Patents
Literature
14547results about "Tissue regeneration" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
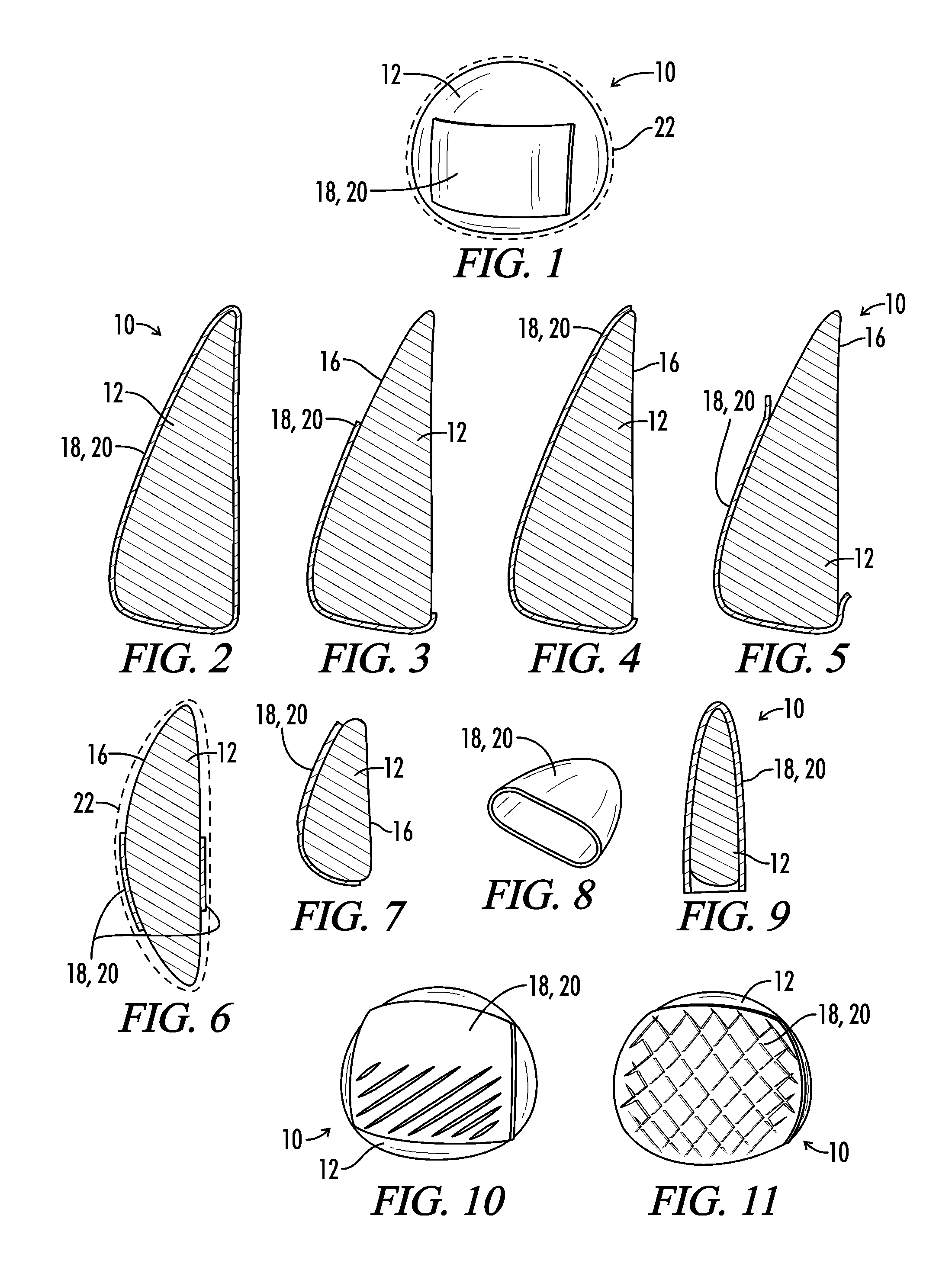
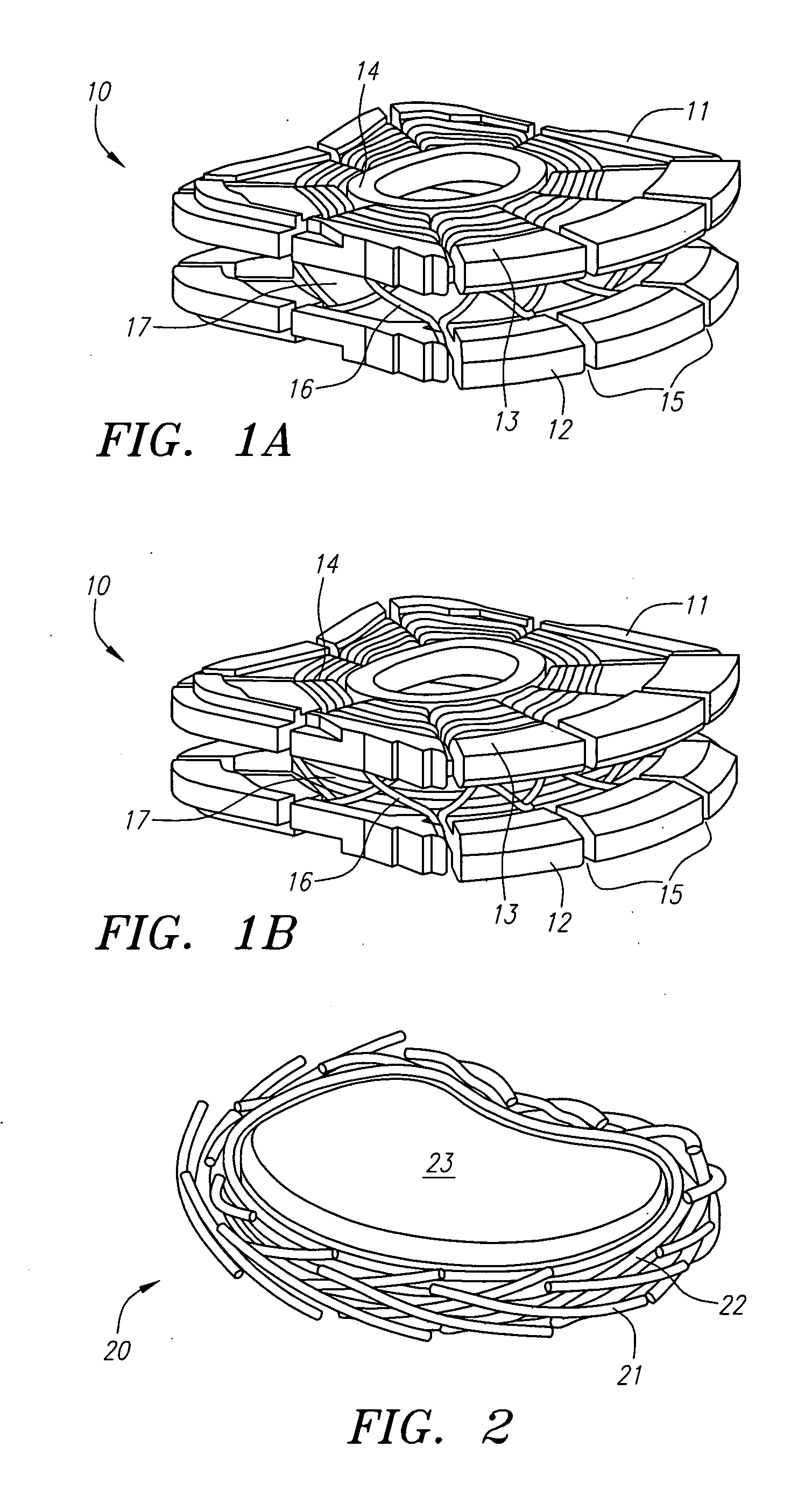
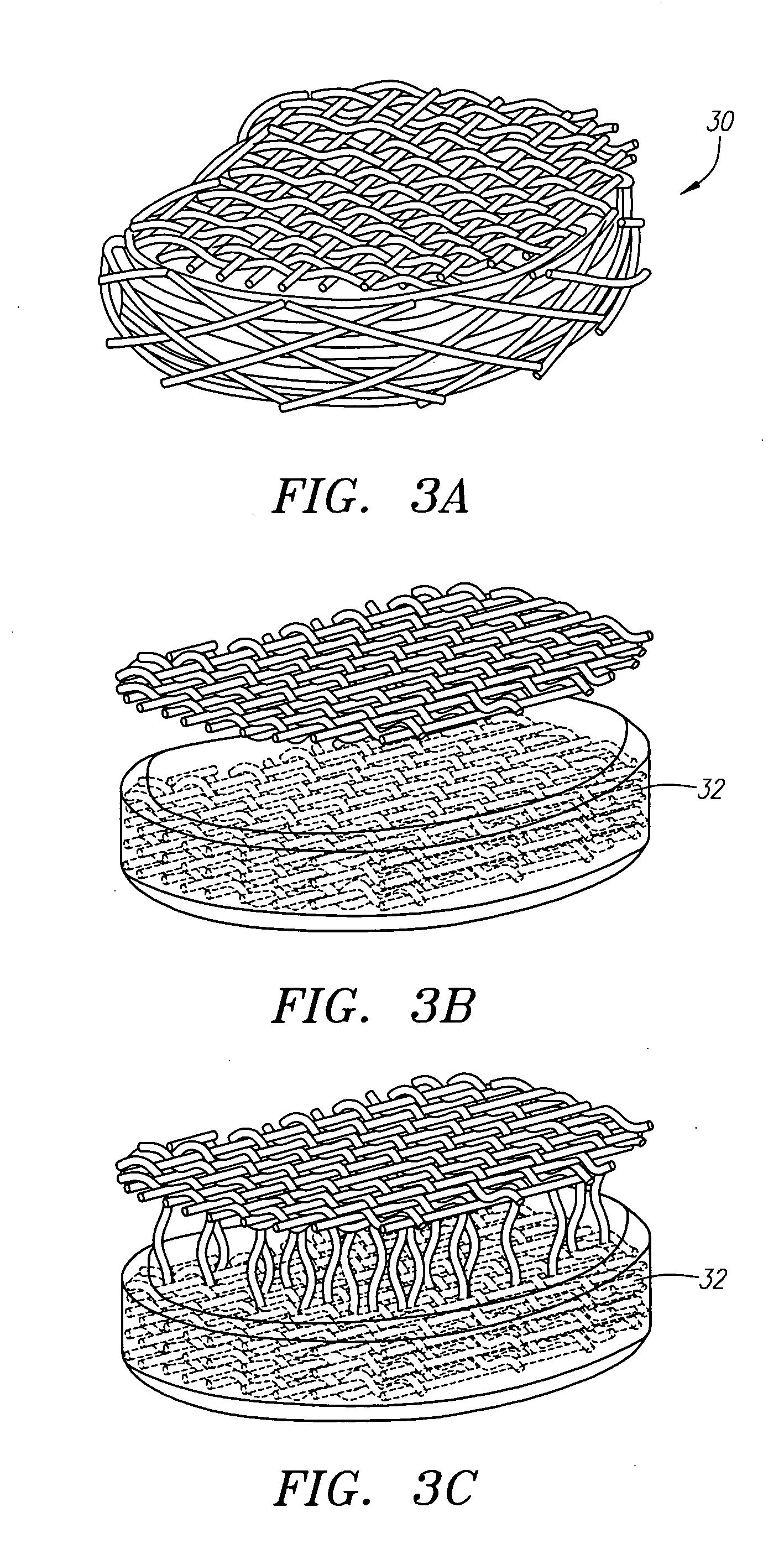
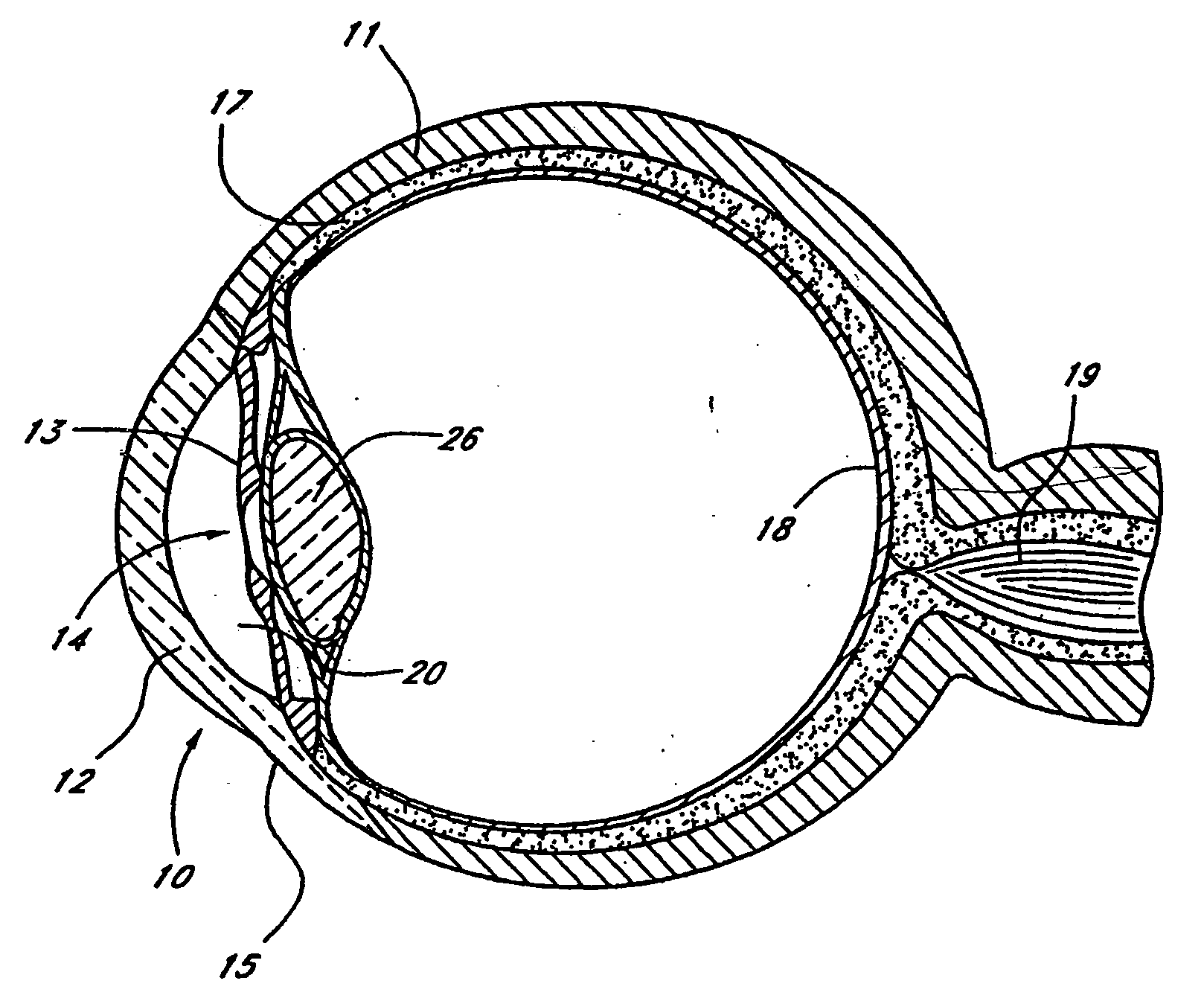
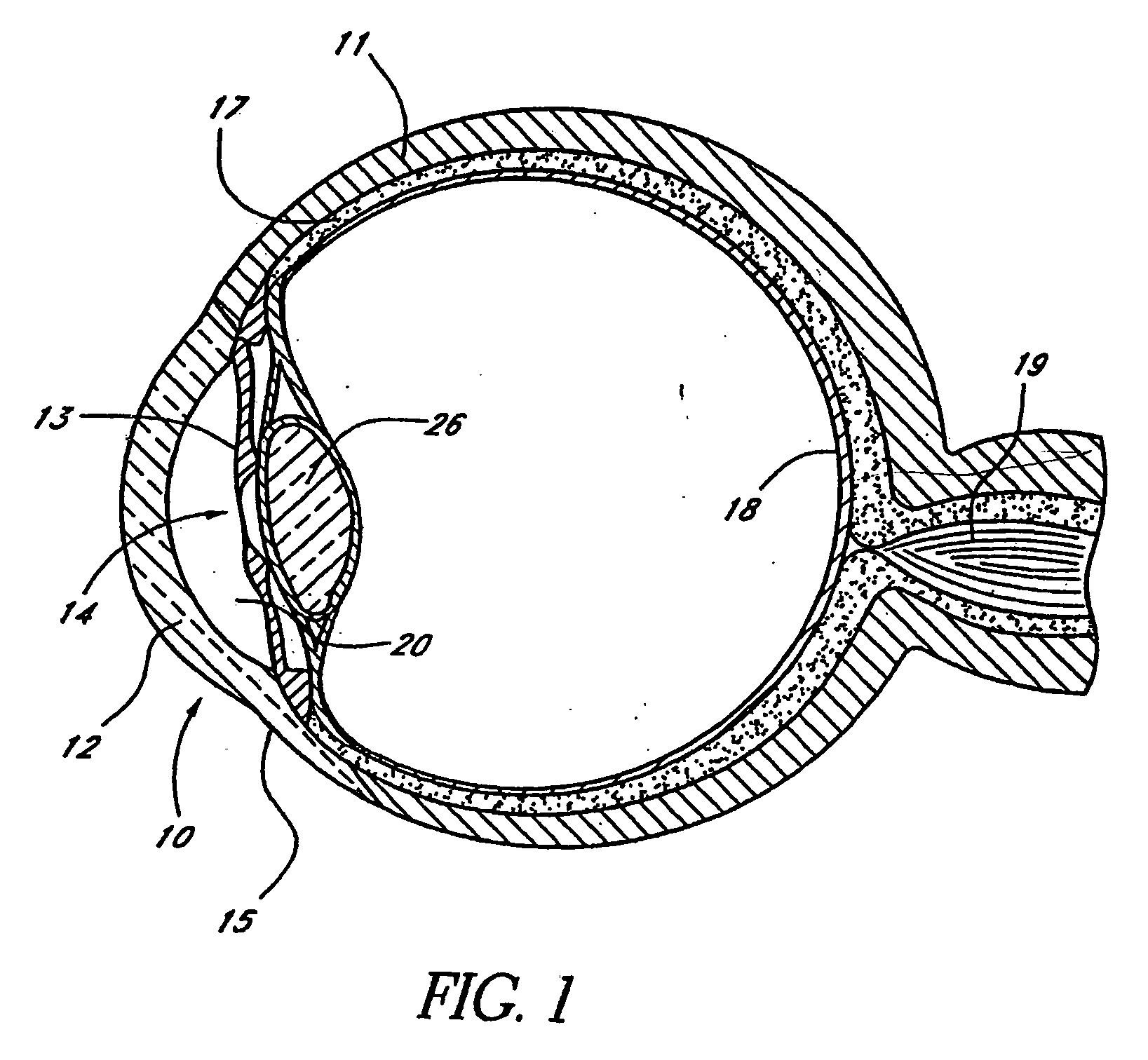
Extended wear ophthalmic lens
InactiveUS5760100AExcellent ion permeabilityGood water permeabilityLiquid surface applicatorsEye implantsExtended wear contact lensesIon permeation
Owner:NOVARTIS AG
Osteogenic implants derived from bone
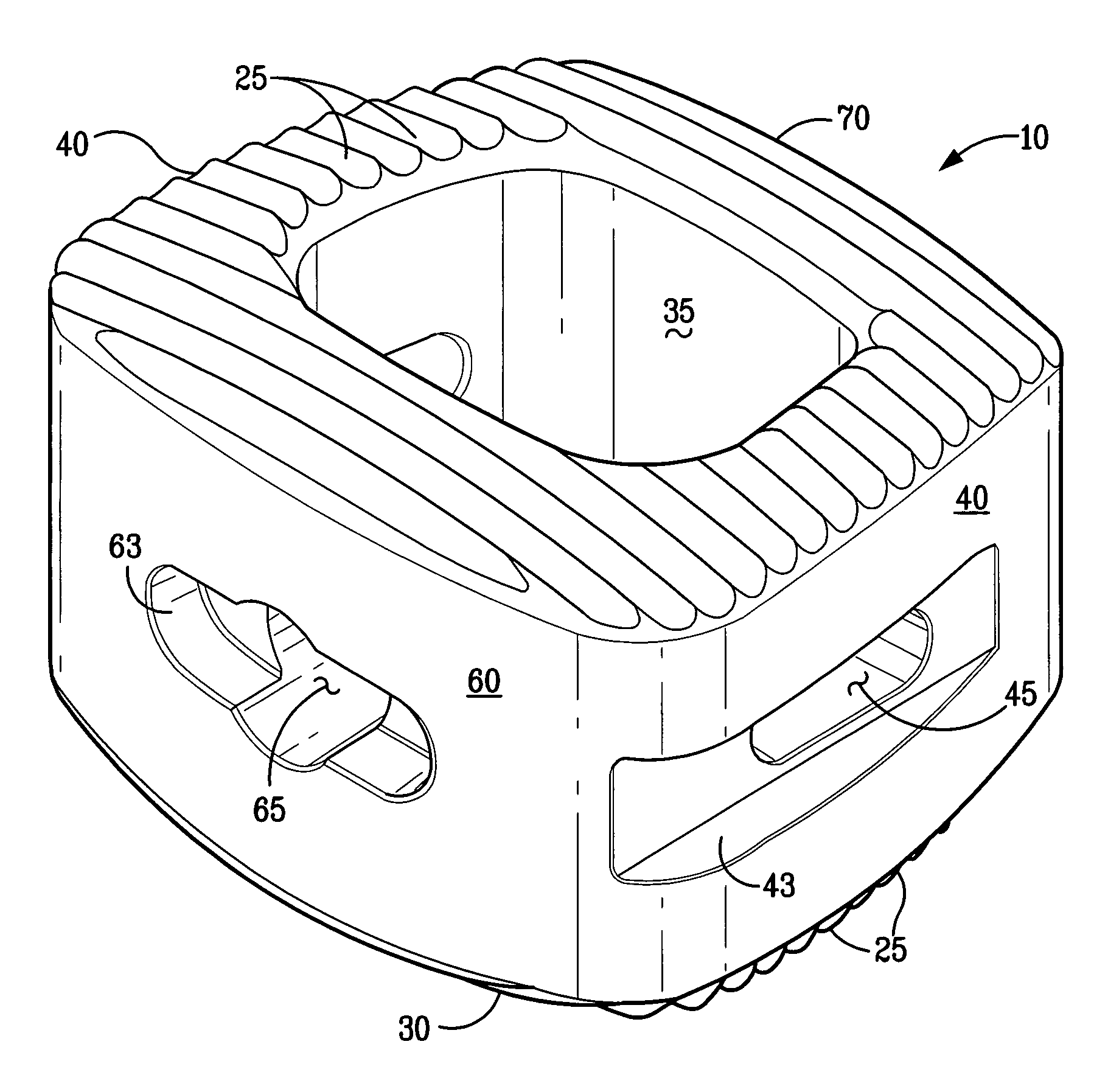
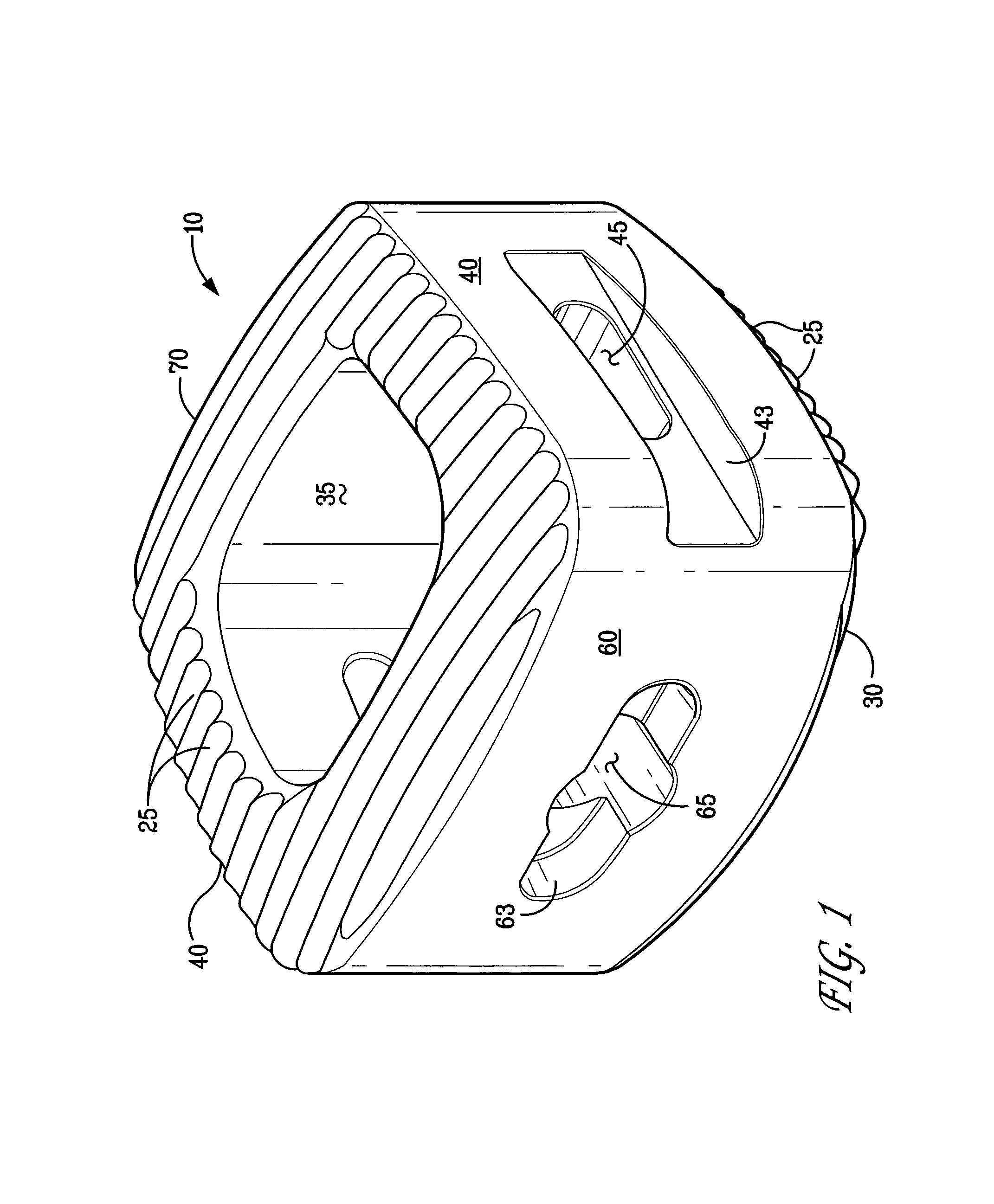
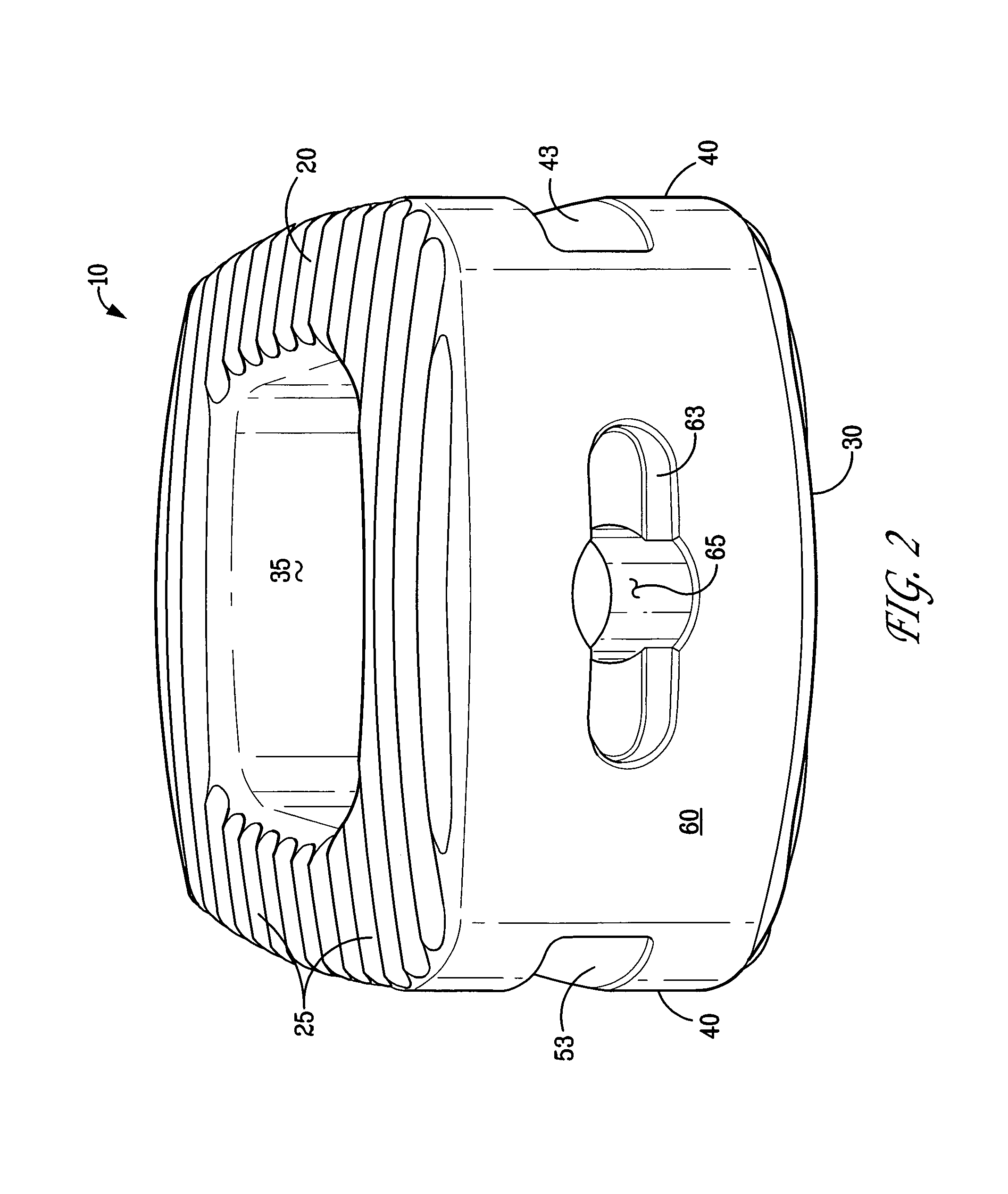
An osteogenic osteoimplant in the form of a flexible sheet comprising a coherent mass of bone-derived particles, the osteoimplant having a void volume not greater than about 32% and a method of making an osteogenic osteoimplant having not greater than about 32% void volume, the method comprising: providing a coherent mass of bone-derived particles; and, mechanically shaping the coherent mass of bone-derived particles to form an osteogenic osteoimplant in the form of a flexible sheet.
Owner:WARSAW ORTHOPEDIC INC
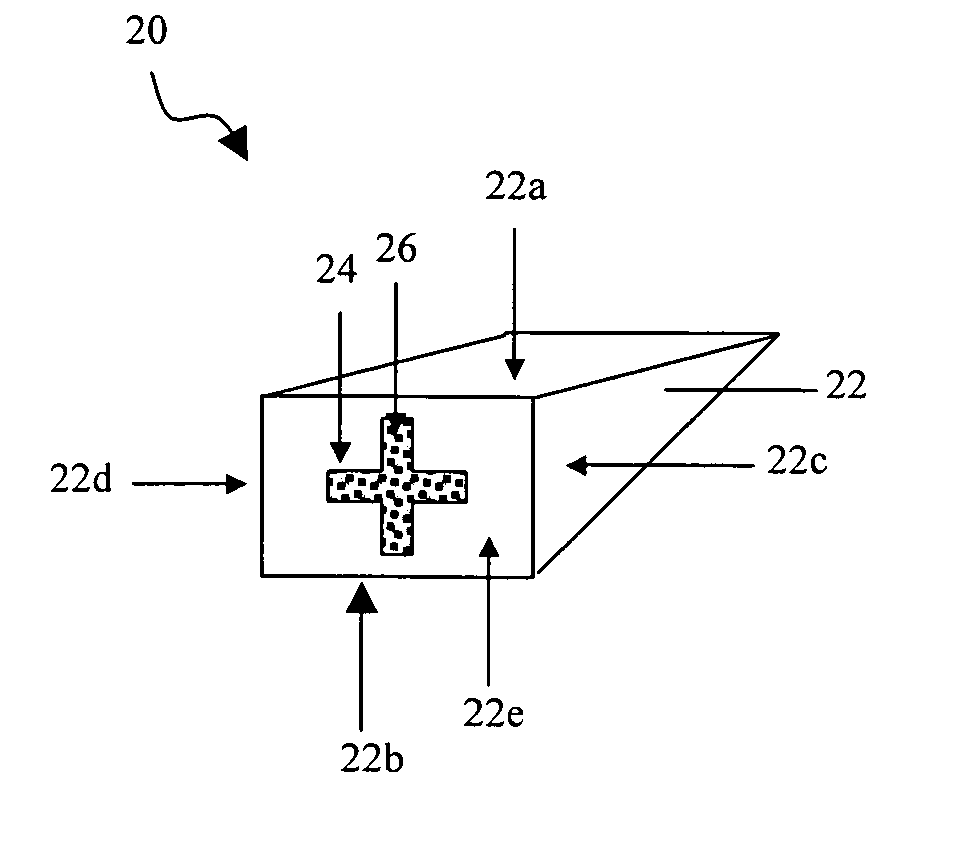
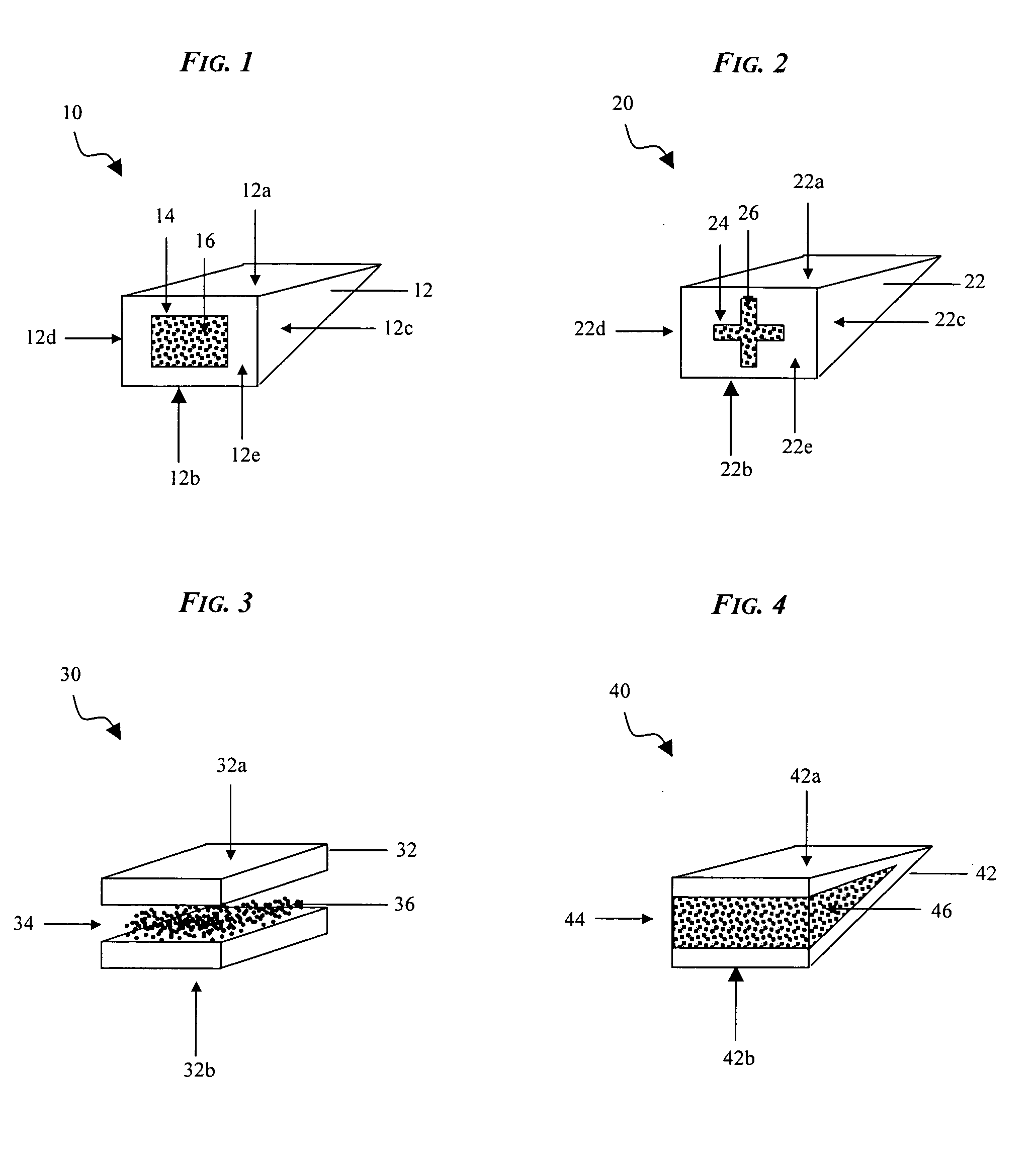
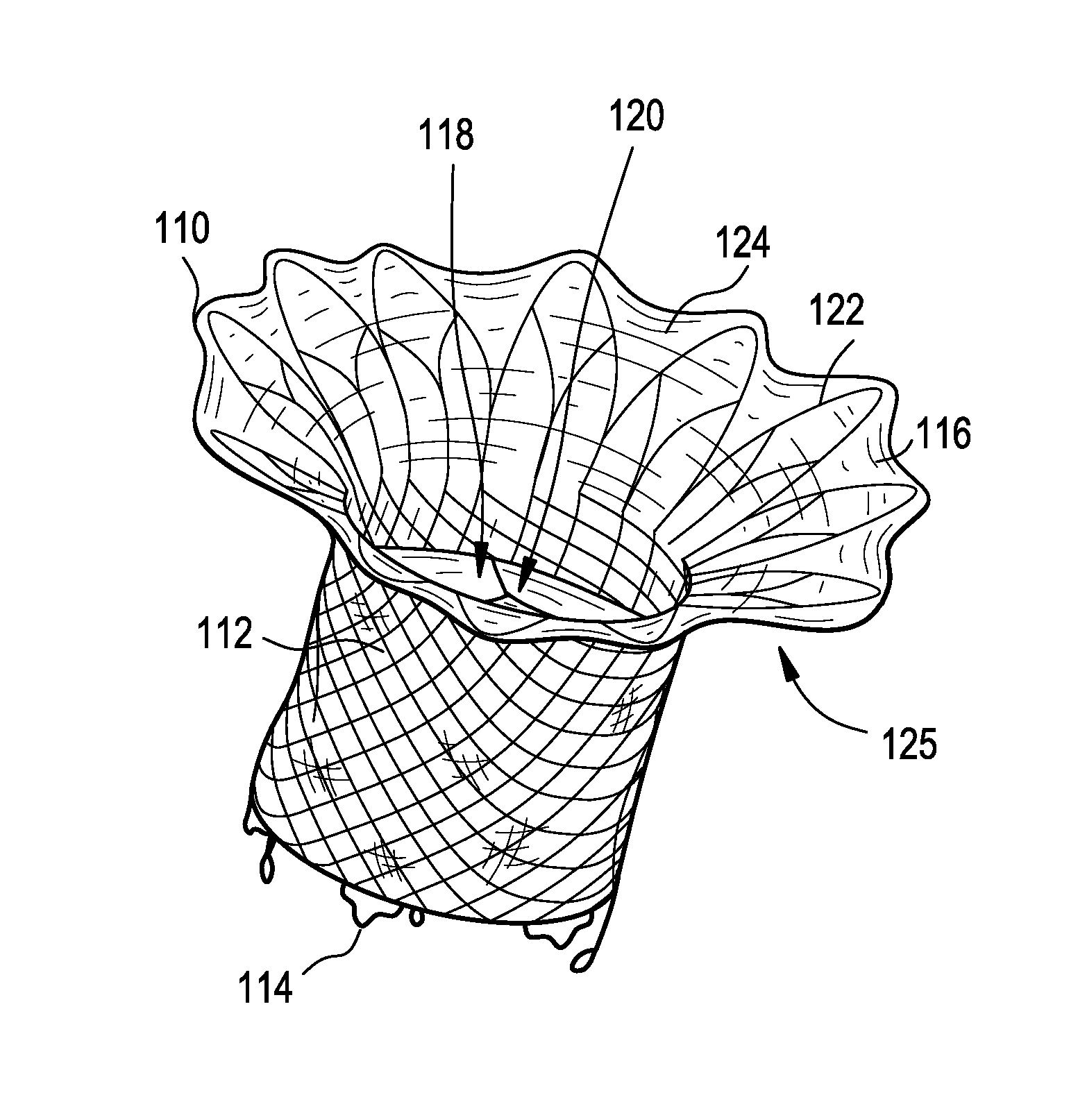
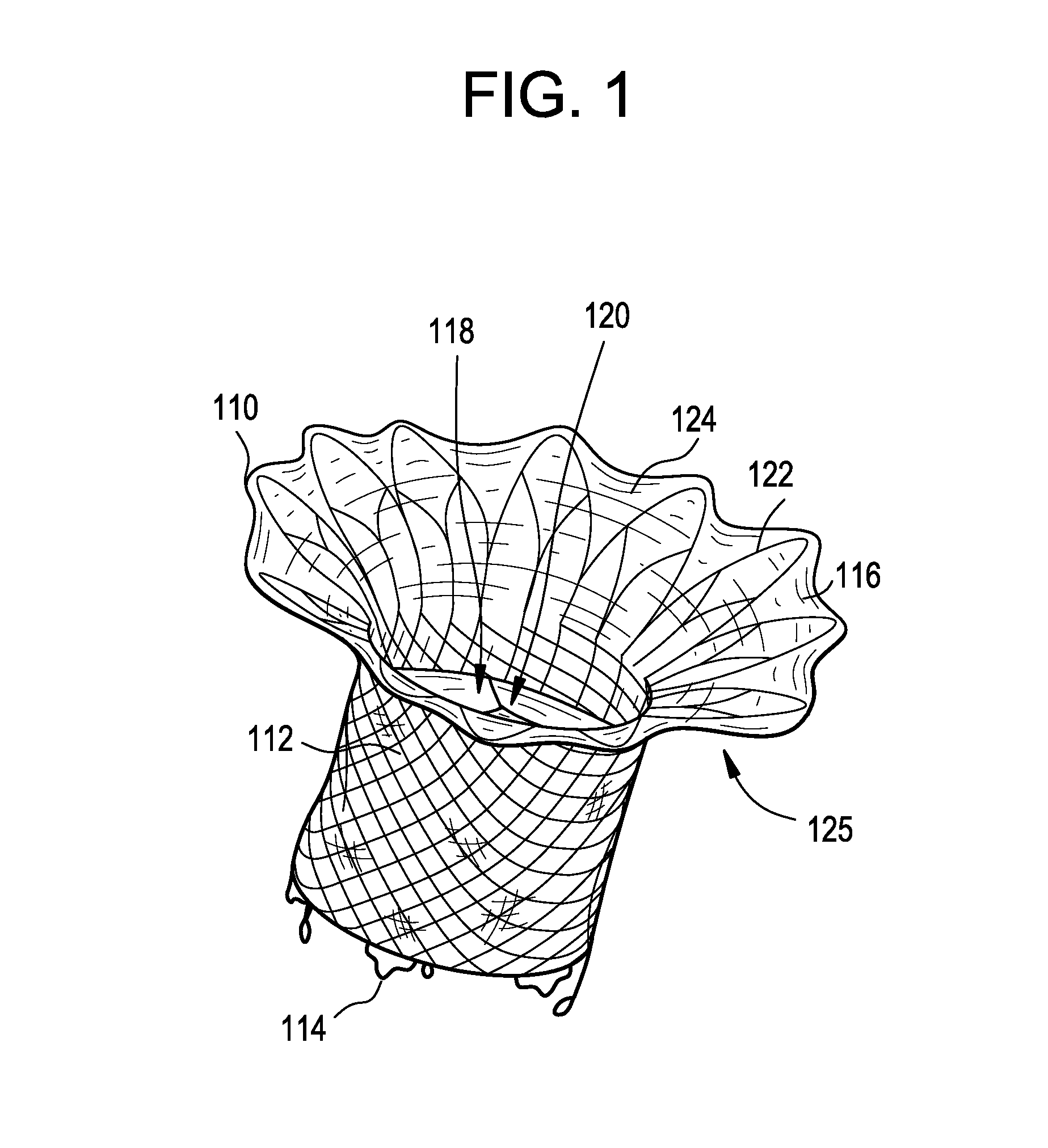
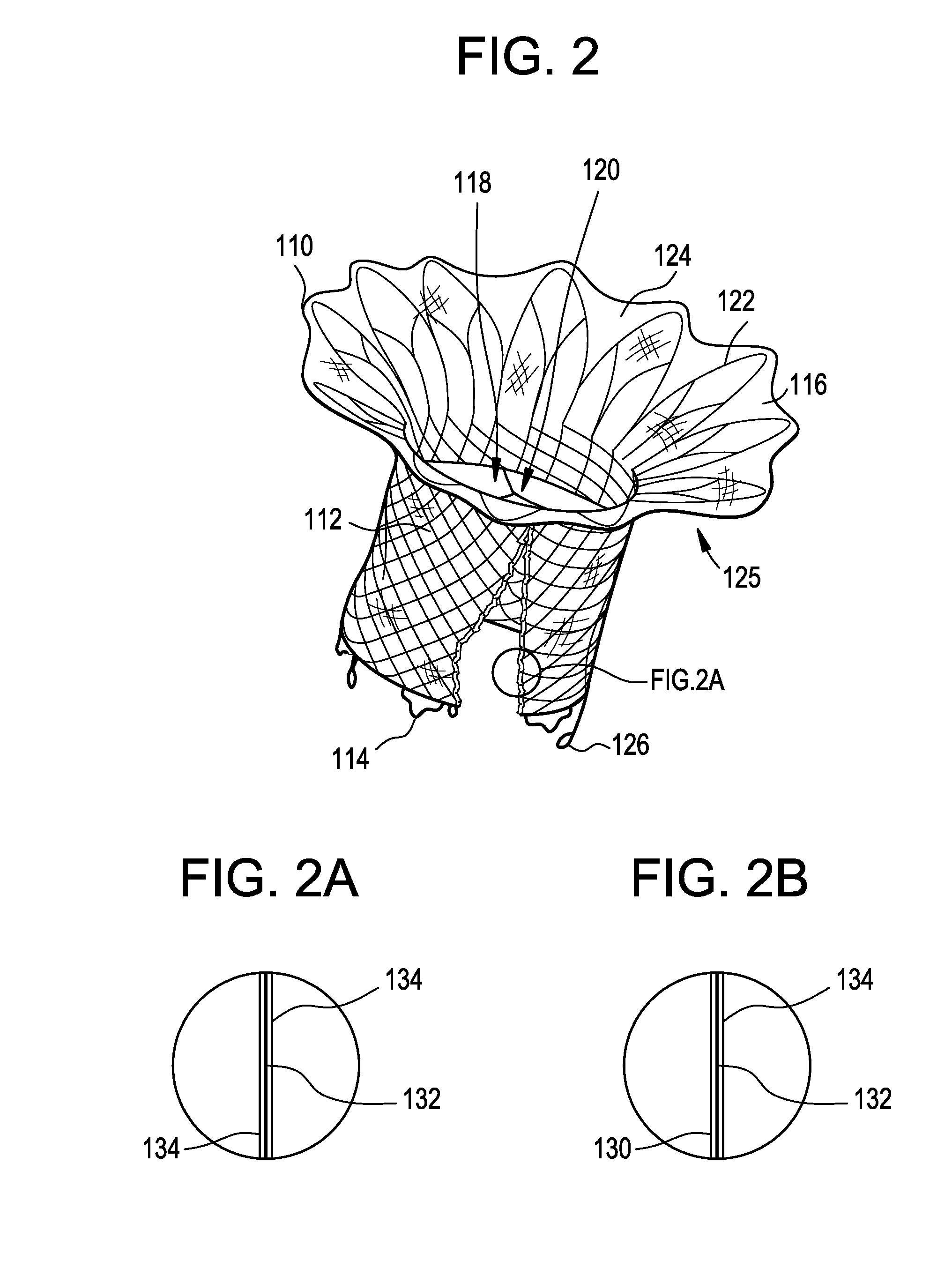
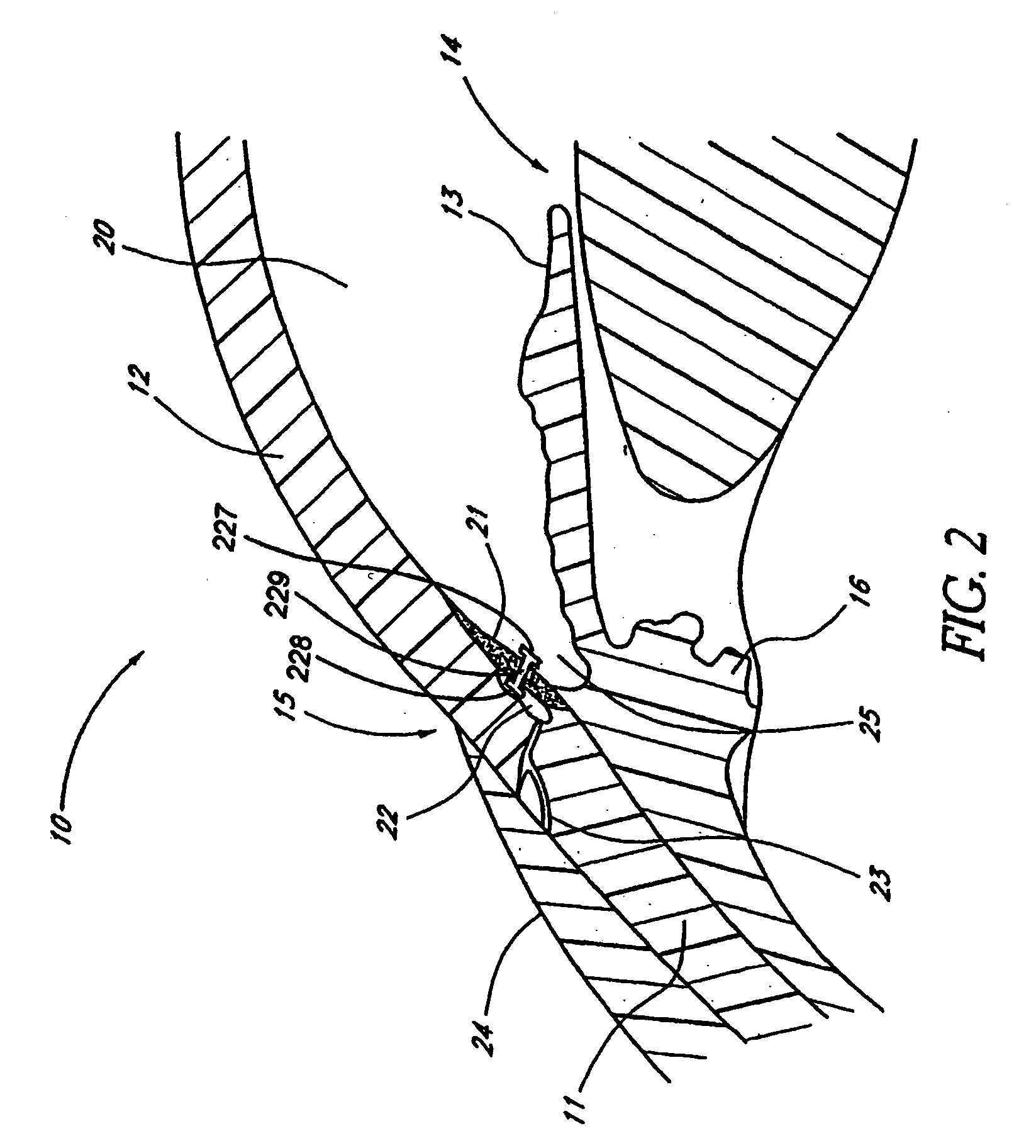
Interfaced medical implant assembly
ActiveUS8425600B2Reduce and eliminate capsular contracturePromote formationMammary implantsTissue regenerationMedicineBreast prostheses
A medical implant assembly and method having a medical implant, e.g. a breast prostheses, attached to a biological interface. The biological interface is comprised of a dermal material with capsular contracture inhibiting properties so that once the medical assembly is inserted into the host, the biological interface, which is intimately coupled to the implant, prevents / reduces capsular contracture formation around the implant. The biological interface comprises a plurality of apertures along its periphery, and attaches to the medical implant by receiving a plurality of attachment flaps or appendages located on the exterior surface of the medical implant within or through the apertures. The attachment of the biological interface is such that the assembly remains intact even where the attachment flaps loosen upon expansion of the implant after insertion into a host, as where the implant is therein injected to a desired dimension.
Owner:MAXWELL G PATRICK
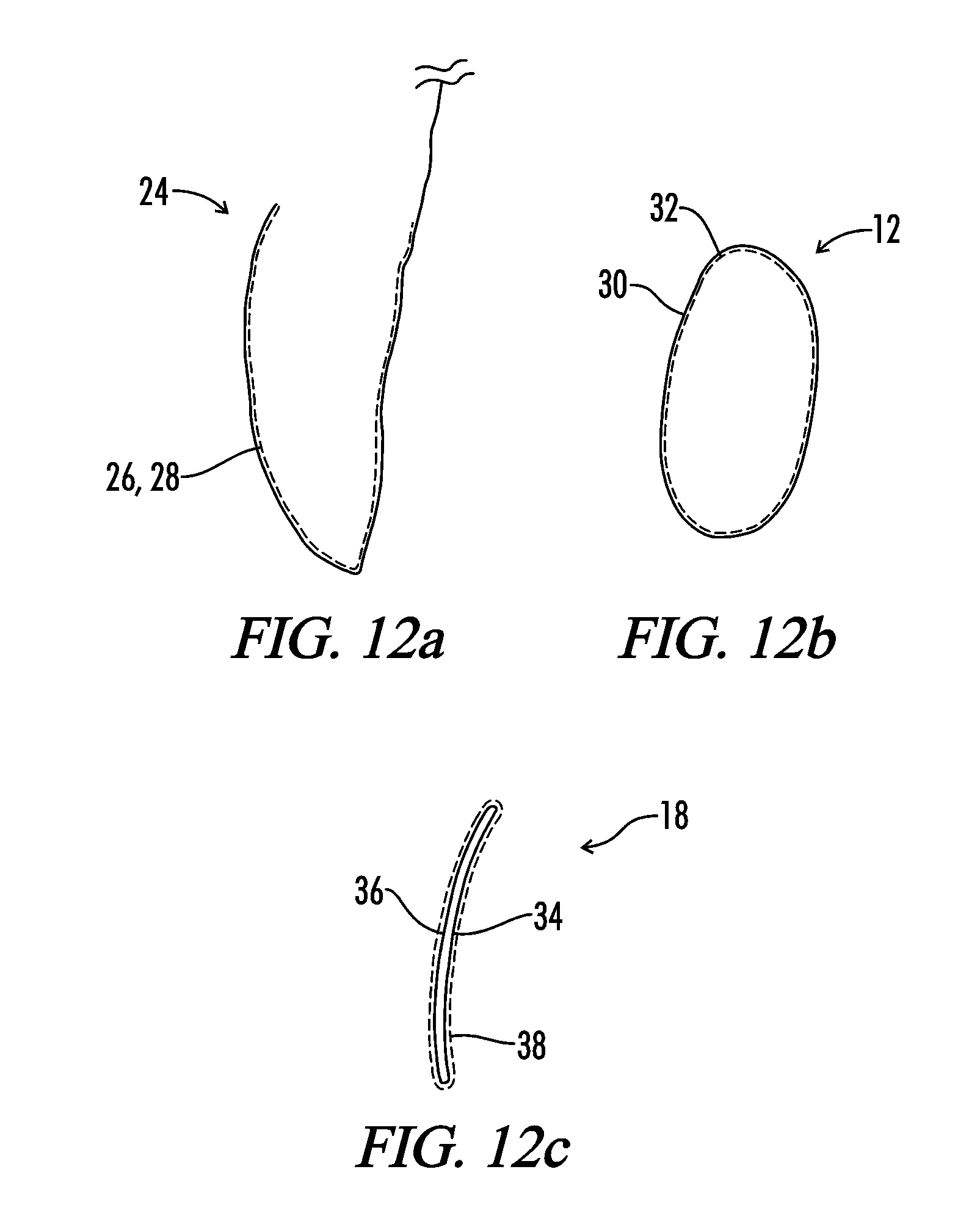
Scaffolds with viable tissue
ActiveUS20050177249A1Promote cell growthProsthesisTissue regenerationTissue defectBiomedical engineering
A composite implant is provided for repairing a tissue defect in a patient. In one embodiment, the implant is a porous tissue scaffold having at least one pocket formed therein and adapted to contain a viable tissue. The tissue scaffold can have a variety of configurations, and in one embodiment it includes top and bottom portions that can be at least partially mated to one another, and in an exemplary embodiment that are heated sealed to one another around a perimeter thereof to form an enclosed pocket therebetween. The pocket is preferably sealed with a viable tissue disposed therein. In another embodiment, the tissue scaffold is substantially wedge-shaped and the pocket comprises a hollow interior formed in the tissue scaffold, and / or at least one lumen extending into the tissue scaffold. The tissue scaffold can also optionally include at least one surface feature formed thereof to promote blood vessel formation.
Owner:DEPUY SYNTHES PROD INC
Unitary surgical device and method
Unitary surgical devices (10) are disclosed. One group of the illustrated devices has a pair of biocompatible, bioresorbable anchors (16,18) connected to fixed lengths suture. The anchors (16,18) and fixed length of suture are connected to each other prior to surgery. Another group of unitary surgical devices has a pair of fixating mechanisms (15,17) connected to a base (21) prior to surgery. The second group of illustrated devices generally includes extracellular matrix material either as part of the base (21) or supported on the base (21). The extracellular matrix material serves as tissue regenerating material. In the second group of unitary surgical devices, the fixating mechanisms illustrated generally comprise suture, anchors or pre-formed holes in the base. All of the illustrated unitary surgical devices are useful in repairing a damaged meniscus. The first group of unitary surgical devices can be used to approximate inner surfaces of a tear in the meniscus. The second group of devices can be used either as an insert to be placed between and approximated to the inner surfaces of the tear or as an insert to replace a void in the meniscus left after a meniscectomy.
Owner:DEPUY SYNTHES PROD INC
Implantable prosthetic valve
InactiveUS20060025857A1Minimize contactFirmly attachedHeart valvesTubular organ implantsEngineeringCatheter
Owner:MEDTRONIC 3F THERAPEUTICS
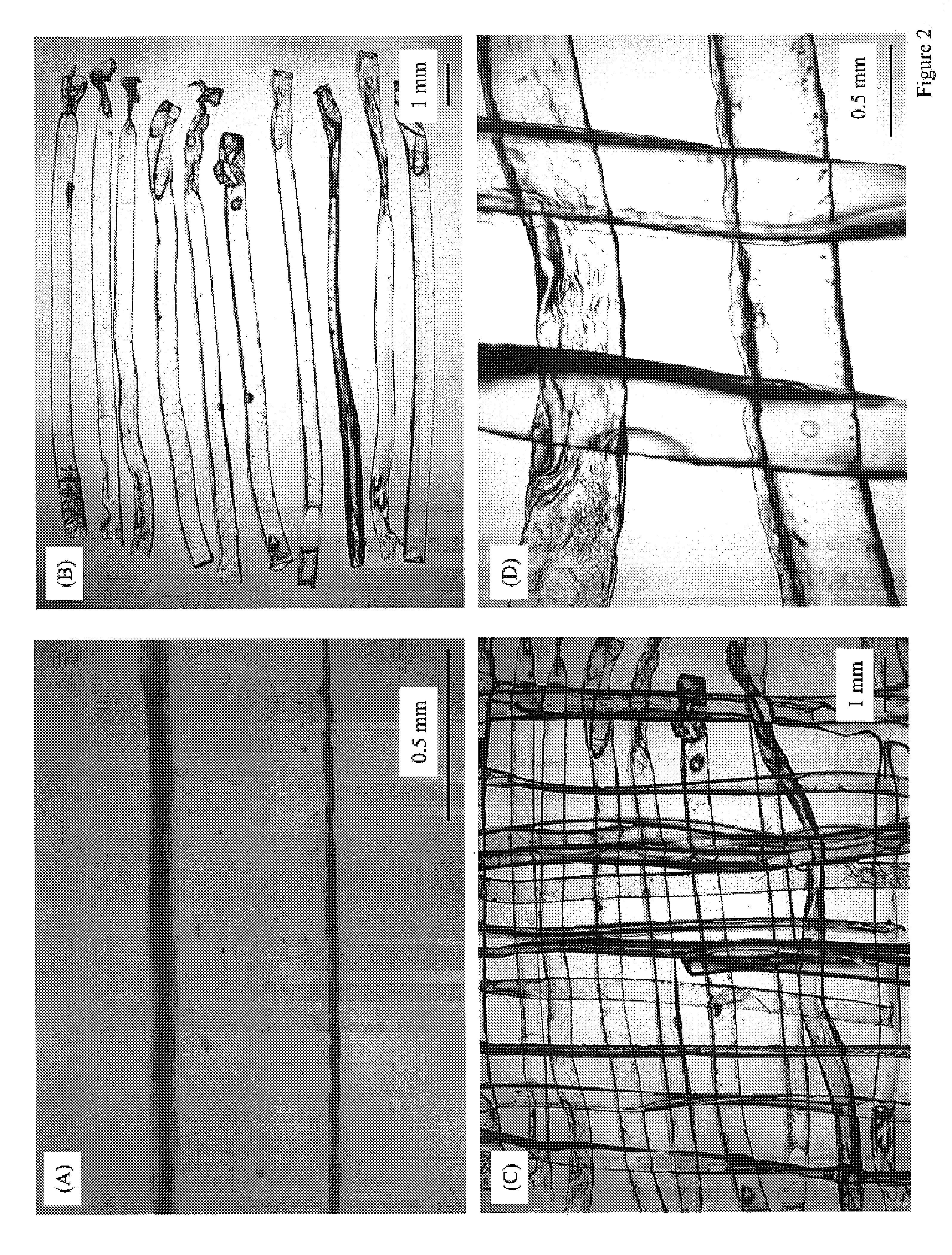
Method of preparation of bioabsorbable porous reinforced tissue implants and implants thereof
A biocompatible tissue implant. The tissue implant may be bioabsorbable, consists of a biocompatible polymeric foam. The tissue implant also includes a biocompatible reinforcement member. The polymeric foam and the reinforcement member are soluble in a lyophilizing solvent. The reinforcement may be annealed and / or coated.
Owner:DEPUY MITEK INC
Device and method for replacing mitral valve
InactiveUS20090276040A1Prevent movementRelieve pressureSuture equipmentsStentsNative tissueProsthesis
A prosthetic mitral valve assembly and method of inserting the same is disclosed. In certain disclosed embodiments, the prosthetic mitral valve assembly has a flared upper end and a tapered portion to fit the contours of the native mitral valve. The prosthetic mitral valve assembly can include a stent or outer support frame with a valve mounted therein, The assembly can be adapted to expand radially outwardly and into contact with the native tissue to create a pressure fit. One embodiment of a method includes positioning the mitral valve assembly below the annulus such that the annulus itself can restrict the assembly from moving in an upward direction towards the left atrium. The mitral valve assembly is also positioned so that the leaflets of the mitral valve hold the assembly to prevent downward movement of the assembly towards the left ventricle.
Owner:EDWARDS LIFESCIENCES CORP
Method of preparation of bioabsorbable porous reinforced tissue implants and implants thereof
Owner:DEPUY SYNTHES PROD INC
Multilayer scaffold
InactiveUS20170182211A1Avoid disease riskAvoids the potential ethical and religious barriers to the useSkin implantsTissue regenerationMedicinePlla scaffold
Owner:SMITH & NEPHEW PLC
At least partially resorbable reticulated elastomeric matrix elements and methods of making same
ActiveUS9050176B2Small volumeIncrease in sizePharmaceutical delivery mechanismPharmaceutical non-active ingredientsElastomerBiomedical engineering
The present disclosure relates to reticulated elastomeric matrices, and more particularly to at least partially degradable elastomeric elements that are compressible and exhibit resilience in their recovery and that can be employed in diverse applications including, without limitation, biological implantation, especially in humans.
Owner:DSM IP ASSETS BV
Intervertebral disc implant
InactiveUS6187048B1Speed up the flowReduce and eliminate any adverse effectJoint implantsSpinal implantsIntervertebral discProsthesis
An implant for forming an intervertebral disc nucleus pulposus prosthesis includes a conformable material adapted to fill cavities within the disc and to at least partially polymerize in-situ to form a shaped, resiliently deformable prosthesis.
Owner:HOWMEDICA OSTEONICS CORP
Collagen biofabric and methods of preparation and use therefor
InactiveUS20040048796A1Improved biophysical propertyImprove featuresSenses disorderPeptide/protein ingredientsSurgical GraftWound dressing
The present invention relates to collagenous membranes produced from amnion, herein referred to as a collagen biofabric. The collagen biofabric of the invention has the structural integrity of the native non-treated amniotic membrane, i.e., the native tertiary and quaternary structure. The present invention provides a method for preparing a collagen biofabric from a placental membrane, preferably a human placental membrane having a chorionic and amniotic membrane, by decellularizing the amniotic membrane. In a preferred embodiment, the amniotic membrane is completely decellularized. The collagen biofabric of the invention has numerous utilities in the medical and surgical field including for example, blood vessel repair, construction and replacement of a blood vessel, tendon and ligament replacement, wound-dressing, surgical grafts, ophthalmic uses, sutures, and others. The benefits of the biofabric are, in part, due to its physical properties such as biomechanical strength, flexibility, suturability, and low immunogenicity, particularly when derived from human placenta.
Owner:CELLULAR THERAPEUTICS DIV OF CELGENE +1
Biphasic osteochondral scaffold for reconstruction of articular cartilage
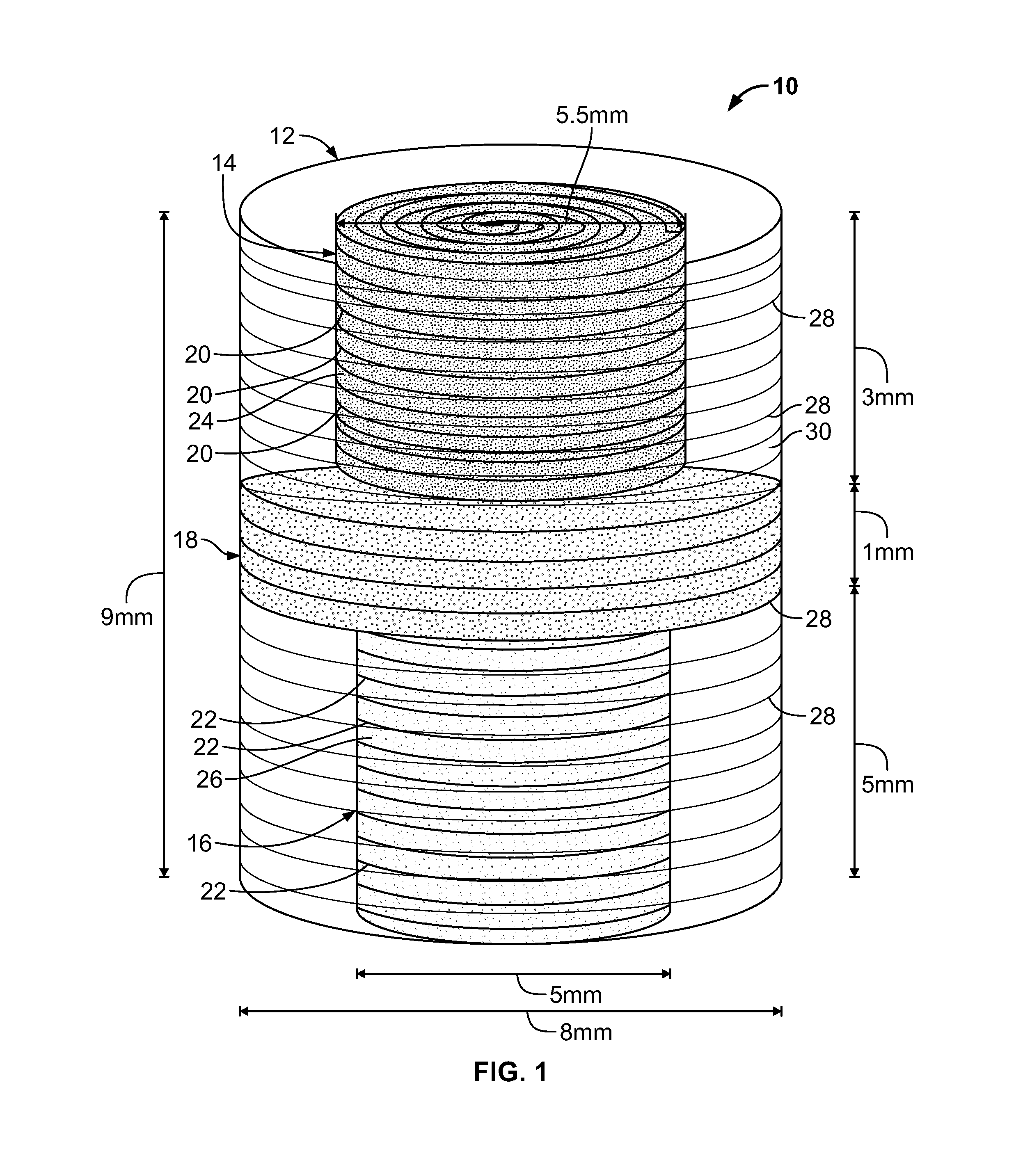
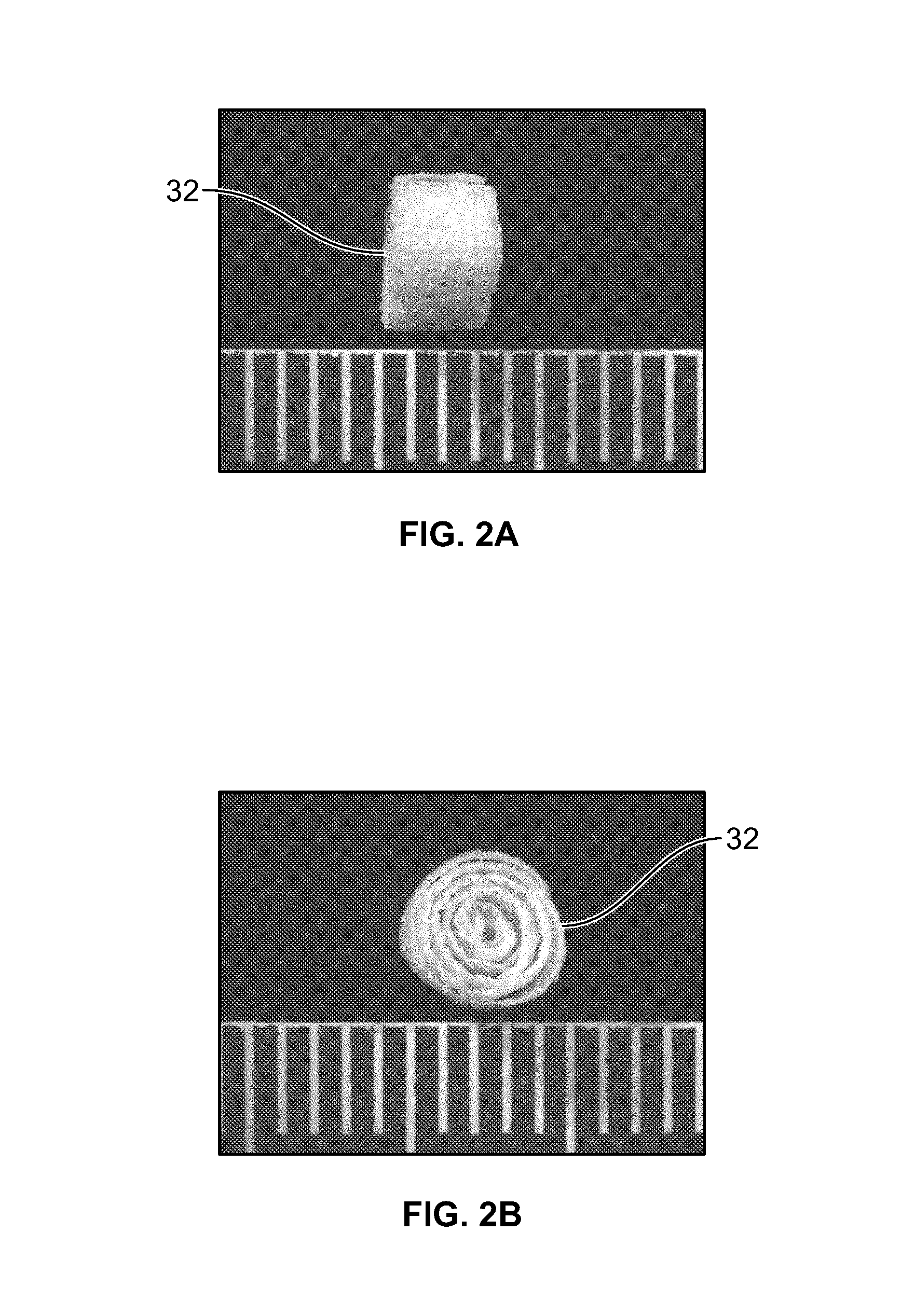
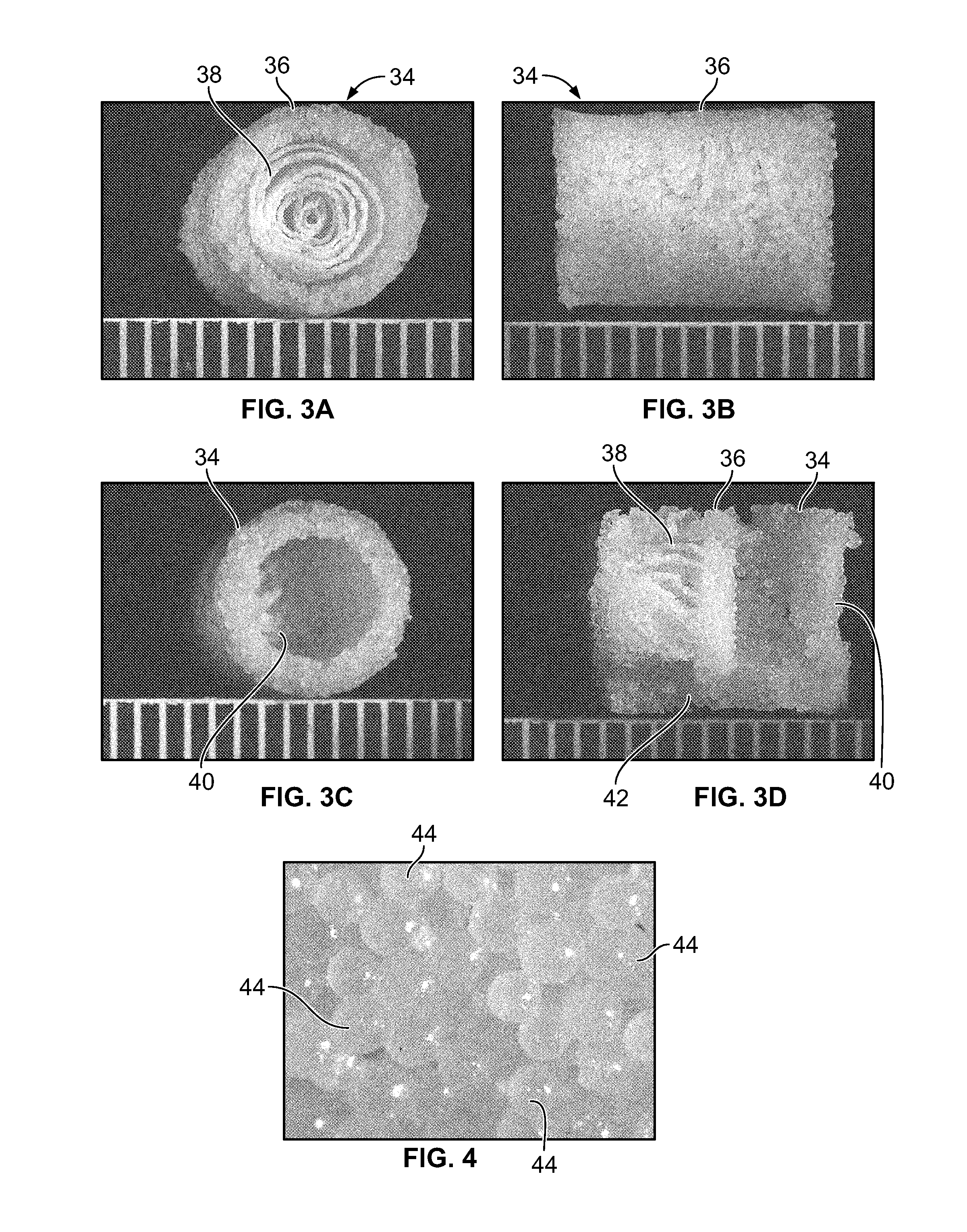
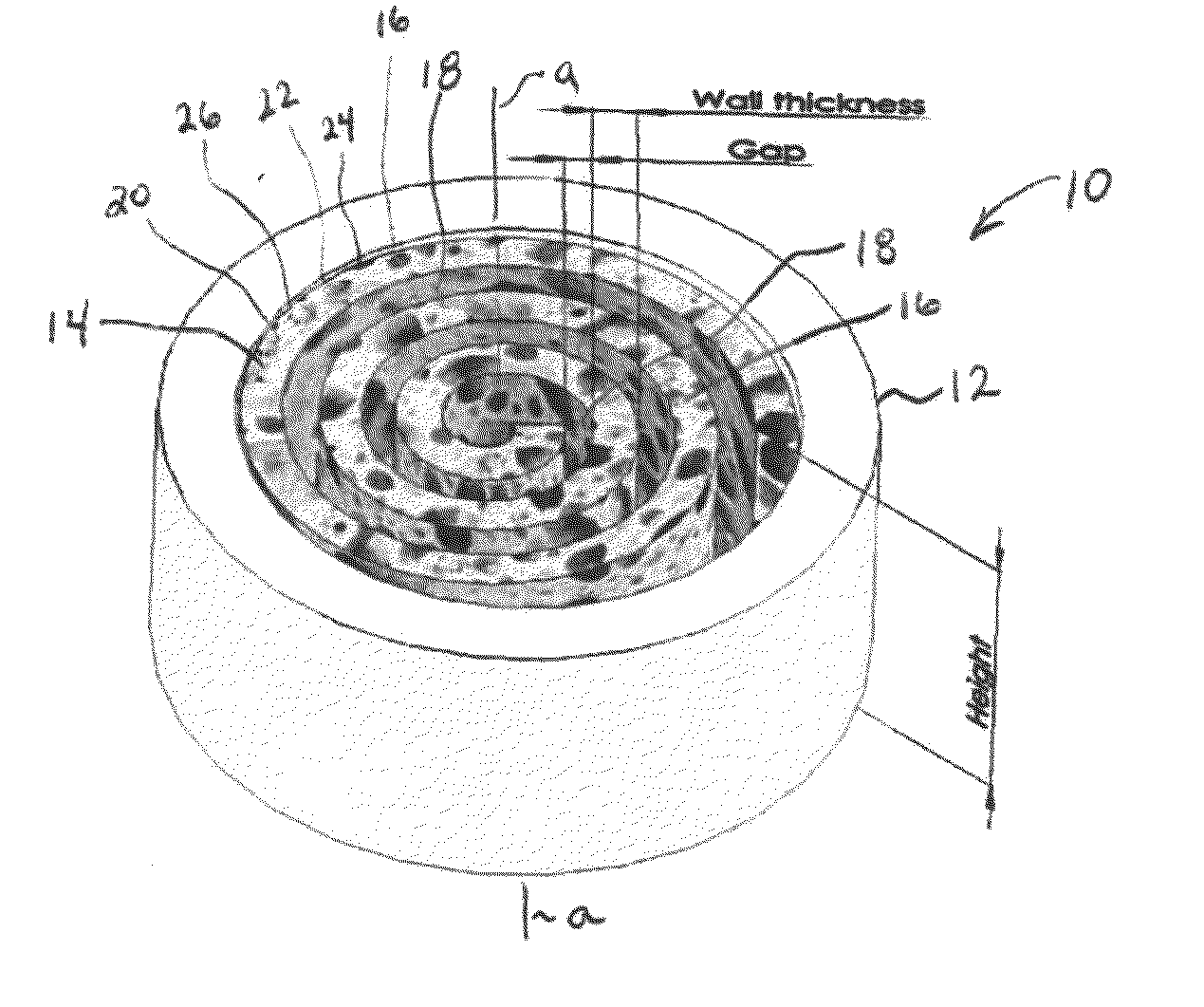
An osteochondral scaffold has a chondrogenic spiral scaffold in one end of an outer shell made of sintered microspheres, and an osteogenic spiral scaffold in the other end of the outer shell. Each spiral scaffold has nanofibers of a composition selected to promote attachment and proliferation of the desired types of cells. The nanofibers for the chondrogenic spiral scaffold have a different composition than the nanofibers for the osteogenic spiral scaffold. The nanofibers of each spiral scaffold are aligned to orient the attached cells so as to recreate the structure of the native tissue.
Owner:STEVENS INSTITUTE OF TECHNOLOGY
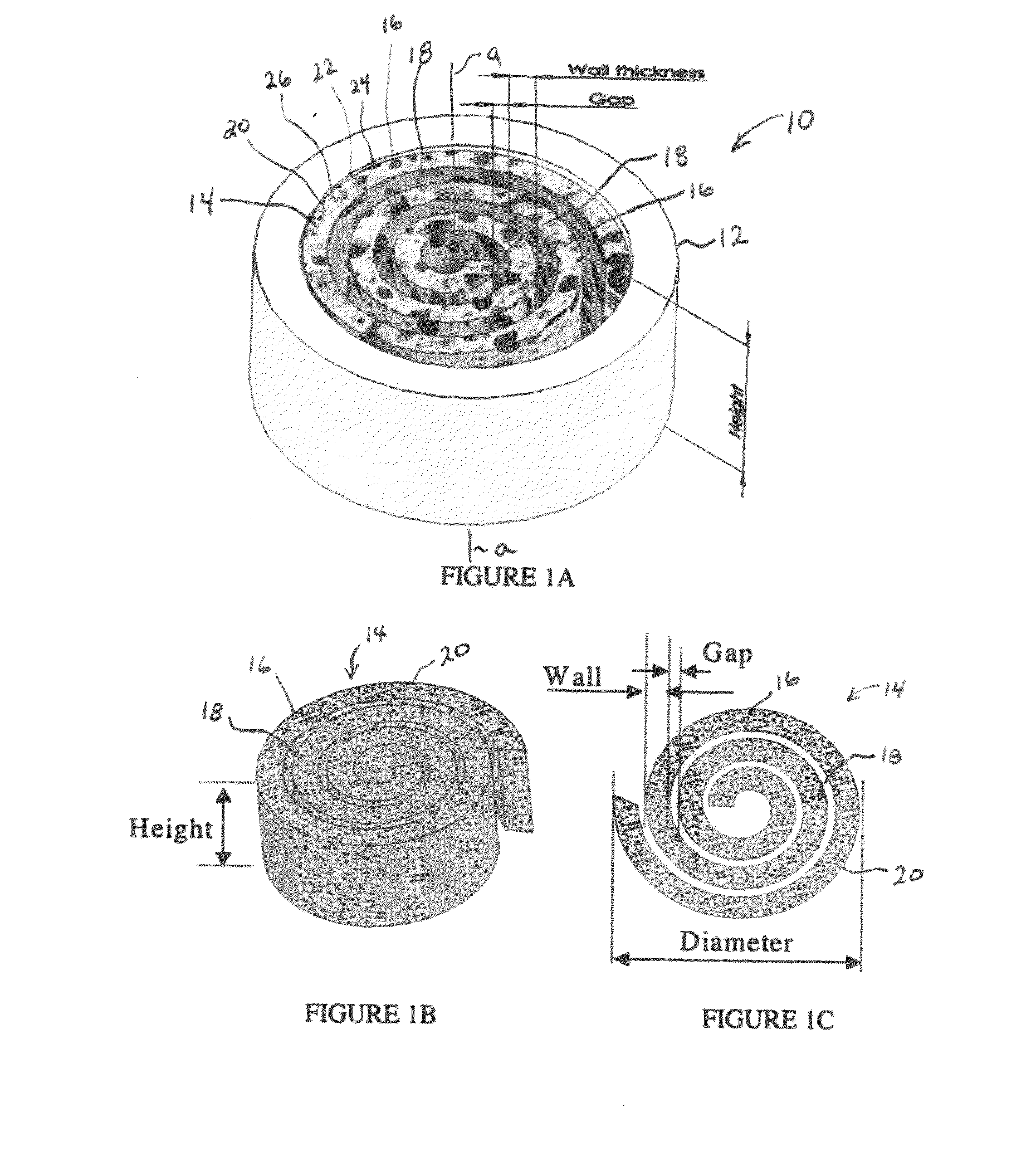
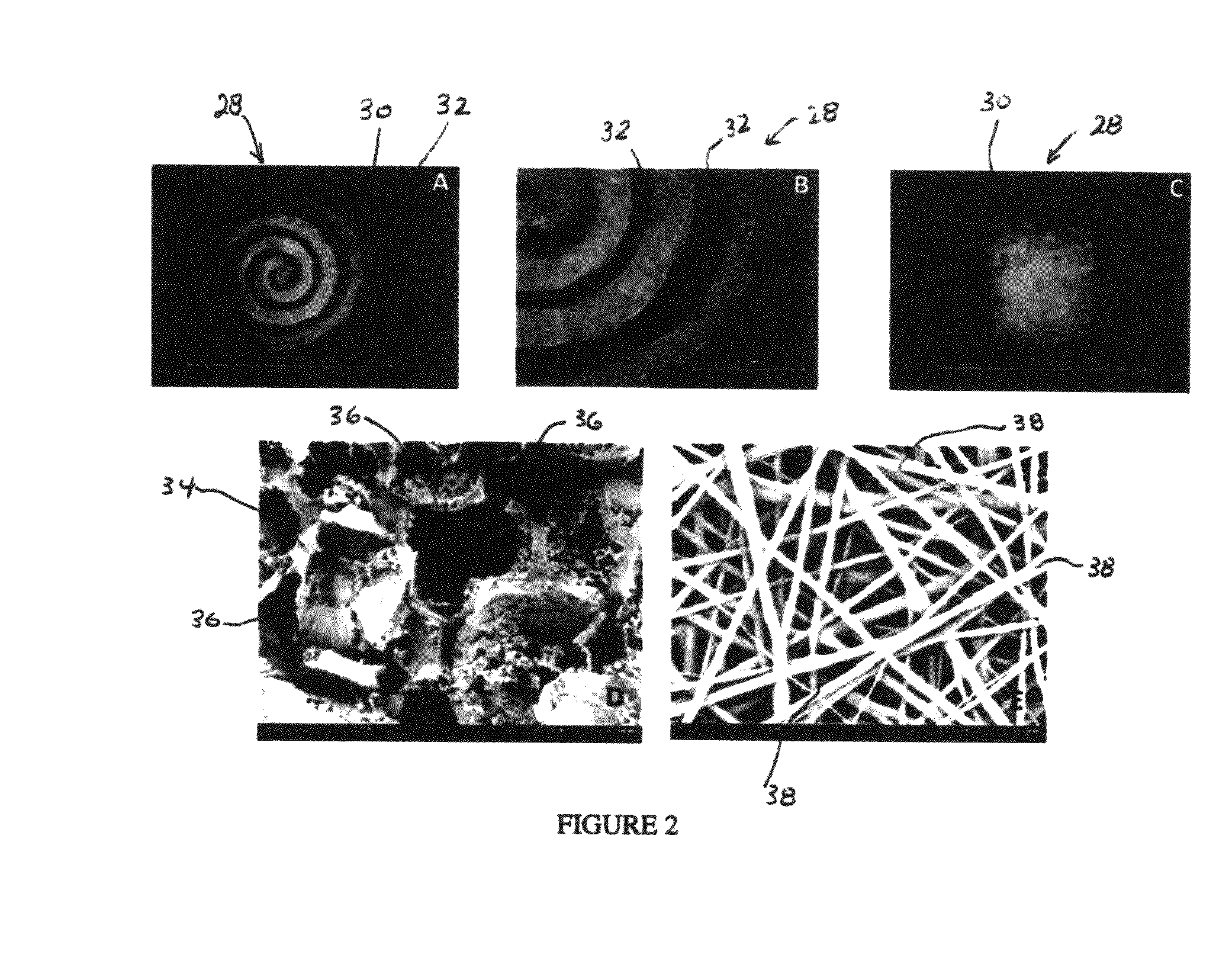
Synergetic functionalized spiral-in-tubular bone scaffolds
InactiveUS20100310623A1Increase the number ofIncrease alkaline phosphatase activityPeptide/protein ingredientsBone implantPorous sheetCell seeding
An integrated scaffold for bone tissue engineering has a tubular outer shell and a spiral scaffold made of a porous sheet. The spiral scaffold is formed such that the porous sheet defines a series of spiral coils with gaps of controlled width between the coils to provide an open geometry for enhanced cell growth. The spiral scaffold resides within the bore of the shell and is integrated with the shell to fix the geometry of the spiral scaffold. Nanofibers may be deposited on the porous sheet to enhance cell penetration into the spiral scaffold. The spiral scaffold may have alternating layers of polymer and ceramic on the porous sheet that have been built up using a layer-by-layer method. The spiral scaffold may be seeded with cells by growing a cell sheet and placing the cell sheet on the porous sheet before it is rolled.
Owner:UNIV OF CONNECTICUT
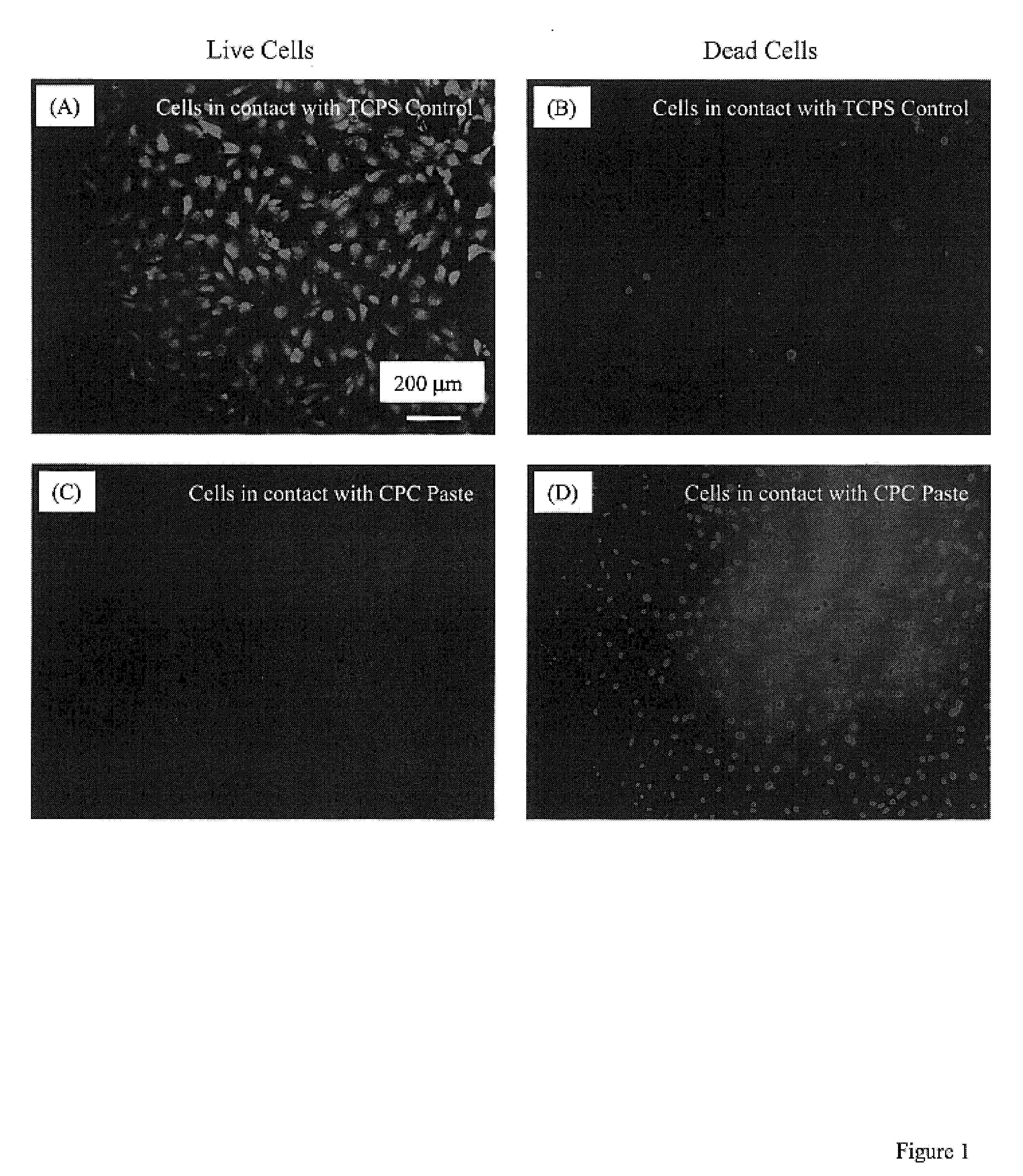
Three dimensional cell protector/pore architecture formation for bone and tissue constructs
InactiveUS7713542B2Greatly multiplied in vitroProtection from damagePowder deliveryBiocideIn vivoLiving cell
Living cellular material is encapsulated or placed in a protective material (cell protector) which is biocompatible, biodegradable and has a three-dimensional form. The three dimensional form is incorporated into a matrix that maybe implanted in vivo, ultimately degrade and thereby by replaced by living cell generated material.
Owner:ADA FOUND
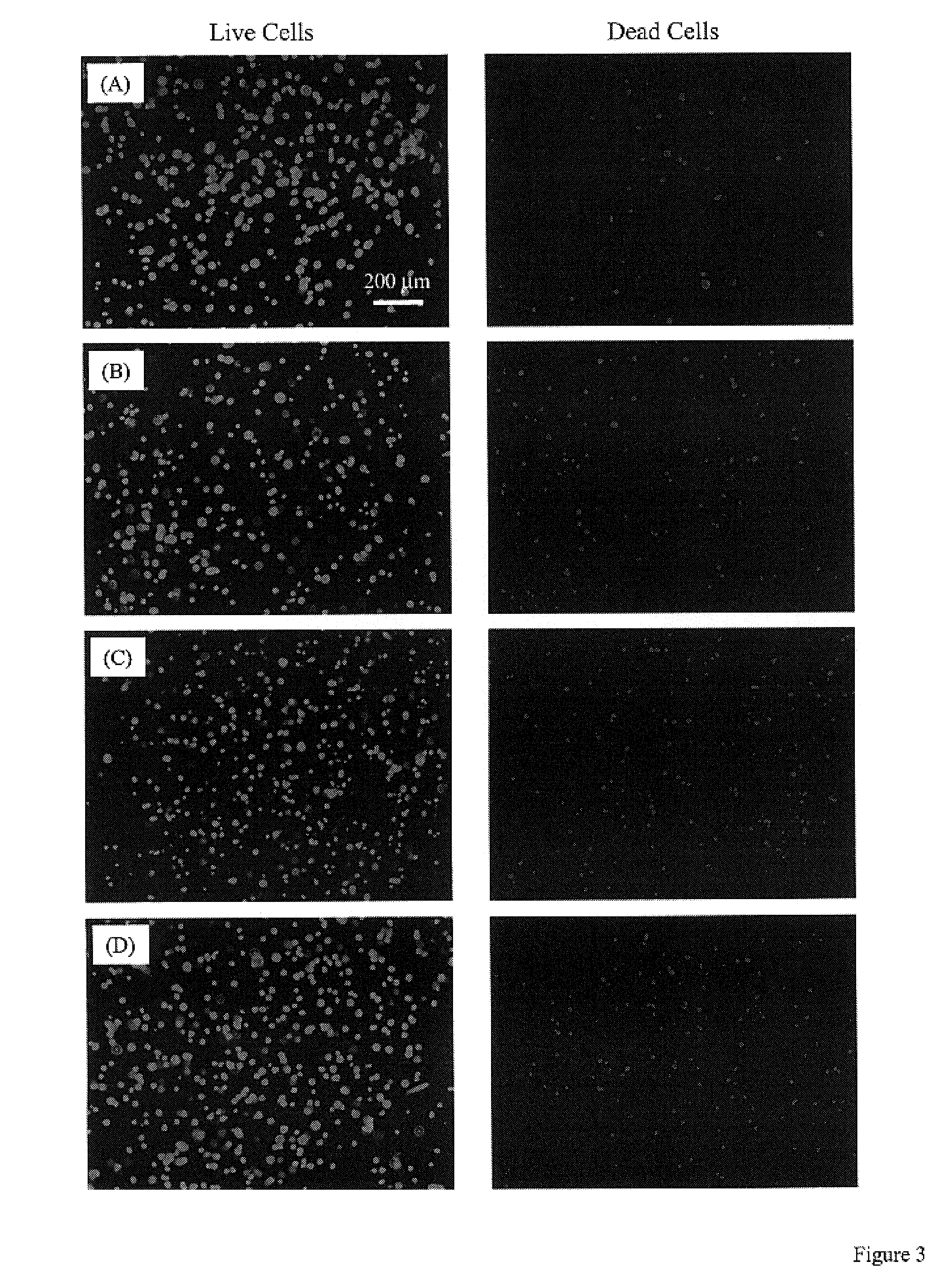
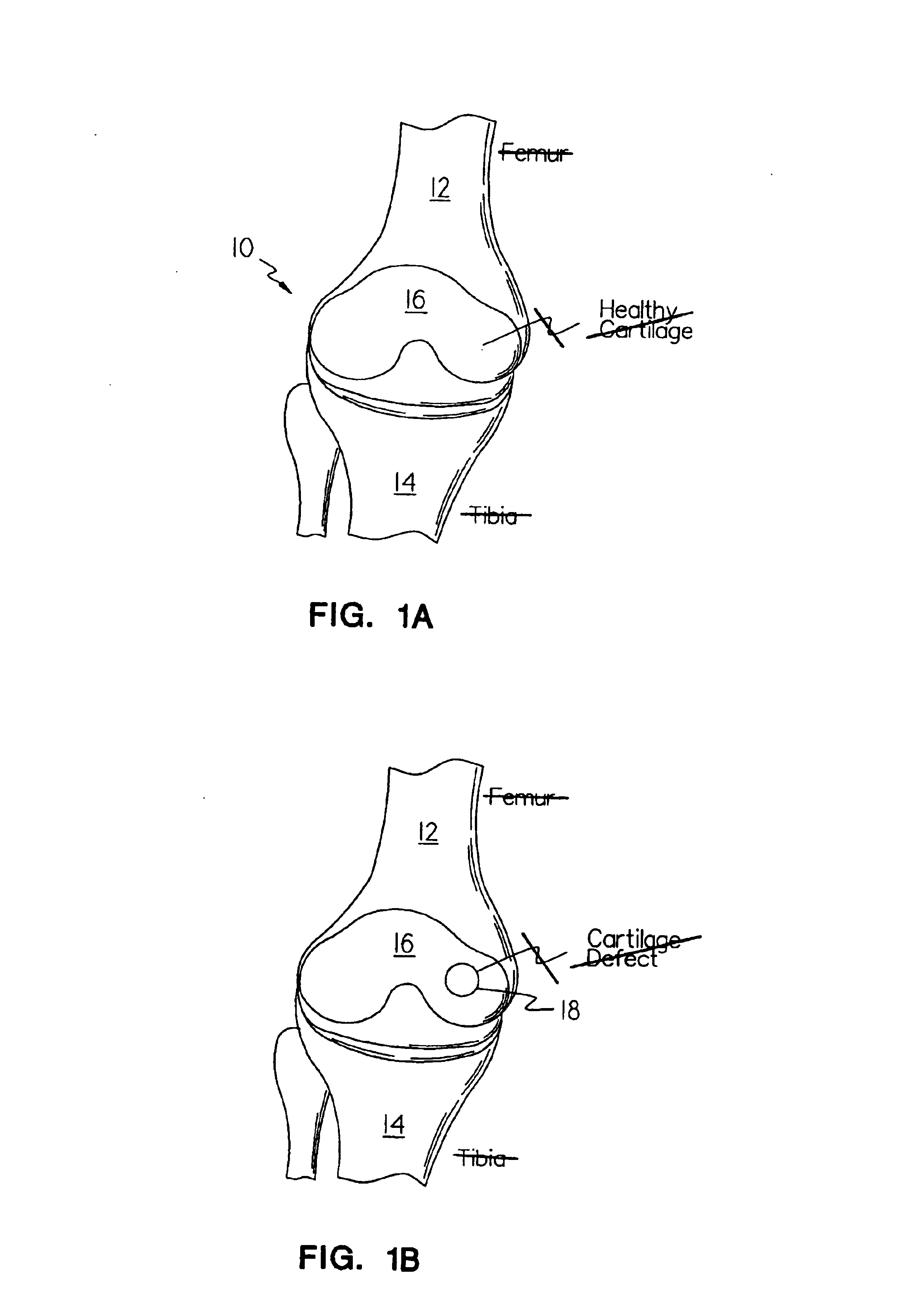
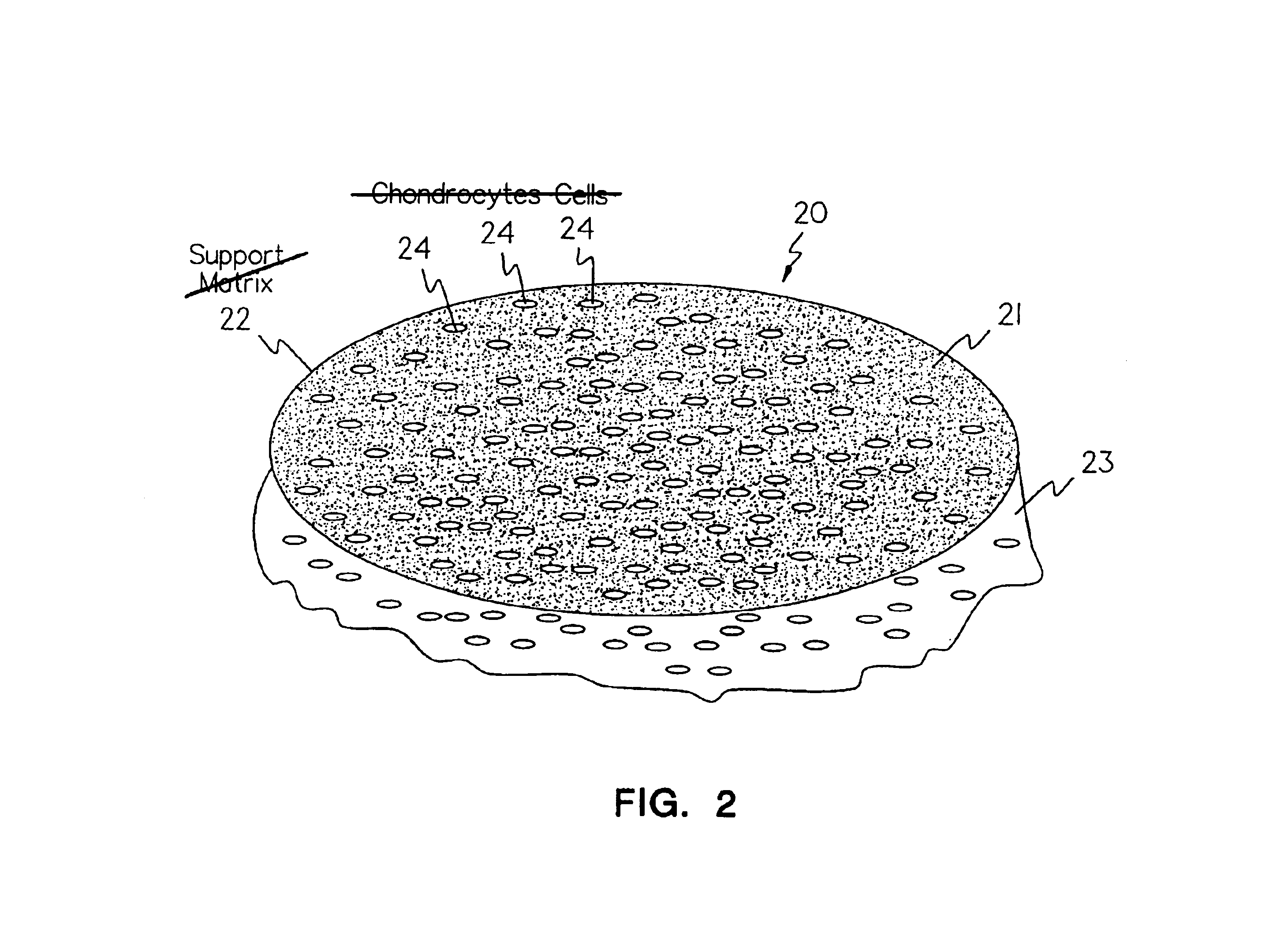
Methods, instruments and materials for chondrocyte cell transplantation
InactiveUS6866668B2Effective treatmentSuture equipmentsSurgical adhesivesSupport matrixTreated animal
A method for the effective treatment of articulating joint surface cartilage in an animal by the transplantation of an implantable article including chondrocyte cells retained to an absorbable support matrix. An instrument for placing and manipulating the implantable article at the site of implantation, and a retention device for securing the implantable article to the site of implantation. An implantable article for cartilage repair in an animal, the implantable article including chondrocyte cells retained on an absorbable support matrix, and a method of making same. An article comprising an absorbable flexible support matrix for living cells grown and adhered thereto.
Owner:VERIGEN TRANSPLANTATION SERVICE INT
Bioactive spinal implants and method of manufacture thereof
InactiveUS7238203B2Facilitating radiographic assessmentEnhance bone contact and stability and fusionInternal osteosythesisJoint implantsLumbar vertebraeCervical fusions
A bioactive spinal implant used in cervical fusion, Anterior Lumbar Interbody Fusion (ALIF), Posterior Lumbar Interbody Fusion (PLIF), and Transforaminal Interbody Fusion (TLIF), having properties and geometries that enhance bone contact, stability, and fusion between adjacent vertebral bodies.
Owner:VIA SPECIAL PURPOSE CORP +1
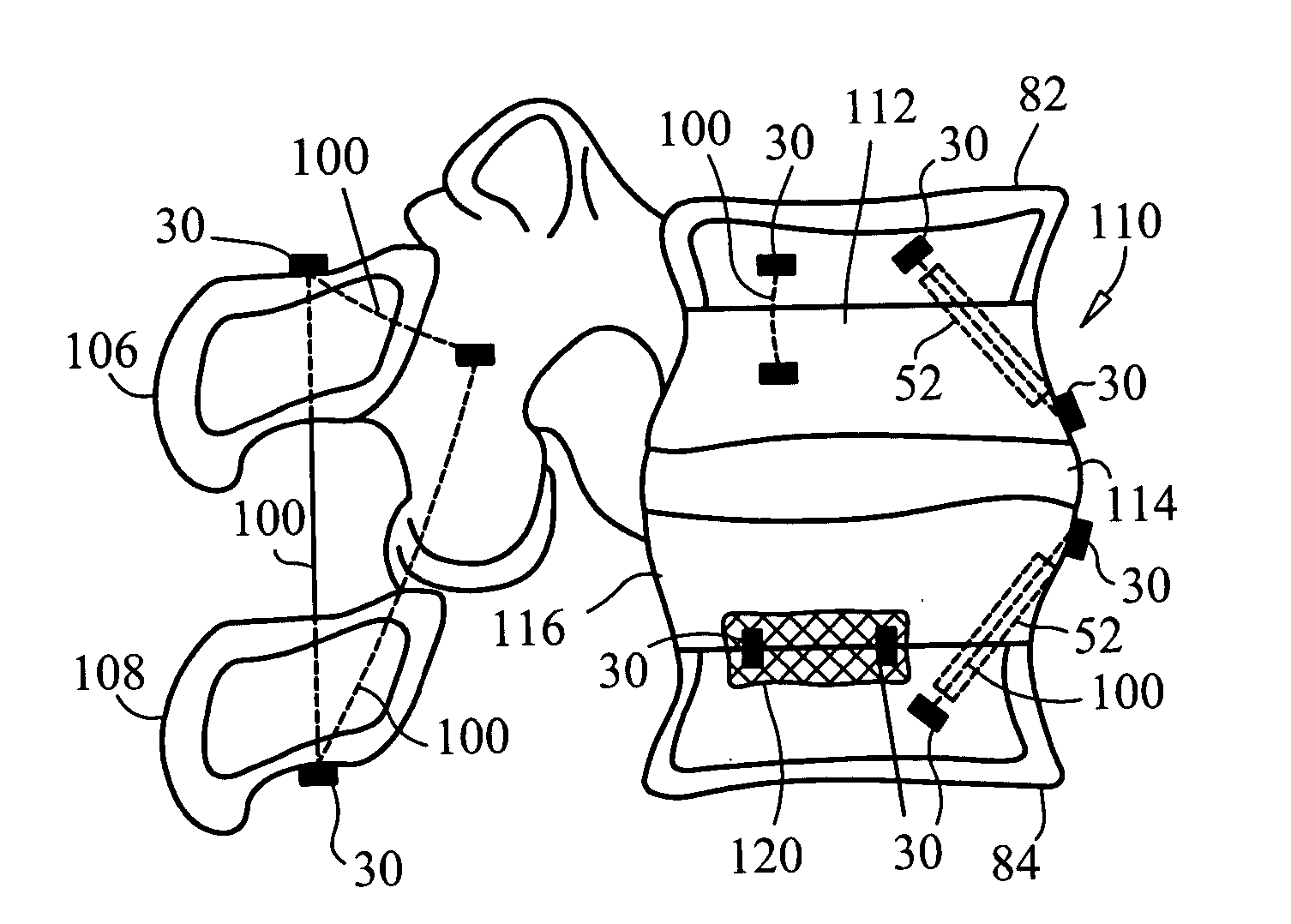
Devices and methods for stabilizing tissue and implants
InactiveUS20060089646A1Reduce traumaMinimized dimensionSuture equipmentsInternal osteosythesisIntervertebral discFibrosis
The present invention provides a method for intervertebral disc surgery. The method includes creating a passage in a vertebral body adjacent to the intervertebral disc. The passage extends from a side surface of the vertebral body to a nucleus pulposus of the intervertebral disc. Annulus fibrosis tissue of the intervertebral disc is not incised during the surgery.
Owner:P TECH
Shaped load-bearing osteoimplant and methods of making same
InactiveUS20030039676A1Promotes new host bone tissue formationPermit of mechanical propertySuture equipmentsDental implantsMedicineHard tissue
A load-bearing osteoimplant, methods of making the osteoimplant and method for repairing hard tissue such as bone and teeth employing the osteoimplant are provided. The osteoimplant comprises a shaped, coherent mass of bone particles which may exhibit osteogenic properties. In addition, the osteoimplant may possess one or more optional components which modify its mechanical and / or bioactive properties, e.g., binders, fillers, reinforcing components, etc.
Owner:WARSAW ORTHOPEDIC INC
Intervertebral disc prosthesis
InactiveUS7001431B2Improved polymer cureImproved implant characteristicInternal osteosythesisAnkle jointsIntervertebral discPolymer
A system for repairing an intervertebral disc by delivering and curing a biomaterial in situ within the disc. The system includes both a device, having an insertable balloon and related lumen, controls and adapters, as well as an in situ curable biomaterial (and related biomaterial delivery means). The system can allow the doctor to determine a suitable endpoint for biomaterial delivery, by controlling distraction and / or biomaterial delivery pressure, and in turn, to deliver a desired quantity of biomaterial to the balloon in order to achieve improved polymer cure and implant characteristics. Also provided is a related method for repairing an intervertebral disc by using such a system to deliver and cure the biomaterial in situ. The system can be used to implant a prosthetic total disc, or a prosthetic disc nucleus in a manner that leaves the surrounding disc tissue substantially intact.
Owner:DISC DYNAMICS
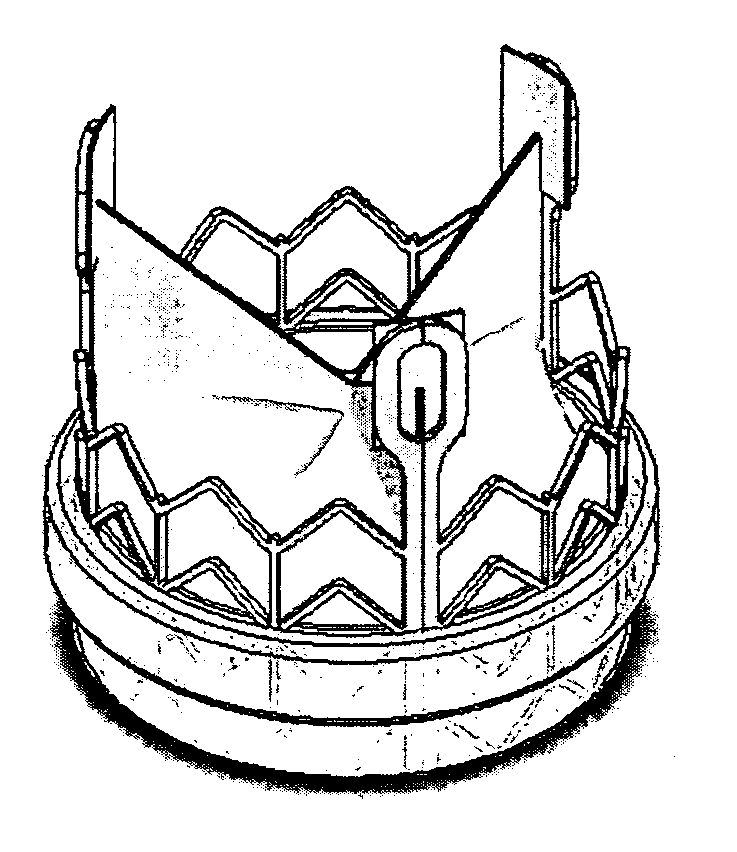
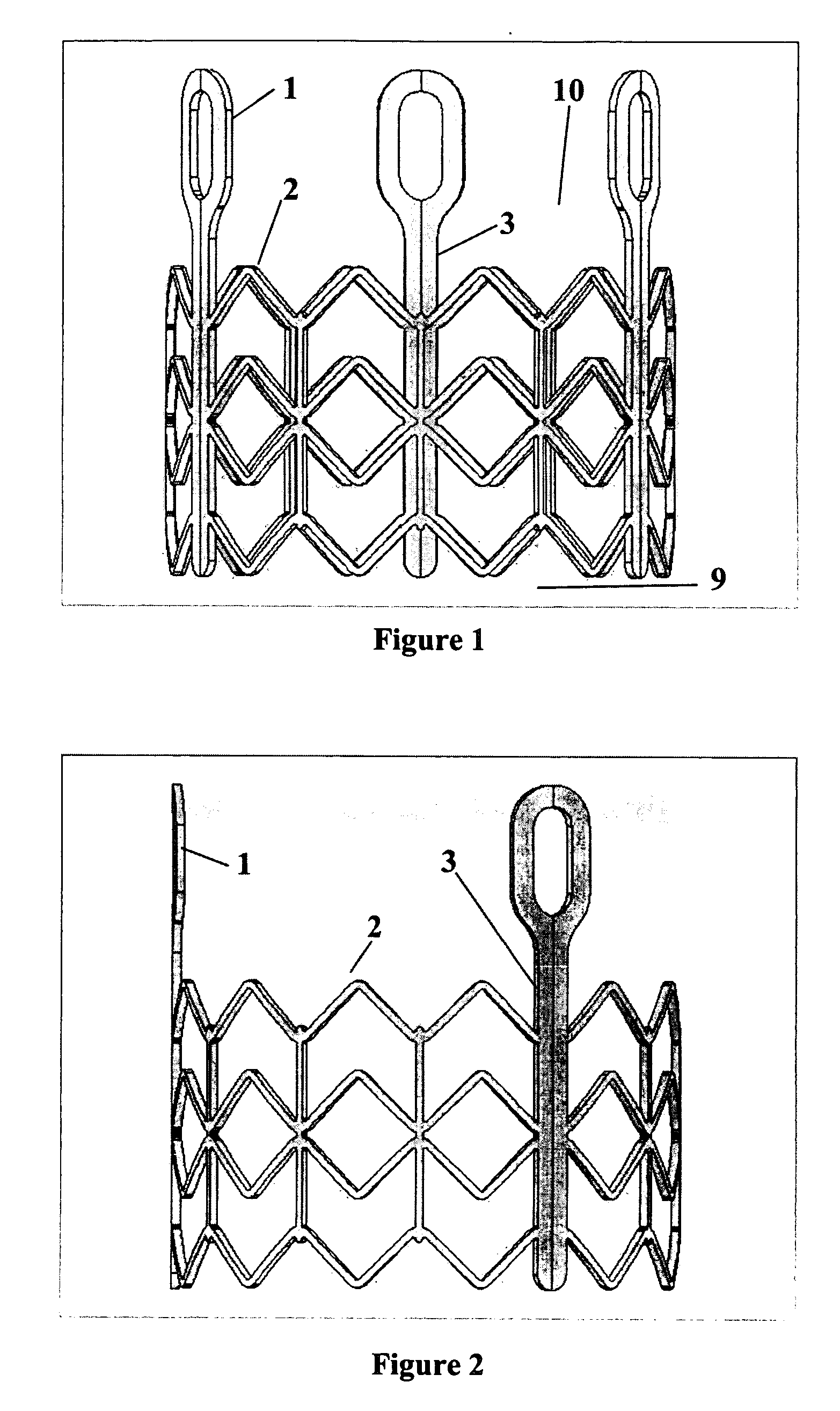
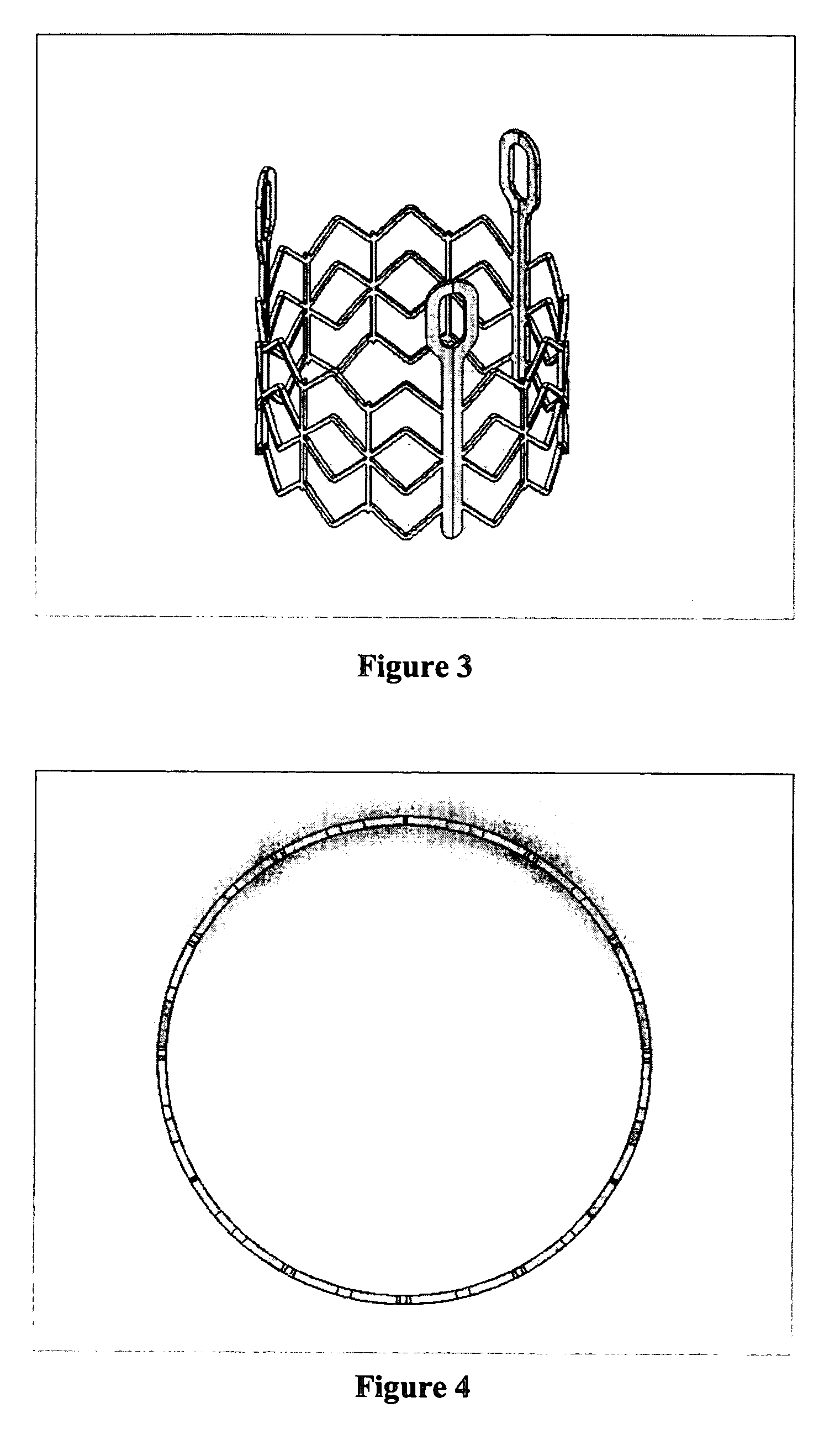
Prosthetic valves and related inventions
ActiveUS20140214159A1Low height to width profilePrevent perivalvular leakSuture equipmentsHeart valvesExtracorporeal circulationProsthetic valve
This invention relates to the design and function of a compressible valve replacement prosthesis, collared or uncollared, which can be deployed into a beating heart without extracorporeal circulation using a transcatheter delivery system. The design as discussed focuses on the deployment of a device via a minimally invasive fashion and by way of example considers a minimally invasive surgical procedure preferably utilizing the intercostal or subxyphoid space for valve introduction. In order to accomplish this, the valve is formed in such a manner that it can be compressed to fit within a delivery system and secondarily ejected from the delivery system into the annulus of a target valve such as a mitral valve or tricuspid valve.
Owner:TENDYNE HLDG
Radiation and melt treated ultra high molecular weight polyethylene prosthetic device and method
InactiveUS6641617B1Reduce productionReduce osteolysis and inflammatory reactionBone implantJoint implantsOxidation resistantPeriprosthetic
A medical prosthesis for use within the body which is formed of radiation treated ultra high molecular weight polyethylene having substantially no detectable free radicals, is described. Preferred prostheses exhibit reduced production of particles from the prosthesis during wear of the prosthesis, and are substantially oxidation resistant. Methods of manufacture of such devices and material used therein are also provided.
Owner:CENTPULSE ORTHOPEDICS +1
Cartilage repair plug
A cartilage plug, which is made from a biocompatible, artificial material, that is used to fill a void in natural cartilage that has been resected due to traumatic injury or chronic disease. Alternatively, the plug may be relied upon to anchor a flowable polymer to subchondral bone. The plug is prefabricatable in any size, shape, and contour and may be utilized either singly or in a plurality to fill any size void for any application. The plug may be formed of a laminated structure to match the physiological requirements of the repair site. A plurality of anchoring elements may share a single upper layer.
Owner:ABS
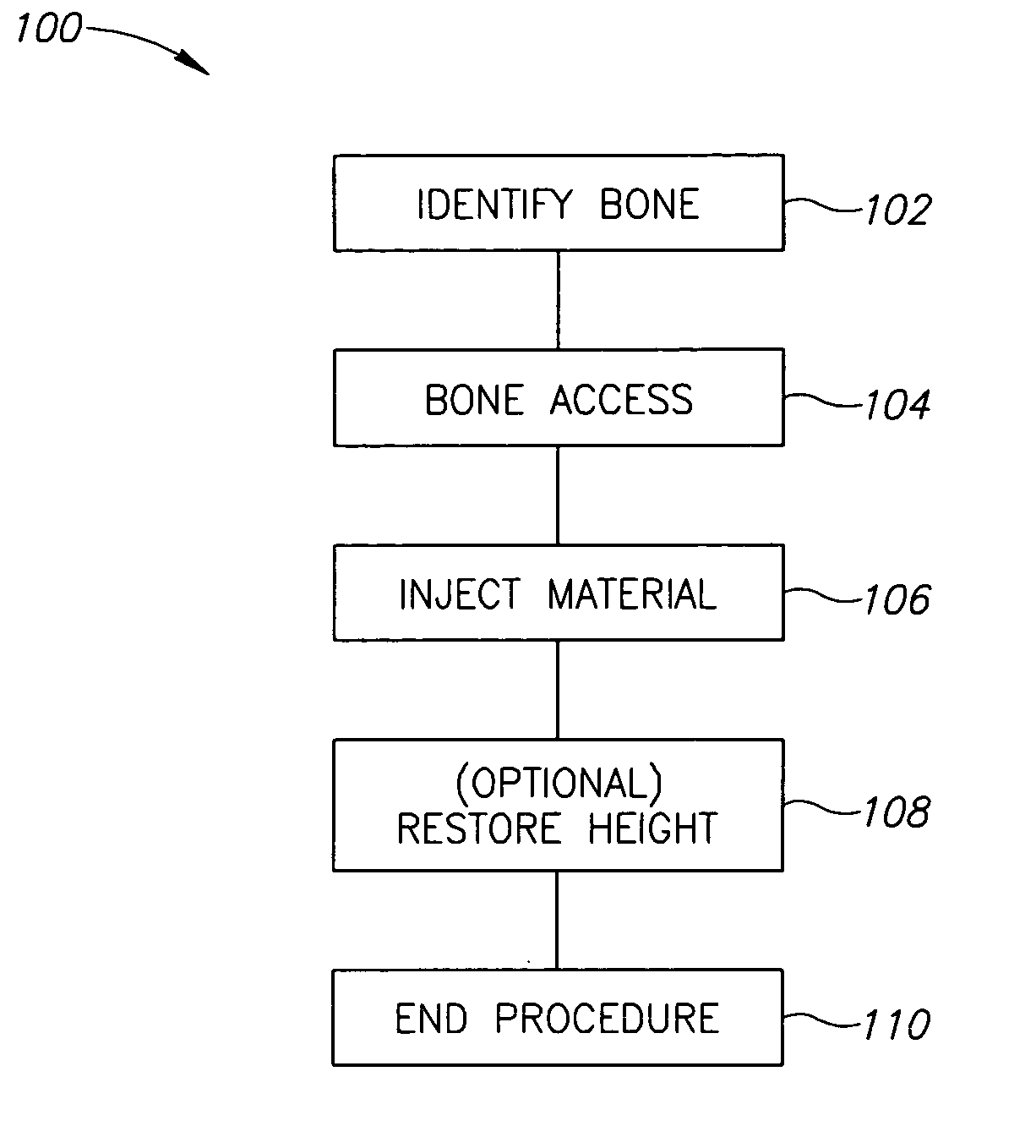
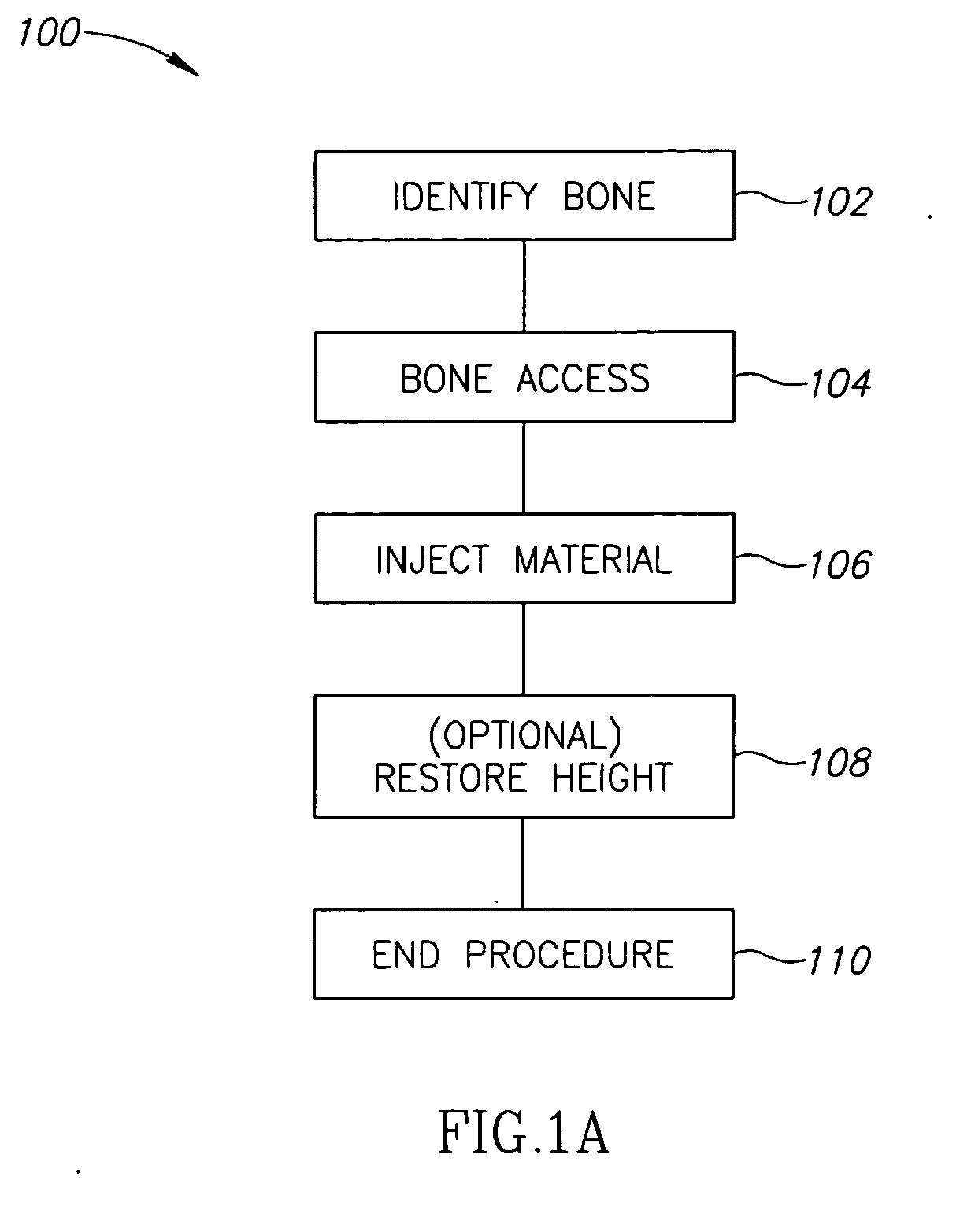
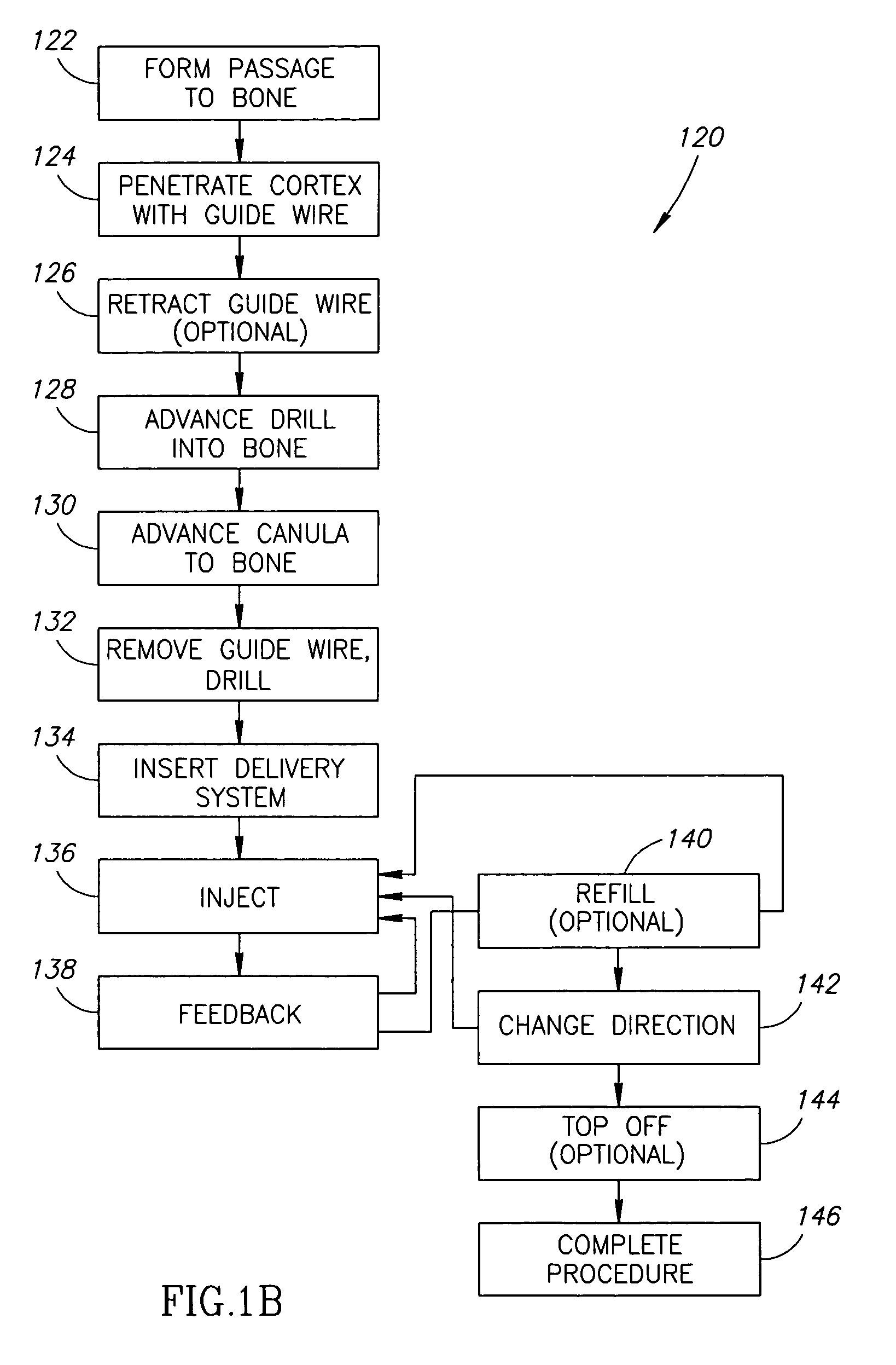
Methods, materials and apparatus for treating bone and other tissue
ActiveUS20060079905A1Avoid less flexibilityEasy to useImpression capsSurgical adhesivesBiomedical engineeringVertebra
A method of treating a vertebra, comprising: (a) accessing an interior of a vertebra; and (b) introducing a sufficient amount of artificial biocompatible material which does not set to a hardened condition in storage, into said bone, with sufficient force to move apart fractured portions of said bone.
Owner:DEPUY SYNTHES PROD INC
Prosthetic intervertebral disc and methods for using same
InactiveUS20050228500A1Improve toughnessHigh modulusJoint implantsSpinal implantsSurgical operationIntervertebral disc
Prosthetic intervertebral discs and methods for using the same are described. The subject prosthetic discs include upper and lower endplates separated by a compressible core member. The prosthetic discs described herein include one-piece, two-piece, three-piece, and four-piece structures. The subject prosthetic discs exhibit stiffness in the vertical direction, torsional stiffness, bending stiffness in the saggital plane, and bending stiffness in the front plane, where the degree of these features can be controlled independently by adjusting the components of the discs. The interface mechanism between the endplates and the core members of several embodiments of the described prosthetic discs enables a very easy surgical operation for implantation.
Owner:SPINAL KINETICS
Glaucoma implant with therapeutic agents
InactiveUS20040127843A1Convenient treatmentReduce and inhibit and slow effectEye surgeryWound drainsAqueous humorSchlemm's canal
Devices and methods are provided for the treatment of glaucoma. An ocular implant is adapted such that aqueous humor flows controllably from the anterior chamber of the eye to Schlemm's canal, bypassing the trabecular meshwork. The implant may utilize one or more bioactive agents effective in treating glaucoma or other pathology.
Owner:DOSE MEDICAL CORP
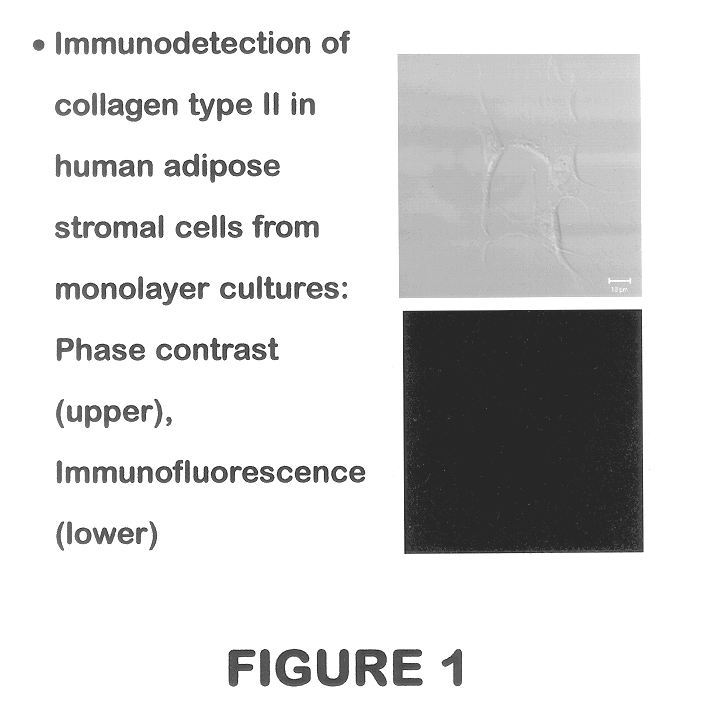
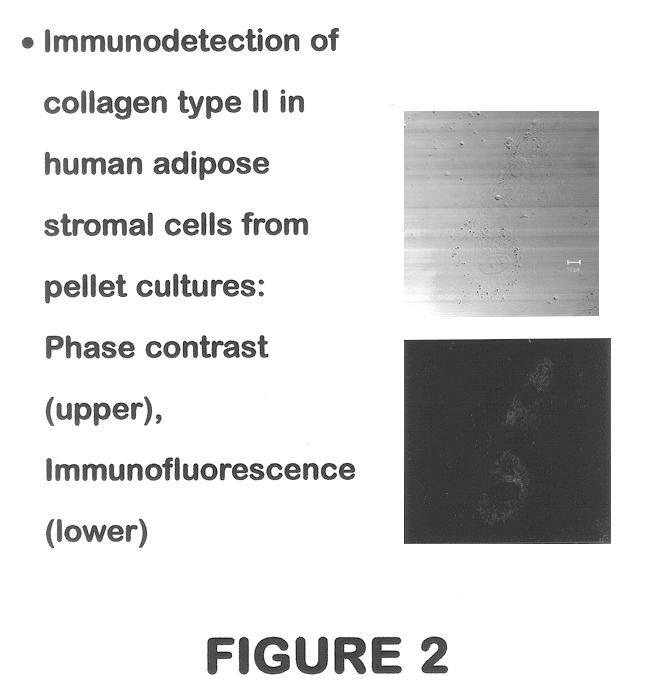
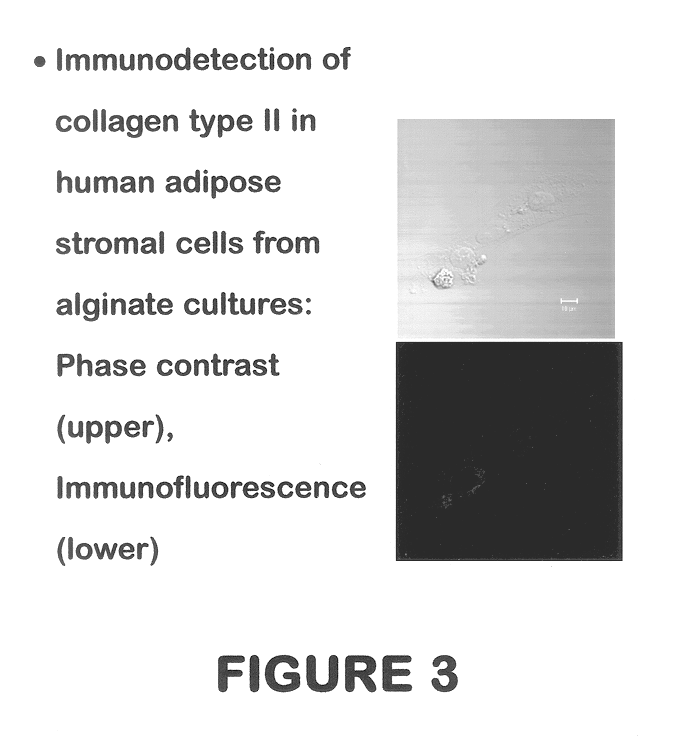
Use of adipose tissue-derived stromal cells for chondrocyte differentiation and cartilage repair
Methods and compositions for directing adipose-derived stromal cells cultivated in vitro to differentiate into cells of the chondrocyte lineage are disclosed. The invention further provides a variety of chondroinductive agents which can be used singly or in combination with other nutrient components to induce chondrogenesis in adipose-derived stromal cells either in cultivating monolayers or in a biocompatible lattice or matrix in a three-dimensional configuration. Use of the differentiated chondrocytes for the therapeutic treatment of a number of human conditions and diseases including repair of cartilage in vivo is disclosed.
Owner:COGNATE BIOSERVICES
Method and apparatus for resurfacing an articular surface
A biocompatible, bioresorbable tissue repair implant or scaffold device is provided for use in repairing a variety of cartilage tissue injuries, and particularly for resurfacing and / or repairing damaged or diseased cartilage. The repair procedures may be conducted with tissue repair implants that contain a biological component that assists in delaying or arresting the progression of degenerative joint diseases and in enhancing tissue healing or repair. The biocompatible, bioresorbable tissue repair implants include a scaffold and particles of viable tissue derived from cartilage tissue, such that the tissue and the scaffold become associated. The particles of living tissue contain one or more viable cells that can migrate from the tissue and populate the scaffold.
Owner:DEPUY SYNTHES PROD INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com