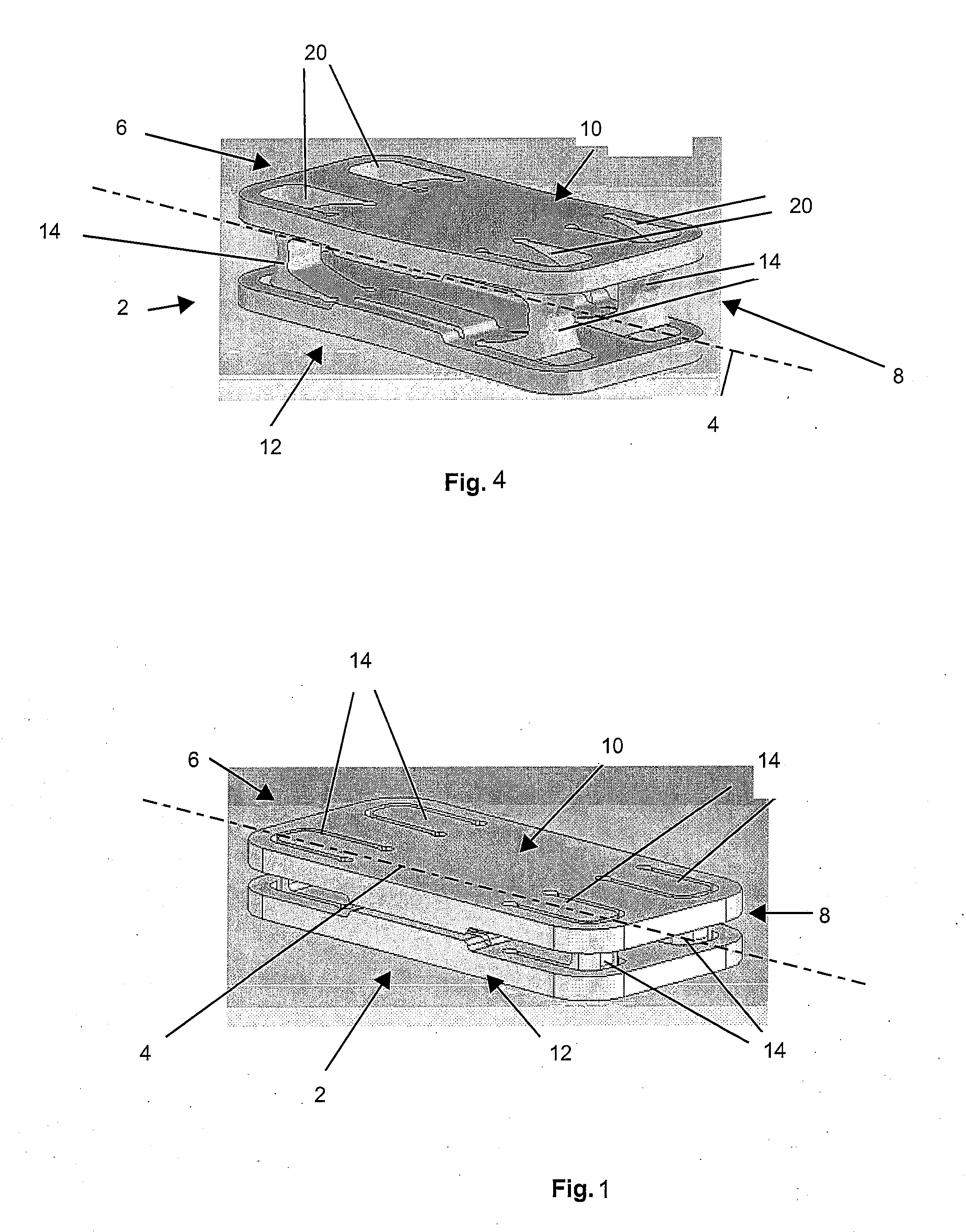
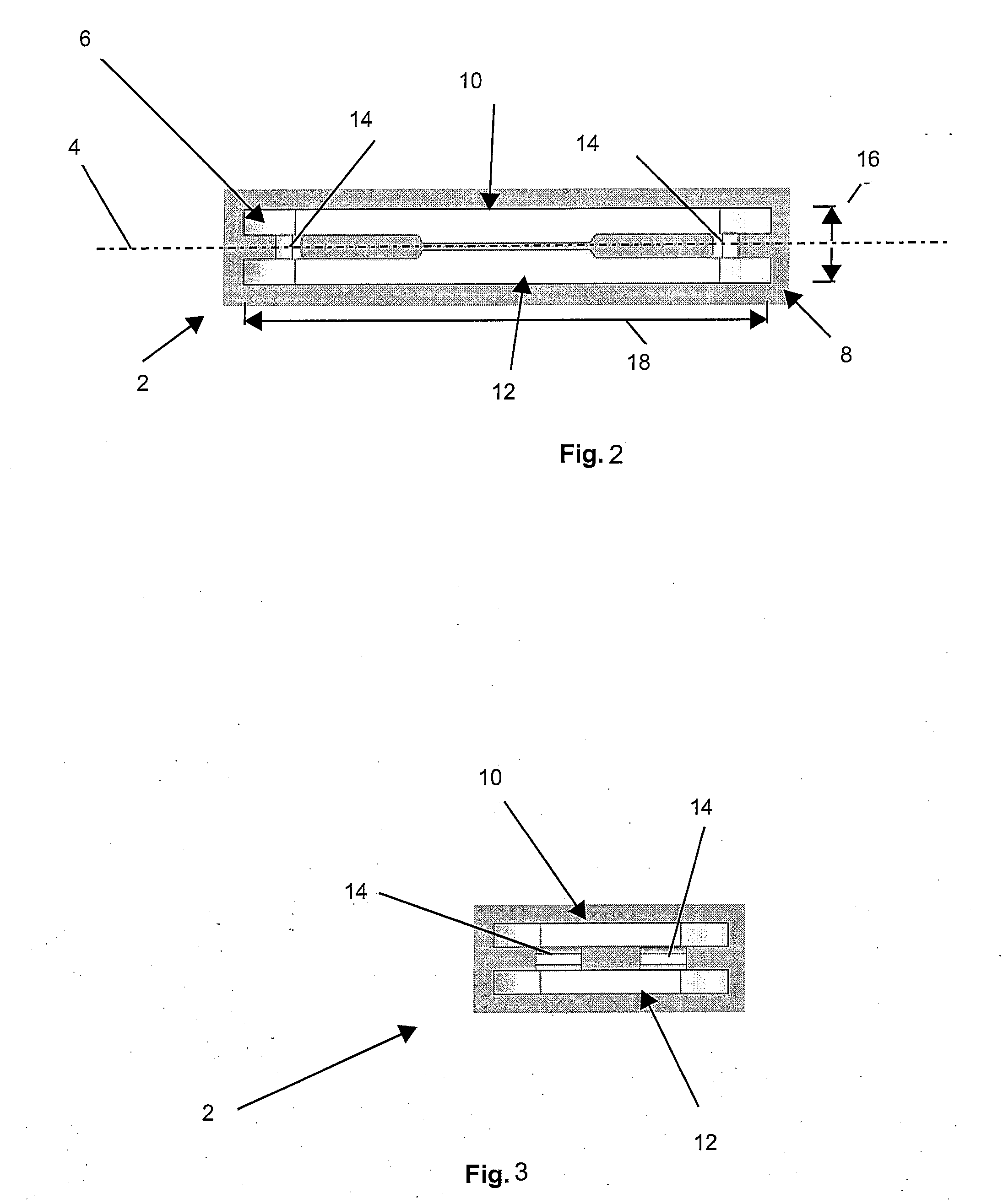
A biocompatible tissue repair stimulating implant or "scaffold" device is used to repair tissue injuries, particularly injuries to ligaments, tendons, and nerves. Such implants are especially useful in methods that involve surgical procedures to repair injuries to ligament, tendon, and nerve tissue in the hand and foot. The repair procedures may be conducted with implants that contain a biological component that assists in healing or tissue repair.
Owner:DEPUY MITEK INC
Reinforced foam implants with enhanced integrity for soft tissue repair and regeneration
InactiveUS6852330B2Sufficient structural integritySufficient propertyPowder deliveryOrganic active ingredientsTissue repairSoft tissue repair
A biocompatible tissue repair stimulating implant or “scaffold” device, and methods for making and using such a device, are provided. The implant includes one or more layers of a bioabsorbable polymeric foam having pores with an open cell pore structure. A reinforcement component is also present within the implant to contribute enhanced mechanical and handling properties. The implant houses a biological component that may be released to tissue adjacent the location in which the implant is implanted to faciliate and / or expedite the healing of tissue. This biological component resides primarily within the foam component of the implant, being incorporated within pores formed within the foam.
Owner:DEPUY SYNTHES PROD INC
Attachment device and methods of using the same
Devices for attaching a first mass and a second mass and methods of making and using the same are disclosed. The devices can be made from an resilient, elastic or deformable materials. The devices can be used to attach a heart valve ring to a biological annulus. The devices can also be used for wound closure or a variety of other procedures such as anchoring a prosthesis to surrounding tissue or another prosthesis, tissue repair, such as in the closure of congenital defects such as septal heart defects, tissue or vessel anastomosis, fixation of tissue with or without a reinforcing mesh for hernia repair, orthopedic anchoring such as in bone fusing or tendon or muscle repair, ophthalmic indications, laparoscopic or endoscopic tissue repair or placement of prostheses, or use by robotic devices for procedures such as those above performed remotely.
Owner:MEDTRONIC INC
Selectively absorbable/biodegradable, fibrous composite constructs and applications thereof
A family of selectively absorbable / biodegradable, fibrous composite constructs includes different combinations of biostable and absorbable / biodegradable yarns assembled as initially interdependent, load-bearing components, transitioning to exhibit independent functional properties during in vivo end-use. The family of constructs consists of two groups, one group is made of fiber-reinforced composites of high compliance, absorbable matrices of segmented polyaxial copolyesters reinforced with multifilament yarn constructs, which are combinations of ultrahigh molecular weight polyethylene fibers and at least one absorbable / biodegradable fiber selected from silk fibers and multifilament yarns made from linear segmented, l-lactide copolyesters and poly (3-hydroxyalkanoates, are useful in orthopedic, maxillofacial, urological, vascular, hernial repair and tissue engineering applications. The second group is made of coated and uncoated, warp-knitted mesh constructs for use in hernial, vascular, and urological tissue repair and tissue engineering.
Owner:POLY MED
Expandable support device and method of use
An expandable support device for tissue repair is disclosed. The device can be used to repair hard or soft tissue, such as bone or vertebral discs. The device can have multiple flat sides that remain flat during expansion. A method of repairing tissue is also disclosed. Devices and methods for adjusting (e.g., removing, repositioning, resizing) deployed orthopedic expandable support devices are also disclosed. The expandable support devices can be engaged by an engagement device. The engagement device can longitudinally expand the expandable support device. The expandable support device can be longitudinally expanded until the expandable support device is substantially in a pre-deployed configuration. The expandable support device can be then be physically translated and / or rotated.
Owner:STOUT MEDICAL GROUP
Methods for making and using composites, polymer scaffolds, and composite scaffolds
InactiveUS20070187857A1Promote safe productionTime-consumeDomestic articlesProsthesisOrganic solventTissue repair
The present invention relates to methods of making and using composites and scaffolds as implantable devices useful for tissue repair, guided tissue regeneration, and tissue engineering. In particular, the present invention relates to methods of making and using compression molded resorbable thermoplastic polymer composites which can be subsequently processed with non-organic solvents to create porous, resorbable thermoplastic polymer scaffolds or composite scaffold with interconnected porosity. Furthermore, these composites or scaffolds can be coated with an organic and / or inorganic material.
Owner:LOREM VASCULAR PTE LTD
Methods and devices for tissue repair
Owner:SMITH & NEPHEW INC
Expandable support device and method of use
An expandable support device for tissue repair is disclosed. The device can be used to repair hard or soft tissue, such as bone or vertebral discs. A method of repairing tissue is also disclosed. The device and method can be used to treat compression fractures. The compression fractures can be in the spine. The device can be deployed by compressing the device longitudinally resulting in radial expansion.
Owner:NUVASIVE
Expandable support device and method of use
An expandable support device for tissue repair is disclosed. The device can be used to repair hard or soft tissue, such as bone or vertebral discs. A method of repairing tissue is also disclosed. The device and method can be used to treat compression fractures. The compression fractures can be in the spine. The device can be deployed by compressing the device longitudinally resulting in radial expansion.
Owner:STOUT MEDICAL GROUP
Method and apparatus for resurfacing an articular surface
A biocompatible, bioresorbable tissue repair implant or scaffold device is provided for use in repairing a variety of cartilage tissue injuries, and particularly for resurfacing and / or repairing damaged or diseased cartilage. The repair procedures may be conducted with tissue repair implants that contain a biological component that assists in delaying or arresting the progression of degenerative joint diseases and in enhancing tissue healing or repair. The biocompatible, bioresorbable tissue repair implants include a scaffold and particles of viable tissue derived from cartilage tissue, such that the tissue and the scaffold become associated. The particles of living tissue contain one or more viable cells that can migrate from the tissue and populate the scaffold.
Owner:DEPUY SYNTHES PROD INC
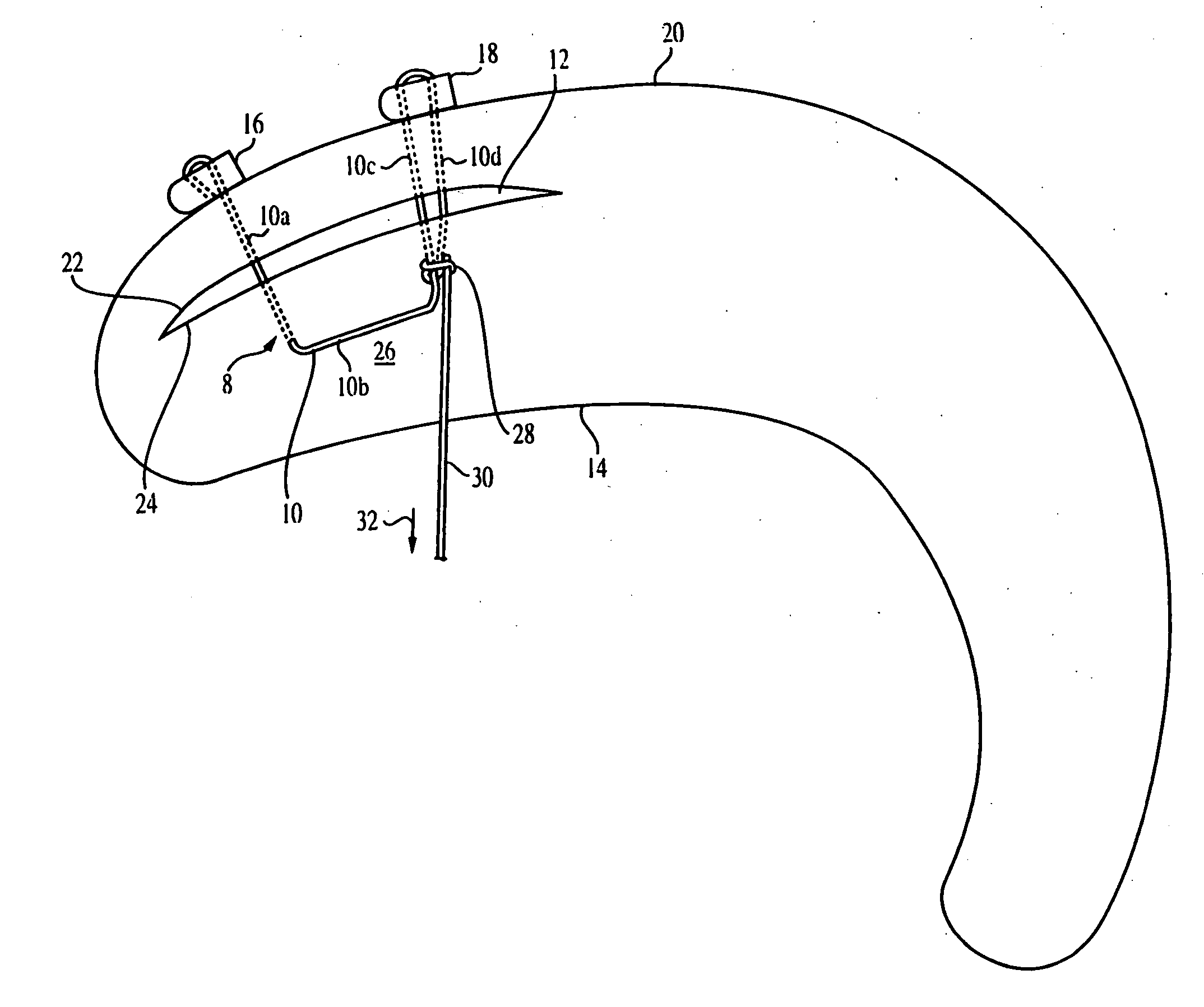
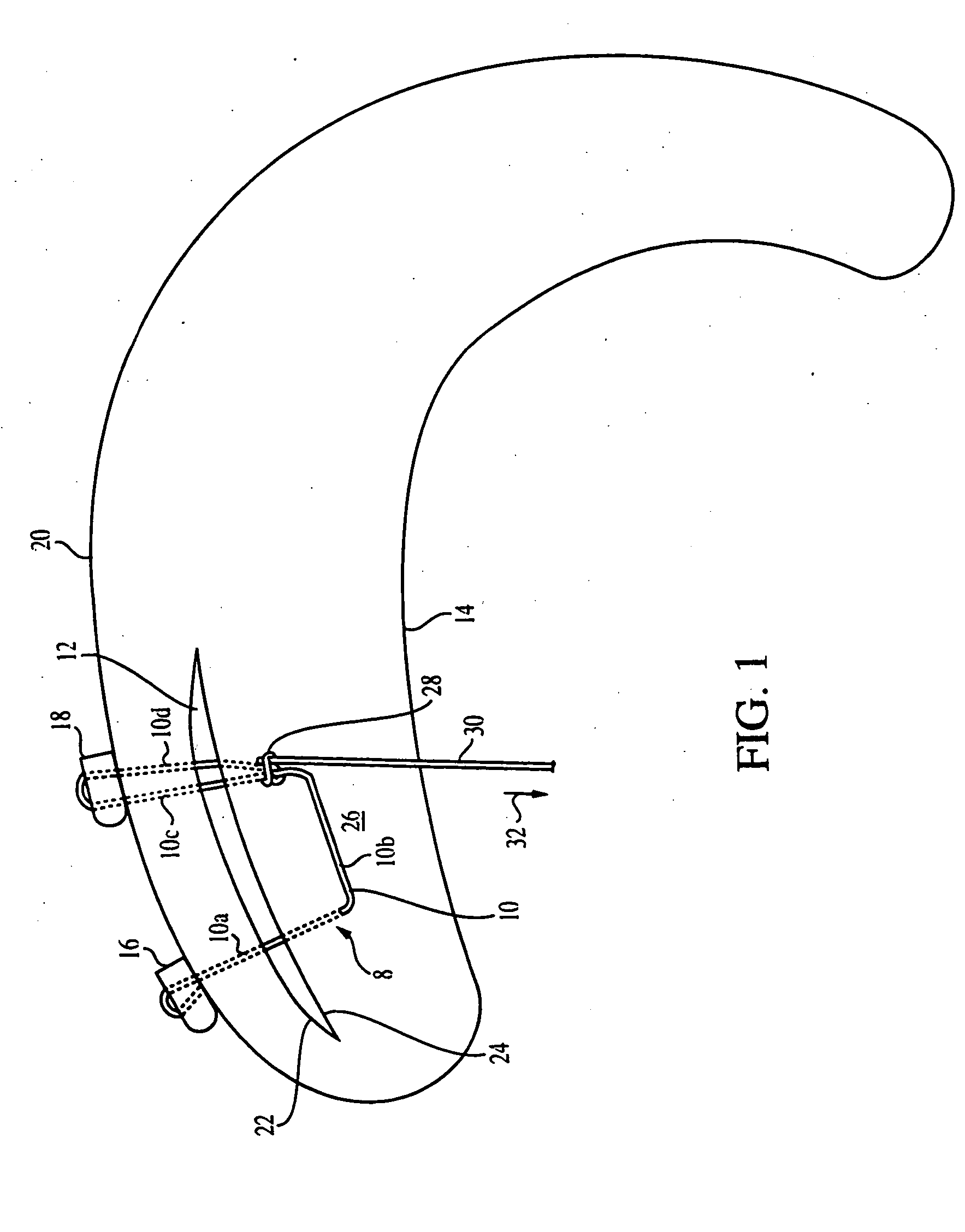
Meniscal suturing instrument and method
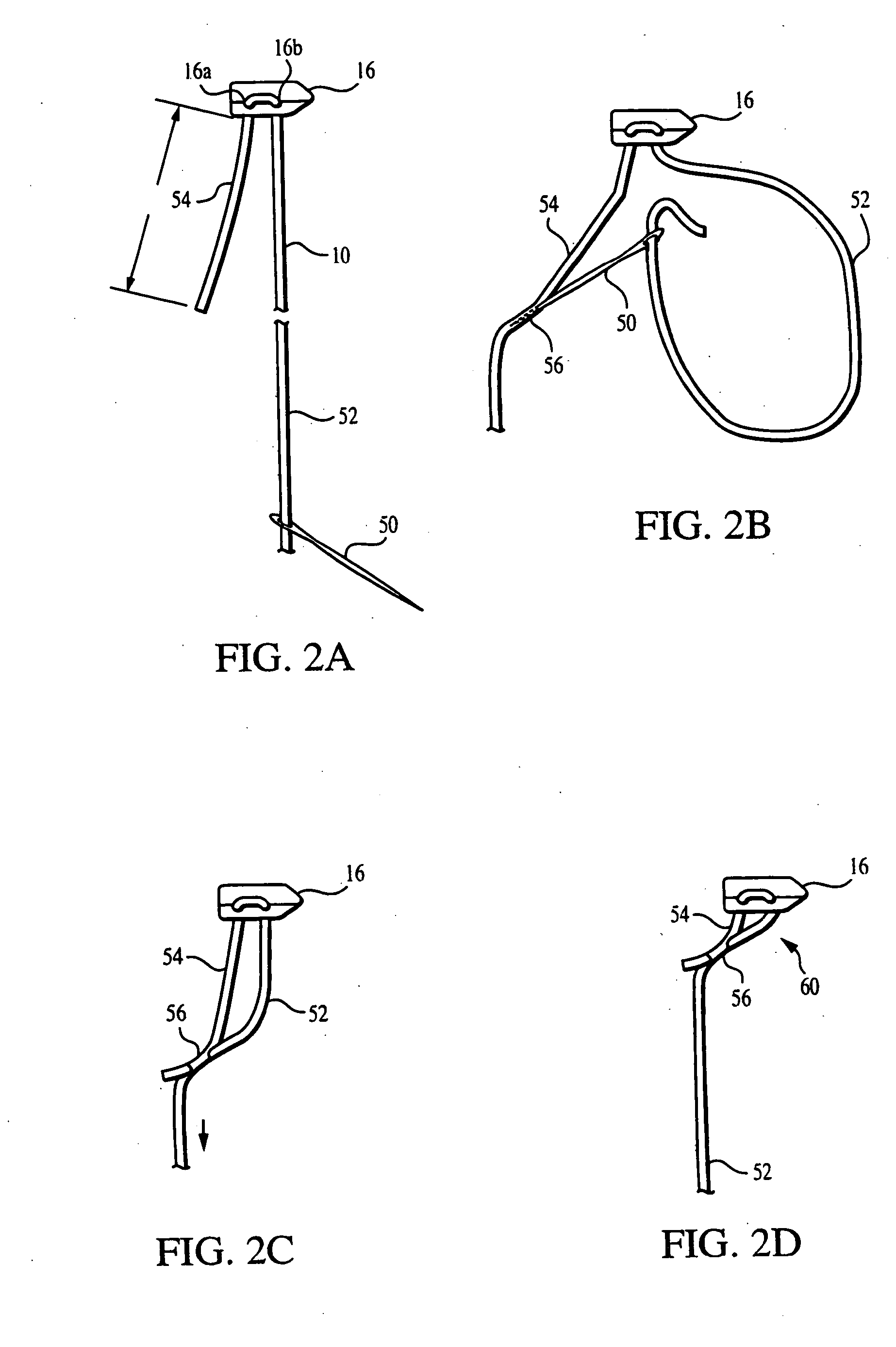
ActiveUS7118583B2Easy withdrawalEasy to operateSuture equipmentsSurgical needlesTissue repairMeniscal suture
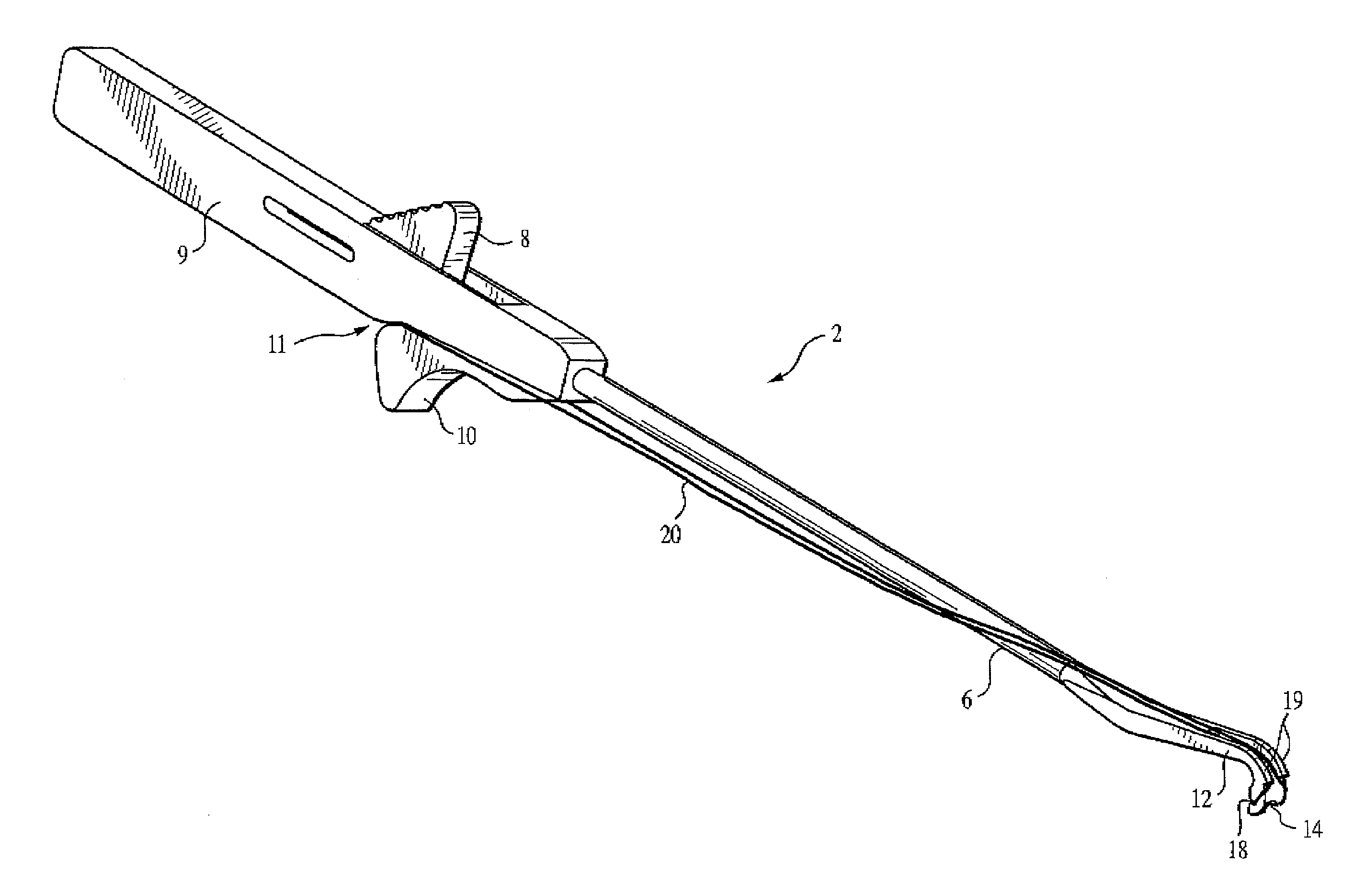
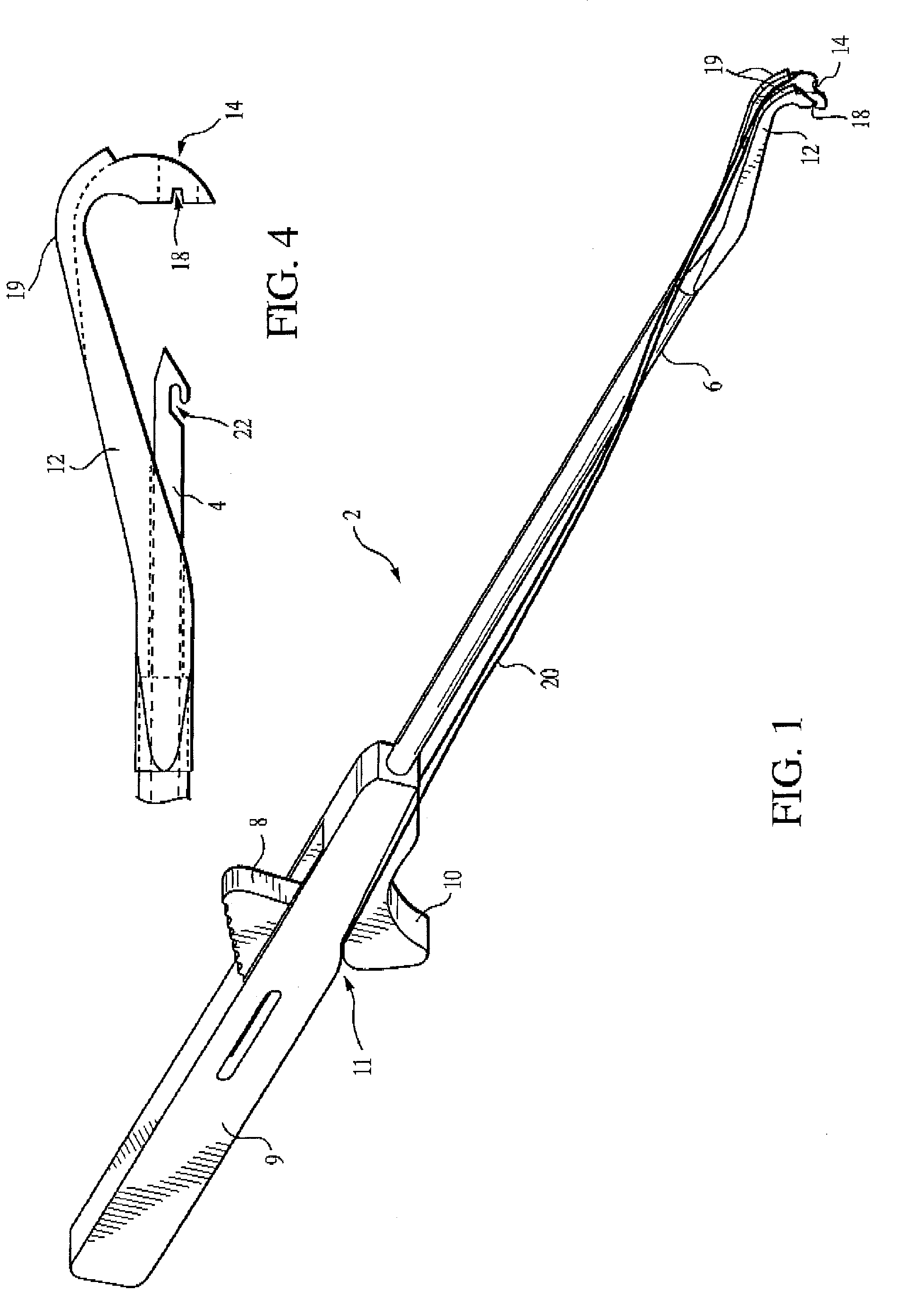
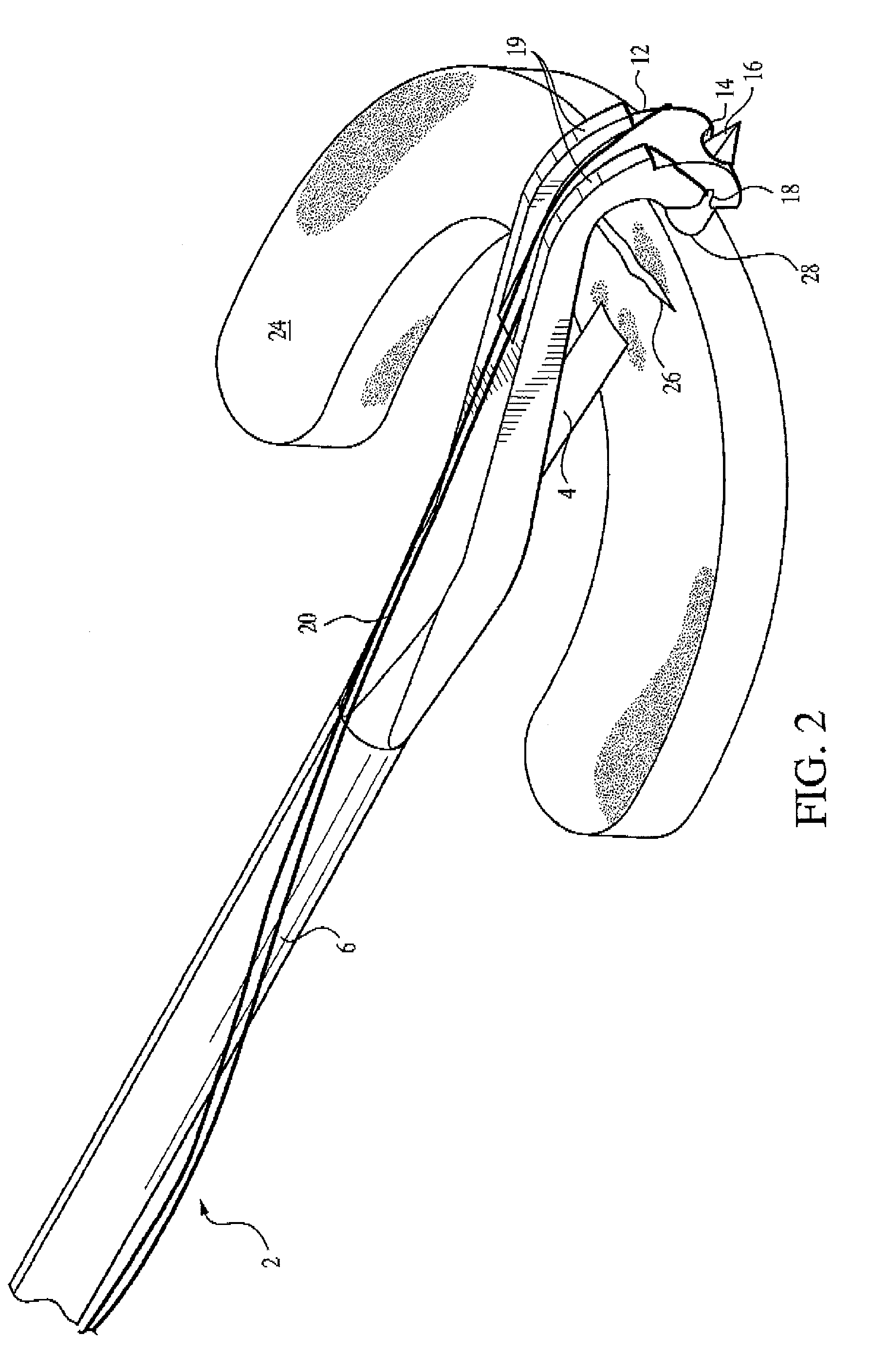
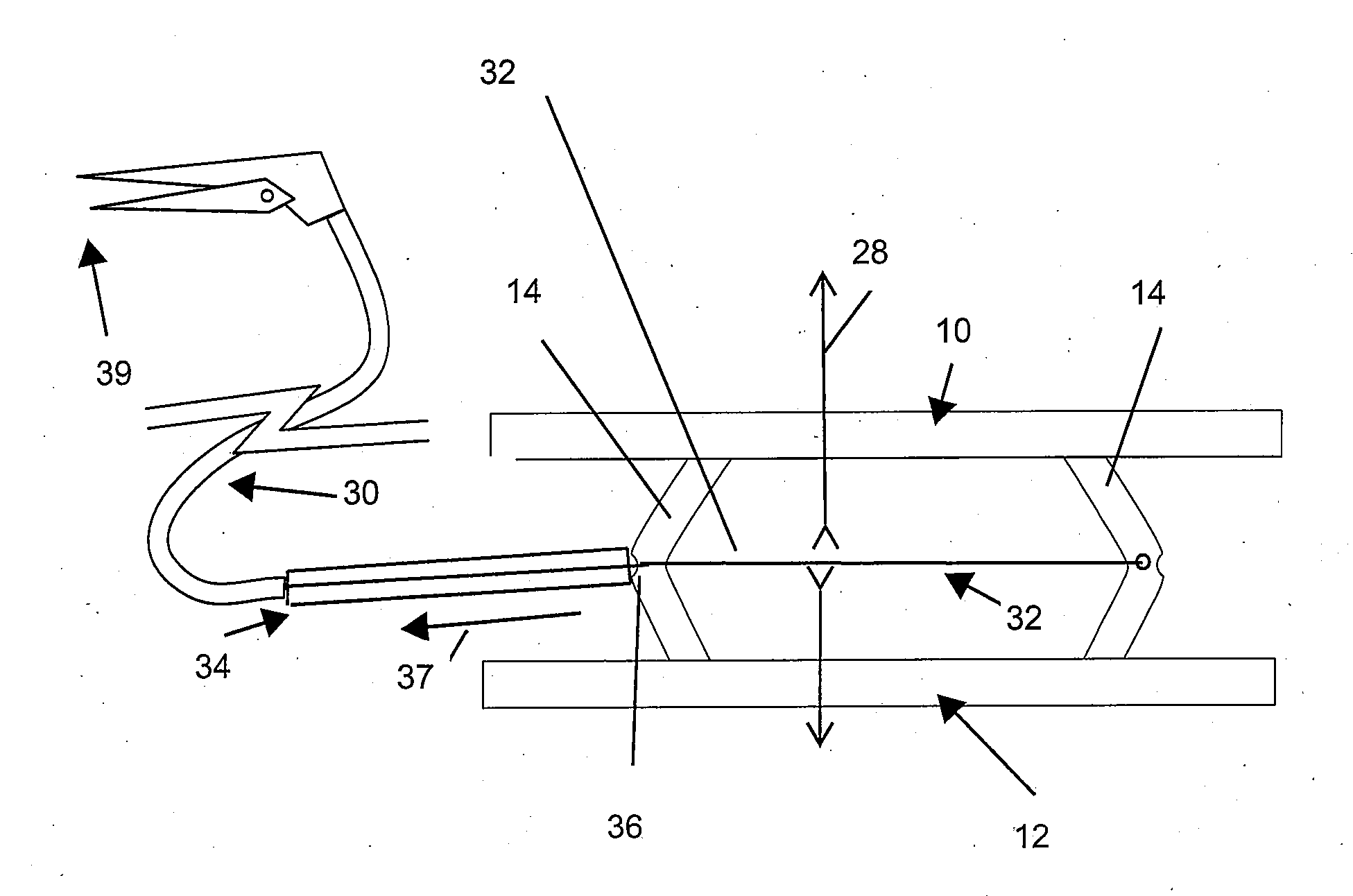
An instrument for surgical suturing includes a shaft, and a needle disposed slidably for longitudinal travel with respect to the shaft. A prong formed on one end of the shaft projects across a longitudinal axis of the needle and has an opening formed through the prong that provides a clearance through which a tip portion of the needle passes when it is advanced. Slots formed on a surface of the prong facing the shaft releasably hold suture across the opening. Advancing the needle through the clearance allows a hook formed on the tip of the needle to capture the suture. Once withdrawn, the needle draws the suture back through tissue pierced during advancement. The suture is released from the slots and drawn back through the tissue for further knot tying and suturing to effect the tissue repairs.
Owner:ARTHREX
Expandable support device and method of use
An expandable support device for tissue repair is disclosed. The device can be used to repair hard or soft tissue, such as bone or vertebral discs. A method of repairing tissue is also disclosed. The device and method can be used to treat compression fractures. The compression fractures can be in the spine. The device can be deployed by compressing the device longitudinally resulting in radial expansion.
Owner:NUVASIVE
Endoscopic capsular suture plication instrument and method
InactiveUS6893448B2Easy to operateEasy accessSuture equipmentsSurgical needlesTissue repairKnot tying
An instrument for surgical suturing includes a shaft, and a needle disposed slidably for longitudinal travel with respect to the shaft. A prong formed on one end of the shaft projects across a longitudinal axis of the needle and has an opening formed through the prong that provides a clearance through which a tip portion of the needle passes when it is advanced. Slots formed on a surface of the prong facing the shaft releasably hold suture across the opening. The suture is held in the slots by passing the suture strands separately back around the sides of the prong, then together up through a channel formed on the prong above the slots, and securing the strands in a pinch slot formed in a handle of the instrument. Advancing the needle through the clearance allows a hook formed on the tip of the needle to capture the suture. Once withdrawn, the needle draws the suture back through tissue pierced during advancement. The suture is released from the slots and drawn back through the tissue for further knot tying and suturing to effect the tissue repairs.
Owner:ARTHREX
Medical devices and applications of polyhydroxyalkanoate polymers
InactiveUS6867247B2Reduce probabilityHigh porositySuture equipmentsStentsTissue repairBiocompatibility Testing
Devices formed of or including biocompatible polyhydroxyalkanoates are provided with controlled degradation rates, preferably less than one year under physiological conditions. Preferred devices include sutures, suture fasteners, meniscus repair devices, rivets, tacks, staples, screws (including interference screws), bone plates and bone plating systems, surgical mesh, repair patches, slings, cardiovascular patches, orthopedic pins (including bone filling augmentation material), adhesion barriers, stents, guided tissue repair / regeneration devices, articular cartilage repair devices, nerve guides, tendon repair devices, atrial septal defect repair devices, pericardial patches, bulking and filling agents, vein valves, bone marrow scaffolds, meniscus regeneration devices, ligament and tendon grafts, ocular cell implants, spinal fusion cages, skin substitutes, dural substitutes, bone graft substitutes, bone dowels, wound dressings, and hemostats. The polyhydroxyalkanoates can contain additives, be formed of mixtures of monomers or include pendant groups or modifications in their backbones, or can be chemically modified, all to alter the degradation rates. The polyhydroxyalkanoate compositions also provide favorable mechanical properties, biocompatibility, and degradation times within desirable time frames under physiological conditions.
Owner:TEPHA INC
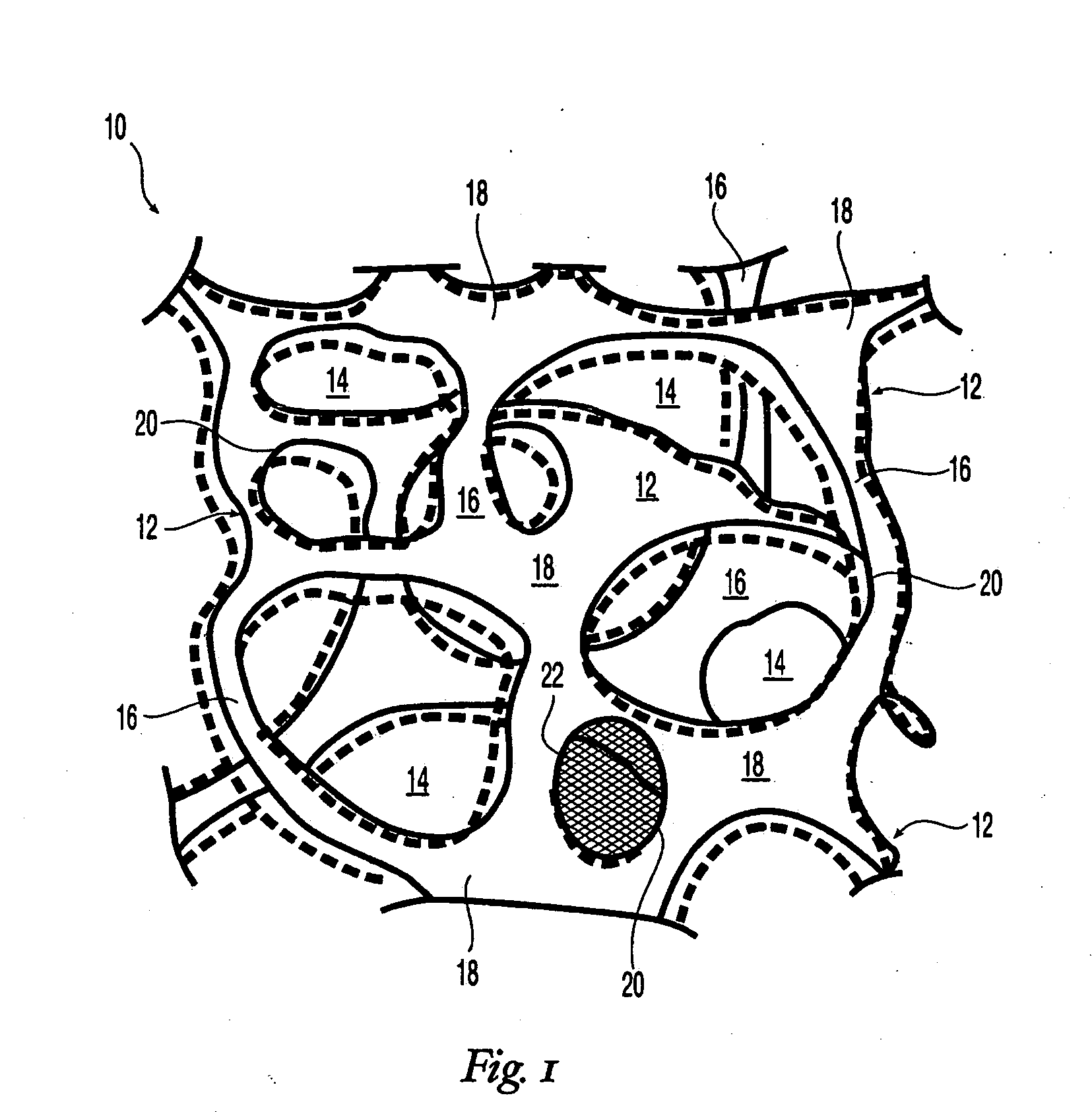
High performance reticulated elastomeric matrix preparation, properties, reinforcement, and use in surgical devices, tissue augmentation and/or tissue repair
InactiveUS20070190108A1Different and simple configurationEfficiently employedProsthesisElastomerSurgical operation
This invention relates to reticulated elastomeric matrices, their manufacture, their post-processing, such as their reinforcement, compressive molding or annealing, and uses including uses for implantable devices into or for topical treatment of patients, such as humans and other animals, for surgical devices, tissue augmentation, tissue repair, therapeutic, nutritional, or other useful purposes.
Owner:BIOMERIX CORP
Tissue engineering devices for the repair and regeneration of tissue
Tissue engineering devices for use in the repair or regeneration of tissue made of support scaffolds and cell sheets.
Owner:ETHICON INC
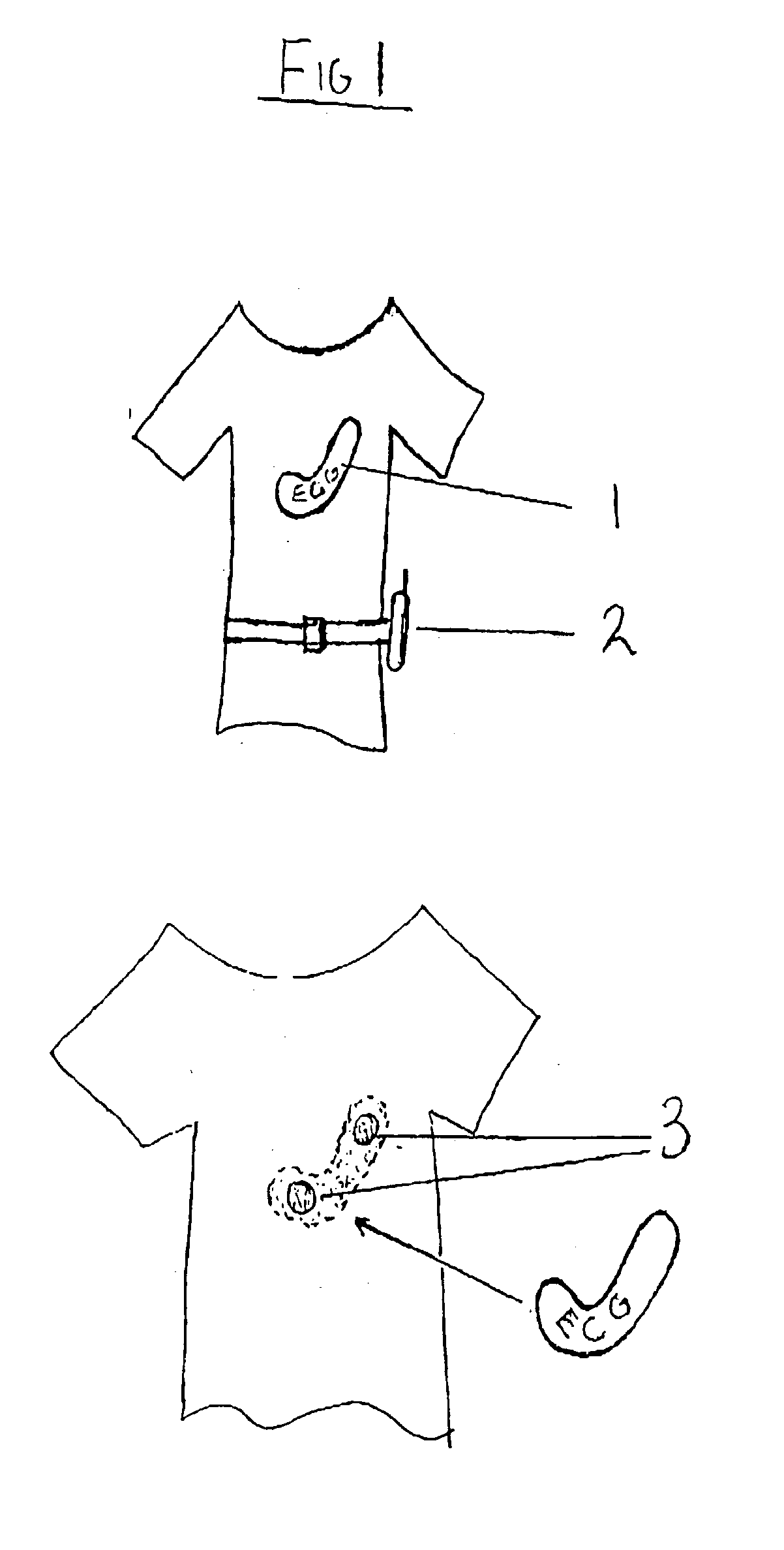
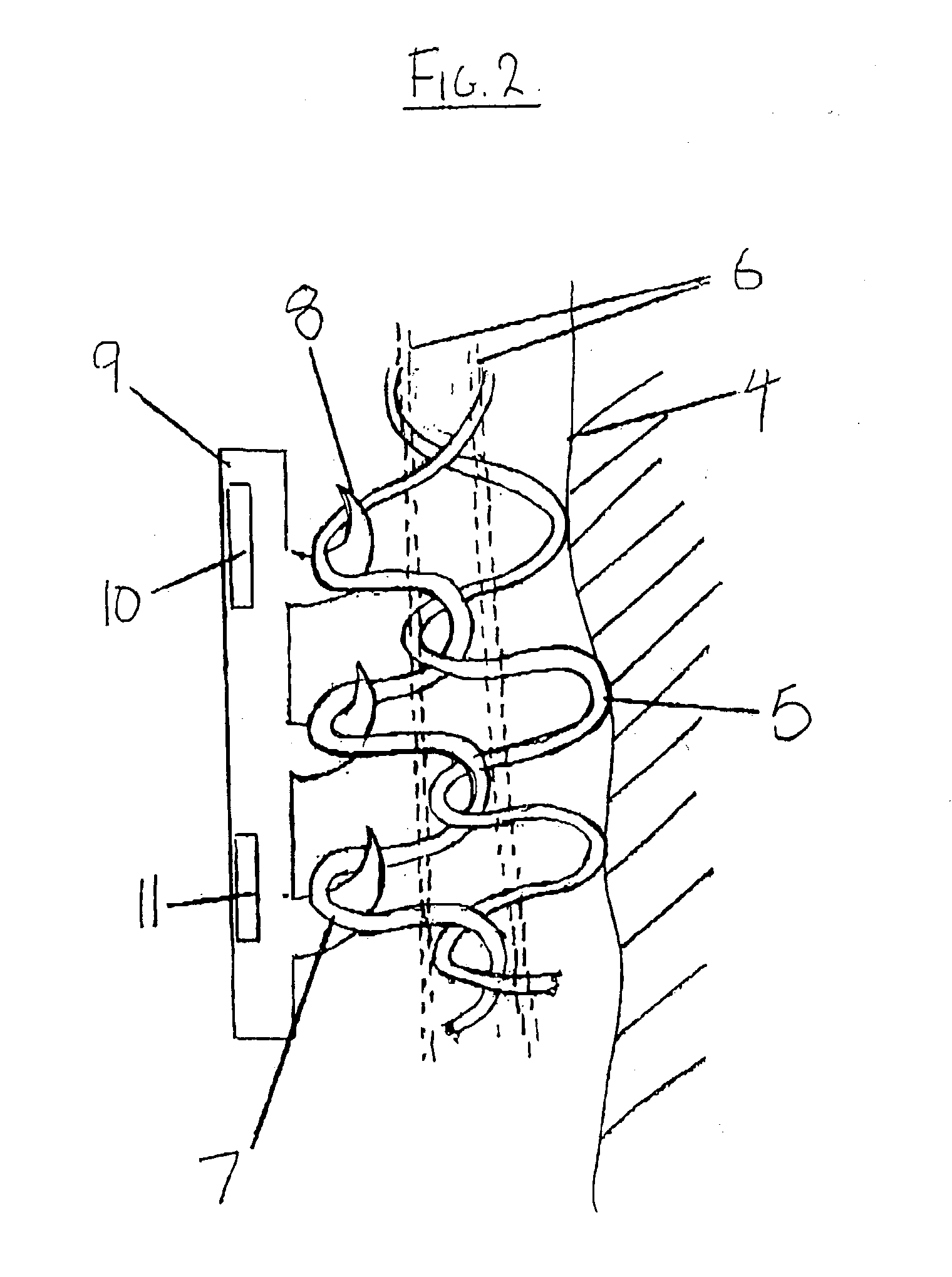
Health monitoring garment
InactiveUS20030212319A1Increase the conductive areaConductivity of contactElectrocardiographyWeft knittingFiberElectricity
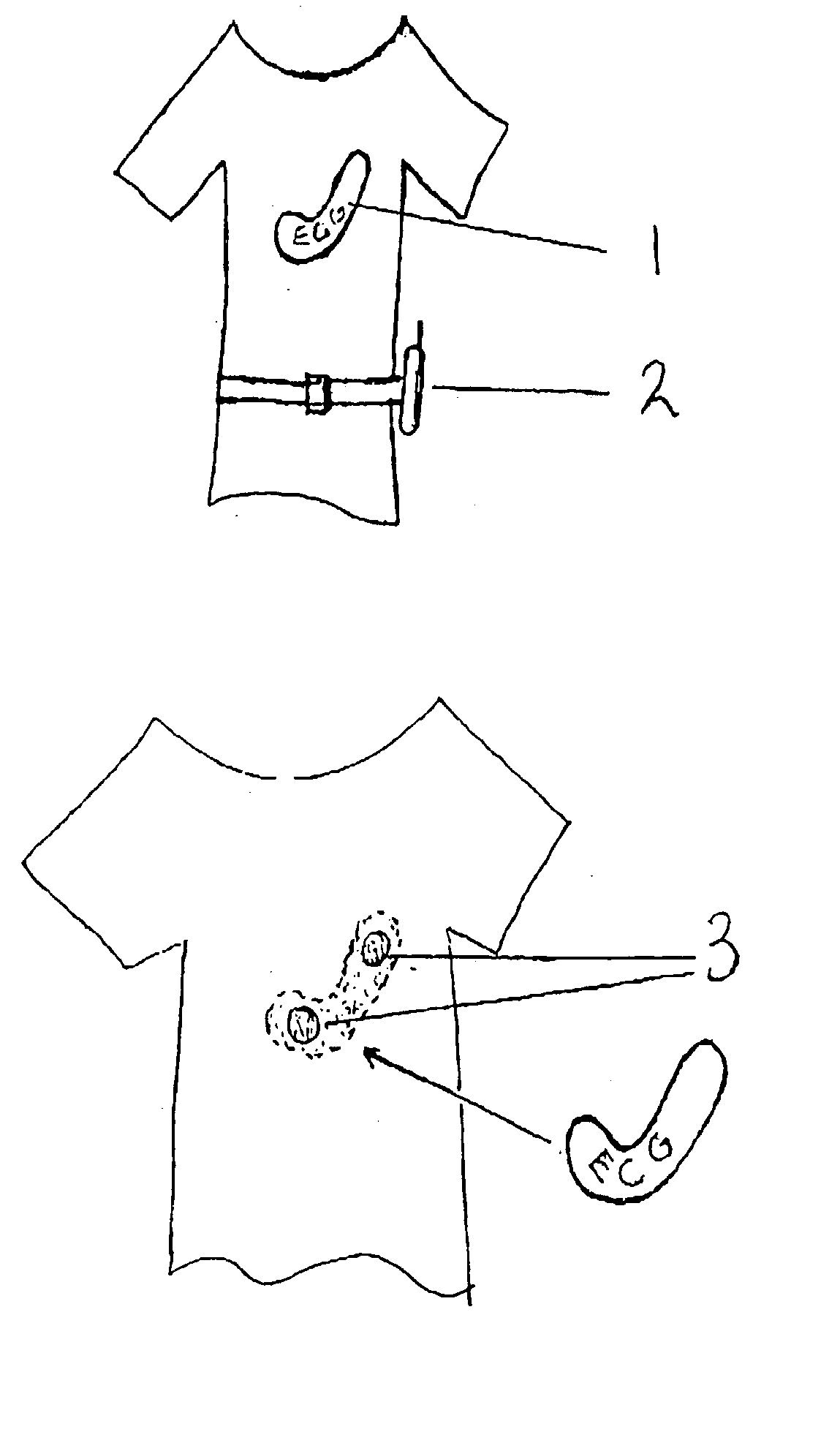
A health monitoring garment which employs a means of conducting electricity from the surface of the skin, through the fibres of a fabric to another fabric which is removably attached to it and contains a microprocessor, telemetry and a power source to monitor and transmit EKG data of a person wearing the clothing, as illustrated in FIG. 2. Removability enables tile garment to be washed and the electronics to be kept separate from the washing and tumble drying process. The same system can be used in reverse to effect cardiac pacing or defibrillation or to deliver other forms of electronically conveyed healing such as tissue repair.
Owner:MAGILL ALAN REMY
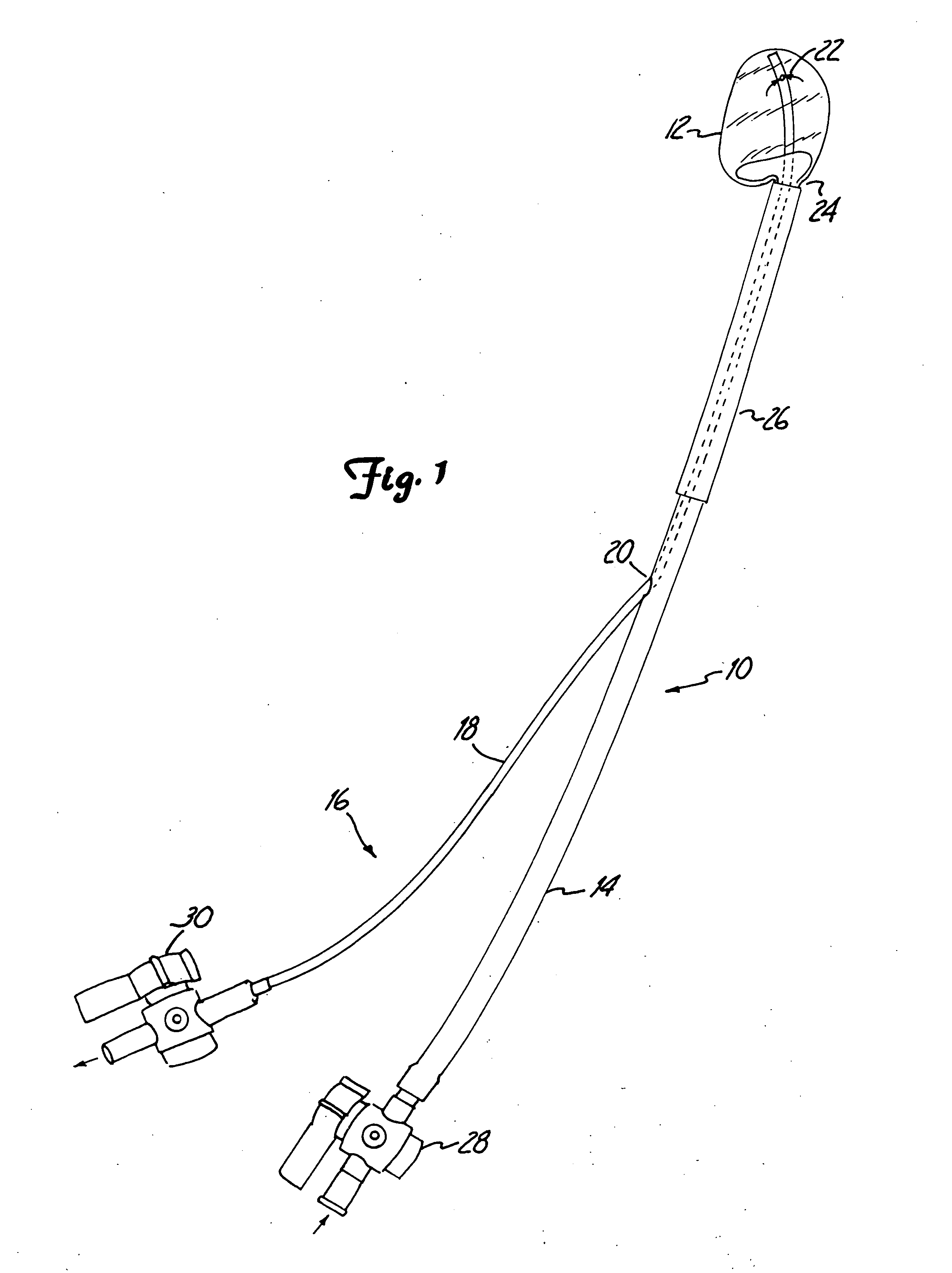
Knee joint prosthesis
InactiveUS20050043808A1Eliminate the effects ofImprove propertiesImpression capsAnkle jointsTissue repairProsthesis
A method, and related composition and apparatus for repairing a tissue site. The method involves the use of a curable polyurethane biomaterial composition having a plurality of parts adapted to be mixed at the time of use in order to provide a flowable composition and to initiate cure. The flowable composition can be delivered using minimally invasive means to a tissue site and there fully cured provide a permanent and biocompatible prosthesis for repair of the tissue site. Further provided are a mold apparatus, e.g., in the form of a balloon or tubular cavity, for receiving a biomaterial composition, and a method for delivering and filling the mold apparatus with a curable composition in situ to provide a prosthesis for tissue repair.
Owner:ADVANCED BIO SURFACE
Viable tissue repair implants and methods of use
InactiveUS20050125077A1Good retention of cellEasy to integrateBone implantSkeletal disorderTissue repairRepair tissue
Biocompatible tissue implants are provided for repairing a tissue injury or defect. The tissue implants comprise a biological tissue slice that serves as a source of viable cells capable of tissue regeneration and / or repair. The biological tissue slice can be harvested from healthy tissue to have a geometry that is suitable for implantation at the site of the injury or defect. The harvested tissue slice is dimensioned to allow the viable cells contained within the tissue slice to migrate out and proliferate and integrate with tissue surrounding the injury or defect site. Methods for repairing a tissue injury or defect using the tissue implants are also provided.
Owner:DEPUY SYNTHES PROD INC
Expandable support device and method of use
An expandable support device, such as an ultra thin expanding stent device, for tissue repair is disclosed. The expandable support device can be used to repair hard or soft tissue, such as bone or vertebral discs. A method of repairing tissue is also disclosed. The expandable support device can have a substantially flat top plate and a substantially flat bottom plate. The top plate can be attached to the bottom plate by expandable struts.
Owner:STOUT MEDICAL GROUP
Meniscal repair device and method
InactiveUS20060280768A1Increase the itineraryEncourage healingBone implantTissue regenerationTissue repairInsertion stent
Methods and apparatus for treating meniscal tissue damage are disclosed, including a biocompatible meniscal repair device comprising a stent. The tissue repair device is adapted to be placed in contact with a defect in the meniscus and can preferably provide a structure for supporting meniscal tissue and / or encouraging tissue growth through contact with vascularized portions of the meniscus or as a conduit for introduction of exogenous healing therapies.
Owner:DEPUY SYNTHES PROD INC +1
Nanoparticle Mediated Ultrasound Therapy and Diagnostic Imaging
InactiveUS20080045865A1Enhanced and localized and targeted hyperthermiaMinimizing collateral damageUltrasonic/sonic/infrasonic diagnosticsUltrasound therapyDiseaseTissue repair
The present invention relates to systems and methods for localized delivery of heat, useful for localized imaging and treatment of a biological material. The systems and methods of the invention can be utilized for localized treatment of cancer, inflammation or other disorders involving over proliferation of tissue, and for tissue repair. The method comprises exposing nanoparticles to electromagnetic radiation under conditions wherein the nanoparticles generate microbubbles which emit heat when exposed to ultrasonic radiation.
Owner:KISLEV HANOCH
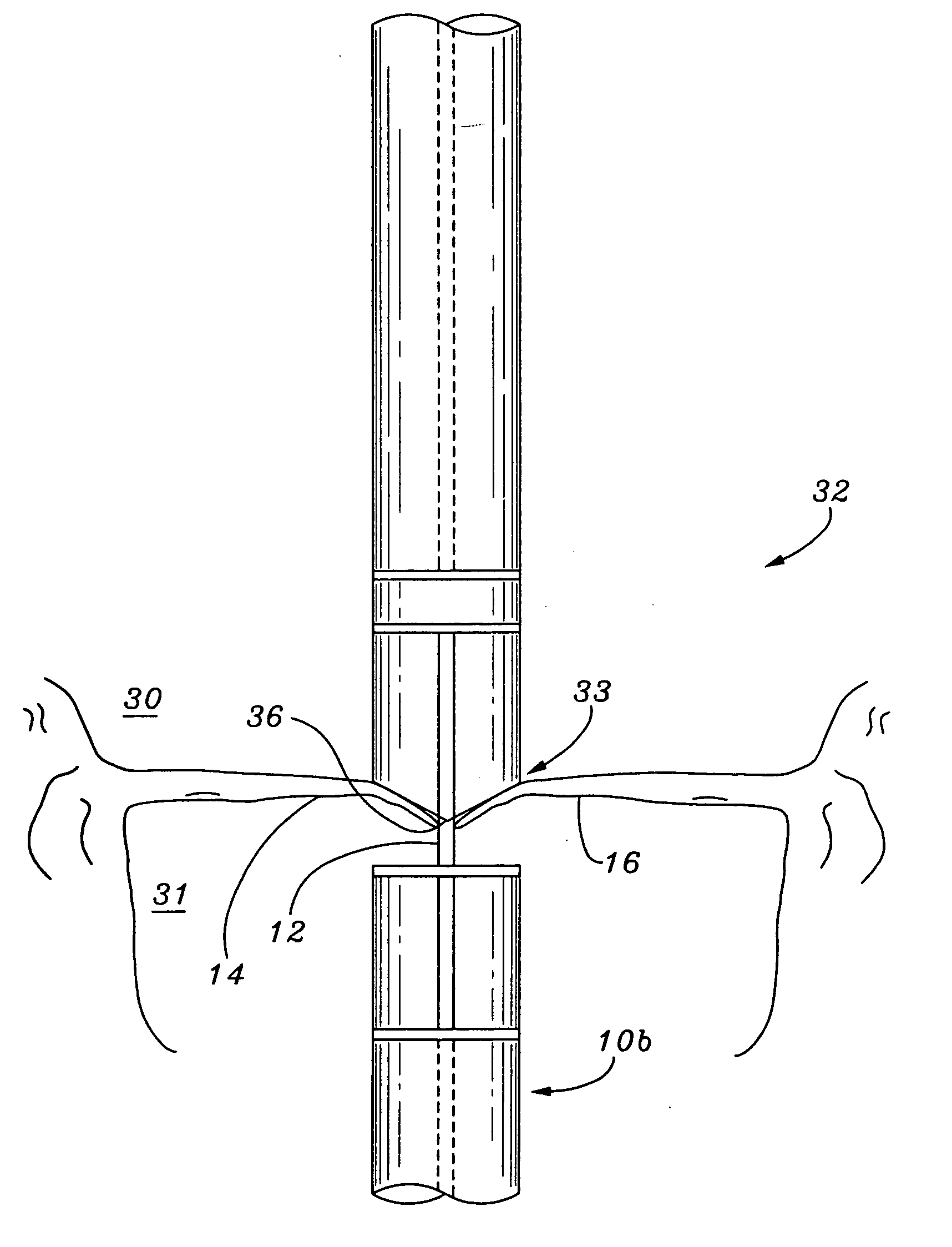
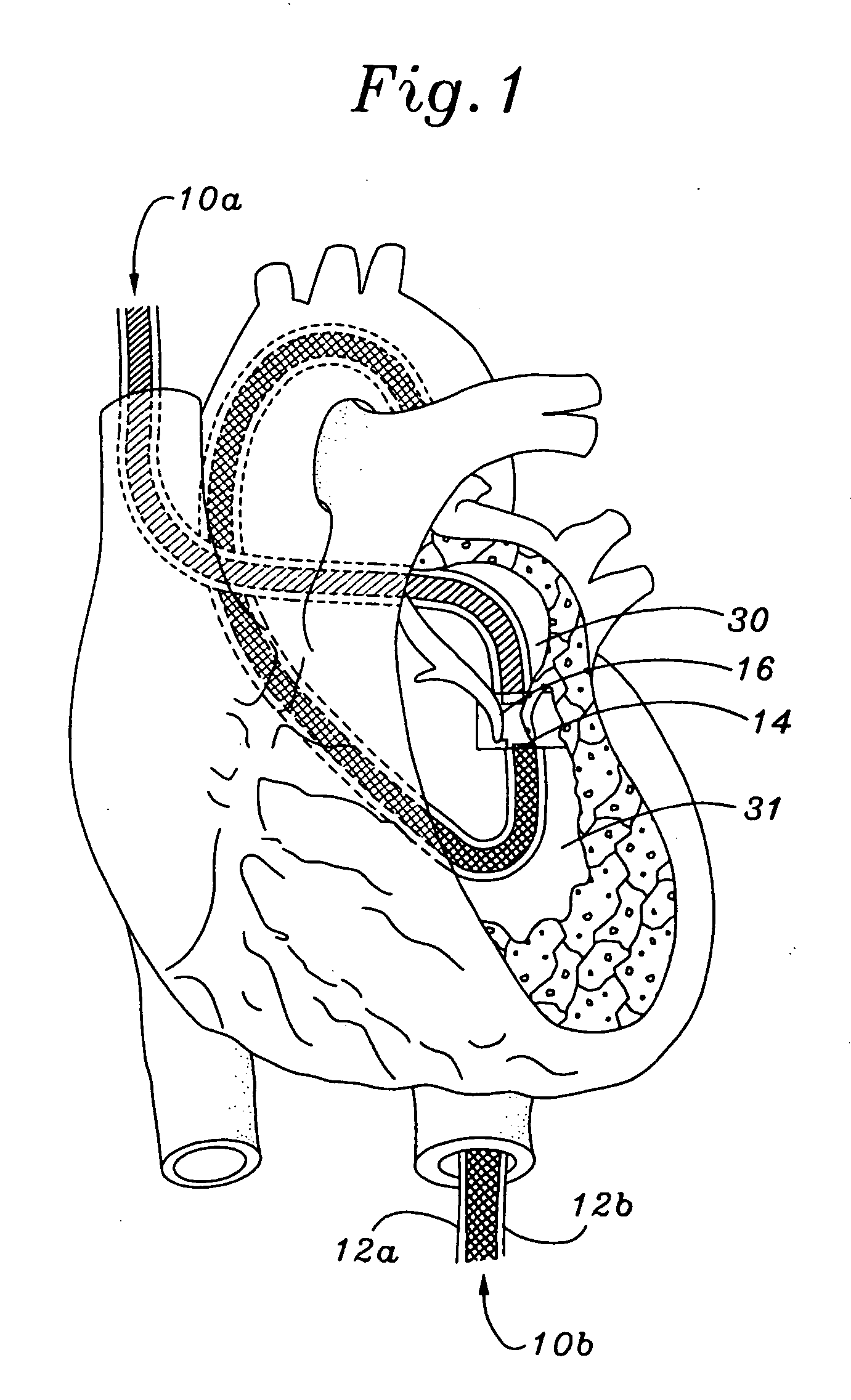
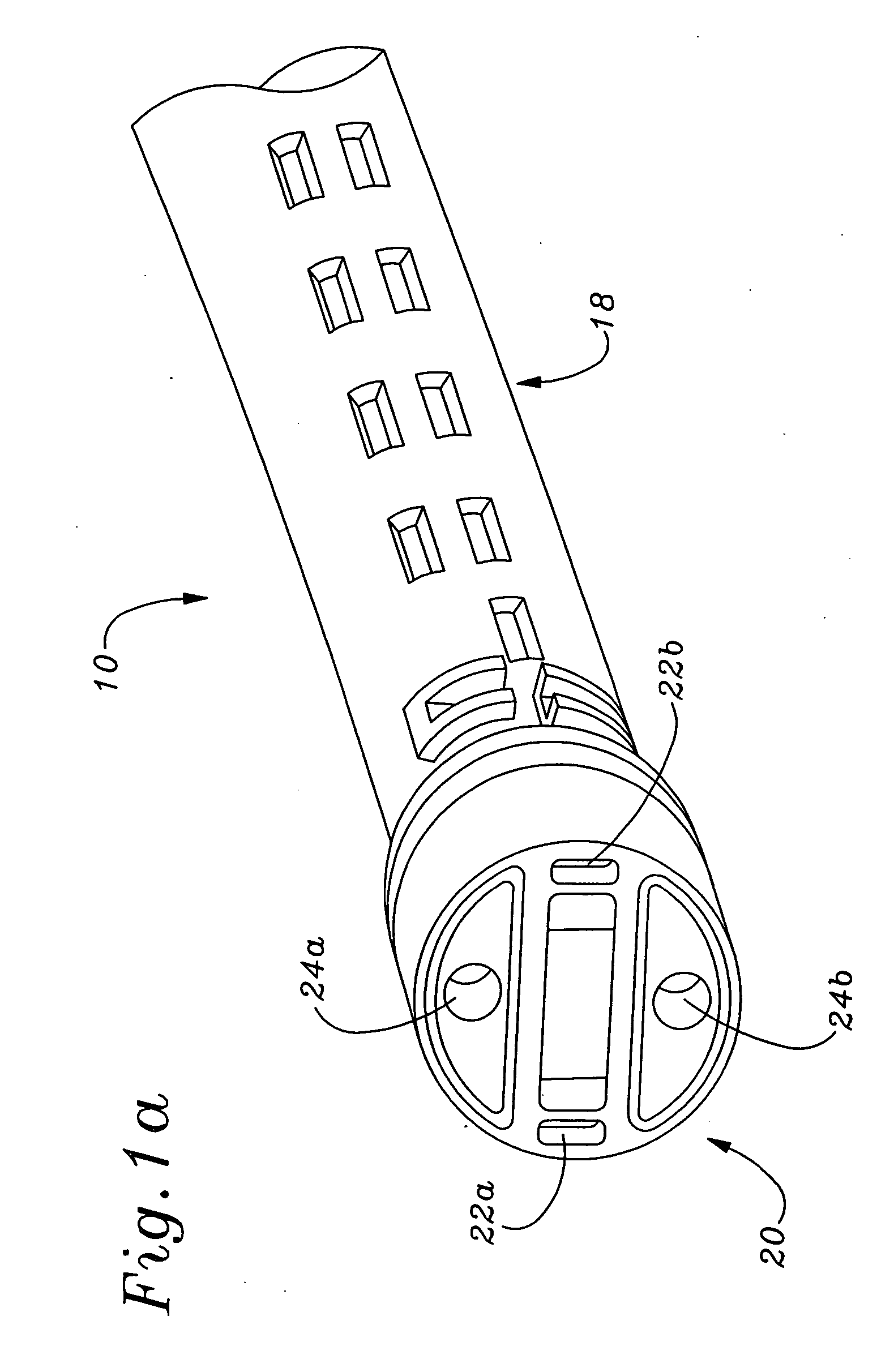
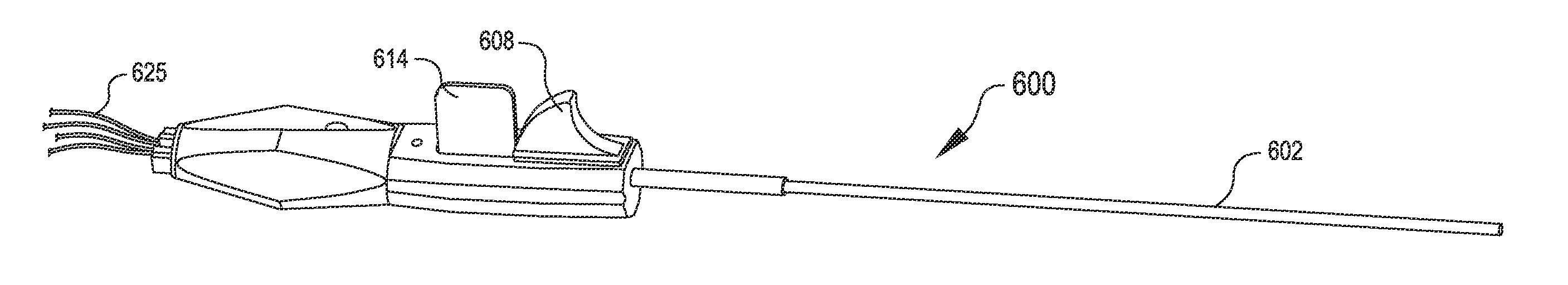
Method and system for tissue repair using dual catheters
The present system is directed to a method and system to stabilize and repair tissue. At least two opposing devices may be used to stabilize and repair the tissue, with the two devices cooperatively engaging the tissue interposed therebetween. Stabilization may be accomplished by opposing force, vacuum force, or mechanical devices disposed at the distal portion of one or both devices. After the tissue has been stabilized, fasteners may be deployed into the tissue. Fasteners include sutures, clips, and staples. Also disclosed is a minimally invasive method of accessing tissue located within a body and conducting a repair of the area using the system disclosed herein.
Owner:SCHRECK STEFAN G +7
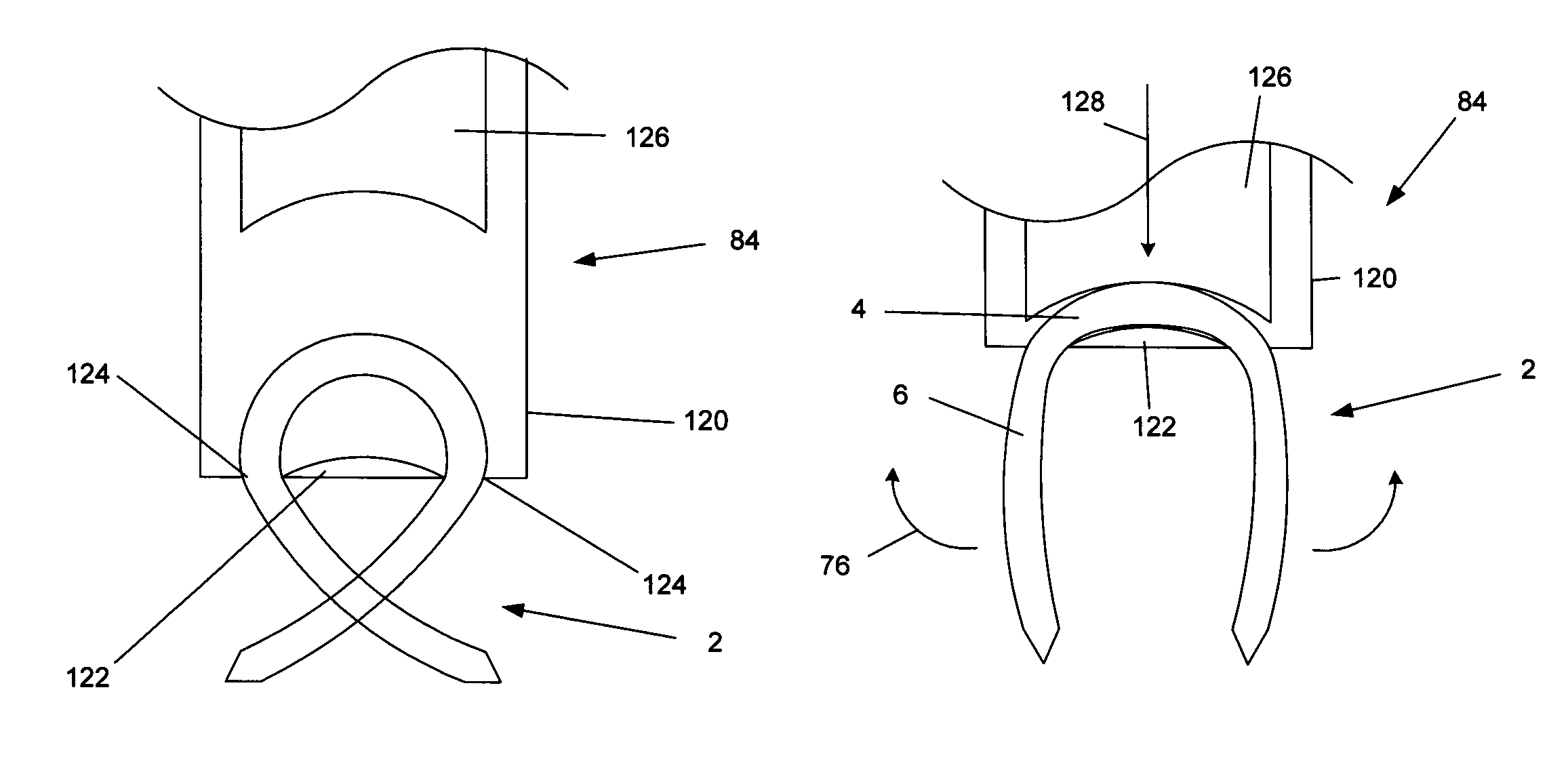
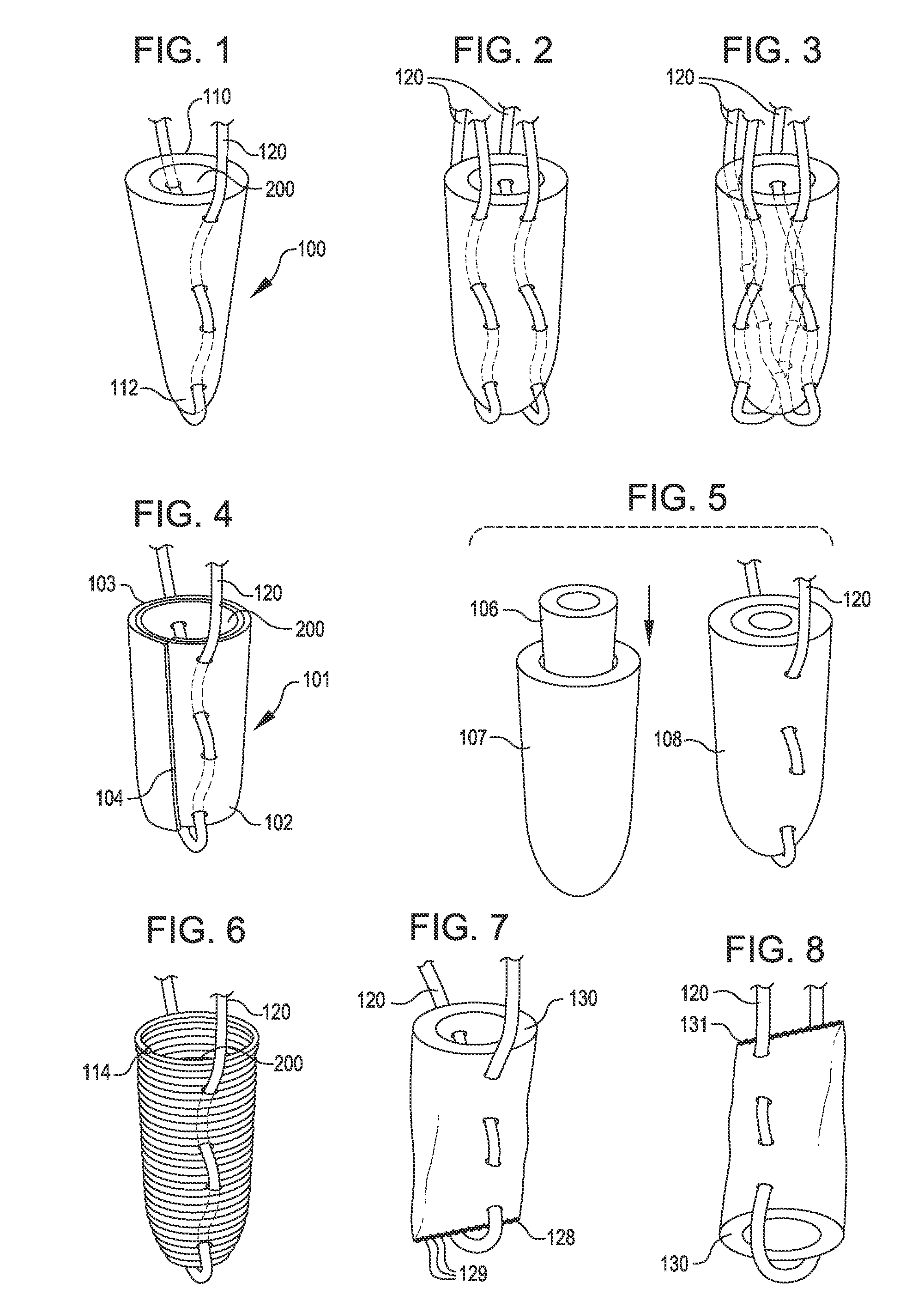
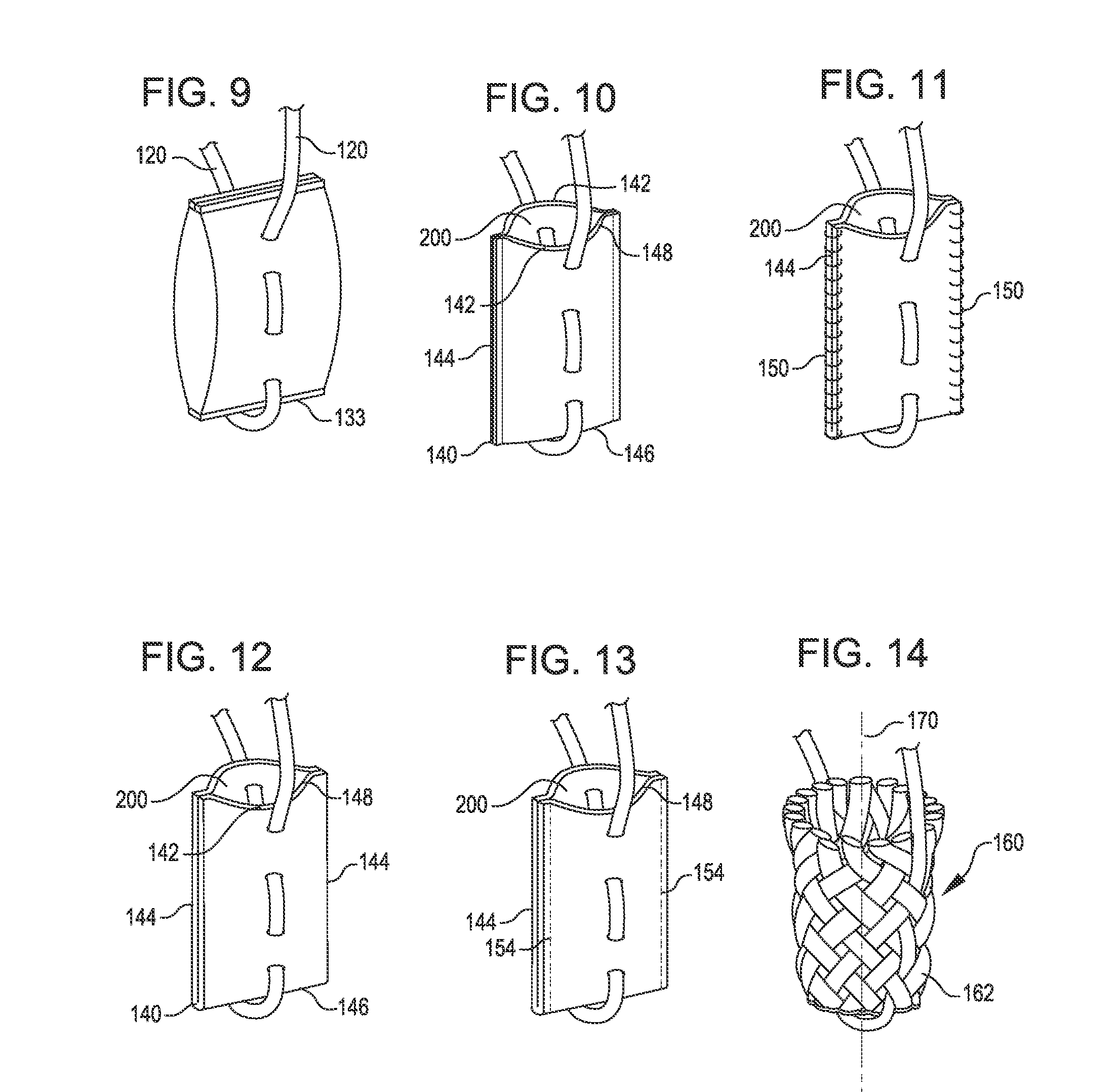
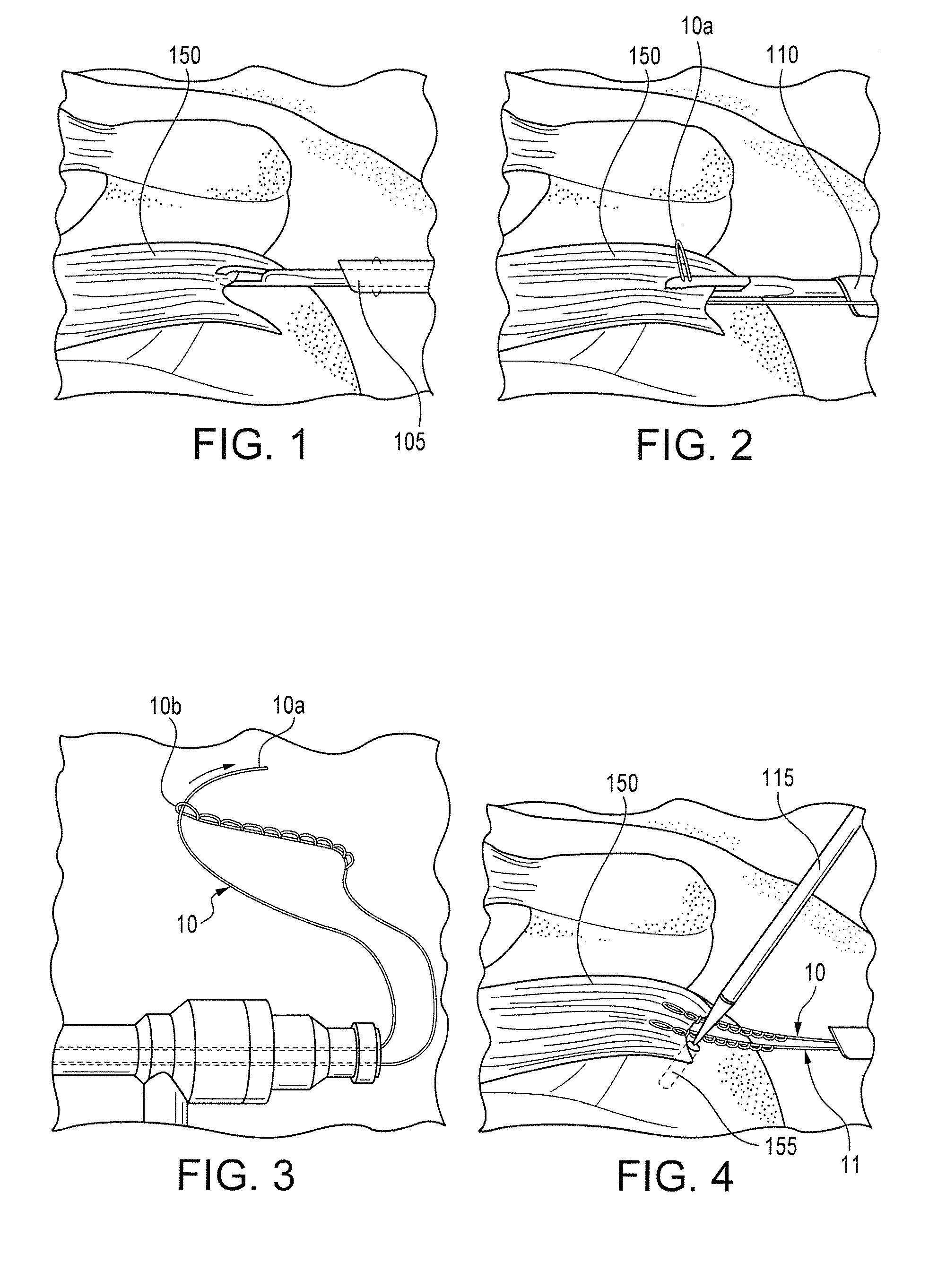
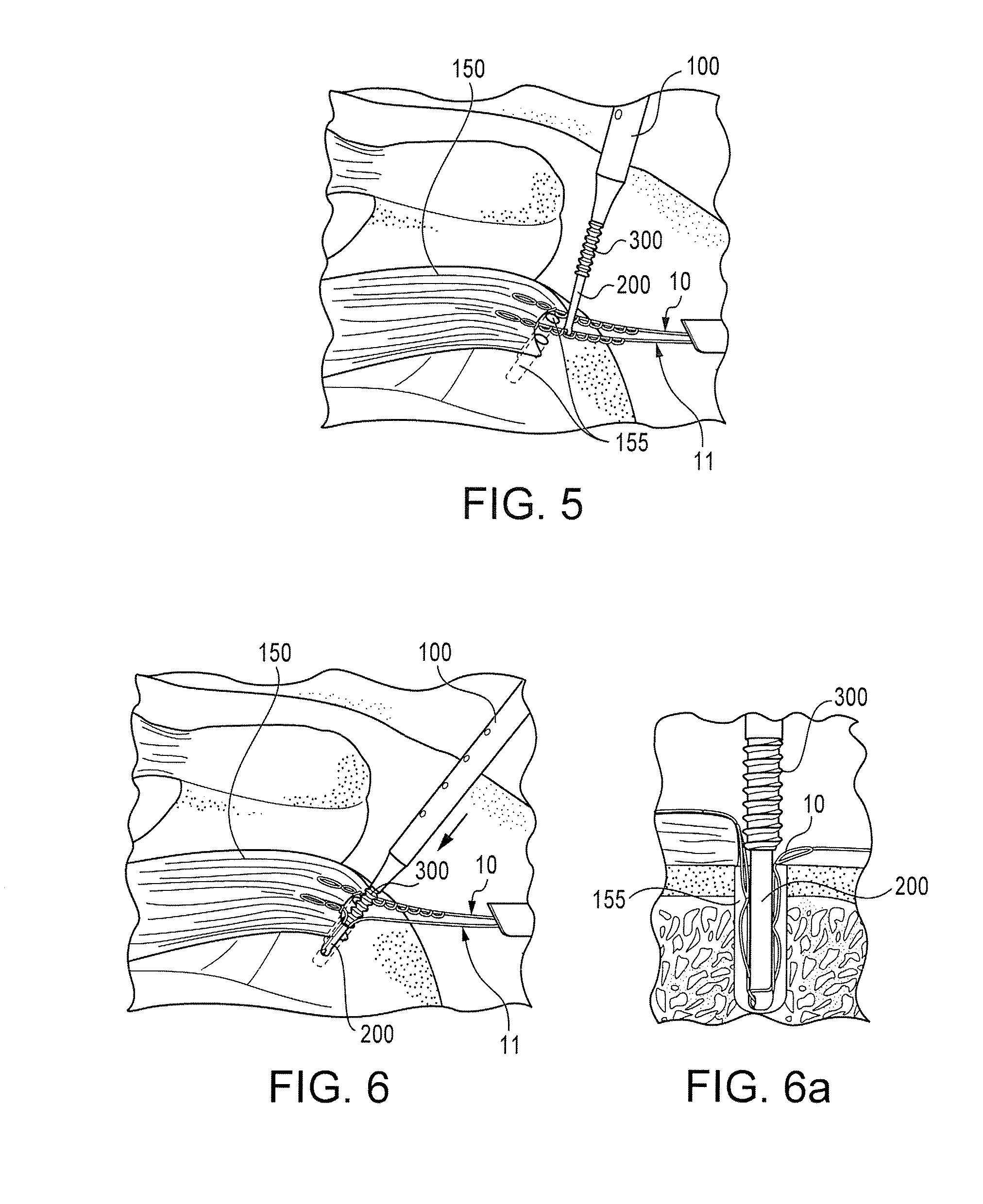
Tissue repair assembly
ActiveUS20130123810A1Reduce the radial distanceIncrease the angleSuture equipmentsDiagnosticsTissue repairBiomedical engineering
A tissue repair assembly for attachment of tissue to bone or tissue to tissue having a soft anchoring implant 100 with a length of suture 120 there through for tensioning the implant and facilitating attachment of other tissue. The implant 100 is a soft, flexible, three-dimensional structure that has a resident volume 200. An inserter tube 310 facilitates the placement of the implant 100 into bone or adjacent soft tissue where it may be deployed. Upon deployment, the soft anchoring implant 100 shortens axially and expands radially, achieving a larger diameter than the hole through which it was placed, thus resisting pull out.
Owner:ARTHROCARE
Swivel anchor and method for knotless fixation of tissue
A method and device for knotless fixation of tissue. A swivel anchor having a rotatable forked anchor tip is used to capture suture, such as a suture chain, for surgical tissue repair without requiring suture knots. Tension on the repair constructs is adjustable through the selection of the specific chain link or links of the suture chain captured by the forked anchor tip of the swivel anchor. The swivel anchor is secured in a hole in bone by advancing a fixation device, such as a cannulated interference screw, over the body of the anchor.
Owner:ARTHREX
Conformable tissue repair implant capable of injection delivery
A conformable tissue implant is provided for use in repairing or augmenting a tissue defect or injury site. The tissue implant contains a tissue carrier matrix comprising a plurality of biocompatible, bioresorbable granules and at least one tissue fragment in association with the granules. The tissue fragment contains one or more viable cells that can migrate from the tissue and populate the tissue carrier matrix. Also provided is a method for injectably delivering the tissue implant.
Owner:DEPUY SYNTHES PROD INC
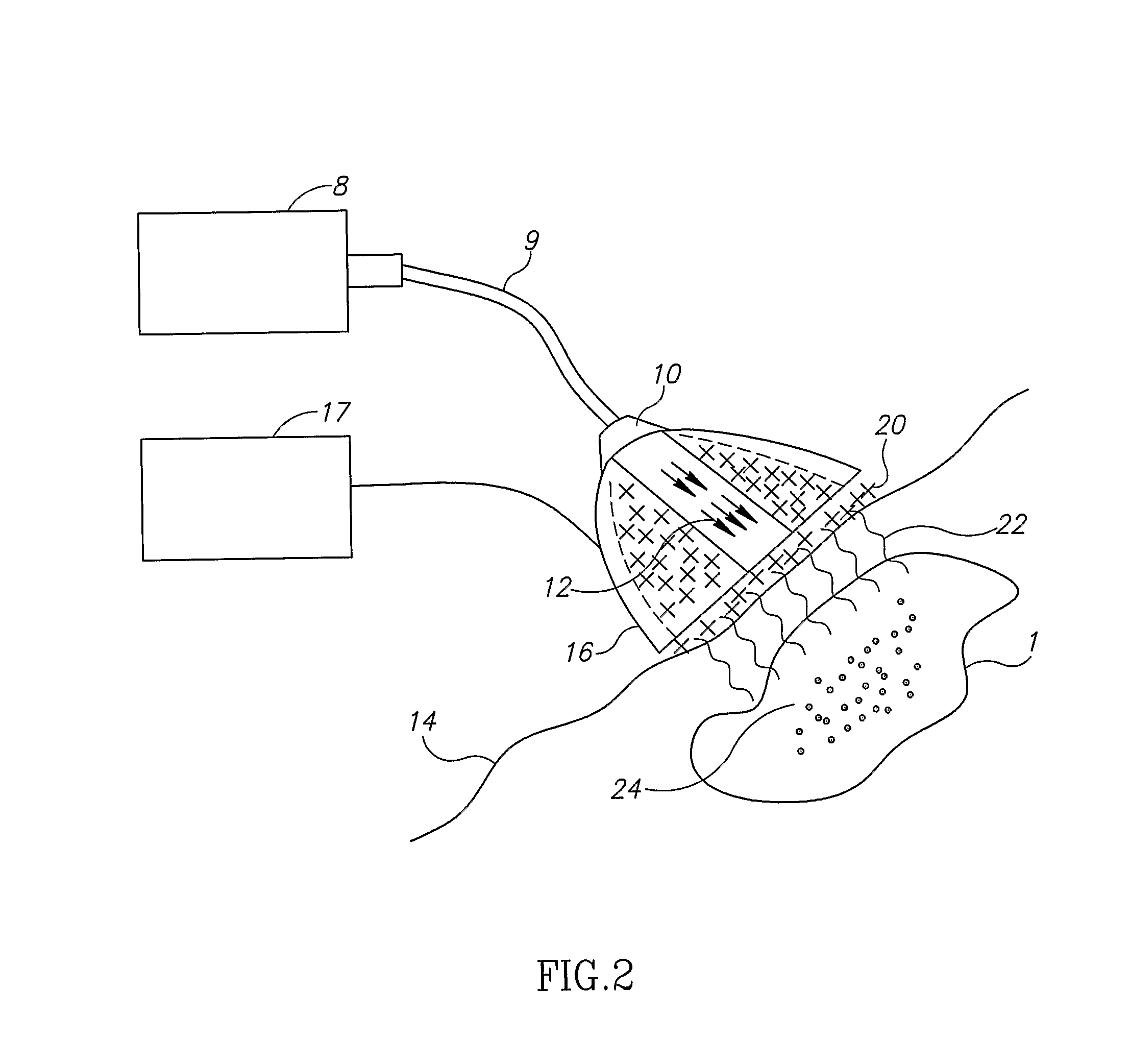
Sequential heart valve leaflet repair device and method of use
InactiveUS20060030866A1Effective and stableReduce distanceSuture equipmentsHeart valvesVacuum pressureTissue repair
A heart valve and tissue repair device for independently, selectively and sequentially grasping heart valve leaflets and independently, selectively and sequentially applying one or more fasteners thereto is disclosed. The device includes a leaflet engaging tip having one or more graspers capable of individually and sequentially grasping leaflets, and one or more deployable fasteners capable of fastening the leaflets. An actuation system for the device individually and selectively controls the graspers and deploys the one or more fasteners. Vacuum pressure from an external vacuum source can be used to grasp the leaflets via a selector system that controls the actuation system so as to individually and sequentially apply vacuum force to the graspers.
Owner:SCHRECK STEFAN
Non-destructive tissue repair and regeneration
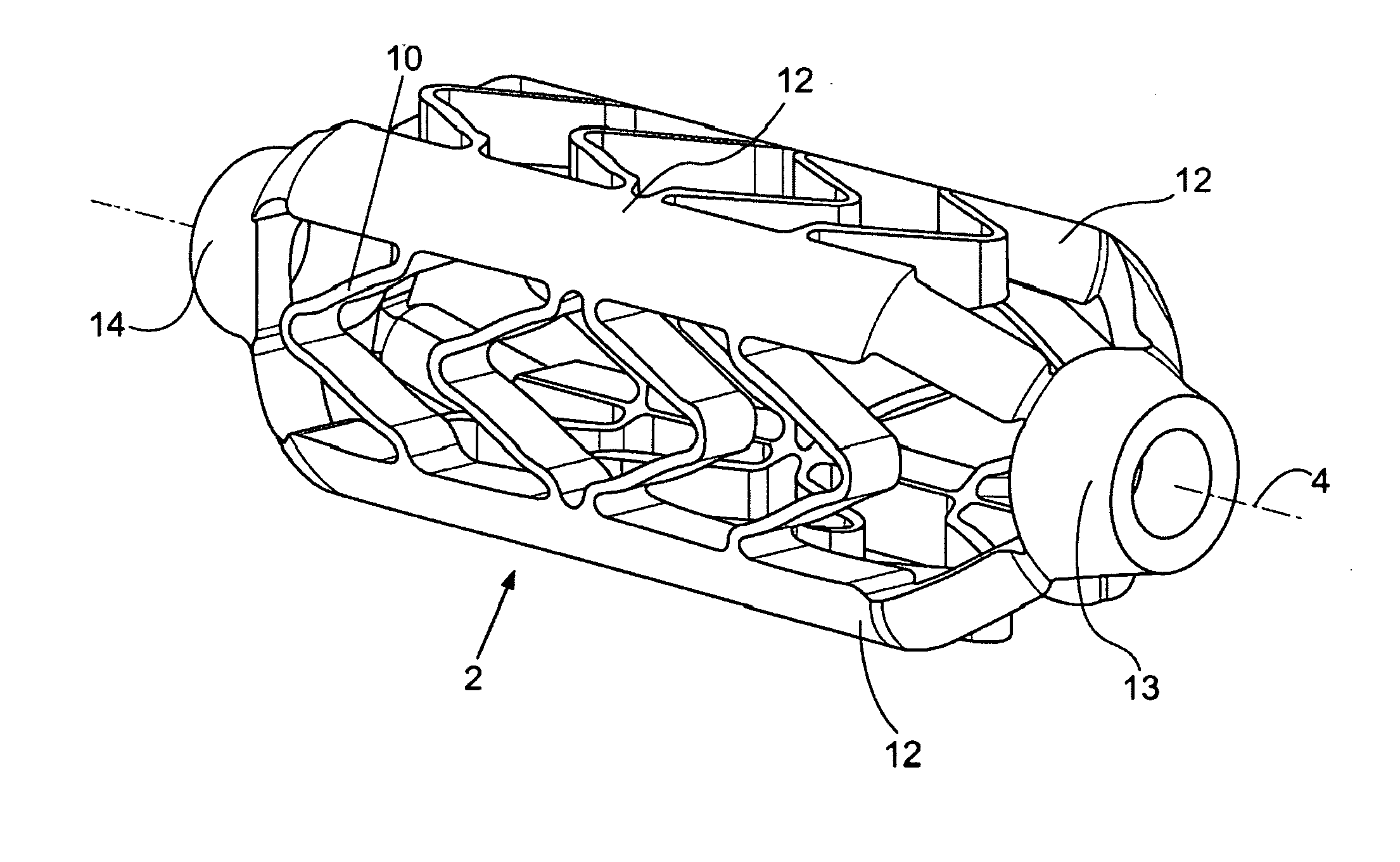
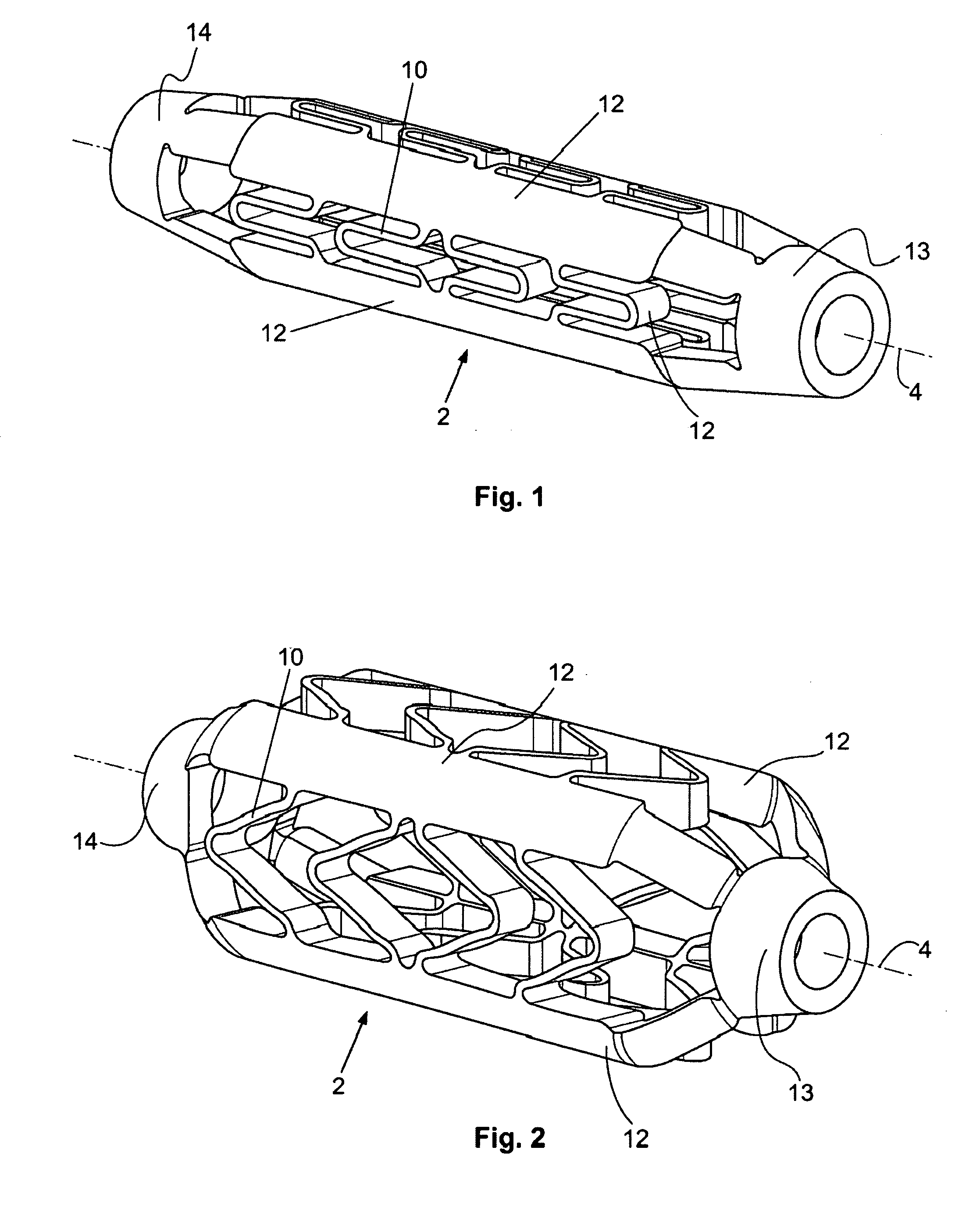
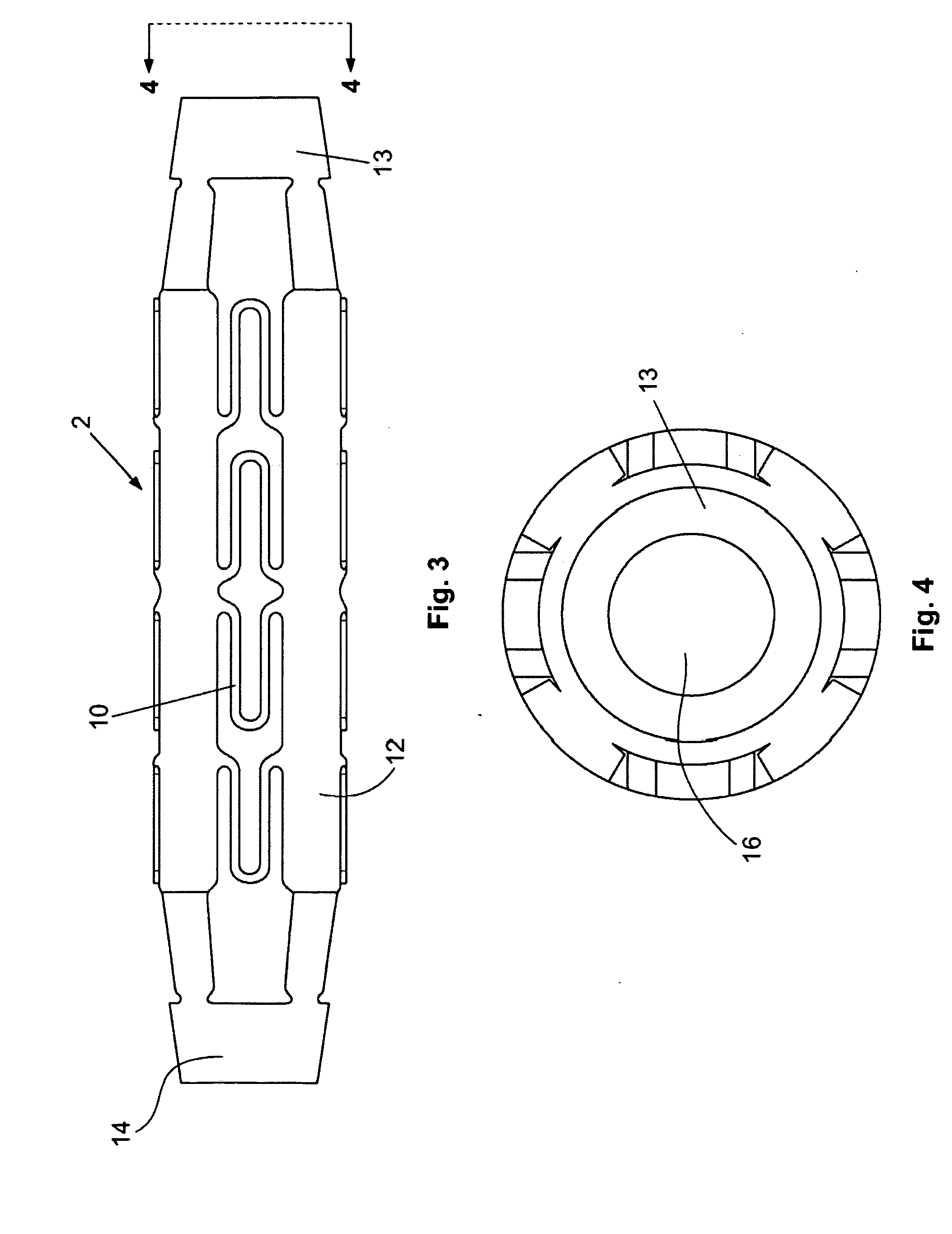
ActiveUS20070185568A1Facilitate healing and regenerationEasy to fixStentsAdditive manufacturing apparatusNon destructiveTissue repair
A surgical stent made of biocompatible material for implantation in human tissue to enable blood and nutrients to flow from an area of vascular tissue to an area of tissue with little or no vasculature.
Owner:HOWMEDICA OSTEONICS CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com