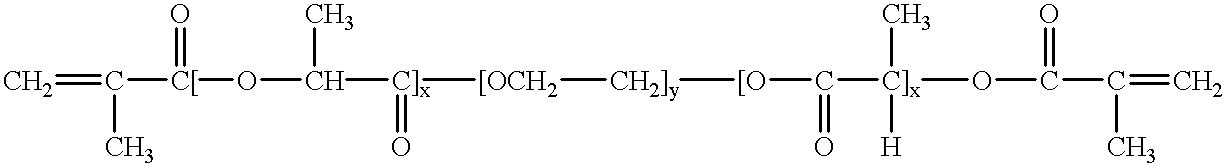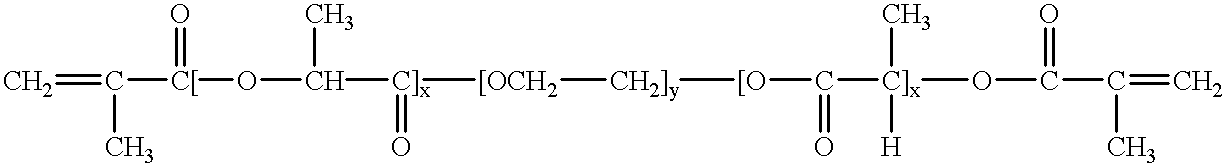Patents
Literature
887results about "Mammary implants" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
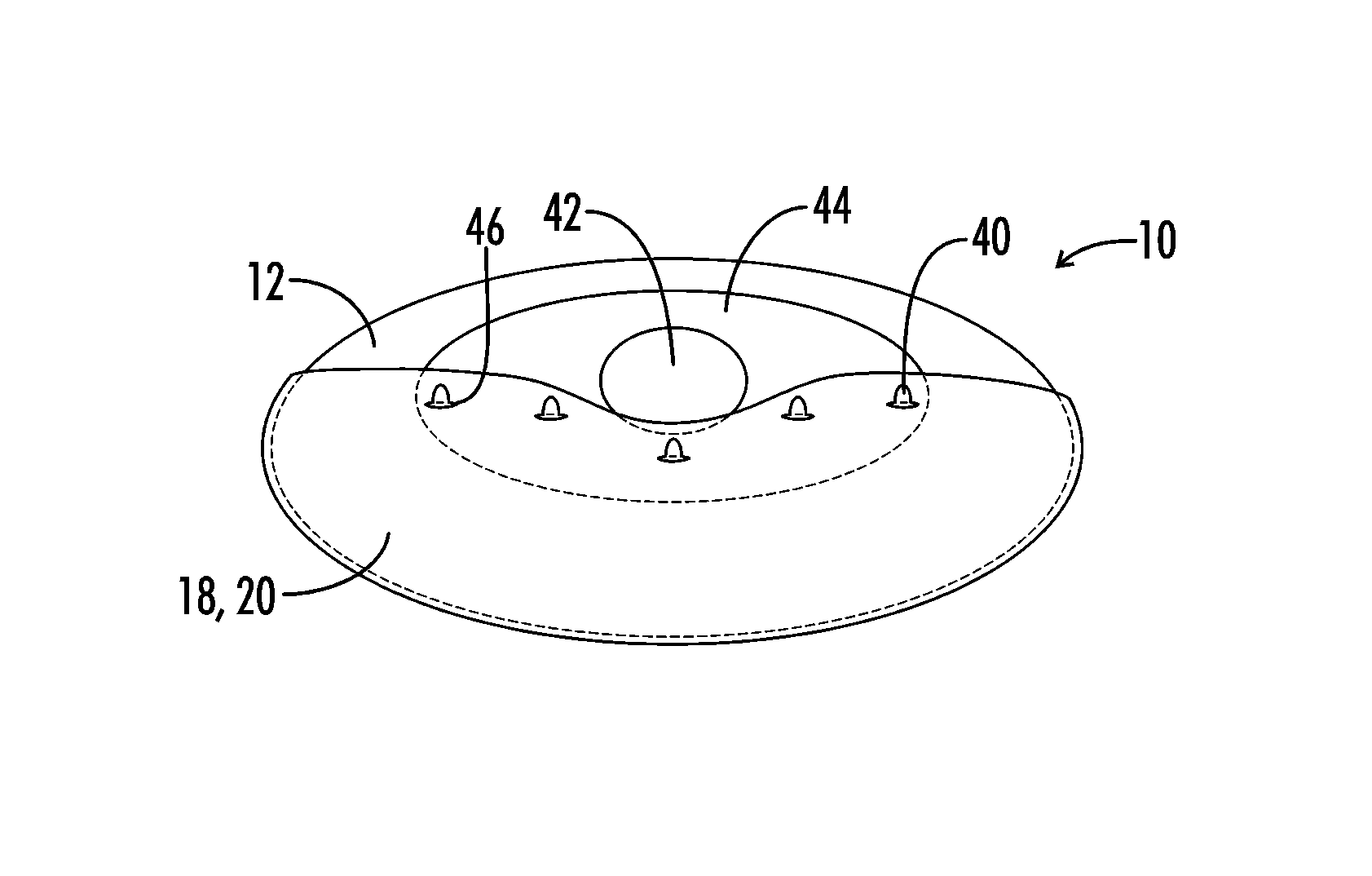
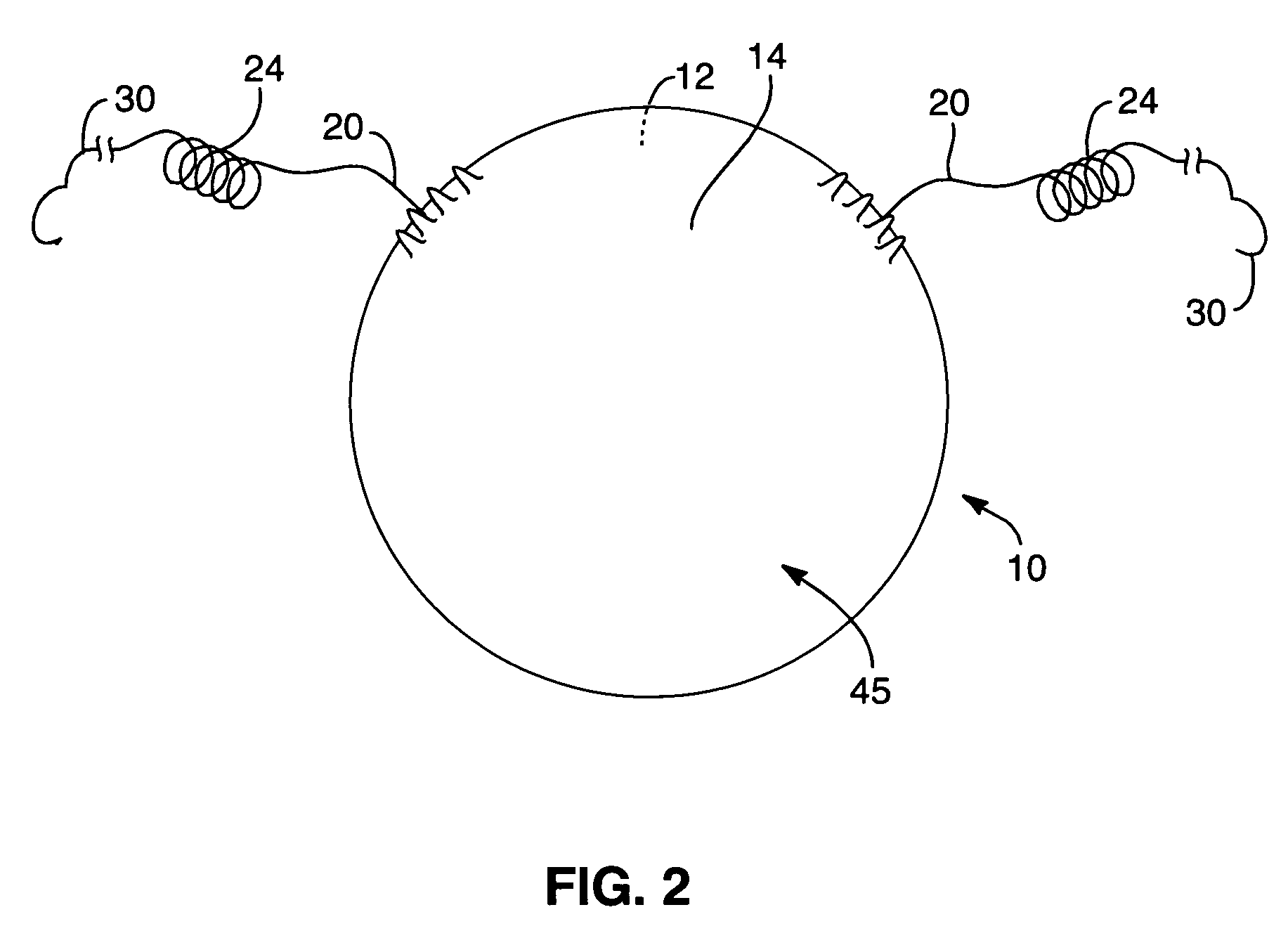
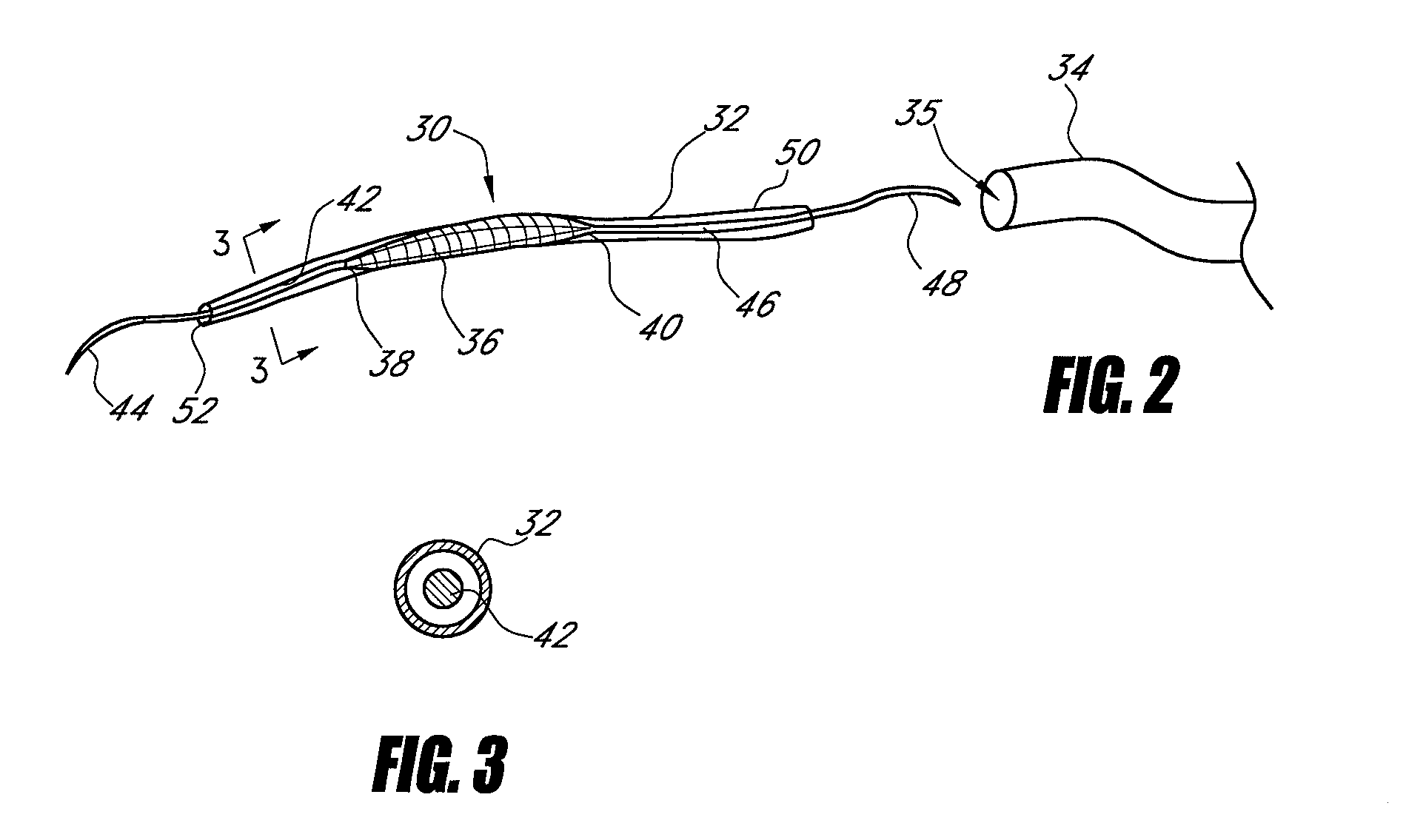
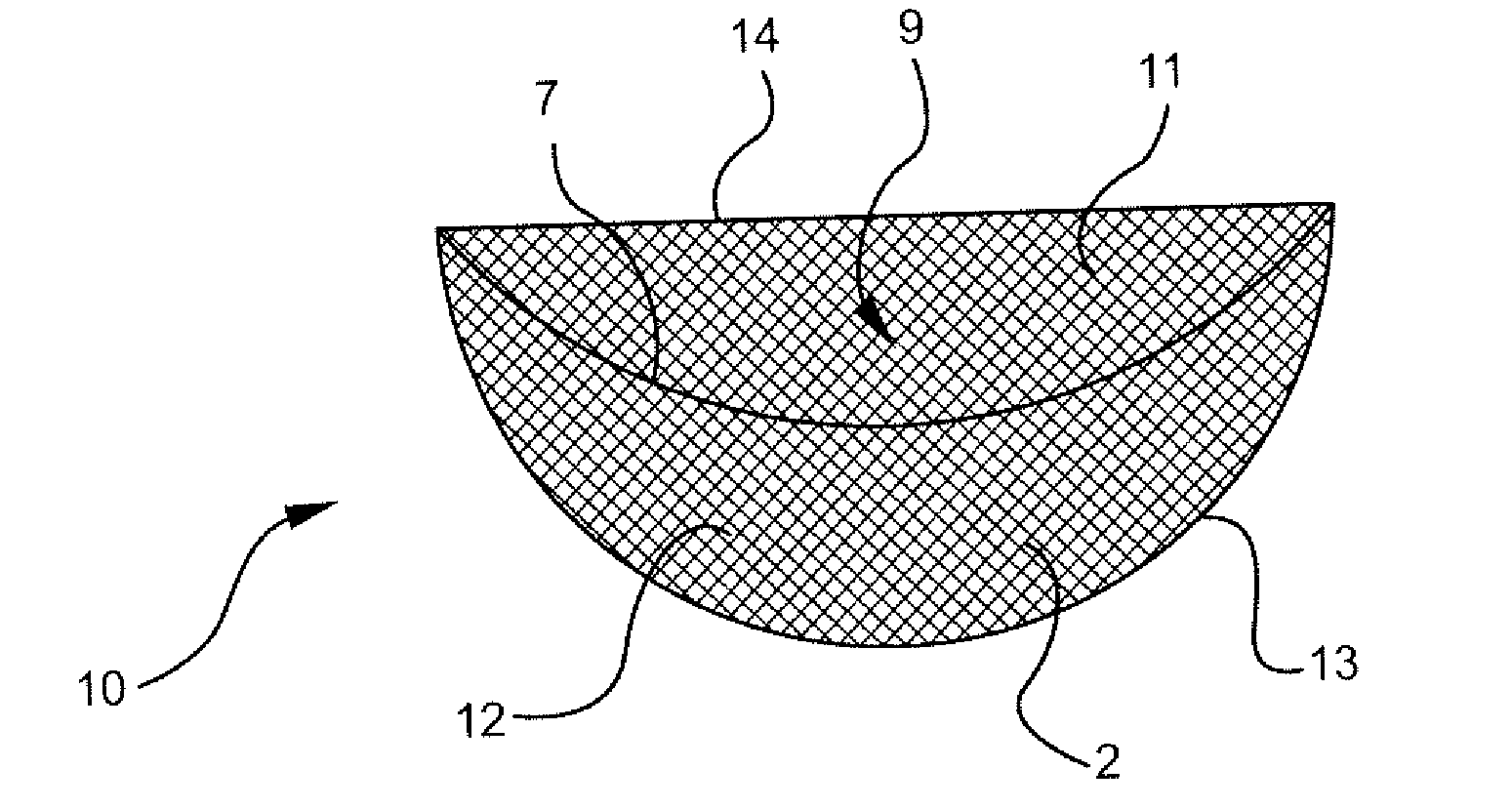
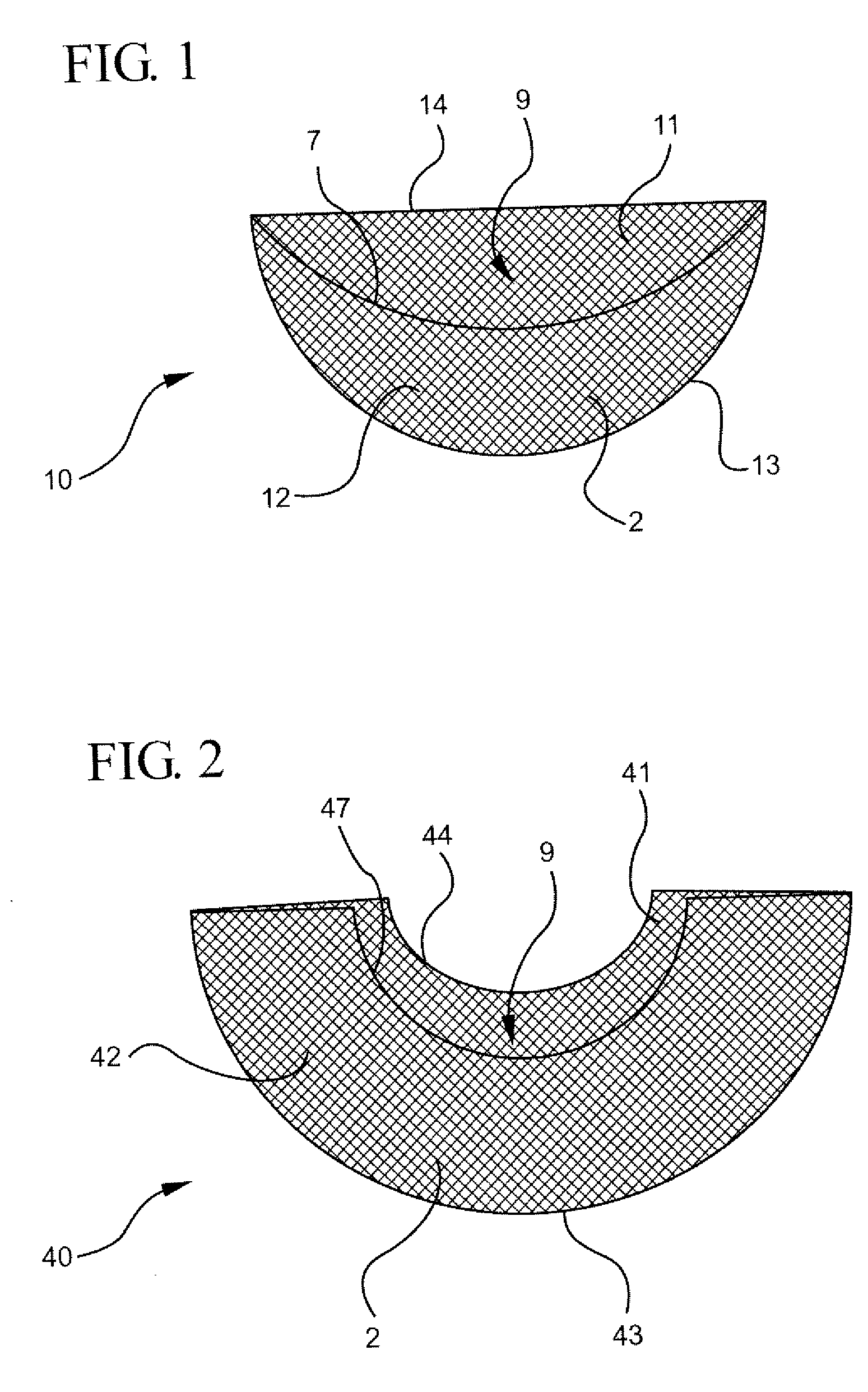
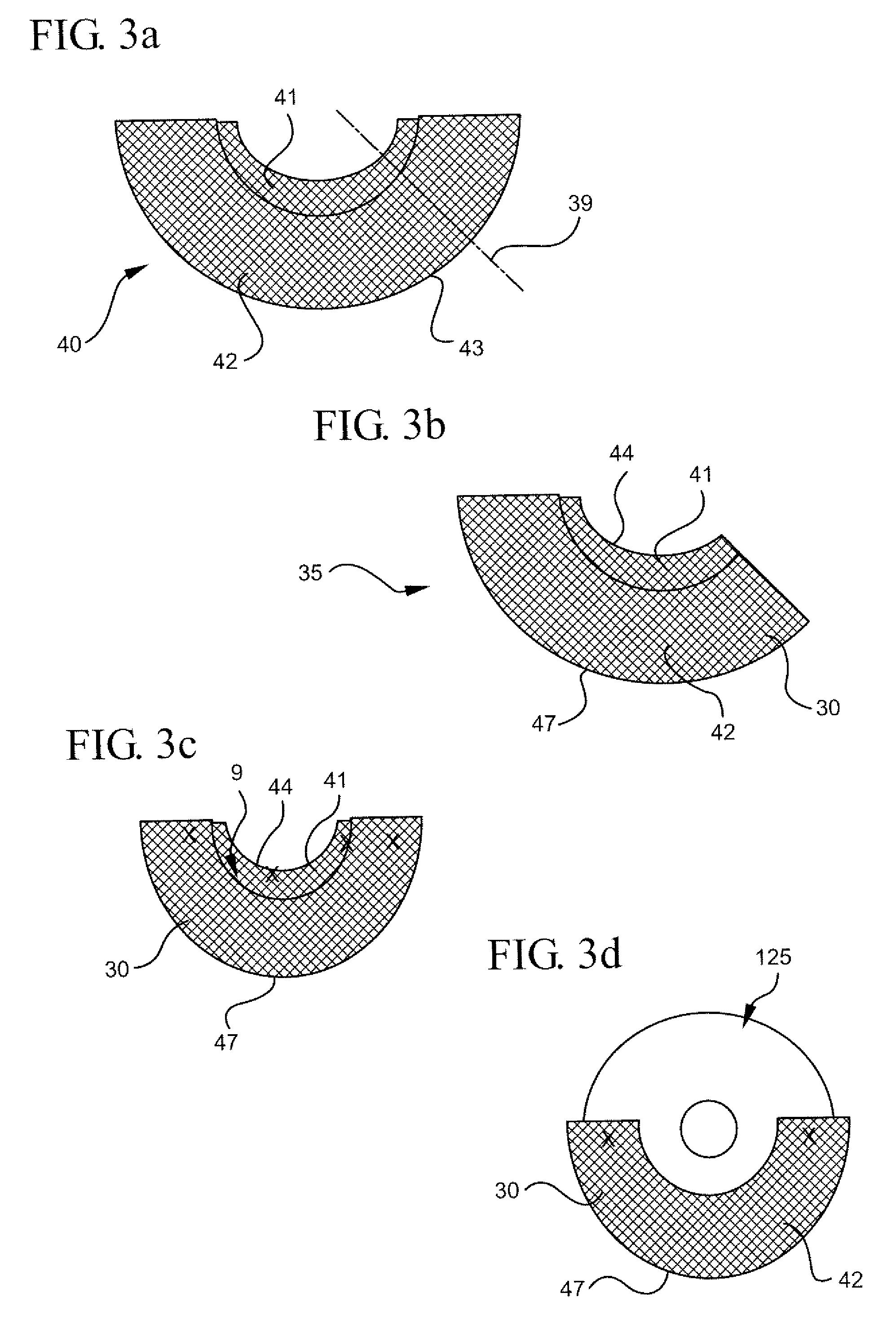
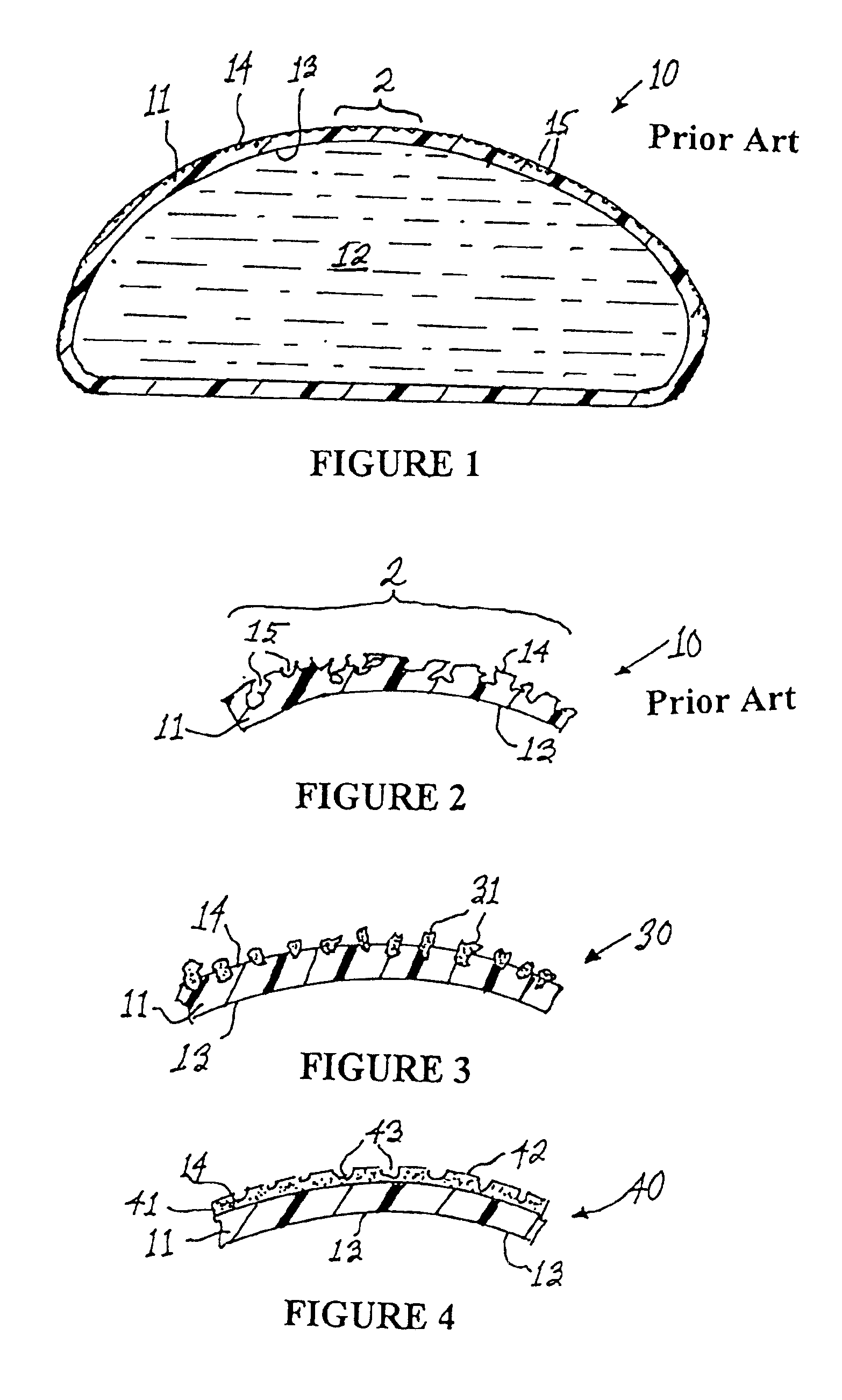
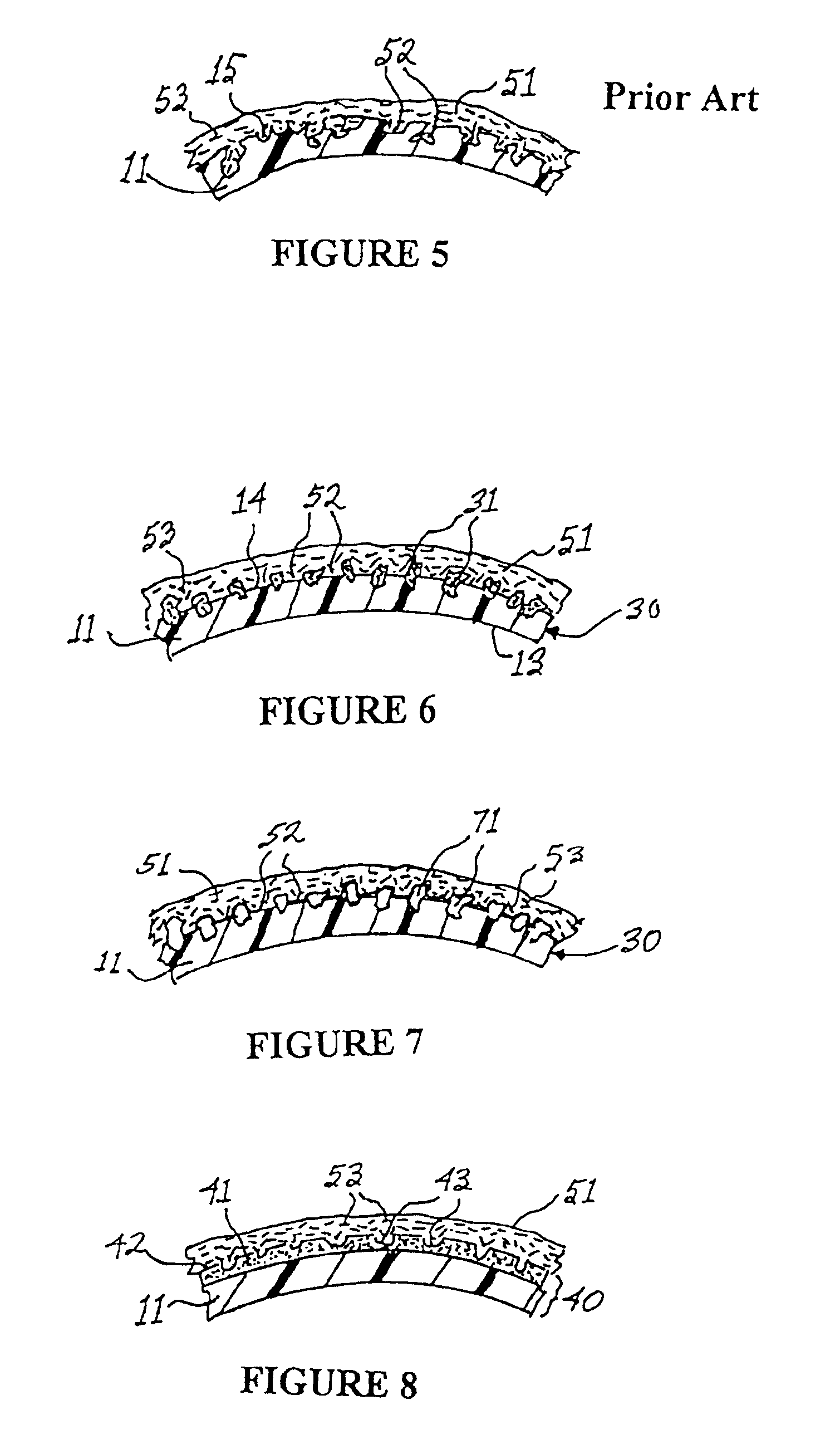
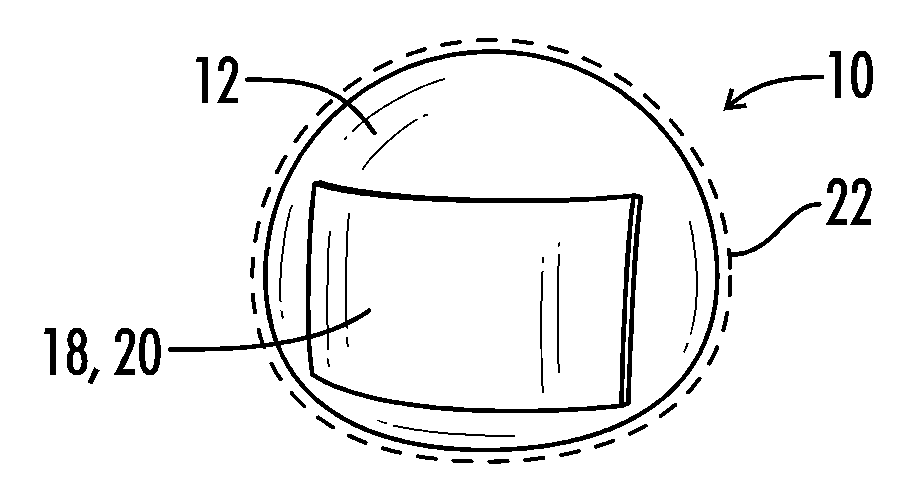
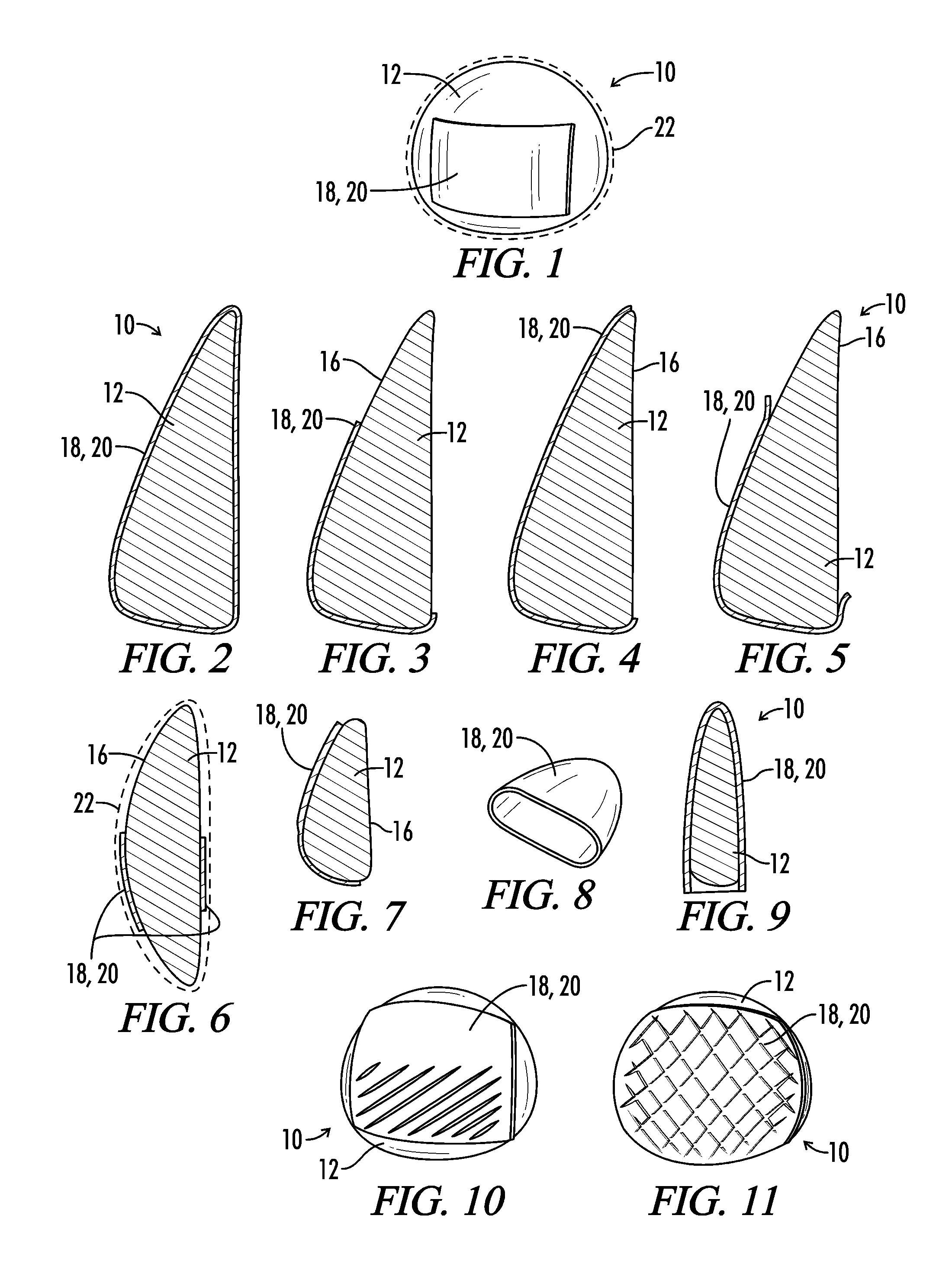
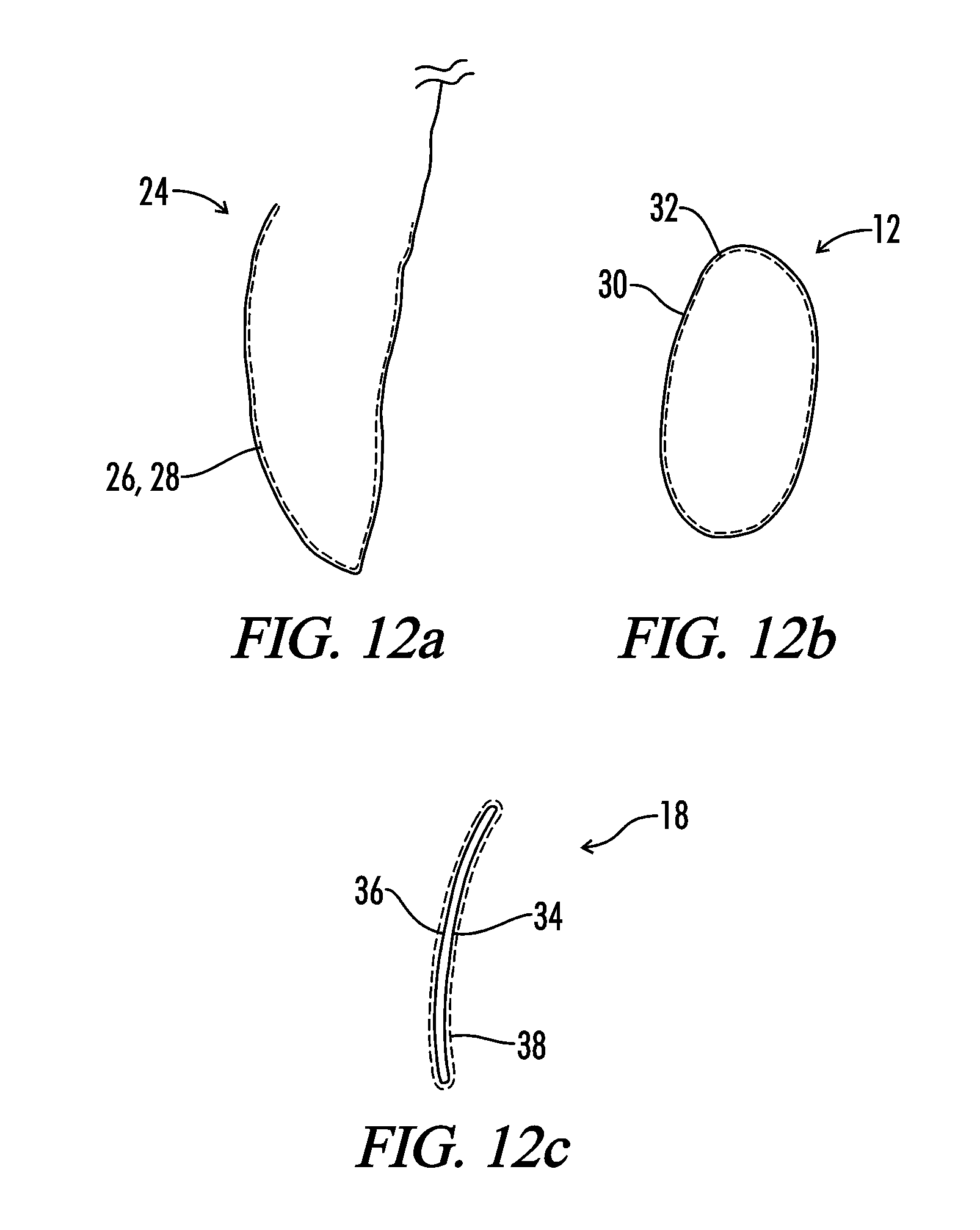
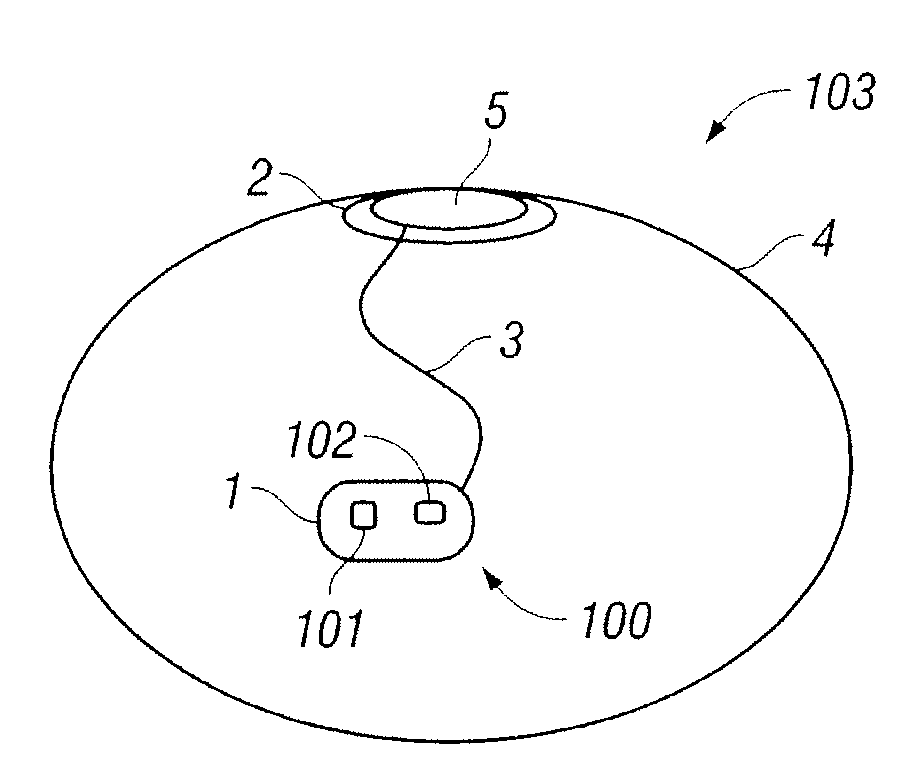
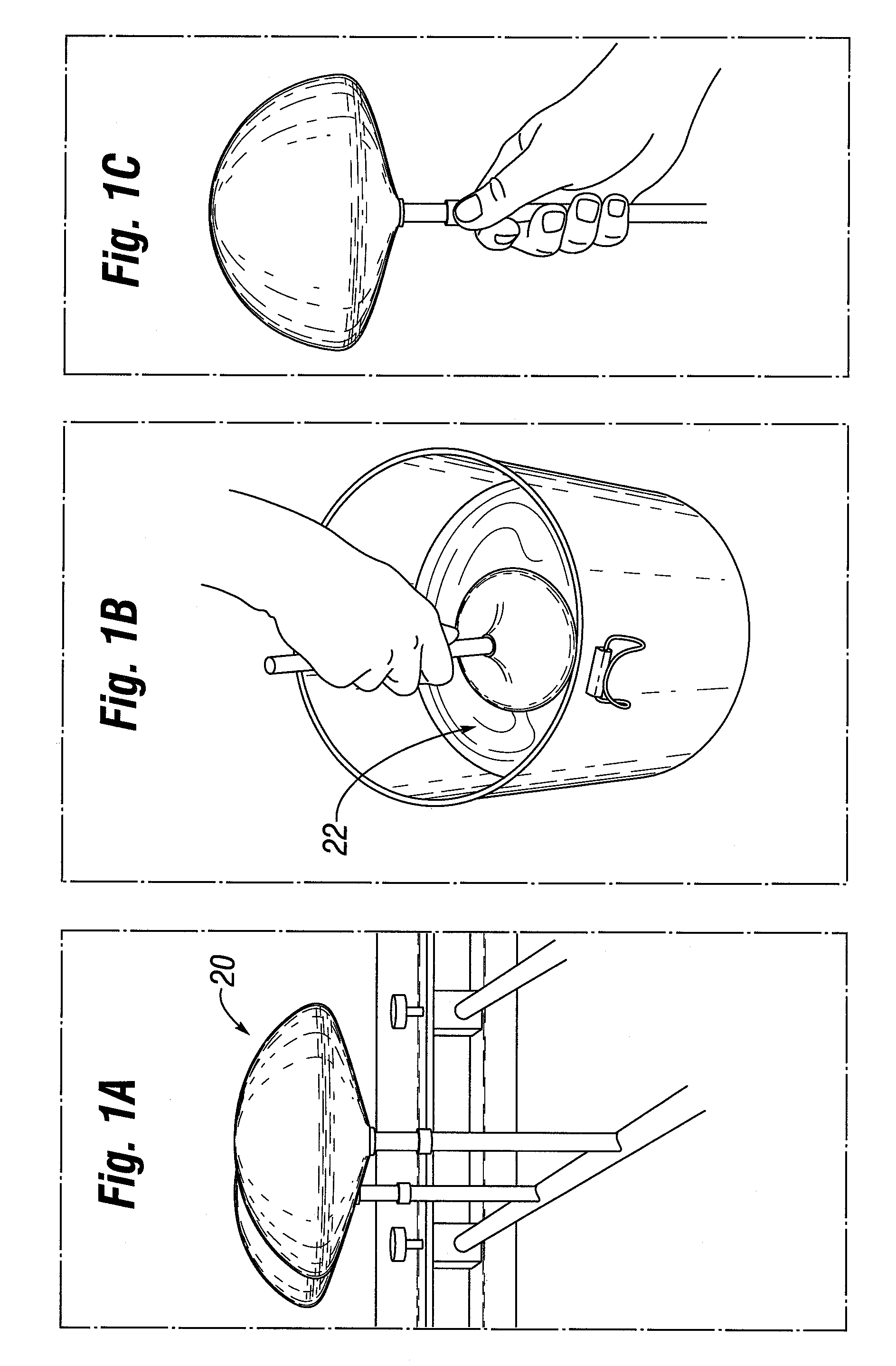
Interfaced medical implant assembly
ActiveUS8425600B2Reduce and eliminate capsular contracturePromote formationMammary implantsTissue regenerationMedicineBreast prostheses
A medical implant assembly and method having a medical implant, e.g. a breast prostheses, attached to a biological interface. The biological interface is comprised of a dermal material with capsular contracture inhibiting properties so that once the medical assembly is inserted into the host, the biological interface, which is intimately coupled to the implant, prevents / reduces capsular contracture formation around the implant. The biological interface comprises a plurality of apertures along its periphery, and attaches to the medical implant by receiving a plurality of attachment flaps or appendages located on the exterior surface of the medical implant within or through the apertures. The attachment of the biological interface is such that the assembly remains intact even where the attachment flaps loosen upon expansion of the implant after insertion into a host, as where the implant is therein injected to a desired dimension.
Owner:MAXWELL G PATRICK
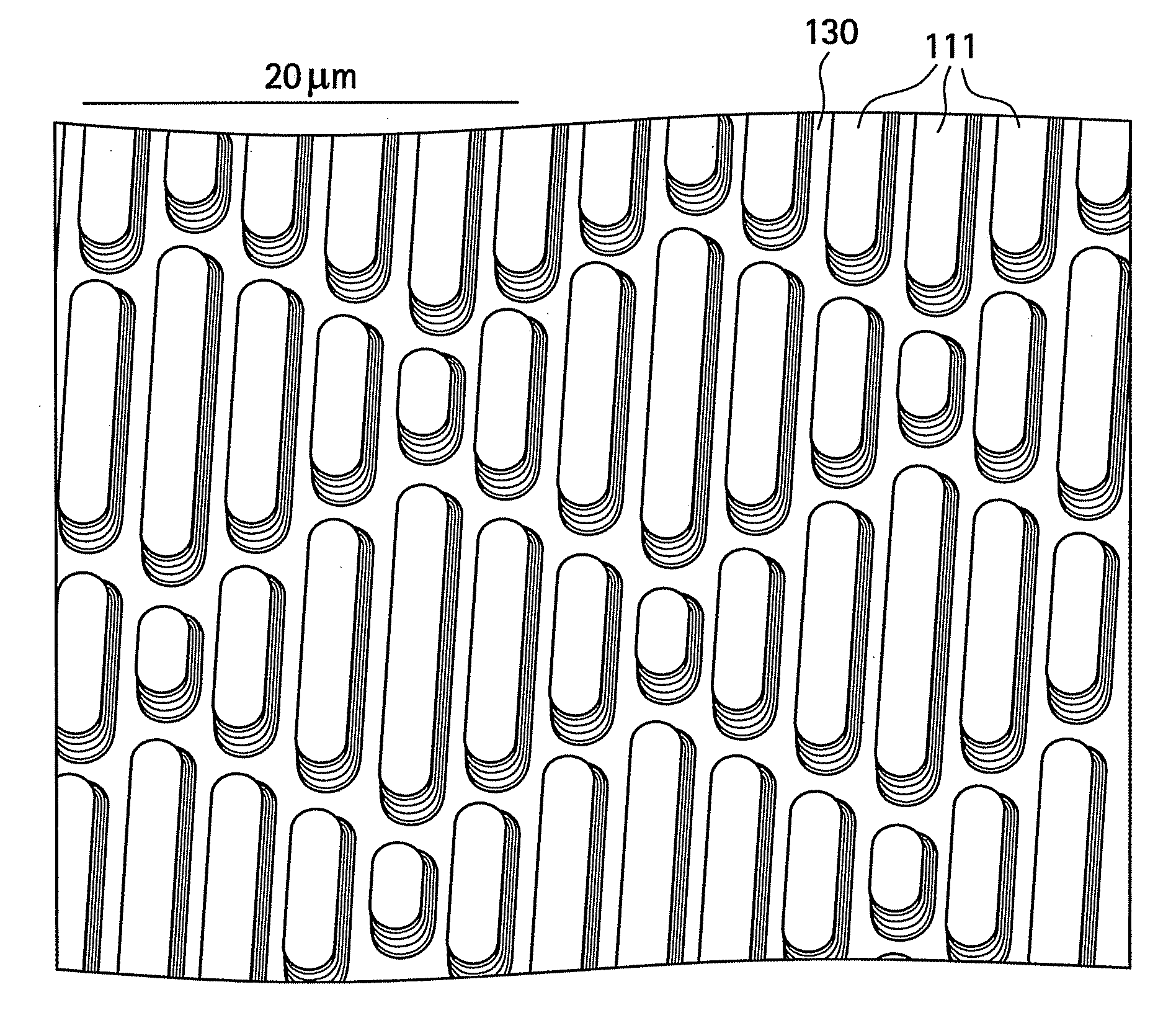
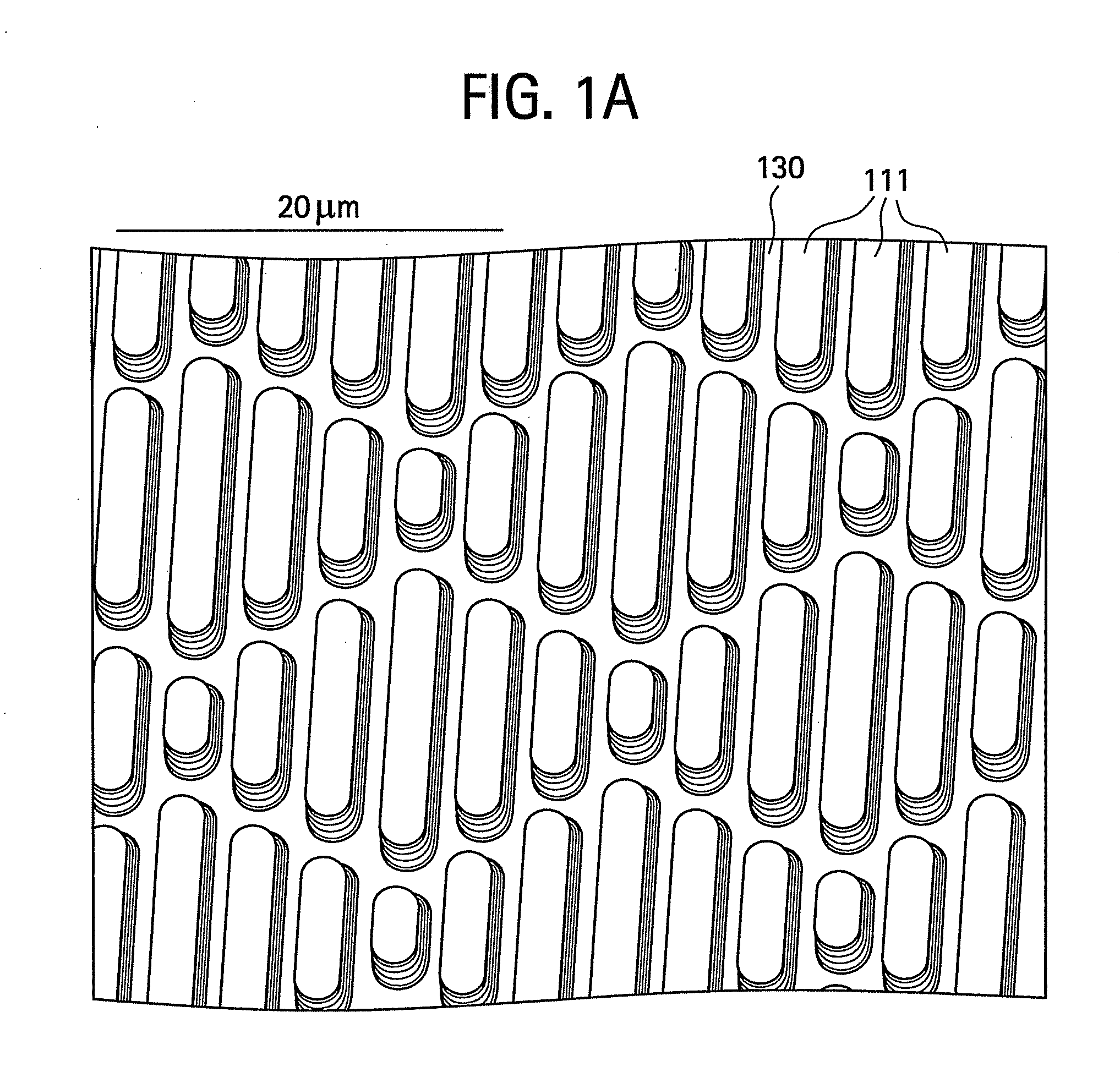
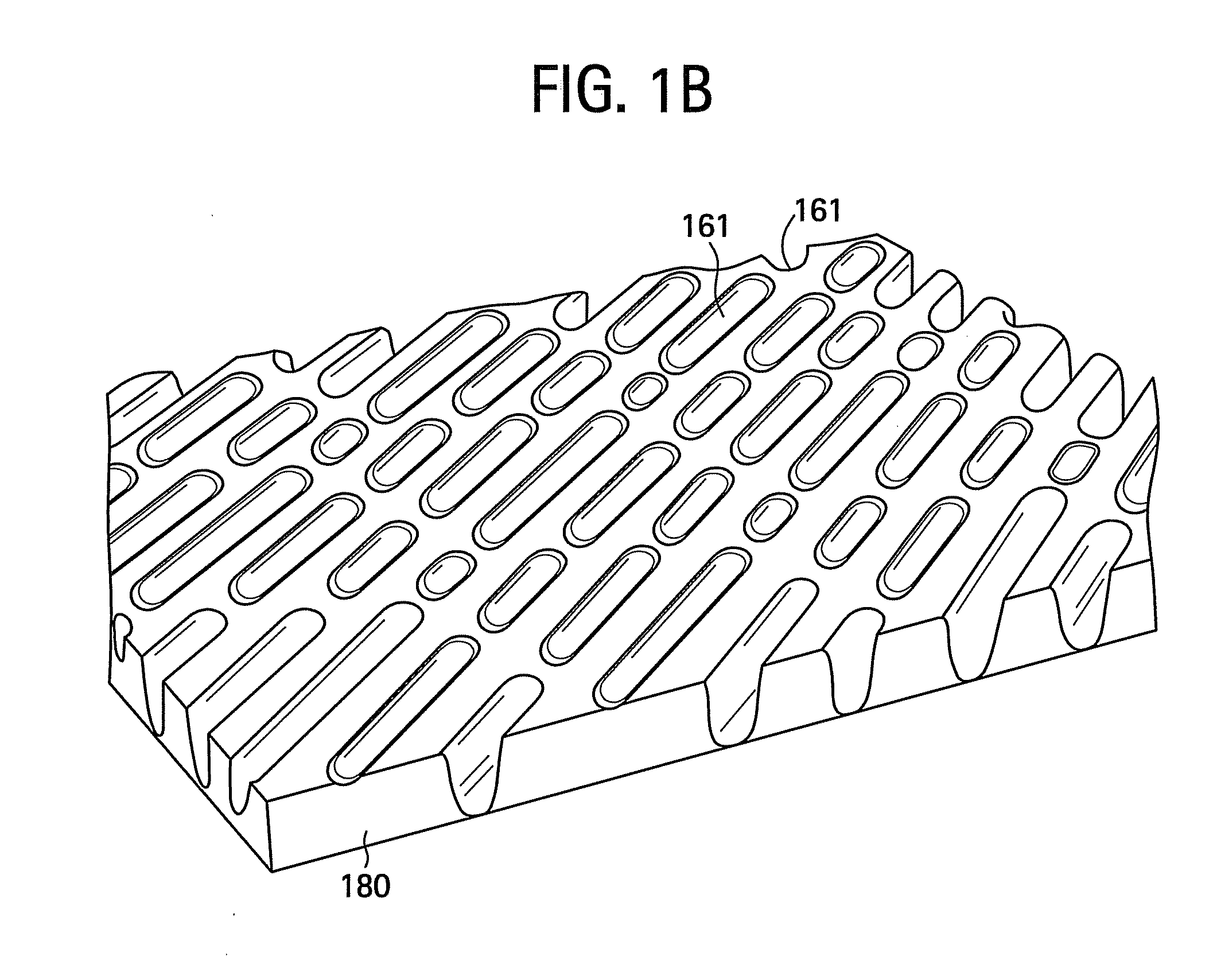
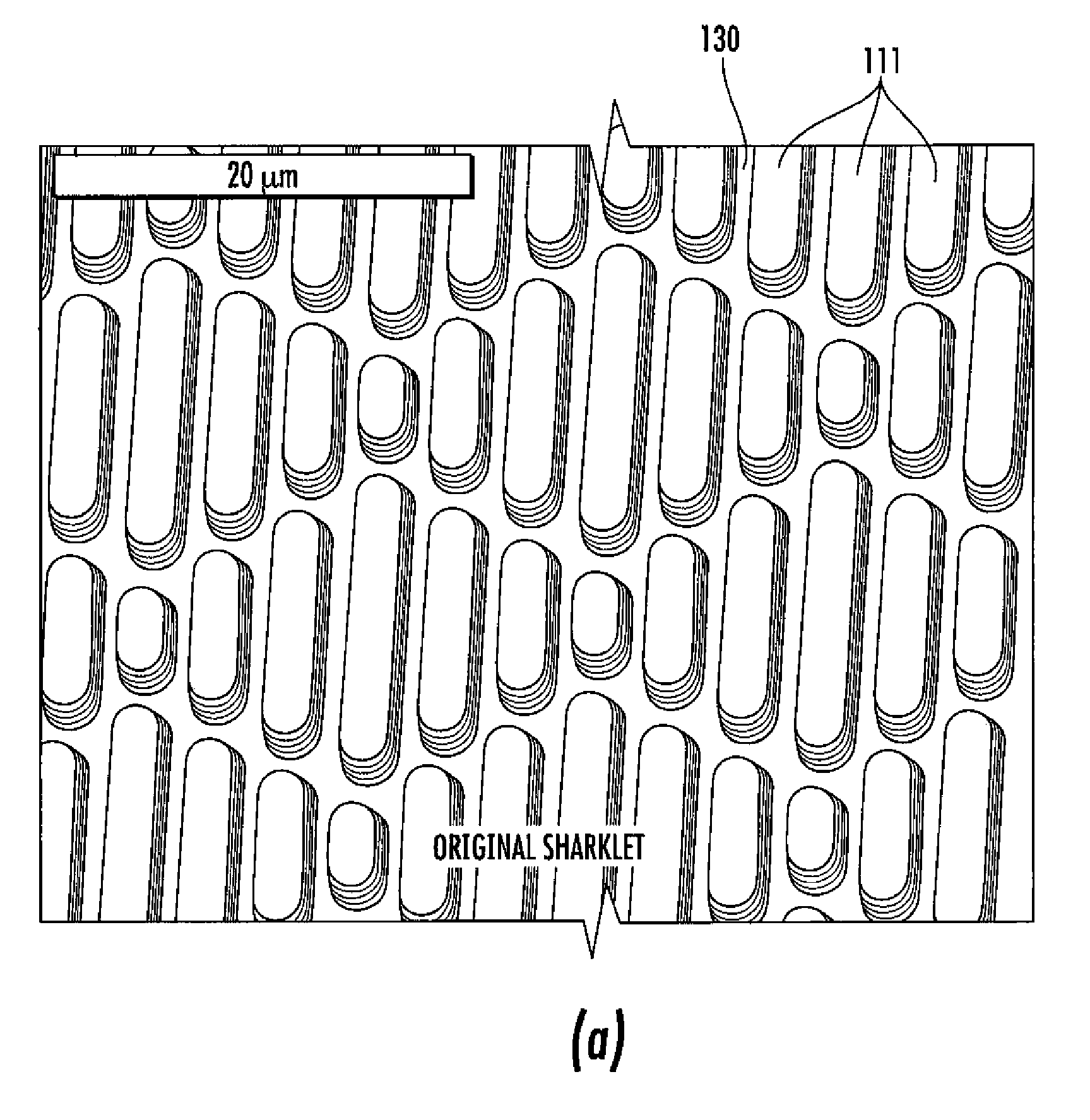
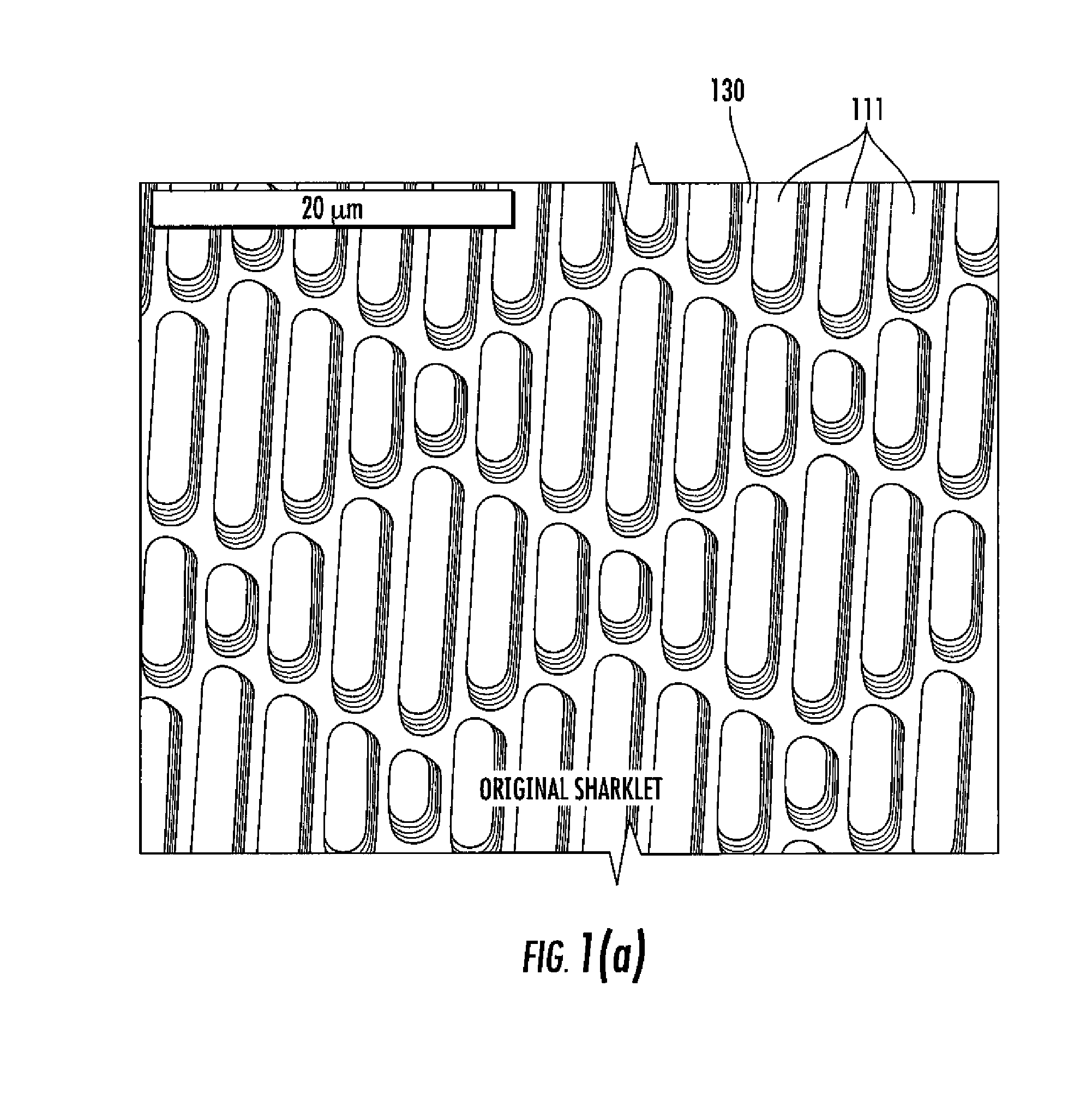
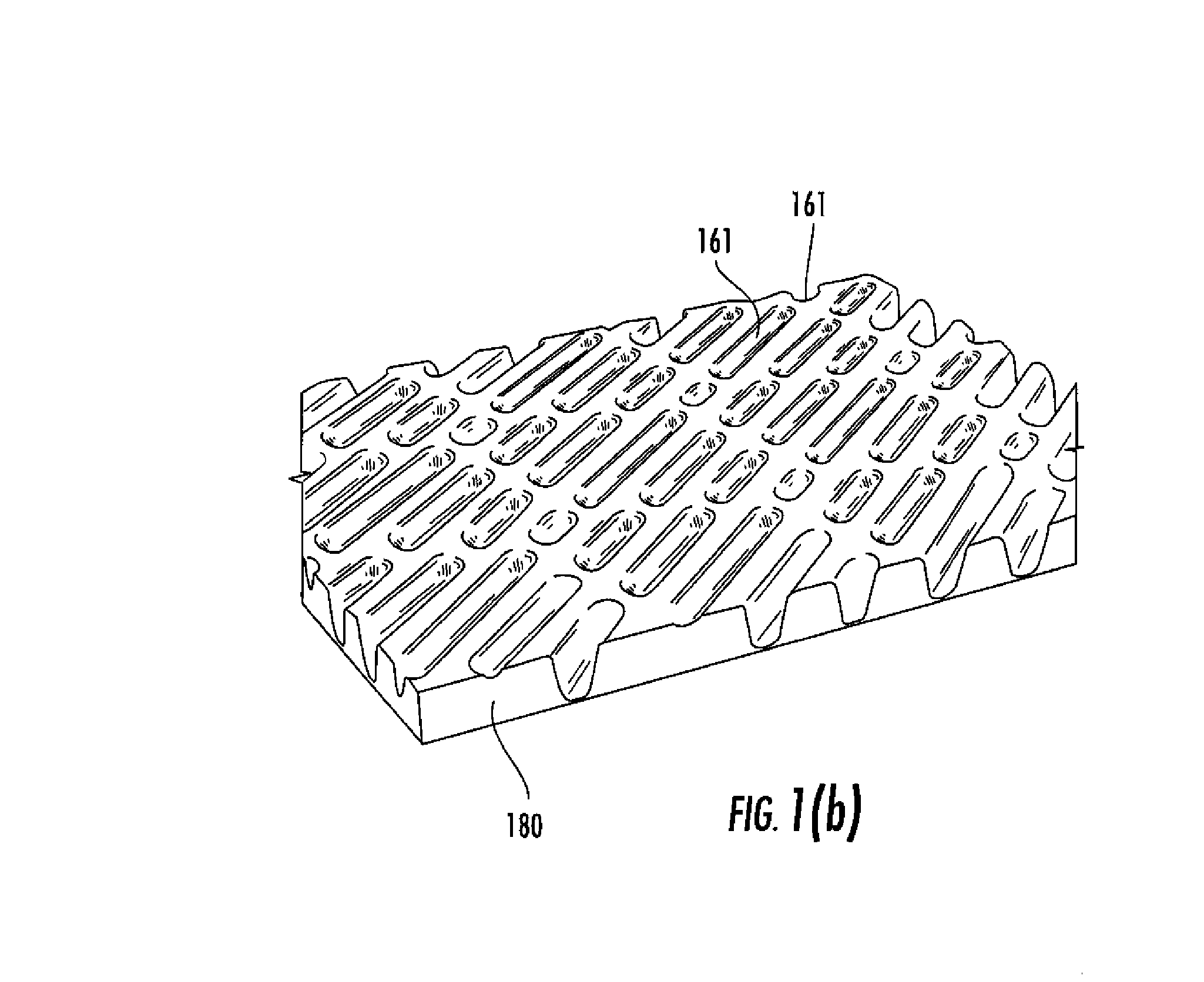
Surface topographies for non-toxic bioadhesion control
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Use of glucomannan hydrocolloid as filler material in prostheses
A prosthetic device for implantation into a mammalian body comprised of a non-absorbable biocompatible flexible material shell or sac filled with various biocompatible gel filler materials. The gel filler materials are comprised of biocompatible glucomannan obtained from konjac hydrocolloid flour and other biocompatible hydrocolloids, producing a natural look and feel for the prosthetic implants, especially reconstructive prostheses such as breast implants.
Owner:KONJAC TECH
Bioresorbable hydrogel compositions for implantable prostheses
Crosslinked compositions formed from water-insoluble copolymers are disclosed. These compositions are copolymers having a bioresorbable region, a hydrophilic region and at least two cross-linkable functional groups per polymer chain. Crosslinking of these polymers can be effected in solution in organic solvents or in solvent-free systems. If crosslinking occurs in a humid environment, a hydrogel will form. If crosslinking occurs in a non-humid environment, a xerogel will form which will form a hydrogel when exposed to a humid environment and the resulting crosslinked materials form hydrogels when exposed to humid environments. These hydrogels are useful as components in medical devices such as implantable prostheses. In addition, such hydrogels are useful as delivery vehicles for therapeutic agents and as scaffolding for tissue engineering applications.
Owner:LIFESHIELD SCI
Treatment of bioprosthetic tissues to mitigate post implantation calcification
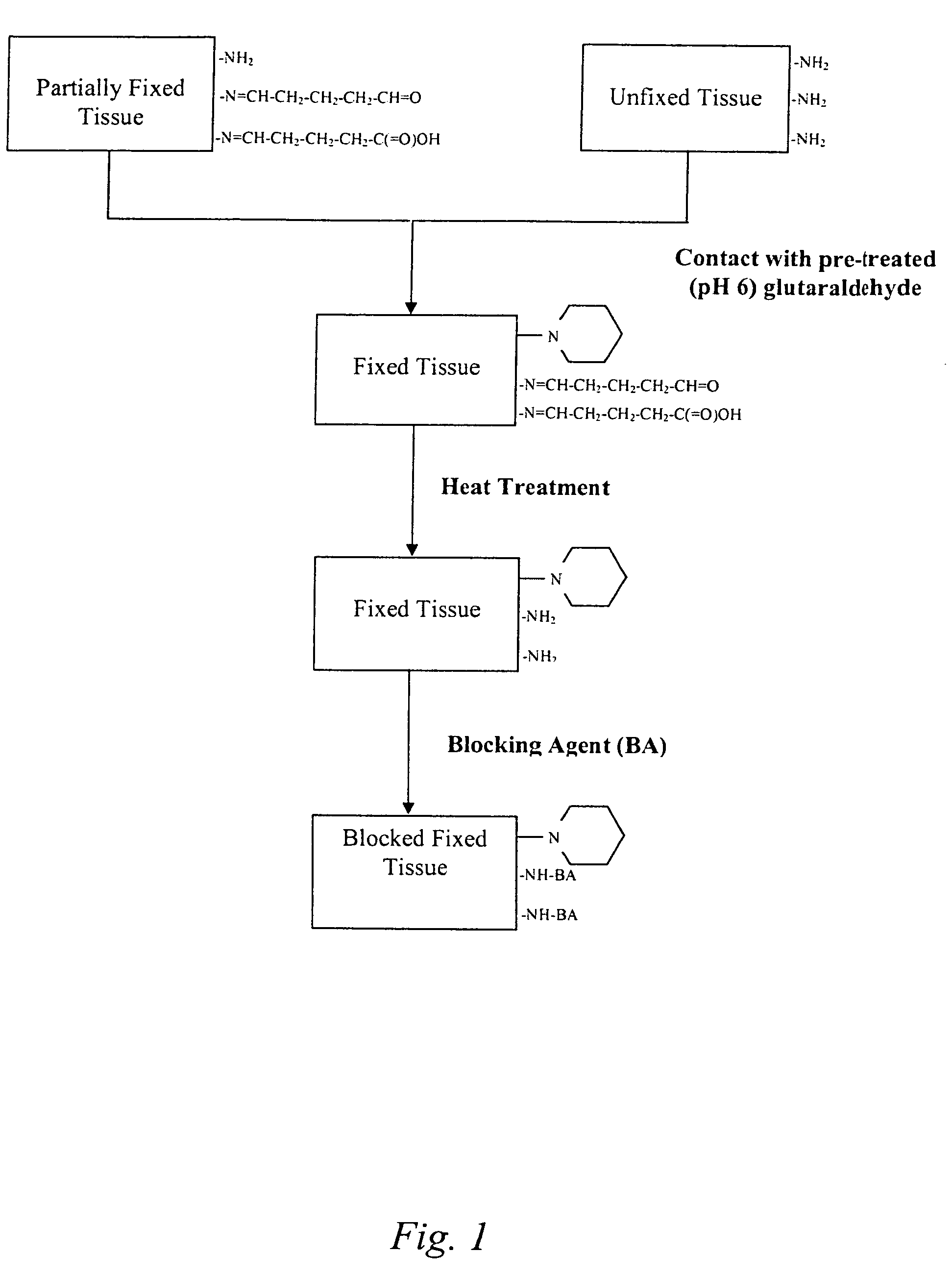
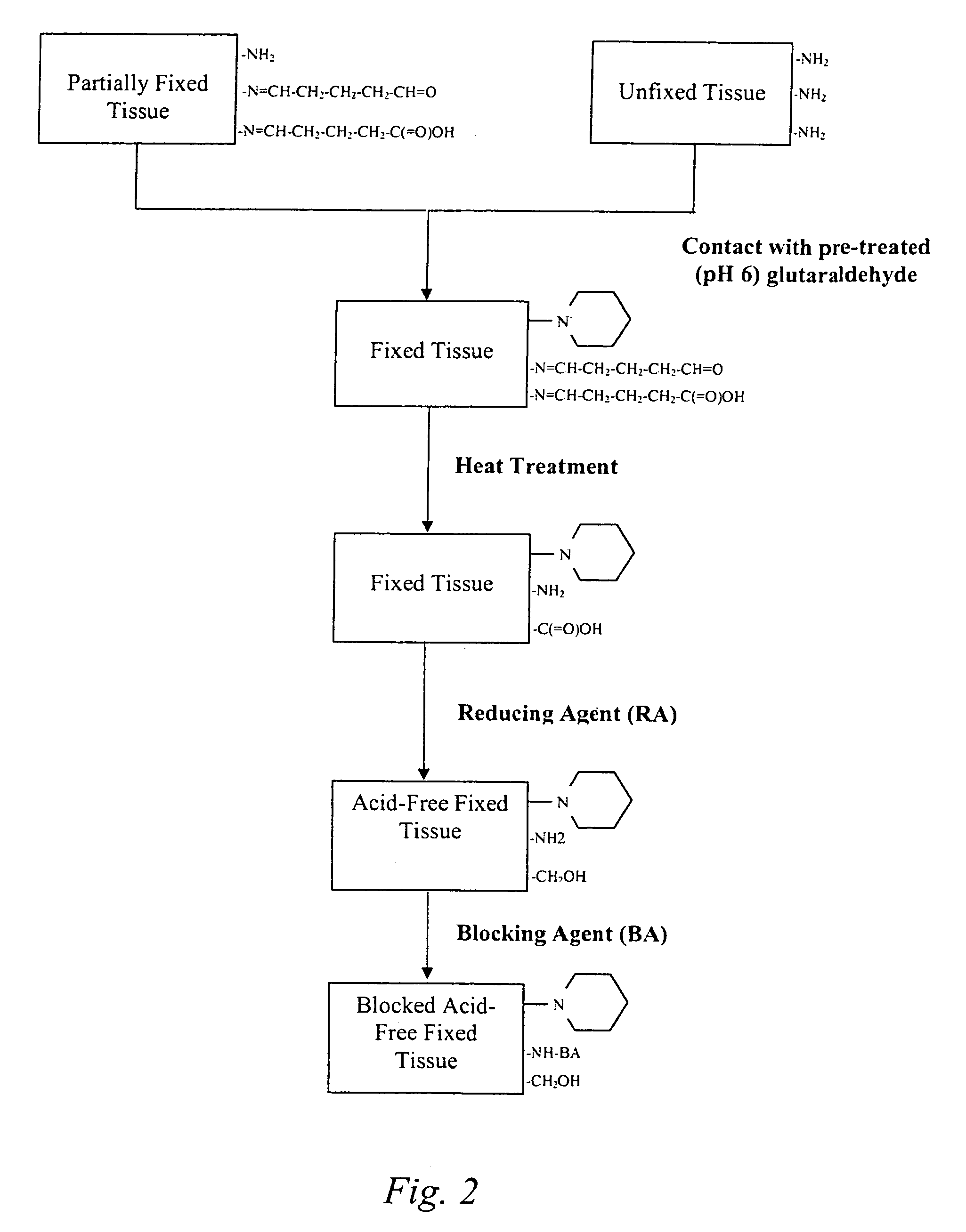
The present invention provides methods for treating tissue to inhibit post-implant calcification of a biological tissue. In one method of this invention, a tissue is immersed in or otherwise contacted with a pretreated glutaraldehyde solution, i.e., a heat-treated or pH-adjusted glutaraldehyde solution. The tissue may be partially fixed with glutaraldehyde prior to, after, or concurrently with the step of contacting the tissue with the pretreated gluteraldehyde. Contact with the pretreated gluteraldehyde produces free amine groups on the tissue, which are subsequently blocked by contacting the crosslinked tissue with a blocking agent. In another embodiment, a tissue is contacted with either a non-pretreated glutaraldehyde or a pH-adjusted glutaraldehyde solution for a period of time sufficient to crosslink the tissue. The crosslinked tissue is then treated with a reducing agent that reduces aldehyde and carboxylic acid groups on the fixed tissue.
Owner:EDWARDS LIFESCIENCES CORP
Biodegradable/bioactive nucleus pulposus implant and method for treating degenerated intervertebral discs
A bioactive / biodegradable nucleus implant for repairing degenerated intervertebral discs that is inflated inside the nucleus space after the degenerated nucleus has been removed to re-pressurize the nuclear space within the intervertebral disc. The implant is inflated with a high molecular weight fluid, gel or combination of fluid and elastomer, preferably an under-hydrated HA hydrogel / growth factor mixture with or without host cells. The implant includes an internal, integral, self-sealing valve that allows one-way filling of the implant after it is placed within the disc, and is made from a material that allows fibrous in growth thereby stabilizing the implant. A variety of substances can be incorporated into the implant to promote healing, prevent infection, or arrest pain.
Owner:RGT UNIV OF CALIFORNIA
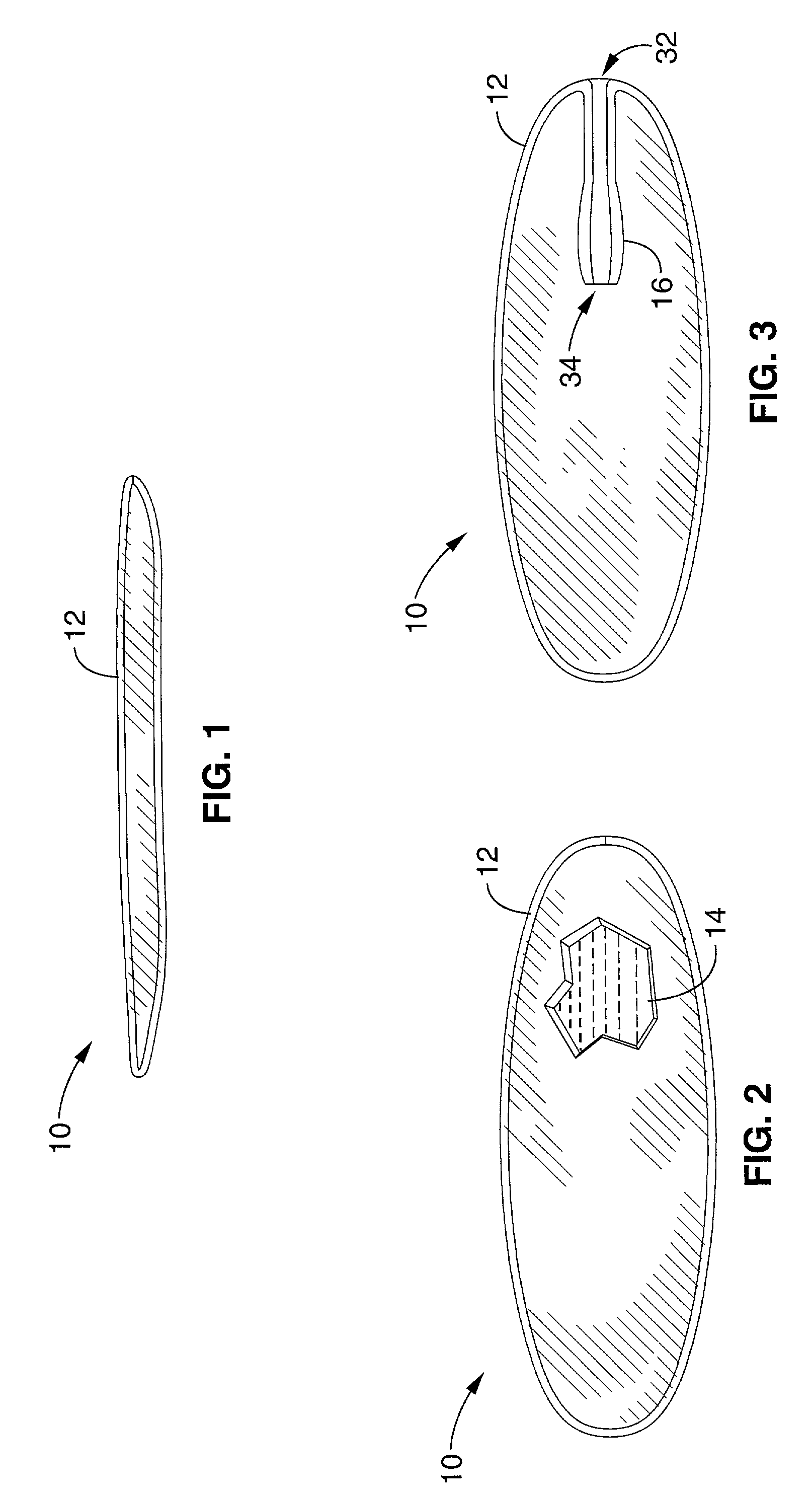
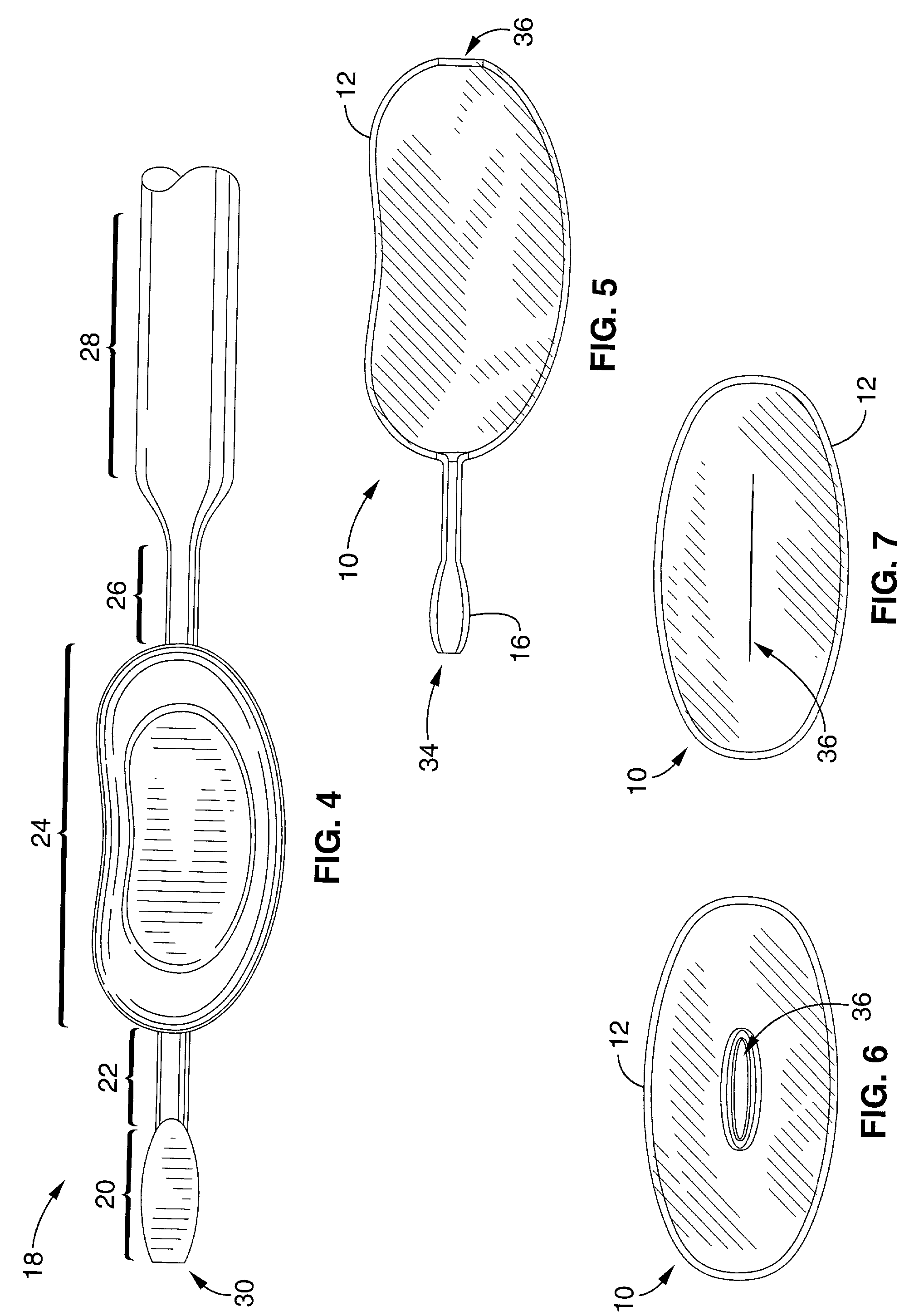
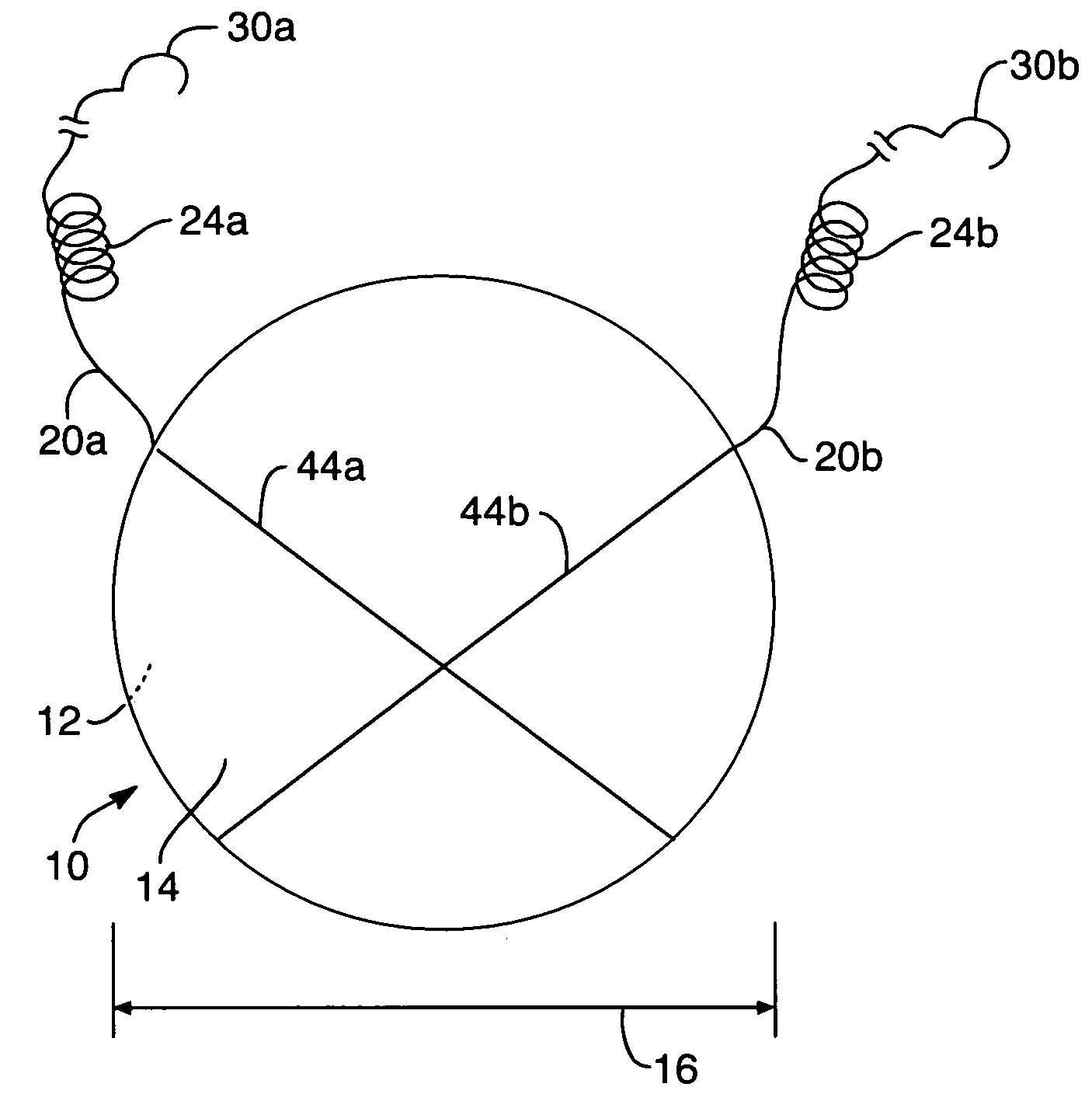
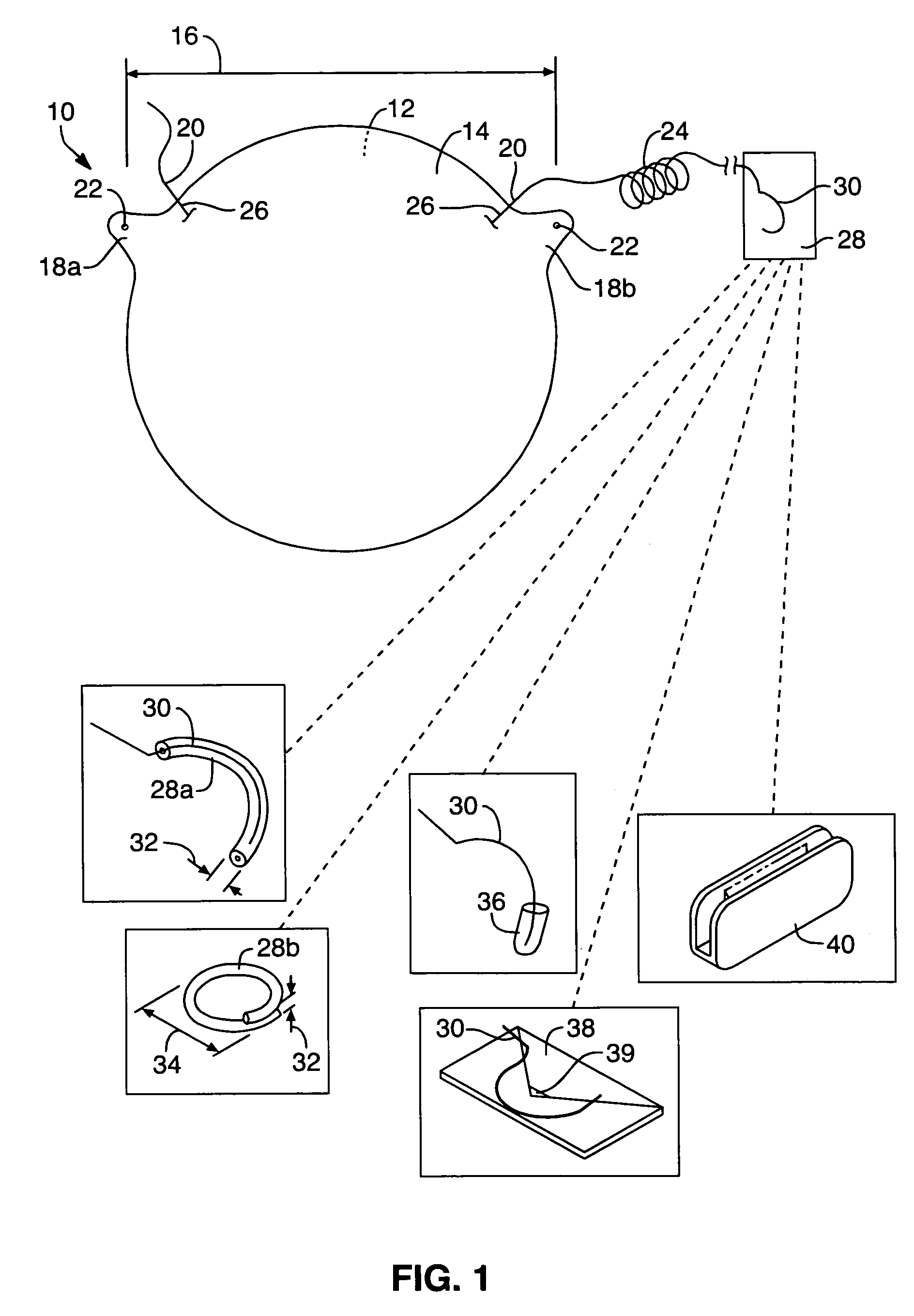
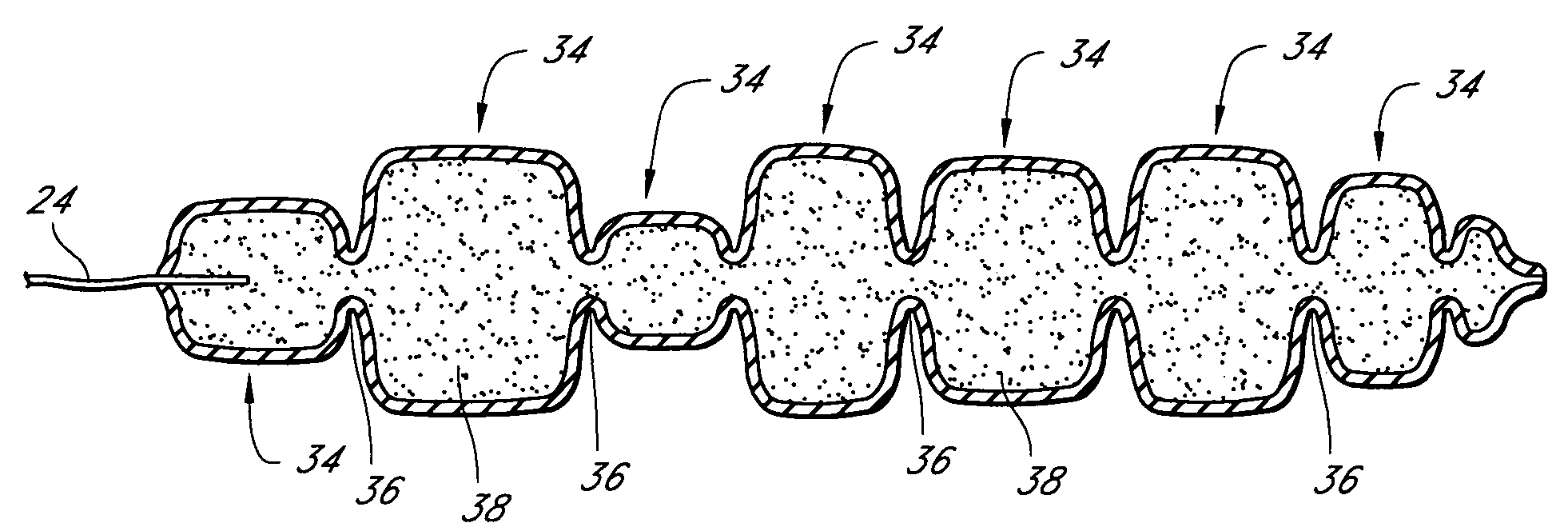
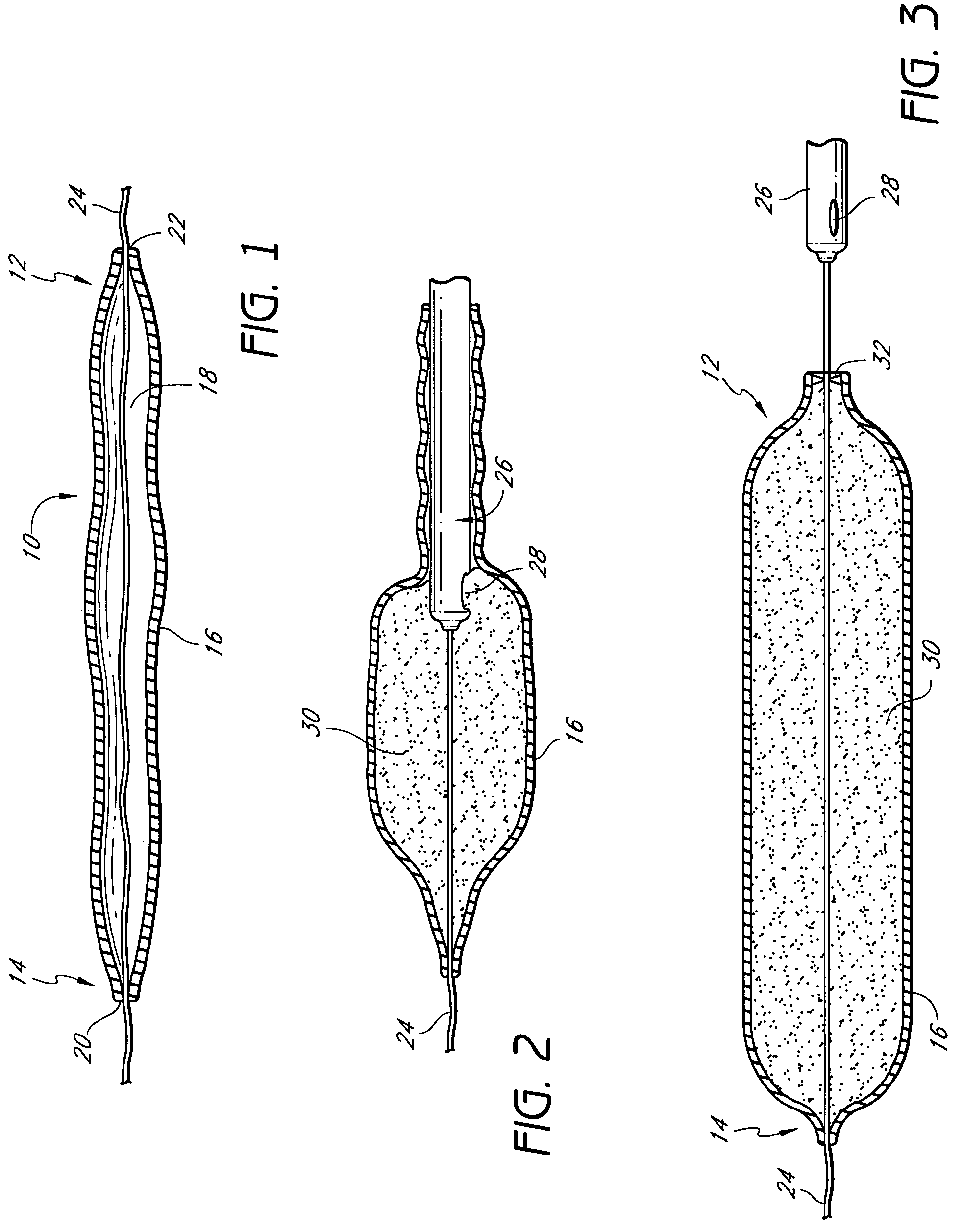
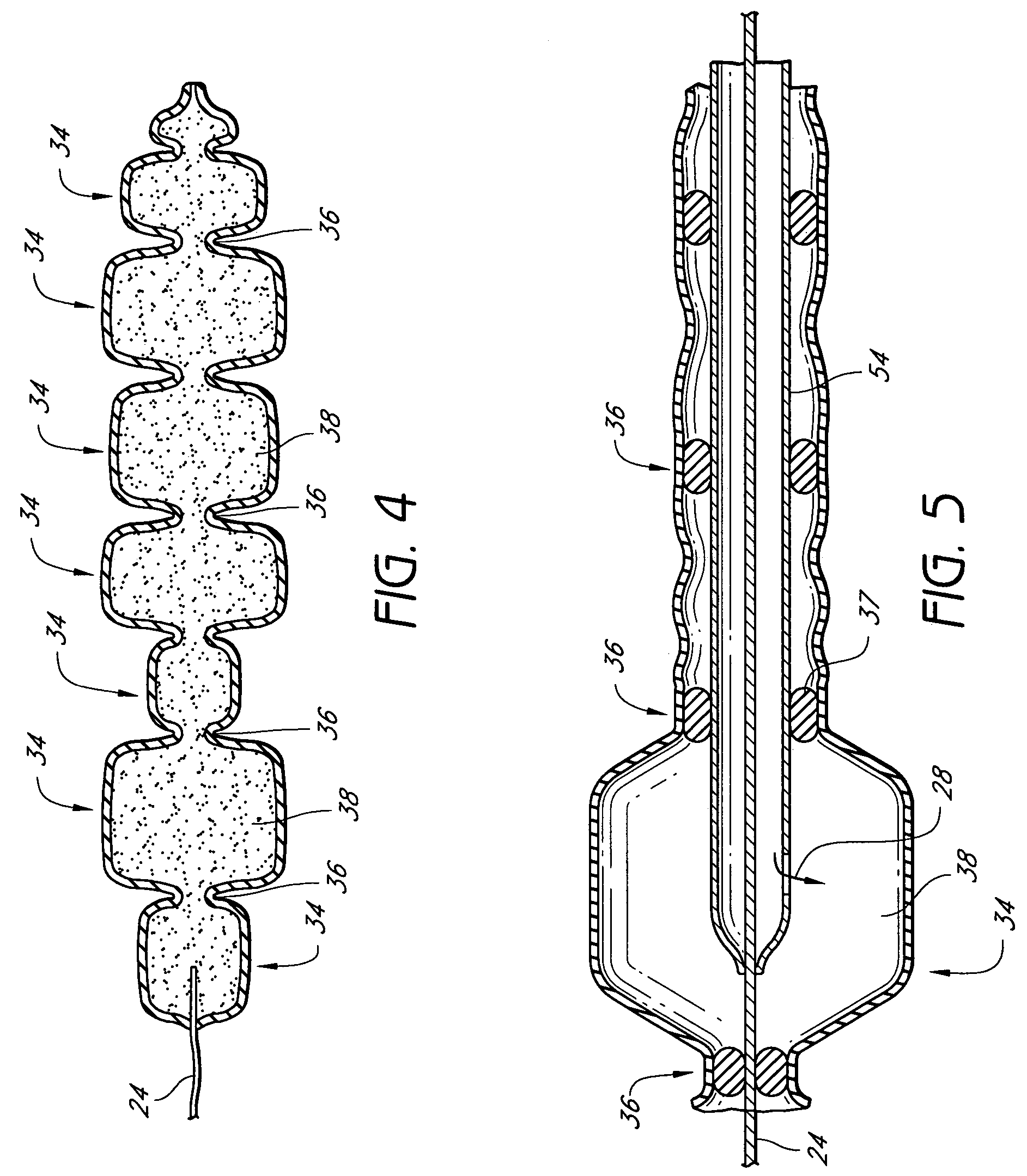
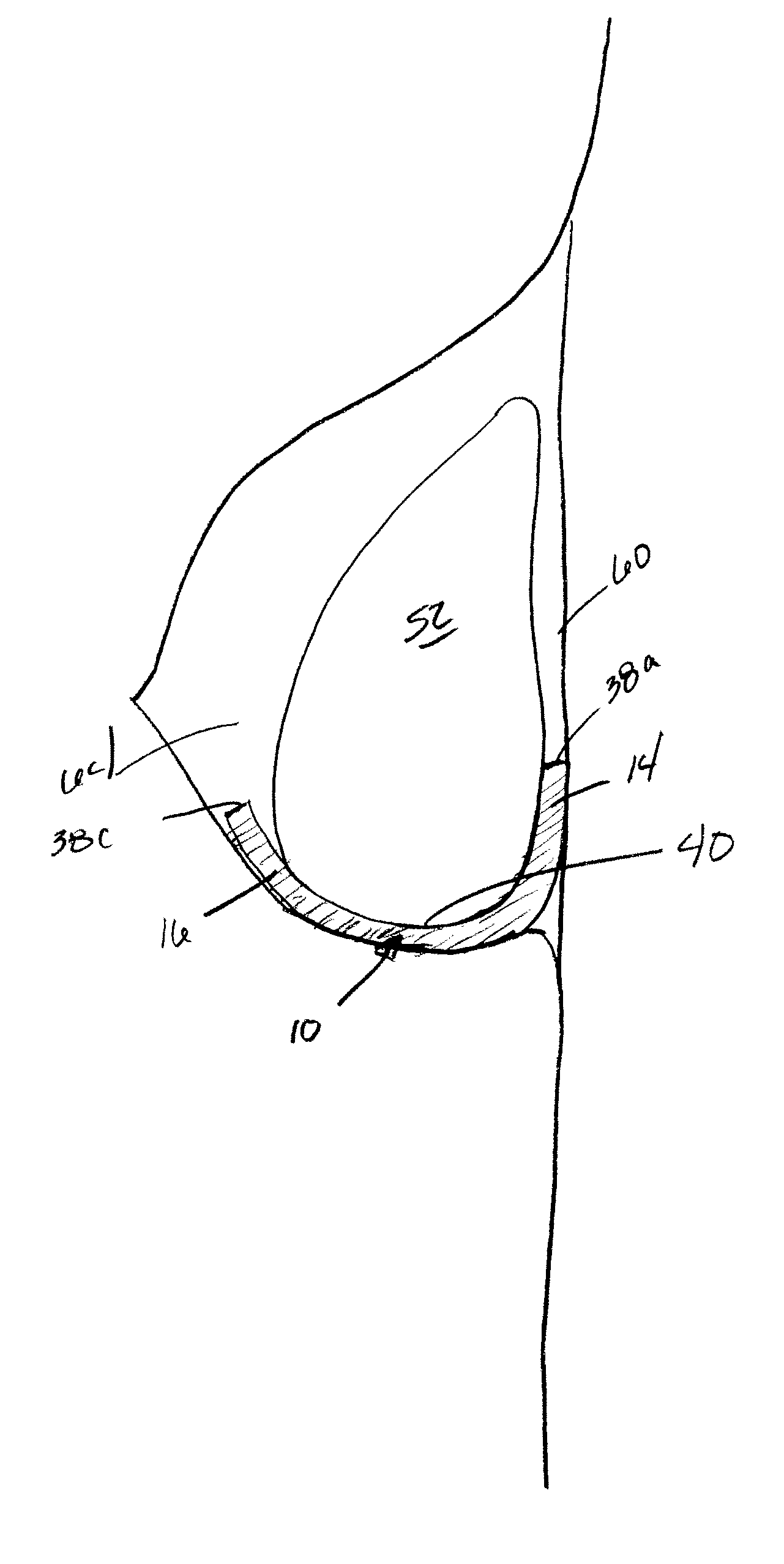
Mastopexy stabilization apparatus and method
An apparatus and method for mastopexy surgeries correcting a ptosis condition caused by tissue stretching, in the breast as a result of pregnancy, time, aging, and the effects of gravity and athletic activity provide an implant having homogeneously formed connectors extending from inside an implant wall for anchoring to the chest wall or chest muscles of a patient. Embedded reinforcements and anchoring tabs or sutures may be readily oriented along a rib or other defining physiological location in order to provide immediate, permanent, and symmetric installation of implants in a mastopexy reconstruction.
Owner:SMITH LANE FIELDING +1
Surface topographies for non-toxic bioadhesion control
An article has a surface topography for resisting bioadhesion of organisms and includes a base article having a surface. A composition of the surface includes a polymer. The surface has a topography comprising a pattern defined by a plurality of spaced apart features attached to or projected into the base article. The plurality of features each have at least one microscale dimension and at least one neighboring feature having a substantially different geometry. An average feature spacing between adjacent ones of the features is between 10 μm and 100 μm in at least a portion of the surface. The surface topography can be numerically represented using at least one sinusoidal function. In one embodiment, the surface can comprise a coating layer disposed on the base article.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Systems and devices for soft tissue augmentation
Medical devices, systems and kits for filling tissue are disclosed. The device has a first configuration wherein the device can pass through a small catheter or needle placed in the tissue to be filled and a second configuration in which the device is expandable to a predetermined or customizable shape. In one application, the device is adapted to be placed into the skin to reduce facial wrinkles or augment facial features such as the lips. Kits and systems include multiple device sizes, filler tubes, and filler media.
Owner:EVERA MEDICAL
Implant with high vapor pressure medium
InactiveUS20100222802A1Promote recoveryImprove the immunitySuture equipmentsUrinary bladderHydrostatic pressureProduct gas
An implant for use in a human or animal body can include a flexible housing with an outer wall and having a chamber therein. The implant can have at least one high vapor pressure medium within the chamber. The at one high vapor pressure medium can have a combined vapor pressure equal to or greater than about the average value of the hydrostatic pressure of the implantation site plus the skin tension of the housing minus the gas tension of the dissolved gasses present at the implantation site.
Owner:SOLACE THERAPEUTICS
Nonaugmentive mastopexy
Disclosed are methods and devices for minimally invasive mastopexy, or other soft tissue suspension, which may be accomplished with our without augmentation.
Owner:SINCLAIR PHARMA
High capacity debulking catheter with razor edge cutting window
InactiveUS20080065124A1Quicker and precise procedureSpeed up procedure timeMammary implantsCannulasAtherectomyNose
The present invention is an atherectomy catheter with a hollow head. The head has a window with at least one internal bladed edge, a plunger, and an adjustable angle nose. The angle of the nose can be manipulated by the operator to apply pressure to an artery wall, thereby forcing the window and the window cutting edge up against a plaque target on the opposite side of the artery wall. The position of the plunger can be manipulated by the operator to open or close the window, thereby exposing or not exposing the bladed window edge, and optionally also pinching off dangling plaque fragments. Cut plaque enters the hollow catheter head through the open window, and is stored inside the catheter for removal from the body and subsequent analysis. In some embodiments, the catheter head may have optional sensors, or the plunger may also serve as a rotary cutter.
Owner:TYCO HEALTHCARE GRP LP
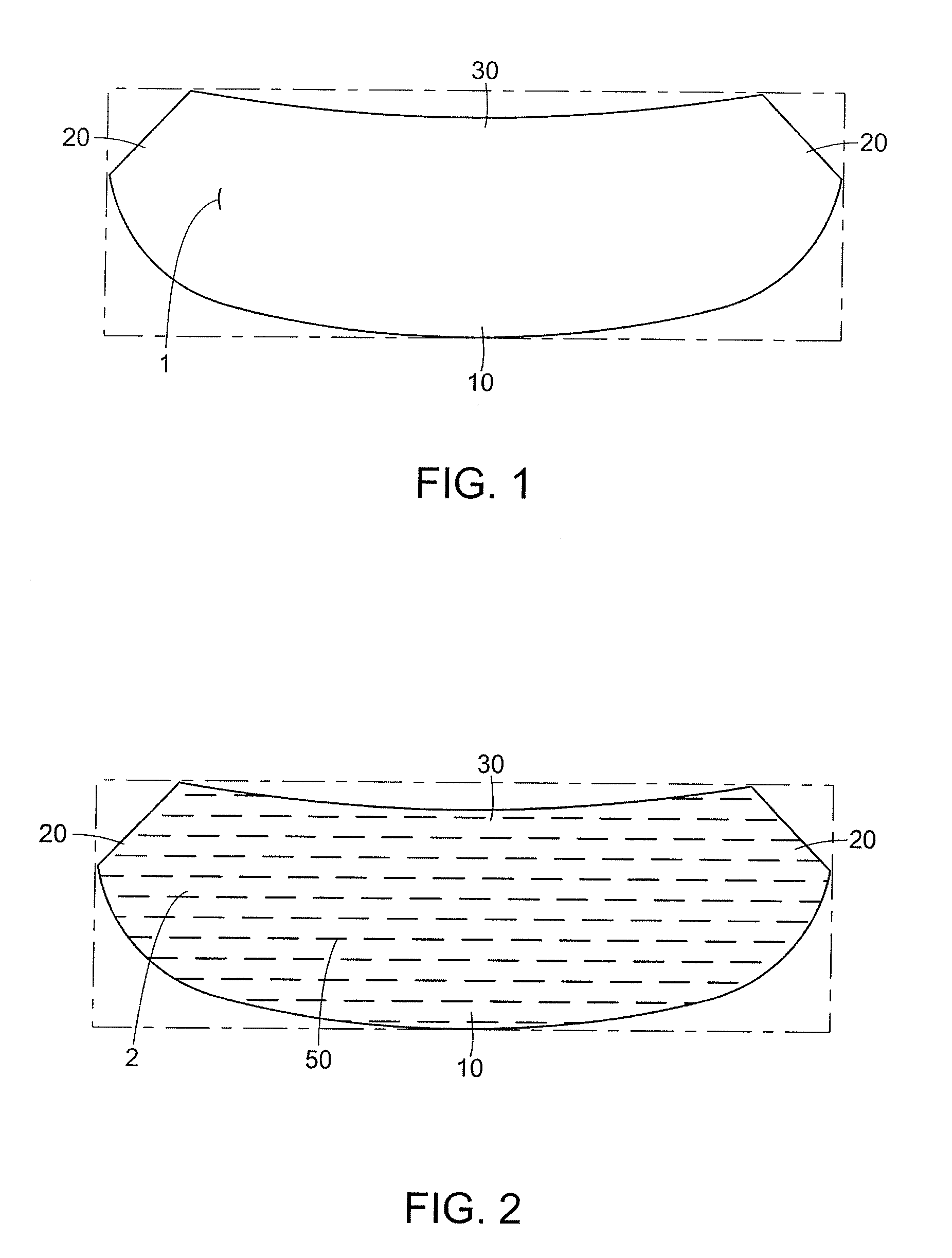
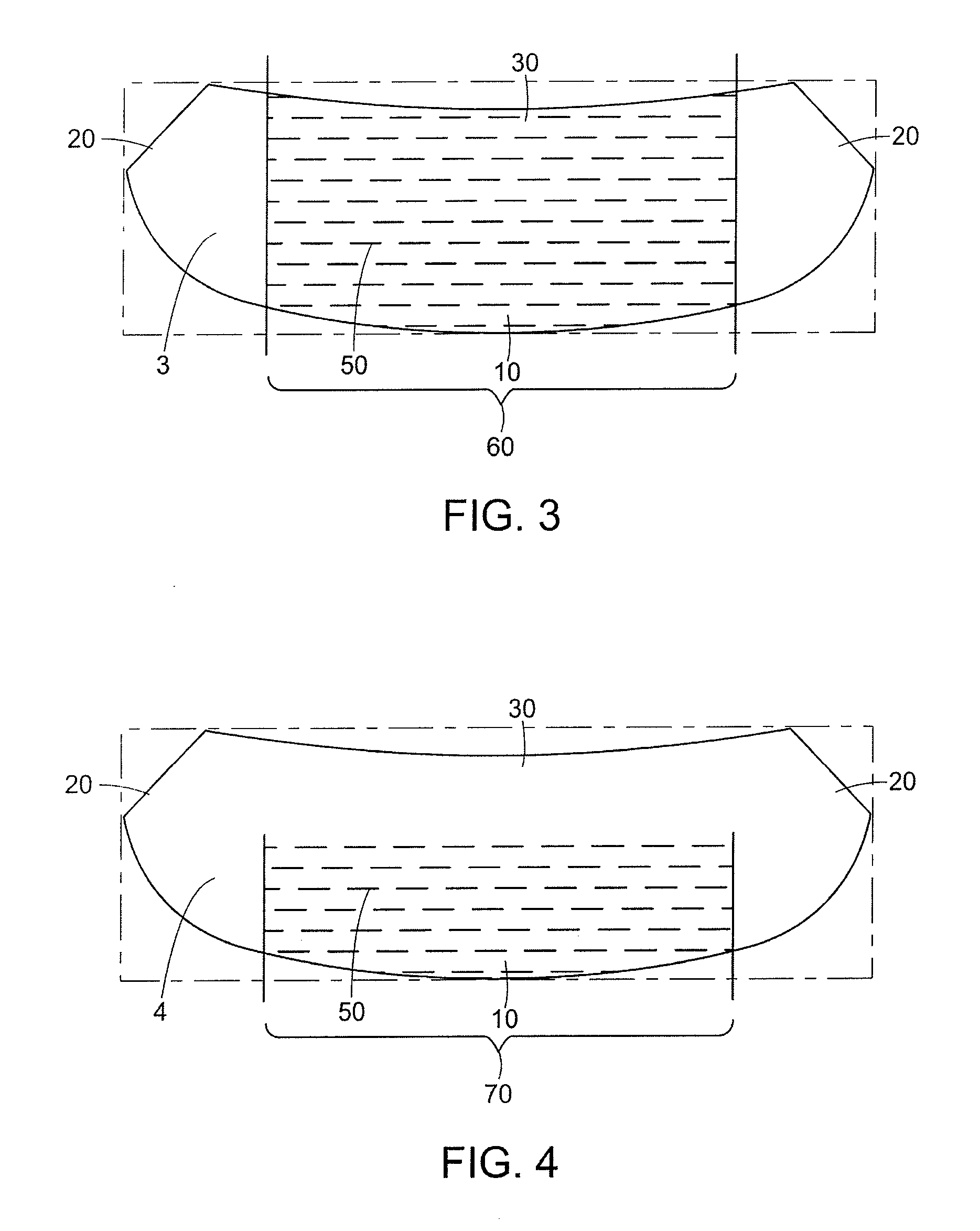
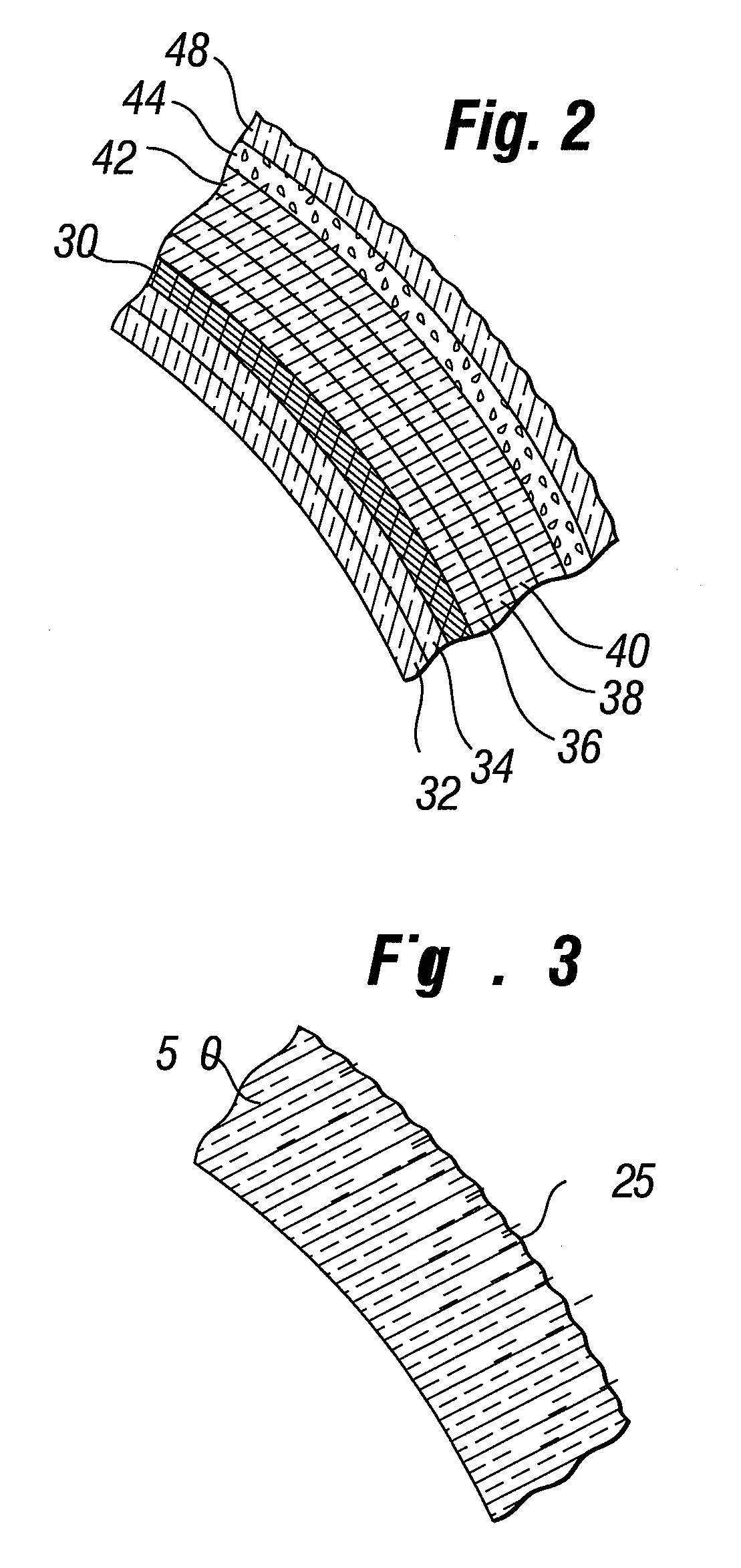
Naturally contoured, preformed, three dimensional mesh device for breast implant support
ActiveUS20090082864A1Easy to deployInherent disadvantageMammary implantsWound clampsWrinkle skinBreast implant
A preformed, seamless, three-dimensional, anatomically contoured prosthetic device for reinforcing breast tissue and supporting a breast implant includes a flat back wall, a concave front wall and a curved transitional region between the flat back wall and the front wall defining a smoothly curved bottom periphery. A concave receiving space is defined by the back wall and the front wall for at least partially receiving and supporting the breast implant therein. The three-dimensional prosthetic device is free of wrinkles, creases, folds or seams, which may have otherwise caused potential tissue irritation, bacteria hosting, infection and palpability problems.
Owner:ETHICON INC
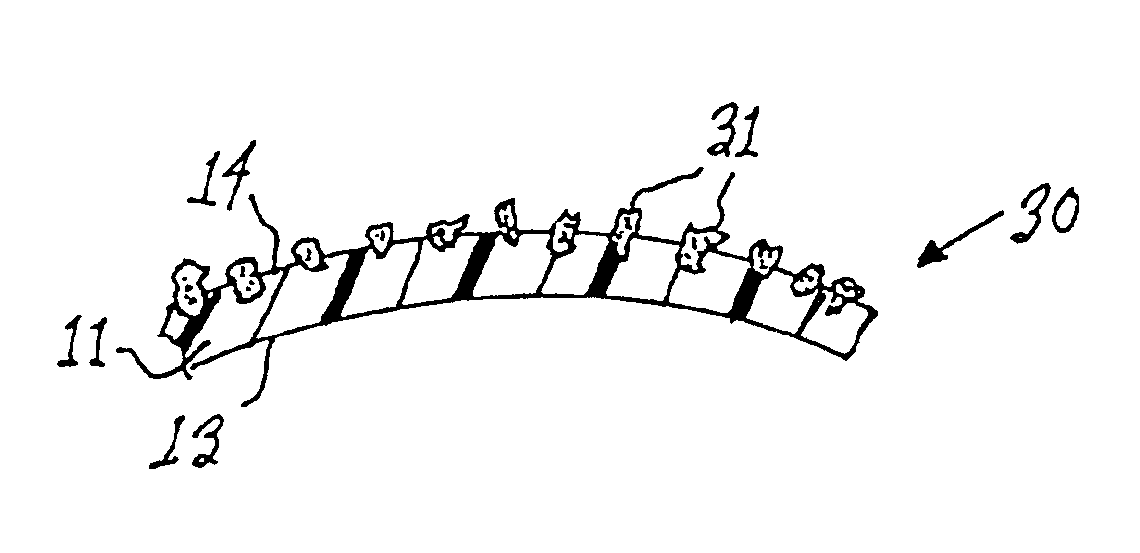
Mastopexy and Breast Reconstruction Prostheses and Method
InactiveUS20080097601A1Easy to handleResistant to biodegradationMammary implantsBandagesMastopexyCell-Extracellular Matrix
Mastopexy and breast reconstruction prostheses and implantation method that allow for radiographic imaging of the breast tissue. The prostheses are arcuate and elongate optionally meshed to conform with breast tissue when implanted. Prostheses are made from naturally occurring extracellular matrix, primarily collagen, that, allows for mammographic imaging without interference as is expected from synthetic materials.
Owner:ORGANOGENESIS
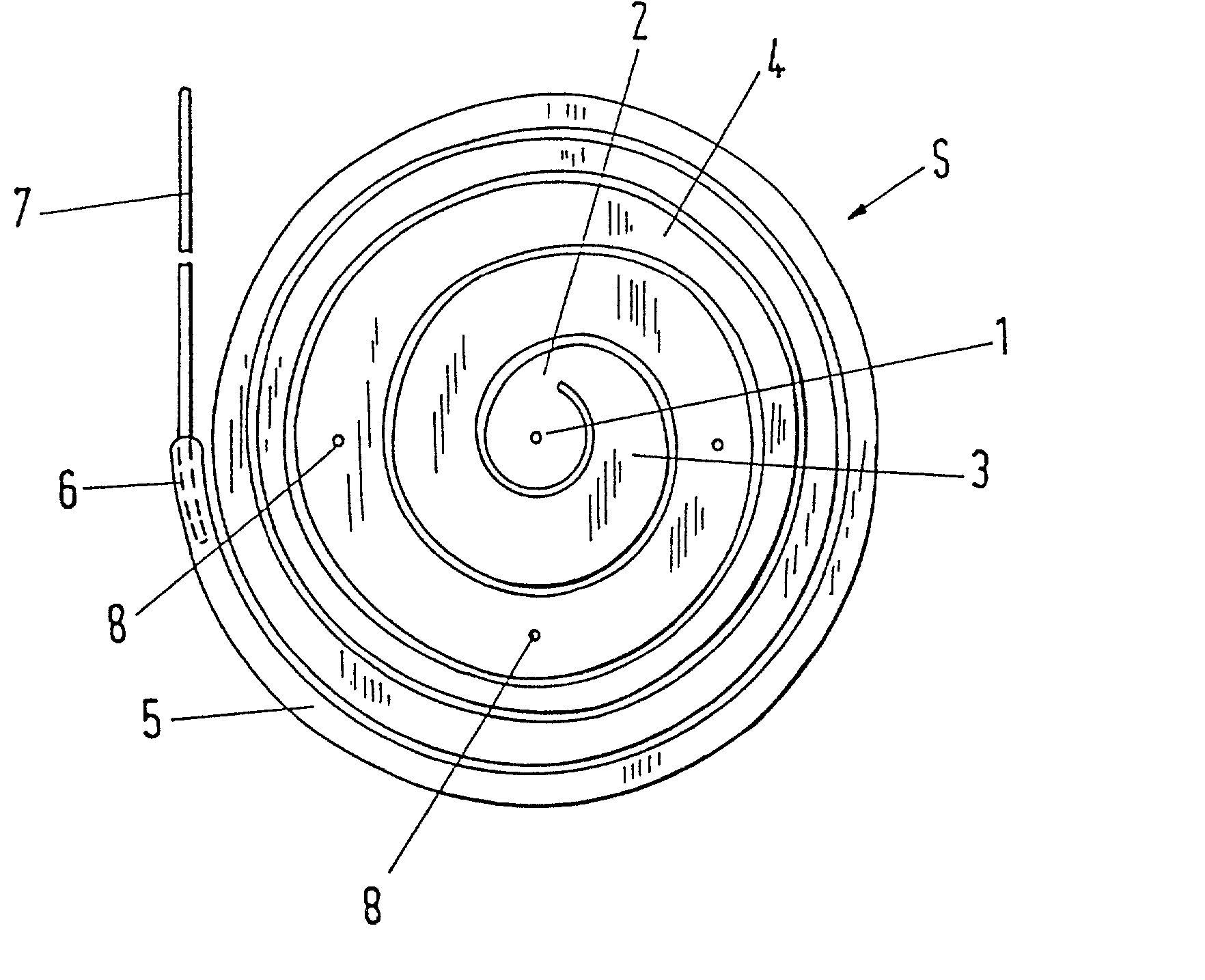
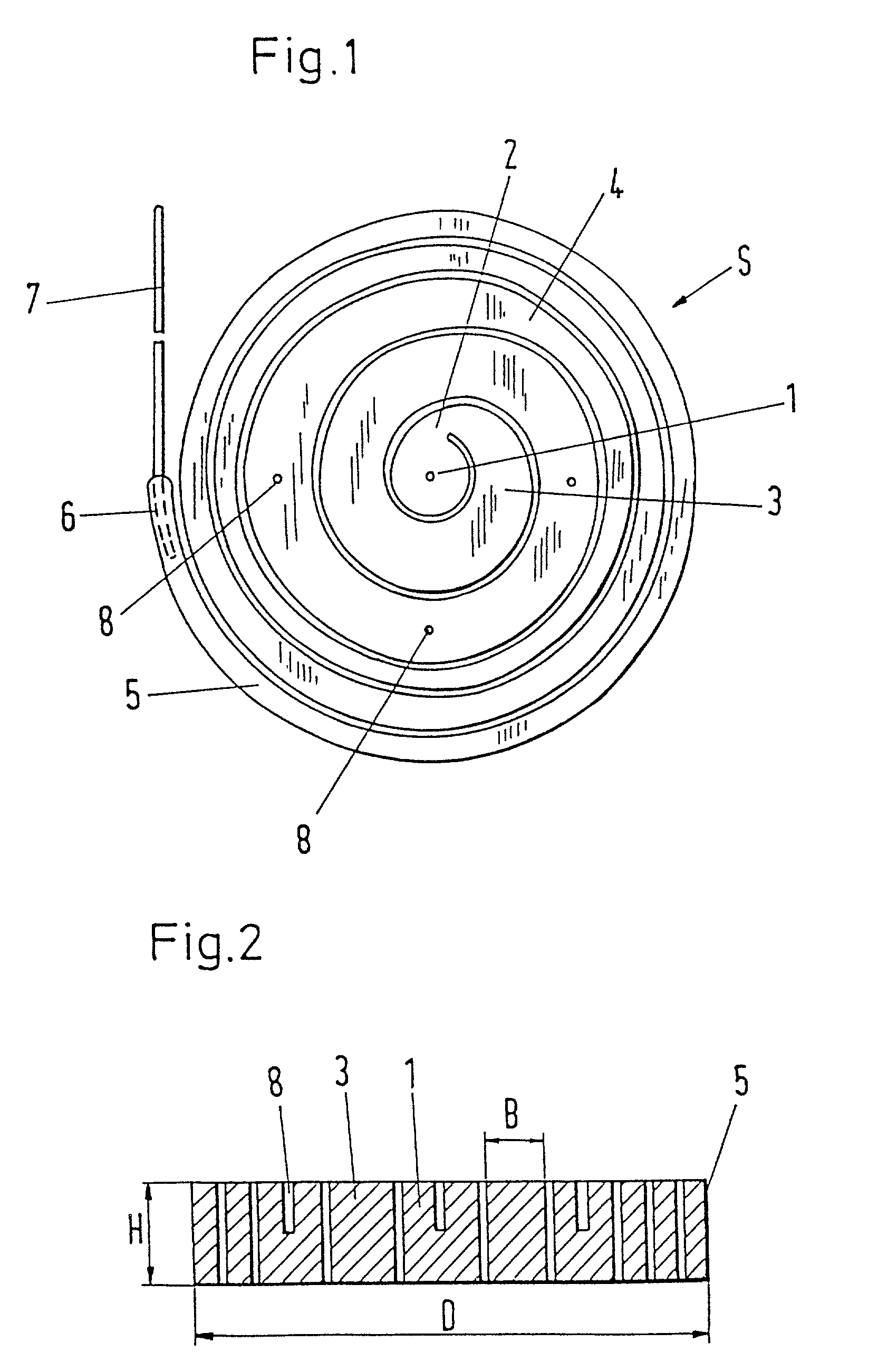
Intervertebral prosthesis
InactiveUS20030018390A1Evenly distributedMammary implantsSurgical scissorsInterior spaceIntervertebral disc
The invention deals with an implant, in particular, an intervertebral prosthesis, which consists of an elongated elastic body which is form-elastic and takes on the form of a spiral S in the force-free state. The spiral can be drawn by a reverse winding up into an insertion instrument which is only unsubstantially larger in the insertion region than the cross-section of the elongated elastic body in order to reach the inner space of an intervertebral disc through a small opening in the annulus fibrosus and to push in and sever off the self-winding spiral when the interior is filled. This has the advantage that inner spaces of differing sizes can be filled with the same spiral.
Owner:ZIMMER GMBH
Biodegradable, Polymer Coverings for Breast Implants
ActiveUS20080241212A1Inhibit and reduce formation of scar tissueInhibiting and reducingAntibacterial agentsBiocideBreast implantMedicine
A biodegradable, flexible covering for a breast implant is provided which comprises one or more biodegradable polymer layers dimensioned and shaped to cover at least a portion of the breast implant. The implant can be inserted into an opening of the covering immediately prior to surgery, but alternate configurations and times of insertion are contemplated as well as open or sheet type devices. The coverings can optionally contain one or more drugs for delivery at the surgical site, particularly for treating or preventing infection, pain, inflammation, capsular contracture, scarring or other complications associated with breast augmentation or breast reconstruction.
Owner:MEDTRONIC INC
Soft tissue implants and anti-scarring agents
InactiveUS20050142162A1Guaranteed functionImprove clinical outcomesPeptide/protein ingredientsAntipyreticChinBiomedical engineering
Soft tissue implants (e.g., breast, pectoral, chin, facial, lip, and nasal implants) are used in combination with an anti-scarring agent in order to inhibit scarring that may otherwise occur when the implant is placed within an animal.
Owner:ANGIOTECH INT AG (CH)
Medical implant having bioabsorbable textured surface
A hybrid medical implant having a biocompatible, nonabsorbable core portion and a bioabsorbable textured outer surface portion overlying the core portion. The hybrid implant is useful as a prosthesis for tissue augmentation and / or reconstruction. The core portion of the implant includes a body formed from a nonabsorbable, biocompatible implantable material such as silicone or urethane elastomer. The core portion may be either a solid body, a viscous gel body or a fluid-filled shell. The textured outer surface portion envelops the core portion and presents an irregular, bioabsorbable textured surface to the exterior environment. As a capsule forms around the implant following implantation, the irregular contour of the outer surface of the implant disorients structural proteins in the capsule to impede spherical contraction thereof. Either during the formation of the capsule and / or after the capsule is formed, the outer bioabsorbable surface portion of the implant is absorbed by the body of the host. After bioabsorbtion of the bioabsorbable outer surface portion, the remaining core portion of the implant remains enveloped by the capsule but unattached to capsular tissue. The outer bioabsorbable portion of the hybrid implant may include more than one biocompatible, bioabsorbable material.
Owner:WHD ELEKTRONISCHE PRUEFTECHN
Bioabsorbable breast implant
InactiveUS6881226B2Reduce scarsOvercome deficienciesMammary implantsPharmaceutical delivery mechanismBreast implantBiomedical engineering
A breast implant has at least an outer shell which is composed of a resorbable material. The implant, which can be formed entirely of bioresorbable material such as a collagen foam, is sized and shaped to replace excised tissue. The implant supports surrounding tissue upon implantation, while allowing for in-growth of fibrous tissue to replace the implant. According to various alternative embodiments, the implant is elastically compressible, or can be formed from self-expanding foam or sponges, and can be implanted through a cannula or by injection, as well as by open procedures. The implant can carry therapeutic and diagnostic substances.
Owner:SENORX
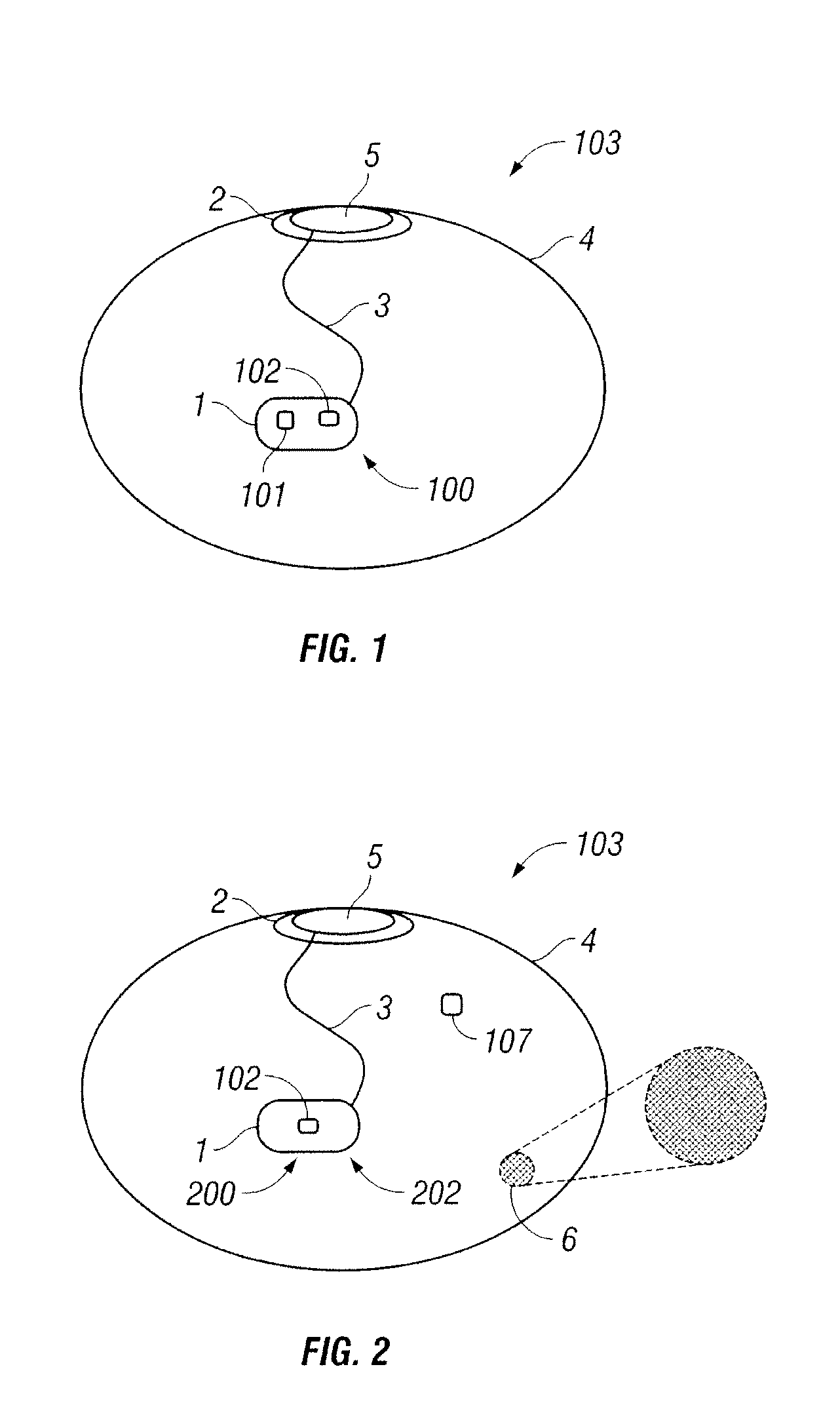
Interfaced Medical Implant Assembly
ActiveUS20090125107A1Reduce and eliminate capsular contracturePromote formationMammary implantsTissue regenerationBreast prosthesesAppendage
A medical implant assembly and method having a medical implant, e.g. a breast prostheses, attached to a biological interface. The biological interface is comprised of a dermal material with capsular contracture inhibiting properties so that once the medical assembly is inserted into the host, the biological interface, which is intimately coupled to the implant, prevents / reduces capsular contracture formation around the implant. The biological interface comprises a plurality of apertures along its periphery, and attaches to the medical implant by receiving a plurality of attachment flaps or appendages located on the exterior surface of the medical implant within or through the apertures. The attachment of the biological interface is such that the assembly remains intact even where the attachment flaps loosen upon expansion of the implant after insertion into a host, as where the implant is therein injected to a desired dimension.
Owner:MAXWELL G PATRICK
Implantable sensors and implantable pumps and anti-scarring agents
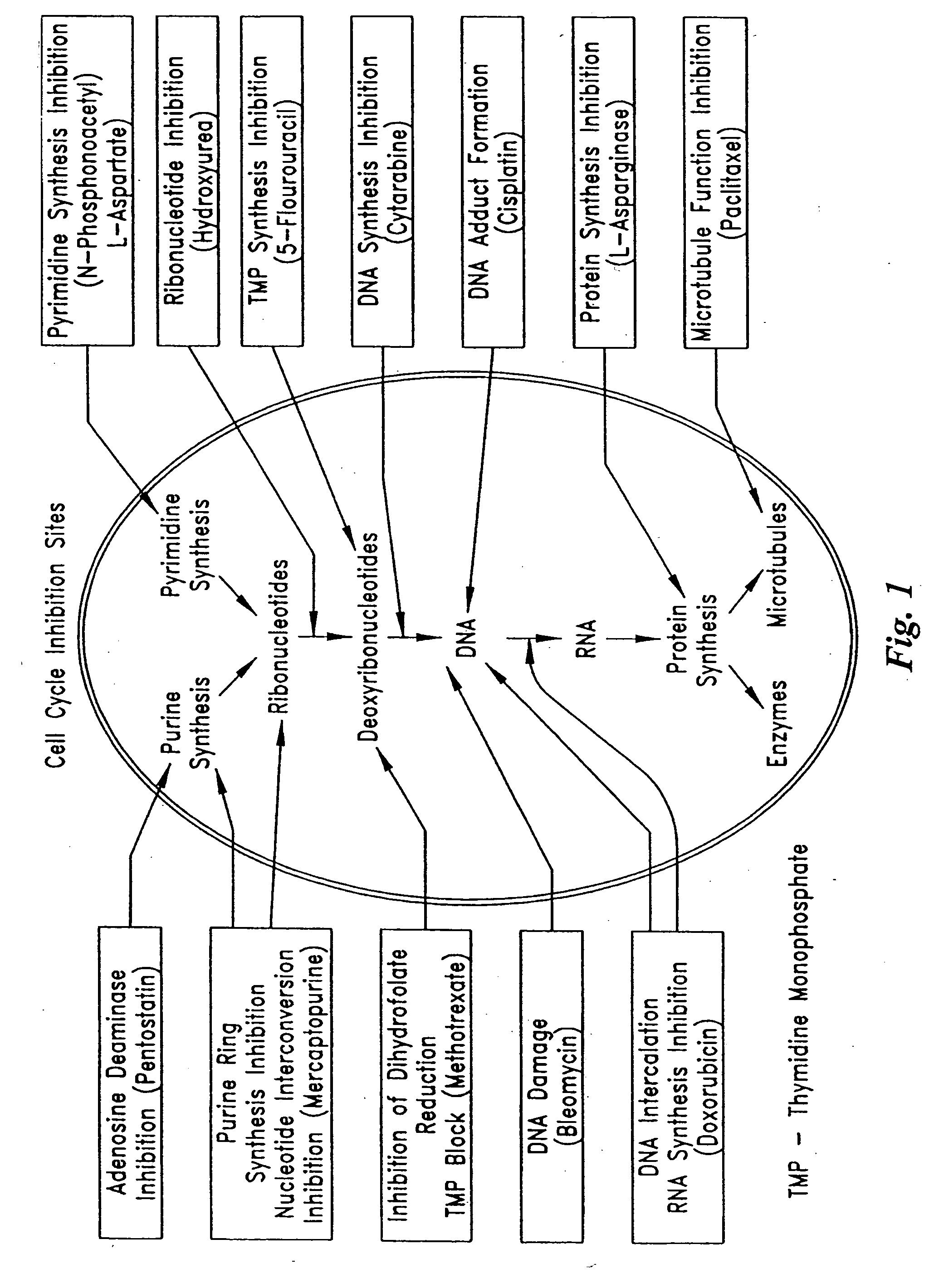
InactiveUS20050154374A1Function increaseImprove clinical outcomesPeptide/protein ingredientsAntipyreticMedicineCell Cycle Inhibition
Pumps and sensors for contact with tissue are used in combination with an anti-scarring agent (e.g., a cell cycle inhibitor) in order to inhibit scarring that may otherwise occur when the pumps and sensors are implanted within an animal.
Owner:ANGIOTECH INT AG (CH)
Silk Fibroin Hydrogels and Uses Thereof
InactiveUS20110008406A1Increase profitGood biocompatibilityBiocideCosmetic preparationsDiseaseFibronectin binding
The present specification provides for methods for purifying fibroins, purified fibroins, methods of conjugating biological and synthetic molecules to fibroins, fibroins conjugated to such molecules, methods of making fibroin hydrogels, fibroin hydrogels and fibroin hydrogel formulations useful for a variety of medical uses, including, without limitation uses as bulking agents, tissue space fillers, templates for tissue reconstruction or regeneration, cell culture scaffolds for tissue engineering and for disease models, surface coating to improve medical device function, or drug delivery devices.
Owner:ALLERGAN INC
Silk Fibroin Hydrogels and Uses Thereof
InactiveUS20110008437A1Increase profitGood biocompatibilityPowder deliveryCosmetic preparationsDiseaseFibronectin binding
The present specification provides for methods for purifying fibroins, purified fibroins, methods of conjugating biological and synthetic molecules to fibroins, fibroins conjugated to such molecules, methods of making fibroin hydrogels, fibroin hydrogels and fibroin hydrogel formulations useful for a variety of medical uses, including, without limitation uses as bulking agents, tissue space fillers, templates for tissue reconstruction or regeneration, cell culture scaffolds for tissue engineering and for disease models, surface coating to improve medical device function, or drug delivery devices.
Owner:ALLERGAN INC
External sensing for implant rupture
InactiveUS20090012372A1The process is convenient and fastMammary implantsEndoradiosondesBreast implantOptical property
The present invention relates to a system and a method for sensing for the rupture of an implant (such as a breast implant) that has been implanted in body tissues or in an organ of a patient. In one embodiment, a system according to the present invention includes, among other possible things, a sensor coupled to an outer surface of the implant and configured to measure a property at the outer surface of the implant, for example, electrical conduction, chemical composition, or an optical property that is indicative of whether an implant rupture has occurred. The sensor is also configured to transmit a wireless signal to a device external to the body, which alerts the patient or a healthcare provider whether the measured property indicates that the implant rupture may have occurred.
Owner:NOVALERT
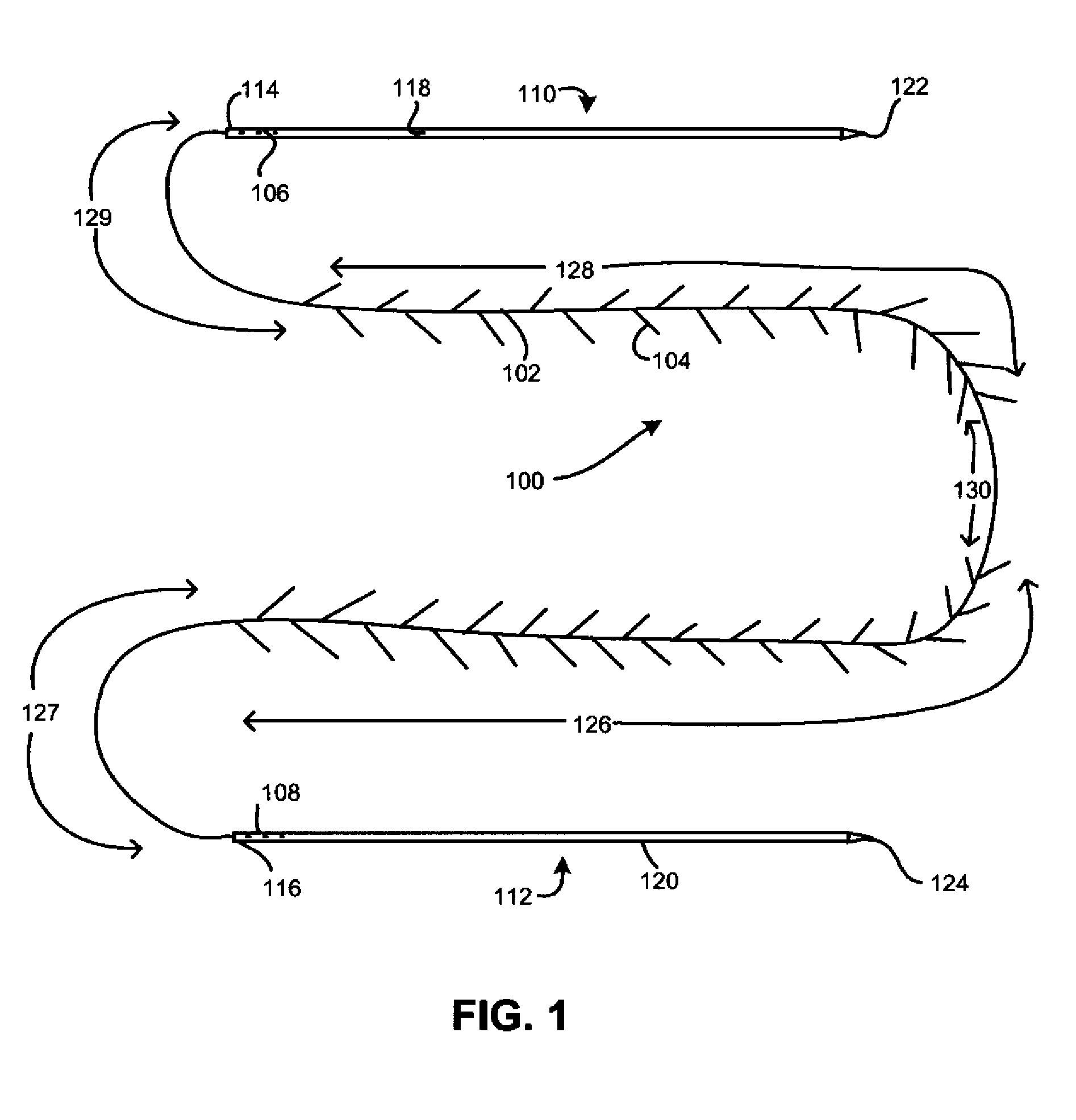
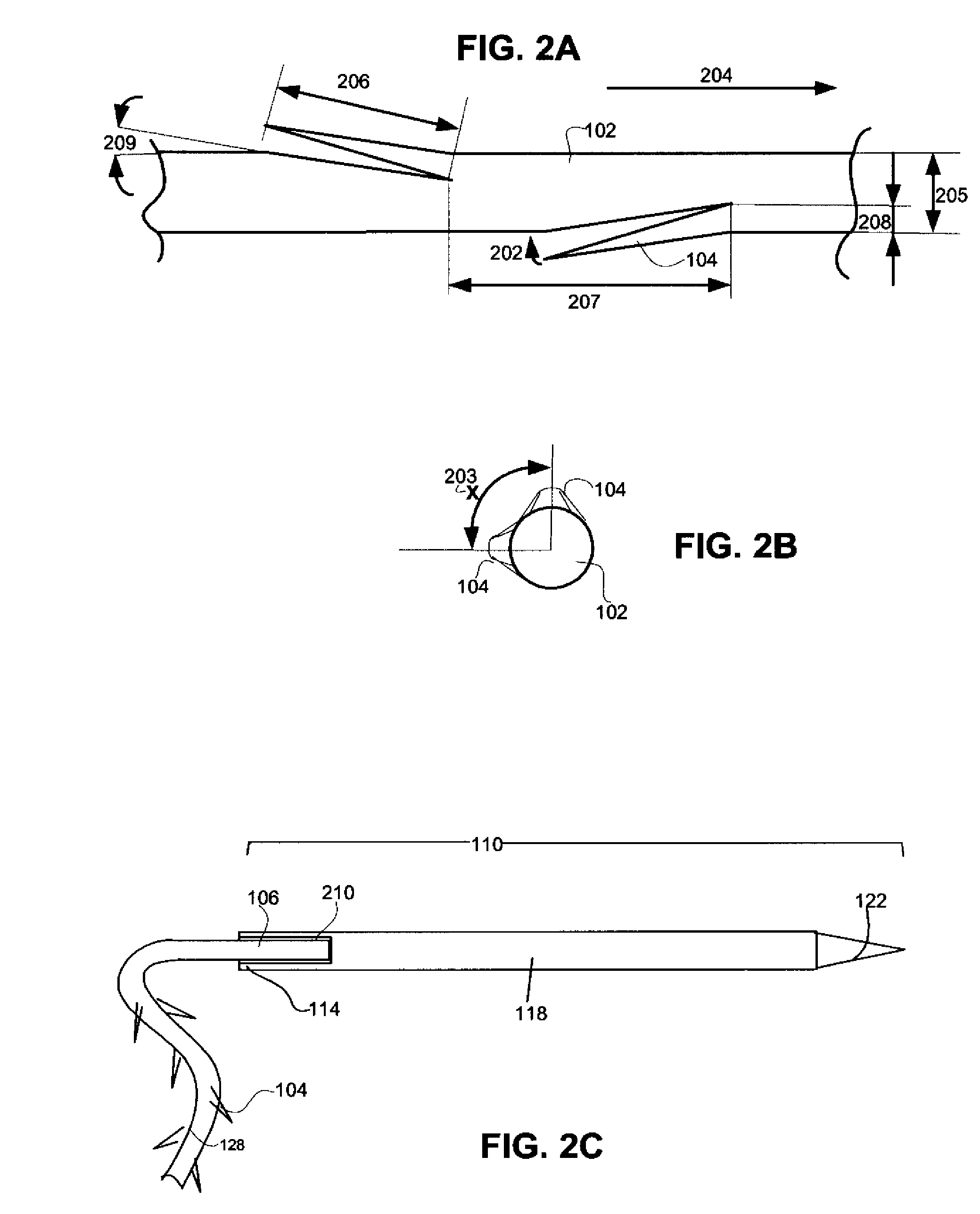
Minimally-invasive nipple-lift procedure and apparatus
Medical devices and methods are provided for a minimally-invasive mastoplasty procedure. In the procedure, barbed sutures are used to accomplish a nipple-lift by deploying the sutures cranially from the nipple-areolar complex to stable anatomical features higher on the chest. Additional barbed sutures may be used to accomplish a breast-lift and / or breast contouring by deploying the sutures caudally from stable anatomical features into the breast tissue.
Owner:ETHICON INC
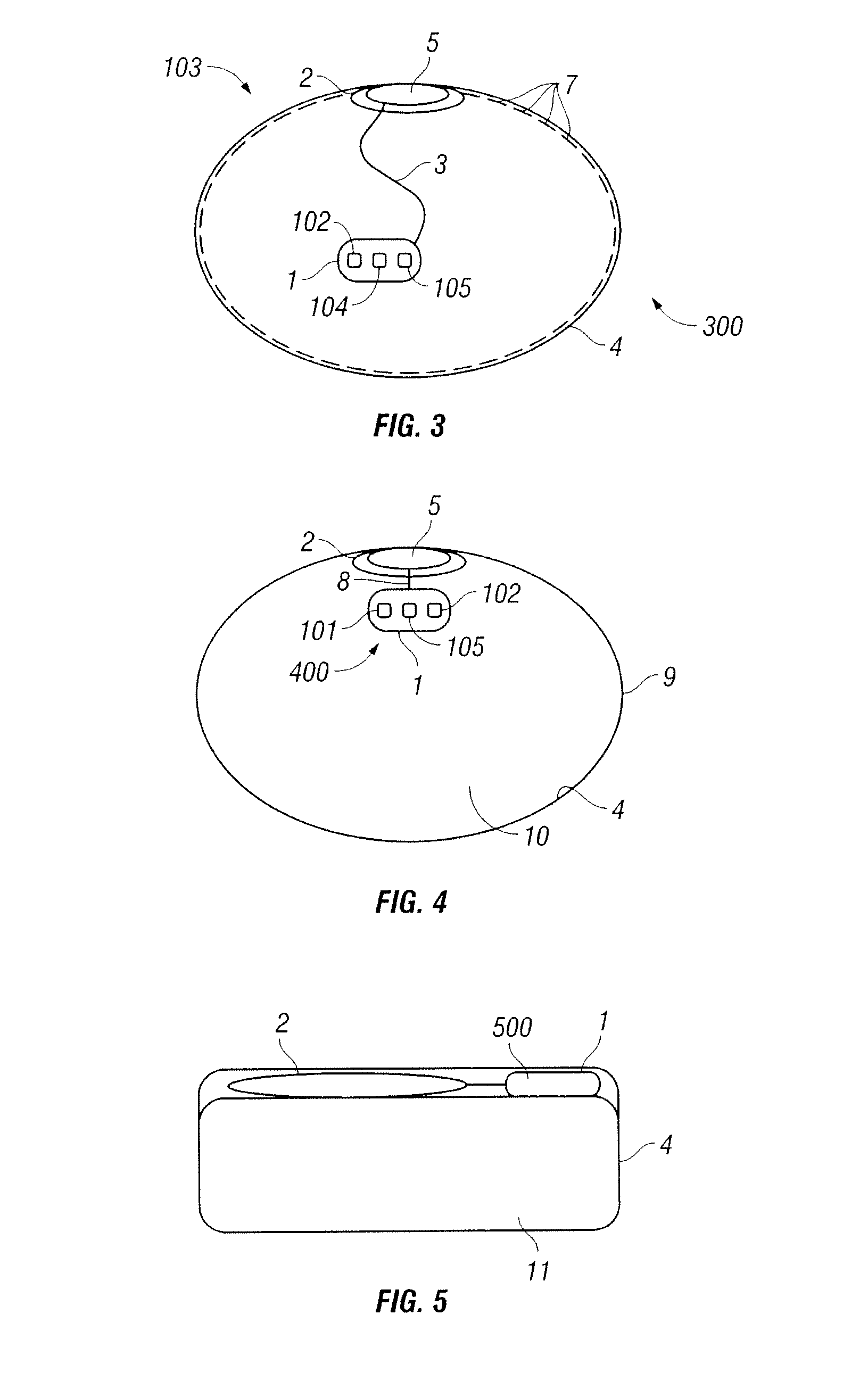
Implantable prosthesis for positioning and supporting a breast implant
ActiveUS20070088434A1Promote growthFixed securityMammary implantsInferior displacementBiomedical engineering
An implantable prosthesis for use in positioning a breast implant comprising a sheet of a prosthetic material configured to form a sling-shaped receiving area for receiving and supporting the breast implant. The surface area of the implantable prosthesis contacting the breast implant comprises a biocompatible or chemically inert material to prevent abrasion of or reaction with the breast implant. The implantable prosthesis of the present invention can be used during corrective procedures to reposition and support a malpositioned breast implant or during reconstructive or cosmetic procedures at the time the implant is first positioned within the patient. The prosthesis is used with implants in the partial sub-muscular, completely sub-muscular, and sub-glandular position and is used to prevent medial, lateral and inferior displacement of the implant.
Owner:LIFECELL
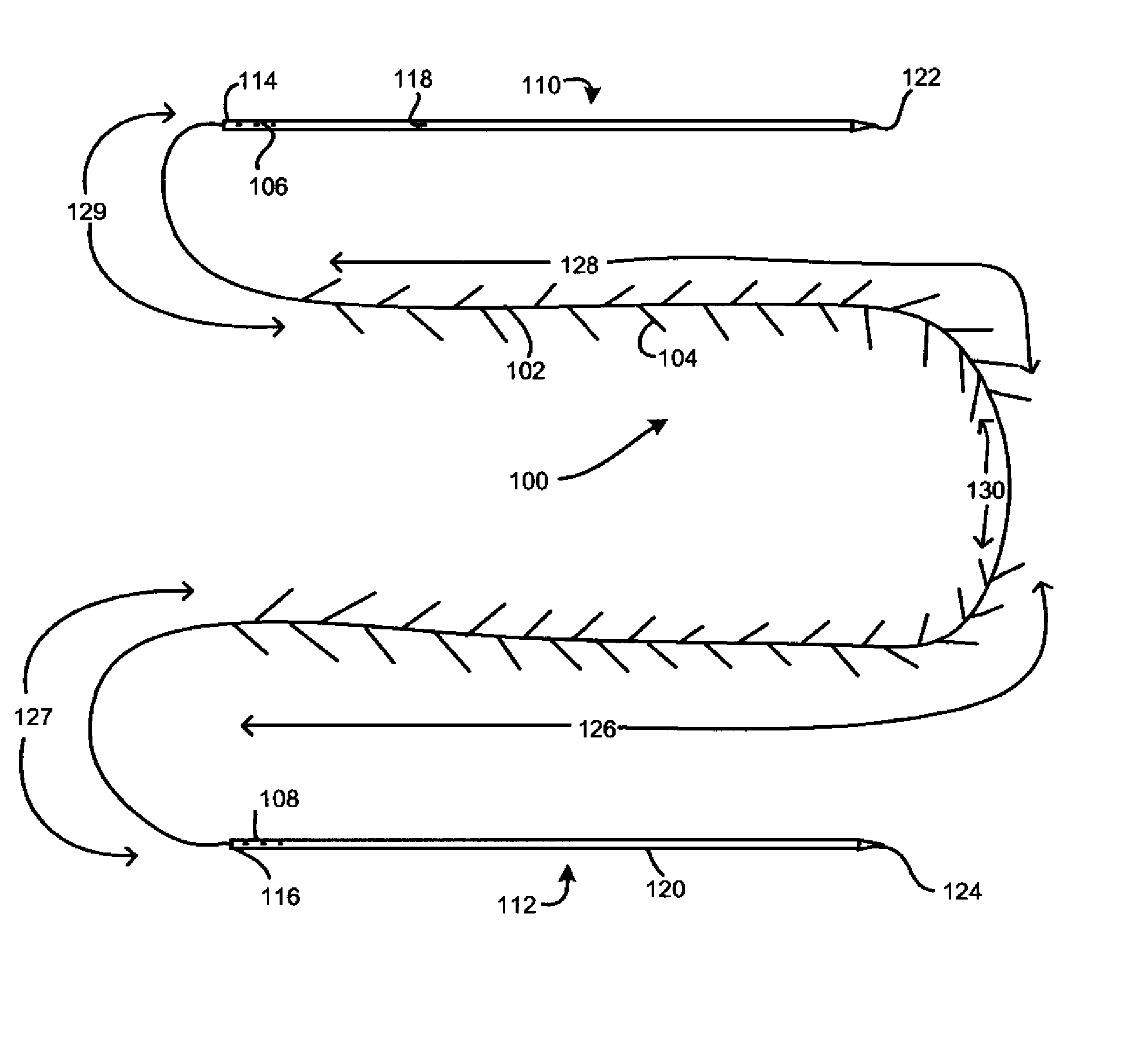
Tissue augmentation device
InactiveUS20060161253A1Flatten nasolabial foldEnhance lipMammary implantsCosmetic implantsTissue augmentationVALVE PORT
Disclosed are implantable tissue augmentation devices, methods, and associated tools. The devices include an inflatable body, having an inner layer and an outer layer. A valve is provided for permitting the introduction of and retaining inflation media. At least one pull tab is provided on an end of the implant, to assist in positioning the implant. Kits and systems are also disclosed.
Owner:EVERA MEDICAL
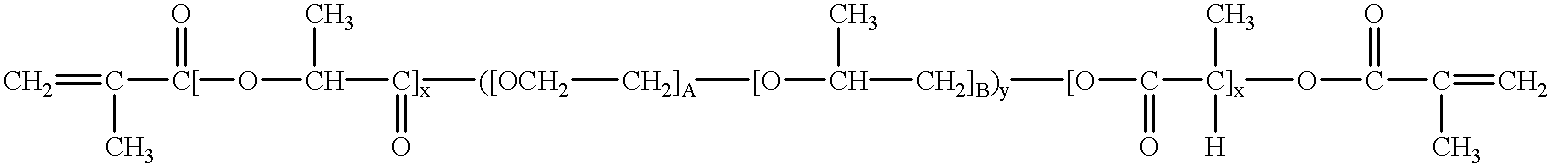
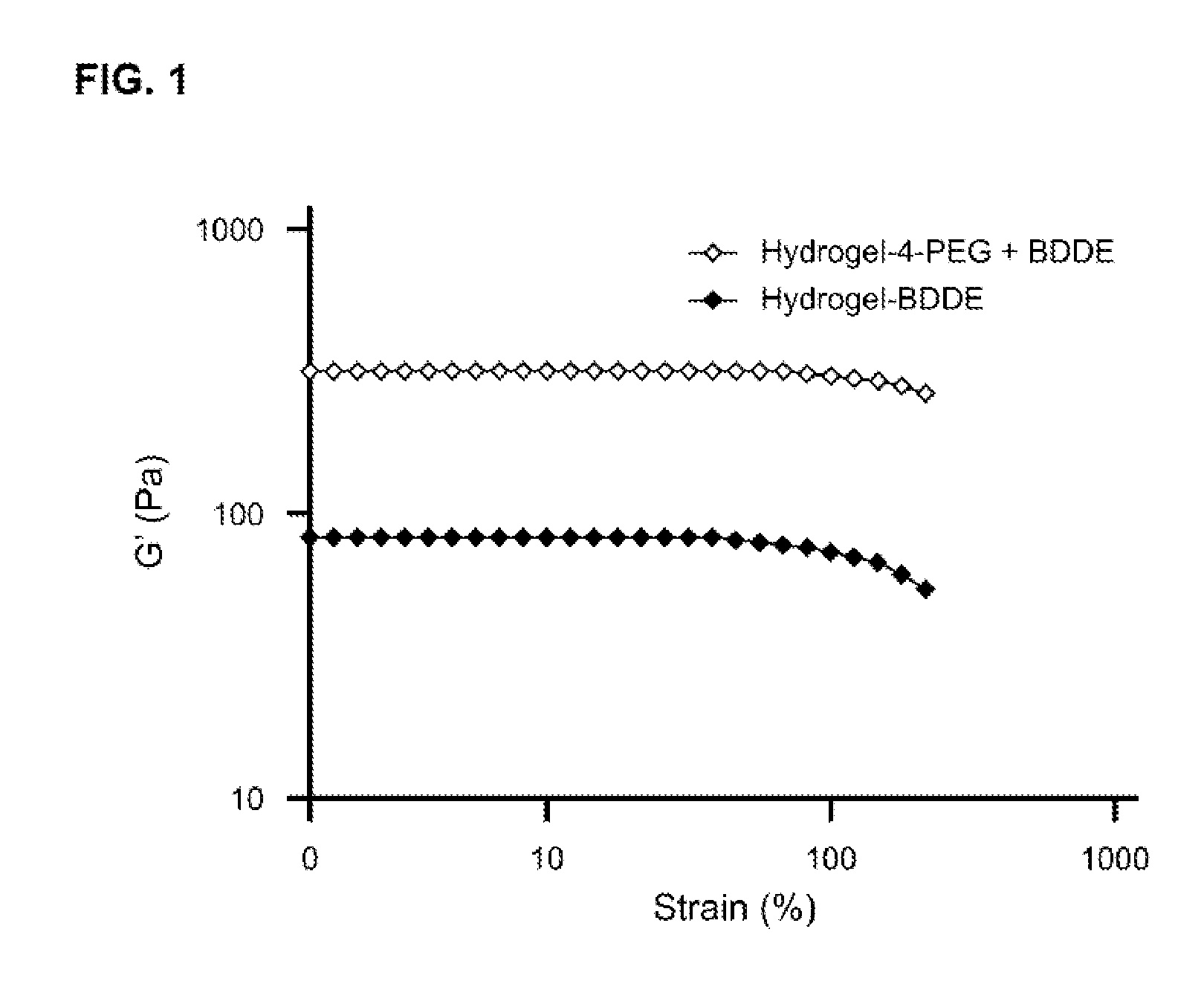
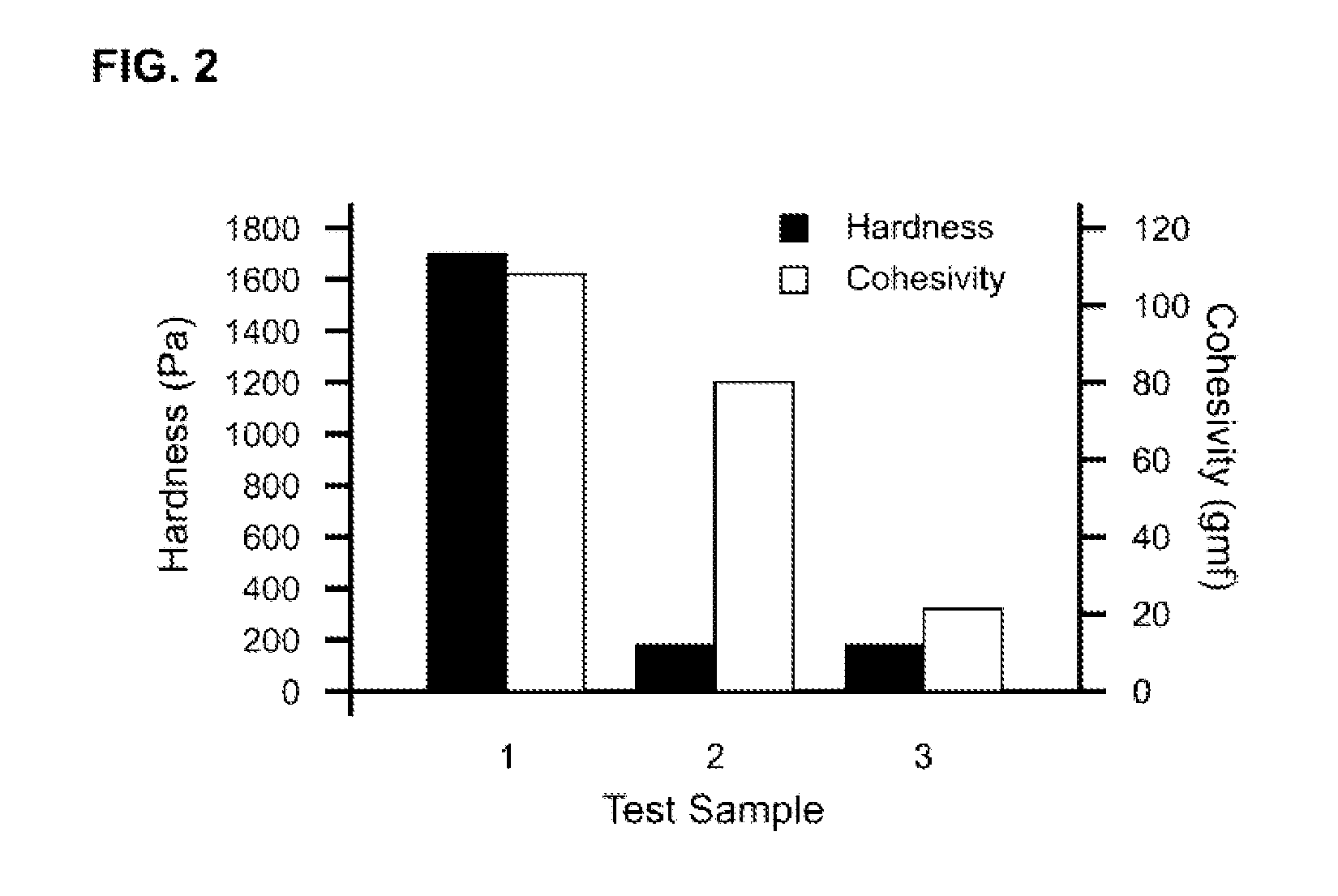
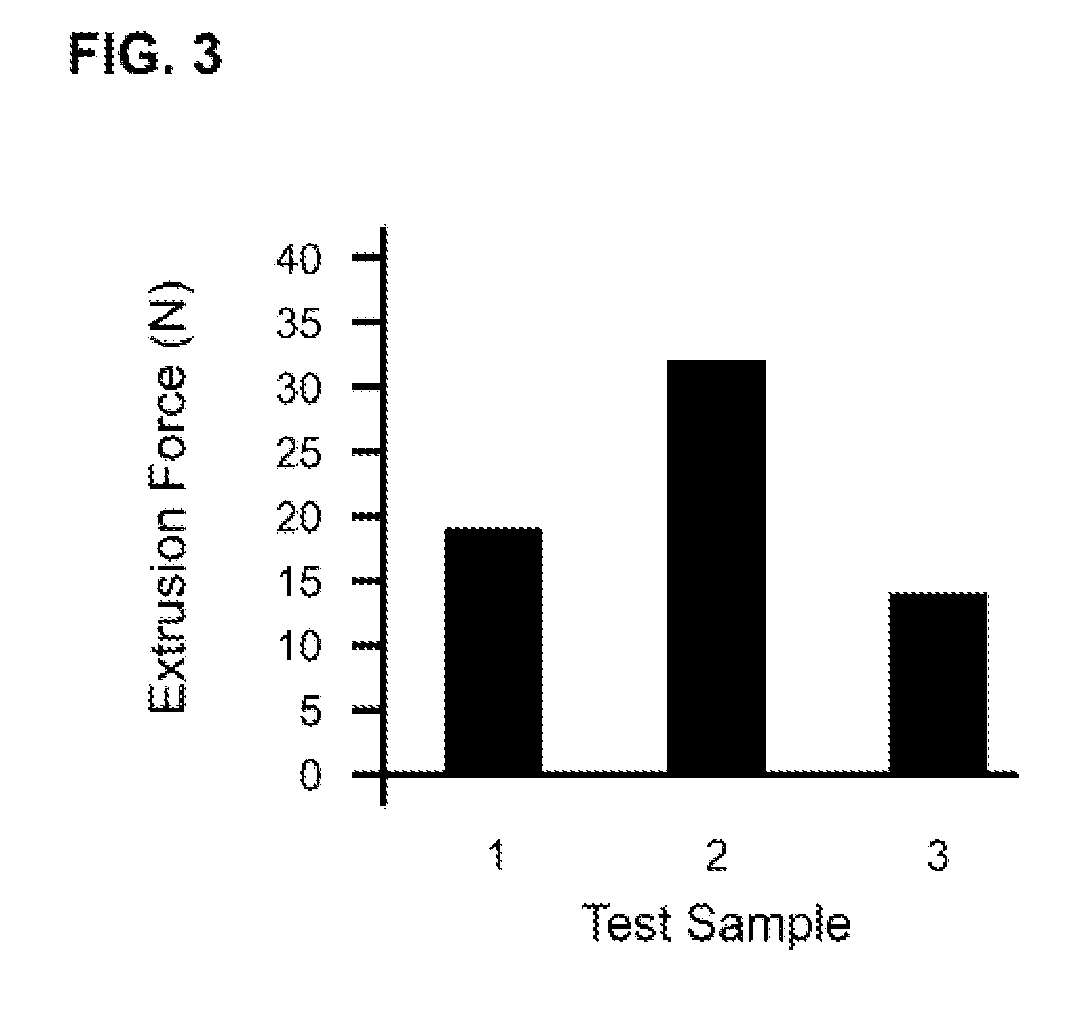
Tunably Crosslinked Polysaccharide Compositions
The present specification generally relates to multifunctional polyethylene glycol-based crosslinking agents, hydrogel compositions comprising a matrix polymer crosslinked with such crosslinking agents, and methods of treating a soft tissue condition using such hydrogel compositions.
Owner:ALLERGAN INC
Soft prosthesis shell texturing method
A method of texturing a soft prosthetic implant shell, such as a silicone breast implant shell. A soft prosthetic implant with a textured external surface layer of silicone elastomer and having an open-cell structure is made by adhering and then dissolving round salt crystals. The resulting roughened surface has enhanced physical properties relative to surfaces formed with angular salt crystals. An implant having such a textured external surface layer is expected to help prevent capsular contraction, to help prevent scar formation, and to help in anchoring the implant within the body.
Owner:ALLERGAN INC
Naturally contoured, preformed, three dimensional mesh device for breast implant support
ActiveUS7875074B2Shorten the construction periodOvercomes inherent disadvantageMammary implantsWound clampsWrinkle skinBreast implant
A preformed, seamless, three-dimensional, anatomically contoured prosthetic device for reinforcing breast tissue and supporting a breast implant includes a flat back wall, a concave front wall and a curved transitional region between the flat back wall and the front wall defining a smoothly curved bottom periphery. A concave receiving space is defined by the back wall and the front wall for at least partially receiving and supporting the breast implant therein. The three-dimensional prosthetic device is free of wrinkles, creases, folds or seams, which may have otherwise caused potential tissue irritation, bacteria hosting, infection and palpability problems.
Owner:ETHICON INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com