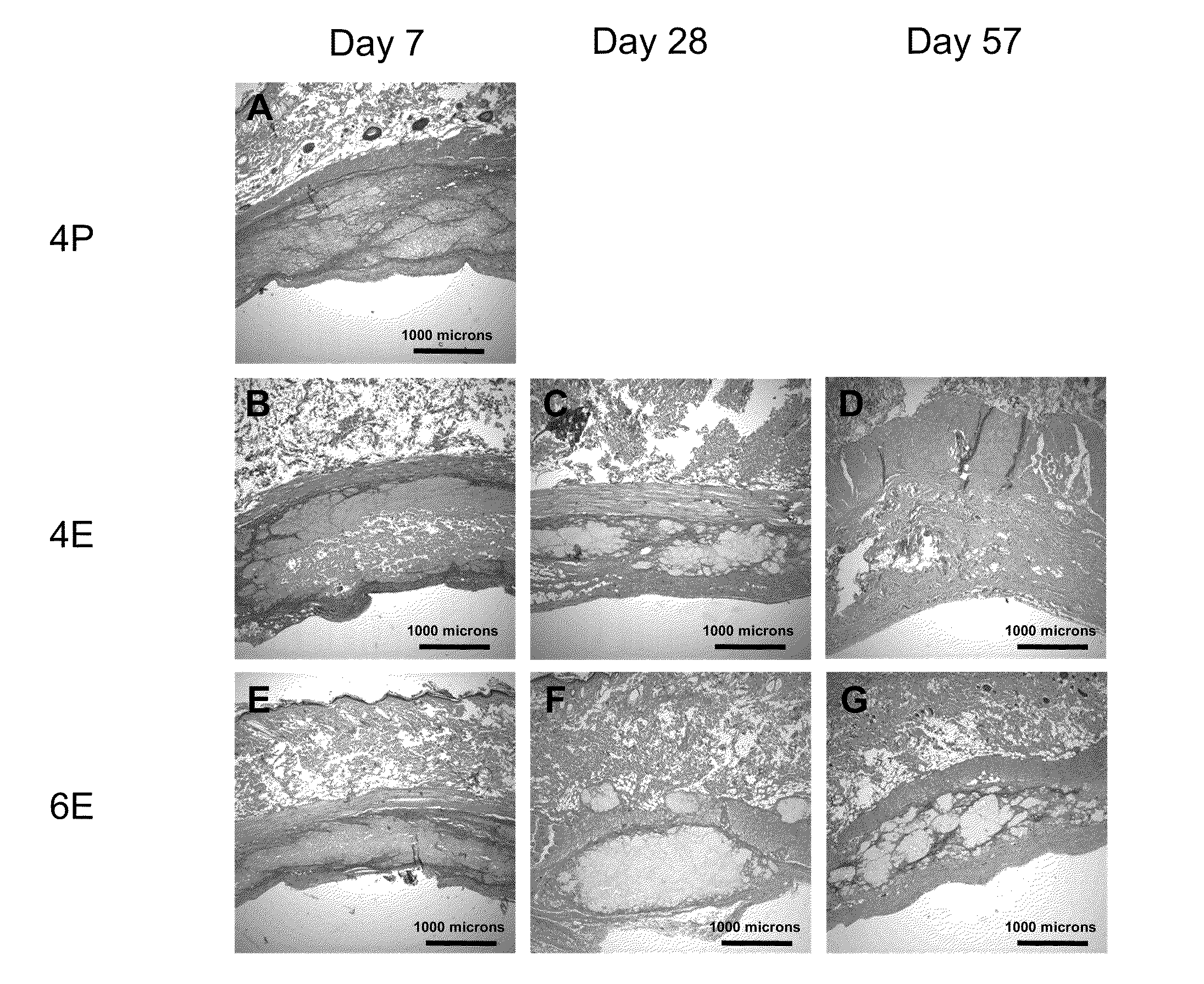
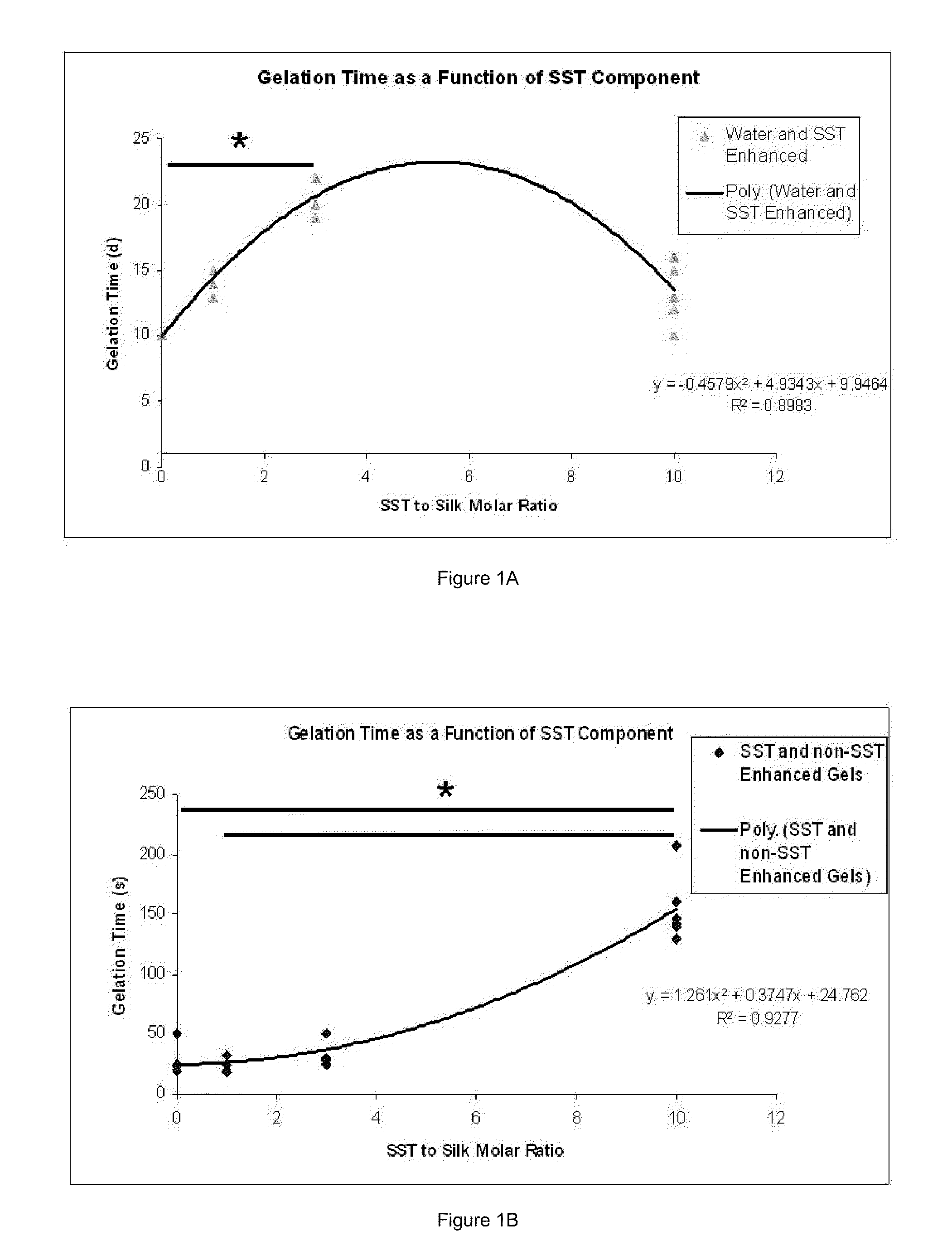
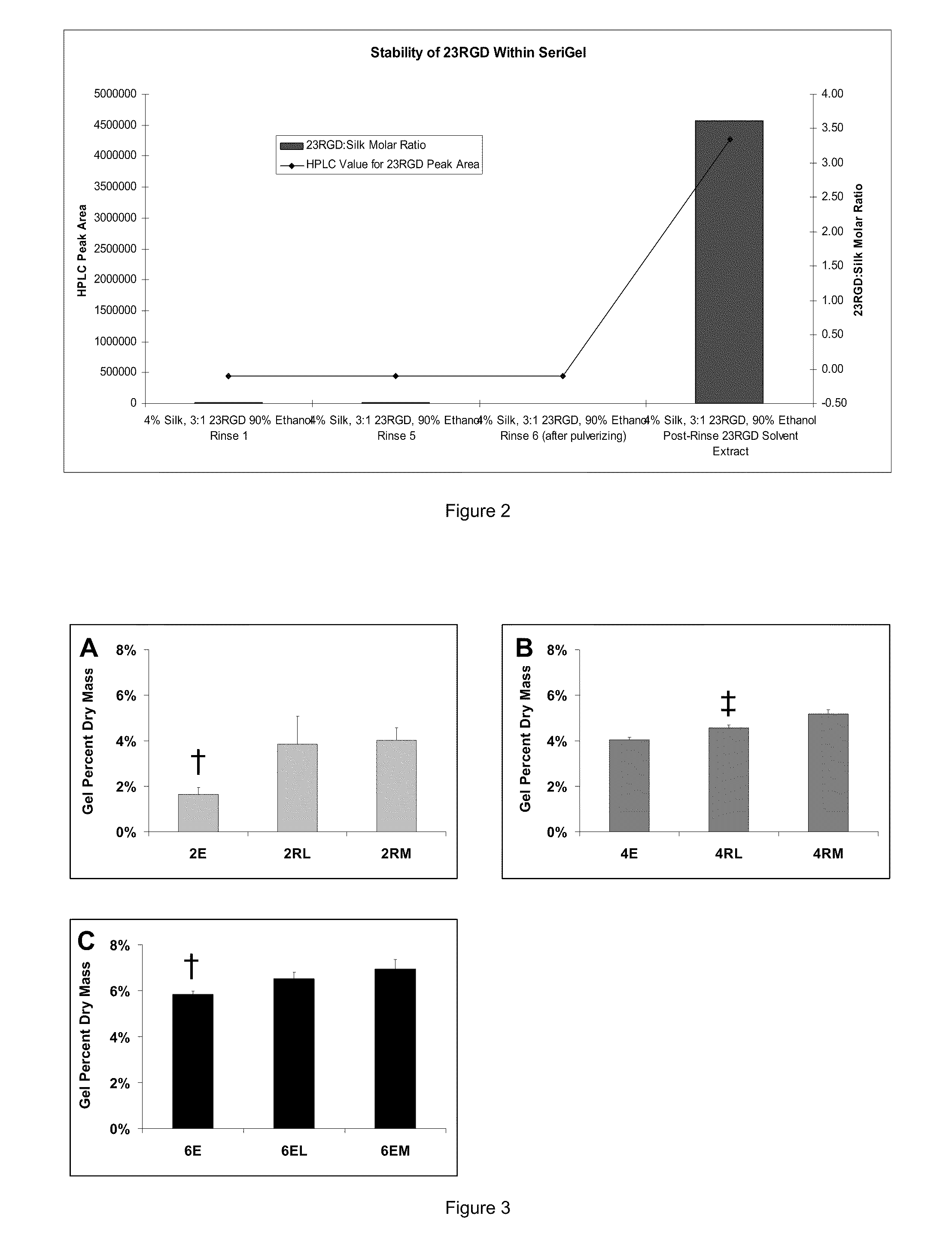
Silk Fibroin Hydrogels and Uses Thereof
a technology of silk fibroblasts and hydrogels, which is applied in the field of silk fibroblast hydrogels, can solve the problems of limiting the capacity of native host cells to bind to and interact with implanted silk devices, adverse tissue reactions, and heightened immune responses, so as to improve the utility and biocompatibility of silk formulations, improve the utility and function of silk formulations, and improve the function of medical devices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Silk Sericin Extraction
[0236]Silk fibroin for generation of the hydrogel was obtained in the form of degummed B. mori silk at a size of 20 denier-22 denier (38 μm±5.6 μm diameter). This degummed silk was further processed in order to remove the inherently present and potentially antigenic protein glue, sericin that conjoins independent fibroin filaments. This was done as described previously herein. Following removal of sericin, the pure fibroin was dried carefully to ambient humidity levels using a laminar flow hood.
example 2
Generation of Silk Fibroin Solution
[0237]Silk fibroin filaments, cleaned of their sericin and rinsed free of insoluble debris and ionic contaminants were used for the generation of an aqueous silk solution. These silk fibers were added to a solution of 9.3M LiBr and purified water (e.g., MILLI-Q® Ultrapure Water Purification Systems) (Millipore, Billerica, Mass.) to make a solution consisting of 20% pure silk (% w / v). This mixture was then heated to a temperature of 60° C. and digested for a period of four hours. A total of 12 mL of the resultant solution was then loaded into a 3 mL-12 mL Slide-A-Lyzer dialysis cassette (Pierce Biotechnology, Inc., Rockford, Ill.) (molecular weight cutoff of 3.5 kD) and placed into a beaker containing purified water as a dialysis buffer at a volume of 1 L water per 12 mL cassette of silk solution. The beakers were placed on stir plates and stirred continuously for the duration of the dialysis. Changes of dialysis buffer occurred at 1, 4, 12, 24, and...
example 3
Induction of Gelation
[0239]A variety of different methods were employed in the course of hydrogel development for the purposes of contrasting and comparing certain relevant properties of various formulae. Regardless of the nature in which the gelation process was carried out, the final determination that a “gel” state had been reached was applied uniformly to all groups. A solution or composite of solutions (i.e., silk solution blended with an enhancer or enhancer solution) was considered a gel after observing formation of a uniform solid phase throughout the entire volume, generally opaque and white in appearance.
[0240]Samples to be produced by passive gelation were not exposed to any enhancer additives. These gels were produced by measuring a volume of silk solution into a casting vessel, for the purposes of these experiments, polypropylene tubes sealed against air penetration and water loss, and the sample allowed to stand under ambient room conditions (nominally 20-24° C., 1 atm...
PUM
| Property | Measurement | Unit |
|---|---|---|
| cross-sectional area | aaaaa | aaaaa |
| cross-sectional area | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com